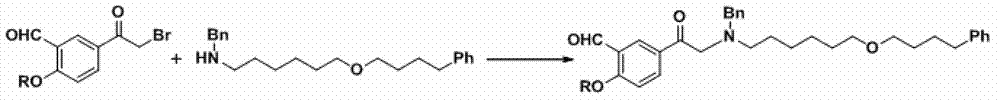
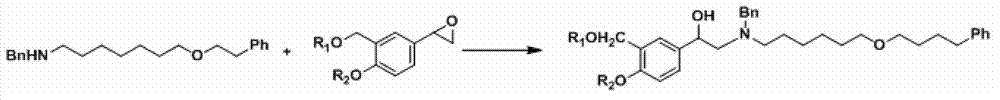
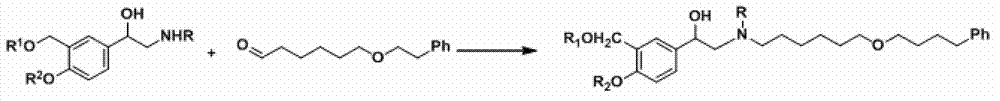
Salmeterol preparation method
A technology for preparation steps and compounds, applied in the preparation of carboxylates, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of excessive use of protective groups, large losses, long reaction steps, etc., to avoid high vacuum or Column chromatographic separation, lower raw material cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: a kind of method for preparing salmeterol, it is characterized in that concrete preparation steps are as follows:
[0039] (1) Alkylation reaction: Control the temperature at 25±5°C, add 823.5kg (5mL / g) of tetrahydrofuran, 132.1kg (1Kmol, 1.0equiv.) of dimethyl malonate and 60.0kg of sodium hydride into a 2000L reactor (60%, 1.5Kmol, 1.5 equiv.). Stir the system for 1-2 hours, slowly add 185.0kg (1Kmol, 1.0equiv.) 2-bromoethylbenzene dropwise and keep the system temperature at 25±5°C (if necessary, add an external bath to cool down) until the reaction is complete. Press the system into 1520.0 kg of saturated ammonium chloride aqueous solution (8 mL / g) to terminate the reaction. Let stand, separate the liquid, concentrate the organic phase, and directly use it in the next step.
[0040] (2) Decarboxylation reaction: control the temperature at 25±5°C, add DMSO1300kg (10mL / g) and purified water 31.5kg (1.75kmol, 3.5equiv.) 118.1kg of methyl 2-phenylethylmal...
Embodiment 2
[0047] Embodiment 2: a kind of method for preparing salmeterol, it is characterized in that concrete preparation steps are as follows:
[0048] (1) Alkylation reaction: Control the temperature at 25±5°C, add 1.65kg of tetrahydrofuran (10mL / g), 192.2g of diethyl malonate (1.2mol, 1.2equiv.) and tert-butanol into a 10L reactor Sodium 115g (1.2mol, 1.2equiv.). Stir the system for 1-2 hours, slowly add 184.0g (1mol, 1.0equiv.) 2-bromoethylbenzene (compound 2) dropwise and keep the system temperature at 25±5°C (if necessary, add an external bath to cool down) until the reaction is complete. The system was pressed into 1.9 kg of saturated ammonium chloride aqueous solution (10 mL / g) to terminate the reaction. Let stand, separate the liquid, concentrate the organic phase, and directly use it in the next step.
[0049] (2) Decarboxylation reaction: Control the temperature at 25±5°C, add 1.5kg (5mL / g) of DMSO and 54g (3mol, 3.0equiv.) of purified water into the 5L reactor 264g (1mol...
Embodiment 3
[0056] Embodiment 3: a kind of method for preparing salmeterol, it is characterized in that concrete preparation steps are as follows:
[0057] (1) Alkylation reaction: Control the temperature at 25±5°C, add 2.04Kg (15mL / g) of methyl tert-butyl ether and 282g of diisopropyl malonate (1.5mol, 1.5equiv. ) and potassium tert-butoxide 224g (2.0mol, 2.0equiv.). The system was stirred for 1-2 hours, and 184.0 g (1 mol, 1.0 equiv.) of 2-bromoethylbenzene was slowly added dropwise and the temperature of the system was kept at 25±5°C until the reaction was completed. Press the system into 2.9 kg of saturated ammonium chloride aqueous solution (15 mL / g) to terminate the reaction. Let stand, separate the liquid, concentrate the organic phase, and directly use it in the next step.
[0058] (2) Decarboxylation reaction: control the temperature at 25±5°C, add 3.6kg (15mL / g) of n-butanol, 90g (5mol, 5.0equiv.) of purified water, 2-phenylmalonic acid diiso Propyl ester 292g (1mol, 1.0equiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com