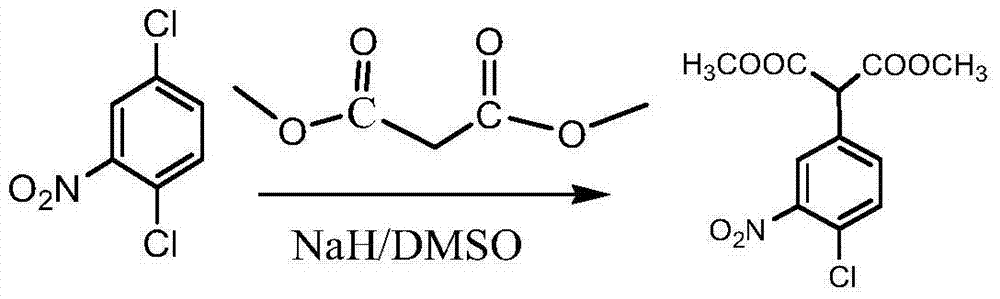
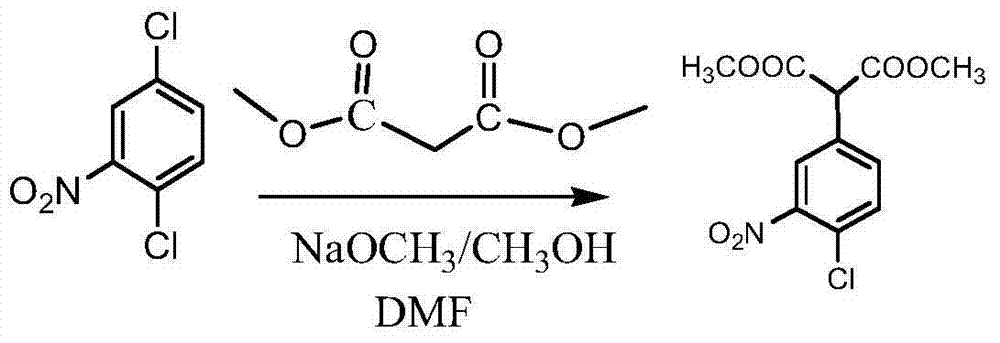
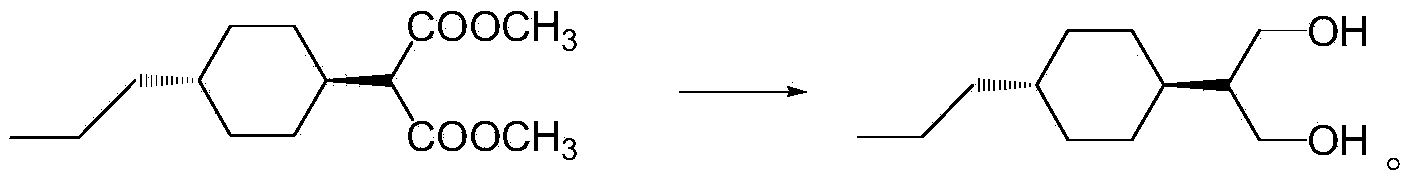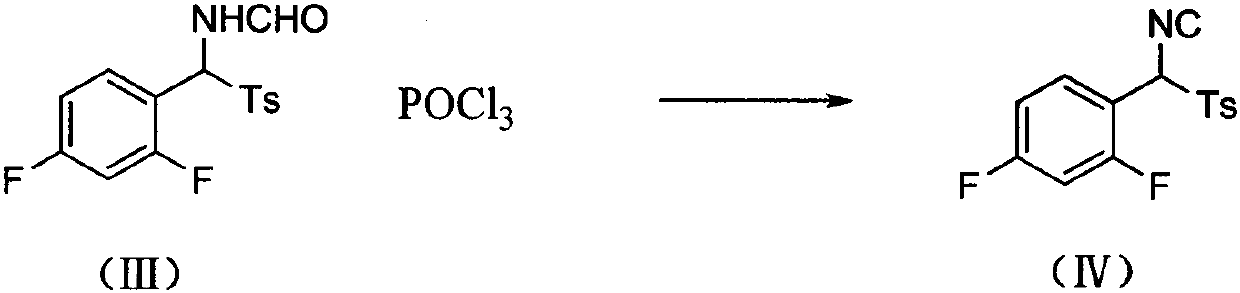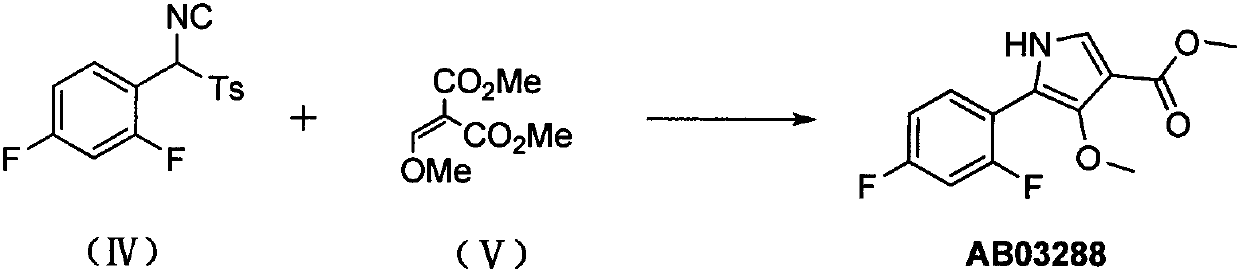Patents
Literature
113 results about "Dimethyl malonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
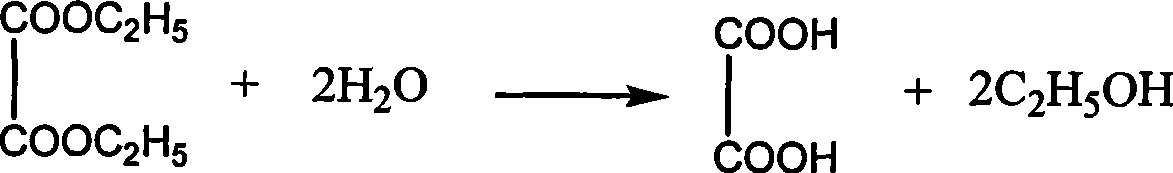
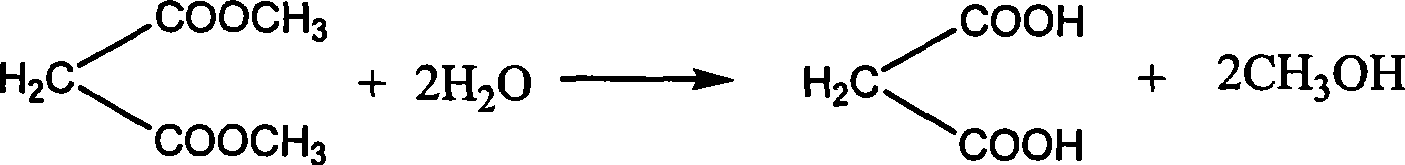
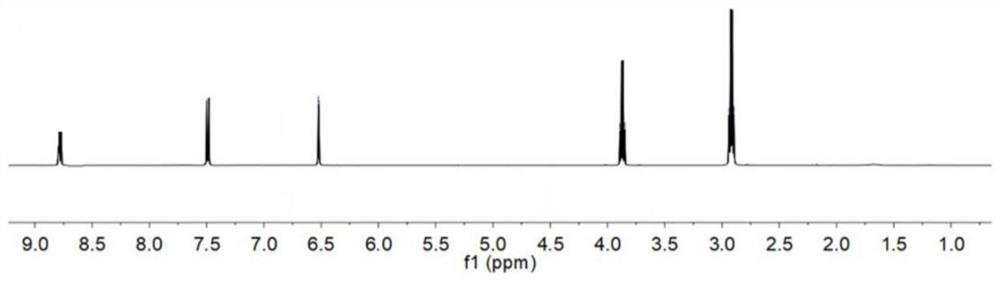
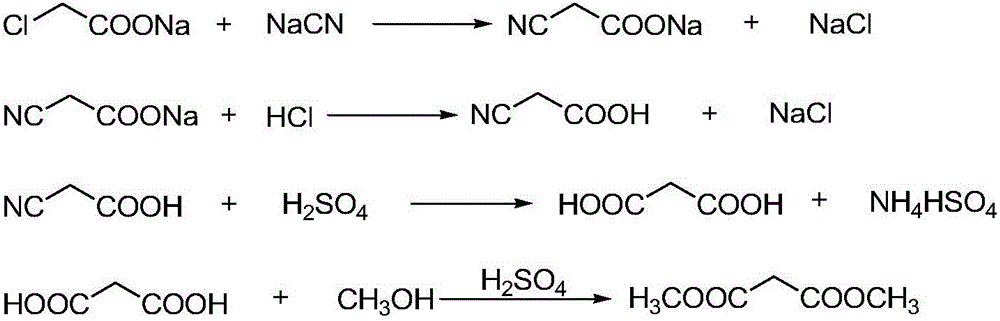
Dimethyl malonate is a diester derivative of malonic acid. It is a common reagent for organic synthesis used, for example, as a precursor for barbituric acid. It is also used in the malonic ester synthesis. It can be synthesized from dimethoxymethane and carbon monoxide. H₂C(OCH₃)₂+2 CO⟶CH₂(CO₂CH₃)₂ Dimethyl malonate is used extensively in the fragrance industry as a raw material in the synthesis of jasmonates.
Method for preparing ester by catalytic oxidization of 1,3-propanediol
InactiveCN101906038AHigh selectivityEasy to separate and purifyMolecular sieve catalystsOrganic compound preparation3-Hydroxypropionic acidGas phase
The invention discloses a method for preparing an ester by catalytic oxidization of 1,3-propanediol. The catalyst adopted is a supported noble metal catalyst (gold or palladium); a carrier is made from metal oxides of different shapes and sizes or mixed oxides (such as CeO2, gamma-Al2O3, CuO, MgO, ZnO, ZSM-5, MoO3, V2O5 and the like), or a silicon dioxide-containing material or a carbon material; after the catalyst is treated by different preparation and treatment methods, the selective oxidization reaction of the 1,3-propanediol for preparing 3-methyl lactate, dimethyl malenate, 3-methoxypropionate and methyl acrylate is performed under a mild liquid-phase or liquid- and gas-phase reaction condition. The highest yield of the produced 3-methyl lactate is 81.5 percent (the conversion rate is 97.0 percent and the selectivity is 84.0 percent); the highest yield of the produced dimethyl malenate is 58.2 percent ( the conversion rate is 99.6 percent and the selectivity is 58.4 percent); and the highest yield of produced 3-methoxypropionate is 53.0 percent (the conversion rate is 97.0 percent and the selectivity is 58.4 percent) and the highest yield of produced methyl acrylate is 38.4 percent (the conversion rate is 92.2 percent and the selectivity is 41.6 percent).
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
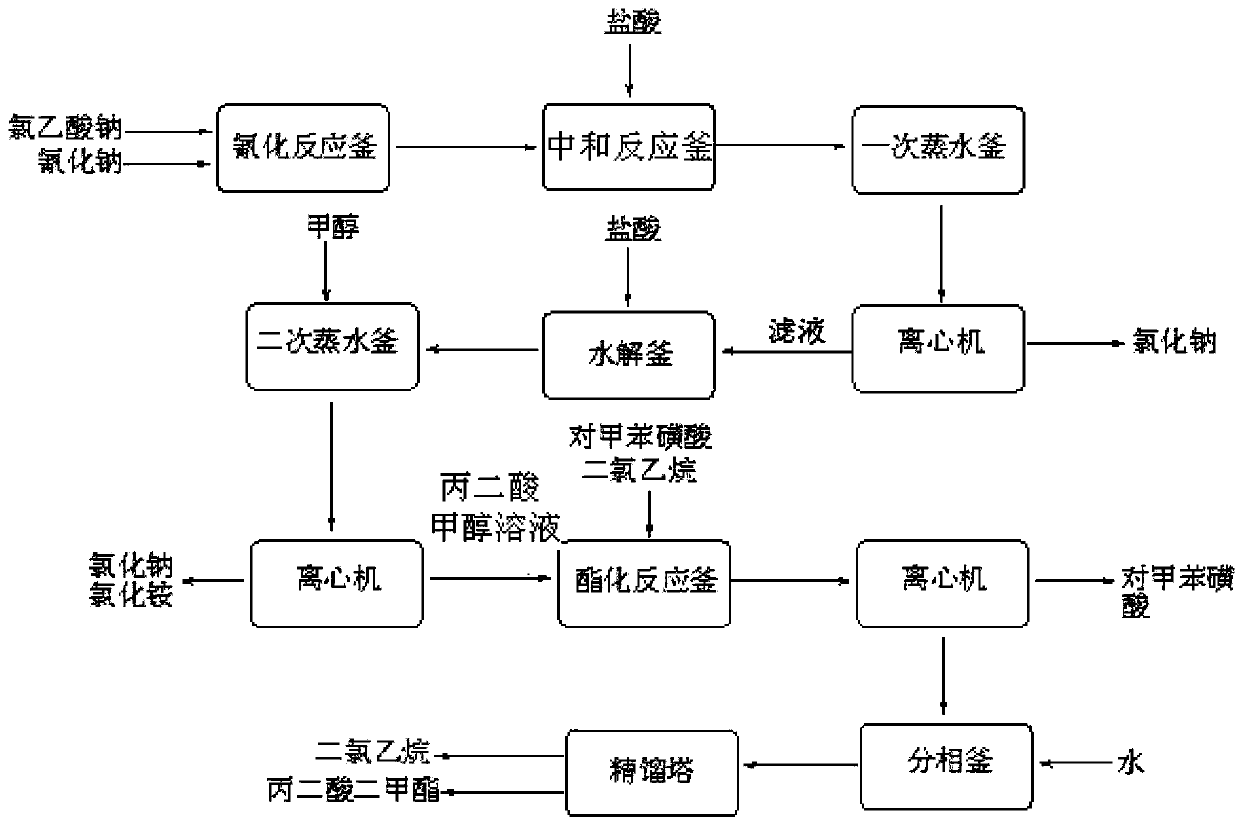
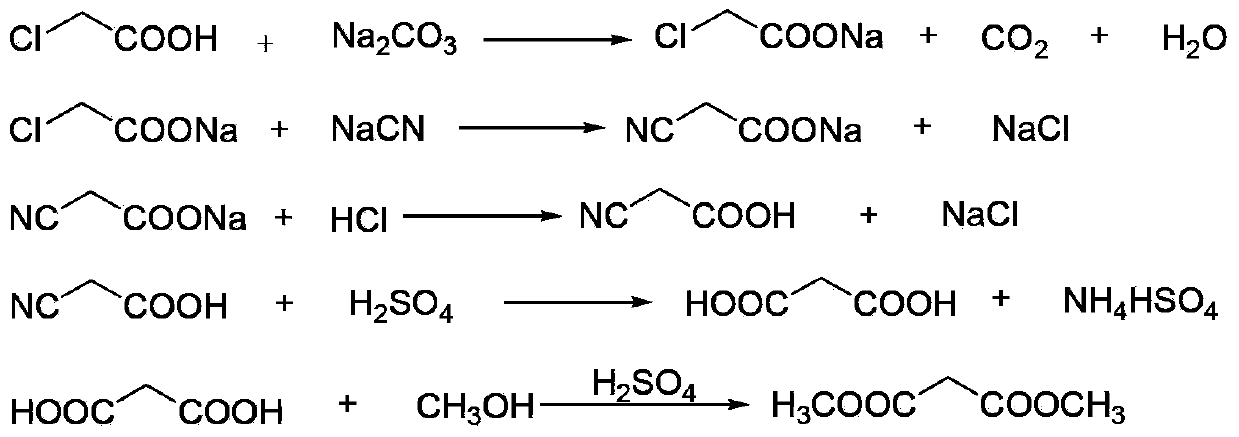
Environment-friendly clean method for preparing dimethyl malonate
InactiveCN103420834AAvoid decompositionAvoid reactionOrganic compound preparationCarboxylic acid esters preparationInorganic saltsWater desalination
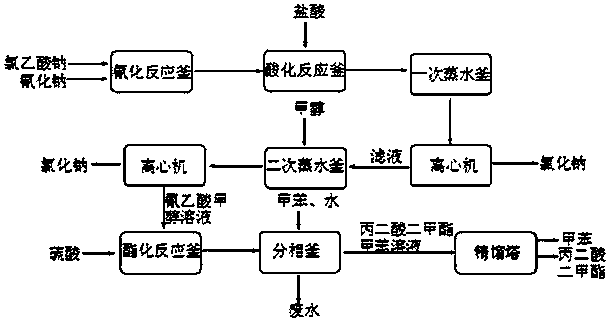
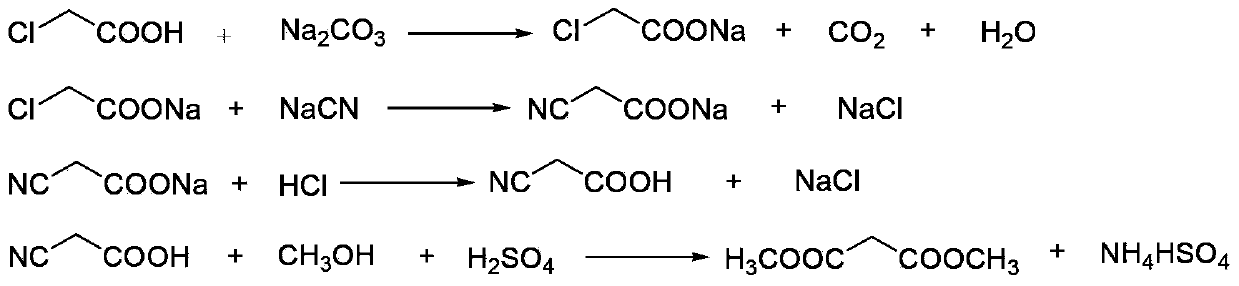
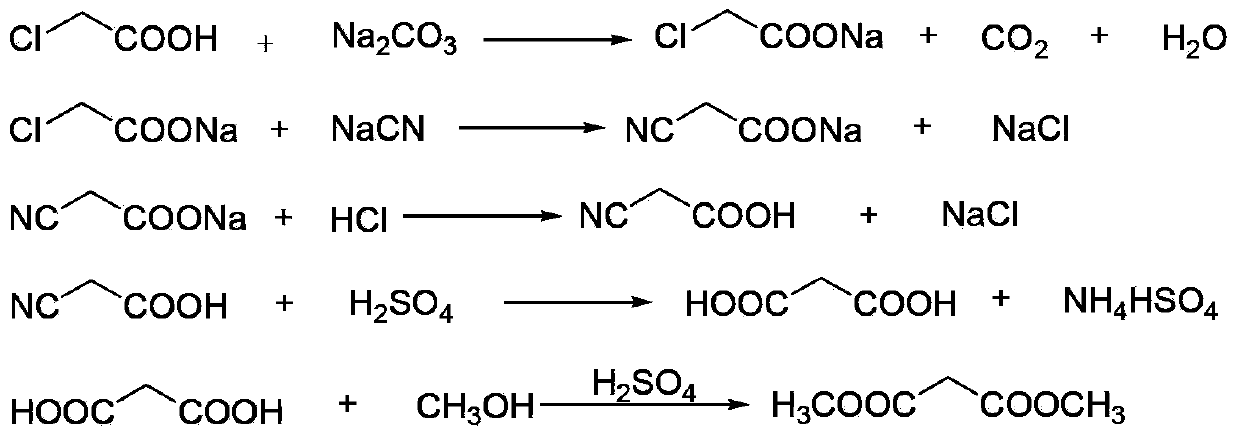
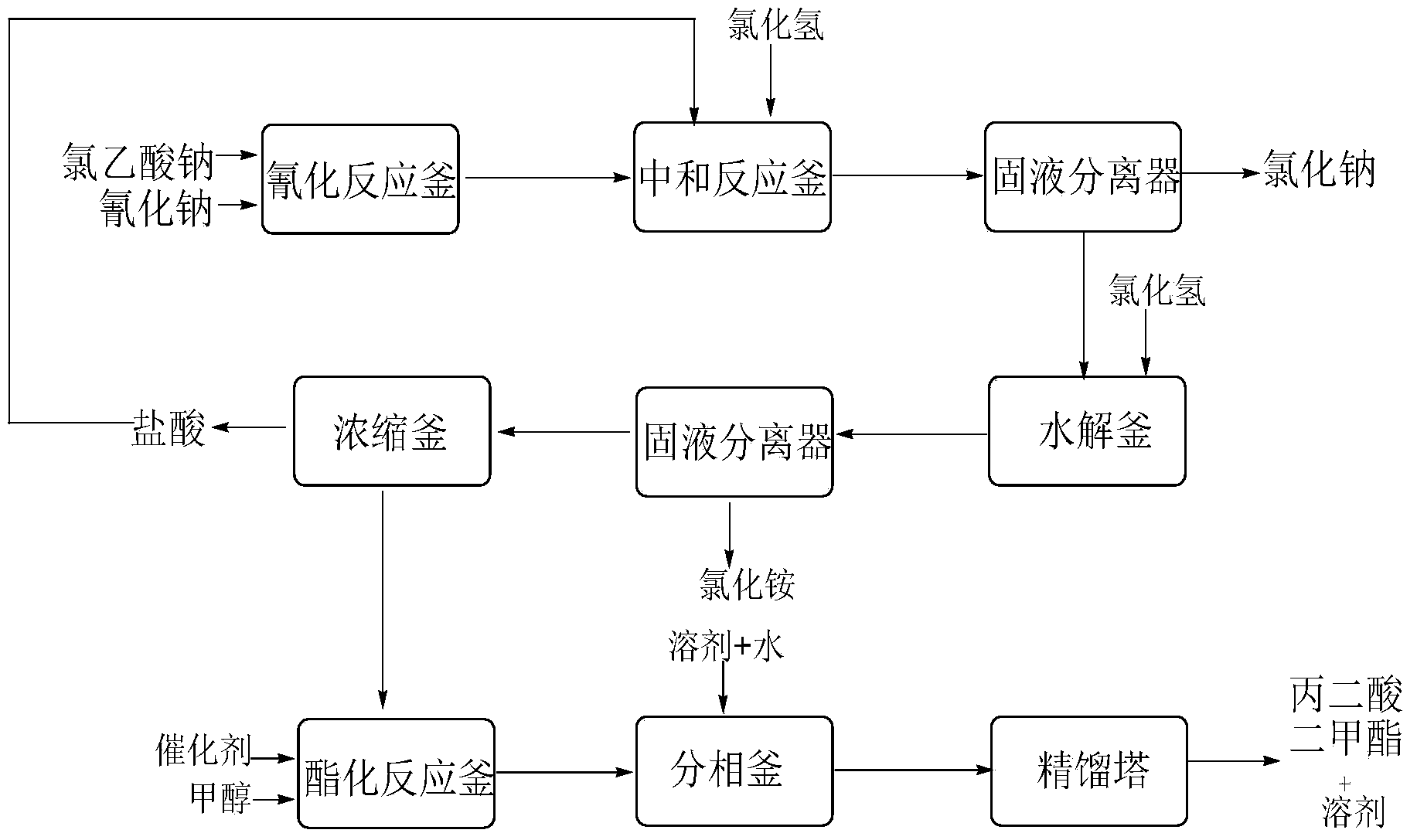
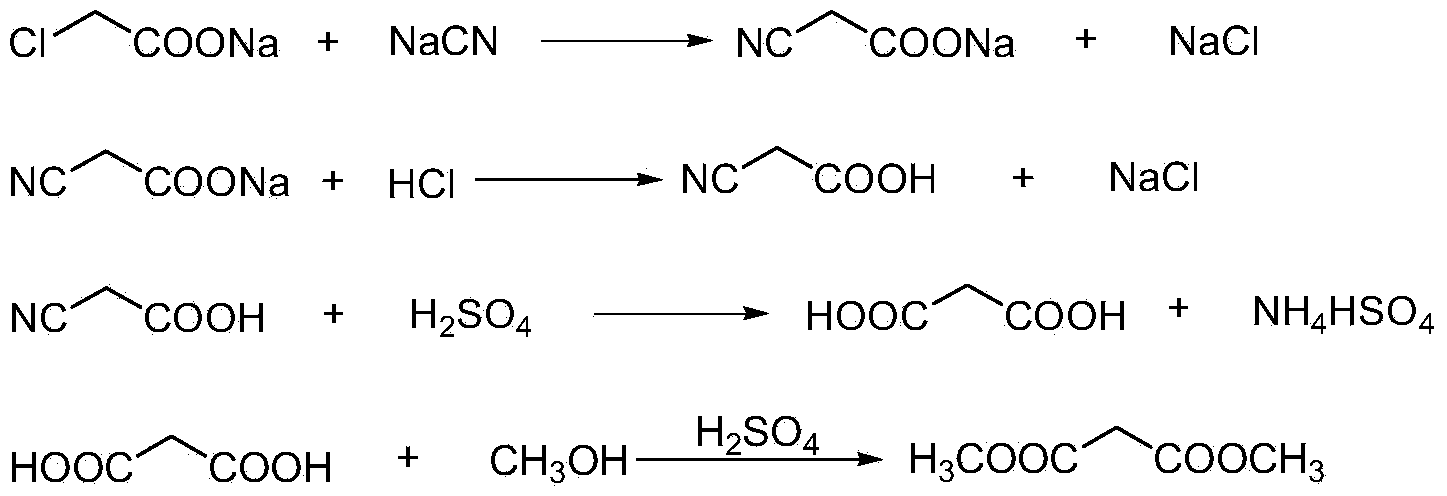
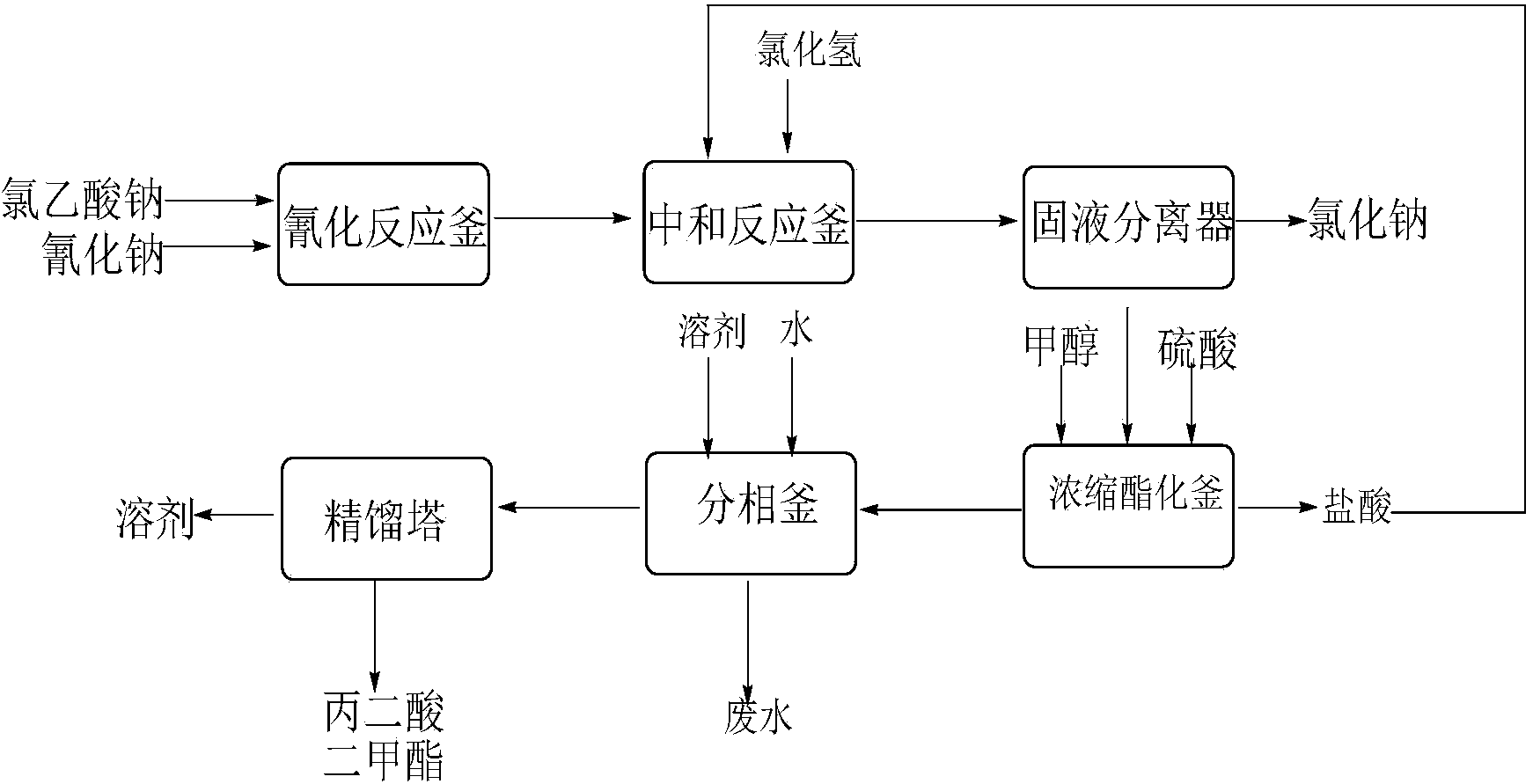
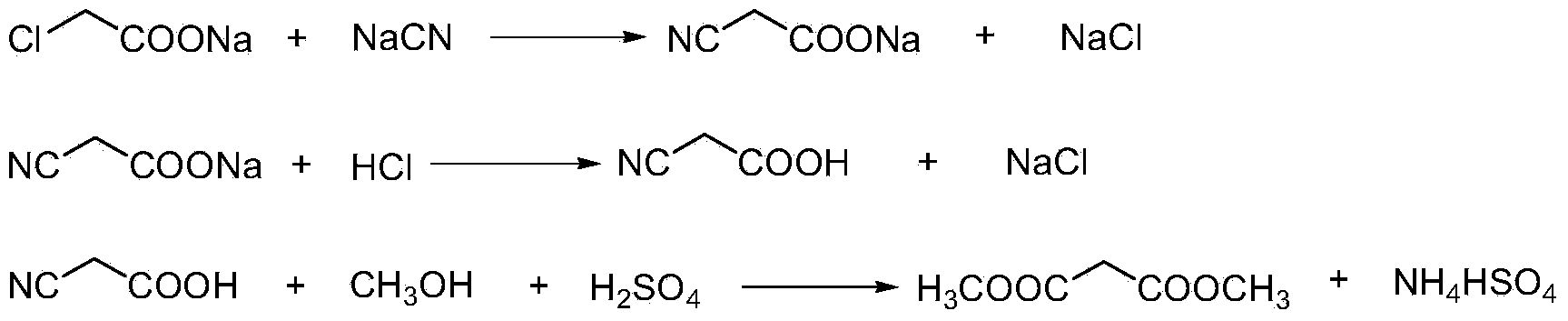
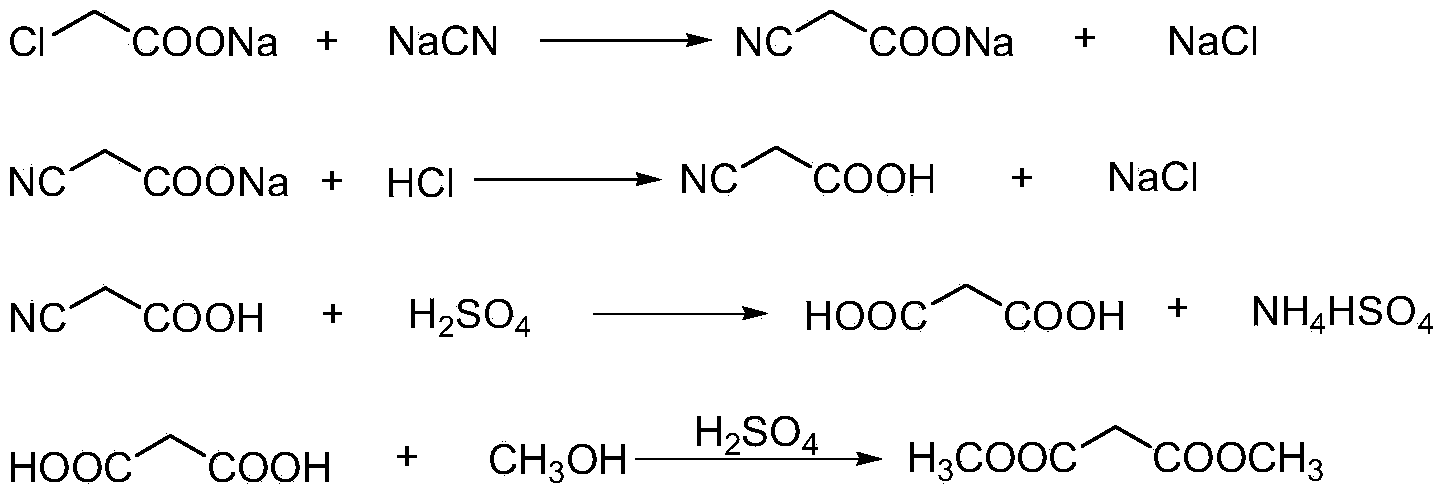
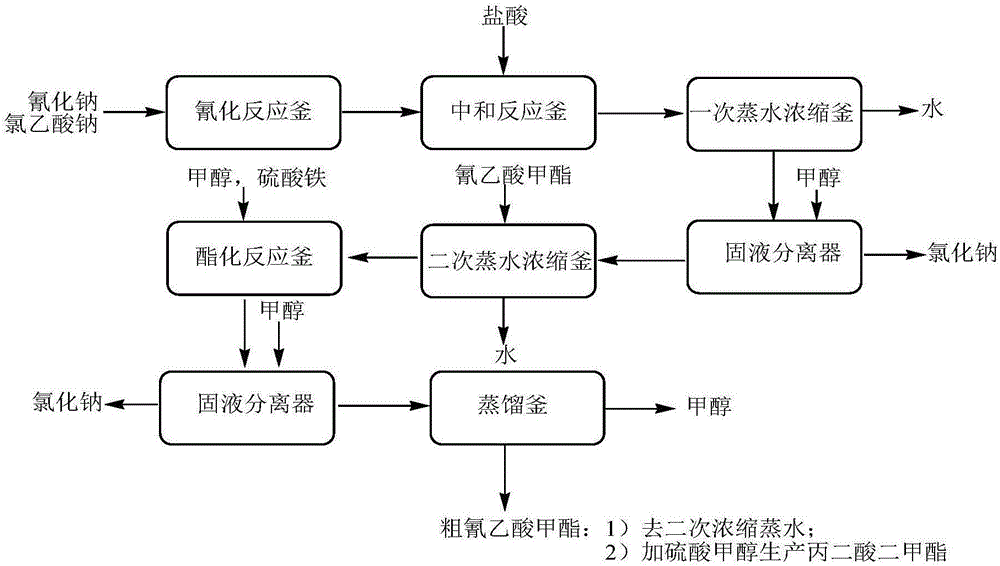
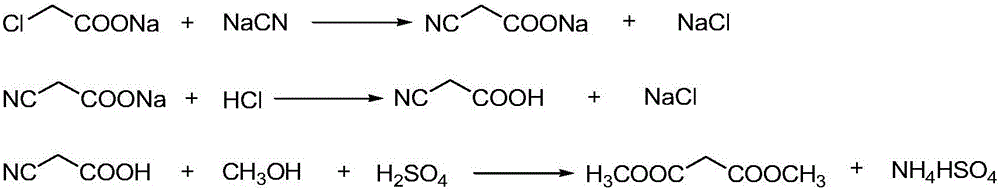
The invention discloses an environment-friendly clean method for preparing dimethyl malonate. The method comprises steps of neutral reaction, cyaniding reaction, acidizing reaction, steaming water desalination, esterification reaction and the like, wherein inorganic salt in a cyanoacetic acid aqueous solution is removed in the step of steaming water desalination, and the preferable technical scheme of steaming water desalination is carried out twice. The inorganic salt-sodium chloride mixed in cyanoacetic acid is removed through steaming water desalination before the esterification reaction, the reaction of sulfuric acid and sodium chloride are avoided in the esterification process, and the consumption of sulfuric acid and the waste water generating capacity are reduced, so that the method for preparing the dimethyl malonate is environment-friendly and clean. The steaming water desalination is carried out twice, so that the phenomenon that cyanoacetic acid is decomposed due to local overtemperature caused by large salt content in the later stage of one-time steaming concentration is avoided.
Owner:CHONGQING UNISPLENDOUR CHEM
Dimethyl malonate preparation method
InactiveCN103724191AReduce productionReduce dosageOrganic compound preparationCarboxylic acid esters preparationCyanoacetic acidDecomposition
The invention discloses a dimethyl malonate preparation method. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided by introducing hydrogen chloride into cyanoacetic acid reaction liquid to separate out sodium chloride; meanwhile, malonic acid and ammonium chloride are obtained by hydrolysis in a closed system, and hydrogen chloride gas is introduced to separate out ammonium chloride; after desalination, an esterification reaction can reduce the catalyst dosage and wastewater generation while a byproduct ammonium chloride is obtained. The method disclosed by the invention is simple to operate, low in production cost and high in product yield and generates a small quantity of three wastes, thereby being an environment-friendly and clean production method.
Owner:CHONGQING UNISPLENDOUR CHEM
Steel surface treatment paint
InactiveCN104073041APrevent infiltrationHigh densityAnti-corrosive paintsGlycidyl methacrylateMaterials science
The invention discloses a steel surface treatment paint which comprises the following raw material ingredients in parts: 3-5 parts of phenyl hydroxide, 20-22 parts of epoxy resin, 18-20 parts of polyester resin, 12-15 parts of rosin resin, 10-12 parts of terpene resin, 8-10 parts of alkyd resin, 10-12 parts of amino resin, 15-18 parts of acrylic resin, 5-6 parts of a lacquer thinner, 8-10 parts of organosilicon oil, 5-8 parts of glycerine, 3-5 parts of ethyl acetate, 5-6 parts of activated zinc oxide, 5-6 parts of fast-dissolving sodium silicate, 3-6 parts of dimethyl malonate, 3-5 parts of glycidyl methacrylate, 5-8 parts of ferric hydroxide, 2-3 parts of polyvinyl alcohol, 20-25 parts of vinyl acetate-acrylic emulsion and 20-30 parts of acrylic emulsion. The steel surface treatment paint mainly plays a wetting and permeating effect on loose rust to separate and wrap the rust to prevent further development of rusting; meanwhile, after forming a film, the paint interacts with the rust by virtue of slow hydrolysis to form an acid complex to achieve the purpose of rust removal; the paint has stable properties, and increases the density of the paint film to prevent permeation of water and oxygen in air.
Owner:周彩球
Dimethyl malonate preparation method
InactiveCN103724196AReduce dosageReduce productionOrganic compound preparationCarboxylic acid esters preparationDecompositionCyanoacetic acid
The invention discloses an environment-friendly and clean dimethyl malonate preparation method. Hydrogen chloride gas is introduced into the mixed liquid of cyanoacetic acid and sodium chloride, and then methanol and sulfuric acid are added for an esterification reaction to obtain dimethyl malonate. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided; meanwhile, after desalination, the esterification reaction can reduce the dosage of sulfuric acid and wastewater generation. The method is simple to operate, low in production cost, high in product yield and suitable for large-scale industrial production.
Owner:CHONGQING UNISPLENDOUR CHEM
Formaldehyde-removing smoke agent and preparation method thereof
The invention provides a formaldehyde-removing smoke agent and a preparation method thereof. The formaldehyde-removing smoke agent provided by the invention comprises the following components: 0 to 15 parts of dimethyl malonate, 0 to 15 parts of ethyl acetoacetate, 0.8 to 12 parts of diglycol, 5 to 30 parts of glycerol, 0.005 to 0.1 part of a catalyst and the balance of pure water. The invention also provides the preparation method of the formaldehyde-removing smoke agent. The preparation method comprises the following steps: putting dimethyl malonate or ethyl acetoacetate, diglycol and the catalyst into a reaction kettle according to a ratio, heating, performing constant-temperature reaction, collecting fractions, cooling to room temperature, and adding glycerol and the balance of pure water to obtain the formaldehyde-removing smoke agent provided by the invention. The formaldehyde-removing smoke agent provided by the invention can be applied to pollution treatment of the environment inside a living room or a vehicle and can react with formaldehyde rapidly to fulfill the aim of removing the formaldehyde pollution. The formaldehyde-removing smoke agent is low in product cost, simple in process operation and convenient to use, and avoid corrosion pollution.
Owner:南京双全科技有限公司
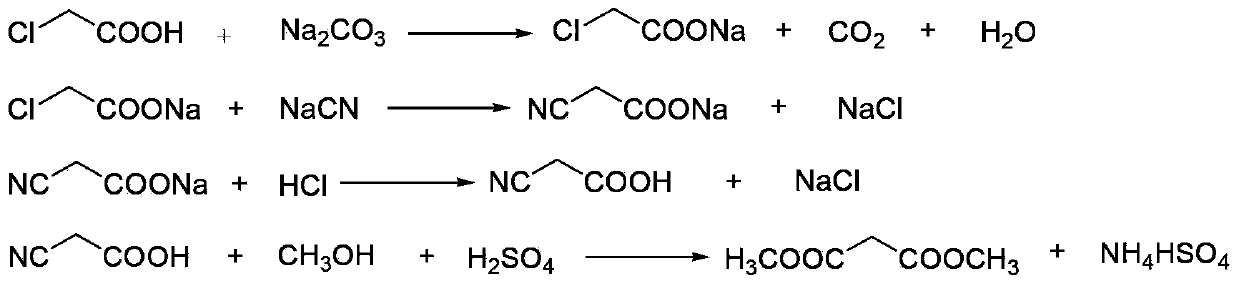
Method for synthesizing p-toluenesulfonic acid-catalyzed dimethyl malonate
ActiveCN103420833AReduce dosageImprove solubilityOrganic compound preparationCarboxylic acid esters preparationPtru catalystChloroacetic acids
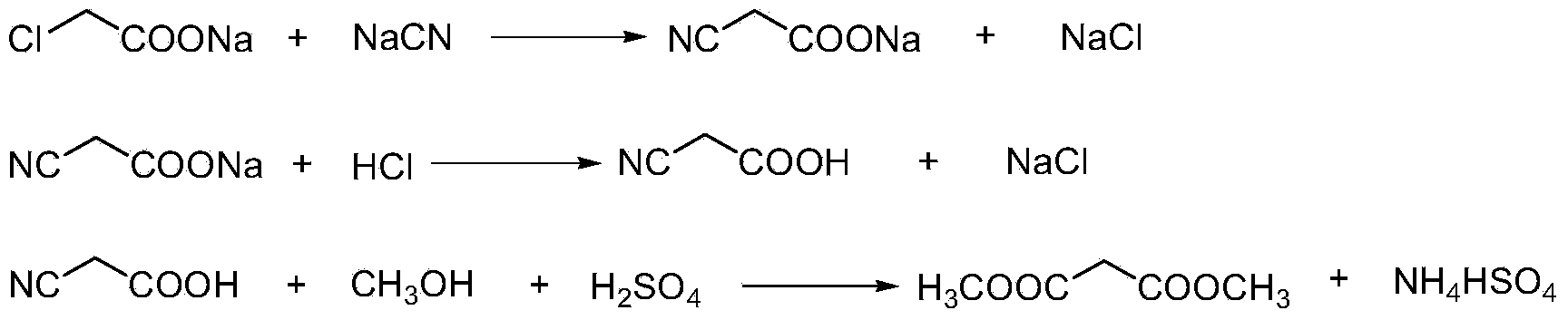
The invention discloses a method for synthesizing p-toluenesulfonic acid-catalyzed dimethyl malonate. Chloroacetic acid is taken as a raw material, dimethyl malonate is generated through a neutralization reaction, a cyanation reaction, an acidification reaction, a hydrolysis reaction and an esterification reaction, the esterification reaction is performed under the catalytic effect of p-toluenesulfonic acid, a catalyst is recovered after the reactions, and the recovered catalyst can be recycled, so that the usage amount of the catalyst can be greatly reduced and sulfuric acid-containing wastewater cannot be produced by selecting p-toluenesulfonic acid instead of sulfuric acid in a traditional process as the catalyst in the esterification reaction; meanwhile, dichloroethane is added in the esterification reaction, has a good dissolving property for dimethyl malonate, is easily separated from dimethyl malonate through rectification and forms a ternary azeotropic system along with methanol and water, and the water generated in the reactions is continuously discharged from the system through azeotropy, so that the esterification reaction can be more thoroughly produced.
Owner:CHONGQING UNISPLENDOUR CHEM
Liquid sustained-release acid breaker
InactiveCN101508891ALittle impact on performanceReduce secondary damageDrilling compositionOxalateOrganic solvent
The invention belongs to the field of a preparation process of oilfield chemicals and relates to an oilfield gel breaking technique, in particular to a liquid slowly-releasing acid gel breaker which aims at overcoming the problems that the operability is bad, the influence to the contracture effect is larger and the oil well can disable seriously in the prior art. In order to solve the problems of the prior art, the invention provides the technical schemes that the liquid slowly-releasing acid gel breaker contains ester acid-supply components and organic solvent protective solute components according to the mass ratio of 1 to 1-7, wherein the ester is a single component or the combination of a plurality of components of methyl formate, ethyl formate, diethy-aceto oxalate, dimethyl malonate and / or ethyl malonate; and the organic solvent is alkane or liquid ester. As the product of the invention can be released slowly, the construction operability and the effectiveness can be greatly improved; after the fracturing operation is finished, the product can be finally released completely, the viscosity of the gel breaking product is low, and the aims of completely breaking gel and flowing back in time can be achieved.
Owner:SHAANXI UNIV OF SCI & TECH
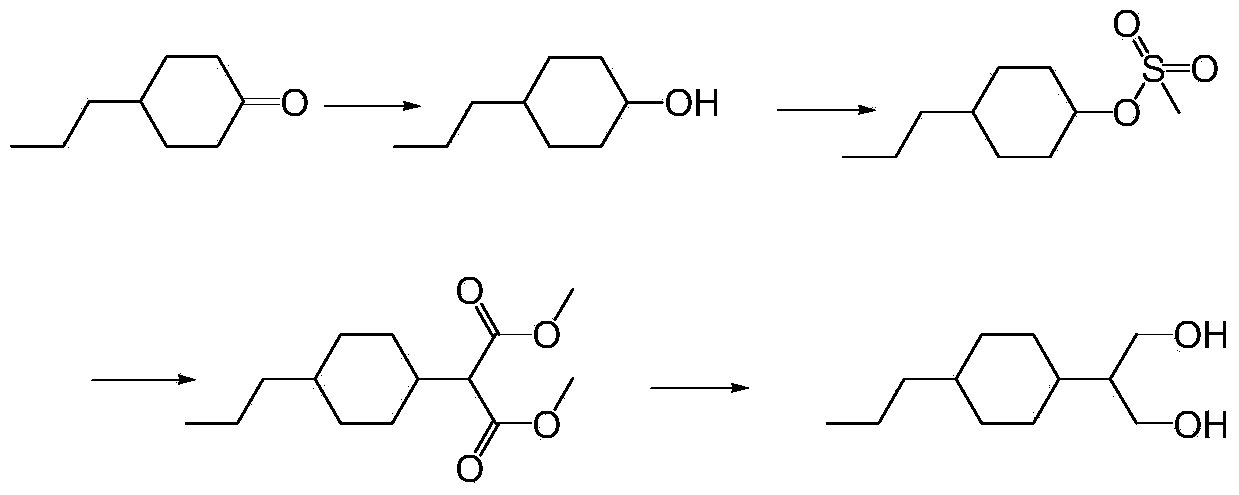
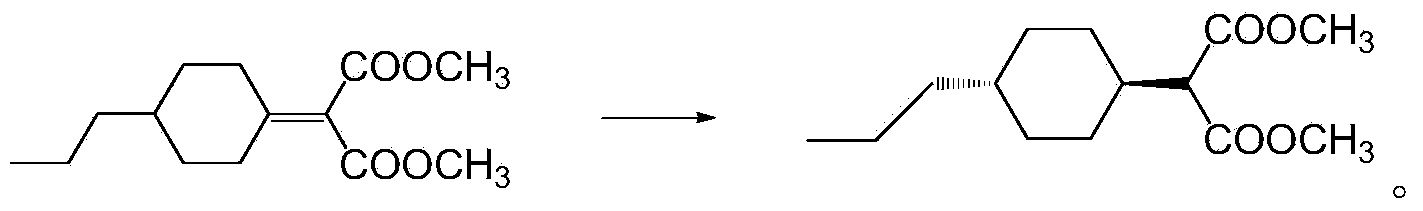
Preparation method of 1,3-propanediol derivatives and intermediates
ActiveCN103524305ASimple processMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationCyclohexanoneLithium chloride
The invention discloses a preparation method of 1,3-propanediol derivatives and intermediates. The preparation method of trans-2-(4-n-propylcyclohexyl)-1,3-propanediol comprises the following steps: (1) carrying out coupling reaction on propyl cyclohexanone and dimethyl malonate in a solvent under the action of organic alkali and titanium tetrachloride in an inert gas protective atmosphere; (2) reacting the product of the step (1) with hydrogen in an organic solvent under the catalytic action of palladium-carbon; and (3) carrying out reduction reaction on the product of the step (2) with sodium borohydride and lithium chloride in an ethanol-water mixed solution. The preparation method avoids using pyridine, THF (tetrahydrofuran) and other expensive raw materials, reduces the cost, lowers the requirements for wastewater treatment equipment, has the advantages of high safety, high efficiency and environmental protection, and can easily implement industrial production.
Owner:LIANHE CHEM TECH +3
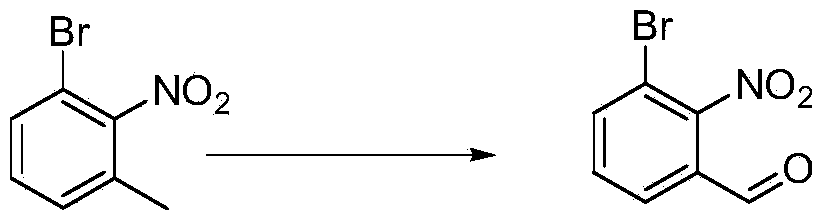
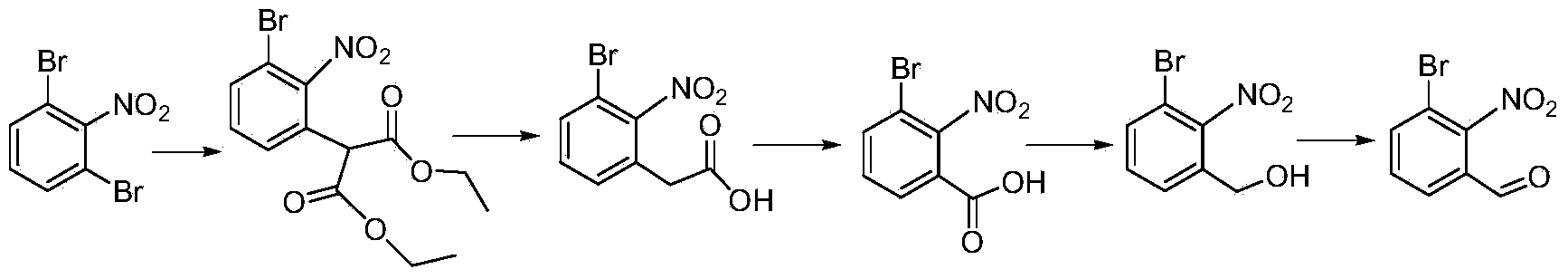
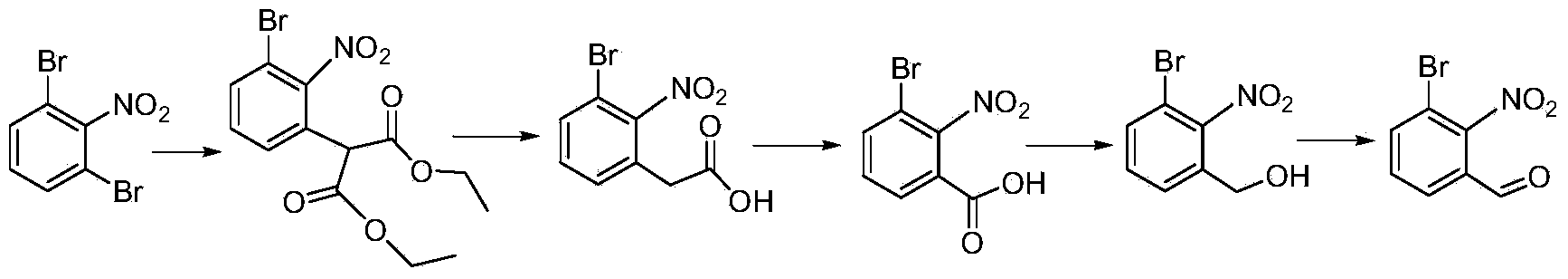
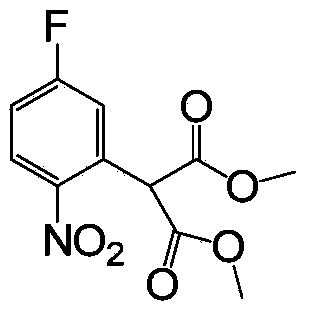
Chemical synthesis method of 3-bromo-2-nitrobenzaldehyde
ActiveCN103880683AHigh yieldEfficient synthesisOrganic chemistryOrganic compound preparationChemical synthesisNitrobenzene
The invention relates to a chemical synthesis method of 3-bromo-2-nitrobenzaldehyde. The chemical synthesis method is characterized by using 1,3-dibromo-2-nitrobenzene as a raw material and carrying out five-step reactions including substitution, decarboxylation, oxidation, reduction and hydroformylation, and comprises the following steps of adding 1,3-dibromo-2-nitrobenzene, dimethyl malonate and alkali to an organic solvent to react to prepare 2-(3-bromo-2-nitrobenzaldehyde)-dimethyl malonate; dissolving 2-(3-bromo-2-nitrobenzaldehyde)-dimethyl malonate obtained in the former step in a tetrahydrofuran or acetone solvent, and adding a hydrochloric acid solution to react to prepare 2-(3-bromo-2-nitrobenzaldehyde)-acetic acid; adding a sodium hydroxide water solution and potassium permanganate to react to obtain 3-bromo-2-nitrobenzoic acid; dissolving 3-bromo-2-nitrobenzoic acid in the tetrahydrofuran solvent, and adding borane tetrahydrofuran to react to obtain 3-bromo-2-nitrobenzyl alcohol; adding 3-bromo-2-nitrobenzyl alcohol to a dichloromethane or 1,2-dichloroethane solvent and adding manganese dioxide to react to prepare 3-bromo-2-nitrobenzaldehyde. The synthetic route has the advantages of low cost and high yield.
Owner:唐山金硕化工有限公司
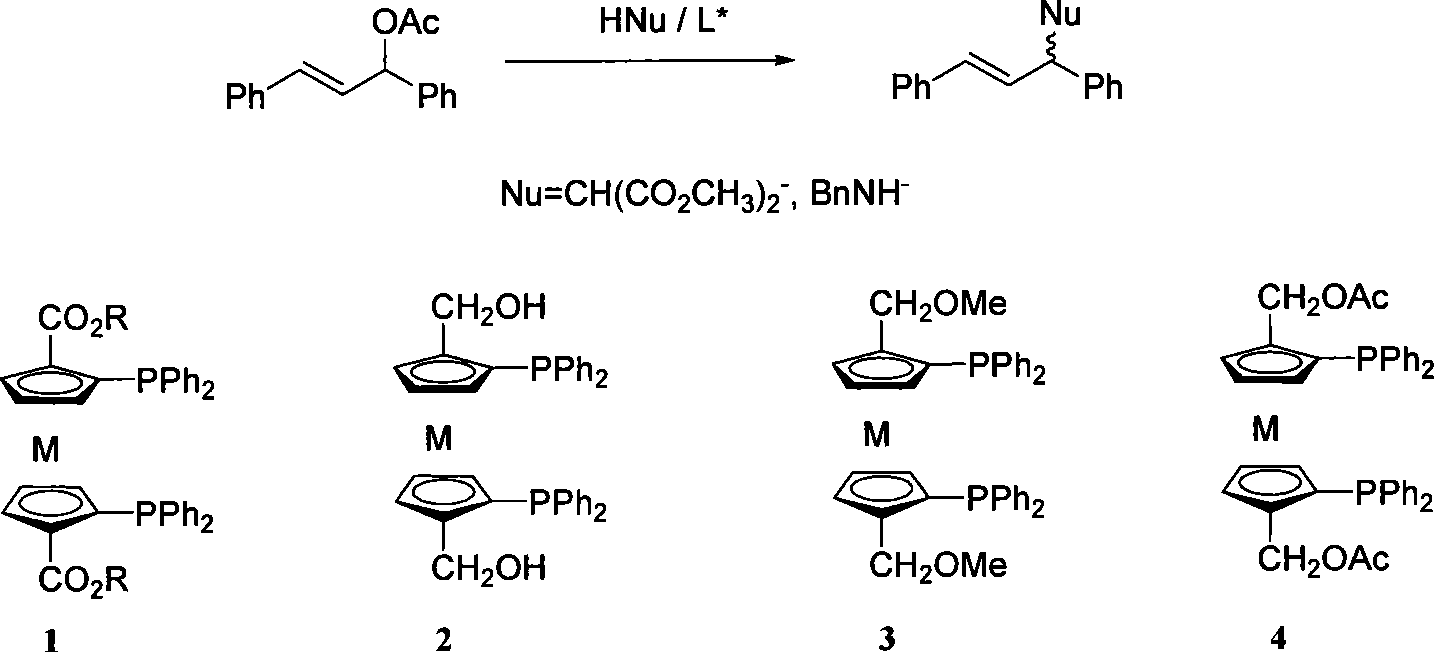
Enantioselectivity reversion method for allyl substitution reaction catalyzed by Pd
InactiveCN101143818AEasy to predict stereo configurationSimple and fast operationOrganic compound preparationOrganic chemistry methodsAllyl acetateOrtho position
The invention relates to a method of enantioselective reversion in the Pd-catalyzed allylic substitution reaction in the field of chemical technique. Metallocene diphosphine ligand with the same chiral skeleton is applied to the Pd-catalyzed allylic asymmetric substitution reaction of 1, 3-diphenyl allyl acetate, and the used nucleophile is dimethyl malonate or benzylamine. When the group at the ortho position of diphenylphosphine is ester group, a S-shaped product is produced by catalysis. If the ester group is reduced into hydroxymethyl group, then a R-shaped product is induced, and the etherified or esterified hydroxyl group has the same inductive function. The method has the advantages of convenient operation, convenient spatial configuration of predicted products and wide screenable range.
Owner:SHANGHAI JIAO TONG UNIV +1
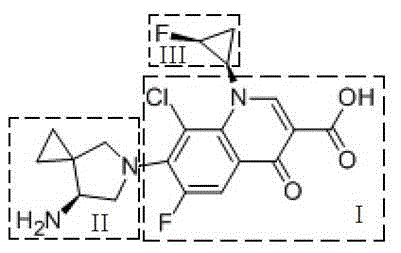
Preparation method for three-membered ring intermediate of sitafloxacin hydrate
InactiveCN104803857AHigh yieldHigh purityOrganic compound preparationSulfonic acids salts preparationDimethyl malonateBromine
The invention discloses a preparation method for a three-membered ring intermediate of sitafloxacin hydrate. The preparation method comprises the following steps: dimethyl malonate and 1,1,2-tribromo-2-fluoroethane react to synthesize a solid A, the solid A is subjected to debromination to prepare an oily liquid B, the oily liquid B is subjected to branched chain removal to prepare a solid C, the solid C is hydrolyzed to prepare a solid D, the solid D is subjected to chiral resolution with L-leucinamide and reduced to prepare a solid F, the solid F is introduced to an amino group and combined with p-toluenesulfonic acid to prepare the three-membered ring intermediate of sitafloxacin hydrate. The preparation method has fewer steps for preparing the three-membered ring intermediate of sitafloxacin hydrate, unnecessary isomer is separated by one step with the chiral resolution method, the product yield and purity are higher, and scale production is easier.
Owner:SUZHOU NACHI BIOTECH CO LTD
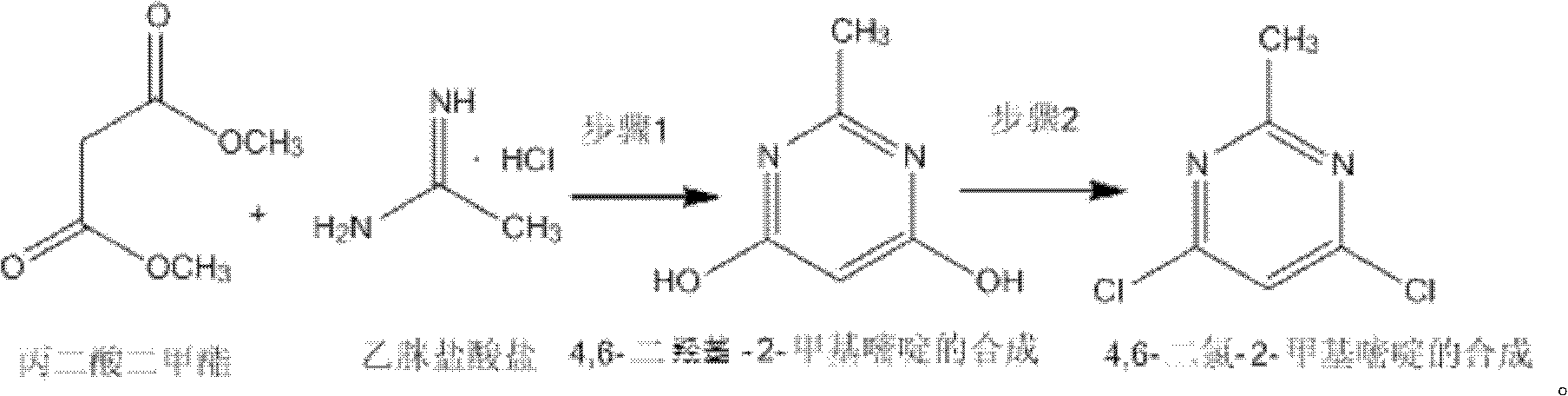
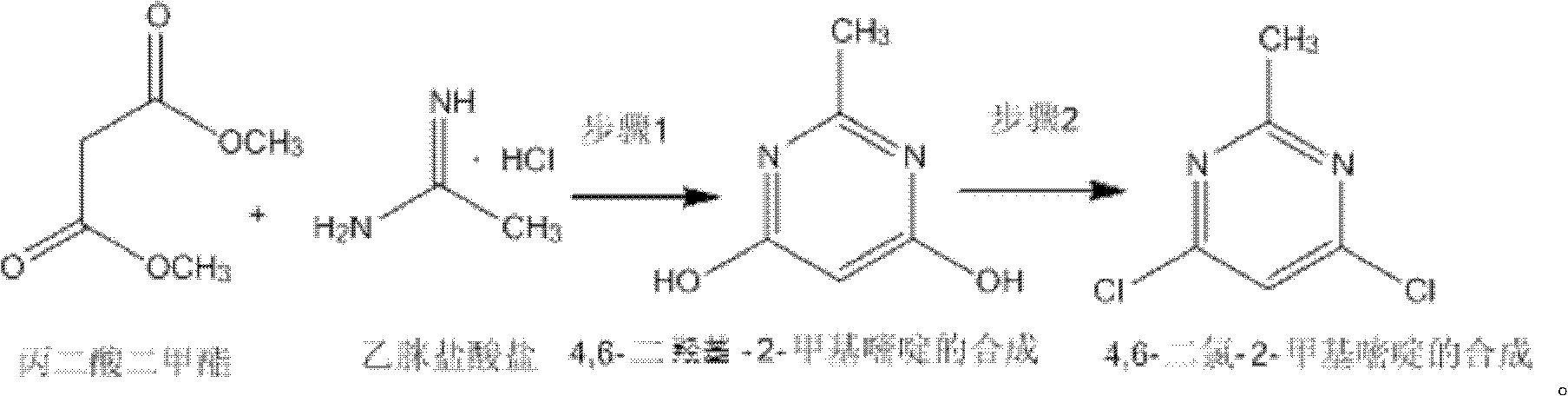
Method for synthetizing 4,6-dichloro-2-methyl pyridine
InactiveCN102432547AEasy to operateThe synthesis process is simpleOrganic chemistrySodium methoxideDistillation
The invention discloses a method for synthetizing 4,6-dichloro-2-methyl pyridine, which comprises the following steps of: under the ice-bath condition, adding sodium methoxide, dimethyl malonate and acetamidine hydrochloride into methanol; removing the ice bath, heating to a temperature of 18 to 25 DEG C and performing a reaction for 3 to 5 hours; carrying out reduced pressure distillation to remove the methanol; adding water for dissolving; regulating a pH value of the obtained solution to the range of 1 to 2; under the condition of a temperature of 0 DEG C, carrying out stirring and crystallization for 3 to 5 hours; carrying out extraction filtering, washing and drying to obtain white solid 4,6-dyhydroxyl-2-methyl pyridine; adding N,N-diethyl aniline and dichloroethane into the obtained 4,6-dyhydroxyl-2-methyl pyridine; heating to the reflux condition; then slowly adding a solution of triphosgene dichloroethane; performing a reflux reaction for 6 to 8 hours; washing reaction liquid; drying, filtering and concentrating an organic layer; and carrying out recrystallization and decolorization treatment on the obtain solid 4,6-dichloro-2-methyl pyridine. In the method, triphosgene is adopted to replace reagents with serious pollution to the environment and large toxicity, such as POC13, phosgene and the like. The method is safe, is easy to operate, has a simple synthetic process and is suitable for the industrial production.
Owner:太仓市运通化工厂
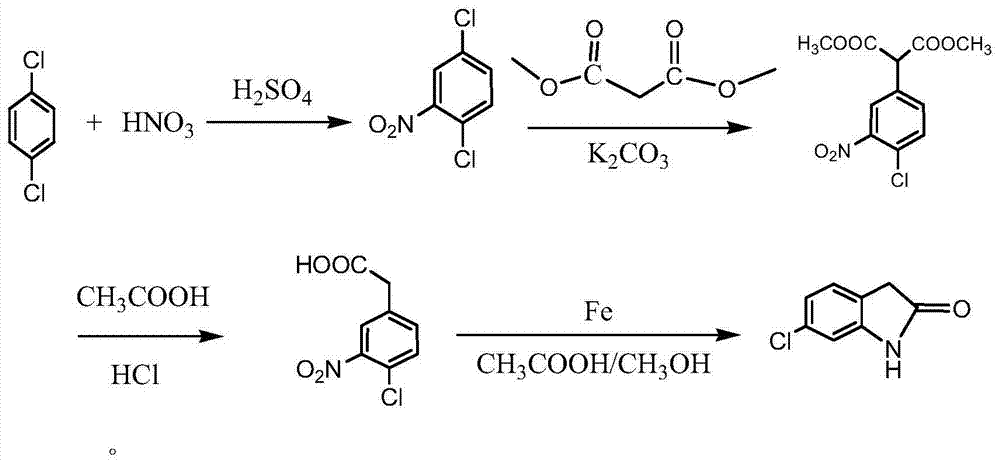
Method for synthesizing 6-chlorhydroxyl indole
The invention relates to a method for synthesizing 6-chlorhydroxyl indole. The synthesizing steps are as follows: (1) preparing 2, 5-dichloronitrobenzene through paradichlorobenzene nitration; (2) synthesizing 2-(4-chlorine-2-nitrobenzophenone) dimethyl malonate by 2, 5-dichloronitrobenzene; (3) synthesizing 4-chlorine-2-nitrophenyl acetic acid by 2, 5-dichloronitrobenzene; and (4) preparing 6-chlorhydroxyl indole by 4-chlorine-2-nitrophenyl acetic acid. By adopting the method, the whole reaction process is mild in condition and easy to control; the safety in the reaction process is increased, the operation is simple and the industrial production can be realized; and the raw materials are simple and easy to obtain, the production cost is reduced, the yield is obviously increased and a foundation for the industrial production is laid.
Owner:天津维智精细化工有限公司
Low-carbon denatured alcohol gasoline and application
InactiveCN101597523ALow costSave resourcesLiquid carbonaceous fuelsFuel additivesDenatured alcoholMethanol fuel
The invention discloses a low-carbon denatured alcohol gasoline and application. The low-carbon denatured alcohol gasoline comprises the following components in percentage by weight: 60-80% of alcohol, 5-15% of fuel ethanol and 5-26% of additive, wherein the additive is composed of the following components in percentage by weight: 45-55% of ortho-nitrotoluene, 20-37% of dimethoxymethane, 5-8% of dimethyl malenate, 0.4-.06% of corrosion inhibitor for metal and 0.4% of antiswelling inhibitor for rubber. The low-carbon denatured alcohol gasoline is added into the national standard gasoline in percentage by weight of 20-85% to be applied. The low-carbon denatured alcohol gasoline is a motor gasoline with better cleanability, lower gasoline consumption rate, larger mixing amount and lower raw material cost; the cost of the motor gasoline is obviously lowered, resource is saved, and engine emission is further improved; at the same time, the motor gasoline solves the problems of high gasoline consumption, low-temperature layering, and the like, lowers saturation vapour pressure and prevents the air blocking phenomenon.
Owner:莫春福
Thermally-stimulated fluorescence/visible light color double-response capsule and synthesis method thereof
ActiveCN112662394AImprove performanceStrong performance and stabilityVegetal fibresLuminescent compositionsChemical synthesisSalicylaldehyde
The invention discloses a thermal-stimulated fluorescence / visible light color double-response capsule and a synthesis method thereof, and belongs to the technical field of chemical synthesis. Through specific molecular structure design, 4-dimethylamino salicylaldehyde and dimethyl malonate are subjected to grafting reaction to prepare chromophoric molecules with a plurality of unsaturated functional groups, the chromophoric molecules are mixed with a phase change material to realize optical double-response reversible output of solvent phase state regulation, and compared with a conventional three-component organic thermal response material, the product only needs two components, namely chromophoric body molecules and a phase change solvent, so that the preparation process is simplified, and the performance stability is high. After microcapsule coating, the material is free from influence of adverse environment, and the performance is stably maintained, so that long-term stable use is realized.
Owner:JIANGNAN UNIV
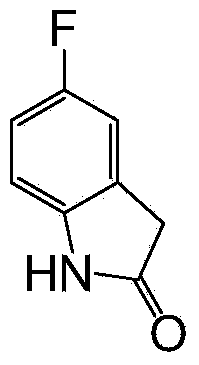
5-fluoroindole-2-one preparation method
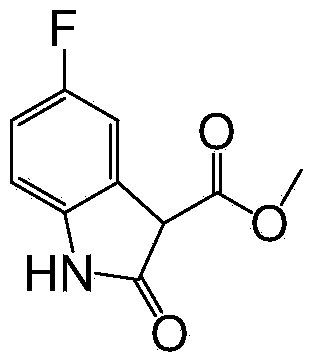
InactiveCN104045592AHigh yieldRaw materials are cheap and easy to getOrganic chemistryIron powderDimethyl malonate
The invention relates to a 5-fluoroindole-2-one preparation method. The method includes the steps of (1) reaction of 2, 4-difluornitrobenzene and dimethyl malonate to prepare 4-fluoro-2-(methyl malonate) nitrobenzene; and (2) reduction cyclization reaction of the 4-fluoro-2-(methyl malonate) nitrobenzene by iron powder to obtain target object 5-fluoroindole-2-one, or palladium / carbon reduction of the 4-fluoro-2-(methyl malonate) nitrobenzene to obtain target object 3-methoxycarbonyl-5-fluoroindole-2-one, and then hydrolysis reaction of the 3-methoxycarbonyl-5-fluoroindole-2-one to obtain the target object 5-fluoroindole-2-one. The method has the advantages of easily obtained raw materials, mild reaction conditions, high yield, less equipment investment, easy industrial production and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Architectural ornament coating
InactiveCN106928830AGood coloring effectAvoid crackingPolyurea/polyurethane coatingsAcrylonitrileRare earth
The invention discloses an architectural ornament coating which comprises the following components in parts by weight: 30-50 parts of waterborne polyurethane, 3-8 parts of AS resin, 0.5-1.5 parts of carboxymethylcellulose, 30-60 parts of rare-earth sulfur oxide, 85-100 parts of fluorine halide, 3-6 parts of heat stabilizer, 0.9-4.9 parts of chemical resisting modifier, 15-20 parts of 4-hydroxy-3-metoxybenzene alcohol, 10-25 parts of heat-conductive filler, 10-18 parts of 2-hydroxy citric acid, 0.75-2.65 parts of infrared reflecting titanium dioxide, 0.4-0.6 part of light stabilizer, 0-0.8 part of coupling agent, 0.7-4.3 parts of accelerant, 10.4-23.6 parts of color paste, 4-8 parts of carboxymethylcellulose, 7-13 parts of toluene diisocynate, 25-30 parts of 3-cyclohexene-1-methyl alcohol, 5-8 parts of allyl dimethyl malonate and 3-10 parts of additives. The weight-average molecular weight of AS resin is 3-10W. The content of acrylonitrile in the AS resin is 20-35%. The fluorine-containing additive is selected as the chemical resisting modifier. The architectural ornament coating has excellent coloring property and is difficult to crack and fade.
Owner:JIANGXI UNIV OF TECH
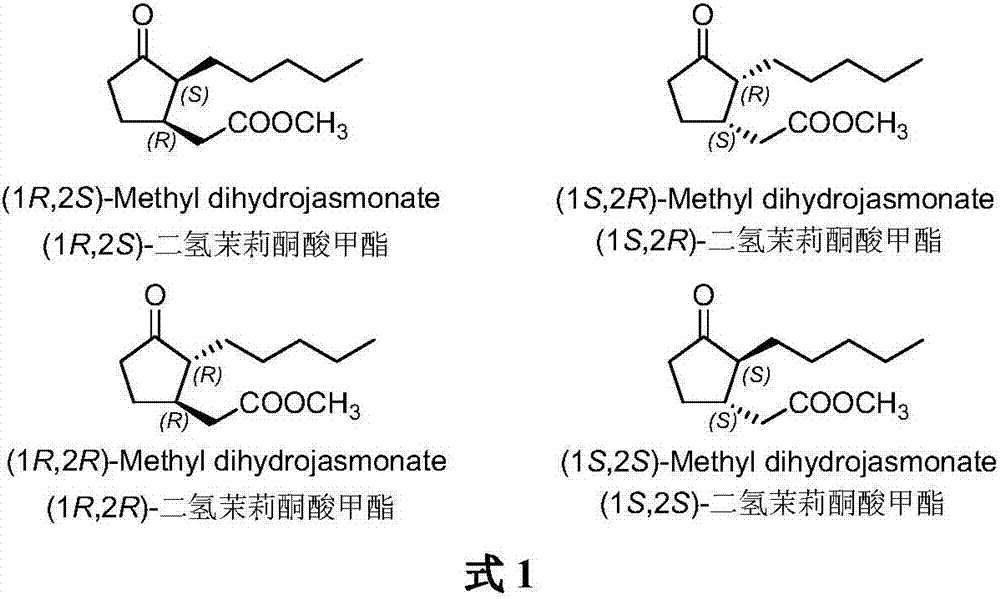
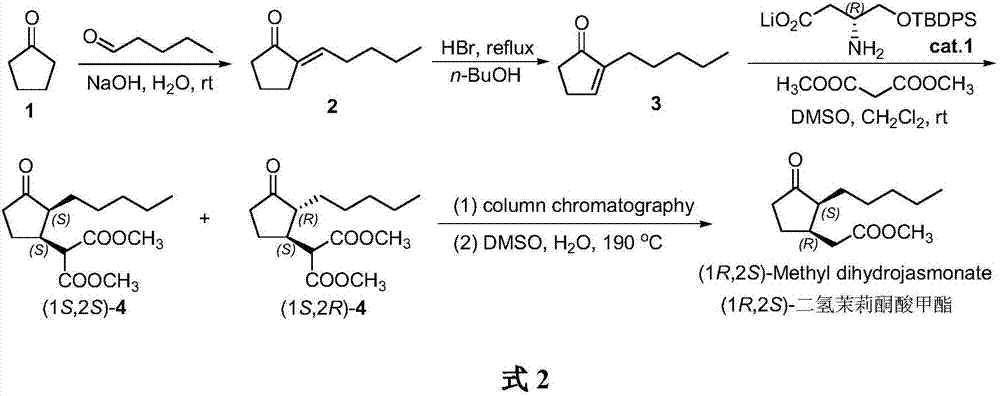
Method for synthesizing (1R,2S)-methyl dihydrojasmonate
ActiveCN106946705AOrganic compound preparationOrganic chemistry methodsChromatographic separationDouble bond
The invention discloses a novel method for synthesizing (1R,2S)-methyl dihydrojasmonate by using an asymmetric Michael addition reaction. The method comprises the steps: firstly, subjecting cyclopentanone, which serves as a starting raw material, to an aldol reaction with n-valeraldehyde under alkaline conditions to produce 2-pentylidene cyclopentanone 2, and then, carrying out double-bond transposition under acidic conditions, so as to obtain 2-n-pentyl-2-cyclopentenone 3; then, carrying out a Michael addition reaction with dimethyl malonate in the presence of a chiral amino-acid lithium salt, and carrying out silicagel-column chromatographic separation twice, so as to obtain (1S,2S)-2-n-pentyl-3-dimethyl malonate cyclopentanone 4; and finally, carrying out a hydrolyzed decarboxylation reaction, thereby obtaining (1R,2S)-methyl dihydrojasmonate. According to the method, the synthesis route is simple and direct, the reaction conditions are mild, and the target compound can be prepared by only four-step reactions.
Owner:北京安胜瑞力科技有限公司
Synthesis method of 1,3-disubstituted allene with high optical activity
ActiveCN104193568AReduce dosageWide applicabilityCarbamic acid derivatives preparationCarboxylic acid esters preparationSynthesis methodsAlkyne
The invention discloses a synthesis method of 1,3-disubstituted allene with high optical activity, namely a method for preparing 1,3-disubstituted allene with high optical activity in one step of functionalizing terminal alkyne, aldehyde and chiral alpha, alpha-diphenyl-L-prolinol under catalysis of a bivalent copper salt. The method is simple to operate, adopts easily available raw materials and reagents, uses a substrate with wide universality, can be compatible with a plurality of functional groups, such as a plurality of glycosidic units, primary alcohol, secondary alcohol, tertiary alcohol, amides and dimethyl malonate, and does not need further protection; and the obtained axial-chirality allene is moderate to good in yield and excellent in diastereoselectivity or enantioselectivity.
Owner:ZHEJIANG UNIV
Binder matrix for gas generants and related compositions and methods
InactiveCN1761636ANitrated acyclic/alicyclic/heterocyclic amine explosive compositionsPressure gas generationCellulose acetateDimethyl malonate
A binder matrix suitable for use in a gas generant composition, a gas generant composition including the binder matrix, and a related method of igniting such a gas generant composition are provided. The binder matrix includes a non-energetic binder material and dimethyl malonate non-energetic plasticizer and has a softening temperature above at least about 125 DEG C. A gas generant composition including the binder matrix includes a non-energetic binder material such as cellulose acetate, dimethyl malonate non-energetic plasticizer, a fuel such as a nitramine fuel such as RDX, HMX or a combination thereof, and a stabilizer such as N-methyl-p-nitroaniline.
Owner:AUTOLIV ASP INC
Synthetic process of 1, 1-cyclopropane dicarboxylic acid dimethyl ester
InactiveCN103864618AGuaranteed yieldGuaranteed purityOrganic compound preparationCarboxylic acid esters preparationPtru catalystMalonic acid
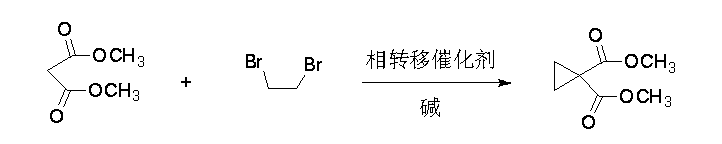
The invention relates to a synthetic process of 1, 1-cyclopropane dicarboxylic acid dimethyl ester, and belongs to the technical field of pharmaceutical raw material intermediate synthesis. The synthetic process is characterized in that dimethyl malonate reacts with dibromoethane under the effect of a phase transfer catalyst under an alkaline condition, and thus 1, 1-cyclopropane dicarboxylic acid dimethyl ester is obtained. The preparation method has the advantages that the raw materials are easy to obtain, conditions are mild, technological operation and controllability are strong, the cost is low, and the yield is high.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
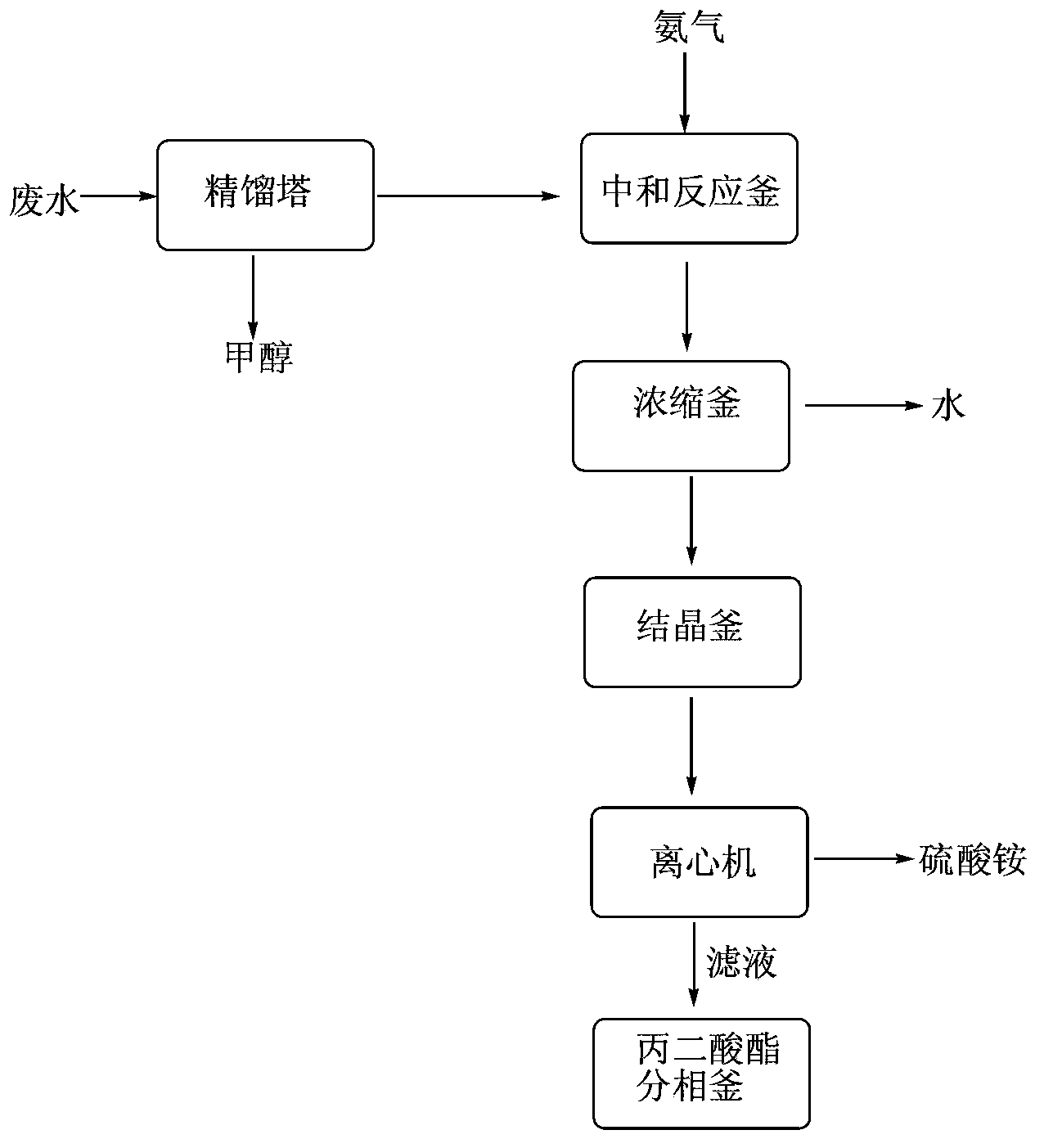
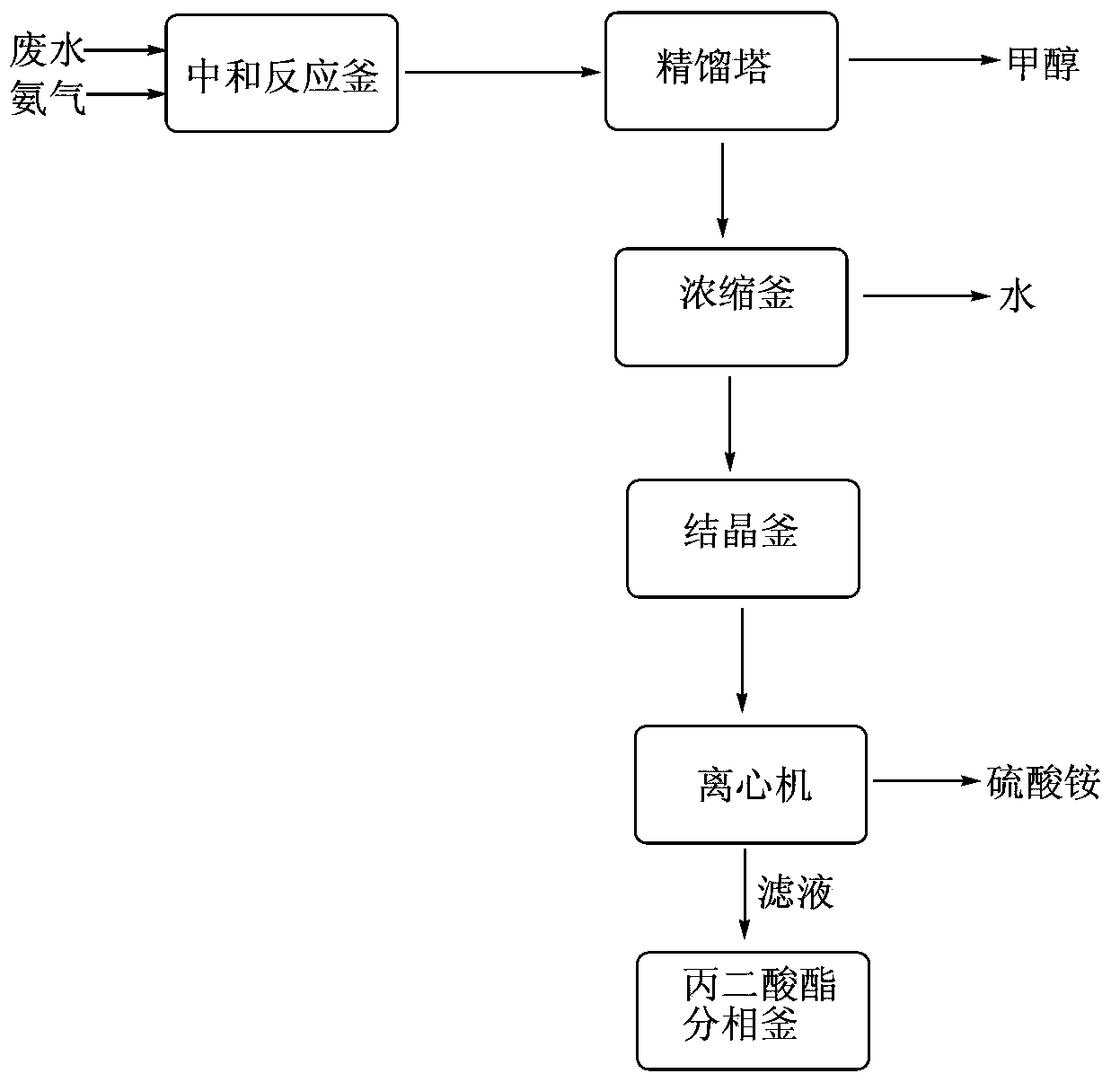
Dimethyl malonate industrial wastewater treatment and resource recovery method
InactiveCN103435441AEfficient use ofReduce pressure on environmental protectionOrganic compound preparationHydroxy compound preparationHigh concentrationAmmonium sulfate
The invention discloses a dimethyl malonate industrial wastewater treatment and resource recovery method which comprises the steps of recovering methyl alcohol through rectifying, neutralizing ammonia and recovering ammonium sulfate through concentrating and crystalizing, or comprises the steps of neutralizing ammonia, recovering methyl alcohol through rectifying, and recovering ammonium sulfate through concentrating and crystalizing. According to the invention, the recovery rate of the methyl alcohol in one batch is more than 85%, the recovered methyl alcohol can be used indiscriminately in the production of dimethyl malonate, the recovery rate of the ammonium sulfate in one batch is more than 70%, the ammonium sulfate can be sold as a by-product, the raffinate left after the methyl and the ammonium sulfate are recovered can be continuously used indiscriminately in the extraction split-phase stage in the production of the dimethyl malonate, due to the treatment with the method provided by the invention, no high-concentration wastewater is discharged in the production of the dimethyl malonate, the useful resources are effectively utilized, the environmental pressure and the production cost are reduced, and the economical effect and the environmental protection effect are remarkable.
Owner:CHONGQING UNISPLENDOUR CHEM
Preparation method of 4-methoxyl pyrrole intermediate
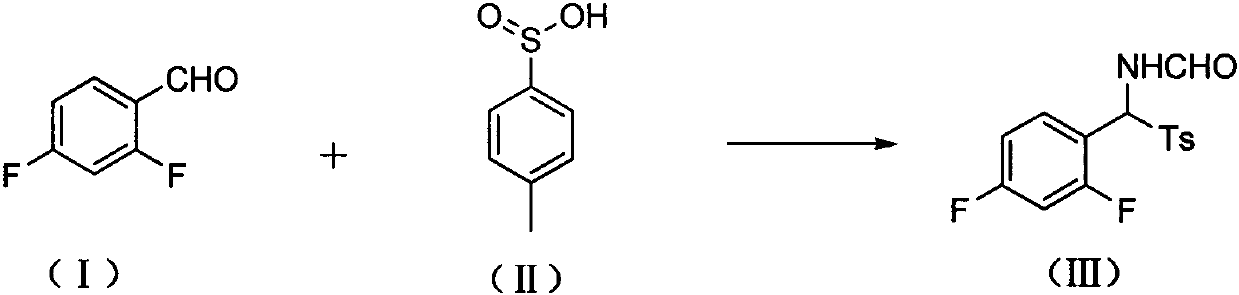
The invention provides a preparation method of a 4-methoxyl pyrrole intermediate. The preparation method comprises the following steps: (1) carrying out a reaction on a compound 2,4-difluorobenzaldehyde represented as in a formula (I) with a compound p-toluenesulfonic acid represented in a formula (II) under a condition of an alkaline solvent and acid catalysis to obtain a compound represented asin a formula (III); (2) carrying out a reaction on the compound represented as in the formula (III) with a compound phosphorus oxychloride in the presence of an alkali to obtain a compound representedas in a formula (IV); and (3) carrying out a reaction on the compound represented as in the formula (IV) with a compound 2-(methoxymethylene)dimethyl malonate represented as in a formula (V) to obtain a compound (4-methoxyl pyrrole intermediate) represented as in a formula (AB03288). According to the method, the raw materials for preparing the 4-methoxy pyrrole intermediate are easy to obtain, the reaction conditions of all the steps are mild, purification is easy, operation is simple, and the yield is high.
Owner:NANJING GEAR PHARMA & TECH CO LTD
Method for increasing yield of dimethyl malonate
InactiveCN106496031AHigh yieldAvoid reactionOrganic compound preparationCarboxylic acid esters preparationDecompositionCyanoacetic acid
The invention provides a method for increasing the yield of dimethyl malonate. The method includes the steps of: 1) acidification reaction: adding acid or hydrogen chloride gas into sodium cyanoacetate to carry out reaction to obtain a cyanoacetic acid aqueous solution; 2) distillation and concentration: adding methyl cyanoacetate into the cyanoacetic acid aqueous solution, conducting distillation and concentration until no water flows out, and removing inorganic salt solid; 3) first esterification reaction: adding methanol and a catalyst into the desalted solution of step 2) to carry out esterification reaction, thus obtaining a solution containing methyl cyanoacetate; 4) desalting: removing inorganic salt solid from the solution; and 5) second esterification reaction: dividing a part of the desalted solution in step 3) and reusing it to step 2), adding methanol to the other part of solution, and carrying out esterification reaction under an acidic condition, thus obtaining dimethyl malonate. The method provided by the invention reduces the consumption of sulfuric acid and generation of wastewater, avoids the decomposition of cyanoacetic acid in the process of concentration and dehydration, and effectively improves the yield of dimethyl malonate.
Owner:内蒙古紫光化工有限责任公司
Novel synthesis method of lobaplatin intermediate
ActiveCN113416150ALow priceReduce manufacturing costOrganic compound preparationOrganic chemistry methodsHydrolysisReaction step
The invention relates to the technical field of medicine synthesis, in particular to a novel synthesis method of a lobaplatin intermediate, the method comprises the following steps: taking low-toxicity dimethyl malonate as an initial raw material, carrying out coupling, bromination, cyclization, hydrolysis, amidation and dehydration reaction to synthesize high-purity trans-1, 2-dicyanocyclobutane. In the whole route synthesis, all adopted raw and auxiliary materials are easy to purchase, low in price and low in toxicity, the reaction conditions are not harsh and uncontrollable, the reaction conditions of each step can be suitable for amplified production, although the reaction steps are increased, the yield of the product is greatly improved, so that the cost is greatly reduced, and the purity of the obtained trans-1,2-dicyanocyclobutane is improved and completely meets the subsequent use requirements, and the market competitiveness is greatly improved.
Owner:上海寻科生物医药科技有限公司
Synthesis method of 4,6-dichloropyrimidine with 4,6-dihydroxypyrimidine serving as midbody
The invention provides a synthesis method of 4,6-dichloropyrimidine with 4,6-dihydroxypyrimidine serving as a midbody. The synthesis method mainly comprises the following steps: adding sodium methylate, dimethyl malonate and formamide into a reaction kettle, fully stirring, then increasing the system temperature, performing heat preservation reaction to remove byproducts, slowly adding sodium methylate drop by drop again, keeping the temperature for half an hour after completion of adding, and performing desolventizing; adding water when the solution is hot, and stirring to cool the solution to realize crystallization; performing centrifugal spin-drying to obtain centrifugal filtrate and crystallized solids, intensively refining and recycling the filtrate, and directly esterifying solid sodium salt; transferring for alkaline hydrolysis, and filtering to obtain a 4,6-dihydroxy pyridine solution; adding trichloromethane and a pyridine catalyst; stirring, slowly feeding phosgene and controlling the reaction temperature; decompressing to distill redundant trichloromethane, and continuously performing suction filtration, concentration and crystallization to obtain white needle-shaped products. The preparation method disclosed by the invention is simple; production of a large amount of wastewater due to multiple acid modulations is avoided; the product yield is high, and the synthesis method is suitable for industrial production.
Owner:ANHUI GUANGXIN AGROCHEM
Preparation method of 6-chlorine-3-methyluracil
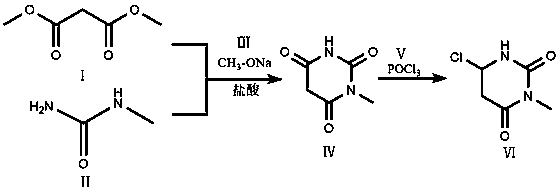
ActiveCN108586360AThe reaction process is green and environmentally friendlyImprove responseOrganic chemistryDimethyl malonateEthyl Chloride
The invention discloses a preparation method of 6-chlorine-3-methyluracil. The preparation method comprises the following steps: cyclizing dimethyl malonate and N-methylurea under an alkaline condition of sodium methylate, acidifying and purifying the mixture to obtain an intermediate 1- methylbarbituric acid, and then chloridizing the intermediate through phosphorus oxychloride to obtain the product. The preparation method has the beneficial effects that the reaction process is more environmentally friendly, and a small number of byproducts are produced; furthermore, no reagents with heavy pollution are used; the reaction process is simple, high in safety and favorable for industrial production; finally, the yield of the finished product is higher.
Owner:EMEISHAN HONGSHENG PHARMA
Energy-saving gasoline additive and preparation method and application thereof
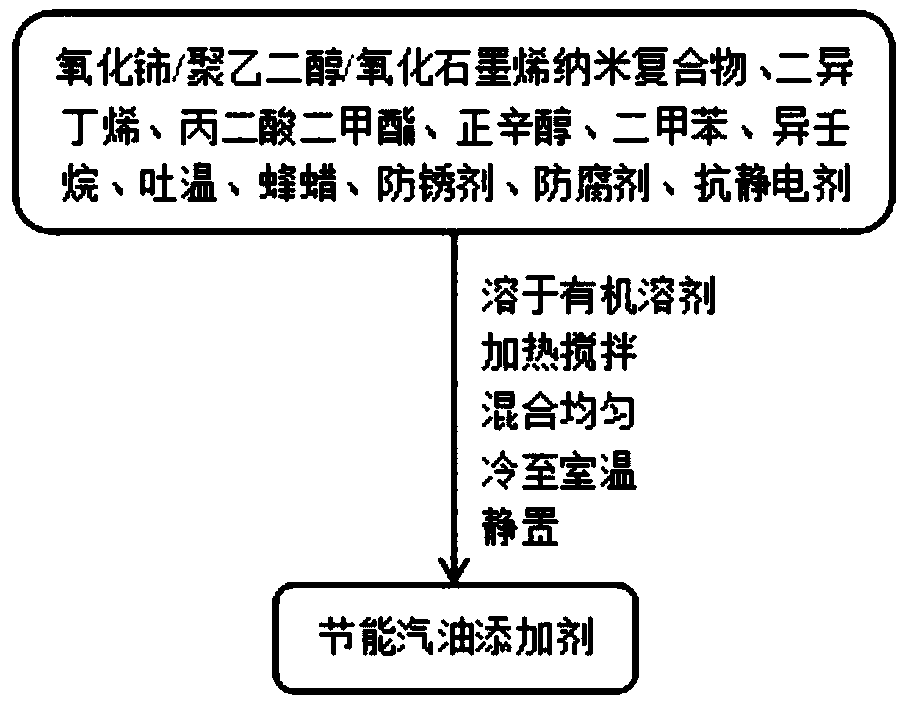
InactiveCN109266404ABurn fullyReduce burnLiquid carbonaceous fuelsFuel additivesPolyethylene glycolOil additive
The invention provides an energy-saving gasoline additive. The energy-saving gasoline additive is prepared from the following raw materials in parts by weight: 30-50 parts of cerium oxide / polyethyleneglycol / graphene oxide nano compound, 10-30 parts of diisobutylene, 10-15 parts of dimethyl malonate, 20-30 parts of n-octanol, 10-30 parts of xylene, 5-15 parts of isononane, 5-15 parts of tween, 10-30 parts of beewax, 10-15 parts of antirust agent, 5-15 parts of preservative, 5-15 parts of anti-static agent and 30-50 parts of organic solvent. Fuel oil can burn more sufficiently by adding the product into the fuel oil at the proportion of 1 to 5000, and the effects of fuel oil conservation, power increasing and pollutant emission reduction can be achieved. The distilling range temperature ofthe gasoline is lowered, the burning performance is improved, the driving performance index of a car is improved, and environmental pollution is reduced.
Owner:宁波蒙曼生物科技有限公司
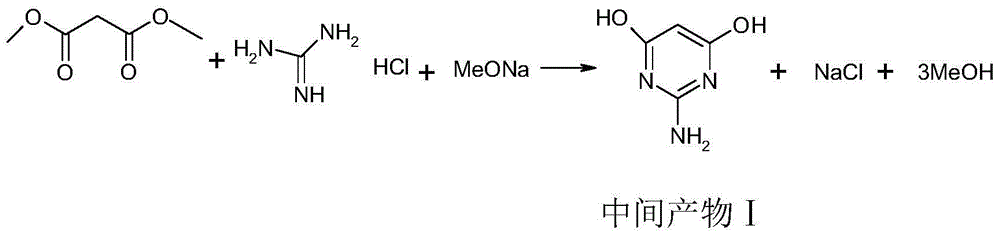
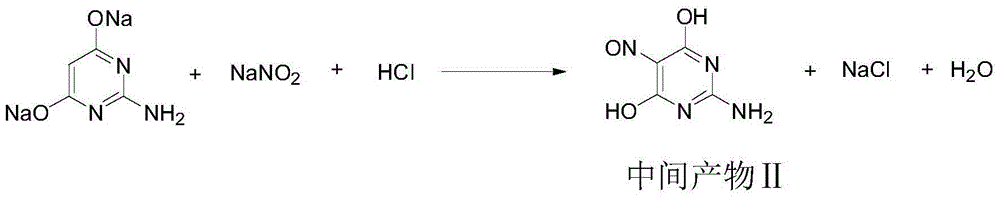
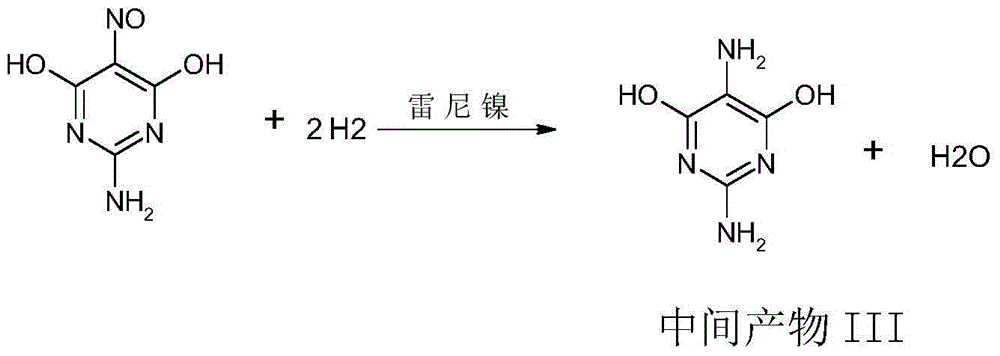
Synthetic method for 2,5-diamino-4,6-dihydroxypyrimidine hydrochloride
The invention discloses a synthetic method for 2,5-diamino-4,6-dihydroxypyrimidine hydrochloride. The synthetic method comprises the following steps: adding guanidine hydrochloride into a sodium methylate solution with a concentration of 28 to 30%, carrying out heating and stirring and adding dimethyl malonate drop by drop; after completion of heat preservation, successively carrying out pressure-reduced distillation, addition of water for dissolving, addition of acid for adjustment of a pH value, cooling, pumping filtration, drying and the like to prepare an intermediate product; and subjecting the primarily prepared intermediate product, a Raney nickel catalyst and the like to continuous conversion so as to prepare 2,5-diamino-4,6-dihydroxypyrimidine hydrochloride. According to the invention, total yield reaches 75% and product purity is up to 99.0%; and the synthetic method provided by the invention can simplify operation, reduce cost and improve reaction yield and is more applicable to industrial production.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com