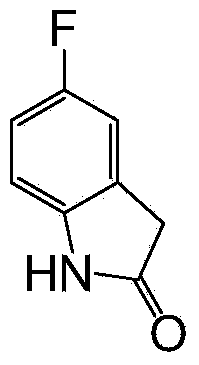
5-fluoroindole-2-one preparation method
A technology of fluoroindole and difluoronitrobenzene, which is applied in the field of preparation of indole derivatives, can solve the problems of expensive raw materials, harsh reaction conditions, unsatisfactory yields, etc., and achieve high yields and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
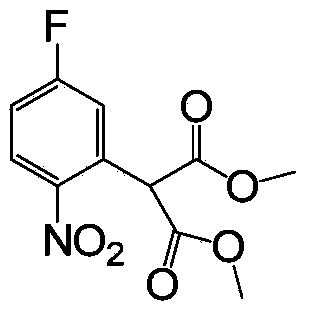
[0021] (1) Preparation of 4-fluoro-2-(dimethylmalonate) nitrobenzene
[0022]
[0023] in N 2 Under the protective atmosphere, add 6.48g (120.0mmol) NaOCH in reaction bottle 3 and dried dimethyl sulfoxide (30mL), add 15.85g (120.0mmol) dimethyl malonate dropwise at room temperature (the dropping time is more than 10min) and stir, then cool to 8°C and slowly add dropwise 6.36g (40.0mmol) 2,4-difluoronitrobenzene (dropping time greater than 40min). Continue to react, TLC tracks the reaction, after the completion of the reaction, add 14mL (80.0mmol) of 6M hydrochloric acid solution under constant stirring to quench, then add ethyl acetate and water to extract, combine the organic layers and wash 2 times with saturated brine, no Dried over sodium sulfate, spin-dried, and separated by column chromatography (eluted with ethyl acetate:petroleum ether=10:1) to obtain 4-fluoro-2-(dimethylmalonate)nitrobenzene, the yield was 88%.
[0024] (2) Preparation of 5-fluoroindol-2-one (t...
Embodiment 2
[0028] (1) The preparation of 4-fluoro-2-(dimethylmalonate)nitrobenzene was the same as step (1) in Example 1.
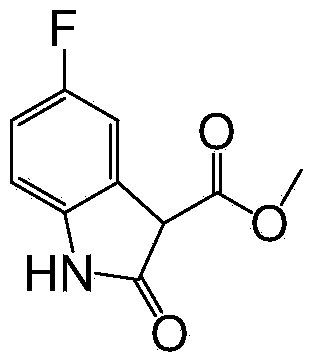
[0029] (2) Preparation of 3-methoxycarbonyl-5-fluoroindol-2-one
[0030]
[0031] in N 2 Under the atmosphere, add 2.71g (10.0mmol) 4-fluoro-2-(dimethylmalonate base) nitrobenzene and 0.24g (10% pd, 55% H 2 (0) palladium carbon, after replacing the hydrogen, add 50mL of ethyl acetate, adjust the temperature to 20°C for reaction, TLC tracking, add diatomaceous earth to filter after the reaction is complete, directly concentrate, column chromatography separation, use ethyl acetate Ester: petroleum ether = 1:1 (V / V) elution, the compound 3-methoxycarbonyl-5-fluoroindol-2-one was obtained with a yield of 92%.
[0032] (3) Preparation of 5-fluoroindol-2-one (target object)
[0033] Add 2.09g (10.0mmol) of 3-methoxycarbonyl-5-fluoroindol-2-one and 50mL of methanol in the reaction flask, add 5mL (30.0mmol) of 6M hydrochloric acid, and turn to reflux for reaction, TLC...
Embodiment 3
[0035] (1) Preparation of 4-fluoro-2-(dimethylmalonate) nitrobenzene
[0036] in N 2 Under the protective atmosphere, add 6.48g (120.0mmol) NaOCH in reaction bottle 3 and dried dimethyl sulfoxide (30mL), add 13.21g (100.0mmol) dimethyl malonate dropwise at room temperature (dropping time is more than 10min) and stir, then cool to 15°C and slowly add dropwise 6.36g (40.0mmol) 2,4-difluoronitrobenzene (dropping time greater than 40min). Continue to react, TLC tracks the reaction, after the completion of the reaction, add 14mL (80.0mmol) of 6M hydrochloric acid solution under constant stirring to quench, then add ethyl acetate and water to extract, combine the organic layers and wash 2 times with saturated brine, no Dry over sodium sulfate, spin dry, and separate by column chromatography (eluted with ethyl acetate:petroleum ether=10:1 (V / V)) to obtain 4-fluoro-2-(dimethylmalonate) nitrate Base benzene, the yield is 80%.
[0037] (2) Preparation of 5-fluoroindol-2-one (target ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com