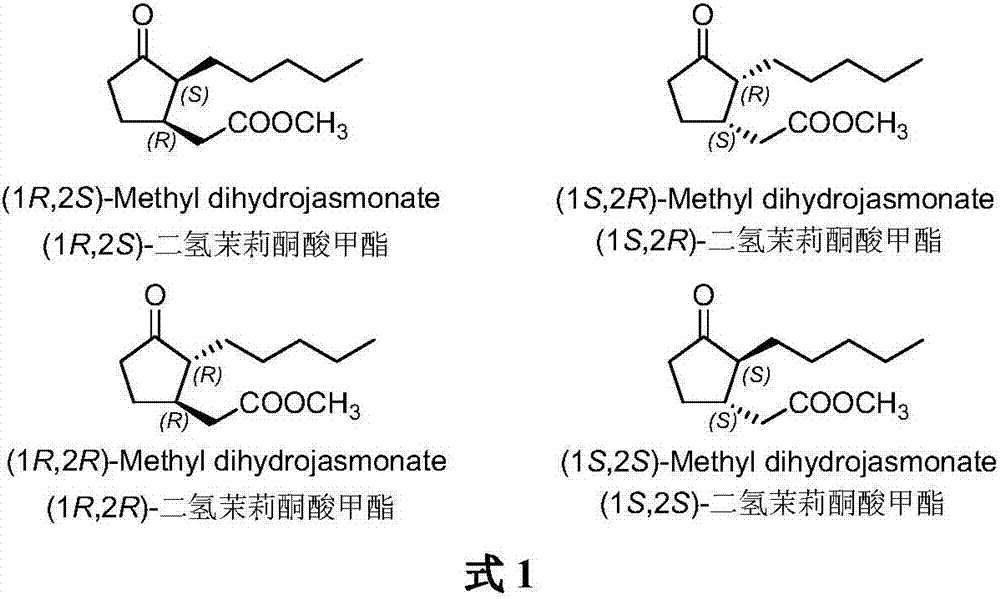
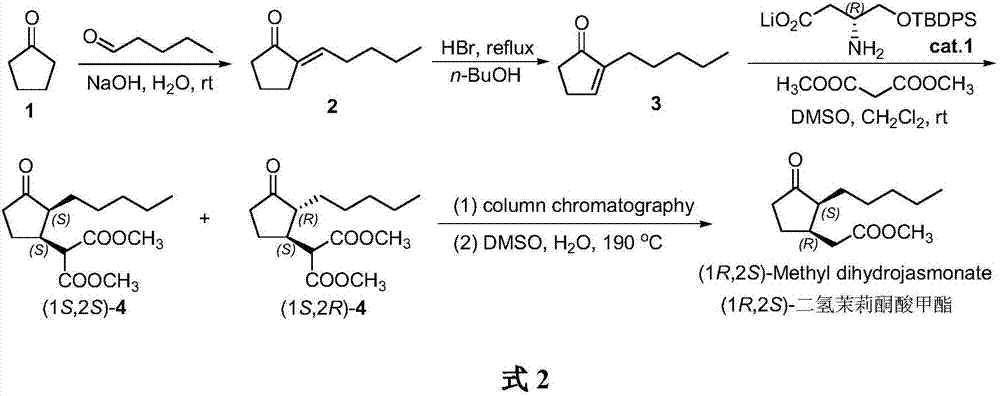
Method for synthesizing (1R,2S)-methyl dihydrojasmonate
A technology of methyl dihydrojasmonate and dimethyl malonate cyclopentanone is applied in the field of fragrance and essence chemistry, and can solve problems such as complicated synthesis routes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of 2-pentylidene cyclopentanone 2
[0022] Under nitrogen protection, NaOH (0.20 g, 0.5 mmol) and 15 mL of water were added to a 100 mL four-necked flask, and stirred to dissolve. Slowly add cyclopentanone 1 (8.40 g, 100 mmol) at room temperature, and after stirring evenly, add n-valeraldehyde (5.18 g, 60 mmol) dropwise through the dropping funnel. After the dropwise addition, the stirring reaction was continued for 1 h at room temperature. After the reaction, the reaction solution was adjusted to neutrality with acetic acid. Distillation under reduced pressure gave 2-pentylidene cyclopentanone 2 (7.30 g, yield 80%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δ6.20(t,J=6.1Hz,1H),2.80-2.71(m,2H),2.50-2.40(m,2H),2.23-2.14(m,2H),1.51-1.44(m,2H) ,1.43-1.30(m,4H),0.89(t,J=6.7Hz,3H).HRMS(APCI-TOF)calcd for C 10 h 17 O[M+H] + 153.1279, found 153.1272.
Embodiment 2
[0024] Synthesis of 2-n-pentyl-2-cyclopentenone 3
[0025] 2-Pentylidene cyclopentanone 2 (4.57 g, 30 mmol) was added into a 100 mL reaction flask, and 30 mL of a n-butanol solution containing 5% HBr was added with stirring. The reaction mixture was heated to reflux, and the stirring reaction was continued for 2h. After the reaction finished, the temperature of the reaction solution was down to room temperature, and the 2 CO 3 Aqueous solution was used to adjust the reaction solution to neutrality. Distillation under reduced pressure gave 2-n-pentyl-2-cyclopentenone 3 (3.88 g, yield 85%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δ6.22(t,J=6.0Hz,1H),2.78-2.69(m,2H),2.50-2.42(m,2H),2.21-2.12(m,2H),1.51-1.43(m,2H) ,1.42-1.28(m,4H),0.88(t,J=6.7Hz,3H).HRMS(APCI-TOF)calcd for C 10 h 17 O[M+H] + 153.1279, found 153.1274.
Embodiment 3
[0027] Synthesis of (1S,2S)-2-n-pentyl-3-malonate dimethylcyclopentanone 4
[0028]At room temperature, add 5mL dimethyl sulfoxide and 10mL dichloromethane into a 50mL Schlenk reaction flask, add chiral amino acid lithium salt Cat.1 (0.72g, 2mmol) and 2-n-pentyl-2- Cyclopentenone 3 (1.52g, 10mmol), stir well. Dimethyl malonate (2.64 g, 20 mmol) was then added, and the reaction mixture was stirred at room temperature for 48 h. After the reaction was completed, 15 mL of saturated NaCl aqueous solution was added to extract the reaction. The organic phase was separated and the aqueous phase was extracted with ether (3 x 20 mL). The combined organic phases were washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. Purify by silica gel column chromatography (petroleum ether / ethyl acetate 10:1) to obtain light yellow oil (1S,2S)-2-n-pentyl-3-malonate dimethyl cyclopentanone and (1S,2S) 2R) - Mixture of 2-n-pentyl-3-malonate dimethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com