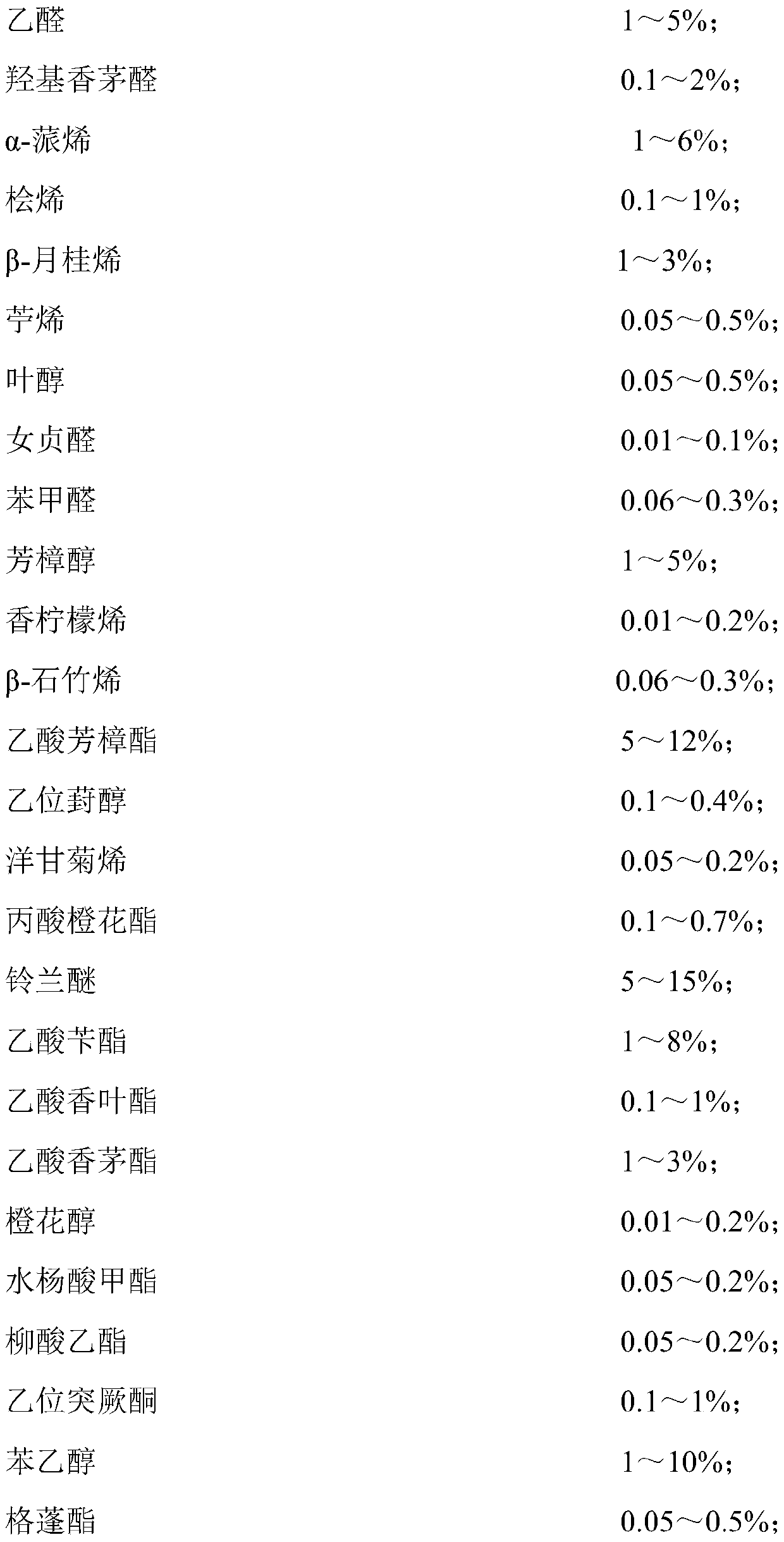
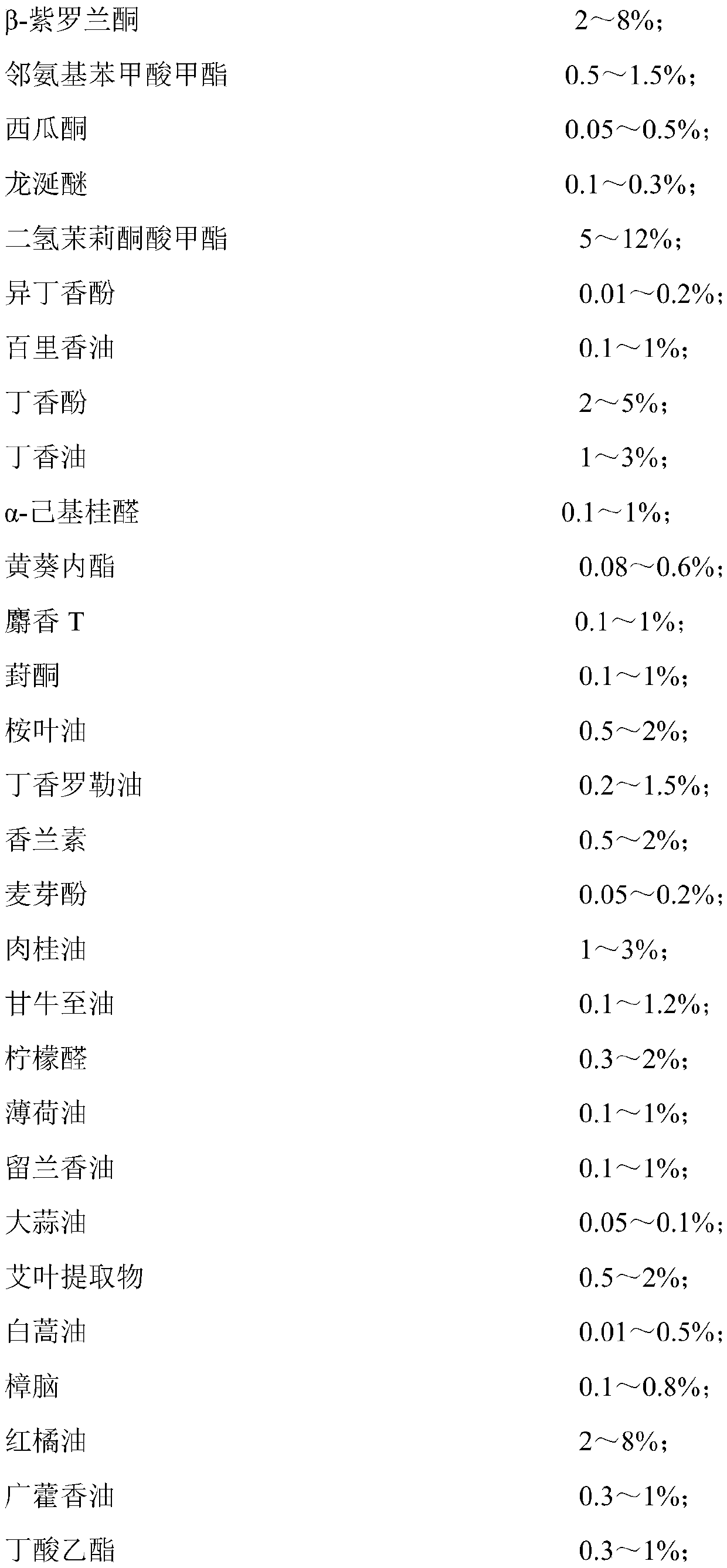
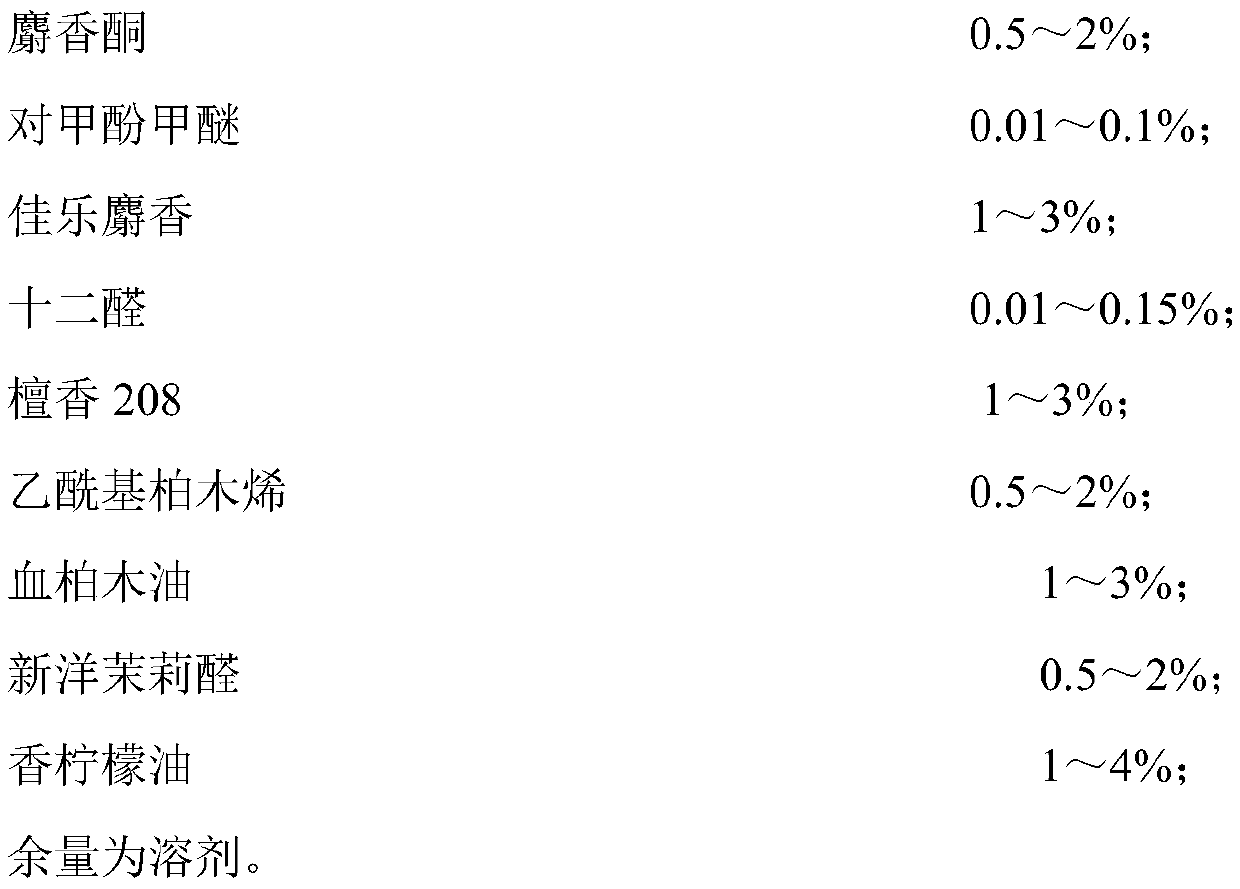
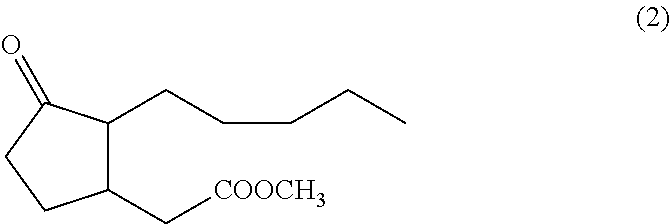Patents
Literature
127 results about "Methyl dihydrojasmonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
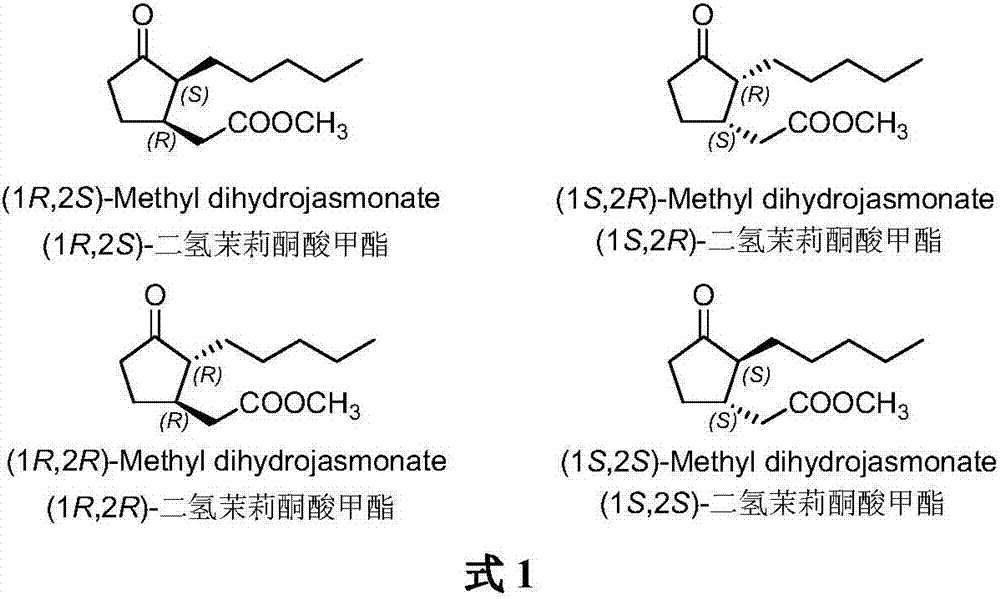
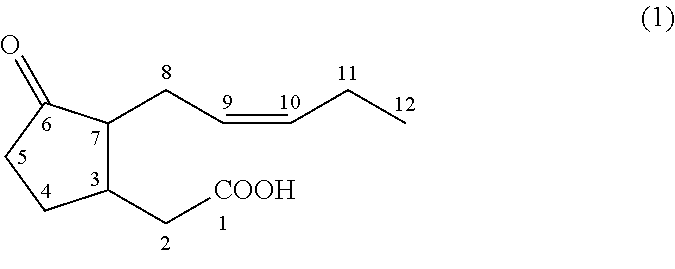
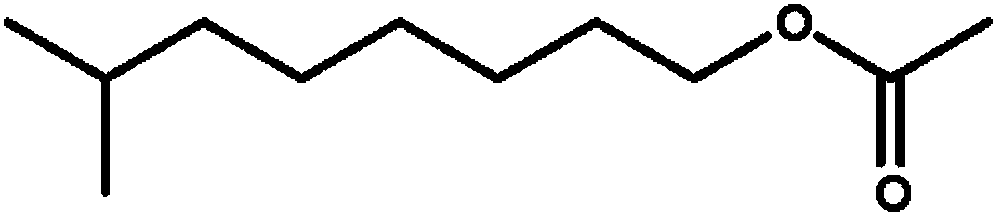
Methyl dihydrojasmonate is an aroma compound, that smells similar to jasmine. In racemic mixtures the odor is floral and citrus while epimerized mixtures exhibit a dense fatty floral odor with odor recognition thresholds of 15 parts per billion.
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
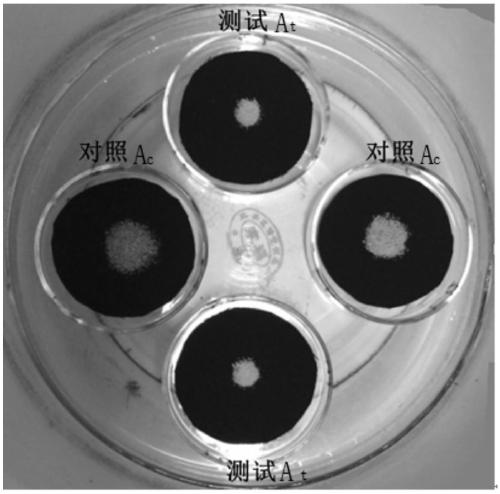
Exogenous Methyl Dihydrojasmonate for Prevention and Control of Biotic Attack in Plants
InactiveUS20090082453A1Preventing and controlling attackBiocideAnimal repellantsDiseaseMethyl dihydrojasmonate
Formulations and methods for treating and preventing biotic attack, including disease and insect infestation, in plants are disclosed. The formulations include methyl dihydrojasmonate:Formulations according to embodiments of the invention are particularly suitable for controlling insect infestation and disease in roses.
Owner:NEW BIOLOGY
Flavoring rose essence
This invention discloses a rose essence, which comprises: crimson glory flower concrete 0.5-2.0 wt.%, cinnamyl alcohol 1.0-3.0 wt.%, phenylethanol 5.0-15.0 wt.%, rose absolute oil 0.5-2.0 wt.%, Jasminum sambac concrete 0.5-2.0 wt.%, linalool 1.5-3.0 wt.%, pearl everlasting oil 1.0-3.0 wt.%, patchouly oil 0.5-3.0 wt.%, 92% ionone 6.0-12.0 wt.%, geranium oil 7.0-15.0 wt.%, 208# sandalwood 2-7.0 wt.%, ylang oil 1.5-5 wt.%, lilial 1.5-5 wt.%, damascene 6.0-15.0 wt.%, methyl dihydrojasmonate 3.0-7.0 wt.%, Jiale muskiness 1.0-6 wt.%, Petitgrain oil 1.5-5 wt.%, citronellol 8.0-20.0 wt.%, rose oil 0.5-2.0 wt.%, 10% delta-decalactone 0.2-1.0 wt.%, rose nitrile 1.0-3.5 wt.%, geraniol 8.0-15.0 wt.%, lemon oil 2.0-6.0 wt.%, and methyl ionone 0.5-3.0 wt.%. The rose essence can be used in fragrant products, such as white cream, and emulsion, and has such advantages as lasting color, pure and lasting fragrance, and small dosage.
Owner:卢伯奇
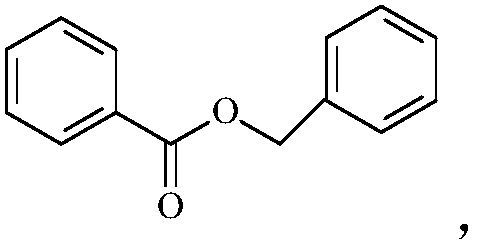
Detergent composition with mite control function
PendingCN109234055AGood anti-mite effectPrevent intrusionInorganic/elemental detergent compounding agentsSoap detergents with organic compounding agentsBenzyl benzoatActive agent
The invention relates to the technical field of daily chemical fabric washing products, in particular to a detergent composition with a mite control function. As benzyl benzoate and methyl dihydrojasmonate with certain content are added in the detergent composition, the detergent has the effect of inhibiting mites from invading washed textile; and moreover, as appropriate amount of surfactant anddetergent assistant are cooperated with benzyl benzoate and methyl dihydrojasmonate, the coordination effect among components is increased, and as a result, the detergent composition has a better mitecontrol effect.
Owner:GUANGZHOU LIBY
Antibacterial plastics for toys and preparation method for antibacterial plastics
InactiveCN106589708AImprove impact resistanceImprove antibacterial propertiesEngineering plasticPolyvinyl chloride
The invention discloses antibacterial plastics for toys and a preparation method for the antibacterial plastics. The antibacterial plastics are prepared from the following raw materials in parts by weight: 30-45 parts of polyvinyl chloride, 10-25 parts of ABS engineering plastics, 1-5 parts of tin di-n-octyl dilaurate, 0.5-2 parts of calcium phosphate, 0.5-2 parts of methyl dihydrojasmonate, 1-4 parts of expandable graphite, 1-3 parts of chlorinated lauryl trimethyl ammonium, 3-5 parts of bamboo-leaf phenolic ketone and 1-5 parts of plasticizer. The antibacterial plastics for the toys have good impact resistance and play a role in inhibiting or killing bacteria, molds, yeasts, algae, even viruses and the like, which are stained to toy plastics, in operating environments, the plastics are kept clean through inhibiting the propagation of microbes, and the antibacterial effect is good; the preparation process is simple, and the production cost is relatively low; and the plastics are safe and non-toxic, so that the physical health of children is protected.
Owner:ZHENGZHOU ZHANGMENG NETWORK TECH CO LTD
Lily essence for daily chemicals and preparation method thereof
ActiveCN104946391AIncrease momentumStrong floral fragranceEssential-oils/perfumesBiotechnologyPropanoic acid
The invention provides a lily essence for daily chemicals and a preparation method thereof. The lily essence is prepared from the following raw materials: cis-6-nonenol; kalong; ethyl vanillin; florhydral; cyanine; allyl cyclohexyloxyacetate; prenylacetate; pino acetaldehyde%; melonal%; allyl heptylate; styralyl acetate; matricaria ester; fructone; cis-3-hexenyl acetate; ligustral; geranyl acetate; geranyl acetate; Delta-Damascone; hexyl acetate; cis-3-Hexenyl salicylate; sandalore; ethyl-2-methylbutyrate; vanillyl alcohol; phenoxyethyl isobutyrate; allyl cyclohexanepropionate; methyl decenol; phenethyl alcohol; cyclamen aldehyde; verdyl acetate; isomethyl ionone; lemon oil; benzyldimethylcarbinyl butyrate; 2-tert-butylcyclohexanol acetate; iso E super; galaxolide stock solution; peach aldehyde; hexyl salicylate; Lily aldehyde; linalool; methyl dihydrojasmonate; and dipropylene glycol. The lily essence has fresh and penetrating top note, strong natural sense, full body note, good floral sense, and lasting basic note.
Owner:广东铭康香精香料有限公司
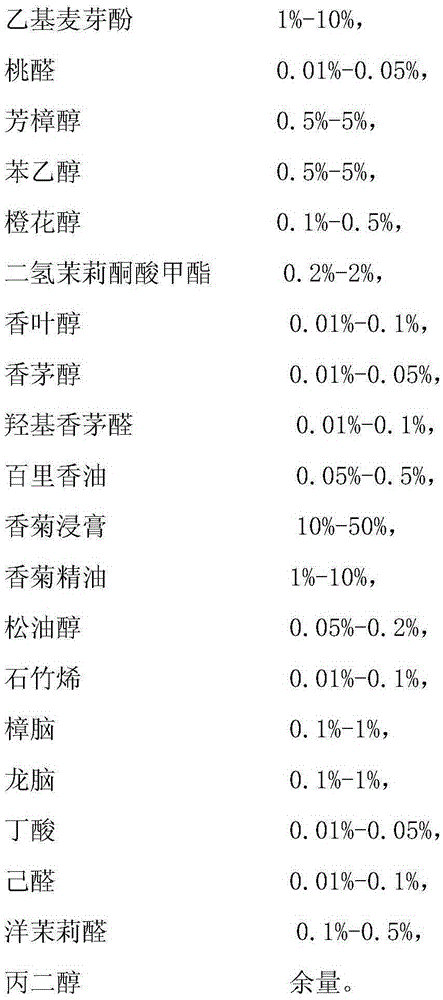
Mixed type chrysanthemum essence and preparation method thereof
InactiveCN105349263AStrong aromaDelicate aromaEssential-oils/perfumesCrossostephiumPhenethyl alcohol
The invention discloses mixed type chrysanthemum essence. The mixed type chrysanthemum essence is prepared from bergamot oil, sweet orange oil, cis-3-hexenyl acetate, citral, ligustral, ethyl acetate, tetrahydrogeraniol, dimethyl benzyl orthoester, myristica oil, alpha-damascone, ethyl maltol, peach aldehyde, linalool, phenethyl alcohol, nerol, methyl dihydrojasmonate, geraniol, citronellol, hydroxycitronellal, thyme oil, crossostephium leaf extract, crossostephium leaf essence, terpilenol, caryophyllene, camphor, borneol, butyric acid, hexanal, heliotropin and propylene glycol. The invention further provides a preparation method of mixed type chrysanthemum essence. Chrysanthemum essence which is strong in fragrance, natural and vivid is obtained by conducting mixing according to the mass percent.
Owner:SHANGHAI INST OF TECH
Microcapsule flavor for anti-depression pleasant laundry detergent
InactiveCN106148006AUniform size distributionFull shapeEssential-oils/perfumesDetergent perfumesLaundry detergentEssence oil
The invention discloses a microcapsule flavor for an anti-depression pleasant laundry detergent. The microcapsule flavor is prepared from the following raw materials in parts by weight: 50-60 parts of pomelo peel, 45-50 parts of lemon peel, appropriate amount of distilled water, 2-3 parts of petitgrain oil, 1-1.2 parts of methyl dihydrojasmonate, 3.5-4 parts of ambrotone, 0.3-0.4 part of arabic gum, appropriate amount of deionized water, 2.5-3 parts of ethylene glycol dimethacrylate, 0.1-0.11 part of ammonium persulfate, 0.13-0.14 part of sodium metabisulfite and 1-1.2 parts of sodium carboxymethylcellulose. According to the invention, by extracting the mixed essential oil of lemon and pomelo and mixing with the petitgrain oil and methyl dihydrojasmonate and mixing the fragrant and sweet citrus aroma with similar jasmine flower aroma and in cooperation with the addition of ambrotone, the aroma is vivid, harmonic, refreshing and lasting; and in a washing process, the microcapsule flavor can effectively cover the bad smell emitted by the base material dissolved in water, offers lasting aroma on the fabric surface and brings refreshing and pleasant feeling to people.
Owner:ANHUI SANHUAN PAPER GRP SPICE SCI & TECH DEV
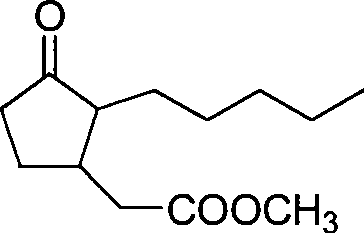
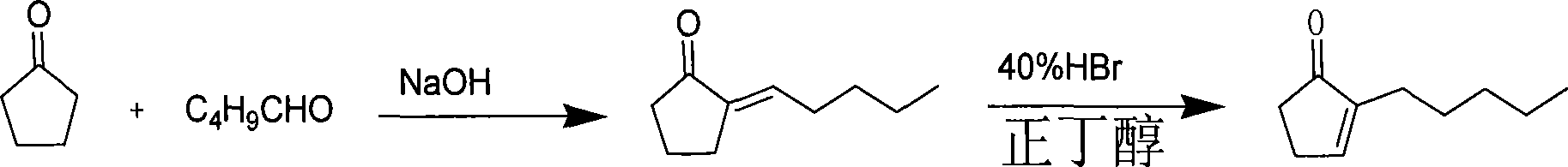
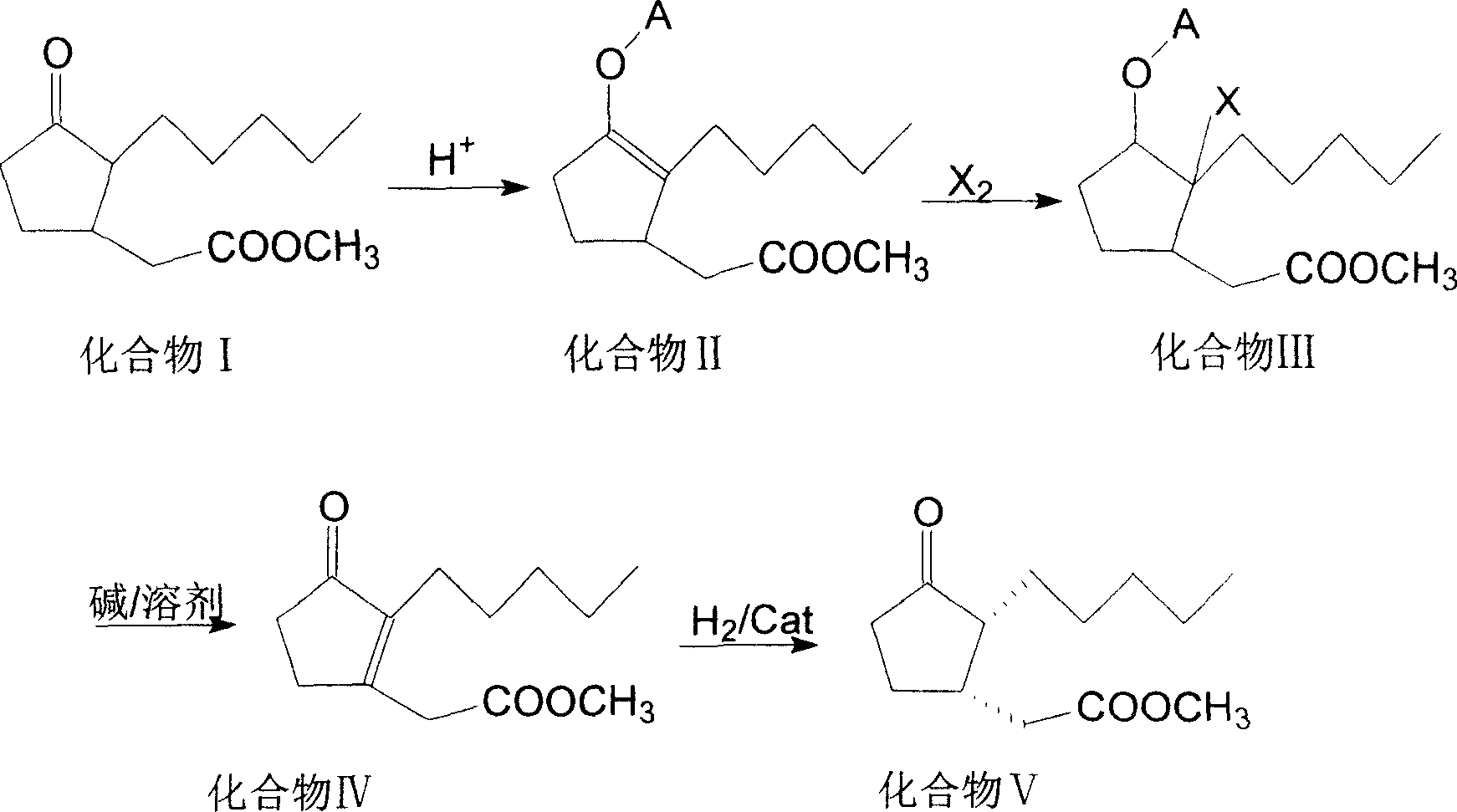
Method for preparing methyl dihydrojasmonate
InactiveCN101519355AShort synthetic routeShort synthesis cycleOrganic compound preparationCarboxylic acid esters preparationZinc bromideMethyl dihydrojasmonate
The invention provides a method for synthesizing methyl dihydrojasmonate. 2-pentyl cyclopentenone is adopted as a raw material; the raw material and allyl zinc bromide are subjected to 1,4-Michael addition to form 2-pentyl-3-allyl cyclopentanone; the 2-pentyl-3-allyl cyclopentanone is oxidized by sodium periodate in the presence of a ruthenium trichloride hexahydrate catalyst to synthesize dihydrojasmonic acid; and then the dihydrojasmonic acid is esterified to form the methyl dihydrojasmonate. The raw material of the method is cheap and low in cost; the process is simple and convenient in operation; the reaction conditions are mild and easy to control; the synthesis route is short and the synthesis period is reduced greatly; and the reaction product is single, high in yield (up to 65 to 85 percent), less in environmental pollution, and environmentally-friendly.
Owner:NORTHWEST NORMAL UNIVERSITY
Method for producing 2-alkylidene cyclopentanone
ActiveCN101654404ATaking care of protectionSave resourcesOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBoiling pointWastewater
The invention discloses an environment-friendly method for producing 2-alkylidene cyclopentanone, which uses cyclopentanone and fatty aldehyde as raw materials and uses amine type weak-base ion-exchange resin for reaction under the condition of existence of fatty acid to produce 2-alkylidene cyclopentanone. The resin and the fatty acid which are separated can be recycled. The 2-alkylidene cyclopentanone is a key intermediate for synthesizing many spices (such as methyl dihydrojasmonate and delta-lactone). The traditional technology of the 2-alkylidene cyclopentanone has the outstanding problems that the acid-base solution catalytic system is adopted, which causes that the catalyst is very difficult to be recycled, the product yield is low, many by-products with high boiling points are generated, the reaction selectivity is poor, and a large amount of waste water is generated, thereby wasting resources and polluting environment. The method of the invention uses a catalytic system whichcan be recycled to replace the traditional solution catalytic system which can not be recycled, uses the cleaning production technology to improve the original technology, and simultaneously achievesthe purposes of saving and fully utilizing resources and protecting the environment.
Owner:河南蔚源生物科技有限公司
Natural strawberry essence and preparation method thereof
InactiveCN102408945AStrong sense of natureRealistic aromaTobacco preparationEssential-oils/perfumesIrritationLinalool
The invention relates to tobacco essence and a production method thereof, in particular to natural strawberry essence and a preparation method thereof. The tobacco essence is prepared by mixing the following raw materials by weight percent: 10-15% of strawberry concentrate, 0.1-0.3% of acetic acid, 0.5-1.5% of leaf alcohol, 0.01-0.03% of acetaldehyde, 0.1-0.3% of hexanoic acid, 0.1-0.5% of ethyl maltol, 0.8-1.2% of methyl cinnamate, 0.03-0.07% of methyl dihydrojasmonate, 0.1-0.4% of deoxy linalool, 0.1-0.5% of gamma-decalactone, 0.3-0.5% of gamma-octalactone, 0.2-0.4% of delta-dodecalactone and the balance of propylene glycol. The natural strawberry essence has stronger natural sense, is purer, has real fragrance and can improve the cigarette fragrance, reduce irritation, mellow the smoke and ensure sweet aftertaste in the oral cavity after being added to the cigarettes.
Owner:WUHAN HUANGHELOU FLAVOR & SPICES
Strawberry essence for oil-based ink and preparation method of strawberry essence
InactiveCN105132175AIncrease added valueEasy to useInksEssential-oils/perfumesSolubilityCinnamyl acetate
The invention relates to strawberry essence for oil-based ink. The strawberry essence consists of strawberry essence, maltodextrin and starch sodium octenylsuccinate, wherein the strawberry essence consists of ethyl acetate, isoamyl acetate, ethyl caproate, ethyl butyrate, allyl hexanoate, leaf alcohol, cis-3-hexenyl acetate, decalactone, peach aldehyde, ethyl benzoate, geranyl acetate, citronellyl acetate, dihydrojasmonic acid methyl ester, hexyl salicylate, linalool oxide, trans-2-hexenol, eugenol, cinnamyl alcohol, cinnamyl acetate, beta-Ionone, vanillin, methyl cyclopentenolone, furanone, raspberry ketone, ethyl methylphenylglycidate, ethyl palmitate, benzyl benzoate and glycerol triacetate. The invention also provides a preparation method of the strawberry essence for the oil-based ink. The strawberry essence, the maltodextrin, the starch sodium octenylsuccinate and water are mixed; homogenizing and emulsification are performed; then, spray drying is carried out; particular strawberry essence is formed, so that the aroma retaining effect and the oil solubility of the essence are improved; the strawberry essence is applicable to perfuming of the oil-based ink.
Owner:SHANGHAI INST OF TECH
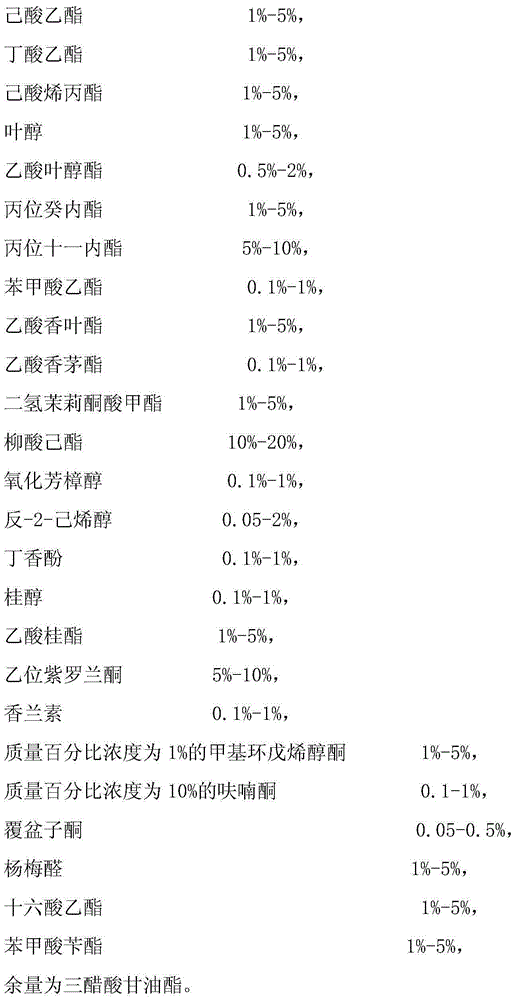
Perfume essence added with evertal
The invention discloses a perfume essence added with evertal. The perfume essence mainly comprises the following components in mass percent: 10% of leaf alcohol, 1.2% of D-limonene, 0.2% of liffarome iff, 4.2% of import linalool, 0.1% of evertal, 1% of ethyl maltol, 5% of linalyl acetate, 5% of methyl violet, 2% of cashmeran, 5% of helional, 6% of methyl cedryl ether, 15% of methyl dihydrojasmonate, 8% of methyl cedryl ketone, 15% of galaxolide, 2% of exaltolide, 23.6% of dipropylene glycol and the like. The essence has the characteristics of pervaporation, agreeability and lasting aroma because of the raw material combination.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Method for preparing jasmine-fragrant master batch for perfuming composite fiber
The preparation method of jasmin essence mother granules for perfuming composite fibre includes the following steps: using the components of methyl dihydrojasmonate, hexyl cinnamic ester, licarcoal, dihydromyrcenol, leaf alcohol and other 10 components to prepare jasmin essence according to its formula; mixing said jasmin essence together with ethylene vinyl acetate copolymer (EVA) granules and heating to make the essence be eneloped in the EVA resin so as to obtain the invented mother granules with jasmin fragrance. The polyester / polypropylene and polypropylene / polyethylene core-sheath composite aromatic short fibre made up by said mother granules can be used as filling material oil quilt, pillow, cushion and toys, etc. or can be used as dress material and internal decorative material.
Owner:上海香料研究所
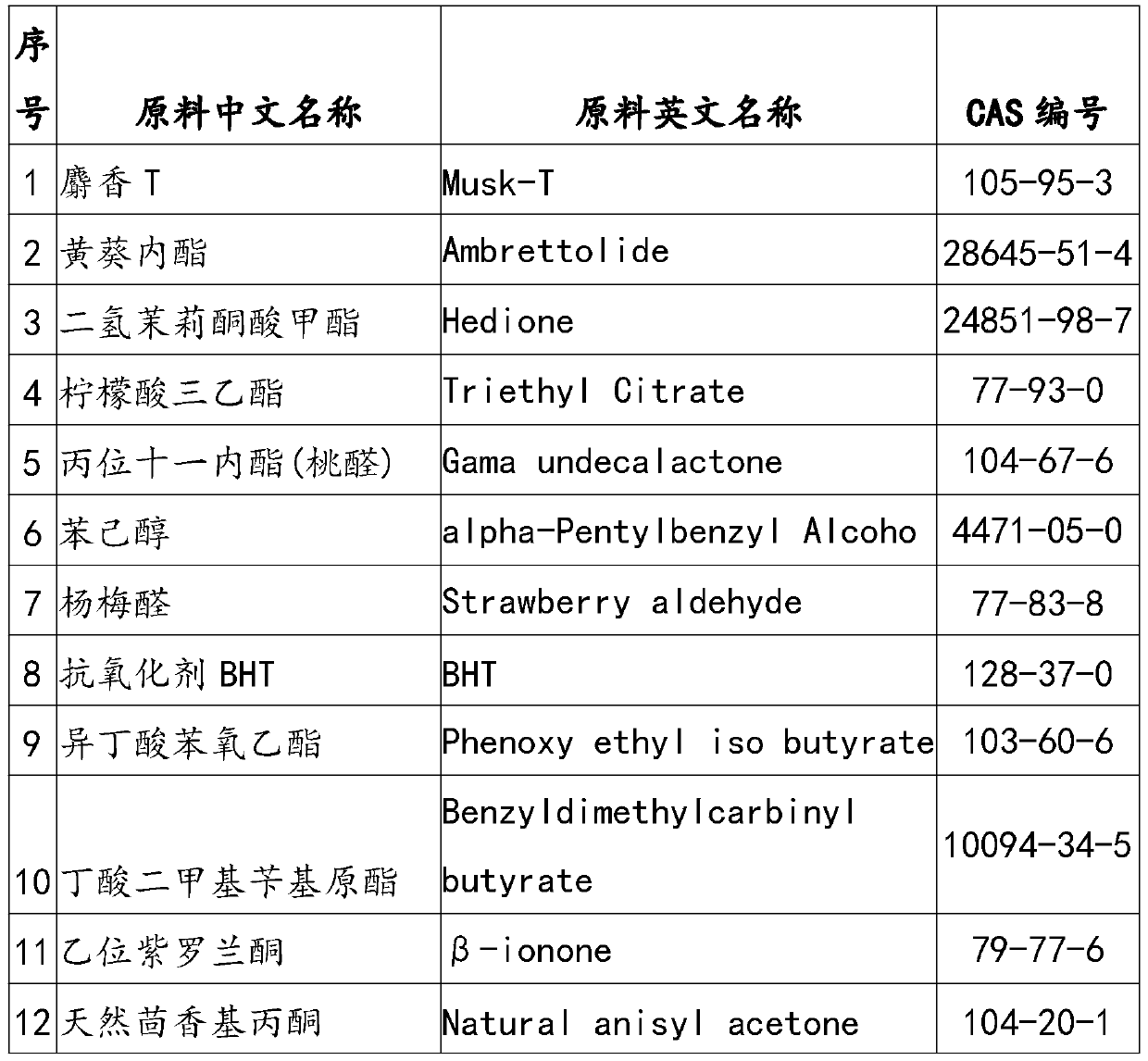
Cherry essence and essence microcapsules
ActiveCN111205924AImprove general performanceInstabilityEssential-oils/perfumesMicroballoon preparationBenzaldehydeHexyl acetate
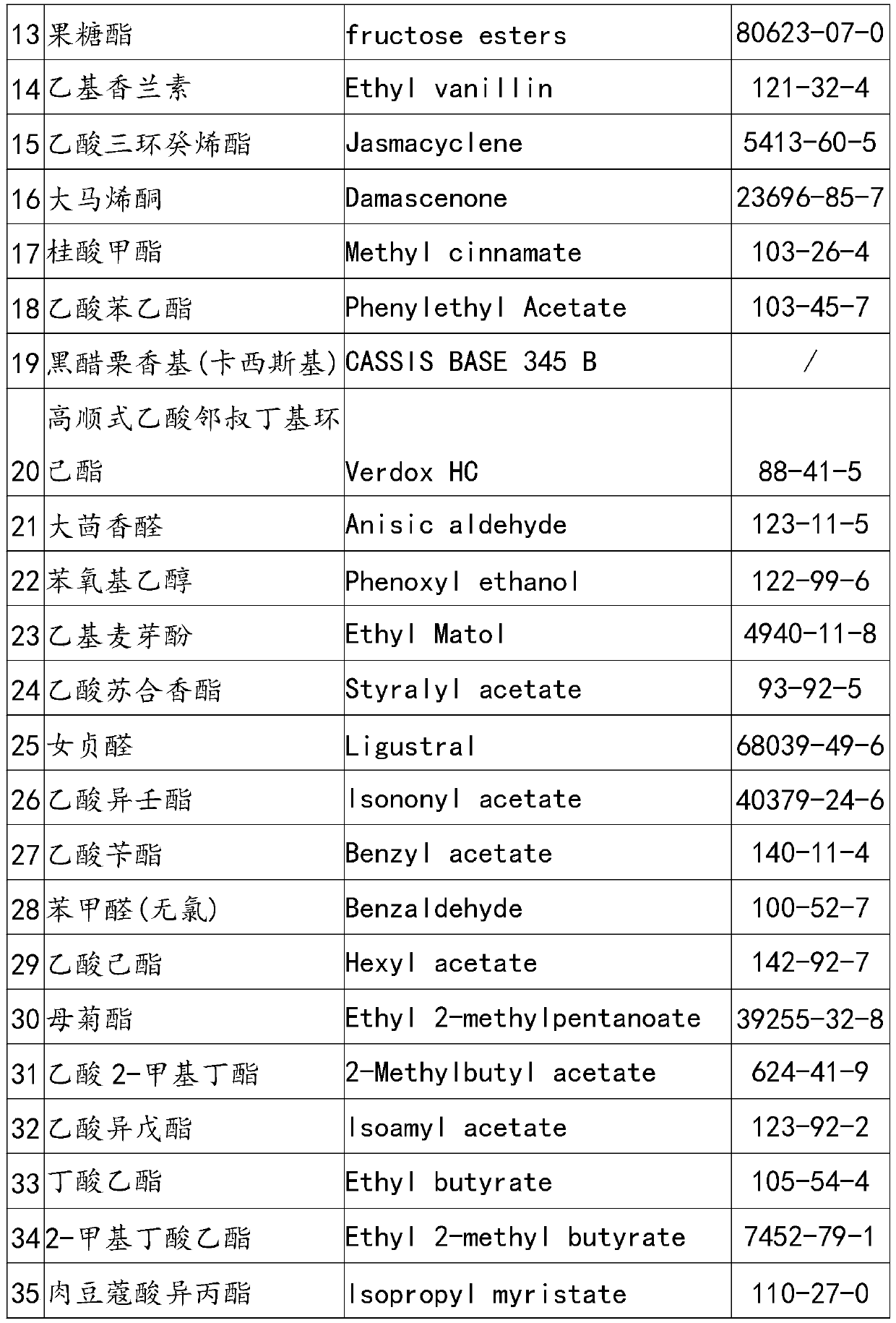
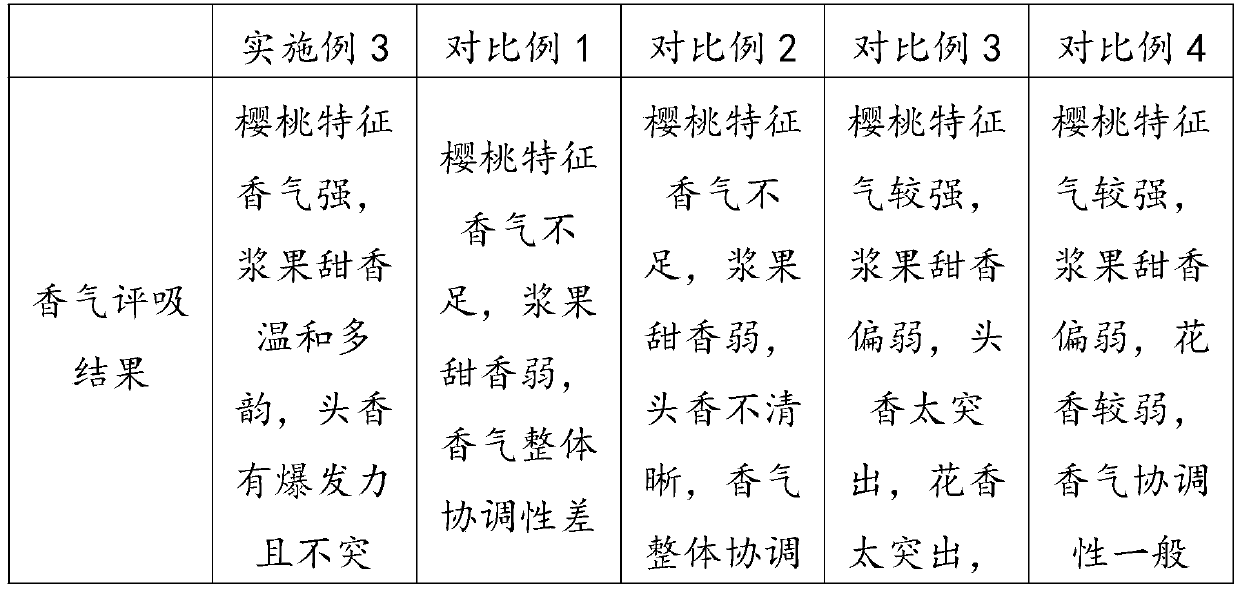
The invention discloses cherry essence and essence microcapsules. The cherry essence comprises musk, abelmoschus esculentus lactone, methyl dihydrojasmonate, triethyl citrate, gamma-undecalactone, phenyl-1-hexanol, 3-Methyl-3-phenylglycidic acid ethyl ester, phenoxyethyl isobutyrate, dimethyl benzyl carbinyl butyrate, beta-ionone, natural anisylacetone, fructose ester, ethyl vanillin, tricyclodecenyl acetate, damascenone, methyl cinnamate, phenylethyl acetate, blackcurrant essence, high-cis o-tert-butyl cyclohexyl acetate, anisic aldehyde, phenoxyethanol, ethyl maltol, styrallyl acetate, ligustral, isononyl acetate, benzyl acetate, benzaldehyde, hexyl acetate, ethyl 2-methylvalerate, 2-methyl butyl acetate and the like. The cherry essence prepared by the invention not only has strong cherry fragrance, but also has natural soft feeling and high harmony, and the cherry essence microcapsules prepared by taking the cherry essence as a raw material can keep excellent flavor quality of the cherry essence in long-term storage.
Owner:宁波芬畅凝科香精香料有限公司
Perfume essence added with musk and fruit ester
InactiveCN103103023AHigh strengthSteady patternCosmetic preparationsToilet preparationsFuranGeraniol
The invention discloses a perfume essence added with musk and fruit ester. The perfume essence mainly comprises the following components by mass percent: 15% of methyl dihydrojasmonate, 2% of alpha-hexylcinnamaldehyde, 4% of lilialdehyde, 40% of ambrotone, 2% of phenylethyl alcohol, 1% of dihydromyrcenol, 1% of cinnamic alcohol, 0.01% of leaf alcohol, 0.8% of vanillyl alcohol, 0.02% of linalool, 1% of geraniol, 5% of ethyl linalool, 6% of florol, 0.2% of dodecahydro-3a, 6, 6, 9a-tetramethyl-naphtol [2, 1-b] furan-3a, 6, 6, 9a-tetrame, 10% of musk and fruit ester and the like. The essence added with musk and fruit ester has the characteristics of stable structure, compound aroma, gradually-released fragrance, novel aroma and lasting fragrance.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Quality modifier for improving red fruits, method for preparing quality modifier and application thereof
InactiveCN105613496APromotes the accumulation of sugarGood colorBiocidePlant growth regulatorsFruit treePotassium
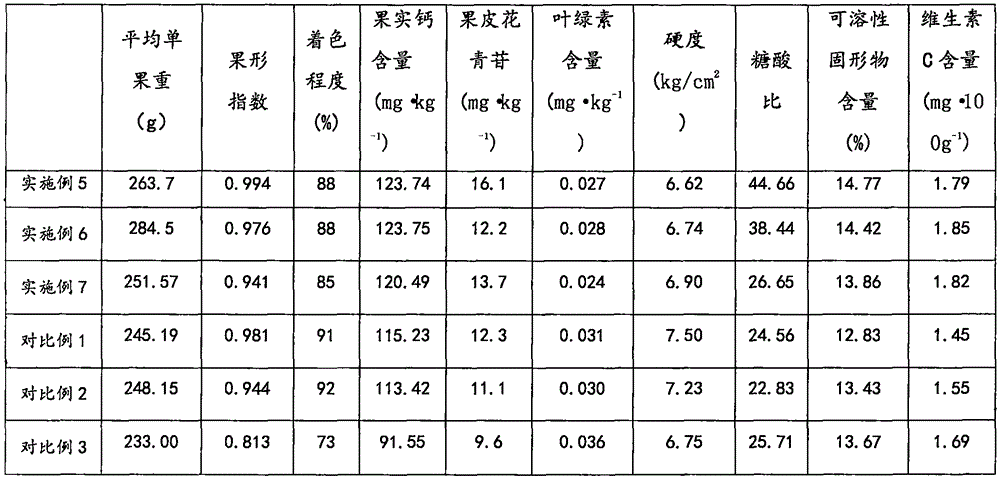
The invention provides a novel quality modifier for improving the intrinsic and extrinsic quality of red fruits. The novel quality modifier comprises aqueous solution. The aqueous solution comprises 2g / L-5g / L of methyl dihydrojasmonate, 0.070g / L-0.152g / L of vanillin, 1.5g / L-5g / L of salt-tolerant emulsifiers, 3g / L-6g / L of film-forming agents and 80g / L-150g / L of inorganic components, the methyl dihydrojasmonate, the vanillin, the salt-tolerant emulsifiers, the film-forming agents and the inorganic components are mixed with one another to obtain the aqueous solution, and the inorganic components contain phosphorus and potassium which are nutrient elements. The novel quality modifier has the advantages that the novel quality modifier is a pollution-free and non-residue improved preparation, obvious quality improving effects can be realized for fruit trees of apples and all red fruits, and the novel quality modifier can be popularized and applied to fruit tree planting, in particular to apple planting, on a large area.
Owner:NORTHWEST A & F UNIV
Daily chemical essence with flower fragrance and preparation method thereof
PendingCN112210438AReduce volatilityReduce evaporation rateEssential-oils/perfumesBenzoic acidPhenethyl alcohol
The invention relates to the field of daily chemical essences, and particularly discloses a daily chemical essence with flower fragrance and a preparation method thereof. The daily chemical essence with the flower fragrance comprises 0.6%-1.4% of B ionone. 0.2%-0.7% of n-amyl salicylate; 0.05%-0.15% of cis-jasmonic ketone; 0.5%-1.5% of convallaldehyde; 6%-10% of benzyl acetate; 1%-3% of methyl dihydrojasmonate; 0.1%-0.5% of methyl benzoate; 0.5%-1.5% of indole; 0.1%-0.3% of leaf alcohol; 0.1%-0.5% of salicylic acid leaf alcohol ester; 1%-3% of phenethyl alcohol; and the balance of dipropyleneglycol, wherein the total mass percentage is 100%. The preparation method comprises the following steps: mixing B-ionone, n-amyl salicylate, cis-jasmonate, convaleraldehyde, benzyl acetate, methyl dihydrojasmonate, methyl benzoate, indole, leaf alcohol, geraniol, leaf alcohol salicylate, phenethyl alcohol and dipropylene glycol in proportion, heating to 45-55 DEG C while stirring, and standing for45-55 minutes to obtain the daily chemical essence with flower fragrance. The daily chemical essence with the flower fragrance has the advantages that the fragrance lasting time of the daily chemicalessence is prolonged, and the cost is relatively low.
Owner:广东芬豪生物科技有限公司
Fragrance essence with antibacterial effect components and preparation method thereof
InactiveCN110129134AHas antibacterial effectAroma harmonyBiocideDead animal preservationEthyl salicylateBenzaldehyde
The invention provides a fragrance essence with bacteriostatic efficacy components and a preparation method thereof. The fragrance essence with bacteriostasis effect is prepared from acetaldehyde, hydroxycitronellal, pinene, sabinene, beta-myrcene, limonene, leaf alcohol, ligustral, benzaldehyde, linalool, Bergapten, caryophyllene, linalyl acetate, beta fenchyl alcohol, anthemene, Neryl propionate, Florol, benzyl acetate, geranyl acetate, citronellyl acetate, nerol, methyl salicylate, ethyl salicylate, ethyl salicylate, Beta Damascone, phenethyl alcohol, allyl amyl glycolate, ionone, methyl anthranilate, watermelon ketone, Ambrox, Methyl dihydrojasmonate, isoeugenol, thyme oil, eugenol and the like. The fragrant raw materials used are convenient in sources, and the obtained essence has thefragrance characteristics of fresh fragrance, flower fragrance, fruit fragrance and pungent fragrance, and also has the fresh feeling of forests and nature, the overall fragrance is fine and smooth,and the fragrance essence has bacteriostatic effect.
Owner:SHANGHAI INST OF TECH
Cat-driving essence as well as preparation method and application thereof
ActiveCN103911214AEffective in repelling catsEssential-oils/perfumesAnimal repellantsLemon oilYlang-Ylang oil
The invention discloses cat-driving essence as well as a preparation method and an application thereof. The cat-driving essence is obtained by adding a certain amount of bergamot oil, lemon oil, citral, linalyl acetate, sweet orange oil, terpilenol, terpinyl acetate, methyl ortho-aminobenzoate, decanal, geranyl acetate, lemonile, geranium oil, menthol, benzyl acetate, benzyl alcohol, linalool, dihydro jasmine with mass fraction of 10%, hydroxycitronellal, benzpyrole with mass fraction of 10%, dihydromyrcenol, methyl ionone, lyral, oil of daidai leaf, ylang ylang oil, gamma-delta-lacton, leaf alcohol, benzyl benzoate, benzyl propionate, methyl dihydrojasmonate, p-cresyl acetate, jasmonyl, phenylacetic acid, eugenol, myracaldehyde, absolute of jasmine, geraniol, cis-3-hexenyl benzoate, galaxolide with mass fraction of 50% and propylene glycol into a container in sequence, shaking uniformly, standing and ageing for two weeks. The cat-driving essence is remarkable in cat-driving effect.
Owner:福建中益制药有限公司
Antibacterial plastic for toys and preparation method of antibacterial plastic
InactiveCN106479090AImprove impact resistanceImprove antibacterial propertiesBetaineEngineering plastic
The invention discloses antibacterial plastic for toys and a preparation method of the antibacterial plastic. The antibacterial plastic is prepared from raw materials in parts by weight as follows: 30-45 parts of polyvinyl chloride, 10-25 parts of ABS engineering plastic, 1-5 parts of dioctyldilauryltin, 0.5-2 parts of calcium phosphate, 0.5-2 parts of methyl dihydrojasmonate, 1-4 parts of polysaccharide, 1-3 parts of chlorinated dodecyl trimethyl ammonium, 3-5 parts of bamboo leaf phenolic ketone and 1-5 parts of betaine. The antibacterial plastic for toys has good impact resistance, have inhibition or killing functions on bacteria, mildew, yeast, algae and even viruses staining toy plastic in the use environment, keeps clean by inhibiting breeding of microorganisms and has a good antibacterial effect; a preparation process is simple, and the production cost is lower; the antibacterial plastic is safe and non-toxic, and physical health of children is protected.
Owner:ZHENGZHOU CHENGHE INFORMATION TECH CO LTD
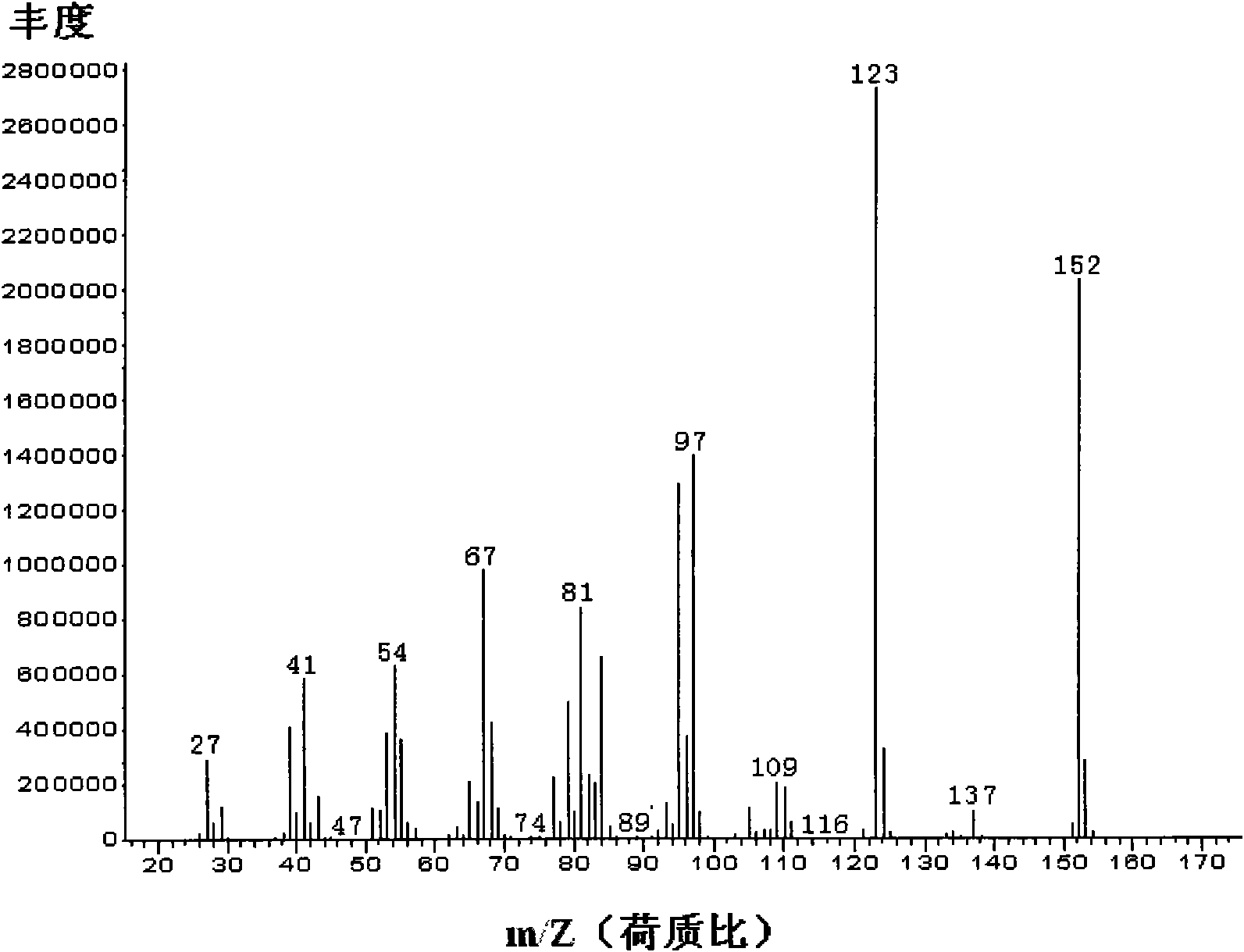
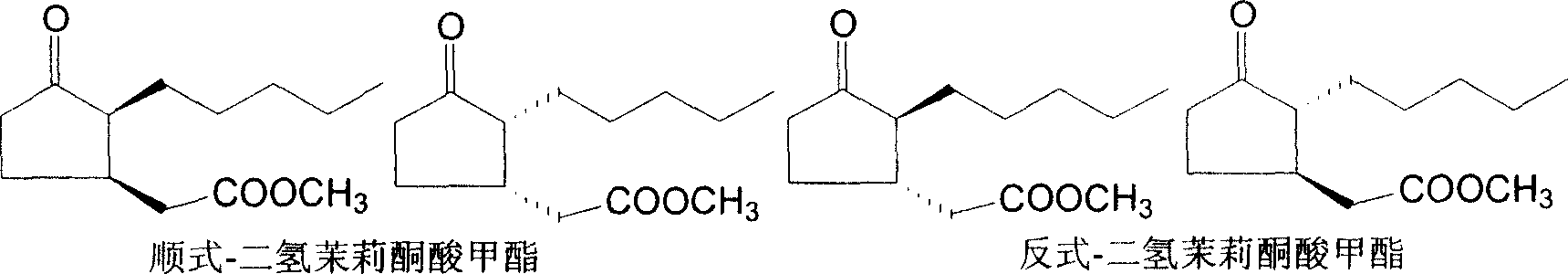
Synthesizing process of cis-dihydro jasmine keto-acid methyl ester
ActiveCN100999466AIncrease contentEasy to operateOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsSolvent free
This invention involves a cis-dihydrojasmone acid methyl ester synthetic method. The existing multi-step synthesis method is low yield and product content. This invention takes ordinary dihydrojasmone acid methyl ester as raw material, by enolase reaction and halogenation reaction, to dehalogenate to prepare double bonds jasmonate methyl ester; finally, under the solvent-free state through hydrogenation to obtain cis-dihydrojasmone acid methyl ester.
Owner:ZHEJIANG NHU CO LTD
Heat dissipation plastic for mobile phone shell and preparation method thereof
The invention discloses a heat dissipation plastic for a mobile phone shell. The heat dissipation plastic comprises the following raw materials in parts by weight: 60 to 100 parts of thermoplastic polyurethane elastomer, 10 to 20 parts of pyrolusite, 4 to 8 parts of nanometer boron fiber, 4 to 6 parts of copper powder, 3 to 6 parts of aluminum powder, 3 to 6 parts of mullite, 2 to 5 parts of monopotassium phosphate, 2 to 4 parts of zinc carbonate, 4 to 8 parts of rice husk carbon dust, 3 to 6 parts of pantothenic acid, 2 to 5 parts of borax, 1 to 3 parts of diatomite, and 1 to 2 parts of methyl dihydrojasmonate. The heat dissipation plastic has excellent comprehensive properties, is beneficial to the protection for mobile phones, good in heat dissipation effect, and beneficial to heat dissipation of mobile phones; the preparation material is available, the preparation method is simple, the plastic is beneficial to industrial production, and has good economic value and market value.
Owner:WUHAN MATENG TECH DEV
Lemon essence used in oil-based ink and preparation method of lemon essence
InactiveCN105176684AImprove fragranceGood oil solubilityInksEssential-oils/perfumesSolubilityLimonium
The invention provides lemon essence used in oil-based ink. The lemon essence is prepared from a lemon essence body, maltodextrin and starch sodium octenylsuccinate, wherein the lemon essence body is prepared from triethyl citrate, methyl dihydrojasmonate, peach aldehyde, beta-naphthol ethyl ether, dodecanenitrile, beta-ionone, gamma-decalactone,floropai, allyl cyclohexanepropionate, verdyl acetate, damacscone alpha, vanillin acetate, neryl acetate, undecanal, lauraldehyde, citronellyl formate, geranyl formate, geraniol, citronellol, nerol, citral, capraldehyde, styralyl acetate, citriodora oil, isocyclocitral, triplal, dihydromyrcenol, linalool, allyl hexanoate, ethyl-2-methylbutyrate, terpinolene, Brazil orange oil, lemon oil terpene, octyl aldehyde, paracymene, tangerine aldehyde, vanillic aldehyde, grapefruit oil and glyceryl triacetate. The invention further provides a preparation method of the lemon essence used in the oil-based ink. By means of the lemon essence used in the oil-based ink and the preparation method of the lemon essence, the fragrance retention effect and oil solubility of the essence are improved.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
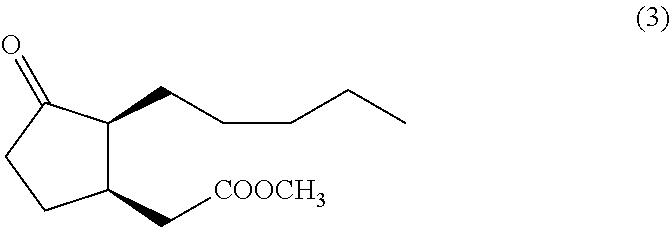
Method for synthesizing (1R,2S)-methyl dihydrojasmonate
ActiveCN106946705AOrganic compound preparationOrganic chemistry methodsChromatographic separationDouble bond
The invention discloses a novel method for synthesizing (1R,2S)-methyl dihydrojasmonate by using an asymmetric Michael addition reaction. The method comprises the steps: firstly, subjecting cyclopentanone, which serves as a starting raw material, to an aldol reaction with n-valeraldehyde under alkaline conditions to produce 2-pentylidene cyclopentanone 2, and then, carrying out double-bond transposition under acidic conditions, so as to obtain 2-n-pentyl-2-cyclopentenone 3; then, carrying out a Michael addition reaction with dimethyl malonate in the presence of a chiral amino-acid lithium salt, and carrying out silicagel-column chromatographic separation twice, so as to obtain (1S,2S)-2-n-pentyl-3-dimethyl malonate cyclopentanone 4; and finally, carrying out a hydrolyzed decarboxylation reaction, thereby obtaining (1R,2S)-methyl dihydrojasmonate. According to the method, the synthesis route is simple and direct, the reaction conditions are mild, and the target compound can be prepared by only four-step reactions.
Owner:北京安胜瑞力科技有限公司
Improved method for producing 2-alkylene alicyclic ketone
InactiveCN101851154ATaking care of protectionSave resourcesOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetoneImproved method
The invention discloses an improved method for producing 2-alkylene alicyclic ketone. In the method, alicyclic ketone and aliphatic aldehyde are used as raw materials, and the reaction of amine type alkalescent ion exchange resin in the presence of an acid compound and metal ions is utilized to generate the 2-alkylene alicyclic ketone. After an organic phase is separated out, a catalyst system can be recycled. The 2-alkylene alicyclic ketone is an important intermediate for synthesizing a plurality of spices (such as methyl dihydrojasmonate and delta-lactone). The method gets rid of a traditional unrecoverable solution catalysis system, replaces the traditional unrecoverable solution catalysis system by a recoverable and recyclable catalysis system, reconstructs an original process by a clean production technology and also realizes the saving and the sufficient utilization of resources and environmental protection.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Strawberry essence and preparation method therefor
InactiveCN105368577ALong lasting fragranceRealistic aromaEssential-oils/perfumesFragariaOrganic solvent
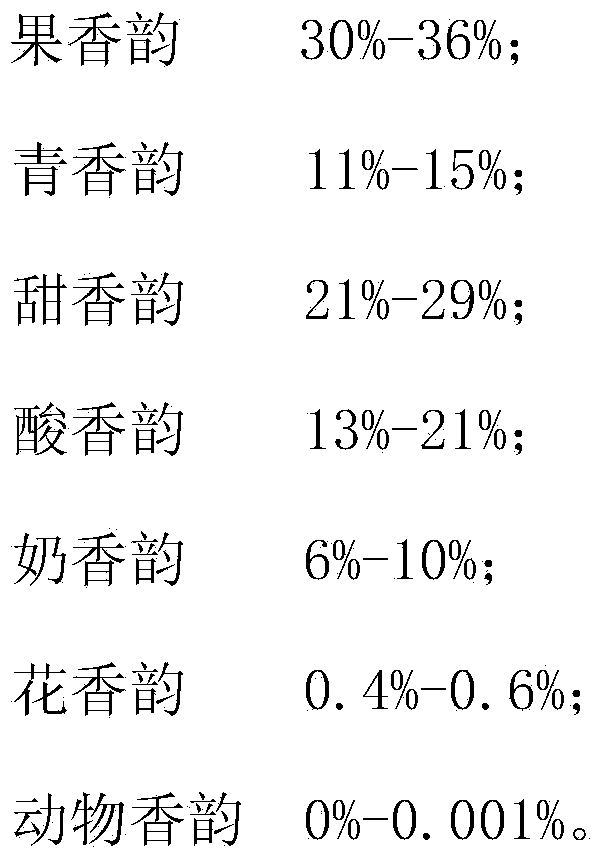
The present invention provides strawberry essence which comprises a scent raw material and an organic solvent with a mass ratio of (4-8):95. According to fragrance classification, calculated by percentage by mass, the scent raw material comprises: 30%-36% of fruit fragrance; 11%-15% of grass fragrance; 21%-29% of sweet fragrance; 13%-21% of acid fragrance; 6%-10% of milk fragrance; 0.4%-0.6% of flower fragrance; and 0%-0.001% of animal fragrance. The invention further provides a preparation method for the strawberry essence. The method comprises: prolonging lasting time of the essence through raw materials such as methyl cinnamate, lauric acid, myristic acid, methyl dihydrojasmonate, galaxolide; and simulatively preparing strawberry essence with a vivid scent and a good scent lasting effect from fruit fragrance, grass fragrance, sweet fragrance, acid fragrance, flower fragrance and animal fragrance by combining scent branching of strawberries. The strawberry essence and the preparation method therefor can be used for flavoring of life supplies such as daily chemical supplies.
Owner:SHANGHAI INST OF TECH
Methods of reducing leaf senescence using methyl dihydrojasmonate
Methods for reducing leaf senescence in plants or plant portions by treating them with methyl dihydrojasmonate are disclosed. The methyl dihydrojasmonate may be in the form of an aqueous foliar spray, which may also include additives such as wetting agents, adjuvants, emulsifiers, dispersants, spreaders, surfactants, anchorage, disintegrants, and plant nutrients.
Owner:BEDOUKIAN RES
Mosquito-repelling essential oil and its preparation method
InactiveCN107638313AEffective time is shortProlong the time of mosquito repellent effectCosmetic preparationsBiocideMethyl dihydrojasmonateAminobenzoic acid
The invention relates to a mosquito-repelling essential oil and its preparation method. The mosquito-repelling essential oil is prepared from, by weight, 5-10 parts of vanillin, 5-10 parts of di-o-aminobenzoic acid, 5-7 parts of methyl dihydrojasmonate, and 5-7 parts of hexylcinnamaldehyde. The preparation method includes steps of mixing every component together at mixture ratio. The mosquito-repelling essential oil takes pure plant essential oil as the main raw material; through selecting proper raw material and ratio of raw materials, the mosquito-repelling essential oil has good mosquito-repelling effect and long mosquito-repelling time; the mosquito-repelling essential oil is applicable to children, pregnant women, and people with hypoimmunity and ethyl alcohol sensitivity.
Owner:上海千紫香料有限公司
Hair growth promoting composition containing plant extracts
ActiveCN105168046AImprove microcirculationPromote growthCosmetic preparationsHair cosmeticsLepidium sativumSoybean germ extract
The invention relates to a hair growth promoting composition containing plant extracts. The composition is characterized by consisting of a wild soybean germ extract, a bistort extract, a lepidium sativum sprout extract and methyl dihydrojasmanate according to mass ratio of (3-10) to (1-5) to (1-5) to (0.05-0.3). The composition disclosed by the invention has a beneficial effect of promoting hair growth.
Owner:PROYA COSMETICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com