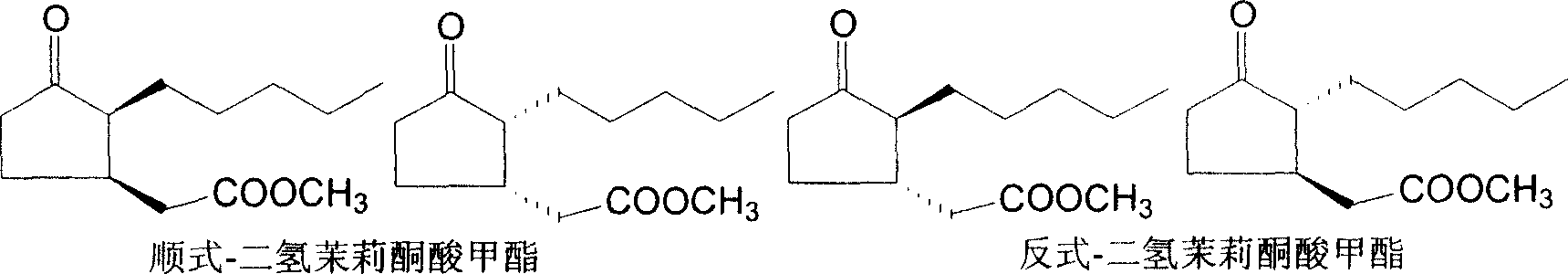
Synthesizing process of cis-dihydro jasmine keto-acid methyl ester
A technology of methyl dihydrojasmonate and methyl jasmonate, which is applied in the field of preparation of fragrance compounds, can solve the problem of low content of cis-methyl dihydrojasmonate, thermodynamic instability of cis structure, reaction There are many steps to achieve the effect of easy industrial production, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
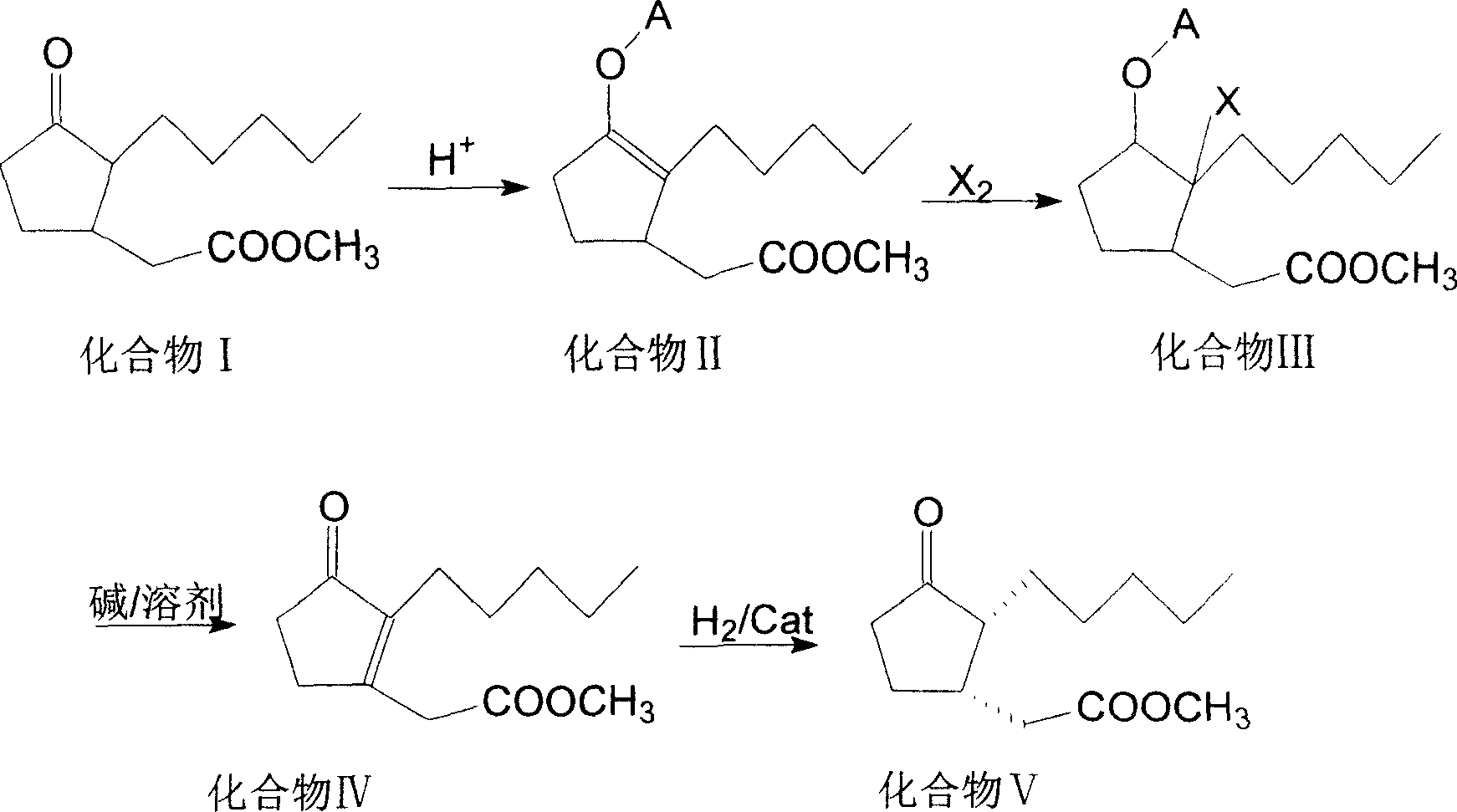
[0023] Add 113.60g (0.50mol) of ordinary methyl dihydrojasmonate, 60.00g (0.55mol) of trimethylchlorosilane, and 1.14g of acetic acid into a 250ml four-necked flask in turn, and heat the mixture to 60°C under stirring. 8hr, during the reaction, monitor the content of trans-methyl dihydrojasmonate, when the content of trans-methyl dihydrojasmonate drops below 1%, add an appropriate amount of sodium carbonate to neutralize, stop reaction. After the reaction, recover excess trimethylchlorosilane, control the temperature not to exceed 60°C, and cool naturally after recovery to obtain compound II for future use.
[0024] Heat the compound II prepared above to 95°C, add 20.00g of sodium acetate first, then start to add 42.00g of bromine dropwise, finish dropping in 120min, keep it warm for 260min, then cool down to room temperature to obtain compound III, set aside.
[0025] In a 500ml four-neck flask, add 200ml200 # Solvent oil and 15.00g sodium carbonate, stir and heat up to 185...
Embodiment 2
[0028] 227.20g (1.0mol) common methyl dihydrojasmonate, 122.20g (1.2mol) isopropenyl acetate, 1.38g p-toluenesulfonic acid and 1.00g acetic acid mixed acid were added successively in a 500ml four-necked flask, and the mixture Heating to 80°C under stirring for 6 hours, during the reaction, monitor the content of trans-methyl dihydrojasmonate, when the content of trans-methyl dihydrojasmonate drops below 1%, add an appropriate amount The reaction stopped after neutralization with potassium oxalate. After the reaction, cool naturally, then reclaim excess isopropenyl acetate under vacuum conditions, control the temperature not to exceed 80°C, cool naturally after recovery, and set aside.
[0029] Cool the compound II prepared above to 0°C, add 30.00g of potassium oxalate first, then start to pass through 1.0mol of chlorine gas for 120min, then keep it warm for 60min and then naturally warm up to room temperature for later use.
[0030] Add 180ml of ethyl acetate and 25.00g of ca...
Embodiment 3
[0033] Add 113.60g (0.5mol) of ordinary methyl dihydrojasmonate, 77.00g (0.55mol) of phosphorus trichloride, and 1.14g of benzenesulfonic acid into a 250ml four-necked flask in sequence, and heat the mixture to 60°C under stirring React for 8 hours, during the reaction, monitor the content of trans-methyl dihydrojasmonate, when the content of trans-methyl dihydrojasmonate drops below 1%, add an appropriate amount of sodium citrate for neutralization , stop responding. After the reaction, recover excess phosphorus trichloride, control the temperature not to exceed 60°C, and cool naturally after recovery to obtain compound II for future use.
[0034] Heat up the compound II prepared above to 55°C, first add 30.00g sodium citrate, then start to add 128.00g iodine, finish adding in 30 minutes, then keep warm for 30 minutes and then cool to room temperature to obtain compound III, which is set aside.
[0035] Add 120ml of xylene and 15.00g of sodium hydroxide in turn to a 500ml fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com