Patents
Literature
68 results about "Isopropenyl acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
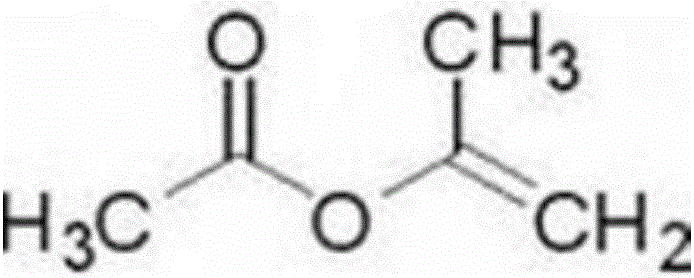
Isopropenyl acetate is an organic compound, which is the acetate ester of the enol tautomer of acetone. This colorless liquid is significant commercially as the principal precursor to acetylacetone. In organic synthesis, it is used to prepare enol acetates of ketones and acetonides from diols.
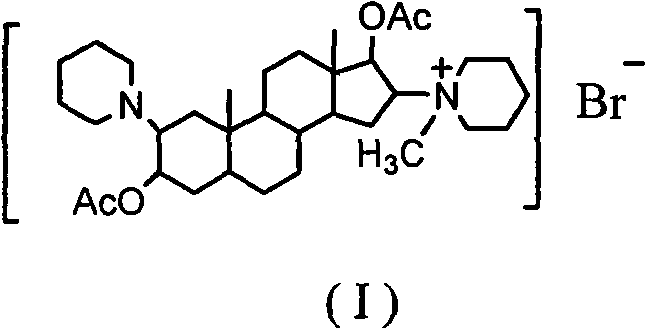
Synthesis process of vecuronium bromide
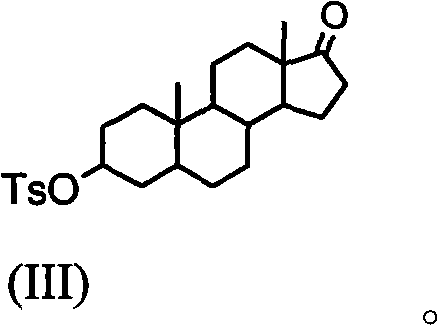
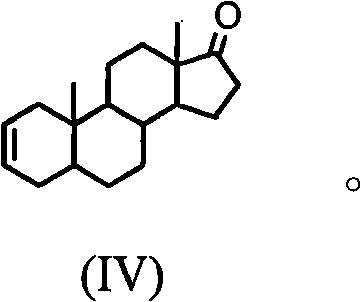
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
Method of preparing sucralose
InactiveCN101121736AHigh yieldHigh content of finished productsSugar derivativesFood sweetenersSucrose
The invention relates to a preparation method of food sweetener-sucralose, comprising the steps of preparing sucrose-6-ester from sucrose, and preparing 1',4,6'-trichlorosucrose by chlorination of sucrose-6-ester -1',4,6'-trideoxygalactosucrose-6-ester step and deesterification step to prepare sucralose; it is characterized in that the preparation of sucrose-6-ester is that sucrose is in DMF solvent, in acidic React with enol ester in the presence of catalyst to obtain sucrose-6-ester. The acidic catalyst is any one of benzenesulfonic acid, p-toluenesulfonic acid, and dibutyltin oxide. Enol esters include vinyl acetate and isopropenyl benzoate. The chlorination reaction is carried out under the conditions of using organic tertiary amine as a catalyst and changing the polarity of the solvent. The yield of each reaction step of the invention is high, the content of the obtained finished product is as high as 99%, the production cost is low, and it is suitable for industrialized production.
Owner:JIANGSU JUBANG PHARMA
Method for synthesizing cane sugar-6-acetic ester by using lipase for catalyzing
The invention discloses a method for synthesizing a cane sugar-6-acetic ester by using lipase for catalyzing. The method comprises the following steps of: based on 72,000 to 150,000 enzyme activity unit enzyme in 100 ml buffer solution, adding the lipase into buffer solution with a pH value of between 8.1 and 8.7 to obtain enzyme solution; adding an organic solvent into the enzyme solution; adding cane sugar until the cane sugar in reaction solution is saturated; stirring until the cane sugar is solved and adding an acetic ester compound; reacting at the temperature of between 40 and 65 DEG C with stirring; adding the acetic ester compound after reacting for 11 to 13 hours; ending an reaction after reacting totally for 23 to 25 hours; and washing the reaction product with distilled water, decompressing and distilling to obtain cane sugar-6-acetic ester, wherein the acetic ester compound is vinyl acetate or isopropenyl acetate; the volume ratio of the enzyme solution to the organic solvent is 0.1-1:100; and the organic solvent is tertiary amyl alcohol, sec-butyl alcohol or tetrahydrofuran. The organic solvent is selected and the water content of a reaction system is less than 1.0 percent, so that the purity of the obtained high-purity cane sugar-6-acetic ester is over 75 percent, and 99 percent in maximum.
Owner:ZHEJIANG UNIV OF TECH
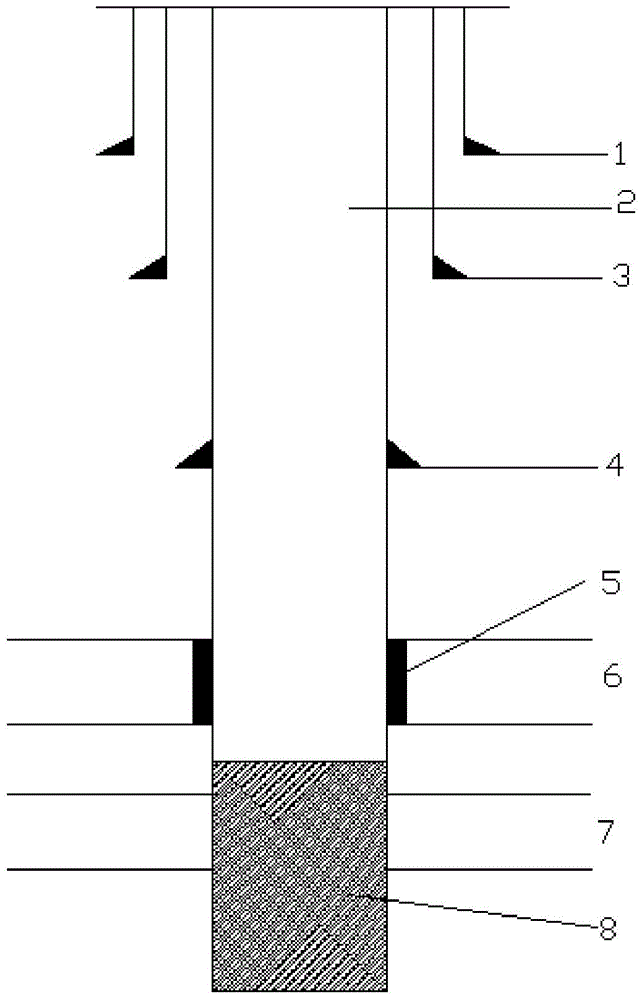
Liquid glue stopper for handling borehole wall collapsing
InactiveCN105086971AImprove work efficiencyAvoid serious leaksDrilling compositionCross-linkIsopropenyl acetate
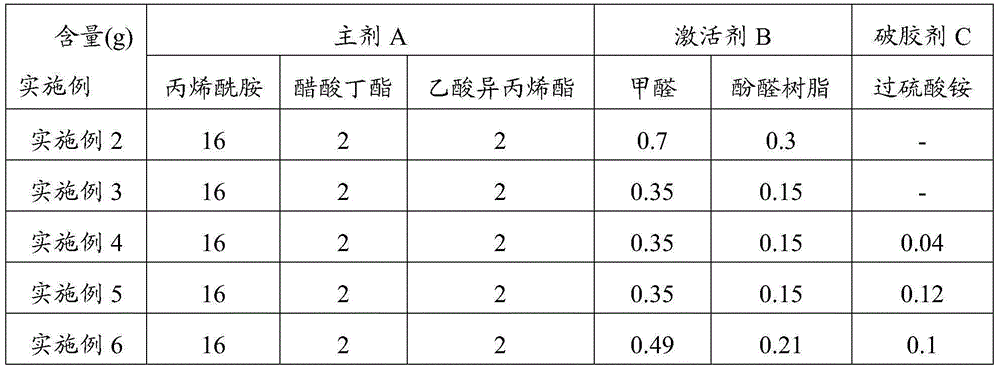
The invention relates to a liquid glue stopper for handling borehole wall collapsing. The liquid glue stopper comprises, by weight, 200 parts of main agent A, 1-10 parts of activating agent B and 750 parts of water, and preferably, the liquid glue stopper comprises, by weight, 200 parts of main agent A, 5 parts of activating agent B and 750 parts of water. The main agent A is composed of, by weight, 8 parts of acrylamide, 1 part of n-butyl acetate and 1 part of isopropenyl acetate. The activating agent B is composed of, by weight, 7 parts of methanal and 3 parts of phenolic resin. The liquid glue stopper is prepared through the cross-linking reaction of the main agent A and the activating agent B, and the cross-linking time is 5-90 minutes. The liquid glue stopper further comprises a gel breaker C of ammonium persulfate, and the liquid glue stopper comprises, by weight, 200 parts of main agent A, 1-10 parts of activating agent B, 0.4-1.2 parts of gel breaker C and 750 parts of water. The liquid glue stopper is large in hardness and capable of bearing construction with the 40 MPa pressure difference and withstanding the temperature of 150 DEG C. Permanent leaking stoppage can be achieved without adding the gel breaker in the liquid glue stopper, self-gel breaking can be achieved when the gel breaker is added, flowback of the liquid glue stopper is promoted, and pollution to stratums is avoided.
Owner:CHINA PETROLEUM & CHEM CORP
Method for preparing starch-based water-resistant adhesive for corrugated board production
InactiveCN102533182AAvoid damageImprove water resistanceMechanical working/deformationGraft polymer adhesivesPolymer scienceAdhesive
The invention relates to a method for preparing a starch-based water-resistant adhesive for corrugated board production, and belongs to the technical field of paper coating adhesives. The method comprises the following steps of: performing grafting copolymerization modification on starch serving as a main raw material by using two monomers, namely isopropenyl acetate and butyl acrylate, and performing crosslinking modification; and heating for pasting, and adding aids to obtain a good-performance starch-based water-resistant adhesive for corrugated board production. The prepared adhesive improves the defect of low water resistance of a starch adhesive, has the advantages of wide material sources, no pollution, high adhesive strength and high water resistance, and can be widely applied to the corrugated board production.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
Method for preparing waterproof adhesive for corrugated paper production
InactiveCN101979452AAvoid damageImprove water resistanceMechanical working/deformationStarch derivtive adhesivesCross-linkAdhesive
The invention relates to a method for preparing waterproof adhesive for corrugated paper production, and belongs to the technical field of paper coating adhesive. A starch-isopropenyl acetate cross linked graft copolymer is synthesized, and then the waterproof adhesive for the corrugated paper production is prepared. The method for preparing the waterproof adhesive for the corrugated paper production can effectively reduce the damage of water molecules to the adhesive so that the waterproof property of the adhesive is greatly improved; and because the hydrophilic property of the modified adhesive declines, the adhesive has good quick drying property and is favorable to be used on high-speed corrugated lines.
Owner:ZHEJIANG GREAT SHENGDA PACKING CO LTD
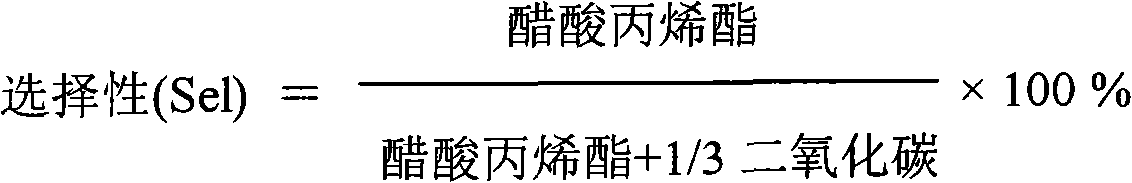
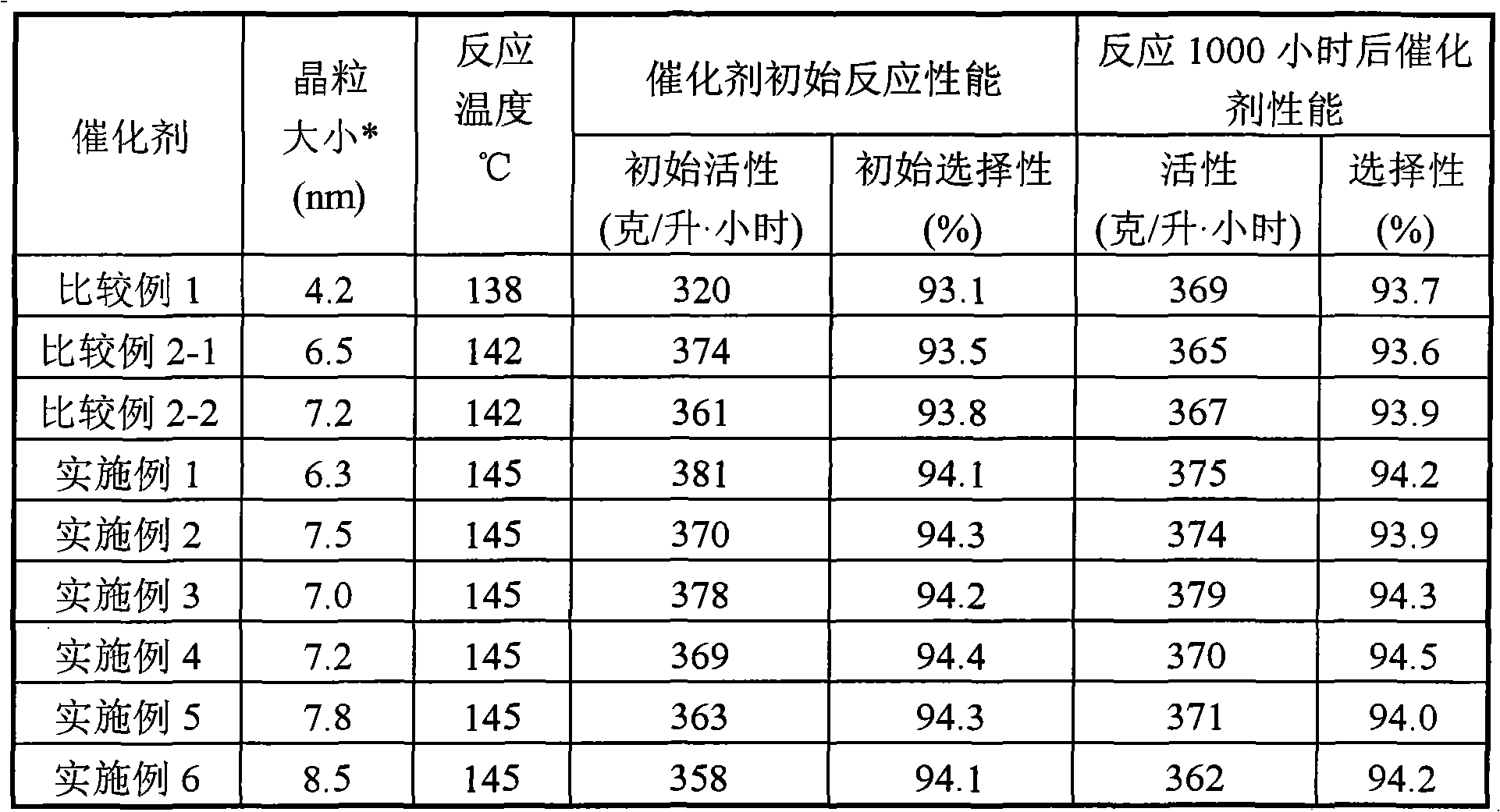
Catalyst for synthesizing isopropenyl acetate and preparation method thereof
ActiveCN102463139AGood activity and stabilityHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsopropenyl acetateReaction temperature
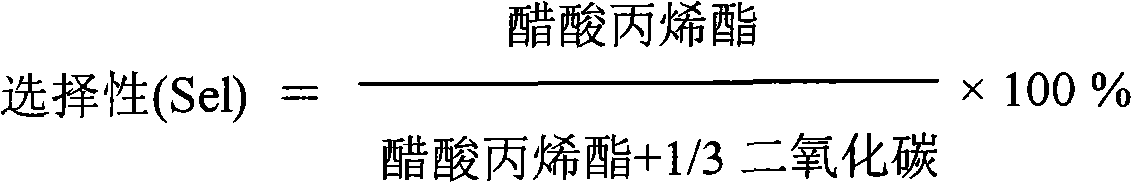
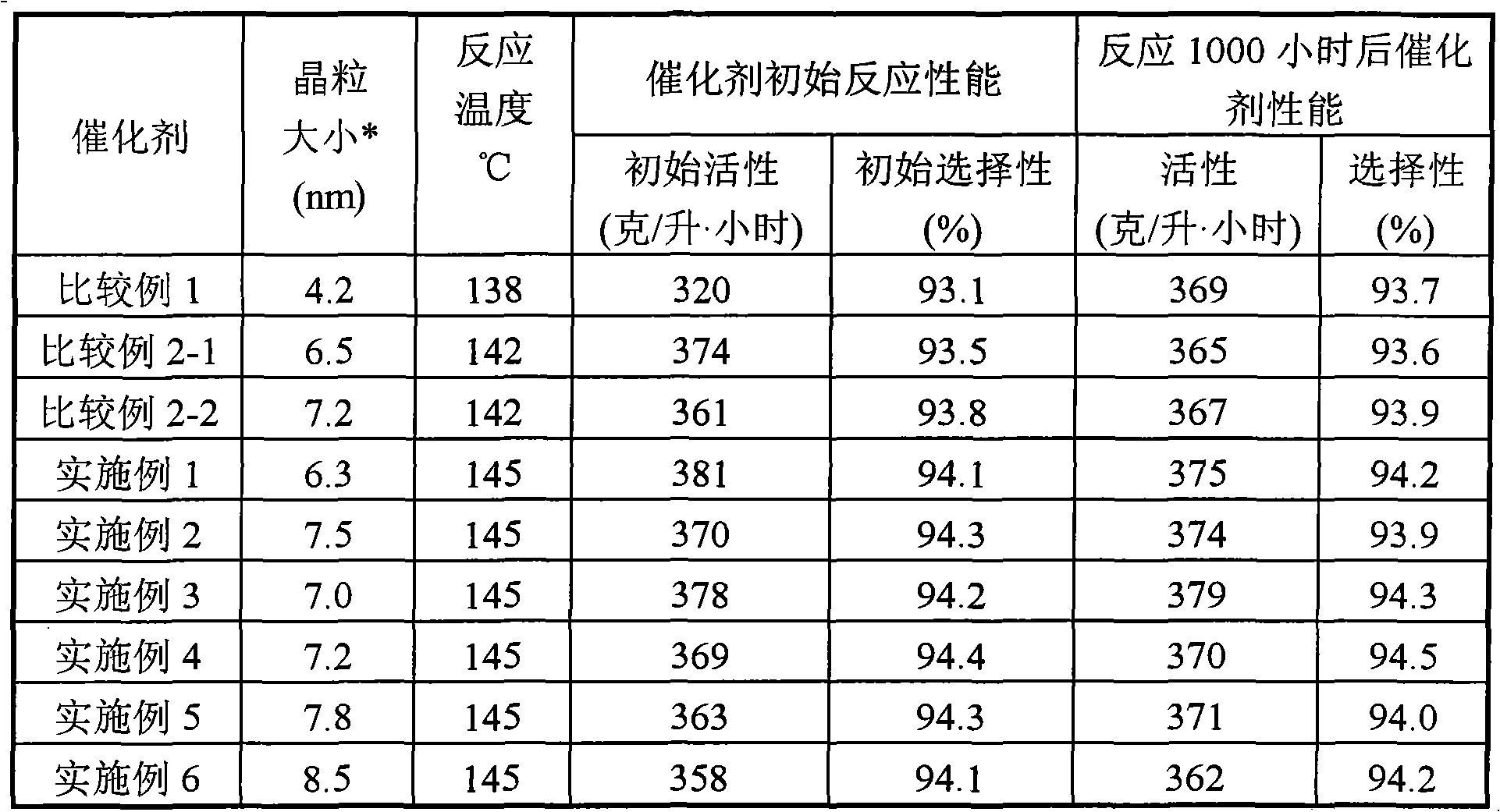
The invention relates to a catalyst for synthesizing isopropenyl acetate and a preparation method thereof, which mainly solve the problems that in the prior art, the Pd-Cu alloy crystal grains are too small, so the initial activity of the catalyst is too high, the catalyst is in a lower reaction temperature for a long time, the induction period of the catalyst is prolonged, and the yield of the isopropenyl acetate is reduced. The catalyst and the preparation method have the technical scheme that Pd (palladium), Cu (copper) and alkali metal acetates are loaded on silicon dioxide, aluminum oxide or a mixture of the silicon dioxide and the aluminum oxide, and the Pd-Cu alloy crystal grain size is 5 to 15nm. The problems are perfectly solved, and the catalyst and the preparation method can be used for industrial production of isopropenyl acetate catalysts.
Owner:CHINA PETROLEUM & CHEM CORP +1
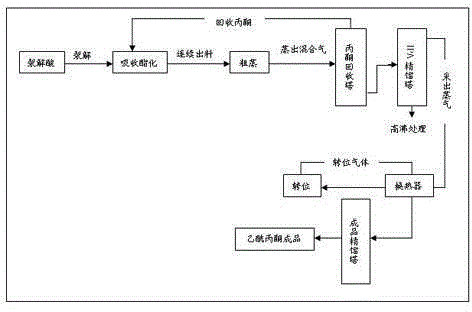
Energy-saving efficient isopropenyl acetate synthesis method
ActiveCN105777540AImprove responseIncrease productivityChemical industryPreparation from ketenes/polyketenesSynthesis methodsEthenone
The invention discloses an energy-saving efficient isopropenyl acetate synthesis method which comprises the following steps: (1) feeding acetic acid into a container, performing heating treatment on the acetic acid so as to generate an ethenone gas from the acetic acid in a catalytic cracking manner, introducing the ethenone gas into a cooler; (2) feeding an acetone solution into a mixing dish, simultaneously feeding a catalyst into the mixing dish, mixing, uniformly stirring, pumping the mixed liquid into the cooler by using a metering pump; (3) conveying the mixed gas in the cooler into a synthesis reaction tower, and enabling the mixed gas to generate a reaction gas in the synthesis reaction tower; and (4) condensing the generated reaction gas so as to obtain a coarse product, refining the coarse product, cooling, crystallizing and drying, thereby obtaining a product. According to the energy-saving efficient isopropenyl acetate synthesis method disclosed by the invention, the ethenone gas and the acetone steam are enabled to have homogeneous reaction directly, so that the reaction efficiency is effectively improved, the transfer amount of acetone is greatly reduced, and the comprehensive utilization rate is increased.
Owner:衢州伟荣药化股份有限公司
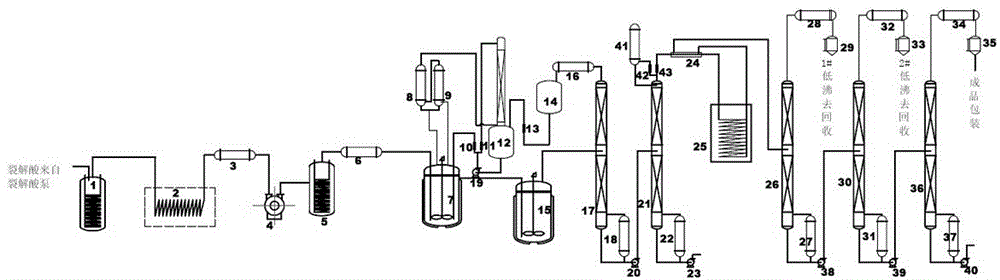
Process method for processing isopropenyl acetate absorption liquid
ActiveCN102442900AHigh yieldNot easy to break downOrganic compound preparationCarboxylic acid esters preparationLiquid stateDecomposition
The invention discloses a process method for processing isopropenyl acetate absorption liquid, which comprises the following steps that: 10 DEG C to 90 DEG C isopropenyl acetate absorption liquid is fed into a film evaporator, the film evaporation is carried out at the temperature being 40 DEG C to 200 DEG C, the vacuum degree being 0.001MPa to 0.099MPa and the rotating speed being 10r / min to 800r / min, substances such as acetone, isopropenyl acetate and the like are collected, and then, the residue liquid is subjected to continuous material discharging. The process method has the advantages that the extraction rate of the isopropenyl acetate is high, thermal sensitive substances in the absorption liquid can not easily generate decomposition or polymerization reaction, in addition, the residue liquid quantity is small, the liquid state material discharging can be ensured, the coking in the evaporator can not be caused, the work intensity is reduced, and the on-site environment is improved.
Owner:HYEC +1
Lewis acid catalyst as well as preparation method and application thereof in technique for producing acetylacetone by isopropenyl acetate displacement method
InactiveCN101485999AHigh reaction conversion rateReduce decompositionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDecompositionIsopropenyl acetate
The invention provides a Lewis acid catalyst, a preparation method and application thereof in a process for producing acetylacetone by an isopropenyl acetate dislocation method. The catalyst consists of a complex of aluminum, a complex of tin or a complex of iron and a sulfur element, wherein ratio of the complex of aluminum to the complex of tin or the complex of iron to the sulfur element is 80-20:50-5:20-1. The Lewis acid catalyst can be used for replacing the prior lead tetraethyl or tetramethyllead catalyst with stronger toxicity in a process for preparing the acetylacetone by the isopropenyl acetate dislocation method so as to improve production condition and working environment, improve reaction conversion rate of isopropenyl acetate to 4 to 10 percent, effectively reduce charring coke amount and content of a side product of a high-boiling product in the reaction, and improve quality of the product. The application of the catalyst does not adopt water as a reaction addition agent, so that decomposition of the isopropenyl acetate can be reduced.
Owner:HUZHOU XINAOTE PHARMA & CHEM +1
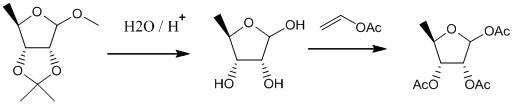
Preparation method of 1,2,3-tri-O-acetyl-5-deoxidization-beta-D-ribose
ActiveCN101824056ASimple and relaxed conditionsHigh yieldEsterified saccharide compoundsSugar derivativesFuranIsopropenyl acetate
The invention discloses a preparation method of 1,2,3-tri-O-acetyl-5-deoxidization-beta-D-ribose. The preparation method comprises the following steps of: (1) carrying out a hydrolysis reaction on 2,3-O-isopropylidene-5-deoxidization-D-furan glucoside and aqueous acid; (2) adding an acetylation reagent to the aqueous solution obtained in the step (1) to carry out an acetylation reaction, wherein the acetylation reagent is vinyl acetate or isopropenyl acetate; (3) post-treating the aqueous solution obtained in the step (2) to obtain a crude 1,2,3-tri-O-acetyl-5-deoxidization-D-ribose product; and (4) separating the crude product to obtain the 1,2,3-tri-O-acetyl-5-deoxidization-beta-D-ribose the purity of which is not lower than 99.5 percent. In the invention, the vinyl acetate or the isopropenyl acetate is adopted to directly carry out the acetylation reaction with 5-deoxidization-D-ribose in the aqueous solution, and the 1,2,3-tri-O-acetyl-5-deoxidization-D-ribose with higher beta isomer purity is finally obtained.
Owner:SUQIAN KEYLAB BIOCHEMICAL CO LTD
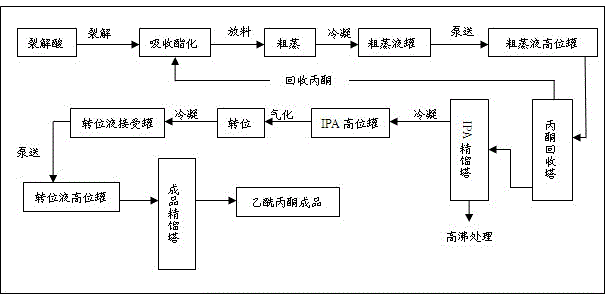
High-efficiency energy-saving preparation method and preparation device of acetylacetone
ActiveCN103333063AImproved Absorption Esterification Production MethodIncrease productivityOrganic compound preparationChemical industryIsomerizationDistillation
The invention relates to a preparation method of a chemical or medical intermediate, and in particular relates to a high-efficiency energy-saving preparation method and preparation device of acetylacetone. The method sequentially comprises the steps of: adding acetone and introducing ketene simultaneously, proceeding with the reaction through continuously introducing isopropenyl acetate esterification liquid, continuously discharging the isopropenyl acetate esterification liquid to a simple distillation still, continuously distilling off mixed vapor containing the acetone and isopropenyl acetate, enabling the simply distilled mixed gas to enter an acetone recycling tower, carrying out rectification, recycling the acetone, enabling tower bottoms of the acetone recycling tower to enter an isopropenyl acetate rectifying tower, carrying out rectification, enabling the isopropenyl acetate gas from the tower top to enter into a transposition process after passing through a heat exchanger, carry out an isomerization reaction, enabling the produced transposition mixed gas to enter an end product rectifying tower after exchanging heat with the isopropenyl acetate gas, carrying out rectification and obtaining end products of the acetylacetone. The method has the advantages of realizing the continuous production, greatly reducing the energy consumption, reducing the production cost and achieving the effects of energy conservation and emission reduction.
Owner:JILIN BEISHA PHARMA
Process for preparing acetylacetone by conversion of isopropenyl acetate and special-purpose equipment thereof
InactiveCN101417932AGreat operating flexibilityShorten the cycle timeOrganic compound preparationCarbonyl compound preparationAcetic acidCombined use
The invention discloses a process for preparing acetylacetone by transforming acetic acid propylene and comprises the following steps of: steaming the acetic acid propylene into gas by a vaporizer; then, putting the gas to a first-way carrier gas and / or using a self heat interchanger, then leading the gas come into a reformer to get reforming gas, and finally cooling down the reforming gas. The invention also discloses a special device for the process. In the process for preparing acetylacetone by transforming acetic acidpropylene, the flow quantity of carrier gas can be adjusted according to the different inventory ratings, thereby reaching to the optimal residence time, greatly reducing carbon deposition and prolonging carbon blowing period, realizing optimal conversion rate and selectivity, and having large work condition operation window. The self heat interchanger can save energy, realize precise temperature raising in section, and raising the selectivity and yield of the transformation reaction. The combination of using the first-way carrier gas and the self heat interchanger, which are cooperated to work, can raise reaction selectivity and yield, and increase the operation flexibility in the best working conditions.
Owner:SHANGHAI WUJING CHEM
High-pressure-resistant carbonized fiber metal composite material and preparation method thereof
The invention discloses a high-pressure-resistant carbonized fiber metal composite material which is prepared from, by weight, 10-15 parts of nickel alloy, 2-3 parts of germanium sulfide, 2-4 parts of indium selenide, 4-6 parts of tin chloride, 3-5 parts of zinc arsenide, 2-6 parts of potassium oxide, 5-7 parts of nickel acetylacetonate, 2-3 parts of cerium dipivaloylmethane, 4-8 parts of silicon carbide, 3-6 parts of silicon nitride, 3-7 parts of graphite, 10-15 parts of polyacrylonitrile-based carbon fiber, 3-7 parts of acetate fiber, 3-10 parts of polyarylsulfone, 3-6 parts of methyl acrylate, 5-9 parts of p-chloroaniline, 3-7 parts of isopropenyl acetate, 2-4 parts of furanmethanol, 3-5 parts of p-aminotoluene o-sulfonyl aniline, 5-8 parts of a modifier and 5-10 parts of a thermal stabilizer. The composite material is high in pressure resistance and hardness. In addition, the invention further discloses a corresponding preparation method.
Owner:SUZHOU HONGKE METAL PROD CO LTD
High-temperature superconducting metal material and preparation method thereof
InactiveCN106048370AStrong superconductivityMeet industry requirementsSuperconductors/hyperconductorsSuperconductor devicesPolypyrroleRare earth
The invention discloses a high-temperature superconducting metal material which is prepared from the following raw materials in parts by weight: 10-20 parts of magnesium diboride, 8-14 parts of molybdenum-niobium alloy, 8-12 parts of bismuth oxide, 7-13 parts of copper oxide, 5-9 parts of calcium carbonate, 4-9 parts of titanium oxide, 3-7 parts of zirconium oxide, 3-8 parts of indium tin oxide, 5-9 parts of tin oxide, 5-7 parts of magnesium silicide, 3-6 parts of zinc sulfide, 7-10 parts of rare earth, 6-12 parts of nano ceramic powder, 4-10 parts of polyurethane, 3-9 parts of polyphenylene sulfide, 3-6 parts of methyl acrylate, 5-8 parts of parachloroaniline, 4-7 parts of isopropenyl acetate, 2-4 parts of polypyrrole, 5-8 parts of a denaturing agent and 5-10 parts of a thermal stabilizer. The high-temperature superconducting metal material is resistant to a low temperature, is high in superconducting property, and has zero resistance. Meanwhile, the invention further discloses a corresponding preparation method.
Owner:SUZHOU HONGKE METAL PROD CO LTD
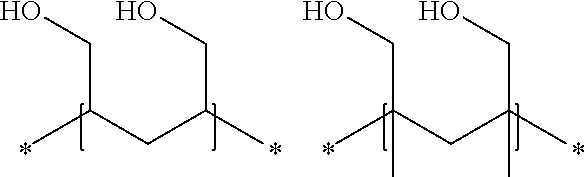
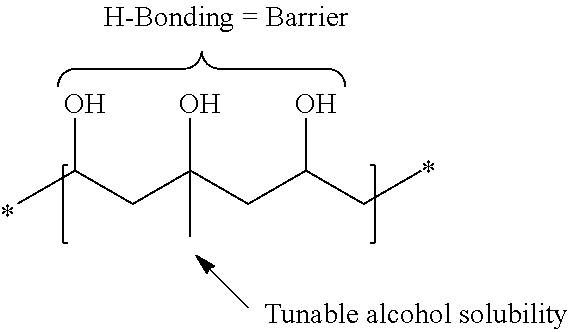
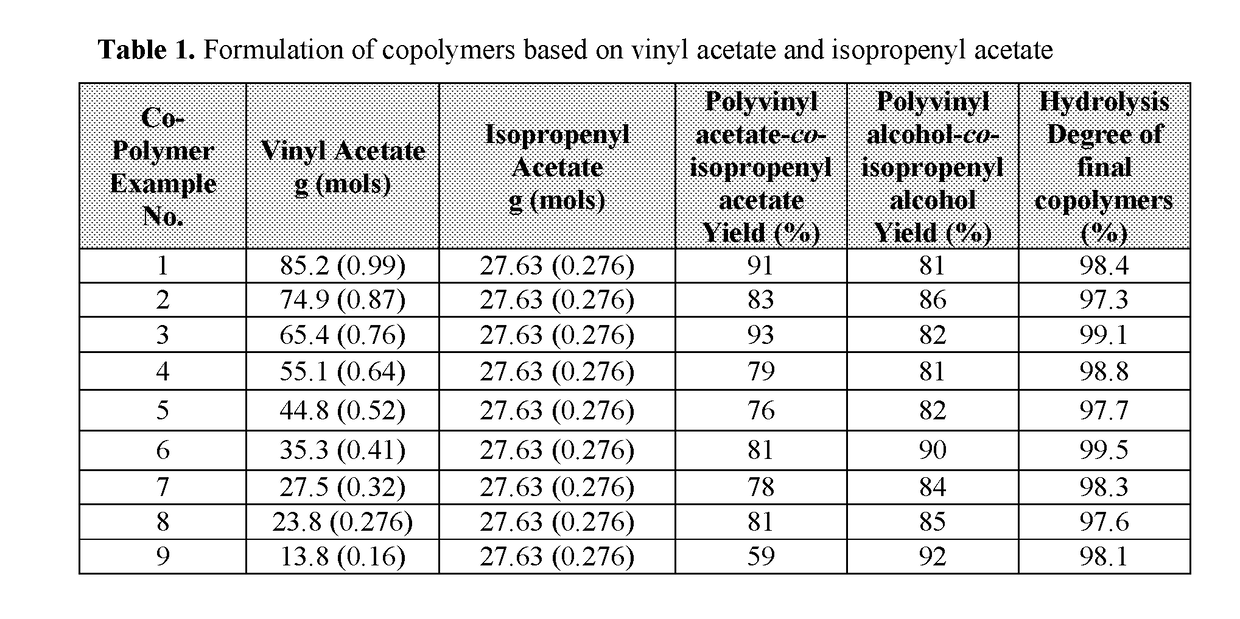
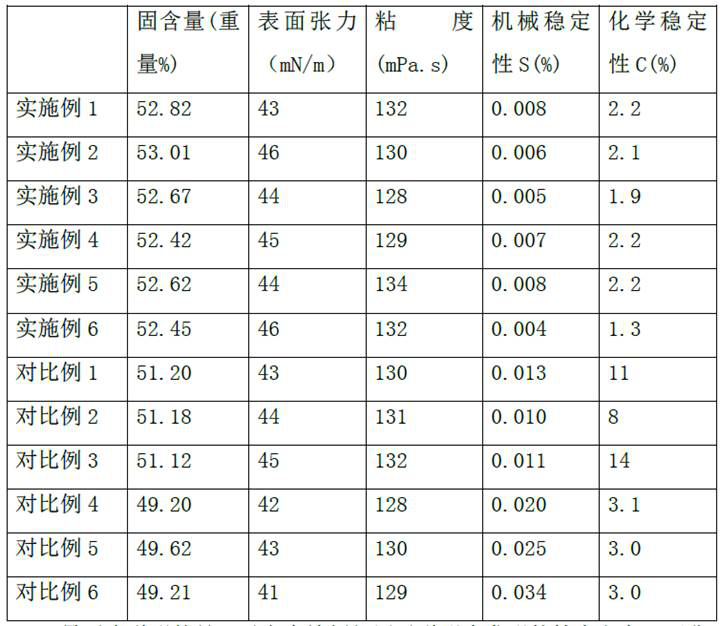
High alcohol tolerant copolymers and terpolymers based on polyvinyl alcohols
InactiveUS20180079839A1Use in some applicationHigh alcohol tolerantCoatingsPolyvinyl alcoholIsopropenyl acetate
The present application describes barrier coatings and barrier films comprising copolymers and terpolymers that are soluble in aqueous solutions containing greater than or equal to 50% alcohol. The copolymers and terpolymers comprise at least one olefin derived from isopropenyl acetate and at least one unit derived from vinyl acetate and derivatives thereof. The copolymers and terpolymers are greater than 90% hydrolyzed. Barrier compositions prepared with the copolymers and terpolymers exhibit reduced oxygen transmission rates, good adhesion to substrates, good lamination bond strength, as well as other properties desirable in barrier coatings and barrier films.
Owner:SUN CHEM CORP
A kind of carboxylated nitrile latex for gloves and preparation method thereof
ActiveCN113480691BGood chemical stabilityImprove mechanical stabilityPolymer scienceFunctional monomer
The invention relates to a carboxyl nitrile latex for gloves and a preparation method thereof. Specifically, in parts by mass, the raw materials include 80-100 parts of deionized water, 1-3 parts of a molecular weight regulator, and 0.2-0.4 parts of a pH buffer. , 2-6 parts of emulsifier, 40-60 parts of butadiene, 15-40 parts of acrylonitrile, 10-20 parts of functional monomer, 2-5 parts of unsaturated carboxylic acid, 0.3-1.5 parts of initiator, 0.4 part of auxiliary ~0.6 parts; the functional monomers include ethylene glycol diacrylate, diallyl maleate and isopropenyl acetate in a mass ratio of 1:(1-1.2):(0.5-1). The carboxylated nitrile latex of the present invention has excellent chemical stability and mechanical stability.
Owner:河北中宫睿宸医用新材料科技有限公司
Super-abrasion-resistant metal mixing nano material and preparation method thereof
InactiveCN106077611AImprove wear resistanceImprove stress resistanceMaterial nanotechnologyTransportation and packagingP-chloroanilineCerium
The invention discloses a super-abrasion-resistant metal mixing nano material. The super-abrasion-resistant metal mixing nano material is made of, by weight, 10-15 parts of nickel alloy, 2-9 parts of nano nickel, 2-8 parts of nano titanium, 4-7 parts of nano copper, 3-6 parts of nano zinc, 2-6 parts of nano selenium, 5-7 parts of nano molybdenum, 3-5 parts of nano magnesium, 2-3 parts of dipivaloylmethane cerium, 8-15 parts of nano carbon, 3-6 parts of silicon nitride, 3-6 parts of methyl acrylate, 5-9 parts of p-chloroaniline, 3-7 parts of isopropenyl acetate, 5-9 parts of sodium benzoate, 3-6 parts of 3,4-diphenylamine oxide, 5-8 parts of terephthaloyl chloride, 5-8 parts of denaturants and 5-10 parts of heat stabilizers. The prepared super-abrasion-resistant metal mixing nano material is good in abrasion resistance and high in compression resistance, and meanwhile, a corresponding preparation method is further disclosed.
Soil stabilizing agent
InactiveCN108069681AIncreased unconfined compressive strengthImprove water stabilityTetramethylammonium hydroxideIsopropenyl acetate
The invention discloses a soil stabilizing agent, and belongs to the technical field of land control. The soil stabilizing agent is prepared from the following raw materials: modified diatomite, fly ash, cement, gypsum powder, perlite powder, titanium oxide, surface active agents, tetramethylammonium hydroxide, polyacrylamide, hydroxypropyl methylcellulose, polycarboxylate, polyoxyethylene octylphenol ether, and isopropenyl acetate-ethyl isothiocyanate copolymers. The soil stabilizing agent provided by the invention has the advantage that the soil intensity, the no confined compressive strength, the water stability and the flushing resistance can be obviously improved.
Owner:CHANGSHA WUDAO IND DESIGN CO LTD
Preparation method of pydiflumetofen
The invention provides a preparation method of pydiflumetofen, and belongs to the field of preparation of technical compounds. The preparation method of pydiflumetofen comprises the following steps: (1) performing a reaction on p-chloroaniline and isopropenyl acetate to obtain 4'-chloropropiophenone; (2) performing benzene ring chlorination to obtain 2',4',6'-trichloropropiophenone; (3) performinga carbonyl reduction reaction to obtain 1-(2',4',6'-trichlorophenyl)-2-propanol; (4) performing hydroxyl halogenation to obtain 1-(2',4',6-trichlorophenyl)-2-chloropropane; (5) performing amination on 1-(2,4,6-trichlorophenyl)-2-chloropropane and O-methylhydroxylamine to obtain O-methyl N-[1-methyl-2-(2,4,6-trichlorophenyl)-ethyl]-hydroxylamine; (6) performing a substitution reaction on the product obtained in step (5) and 3-difluoromethyl-1-methyl-1H-pyrazole-4-carbonyl chloride under the action of an acid binding agent to obtain pydiflumetofen. By improving the preparation process of pydiflumetofen and changing a synthetic route of an intermediate, a traditional reduction process of sodium cyanoborohydride for oxime is omitted, and production cost is reduced.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
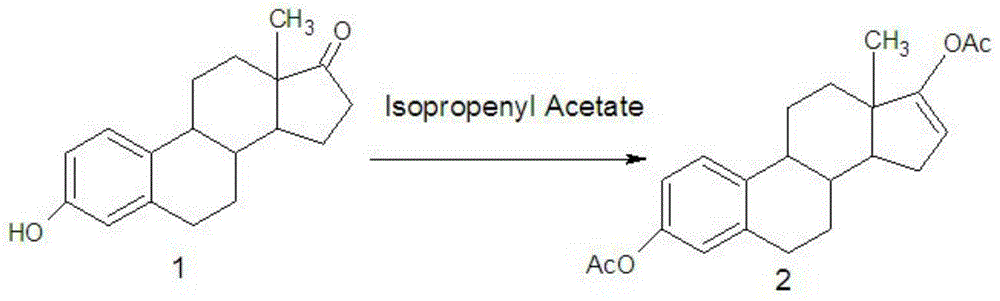
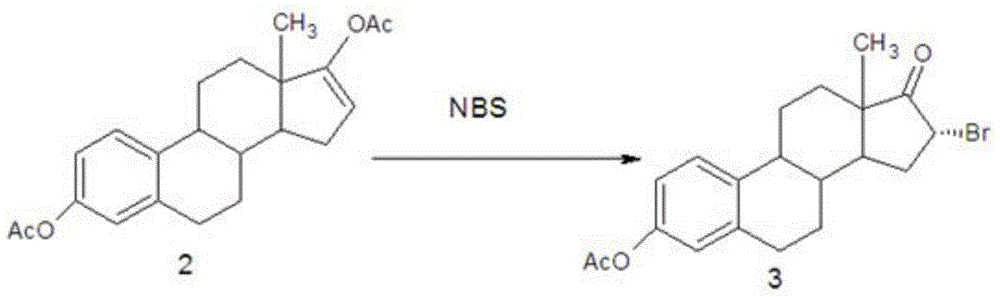
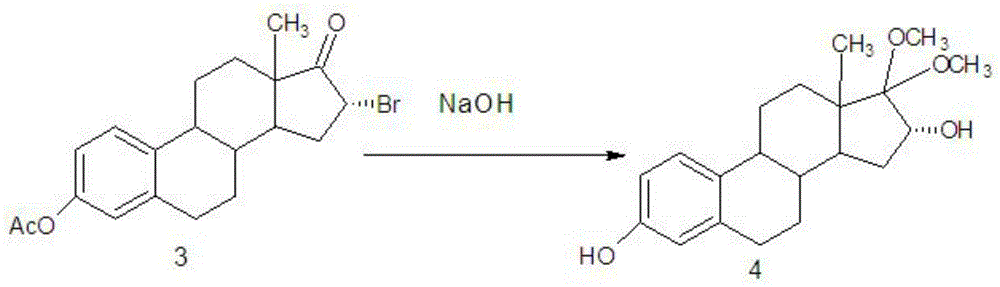
Novel estriol preparing method
The invention relates to a novel estriol preparing method. The novel estriol preparing method includes the following steps: 1, estrone is reacted with isopropenyl acetate to prepare an estraene diacetate compound; 2, the estraene diacetate compound is reacted with N-bromosuccinimide to prepare 16-bromo-acetic-acid estrone; 3, the 16-bromo-acetic-acid estrone is reacted in the environment of sodium hydroxide and methyl alcohol to prepare 17,17-dimethoxy-estradiol; 4, a hydrolysis reaction is carried out on the 17,17-dimethoxy-estradiol and hydrochloric acid, and 16alpha-hydroxy-estrone is obtained; 5, a reduction reaction is carried out on the 16alpha-hydroxy-estrone, and estriol can be obtained. When the estriol is prepared with the novel estriol preparing method, the reagents used for preparing are low in price, the reaction time is short, consumption of the reagents required by the reaction is small, and the content of the prepared estriol is high; meanwhile, epiestriol serving as impurities is effectively controlled, and the novel estriol preparing method is suitable for industrial production.
Owner:湖北三晶生物科技股份有限公司
Oil sludge diluent
An oil sludge diluent includes the following components by mass: 5%-20% of dichloropropane; 10%-15% of methanol; 10%-15% of an alcohol acid; 20%-25% of nitro isopropenyl acetate and 40%-50% of ytterbium hexafluoro-acetylacetone, and has the advantage of improving of the processing capacity.
Owner:SHAANXI SANYUAN JINTAI IND DEV
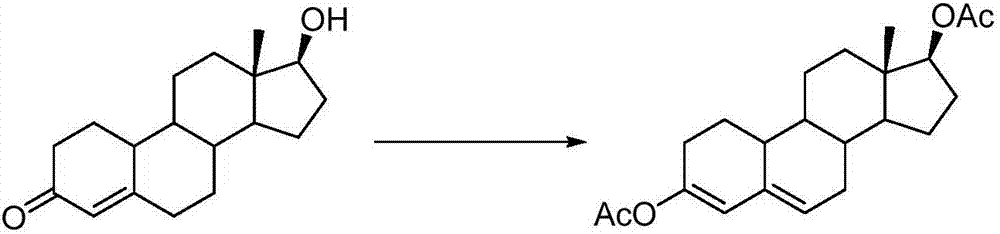
Preparation method for 3,5-estradiene-3,17beta-diacetate
The invention relates to a preparation method for 3,5-estradiene-3,17beta-diacetate. The preparation method comprises the following steps: sequentially adding nandrolone, p-toluenesulfonic acid monohydrate, isopropyl acetate and magneton No. 15 into a reactor, introducing condensed water, carrying out a heating reaction in an oil-bath pot and slowing adding isopropenyl acetate when reflux condensation of the obtained system begins; after completion of a reaction, carrying out evaporation to remove a solvent; cooling the system, adding pyridine, and adding isopropanol drop by drop until a white solid product is precipitated; carrying out low-temperature cooling and pumping filtration and then carrying out cleaning with cold isopropanol; collecting the white solid product and then carrying out drying so as to obtain a pure 3,5-estradiene-3,17beta-diacetate product. According to the invention, nandrolone is used as a substrate and a one-pot method is employed for carbonyl and hydroxyl acetylation protection so as to synthesize the product; the method is simple in process, mild in conditions and friendly to environment and has yield of up to 85%; and the synthesized product has critical application value in the fields of medicines, veterinary drugs and pesticides and is an important pro-drug for tibolone.
Owner:HUBEI UNIV OF TECH
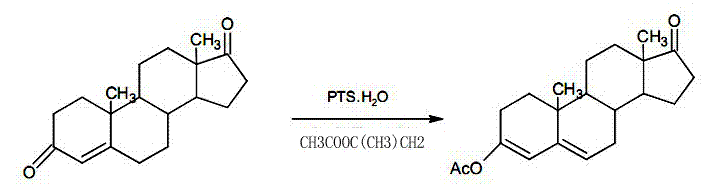
Preparation method of DHEA (dehydroepiandrosterone) intermediate 3 beta- acetoxyl- androstane-3, 5- diene-17-ketone
ActiveCN103694296AReduce manufacturing costFacilitate the implementation of large-scale industrial productionSteroidsPtru catalystIsopropenyl acetate
The invention discloses a preparation method of DHEA (dehydroepiandrosterone) intermediate 3 beta-acetoxyl-androstane-3,5-diene-17-ketone. The preparation method is characterized in that esterification reaction reagent is isopropenyl acetate. The preparation method comprise the following steps of dissolving 1W of 4AD (4-androstenediol) into 3-8V of organic solvent, wherein W is weight g, and V is volume ml; adding 1-5w% strong acidic catalyst, stirring and heating up to the reaction temperature of 40-80 DEG C; dripping 0.5-1.2 V of isopropenyl acetate, preserving heat and reacting for 1-2 hours after dripping, until displaying completion of reaction of raw materials by TLC (Thin Layer Chromatography); adding 2-6% V triethylamine for neutralization after reaction, and concentrating the solvent to dry; adding 1V of methyl alcohol, dissolving while stirring, cooling to crystallize for 2-3 hours, filtering, performing solvent washing, and baking to dry below 70 DEG C, thereby obtaining the DHEA intermediate 3 beta- acetoxyl- androstane-3, 5- diene-17-ketone. The DHEA intermediate prepared by the invention has high yield, high purity and steady mass, and can be baked under normal pressure; more importantly, solvent recovery rate is high, and the preparation method almost produces no wastewater, is economic and environmental friendly, and is very beneficial to industrial production.
Owner:HUNAN KEREY BIOTECH
Method for preparing waterproof adhesive for corrugated paper production
InactiveCN101979452BAvoid damageImprove water resistanceMechanical working/deformationStarch derivtive adhesivesCross-linkAdhesive
The invention relates to a method for preparing waterproof adhesive for corrugated paper production, and belongs to the technical field of paper coating adhesive. A starch-isopropenyl acetate cross linked graft copolymer is synthesized, and then the waterproof adhesive for the corrugated paper production is prepared. The method for preparing the waterproof adhesive for the corrugated paper production can effectively reduce the damage of water molecules to the adhesive so that the waterproof property of the adhesive is greatly improved; and because the hydrophilic property of the modified adhesive declines, the adhesive has good quick drying property and is favorable to be used on high-speed corrugated lines.
Owner:ZHEJIANG GREAT SHENGDA PACKING CO LTD
Method for synthesizing cane sugar-6-acetic ester by using lipase for catalyzing
The invention discloses a method for synthesizing a cane sugar-6-acetic ester by using lipase for catalyzing. The method comprises the following steps of: based on 72,000 to 150,000 enzyme activity unit enzyme in 100 ml buffer solution, adding the lipase into buffer solution with a pH value of between 8.1 and 8.7 to obtain enzyme solution; adding an organic solvent into the enzyme solution; adding cane sugar until the cane sugar in reaction solution is saturated; stirring until the cane sugar is solved and adding an acetic ester compound; reacting at the temperature of between 40 and 65 DEG Cwith stirring; adding the acetic ester compound after reacting for 11 to 13 hours; ending an reaction after reacting totally for 23 to 25 hours; and washing the reaction product with distilled water,decompressing and distilling to obtain cane sugar-6-acetic ester, wherein the acetic ester compound is vinyl acetate or isopropenyl acetate; the volume ratio of the enzyme solution to the organic solvent is 0.1-1:100; and the organic solvent is tertiary amyl alcohol, sec-butyl alcohol or tetrahydrofuran. The organic solvent is selected and the water content of a reaction system is less than 1.0 percent, so that the purity of the obtained high-purity cane sugar-6-acetic ester is over 75 percent, and 99 percent in maximum.
Owner:ZHEJIANG UNIV OF TECH
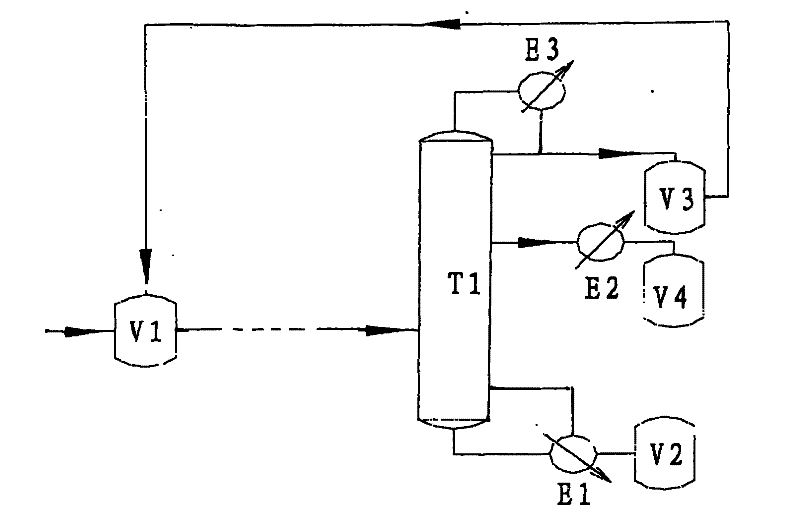
Method for refining acetylacetone
InactiveCN101514151BStable mass contentHigh purityCarbonyl compound separation/purificationDecompositionIsopropenyl acetate
A method for refining high-purity acetylacetone comprises procedures of devolatiligation, deacidification and deanhydride to coarse acetylacetone which later on enters a refining tower T1. The method is characterized in that the refining tower T1 is a pressure relief refining tower, the high-purity acetylacetone is extracted from the upper side line of the rectification section in liquid phase, acetone generated by the decomposition of acetylacetone and low-boiling mixed components, such as isopropenyl acetate, which are not completely removed due to instability of the initial stage of the process, are collected at the top of the tower, returned to a coarse acetylacetone groove V1, placed in the refining tower T1 after the procedures of devolatiligation, deacidification and deanhydride, and the tower kettle uses high-boiling components. The invention has the advantages that the refining tower T1 has the function of removing a small mount of low-boiling components because of liquid phase side line discharge way thereof, meanwhile, the acetone and isopropenyl acetate obtained from the top of the tower can be recycled, and the tower kettle uses high-boiling components, so that the whole rectification section is easy to control, flexible in operation, high in purity of the finished products, and stable in mass content of 99.9%.
Owner:SHANGHAI WUJING CHEM +1
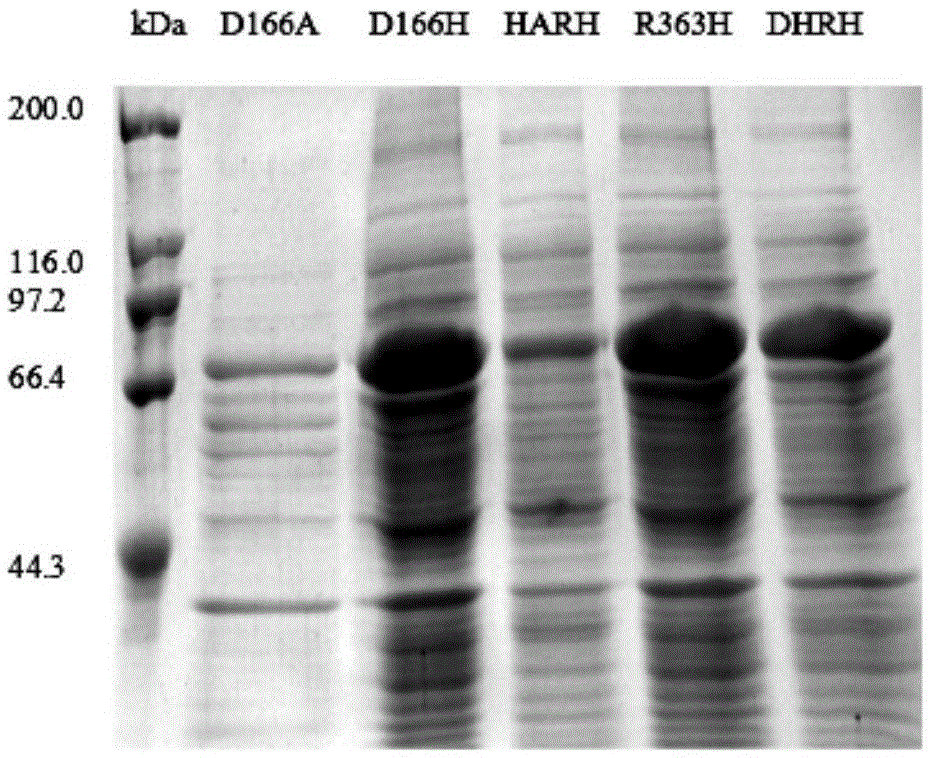
DBAT mutation enzyme D166H and application thereof
ActiveCN105524893AIncrease usageIncrease productionBacteriaMicroorganism based processesIsopropenyl acetateAmyl acetate
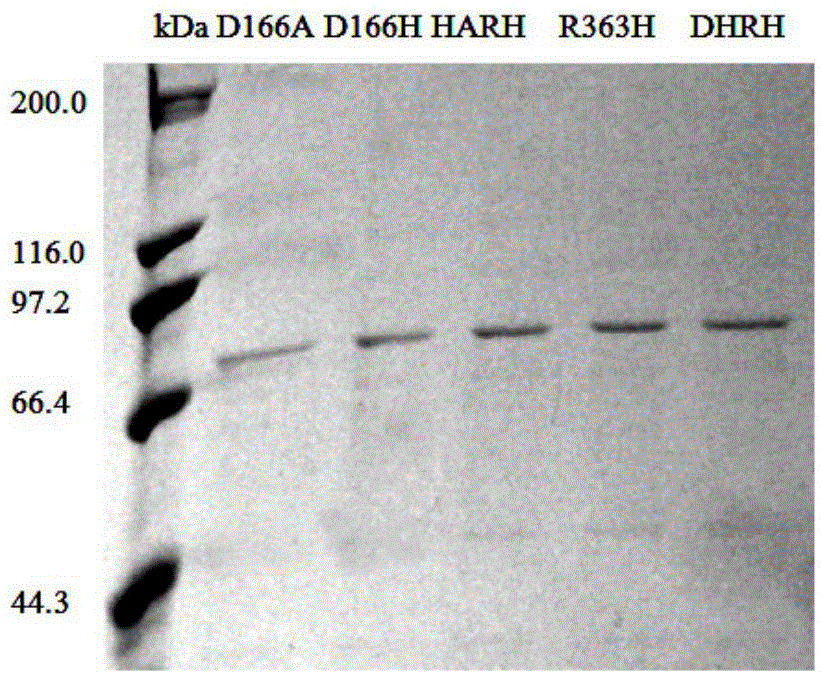
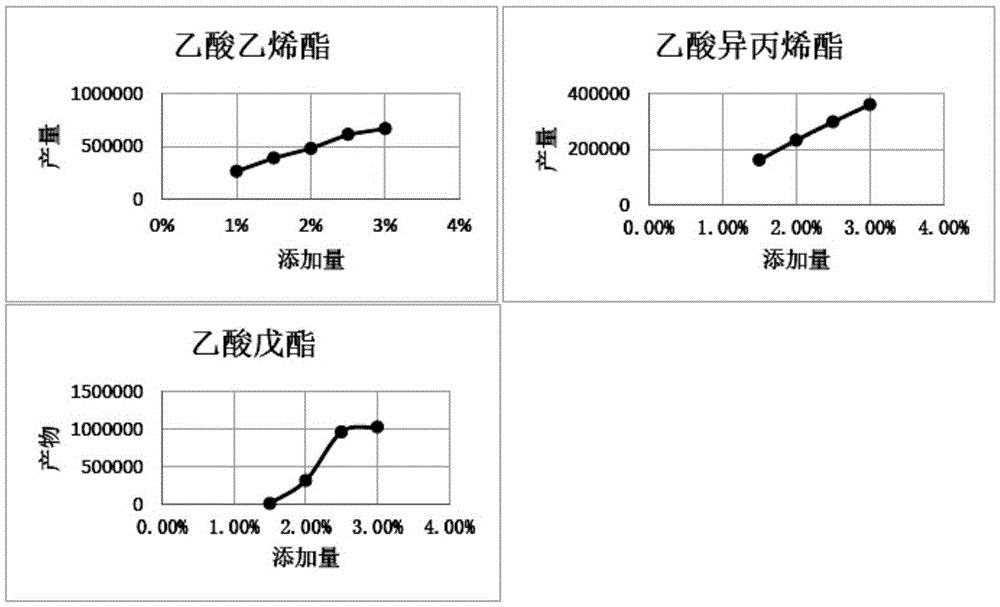
The invention discloses a DBAT mutation enzyme D166H and application thereof. The mutation enzyme D166H is obtained by changing a 166th amino acid of a DBAT enzyme with an amino acid sequence of SEQ ID NO: 2 from Asp to His. The amino acid sequence of the mutation enzyme D166H is SEQ ID NO: 6. The catalytic efficiency of utilizing acetyl coenzyme A of the mutation enzyme is improved by 11 times compared with that of a wild type enzyme, and the usage rate of acetyl coenzyme A and the yield of a product baccatin III are increased greatly. Meanwhile, in order to reduce the production cost, seven novel acyl donors are found, and acetic acid vinyl ester, amyl acetate and isopropenyl acetate can be utilized efficiently by the mutation enzyme. The utilization rate of acetic acid vinyl ester is improved by 74 times, and the mutation enzyme can replace an expensive acetyl coenzyme A substrate to be used for preparing baccatin III. So far, in research for baccatin III biosynthesis, research for using non-plant coenzymes as the acyl donors and achieving the high utilization rate is not found.
Owner:SOUTH CHINA AGRI UNIV
Catalyst for synthesizing isopropenyl acetate and preparation method thereof
ActiveCN102463139BInitial activity inhibitionShort acclimatization periodOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperatureIsopropenyl acetate
The invention relates to a catalyst for synthesizing isopropenyl acetate and a preparation method thereof, which mainly solve the problems that in the prior art, the Pd-Cu alloy crystal grains are too small, so the initial activity of the catalyst is too high, the catalyst is in a lower reaction temperature for a long time, the induction period of the catalyst is prolonged, and the yield of the isopropenyl acetate is reduced. The catalyst and the preparation method have the technical scheme that Pd (palladium), Cu (copper) and alkali metal acetates are loaded on silicon dioxide, aluminum oxide or a mixture of the silicon dioxide and the aluminum oxide, and the Pd-Cu alloy crystal grain size is 5 to 15nm. The problems are perfectly solved, and the catalyst and the preparation method can be used for industrial production of isopropenyl acetate catalysts.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nail polish diluting agent
A nail polish diluting agent is prepared from 40-60% of isopropenyl acetate, 20-30% of chromium fluoride, 10-15% of tetra-n-ammonium perchlorate, 1-5% of polyvinyl alcohol and the balance inevitable impurities. The nail polish diluting agent has the advantage of being good in use effect.
Owner:SHENYANG XINDA INFORMATION SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com