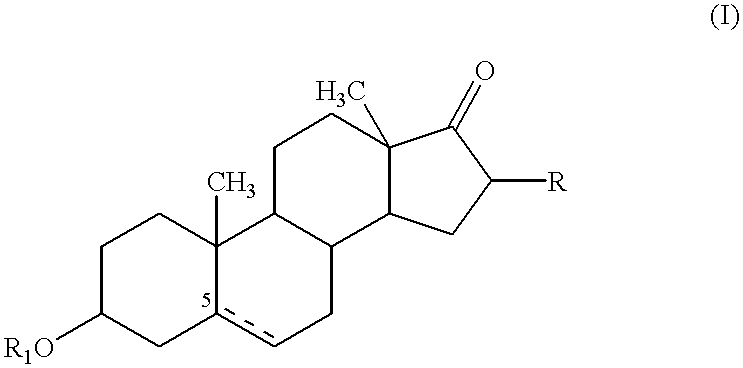
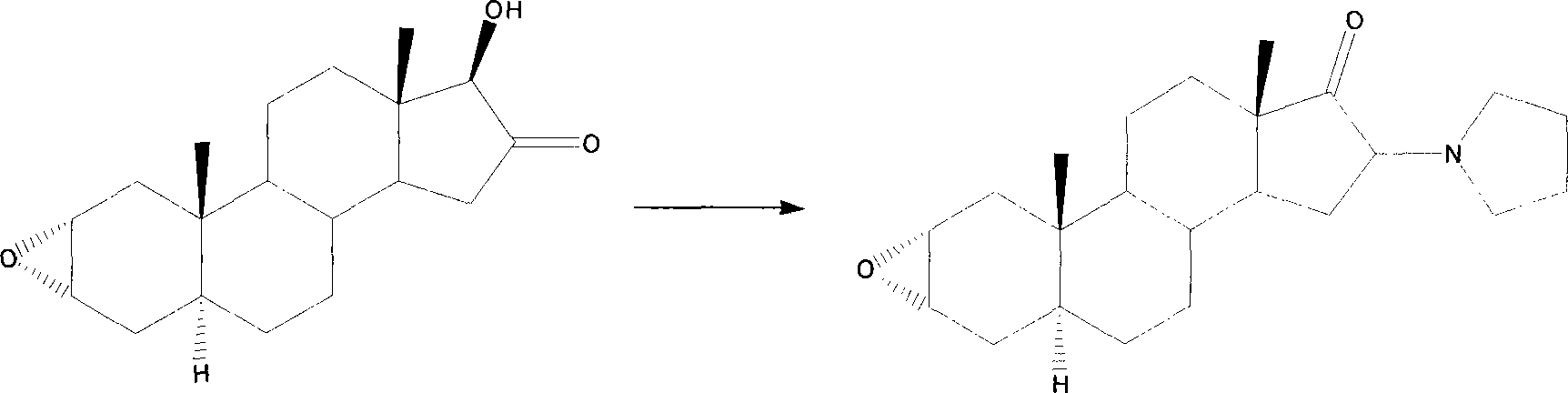
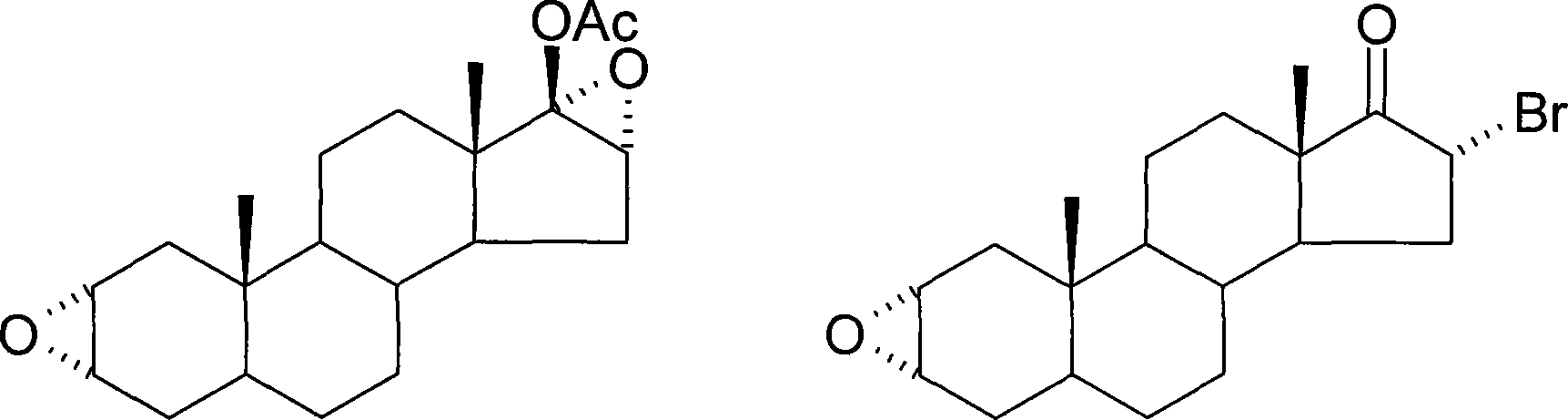
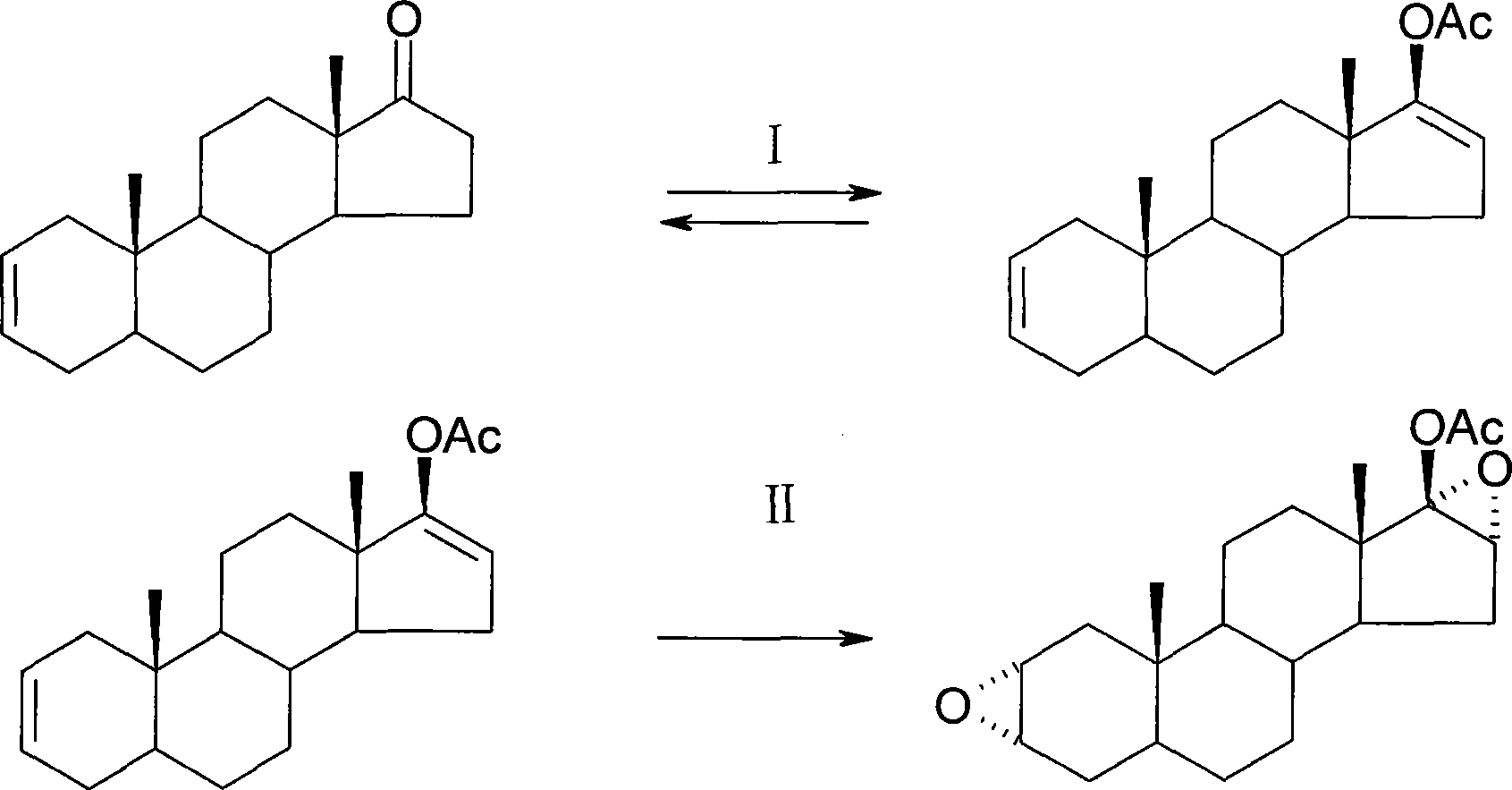
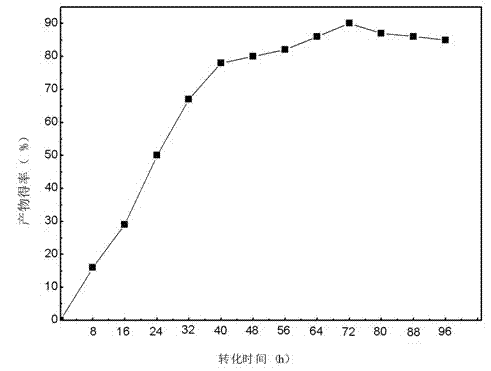
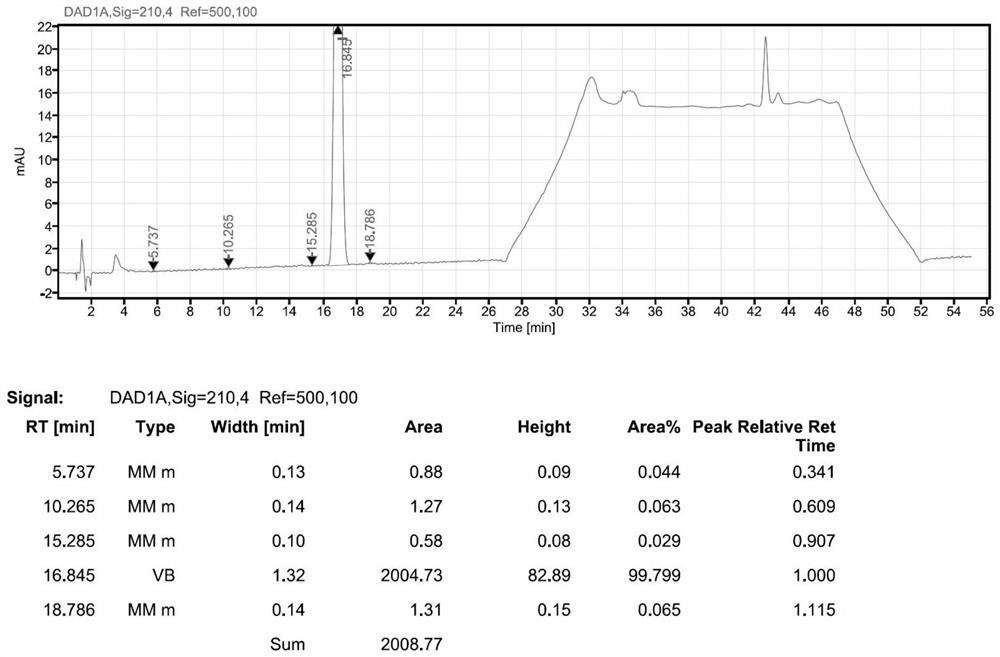
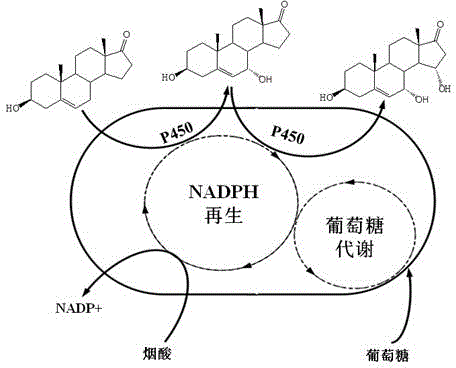
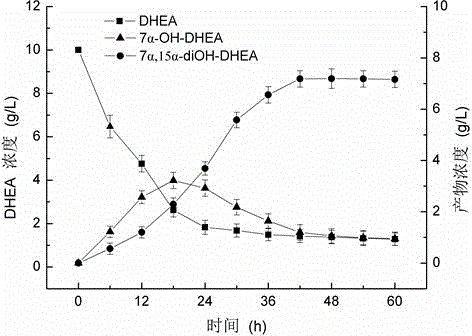
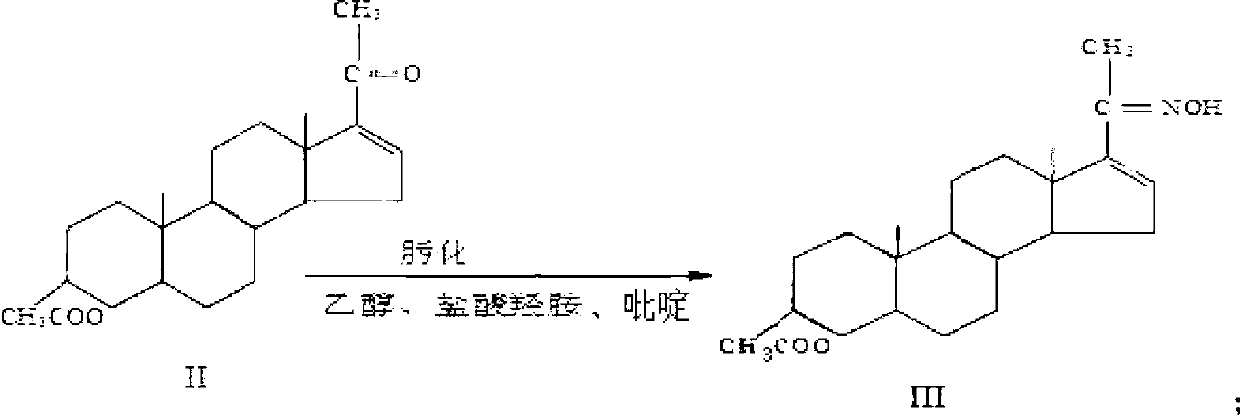
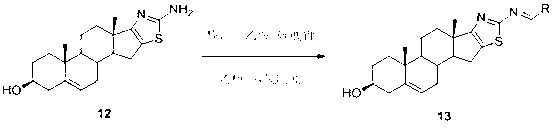Patents
Literature
93 results about "Epiandrosterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
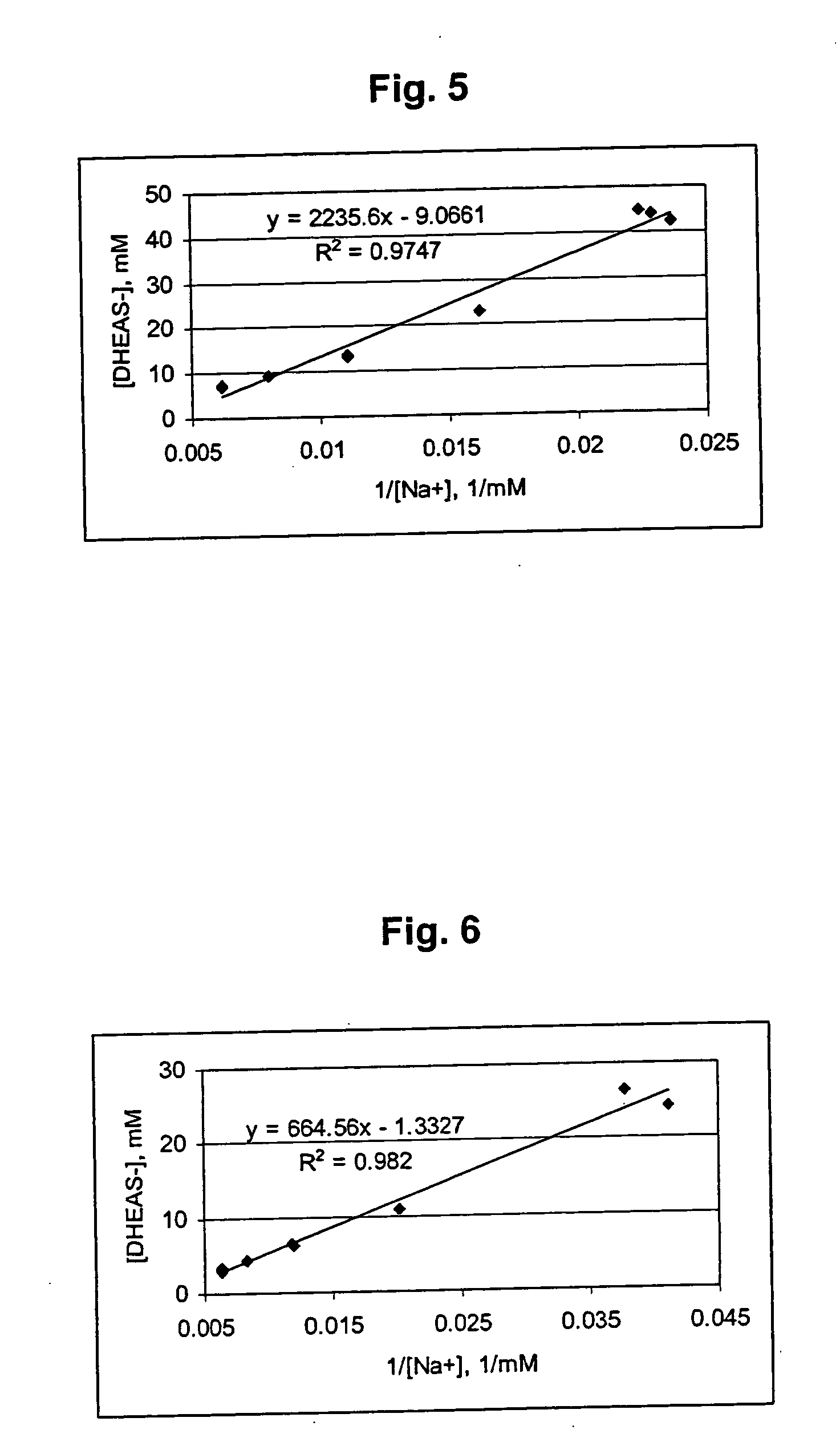
Epiandrosterone, or isoandrosterone, also known as 3β-androsterone, 3β-hydroxy-5α-androstan-17-one, or 5α-androstan-3β-ol-17-one, is a steroid hormone with weak androgenic activity. It is a metabolite of testosterone and dihydrotestosterone (DHT). It was first isolated in 1931, by Adolf Friedrich Johann Butenandt and Kurt Tscherning. They distilled over 17,000 litres of male urine, from which they got 50 milligrams of crystalline androsterone (most likely mixed isomers), which was sufficient to find that the chemical formula was very similar to estrone.
Compositions & formulations with an epiandrosterone or a ubiquinone & kits & their use for treatment of asthma symptoms & for reducing adenosine/adenosine receptor levels
A composition and various formulations comprise preventative or therapeutic amounts of an epiandrosterone, analogue thereof or salt thereof, and / or a ubiquinone or salt thereof, and a pharmaceutically or veterinarily acceptable carrier or diluent. The composition and formulations are useful for treating bronchoconstriction, respiratory tract inflammation and allergies, asthma, and cancer. A method of treating diseases associated with low adenosine levels or adenosine depletion comprises administering folinic acid or a pharmaceutically acceptable salt hereof in a preventative or therapeutic amount, or an amount effective to treat adenosine depletion.
Owner:EAST CAROLINA UNIVERISTY
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026850A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
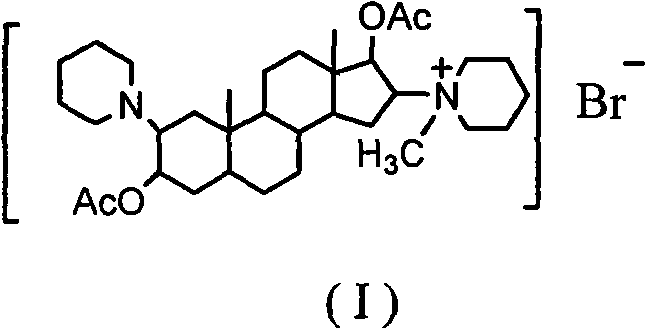
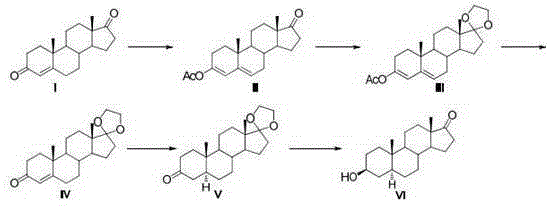
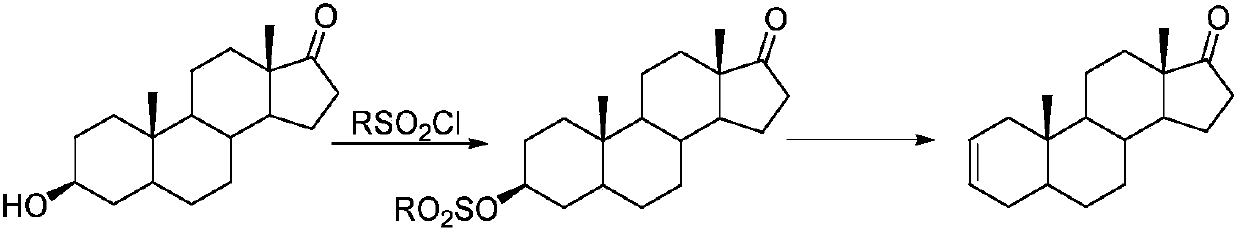
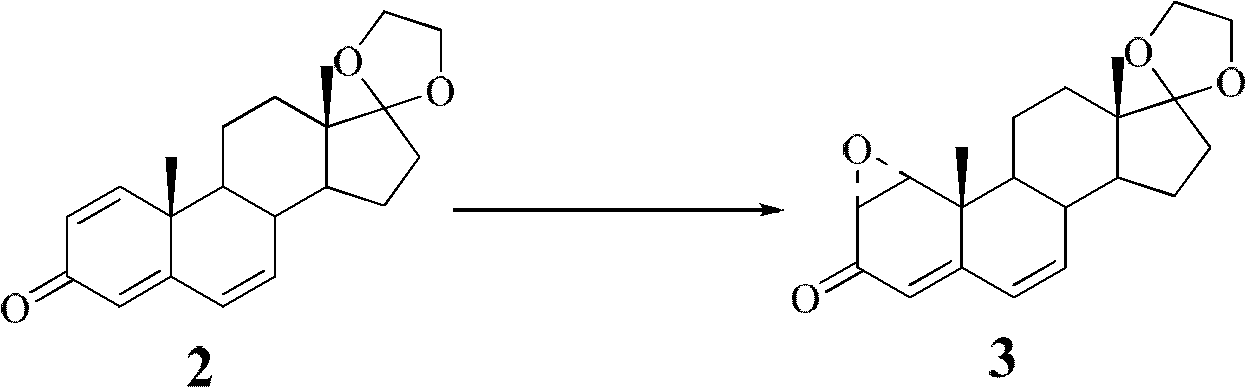
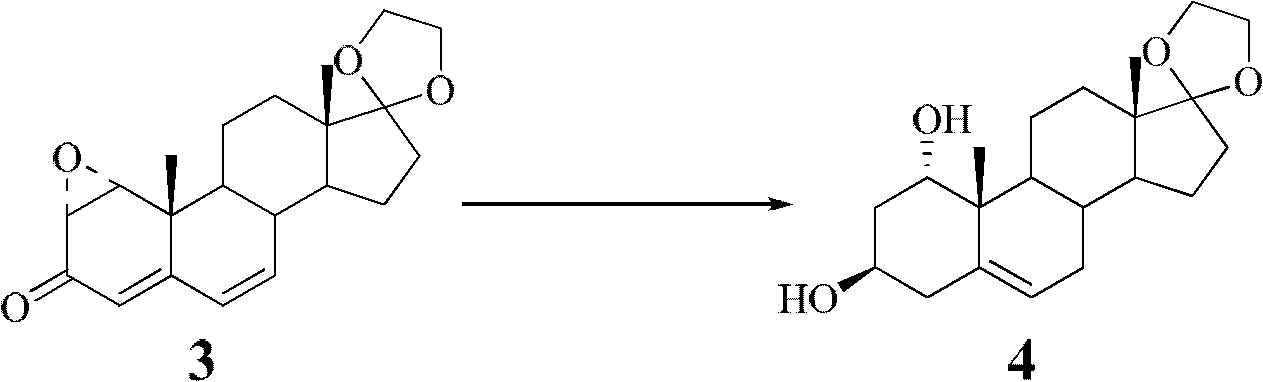
Synthesis process of vecuronium bromide
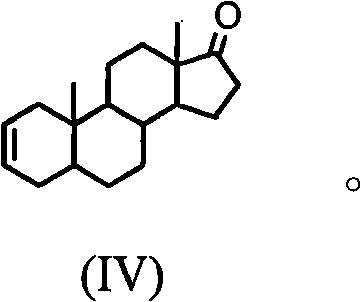
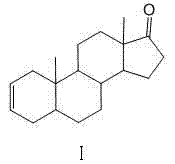
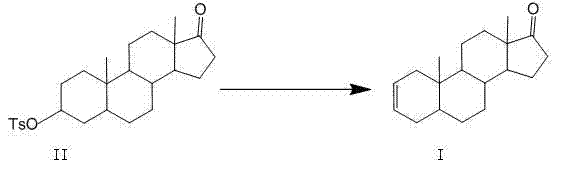
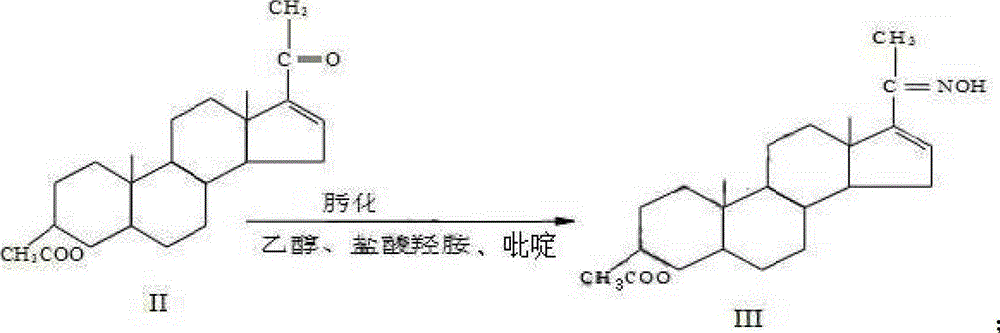
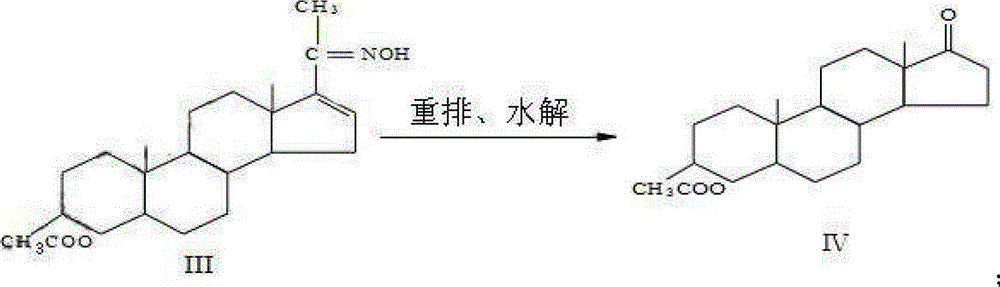
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
Pharmaceutical compositions
ActiveUS20090054383A1Achieve beneficial effectPrevent adverse side effectsBiocideOrganic active ingredientsDiseaseEstrogenic Effects
Novel methods for treating or reducing the likelihood of acquiring symptoms or diseases due to the menopause, in postmenopausal women, particularly osteoporosis, vaginal atrophy and dryness, hypogonadism, diminished libido, skin atrophy, connective tissue disease, urinary incontinence, breast, endometrial, ovarian and uterine cancers, hot flashes, loss of muscle mass, insulin resistance, fatigue, loss of energy, aging, physical symptoms of menopause, in susceptible warm-blooded animals including humans involving administration of a sex steroid precursor are disclosed. Said method comprising novel ways of administering and dosing dehydroepiandrosterone (DHEA) in order to take advantage of positive androgenic effects in the vaginal layers lamina propia and / or the layer muscularis, without undesirably causing systemic estrogenic effects in order to avoid the risk of breast and uterine cancer. Pharmaceutical compositions for delivery of active ingredient(s) useful to the invention are also disclosed.
Owner:MYRIEL PHARM LLC
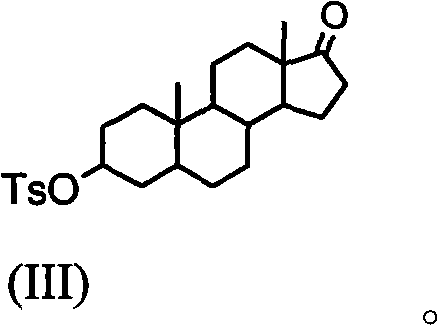
Method of synthesizing 2alpha,3alpha-epoxy-16alpha-bromo-5alpha-androsterone-17-one
The invention discloses a making method of 2 alpha, 3 alpha-epoxy-16 alpha-bromine-5 alpha-androstane-17-ketone as intermediate of steroidal muscular relaxation drug, which comprises the following steps: dissolving epiandrosterone in the benzene; adding catalyst; stirring; heating; refluxing 8-12h; preparing 5 alpha-androst-2-olefin-17-ketone; reacting the 5 alpha-androst-2-olefin-17-ketone and copper bromide in the carbinol to produce 16 alpha-bromine-5 alpha-androst-2-olefin-17-ketone; reacting the 16 alpha-bromine-5 alpha-androst-2-olefin-17-ketone and m-perbenzoic acid in the composite solvent of water and dichloromethane; producing the product through eliminating, bromizing and epoxidising.
Owner:WUHAN UNIV
Strain for biosynthesis of 3beta, 7alpha, 15alpha-trihydroxyandrost-5-ene-17-one and application thereof
Owner:JIANGNAN UNIV
Composition and preparation for resisting ageing and improving male energy, preparation method of preparation and application of composition
InactiveCN109045059AReasonable compositionAppropriate compatibilityOrganic active ingredientsInorganic active ingredientsSexual functionProtopanaxadiol
The invention discloses a composition for resisting ageing and improving male energy. The composition is prepared from a raw material and an auxiliary material; the raw material is prepared from the following components in parts by weight: 1-15 parts of nicotinamide mononucleotide, 1-10 parts of protopanoxadiol, 1-9 parts of icarisid I, 2-8 parts of baohuoside I, 3-7 parts of dehydroepiandrosterone and 2-10 parts of ursodesoxycholic acid. The composition is reasonable in composing prescription and proper in compatibility; in the composition, the four of the nicotinamide mononucleotide (NMN), the icarisid I, the baohuoside I and protopanoxadiol are used as monarch drugs together, the composition is capable of comprehensively regulating gonad axis and invigorating kidney-yang and liver, candelay ageing period of the gonad axis and improve the sexual function after being taken for a long time; through intercoordination and synergistic effect of various components, the composition is comprehensive in nutrition, has very good palatability, is particularly suitable for males to take and thus relieving the symptoms of male ageing syndrome and improving the male energy. The invention alsoprovides a preparation containing the composition and a preparation method of the preparation. The preparation method is simple, is moderate in condition and is suitable for industrial batch production.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Preparation method for dehydroepiandrosterone, and enzyme for preparation thereof
InactiveCN109312382AHigh yieldSimple processMicroorganismsMicroorganism based processesSodium ascorbate4-Androstenedione
A preparation method for dehydroepiandrosterone, comprising: in a protective atmosphere, adding potassium tert-butoxide to tert-butanol, stirring evenly, adding 4-androstenedione to obtain a mixture,and adding the mixture dropwise to a sodium ascorbate-containing acetic acid solution for a reaction to obtain 5-androstenedione; dissolving the 5-androstenedione in an organic solvent, adding a ketone reductase, a glucose dehydrogenase, glucose and a redox coenzyme to obtain a mixture, controlling the pH of the mixture to be 6.0-6.3, stirring and reacting for 1-6 hours at 22-26 DEG C to obtain areaction solution, and performing separation and purification on the reaction solution to obtain dehydroepiandrosterone, the ketone reductase and the glucose dehydrogenase being coexpressed by a microbial strain and added in the form of a crude enzyme solution. The synthesis process of the preparation method has few steps, simple operations, high yields and low costs, and may be widely applied toindustrial scale production. Also provided is an enzyme for preparation.
Owner:BONTAC BIO ENG SHENZHEN
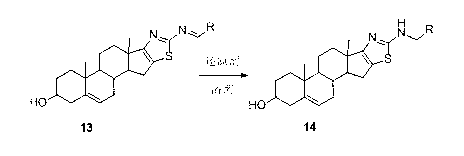
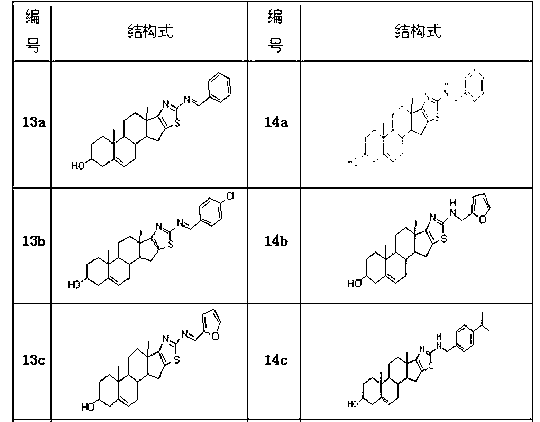
Dehydroepiandrosterone D cyclobenzo-aminothiazole ring compounds as well as preparation method and application thereof
ActiveCN103193859AThe synthesis method is simpleSuitable for industrial productionOrganic active ingredientsSteroidsCancer cellEpiandrosterone
The invention belongs to the field of medicinal chemistry and in particular relates to dehydroepiandrosterone D cyclobenzo-aminothiazole ring imine and imine reducing compounds as well as a preparation method and application thereof. The compounds are shown in a formula I in the specification. An in-vitro antineoplastic activity experiment indicates that the compounds have better broad-spectrum antineoplastic activity, have a favorable inhibition effect on multiple human cancer cells, such as EC109 and EC9706 and are suitable for research of antineoplastic lead compounds or preparation of novel antineoplastic medicaments. The preparation method is simple and convenient and suitable for industrial production.
Owner:ZHENGZHOU UNIV
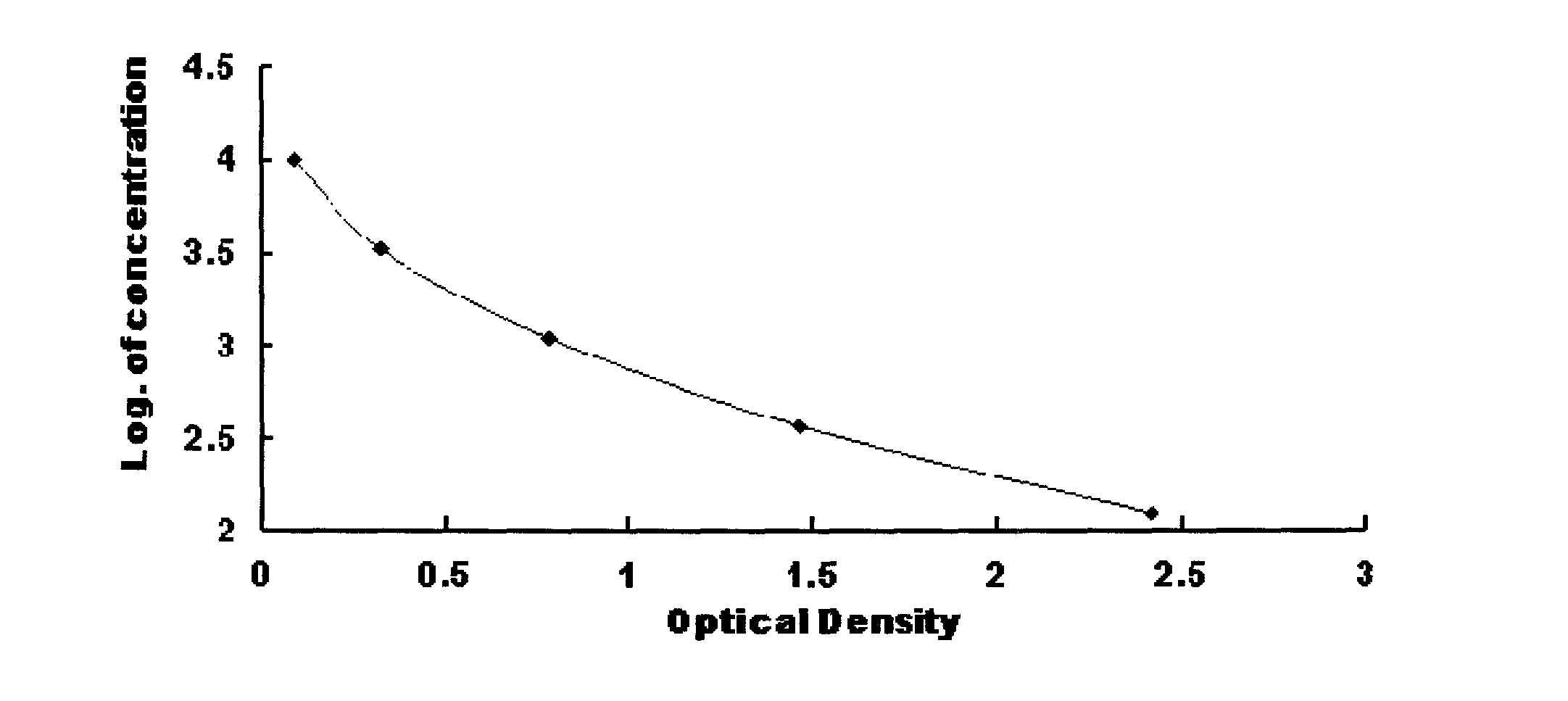
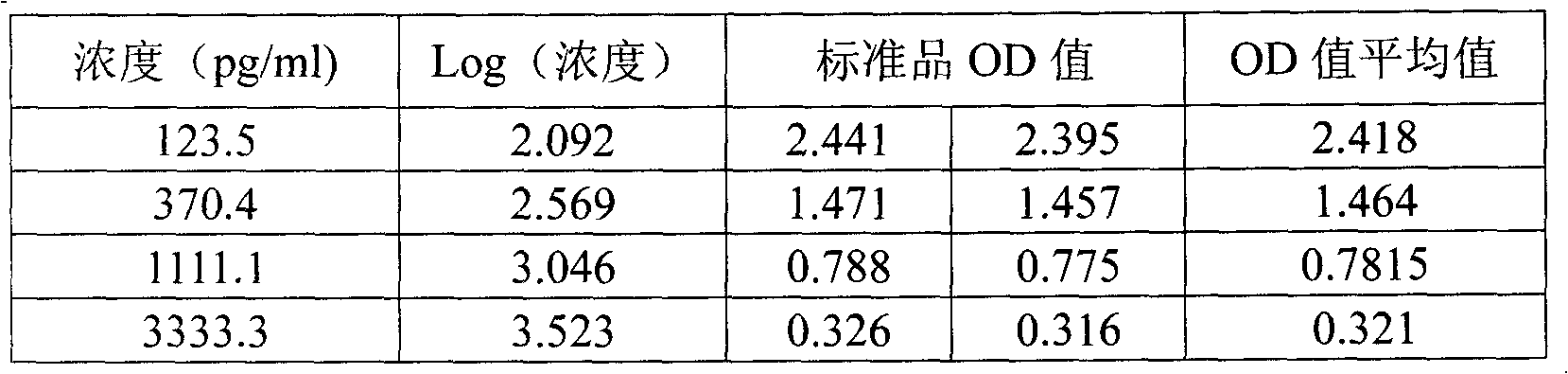
Dehydroepiandrosterone enzyme-linked immunosorbent assay kit development method
InactiveCN102998464ASimplify operation stepsImprove stabilityBiological testingElisa methodDehydroepiandrosterone
The present invention discloses a dehydroepiandrosterone (DHEA) enzyme-linked immunosorbent assay kit development method, which comprises: adopting dehydroepiandrosterone and succinic anhydride as raw materials, and adopting an anhydride mixing method to respectively prepare dehydroepiandrosterone artificial antigen (DHEA-BSA and DHEA-OVA) and anti-dehydroepiandrosterone (DHEA) monoclonal antibody so as to prepare an enzyme-linked immunosorbent assay (ELISA) kit. Compared with the high performance liquid chromatography (HPLC) method, the method of the present invention has the following advantages that: operation steps are simple, a plurality of samples can be detected in one time, expensive instrument is not required, and detection sensitivity, the required time and other aspects are better than the HPLC method. Compared to the HPLC method, the ELISA method has the following characteristics that: uses of organic reagents such as n-hexane, acetonitrile, methanol and the like are not required, poison on experimenters and environment can be reduced, and an environmental protection characteristic is provided.
Owner:WUHAN CLOUD CLONE CORP
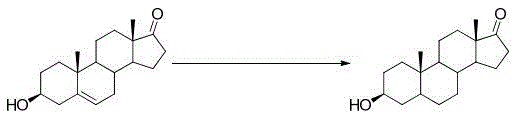
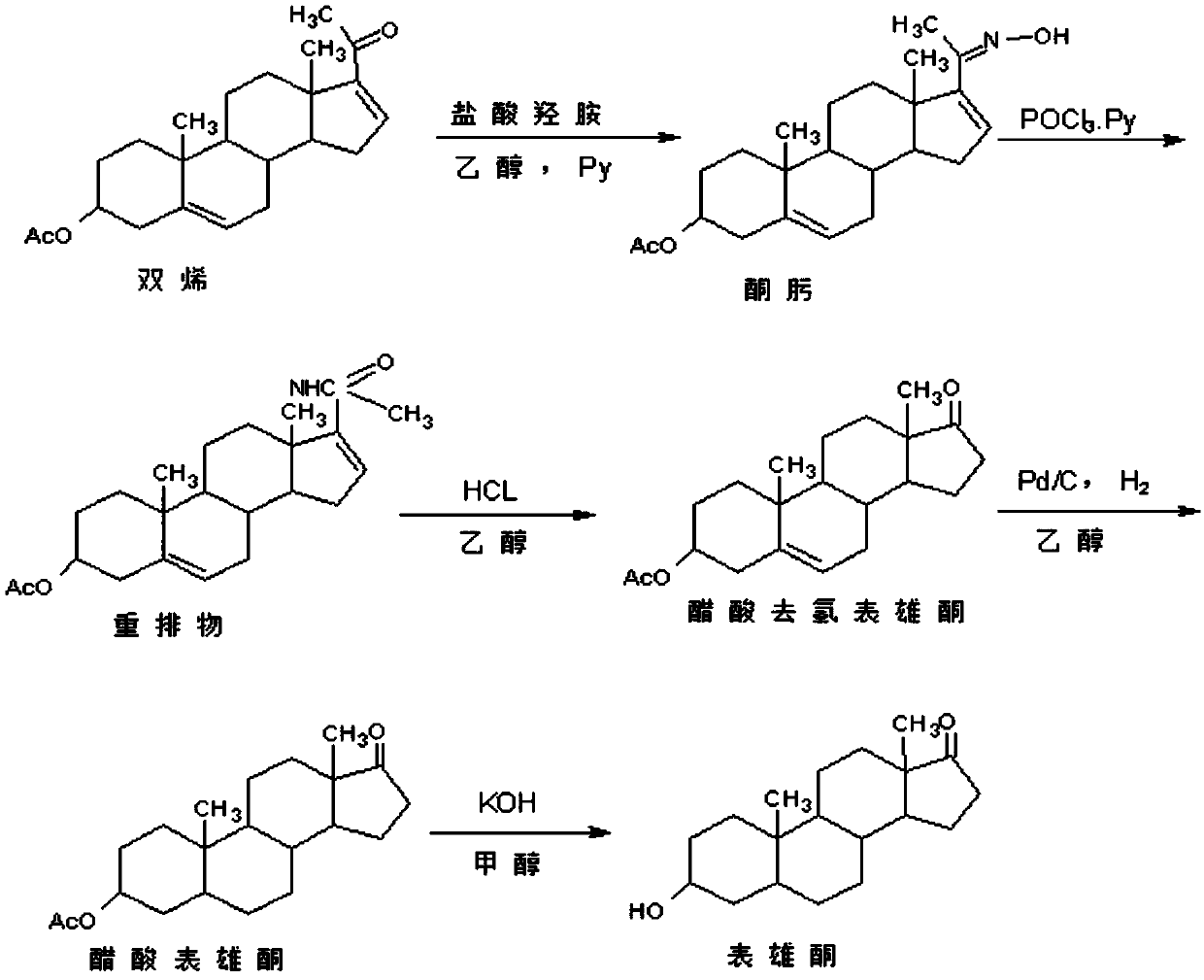
Method for preparing epiandrosterone
The invention discloses a method for preparing epiandrosterone. 4-androstenedione (I) is used as a raw material to prepare the epiandrosterone. The reaction formula is shown in the description. The low-cost 4-androstenedione is used as the raw material for the first time, and the epiandrosterone is obtained through synthesis on the mild reaction conditions, so that the production cost is greatly lowered, and the method is suitable for large-scale industrial production.
Owner:仙琚(嘉兴)医药科技有限公司 +1
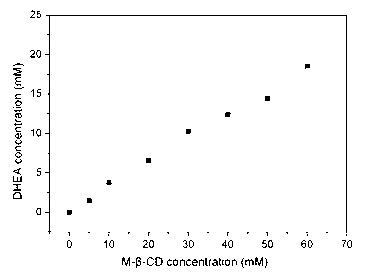
Method for hydroxylating dehydroisoandrosterone by using colletotrichumlini
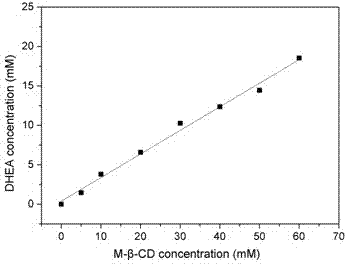
ActiveCN102703558AHigh feeding concentrationHigh substrate conversion rateMicroorganism based processesFermentationChemistryWater soluble
The invention relates to a method for hydroxylating dehydroisoandrosterone (DHEA) by using colletotrichumlini ST-1. The method for hydroxylating the dehydroisoandrosterone by using the colletotrichumlini ST-1 is characterized in that: in a conversion process, a way of pre-including the DHEA and methyl-beta-cyclodextrin with an equimolar ratio first and then feeding is adopted, so that the water solubility and the conversion efficiency of the DHEA are significantly improved. Under a condition that a substrate feeding amount is 10g / L, the conversion rate is as high as 95% and the total yield of products 7 alpha-OH-dehydroepiandrosterone and 7,15 alpha-OH-dehydroepiandrosterone is 80.94%.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
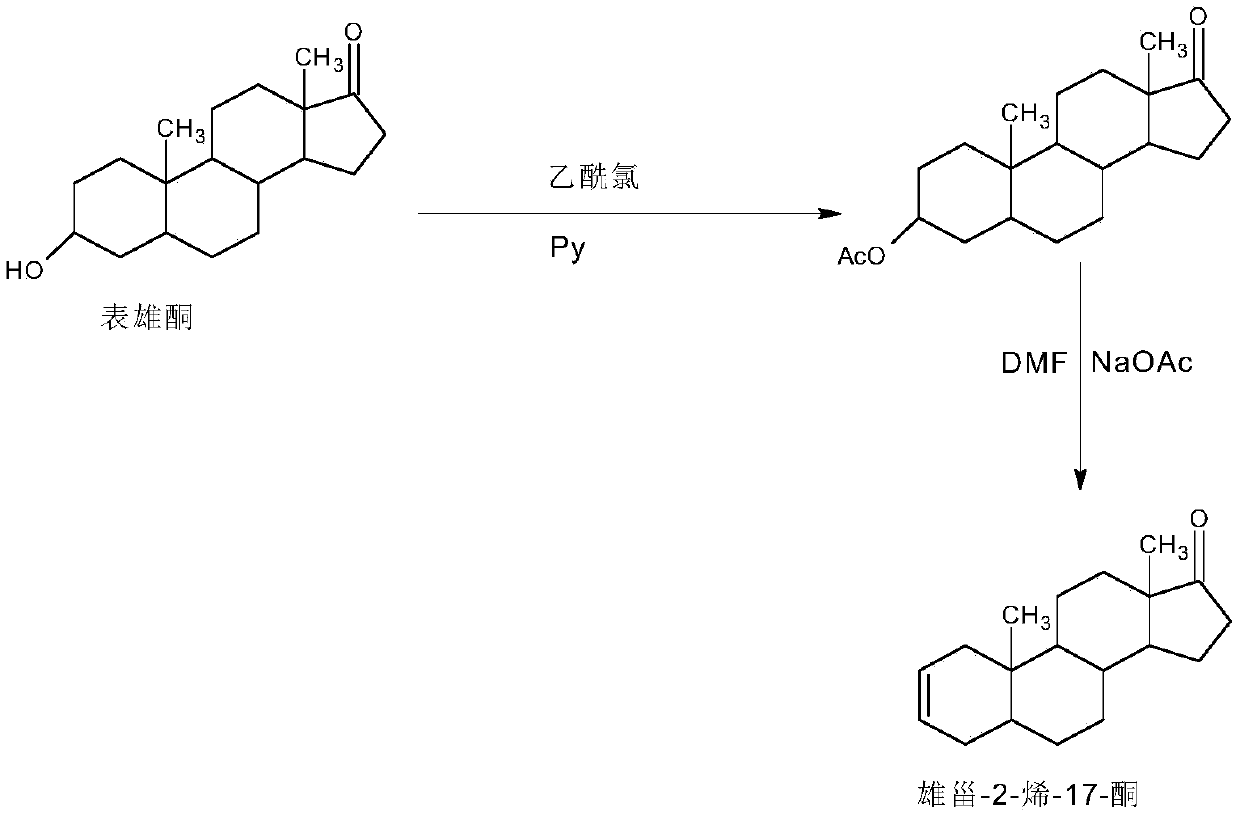
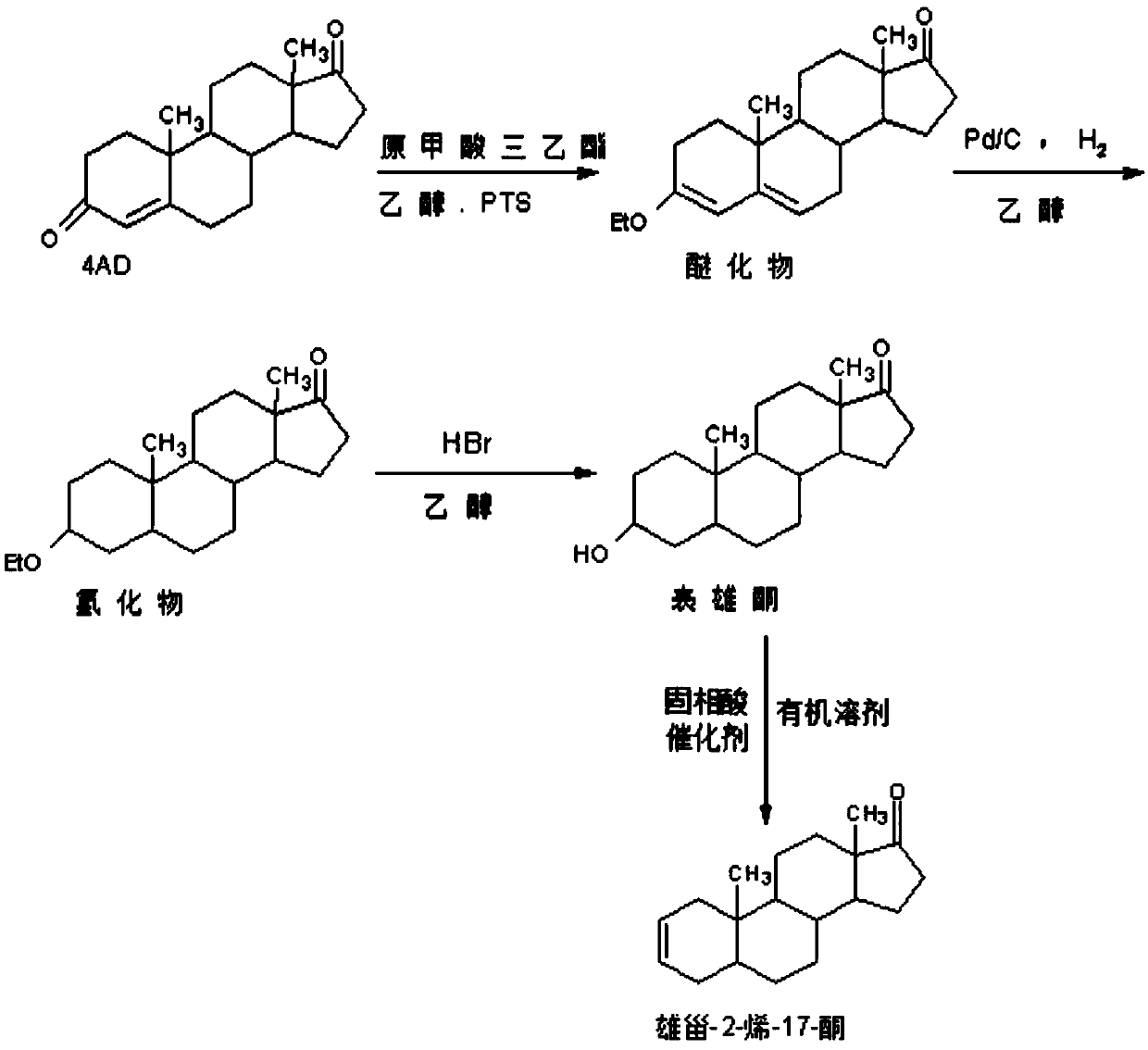
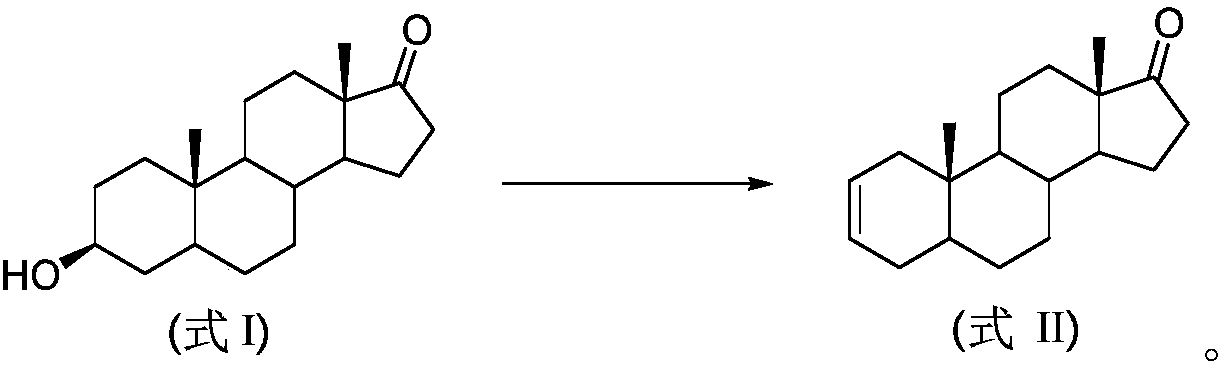
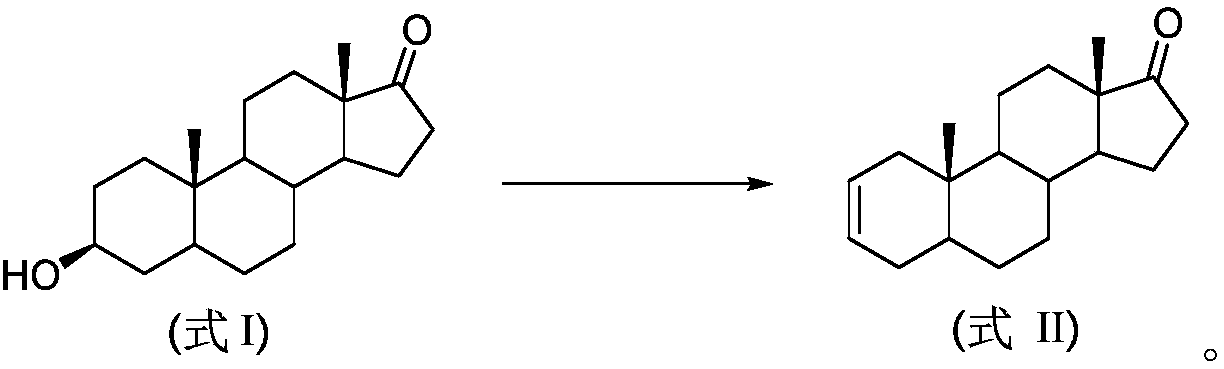
Preparation method of androst-2-en-17-one
The invention discloses a preparation method of androst-2-en-17-one. The method comprises the steps that 4-androstenedione is adopted as a raw material, three-step reactions of a protective reaction,a reduction reaction and a deprotection reaction are adopted for synthesizing epiandrosterone, in an organic solvent, a carrier solid phase acid catalyst soaked with acid is used for catalyzing, epiandrosterone dehydration is performed to eliminate 3-bit hydroxyls in molecules, and a target product is obtained. 4-androstenedione obtained by phytosterol extracted from a soybean oil deodorization distillate through the modern fermentation technology is adopted as a raw material; 4-androstenedione is subjected to the three-step reactions to synthesize a key intermediate epiandrosterone, and raw materials are abundant in source and low in price. The epiandrosterone is used for preparing a target product, compared with a traditional method, a synthetic route is cut into a one-step reaction fromtwo-step reactions, the method is economical and environmentally friendly, and production and operation are easier. By adopting the carrier solid phase acid catalyst soaked with acid for catalyzing,an oligomer makes indirect contact with the acid catalyst, the reactions are mild and stable, the dehydration elimination reactions are performed in the thermodynamics stable 2,3-bit directions, and the reaction selectivity is good.
Owner:HUNAN KEREY BIOTECH
Preparation method of 5 alpha-androstane-2-ethylene-17-ketone
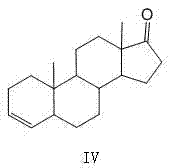
The invention discloses a preparation method of 5 alpha-androstane-2-ethylene-17-ketone. The preparation method comprises the steps of taking epiandrosterone (formula I as shown in the specification)as a raw material to give a dehydration reaction by joint catalysis of protonic acid and trifluoromethanesulfonate, and performing post-treatment, recrystallization and separation on a reaction product to form 5 alpha-androstane-2-ethylene-17-ketone (formula II as shown in the specification). The preparation method avoids the use of much organic base compound in the traditional method, has the advantages of high reaction yield, short procedure, good selectivity, low cost, less waste gas, waste water and industrial residue and the like and is a synthesis method suitable for industrial production.
Owner:台州仙琚药业有限公司
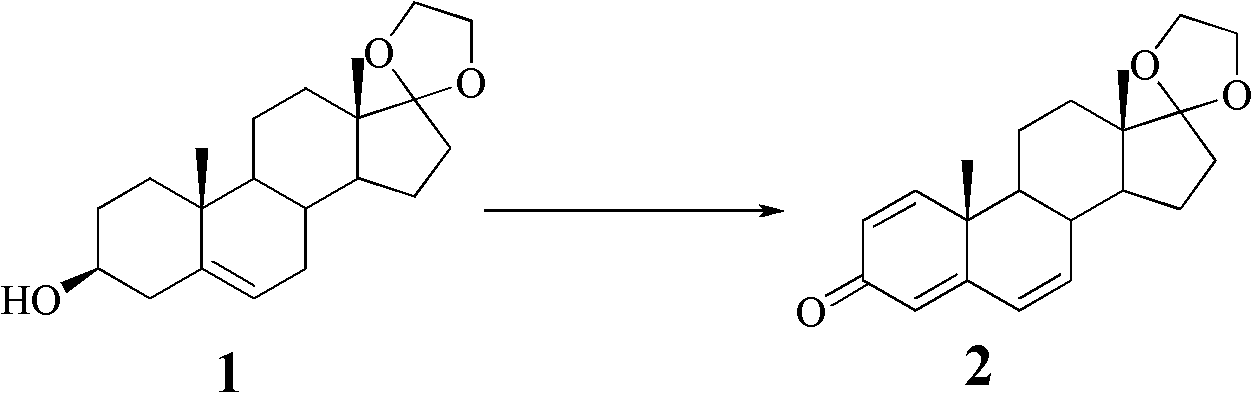
Preparation method and application of 1alpha-dehydroepiandrosterone
The invention relates to a preparation method of 1alpha-dehydroepiandrosterone, belonging to a preparation method of steroide compounds. The preparation method is characterized by comprising the following steps of: carrying out a dehydrogenated oxidizing reaction on glycol-condensed dehydroepiandrosterone as a raw material under the action of a metallic palladium catalyst, an allyl phosphodiester ligand and alkali to generate conjugated ketene; and carrying out epoxidation and liquid ammonia-metal reduction and deprotecting reaction to obtain the 1alpha-dehydroepiandrosterone. The invention has the advantages of simple process flow, short production cycle, high product yield, low production cost, low price and easy accessibility of the raw material and a reagent, simple operation, no need of column chromatography and other operations and suitability for large-scale preparation and production. In addition, the invention solves the problems of complicated process, long production cycle, low yield and high production cost existing in the method for fermenting microorganisms in the prior art.
Owner:REYOUNG PHARMA
Treatment of hot flushes, vasomotor symptoms, and night sweats with sex steroid precursors in combination with selective estrogen receptor modulators
PendingUS20100317635A1Easily measurable changeGood effectBiocideNervous disorderVasomotor symptomDisease
Novel methods for reduction or elimination the incidence of hot flushes, vasomotor symptoms, and night sweats while decreasing the risk of acquiring breast, uterine or endometrial cancer and furthermore having beneficial effect by inhibiting the development of osteoporosis, hypercholesterolemia, hyperlipidemia, atherosclerosis, hypertension, insulin resistance, diabetes type 2, loss of muscle mass, adiposity, Alzheimer's disease, loss of cognition, loss of memory, or vaginal dryness in susceptible warm-blooded animals including humans involving administration of an amount of a sex steroid precursor, particularly dehydroepiandrosterone (DHEA) and an antiestrogen or a selective estrogen receptor modulator, particularly compounds having the general structure:Pharmaceutical compositions for delivery of active ingredient(s) and kit(s) useful to the invention are also disclosed.
Owner:ENDORES & DEV
Industrial production method of 5 alpha-androst-2-ene-17-one
InactiveCN103360455ARaw materials are easy to obtainSimple and fast operationSteroidsIsomerizationMeth-
The invention discloses an industrial production method of 5 alpha-androst-2-ene-17-one. The method comprises the following steps of: dissolving epiandrosterone p-toluenesulfonates into a monomethylpyridine solvent, carrying out beta-elimination reaction within a certain temperature range, and removing p-toluenesulfonates to obtain the 5 alpha-androst-2-ene-17-one. The industrial production method has the advantages of simple and easily-obtained raw materials, simplicity and convenience in operation, no 3,4-double bond isomerization impurities in the finally-obtained product, safety in production, high yield and little pollution from three wastes so as to be suitable for industrial production.
Owner:CHONGQING KANGLE PHARMA +1
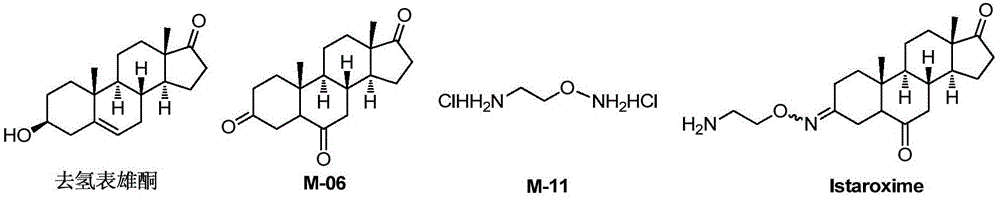
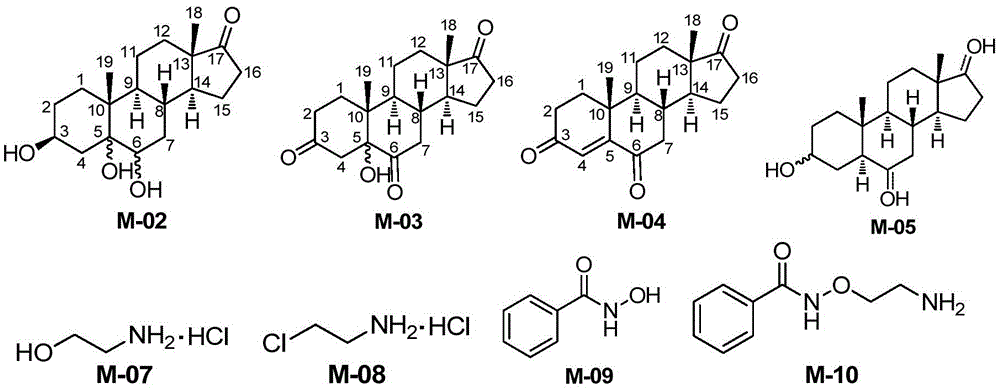
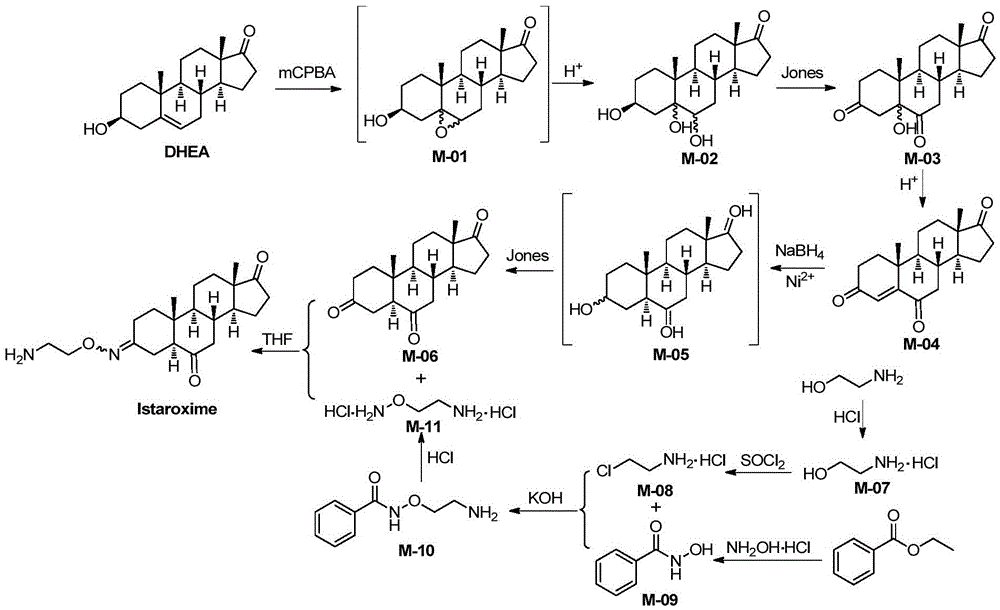
Novel synthesis method of Istaroxime
InactiveCN105585607AEasy to operateRaw materials are easy to getSteroidsBenzoic acidChemical reaction
The invention belongs to the field of pharmaceutical chemistry, and discloses a novel synthesis method of Istaroxime. The method comprises the following steps: by using dehydrogenated epiandrosterone as an initial raw material, carrying out epoxidation, ring opening, reduction, oxidation and other reactions to prepare an intermediate M-06; by using ethyl benzoate as an initial raw material, reacting the ethyl benzoate with hydroxylamine hydrochloride to obtain phenyl hydroximic acid, carrying out hydrochlorination and chlorination by using ethanolamine as a raw material to obtain dichloroacetate, and carrying out substitution, hydrolysis and other reactions on the dichloroacetate and the phenyl hydroximic acid to obtain an intermediate M-11; and finally, reacting the M-06 with the M-11 to obtain the end product Istaroxime. According to the method, in the intermediate M-06 synthesis process, the polarity of all the intermediates has great differences from that of the impurities and reaction reagents; and in the intermediate 11 synthesis process, the active spots capable of participating in the chemical reaction in the reaction substrate are simple. Therefore, the method can achieve the requirements without carrying out column chromatography purification, thereby simplifying the synthesis after-treatment process.
Owner:JINAN UNIVERSITY
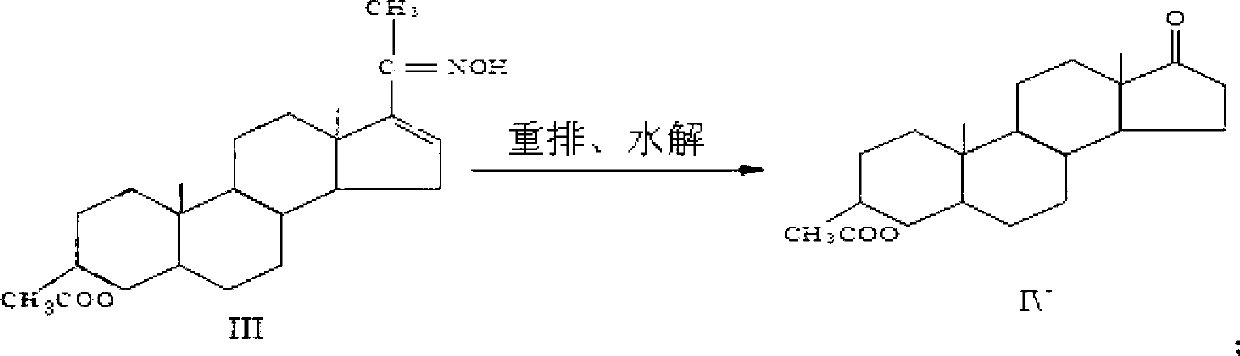
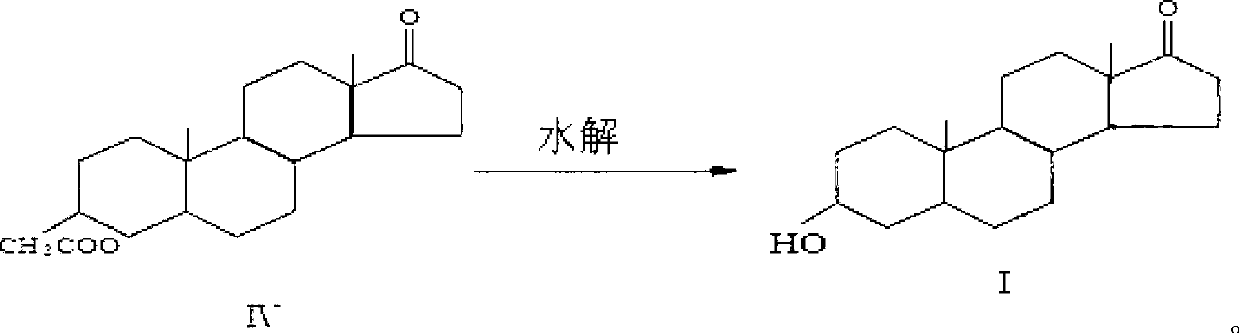
A kind of production method of synthesizing epiandrosterone by monoenolone acetate
The invention provides a production method for synthetizing epiandrosterone from single enol ketone acetate. The production method comprises the steps of: taking single enol ketone acetate as a material, and obtaining epiandrosterone by oximating, rearrangement and hydrolysis reaction, wherein the purity can be up to over 98.5%; and the yield achieves over 68%. Hydrogenation is saved; the problem of high industrial cost due to the fact that palladium chloride-calcium carbonate is taken as a catalyst in the traditional method is solved; the product yield is improved; and the process has the characteristics of being simple to operate, small in pollution, high in yield and the like, and is suitable for industrial production.
Owner:湖北民生生物医药有限公司
Novel method for preparing dehydroepiandrosterone
The invention provides a novel method for preparing dehydroepiandrosterone, discloses a preparation method of dehydroepiandrosterone, and belongs to the technical field of preparation and processing of compounds. The method comprises the following steps of: taking 4-androstenedione (I) as a starting raw material, carrying out an acetylation reaction to obtain 3-acetoxyl-delta 3, 5-androstadiene-17-ketone (II), and then carrying out a biological enzyme method reaction on the obtained intermediate compound (II) to obtain the product dehydroepiandrosterone. The compound II in the invention is used as a key intermediate, the property of the compound II is more stable than that of 5-AD, and the purity is high, so that the stability of entering the next step of the biological enzyme method is effectively ensured; the biological enzyme method of the reaction route adopts a composite biological enzyme with a specific ratio, and the composite biological enzyme with the specific ratio acts on the compound II and has a better and more stable conversion effect; and the single-batch yield of the route is larger than 90%, the purity of the product compound III is high, the purity is larger than 99%, and the industrial application value is higher.
Owner:ZHEJIANG SHENZHOU PHARMA
Method of utilizing coenzyme regeneration and resin in-situ extraction to promote hydroxylation of DHEA by Colletotrichum lini ST-1
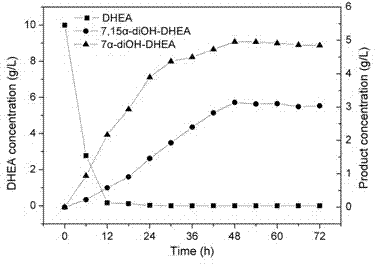
InactiveCN104611400AHigh feeding concentrationHigh yieldMicroorganism based processesFermentationColletotrichum liniOrganic chemistry
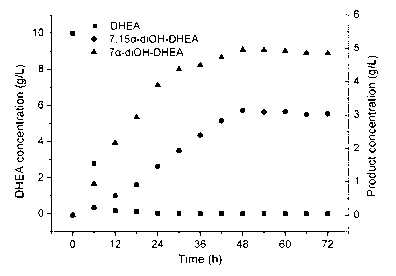
Belonging to the field of biotechnology, the invention relates to a method of utilizing a coenzyme regeneration and resin in-situ extraction integrated strategy to promote hydroxylation of DHEA (dehydroepiandrosterone) by Colletotrichum lini ST-1. The method is characterized in that in the conversion process, a 10g / L cosubstrate is added to conduct NADPH regeneration, and simultaneously D101 type macroporous adsorption resin is employed for in-situ extraction of the product, thus significantly enhancing the conversion rate of C. lini ST-1 on DHEA. Under the condition of a substrate feeding amount of 10g / L, the conversion rate of the substrate DHEA is 93%, and the yield of 7 alpha, 15 alpha-diOH-DHEA can reach 7.12 g / L.
Owner:JIANGNAN UNIV +1
Production method for synthetizing epiandrosterone from single enol ketone acetate
The invention provides a production method for synthetizing epiandrosterone from single enol ketone acetate. The production method comprises the steps of: taking single enol ketone acetate as a material, and obtaining epiandrosterone by oximating, rearrangement and hydrolysis reaction, wherein the purity can be up to over 98.5%; and the yield achieves over 68%. Hydrogenation is saved; the problem of high industrial cost due to the fact that palladium chloride-calcium carbonate is taken as a catalyst in the traditional method is solved; the product yield is improved; and the process has the characteristics of being simple to operate, small in pollution, high in yield and the like, and is suitable for industrial production.
Owner:湖北民生生物医药有限公司
Steroid hormone detection method
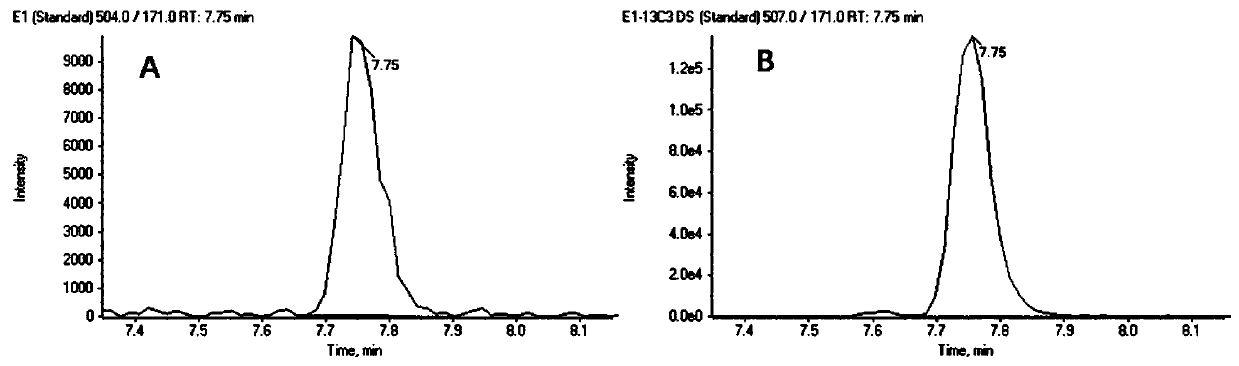
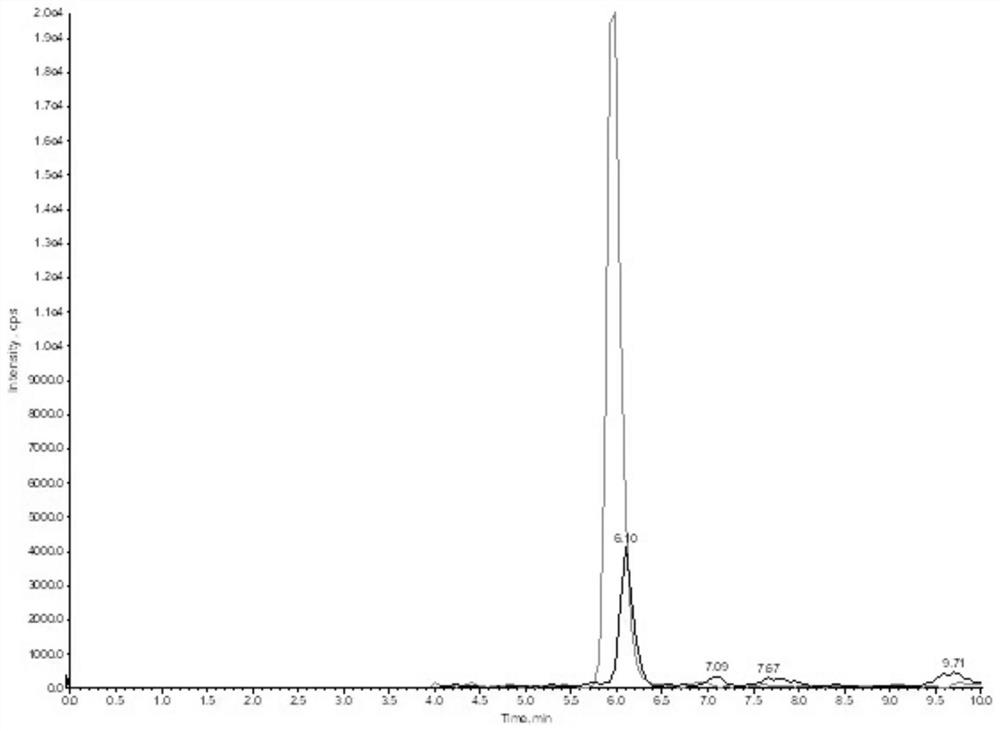
The invention discloses a steroid hormone detection method that comprises the following steps: adding an internal standard substance into a sample to be detected; carrying out a derivatization reaction; detecting an analyte by using chromatography tandem mass spectrometry; the analyte comprises a first steroid hormone and a second steroid hormone at the same time; the first steroid hormone is selected from at least one of the following steroid hormones: estradiol and estriol; and the second steroid hormone is selected from at least one of the following steroid hormones: dehydroepiandrosterone,dehydroepiandrosterone sulfate, testosterone, dihydrotestosterone, androstenedione, cortisol, cortisone, 11-deoxycortisol, 17alpha-hydroxyprogesterone, 17alpha-hydroxypregnenolone, aldosterone, cortisol, deoxycortisol, progesterone and pregnenolone. The detecting method has the advantages of few operation steps and short detection time, and can specifically, sensitively, accurately and quantitatively analyze the analyte substance.
Owner:杭州度安医学检验实验室有限公司
Kits for dhea and dhea-sulfate for the treatment of chronic obstructive pulmonary disease
Kits for treating or preventing chronic obstructive pulmonary disease (COPD) by using as active agent a non-glucorticoid steroid, analogue thereof, such as dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S), or their salts, in an amount effective for preventing or treating COPD.
Owner:EAST CAROLINA UNIVERISTY
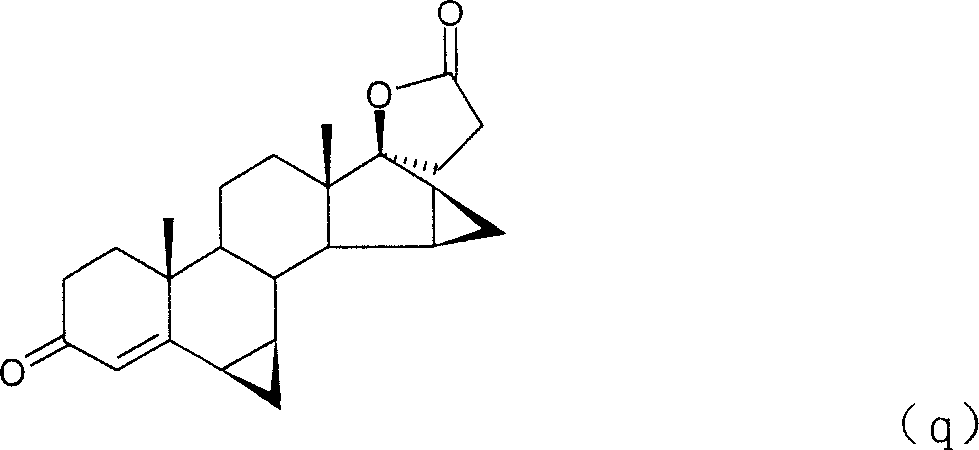
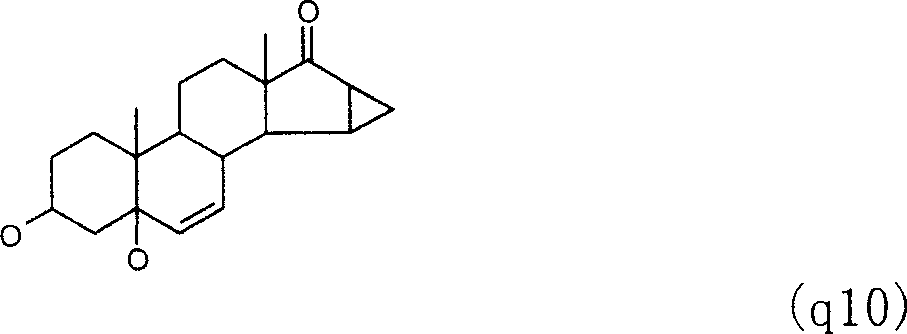
Method for preparing drospirenone intermediate 3beta,5-dihydroxy-15beta,16beta-methylene-5beta-androst-6-en-17-one
The invention provides a method for preparing an intermediate of eplerenone 3 Beta, 5- dihydroxy-15 Beta, 16 Beta-methylene-5 Beta-androstane-6-alkene-17-ketone (q10) from dehydroepiandrosterone acetate.
Owner:王爱玲 +3
Method for detecting content of DHEA in human body fluid
The invention discloses a method for detecting the content of DHEA in human body fluid. The method comprises the steps of detecting dehydroepiandrosterone in saliva by using a high performance liquid chromatography-tandem mass spectrometry technology, specifically, S1, adding the following reagents into a kit: a mobile phase I, a mobile phase II, a standard substance mother solution, an internal standard solution, a diluent, an extracting solution, a quality control substance and a redissolving solvent; S2, detecting the dehydroepiandrosterone in the pretreated saliva by adopting the high performance liquid chromatography-tandem mass spectrometry technology; S3, separating the dehydroepiandrosterone from the interferent by using high performance liquid chromatography; S4, quantifying by using an isotope internal standard method; S5, taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis; and S6, establishing a standard curve, and calculating the content of the dehydroepiandrosterone in the sample to be detected. The method is high in sensitivity and good in specificity, saliva sample treatment is simple, methodology verification meets requirements, and it is indicated that the method is reliable and capable of being applied to quantitative detection of dehydroepiandrosterone in human saliva.
Owner:艾可泰科(浙江)控股有限公司
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a PDE-4 inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026883A1Alleviate different aspectConvenient treatmentBiocidePowder deliveryPhosphodiesteraseDisease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a phosphodiesterase-4 inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Method for hydroxylating dehydroisoandrosterone by using colletotrichumlini
ActiveCN102703558BHigh feeding concentrationHigh yieldMicroorganism based processesFermentationPhysiologyCyclodextrin
The invention relates to a method for hydroxylating dehydroisoandrosterone (DHEA) by using colletotrichumlini ST-1. The method for hydroxylating the dehydroisoandrosterone by using the colletotrichumlini ST-1 is characterized in that: in a conversion process, a way of pre-including the DHEA and methyl-beta-cyclodextrin with an equimolar ratio first and then feeding is adopted, so that the water solubility and the conversion efficiency of the DHEA are significantly improved. Under a condition that a substrate feeding amount is 10g / L, the conversion rate is as high as 95% and the total yield of products 7 alpha-OH-dehydroepiandrosterone and 7,15 alpha-OH-dehydroepiandrosterone is 80.94%.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
Method for preparing dehydroepiandrosterone through conversion of plant sterols by resting cells
InactiveCN110656148ARaw materials are easy to getReduce manufacturing costBacteriaMicroorganism based processesBiotechnologyPlant sterol
The invention relates to a method for producing a steroidal drug intermediate, in particular to a method for preparing dehydroepiandrosterone through conversion of plant sterols by resting cells. Themethod includes the steps of 3-position hydroxyl protective reaction, bioconversion of the resting cells, hydrolysis and purification. According to the method, the plant sterols are used as raw materials to produce dehydroepiandrosterone, the raw materials are easily available, the production cost is reduced, the yield is higher, the reaction route is shorter, and reaction steps and post-treatmentsteps required by most traditional preparation methods are eliminated; and a resting cell conversion method is adopted, the conversion system is single in nutrition and low in risk of infection and has an adjustable bacterial volume, and good conversion ability can be ensured.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
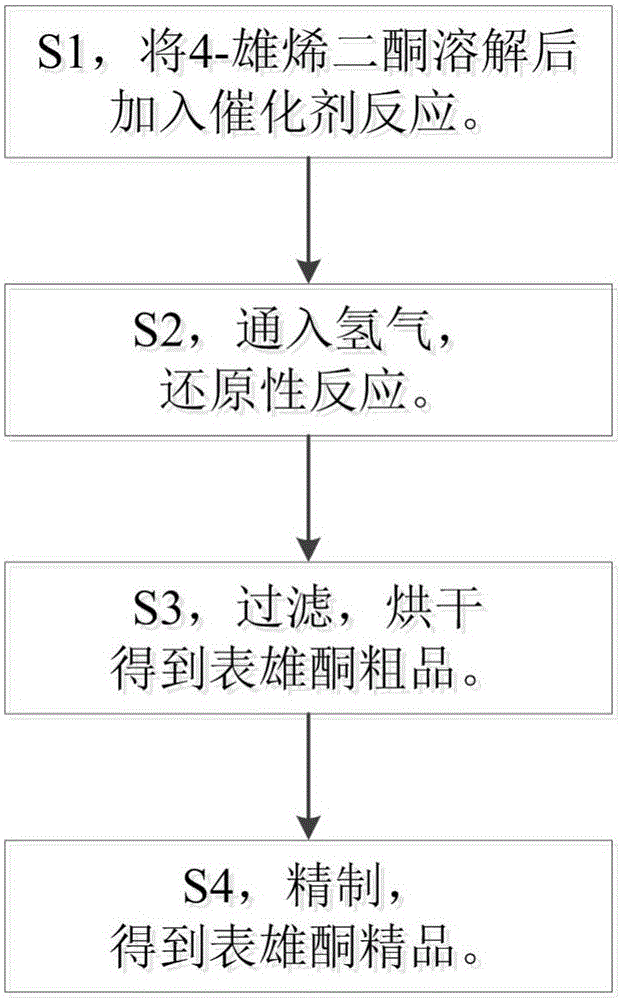
Novel technology for synthesizing epiandrosterone by adopting 4-androstenedione through selective hydrogenation reducing
The invention provides a novel technology for synthesizing epiandrosterone by adopting 4-androstenedione through selective hydrogenation reducing.Epiandrosterone is synthesized by adopting 4-androstenedione through selective hydrogenation reducing, therefore, the reaction path is greatly shortened, generation of an intermediate reaction by-product is prevented, and the product purity and yield are high; in addition, the reaction raw material consumption is lower, the cost is reduced, and the concept requirement for green production is met.
Owner:湖北省丹江口开泰激素有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com