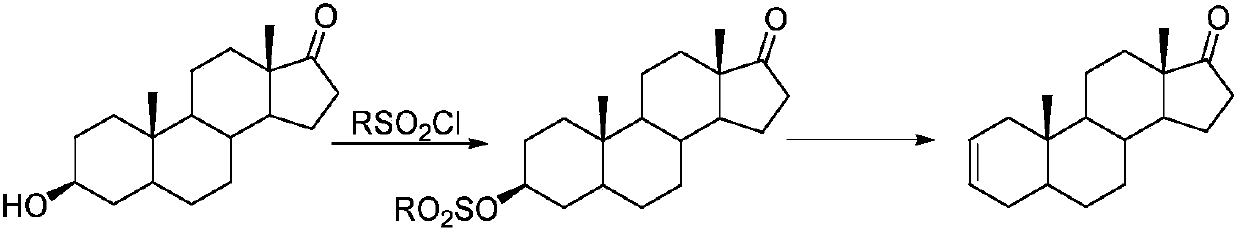
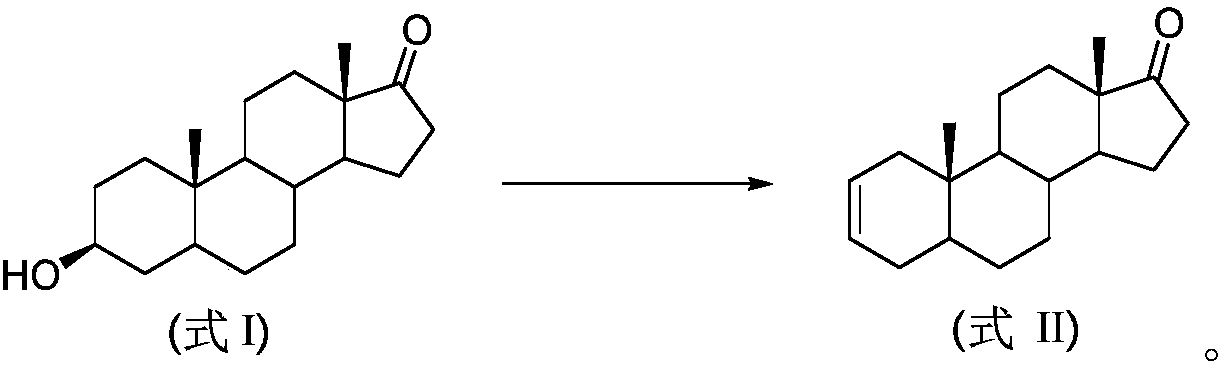
Preparation method of 5 alpha-androstane-2-ethylene-17-ketone
A technology of androsterone and epiandrosterone, which is applied in the field of chemical preparation, can solve the problems of complicated operation, low atom economy, large amount of three wastes, etc., and achieves the effects of shortening reaction steps, simple post-processing and reducing discharge amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add epiandrosterone (10mmol, 2.9g), p-toluenesulfonic acid (2mmol, 0.34g) and ytterbium trifluoromethanesulfonate (0.5mmol, 310mg) into a 50mL single-port reaction flask, toluene (20mL) as a solvent, 110°C Reaction 16h. After cooling the reaction solution to room temperature, pour it into 100mL of ice water, add 50mL of dichloromethane for extraction, wash the organic layer with saturated sodium bicarbonate solution and dry (anhydrous sodium sulfate), then distill and concentrate under reduced pressure to obtain a dark brown oil , the residue was recrystallized by 80% aqueous ethanol to obtain 2.4 g of 5α-androst-2-en-17-one, with a yield of 88%.
[0023] After testing, the specific characteristics of the product are as follows:
[0024] Melting point: 108.2-110.1℃, 1 H NMR (400MHz, CDCl 3 )δ5.67-5.55(m,2H),2.46(dd,J 1 =6.0Hz,J 2 =12.8Hz,1H),2.12-2.06(m,1H),1.99-1.88(m,2H),1.86-1.80(m,2H),1.78-1.70(m,2H),1.68-1.59(m,2H ),1.57-1.50(m,3H),1.46-1.40(m,2H),1.32-1.25(m...
Embodiment 2
[0026] According to the method and steps of Example 1, the only difference is that the molar ratio of epiandrosterone and p-toluenesulfonic acid was adjusted to 1:0.1, the reaction time was adjusted to 18 hours, and the yield was 78%.
Embodiment 3
[0028] According to the method and steps of Example 1, the only difference is that the molar ratio of epiandrosterone and ytterbium trifluoromethanesulfonate was adjusted to 1:0.1, the reaction time was adjusted to 8 hours, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com