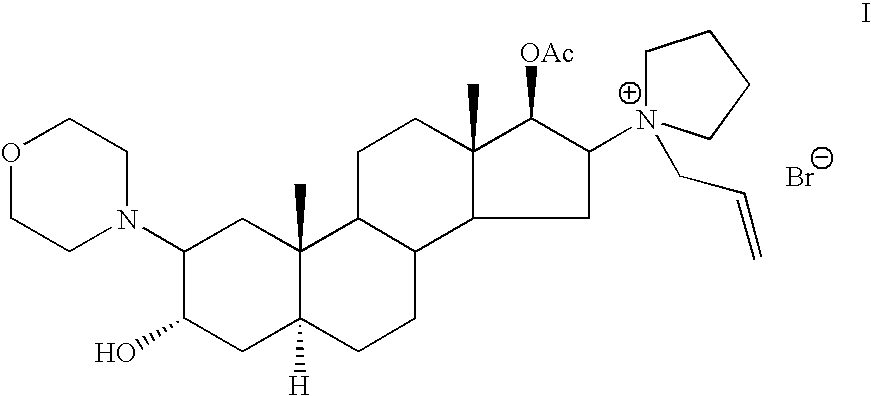
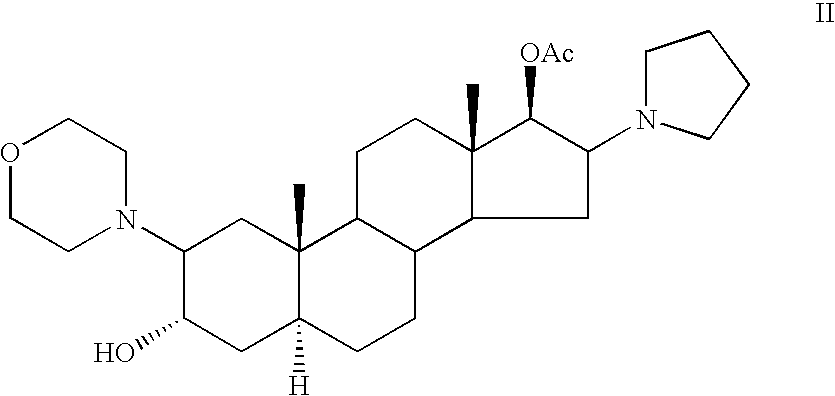
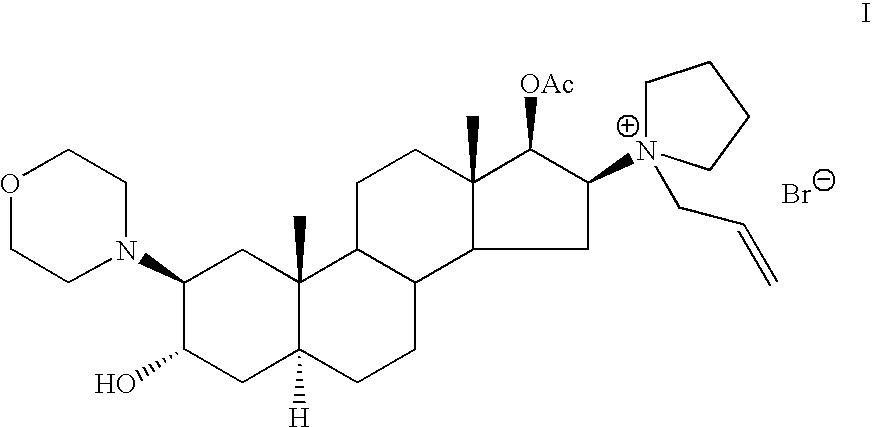
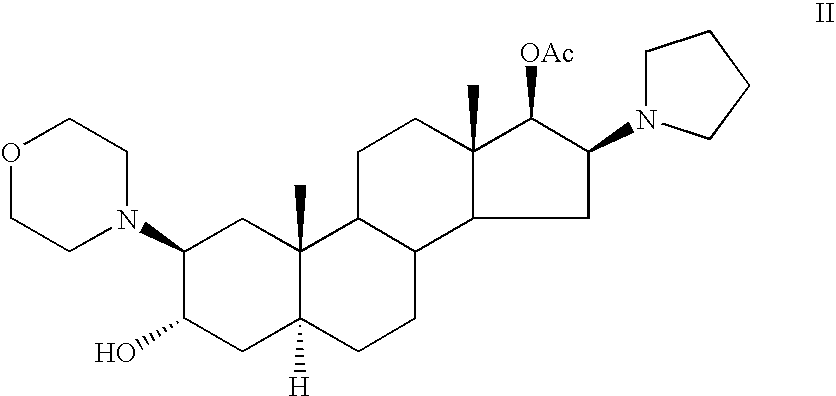
Patents
Literature
37 results about "Rocuronium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
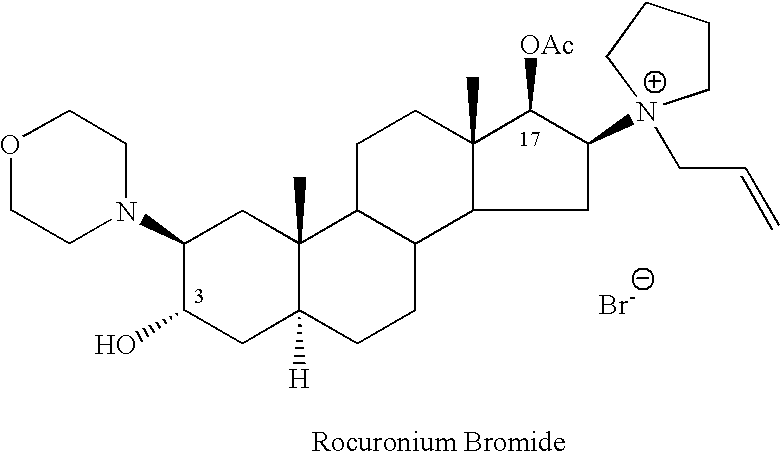
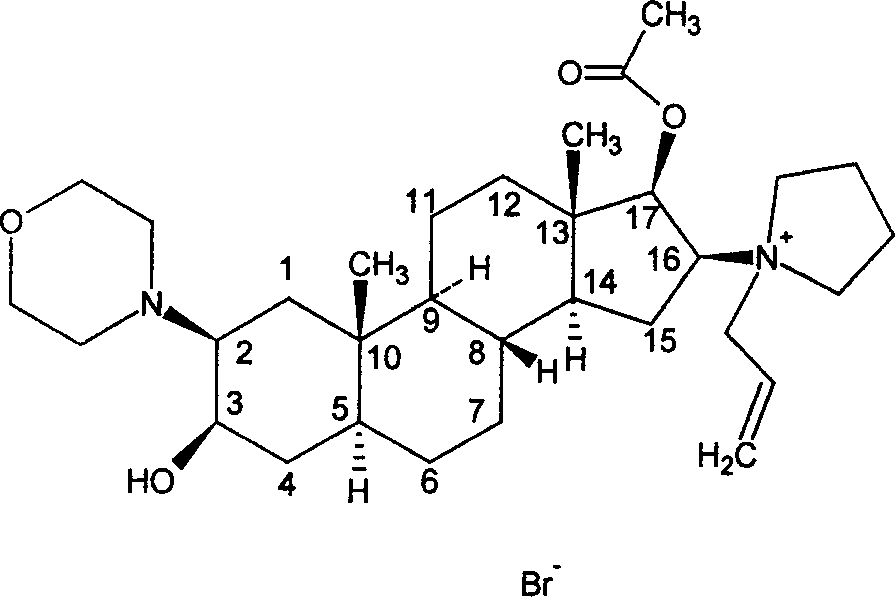
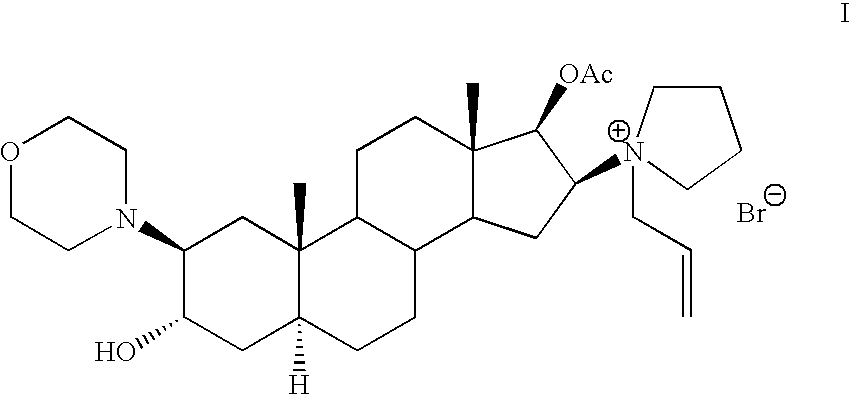
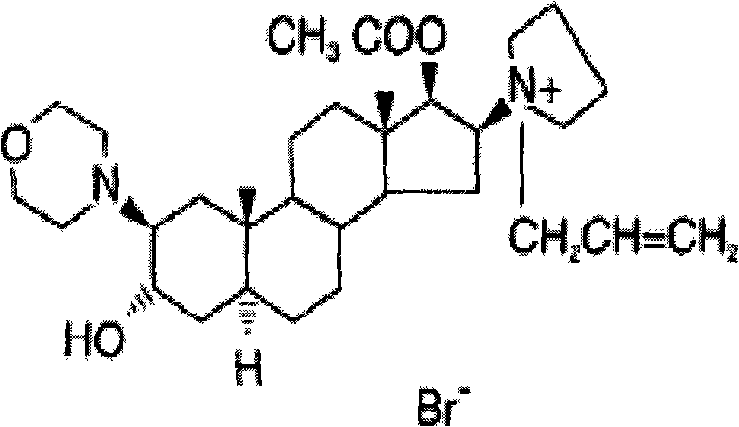
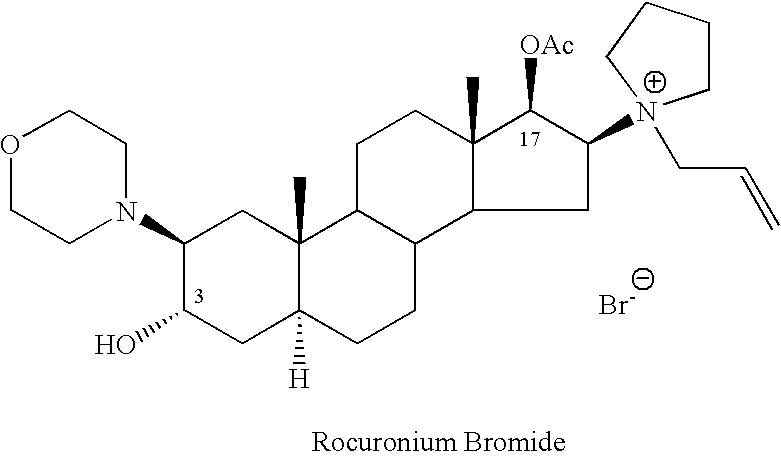
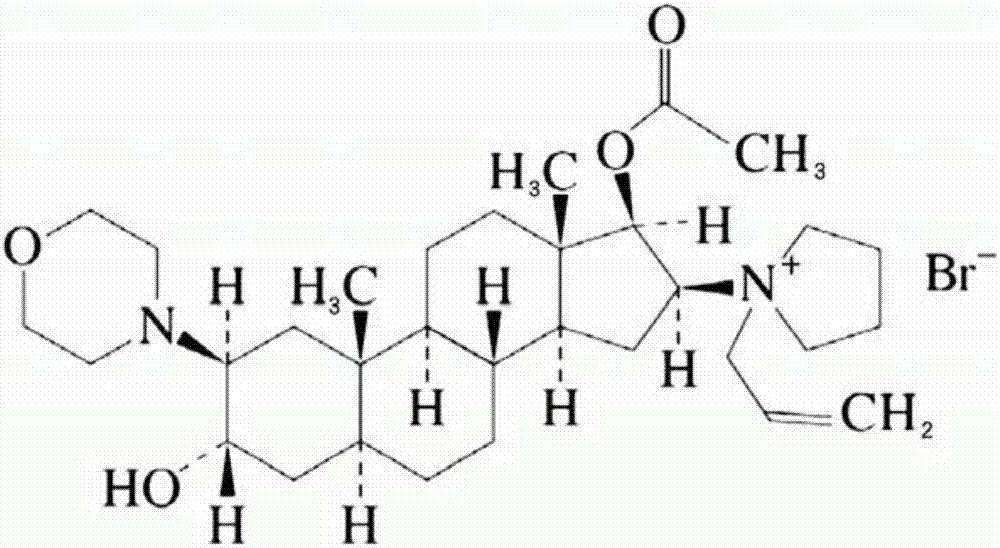
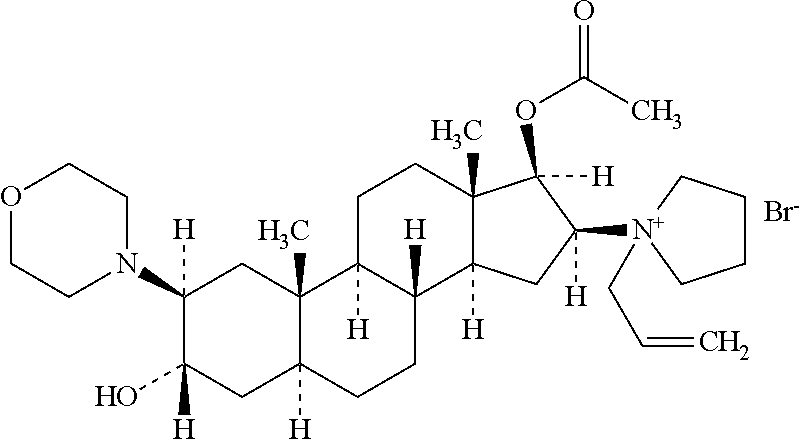
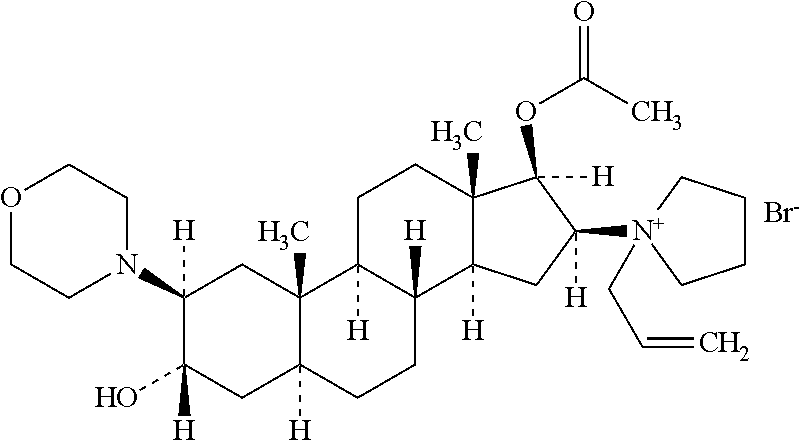
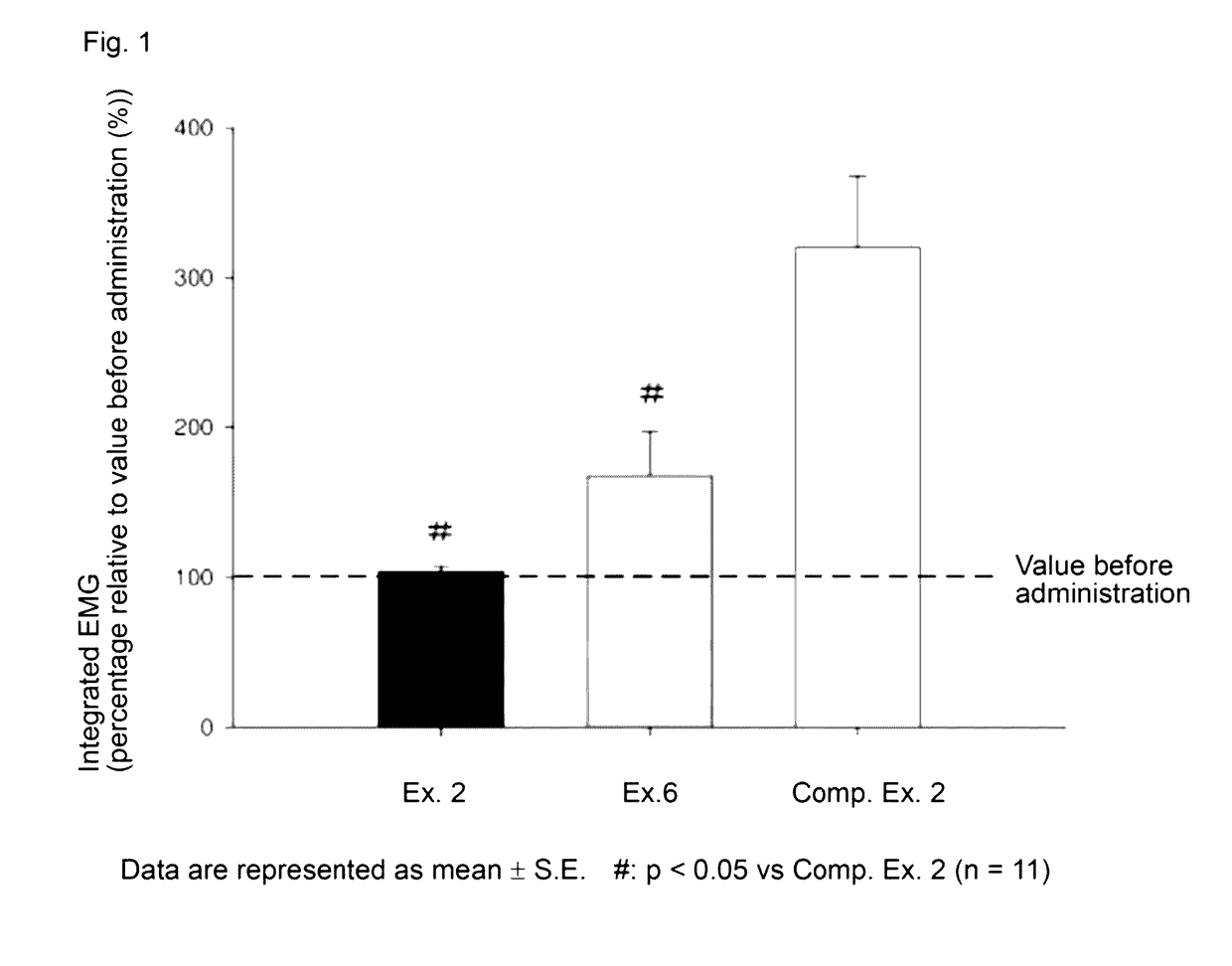
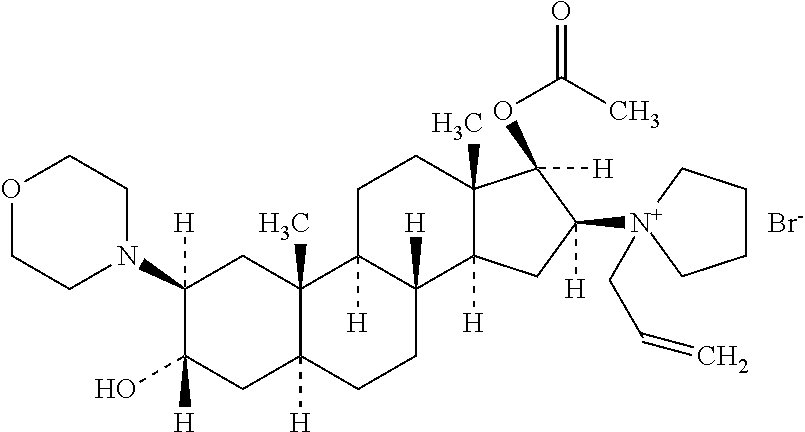
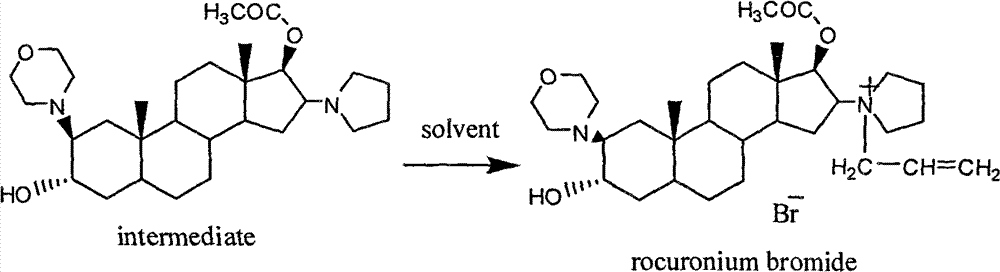
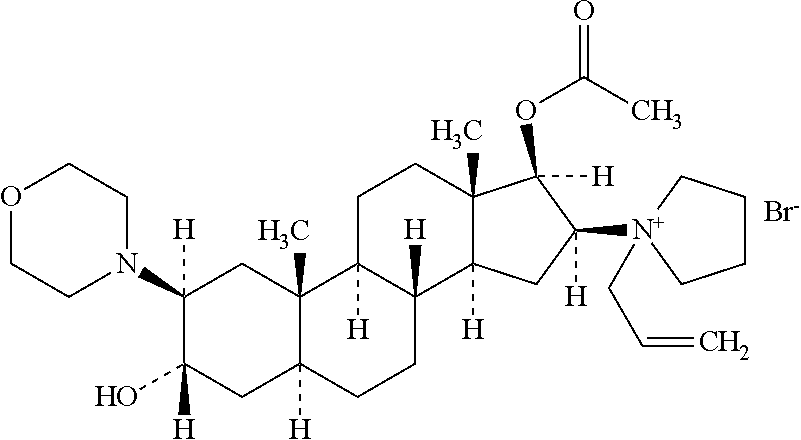
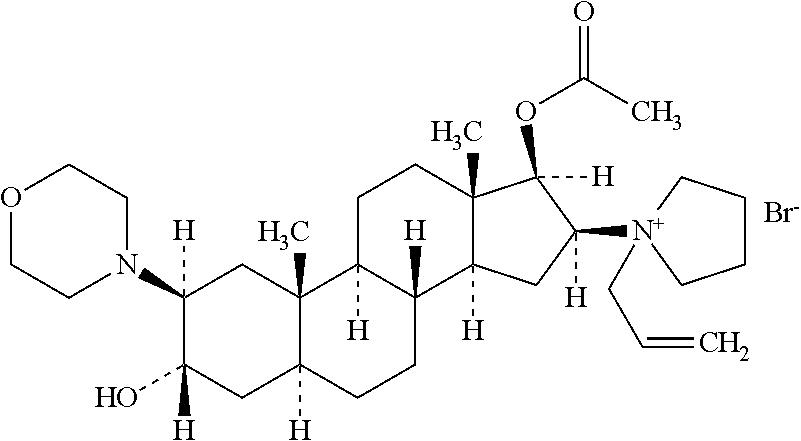
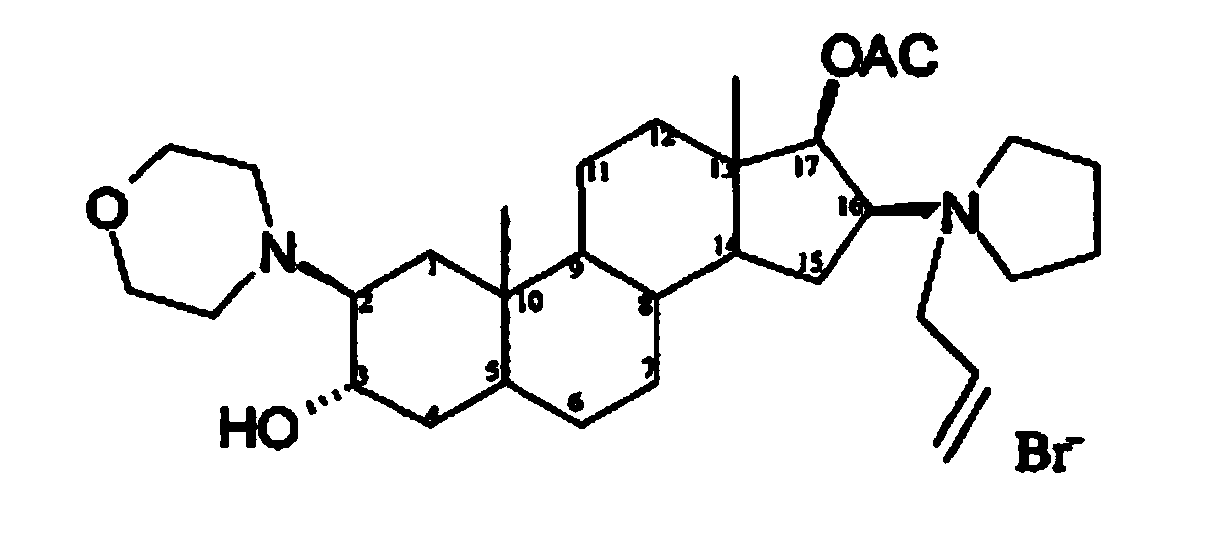
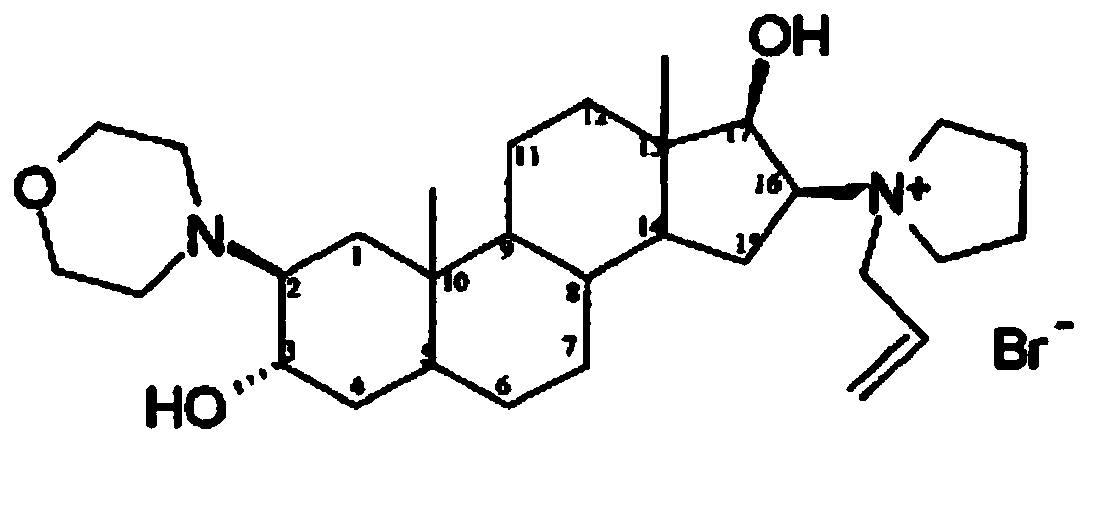
Rocuronium bromide (brand names Zemuron, Esmeron) is an aminosteroid non-depolarizing neuromuscular blocker or muscle relaxant used in modern anaesthesia to facilitate tracheal intubation by providing skeletal muscle relaxation, most commonly required for surgery or mechanical ventilation. It is used for standard endotracheal intubation, as well as for rapid sequence induction (RSI).
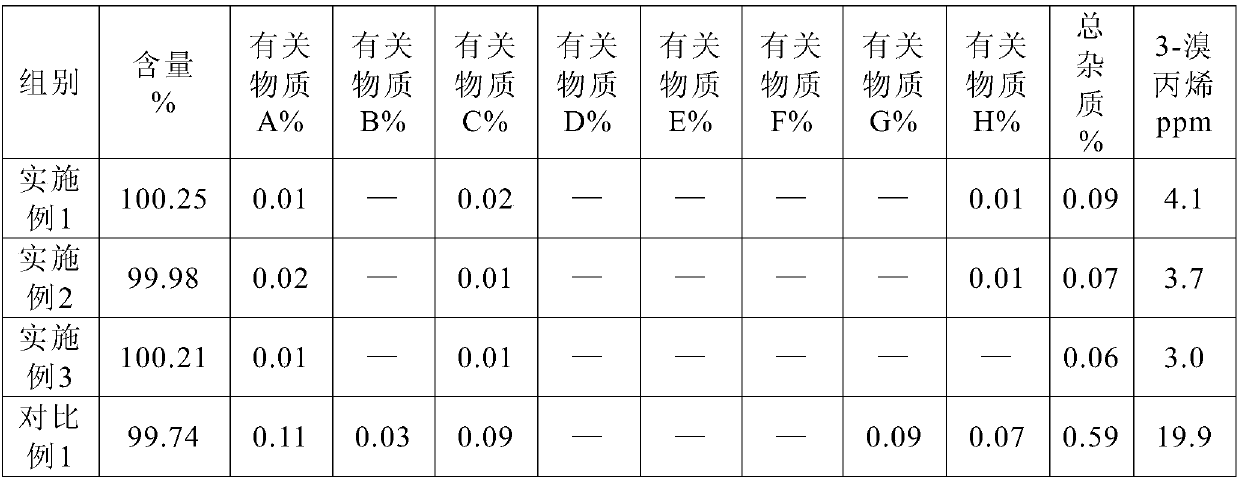
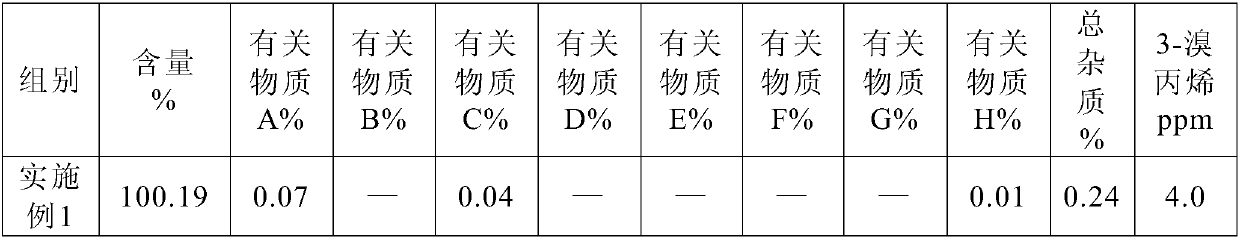
Processes for preparing stabilized, highly pure rocuronium bromide
InactiveUS20060058275A1High yieldSpeed up the processOrganic active ingredientsOrganic chemistryRocuronium
Processes are provided herein for the preparation and purification of stable, powdered solids comprising substantially pure rocuronium bromide.
Owner:CHEMAGIS
Method of reprocessing quaternary ammonium-containing neuromuscular blocking agents
Provided is a method for reprocessing neuromuscular blocking agents containing a quaternary ammonium salt, e.g., Rocuronium bromide, using a novel dealkylation method. The process is effective in obtaining a highly pure product from a contaminated starting material by heating, optionally in the presence of an organic solvent, to produce a dealkyated product. The dealkylated product is purified, e.g., by crystallization, and converted by any known method to a stable, highly-pure neuromuscular blocking agent.
Owner:CHEMAGIS
Processes for the preparation of rocuronium bromide and intermediates thereof
InactiveUS20050159398A1Efficient and cost-effectiveOrganic active ingredientsNervous disorderAndrostanesCombinatorial chemistry
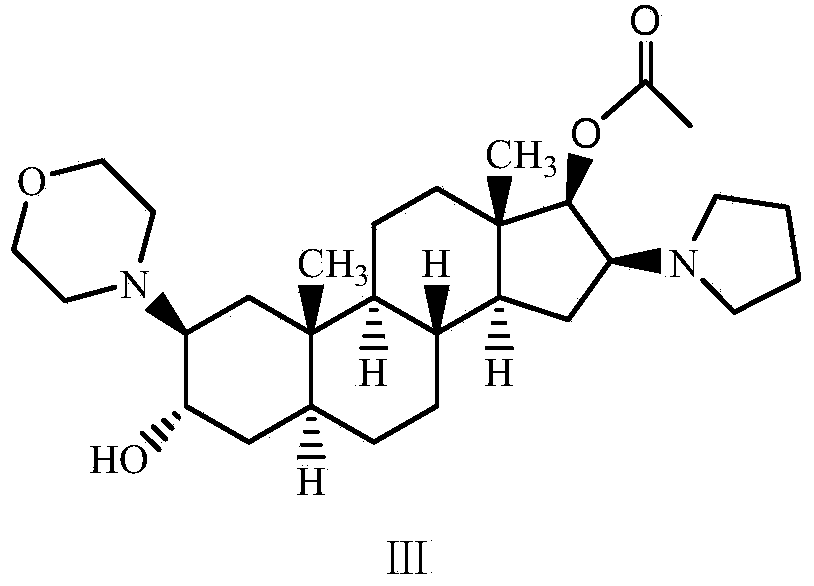
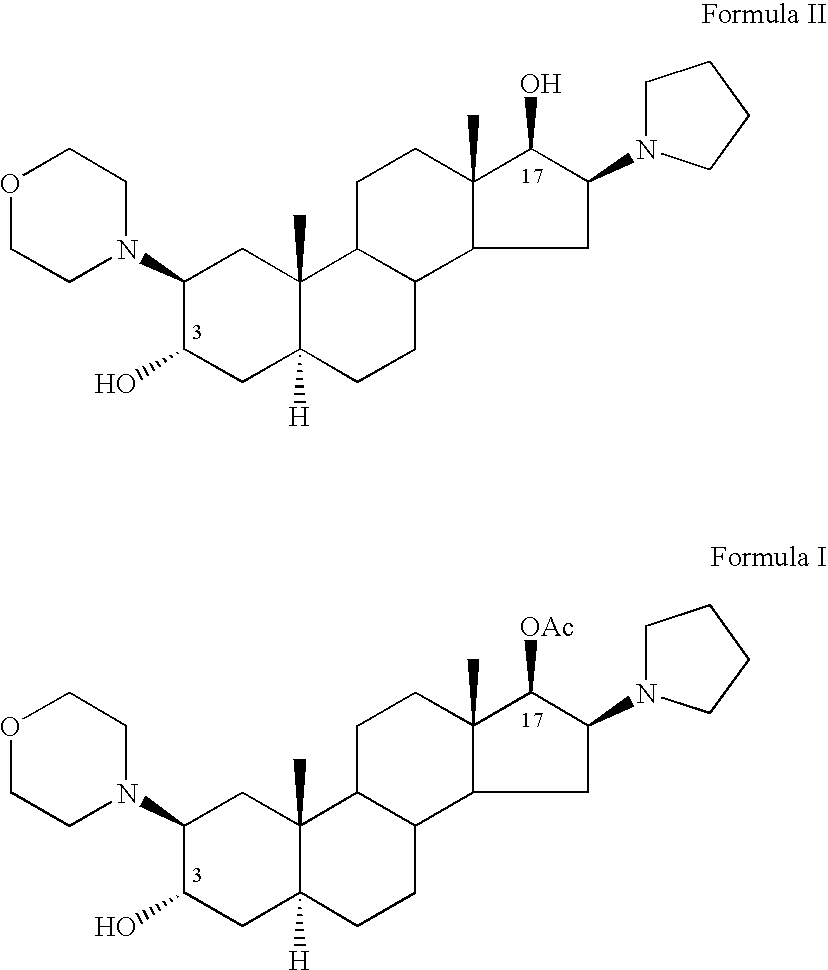
A novel process for preparing (2β,3α,5α,16β,17β)-17-acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane, a known intermediate in the synthesis of the skeletal muscle relaxant rocuronium bromide, is disclosed.
Owner:WAVELENGTH ENTERPRISES LTD
Stable rocuronium injection preparation and preparation method thereof
InactiveCN103462885AOrganic active ingredientsInorganic non-active ingredientsMuscle relaxationSterility
The invention discloses a stable rocuronium injection preparation prescription and a preparation method thereof. The injection contains the active ingredient rocuronium, a stabilizer, an osmotic pressure regulator, a pH (potential of Hydrogen) regulator and injection water, and has two specifications, i.e., 2.5 ml:25 mg and 5ml:50 mg. Rocuronium is added into the proper stabilizer, so that the problem of stability is solved; autoclave sterilization of 121 DEG C is adopted with the help of a filtration sterilization mode to guarantee the sterility. The preparation has the advantages of reasonable prescription, simple process and stable quality and is suitable for industrial production. The injection is used for muscular relaxation of surgical patients.
Owner:河北凯盛医药科技有限公司
A stable lyophilized preparation of rocuronium bromide and preparation method thereof
The present invention relates to stable freeze dried rocuronium bromide preparation and its preparation process. The freeze dried rocuronium bromide preparation includes rocuronium bromide and freeze drying excipient in the weight ratio of 0.2-1. The freeze dried rocuronium bromide preparation needs no low temperature preservation and convenient storing, and is used for muscular relaxation of patient in surgical operation.
Owner:CHONGQING PHARMA RES INST +1
Stable rocuronium bromide composition for injection
InactiveCN101653412AReduce hydrolysisReduce generationOrganic active ingredientsMuscular disorderControllabilityHydrolysis
The invention provides a stable rocuronium bromide composition for vein, which contains rocuronium bromide with treating effective dose, EDTA-2Na-Ca or EDTA-2Na and a pH value buffer system. The EDTA-2Na-Ca or the EDTA-2Na delays the hydrolysis of a rocuronium bromide 17-bit ester bond of the composition in the processes of preparation and storage; and on one hand, medicine liquid can resist the high temperature in the processes of hot press and sterilization, on the other hand, the content of rocuronium bromide deacetyl impurities in the composition in the process of storage is reduced. The invention also provides a reasonable method for preparing the medicine liquid, which can reduce the generation of the rocuronium bromide hydrolysis impurities in the process of preparation. The reduction of the rocuronium bromide hydrolysis impurities improves the quality controllability of the composition and ensures the safety and effectiveness of clinical use.
Owner:尹双保 +2
Preparation method of high-purity high-stability rocuronium bromide
ActiveCN103435674AReduce generationAvoid the problem that the residue on ignition seriously exceeds the standardSteroidsFreeze-dryingAcetic acid solution
The invention belongs to the technical field of medical chemistries, and particularly discloses a preparation method of high-purity high-stability rocuronium bromide. The preparation method comprises the following steps: performing pretreatment on a rocuronium bromide crude product by adopting aluminium oxide for removing most 3-bromopropylene, filtering, dropwise adding an obtained filtrate into diethyl ether stirred violently, wherein during adding process, a great amount of white solid separates out, dissolving the obtained white solids into a dilute acetic acid solution, rapidly freezing the solution into ice, and freeze drying, thus forming the rocuronium bromide. The preparation method provided by the invention has the advantages that the problems that a residual solvent in the prior art exceeds standard, the 3-bromopropylene is hard to remove, and the impurity content is high are successfully solved, and the obtained rocuronium bromide has the high purity and good stability.
Owner:SHANDONG XINHUA PHARMA CO LTD
Preparation of rocuronium
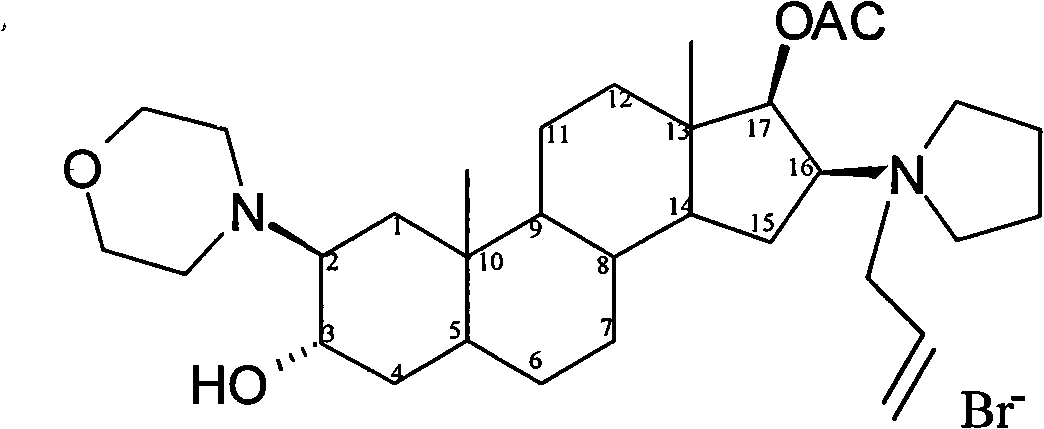
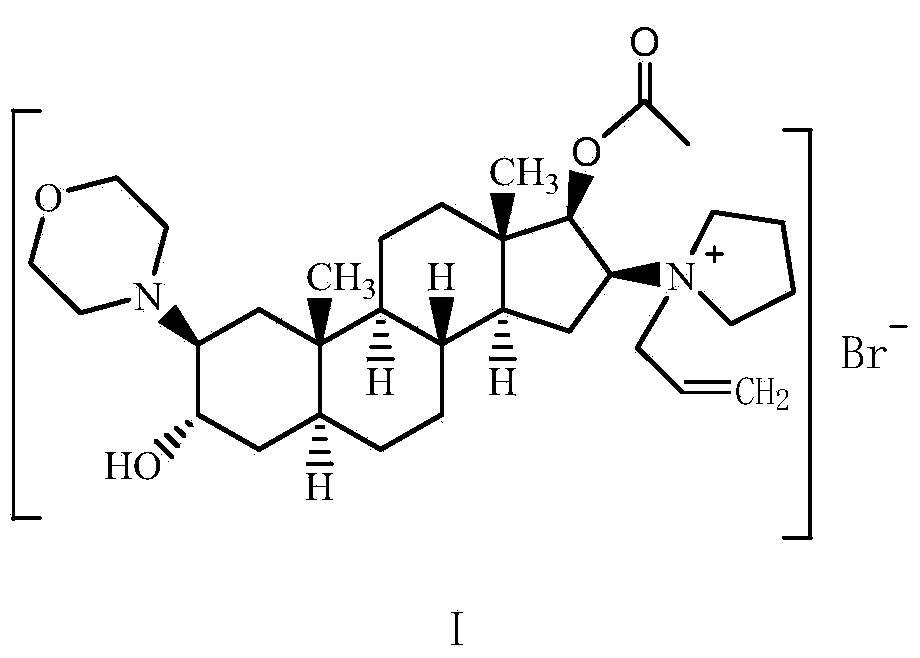
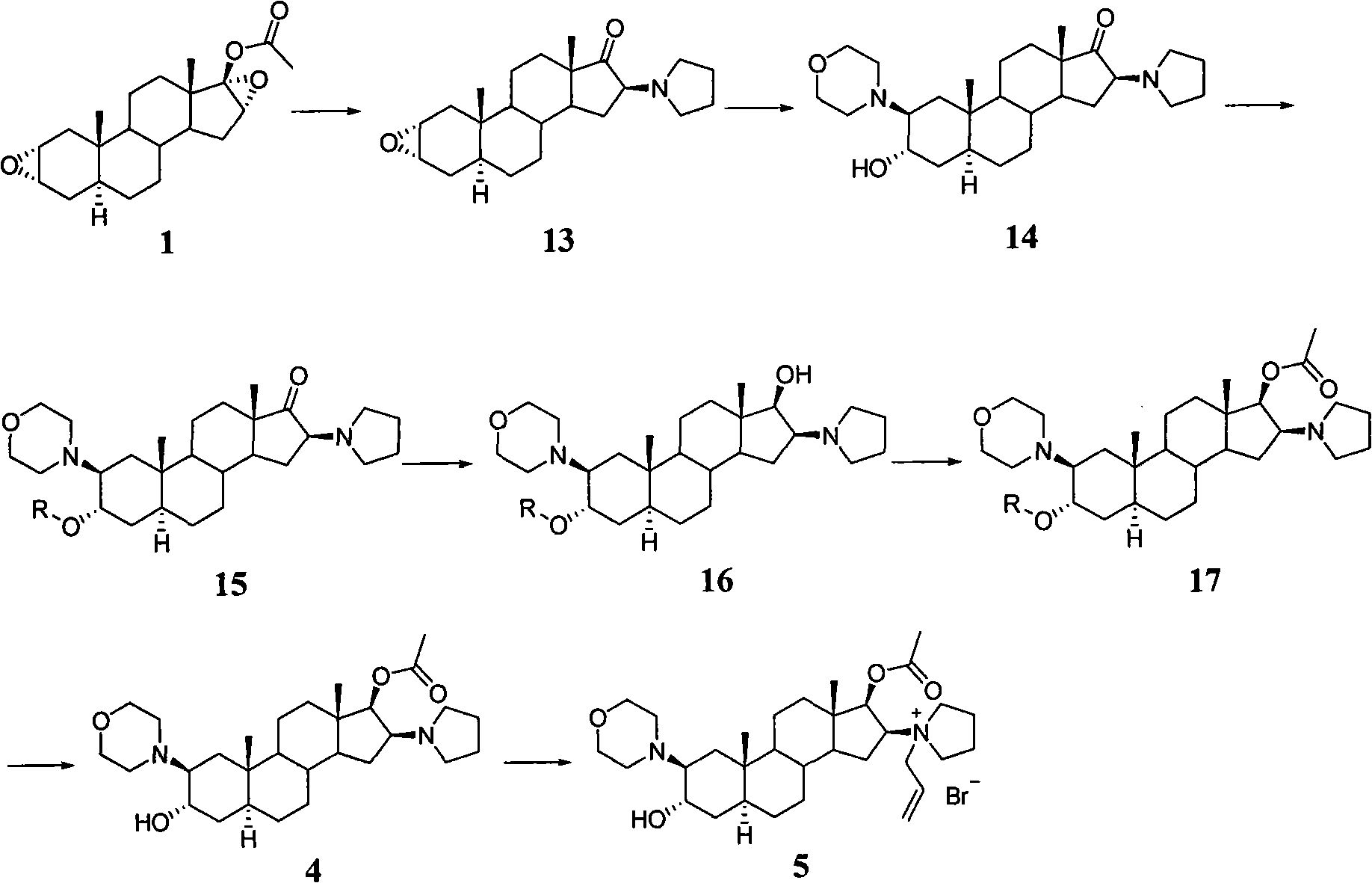
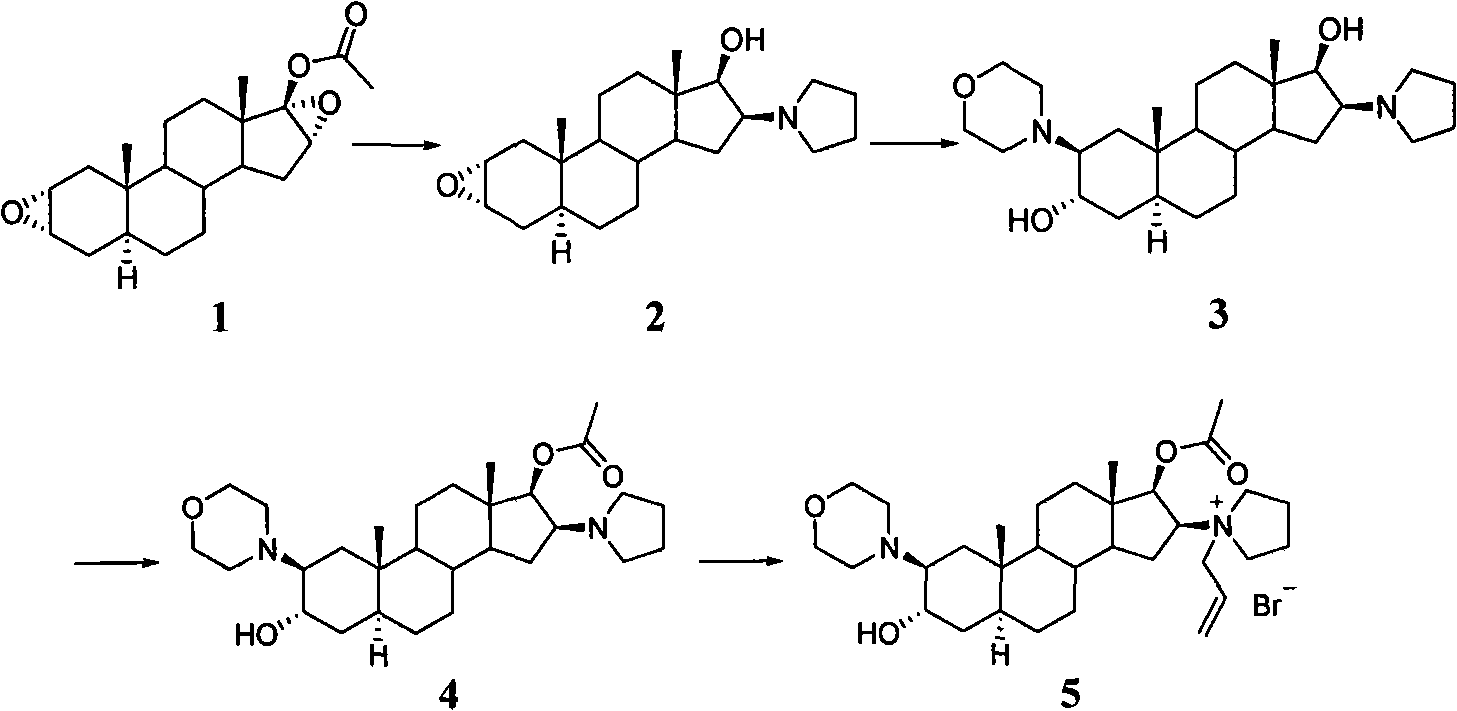
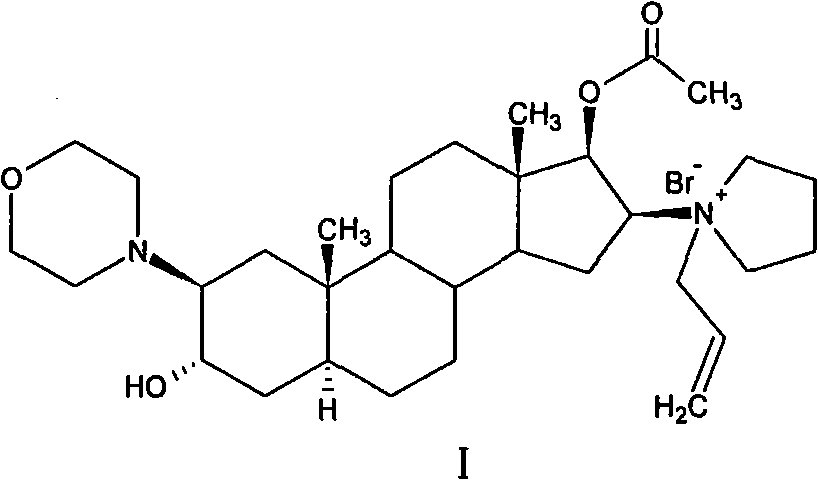
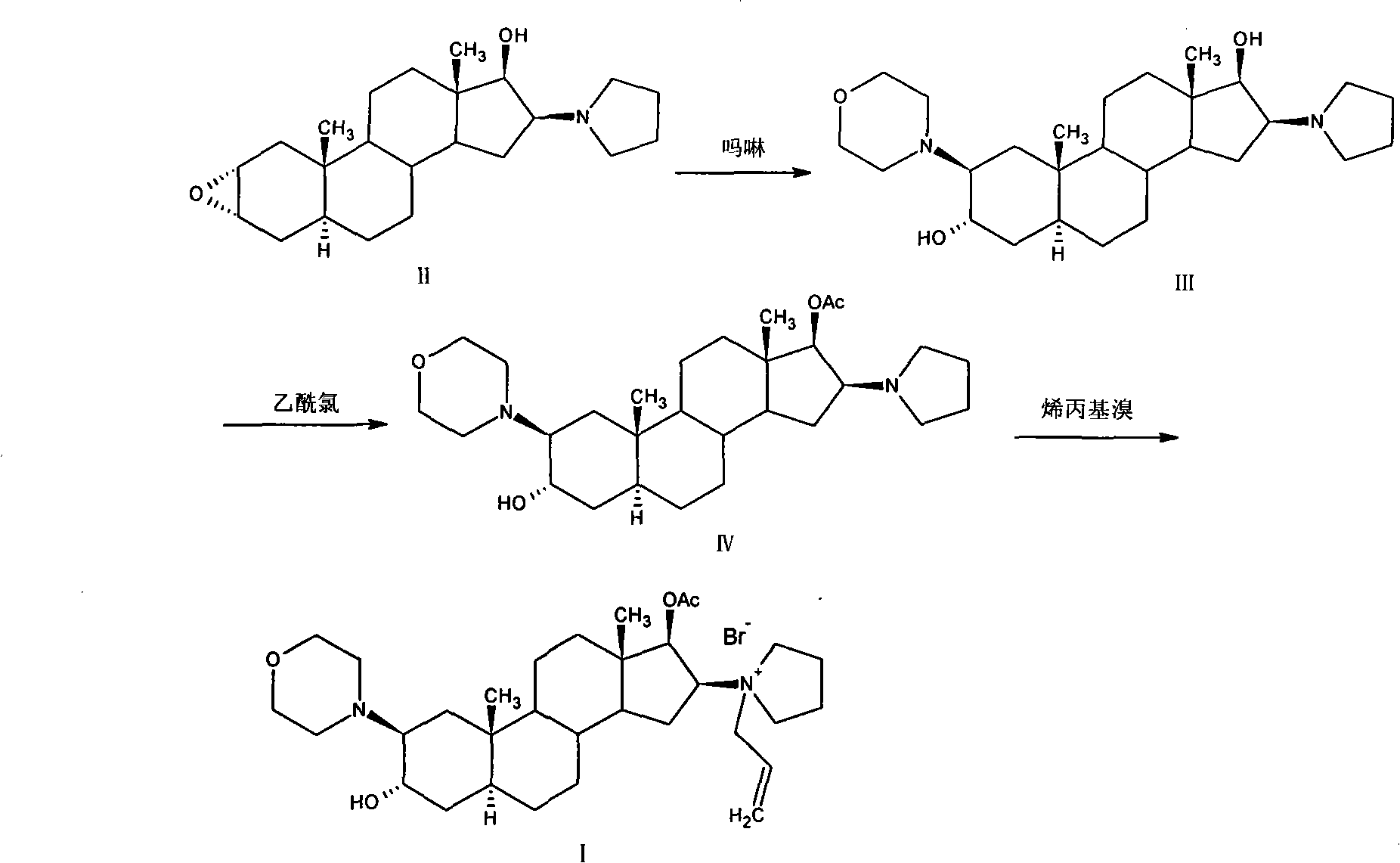
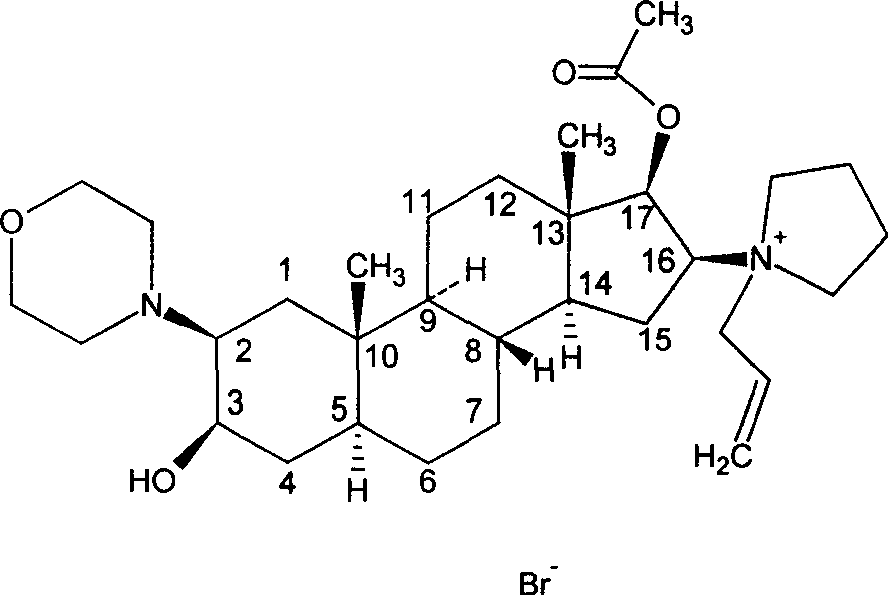
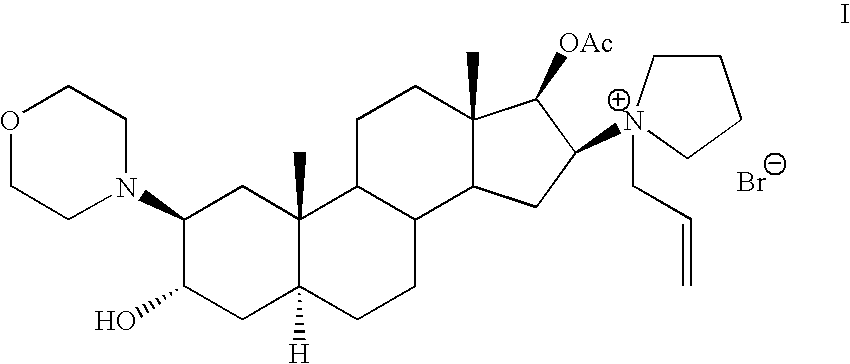
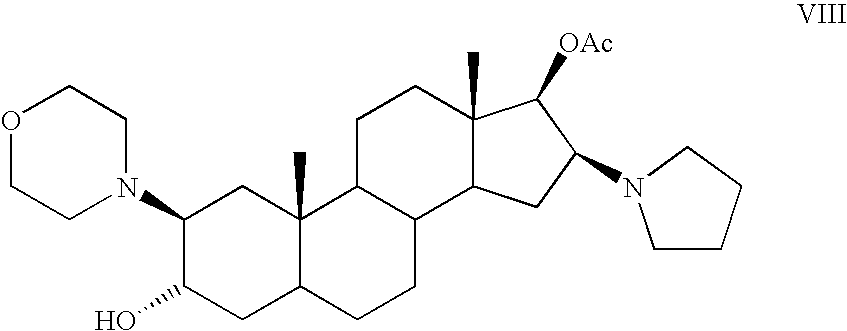
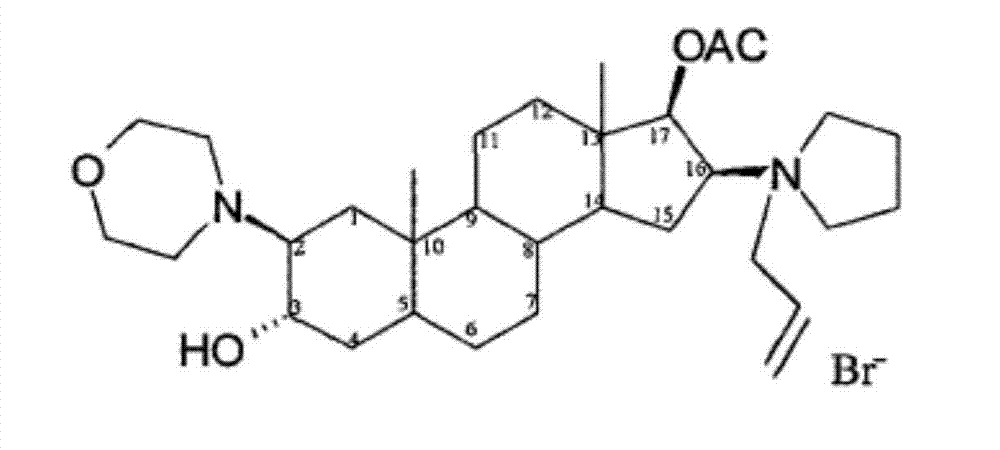
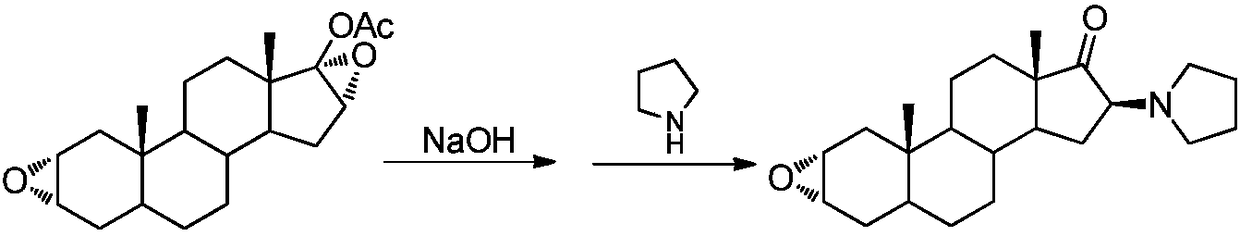
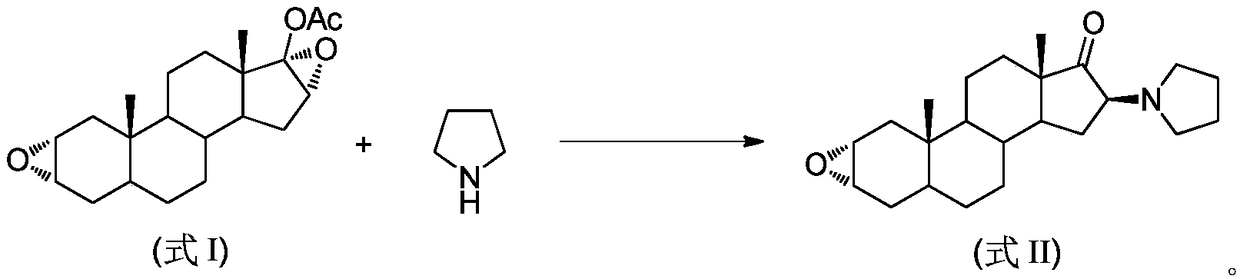
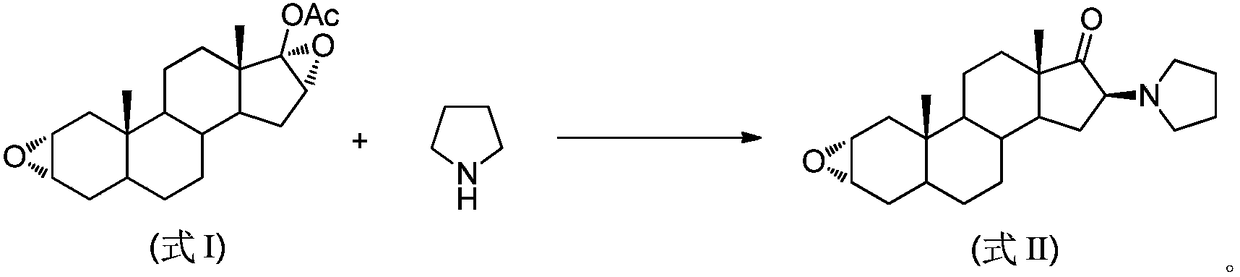
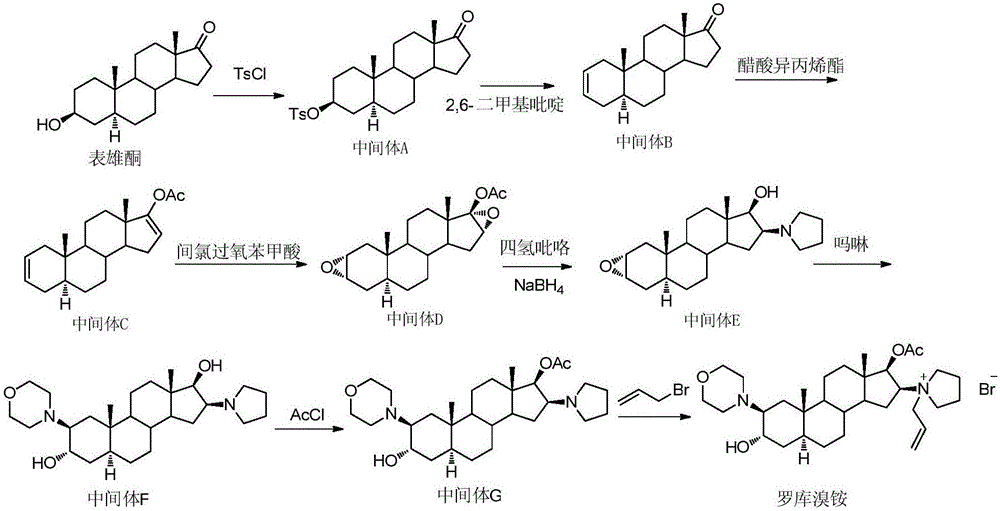
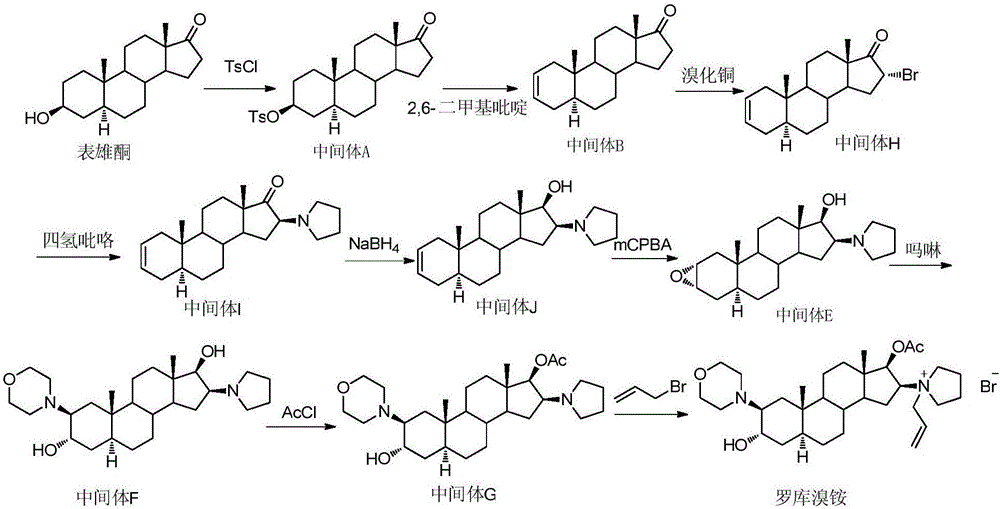
The invention pertains to the medicinal chemosynthesis field and relates to the preparation method of 1-square bracket 17Beta-acetoxyl-3Alpha-oxhydryl-2Beta-(4-morpholinyl)-androstane-16Beta-group square bracket-1-(2-propenyl) pyrrole bromide (rocuronium). The preparation method adopts 2Alpha, 3Alpha, 16Alpha, 17Alpha-diepoxy-5Alpha-androstane-17Beta-glycol acetate as the raw material from which the rocuronium is obtained after seven reaction steps including hydrolysis, pyrrole reaction, morpholinyl reaction, etherification, reduction, acetylation, hydrolysis and quaterisation. The preparation method of the invention has the advantages that the hydrolysis of 16Alpha, 17Alpha-epoxy-17Beta-acetoxyl takes place at a comparatively low temperature, which can avoid the ring opening of the epoxy and further the inhibition of the formation of 17Alpha-(1-pyrrolidine)-16-ketone; the synthesis avoids the optional acetylation of site 3 and site 17 oxhydryls and clearly simplifies the separation after reaction.
Owner:FUDAN UNIV
Method for preparing rocuronium bromide injection
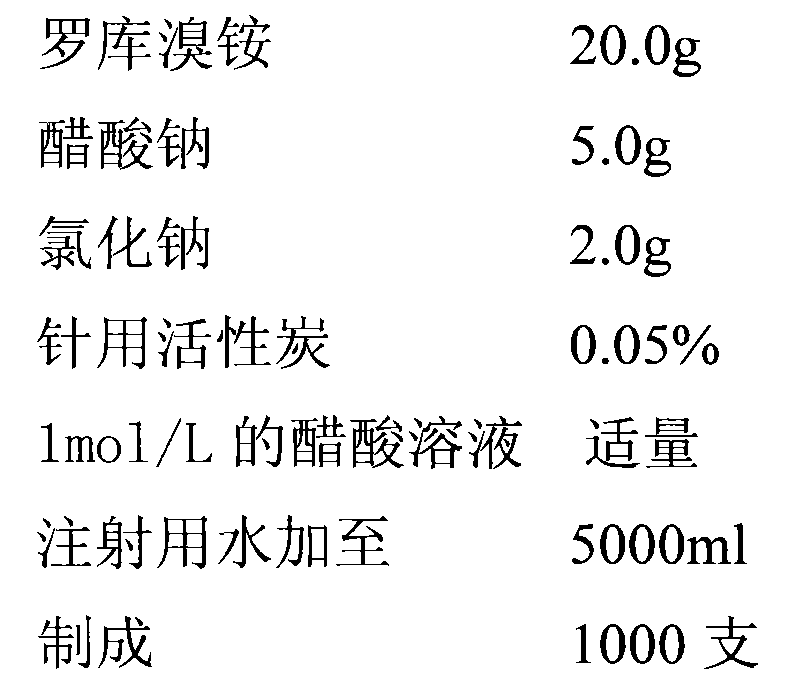
InactiveCN102048684AQuality improvementSimple processOrganic active ingredientsMuscular disorderAcetic acidSodium acetate
The invention discloses a method for preparing rocuronium bromide injection, which comprises the following steps of: making each 50,000ml of injection contain the following components by weight: 20 to 70.0g of rocuronium bromide, 1 to 20.0g of sodium acetate and 0.5 to 2.0g of sodium chloride; cooling water for injection to room temperature; adding acetic acid-sodium acetate buffer solution, and mixing uniformly; dissolving the sodium chloride and the rocuronium bromide; regulating the pH value of the solution to be 4.0 by using 1mol / L acetic acid; adding active carbon, removing carbon, filtering, and filtering to remove bacteria through a 0.22 mu m microporous filtering film; and filling and sterilizing. The method is simple in process, and the product quality is stable.
Owner:华北制药集团制剂有限公司
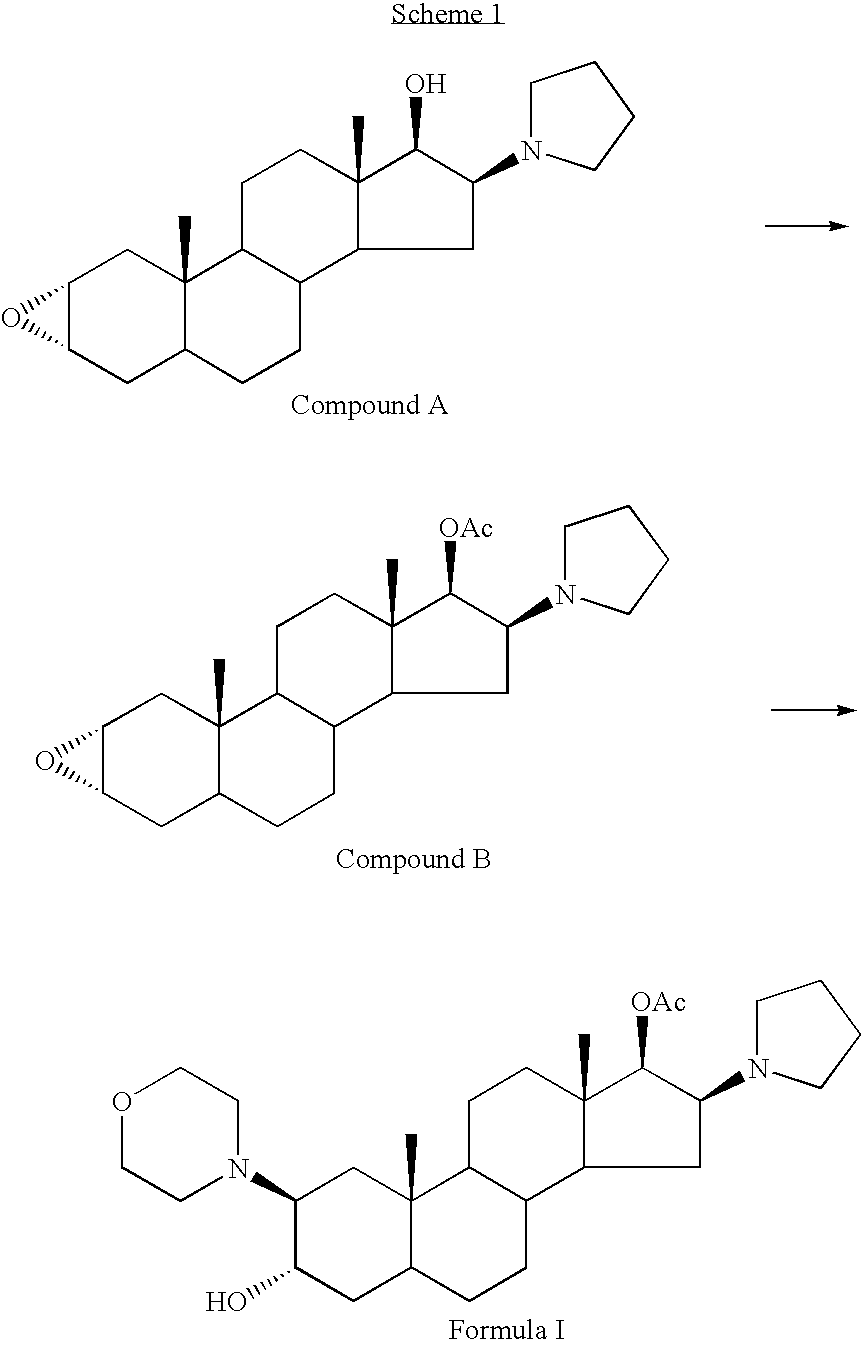
High-purity (2 beta, 3 alpha, 5 alpha, 16 beta, 17 beta)-2-(4-morpholinyl)-16-(1-pyrrolidinyl)-androstane-3,17-diol or composition thereof and preparation method thereof
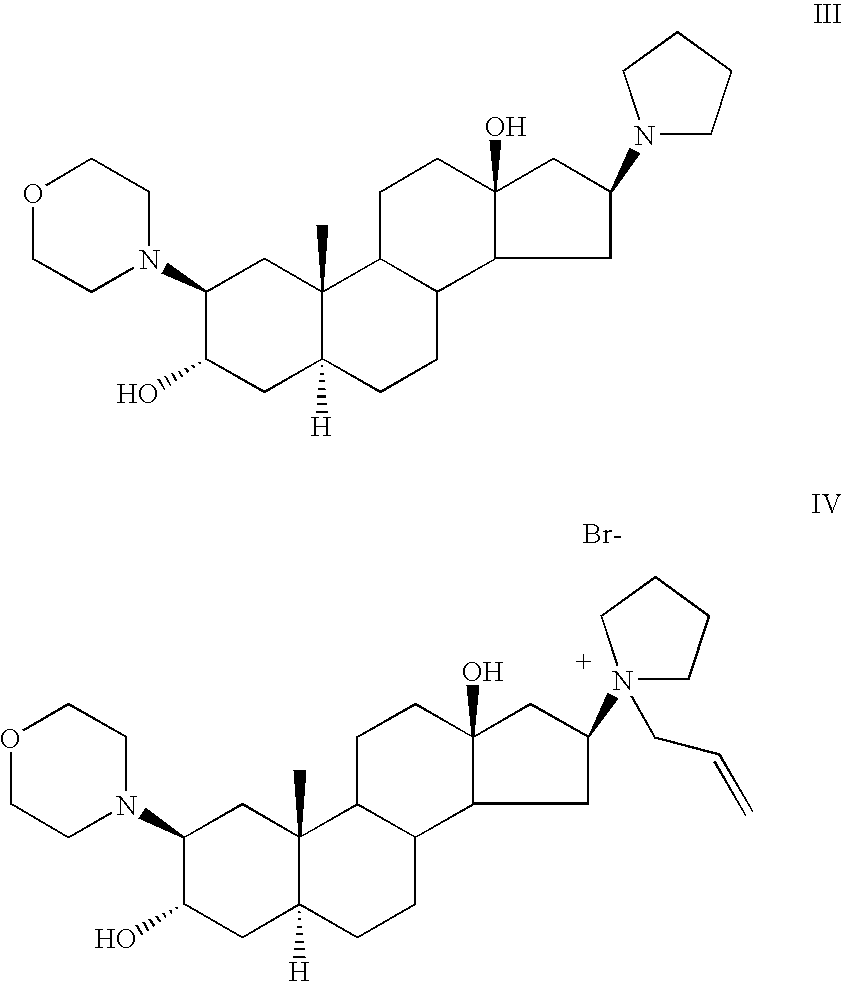
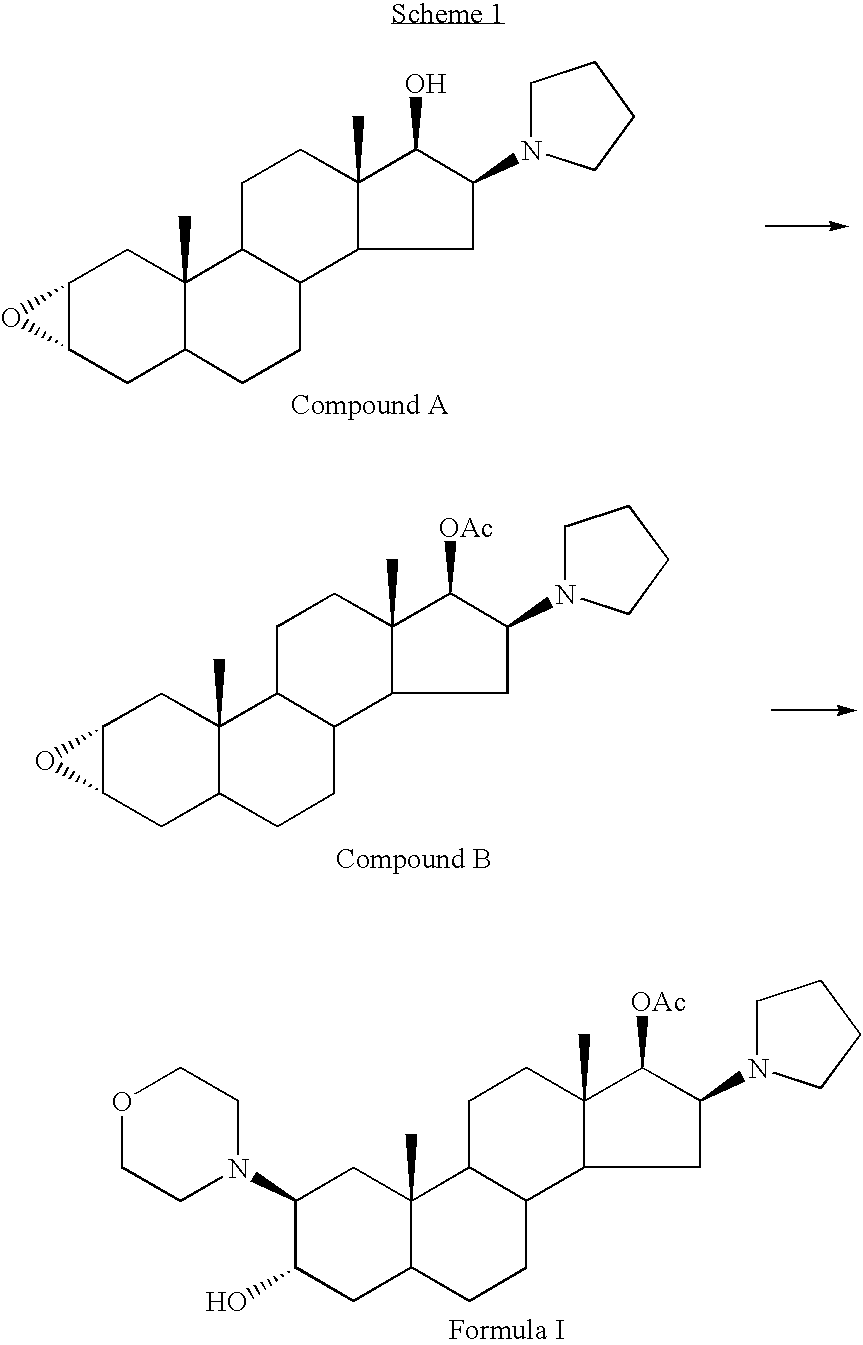
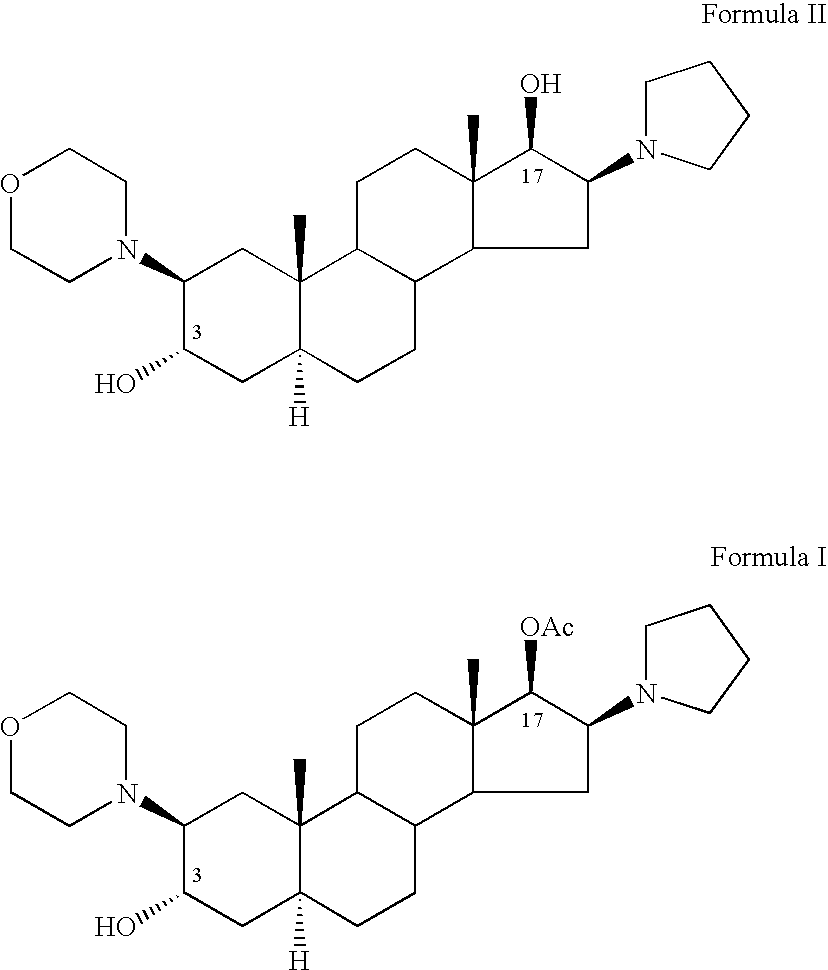
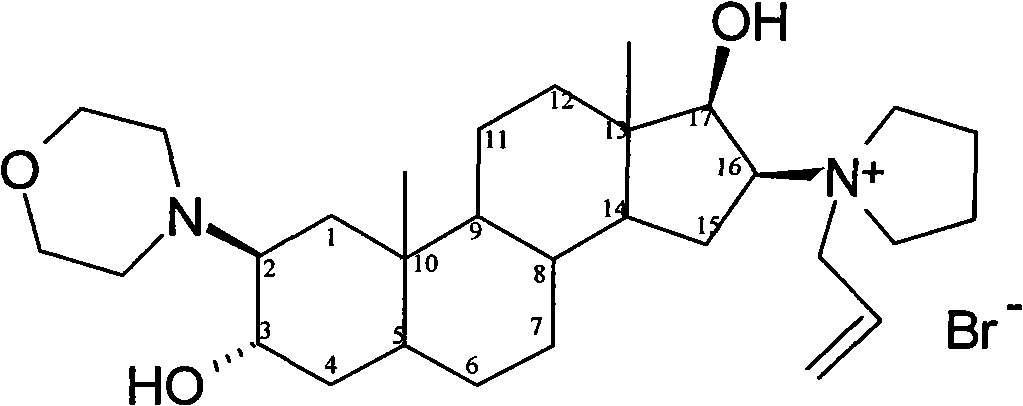
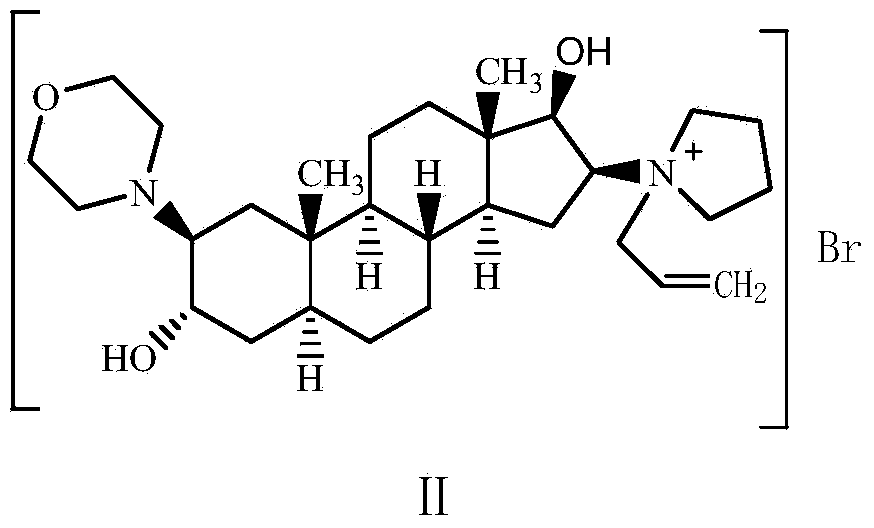
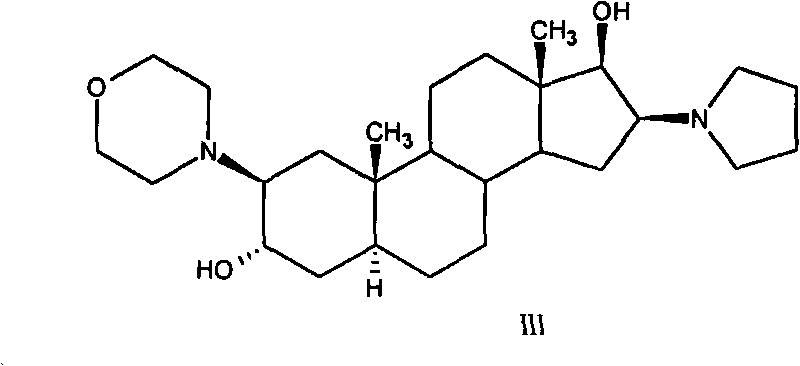
The invention relates to a high-purity (2 beta, 3 alpha, 5 alpha, 16 beta, 17 beta)-2-(4- morpholinyl)-16-(1-pyrrolidinyl)-androstane-3,17-diol or a composition thereof, a preparation method thereof (a compound shown in a formula III) or a composition thereof and application thereof to preparing rocuronium (a compound shown in a formula I). The method has good effect, low cost, high quality and simple and convenient operation and is suitable for large-scale industrial production; and the obtained product has high purity and stable properties.
Owner:重庆凯林制药有限公司 +1
A stable lyophilized preparation of rocuronium bromide and preparation method thereof
The present invention relates to stable freeze dried rocuronium bromide preparation and its preparation process. The freeze dried rocuronium bromide preparation includes rocuronium bromide and freeze drying excipient in the weight ratio of 0.2-1. The freeze dried rocuronium bromide preparation needs no low temperature preservation and convenient storing, and is used for muscular relaxation of patient in surgical operation.
Owner:CHONGQING PHARMA RES INST +1
Pure rocuronium bromide
Owner:SICOR INC
Rocuronium bromide-containing injection
ActiveCN102949339AReduce hydrolysisReduce hydrolysis reactionOrganic active ingredientsMuscular disorderBULK ACTIVE INGREDIENTActive ingredient
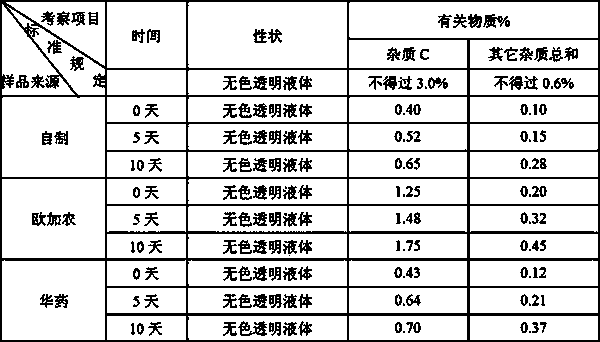
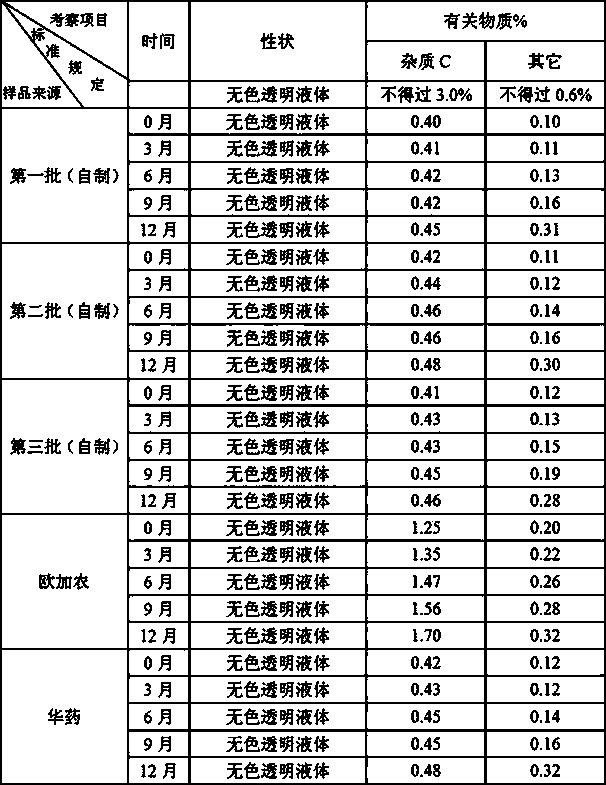
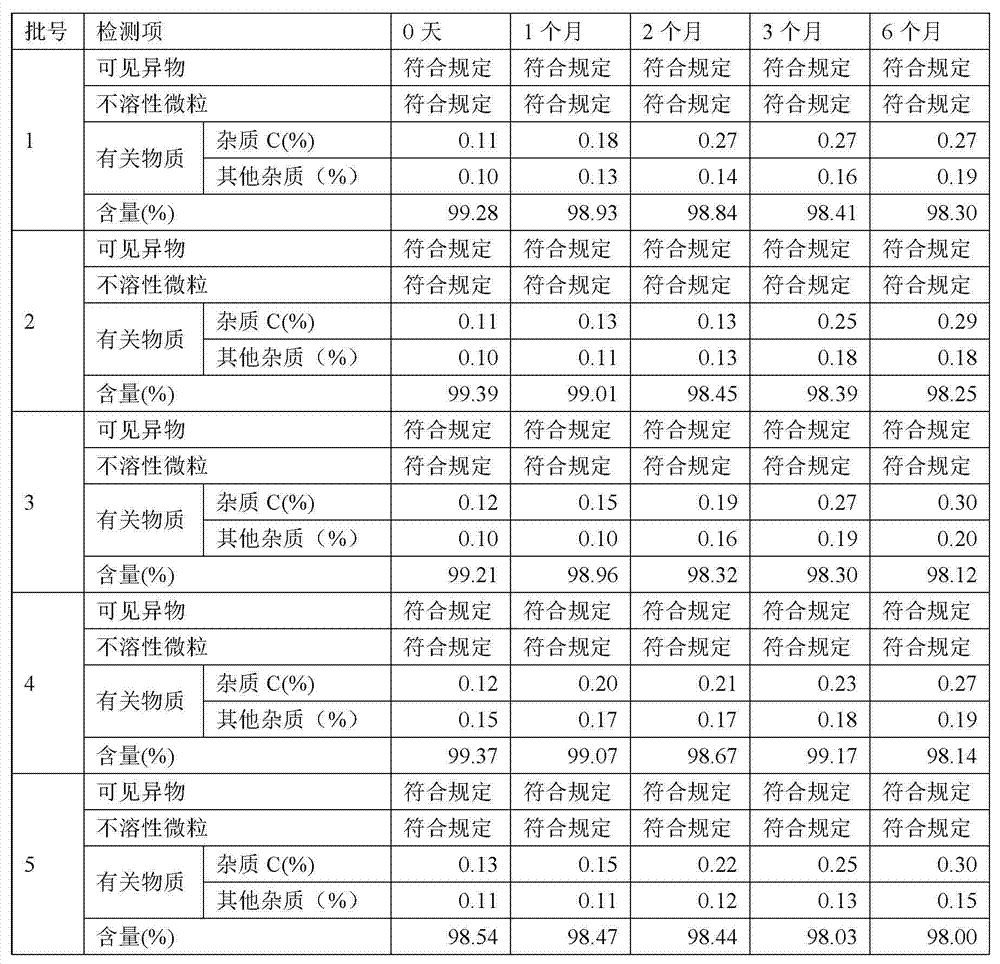
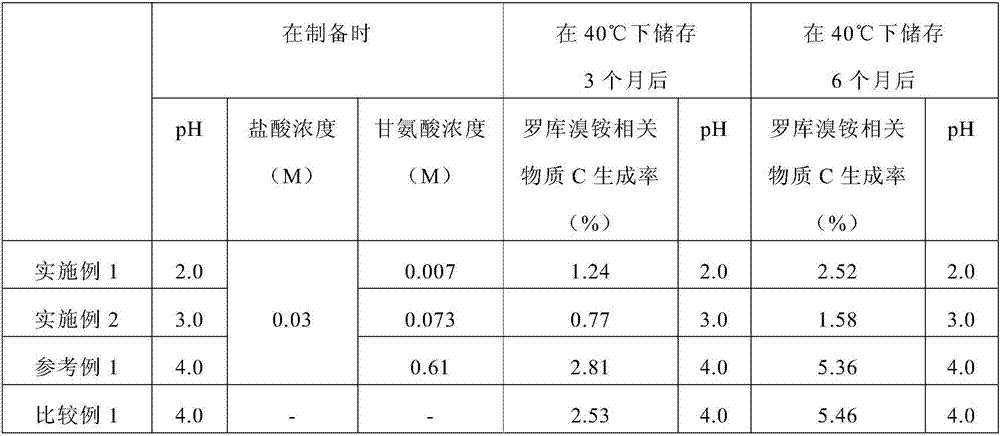
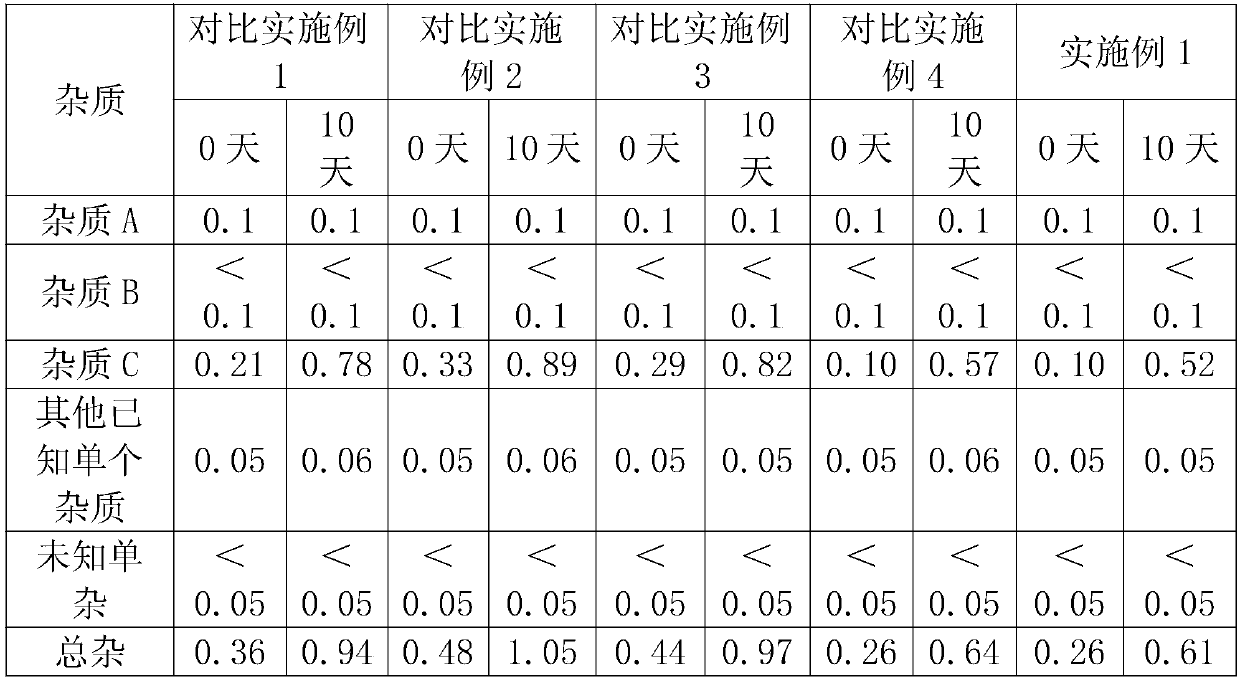
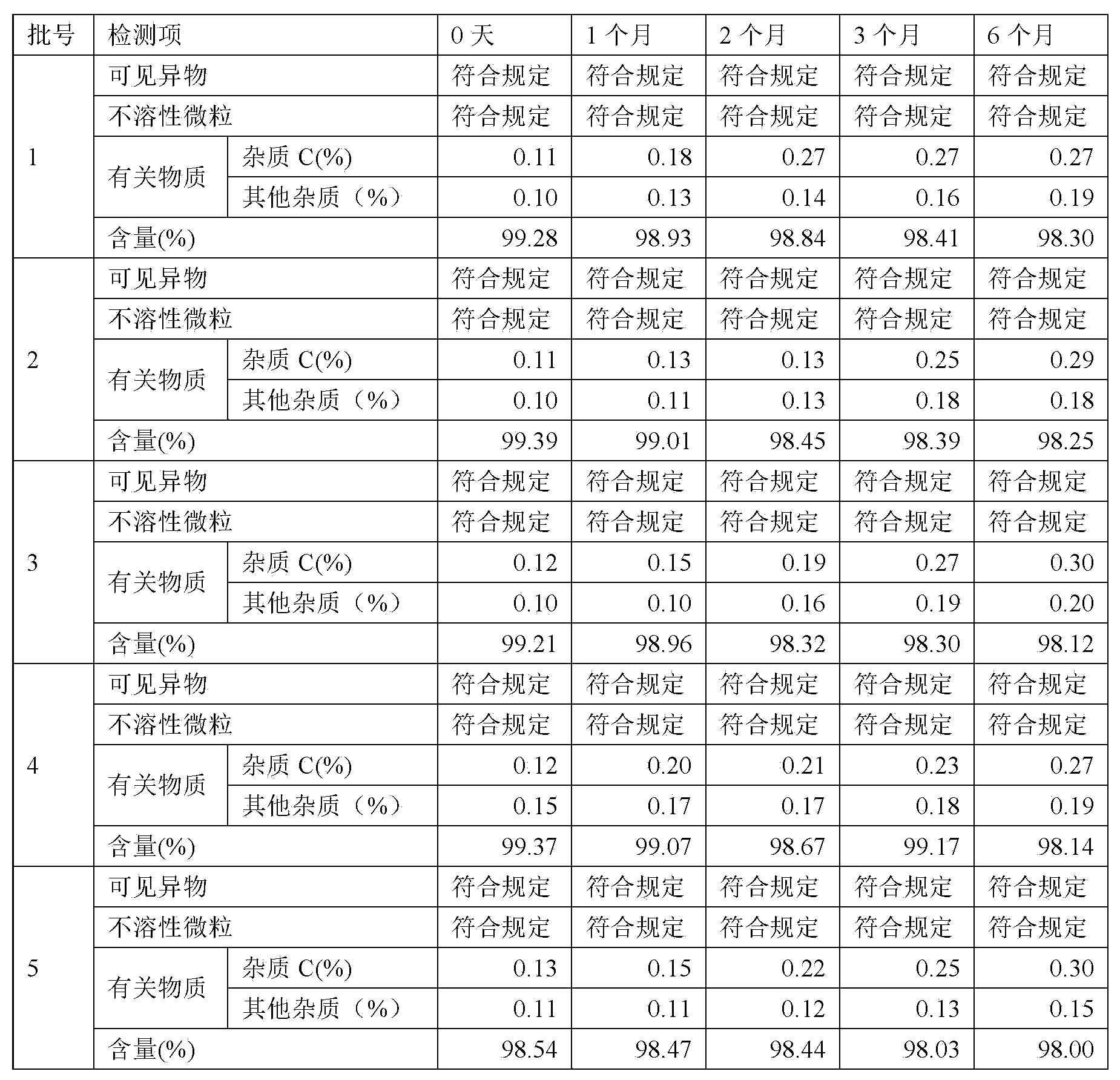
The invention relates to a rocuronium bromide-containing injection. The injection is prepared from rocuronium bromide serving as an active ingredient and a pharmaceutically-acceptable carrier as accessories. The pH value of a system is maintained by adding a 1-5 percent acetate buffer solution, so that the stability of the solution is enhanced, the pH value of a system is maintained at the same time, degradation of main medicaments in a preparation process is suppressed, the stability of the main medicaments is enhanced, and the quantity of rocuronium bromide impurities C in an autoclave sterilizing process is reduced. Finally, a rocuronium bromide injection which has high stability and stable quality and is suitable for storing for a long time is obtained.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Method for preparing rocuronium bromide injection
ActiveCN102860980ASimple production processImprove product qualityOrganic active ingredientsMuscular disorderSodium acetateSodium acetrizoate
The invention discloses a method for preparing rocuronium bromide injection. The method includes steps of cooling injection liquid to the temperature lower than 25 DEG C, adding and dissolving sodium acetate in the injection and regulating a pH (potential of hydrogen) value of the solution to range from 3.8 to 4.2 by glacial acetic acid; adding and dissolving a prescription amount of rocuronium bromide crude drugs into the solution and regulating a pH value of the solution to range from 3.8 to 4.2; adding sodium chloride to condition the solution until the solution is hypertonic; and filtering the solution via a microporous membrane with the aperture of 0.22 micrometer. 1ml of the injection contains, by weight, 10mg of the rocuronium bromide, 2.0mg of sodium acetate, a proper amount of sodium chloride and a proper amount of glacial acetic acid. The method is simple in process, and the quality of the product is stable.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
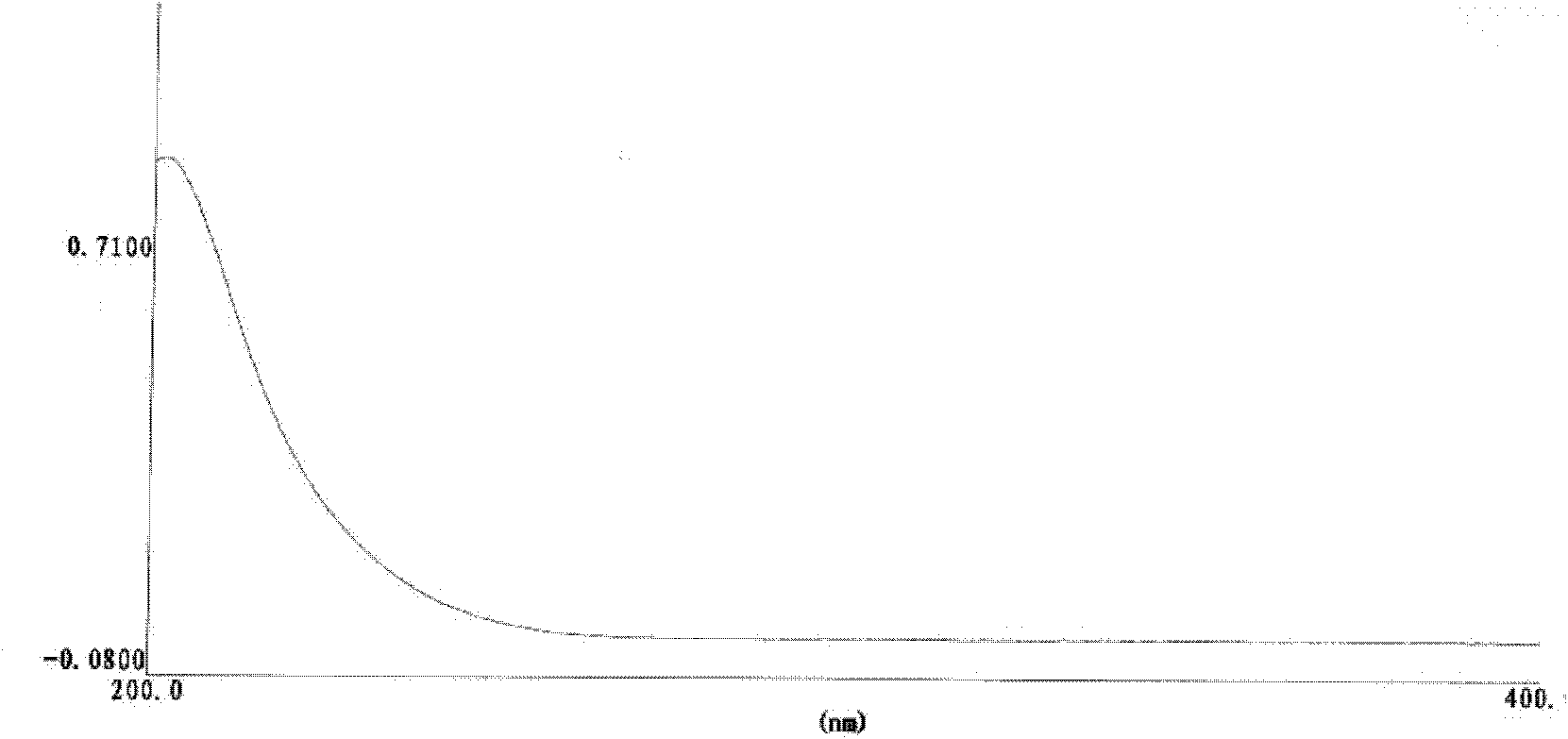
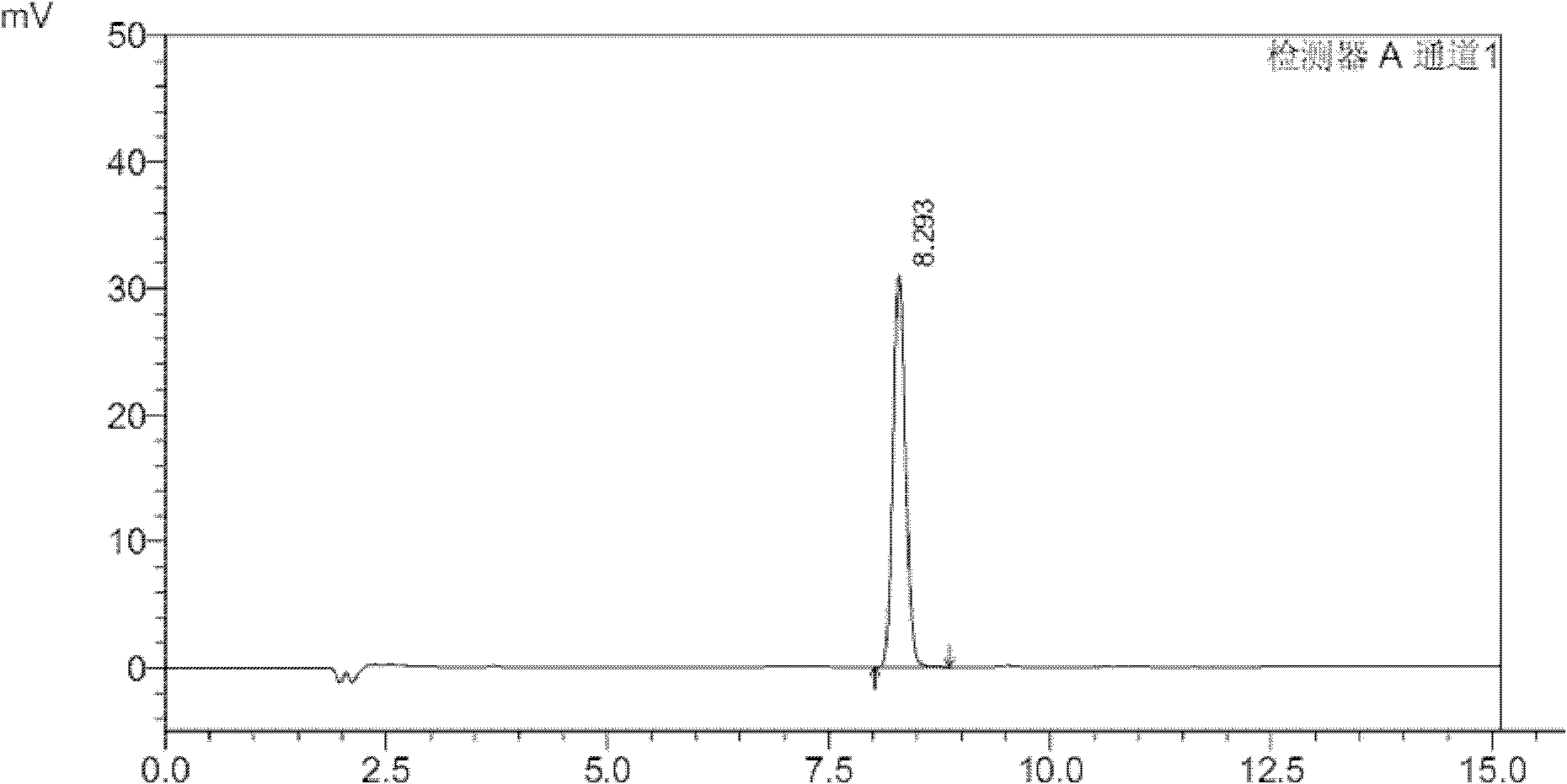
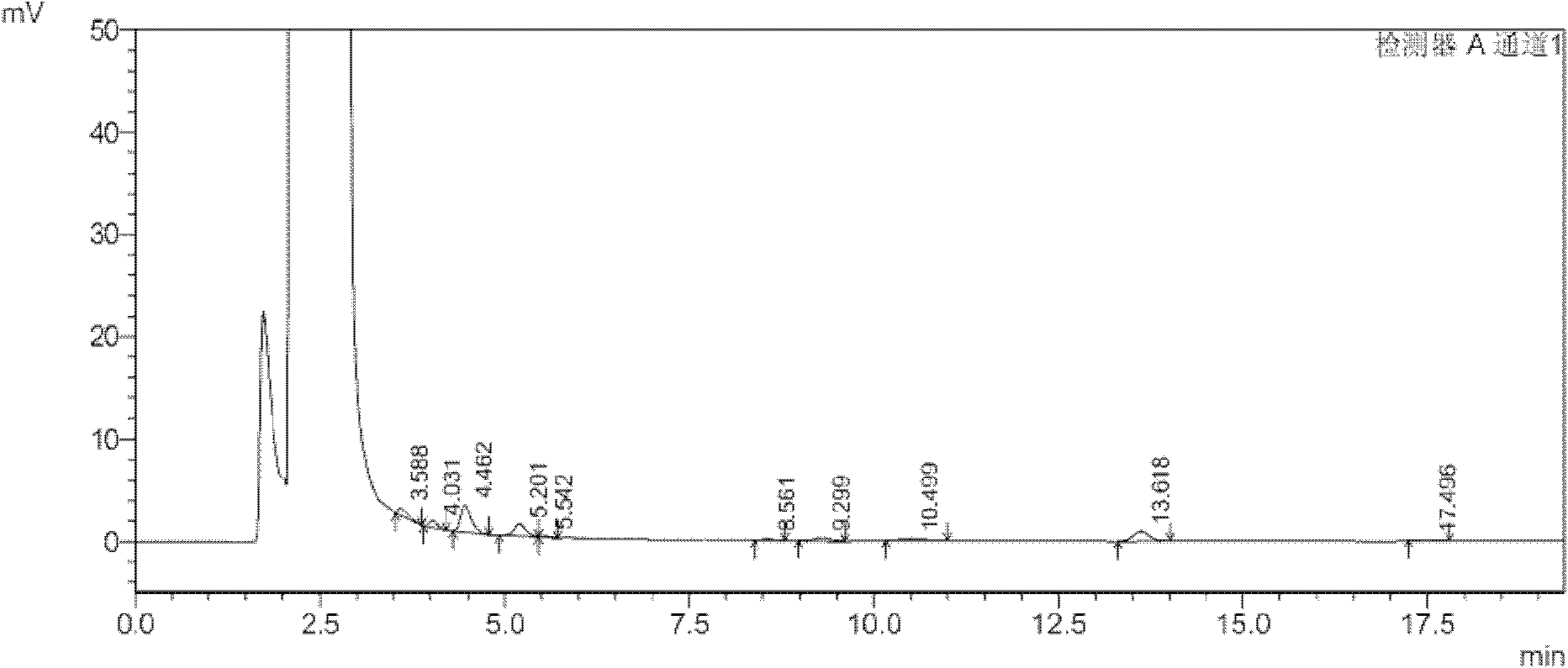
A kind of method that detects propylene bromide content in rocuronium bromide
The invention relates to the field of analytical chemistry and discloses a method for detecting the content of bromopropylene in rocuronium bromide. Particularly, the method comprises the following steps of: taking a rocuronium bromide sample and dissolving the rocuronium bromide sample by using mixed solution with a volume ratio of acetonitrile to water of (9-6):(1-4) as a solvent to obtain tested solution; taking a bromopropylene reference substance and dissolving the bromopropylene reference substance by taking the mixed solution with the volume ratio of acetonitrile to water of (9-6):(1-4) as the solvent to obtain reference substance solution; respectively carrying out high-efficiency liquid chromatography detection on the reference substance solution and the tested solution; and analysing a measuring result by an external standard method. The method for detecting the content of the bromopropylene in the rocuronium bromide can be used for detecting the content of the bromopropylene in a rocuronium bromide product and has the limit of detection of 0.044ng. Due to the establishment of the method, in the process of preparing the rocuronium bromide, the residual amount of the bromopropylene can be detected. The method has high sensitivity, strong specificity and accurate and reliable results, so that the quality of the rocuronium bromide product is accurately evaluated and thequality of the rocuronium bromide product is ensured.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
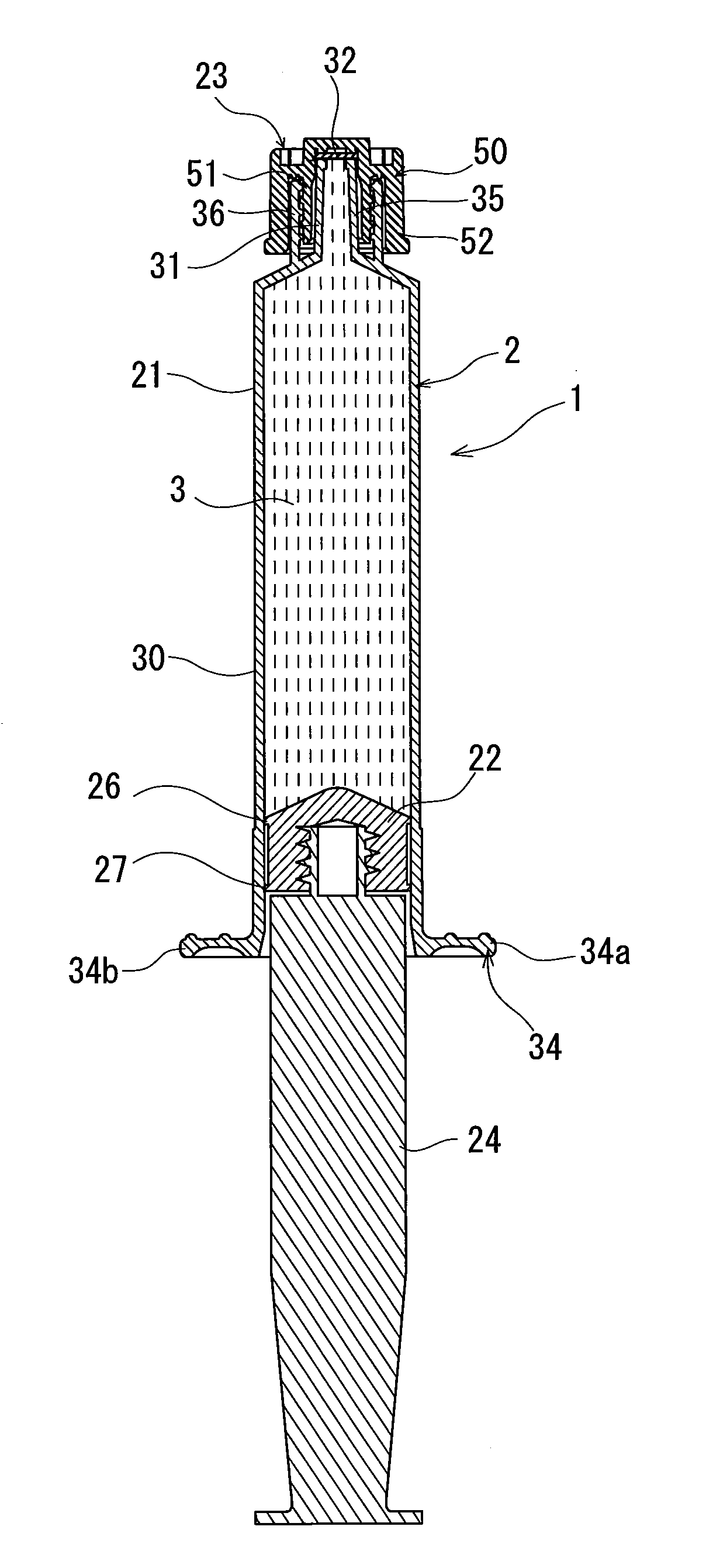
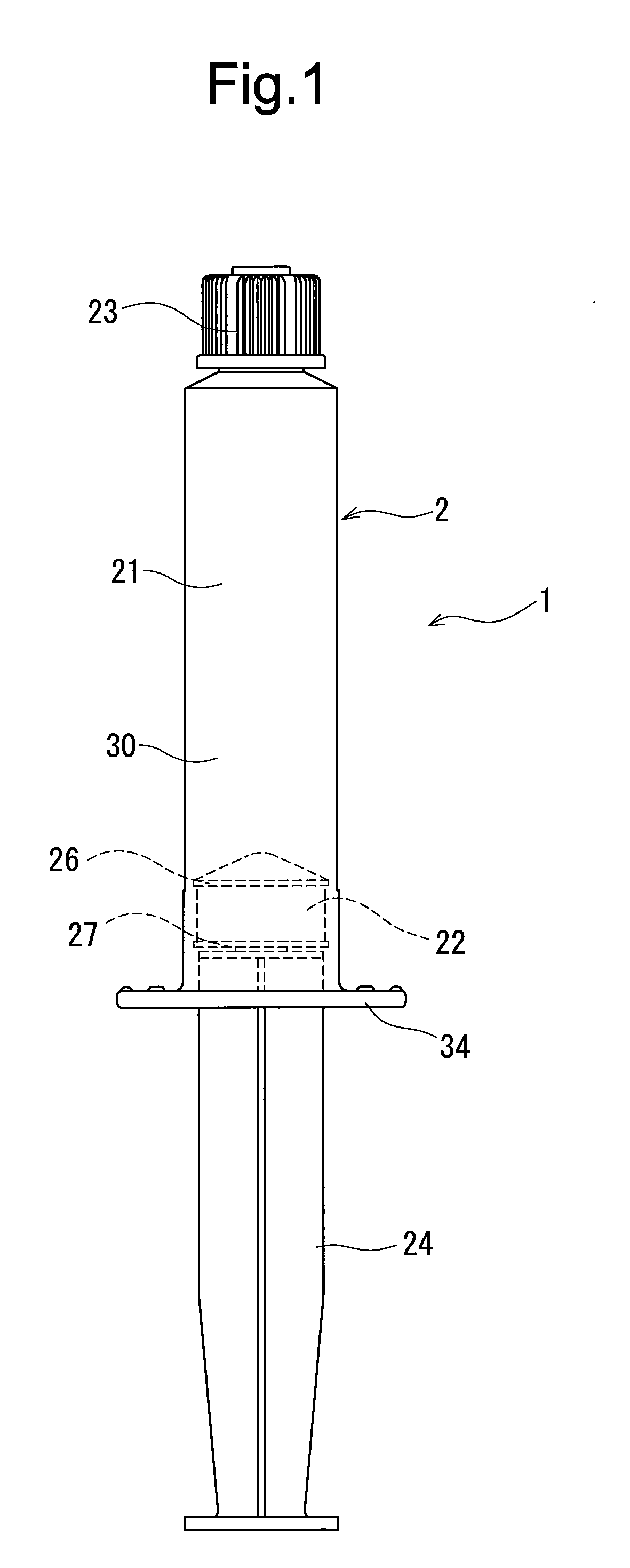
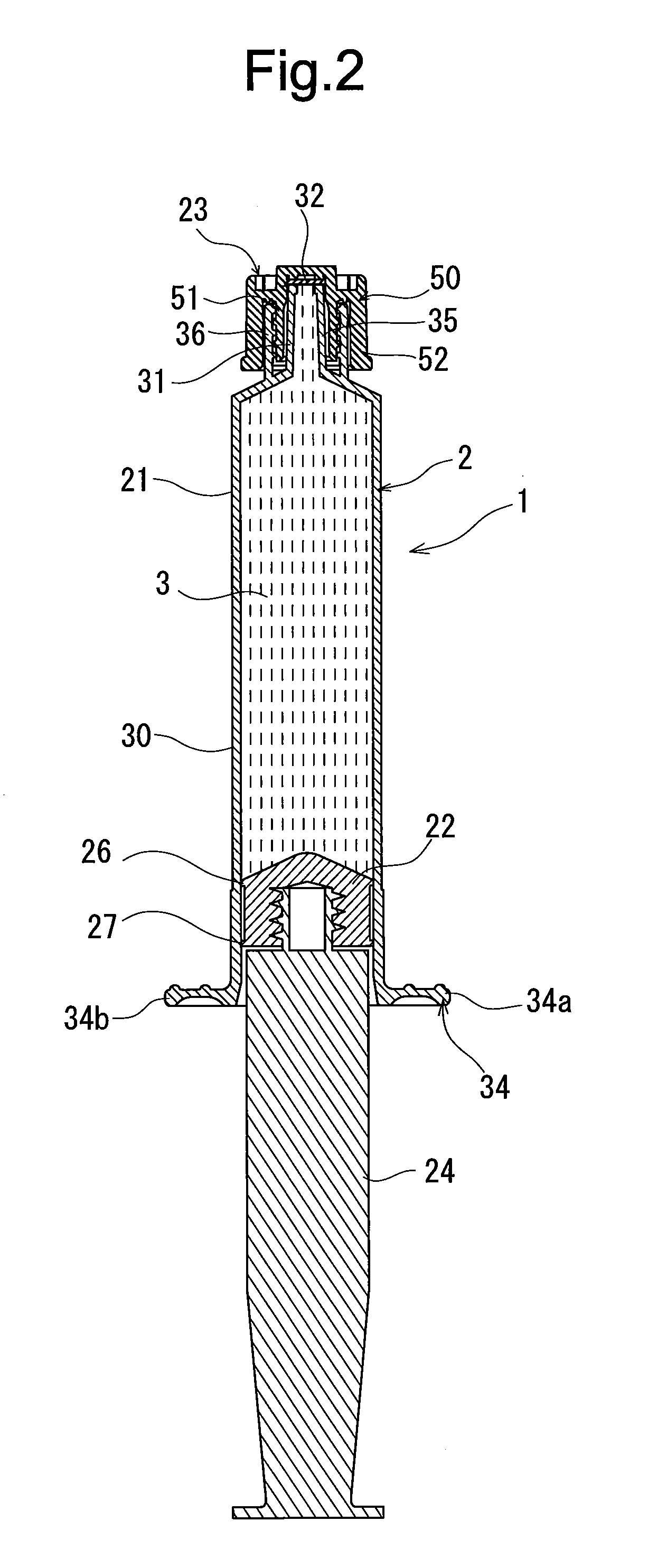
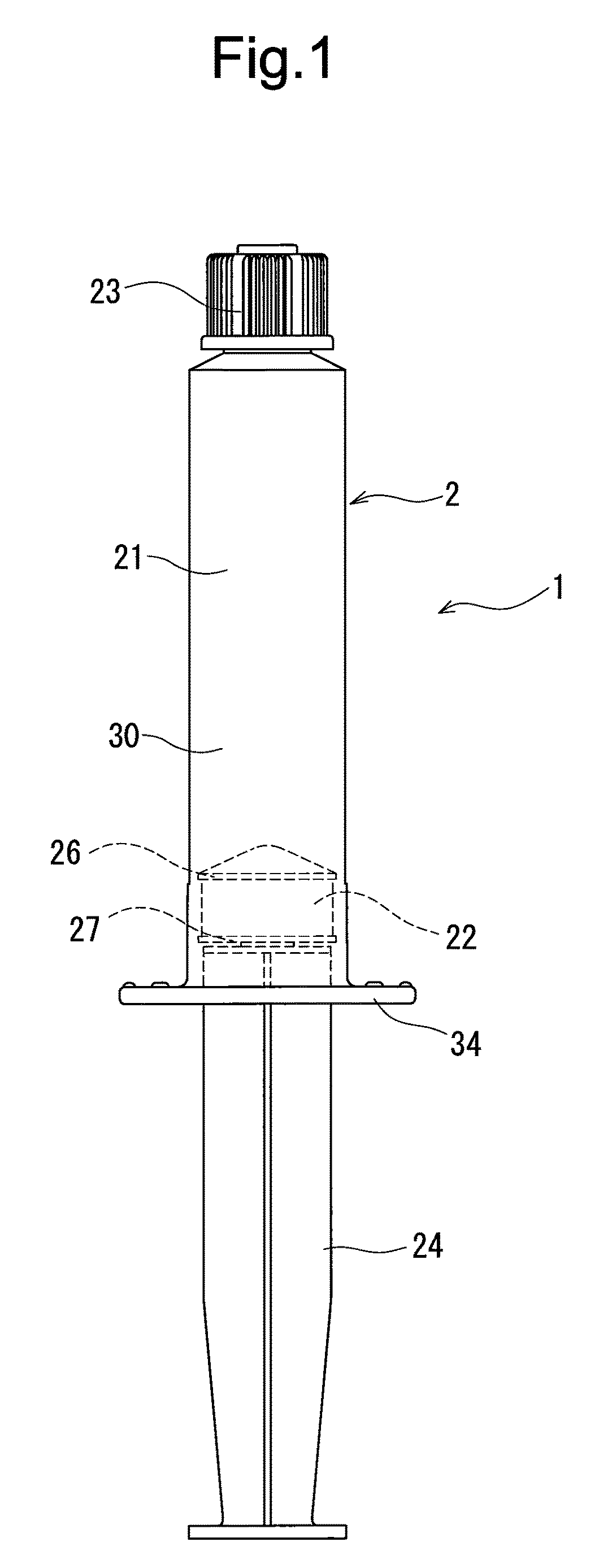
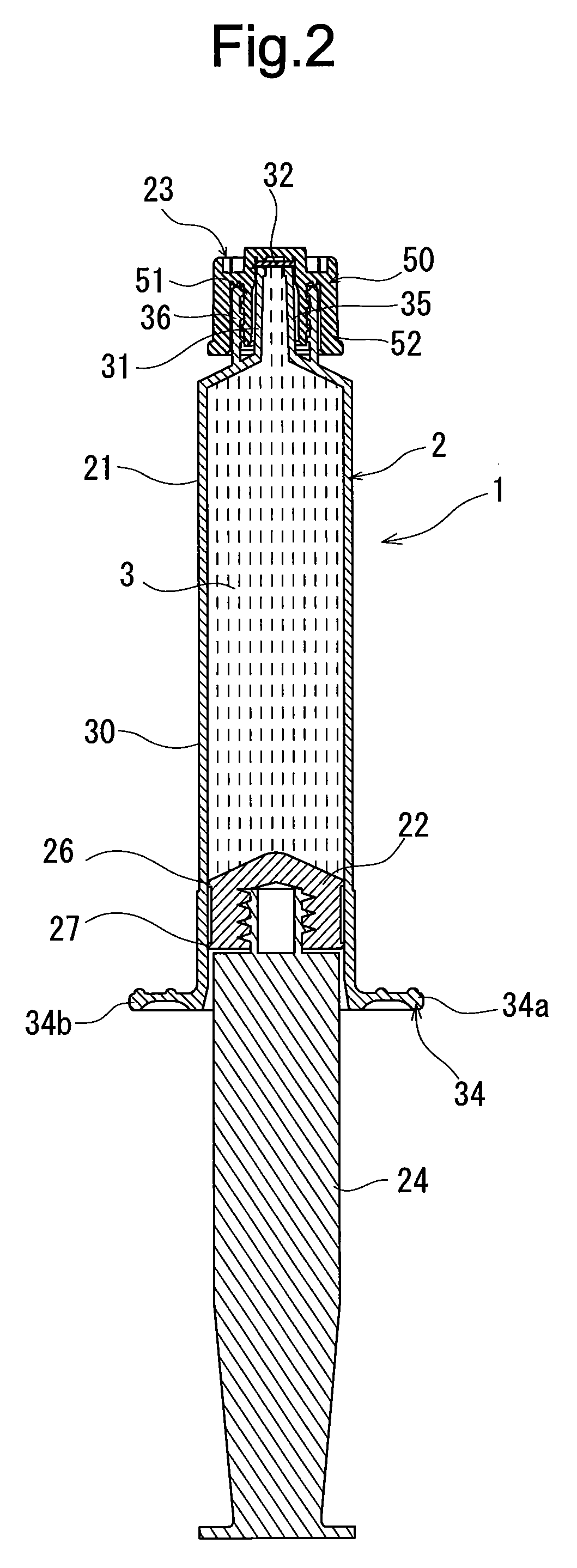
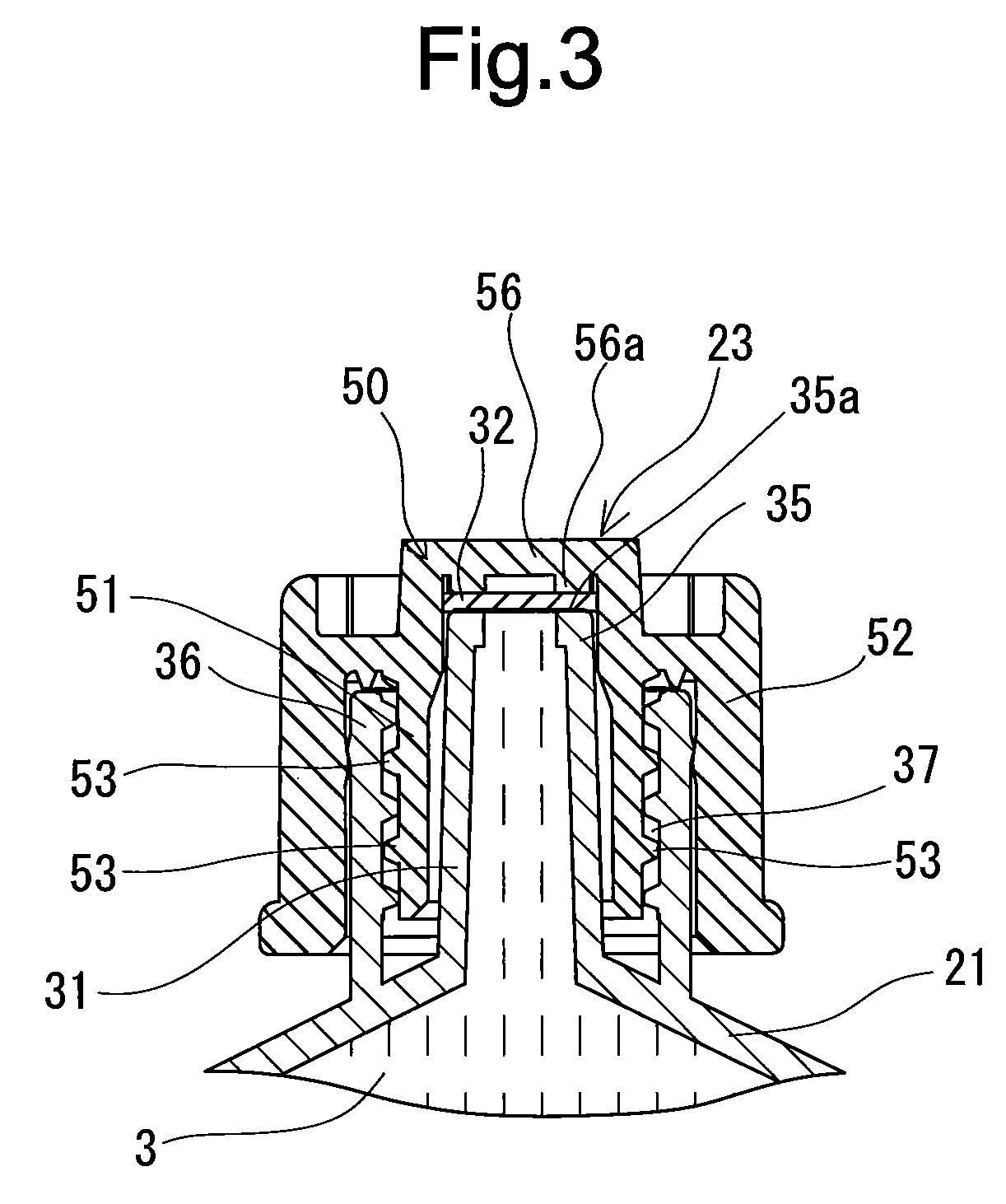
Rocuronium bromide injection solution-filled prefilled syringe
ActiveUS20170014431A1Keep for a long timeOrganic active ingredientsAmpoule syringesPrefilled SyringeButyl rubber
A rocuronium bromide injection solution-prefilled syringe of the present invention includes a syringe comprising an outer cylinder made of synthetic resin, a gasket which is accommodated inside the outer cylinder and liquid-tightly slidable inside the outer cylinder, and a seal member for sealing a distal end opening of the outer cylinder; and a rocuronium bromide injection solution filled inside the syringe. The gasket is formed by vulcanized chlorinated butyl rubber added calcined clay, and said calcined clay is only added as an inorganic filler classified as an inorganic reinforcing agent and an inorganic filling agent.
Owner:TERUMO KK
Processes for the preparation of rocuronium bromide and intermediates thereof
InactiveUS7579461B2Efficient and cost-effectiveOrganic active ingredientsNervous disorderAndrostanesCombinatorial chemistry
A novel process for preparing (2β,3α,5α,16β,17β)-17-acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl) androstane, a known intermediate in the synthesis of the skeletal muscle relaxant rocuronium bromide, is disclosed.
Owner:WAVELENGTH ENTERPRISES LTD
Rocuronium formulation with improved stability
InactiveCN107073007AImprove stabilityImprove thermal stabilityOrganic active ingredientsInorganic non-active ingredientsGlycineGeneration rate
Owner:MARUISHI PHARMACEUTICAL CO LTD
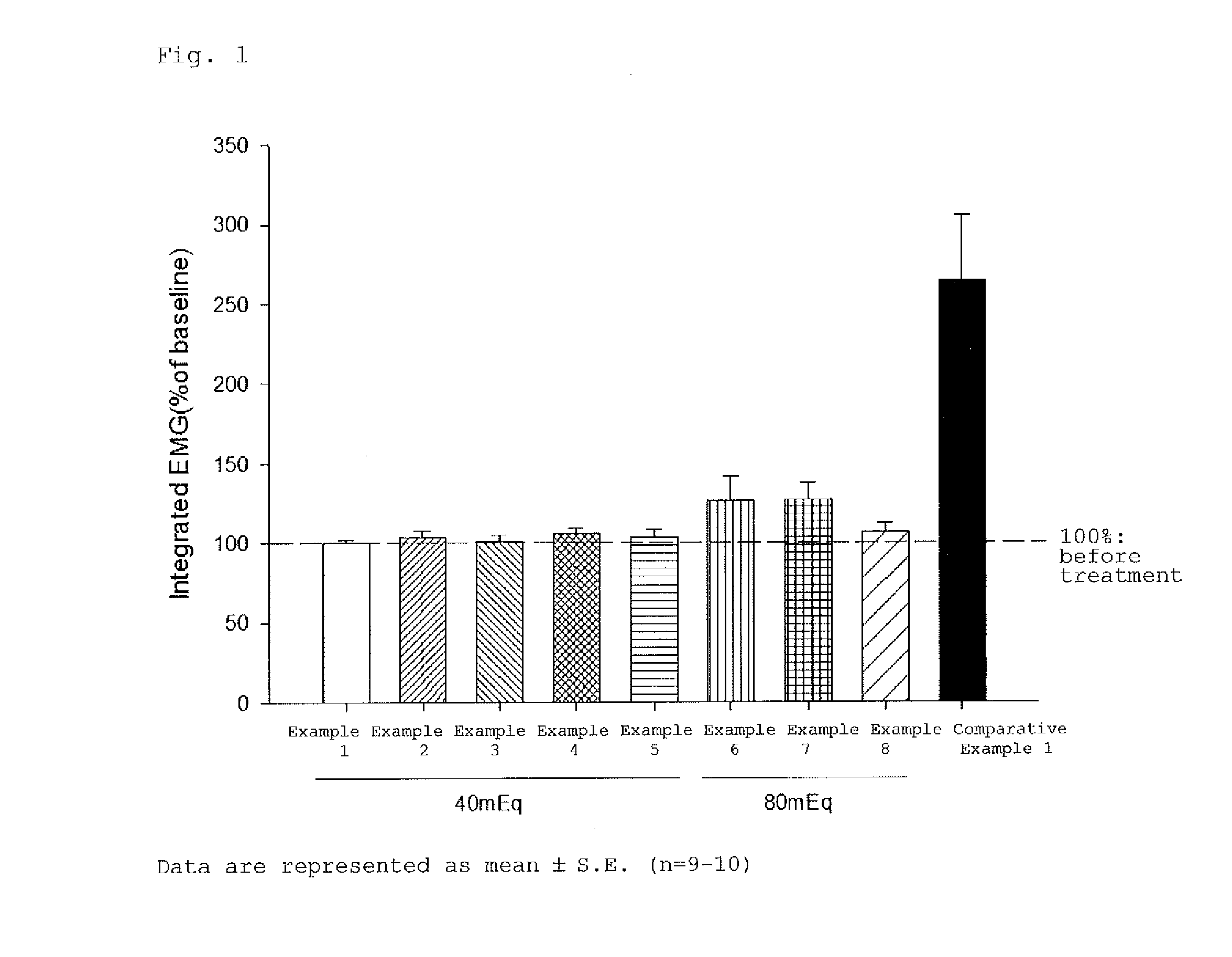
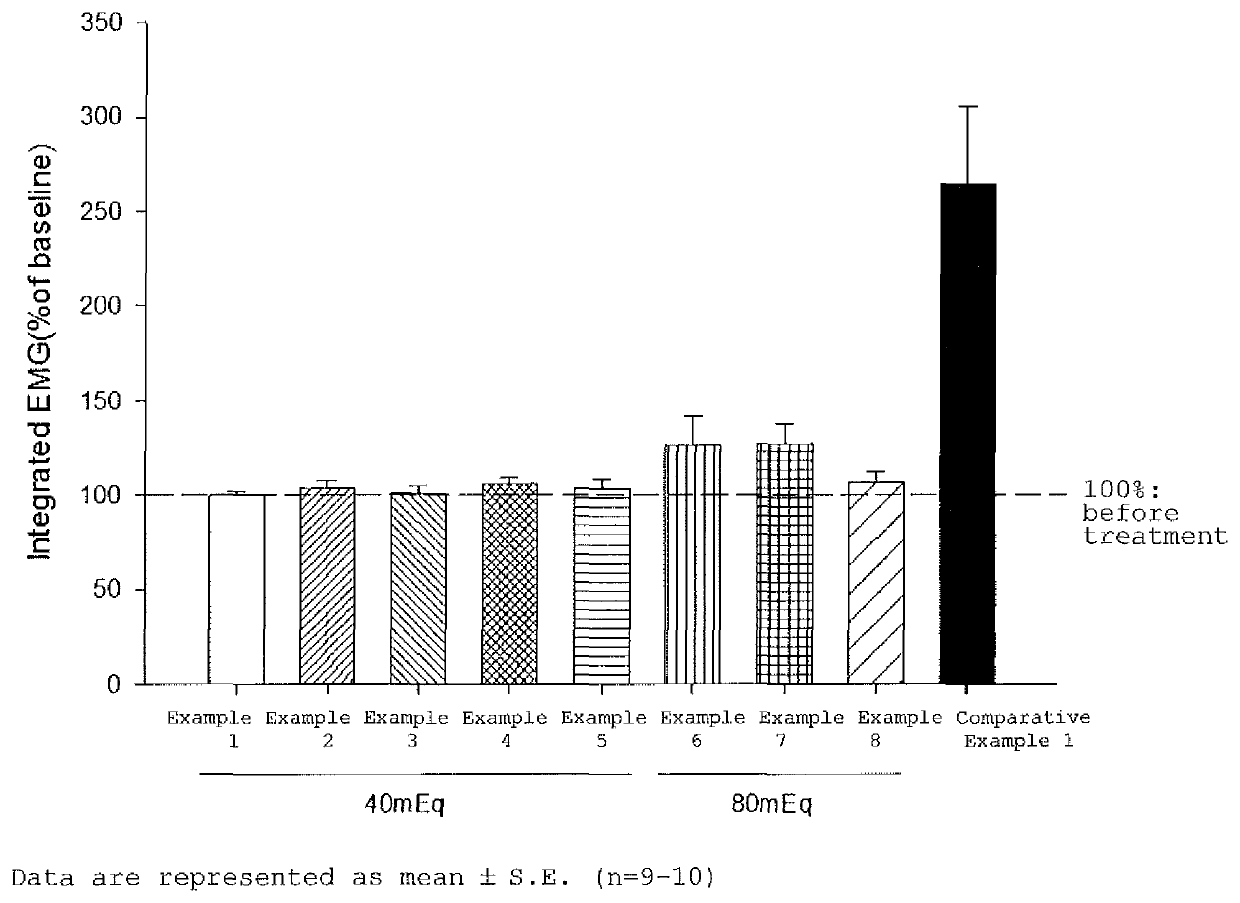
Rocuronium preparation causing less pain, method for producing the same, and method for reducing and/or alleviating vascular pain to be induced using the same
ActiveUS20160143919A1Relieve painOrganic active ingredientsInorganic non-active ingredientsAcetic acidMedicine
Provided is a rocuronium preparation designed to reduce vascular pain to be induced. The rocuronium preparation contains rocuronium and a buffer solution, and has titratable acidity of 100 mEg or less. The buffer solution may be an acetate buffer solution, a citrate buffer solution, a formate buffer solution, a tartrate buffer solution, a phosphate buffer solution, a glycine-hydrochloric acid buffer solution, or a citric acid-phosphate buffer solution.
Owner:MARUISHI PHARMACEUTICAL CO LTD
Drying method of rocuronium raw material medicine for injection
The invention discloses a drying method of a rocuronium raw material medicine for injection, and belongs to the technical field of raw material medicine preparation. The drying method of the rocuronium raw material medicine for the injection is characterized in that a rocuronium crude product is dissolved in an organic solvent with the boiling point lower than 60 DEG C to form a crude product solution, neutral alumina with the weight being 0.9 to 1.2 times of that of the rocuronium crude product is added into the crude product solution and stirred for 1h to 2h, then a filtrate filtered through a coarse filter and a fine filter for two times enters a temperature control tank, the temperature in the temperature control tank is kept at 10 to 35 DEG C, the residence time of the temperature control tank is 5min to 15min, then the filtrate is atomized into droplets through an atomizer and blown into a drying tower at the temperature of 50 to 80 DEG C to evaporate the solvent and form fine powder, and the fine powder is collected from a bottom discharge port after being separated through a cyclone separator to obtain the rocuronium raw material medicine for the injection. The drying method of the rocuronium raw material medicine for the injection successfully uses a spray drying process to replace the traditional vacuum oven drying and vacuum freeze-drying production process.
Owner:SHANDONG VOCATIONAL COLLEGE OF LIGHT IND
Preparation method for rocuronium bromide injection in low impurity level
InactiveCN108670949AIncrease contentReduce contentOrganic active ingredientsInorganic non-active ingredientsActivated carbonNitrogen
The invention discloses a preparation method for rocuronium bromide injection in a low impurity level. In a preparation process of the rocuronium bromide injection, the content of an impurity C is reduced to 0.1% as possible, and the condition that the impurities of the rocuronium bromide injection do not exceed a standard (a shelf life standard: 1.5%) within a validity period is guaranteed. Whilebacterial endotoxin of a raw material and an auxiliary material is controlled, activated carbon does not need to be added in a prescription process for performing a pyrogen removing operation, and the bacterial endotoxin of a finished product of the rocuronium bromide injection is still qualified. A temperature of prepared chemical is reduced, dissolved oxygen in solution is removed by nitrogen aeration and a solution pH value in the preparation process is reduced, the content of the impurity C is helpful to reduce, and the stability of the rocuronium bromide injection is helpful to improve.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Rocuronium preparation with improved stability
ActiveUS20170128463A1Improve stabilityChange controlOrganic active ingredientsInorganic non-active ingredientsGeneration ratePotassium hydrogen phthalate
The present invention provides a rocuronium preparation with an excellent stability. The rocuronium preparation contains rocuronium and a buffer solution and having an adjusted pH of 3.5 or less (for example, 2.5 to 3.5). The buffer solution may be a citric acid-sodium hydroxide buffer solution, a tartaric acid-sodium hydroxide buffer solution, a potassium hydrogen phthalate-hydrochloric acid buffer solution, a glycine-hydrochloric acid buffer solution, or the like. Such a rocuronium preparation has, for example, after 6-month storage at 40° C., a generation rate of rocuronium-related substance C of 5% or less.
Owner:MARUISHI PHARMACEUTICAL CO LTD
Preparation method and pharmaceutical composition of rocuronium bromide
ActiveCN108676052AIncrease contentReduce contentOrganic active ingredientsPharmaceutical delivery mechanismStability studyDiethyl ether
The invention belongs to the technical field of biological medicine, and specifically relates to a preparation method and a pharmaceutical composition of rocuronium bromide. The preparation method ischaracterized by adding trichloromethane and water under a low-temperature condition, adding a certain amount of CO2, and adding diethyl ether for crystallization, thus obtaining a purified rocuroniumbromide raw material. After stability study is carried out on the raw material, increment of the contents of an impurity A and an impurity C of the raw material is less; in addition, the content of 3-bromopropylene (allyl bromide) in the raw material is smaller than 10ppm; an injection solution is prepared from the rocuronium bromide raw material obtained through the preparation method, the injection solution accords with all standards of a pharmacopoeia, and the increment of the impurity A, the impurity C and total impurity is remarkably lower than those in a commercially available product after cold-thermal cycling.
Owner:北京市新里程医药科技有限公司
Synthesis method of 16beta-tetrahydropyrrolyl androstan-2alpha-epoxy-17-one
The invention relates to the technical field of medicines and chemical industry, discloses a preparation method of an important intermediate of rocuronium bromide, namely 16beta-tetrahydropyrrolyl androstan-2alpha-epoxy-17-one. The preparation method comprises the following steps: (1), in a ball milling tank, adding 17-acetoxy androstan-2alpha,16alpha-bisepoxide, pyrrolidine and silica gel, and reacting at a certain mechanical grinding frequency; and (2), post-treating a reaction mixture, and recrystallizing to obtain the 16beta-tetrahydropyrrolyl androstan-2alpha-epoxy-17-one. The preparationmethod has the advantages of high reaction yield, short time, high selectivity, simple and convenient operation, less pollution and the like, and is an environment-friendly chemical synthesis methodwith relatively good popularization and application prospects.
Owner:台州仙琚药业有限公司
Method for detecting content of bromopropylene in rocuronium bromide
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Process for preparing high-purity rocuronium
ActiveCN101993470BEasy to operateNo pollution in the processMuscular disorderNeuromuscular disorderChlorotrifluoromethaneNitrogen gas
The invention discloses a process for preparing high-purity rocuronium, comprising the following steps of: mixing a low-boiling anti-solvent with the rocuronium containing organic solvents or / and impurities; and removing the organic solvents or / and the impurities which are contained in the rocuronium by utilizing a principle that the organic solvents or / and the impurities are soluble in the low-boiling anti-solvent and the rocuronium is not or hardly soluble in the low-boiling anti-solvent so that the rocuronium is precipitated or crystallized so as to obtain the high-purity rocuronium without containing the organic solvents or / and the impurities, wherein the low-boiling anti-solvent is a mixture of any one or a few of ethane, propane, butane, ethylene, propylene, trifluoromethane, trifluorochloromethane, carbon dioxide, sulfur dioxide and nitrogen in any proportion. Compared with the traditional extraction method, the process can be used for extracting and separating under the condition of adjacent normal temperature and almost retains all effective components of a product; the quantity of organic solvent residuals can be low not to be detected, and the obtained product has high purity; and in addition, the process is easy to operate and green and saves the energy.
Owner:刘志亭
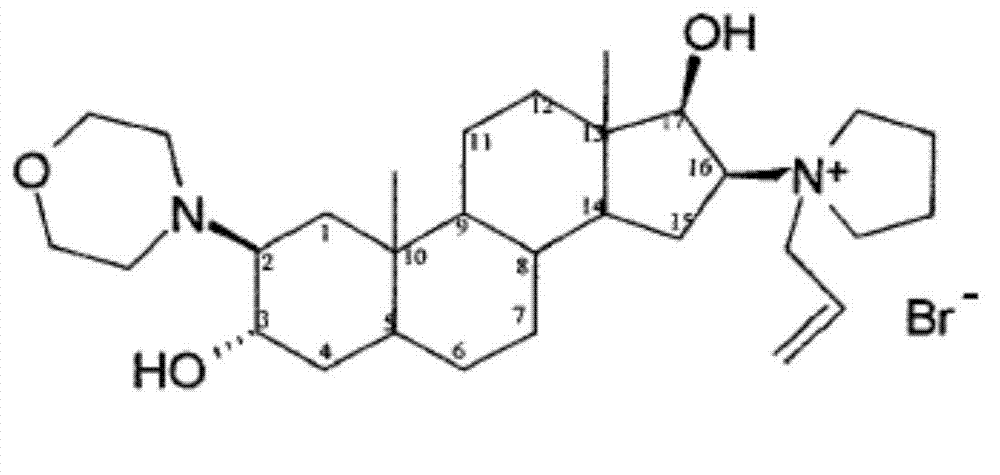
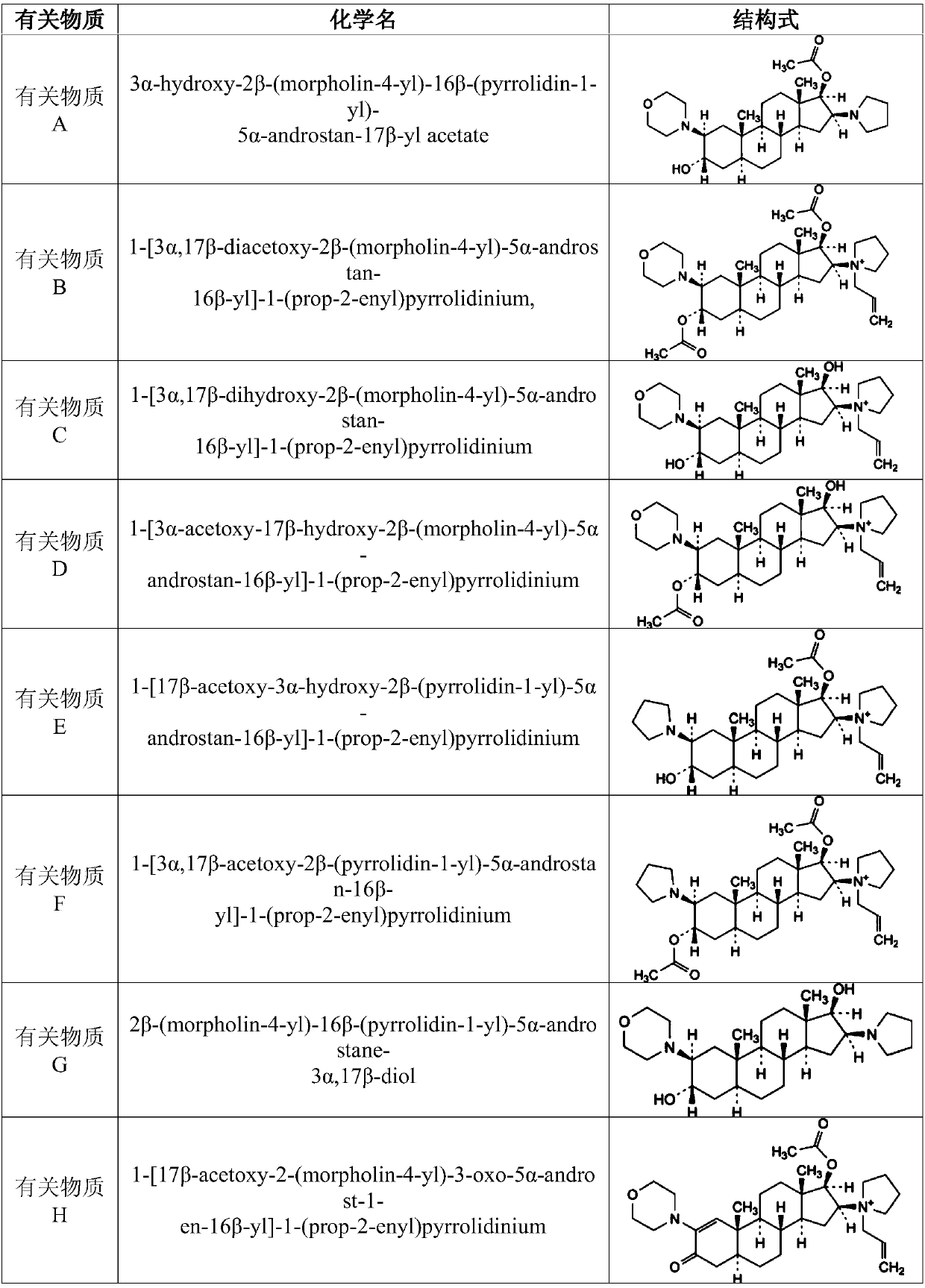
Rocuronium bromide enantiomeric impurity, or salt thereof and method for preparing rocuronium bromide enantiomeric impurity or salt
InactiveCN106810589AShort synthetic routeMild reaction conditionsOrganic chemistry methodsSteroidsEnantiomerDiastereomer
The invention discloses a rocuronium bromide enantiomeric impurity, a salt, a solvate, an enantiomer or a diastereoisomer of the rocuronium bromide enantiomeric impurity and a method for preparing the same. The rocuronium bromide enantiomeric impurity is shown as a formula V. An R1 and an R2 in the formula V are independently selected from H and acetyl; an R3 is selected from N-pyrrolidyl and N-allyl pyrrolidyl. The invention further provides application of the rocuronium bromide enantiomeric impurity to detecting the quality of rocuronium bromide intermediates, crude drugs or preparations. The rocuronium bromide enantiomeric impurity, the salt, the solvate, the enantiomer or the diastereoisomer, the method and the application have the advantages that gaps in the aspect of research on rocuronium bromide impurities can be filled, and foundations and bases can be provided for controlling the quality of rocuronium bromide materials or drugs.
Owner:CHENGDU SINO STRONG PHARMA
Rocuronium preparation causing less pain, method for producing the same, and method for reducing and/or alleviating vascular pain to be induced using the same
ActiveUS10821119B2Relieve painOrganic active ingredientsInorganic non-active ingredientsTitratable acidPharmacology
Provided is a rocuronium preparation designed to reduce vascular pain to be induced. The rocuronium preparation contains rocuronium and a buffer solution, and has titratable acidity of 100 mEg or less. The buffer solution may be an acetate buffer solution, a citrate buffer solution, a formate buffer solution, a tartrate buffer solution, a phosphate buffer solution, a glycine-hydrochloric acid buffer solution, or a citric acid-phosphate buffer solution.
Owner:MARUISHI PHARMACEUTICAL CO LTD
Rocuronium bromide injection solution-filled prefilled syringe
ActiveUS10342808B2Keep for a long timeOrganic active ingredientsAmpoule syringesButyl rubberPrefilled Syringe
A rocuronium bromide injection solution-prefilled syringe of the present invention includes a syringe comprising an outer cylinder made of synthetic resin, a gasket which is accommodated inside the outer cylinder and liquid-tightly slidable inside the outer cylinder, and a seal member for sealing a distal end opening of the outer cylinder; and a rocuronium bromide injection solution filled inside the syringe. The gasket is formed by vulcanized chlorinated butyl rubber added calcined clay, and said calcined clay is only added as an inorganic filler classified as an inorganic reinforcing agent and an inorganic filling agent.
Owner:TERUMO KK
Rocuronium bromide-containing injection
ActiveCN102949339BReduce hydrolysisReduce hydrolysis reactionOrganic active ingredientsMuscular disorderBULK ACTIVE INGREDIENTBuffer solution
Owner:TIANJIN CHASE SUN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com