Stable rocuronium injection preparation and preparation method thereof
A technology of rocuronium bromide and injection, which is applied in directions such as medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
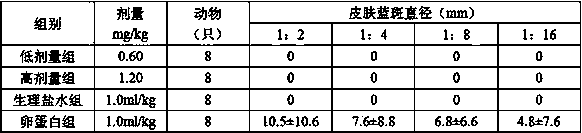
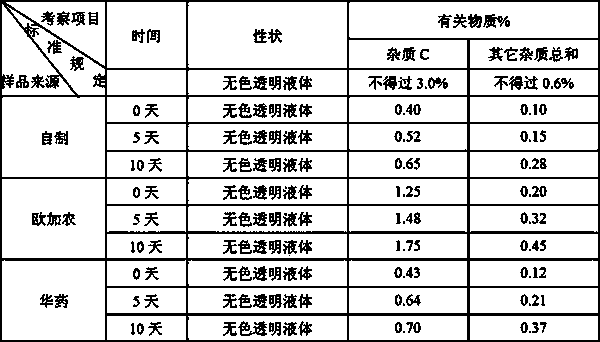
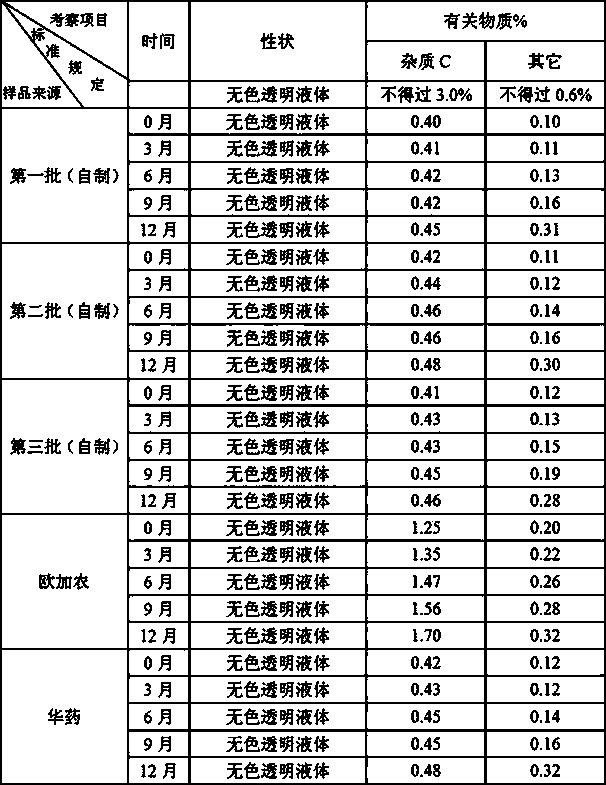
[0139] Implementation 1: Accurately measure the citric acid-sodium dihydrogen phosphate buffer solution with a pH of 3.7, add an appropriate amount of water for injection, and mix well; add sodium chloride, stir or shake to completely dissolve; add rocuronium bromide, and stir Or shake until completely dissolved, adjust the pH to 3.7 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide; then add 0.1% (w / v) activated carbon for needles, stir at room temperature for 30min, and coarsely filter; quantitatively, after 0.22 Sterilize by microporous membrane filtration; filling, 2.5ml or 5ml per bottle, fully pressed rubber stopper, crimping cap; 121°C autoclaving for 12 minutes; inspection, printing, packaging, and storage in the refrigerator at 2-8°C .
Embodiment 2
[0140] Implementation 2: Accurately measure the citric acid-trisodium citrate buffer solution with a pH of 3.8, add an appropriate amount of water for injection, and mix well; add sodium chloride, stir or shake to completely dissolve; add rocuronium bromide, stir or After shaking until completely dissolved, adjust the pH to 4.0 with 1 mol / L citric acid or 1 mol / L sodium hydroxide; then add 0.05% (w / v) activated carbon for needles, stir at room temperature for 30 min, and coarsely filter; quantitatively, after 0.22 Sterilize by microporous membrane filtration; filling, 2.5ml or 5ml per bottle, fully pressed rubber stopper, rolling cap; 121℃ autoclaving for 20 minutes; inspection, printing, packaging, and storage in the refrigerator at 2-8℃ .
Embodiment 3
[0141]Implementation 3: Accurately measure the phosphoric acid-sodium dihydrogen phosphate buffer solution with a pH of 4.0, add an appropriate amount of water for injection, and mix well; add sodium chloride, stir or shake to dissolve completely; add rocuronium bromide, stir or shake After shaking until completely dissolved, adjust the pH to 4.3 with 1mol / L phosphoric acid or 1mol / L sodium hydroxide; then add 0.15% (w / v) activated carbon for needles, stir at room temperature for 30min, and filter coarsely; quantify to 50ml; Sterilize by microporous membrane filtration; filling, 2.5ml or 5ml per bottle, fully pressed rubber stopper, crimping cap; 121°C autoclaving for 15 minutes; inspection, printing, packaging, and storage in a refrigerator at 2-8°C .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com