Patents
Literature
40 results about "Rocuronium Bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
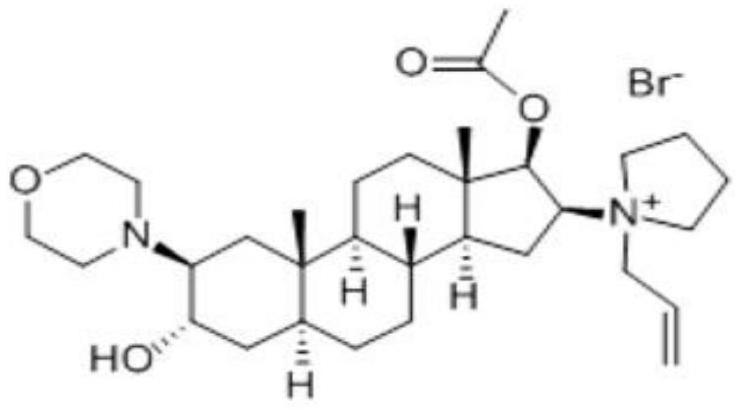
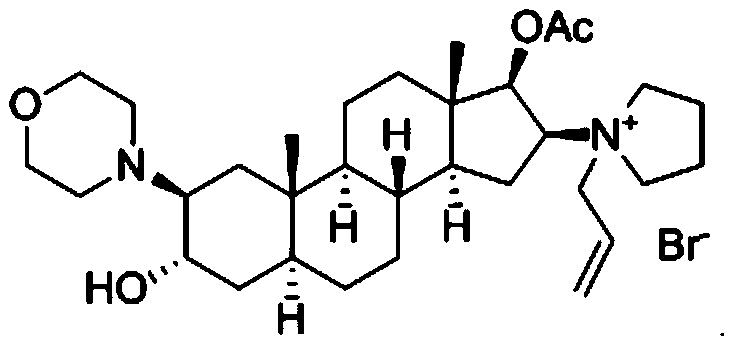
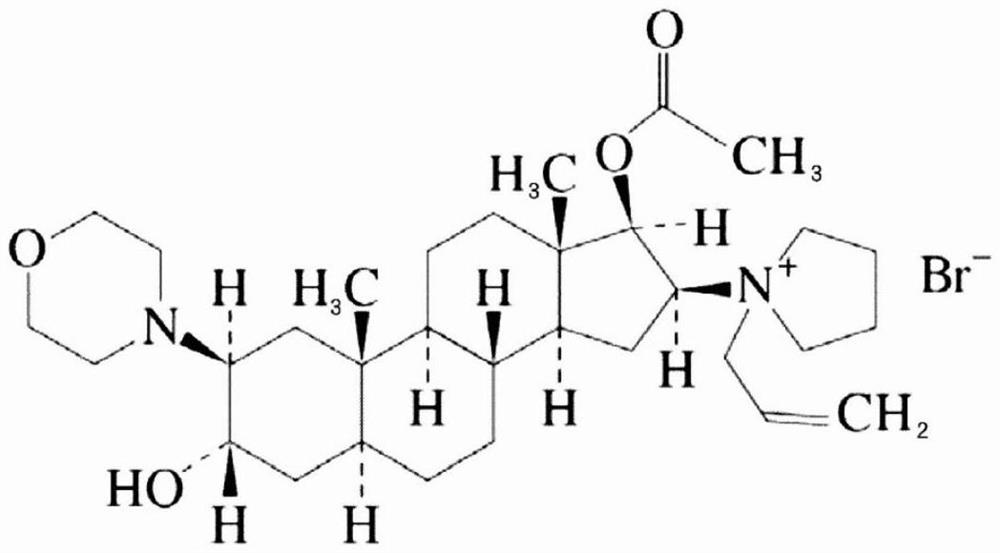
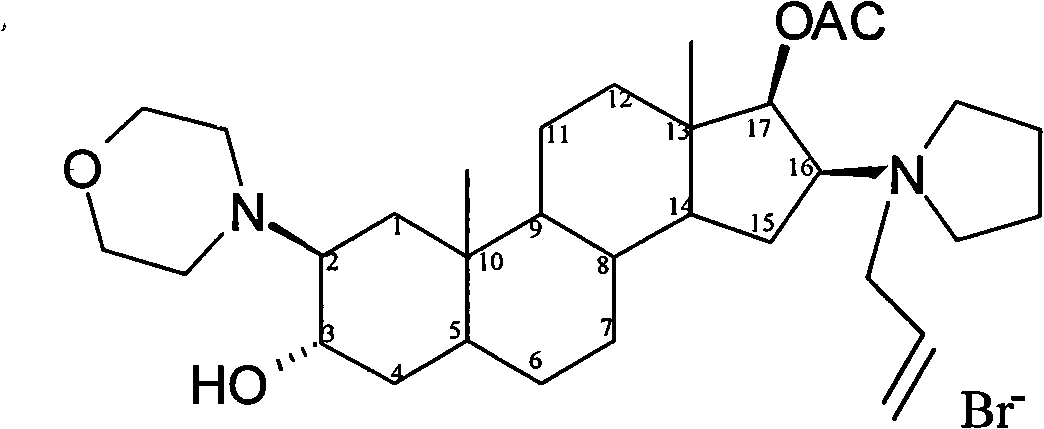
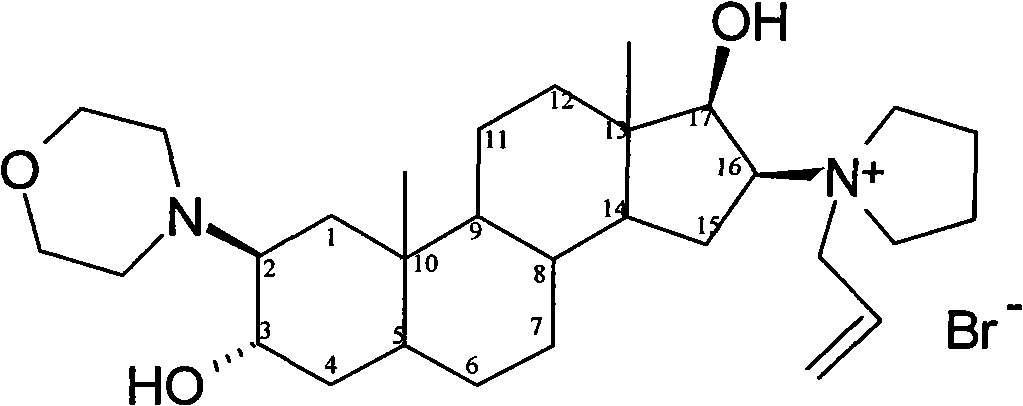
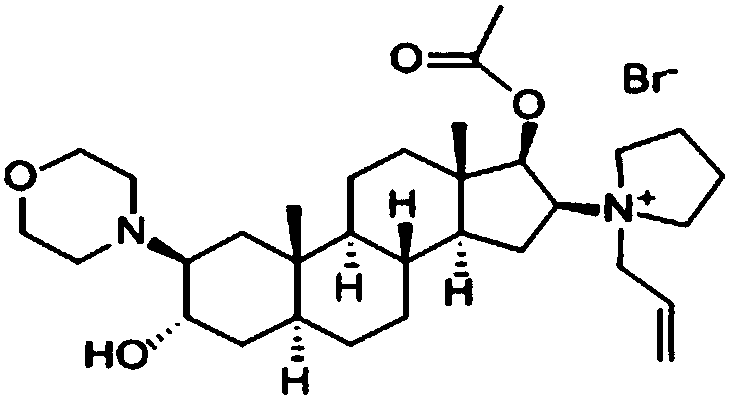
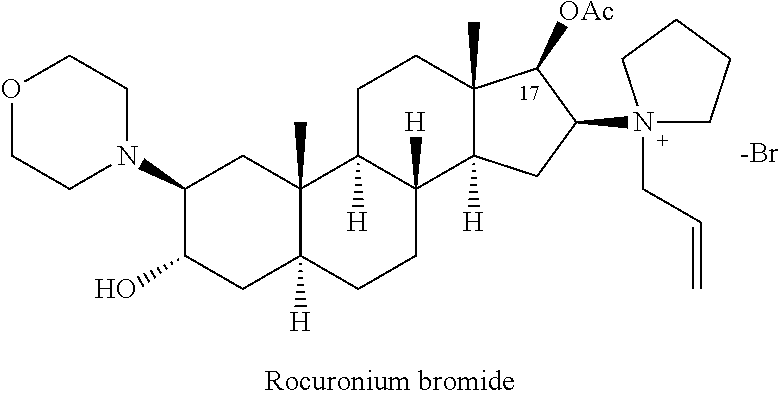
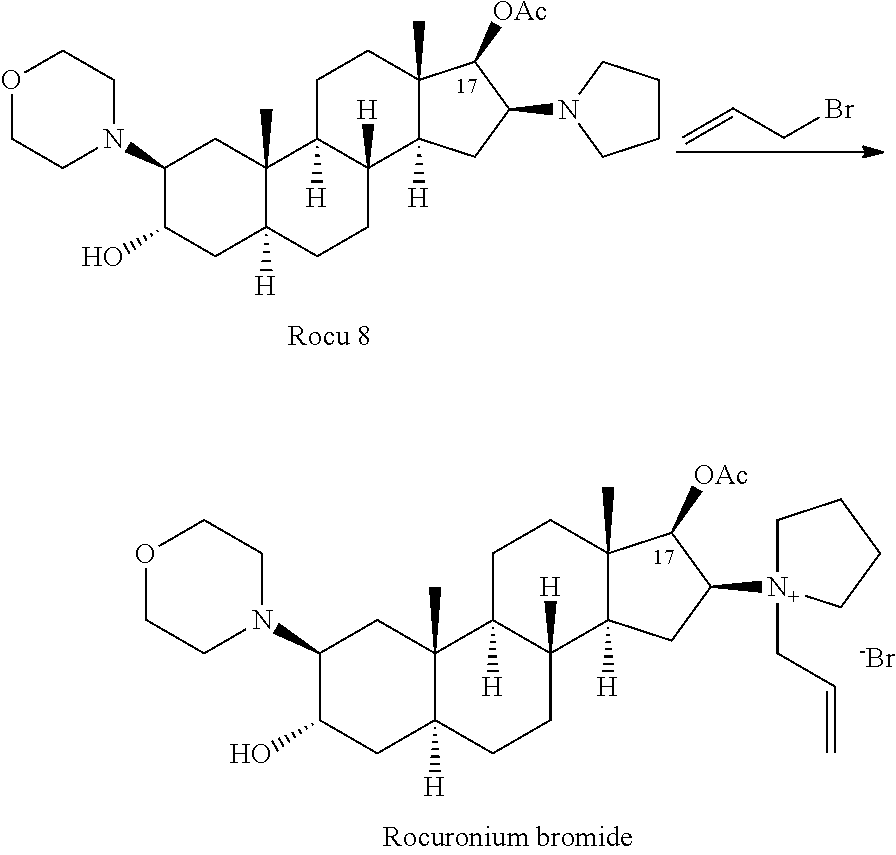
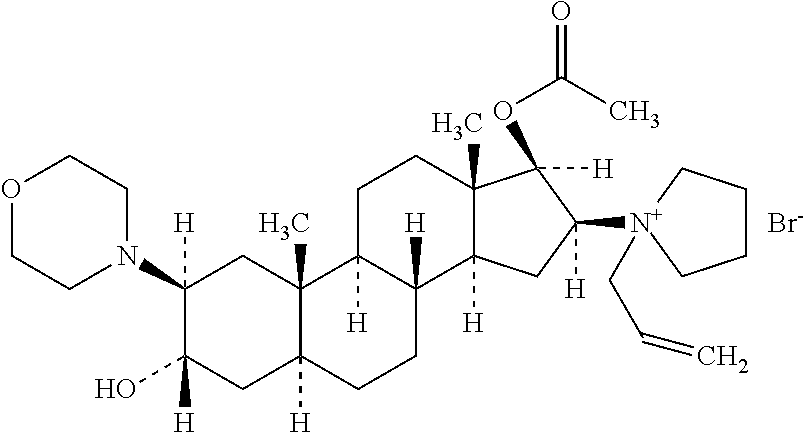
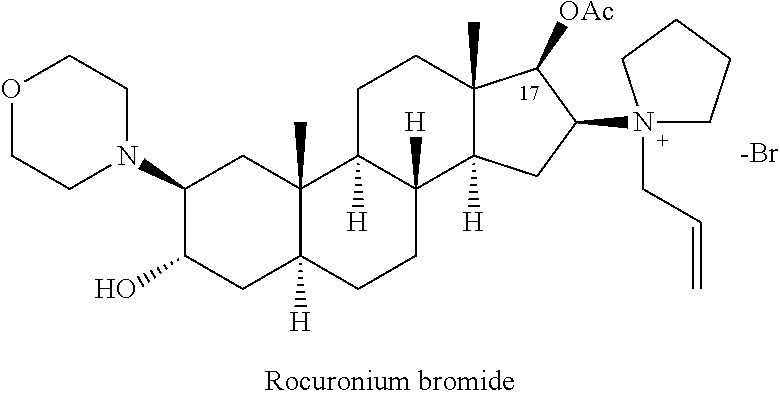
The bromide salt form of rocuronium, an intermediate-acting quaternary aminosteroid with muscle relaxant property. Rocuronium bromide competitively binds to the nicotinic receptor at the motor end plate, and antagonizes acetylcholine binding, which results in skeletal muscle relaxation and paralysis.
Synthetic method of bromamines muscle relaxant
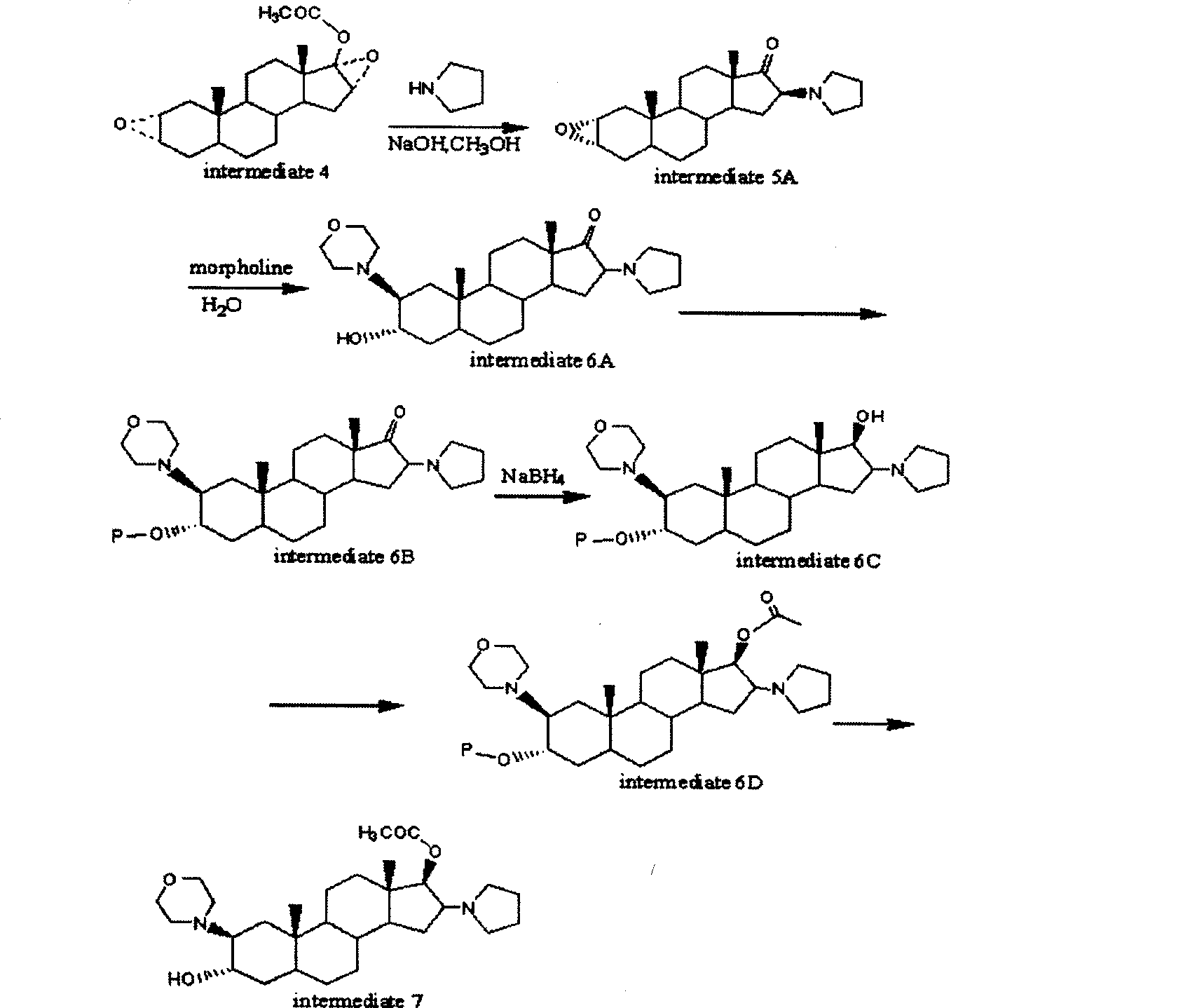
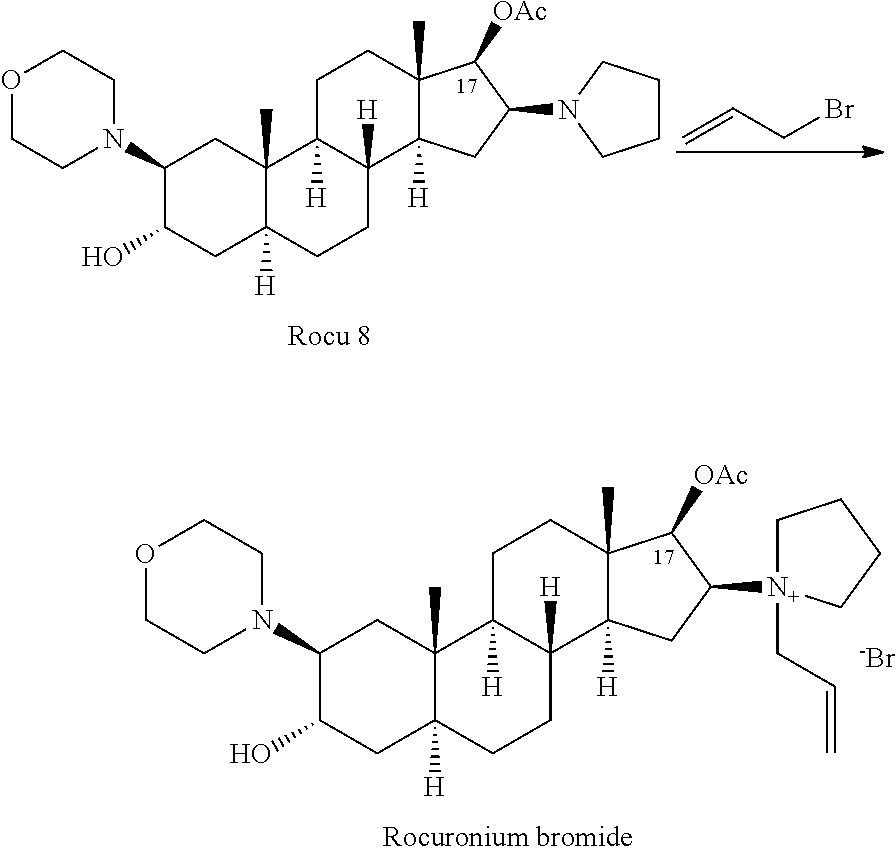
The invention provides a bromide type muscle relaxant, which mainly comprises a method for synthesizing rocuronium bromide, vecuronium bromide, pancuronium bromide and pipecuronium bromide , and is mainly characterized by adopting a ketene as an acylating agent during the process of transforming 5Alpha-androstane -2-alkene-17-alkone to 17-acetoxyl group-5Alpha- androstane-2, and 16-diene, adopting a stress kettle as a reactor when realizing the step of ring-opening and condensation of the 2 Alpha and 3 Alpha-epoxy compound, and adding a solid catalyst and a desiccant in the last step of operation of rocuronium bromide to greatly improve the reaction yield and the quality of products. The technology provided by the invention can shorten the synthesis time of the bromide type muscle relaxant, simplify the operation steps, improve the quality of products and reduce the production cost.
Owner:王加旺
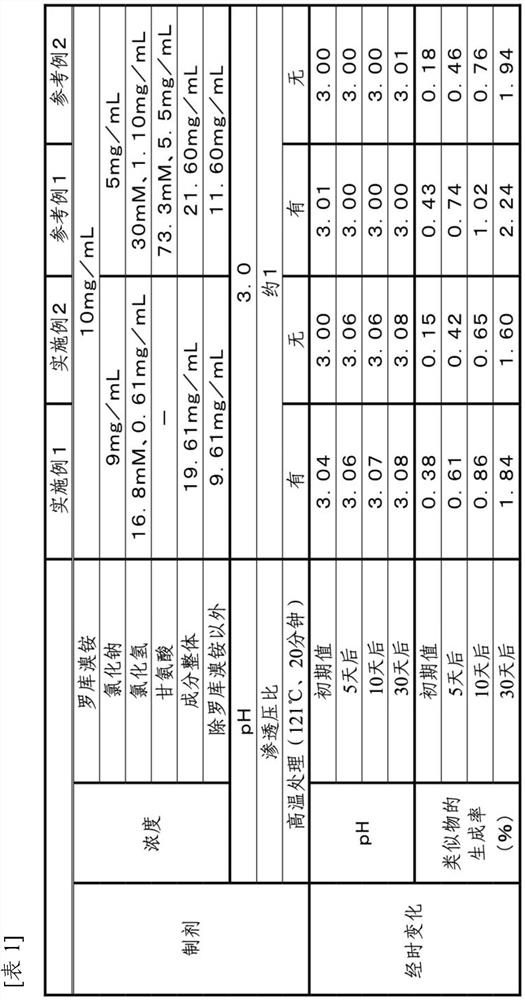
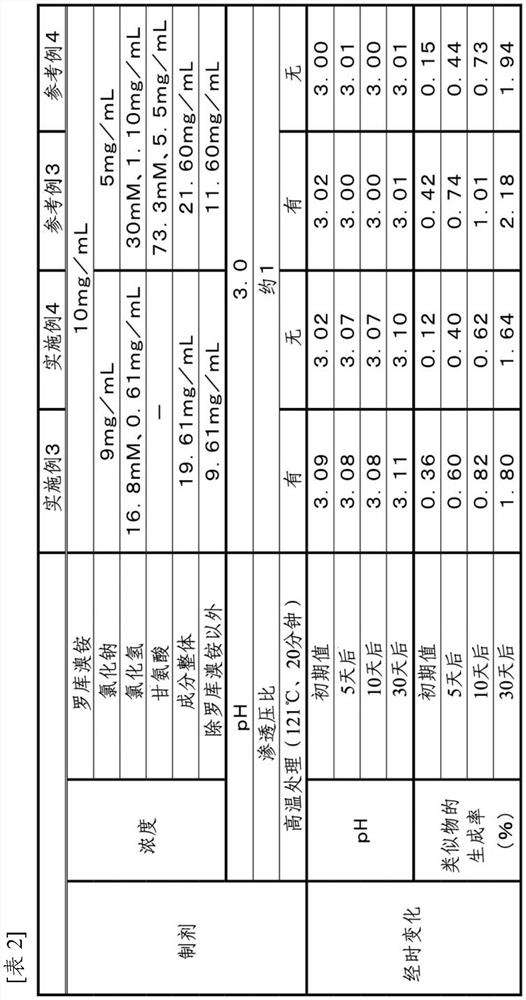
Stable rocuronium bromide composition for injection
InactiveCN101653412AReduce hydrolysisReduce generationOrganic active ingredientsMuscular disorderControllabilityHydrolysis
The invention provides a stable rocuronium bromide composition for vein, which contains rocuronium bromide with treating effective dose, EDTA-2Na-Ca or EDTA-2Na and a pH value buffer system. The EDTA-2Na-Ca or the EDTA-2Na delays the hydrolysis of a rocuronium bromide 17-bit ester bond of the composition in the processes of preparation and storage; and on one hand, medicine liquid can resist the high temperature in the processes of hot press and sterilization, on the other hand, the content of rocuronium bromide deacetyl impurities in the composition in the process of storage is reduced. The invention also provides a reasonable method for preparing the medicine liquid, which can reduce the generation of the rocuronium bromide hydrolysis impurities in the process of preparation. The reduction of the rocuronium bromide hydrolysis impurities improves the quality controllability of the composition and ensures the safety and effectiveness of clinical use.
Owner:尹双保 +2
Novel method for preparing rocuronium bromide
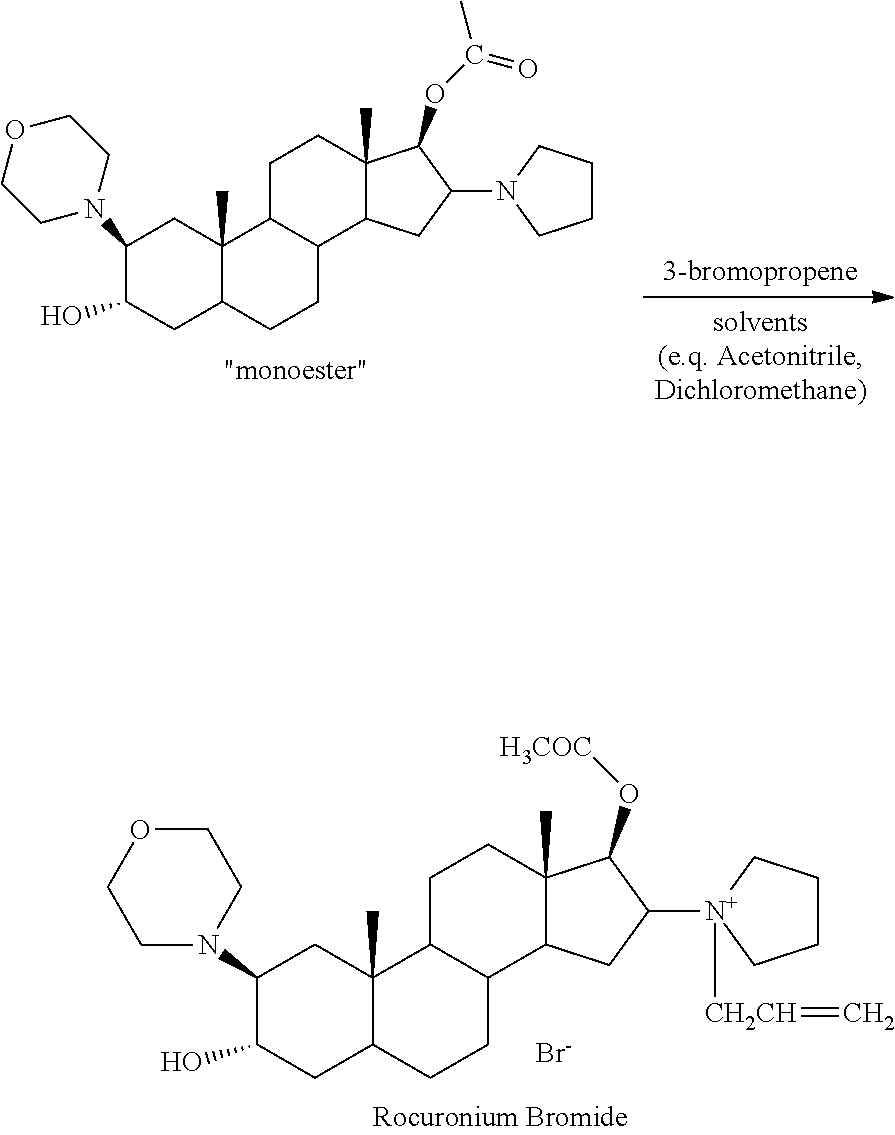
The invention relates to a novel method for preparing rocuronium bromide 1-[17beta-acetoxyl-3alpha-hydroxyl-2beta-(4-morpholinyl)-androstane-16beta-yl]-1-(2-propenyl) pyrrole bromide, the problem of chemoselectivity of pyrrolidine open epoxy in an original line is solved, generation of byproducts is avoided, reaction yield is greatly improved, the production cost is reduced, column chromatography separation is avoided, and aftertreatment purification is implemented easily.
Owner:JIANGSU QINGJIANG PHARMA
Method for preparing rocuronium crystalline hydrate
The invention relates to a preparation method of bulk drugs, in particular to a preparation method of rocuronium bromide crystalline hydrate, which comprises the following steps of: preparing the rocuronium bromide aqueous solution with 2-15 percent of weight volume percent concentration, freezing the aqueous solution to ice under -50-30 DEG C, decompressing and heating up to 30-50 DEG C under vacuum state, keeping 10-20 hours of temperature-rise period and preserving heat for 10-30 hours in vacuum at the temperature. The rocuronium bromide crystalline hydrate prepared by the method enhances the stability of the rocuronium bromide bulk drugs, improves the particle exterior of the rocuronium bromide and effectively reduces the excessive organic residue of the rocuronium bromide bulk drugs.
Owner:重庆人本药物研发有限责任公司
Preparation method of high-purity rocuronium bromide
The invention provides a preparation method of high-purity rocuronium bromide. The preparation method comprises the steps of enabling a rocuronium bromide crude product to be dissolved in acetone water solution, filtering, vacuum-concentrating, adding normal heptane for homogenizing, pulping and the like. The provided preparation method of the high-purity rocuronium bromide has many advantages ofextremely low solvent residual quantity, extremely high HPLC purity, extremely low related substance content, extremely high stability and the like.
Owner:江苏盈科生物制药有限公司
Processes for the synthesis of rocuronium bromide
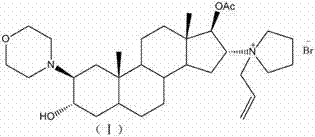
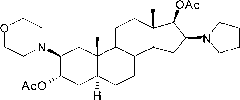
The invention encompasses processes for synthesizing 1-[17β-acetyloxy-3α-hydroxy-2β-(4-morpholinyl)-5α-androstan-16β-yl]-1-(2-propenyl)pyrrolidinium bromide (rocuronium bromide) and intermediates thereof.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
Method for purifying rocuronium bromide
Provided is a method for purifying rocuronium bromide, which comprises: formulating crude rocuronium bromide to be purified into an aqueous solution, distilling off excess residue solvents at reduced pressure, absorbing by adding active carbon or silica gel, then filtrating, quick freezing the filtrate into ice, and then lyophilizing to obtain rocuronium bromide.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for preparing rocuronium bromide midbody compound crystal
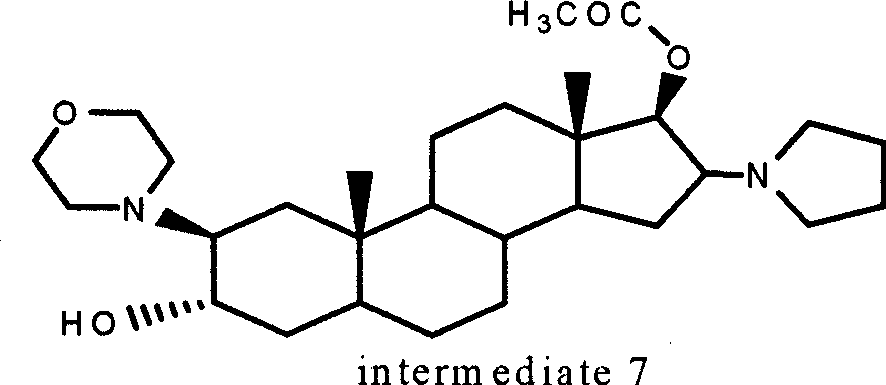
The invention discloses a method for preparing rocuronium bromide midbody compound crystal, which is characterized in that compound 2beta-(4-morpholinyl)-16beta-(1-pyrrolidyl)-5alpha-androstane-3alpha, 17beta-glycol is adopted as raw material to be proceeded with acylation reaction and then to be refined to obtain crystal powder compound 2beta-(4 - morpholinyl)-16beta-(1 - pyrrolidinyl)-5alpha-Androstane-3alpha, 17beta-diol - 2 acetate with high purity. With the method, the fallow crystal powder compound with high purity can be prepared, and conditions are created for synthesizing high-quality rocuronium bromide.
Owner:ZHEJIANG XIANJU PHARMA
Preparation method for rocuronium bromide key intermediate 2alpha, 3alpha-epoxy-16beta-(1-pyrrolidyl)-5alpha- androstane-17 hydroxy
The invention belongs to the field of pharmaceutical chemistry synthesis, and relates to a preparation method for a key intermediate 2alpha, 3alpha-epoxy-16beta-(1-pyrrolidyl)-5alpha- androstane-17 hydroxy of rocuronium bromide of steroid muscle relaxant, which includes the steps: leading inexpensive and available 5alpha-androstane-2-alkene-17 ketone and isopropenyl acetate to undergo ester exchange to generate cresyl violet acetate, oxidizing the cresyl violet acetate by the meta-chloroperoxybenzoic acid to obtain an epoxy compound, carrying out an open-loop substitution reaction between the meta-chloroperoxybenzoic acid and pyrrolidine under the alkaline condition, and reducing via sodium borohydride so that the rocuronium bromide key intermediate 2alpha, 3alpha-epoxy-16beta-(1-pyrrolidyl)-5alpha-androstane-17 hydroxy is prepared. An ester group with large steric hindrance is generated at the 17th position when the 5alpha-androstane-2-alkene-17 ketone and the isopropenyl acetate undergo ester exchange in the process to lead stereoscopic steric hindrance of a whole steroid ring to be large, so that attack on the lower portion of the steroid ring can be performed when epoxidation is carried out, and further stereoselectivity is high when an epoxide is formed, and then a beta configuration can be directly formed when the pyrrolidine is used for substitution. The synthesis method is good in stereoselectivity.
Owner:LIANYUNGANG GUIKE PHARMA
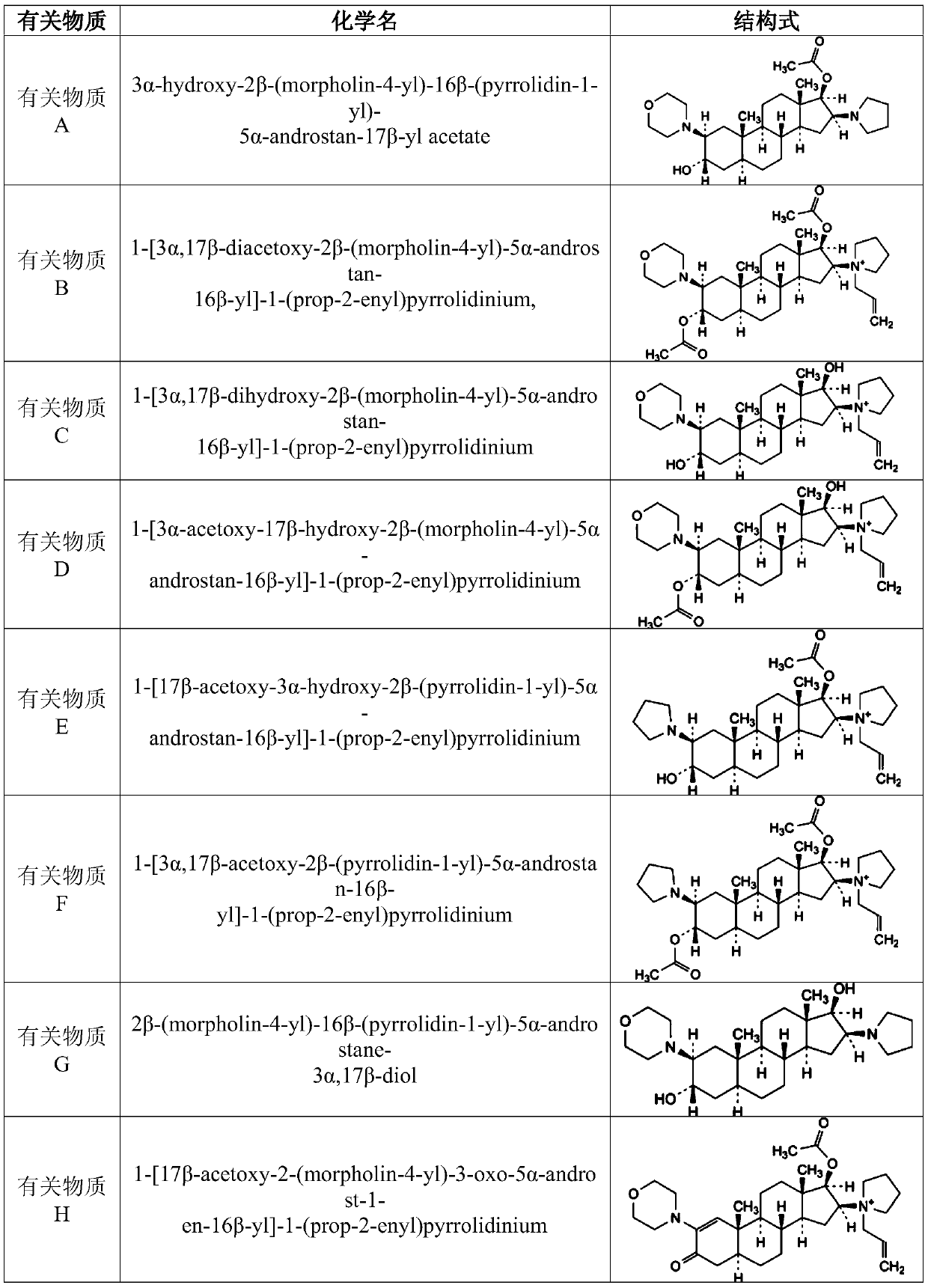
Rocuronium bromide enantiomeric impurity, or salt thereof and method for preparing rocuronium bromide enantiomeric impurity or salt
InactiveCN106810589AShort synthetic routeMild reaction conditionsOrganic chemistry methodsSteroidsEnantiomerDiastereomer
The invention discloses a rocuronium bromide enantiomeric impurity, a salt, a solvate, an enantiomer or a diastereoisomer of the rocuronium bromide enantiomeric impurity and a method for preparing the same. The rocuronium bromide enantiomeric impurity is shown as a formula V. An R1 and an R2 in the formula V are independently selected from H and acetyl; an R3 is selected from N-pyrrolidyl and N-allyl pyrrolidyl. The invention further provides application of the rocuronium bromide enantiomeric impurity to detecting the quality of rocuronium bromide intermediates, crude drugs or preparations. The rocuronium bromide enantiomeric impurity, the salt, the solvate, the enantiomer or the diastereoisomer, the method and the application have the advantages that gaps in the aspect of research on rocuronium bromide impurities can be filled, and foundations and bases can be provided for controlling the quality of rocuronium bromide materials or drugs.
Owner:CHENGDU SINO STRONG PHARMA
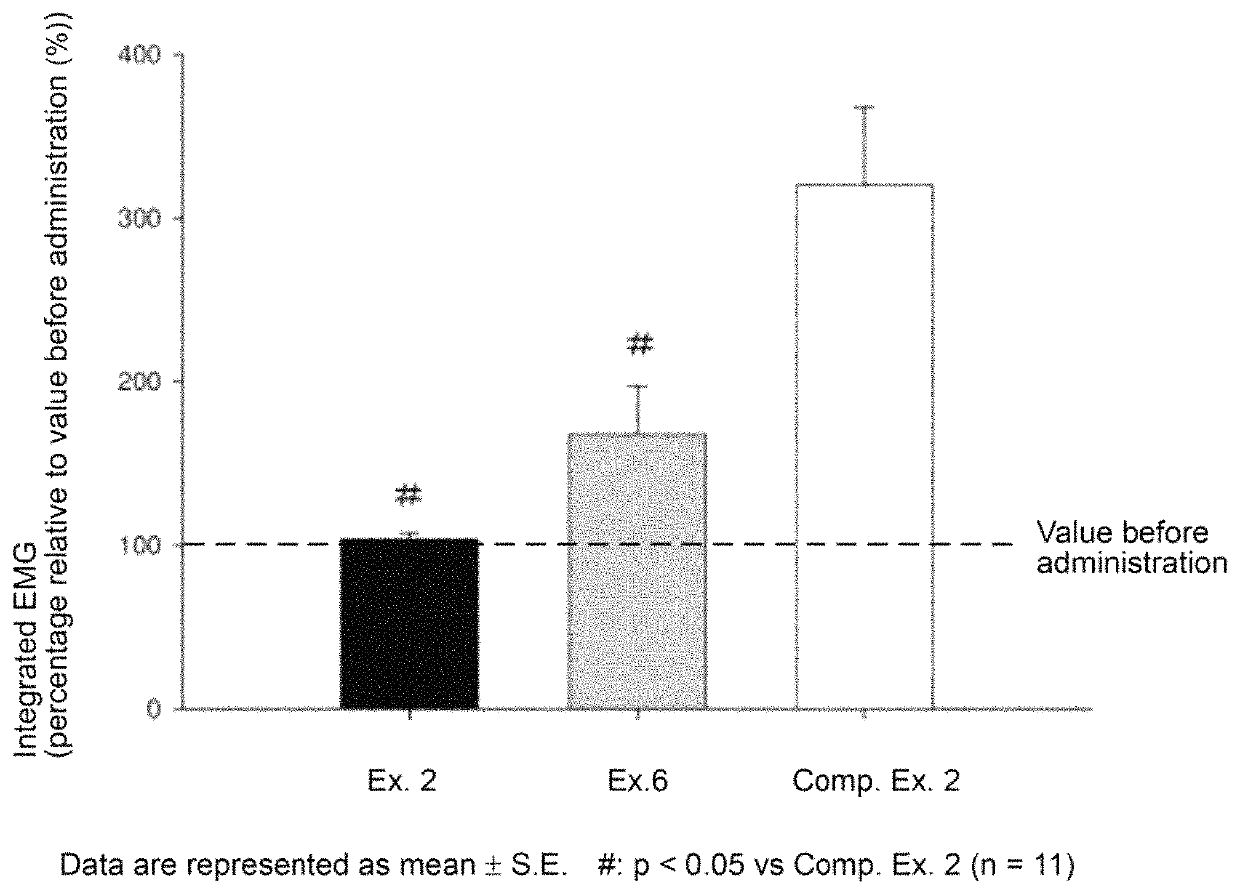
Rocuronium preparation with improved stability
ActiveUS10869876B2Improve stabilityChange controlOrganic active ingredientsInorganic non-active ingredientsGlycinePotassium hydrogen phthalate
Owner:MARUISHI PHARMACEUTICAL CO LTD
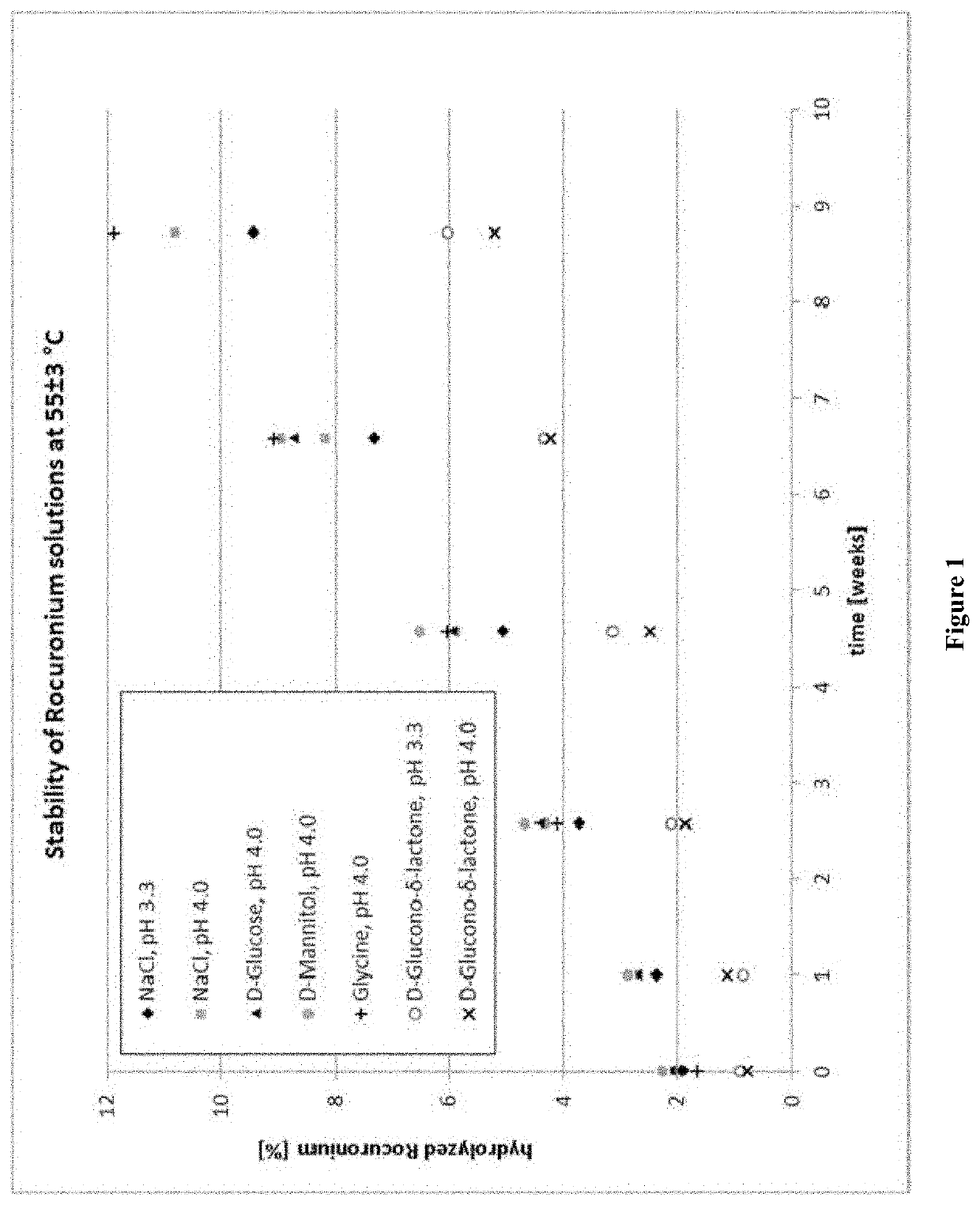
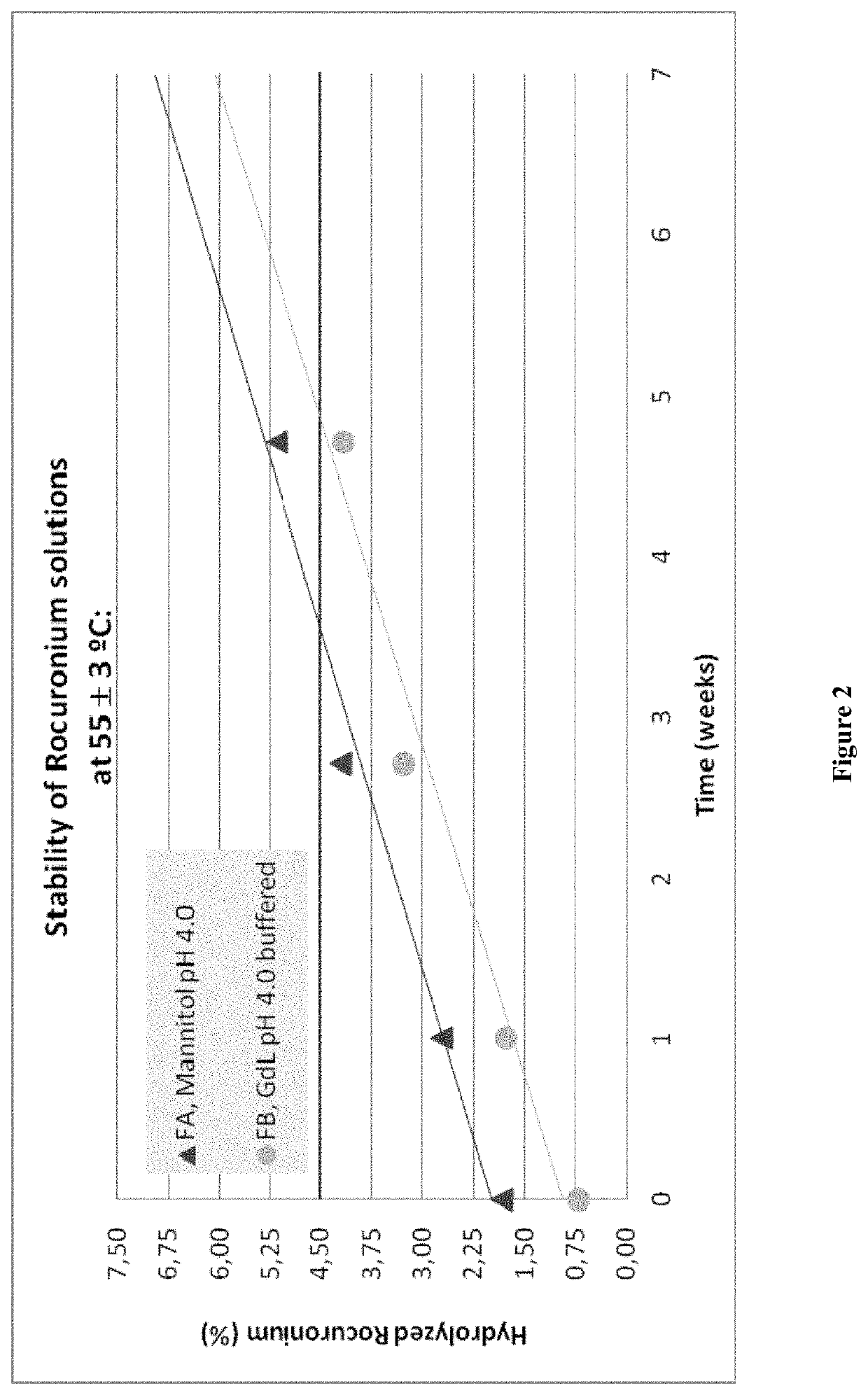
Stabilized Aqueous Compositions of Neuromuscular Blocking Agents
PendingUS20200000925A1Organic active ingredientsNervous disorderGluconic acidPharmaceutical Substances
The present invention relates to an aqueous composition comprising:(i) a steroidal neuromuscular blocking agent that is a Rocuronium salt, further wherein the steroidal neuromuscular blocking agent is present at a concentration ranging from 5 mg / mL to 20 mg / mL based on the total volume of the aqueous composition; and(ii) an excipient that includes D-gluconic acid, D-glucono-delta-lactone, or a combination thereof, where the steroidal neuromuscular blocking agent and the excipient are dissolved in a solution consisting of water.Furthermore the present invention relates to a liquid pharmaceutical composition comprising or consisting of said aqueous composition and a method for producing said aqueous composition.
Owner:B BRAUN MELSUNGEN AG
Method for purifying rocuronium bromide
Provided is a method for purifying rocuronium bromide, which comprises: formulating crude rocuronium bromide to be purified into an aqueous solution, distilling off excess residue solvents at reduced pressure, absorbing by adding active carbon or silica gel, then filtrating, quick freezing the filtrate into ice, and then lyophilizing to obtain rocuronium bromide.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof
ActiveCN113563352AExcellent relaxationExcellent effect Reversal of relaxationOrganic active ingredientsOrganic chemistrySulfonateAntagonism
The invention belongs to the technical field of biological medicine, and relates to a water-soluble cucurbit [8] urea sulfonate derivative serving as a neuromuscular blocker antagonist as well as a preparation method and application thereof. The water-soluble cucurbit [8] urea sulfonate derivative is obtained by taking cucurbit [8] urea as a raw material and introducing a water-soluble side chain group through a chemical reaction; a hydrophobic cavity of the water-soluble cucurbit [8] urea sulfonate derivative can be combined with neuromuscular blockers including cis-asatracurium besylate, rocuronium bromide, vecuronium bromide and pantocuronium bromide in a water phase through interaction of hydrophobic agents, so that antagonism is achieved. Experiments show that the water-soluble cucurbit [8] uril derivative can efficiently antagonize various neuromuscular blockers in a living body, is high in universality and low in biotoxicity, and is an ideal neuromuscular blocker antagonist.
Owner:FUDAN UNIV
A kind of preparation method of rocuronium bromide
Owner:武汉华龙生物制药有限公司
A kind of preparation method of high-purity rocuronium bromide
The invention provides a preparation method of high-purity rocuronium bromide, which comprises the steps of dissolving crude rocuronium bromide in aqueous acetone, filtering, concentrating under reduced pressure, adding n-heptane for homogenization, beating and the like. The preparation method of the high-purity rocuronium bromide provided by the invention has many advantages such as extremely low solvent residue, extremely high HPLC purity, extremely low content of related substances, and high stability.
Owner:江苏盈科生物制药有限公司
A preparation method of rocuronium bromide and its pharmaceutical composition
ActiveCN108676052BIncrease contentReduce contentOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical drugDiethyl ether
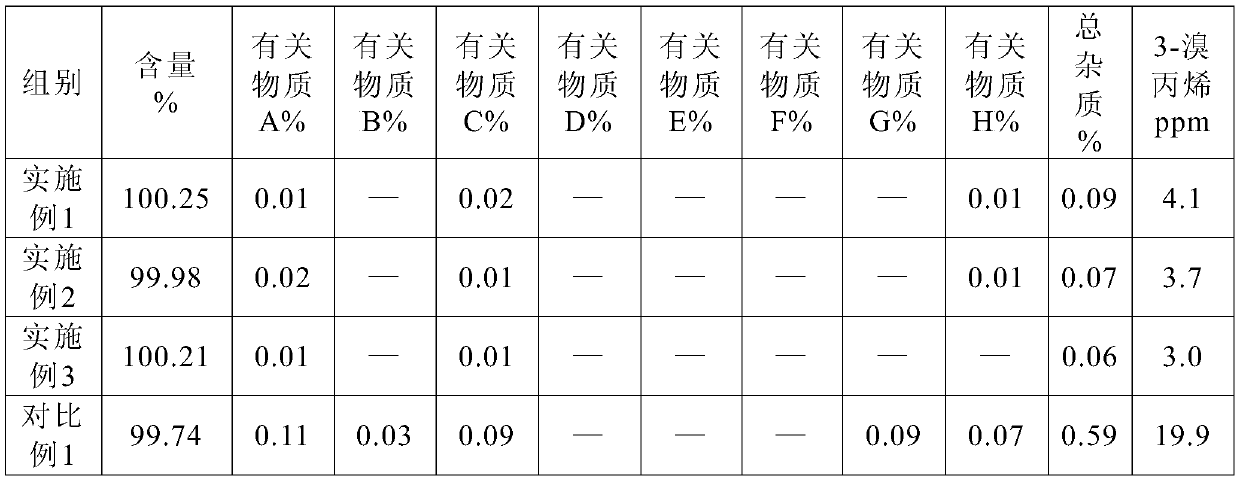
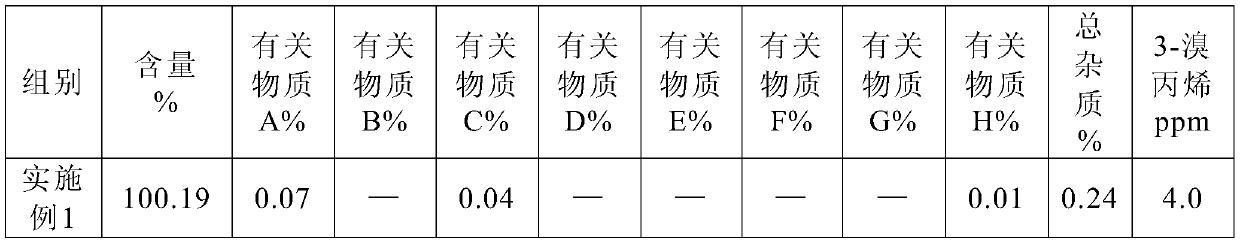
The invention belongs to the technical field of biological medicine, and specifically relates to a preparation method and a pharmaceutical composition of rocuronium bromide. The preparation method ischaracterized by adding trichloromethane and water under a low-temperature condition, adding a certain amount of CO2, and adding diethyl ether for crystallization, thus obtaining a purified rocuroniumbromide raw material. After stability study is carried out on the raw material, increment of the contents of an impurity A and an impurity C of the raw material is less; in addition, the content of 3-bromopropylene (allyl bromide) in the raw material is smaller than 10ppm; an injection solution is prepared from the rocuronium bromide raw material obtained through the preparation method, the injection solution accords with all standards of a pharmacopoeia, and the increment of the impurity A, the impurity C and total impurity is remarkably lower than those in a commercially available product after cold-thermal cycling.
Owner:北京市新里程医药科技有限公司
Preparation method of rocuronium bromide intermediate 5alpha-sterane-2-ene-17-one
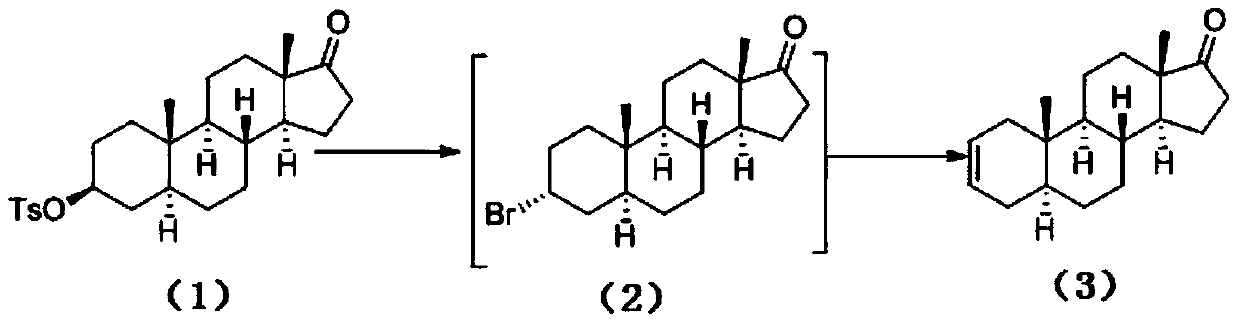
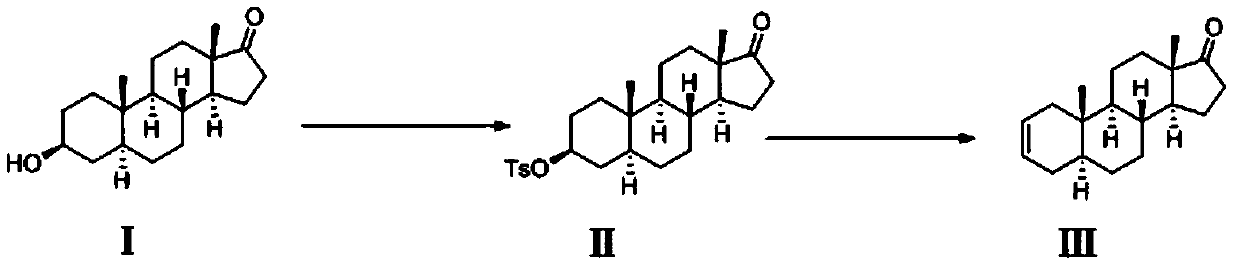
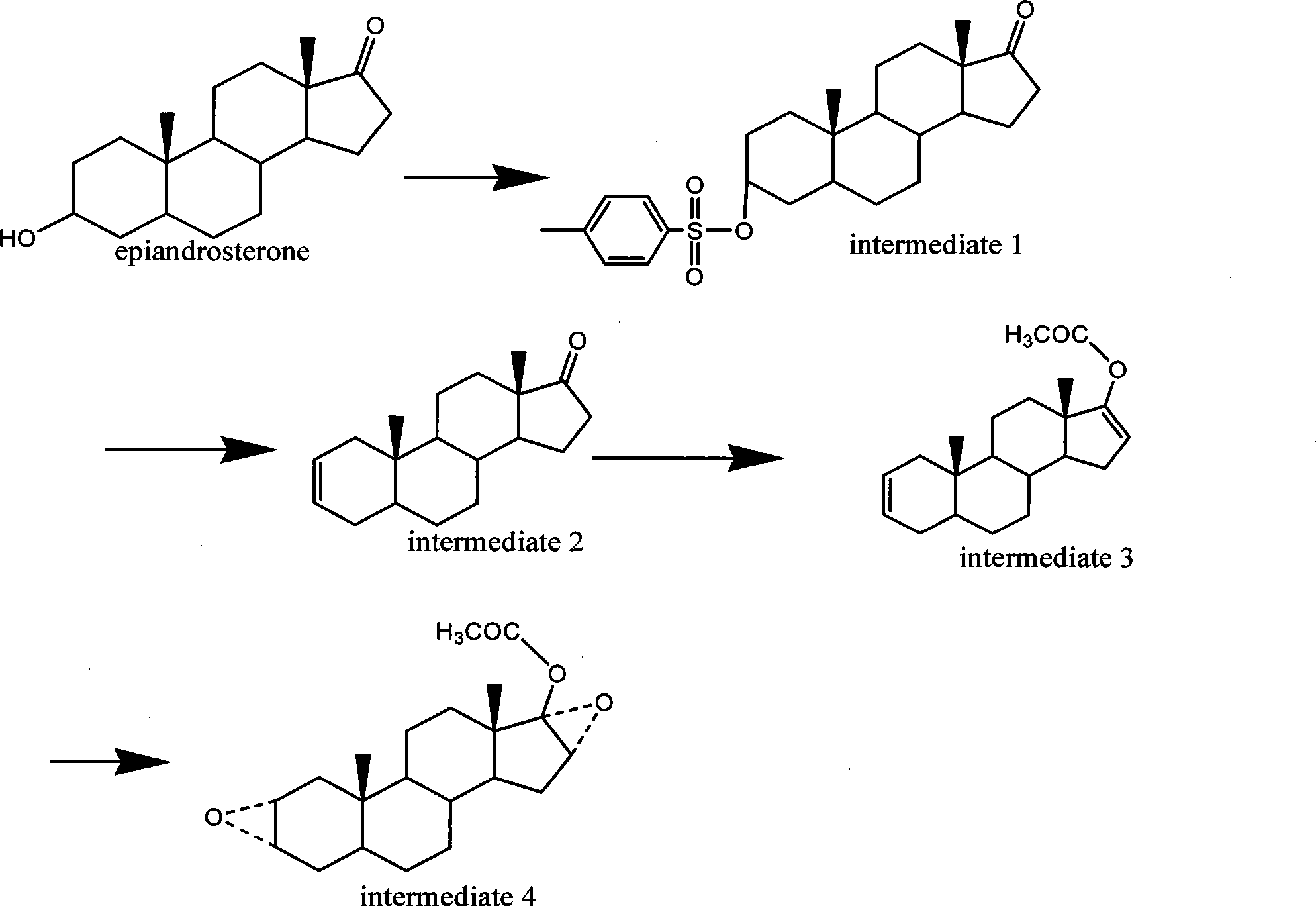
The invention discloses a preparation method of a rocuronium bromide intermediate 5alpha-sterane-2-ene-17-one. The specific steps include: sequentially adding epiandrosterone TS, a solvent and a phasetransfer catalyst into a reaction bottle, performing heating to a reaction temperature of 90-120DEG C, carrying out stirring reaction, and conducting post-treatment purification to obtain the 5alpha-sterane-2-ene-17-one. The method provided by the invention greatly shortens the reaction time, reduces byproducts, enhances the yield, reduces three wastes, lowers the production cost, is suitable forindustrial production, and has obvious social and economic benefits.
Owner:ZENJI RES LAB
Method for purifying crude rocuronium bromide
ActiveUS11466049B2Meet the requirementsMuscular disorderNeuromuscular disorderPhysical chemistryEngineering
Owner:JINAN GOOD MEDICAL TECH CO LTD
Preparation method of rocuronium bromide intermediate
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of a rocuronium bromide intermediate. The preparation method comprises the following steps: by taking epiandrosterone as shown in a formula I as a raw material, carrying out dehydration reaction under the catalysis of an Eaton reagent, and then washing, extracting and concentrating to obtain the rocuronium bromide intermediate (5alpha-androstane-2-ene-17-ketone). According to the preparation method, the reaction temperature is reduced to 40 DEG C, byproducts of high-temperature reaction are reduced, white-like solids can be obtained by simply washing, extracting and concentrating reaction liquid, and main product loss caused when the byproducts are removed through recrystallization is reduced. And the process is simpler, the yield is lower, and the emission of three wastes is less.
Owner:福安药业集团重庆博圣制药有限公司
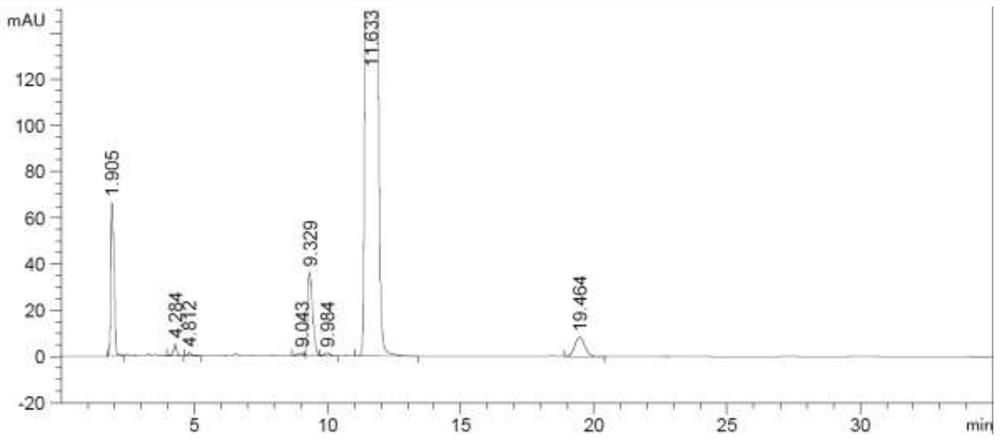
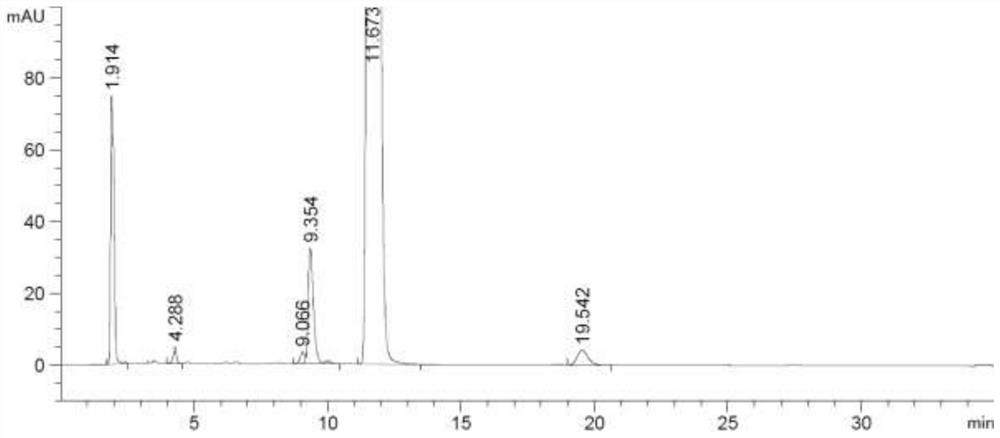
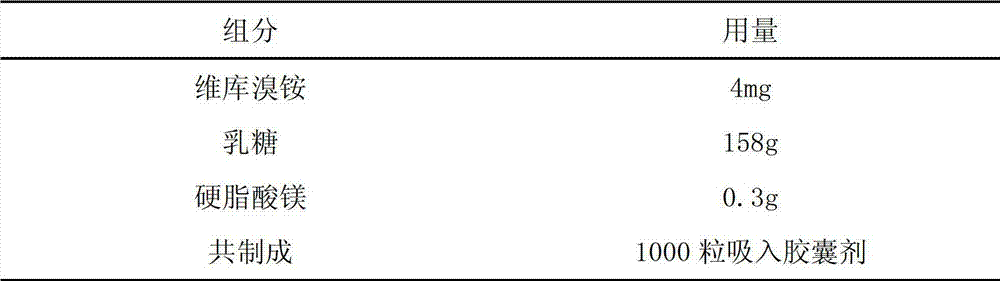
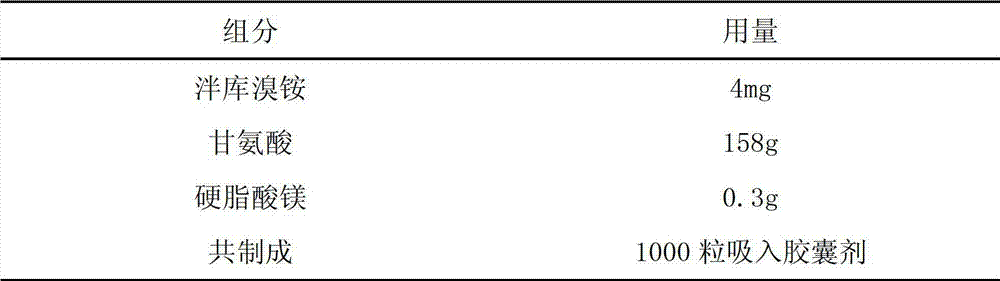
Inhalation preparation for treating asthma
InactiveCN102961366ARich choiceOrganic active ingredientsPharmaceutical delivery mechanismPowder InhalerPharmaceutical medicine
The invention relates to an inhalation preparation for treating asthma. The inhalation preparation is prepared by taking one of otilonium bromide, oxyphenonium bromide, pipecuronium bromide, rocuronium bromide, pancuronium bromide, pinaverium bromide and vecuronium bromide as active ingredient and mixing pharmaceutical-accepted auxiliary materials. The inhalation preparation includes but is not limited to dry powder inhaler, aerosol and spray.
Owner:FOSHAN YIBAOSHENG PHARMA CO LTD
New compound for muscle relaxation antagonism
PendingCN112279937AReduce humidityImprove controllabilityOrganic active ingredientsNervous disorderMuscle relaxationVercuronium
The invention discloses a new compound for muscle relaxation antagonism. The new compounds of theta type, beta type, gamma type, lambda type, kappa type, nu type, tau type, omega type, upsilon type and the like of sugammadex sodium have less hygroscopicity and better storage stability, are more beneficial to quality control of medicines and preparations and the like, and are suitable for preparingspecific binding neuromuscular block antagonists. And the compound can be applied to drugs for antagonizing treatment or prevention of neuromuscular block induced by rocuronium bromide or vecuroniumbromide and the like.
Owner:刘力
A kind of preparation method of rocuronium bromide intermediate
The invention belongs to the technical field of drug synthesis, in particular to a preparation method of a rocuronium bromide intermediate. The preparation method takes the epiandrosterone shown in formula I as a raw material, carries out a dehydration reaction under the catalysis of Eaton reagent, and then obtains a rocuronium bromide intermediate (5α-androst-2-ene-17- after washing, extraction and concentration) ketone). The preparation method reduces the reaction temperature to 40°C, reduces the by-products of the high-temperature reaction, and the reaction solution can be simply washed with water, extracted, and concentrated to obtain an off-white solid, which reduces the loss of the main product caused by the removal of by-products by recrystallization. . The process is simpler, the yield is lower, and the discharge of three wastes is less.
Owner:福安药业集团重庆博圣制药有限公司
Rocuronium bromide formulations
PendingCN114558021APracticalImprove stabilityOrganic active ingredientsInorganic non-active ingredientsBuffering agentMedicinal chemistry
The invention provides a novel rocuronium bromide preparation. The rocuronium bromide preparation contains rocuronium bromide, has a pH of 4 or less, and does not contain a buffering agent.
Owner:MARUISHI PHARMACEUTICAL CO LTD
Rocuronium bromide crystal form
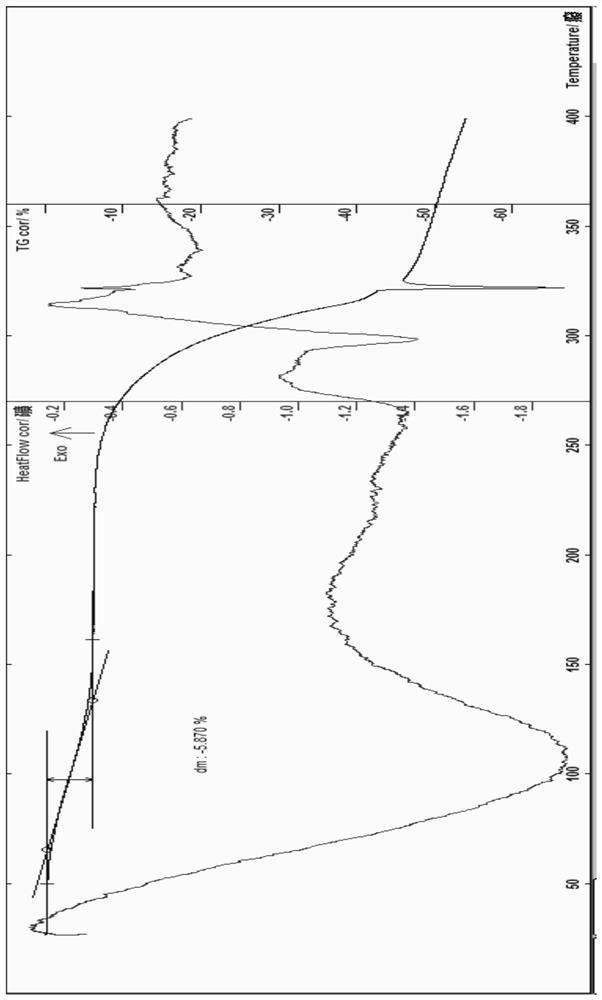
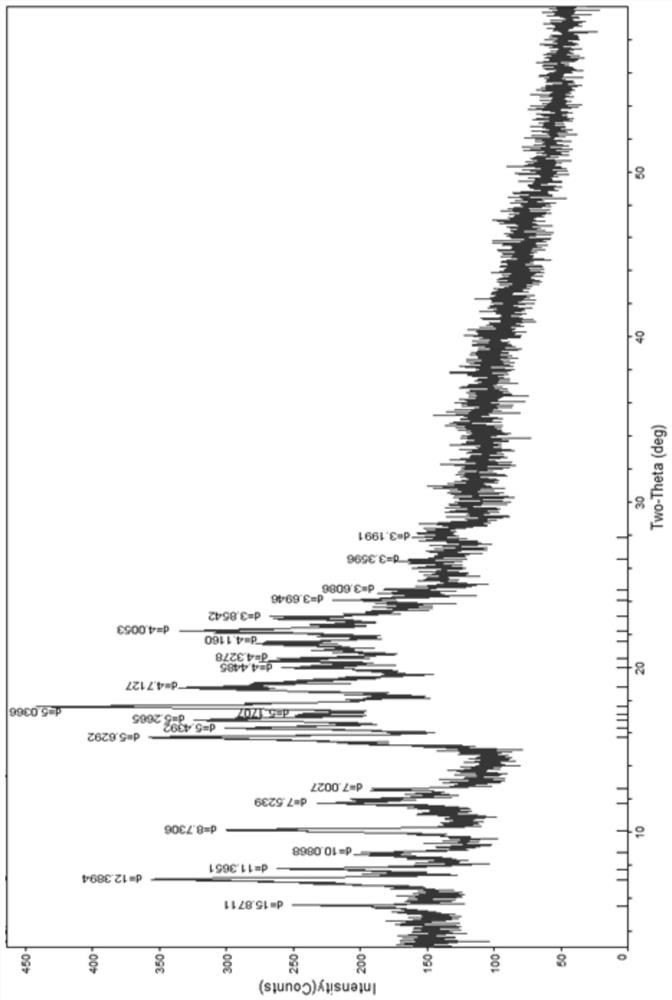
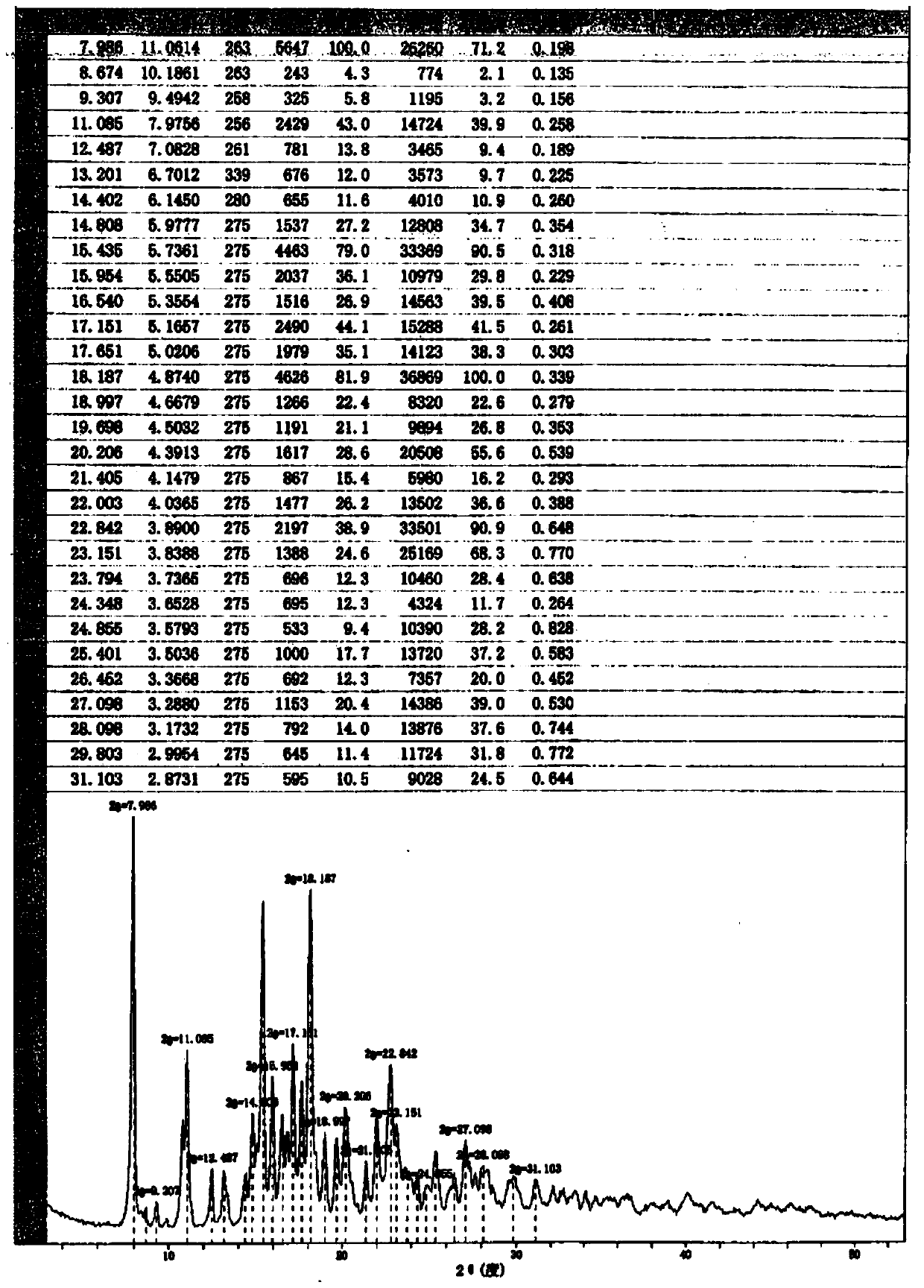
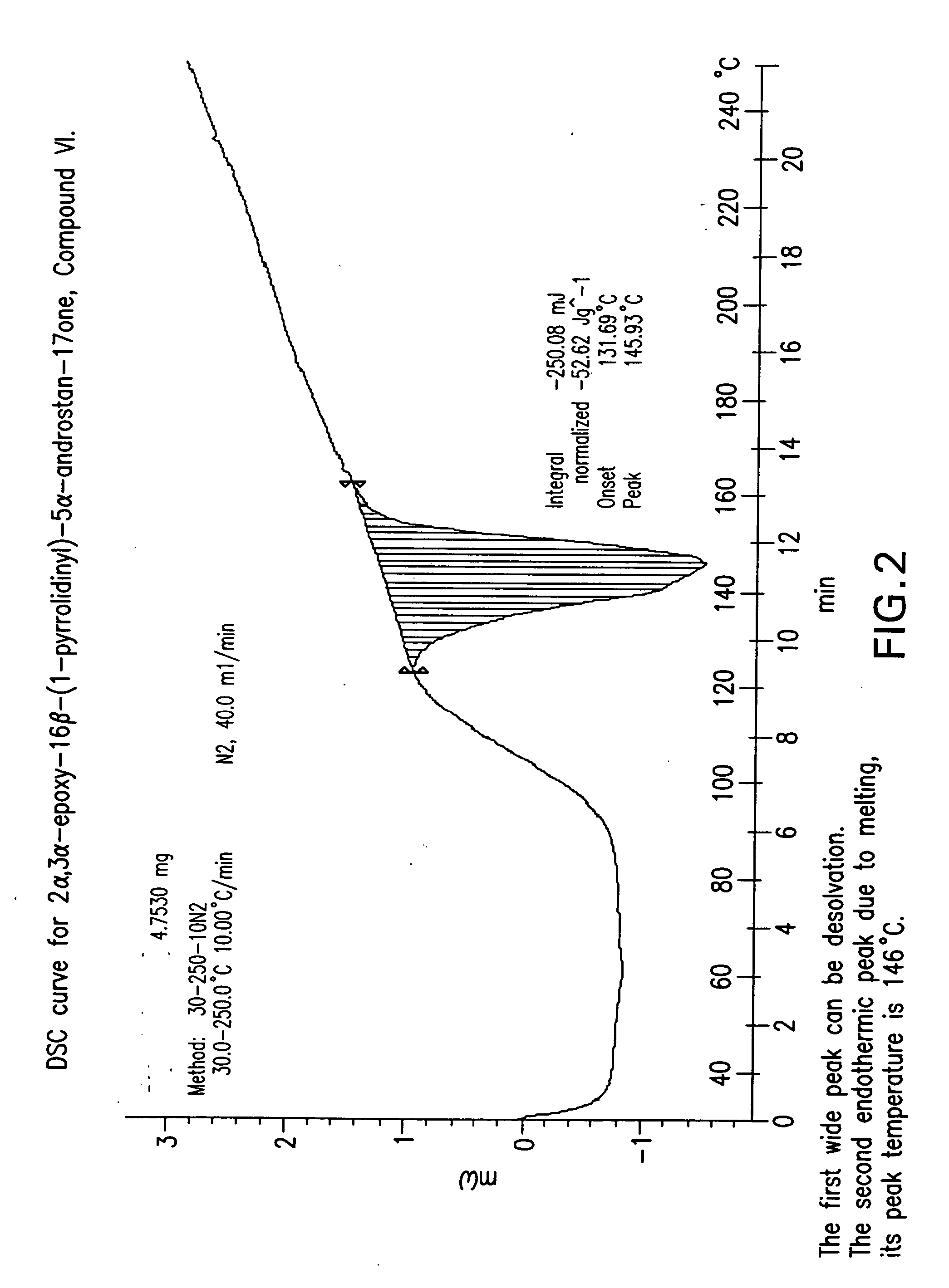
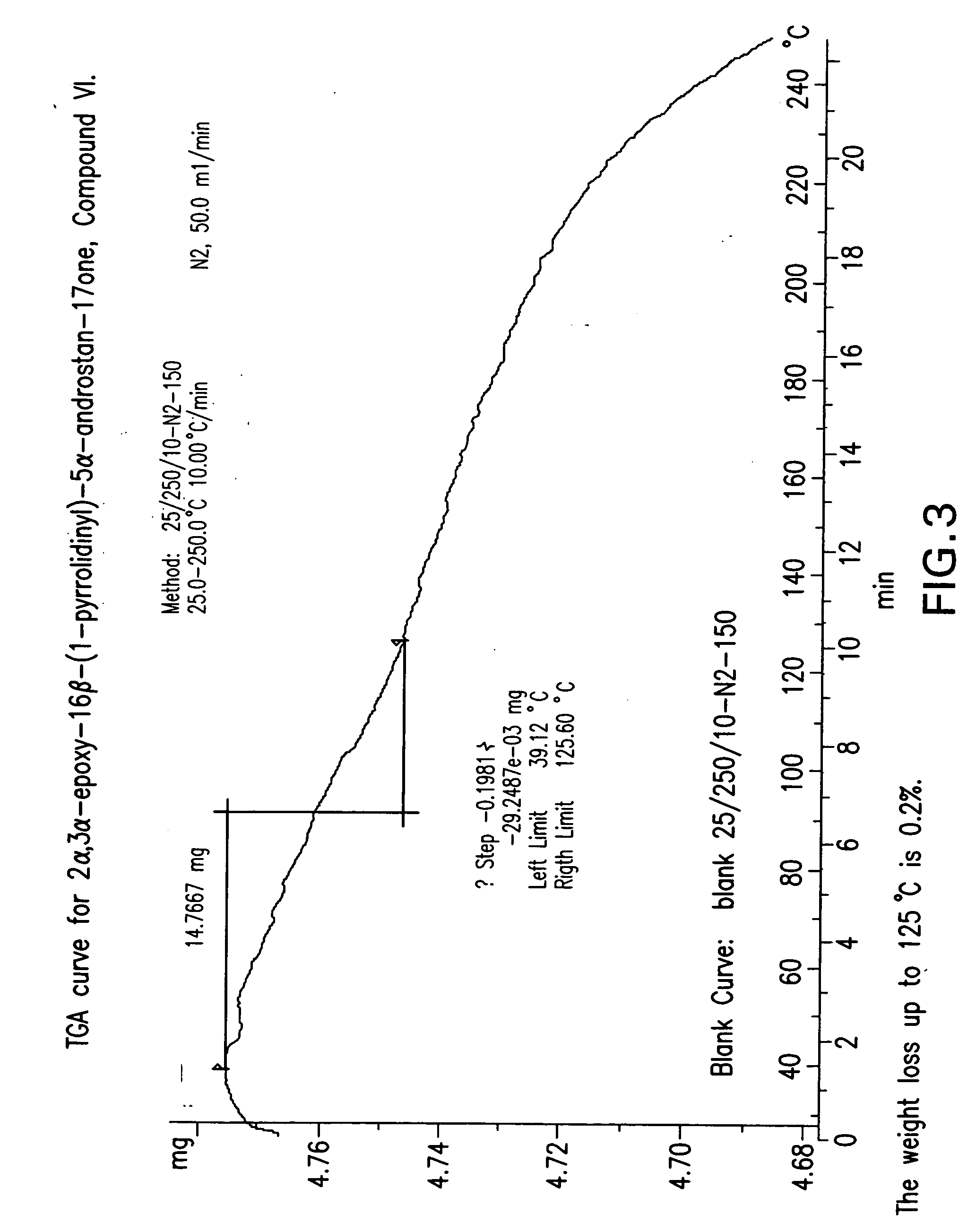
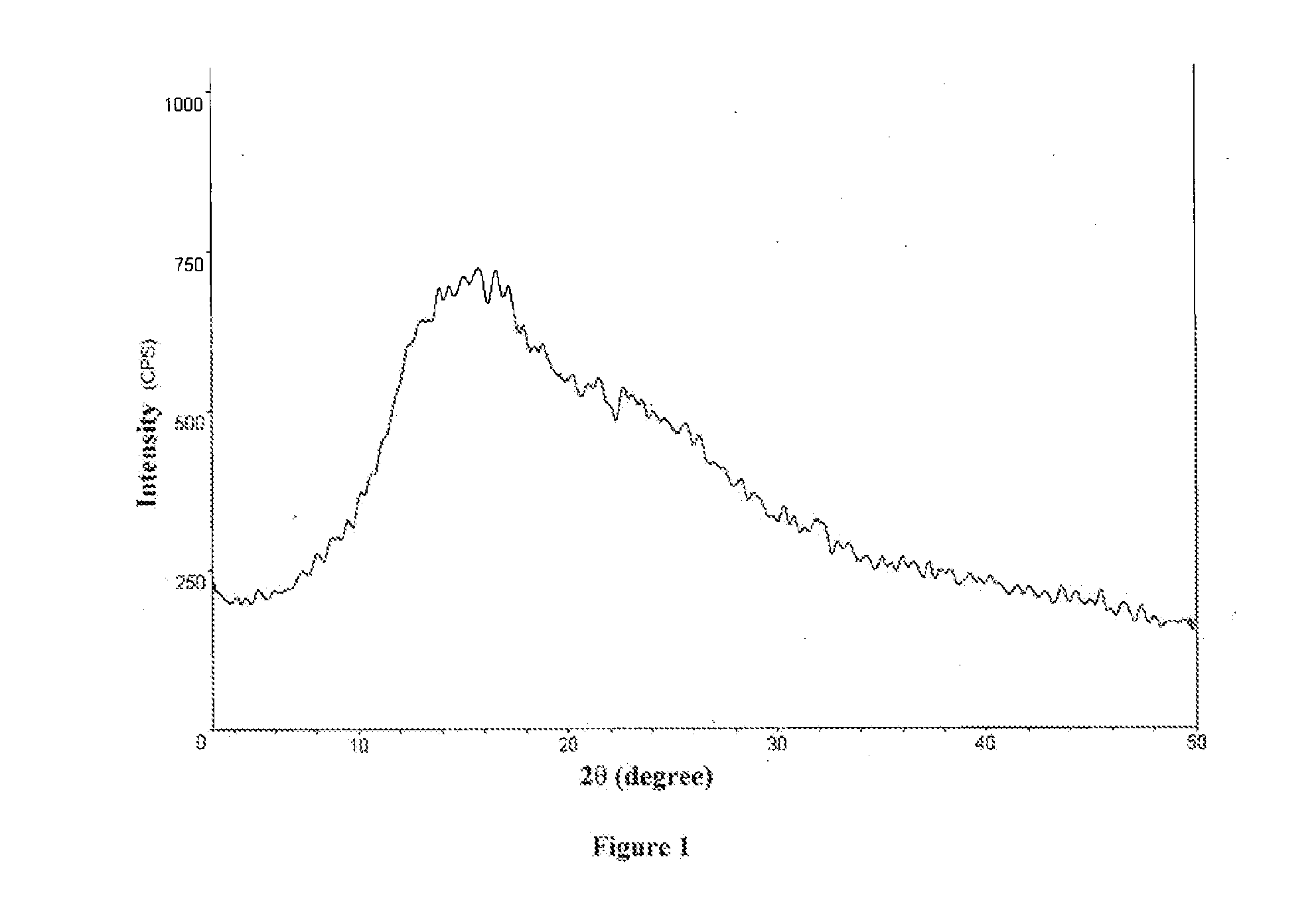
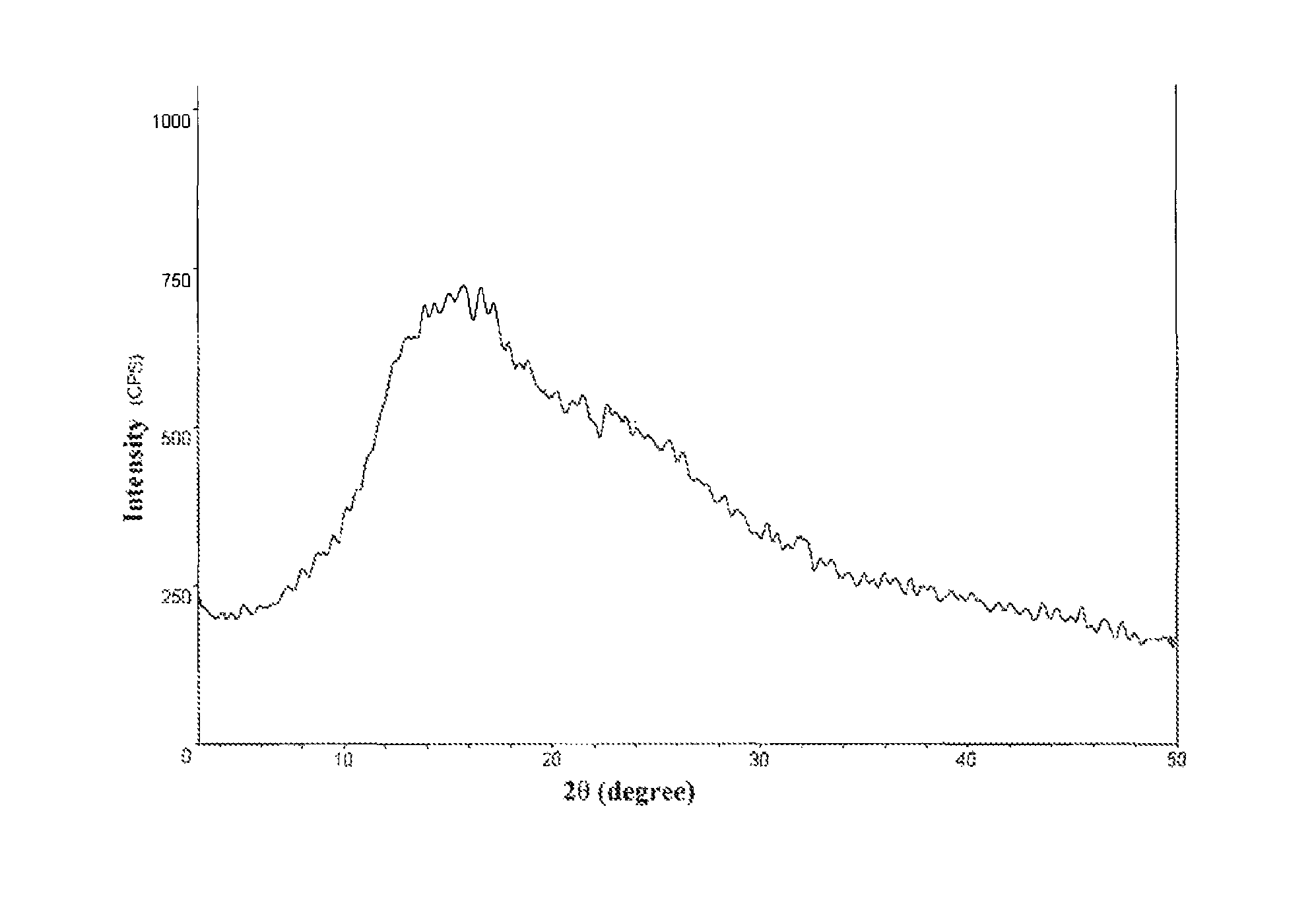
ActiveCN111196835AStability impactSolubility effectOrganic active ingredientsOrganic chemistry methodsPhysical chemistryPowder diffraction
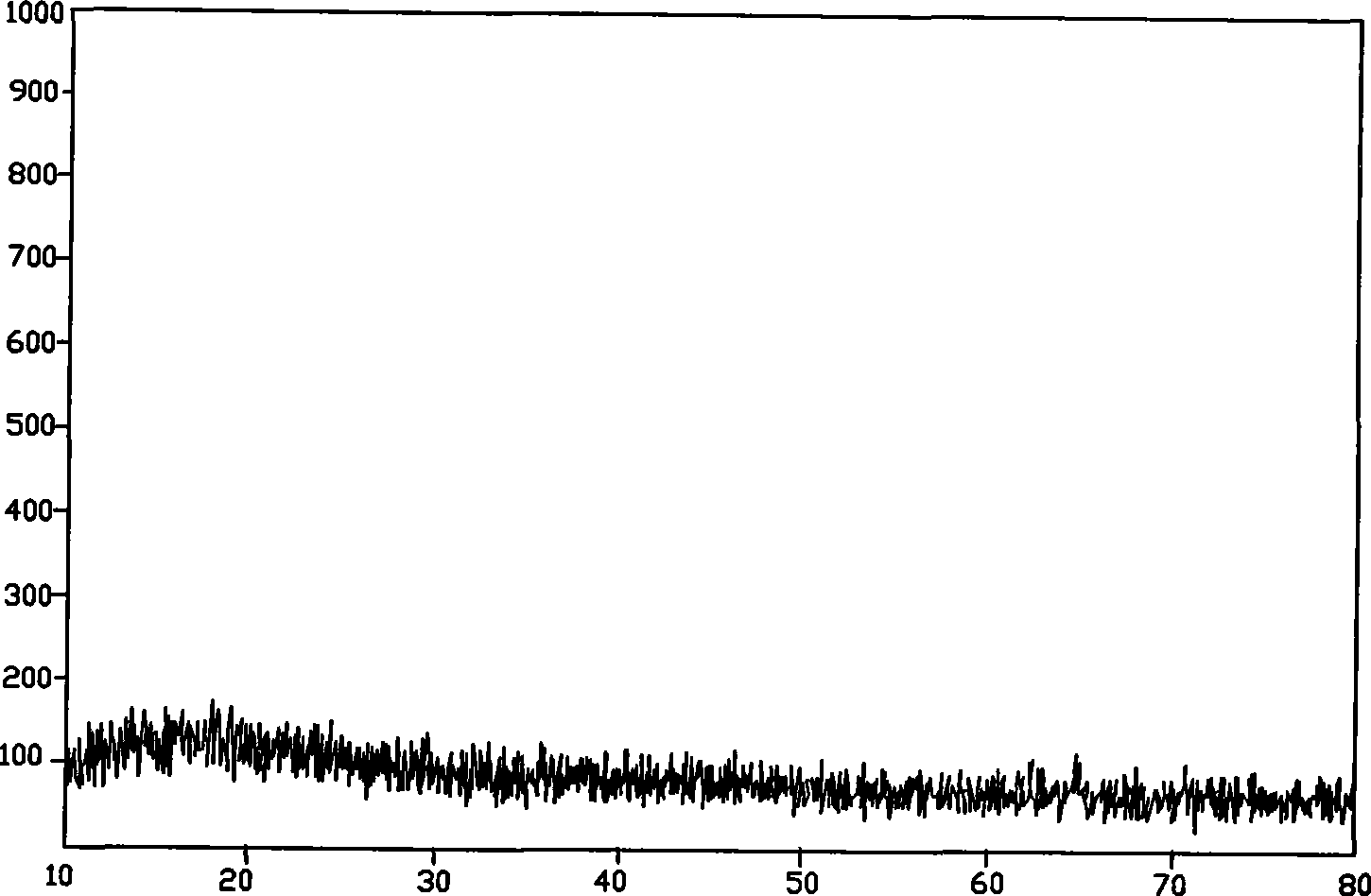
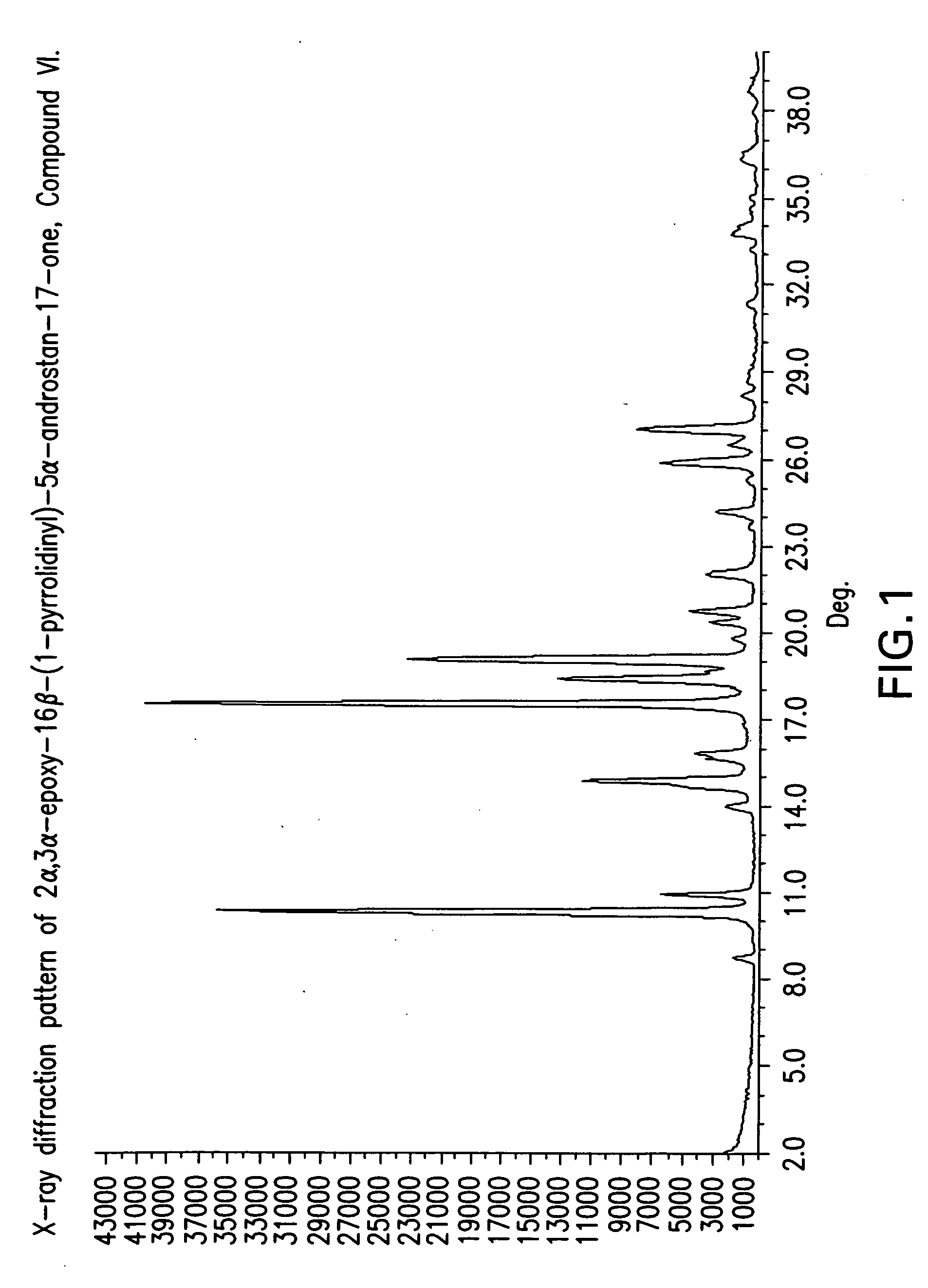
The invention discloses a crystal form A of rocuronium bromide. Cu-Kalpha radiation is used; an X-ray powder diffraction pattern expressed by a 2theta angle has characteristic diffraction peaks at positions of 8.0 + / - 0.2 degrees, 11.1 + / - 0.2 degrees, 15.4 + / - 0.2 degrees, 15.9 + / - 0.2 degrees, 17.1 + / - 0.2 degrees, 18.2 + / - 0.2 degrees, 19.0 + / - 0.2 degrees, 19.6 + / - 0.2 degrees, 20.2 + / - 0.2 degrees, 22.0 + / - 0.2 degrees, 22.8 + / - 0.2 degrees and 25.4 + / - 0.2 degrees. The preparation method of the rocuronium bromide crystal form is simple, and the rocuronium bromide crystal form has good stability and is more beneficial to production, storage and transportation.
Owner:CHENGDU SINO STRONG PHARMA
Processes for the synthesis of rocuronium bromide
The invention encompasses processes for synthesizing 1-[17β-acetyloxy-3α-hydroxy-2β-(4-morpholinyl)-5α-androstan-16β-yl]-1-(2-propenyl)pyrrolidinium bromide (rocuronium bromide) and intermediates thereof.
Owner:MENDEZ JUANA ARACELI +5
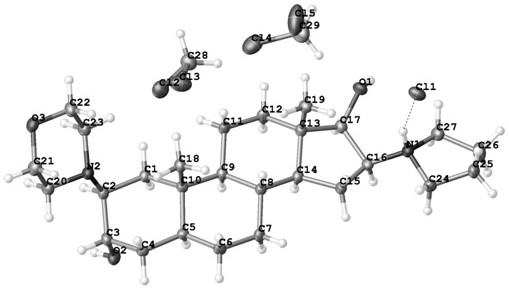
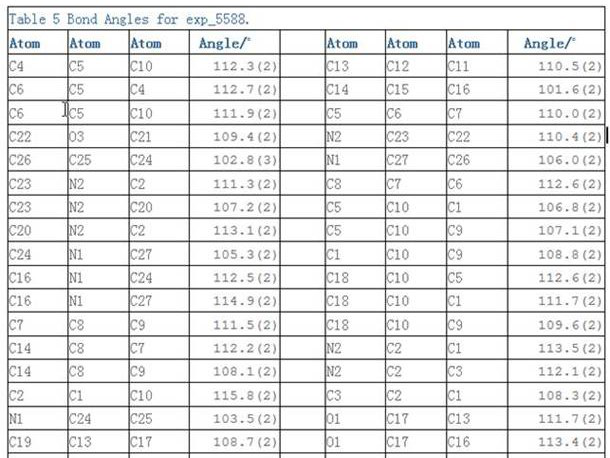
Preparation method of rocuronium bromide starting material (LK-7) single crystal
PendingCN114685594AProof space structurePolycrystalline material growthFrom normal temperature solutionsOrganic solventAbsolute configuration
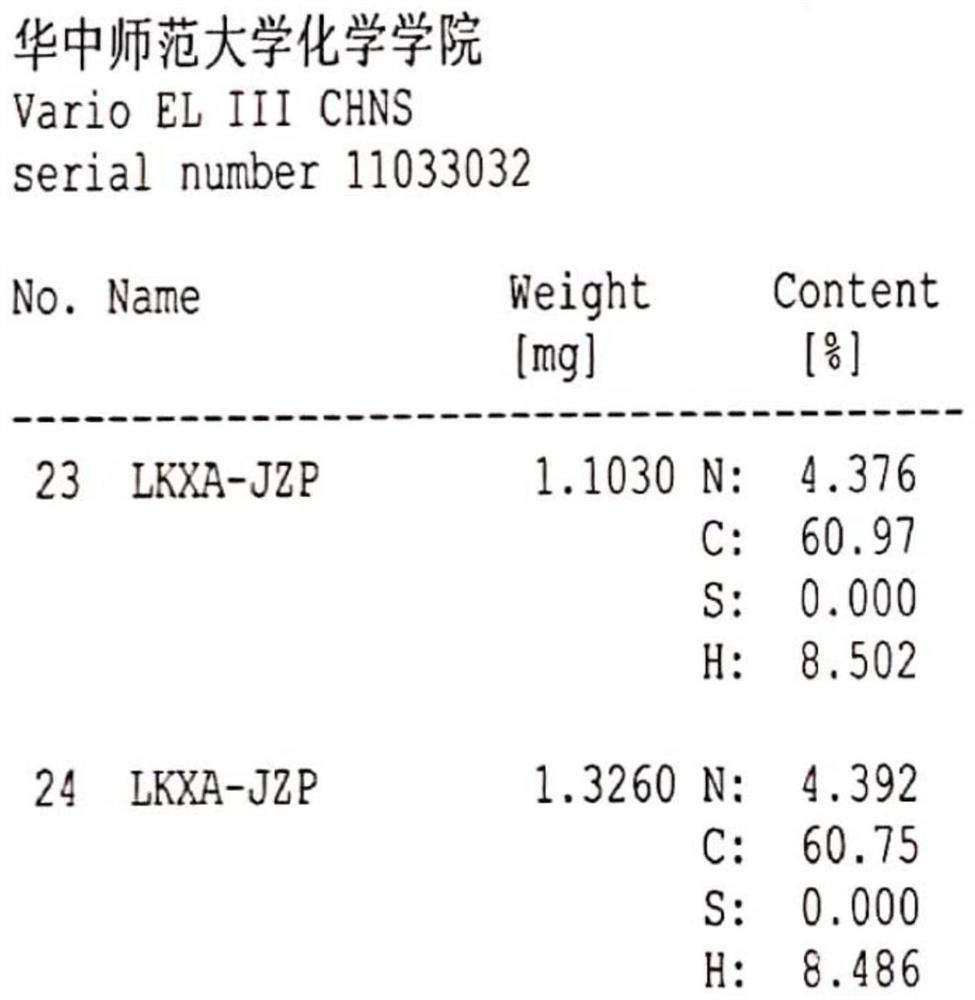
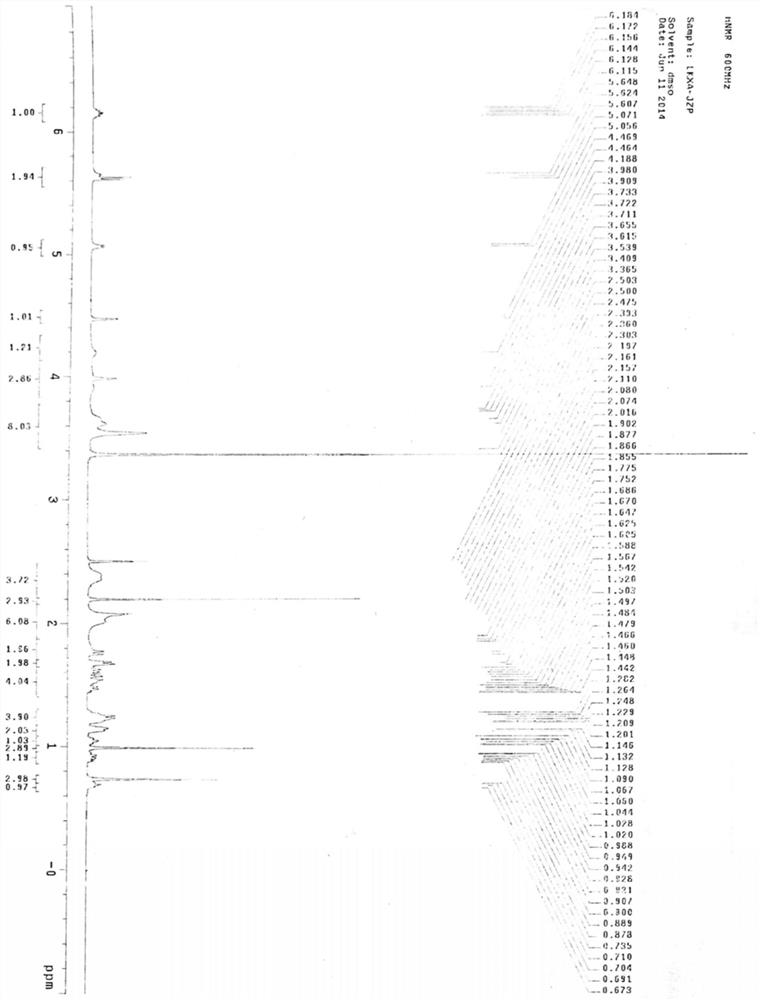
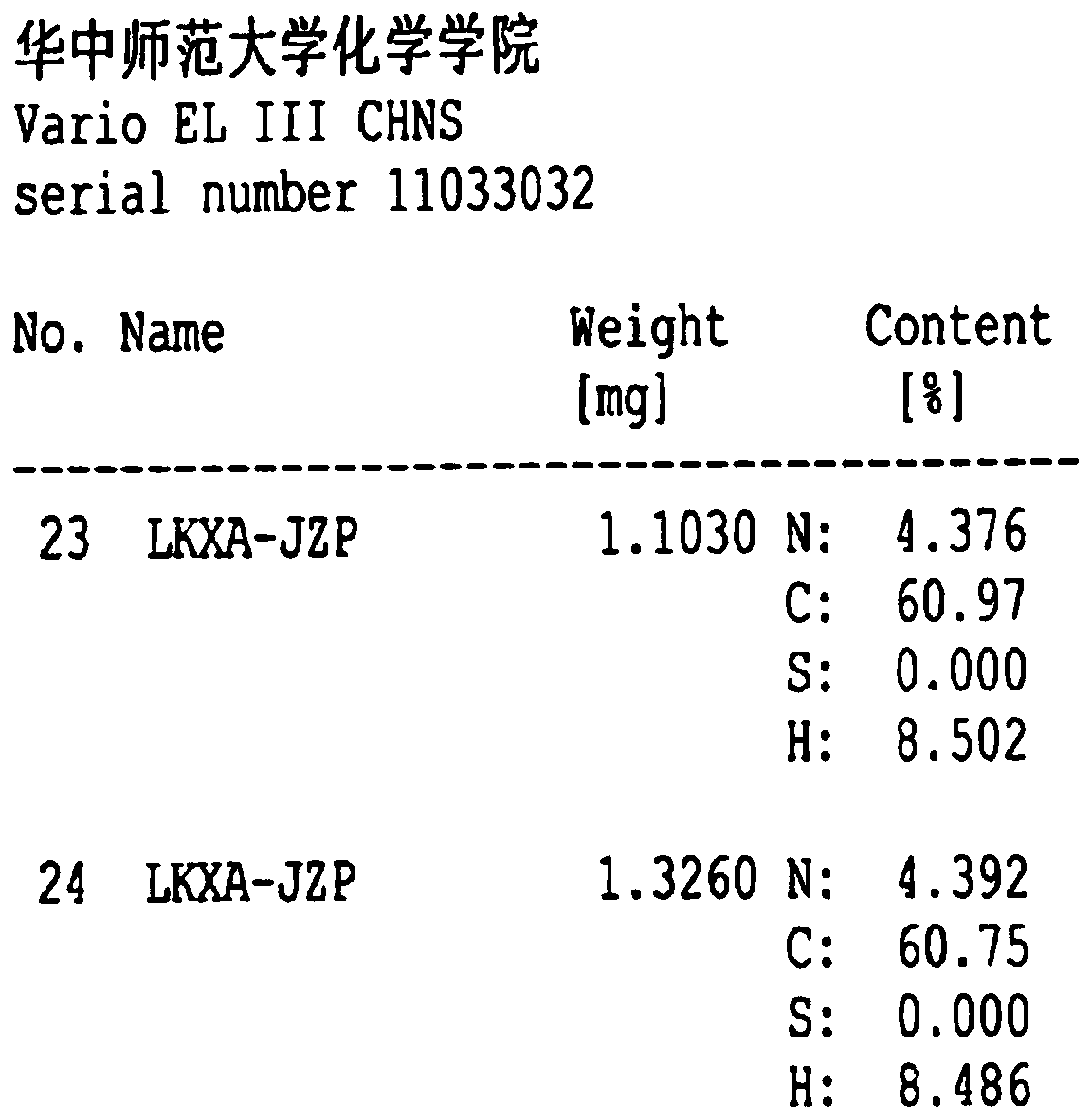
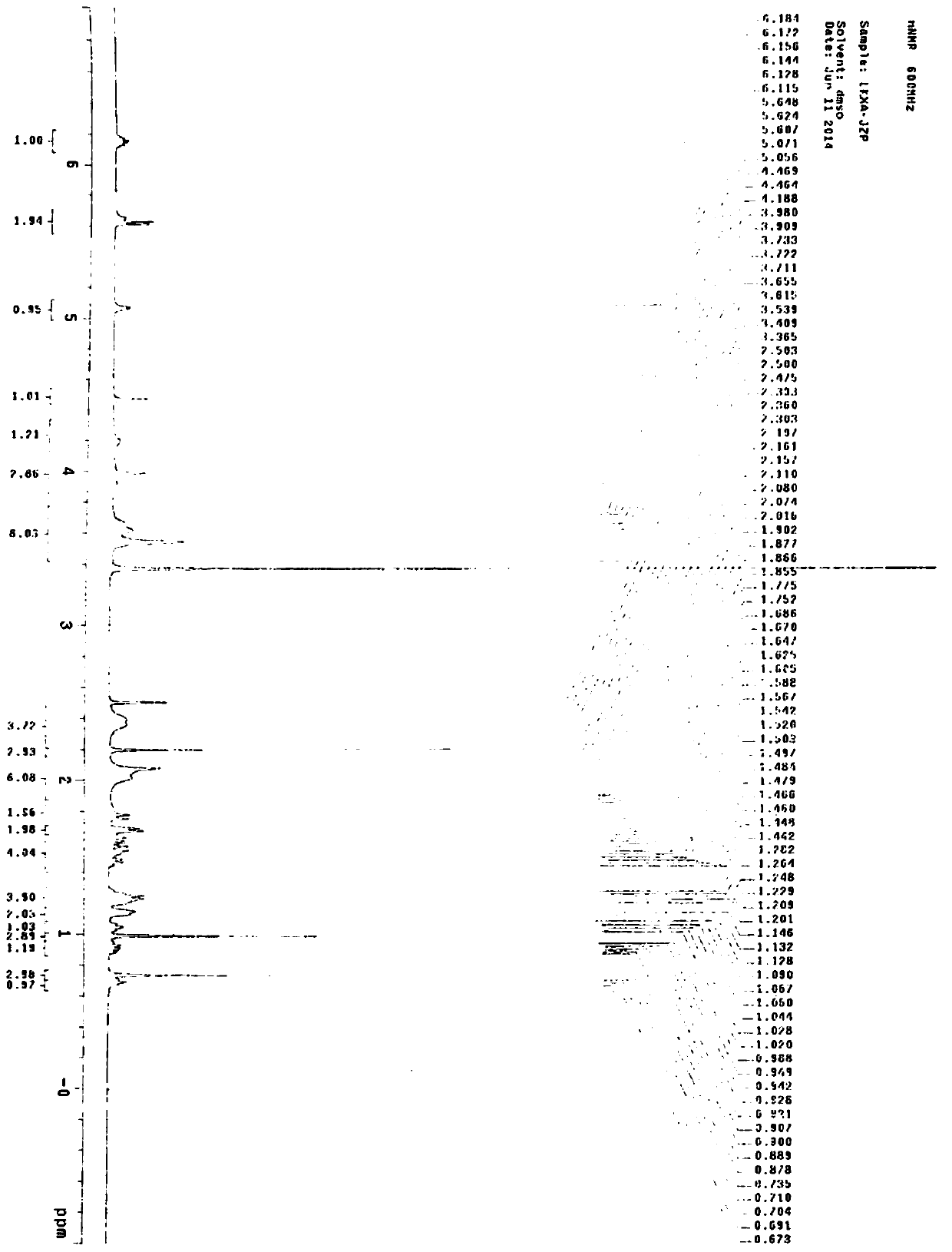
The invention belongs to the technical field of raw material medicine preparation, and particularly relates to a single crystal culture method for a rocuronium bromide starting raw material (LK-7). According to the technical scheme, the method comprises the following steps: firstly, dissolving a certain amount of LK-7 solid in a proper amount of organic solvent at a certain temperature to obtain an LK-7 solution with a certain concentration, and meanwhile, ensuring that the obtained solution is clear and transparent without any macroscopic particles; and culturing the LK-7 single crystal by adopting a volatile solvent method. The invention provides a culture method of an LK-7 single crystal, and provides a scientific method for quality control of an initial bulk drug LK-7, especially for confirmation of an absolute configuration of a chiral center.
Owner:北京满格医药科技有限公司 +1
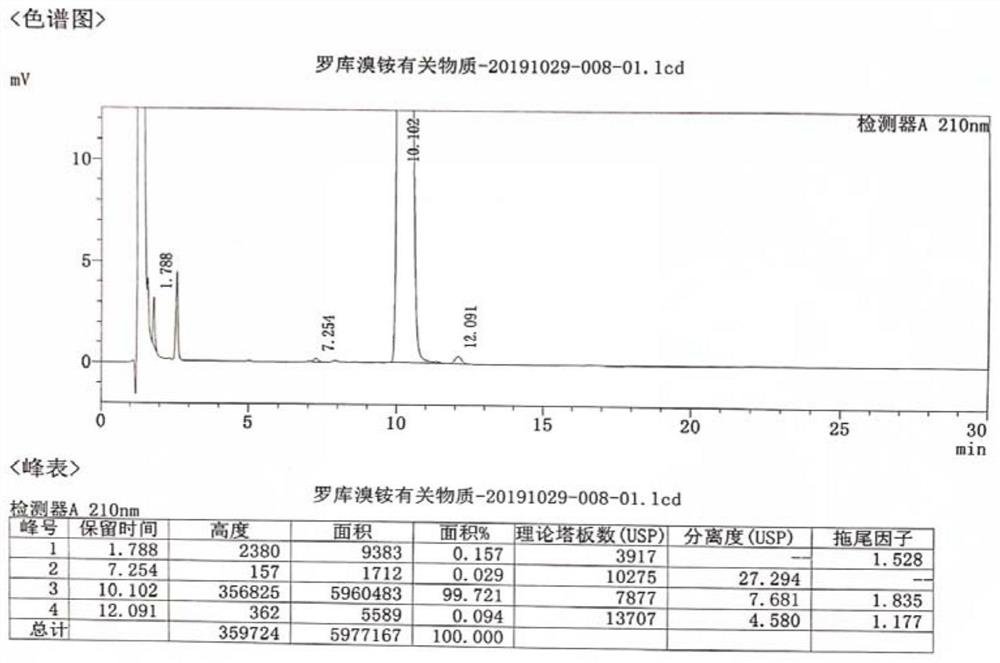
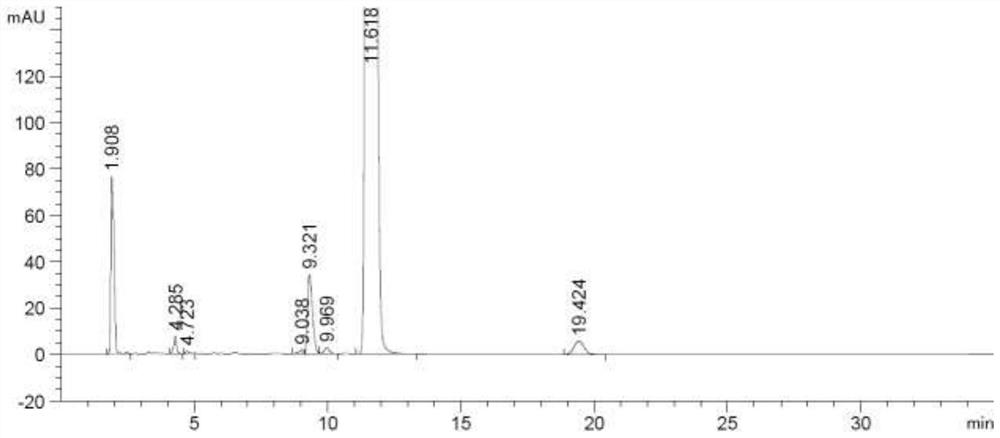
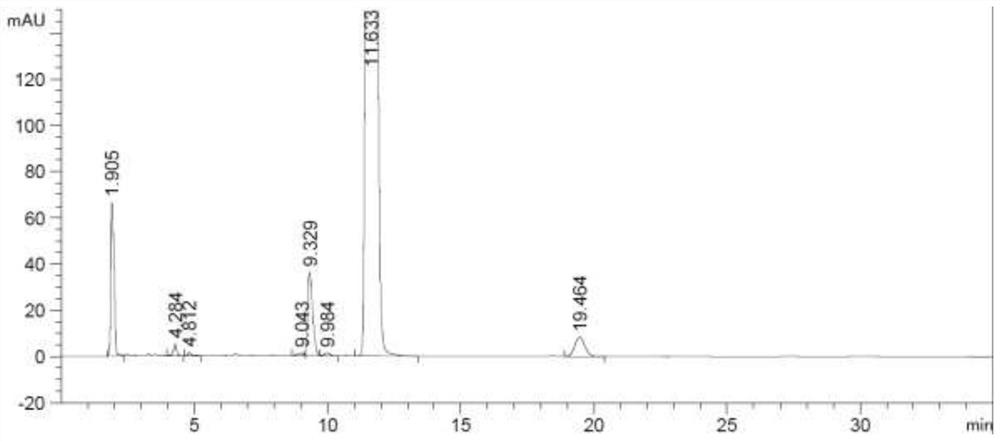
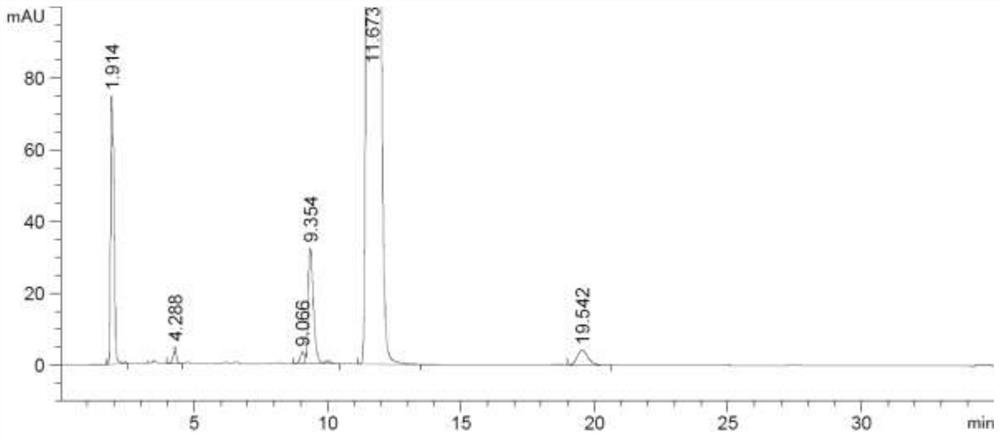
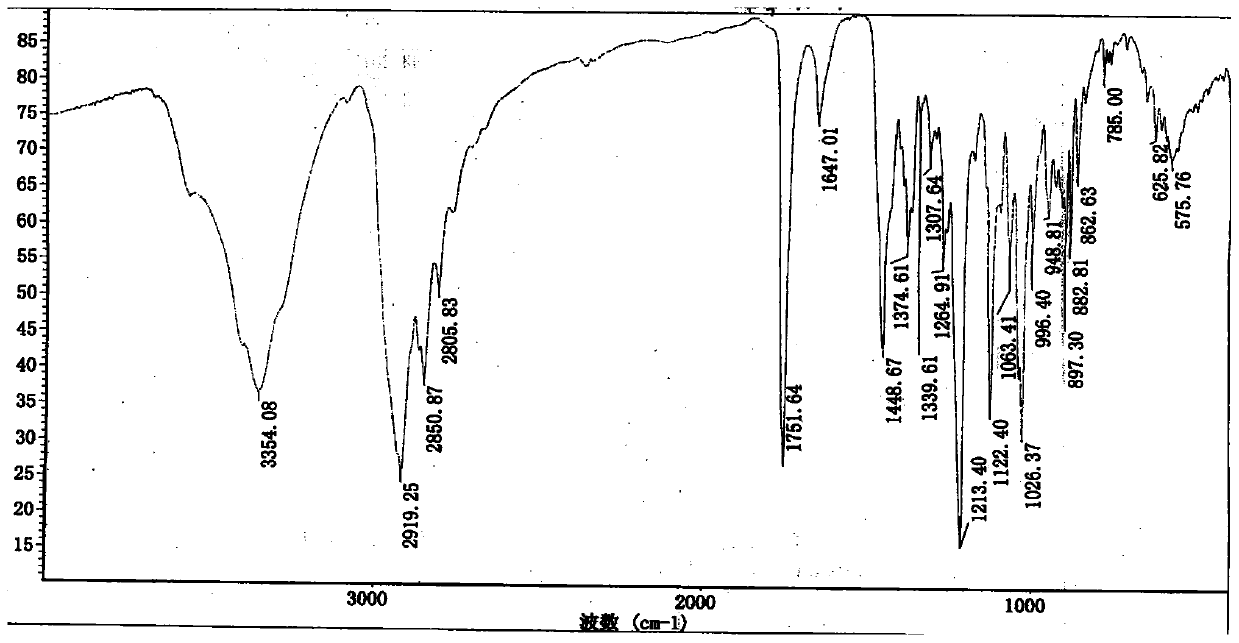
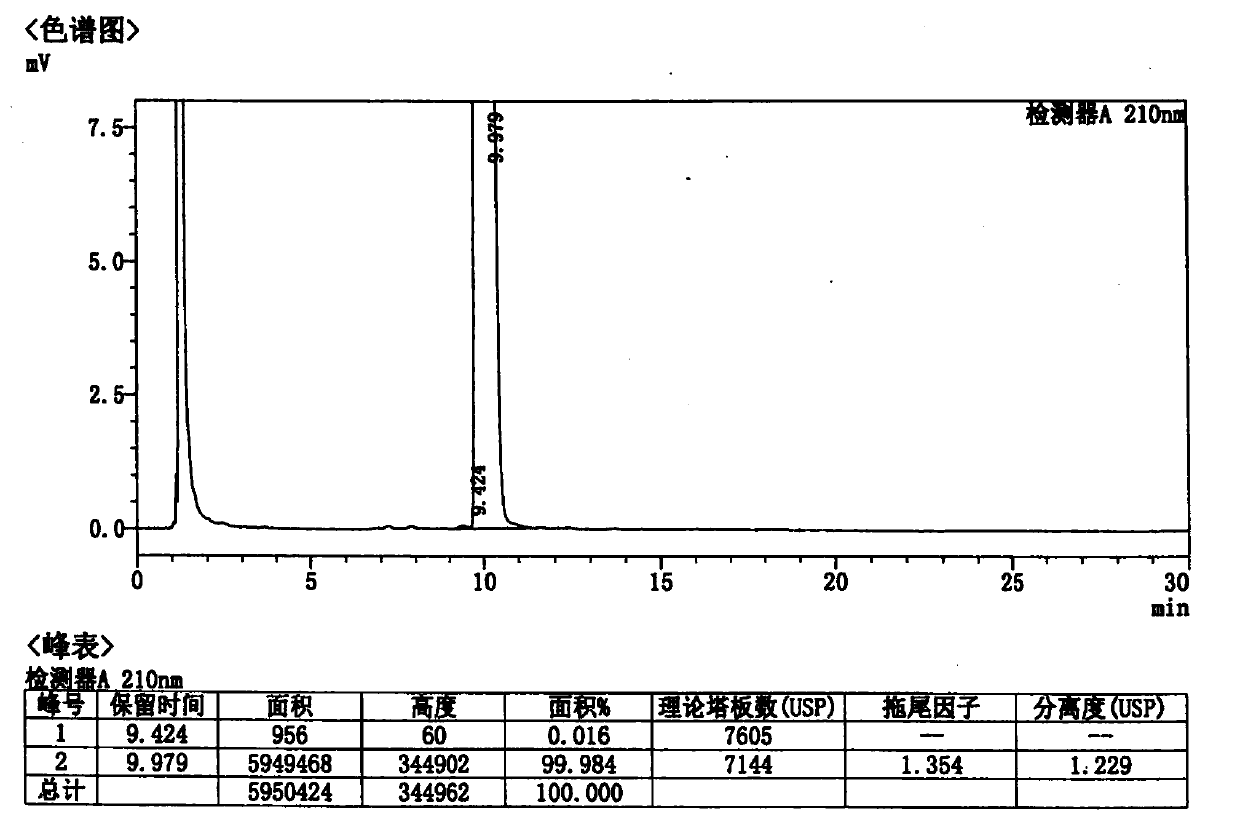
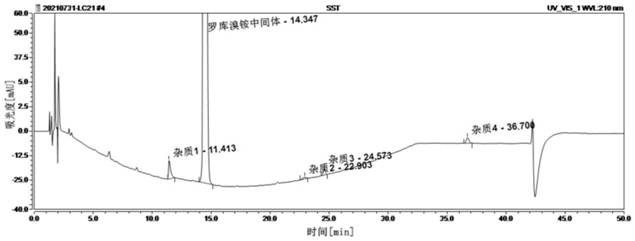
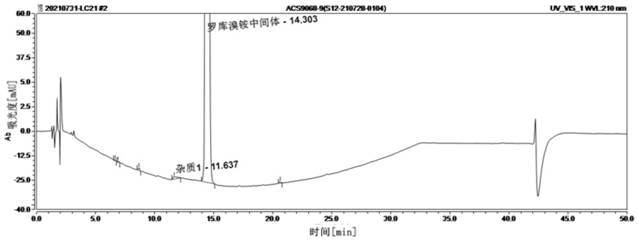
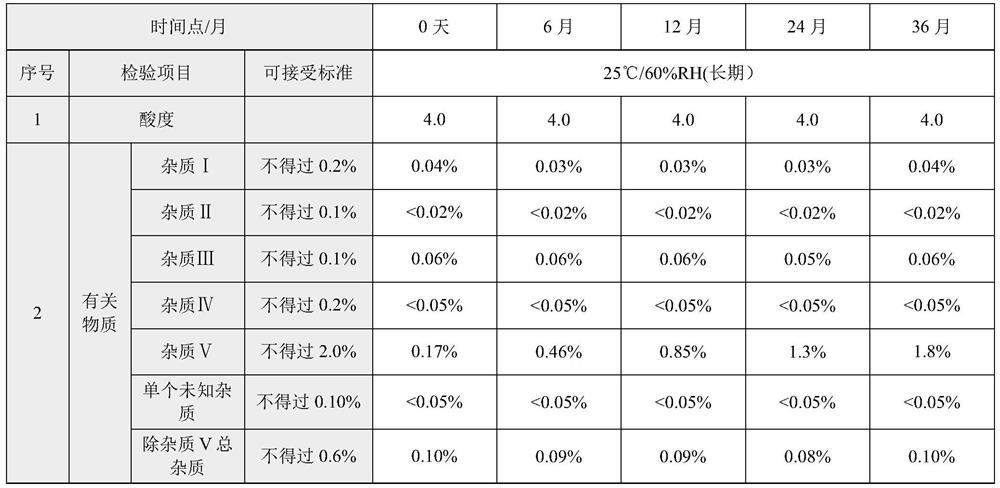
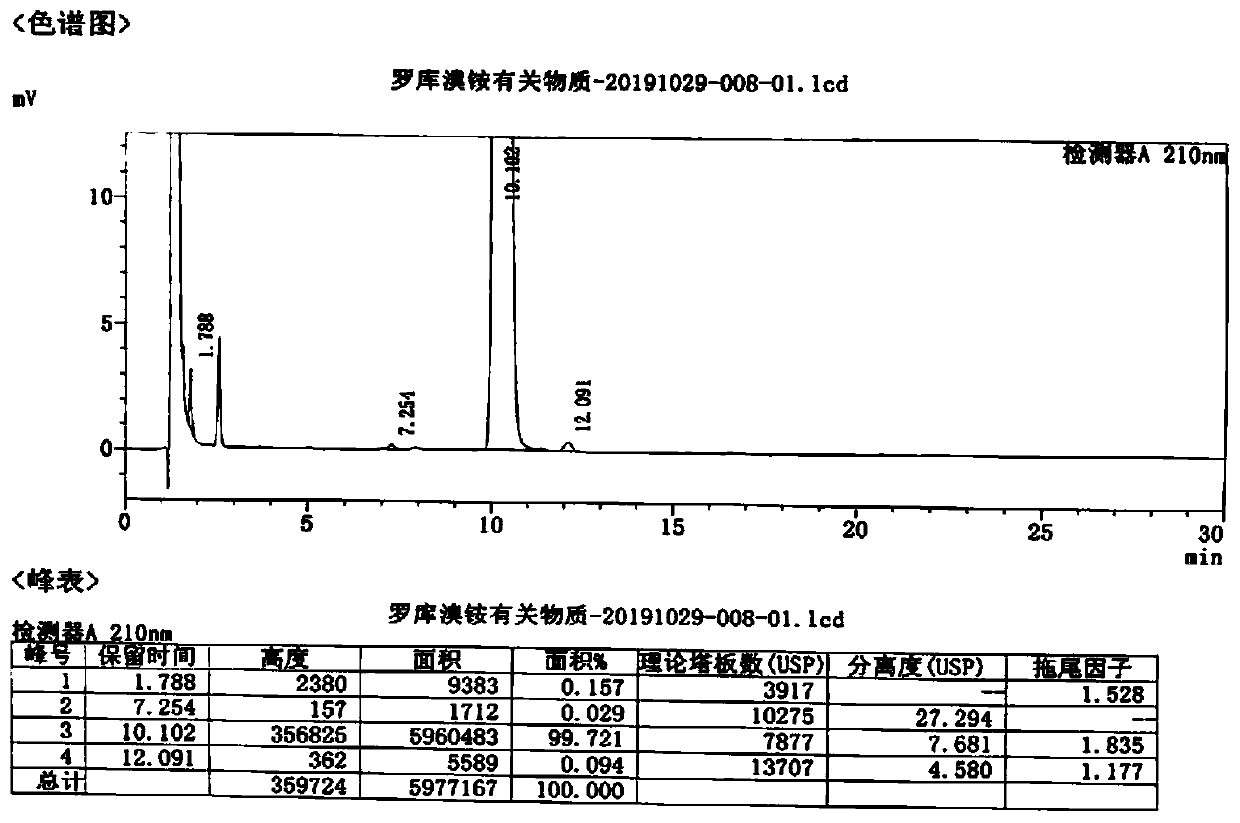
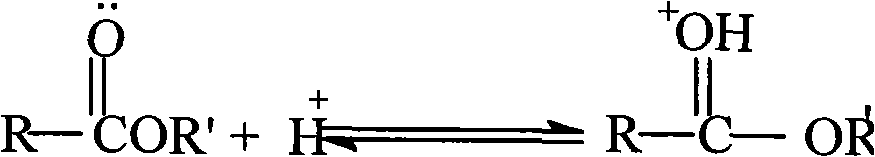
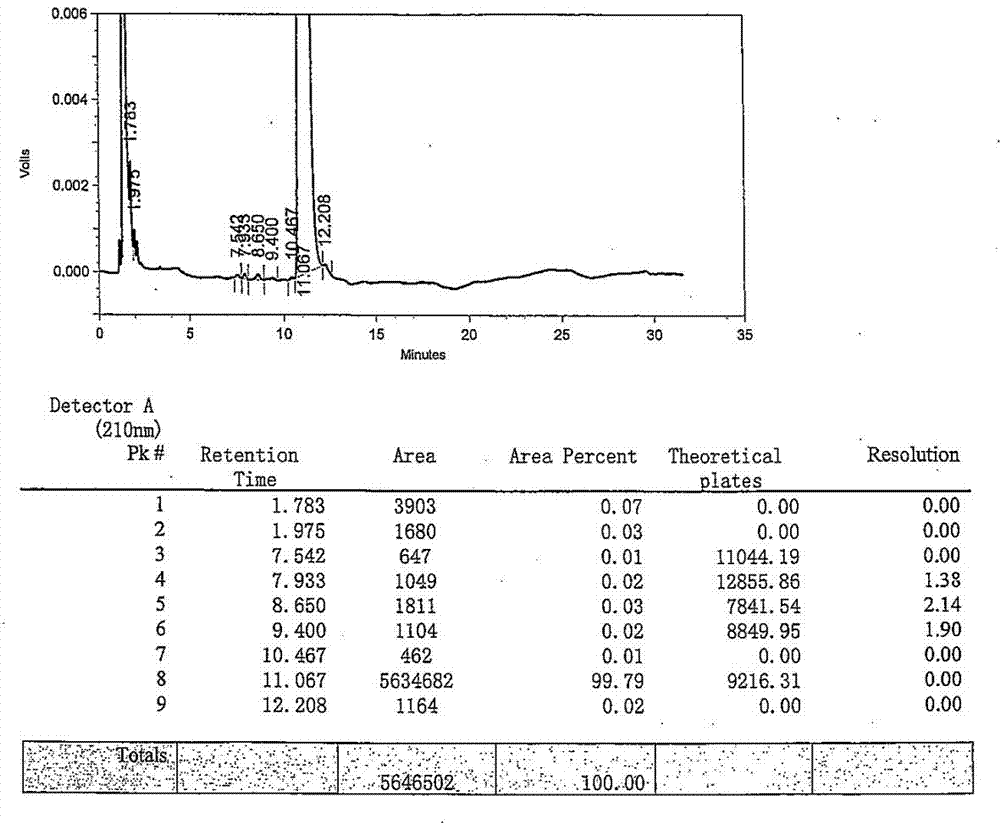
Method for detecting rocuronium bromide intermediate and impurities
ActiveCN114088842AStrong retentionRealize detectionComponent separationFluid phasePhysical chemistry
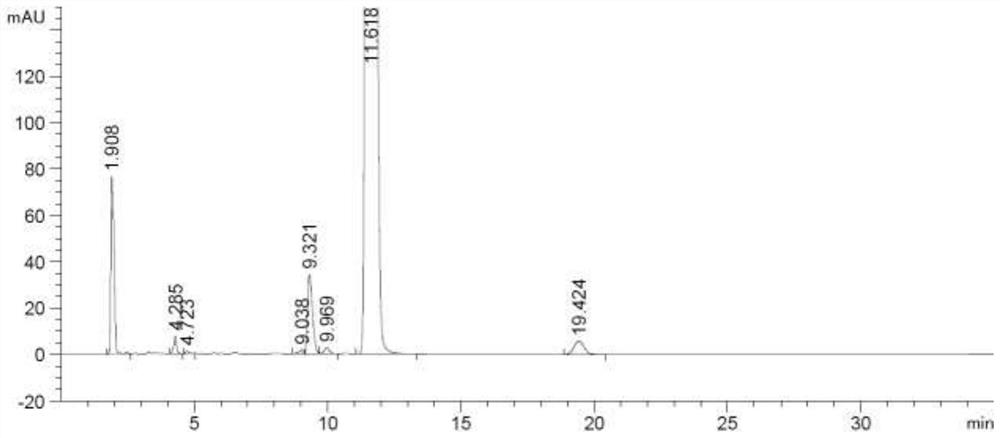
The invention provides a method for detecting a rocuronium bromide intermediate and impurities, which adopts a high performance liquid chromatography to detect and analyze the rocuronium bromide intermediate, an impurity 1, an impurity 2, an impurity 3 and an impurity 4. According to the method, known impurities can be accurately detected, the main peak of the intermediate and the peak of the known impurities can be effectively separated, and the method is a brand-new detection and analysis method beneficial to quality control of the rocuronium bromide intermediate. The method is high in sensitivity, wide in linear range, good in specificity and repeatability and easy and convenient to operate.
Owner:JIANGSU LONG HEALTHCARE
Rocuronium bromide injection preparation and preparation method thereof
PendingCN114404363AImprove stabilityOrganic active ingredientsInorganic non-active ingredientsPharmaceutical AidsOrganic chemistry
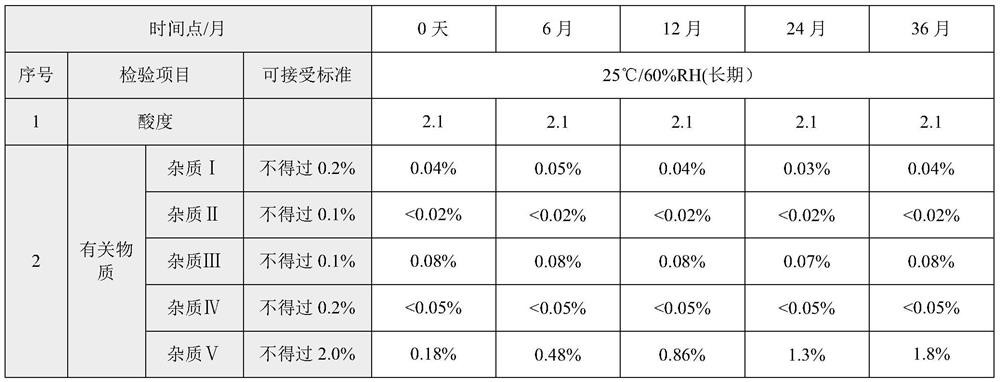
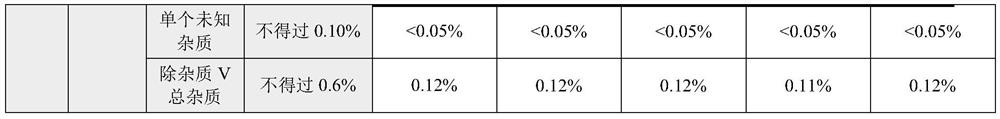
The invention discloses a rocuronium bromide injection preparation. The pH (Potential of Hydrogen) value of the rocuronium bromide injection preparation is between 2.0 and 2.2 or between 3.8 and 4.2; comprising main medicines and auxiliary materials, wherein the content of the main drug in the rocuronium bromide injection preparation is 10 mg / mL; the main drug is rocuronium bromide; the auxiliary material is a pH regulator. The rocuronium bromide injection prepared by the preparation method provided by the invention has better stability, and can still meet the standard of the product in Chinese pharmacopoeia after being stored for 36 months at normal temperature.
Owner:杭州泓友医药科技有限公司
Preparation method of rocuronium bromide
Owner:武汉华龙生物制药有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
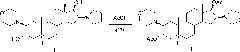
![Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/cb6e2100-358f-4a9f-a3cc-a65af10ad4f8/HDA0003180832410000011.png)
![Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/cb6e2100-358f-4a9f-a3cc-a65af10ad4f8/HDA0003180832410000012.png)
![Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof Water-soluble cucurbit [8] urea sulfonate derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/cb6e2100-358f-4a9f-a3cc-a65af10ad4f8/HDA0003180832410000021.png)