Inhalation preparation for treating asthma
A technology for inhalation preparations, asthma, applied in the field of medicine, which can solve problems such as little research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
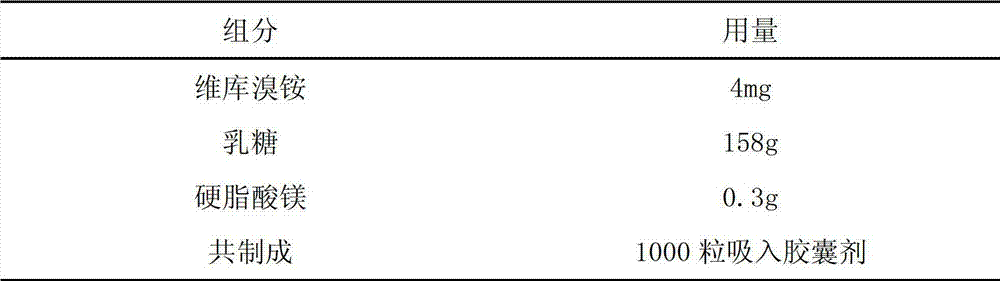
[0011] Embodiment 1 dry powder inhaler
[0012] prescription:
[0013]
[0014] Preparation:
[0015] Mix vecuronium bromide, lactose 8g, and magnesium stearate thoroughly and evenly. The particle size of the micropowder is D90 less than 7 microns, and the obtained mixed powder is baked at 70 degrees for 12 hours, and filled into the No. 3 capsule shell.
Embodiment 2
[0016] Embodiment 2 dry powder inhaler
[0017] prescription:
[0018]
[0019] Preparation:
[0020] Mix pancuronium bromide, lactose 8g, and magnesium stearate thoroughly and evenly. The particle size of the micropowder is D90 less than 7 microns, and the obtained mixed powder is baked at 70 degrees for 12 hours, and filled into the No. 3 capsule shell.
Embodiment 3
[0021] Embodiment 3: Aerosol
[0022] prescription:
[0023]
[0024] Preparation:
[0025] Dissolve pancuronium bromide in ethanol, dispense quantitatively into aerosol containers after dissolving, install valves, tighten caps, and fill propellants to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com