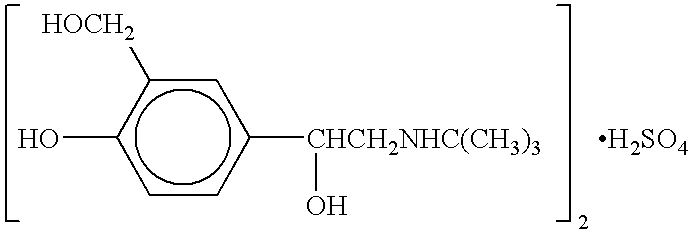
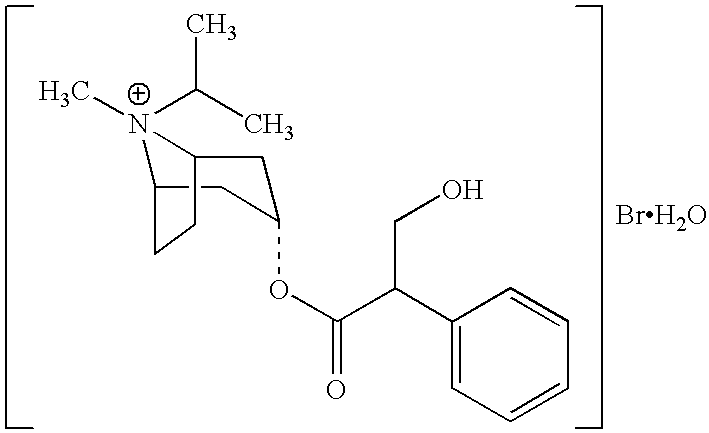
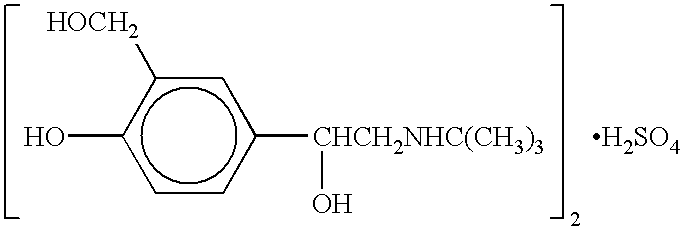
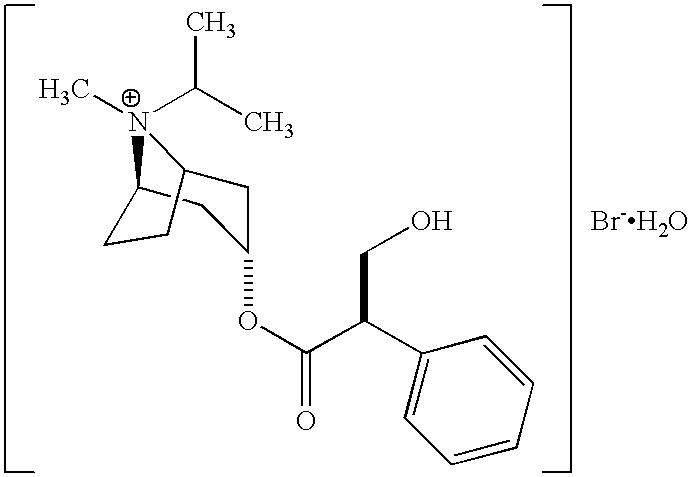
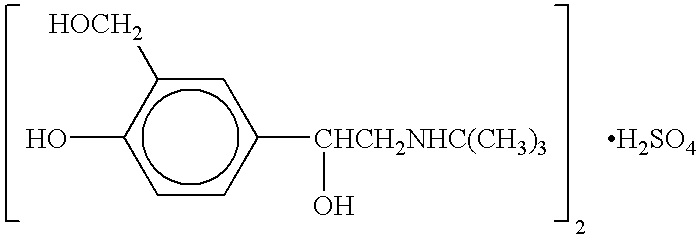
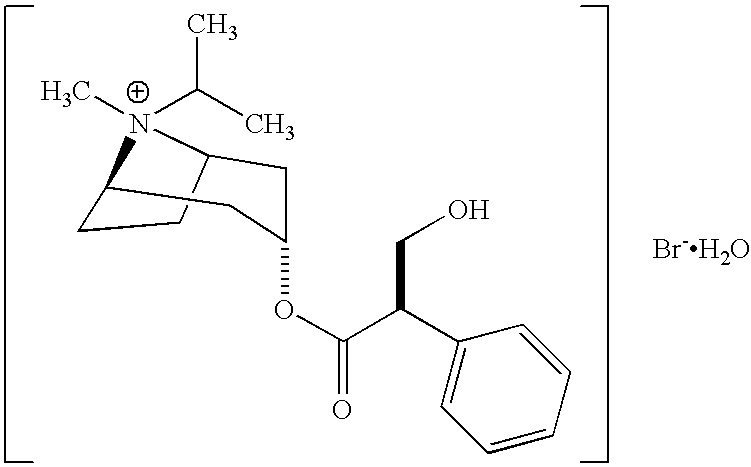
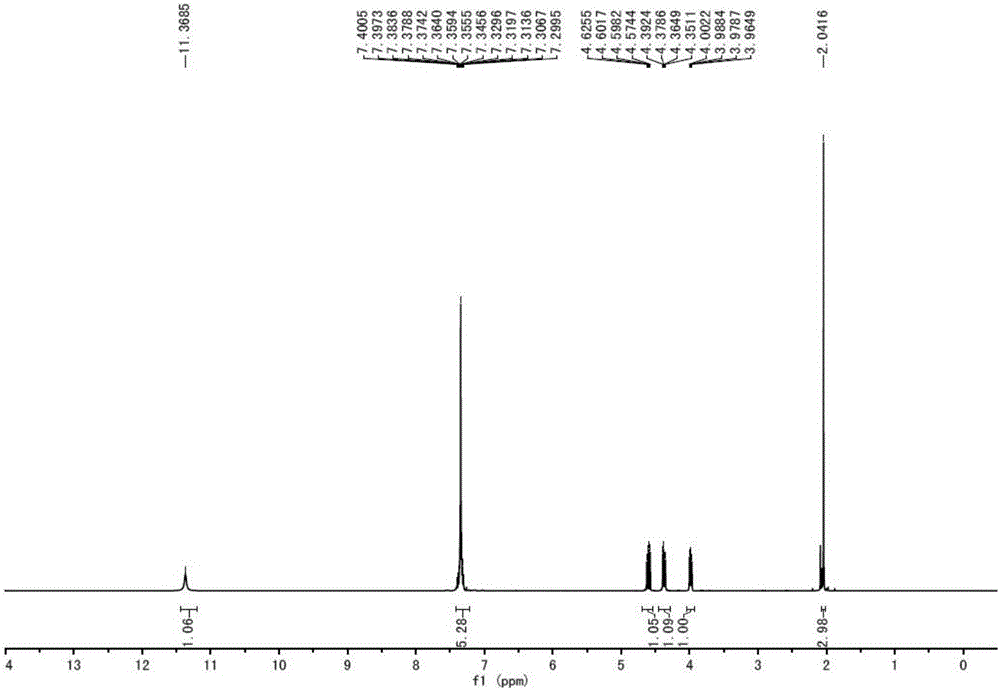
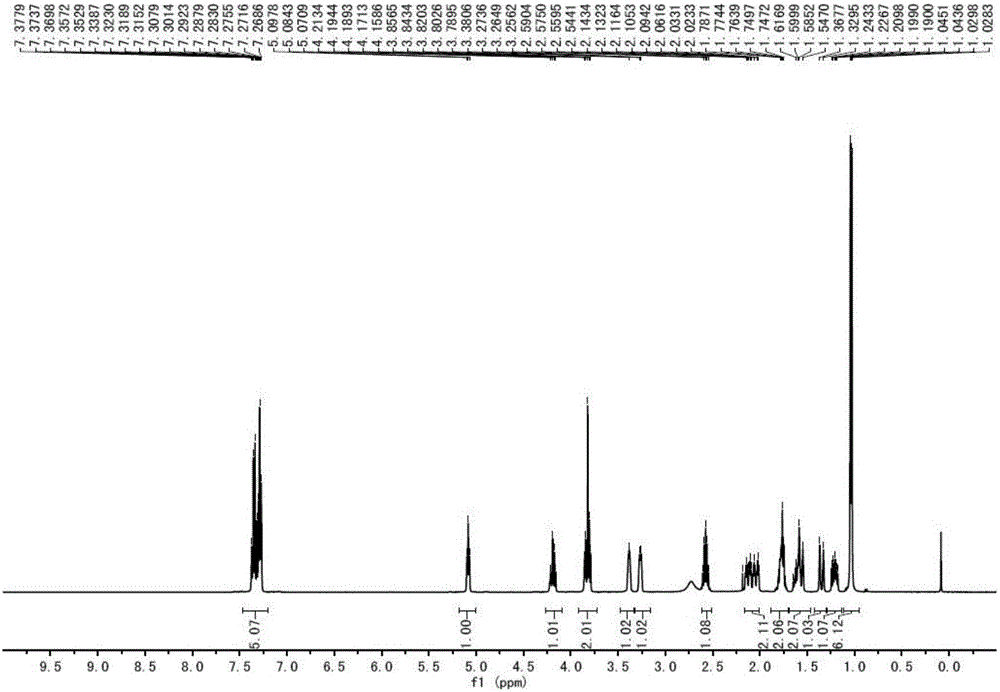
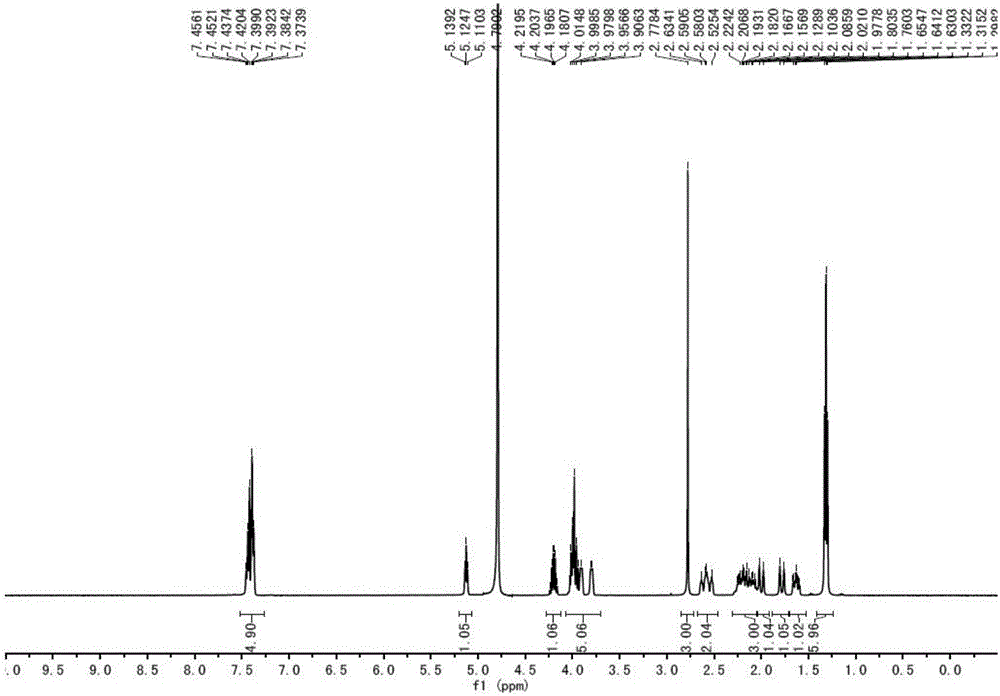
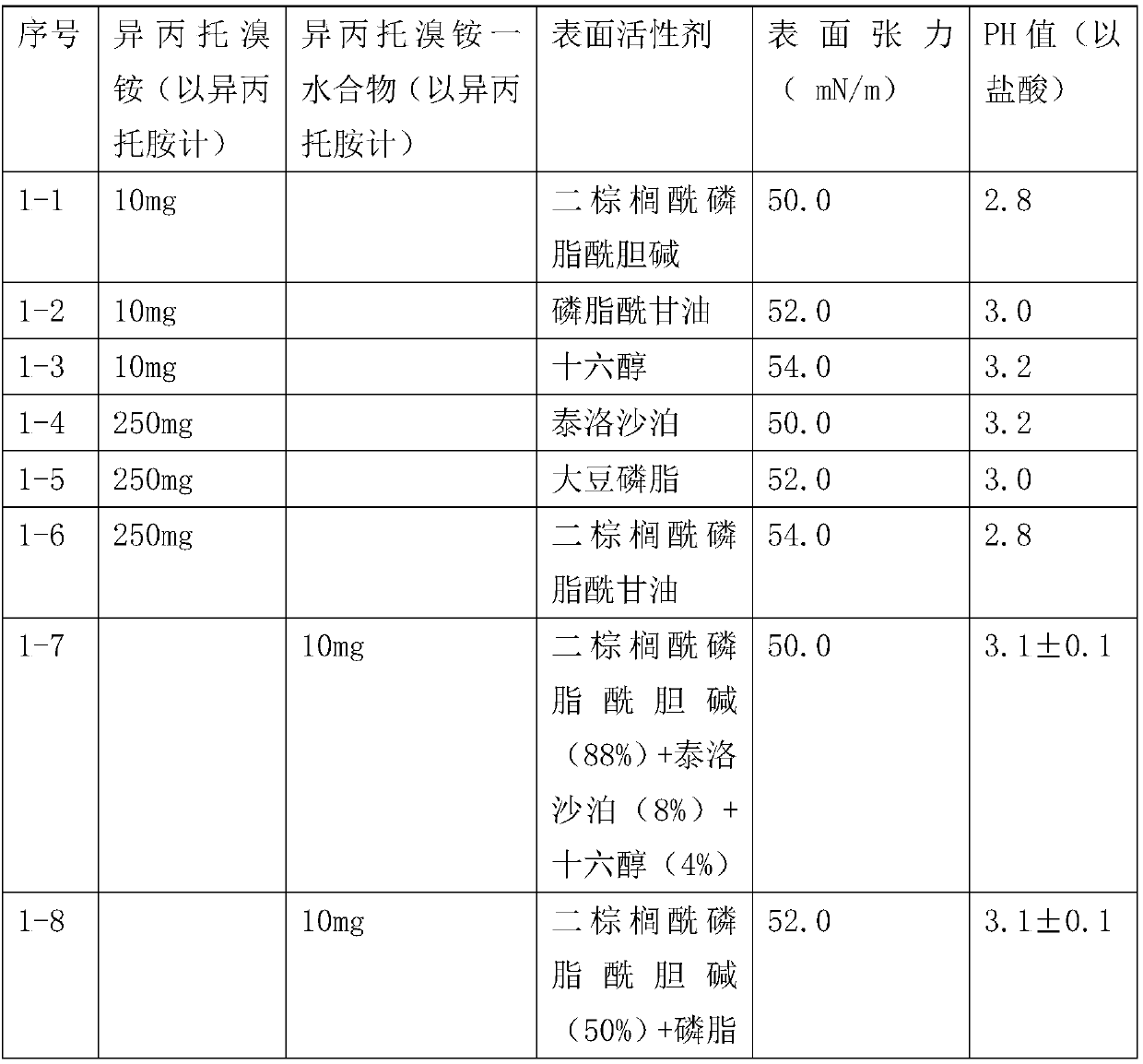
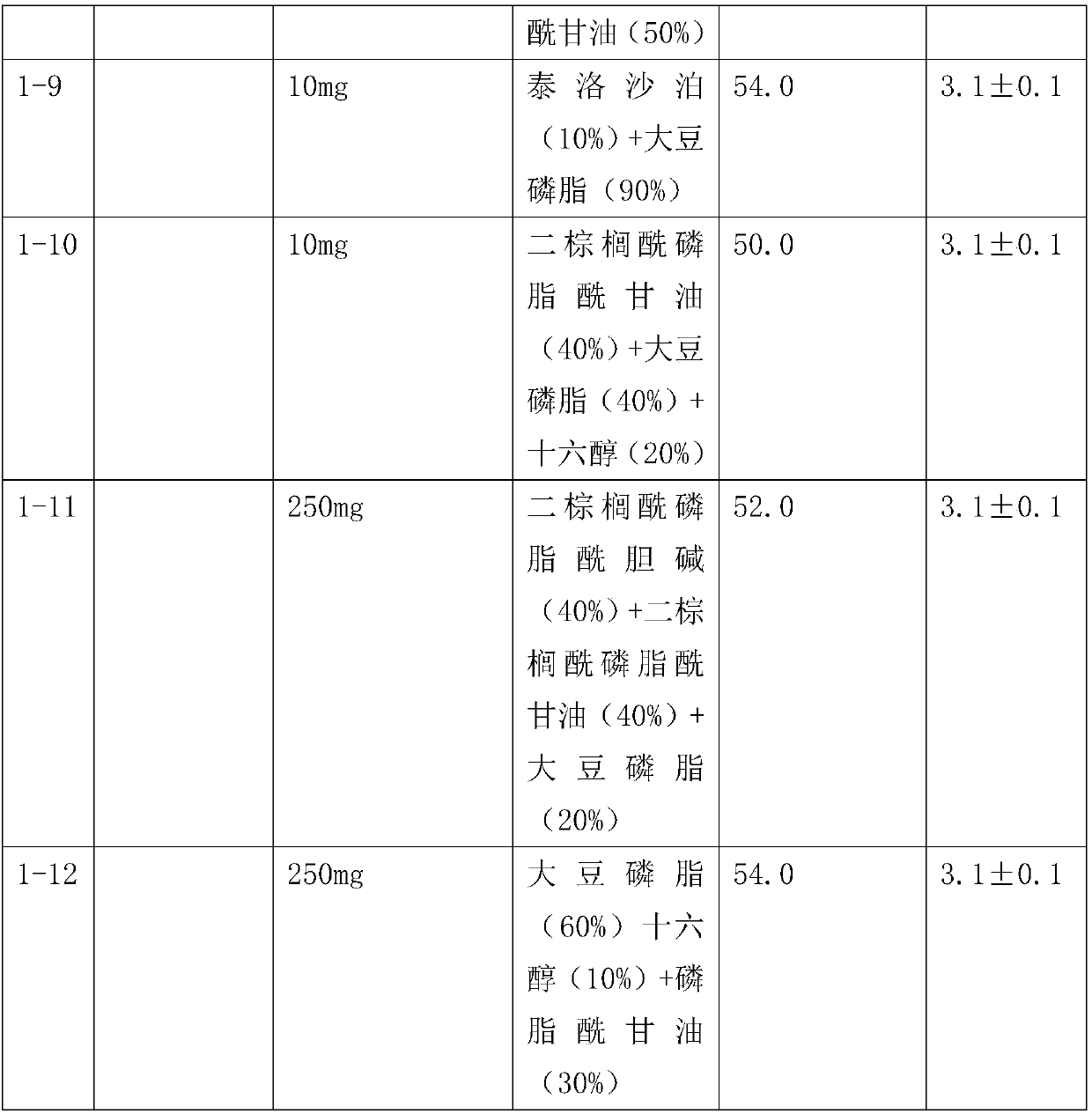
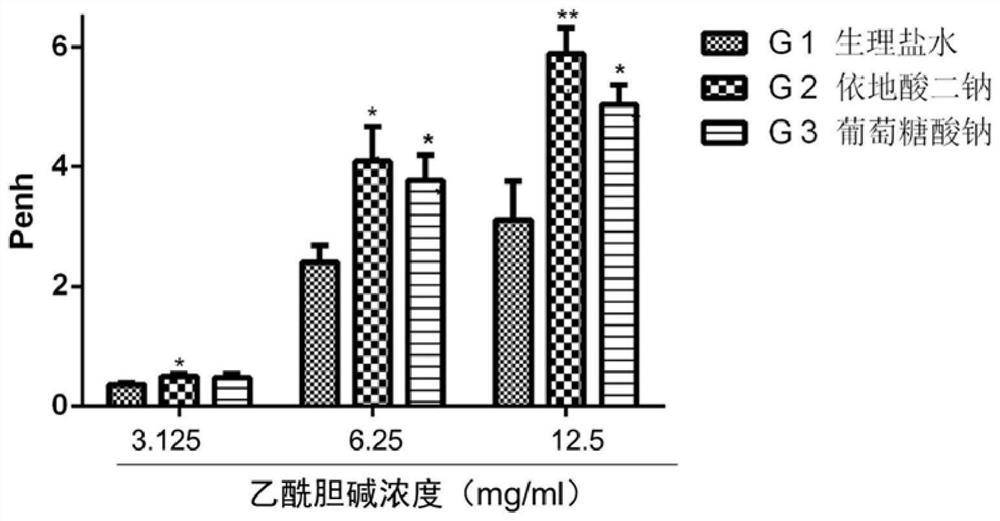
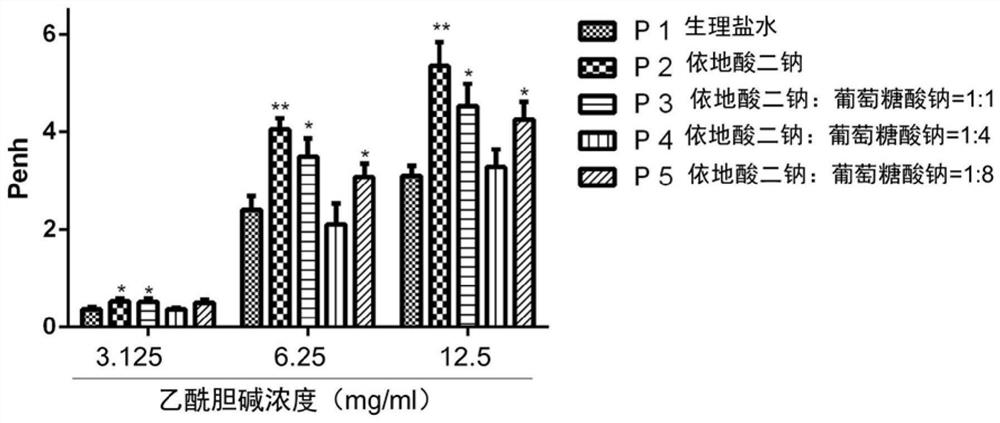
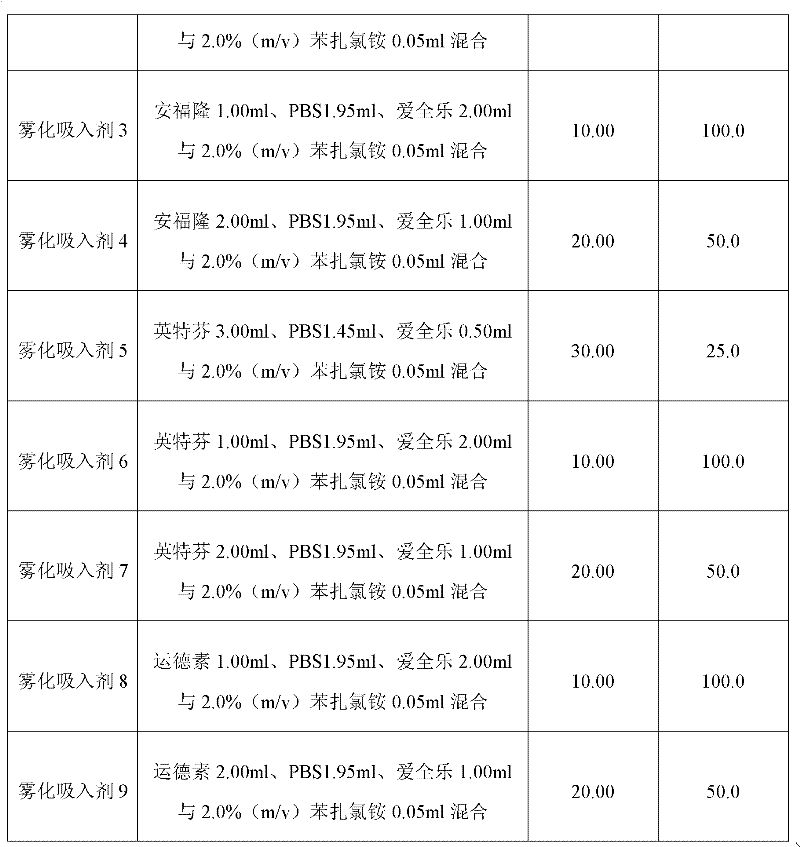
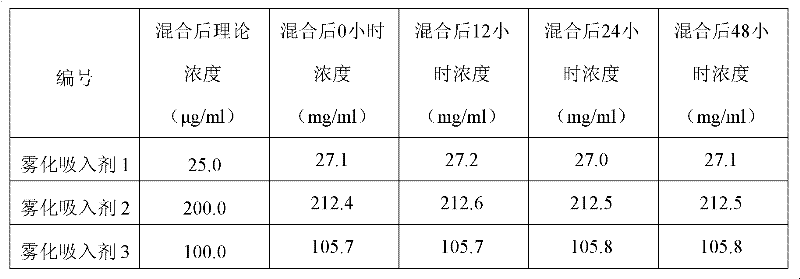
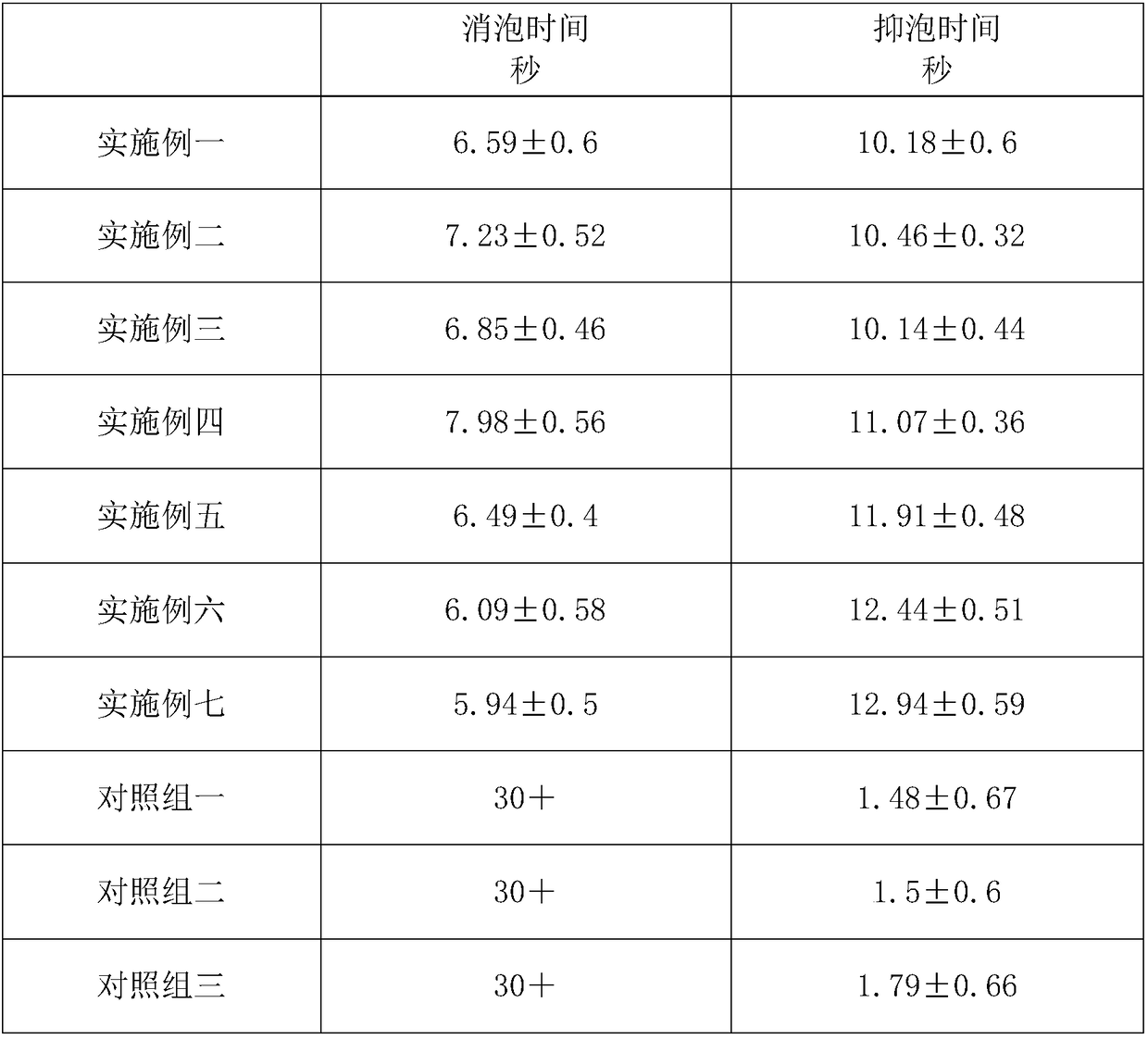
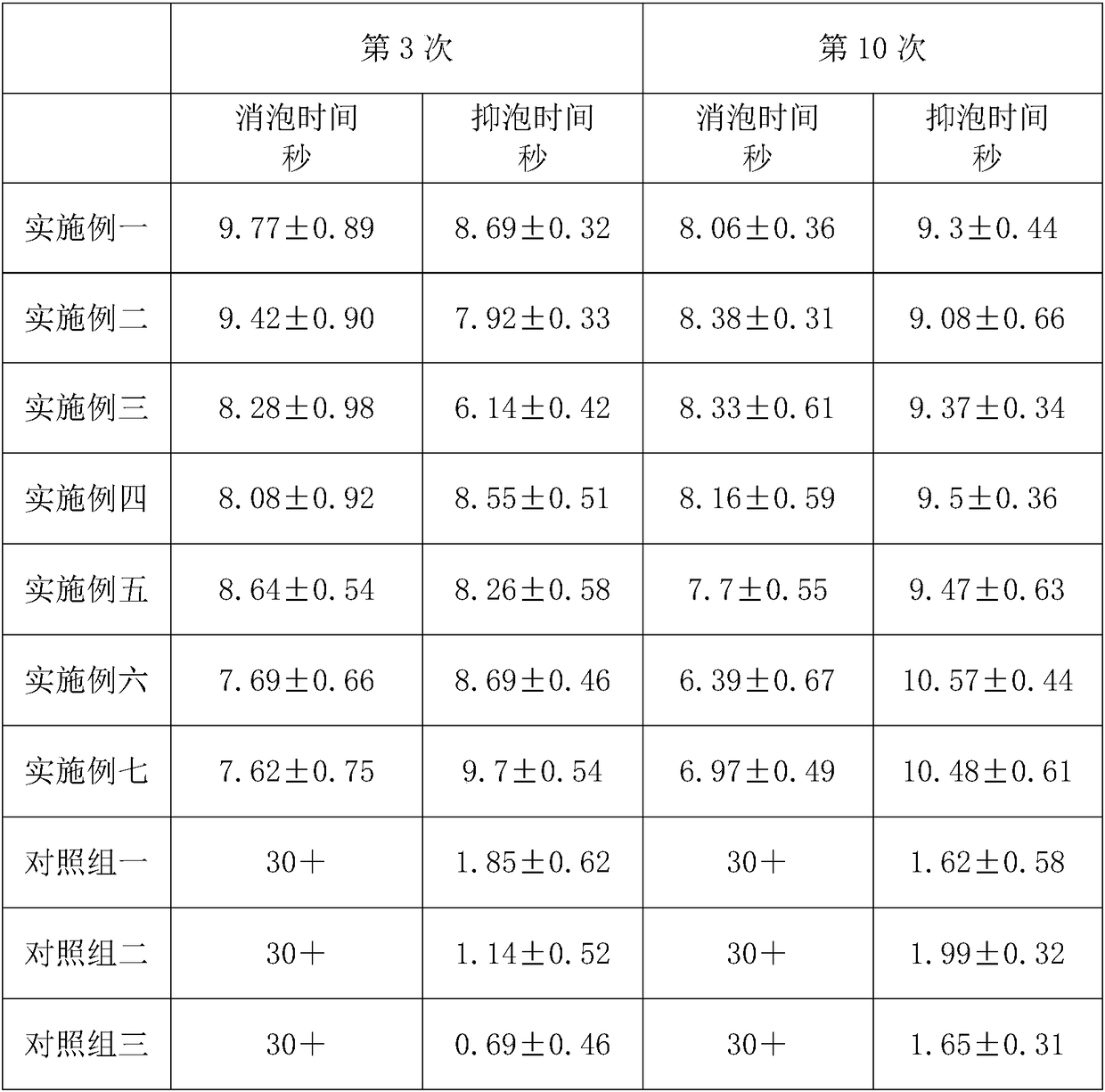
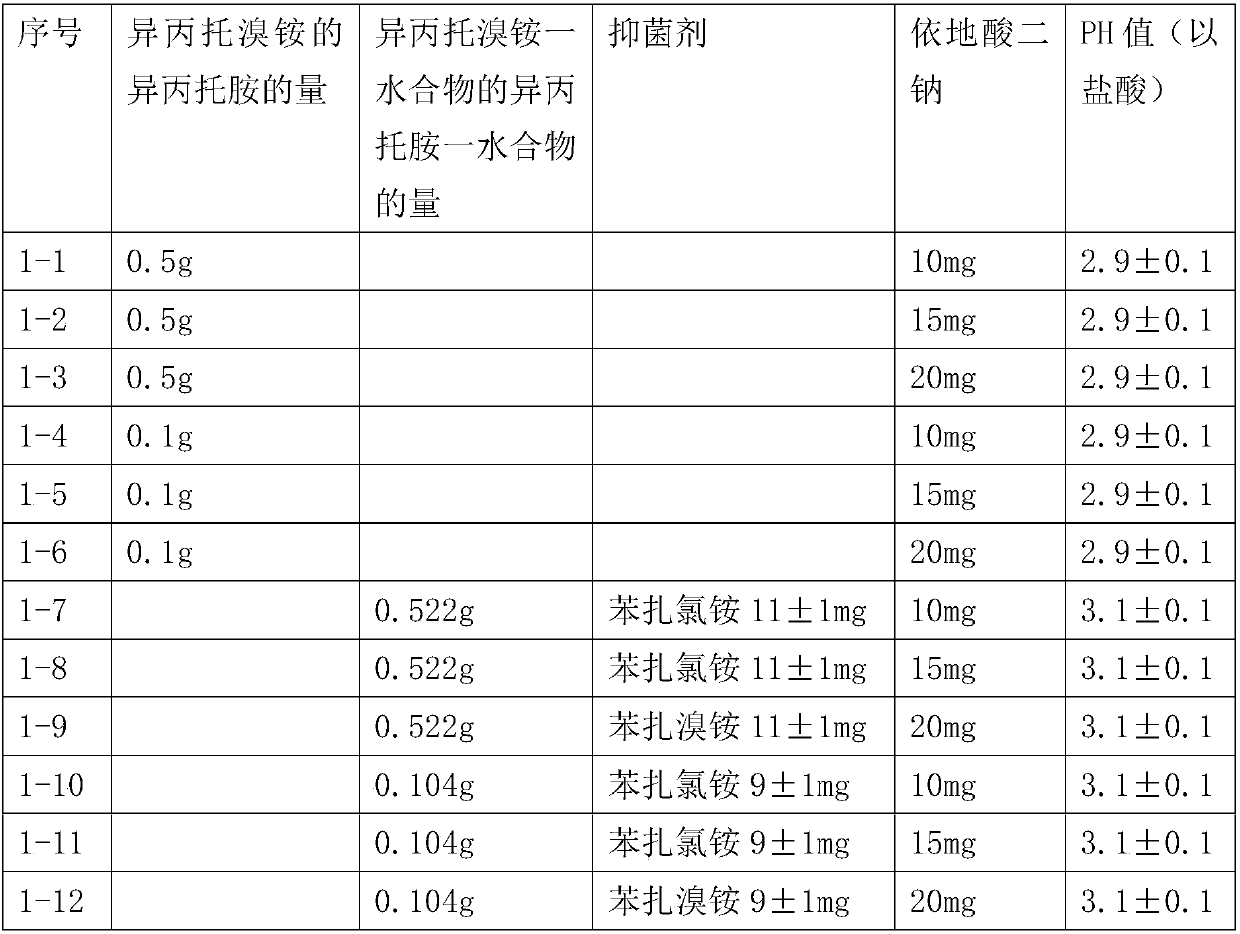
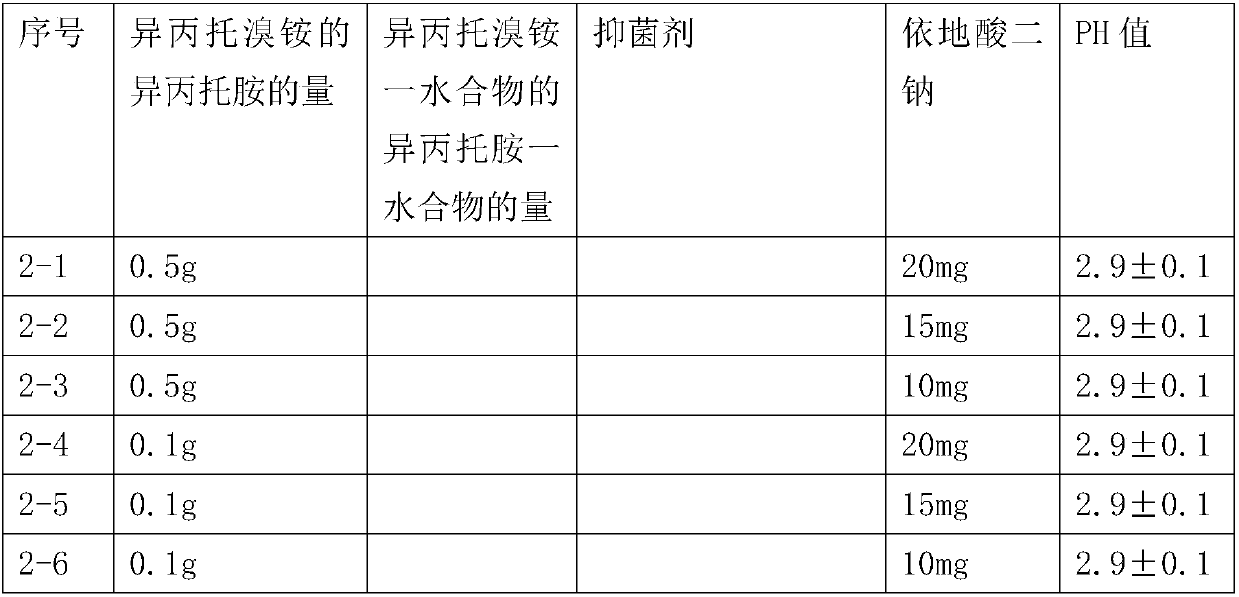
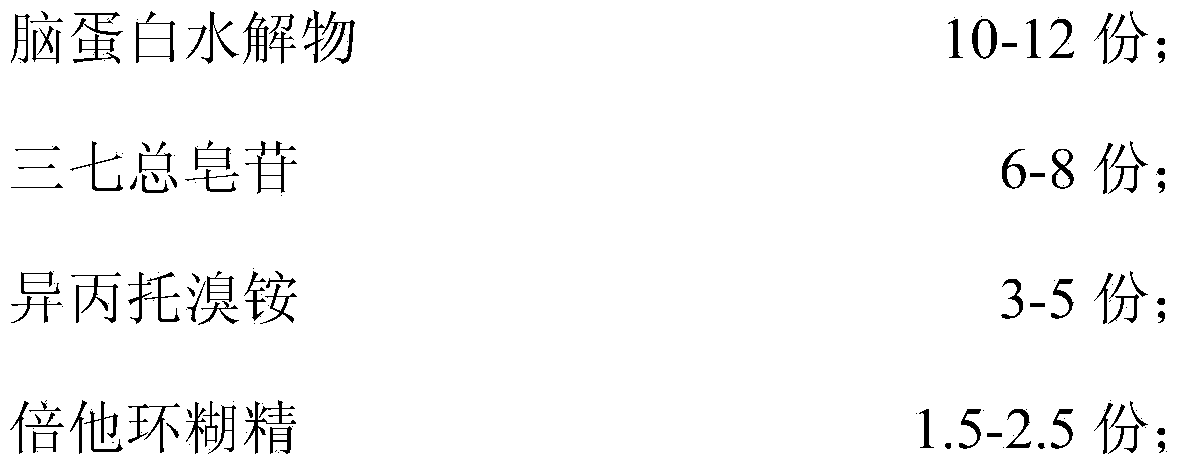Patents
Literature
51 results about "Ipratropium bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ipratropium is used to treat a runny nose caused by the common cold or seasonal allergies.
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030191151A1Relieve bronchospasmBiocideDispersion deliveryBenzalkonium chlorideSalbutamol
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS6632842B2Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030149007A1Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:MYLAN SPECIALTY
Synthesis method of ipratropium bromide
ActiveCN106831753ASuitable for industrial productionEasy to purifyOrganic chemistryOrganic acidAlcohol
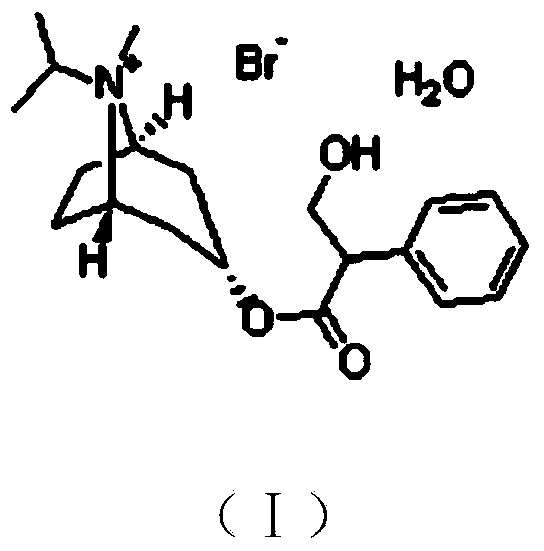
The invention provides a synthesis method of ipratropium bromide. The method comprises the steps of (1) carrying out acetyl protection reaction on tropic acid as a raw material to obtain a compound 1; (2) carrying out chloroformylation reaction on the compound 1 to obtain a compound 2; (3) dissolving isopropyl tropine and organic acid into dichloromethane, adding obtained solution to the compound 2 and carrying out acylation reaction to obtain a compound 3; (4) carrying out alcoholysis reaction on the compound 3 to obtain a compound 4; and (5) carrying out bromine methylation reaction on the compound 4 to obtain the ipratropium bromide. The synthesis method of the ipratropium bromide is simple and raw materials are easily available, the operation is simple and stable, various steps are mild in reaction conditions, reaction products of various steps are easy to purify, the purity of the obtained ipratropium bromide product reaches over 97% and the synthesis method is suitable for industrial production of ipratropium bromide.
Owner:WUHAN LEADPHARM TECH CO LTD
Preparation method of ipratropium bromide
The invention discloses a preparation method of ipratropium bromide. The method comprises the following steps that as a starting material, methyl phenylacetate (III) undergoes the substitution reaction to produce alpha-formyl methyl phenylacetate (IV); tropic alcohol reacts with bromomethane to produce a compound (II); the compound (II) reacts with a compound (IV) to produce a compound (V); the compound (V) undergoes a reductive reaction to produce ipratropium bromide anhydrous substance, and then ipratropium bromide (VII) is produced through refining preparation. The method has the advantages of simple operation, high safety, a low cost and suitability for industrial production.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Preparation method of ipratropium bromide
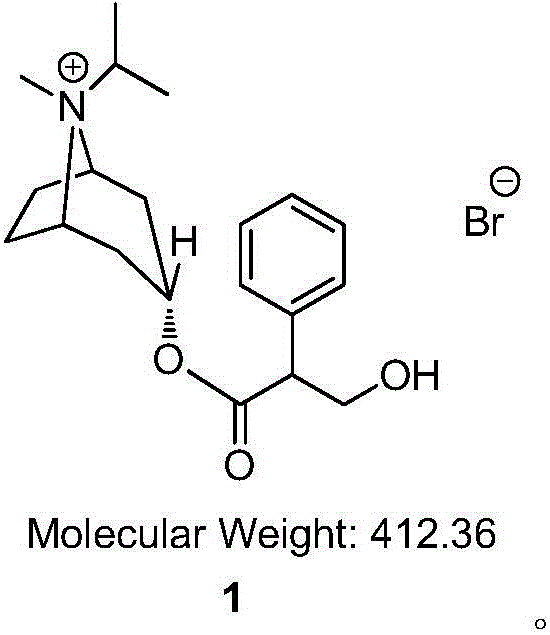
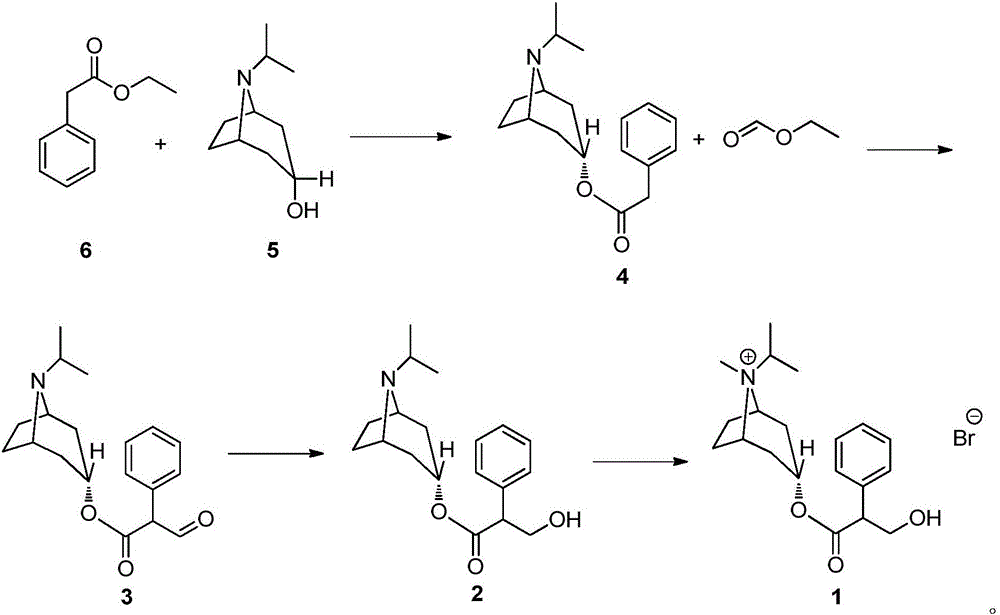
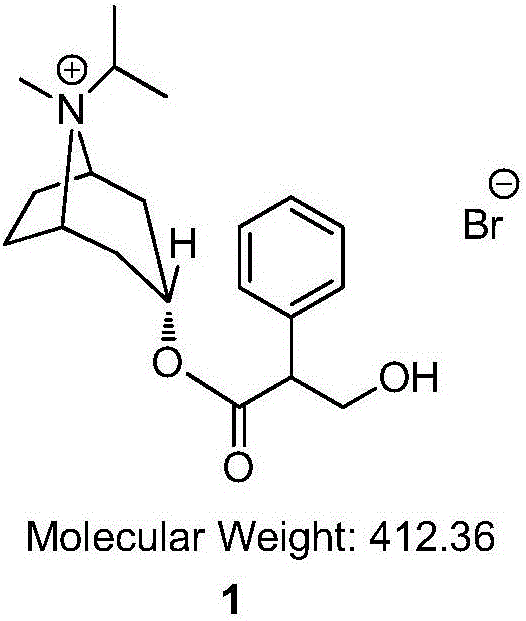
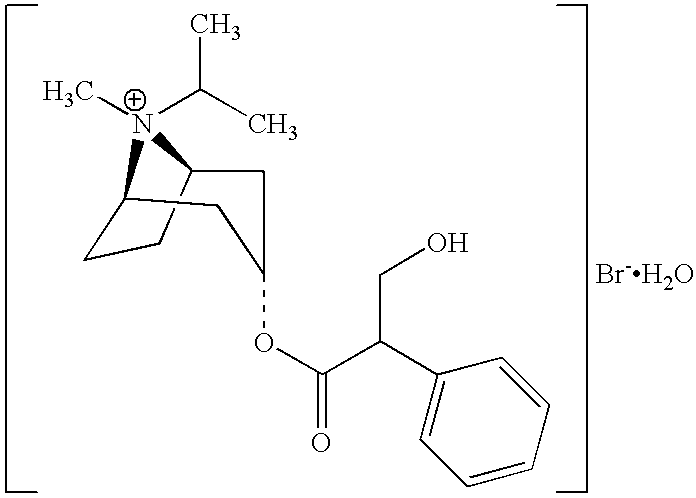
The invention relates to a preparation method of an M-choline receptor blocking agent of ipratropium bromide 1. The preparation method comprises the following steps of using ethyl phenylacetate as starting raw materials; performing reaction with isopropyl tropanol; generating phenylacetate isopropyl tropeine; then, performing substitution, reduction and addition reaction to obtain the ipratropium bromide. The method has the advantages that the operation is simple; safety and controllability are realized; the work protection is low; the preparation method is suitable for industrial production. The formula is shown as the accompanying drawing, and the molecular weight is 412.36.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030203930A1Relieve bronchospasmBiocideDispersion deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Electroplating solution with excellent performance
InactiveCN104911655AImprove performanceImprove corrosion resistanceDipotassium hydrogen phosphateCocamidopropyl betaine
The invention discloses an electroplating solution with excellent performance. The electroplating solution consists of the following raw material components: 20-25 parts of antimony trioxide, 150-200 parts of ferrous sulfate, 100-120 parts of copper sulfate, 130-150 parts of nickel sulfate, 100-120 parts of stannous chloride, 30-50 parts of aluminum sulfate, 30-40 parts of zinc carbonate, 8-10 parts of ammonium citrate, 6-8 parts of dipotassium hydrogen phosphate, 8-10 parts of potassium tartrate, 5-8 parts of potassium citrate, 8-10 parts of cocamidopropyl betaine, 6-8 parts of sodium lauryl sulfate, 3-5 parts of ipratropium bromide and 100-150 parts of deionized water. The electroplating solution with the excellent performance is strong in corrosion resistance, can reduce pollution, is an ideal plated layer, and can maintain the metal concentration of the plated layer used by the electroplating solution, and an obtained plated layer is high in brightness, strong in corrosion resistance, and capable of meeting use requirements of severe environments.
Owner:张慧玲
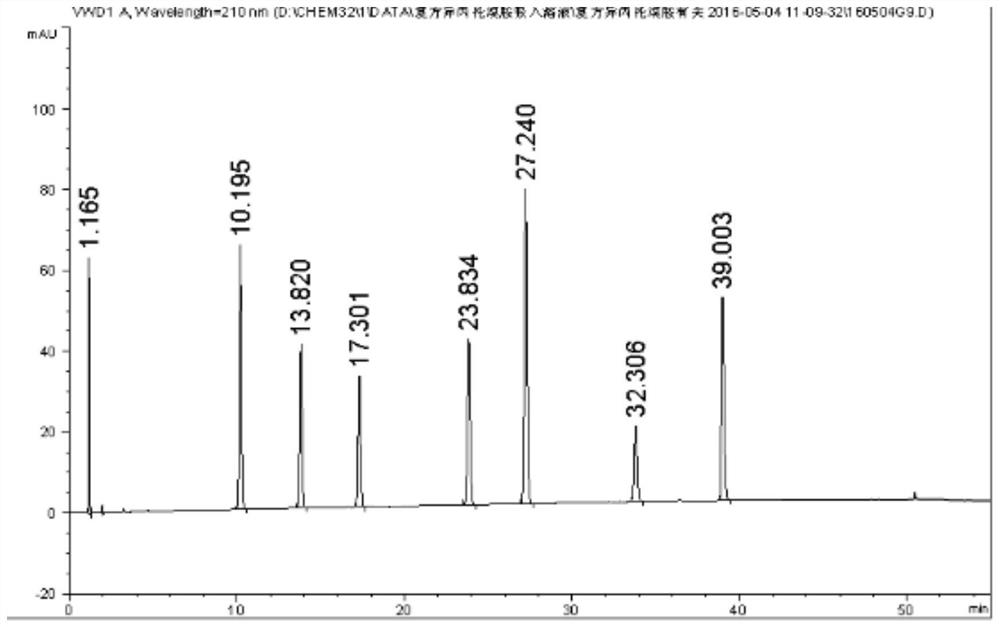
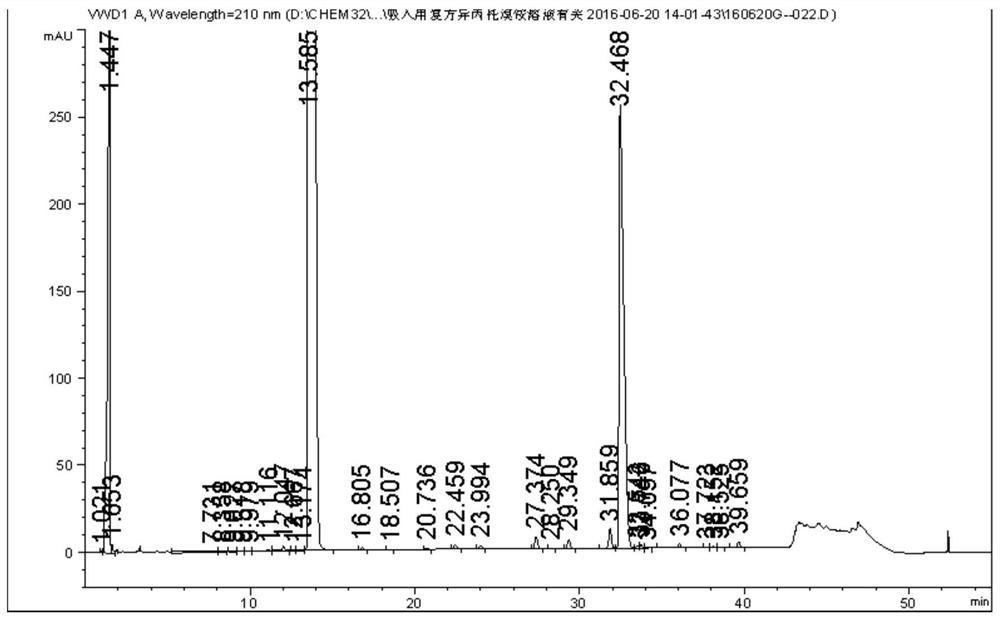
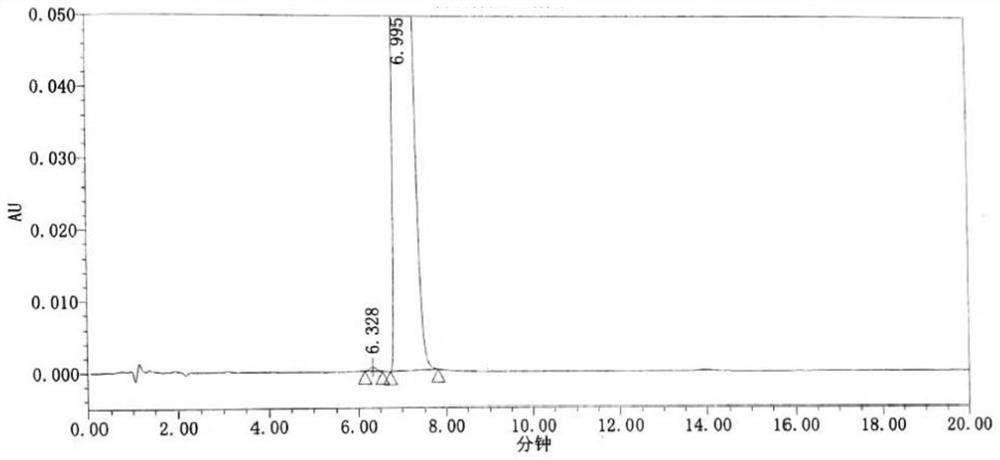
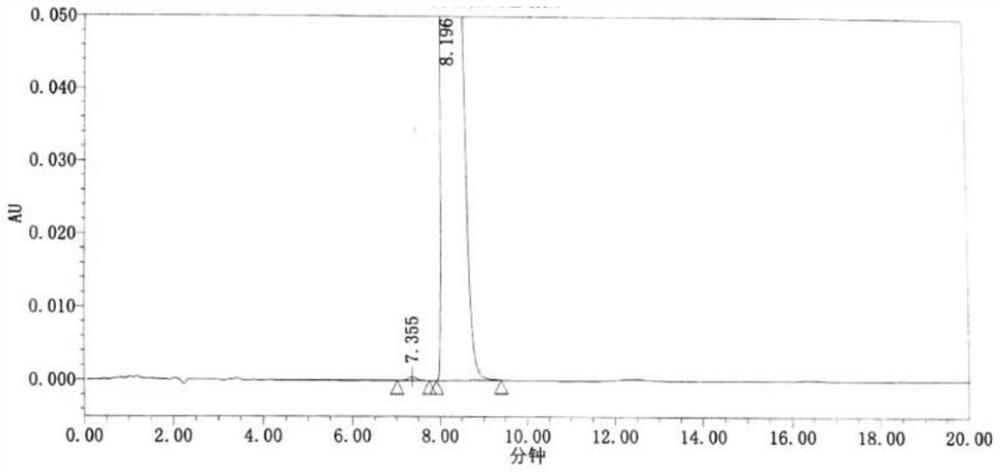
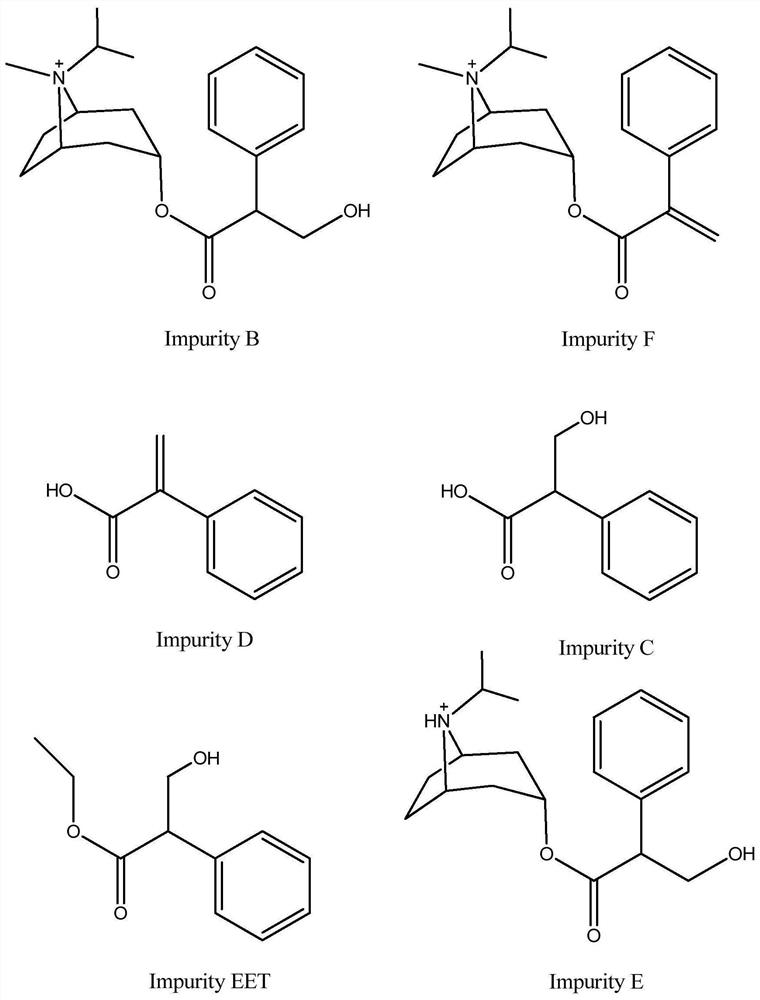
Method for synchronously detecting five related substances in compound ipratropium bromide solution for inhalation
ActiveCN111721845AMeet the requirementsAvoid replacementComponent separationAgainst vector-borne diseasesPhosphateSilica gel
The invention relates to a method for synchronously detecting five related substances in a compound ipratropium bromide solution for inhalation, and belongs to the technical field of drug quality determination methods. High performance liquid chromatography is adopted for detection, a chromatographic column with alkyl bonded silica gel is adopted as a filler, a phosphate buffer solution containingsodium heptanesulfonate is used as a mobile phase A, an organic phase is used as a mobile phase B, and a detection wavelength is 205-300nm. The detection method provided by the invention can effectively separate ipratropium bromide and salbutamol sulfate from other impurities, and has characteristics of simple operation, a comprehensive result, accuracy, reliability and strong specificity.
Owner:LUNAN PHARMA GROUP CORPORATION
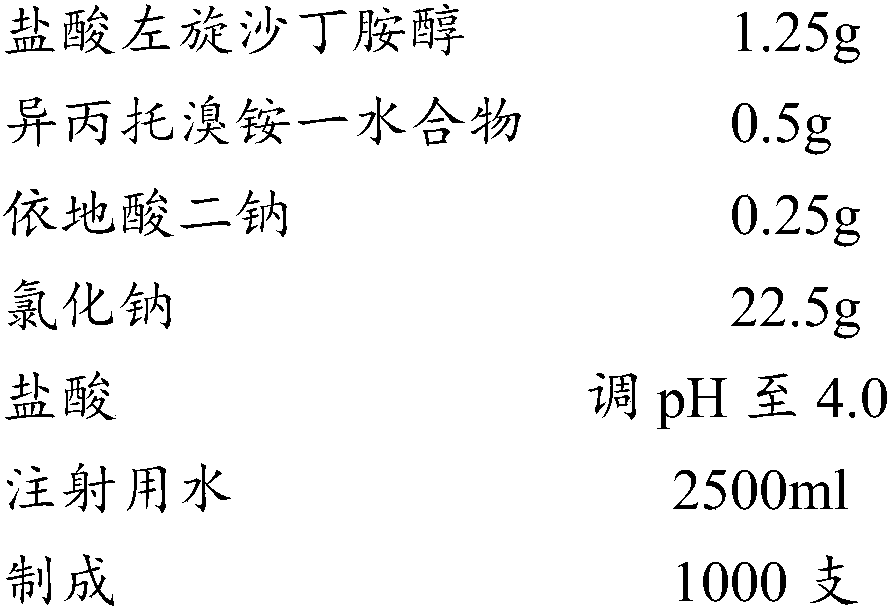
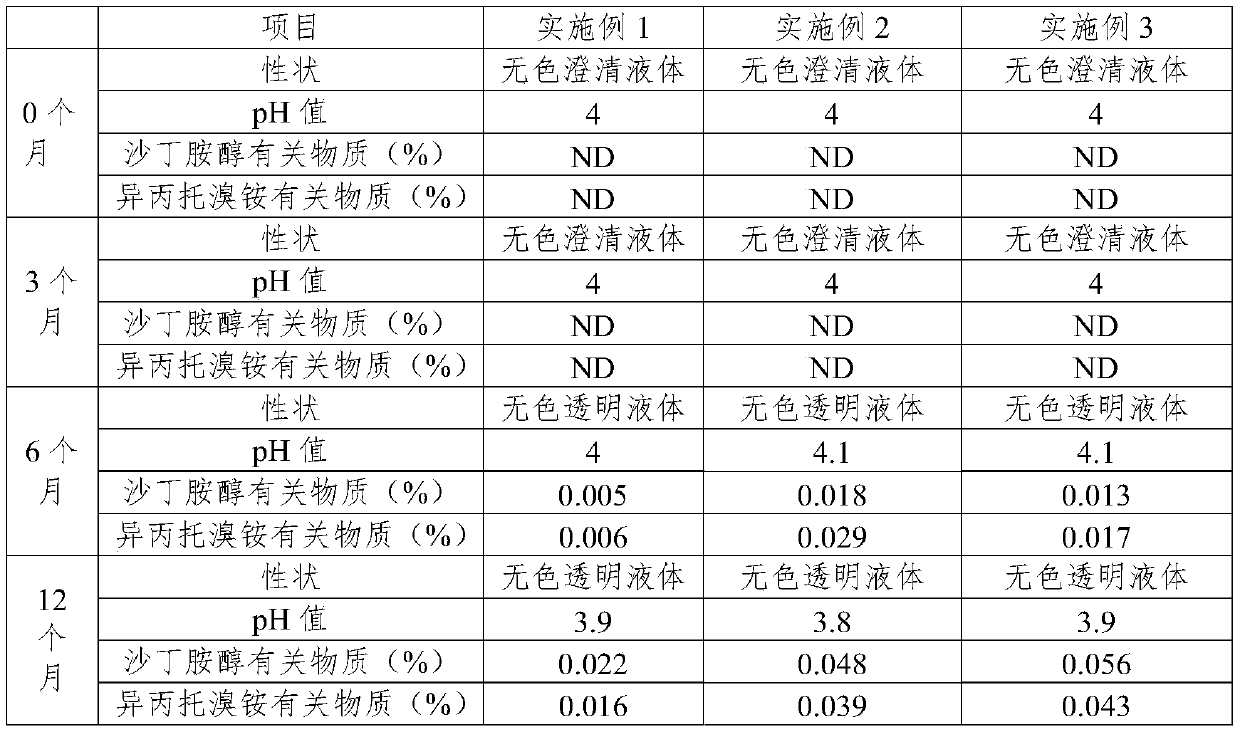
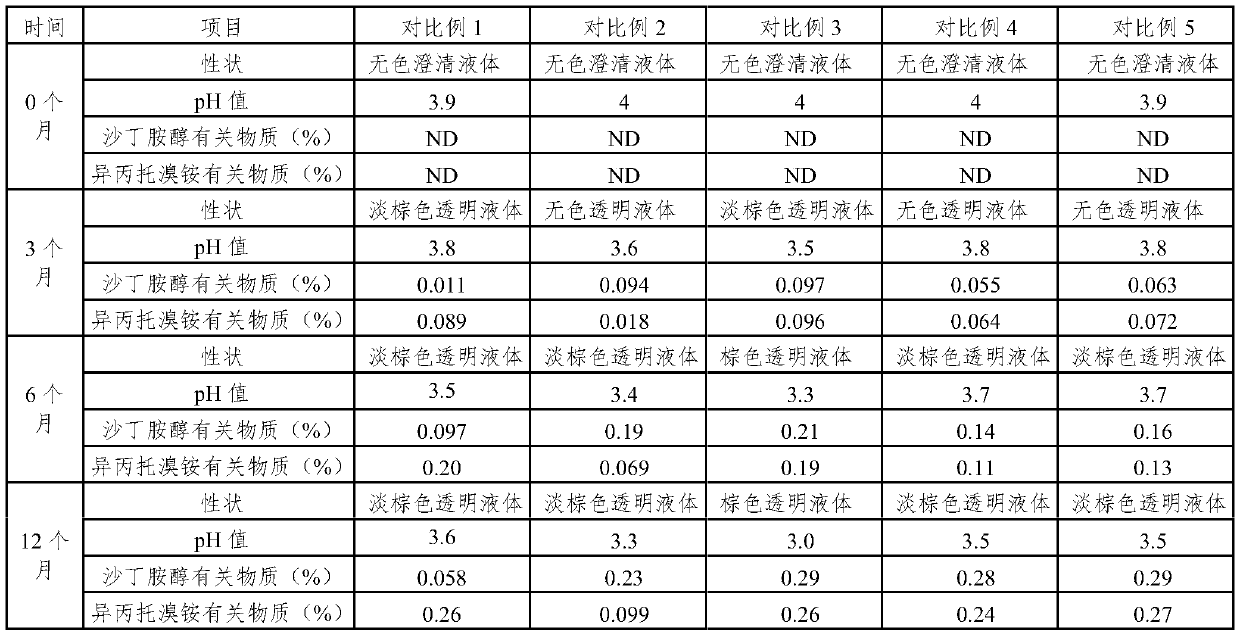
Medicine composition for treating respiratory diseases and preparation method thereof
PendingCN109602910AReduce riskImprove stabilityOrganic active ingredientsDispersion deliveryDiseaseSodium edetate
The present invention relates to a medicine composition for treating a respiratory disease, and a preparation method thereof. The medicine composition comprises a beta-2 receptor agonist with a singleconfiguration, ipratropium bromide or a monohydrate thereof, a stabilizer, an osmotic pressure adjusting agent, a pH adjusting agent and a dispersion medium, wherein the stabilizer is one or more selected from sodium edetate and ascorbic acid, and the usage amount of the stabilizer is 0.002% to 0.02%.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
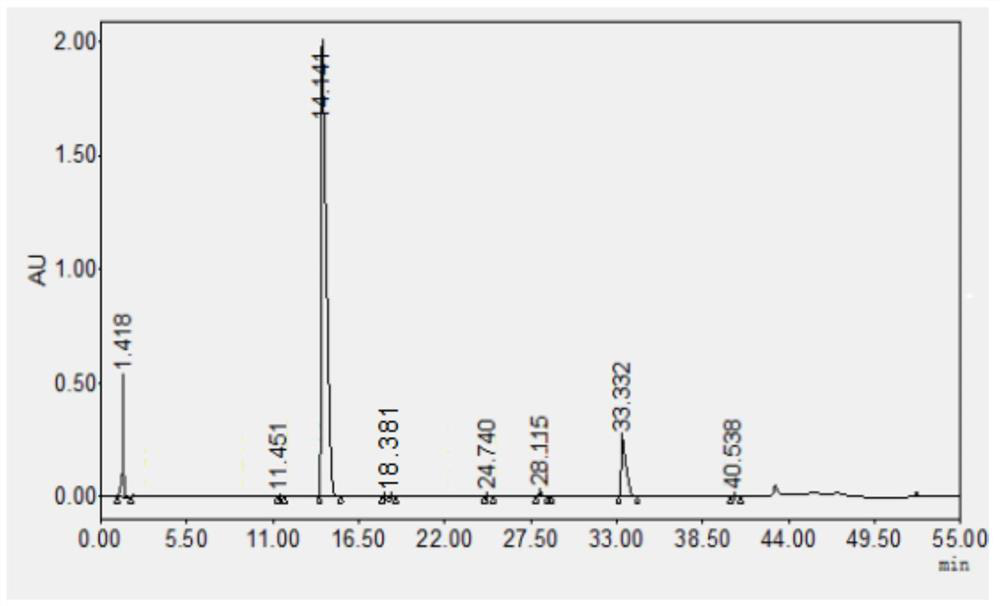
Method for detecting content of ipratropium bromide intermediate I and related substances
PendingCN111751454AAccurate detectionReduce smearingComponent separationUltraviolet detectorsFluid phase
The invention provides a method for detecting the content of an ipratropium bromide intermediate I and related substances. High performance liquid chromatography is adopted, propyl silane bonded silica gel serves as a chromatographic column, a mobile phase is composed of a phase A and a phase B, a phosphate buffer solution containing cation pairs serves as a mobile phase A, acetonitrile serves asa mobile phase B, the ratio of the mobile phase A to the mobile phase B is 90-98: 2-10, an ultraviolet detector is adopted, and the detection wavelength ranges from 210 nm to 230 nm. The method disclosed by the invention has the beneficial effects that the method can be used for rapidly and accurately detecting the exterior isomer impurities in the reduction product and has good specificity and sensitivity, the mobile phase is cheap and easy to obtain, the operation process is simple and convenient, and the accuracy and repeatability are good.
Owner:TIANJIN PHARMA GROUP CORP
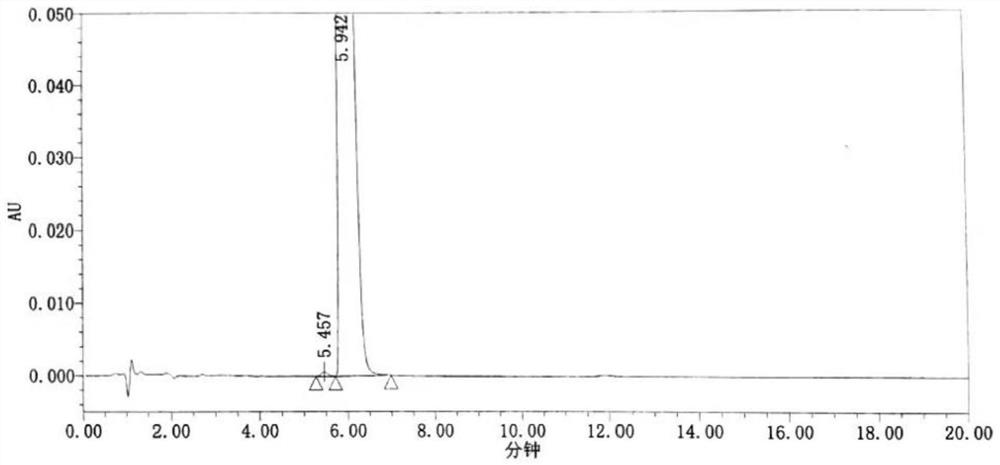
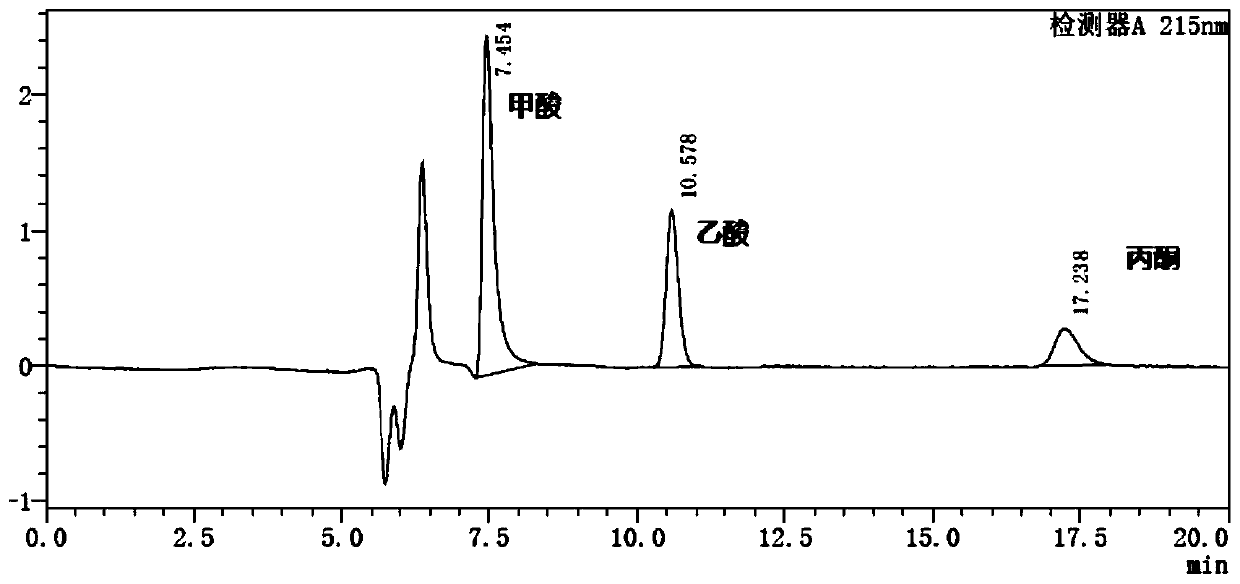
Method for detecting formic acid, acetic acid and acetone in ipratropium bromide solution for inhalation
ActiveCN110632221ARapid determinationAccurate measurementComponent separationSilica gelTetrahydrofuran
The invention discloses a method for detecting formic acid, acetic acid and acetone in an ipratropium bromide solution for inhalation, and solves the problem that a method for simultaneously determining formic acid, acetic acid and acetone in the ipratropium bromide solution for inhalation is unavailable in the prior art. According to the detection method disclosed by the invention, reversed-phasehigh-performance liquid chromatography is adopted for detection, diisopropyl substituted octadecylsilane bonded silica is used as a filler, a buffer salt solution with the pH value of 2.0-3.3 is usedas a mobile phase A, and an organic solvent is used as a mobile phase B, formic acid and acetic acid are eluted according to a ratio of A to B being (90-100):(0-10) to generate peaks, and then acetone is eluted according to a ratio of A to B being (75-100):(0-25) to generate peaks; the organic solvent is selected from one or more than two of acetonitrile, isopropanol, ethanol, tetrahydrofuran andmethanol. According to the method, the formic acid, the acetic acid and the acetone in the ipratropium bromide solution for inhalation can be rapidly and accurately measured, the product quality of the ipratropium bromide solution for inhalation is effectively guaranteed, and the medication safety is guaranteed.
Owner:SICHUAN PURITY PHARM CO LTD
Salbutamol sulfate solution for inhalation and preparation method thereof
ActiveCN110898042AImprove toleranceIncreased maximum relaxation ratePharmaceutical delivery mechanismPharmaceutical non-active ingredientsInhalationIpratropium bromide
The invention provides a salbutamol sulfate solution for inhalation and a preparation method thereof. The prescription of the salbutamol sulfate solution comprises salbutamol sulfate, ipratropium bromide, an isoosmotic adjusting agent, a pH adjusting agent, sodium hyaluronate, carboxymethyl chitosan oligosaccharide and water for injection. The preparation process comprises the following steps: weighing, preparing a solution, adjusting the pH value, filtering, encapsulating, carrying out quality inspection and the like. According to the prescription and the preparation method, the stability ofthe preparation can be improved, meanwhile, the preparation has a good permeation effect, the salbutamol sulfate tolerance can be improved, and the preparation can better play a role.
Owner:深圳大佛药业股份有限公司
Synthesis method of ipratropium bromide
The invention relates to the technical field of medicine synthesis, and particularly discloses a synthesis method of ipratropium bromide. The synthesis method of the ipratropium bromide comprises thefollowing steps: carrying out an acylating chlorination reaction on 2-phenyl-3-acetoxypropionic acid and oxalyl chloride in an organic solvent, then adding an isopropylnortropine mesylate solution, performing a reaction, removing the organic solvent, and adding an inorganic acid into the remaining reaction solution for hydrolysis; and extracting and separating out a reaction product in the hydrolysate, and carrying out a bromomethylation reaction on the reaction product and added bromomethane to obtain the ipratropium bromide. The method can be carried out at low temperature, and the product obtained by the reaction has the advantages of high purity, high yield, low enantiomer content and higher quality.
Owner:SHIJIAZHUANG NO 4 PHARMA +1
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide in a 0.5 ml volume.
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:德艾公司
Ipratropium bromide spray containing surfactant
The invention relates to a drug ipratropium bromide spray for treating respiratory diseases, especially asthma, chronic obstructive pneumonia, trachitis and the like. An inhalable surfactant can makethe spray particle size, the spray time and the spray speed of the composition more suitable by adjusting the surface tension of the ipratropium bromide spray pharmaceutical composition without a propellant.
Owner:TIANJIN JINYAO GRP
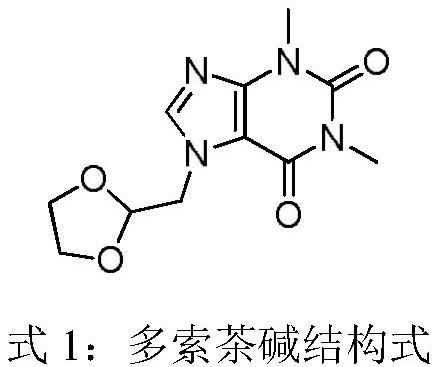
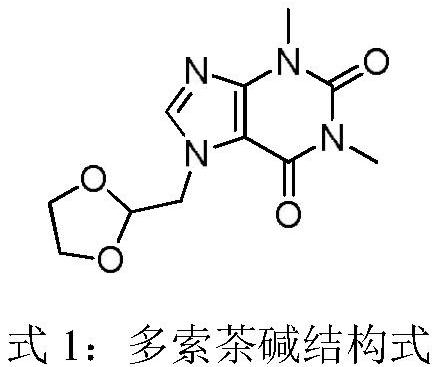
Compound doxofylline solution for inhalation and preparation method thereof
ActiveCN112618519APrevent shrinkageDispersion deliveryInorganic non-active ingredientsDoxofyllineUse medication
The invention discloses a compound doxofylline solution for inhalation and a preparation method thereof. The compound doxofylline solution for inhalation comprises the following components: an active pharmaceutical ingredient, an isoosmotic adjusting agent, an antioxidant, a pH regulator and water for injection, and the active pharmaceutical ingredient comprises a mixture of doxofylline and ipratropium bromide. The compound doxofylline solution for inhalation disclosed by the invention is suitable for patients needing combined application of multiple bronchodilators, is used for treating reversible bronchospasm related to airway obstructive diseases, and has more stable quality and is safer and more convenient to use in comparison with the prior art.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Preparation method of compound ipratropium bromide solution for inhalation
PendingCN114699393AChange pH valueReduce contentInorganic non-active ingredientsPharmaceutical delivery mechanismInhalationPhysical chemistry
The invention relates to a preparation method of a compound ipratropium bromide solution for inhalation, which comprises the following steps: S1, adding injection water accounting for 75% of the total preparation amount into a liquid preparation tank, and cooling the injection water to below 40 DEG C; s2, taking injection water accounting for 5% of the total preparation amount, cooling to 50-70 DEG C, adding sodium chloride, stirring, pouring into a liquid preparation tank, uniformly stirring, and adding diluted hydrochloric acid to obtain a first acidic solution; s3, taking injection water which accounts for 1-3% of the total preparation amount and is cooled to room temperature, adding ipratropium bromide, stirring, pouring into a liquid preparation tank, and uniformly stirring; s4, taking the injection water which accounts for 7-15% of the total preparation amount and is cooled to room temperature, adding diluted hydrochloric acid to obtain a second acidic solution, adding salbutamol sulfate while stirring, stirring and dissolving until the solution is clear, and pouring the solution into a liquid preparation tank for stirring; and S5, adding the remaining water for injection according to the prescription amount to the total preparation amount, stirring until the solution is uniformly stirred, adjusting the pH value of the solution to 3.0-3.5, and uniformly stirring.
Owner:成都普什制药有限公司
Composition containing cerebroprotein hydrolysate and application of composition
ActiveCN104189036AImprove memory lossEnhance memoryNervous disorderHydrolysed protein ingredientsBiotechnologyPANAX NOTOGINSENG ROOT
The invention discloses a composition containing cerebroprotein hydrolysate and application of the composition. The composition comprises the following components in parts by weight: 10-12 parts of cerebroprotein hydrolysate, 6-8 parts of panax notoginseng saponins, 3-5 parts of ipratropium bromide and 1.5-2.5 parts of betadex. According to the composition containing cerebroprotein hydrolysate, the panax notoginseng saponins, the ipratropium bromide, the betadex and the cerebroprotein hydrolysate are firstly combined to achieve effects of improving the memory loss condition and strengthening the memory by virtue of proper proportion regulation, and can be used for preparing a drug or a health product for strengthening the memory.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20110247613A1Relieve bronchospasmDispersion deliverySolution deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide in a 0.5 ml volume.
Owner:MYLAN SPECIALTY
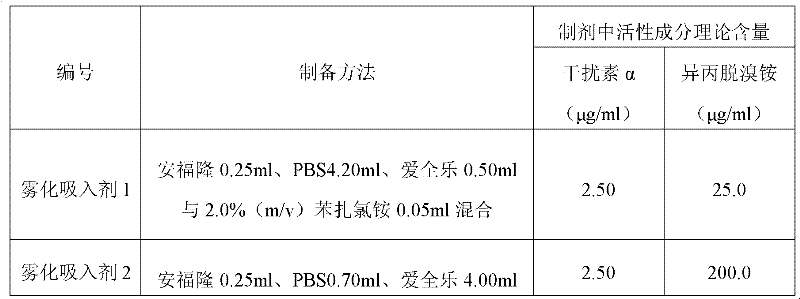
Aerosol inhalant containing interferon alpha and ipratropium bromide
ActiveCN102416166AGood treatment effectPeptide/protein ingredientsPharmaceutical delivery mechanismAntiviral drugCurative effect
The invention belongs to the field of medicinal compositions for resisting virus, and relates to an aerosol inhalant containing interferon alpha and ipratropium bromide. The aerosol inhalant contains a therapeutically effective amount of interferon alpha, a therapeutically effective amount of ipratropium bromide and an appropriate amount of pharmaceutic auxiliary materials; preferably, single dose of the aerosol inhalant contains 2.5 to 30mu g of interferon alpha, 0.025 to 0.2mg of ipratropium bromide and an appropriate amount of pharmaceutic auxiliary materials; and more preferably, single dose of the aerosol inhalant contains 10 to 20mu g of interferon alpha, 0.05 to 0.1mg of ipratropium bromide and an appropriate amount of pharmaceutic auxiliary materials. Compared with the interferon alpha or the ipratropium bromide, the aerosol inhalant containing the interferon alpha and the ipratropium bromide has the advantages that the effect of treating viral pneumonia can be improved obviously.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
A kind of propofol phosphate gas defoamer and its preparation method and use method
ActiveCN106731031BGood defoamingGood dispersionFoam dispersion/preventionPhosphoric Acid EstersPhosphate
The invention belongs to the technical field of textile auxiliaries and discloses a propofol phosphate gas defoamer. The propofol phosphate gas defoamer is prepared from the following components in parts by weight: 0.1 to 0.8 part of propofol phosphate, 0.5 to 1.7 parts of ipratropium bromide, 100 to 150 parts of nitrogen, 15 to 20 parts of helium, 1 to 4 parts of oxygen, 0.1 to 1.5 parts of carbonic oxide, 12 to 14 parts of argon and 10 to 15 parts of ethanol. The preparation method of the propofol phosphate gas defoamer comprises the following steps: dissolving propofol phosphate into an ethanol solution, heating and stirring at the temperature of 30 to 40 DEG, collecting evaporation gas and mixing the evaporation gas with nitrogen; then mixing the mixed gas with isopropyl ammonium bromide aerosol, subsequently carrying out ultraviolet irradiation treatment for 1 to 2 hours, and mixing a treated mixture with other gases, thus obtaining a finished product. The gas defoamer is introduced into the bottom of a working bath for use, wherein the use amount of working solution per liter is 1 to 2L per minute; by means of adding gas, the dispersion uniformity of the working bath is improved; at the same time, a good foam eliminating and inhibiting effect is achieved; the propofol phosphate gas defoamer can be recycled and is high in economic benefit.
Owner:浙江伟丰新材料有限公司
UPLC (Ultra Performance Liquid Chromatography) analysis method of ipratropium bromide aerosol
ActiveCN113820404AGood precisionImprove linearityComponent separationNuclear energy generationIsocratic elutionFluid phase
According to the UPLC analysis method for determining the ipratropium bromide aerosol, the analysis efficiency is remarkably improved, and the detection process is completed within 8 minutes. According to the method, ultra-high performance liquid chromatography is adopted, and the chromatographic conditions are as follows: octadecylsilane chemically bonded silica is used as a filler; a sodium heptanesulfonate aqueous solution (A) with a pH value of 2.6 and acetonitrile (B) with a ratio of 72: 28 are taken as a mobile phase; the detection wavelength is 210 nm; the column temperature is 40 DEG C; the flow velocity is 0.6 ml / min; and isocratic elution is carried out.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Ipratropium bromide aerosol composition without propellant and preparation method thereof
The invention relates to a drug ipratropium bromide for treating respiratory diseases, especially asthma, chronic obstructive pneumonia, bronchitis and the like, the drug is the inhalable aerosol without a propellant, and the invention also relates to a preparation method of the drug.
Owner:TIANJIN JINYAO GRP
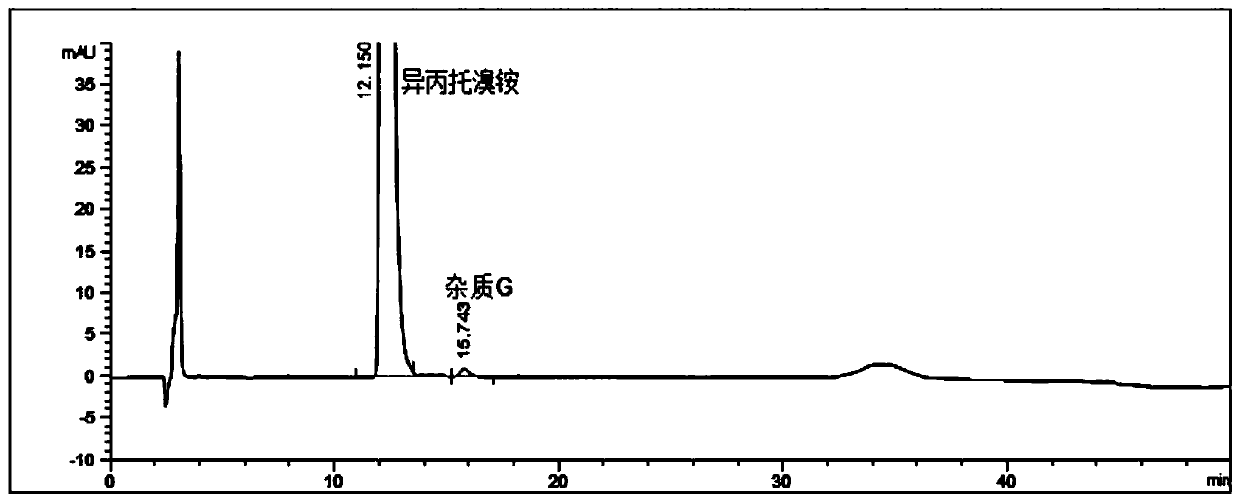
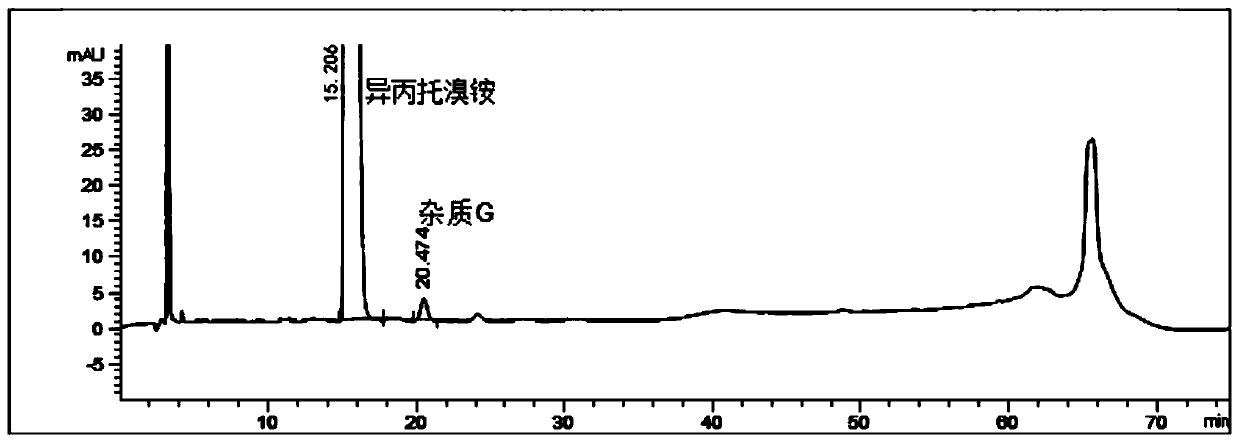
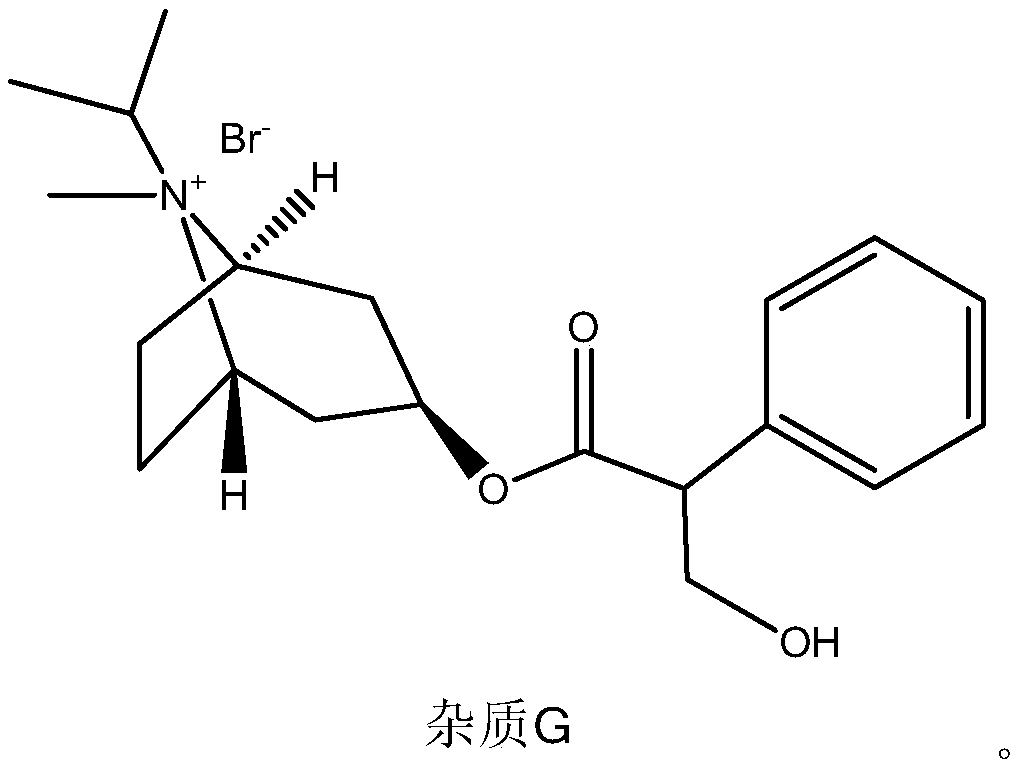
Detection method for impurities G in ipratropium bromide aerosol
ActiveCN110361494AMake up for the lack of quality controlRealize synchronous detectionComponent separationGradient elutionIpratropium bromide
The present invention discloses a detection method for impurities G in ipratropium bromide aerosol. According to the detection method, double flow phases and more than three sections of gradient elution programs are adopted; and a liquid phase chromatography method is adopted to measure the content of the impurities G in the ipratropium bromide aerosol. With the detection method of the invention adopted, an accurate and efficient solution can be provided for the defect that effective detection means are not available for the detection of impurities G in the field.
Owner:SICHUAN PURITY PHARM CO LTD
Continuous process for preparation of anticholinergic agents
Owner:HOVIONE SCIENTIA
Preparation method of choline receptor antagonist
ActiveCN113943286AMild reaction conditionsEasy to operateOrganic chemistryIsopropylPhenylacetic acid
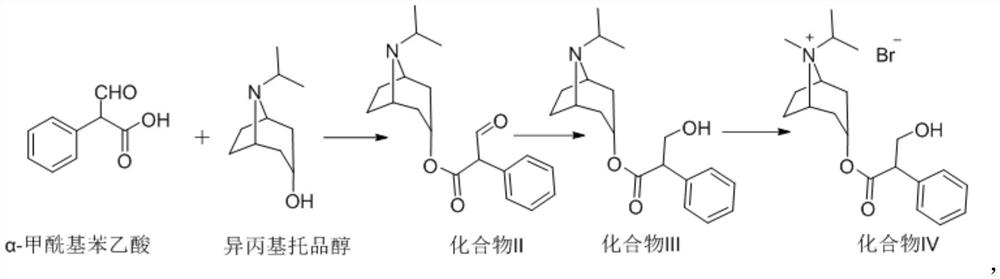
The invention provides a preparation method of a choline receptor antagonist. The choline receptor antagonist is ipratropium bromide. According to the method provided by the invention, alpha-formyl phenylacetic acid is taken as an initial raw material and reacts with isopropyl tropine alcohol to generate a compound II, the compound II is subjected to a reduction reaction to generate a compound III, and the compound III reacts with bromomethane to generate ipratropium bromide. Compared with the prior art, the method is easy to operate, high in safety, low in cost, high in yield and product purity and more suitable for industrial production.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
A kind of detection method of impurity a in different third detropammonium bromide
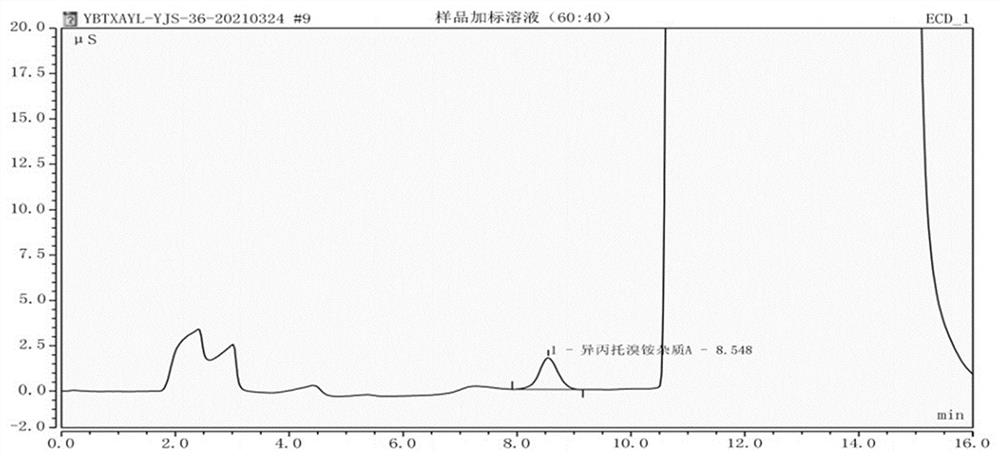
ActiveCN112986450BAvoid inaccuraciesRapid determinationComponent separationPhysical chemistryIpratropium bromide
The invention provides a method for detecting impurity A in ipratropium bromide, said method comprising the following steps: (1) preparing a reference substance solution: accurately weighing an appropriate amount of ipratropium bromide impurity A reference substance, and using ultra-pure Dissolve and dilute with water to make a reference substance solution containing about 5 μg of ipratropium bromide impurity A in every 1ml; Dissolve in pure water and dilute to the mark, shake well, and you get it; (3) Detection: Precisely draw 500 μl of the reference solution prepared in step (1) and the test solution prepared in step (2) and inject it into a chromatographic column equipped with a cation measured in an ion chromatograph.
Owner:JOINCARE HAIBIN PHARM CO LTD
A kind of compound doxofylline solution for inhalation and preparation method thereof
ActiveCN112618519BPrevent shrinkageDispersion deliveryInorganic non-active ingredientsDoxofyllineUse medication
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com