Aerosol inhalant containing interferon alpha and ipratropium bromide
A technology of aerosol inhalation and isopropyl deammonium bromide, which is applied in the directions of antiviral agents, respiratory diseases, active ingredients of heterocyclic compounds, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
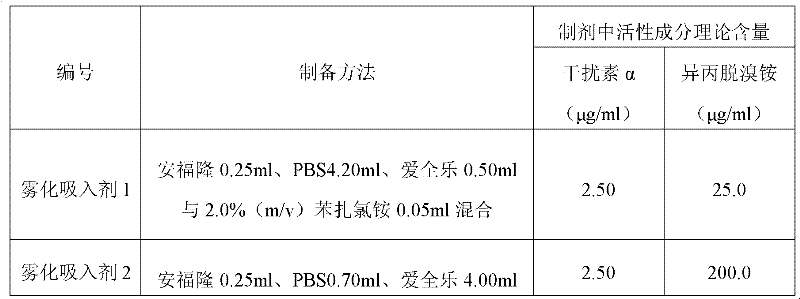
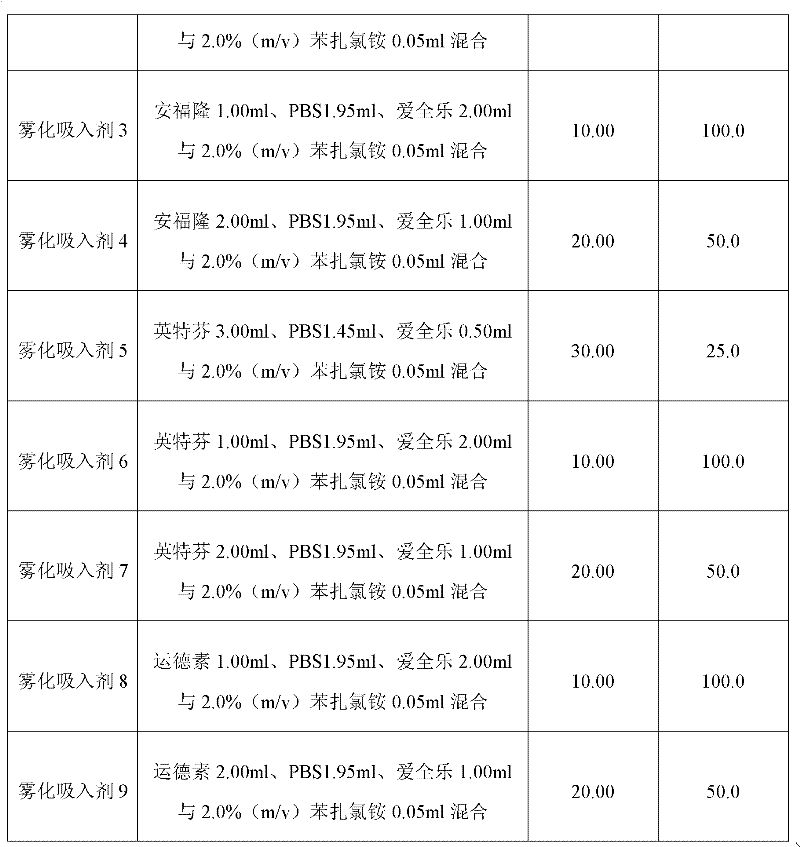
[0028] Embodiment 1: the preparation of the atomized inhalation of interferon α and isopropyl debromide
[0029] The nebulized inhalation of interferon α and isopridine bromide was prepared according to the method in Table 1 below. Among them, "Anfulong" (trade name) is the recombinant human interferon α2b injection with a specification of 50 μg / ml / bottle produced by Tianjin Hualida Bioengineering Co., Ltd.; "Interfern" (trade name) is Shenyang Sansheng Pharmaceutical Co., Ltd. Recombinant human interferon α2a injection with a specification of 50 μg / ml / bottle produced by the limited liability company; Interferon α1b injection, "Aiquanle" (trade name) is the iprodeammonium bromide solution for inhalation with a specification of 0.5mg / 2ml / bottle produced by Shanghai Boehringer Ingelheim Pharmaceutical Co., Ltd.; "PBS" is the solution containing 0.15mol / L NaCl in 25mmol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer (pH7.0). Unless otherwise specified in the s...
Embodiment 2
[0033] Embodiment 2: the purity detection of isopropyl debromium after mixing interferon alpha and isopropyl debromium
[0034] The aerosolized inhalation of each interferon α and isopropyl debromide prepared by the method of Example 1 was carried out ultrafiltration treatment with Millipore ultrafiltration centrifuge tubes with a molecular weight cut-off of 3000Da after placing the preset time, and filtered Dilute the supernatant with deionized water to the same volume as before ultrafiltration and centrifugation, and then take 20 μl of KromasilC 8 Reversed-phase high-performance liquid chromatography column (5μm filler particle size, column size 4.6×150mm), chromatographic operation was performed at a column temperature of 35°C, the mobile phase was acetonitrile-0.25% sodium heptanesulfonate (29:71), and phosphoric acid was used Adjust the pH value to 3.2, the flow rate is 1.0ml / min, and the detection wavelength is 210nm. The measurement results are shown in Table 2 below. ...
Embodiment 3
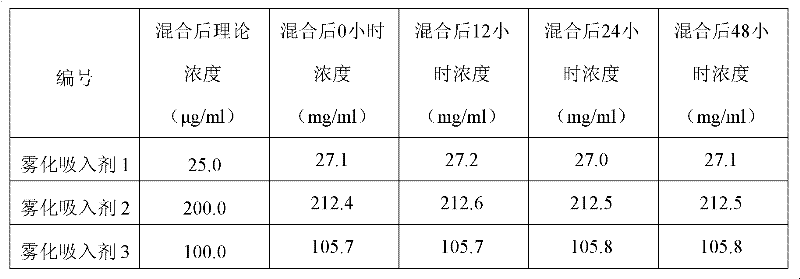
[0038] Embodiment 3: Interferon alpha and isopropyl debromium content detection after mixing isopropyl debromium
[0039] The isopropyl debrominated ammonium reference substance (prepared by China Institute for the Control of Pharmaceutical and Biological Products, batch number 100522-200601) was dissolved and diluted with water respectively to a concentration of 0.0258, 0.0515, 0.1030, 0.2060, 0.3090 mg / ml and then according to the high-efficiency liquid phase of Example 2 Chromatographic conditions were applied to determine the peak area of the main peak. Use the peak area (y) to perform linear regression on the concentration (x) to obtain a working curve, the equation is y=19000000x+11396, and the regression coefficient is r=0.9999.
[0040] The aerosolized inhalation of each interferon α and isopropyl debromide prepared by the method of Example 1 is carried out ultrafiltration centrifugation according to the method of Example 2 respectively after placing the preset time,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com