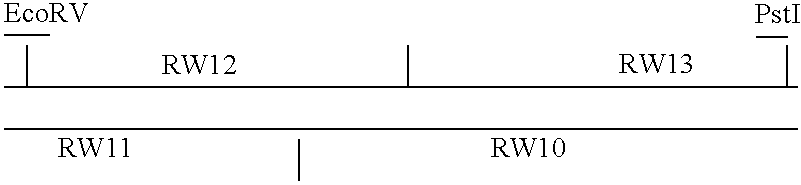Patents
Literature
1323 results about "Interferon alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alpha interferon. noun. : an interferon produced by white blood cells that inhibits viral replication, suppresses cell proliferation, and regulates immune response and that is used in a form obtained from recombinant DNA to treat various diseases — compare beta interferon, gamma interferon.
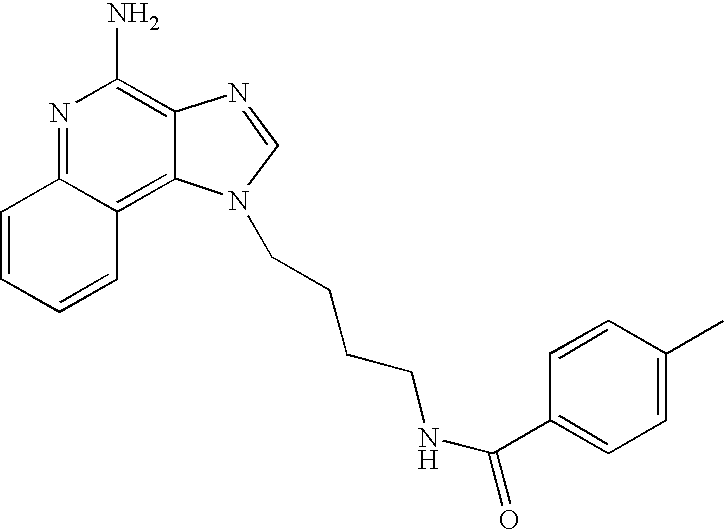
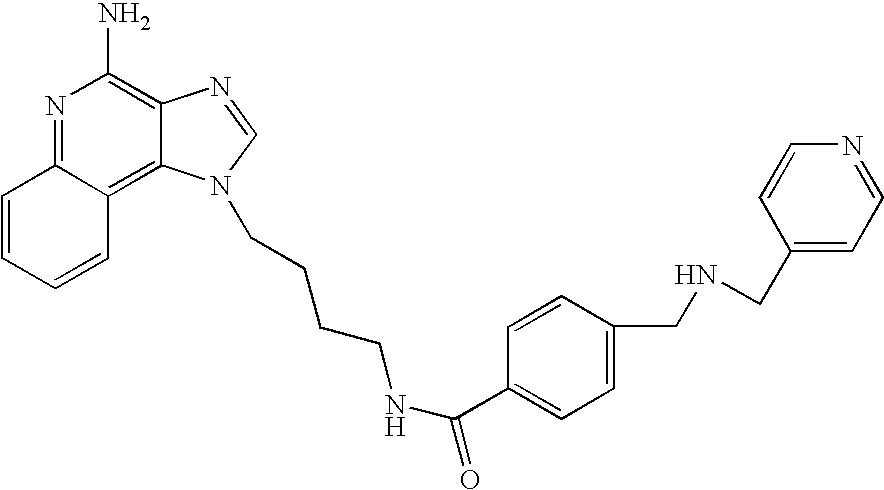
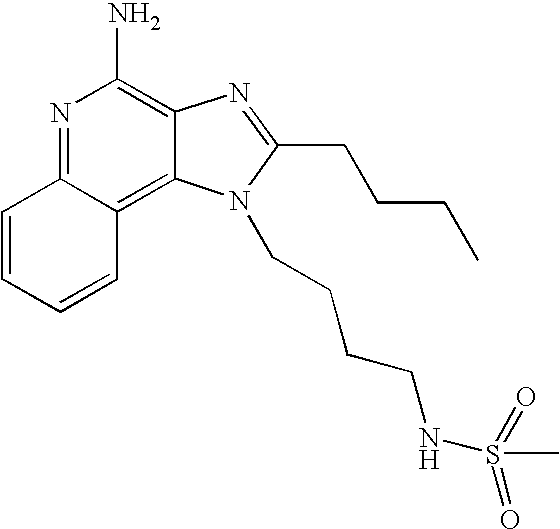
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
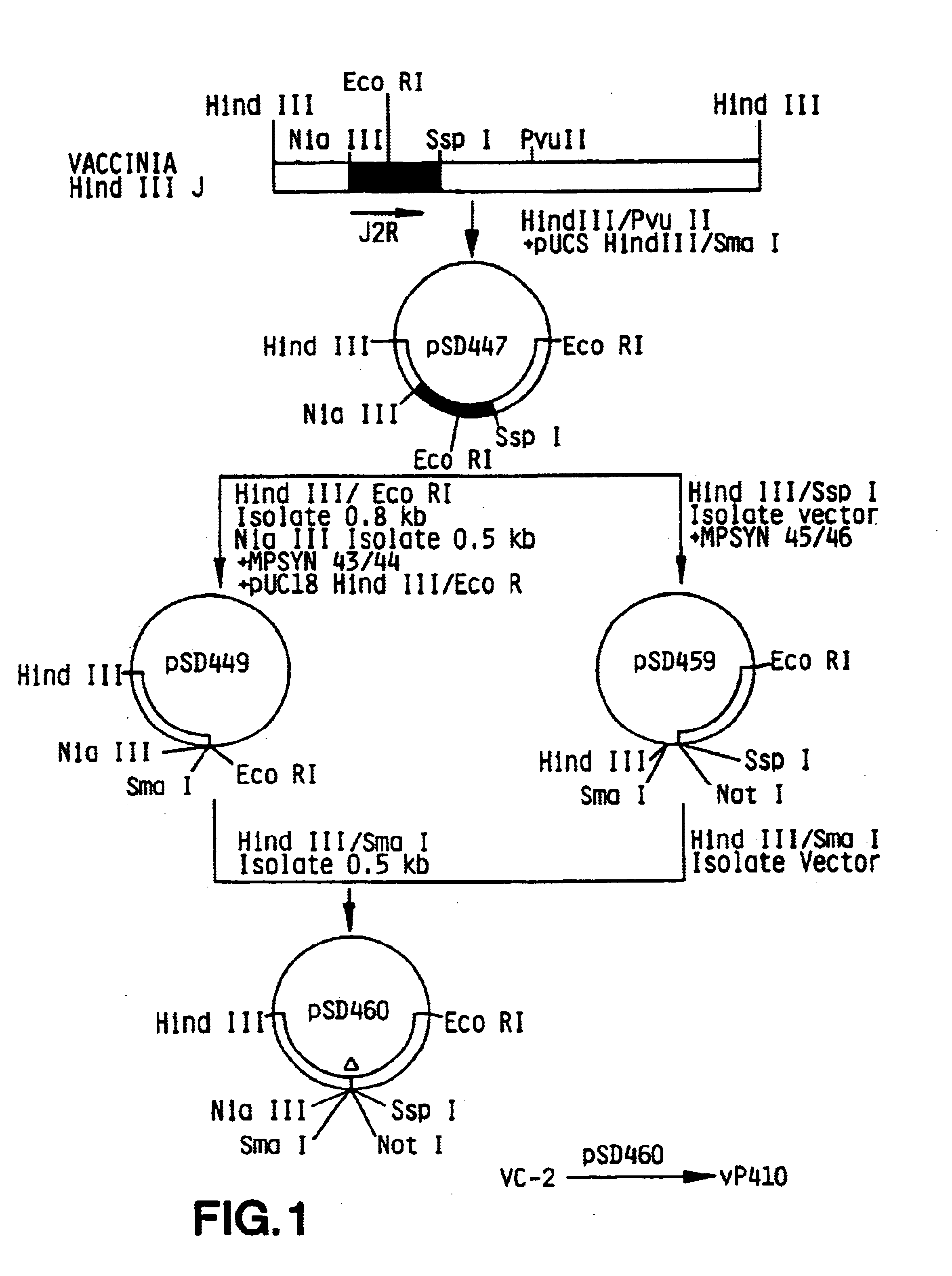
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
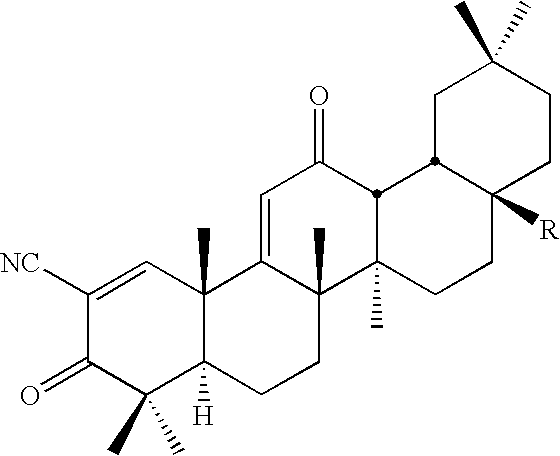
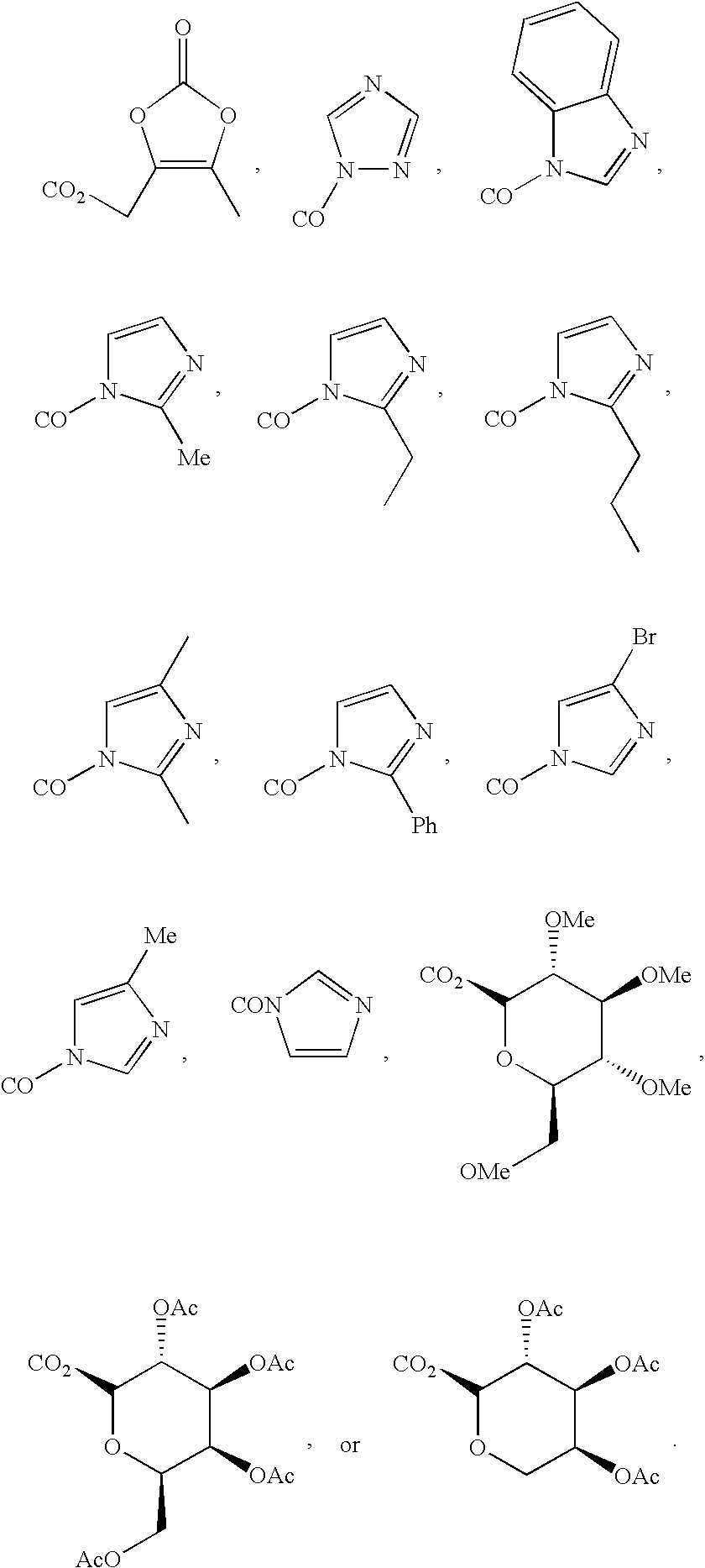
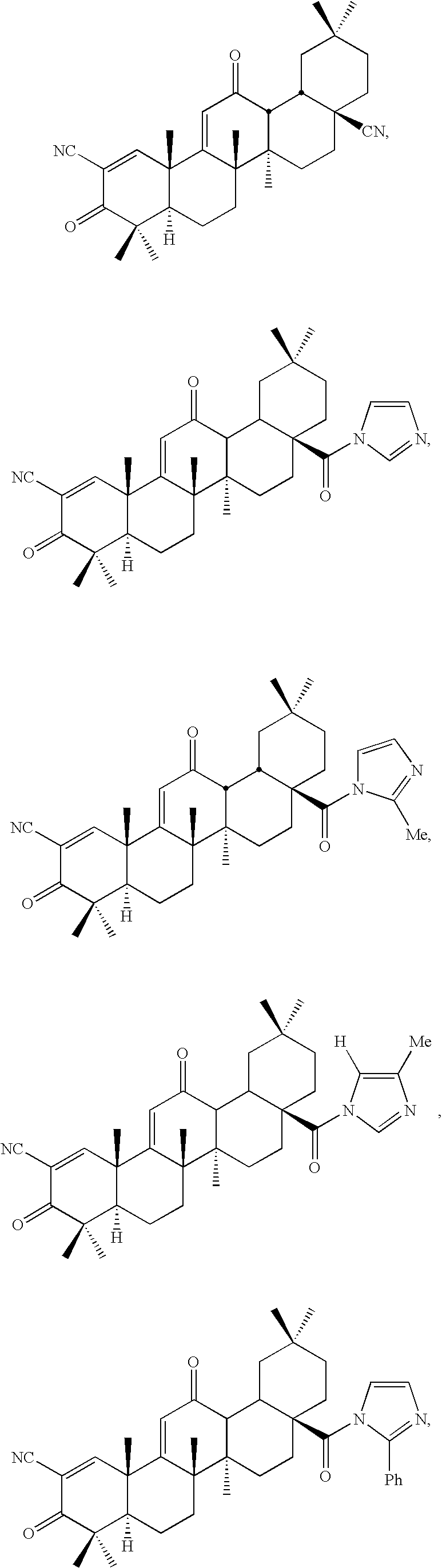
Inhibitors and methods of use thereof
New triterpenoid derivatives with various substituents at the C-17 position of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were synthesized. Among them, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (CNDDO), 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl) imidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-2-methylimidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-4-methylimidazole show extremely high inhibitory activity (IC50=0.01-1 pM level) against production of nitric oxide induced by interferon-γ in mouse macrophages. These compounds can be used in the prevention or treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, multiple sclerosis, rheumatoid arthritis, and other inflammatory diseases. All the new triterpenoid derivatives are more potent than previously known CDDO.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Fully human antibody Fab fragments with human interferon-gamma neutralizing activity
InactiveUS7084257B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsDNA-binding domainAntigen binding
Selective binding agents of interferon-gamma (IFNγ) are provided by the invention. More particularly, the invention provides for antibodies and antigen binding domains which selectively bind to IFNγ and may be used to prevent or treat conditions relating to autoimmune and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis. Nucleic acid molecules encoding said antibodies and antigen binding domains, and expression vectors and host cells for the production of same are also provided.
Owner:AMGEN INC
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Media and method for treating pathological syndrome
InactiveUS7572441B2Energy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsNatural antibodyUltra low dose
A medicament based on antibodies contains an activated form of monoclonal, polyclonal, or natural antibodies to interferon in low or ultra-low doses prepared by multiple consecutive dilutions and exposure to external factors, preferably in accordance with homeopathic technology. In order to obtain antibodies, human or heterologous interferon alpha, beta, or gamma, including recombinant interferon, is used; a mixture of various, mostly centimal, homeopathic dilutions being employed. A method of treating a pathologic syndrome, whose formation is affected by interferon, consists in the use of activated forms of antibodies to interferon alpha, beta, or gamma in low or ultra-low doses obtained by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Glycopegylated Interferon Alpha
InactiveUS20090028822A1Improved pharmacokinetic propertiesPeptide/protein ingredientsDepsipeptidesInterferon alphaSugar
The present invention provides IFN-α conjugates including IFN-α peptides and modifying groups such as PEG moieties. The IFN-α peptide and modifying group are linked via an intact glycosyl linking group interposed between and covalently attached to the IFN-α peptide and the modifying group. The IFN-α conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar onto an amino acid or a glycosyl residue on the IFN-α peptide. Also provided are methods for preparing the IFN-α conjugates, methods for treating various disease conditions with the IFN-α conjugates, and pharmaceutical formulations including the IFN-α conjugates.
Owner:NOVO NORDISK AS
Interferon alpha antibodies and their uses
ActiveUS20070014724A1Inhibit biological activityInhibiting surface expressionPeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides isolated anti-interferon alpha monoclonal antibodies, particularly human monoclonal antibodies, that inhibit the biological activity of multiple interferon (IFN) alpha subtypes but do not substantially inhibit the biological activity of IFN alpha 21 or the biological activity of either IFN beta or IFN omega. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting the biological activity of IFN alpha using the antibodies of the invention, as well as methods of treating disease or disorders mediated by IFN alpha, such as autoimmune diseases, transplant rejection and graft versus host disease, by administering the antibodies of the invention.
Owner:MEDAREX LLC
Method for administering a cytokine to the central nervous system and the lymphatic system
InactiveUS6991785B2Provide effectModulate immune and inflammatory responseBiocideNervous disorderImmunologic disordersInterferon alpha
The present invention is directed to a method for delivering cytokines to the central nervous system and the lymphatic system by way of a tissue innervated by the trigeminal nerve and / or olfactory nerve. Cytokines include tumor necrosis factors, interleukins, interferons, particularly interferon-β and its muteins such as IFN-βser17. Such a method of delivery can be useful in the treatment of central nervous system disorders, brain disorders, proliferative, viral, and / or autoimmune disorders such as Sjogren's disorder.
Owner:CHIRON CORP
Drug for reducing side effects in ribavirin interferon combination therapy
InactiveUS20060088502A1Eliminate side effectsReduces side effects found in ribavirin/IFNBiocideElcosanoid active ingredientsChronic viral hepatitis CSide effect
There is provided a drug for reducing side effects, anemia in particular, in combination therapy of chronic hepatitis C with ribavirin and interferon, which contains as the active ingredient at least one member selected from the group consisting of eicosapentaenoic acid (EPA) and pharmaceutically acceptable salts and esters thereof.
Owner:MOCHIDA PHARM CO LTD
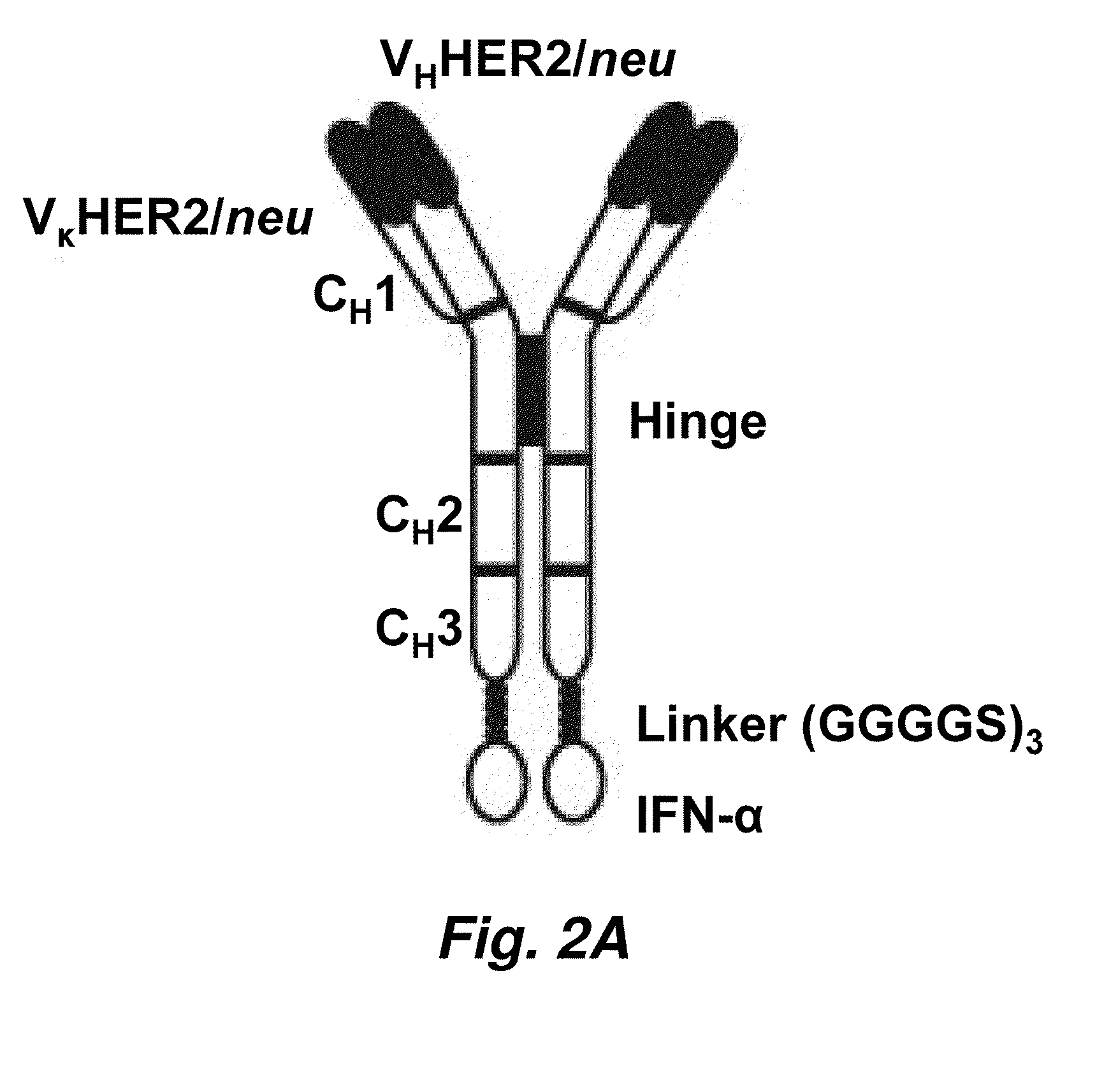
Targeted interferons demonstrate potent apoptotic and Anti-tumor activities
ActiveUS20100172868A1Peptide/protein ingredientsPharmaceutical delivery mechanismInterferon alphaAntitumor activity
Novel chimeric moieties that show significant efficacy against cancers are provided. In certain embodiments the chimeric moieties comprise a targeting moiety attached to an interferon. In certain embodiments, the chimeric moieties comprise fusion proteins where an antibody that specifically binds to a cancer marker is fused to interferon alpha (IFN-α) or interferon beta (IFN-β).
Owner:RGT UNIV OF CALIFORNIA
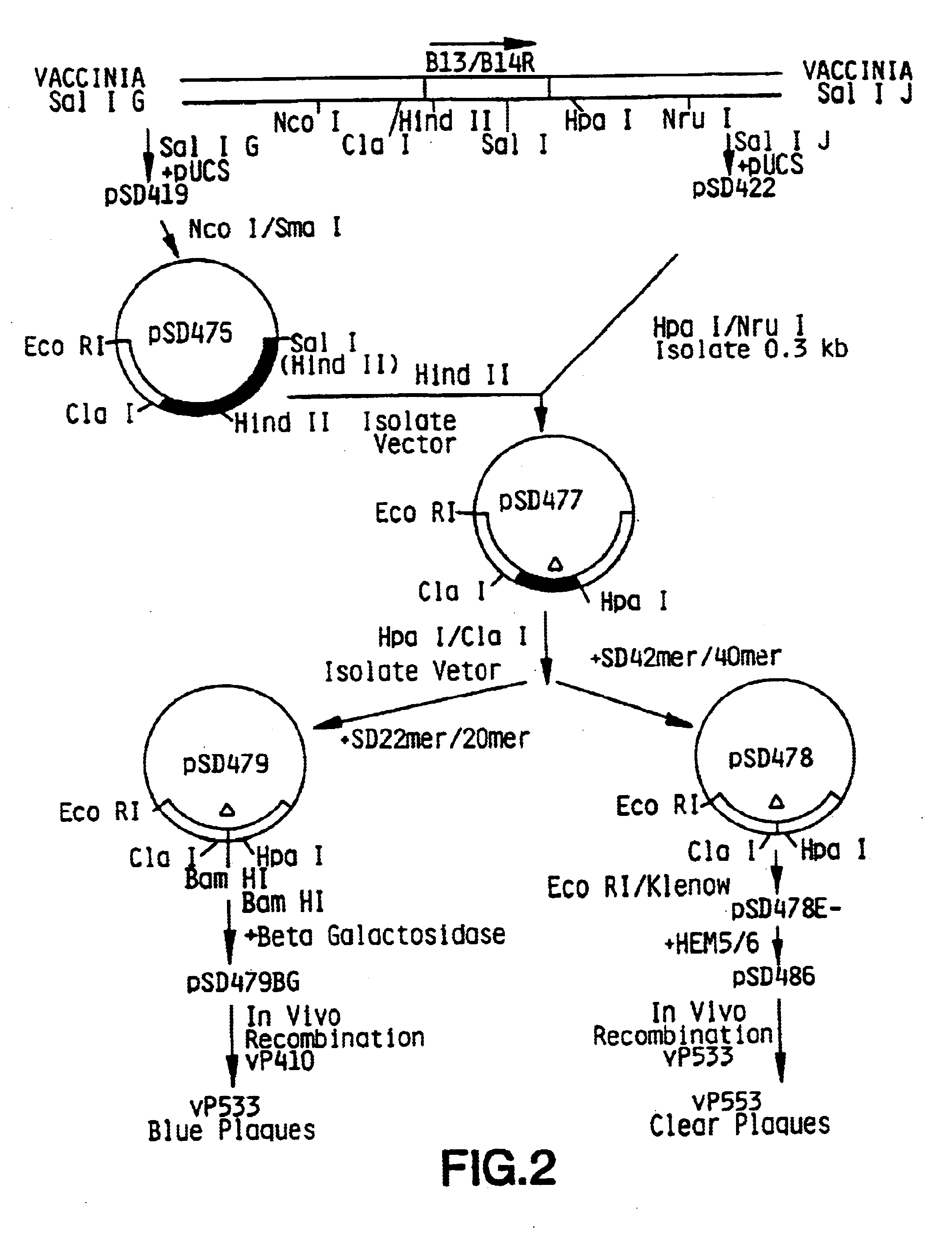
Vaccina virus comprising cytokine and/or tumor associated antigen genes
InactiveUS6537594B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Owner:VIROGENETICS
Targeted interferon demonstrates potent apoptotic and Anti-tumor activities
ActiveUS20100297076A1Peptide/protein ingredientsPharmaceutical delivery mechanismInterferon alphaWilms' tumor
This invention provides novel chimeric moieties that show significant efficacy against cancers. In certain embodiments the chimeric moieties comprise a targeting moiety attached to an interferon. In certain embodiments, the chimeric moieties comprise fusion proteins where an antibody that specifically binds to a cancer marker is fused to interferon alpha (IFN-α).
Owner:RGT UNIV OF CALIFORNIA
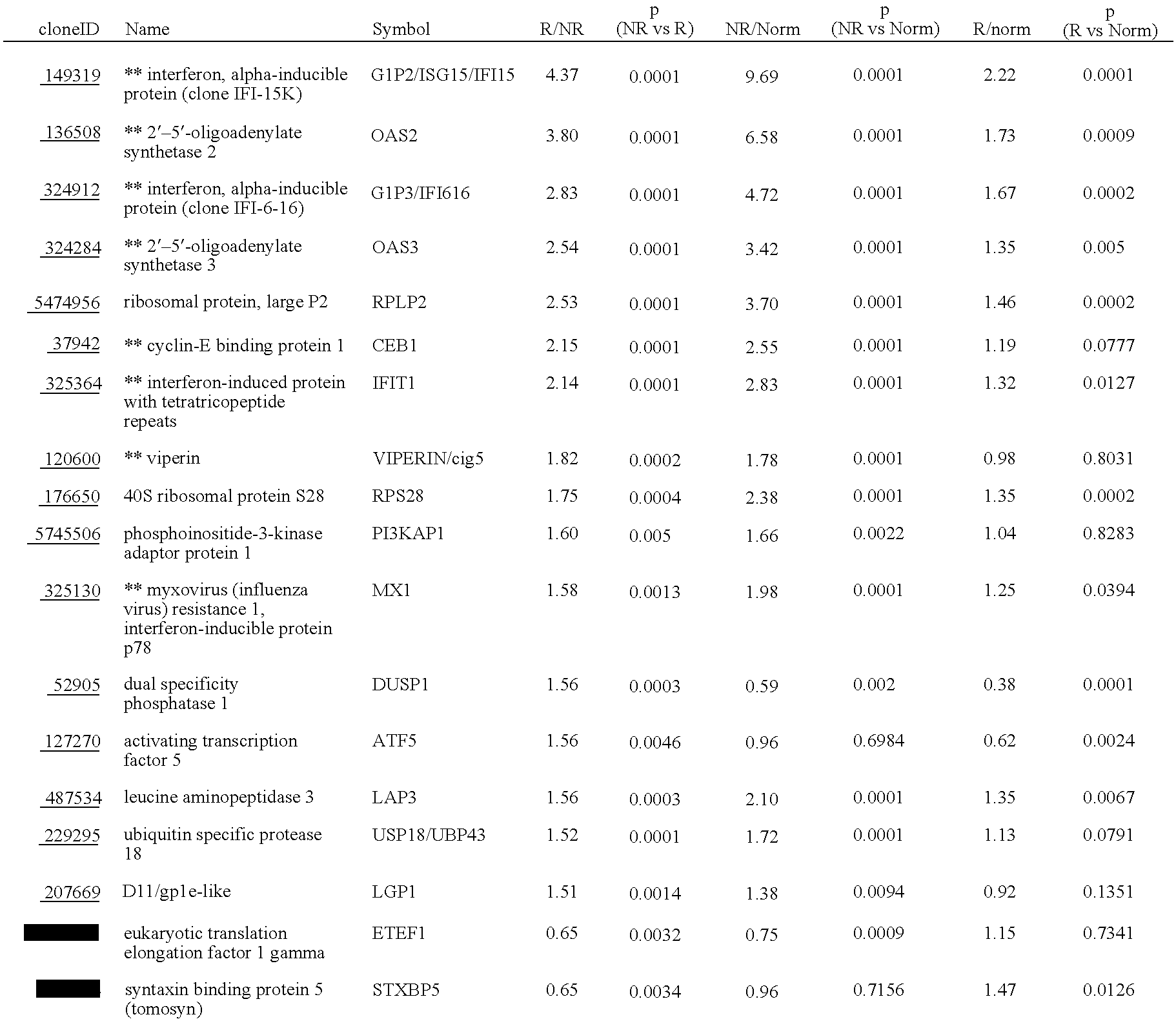
Systems and methods for identifying diagnostic indicators
InactiveUS20060177837A1Enhance and improve therapeutic effectReduces liver disease activityMicrobiological testing/measurementDrug and medicationsHepatitis c viralRegimen
Systems and methods are provided for predicting patient response to a therapy regimen for a liver disease or a disease that is treatable with an immunomodulatory disease therapy using gene expression classifiers. Systems and methods for screening for modulators of target gene expression are also provided. Systems and methods for developing therapeutics against one or more of the proteins coded for by genes of the present invention are also provided. Systems and methods for predicting a patient response to a regimen of pegylated interferon alpha and ribavirin in a therapy for hepatitis C viral infection are also provided.
Owner:JAGUAR BIOSCI
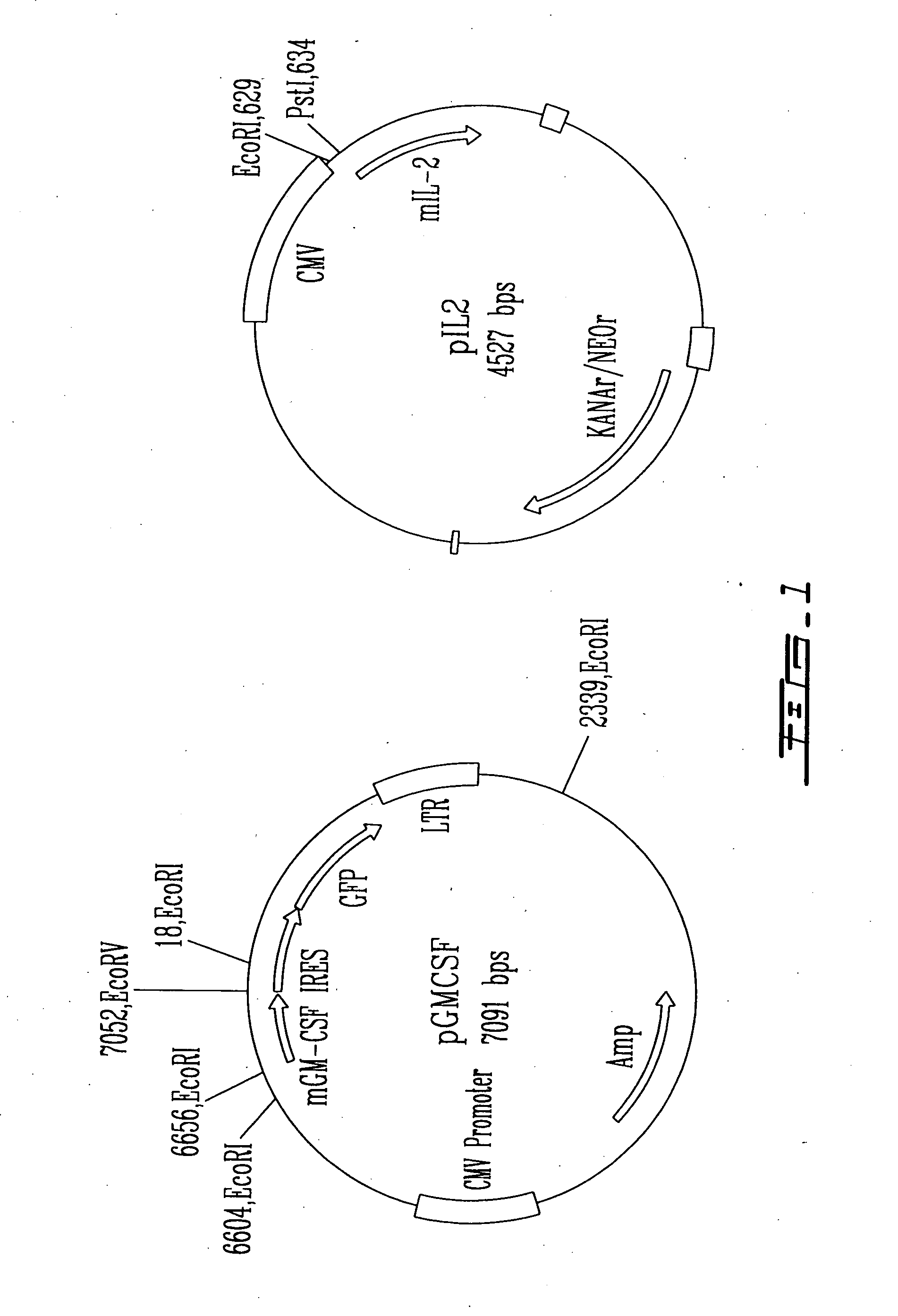
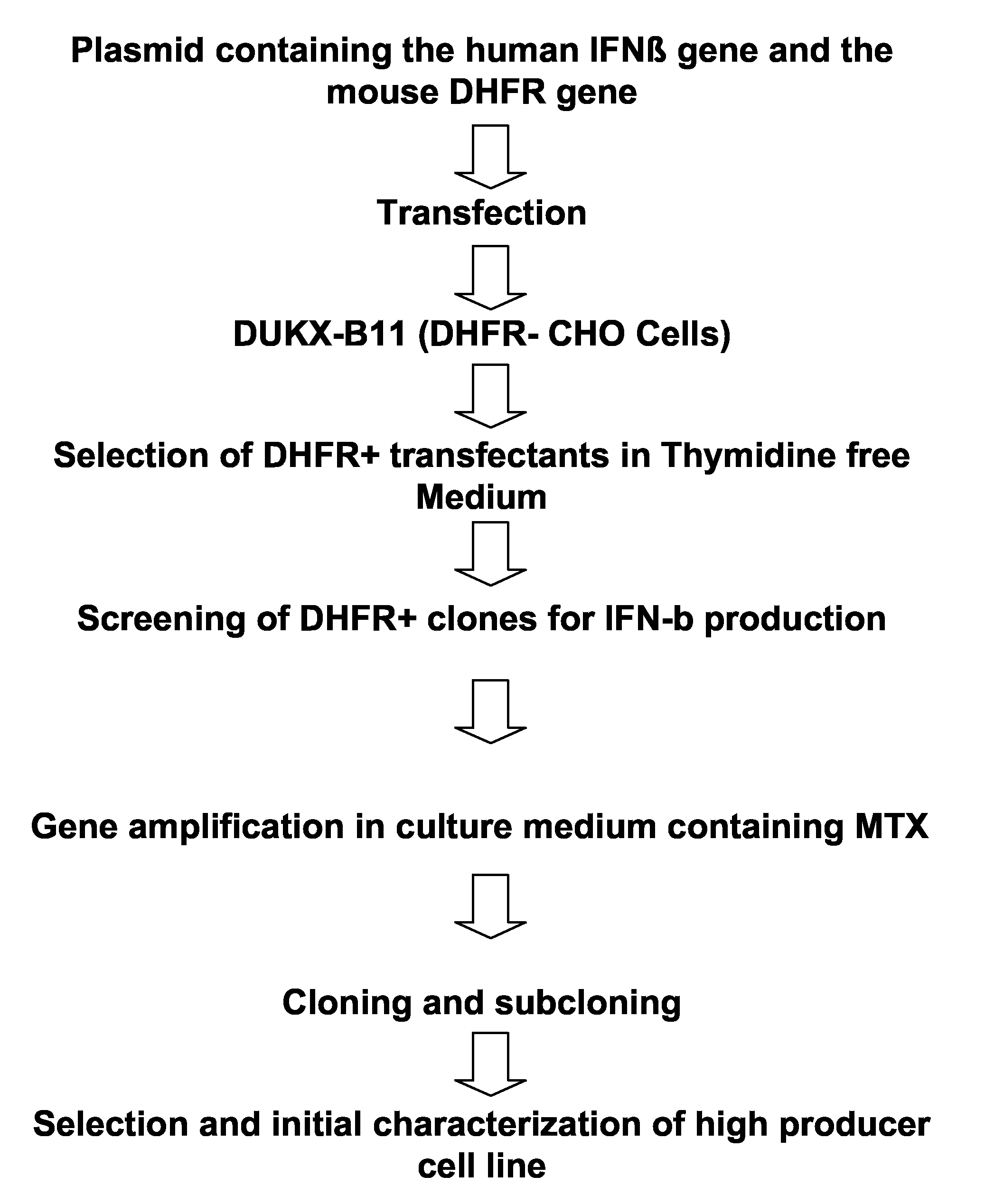
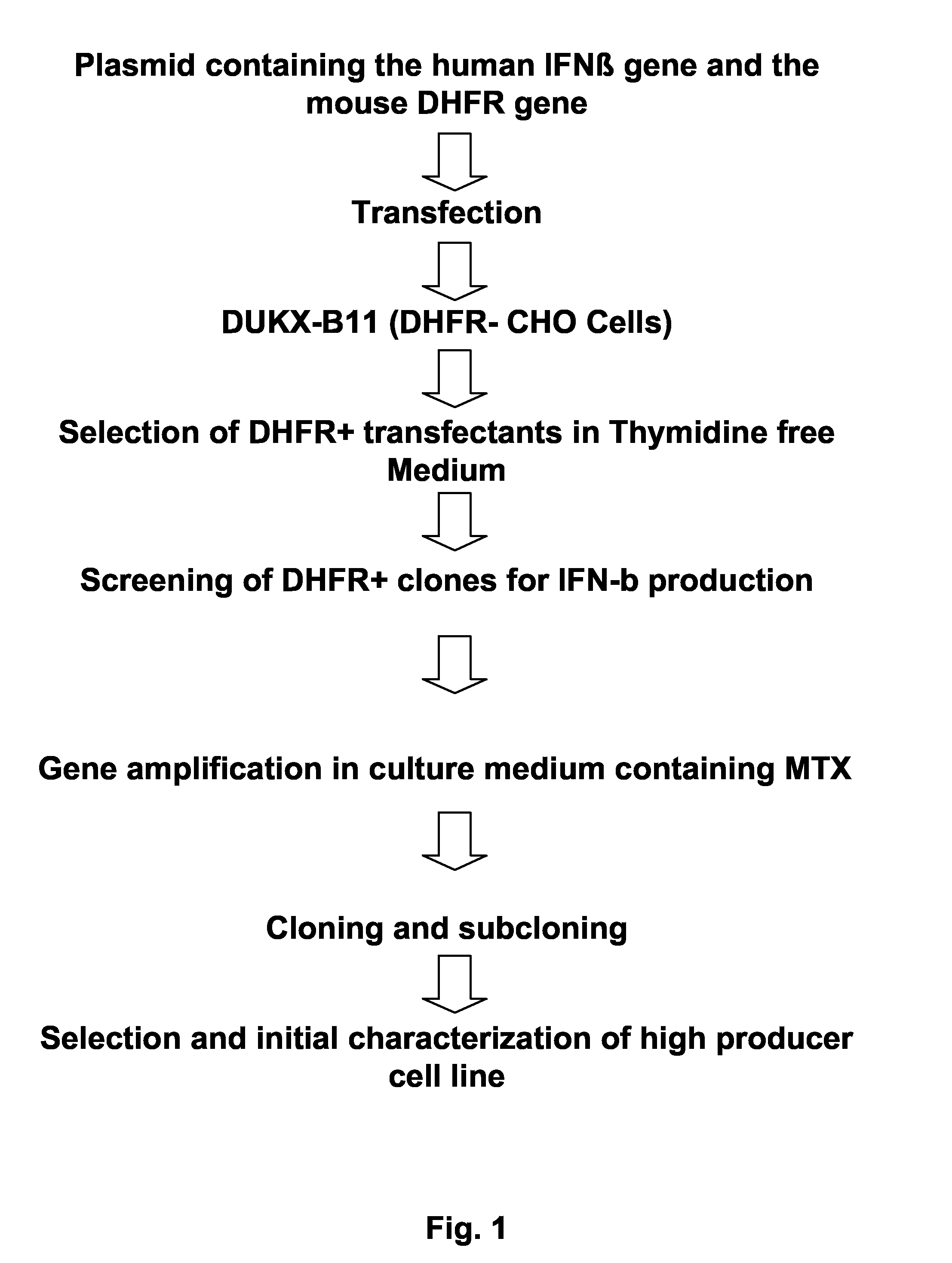
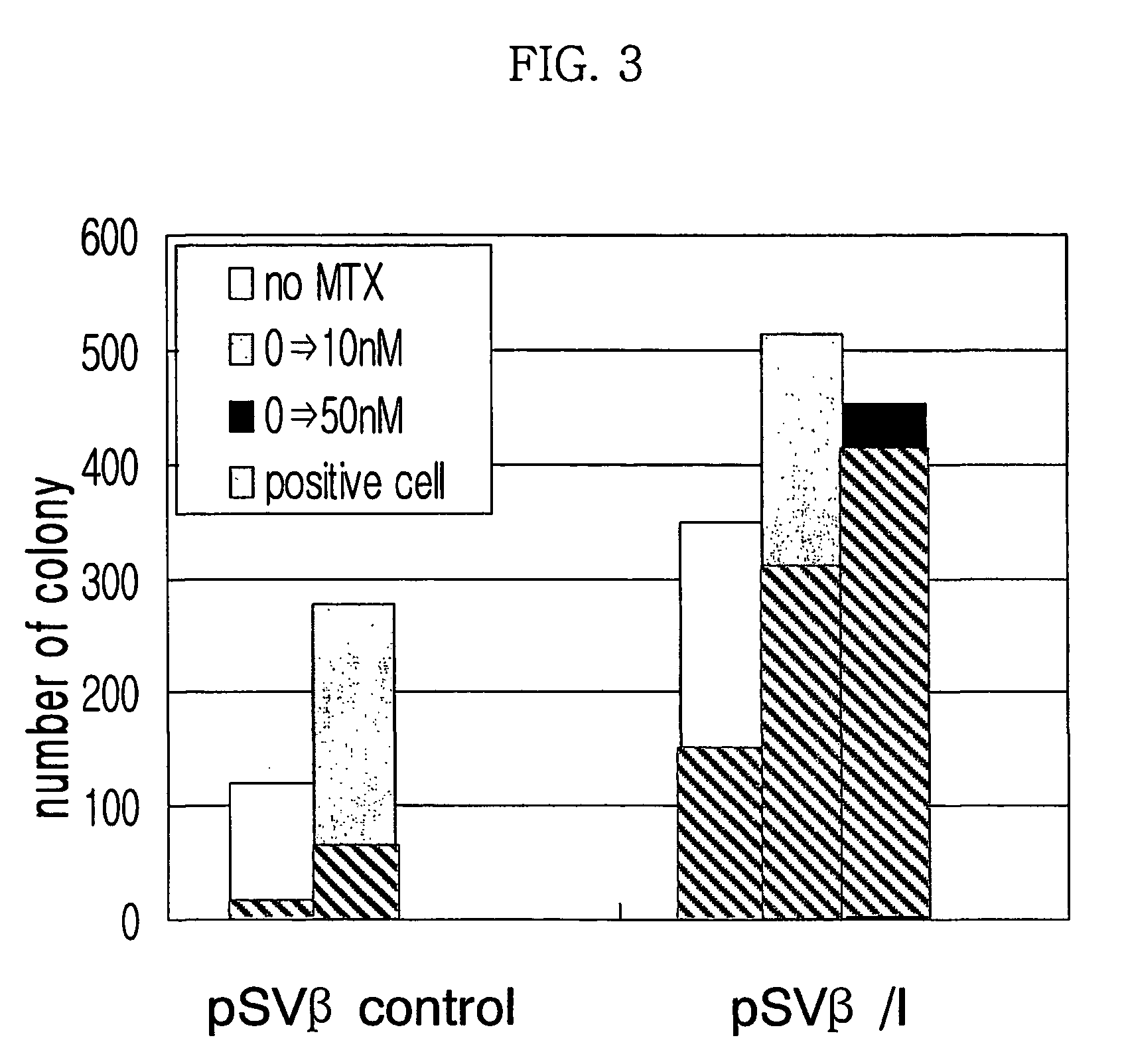
Expression vector for animal cell containing nuclear matrix attachment region of interferon beta
InactiveUS7259010B2Increased foreign protein expression efficiencyReduce the amount of solutionVectorsSugar derivativesMammalNuclear matrix
The present invention relates to mammalian expression vectors including nuclear matrix attachment region of human interferon β, and more particularly to pPGM-1, pPGM-2 and pPGM-3 including nuclear matrix attachment region of interferon β gene. Those expression vectors confer position independent expression of the introduced foreign gene, thus increasing the frequency of colonies which efficiently express the recombinant protein.
Owner:PANGEN BIOTECH
Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
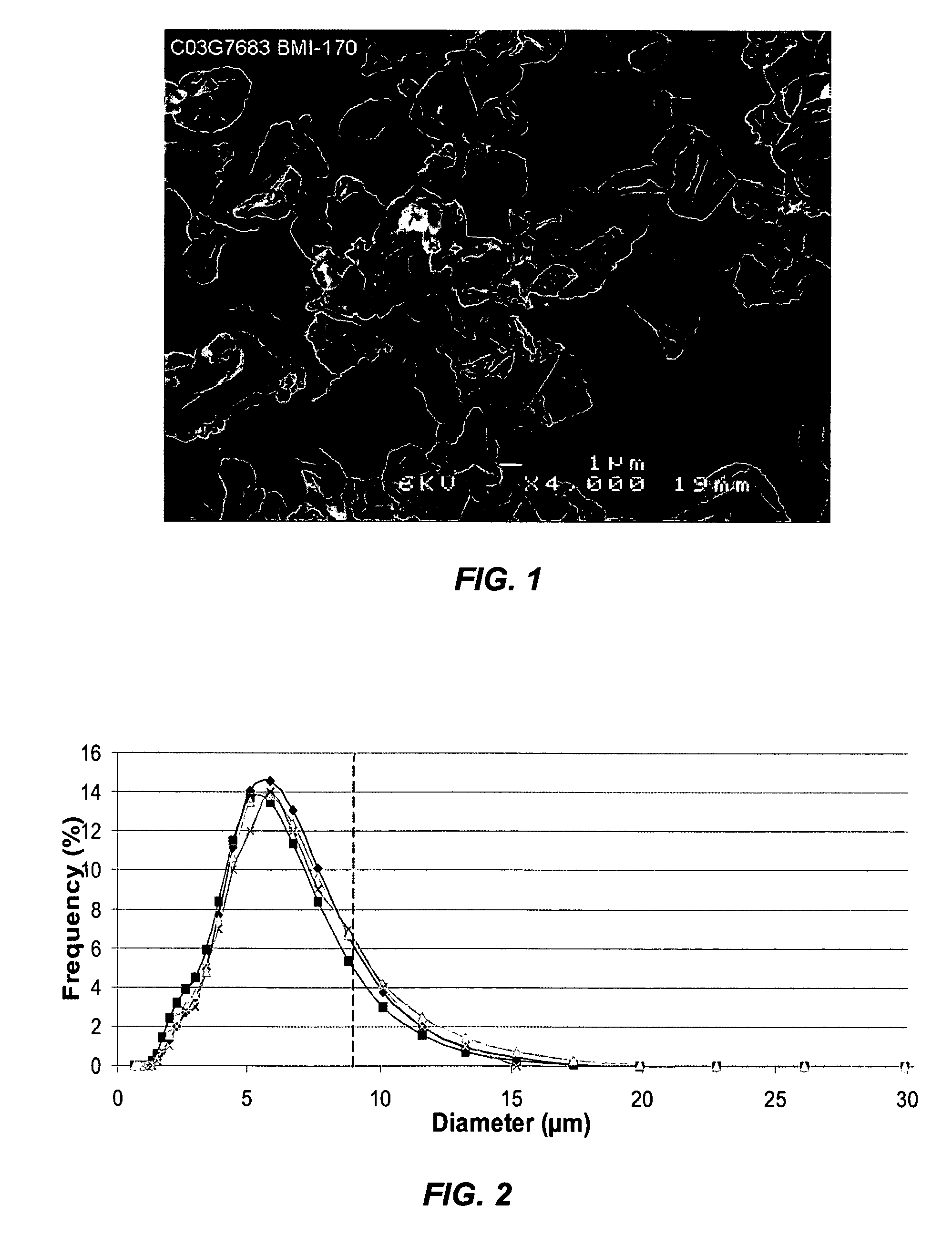
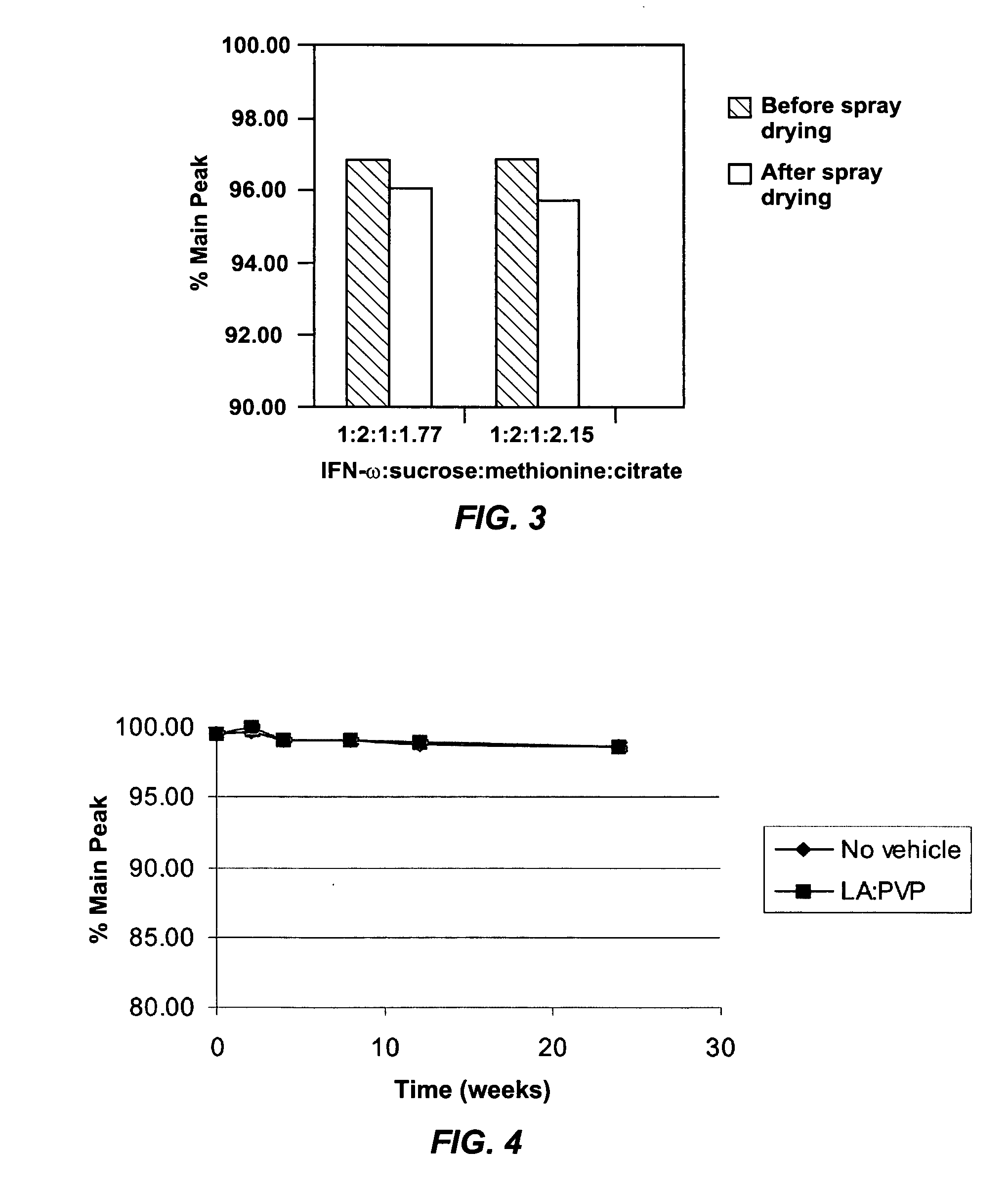
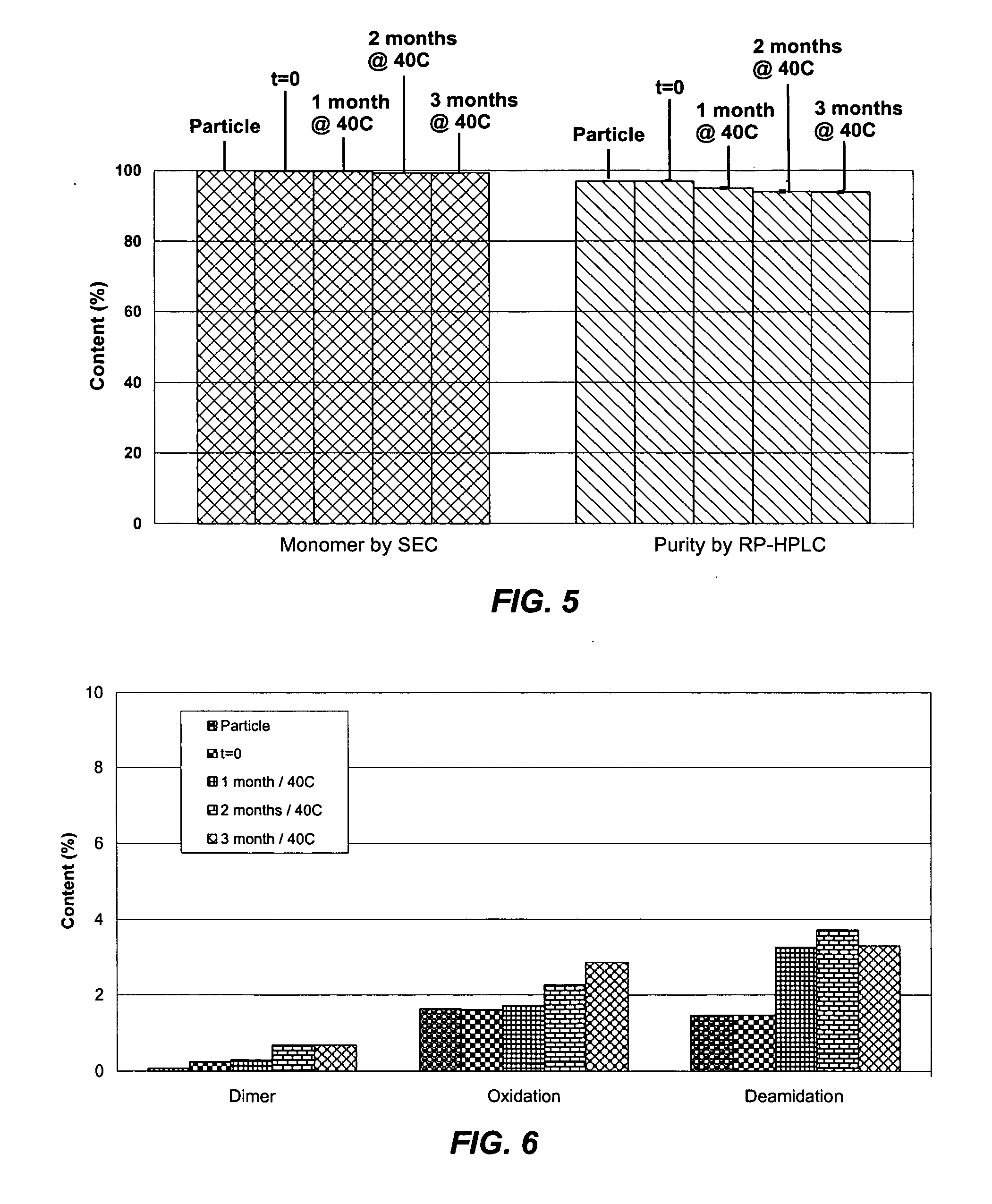
Suspension formulation of interferon
A suspension formulation of interferon includes a non-aqueous, single-phase vehicle including at least one polymer and at least one solvent, the vehicle exhibiting viscous fluid characteristics, and an interferon contained in a particle formulation dispersed in the vehicle. The particle formulation includes a stabilizing component comprising one or more stabilizers selected from the group consisting of carbohydrates, antioxidants, and amino acids. The suspension formulation is characterized in that less than 10% of the interferon degrades over 3 months under an accelerated storage condition.
Owner:INTARCIA THERAPEUTICS INC
Novel synthetic chimeric fusion transgene with immuno-therapeutic uses
InactiveUS20050053579A1Reducing tumorigenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInterferon alphaWilms' tumor
The present invention relates to an immuno-therapy conjugate which comprises A-c-B wherein: A and B are different and are compounds selected from the group consisting of cytokines, chemokines, interferons, their respective receptors or a functional fragment thereof; and c is a linker consisting of a bond or an amino acid sequence containing from 1 to 100 residues. The present invention also relates to a vaccine adjuvant comprising the immuno-therapy conjugate of the present invention. The present invention further relates to a method of reducing tumor growth, for inhibiting a viral infection and for improving immune response in a patient.
Owner:GALIPEAU JACQUES +1
Interferon alpha receptor 1 antibodies and their uses
The present invention provides isolated human monoclonal antibodies that bind to IFNAR-1 and that are capable of inhibiting the biological activity of Type I interferons. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting Type I interferon-mediated disorders using the antibodies of the invention, including methods for treating autoimmune disorders, transplant rejection or Graft Versus Host Disease using the antibodies of the invention.
Owner:ER SQUIBB & SONS INC
Method of treating tuberculosis with interferons
InactiveUS20100098660A1Increase appetiteReduction in wheezingAntibacterial agentsBiocideInterferon therapyInterferon alpha
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Further, a method of reducing the infectivity of tuberculosis or reducing the number of infectious organisms present in the lungs of a patient suffering from tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Methods for Treating HCV
ActiveUS20130102526A1Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Adoptive immunotherapy using macrophages sensitized with heat shock protein-epitope complexes
InactiveUS6156302AEnhancing host 's immunocompetenceHigh activityBiocideOrganic active ingredientsDiseaseInterleukin 6
The present invention relates to methods and compositions for enhancing immunological responses and for the prevention and treatment of infectious diseases or primary and metastatic neoplastic diseases based on the administration of macrophages and / or other antigen presenting cells (APC) sensitized with heat shock proteins non-covalently bound to peptide complexes and / or antigenic components. APC are incubated in the presence of hsp-peptide complexes and / or antigenic components in vitro. The sensitized cells are reinfused into the patient with or without treatment with cytokines including but not limited to interferon- alpha , interferon- alpha , interleukin-2, interleukin-4, interleukin-6 and tumor neurosis factor.
Owner:FORDHAM UNIVERSITY
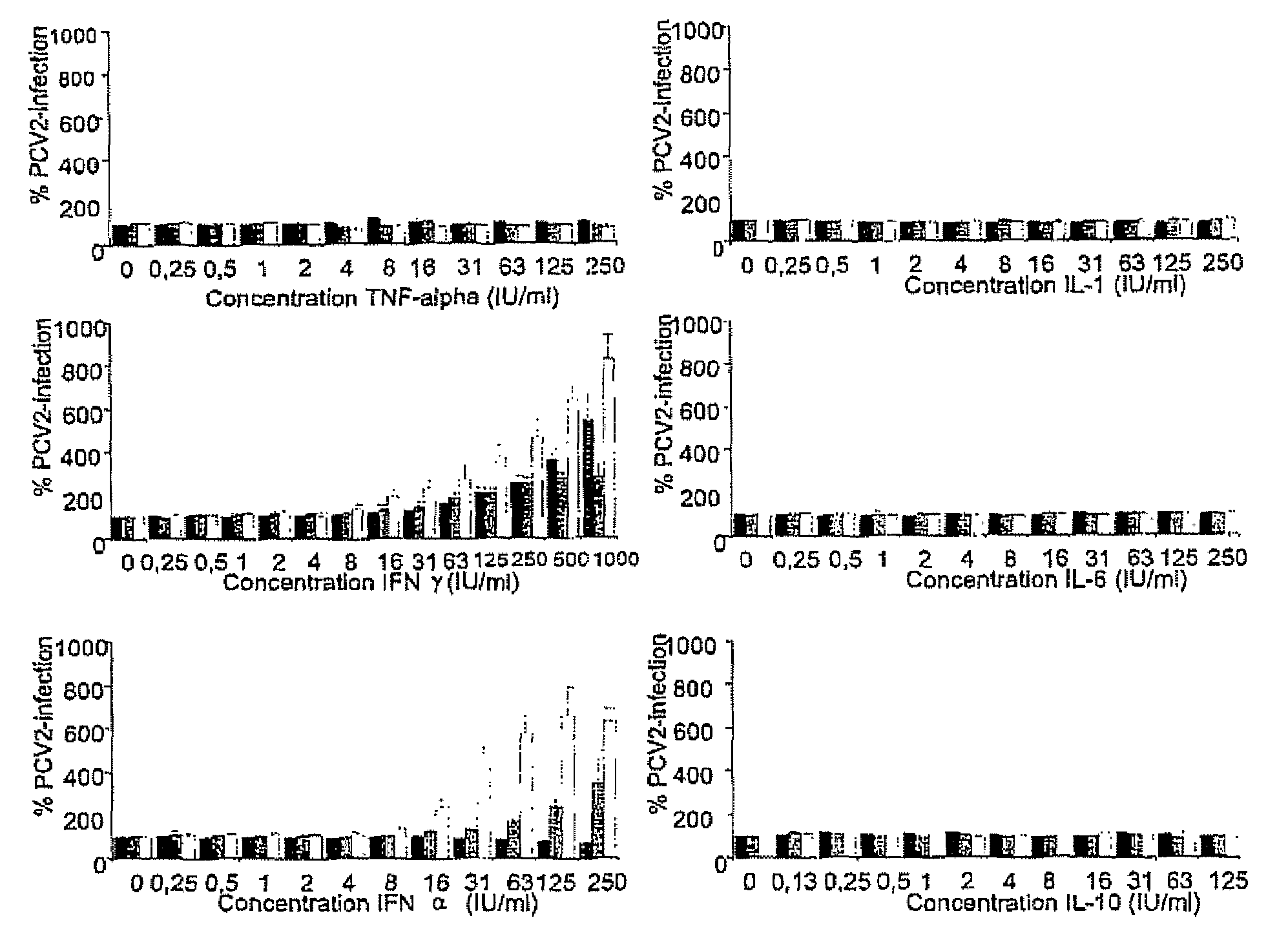
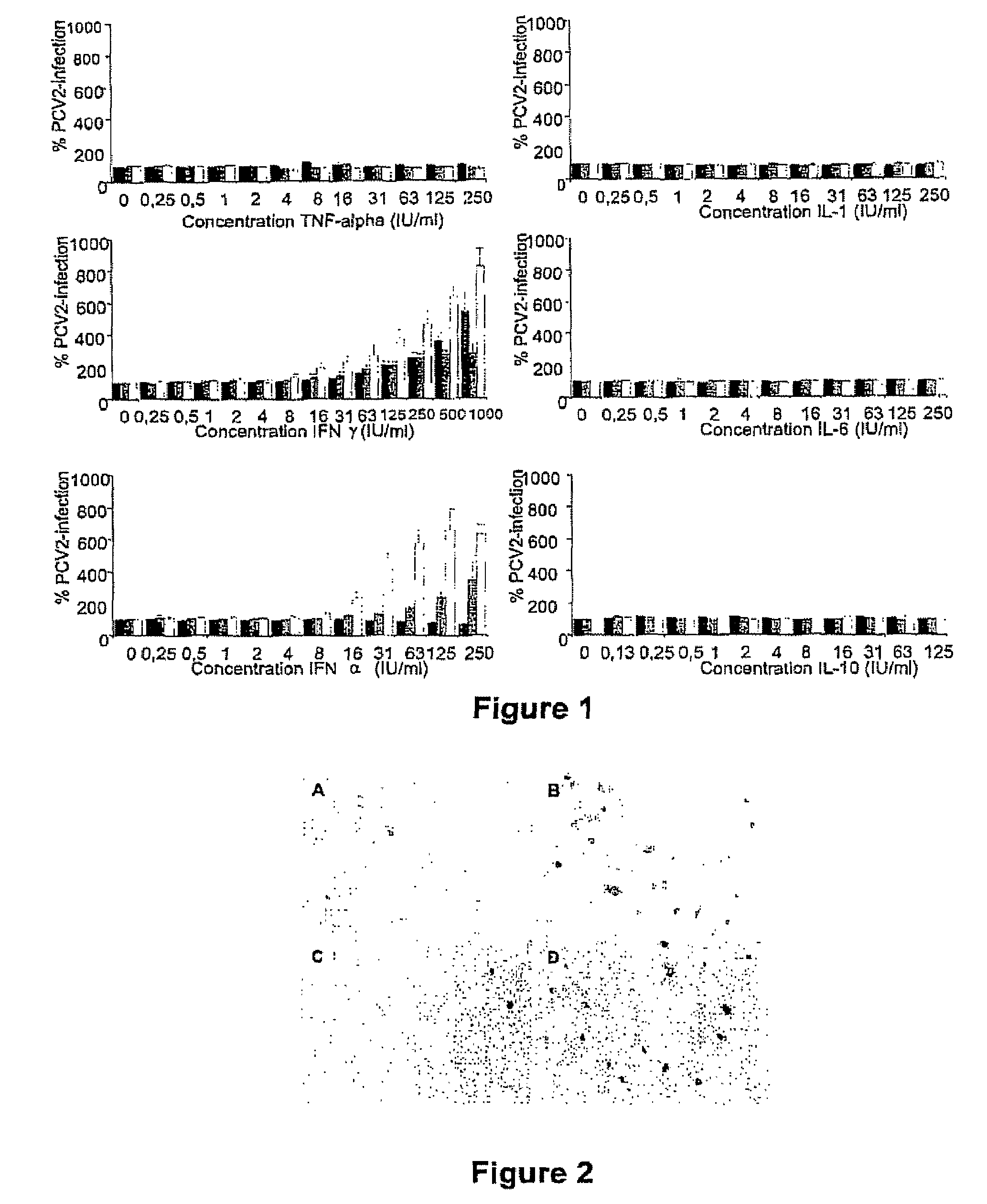
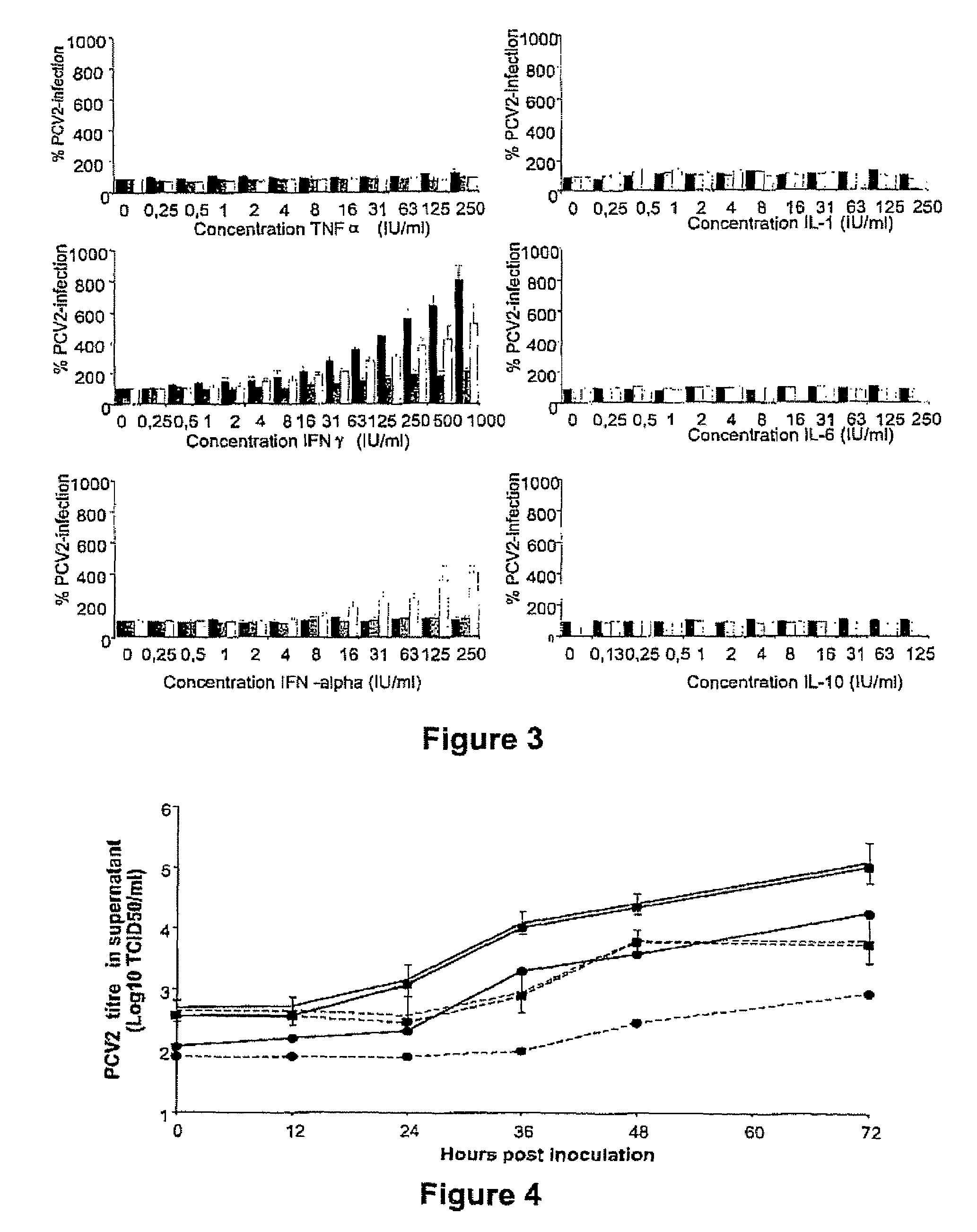
Culturing circular ssDNA viruses for the production of vaccines
InactiveUS7300785B2Increase virus titresIncreased virus titresViral antigen ingredientsMicrobiological testing/measurementSsDNA virusesInterferon alpha
The present invention relates to the use of interferon in the in vitro cultivation of animal circular ssDNA virus such as Porcine Circovirus 2 or human TT virus in an animal cell line. Increased titres of animal circular ssDNA virus are obtained by addition of interferons or agents which ensure the production of endogenous interferons by said cell line.
Owner:UNIV GENT
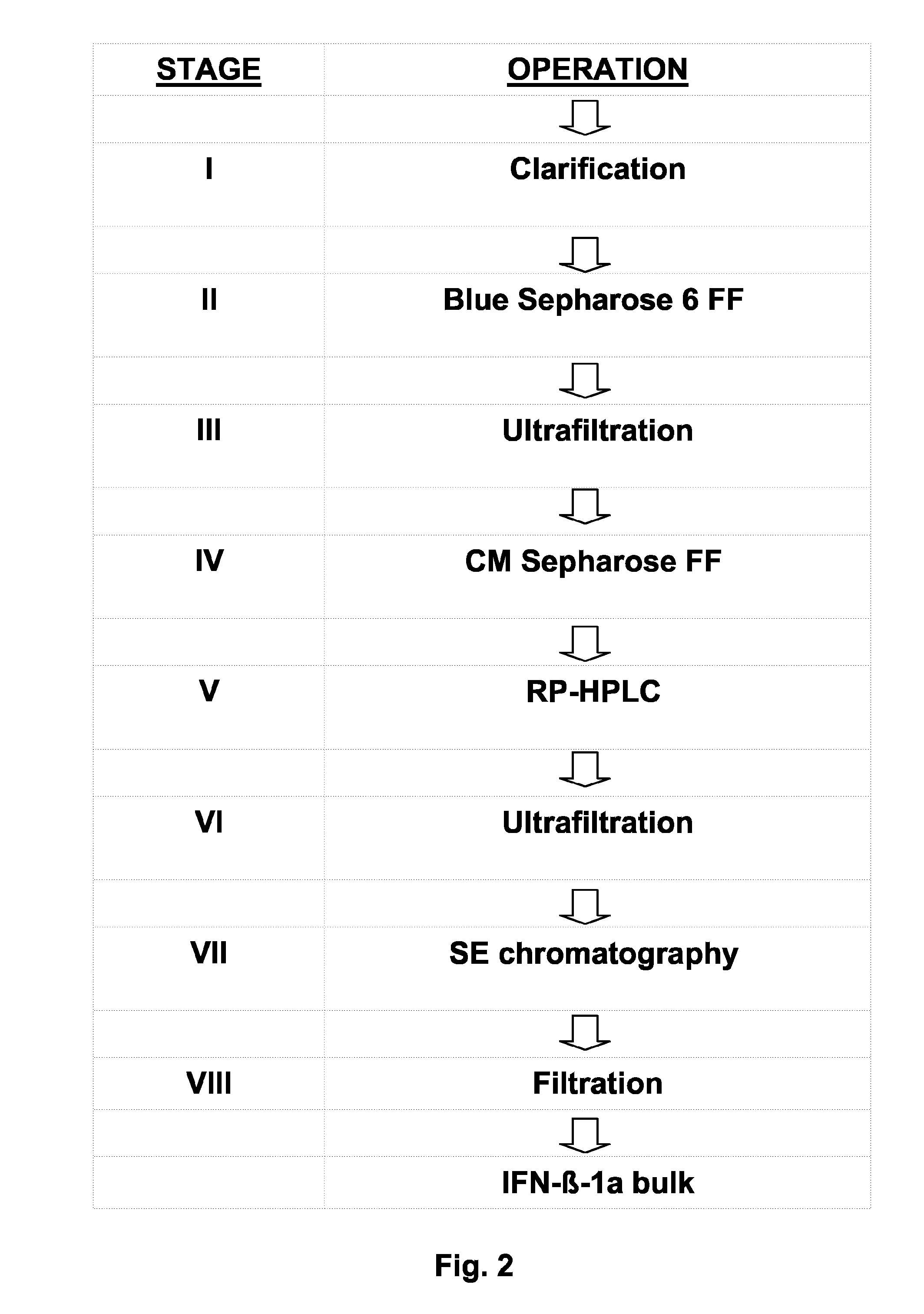
Process For the Preparation of Glycosylated Interferon Beta
The present invention relates to a process for the production of interferon beta, and to an interferon beta composition having a unique glycosylation pattern.
Owner:ARES TRADING SA
Pox virus comprising DNA sequences encoding CEA and B7 antigen
Attenutated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: human tumor necrosis factor; nuclear phosphoprotein p53, wiltype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GMCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:AVENTIS PASTEUR LTD
HCV combination therapy
InactiveUS6849254B1Ameliorate ribavirin-related hemolysisLow viral-RNABiocidePeptide/protein ingredientsChronic viral hepatitis CHemolysis
Methods of treating patients having susceptible viral infections, especially chronic hepatitis C infection by administering to said patient a therapeutically effective amount of a combination therapy of interferon-alfa and ribavirin for a time sufficient to lower HCV-RNA in association with a therapeutically effective amount of an antioxidant for a time sufficient to ameliorate ribavirin-related hemolysis are disclosed.
Owner:MERCK SHARP & DOHME CORP
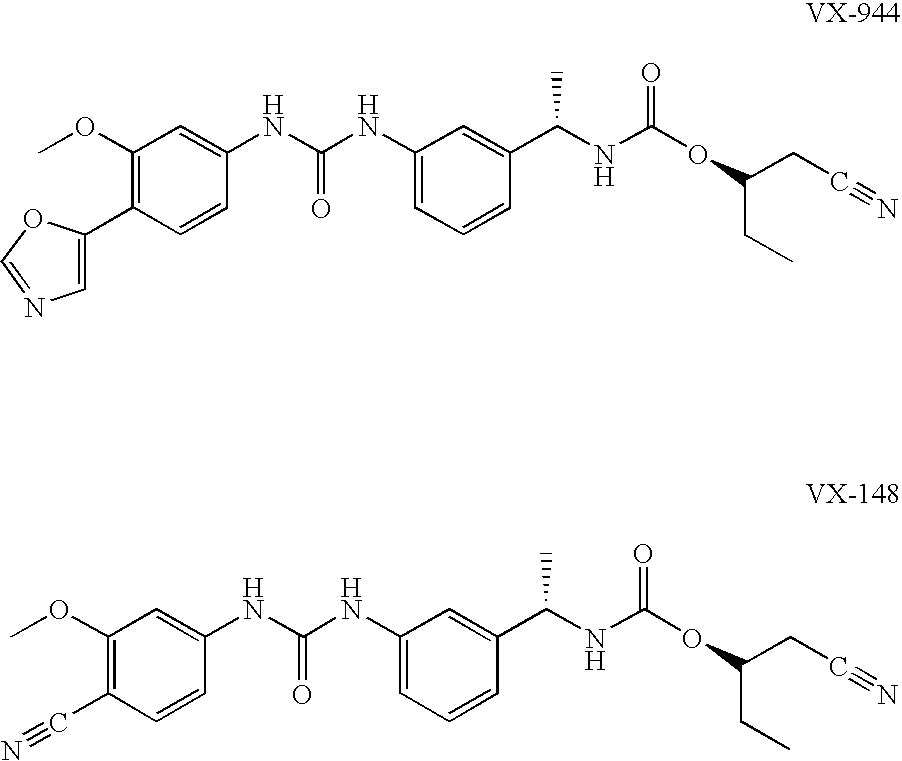
Compositions comprising IMPDH inhibitors and uses thereof for treating HCV infection
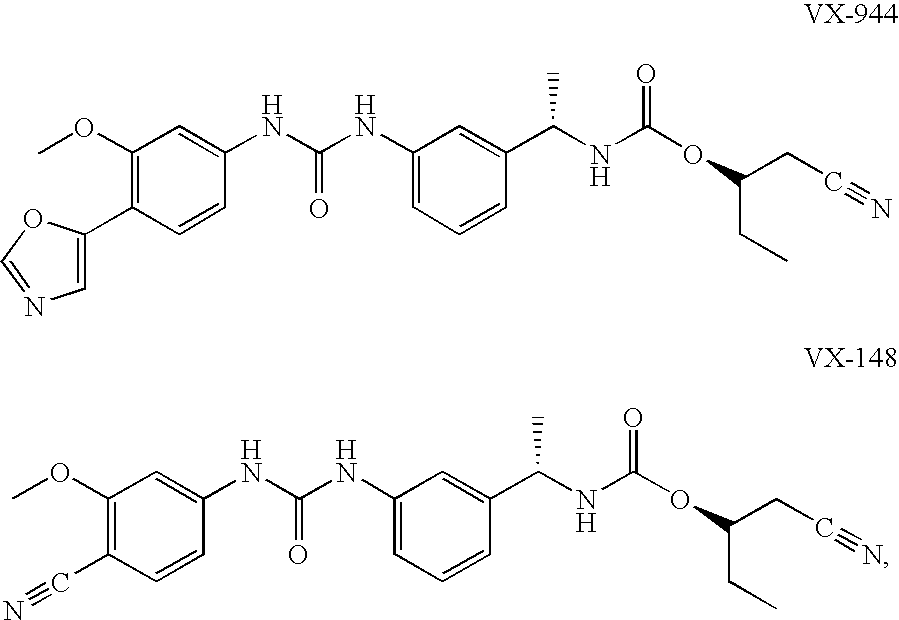
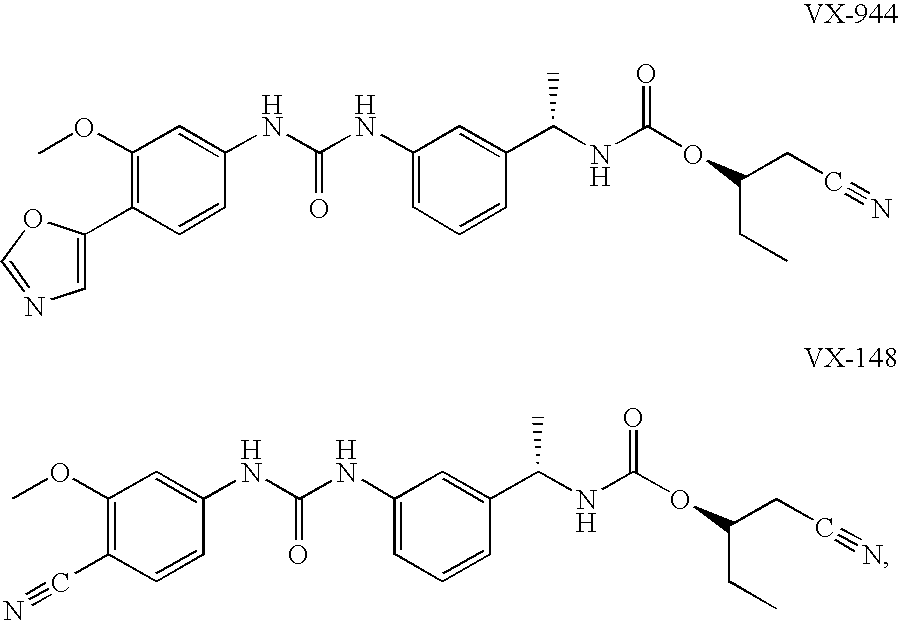
The present invention relates to optimal compositions useful in treating HCV infections in humans. These compositions comprise alpha-interferon or pegylated alpha-interferon and an IMPDH inhibitor selected from VX-148 or VX-944, wherein the IMPDH inhibitor is present in an amount such that a ratio of Cavg / Cmin is between 1 to 10, wherein:Cavg is average plasma concentration produced by said IMPDH inhibitor in said human; andCmin is estimated trough concentration produced by said IMPDH inhibitor in said human.The present invention also relates to methods of producing and using the optimal compositions to treat HCV infections in humans.
Owner:VERTEX PHARMA INC
Screening method for identifying compounds that selectively induce interferon alpha
Methods for screening for compounds that selectively induce IFN-α production and methods for ameliorating conditions in a patient using a small molecule that selectively induces the production of IFN-α are disclosed.
Owner:COLEY PHARM GRP INC
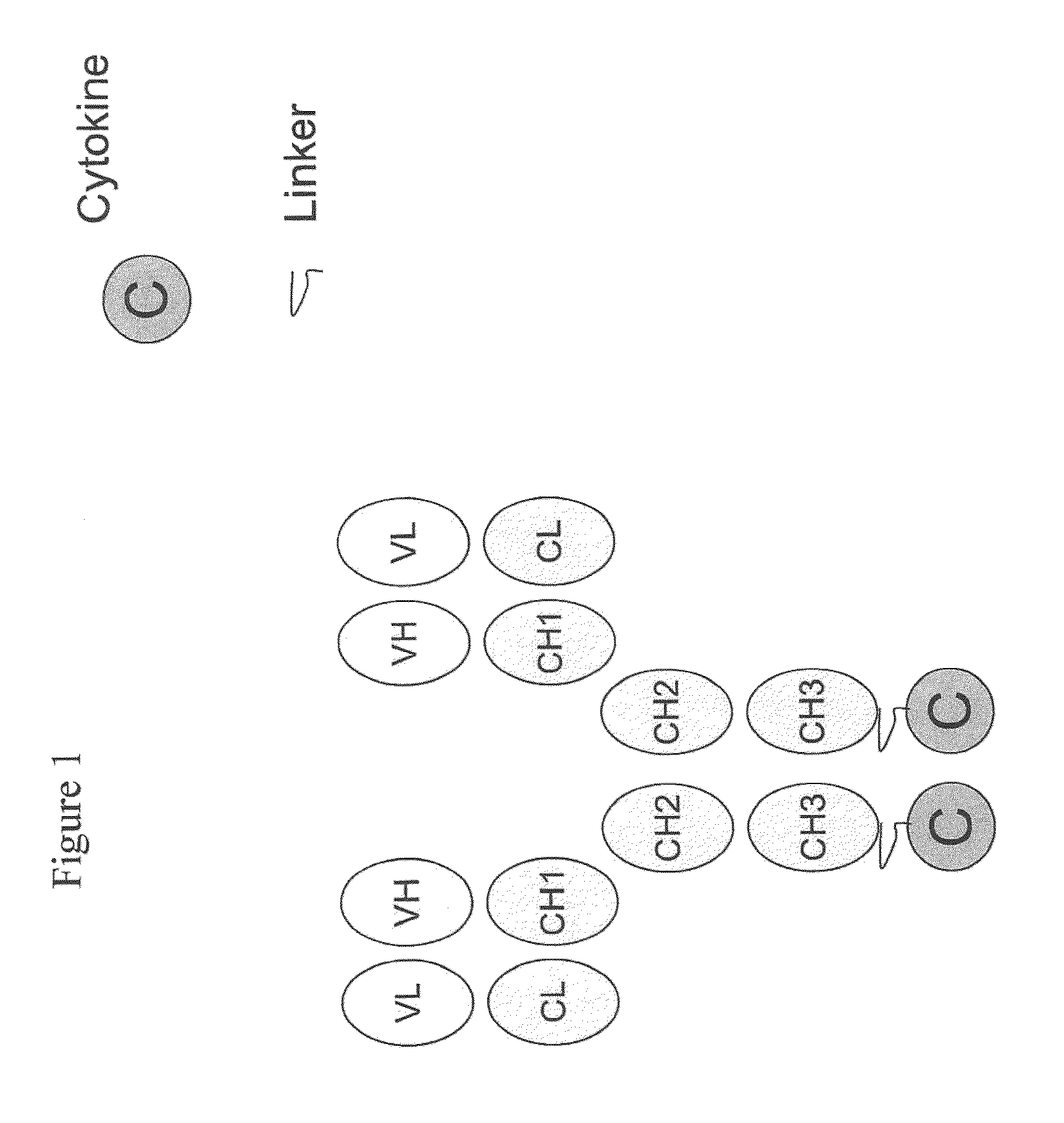
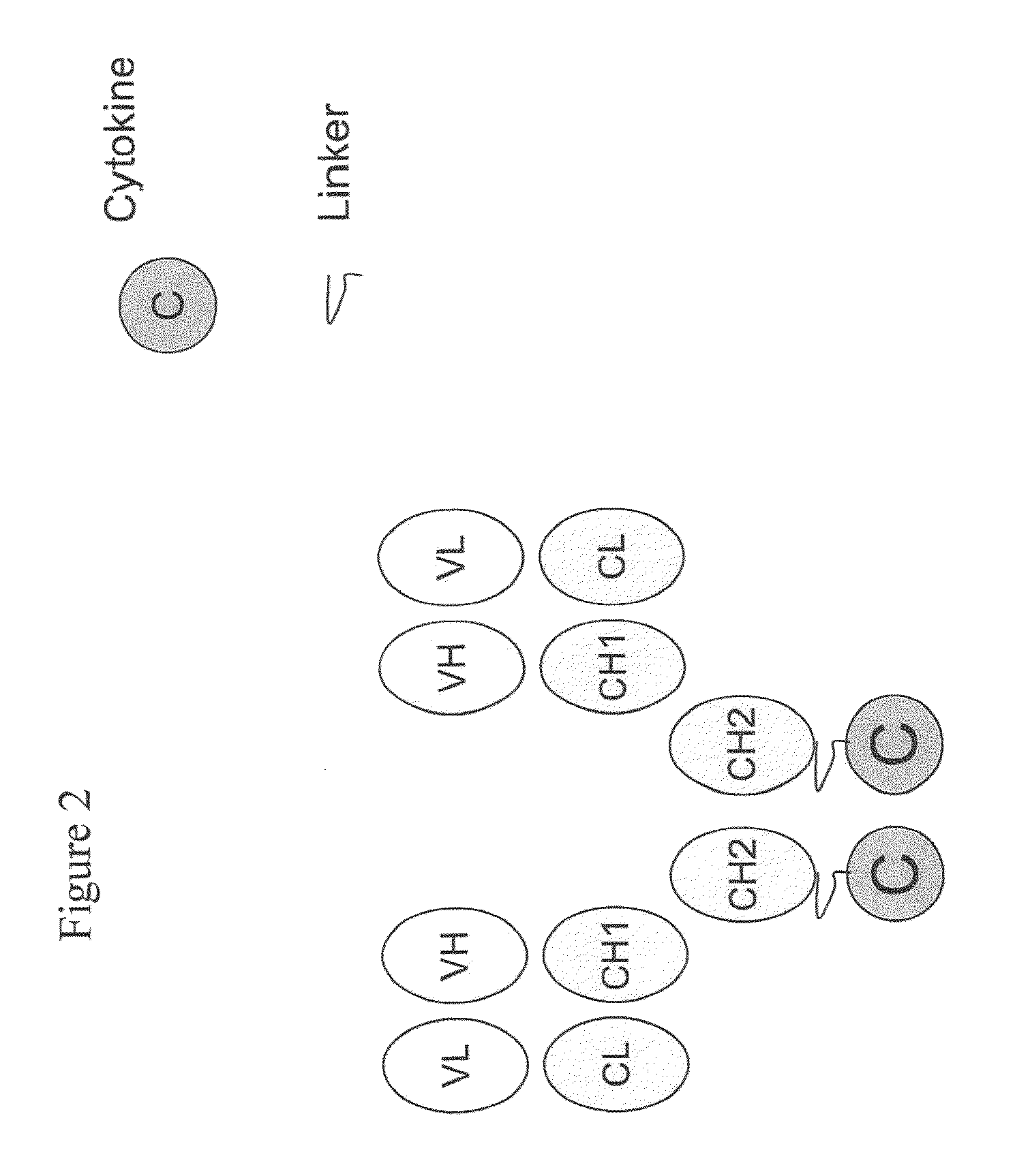
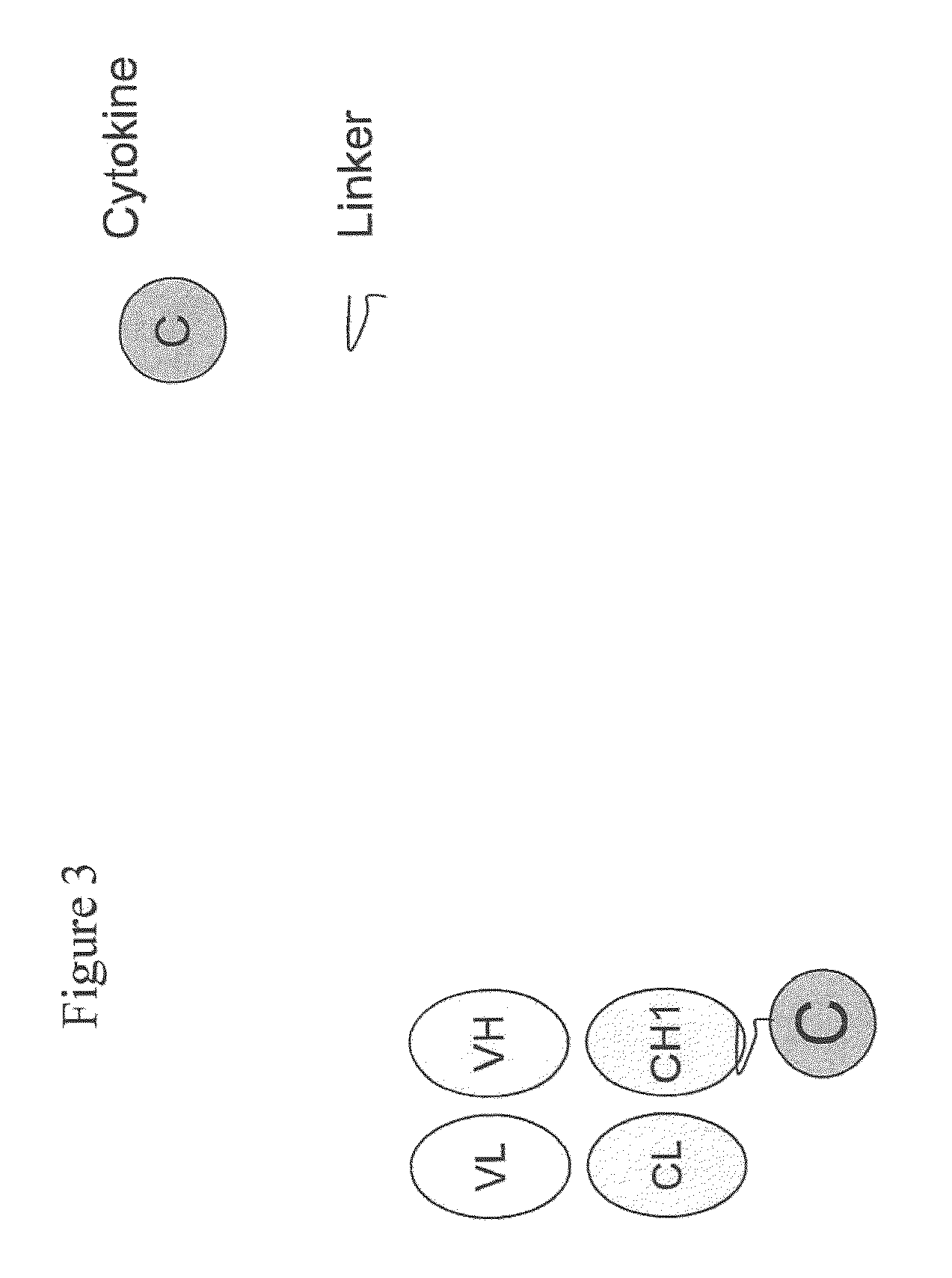
Engineered antibody-interferon mutant fusion molecules
The field of the present invention relates to genetically engineered fusion molecules, methods of making said fusion molecules, and uses thereof in anti-tumor immunotherapies. More specifically, the present invention relates to fusion molecule constructs wherein a tumor associated antigen (TAA) antibody (Ab) serves as a targeting moiety to selectively deliver a cytokine to a tumor cell for purposes of killing or inhibiting the growth or proliferation of said tumor cell. In various embodiments, the engineered fusion molecules comprise a TAA Ab fused to an interferon-alpha (IFN-α) mutant molecule. The engineered Ab-IFN-α mutant fusion molecules of the present invention demonstrate improved therapeutic index and preserved or increased efficacy as compared to Ab-wildtype IFN-α fusion molecules, and / or demonstrate improved PK properties as compared to Ab-wildtype IFN-α fusion molecules.
Owner:IMMUNGENE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka.patsnap.com/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00001.png)
![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka.patsnap.com/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00002.png)
![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka.patsnap.com/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00003.png)