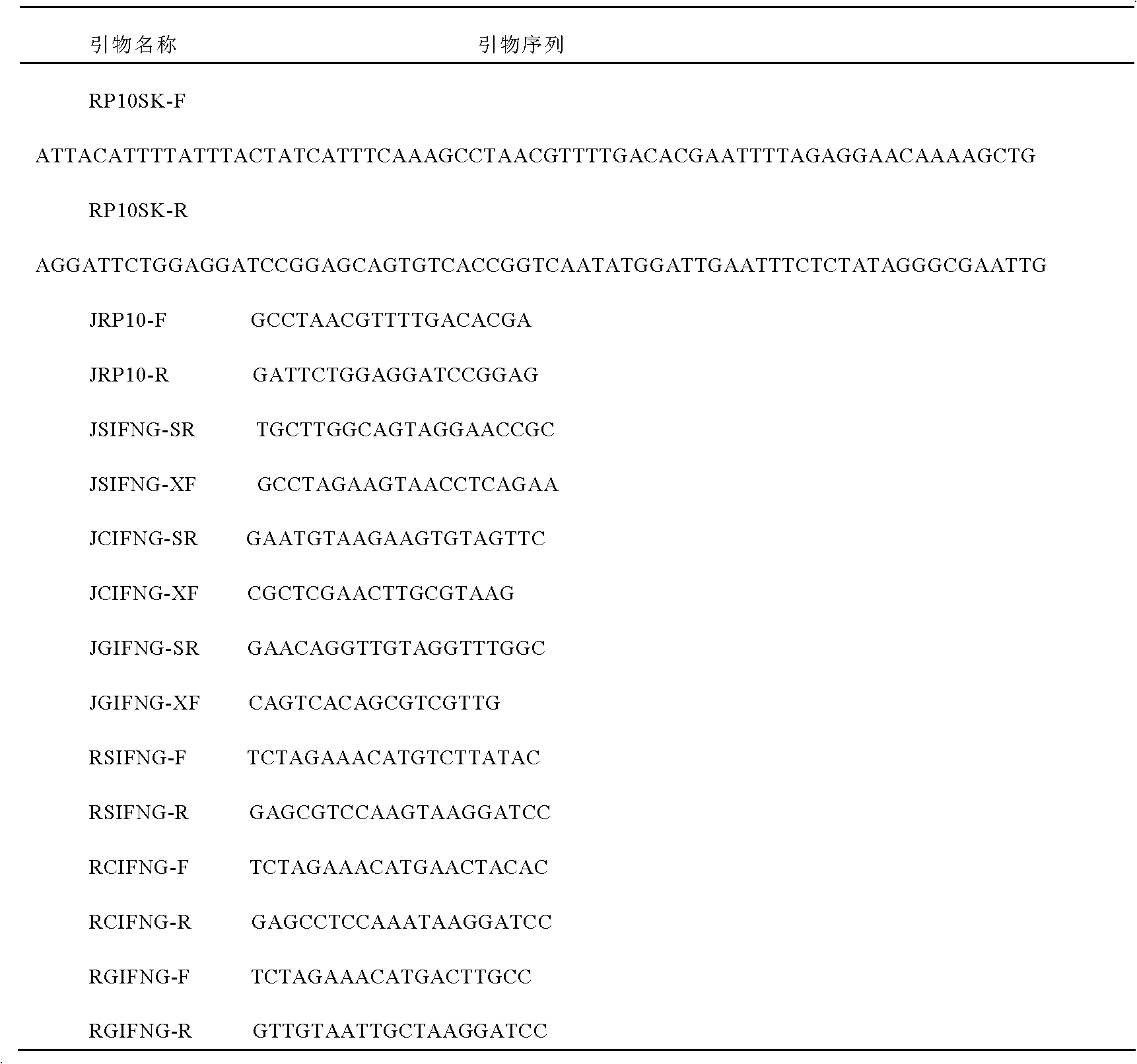
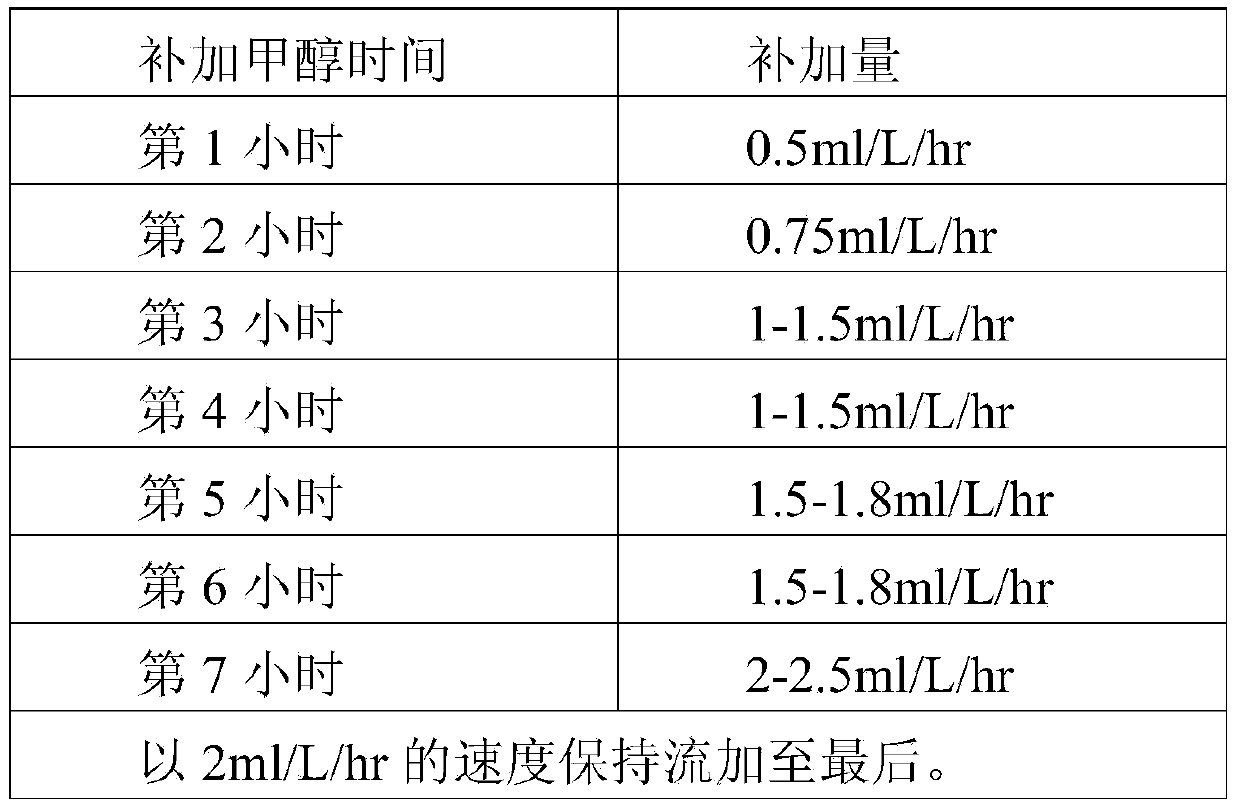
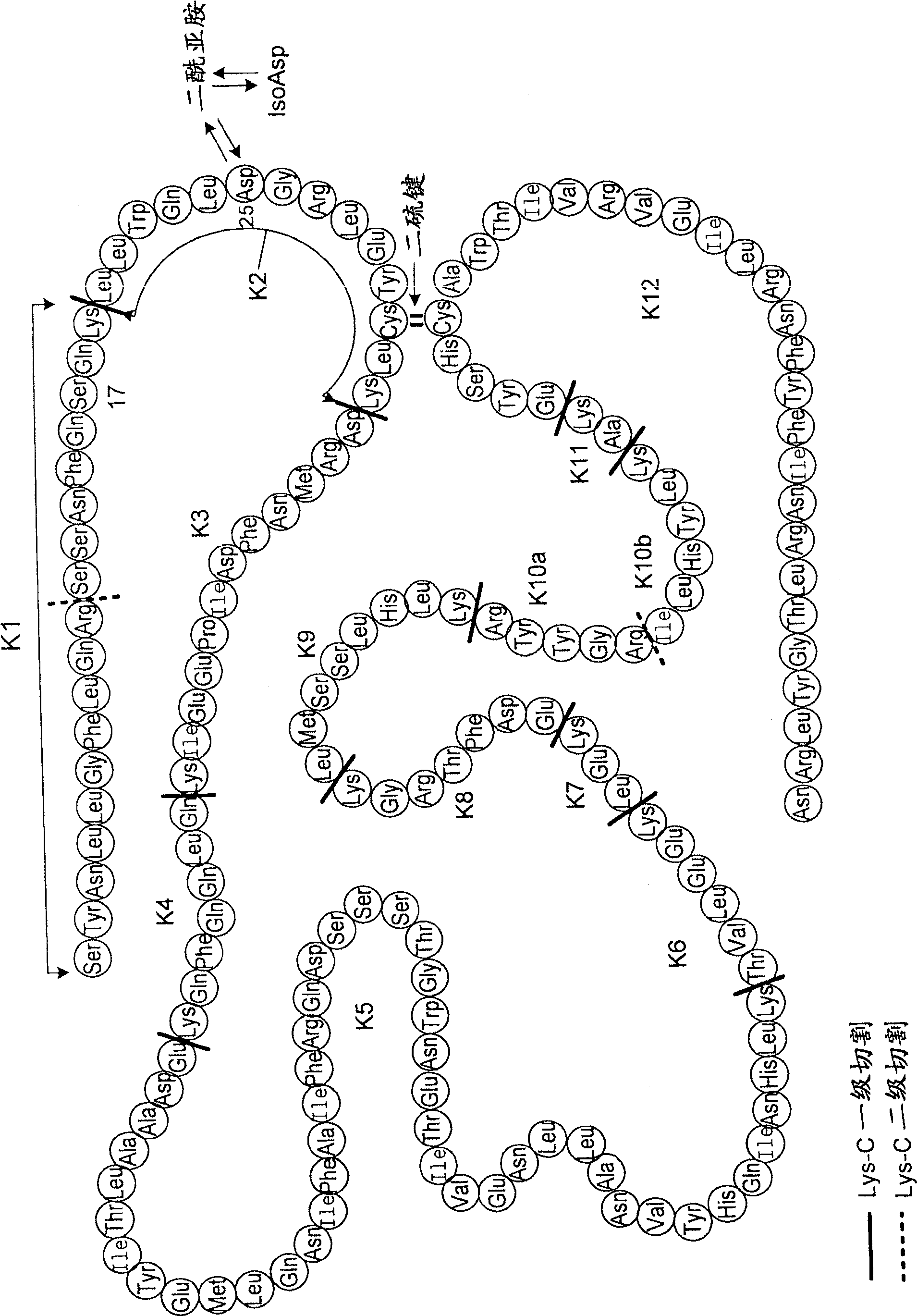Patents
Literature
63 results about "Recombinant interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant interferon refers to interferon compounds that are produced by recombinant techniques. In recombinant technology, a gene of interest is placed into the genome of a system, such as a cell culture or a specific animal, in which manipulation can lead to increased production of the gene product of interest.
Media and method for treating pathological syndrome
InactiveUS7572441B2Energy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsNatural antibodyUltra low dose
A medicament based on antibodies contains an activated form of monoclonal, polyclonal, or natural antibodies to interferon in low or ultra-low doses prepared by multiple consecutive dilutions and exposure to external factors, preferably in accordance with homeopathic technology. In order to obtain antibodies, human or heterologous interferon alpha, beta, or gamma, including recombinant interferon, is used; a mixture of various, mostly centimal, homeopathic dilutions being employed. A method of treating a pathologic syndrome, whose formation is affected by interferon, consists in the use of activated forms of antibodies to interferon alpha, beta, or gamma in low or ultra-low doses obtained by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Recombination interferon with new space conformation and enhanced effect, its preparing method and application
ActiveCN1740197AStrong antiviral activityLittle side effectsPeptide/protein ingredientsAerosol deliveryAntigenSide effect
The invention provides recombination interferon (rSIFN-co) or the functional analog with new space conformation, enhanced effect, and low side effect. It is showed by the external pharmacodynamics, it can not only restrain the DNA replication of the hepatitis b virus, but also restrain the exudation of the surface antigen (HBsAg) and e antigen (HBeAg). The cytology toxicity is 1 / 8 of the clinical interferon and the antiviral activity is 5-20 times to the clinical interferon and has a higher, a more board spectrum and longer time biological response reaction, and prevent the tumor hyperplasia and transmission. The invention also provides the synthetic gene code, gene carrier, expression system of the synthetic gene of the recombination interferon or the functional analog. Finally, the invention also provides the preparing method and application thereof.
Owner:SUPERLAB FAR EAST LTD
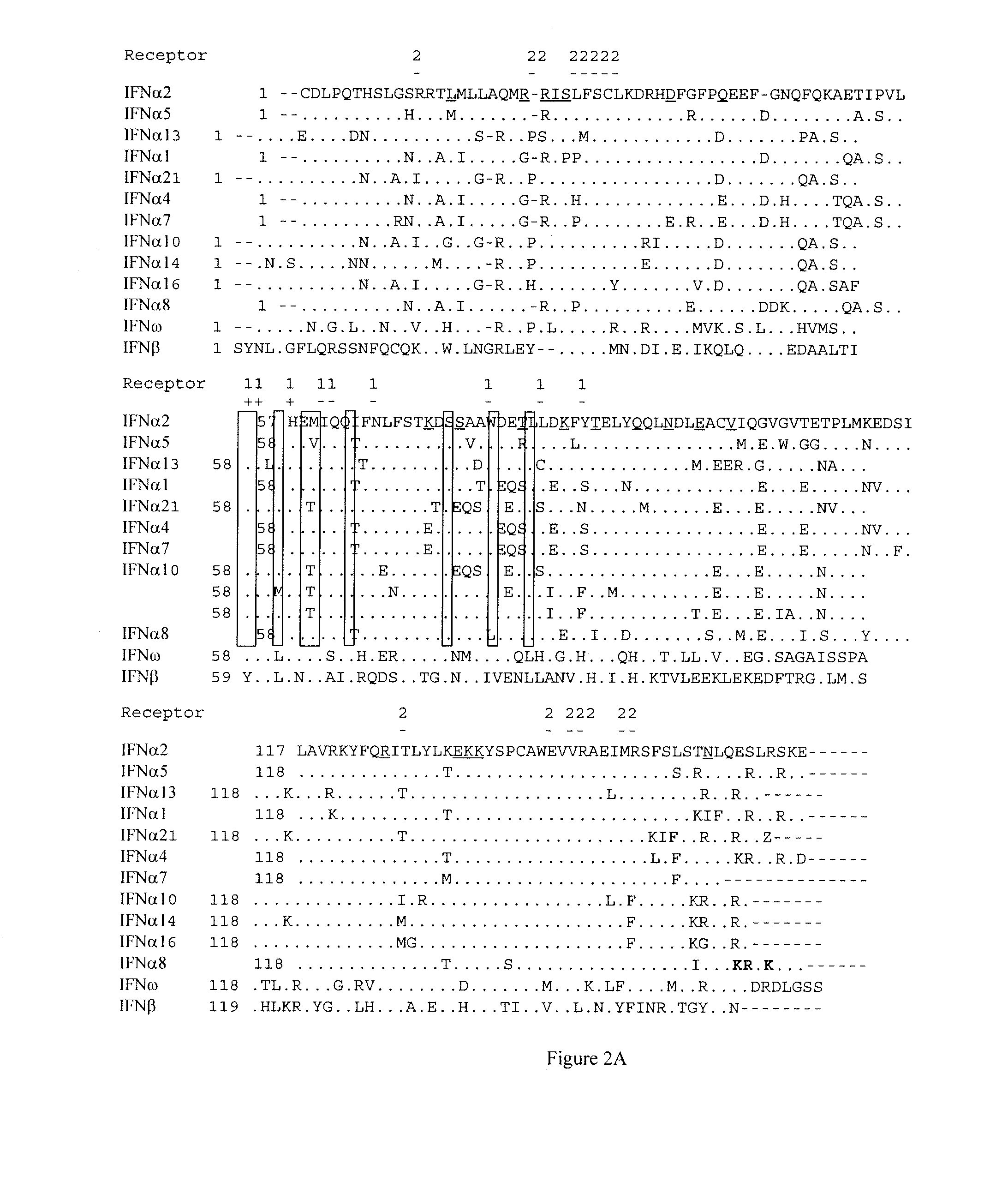
Recombinant interferon alpha2 (ifnalpha2) mutants and methods of use thereof
InactiveUS20080166319A1High affinityAntibacterial agentsNervous disorderAutoimmune diseaseBiochemistry
The present invention provides IFNα2 mutants and active fragments, analogs, derivatives, and variants thereof, nucleotide molecules encoding same, pharmaceutical compositions containing the same, and methods utilizing the same for treating cancer, infectious diseases, and autoimmune diseases.
Owner:YEDA RES & DEV CO LTD
Expression method of animal alpha interferon and gamma interferon
ActiveCN102628062AConvenient timeIncrease resistanceFermentationGenetic engineeringGamma interferonInterferon alpha
The invention discloses an expression method of an animal alpha interferon and a gamma interferon. The method comprises the following step of: performing combined expression or co-expression on the alpha interferon and gamma interferon of a human being or an animal of the same type in a bioreactor. The alpha interferon gene and gamma interferon gene of a human being or an animal of the same type are subjected to combined expression or co-expression in the same insect rhabdovirus bioreactor, and an expressed recombinant alpha interferon product and recombinant gamma interferon have cooperative and synergistic actions, so that the valence and stability of a recombinant interferon product can be enhanced. The method has the advantages of high expression efficiency, high product valence, high stability and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
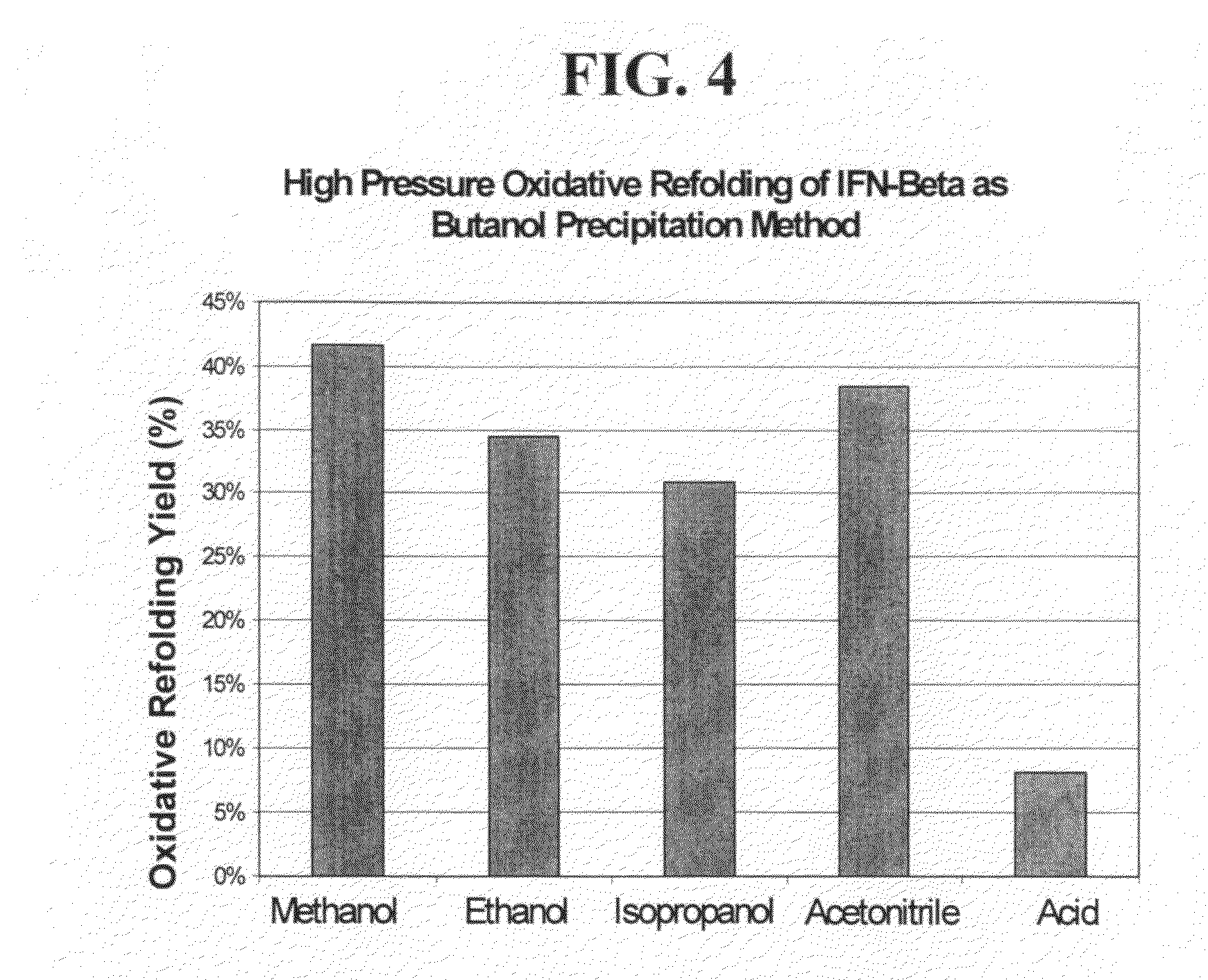
High pressure treatment of aggregated interferons
InactiveUS20090208453A1High yieldImprove efficiencyPeptide/protein ingredientsDepsipeptidesInclusion bodiesCell Aggregations
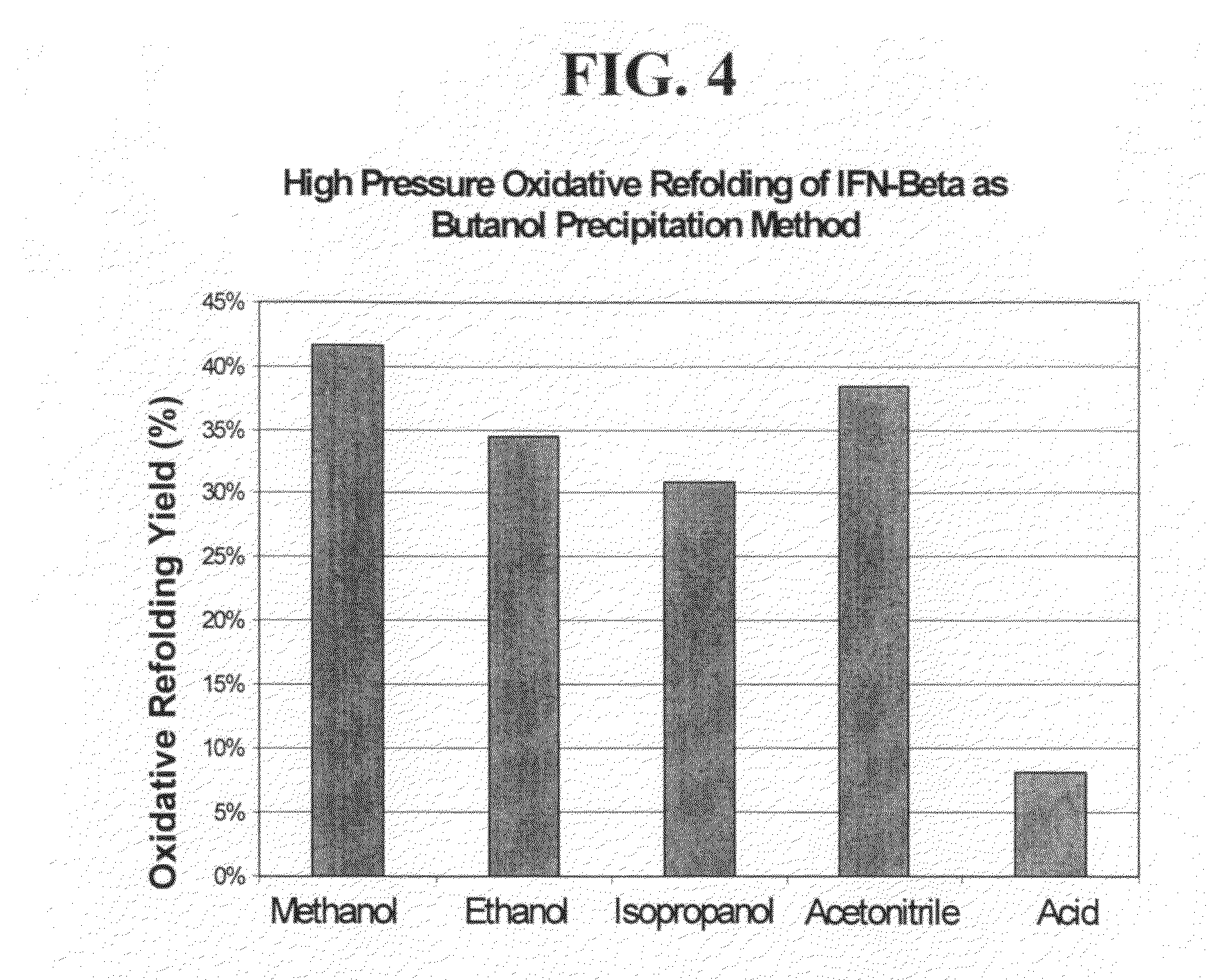
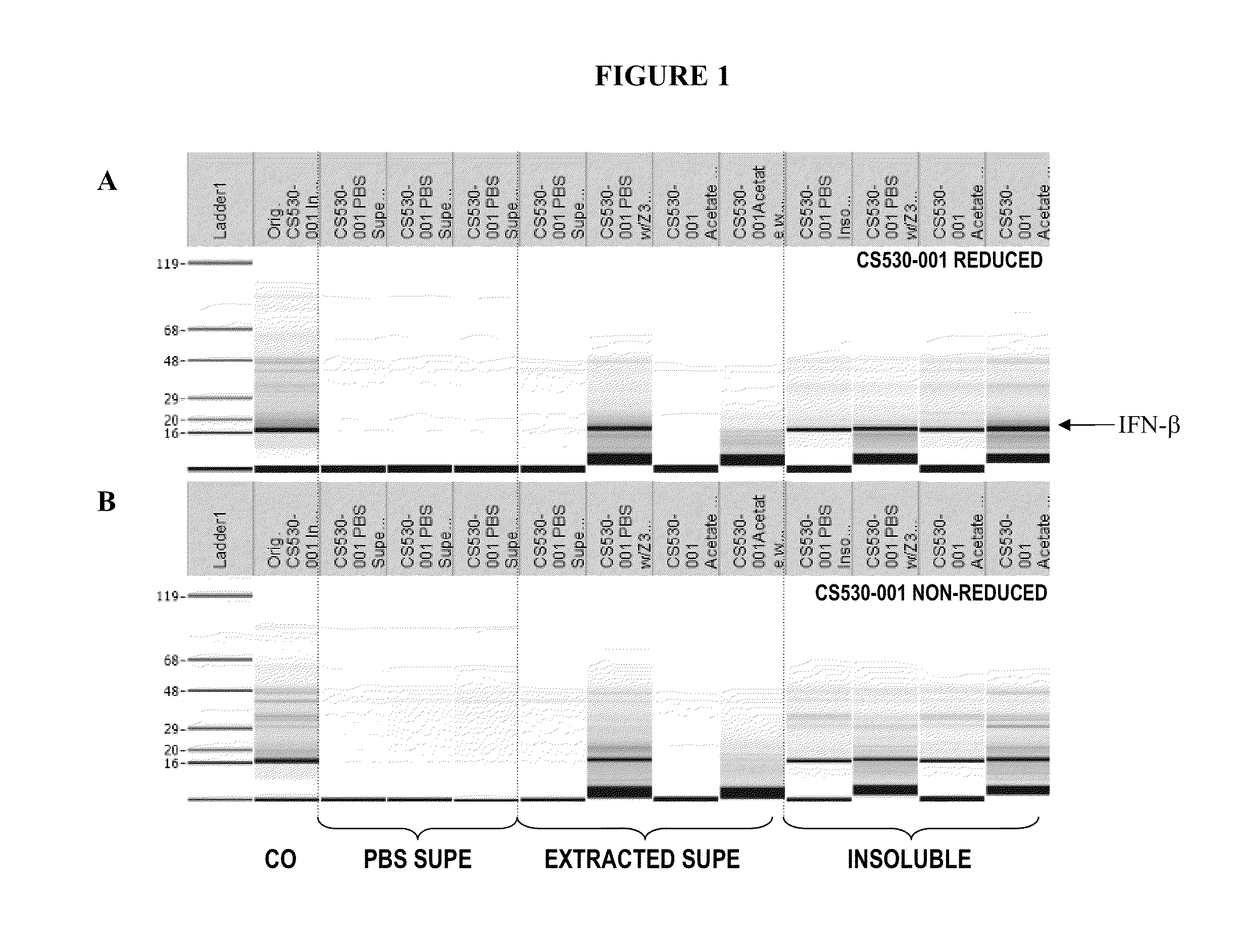
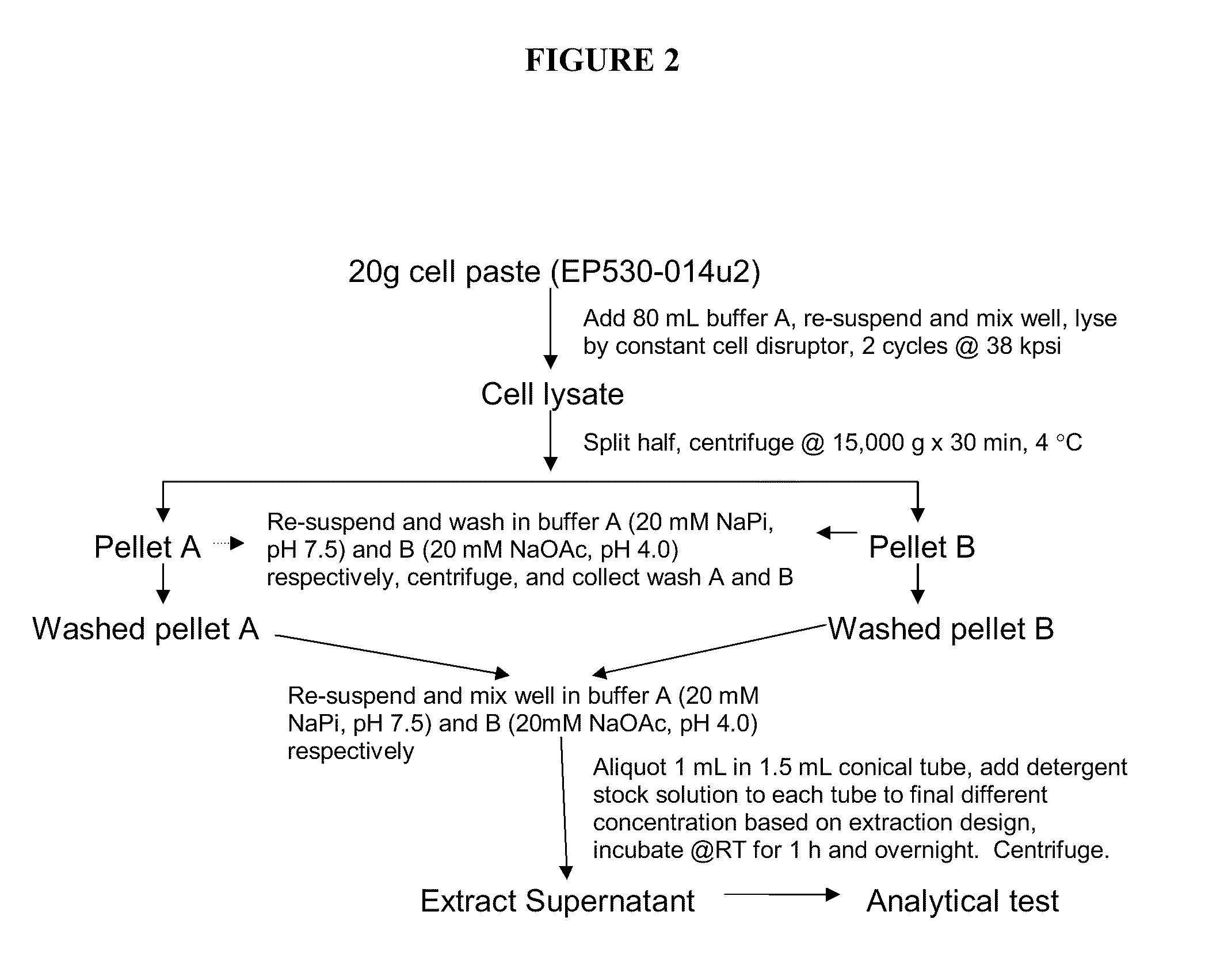
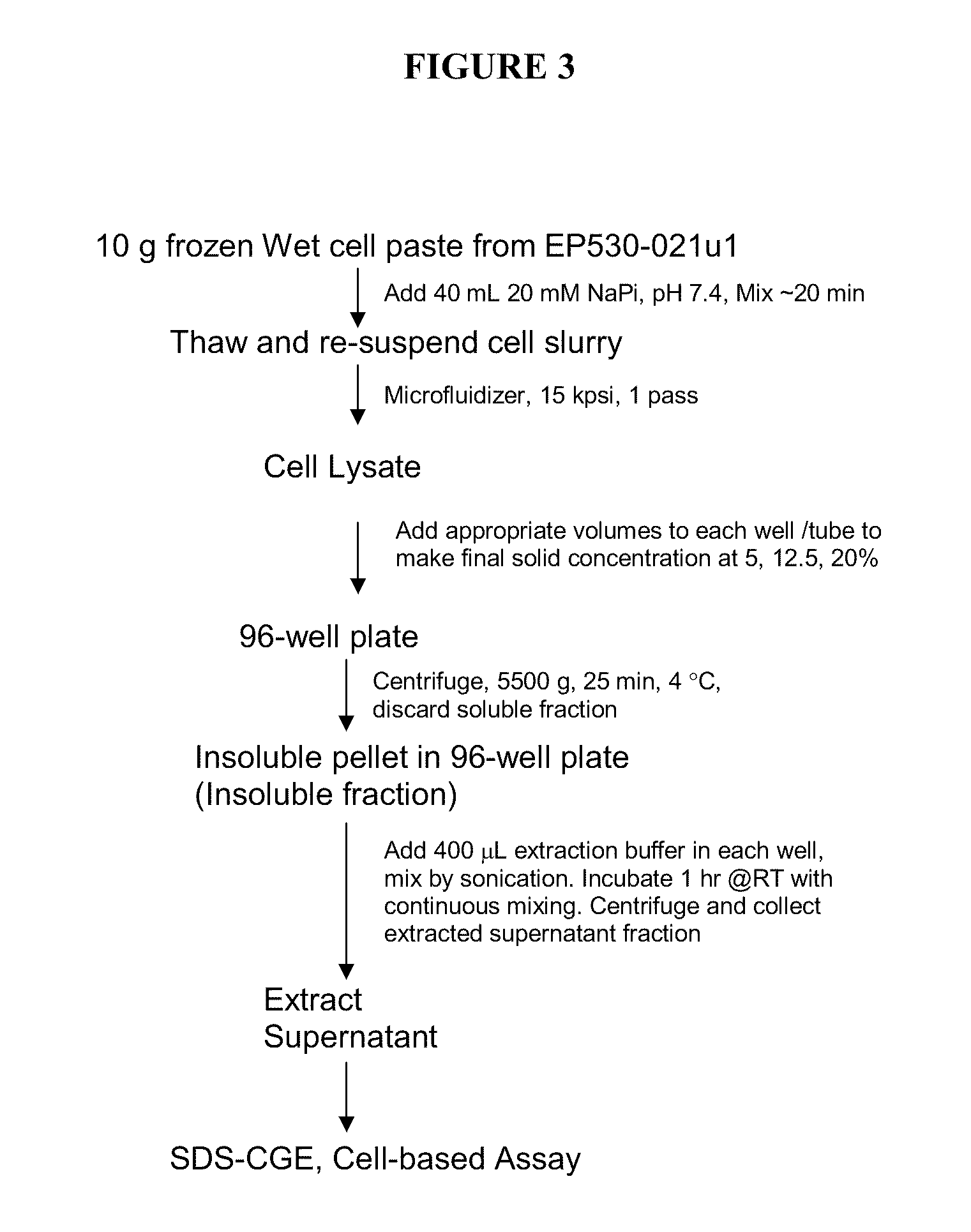
High pressure to treat aggregated interferons, particularly recombinant human interferon-β, to reduce the aggregate content of interferon material. Highly pure, soluble monomeric recombinant interferon-β is prepared in representative embodiments. Multiple strategies may be used in combination that make nonglycosylated IFN-β more amenable to high pressure treatment. It has been found that refolding yields of high pressure treatment can be significantly improved by use of a combination of strategies, including, or example a pre-treatment of the IFN-β that involves solubilizing and then precipitating the protein. This pre-treatment is particularly effective with respect to recombinant IFN-β inclusion bodies recovered from host cells such as E. coli cells. According to another strategy, refolding under high pressure is much more effective when the refolding reagent incorporating the IFN-β incorporates a zwitterionic surfactant and / or a cholate salt. When a solubilization and precipitation pre-treatment is used, the effectiveness of the high pressure treatment is further enhanced when the refolding reagent incorporating the protein incorporates a disulfide shuffling chemistry such as cysteine / cystine. According to still yet another strategy, high pressure treatment is more effective when using atypically high treatment pressures. When coupled with purification techniques, these strategies singly or in combination provide a low aggregate or substantially aggregate free, biologically active solution. Biologically active solutions comprising nonglycosylated interferon, said interferon comprising less than about 5 weight percent of protein aggregation has been found to exhibit improved PK / PD characteristics.
Owner:NURON BIOTECH
Recombinant Interferon 2alpha (Infalpha2) Mutants
InactiveUS20080279823A1Strong specificityAntibacterial agentsNervous disorderWild typeAutoimmune disease
The present invention provides IFNα2 mutants and active fragments, analogs, derivatives, and variants thereof that have improved specific agonist or antagonist activity as compared to wild-type IFNα2. The present invention further provides pharmaceutical compositions comprising IFNα2 mutants useful for treating or preventing cancer, autoimmune diseases, infectious diseases or disorders associated with increased expression of IFNα2.
Owner:YEDA RES & DEV CO LTD
High pressure treatment of aggregated interferons
InactiveUS8273561B2High yieldImprove efficiencyPeptide/protein ingredientsDepsipeptidesInclusion bodiesCell Aggregations
High pressure to treat aggregated interferons, particularly recombinant human interferon-β, to reduce the aggregate content of interferon material. Highly pure, soluble monomeric recombinant interferon-β is prepared in representative embodiments. Multiple strategies may be used in combination that make nonglycosylated IFN-β more amenable to high pressure treatment. It has been found that refolding yields of high pressure treatment can be significantly improved by use of a combination of strategies, including, or example a pre-treatment of the IFN-β that involves solubilizing and then precipitating the protein. This pre-treatment is particularly effective with respect to recombinant IFN-β inclusion bodies recovered from host cells such as E. coli cells. According to another strategy, refolding under high pressure is much more effective when the refolding reagent incorporating the IFN-β incorporates a zwitterionic surfactant and / or a cholate salt. When a solubilization and precipitation pre-treatment is used, the effectiveness of the high pressure treatment is further enhanced when the refolding reagent incorporating the protein incorporates a disulfide shuffling chemistry such as cysteine / cystine. According to still yet another strategy, high pressure treatment is more effective when using atypically high treatment pressures. When coupled with purification techniques, these strategies singly or in combination provide a low aggregate or substantially aggregate free, biologically active solution. Biologically active solutions comprising nonglycosylated interferon, said interferon comprising less than about 5 weight percent of protein aggregation has been found to exhibit improved PK / PD characteristics.
Owner:NURON BIOTECH
Method of treatment for feline leukemia virus infections
InactiveUS6350443B1Reduce feverReduce in quantityBiocidePeptide/protein ingredientsGranulocytopeniasInterferon alpha
A method of treatment for feline leukemia virus infections by continuously administering a feline interferon preparation containing a feline interferon as a main component daily to a cat is disclosed. As a feline interferon, a feline omega (omega)-interferon is preferably used, and more particularly, a recombinant interferon is preferably used. A method of treatment using a therapeutic agent containing a feline omega-interferon as a main component in accordance with the present invention is a novel and superior method suitable for treating feline leukemia virus infections, and in particular, for treating neutropenia.
Owner:TORAY IND INC
Method for producing soluble recombinant interferon protein without denaturing
ActiveUS20110217784A1Peptide/protein ingredientsPeptide preparation methodsType 1 interferonBacteria
The present invention relates to the field of recombinant protein production in bacterial hosts. It further relates to extraction of soluble, active recombinant protein from an insoluble fraction without the use of denaturation and without the need for a refolding step. In particular, the present invention relates to a production process for obtaining high levels a soluble recombinant Type 1 interferon protein from a bacterial host.
Owner:PFENEX
Chicken interferon gamma, preparing process and use thereof
A chicken interferon gamma used for preparing medicines, immunopctentiator, feed additive, etc is prepared through configuiring the recombinant carrier of the recombinant chicken interferon, screening, and separating and purifying the expression product. Its advantages are high purity and low cost.
Owner:蔡中华 +2
Canine recombinant interferon alpha and preparation method
InactiveCN102321169AMicroorganism based processesPeptide preparation methodsCanine kidneyEscherichia coli
The invention discloses canine recombinant interferon alpha, and also provides a preparation method of the canine recombinant interferon alpha. Recombinant genes of canine interferon alpha are expressed in escherichia coli, and canine interferon alpha with a purity of more than 98% is obtained through processes of renaturation and purification. The biological activity is determined by a MDCK (canine kidney cell)-VSV (vesicular stomatitis virus) virus system, and the specific activity reaches 2-3*107 IU / mg.
Owner:CHANGCHUN UNIV OF TECH
BCG vaccine strain of recombined interferon and its preparation process
The invention discloses a recombinant interferon--BCG Vaccine bacterial strain and its preparing method, transferring the composed pshuttle phIFN-alpha-2B into the BCG to compose Rbcg-hIFN-alpha-2B bacterial strain. Because it can secrete the interferon IFN-alpha-2B, it can reduce the usage of BCG as compared with that of the wild BCG on the condition of reaching the same and even increased immune effect, thus reducing toxic and side effect; and it can secrete cell factors at the properest time and part. Therefore, it lays the foundation of application to curing cystic tumors.
Owner:天津市泌尿外科研究所 +5
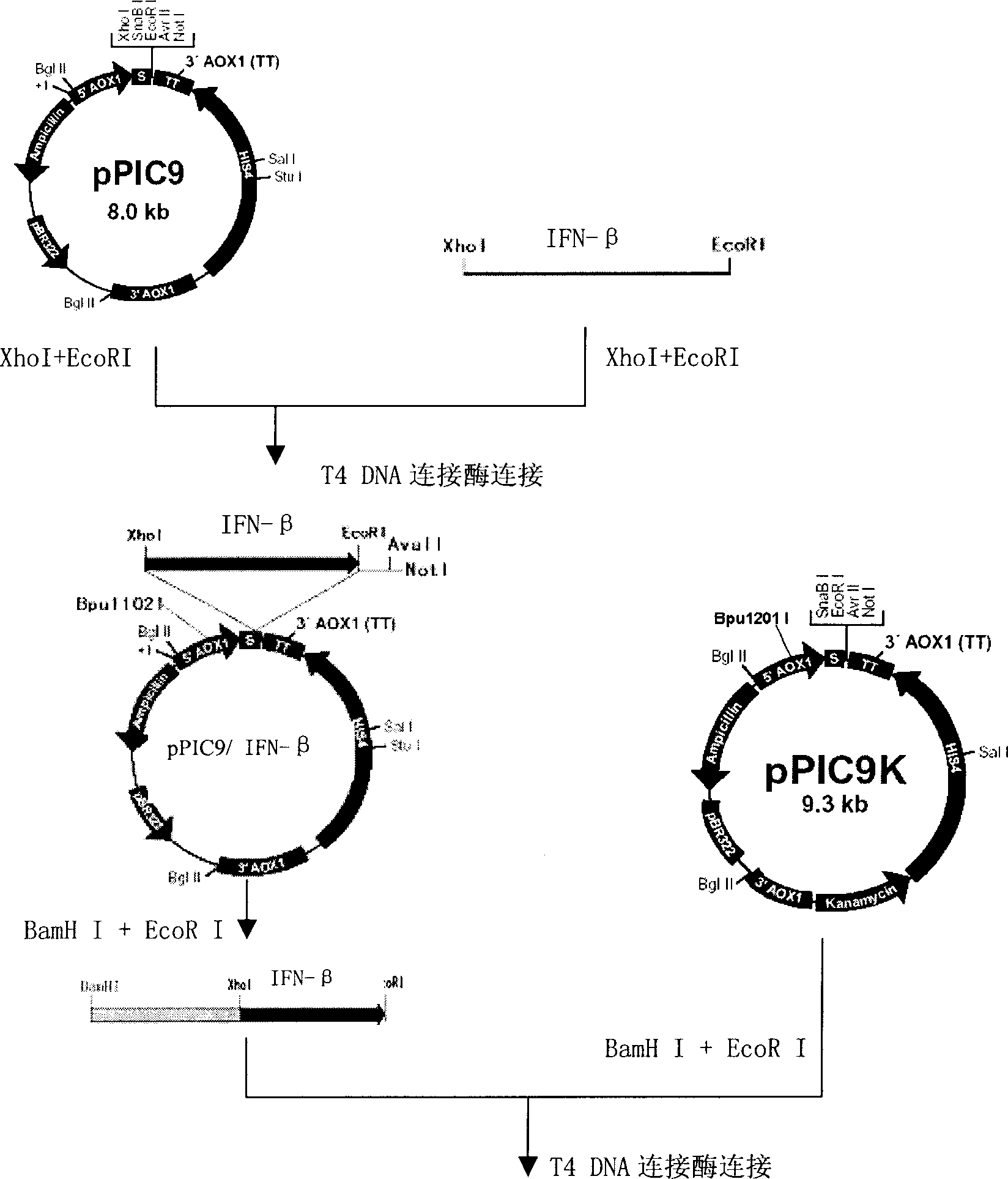
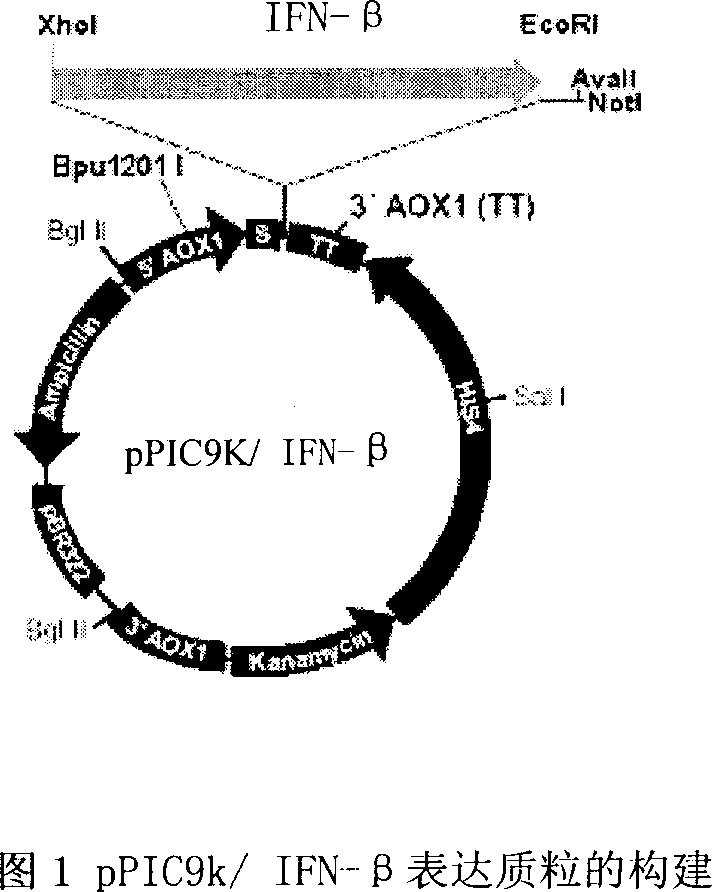
Production of recombinant human interferon beta
InactiveCN1854296AEfficient and/or easy productionEasy to produceFungiFermentationPichia pastorisNucleotide
The invention provides a nucleotide sequence of recombinant human interferon beta, a production method for efficiently producing recombinant human interferon beta, and related engineering cell construction, expression and purification techniques. The optimized recombinant human interferon beta protein gene is very suitable for expression in Pichia pastoris. At the same time, through optimization of fermentation and purification process, the expression level has been increased, and it has the advantages of high expression and high stability. The invention can obtain the pure product of recombinant human interferon beta efficiently, conveniently and at low cost.
Owner:SHANGHAI NEWSUMMIT BIOPHARMA
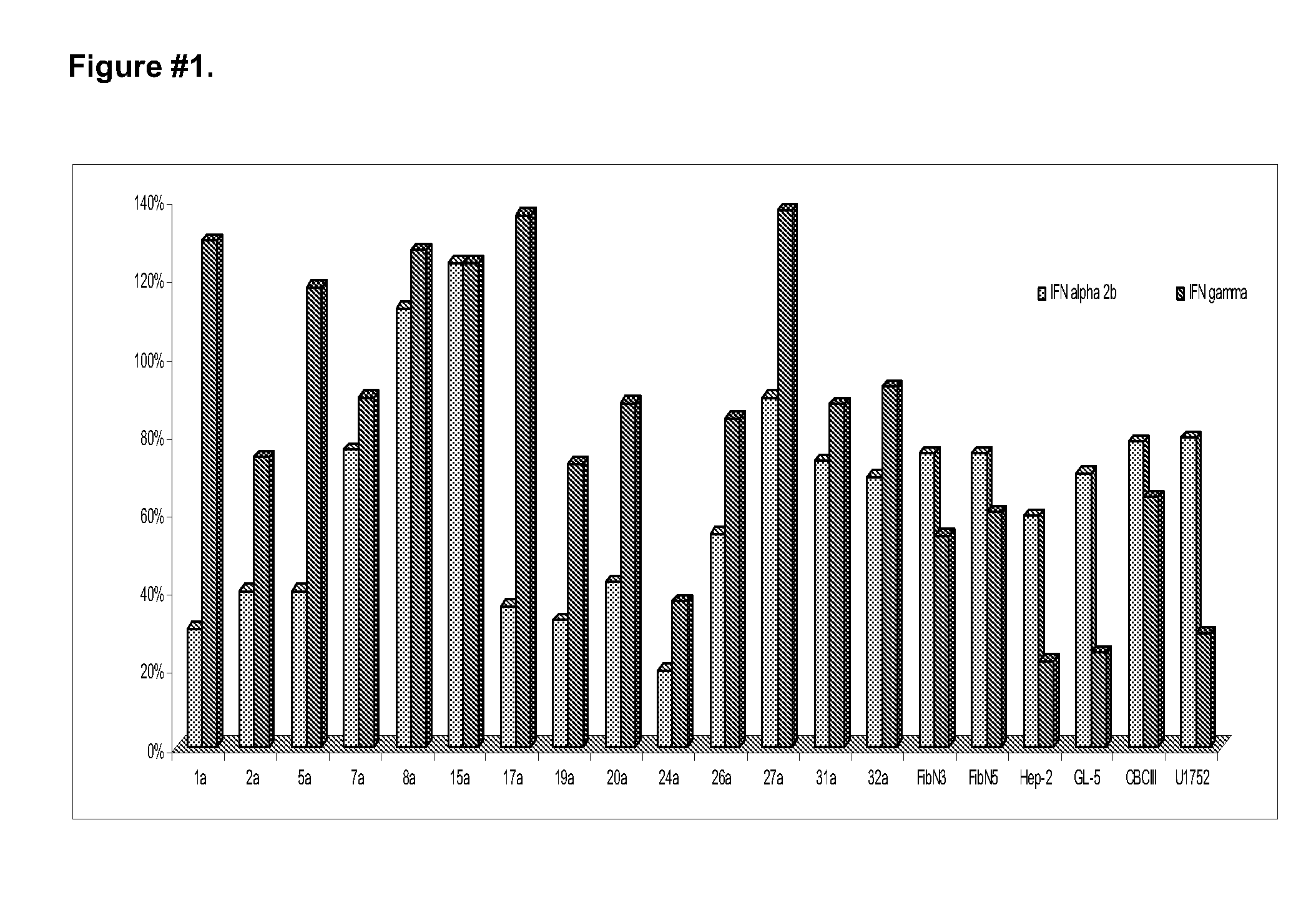
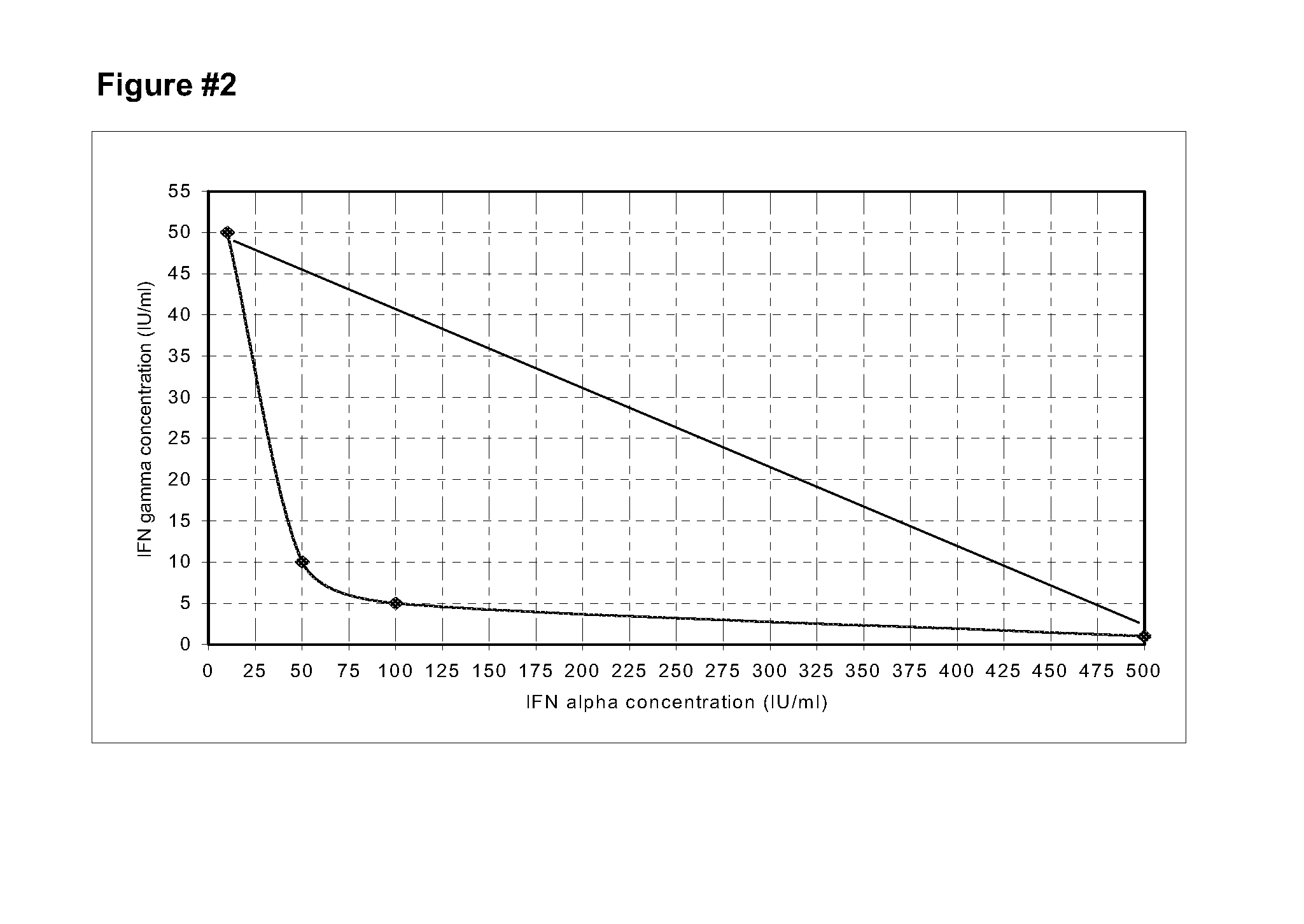
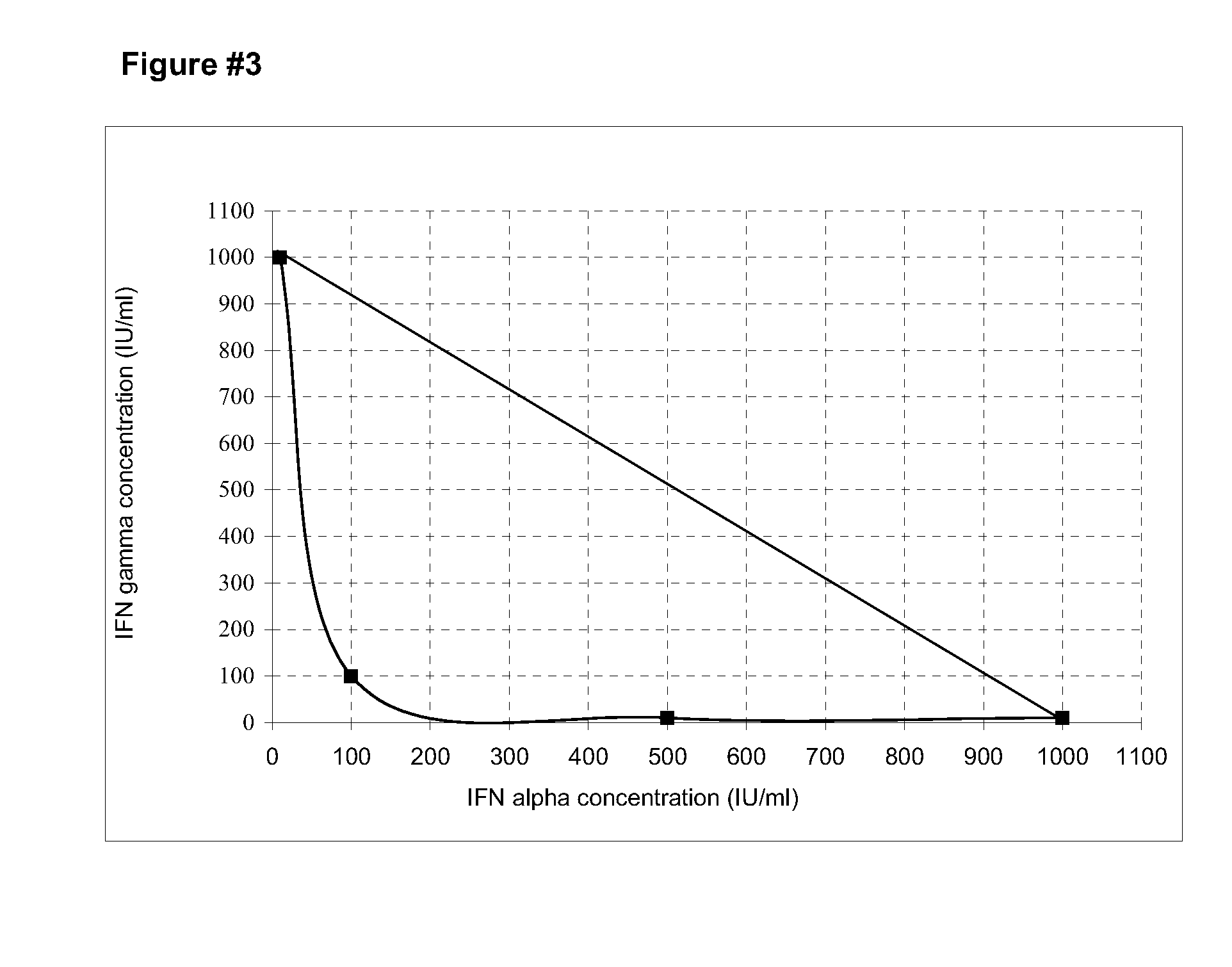
Stabilized pharmaceutical formulations that contain the interferons gamma and alpha in synergistic proportions
ActiveUS20090304628A1Little riskInhibit aggregationPeptide/protein ingredientsPharmaceutical non-active ingredientsFreeze-dryingMedicine
The present invention is related to stable pharmaceutical formulations to be applied by parenteral (liquids or freeze-dried), or topic way (gel, unguent or cream) that contain different quantities of the recombinant interferons gamma and alpha in synergistic proportions for the treatment of pathological events that contemplate the malignant or benign not-physiological growth of cells in tissue or organs.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Oral liquid synergistic agent for livestock, poultry and aquatic product, and preparation method thereof
InactiveCN101314033AImprove immunityImprove disease resistanceAntibacterial agentsPeptide/protein ingredientsAquatic animalOrganism
The invention discloses an oral liquid synergistic agent for vaccines of livestock, poultry and aquatic animals and a preparation method thereof. The oral liquid synergistic agent for the vaccines is prepared from the following materials by weight portion: 10 to 25 portions of ginkgo leaves, 15 to 25 portions of Japanese honeysuckle stem, 5 to 20 portions of echinacea purpurea root, 10 to 20 portions of cogongrass rhizome, 8 to 20 portions of purslane, 10 to 20 portions of plantain, 15 to 25 portions of rehmanniae, 2 to 5 portions of snake gall, 10 to 20 portions of ox gall, 40 to 60 portions of aloe, 25 to 40 portions of rock candy, 10 to 20 portions of gene recombinant interferon and 5 to 15 portions of amino vitamin. The oral liquid synergistic agent for the vaccines disclosed by the invention can be directly mixed with normal feed of animals or directly mixed with water drunk by the animals, thereby effectively improving the immunity of an organism and disease resistant capability of the livestock, the poultry and the aquatic animals and eliminating the mutual interference among the vaccines, strengthening the immune effect of the combined vaccine, improving the immunity of the vaccines and making organisms of the animals have good immune effects for the vaccines.
Owner:雷清莲
Pig interferon inducer and use
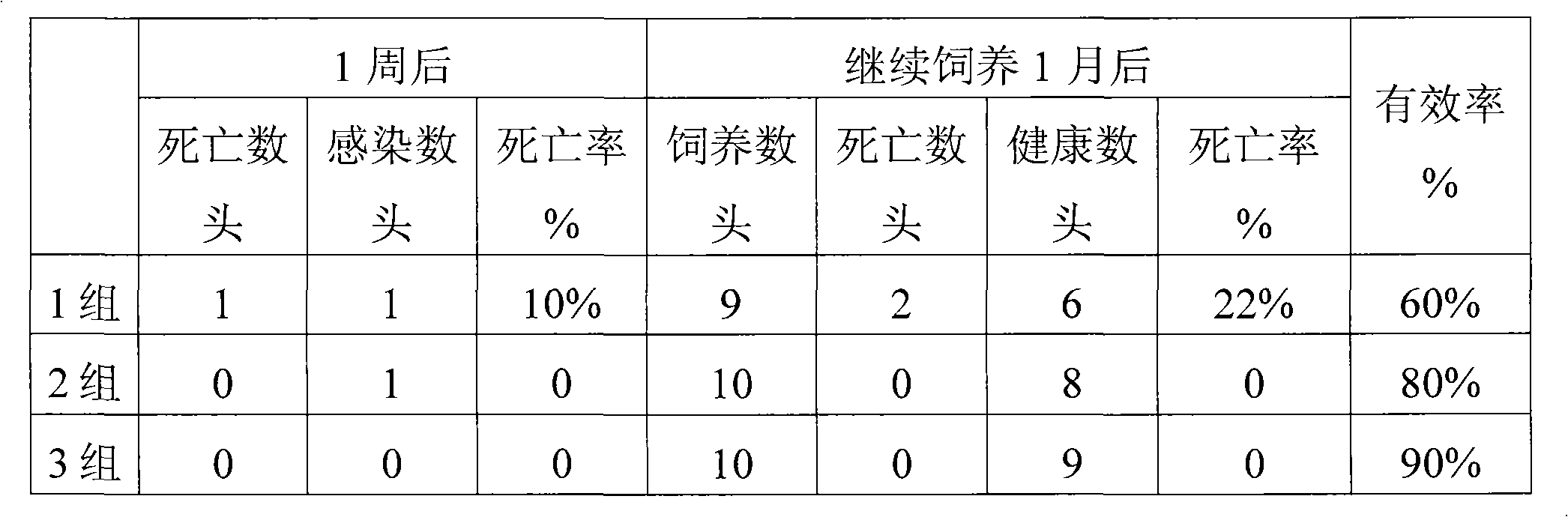
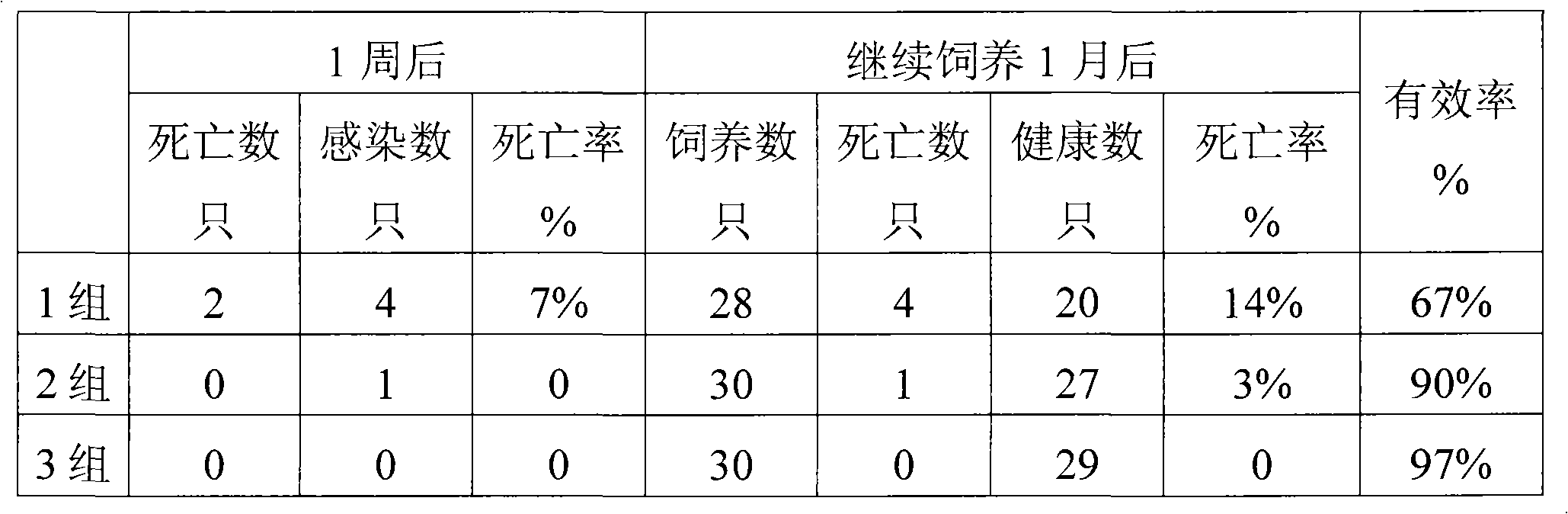
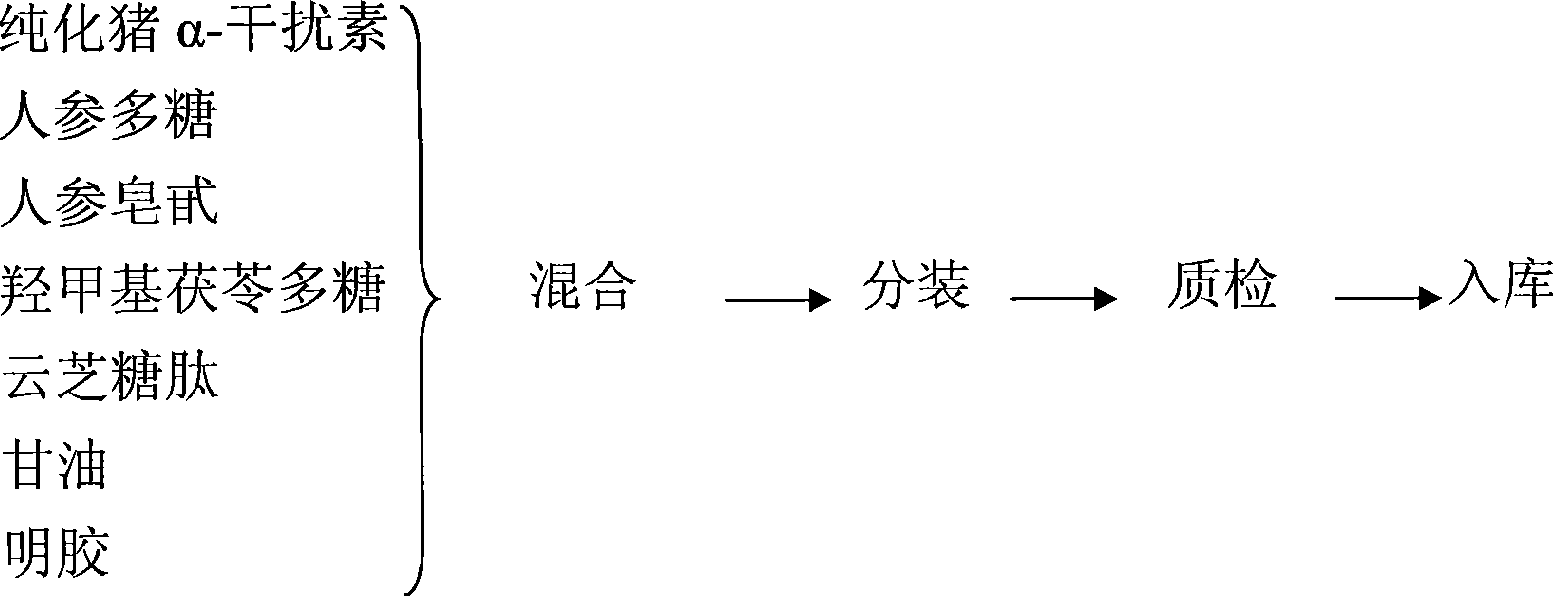
InactiveCN101461937AHigh activityImprove immunityOrganic active ingredientsPeptide/protein ingredientsDiseaseGlycerol
The invention provides a pig interferon inducer. The active components of the pig interferon inducer consist of purified pig alpha-interferon, ginseng polysaccharide, ginsenoside, hydroxymethyl tuckahoe polysaccharide and polysaccharide-peptide. By weighing up the active components and adding with glycerin and gelatin for fully mixing, a suspending liquid is prepared, and the suspending liquid needs to be stored in light-shading places. For porcine reproductive respiratory syndrome, hog cholera and pseudorabies, the effective prevention rate of the pig interferon inducer provided by the invention is more than 70 percent. The invention provides a veterinary medicine which is safe, effective and low-priced, and can prevent diseases, and the cells are induced to generate the interferon by stimulating an animal body, so that the veterinary medicine has various biological activities of resisting virus in the broad spectrum, improving body immunity and the like, and the aims of preventing and curing viral diseases are achieved. The pig interferon inducer has the advantages of solving the problems of high production cost and inconvenient use existing in the leukocyte interferon and the recombination interferon of the pig, along with simple production process, low production cost and convenient popularization.
Owner:HANGZHOU WONDERFUL BIOTECH
Recombinant strain of mink IFN-Gamma gene
InactiveCN101531986AHas antiviral activityBacteriaMicroorganism based processesEscherichia coliBiotechnology
The invention relates to a recombinant strain of a mink IFN-Gamma gene, which is characterized by being an IFN-Gamma gene sequence which is cloned from peripheral mink bloodlymph cells (PMBC) simulated by phytohemagglutinin (PHA) and being a recombinant plasmid vector containing a nucleotide sequence; i.e., using a plasmid vector pGM-T which is formed by the cutting of a cloning vector by taking an EcoR V enzyme as the cutting point and then adding a 'T' from the '3' end at both sides; and TA cloning is carried out on the nucleotide sequence of a coding Gamma-interferon which is amplified from the peripheral mink bloodlymph cells so as to construct the recombinant plasmid of the nucleotide sequence of the mink Gamma-interferon. In addition, Escherichia coli conversed from the plasmid vector is used as a host. The recombinant strain aims at laying down foundation for researching and developing genetically engineered mink recombinant interferons biological products with anti-viral activity and immunomodulatory activity.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Preparation method of recombinant pig alpha interferon
ActiveCN103695457AShorten the production cycleOmit feed growth periodMicroorganism based processesFermentationEscherichia coliPichia pastoris
The invention constructs and screens a recombinant pichia pastoris strain PoIFN-alpha / GS115 which can efficiently perform secretory expression of pig alpha interferon, overcoming the problems of complex purification process and low biology activity and the like when a recombinant interferon is expressed and prepared by using escherichia coli. According to the invention, a specific fermenting technology which is different from a traditional high-density fermenting technology and is suitable for efficient expression of a target gene is established. The fermenting technology is short in production cycle, is simple in purification process and is low in cost.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
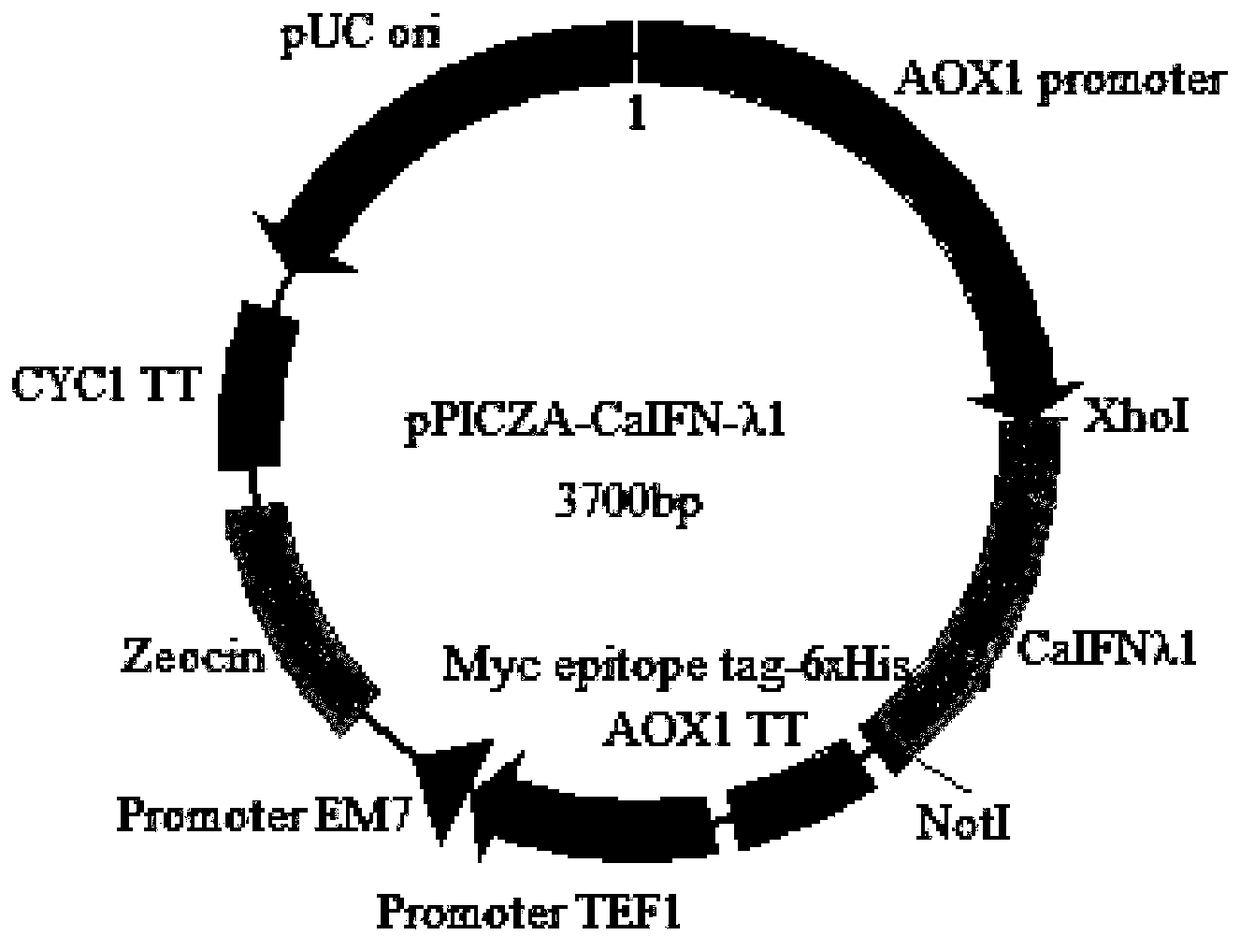
Canine recombinant interferon-lambda 1, and preparation method and application thereof
The invention provides a canine recombinant interferon-lambda 1, and a preparation method and an application thereof. The canine recombinant interferon-lambda 1 has a nucleotide sequence as shown in SEQ ID NO. 1. The invention also provides a preparation method for the canine recombinant interferon-lambda 1. According to the invention, a pichia pastoris strain expression system is utilized to express a recombinant yeast engineering strain containing a codon-optimized canine recombinant interferon-lambda 1 gene, so secretion-expressed canine recombinant interferon-lambda 1 with high activity isobtained. The canine recombinant interferon-lambda 1 prepared by using the method provided by the invention has high purity, can reach a purity of 0.86 mg / ml after purification, has a protein yield of 42 mg in 1 L of recombinant yeast engineering strains, has a specific antiviral activity of 2.85 x 10<7> IU / mg, can effectively prevent and treat infectious diseases like canine distemper, canine parvovirus, canine parainfluenza virus infection and canine infectious hepatitis, and has high safety and good application prospects.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Canine recombinant interferon alpha7, and preparation method and application thereof
ActiveCN111004317AIncrease productionHigh activityFungiPeptide/protein ingredientsPichia pastorisInterferon alpha
The invention discloses a canine recombinant interferon alpha7, and a preparation method and an application thereof. The nucleotide sequence of the canine recombinant interferon alpha7 is obtained through codon optimization, a Pichia pastoris strain expression system is used for expressing recombinant yeast engineering bacteria containing the canine recombinant interferon alpha7 gene with the optimized codon, and the secreted and expressed high-activity canine recombinant interferon alpha7 is obtained. The canine recombinant interferon alpha7 prepared by the method is high in purity, the protein concentration after purification reaches 0.55 mg / ml, the protein yield of 1 L of recombinant yeast engineering bacteria reaches 150 mg, the antiviral specific activity of the recombinant protein onVSV on MDCK cells can reach 1.85 * 10<6> IU / mg, studies on the expression, fermentation and activity of CaIFN-alpha7 in an eukaryotic yeast system are of great significance in early industrial production of canine interferon alpha.
Owner:ANHUI JIUCHUAN BIOTECH +1
Application of recombinant alpha-interferon in preparing medicine for preventing and treating respiratory system infection
InactiveCN1548149AEnhanced inhibitory effectPeptide/protein ingredientsDigestive systemDiseaseInterferon alpha
The alpha-interferon has been used mainly in treating chronic viral infection. The present invention uses coronavirus or rhinovirus infected human cervical cancer cell as estimation model in observing the inhibition effect of alpha-interferon on common virus causing respiratory system infection. The results show that alpha-interferon may be used in preventing and treating coronavirus and rhinovirus caused diseases, including SARS. The integrated interferon, its mutant and interferon alpha-1b have even high inhibiting effect on new SARS virus.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD +1
Method for purifying interferon protein
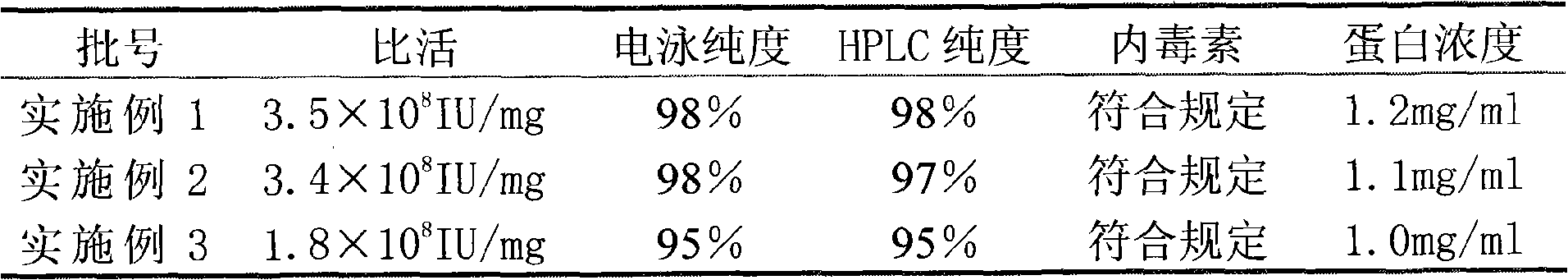
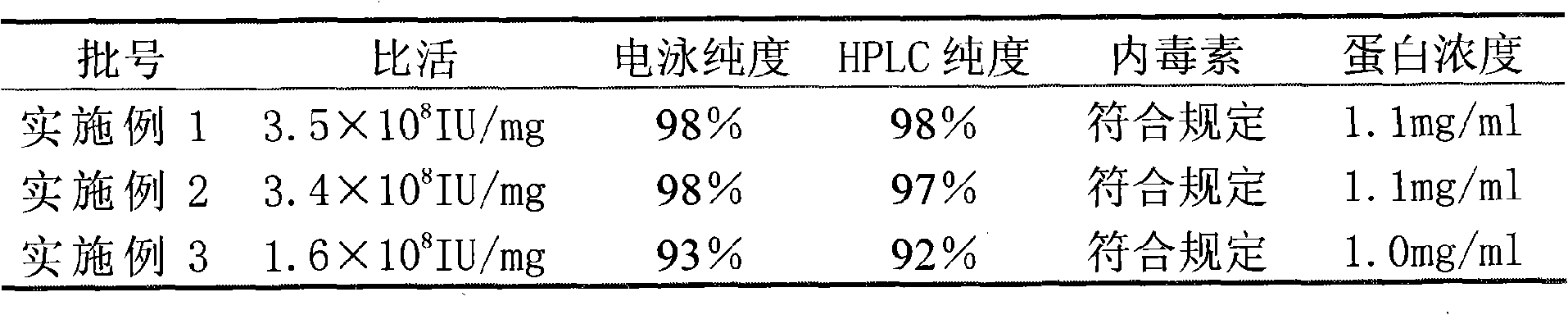
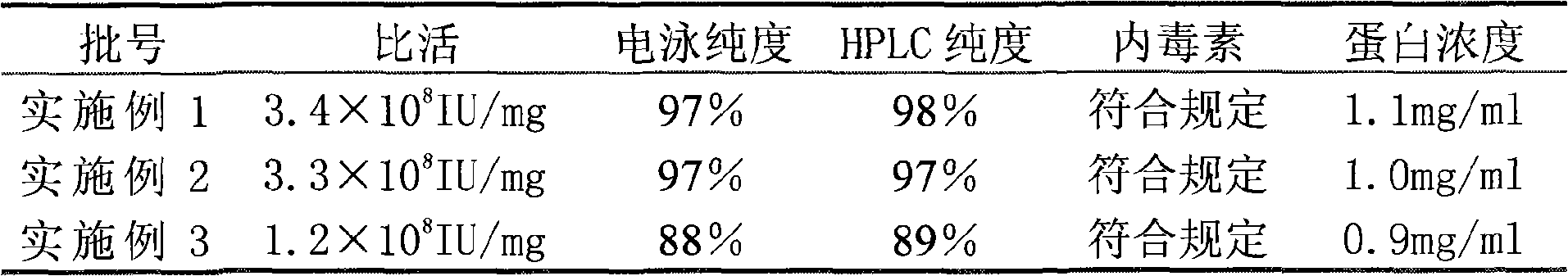
InactiveCN101659690ALittle side effectsHigh purityPeptide preparation methodsElectrophoresesSpecific activity
The invention relates to a method for purifying interferon protein. The method adopts a reverse phase filler refining and separating purification step in a purification process. Compared with the prior art, the method purifies an interferon by using a reverse phase filler refining and separating method for the first time and improves the electrophoresis purity of the interferon to 98 percent from90 percent, HPLC purity to 98 percent from 90 percent and specific activity of the interferon to 3.5*10<8>U / mg to 0.9*10<7>U / mg. The purification process of the recombinant interferon can greatly improve the purity, specific activity and stability of human interferons and effectively reduce the side effects of the interferons in clinic.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
A method for stably producing porcine alpha interferon using recombinant Pichia pastoris
Owner:SHANGHAI ACAD OF AGRI SCI +1
High-efficiency expression and application of human recombinant interferon B
InactiveCN1325651CRelatively largeHigh purityMetabolism disorderAntipyreticInterferon-BAutoimmune disease
The present invention relates to high-effective expression of human recombinant interferon B and its application, further relates to adoption of biological gene engineering technique to make expression and produces a recombinant human interferon B, and utilizes eukaryocyte expression vector to convert host cell and produces a recombinant human interferon B. The recombinant human human interferon B which is high in yield and is obtained by means of expression and production of eukaryocyte system is a glycosylated protein modified after protein translation, and is similar to natural human interferon B in structure and function. The amount of protein produced by this invented expression system is large, and its purity is high, it can extensively be used for clinical curing autoimmune diseases.
Owner:JINWEI GENE TECHN JINWEI
Treatment of tumors and viral diseases with recombinant interferon alpha
This invention provides a recombinant interferon (rSIFN-co) and its equivalent with changed spatial configuration, high efficacy and low side effects. Therefore, high dose of rSIFN-co may be used. The cytotoxic effect of rSIFN-co is only one-eighth (⅛) of currently clinically available interferons but its anti-viral effect is approximately five to twenty (5-20) times higher, and when used in vivo it has a broader spectrum of clinical applications and longer biofeedback response. This invention further provides methods of using the recombinant interferon to treat proliferative disorders such as cancer or viral diseases.
Owner:SUPERLAB FAR EAST LTD
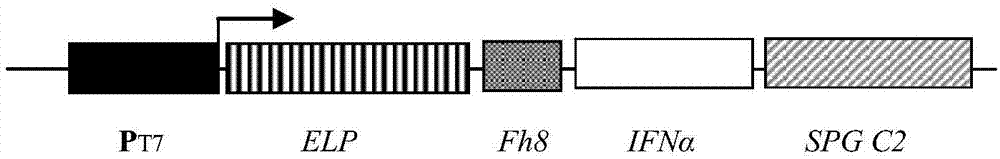
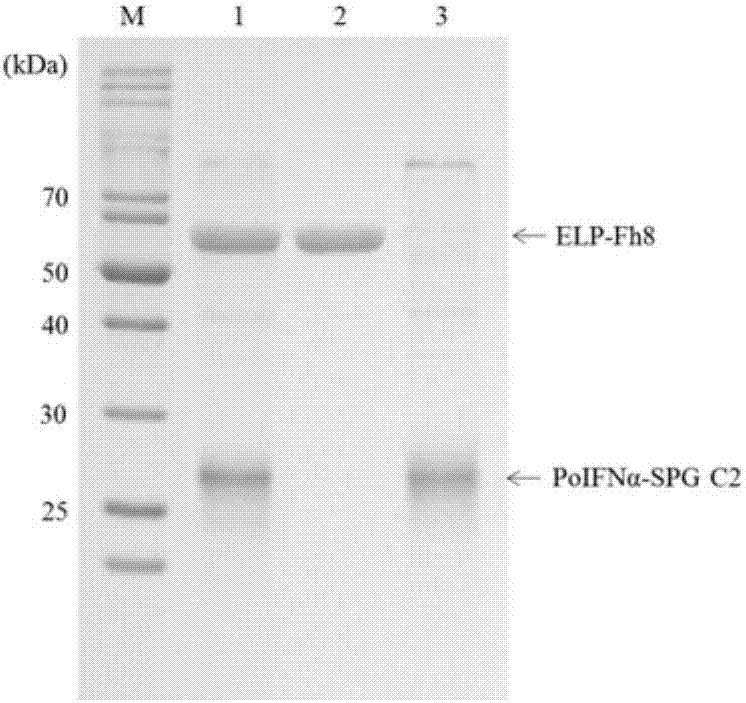
Long-acting interferon and preparation method thereof
InactiveCN107082814AExtended half-lifeEasy to prepareNucleic acid vectorFusions for plasma life prolongingBiotechnology researchHalf-life
The invention belongs to the field of biotechnological research, and particularly relates to long-acting interferon and a preparation method thereof. The long-acting interferon is composed of interferon and streptococcal G protein C2 structural domain, and the preparation method includes the steps of fusion expression vector, fusion interferon expression and purification, purification tag removal and fusion interferon recovery. Compared with the existing recombinant interferon, the long-acting interferon has the advantages of being simple in preparation process, low in cost, long in half-life period and the like, and can be used for the preparation of long-acting interferon for human and animals.
Owner:YANGZHOU UNIV
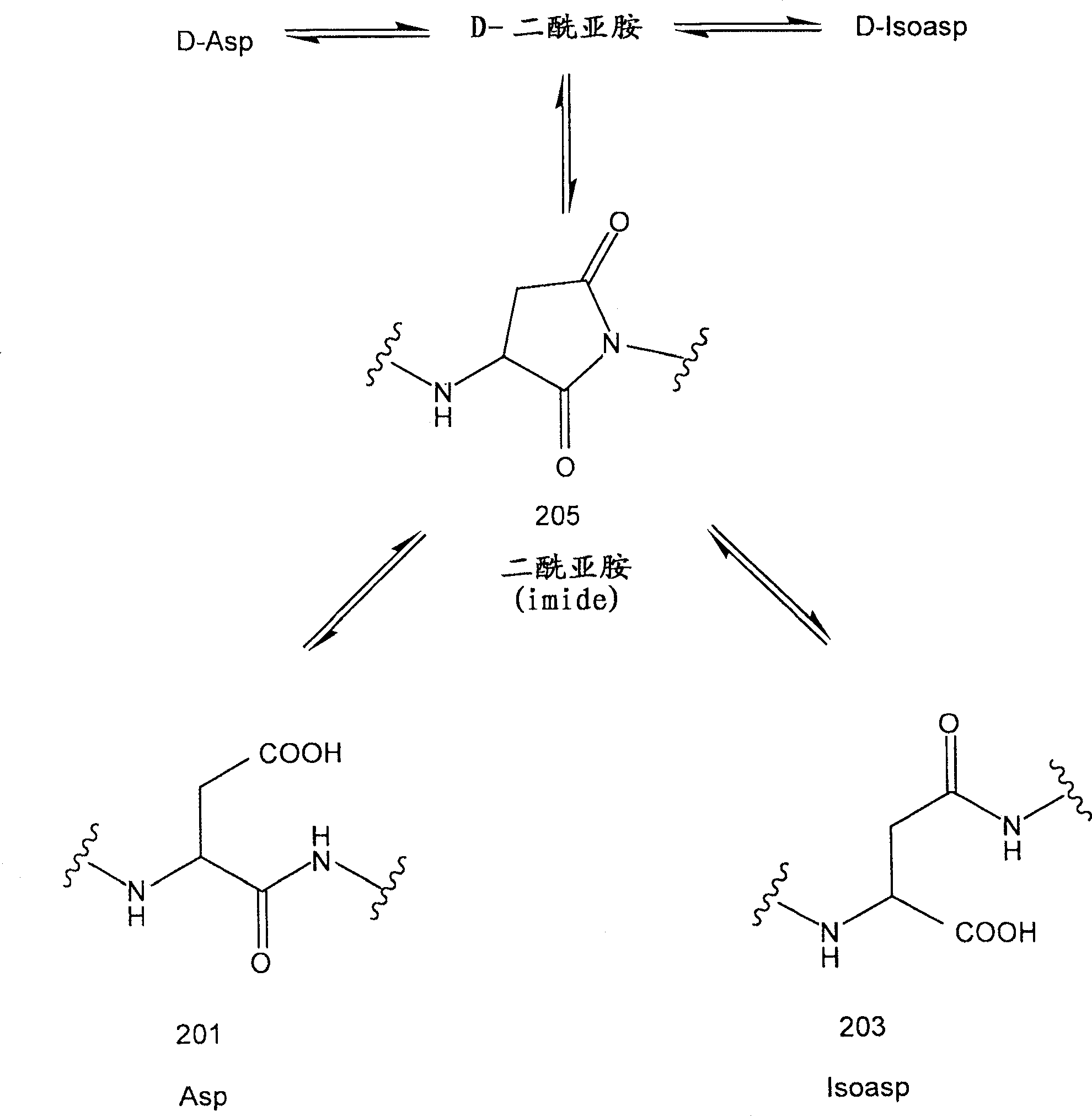
Recombinant interferon-beta with enhanced biological activity
Human interferon-ss protein analogs in which the asparagine at position 25, numbered in accordance with native human interferon-ss, is recombinantly replaced with an aspartate residue exhibit a biological activity of human interferon-ss (e.g. IFN-ss 1b) at an increased level relative to IFN-ss 1b. These analogs are obtained by introducing a gene coding for Asp25 IFN-ss into a cell and expressing the recombinant protein. The resulting IFN-ss protein analog is suitable for large scale manufacturing for incorporation in HA-containing or HA-free therapeutics for treatment of diseases including multiple sclerosis. A reduced Lys endoproteinase-C peptide map technique that produces a fingerprint profile for proteins using an enzymatic digest followed by RP-HPLC is also useful in quality control as an ID test for the IFN-ss protein analog products.
Owner:NOVARTIS AG
Immunization magnetic separation technology for purifying genetic engineering recombinant interferon
InactiveCN1229389CHigh puritySimple and fast operationPeptide preparation methodsInterferonsBiotechnologyMicrosphere
The invention relates to an isolation process for recombination protein in gene engineering, in particular a process for isolating purification gene engineering recombination interferon (IFN) by using immunity isolation technique, which belongs to the field of biological technology, wherein dissimilar materials are employed for preparation of magnetic micro-balloons containing hydroxyl, after epichlorohydrin activating, active amino is introduced and glutaric dialdehyde is used as link arm for joining the antiinterferon antibody.
Owner:NANKAI UNIV
Industrial preparation method and application of recombinant interferon Alpha1
PendingCN106498013AHigh target protein expressionFacilitate downstream processingMicroorganism based processesPeptide preparation methodsEscherichia coliHigh density
The invention provides an industrial production method for Escherichia coli expression, inclusion denaturation and renaturation and purification of recombinant interferon Alpha1 (rpIFNAlpha1). Specifically related are 1, construction of rpIFNAlpha1 Escherichia coli expression bacteria; 2, the development of high-density fermentative processes of rpIFNAlpha1; 3, rpIFNAlpha1 inclusion denaturation and complex process development; 4, purification preparation of denatured rpIFNAlpha1 samples. Through four levels of technical breakthrough, the rpIFNAlpha1 produced by using the method has high bioactivity; the method has high yield, low cost and low amplification difficulty.
Owner:GENSUN INST OF BIOMEDICINE
Animal fusion recombinant interferon
ActiveCN103214579APeptide/protein ingredientsAntibody mimetics/scaffoldsAntiviral drugPolyribonucleotides
Provided is a fusion protein containing an animal interferon and an animal immune globulin Fc section. The fusion protein further comprises a linker for linking the interferon and the immune globulin Fc section. Further provided are a polyribonucleotide for encoding the fusion protein, a method for preparing the fusion protein, and an application of the fusion protein in the preparation of an antiviral drug.
Owner:维克香港贸易有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com