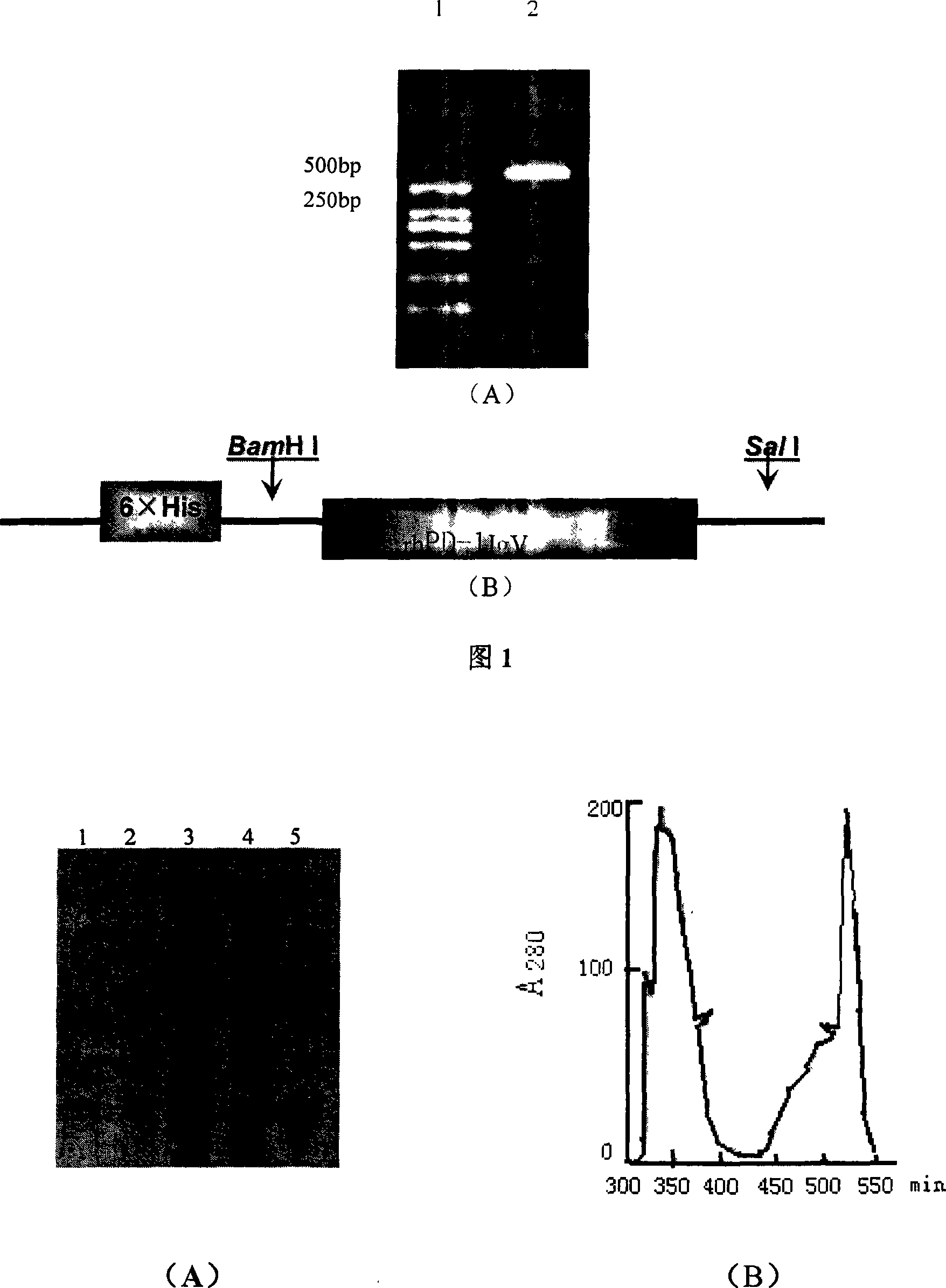
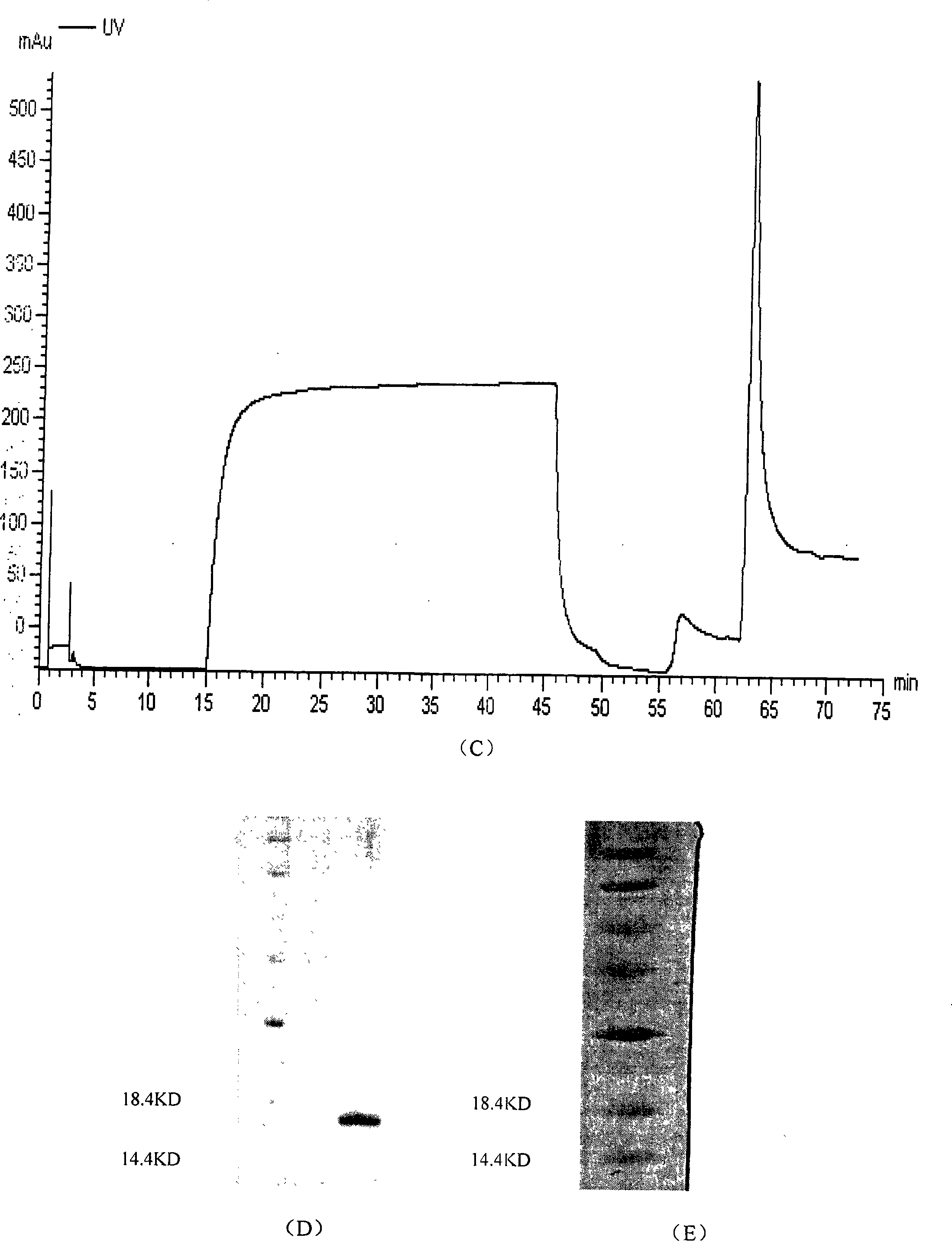
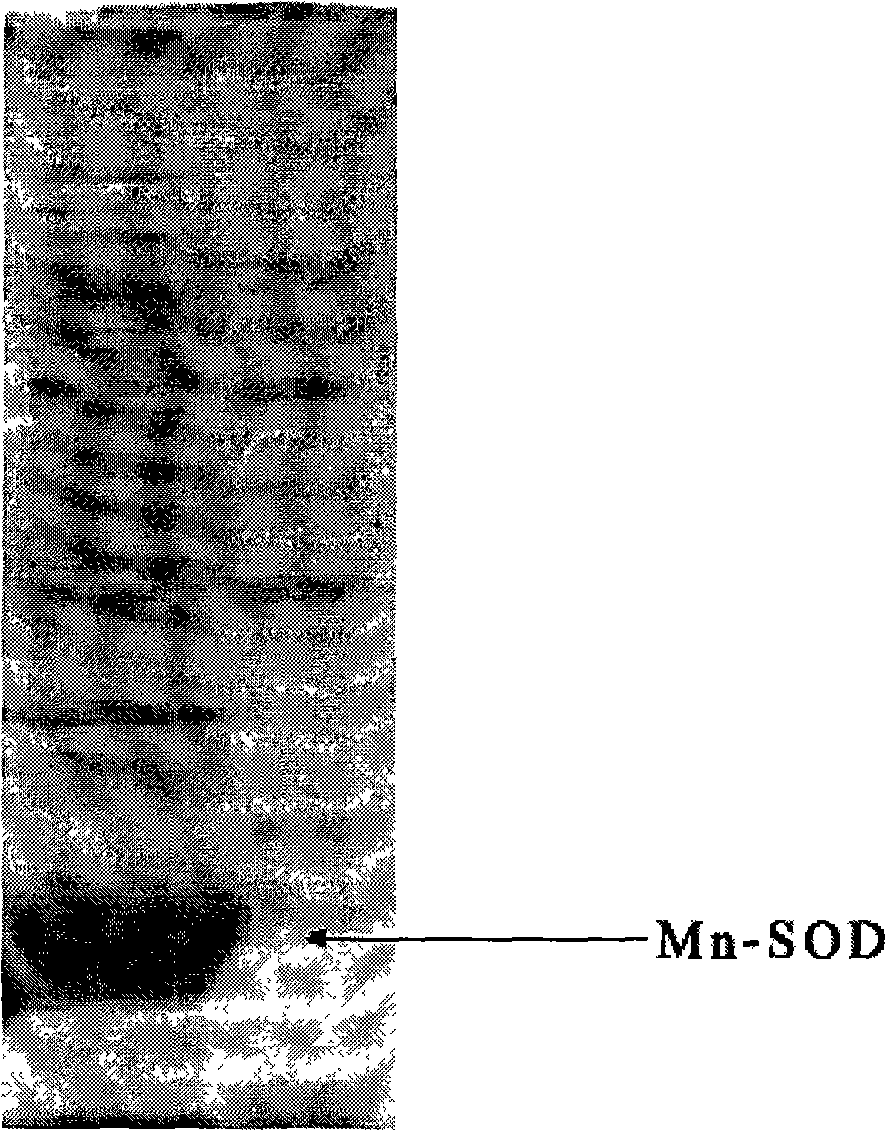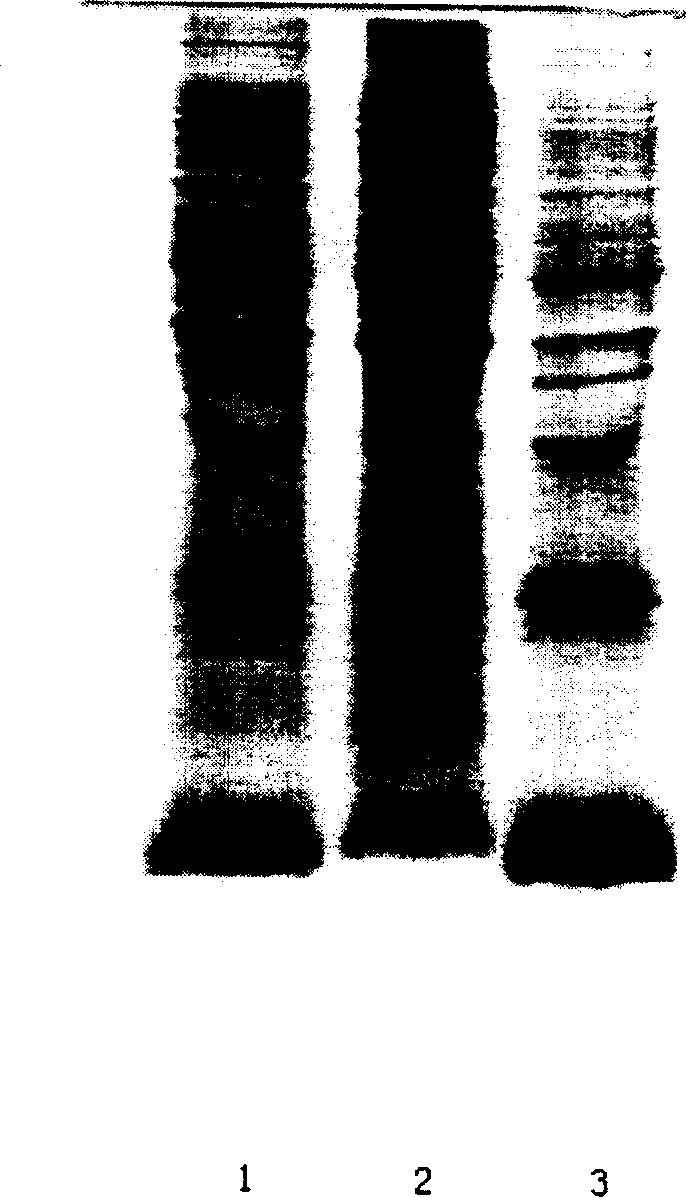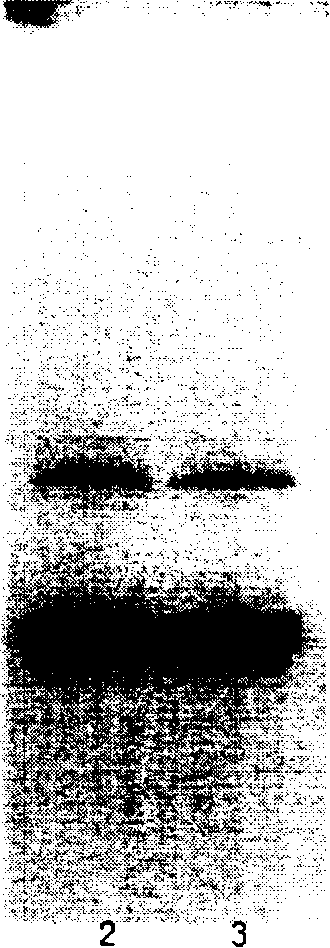Patents
Literature
960 results about "Inclusion bodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inclusion bodies , sometimes called elementary bodies, are nuclear or cytoplasmic aggregates of stable substances, usually proteins. They typically represent sites of viral multiplication in a bacterium or a eukaryotic cell and usually consist of viral capsid proteins. Inclusion bodies can also be hallmarks of genetic diseases, as in the case of neuronal inclusion bodies in disorders like frontotemporal dementia and Parkinson's disease.
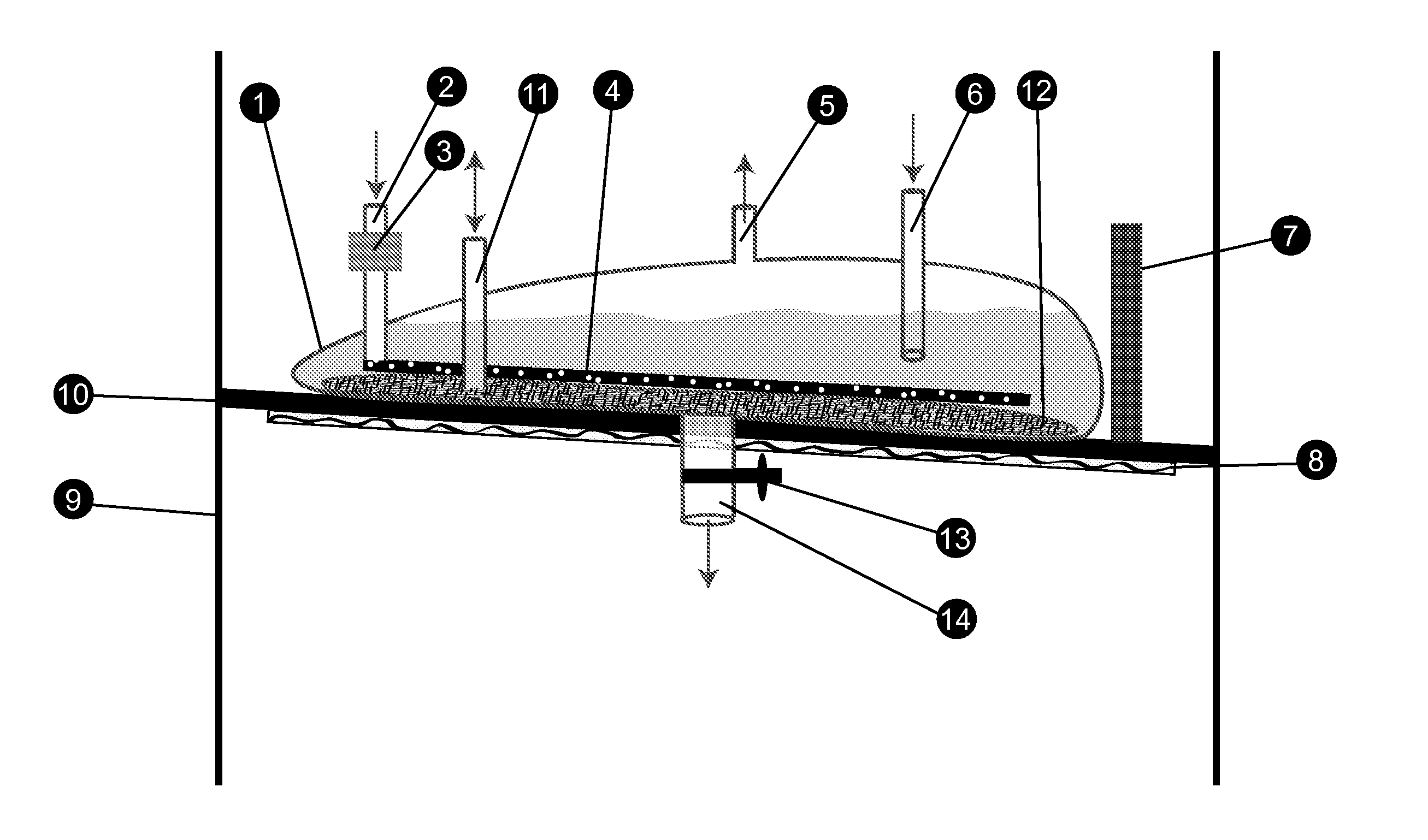
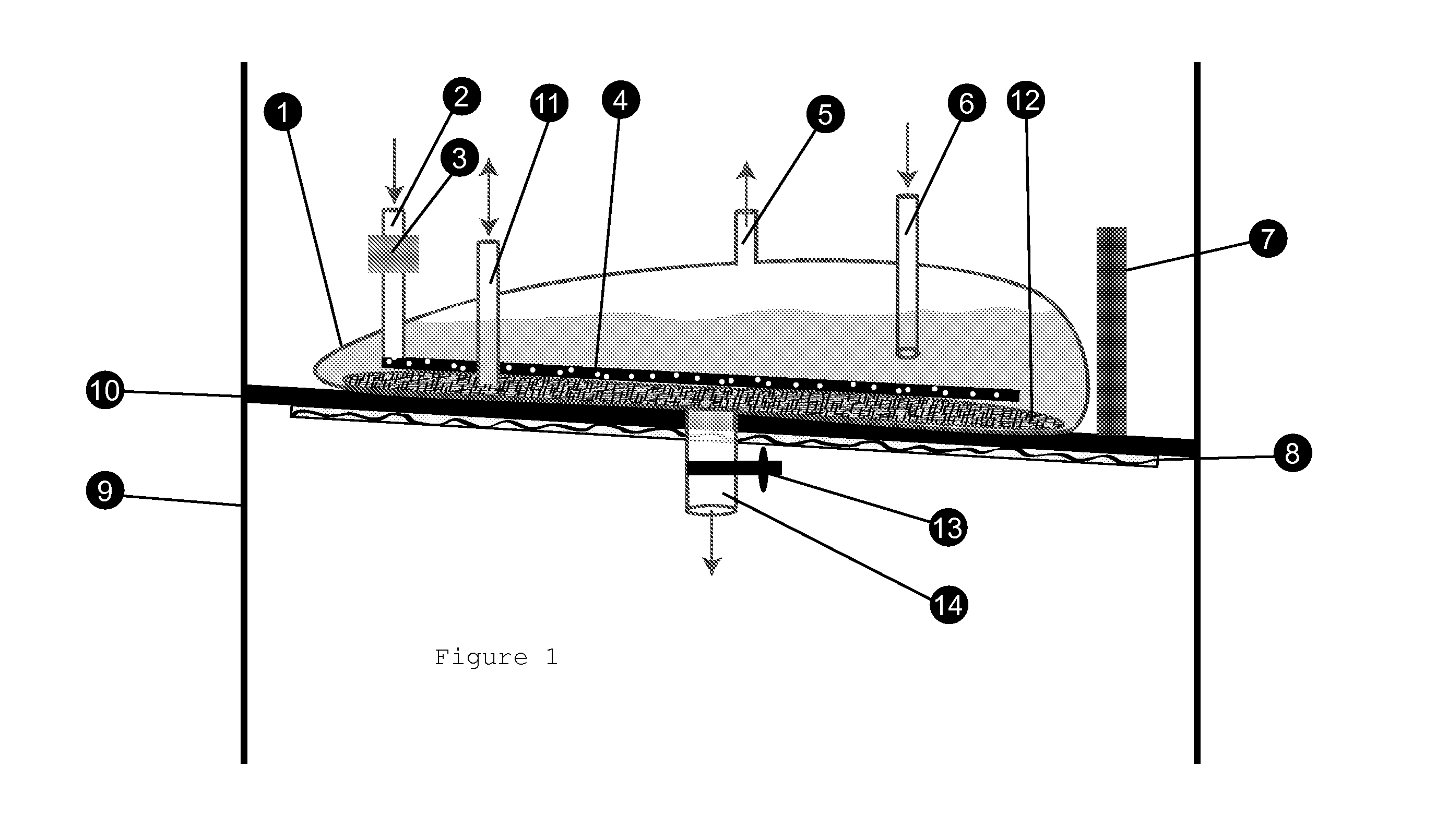
Separative Bioreactor
InactiveUS20110198286A1Improve abilitiesLow costSolvent extractionLighting and heating apparatusInclusion bodiesPerfusion
A bioreactor that combines the steps of recombinant expression and separation of a biological product by binding the secreted biological product with a resin, discarding the nutrient medium and eluting the biological product as a concentrated solution, eliminating the steps of sterile filtration and volume reduction. The method also allows loading of resin for column-purification, eliminating all steps of perfusion process and maintaining a sink condition of a toxic product in nutrient medium to optimize productivity of host cells. The instant invention also allows harvesting of solubilized inclusion bodies after the cells have been lysed and refolding of proteins inside the bioreactor.
Owner:NIAZI SARFARAZ K
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
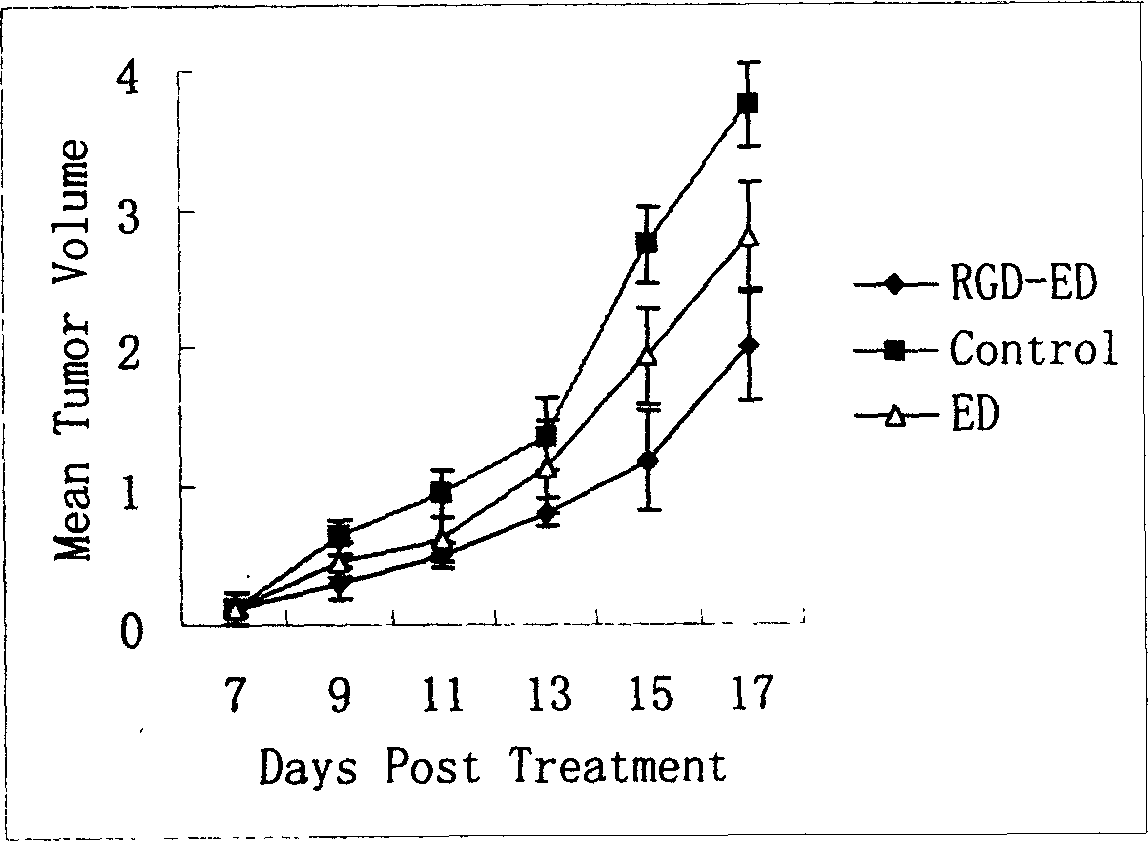
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
Methods and DNA constructs for high yield production of polypeptides
ActiveUS20050221444A1Improve usabilityImprove purification effectPeptide/protein ingredientsTissue cultureInclusion bodiesDNA construct
Owner:MEDTRONIC INC
Method for preparing recombinant human insulin and analogs of recombinant human insulin
InactiveCN101519446AHigh expressionBacteriaMicroorganism based processesInsulin activityEscherichia coli
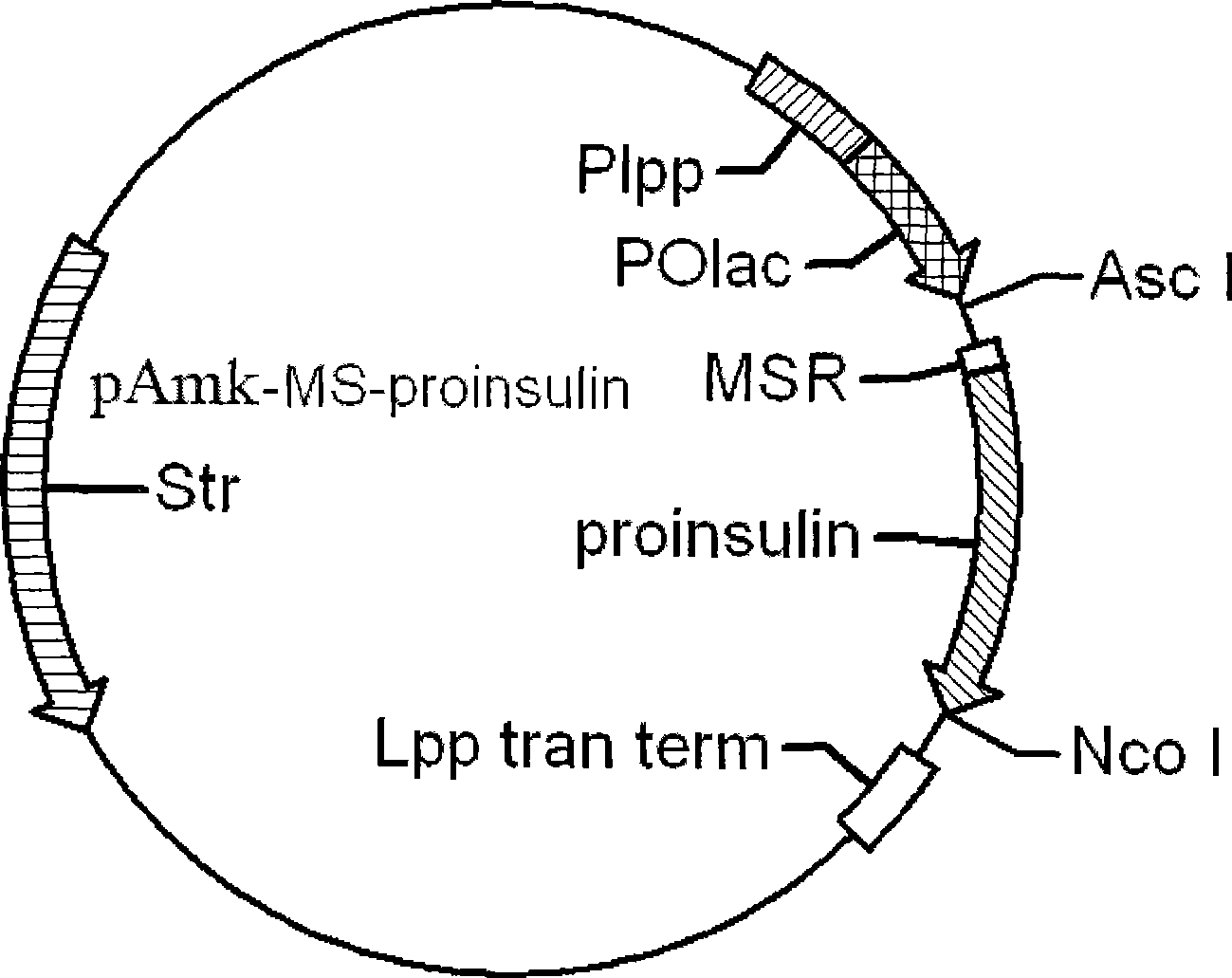
The invention provides a molecule (Preproinsulin) of human proinsulin with a novel N-terminal expressed peptide sequence or analogs of the human proinsulin, a method for producing human insulin by using the molecule, and processes for building related expression vectors and engineering cells and expressing and purifying human proinsulin. The DNA sequence of the human proinsulin coded by the N-terminal expressed peptide sequence or the analogs of the human proinsulin is first introduced into a prokaryotic expression vector and then transferred into an escherichia coli to express the molecule in form of an inclusion body. The invention has the advantages that: the product has high expression amount and is easy to purify; the preparation method avoids the use of CNBr; and the process for processing the recombinant insulin is simple.
Owner:AMTEK PHARMA
Peptide tags for the expression and purification of bioactive peptides
Owner:EI DU PONT DE NEMOURS & CO
Resonance driven changes in chain molecule structure
InactiveUS6060293AEfficient inductionPeptide preparation methodsElectrical/wave energy microorganism treatmentChemical industryDisease
PCT No. PCT / DK96 / 00158 Sec. 371 Date Nov. 26, 1997 Sec. 102(e) Date Nov. 26, 1997 PCT Filed Apr. 1, 1996 PCT Pub. No. WO96 / 30394 PCT Pub. Date Oct. 3, 1996The invention relates to the technical application of electromagnetic radiation such as microwaves and radiowaves and application of ultra sound to chain molecules. In particular, the present invention relates to the utilization of topological excitations such as wring, twist and torsional modes, e.g., for generating structure, such as in folding, refolding or renaturation, and denaturation or unfolding of peptides, polypeptides, proteins, and enzymes; for generating changes in molecular affinity; for stimulating drug receptor interactions; and for changing molecular communication, is described. The technique is based on a new understanding of the underlying physical phenomenon and can also be applied to other chain molecules and biologically active biomolecules and tailored polymers such as glucoproteins, antibodies, genomic chain molecules such as DNA and RNA as well as PNA, carbohydrates, and synthetic and natural organic polymers. The invention is especially applicable for solving problems related to inclusion bodies and aggregation when using recombinant DNA and protein engineering techniques. Furthermore, the invention can be utilized in therapeutic treatment and in development and production of pharmaceuticals. The area of applicability ranges from biotechnological industry, food industry, drug industry, pharmacological industry, chemical industry, and concerns, e.g., the treatment of conditions and diseases related to influenza, hepatitis, polio, malaria, borrelia, diabetes, Alzheimer's disease, Creutzfeldt Jakob disease, other prion related diseases, multiple sclerosis, cataract, heart diseases, cancer, and aging.
Owner:PROKYON
Method for obtaining single chain antibodies to human interferon alpha2b
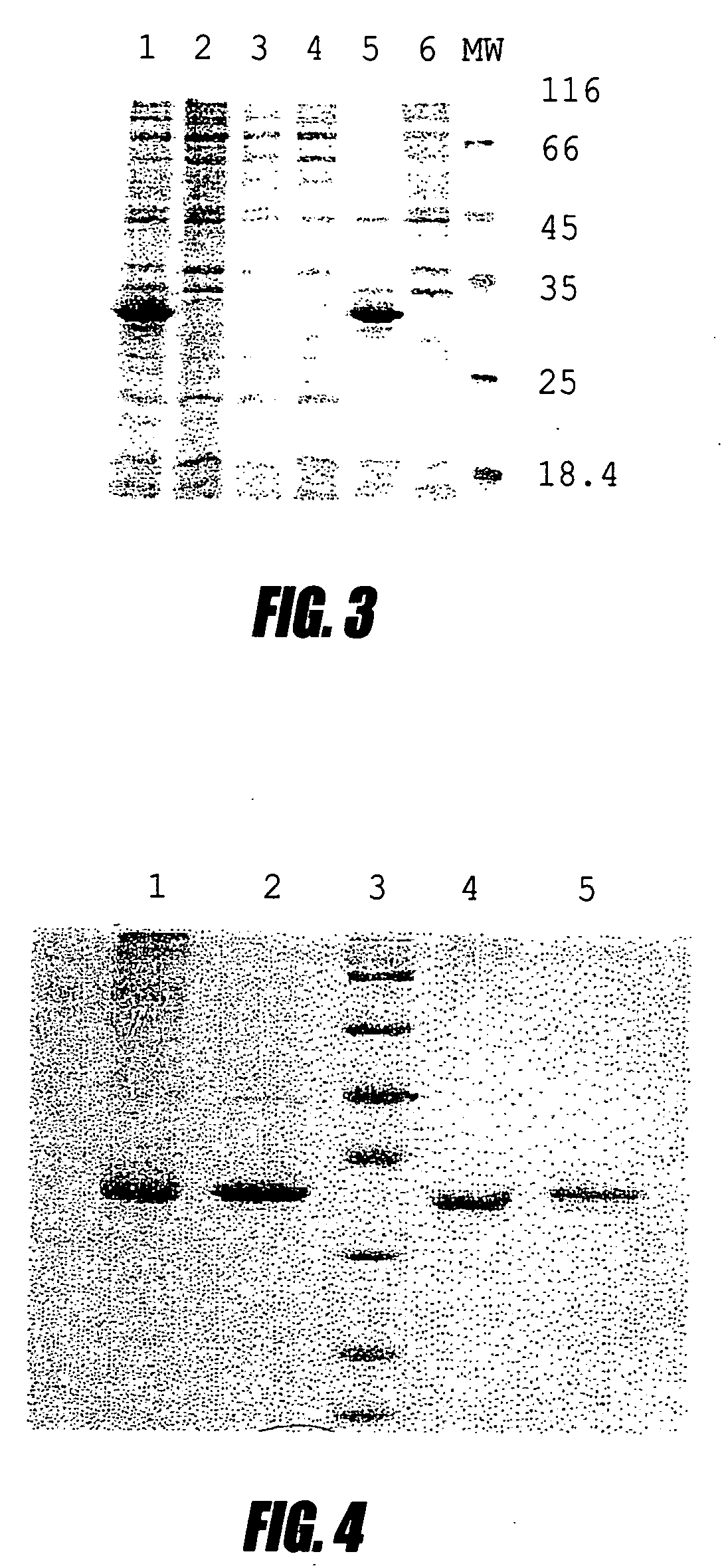
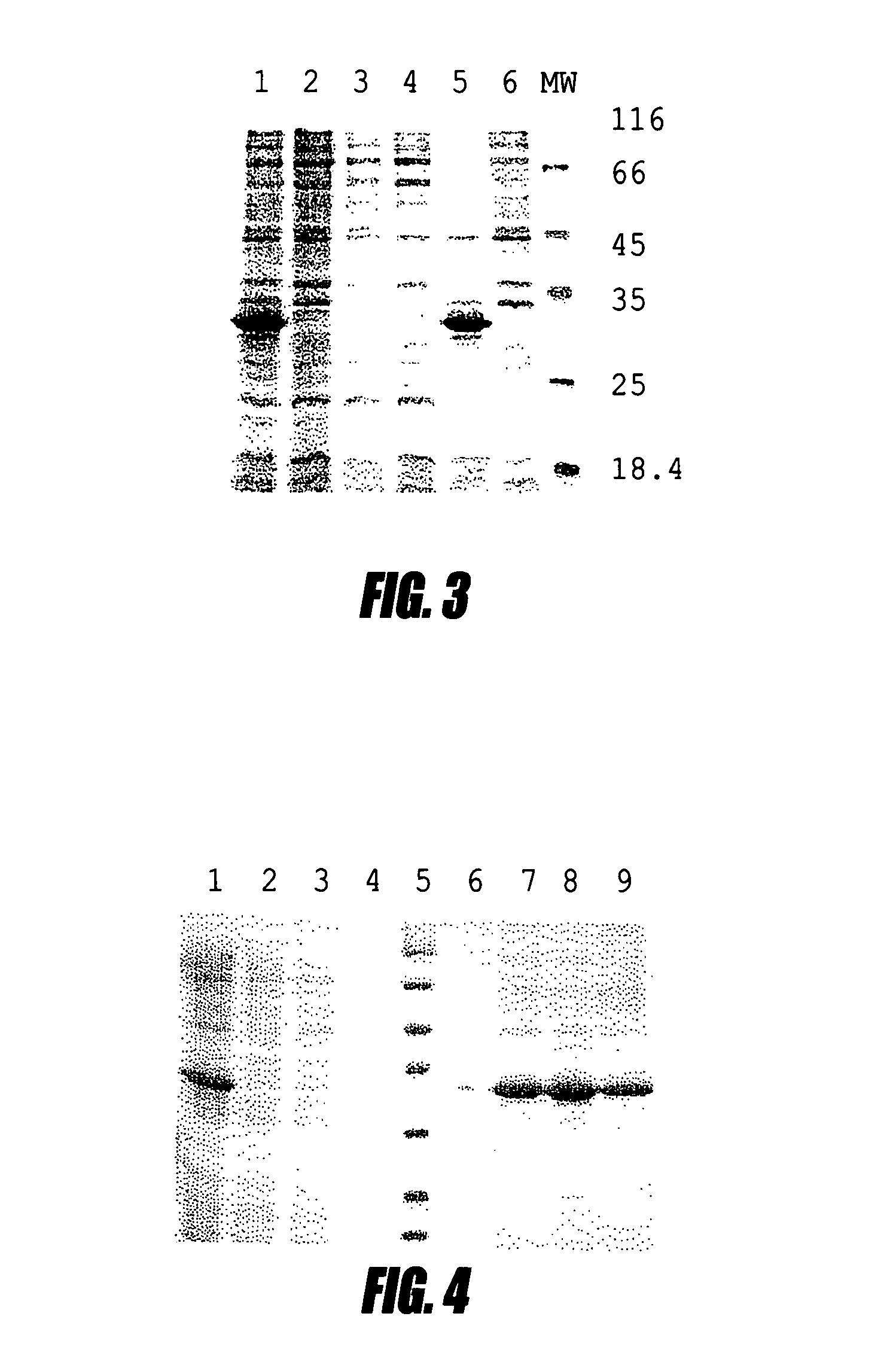
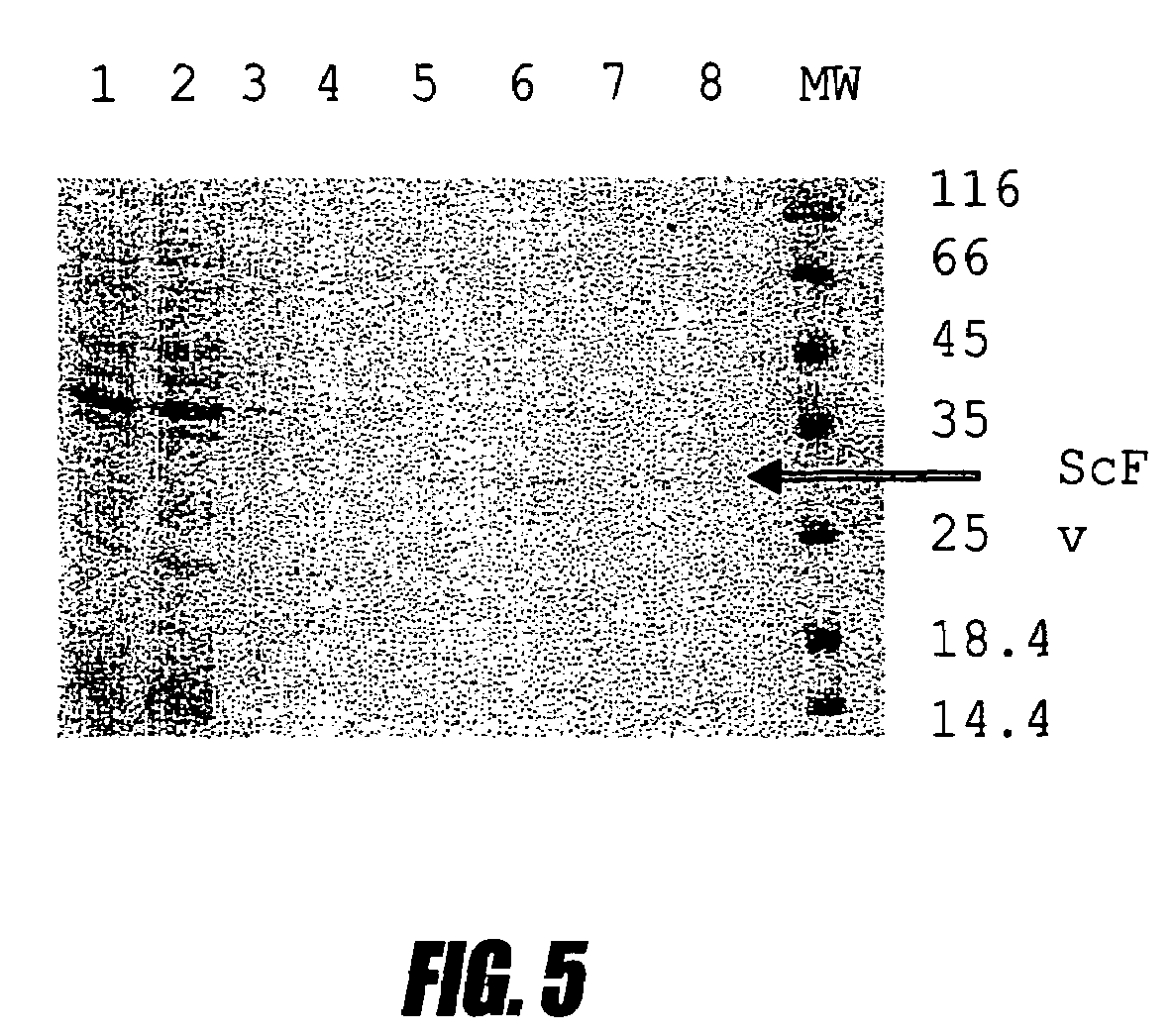
A bacterial high-expression system which is applicable for simultaneous screening of large numbers of recombinant clones from combinatorial antibody libraries is disclosed. The method pertains to screening of single chain antibodies from libraries expressed in the periplasm of E. coli by secretion. By this approach, approximately 104 clones can be screened in a single round. After screening, the clones, which express the recombinant antibodies to the desired antigen, can be directly used for production of large quantities of antibodies from microorganism culture. The system is especially attractive for fast screening of antibody libraries from a hybridoma source. A refolding method for the large-scale production of biologically active scFv-6 his proteins from bacterial inclusion bodies is also disclosed.
Owner:NEW TECH HLDG
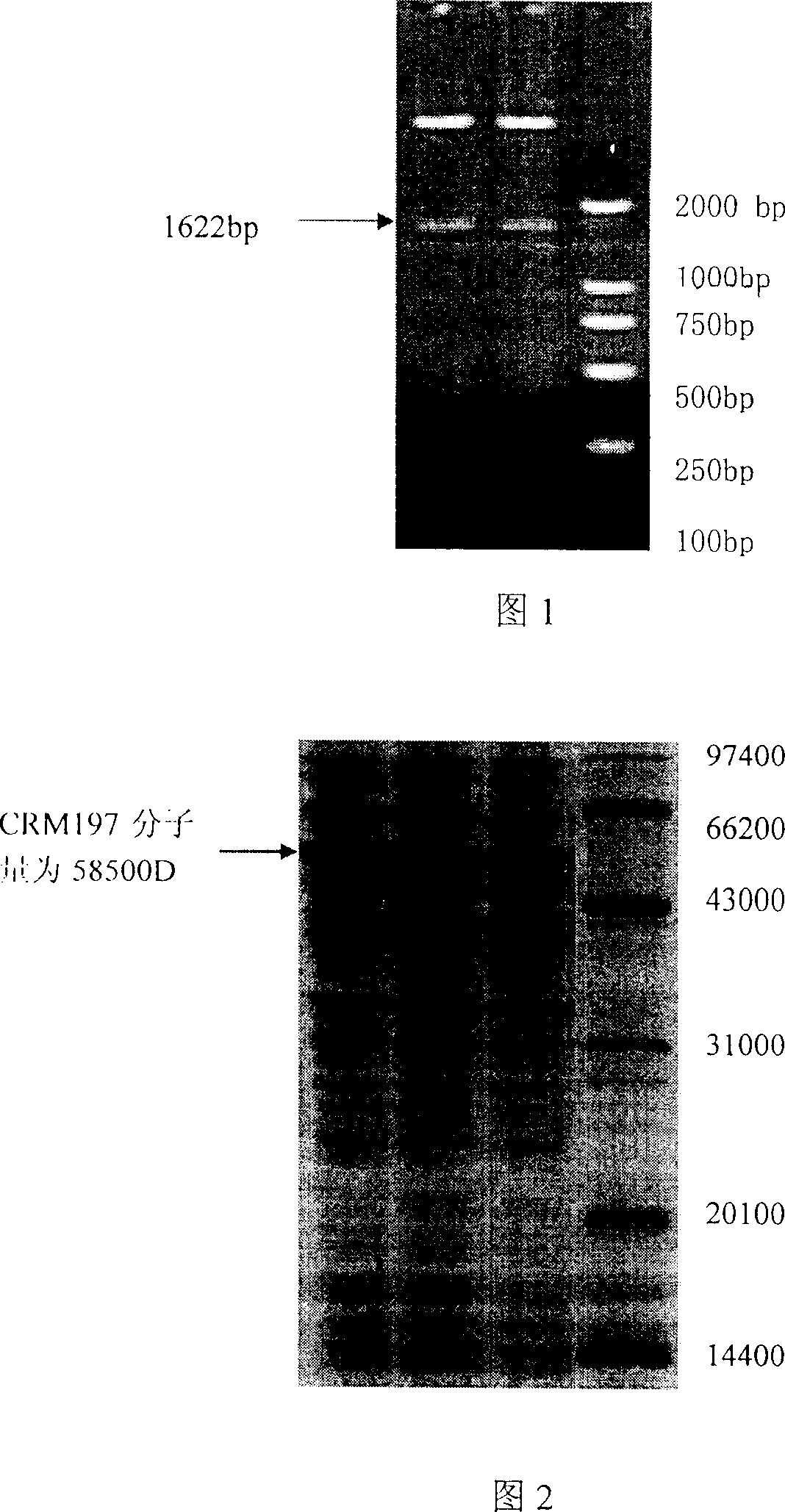
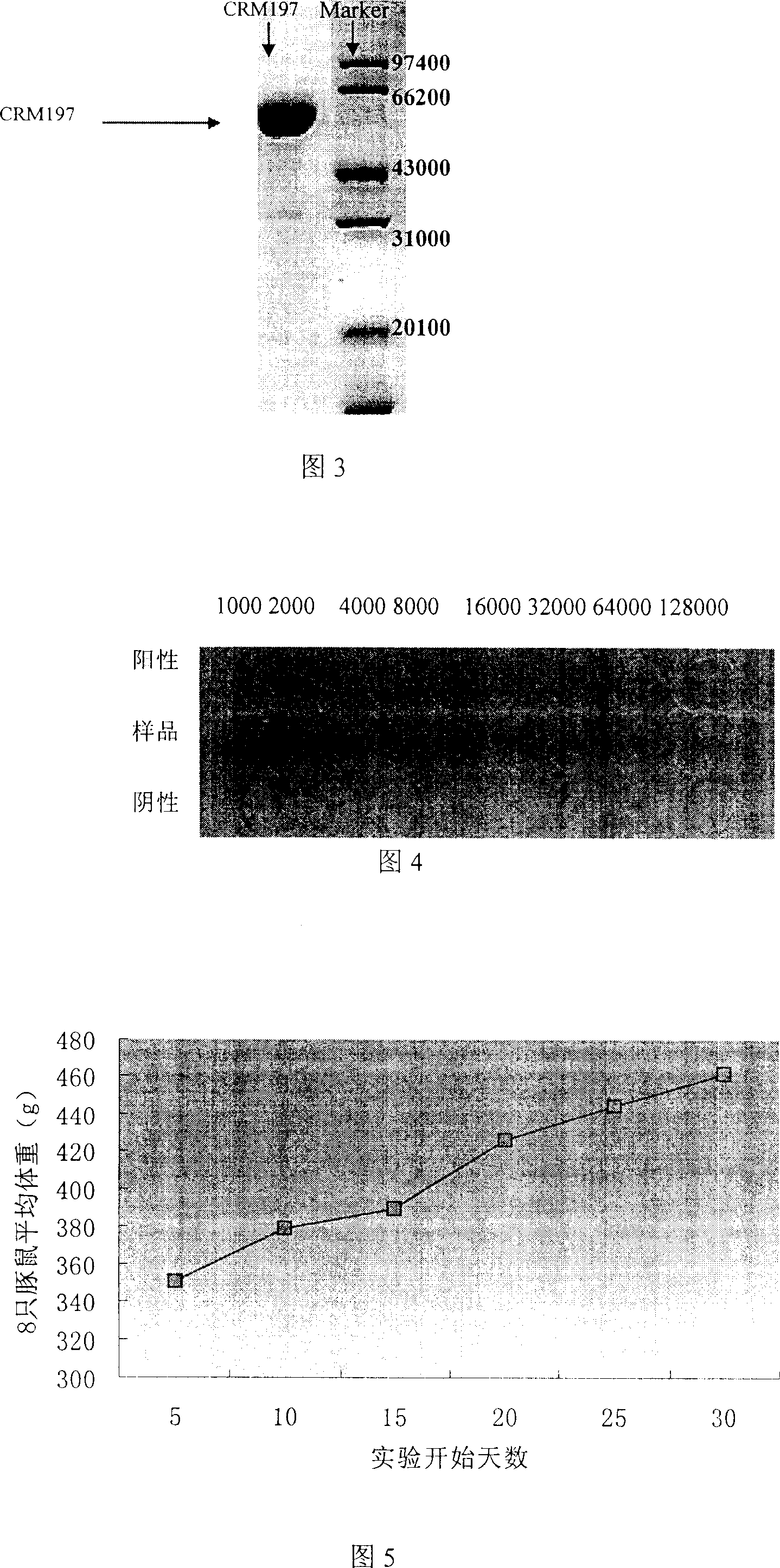
Diphtheria toxin muton CRM197 and its preparation process
The present invention is diphtheria toxin mutant CRM197 and its preparation process, and belongs to the field of immune protein carrier technology. The diphtheria toxin mutant CRM197 is expressed in colibacillus in the form of inclusion body, and the target product has sequence length of 536 amino acids, the immunogenicity the same as that of diphtheria toxin and no toxicity similar to that of diphtheria toxin. The present invention clones gene coding CRM197, constructs recombinant expression plasmid expressing CRM197 and transforms to colibacillus host for expression in the form of inclusion body. The present invention has target protein accounting for over 24 % of total protein content, high CRM197 purity over 95 % and high target protein recovering rate. The preparation process is simple, low in cost and suitable for industrial production.
Owner:QILU PHARMA HAINAN
Soluble human program death protein-1-lgV and preparation method thereof
InactiveCN101215329ACell receptors/surface-antigens/surface-determinantsFermentationEscherichia coliProtein target
The invention discloses soluble programmed dead protein-1-IgV and a preparation process. The protein sequence of is showed as followed: PPTFFPAL LVVTEGDNAT FTCSFSNTSE SFVLNWYRMS PSNQTDKLAA FPEDRSQPGQ DSRFRVTQLP NGRDFHMSVV RARRNDSGTY LCGAISLAPK AQIKESLRAE LRVTERRAEV PTAHPSP. The invention obtains gene of Hpd-1-IgV through applying artificial synthesis according to the structure of human PD-1, target gene is cloned into pQE-30 pronucleus expression carrier to transform colibacillus and is expressed in colibacillus with high efficiency, bacterioclasis shows that target protein is existed in inclusion body form, and purified soluble programmed dead protein-1-IgV is finally obtained through inclusion body scouring, affinity chromatography, gel column chromatography and dialysis renaturation. The soluble programmed dead protein-1-IgV can respectively combine with mPD-L1 / Fc and hPD-L1 / Fc in extra-corporal shPD-1IgV and on cell level and molecular level, and has certain tumor killing effect in internal shPD-1IgV. Therefore, shPD-1IgV has excellent internal and external biological activity, provides experiment basis for curing tumor, establishes experiment foundation, and has potential application prospect.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
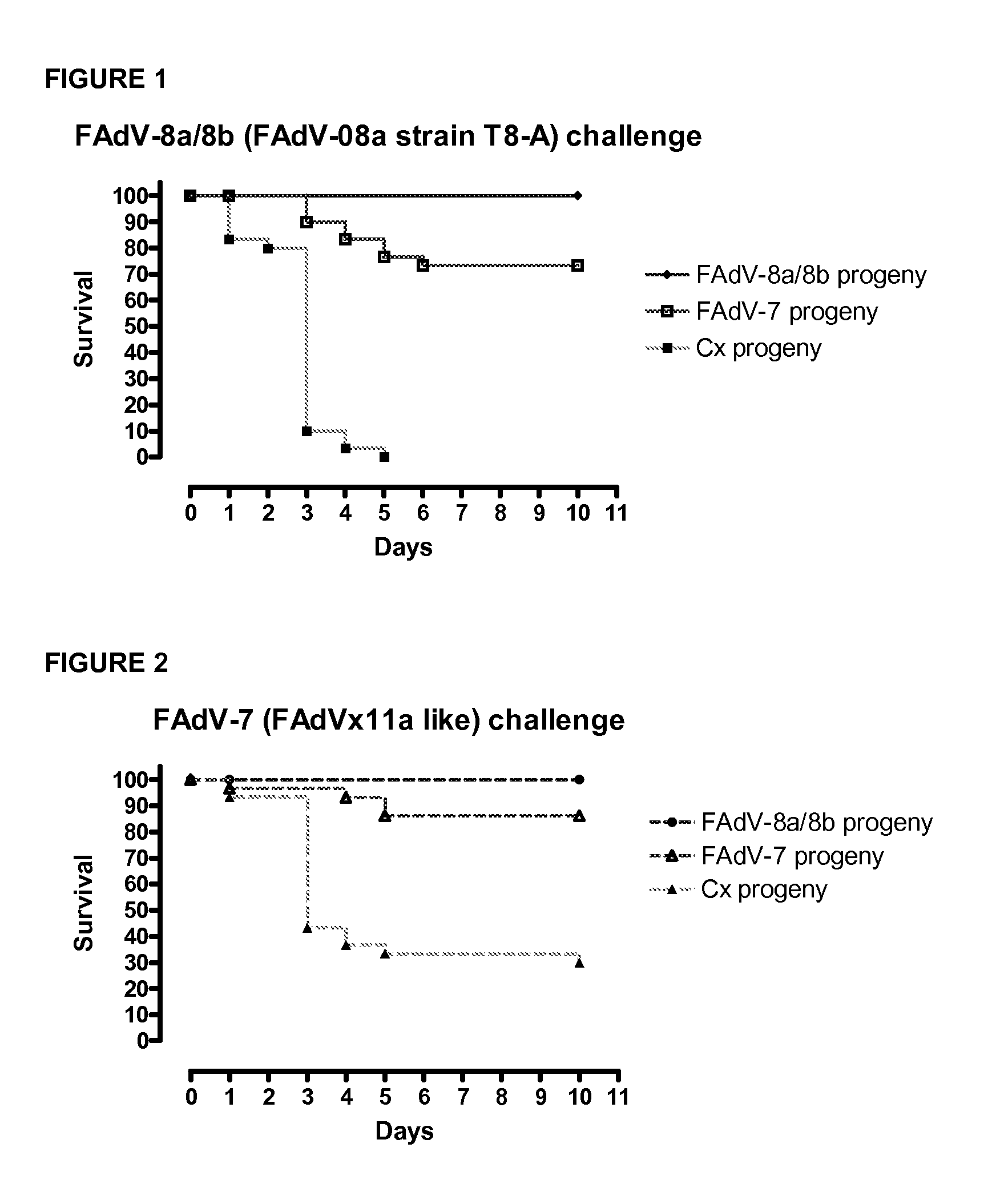
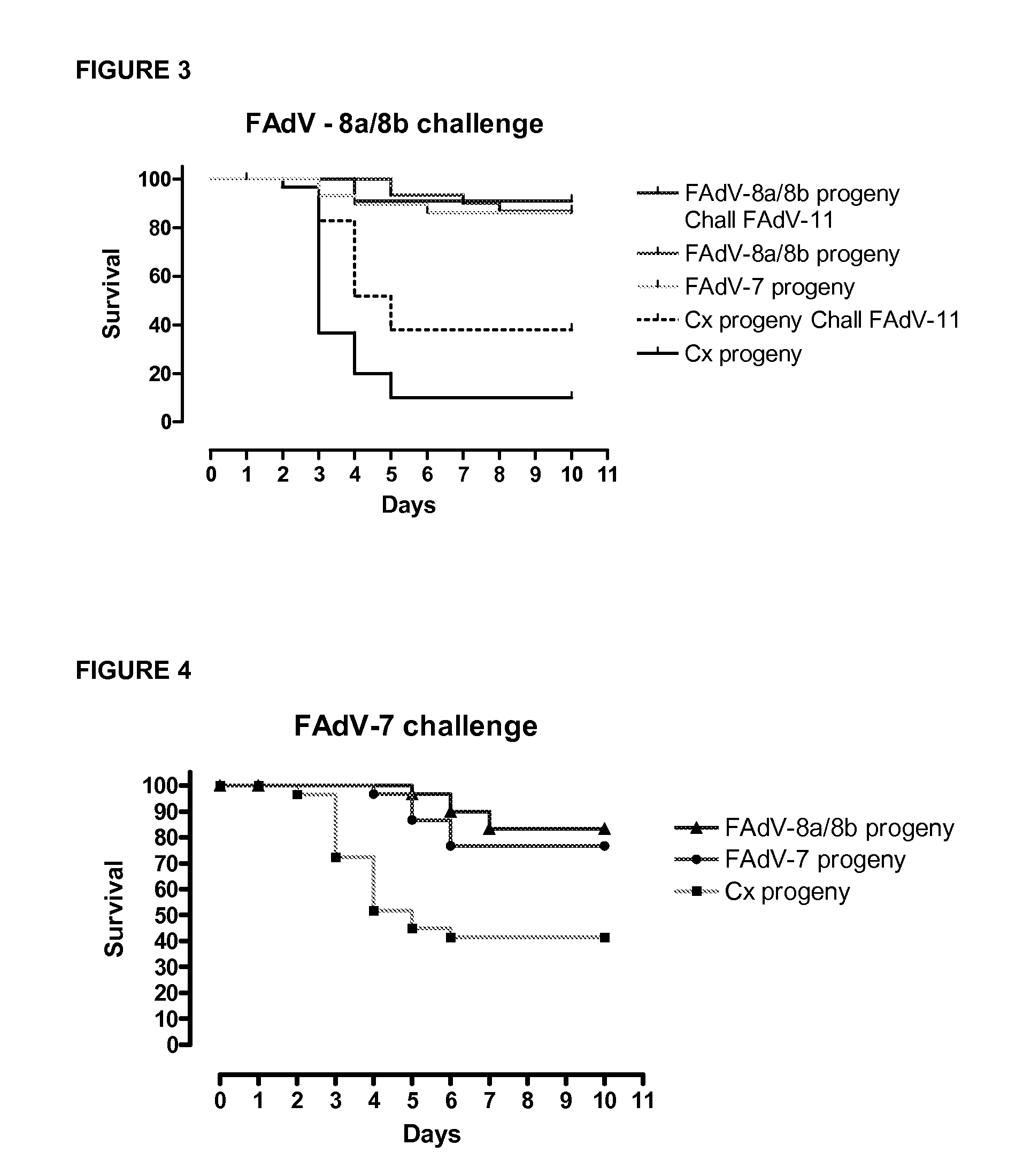
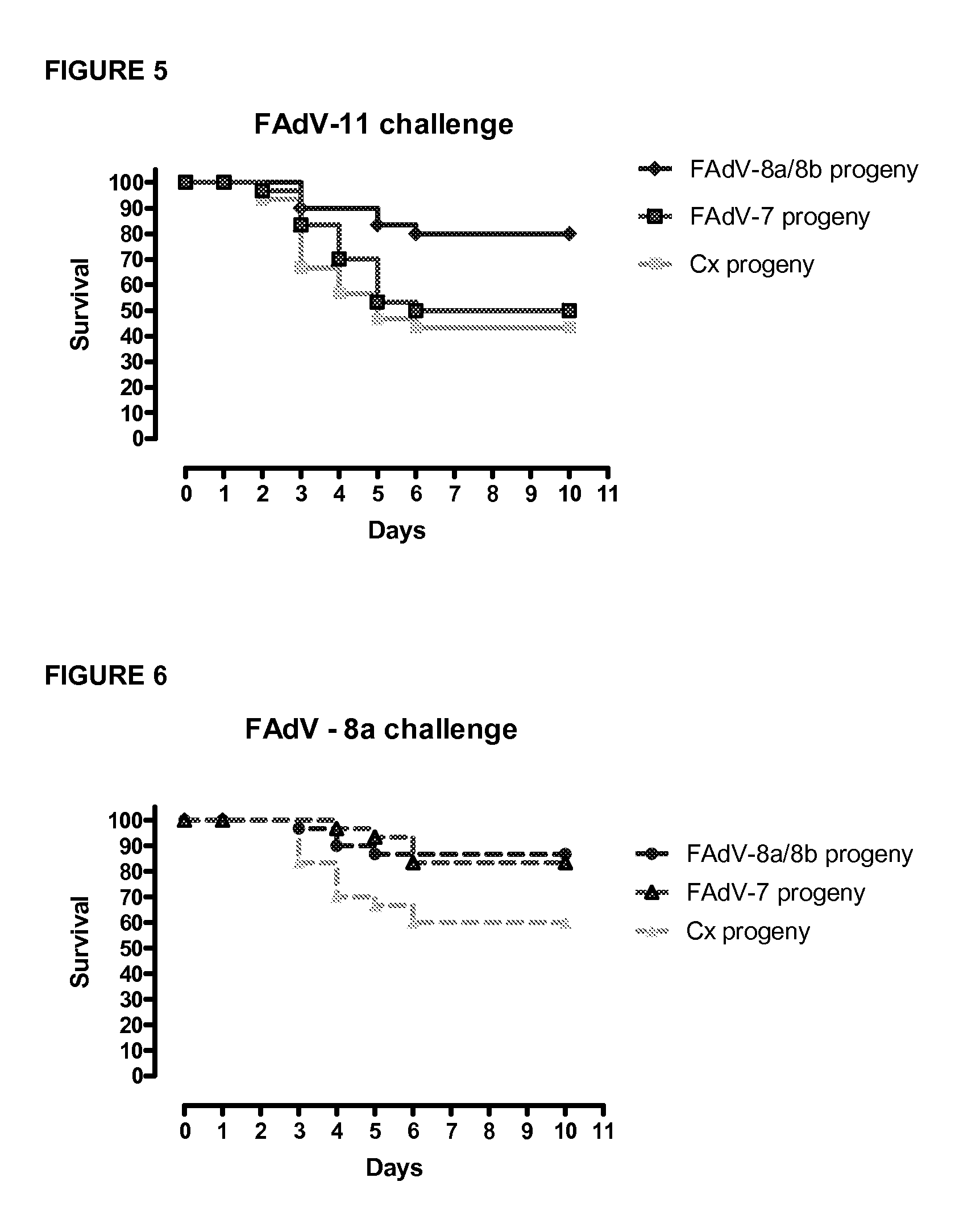
Vaccines for inclusion body hepatitis
A composition comprising an isolated fowl adenovirus (FAdV), wherein the FAdV is a strain selected from FAdV-2, FAdV-7, FAdv-8a, FAdV-8b, FAdV-8a / 8b or FAdV-11 serotype strains; and a suitable carrier and methods for inducing protective immunity in a subject and / or its progeny.
Owner:UNIVERSITY OF GUELPH +1
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof
ActiveCN106946995AHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
High-density fermentation and purification process for recombination high temperature-resistant hyperoxide dismutase
InactiveCN101275144AAvoid pollutionSimple purification processBacteriaMicroorganism based processesEscherichia coliDismutase
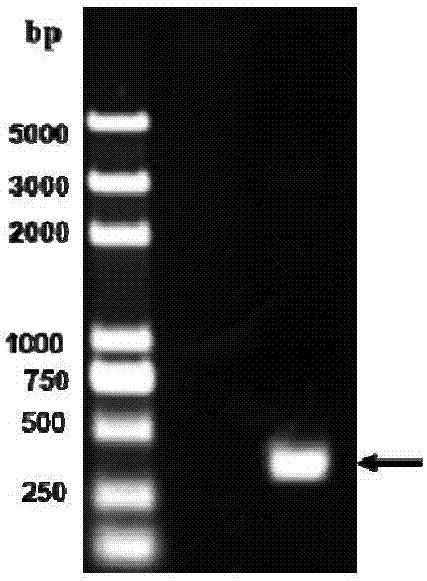
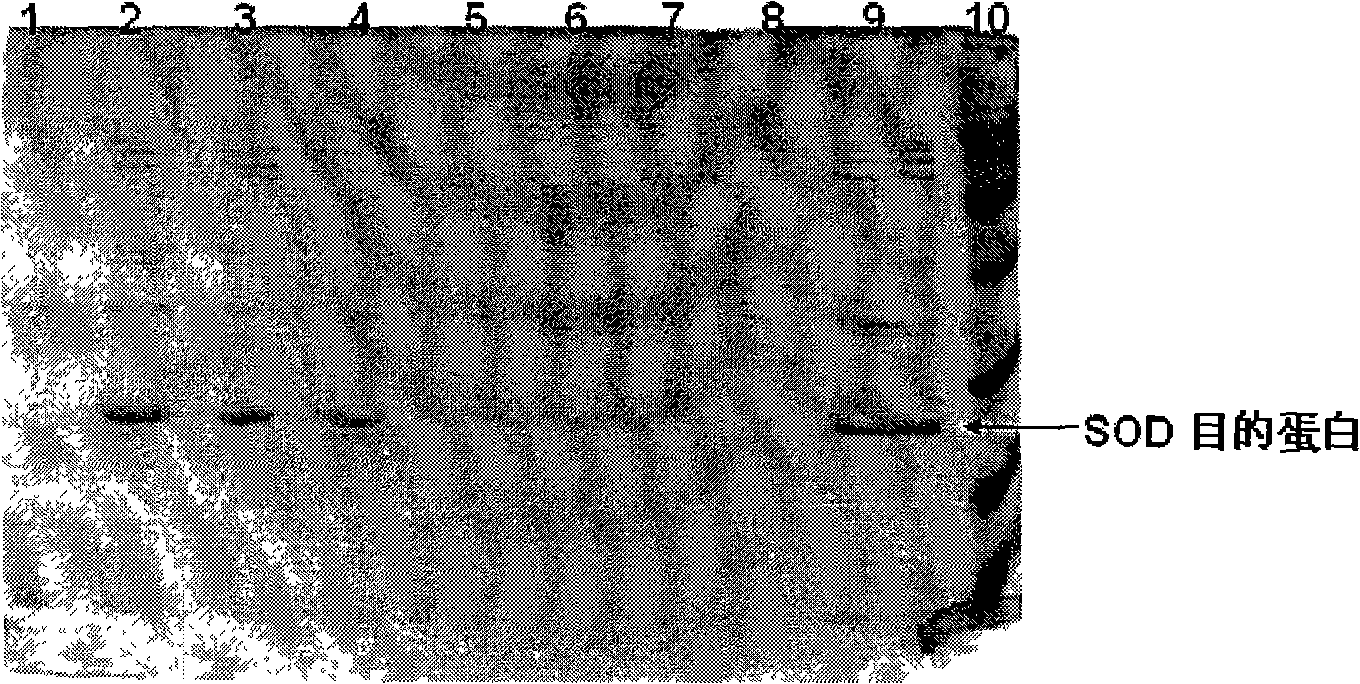
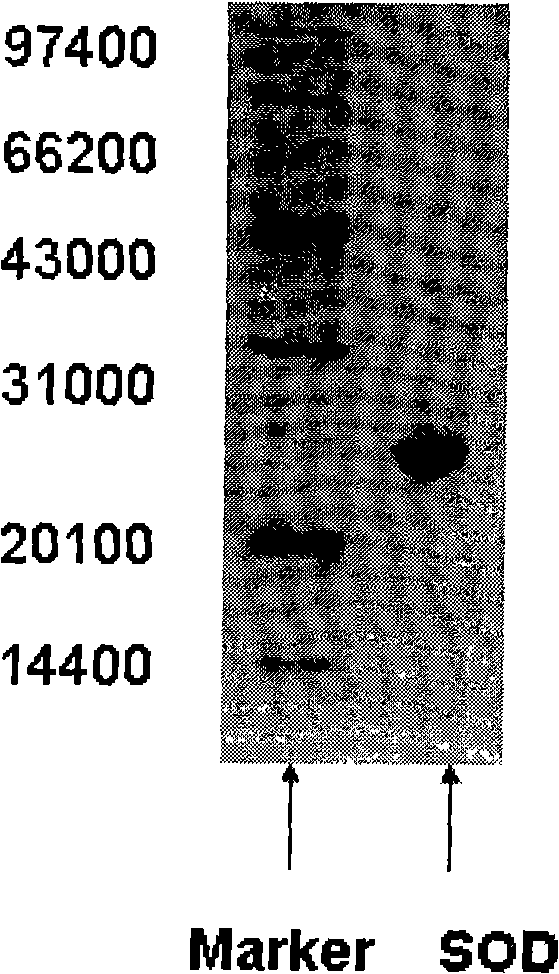
The present invention provides a high density fermentation and a purification process of a recombination high temperature resistance superoxide dismutase, the construction method of the invention includes: using gene coded for SOD in a thermophilic bacteria as a template, designing specific primer amplification target gene having restriction enzyme sites, after double digestion, connecting to plasmid vector pET28a after the same double digestion, constructing a recombinant plasmid, named for pSOD, transforming plasmid pSOD to competence escherichia coli BL21(DE3) by chemical transformation method, obtaining strain having high SOD yield after screening, completing the construction of SOD engineering bacteria; the fermentation process includes four steps of first order seed culture, secondary order feed culture, batch fermentation and induced expression, fermentation product SOD is finally obtained; the fermentation process realizes high level expression of SOD, the expression of the target protein is more than 60% of the bacterial protein total; SOD has excellent thermal stability and heat resistance, the expression product accounts for more than 60% of the whole proteins, and fully soluble protein, avoiding any trouble in the course of inclusion body renaturation; the purification process is simple, having high yield, lower cost, the final product SOD has high purification, high activity and strength stability.
Owner:YANGTZE DELTA REGION INST OF TSINGHUA UNIV ZHEJIANG +1
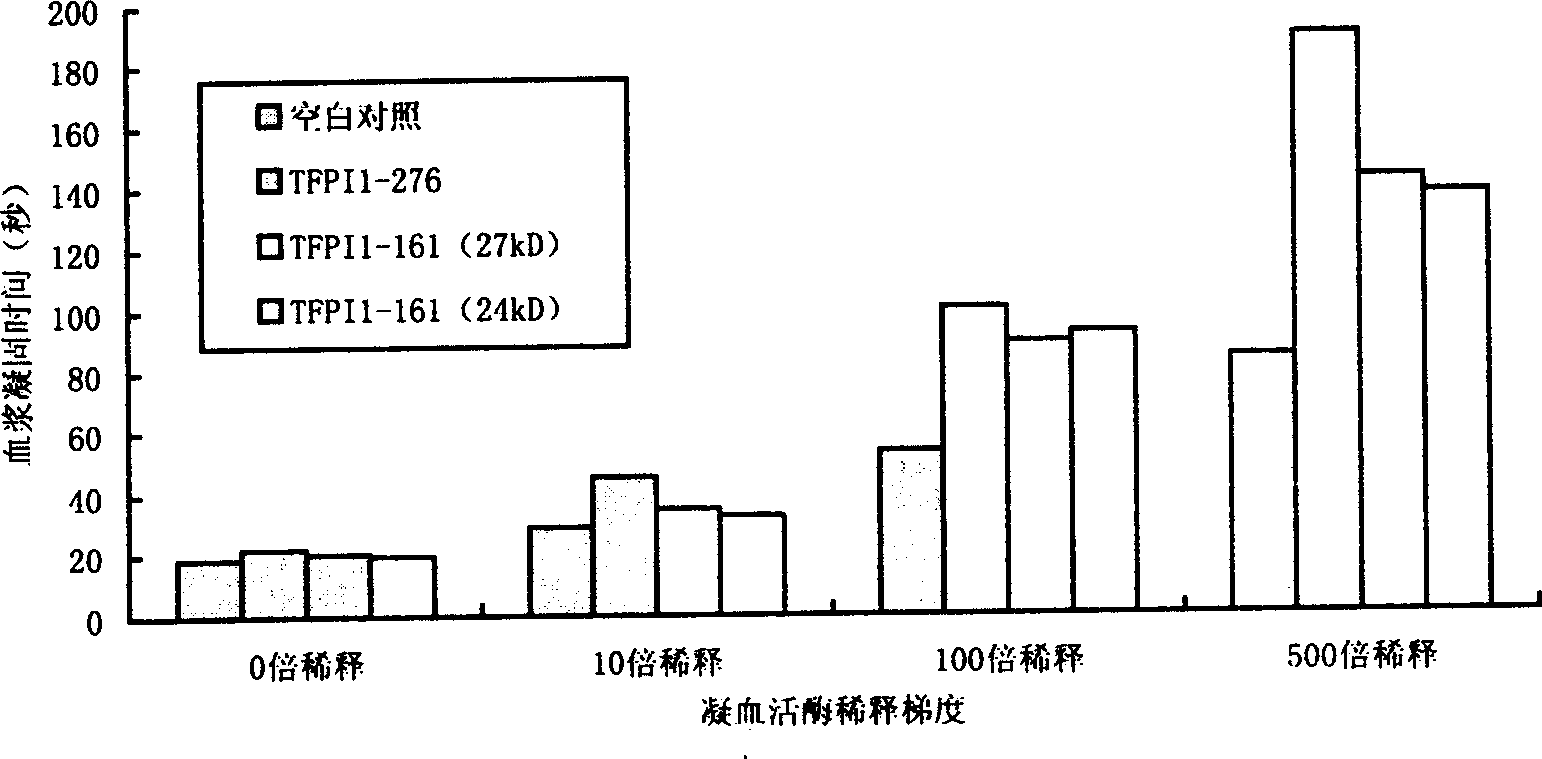
Long-acting reconbinant tissue factor channel inhibitor and preparing method thereof
InactiveCN1528894AExtended half-lifeHighly effective anticoagulantFermentationVector-based foreign material introductionBiotechnologyInclusion bodies
The invention belongs to biology technology field, which concretely refers to controlled release tissue factor path inhibitor (LTFPI) and the manufacturing method, and the application. The invention analyzes and experiments the biology information science and structure molecular biology science of tissue factor path inhibitor (TFPI) and its acceptor low density lipoprotein acceptor correspondent protein (LRP), it ascertains the part where the TFPI carboxy end combines with LRP and is eliminated. It designs TFPI carboxy end mutant, which is recombined with primary nucleus or eukarya expressing carrier after constructing LTFPI gene through PCR location, it converts bacillus coli or bici yeast, sifts the high expression project fungus. The primary project fungus are yeasted and expanded, crushed, then they are centrifugated and collects the inclusion body, purifies the LTFPI through molecular sift and ion interchanging two-step method; the eukarya project fungus are carried on with two-step purifying directly. The half-life are prolonged, it has good pour-depressant function.
Owner:海菲尔(辽宁)生物科技有限公司
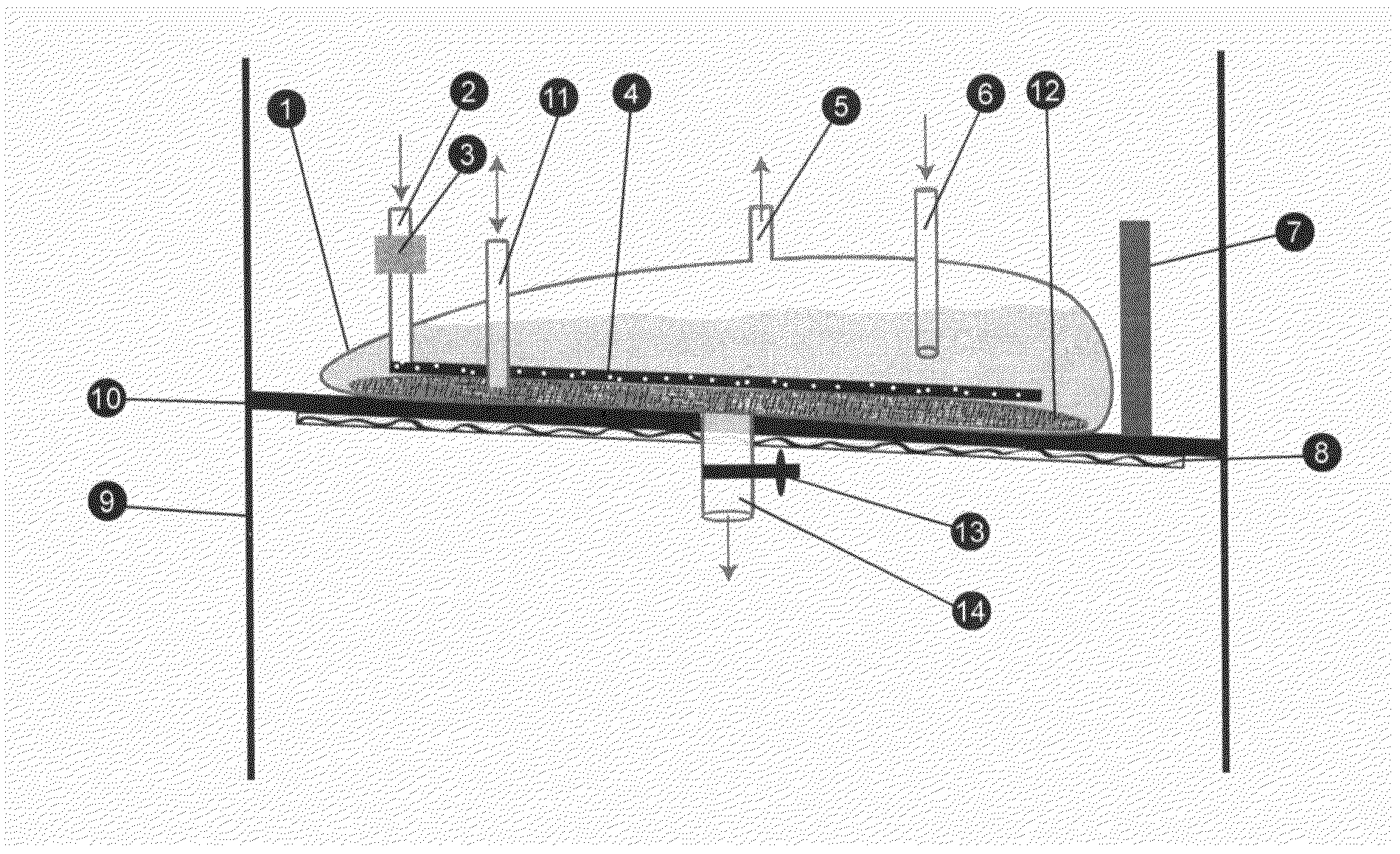
Separative harvesting device
InactiveUS20130143313A1Low costReduce manufacturing costBioreactor/fermenter combinationsBiological substance pretreatmentsInclusion bodiesBiochemistry
A harvesting device for capturing a biological product directly by binding the secreted biological product with a resin, discarding the nutrient medium and eluting the biological product as a concentrated solution, eliminating the steps of sterile filtration and volume reduction, thus allowing one to combine the steps of recombinant expression and separation of a biological product. The method allows loading of resin for column-purification, eliminating all steps of perfusion process and maintaining a sink condition of a toxic product in nutrient medium to optimize productivity of host cells. The instant invention also allows harvesting of solubilized inclusion bodies after the cells have been lysed and refolding of proteins inside the bioreactor.
Owner:THERAPEUTIC PROTEINS INT
Cystatin-based peptide tags for the expression and purification of bioactive peptides
InactiveUS20080096245A1High expressionImprove purification effectFungiBacteriaInclusion bodiesBioactive peptide
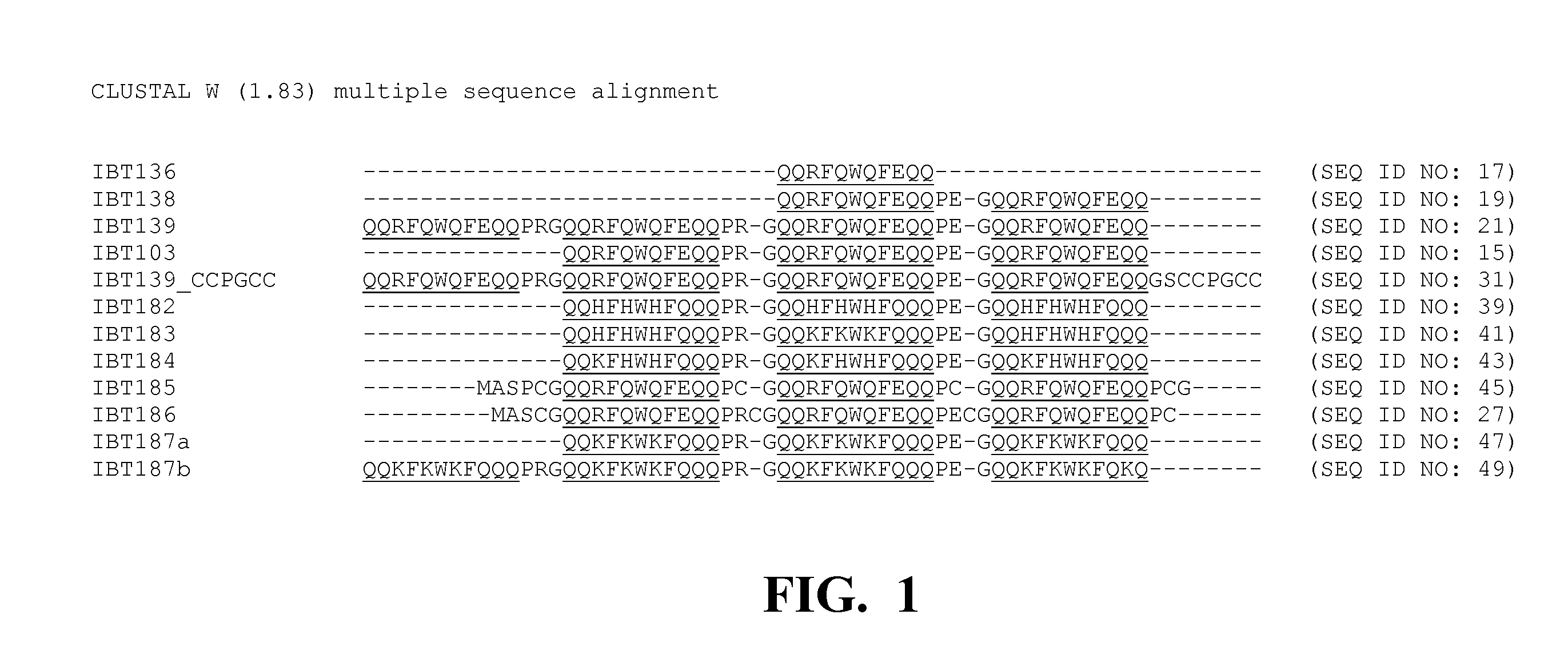
Cystatin-based peptide tags, referred to here as inclusion body tags (IBTs), are disclosed useful for the generation of insoluble fusion peptides. The fusion peptides comprise at least one inclusion body tag operably linked to a peptide of interest. Expression of the fusion peptide in a host cell results in a product that is insoluble and contained within inclusion bodies in the cell and / or cell lysate. The inclusion bodies may then be purified and the protein of interest may be isolated after cleavage from the inclusion body tag.
Owner:EI DU PONT DE NEMOURS & CO
Solubility tags for the expression and purification of bioactive peptides
InactiveUS7678883B2Improve purification effectHigh expressionBacteriaPeptide/protein ingredientsSolubilityInclusion bodies
Peptide tags, referred to here as inclusion body tags, are disclosed useful for the generation of insoluble fusion peptides. The fusion peptides comprise at least one inclusion body tag operably linked to a peptide of interest. Expression of the fusion peptide in a host cell results in a product that is insoluble and contained within inclusion bodies in the cell and / or cell lysate. The inclusion bodies may then be purified and the protein of interest may be isolated after cleavage from the inclusion body tag.
Owner:DUPONT US HLDG LLC
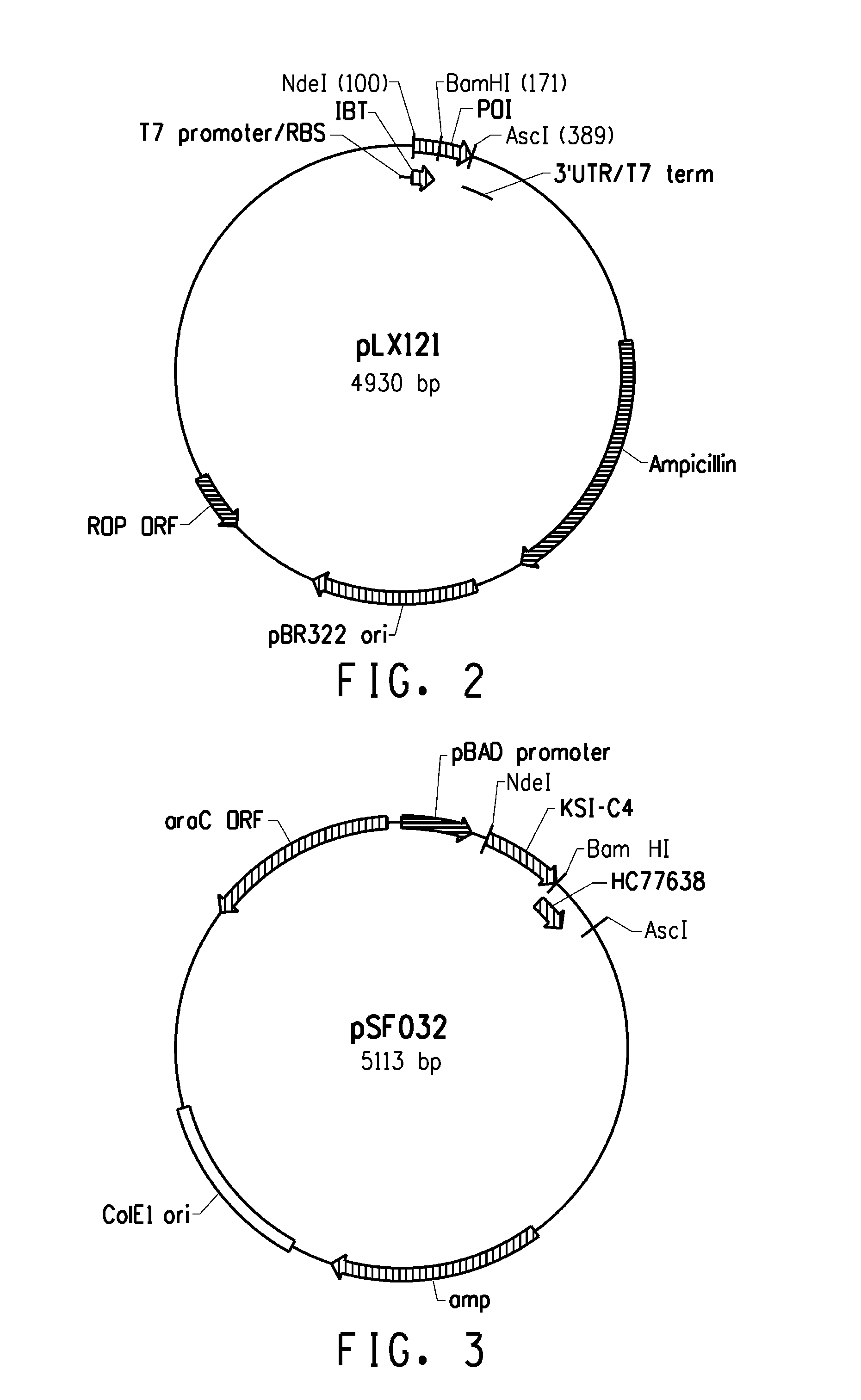
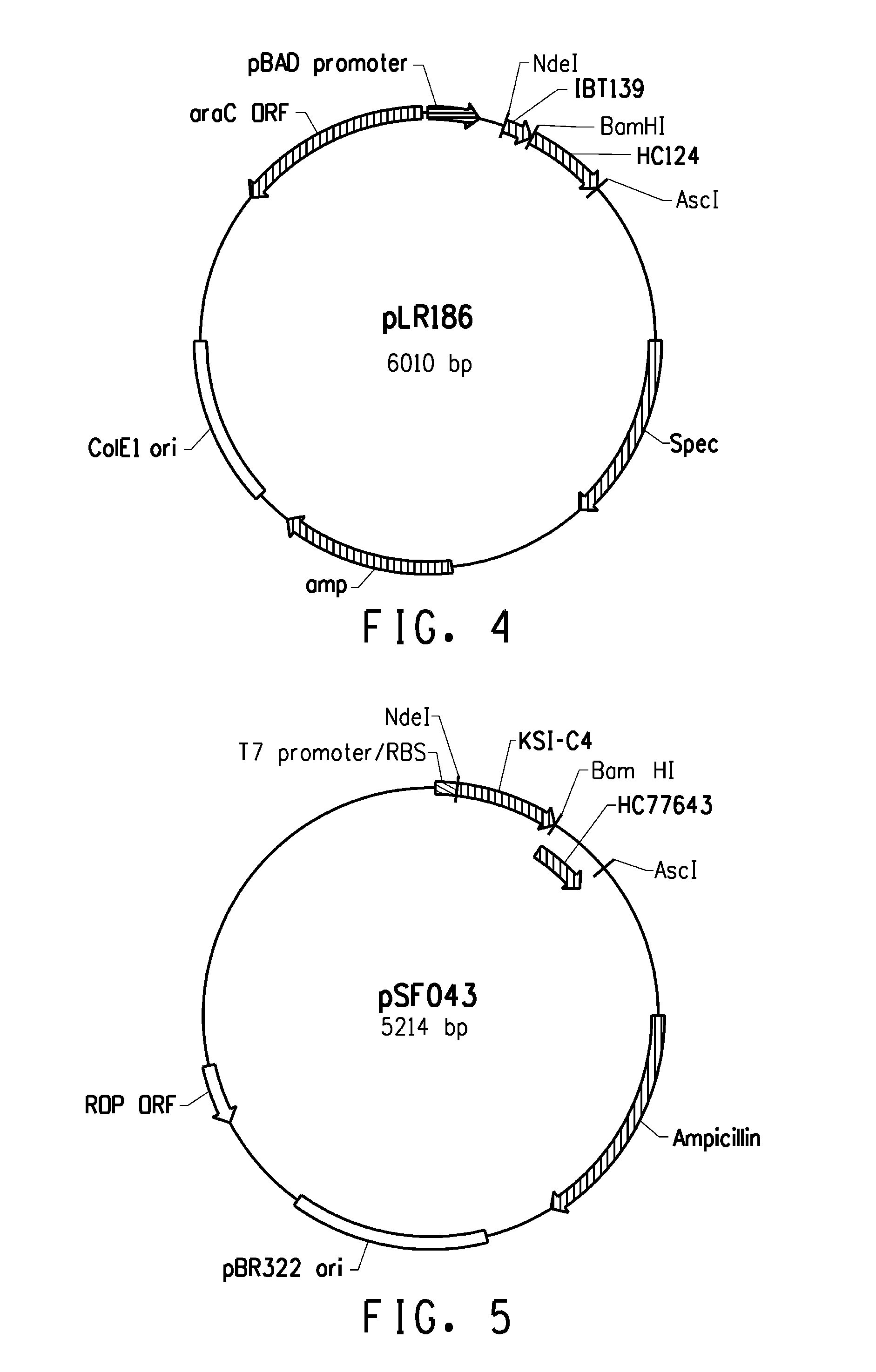
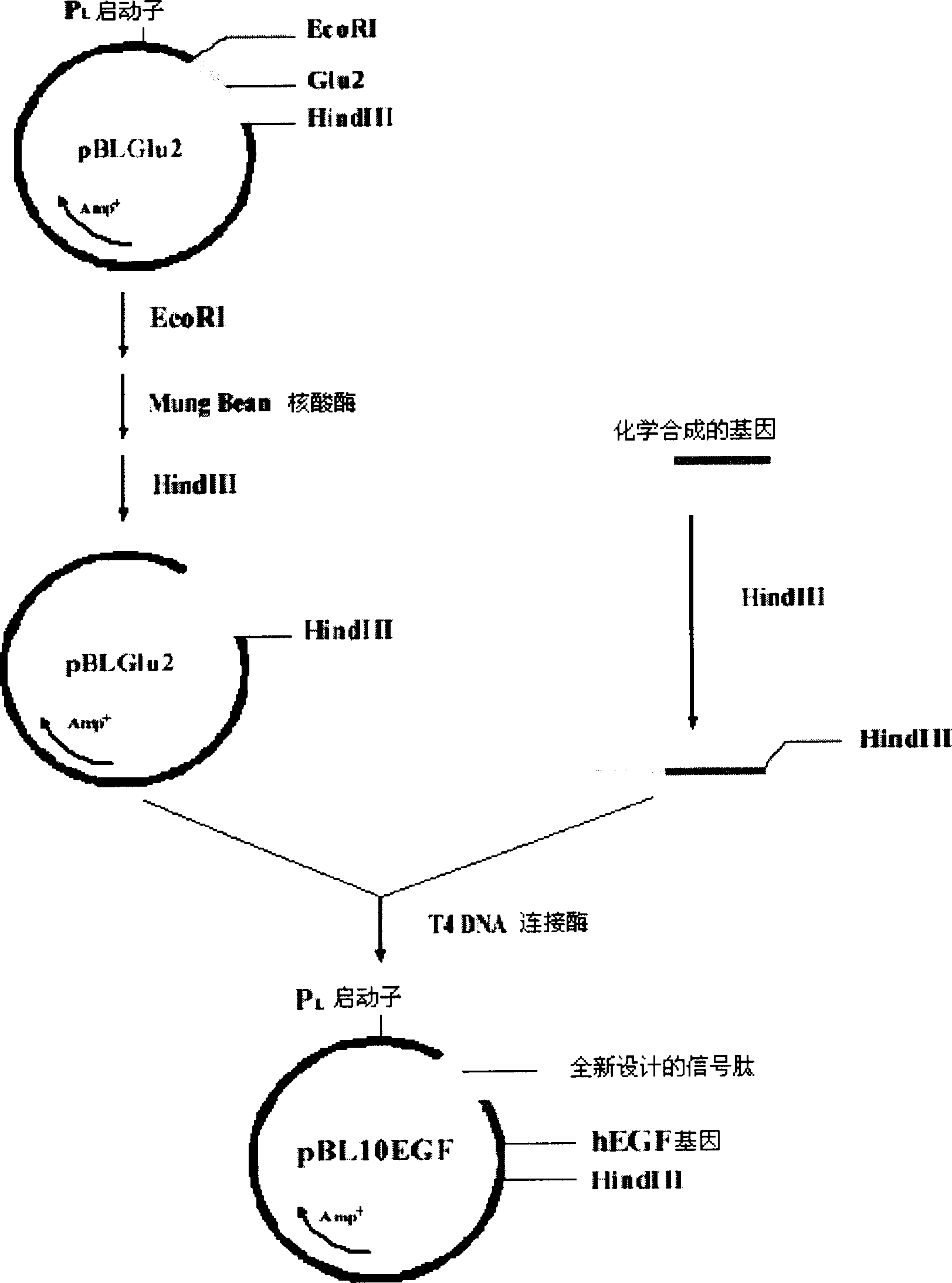
Escherichia expression system of secretory expression recombinant human epidermal growth factor
This invention includes signal peptide gene phoAspL10, expression plasmid pBL10EGF, host cell of bacillus coli BL21 (DE3) inverted by expression plasmid pBL10EGF, engineering strain BE-2 screened from the host cell and preparation method of rhEGF. The strain BE-2 can excrete and express rhEGF directly into culture after fermentation and the quantity is 384mg / l.The purity is more than 95% and specific activity more than 1.0*106IU / mg protein. The product rhEGF reserves crude space configuration and shows high biological activity.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
High-pressure inclusion body solubilization and protease clipping of recombinant fusion proteins
Described herein are methods for the solubilization and proteolytic cleavage of fusion protein aggregates, including autocatalytic fusion proteins, at pressures greater than atmospheric pressure to yield soluble target polypeptides.
Owner:BARFOLD INC
Recombination of human soluble TRAIL protein, the preparing method and the application in preparing antineoplastic medicine
InactiveCN1500808AHigh purityInduce apoptosisPeptide/protein ingredientsFermentationCancer cellTRAIL Protein
The present invention discloses recombinant soluble human tumore necrosis factor related death inducing ligand and its preparation process and application in preparing antitumor medicine. The present invention adopts human tonsil tissue mRNA as template to amplify TRAIL coding sequence, constitute engineering colibacillus for expressing rhsTRAIL and engineering saccharomycete, establish the rhsTRAIL engineering colibacillus fermentation, occlusion body washing and destination protein purification process and the engineering saccharomycete and destination protein purification process separately, and obtain pure rhsTRAIL product, which has molecular weight of 19.6-24 KD and exists in both monomer and dimmer. The rhsTRAIL can induce death of several kinds of cancer cells outside body, inhibit amplification of liver cancer cell in tumor loading mouse obviously and kill tumor cell selectively, so that it may be used in preparing effective antitumor medicine.
Owner:李宏
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Colibacillus and method for performing soluble expression of transglutaminase proenzyme thereof
InactiveCN101691560ASimplify the fermentation production processOmit transgenderBacteriaTransferasesBiotechnologyEscherichia coli
The invention discloses colibacillus and a method for performing soluble expression of transglutaminase proenzyme thereof. The method comprises the following steps: (1) designing PCR primer to perform PCR amplification according to the nucleotide sequence of transglutaminase proenzyme of streptomyces mobaraensis, cloning the related gene to prokaryotic expression vector pET22b (+),constructing expression vector pET22b-proMTG to conform to E.coli BL21 / proMTG of which strain preservation number is CCTCC M 208240; (2) performing soluble expression of transglutaminase proenzyme; (3) activating transglutaminase proenzyme; (4) performing high density fermentation; and (5) performing protein purification technology. In the method of the invention, the yield and specific activity of transglutaminase proenzyme can reach the level of that of transglutaminase proenzyme which is produced by firstly performing direct expression in colibacillus to form inclusion body and then performing denaturation and renaturation and the fermentation process is easier.
Owner:SOUTH CHINA UNIV OF TECH
Method for obtaining single chain antibodies to human interferon alpha2b
A bacterial high-expression system which is applicable for simultaneous screening of large numbers of recombinant clones from combinatorial antibody libraries is disclosed. The method pertains to screening of single chain antibodies from libraries expressed in the periplasm of E. coli by secretion. By this approach, approximately 104 clones can be screened in a single round. After screening, the clones, which express the recombinant antibodies to the desired antigen, can be directly used for production of large quantities of antibodies from microorganism culture. The system is especially attractive for fast screening of antibody libraries from a hybridoma source. A refolding method for the large-scale production of biologically active scFv-6 his proteins from bacterial inclusion bodies is also disclosed.
Owner:NEW TECH HLDG
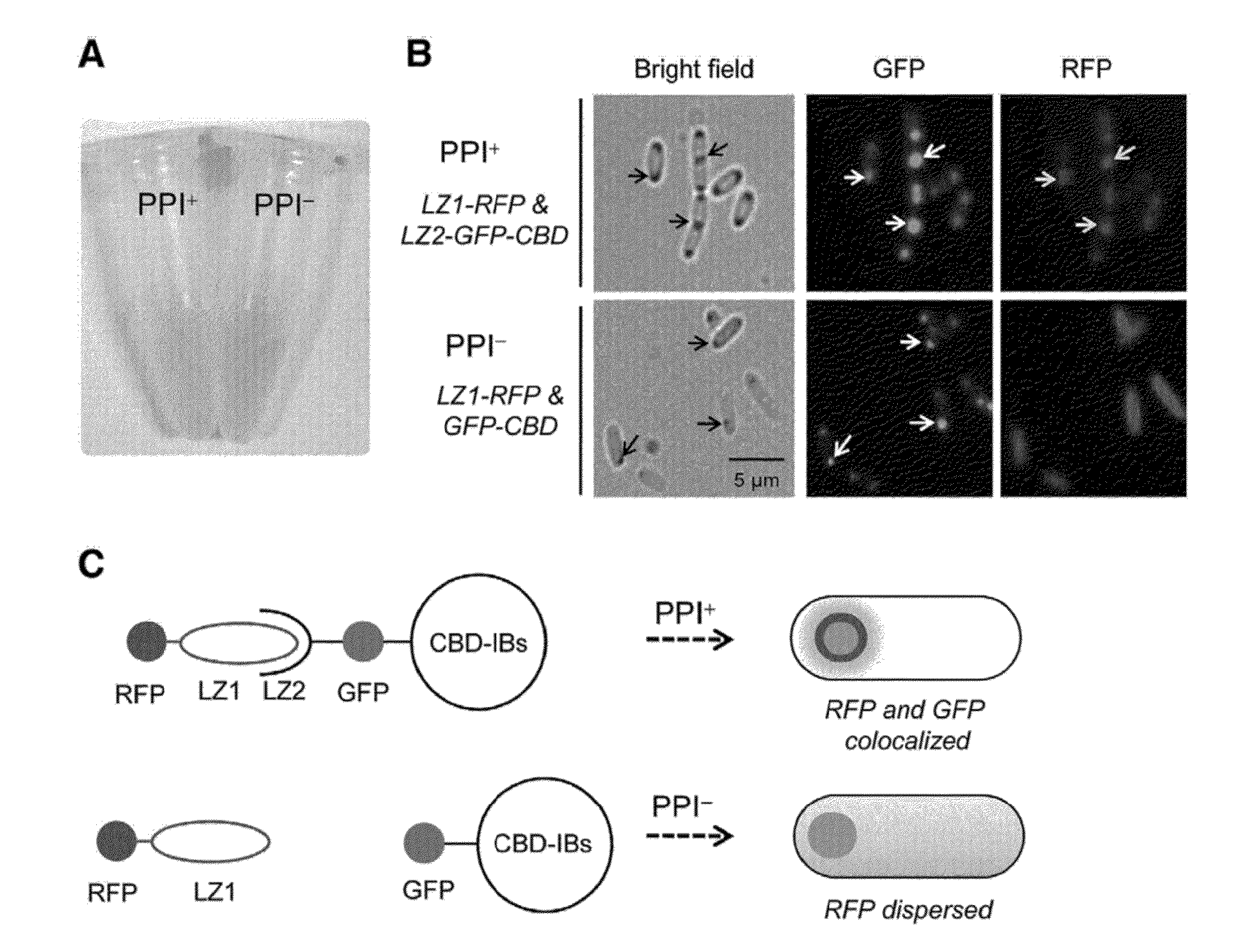
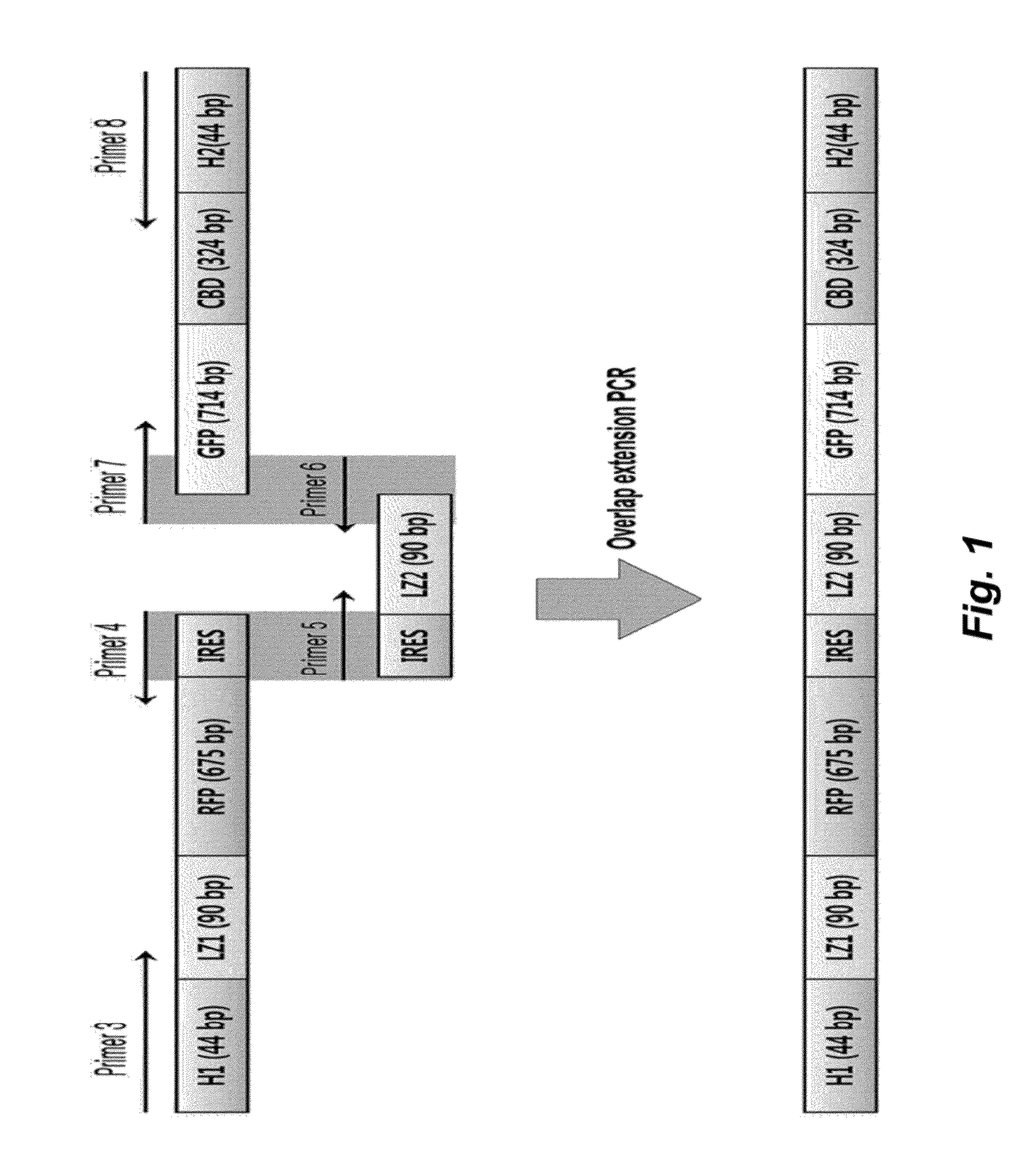
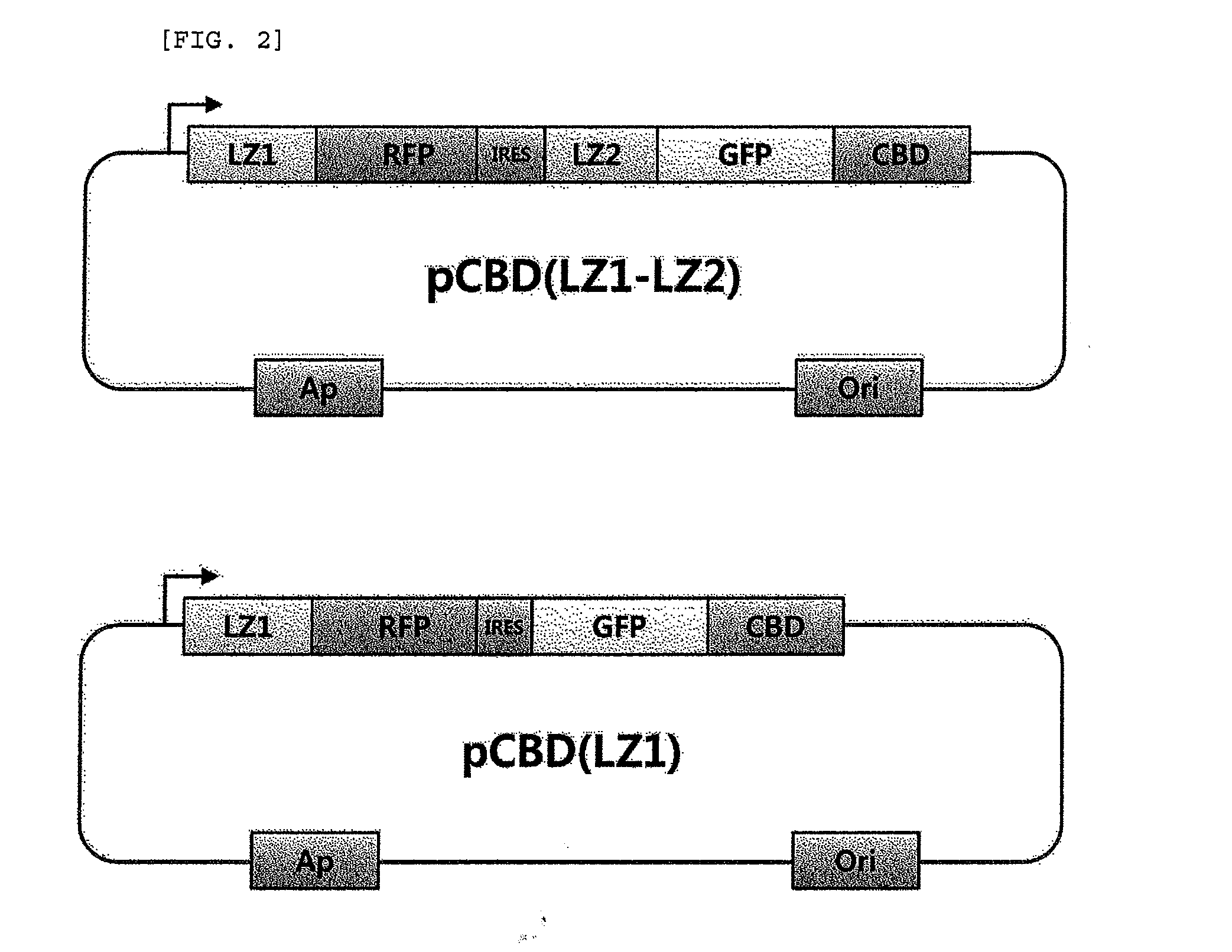
Method for detecting protein-protein interactions in cells
The present invention relates to a method for detecting protein-protein interactions in living cells, and more particularly, to a method for providing cells comprising a first construct and a second construct, wherein the first construct comprises a polynucleotide encoding a first fusion protein which comprises a bait protein, a first fluorescent protein and a CBD (cellulose-binding domain) protein, and wherein the second construct comprises a polynucleotide encoding a second fusion protein which comprises a prey protein and a second fluorescent protein so as to allow formation of inclusion bodies, and detecting interactions between the bait protein and the prey protein that are displayed by inclusion bodies, a method for isolating the prey protein bound to the bait protein using the cells comprising the constructs, the cells, and a kit for detecting protein-protein interactions, comprising the constructs.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Soluble cytoplasmic expression of heterologous proteins in escherichia coli
InactiveUS20130273585A1Specific activityPeptide/protein ingredientsMicrobiological testing/measurementEscherichia coliHeterologous
Soluble variants of recombinant proteins produced in a prokaryotic host cell, where the high expression levels often cause the original proteins to aggregate into insoluble inclusion body aggregates. The variant polypeptides retain biological function while increasing protein solubility with comparable or higher recoverable levels of biologically active protein when expressed in a suitable expression host. Methods of identifying critical residues and substituting them are provided to produce the variants.
Owner:GANGAGEN
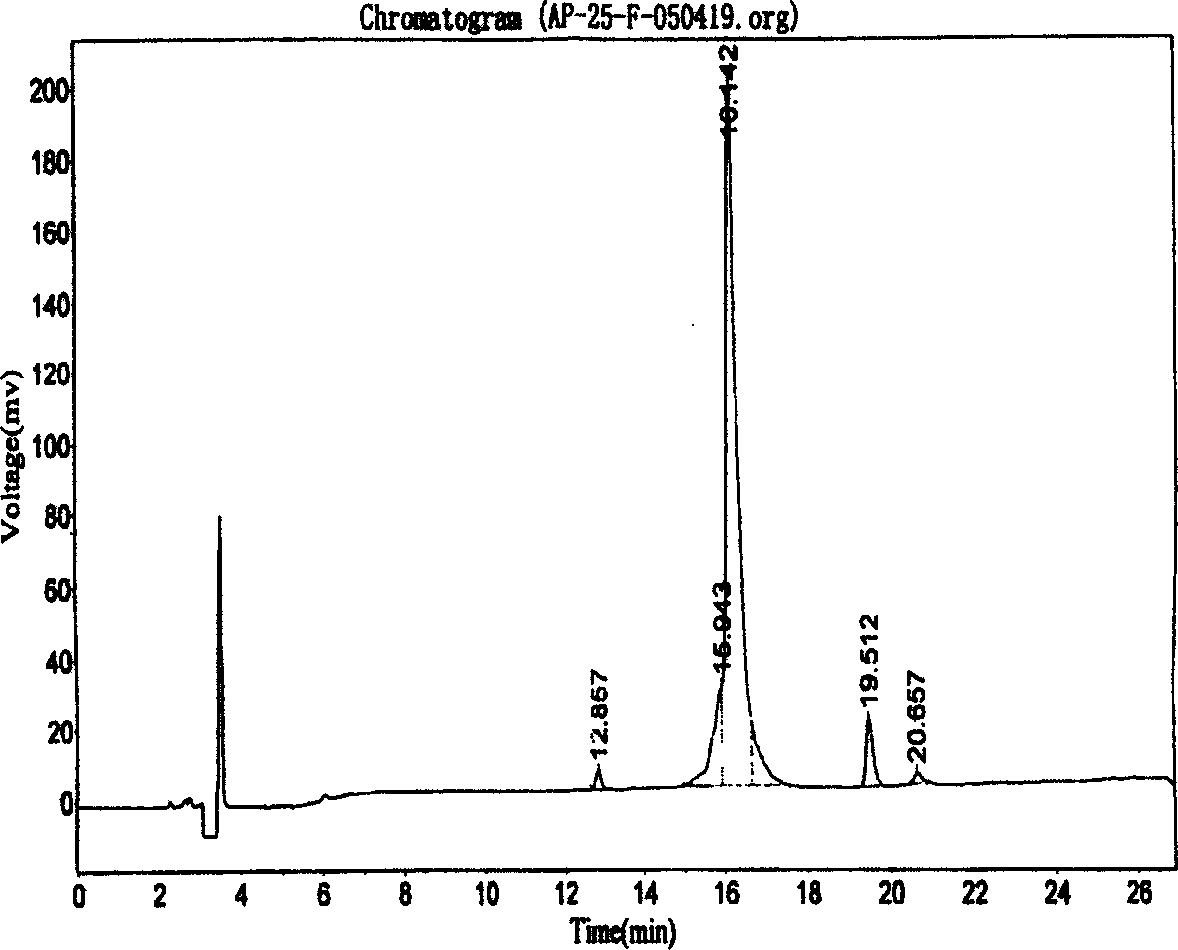
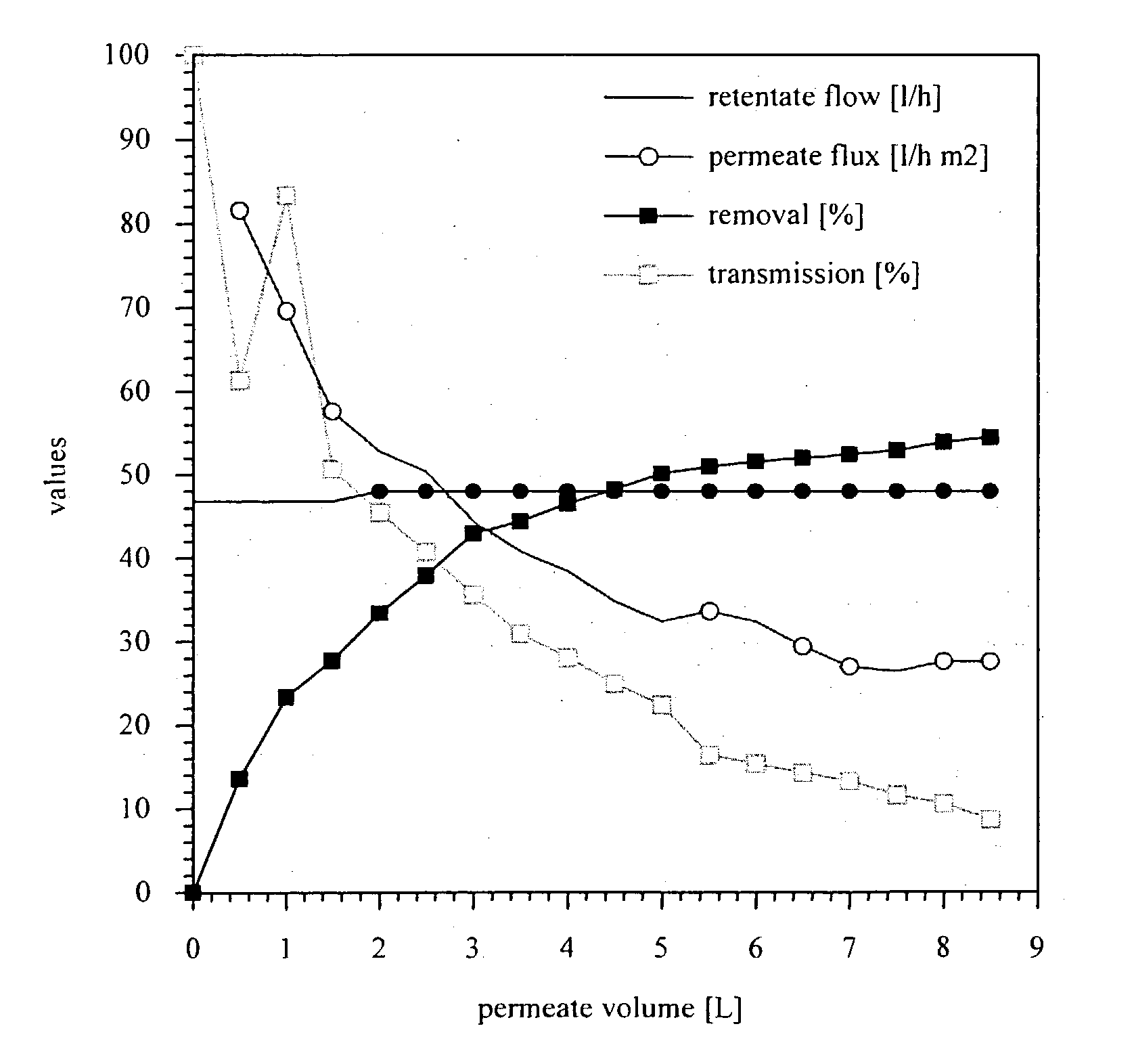
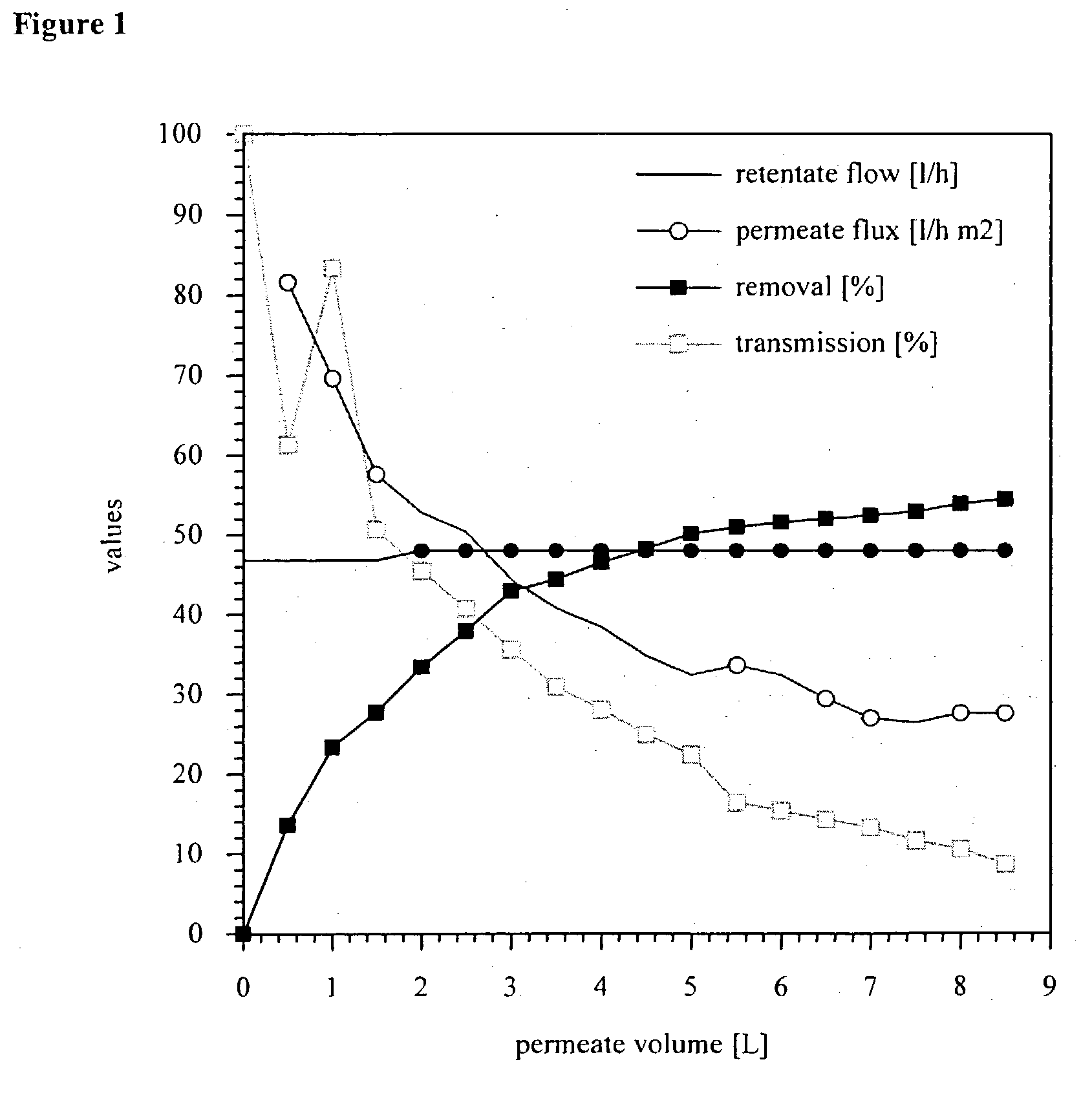
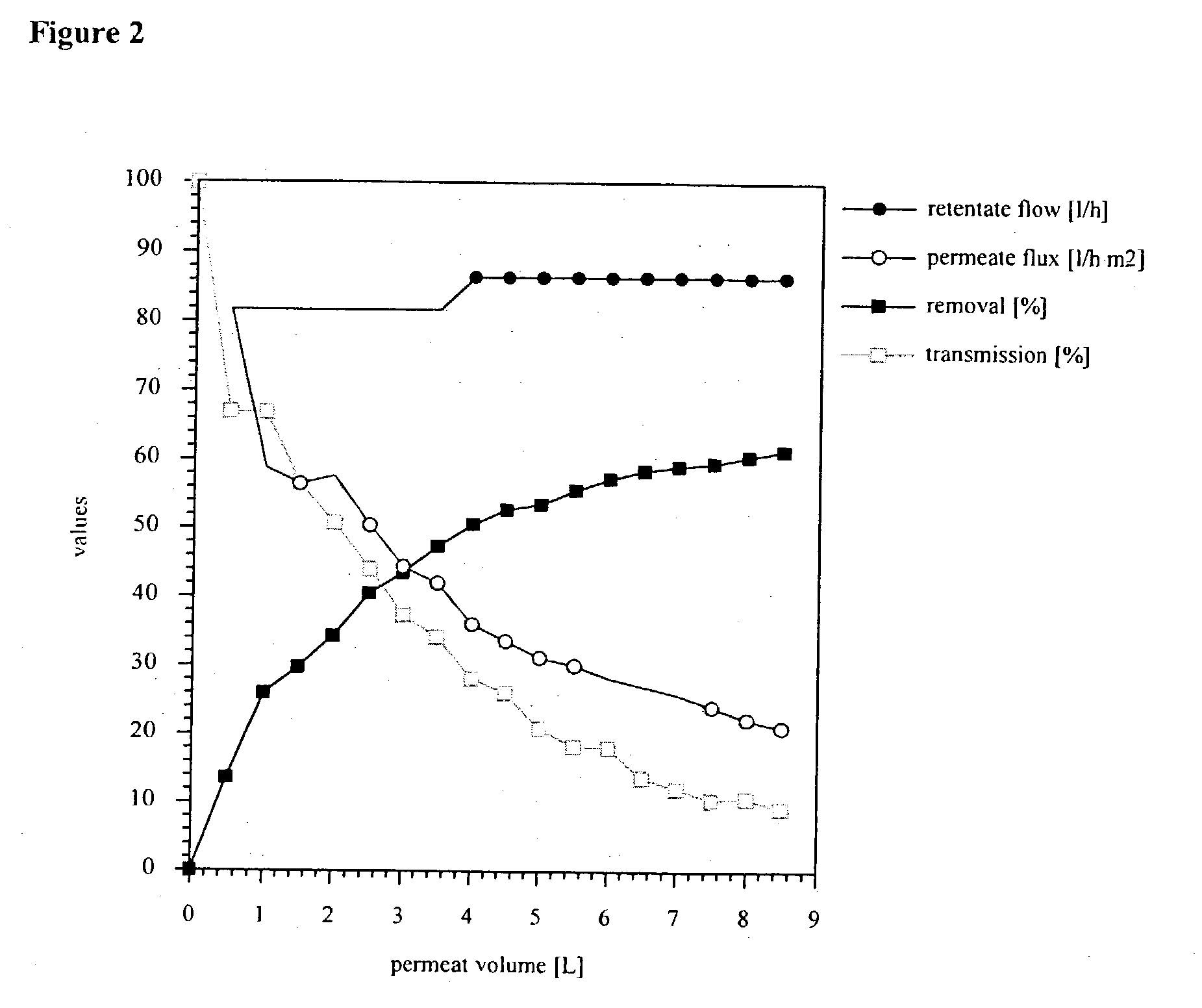
Purification of protein inclusion bodies by crossflow microfiltration
InactiveUS20030171560A1Reduce concentrationPromote fermentationMicroorganismsDepsipeptidesInclusion bodiesSemipermeable membrane
The invention relates to a process for purifying and / or concentrating inclusion bodies (refractile bodies) in a highly concentrated particle-containing solution, in which process the inclusion body-containing suspension is directed tangentially past one or more semipermeable membranes, so that the inclusion bodies are retained behind the membranes and that substances having a relatively small molecular weight may pass through the membrane and / or be adsorbed to the membrane, and a purified and / or concentrated inclusion body suspension is obtained. In the same manner, cell wall particles are removed from a inclusion body suspension. The invention further relates to the use of a crossflow microfiltration unit for purifying and / or concentrating inclusion bodies in a suspension and for removing cell wall particles from a inclusion body preparation.
Owner:BAYER AG
Method for producing recombined insulin human
ActiveCN101173006ASimple processReduce manufacturing costPeptide preparation methodsInsulinsEscherichia coliInsulin activity
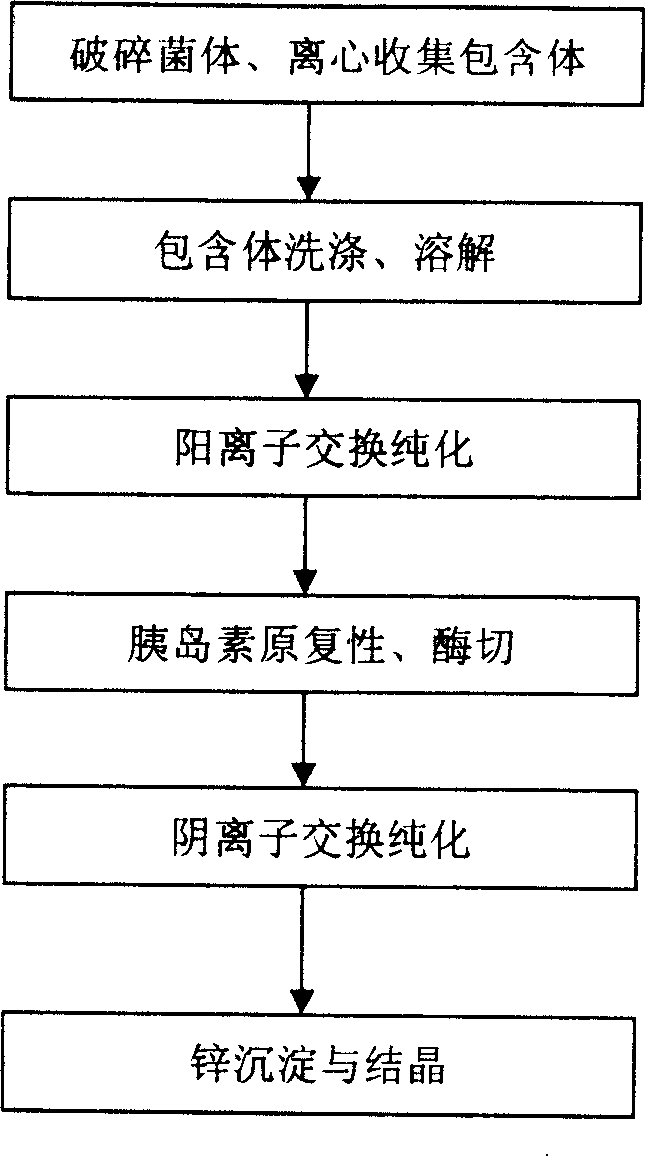
The invention discloses a preparation method of recombinant human insulin, which relates to the protein polypeptide type medicine field. The technical problem needed to be solved by the invention aims at providing a preparation method for Escherichia coli to express the recombinant human insulin in order to overcome the technical disadvantages in the prior art of using the poisonous and harmful substance CNBr and having a complex production process. The preparation method for the recombinant human insulin of the invention comprises the steps of: thallus crashing, inclusion body dissolving, proinsulin sulfonating, purifying proinsulin, and proinsulin renaturation, enzyme cutting transmission, etc., and the preparation method is characterized in that during the inclusion body dissolving process, the -SH of proinsulin is transformed into -SSO3<->, and then human insulin is obtained through purifying the sulfonated proinsulin, reduction renaturation and enzyme cutting transmission by positive ion, and then through the negative ion exchange layer purification. The preparation method of the recombinant human insulin of the invention does not use the CNBr and has simple technique with high human insulin yield coefficient and purity.
Owner:JIANGSU WANBANG BIOPHARMLS +1
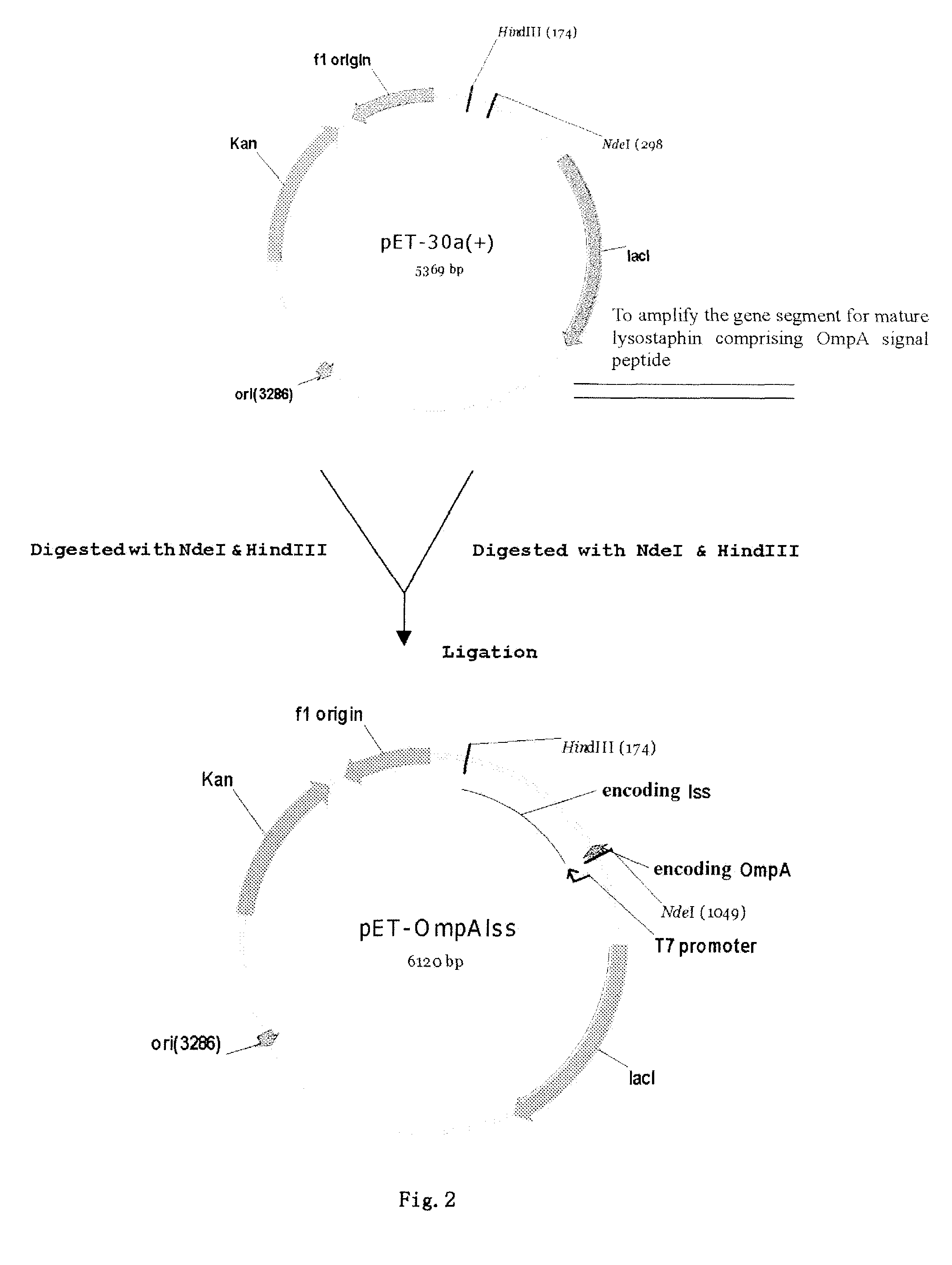
Method of secretory expression of lysostaphin in Escherichia coli at high level
ActiveUS8241901B2Economical and social benefitSpeed up the processBacteriaPeptide/protein ingredientsEscherichia coliInclusion bodies
A method of secretory expression of lysostaphin in Escherichia coli at high level, which comprises constructing a expression vector by cloning a sequence encoding a signal peptide which is suitable for secretory expression in Escherichia coli before part or whole gene sequence which encodes mature lysostaphin, and ligating the cloned sequence with a promoter; and transforming Escherichia coli with the expression vector, culturing and fermenting, and then isolating lysostaphin from the supernatant of the fermentation broth. The advantage of secretory expression is that the expression product can exist in the medium in an active form, and thus does not need the process for renaturation of the inclusion body; it is more easily to purify from the supernatant of the fermentation broth with high rate of recovery; and there is less contamination from the host's proteins.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Production method for recombinant human granulocyte colony-stimulating factor
ActiveCN103233053ALarge amount of processingReduce difficultyMicroorganism based processesPeptide preparation methodsEscherichia coliYeast
The invention discloses production method for a recombinant human granulocyte colony-stimulating factor. According to the invention, an engineered strain of an escherichia coli expressed recombinant human granulocyte colony-stimulating factor is cultured to obtain an inclusion body, the engineered strain of the escherichia coli expressed recombinant human granulocyte colony-stimulating factor is pKG931 / HB101, a culture method comprises multiplication culture and culture in a fermentation cylinder, an antibiotic-free medium containing yeast and peptone is employed in both culture, a high pressure homogenizer is used for breaking of bacteria, and denaturation, renaturation and chromatography are carried out so as to obtain a high purity G-CSF stock solution. The production method provided by the invention has the advantages of a short production period, high production efficiency, a great production scale, especial suitability for industrial production and reduction in production cost.
Owner:BEIJING FOUR RINGS BIOPHARM
Recombined porcine interferon Gamma, coding gene thereof and expressing method
ActiveCN103059124AImprove defenseEnhance immune responseBacteriaPeptide/protein ingredientsBiotechnologyHeterologous
The invention provides a recombined porcine interferon Gamma, a coding gene thereof, and an expressing, purifying and inclusion body refolding method, and belongs to the field of biological gene engineering. The recombined porcine interferon Gamma serves as a non-specific broad-spectrum anti-viral biological preparation, and has a wide medicinal prospect in the field of veterinary medicines; but as most of gene engineering veterinary medicines, the porcine interferon Gamma has the problems of underproduction, high prices, non-uniform quality and the like. According to the recombined porcine interferon Gamma, the coding gene thereof and the expressing method, an escherichia coli expression system is used for performing heterologous expression for the recombined porcine interferon Gamma subjected to codon optimization. Besides, aiming at the problem that the porcine interferon Gamma in a prokaryotic expression system is usually expressed in a manner of an inclusion body, the invention further provides the purifying and refolding method for the inclusion body of the recombined porcine interferon Gamma, so that the prepared recombined porcine interferon Gamma has high activity and meets a standard for industrialized production.
Owner:GENSUN INST OF BIOMEDICINE
Preparation method of liraglutide intermediate polypeptide
ActiveCN108191981ALow costHigh expressionPolypeptide with localisation/targeting motifBacteriaHigh concentrationEscherichia coli
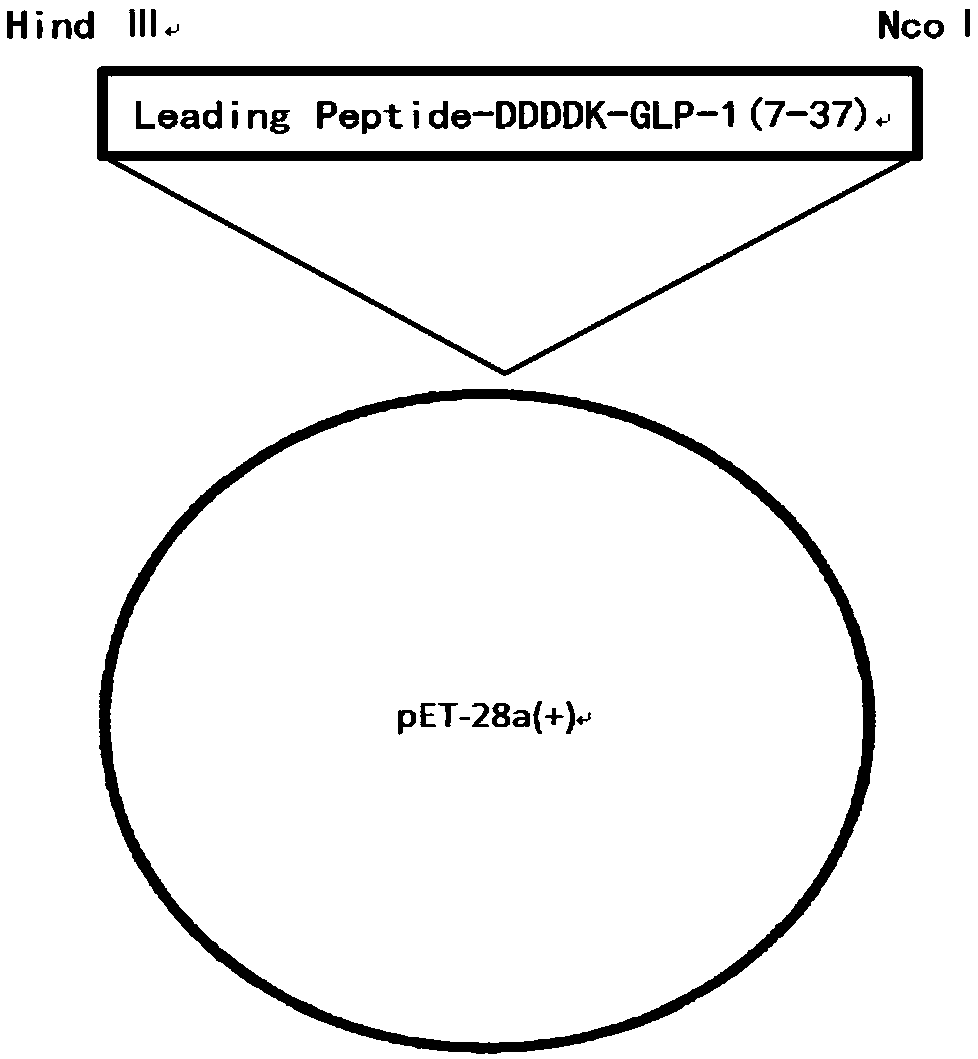
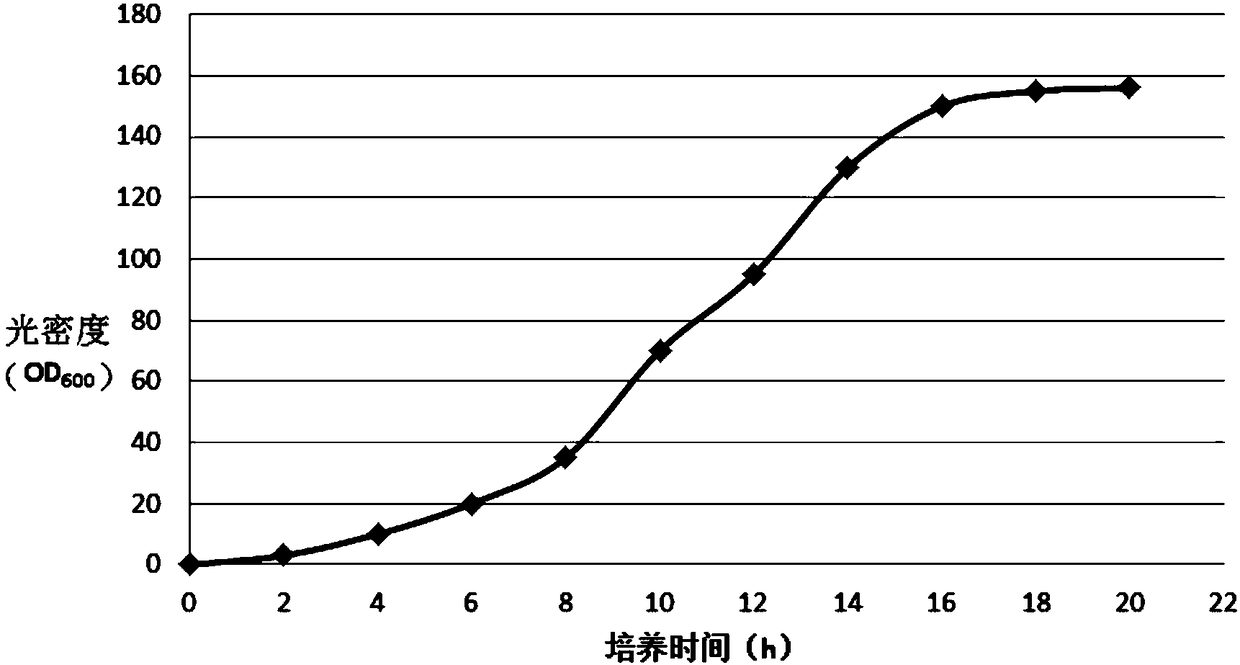
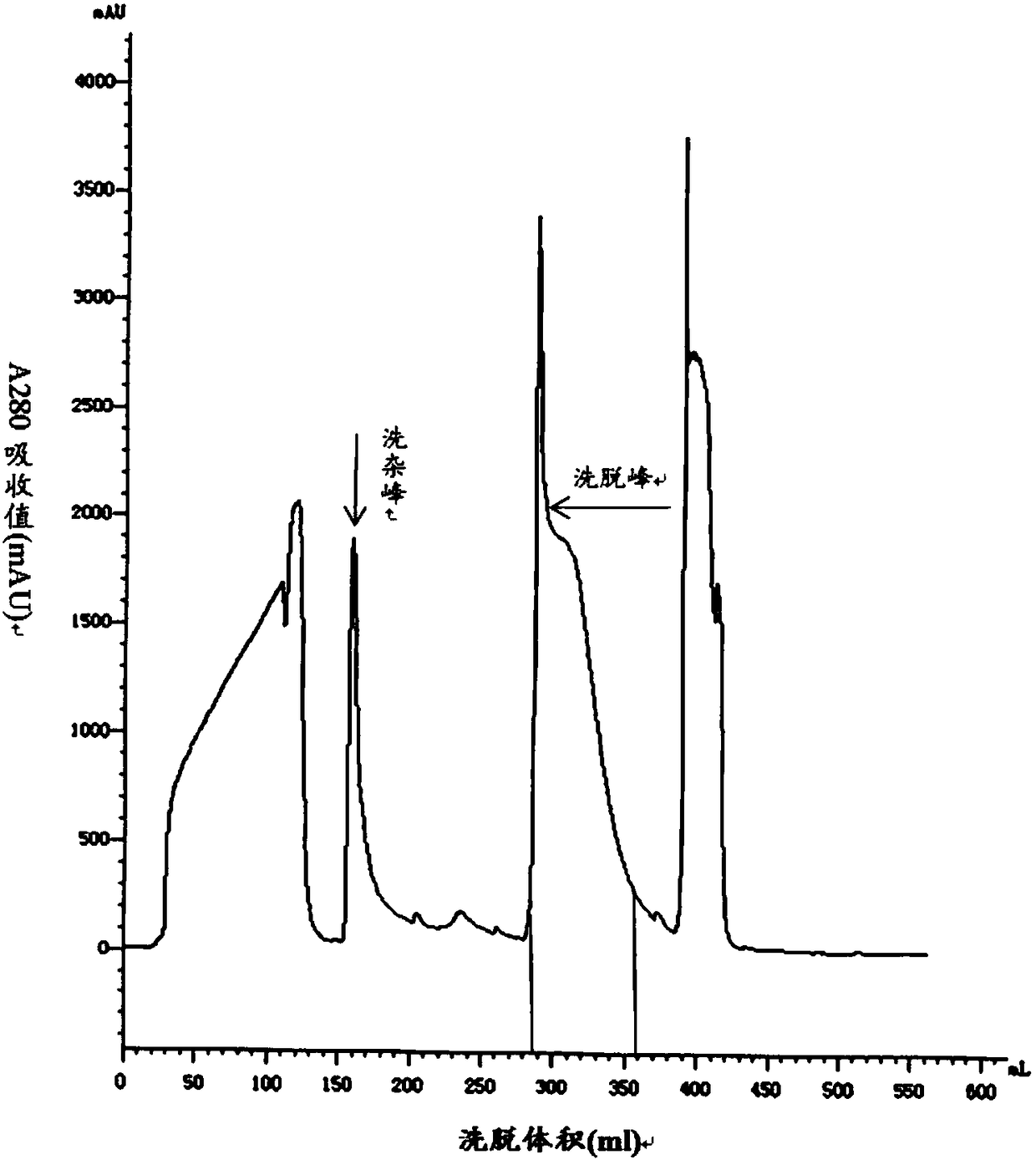
The invention belongs to the technical field of preparation methods of polypeptides and particularly relates to a preparation method of liraglutide intermediate polypeptide GLP-1 (glucagon-like peptide-1) (7-37). The method comprises the following main steps: constructing recombinant liraglutide engineering bacteria, expressing liraglutide intermediate fusion protein in a form of an inclusion bodyunder Echerichia coli induction, and performing denaturation, renaturation, enzyme digestion, separation and purification to obtain the liraglutide intermediate polypeptide GLP-1 (7-37). Expression is changed into intracellular insoluble inclusion body expression by changing recombinant sequence signal peptides, and expression quantity is increased greatly; the washed inclusion body is subjectedto alkali dissolution, a large quantity of denaturant is not needed, the inclusion body with high concentration of protein concentration being 20-30 g / L is added to an inclusion body dissolution buffer, denaturation and renaturation time does not exceed 1 h, and enzyme digestion can be performed after dissolution; procedures are reduced, operation volume is reduced, reagent cost is reduced, and industrialized enlargement is facilitated; UniSP-50XS cation exchange is adopted for separation and purification, and separation degree is high. The purity of the liraglutide intermediate polypeptide prepared with the method reaches 87% or higher, and the yield is higher than 85%.
Owner:AMPHASTAR NANJING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com