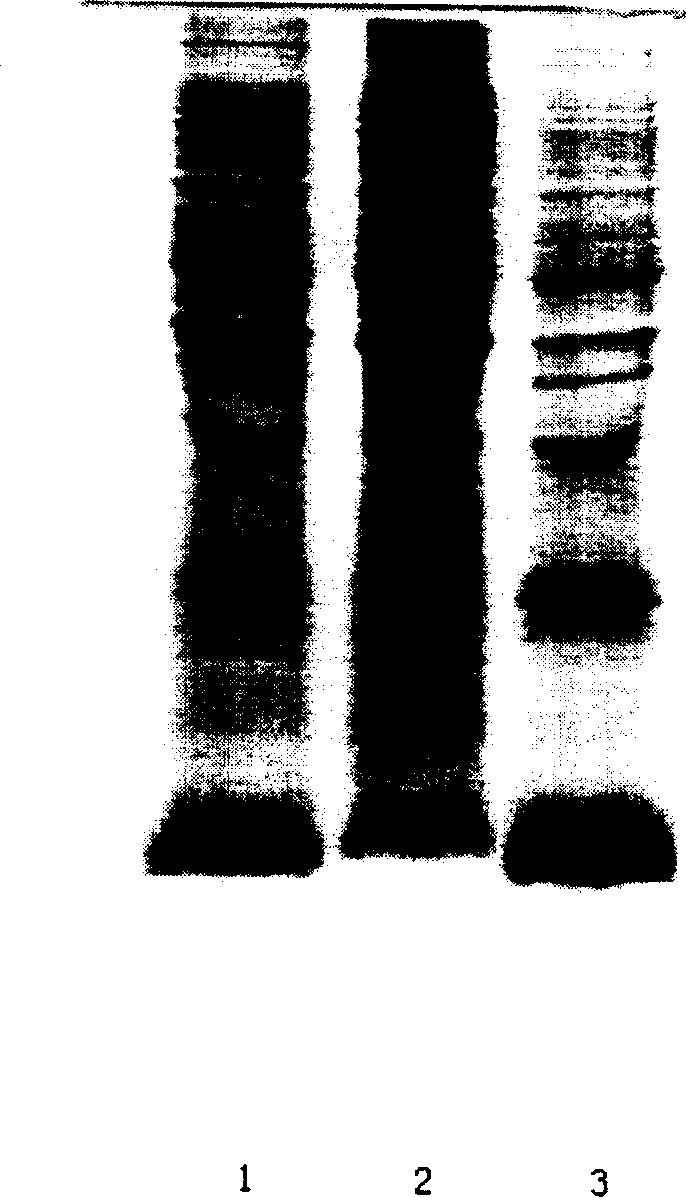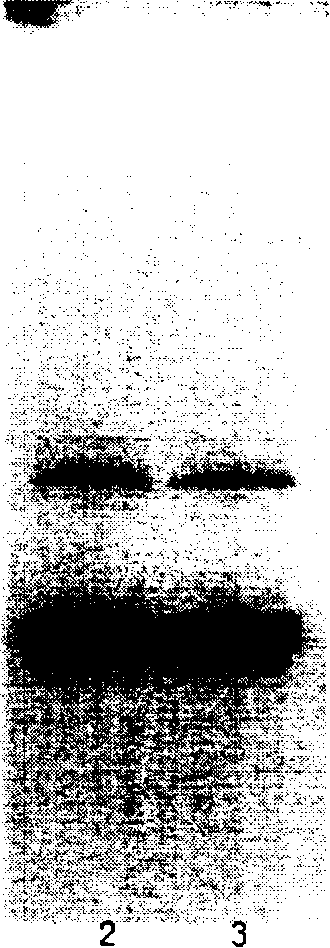Method for producing recombined insulin human
A technology of recombinant human insulin and human insulin, which is applied to the preparation methods of peptides, insulin, chemical instruments and methods, etc., can solve the problems of complex production process, and achieve the effect of simple process and lower production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 recombinant human insulin
[0029] Step 1: Cell disruption and inclusion body collection
[0030] Add 1 liter of TE buffer solution [5mM EDTA; 50mM Tris-HCl (pH9.0)] for every 100g of wet bacteria, mix well, crush in a homogenizer at 700-750 bar, until the bacteria are completely broken, use a centrifuge Centrifuge to collect the precipitate. The obtained precipitates are mainly inclusion bodies, which are washed three times as follows to remove most of the impurities: 1 L of STET buffer [5mM EDTA; 50mM Tris-HCl (pH 9.0); Triton-X100, 3% (V / V) per 100g of precipitate ] and mix well, and stir at room temperature for 30 minutes; centrifuge again to collect the precipitate; the inclusion bodies after washing are detected by SDS-PAGE, see attached figure 1 As shown, it can be seen that its components are less, the purity is higher, and inclusion body dissolution can be performed.
[0031] Step 2: Inclusion Body Solubilization
[0032]Add ...
Embodiment 2
[0042] The preparation of embodiment 2 recombinant human insulin
[0043] Proinsulin S-sulfonate purified by SP column was diluted with 50mM Gly buffer solution (pH11.5) containing 1M urea to make the protein concentration reach 0.75mg / ml, degas immediately, fill with nitrogen, and add the prepared mercaptoethanol solution, so that the molar ratio of its concentration to proinsulin is 1:3, and stirred at 4°C for 12 hours; through this step of folding, at least 60% of proinsulin S-sulfonate can be folded into the correct conformation. Other operations are the same as in Example 1. The purity of the insulin reaches over 99.0%, and the SDS-PAGE test has the same molecular weight as the insulin standard. Has the same RP-HPLC retention characteristics as human insulin standards.
Embodiment 3
[0044] Embodiment 3 Redo the preparation of human insulin
[0045] Proinsulin S-sulfonate purified by SP column was diluted with 50mM Gly buffer solution (pH11.5) containing 1M urea to make the protein concentration reach 1.0mg / ml, immediately degassed, filled with nitrogen, and added to the prepared A solution of mercaptoethanol so that the molar ratio of its concentration to proinsulin was 1:6 was stirred at 4°C for 12 hours.
[0046] Others are the same as implementation 1. The purity of the insulin reaches over 99.0%, and the SDS-PAGE detection has the same molecular weight as the insulin standard product, and has the same RP-HPLC retention characteristics as the human insulin standard product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com