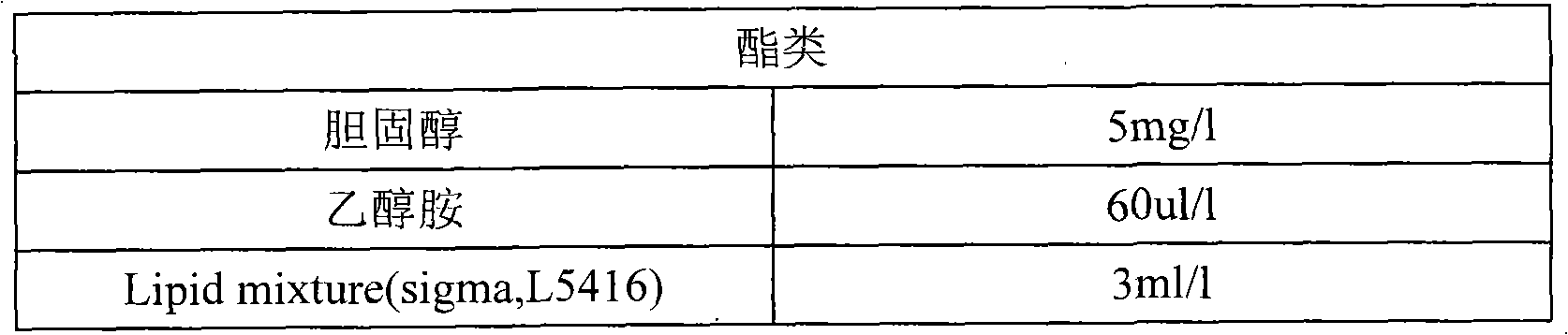
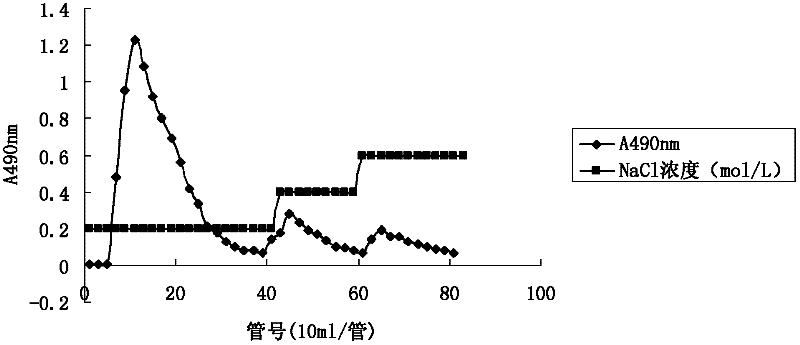
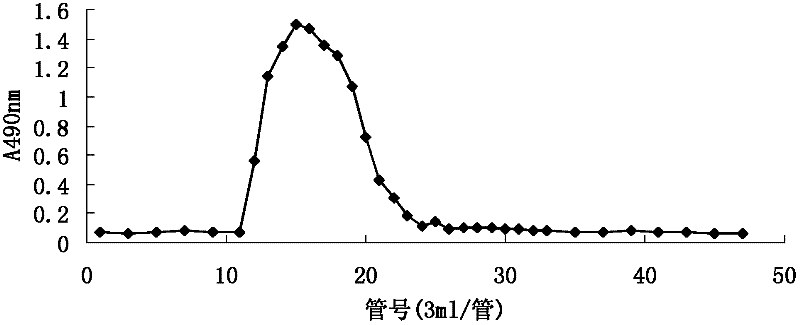
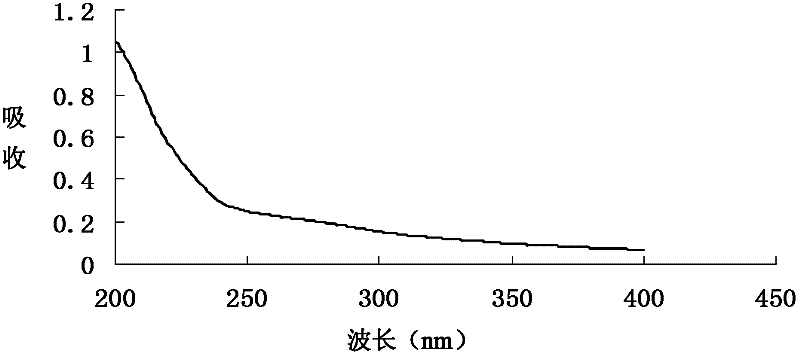
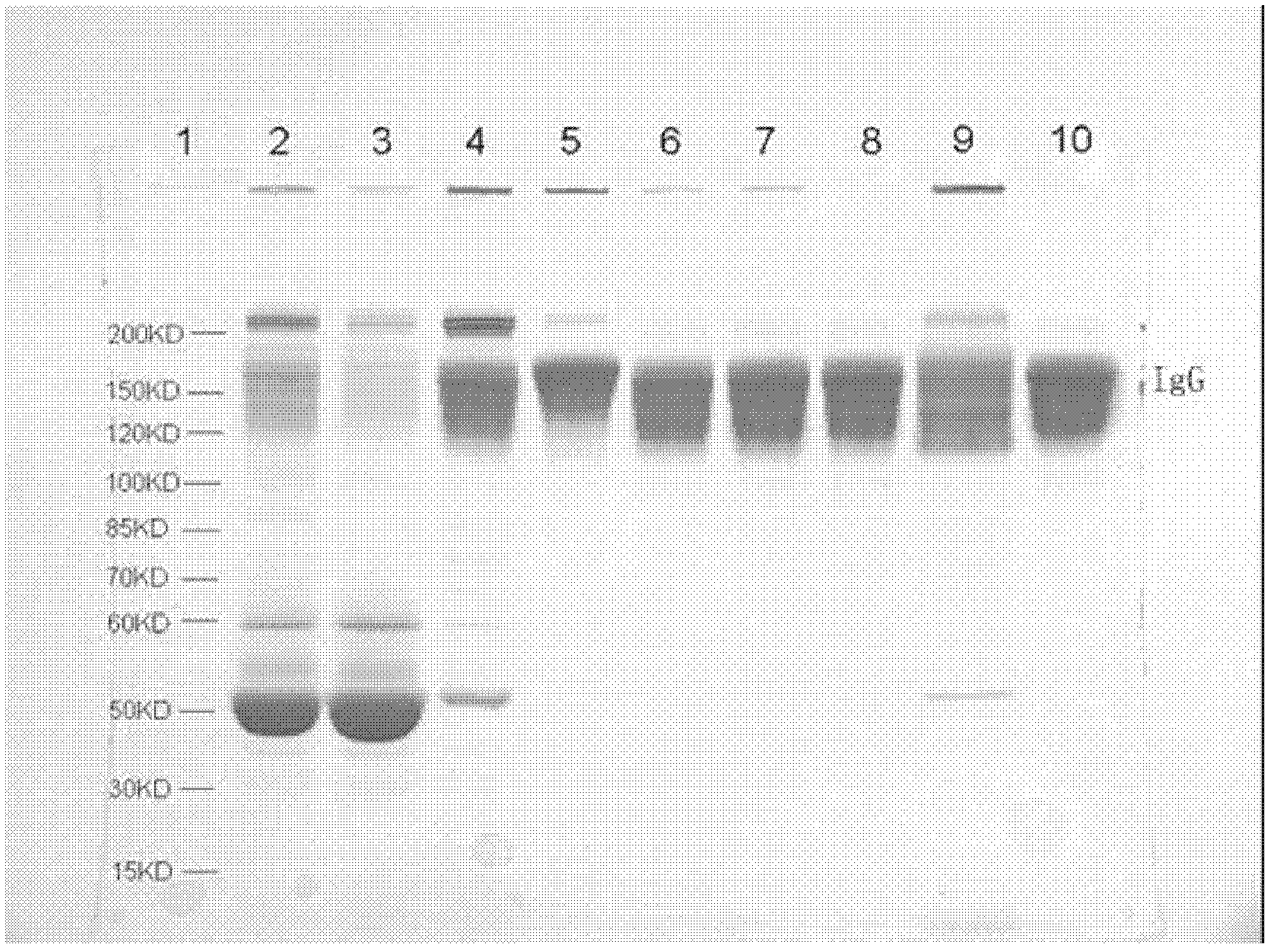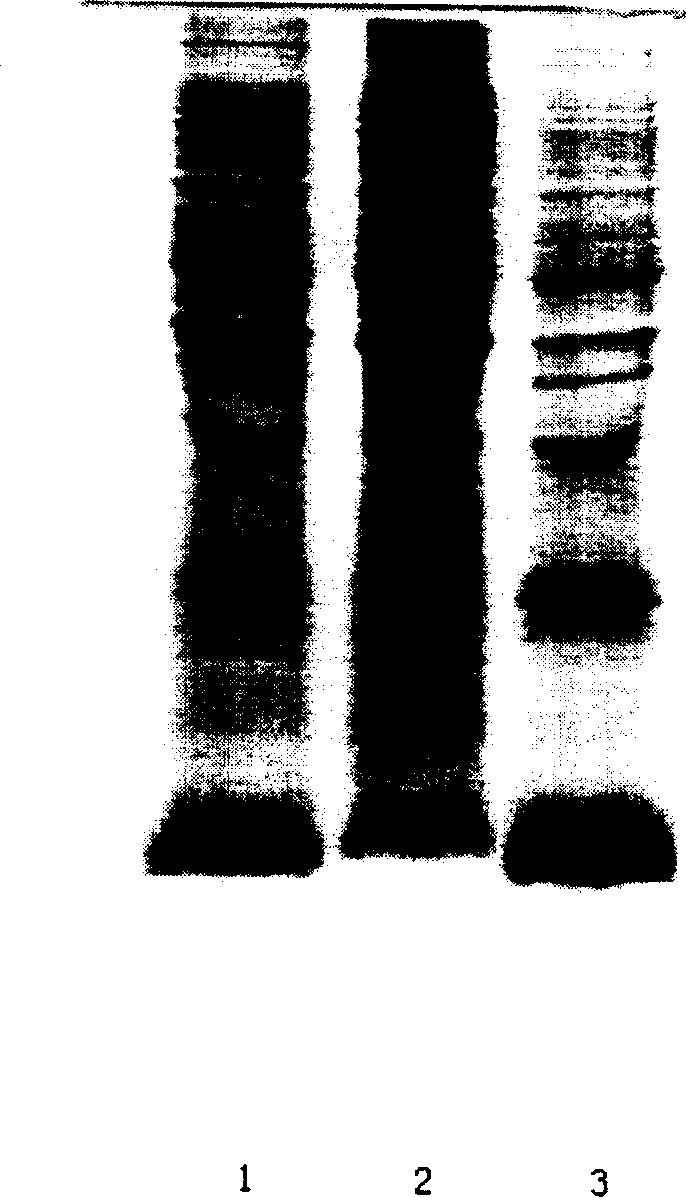Patents
Literature
603 results about "Anion Exchange Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anion exchange resins will bind to negatively charged molecules, displacing the counter-ion. Anion exchange chromatography is commonly used to purify proteins, amino acids, sugars/carbohydrates and other acidic substances with a negative charge at higher pH levels.
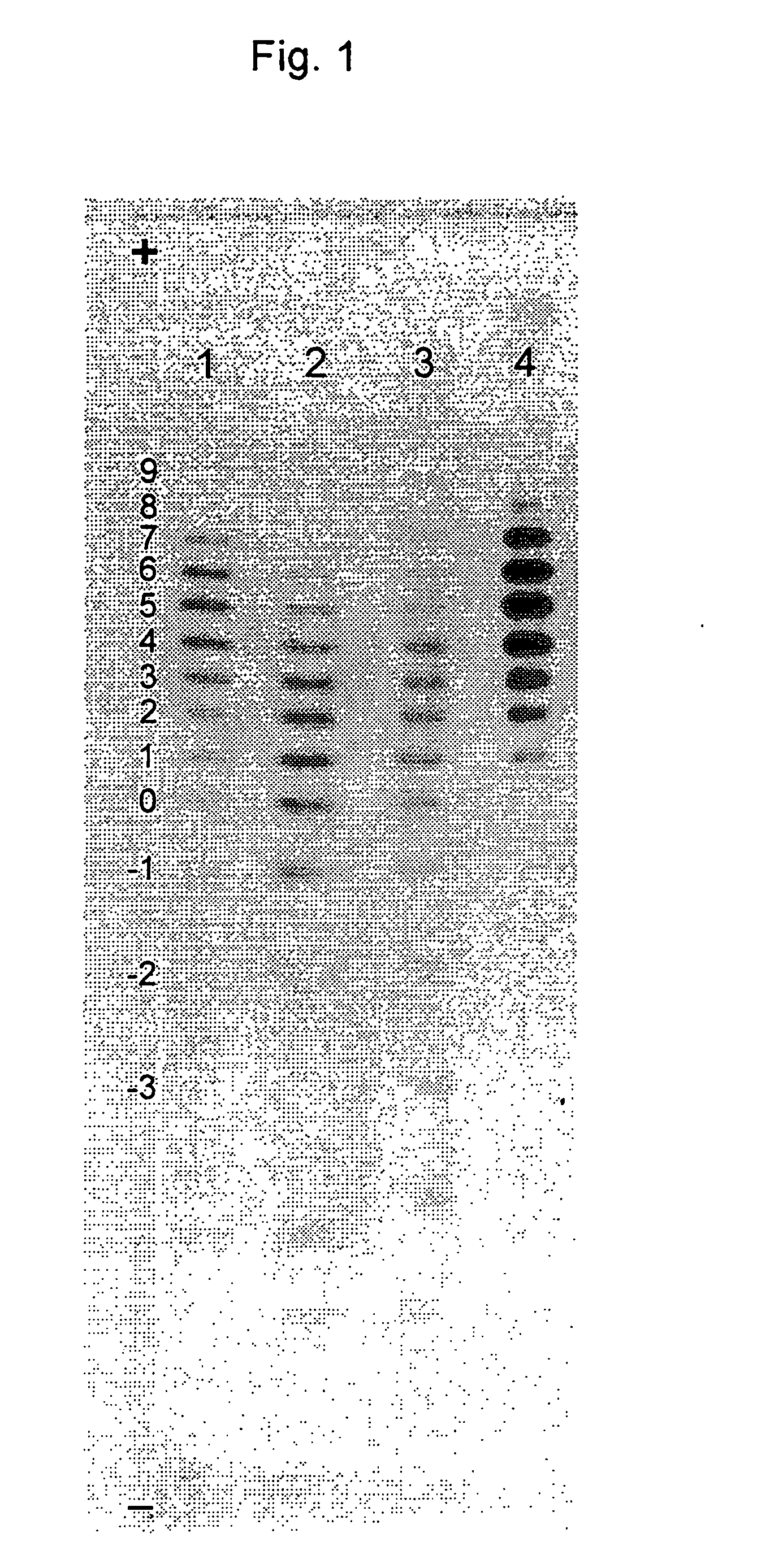
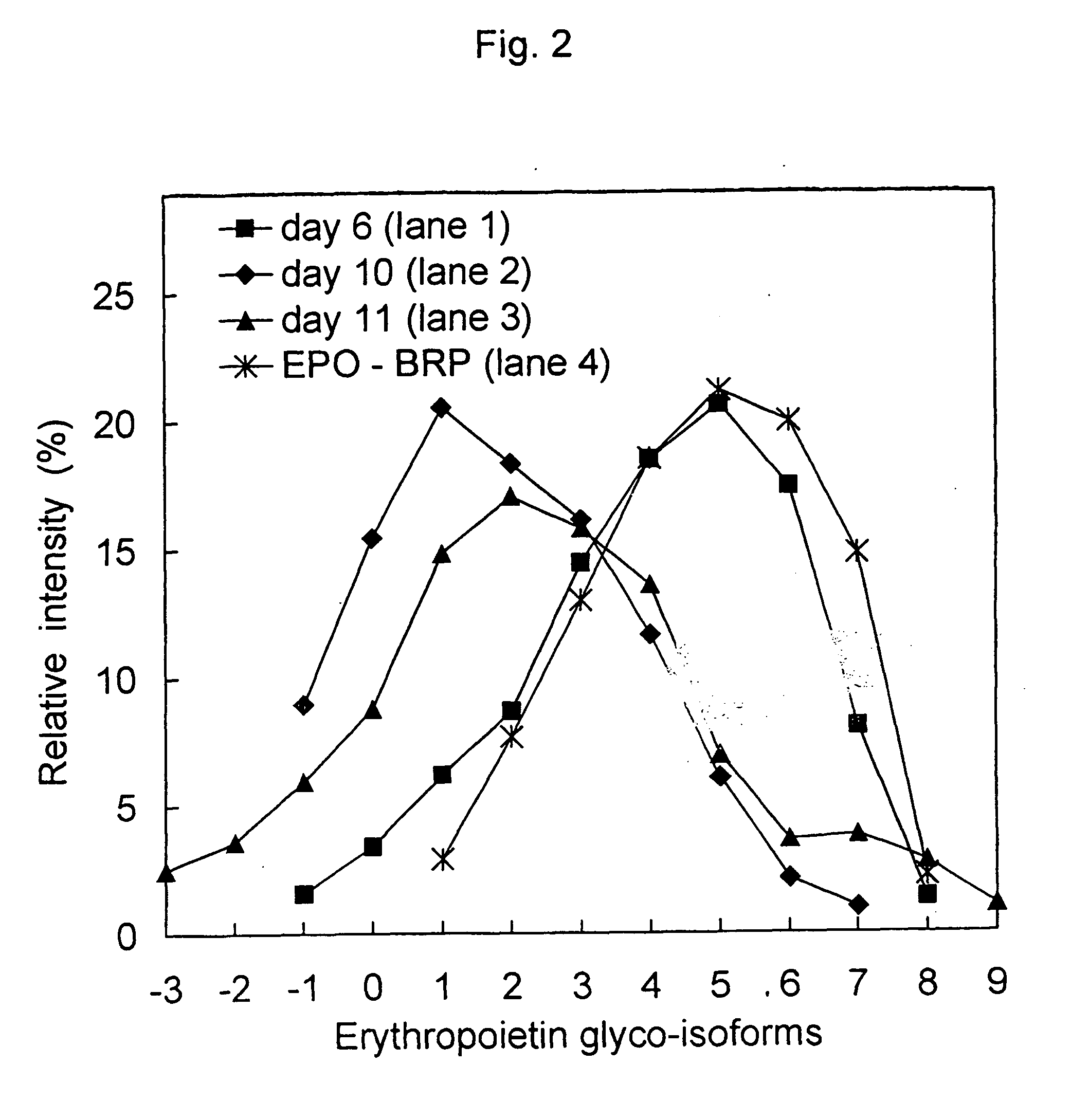
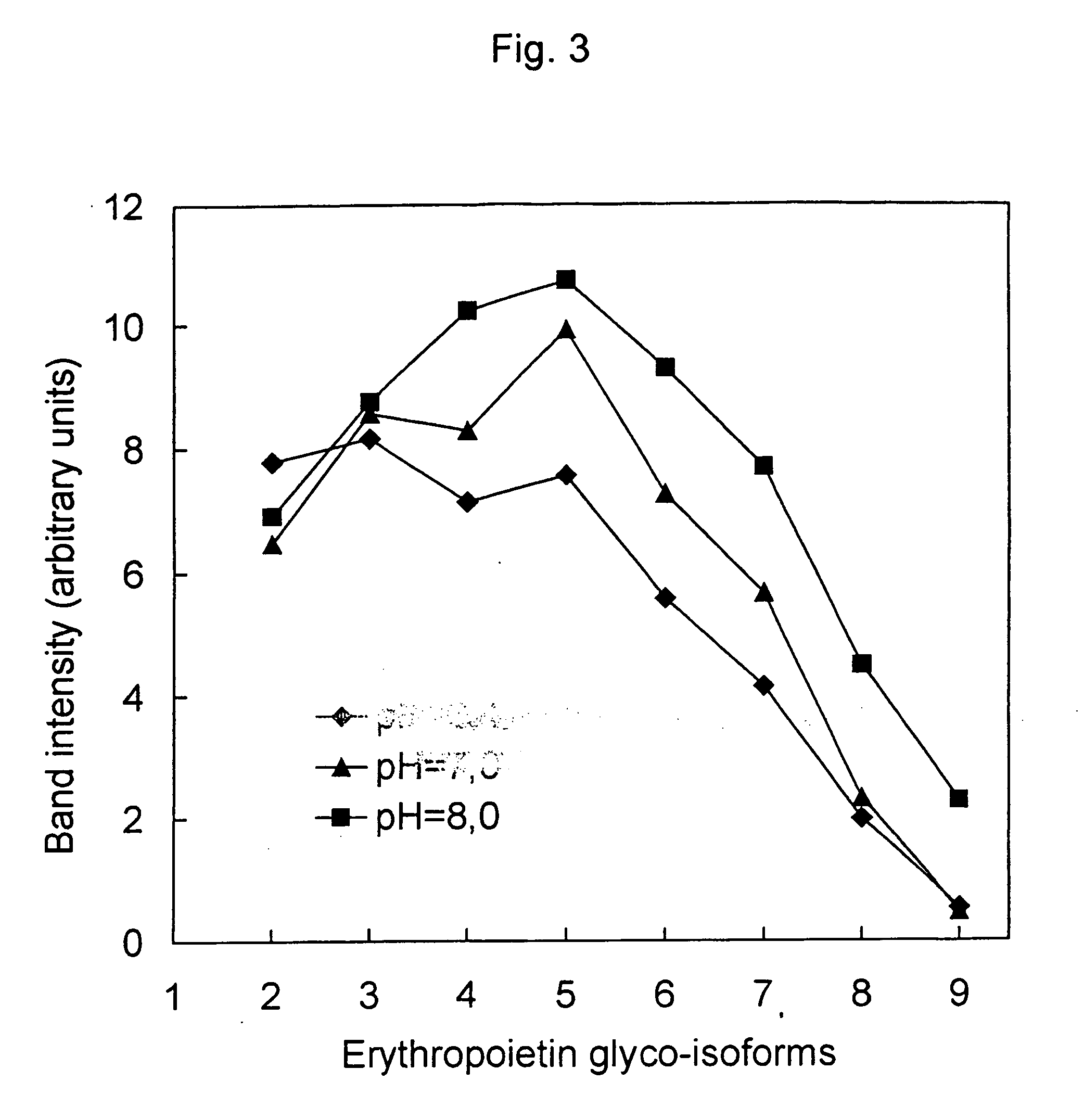
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
Method for preparing human immunoglobulin concentrates for therapeutic use
InactiveUS7186410B2Simple processHighly compatibleAntibacterial agentsSerum immunoglobulinsAnion-exchange chromatographyBlood plasma
The invention concerns a method for preparing human immunoglobulin concentrates for therapeutic use, from plasma or a plasma fraction. The method comprises pre-purification and a single anion-exchange chromatography carried out at alkaline pH, thereby enabling the immunoglobulins to be retained on the chromatographic support and fractionated. The method enables to obtain IgG, IgA and IgM concentrates.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
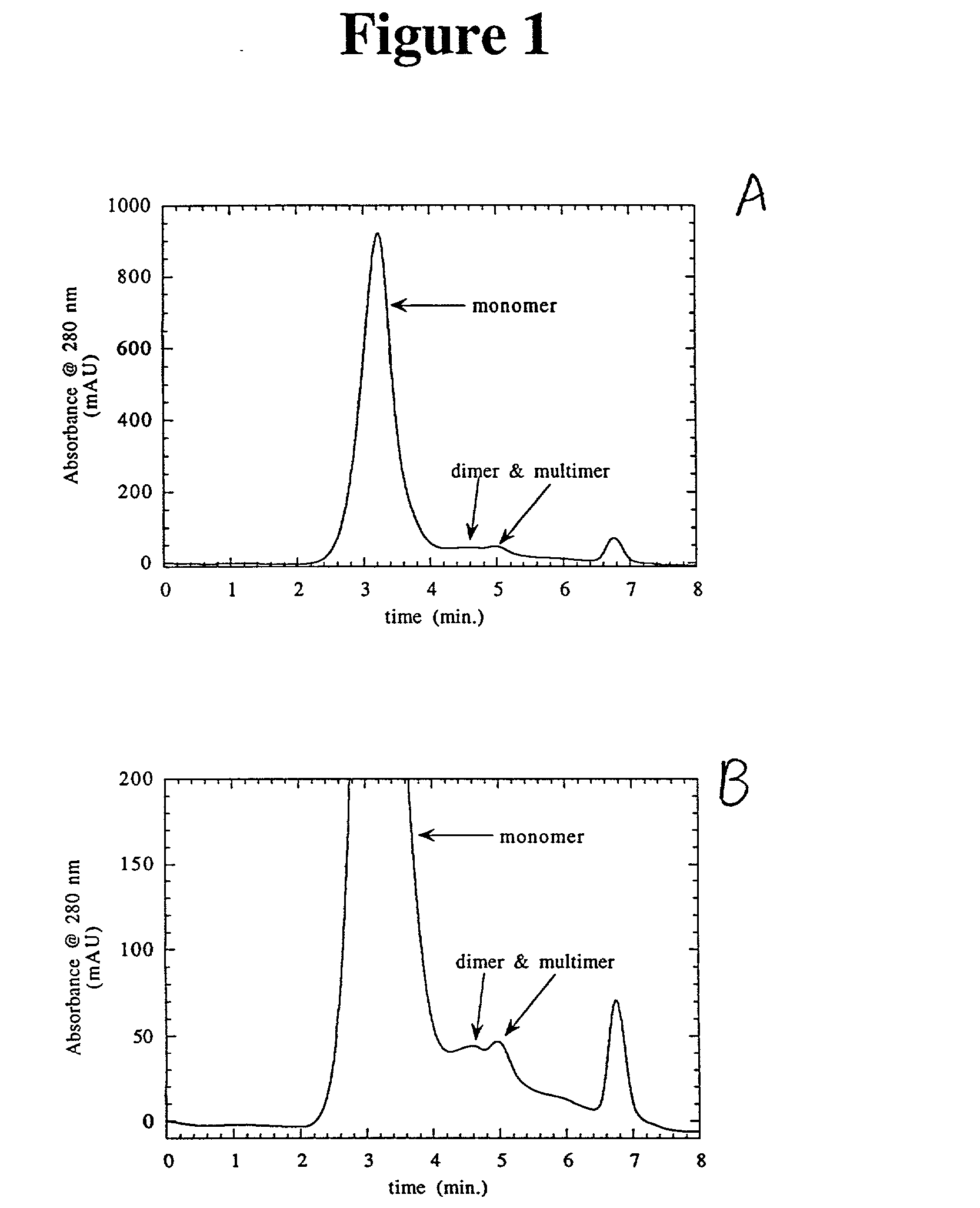
Separation of polypeptide monomers
InactiveUS20020010319A1Easy to separateInexpensiveComponent separationOther chemical processesAnion-exchange chromatographyAnion Exchange Proteins
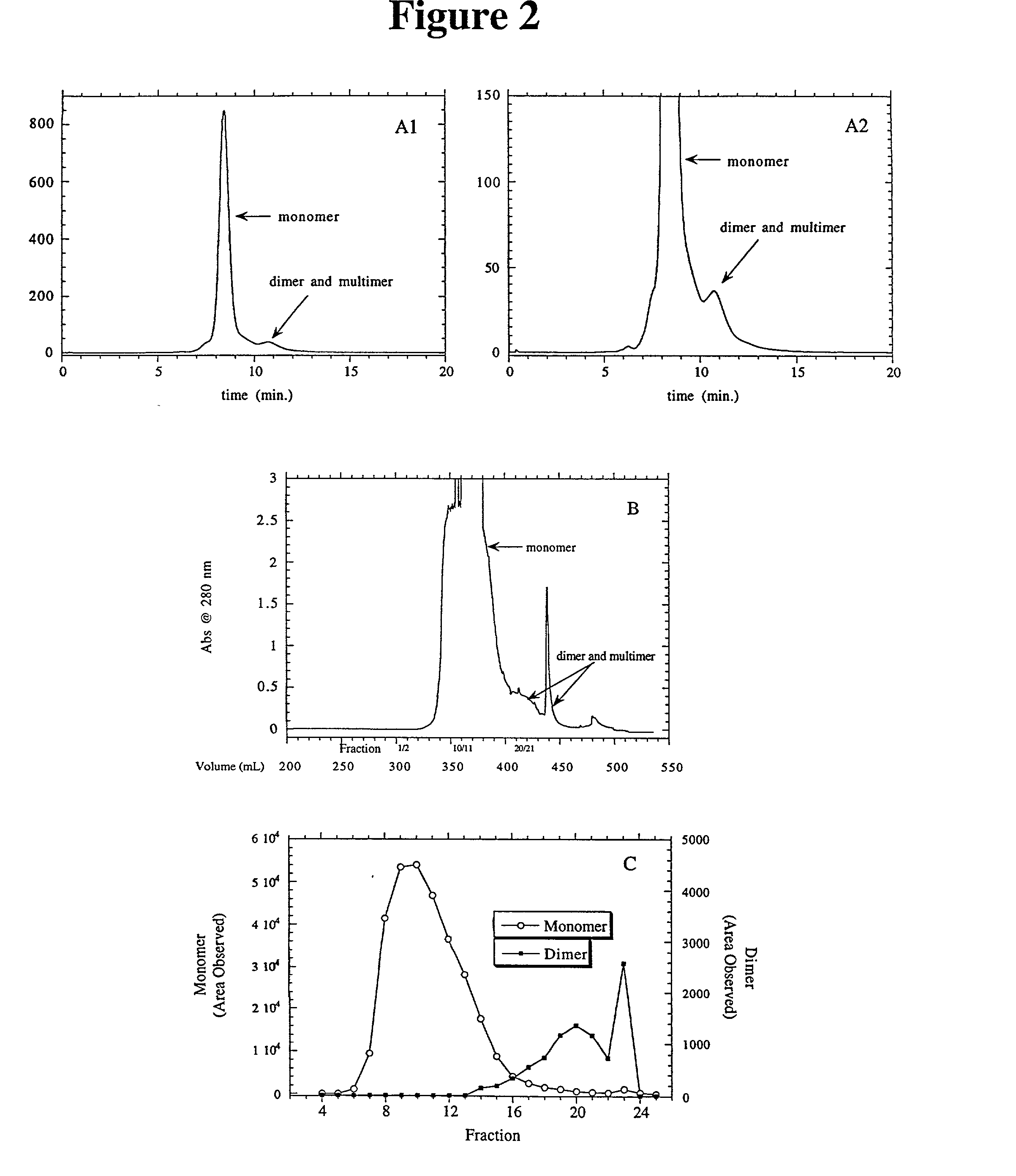
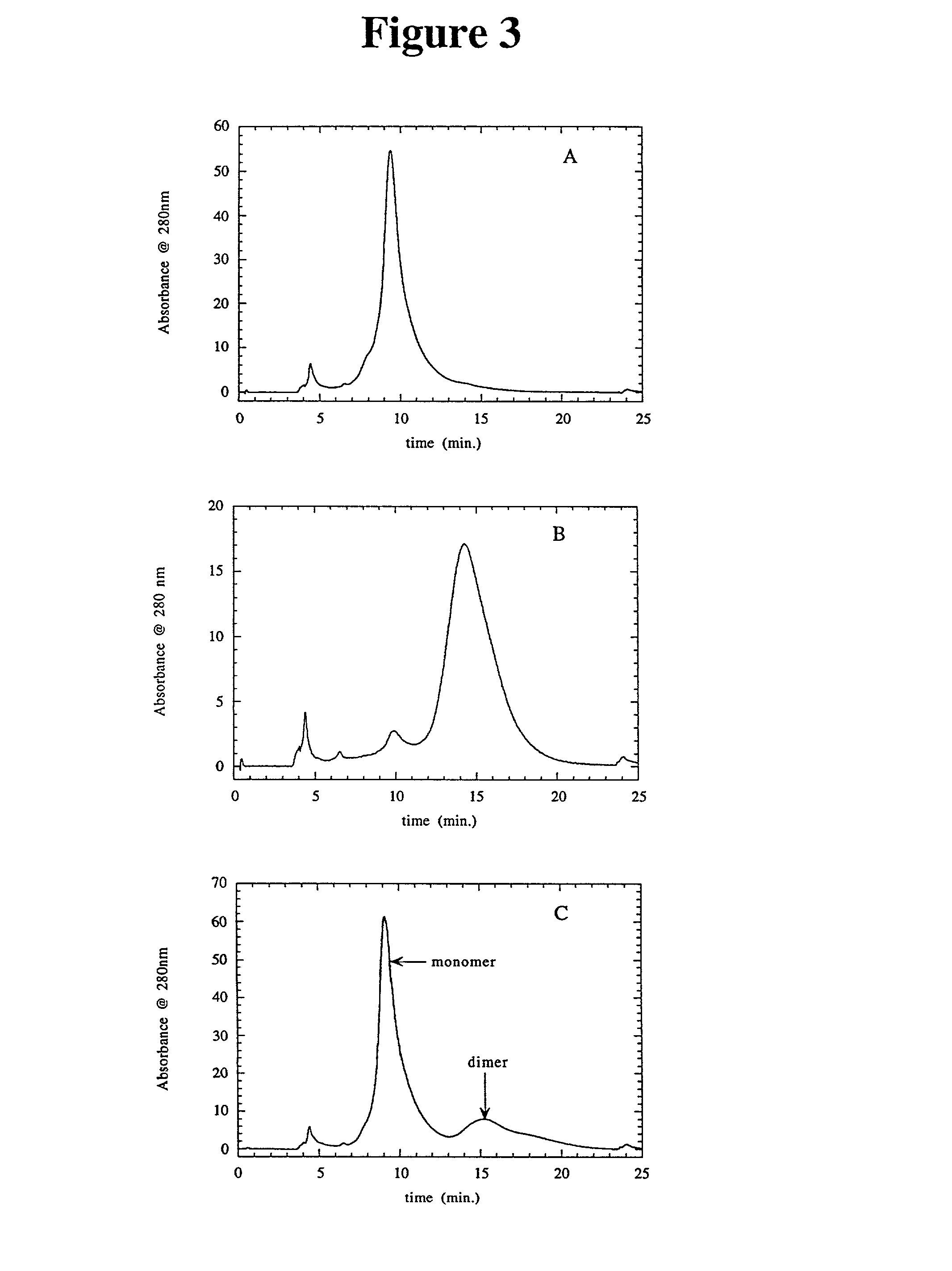
A method is disclosed for separating a polypeptide monomer from a mixture comprising dimers and / or multimers. The method comprises applying the mixture to either a cation-exchange chromatography resin or an anion-exchange chromatography resin and eluting the mixture at a gradient of about 0-1 M of an elution salt, wherein the monomer is separated from the dimers and / or multimers present in the mixture.
Owner:GENENTECH INC
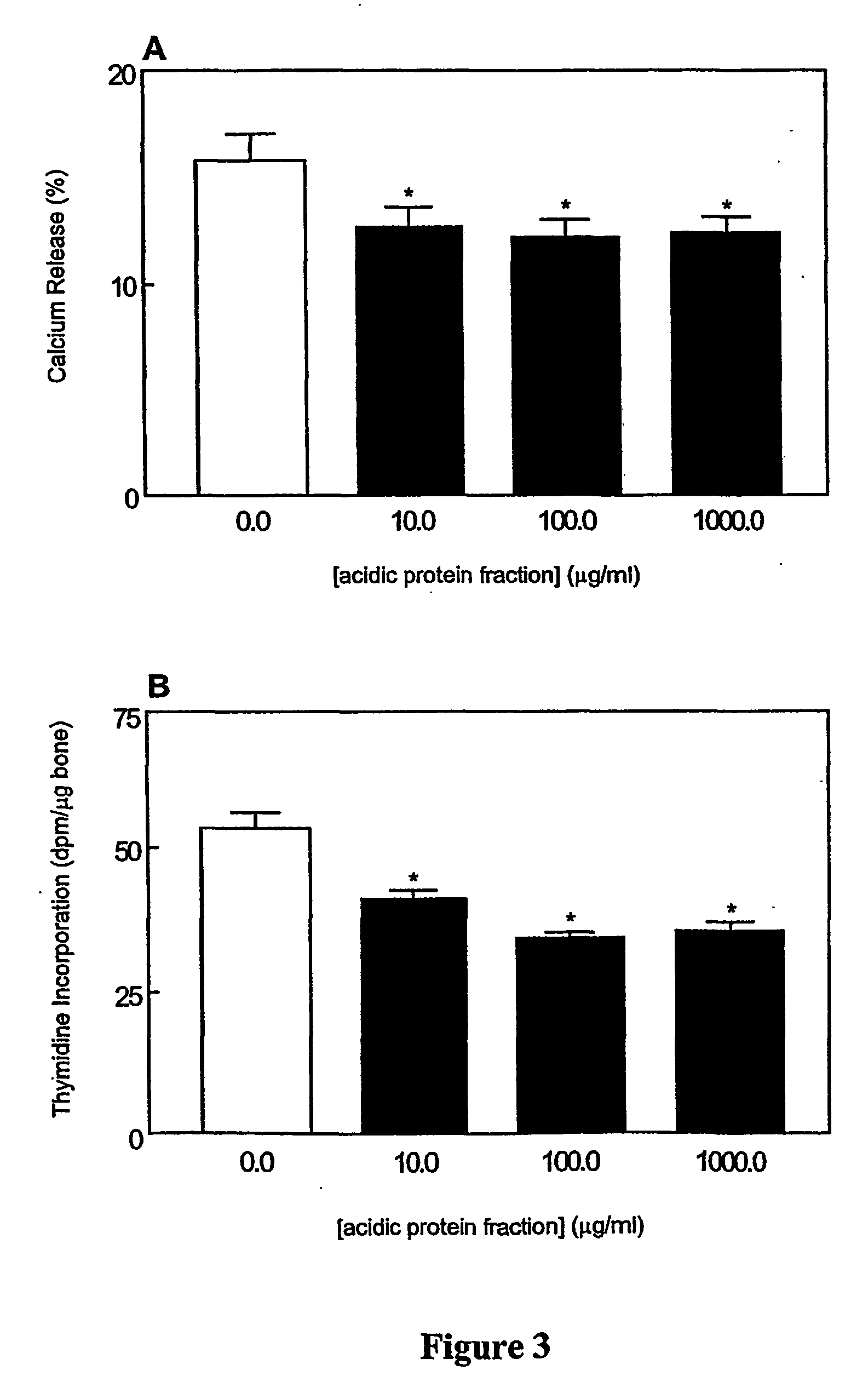
Bone health compositions derived from milk
InactiveUS20040052860A1Reducing net bone lossMetabolism disorderProtein composition from milkHydrolysatePhysiology
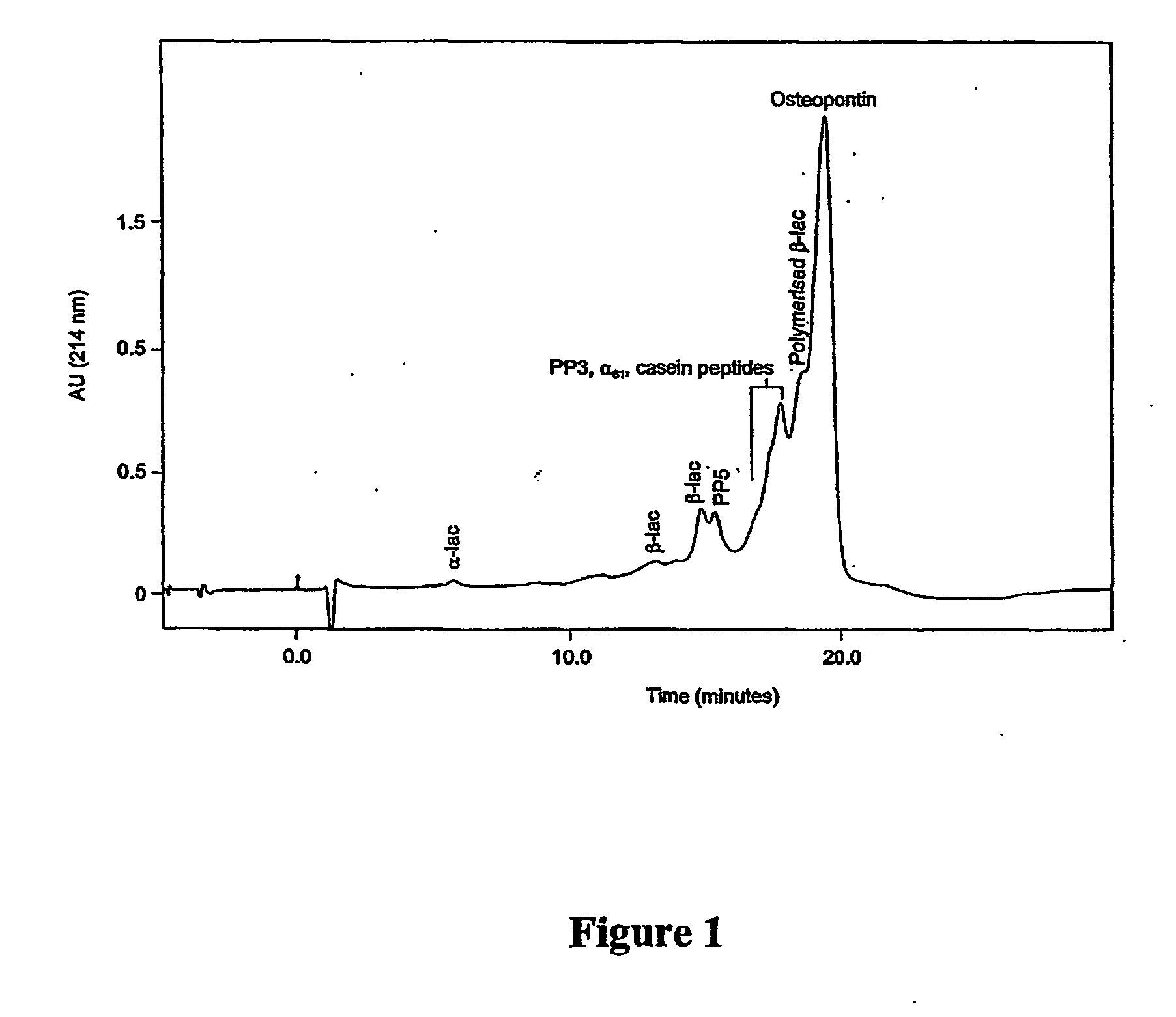
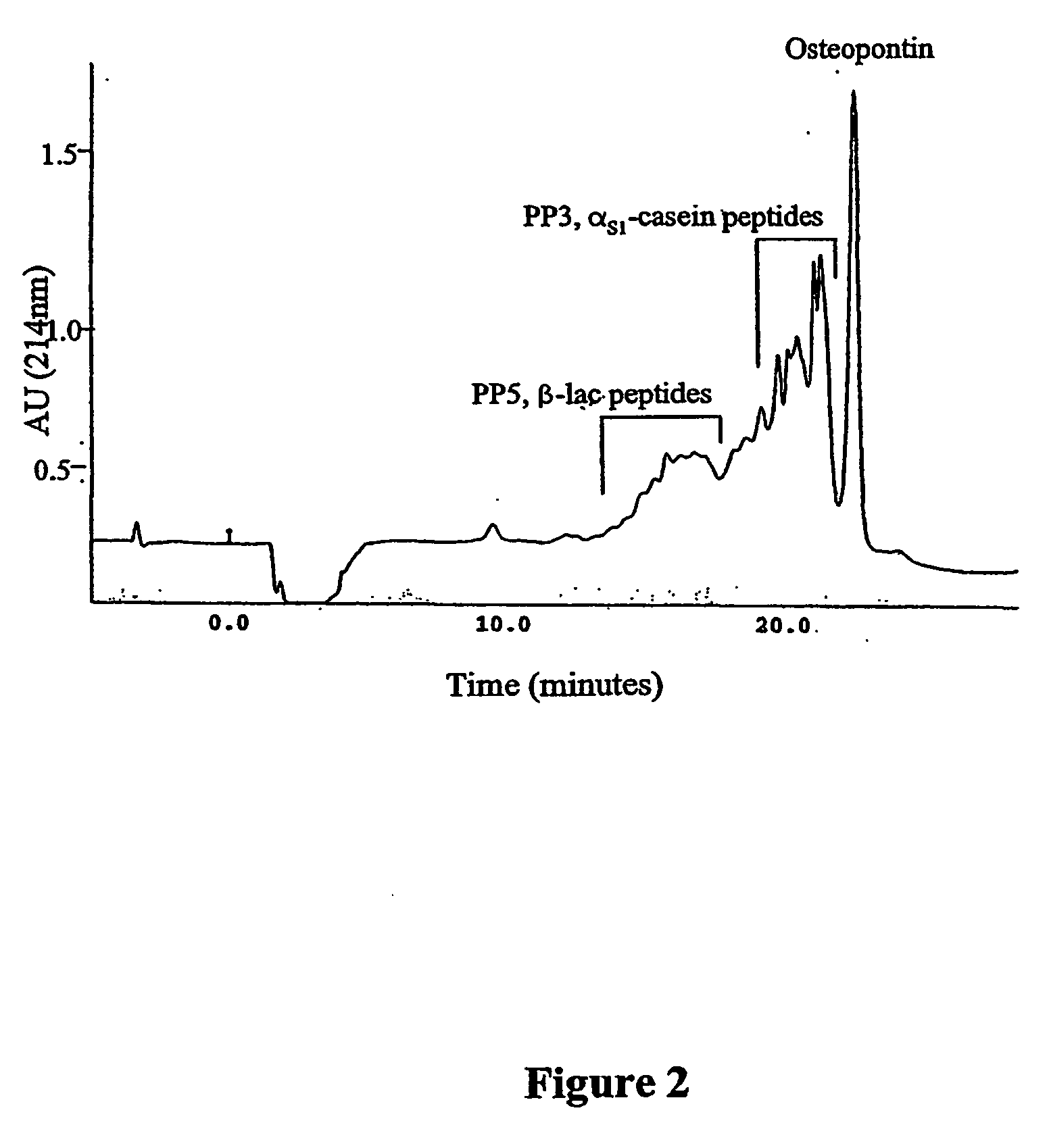
The invention relates to bone health compositions comprising an acidic protein fraction of milk, to a method of producing said bone health composition, to methods of treatment comprising said bone health compositions and to medicinal uses of said bone health compositions. One broad aspect of the invention provides a bone health composition comprising an acidic protein fraction derived from milk, from a component of milk, from whey, from hydrolysates thereof, or from a combination thereof, or from a combination thereof wherein the composition does not comprise caseinoglycomacropeptide (CGMP). Another broad aspect provides a method of manufacturing the composition of the invention using anion exchange chromatography.
Owner:NEW ZEALAND DAIRY BOARD
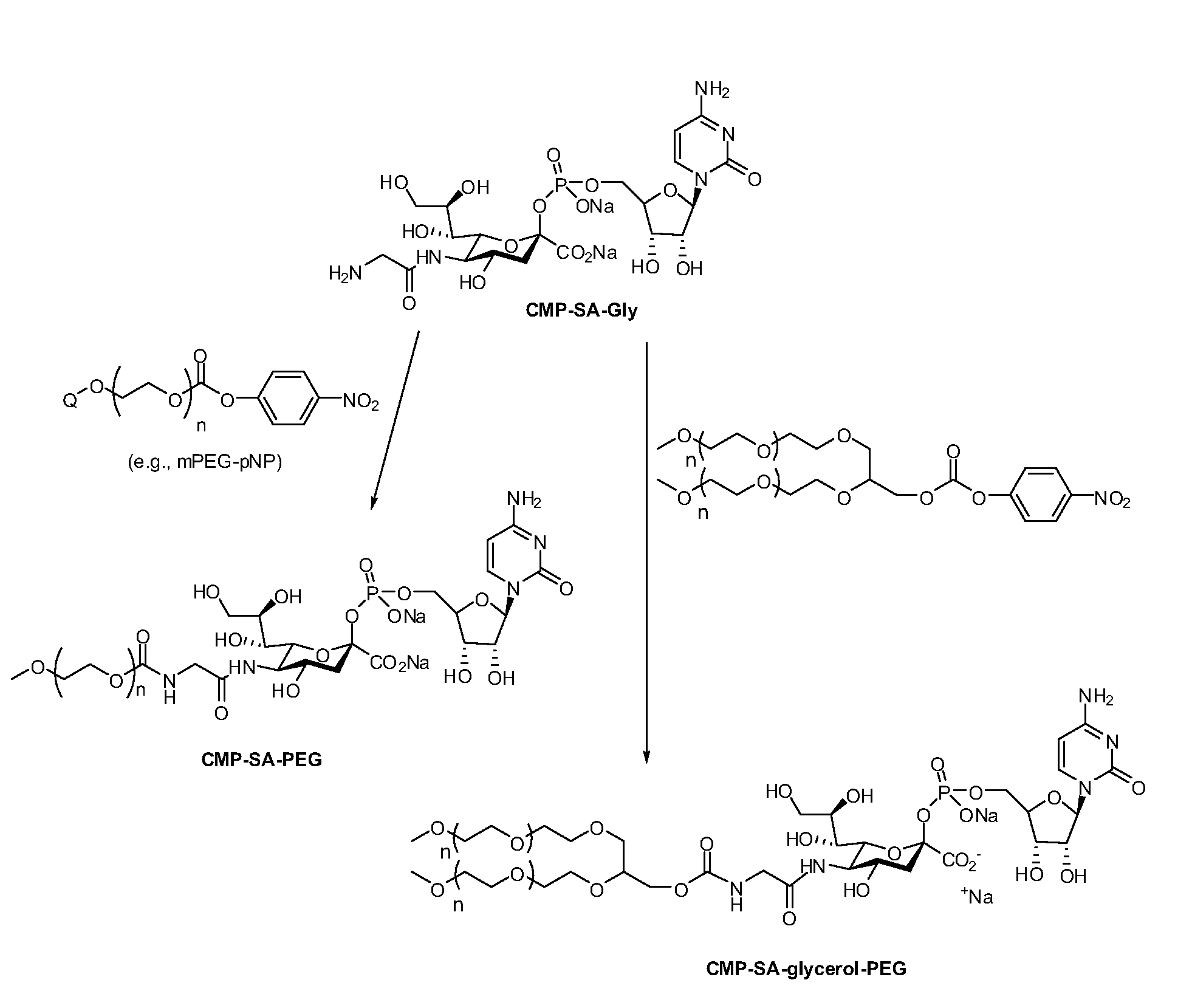
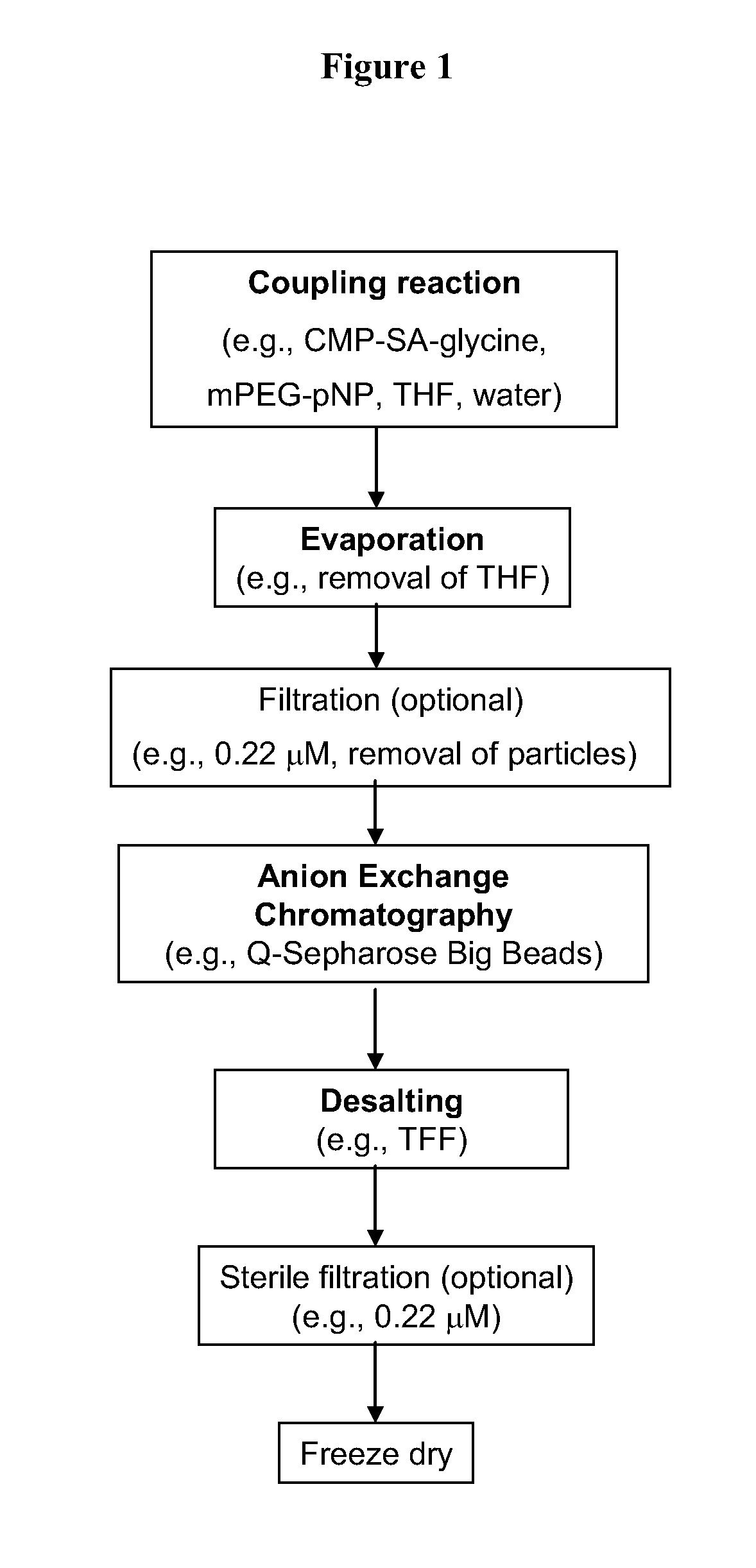
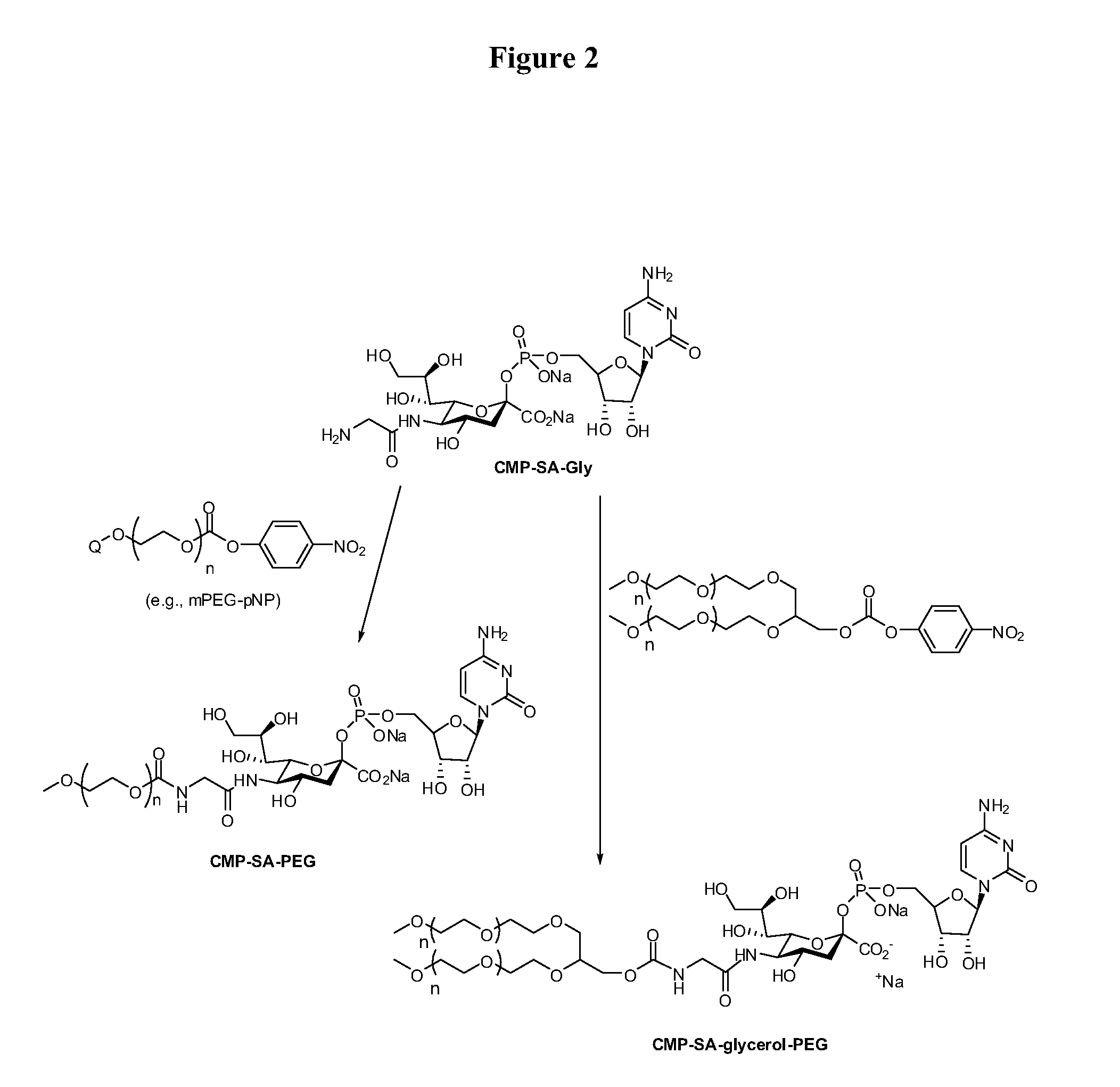
Process for the production of nucleotide sugars
InactiveUS20100174059A1Reduce formationHigh puritySugar derivativesSugar derivatives preparationFiltrationPolyethylene glycol
The current invention provides methods (e.g., large-scale processes) for the production of nucleotide sugars, which are modified with a polymeric modifying group, such as poly(alkylene oxide) moieties (e.g., poly(ethylene glycol) or poly(propylene glycol)) moieties. A typical process of the invention includes anion exchange chromatography followed by an ultrafiltration procedure, such as tangential flow filtration. The process of the invention provides modified nucleotide sugars in unexpectedly high purity and high overall yields.
Owner:RATIOPHARM GMBH
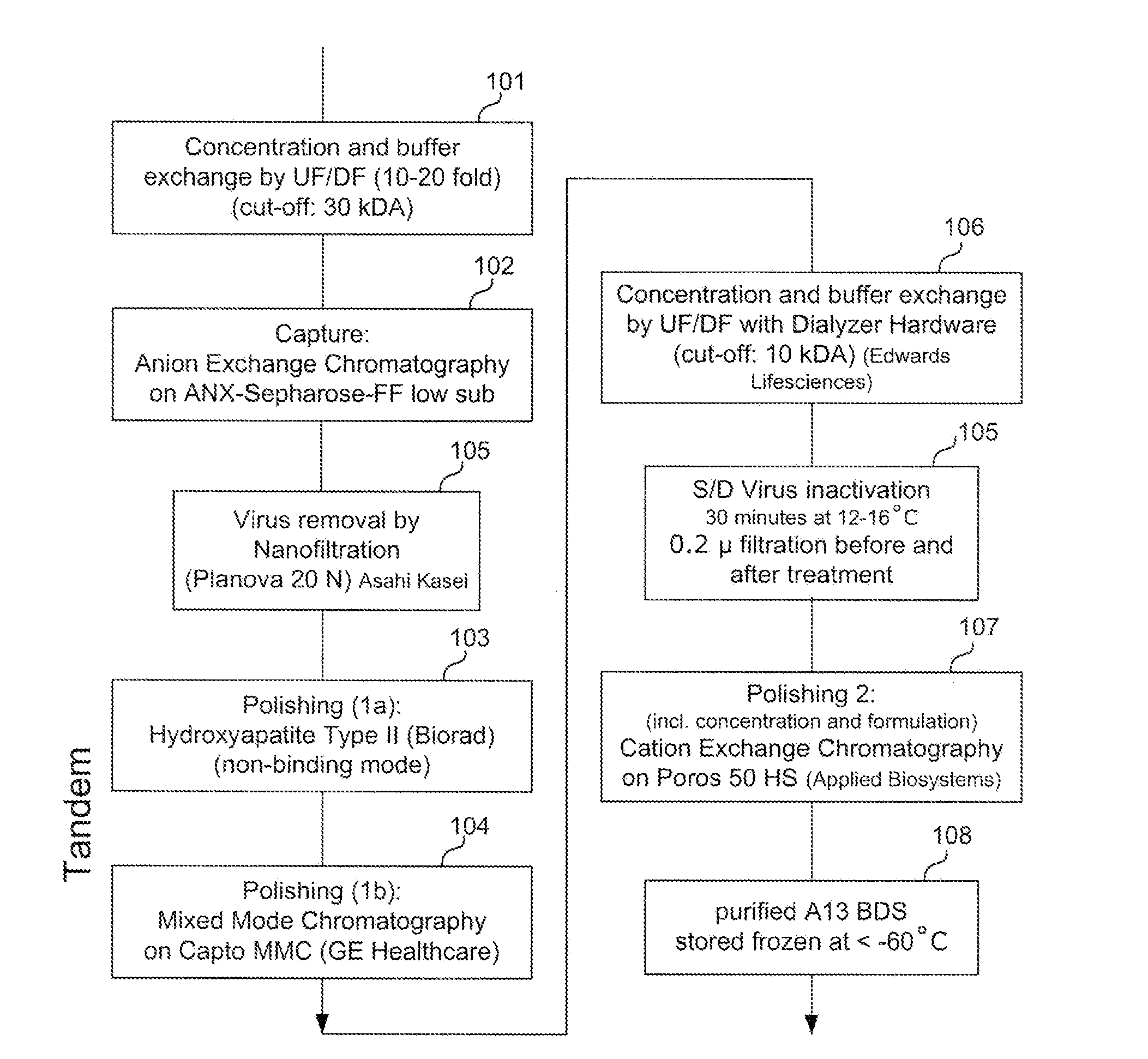
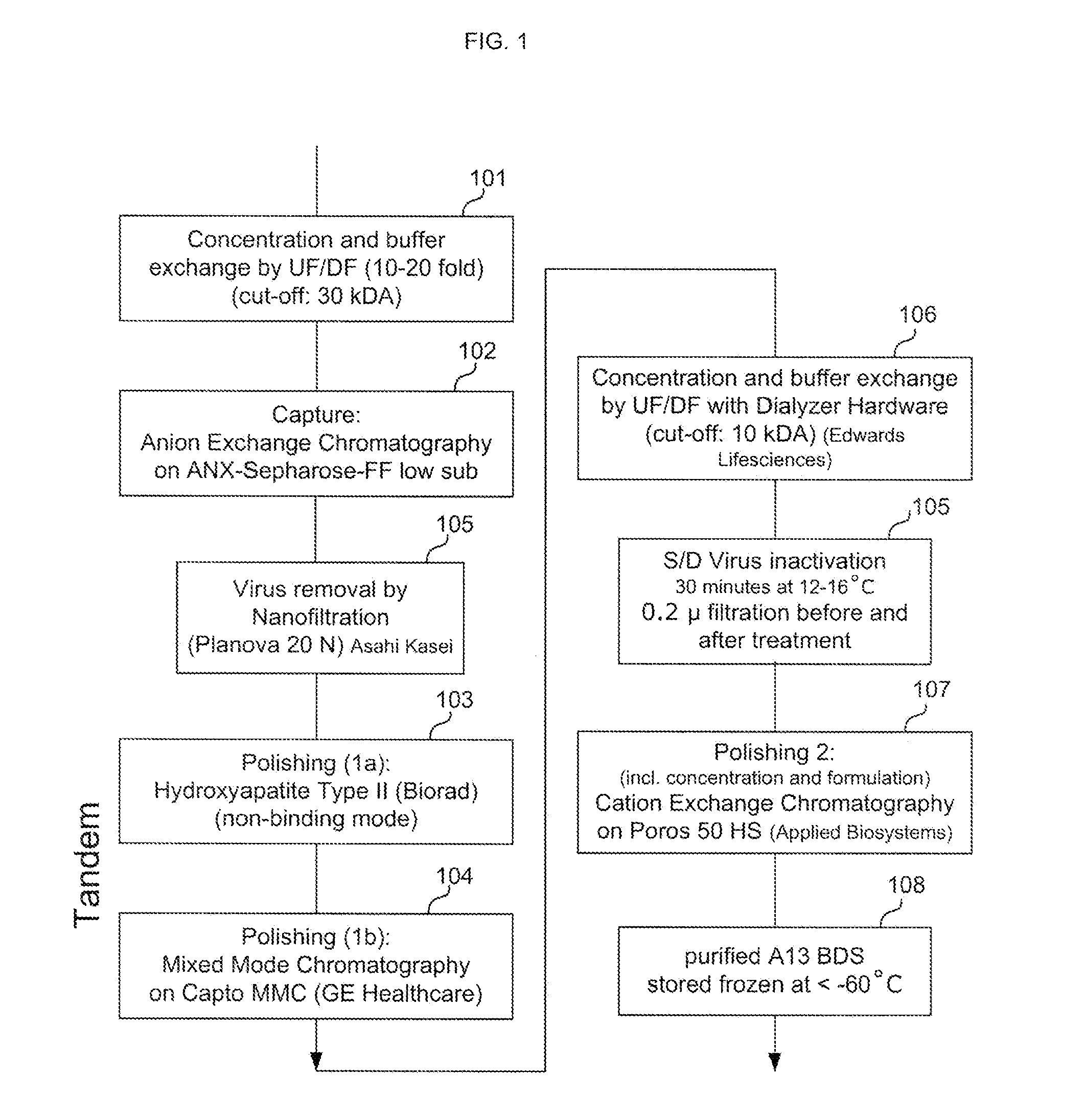
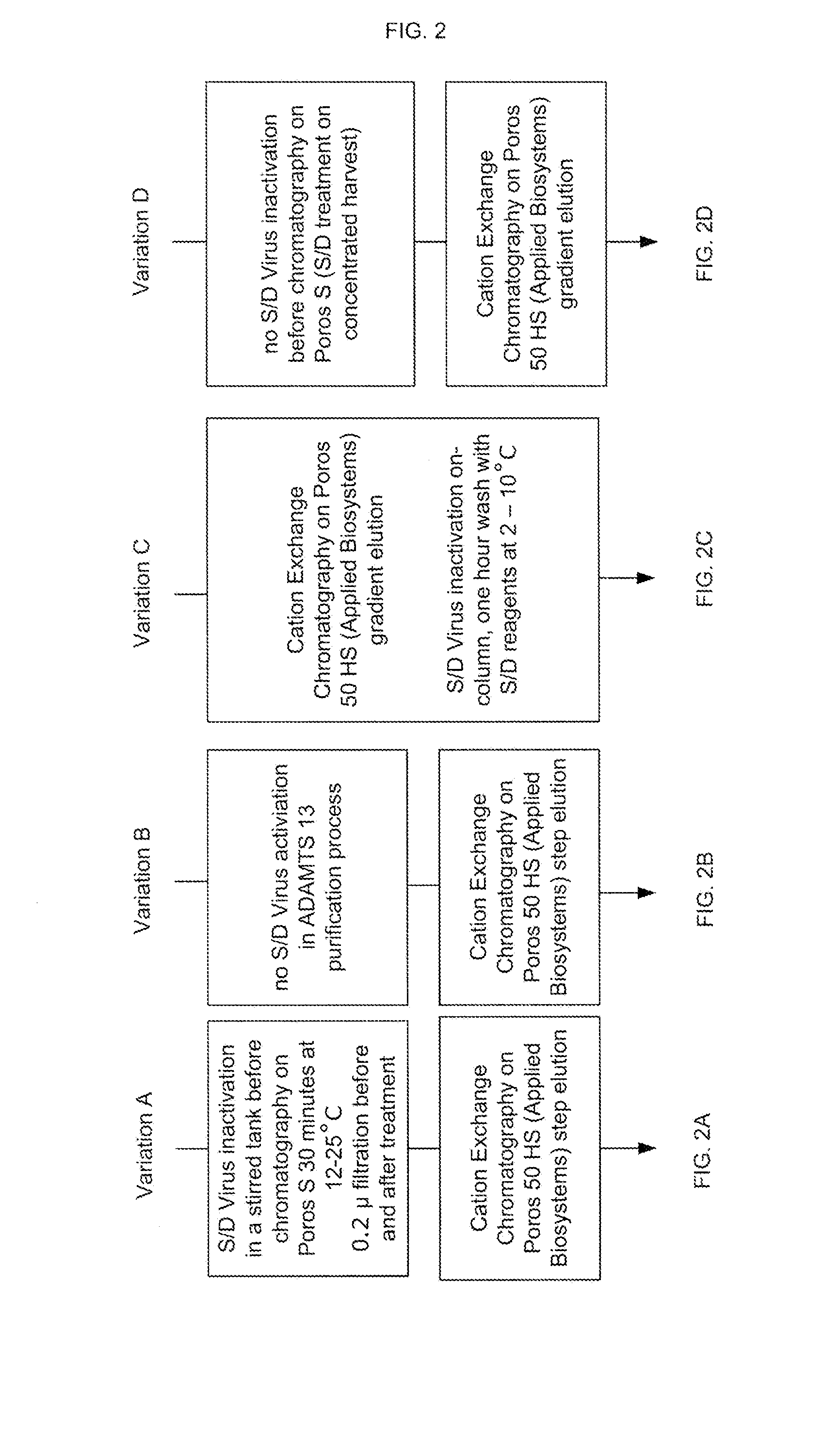
Methods of Purifying Recombinant Adamts13 and Other Proteins and Compositions Thereof
ActiveUS20110081700A1Reduce formationExtension of timeInactivation/attenuationBiomass after-treatmentHydroxylapatiteAnion-exchange chromatography
Provided herein are methods for purifying recombinant A Disintegrin-like and Metallopeptidase with Thrombospondin Type 1 Motif 13 (ADAMTS13) protein from a sample. The method comprises enriching for ADAMTS13 protein by chromatographically contacting the sample with hydroxyapatite under conditions that allow ADAMTS13 protein to appear in the eluate or supernatant from the hydroxylapatite. The methods may further comprise tandem chromatography with a mixed mode cation exchange / hydrophobic interaction resin that binds ADAMTS13 protein. Additional optional steps involve ultrafiltration / diafiltration, anion exchange chromatography, cation exchange chromatography, and viral inactivation. Also provided herein are methods for inactivating virus contaminants in protein samples, where the protein is immobilized on a support. Also provided herein are compositions of ADAMTS13 prepared according to said methods.
Owner:TAKEDA PHARMA CO LTD
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Production of organic and inorganic selenium compounds by lactic acid bacteria
InactiveUS20070077238A1Improve feed conversionEnhanced Glutathione Peroxidase (GPx) activityBiocideBacteriaHigh concentrationAnion-exchange chromatography
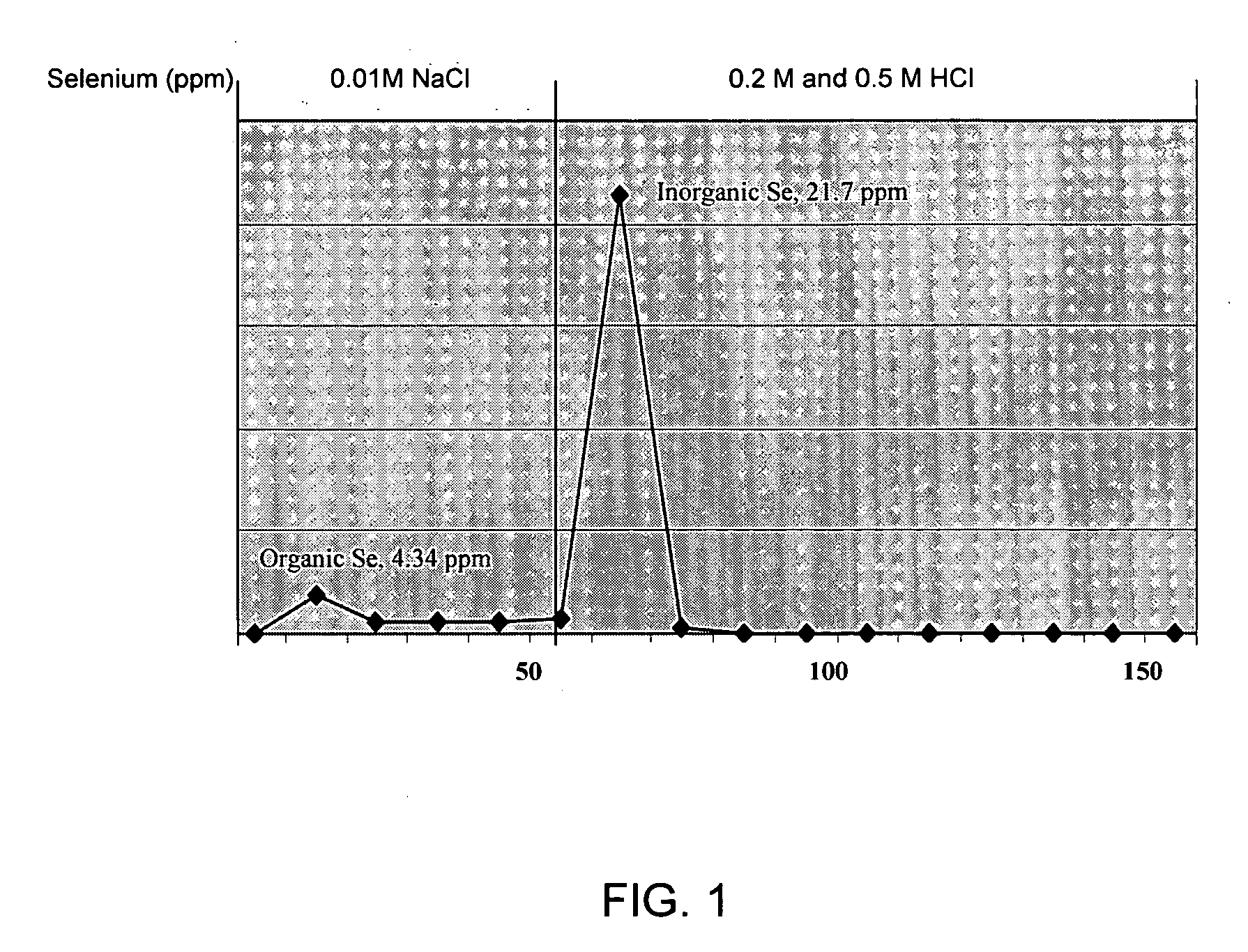
A novel strain of lactic acid bacteria was found to be heat resistant and able to grow in a sulfur-limiting medium (SLM) containing a high concentration of sodium selenite. The microorganism is a non-spore forming and Gram-positive coccus, which is identified with >90% confidence using the API biochemical and sugar fermentation tests, ribotyoing and 16S rRNA sequencing as Pediococcus pentosaceus SP80. In the current study, P. pentosaceus SP80 grown on SLM containing 250 ppm sodium selenite produced both organic and inorganic forms of selenium. These selenium compounds can be separated using an anion exchange chromatography technique. The concentrations of selenium detected in the organic and inorganic fractions were 4.34 and 21.7 ppm, respectively. Selenium-enriched bacteria are useful as a source of selenium for supplementing the diets of animals and humans. Animals fed efficacious amounts of the selenium-enriched bacteria show improved feed conversion rates and higher levels of glutathione peroxidase (GPx) activity in heart, kidney and liver tissues indicating an increased absorption and retention of selenium over control diets.
Owner:KEMIN IND INC
Purification of human monoclonal antibody and human polyclonal antibody
InactiveUS20060257972A1Serum immunoglobulinsImmunoglobulins against animals/humansAntiendomysial antibodiesAnion-exchange chromatography
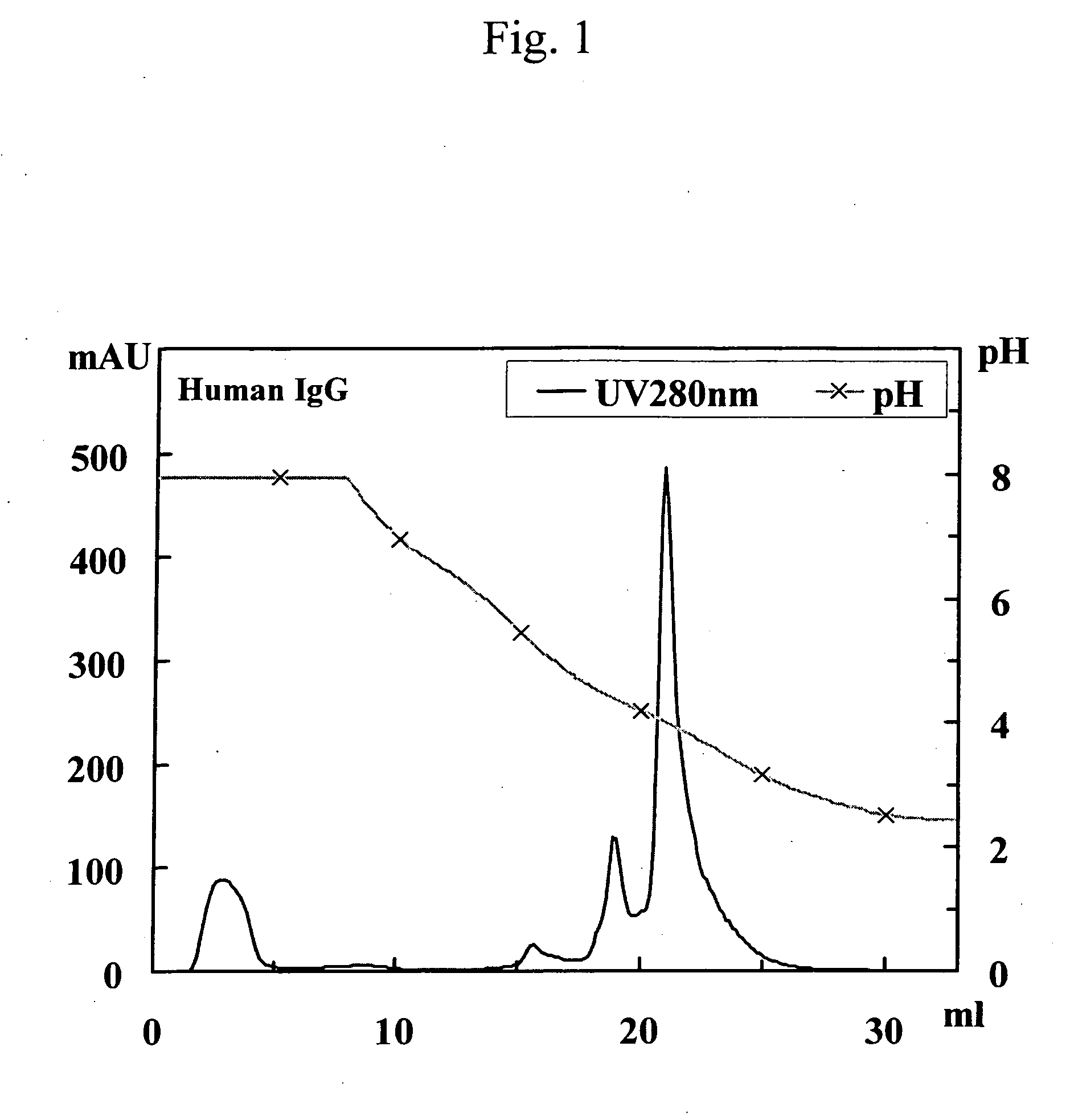
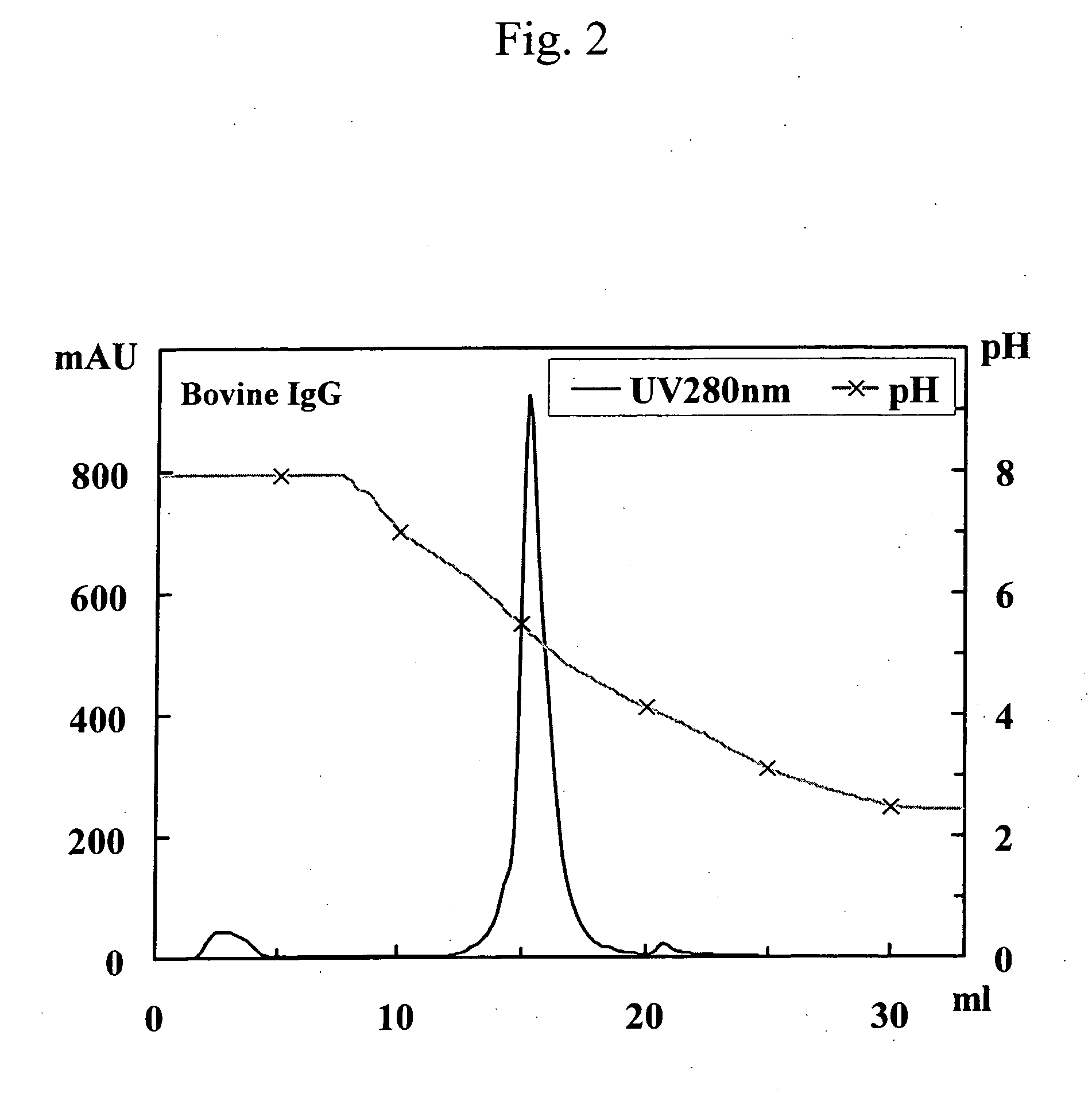
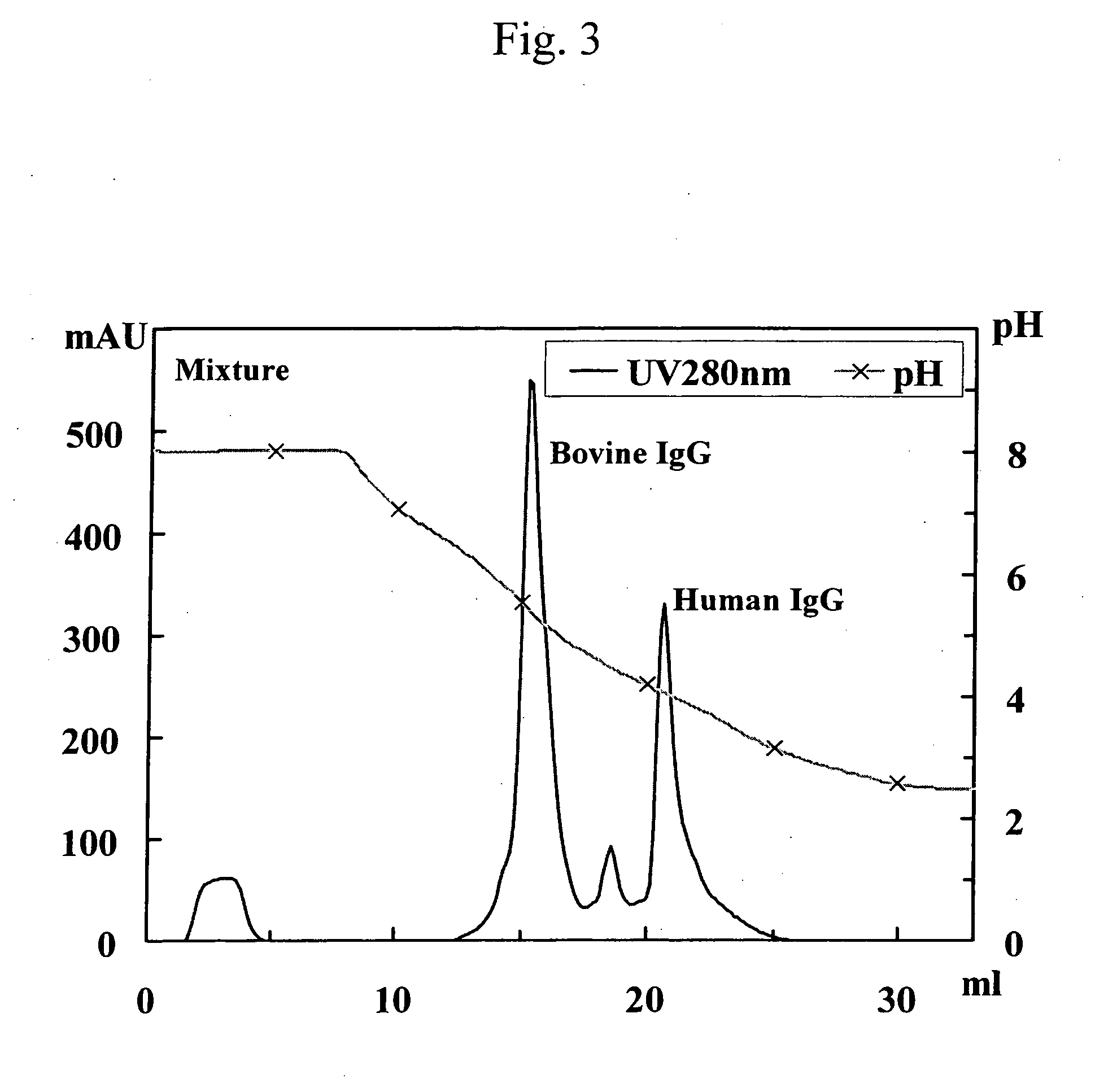
This invention provides a method for separating and purifying human monoclonal antibodies and human polyclonal antibodies from an antibody-containing mixture where such antibodies are primarily used for therapeutic applications and are occasionally used for diagnostic and reagent applications. This method comprises at least the following steps of: (1) purification via anion exchange chromatography; (2) purification via cation exchange chromatography following step (1); and separation of human antibodies from artiodactyl antibodies from a mixture that contains human antibodies and artiodactyl antibodies via protein A affinity chromatography that employs pH gradient elution.
Owner:KIRIN BREWERY CO LTD
Method for purifying anti-HER2 or/and anti-HER3 antibody proteins
ActiveCN102492040ASpeed up filteringReduce manufacturing costImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsVirus inactivationPurification methods
The invention belongs to the biochemistry technical field, concretely relates to a method for purifying anti-HER2 or / and anti-HER3 antibody proteins. The method of the invention comprises the following the steps: clarifying a broth, carrying out affinity chromatography, carrying out inactivation on virus by acidic pH value, carrying out cation exchange chromatography, carrying out anion exchange chromatography; and filtering virus; wherein, the step of virus inactivation by acidic pH value and the step of virus filtration can be inserted at any position of the step after the affinity chromatography. The method of the invention comprises the following advantages that: 1) the chromatography step can be reduced to three steps, thereby the production efficiency can be enhanced and the production cost can be minimized. 2) the step of virus filtration is added, thereby the security can be raised.
Owner:GENOR BIOPHARMA +1
Method of preparing alpha-1 proteinase inhibitor
InactiveUS20110237781A1High yieldHigh purityDepsipeptidesPeptide preparation methodsAnion-exchange chromatographyBlood plasma
Purification of α-1 proteinase inhibitor (α-1 PI) from solutions comprising α-1 PI is accomplished using hydrophobic interaction chromatography (HIC). In some embodiments, purification of α-1 PI is accomplished by precipitation of contaminating proteins from a starting solution comprising α-1 PI, such as human plasma, followed by anion exchange resin chromatography prior to HIC. Further purification may be accomplished by an optional cation exchange chromatography subsequent to anion exchange chromatography but prior to HIC. Some embodiments of the invention also include virus removal and / or inactivation by methods such as nano filtration and such as contact with a non-ionic detergent. The methods of the present invention result in greater yield, purity, and pathogenic clearance of plasma fractions than known methods.
Owner:GRIFOLS THERAPEUTICS INC
Intravenous injection of cytomegalovirus human immunoglobulin and its preparation method
ActiveCN102286099ASteps to reduce precipitationKeep aliveImmunoglobulins against virusesAntiviralsEthanol precipitationAnion-exchange chromatography
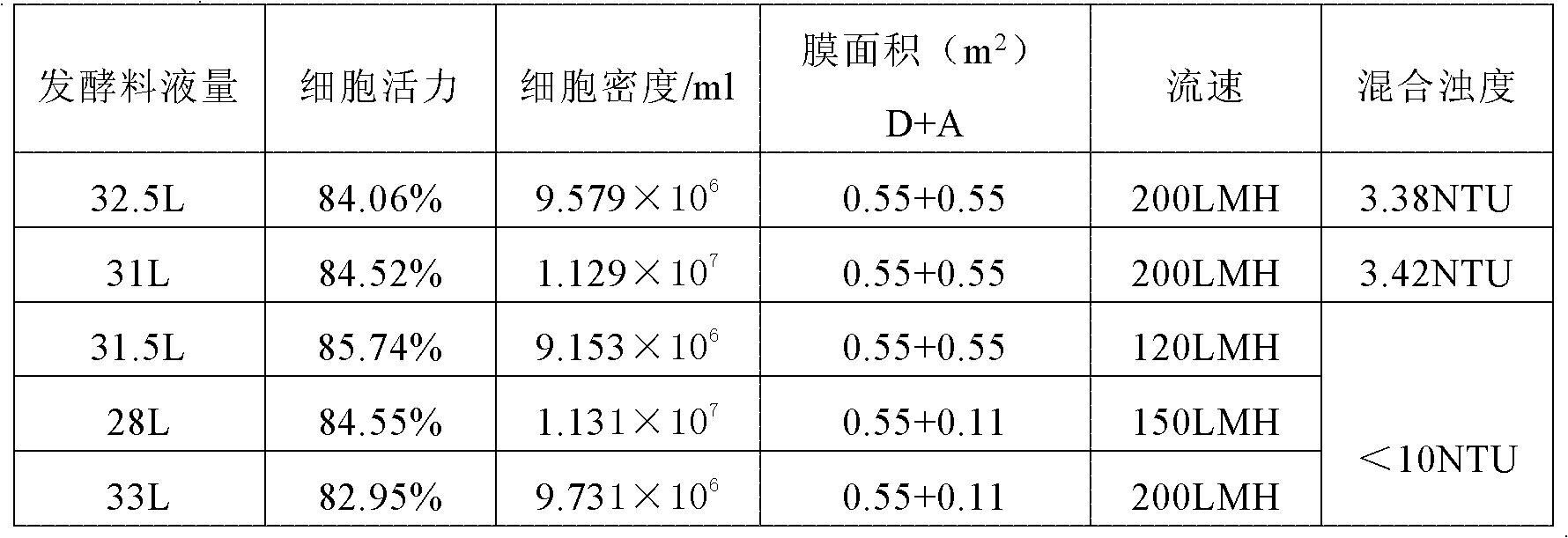
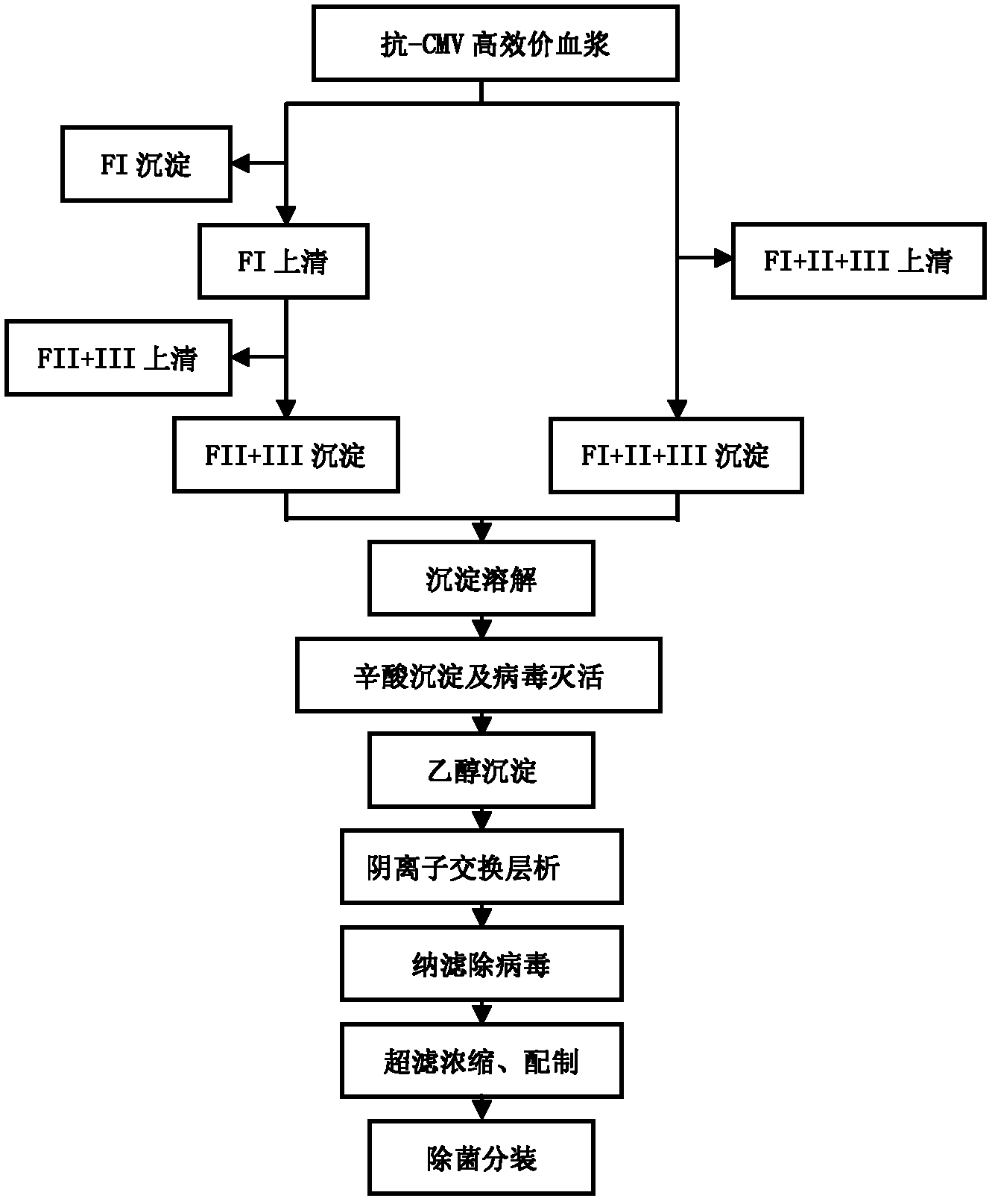
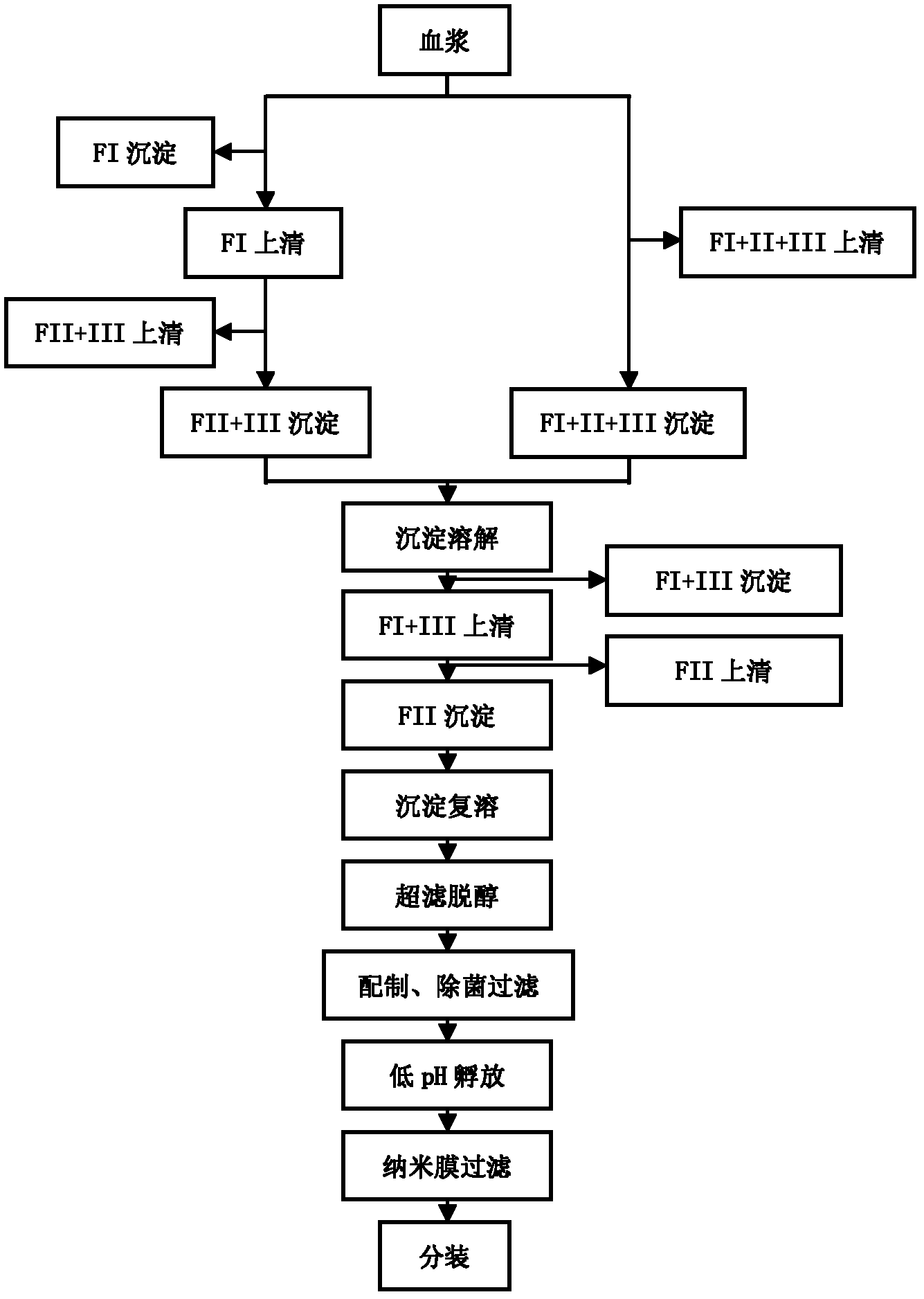
The invention discloses a human cytomegalovirus immunoglobulin for intravenous injection and a preparation method thereof, and aims to improve the purity, yield and safety of the product. In the invention, the specific activity of the human cytomegalovirus immunoglobulin for intravenous injection is not less than 2.5 PEI-U / mg, the anti-CMV titer is not less than 100 PEI-U / ml, the purity is greater than 98.2%, and the protein content is 51-55 mg / ml. Caprylic acid precipitation and anion exchange chromatography are used instead of the partial ethanol precipitation step in the traditional low-temperature ethanol method, thereby keeping IgG in the supernate all the time so as to keep the IgG activity; and processes of caprylic acid virus inactivation and nano film virus removal are used, thereby effectively ensuring the safety of the product. Researches show that the preparation method disclosed by the invention improves the purity, yield and safety of the product, saves the energy and reduces the production cost.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
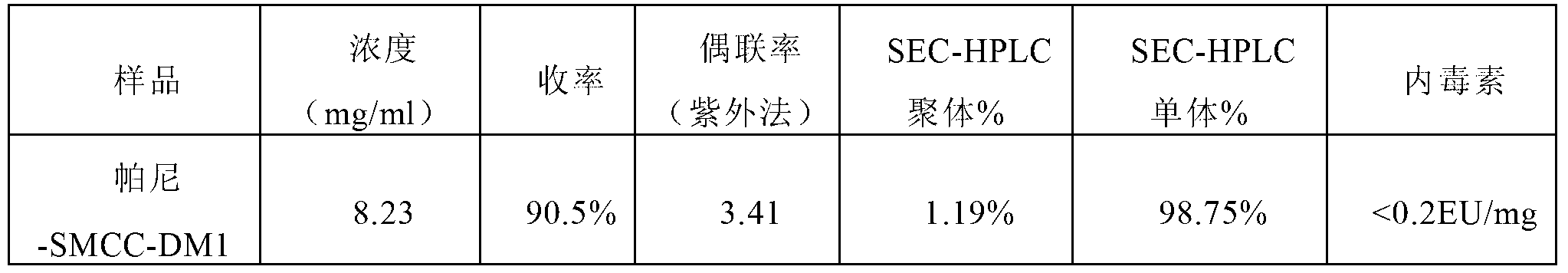
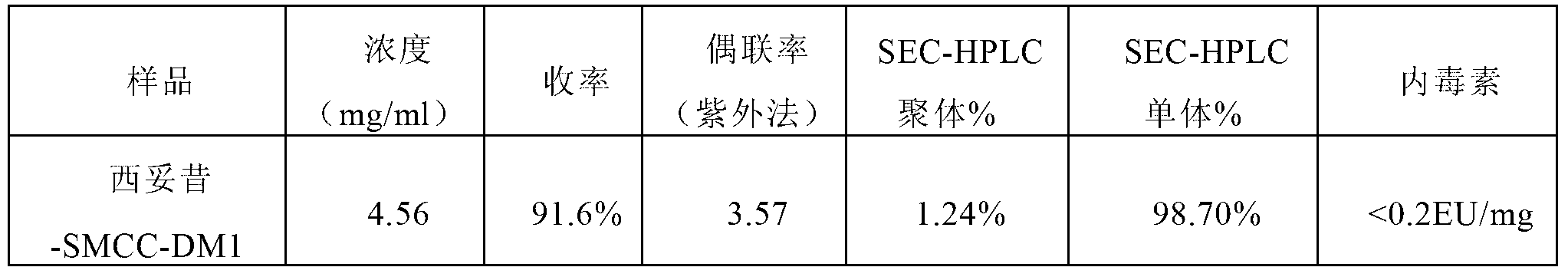
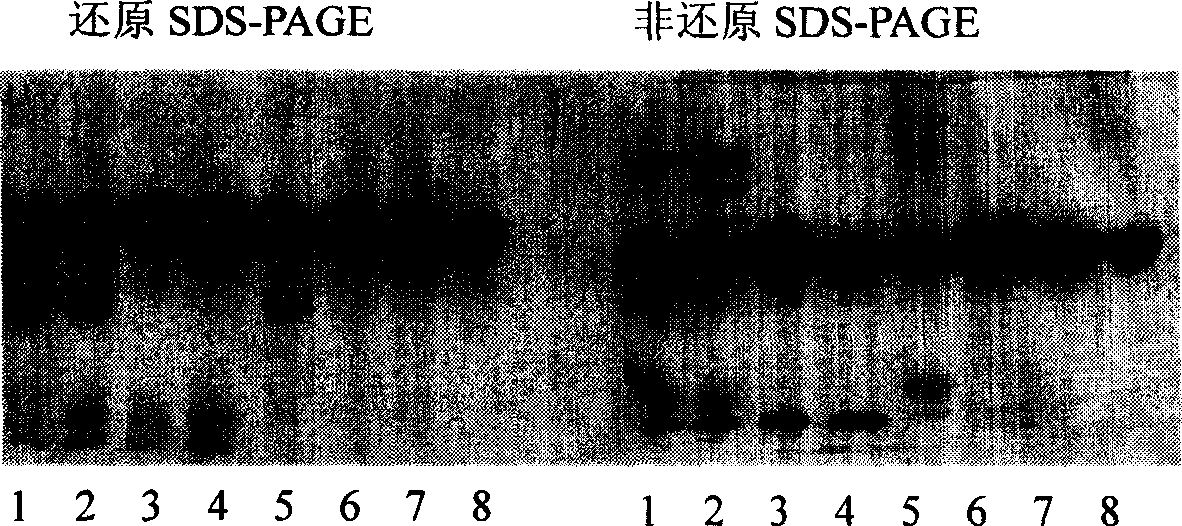
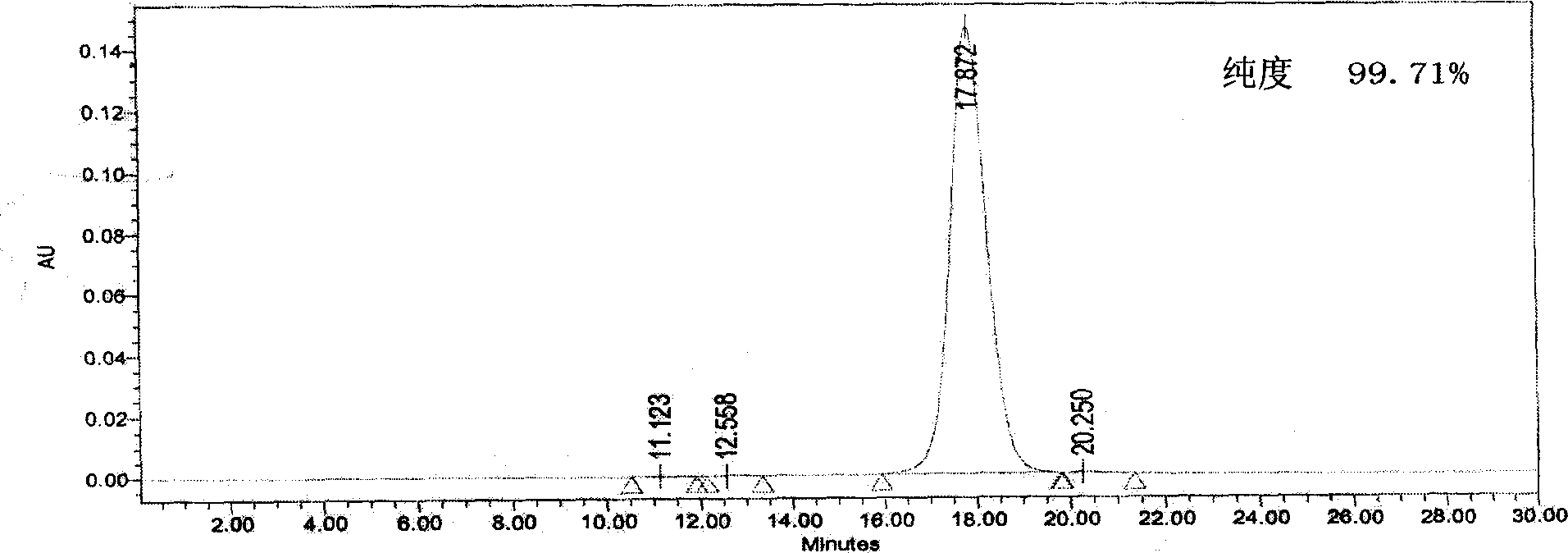
Method for preparing antibody-maytansine alkaloid medicine conjugate
ActiveCN103254311AReduce hidden dangersImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsAnion-exchange chromatographyAlkaloid
The invention relates to a method for preparing antibody-maytansine alkaloid medicine conjugate. The method comprises the following steps of replacing the antibody into a reaction buffer solution; dissolving a dual-function bridging agent-maytansine alkaloid with an organic solvent so as to prepare the mother liquor of maytansine alkaloid medicine; mixing the replaced antibody with the mother liquor of maytansine alkaloid medicine for coupled reaction for 1-4 hours at 20-30 DEG C; and carrying out anion exchange chromatography on the reaction liquid, conducting Sephadex TMG25 desalination chromatographic column purification on collected flow liquid, and collecting a first peak sample as the prepared antibody-maytansine alkaloid medicine conjugate. The prepared antibody-maytansine alkaloid medicine conjugate is proper in coupling rate, high in purity and low in endotoxin content.
Owner:QILU PHARMA CO LTD
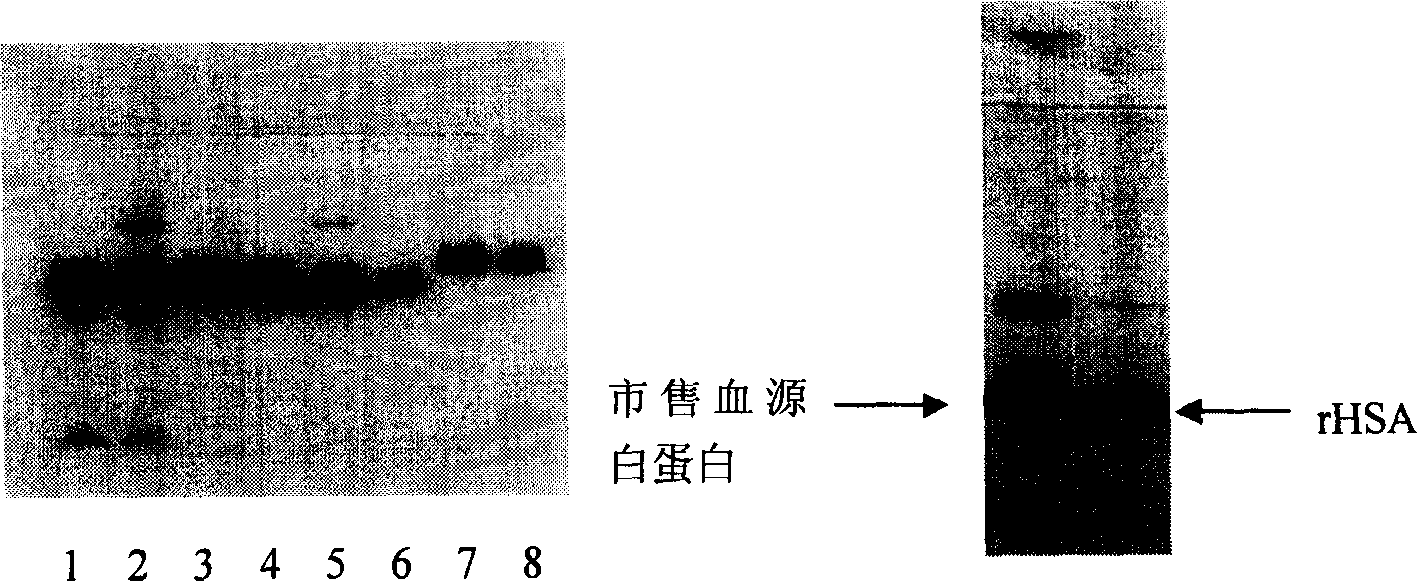
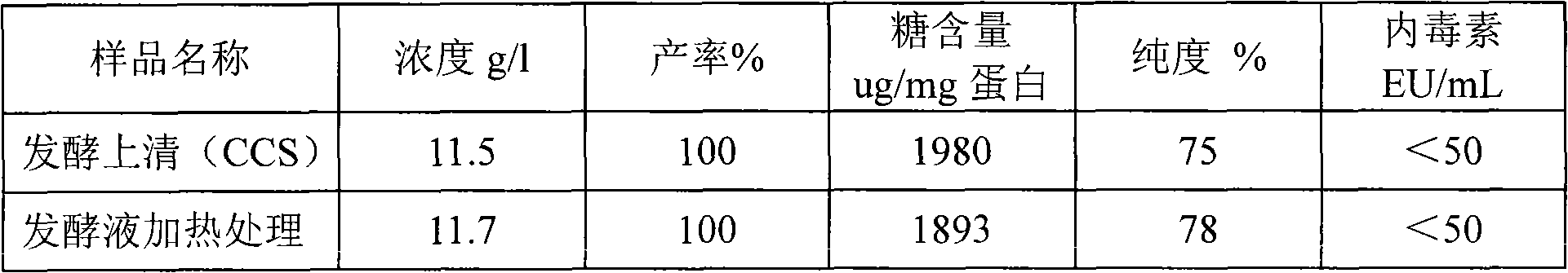
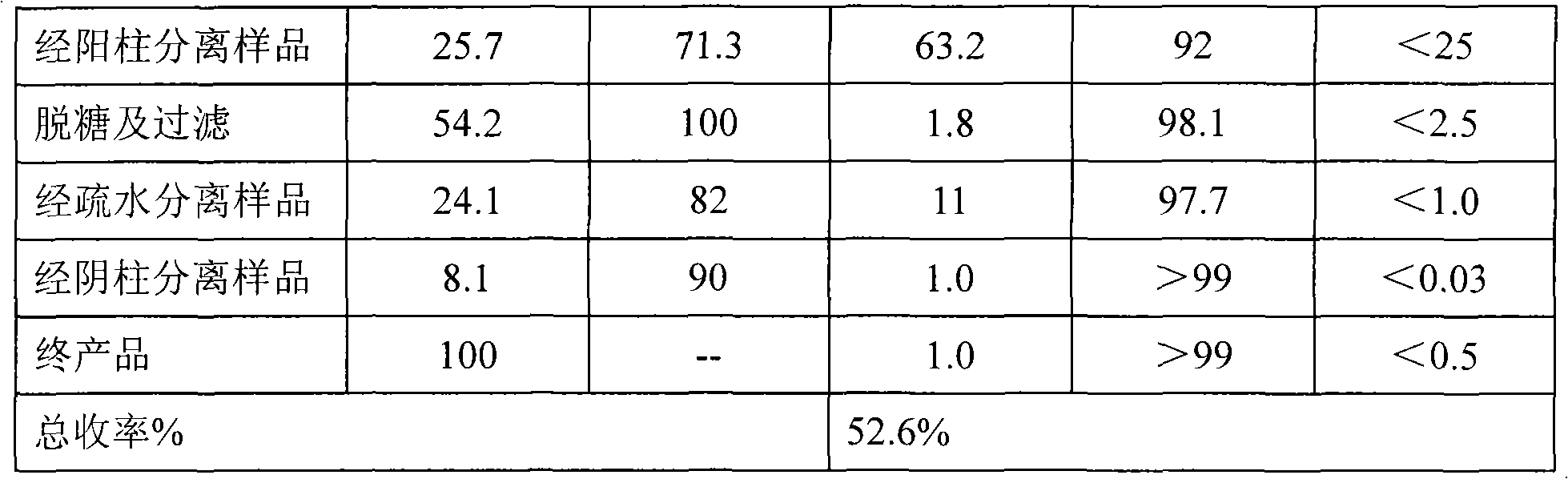
Purification of rHSA
ActiveCN1854155AGuaranteed stabilityEfficient removalAlbumin peptidesPeptide preparation methodsAnion Exchange ProteinsHeat treating
Purification of rHSA is carried out by heat treating for fermented supernatant containing rHSA at 60-75degree under existence of stabilizer, decolorant and proteinase inhibitor, adjusting solution pH4.0-5.5, hypersaline cation exchange chromatographying for fermented supernatant containing rHSA, hydrophobic exchange chromatographying and weak anion exchange chromatographying to obtain purified rHSA and borate treating. It has short experimental period and higher purity.
Owner:NCPC NEW DRUG RES & DEV
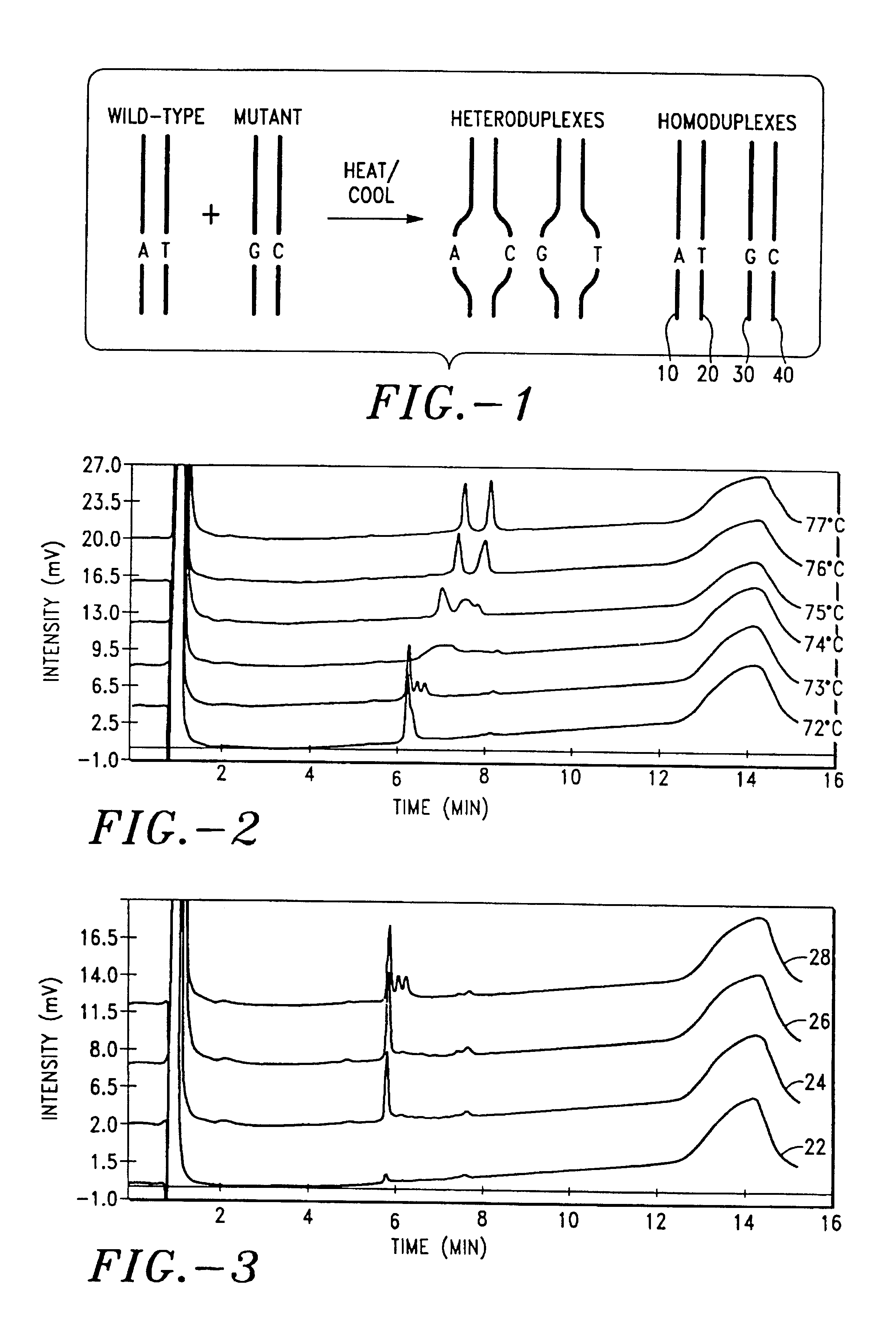
Detection of nucleic acid heteroduplex molecules by anion-exchange chromatography
InactiveUS6969587B2Easy to useGuaranteed uptimeImmobilised enzymesBacteriaOrganic solventAnion-exchange chromatography
The present invention describes a method for separating or partially separating heteroduplex and homoduplex DNA molecules in a mixture. In the method, the mixture is applied to an anion-exchange chromatography medium. The heteroduplex and homoduplex molecules are eluted with a mobile phase containing an eluting salt, including an anion and a cation, a buffer, and preferably including an organic solvent. The eluting is carried out under conditions effective to at least partially denature the heteroduplexes (e.g., thermal or chemical denaturing) resulting in the separation of the heteroduplexes from the homoduplexes. The method has many applications including, but not limited to, detecting mutations and comparative DNA sequencing.
Owner:ADS BIOTEC INC
Method for purifying recombinant human serum albumin protein and application thereof
The present invention relates to a method for purifying recombinant human serum albumin (rHSA) protein. The method comprises the following steps: fermented liquid containing rHSA is processed by a ceramic membrane, supernatant liquid is orderly purified by high salt cation exchange chromatography, hydrophobic layer exchange chromatography and weak anion exchange chromatography, and purified rHSA is obtained. The present invention is characterized in that solution processed by high salt cation exchange chromatography is processed by borate and then filtered by hollow fibers. The rHSA obtained can be used for producing vaccines for humans against viruses with a cell culture method, particularly rabies vaccines.
Owner:NCPC NEW DRUG RES & DEV
Method for co-producing biodiesel, phytosterol and tocopherol by using grease deodorized distillate
The invention discloses a method for co-producing biodiesel, phytosterol and tocopherol by using grease deodorized distillate. Aiming at the characteristics of high acid value and complex components of the grease deodorized distillate, fatty acid and glyceride in the deodorized distillate are reacted with methanol to generate fatty acid methyl ester (biodiesel) by adopting p-toluenesulphonic acid as a catalyst. The method comprises the following steps of: adding a small amount of sterol powder serving as crystal seeds into a reaction mixture after methyl esterification reaction, then promoting the sterol in the reaction system to form crystals through a temperature program consisting of three steps in turn, and primarily separating the sterol by methods of centrifuging, filtering and the like; and separating fatty acid methyl ester (light phase) from the reaction mixture after the primary separation of the sterol by adopting primary molecular distillation, removing triglyceride, diglyceride and glycerol (heavy phase) from the reclaimed heavy phase through molecular distillation again, performing sterol crystallization and separation on the reclaimed light phase again, and reclaiming the tocopherol from the mixture after the sterol is re-separated through anion exchange chromatography. The method has the characteristics of high extraction efficiency, mild conditions and the like.
Owner:JIANGNAN UNIV
Triple-helical Tremellan, preparation method and application thereof
InactiveCN102336840AHigh viscosityStrong ability to scavenge hydroxyl radicalsOrganic active ingredientsAntinoxious agentsFiltrationFreeze-drying
The invention discloses triple-helical Tremellan, a preparation method and application thereof. The preparation method comprises the following steps of: extracting Tremellan components through water extraction and alcohol precipitation, removing protein by using an enzyme-Sevage combined method, performing dialysis, and purifying by using anionexchange chromatography and gel filtration chromatography, and performing vacuum freeze drying, separation and purification on the components to obtain the Tremellan. By the method for preparing the Tremellan, the natural structure and activity of the Tremellan are not influenced; and the method has low requirement on equipment and low cost, and is suitable for large-scale promotion, development and use in industrial production. The purified Tremellan is subjected to component analysis, structure identification and antioxidant activity research, and results show that the Tremellan has high activity of removing hydroxyl radicals and is a potential antioxidant substance.
Owner:ZHEJIANG UNIV
Method for preparing bonito stick protein hydrolysate with effect of reducing uric acid
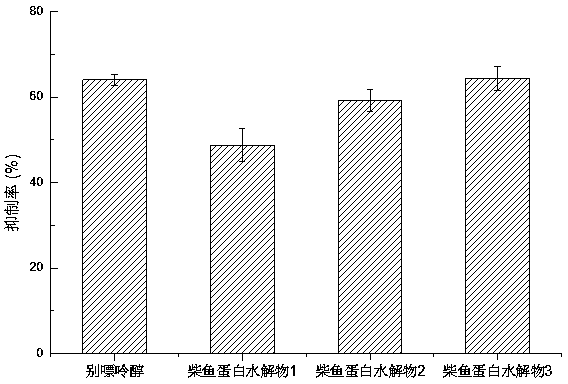
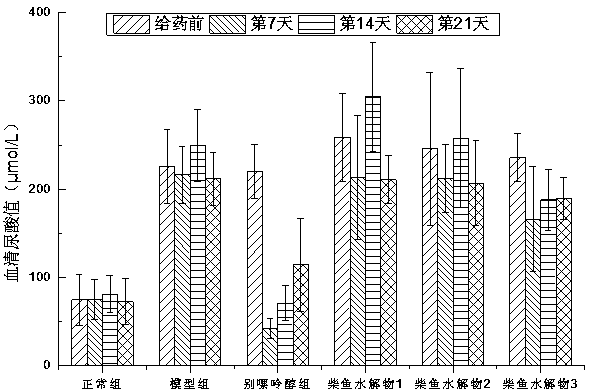
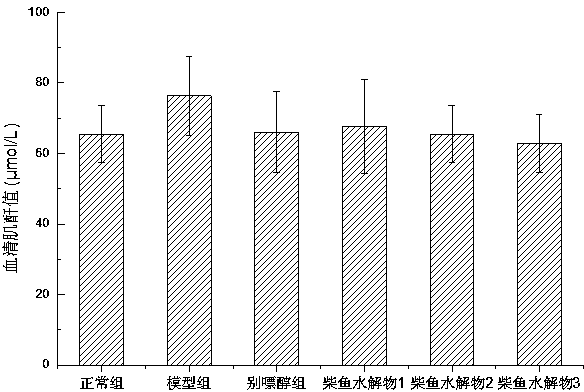
ActiveCN104337836AGood effectHigh purityHydrolysed protein ingredientsSkeletal disorderSerum uric acidArginine
The invention relates to a method for preparing bonito stick protein hydrolysate with an effect of reducing uric acid. The method comprises the following main steps of raw material heat treatment, restriction digestion, membrane separation-anion exchange chromatography-gel filtration chromatography separation, concentration and spray drying so as to obtain the bonito stick protein hydrolysate with an effect of reducing uric acid. The amino acid analysis indicates that the zymolyte peptide fragment primary amino acid sequence contains four amino acids, namely histidine, arginine, lysine and threonine, the total mass content of the four amino acids is 70 percent of the total amino acid content of zymolyte. MALDI-TOF-MS mass spectrum determines that the molecular weight of the main peptide effective ingredient is less than 700Da. In-vitro uric acid reduction experiments prove that the bonito stick protein hydrolysate has a remarkable effect of inhibiting generation of uric acid, and has an inhibition rate over 50 percent; an oteracil potassium molded hyperuricemic rat animal model indicates that the bonito stick protein hydrolysate can be used for remarkably reducing the level of serum uric acid and serum creatinine of rats, and shows a relatively good kidney protecting effect.
Owner:SOUTH CHINA UNIV OF TECH
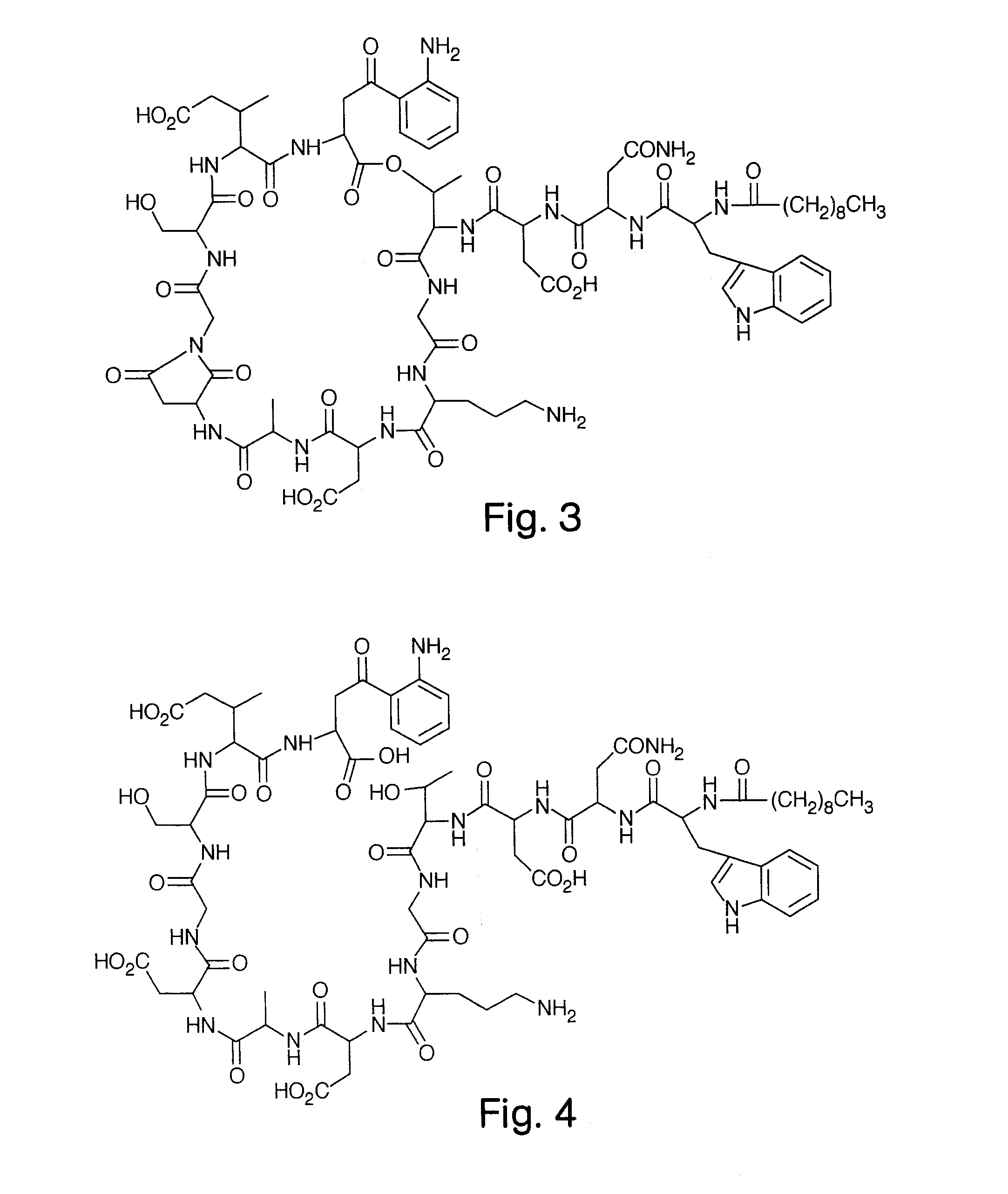
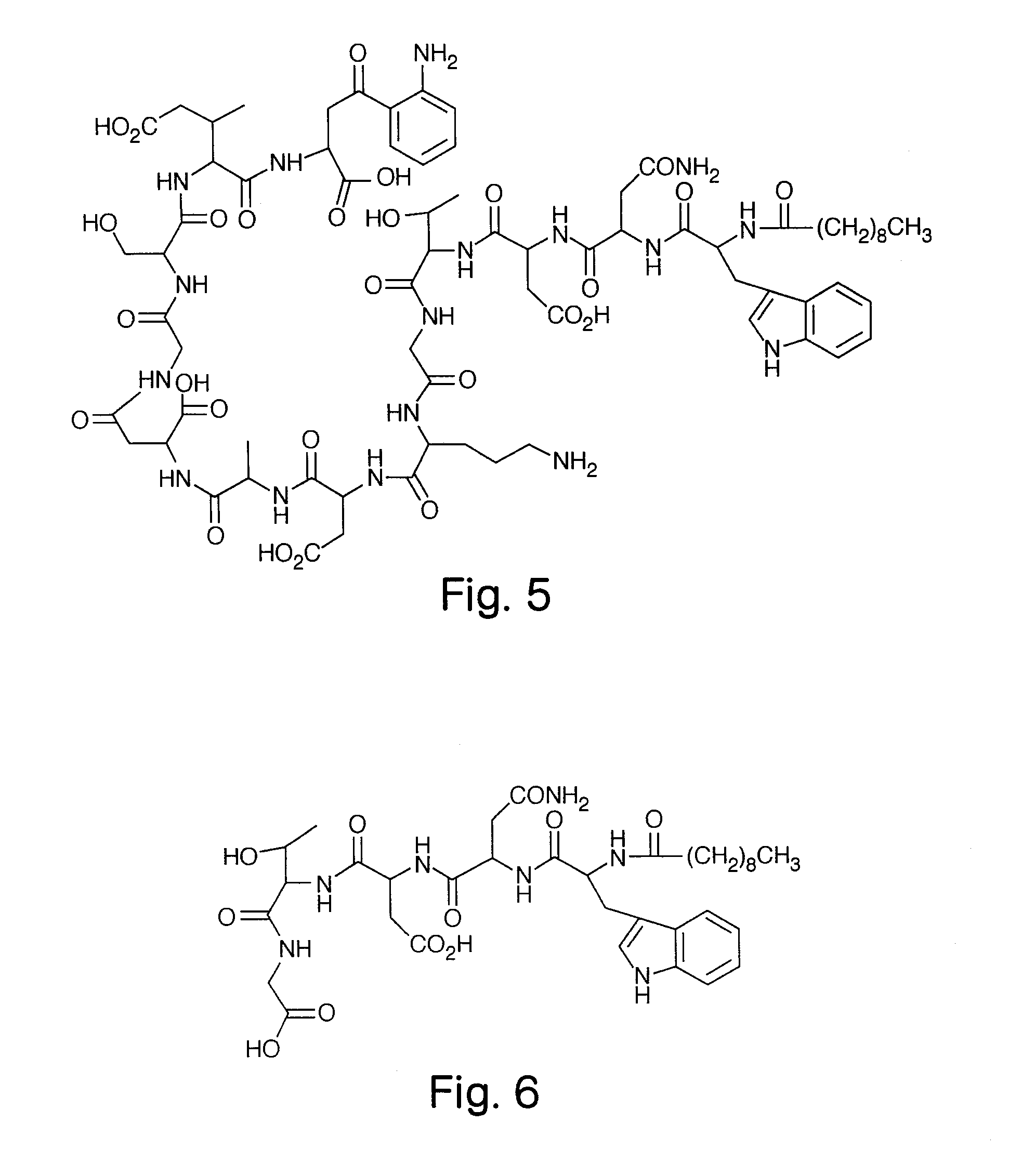
High Purity Lipopeptides
The invention discloses highly purified daptomycin and to pharmaceutical compositions comprising this compound. The invention discloses a method of purifying daptomycin comprising the sequential steps of anion exchange chromatography, hydrophobic interaction chromatography and anion exchange chromatography. The invention also discloses a method of purifying daptomycin by modified buffer enhanced anion exchange chromatography. The invention also discloses an improved method for producing daptomycin by fermentation of Streptomyces roseosporus. The invention also discloses high pressure liquid chromatography methods for analysis of daptomycin purity. The invention also discloses lipopeptide micelles and methods of making the micelles. The invention also discloses methods of using lipopeptide micelles for purifying lipopeptide antibiotics, such as daptomycin. The invention also discloses using lipopeptide micelles therapeutically.
Owner:CUBIST PHARMA INC
Production method of Sublancin antibacterial peptide
InactiveCN102851339AIncrease productionHigh purityMicroorganism based processesDepsipeptidesCentrifugationUltrafiltration
The invention provides a preparation method of Sublancin antibacterial peptide, which comprises the following steps: (1) inoculating bacillus subtilis into a medium for fermentation, filtering the fermentation liquor; (2) orderly performing centrifugation, cation exchange chromatography, hydrophobic chromatography, size exclusion chromatography, and anion exchange chromatography of the fermentation liquor in step (1), finally performing ultrafiltration concentration and drying to obtain the Sublancin antibacterial peptide. The method of the invention is low in cost, increased in production efficiency, and high in product purity; the prepared Sublancin antibacterial peptide has purity of up to more than 99%; the process is simple, and easy to popularize and apply, and can realize large-scale production.
Owner:中农颖泰林州生物科园有限公司
Process for the purification of fc-fusion proteins
InactiveUS20100267932A1Peptide preparation methodsFermentationAnion-exchange chromatographyAnion Exchange Proteins
The invention relates to a process for the purification of an Fc-fusion protein having a pI between 6.9 and 9.5 comprising protein A or G affinity chromatography, cation exchange chromatography, anion exchange chromatography and hydroxyapatite chromatography.
Owner:EON DUVAL ALEX +1
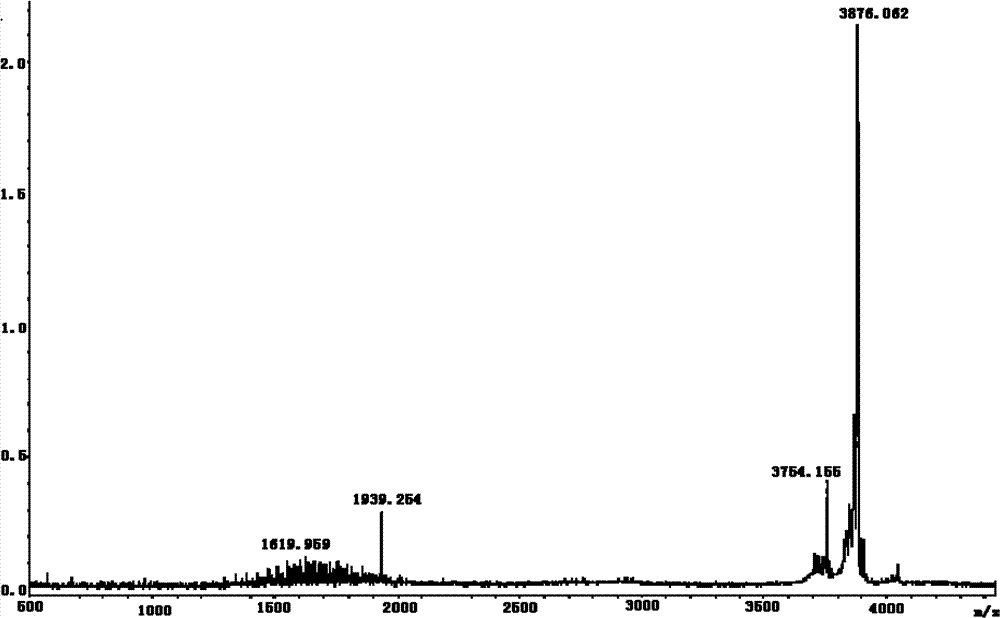
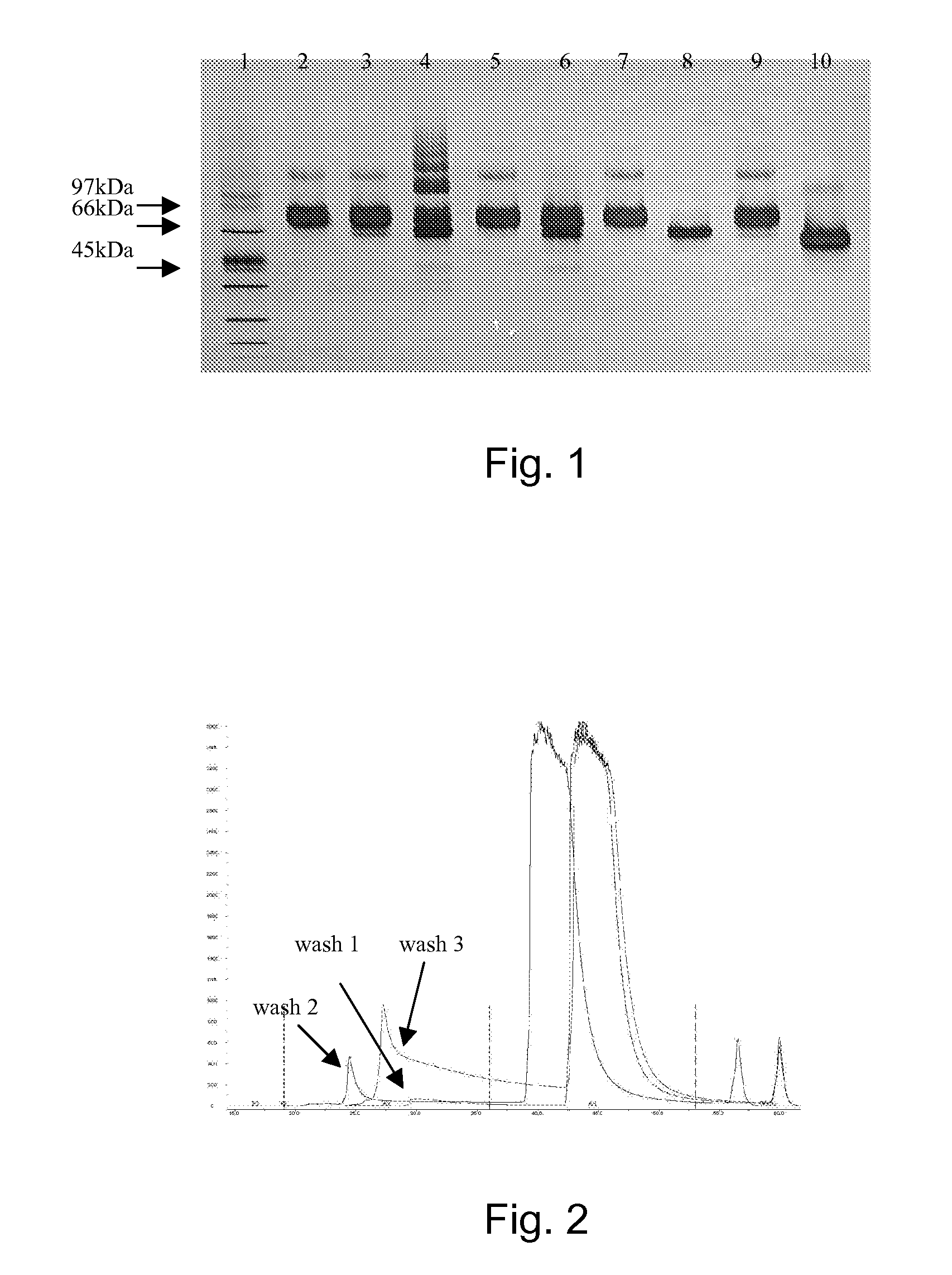
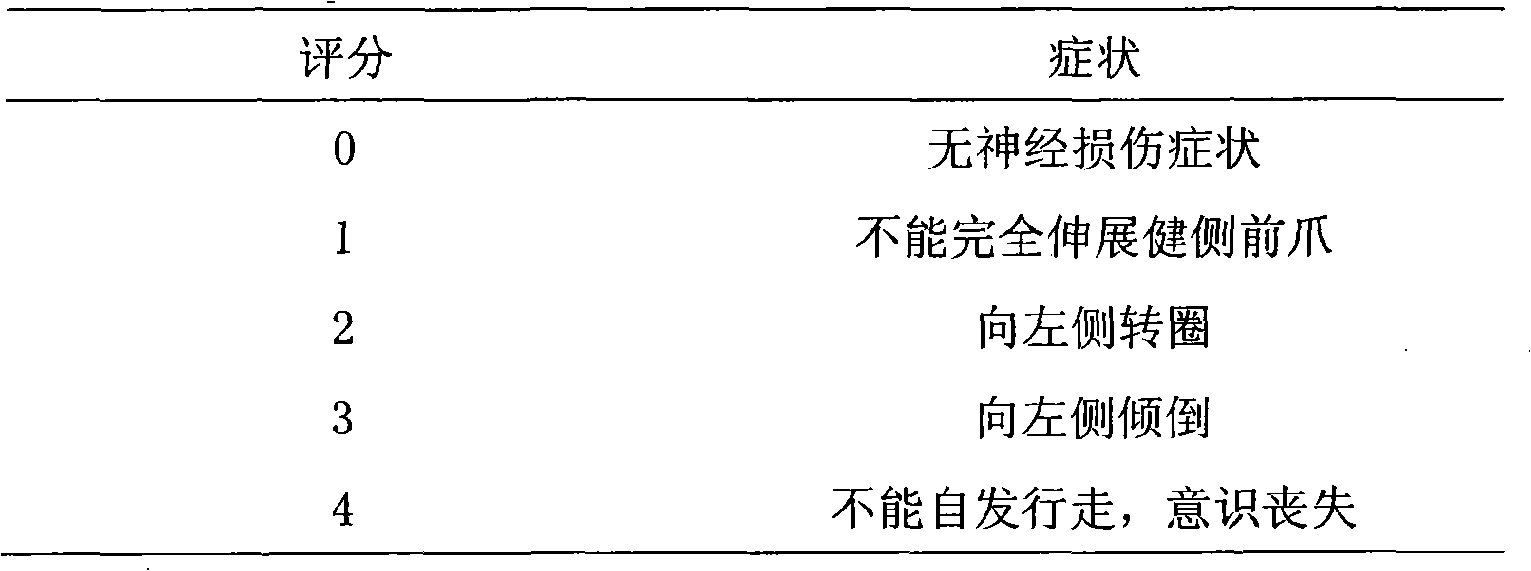
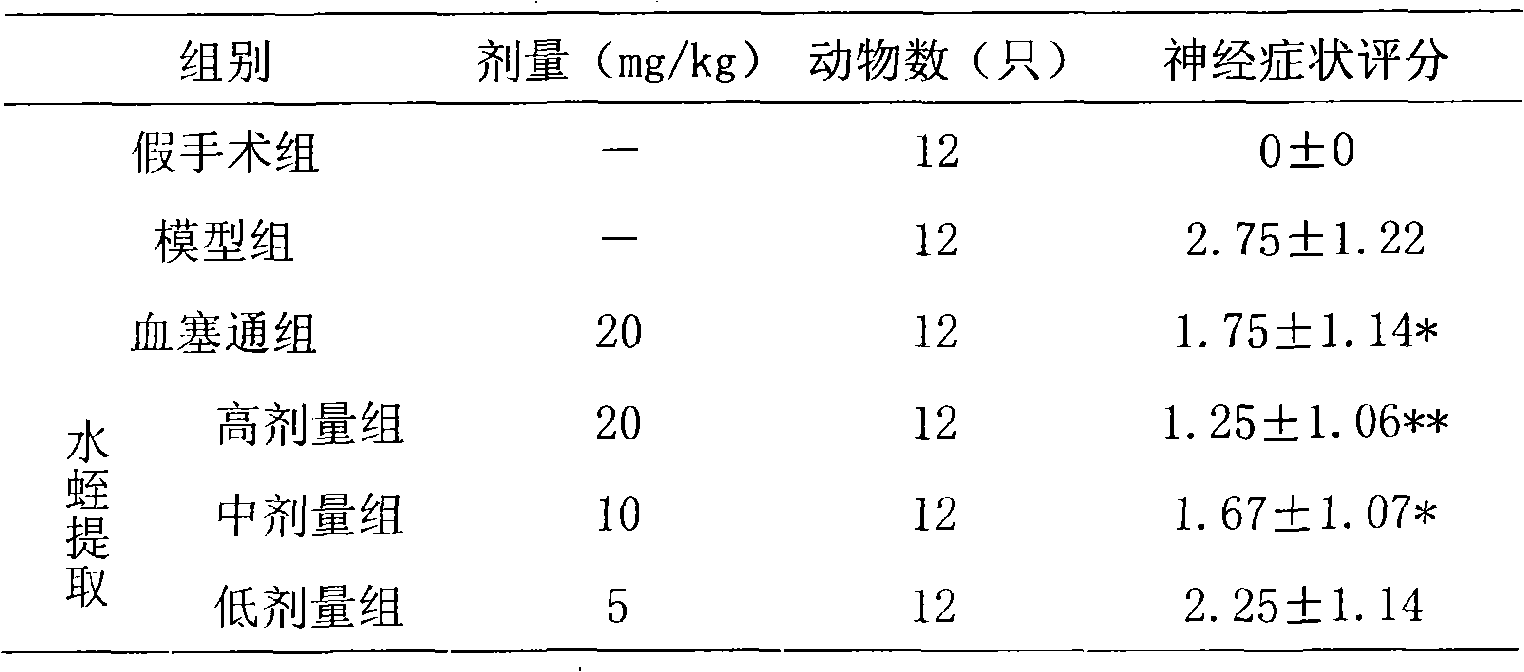
Leech extract and preparation method and use thereof
InactiveCN101332211AHigh purityHigh activityNervous disorderBlood disorderPharmacy technologyUltrafiltration
The present invention discloses a preparation method of bloodsucker extract, in which the bloodsucker is added with water and homogenized, and then the bloodsucker extract is obtained by controllable enzymolysis, organic solvent precipitation, ultrafiltration and anion-exchange chromatography. The bloodsucker extract can be adopted to prepare a medicine with anticoagulation, thrombolytic ability, inhibition on platelet aggregation and neuroprotection effect. The present invention, according to the modern technological requirements of Chinese medicine, adopts the technology of controllable enzymolysis for extracting the peptide component, the separating technology of ultrafiltration and gel chromatography for purification further, and the biological pharmacy technology, thus obtaining the extract with higher pureness and stronger activity than the traditional bloodsucker extract. The method breaks through the bottleneck of the traditional animal medicine in extraction and separation, solves the problem of the traditional animal medicine that the amount of raw powder is large and the taste is fishy, improves the activity and quality controllability of the animal medicine extract further and greatly improves the utilization rate of medicinal materials.
Owner:SHANDONG UNIV
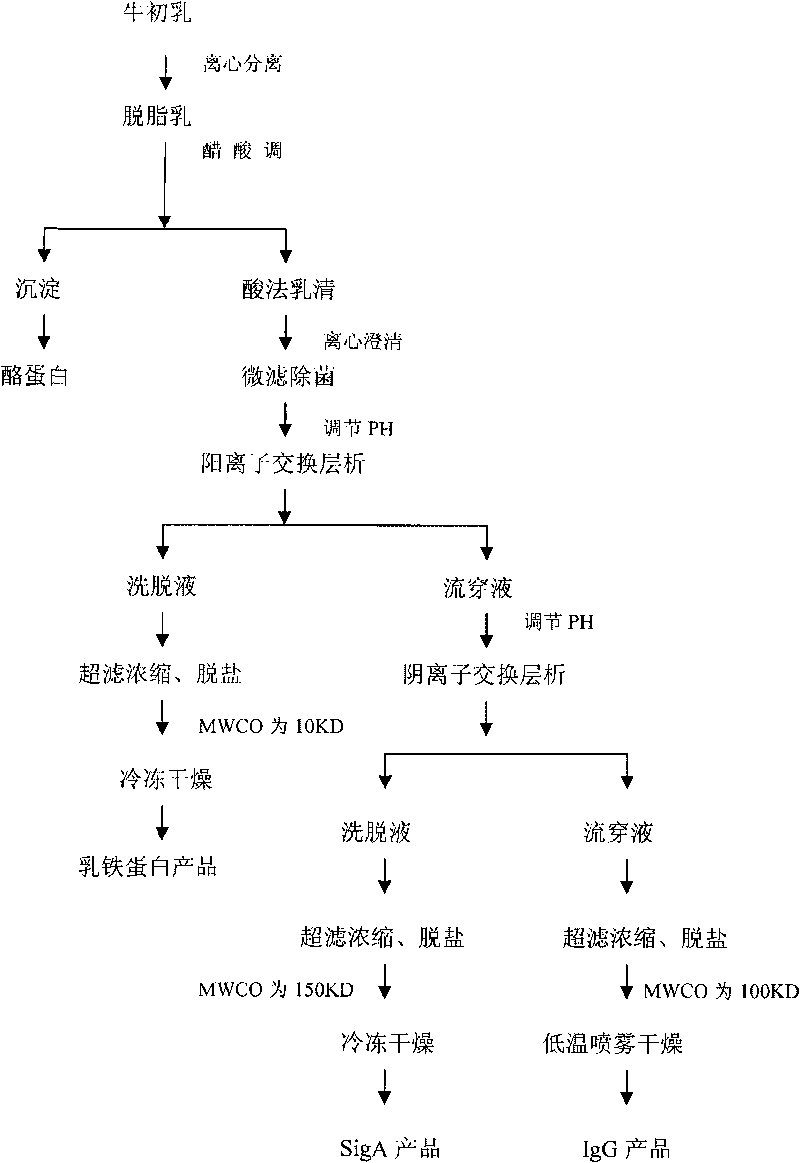
Method for separating and purifying immunoglobulin A, immunoglobulin G and lactoferrin from bovine colostrum in industrializing way
ActiveCN101724013AReduce pollutionIncrease productivityTransferrinsMilk immunoglobulinsAnion-exchange chromatographyLactoferrin
The invention provides a method for separating and purifying immunoglobulin A, immunoglobulin G and lactoferrin from bovine colostrum in an industrializing way, which comprises the following steps of: (1) degreasing the bovine colostrum and separating casein to prepare whey; (2) after micro-filtering the whey for degerming, carrying out cation exchange chromatography to obtain a first fast flow liquid, eluting a chromatographic column to obtain eluent, ultra-filtering, concentrating and desalting the eluent by using a first ceramic membrane, and freezing and drying to obtain the lactoferrin; (3) carrying out anion exchange chromatography on the first fast flow liquid to obtain a second fast flow liquid, eluting the chromatographic column to obtain eluent, ultra-filtering, concentrating and desalting the eluent by using a second ceramic membrane, and freezing and drying to obtain the immunoglobulin A; and (4) ultra-filtering, concentrating and desalting the second fast flow liquid by using a third ceramic membrane, and obtaining the immunoglobulin G by using low-temperature spray drying. By utilizing the method, the immunoglobulin A, the immunoglobulin G and the lactoferrin can be separated and purified from the bovine colostrumin efficiently and continuously in the industrializing way.
Owner:HEILONGJIANG KANPURE BIOTECH
Separation and purification method of bacillus amyloliquefaciens antimicrobial proteins
InactiveCN102153618AHigh purityHigh antibacterial activityPeptide preparation methodsPurification methodsAntibiotic resistance
The invention discloses a separation and purification method of bacillus amyloliquefaciens antimicrobial proteins, relating to a separation and purification method of bacillus antimicrobial proteins. The method solves the problems that the existing separation and purification method of proteins can not acquire bacillus amyloliquefaciens TF28 antimicrobial proteins with high purity, generates various impurity proteins and is low in the yield of antimicrobial proteins. The method includes the following steps: I. obtaining the crude extract of antimicrobial proteins; II. obtaining protein concentrate by anion exchange chromatography; and III. performing gel filtration chromatography through a molecular sieve to obtain bacillus amyloliquefaciens antimicrobial proteins. The separation and purification method is used for the separation and purification of bacillus amyloliquefaciens antimicrobial proteins.
Owner:INST OF MICROBIOLOGY HEILONGJIANG ACADEMY OF SCI
Method for producing recombined insulin human
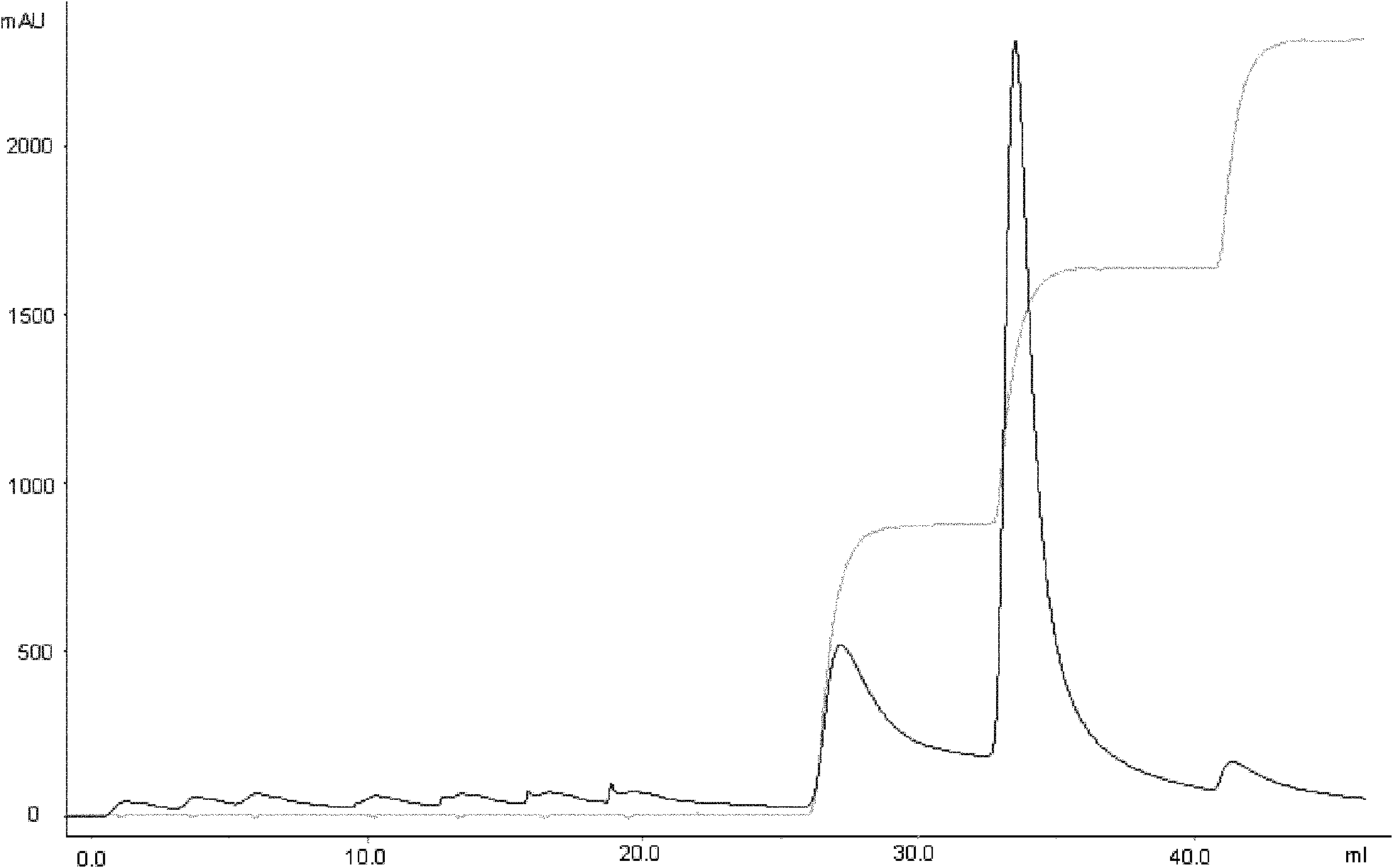
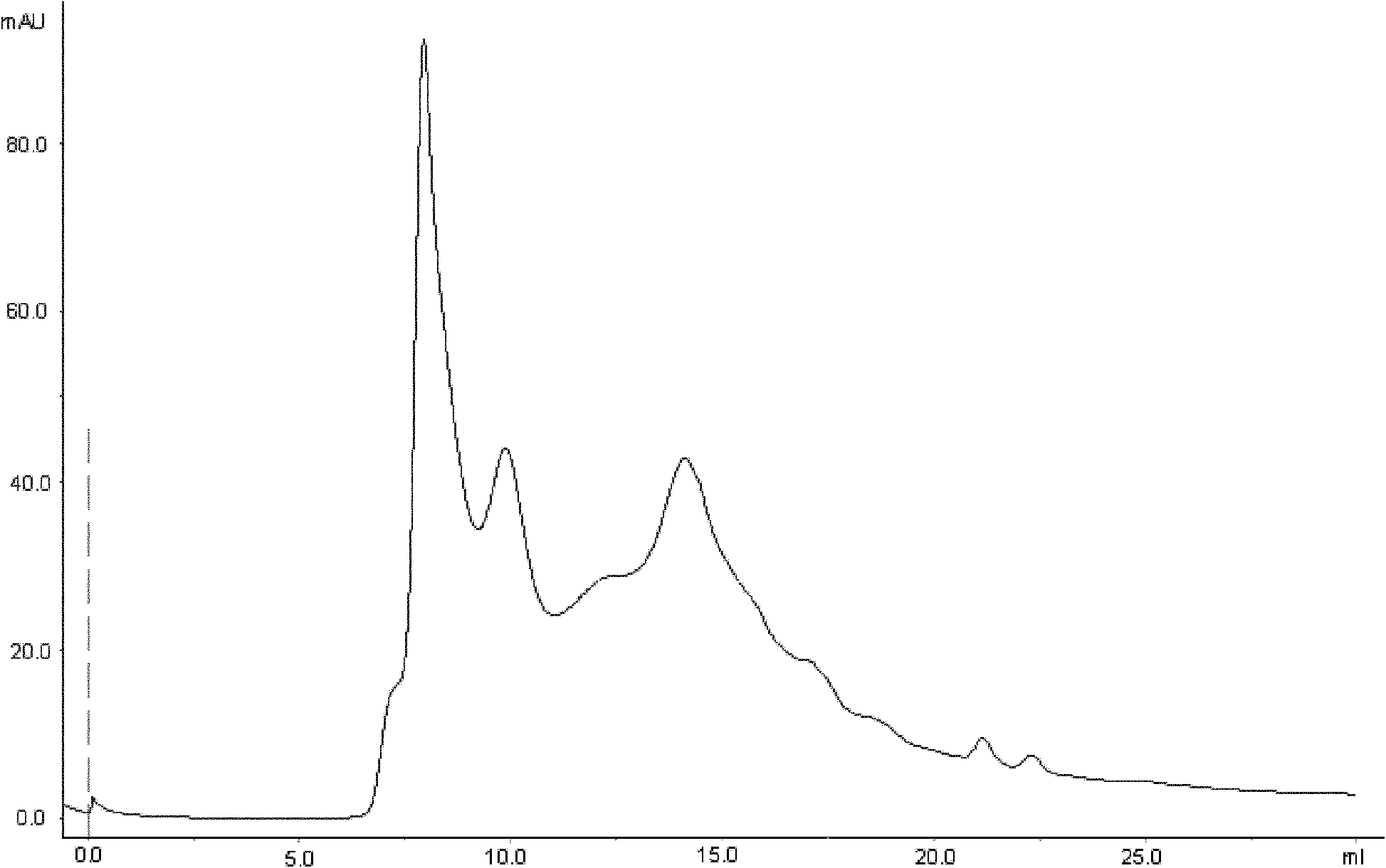
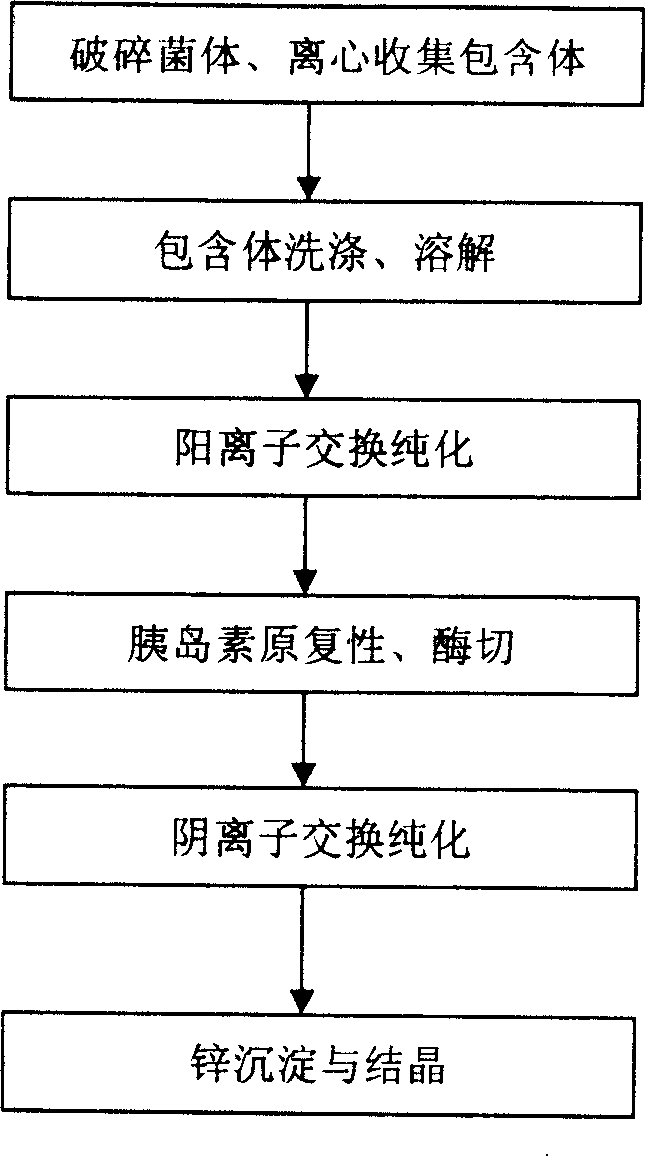
ActiveCN101173006ASimple processReduce manufacturing costPeptide preparation methodsInsulinsEscherichia coliInsulin activity
The invention discloses a preparation method of recombinant human insulin, which relates to the protein polypeptide type medicine field. The technical problem needed to be solved by the invention aims at providing a preparation method for Escherichia coli to express the recombinant human insulin in order to overcome the technical disadvantages in the prior art of using the poisonous and harmful substance CNBr and having a complex production process. The preparation method for the recombinant human insulin of the invention comprises the steps of: thallus crashing, inclusion body dissolving, proinsulin sulfonating, purifying proinsulin, and proinsulin renaturation, enzyme cutting transmission, etc., and the preparation method is characterized in that during the inclusion body dissolving process, the -SH of proinsulin is transformed into -SSO3<->, and then human insulin is obtained through purifying the sulfonated proinsulin, reduction renaturation and enzyme cutting transmission by positive ion, and then through the negative ion exchange layer purification. The preparation method of the recombinant human insulin of the invention does not use the CNBr and has simple technique with high human insulin yield coefficient and purity.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Antibacterial lipopeptide of endophytic Bacillus subtilis and separation and purification method
InactiveCN101724014ABroad antibacterial spectrumImprove thermal stabilityMicroorganism based processesFungicidesPesticide residueAntifungal drug
The invention relates to an extracellular antibacterial lipopeptide of plant endophytic Bacillus subtilis Jaasedl and a separation and purification method. The distribution range of the molecular weight of the extracellular antibacterial lipopeptide is mainly between 1,000Da and 2,200Da. The extracellular antibacterial lipopeptide contains an Iturin homologue, a Fengycin homologue and a Surfactin-like Compound homologue. The extracellular antibacterial lipopeptide is a group of uncommon antibacterial lipopeptide mixture produced by a single bacterial strain. The separation and purification method of the extracellular antibacterial lipopeptide comprises the following steps of: after salting out to obtain the crude extract of the antibacterial lipopeptide by using ammonium sulfate from the fermentation liquor of the endophytic Bacillus subtilis Jaasedl, performing Sephedex G-25 molecular sieve chromatography, Cellulose DEAE-52 anion exchange chromatography and FPLC300SB-C18 column chromatography successively, wherein 34 to 37 min of collecting peak has bacteriostatic activity; detecting by Tricine-SDS-PAGE after concentrating; and achieving electrophoretically pure at only one strip to obtain pure extracellular antibacterial lipopeptide. The antibacterial lipopeptide has extremely high research and application value for the development of broad-spectrum antifungal medicaments, and has wide application prospect for natural quality protection of crops and pesticide residue reduction.
Owner:JIANGSU ACAD OF AGRI SCI
Process for The Manufacture of Virus Safe Immunoglobulin
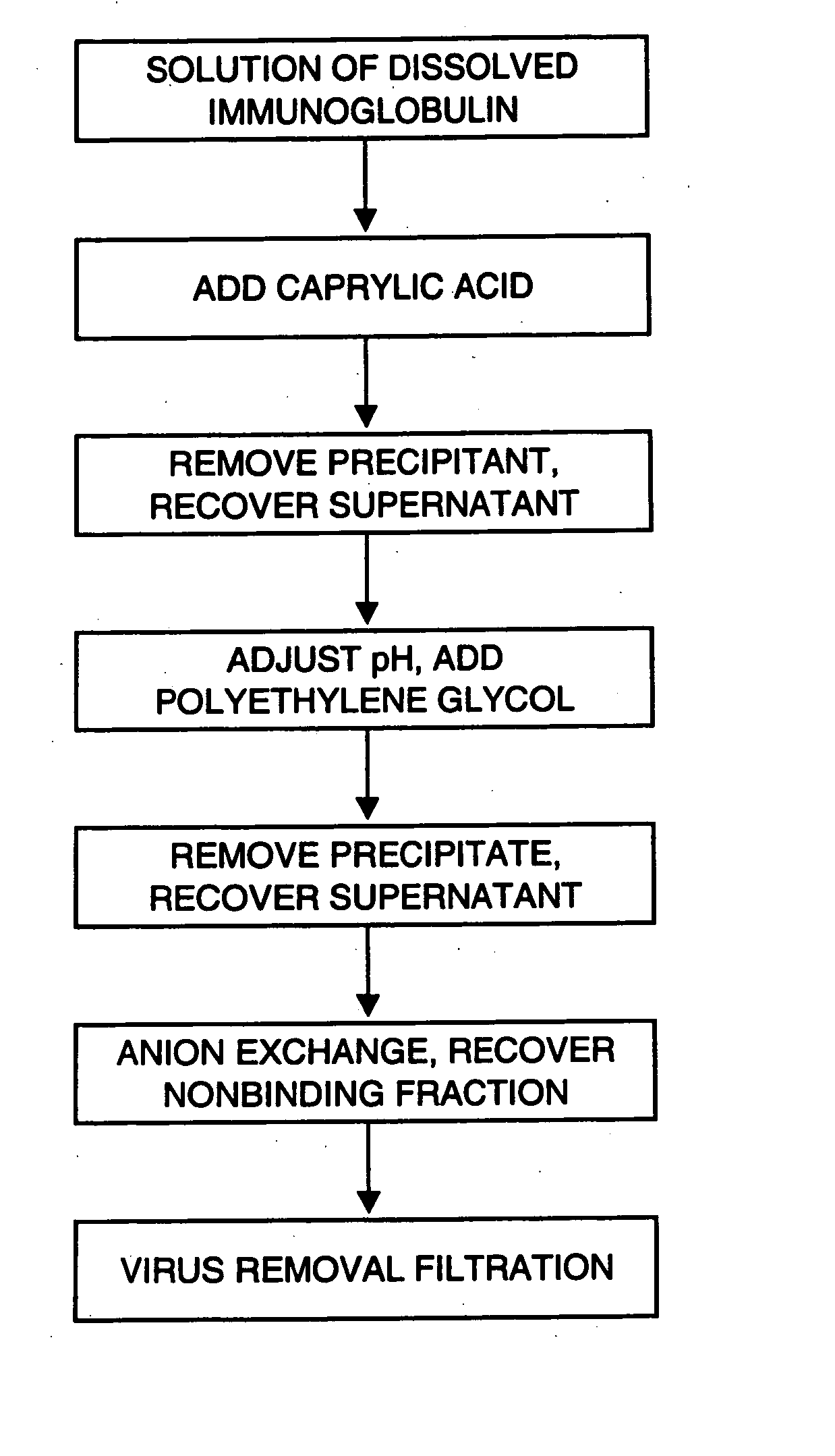
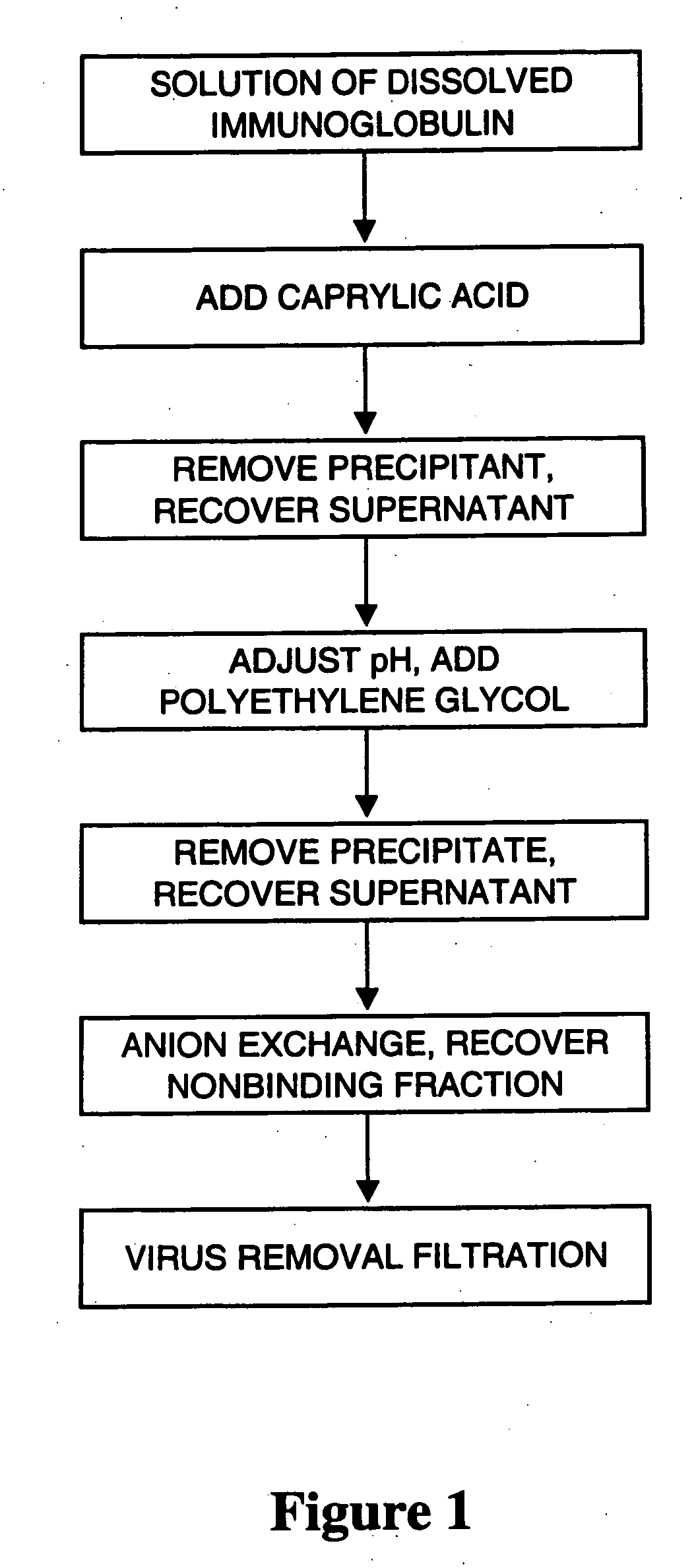
InactiveUS20070244305A1Eliminate the problemEfficient precipitationSerum immunoglobulinsPeptide preparation methodsProtein aggregationAnion-exchange chromatography
Process for preparing a purified immunoglobulin preparation. The process comprises the steps of subjecting a crude immunoglobulin solution to caprylic acid treatment, removing protein aggregates and viruses from the immunoglobulin solution, subjecting the immunoglobulin solution to anion exchange chromatography in order to purify the immunoglobulin, filtering the immunoglobulin solution thus obtained on a virus-removal filter to produce an eluate containing immunoglobulin, and recovering the immunoglobulin. By combining caprylic acid treatment and precipitation with a protein precipitant the level of aggregated proteins and viruses is effectively reduced and a truly virus safe preparation is provided after filtration.
Owner:SUOMEN PUNAINEN RISTI VERIPALVELU
Method for extracting rabies virus
InactiveCN101270350AImprove removal efficiencyGood removal effectSsRNA viruses negative-senseMicroorganism based processesHollow fibreFiber
The invention provides a method for extracting rabies virus, and is to solve the defects that great discrepancy of quality indices of different batches occurs; removal of remaining DNA becomes difficult; the protein content of the remaining host is too high; and great side effects appear clinically when the single method of molecular sieve gel chromatography is adopted for extracting rabies virus vaccine. The essential of the invention is that a hollow fiber ultrafiltration column or ultrafiltration membrane with the molecular weight cut-off of 750KD or 500KD is used to condense and partially purify harvested liquid of virus; anion exchange chromatography or molecular sieve gel chromatography is adopted to separate and purify samples; molecular sieve gel chromatography or anion exchange chromatography is adopted to separate and purify samples got in step (2). The method for extracting rabies virus has the characteristics of great productive capacity, high product quality, excellent batch stability, being remarkably effective in removal of remaining DNA and HCP, and reducing the potential safety hazard of vaccine.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Method for purifying plasmid DNA
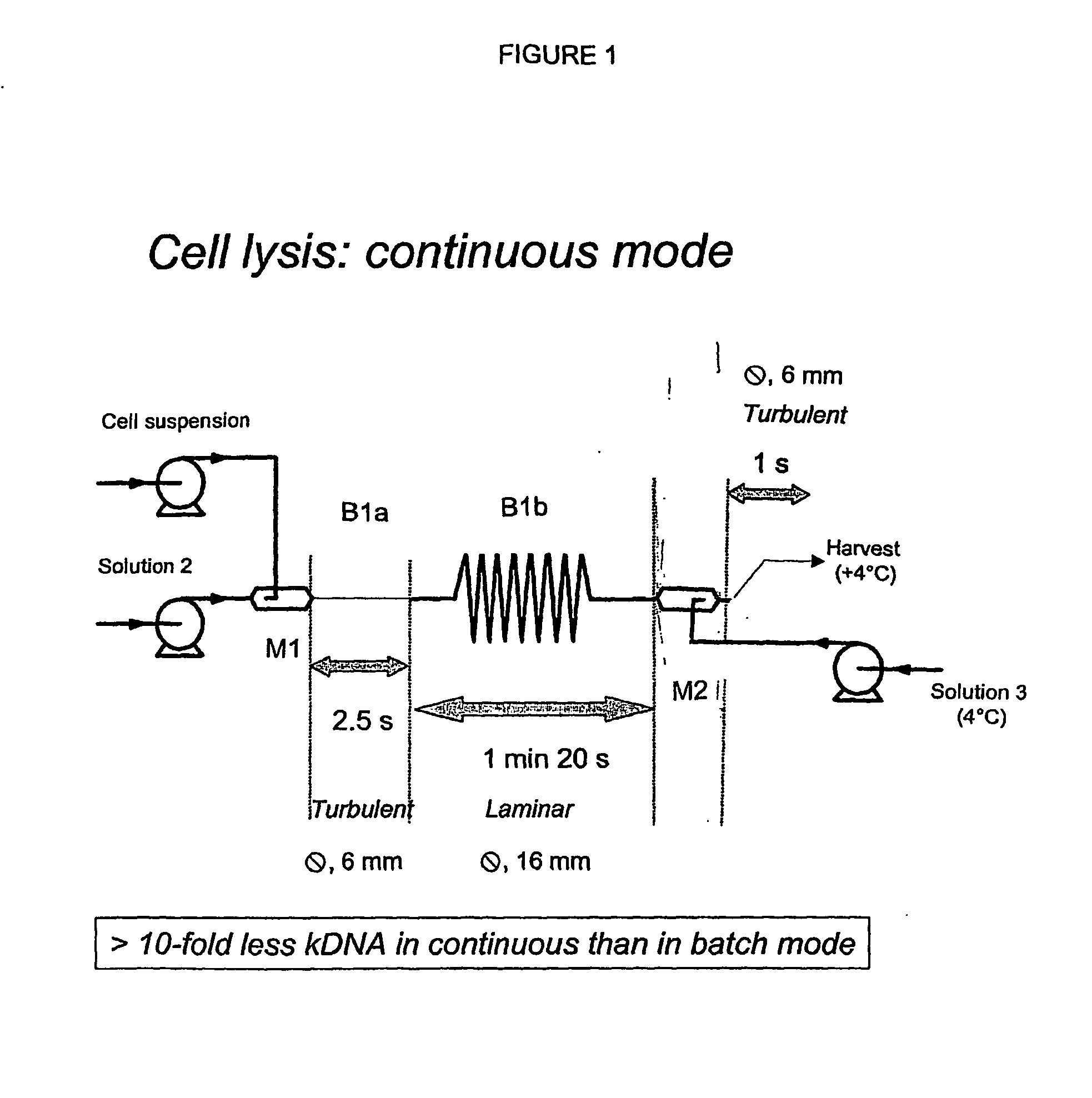
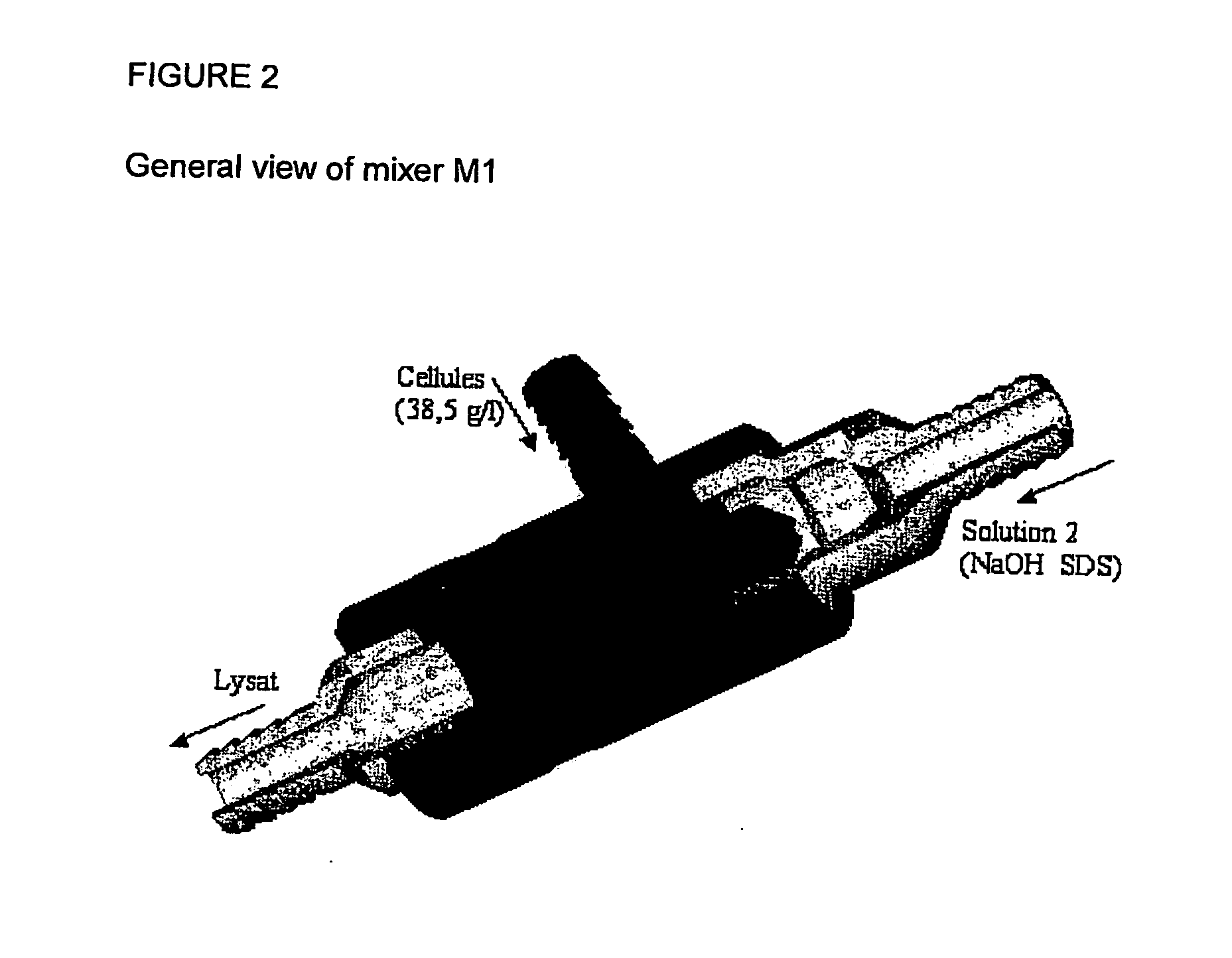
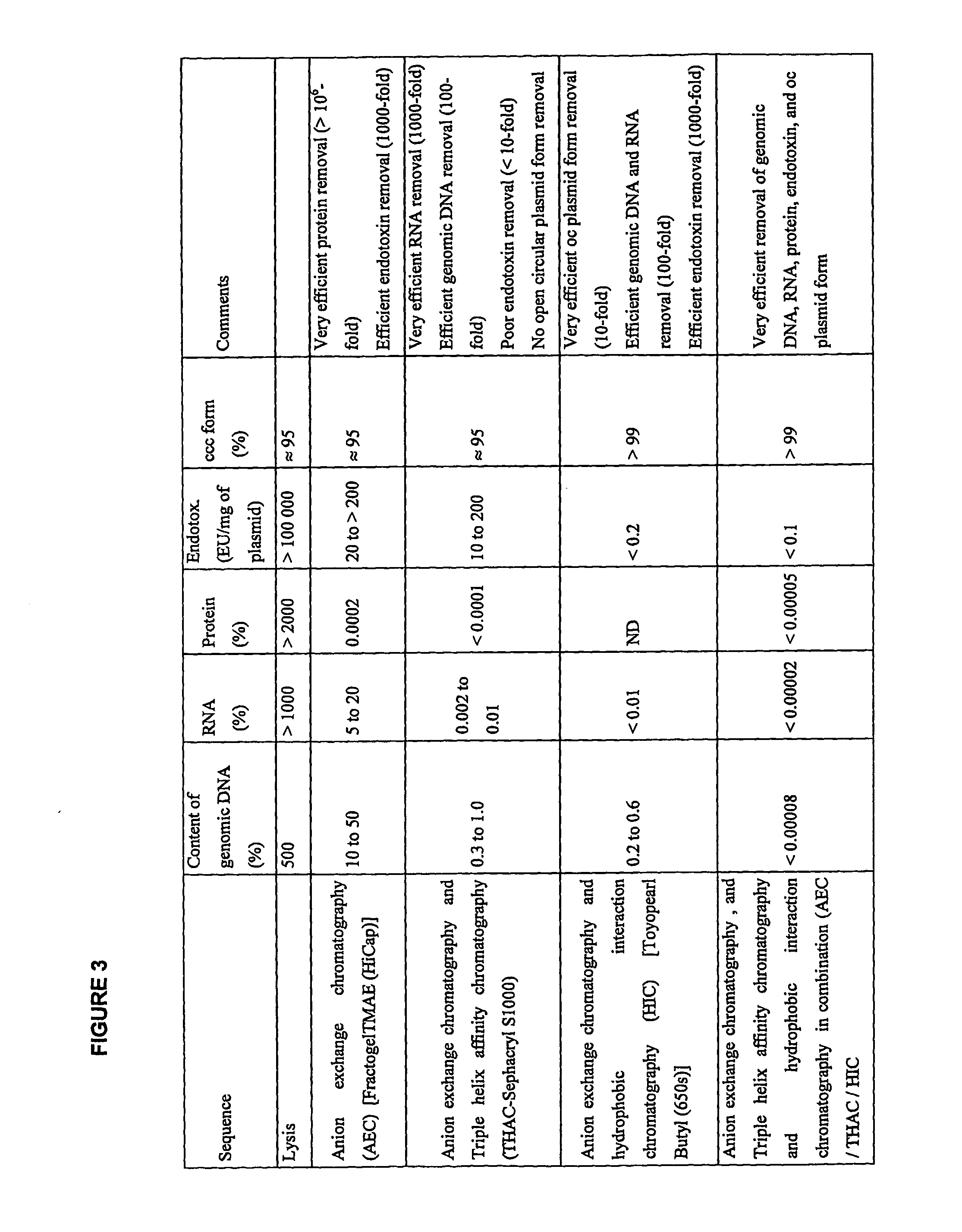
InactiveUS20070213289A1Yield maximizationProtozoaGenetic therapy composition manufactureLysisFiltration
This invention provides a process for the continuous alkaline lysis of a bacterial suspension in order to harvest pDNA. It further provides for optional additional purification steps, including lysate filtration, anion exchange chromatography, triplex affinity chromatogragphy, and hydrophobic interaction chromatography. These optional purification steps can be combined with the continuous lysis in order to produce a highly purified pDNA product substantially free of gDNA, RNA, protein, endotoxin, and other contaminants.
Owner:AVENTIS PHARMA SA (US)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com