Method for purifying recombinant human serum albumin protein and application thereof
A technology of supernatant and application, applied in the purification of recombinant human serum albumin and its application field, can solve the problems of reduction and difficulty in controlling endotoxin levels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
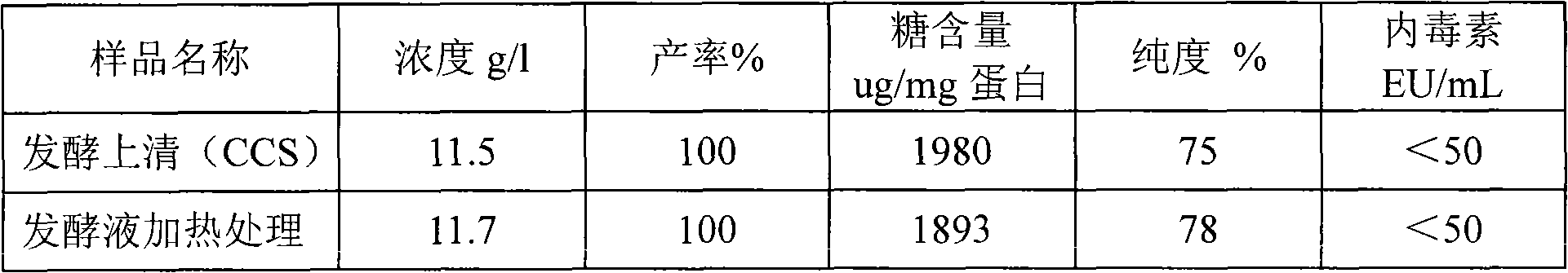
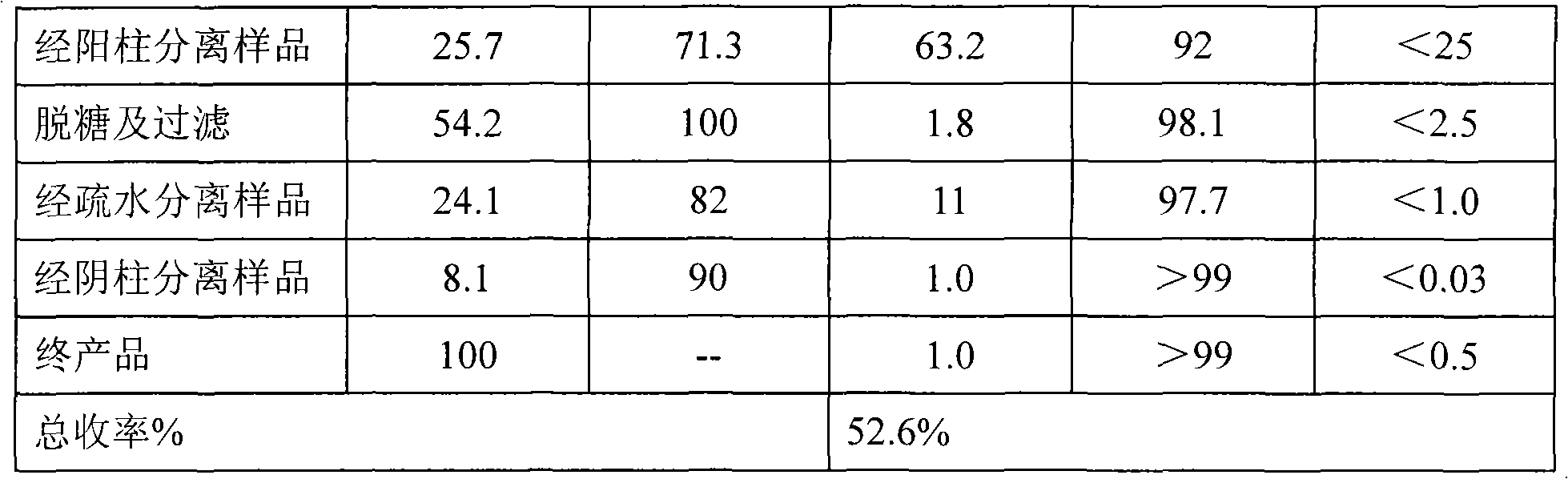
[0024] The purification of embodiment 1 recombinant human serum albumin (rHSA)
[0025] The various buffers used in the purification process are shown in the table below:
[0026] name
Buffer formulation
Solution A
25mM Disodium Hydrogen Phosphate - Sodium Dihydrogen Phosphate at pH 4.5
Solution B
50mM Disodium Hydrogen Phosphate - Sodium Dihydrogen Phosphate at pH 7.0,
0.1M NaCl, 10mM Sodium Octanoate
Solution C
50mM Disodium Hydrogen Phosphate - Sodium Dihydrogen Phosphate, 0.1M Sodium Chloride, pH 6.0
Solution D
50mM Disodium Hydrogen Phosphate - Sodium Dihydrogen Phosphate, 0.2M Sodium Chloride, pH 6.0
[0027] a) Clarification and heat treatment of fermentation broth
[0028] The fermentation broth was clarified using a ceramic membrane with a pore size of 0.5um, and 80L of the fermentation supernatant was taken, added with 5mM sodium octanoate and 5mM EDTA, heated at 70°C for 20 minutes, and ...
Embodiment 2
[0046] Example 2 The application of rHSA in the rabies vaccine rotary bottle culture process
[0047] a) Cell preparation:
[0048] Vero cells (derived from ATCC CRL-1586 in the United States) were revived, expanded in spinner bottles, and passaged into 3.5L double-layer spinner bottles. Culture conditions 37°C, CO 2 5% (v / v).
[0049] b) Virus inoculation:
[0050] When the Vero cells were cultured into a monolayer, the virus seed of CTN strain (National Institute for the Control of Pharmaceutical and Biological Products) was inoculated at an MOI of 0.025-0.125. After inoculation, the DMEM medium maintenance solution supplemented with 0.35% rHSA for vaccine production was replaced. The culture temperature was 34°C.
[0051] c) Virus collection liquid:
[0052] After changing to the maintenance solution, the solution was changed every three days, and after the solution was collected, the fresh maintenance solution was continued, and the solution was collected three times ...
Embodiment 3
[0060] Example 3 Application of rHSA in Rabies Vaccine NBS Reactor Cultivation Process
[0061] a) Cell preparation:
[0062] The Vero cells were revived and expanded in spinner bottles at 4×10 5 The density of cells / ml was inoculated in NBS5L bioreactor, with 160g polyester sheet in the reactor ester sheet basket, working volume 2.5L, culture condition 37°C, dissolved oxygen 45%, stirring speed 70rpm, pH value 7.4.
[0063] b) Virus inoculation:
[0064] When the Vero cells were cultured for 4-5 days, the DMEM medium supplemented with 0.35% rHSA for vaccine production was replaced, and the CTN strain virus seed was inoculated at an MOI of 0.025-0.125. The culture temperature is 34° C., the dissolved oxygen is 45%, the stirring speed is 70 rpm, and the pH value is 7.4.
[0065] c) Virus collection liquid:
[0066] After continuous perfusion for 20 days, the collection volume was 24L, and the virus titer of the mixed virus collection reached 6.51gLD50.
[0067] d) Purifica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com