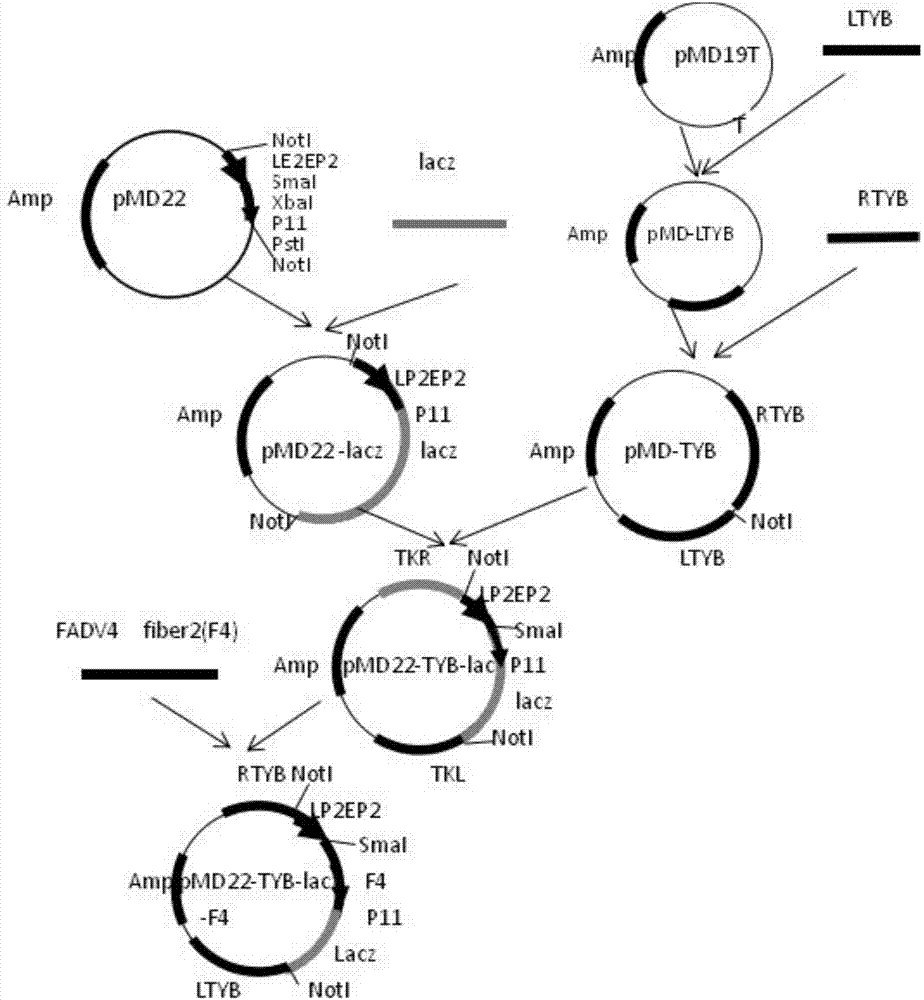
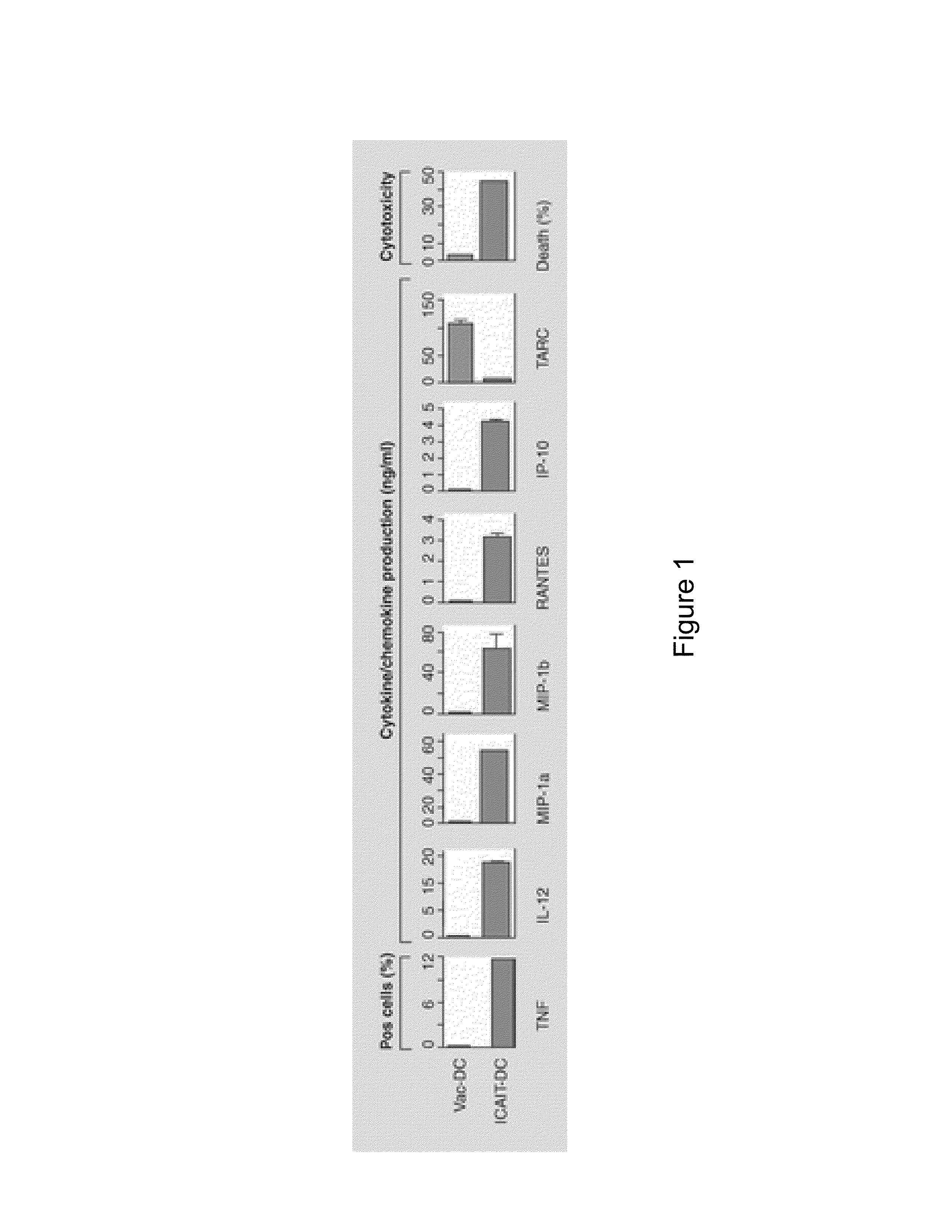
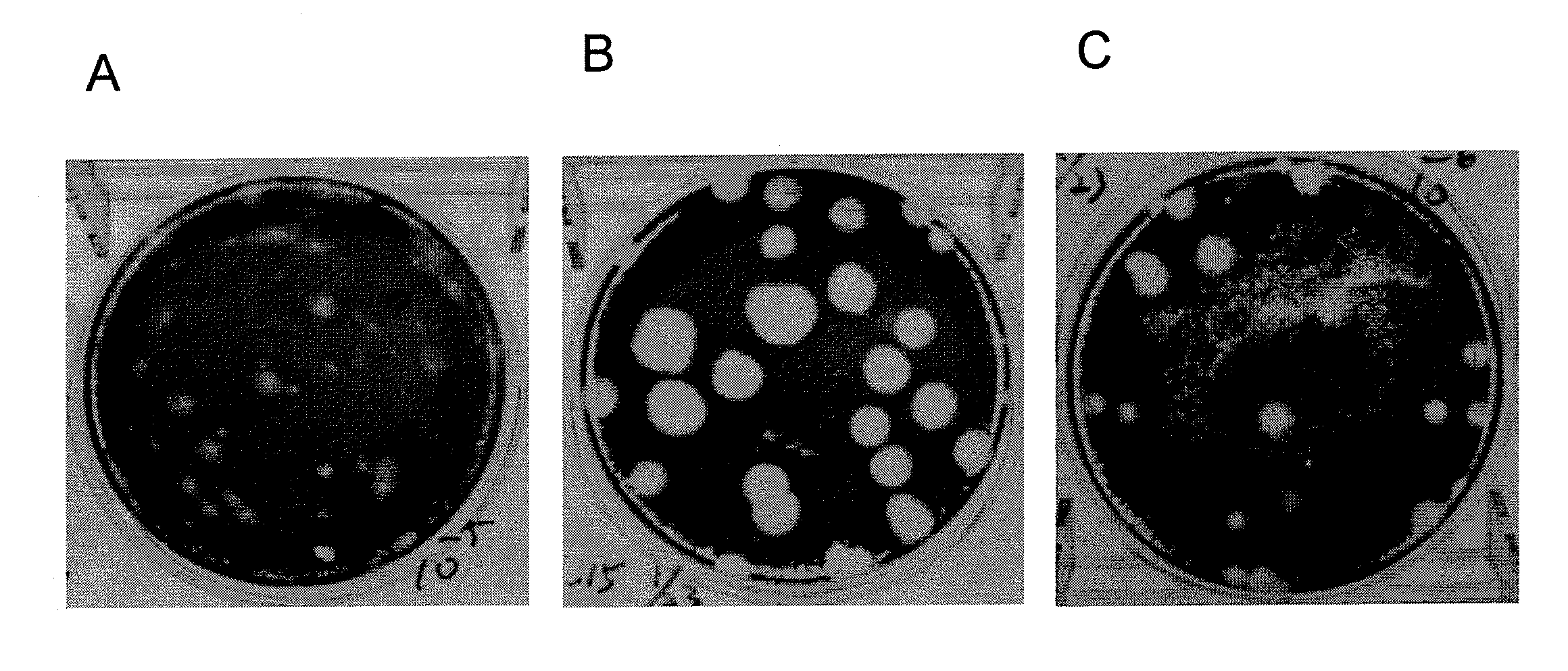
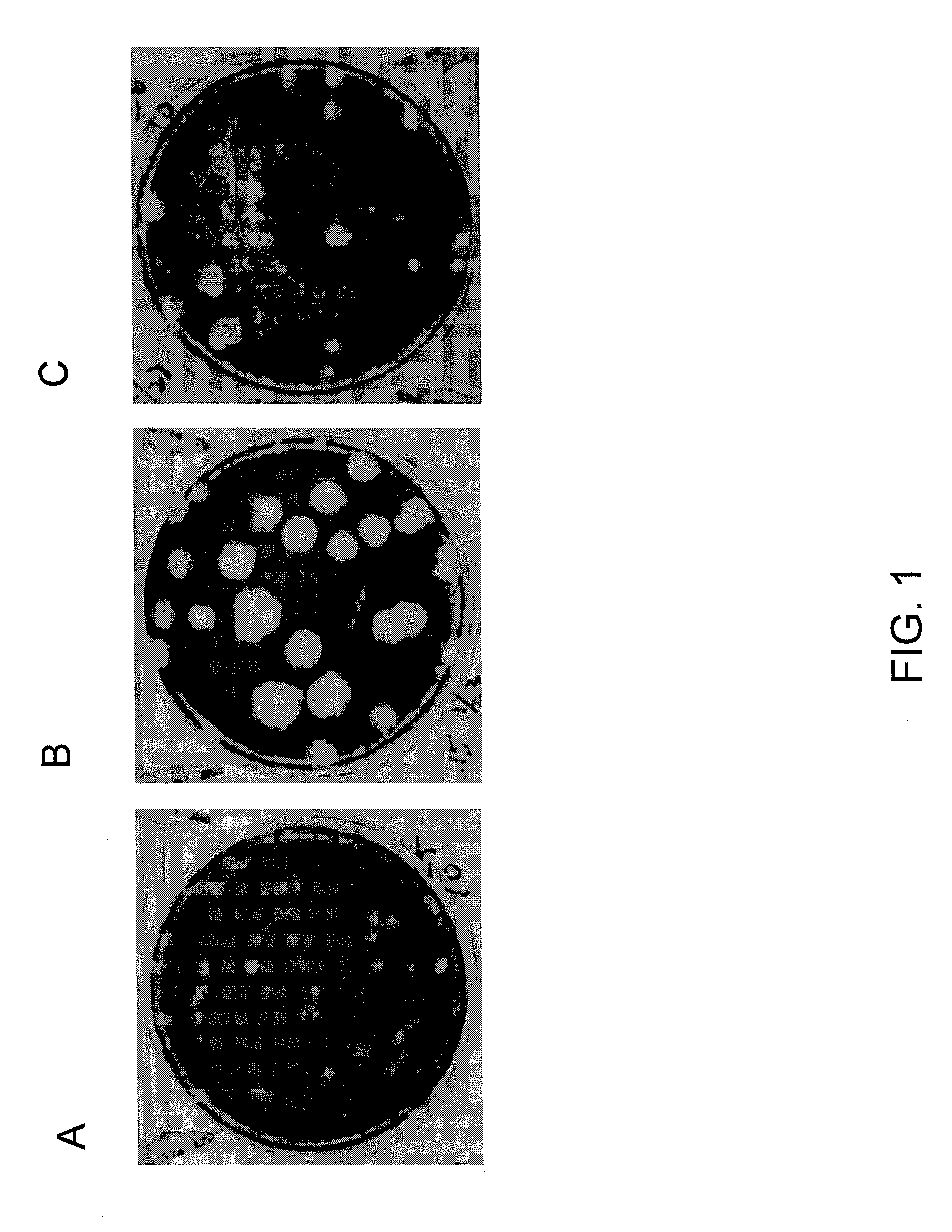
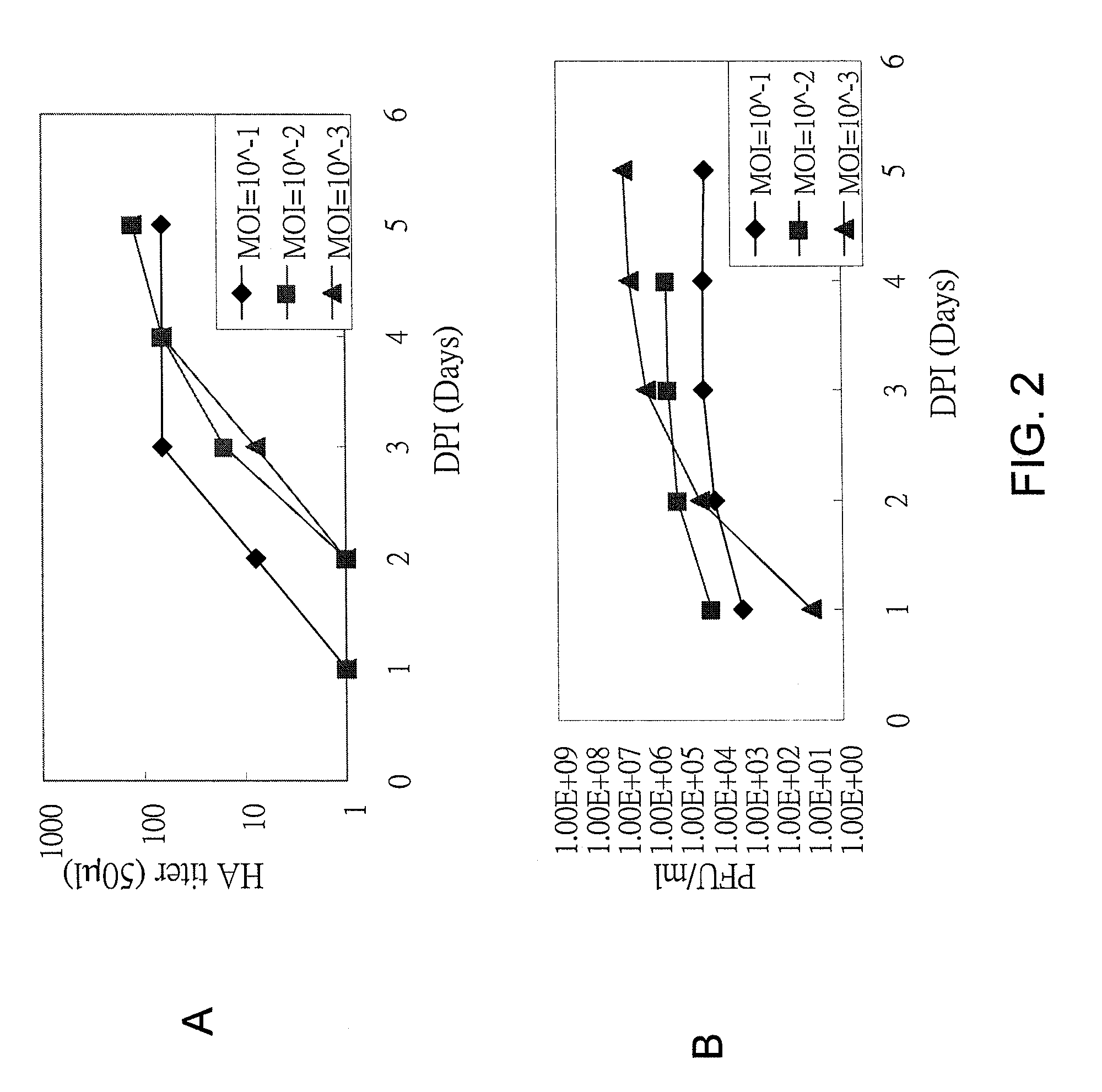
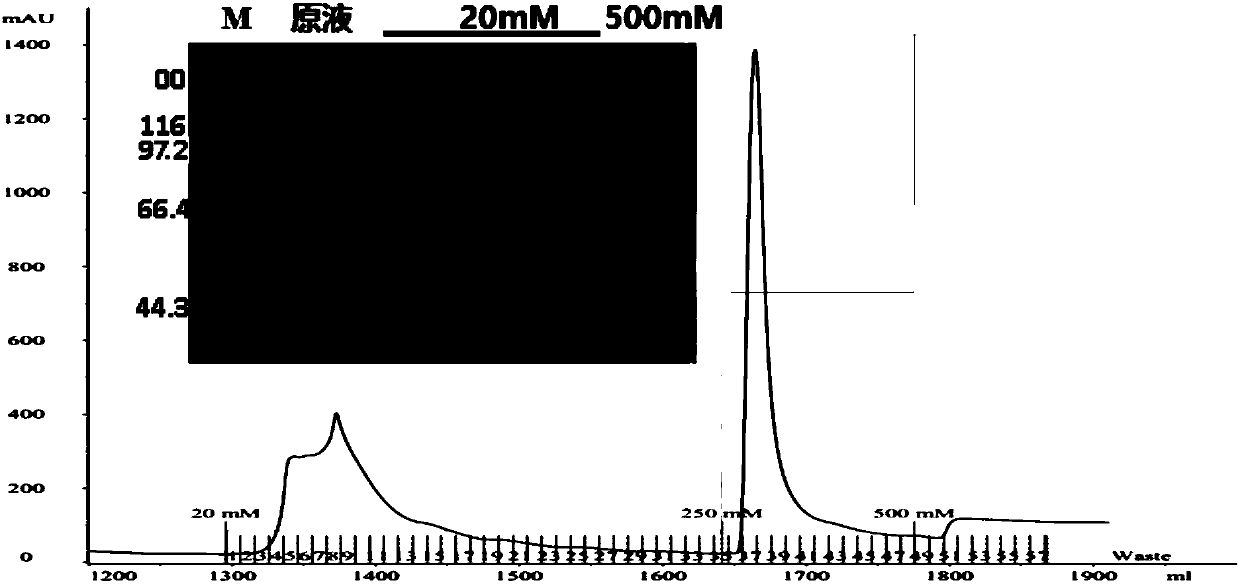
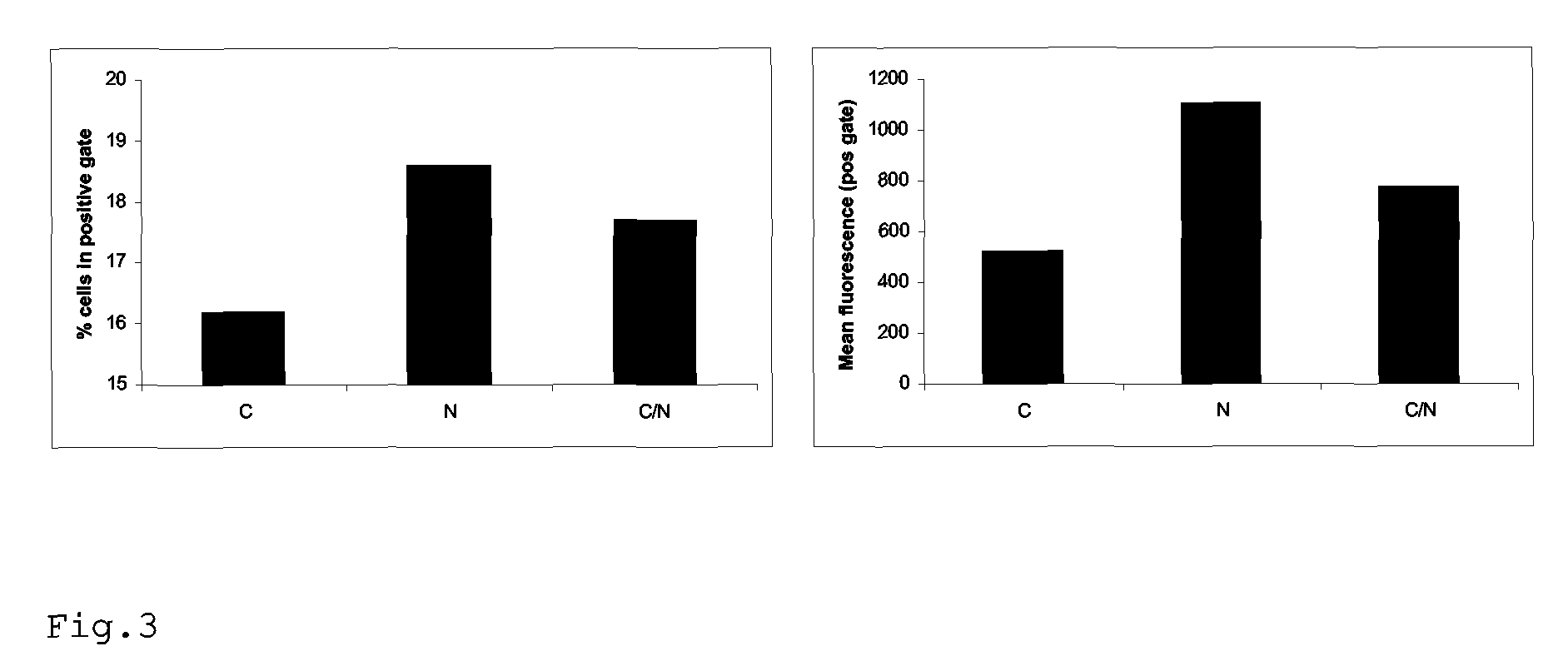Patents
Literature
570 results about "Vaccine Production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
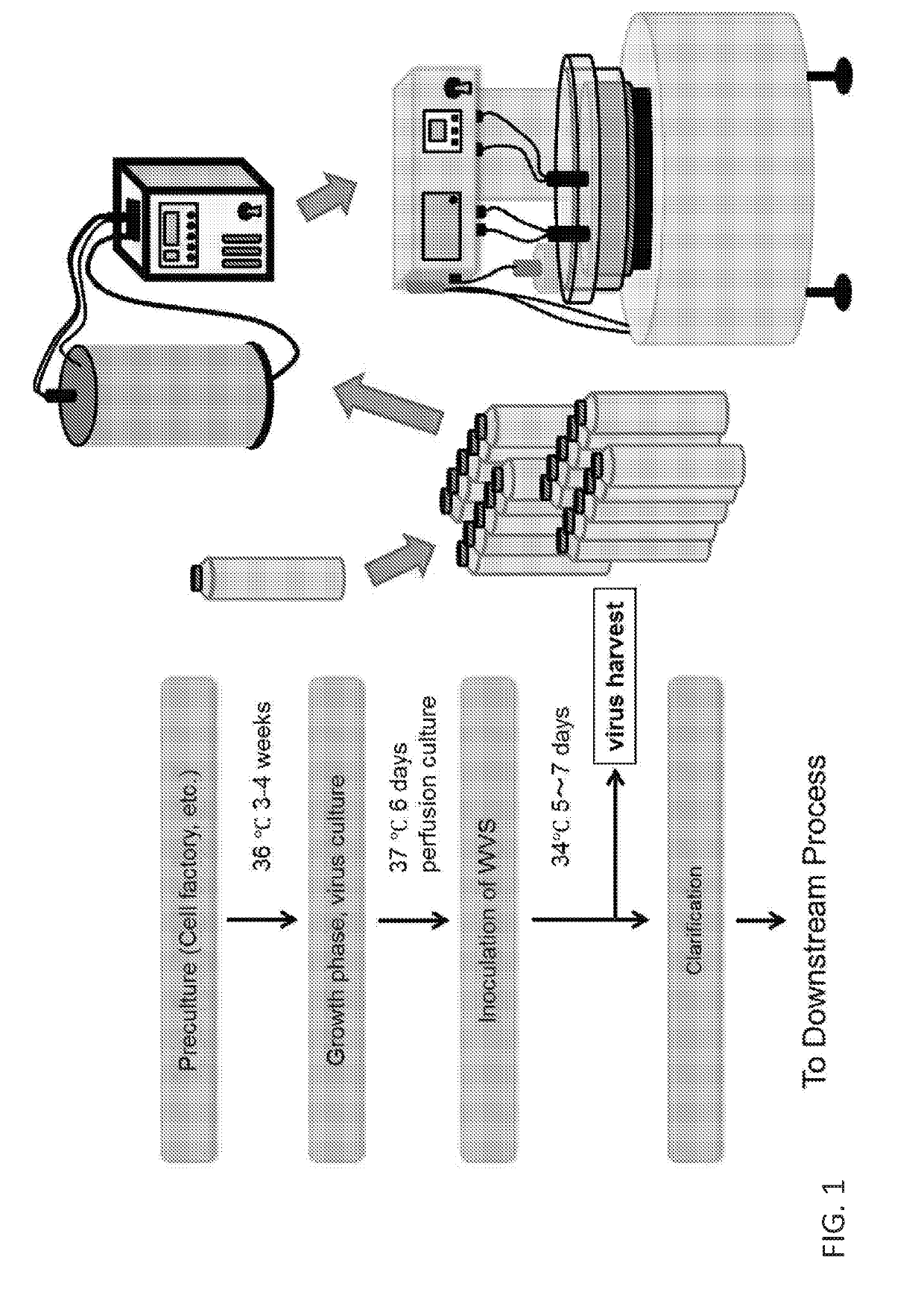
Vaccine production has several stages. Process of vaccine manufacture has the following steps: Inactivation – This involves making of the antigen preparation Purification – The isolated antigen is purified Formulation – The purified antigen is combined with adjuvants, stabilizers and preservatives to form the final vaccine preparation.
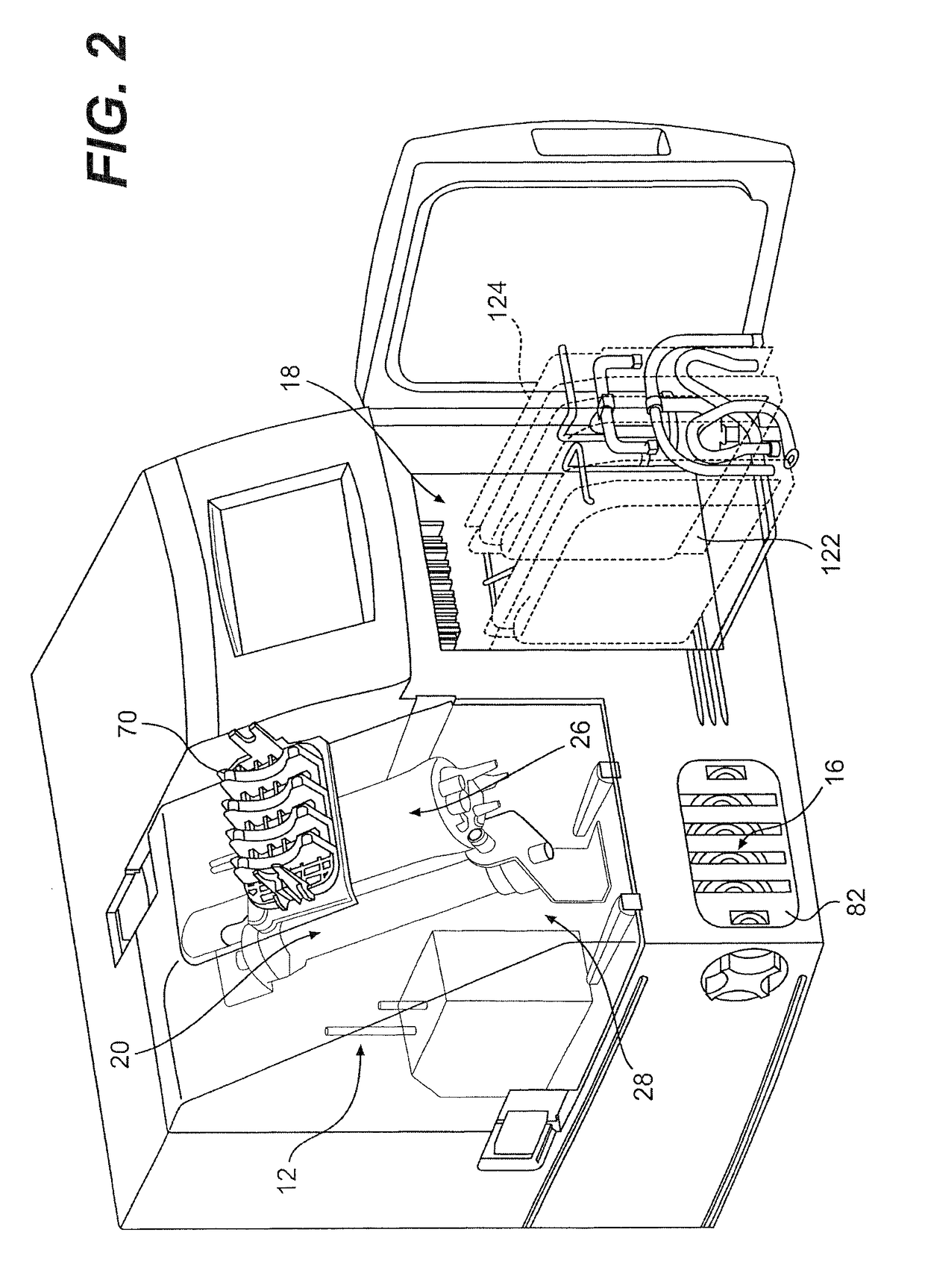
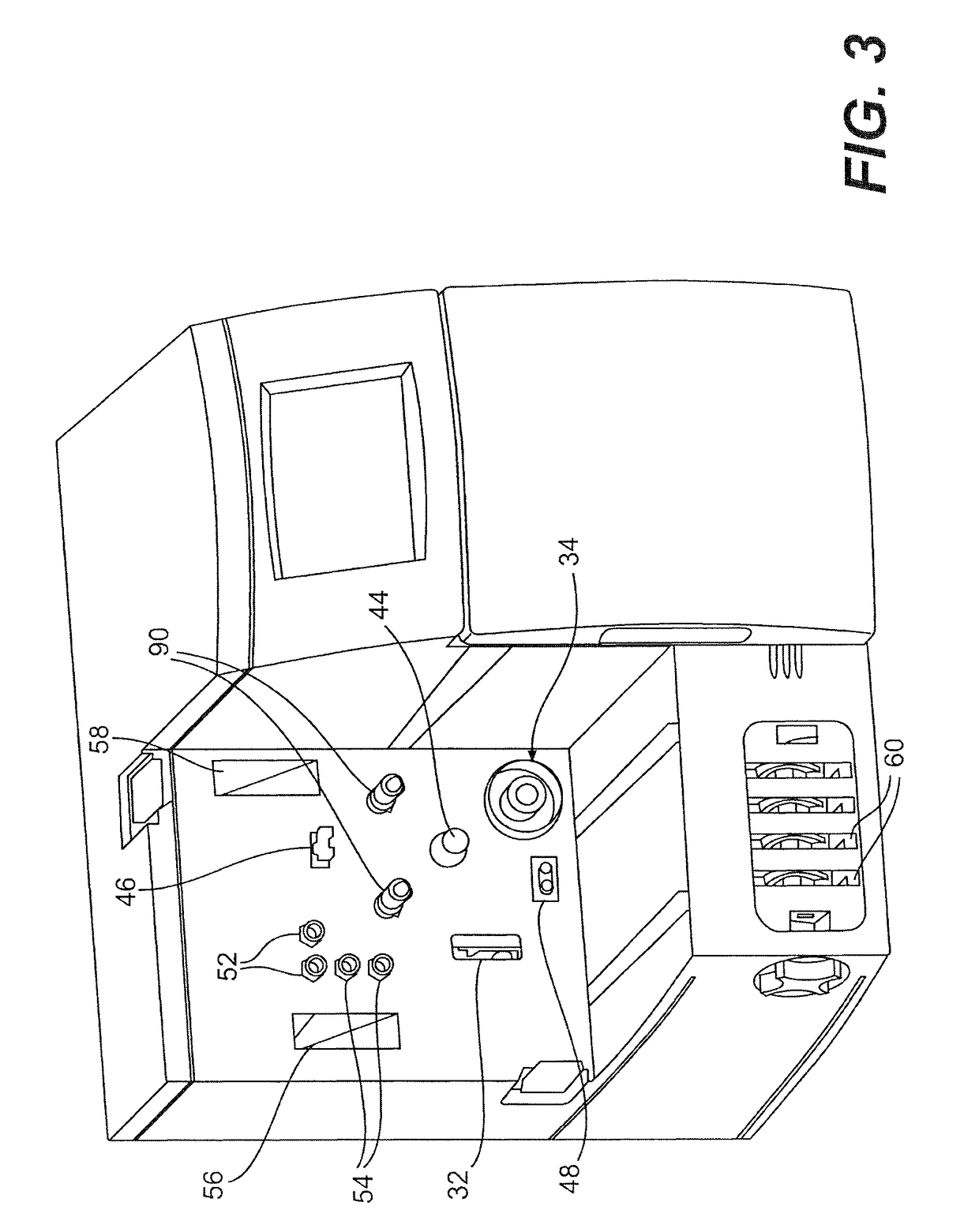
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
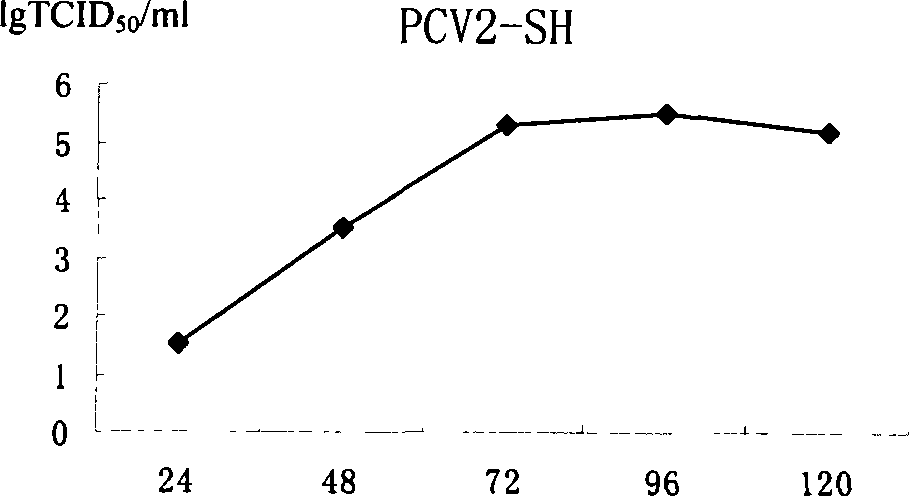
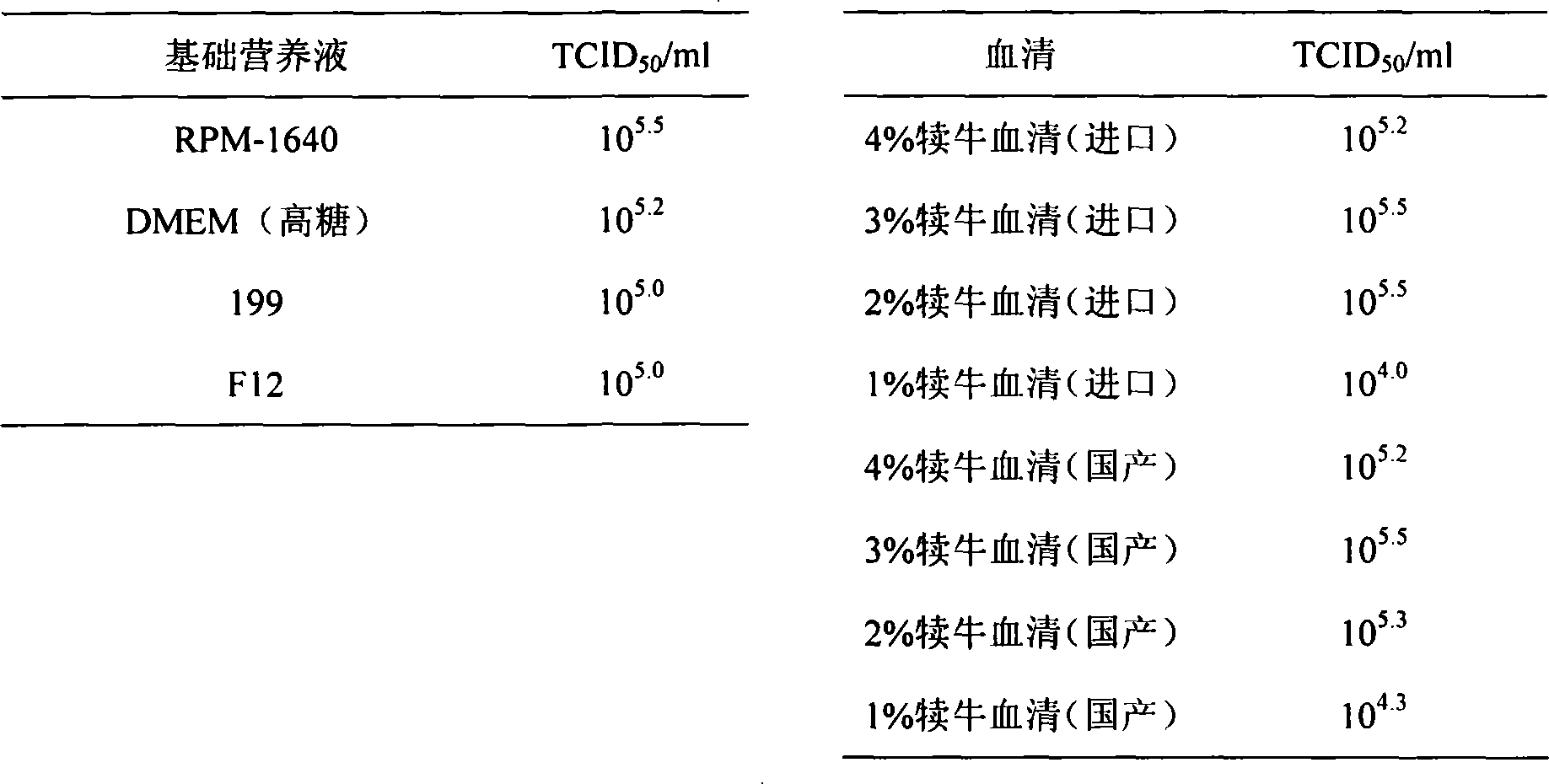
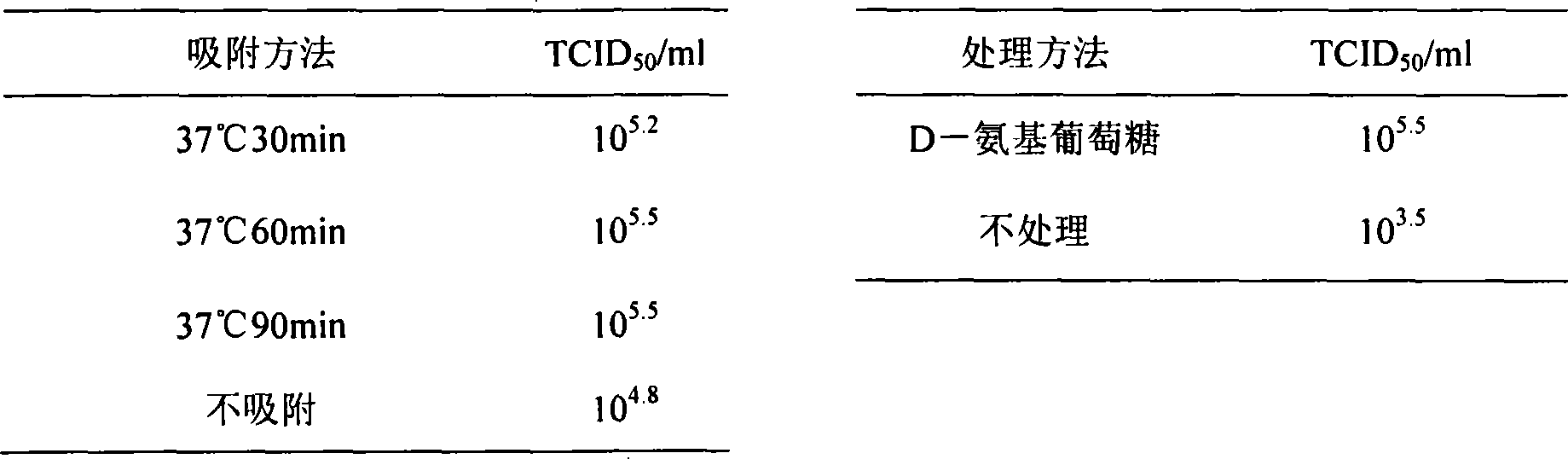
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Enhancement of in vitro culture or vaccine production using electromagnetic energy treatment
InactiveUS20050009161A1Enhanced and accelerated formationEnhancement and prolongation of life of cellBioreactor/fermenter combinationsBiological substance pretreatmentsVaccine ProductionLight energy
Disclosed are apparatus and methods for enhancing or improving cell cultures, including cell cultures for the production of monoclonal antibodies, using electromagnetic energy treatment, primarily using light in the near infrared to visible region of the spectrum. The delivery of light energy to a culture, in accordance with preferred embodiments, enhances or improves the cell culture such as by providing for enhanced and accelerated formation of important biological macromolecules, including, but not limited to, antibodies, proteins, collagen, and polysaccharides, and also providing for accelerated cellular replication and an enhancement or prolongation of the life of cells so treated.
Owner:PHOTOTHERA
Apparatuses and methods for the production of haematophagous organisms and parasites suitable for vaccine production
InactiveUS7229627B2Efficient collectionAvoid pollutionViral antigen ingredientsSurgerySporeParasitic life cycles
Disclosed are apparatuses and methods for the production of attenuated aseptic parasites in hematophagous insects generally, and production of Plasmodium species sporozoites in Anopheles species mosquitoes specifically; apparatuses and methods for the production of strains of hematophagous insects with desired properties such as hypoallergenicity or hyperinfectivity; methods of producing a parasite strain that is capable of withstanding cyropreservation at temperatures close to freezing; apparatuses and methods for the injection of an attenuated parasite vaccine; production of parasites and hematophagous insects that are free from contamination by unwanted biological agents; apparatuses for the reconstruction of complex parasitic life cycles aseptically to avoid the contamination of the parasite or the insect vector host with unwanted biological agents.
Owner:SANARIA INC
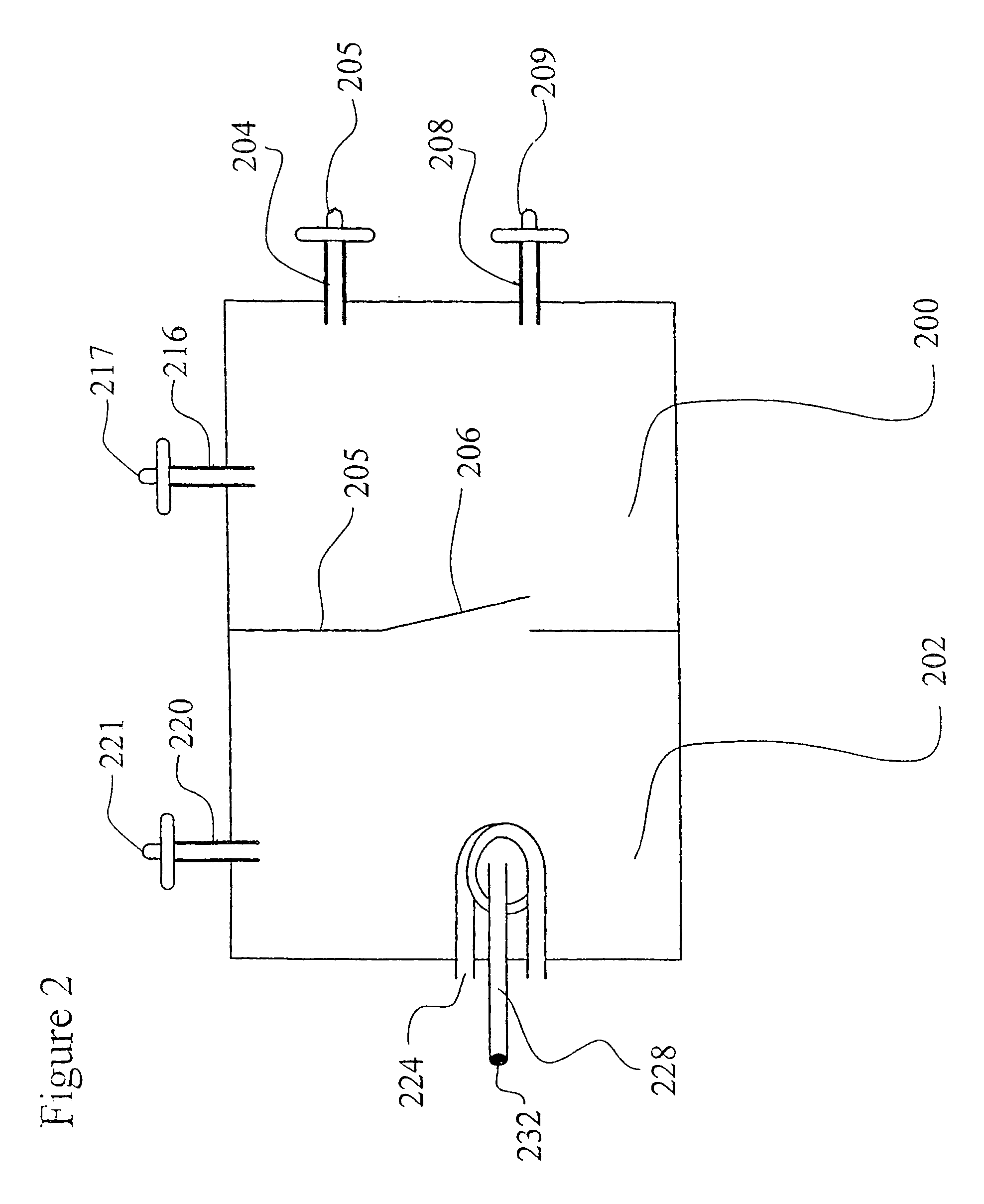
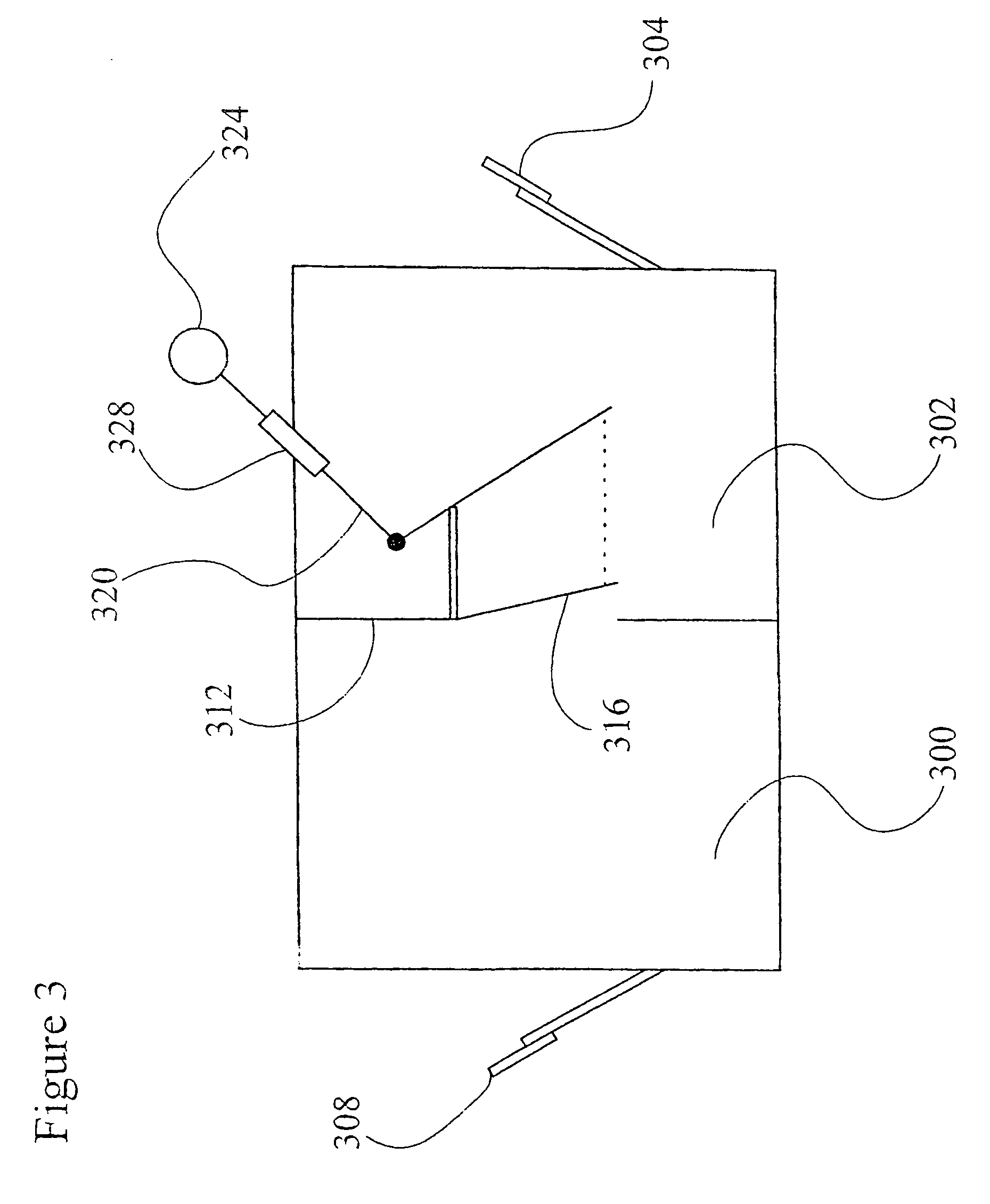
Recombinant avian herpesvirus useful in vaccine production
InactiveUS6913751B2SsRNA viruses negative-senseSsRNA viruses positive-senseInfectious laryngotracheitisInfectious laryngotracheitis virus
The present invention provides a novel avian herpesvirus (NAHV) vector and recombinant vaccines made therefrom that are useful to immunize avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease. Methods of immunizing an avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease are also provided.
Owner:SCHERING PLOUGH ANIMAL HEALTH
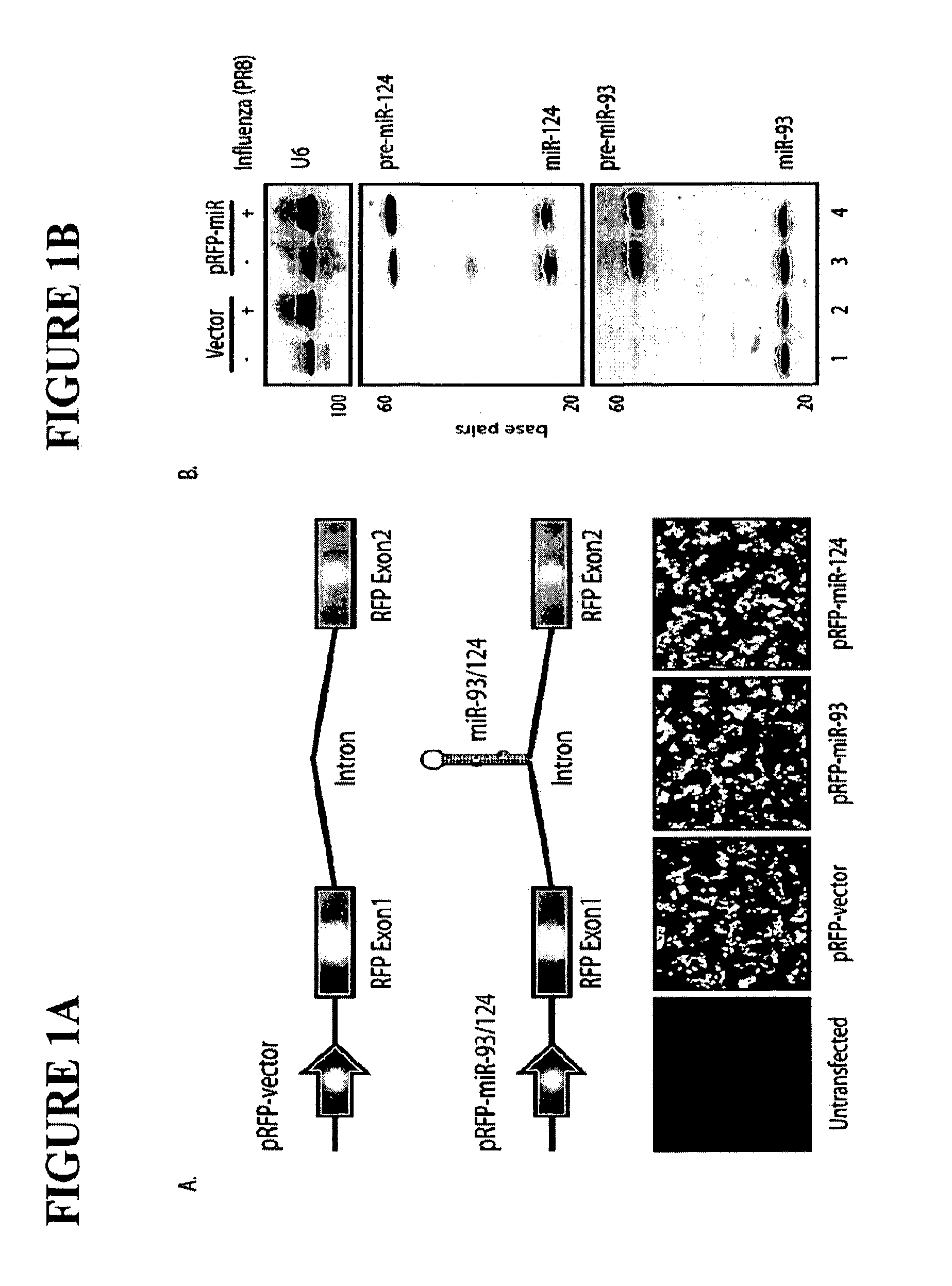
Live Attenuated Influenza Virus Vaccines Comprising Microrna Response Elements
ActiveUS20120148622A1Increase vaccine safetyQuick buildSsRNA viruses negative-senseSugar derivativesUltrasound attenuationInfluenza virus vaccine
The invention is directed to novel live attenuated influenza virus (LAIV) vaccines comprising one or more microRNA (miRNA) Response Element(s) (MRE) within an influenza virus genome. The MREs useful for the present invention can be derived from any miRNA which is highly expressed in influenza-targeted cells of an animal in need of vaccination but are not expressed or are expressed at very low levels in species (e.g., embryonated chicken eggs) or cell lines used for a large-scale vaccine production. This allows efficient vaccine production but renders the vaccine virus susceptible to attenuation in the influenza-targeted cells of vaccinated animals expressing a cognate miRNA.
Owner:MT SINAI SCHOOL OF MEDICINE
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
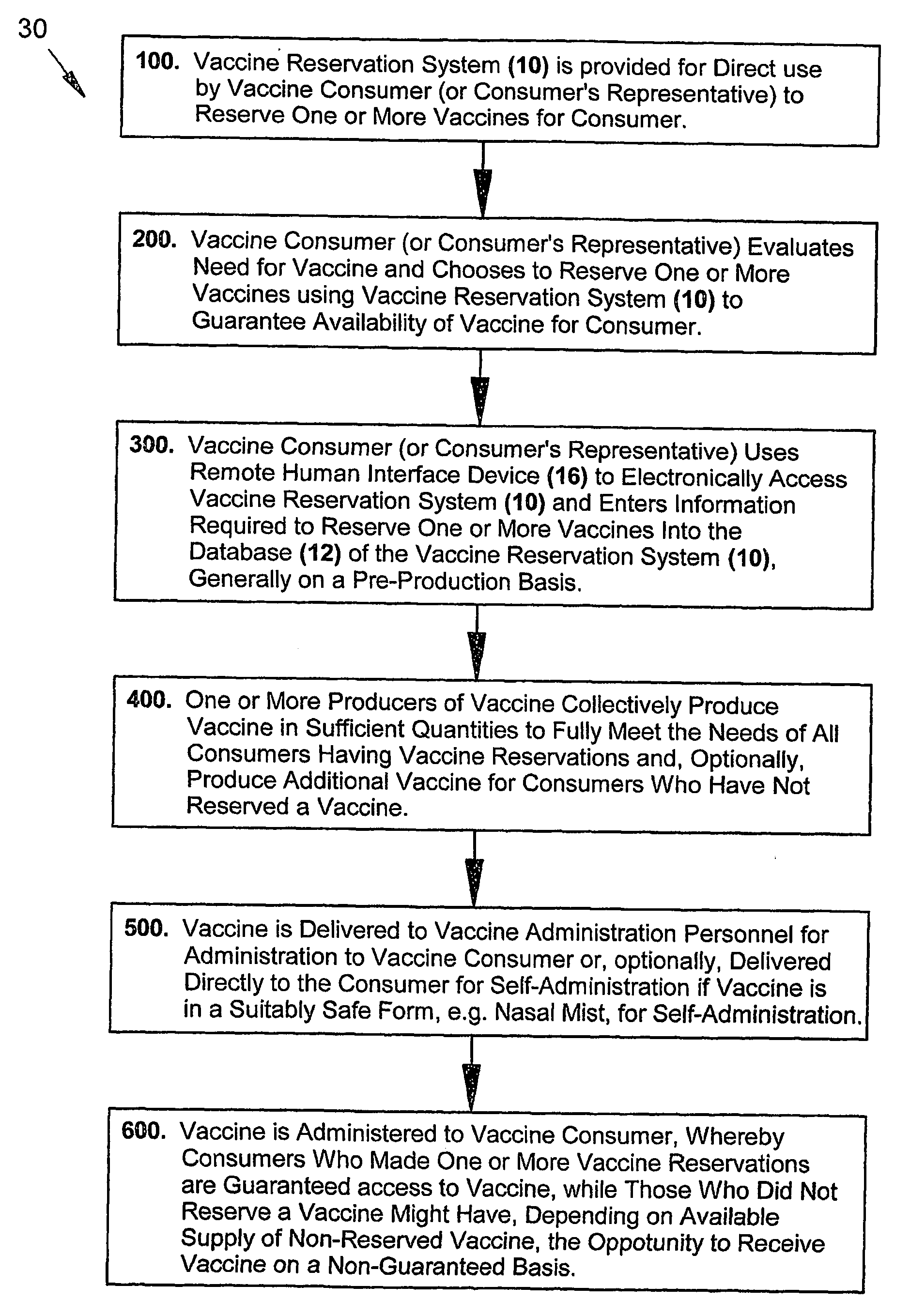
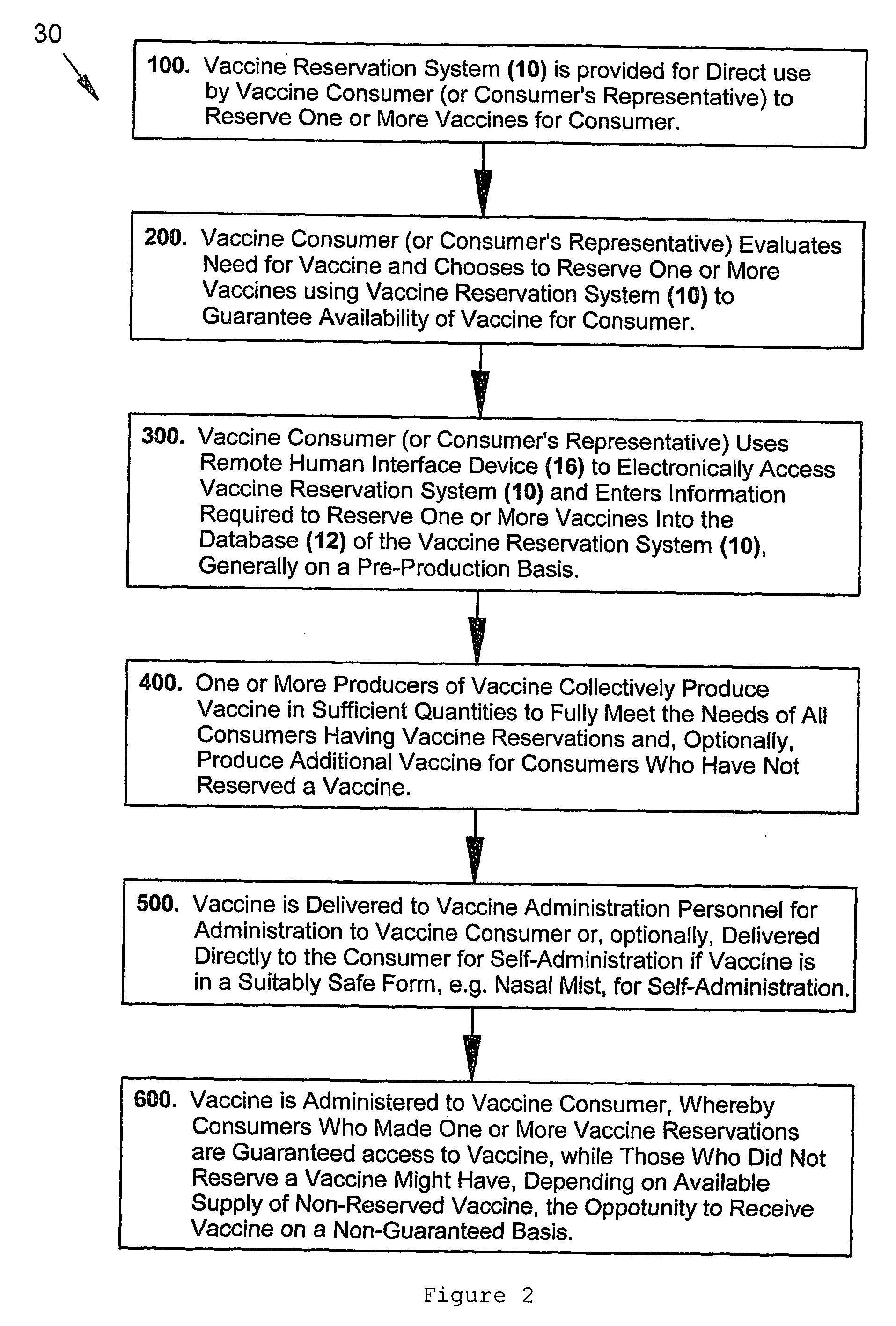
Consumer-driven pre-production vaccine reservation system and methods of using a vaccine reservation system
The present inventive subject matter relates to an automated, consumer-driven, pre-production, seasonal vaccine reservation system for facilitating the efficient production, distribution, and / or administration of health-critical, long-production-lead-time seasonal vaccines, or other pharmacological agents, whereby use of such a consumer-driven vaccine reservation system provides a highly effective means for substantially ensuring or guaranteeing that consumer demand for seasonal vaccine can be fully and consistently met, but without subjecting the producers of vaccine and / or other cognizant parties associated with the healthcare industry to undue financial risk. In addition to the physical elements comprising such a consumer-driven vaccine reservation system, the present invention also represents various unique operational methods for accurately matching seasonal vaccine supply with seasonal consumer demand, thereby substantially facilitating production, distribution, and / or timely administration of vaccine.
Owner:ESSIG JOHN R +3
Enhancement of in vitro culture or vaccine production in bioreactors using electromagnetic energy
InactiveUS20060223155A1Bioreactor/fermenter combinationsBiological substance pretreatmentsVaccine ProductionElectromagnetic radiation
Disclosed are apparatus and methods for enhancing or improving cell cultures, including cell cultures for the production of monoclonal antibodies, using electromagnetic energy treatment, primarily using electromagnetic radiation in the near infrared to visible region of the spectrum. The delivery of electromagnetic energy to a culture, in accordance with preferred embodiments, enhances or improves the cell culture such as by providing for enhanced and accelerated formation of important biological macromolecules, including, but not limited to, antibodies, proteins, collagen, and polysaccharides, and also providing for accelerated cellular replication and an enhancement or prolongation of the life of cells so treated.
Owner:PHOTOTHERA
Method for rapidly screening recombinant fowlpox virus by means of CRISPR/Cas9 system and application thereof
ActiveCN107446951AIncrease productivityImprove efficiencyViral antigen ingredientsStable introduction of DNADouble strandedVaccine Production
The invention relates to the field of biotechnology, in particular to a method for rapidly screening the recombinant fowlpox virus by means of a CRISPR / Cas9 system and application thereof. The method comprises the steps of designing an sgRNA (short guide RNA) double-stranded oligonucleotide sequence of a target gene, ligating the sequence with a linearized plasmid vector to obtain an sgRNA expression vector, co-transfecting chicken embryo fibroblasts (CEF) with the sgRNA expression vector, recombinant fowlpox virus plasmids expressing a foreign gene and the fowlpox virus, and conducting purification and verifying the purification effect. By means of the CRISPR / Cas9 method, the number of recombinant fowlpox virus screening generations is reduced to 3-4, the efficiency of vaccine production is greatly improved, operation is easy, and the production cost is reduced.
Owner:WENS FOOD GRP CO LTD
System and Method of Preparing and Storing Activated Mature Dendritic Cells
InactiveUS20130183343A1Promote recoveryImprove viabilityAntibacterial agentsBacterial antigen ingredientsDendritic cellVaccine Production
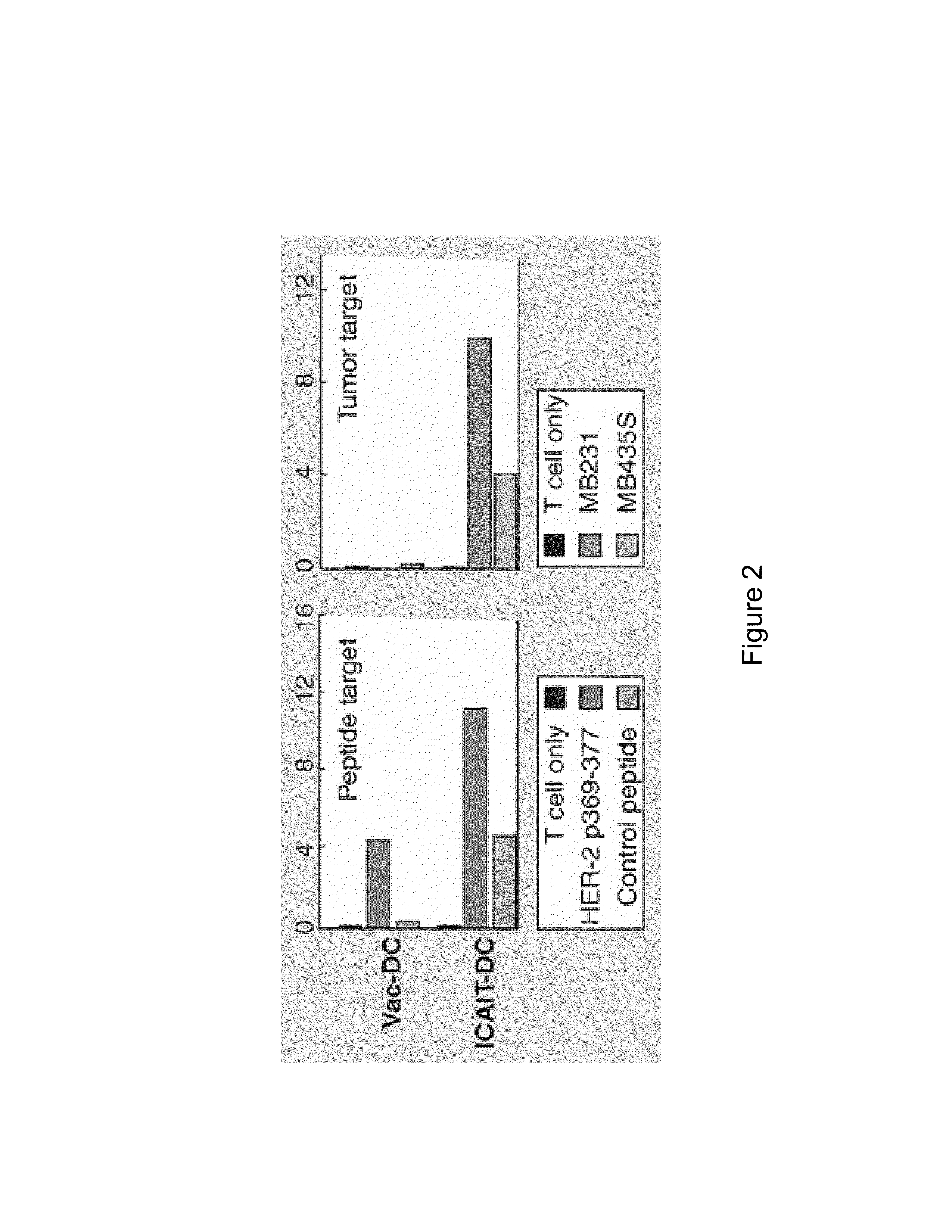
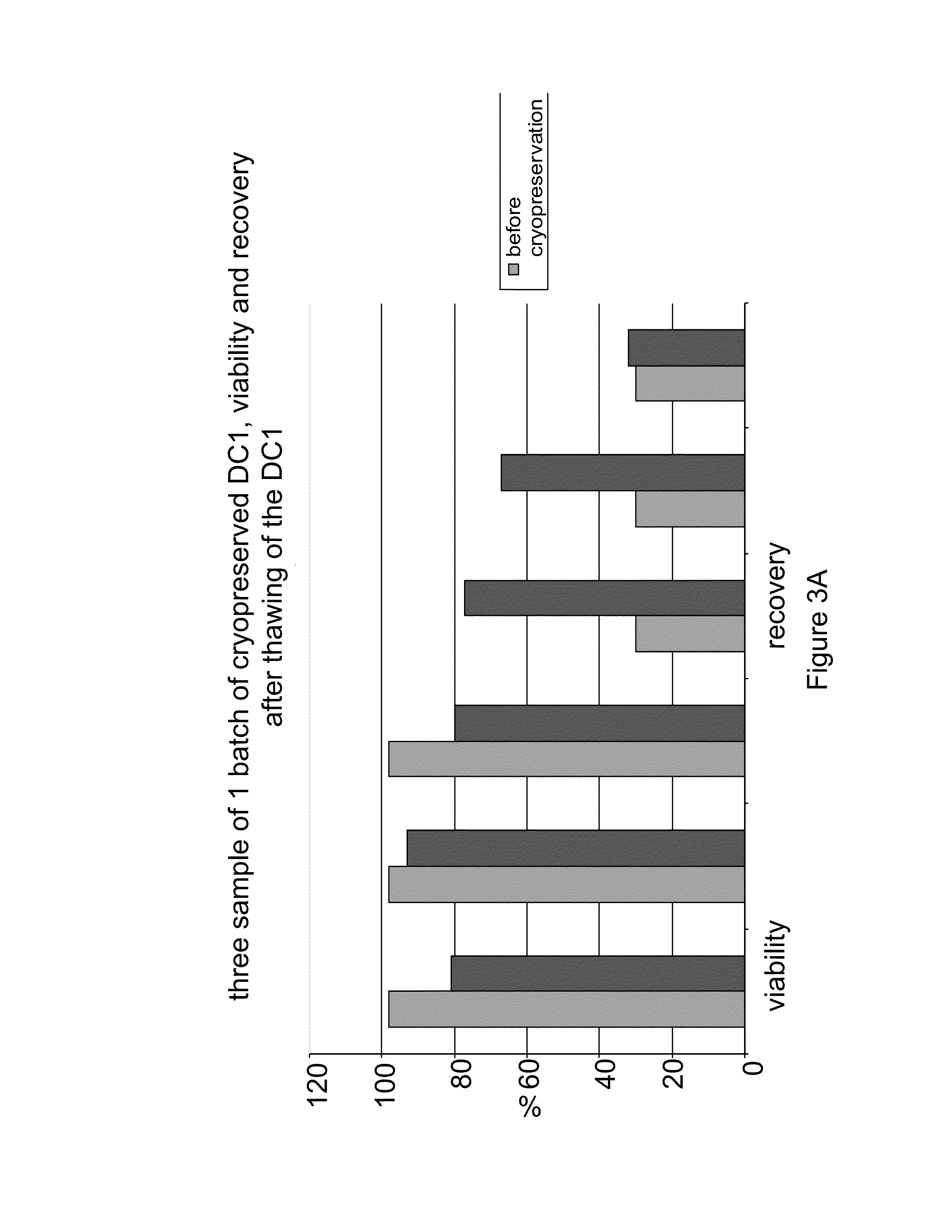
The present invention provides compositions and methods for generating and cryopreserving dendritic cells with superior functionality in producing stronger signals to T cells, resulting in a more potent DC-based anti-tumor vaccine. The present invention includes mature, antigen loaded DCs activated by Toll-like receptor agonists that induce clinically effective immune responses, preferably when used earlier in the disease process. The DCs of the present invention produce desirable levels of cytokines and chemokines, and further have the capacity to induce apoptosis of tumor cells. The cells can be cryopreserved and thawed for later use, thereby reducing the need for repeated pheresis and elutriation processes during vaccine production. These methods can also be utilized to directly target molecules involved in carcinogenetic signaling pathways and cancer stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Establishing method of pig immunoglobulin Fc fragment-swine classical fever E2 fusion protein in CHO cell strain, as well as preparation method and application of fusion protein
ActiveCN106519041ASsRNA viruses positive-senseAntibody mimetics/scaffoldsFusion Protein ExpressionVaccine Production
The invention relates to a vaccine production technology in the technical field of biology, in particular to a CHO cell strain which is established by utilizing a gene engineering means and is used for expressing recombinant protein PigFC-pigSCFVE2, and a preparation method and application of the recombinant protein. The recombinant fusion protein PigFC-pigSCFVE2 provided by the invention is A1) or A2) shown as follows, wherein A1) is protein of which the amino acid sequence is as shown in SEQ ID No.2, and A2) is protein which is obtained by substituting, losing and / or adding one or several amino acid residues in the amino acid sequence of the protein of the A1) and has PigFC-pigSCFVE2 activity. A monoclonal cell strain which is obtained through the method and capable of carrying out secretory expression on PigFC-pigSCFVE2 is higher in fusion protein expression quantity, fusion protein obtained through affinity separation and purification of an antibody can be combined with a monoclonal antibody, animals can be immunized, the immunity of a generated neutralizing antibody is higher than that of a present market product, the fusion protein can be used for swine classical fever preventive vaccine, and the production cost and the immunity failure loss can be reduced.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
Production of poliovirus at high titers for vaccine production
ActiveUS8546123B2High potencyShort processNervous disorderSsRNA viruses positive-senseSerum freeVaccine Production
Provided is a process for the production of poliovirus, comprising the steps of:a) providing a serum-free suspension culture of cells, which are primary human retina (HER) cells that have been immortalized by expression of adenovirus E1 sequences,b) infecting the cells with poliovirus, at a cell density of between 2×106 cells / ml and 150×106 cells / ml, and c) harvesting poliovirus at a time of between 12 and 48 hours after infection.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
Apparatuses and methods for the production of haematophagous organisms and parasites suitable for vaccine production
InactiveUS20070169209A1Avoid pollutionEfficient collectionProtozoaAgainst vector-borne diseasesSporeParasitic life cycles
Disclosed are apparatuses and methods for the production of attenuated aseptic parasites in haematophagous insects generally, and production of Plasmodium species sporozoites in Anopheles species mosquitoes specifically; apparatuses and methods for the production of strains of haematophagous insects with desired properties such as hypoallergenicity or hyperinfectivity; methods of producing a parasite strain that is capable of withstanding cyropreservation at temperatures close to freezing; apparatuses and methods for the injection of an attenuated parasite vaccine; production of parasites and haematophagous insects that are free from contamination by unwanted biological agents; apparatuses for the reconstruction of complex parasitic life cycles aseptically to avoid the contamination of the parasite or the insect vector host with unwanted biological agents.
Owner:SANARIA INC
Virus preparation or vaccine production method by culturing cells with polyester fiber carrier
InactiveCN102406926AHigh culture specific surface areaReduce the chance of infectionAnimal cellsViral antigen ingredientsPolyesterCulture cell
The invention relates to a virus preparation or vaccine production method by culturing cells with a polyester fiber carrier. The method particularly comprises the following steps: 1) preparing a polyester fiber cell culturing carrier by adopting a processing method; 2) in a cell culturing system, culturing proliferation cells in a cell proliferation culture solution with the treated polyester fiber cell culturing carrier; 3) changing a cell maintaining culture solution after the cells grow to a certain density or number, and inoculating virus to infect cells; 4) amplifying virus; 5) harvesting virus fluid; 6) inactivating or implementing living vaccine treatment according to the required preparation type; 7) concentrating, purifying and cracking the virus fluid; 8) adding a stabilizing agent and an immunoadjuvant; and 9) packaging vaccine. According to the method, a material which has low price and is suitable for wall attaching growth of the cells is used to make the cell culturing carrier, carry out cell culture and prepare virus or produce vaccine, so that the cost of the cell carrier is greatly reduced.
Owner:上海泰因生物技术有限公司
Permissive cells and uses thereof
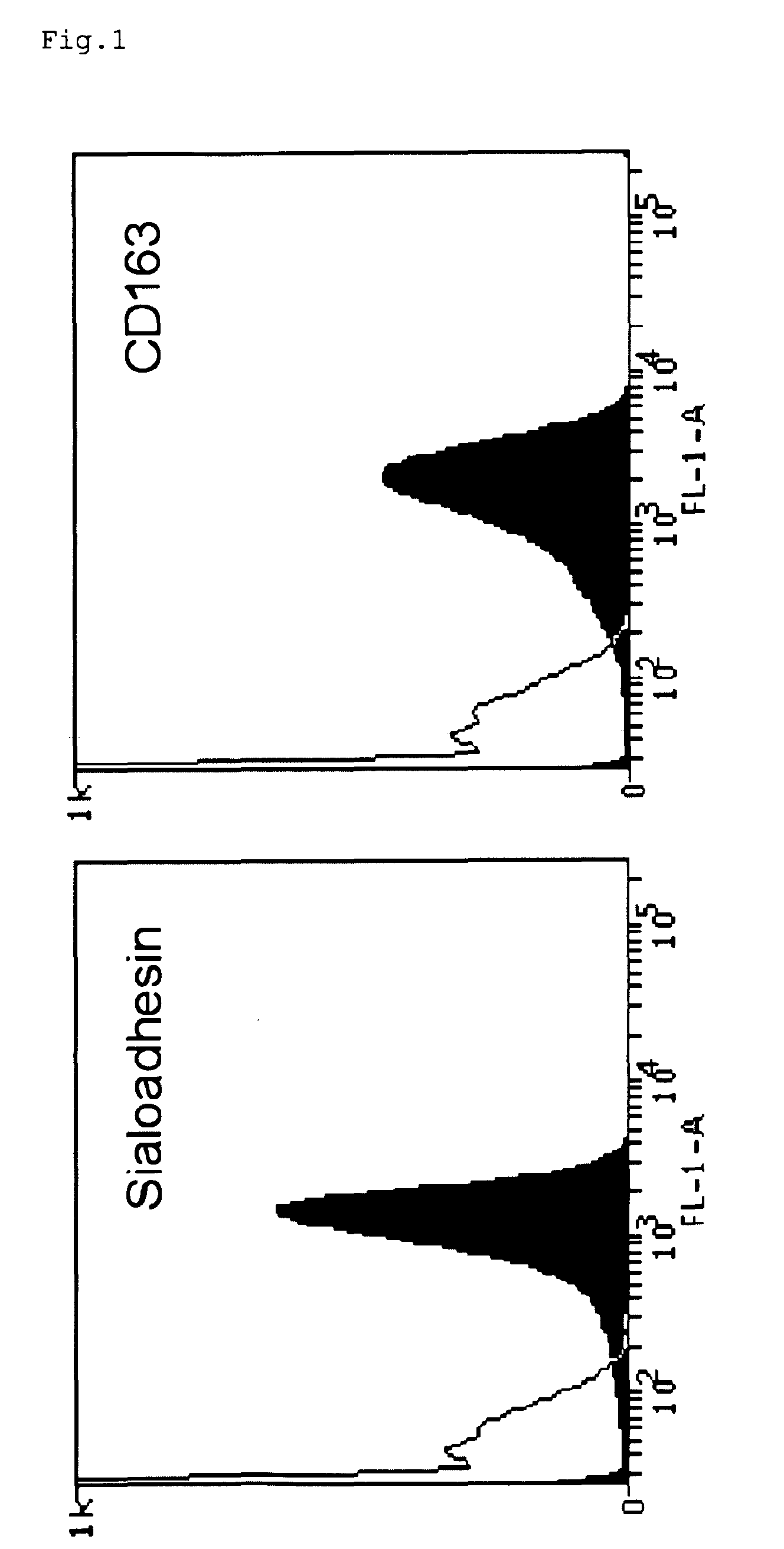
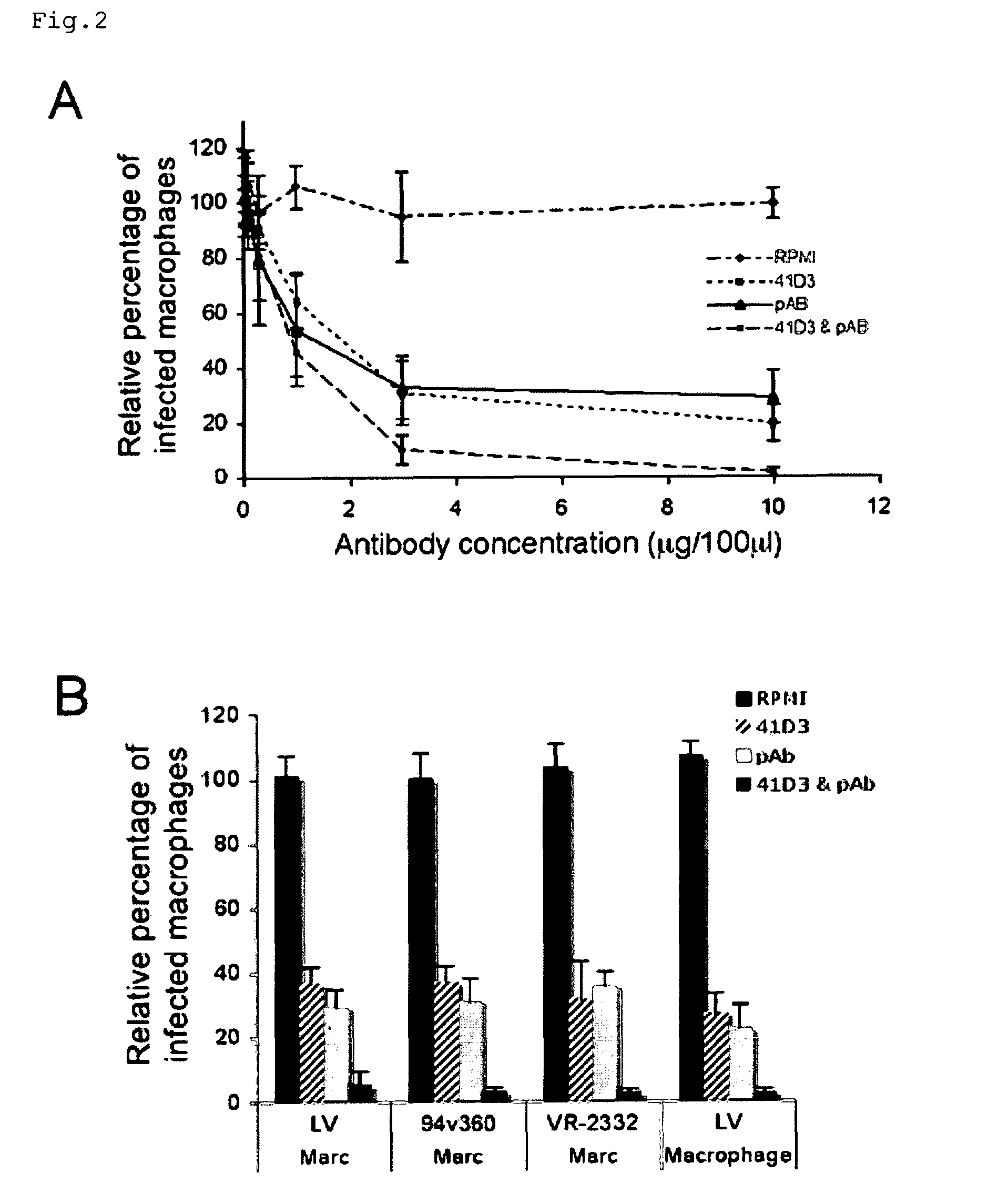
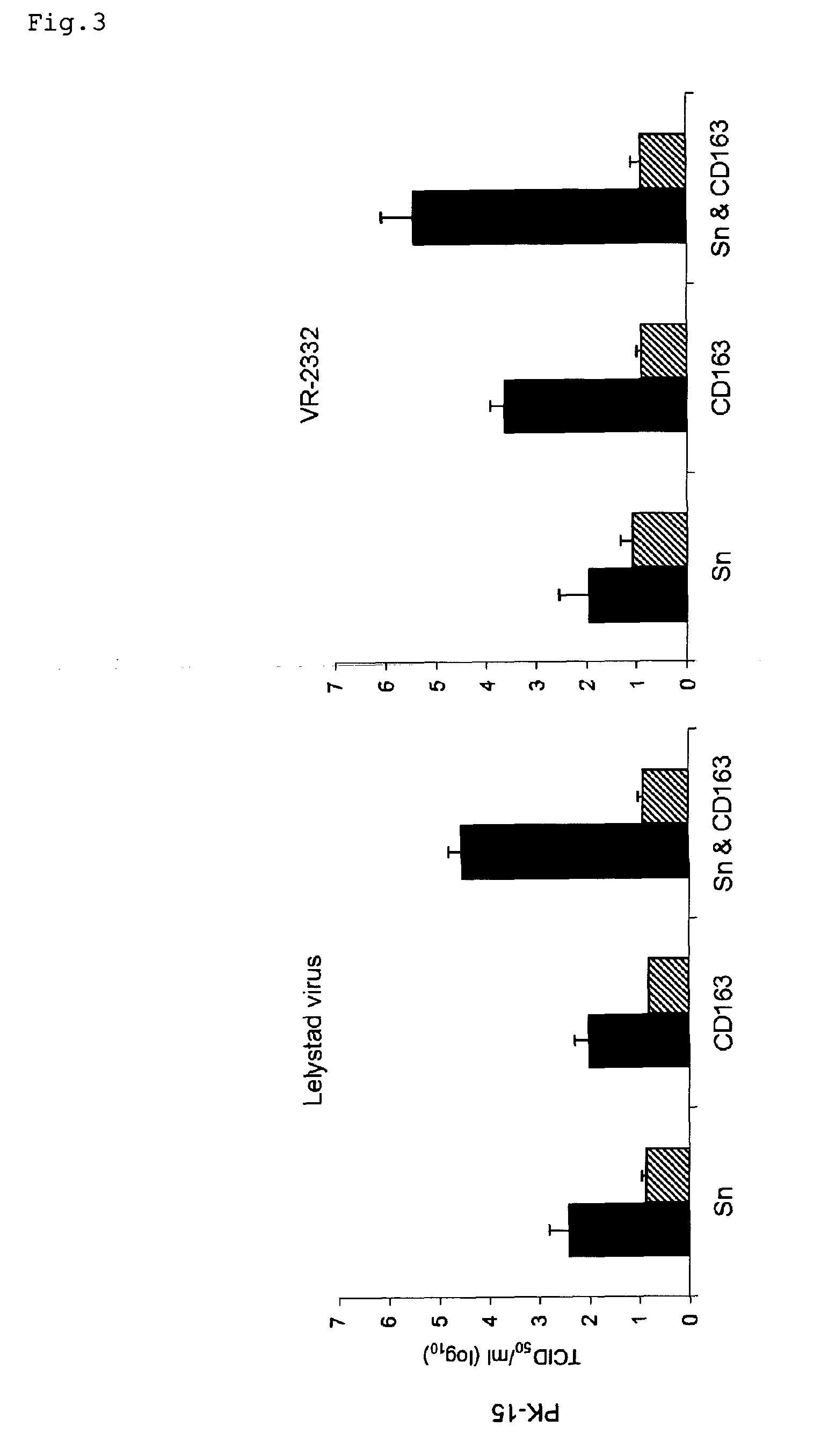
ActiveUS20100158947A1Compound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
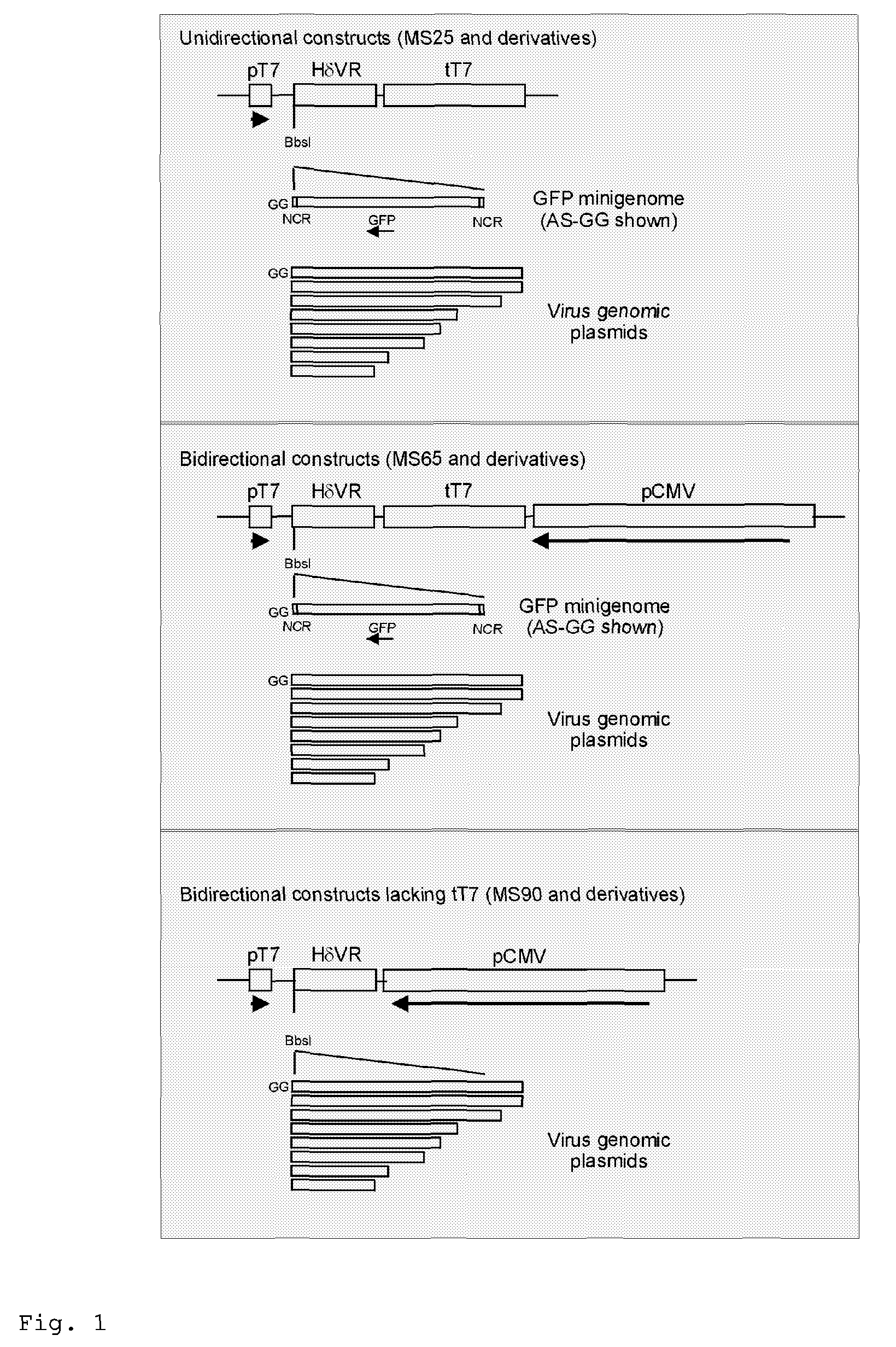
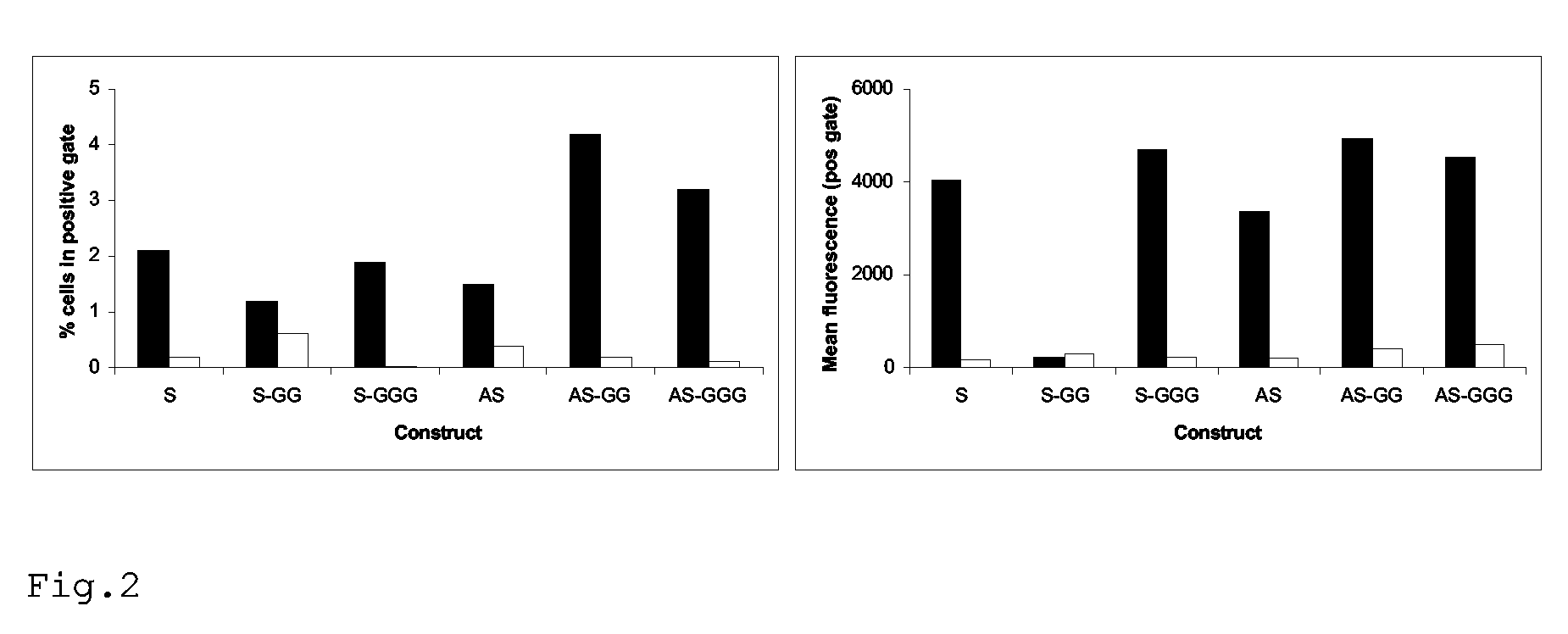
Rescue of influenza virus
The invention relates to the field of influenza vaccine production. Influenza vaccines have been produced in embryonated hens' eggs for over 50 years, but recently there have been considerable efforts to develop cell culture systems for vaccine production. The invention provides a nucleic acid comprising an influenza gene segment and a bacteriophage polymerase promotor or a complementary strand of said nucleic acid, and a cell comprising such a nucleic acid capable of producing desired influenza virus. Furthermore, the invention provides a composition comprising a cell or material derived from a cell according to the invention and a virus or material derived from a viral particle according to the invention.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC +1
Avian influenza virus inactivated vaccine and preparation method thereof
InactiveCN102600464AAchieve mass productionIncrease volumeAnimal cellsAntiviralsAdjuvantVaccine Production
The invention discloses an avian influenza virus inactivated vaccine and a preparation method thereof. Adaptive avian influenza viruses are adaptive to screening and domestication of a continuous cell line, and after the viruses are adaptive to cells, the number of the viruses is continuously increased until the viruses are inoculated into a cell culture bottle or a bioreactor to culture. After the obtained culture is inactivated, the obtained culture and mineral oil adjuvant are mixed and emulsified so as to prepare the vaccine. A virus reproduction method for producing an avian vaccine by means of utilizing a large quantity of chicken embryo is omitted, resource consumption is reduced, environmental pollution is decreased, biosafety is guaranteed, cost is lowered, and production efficiency is improved. By the aid of the preparation method, high-potency viruses can be produced to prepare corresponding vaccines, and requirements of fast and high-efficient vaccine production are met.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD +1
Enterovirus type 71 and use thereof
ActiveCN101717754AViral/bacteriophage medical ingredientsHybrid cell preparationEnterovirusHand-foot-and-mouth disease
The invention relates to an enterovirus type 71 (abbreviated as EV71) and use thereof, in particular to a monoclonal virus strain which is separated by a plaque assay method. The progeny viruses of the monoclonal virus strain show genetic stability. The monoclonal virus strain can be used in the production of a vaccine (particularly the production of a vaccine for infant). The virus strain or the vaccine produced by using the same can be used in the prevention of diseases (such as hand-foot-and-mouth diseases, particularly hand-foot-and-mouth diseases in children) caused by the EV71 and has the characteristics of stable titer, high immunogenicity and small immunizing dose.
Owner:SINOVAC BIOTECH
Method for improving proliferation capability of mycoplasma gallisepticum in vaccine production
ActiveCN102168073AImprove proliferative abilityHigh titerAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureVaccine Production
The invention relates to a method for improving proliferation capability of mycoplasma gallisepticum in vaccine production, vaccines are prepared through the process steps of preparing seeds, preparing a culture medium of the mycoplasma gallisepticum, preparing vaccine-making bacterial liquid and preparing the inactivated vaccines, after improvement, the content of PPLO (pleuropneumonia-like organism) broth, porcine serum and glucose is increased, MEM (minimum essential medium) ingredients are removed, and the mixture ratio of the ingredients in the culture medium are more scientific and the nutritional value is higher after adjustment. The bacterial liquid concentration of a semi-finished product prepared by the method is as high as 1.0*1013CCU / ml-1.0*1014CCU / ml, and the bacterial liquiddoes not need to be concentrated and can be directly or indirectly used for preparing the inactivated vaccines after dilution, thereby not only simplifying the production process, but also reducing the production cost.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Methods for producing virus for vaccine production
InactiveUS20190194628A1Reducing per-dose costCost-effectiveSsRNA viruses positive-senseViral antigen ingredientsCulture cellVaccine Production
The present disclosure relates to methods of producing Enterovirus C, e.g., for poliomyelitis vaccine production. In some embodiments, the methods include adding polysorbate to the cell culture medium during or prior to inoculation with the virus and / or culturing cells in a fixed bed bioreactor. Further provided herein is an Enterovirus C produced by the methods of production disclosed herein, as well as compositions, immunogenic compositions, and vaccines related thereto.
Owner:TAKEDA PHARMA CO LTD +1
Method for industrially producing pseudorabies vaccine by using bioreactor
InactiveCN102038946AHigh titerHigh degree of automation controlMicroorganism based processesAntiviralsSide effectCytopathic effect
The invention relates to a method for industrially producing a pseudorabies vaccine by using a bioreactor. The method comprises the following steps of: (1) sterilizing a microcarrier and the bioreactor, inoculating cells for vaccine production, culturing the cells, and after the cells on the microcarrier form a compact monolayer, inoculating pseudorabies virus, and continuously culturing to allow the virus to reproduce; (2) after more than 80 percent of cells are subjected to a cytopathic effect, stopping culturing, and obtaining virus liquid to prepare the pseudorabies vaccine. In the method, the pseudorabies vaccine is produced by culturing the cells in high density by the bioreactor microcarrier culture technology; and compared with the conventional roller bottle production process, the invention has the advantages that: the method has high automation control degree, saves manpower, reduces cost and is small in production land, the production can be monitored in real time, the scale is easy to expand, the produced virus has high titer and small difference among batches, and the product has stable quality and small side effect.
Owner:WUHAN CHOPPER BIOLOGY
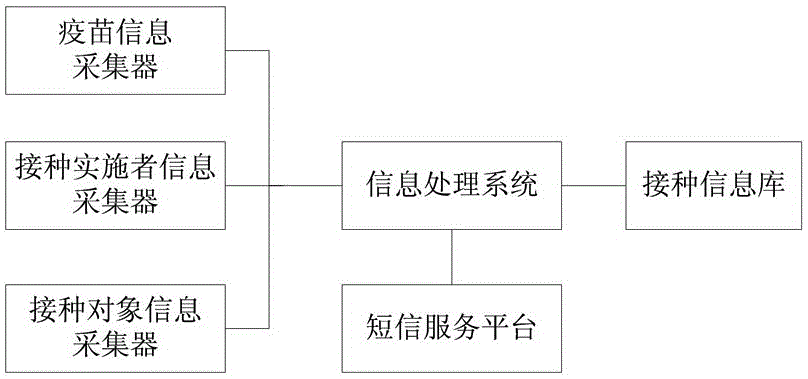
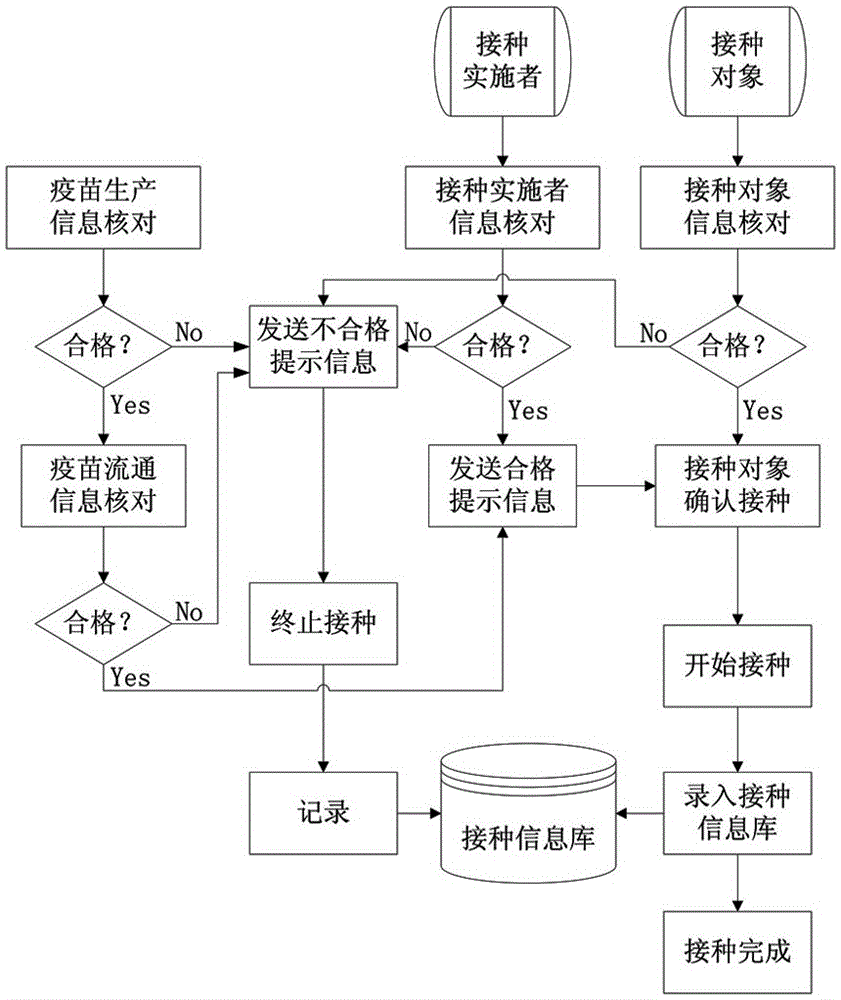
Human vaccine-matched vaccination information acquisition and traceability system
InactiveCN106557793AThe principle is simpleEasy to implementCo-operative working arrangementsInformation processingVaccination
The invention discloses a human vaccine-matched vaccination information acquisition and traceability system, which comprises a vaccine information acquisition device, a vaccination implementer information acquisition device, a vaccination object information acquisition device, a vaccination information bank, a short message service platform, and an information processing system, wherein the vaccine information acquisition device is in charge of acquiring vaccine production and circulation information; the vaccination implementer information acquisition device is used for directly reading and recording the identity information and the occupation information of the vaccination implementer; the vaccination object information acquisition device is used for directly reading and recording the identity information of the vaccination object; the vaccination information bank comprises a server and a database for recording the information of the acquired vaccine, the vaccination implementer and the vaccination object the short message service platform is used for sending vaccine eligibility verification information, and vaccination vaccine type and time type prompt information to the vaccination object and receiving vaccination confirmation information by the vaccination person; and the information processing system is used for being in charge of acquisition, query, matching and storing on human and vaccine information during the vaccination implementation process. The system of the invention has the advantages that the principle is simple; realization is easy; and each vaccine can be ensured to be safely controlled, and the vaccination is ensured to be safe and effective.
Owner:杨斯淋
Vero cell-based influenza virus strains and vaccines
InactiveUS20090074804A1Increase productionSsRNA viruses negative-senseVirus peptidesVaccine ProductionMammal
The present invention relates to isolated influenza virus strains suitable for increased vaccine production for mammals. The influenza virus strains contain at least one modified influenza protein that results in increased production of the influenza virus from a mammalian host cell, such as a vero cell. The present invention also relates to the vaccines produced from the influenza virus strains. The present invention further relates to isolated modified influenza proteins and isolated nucleic acid molecules that encode for the modified influenza proteins.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Application of baby hamster kidney(BHK)-21 cell serum-free suspension culture technology in foot-and-mouth disease vaccine production
ActiveCN102178946AShort cycleIncrease productionMicroorganism based processesAntiviralsSerum igeSerum free
The invention discloses application of a baby hamster kidney(BHK)-21 cell serum-free suspension culture technology in foot-and-mouth disease vaccine production, which comprises the following steps of: 1) performing cell recovery; 2) performing reactor culture and cell amplification culture; and 3) inoculating foot-and-mouth disease virus seed venom and collecting the venom. A process for producing foot-and-mouth disease inactivated vaccines by culturing the BHK-21 cells through serum-free suspension culture and a step-by-step cell amplification method make the production period f the foot-and-mouth disease vaccines shortened and yield increased, and ensure stable quality and obvious benefit. The production process reduces the using amount of a culture medium, and the amount of the collected virus liquid is the culture medium consumption amount, while the culture medium consumption amount in a roller bottle production process is 2 times higher than the amount of the collected virus liquid, and bovine serum which is about 5 percent of the culture medium amount is needed.
Owner:马忠仁 +5
Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
PendingCN107674883AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetVaccine Production
The invention discloses preparation methods and application of recombinant swine fever E2 protein and a subunit vaccine of the recombinant swine fever E2 protein. The preparation method of the recombinant swine fever E2 protein comprises the following steps that (1) a swine fever E2 protein coding gene is cloned into an eukaryotic expression vector to obtain recombinant plasmid containing the swine fever E2 protein coding gene; (2) then, the recombinant plasmid containing the swine fever E2 protein coding gene is transfected into a CHO cell strain; (3) the CHO cell strain obtained in the step(2) is cultured, screened and domesticated; and (4) the cell strain in the step (3) is fermented and cultured; and the recombinant swine fever E2 protein is obtained after purification. The methods provided by the invention have the advantages that the target protein can be obtained from cell culture supernatant; the yield reaches up to 1g / L; the protein purification time is shortened; the vaccineproduction steps are simplified; and the vaccine production cost is also greatly reduced.
Owner:NOVO BIOTECH CORP
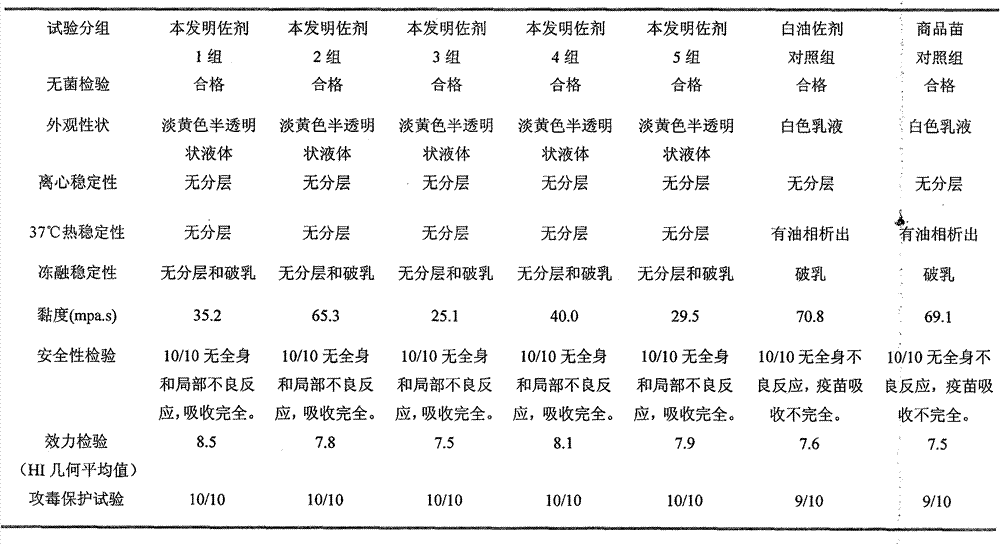
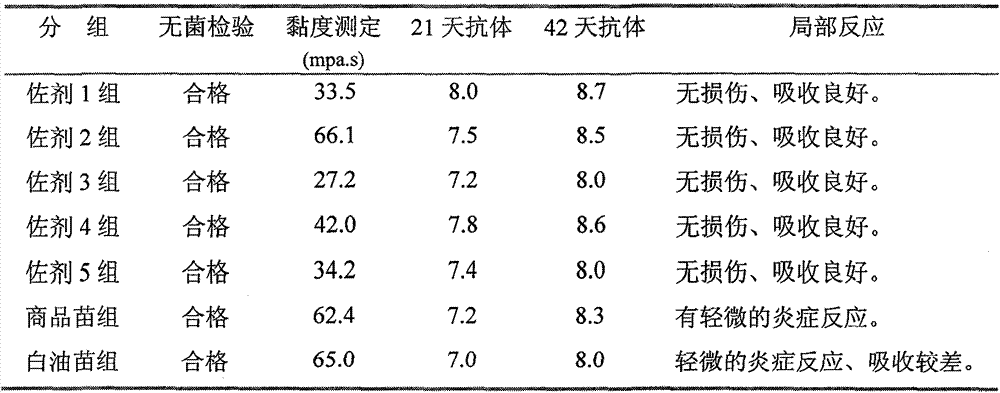
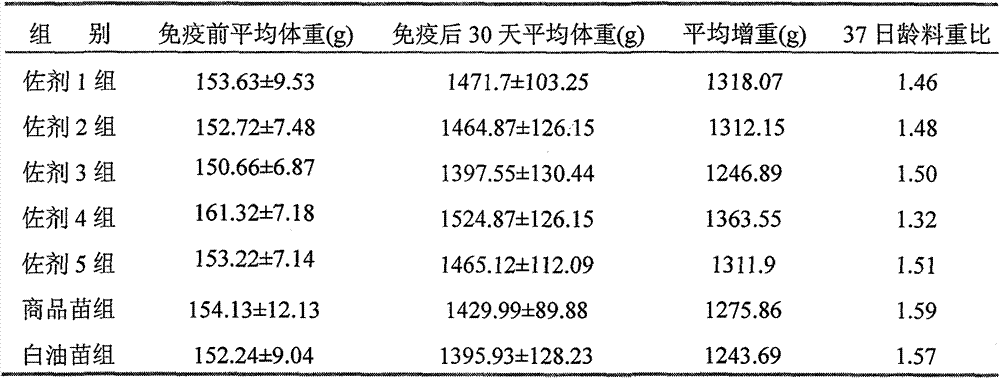
Preparation method and application of novel oil-free adjuvant
ActiveCN103083659AImprove product qualityReduce manufacturing costAntibacterial agentsAntiviralsImmune effectsAdjuvant
The invention relates to a preparation method of a novel oil-free adjuvant (adjuvant 605) compound and an application of the novel oil-free adjuvant in veterinary vaccines. The adjuvant prepared by using the method provided by the invention is capable of completely taking the place of mineral oil adjuvants and alumina gel adjuvants for inactivated vaccine production; the adjuvant can be completely absorbed by organisms within short time without residual; the adjuvant is low in side reaction, and capable of improving the safety of vaccines, and simultaneously simplifying the vaccine production process and reducing the vaccine production cost. The adjuvant prepared by using the method provided by the invention can be also taken as live vaccine diluting protection liquid, and has the characteristics of protecting the biological activity of the vaccine antigen, enhancing the immune effect of the vaccine, prolonging the persistent period of the vaccine antibody and the like.
Owner:BEIJING HUAXIA XINGYANG BIOLOGICAL SCI & TECH
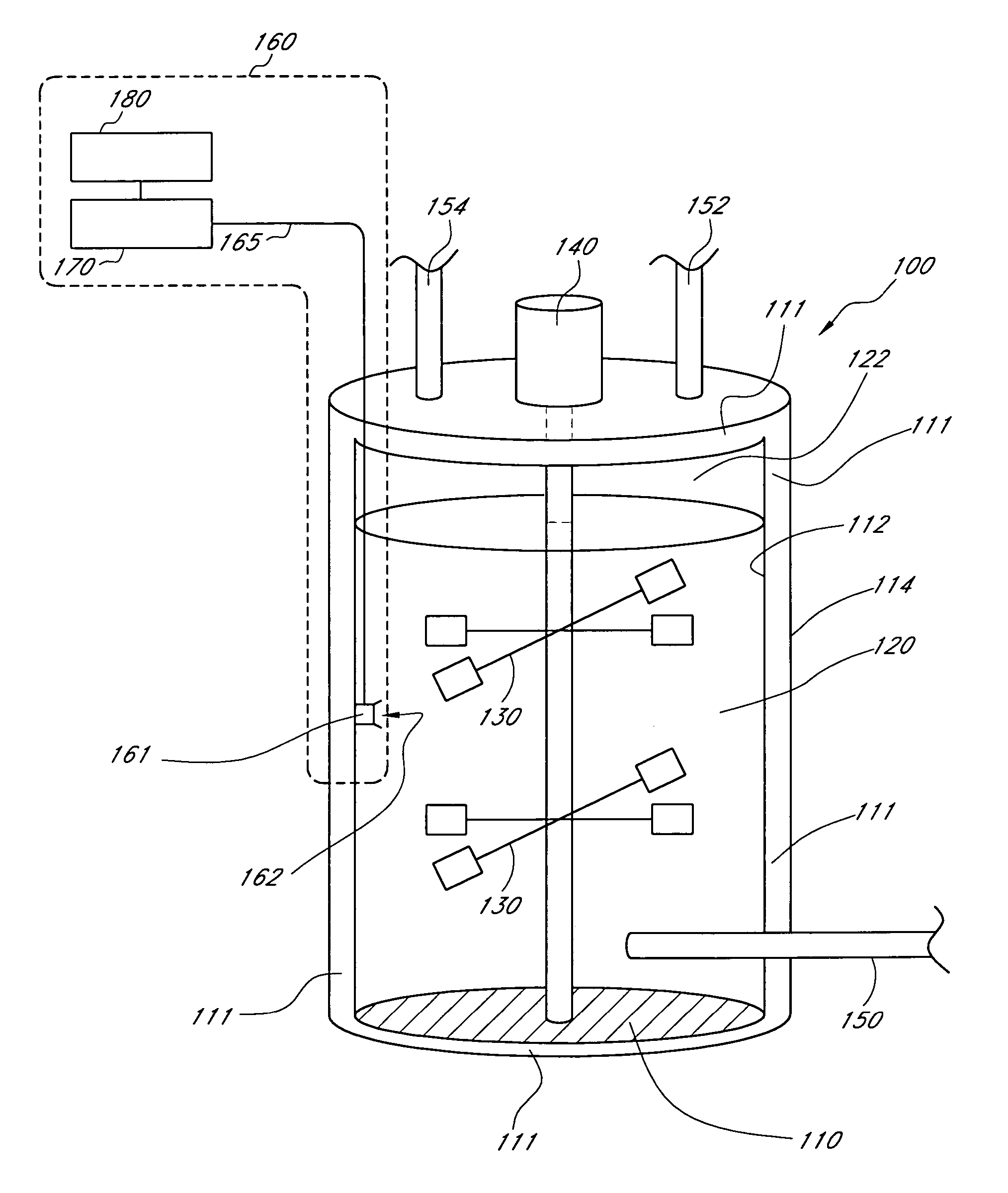
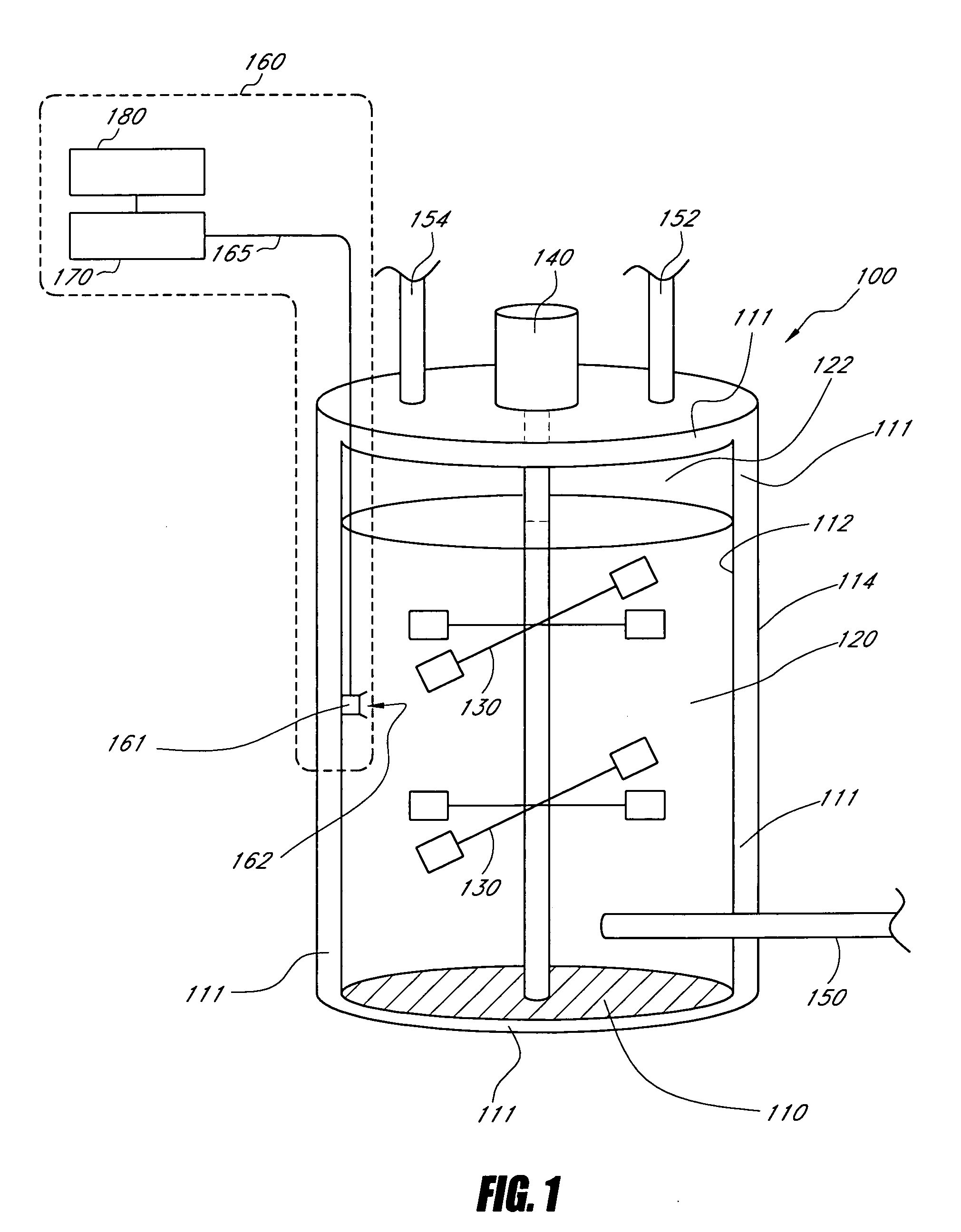
Method and apparatus for virus and vaccine production
ActiveUS9732313B2High yieldIncrease flow rateSsRNA viruses negative-senseBioreactor/fermenter combinationsVaccine ProductionVirus-like particle
Owner:BIOVEST INT
Avian adenovirus 4-type strain, vaccine composition and application of strain
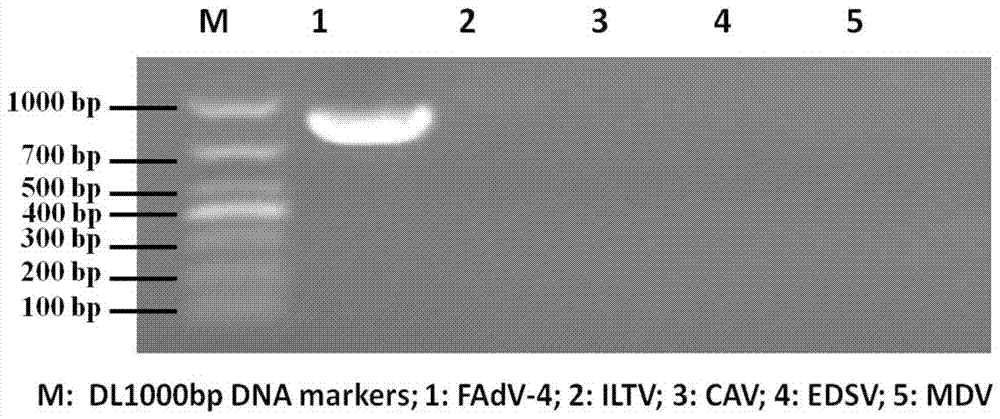
ActiveCN107338226AImprove featuresImproving immunogenicityViral antigen ingredientsAntiviralsFowlMicroorganism
The invention discloses a newly isolated avian adenovirus 4-type HNJZ strain. The microbial preservation number of the newly isolated avian adenovirus 4-type HNJZ strain is CGMCC NO.13385, and the strain has good specificity and immunogenicity, can serve as an inactivated vaccine production strain and an inspection strain and is used for preventing hepatitis-hydropericardium syndromes of poultry. The invention further discloses a vaccine composition prepared from the avian adenovirus 4 type HNJZ strain. The vaccine composition contains the inactivated avian adenovirus 4-type HNJZ strain and pharmaceutically acceptable adjuvant, the vaccine composition has good immunogenicity, high immunity can be generated after immunization, the protection rate is high, prevalence and spread of avian adenoviruses can be effectively prevented, and application prospects are wide.
Owner:HENAN AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com