Avian influenza virus inactivated vaccine and preparation method thereof
An inactivated vaccine and avian influenza technology, which is applied to the preparation method and the product field obtained by the method, can solve the problem of late start of large-scale industrialized animal cell culture, no large-scale production of avian influenza inactivated vaccine, staying in the starting period, problems such as laboratory scale, to achieve the effect of reducing the factors of human operation, improving the uniformity, and improving the degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In the present invention, the preparation method of bird flu inactivated vaccine comprises the steps:
[0040] 1) subculture and cultivation of cells for seedling production;
[0041] 2) The virus adapts to cell domestication;
[0042] 3) Optionally, establishing virus seed batches;
[0043] 4) Cultivation of virus for making seedlings;
[0044] 5) Harvesting of virus liquid for making seedlings;
[0045] 6) Optionally, make a finished vaccine.
[0046] In the present invention, step 3) is optional, that is to say, the preparation method of the present invention may only include steps 1), 2), 4), 5), and 6). Preferably, the present invention includes steps 1) to 6).
[0047] In the present invention, step 6) can adopt the methods well known in the art or the methods described in the present invention, and thus is also optional. Even if step 6) is omitted, it still belongs to the scope of the present invention. Of course, preferably, step 6) is included in the pre...
Embodiment 1
[0132] Selection of bioreactor
[0133] Hangzhou AMP's rapid flow bioreactor, 100L culture volume.
[0134] Selection of cells for seedling production
[0135] (1) Canine kidney cells MDCK (purchased from China Veterinary Drug Administration) were selected as cells for seedling production.
[0136] (2) Cell generation
[0137] The 1st to 10th passage cells are defined as primitive cells, the 10th to 20th passages are defined as basic cells, the 20th to 35th passages are designated as working cells, and the production cells must be controlled within 40 passages. Stored in liquid nitrogen, the shelf life is set at 36 months.
[0138] (3) Cell characteristics
[0139] According to the current "Chinese Veterinary Pharmacopoeia" (2010 Edition) appendix to test, there should be no bacteria, mold, mycoplasma and foreign virus contamination, sensitive to H9 subtype avian influenza virus.
[0140] Poison seeds for making seedlings
[0141] (1) The virus seed used for mak...
Embodiment 2-3
[0161] Except that the African green monkey kidney cell line VERO (purchased from the China Veterinary Drug Control Institute) and the chicken embryo fibroblast cell line DF-1 (purchased from the China Veterinary Drug Control Institute) were selected, other steps were the same as in Example 1, Obtain inactivated bird flu vaccine (H9 subtype, F strain). The finished product inspection results of the obtained product are shown in Table 1.
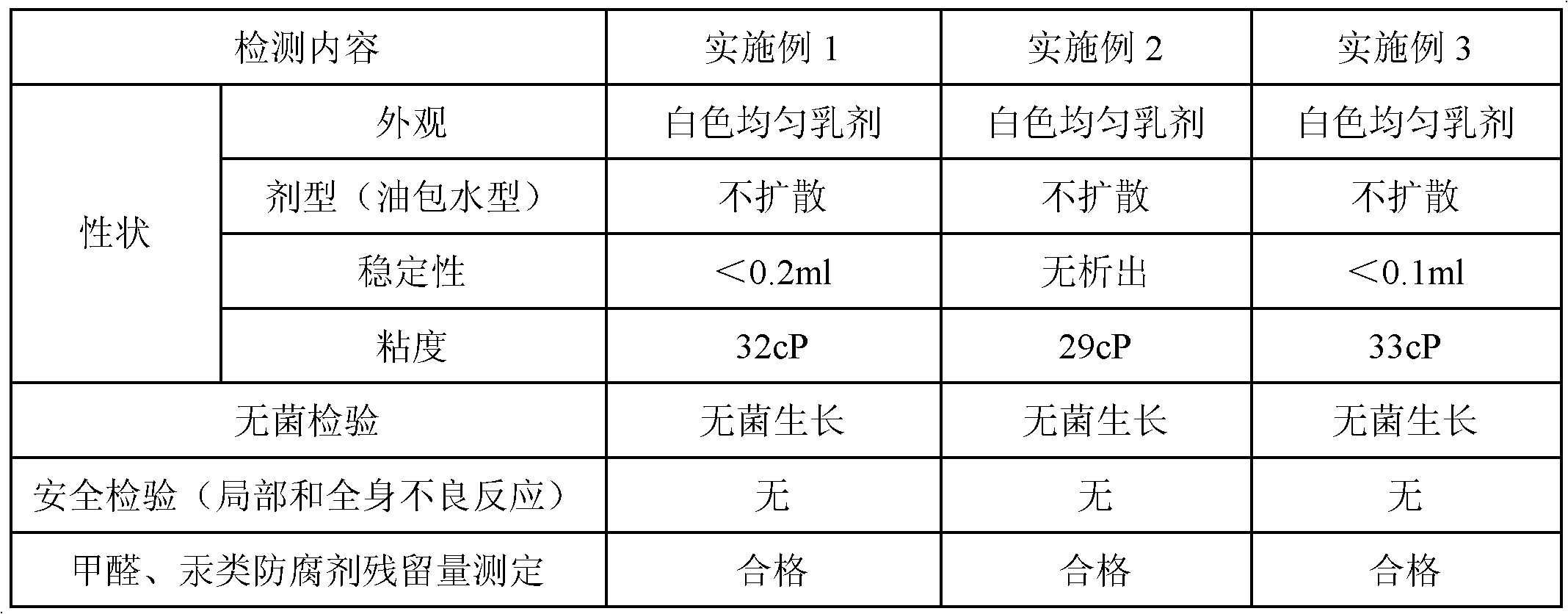
[0162] Table 1. Test results of finished vaccine products
[0163]
[0164] It can be seen from Table 1 that the vaccine of the present invention has stable properties, moderate viscosity and good safety.
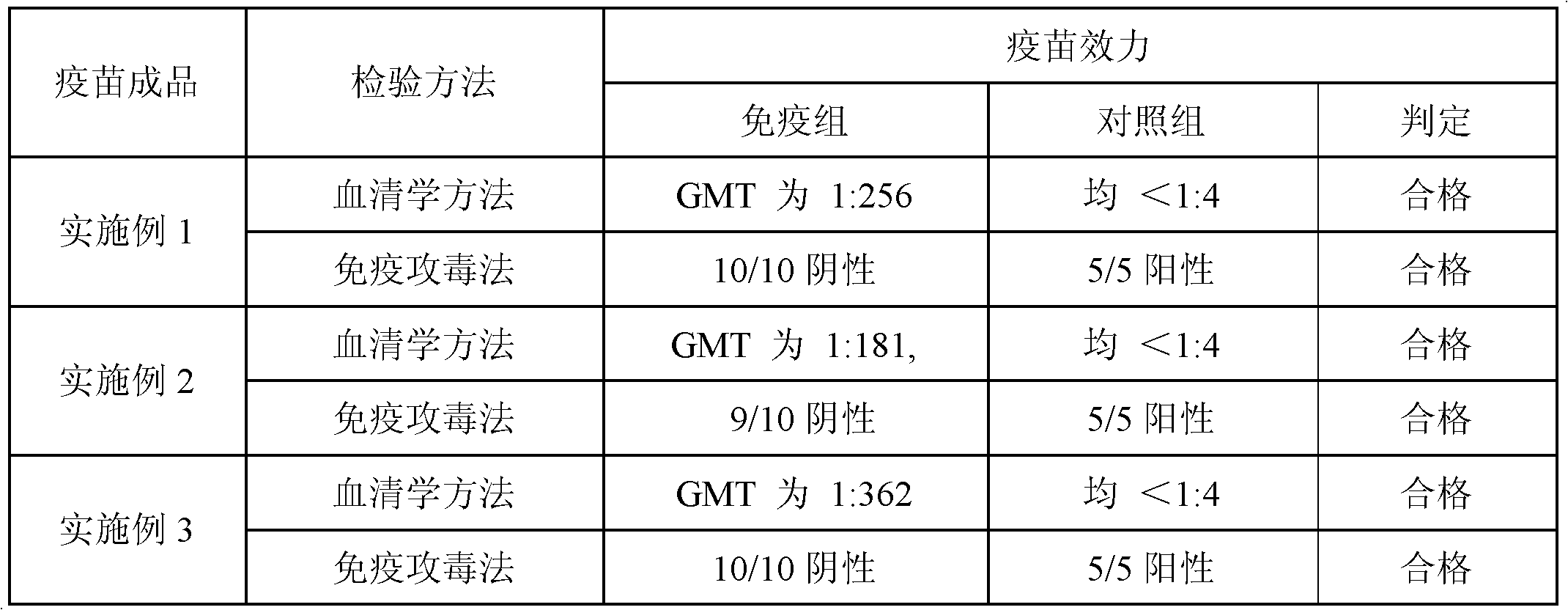
[0165] Table 2. Efficacy test results of finished vaccine products
[0166]
[0167] It can be seen from Table 2 that the vaccine of the present invention has a good immune effect.
[0168] The method for producing an inactivated avian influenza vaccine used in the present invention can not only solve the problem of chicken embryo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com