Patents
Literature
1351 results about "Avian virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The highly pathogenic influenza A virus subtype H5N1 is an emerging avian influenza virus that is causing global concern as a potential pandemic threat. It is often referred to simply as "bird flu" or "avian influenza", even though it is only one of many subtypes.
Functional influenza virus-like particles (VLPs)
ActiveUS20050009008A1SsRNA viruses negative-senseVirus peptidesMultiple copyVirus Structural Proteins
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080069821A1Extended half-lifeReducing/increasing polypeptide antigenicityFungiVirusesHemagglutininNeuraminidase
Owner:MEDIMMUNE LLC +1
Influenza hemagglutinin and neuraminidase variants
InactiveUS20060008473A1SsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:GOVERNMENT OF THE US SEC THE DEPT OF HEALTH SERVICES NAT INSTITTUTE OF HEALTH
Live bacterial vaccines for viral infection prophylaxis or treatment
InactiveUS20080124355A1Enhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsHemagglutininBacteroides
The present invention provides a vaccine, method of use, and kit employing genetically isolated and stabilized, live attenuated bacterial strains including Salmonella that express one or more avian influenza antigens for use in live vaccine compositions that can be orally administered to an individual to protect against avian influenza. Genetic stabilization may be achieved through deletion of IS200 elements and bacteria phage and prophage elements. The bacterial strains may be genetically isolated from external phage infection by constitutive expression of a P22 phage repressor. Nucleic acid sequences encoding antigenic hemagglutinin and neuraminidase avian influenza proteins, having at least one modified codon for optimum expression when transferred into a prokaryotic microorganism for improved immunogenicity
Owner:AVIEX TECH
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
Feline vaccines against avian influenza
InactiveUS20080107687A1Elicit immune responseSsRNA viruses negative-senseViral antigen ingredientsEpitopeViral Vaccine
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a recombinant poxvirus vaccine or an inactivated vaccine. The invention also encompasses recombinant poxvirus vectors encoding and expressing avian influenza antigens, epitopes or immunogens which can be used to protect animals, in particular felids, against avian influenza.
Owner:MERIAL LTD
Composition for widely treating viral diseases and application thereof
The invention relates to a composition for widely treating viral diseases. The composition contains the following raw materials, or extracts of the following raw materials, or derivatives of the extracts of the following raw materials in chemically-acceptable forms, wherein the raw materials include processed products prepared from the raw materials. The constituents of the composition are as follows: 100 parts of Baikal skullcap root, 50-150 parts of radix bupleuri and 50-150 parts of kudzu root; and according to specific circumstances, one or more selected from (but not limited to) the following raw materials can be added on this basis: radix isatidis, honeysuckle flower, Fructus Forsythiae, rhubarb, phellodendron bark, rhizoma anemarrhenae, Cyrtomium fortunei, gardenia, coptis, rhizoma cyperi, fritillaria, balloonflower and licorice root. The composition provided by the invention can be prepared into powder, liquid, paste, granules, capsules or other pharmaceutically-acceptable physical forms. The composition provided by the invention is used for preventing and treating avian influenza, epidemic hemorrhagic fever, epidemic parotitis, nameless hyperpyrexia, Newcastle diseases, swine fever, blue-ear diseases and other multiple human and animal viral diseases characterized by hemorrhage or body temperature rise, and reducing the degree of pathological damage to the body.
Owner:GUANGDONG ZIJIN ZHENGTIAN PHARMA
Functional influenza virus like particles (VLPs)
ActiveUS8080255B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Method for identifying animal-based components in meat and meat products by utilizing SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) method
The invention relates to a method for identifying the animal-based components in meat and meat products by utilizing an SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) method, and belongs to the field of food sanitation inspection. The method provided by the invention is utilized to identify the pig, cattle, sheep, chicken and fish-based components in the meat products; the SDS-PAGE is carried out on the soluble proteins which are extracted from the processed sample by carrying out different temperature thermal treatments on fresh animal muscle tissues; and the pig, cattle, sheep, chicken and fish-based components in the meat products are judged according to an electrophoresis pattern. The method provided by the invention has the characteristics of strong specificity and sensitivity, and visual and reliable result judgment, and is simple to operate and has the extremely important significances on preventing animal disease pathogens such as foot-and-mouth disease, avian influenza and the like from spreading into China, preventing illegal retailers from concealing commodity components and guaranteeing the health of people.
Owner:QINGDAO AGRI UNIV +1
Functional influenza virus like particles (VLPs)
ActiveUS8506967B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
Owner:NOVAVAX
Serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture
ActiveCN103555659AHigh densityClear ingredientsVertebrate cellsArtificial cell constructsCanine kidneyFlu immunization
The invention discloses a serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture. The serum-free medium for MDCK cell full suspension culture comprises an amino acid part, a vitamin part, an inorganic salt part and other additive parts. The serum-free medium has the beneficial effects that the serum-free medium for MDCK cell full suspension culture, provided by the invention, is high in cell culture density and clear in composition and does not contain animal serum, a downstream product is purified, the product quality is improved, and the serum-free medium is convenient to prepare and use and suitable for large-scale production of influenza vaccines and avian influenza vaccines.
Owner:无锡市赛尔百灵生物技术有限公司
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
Avian influenza viruses, vaccines, compositions, formulations, and methods
ActiveUS20080118531A1Reduces and eliminates disease transmissionInhibit growthCompounds screening/testingSsRNA viruses negative-senseHemagglutininAvian influenza virus
A vaccine composition and method which is effective in preventing or ameliorating Avian Influenza Virus infection is set forth herein. The vaccine contains at least two inactivated strains of avian influenza virus, wherein the combined hemagglutinin (HA) total is at least about 200 HA / dose of the vaccine composition, and wherein each of the strains presents at least about 128 HA / dose, and further wherein one of the strains has the same HA subtype as that of a challenge virus, and wherein at least one of the strains has a different NA subtype than the challenge virus.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
Highly efficient influenza matrix (M1) proteins
ActiveUS20130039938A1Improve efficiencyIncreased VLP formation efficiencySsRNA viruses negative-senseSsRNA viruses positive-senseVirus-like particleAvian virus
Owner:NOVAVAX
Avian influenza virus live attenuated vaccine and uses thereof
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Newcastle disease virus vectored avian vaccines
The present invention encompasses engineered Newcastle Disease Virus (NDV) vaccines or compositions. The vaccine or composition may be a recombinant vaccine. The invention also encompasses recombinant vectors encoding and expressing avian pathogen antigens, more specifically avian influenza proteins, epitopes or immunogens. Such vaccines or compositions can be used to protect animals, in particular avian, against disease.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases
ActiveCN103773899AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationDiseaseDuck hepatitis A virus
The invention discloses a GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases. The invention provides a GeXP detection primer group for identifying or assisting to identify duck infectious disease pathogens, wherein the primer group consists of a primer pair A, a primer pair B, a primer pair C, a primer pair D, a primer pair E, a primer pair F, a primer pair G, a primer pair H, a primer pair I, a primer pair J, a primer pair K and a primer pair L. According to the GeXP detection kit, shown by experiments, the primer group, a PCR (Polymerase Chain Reaction) reagent and the primer pairs, provided by the invention, are used for simultaneously differentiating and detecting avian influenza viruses, H5, H7 and H9 subtype avian influenza viruses, duck hepatitis viruses, duck plague viruses, duck flaviviruses, newcastle disease viruses, egg drop syndrome viruses, muscovy duck reoviruses, muscovy duck parvoviruses and duck circoviruses and are good in specificity and high in sensitivity. The detection kit, which is simple and convenient and is high in flux, and a detection system are provided for the detection on common major duck infectious disease pathogens, so that the practical needs are better met, and application prospects are broad.
Owner:GUANGXI VETERINARY RES INST
Primer system and method for detecting and analyzing avian influenza virus
InactiveCN101058833AQuick checkAccurate detectionMicrobiological testing/measurementDNA preparationFowlHypotype
The invention provides a method for detecting fowl influenza virus and parting primer system and augmenting fowl influenza virus RNA asymmetrically. The invention designs and screens 25 pairs multiple asymmetrical RT-PCR primers, a pair general primer, 52 specific probes and 3 quality control probes according to the report fowl influenza virus total hypotype genome sequence in Genebank, wherein every pair in 25 pairs multiple asymmetrical RT-PCR primers is fit for a hypotype of fowl influenza virus, the primer system comprising 25 pairs multiple asymmetrical RT-PCR primers and a pair general primer can be used for detecting and parting fowl influenza virus, the primer system builds the method of asymmetrically augmenting fowl influenza virus RNA and detecting and parting fowl influenza virus. The invention provides strong specificity, high sensibility, which also proceeds the first step application.
Owner:中国检验检疫科学研究院动植物检疫研究所
Functional influenza virus-like particles (VLPS)
InactiveUS20120207786A1SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Method and detection kit used for detecting virus
InactiveCN102435746AEasy separationHigh fluorescence intensityFluorescence/phosphorescenceViral membraneHypotype
The invention relates to a method and a detection kit used for detecting a virus. The method comprises steps that: an antibody of the glycoprotein of a target virus adventitia is coupled with magnetic nano / micro-spheres, such that immunized magnetic nano / micro-spheres are obtained; the immunized magnetic nano / micro-spheres are used for catching the target virus in the sample, such that a magnetic sphere virus composition is obtained; the antibody of the glycoprotein of the adventitia of the biotinylated virus is incubated with the magnetic sphere virus composition; the obtained material is incubated with streptavidin-coupled quantum dots, such that a magnetic sphere-virus-quantum dot composition is obtained; and the virus is detected through the detection upon fluorescence signals of the magnetic sphere-virus-quantum dot composition. With the method, specific recognition, preconcentration, separation, and high-sensitivity detection of H9N2 subtype avian influenza active virus can be realized. The invention also provides a detection kit employed by the method. The kit and the method provided by the invention are characterized by simple operation, high specificity, high sensitivity, and the like. The whole detection process can be finished in one hour.
Owner:WUHAN UNIV
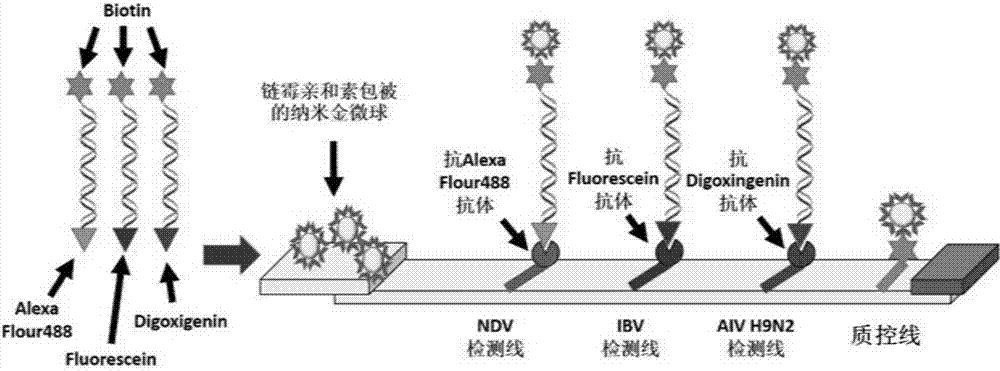
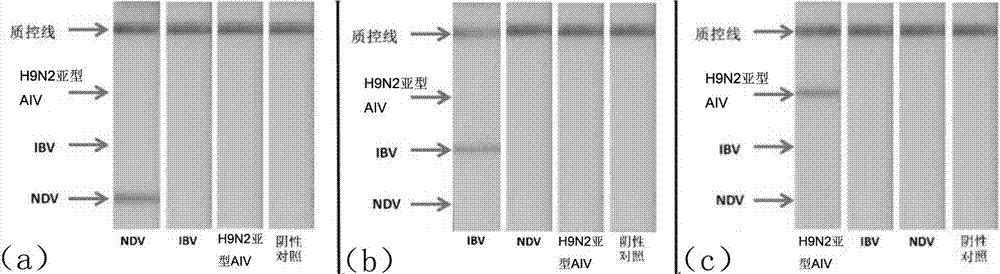
Multi-RPA primer and probe for simultaneously detecting NDV, IBV, H9N2-subtype AIV and detection method of multi-RPA primer and probe
ActiveCN107287349AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMultiplex pcrsRNA extraction
The invention discloses a multi-RPA primer and probe for simultaneously detecting a newcastle disease virus, an infectious bronchitis virus and an H9N2-subtype avian influenza virus. The multi-RPA primer and probe comprise a primer and probe for detecting the newcastle disease virus, a primer and probe for detecting the infectious bronchitis virus and a primer and probe for detecting the H9N2-subtype avian influenza virus. The multi-RPA primer and probe are high in specificity, high in sensitivity and accurate in detection result. The invention further discloses a method for simultaneously detecting the newcastle disease virus, the infectious bronchitis virus and the H9N2-subtype avian influenza virus through multi-RPA. The method comprises the steps of primer synthesis, template RNA extraction, reverse transcription, multi-PCR amplification and amplification product analysis. The detection method is simple in operation and good in stability, and a low-cost, fast and specific field diagnosis method is provided for effective detection and identification of mixed infection of common avian respiratory diseases.
Owner:HENAN AGRICULTURAL UNIVERSITY
Method of inhibiting the transmission of influenza virus
ActiveUS20070280901A1Rapid and antiviral effectivenessHigh activityCosmetic preparationsBiocideOrganic acidAlcohol
Antimicrobial compositions having a rapid and persistent antiviral effectiveness against influenza viruses, including avian flu viruses, are disclosed. The antimicrobial compositions contain (a) a disinfecting alcohol, (b) an organic acid, and (c) water, wherein the composition has a pH of about 5 or less and the nonvolatile components of the composition are capable of forming a barrier film or layer on a treated surface.
Owner:HENKEL KGAA
Monoclonal antibodies binding to avian influenza virus subtype H5 haemagglutinin and uses thereof
Owner:XIAMEN UNIV +1
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for preventing and treating avian influenza in human
A method and composition for preventing and treating Avian Influenza in Humans utilizes an effective quantity of polyphenolic(s) and / or its derivatives in combination with a carrier. The anti-avian influenza ingredient having a composition selected from the group consisting of theaflavin, theaflavin-3,3′-digallate, theaflavin-3-monogallate, theaflavin-3 gallate, theaflavin-3′-gallate, thearubigin, gallic acid, tannic acid, (−)-epigallocatechin gallate (EGCG), (−) epigalloatechin (EGC), (+)-epicatechin (EC), (−)-gallocatechin gallate (GCG), and catechin.
Owner:SICAN VENTURES
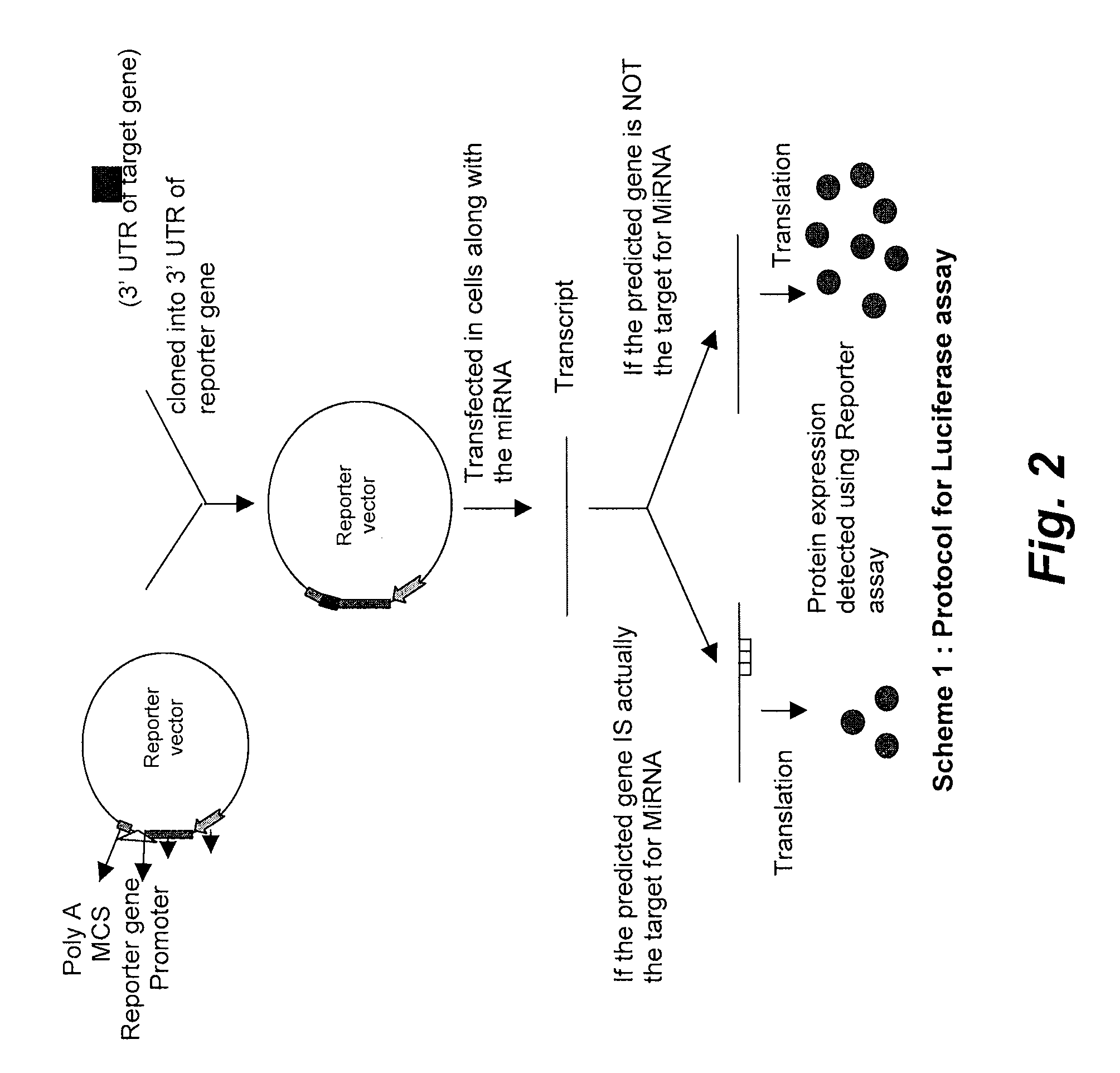
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
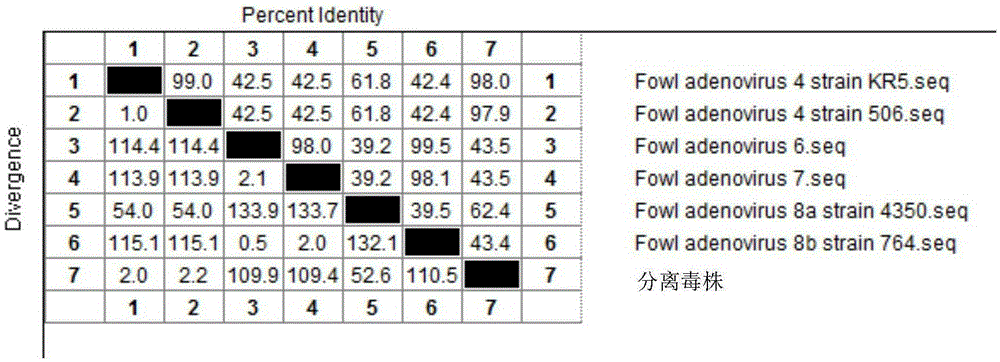
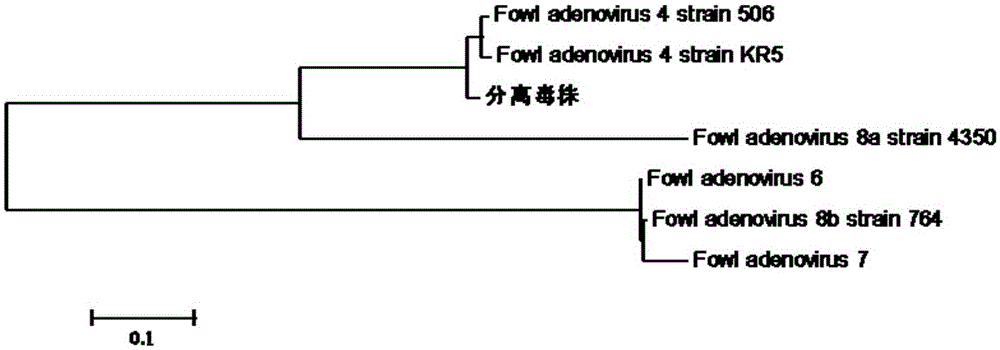
I-colony fowl adenovirus 4 strain and application thereof
ActiveCN105368795AImprove featuresImproving immunogenicityViral antigen ingredientsBiological material analysisInfected cellLaryngotracheitis virus
The invention aims at providing an I-colony fowl adenovirus 4 strain, which is preserved with preservation number of CCTCC No. V201541. The I-colony fowl adenovirus 4 YBAV-4 strain disclosed by the invention is excellent in specificity and immunogenicity; a specific precipitation line does not appear in specific positive serum chicken SPF chicken serum such as infected cell sap and egg drop syndrome resisting virus, chicken infectious bursal disease virus, Newcastle disease virus, chicken infectious laryngotracheitis virus, chicken Marek's disease virus, avian influenza and the like, while an obvious specific precipitation line appears in I-colony fowl adenovirus 4 specific serum. The strain disclosed by the invention, as a vaccine strain which is good in manufacturing effect, is capable of preventing chicken hydropericardium syndrome, and the strain is applicable to identification of virus serotype and investigation on epidemiology.
Owner:YEBIO BIOENG OF QINGDAO
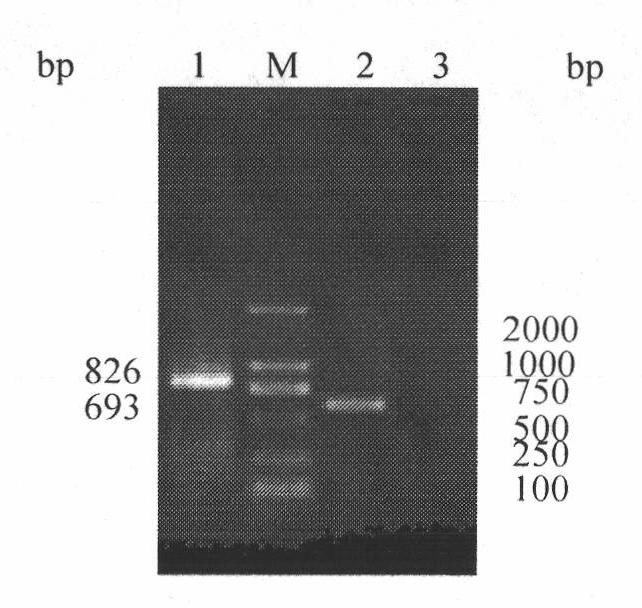
h5, h7, h9 subtype avian influenza virus detection kit
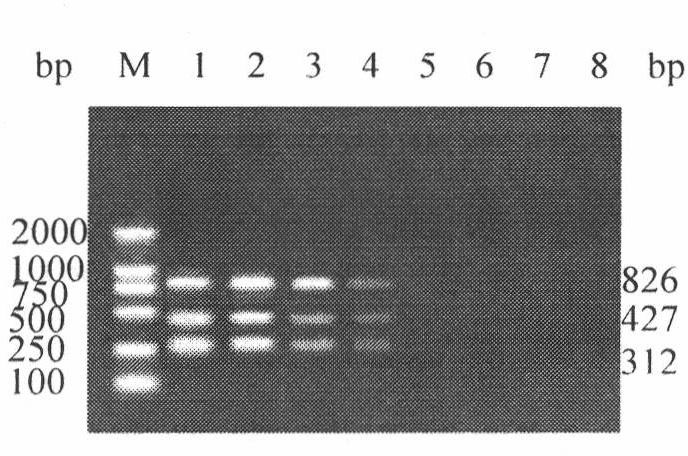
InactiveCN102260749AMicrobiological testing/measurementAvian influenza virusComplementary deoxyribonucleic acid
The invention discloses an H5, H7 and H9 subtype avian influenza virus detection kit. In the GenBank, three kinds of subtype HA gene sequences are searched, and specific primers are designed. By many tests and actual detections, three pairs of primers and reaction systems thereof are screened out and optimized, and the sizes of the amplified cDNA (complementary deoxyribonucleic acid) fragments are respectively 427bp, 312bp and 826bp. The result shows that by optimizing multiple RT-PCR (reverse transcription-polymerase chain reaction) amplification conditions, a multiple RT-PCR detection kit capable of detecting three kinds of subtype avian influenza viruses at the same time is prepared, and the minimum detectable quantity is 10pg. The detection kit does not have cross reaction to various kinds of chicken infectious diseases and normal structures, has strong specificity, and has consistent sensibility to three kinds of subtype detections. The avian influenza can be diagnosed in a shorttime in a primary laboratory and can be positioned in a subtype so as to gain the time for preventing and controlling the avian influenza, thus the spread of the avian influenza can be controlled in a small range as much as possible by adopting measures in time.
Owner:JILIN AGRICULTURAL UNIV
Immunological adjuvant, and its application in preparing vaccine and medicine for anti-virus
InactiveCN1718243AImprove immune activityReach clearAntiviralsAntibody medical ingredientsAnti virusDisease
An immunoadjuvant used to prepare the antiviral vaccine or medicine for increasing the immune activity of the antigens for HBV, HCV, SARS coronavirus, fowl influenza virus, etc is a kind of human or animal's novel heat shock proteins gp96, hsp108 and hsp70.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Preparation method of avian influenza virus growing in serum-free full-suspended cultured MDCK cells and obtained avian influenza virus
ActiveCN106011083AStable characteristicsGet rid of dependenceVertebrate cellsArtificial cell constructsSerum freeF1 generation
The invention discloses a preparation method of an avian influenza virus suitable for growing in a serum-free full-suspended cultured MDCK cell line and the avian influenza virus obtained through the method. The preparation method comprises the following steps of 1 preparation of the MDCK cells to be inoculated; 2 virus seed preparation, wherein a chick embryo source avian influenza virus is prepared; 3 F1 generation virus domestication; 4 F2 generation virus domestication, wherein a supernatant sample retained at the time point when the blood clotting titer of the avian influenza virus obtained in the F1 generation is highest is taken, and the step 3 is repeated; 5 F3 generation virus domestication, wherein the F3 generation virus culturing temperature is 35 DEG C; 6 F4 generation-F10 generation virus domestication, wherein the step 5 is repeated, and the domesticated avian influenza virus is obtained. According to the preparation method, through a domestication method, the avian influenza virus is directly domesticated to completely adapt to be efficiently reproduced on the serum-free full-suspended cultured MDCK cells from the mode of being cultured by a chick embryo, the domestication efficiency is high, the avian influenza virus can be efficiently infected and copied in the MDCK cells, and the virus characteristic is stable.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com