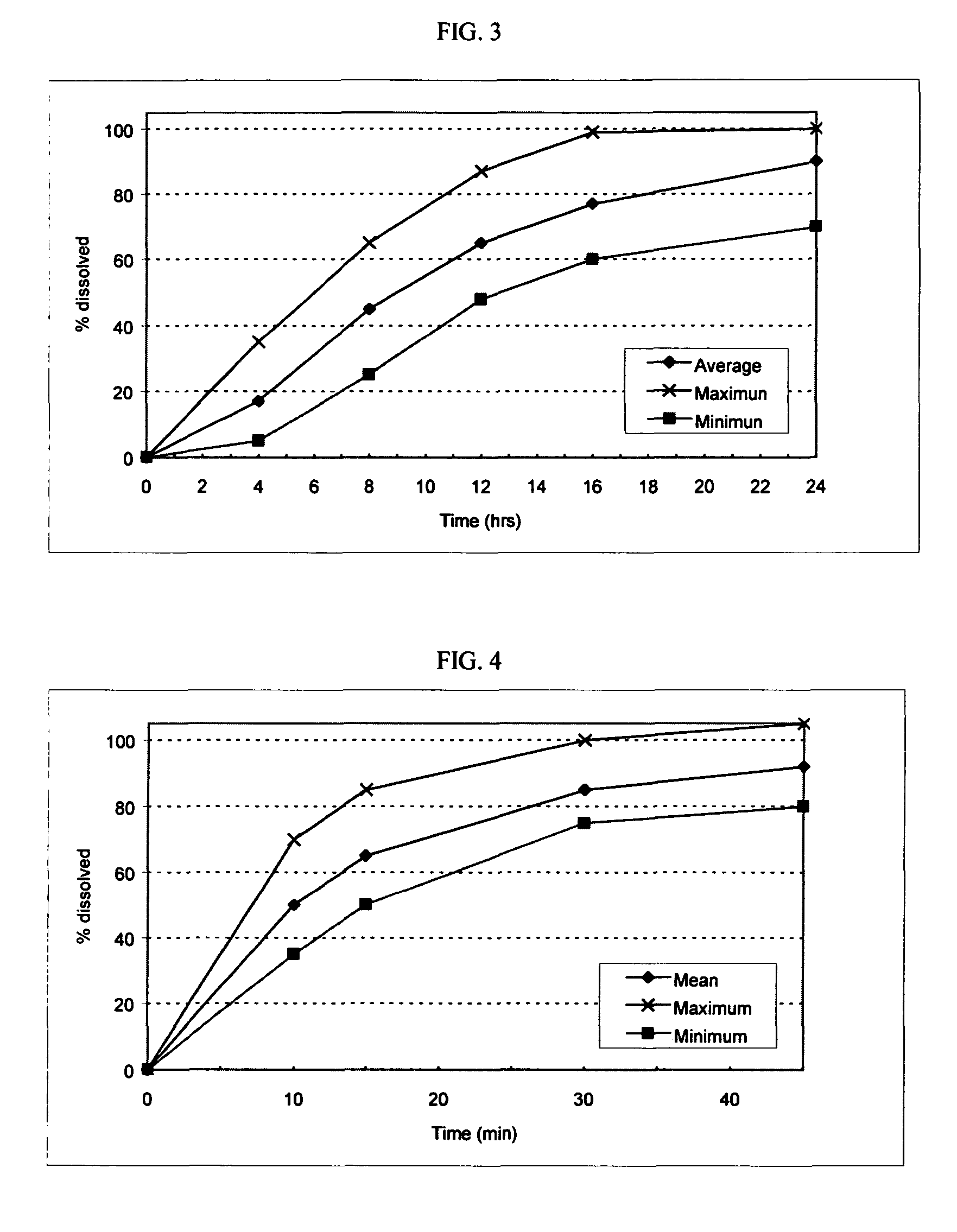
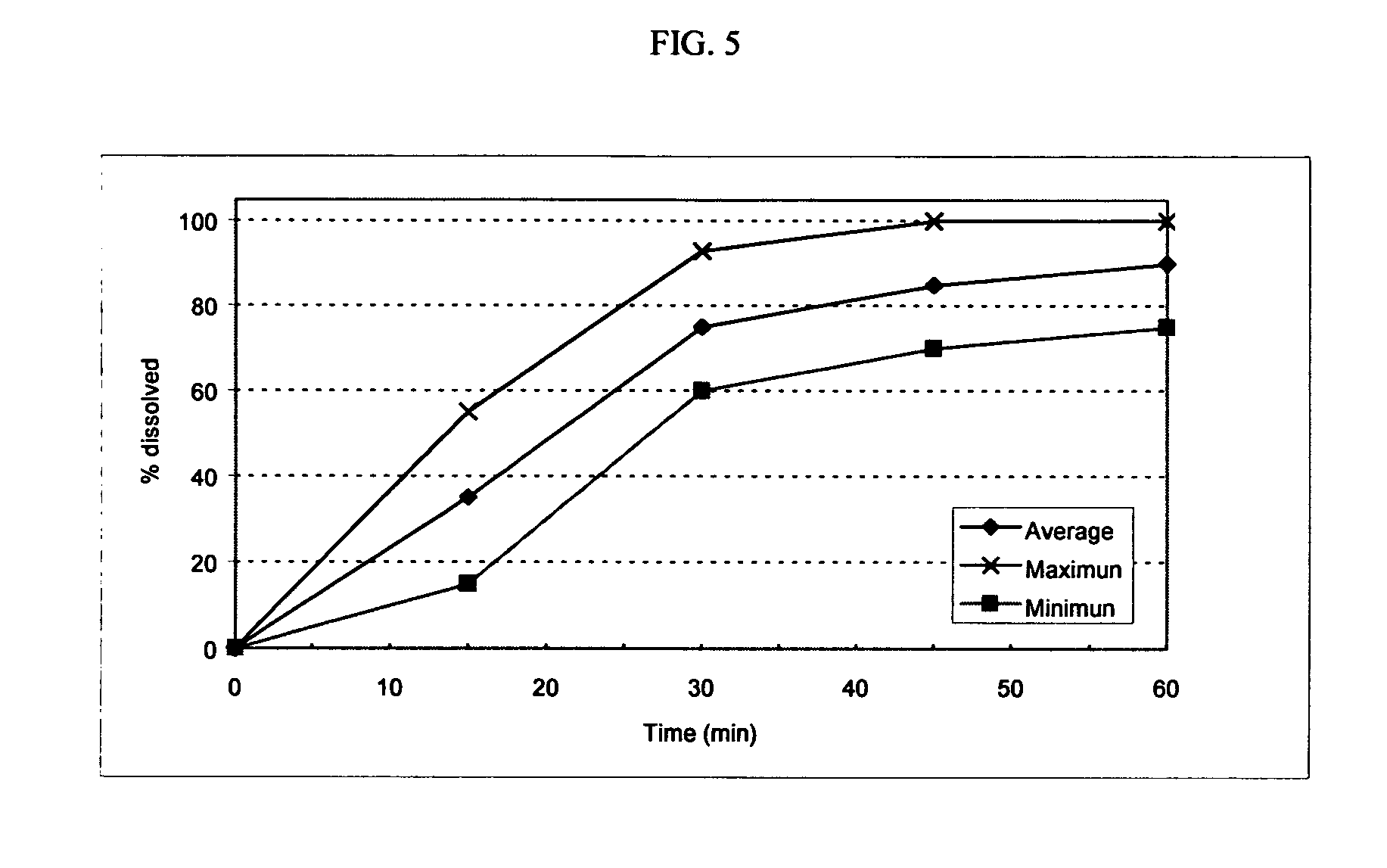
Patents
Literature
159 results about "Oseltamivir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
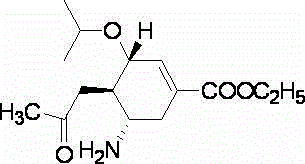
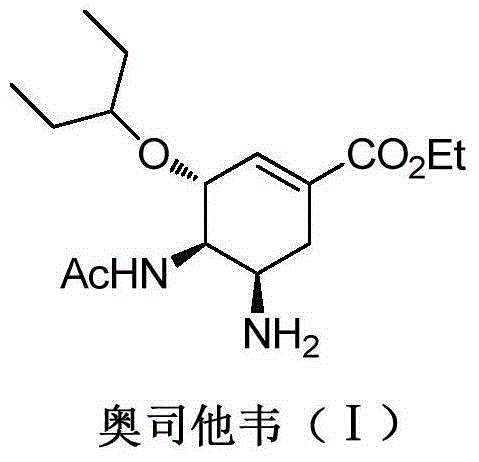
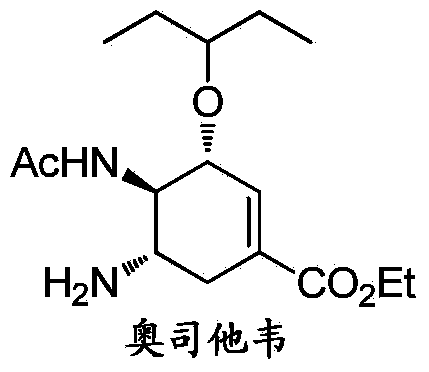
Oseltamivir is used to treat symptoms caused by the flu virus (influenza).
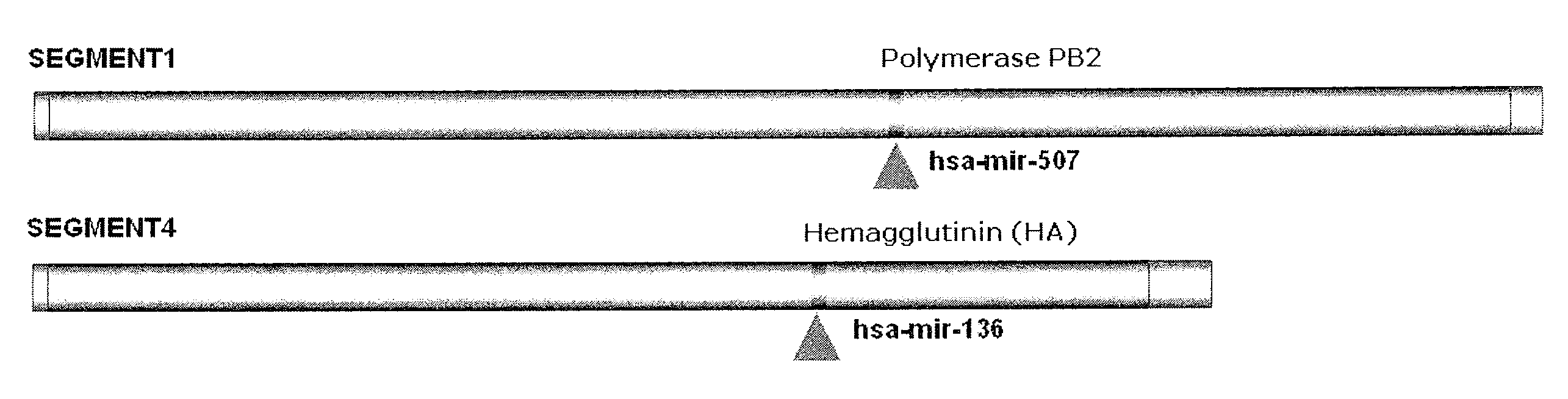
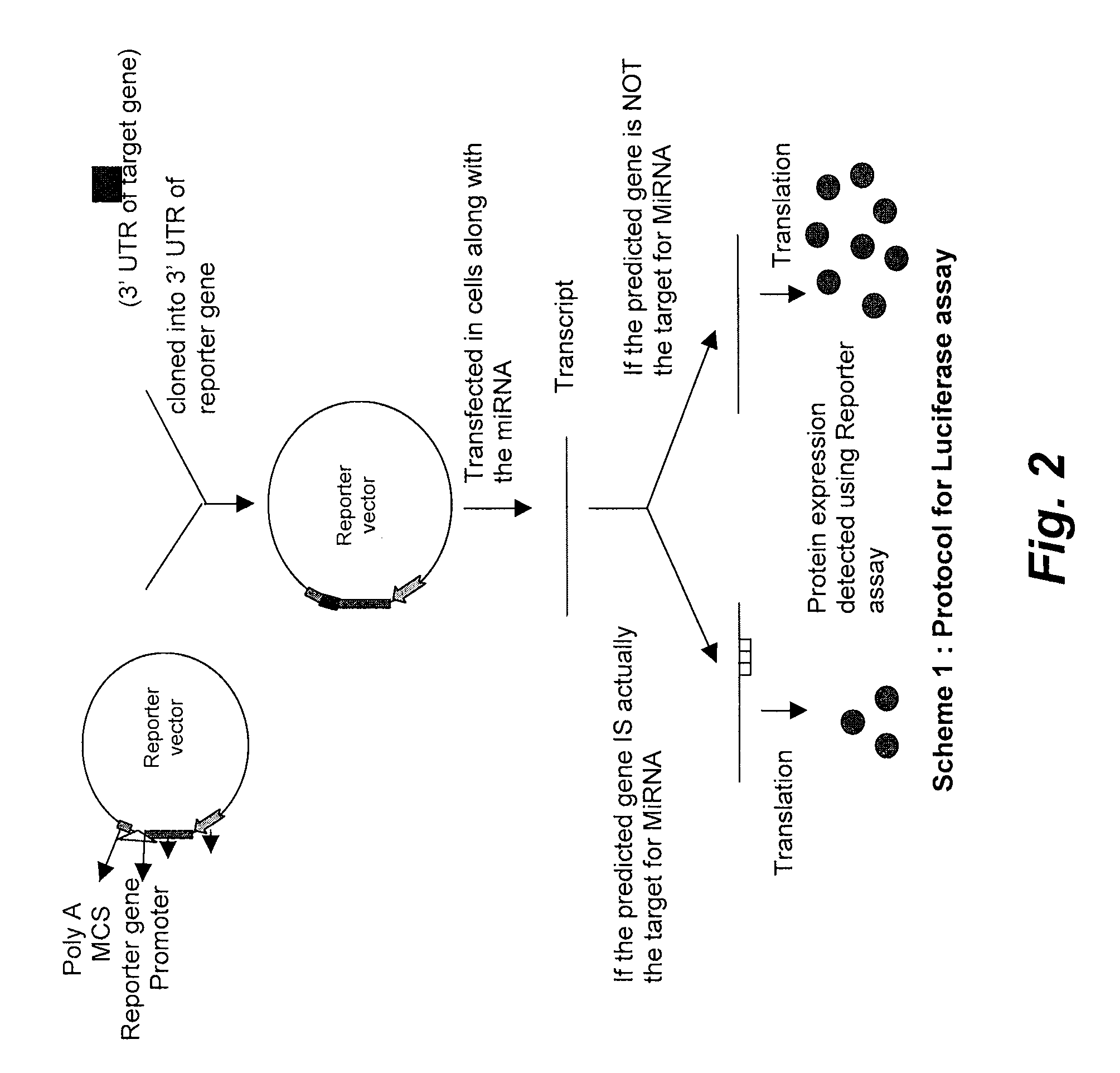
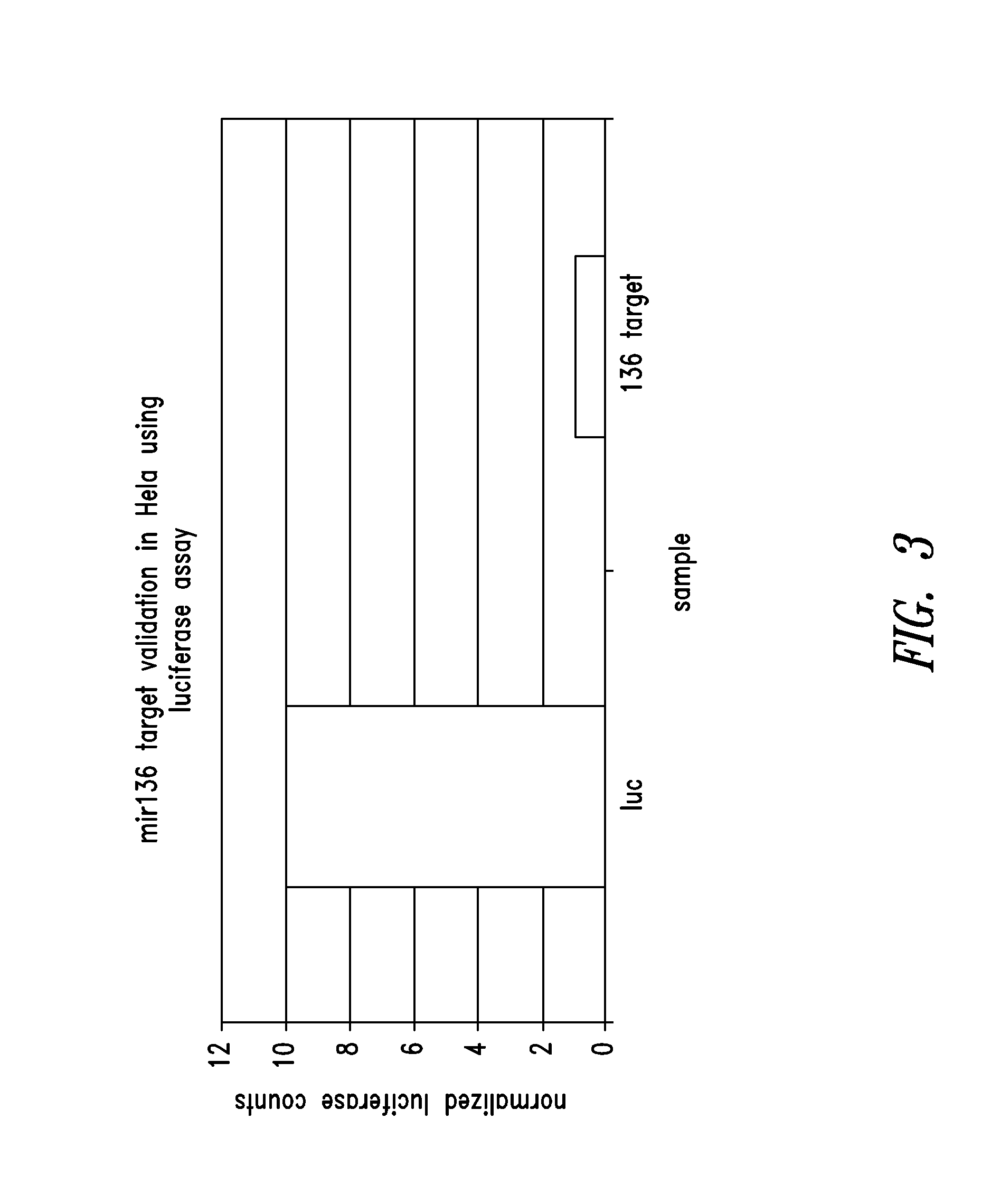
Targets for human micro rnas in avian influenza virus (H5N1) genome
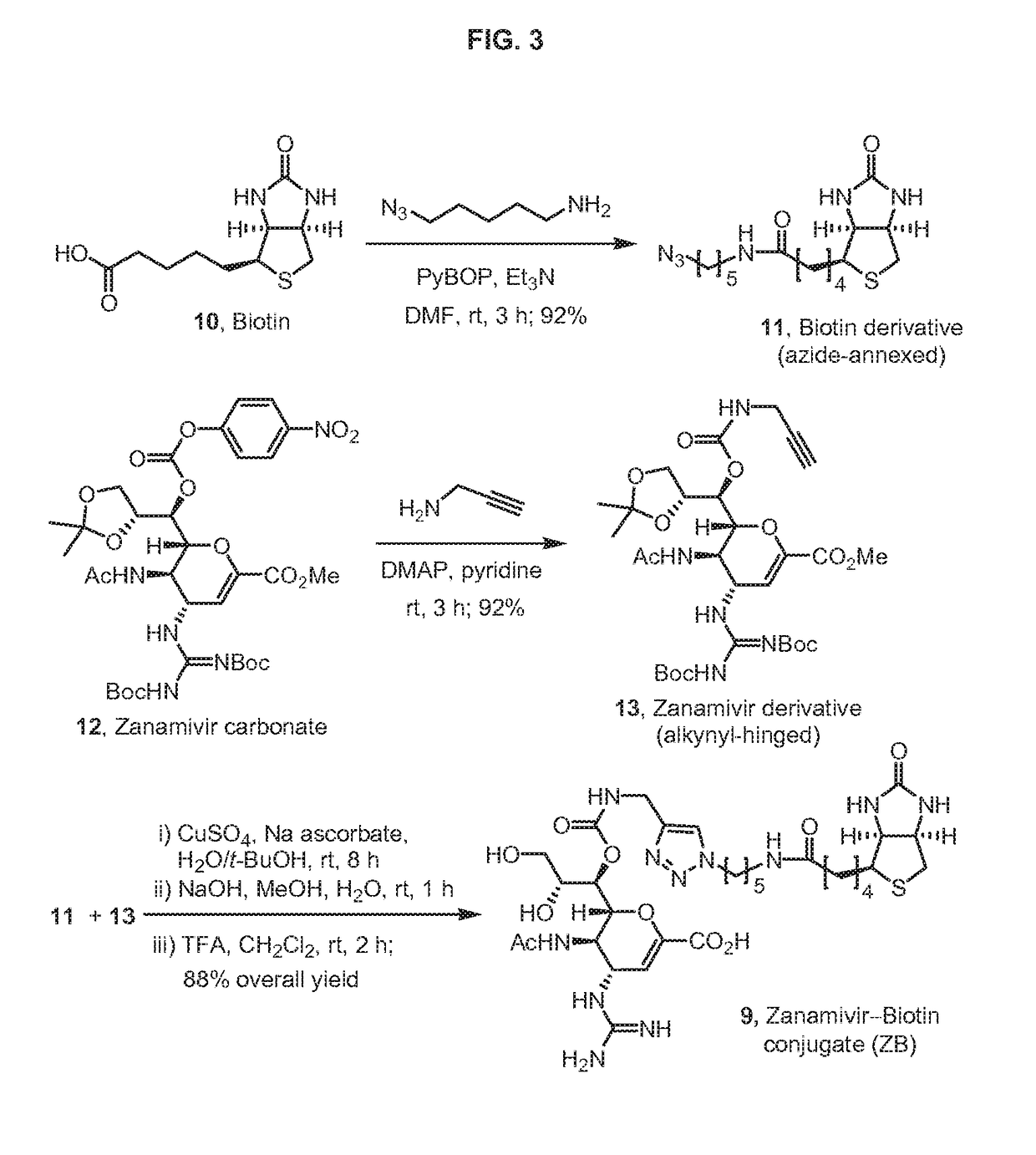
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Bi-Functional Polymer-Attached Inhibitors of Influenza Virus
InactiveUS20090081249A1Inhibiting and preventing development of resistanceAntiviralsCarrier-bound antigen/hapten ingredientsPolyethylene glycolDextran
Antimicrobial compositions containing two or more antiviral agents coupled to a polymer and methods of making and using the compositions, are described herein. In one embodiment, two or more antiviral agents are covalently coupled to the polymer. Suitable antiviral agents include, but are not limited to, sialic acid, zanamivir, oseltamivir, amantadine, rimantadine, and combinations thereof. The polymer is preferably a water-soluble, biocompatible polymer. Suitable polymers include, but are not limited to, poly(isobutylene-alt-maleic anhydride) (PIBMA), poly(aspartic acid), poly(l-glutamic acid), polylysine, poly(acrylic acid), plyaginic acid, chitosan, carboxymethyl cellulose, carboxymethyl dextran, polyethyleneimine, and blends and copolymers thereof. In another embodiment, the compositions contain a physical mixture of polymer containing one antiviral agent and polymer containing a second antiviral agent. The compositions can be formulated for enteral or parenteral administration. Suitable oral / intranasal dosage forms include, but are not limited to, tablets, capsules, solutions, suspensions, emulsions, syrups, and lozenges. Suitable dosage forms for parenteral administration include, but are not limited to, solutions, suspensions, and emulsions. The compositions described herein are effective at treating a variety of infections, including viral infections such as influenza, while inhibiting or preventing the development of microbial resistance.
Owner:MASSACHUSETTS INST OF TECH
Oseltamivir lyophilized orally-disintegrating tablets and preparation method thereof
InactiveCN104367558AOral convenienceEasy to takeOrganic active ingredientsAntiviralsFreeze-dryingOrally disintegrating tablet
The present invention provides oseltamivir lyophilized orally-disintegrating tablets and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The oseltamivir lyophilized orally-disintegrating tablets comprise oseltamivir phosphate or oseltamivir and a matrix, wherein the oseltamivir lyophilized orally-disintegrating tablets contain (calculated as the oseltamivir) 10-75 parts by weight of the effective component, and the matrix contains 1-60 parts by weight of a framework support agent, 1-50 parts by weight of a binder, 0-10 parts by weight of a lyoprotectant, and 0-10 parts by weight of a flavoring agent, and can further contains 0-10 parts by weight of a flavoring agent and 0-69 parts by weight of an inorganic alkali. The oseltamivir lyophilized orally-disintegrating tablet preparation method comprises dissolving, mold injection, rapid freezing, freeze-drying and product packaging. The oseltamivir lyophilized orally-disintegrating tablets of the present invention have characteristics of convenient taking, taking without water, rapid absorption, and first pass effect avoiding.
Owner:BEIJING SUNHO PHARMA
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
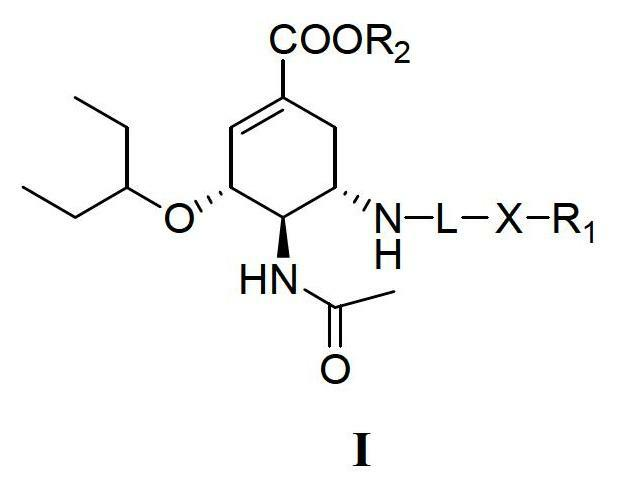
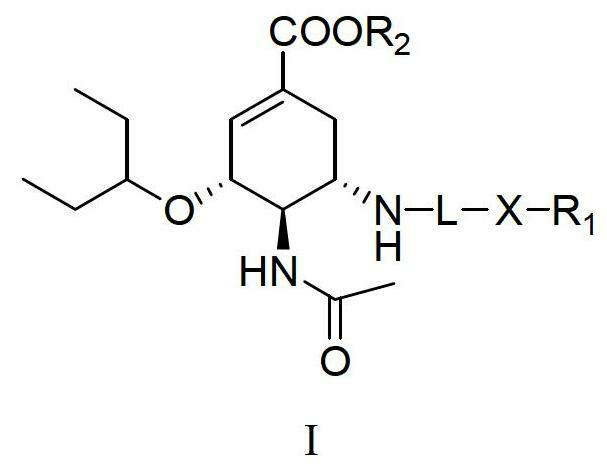
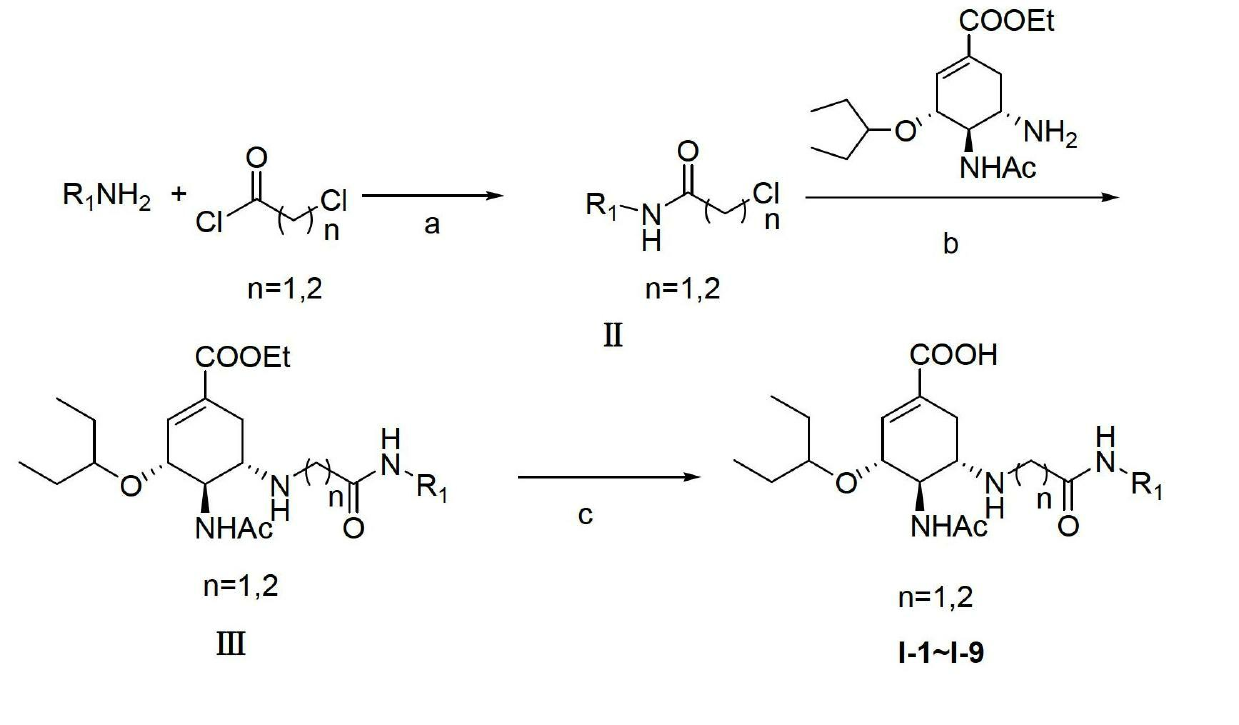
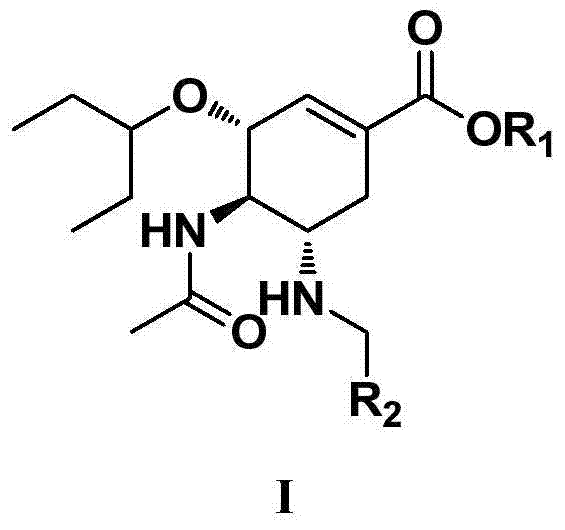
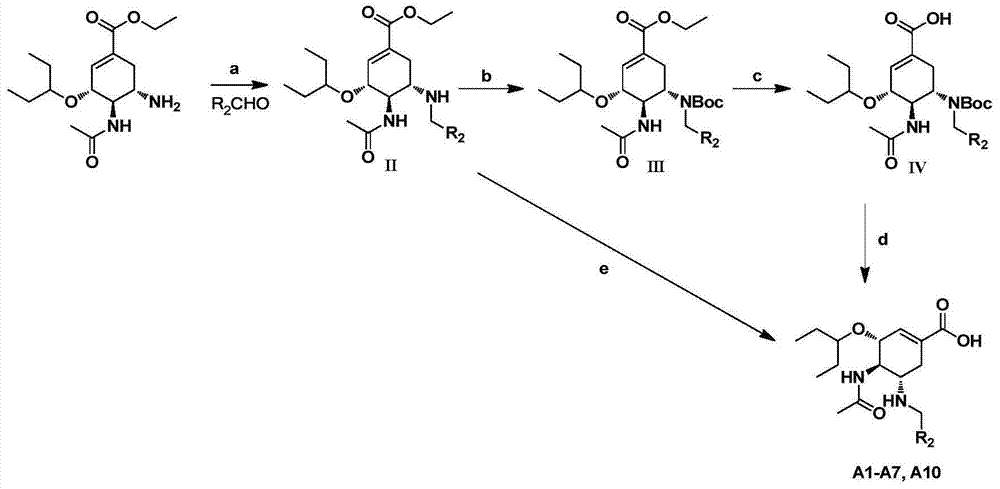
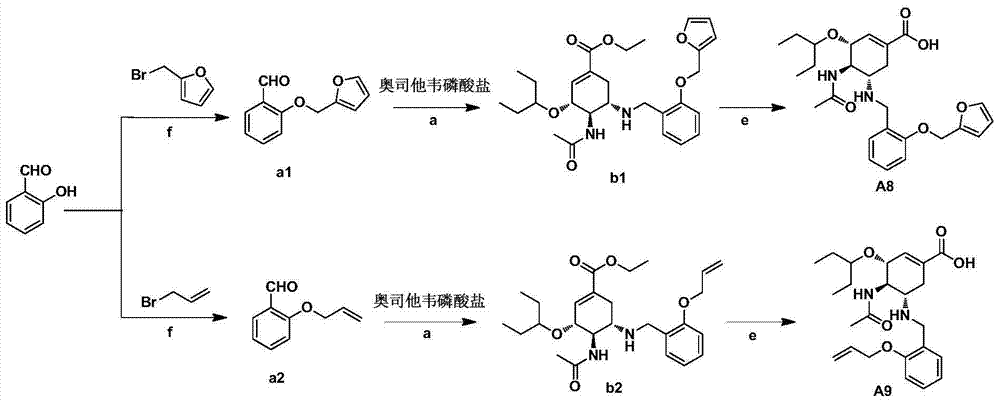
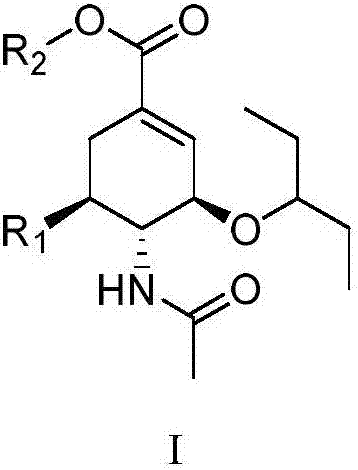
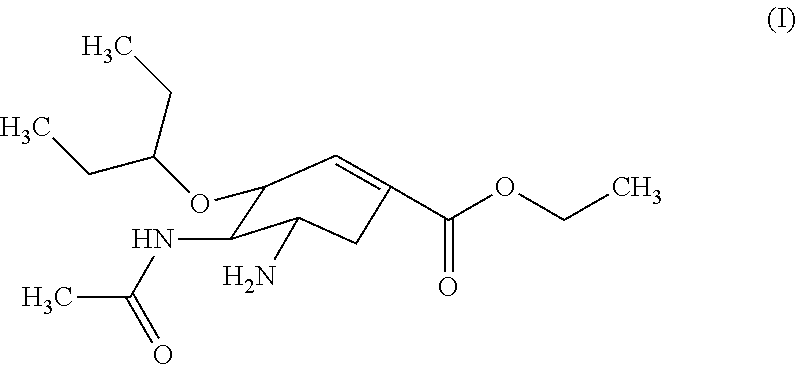
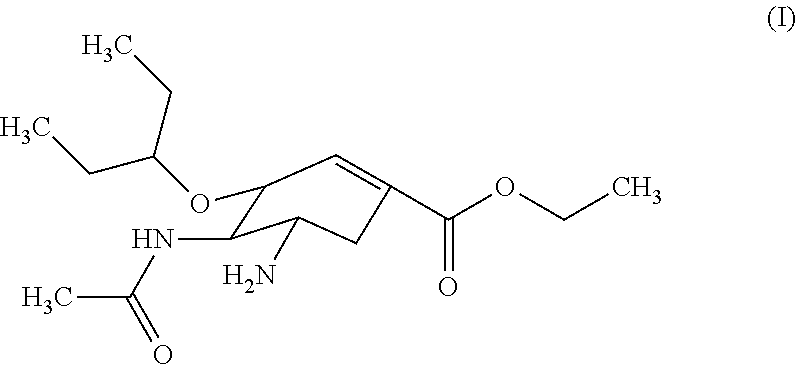
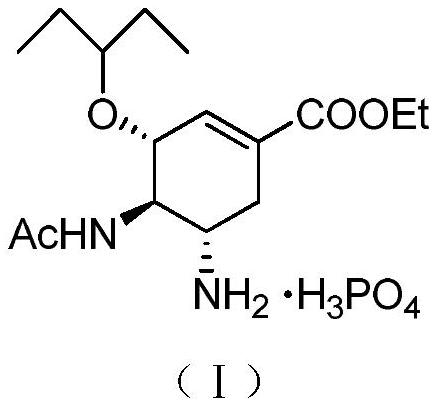
Derivatives of oseltamivir, and method and medical application thereof
InactiveCN102659615AStrong inhibitory activityReduced inhibitory activityOrganic chemistryAntiviralsInfective disorderVirus
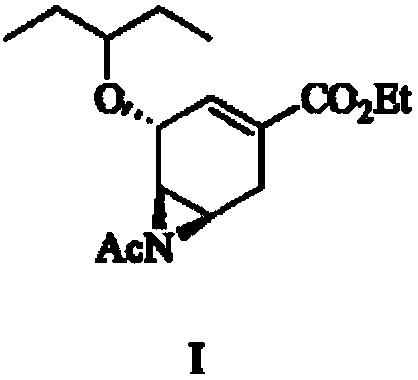
The invention relates to the field of medical chemistry, in particular to derivatives of oseltamivir (I). R1, R2, L and X are explained in the specifications. The invention also discloses a method for preparing the derivatives of oseltamivir and the purpose of the derivatives for treating infectious diseases, particularly the infectious diseases caused by influenza viruses. The oseltamivir is shown in the description.
Owner:CHINA PHARM UNIV +1
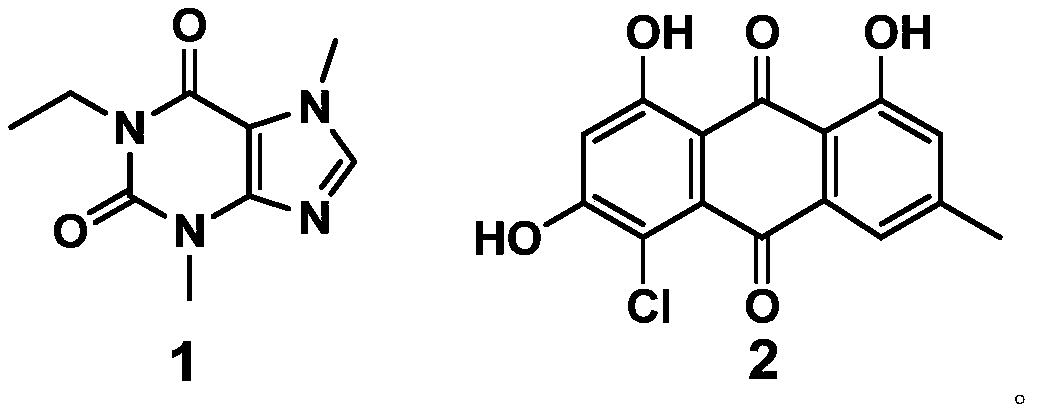
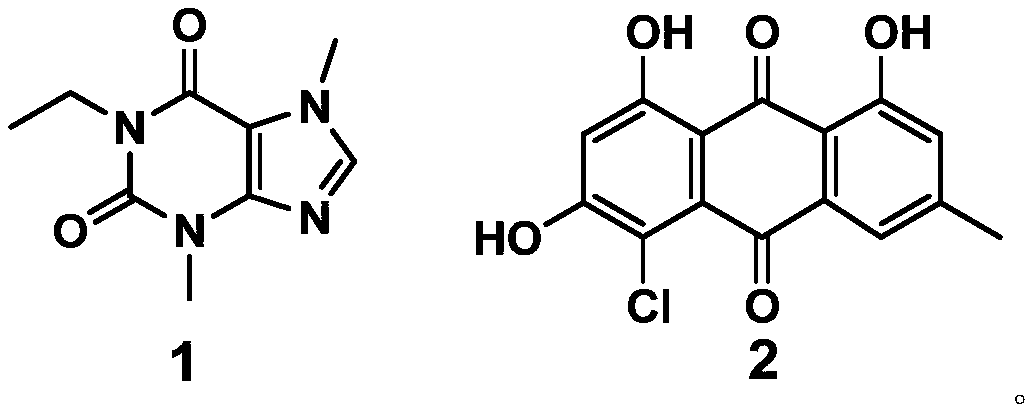
Application of compound to preparation of medicines for treating viral pneumonia
ActiveCN111228275AExcellent antiviral pneumonia effectStrong anti-virusAntiviralsRespiratory disorderPurinePharmacology
The invention discloses an application of a compound to preparation of medicines for treating viral pneumonia, and relates to the technical field of drugs. The technical problem that novel antiviral drugs are deficient in the prior art, can be solved. The compound comprises a purine compound adopting the structure as shown in the formula 1, and / or an anthraquinone derivative adopting the structureas shown in the formula 2. The purine compound adopting the structure as shown in the formula 1, and / or the anthraquinone derivative adopting the structure as shown in the formula 2 both can show predominant effect for resisting viral pneumonia when being separately used or used jointly. Drugs for treating viral pneumonia, are prepared by the compound provided by the invention are more notable inactivity compared with clinical frontline drugs, and are better than drugs of ribavirin, oseltamivir and the like.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Orally disintegrating tablet suitable for infants and children and preparation method thereof
ActiveCN103340835AOrganic active ingredientsAntiviralsOrally disintegrating tabletAdditive ingredient
The invention relates to an oseltamivir orally disintegrating tablet which is used for treating the flu of infants and children. The oseltamivir orally disintegrating tablet is prepared from the following ingredients in percentages by weight: 10-40% of taste masking micropill, 20-80% of filling agent, 1-6% of binding agent, 2-10% of disintegrating agent, 0-5% of corrigent and 0.5-2.5% of lubricating agent, wherein the taste masking micropill comprises a medicine containing pill core and a coating layer, the medicine in the medicine containing pill core is oseltamivir or a pharmaceutically acceptable salt of the oseltamivir, which accounts for 10-40% of the total weight of the micropill, the material of the coating layer is polyacrylic resin IV, which accounts for 1-50% of the total weight of the micropill, and the particle size of the micropill is 0.10-0.50mm.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Oseltamivir derivative as well as preparation method and application thereof
InactiveCN103923060AHigh selectivityHigh activityOrganic active ingredientsOrganic compound preparationHigh selectivityInfluenza a
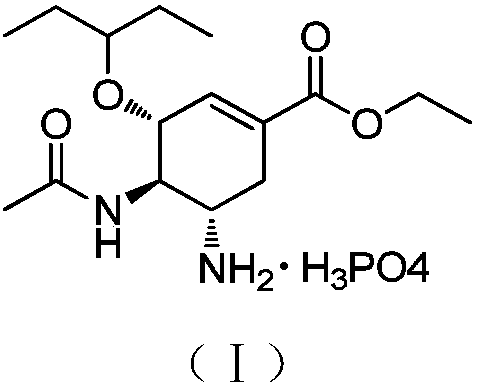
The invention relates to an oseltamivir derivative as well as a preparation method and application thereof. The compound has the structure shown in a formula I. The invention provides an efficient influenza virus neuraminidase inhibitor with high selectivity, which is used for preparing drugs for preventing or curing flu, particularly the diseases caused by N1 influenza virus. The invention also relates to a drug composition comprising the compound shown in the formula I.
Owner:SHANDONG UNIV
Oseltamivir derivative, preparation method and application thereof
ActiveCN107056636AOrganic active ingredientsOrganic compound preparationAvian influenza virusEnzyme inhibitor
The invention discloses an oseltamivir derivative, a preparation method and an application thereof. The derivative has a structure shown as formula I. The invention also discloses the preparation method for the oseltamivir derivative, the application thereof as an avian influenza virus neuraminidase inhibitor and the application of a composition containing one or more compounds in preparing anti-influenza virus drugs.
Owner:SHANDONG UNIV
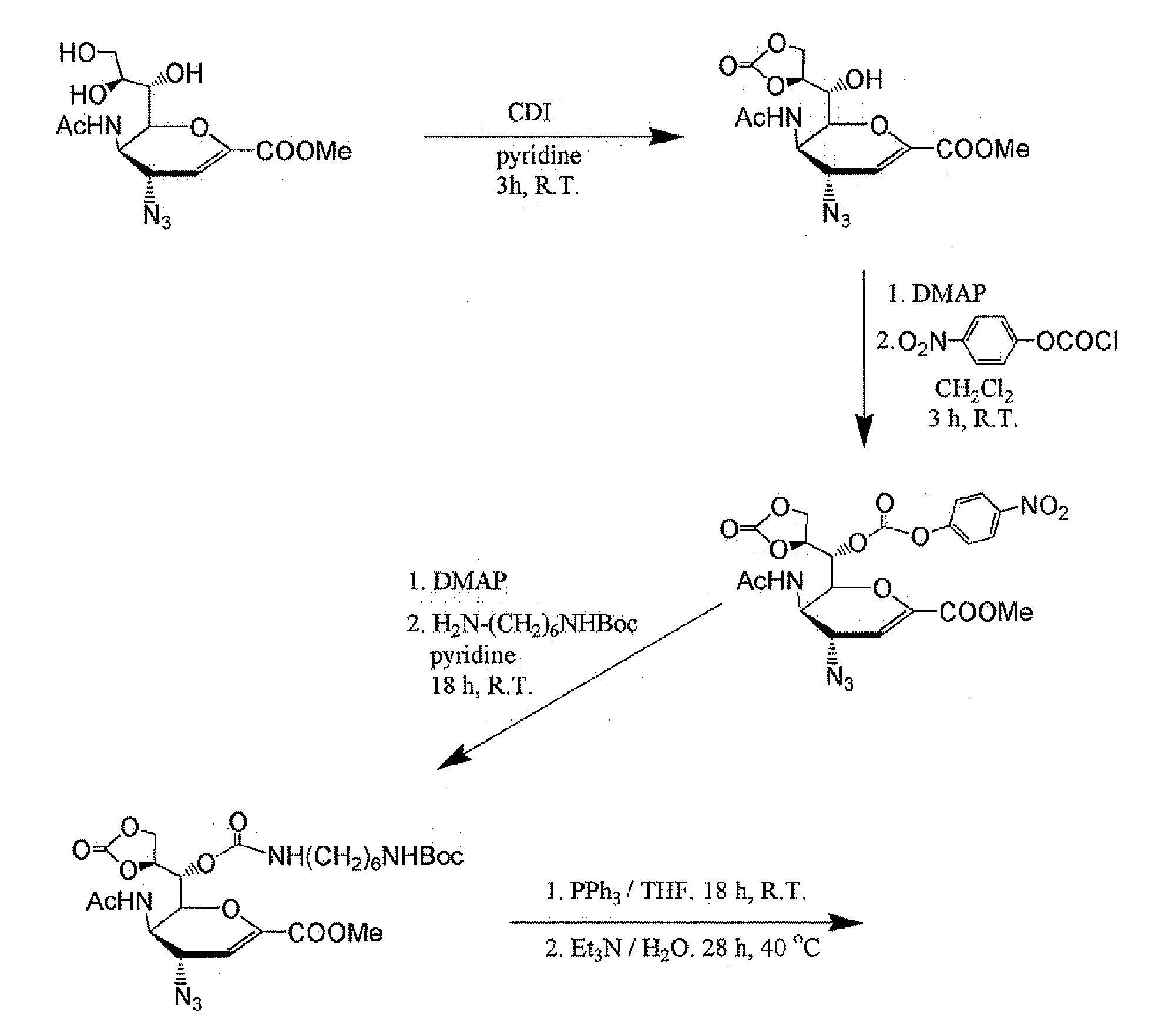
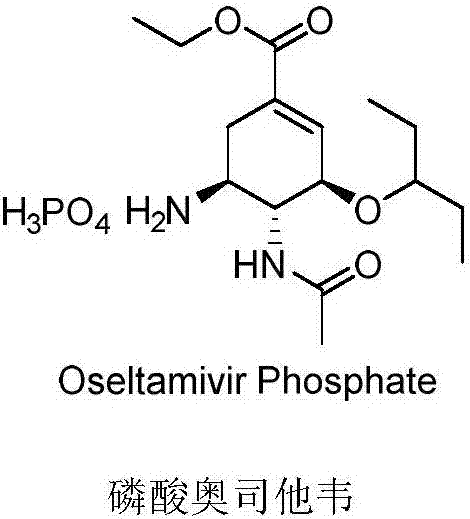
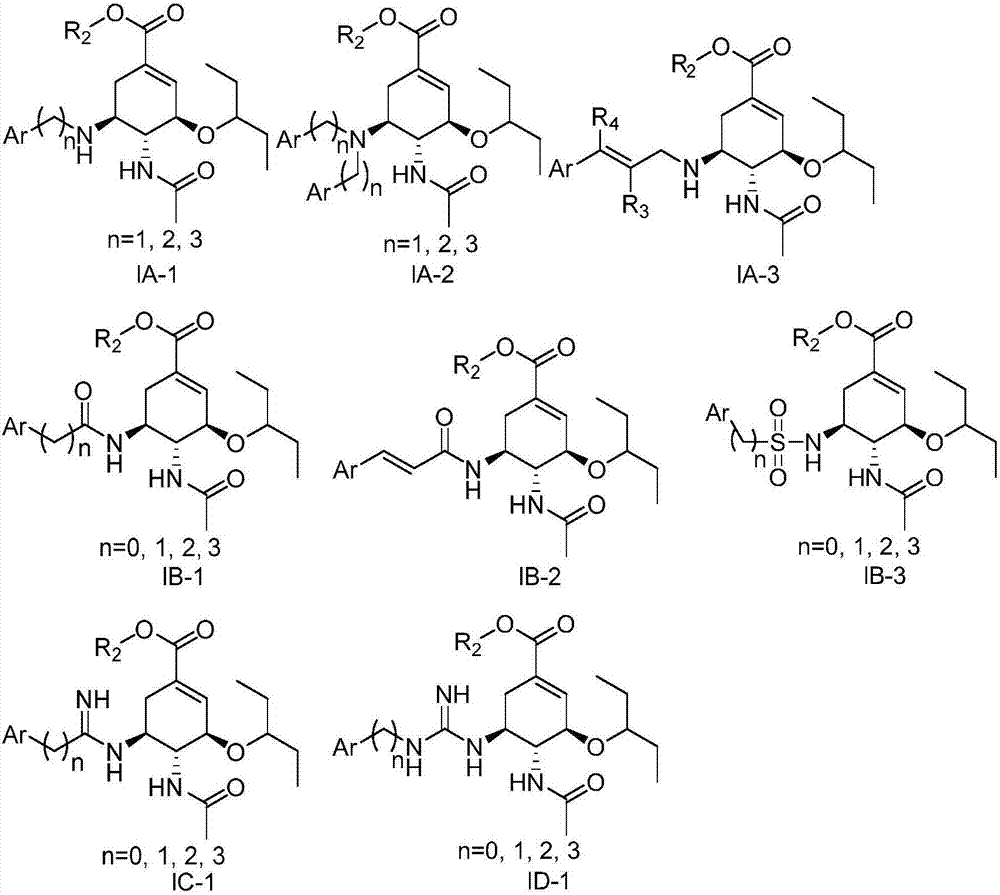
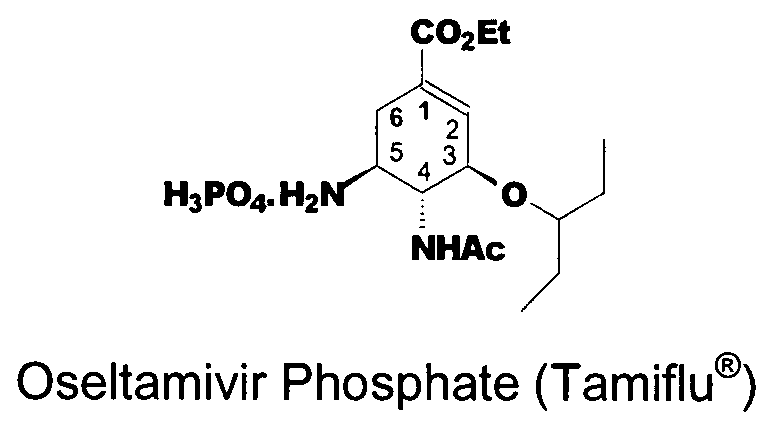
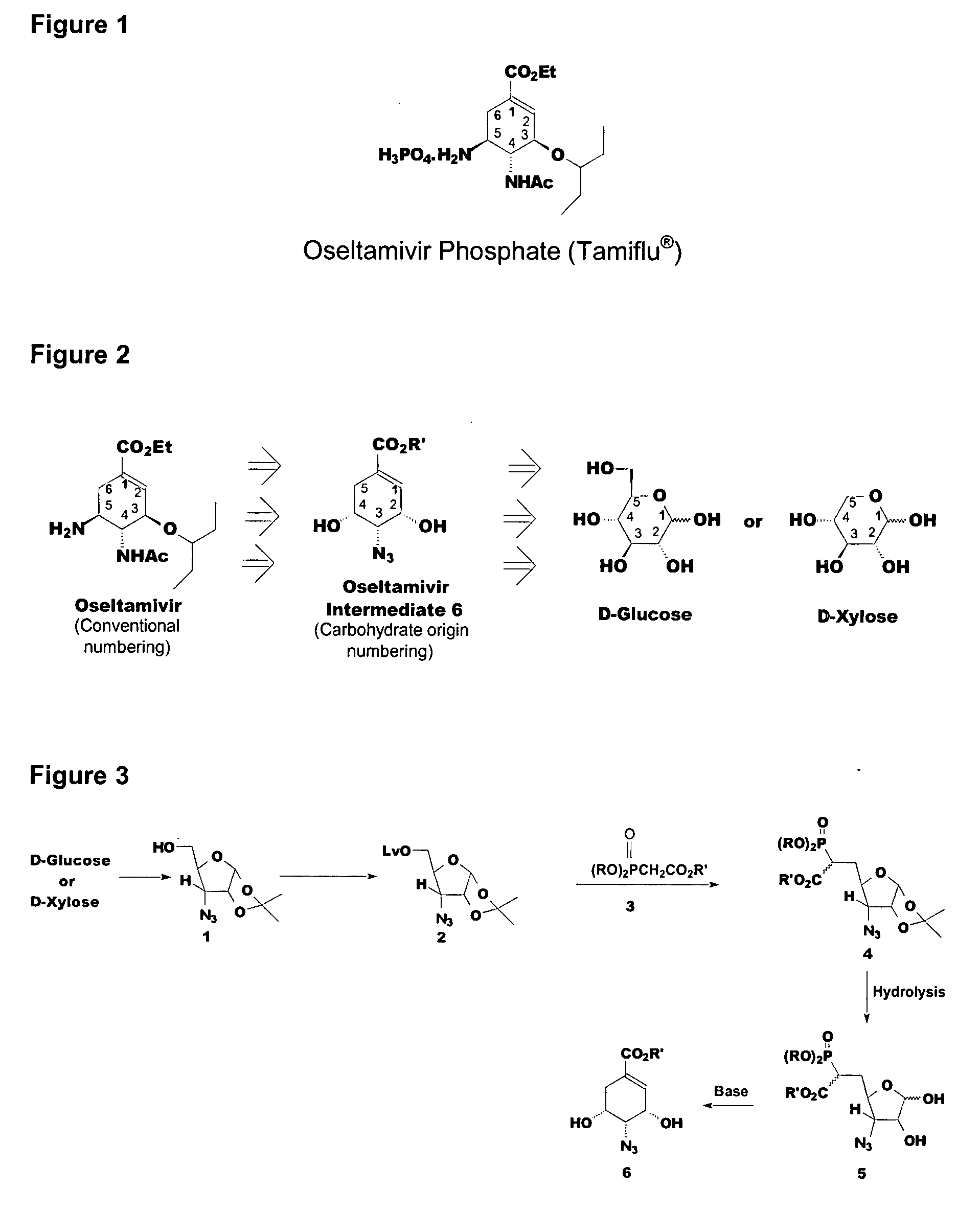
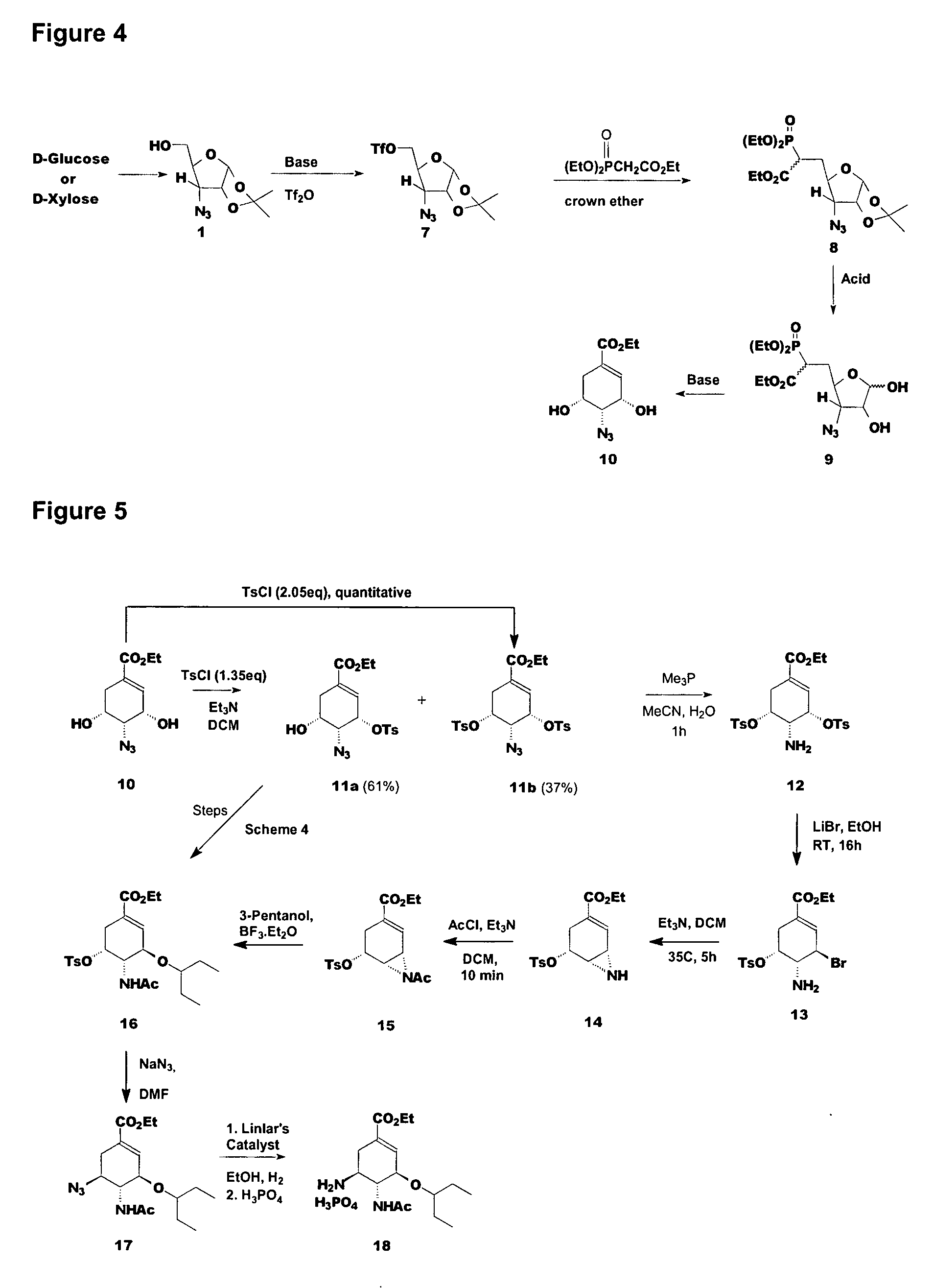
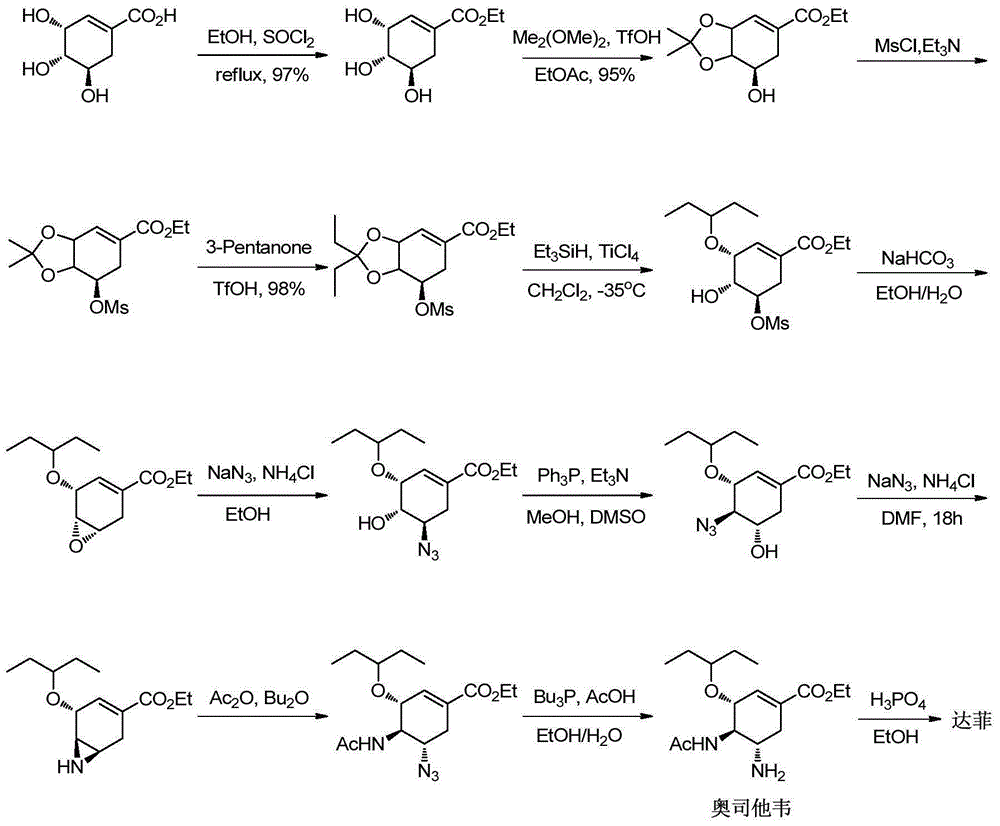
Preparation of oseltamivir phosphate (Tamiflu) and intermediates starting from D-glucose or D-xylose
InactiveUS20080009639A1Marginally expensiveSilicon organic compoundsOrganic compound preparationGlucose polymersD-Glucose
Novel processes for the preparation of the anti-viral agent, Oseltamivir Phosphate and novel intermediates prepared in such processes. The novel processes use as starting materials D-glucose or D-xylose in the preparation of Oseltamivir Phosphate.
Owner:APOTEX PHARMACHEN INC
New preparation method of oseltamivir intermediate
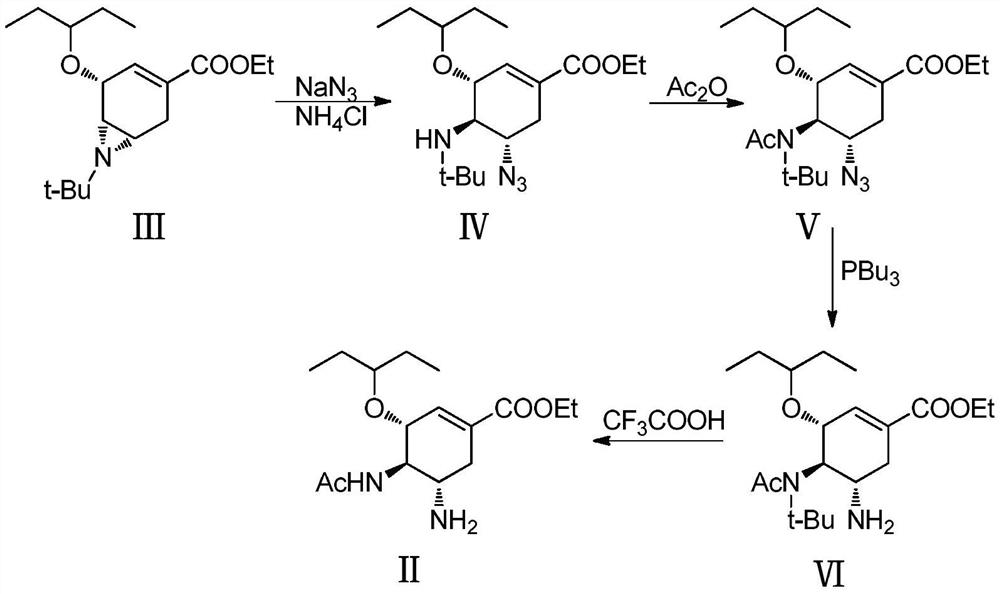
The invention provides a new method for preparing an intermediate (VI) of oseltamivir shown in a formula (I). Starting from a known intermediate (II) reported in a document, chiral acetyl ammonia is built based on a key lossen rearrangement reaction step to obtain a known oseltamivir intermediate structure in the document, so that the use operation of the dangerous sodium azide is avoided, and the high reaction yield is maintained. The method has the advantages that operation is safe and convenient, cheap raw materials are cheap and available. The formula is shown in the specification.
Owner:SUNSHINE LAKE PHARM CO LTD
Synthesis method of oseltamivir
InactiveCN103833570AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationBird fluSynthesis methods
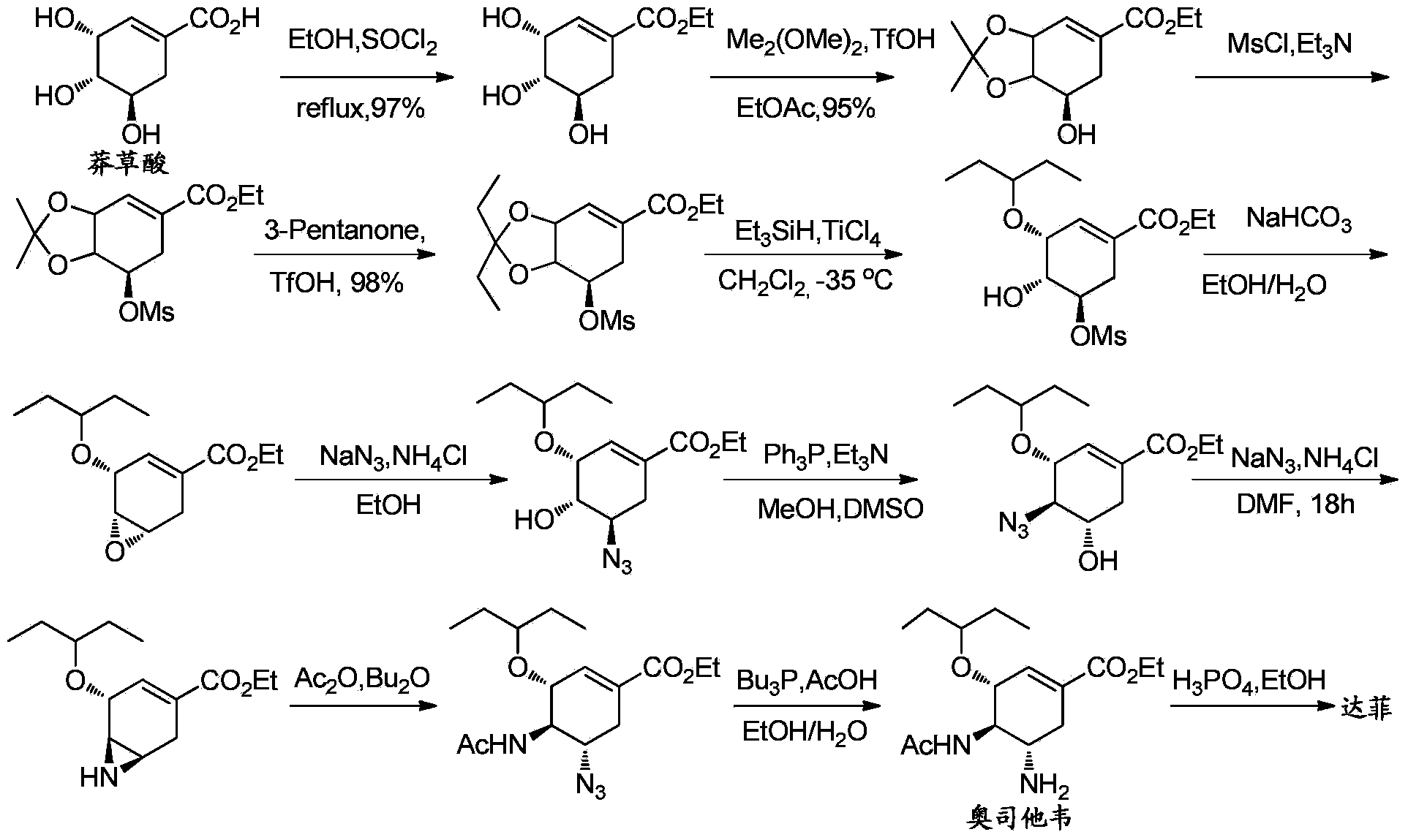
The invention discloses a synthesis method of oseltamivir. The synthesis method of oseltamivir comprises the following steps: starting from a compound 1,3-butadiene-3-amyl ether and compound 3-nitro-ethyl acrylate, carrying out Diels-Alder reaction, then reacting at room temperature in acetonitrile in the presence of a copper catalyst and PhI-NNs to prepare an aziridine compound in a one-pot method, wherein the mole ratio of the 1,3-butadiene-3-amyl ether to 3-nitro-ethyl acrylate to the copper catalyst is 1.1: 1: 0.025-0.1; and finally synthesizing the oseltamivir for preventing bird flu through the aziridine ring opening, nitryl and p-nitrobenzene sulfonyl removal, acetylation and hydrogenation. The method comprises short steps, the used reagent is cheap and easily available, the operation is simple, the total yield is up to 40%, and the method is a simple and efficient synthesis method of oseltamivir.
Owner:ZHEJIANG NORMAL UNIVERSITY
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20170102387A1Organic active ingredientsGroup 5/15 element organic compoundsWild typeDrug resistance
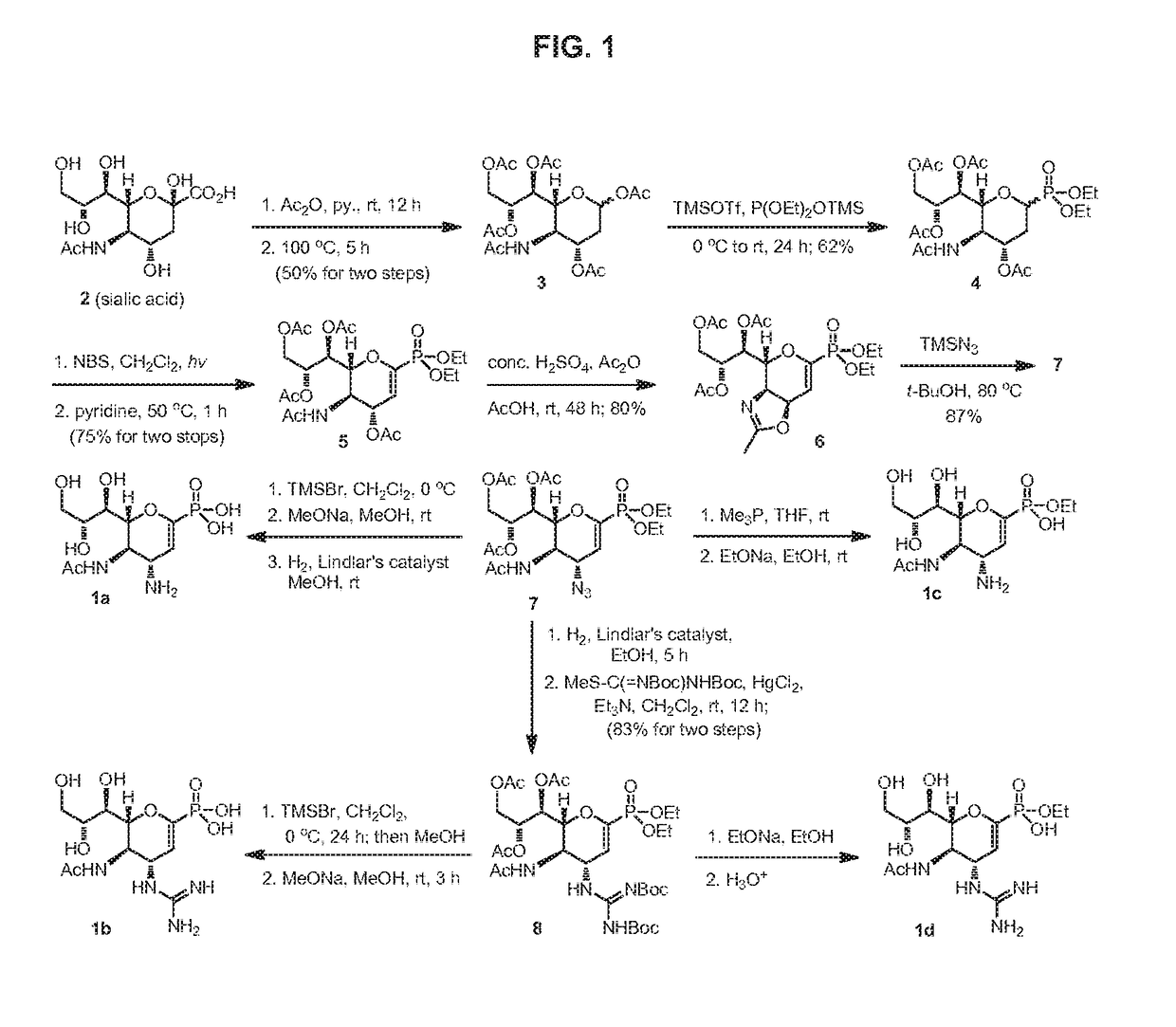
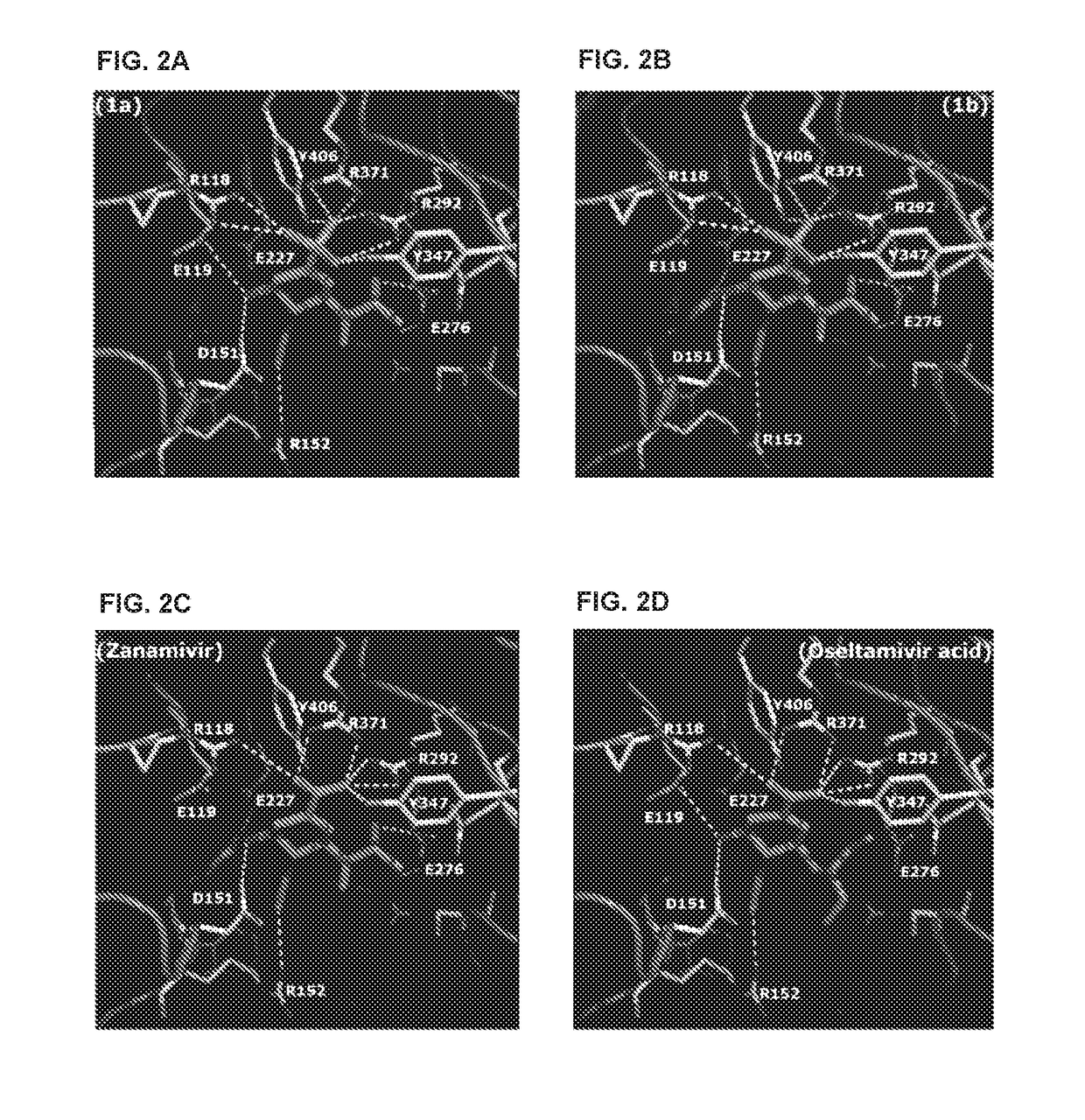
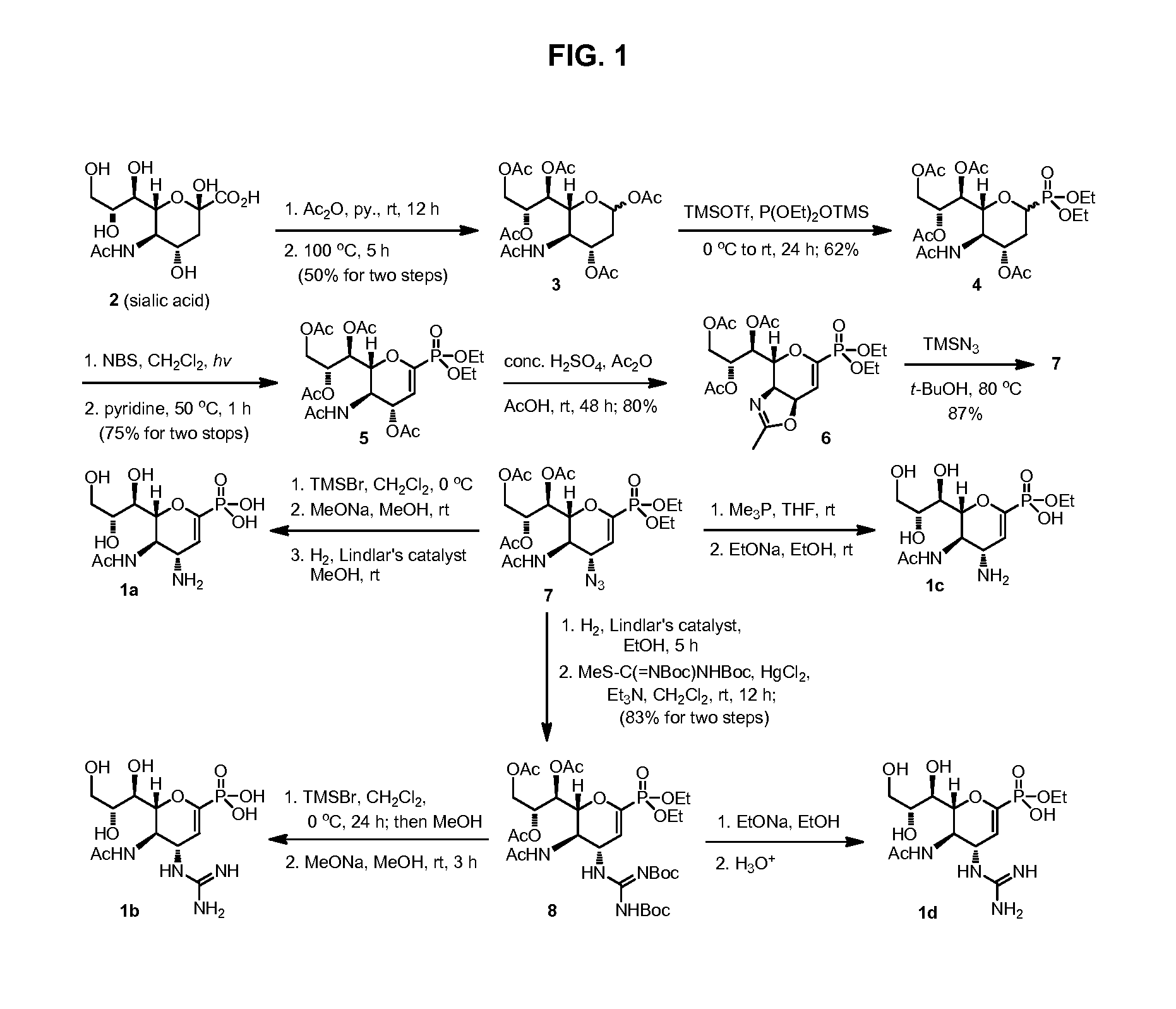
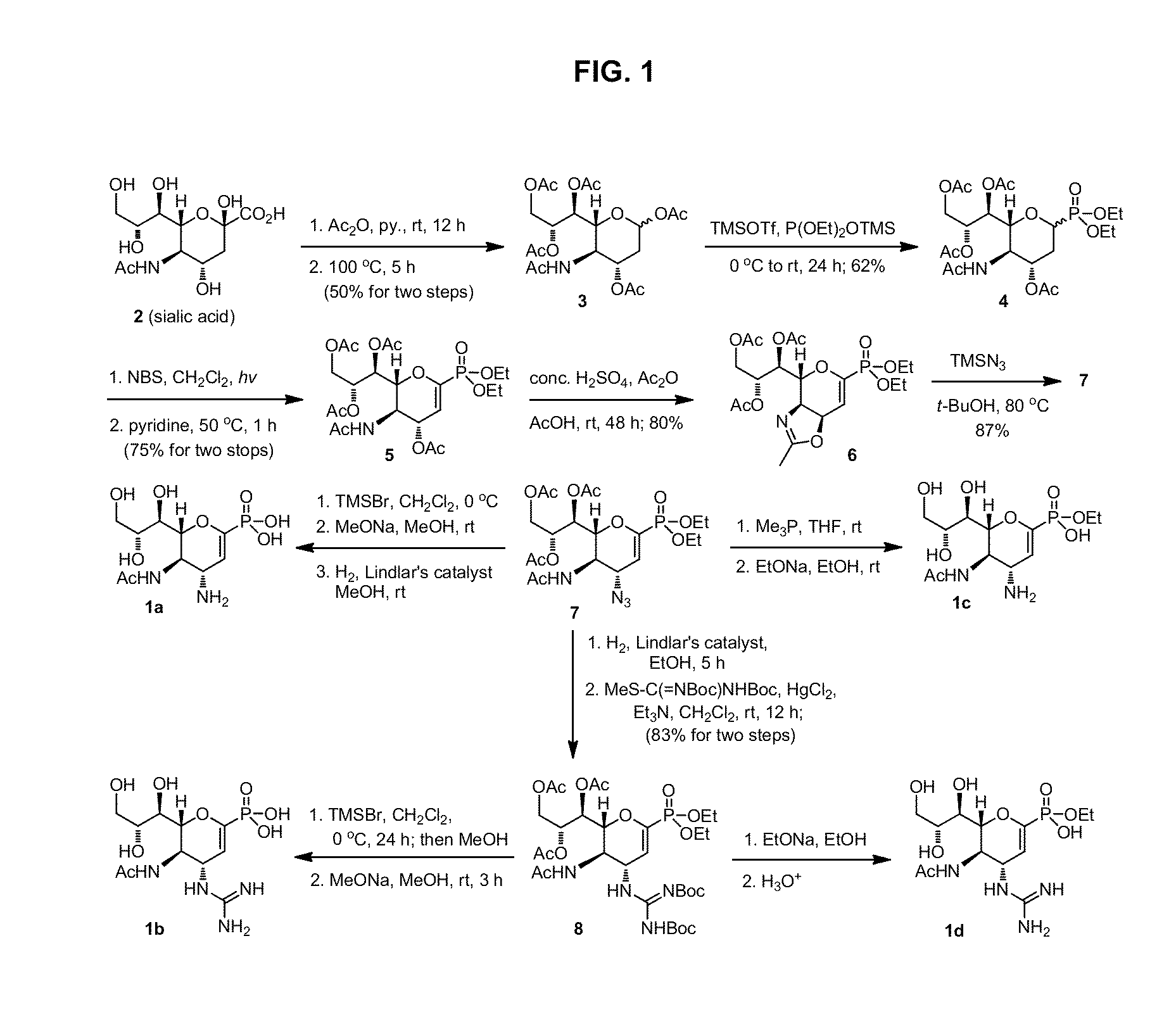
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINICA
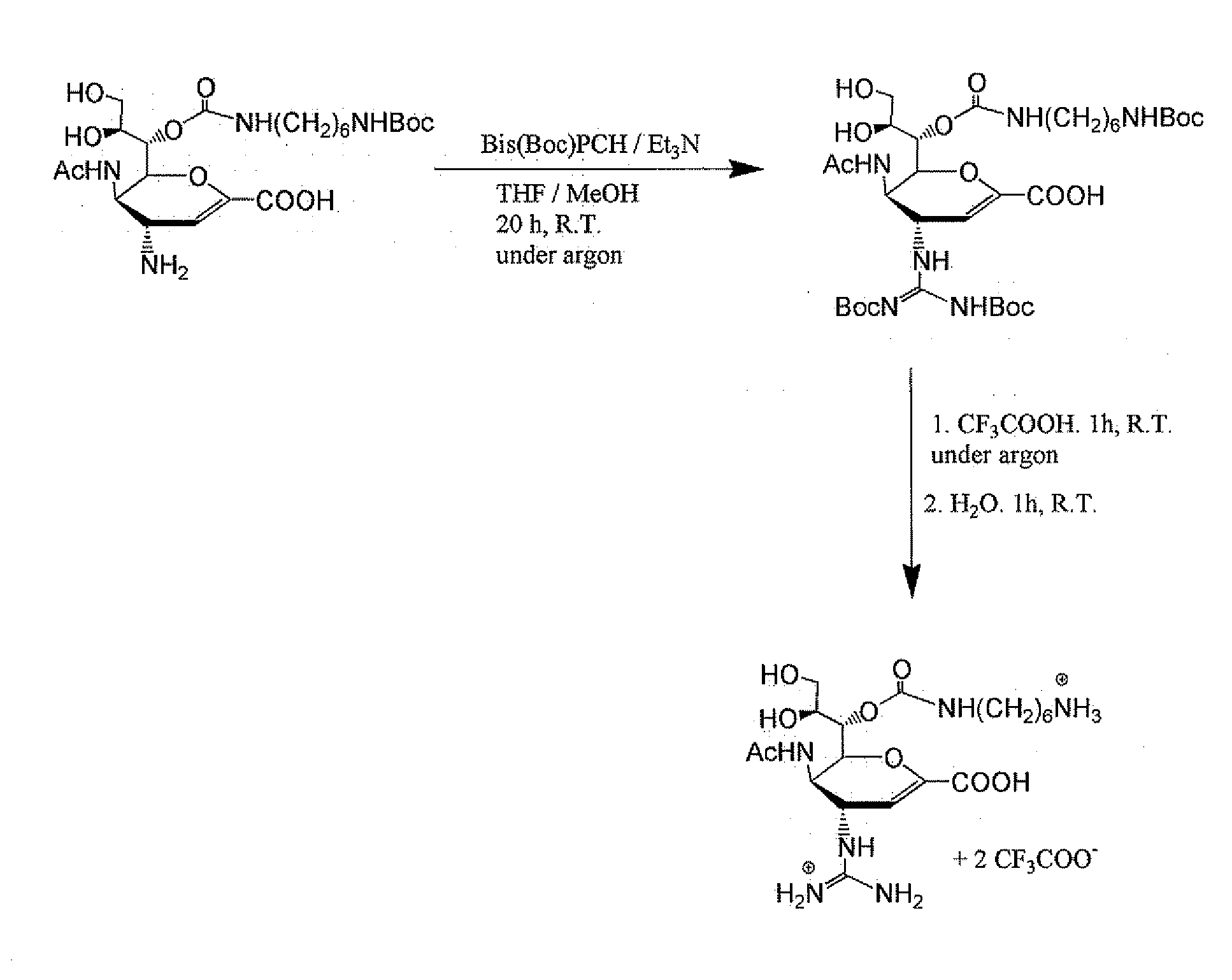
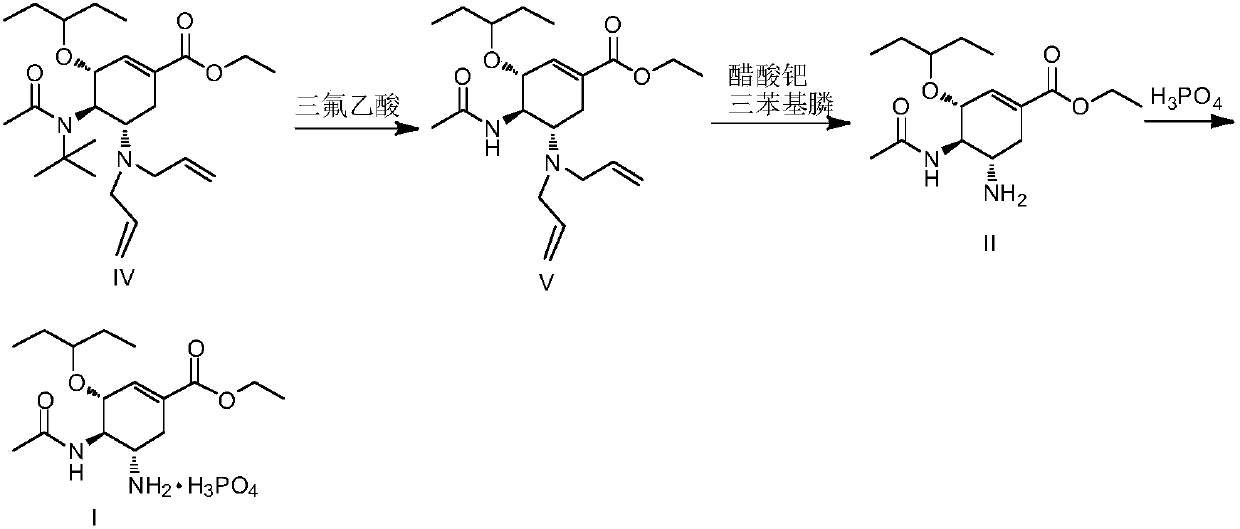
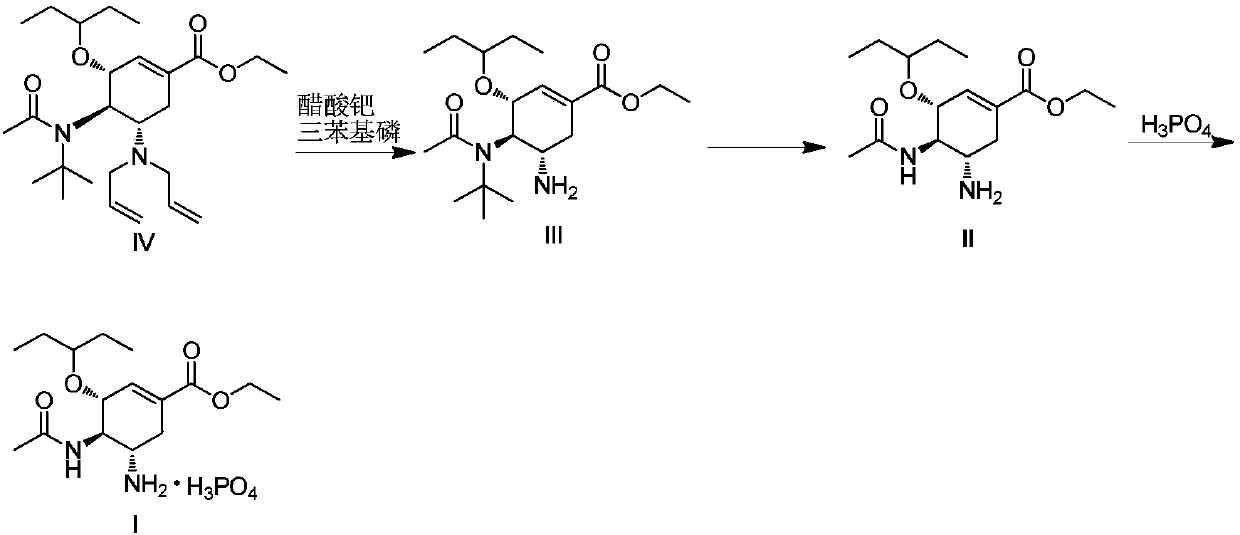
Method for preparing oseltamivir phosphate
ActiveCN109574869AOrganic compound preparationCarboxylic acid amide separation/purificationEquivalence ratioPhosphoric acid
The invention discloses a method for preparing oseltamivir phosphate. The method includes steps of carrying out reaction on intermediates shown as a formula (IV) with palladium acetate, triphenylphosphine and N,N-dimethylbarbituricacid in solvents, and removing allyl to obtain intermediates shown as a formula (III); carrying out acid treatment on the intermediates shown as the formula (III), and removing tertiary butyl to obtain oseltamivir free alkali shown as a formula (II); carrying out reaction on the oseltamivir free alkali with phosphoric acid in solvents, and carrying out crystallization purification. An equivalence ratio of the intermediates shown as the formula (IV) to the palladium acetate to the triphenylphosphineto the N,N-dimethylbarbituricacid is 1:0.01:0.04:1.2. The method has the advantages that the residual quantities of main heavy metal such as palladium, arsenic, cadmium, cobalt, copper, mercury, lithium, nickel, lead, antimony, titanium and vanadium in the oseltamivir phosphate prepared by the aid of the method are within the limit ranges, and accordingly the ICH standards can be met.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20130225532A1Reduce the binding effectBiocideOrganic active ingredientsCompetitive bindingWild type
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
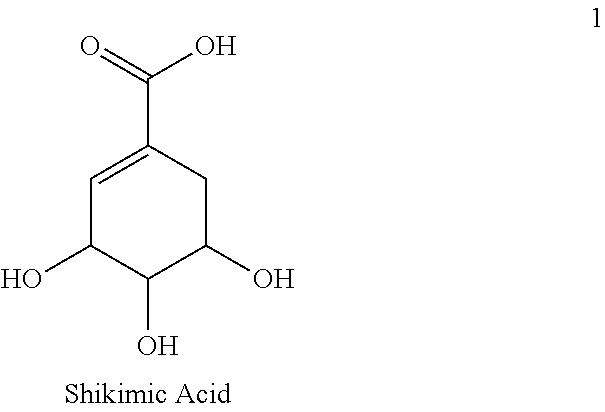
Use of glyphosate to produce shikimic acid in microorganisms
The present invention provides methods for producing shikimic acid. In particular the invention provides methods for producing and isolating shikimic acid from a microorganism. Additionally, the invention provides methods for synthesizing compounds such as oseltamivir and 6-fluoroshikimic acid using shikimic acid produced from microorganisms.
Owner:MONSANTO TECH LLC
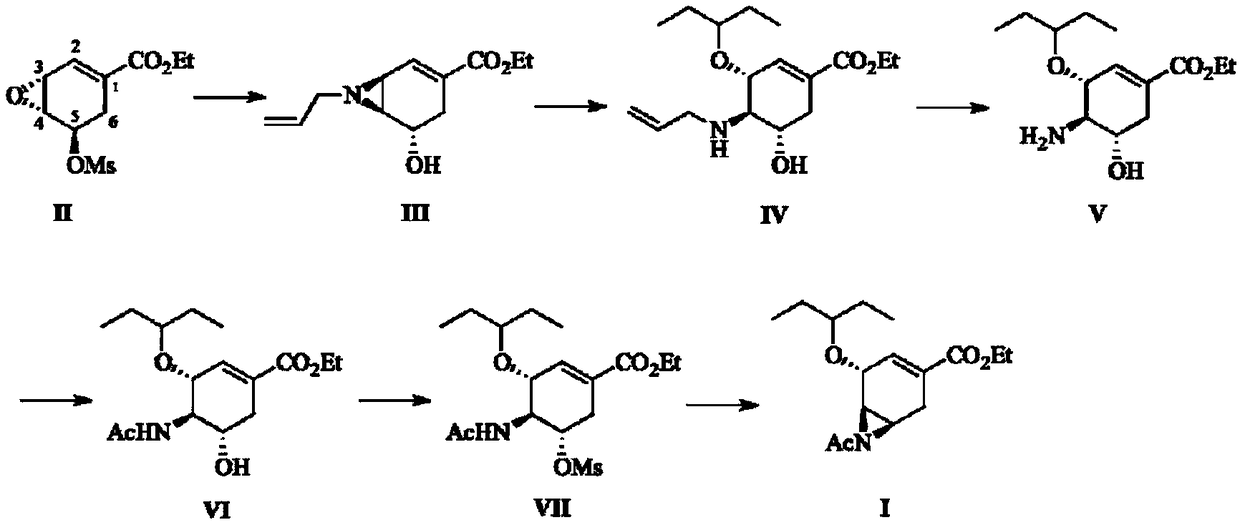
Preparation method of oseltamivir acetyl aziridine intermediate
InactiveCN108484467ARich sourcesIncrease production capacityOrganic chemistryEpoxyChemical synthesis
The invention discloses a preparation method of an oseltamivir acetyl aziridine intermediate I (shown in a figure 1). The preparation method is characterized in that the oseltamivir acetyl aziridine intermediate I is prepared through six chemical synthesis steps (in figure 2) by taking a shikimic acid epoxy derivative II as a raw material, wherein the yield is 44 to 61 percent. The preparation method comprises the following six chemical synthesis steps: (1) carrying out ring opening in a 3-site of an epoxy compound II, and carrying out 'one-pot' three-step tandem reaction, thus forming 3,4-aziridine compound III; (2) selectively attacking the 3-site by 3-pentanol, and carrying out the ring opening, thus obtaining a compound IV; (3) removing an allyl group in a 4-site, thus obtaining a compound V; (4) carrying out amino acetylization in the 4-site, thus obtaining a compound VI; (5) carrying out hydroxyl methyl sulfonylation in a 5-site, thus obtaining a compound VII; (6) attacking 5-site by a 4-acetamino group, and carrying ring closing, thus obtaining the oseltamivir acetyl aziridine intermediate I.
Owner:EAST CHINA UNIV OF SCI & TECH
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Orally-disintegrating oseltamivir tablet and method for preparing the same
InactiveUS20160120802A1Dissolve fastShort disintegration timeOrganic active ingredientsBiocideALLYL SUCROSEBULK ACTIVE INGREDIENT
An orally-disintegrating oseltamivir tablet, including: between 10 and 50 wt. % of a taste-masking pellet, between 30 and 80 wt. % of a first filler, between 1 and 6 wt. % of a first adhesive, between 2 and 10 wt. % of a disintegrant, between 0 and 5 wt. % of a flavoring agent, between 0.5 and 2.5 wt. % of a lubricant. The taste-masking pellet includes: an active ingredient-loaded pellet core and a coating layer. The active ingredient of the pellet core is oseltamivir or a pharmaceutically acceptable salt of oseltamivir and accounts for between 10 and 40 wt. % of a total weight of the taste-masking pellet. The coating layer include a polyacrylic acid resin IV and accounts for between 1 and 50 wt. % of the total weight of the taste-masking pellet. The diameter of the taste-masking pellet is between 0.10 and 0.50 mm.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Compositions and methods for the treatment of viral infections
InactiveUS20080045482A1Reduce viral loadInhibits viral replicationBiocideInorganic phosphorous active ingredientsViral replicationViral infection
The present invention provides compositions and methods for inhibiting the replication of influenza virus by administering thiophosphonoformic acid alone or in combination with a neuraminidase inhibitor, for example, oseltamivir.
Owner:ADVENTRX PHARMA INC
Method for preparing oseltamivir phosphate by azide process
PendingCN111747861AEmission reductionReduce governance costsOrganic compound preparationCarboxylic acid amides preparationO-Phosphoric AcidEthylic acid
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a method for preparing oseltamivir phosphate by an azide process. The method comprises the following steps:reacting a compound shown in a formula (III) with sodium azide and ammonium chloride, opening a nitrogen heterocyclic ring, performing acetylation, reducing an azide group, removing tert-butyl, salifying with phosphoric acid, and purifying to obtain pure oseltamivir phosphate shown in a formula (I). According to the method, diallylamine with strong corrosivity and expensive palladium acetate do not need to be used so that the enterprise cost is reduced.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
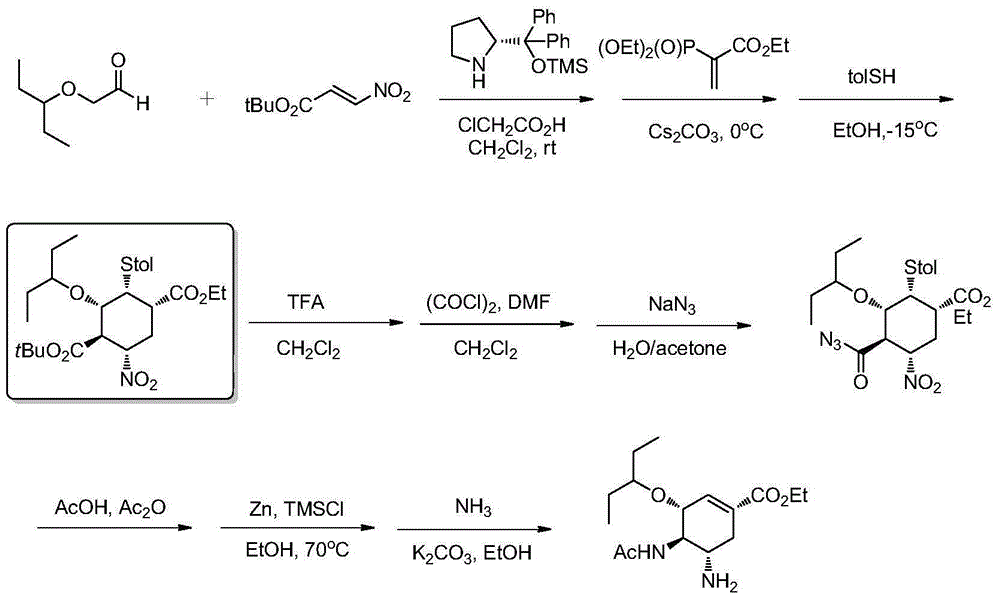
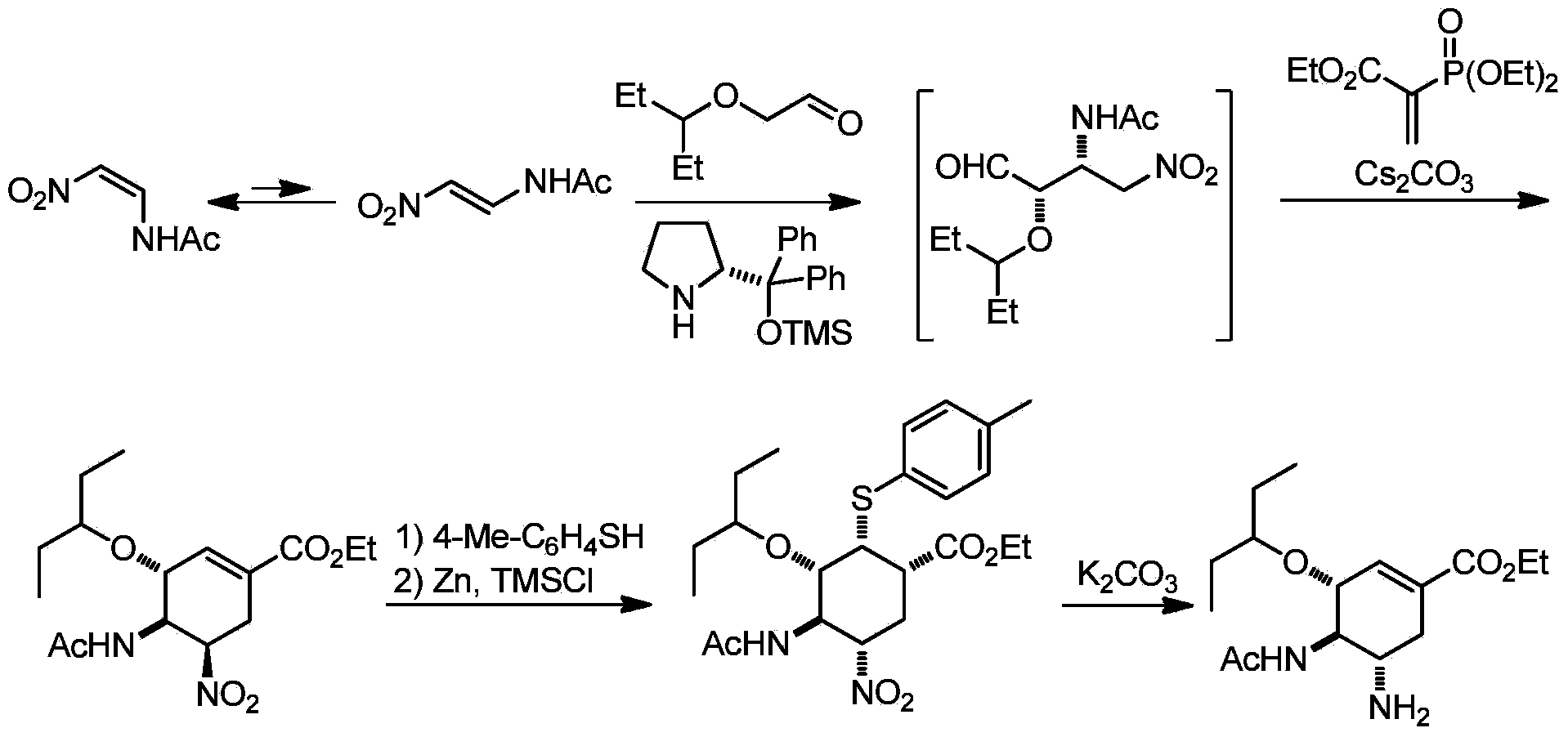
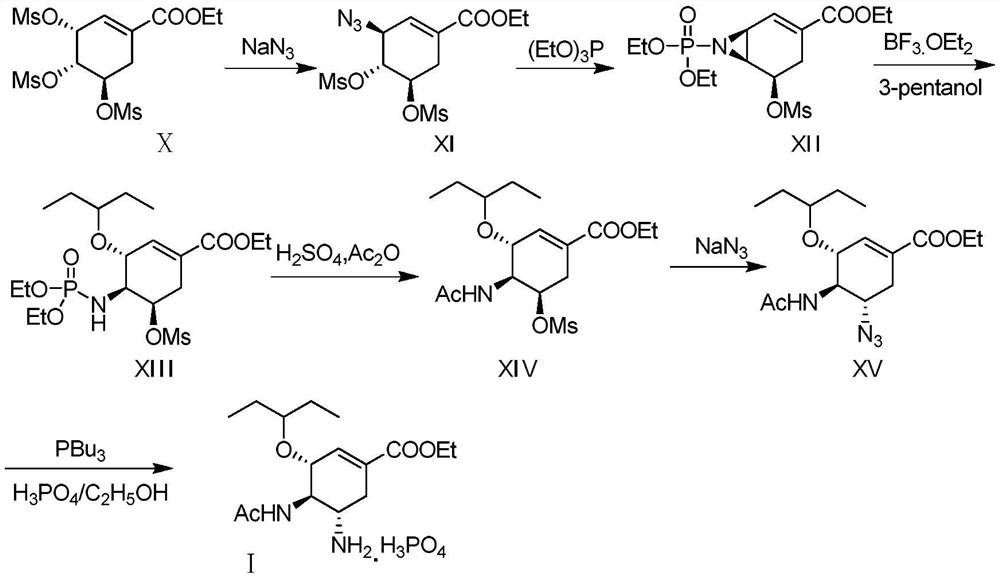
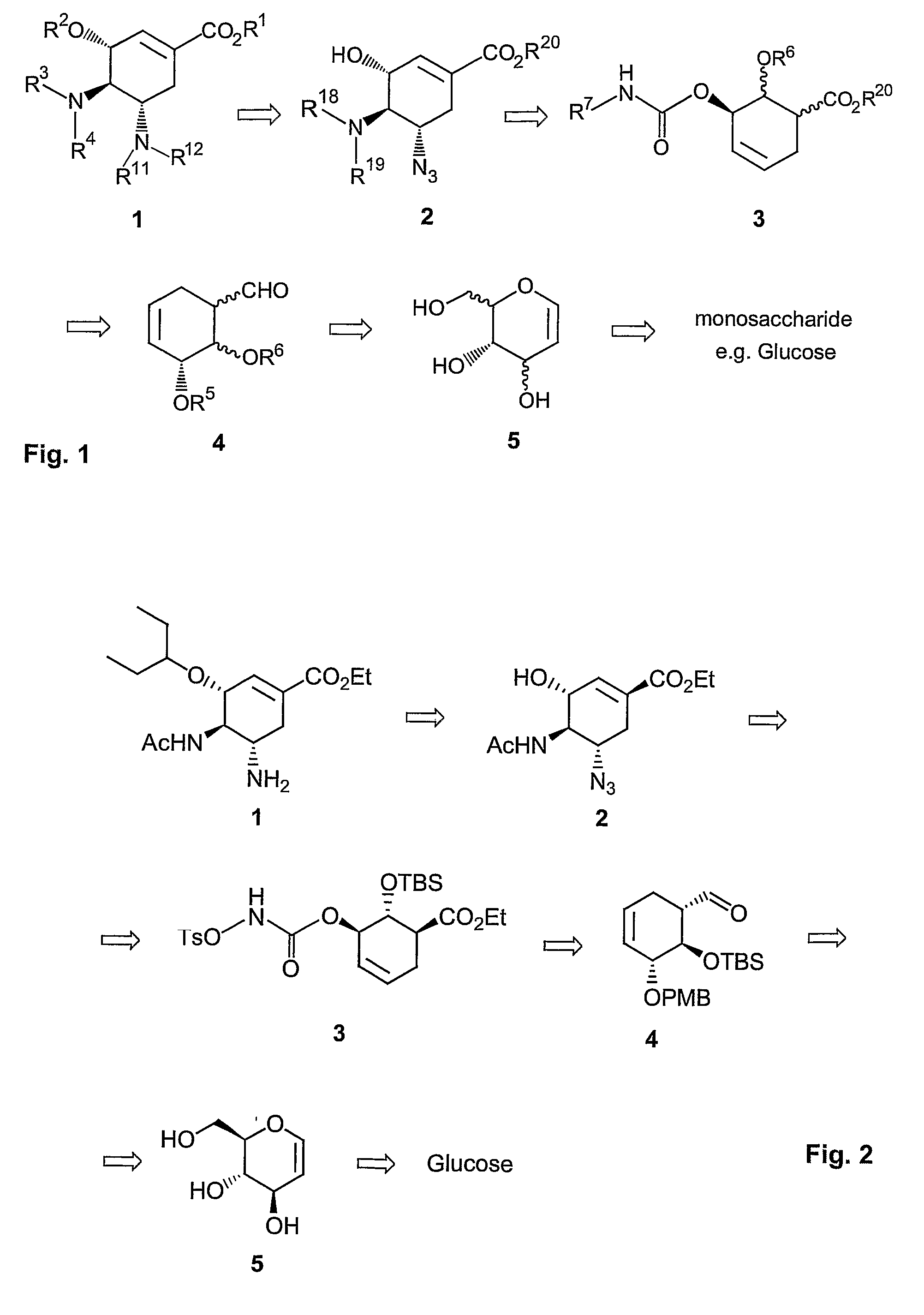
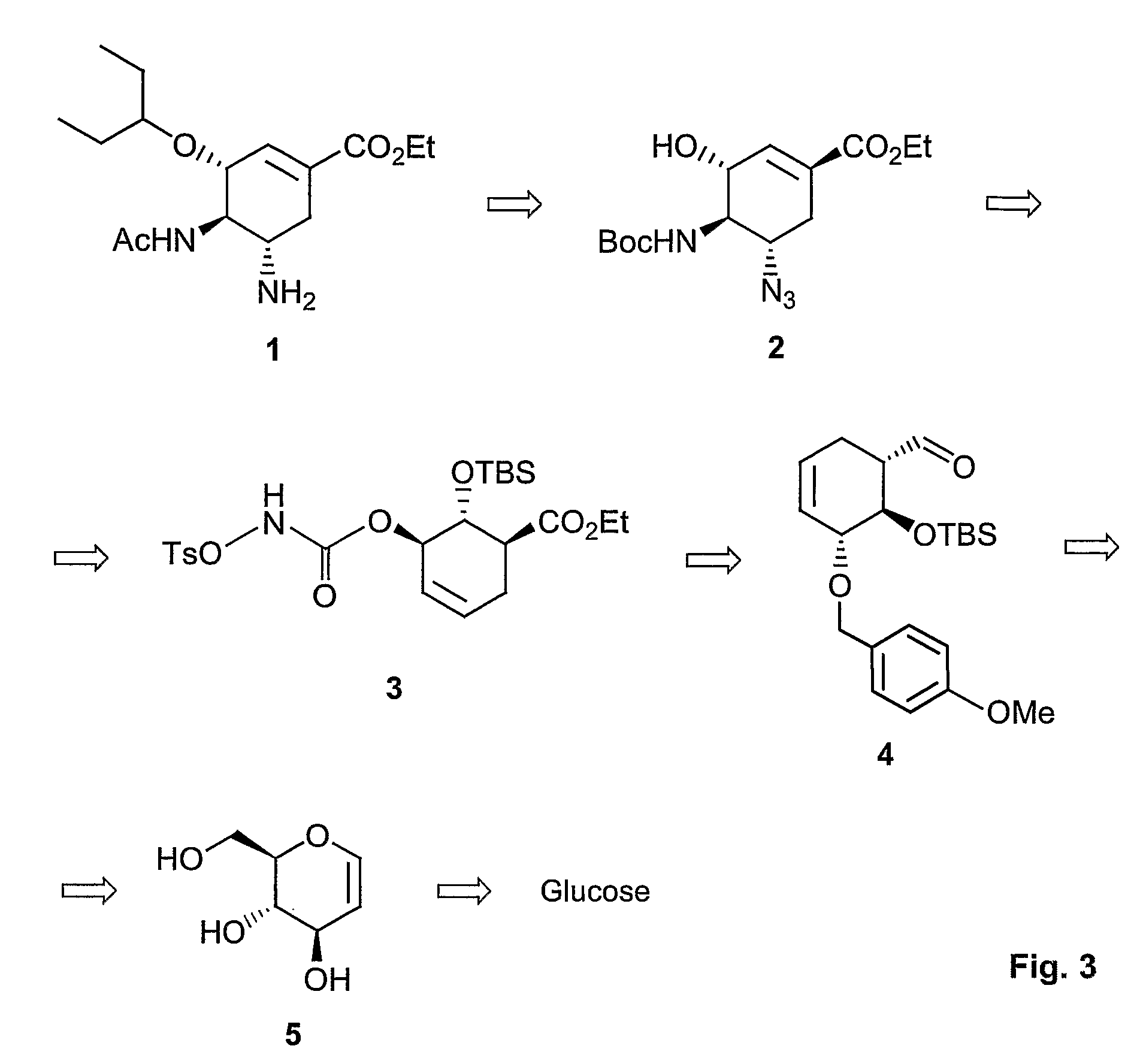
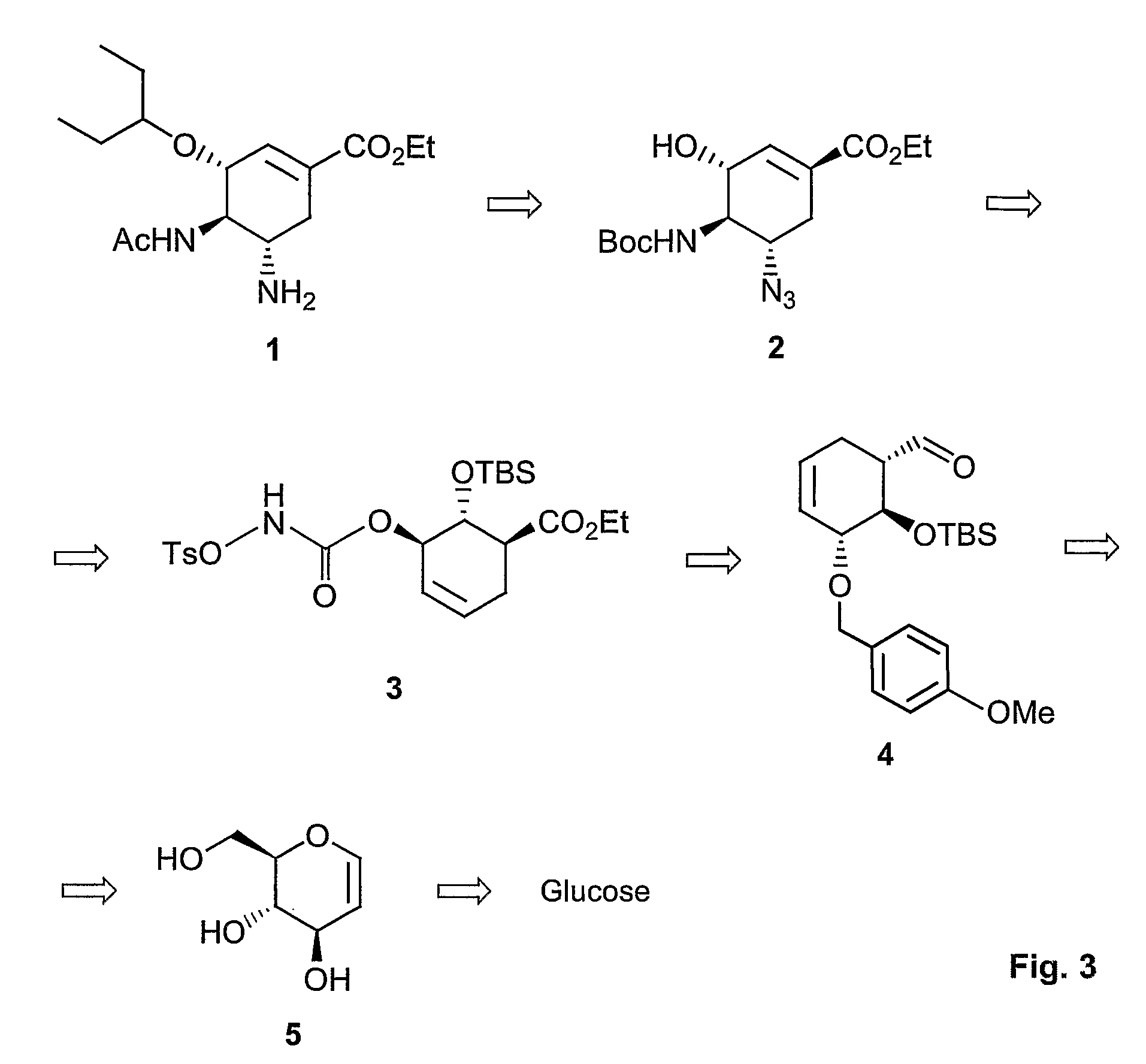
Method of forming oseltamivir and derivatives thereof
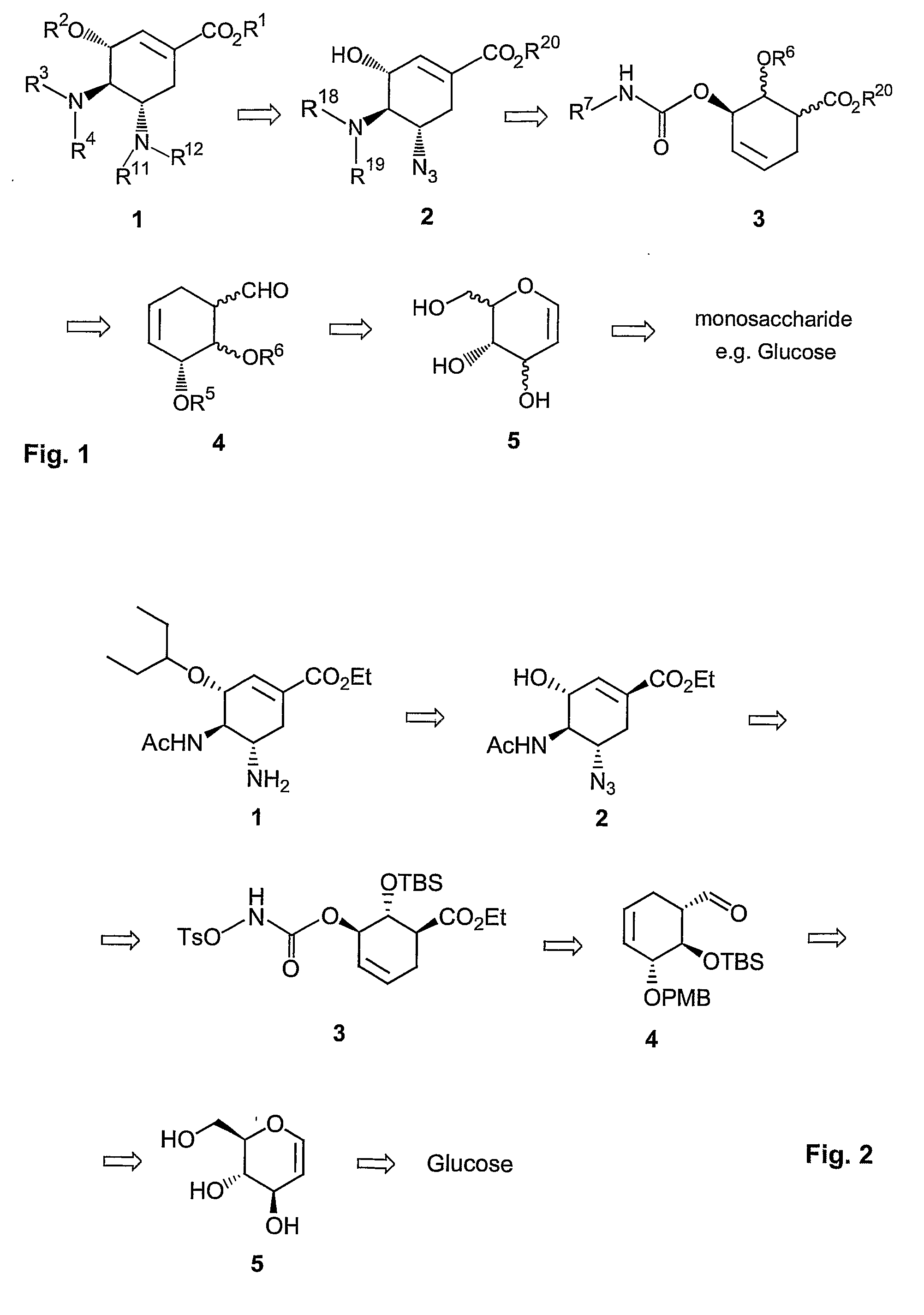
ActiveUS8304553B2Improve efficiencySilicon organic compoundsOrganic compound preparationSilyleneCarbamate
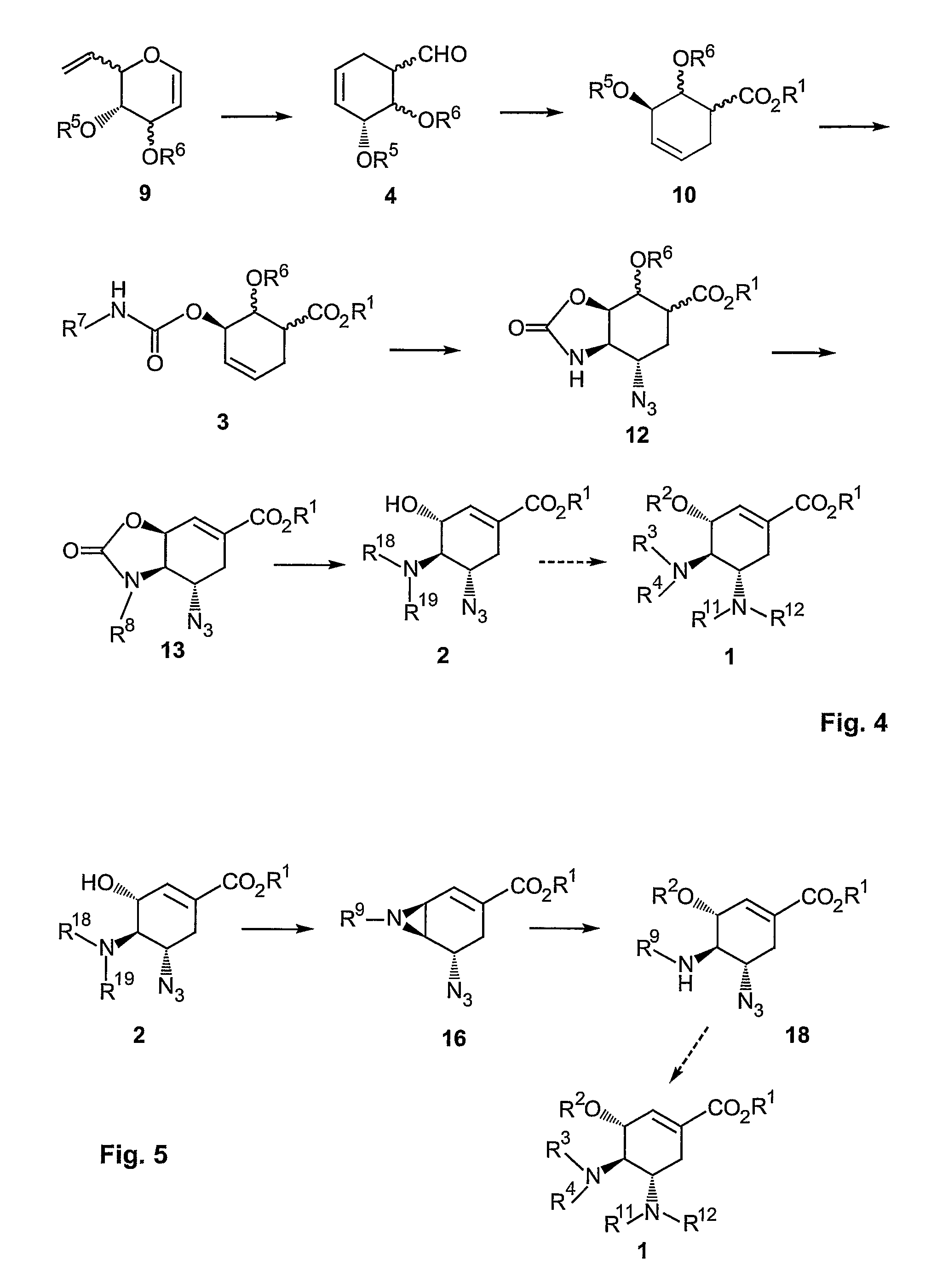
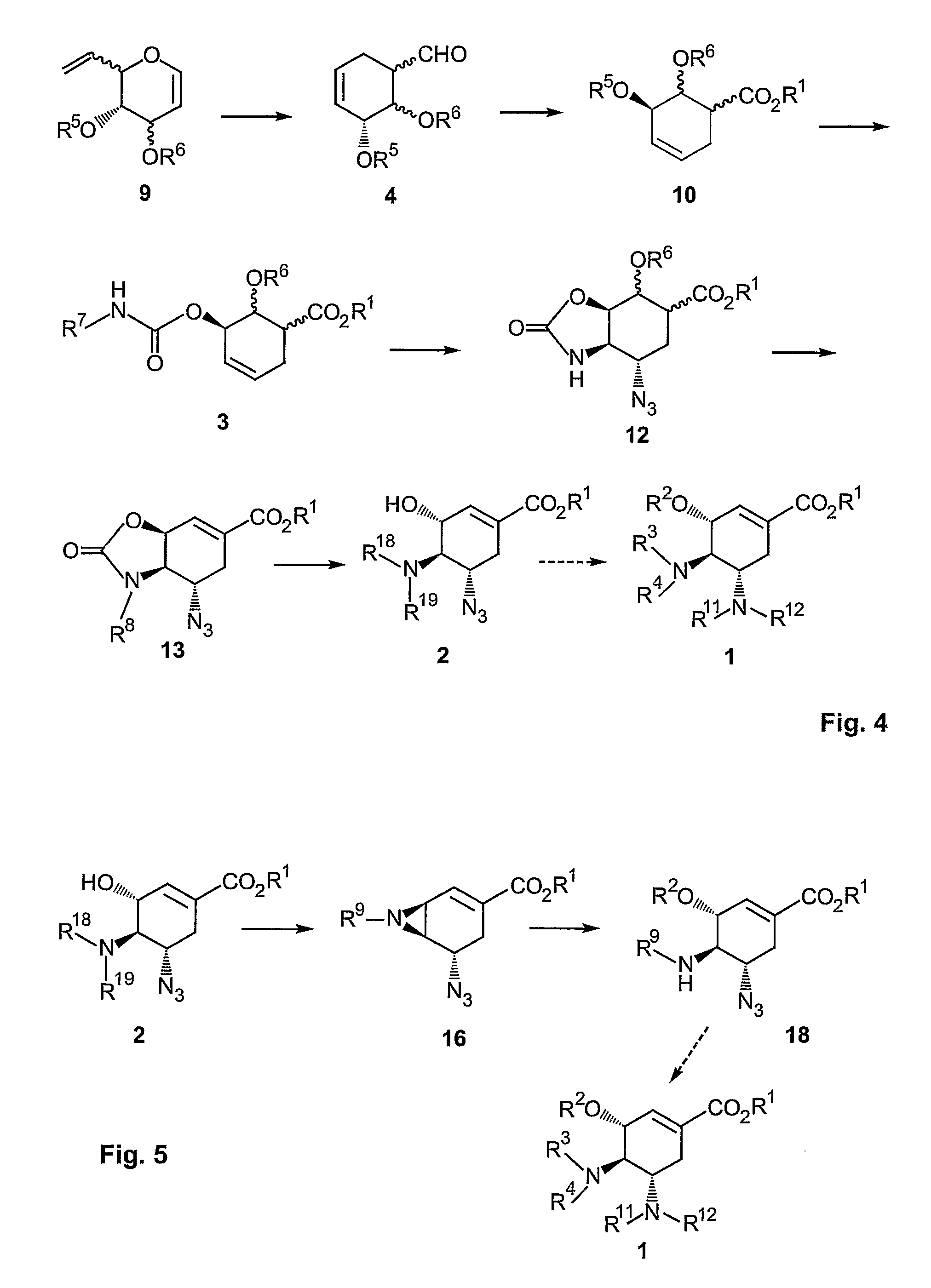
A process is provided for the synthesis of 4,5-diamino cyclohexene carboxylate ester (1): or a pharmaceutically acceptable salt thereof. R1-R3 are a silyl-, an aliphatic, alicyclic, aromatic, arylaliphatic, or an arylalicyclic group. R4, R11 and R12 are H, a silyl-group, an aliphatic, alicyclic, aromatic, arylaliphatic, or an arylalicyclic group. 3,4-Dihydropyran compound (9): with R5 and R6 being suitable protecting groups, is reacted to form aldehyde (4): which is oxidized and converted to N-substituted carbamate (3): with R7 being a suitable protecting group. (3) is, via oxazolinidone (13): converted to azido carboxylate ester (2): and then to 4,5-diamino cyclohexene carboxylate ester (1).
Owner:NANYANG TECH UNIV
Pharmaceutical composition of oseltamivir phosphate coated particles, as well as application and preparation method
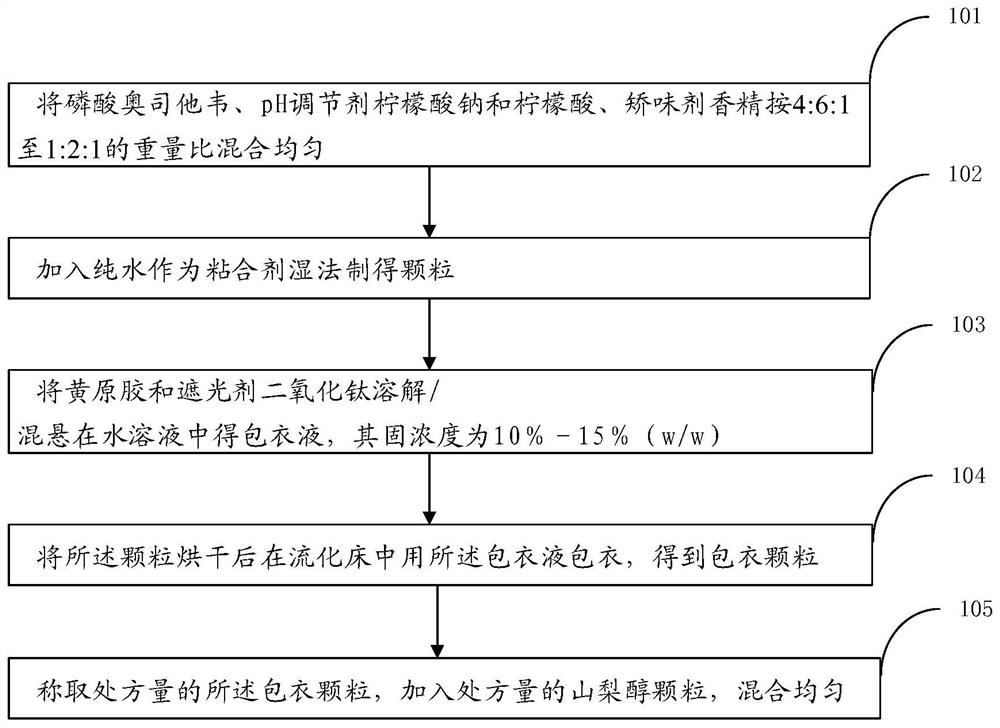
ActiveCN112121027AReady for industrializationFormulation stabilityOrganic active ingredientsInorganic non-active ingredientsCombinatorial chemistrySodium citrate
The invention provides a pharmaceutical composition of oseltamivir phosphate coated particles, as well as application and a preparation method. The pharmaceutical composition comprises coated particles and drug-free particles, and the weight ratio of the coated particles to the drug-free particles is (1: 10) to (1: 2); the coating particles comprise oseltamivir phosphate-containing medicinal particles and a coating layer, and the coating layer comprises xanthan gum and opacifier titanium dioxide; the oseltamivir phosphate-containing medicinal particles comprise oseltamivir phosphate, a pH regulator sodium citrate and citric acid, a flavoring agent essence and an adhesive pure water; and the weight ratio of oseltamivir phosphate to the pH regulator to the flavoring agent is 2: 3: 1. According to the oseltamivir phosphate coated particle pharmaceutical composition, the application and the preparation method provided by the invention, oseltamivir phosphate is prevented from being degradedby related auxiliary materials and external factors, the pharmaceutical composition is ensured to be rapidly dissolved out, and the due preparation effect is ensured; and the preparation operation issimple, the cost is reduced, and the industrial production efficiency is improved.
Owner:北京民康百草医药科技有限公司
Method for the extraction of shikimic acid
InactiveUS20130137895A1Inhibit blood platelet assemblingPrevent thrombosisCarboxylic compound separation/purificationInternational marketPlatelet inhibition
This invention is directed to the production of shikimic acid from Illicium griffithii fruits. The method according to the invention is particularly applicable to the isolation of shikimic acid from Illicium griffithii fruits (seeds and pericarps). Yield is 12-18% w / w. Shikimic acid is useful as raw material for the production of oseltavir (Tamiflu) used against Avian Flu. It is also reported that its triacyl derivatives can inhibit blood platelet assembling and Thrombosis by affecting the metabolism of Arachidonic acid. Hitherto known commercial methods of production of shikimic acid from the fruits of star anise (Illicium verum) and sweet gum (Liquidambar styraciflua) gives only 3-7% and 1.5% respectively. The price of shikimic acid in the international market varies from US$ 45.00 to 1000.00 per Kg depending on demand. Further as per report published at the website www.livemint.com, China Government has imposed restriction on export of shikimic acid.
Owner:COUNCIL OF SCI & IND RES
Oseltamivir Compositions
InactiveUS20170258749A1Powder deliveryOrganic active ingredientsExcipientPharmaceutical preservatives
The present invention relates to pharmaceutical composition comprising two different populations with first population comprising oseltamivir or a pharmaceutical acceptable salt thereof and one or more pharmaceutically acceptable excipients and second population comprising one or more pharmaceutically acceptable excipients. Preferably, the compositions wherein the second population does not contain oseltamivir or a pharmaceutically acceptable salt thereof. The invention also disclose new method of filing the composition into container. The inventors of the present invention surprisingly found that the composition are stable in real-time and long-term stability conditions. Further, the compositions are bioequivalent to marketed suspension formulation of Oseltamivir phosphate.
Owner:LUPIN ATLANTIS HLDG
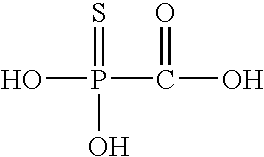
Oseltamivir warning structure impurity and preparation method thereof
PendingCN114456097AHigh yieldOrganic chemistryComponent separationBiochemical engineeringCombinatorial chemistry
The invention discloses an oseltamivir warning structure impurity and a preparation method thereof. The oseltamivir warning structure impurity is shown as a formula I in the specification. The preparation method disclosed by the invention is low in cost and simple and convenient to operate, can be used for large-scale synthesis, and provides a reference substance for qualitative and quantitative analysis of oseltamivir warning structure impurities, thereby laying a solid foundation for quality research of oseltamivir crude drugs and related preparations.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Method of forming oseltamivir and derivatives thereof
A process is provided for the synthesis of 4,5-diamino cyclohexene carboxylate ester (1): or a pharmaceutically acceptable salt thereof. R1-R3 are a silyl-, an aliphatic, alicyclic, aromatic, arylaliphatic, or an arylalicyclic group. R4, R11 and R12 are H, a silyl-group, an aliphatic, alicyclic, aromatic, arylaliphatic, or an arylalicyclic group. 3,4-Dihydropyran compound (9): with R5 and R6 being suitable protecting groups, is reacted to form aldehyde (4): which is oxidized and converted to N-substituted carbamate (3): with R7 being a suitable protecting group. (3) is, via oxazolinidone (13): converted to azido carboxylate ester (2): and then to 4,5-diamino cyclohexene carboxylate ester (1).
Owner:NANYANG TECH UNIV
Non-biological method for screening effective antivirus components in traditional Chinese medicines
InactiveCN103940932AAbundant resourcesExpedited screeningComponent separationFunctional monomerPharmaceutical Substances
The invention discloses a non-biological method for screening effective antivirus components in traditional Chinese medicines. The method comprises the following steps: taking an influenza virus specific drug oseltamivir (OS) as the template molecule, taking acrylamide and 4-vinylpyridine as the functional monomers, carrying out synthesis to obtain non-covalent oseltamivir molecular imprinting polymer (OS-MIP), taking the OS-MIP as the stationary phase to prepare a liquid chromatographic column through a wet method, carrying out affinity screening on effective component groups of traditional Chinese herbals and compounds, identifying the compound components with affinity through mass spectrum, then carrying out in-vitro anti-virus experiments to verify the compound activity, and finally finding the compounds, which have the same antivirus activity as the template molecule, in the traditional Chinese medicines. The method can also be modified to screen and verify other active compounds so as to shorten the screening process of drug entities and promote the research process of replacement drugs, and has a very important meaning and wide application prospect in the field of non-biological drug screening methods.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
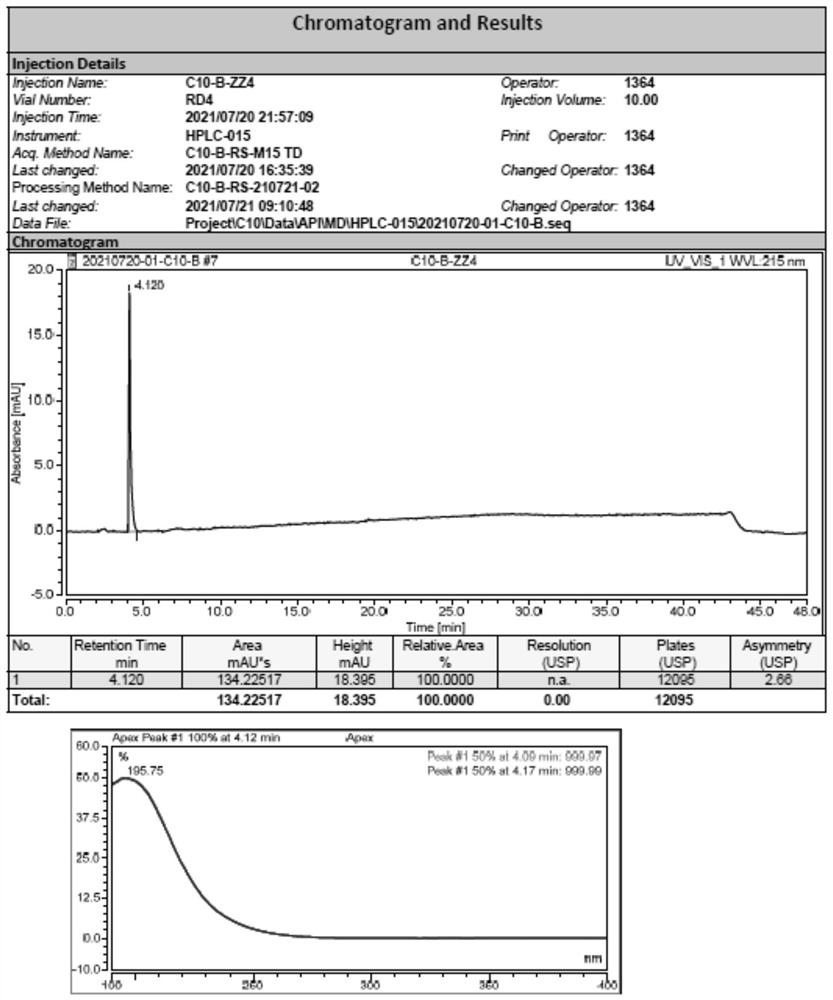
Analysis method for separating and detecting oseltamivir phosphate intermediate and impurities thereof
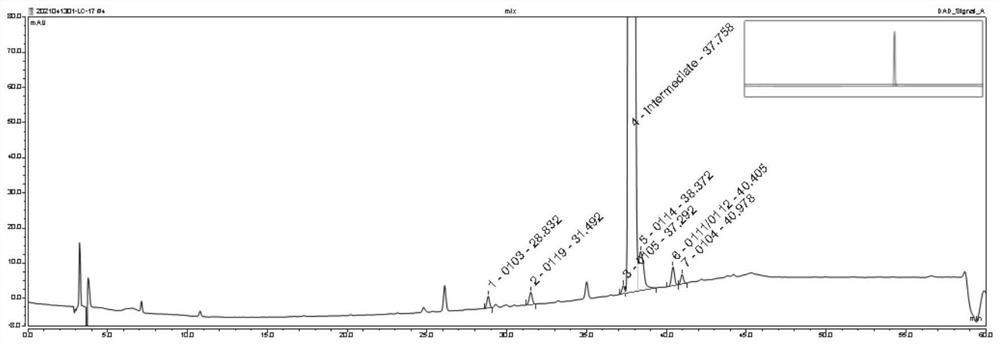
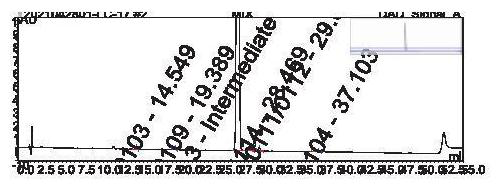
ActiveCN114166983AEfficient separationEasy to detectComponent separationBulk chemical productionSilanesPhosphoric acid
The invention discloses an analysis method for separating and detecting an oseltamivir phosphate intermediate and impurities thereof, belongs to the field of analytical chemistry, and particularly relates to an analysis method for carrying out gradient elution on the oseltamivir phosphate intermediate by taking pentafluorophenylsilane bonded silica gel as a filler, water-perchloric acid as a mobile phase A and acetonitrile-methanol as a mobile phase B. The method can realize effective separation and detection of the oseltamivir phosphate intermediate and the specific impurities thereof, has the characteristics of high detection sensitivity and accurate and reliable detection result, and provides a reliable reference method for quality research on separation and detection of the oseltamivir phosphate intermediate and the specific impurities thereof.
Owner:苏州正济药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com