Method for preparing oseltamivir phosphate by azide process
An oseltamivir phosphate and process technology, which is applied in the field of preparing oseltamivir phosphate by an azide process, can solve problems such as excessive heavy metals in diallylamine, and reduce the cost of environmental treatment of enterprises, with low cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
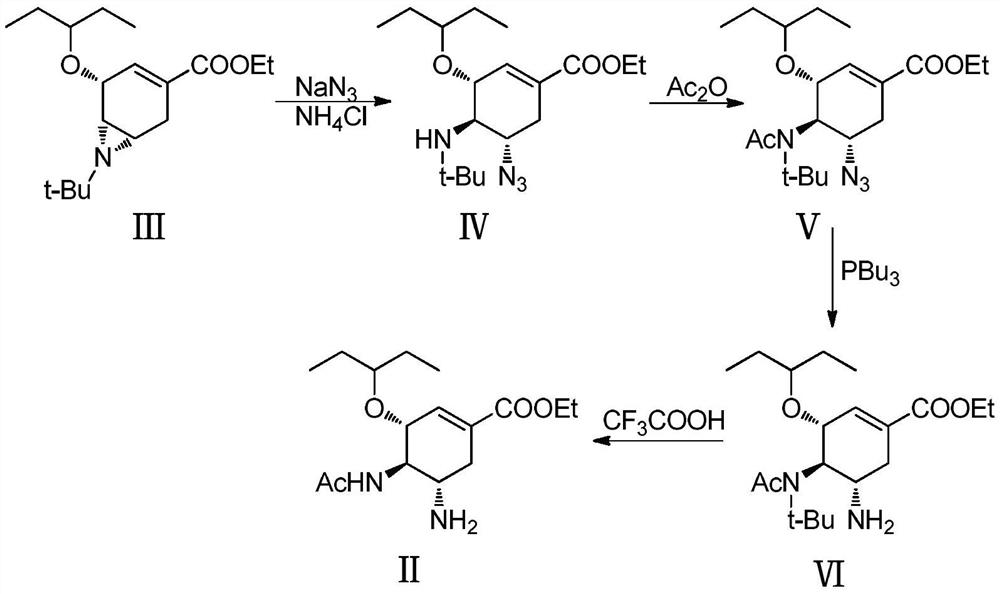
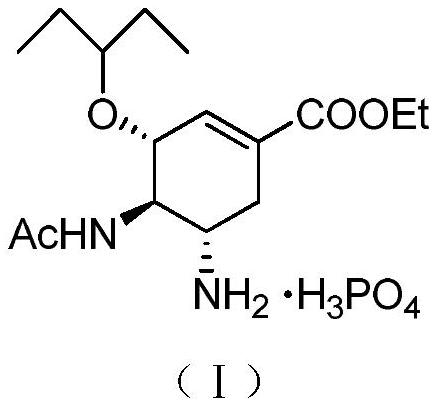
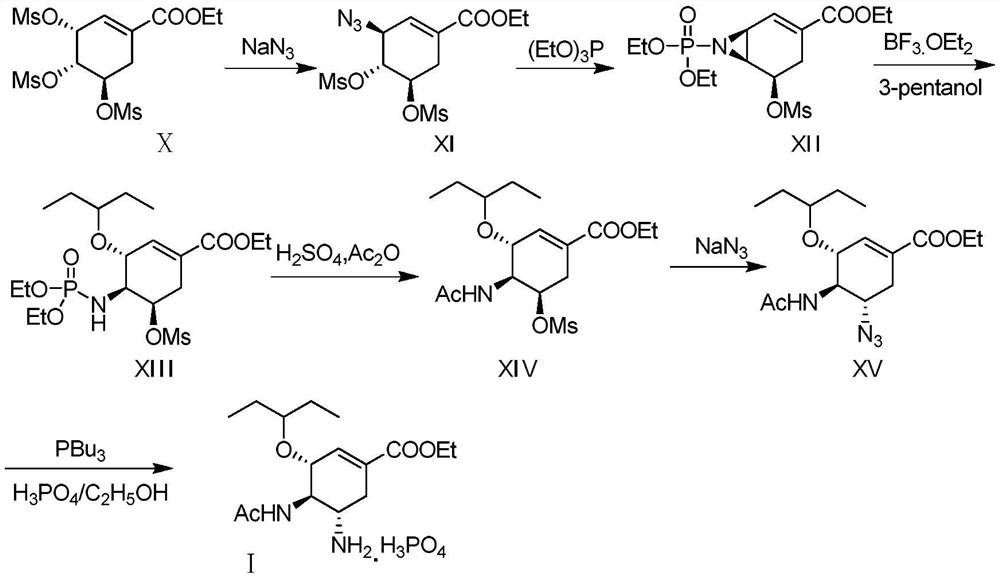
[0046] A method for preparing oseltamivir phosphate by an azide process, the synthetic route of said oseltamivir phosphate is as follows:
[0047]
[0048] Step (1): The intermediate (30.94g, 0.1mol) shown in formula (Ⅲ) was dissolved in 300mL N,N-dimethylformamide, and sodium azide (16.25g, 0.25mol) and ammonium chloride (16.05 g, 0.3mol). The system was heated to 70-75°C for 16 hours, and cooled to room temperature after the reaction was completed. Then the reaction system was poured into 1000mL water, extracted with ethyl acetate (200mL×3), the organic phases were combined, and concentrated on a rotary evaporator to obtain an oily crude product. The crude product was dissolved in 100 mL of methyl tert-butyl ether, stirred at -20°C for 20 h, a white solid was precipitated, and 28.4 g of the intermediate of formula (IV) was obtained by suction filtration, with a yield of 80.6%.
[0049] Step (2): Dissolve the intermediate of formula (IV) obtained in step (1) in 100 mL of...
Embodiment 3
[0056] The intermediate of formula (Ⅴ) was dissolved in 100 mL of ethanol, and 20 mL of water and about 10 drops of glacial acetic acid were added. Cool down to 0°C (±5°C) and add tributylphosphine (25.1g, 0.096mol) dissolved in 50mL of ethanol dropwise in about 1h, and rinse the dropping funnel with a small amount of ethanol. The reaction system continued to stir at this temperature for 90 minutes, then raised the temperature to about 25°C and stirred for 3 hours to release nitrogen. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain an oily crude intermediate of formula (VI).
[0057] The crude intermediate of formula (VI) was dissolved in 100 mL of trifluoroacetic acid, and the temperature was raised to 50° C. for 2 h. After the reaction was completed, it was concentrated on a rotary evaporator, and toluene was added to dry up excess trifluoroacetic acid. Add 100mL toluene and 100mL water to the concentrated solution, stir vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com