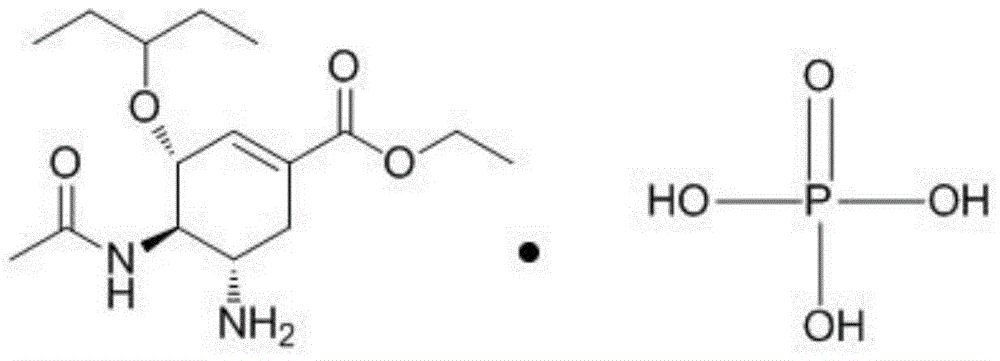
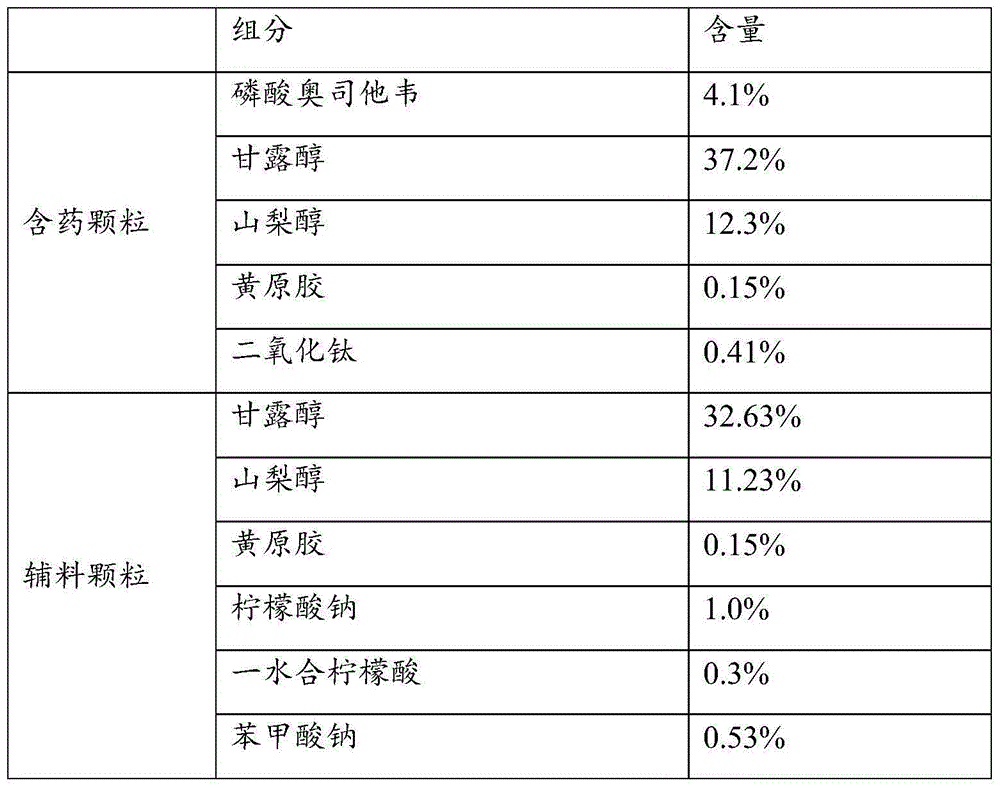
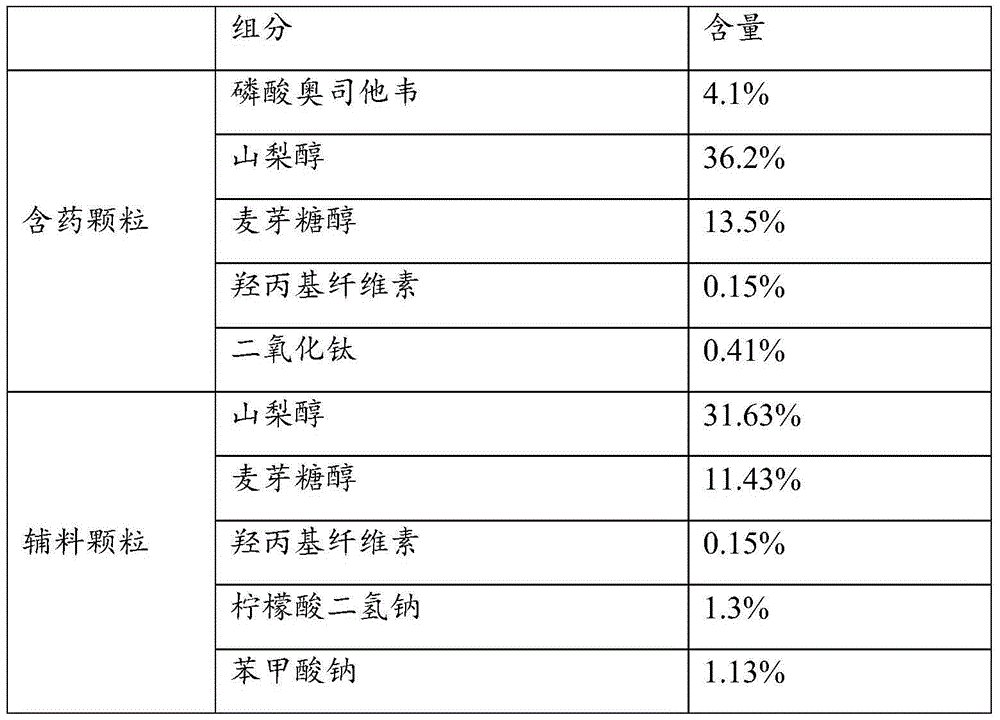
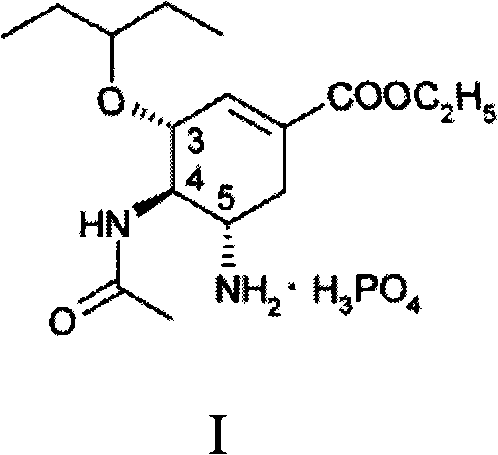
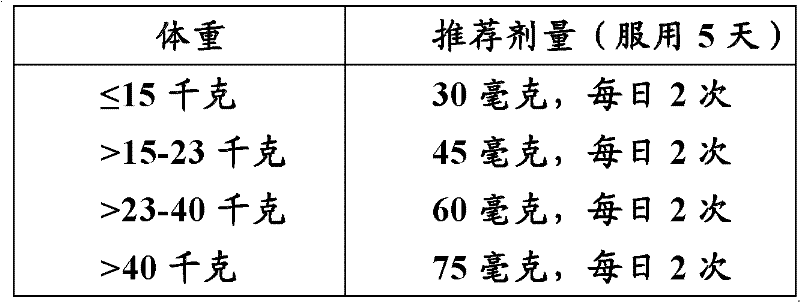
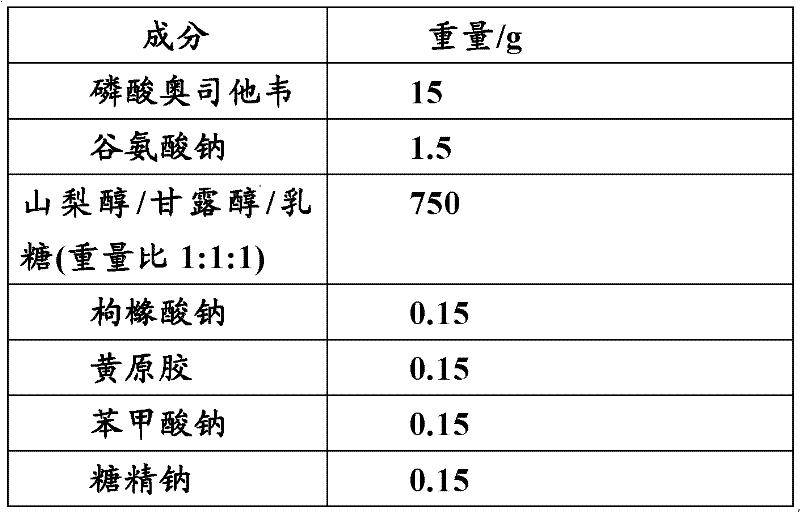
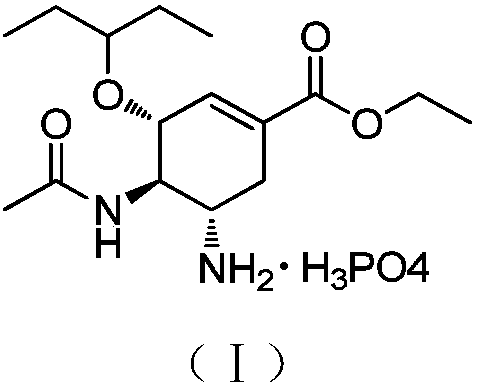
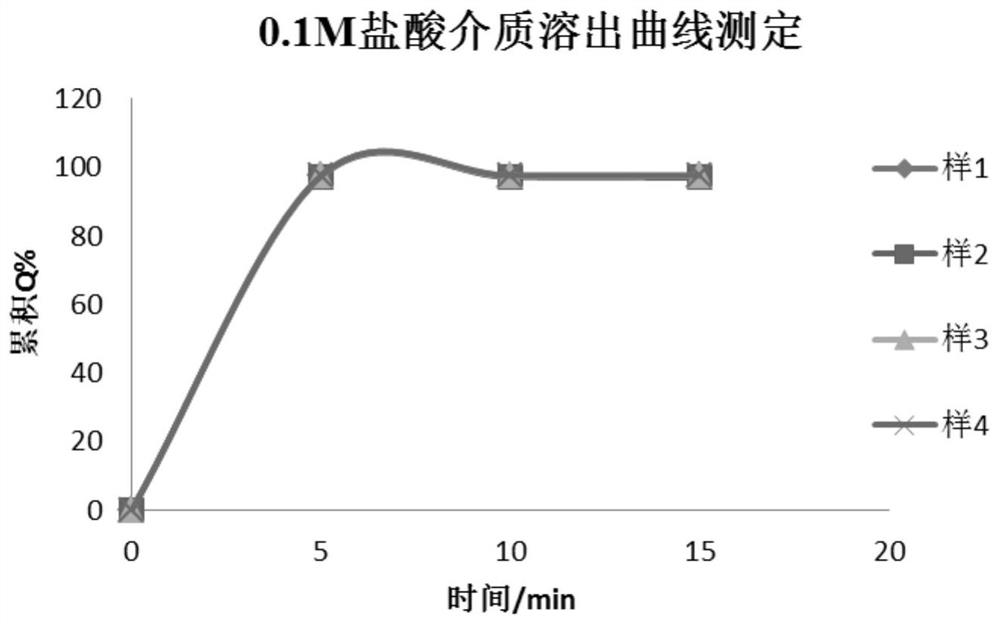
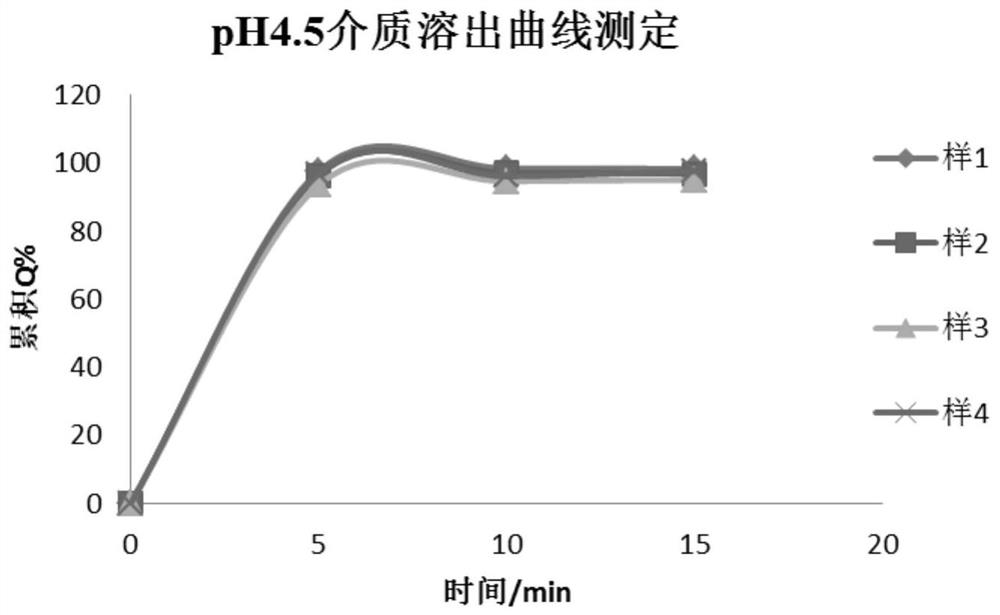
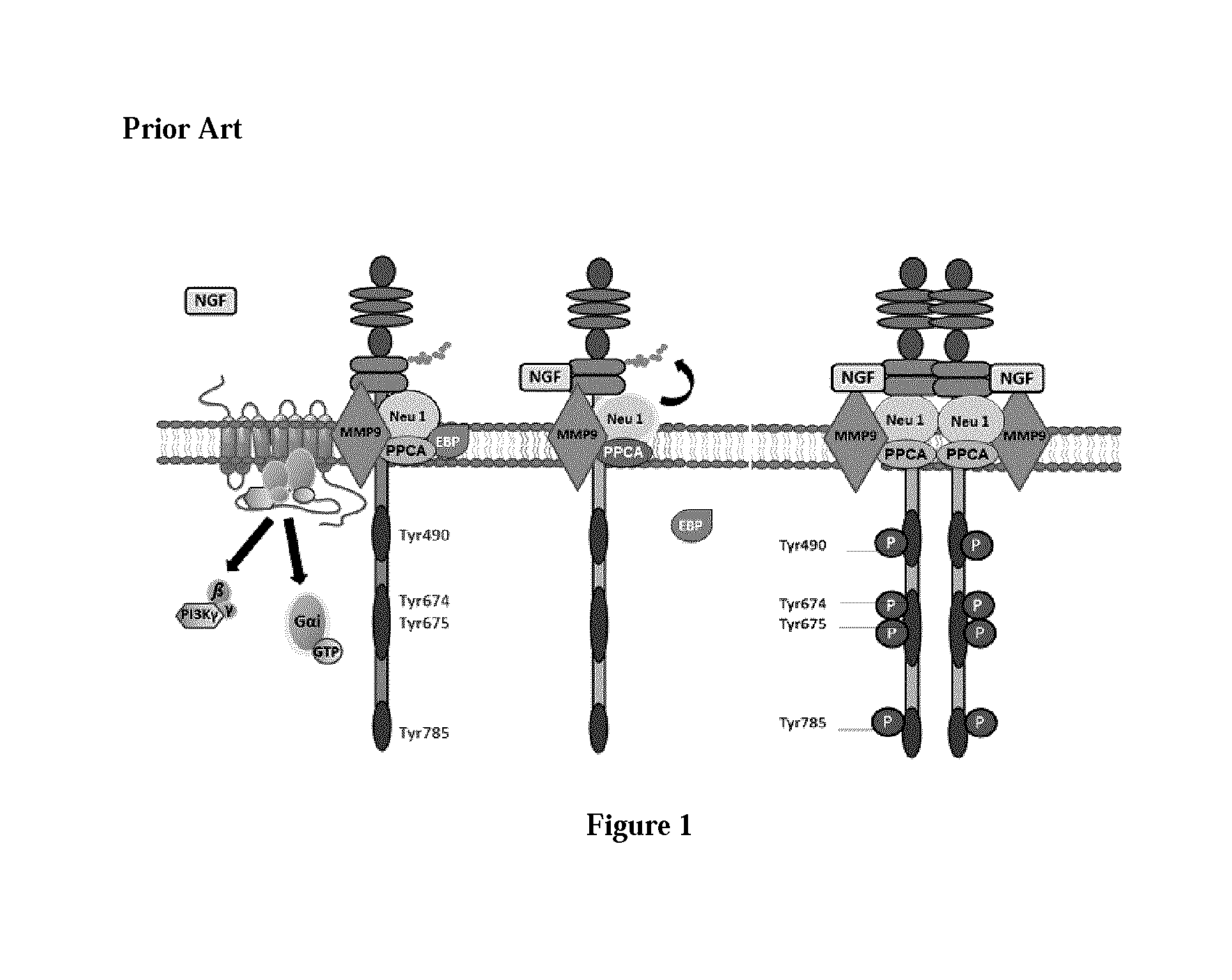
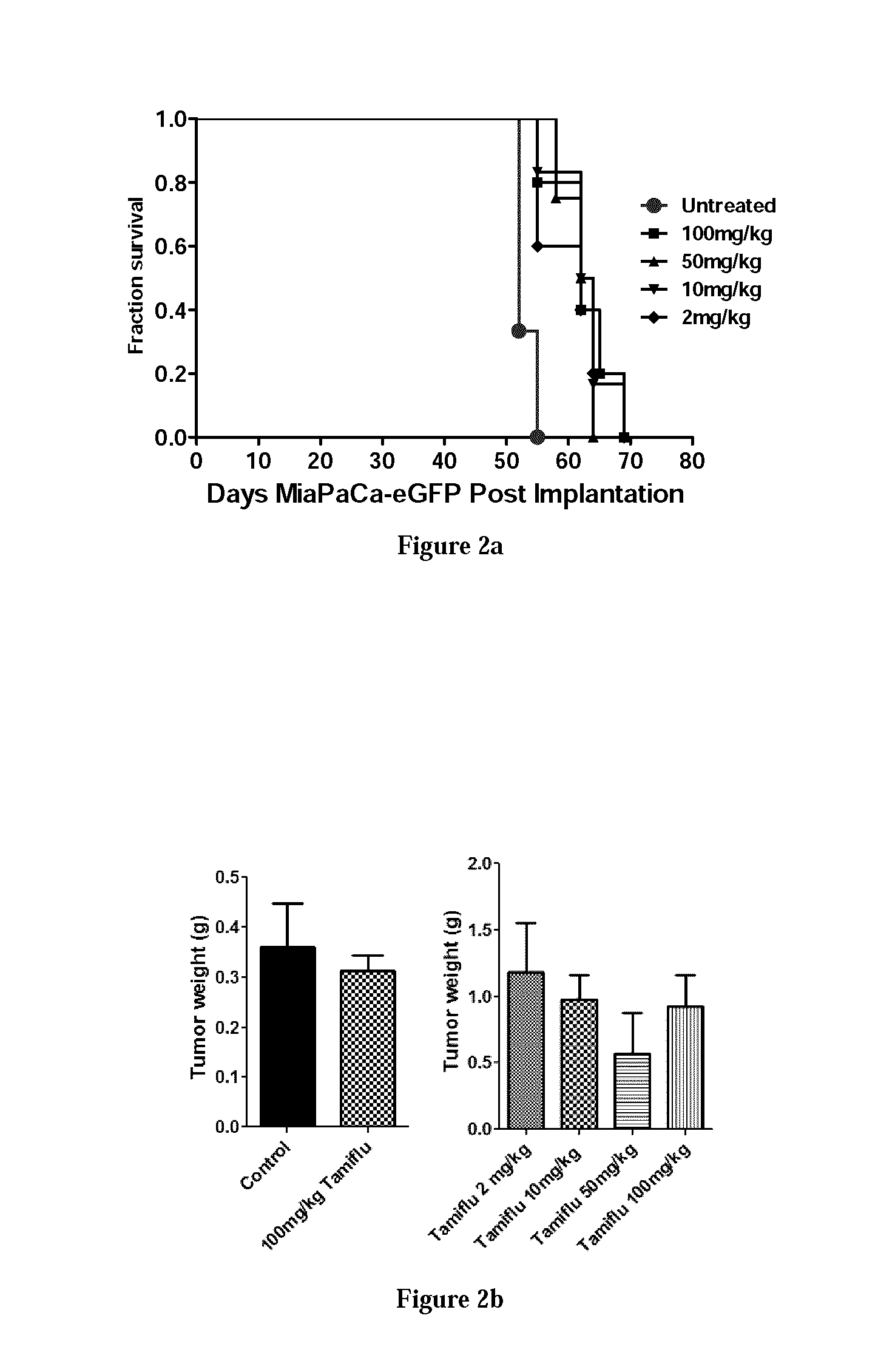
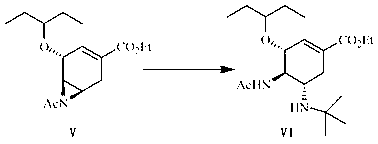Patents
Literature
106 results about "Oseltamivir Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
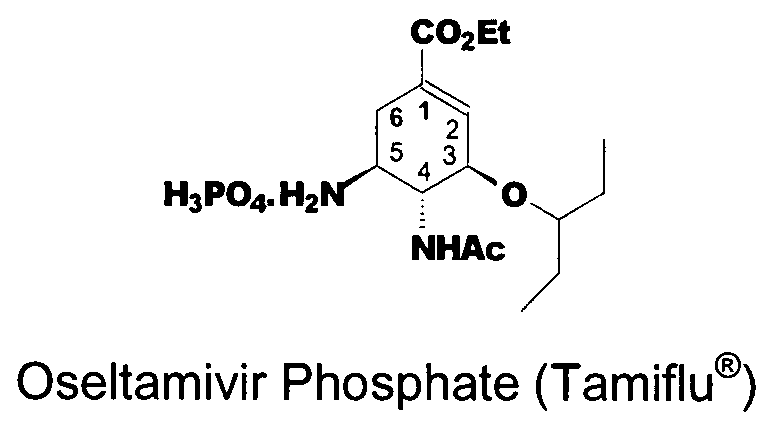
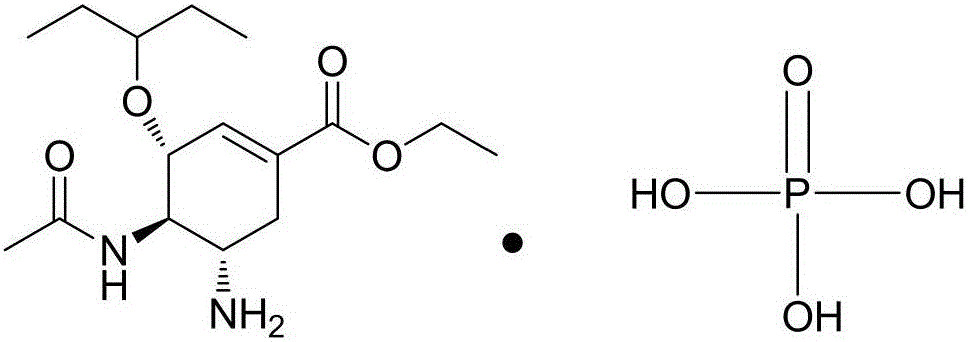
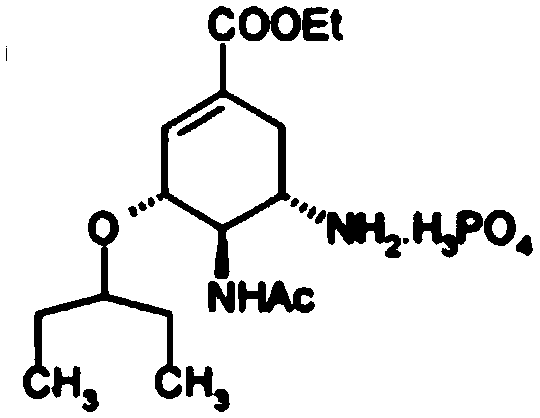
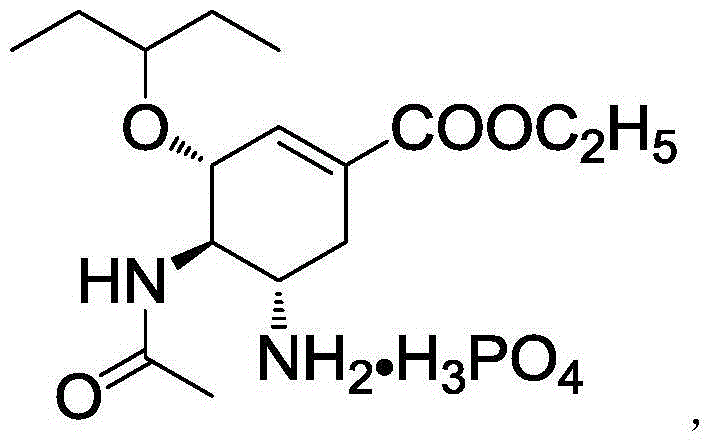
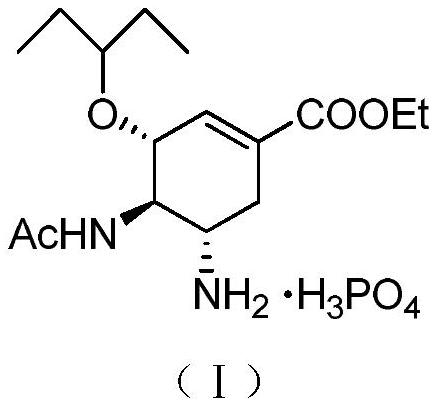
The phosphate salt of oseltamivir, a synthetic derivative prodrug of ethyl ester with antiviral activity. By blocking neuraminidases on the surfaces of influenza viruses, oseltamivir interferes with host cell release of complete viral particles.
Oseltamivir lyophilized orally-disintegrating tablets and preparation method thereof
InactiveCN104367558AOral convenienceEasy to takeOrganic active ingredientsAntiviralsFreeze-dryingOrally disintegrating tablet
The present invention provides oseltamivir lyophilized orally-disintegrating tablets and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The oseltamivir lyophilized orally-disintegrating tablets comprise oseltamivir phosphate or oseltamivir and a matrix, wherein the oseltamivir lyophilized orally-disintegrating tablets contain (calculated as the oseltamivir) 10-75 parts by weight of the effective component, and the matrix contains 1-60 parts by weight of a framework support agent, 1-50 parts by weight of a binder, 0-10 parts by weight of a lyoprotectant, and 0-10 parts by weight of a flavoring agent, and can further contains 0-10 parts by weight of a flavoring agent and 0-69 parts by weight of an inorganic alkali. The oseltamivir lyophilized orally-disintegrating tablet preparation method comprises dissolving, mold injection, rapid freezing, freeze-drying and product packaging. The oseltamivir lyophilized orally-disintegrating tablets of the present invention have characteristics of convenient taking, taking without water, rapid absorption, and first pass effect avoiding.
Owner:BEIJING SUNHO PHARMA
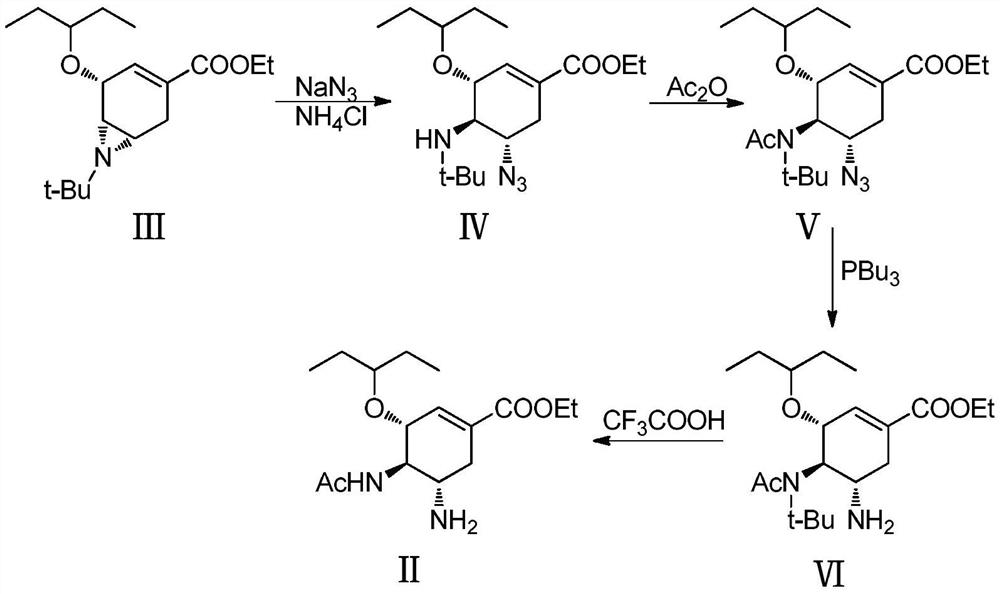
Method for synthesizing oseltamivir phosphate without using nitrine
ActiveCN103304437AIndustrial Production SafetyEfficient industrial productionOrganic compound preparationCarboxylic acid amides preparationEpoxyO-Phosphoric Acid
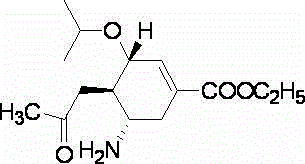
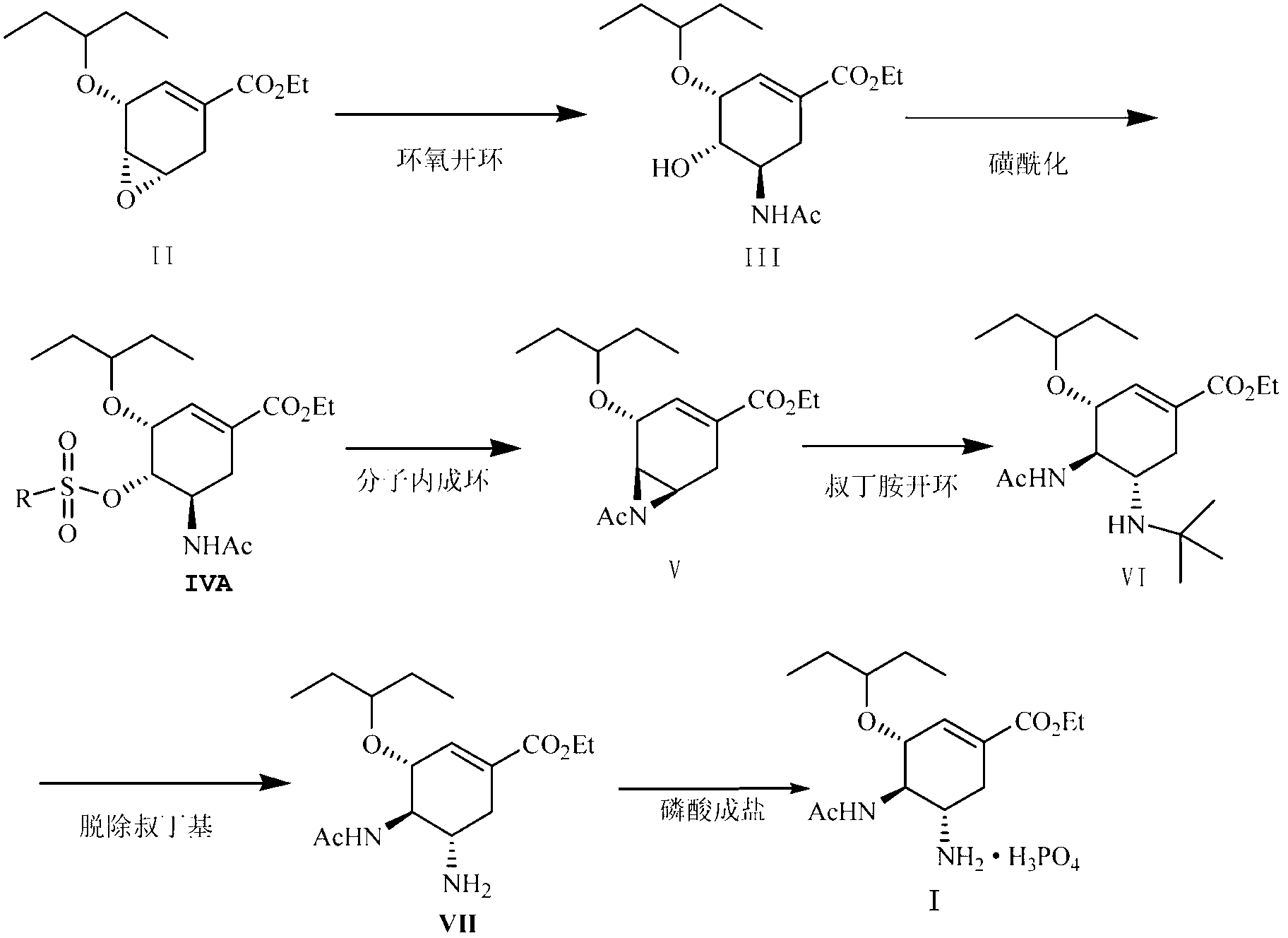
The invention discloses a method for synthesizing oseltamivir phosphate without using nitrine. The method comprises the following steps of: sequentially performing an epoxy ring-opening reaction, a hydroxyl acylation reaction, an intramolecular substitution reaction for ring formation, a selective ring-opening reaction, a reaction for removing tertiary-butyl and a phosphoric acid salt forming reaction on a raw material namely (3R, 4R, 5S)-4,5-epoxy-3-(1-ethyl-propoxy)-1-cyclohexene-1-ethyl ester carboxylate to obtain a product namely oseltamivir phosphate. The method is safe and high in efficiency and yield; compared with the prior art, the method has the biggest advantage that toxic explosive chemicals such as sodium azide and trimethyl-phosphine are not used, and is rich in raw material source and capable of performing large-scale production, so that the method can better meet requirements for strategic reserve of medicaments for preventing and treating bird flus by all human beings.
Owner:GUANGZHOU TROJAN PHARMATEC LTD
Oseltamivir phosphate dry suspension and preparation method thereof
InactiveCN104138355AFine grainFix stability issuesOrganic active ingredientsDispersion deliveryFiller ExcipientMaltitol
The invention provides an oseltamivir phosphate dry suspension. The oseltamivir phosphate dry suspension comprises oseltamivir phosphate, filler not prone to reacting with the oseltamivir phosphate and other pharmaceutically acceptable accessories, wherein the filler is one or more of maltitol, xylitol and mannitol or is the composition of one or more of the maltitol, the xylitol and the mannitol and sorbitol, the content of the sorbitol in the whole composition is X, and X large than or equal to 0% and less than or equal to 75%. Meanwhile, the invention provides a preparation method of the oseltamivir phosphate dry suspension. Preferably, the preparation method is a step-by-step wet preparation method. The oseltamivir phosphate dry suspension is good in stability, low in cost, simple in preparation technology and capable of being produced in an industrial mode easily.
Owner:SUNSHINE LAKE PHARM CO LTD
Purification process for Oseltamivir Phosphate
InactiveCN101343241ASimple and fast operationRaw materials are easy to getCarboxylic acid amide separation/purificationAlcoholPurification methods
The invention relates to a purification method of Oseltamivir phosphate, and the method is to recrystallize an Oseltamivir phosphate crude product acquired by a non-sodium azide process with water, alcohol or an aqueous solution of alcohol for obtaining the Oseltamivir phosphate with the purity higher than 99.0 percent, the maximum single impurity less than 0.1 percent, and the total impurities less than 1.0 percent. The method has advantages of simple operation, high purity of products, stable quality and high overall yield, and is particularly applied to the industrialized production.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
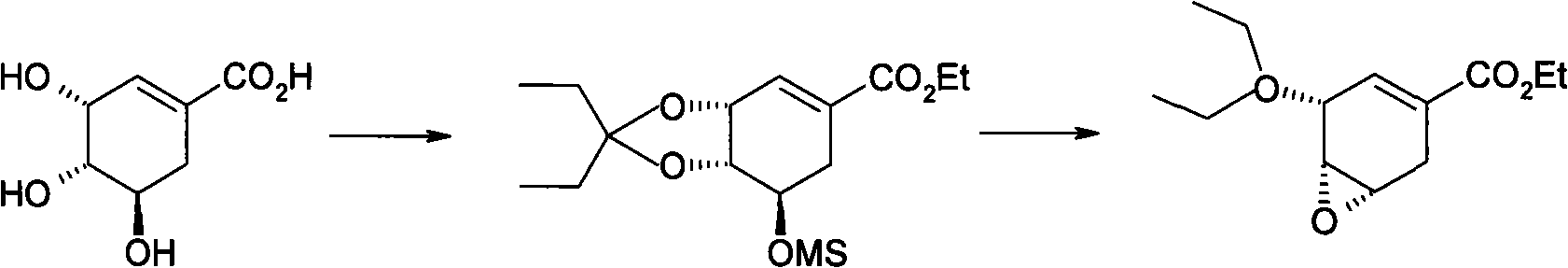
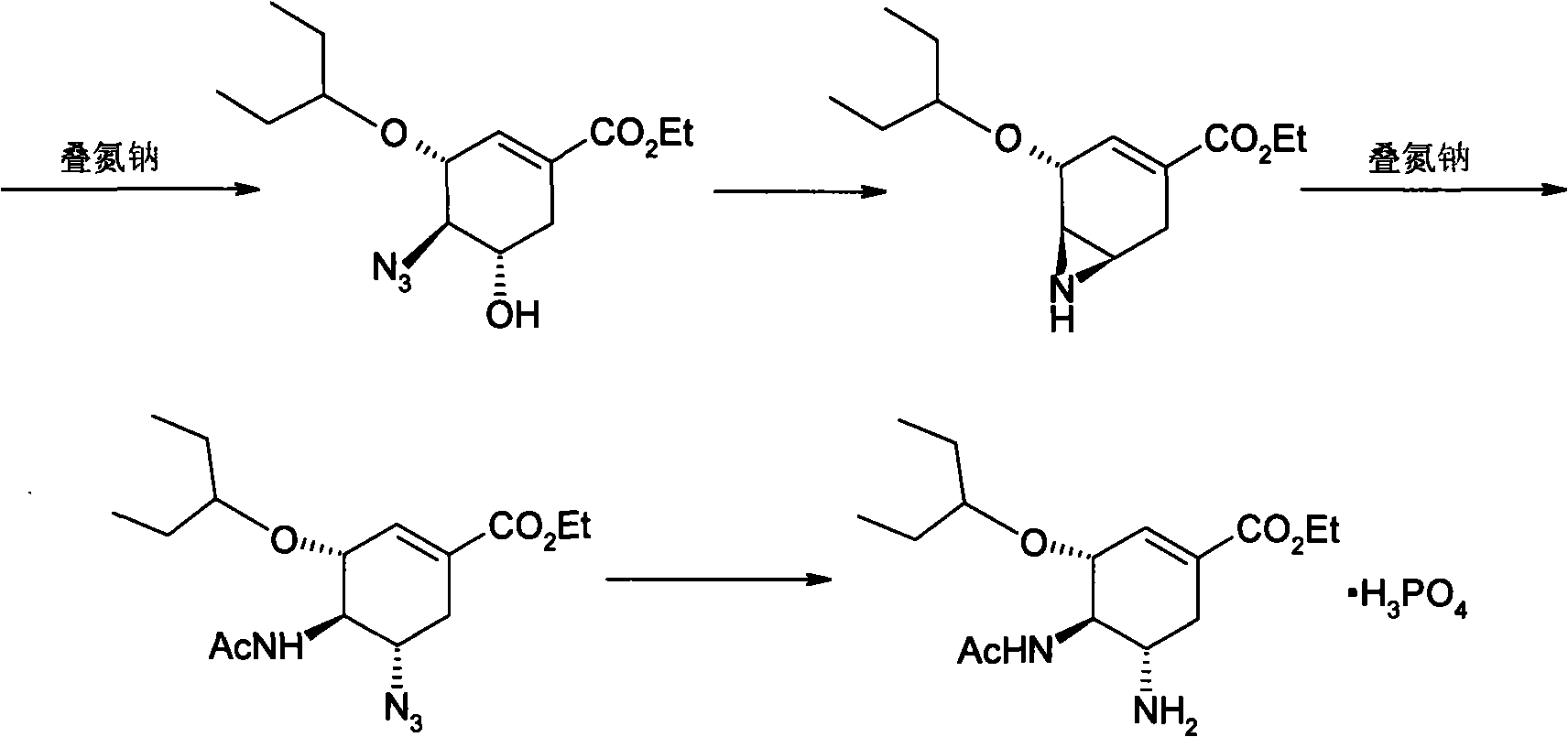
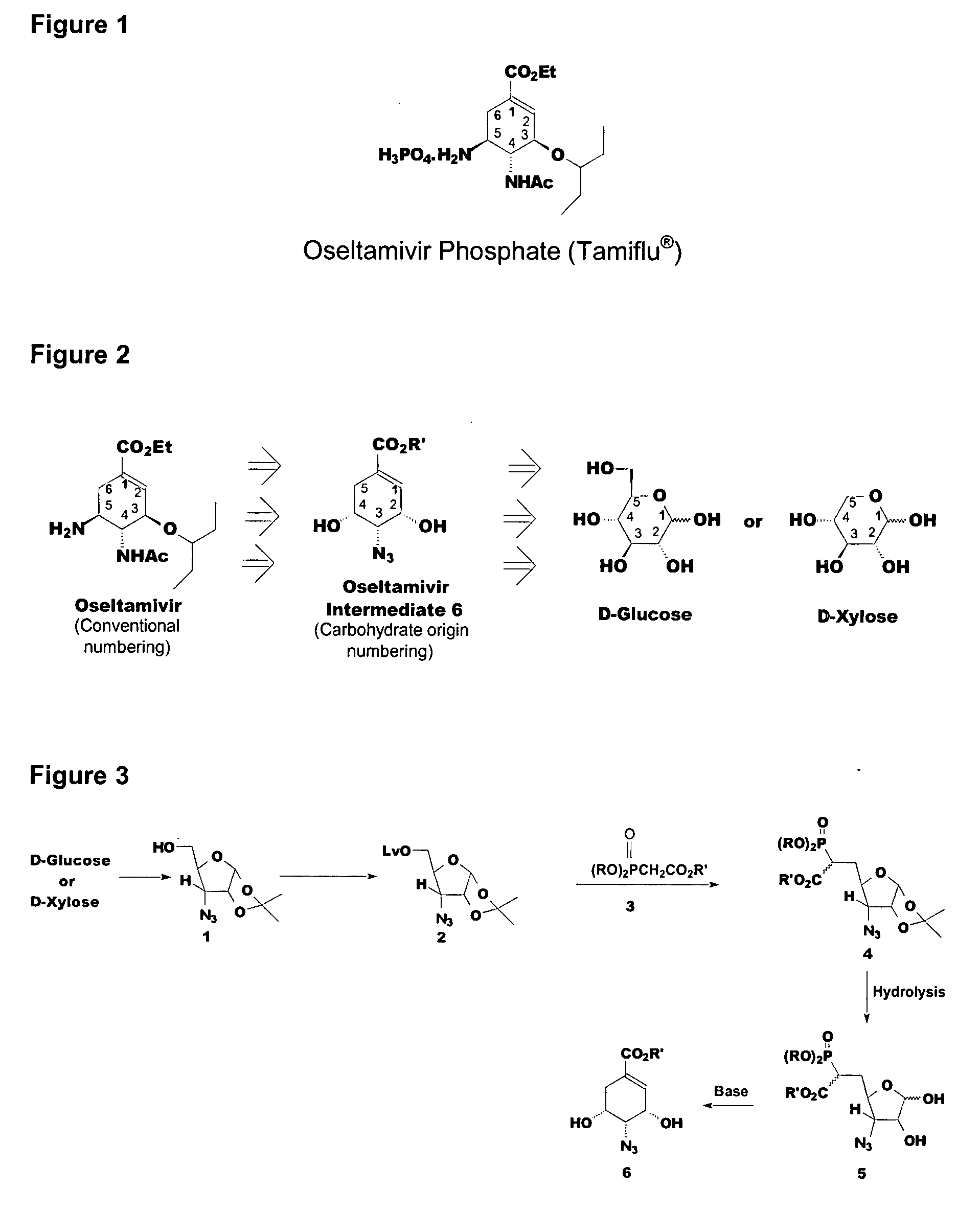
Preparation of oseltamivir phosphate (Tamiflu) and intermediates starting from D-glucose or D-xylose
InactiveUS20080009639A1Marginally expensiveSilicon organic compoundsOrganic compound preparationGlucose polymersD-Glucose
Novel processes for the preparation of the anti-viral agent, Oseltamivir Phosphate and novel intermediates prepared in such processes. The novel processes use as starting materials D-glucose or D-xylose in the preparation of Oseltamivir Phosphate.
Owner:APOTEX PHARMACHEN INC
Oseltamivir phosphate dry powder inhalations and preparation method thereof
The invention relates to the field of pharmaceutical preparations, and particularly relates to oseltamivir phosphate dry powder inhalations for treating influenza A and influenza B and a preparation method thereof. The oseltamivir phosphate dry powder inhalations are characterized in that the inhalations comprise oseltamivir phosphate and a co-atomizing agent in a weight ratio of (8:2)-(6:4). The oseltamivir phosphate dry powder inhalations provided by the invention have a simple preparation method, good fluidity, and good stability. After administration, the medicine can be metabolized to metabolites with anti-influenza virus activity, which can take an antibacterial effect and be used for preventing and treating influenza A and influenza B.
Owner:CHINA PHARM UNIV
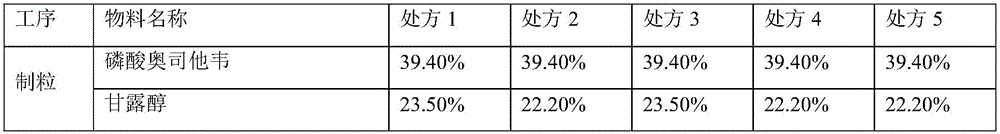
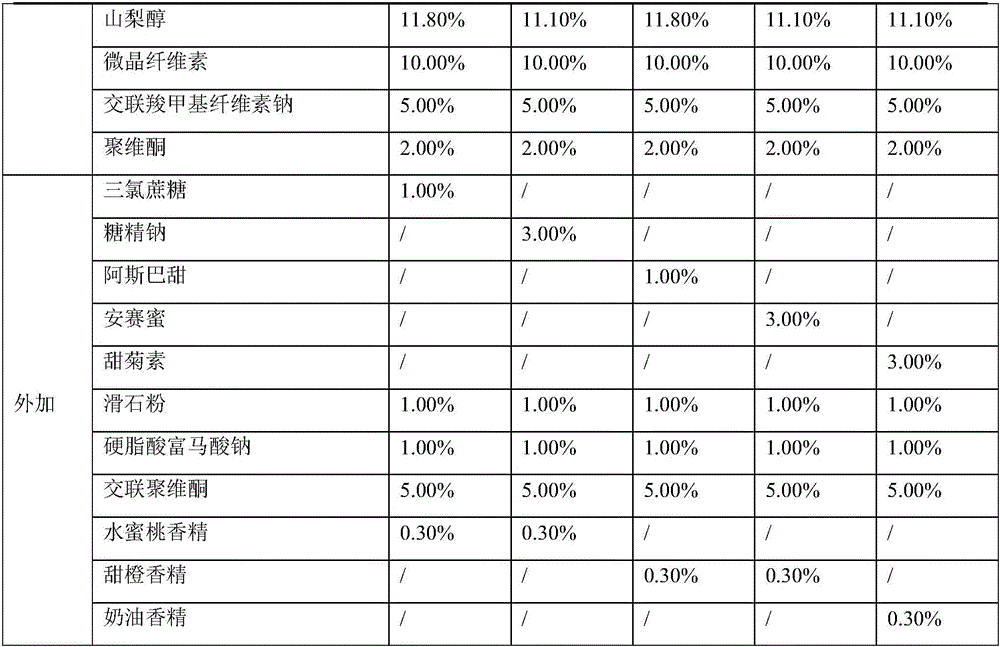
Oseltamivir phosphate tablet and preparation method thereof
The invention provides an oseltamivir phosphate tablet and a preparation method thereof. The oseltamivir phosphate tablet contains sucralose, Aspartame or a mixture of sucralose and Aspartame as a sweetening agent, and also contains pharmaceutically acceptable auxiliary materials. The oseltamivir phosphate tablet has good mouthfeel and good stability and is convenient to carry and take. On the other hand, the invention also provides a preparation method of the oseltamivir phosphate tablet. The method is simple and low-cost, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
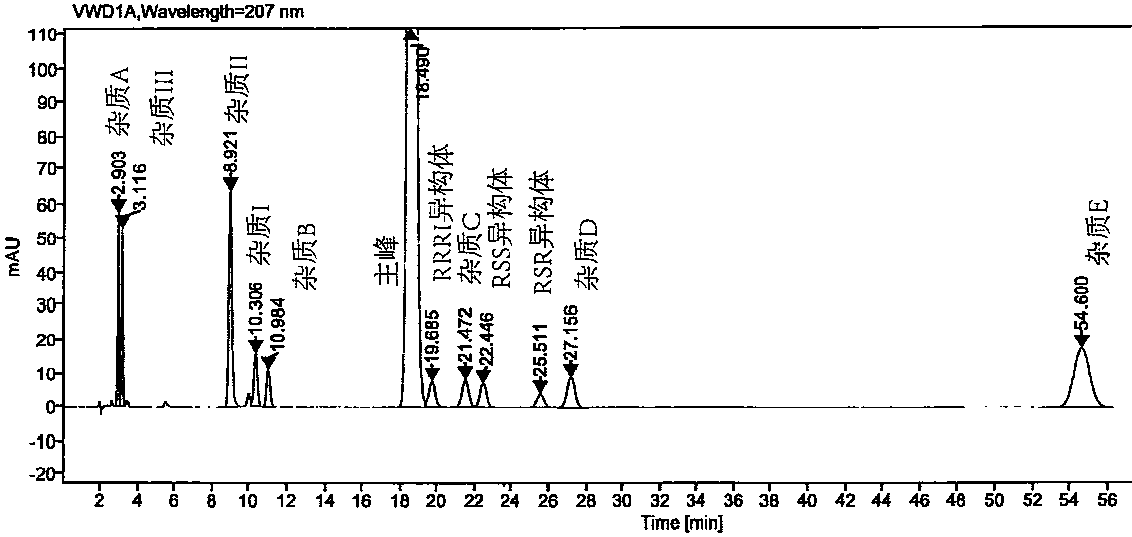
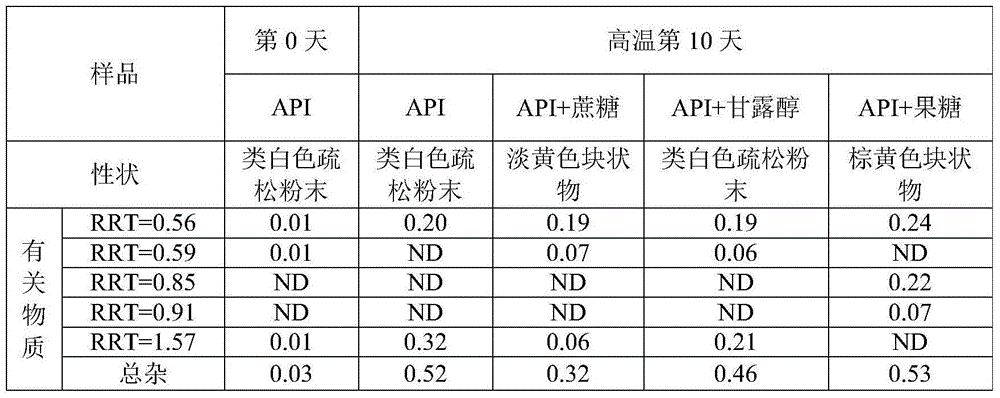
High performance liquid chromatography method for separating and measuring oseltamivir phosphate and specific impurities of oseltamivir phosphate
ActiveCN109580850AThe method is simpleGood reproducibilityComponent separationSilanesColumn temperature
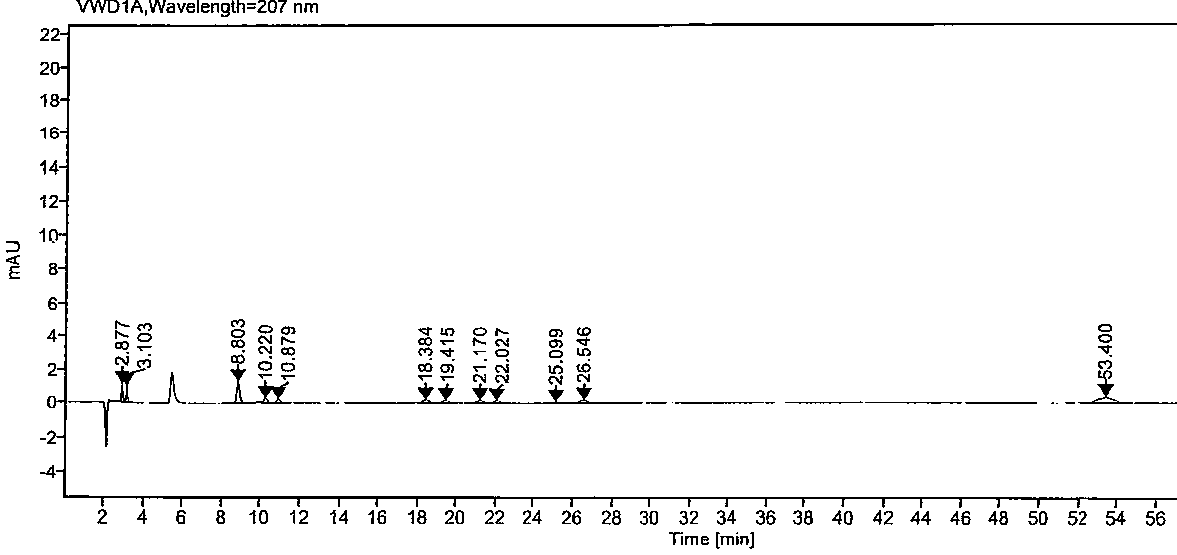
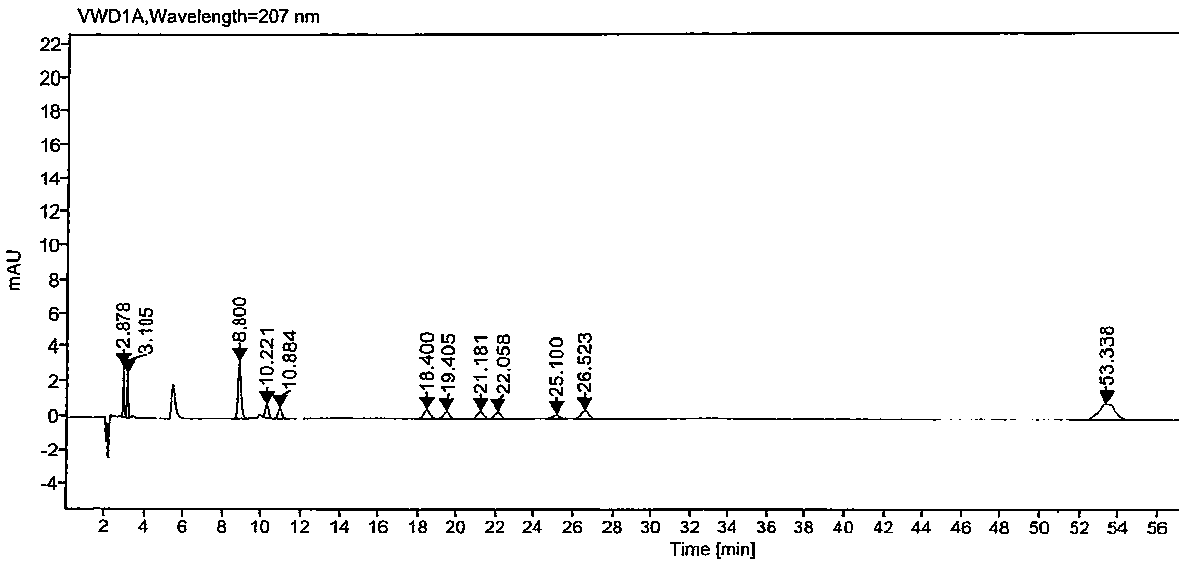
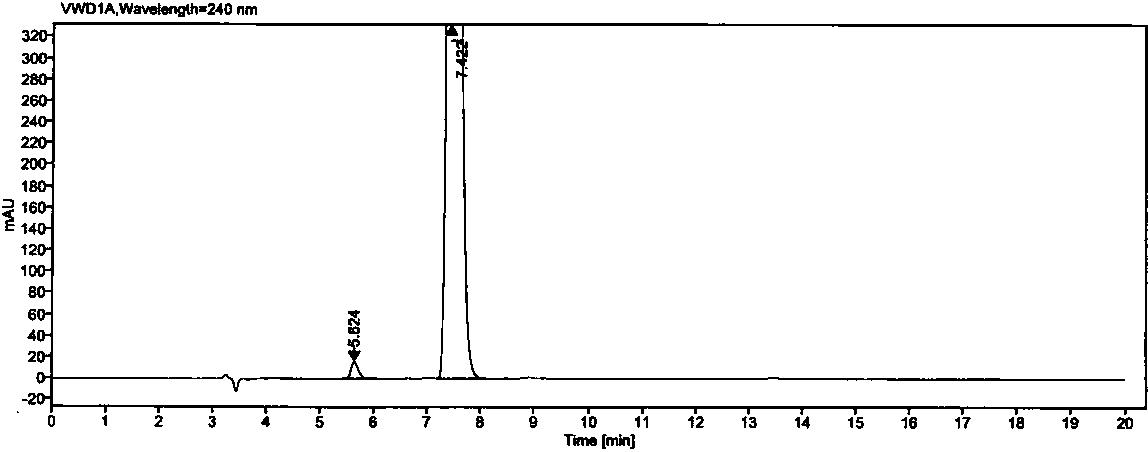
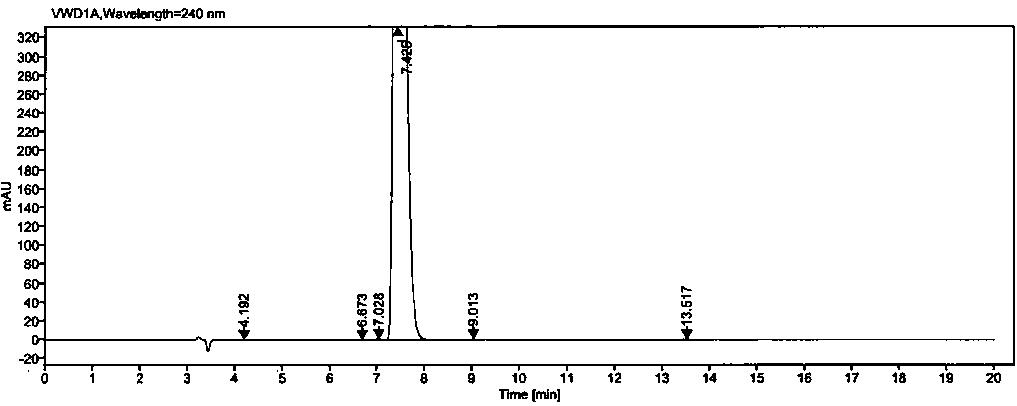
The invention relates to a high performance liquid chromatography method for separating and measuring oseltamivir phosphate and specific impurities of the oseltamivir phosphate. According to the highperformance liquid chromatography method for separating and measuring the oseltamivir phosphate and the specific impurities of the oseltamivir phosphate, octyl silane bonded silica gel columns are adopted, the mobile phase is 0.05 mol / L, the volume ratio of a monopotassium phosphate buffer solution to methyl alcohol to acetonitrile is 620 to 245 to 135, the column temperature is 48 DEG C-50 DEG C,the detection wave length is 207 nm, and the flow rate is 1.1-1.3 ml per minute; and three diastereoisomers of the oseltamivir phosphate, two isomerides (impurity C and impurity D), and a specific technology impurity A, a specific technology impurity B and a specific technology impurity C and the oseltamivir phosphate specific impurity I, the oseltamivir phosphate specific impurity II and the oseltamivir phosphate specific impurity III recorded in the 2015 version of 'Chinese Pharmacopoeia' can be separated totally at the same time by the high performance liquid chromatography method, the method is simple and exact, and is high in sensitivity, and is very important for the quality control over drug products.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
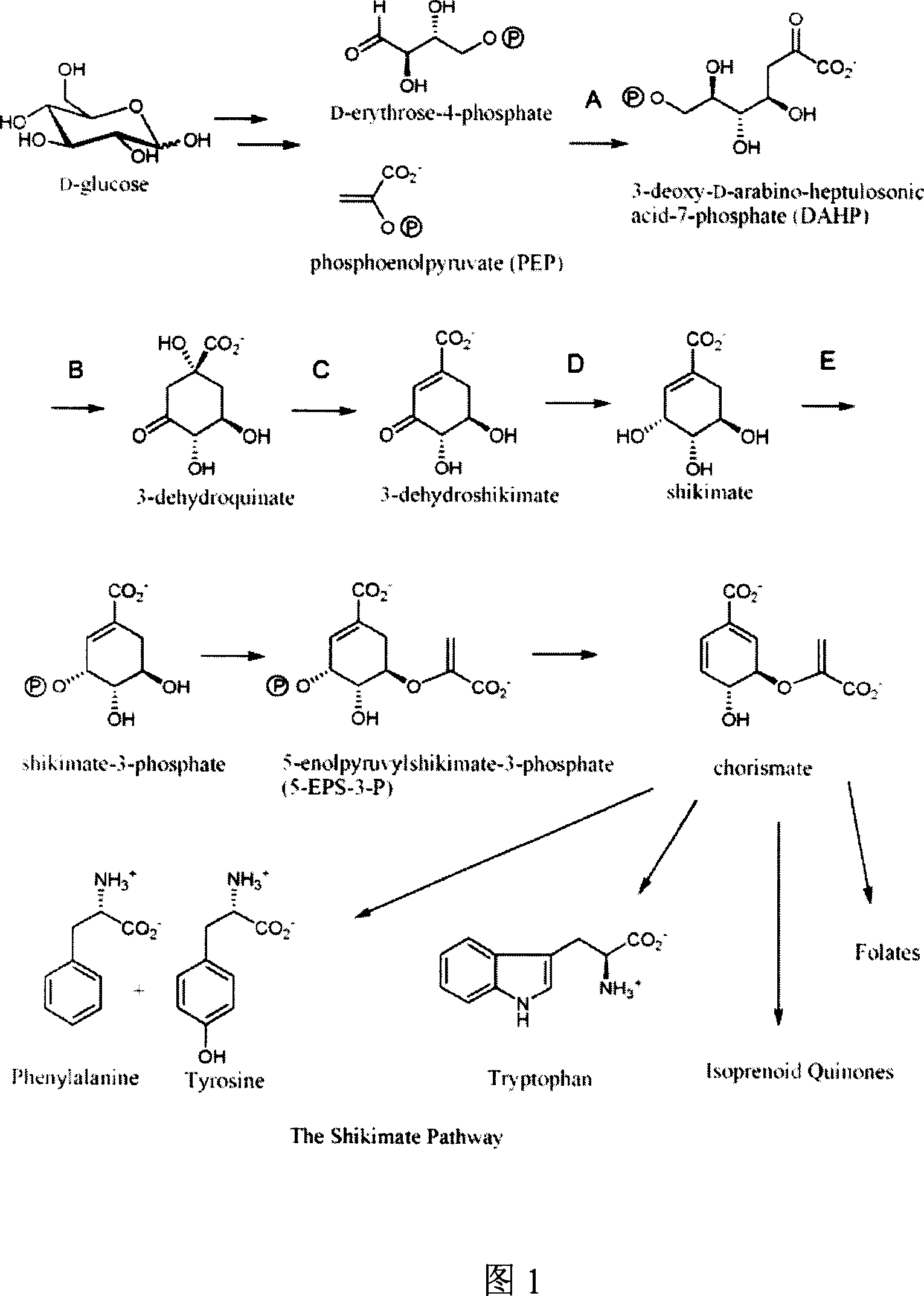
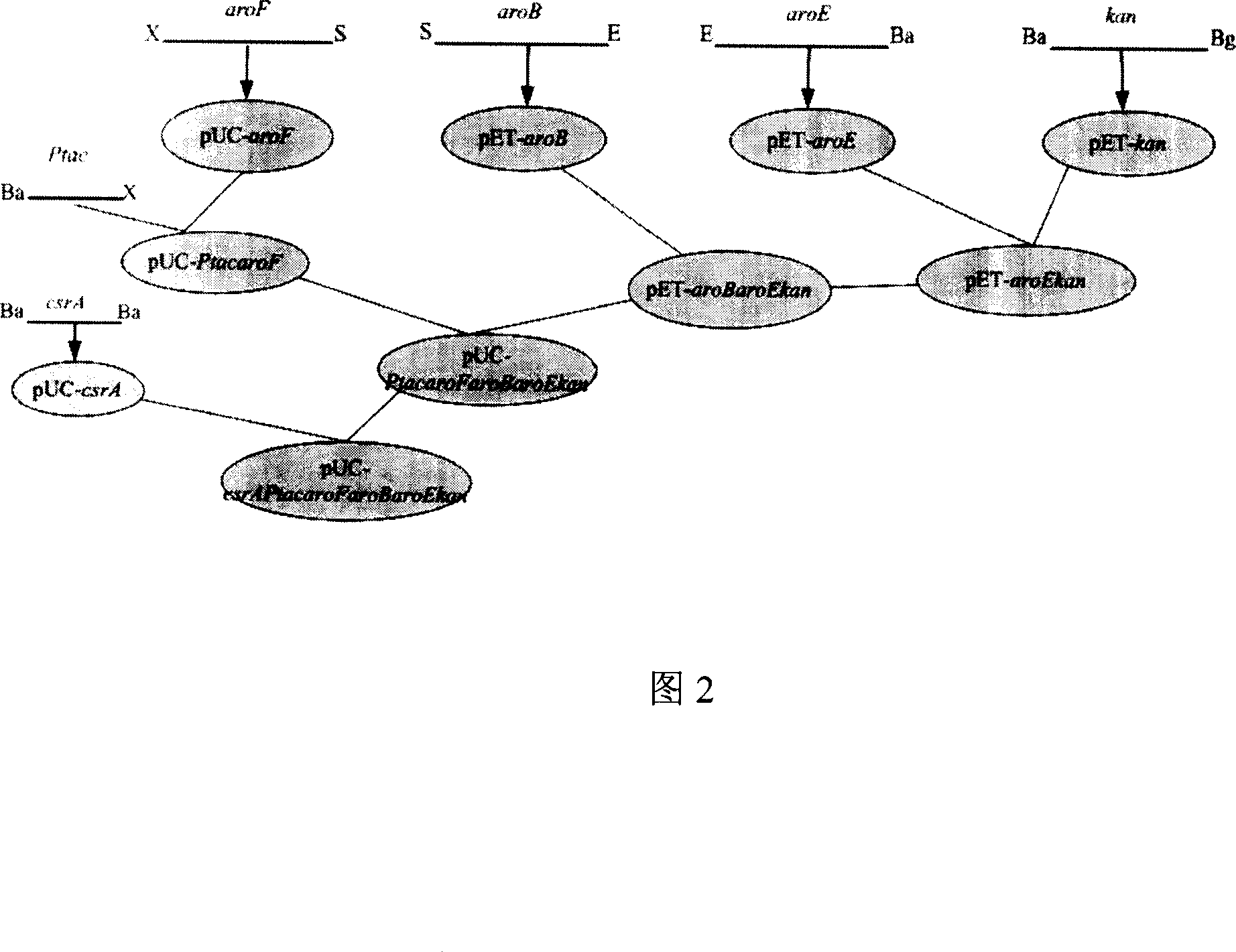
Method for producing shikimic acid by biological engineering invoice method expression and engineering bacterium constructed thereby
InactiveCN101134958AThe operation method is simple and preciseEasy to operateBacteriaFermentationOseltamivir PhosphateBio engineering
The present invention discloses biosynthesis process of preparing shikimic acid and the recombinant colibacillus therefor. The biosynthesis process of preparing shikimic acid includes constituting vector including the polygene kit of shikimic acid metabolizing pathway rate-limiting enzyme, replacing the polygene kit for shiA gene, knocking out shikimate kinase isozyme with Red recombinant system, fermenting recombinant engineering bacteria to obtain shikimic acid, purifying shikimic acid and other steps. The process has high shikimic acid expressing yield and lowered shikimic acid producing cost, and can provide material for preparing Oseltamivir phosphate as influenza preventing and treating medicine.
Owner:SHANGHAI HAITAI PHARMA
Solid oseltamivir phosphate medicinal composition
The invention provides a powdered oseltamivir phosphate medicinal composition, which comprises a therapeutic or prevention effective amount of oseltamivir phosphate, sodium glutamate and any one or more pharmaceutically acceptable excipients. The invention also provides the use of the solid medicinal composition in the preparation of oral liquid medicines and further provides a liquid medicinal composition which comprises the solid medicinal composition provided by the invention and a water-containing medium. When prepared into suspension with water, the solid medicinal composition has a stable advantage.
Owner:HAIKOU QILI PHARMA
Method for preparing oseltamivir phosphate
ActiveCN109574869AOrganic compound preparationCarboxylic acid amide separation/purificationEquivalence ratioPhosphoric acid
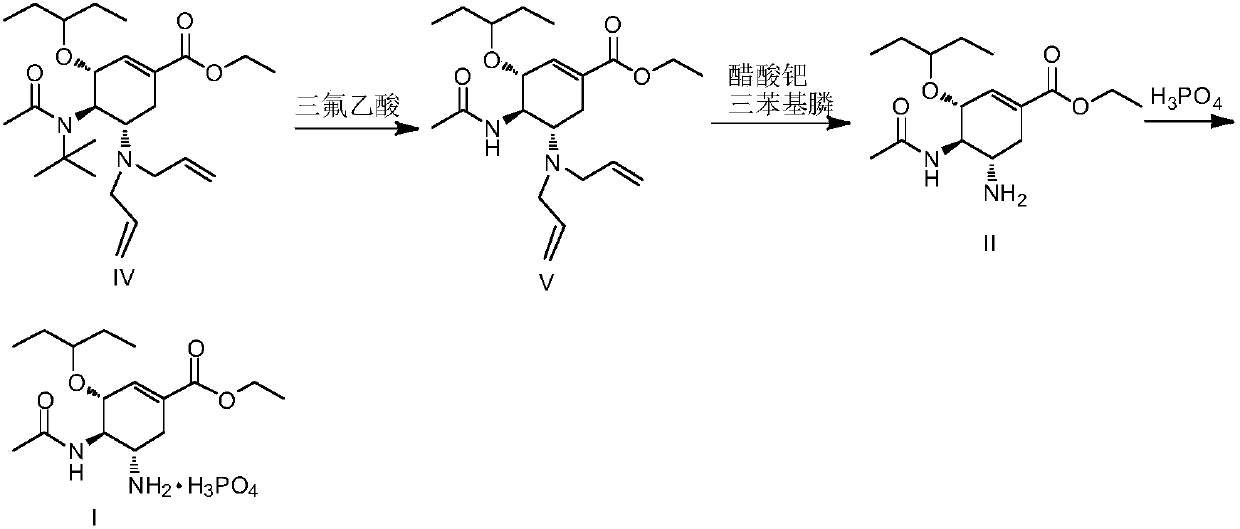
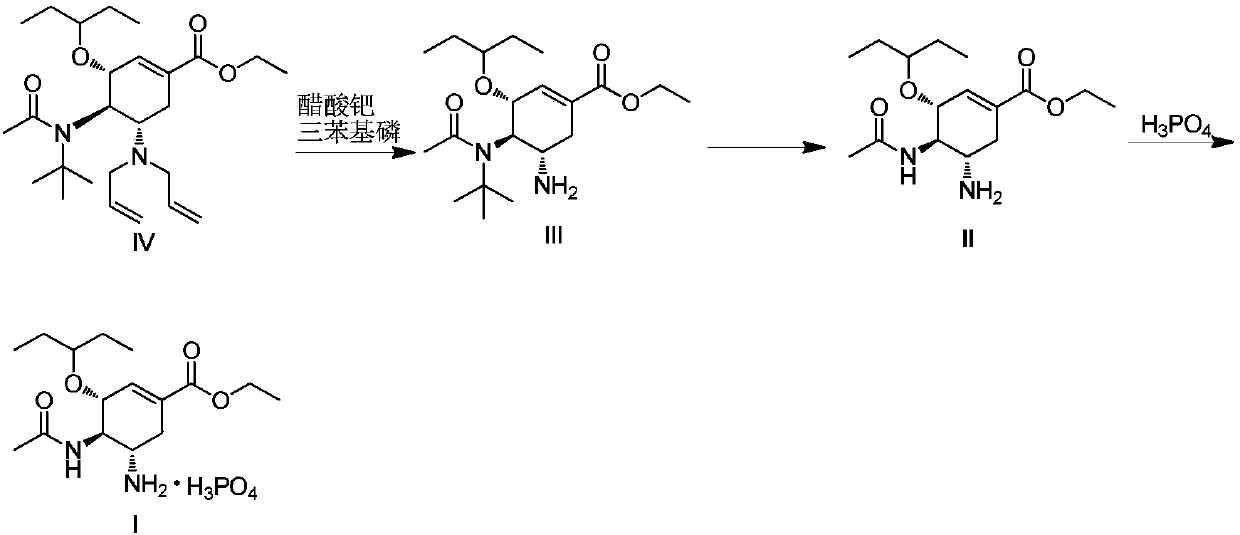
The invention discloses a method for preparing oseltamivir phosphate. The method includes steps of carrying out reaction on intermediates shown as a formula (IV) with palladium acetate, triphenylphosphine and N,N-dimethylbarbituricacid in solvents, and removing allyl to obtain intermediates shown as a formula (III); carrying out acid treatment on the intermediates shown as the formula (III), and removing tertiary butyl to obtain oseltamivir free alkali shown as a formula (II); carrying out reaction on the oseltamivir free alkali with phosphoric acid in solvents, and carrying out crystallization purification. An equivalence ratio of the intermediates shown as the formula (IV) to the palladium acetate to the triphenylphosphineto the N,N-dimethylbarbituricacid is 1:0.01:0.04:1.2. The method has the advantages that the residual quantities of main heavy metal such as palladium, arsenic, cadmium, cobalt, copper, mercury, lithium, nickel, lead, antimony, titanium and vanadium in the oseltamivir phosphate prepared by the aid of the method are within the limit ranges, and accordingly the ICH standards can be met.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Method for separating oseltamivir phosphate and oseltamivir phosphate SSR-isomers through normal-phase chromatography method
ActiveCN109870521AEffective separation assayThe method is simpleComponent separationCelluloseCarbamate
The invention relates to a high-performance liquid chromatography method for separating oseltamivir phosphate and oseltamivir phosphate SSR-isomers through the normal-phase chromatography method. Themethod is characterized in that the surface of silica gel is coated with cellulose-tris(3,5-dichlorophenyl carbamate) columns, a moving phase is a mixture of normal hexane, absolute ethyl alcohol, methanol, trifluoroacetic acid and diethylamine at a volume ratio of 90:8:2:0.4:0.2, the column temperature ranges from 30 DEG C to 40 DEG C, the detection wavelength is 240nm, and the flow velocity is 0.9-1.1ml per minute. The method has the advantages that the oseltamivir phosphate and the oseltamivir phosphate SSR-isomers can be separated; high simplicity, high accuracy and high sensitivity can beachieved; and the method can be used for SSR-isomer detection during oseltamivir phosphate production.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Method for preparing antiviral drug oseltamivir phosphate intermediate tert-butylamine derivative I
ActiveCN111153818APrevent curingAvoid situations where by-products increaseOrganic compound preparationCarboxylic acid amides preparationTert-ButylamineAntiviral drug
The invention discloses a method for preparing a tert-butylamine derivative, and relates to the field of drug synthesis. The method comprises the following steps: 1, preparing a magnesium-amine compound, namely, adding magnesium halide and tert-butylamine A into an aprotic solvent, and carrying out a mixing stirring reaction for 0.5-1.5 h at a temperature of 0-15 DEG C to prepare a mixed solutionA; 2, adding a compound B into the mixed solution A prepared in the step 1, and carrying out a stirring reaction for more than 8 hours to prepare a mixed solution B; and 3, supplementing tert-butylamine D into the mixed solution B prepared in the step 2, and carrying out a stirring reaction for 24-48h at a temperature of 50-70 DEG C to prepare a tert-butylamine derivative I. By controlling the preparation temperature of the compound, the addition mode of tert-butylamine and the time of the ring-opening reaction, the curing phenomenon in the reaction and the increase of by-products can be effectively controlled.
Owner:TIANJIN PHARMA GROUP XINZHENG
Oseltamivir phosphate preparation method
ActiveCN109438276AHigh purityQuality improvementOrganic compound preparationCarboxylic acid amide separation/purificationCyclohexenePhosphoric acid
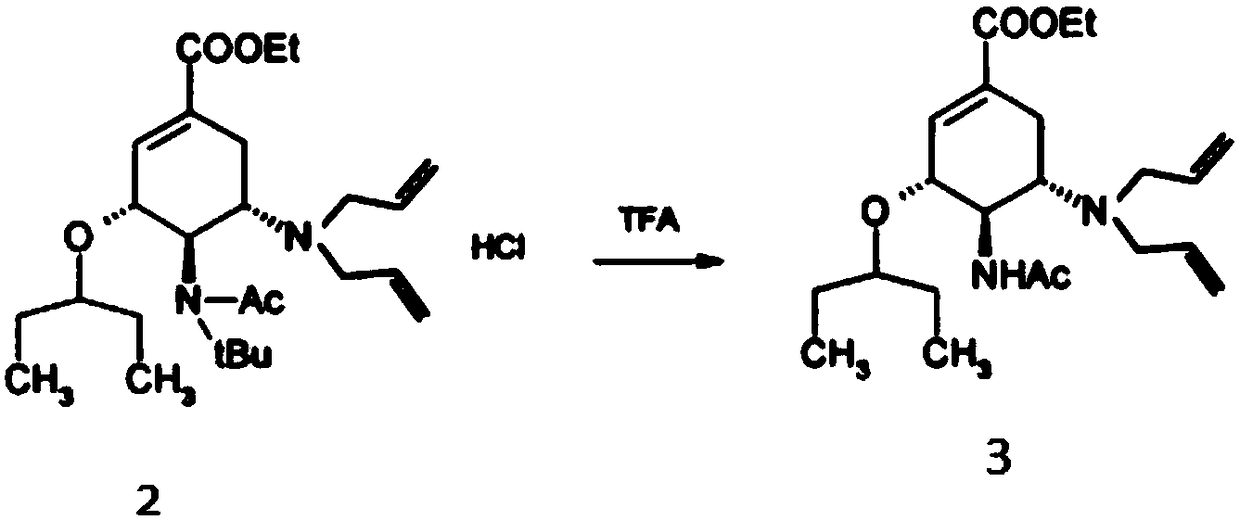
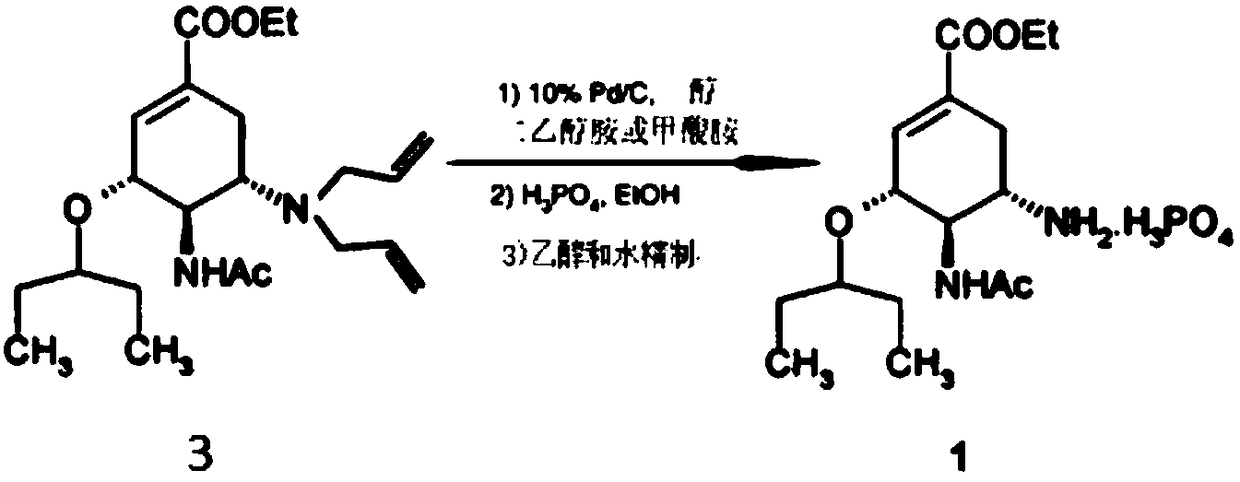
The invention discloses an oseltamivir phosphate preparation method which includes the steps: taking (3R, 4R, 5s)-4-n-acetyl (1, 1-dimethyl ethyl) amino-5-N, N-diallyl amino-3-(1-ethyl propoxy)-1-cyclohexene-1-ethyl carboxylate hydrochloride acquired by non-azide reaction as a starting raw material; removing tertiary butyl and diallyl; enabling the raw material and phosphoric acid to form salt toobtain crude oseltamivir phosphate; refining the crude oseltamivir phosphate by an ethanol water solvent to obtain high-purity oseltamivir phosphate with a crystal form A. The method does not adopt sodium azide, azide and sodium hydride and is safe and environmentally friendly. The purity of the starting raw material is 99.9% or more, the purity of an intermediate and a crude product is 99.5% or more, the purity of the oseltamivir phosphate is 99.9%, the quality and the yield of the oseltamivir phosphate are improved, cost is reduced, and the preparation method is simple, good in repeatabilityand suitable for mass production.
Owner:西安吉泰医药有限公司
Solid preparation of oseltamivir phosphate
InactiveCN104940125AQuality improvementWide range of drug usersOrganic active ingredientsAntipyreticAdhesiveDiluent
The invention provides a solid preparation of oseltamivir phosphate; the solid preparation contains oseltamivir phosphate and a diluent with the effective treatment or prevention amount, wherein the diluent is maltitol; and the solid preparation further contains an adhesive and a selectable sweetening agent and / or edible essence. The solid preparation has the characteristics of being steady in quality, safe, effective, good in patient compliance and wide in drug application crowd. The invention further provides a preparation method of the solid preparation of oseltamivir phosphate. The preparation method is simple and feasible and is applied to industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD +1
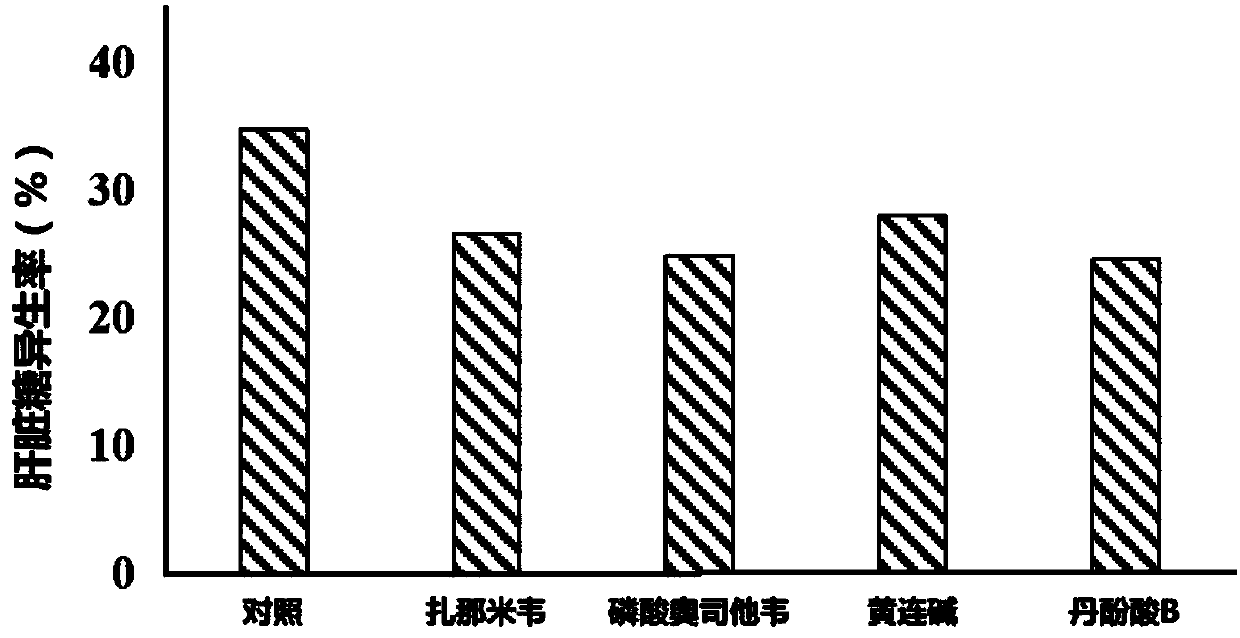
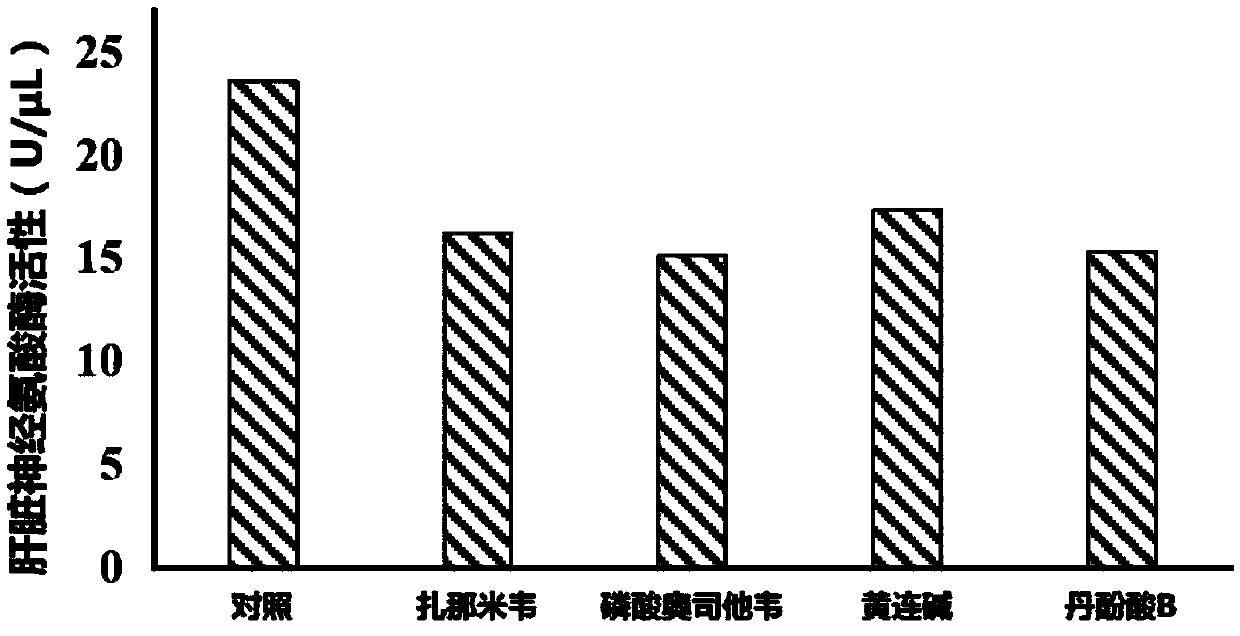
Application of neuraminidase and neuraminidase inhibitors to preparation of medicines for inhibiting hepatic gluconeogenesis
ActiveCN107812182AInhibitory activityOrganic active ingredientsPeptide/protein ingredientsZanamivirOseltamivir Phosphate
The invention discloses application of neuraminidase and neuraminidase inhibitors to preparation of medicines for inhibiting hepatic gluconeogenesis. Zanamivir and oseltamivir phosphate are effectiveinhibitors of the neuraminidase; the inhibition effect of coptisine on neuraminidase is also disclosed in the previous patent application of the applicant. The applicant also discovers that salvanic acid B is capable of inhibiting the activity of the neuraminidase in vitro and is used as an inhibitor of the neuraminidase. In-vivo test proves that the several neuraminidase inhibitors are capable ofeffectively inhibiting the activity of the neuraminidase in the liver and then inhibiting hepatic gluconeogenesis. Therefore, the neuraminidase and the neuraminidase inhibitors can be used for preparing the medicines for inhibiting hepatic gluconeogenesis and can also be used for treating diabetes, obesity and non-alcoholic fatty liver disease.
Owner:CHINA PHARM UNIV
Method for preparing oseltamivir phosphate by azide process
PendingCN111747861AEmission reductionReduce governance costsOrganic compound preparationCarboxylic acid amides preparationO-Phosphoric AcidEthylic acid
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a method for preparing oseltamivir phosphate by an azide process. The method comprises the following steps:reacting a compound shown in a formula (III) with sodium azide and ammonium chloride, opening a nitrogen heterocyclic ring, performing acetylation, reducing an azide group, removing tert-butyl, salifying with phosphoric acid, and purifying to obtain pure oseltamivir phosphate shown in a formula (I). According to the method, diallylamine with strong corrosivity and expensive palladium acetate do not need to be used so that the enterprise cost is reduced.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
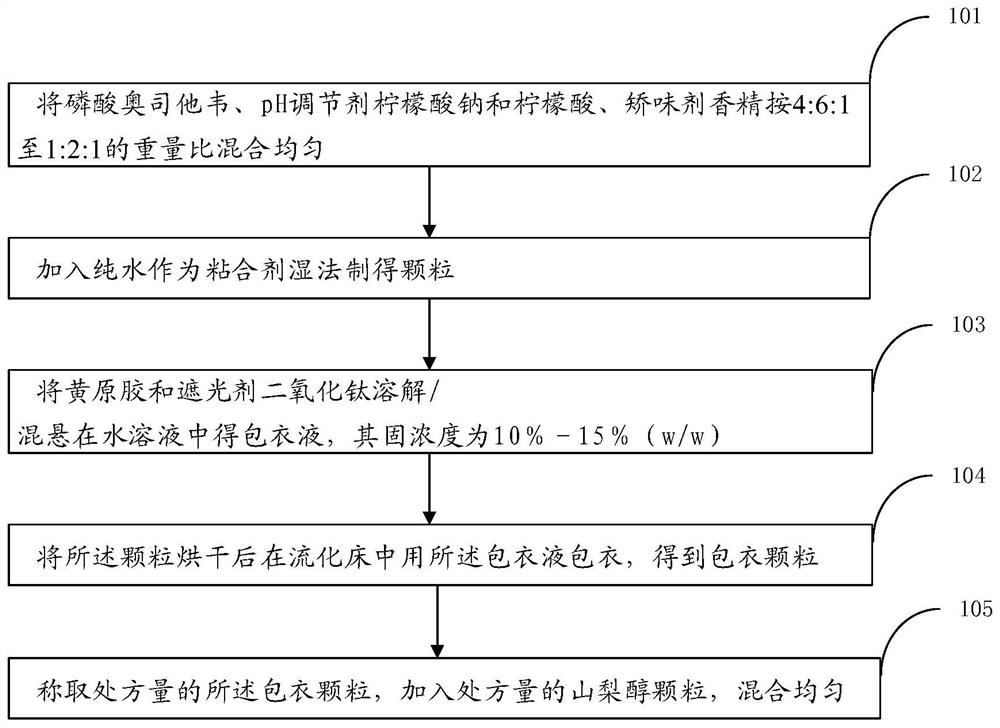
Pharmaceutical composition of oseltamivir phosphate coated particles, as well as application and preparation method
ActiveCN112121027AReady for industrializationFormulation stabilityOrganic active ingredientsInorganic non-active ingredientsCombinatorial chemistrySodium citrate
The invention provides a pharmaceutical composition of oseltamivir phosphate coated particles, as well as application and a preparation method. The pharmaceutical composition comprises coated particles and drug-free particles, and the weight ratio of the coated particles to the drug-free particles is (1: 10) to (1: 2); the coating particles comprise oseltamivir phosphate-containing medicinal particles and a coating layer, and the coating layer comprises xanthan gum and opacifier titanium dioxide; the oseltamivir phosphate-containing medicinal particles comprise oseltamivir phosphate, a pH regulator sodium citrate and citric acid, a flavoring agent essence and an adhesive pure water; and the weight ratio of oseltamivir phosphate to the pH regulator to the flavoring agent is 2: 3: 1. According to the oseltamivir phosphate coated particle pharmaceutical composition, the application and the preparation method provided by the invention, oseltamivir phosphate is prevented from being degradedby related auxiliary materials and external factors, the pharmaceutical composition is ensured to be rapidly dissolved out, and the due preparation effect is ensured; and the preparation operation issimple, the cost is reduced, and the industrial production efficiency is improved.
Owner:北京民康百草医药科技有限公司
Use of neu1 sialidase inhibitors in the treatment of cancer
ActiveUS20150064282A1Good curative effectHigh anticancer activityHeavy metal active ingredientsBiocideNEU1N-Acetylneuraminic acid
Use of Neul sialidase inhibitors for the treatment of cancer as a monotherapy or in combination with known chemotherapeutics. Preferably, Neul sialidase inhibitors are oseltamivir phosphate or 2-deoxy-2,3-dehydro-N-acetylneuraminic acid (DANA) or analogues thereof.
Owner:SZEWCZUK MYRON R
Neuraminidase inhibitor and preparation method thereof
InactiveCN106117077AAvoid excessive protonationIncrease electrophilicityOrganic compound preparationAntiviralsAcetic acidReducing agent
The invention discloses a neuraminidase inhibitor and a preparation method thereof. The preparation method comprises the following steps: with oseltamivir phosphate and biphenyl aldehyde substances as raw materials, carrying out a reaction with a reducing agent under the action of a catalyst so as to obtain the neuraminidase inhibitor, wherein the catalyst is acetic acid. The invention has the following beneficial effects: by adopting a one-pot method, a final product with high purity and high yield can be obtained; meanwhile, practicability and effectiveness in economy and environmental friendliness are realized.
Owner:SHANGHAI INST OF TECH
Oseltamivir Compositions
InactiveUS20170258749A1Powder deliveryOrganic active ingredientsExcipientPharmaceutical preservatives
The present invention relates to pharmaceutical composition comprising two different populations with first population comprising oseltamivir or a pharmaceutical acceptable salt thereof and one or more pharmaceutically acceptable excipients and second population comprising one or more pharmaceutically acceptable excipients. Preferably, the compositions wherein the second population does not contain oseltamivir or a pharmaceutically acceptable salt thereof. The invention also disclose new method of filing the composition into container. The inventors of the present invention surprisingly found that the composition are stable in real-time and long-term stability conditions. Further, the compositions are bioequivalent to marketed suspension formulation of Oseltamivir phosphate.
Owner:LUPIN ATLANTIS HLDG
A process for the preparation of intermediate for the preparation of oseltamivir phosphate
ActiveUS20160222029A1Carbamic acid derivatives preparationOrganic compound preparationCysteine thiolatePhosphate
Owner:COUNCIL OF SCI & IND RES
Dry suspension containing oseltamivir phosphate and preparation method of dry suspension
PendingCN112494434ASolve the problem of easy groupingFine grainOrganic active ingredientsPharmaceutical product form changePharmaceutical AidsSodium benzoate
Owner:江苏万珺医药科技有限公司
Influenza luciferase reporter virus-based animal model building method and application
InactiveCN108815203AShorten experiment timeEasy to measureCompounds screening/testingViral/bacteriophage medical ingredientsTreatment effectLuciferase Gene
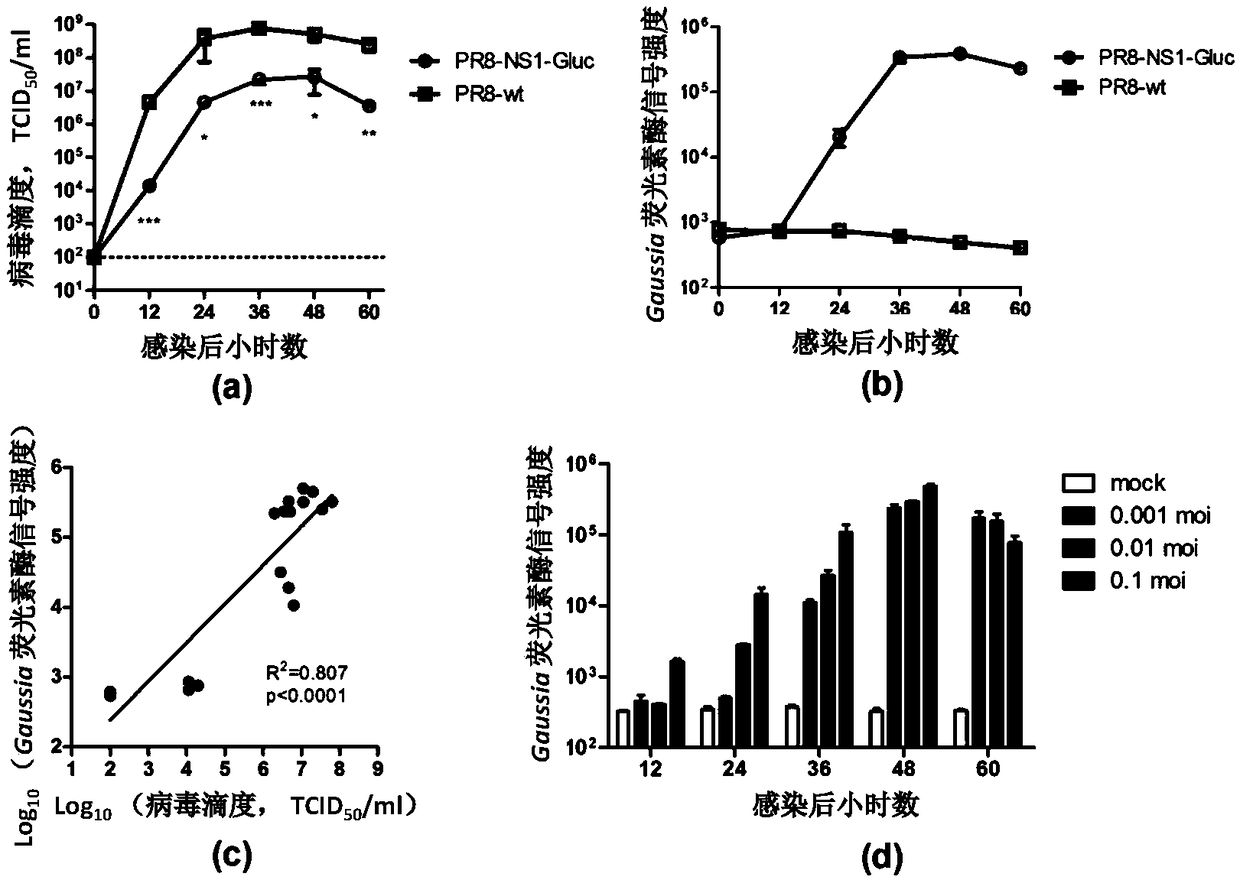
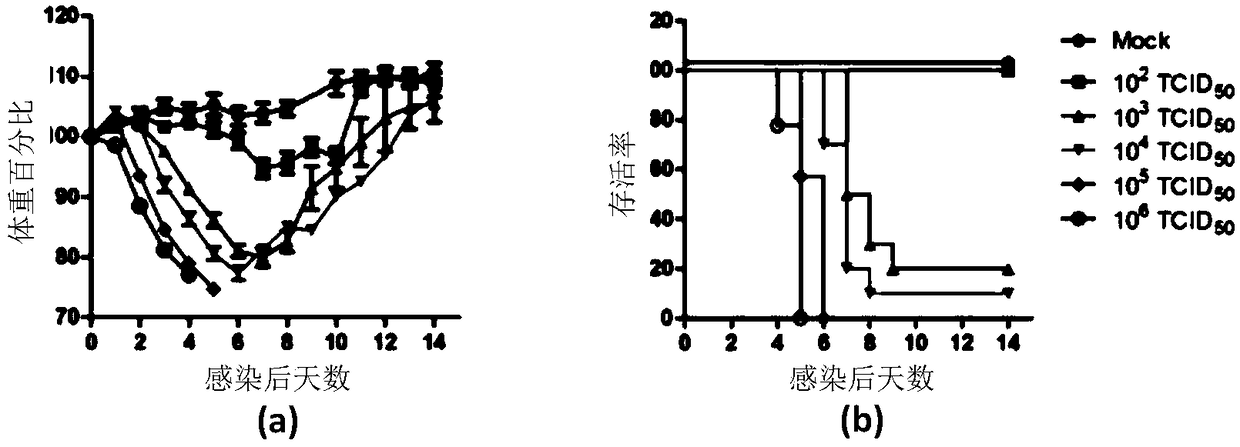
The invention discloses an influenza luciferase reporter virus-based animal model building method and application. In the method, a mouse is mainly infected with Gaussia luciferase gene-carrying recombinant influenza virus, and the infection degree of the mouse is evaluated by detecting the expression level of luciferase in a lung tissue of the mouse. The invention further discloses an influenza luciferase reporter virus technology-based method for screening and evaluating an anti-influenza drug. According to the method, anti-influenza drugs with prevention / treatment effects are screened by mainly detecting the influence of antiviral treatment on the expression level of the luciferase in the lung tissue of the mouse. Detection of positive drugs ribavirin and oseltamivir phosphate shows that the method disclosed by the invention has a wide application value in the aspect of screening, researching, developing and evaluating the novel anti-influenza drug.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
Pharmaceutical composition comprising oseltamivir phosphate
ActiveUS20100222427A1Good storage stabilityInhibit coloringOrganic active ingredientsBiocideAlcohol sugarsGlucose polymers
The present invention provides a pharmaceutical composition comprising: one or more excipients selected from sugars and sugar alcohols in which equilibrium water content is 1% by weight or less at 25° C. and at 70% relative humidity; and oseltamivir phosphate, wherein an amount of each of glucose and mannose contained in the sugars and sugar alcohols as impurities is 0.01% by weight or less.
Owner:CHUGAI PHARMA CO LTD
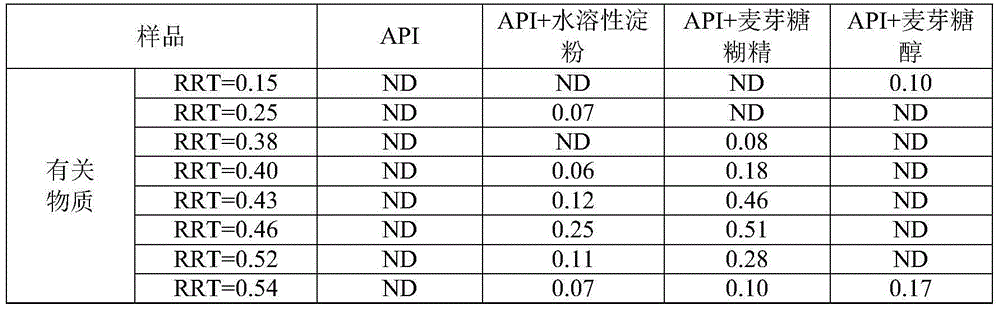
Analysis method for separating and detecting oseltamivir phosphate intermediate and impurities thereof
ActiveCN114166983AEfficient separationEasy to detectComponent separationBulk chemical productionSilanesPhosphoric acid
The invention discloses an analysis method for separating and detecting an oseltamivir phosphate intermediate and impurities thereof, belongs to the field of analytical chemistry, and particularly relates to an analysis method for carrying out gradient elution on the oseltamivir phosphate intermediate by taking pentafluorophenylsilane bonded silica gel as a filler, water-perchloric acid as a mobile phase A and acetonitrile-methanol as a mobile phase B. The method can realize effective separation and detection of the oseltamivir phosphate intermediate and the specific impurities thereof, has the characteristics of high detection sensitivity and accurate and reliable detection result, and provides a reliable reference method for quality research on separation and detection of the oseltamivir phosphate intermediate and the specific impurities thereof.
Owner:苏州正济药业有限公司
Improved oseltamivir phosphate medicinal composition
The invention provides an improved oseltamivir phosphate medicinal composition, which comprises an effective amount of oseltamivir phosphate for treating or preventing diseases, at least one beta cyclodextrin or cyclodextrin derivative and one or more optional pharmaceutically acceptable excipients. The invention also provides the application of a solid medicinal composition to the preparation of an oral liquid medicament. The invention further provides a liquid medicinal composition which comprises the medicinal composition and a water-containing medium. The medicinal composition has an excellent effect as specified in the specifications.
Owner:李春娟
Oseltamivir phosphate dispersible tablet and preparation method thereof
InactiveCN106890146AGood dispersionGreat tasteOrganic active ingredientsDispersion deliveryBed-riddenOlder people
The invention provides an oseltamivir phosphate dispersible tablet and a preparation method thereof. The dispersible tablet is composed of the following components in percentage by weight: 30-50% of oseltamivir phosphate and 50-70% of medicinal excipient. The preparation method provided by the invention comprises the following steps: stirring and mixing oseltamivir phosphate and the proper auxiliary material, granulating, finishing, and tabletting to obtain the oseltamivir phosphate dispersible tablet. The dispersible tablet can be quickly disintegrated and uniformly dispersed in water, and is suitable for children, old people, bed-ridden patients and severely disabled patients; and the dispersible tablet can be prepared into a uniform dispersion solution, and the uniform dispersion solution can be measured by an appropriate measuring vessel to satisfy the application demands of children with different weights. The method is easy to implement and simple for preparation, is suitable for industrial production, and has great application value.
Owner:SHANGHAI SUNTECH PHARMA
Preparation method of oseltamivir phosphate capsule
ActiveCN111297823AGuaranteed dissolution uniformityGuarantee effective timeOrganic active ingredientsAntiviralsPharmaceutical SubstancesMedicinal chemistry
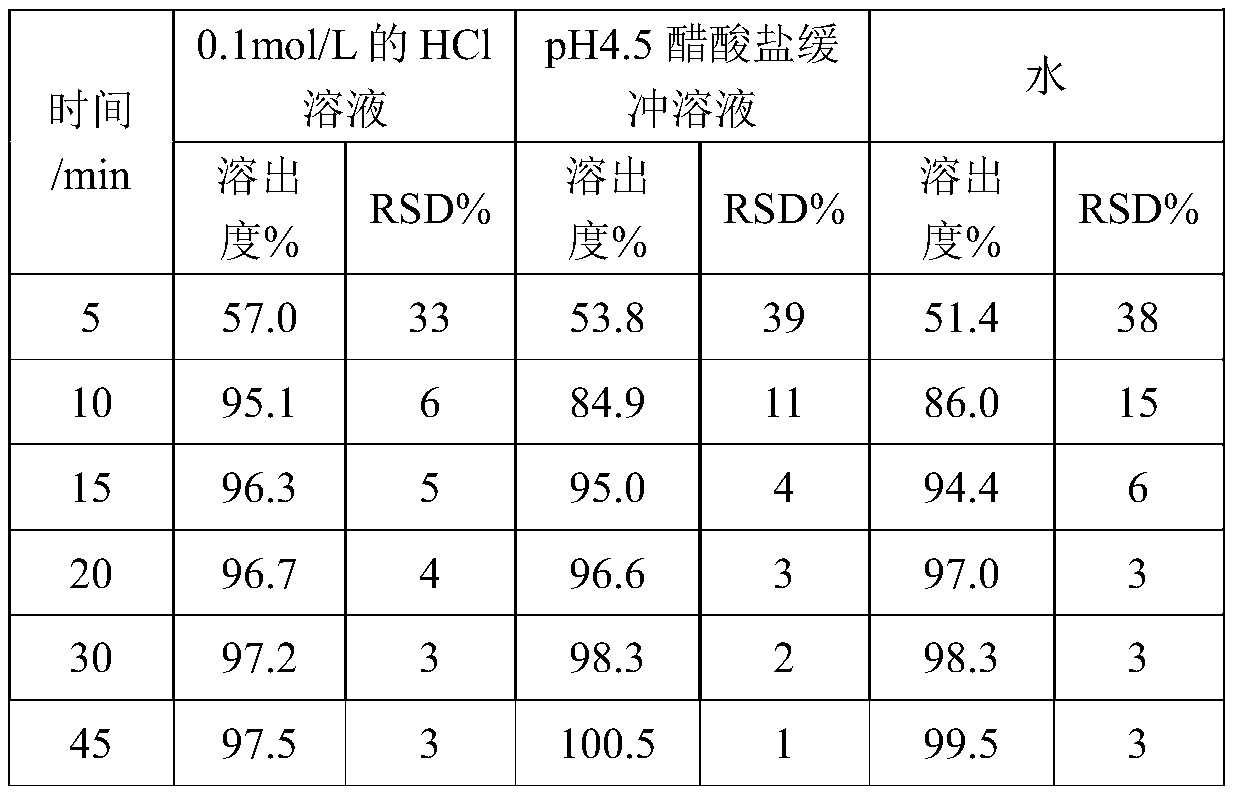
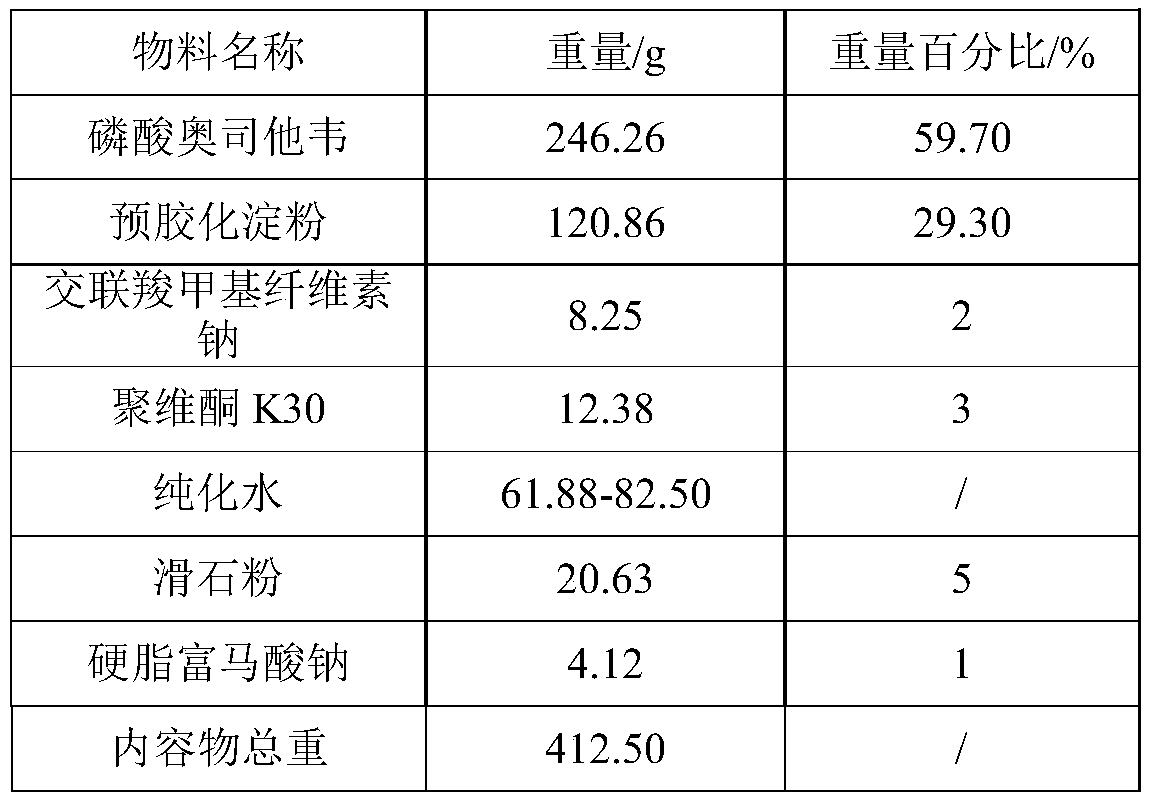
The invention provides a preparation method of an oseltamivir phosphate capsule. By controlling the adding manner and adding amount of a flow aid, the dissolution uniformity of medicines is effectively improved, and the effects of the change of the dissolution speed of the medicines on the effect taking time of the medicines are reduced.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Application of neuraminidase inhibitor to preparation of drug for treating myocarditis
ActiveCN108125942ARemission of lesionEffective therapeutic effectOrganic active ingredientsCardiovascular disorderSerum igeCardiac muscle
The invention discloses application of a neuraminidase inhibitor to preparation of a drug for treating myocarditis. The invention discovers that oseltamivir phosphate or zanamivir as the neuraminidaseinhibitor has an effective treatment effect on a myocarditis model mouse. After the myocarditis model mouse is treated by the oseltamivir phosphate or the zanamivir, the serum cTnI concentration is significantly reduced, so that the degree of myocardial injury is improved; and myocardial case scores are significantly reduced, so that the degree of cardiomyopathy is relieved. Therefore, the oseltamivir phosphate or the zanamivir as the neuraminidase inhibitor can be used for preparing the drug for treating the myocarditis.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com