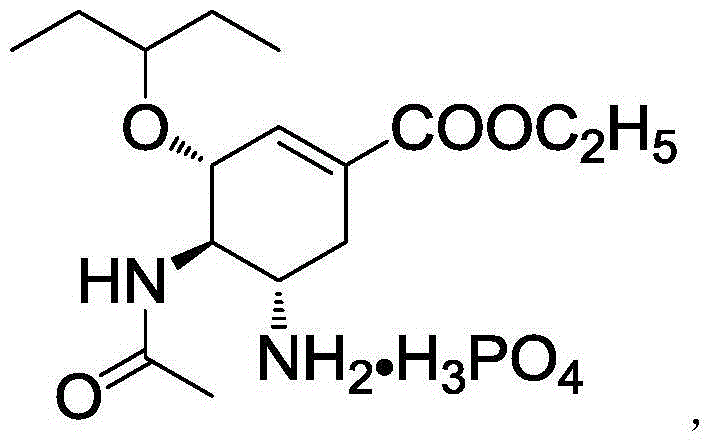
Solid preparation of oseltamivir phosphate
A technology of oseltamivir phosphate and solid preparations, applied in the field of solid preparations of oseltamivir phosphate and its preparation, can solve the problems of easy generation of impurities, unfavorable drug storage, poor compatibility, etc., and achieve stable quality and patient compliance Good sex and a wide range of drug users
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] Another aspect of the present invention provides a preparation method of oseltamivir phosphate solid preparation, said method comprising mixing oseltamivir phosphate, diluent, binder and optional edible essence, and / or sweetener , sieving, adding binder solution to make soft material, sieving and drying, granulation and other steps.
[0044] In some embodiments, the solid preparation of oseltamivir phosphate can be prepared according to the following method:
[0045] 1) Pass oseltamivir phosphate and maltitol through a 100-mesh sieve, and mix well;
[0046] 2) adding the binder into the solvent to form a binder solution;
[0047] 3) Add the binder solution to the mixed material in 1) to make a soft material, and use a 30-mesh screen to make wet granules;
[0048] 4) Optionally add sweeteners and / or food flavors; dry the wet granules at a temperature of 60°C, and control the moisture content of the granules below 2%;
specific Embodiment approach
[0053] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0054] All the reagents used in the present invention can be purchased from the market.
[0055]In the present invention, mg means milligram, g means gram, RT means retention time, and RRT means relative retention time.
Embodiment 1
[0056] Embodiment 1 Oseltamivir Phosphate and Diluent Compatibility Experiment
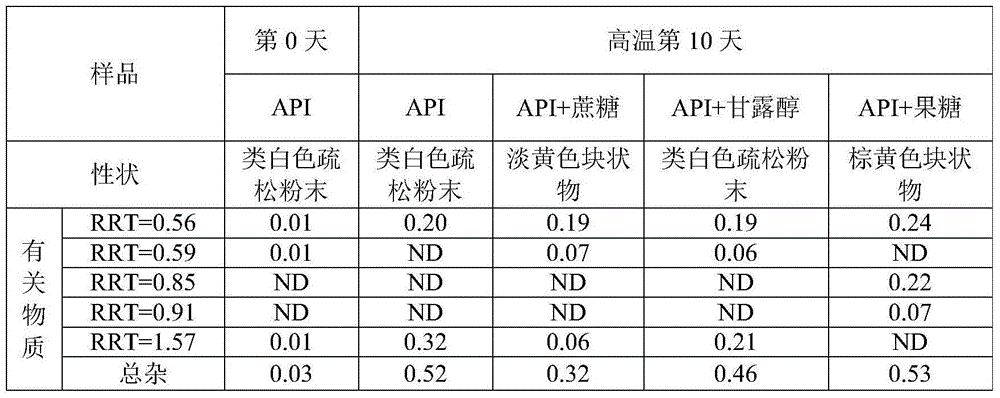
[0057] 1) Compatibility test of API and sucrose, mannitol, fructose raw materials and excipients
[0058] Weigh an appropriate amount of raw materials and a mixture of raw and auxiliary materials into a vial, add an appropriate amount of purified water (w / w), seal it (simulating a high-temperature and high-humidity environment), and place it in an oven at 60°C. Take a sample on the 10th day to detect its related substances (Content data expressed in percentage, %).
[0059] Table 1. Compatibility results of API with sucrose, mannitol, and fructose at high temperature and high humidity for 10 days
[0060]
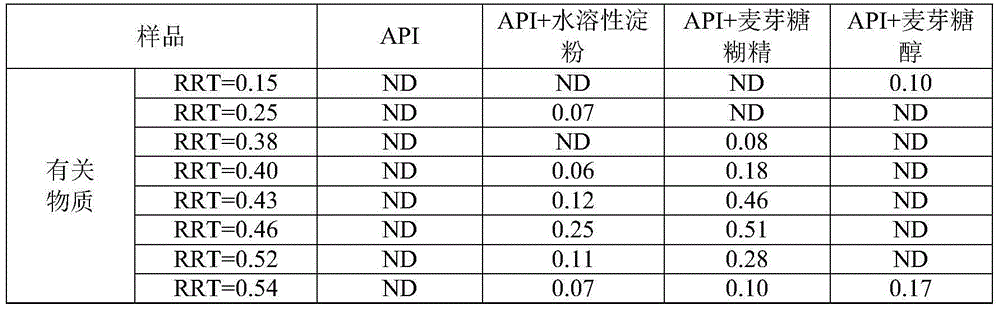
[0061] Table 2. Compatibility results of API with water-soluble starch, maltodextrin, and maltitol at high temperature and high humidity for 10 days
[0062]
[0063]
[0064] Note: ND stands for "Not Detectable"
[0065] discuss:
[0066] The APIs in Table 1 and Table 2 are not th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com