Oseltamivir phosphate dispersible tablet and preparation method thereof
A technology of oseltamivir phosphate and dispersible tablets, which is applied in the direction of dispersion liquid delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of inability to ensure drug safety and high cost of granules , Difficulty in accurate drug administration and other issues, to achieve the effect of high application value, simple process conditions and equipment requirements, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Grind oseltamivir phosphate through 80 meshes, dissolve the prescribed amount of polyvinylpyrrolidone K30 in 50% ethanol water, prepare 5% soft material, and set aside;
[0025] 2) Weigh the raw materials, fillers, disintegrants and sweeteners according to the formula in the table, and mix them evenly in the granulator. Pour into the granulator, during the process of adding liquid and granulating, the stirring speed of the granulator is 200rpm, the speed of the shearing knife is 1800rpm, and the wet mixing time is controlled at 10 minutes in total;
[0026] 3) Pass the soft material through a 20-mesh sieve, dry in a fluidized bed (Glatt, GPCG2) (inlet air temperature 60°C, dry for 2 hours), 24-mesh granulation, add lubricant and lemon essence during the granulation process, Type mixer (Yali Machinery, DGN-II) after mixing and tableting to obtain 1000 oseltamivir phosphate dispersible tablets.
[0027] The hardness of the tablets was measured with a hardness tester (...
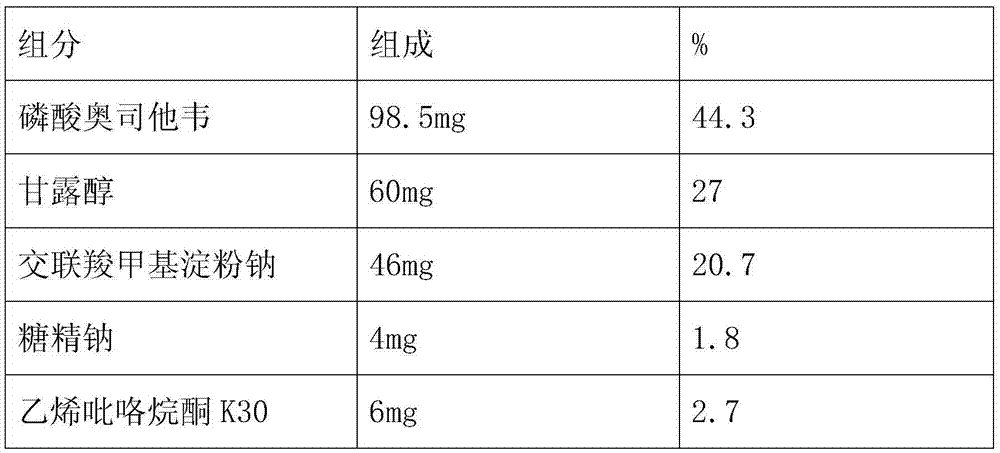
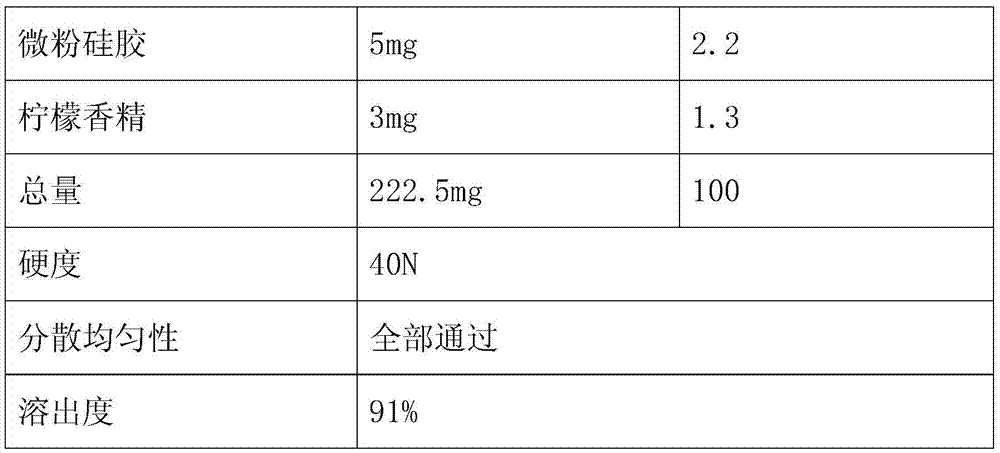
Embodiment 2
[0037] The preparation method is the same as in Example 1, and the components and detection results of the oseltamivir phosphate dispersible tablet are as follows:
[0038] Formula and test results:
[0039]
[0040]
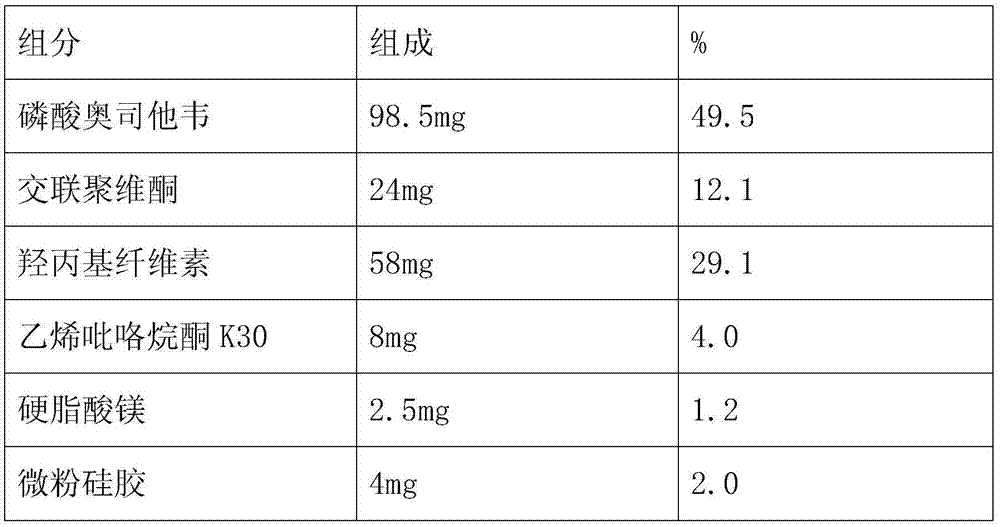
Embodiment 3
[0042] The preparation method is the same as in Example 1, and the components and detection results of the oseltamivir phosphate dispersible tablet are as follows:
[0043] Formula and test results:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com