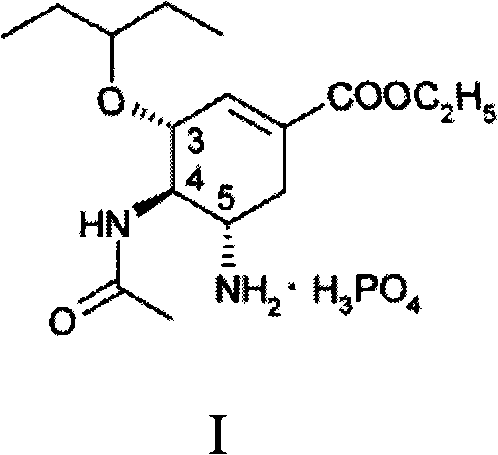
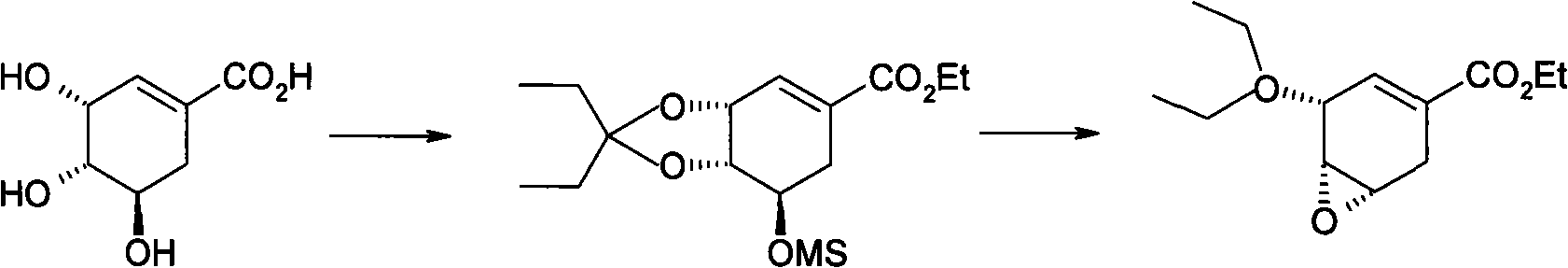
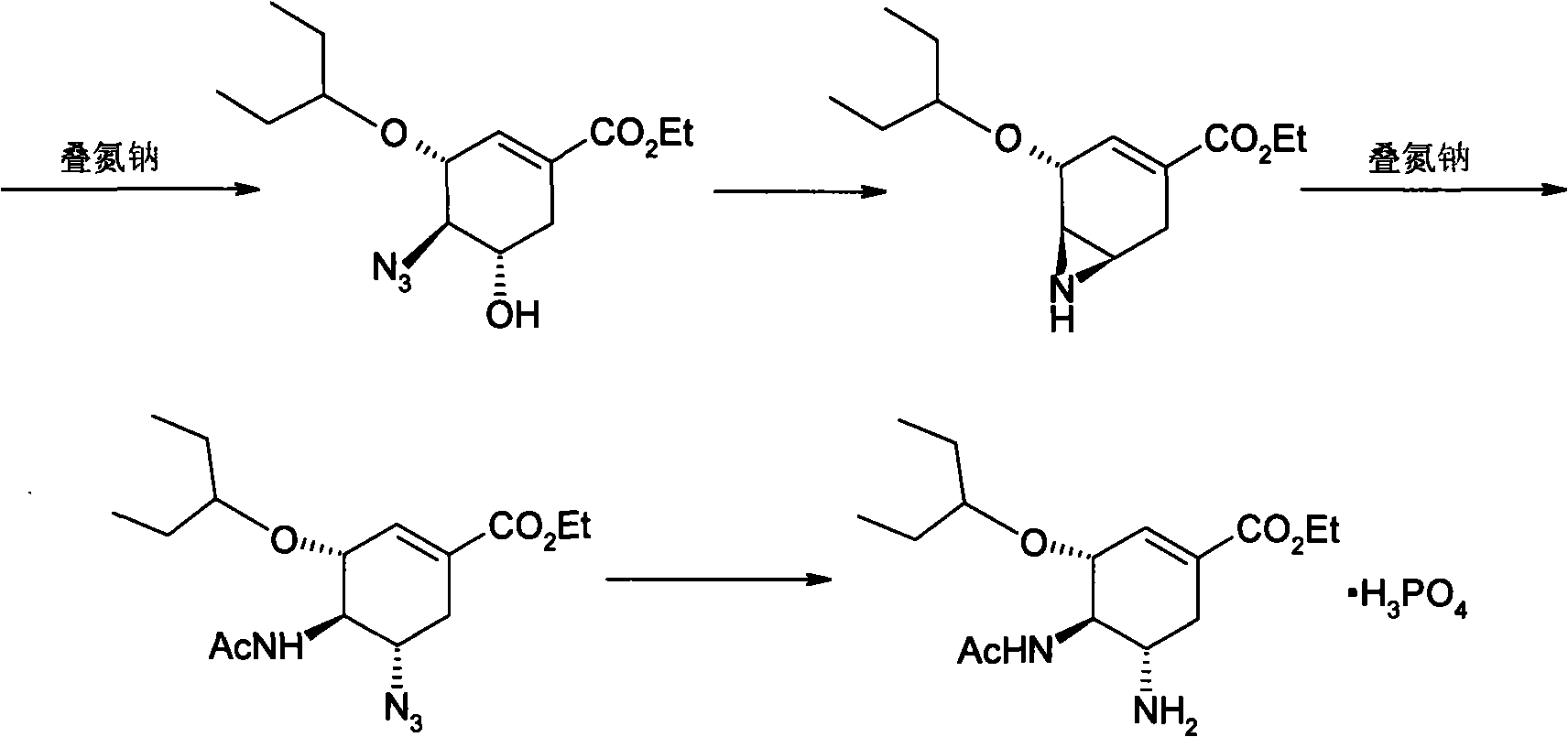
Purification process for Oseltamivir Phosphate
A technology of oseltamivir phosphate and purification method, which is applied in the field of purification of oseltamivir phosphate, can solve the problems of increased reaction steps, undisclosed final product purification method, and difficulty in achieving the quality of finished products, and achieves simple operation and high product quality. The effect of stability and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under nitrogen protection, ethyl (3R, 4R, 5S)-4-N-acetylamino-5-N, N-bisallylamino-3-(1-ethylpropoxy)- 12 grams of 1-cyclohexene-1-carboxylic acid, 5 grams of 1,3-dimethylbarbital, 0.3 grams of triphenylphosphine, 0.07 grams of palladium acetate and 55 grams of absolute ethanol, stirring and heating to 35 ° C React for 2 hours. Then the hydrogenolysis reaction solution was added dropwise to another reaction kettle containing 3.5 grams of phosphoric acid and 36.5 grams of absolute ethanol at 50°C, stirred and reacted for 2 hours, cooled to -17°C to -18°C, and waited for complete crystallization ,filter. Wash 20 g x 3 with acetone in turn; wash 20 g x 3 with n-heptane, and dry to obtain crude oseltamivir phosphate.
Embodiment 2
[0022] Put 12.5 g of crude oseltamivir phosphate obtained in Example 1 and 270 g of absolute ethanol into the refining kettle, heat to 60°C, add 1 g of activated carbon, continue heating to about 80°C, and decolorize and reflux for 60 minutes. Filtrate while hot, and the filtrate is cooled to precipitate crystals, and placed in the refrigerator overnight. The next day, it was filtered and dried to obtain 11.2 grams of oseltamivir phosphate finished product. The purity (content HPLC) is 99.7712%, the largest single impurity is 0.0843%, the total impurity is 0.2248%, and the total yield is 89.15%.
Embodiment 3
[0024] Put 12.5 g of crude oseltamivir phosphate obtained in Example 1 and 80 g of anhydrous methanol into the refining kettle, heat to 50°C, add 1 g of activated carbon, continue heating to about 60°C, and decolorize and reflux for 45 minutes. Filtrate while hot, and the filtrate is cooled to precipitate crystals, and placed in the refrigerator overnight. The next day, it was filtered and dried to obtain 10.5 grams of oseltamivir phosphate finished product. The purity (content HPLC) is 99.6684%, the largest single impurity is 0.0837%, the total impurity is 0.3316%, and the total yield is 83.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com