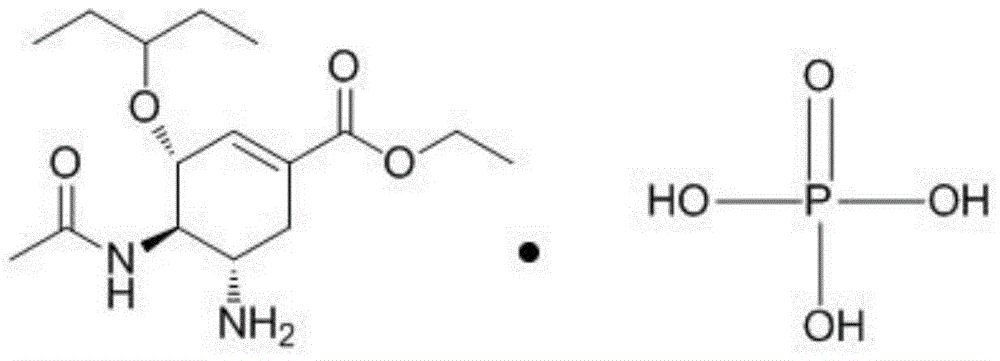
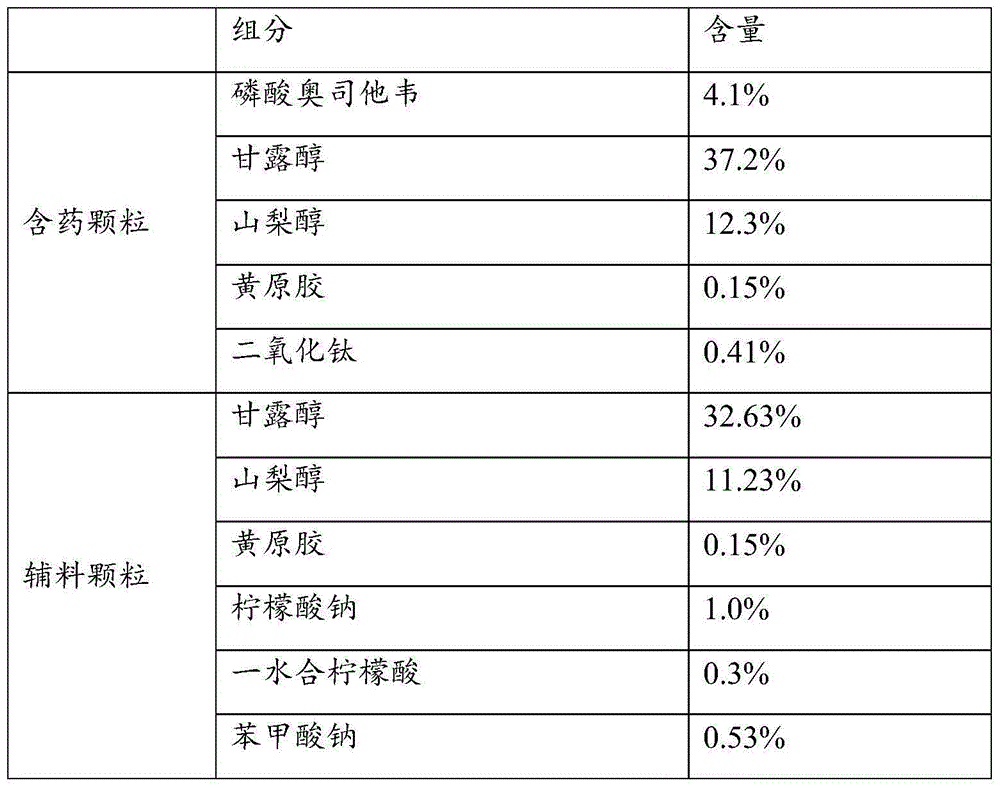
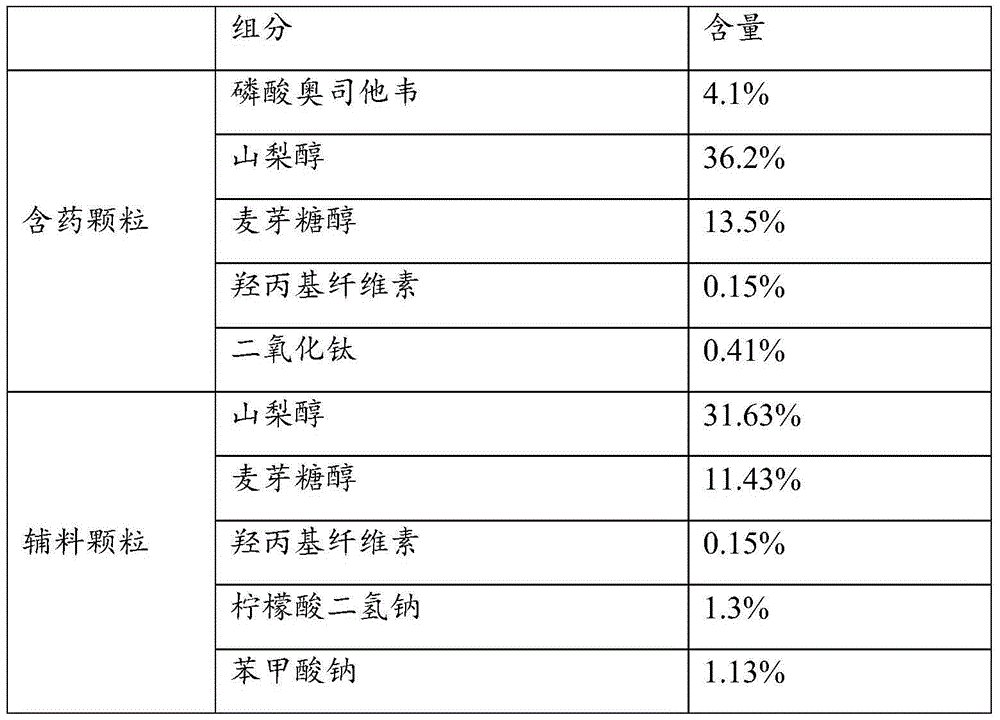
Oseltamivir phosphate dry suspension and preparation method thereof
A technology of oseltamivir phosphate and dry suspension, which is applied in the field of medicine and chemical industry, and can solve the problems of high cost, high control standard of excipients, and difficult to control or achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0068] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0069] In this embodiment, the percentages described in the specific embodiments are by mass ratio.
[0070] The reagents used in this example can be purchased from the market or can be prepared by the methods described in the present invention.
[0071] In this embodiment, sorbitol is purchased from Roquette, which meets the USP-NF excipient standard, wherein the limit of reducing sugar is less than 0.3% (calculated as sucrose)
Embodiment 1
[0073] prescription:
[0074] components
content
3.5%
49.6%
34.1%
4.5%
Titanium dioxide
2.5%
Sodium citrate
4.0%
Citric Acid Monohydrate
0.8%
[0075] sodium benzoate
1.0%
total
100.0%
[0076] Preparation:
[0077]Weigh an appropriate amount of oseltamivir phosphate and auxiliary materials according to the prescription amount, add 3.5% oseltamivir phosphate, 34.1% sorbitol, 49.6% mannitol, 2.5% titanium dioxide, 4.0% sodium citrate, 0.8% a Hydrated citric acid, 1.0% sodium benzoate and 4.5% xanthan gum were uniformly mixed in a granulator, the blade speed of the granulator was 150rpm, the cutter speed was 3000rpm, and then passed through a 30-mesh sieve for 3 times.
Embodiment 2
[0079] prescription:
[0080] components
content
3.8%
70.8%
23.5%
0.1%
Titanium dioxide
0.5%
Sodium citrate
1.0%
Citric Acid Monohydrate
0.2%
0.1%
total
100.0%
[0081] Preparation Process:
[0082] Weigh an appropriate amount of oseltamivir phosphate and auxiliary materials according to the prescription amount, add 3.8% oseltamivir phosphate, 23.5% sorbitol, 70.8% mannitol, 0.5% titanium dioxide, 1.0% sodium citrate, 0.2% a Hydrated citric acid, 0.1% sodium benzoate and 0.1% hydroxypropyl cellulose are mixed uniformly in a granulator, the blade speed of the granulator is 150rpm, the cutter speed is 3000rpm, and then passed through a 30-mesh sieve for 3 times.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com