Patents
Literature
1038 results about "Filler Excipient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A therapeutically inactive ingredient of a solid pharmaceutical dosage form that is used to increase the volume of the material to accommodate easier processing of the drug and to make it a suitable size for patient consumption Typical fillers include calcium phosphate (also an added source of both minerals), lactose, powder, sucrose powder.
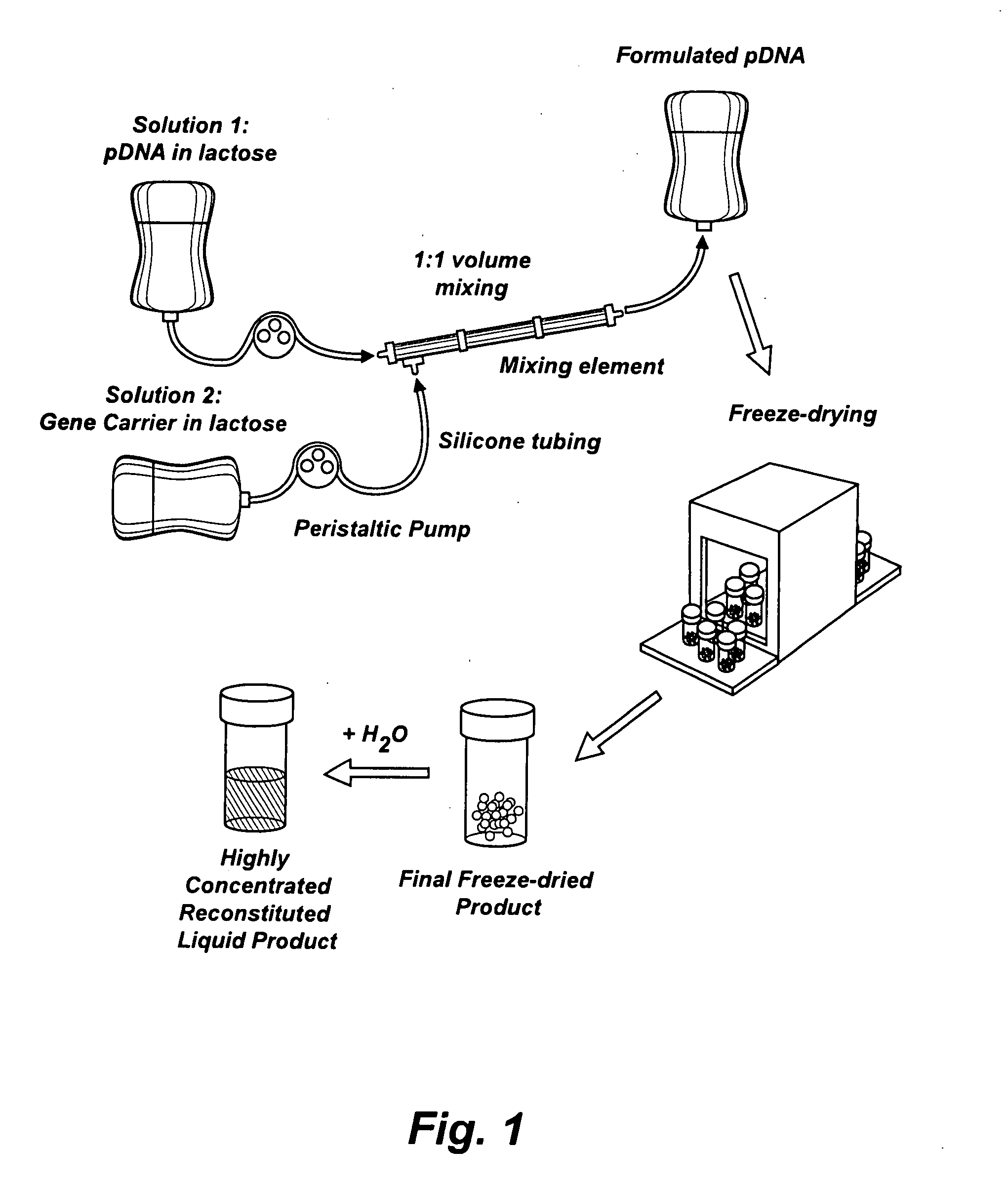
Nucleic Acid-Lipopolymer Compositions
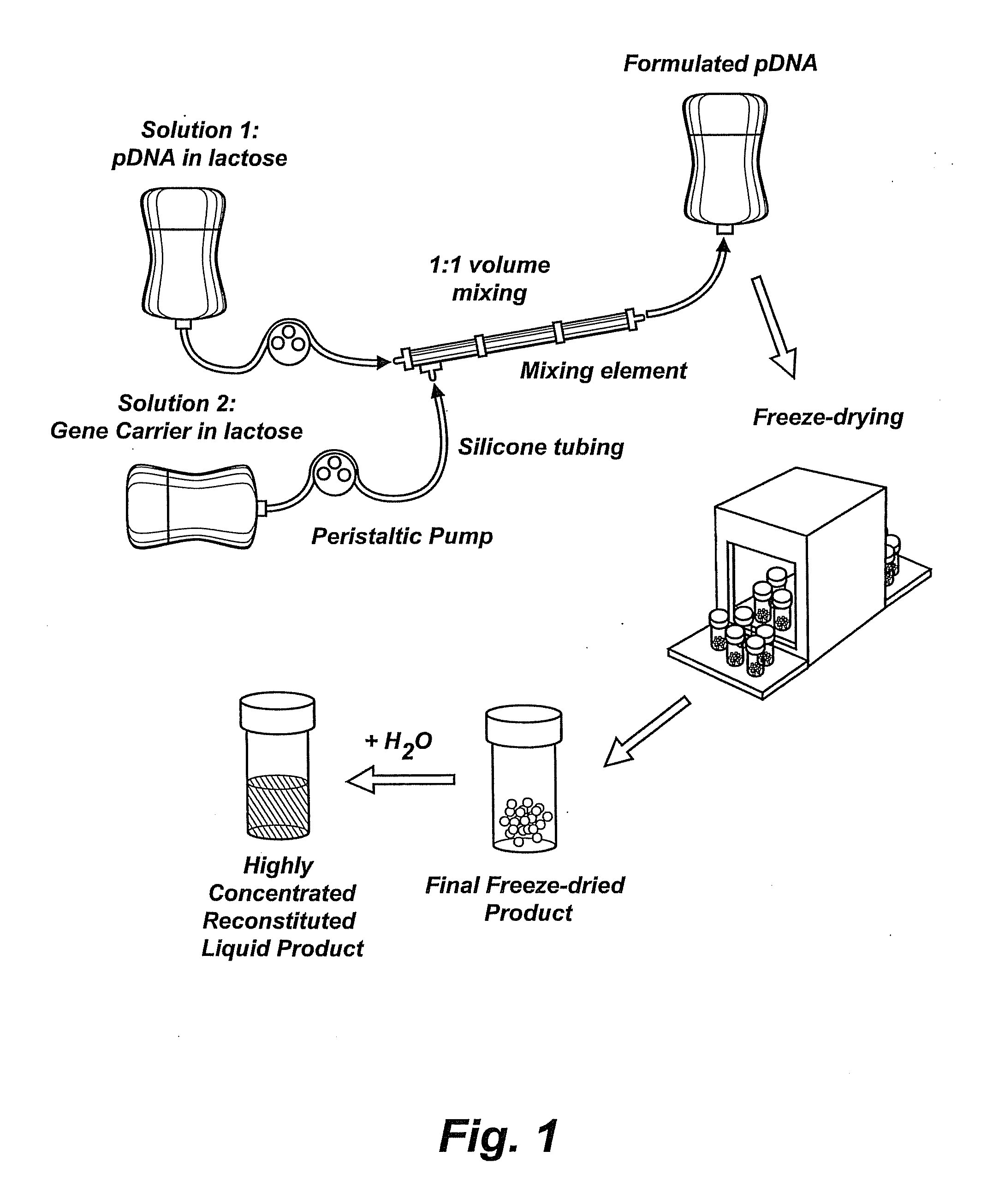
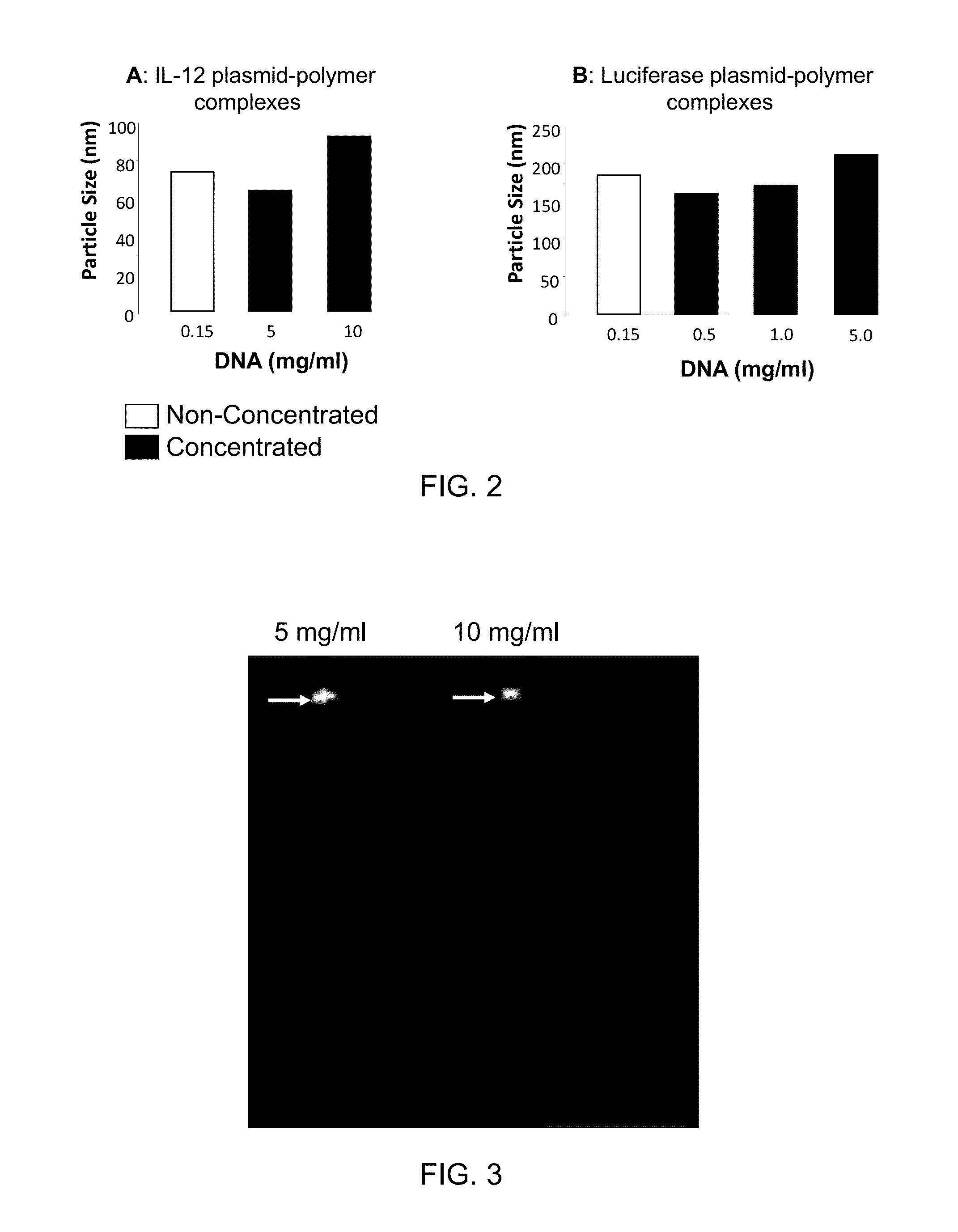
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
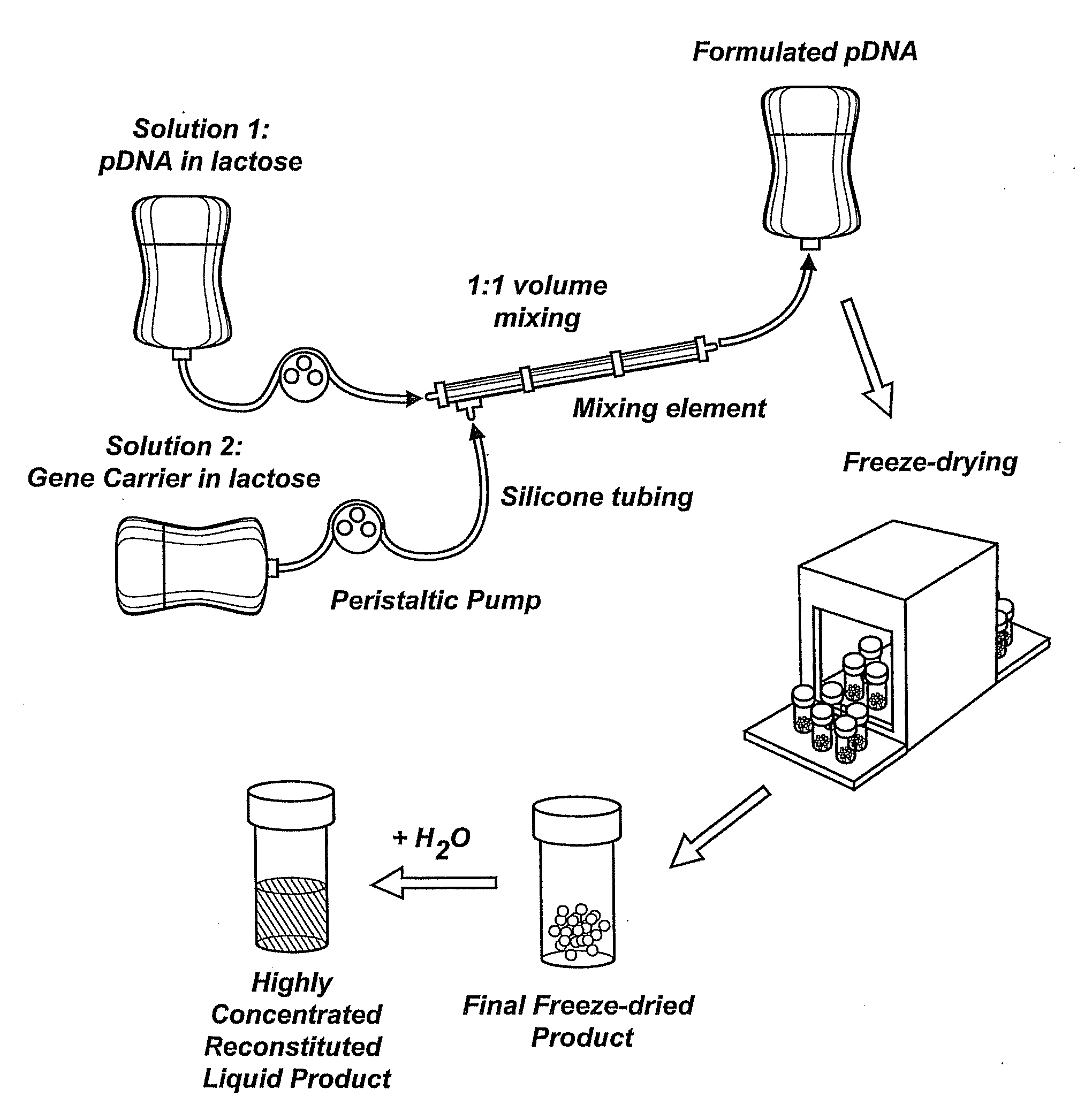
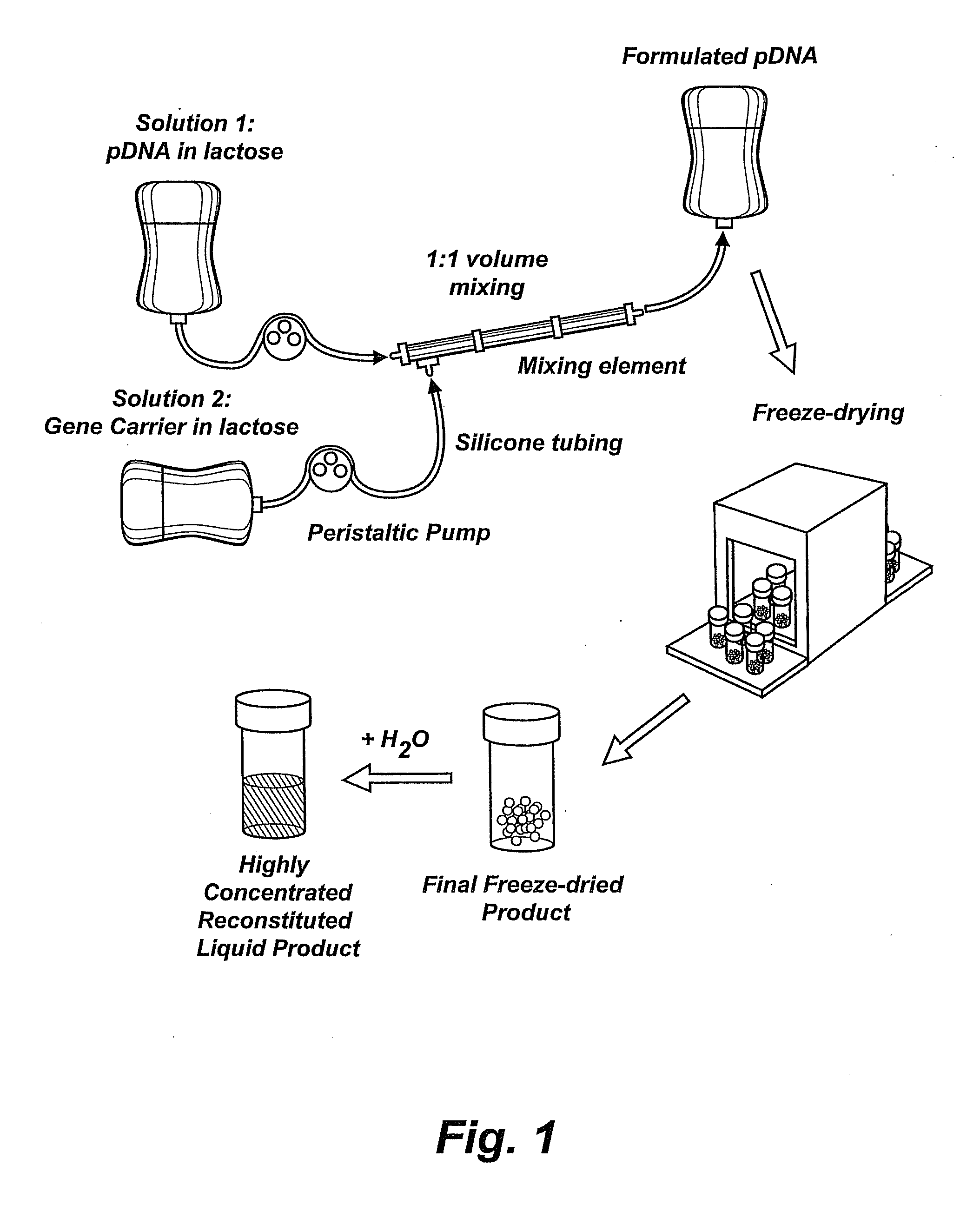
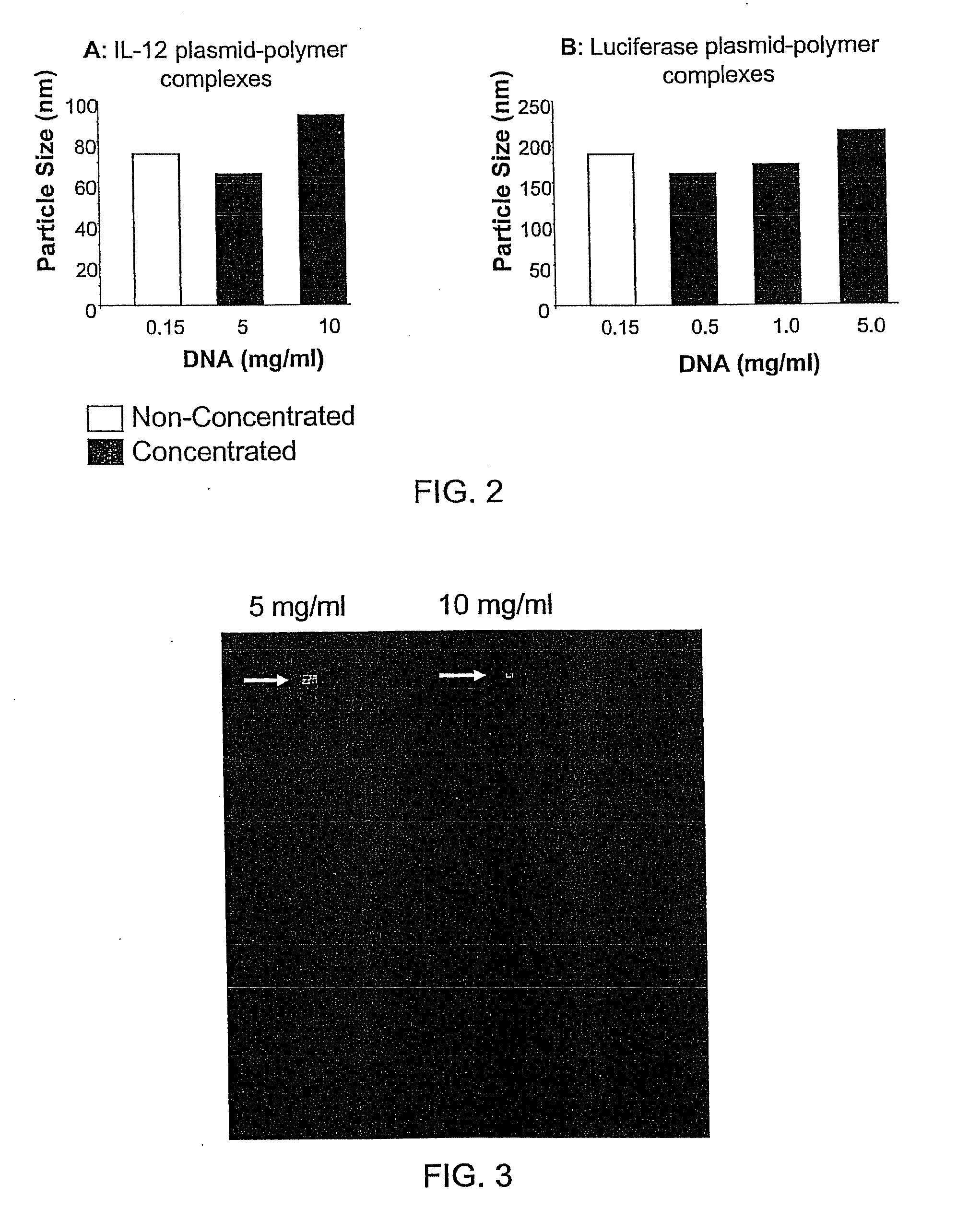
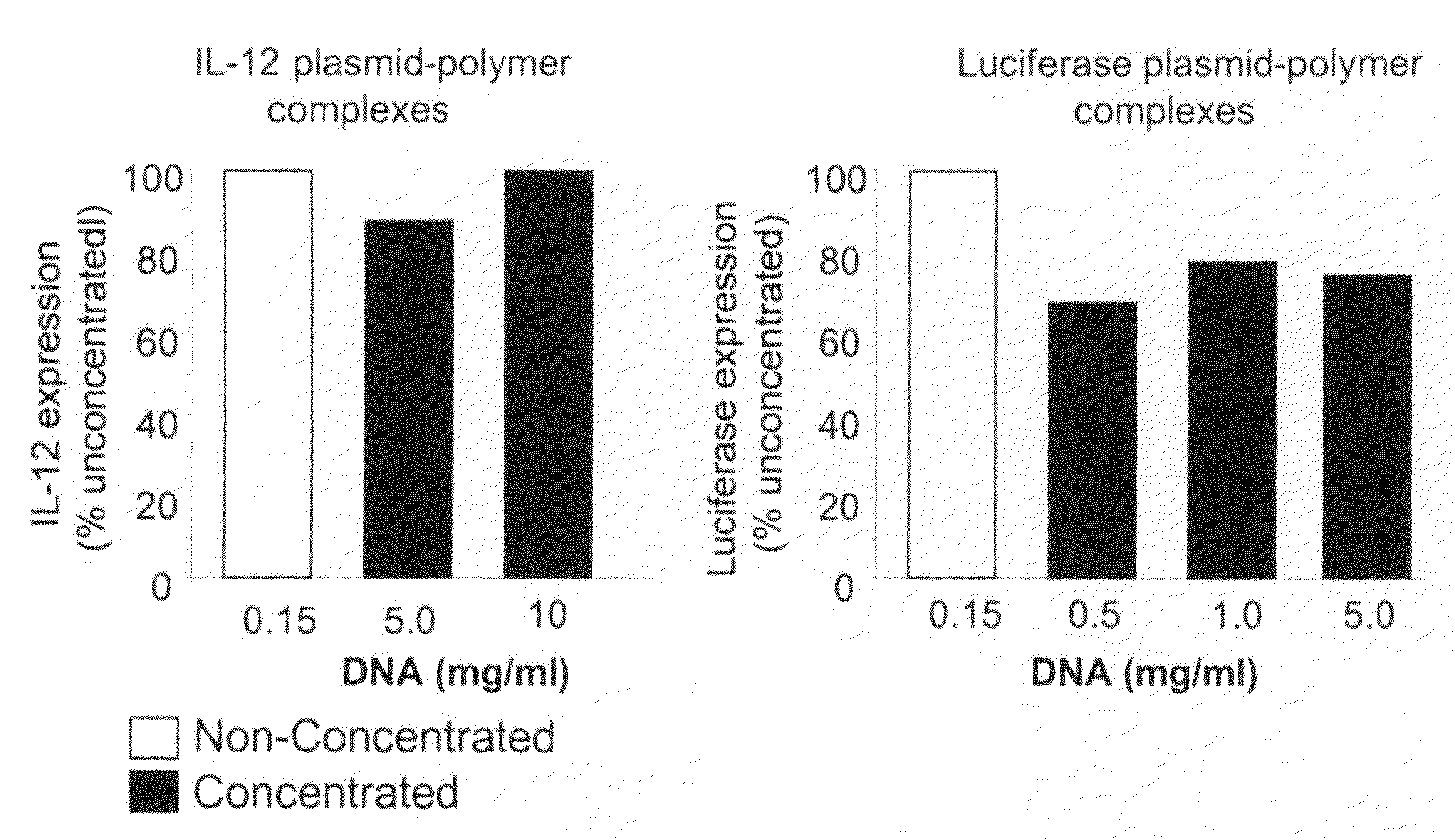
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Composition, method of preparation & application of concentrated formulations of condensed nucleic acids with a cationic lipopolymer
UndeterminedUS20090042825A1Increase efficiency and dosing flexibilitySpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:EXPRESSION GENETICS INC
Biodegradable oxidized cellulose esters and their uses as microspheres
InactiveUS7649089B2Extended durationOrganic active ingredientsGranular deliveryControlled releaseAlcohol
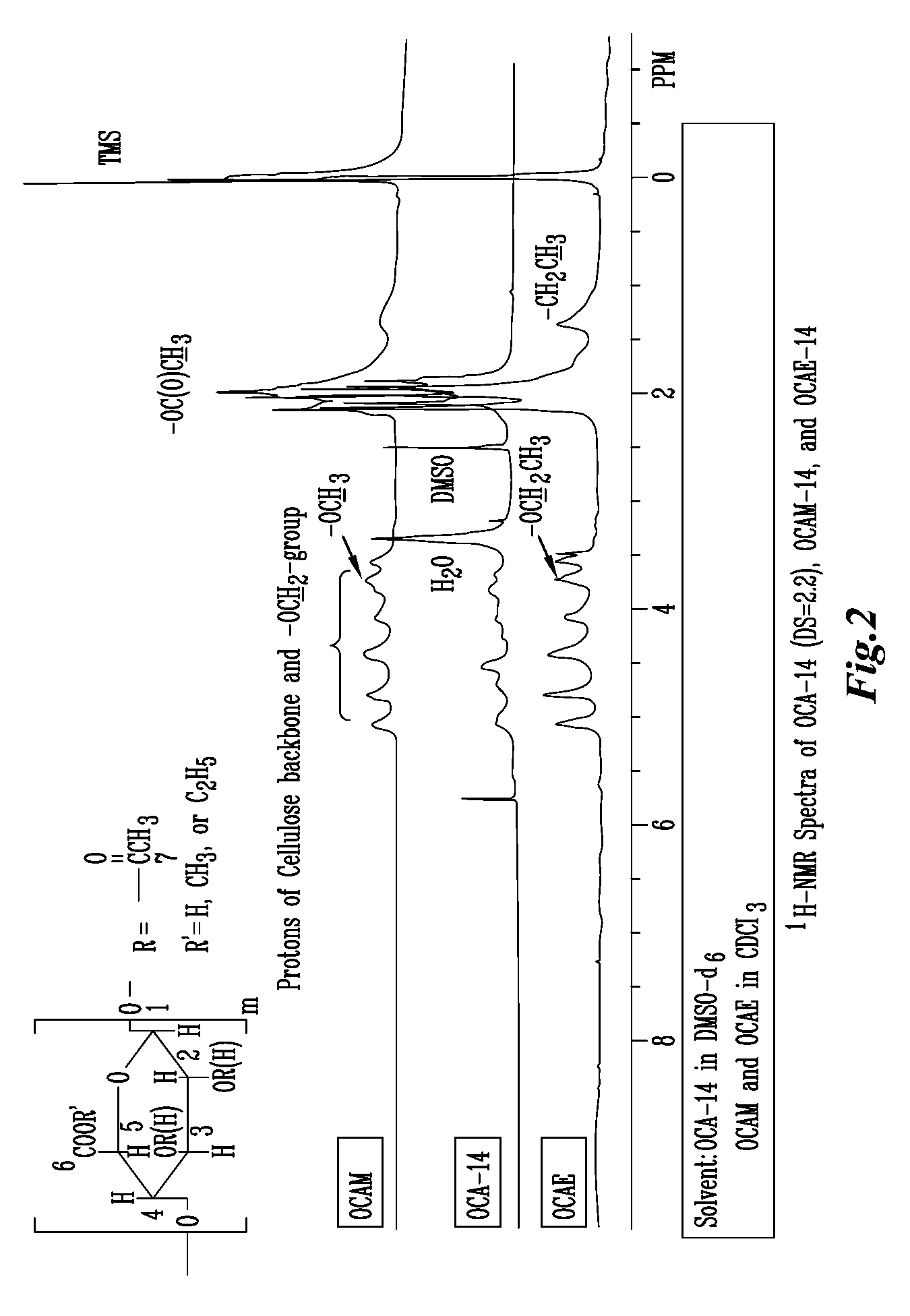
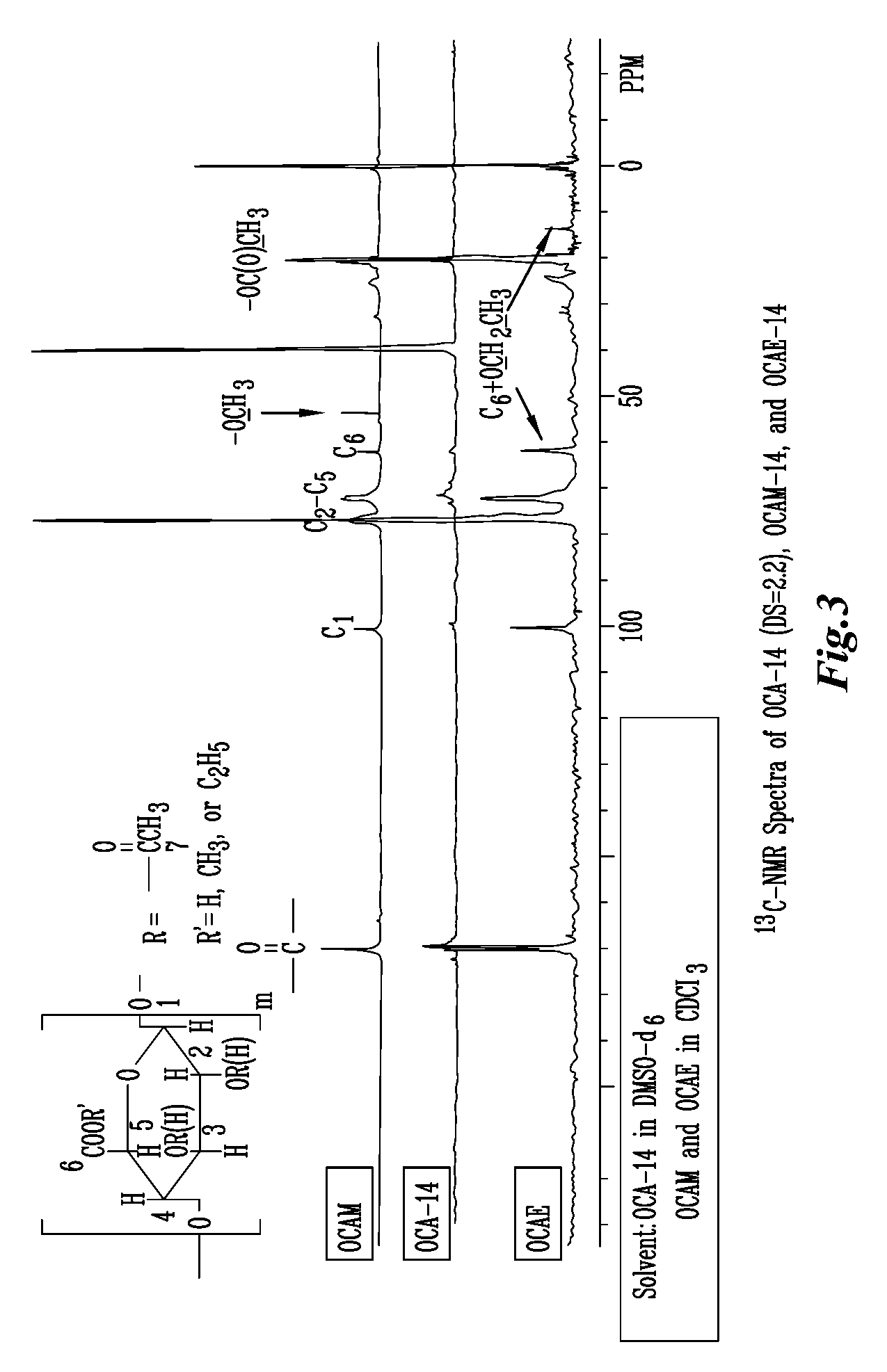
A new cellulose excipient, OCCAE, suitable for use as a binder, filler, and / or disintegrant in the development of solid dosage forms and as a bodying agent or a drug carrier in the preparation of topical formulations is described. The cellulose excipient is formed by reacting an oxidized cellulose ester with an alcohol in the presence of a catalyst. The invention also describes the formation of controlled release microspheres using OCCAE and / or oxidized cellulose esters that may be used to control the release of drug in a patient over a time period of several hours to several days.
Owner:UNIV OF IOWA RES FOUND
Alloplastic injectable dermal filler and methods of use thereof
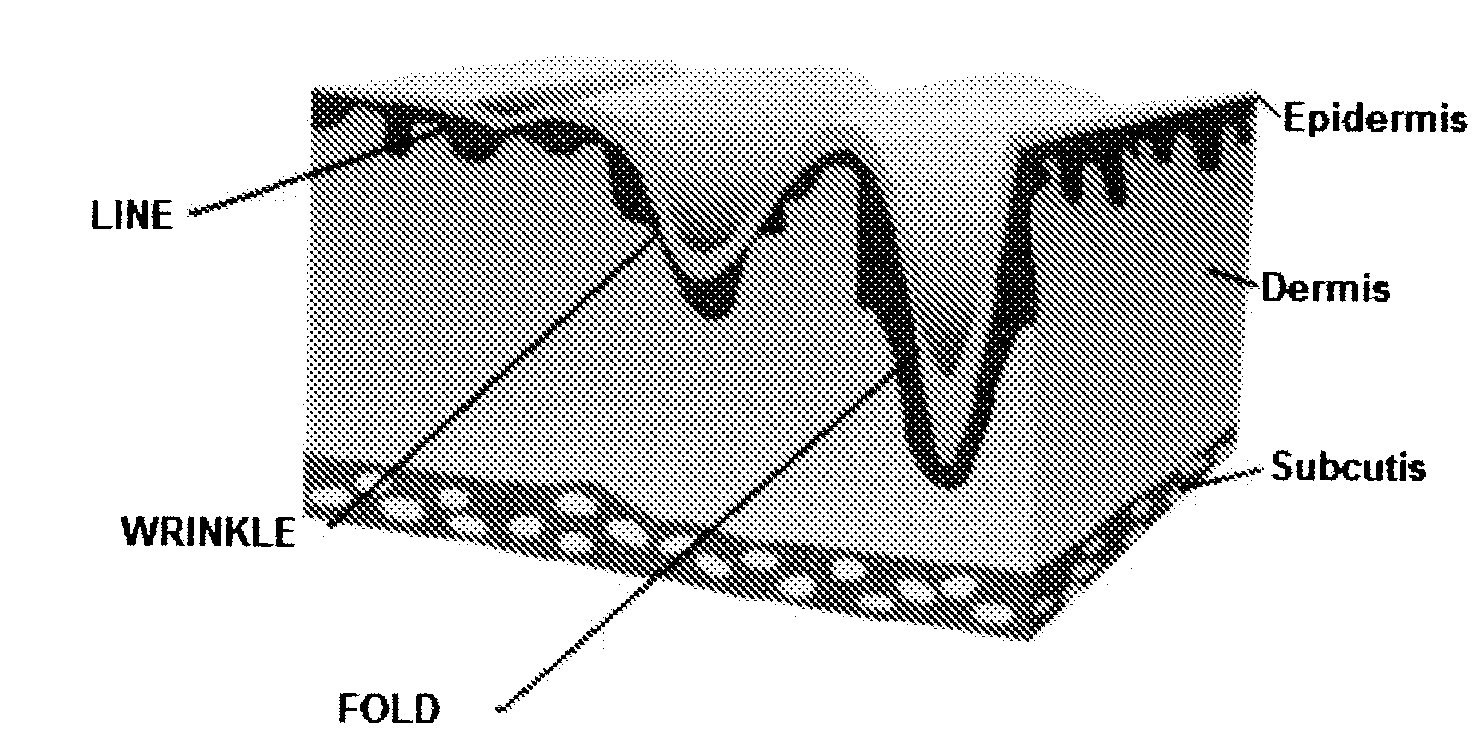
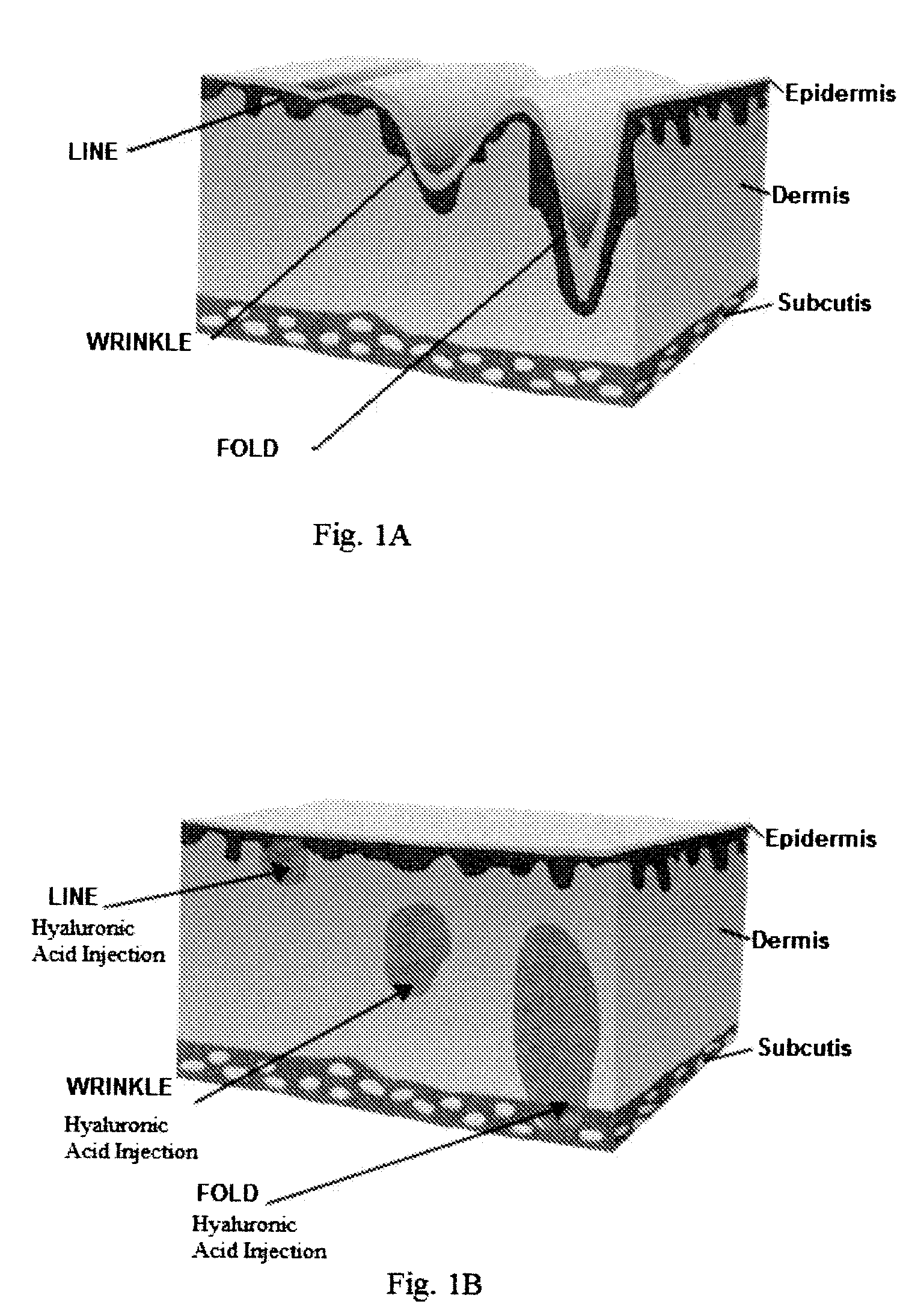
InactiveUS20090110736A1Reduce wrinklesReduce scarsPowder deliveryCosmetic preparationsFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Coated Hyaluronic Acid Particles
The present invention generally relates to particles comprising hyaluronic acid, wherein the particles are coated or encapsulated with a coating. The coating preferably comprises a polymer, protein, polysaccharide, or combination thereof that decreases the rate of degradation of the hyaluronic acid once the particles are placed in an aqueous environment, such as inside mammalian skin. The compositions of the present invention comprising such coated hyaluronic acid are useful for soft tissue augmentation, and are particularly useful as dermal fillers.
Owner:ALLERGAN INC
Nucleic Acid-Lipopolymer Compositions
ActiveUS20130065942A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Regeneration method of waste elastic body
A process for regenerating the used or rejected elastomer features that the non-odor softening agent prepared from the kernel of plant fruit, oil-containing decolored mud and / or frequently used softener, non-odor activating agent, anti-oxidizing-antiageing agent, adsorbent, industrial refuse, the powder of bone, shell, or straw, and / or reinforcing filler are used to treat said used or rejected elastomer to obtain fine (80 meshes) powder, which can be used as the filler of rubber or plastics.
Owner:陈民铎
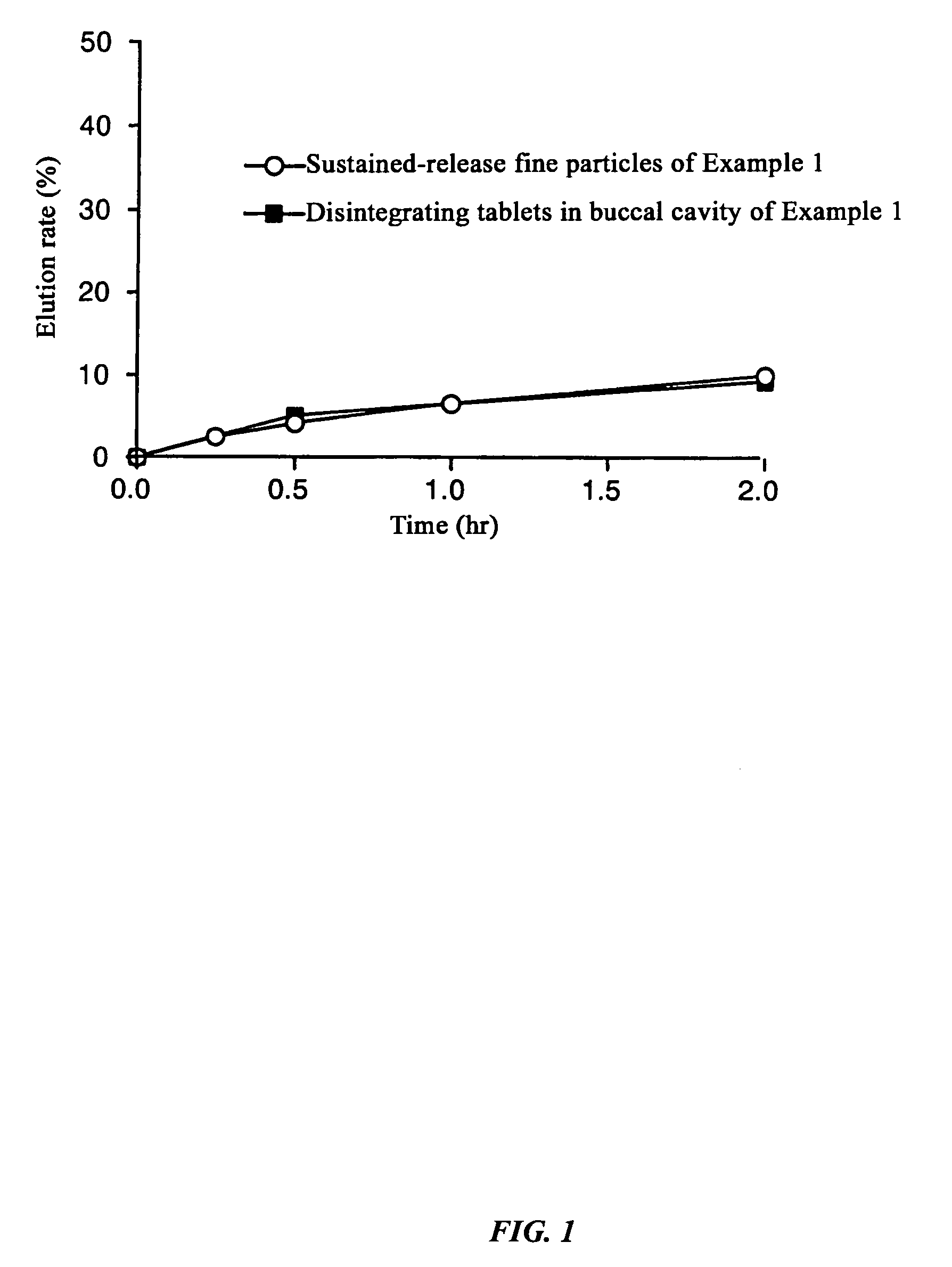
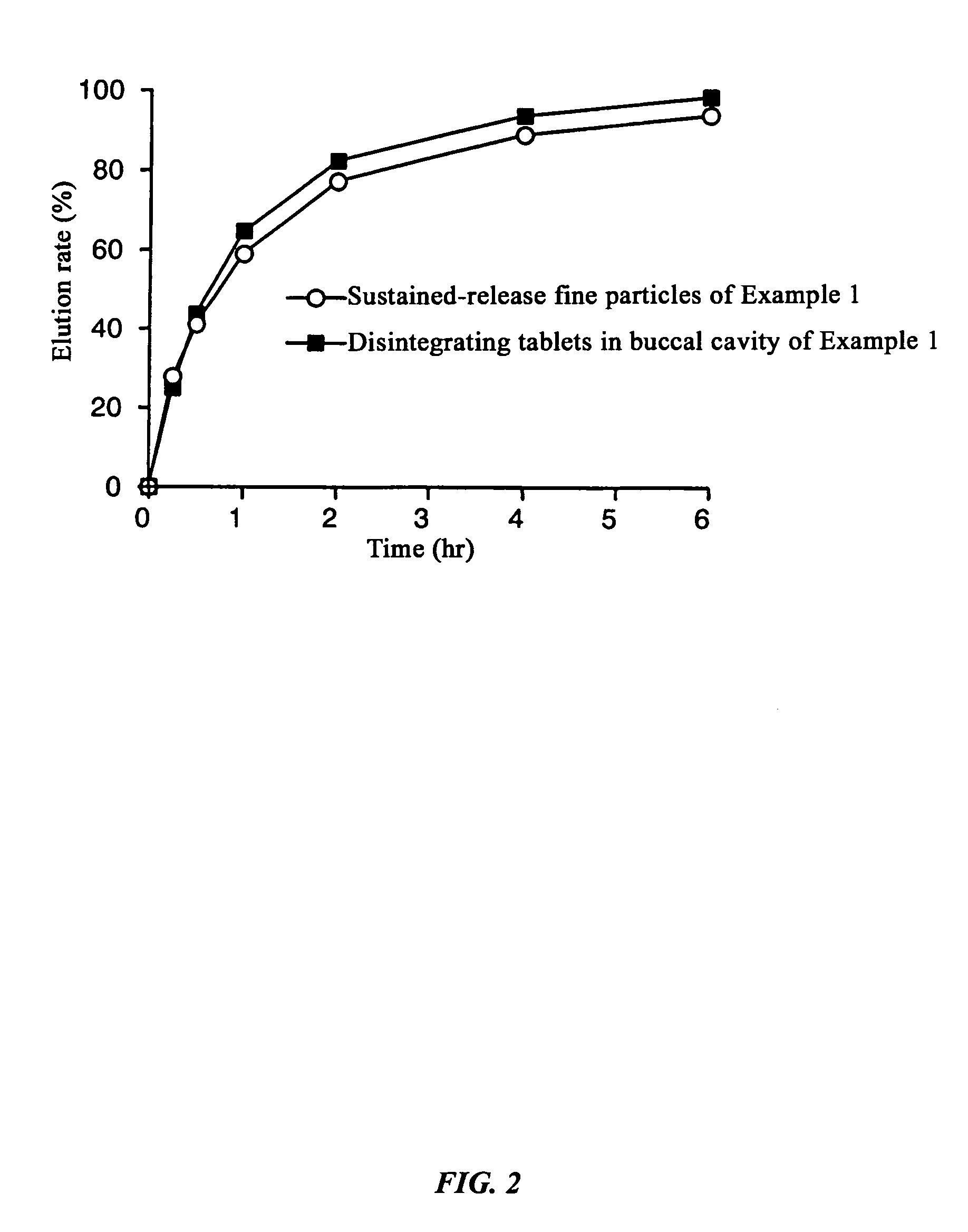
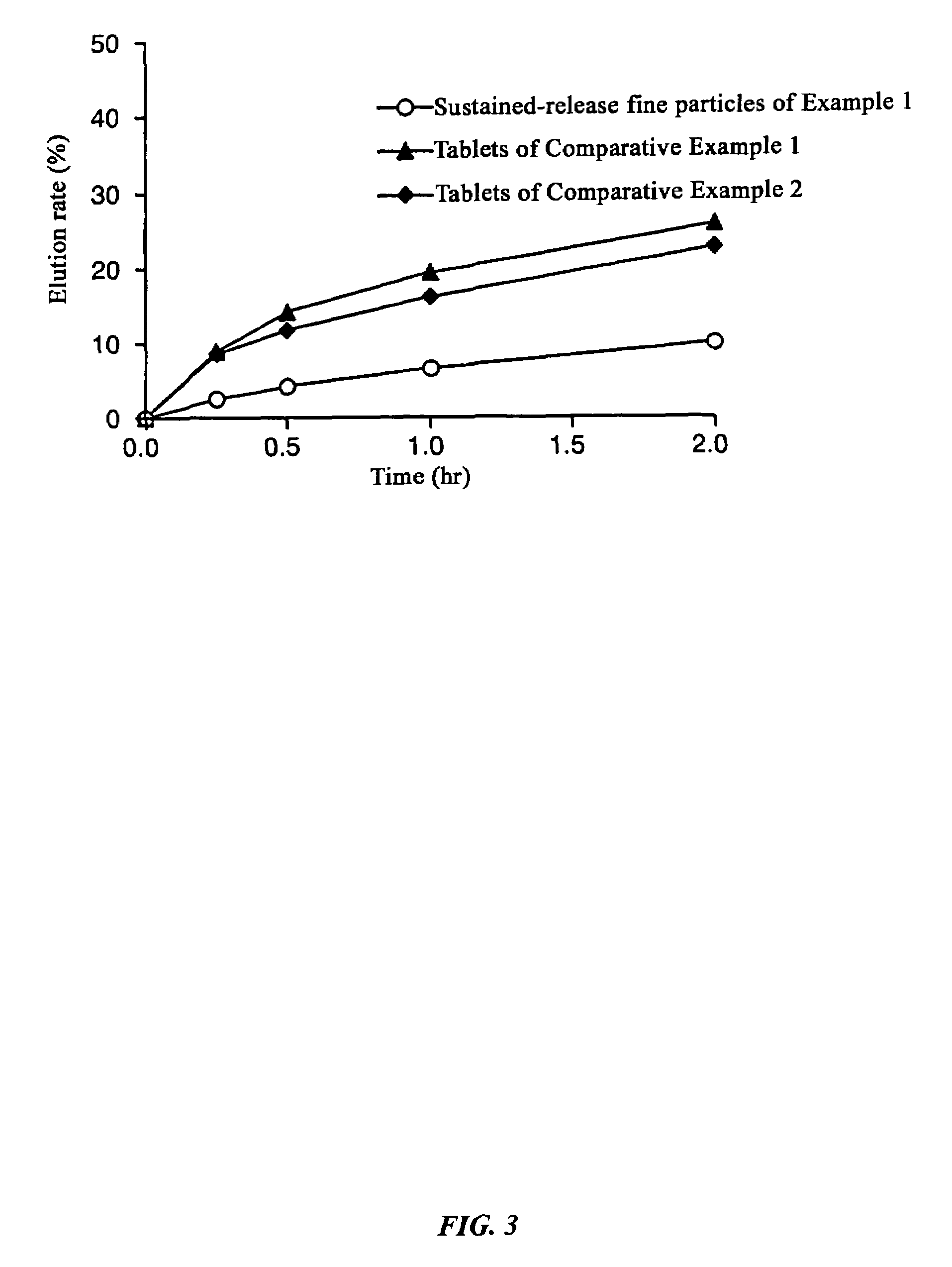
Composition comprises sustained-release fine particles and manufacturing method thereof
InactiveUS7255876B2Good reproducibilityAvoid separationBiocidePharmaceutical non-active ingredientsFiller ExcipientAlcohol sugars
The present invention relates to a composition comprising sustained-release fine particles, characterized in that it contains sustained-release fine particles that can be used in quick-disintegrating tablets in the buccal cavity, one or more fillers selected from the group consisting of sugars or sugar alcohols, and one or more binders for quick-disintegrating tablets in the buccal cavity selected from the group consisting of sugars of high moldability and water-soluble polymer substances, and in that the sustained-release fine particles are granulated with filler and binder for quick-disintegrating tablets in the buccal cavity, and a manufacturing method thereof.
Owner:ASTELLAS PHARMA INC
Pharmaceutical Formulation and Process
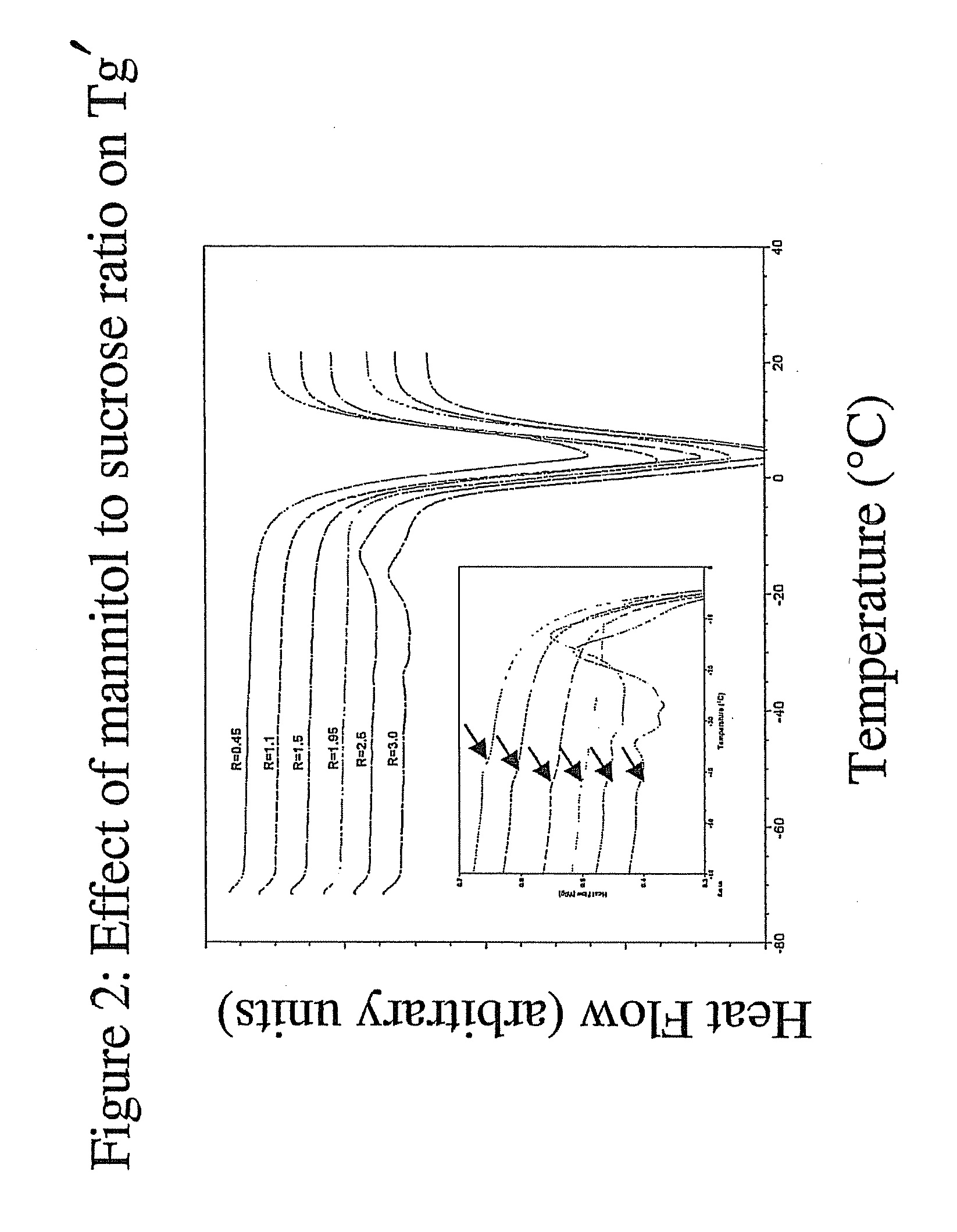
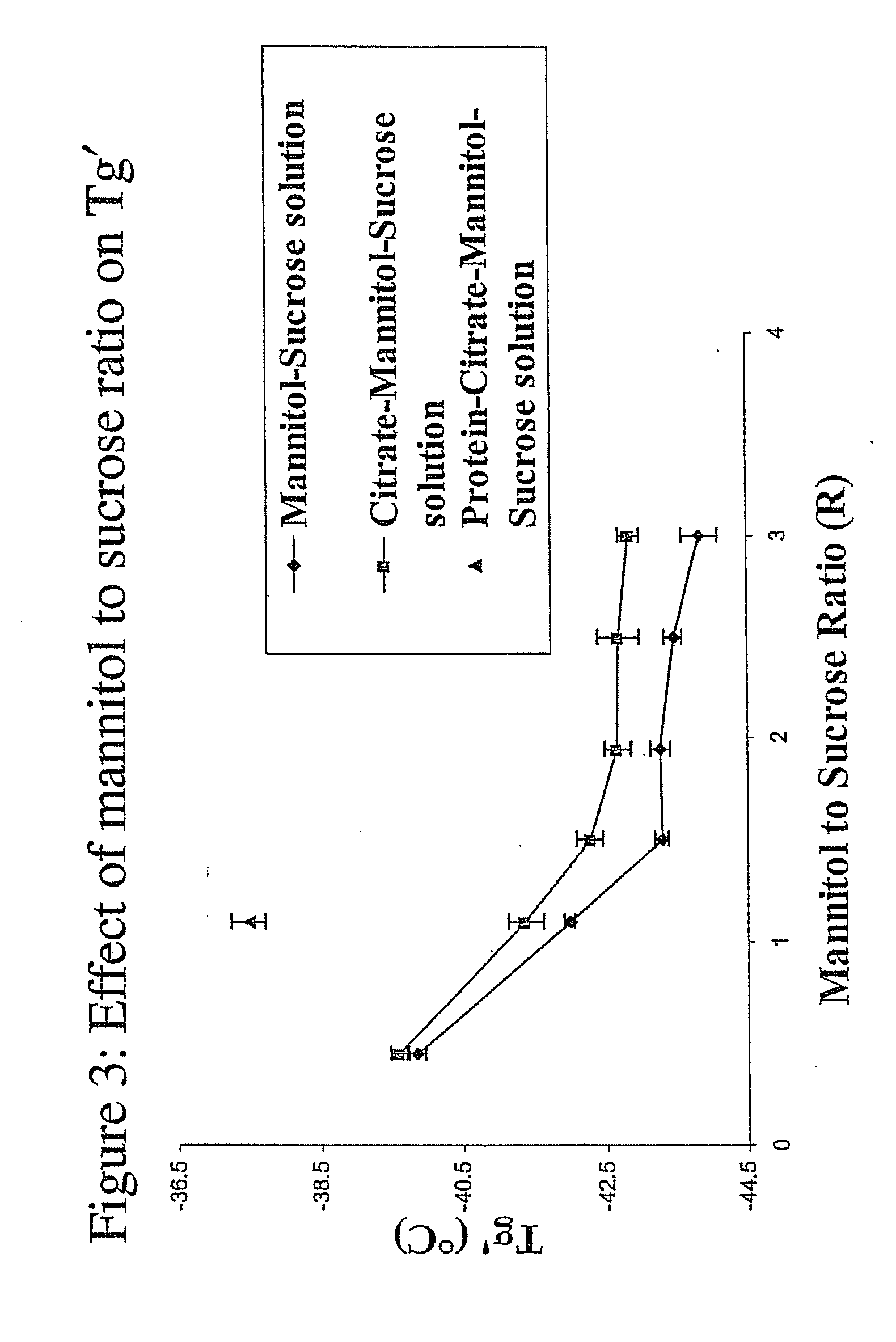
InactiveUS20070196364A1Increased Tg′Less-dramatic effectOrganic active ingredientsBiocideSucroseActive agent
A process for lyophilization or freeze-drying of a pharmaceutical product is provided and a liquid formulation suitable for lyophilization. In particular, a process for lyophilization or freeze-drying a liquid formulation that includes a protein active agent, a bulking agent and a saccharide stabilizing agent is provided. The saccharide to bulking agent ratio and the protein concentration of the formulation are important factors that affect crystallization of the bulking agent during lyophilization and storage as are some processing conditions. In one embodiment, the saccharide is a disaccharide, such as sucrose and the crystalline bulking agent is mannitol. The protein can be an antibody or a non-antibody protein.
Owner:HUMAN GENOME SCI INC
Lutein ester microcapsule powder and preparation method thereof
ActiveCN102389108AInstant highGood self-emulsifying performanceFood preparationAntioxidantFiller Excipient
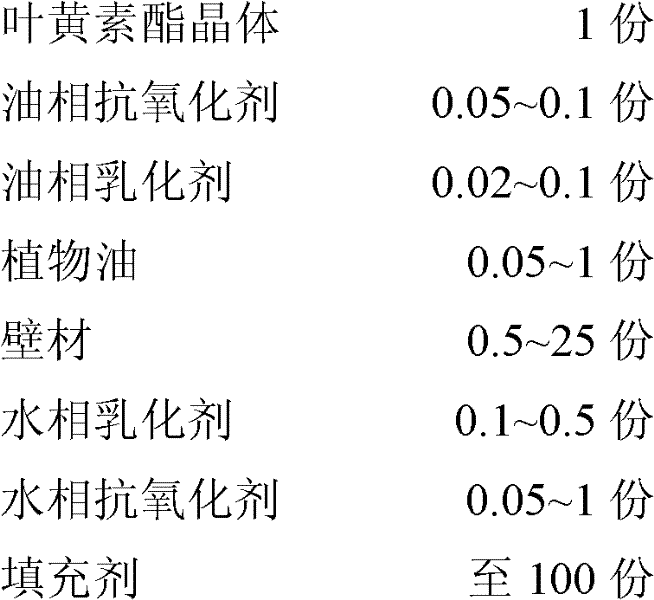
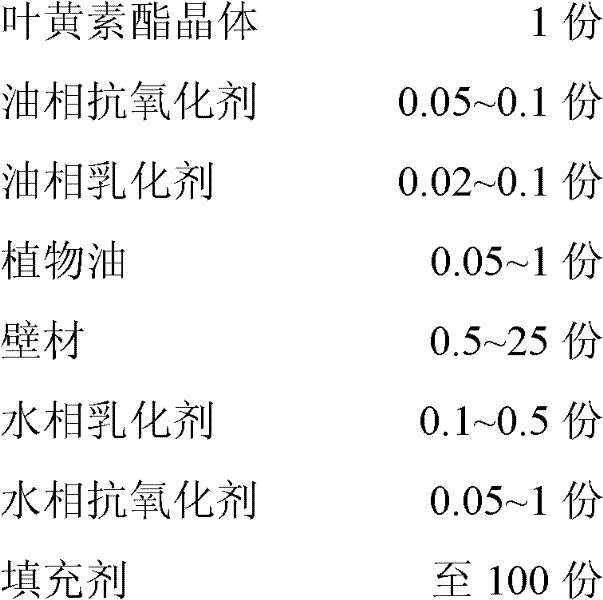
The invention discloses lutein ester microcapsule powder and a preparation method thereof. The preparation method comprises the following steps of: preparing an oil phase solution and a water phase solution respectively, and mixing and emulsifying the oil phase solution and the water phase solution; and non-water raw materials comprise the following components in part by weight: 1 part of lutein ester crystal, 0.05 to 0.1 part of oil phase antioxidant, 0.02 to 0.1 part of oil phase emulsifying agent, 0.05 to 1 part of plant oil, 0.5 to 25 parts of wall material, 0.1 to 0.5 part of water phaseemulsifying agent, 0.05 to 1 part of water phase antioxidant, and the balance of filler. In the preparation method for the lutein ester microcapsule powder, the oil-soluble lutein ester is fully dispersed in cold water in the form of sub nanometer particles, and the product can be self-emulsified in water, and is good in instant capacity and self-emulsification capacity, and high in stability, and has uniform and durable color; and the phenomena of oil floating, precipitation, adhesion to a bottle wall and the like are avoided during application. Organic solvents are not introduced in the powder, so solvent residues are avoided in the product. The production process is environment-friendly, simple and practicable, expensive equipment is not needed, and the microcapsule powder can be industrially produced easily and conveniently.
Owner:INNOBIO CORP LTD
Natural high-potency tabletop sweetener compositions with improved temporal and/or flavor profile, methods for their formulation, and uses
The present invention relates generally to tabletop sweetener compositions comprising non-caloric or low-caloric natural high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different forms of tabletop sweetener compositions comprising at least one non-caloric or low-caloric natural high-potency sweeteners in combination with at least one bulking agent, or at least one sweet taste improving composition, or at least one anti-caking agent, or combinations thereof. The present invention also relates to tabletop compositions and methods that can improve the tastes of non-caloric or low-caloric natural high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the tabletop compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA COLA CO
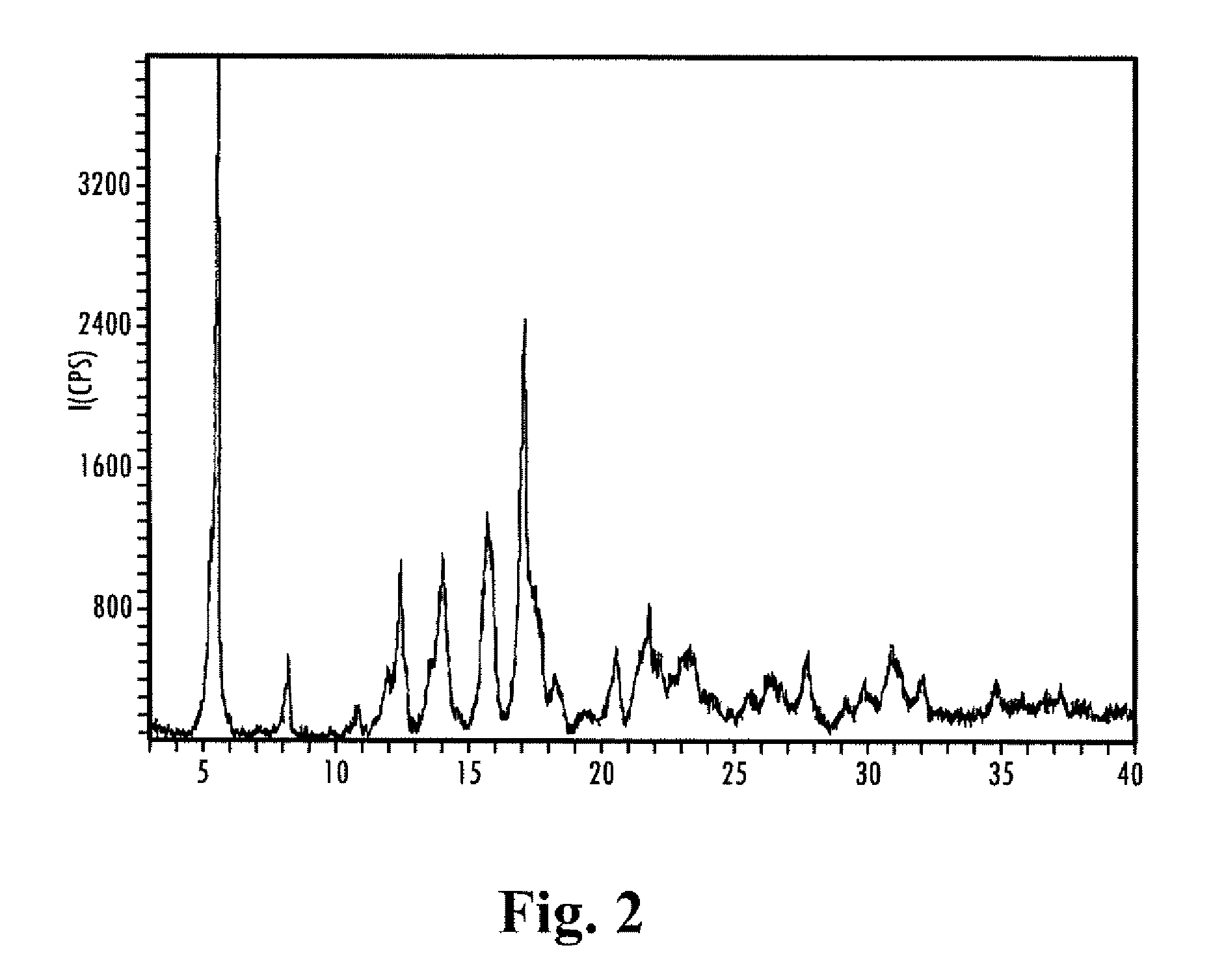
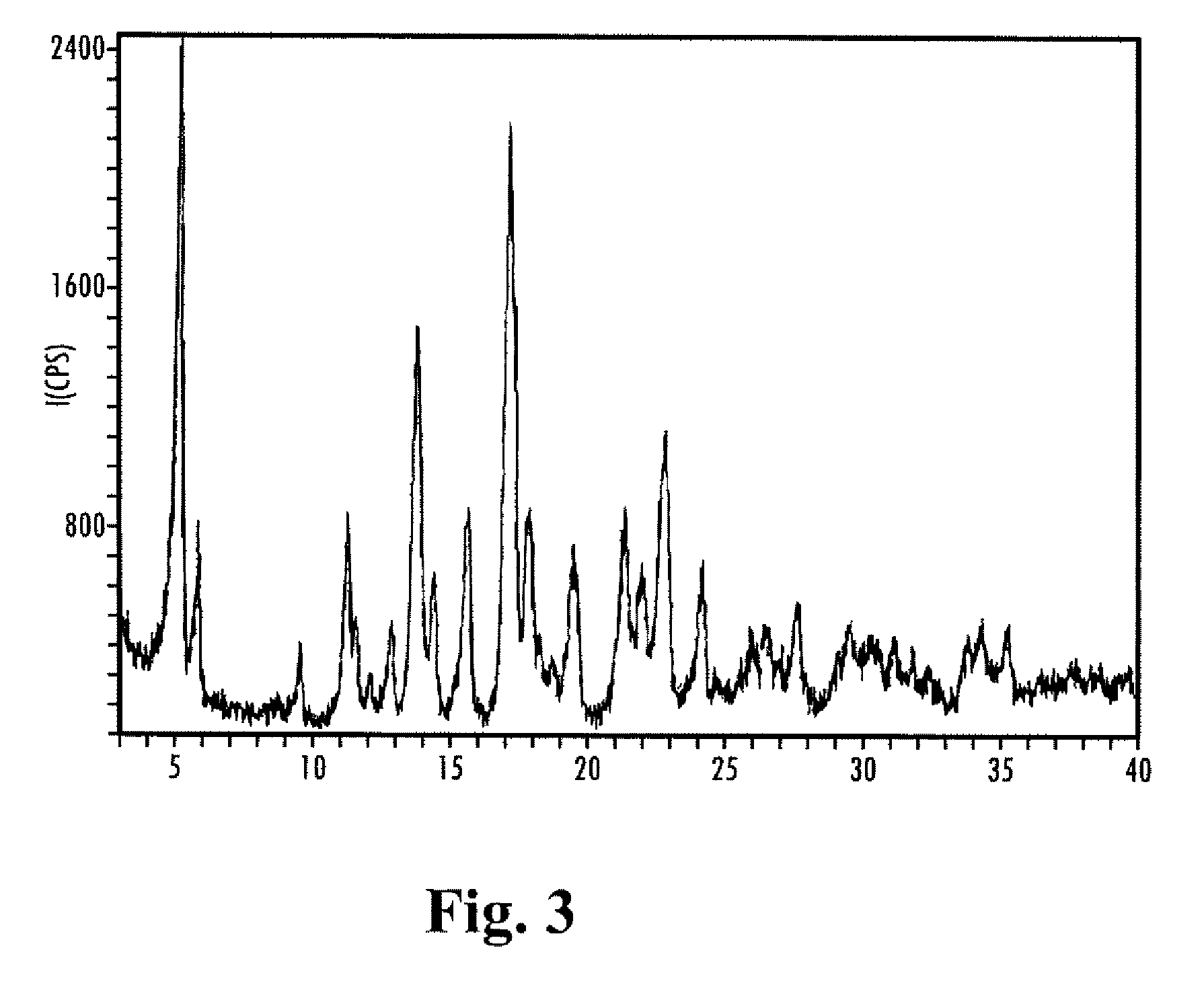
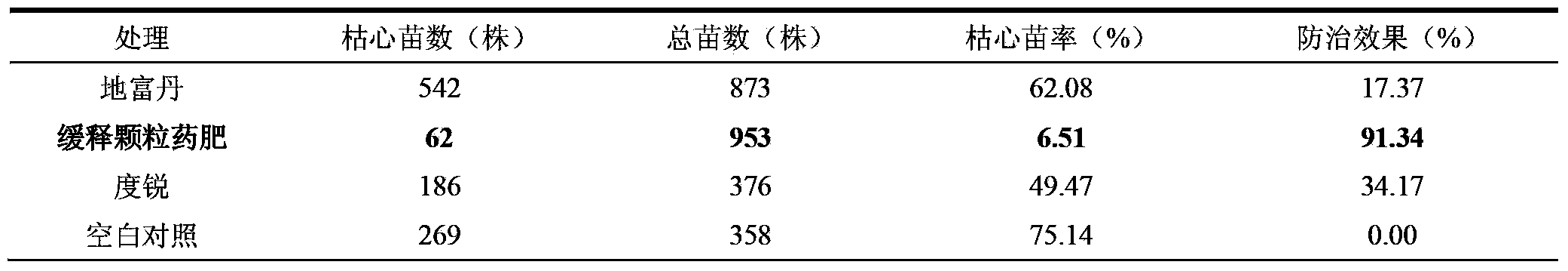
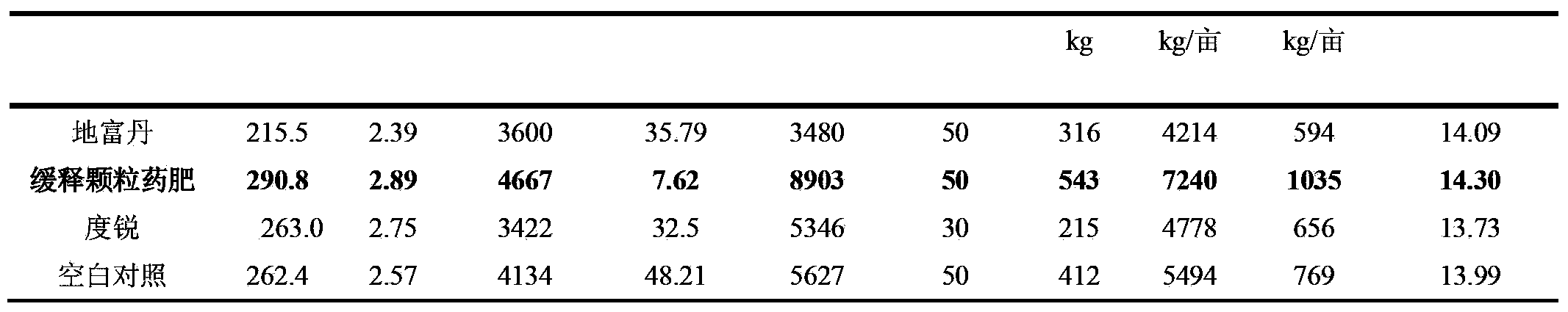
Sugarcane sustained-release granular medicinal fertilizer
ActiveCN103360162AIncrease profitReduce planting costsFertilizer mixturesModified-release granulesFiller Excipient
The invention provides a sugarcane sustained-release granular medicinal fertilizer comprising the components of, by mass: 0.01-3% of a pesticide, 20-50% of fertilizer effective components, 0.01-2% of trace element fertilizer effective components, 0.001-0.1% of beneficial element fertilizer effective components, 0.1-5% of a fertilizer sustained-release agent, and balance of a filling agent. The fertilizer effective components comprise N, P2O5, and K2O. The trace element fertilizer effective component comprises boron, zinc, manganese, molybdenum, copper and iron. The beneficial element fertilizer effective components comprise silicon and selenium. The sugarcane special-purposed sustained-release granular medicinal fertilizer provided by the invention can provide nutrients for sugarcane, and can control pests effectively. With the medicinal fertilizer, application effects of pesticide and fertilizer are improved, labor intensity is saved, and plantation cost is reduced.
Owner:ZHANJIANG SUGARCANE RES CENT GUANGZHOU SUGARCANE RES INST
Tablets immediately disintegrating in the oral cavity
The present invention provides an intraoral quickly disintegrating tablet containing a phosphodiesterase inhibitor having an effect of improving the erectile dysfunction and a method for manufacturing the tablet. The present invention also provides an intraoral quickly disintegrating tablet containing a slightly soluble pharmaceutical agent having an improved solubility and a method for manufacturing the tablet. That is, it is an intraoral quickly disintegrating tablet containing a cyclic GMP phosphodiesterase inhibitor and a saccharide, and a method for manufacturing the tablet. Further, it is a method for manufacturing an intraoral quickly disintegrating tablet, which comprises dissolving a slightly soluble pharmaceutical agent in an organic solvent or an aqueous organic solvent together with a surfactant and / or a water-soluble polymer, coating the solution on a filler or granulating it with a filler to obtain molded products, mixing a saccharide with them, adding an organic solvent, water or an aqueous organic solvent thereto, followed by kneading, and subjecting it to a compression-molding.
Owner:EISIA R&D MANAGEMENT CO LTD
Dry mix for a low-calorie slush
A dry mix for producing a low-calorie slush when combined with an aqueous fluid and ice in an electric blender. The dry mix contains 62-87% bulking agent, such as maltodextrin, 2-20% food acid, 3-15% low-viscosity hydrocolloid, 1-8% non-gelling hydrocolloid, one or more intensive sweeteners and optionally a carbonating salt. Preferably, the mix is sugar-free and produces a slush, when combined with water and ice, having a calorie content of less than 60 calories per 8-ounce serving.
Owner:KRAFT FOODS INC
Mouth Dissolving Pharmaceutical Composition and Process for Preparing the Same
ActiveUS20080317853A1Maintain good propertiesFast oral disintegration timeBiocidePill deliveryMedicineAdditive ingredient
Disclosed herein is a orally disintegrating and / or dissolving oral pharmaceutical composition, comprising one or more active pharmaceutical ingredients, one or more fillers having particle size of 100 microns or above, a high and desirable amount of silicon dioxide, one or more disintegrating agents, optionally effervescent couple, wherein said composition has good organoleptic properties like desired mouth feel and fast oral disintegration time.
Owner:JUBILANT GENERICS
Method for preparing solid delivery system for encapsulated and non-encapsulated pharmaceuticals
InactiveUS6541025B1Maintain good propertiesMinimized surface tensionBiocideSolution deliveryLipid formationFiller Excipient
The present invention is an oral drug delivery system for delivering unpalatable pharmaceuticals, wherein the pharmaceutical delivery system comprises a lipid, dry particles including at least one pharmaceutical and at least one filler, and a surfactant, wherein the dry particles are continuously coated by the lipid and form a suspension with the lipid, making the pharmaceutical more palatable.
Owner:SHEARKERSHMAN LAB
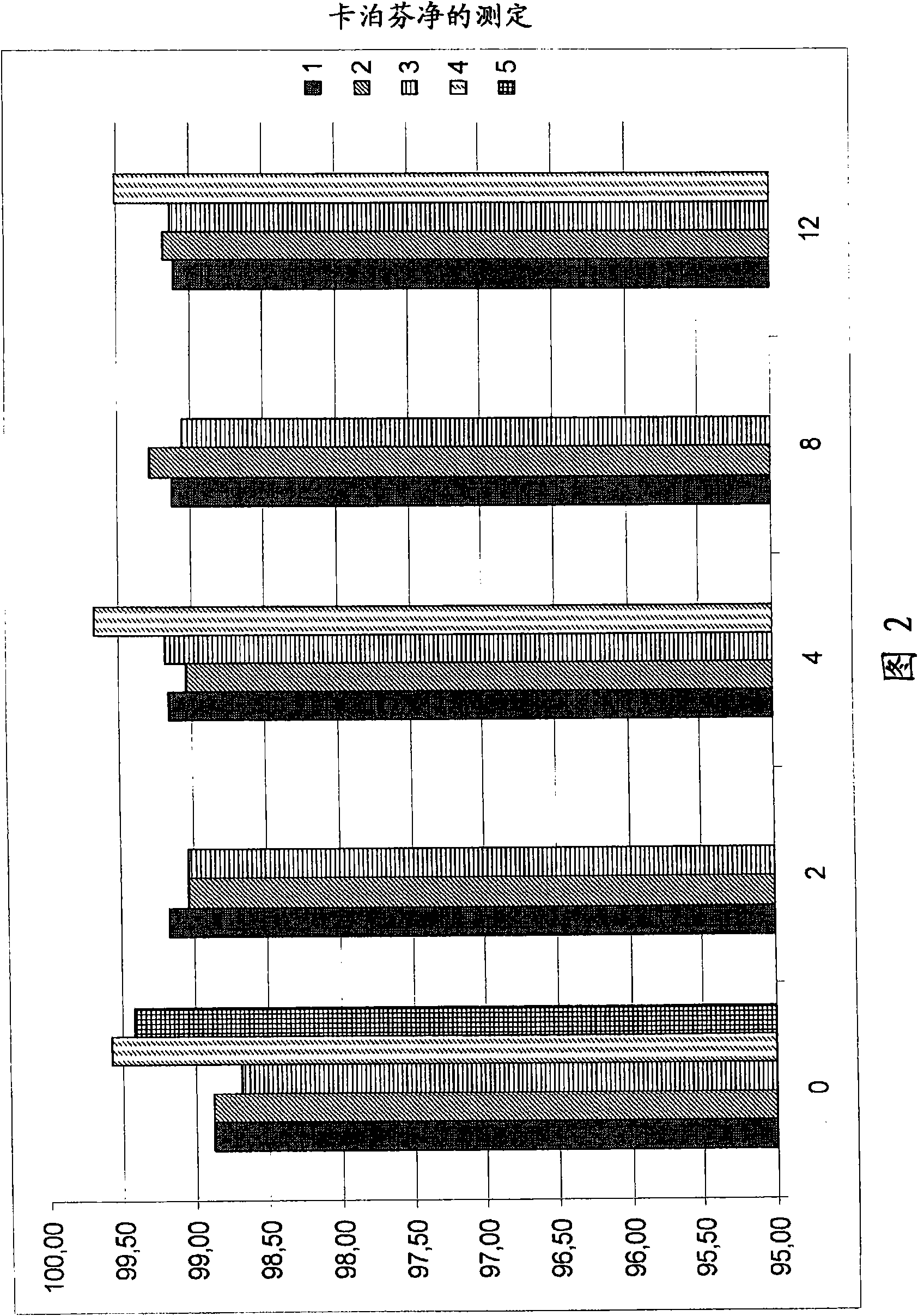
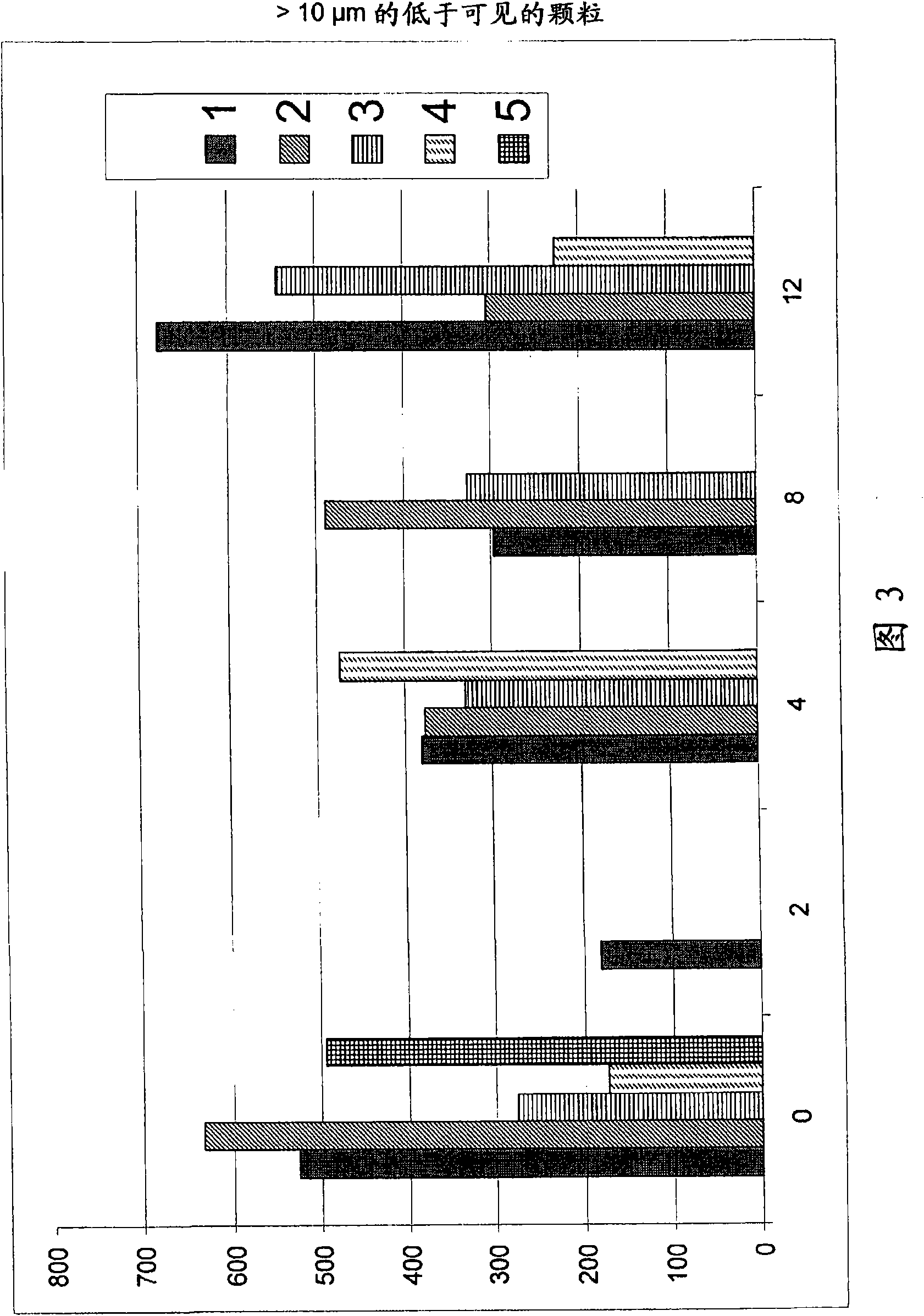
Caspofungin formulations
The present invention relates to pharmaceutical compositions comprising a pharmaceutically acceptable salt of caspofungin as active ingredient being useful for the prevention and / or treatment of fungal infections. Said compositions additionally comprise specific bulking agents and small amounts or no amounts of an additional pH modifier and may be in a liquid or solid form, e.g. may be lyophilized compositions. Said compositions show good stability and reduced amounts of sub-visible particulate matter formed in solutions which are reconstituted from the lyophilized product.
Owner:SANDOZ AG
Aqueous glass frosting powder chemical composition and preparation method thereof
The invention discloses an aqueous glass frosting powder chemical composition containing a mixed material of an inorganic fluorine compound capable of generating fluorine ions in water, a solid acid compound capable of dissolving in water, an assistant and a filling agent, wherein the mixed material is doped with at least one surfactant; the adding amount of the inorganic fluorine compound is 20 to 90 percent of the total weight percentage of the frosting powder; the adding amount of the solid acid compound is 10 to 60 percent of the total weight percentage of the frosting powder; the adding amount of the filling agent is 1 to 30 percent of the total weight percentage of the frosting powder; the adding amount of the surfactant is 0.1 to 10 percent of the total weight percentage of the frosting powder; and during use, the water is 20 to 100 percent of the total weight percentage of the frosting powder to prepare frosting powder liquid. The technical scheme can avoid acid pollution of the production environment and natural environment because of the inevitable strong acid medium such as sulphuric acid and hydrochloric acid when frosting a glass product by using the prior glass frosting liquid.
Owner:上海多林玻璃技术有限公司
Krill oil microcapsual powder and its preparation method
The invention discloses a krill oil microcapsual powder and its preparation method. The preparation method comprises steps of: preparation of an oil phase solution and an aqueous phase solution, mixing and emulsification of the two solutions, homogenizing, drying and the like. The krill oil microcapsual powder is prepared from the following raw materials of: by weight, 0.001-90% of krill oil, 0.5-90% of sodium caseinate and 0-99% of a filler. In addition, 0.001-10 wt% of an oil phase antioxidant, 0.001-5 wt% of an aqueous phase antioxidant, 0.001-5 wt% of an aqueous stabilizer and 0.001-5 wt% of an anti-caking agent can also be added. The krill oil microcapsual powder has high content of the encapsuled krill oil and has good stability and fluidity. After rehydration, krill oil can be dispersed into water completely in the form of particles (nm); and krill oil is stable if stored for a long time, and has no phenomenon such as floating oil, precipitation, adhesion to bottles and the like. therefore, the application range of krill oil is broadened, and krill oil is applied to fields such as dry mixing, drinks, dairy products, bakery, tablets and the like.
Owner:辽渔集团有限公司
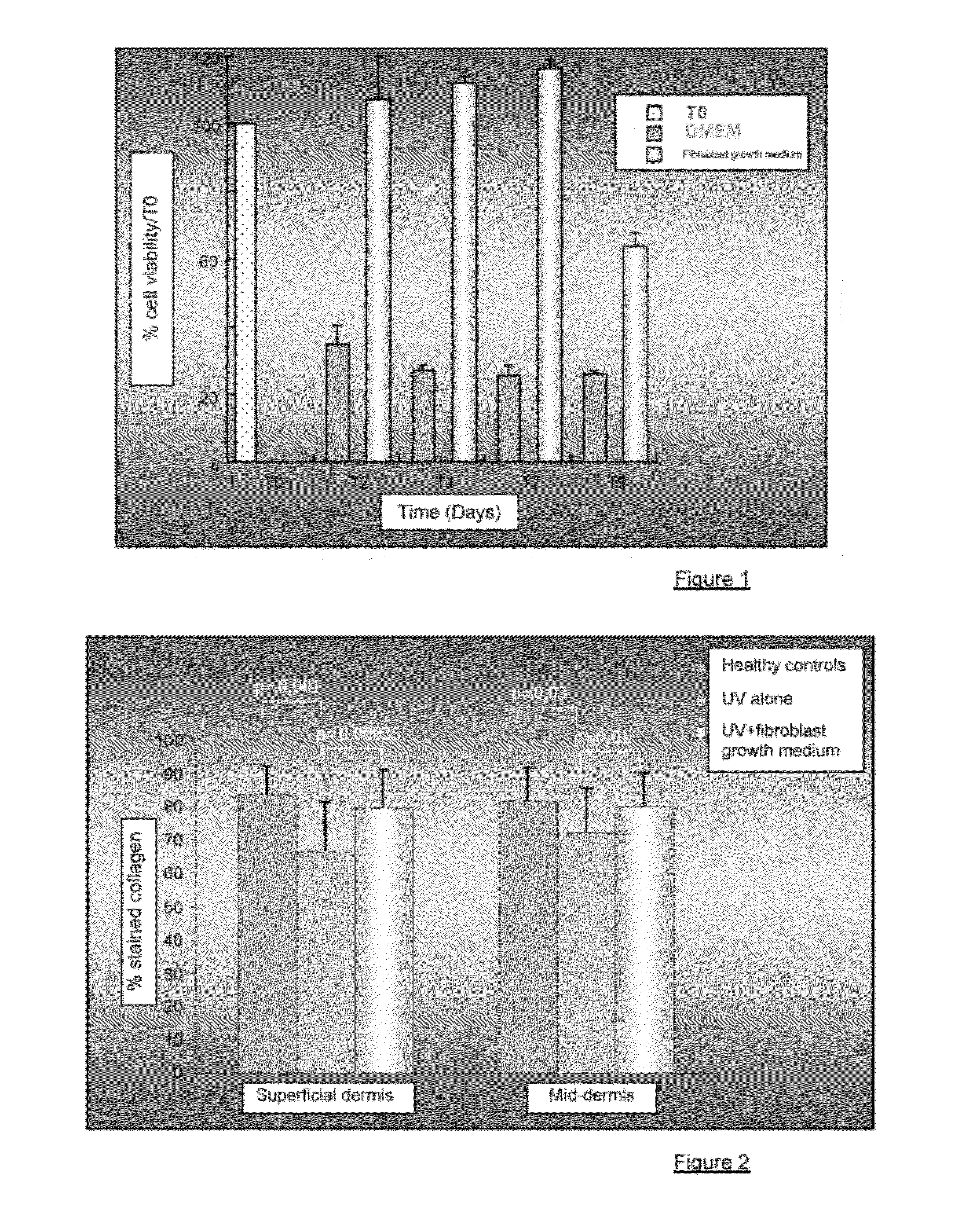
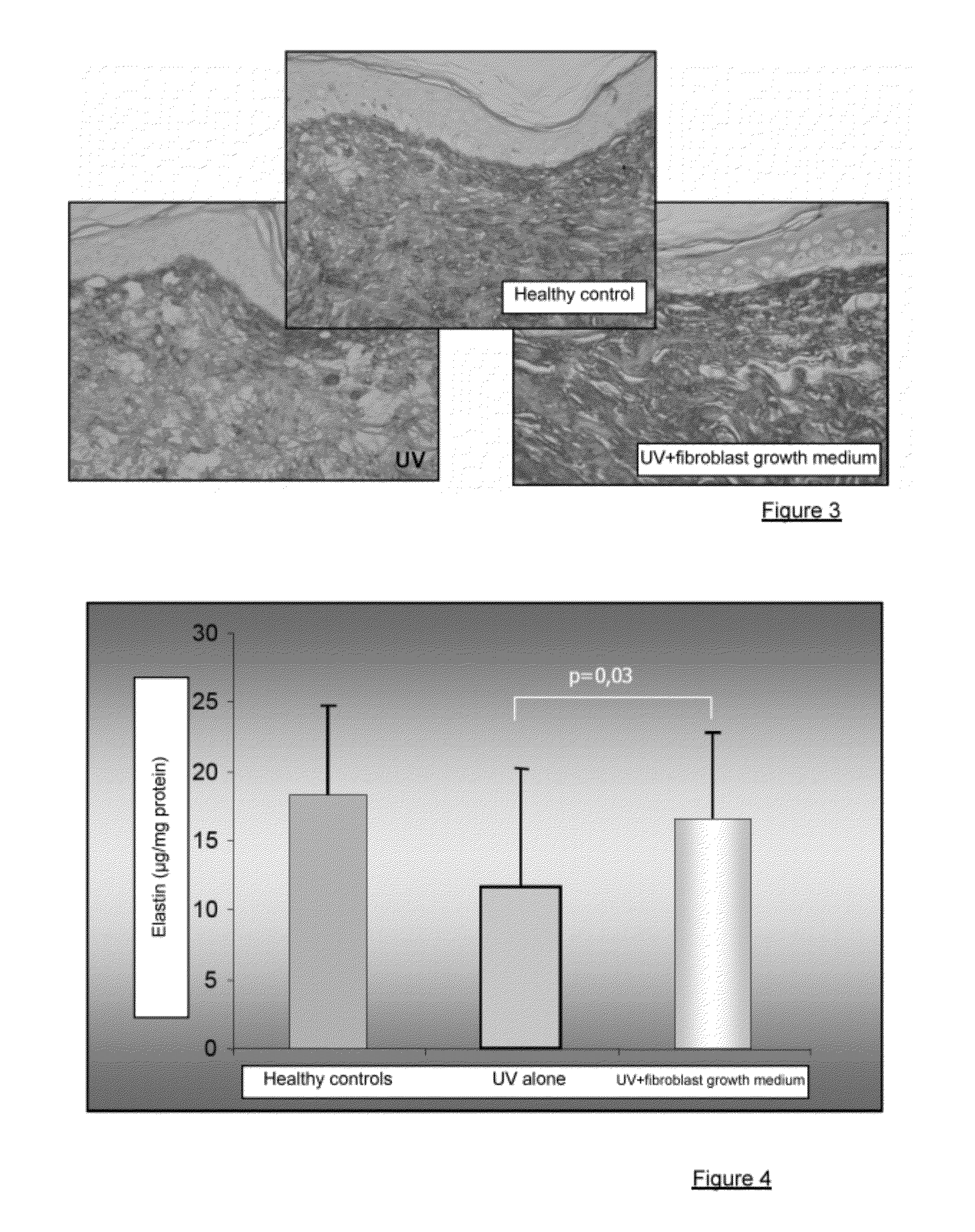
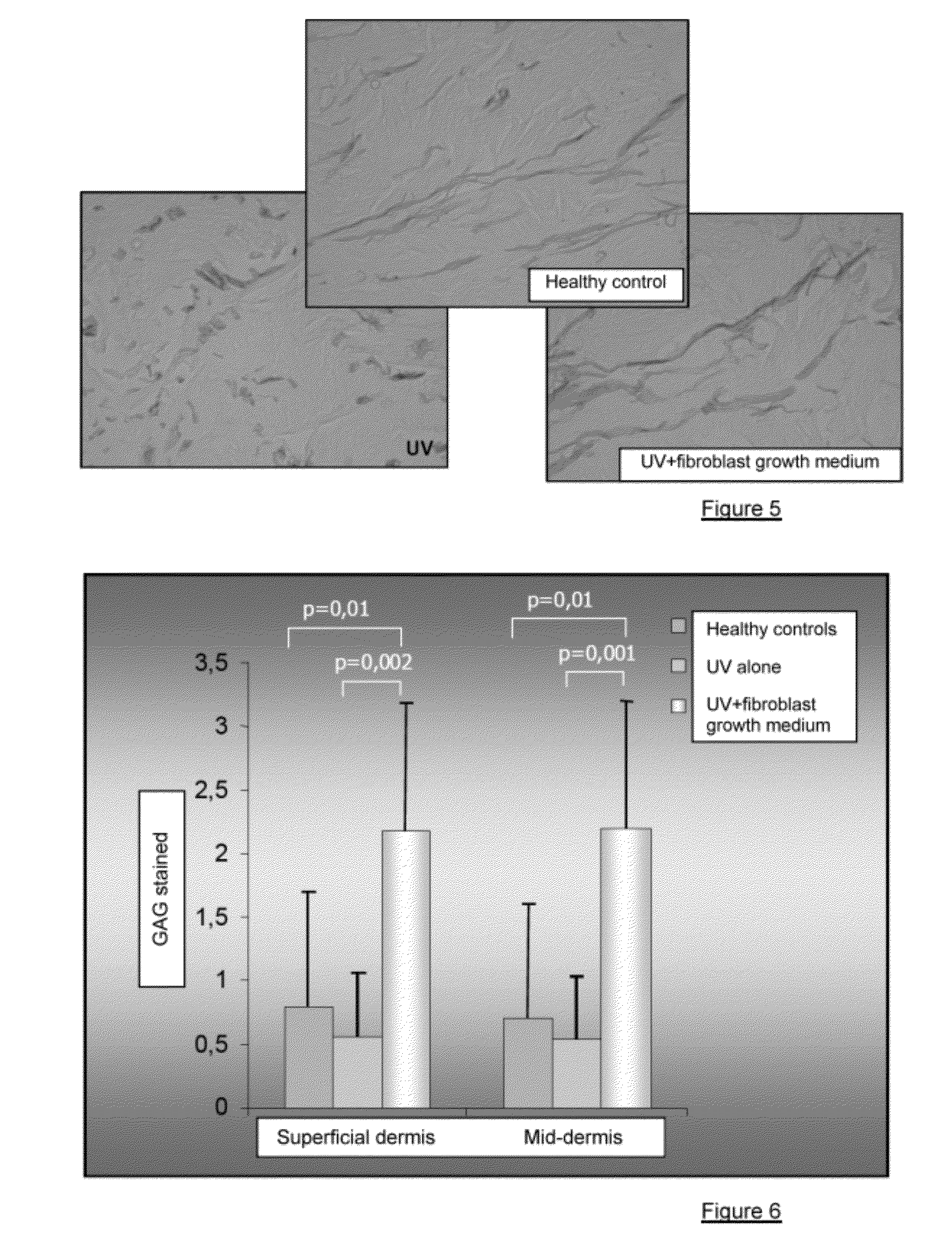
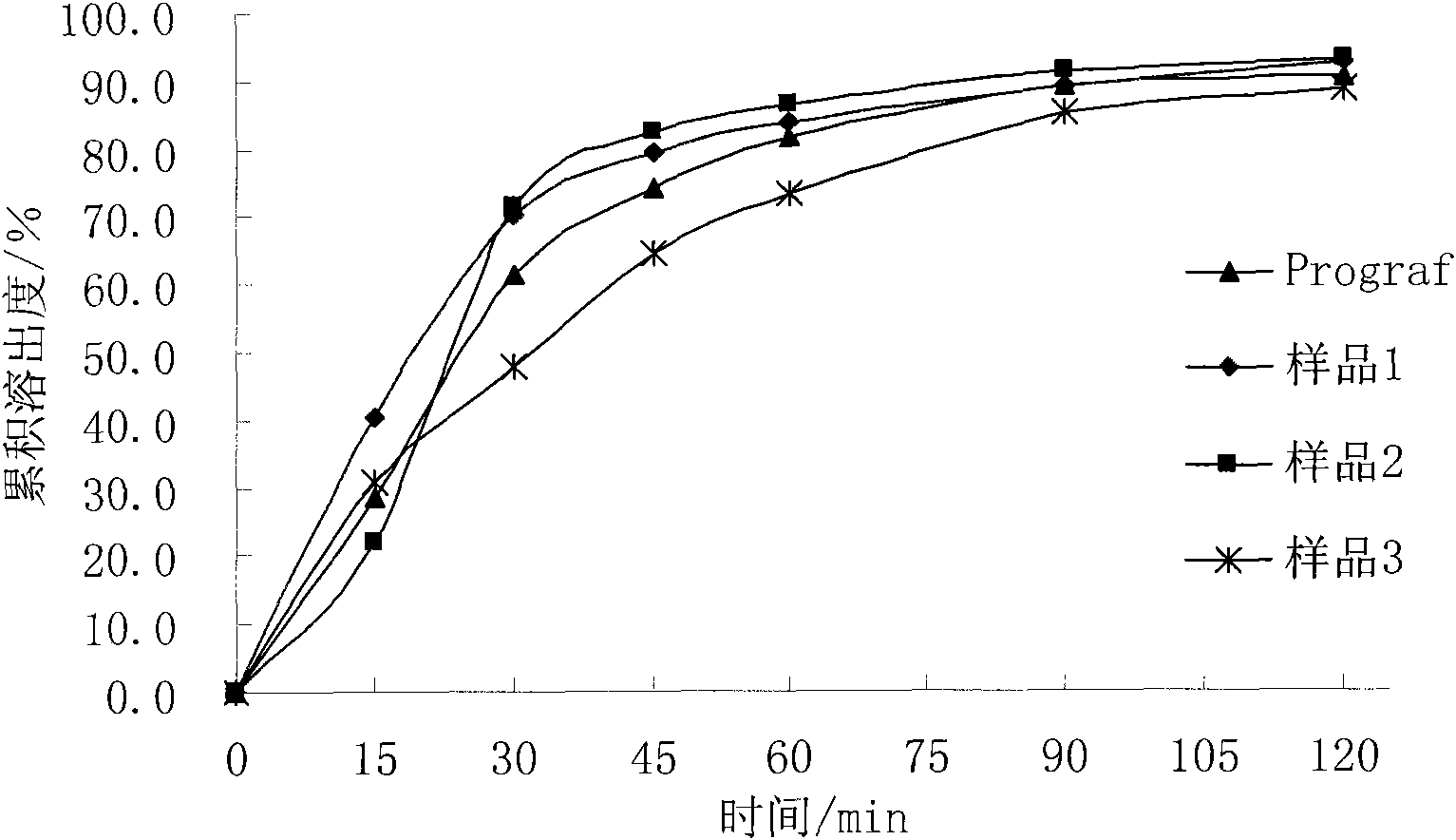
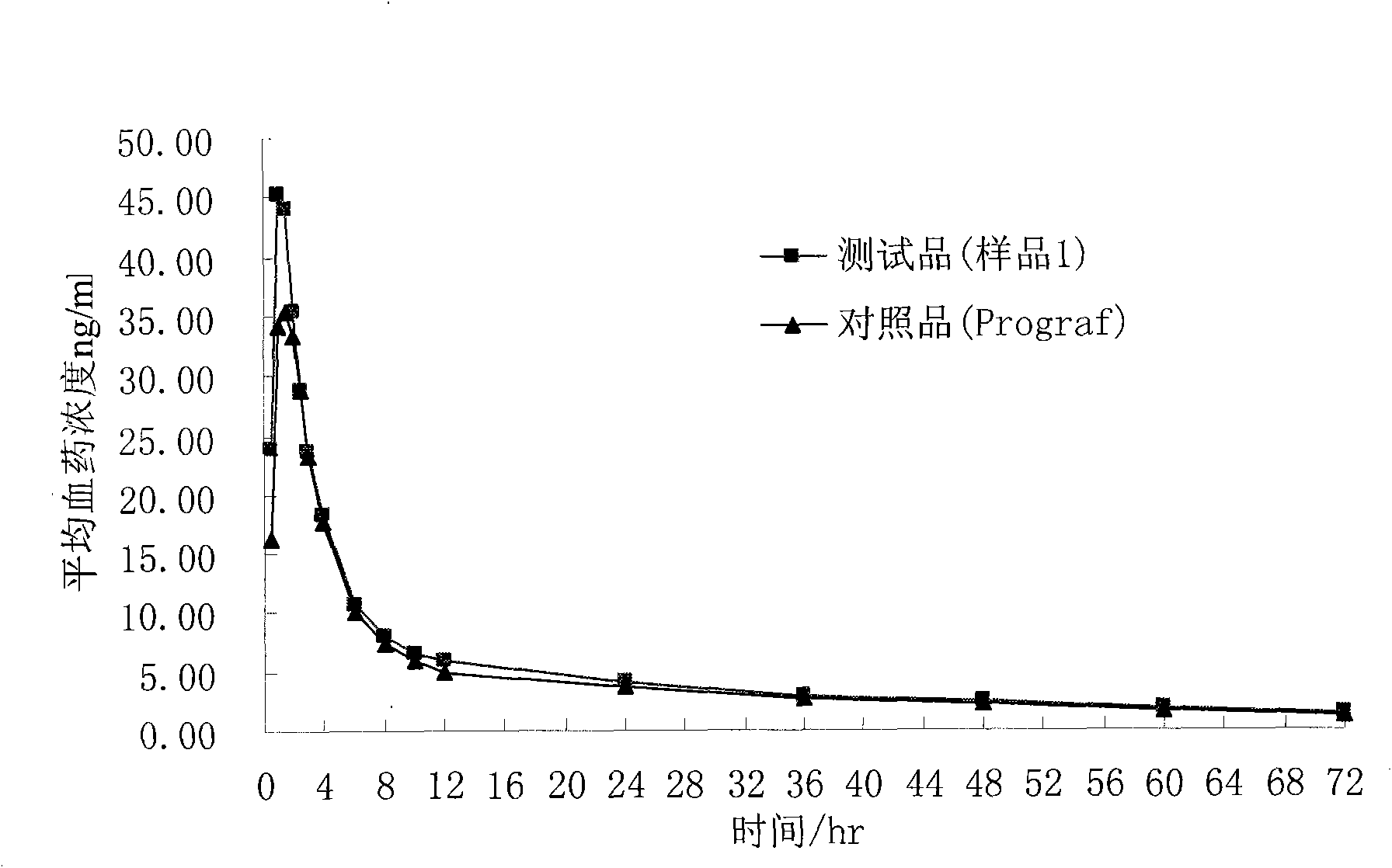
Injectable composition combining a filling agent and a fibroblast growth medium
ActiveUS20120121534A1Improve skin appearanceReduce riskOrganic active ingredientsBiocideFiller ExcipientCell culture media
The invention relates to a composition that can be subcutaneously or intradermally injected, comprising: a filling agent; and a fibroblast growth medium.
Owner:THOREL JEAN NOEL
Composition of composite potassium peroxymonosulfate disinfection tablet and preparation method thereof
ActiveCN101828550AEasy to useDisintegrates quicklyBiocideDisinfectantsSodium bicarbonateFiller Excipient
The invention discloses acomposite potassium peroxymonosulfate disinfection tablet which comprises 10.0-70.0 parts by weight of potassium monopersulfate, 1.0-65.0 parts by weight of fillers, 1.0-20.0 parts by weight of adhesives and 0.01-10.0 parts by weight of lubricant, wherein the fillers is formed by one or more then one of sodium sulfate, sodium chloride, potassium chloride, sodium carbonate, sodium bicarbonate, phosphate, inorganic acid, organic acid, starch, dextrin, glucose, solid emulsifier, xylitol and a metal ion complexing agent, the adhesives comprise cellulose, polyvinylpyrrolidone and gelatin; the lubricant is formed by one or more than one of magnesium stearate, talcum powder, micro-powder silica gel, sodium benzoate and polyethylene glycol; in addition, sodium percarbonate, calcium percarbonate, sodium perborate or calcium peroxide 1-2 are added. The invention is convenient to use, has the advantages of quick disintegration and dissolution, can significantly increase the effect of the disinfection tablet and can adjust pH value.
Owner:镇江威特药业有限责任公司 +1
Calcium sulfate whisker reinforced resin grinding wheel and preparation method thereof
InactiveCN104669131AReasonable designInnovative designOther chemical processesAbrasion apparatusFiberEpoxy
The invention discloses a calcium sulfate whisker reinforced resin grinding wheel, which comprises a grinding wheel body, wherein the grinding wheel body is formed by adhering and curing a grinding material, a reinforced mesh, a binding agent and a filling agent according to the following weight proportions; the grinding material comprises 50-60 parts of 20# brown corundum, 20-30 parts of 24# white corundum and 10-20 parts of silicon carbide; the reinforced mesh is a glass fiber and / or carbon fiber reinforced grinding wheel mesh; the binding agent comprises 5-7 parts of resin liquid and / or 10-12 parts of phenolic resin powder; the filling agent comprises 3-5 pars of calcium sulfate whisker; the resin liquid is prepared by phenolic resin liquid and epoxy resin according to the mass proportion of 8:(2-3); the calcium sulfate whisker is fiber-shaped single crystal, the diameter is 1-4mum, and the length is 20-80mum. The calcium sulfate whisker reinforced resin grinding wheel has the advantages that the grinding ratio is good, the safety speed is high, the impact-resistant property is strong, and the pollution to the working environment is little.
Owner:JIANGXI FENGZHU NEW MATERIAL TECH
Medicinal or health care composition containing natural antimicrobials
The invention provides a medical or health-care compound and a preparation method. The compound contains at least one natural anti-bacterial agent which is separated for plants and is the soluble salt or ester formed through the extraction of the non-soluble anti-bacterial substance from plants. Further, the compound of the invention can also contain inflammation-diminishing, detumescence and anti-oxidation ingredients, as well as the preparation type auxiliary substances, Chinese medicine padding agent, etc, which are produced into a series of medicines or health-care medicines which restrain the anaerobic bacteria and effectively prevent the oxidation or aging of the human body cells. The compound of the invention can be produced into various forms, not only confined in the liquid form (for example the orally taken liquid), but also including spraying agent, liquid spraying agent, pills( e.g. buccal tablets), capsules, toothpaste, mouth clearing, etc. Moreover, the invention can be used for the health care for mouth, heart, gastrointestinal tract and joint or the treatment of the related diseases.
Owner:GUANGXI HEBABIZ PHARM TECH CO LTD
Composition containing water-insoluble high-activity drug and preparation method thereof
ActiveCN101537184ASolving DissolutionSolve the two major problems of dustOrganic active ingredientsPowder deliveryOrganic solventWater insoluble
The invention discloses a composition containing a water-insoluble high-activity drug and a preparation method thereof. The water-insoluble high-activity drug is dissolved in an organic solvent containing a solid dispersion carrier, evenly sprayed on the surface of filler or blank pellets by adopting a fluidized bed and dried. The invention realizes the one-step completion of preparing the solid dispersion of the water-insoluble high-activity drug, pelleting and drying, has simple and convenient process, and no dust pollution, and effectively avoids the adverse influence of the high-activity drug on the health of production staff. The granules prepared by the method has even granularity, and good liquidity and compression formability, and can be further prepared into oral solid preparation. The composition prepared by the method has good drug dissolvability, and high oral absorption and bioavailability.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA +1
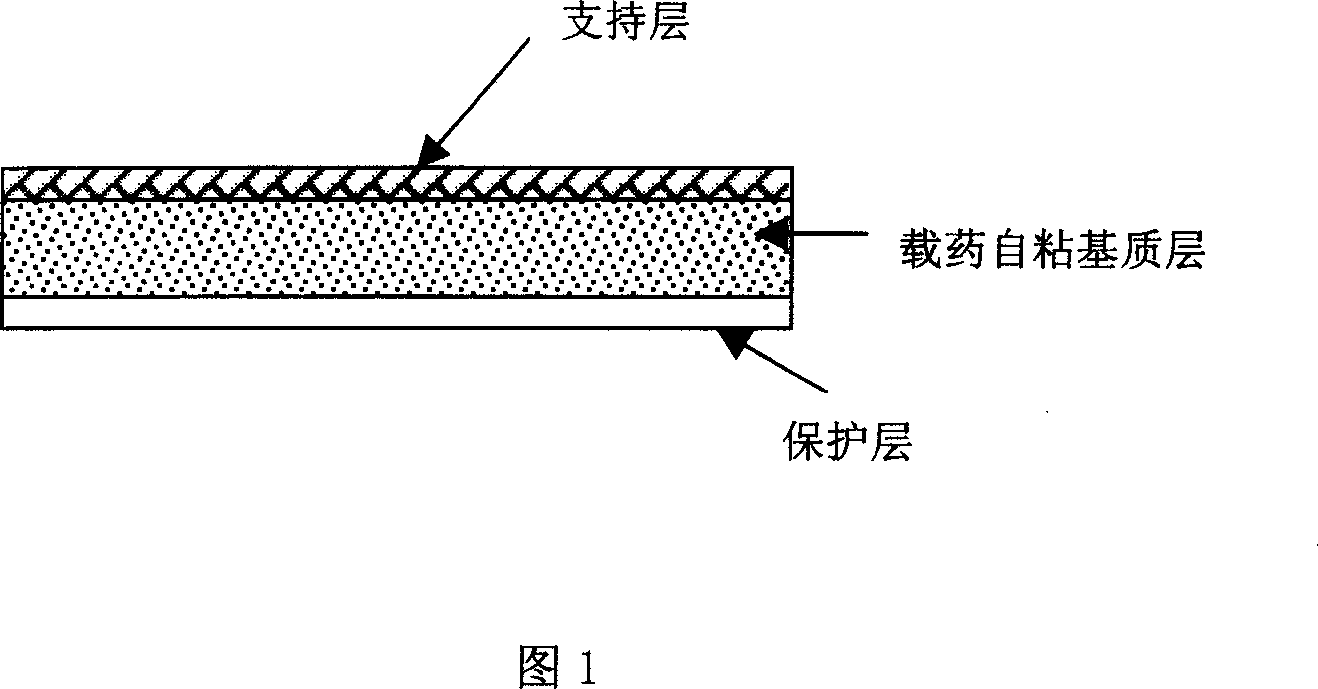
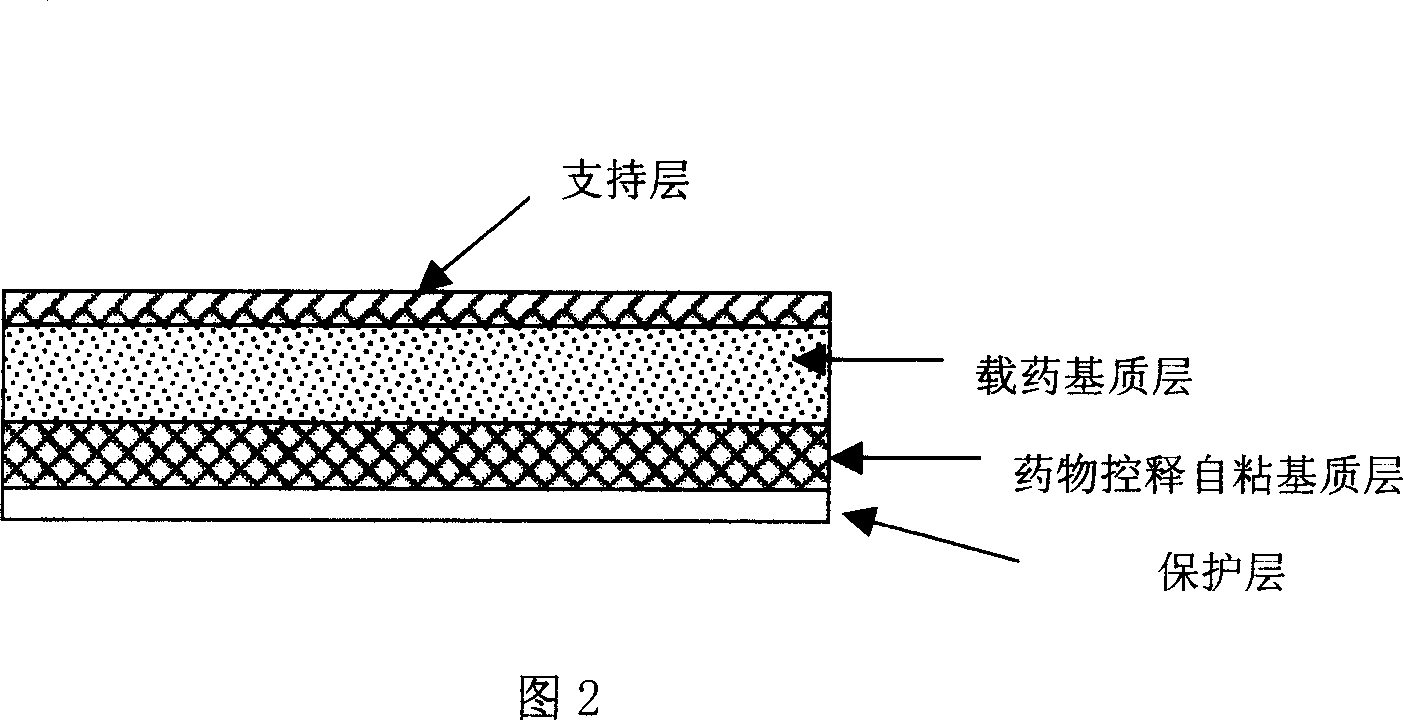
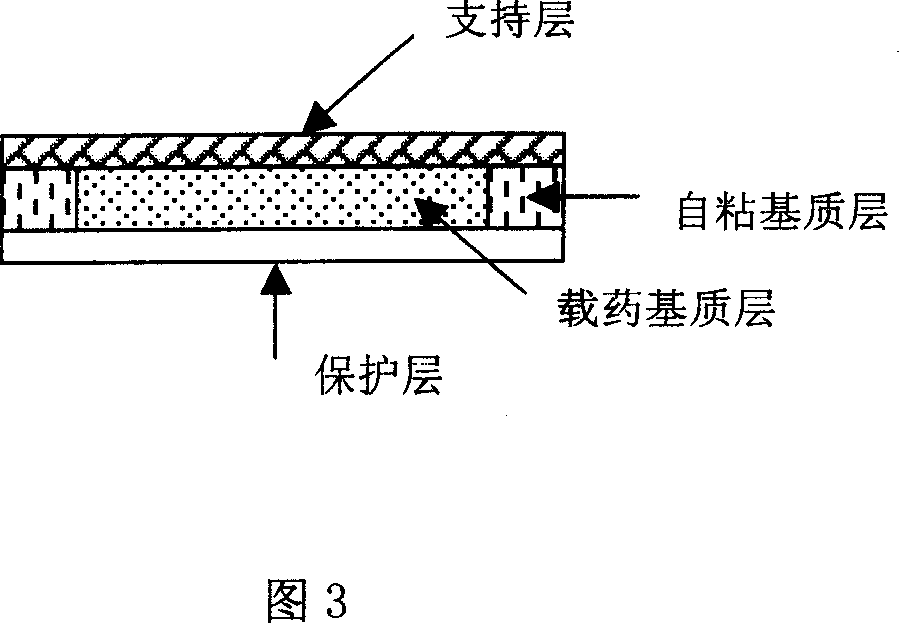
Rasagiline transparent patch for curing and preventing neurological diseases and the preparing method thereof
The present invention relates to one kind of transdermal rasagiline medicine plaster for preventing and treating neurological diseases and its preparation process. The transdermal rasagiline medicine plaster includes one support layer without chemical reaction with the matrix components, one matrix layer containing rasagiline or its pharmaceutically acceptable salt, and operate protecting layer being torn off before the plaster is used. It features the matrix layer with polymer as the basic material, adhering system with inorganic or organic filler, stored rasagiline and matter(s) to promote the transdermal absorption of rasagiline.
Owner:CHONGQING PHARMA RES INST +1
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
Mouth dissolving pharmaceutical composition and process for preparing the same
InactiveUS8048449B2Improved orally disintegrating and dissolvingMaintain good propertiesBiocidePill deliveryAdditive ingredientMedicine
Disclosed herein is a orally disintegrating and / or dissolving oral pharmaceutical composition, comprising one or more active pharmaceutical ingredients, one or more fillers having particle size of 100 microns or above, a high and desirable amount of silicon dioxide, one or more disintegrating agents, optionally effervescent couple, wherein said composition has good organoleptic properties like desired mouth feel and fast oral disintegration time.
Owner:JUBILANT GENERICS
Process for preparing a rapidly dispersing solid drug dosage form
InactiveUS7939105B2Minimizing any tendencyGood contactPowder deliveryPeptide/protein ingredientsParticulatesFiller Excipient
Rapidly dispersing solid dry therapeutic dosage form comprised of a water insoluble compound existing as a nanometer or micrometer particulate solid which is surface stabilized by the presence of at least one phospholipid, the particulate solid being dispersed throughout a bulking matrix. When the dosage form is introduced into an aqueous environment the bulking matrix is substantially completely dissolves within less than 2 minutes thereby releasing the water insoluble particulate solid in an unaggregated and / or unagglomerated state. The matrix is composed of a water insoluble substance or therapeutically useful water insoluble or poorly water soluble compound, a phospholipid and optionally also at least one non-ionic, anionic, cationic or amphipathic surfactant, together with a matrix or bulking agent and if needed a release agent. The volume weighted mean particle size of the water insoluble particle is 5 micrometers or less.
Owner:JAGOTEC AG
Preparation method of highly-stable and cold water-soluble natural astaxanthin microcapsule preparation
ActiveCN105596314AHigh embedding rateOvercoming poor pressure resistanceCosmetic preparationsOrganic active ingredientsFluidized bed dryingAntioxidant
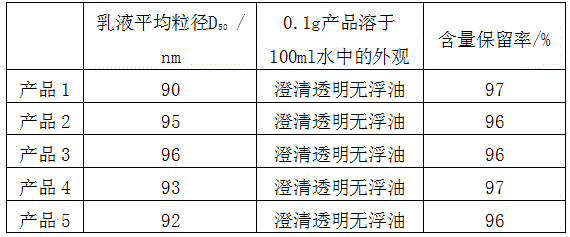
The invention relates to a preparation method of a highly-stable and cold water-soluble natural astaxanthin microcapsule preparation. The method concretely comprises the following steps: uniformly mixing astaxanthin oil, an oil-soluble antioxidant and a water-soluble emulsifier under a heating condition to prepare an oil phase; adding a wall material, a water-soluble antioxidant and a filler to purified water, stirring and dissolving above added materials, and adjusting the pH value of the obtained solution to prepare a water phase; and adding the oil phase to the water phase, uniformly stirring the water phase and the oil phase, grinding the obtained mixture through a sand mill, and carrying out one-step granulation on the obtained emulsion through a cold spraying-starch fluidized bed drying technology to obtain the microcapsule preparation with the particle size of 40-100 meshes. The content reservation rate of the microcapsule preparation disposed at 40DEG C under RH of 75% for 6 months is greater than 95%, and 0.1g of the microcapsule preparation is dissolved in 100ml of water to form a clear, transparent and floating oil-free solution, so the microcapsule preparation can be applied in beverages and other aqueous foods.
Owner:CHENGUANG BIOTECH GRP HANDAN CO LTD
Calcium supplementing preparation with functions of improving bones and joints
The invention relates to a novel calcium supplementing preparation with functions of improving bones and joints, which is prepared from the following raw materials in parts by weight: 10-42 parts of calcium source components, 5-25 parts of amino sugar components, 0.1-5 parts of casein phosphopeptide, 0.5-5 parts of bone collagen protein, 0-8 parts of oxidation resistant components for human bodies and 0-3 parts of filling agents. The novel calcium supplementing preparation not only can supplement a calcium source to increase the bone hardness, but also can supplement bone collagen protein to increase the bone toughness for preventing calcium loss; the casein phosphopeptide is added to promote the calcium absorption, thereby solving the problem of the calcium absorption in the calcium supplementing process; and simultaneously, the oxidation resistant components and the components for promoting osteoblast proliferation are added to enable the bone reconstruction process formed by bone formation and bone absorption to balance again, thus the supplemented calcium can be reserved and firmly deposited on the bones. The related components in the novel calcium supplementing preparation can restore cartilages, improve the joint structure and interdict the deterioration of osteoarthritis.
Owner:洛阳新春都生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com