Caspofungin formulations
A technology of caspofungin and regulator, which is applied in the directions of antifungal agents, medical preparations with non-active ingredients, and pharmaceutical formulas, and can solve the problems of increased risk of embolism and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
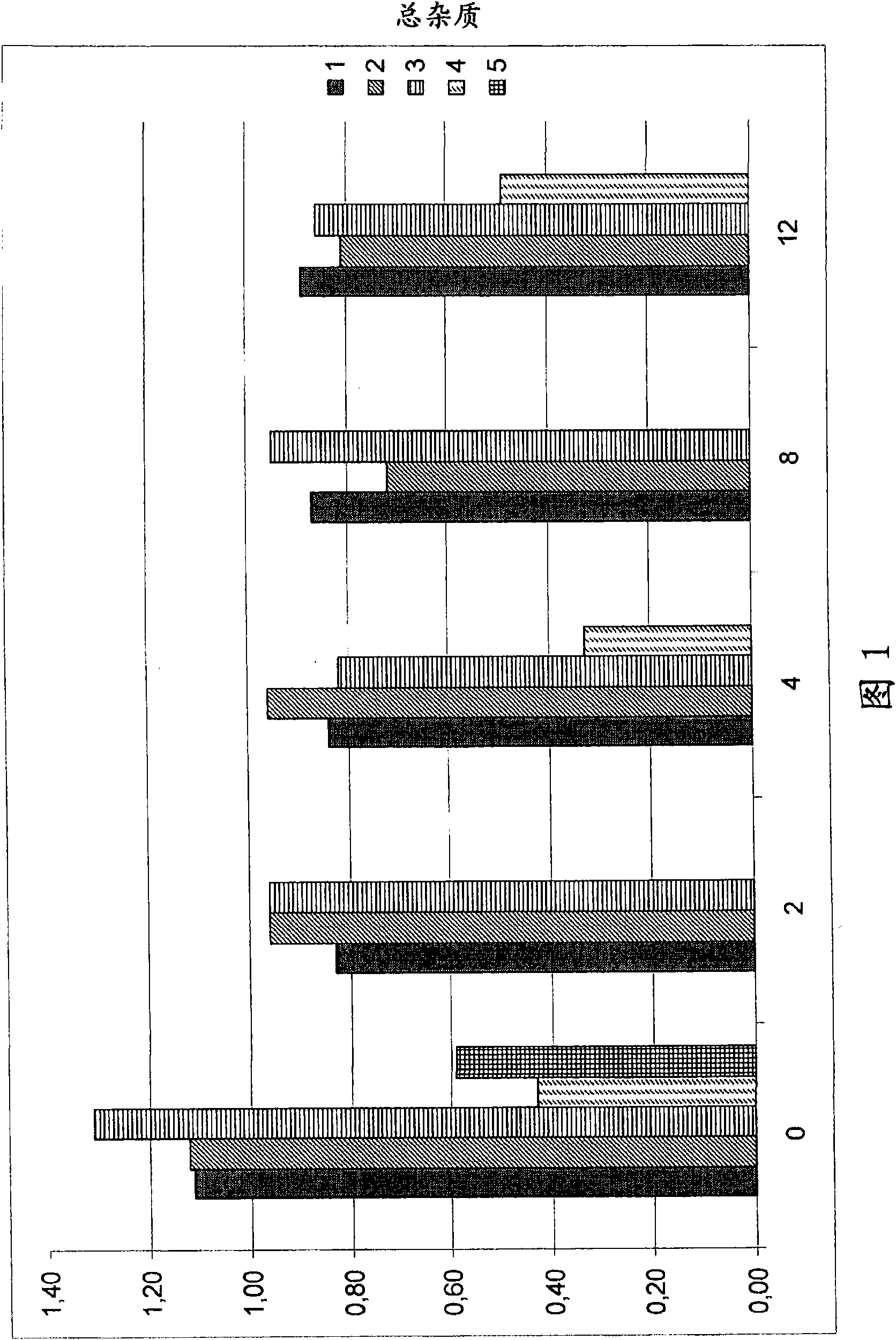
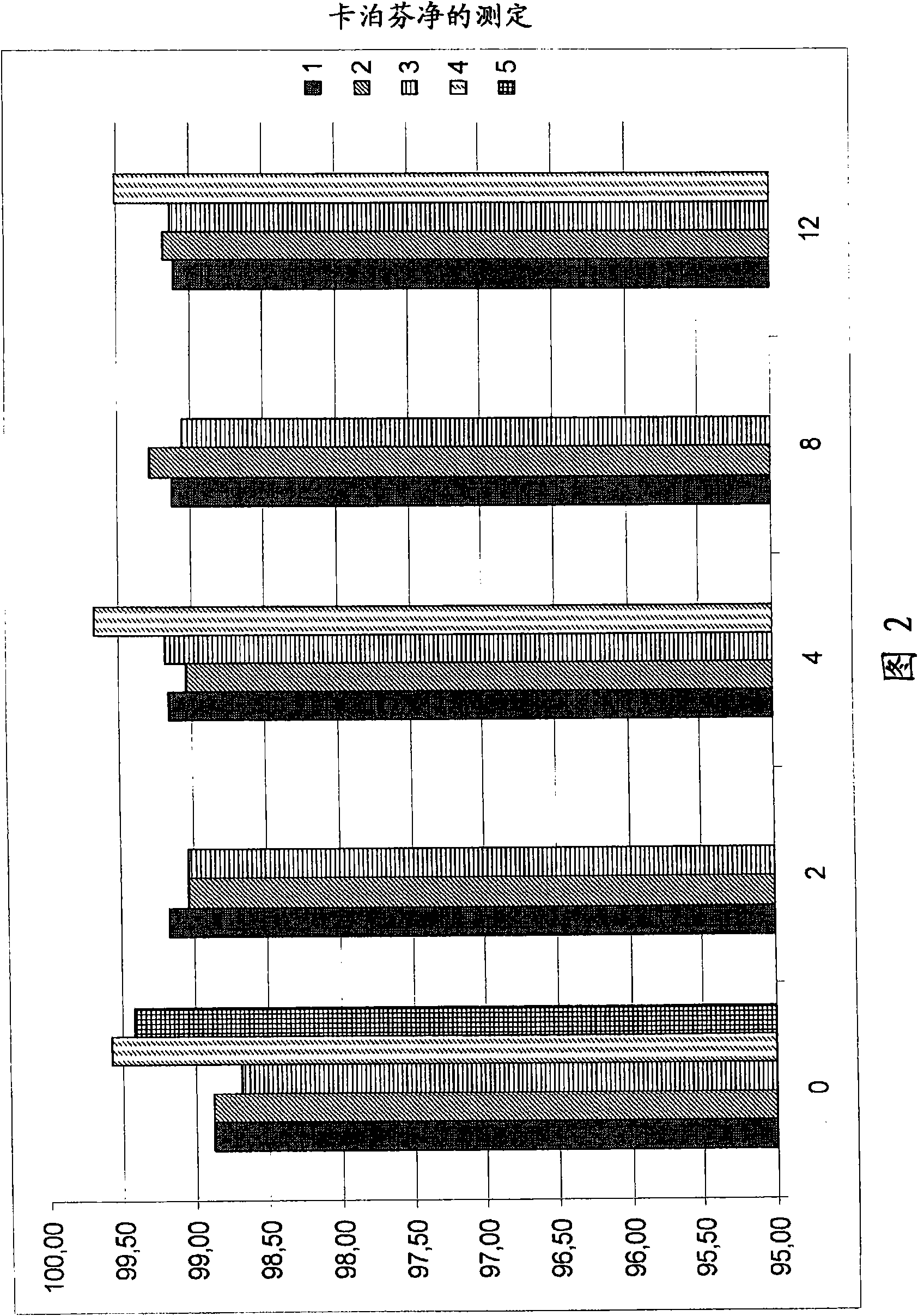
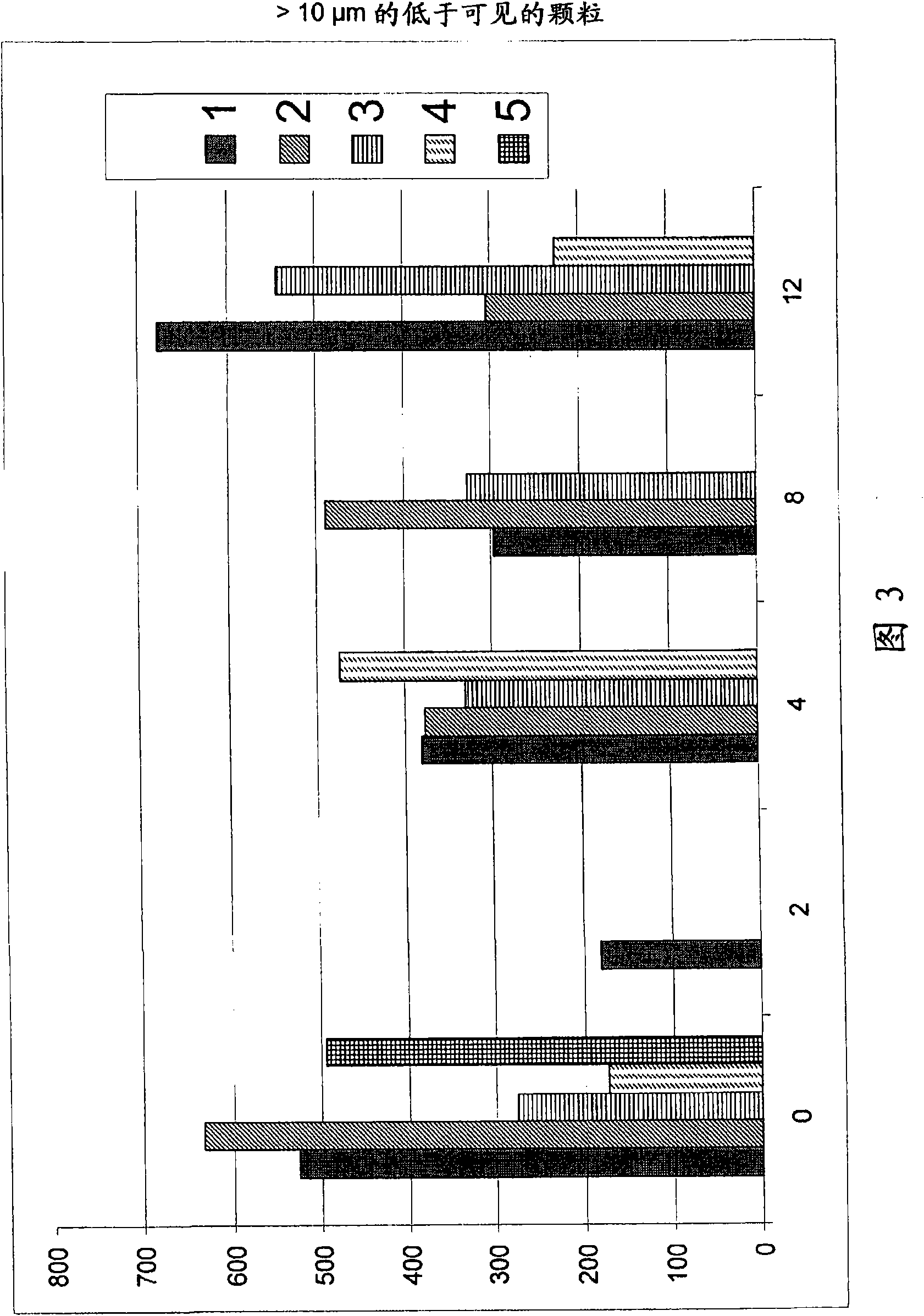
[0246] Embodiment 1 (comparison) :
[0247] Preparation of composition 1 comprising caspofungin diacetate and additional amount of acetate buffer according to example 1 of EP 0 904 098 B1
[0248] Table 1:
[0249]
[0250] A liquid formulation of Composition 1 was prepared by dissolving 5 g of mannitol and 7.5 g of sucrose in about 200 ml of water. The pH was then measured and acetic acid was added to a final concentration of 1.5 mg / ml and the pH was adjusted to pH 3.7 with 1 N NaOH. Then 11.7 g of caspofungin diacetate were added, corresponding to 46.6 mg / ml caspofungin diacetate or 42 mg / ml caspofungin calculated as base, and the pH was adjusted to pH 6.0 using 1 N NaOH. Adjust volume to 250ml with water and pass through Millex TM -GV injector-driven with Durapore TM - The solution is filtered with a membrane and a filter device with a diameter of 0.22 μm and filled with 1.25 ml each into 15 ml glass vials. The vials were partially stoppered with lyophilization st...
Embodiment 2 and 3
[0252] Preparation of Composition 2 and Composition 3 comprising caspofungin and an additional pH adjuster, namely acetic acid:
[0253] Table 2:
[0254] components
[0255] Liquid formulations of compositions 2 and 3 were prepared according to Example 1 by dissolving mannitol and sucrose using a 100 ml batch size. Subsequently 46.6 mg / ml caspofungin diacetate was added, corresponding to 42 mg / ml caspofungin base, the pH was measured at 6.59 and adjusted to pH 6.0 or pH 6.5 with 1N acetic acid, respectively. For composition 2, 0.1315 mg / ml acetic acid was added (calculated based on the final volume of the liquid formulation), corresponding to a final molar concentration of 2.19 mmol / l of additional acetic acid or a molar ratio of additional acetic acid to caspofungin of 0.0569 . After adjusting the volume with water, a pH of 6.05 was obtained. For composition 3, 0.0188 mg / ml of acetic acid was added (calculated based on the final volume of the liquid formulation)...
Embodiment 4
[0257] Preparation of composition 4 comprising caspofungin without any additional pH adjuster:
[0258] table 3:
[0259]
[0260] A 200 ml batch size liquid formulation of Composition 4 was prepared by dissolving mannitol and sucrose according to Examples 2 and 3. Then 42 mg / ml caspofungin base, ie 46.6 mg / ml caspofungin diacetate, was added without further pH adjustment. The volume was adjusted with water in a similar manner to Example 1, ie to a final volume of 200 ml, whereby a pH value of 5.96 was obtained, the solution was filtered, filled into vials and lyophilized. Redissolve and / or dilute lyophilized composition 4 in a similar manner to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com