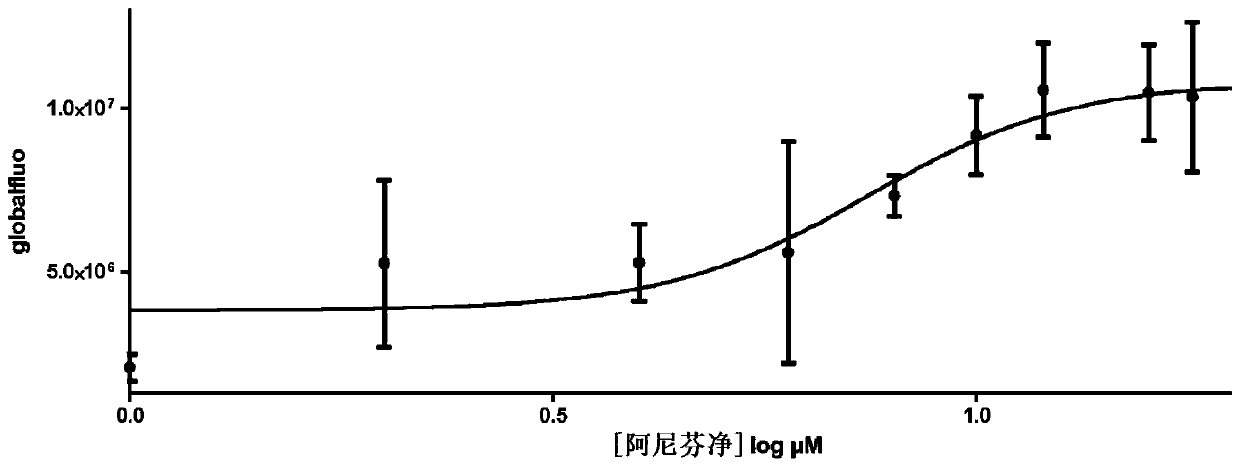
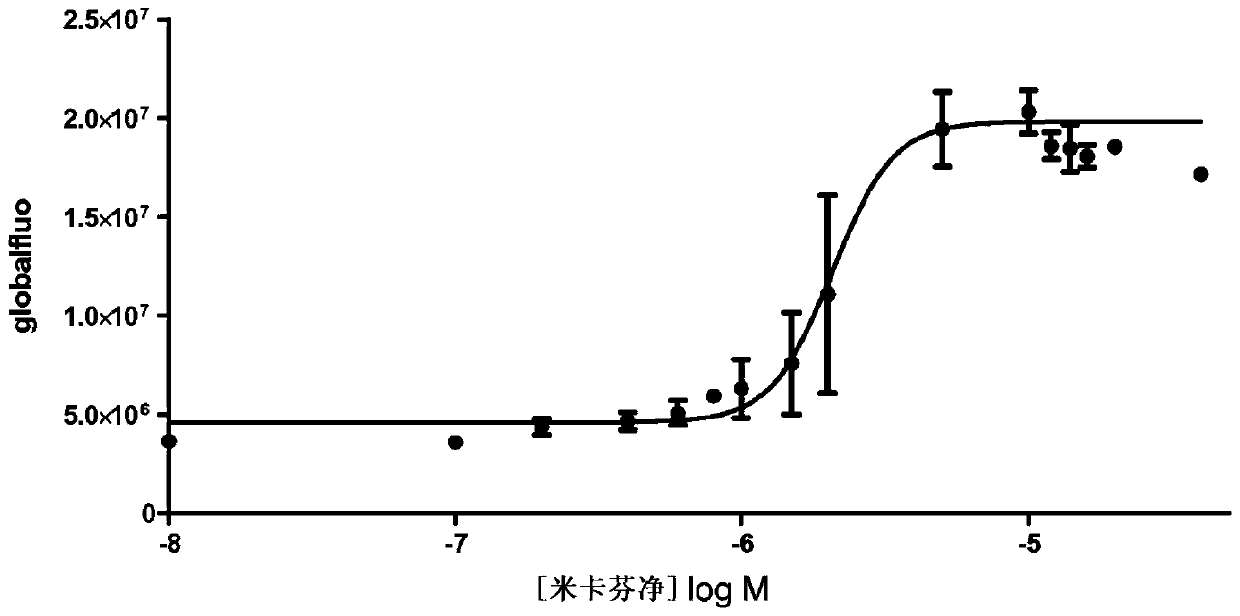
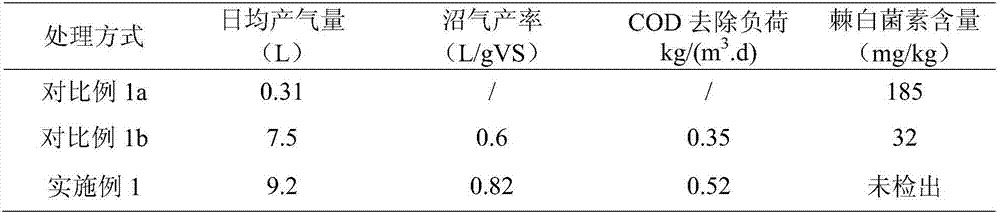
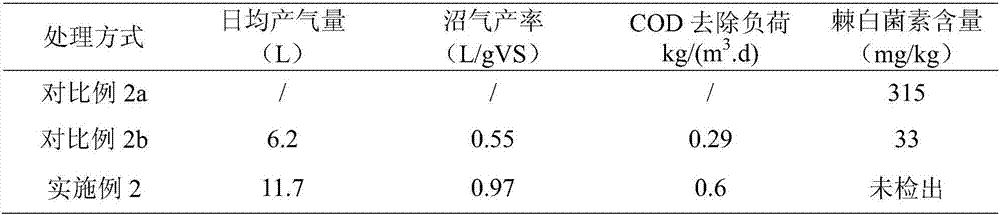
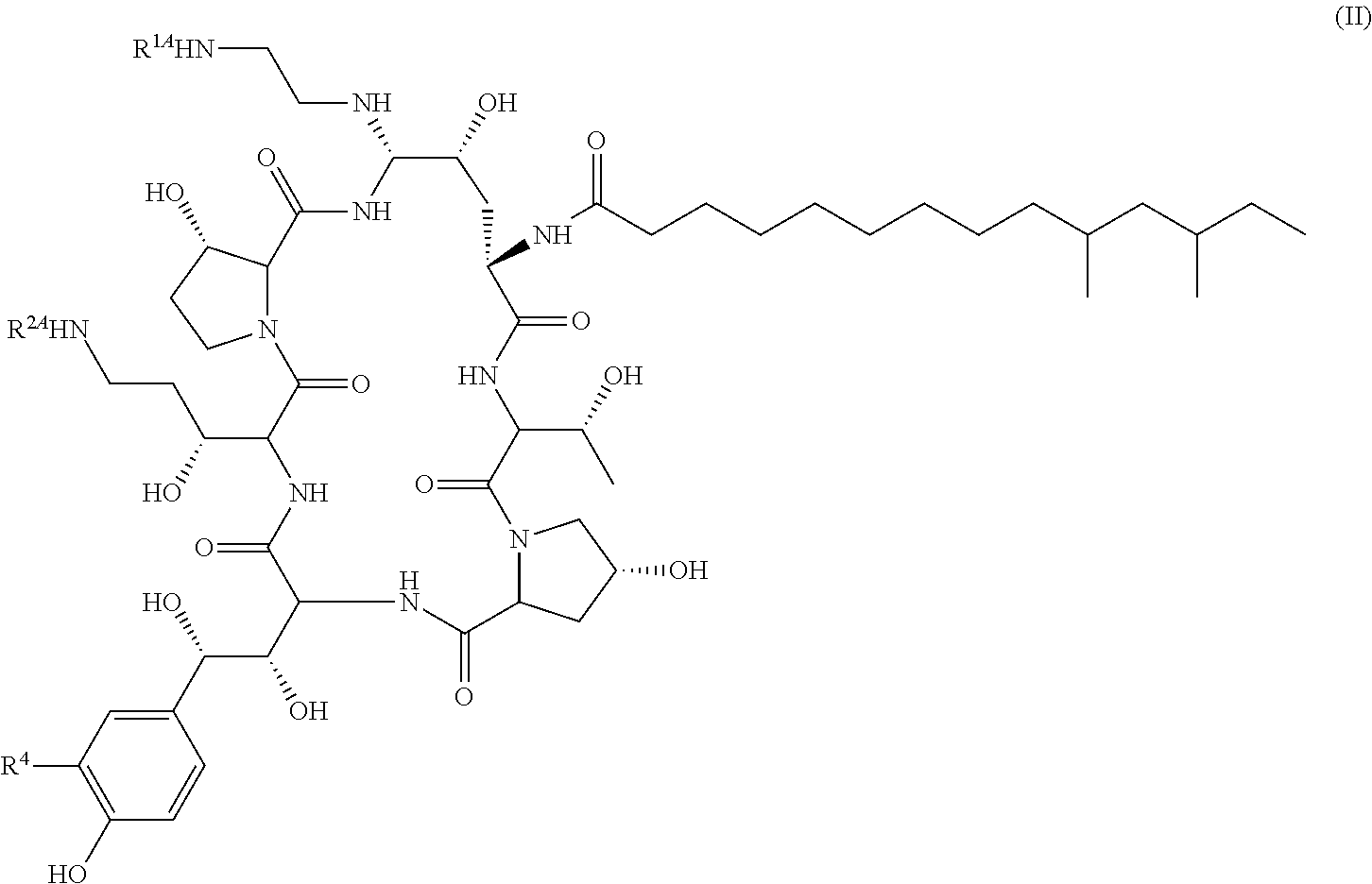
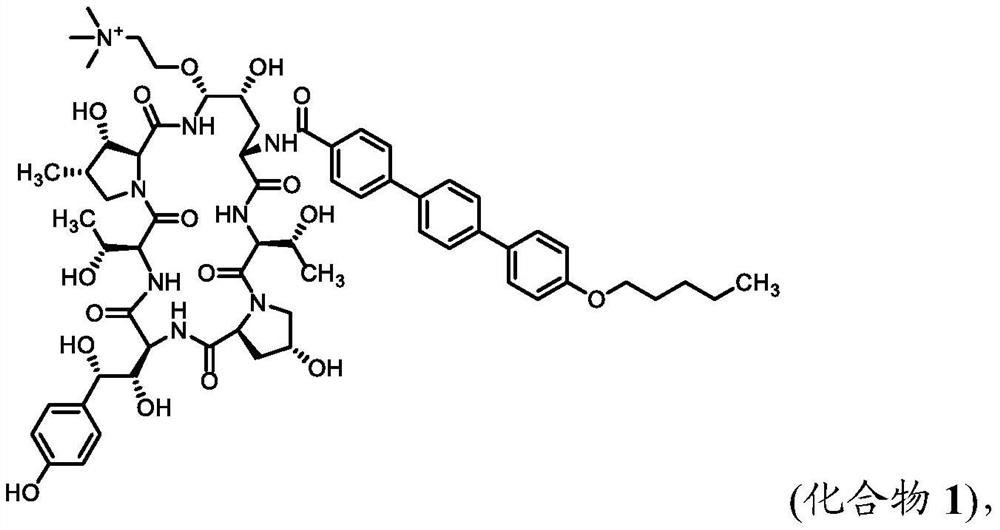
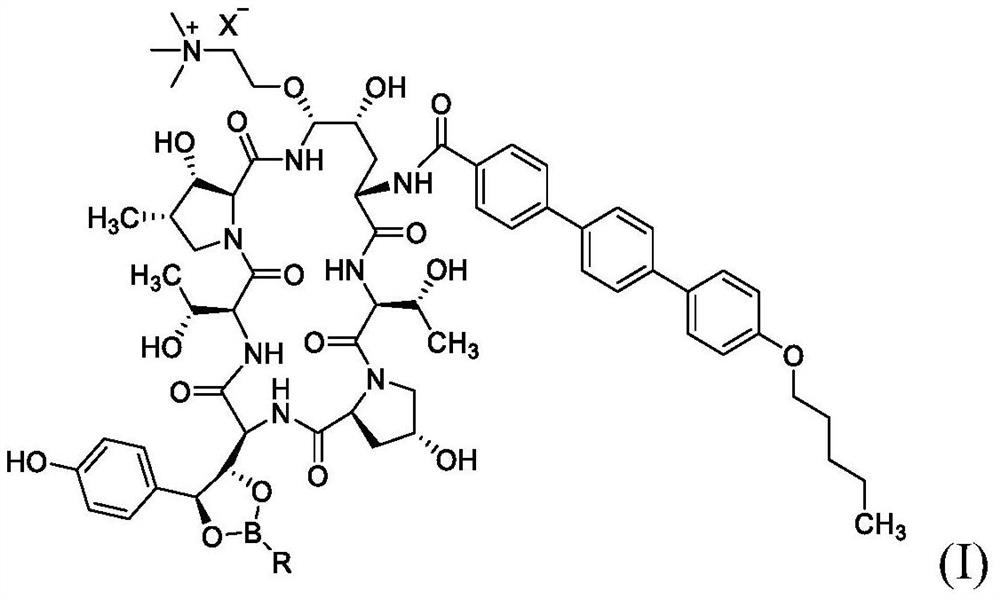
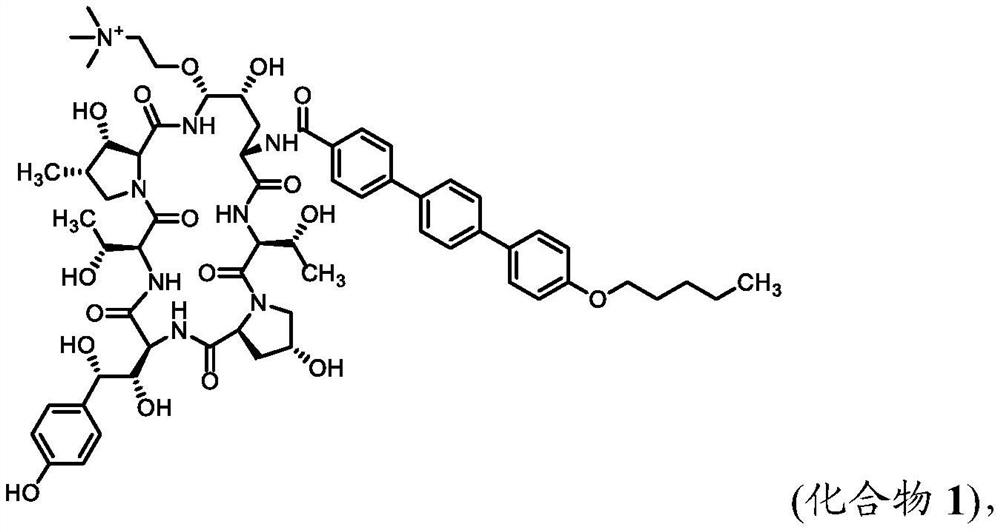
Patents
Literature
52 results about "Echinocandin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
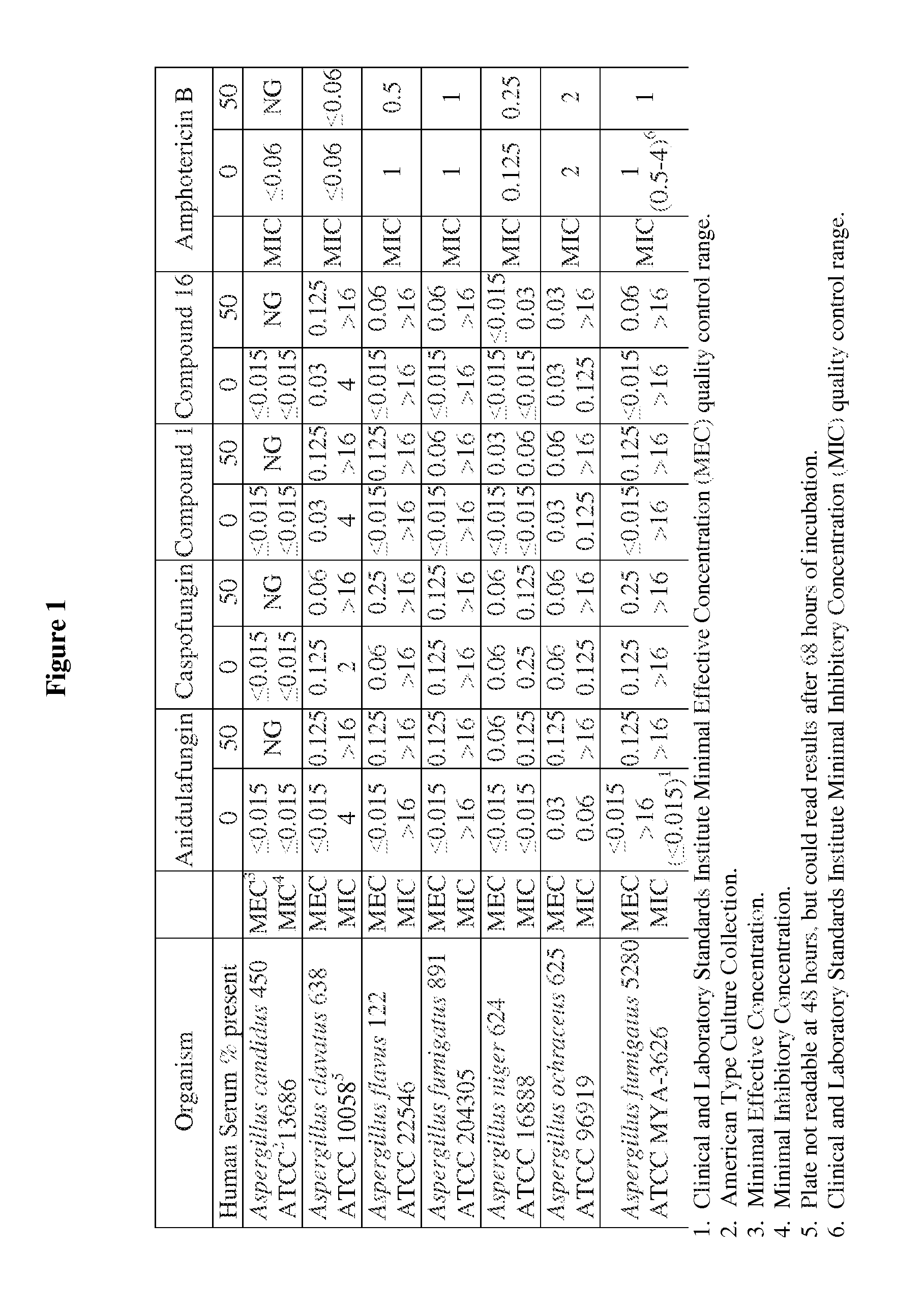
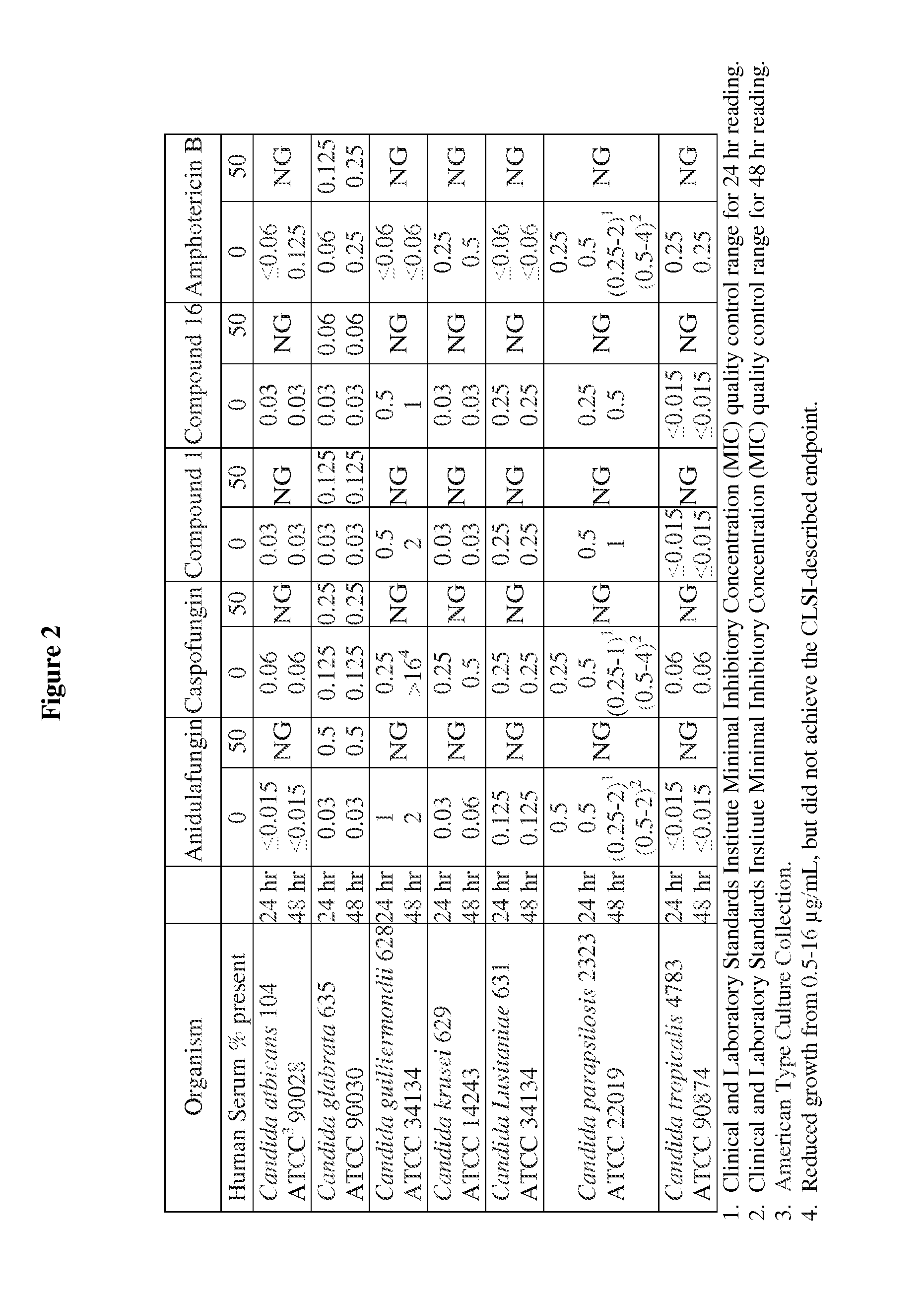
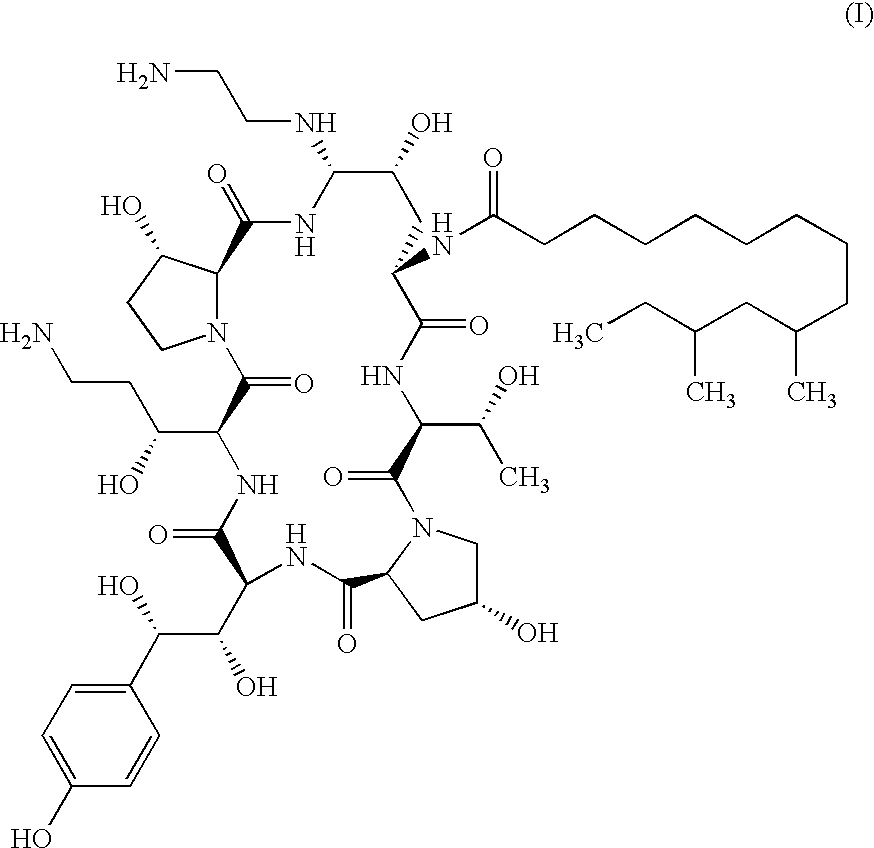
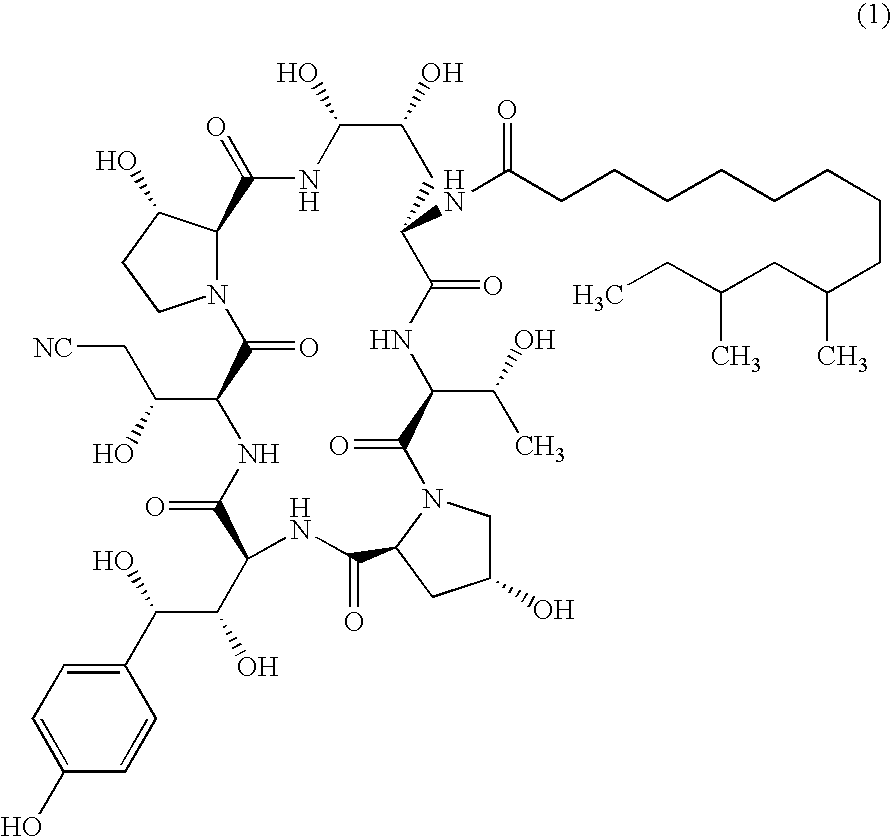
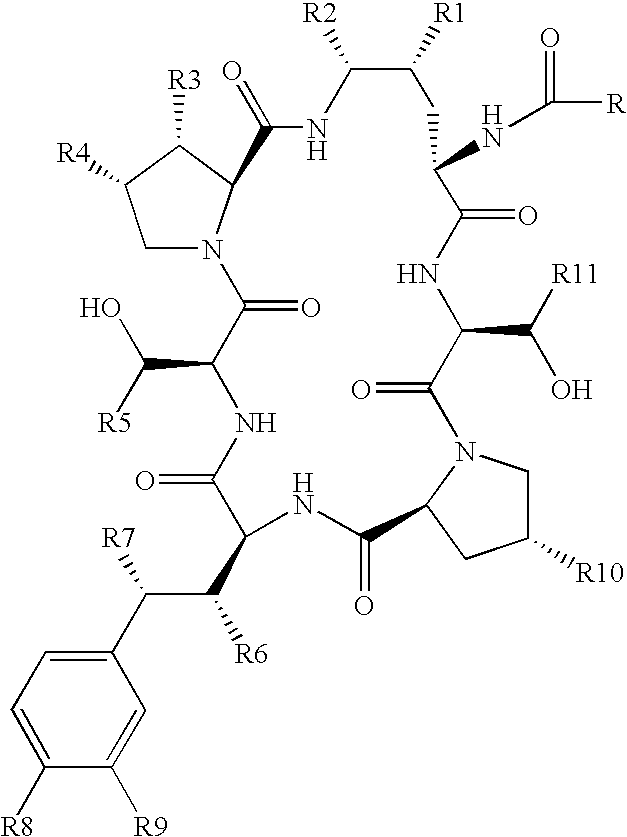
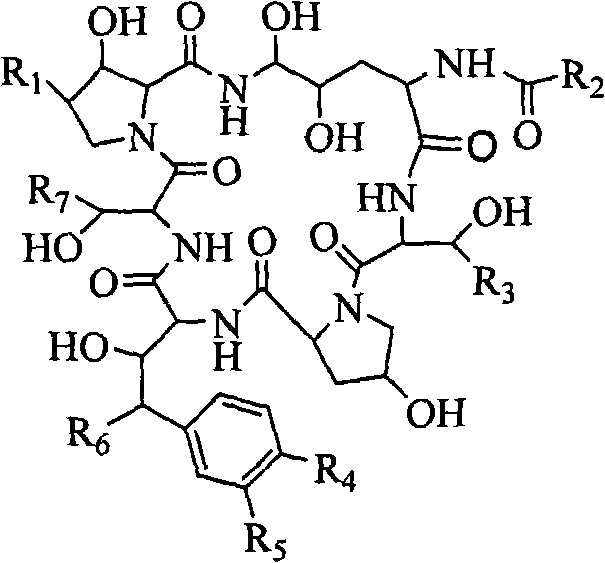
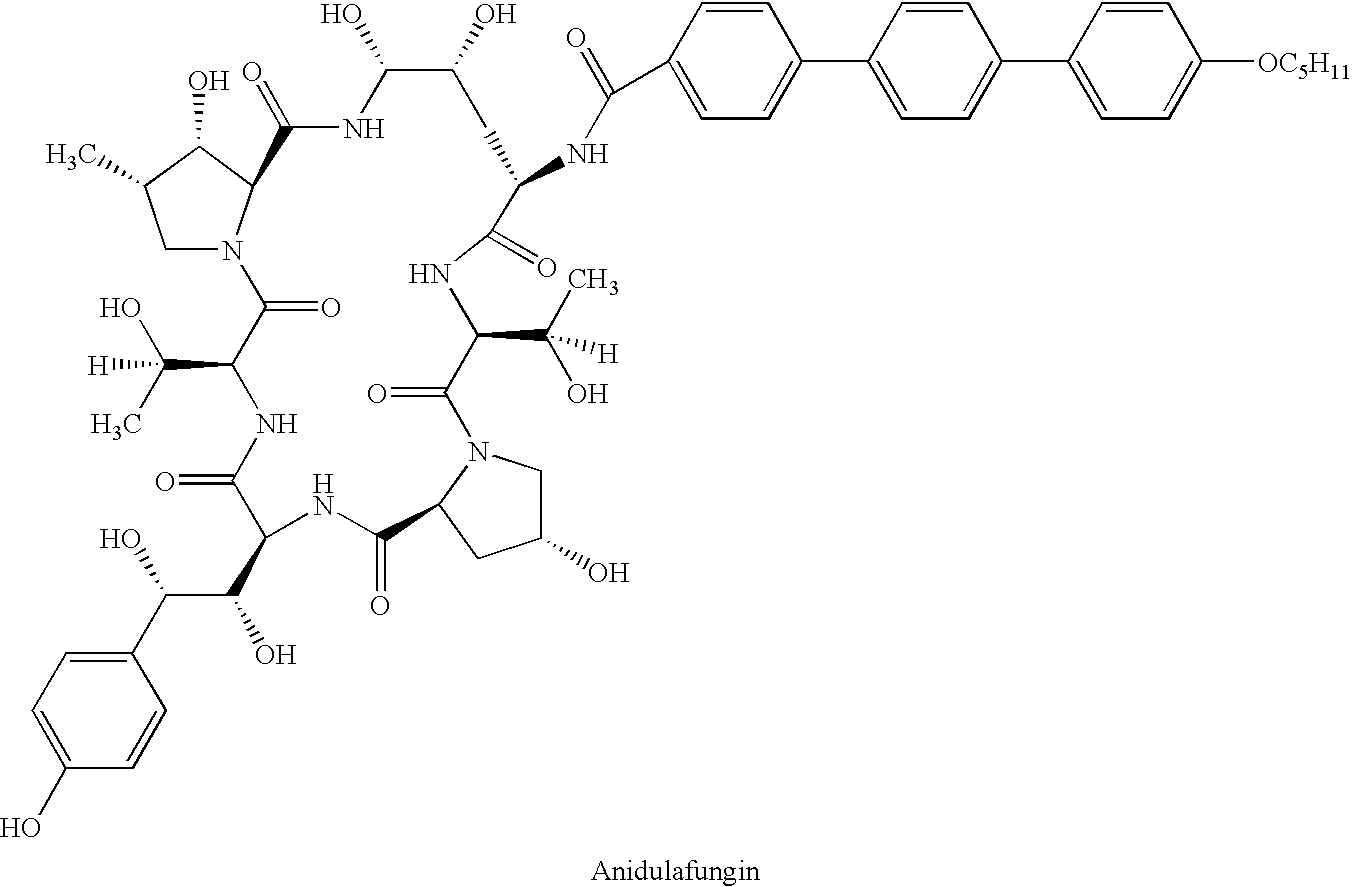
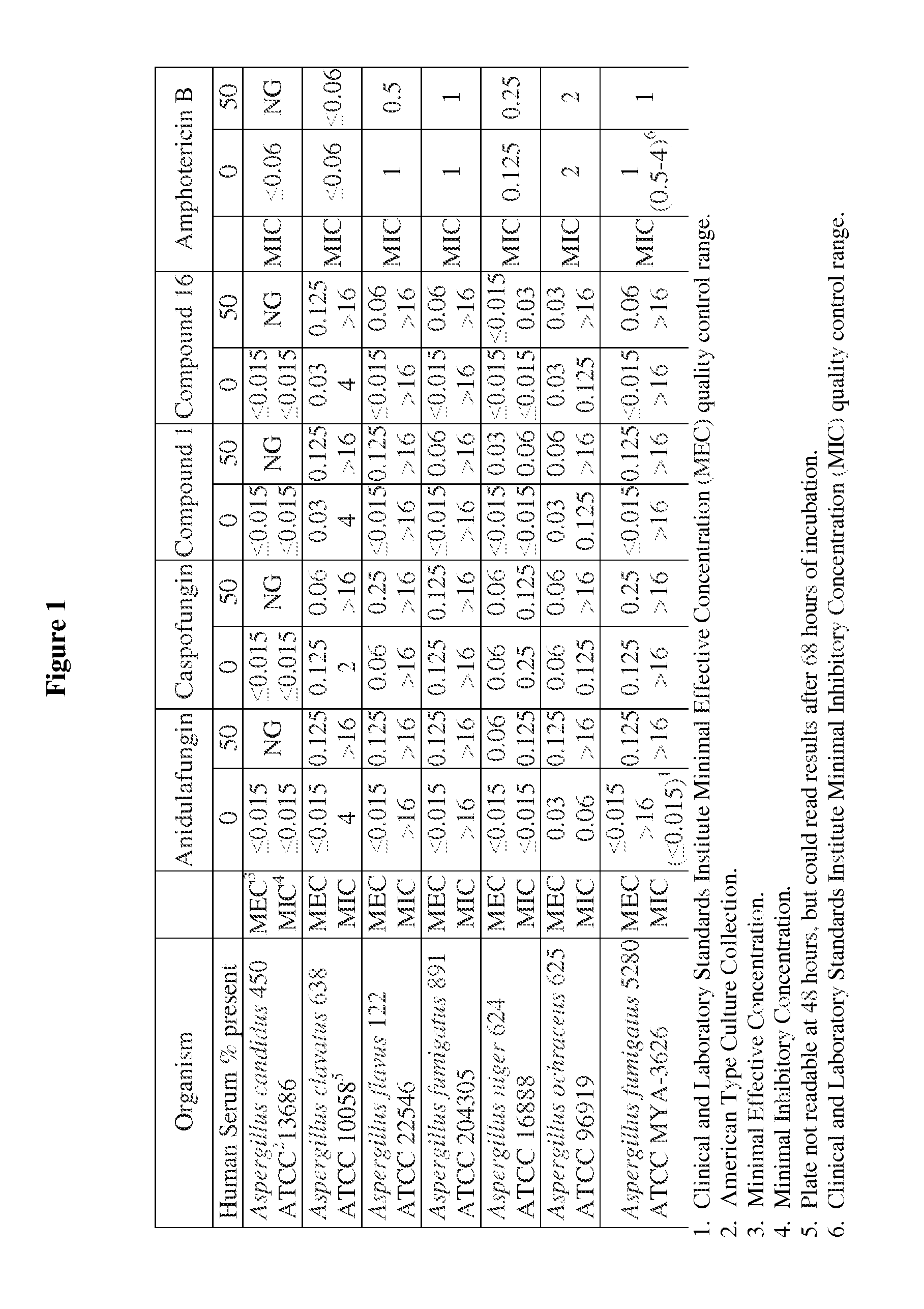
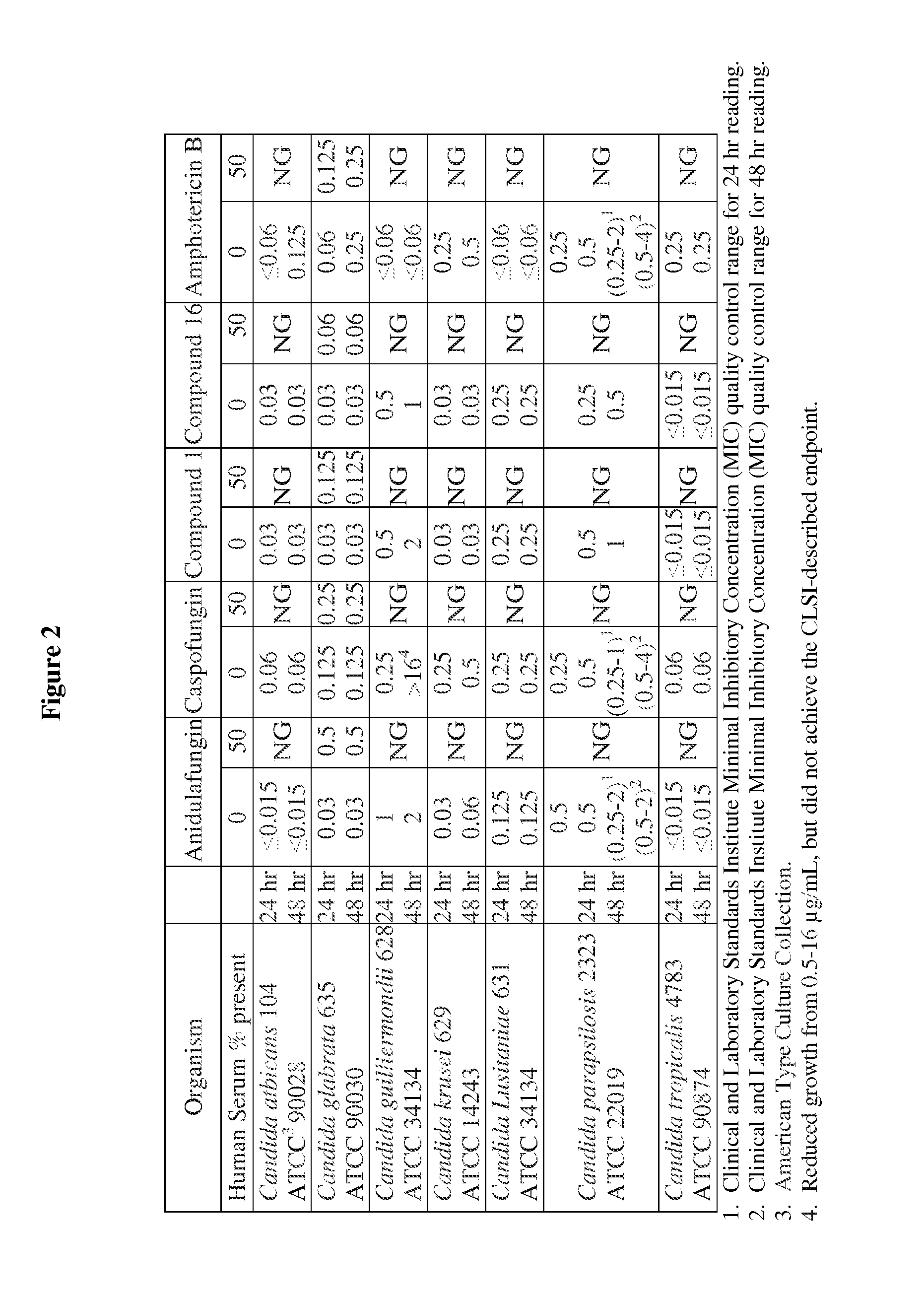
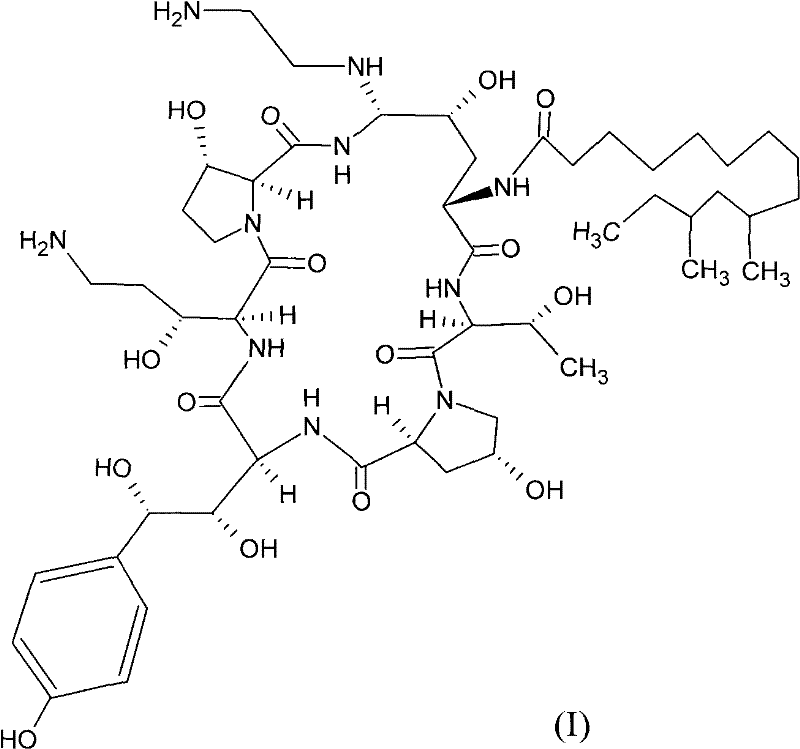
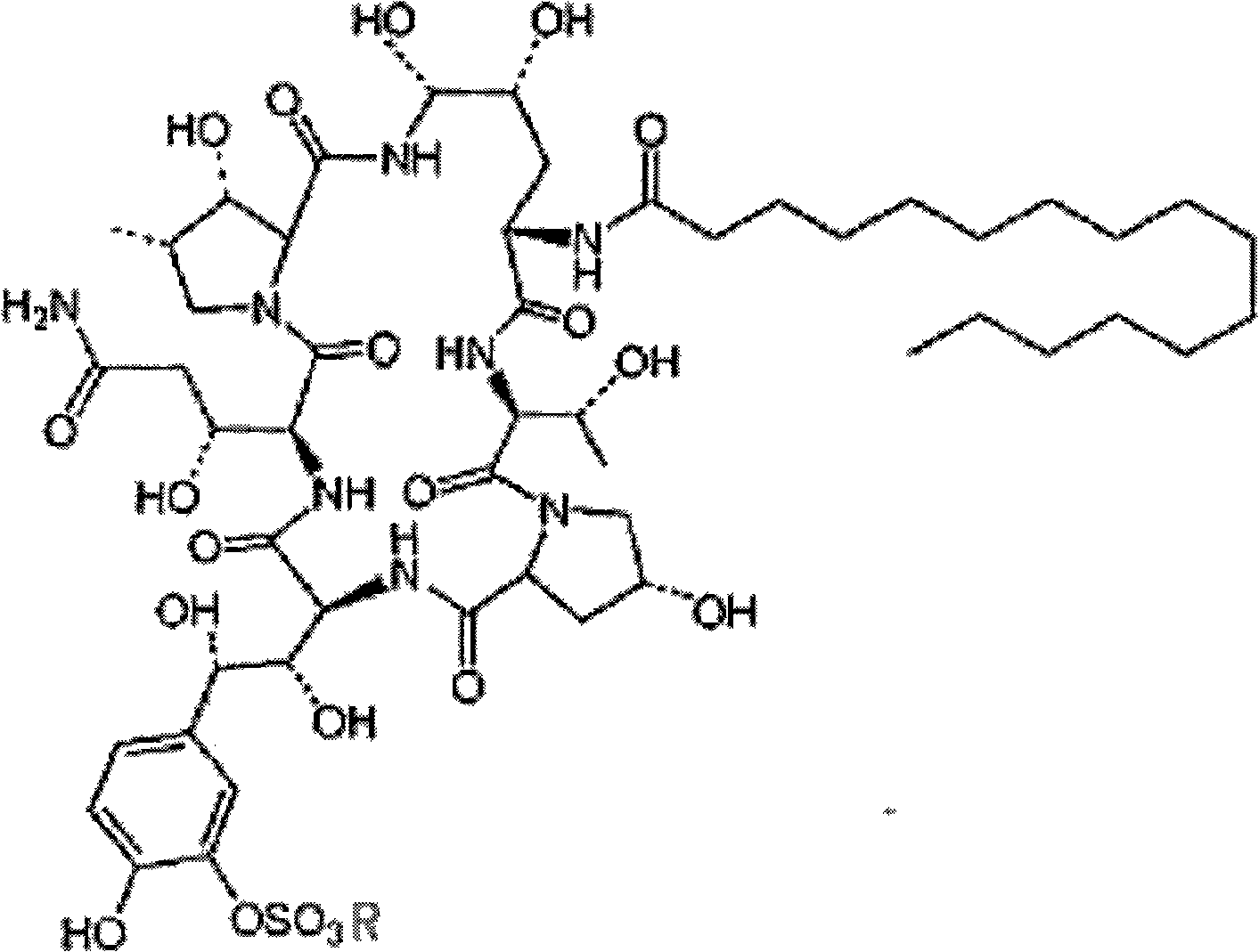
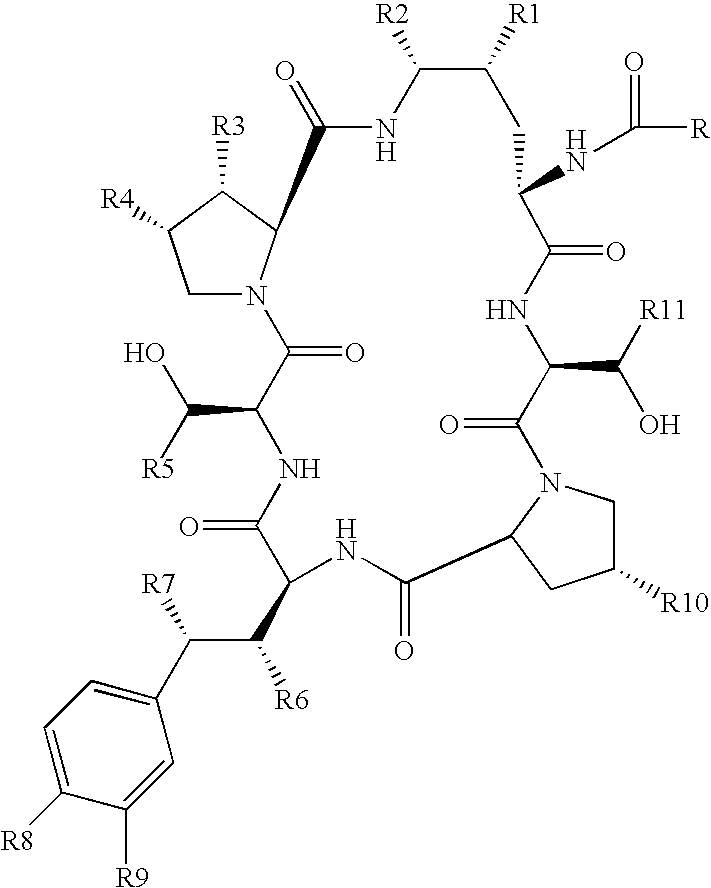
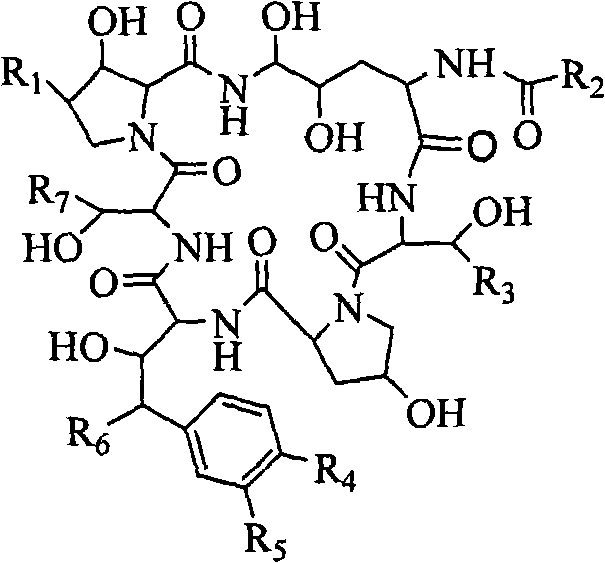
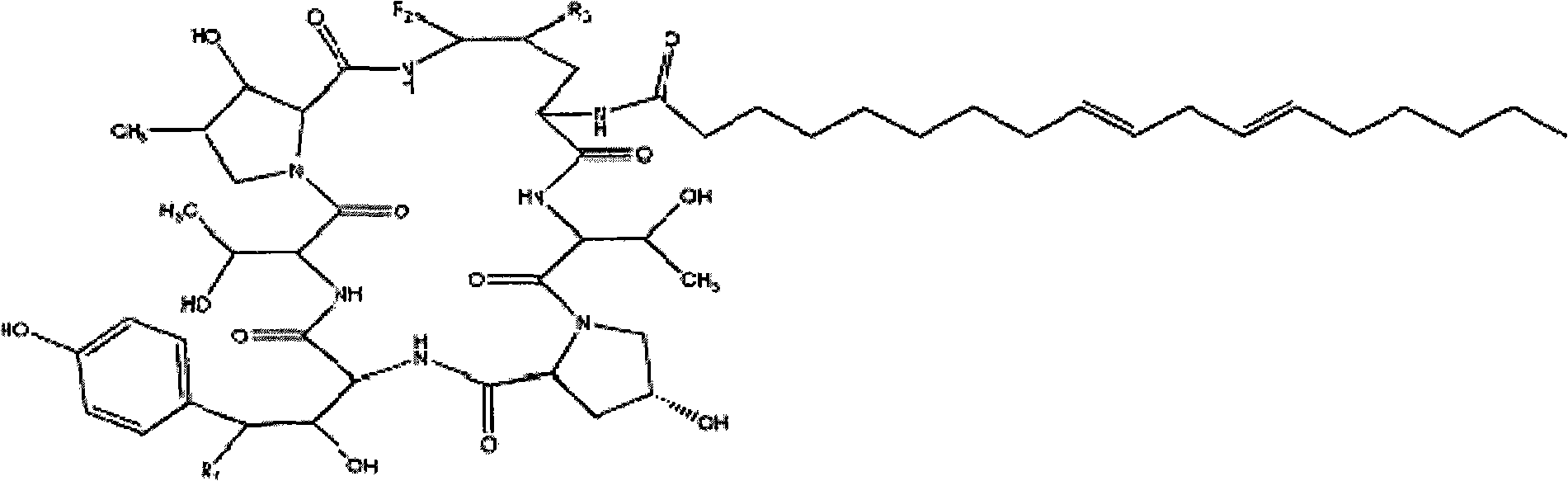
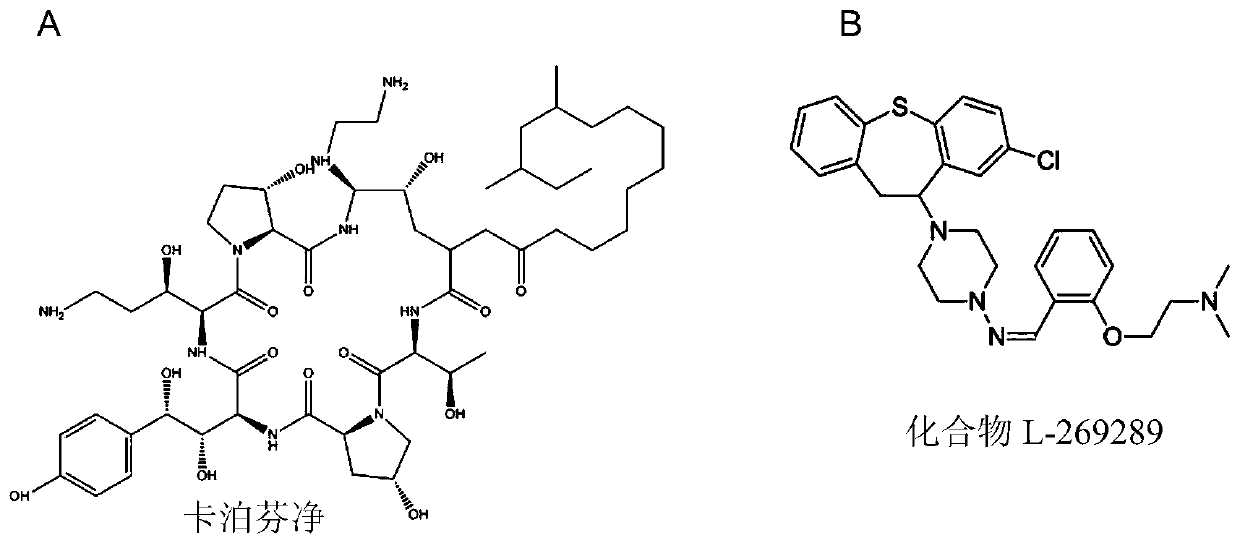
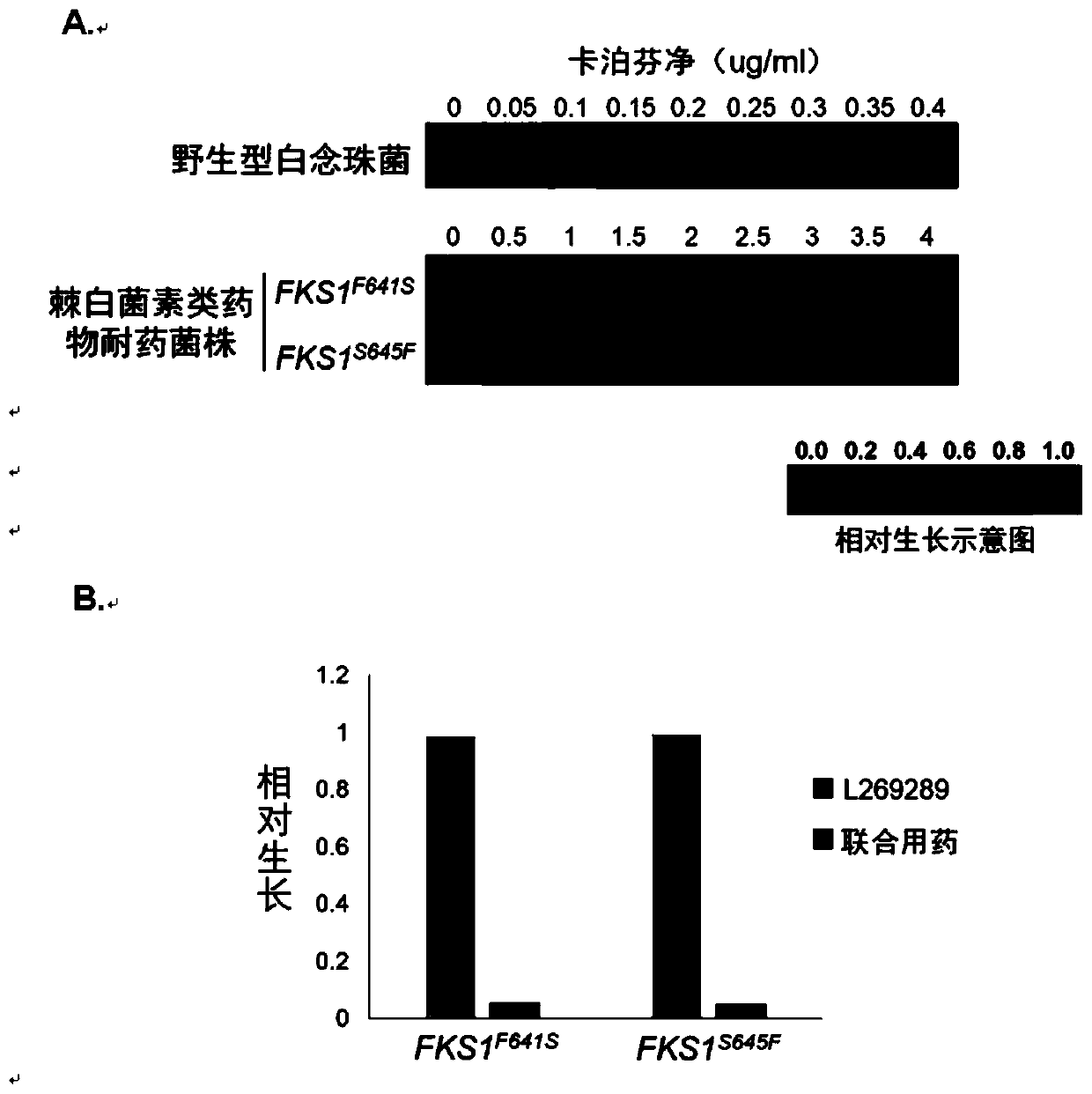
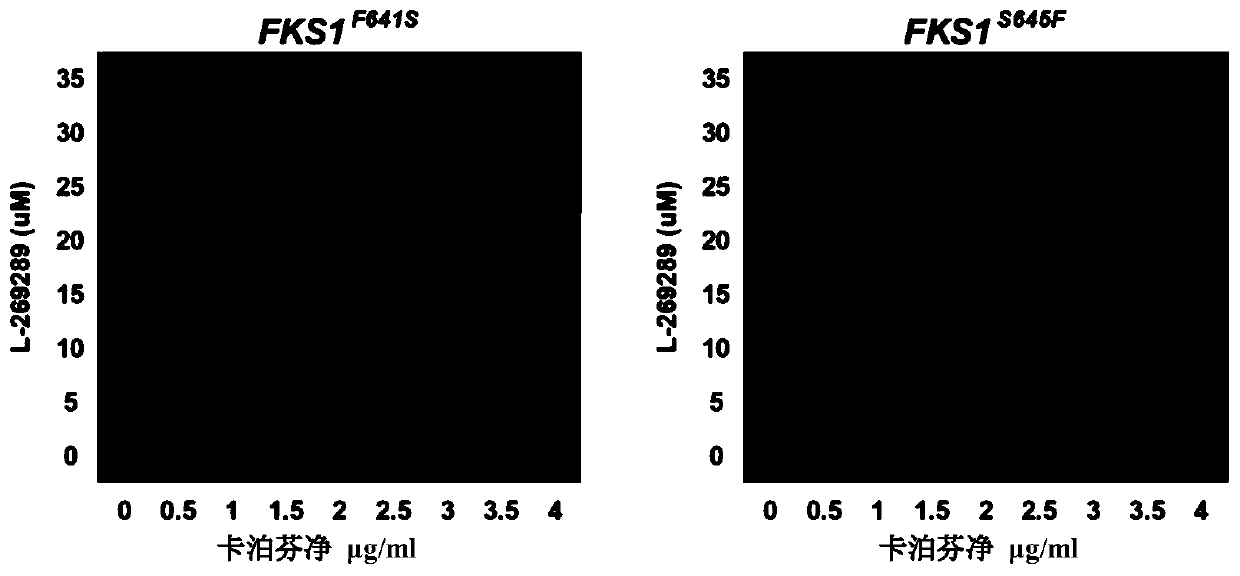
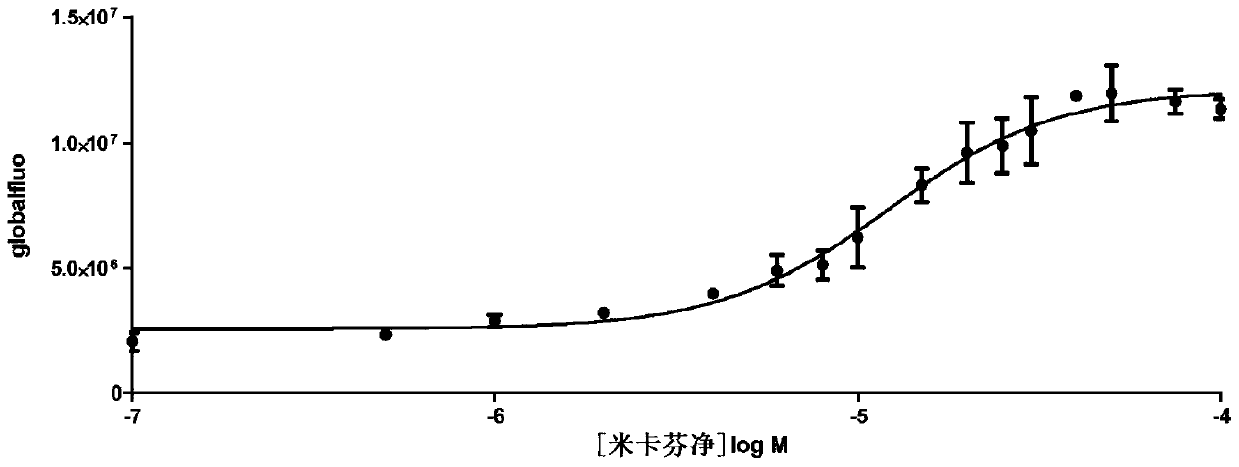
Echinocandins are a new class of antifungal drugs that inhibit the synthesis of β-glucan in the fungal cell wall via noncompetitive inhibition of the enzyme 1,3-β glucan synthase. The class has been termed the "penicillin of antifungals," along with the related papulacandins, as their mechanism of action resembles that of penicillin in bacteria. β-glucans are carbohydrate polymers that are cross-linked with other fungal cell wall components, equivalent to bacterial peptidoglycan. Caspofungin, micafungin, and anidulafungin are semisynthetic echinocandin derivatives with clinical use due to their solubility, antifungal spectrum, and pharmacokinetic properties.
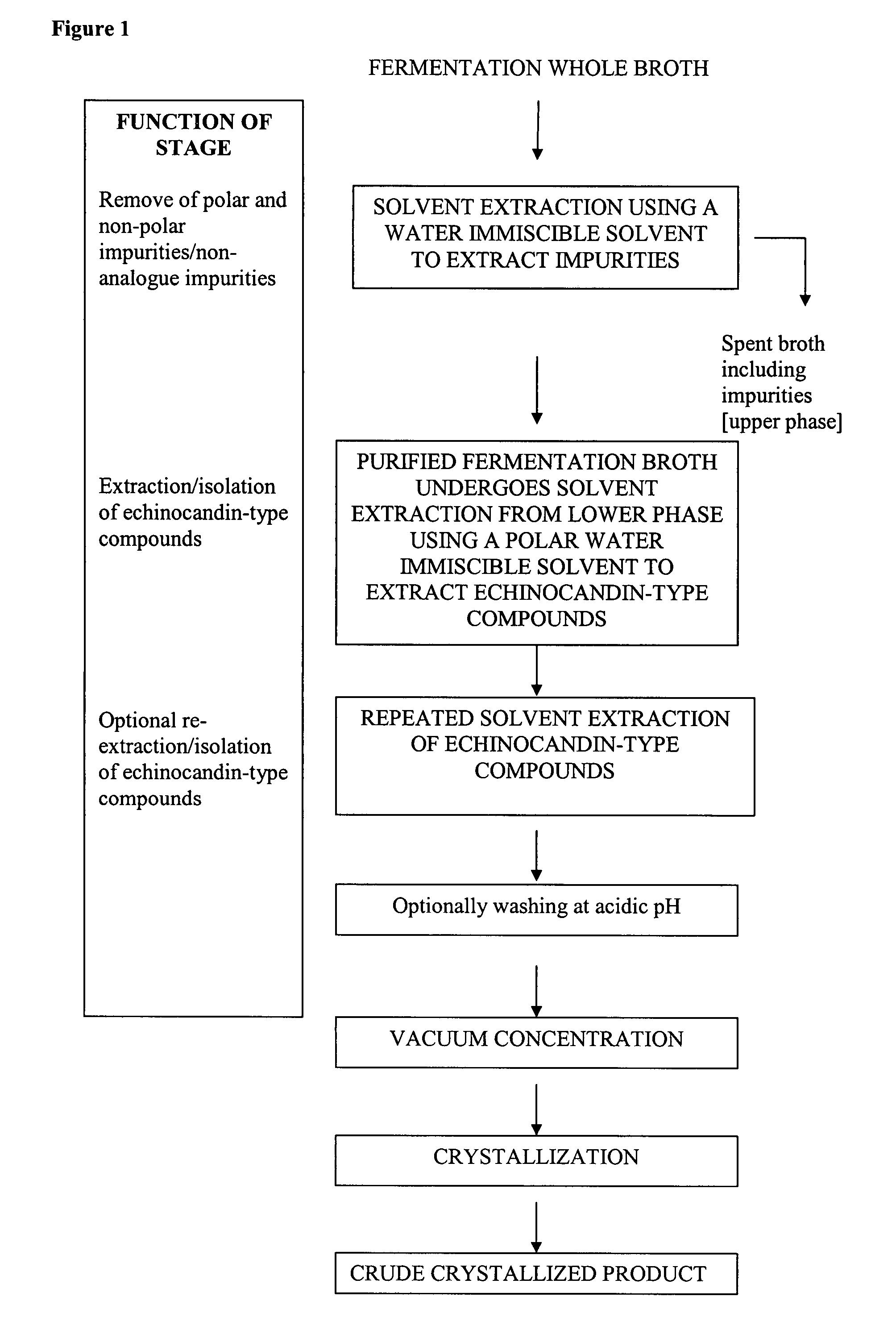
Method for preparing pneumocandin B0
The invention discloses a method for preparing pneumocandin B0. The method comprises the following steps of: a) centrifuging the fermentation liquid of echinocandin B0, taking mycelium, leaching the echinocandin B0 in the mycelium with a first solvent and filtering and removing the mycelium; b) distilling the solvent in the first solvent leaching solution of echinocandin B0 to dryness, soaking with a second solvent and then filtering and removing the insoluble substances; c) distilling the second solvent soak solution of echinocandin B0 to dryness and then dissolving in the first solvent, andcollecting effluent liquid through overly acidic alumina column; d) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent, utilizing adsorbent resin,performing eluting with the first solvent and then collecting the part with higher purity; e) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent,utilizing inverse resin, performing eluting with the first solvent and collecting the part with higher purity; and f) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent, adding in a small amount of water by dripping to achieve the purpose of separation by crystallization after supersaturation, and then preparing the echinocandin B0. The methodfor preparing the echinocandin B0 not only can well remove the pigment, but also leads the purity of the echinocandin B0 to be improved by more than 96 percent.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method for high-purity echinocandin compound
ActiveCN102952178AQuality improvementHigh purityPeptide preparation methodsChromatographic separationEchinocandin
The invention discloses a preparation method for an echinocandin compound by using a Coleophoma sp. fermentation culture. The method comprises the following steps: carrying out filtration, macroporous resin adsorption, desorption, condensation, crystallization and the like on the Coleophoma sp. fermentation culture so as to obtain a crude extract of the echinocandin compound FR179642; and carrying out chromatographic separation on the crude extract of the echinocandin compound FR179642 by using a reversed-phase filling material so as to obtain an echinocandin compound FR179642 product with high purity. The invention has the following advantages: a macroporous resin is used to extract and separate the echinocandin compound FR179642 in the method, and the method is simple and feasible and is suitable for industrial production; and through application of the reversed-phase filling material in chromatographic separation of the echinocandin compound FR179642, the echinocandin compound FR179642 product with high purity is prepared.
Owner:NCPC NEW DRUG RES & DEV
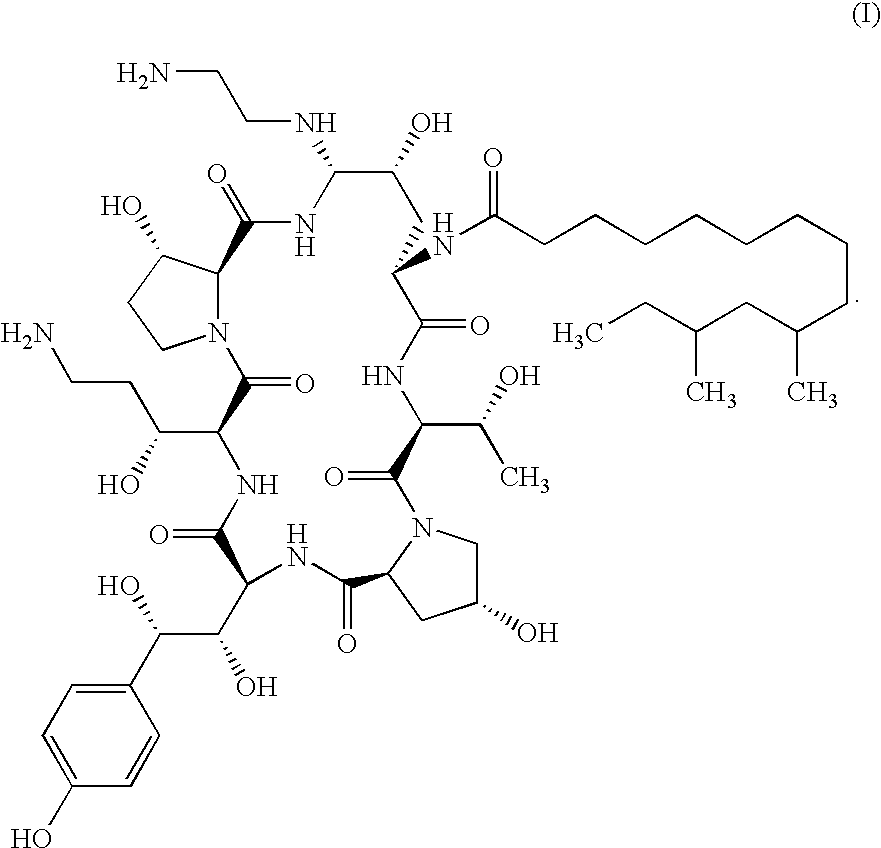
Antifungal agents and uses thereof
The invention features echinocandin class compounds. The compounds can be useful for the treatment of fungal infections.
Owner:CIDARA THERAPEUTICS
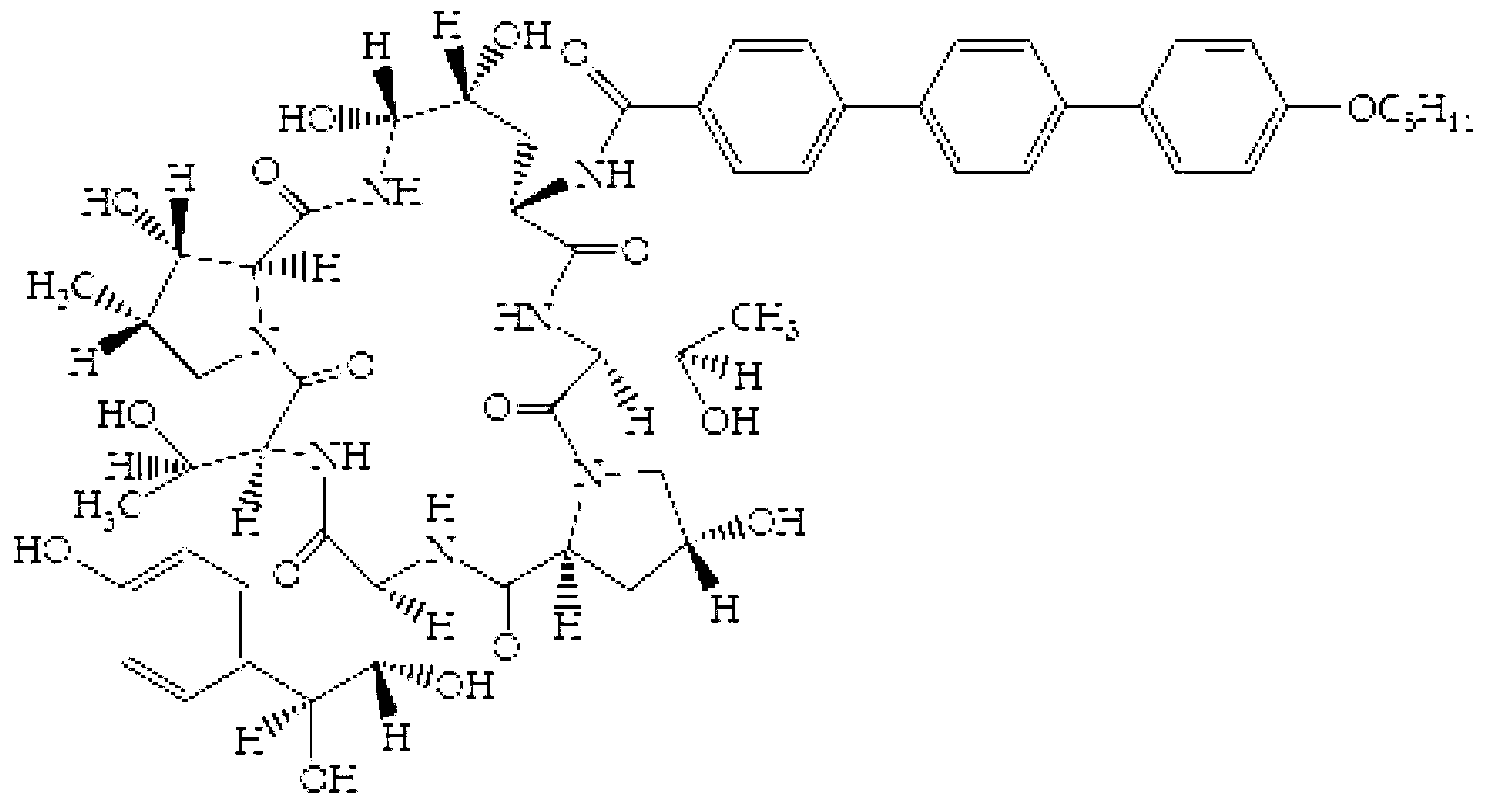
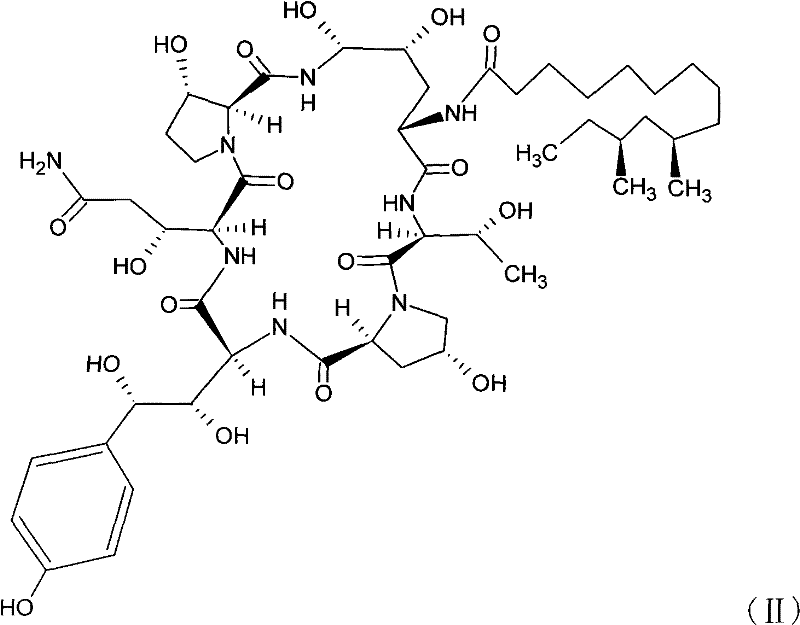
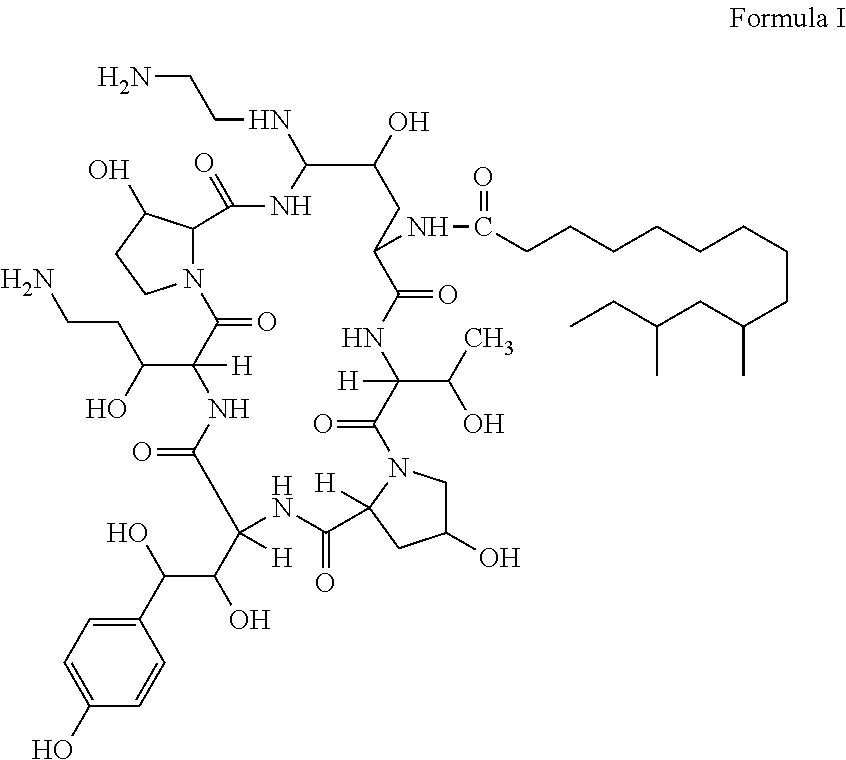
Pharmaceutical composition containing echinocandin antifungal agent micafungin, its preparation method and application
The invention discloses a pharmaceutical composition containing an annular polypeptide compound micafungin of formula (I), or its salt and trehalose as a stabilizer. The pharmaceutical composition provided by the invention has good stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
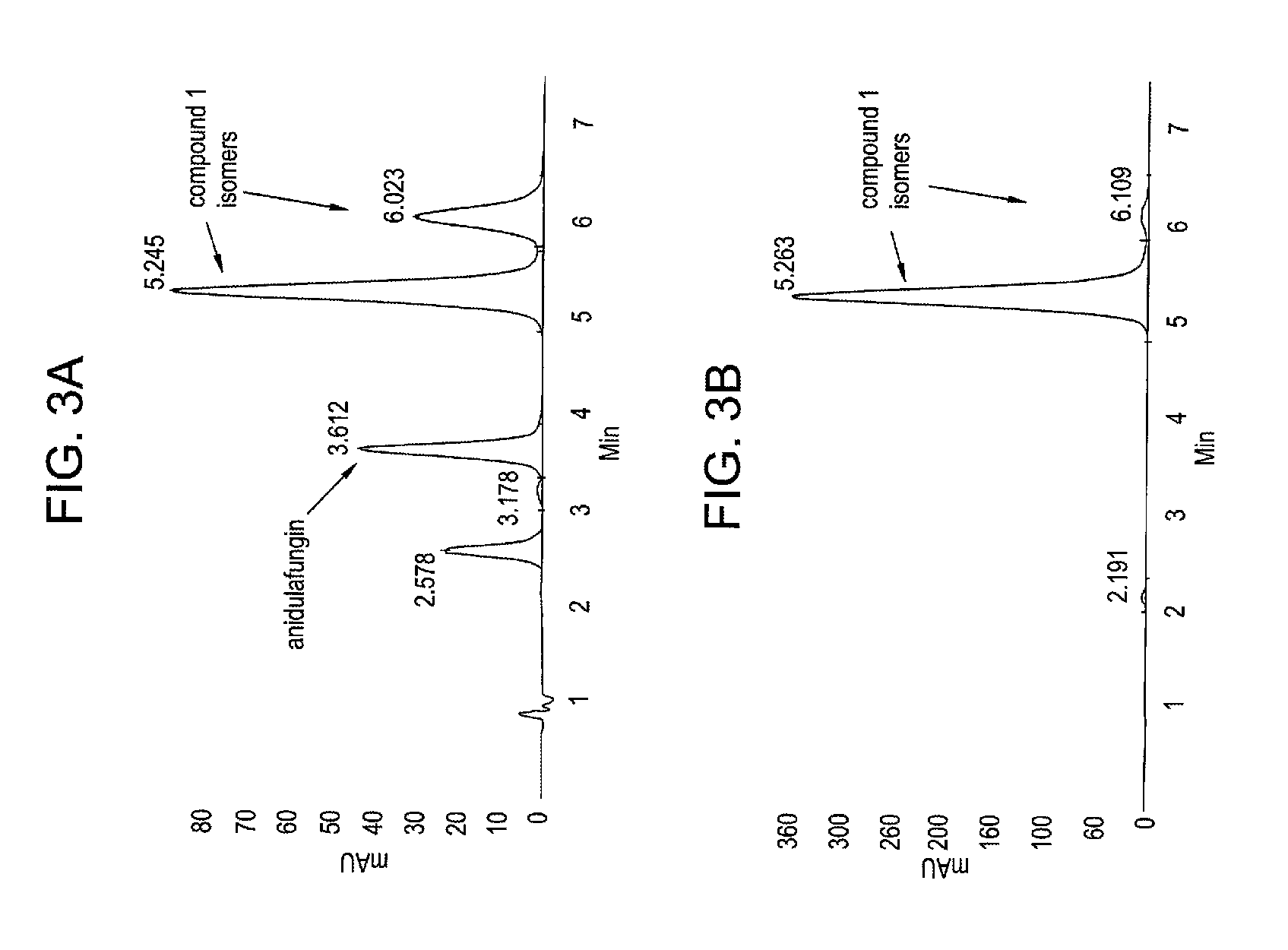
Purification method of echinocandins antifungal drug anidulafungin
ActiveCN103193868ASimple stepsEasy to recyclePeptide preparation methodsEchinocandinPurification methods
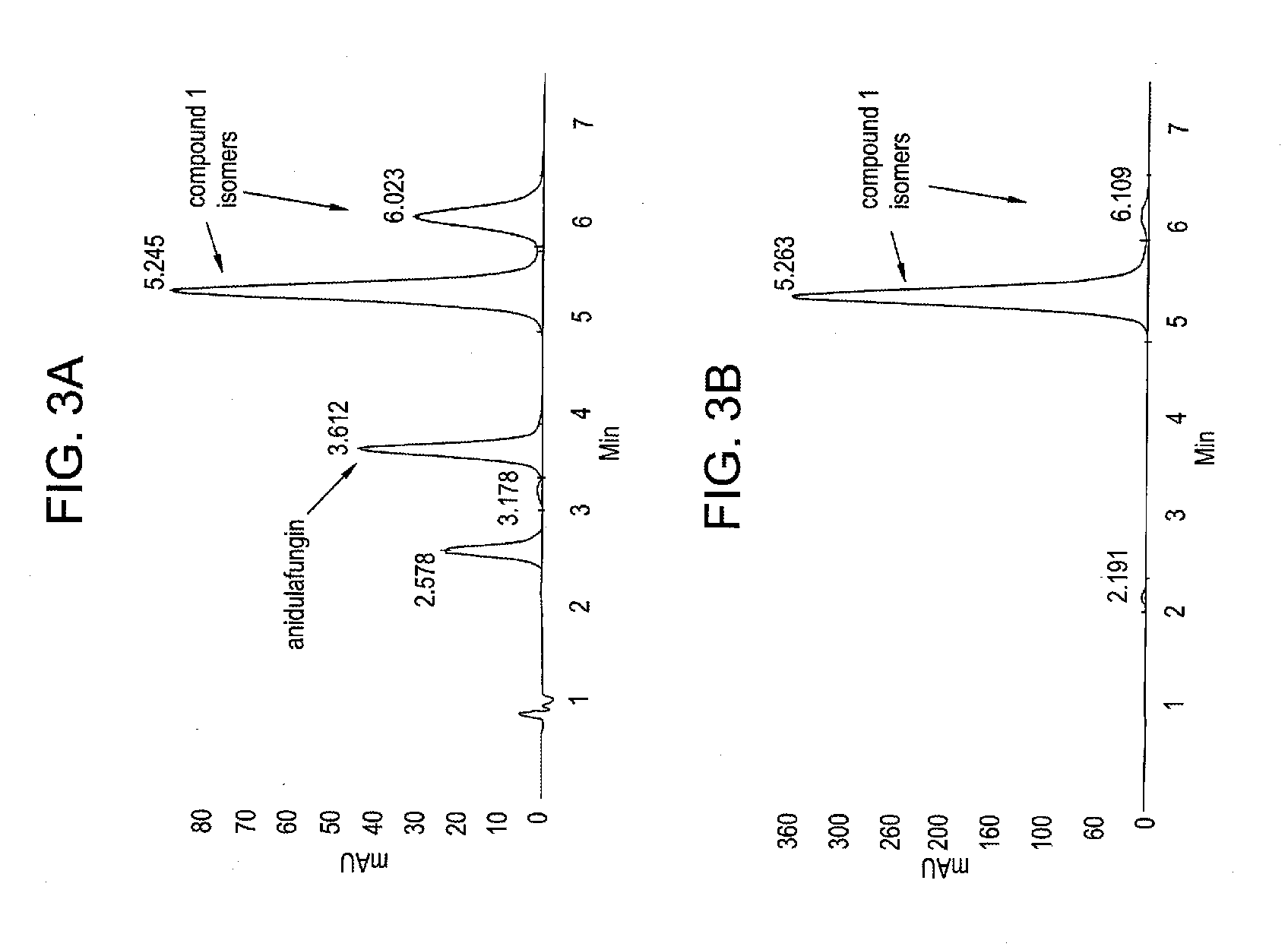
The invention discloses a purification method of echinocandins antifungal drug anidulafungi. The method comprises the following steps of: (1) preparing a dry sample: adding organic solvent in a crude product of the anidulafungin to dissolve the crude product, adding silica gel after the crude product of the anidulafungin is adequately dissolved, and uniformly mixing and drying the mixture to obtain the anidulafungin dry sample; (2) pressurizing and eluting: uniformly filling the anidulafungin dry samples in the top end of a chromatographic column with silica gel, pressurizing and eluting the chromatographic column with the anidulafungin dry samples by adding elution solvent, utilizing a high-effective liquid phase chromatography to monitor, and collecting the elution solution with the anidulafungin content being greater than 98 percent; and (3) concentrating: concentrating the elution solution with the anidulafungin content being greater than 98 percent until dryness to obtain a pure product with the anidulafungin content being greater than 98 percent. By adopting the column chromatography, simplicity in operation is realized, and the equipment cost is low; the organic solvent with low toxicity and low boiling point is adopted as the elution solution, so that the subsequent recycling treatment is simple, and the environmental pressure can be greatly reduced; and the purification separation time is short, the purification effect is good, the purification yield is high, and the purification method is applicable to industrialized mass production.
Owner:NCPC NEW DRUG RES & DEV
Echinocandin process
InactiveUS7214768B2Useful in treatmentPeptide preparation methodsImmunoglobulinsEchinocandinBoronic acid
This invention relates to an improved process for the minimization of acid-catalyzed reactions of certain echinocandins of the kind disclosed in U.S. Pat. No. 5,378,804. The process involves the use of a boronic acid.
Owner:MERCK SHARP & DOHME CORP
Echinocandin pharmaceutical formulations containing micelle-forming surfactants
Pharmaceutical formulations are described comprising an echinocandin compound or echinocandin / carbohydrate complex and a pharmaceutically acceptable micelle-forming surfactant in a non-toxic aqueous solvent such that the solubilization of the echinocandin compound is optimized and the ability to freeze-dry the solution is maintained. Both the solution and freeze-dried formulations have increased stability. A bulking agent, tonicity agent buffer and / or a stabilizing agent may optionally be added to the formulations to further enhance the stability of the formulation.
Owner:ELI LILLY & CO
Echinocandin biotransformation method
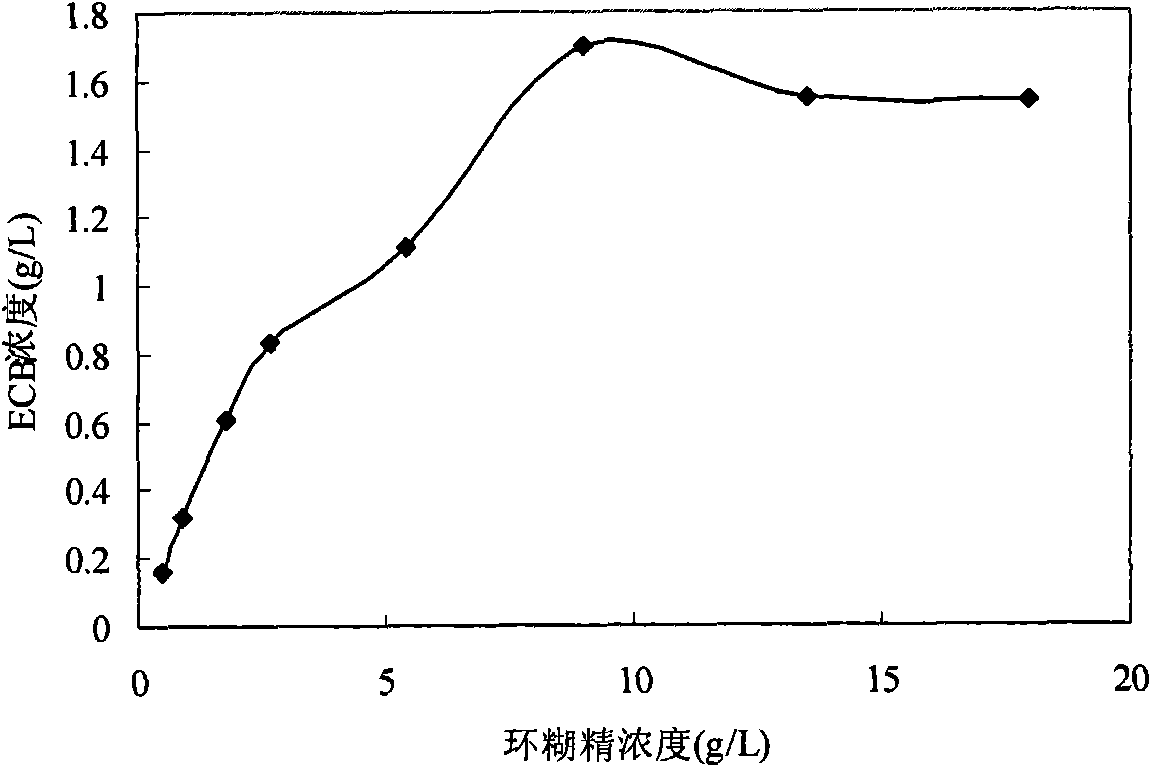
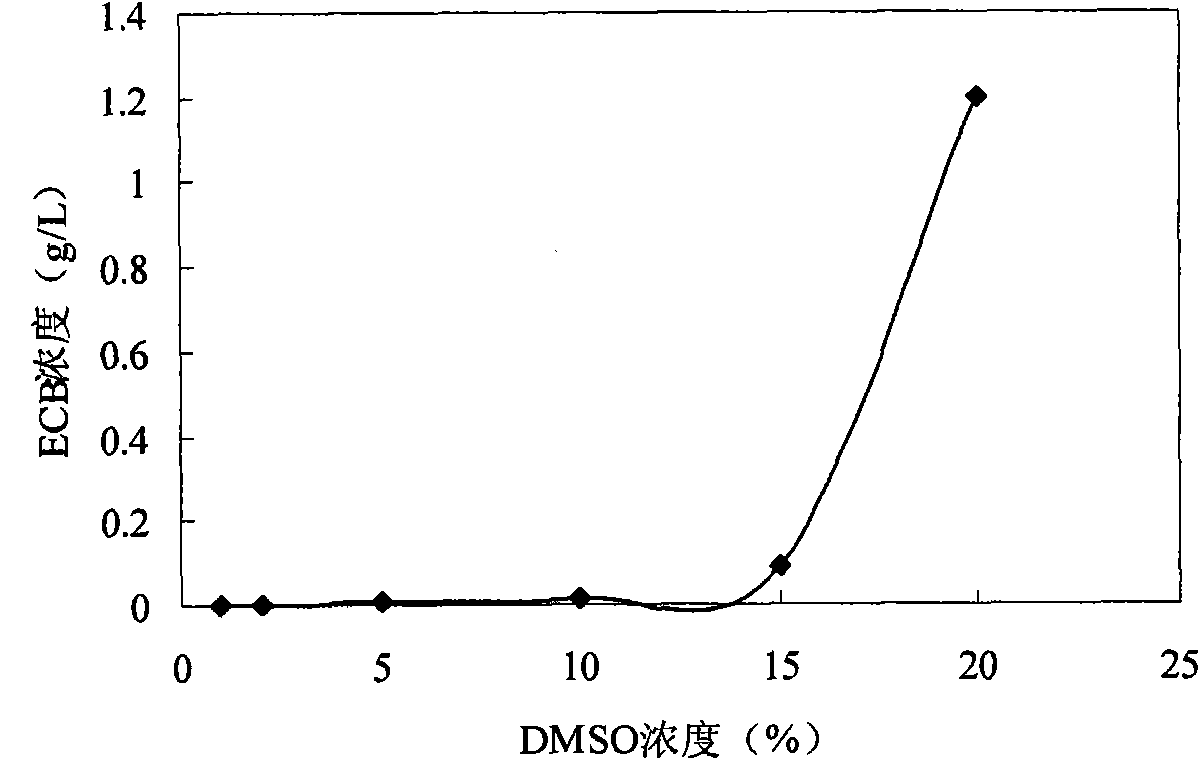
ActiveCN102618606AAvoid steps such as separation and purificationReduce manufacturing costMicroorganism based processesFermentationEchinocandinSolubility
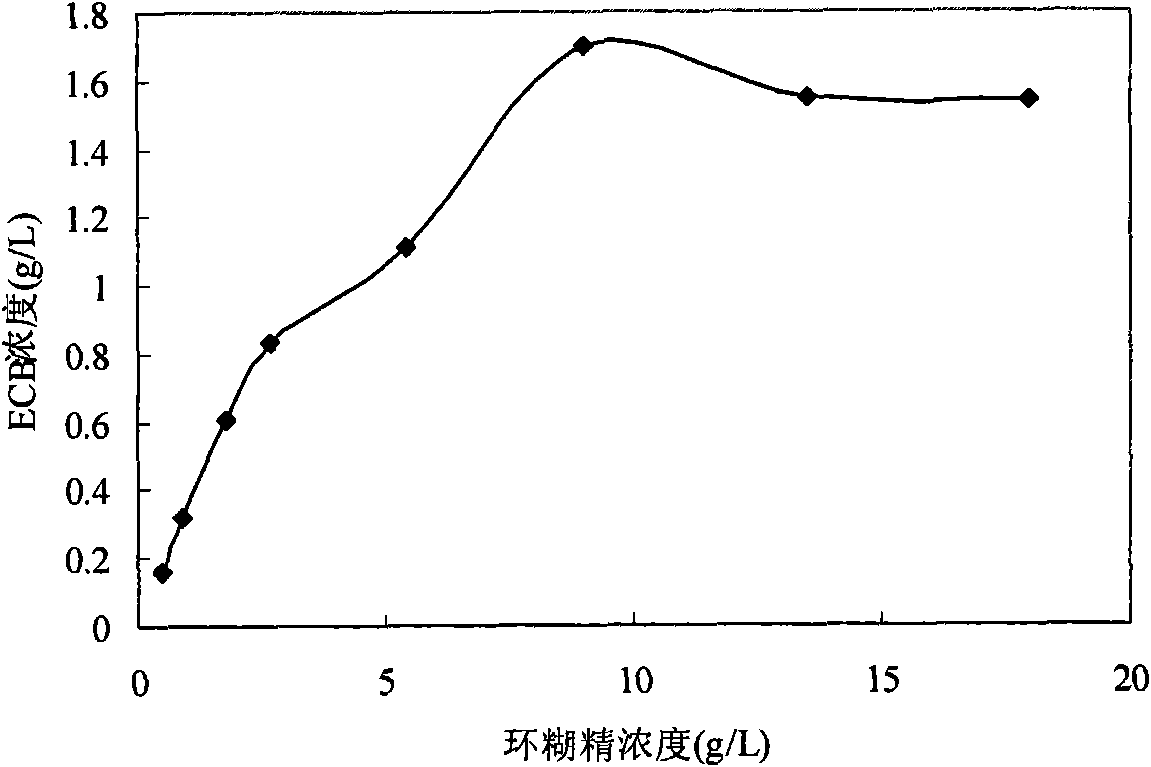
The invention discloses an echinocandin biotransformation method using an actinomycete whole cell or fermentation broth as a catalyst. The invention also provides an echinocandin biotransformation method using cyclodextrin and its derivative or other cosolvents. A transformation system involved in the invention is characterized in that the system can effectively improve the solubility of echinocandin compounds in an aqueous solution, improve the utilization rate and the reaction rate of substrates, and improve the transformation rate of products. Actinoplanes involved in the invention and their mutant strains are in a nutritional medium containing an assimilable carbon and nitrogen source and can generate whole-cell deacylated enzymes under ventilation conditions, and the whole cell deacylated enzymes can be used for the echinocandin biotransformation.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Antifungal parenteral products
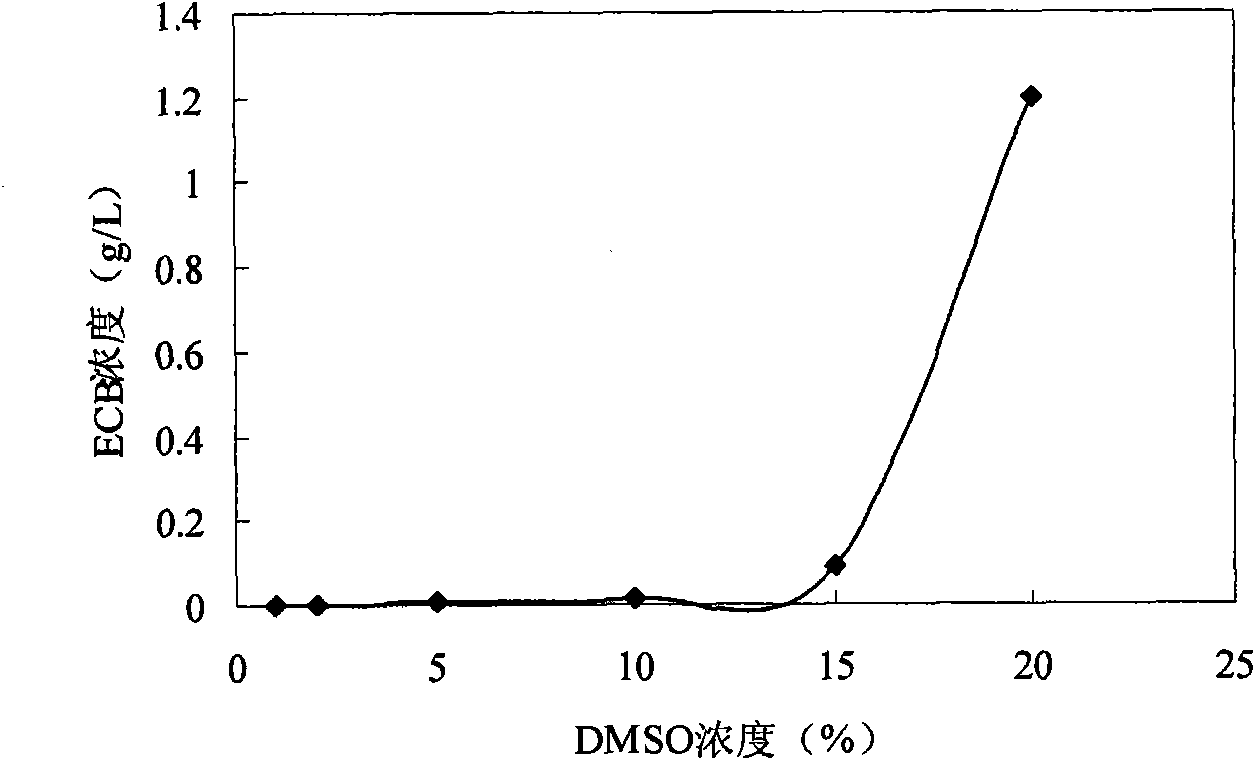
Parenteral pharmaceutical formulations containing an echinocandin antifungal compound and an aqueous solvent are provided, wherein the formulation includes ethanol, for example about 20% w / v ethanol. The parenteral pharmaceutical formulation may further include one or more additives, such as a stabilizing agent, buffer or tonicity agent. The parenteral pharmaceutical formulations are useful in extending the shelf life and improving the solubility of the echinocandin antifungal compound.
Owner:VICURON HLDG
Antifungal agents and uses thereof
The invention features echinocandin class compounds. The compounds can be useful for the treatment of fungal infections.
Owner:CIDARA THERAPEUTICS
Safer preparation method of echinocandin antifungal medicament
InactiveCN102219833ASafe preparationSuitable for industrial productionPeptidesPhenylboronic acidEchinocandin
The invention belongs to the technical field of organic synthesis and provides a method for preparing caspofungin. The preparation method of the caspofungin comprises the following steps: a) reacting a compound II with phenylboronic acid and a stereochemical structure selecting agent so as to generate a compound III, wherein the stereochemical structure selecting agent is 2-sulfydryl benzothiazole or 1-phenyl-5-sulfydryl-tetrazole; b) reducing the compound III into amine, namely, a compound IV; and c) finally reacting the compound IV with quadrol for substitution, so as to obtain a target compound. According to the preparation method of the caspofungin, the non-toxic stereochemical structure selecting agent is adopted, so that the preparation process is safer and the preparation method is more suitable for industrial production.
Owner:SHENZHEN JYMED TECH
Dosing regimens for echinocandin class compounds
The invention features pharmaceutical compositions, methods, and kits featuring dosing gimens and oral dosage formulations for administration of echinocandin class compounds.
Owner:CIDARA THERAPEUTICS
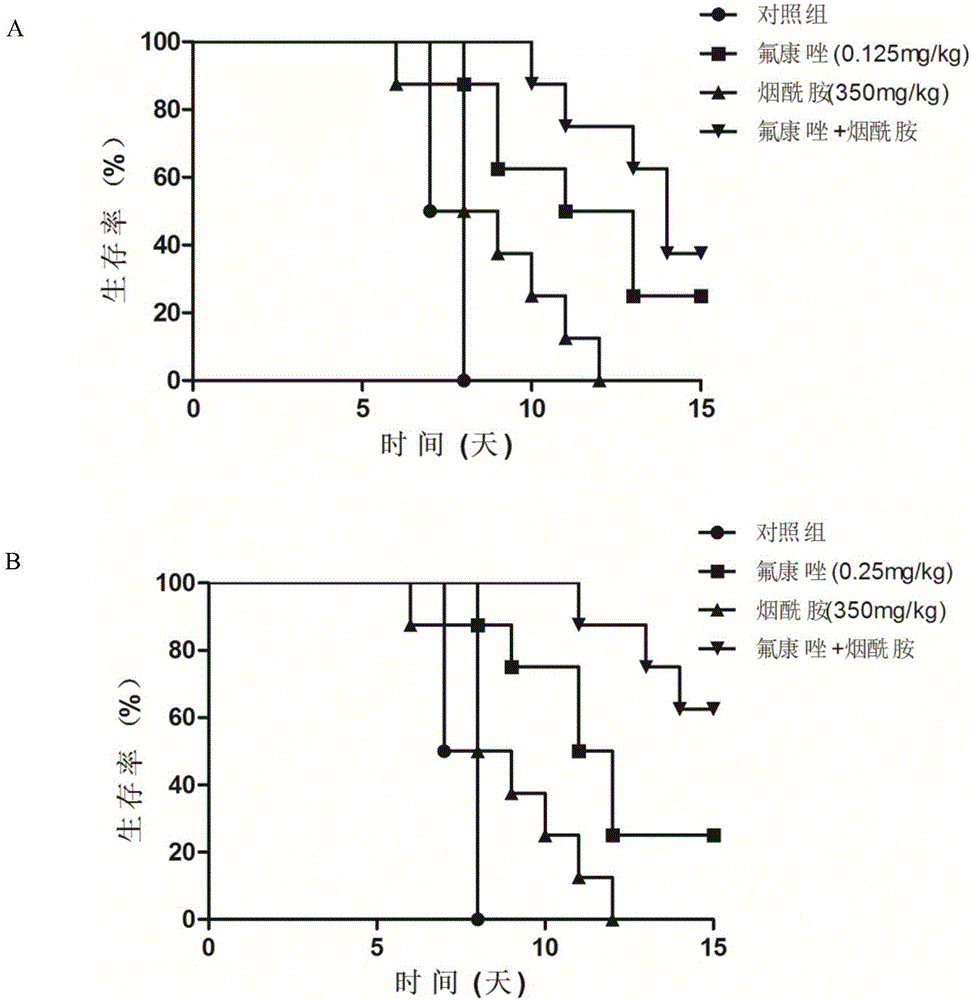
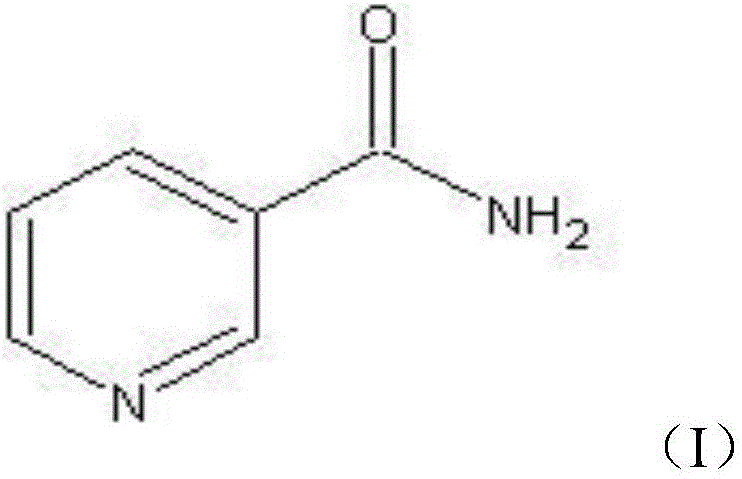
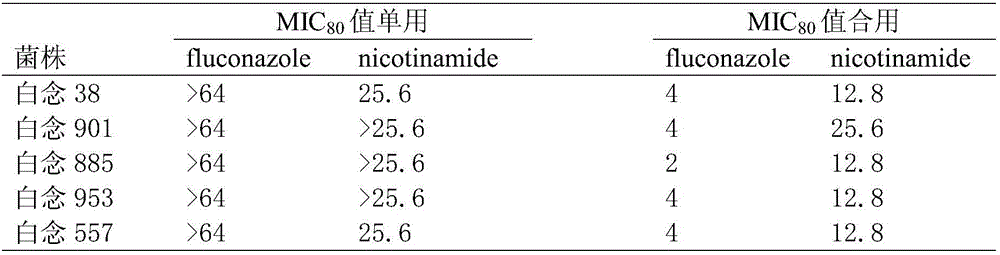
Applications of nicotinamide as antifungal drug synergist
ActiveCN106390130AEliminate side effectsLow toxicityAntibacterial agentsOrganic active ingredientsEchinocandinSide effect
The invention belongs to the technical field of medicine, and more specifically provides applications of nicotinamide in preparing an antifungal drug synergist. Novel applications of nicotinamide are provided; nicotinamide is capable of reducing dosages of antifungal drugs such as azoles, echinocandins, and polyenes as an antifungal drug synergist, and reducing toxic and side effect of drugs, especially fluconazole, voriconazole, caspofungin, and amphotericin B, accordingly. Nicotinamide is capable of recovering the effect of antifungal drugs on drug resistance funguses as a antifungal drug synergist, treating fungal infection especially drug resistance fungus infection effectively, reducing drug toxicity, and reducing economic burden of patients in drug therapy; and in addition, nicotinamide is capable of improving the effect of antifungal drugs on candida tropicalis and cryptococcus neoformans, and possesses high clinical application value.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Echinocandin antifungal pharmaceutical composition sustained release microsphere preparation and preparation method thereof
The invention belongs to the field of pharmaceutical preparations and relates to an echinocandin antifungal pharmaceutical composition sustained release microsphere preparation and a preparation method thereof. The pharmaceutical active ingredients of the echinocandin antifungal pharmaceutical composition comprises caspofungin acetate and antifungal agents respectively being polyenes and triazoles; and the sustained release microsphere comprises the following materials in percentage by total weight of microsphere: 0.1%-30% (w / w) of active ingredients, 60%-99.9% daltonian biodegradable and biocompatible high polymer material with molecular weights of 5,000-200,000 and 0%-10% of pharmaceutically acceptable other auxiliary materials. The average grain diameter of the sustained release microsphere is 5-20 microns, and the encapsulation efficiency is more than 85%. The sustained release period of the sustained release microsphere is up to 50 days, the drug use frequency is significantly reduced, the bioavailability is improved, and the toxic and side effects of the drug are reduced, thus being beneficial to clinical treatment. The product is good in reproducibility of production technology and good in feasibility.
Owner:南京星银药业集团有限公司
Echinocandin antifungal agent caspofungin containing medicinal composition, a preparation method and application thereof
The invention discloses an echinocandin antifungal agent caspofungin containing medicinal composition, which contains cane sugar used as an excipient. The medicinal composition provide by the invention has good stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Echinocandin compound purifying method
The invention relates to an echinocandin compound purifying method, and especially relates to a WF11899A purifying method. The method comprises the processes of pre-purifying solution preparation, resin chromatography separation, crystallization and the like. The method has the advantages of simplicity, no use of a mixed solvent, high sample purity and the like.
Owner:ZHEJIANG HISUN PHARMA CO LTD
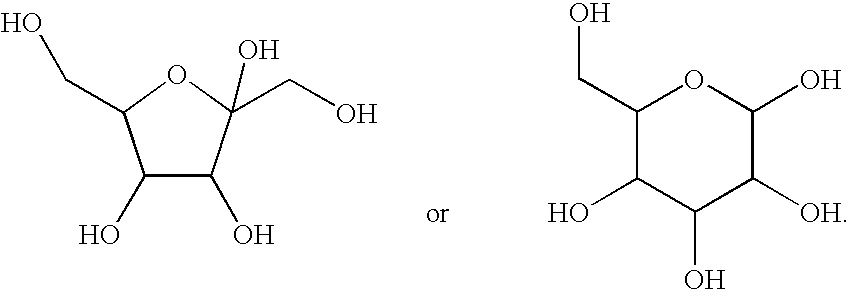
Echinocandin/carbohydrate complexes
InactiveUS7041637B2Improve thermal stabilityGood water solubilityBiocideAntimycoticsSolubilityEchinocandin
A complex of an echinocandin compound with a carbohydrate is described having improved thermal stability and water solubility. A process for making the echinocandin / carbohydrate complex is also described as well as the use of the complex in pharmaceutical formulations and treatments of fungal infections.
Owner:ELI LILLY & CO
Echinocandin biotransformation method
ActiveCN102618606BAvoid steps such as separation and purificationReduce manufacturing costMicroorganism based processesFermentationSolubilityEchinocandin
The invention discloses an echinocandin biotransformation method using an actinomycete whole cell or fermentation broth as a catalyst. The invention also provides an echinocandin biotransformation method using cyclodextrin and its derivative or other cosolvents. A transformation system involved in the invention is characterized in that the system can effectively improve the solubility of echinocandin compounds in an aqueous solution, improve the utilization rate and the reaction rate of substrates, and improve the transformation rate of products. Actinoplanes involved in the invention and their mutant strains are in a nutritional medium containing an assimilable carbon and nitrogen source and can generate whole-cell deacylated enzymes under ventilation conditions, and the whole cell deacylated enzymes can be used for the echinocandin biotransformation.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Medicinal composition for treating complex infection and preparation method of medicinal composition
InactiveCN104888222AConvenient clinical administrationAntibacterial agentsOrganic active ingredientsEchinocandinOritavancin
The invention relates to a medicinal composition for treating complex infection and a preparation method thereof. The medicinal composition is characterized by comprising an active ingredient selected from one of glycopeptides antibacterial drugs of telavancin, dalbavancin, oritavancin, vancomycin, teicoplanin or a pharmaceutically acceptable salt thereof, and also comprising polyene and echinocandin type antifungal agents, and being prepared into a freeze-dried powder injection by adopting a conventional process. The medicinal composition provided by the invention realizes combined application of the antibacterial drugs and the antifungal agents, can simultaneously give play to an antibacterial infection effect and an antifungal infection effect, and is convenient for clinical use.
Owner:SHENZHEN JYMED TECH
Medicinal composition containing echinocandin antifungal agent and its preparing method and its use
The invention discloses a medicinal composition containing an echinocandin antifungal agent, wherein the pharmaceutical composition contains a pharmaceutically acceptable amount of excipient, and a pharmaceutically acceptable amount of phosphate buffer agent. The invention provides the medicinal composition having good stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Method for obtaining high purity echinocandin D
The invention discloses a method for obtaining high purity echinocandin D. The method comprises the steps of: (1) mixing a solution containing an echinocandin D crude product with a methanol aqueous solution 1 so as to obtain a solution 1; (2) loading the solution 1 to a reversed-phase chromatography column; and (3) conducting elution with a methanol aqueous solution 2, thus obtaining the high purity echinocandin D.
Owner:SHANGHAI INST OF PHARMA IND
Pharmaceutical composition for candida albicans echinocandin drug-resistant bacteria and application of pharmaceutical composition
ActiveCN110859950AEfficient killingFor invasive infectionOrganic active ingredientsAntimycoticsEchinocandinResistant bacteria
Owner:WUHAN UNIV
Novel cytoprotective drugs
The invention relates to compounds of the echinocandin family or a semi-synthetic derivative thereof or a salt thereof or an ester thereof, or an ester salt thereof which is intended to be used as a cytoprotective drug, particularly as a drug for the prevention and / or protection and / or treatment of cell death and / or of degenerative pathological situations or processes which result in cell death, and even more particularly as a drug for the prevention and / or protection and / or treatment of cell death and / or degenerative pathological situations or processes which result in cell death, when they are associated with ischaemia-reperfusion.
Owner:巴勒姆移植
Echinocandin fungi residue and sludge mixing wet-type anaerobic digestion cooperated disposal method
ActiveCN107119079AImprove biodegradabilityReduce residual riskSludge treatment by pyrolysisWaste based fuelEchinocandinEconomic benefits
Owner:NANJING AGRICULTURAL UNIVERSITY
Purification processes for echinocandin-type compounds
Owner:TEVA PHARM USA INC
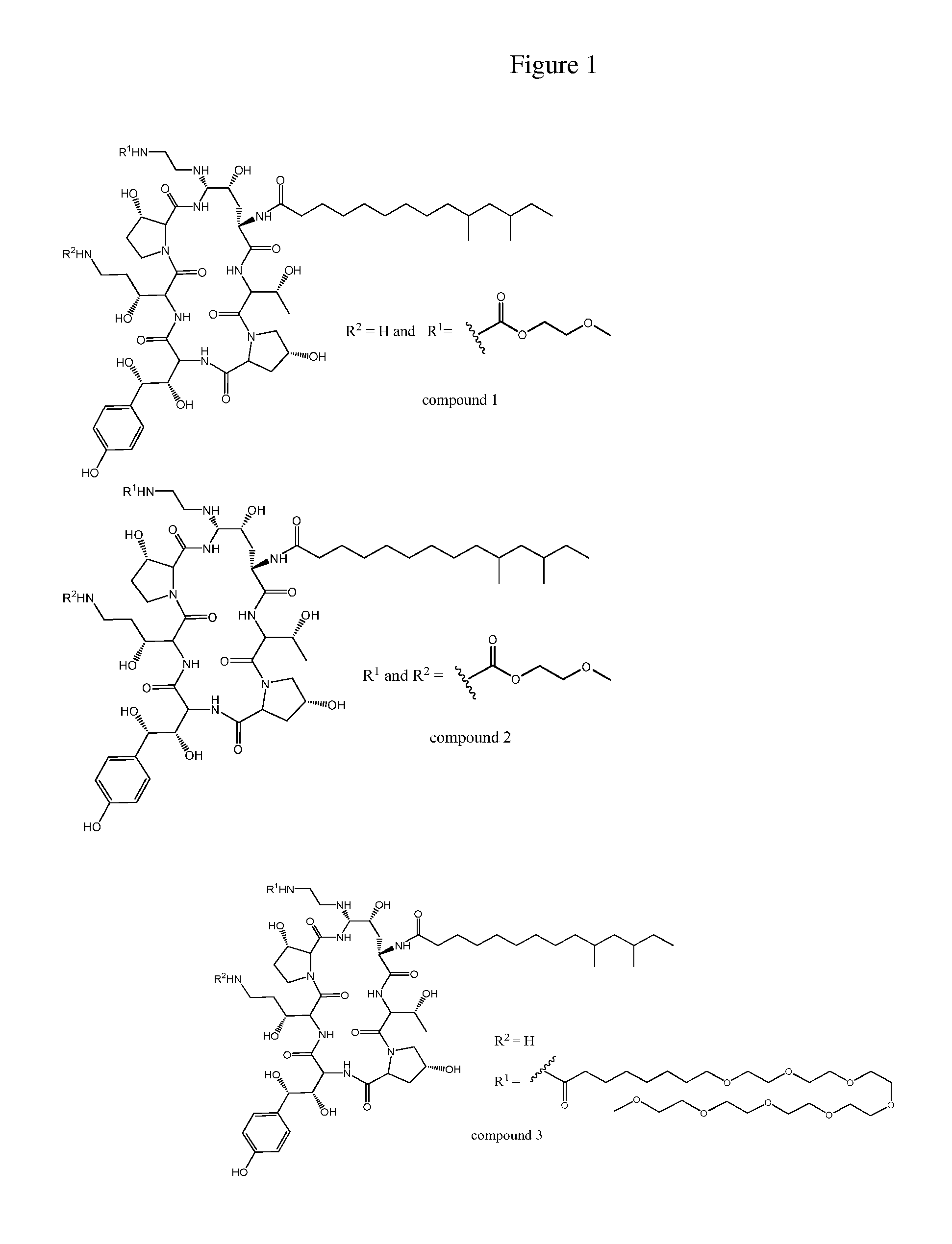
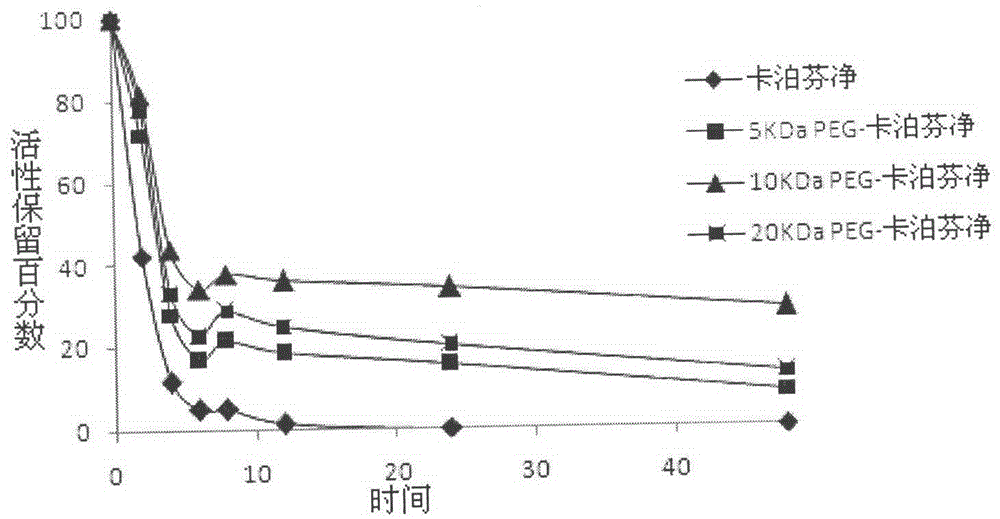
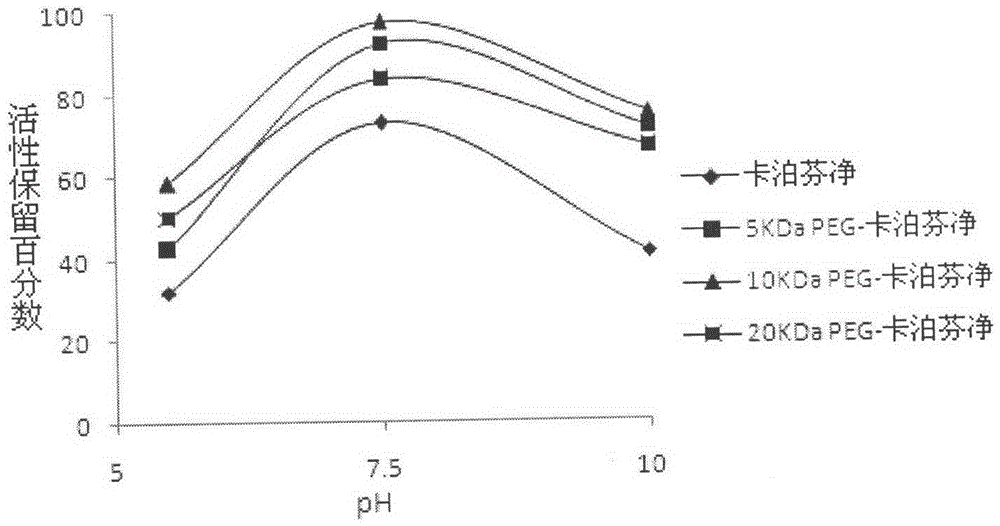
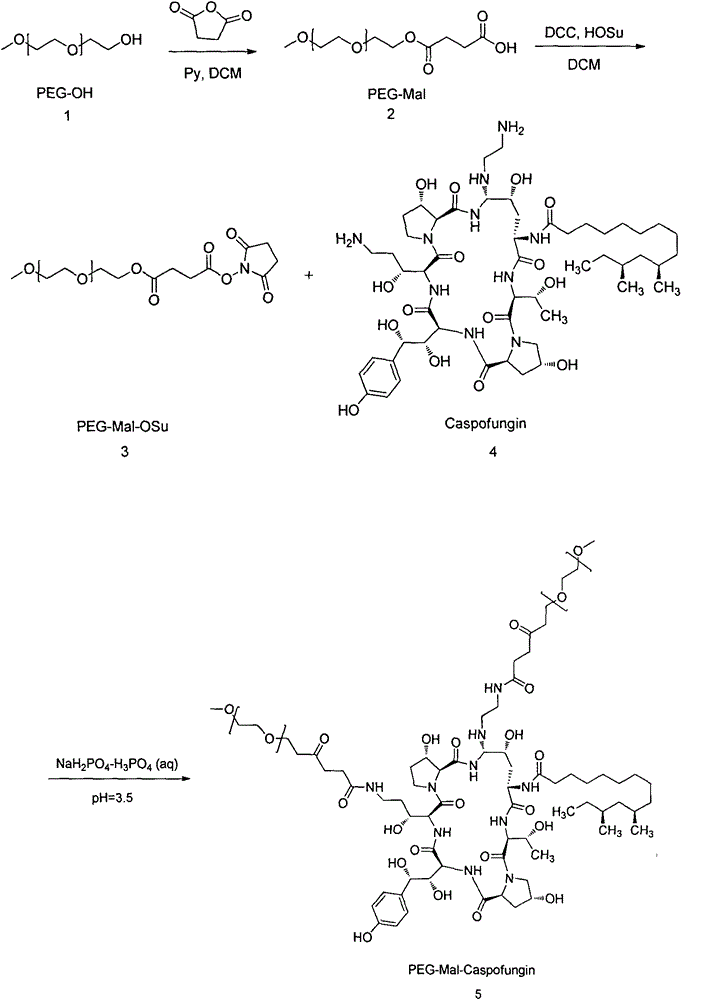
PEG-modified echinocandin antifungal drug complex and preparation thereof
The present invention relates to a PEG-modified echinocandin antifungal drug complex and a preparation method thereof, wherein the preparation method is characterized in that PEG and maleic anhydride are bound to form an active ester, and then the active ester and an echinocandin antifungal drug caspofungin are subjected to two-site PEG modification so as to improve the drug stability, save the pharmaceutical activity, and increase the sustained release effect of the drug.
Owner:南京星银药业集团有限公司 +1
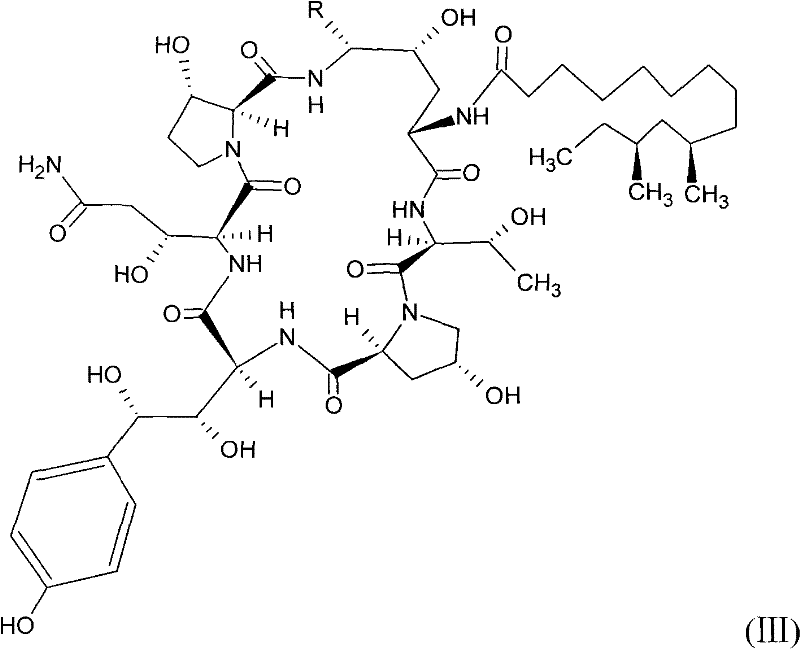
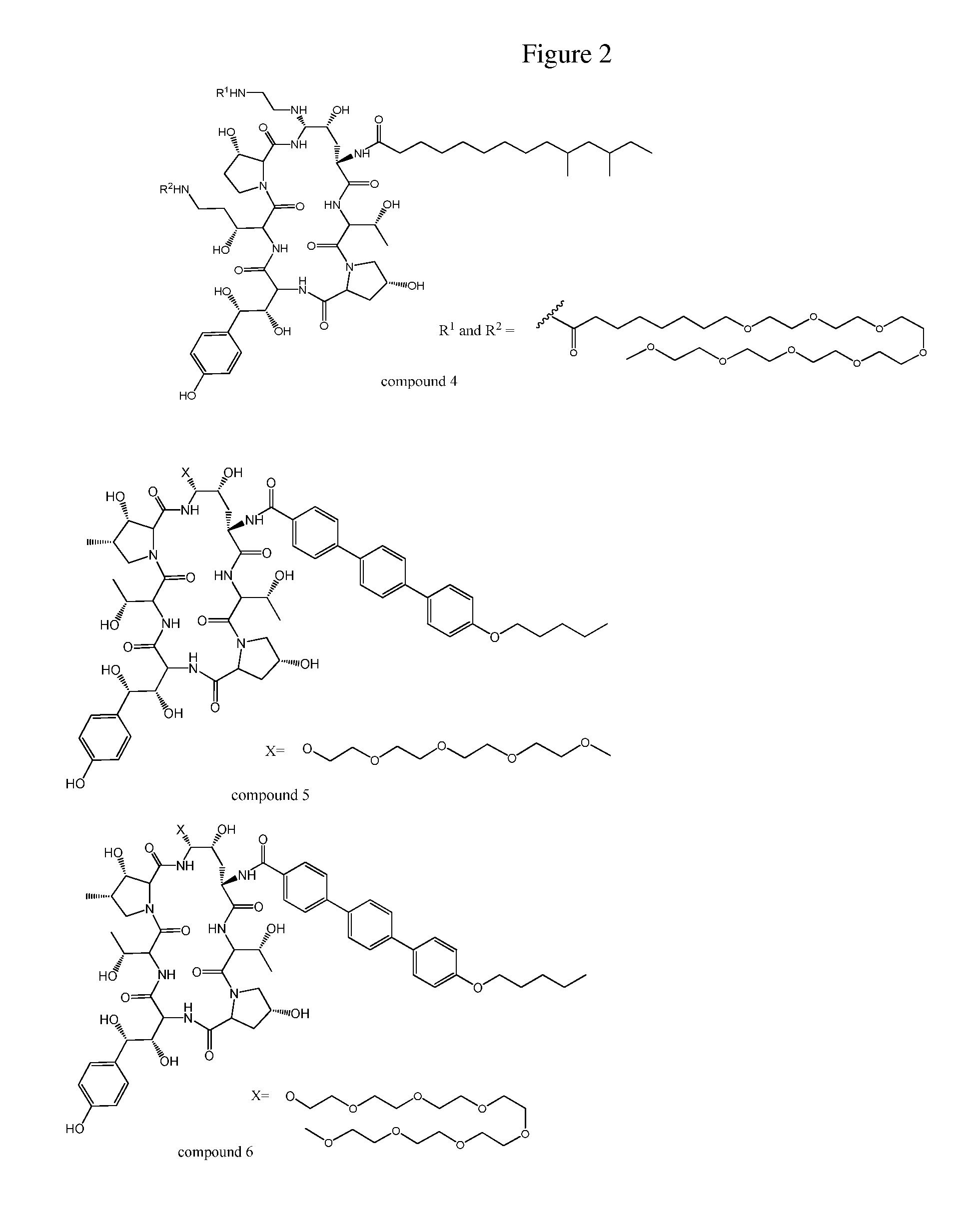
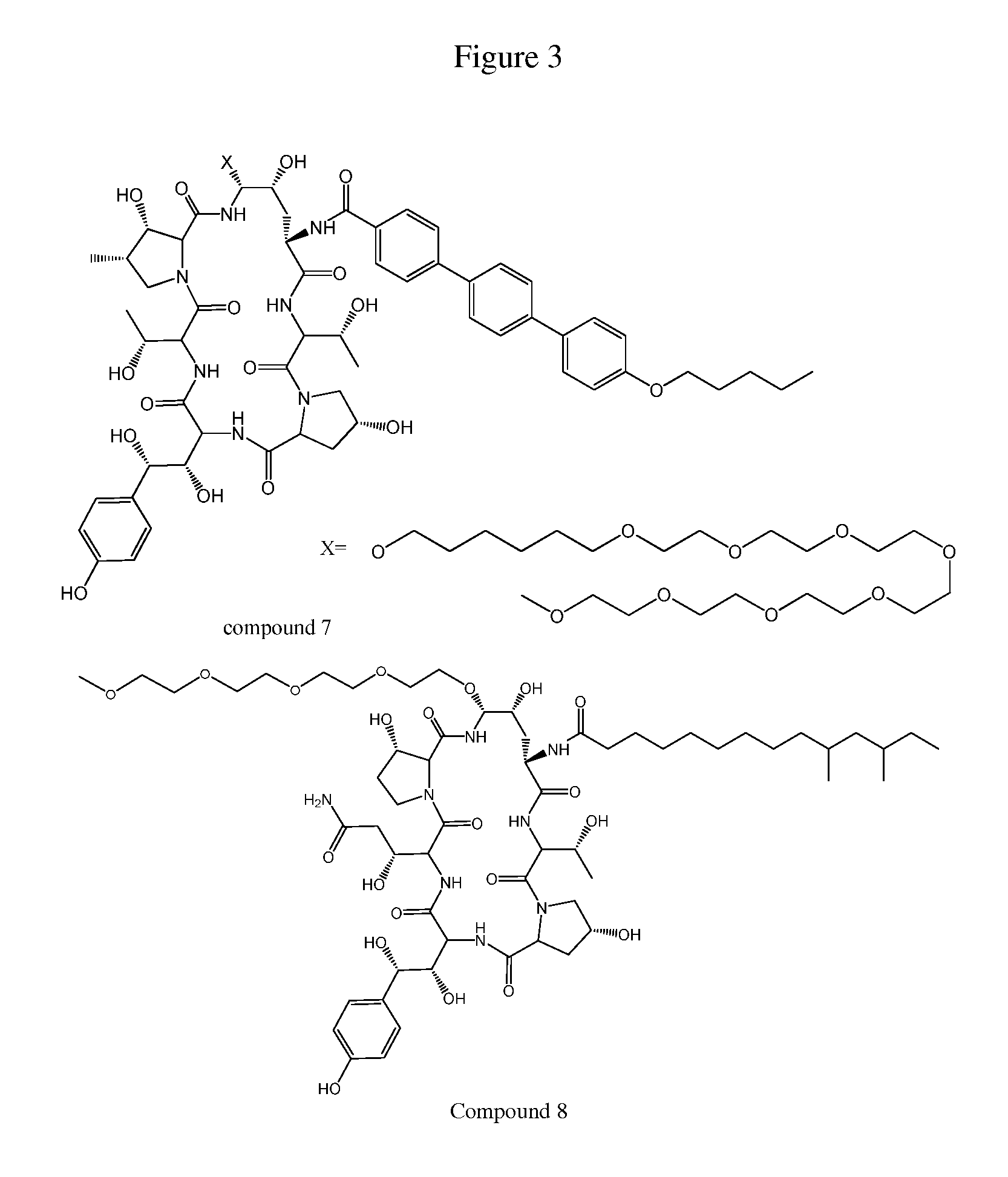
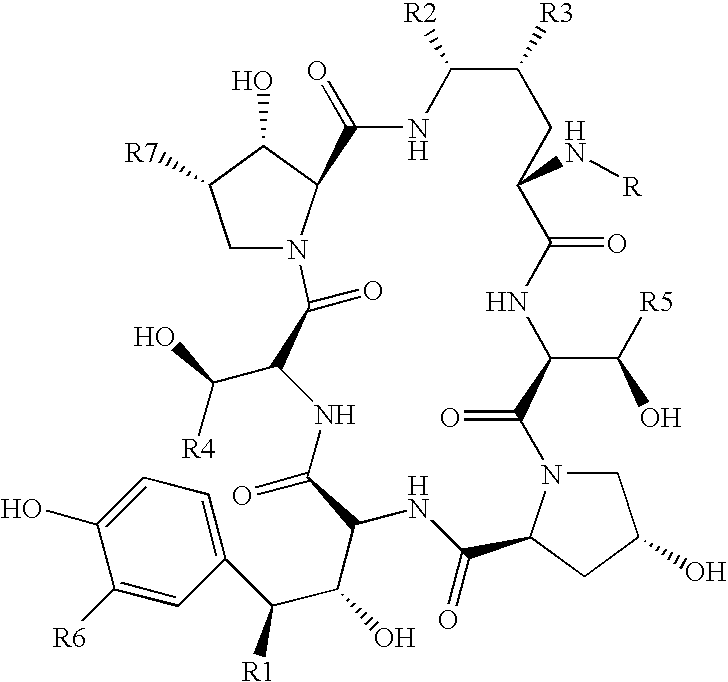
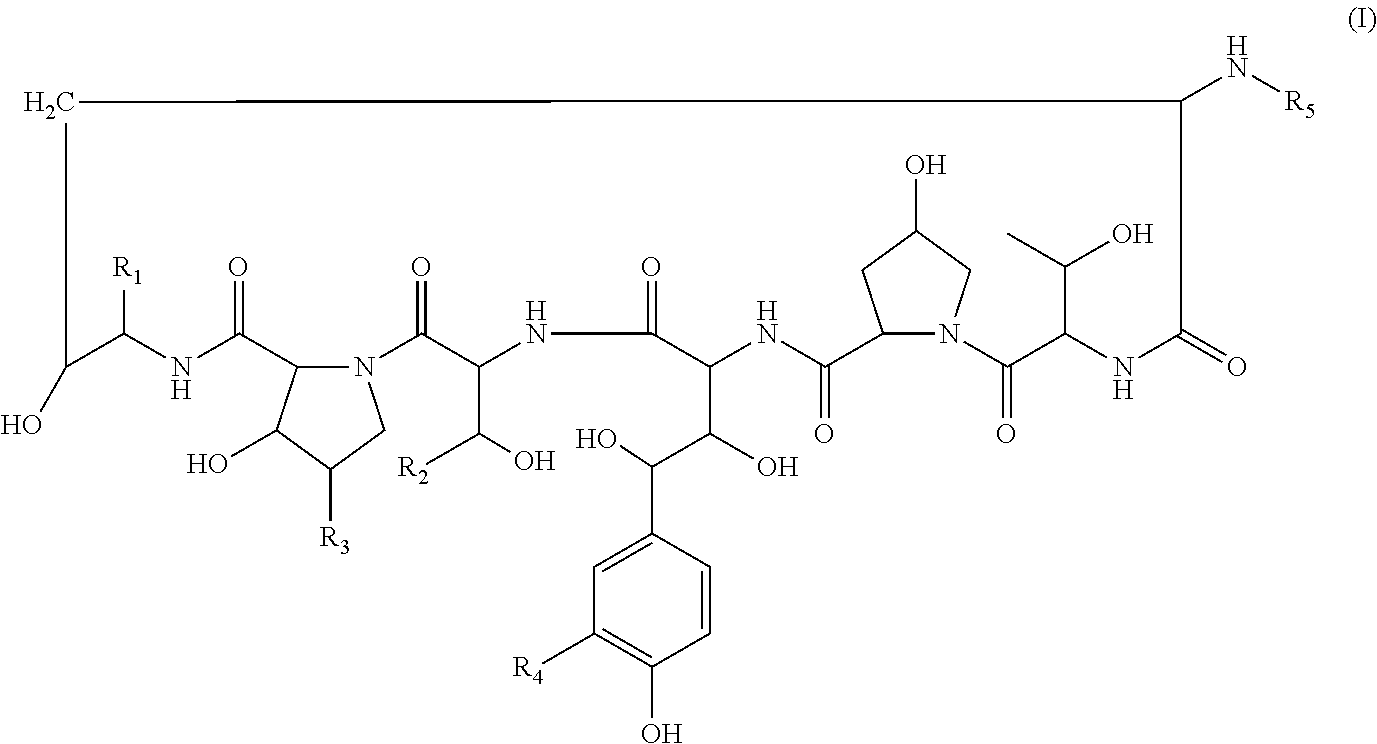
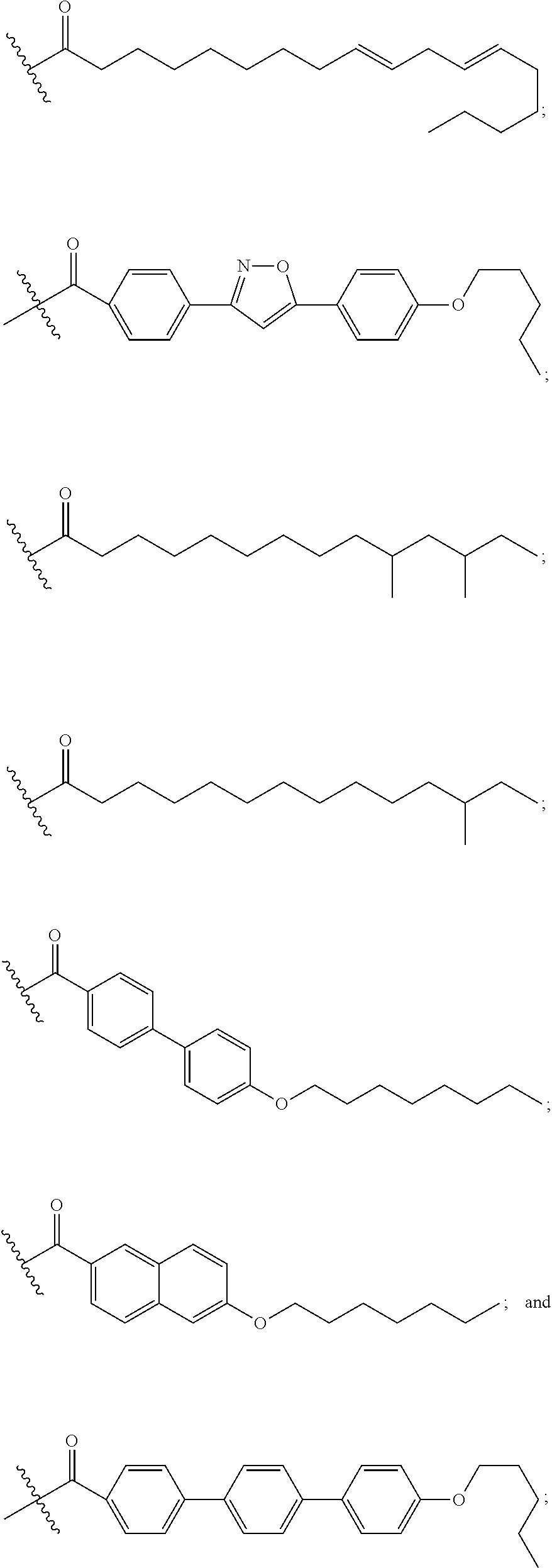
Echinocandin derivatives
InactiveUS20120190613A1Improve oral bioavailabilityImprove bioavailabilityBiocideAntimycoticsEchinocandinAryl
The invention features echinocandin class compounds that have been modified to (i) have activity against one or more fungal species or genera; (ii) have increased aqueous solubility; (iii) have an increased therapeutic index; (iv) be suitable for topical administration; and / or (v) be suitable for oral administration. The echinocandin class compounds of the invention include, for example, a PEG, alkyl-PEG, aryl-PEG, alkaryl-PEG, PEG-alkyl, PEG-aryl, or PEG-alkaryl substituent.
Owner:JAMES JR KENNETH DUKE +1
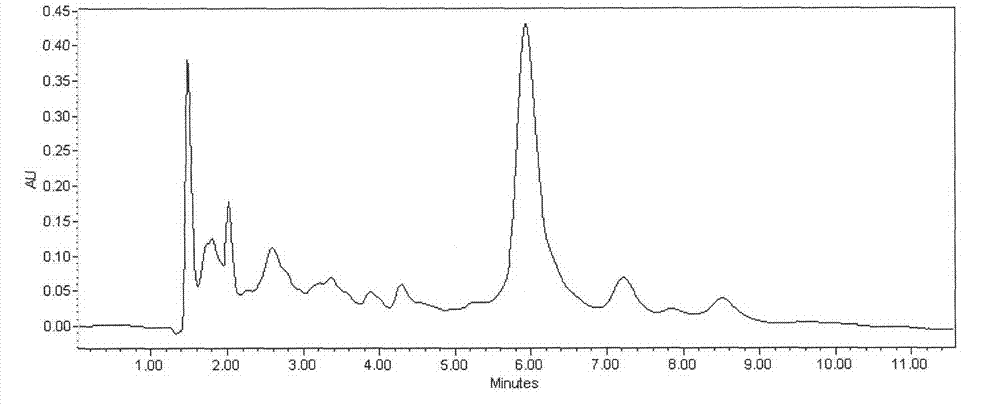
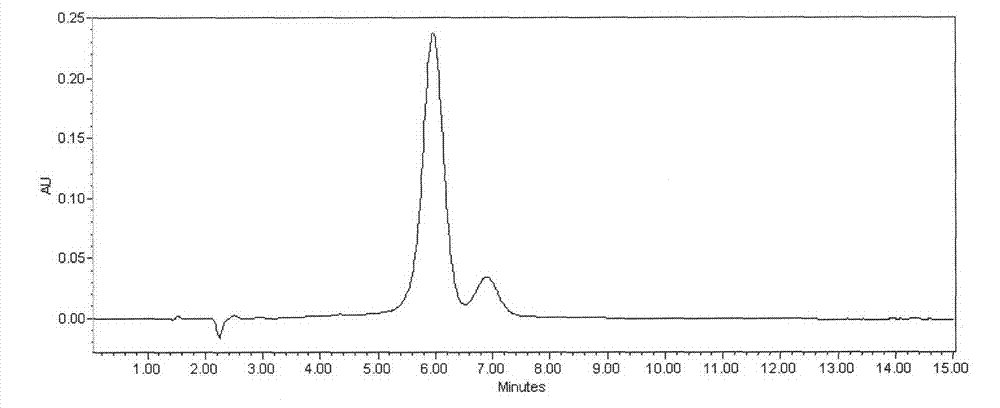
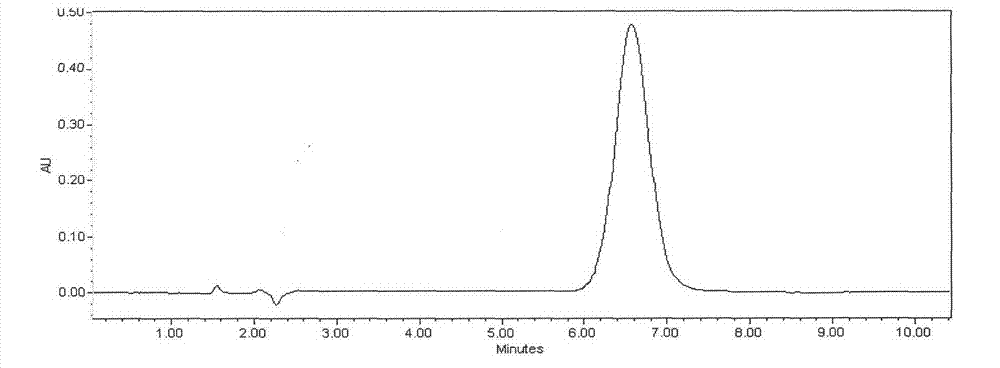
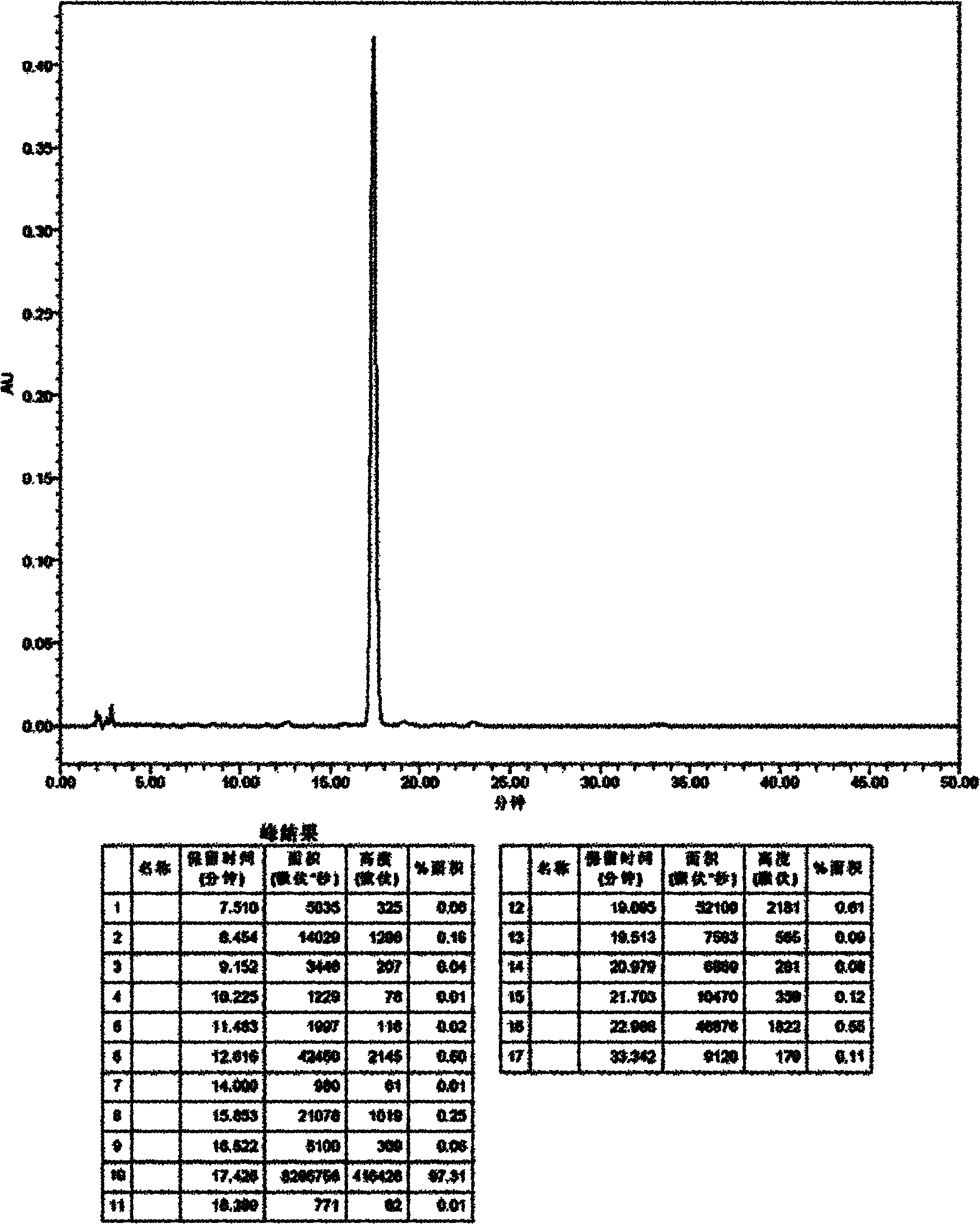
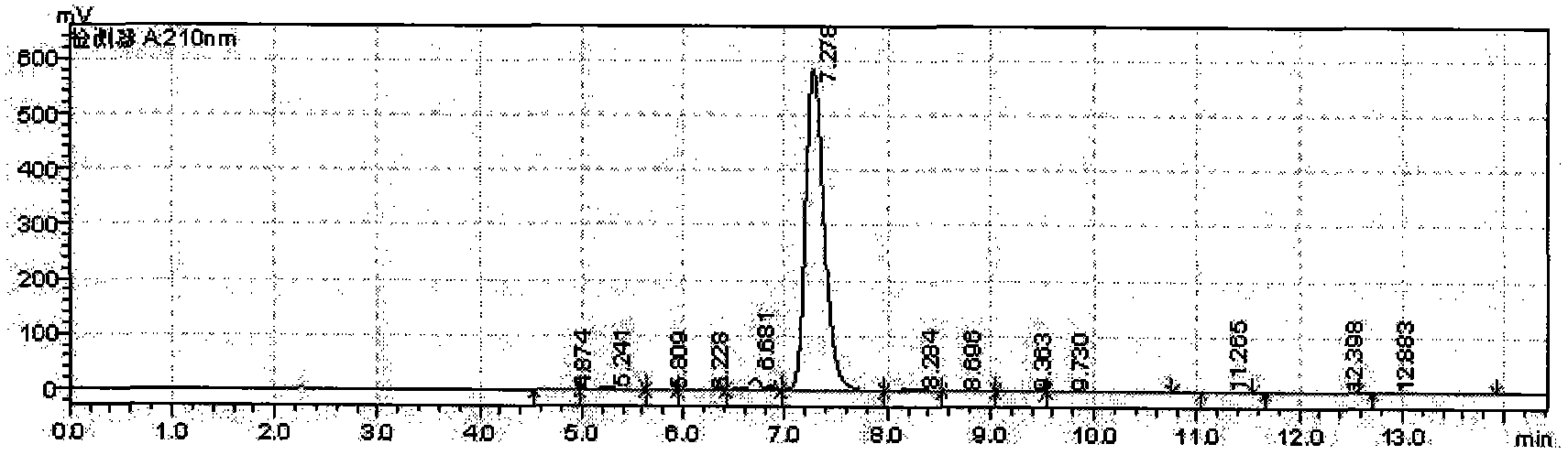
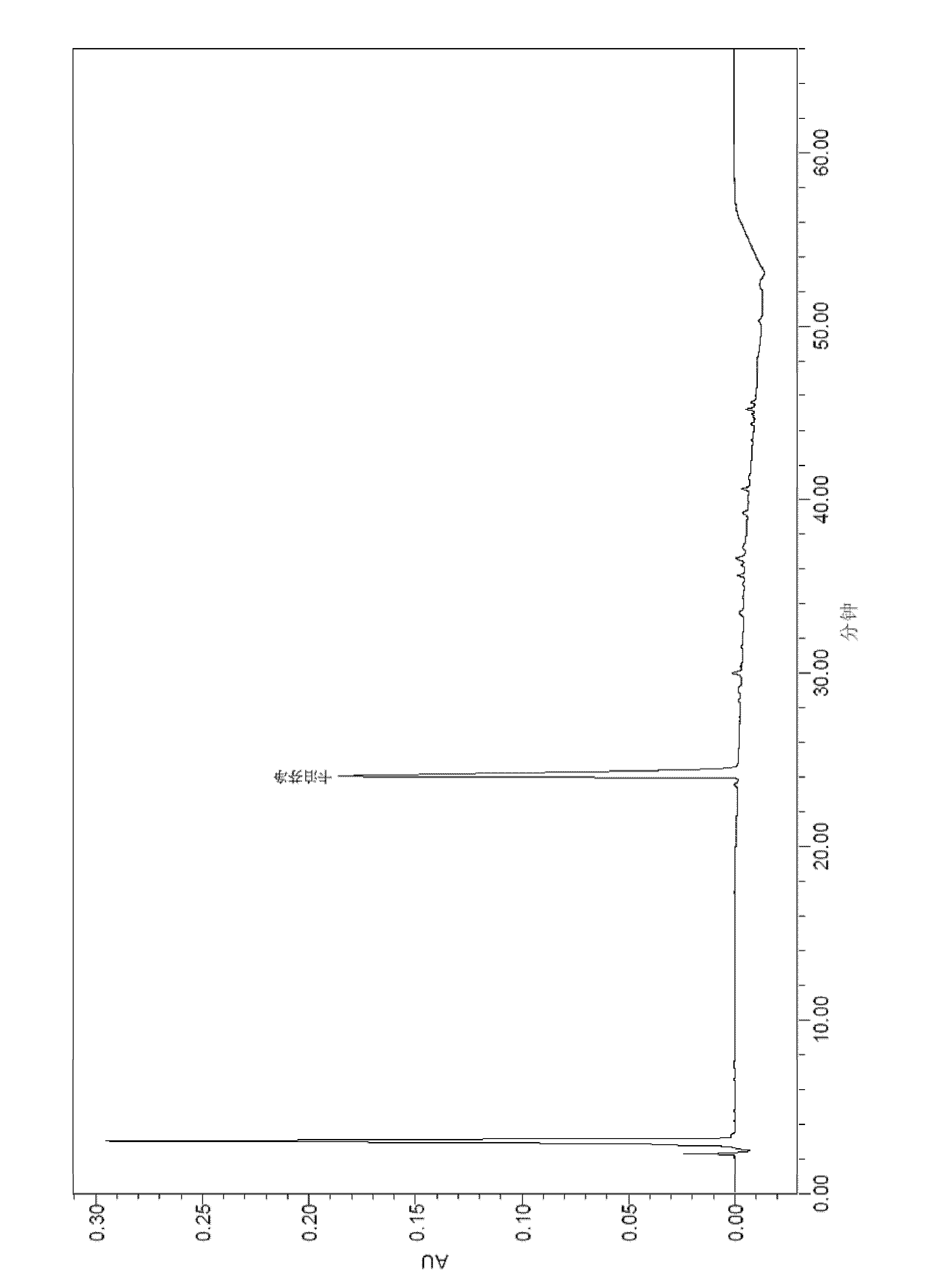
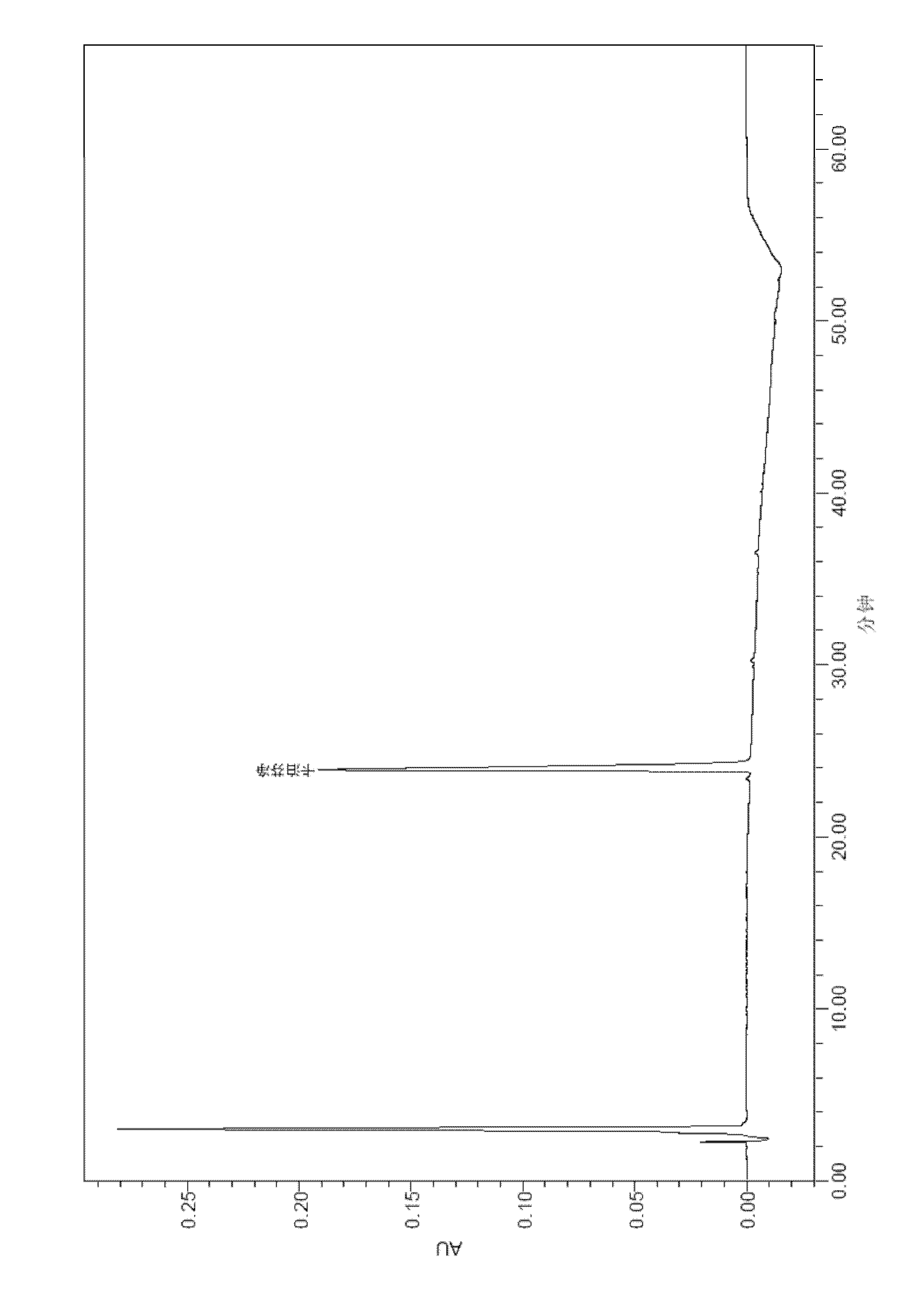
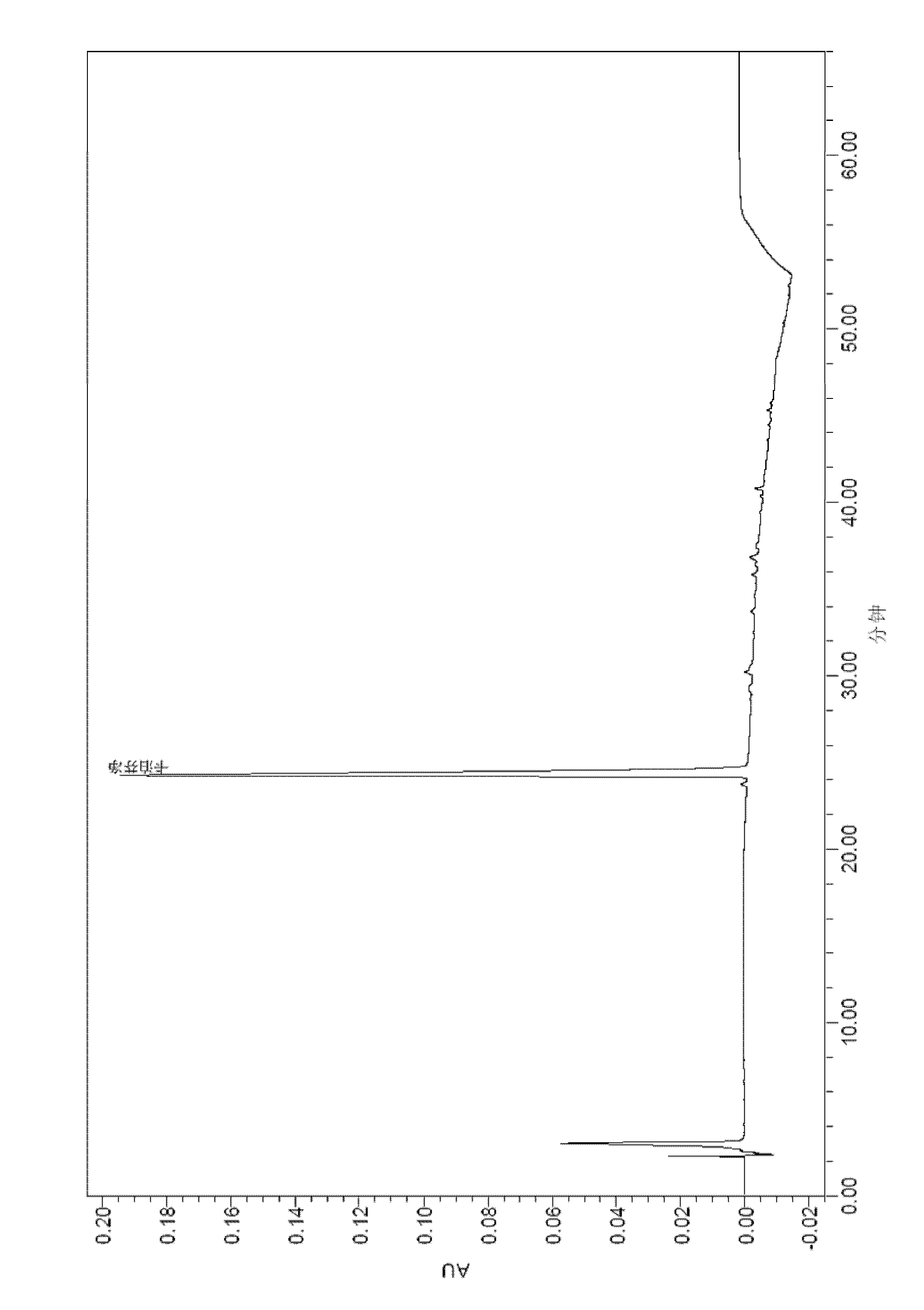
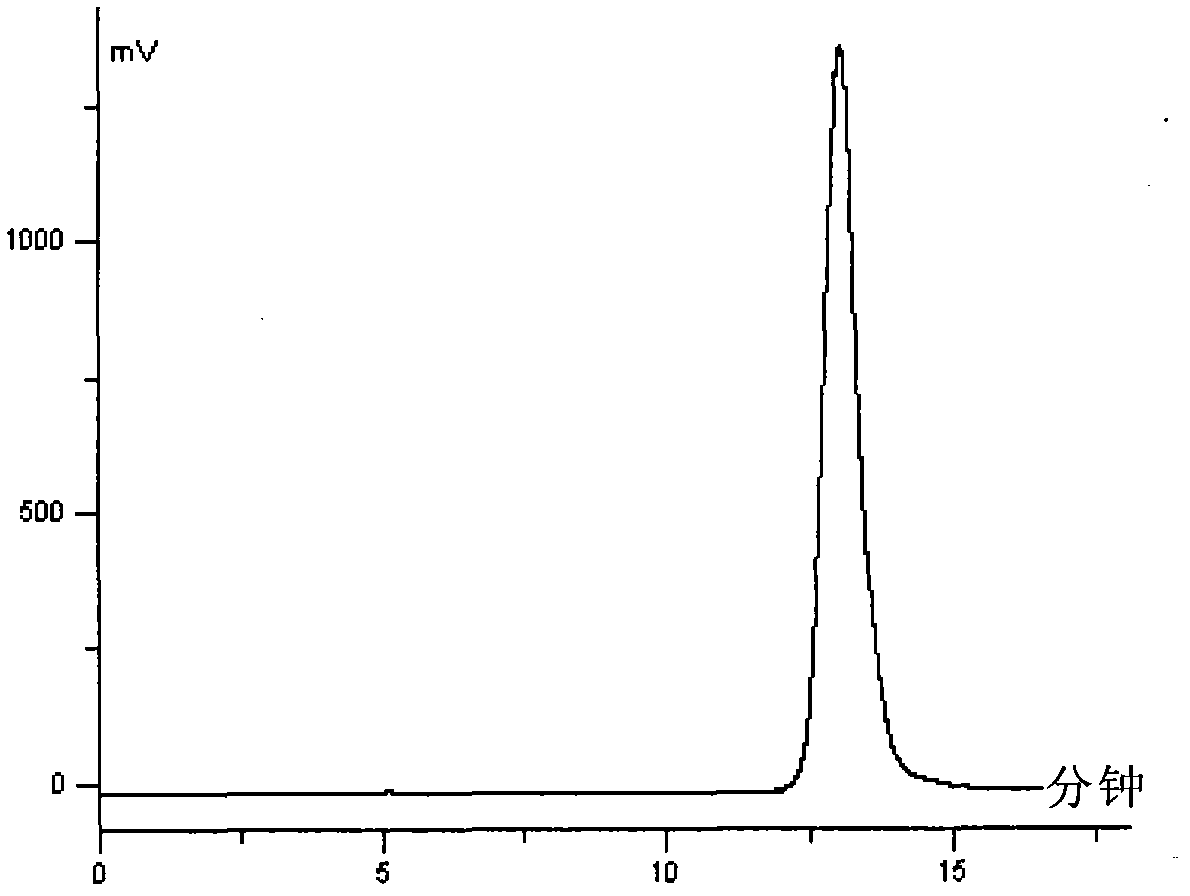
Detection method of echinocandin
The invention belongs to the technical field of medicines, and provides a high performance liquid chromatography method for detecting echinocandin B0 and C0, wherein the detection conditions are as follows: a chromatographic column is Ultra Amide, 4.6 * 250 mm, the filler particle size is 3.5 [mu]m, the column temperature is 30-50 DEG C, the flow rate is 1-2.5 mL / min, the sample injection volume is 10-20 microlitres, the detection wavelength is 200-230 nm, and a mobile phase adopts water and acetonitrile for gradient elution. The detection method is high in precision, high in accuracy, good inreproducibility, simple, convenient and practical, can realize simultaneous detection of echinocandin B0 and echinocandin C0, and has a very wide application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthesis of echinocandin antifungal agent
The present invention relates to echinocandin cyclopeptides and to methods for preparing echinocandin cyclopeptides.
Owner:CIDARA THERAPEUTICS
Purification process for lipopeptides
InactiveUS9394340B2Simple and rapid and efficient methodSimple structurePeptide preparation methodsImmunoglobulinsAntifungalAdditive ingredient
The present invention provides simple, cost effective, rapid, and scalable at industrial scale and provide high purity and yield of Echinocandin-type compounds at low cost as compared to prior art. Moreover the process allows for the removal of impurities by using economical salt-adsorbent complex and provide highly purified Echinocandin type compounds which is highly improved in terms of purity and sufficient for further processing to obtain an active pharmaceutical ingredient such as, the antifungals caspogungin, anidulafungin, and micafungin.
Owner:CADILA HEALTHCARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com