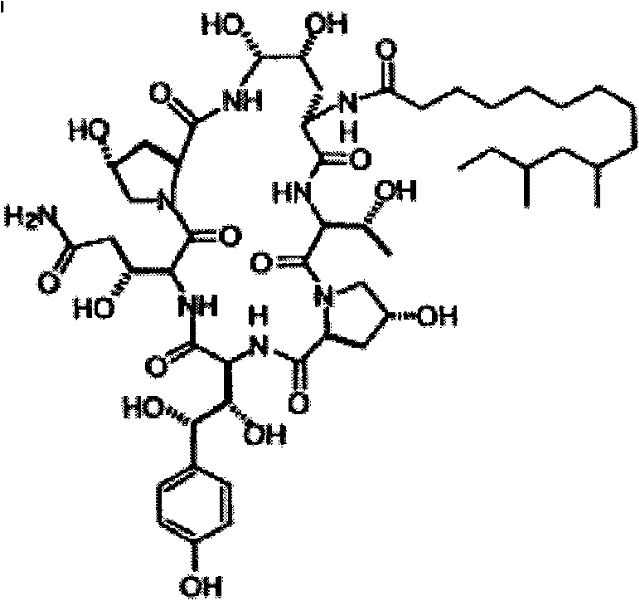
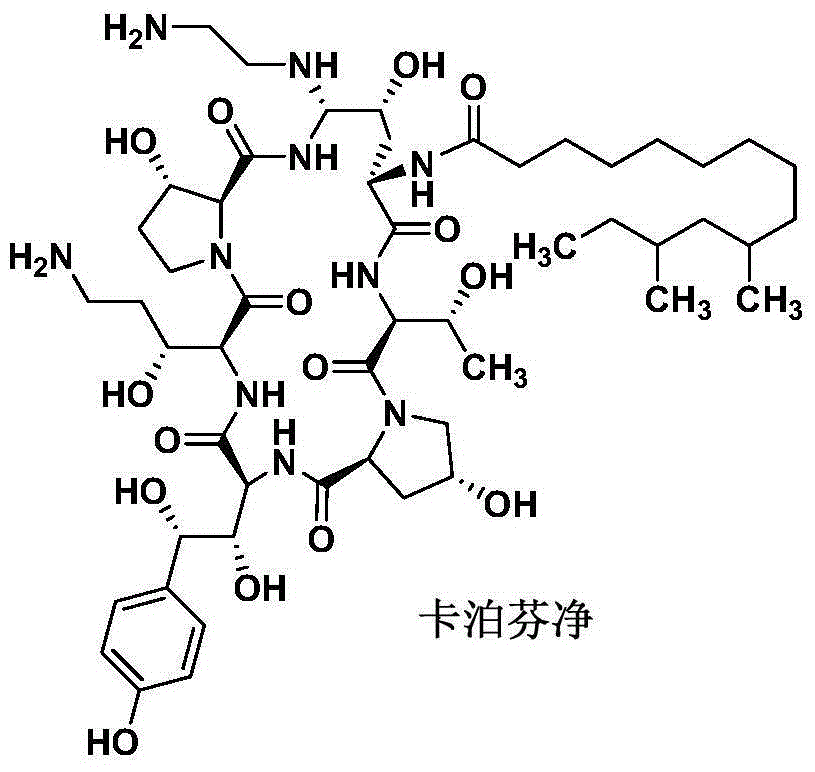
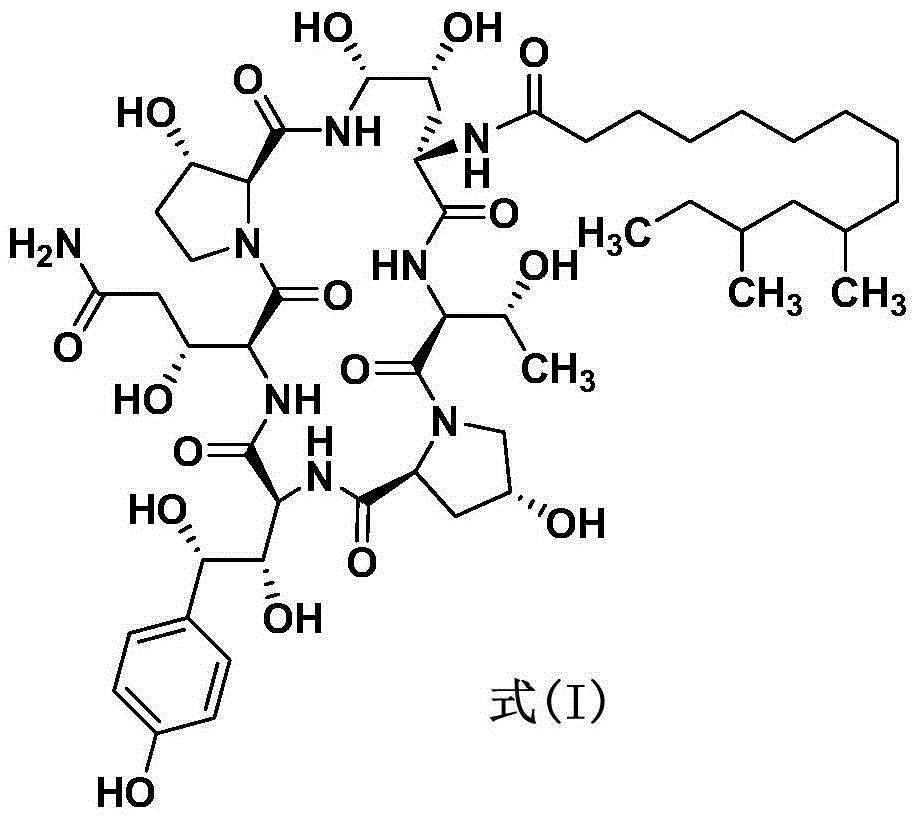
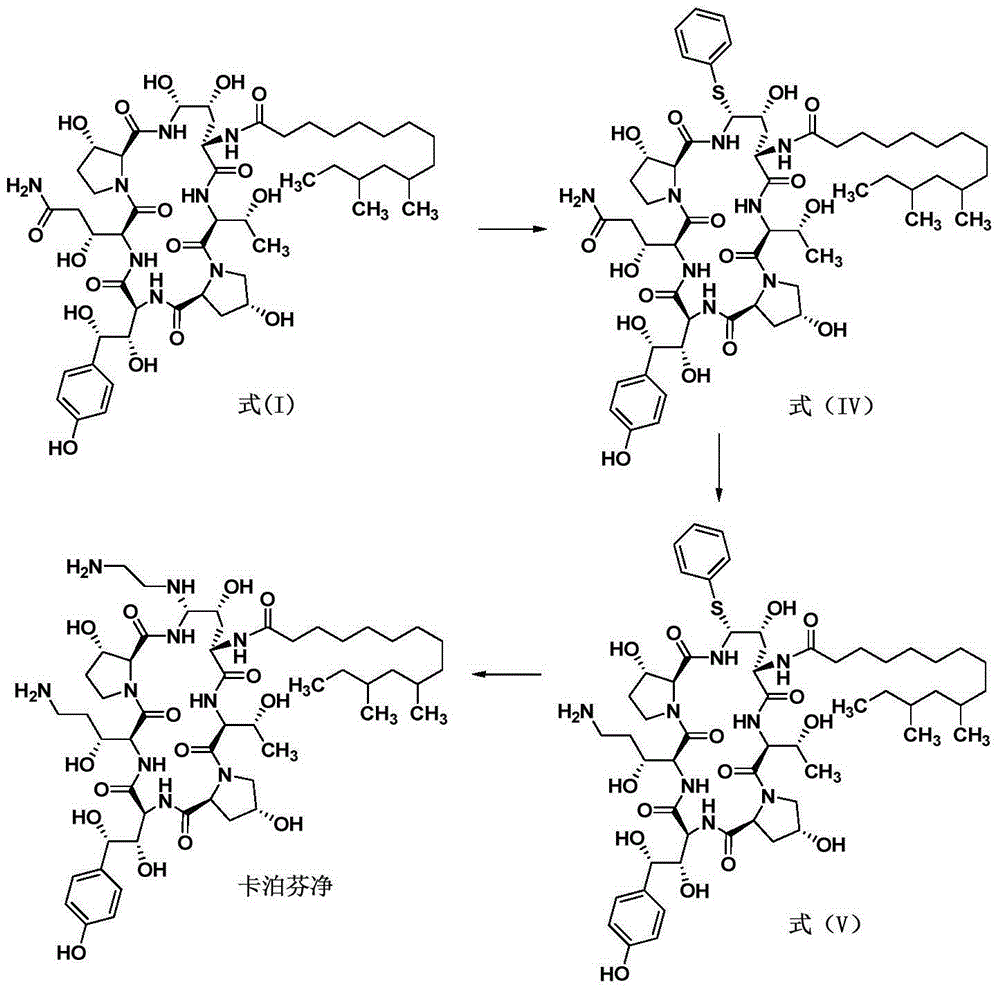
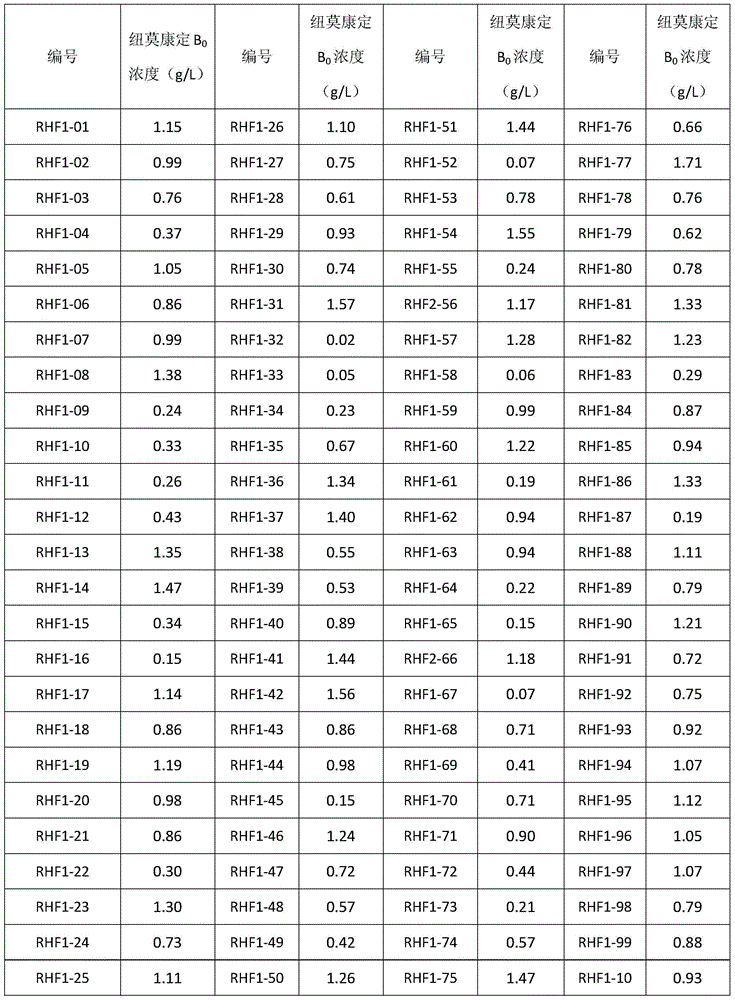
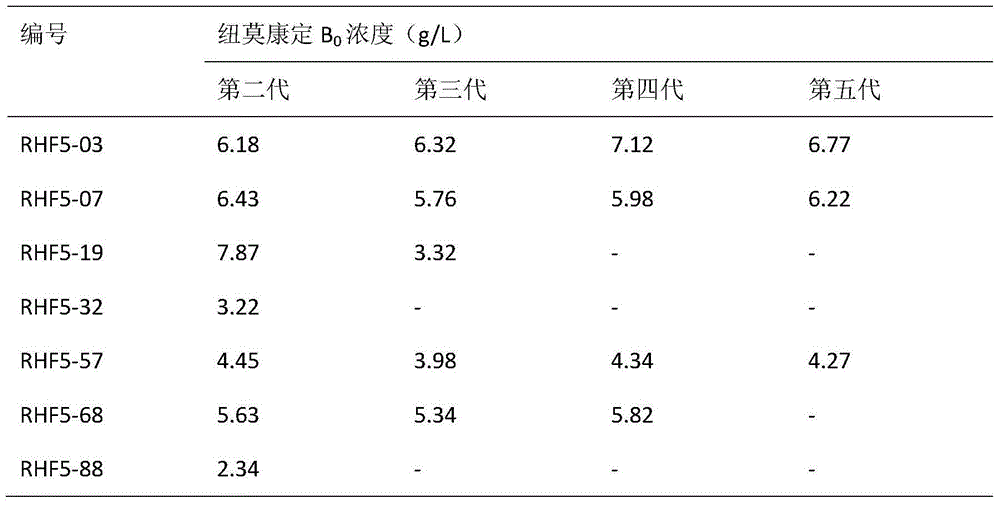
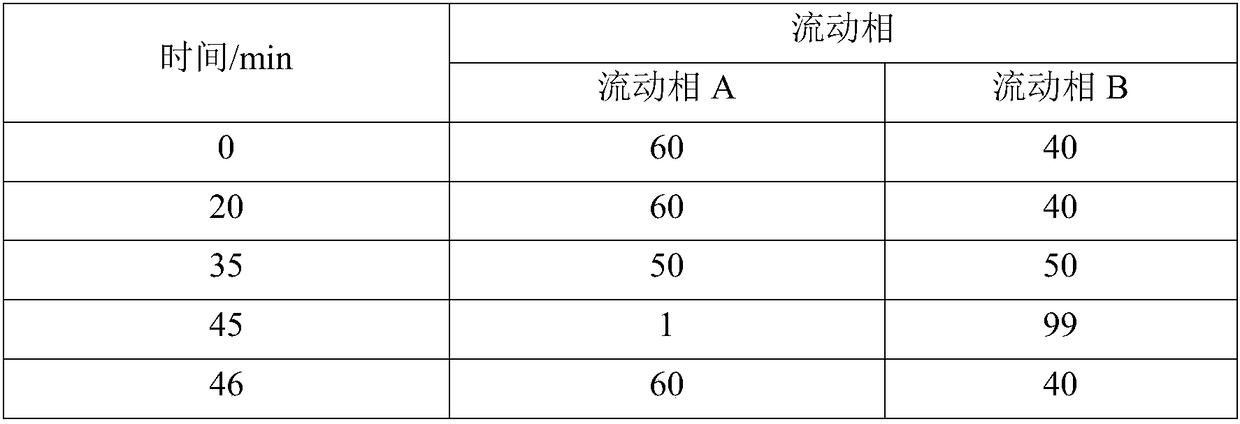
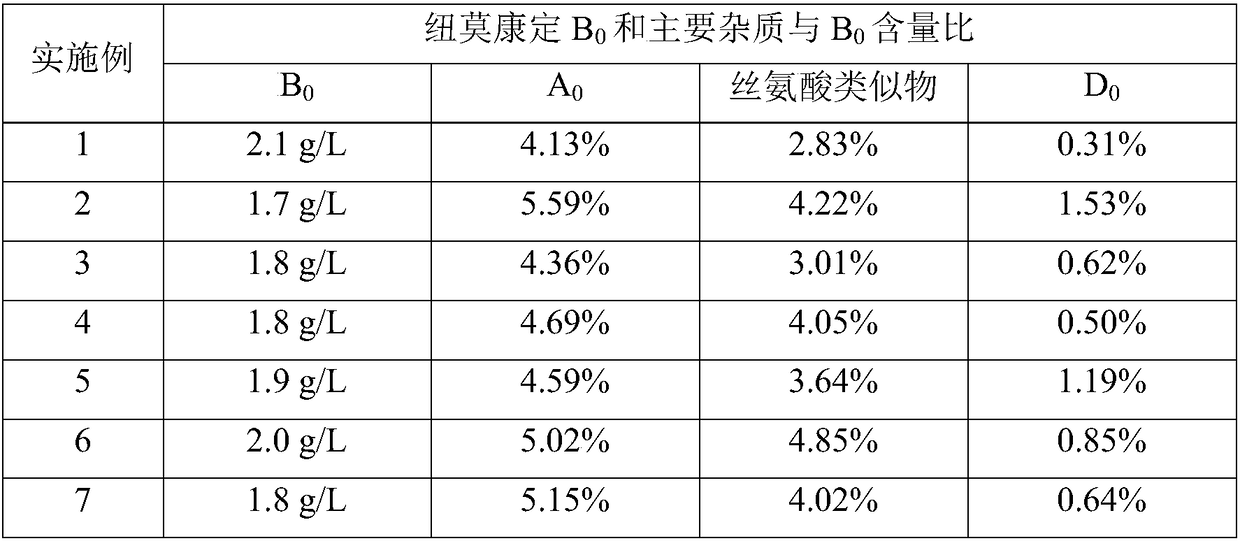
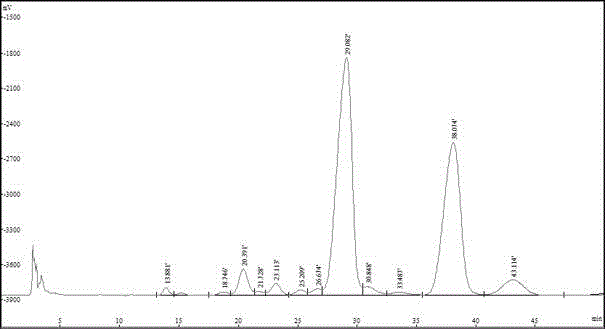
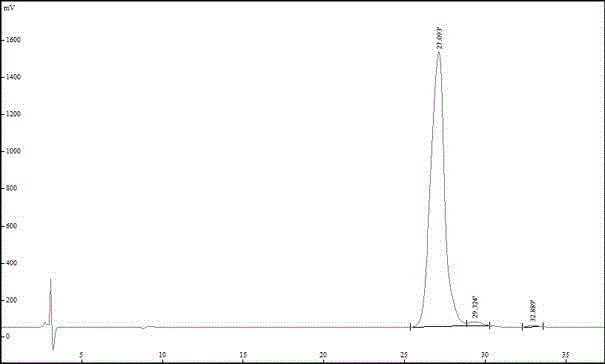
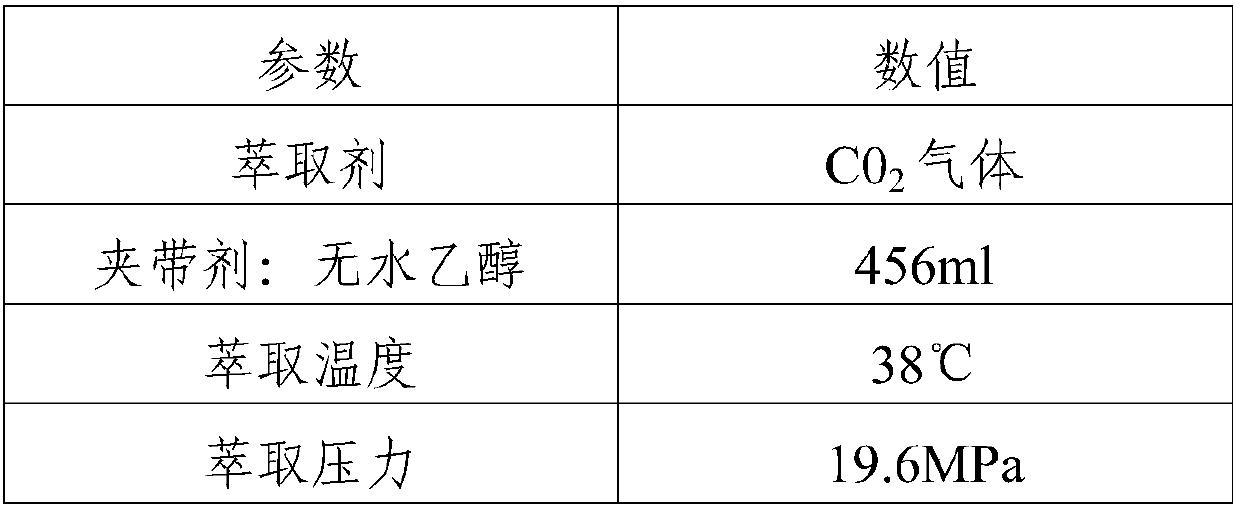
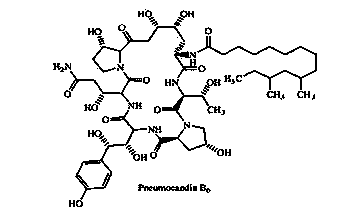
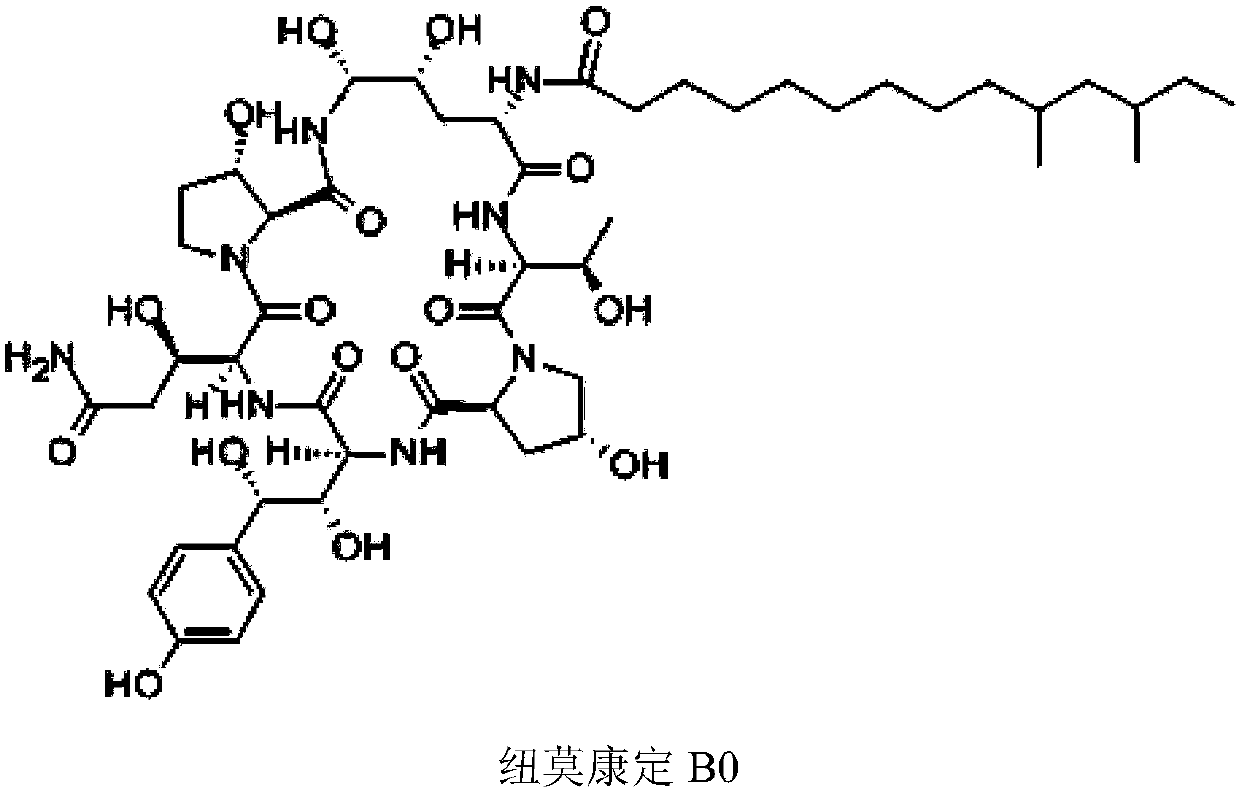
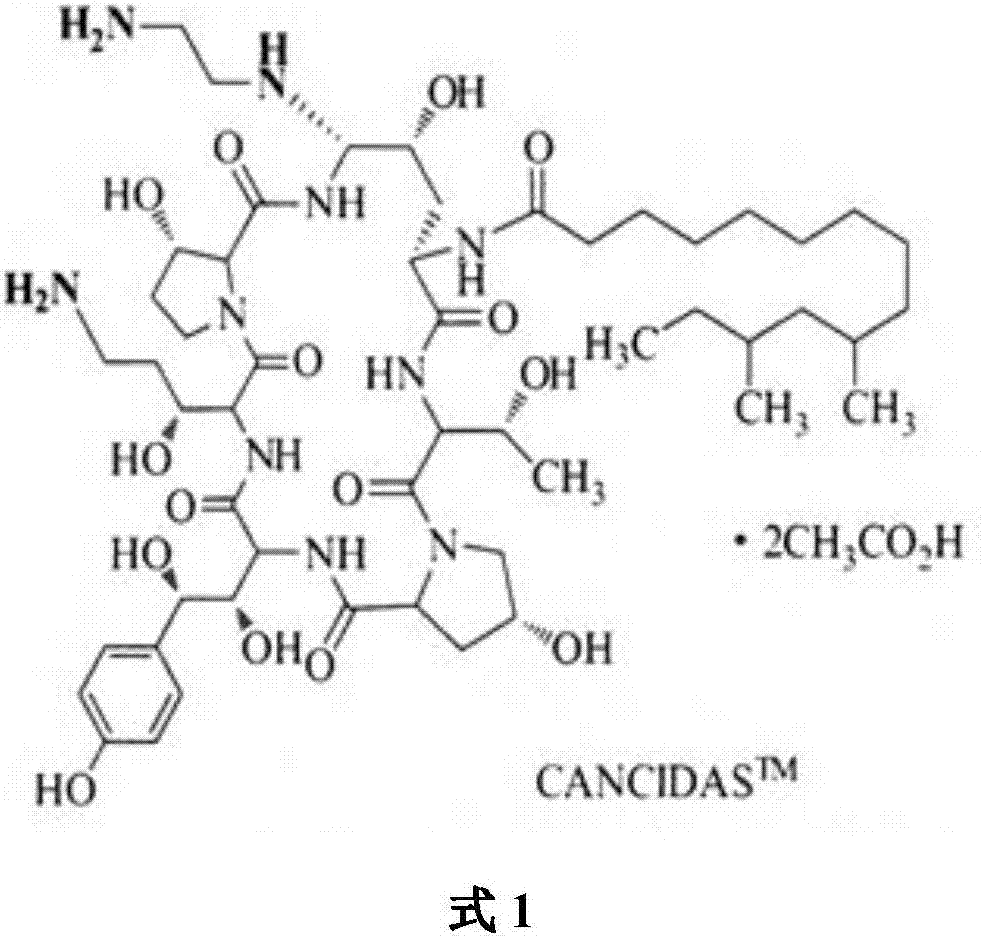
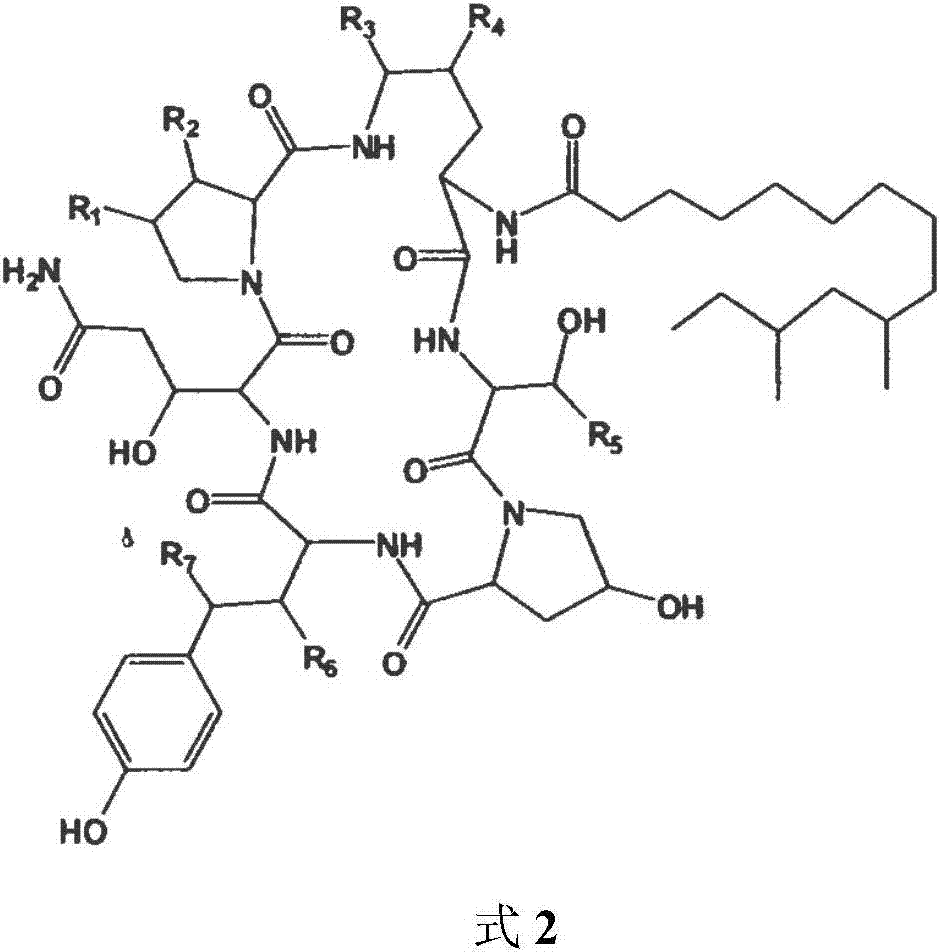
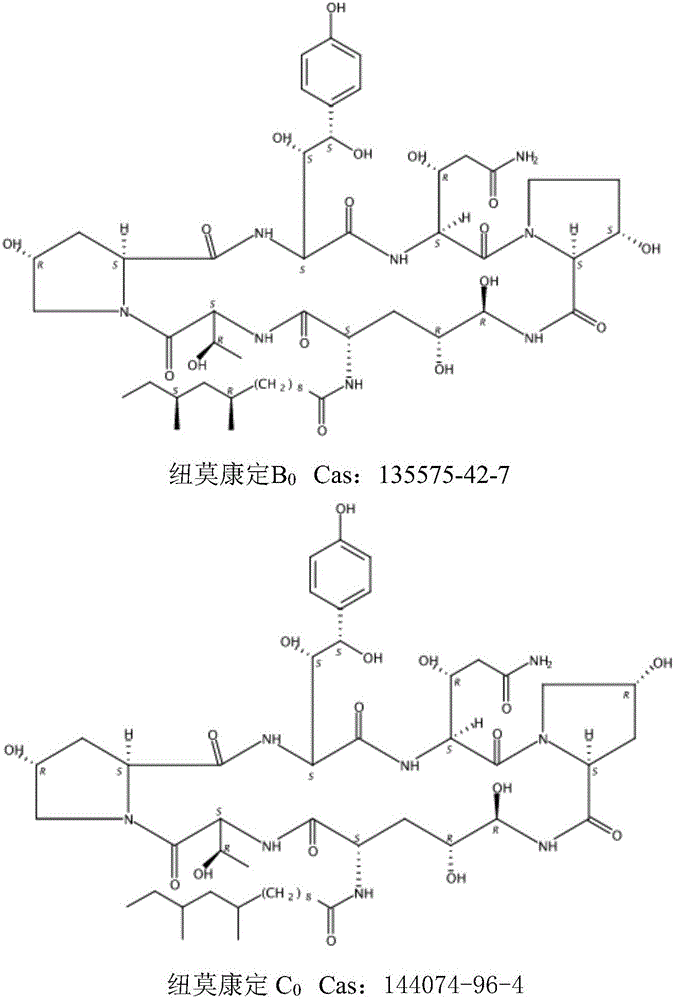
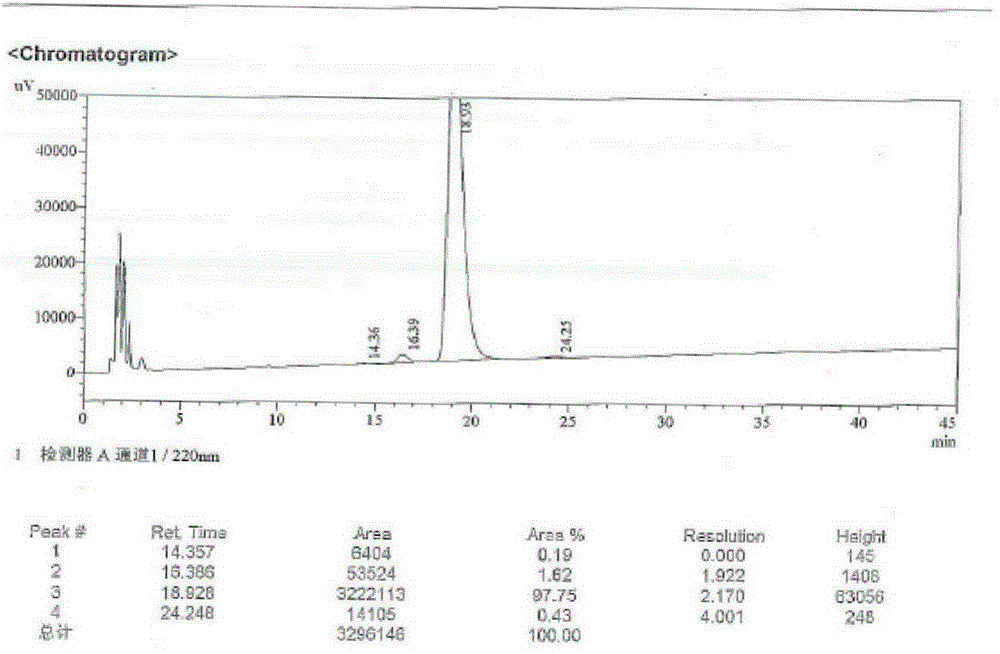
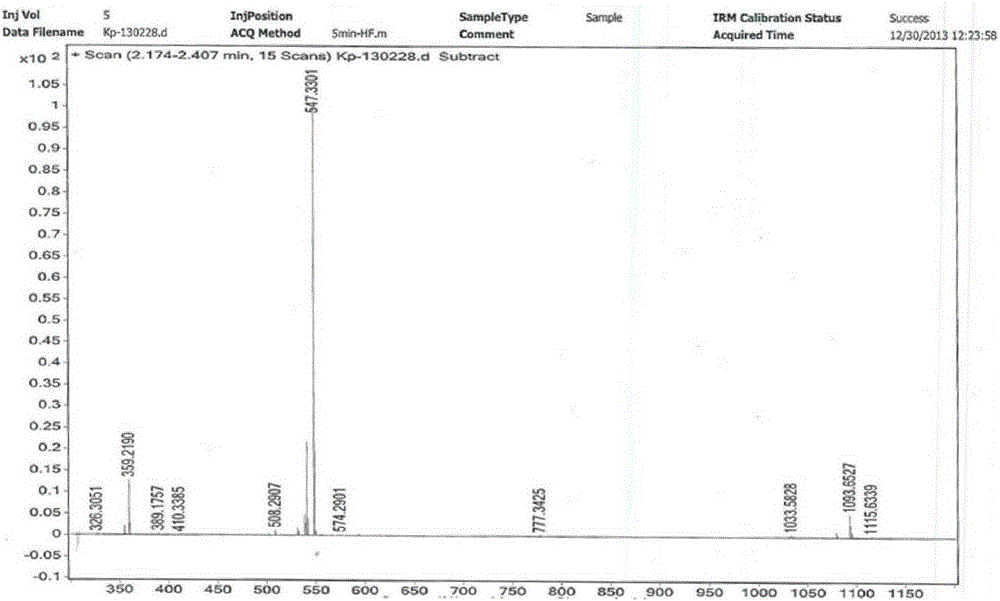
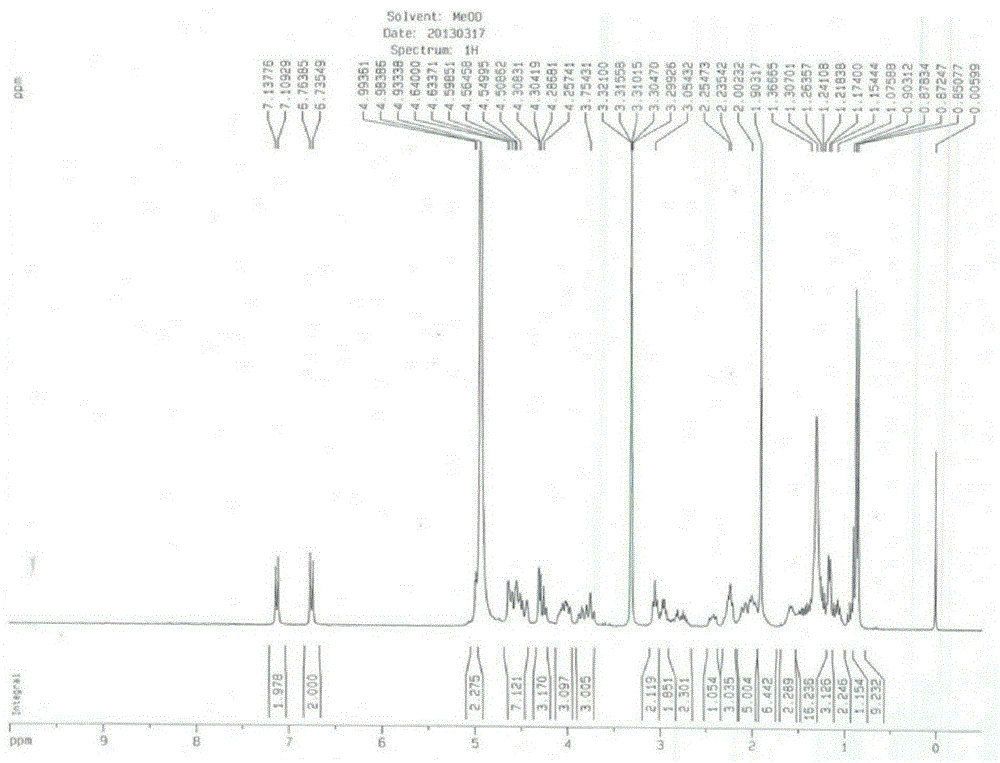
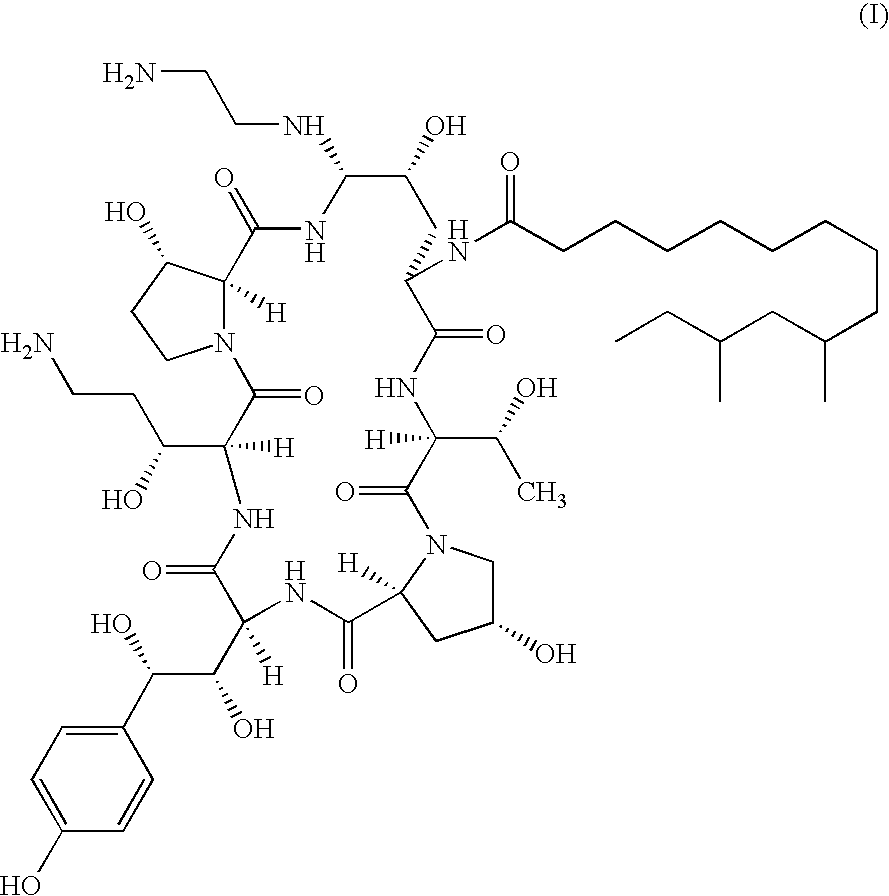
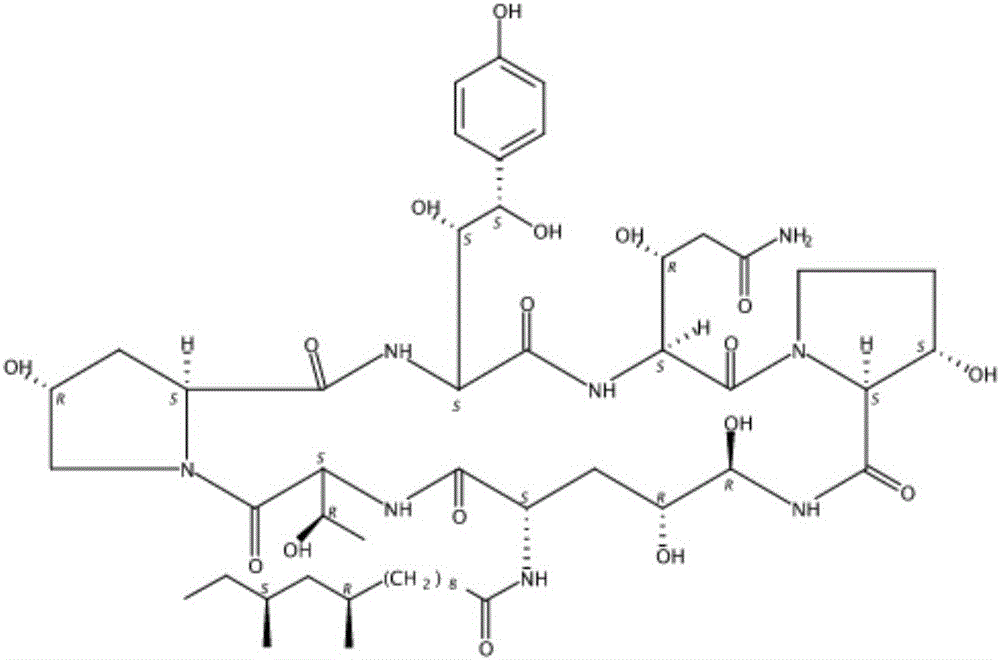
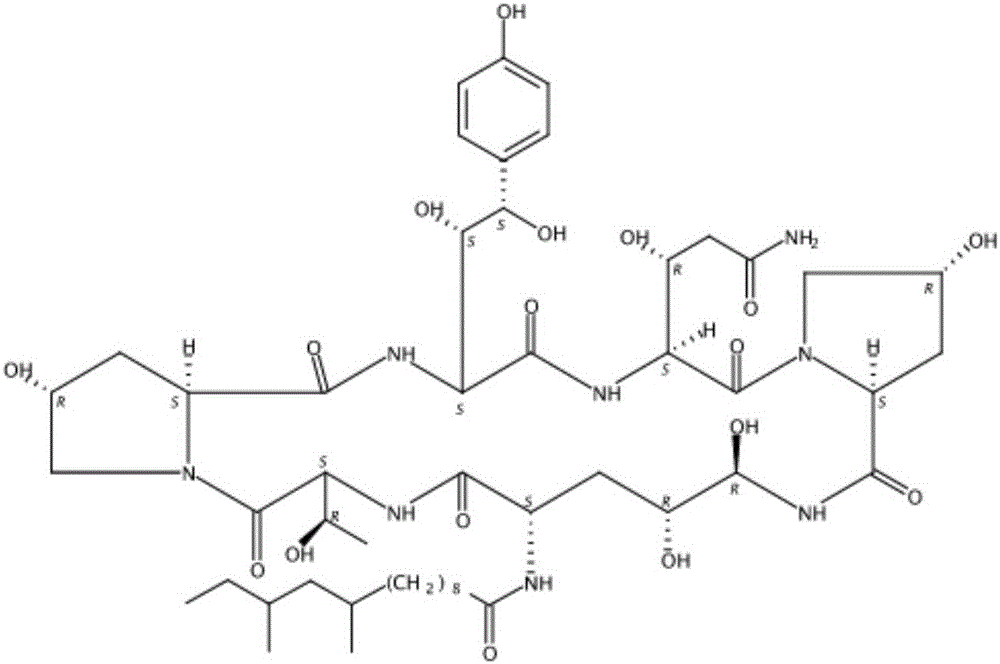
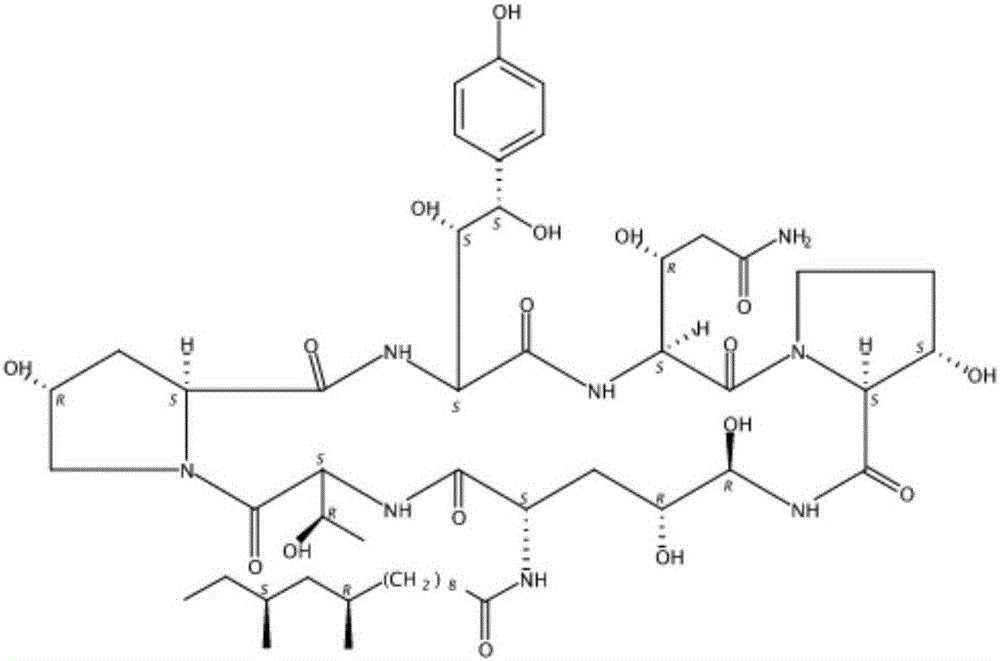
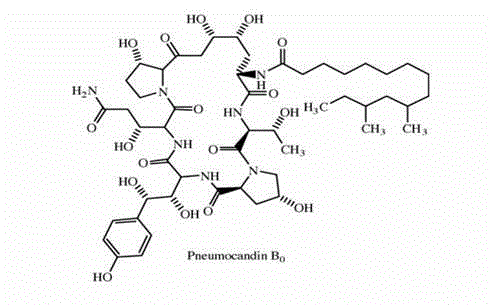
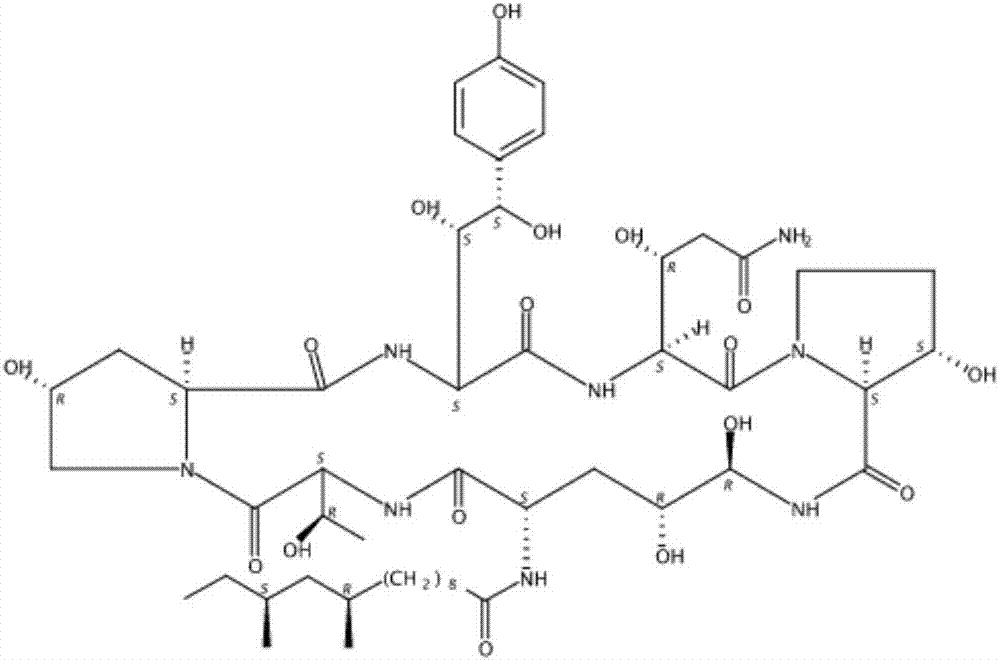
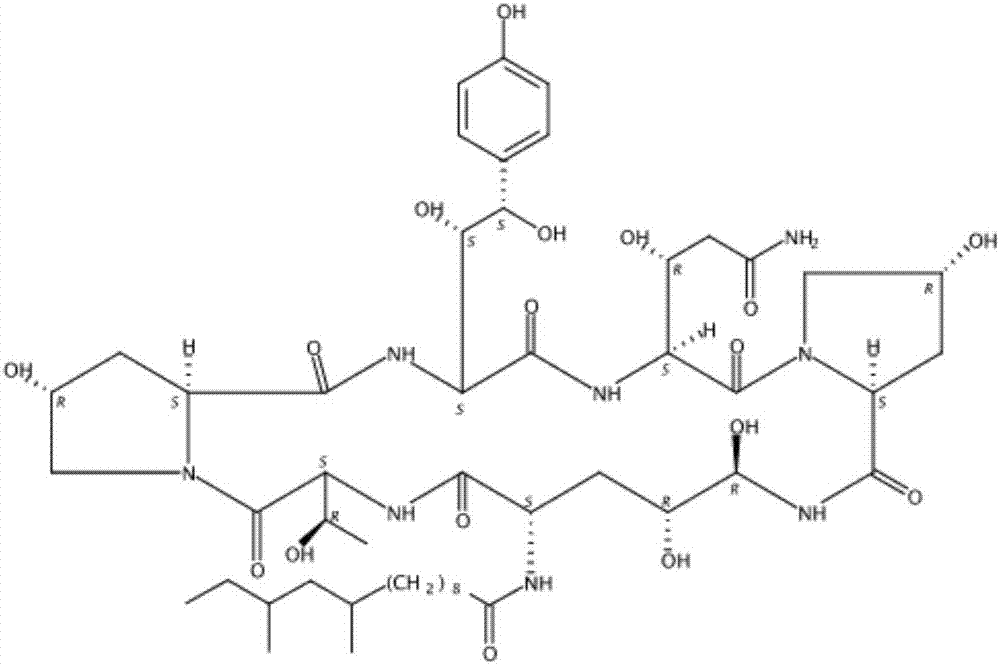
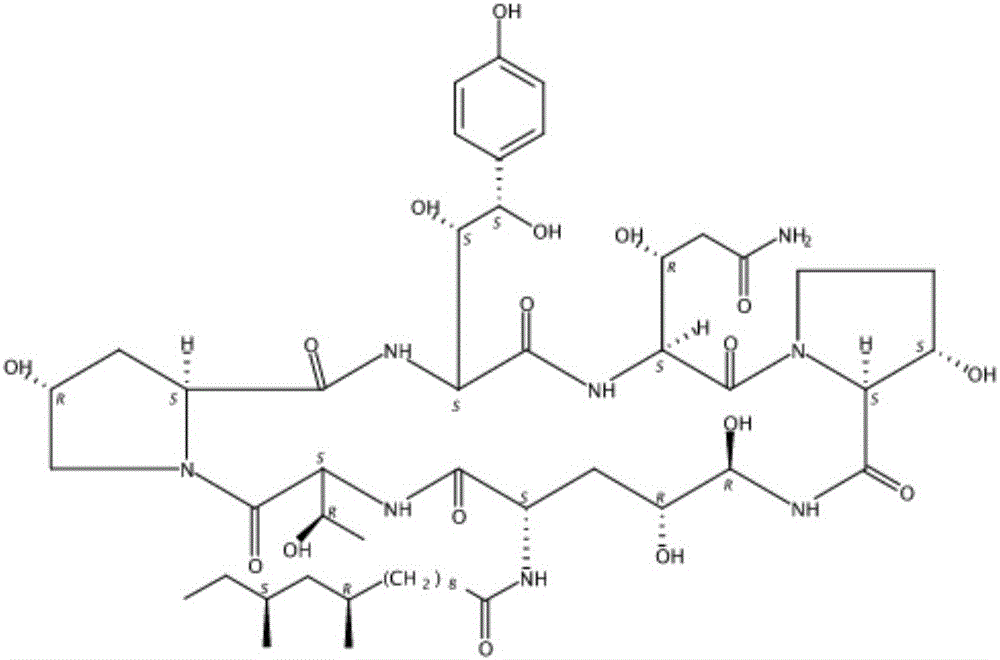
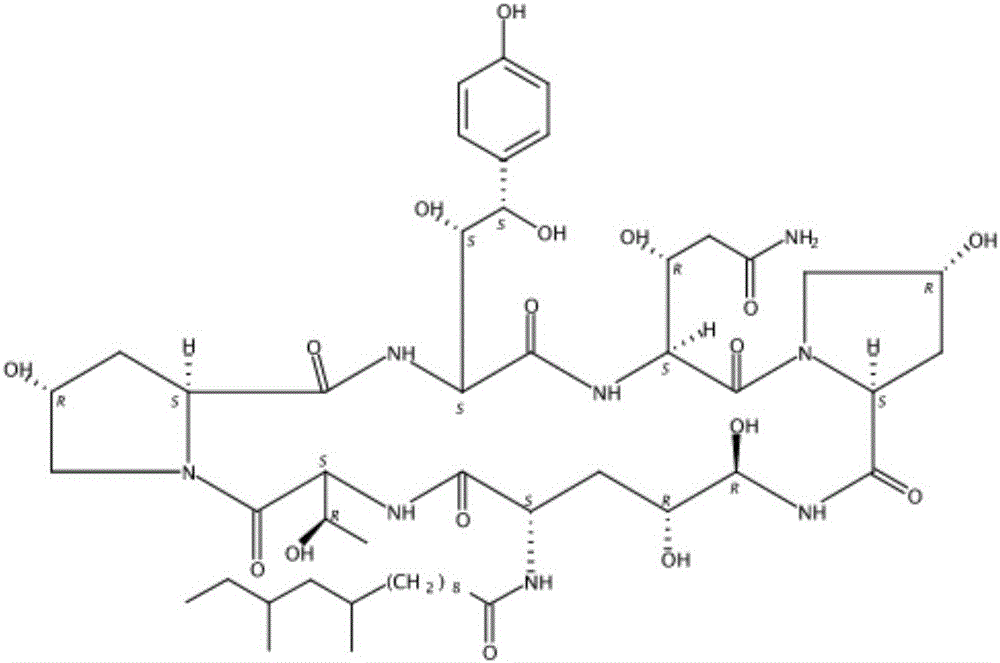
Patents
Literature
38 results about "Pneumocandins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
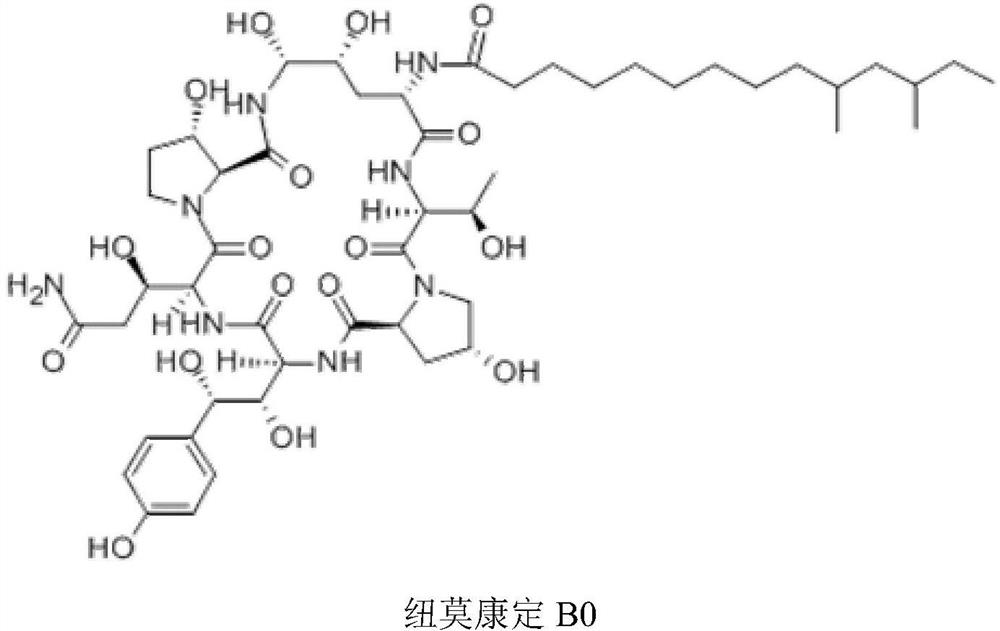
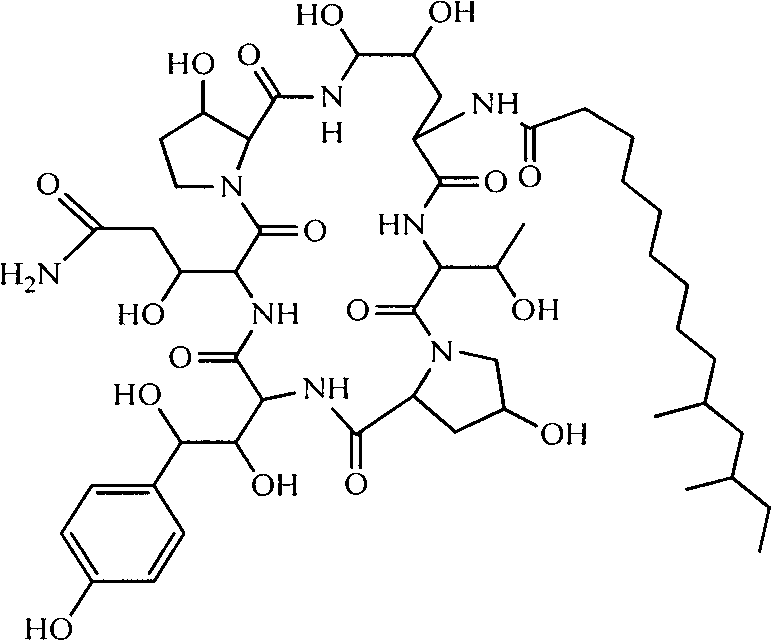
Method for preparing pneumocandin B0
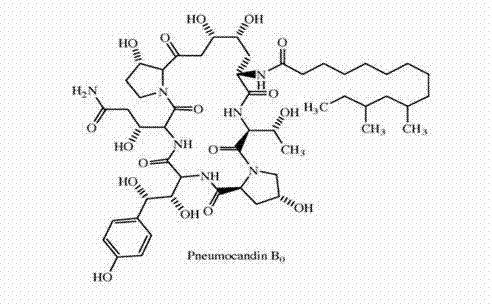
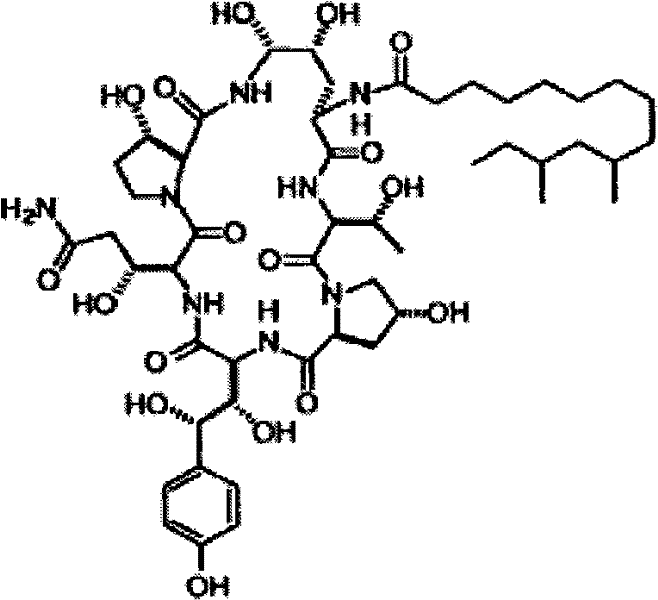
The invention discloses a method for preparing pneumocandin B0. The method comprises the following steps of: a) centrifuging the fermentation liquid of echinocandin B0, taking mycelium, leaching the echinocandin B0 in the mycelium with a first solvent and filtering and removing the mycelium; b) distilling the solvent in the first solvent leaching solution of echinocandin B0 to dryness, soaking with a second solvent and then filtering and removing the insoluble substances; c) distilling the second solvent soak solution of echinocandin B0 to dryness and then dissolving in the first solvent, andcollecting effluent liquid through overly acidic alumina column; d) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent, utilizing adsorbent resin,performing eluting with the first solvent and then collecting the part with higher purity; e) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent,utilizing inverse resin, performing eluting with the first solvent and collecting the part with higher purity; and f) distilling the collected solution of echinocandin B0 to dryness and then dissolving in the first solvent, adding in a small amount of water by dripping to achieve the purpose of separation by crystallization after supersaturation, and then preparing the echinocandin B0. The methodfor preparing the echinocandin B0 not only can well remove the pigment, but also leads the purity of the echinocandin B0 to be improved by more than 96 percent.
Owner:SHANGHAI INST OF PHARMA IND
Method used for high-efficient purification of pneumocandins B0
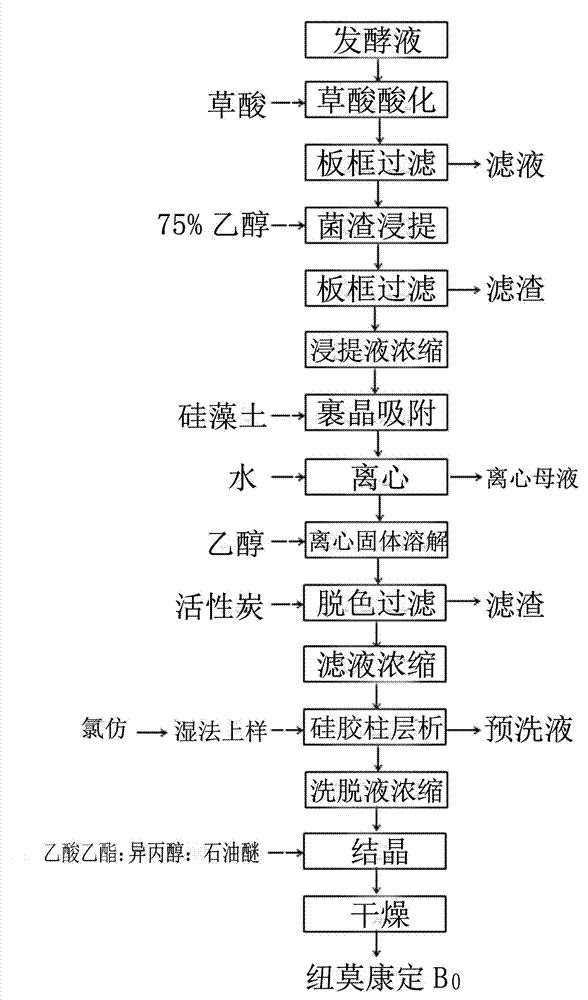
The invention discloses a method used for high-efficient purification of pneumocandins B0. The method comprises following steps: (1) pH value of a fermentation broth containing pneumocandins B0 is adjusted to 2.0 to 4.0, the fermentation broth is filtered, and a pneumocandins B0 extract liquid is obtained via extraction; (2) the extract liquid is condensed, diatomite is added for crystal packaging, water is added, and an obtained mixture is stirred and is subjected to centrifugation; (3) a solid material obtained via centrifugation is dissolved in ethanol, active carbon is added for decolouring, and an obtained mixed material is filtered; (4) an obtained filtrate is condensed, chloroform is added, an obtained solution is delivered through a silica gel column, and a pneumocandins B0 eluant is collected; and (5) the pneumocandins B0 eluant is condensed to be dry, and crystallization is carried out in a multiphase solvent system so as to obtain pneumocandins B0. The method is capable of avoiding application of resin columns and reducing amount of solvents used for elution greatly; active carbon can be used for removing pigments effectively; silica gel column chromatography is capable of removing related substances C0; and extraction purity can be as high as 99%. The method is simple and convenient; cost is reduced greatly; and industrialized production can be easily realized.
Owner:JIANGSU SENRAN CHEM
Method for purifying caspofungin precursor pneumocandin B0 component
ActiveCN102816207AHigh yieldSimplified purification stepsPeptide preparation methodsState of artPurification methods
The invention relates to a method for purifying a caspofungin precursor pneumocandin B0 component, and the method comprises the following steps of: carrying out reversed-phase column chromatography on a PB0 component crude product, collecting and carrying out reduced-pressure concentration to obtain a semi-pure product; carrying out normal phase column chromatography on the PB0 semi-pure product obtained in the step 1 to obtain high-purity PB0 component; and carrying out positive and negative phase liquid phase analysis, wherein the chromatographic purity is more than 95%. Compared with the prior art, the method has the beneficial effects that a normal phase column and a reversed-phase column are used for purifying the PB0 component, so that the purification steps are simple, the purification effect is good, the requirements for equipment are low, large-scale production is realized, and the production feasibility is high; and particularly, the PB0 component separated by the method is high in yield, but is lower in cost.
Owner:CHENGDU YATU BIOLOGICAL TECH
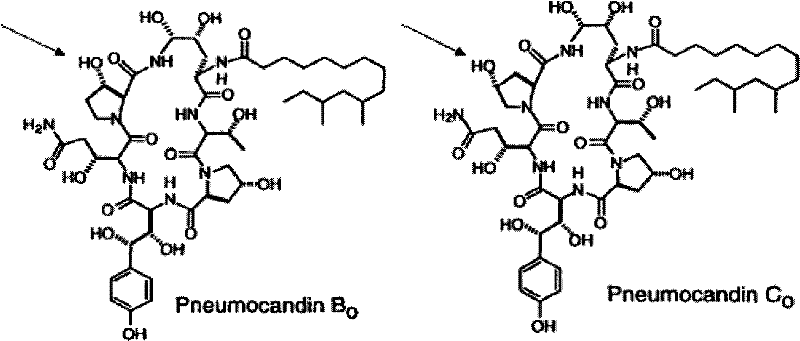
Separation and/or purification of pneumocandin b0 from c0
InactiveCN102481336AIon-exchange process apparatusIon-exchanger regenerationStationary phasePneumocandin C0
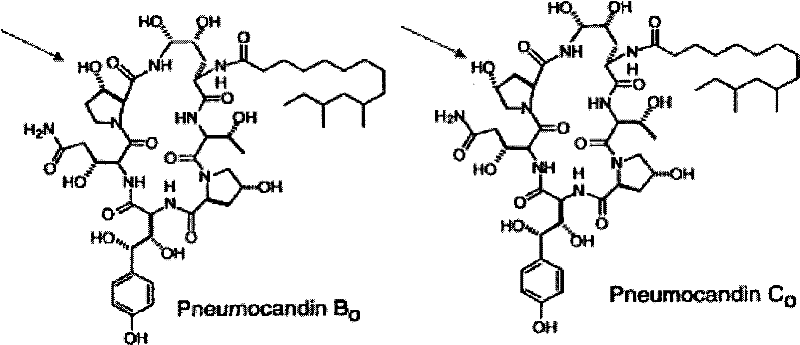
The present invention concerns a method for separation of the antifungal cyclic hexapeptides Pneumocandin B0 from Pneumocandin C0 using a hydrophilic stationary phase and a hydrophobic mobile phase.
Owner:克塞里尔制药公司
High-yield strain for pneumocandin B0 and application for same
InactiveCN103289900AHigh industrial valueStable genetic traitsFungiMicroorganism based processesMicroorganismZalerion arboricola
The invention discloses a high-yield strain for pneumocandin B0, which is the mutant strain of Zalerion arboricola ATCC46001 strain, and collected in the China General Microbiological Culture Collection Centre with a collection number of CGMCC No. 5412. The invention further discloses an application for the same, wherein the yield of pneumocandin B0 prepared from the obtained high-yield strain for pneumocandin B0 achieves about 4500 mg / l.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +1
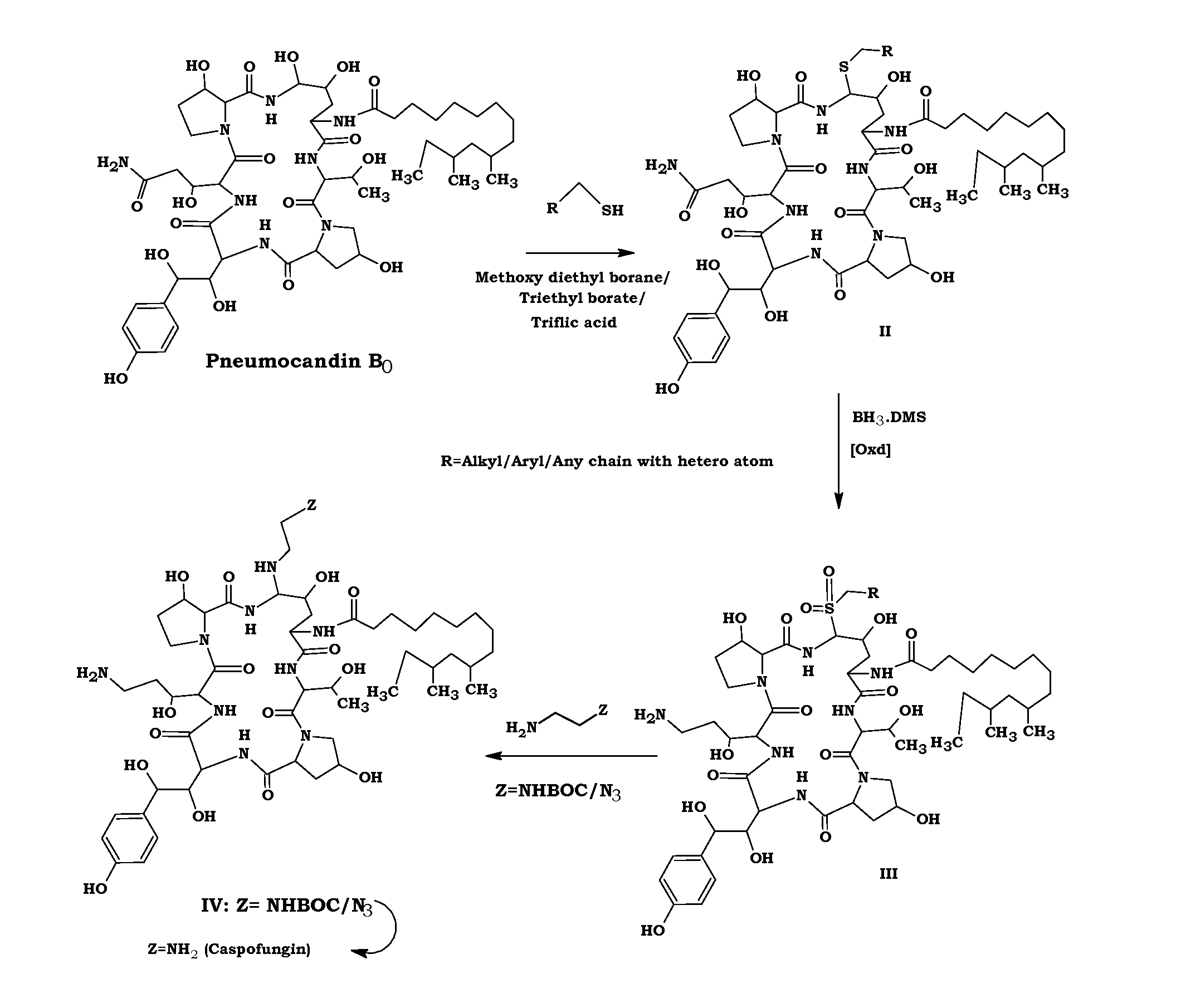
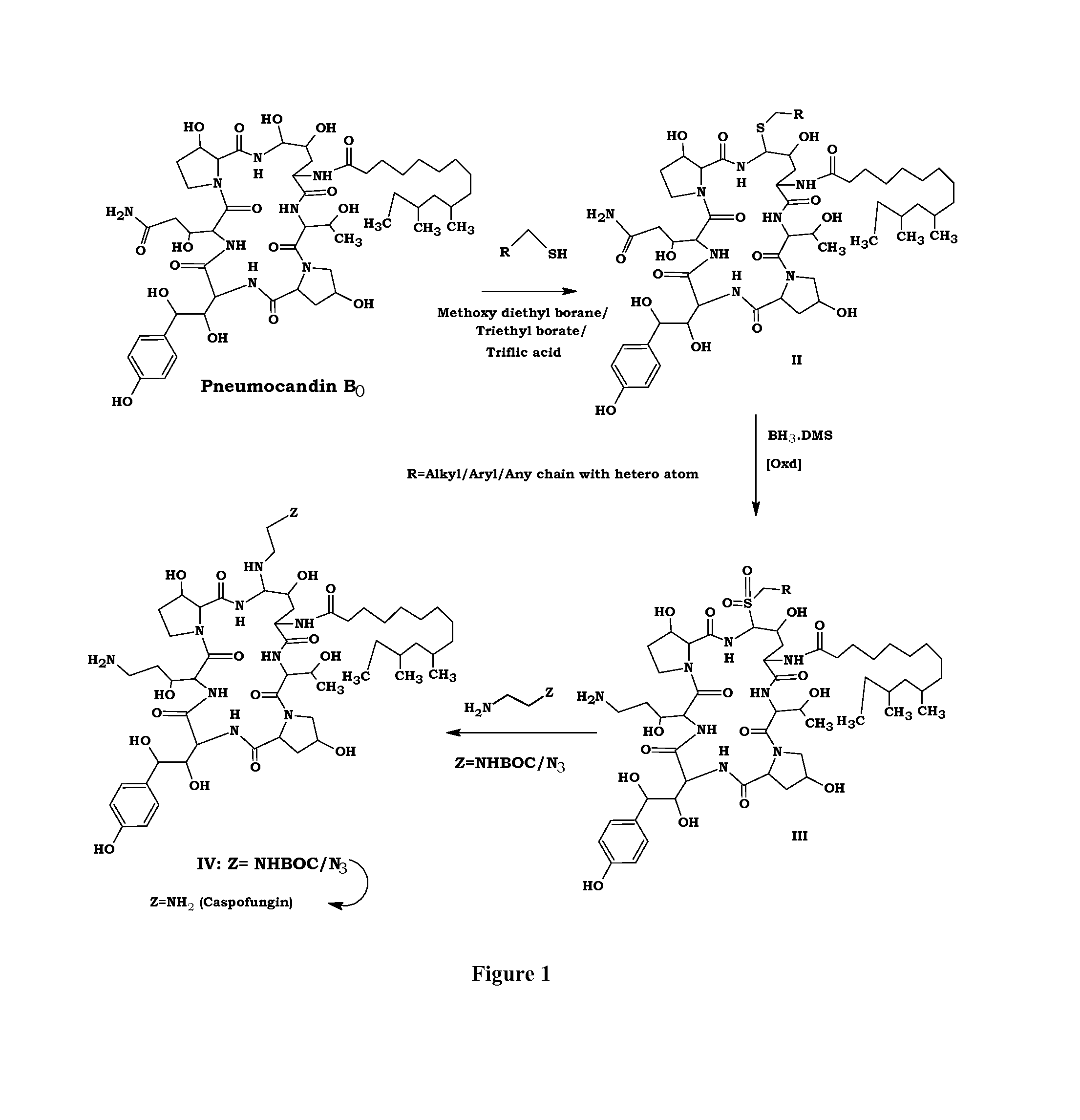
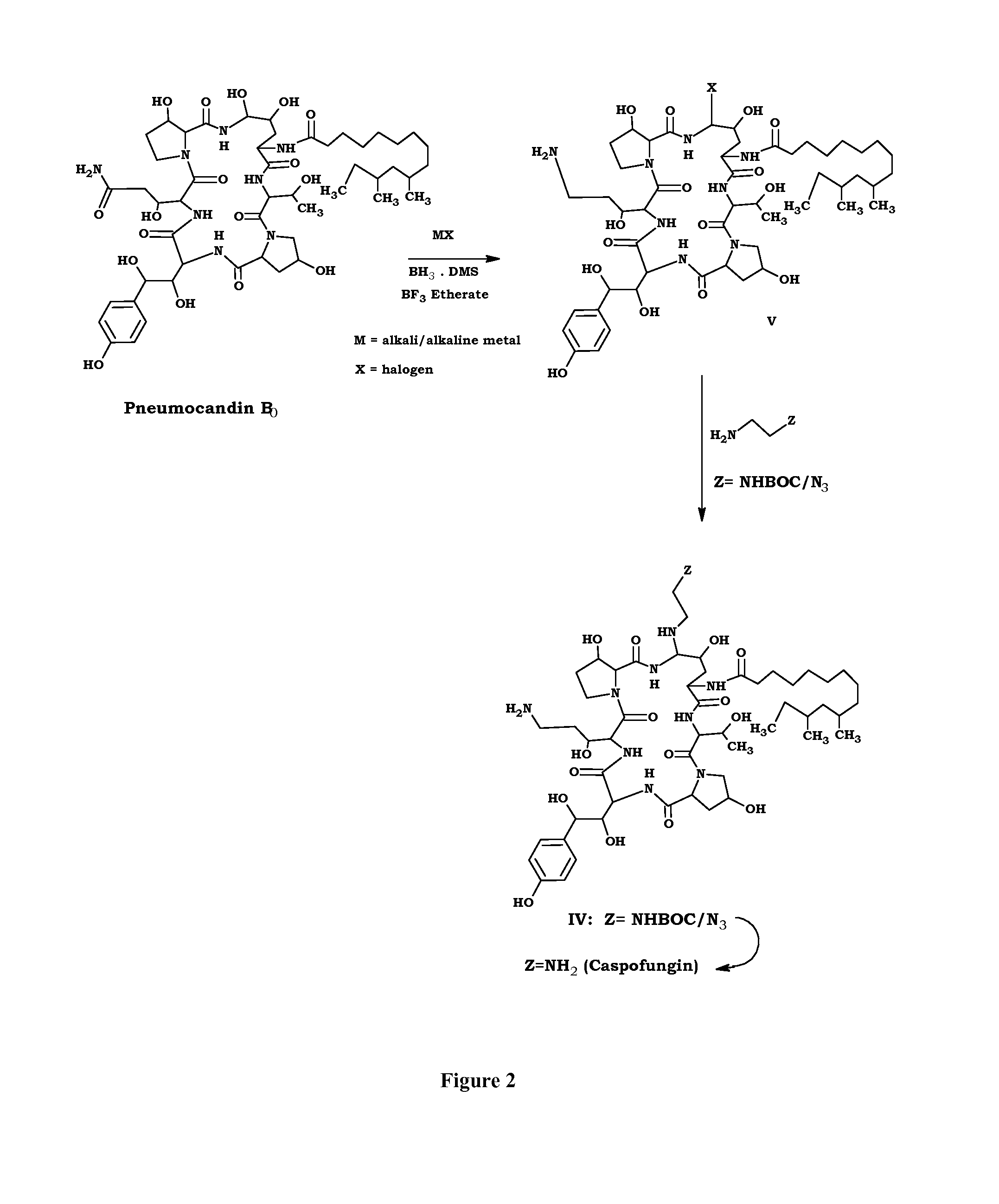
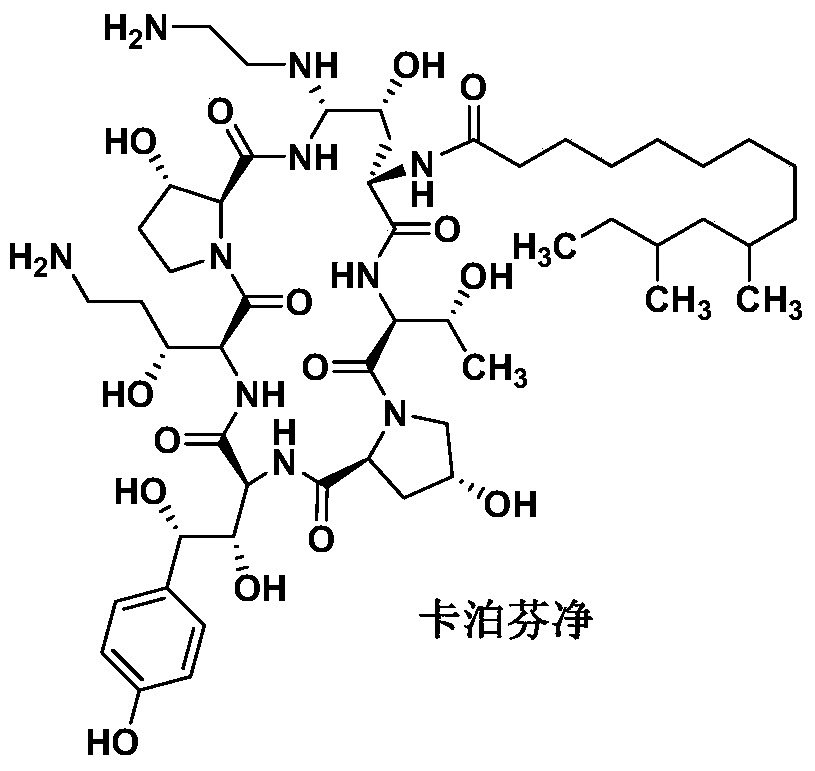
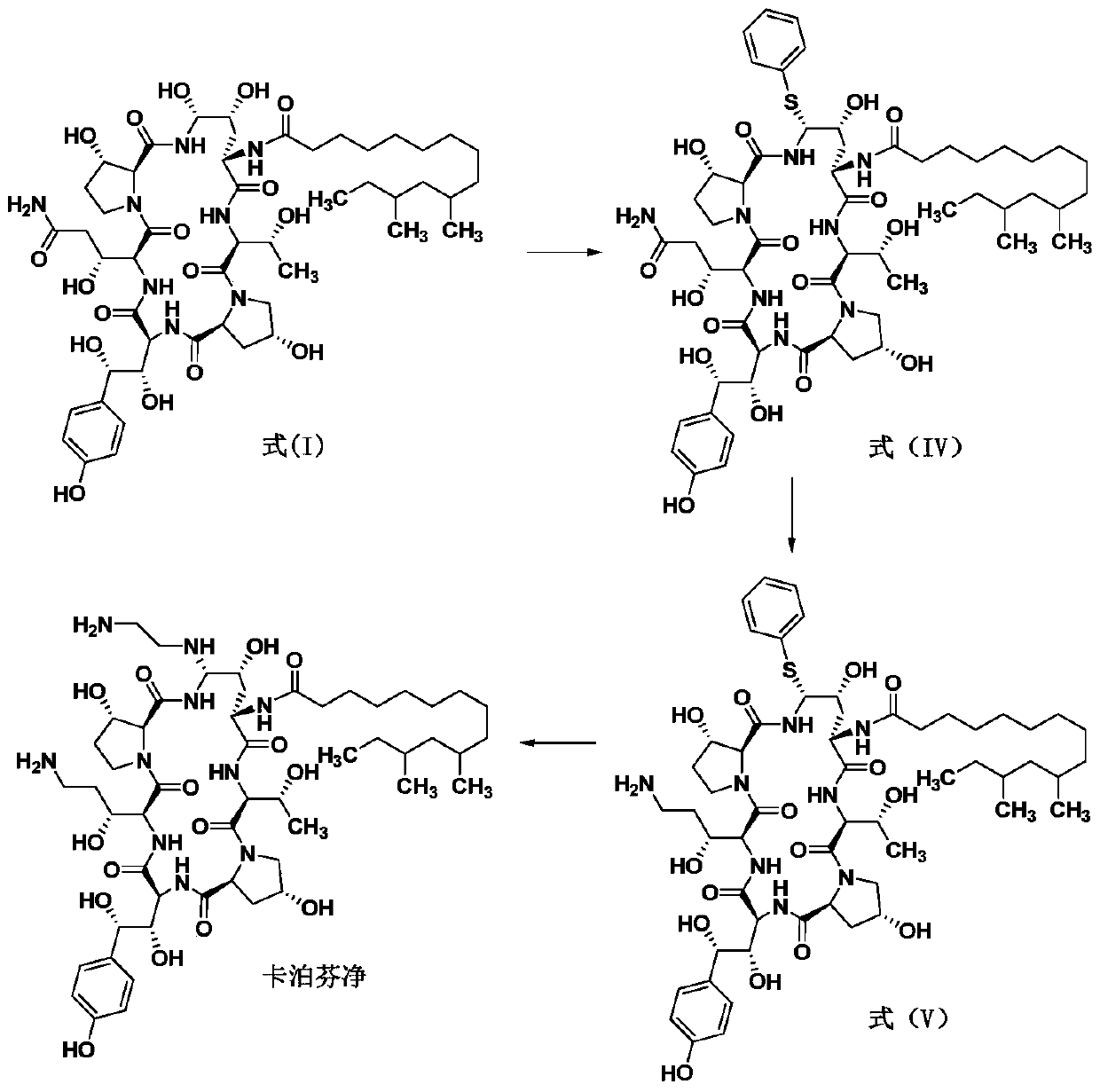
Preparation method of caspofungin
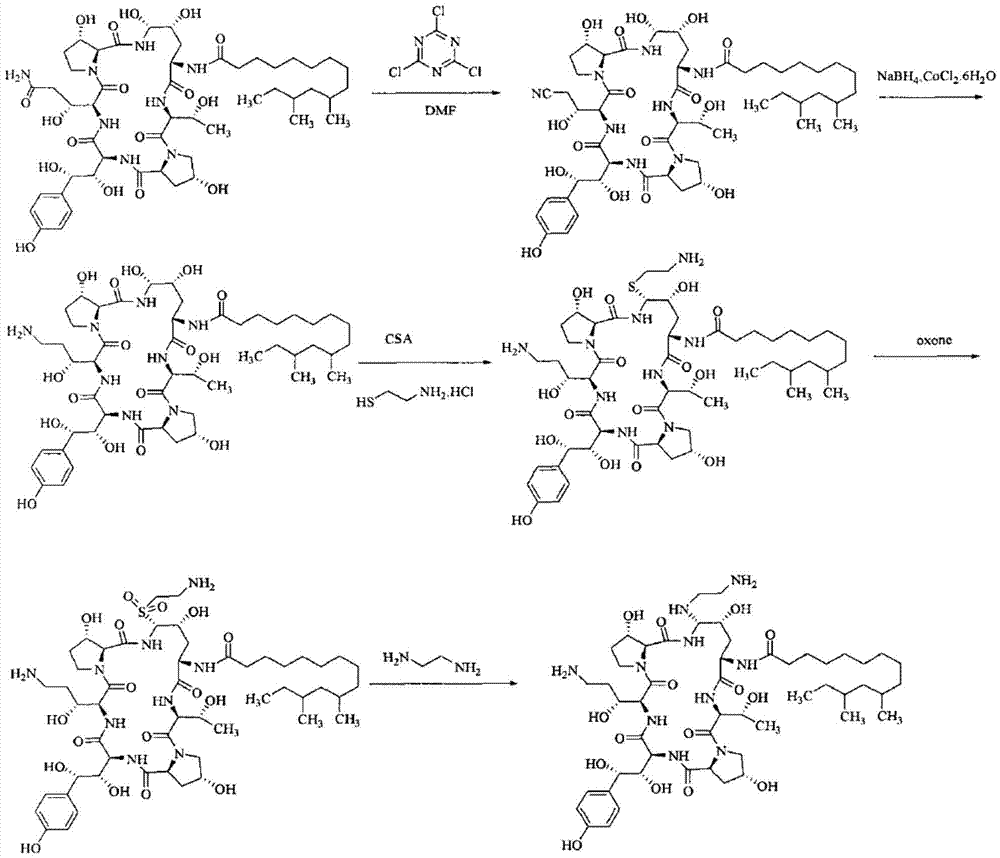
The invention relates to a preparation method of caspofungin. The preparation method comprises the following steps: using p-thiocresol to replace hydroxide radicals of pneumocandins B0, then reducing acylamino groups by using a reducing agent, and finally using ethidene diamine to replace p-sulfonyl toluene, thus obtaining the caspofungin. According to the preparation method disclosed by the invention, an appropriate solvent is adopted for crystallizing and separating an intermediate, repeated separation and purification operation for chromatographic column preparation in the prior art is avoided, the reaction time is shortened, degradation of the intermediate is avoided, the operation is simplified, high-purity and high-yield caspofungin is obtained, and the preparation method is particularly suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
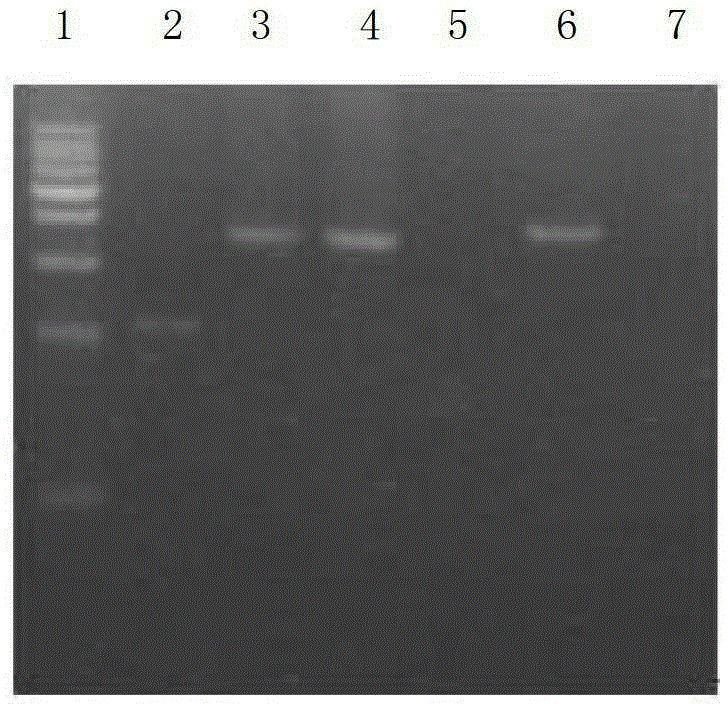
Genetic recombination strain for producing pneumocandins B0, breeding method and application
ActiveCN104531535AShort fermentation cycleEasy to separate and extractFungiMicroorganism based processesLithium chlorideGlarea lozoyensis
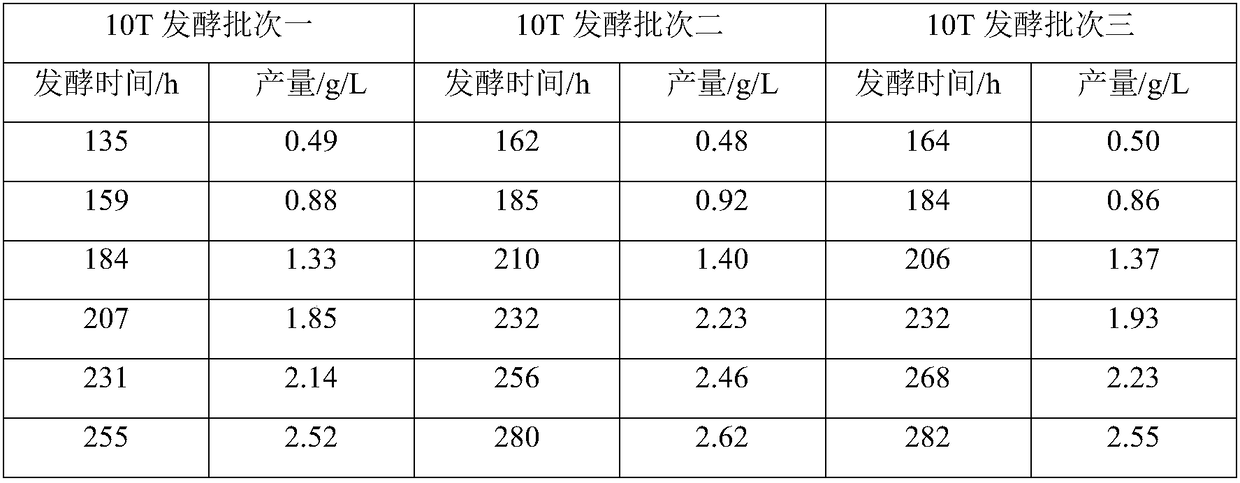
The invention discloses a genetic recombination strain for producing pneumocandins B0, a breeding method and applications. The bacterial strain classification name of the genetic recombination strain is Glarea lozoyensis Q45; the preservation registration number is CCTCC NO: M2014416; and the preservation date is September 14, 2014. The method for breeding the strain includes the steps that an original strain is manufactured into a protoplast; a mutation library composed of a plurality of mutant strains is obtained through ion implantation and lithium chloride processing; the mutation library is manufactured into a protoplast again; random fusion is carried out on the protoplasts after ion implantation inactivation and heat inactivation are carried out; fermentation screening is carried out on high-yield genetic recombinant bacteria; and protoplast preparation and fusion and fusant screening are carried out on the screened genetic recombinant bacteria, and therefore the genetic recombination strain is obtained. The genetic recombination strain is excellent in performance and stable in production capacity; the yield of the pneumocandins B0 obtained through fermentation is 6 g / L; in addition, by-products are few; the post-extraction difficulty of the pneumocandins B0 is reduced; the application to high-quality caspofungin preparation is facilitated; and the genetic recombination strain has the important industrial value.
Owner:NANJING UNIV OF TECH
Method for preparing pneumocandin B0 by microbial fermentation
ActiveCN108265096AEfficient productionStable productionFungiMicroorganism based processesMicroorganismInorganic salts
The invention relates to a method for preparing pneumocandin B0 by microbial fermentation. The method is concretely characterized in that mold Glarea lozoyensi undergoes liquid deep fermentation, eachof a seed medium and a fermentation medium contains a proper carbon source, a proper nitrogen source, a proper inorganic salt and a proper trace element source, wherein the fermentation medium uses sorbitol as a main carbon source, and glucose is added to form a composite carbon source. The fermentation method can realize the stable production in an industrial fermentation tank, and allows the yield of the pneumocandin B0 to reach 2.5 g / L.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method of detecting pneumocandin compounds
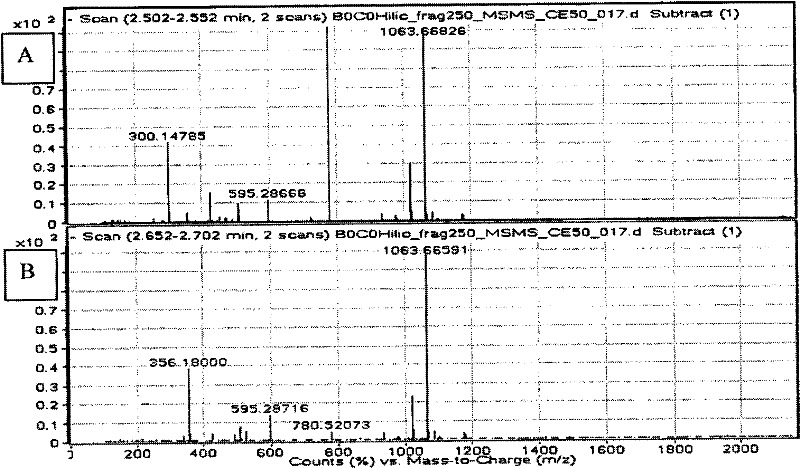
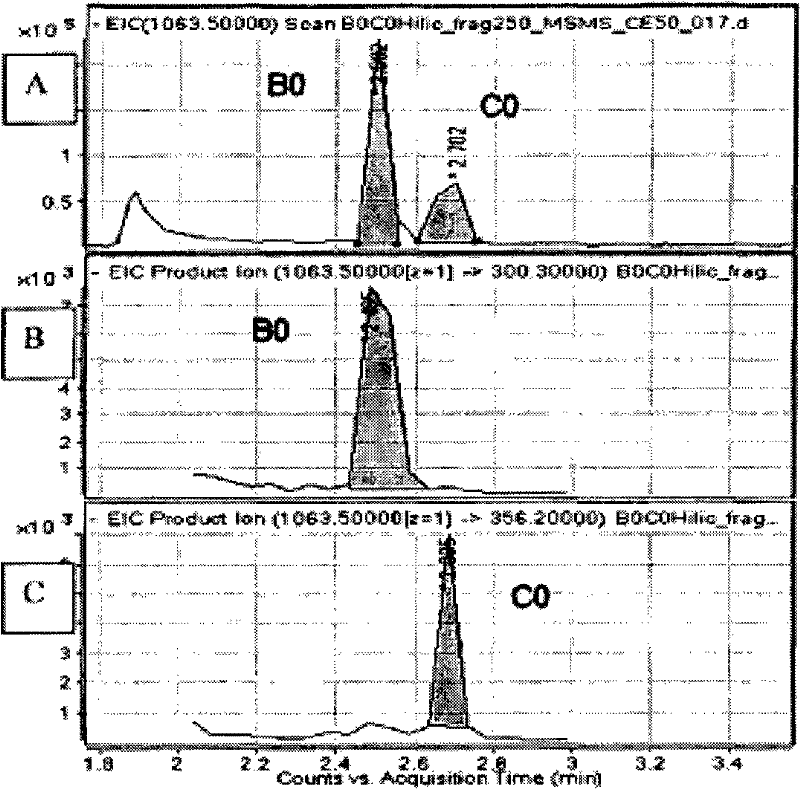
InactiveCN102483421AMaterial analysis by electric/magnetic meansBiological testingPneumocandin C0Chemistry
The present invention concerns a method of detecting the antifungal cyclic hexapeptides Pneumocandin B0 and / or Pneumocandin C0 specific fragment is / are detected using MS in negative mode.
Owner:克塞里尔制药公司
Method for preparing pneumocandins B0 by adopting dynamic axial compression column system
InactiveCN104558123AGood removal effectColumn bed stabilityPeptide preparation methodsUltraviolet detectorsCentrifugation
The invention provides a preparation method of pneumocandins B0. The method comprises the following steps: (1) performing acid precipitation, centrifugation and decoloring pretreatment on a fermentation broth containing pneumocandins B0 to obtain a pneumocandins crude product; (2) packing by adopting a dynamic axial compression column; and (3) pumping the pneumocandins crude product into the dynamic axial compression column, performing online monitoring by using an ultraviolet detector, collecting an objective elution component, and concentrating and drying to obtain pneumocandins B0. The dynamic axial compression column adopted by the method provided by the invention is convenient to operate and can be used for realizing online monitoring, the amount of an elution solvent can be greatly reduced, the elution time is shortened, and the service life of a chromatographic column is ensured at the same time, so that the production cost is reduced, C0 can be effectively removed at the same time, the product recovery rate is high, and the purity can be up to 99% or higher.
Owner:JIANGSU HANBON SCI & TECH CO
Pneumocandin B0 purification method
ActiveCN107674116AEasy to separateLow costPeptide preparation methodsBulk chemical productionPurification methodsFiltration
The present invention provides a pneumocandin B0 purification method, which comprises: extracting a pneumocandin B0 crude product from fermentation broth bacteria residue, carrying out membrane filtration treatment on the crude product, adding an adsorbent to the filtrate to form a solid with adsorbed pneumocandin B0, carrying out CO2 supercritical fluid extraction on the solid, adding a solvent to the obtained extraction solution, crystallizing, carrying out column chromatography separation on the obtained crystal by using an HILIC mode reverse phase silica gel bonded filler as a stationary phase and using an ethanol aqueous solution as a mobile phase, and collecting the pneumocandin B0-containing effluent to obtain the pure product pneumocandin B0. According to the present invention, with the pneumocandin B0 purification method, the separation of PB0 and PC0 can be well achieved, the pneumocandin B0 can be stably and efficiently obtained, the HPLC purity of the pneumocandin B0 can achieve more than 99.4%, and the HPLC purity of the isomer PC0 is as low as 0.01-0.04%; and compared to the existing purification method, the purification method of the present invention has advantagesof simple process operation and good product quality.
Owner:NEW FOUNDER HLDG DEV LLC +2
Method for separating and purifying Pneumocandins B0
InactiveCN104250289ASolve problems that cannot be removedHigh purityPeptide preparation methodsAntifungal drugMedicine
The invention provides a method for separating and purifying Pneumocandins B0, which comprises the steps of acidifying and extracting a broth, separating a leaching solution by resin adsorption, concentrating, and then drying to obtain the Pneumocandins B0. The obtained product with high purity is a good raw material to prepare antifungal drug Pneumocandins B0. The prepared end product has the advantages of high yield, low production cost, and simple operation, and is especially suitable for industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Extraction and purification method of pneumocandins B0
ActiveCN107778357AEasy to realize industrial productionPeptide preparation methodsHigh concentrationActivated carbon
The invention discloses an extraction and purification method of pneumocandins B0. The method comprises the steps of: 1) adjusting a pH (potential of hydrogen) value of fermentation liquid containingpneumocandins B0, adding a filter aid and performing filtration to form bacterial residue, 2) extracting the bacterial residue with a high-concentration aqueous solution of methanol or ethanol, 3) adsorbing extraction liquid with adsorbent resin, eluting with the 80-90(V / V) aqueous solution of methanol (ethanol), and collecting an eluant rich in pneumocandins B0, 4) adding active carbon into the eluant containing pneumocandins B0 for decoloration treatment, 5) adsorbing with the adsorbent resin, eluting the resin with the 80-100% (V / V) aqueous solution of methanol (ethanol) and collecting an eluant rich in pneumocandins B0, and 6) performing vacuum concentration to form a crude product of pneumocandins B0. Due to improvement of the extraction and purification method, a decoloration and purification effect is obvious, the purity of the product is improved obviously, the production cost is low, and the method is simple in technical step and suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Aspergillus and method for producing pneumocandin B0 by using aspergillus
ActiveCN107201316AThe production is stableRaise the fermentation unitFungiMicroorganism based processesAspergillusPneumocandin C0
The invention discloses a novel aspergillus strain and application thereof. The strain is named as Glarea lozoyensis HS-2158, and has the preservation code being CGMCC NO.13367. The strain HS-2158 provided by the invention is a high-yield strain of the pneumocandin B0; the generated byproducts such as pneumocandin C0, E0, B5, B0 serine analogues account for low percentage in the pneumocandin B0; the extraction and separation difficulty of the pneumocandin B0 is reduced, so that the strain HS-2158 has good application values.
Owner:HISUN PHARMACEUTICAL (HANGZHOU) CO LTD
Method of purifying pneumocandins B0
InactiveCN106749543ARaise the pHHigh purityPeptide preparation methodsDevitrificationProcess optimization
The invention discloses a purification method of pneumocandins B0 through process optimization. The method comprises the following steps: (1) acidifying fermenting liquor of pneumocandins B0, filtering the fermenting liquor and performing extraction to obtain extraction liquor; (2) adding diatomite into an extraction liquor concentrated mixture to wrap the crystals, and adding deionized water to centrifugalize so as to obtain a white solid containing pneumocandins B0; (3) dissolving the centrifugalized solid by adding ethanol, fully stirring the mixture, then adding activated carbon, fully stirring the mixture to decolor, and performing filtration to obtain a colorless filtrate; (4) concentrating the colorless filtrate till the mixture is dried, adding a heterogeneous solvent system, dissolving, and performing devitrification to obtain humid crystals; and (5) further adding the obtained humid crystals into the heterogeneous solvent system, dissolving, and performing devitrification to obtain the humid crystals and drying the same. On the basis of keeping the purity unchanged, the method not only simplifies the process steps, but also reduces the production cost, so that industrial scaled production is further facilitated.
Owner:BRIGHTGENE PHARMA
Preparation method of high-purity and high-yield caspofungin impurity CO
ActiveCN105218645ASimple processPeptide preparation methodsChromatographic separationPurification methods
The invention discloses a preparation method of a high-purity and high-yield caspofungin impurity CO. The preparation method comprises step1, an intermediate I is prepared from solids containing pneumocandins BO and CO; step 2, a crude product of an intermediate II is prepared from the intermediate I, chromatography purification and separation are performed with a reversed-phase chromatography method and a normal-phase chromatography method, and the intermediate II is obtained; step3, a crude product of the caspofungin impurity CO is prepared from the intermediate II, chromatographic separation is performed with a reversed phase chromatography method, and the high-purity caspofungin impurity CO is obtained. By means of a specific combination and purification method of raw materials, the caspofungin impurity CO with HPLC (high performance liquid chromatography) purity higher than 97% can be obtained, and a way is provided for preparation of the caspofungin impurity CO. The purity of the product obtained with the method can completely meet the requirements, and a process is relatively simple.
Owner:CHENGDU YATU BIOLOGICAL TECH
Preparation method of caspofungin
ActiveCN105440109AMild reaction conditionsSimple post-processingPeptidesEthylenediamineFreeze-drying
The invention discloses a preparation method of caspofungin. The preparation method comprises steps as follows: pneumocandin B0 and phenylboronic acid are dissolved in acetonitrile, thiophenol is added, the mixture is stirred and uniformly mixed, trifluoromethane sulfonic acid is added dropwise, and an intermediate MD-I is obtained; the intermediate MD-I and phenylboronic acid are added to an anhydrous tetrahydrofuran solution, mixed and dissolved, BSTFA (bis-trimethylsilyl-trifluoroacetamide) is added under protection of nitrogen, the mixture continues to have a reaction, a borane-tetrahydrofuran solution is added dropwise, the mixture has a reaction, tetrahydrofuran is removed through reduced pressure distillation, and an intermediate MD-II is prepared after a product is separated; the intermediate MD-II is dissolved in methanol, the mixture is cooled under the protection of nitrogen, ethylene diamine is added until the mixture reacts sufficiently, and caspofungin is obtained after freezing drying. The new preparation method of caspofungin has the advantages as follows: the route is short, the reaction condition is mild, post-processing is simple, the yield is remarkably improved by comparison with that in the prior art, the total synthesis time can be remarkably shortened, the operative difficulty index and requirements for equipment are reduced to a certain extent, and the production cost is remarkably reduced.
Owner:成都摩尔生物医药有限公司
Antifungal combination therapy
There is described antifungal combination therapy comprising the use of known antifungal agents such as the azoles or polyenes in combination with a pneumocandin derivative antifungal agent. More particularly, the invention relates to antifungal combination therapy comprising the use of azoles such as fluconazole, voriconazole, itraconazole, ketoconazole, miconazole, ER 30346, SCH 56592; polyenes such as amphotericin B, nystatin or liposomal and lipid forms thereof such as Abelcet, AmBisome and Amphocil; purine or pyrimidine nucleotide inhibitors such as flucytosine; or polyoxins such as nikkomycins, in particular nikkomycin Z or other chitin inhibitors, elongation factor inhibitors such as sordarin and analogs thereof, mannan inhibitors such as predamycin, bactericidal / permeability-inducing (BPI) protein products such as XMP.97 or XMP.127 or complex carbohydrate antifungal agents such as CAN-296 in combination with a pneumocandin derivative as described herein.
Owner:MERCK SHARP & DOHME CORP
Fermentation method of caspofungin fermentation intermediate
ActiveCN106755224ALow viscosityImprove ventilationMicroorganism based processesPeptidesLactosePneumocandin C0
The invention relates to a fermentation method of a caspofungin fermentation intermediate and provides a method for reducing content of pneumocandin C0 while increasing the fermentation unit of pneumocandin B0. The strain adopted in the method is glarea lozoyensis. In the invention, a dilute formula is adopted for a culture medium; lactose and ammonia water are fed in the fermentation process, so that the strain viscosity can be effectively lowered, and the ventilation effect is improved; and meanwhile, pH is strictly controlled in the fermentation process. Meanwhile, vitamin b5 is supplemented to fermentation liquid; in the existing fermentation technology of pneumocandin B0, the fermentation unit of pneumocandin B0 is around 800-1,000mg / l; and in the fermentation method provided by the invention, the fermentation unit can be raised to 2,000-2,500mg / l, and the content of pneumocandin C0 is remarkably lowered.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Fermentation method of pneumocandin B0
The invention provides a new fermentation method of pneumocandin B0, which can reduce the content of pneumocandin C0 in the fermentation process and remarkably lower the cost of downstream extraction technology. According to the method, the adopted strain is glarea lozoyensis, vitamin b5 is supplemented in the whole fermentation process, and pH is strictly controlled; and in the fermentation liquid obtained by the method, the content of pneumocandin C0 is reduced to 1.5% from 6%.
Owner:BRIGHTGENE PHARMA
Process for preparation of caspofungin acetate and intermediates
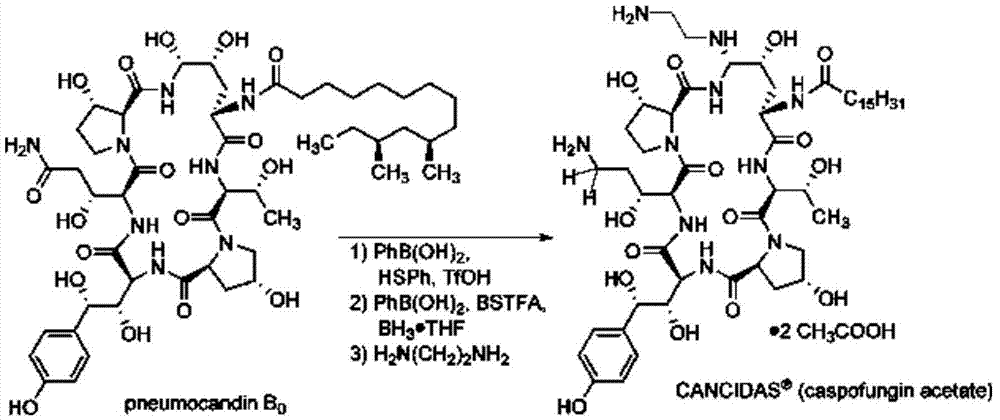
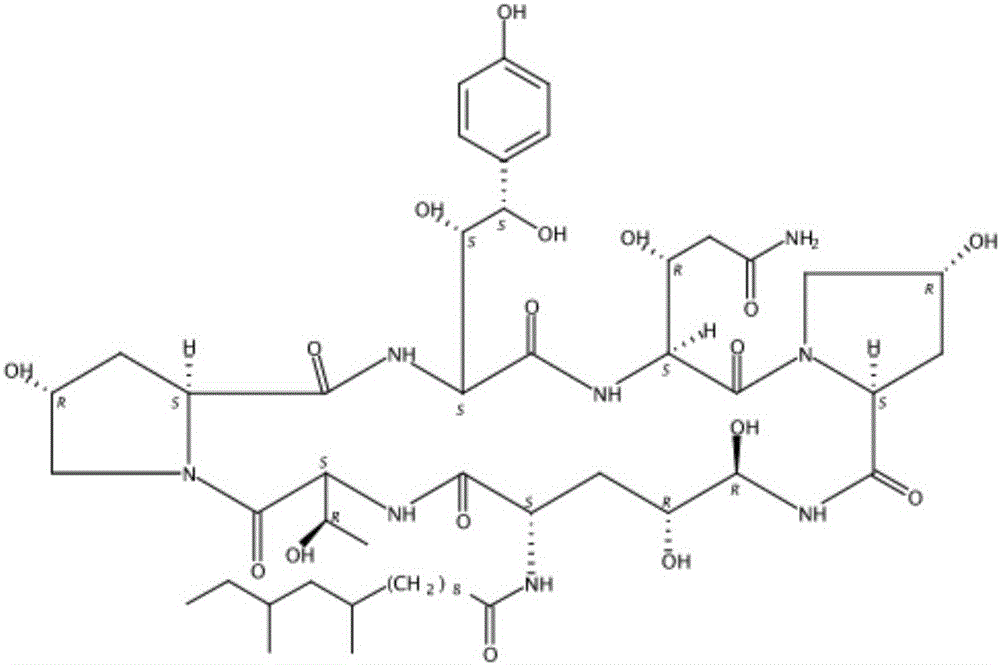
The present invention discloses a process for the preparation of Caspofungin and its intermediates from Pneumocandin B0.
Owner:BIOCON LTD
Method for purifying caspofungin precursor pneumocandin B0 component
ActiveCN102816207BHigh yieldSimplified purification stepsPeptide preparation methodsState of artPurification methods
The invention relates to a method for purifying a caspofungin precursor pneumocandin B0 component, and the method comprises the following steps of: carrying out reversed-phase column chromatography on a PB0 component crude product, collecting and carrying out reduced-pressure concentration to obtain a semi-pure product; carrying out normal phase column chromatography on the PB0 semi-pure product obtained in the step 1 to obtain high-purity PB0 component; and carrying out positive and negative phase liquid phase analysis, wherein the chromatographic purity is more than 95%. Compared with the prior art, the method has the beneficial effects that a normal phase column and a reversed-phase column are used for purifying the PB0 component, so that the purification steps are simple, the purification effect is good, the requirements for equipment are low, large-scale production is realized, and the production feasibility is high; and particularly, the PB0 component separated by the method is high in yield, but is lower in cost.
Owner:CHENGDU YATU BIOLOGICAL TECH
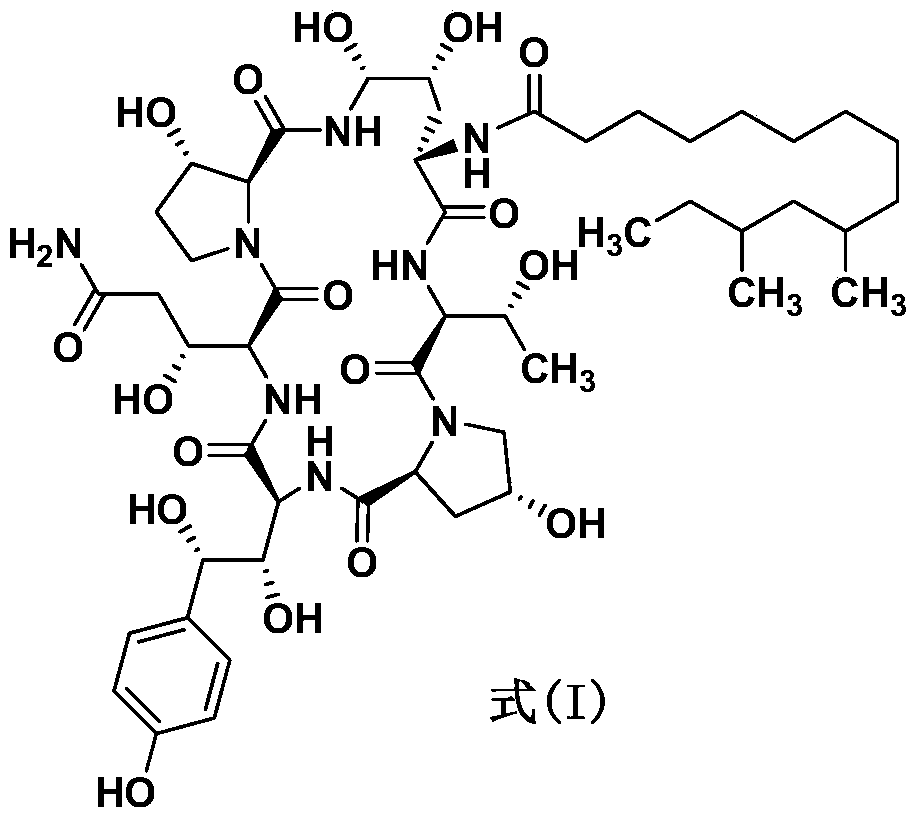
Process for preparing caspofungin and novel intermediates thereof
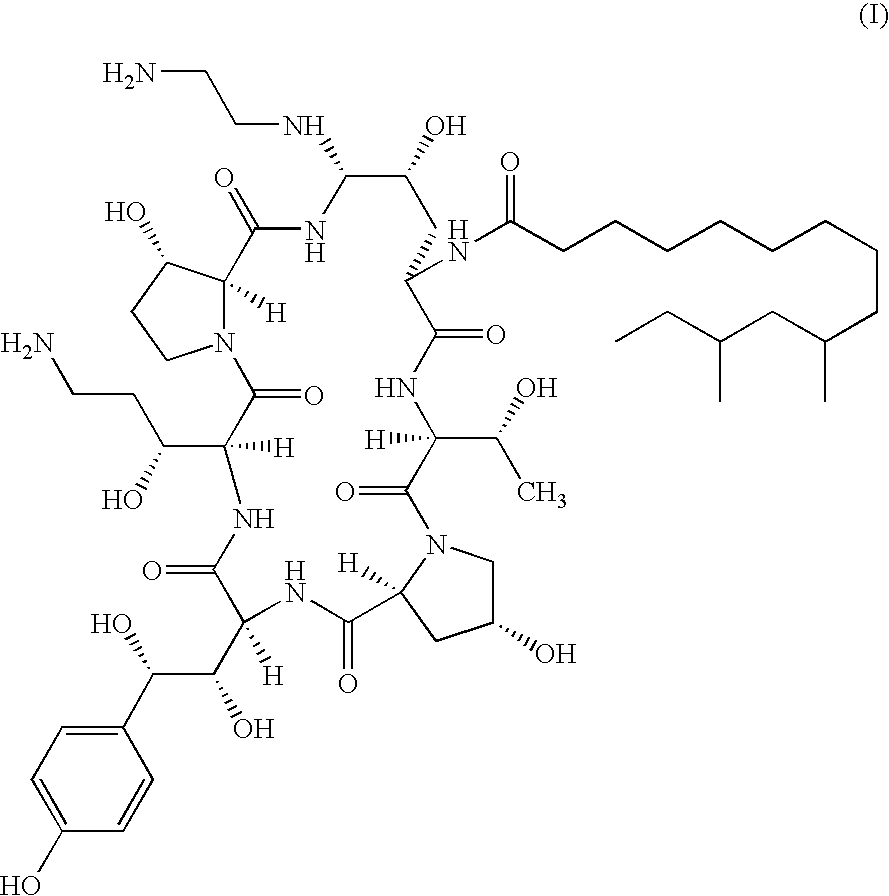
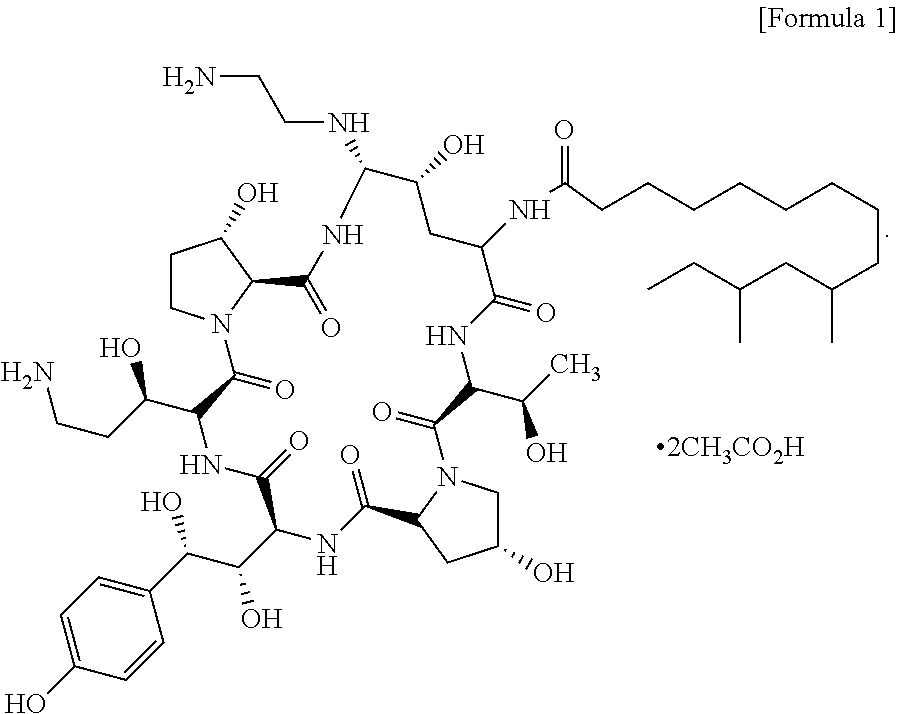
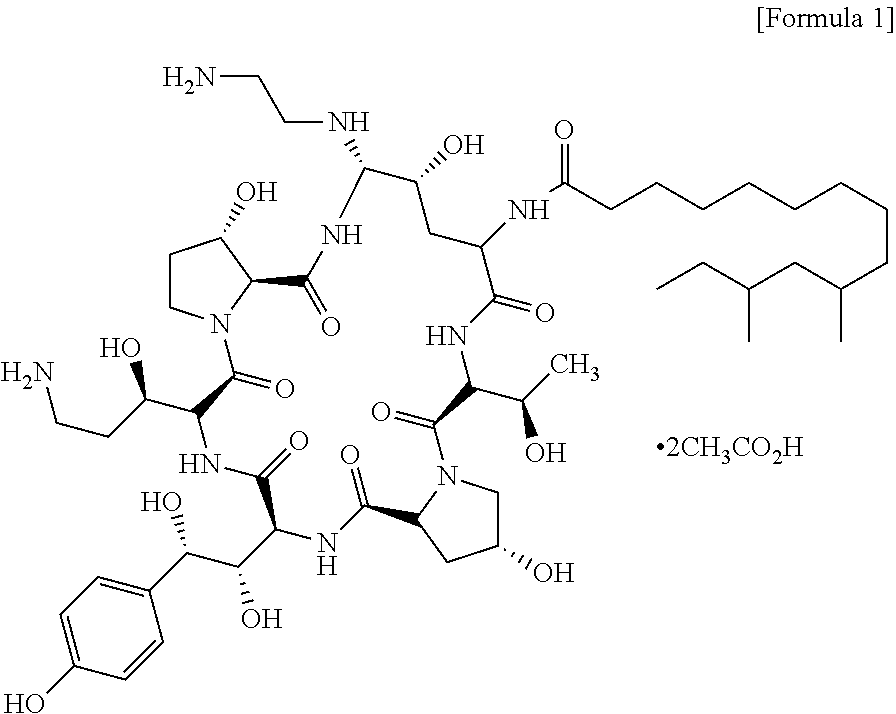
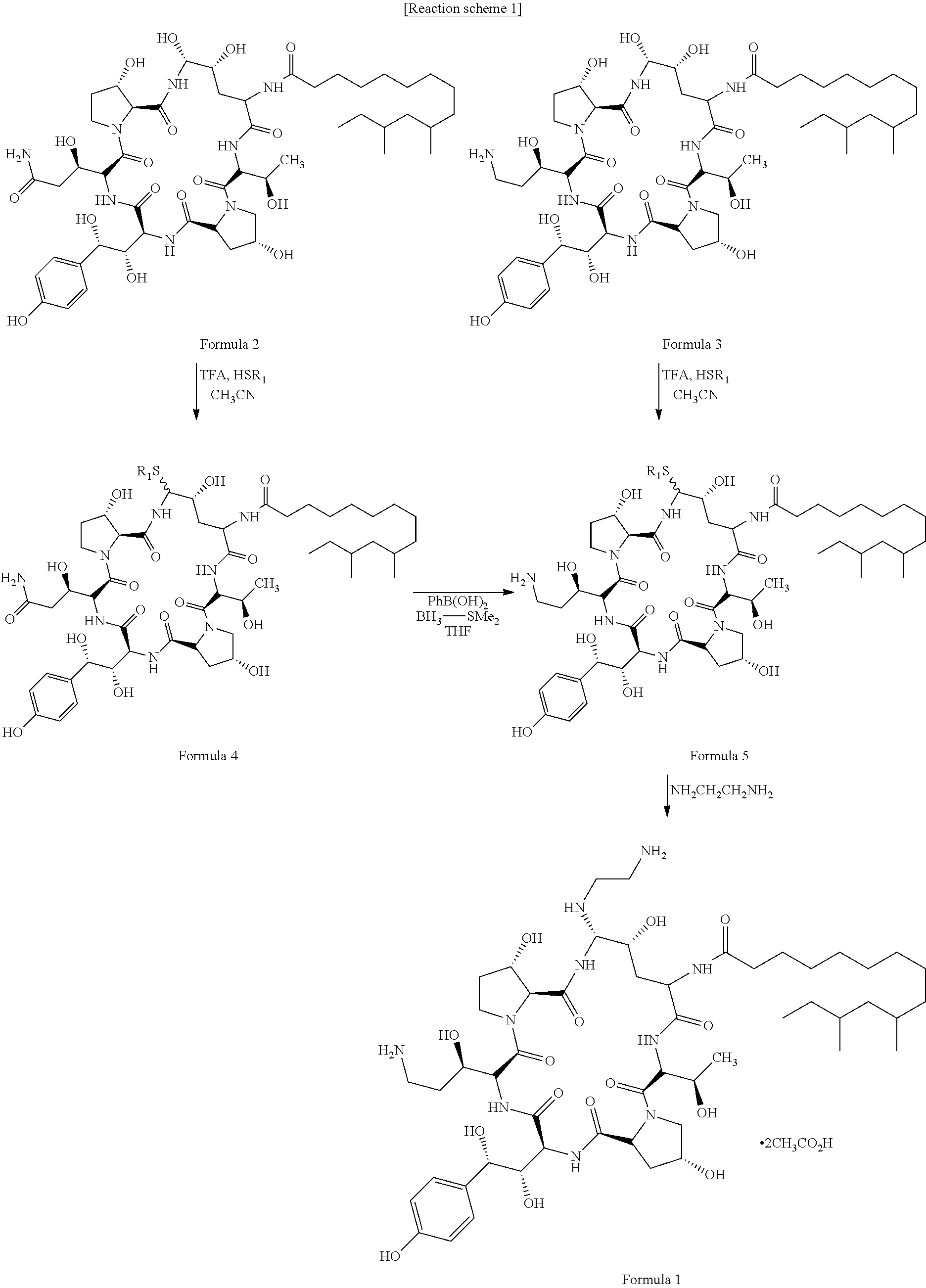
InactiveUS9018349B2Increase productionImmunoglobulinsCyclic peptide ingredientsOrnithine synthesisL-Ornithine
The present invention relates to a novel process for preparing the aza cyclohexapeptide compound 1-[(4R,5S)-5-[(2-aminoethyl)amino]-N2-(10,12-dimethyl-1-oxotetradecyl)-4-hydroxy-L-ornithine]-5-[(3R)-3-hydroxy-L-ornithine]-pneumocandin B0 (caspofungin) represented by the following formula 1, which can improve the problem due to a pungent odor and toxicity during the process and can prepare caspofungin as a final product at high yield compared to conventional processes, and to novel intermediates which are used in the preparation process:
Owner:CHONGKUNDANG BIO
Fermentation method of caspofungin fermentation intermediate
ActiveCN106755224BReduce contentRaise the fermentation unitMicroorganism based processesPeptidesLactosePneumocandin C0
The invention relates to a fermentation method of a caspofungin fermentation intermediate and provides a method for reducing content of pneumocandin C0 while increasing the fermentation unit of pneumocandin B0. The strain adopted in the method is glarea lozoyensis. In the invention, a dilute formula is adopted for a culture medium; lactose and ammonia water are fed in the fermentation process, so that the strain viscosity can be effectively lowered, and the ventilation effect is improved; and meanwhile, pH is strictly controlled in the fermentation process. Meanwhile, vitamin b5 is supplemented to fermentation liquid; in the existing fermentation technology of pneumocandin B0, the fermentation unit of pneumocandin B0 is around 800-1,000mg / l; and in the fermentation method provided by the invention, the fermentation unit can be raised to 2,000-2,500mg / l, and the content of pneumocandin C0 is remarkably lowered.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Pneumocandin B0 purification method
InactiveCN106589073AImprove pHHigh purityPeptide preparation methodsPurification methodsEthyl acetate
The present invention discloses a process-optimizing pneumocandin B0 purification method, which comprises: (1) acidifying a pneumocandin B0 fermentation broth, filtering, and leaching to obtain a leaching liquid; (2) adding diatomite coating crystal to the leaching liquid concentration mixture, adding deionized water, and centrifugating to obtain a white solid containing pneumocandin B0; (3) adding ethanol to the obtained solid, dissolving, completely stirring, adding active carbon, completely stirring and decolorizing, and filtering to obtain a colorless filtrate; (4) concentrating the colorless filtrate to achieve a dry state, adding methanol to dissolve the dried filtrate, adding water, stirring to achieve a dissolved clarification state, adding ethyl acetate, stirring, and crystallizing to obtain a primary wet crystal; and (5) crystallizing the wet crystal again by using the same crystallizing method to obtain a wet crystal, and drying. With the method of the present invention, on the basis of the unchanged purity, the process steps are simplified, the production cost is reduced, and the industrialized mass production is easily achieved.
Owner:BRIGHTGENE PHARMA
A kind of extraction and purification method of pneumocidine b0
ActiveCN107778357BEasy to realize industrial productionPeptide preparation methodsBiotechnologyActivated carbon
The invention discloses an extraction and purification method of pneumocandins B0. The method comprises the steps of: 1) adjusting a pH (potential of hydrogen) value of fermentation liquid containingpneumocandins B0, adding a filter aid and performing filtration to form bacterial residue, 2) extracting the bacterial residue with a high-concentration aqueous solution of methanol or ethanol, 3) adsorbing extraction liquid with adsorbent resin, eluting with the 80-90(V / V) aqueous solution of methanol (ethanol), and collecting an eluant rich in pneumocandins B0, 4) adding active carbon into the eluant containing pneumocandins B0 for decoloration treatment, 5) adsorbing with the adsorbent resin, eluting the resin with the 80-100% (V / V) aqueous solution of methanol (ethanol) and collecting an eluant rich in pneumocandins B0, and 6) performing vacuum concentration to form a crude product of pneumocandins B0. Due to improvement of the extraction and purification method, a decoloration and purification effect is obvious, the purity of the product is improved obviously, the production cost is low, and the method is simple in technical step and suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of surface molecularly imprinted polymer of PN (pneumocandins)B0
InactiveCN105061699AOvercome the defects of complex extraction and purification processHigh selectivityOther chemical processesAlkali metal oxides/hydroxidesMethacrylateStrong acids
The invention relates to a preparation method of a surface molecularly imprinted polymer of PN (pneumocandins)B0. The preparation method comprises the following steps: step 1, SiO2 is taken and placed in a container filled with a strong acid solution, reacts, and is left to stand, washed to neutral with distilled water and dried; step 2, gamma-trimethoxysilyl propyl methacrylate, ethanol and water are added to the substance obtained in the step 1, the mixture reacts, and a product is dried; step 3, water and methacrylic acid are added to the substance obtained in the step 2, N2 is adopted to exhaust air, the temperature is increased, an initiator ammonium persulfate is added, the mixture reacts, and a product is dried; step 4, PNB0, ethanol and ethylene glycol diglycidyl ether are added to the substance obtained in the step 3, pH is regulated, the mixture reacts, PNB0 is removed with a polar solvent, a product is dried, and the PNB0 molecularly imprinted material is prepared. The preparation method adopts a surface molecular imprinting technology and overcomes the defect of complex PNB0 extraction and purification processes in the prior art, the purity of PNB0 is improved, the content of PNB0 is increased, and industrial production of PNB0 is simpler and more convenient.
Owner:SHIHEZI UNIVERSITY
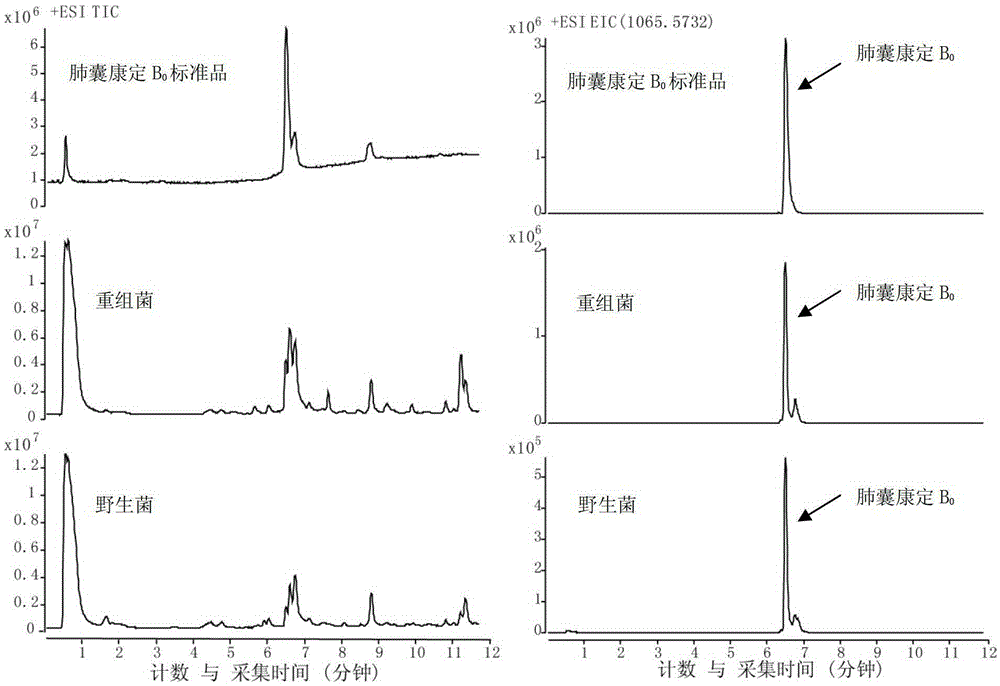
A kind of preparation method of high-purity lung capsule Kangding b0
ActiveCN103073622BQuality improvementHigh recovery ratePeptide preparation methodsChromatographic separationMicrosphere
The invention discloses a method for preparing pneumocandin B0 by using a fermentation culture product of filamentous fungi glarea lozoyensis. According to the method, a filter aid is adopted; with steps such as leaching, discoloring, adsorption, crystallization, and the like, a pneumocandin B0 crude extract is obtained; and the crude extract is subjected to chromatographic separation by using polymer microspheres, such that the high-purity pneumocandin B0 product is obtained. The method provided by the invention has the advantages that: pneumocandin B0 is extracted and separated by using macroporous resin; the process is simple and feasible, and is suitable for industrialized productions; the polymer microspheres are used in chromatographic separation of the product for a first time, and high-purity pneumocandin B0 product can be prepared.
Owner:NCPC NEW DRUG RES & DEV
Glpks3 and its coding gene related to the synthesis of Pneumonia Kangding b0
The invention discloses a method for synthesizing associated protein glpks3 through pneumocandin B0 and an encoding gene of the glpks3. Protein provided in the method can be (a) protein composed of an amino acid sequence presented in a sequence 1 in a sequence table, or (b) protein associated with pneumocandin B0 synthesis, derived from the sequence 1 and formed in the mode that the amino acid sequence presented in the sequence 1 in the sequence table undergoes substitution and / or deletion and / or adding through one or more amino acid residues. By means of expression of the encoding gene for prohibiting the protein in Glarea lozoyensis, the yield of the pneumocandin B0 is improved by four times. Theoretical bases are provided for intensive study of metabolic regulation of pneumocandin biosynthesis, the method can be used for improving the yield of the pneumocandin B0 through gene knockout, and guarantee is provided for high yield of caspofungin.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
The preparation method of caspofungin
The invention relates to a preparation method of caspofungin. The preparation method comprises the following steps: using p-thiocresol to replace hydroxide radicals of pneumocandins B0, then reducing acylamino groups by using a reducing agent, and finally using ethidene diamine to replace p-sulfonyl toluene, thus obtaining the caspofungin. According to the preparation method disclosed by the invention, an appropriate solvent is adopted for crystallizing and separating an intermediate, repeated separation and purification operation for chromatographic column preparation in the prior art is avoided, the reaction time is shortened, degradation of the intermediate is avoided, the operation is simplified, high-purity and high-yield caspofungin is obtained, and the preparation method is particularly suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com