Preparation method of caspofungin
A caspofungin and dripping technology, applied in the direction of peptides, etc., can solve problems such as long time and affecting product production efficiency, and achieve the effects of increasing yield, facilitating quality control, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
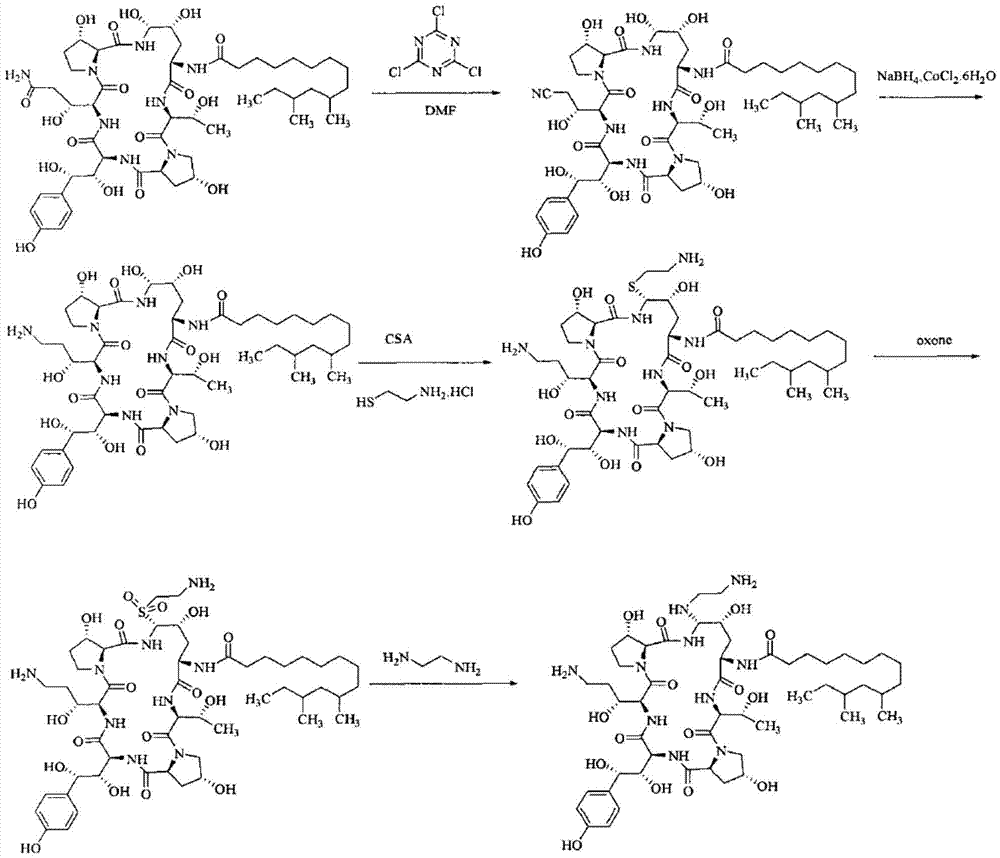
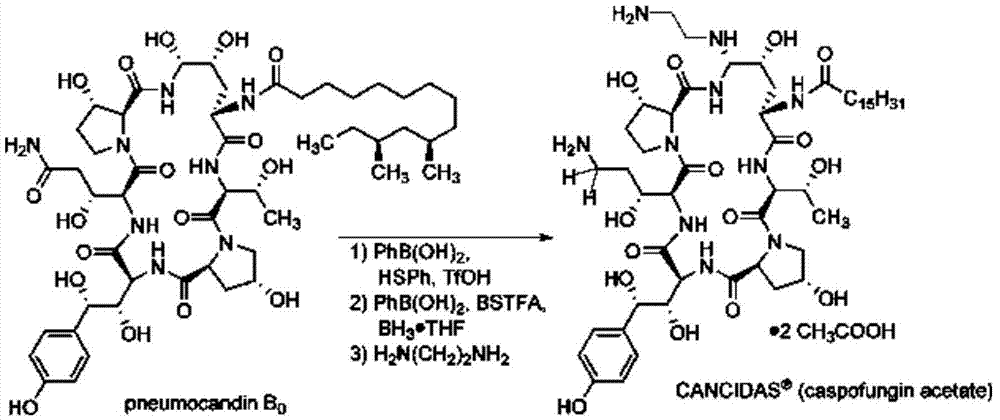
[0028] A kind of preparation method of caspofungin, its synthetic process is:
[0029]
[0030] Specifically include the following steps:
[0031] (1) Add 1 mol neomocontin B 0 After dissolving 2mol phenylboronic acid in 300ml acetonitrile, cool down to -45°C; add 3mol thiophenol and stir to mix evenly, add dropwise 2mol trifluoromethanesulfonic acid, control the temperature below -35°C to pneumocantine B 0 The content is lower than 5%, that is, the reaction is terminated. After the reaction was terminated, sodium acetate acetonitrile aqueous solution was added dropwise, and the volume ratio of acetonitrile and water in the sodium acetate acetonitrile aqueous solution was 4:1. After the dropwise addition, the solution is nearly neutral, and the pH value can be adjusted to about 6.5. Continue to stir, then perform low-temperature filtration, wash the filter cake with pure water until the solution is nearly neutral, and dry after washing to obtain the intermediate MD-I; th...
Embodiment 2
[0035] A preparation method of caspofungin, comprising the following steps:
[0036] (1) Add 1 mol neomocontin B 0 After dissolving 3mol phenylboronic acid in 400ml acetonitrile, cool down to -42°C; add 4mol thiophenol and stir to mix evenly, add 1.5mol trifluoromethanesulfonic acid dropwise, control the temperature below -35°C to pneumocantine B 0 The content is lower than 3%, that is, the reaction is terminated. After the reaction was terminated, sodium acetate acetonitrile aqueous solution was added dropwise, and the volume ratio of acetonitrile and water in the sodium acetate acetonitrile aqueous solution was 4:1. After the dropwise addition, the solution is nearly neutral, and the pH value can be adjusted to about 7. Continue to stir, then perform low-temperature filtration, wash the filter cake with pure water until the solution is nearly neutral, and dry after washing to obtain the intermediate MD-I; the yield is 98.1%.
[0037] (2) Add the dried intermediate MD-I an...
Embodiment 3
[0040] A preparation method of caspofungin, comprising the following steps:
[0041] (1) Add 1 mol neomocontin B 0 After dissolving 3mol phenylboronic acid in 400ml acetonitrile, cool down to -42°C; add 4mol thiophenol and stir to mix evenly, add 2mol trifluoromethanesulfonic acid dropwise, control the temperature below -40°C to pneumocantine B 0The content is 5%, that is, the reaction is terminated. After the reaction was terminated, sodium acetate acetonitrile aqueous solution was added dropwise, and the volume ratio of acetonitrile and water in the sodium acetate acetonitrile aqueous solution was 4:1. After the dropwise addition, the solution is nearly neutral, and the pH value can be adjusted to about 6. Continue to stir, then perform low-temperature filtration, wash the filter cake with pure water until the solution is nearly neutral, and dry after washing to obtain the intermediate MD-I; the yield is 98.3%.
[0042] (2) Add the dried intermediate MD-I and 6mol phenylb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com