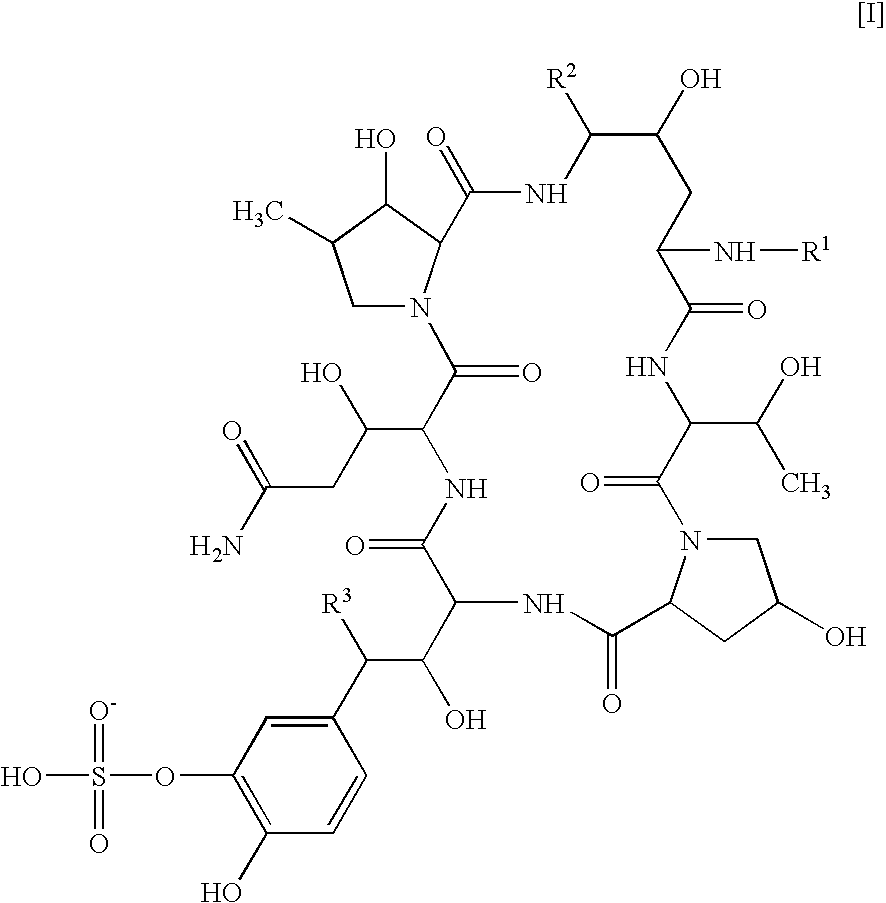
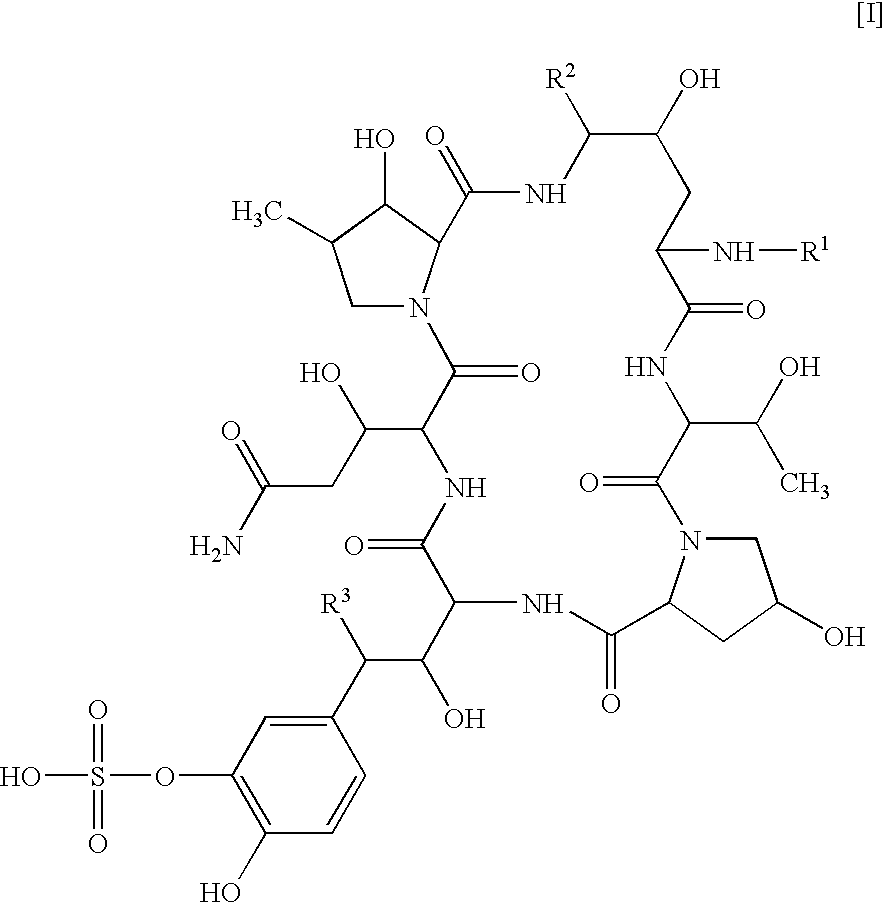
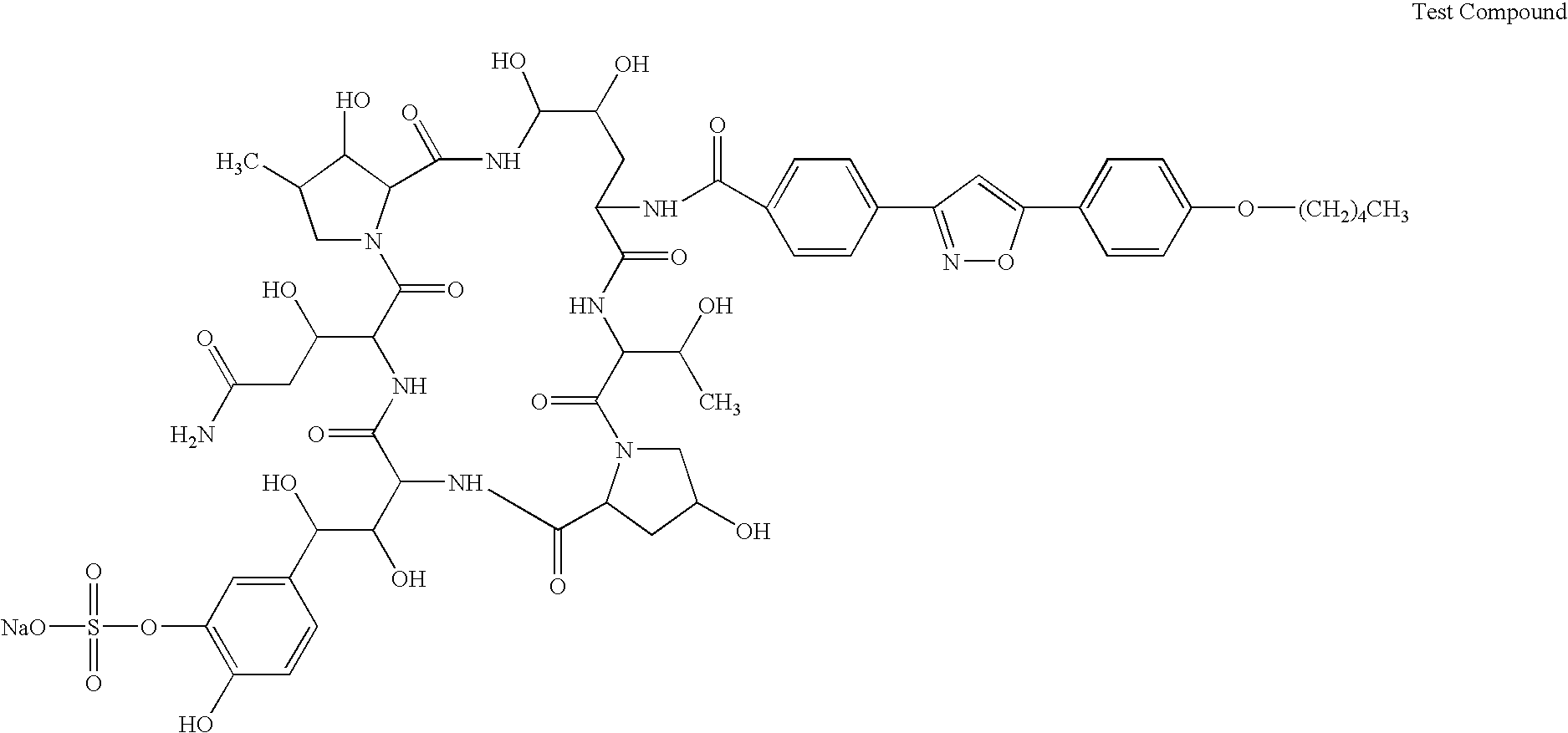
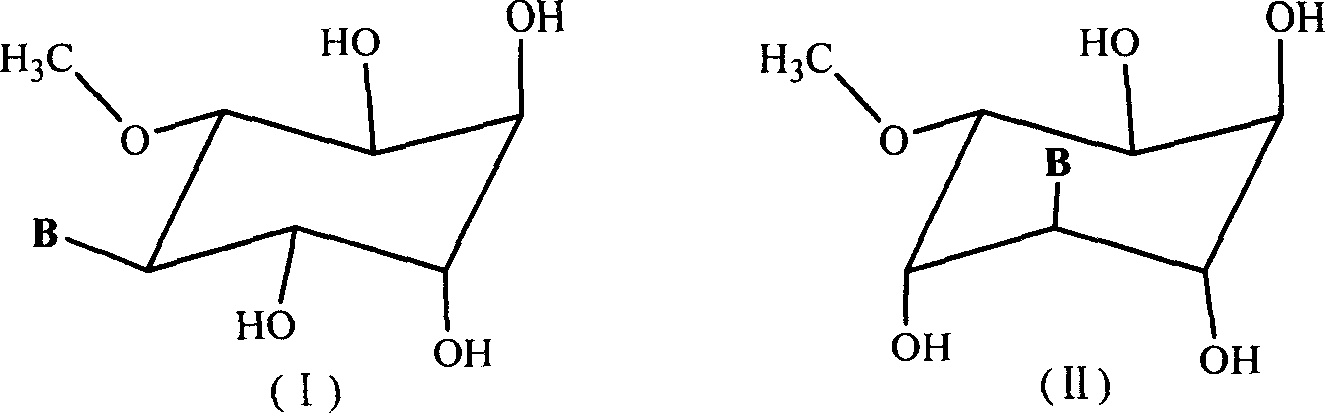
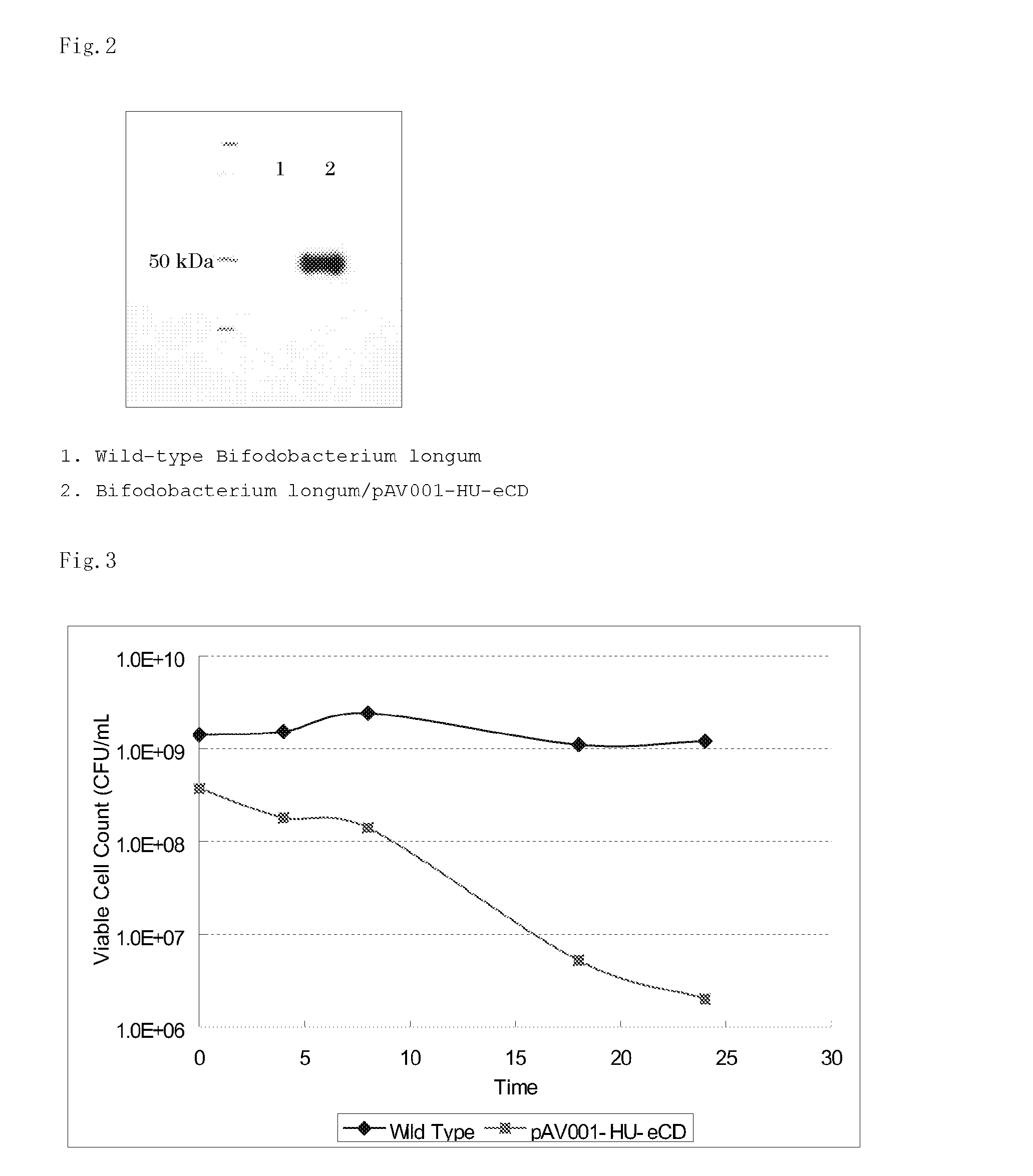
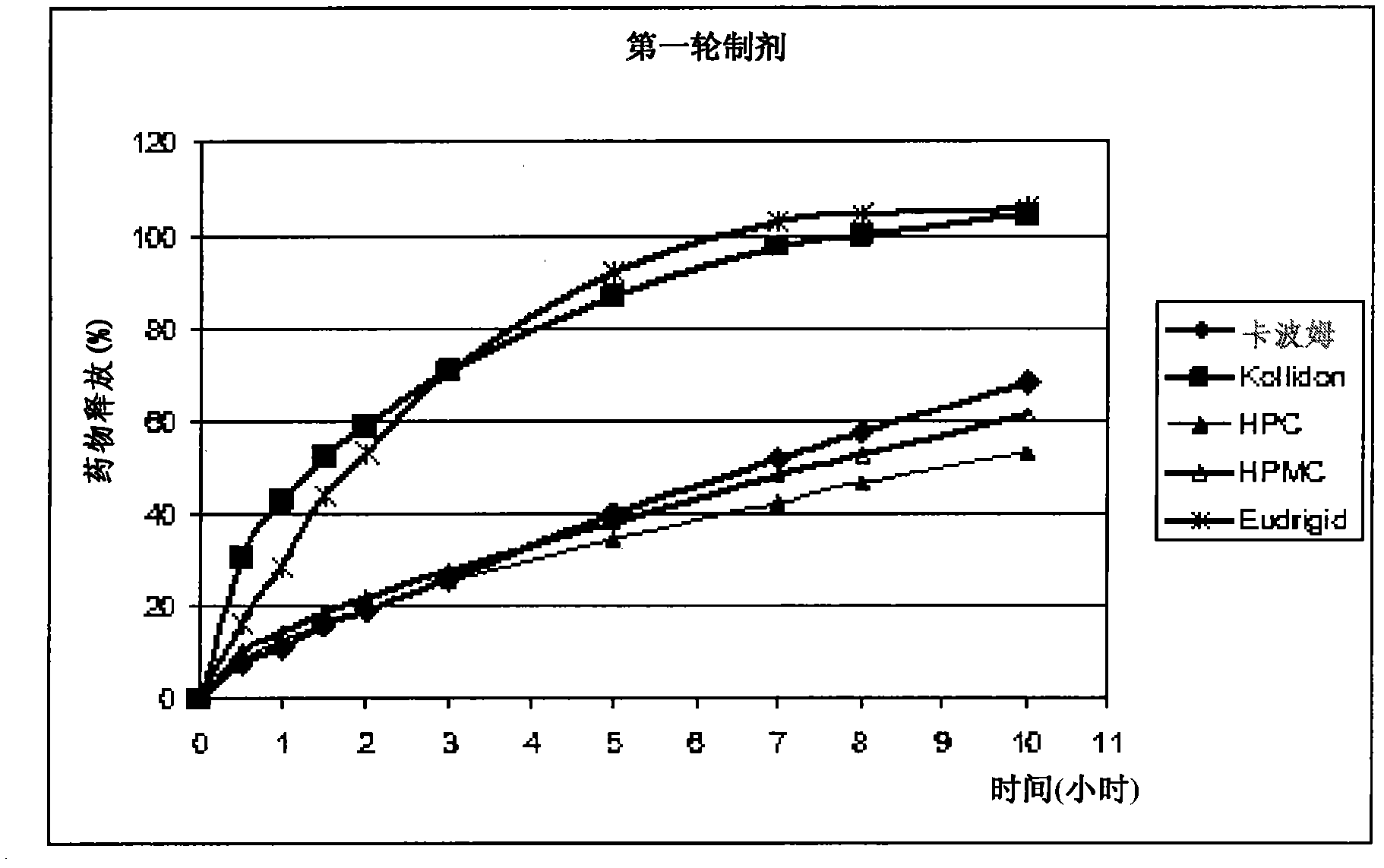
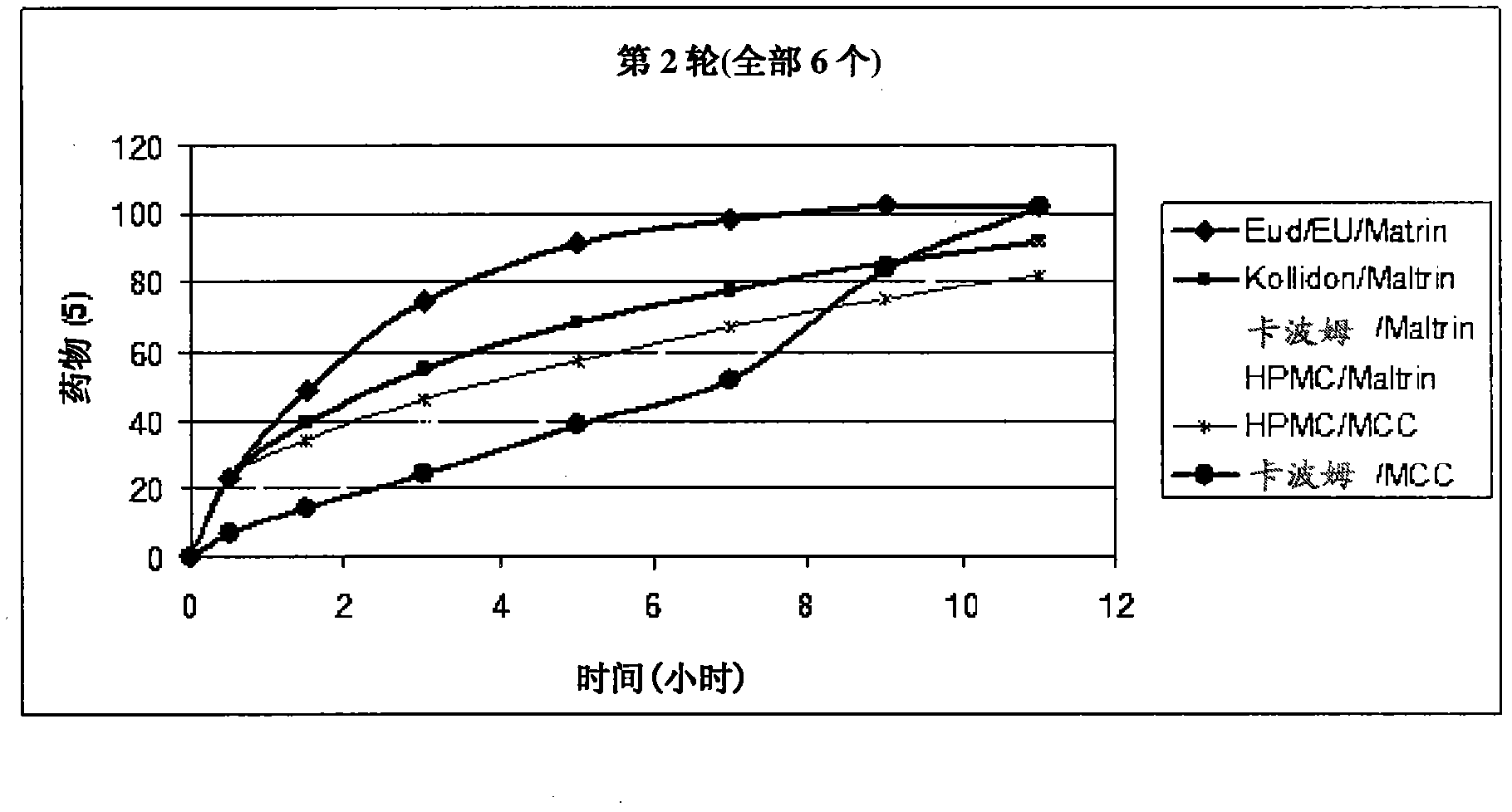
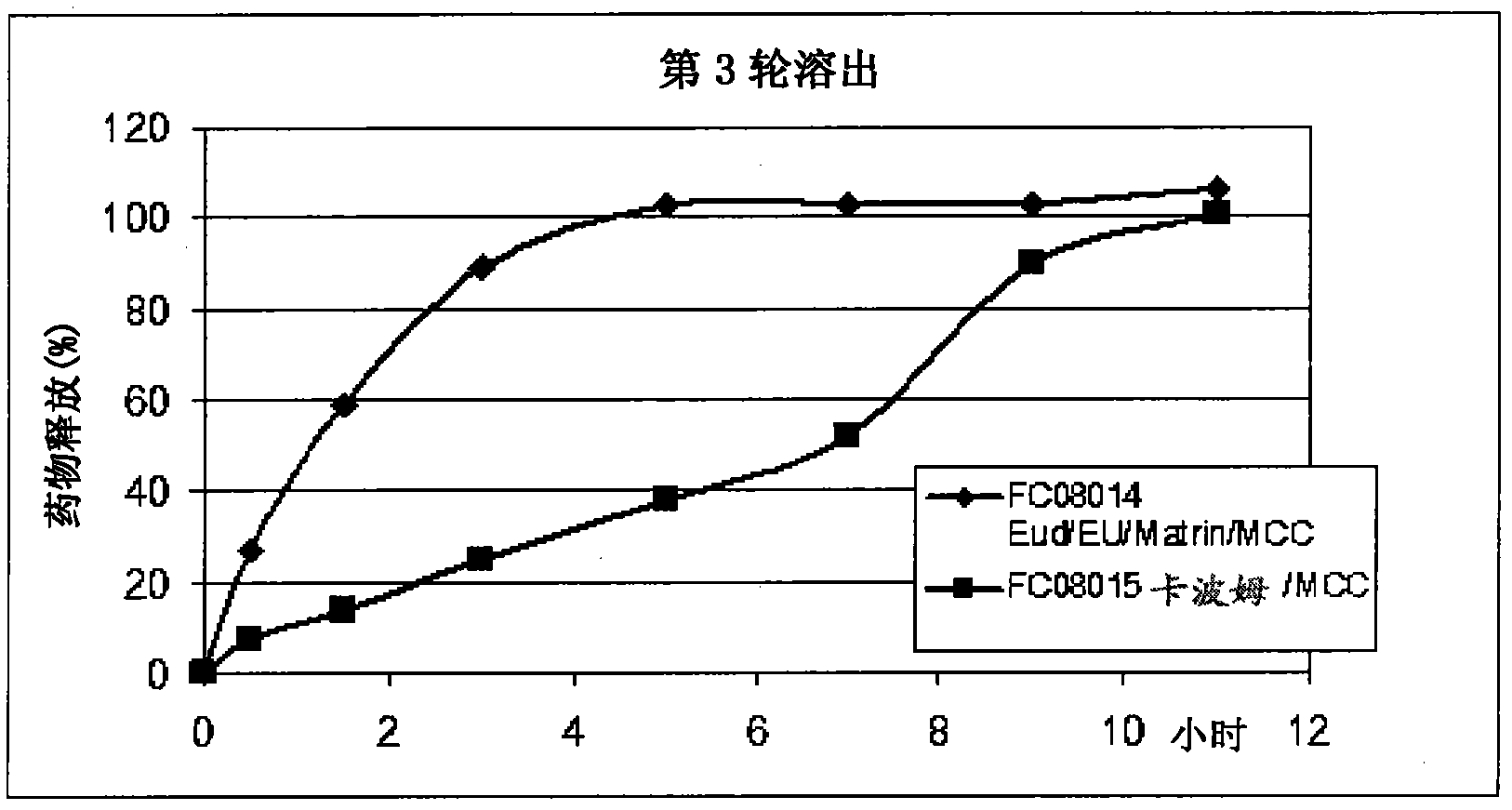
Patents
Literature
49 results about "Flucytosine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
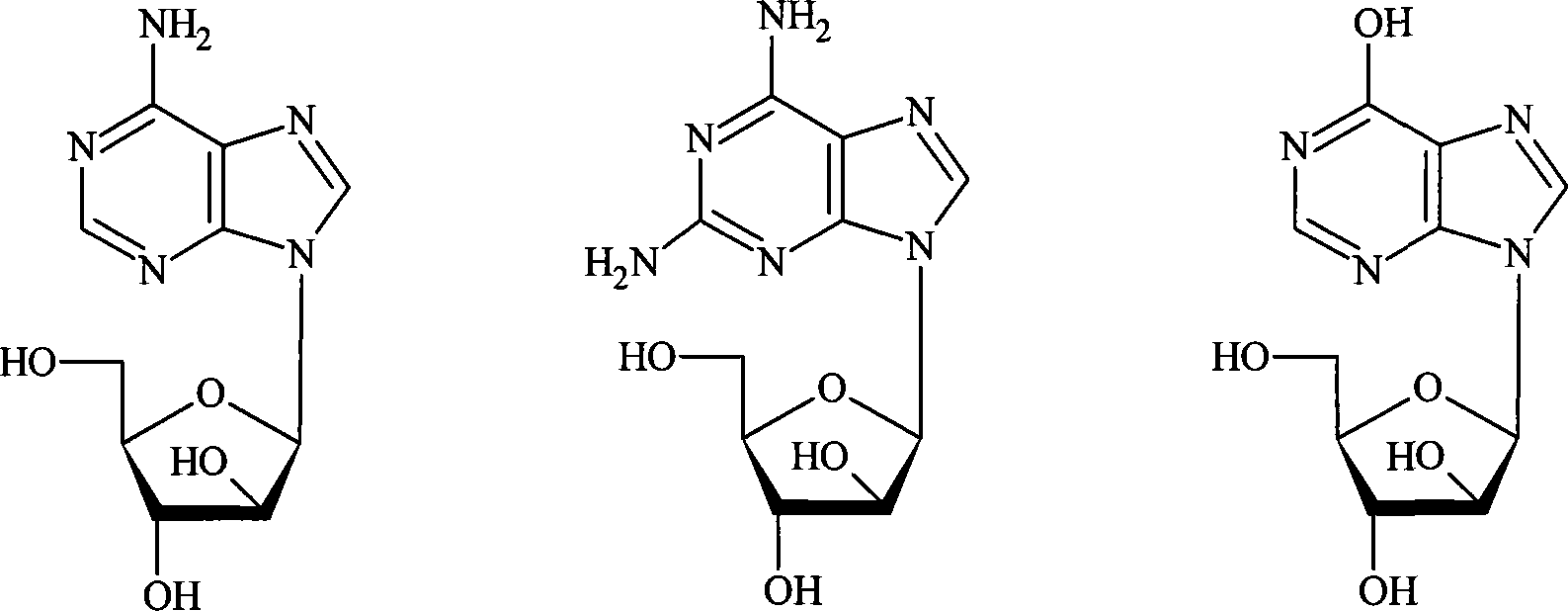
Flucytosine is used to treat serious fungal infections in the body.
Molecular chemotherapy enhancement of radiotherapy
InactiveUS6552005B1Tumour growth inhibitionCurrent is limitedBiocidePeptide/protein ingredientsWhole bodyCytotoxicity
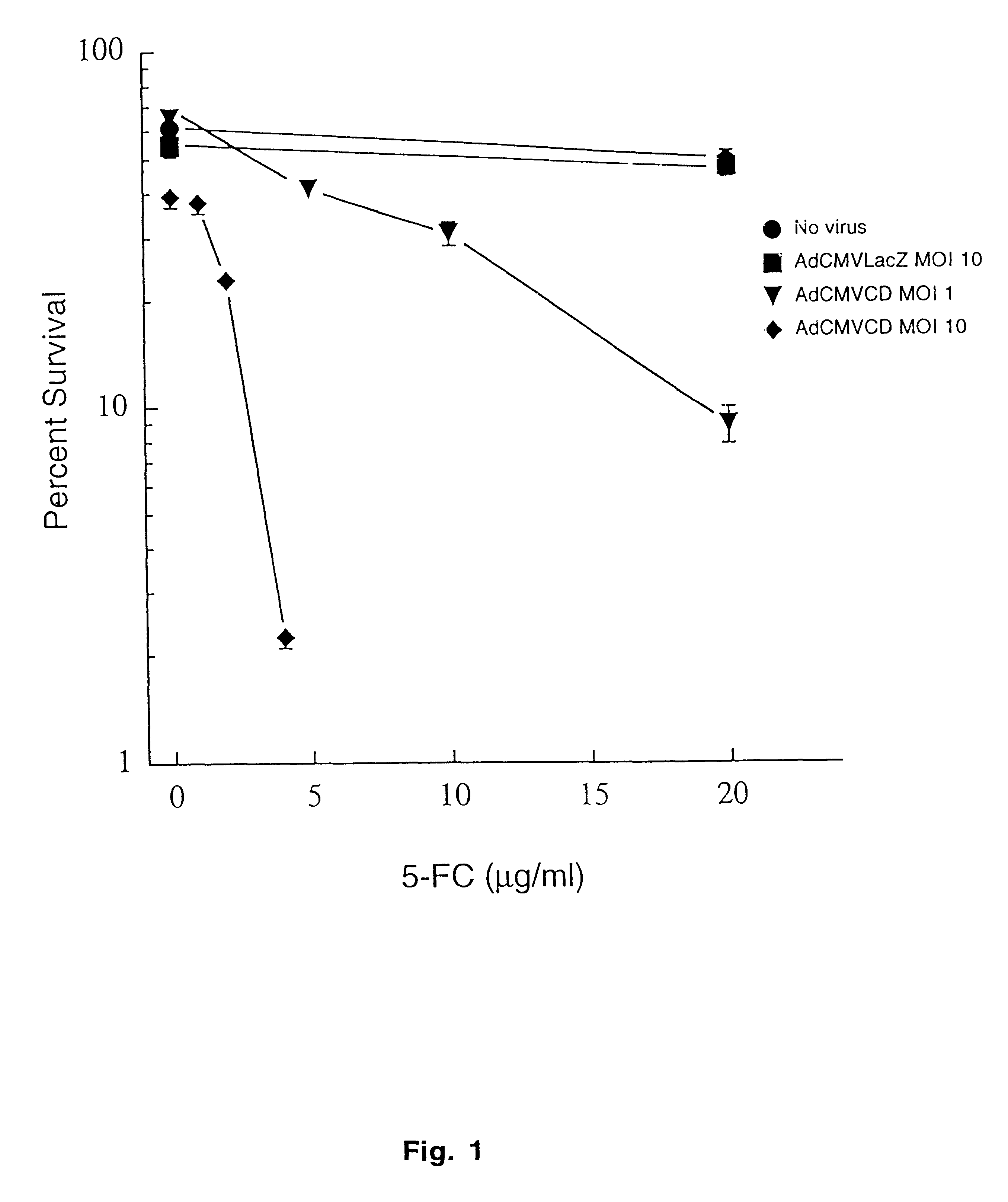
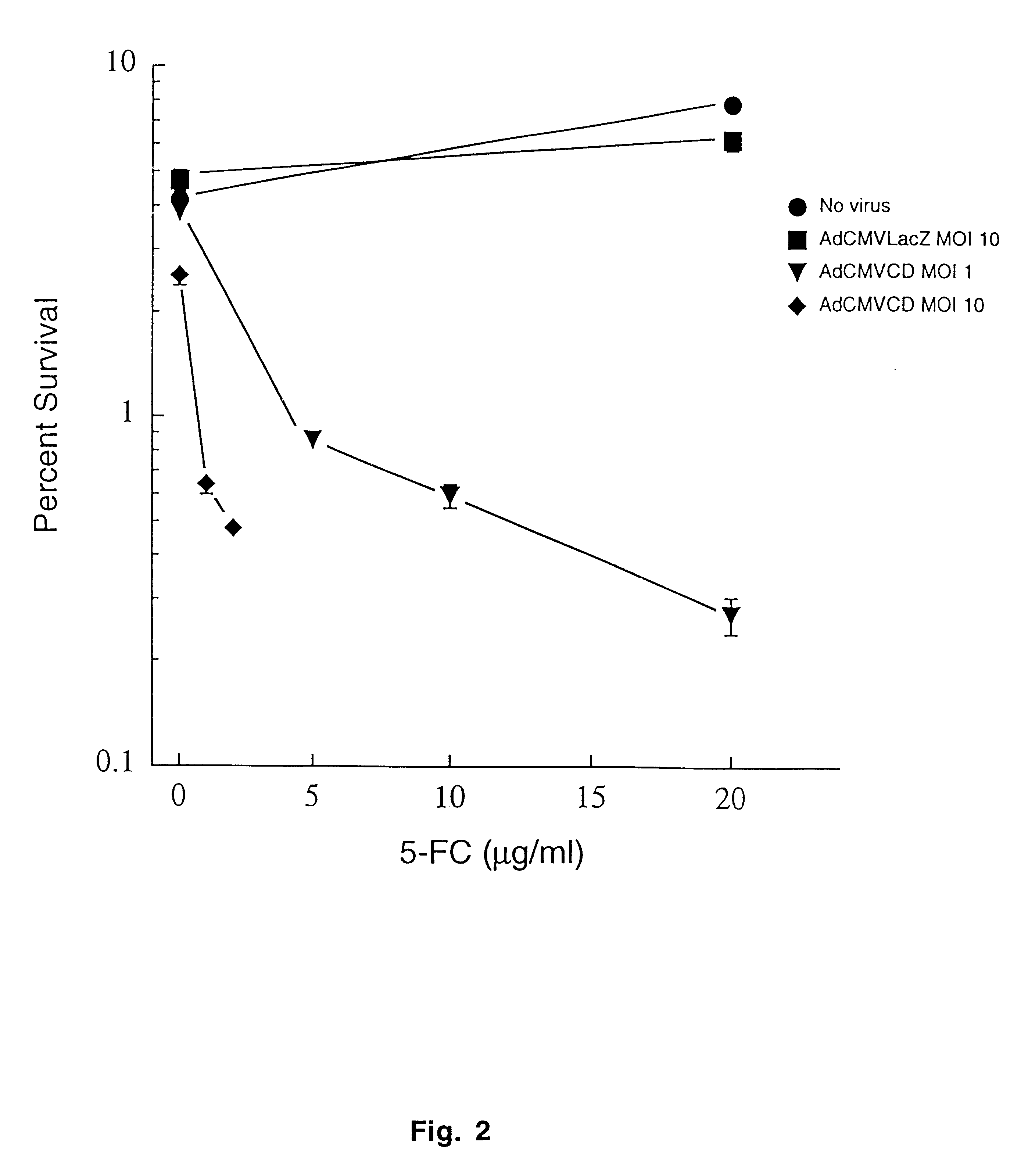
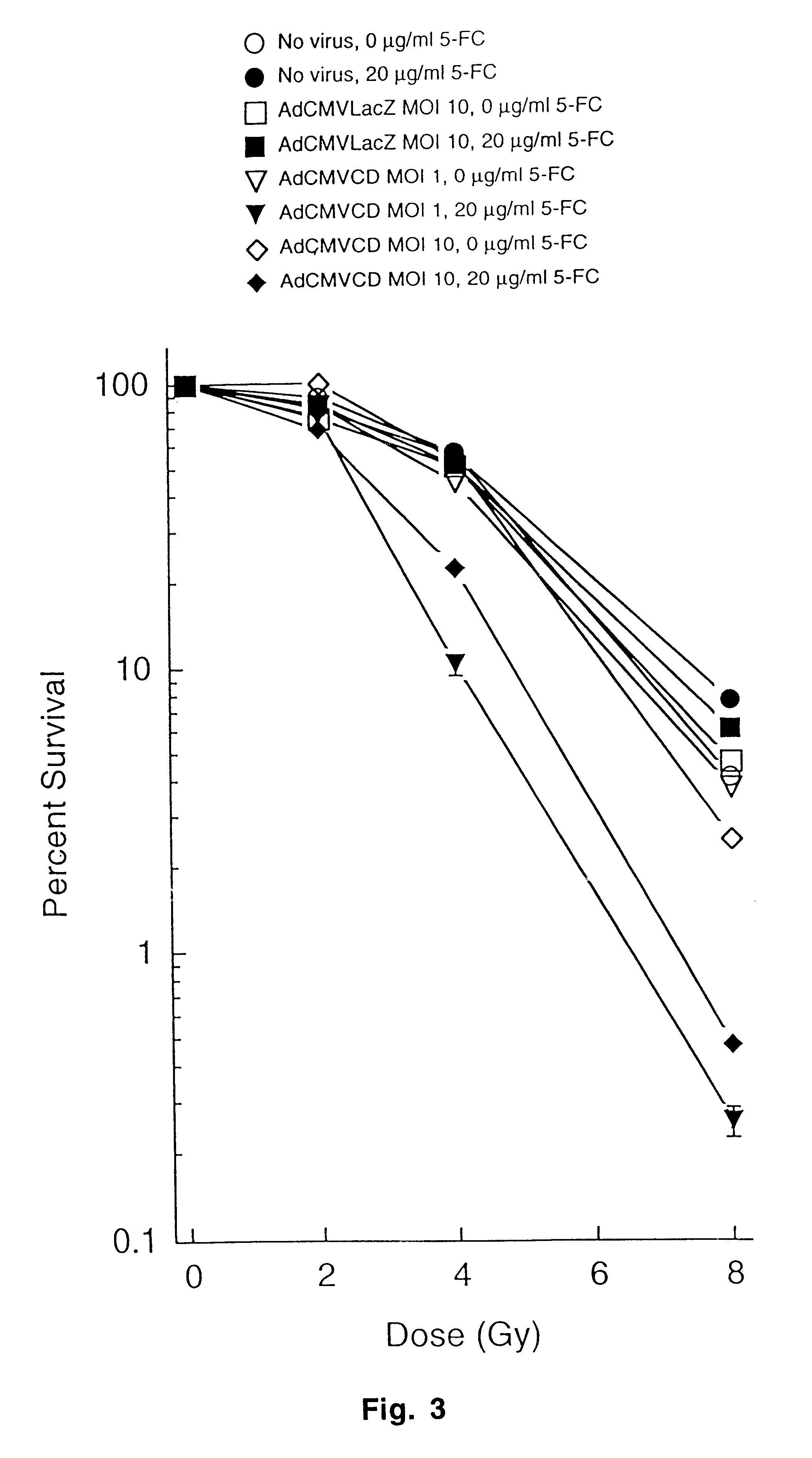
The present invention provides a new approach for cancer treatment by utilizing gene therapy combined with radiation therapy to enhance cytotoxicity in malignant cells. Specifically, the present invention demonstrates that molecular chemotherapy with the cytosine deaminase gene and 5-fluorocytosine is an effective radiosensitizing strategy which may lead to substantial improvement in tumor control, with less normal tissue toxicity than conventional systemic administration of 5-fluorouracil, that would translate into improved cure rates and better survival. A noninvasive method is described for continuous in vivo monitoring of 5-fluorouracil production via magnetic resonance spectroscopy. An adenovirus encoding cytosine deaminase gene which selectively replicates in tumor cells with a defective p53 pathway was constructed. Also provided is an adenovirus which encodes a fusion protein of cytosine deaminase and uracil phosphoribosyltransferase.
Owner:CDEPT
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa, and in particular otitis externa of fungal etiology, using topical medication, including antifungal agents such as, for example fluconazole, voriconazole, itraconazole, clotrimazole, amphotericin B, caspofungin (Cancidas®), micafungin (Mycamine®), terbinafine, naftifine, butenafine, amorolfine, ravuconazole, posaconazole, flucytosine, econazole, enilaconazole, miconazole, oxiconazole, saperconazole, sulconazole, terconazole, tioconazole, nikkomycin Z, anidulafungin (LY303366), nystatin, pimaricin, griseofulvin, ciclopirox, haloprogin, tolnaftate, and undecylenate.
Owner:FAIRFIELD CLINICAL TRIALS
Antifungal phenylethylene
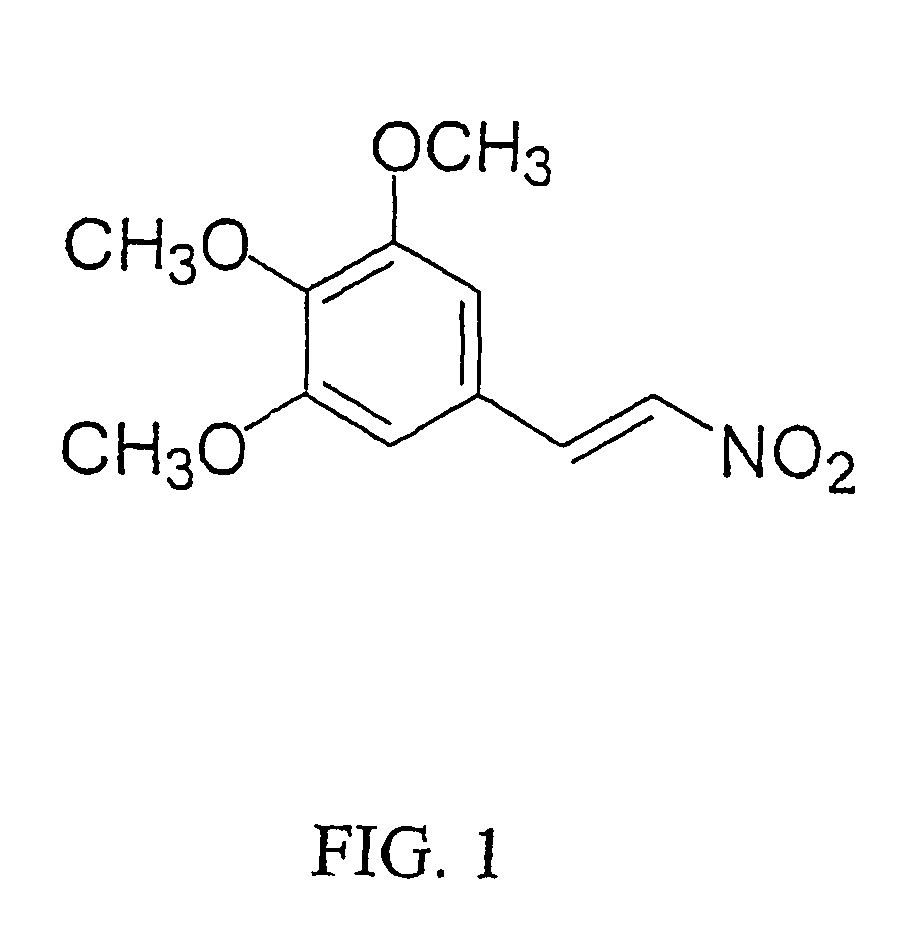
The antifungal and cancer cell growth inhibitory activities of 1-(3′,4′,5′-trimethoxyphenyl)-2-nitro-ethylene (TMPN) were examined. TMPN was fungicidal for the majority of 132 reference strains and clinical isolates tested, including those resistant to fluconazole, ketoconazole, amphotericin B or flucytosine. Minimum fungicidal concentration / minimum inhibitory concentration (MFC / MIC) ratios were ≦2 for 96% of Cryptococcus neoformans clinical isolates and 71% of Candida albicans clinical isolates. TMPN was fungicidal for a variety of other basidiomycetes, endomycetes and hyphomycetes, and its activity was unaffected by alterations in media pH. TMPN was slightly cytotoxic for murine and human cancer cell lines (GI50=1-4 μg / ml), and weakly inhibited mammalian tubulin polymerization (IC50=0.60 μg / ml). TMPN may also be used as a biochemical probe for tubulin and fungal dimorphism studies.
Owner:ARIZONA STATE UNIVERSITY
Novel topical formulations of flucytosine and uses thereof
InactiveUS20090068287A1Promote resultsLimited systemic exposureBiocideOrganic active ingredientsSide effectWhole body
The invention relates to topical formulations and methods of use of flucytosine which demonstrate a clear advantage over currently available therapeutic regimens for the treatment and maintenance of fungal infections, particularly vulvovaginal candidiasis. The invention provides compositions which solve the long-standing need for antimicrobial agents which treat effectively resistant strains of Candida spp., especially C. albicans, C. glabrata, and C. tropicalis, and which pose limited risk of side effects, adverse reactions, or the development of resistant pathogens. The invention provides novel topical formulations of flucytosine designed to allow the active drug to act at the local application area, but which inhibit or moderate transdermal or transmucosal absorption of the drug, thus limiting systemic exposure.
Owner:CAMARGO PHARMA SERVICES
Application of effective part of cockroach extract in preparing drug for inhibiting fungus growth
InactiveCN102973608AEnhanced inhibitory effectIncrease incomeBiocideAntimycoticsMonilinia laxaFreeze-drying
The invention relates to application of an effective part of a cockroach extract in preparing a drug for inhibiting fungus growth, and particularly relates to application of an effective part obtained by refining a crude cockroach extract through macroporous adsorption resin in the aspect of preparing a drug capable of resisting fungal infection. The effective part is refined from a fresh or dried Periplaneta americana L. polypide product through alcohol and water extraction, macroporous adsorption resin column chromatography and alcohol solvent elution, and has an obvious inhibition effect on the growth activity of candida albicans and a fungus growth inhibition capacity 80 times higher than that of fluconazole and 5-fluorocytosine and 800 times higher than that of amphotericin. The effective part of the cockroach extract can be prepared into forms, such as a hydrogel, a frothy gel, a cataplasm, freeze-dried powder, a water aqua, an aerosol, a suppository, a film, an external liniment, an ointment and the like, and is used for preparing pharmaceutical preparations for preventing and curing fungal infection, medical appliances and daily-use chemical products.
Owner:DALI UNIV
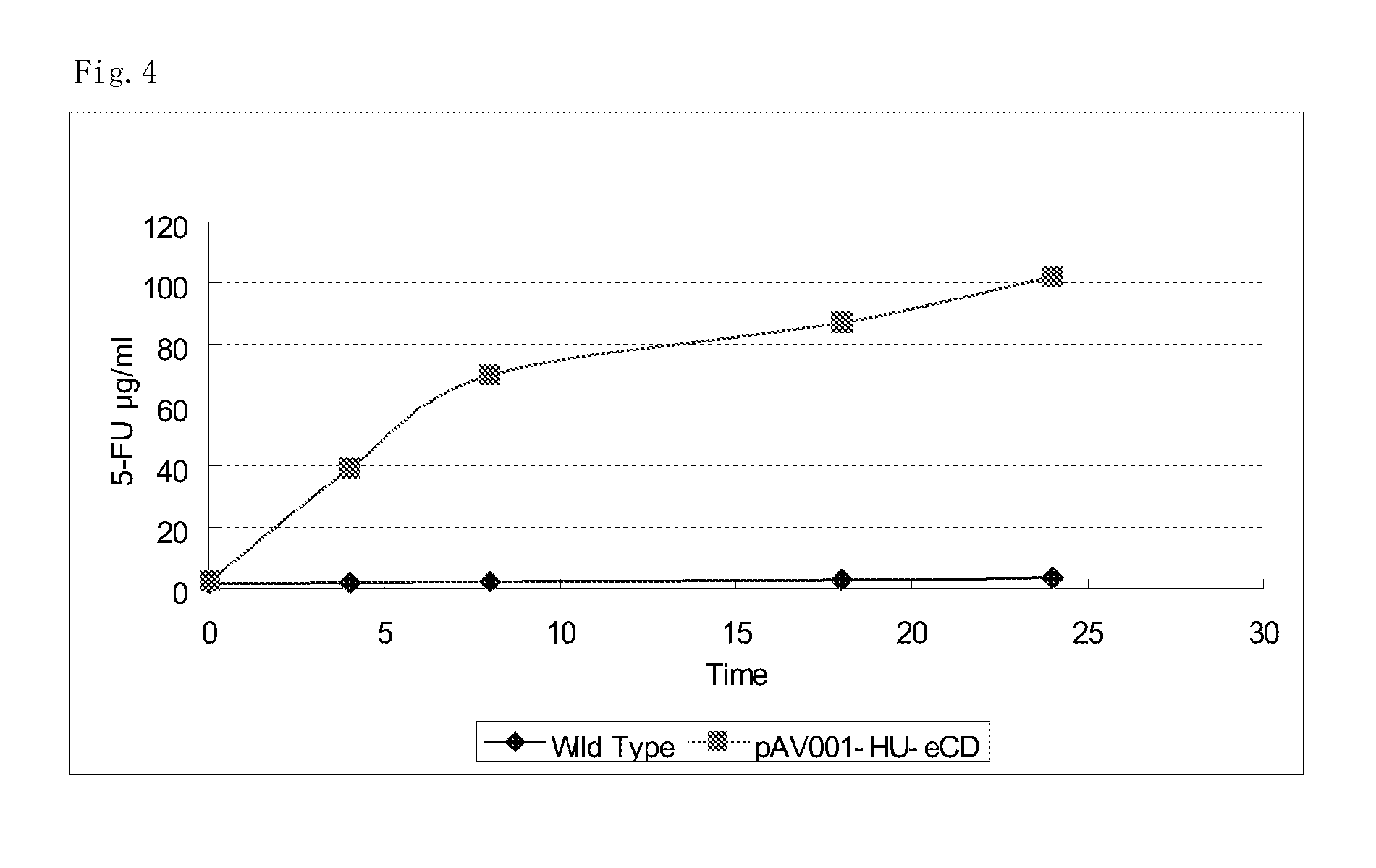
5-fluorouracil-resistant bacteria and method for production thereof
The present invention provides a method for producing a cytosine deaminase (CD)-expressing, 5-fluorouracil (5-FU)-resistant microorganism which can grow in anaerobic tumor tissues, can express CD, and has a resistance to 5-FU at a concentration that is at least effective for antitumor activity. More specifically, the method is a method (A) comprising performing subculture or acclimation culture of a CD-expressing microorganism which can grow in anaerobic tumor tissues, in the presence of 5-fluorocytosine (5-FC), or a method (B) comprising (1) performing subculture or acclimation culture of a microorganism which can grow in anaerobic tumor tissues and does not express CD, in the presence of 5-FU to produce a 5-FU-resistant microorganism and (2) transforming the 5-FU-resistant microorganism by introducing a CD gene. The present invention also provides the CD-expressing, 5-FU-resistant microorganism and a pharmaceutical composition comprising the microorganism.
Owner:ANAEROPHARMA SCI
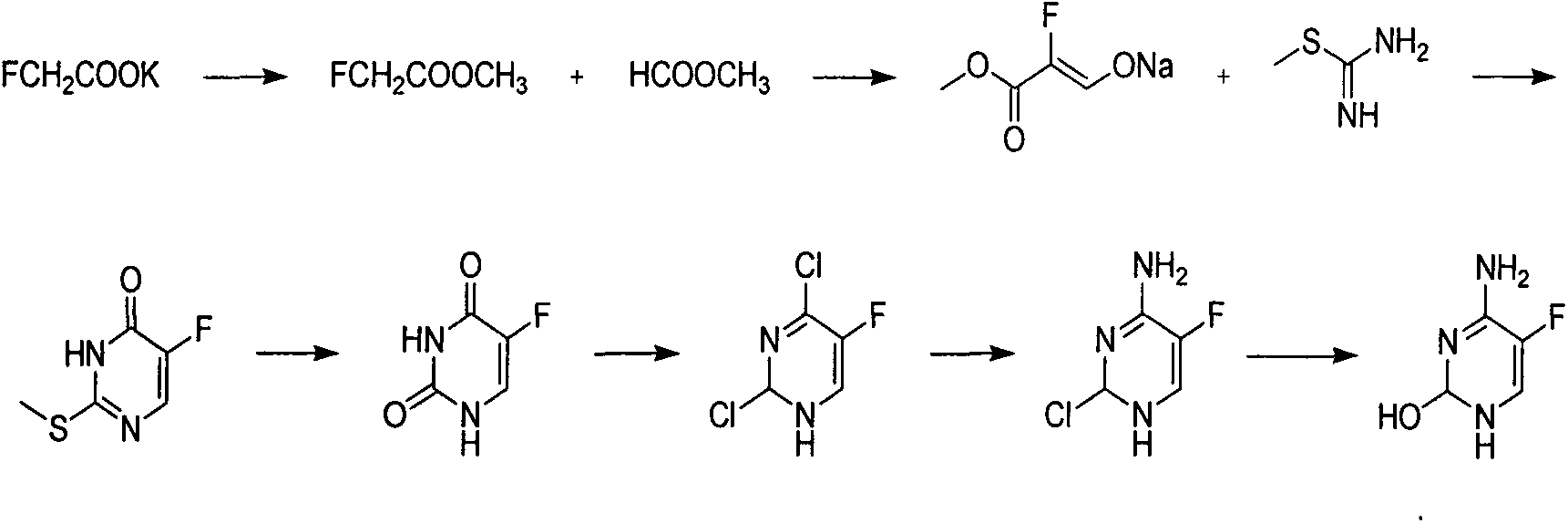
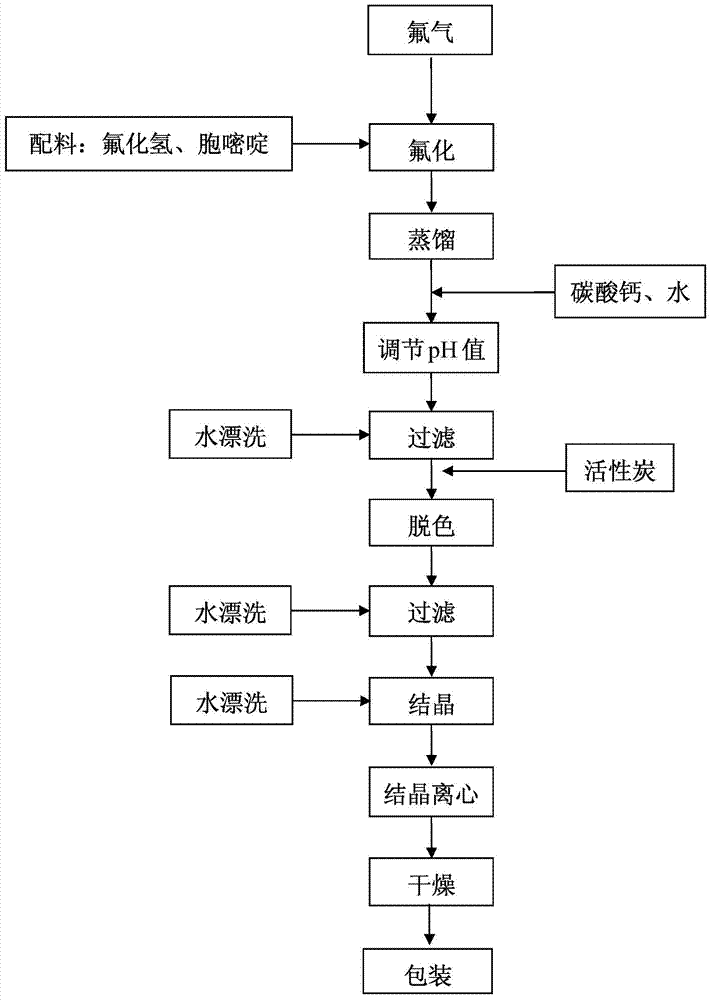
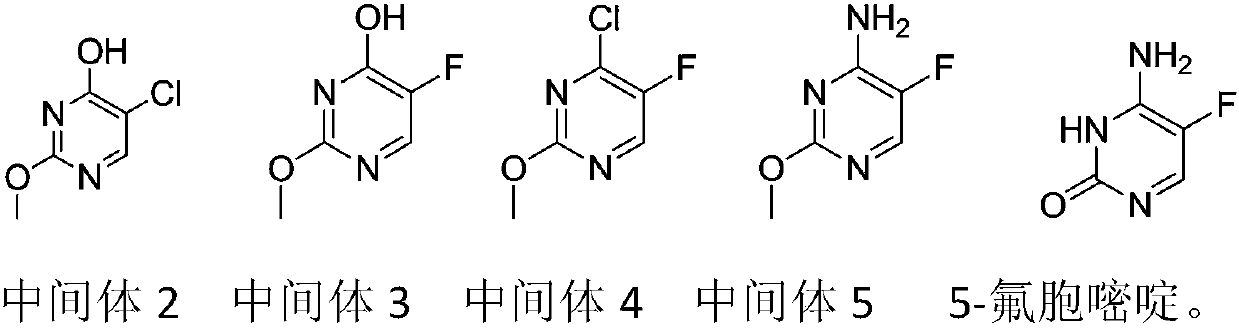
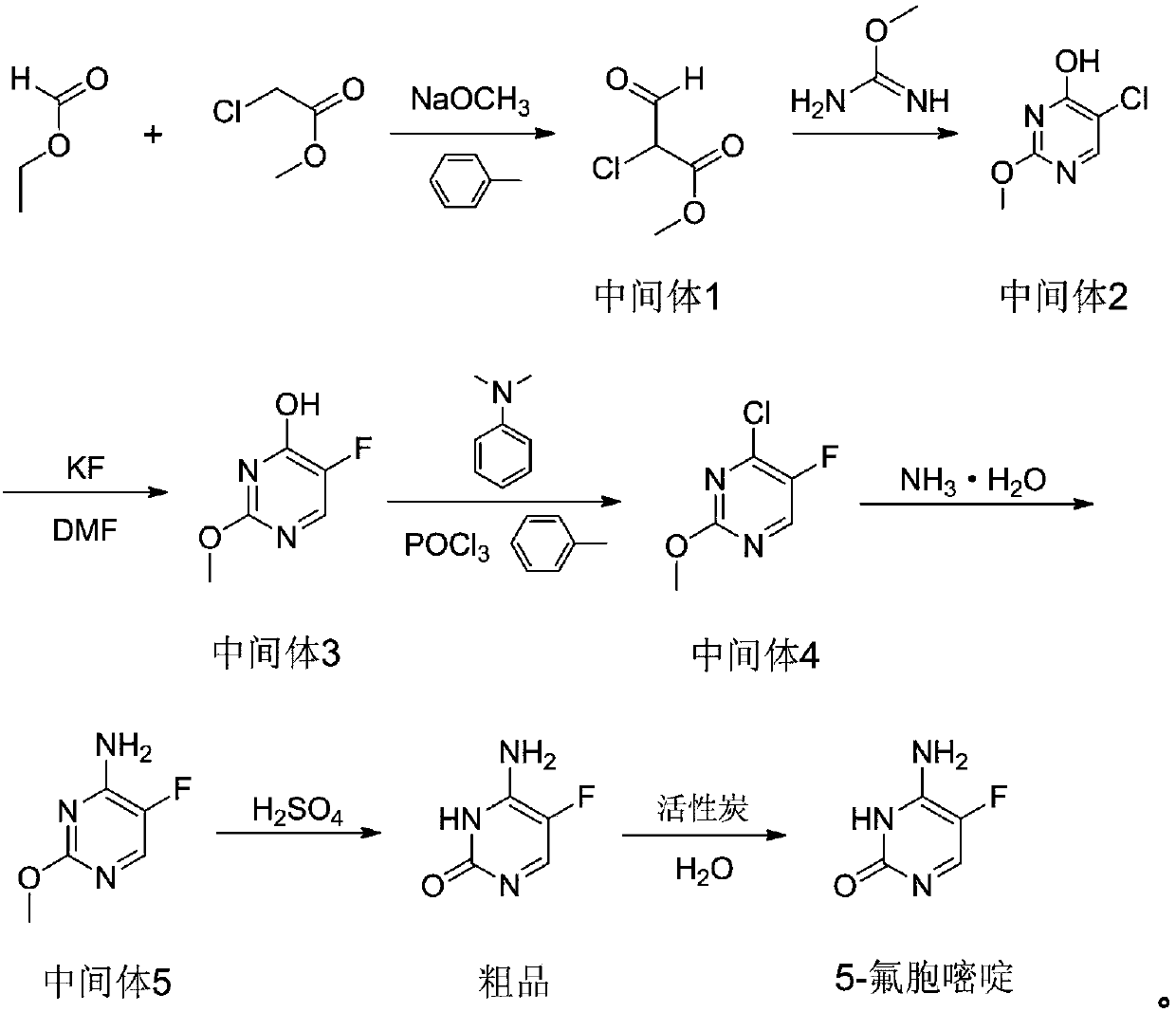
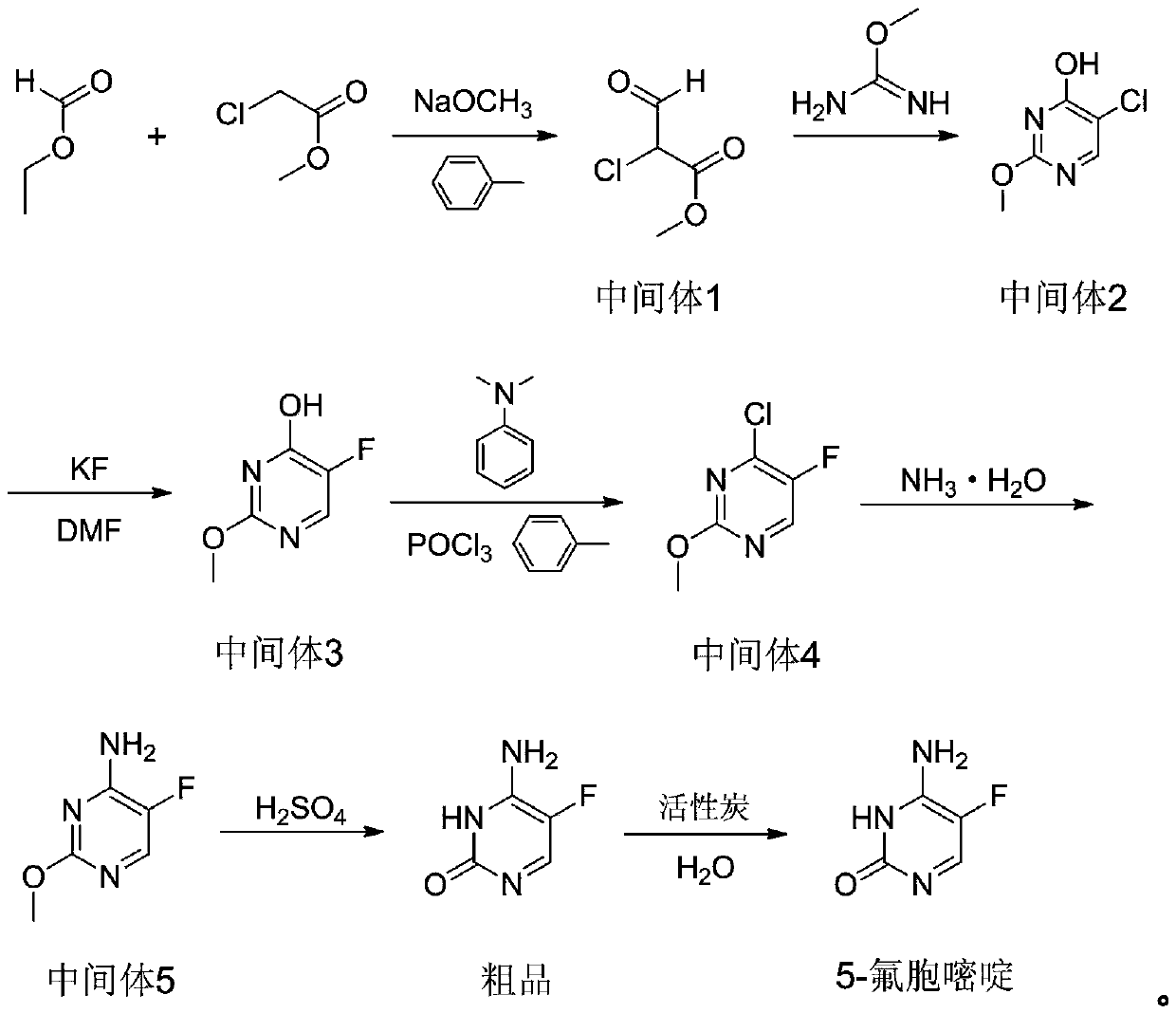
Novel technology for synthesis of 5-flucytosine
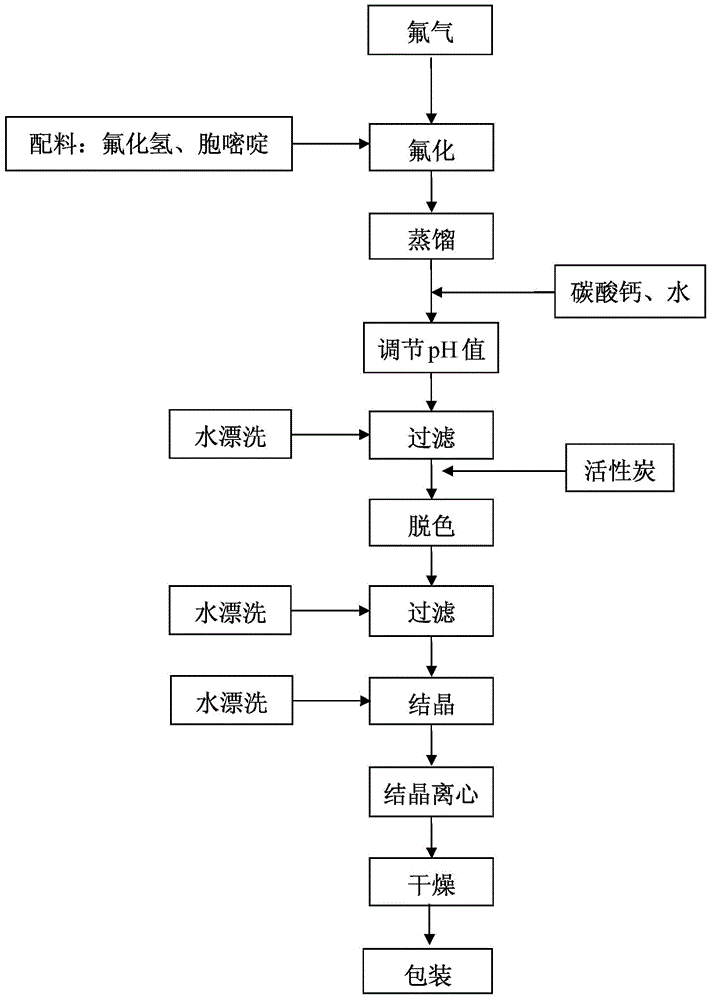
The invention discloses a novel technology for synthesis of 5-flucytosine. The novel technology comprises that through condensation, acidification, cyclisation, fluorination and refining, 5-flucytosine is prepared from acetonitrile, formate, hydrogen chloride low-grade saturated fatty alcohol solution, urea, fluorine gas and anhydrous hydrogen fluoride as main raw materials. The 5-flucytosine has a weight yield of 1.45-1.55 (based on acetonitrile), unknown single impurity content less than 0.1%, total impurity content less than 0.2% and quality satisfying CP2010, USP36 and BP2013 quality standards.
Owner:BEIJING ALLIESYN TECH
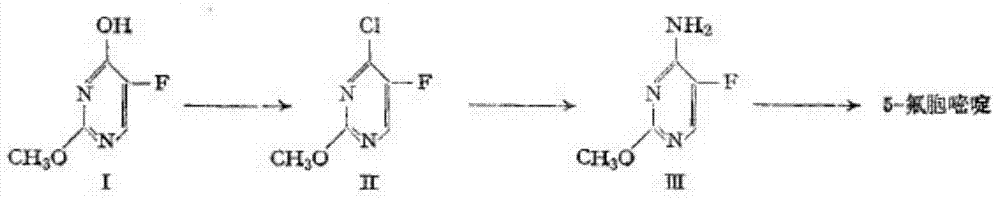
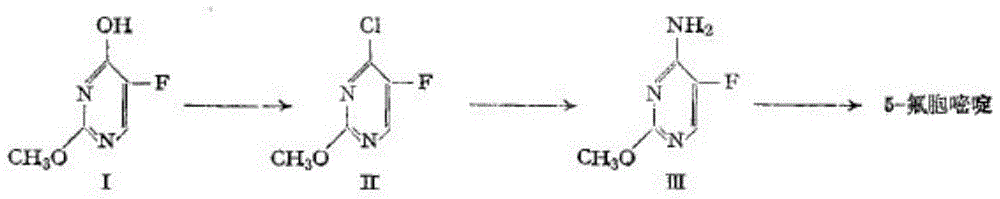
Method for fluoridating and synthesizing 5-flucytosine by cytosine
The invention discloses a method for fluoridating and synthesizing 5-flucytosine by cytosine, which comprises the following steps: 1)under inert gas protection, adding cytosine in anhydrous hydrogen fluoride, introducing fluorine containing gas for a fluorination reaction under -5--20 DEG C, and 2)after reacting for 3-5 hours, distilling and concentrating the reaction solution, adding water for dissolving, adding alkali to adjust the pH value of the reaction solution, and separating to obtain the 5-flucytosine by cytosine. The method has the advantages of short synthesis route and good technology selectivity, no organic solvent is used during the production process, and the synthesized 5-flucytosine by cytosine has the advantages of high purity and high synthesis yield.
Owner:SHANGYU HUALUN CHEM
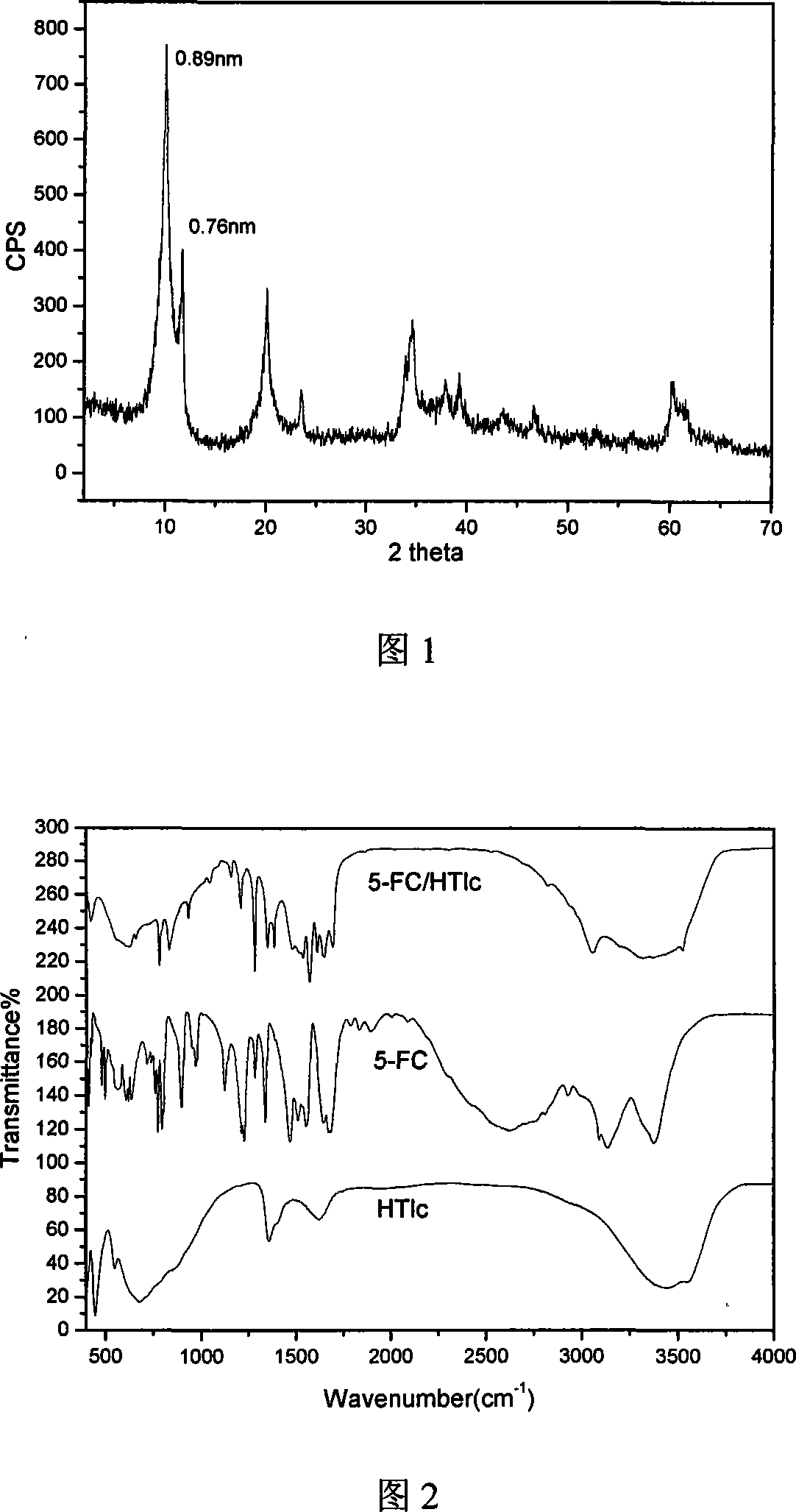
5-flucytosine/hydrotalcite-like NANO hybrid compound and preparation method thereof
InactiveCN101085355AGood slow releaseSimple processOrganic active ingredientsAntimycoticsFlucytosineSynthesis methods
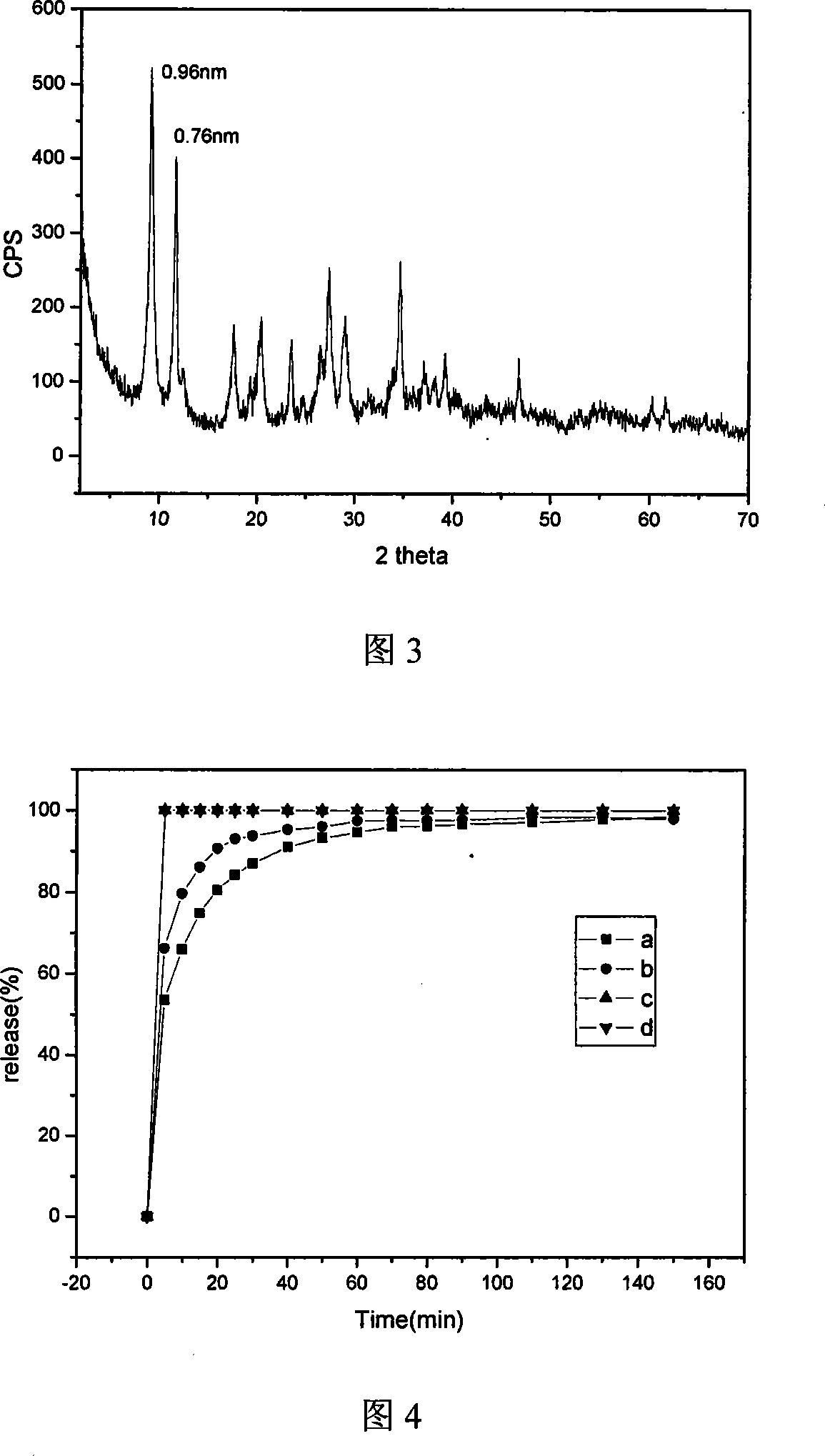
A nano-hybrid of 5-fluctyosine (5-FC) and hydrotalcite-like compounds (HTlc) belongs to technology field of new material and pharmaceutical preparation.5-FC / HTlc nanohybrid is synthesized by mixed salt solution of divalent metal ion trivalent metal ion and 5-fluctyosine alkali solution throug coprecipitation method, and has a chemical constitution general formula of [MII(1-x)MIIIx(OH)2](An-)a(5-FC-)b.nH2O.The inventive 5-FC / HTlc nanohybrid has simple and easy synthetic method, large drug loading dosage, certain sustained release effect compared with raw material of 5-FC, and important significance for clinical application of 5-FC.
Owner:SHANDONG UNIV
Preparation method of 5-flucytosine
ActiveCN103819412AReduce usageReduce harmOrganic chemistryBulk chemical productionFlucytosineHydrogen fluoride
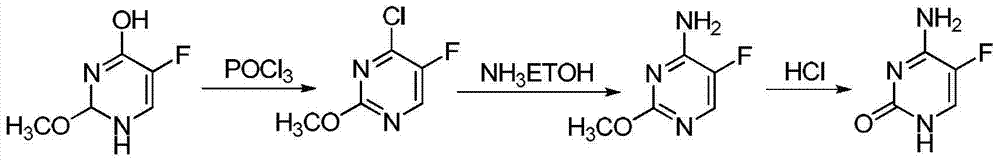
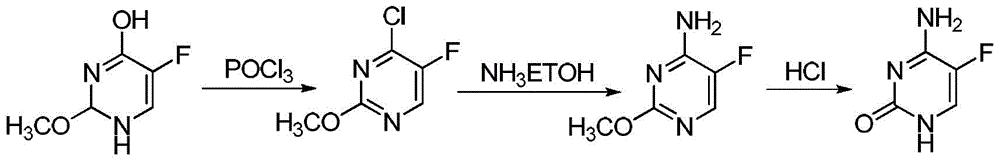
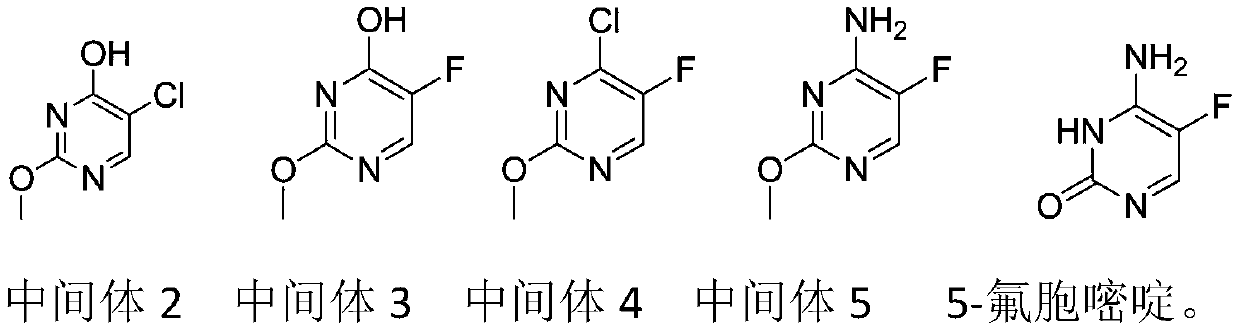
The invention discloses a preparation method of 5-flucytosine. The method comprises the following steps of: 1) cytosine and a halogenating reagent perform halogenating reaction in an organic solvent at 0-80 DEG C to prepare an intermediate (I), 2) the intermediate (I) reacts with an amino protecting agent at 0-120 DEG C to prepare an intermediate (II), and 3) the intermediate (II) and a fluoro reagent perform fluoro reaction in a polar aprotic solvent or hydrogen fluoride at 70-200 DEG C to prepare an intermediate (III), and the intermediate (III) directly performs amino deprotecting reaction, and is separated and purified to prepare 5-flucytosine. According to the method, a process route is reasonable, the product yield is high, the quality is good, the production safety of the fluoro reagent is high, and the method is simple to operate and suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH
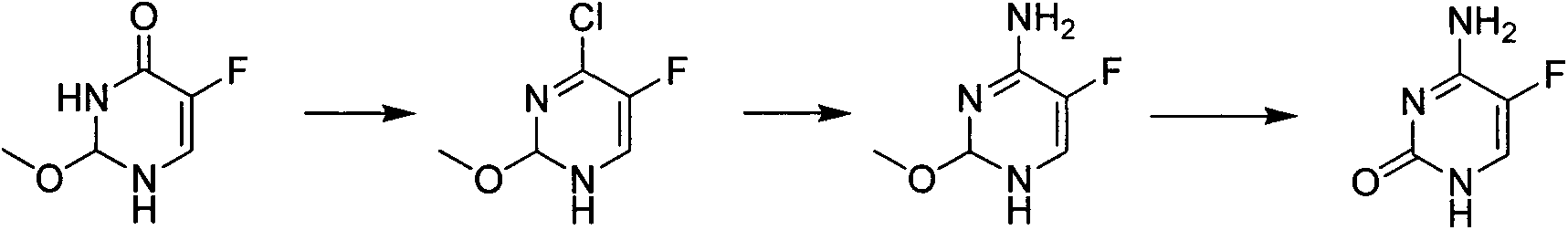
Preparation method of 5-flucytosine
ActiveCN108033917AReduce usageThe process is environmentally friendlyOrganic chemistryChemical synthesisPotassium fluoride
The invention belongs to the technical field of chemical synthesis of medicines and relates to a preparation method of 5-flucytosine. The preparation method comprises the following steps: utilizing ethyl formate and methyl chloroformate to synthesize 2-chloro-3-oxo methyl propionate, then utilizing oxymethylisourea to close rings to obtain pyrimidine rings, utilizing potassium fluoride to substitute chlorine on the pyrimidine rings, utilizing phosphorus oxytrichloride to substitute hydroxyl groups on the pyrimidine rings, then adding ammonia water to lead chloride to be substituted with aminogroups, and hydrolyzing under an acid condition to obtain a product, namely the 5-flucytosine. The preparation method has the beneficial effects that the methyl chloroacetate is adopted for substituting methyl fluoroacetate to be used as a synthetic raw material of the 5-flucytosine, so that the use of highly-toxic chemicals such as the methyl fluoroacetate is avoided; simultaneously, since the price of the methyl chloroacetate is much lower than the price of the methyl fluoroacetate, the production cost can be saved; by utilization of the synthetic route provided by the invention, the higher-purity 5-flucytosine can be prepared without need of complex aftertreatment steps; simultaneously, the preparation method has higher overall yield and obvious industrial value and is worthy of being promoted and used on a large scale.
Owner:ZHEJIANG XIANFENG TECH
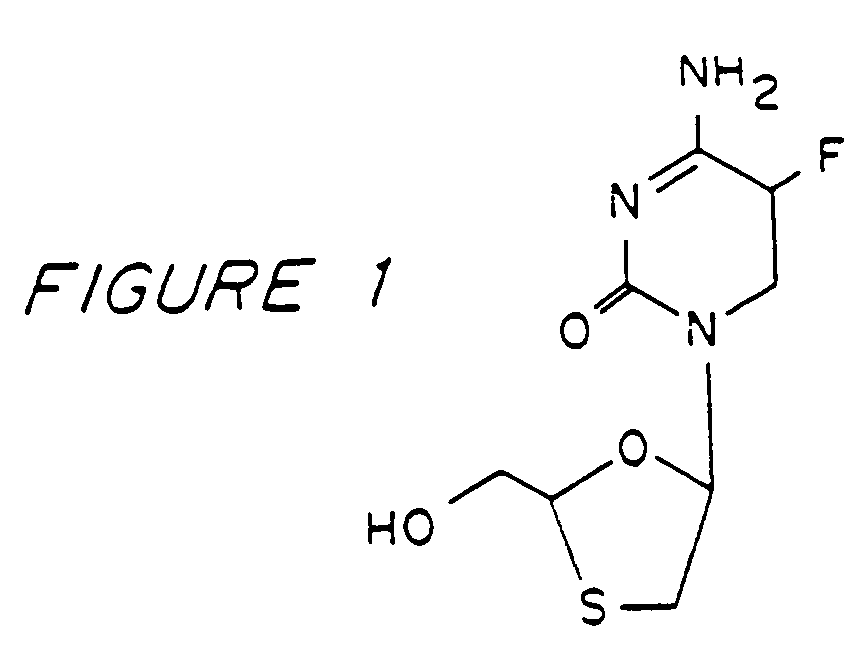
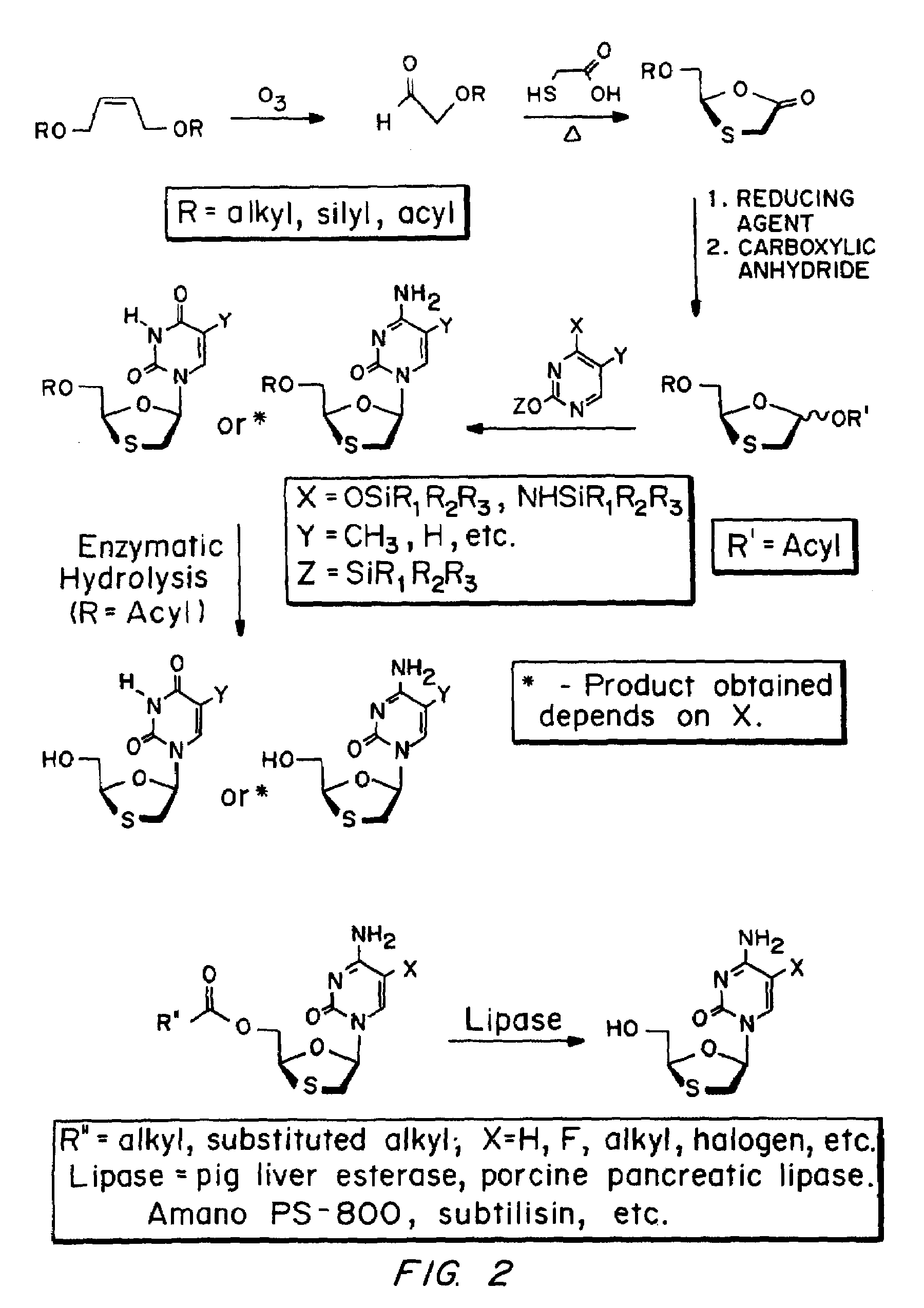
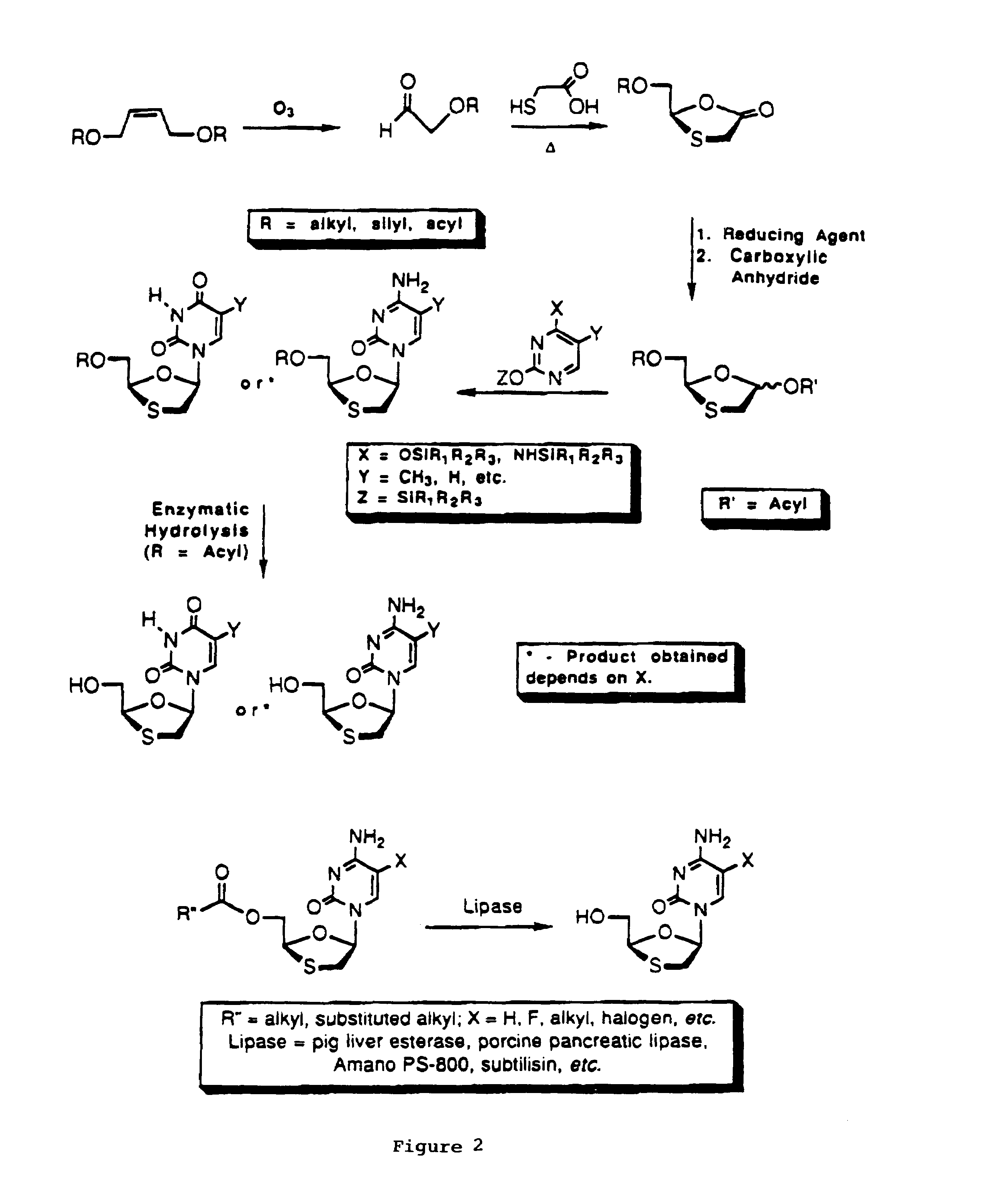
Method of resolution and antiviral activity of 1,3-oxathiolane nucleoside enantiomers
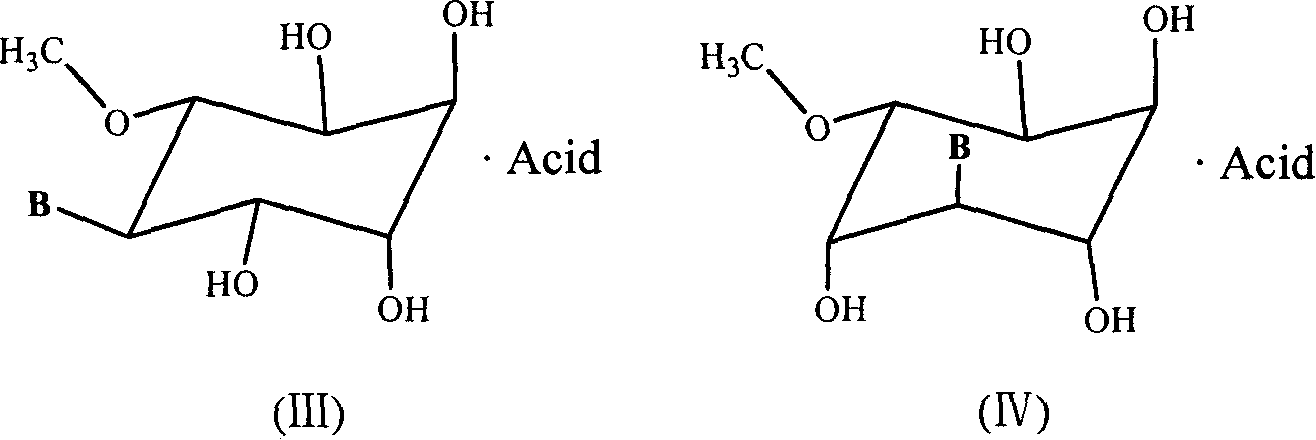
A process for the resolution of a racemic mixture of nucleoside enantiomers that includes the step of exposing the racemic mixture to an enzyme that preferentially catalyzes a reaction in one of the enantiomers. The nucleoside enantiomer (−)-2-hydroxymethyl-5-(5-fluorocytosin-1-yl)-1,3-oxathiolane is an effective antiviral agent against HIV, HBV, and other viruses replicating in a similar manner.
Owner:EMORY UNIVERSITY
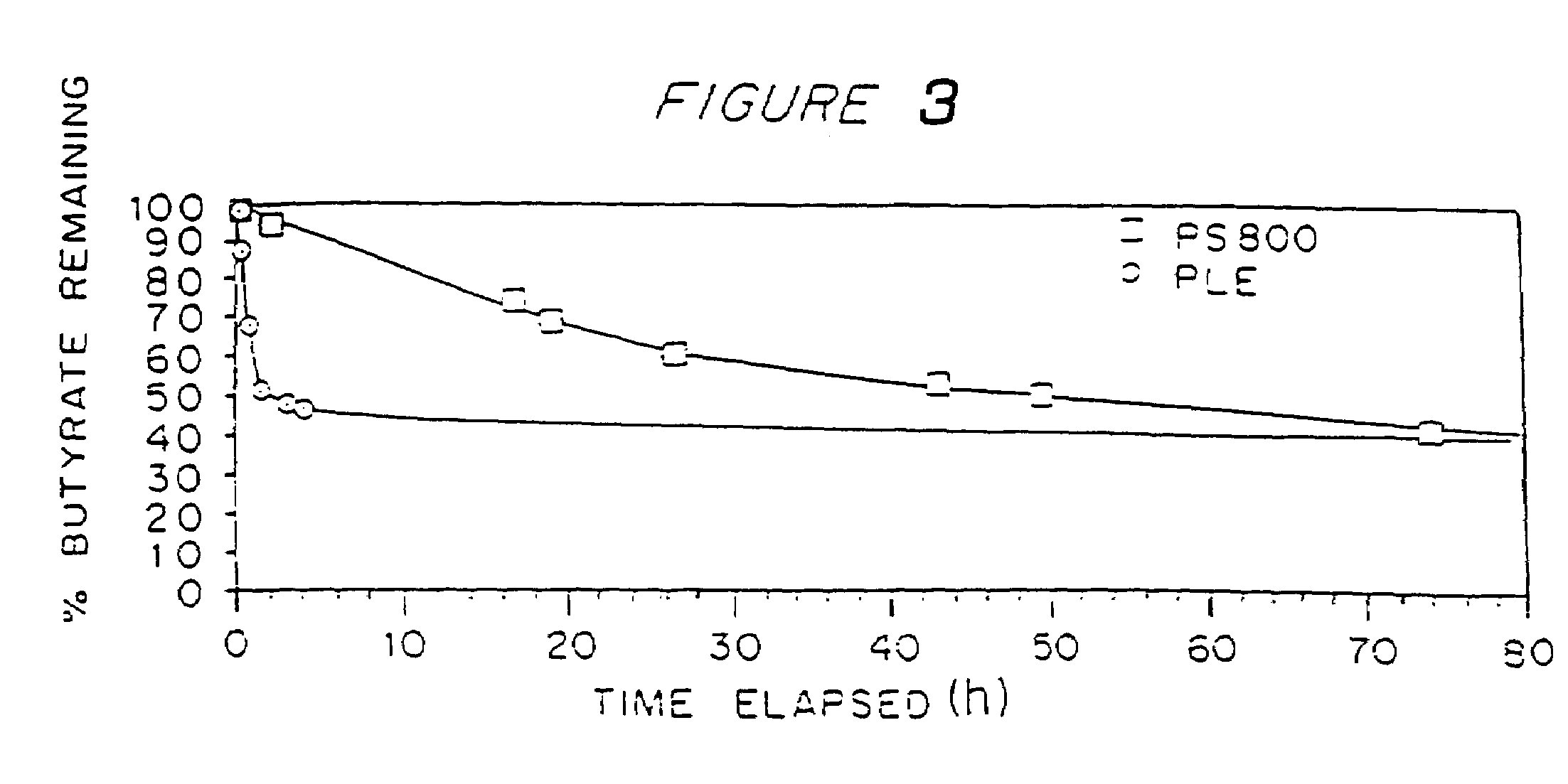
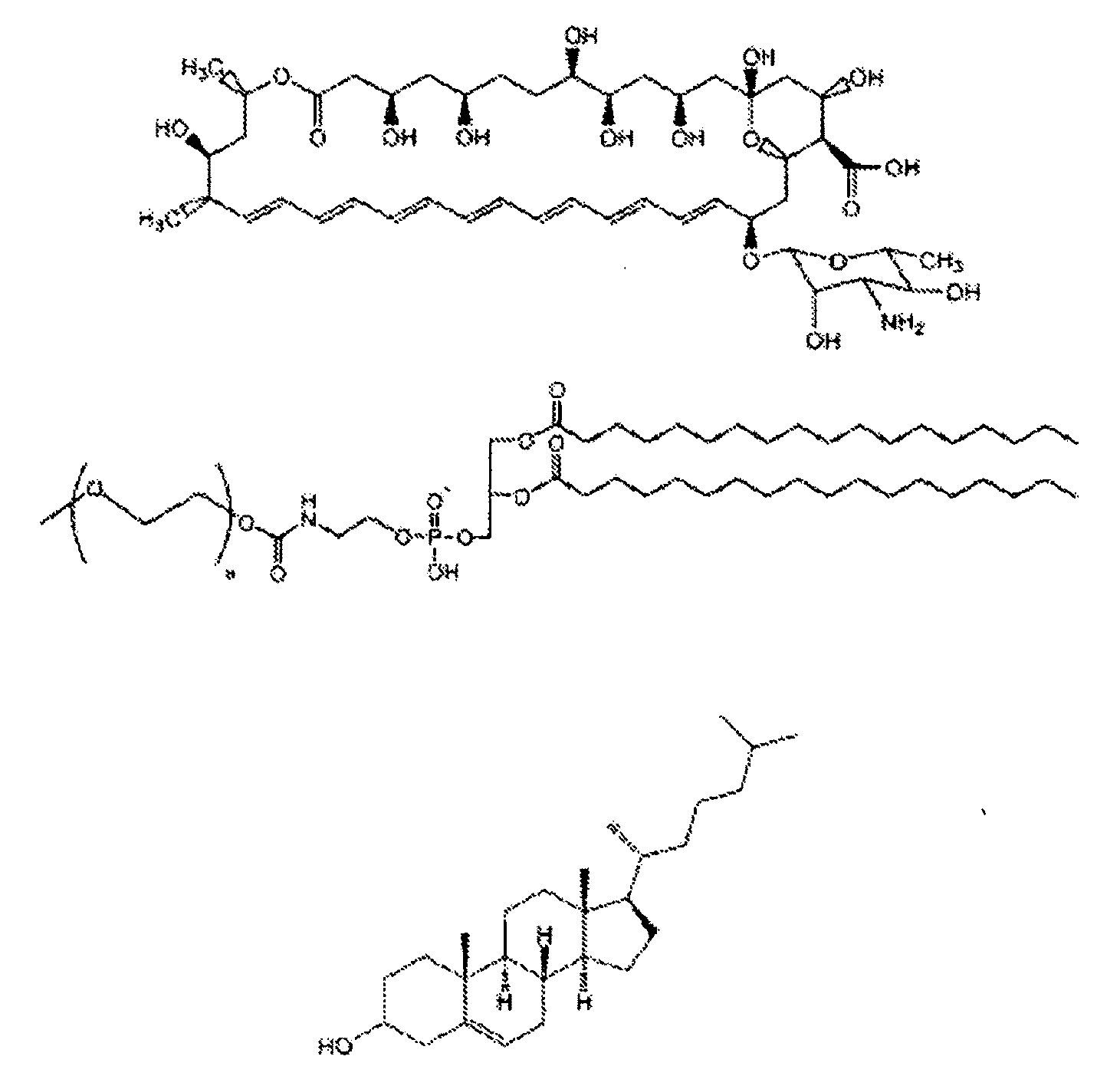
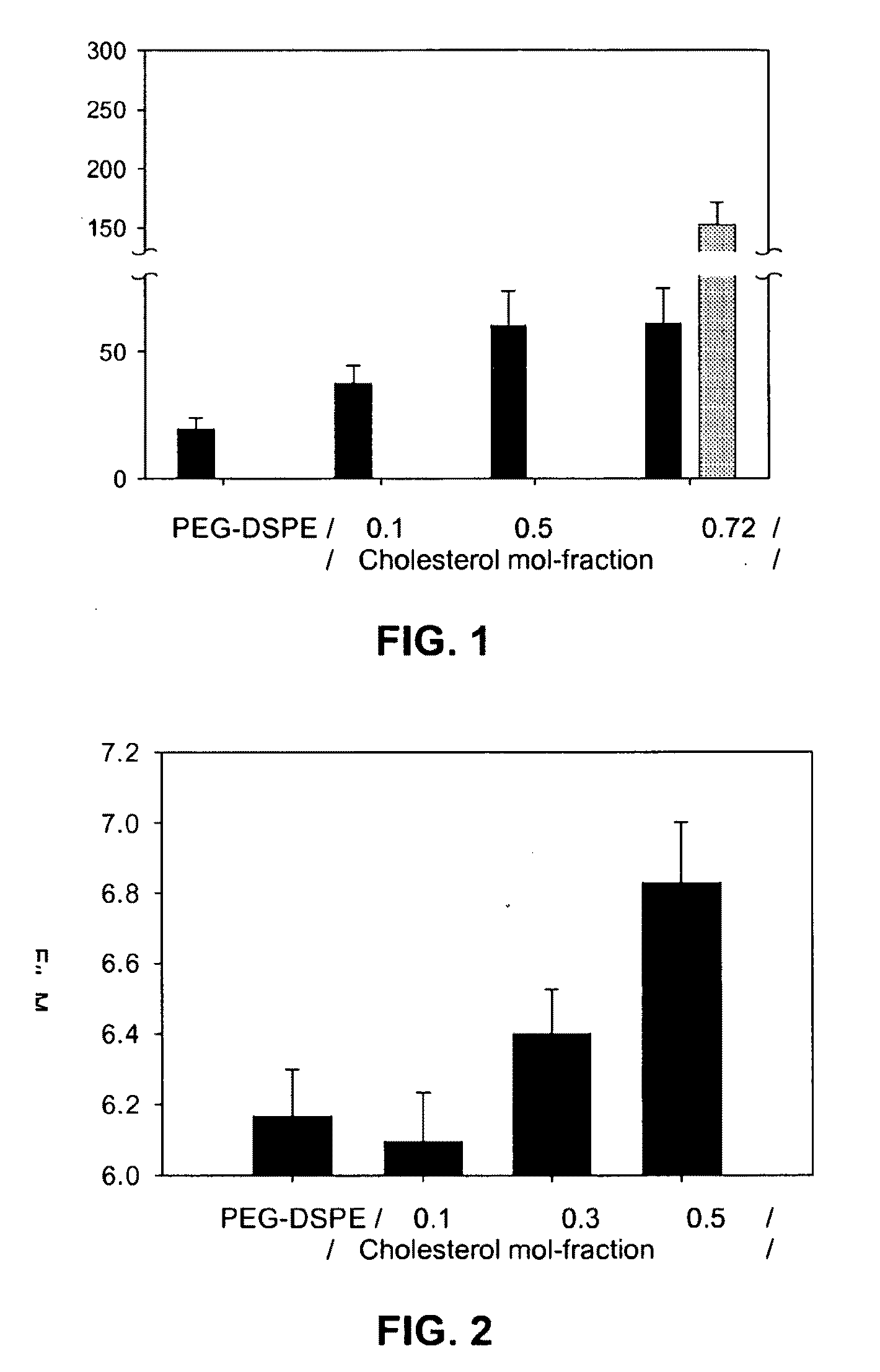
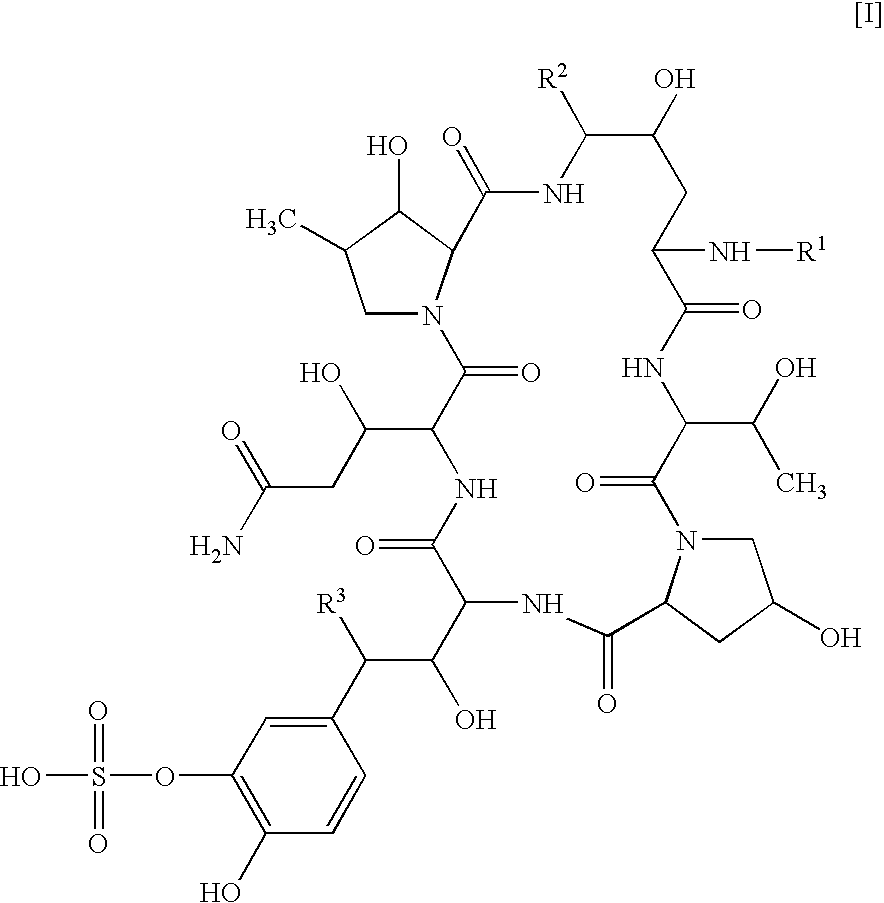
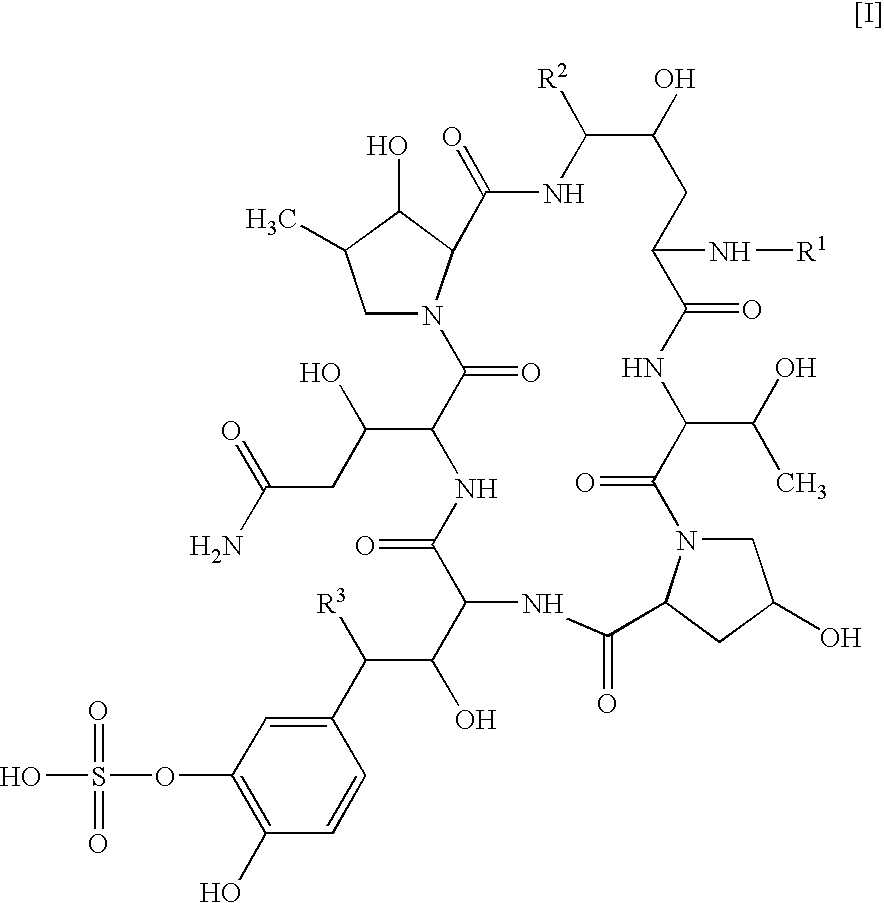
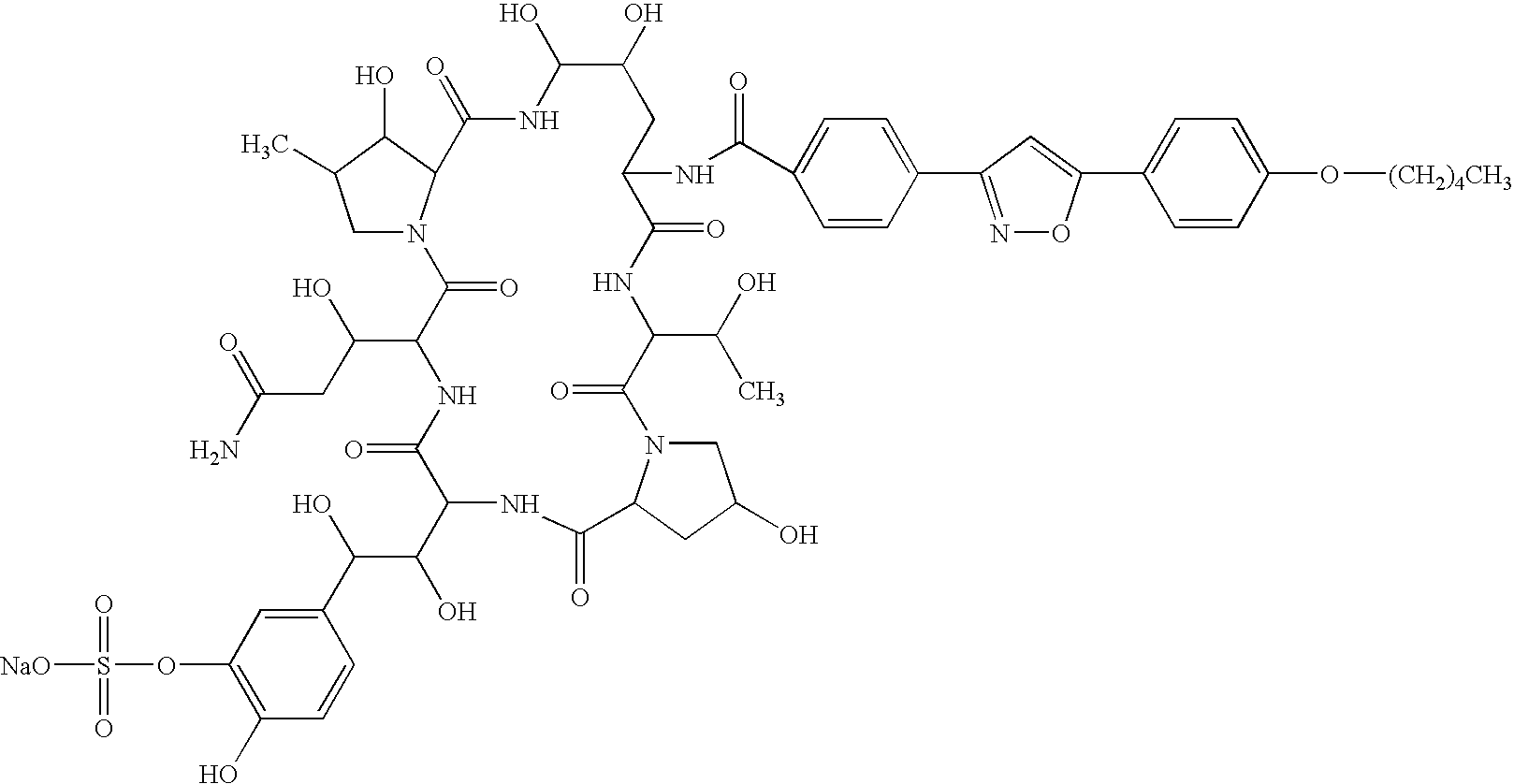
Structuring effect of cholesterol in peg-phospholipid micelles, drug delivery of amphotericin b, and combination antifungals
InactiveUS20100210575A1Reduce releaseReduce AmB-related toxicityBiocideFungicidesSolubilityAntifungal
The disclosure herein relates to embodiments of compositions and methods in connection with polymeric micelles including PEG-phospholipids. Embodiments also relate to the controlled release of pharmaceutical agents in the context of drug delivery. Further disclosed are embodiments of PEG-DSPE / Cholesterol micelle formulations prepared with an antifungal agent, Amphotericin B, with capabilities including slow release of the agent in a deaggregated state. In embodiments, micellar preparations with Amphotericin B are compatible with solubility in aqueous salt solutions, thus allowing for concurrent co-administration of other pharmaceutical agents and / or sodium supplementation. In embodiments, polymeric micelle compositions are employed in combination antifungal therapeutic approaches such as Amphotericin B and other antifungal agents. Also disclosed herein are compositions and methods relating to combinations including AmB:PEG-DSPE, rapamycin:PEG-DSPE, and / or 5-fluorocytosine.
Owner:WISCONSIN ALUMNI RES FOUND
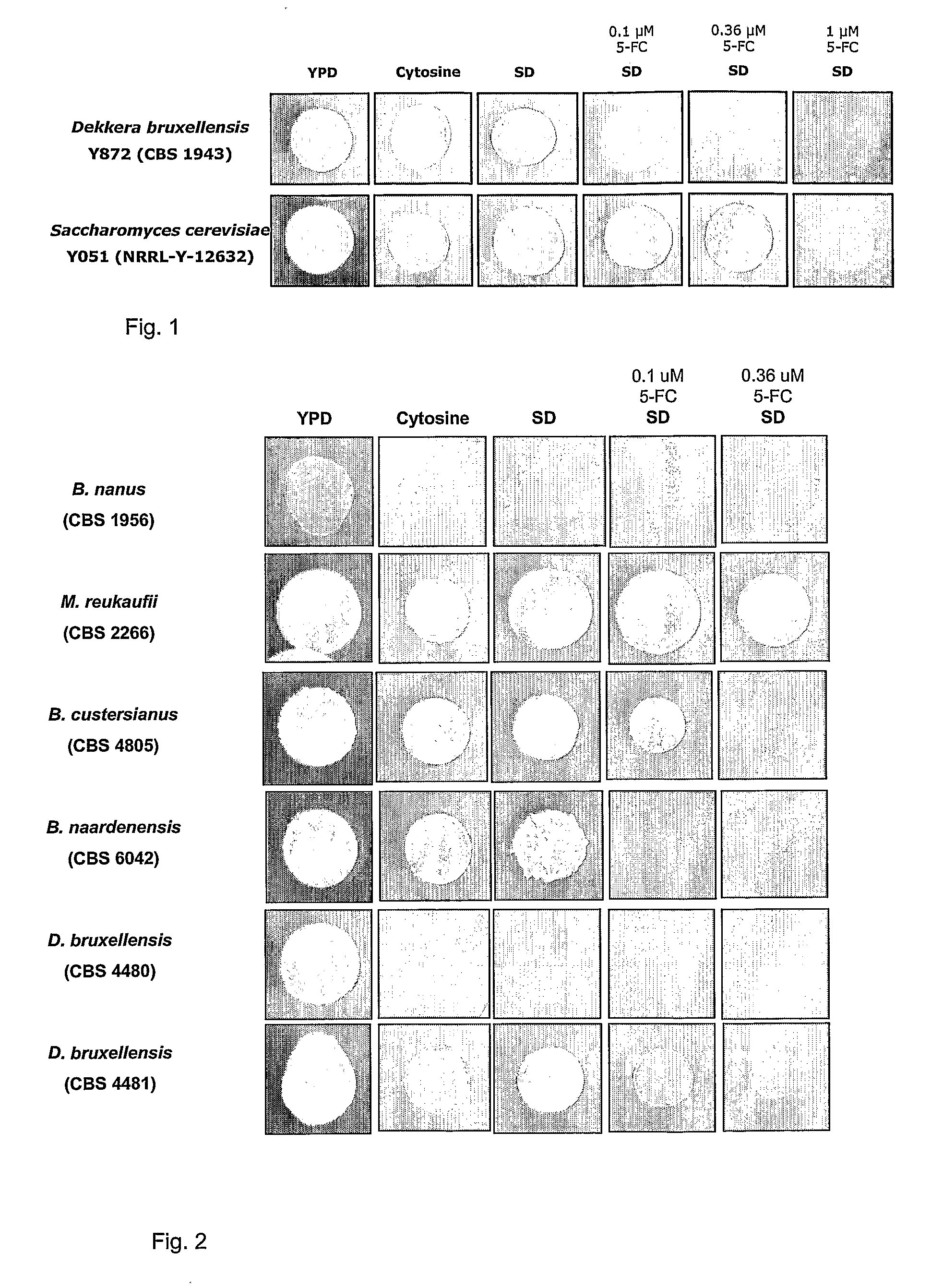
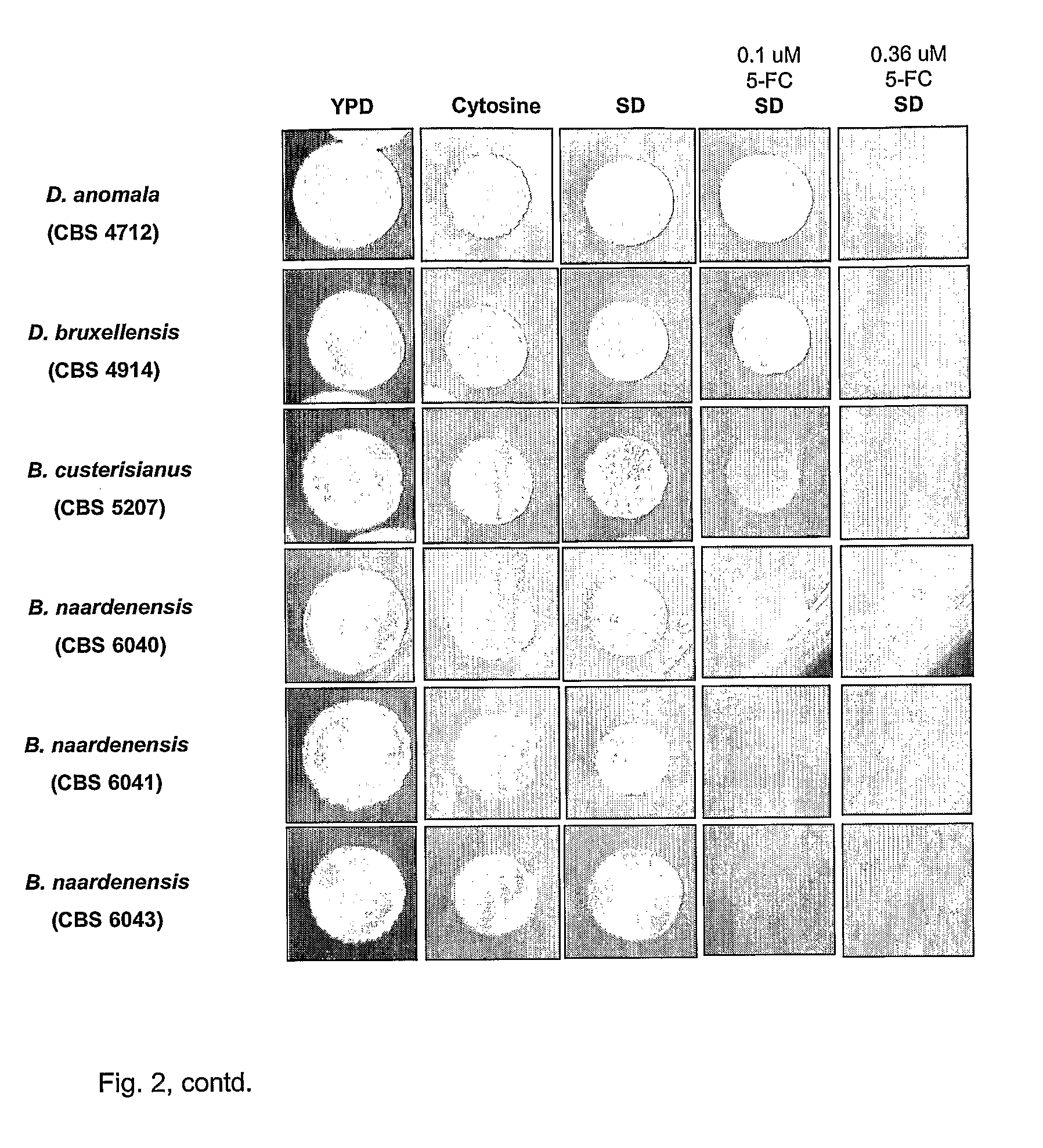
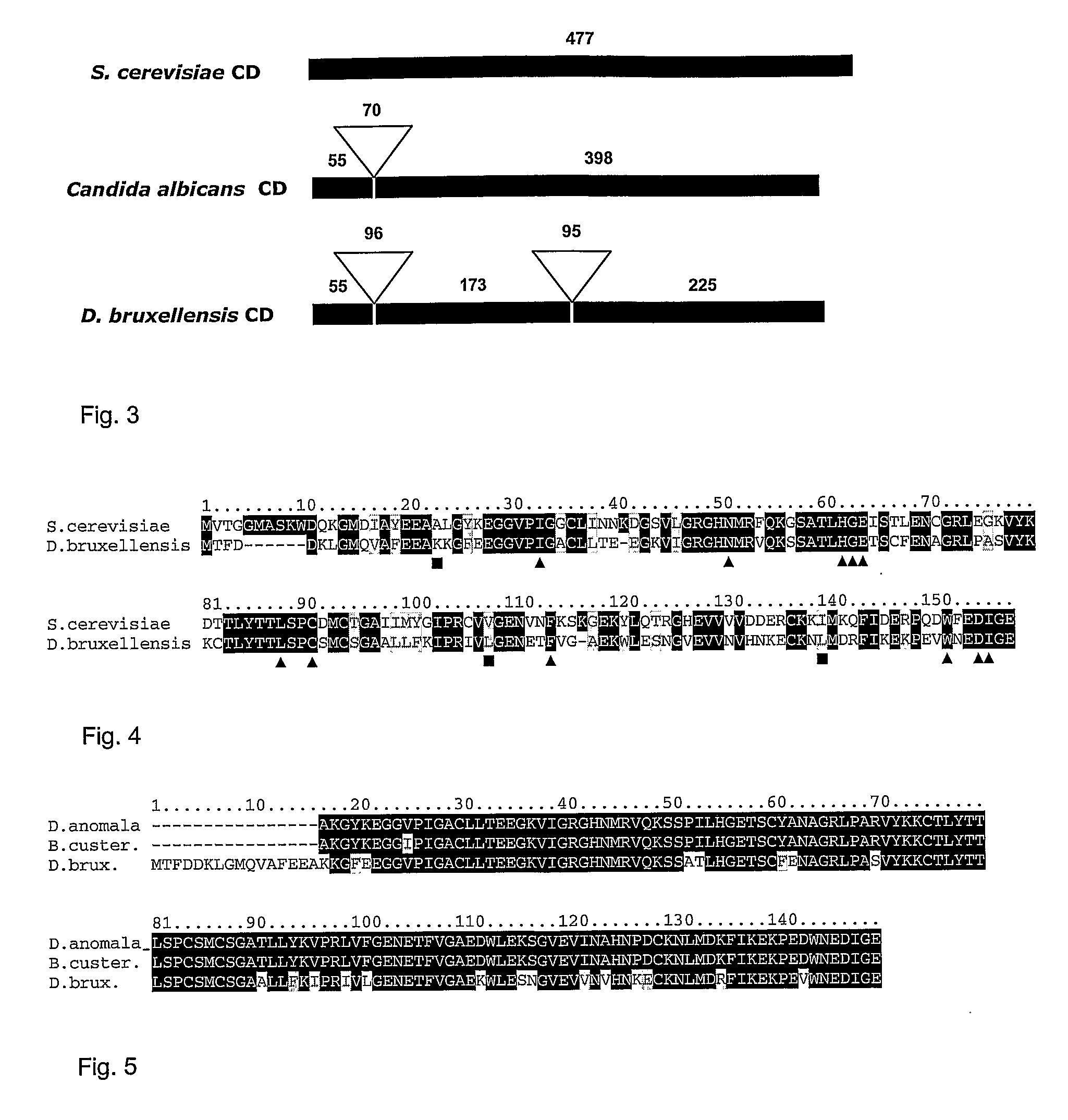
Dekkera/Brettanomyces Cytosine Deaminases And Their Use
The present invention relates to cytosine deaminase protein and cDNA from various species of the yeast genus Dekkera / Brettanomyces. Compared to yeast cytosine deaminase the novel cytosine deaminases are more efficient and have a higher stability. The invention also relates to the field of suicide gene therapy based on activation of a non-toxic prodrug, 5-fluorocytosine to a toxic drug 5-fluorouracil based on the enzymatic activity of novel cytosine deaminses. Finally the invention provides use of 5-fluorocytosine for controlling the growth of Dekkera / Brettanomyces yeast.
Owner:ZGENE
Bacterial with high-yield of nucleoside phosphorylase and method for synthesizing arabinose nucleoside
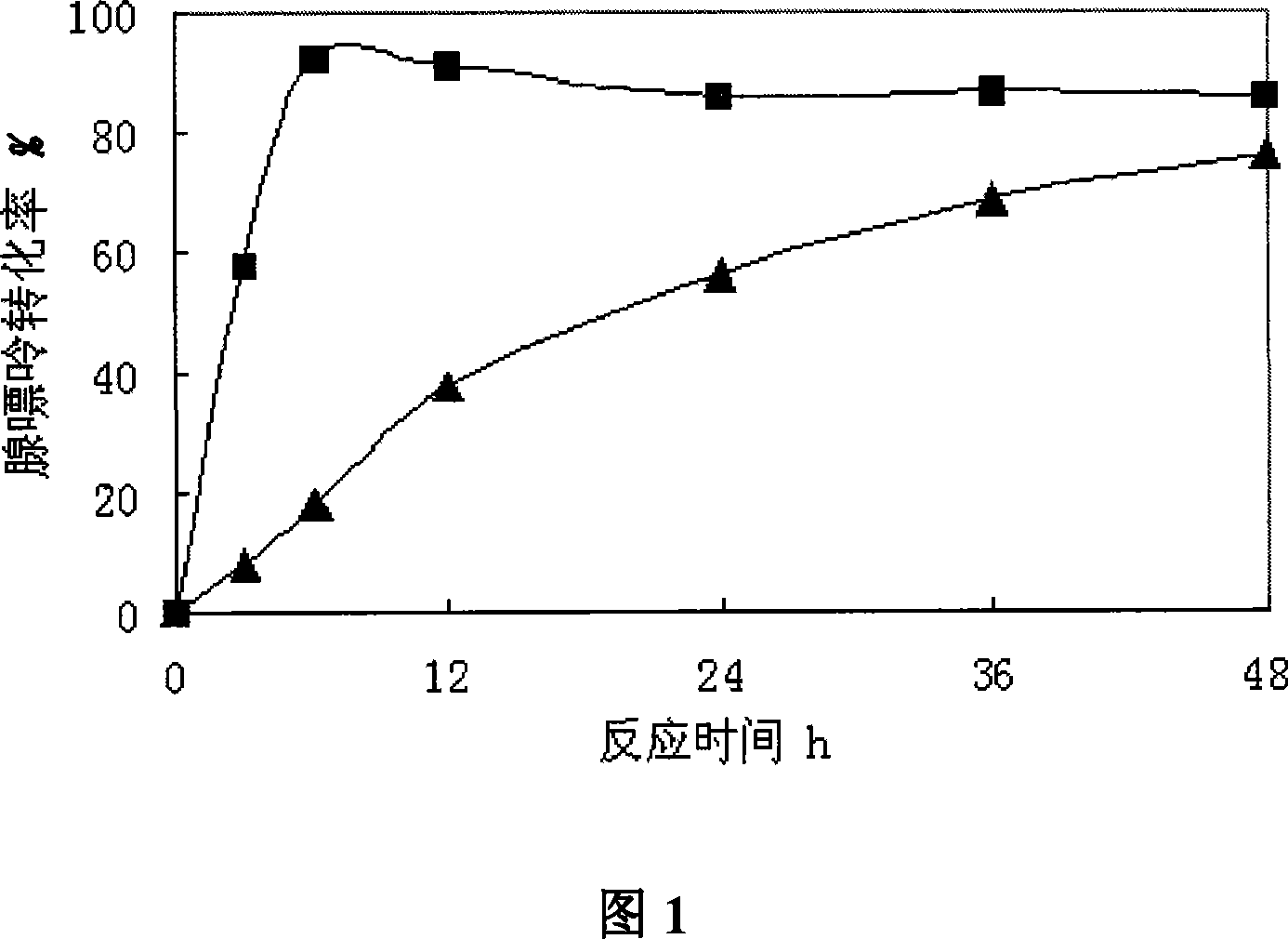
InactiveCN101113420AIncrease vitalityImprove conversion rateBacteriaFermentationFlucytosineNucleoside phosphorylase
A compound method of high-yield nucleoside phosphorylase strains and arabinose nucleoside pertains to biochemical engineering field, in particular to the high-yield nucleoside phosphorylase strains and a method for compounding arabinose purine nucleoside with the strains by an enzyme method. The invention aims at solving a technical problem for providing the strains of the high-yield nucleoside phosphorylase and strains of uridine phosphorylase and the method for producing the arabinose purine nucleoside with the strains. The invention discloses enterobacter aerogenes with a preservation number of CGMCC No.2035 and the method for producing the arabinose purine nucleoside with the strains, and the invention comprises steps that (1) the enterobacter aerogenes DWOQ-58 of the invention is cultured and collected, and (2) the enterobacter aerogenes DWOQ-58 is contacted with arabinose donor and receptors of purine base. The strains of the invention are rich in vigor and resists 5-flucytosine with an average conversion rate of more than 80 percent in general and the reaction time of the invention is shortened to less than 12 hours.
Owner:SHANGHAI WEIPING BIOLOGICAL TECH
Antifungal combination use
Owner:ASTELLAS PHARMA INC
Combination of a cyclic hexapeptide with antifungal drugs for treatment of fungal pathogens
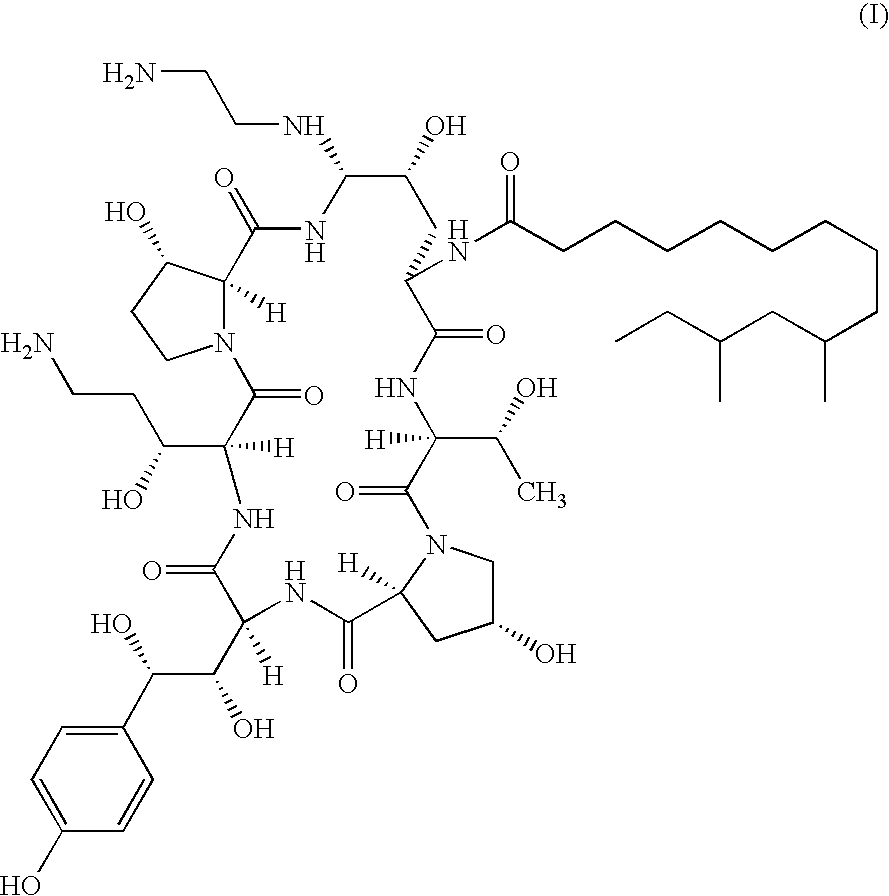
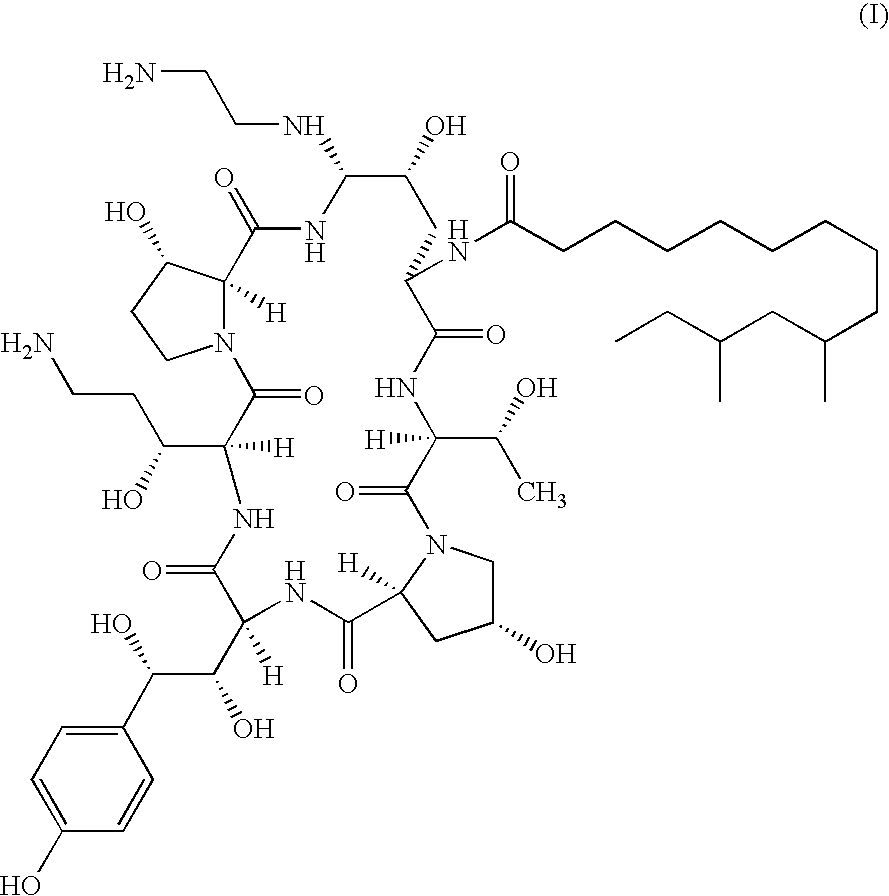
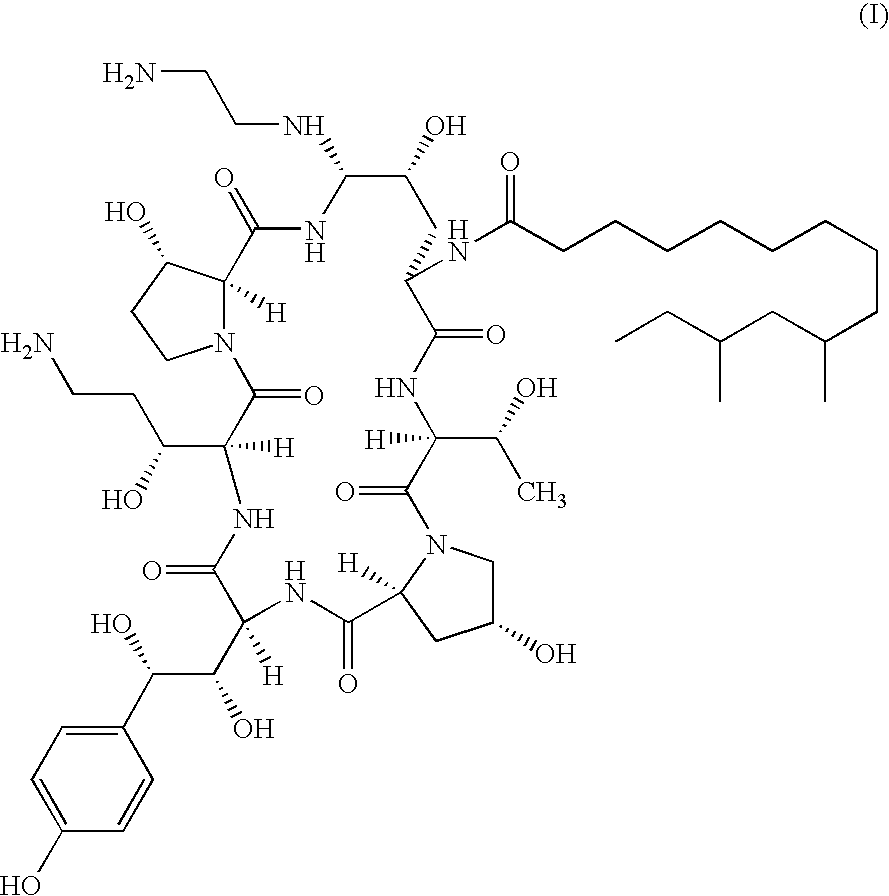
There is described antifungal combination use of known antifungal agents such as the azoles or polyenes in combination with a lipopeptide compound antifungal agent. More particularly, the invention relates to antifungal combination use of azols such as fluconazole voriconazole, itraconazole, ketoconazole, miconazole, ER 30346 and SCH 56592; polyenes such as amphotericin B, nystatin, liposomal and lipid forms thereof such as Abelcet, AmBisome and Amphocil; purine or pyrimidine nucleotide inhibitors such as flucytosine; or polyoxins such as nikkomycins, in particular nikkomycin Z or nikkomycin X; other chitin inhibitors; elongation factor inhibitors such as sordarin and analogs thereof; mannan inhibitors such as predamycin, bactericidal / permeability-inducing (BPI) protein products such as XMP.97 or XMP.127; or complex carbohydrate antifungal agents such as CAN-296; with a lipopeptide compound (I) as described herein.
Owner:ASTELLAS PHARMA INC
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN1803819ASaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
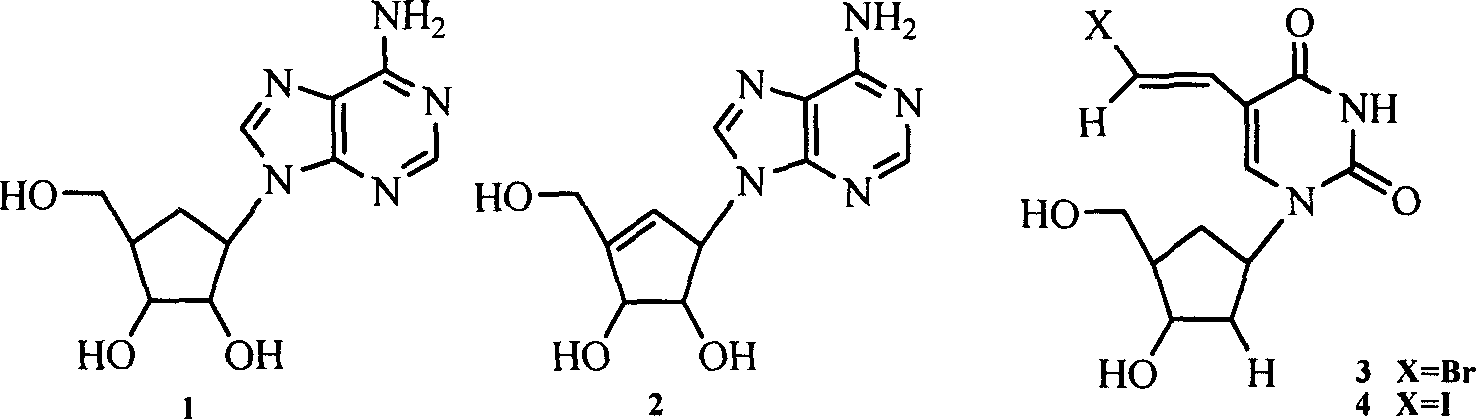
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
5-fluorouracil-resistant bacteria and method for production thereof
ActiveUS20090280091A1Good for survivalImprove the immunityOrganic active ingredientsBiocideCytosine deaminaseResistant bacteria
The present invention provides a method for producing a cytosine deaminase (CD)-expressing, 5-fluorouracil (5-FU)-resistant microorganism which can grow in anaerobic tumor tissues, can express CD, and has a resistance to 5-FU at a concentration that is at least effective for antitumor activity. More specifically, the method is a method (A) comprising performing subculture or acclimation culture of a CD-expressing microorganism which can grow in anaerobic tumor tissues, in the presence of 5-fluorocytosine (5-FC), or a method (B) comprising (1) performing subculture or acclimation culture of a microorganism which can grow in anaerobic tumor tissues and does not express CD, in the presence of 5-FU to produce a 5-FU-resistant microorganism and (2) transforming the 5-FU-resistant microorganism by introducing a CD gene. The present invention also provides the CD-expressing, 5-FU-resistant microorganism and a pharmaceutical composition comprising the microorganism.
Owner:ANAEROPHARMA SCI
Formulations of 5-fluorocytosine and uses thereof
The disclosure provides an extended release formulation of 5- f luorocytosine. In another aspect, a method of treating a fungal disease is provided. The method comprises administering to a subject in need thereof a fungus-treating effective amount of a composition comprising 5-f luorocytosine. In yet another aspect, a method of treating a cancer is provided. The method comprises administering to a subject in need thereof a sufficient amount of an expression vector to induce expression of cytosine deaminase which is capable of converting 5-f luorocytosine to 5-f luorourcail in cells of the cancer and a cancer-treating effective amount of a composition comprising 5-f luorocytosine.
Owner:TOCAGEN
Antifungal combination therapy
There is described antifungal combination therapy comprising the use of known antifungal agents such as the azoles or polyenes in combination with a pneumocandin derivative antifungal agent. More particularly, the invention relates to antifungal combination therapy comprising the use of azoles such as fluconazole, voriconazole, itraconazole, ketoconazole, miconazole, ER 30346, SCH 56592; polyenes such as amphotericin B, nystatin or liposomal and lipid forms thereof such as Abelcet, AmBisome and Amphocil; purine or pyrimidine nucleotide inhibitors such as flucytosine; or polyoxins such as nikkomycins, in particular nikkomycin Z or other chitin inhibitors, elongation factor inhibitors such as sordarin and analogs thereof, mannan inhibitors such as predamycin, bactericidal / permeability-inducing (BPI) protein products such as XMP.97 or XMP.127 or complex carbohydrate antifungal agents such as CAN-296 in combination with a pneumocandin derivative as described herein.
Owner:MERCK SHARP & DOHME CORP
Selective culture medium for gardnerella vaginalis and method for producing the same
The invention discloses a selective culture medium of Gardnerella vaginalis. Preparation method for 1000ml culture medium: 15 to 20g nutrient broth, 35 to 44g Columbia agar bess, 0.2 to 0.4 Tween 80, 0.05-0.075ml Gram's stain I solution (crystal violet), 0.02-0.03g 5-Fluorocytosine, 50-80ml human blood, 30-50ml plasma; distilled water is added to 1000ml. The invention has the advantages of ability to effectively separate the Gardnerella vaginalis from Gram-positive staphylococcus, streptococcus, Gram positive bacilli and various mould, quickened detection of Gardnerella vaginalis, rich nutrient, low cost and convenient preparation.
Owner:绍兴县中心医院
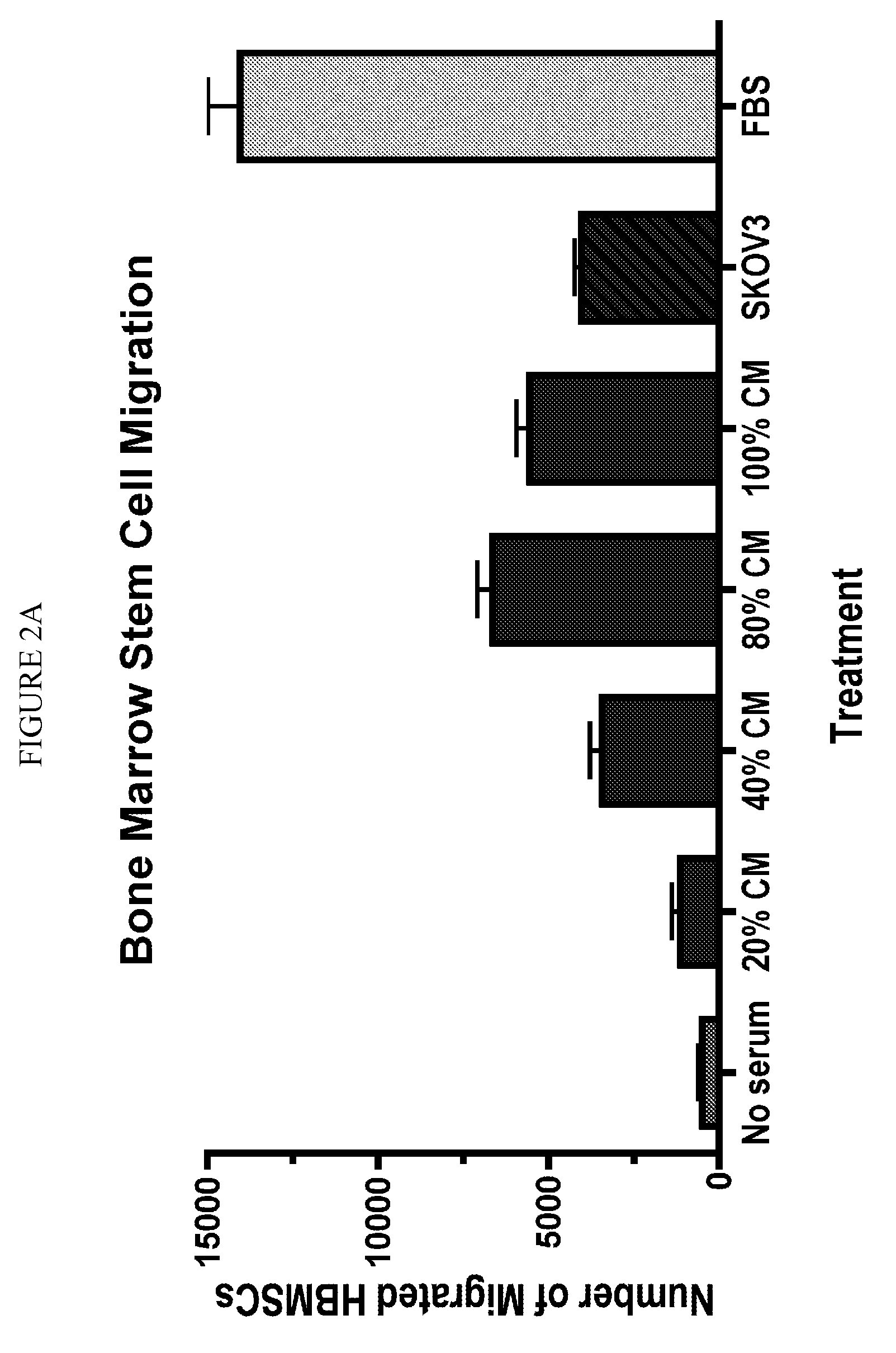
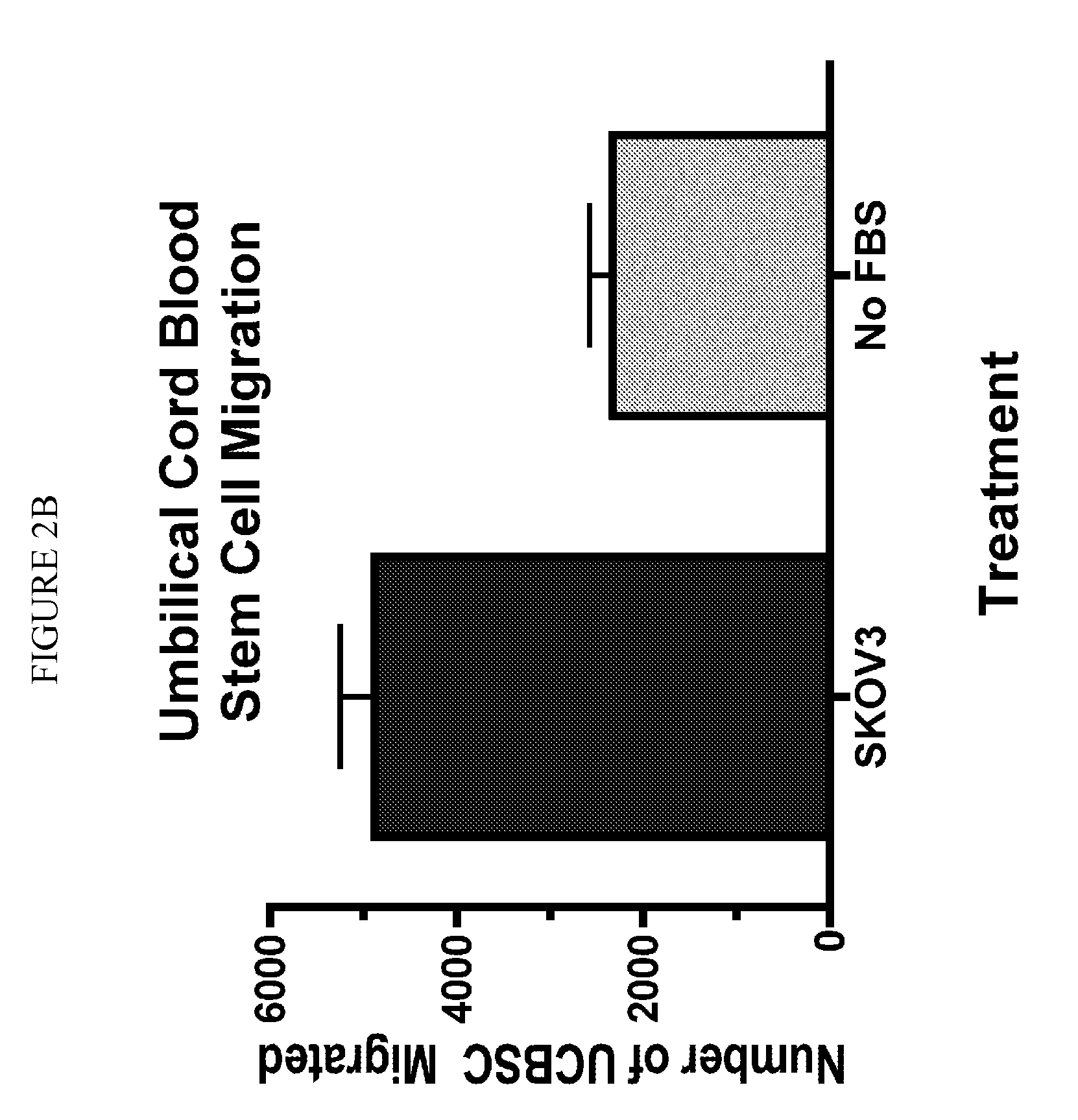
Stem cell targeting of cancer, methods and compositions therefor
Disclosed are methods of detecting and treating a cancer such as an ovarian cancer using stem cells. Detection methods include administering a plurality of labeled stem cells to a subject having, or suspected of having, a cancer; and detecting the distribution of the stem cells. In some configurations, the label can be a nanoparticle such as a mono-crystalline iron oxide, which can be detected by magnetic resonance imaging. Treatment methods include administering a plurality of stem cells comprising a therapeutic agent such as an enzyme which activates a prodrug. In some configurations, the stem cells harbor a nucleic acid sequence encoding a cytosine deaminase, the cells express the enzyme, and the treatment further includes administering the prodrug 5-fluorocytosine, which is converted by the cytosine deaminase to the cytotoxic metabolite, 5-fluorouracil (5-FU).
Owner:BOARD OFTRUSTEES OF SOUTHERN ILLINOIS UNIV
Method of recycling flucytosine chlorination wastewater
InactiveCN108585260AEmission reductionReduce processing costsTreatment involving filtrationMultistage water/sewage treatmentPhosphateOrganic layer
The invention discloses a method of recycling flucytosine chlorination wastewater, which includes the steps of: 1) sending the flucytosine chlorination wastewater to a chlorination wastewater treatment kettle, stirring and cooling the wastewater at rotation speed of 180 rpm, dropwise-adding a Ca(OH)2 solution to neutralize the wastewater and regulate pH value to generate phosphate solid; 2) filtering the wastewater to remove the phosphate solid, and sending filtered wastewater to a chlorination wastewater extraction washing kettle; 3) adding a solvent to perform extraction washing, and layering the mixture to obtain an upper layer being an organic layer and a lower layer being a water layer; 4) removing the organic layer, and regulating pH of the water later to 6.5-7.5 with an 8 mass% hydrochloric acid solution; 5) feeding the water layer to a flucytosine chlorination industrial production line for recycling use. The method is advantaged in that the treated wastewater can be recycled,so that water consumption and wastewater emission during production are greatly reduced, thus reducing wastewater treatment cost and increasing economic benefit of enterprises. The method satisfies the concept of modern green and environment-friendly production.
Owner:JINGHUA PHARMA GRP NANTONG
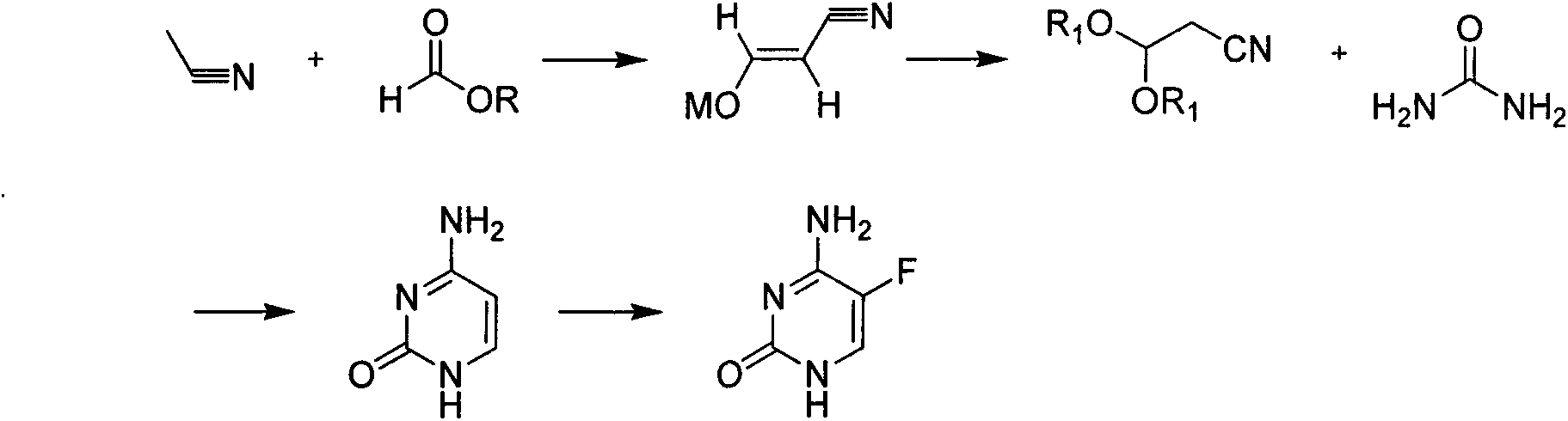
A kind of method for synthesizing 5-fluorocytosine by cytosine fluorination
ActiveCN104326990BSolve the problem of selectivityAvoid dangerOrganic chemistryFlucytosineHydrogen fluoride
The invention discloses a method for fluoridating and synthesizing 5-flucytosine by cytosine, which comprises the following steps: 1)under inert gas protection, adding cytosine in anhydrous hydrogen fluoride, introducing fluorine containing gas for a fluorination reaction under -5--20 DEG C, and 2)after reacting for 3-5 hours, distilling and concentrating the reaction solution, adding water for dissolving, adding alkali to adjust the pH value of the reaction solution, and separating to obtain the 5-flucytosine by cytosine. The method has the advantages of short synthesis route and good technology selectivity, no organic solvent is used during the production process, and the synthesized 5-flucytosine by cytosine has the advantages of high purity and high synthesis yield.
Owner:SHANGYU HUALUN CHEM
A kind of preparation method of 5-fluorocytosine
ActiveCN108033917BReduce usageThe process is environmentally friendlyOrganic chemistryChemical synthesisPotassium fluoride
Owner:ZHEJIANG XIANFENG TECH
Method for using 5-flucytosine for real-time monitoring of deoxyribonucleic acid (DNA) deamination process of human immunodeficiency virus-1 (HIV-1)
InactiveCN102559859AMicrobiological testing/measurementMicroorganism based processesDNA deaminationHuman immunodeficiency virus 1
The invention firstly discloses a method for using 5-flucytosine for real-time monitoring of deoxyribonucleic acid (DNA) deamination process of human immunodeficiency virus-1 (HIV-1). Firstly, a 5-flucytosine precursor is led in a HIV-1 single-stranded DNA sequence through a solid-phase synthesis method to compound a DAN sequence containing the 5-flucytosine; then the synthetic DNA sequence is mixed with a humanized cytosine protein, and the DNA deamination reaction process is monitored through variation of F spectrum signals. The method solves the problem that a reaction process of humanized cytosine protein deamination virus gene cytosine is very fast and not easy to monitor, signal overlapping and monitoring inconvenience exist when hydrogen spectrum monitoring is used and the like, and provides a convenient and fast method for research of inhibition of HIV-1 virus infection.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of flucytosine
InactiveCN113559101ALow costSimple preparation stepsOrganic active ingredientsAntimycoticsFlucytosineCytosine
The invention discloses a preparation method of flucytosine. The preparation method comprises the following steps of S1, material preparation: separately preparing 2-12 parts of organic alkali, 1-10 parts of cytosine, 2-8 parts of a halogenating reagent, 0.5-5 parts of an amino protective agent, 1-5 parts of a fluorinating reagent, 2-9 parts of aztreonam and 0.5-6 parts of compound probiotics; and S2, preparation I: putting a certain amount of organic alkali into a reaction kettle at the temperature of 80 DEG C, then taking a certain amount of cytosine and a certain amount of halogenating reagent, putting the taken cytosine and halogenating reagent into the reaction kettle, and mixing and stirring with the organic alkali to obtain an intermediate I. According to the flucytosine preparation method, the preparation steps are simple, unnecessary tedious steps are omitted, the raw material cost is low, the product quality is very stable, the treatment effect can be improved and is more remarkable through the added aztreonam, the nutritional ingredients can be increased through the added compound probiotics, and the side effects can be reduced.
Owner:德州隆盛化工有限公司
5-fluorocytosine derivative, its preparation method and its application in 5-fluorocytosine immunoassay reagent
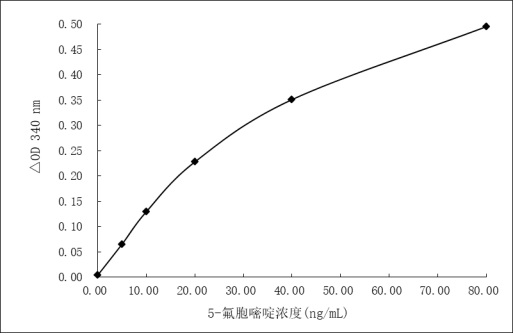
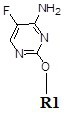
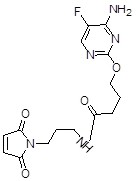
ActiveCN111875587BStrong specificitySpecific antibodies can be used to prepare high sensitivityOvalbuminSerum albuminAntiendomysial antibodiesFluorcytosine
The present invention obtains a brand-new 5-fluorocytosine derivative, and uses the 5-fluorocytosine derivative to prepare a highly immunogenic 5-fluorocytosine artificial antigen, and then immunizes experimental animals to obtain a high-titer anti-antigenic antigen. 5-fluorocytosine-specific antibody; meanwhile, use the 5-fluorocytosine derivative to prepare a 5-fluorocytosine enzyme-labeled conjugate. The 5-fluorocytosine immunoassay reagent containing the above-mentioned anti-5-fluorocytosine specific antibody and 5-fluorocytosine enzyme-labeled conjugate can realize the automatic determination of 5-fluorocytosine content on a fully automatic biochemical analyzer , can high-throughput, rapid and accurate determination of 5-fluorocytosine content in biological samples, and has the advantages of simple operation, high sensitivity, strong specificity, accurate results, etc., effectively reducing the cost of 5-fluorocytosine detection, It is beneficial to widely popularize and use clinically.
Owner:长沙博源医疗科技有限公司
Method of resolution and antiviral activity of 1,3-oxathiolane nucleoside enantiomers
A process for the resolution of a racemic mixture of nucleoside enantiomers that includes the step of exposing the racemic mixture to an enzyme that preferentially catalyzes a reaction in one of the enantiomers. The nucleoside enantiomer (−)-2-hydroxymethyl-5-(5-fluorocytosin-1-yl)-1,3-oxathiolane is an effective antiviral agent against HIV, HBV, and other viruses replicating in a similar manner.
Owner:EMORY UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com