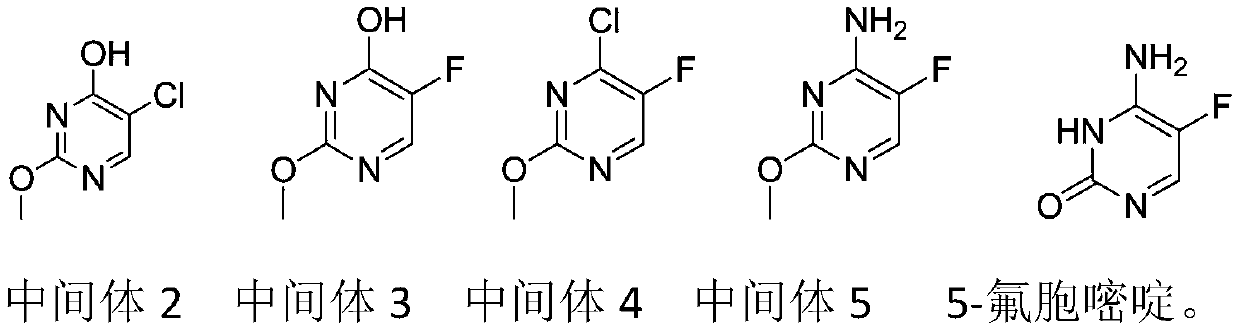
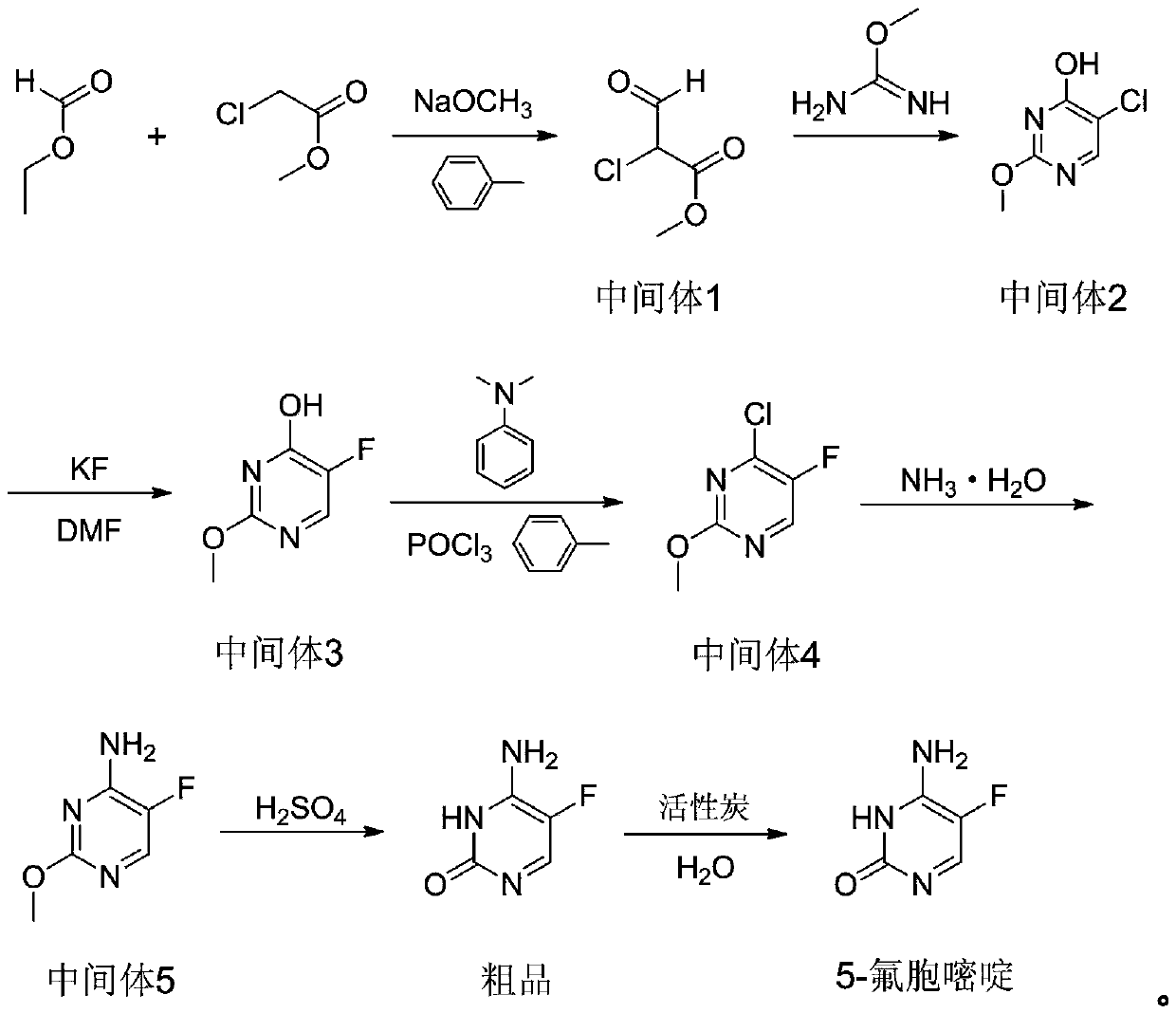
A kind of preparation method of 5-fluorocytosine
A technology of fluorocytosine and intermediates, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of increasing the production cost of 5-fluorocytosine and the high price of methyl fluoroacetate, achieve significant industrial value, reduce production costs, and increase the total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment provides a preparation method of 5-fluorocytosine, specifically, comprising the following steps:
[0031] Step 1: add 600kg toluene in reactor, cool, add 188kg solid sodium methylate under agitation condition, utilize nitrogen to replace the air in reactor, add 282kg ethyl formate in reactor, control temperature during adding to be 10 -20°C, then add 188kg methyl chloroacetate, control the temperature at 10-25°C during the addition process, raise the temperature to 65-75°C after the addition, and react for 12 hours to obtain intermediate 1, cool to below 20°C, and transfer to the intermediate Add 333.7kg of liquid sodium methoxide and 507.6kg of oxymethylisourea to 1, heat up to 35-45°C after the addition, react for 7 hours, remove the solvent from the reaction solution, then dissolve it in 1000kg of water, let it stand for stratification, and adjust the water phase pH value to 3-4, cooled, filtered, washed, dried to obtain 212.9kg of intermediate 2, the...
Embodiment 2
[0038] This embodiment provides a preparation method of 5-fluorocytosine, specifically, comprising the following steps:
[0039] Step 1: add 600kg toluene in reactor, cooling, add 225.6kg solid sodium methylate under stirring condition, utilize nitrogen to replace the air in reactor, add 376kg ethyl formate in reactor, control temperature during adding 10-20°C, then add 188kg methyl chloroacetate, control the temperature at 10-25°C during the addition process, raise the temperature to 65-75°C after the addition, and react for 8 hours to obtain intermediate 1, cool to below 20°C, and transfer to the middle Add 400.4kg of liquid sodium methoxide and 601.6kg of oxymethylisourea to body 1, heat up to 35-45°C after addition, react for 5 hours, remove the solvent from the reaction solution, then dissolve it in 1100kg of water, let it stand and separate, and the water phase Adjust the pH value to 3-4, cool, filter, wash, and dry to obtain 213.0kg of intermediate 2 with a yield of 76....
Embodiment 3
[0046] This embodiment provides a preparation method of 5-fluorocytosine, specifically, comprising the following steps:
[0047] Step 1: Add 600kg of toluene to the reactor, cool it, add 206.8kg of solid sodium methylate under stirring conditions, replace the air in the reactor with nitrogen, add 319.6kg of ethyl formate to the reactor, and control the temperature during the addition process 10-20°C, then add 188kg of methyl chloroacetate, control the temperature at 10-25°C during the addition process, raise the temperature to 65-75°C after the addition, and react for 10 hours to obtain intermediate 1, cool to below 20°C, and send to Add 367.1kg of liquid sodium methoxide and 564kg of oxymethylisourea to intermediate 1, raise the temperature to 35-45°C after the addition, and react for 6 hours, remove the solvent from the reaction solution, then dissolve it in 1100kg of water, let it stand for stratification, and the water phase Adjust the pH value to 3-4, cool, filter, wash, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com