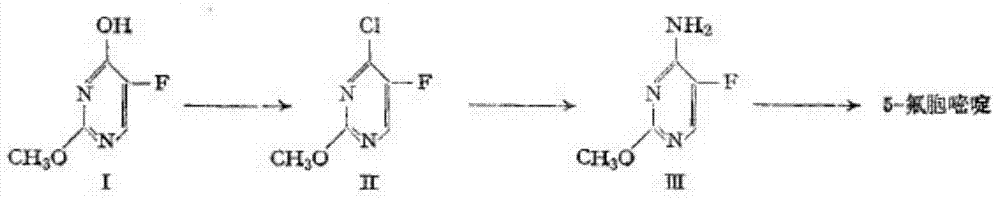
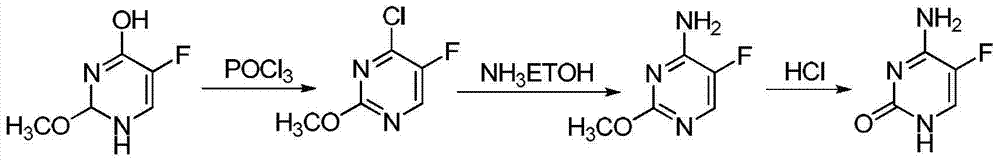
Method for fluoridating and synthesizing 5-flucytosine by cytosine
A technology of fluorocytosine and cytosine, which is applied in the field of fluorination of cytosine to synthesize 5-fluorocytosine, which can solve the problems of unfavorable industrial production, unstable product quality, and the use of organic solvents, and achieve easy temperature control of raw materials and avoid fluorine The effect of high gas concentration and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
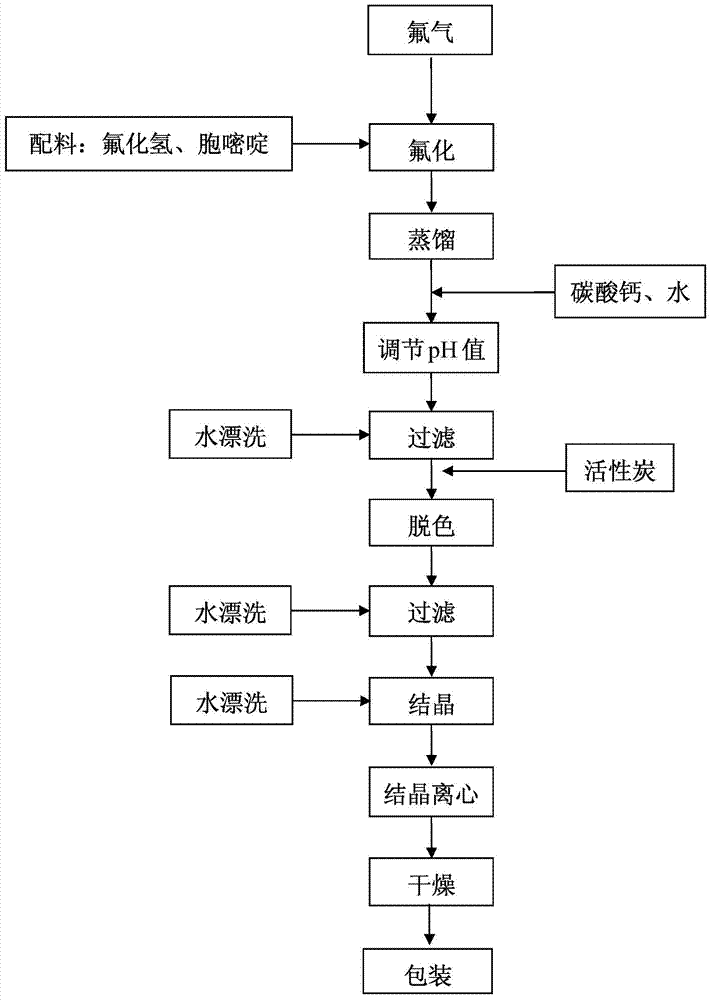
[0043] (1) Under the protection of nitrogen, add 1150g of cytosine to the anhydrous hydrogen fluoride with a temperature of 0°C and a mass of 3450g, and at a temperature of -15°C, feed a mixed gas of fluorine and nitrogen with a fluorine content of 15%, The flow rate is 40g / h, and the fluorination reaction is carried out;
[0044] (2) After reacting for 4 hours, feed nitrogen gas to remove excess fluorine gas, vacuum distill anhydrous hydrogen fluoride from the reaction solution to dryness at -20°C, add 8L of water, and then add calcium carbonate to adjust the pH value to 8;
[0045](3) Heat the reaction solution to 90°C and keep it warm for 1 hour. After hot filtration, add 100g of activated carbon, keep warm at 90°C for 0.5 hour, heat and filter again, cool down to 25°C and stir for 0.5 hour, then cool down to 0°C and stir for 1 hour , filtered to obtain white 5-fluorocytosine wet product;
[0046] (4) After drying the wet product of 5-fluorocytosine at 70° C. for 16 hours,...
Embodiment 2
[0049] (1) Under the protection of nitrogen, add 1150g of cytosine to the anhydrous hydrogen fluoride with a temperature of 0°C and a mass of 4600g, and at a temperature of -15°C, feed a mixed gas of fluorine and nitrogen with a fluorine content of 15%, The flow rate is 40g / h, and the fluorination reaction is carried out;
[0050] (2) After reacting for 3 hours, feed nitrogen to remove excess fluorine gas, vacuum distill anhydrous hydrogen fluoride from the reaction solution to dryness at -20°C, add 8L of water, and then add calcium carbonate to adjust the pH value to 7;
[0051] (3) Heat the reaction solution to 90°C and keep it warm for 1 hour. After hot filtration, add 100g of activated carbon, keep warm at 90°C for 0.5 hour, heat and filter again, cool down to 25°C and stir for 0.5 hour, then cool down to 0°C and stir for 1 hour , filtered to obtain white 5-fluorocytosine wet product;
[0052] (4) After drying the wet product of 5-fluorocytosine at 70° C. for 16 hours, th...
Embodiment 3
[0055] (1) Under the protection of nitrogen, add 1150g of cytosine to the anhydrous hydrogen fluoride with a temperature of 0°C and a mass of 2300g, and at a temperature of -15°C, feed a mixed gas of fluorine and nitrogen with a fluorine content of 20%, The flow rate is 50g / h, and the fluorination reaction is carried out;
[0056] (2) After reacting for 3 hours, feed nitrogen gas to remove excess fluorine gas, vacuum distill anhydrous hydrogen fluoride from the reaction solution to dryness at -20°C, add 8L of water, and then add calcium carbonate to adjust the pH value to 8;
[0057] (3) Heat the reaction solution to 90°C and keep it warm for 1 hour. After hot filtration, add 100g of activated carbon, keep warm at 90°C for 0.5 hour, heat and filter again, cool down to 25°C and stir for 0.5 hour, then cool down to 0°C and stir for 1 hour , filtered to obtain white 5-fluorocytosine wet product;
[0058] (4) After drying the wet product of 5-fluorocytosine at 70° C. for 16 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com