Antifungal combination therapy
a combination therapy and antifungal technology, applied in the field of antifungal combination therapy, can solve the problem that none of these studies have demonstrated the results found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
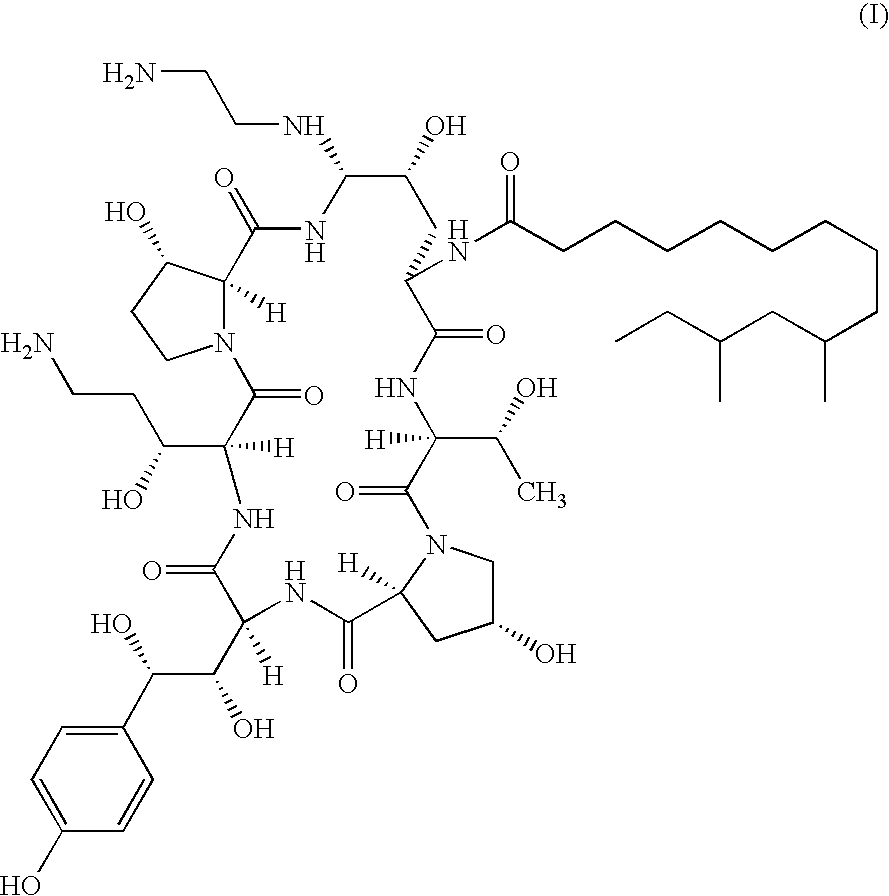
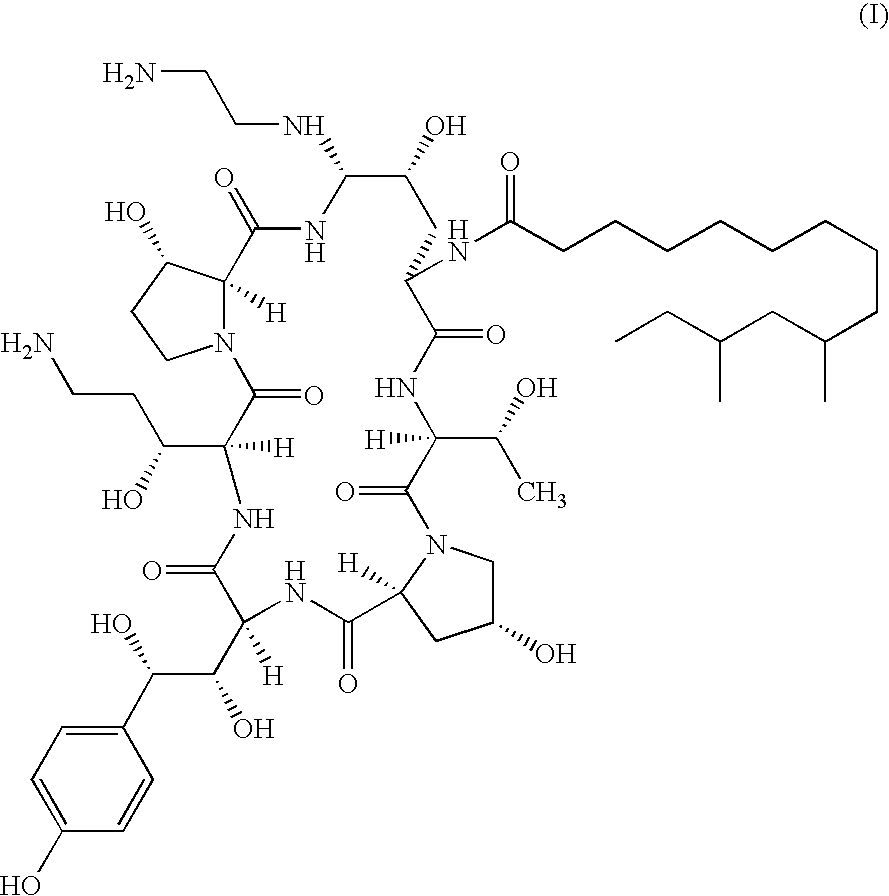
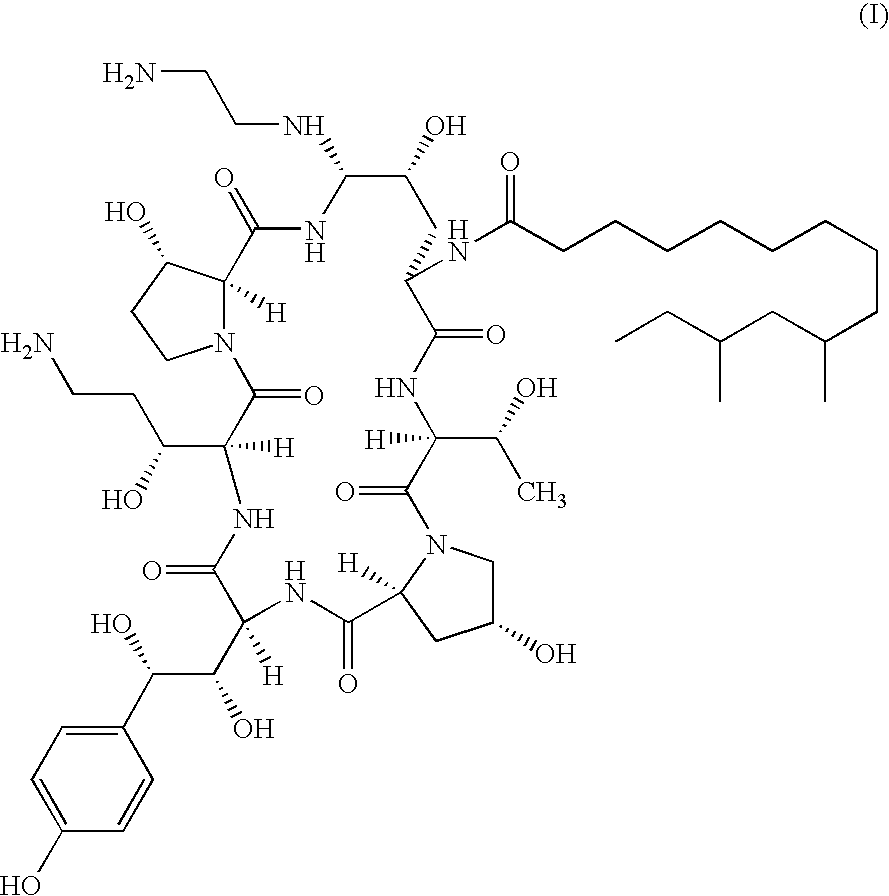
[0014]It has been found that combination therapy of Compound I with AmB and FCZ against C. neoformans results in enhanced activity against strains of C. neoformans in vitro. It has also been found that combination therapy of Compound I with AmB against C. albicans and A. fumigatus results in enhanced activity in vitro. This has been shown using a broth microdilution technique which is the standard method for antifungal susceptibility testing proposed by the NCCLS (protocol M27-T). Sub-inhibitory concentrations of Compound I in combination with sub-inhibitory concentrations of AmB and sub-inhibitory concentrations of FCZ were employed. The minimal inhibitory concentrations (MICS) for AmB and Compound I were defined as the lowest drug concentration at which there was an absence of growth. FCZ MIC was defined as the lowest drug concentration which resulted in a visual turbidity less than or equal to 80% inhibition compared with that produced by the control without antifungal agent.
[001...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com