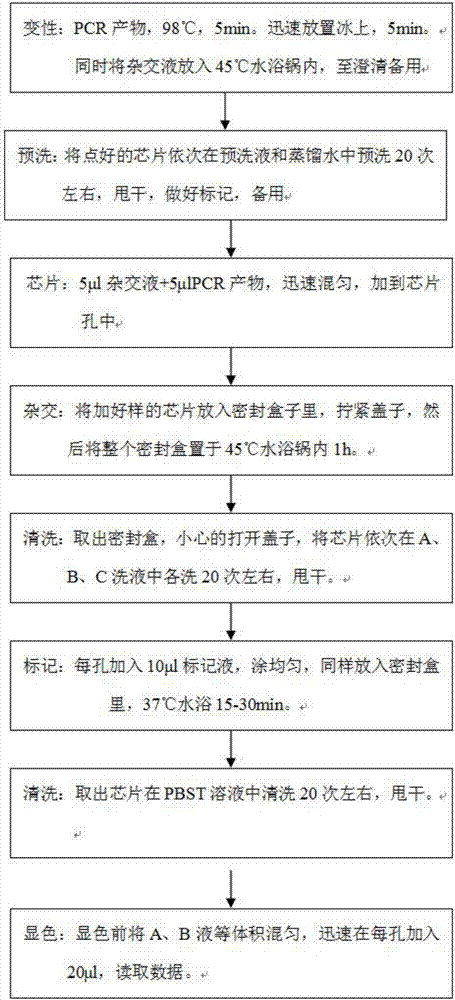
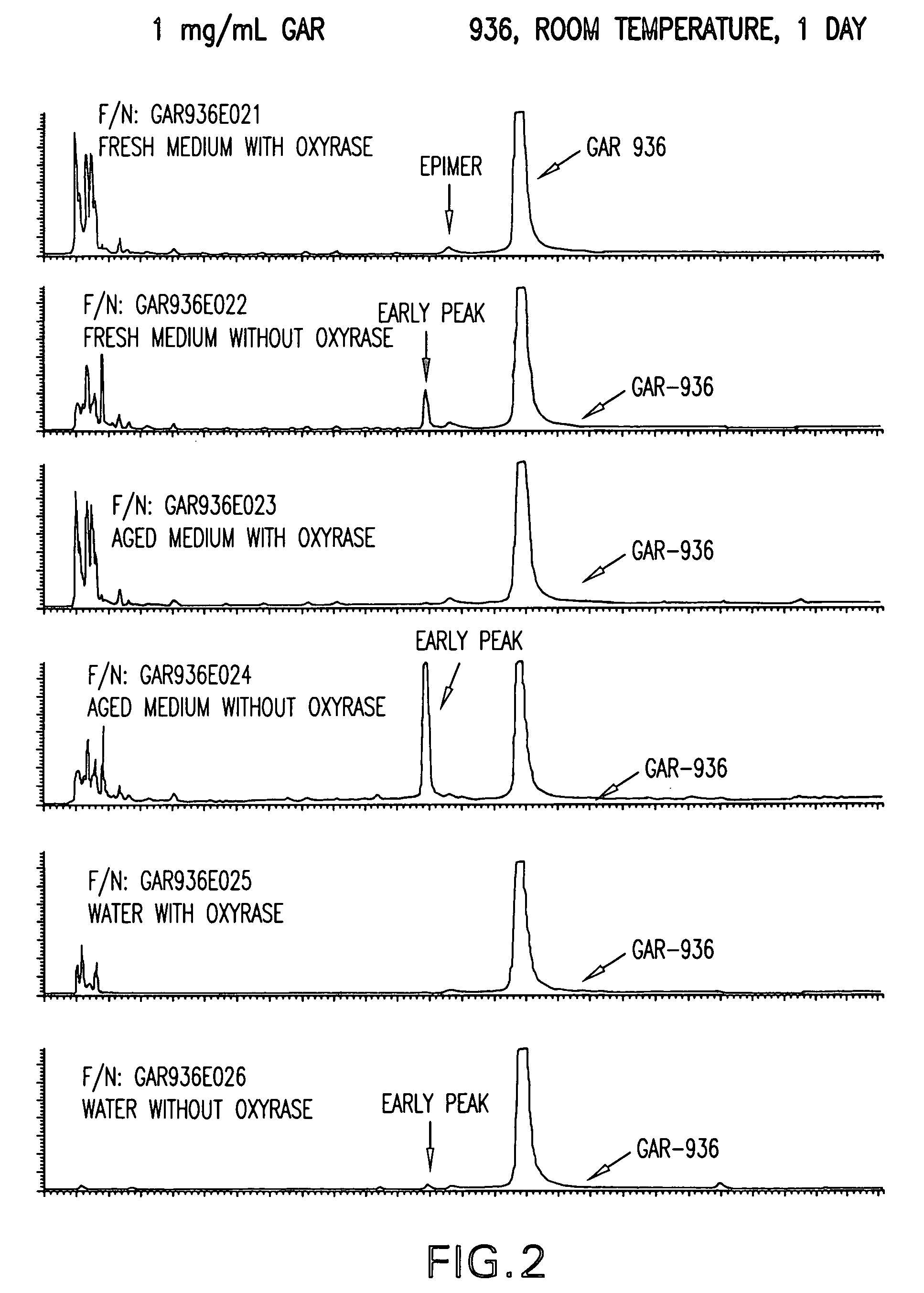
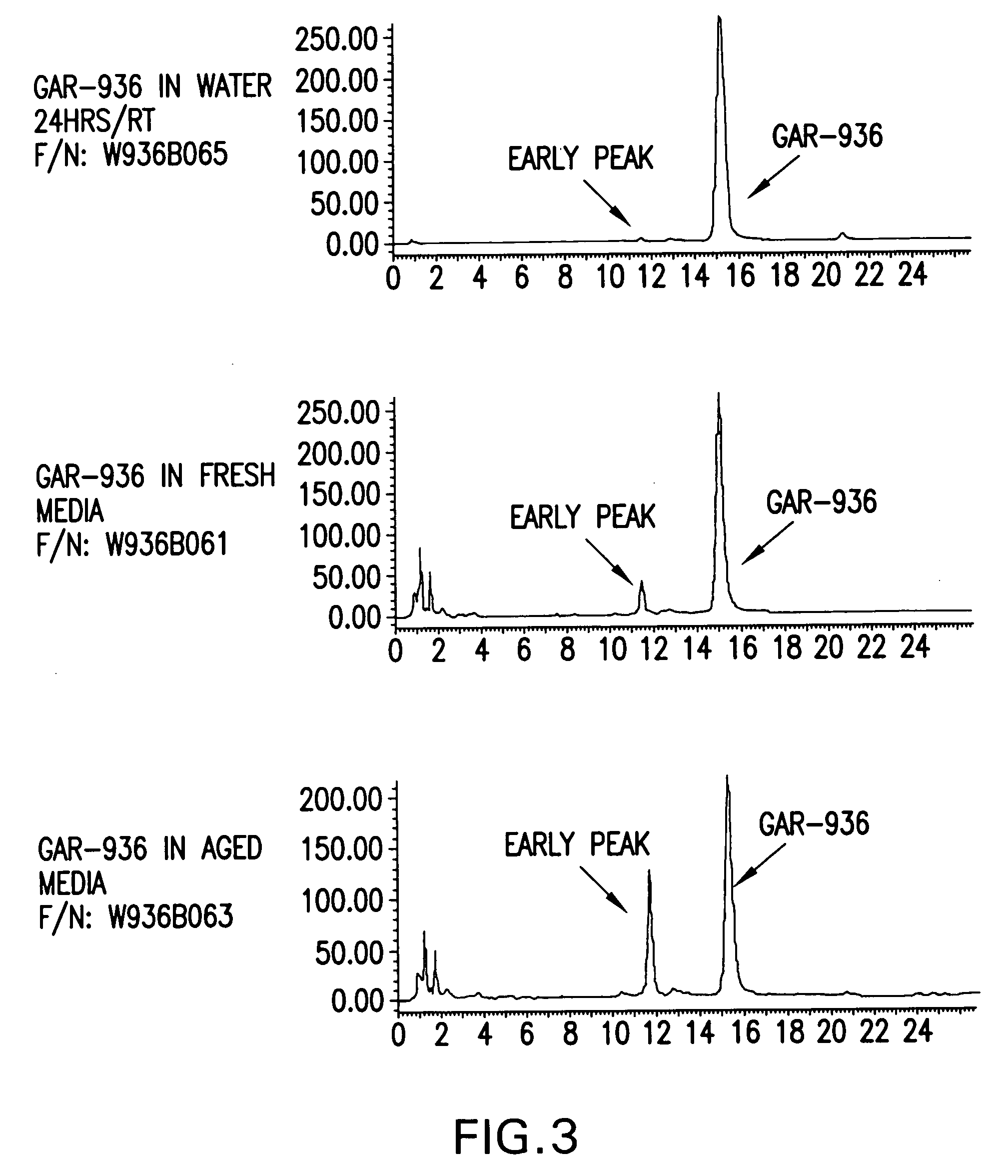
Patents
Literature
60 results about "Susceptibility testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
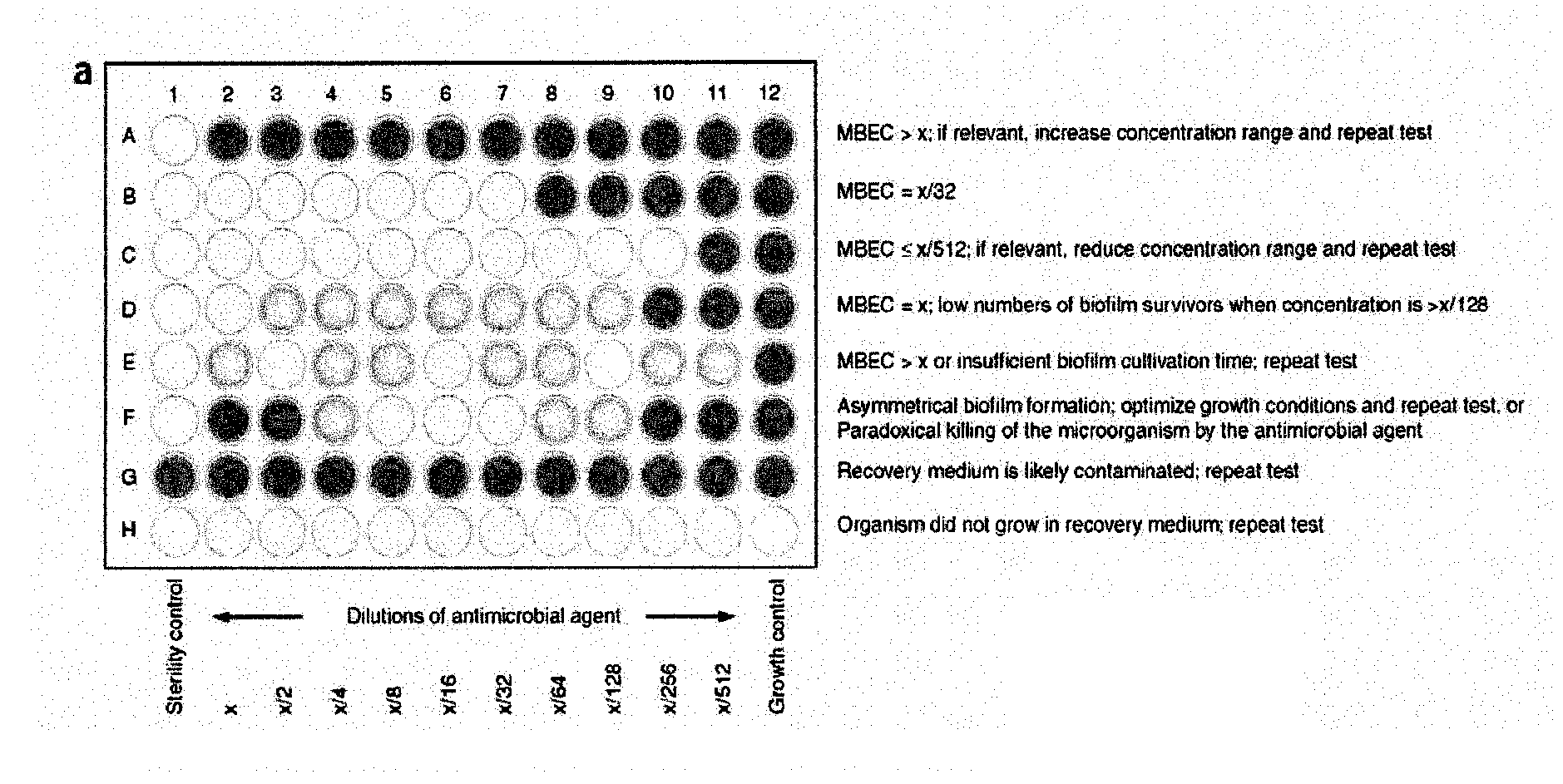
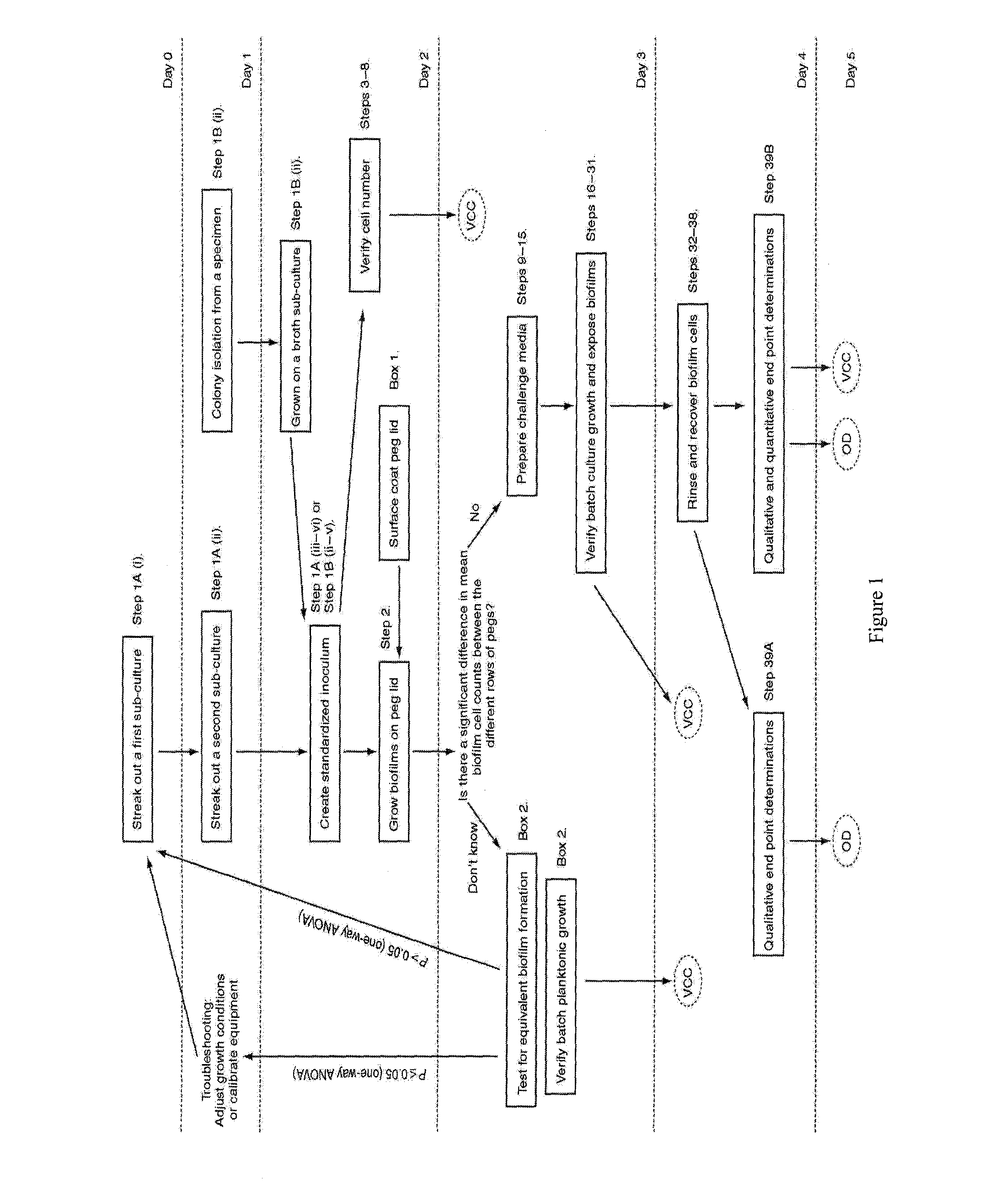
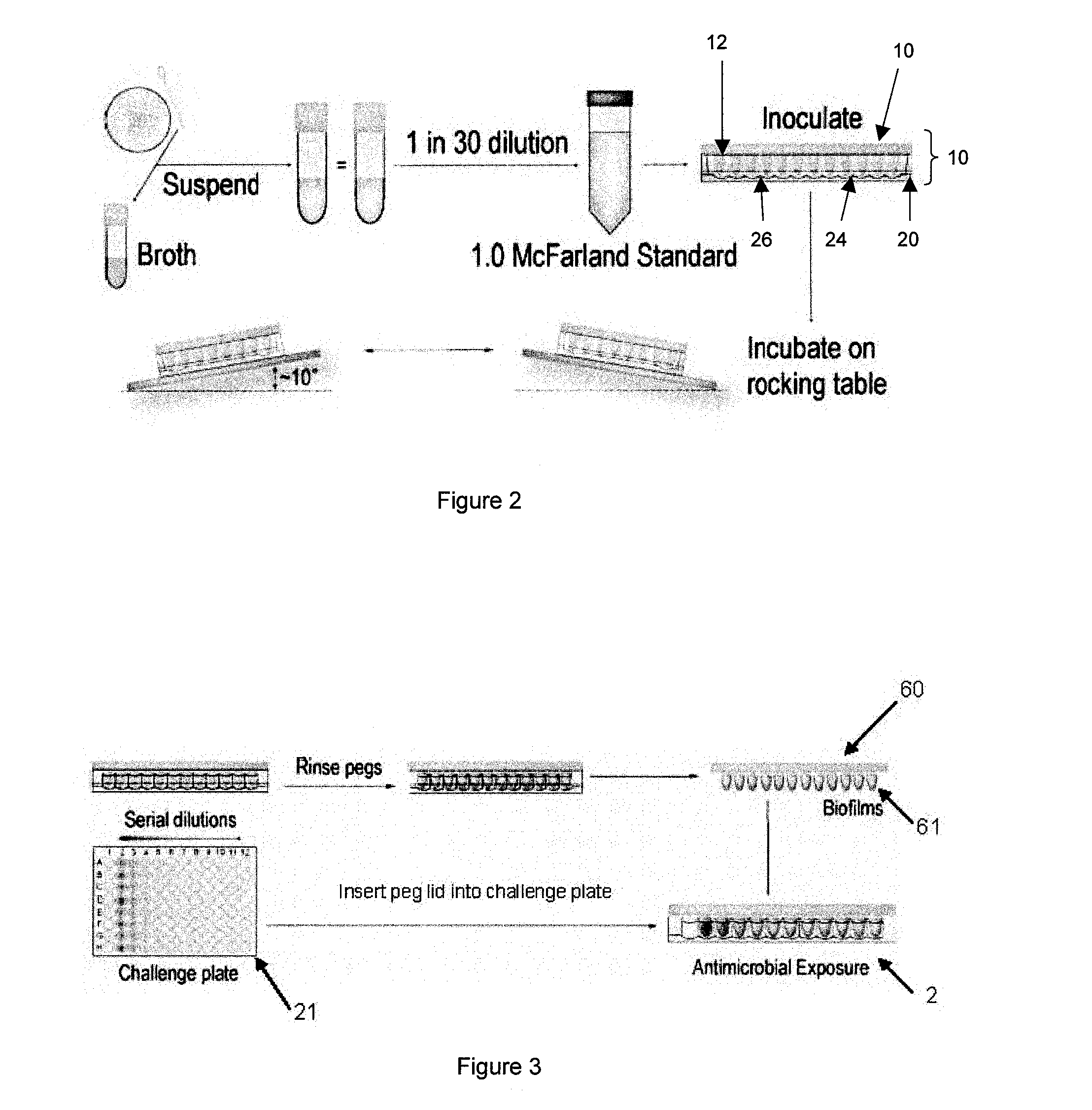
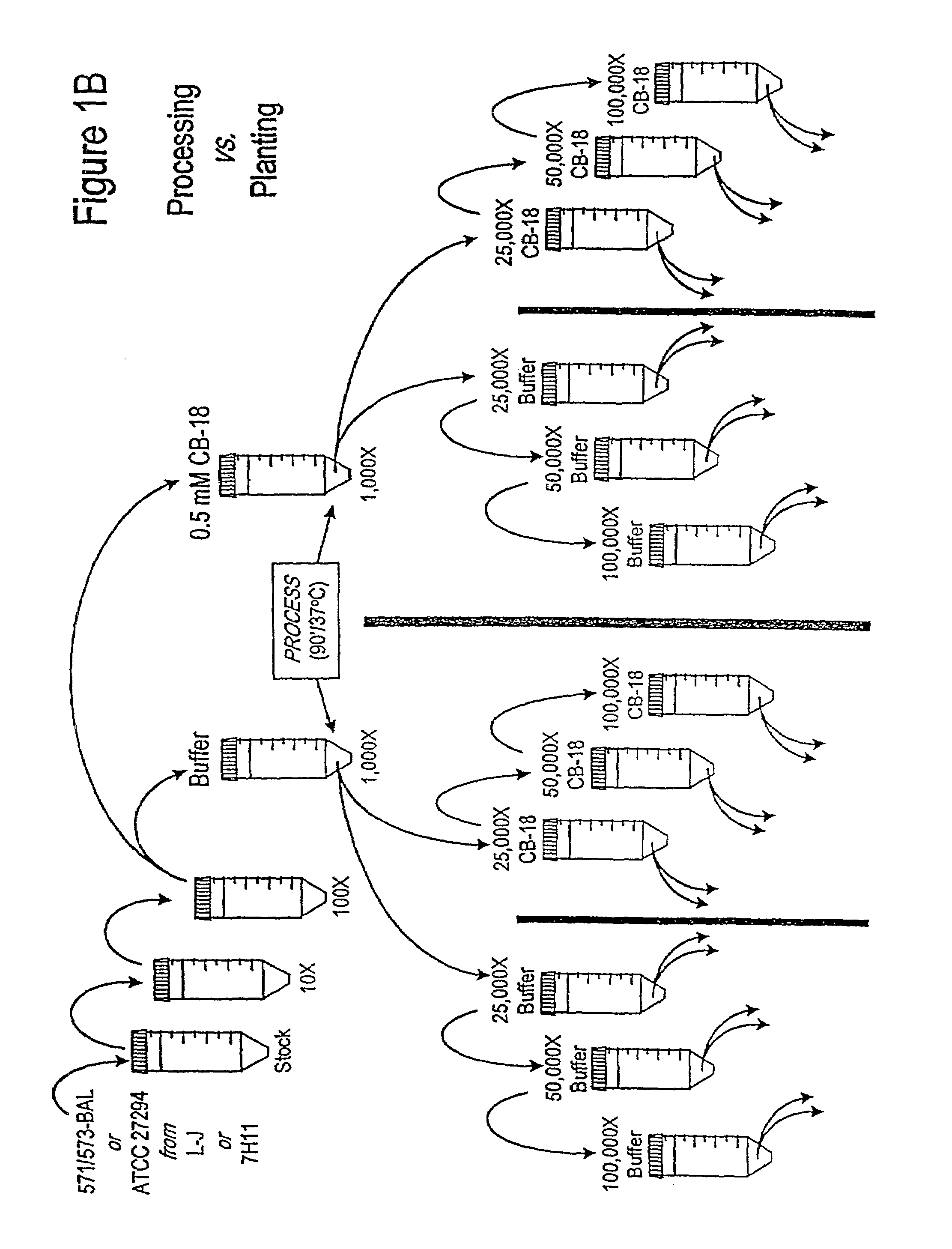
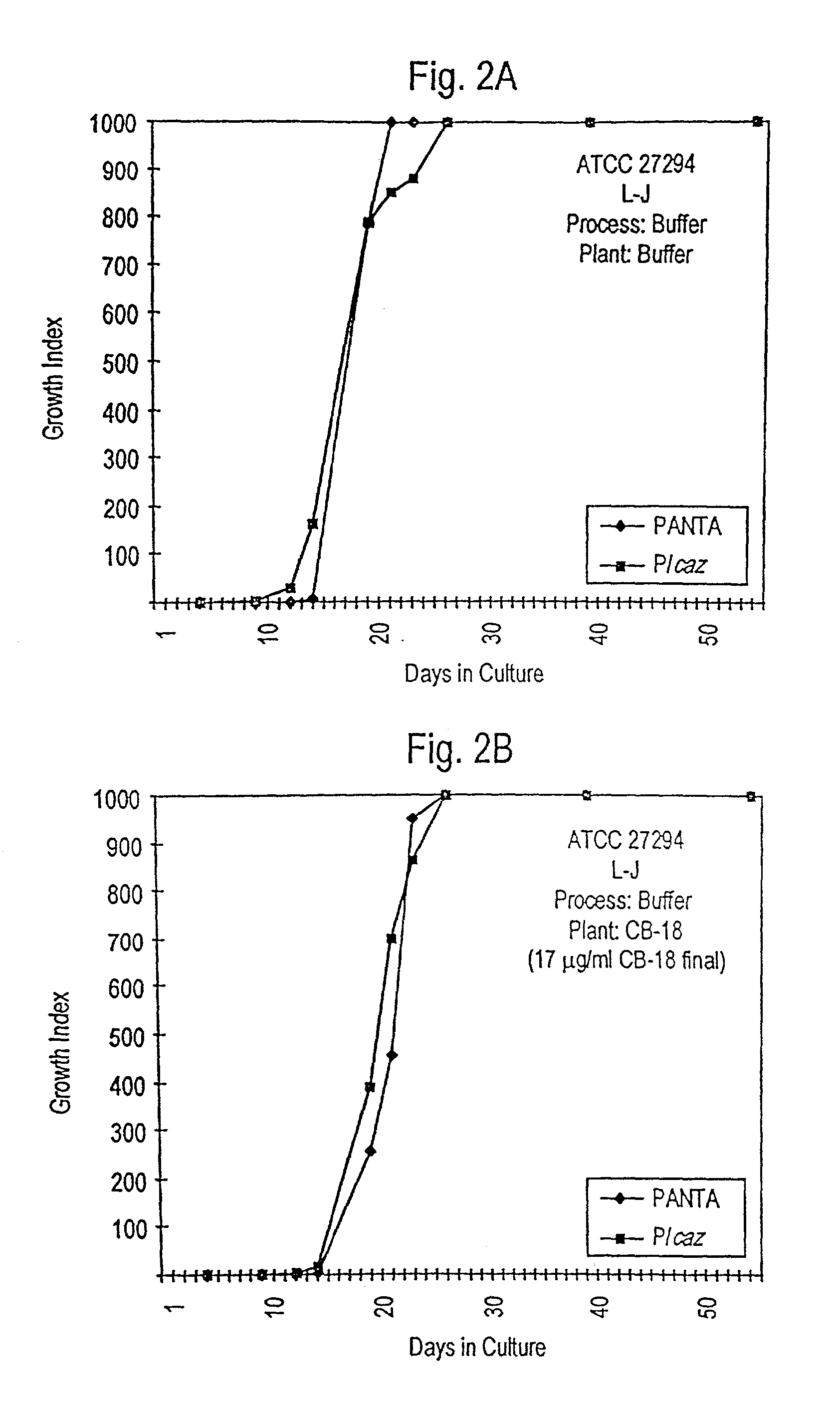
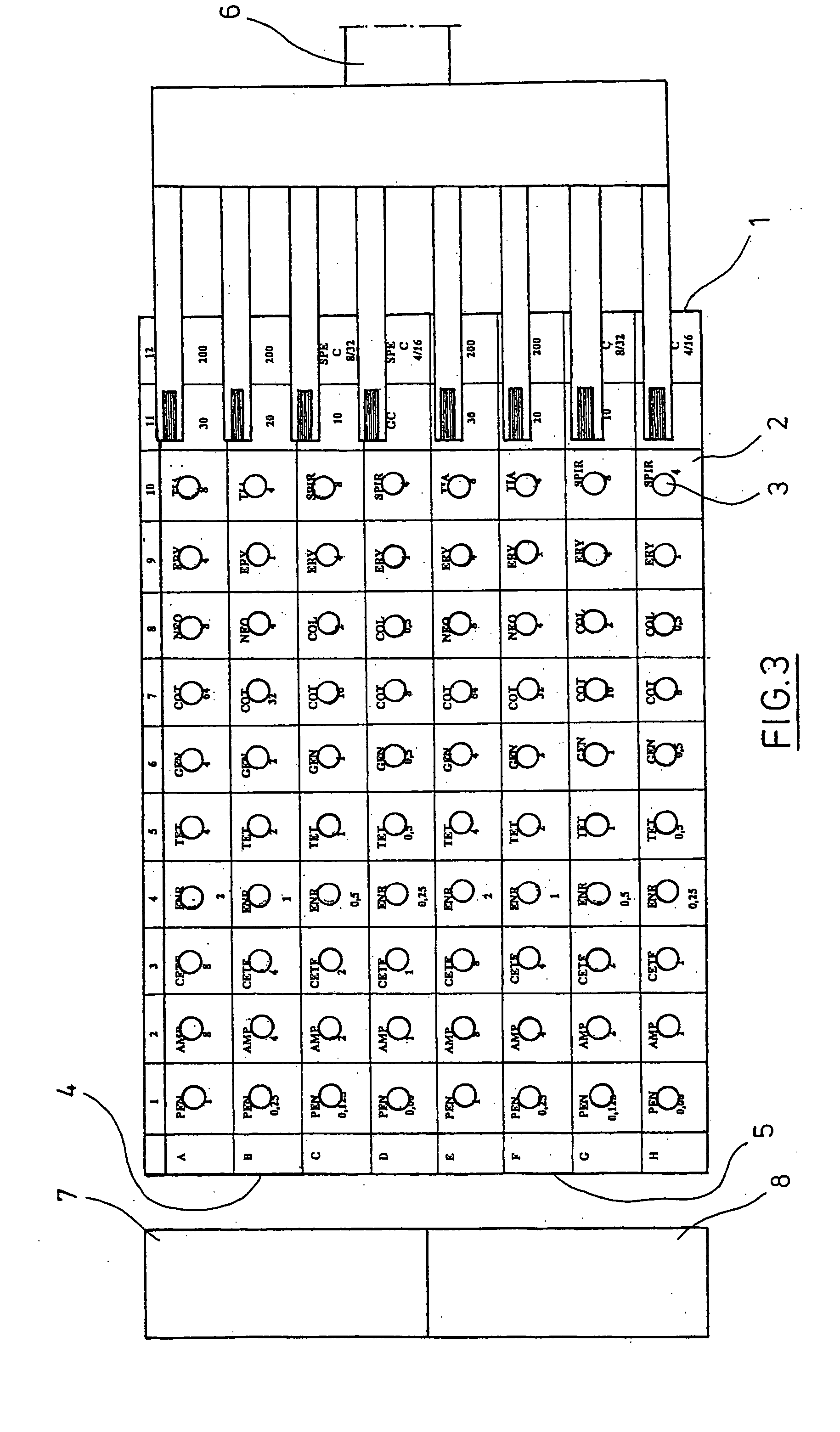
Testing of Biofilm for Anti-microbial Agent Susceptibility
InactiveUS20120329675A1Economical and simpleEfficient and cost-effectiveMicrobiological testing/measurementLibrary screeningSpecific testBiofilm
This invention is an apparatus and method for susceptibility testing one or more biofilms, for selecting one or more anti-microbial combinations with efficacy against the biofilm, and / or in treating a disease or condition mediated by the biofilm The invention includes methods for the selection of antibiotic combinations with efficacy against a specific microbial type and for the formulation of microbe-specific test plates. The invention also includes an assay system to test patient specific isolates for sensitivity to the anti-microbial combinations.
Owner:OLSON MERLE E +1
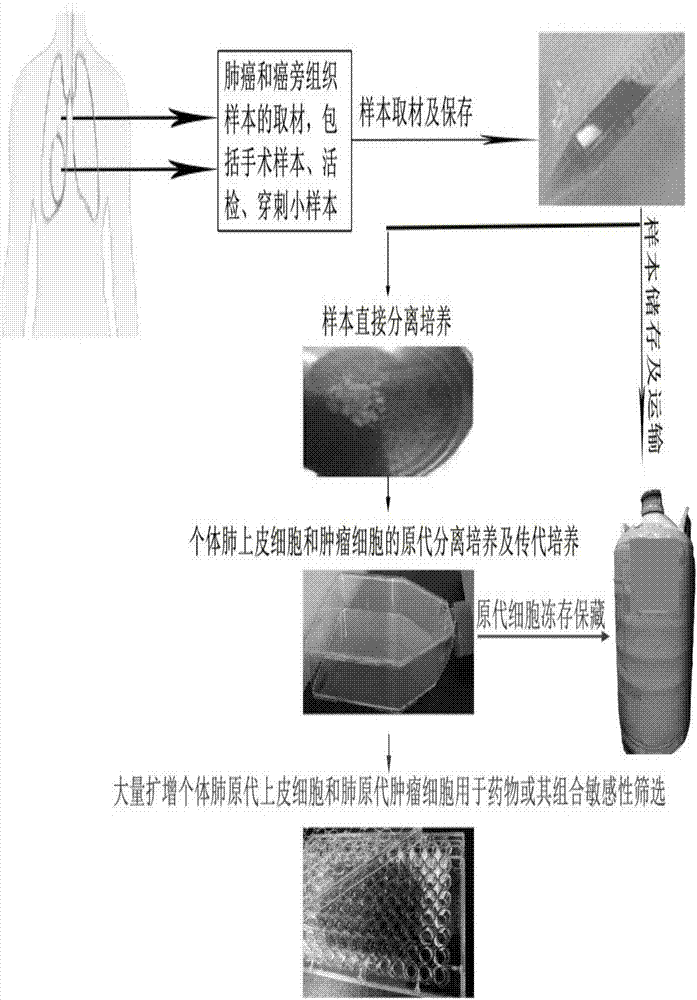
In-vitro individualized medicine test method for lung cancer and culture medium
InactiveCN107151645AProlong survival timeLow toxicityCell dissociation methodsCompound screeningProcess systemsIndividualized treatment
The invention discloses an in-vitro individualized medicine test method for lung cancer and a culture medium, and belongs to the field of cell biology and pharmacology. The in-vitro individualized medicine test method and the culture medium have the advantages that the culture medium M can be applied to normal epithelial cells and primary tumor cells of human or mammals, accordingly, in-vitro culture systems for tumor cells and para-carcinoma cells of lung cancer patients can be established, and primary cells, with continuous passage and quick proliferation functions, of patient individuals can be obtained; the culture systems can be used for detecting the sensitivity and the safety of medicines or combination groups of the medicines, and accordingly stable, accurate and reliable lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be established; individualized sensitive chemotherapy medicines or compositions screened by the aid of the lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be combined with clinical medicine or relevant subjects, accordingly, clinical individualized treatment schemes can be formulated, and the in-vitro individualized medicine test method and the culture medium can ultimately serve for clinical application.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
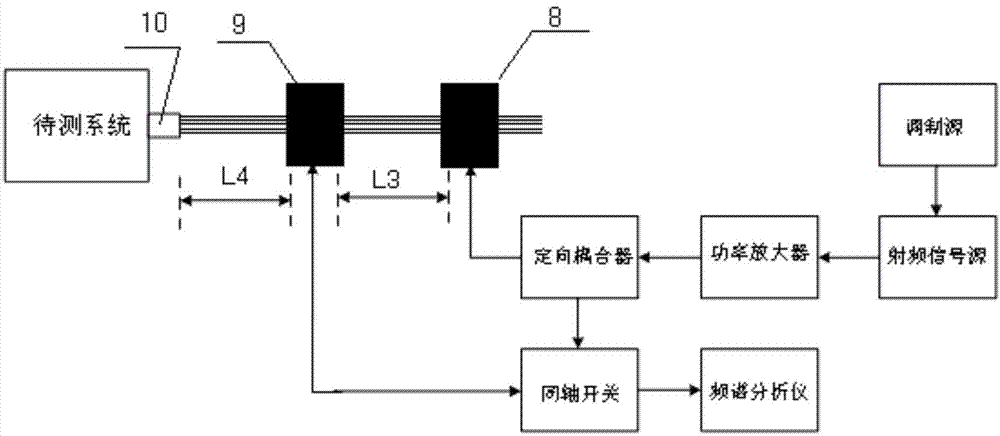
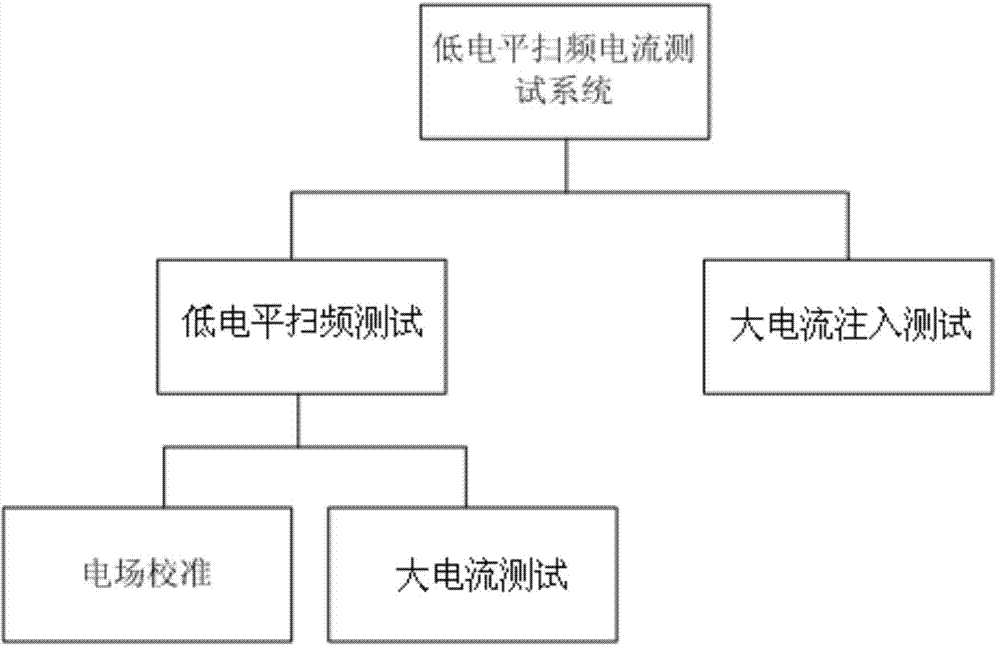
Low-level sweep-frequency current testing system and testing method
Owner:陕西海泰电子有限责任公司
Micro-fluidic chip and application thereof in authentication of pathogene and susceptibility testing
ActiveCN106238112AExpand the range of detection objectsGood application effectMicrobiological testing/measurementLaboratory glasswaresConcentration gradientDigestion
The invention discloses a micro-fluidic chip and application thereof in authentication of pathogene and susceptibility testing. The characteristic that an agar culture-medium has high-temperature digestion and low-temperature solidification is utilized, an authenticating culture medium is placed on the middle layer of the chip, an upper layer chip concentration gradient generator is utilized, and a drug to be studied is introduced; the drug is separated in different culture ponds, multiple pathogene analysis is achieved through the space resolving power of a culture pond array, pathogene authentication is achieved according to the specificity developing result, pathogene quantifying is achieved through real-time developing strength analysis, and the drug susceptibility is determined according to the lowest antibiotic concentration of a developing inhibiting reaction. The micro-fluidic chip is especially suitable for pathogene analysis under the deficient medical resource condition, and has wide application prospects.
Owner:QIQIHAR MEDICAL UNIVERSITY
Bacteria drug-resistance detection system and operation method thereof
ActiveCN106497772AEasy to useReduce abuseBioreactor/fermenter combinationsBiological substance pretreatmentsOnline and offlineBacterial strain
The invention discloses a bacteria drug-resistance detection system and an operation method thereof. The system comprises a drug sensitive test inoculation instrument, drug sensitive test image acquisition conversion equipment, a drug sensitivity detection kit and a bacterial strain culture device. A medium is arranged in the drug sensitivity detection kit. The drug sensitive test inoculation instrument is used for inoculating a bacterial strain into the drug sensitivity detection kit. The bacterial strain culture device provides a growth environment for the bacterial strain in the drug sensitivity detection kit. The drug sensitive test image acquisition conversion equipment is used for acquiring and converting images of the bacterial strain in the drug sensitivity detection kit. Management software is arranged in the drug sensitive test image acquisition conversion equipment, and the management software is connected to the network. With combination of online and offline, clinical medication is directly guided. The system meets on-site detection requirements, is convenient for data analysis and decision-making for a farm, a veterinary medicines factory and an administrative department, is suitable for current high-level drug resistance situation in our country, and satisfies the need of quantitative networked monitoring of drug resistance. A broth dilution method and an agar dilution method are both considered. The system meets different requirements of large-scale monitoring and scattered sample detection.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
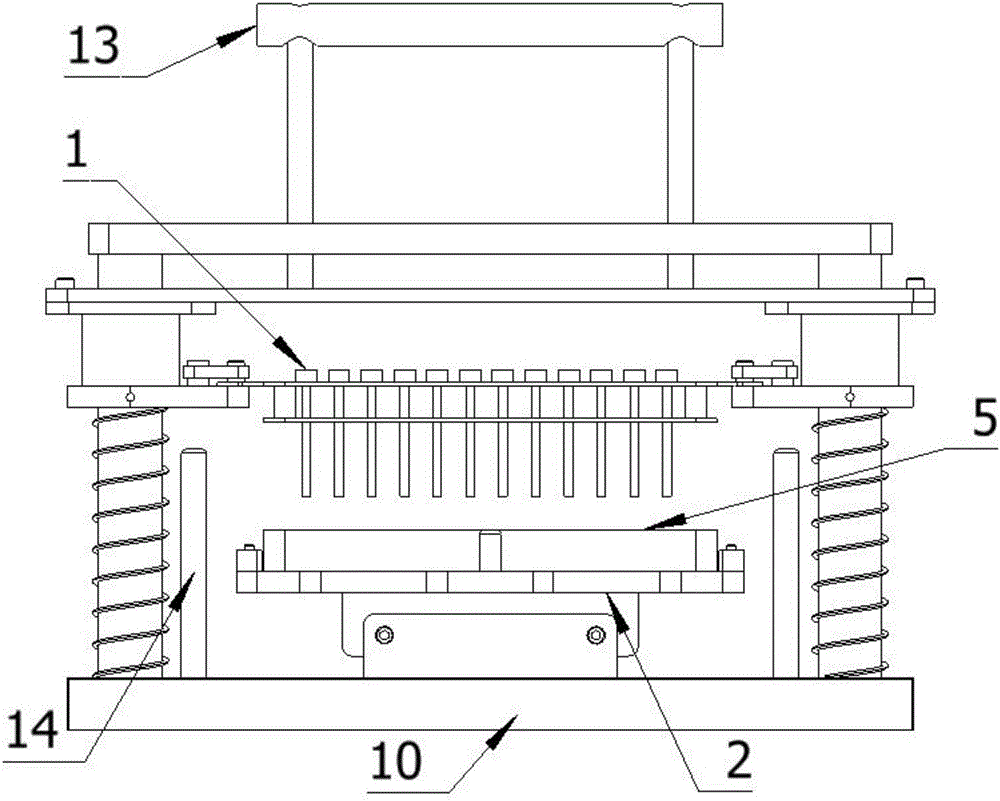
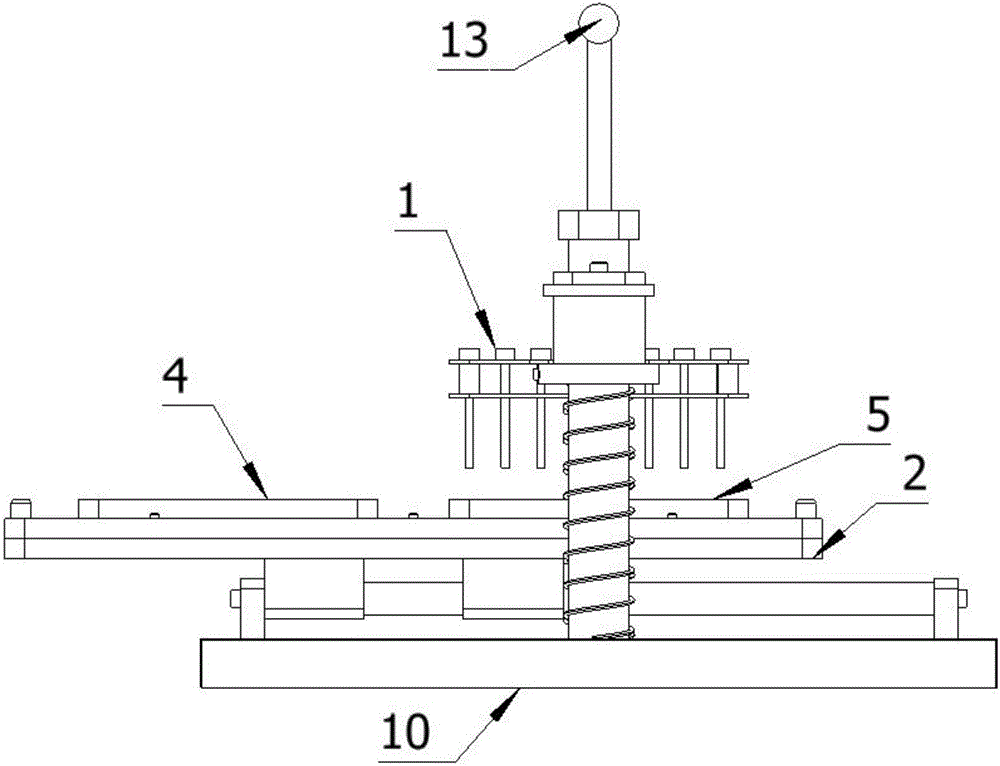
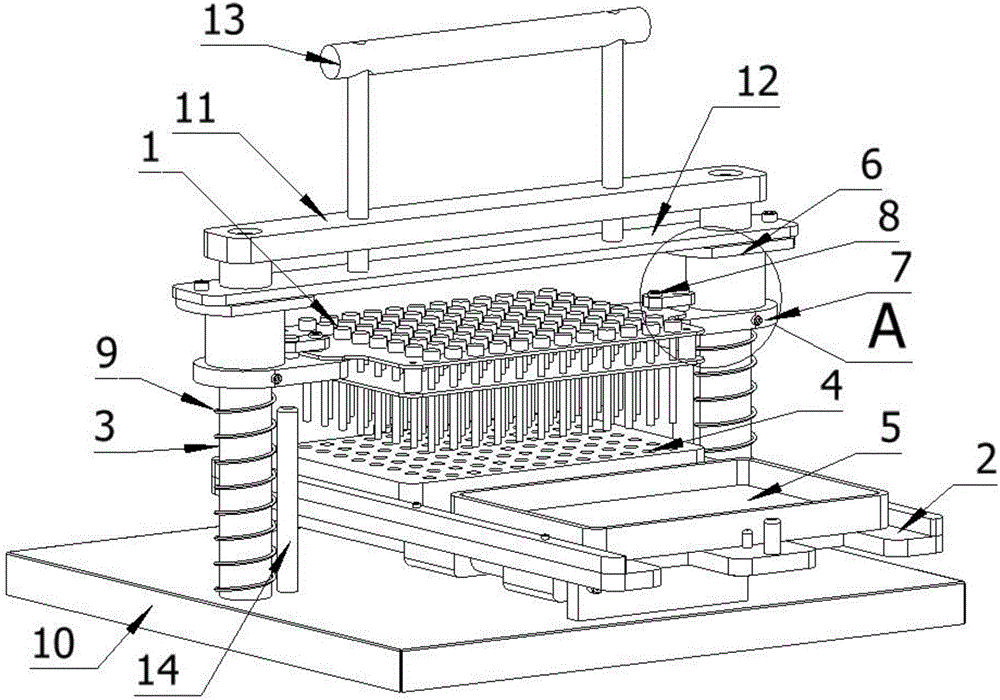
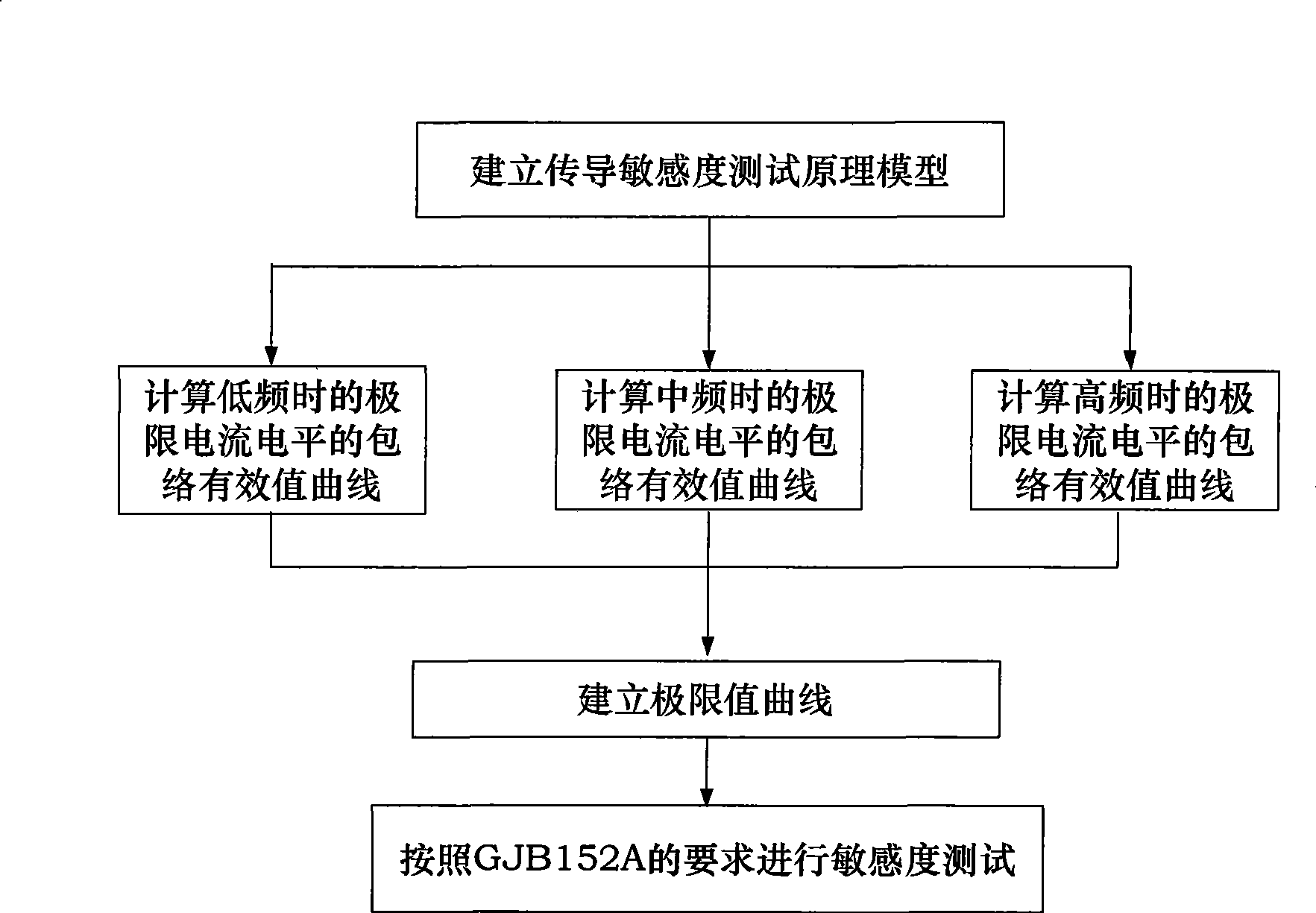
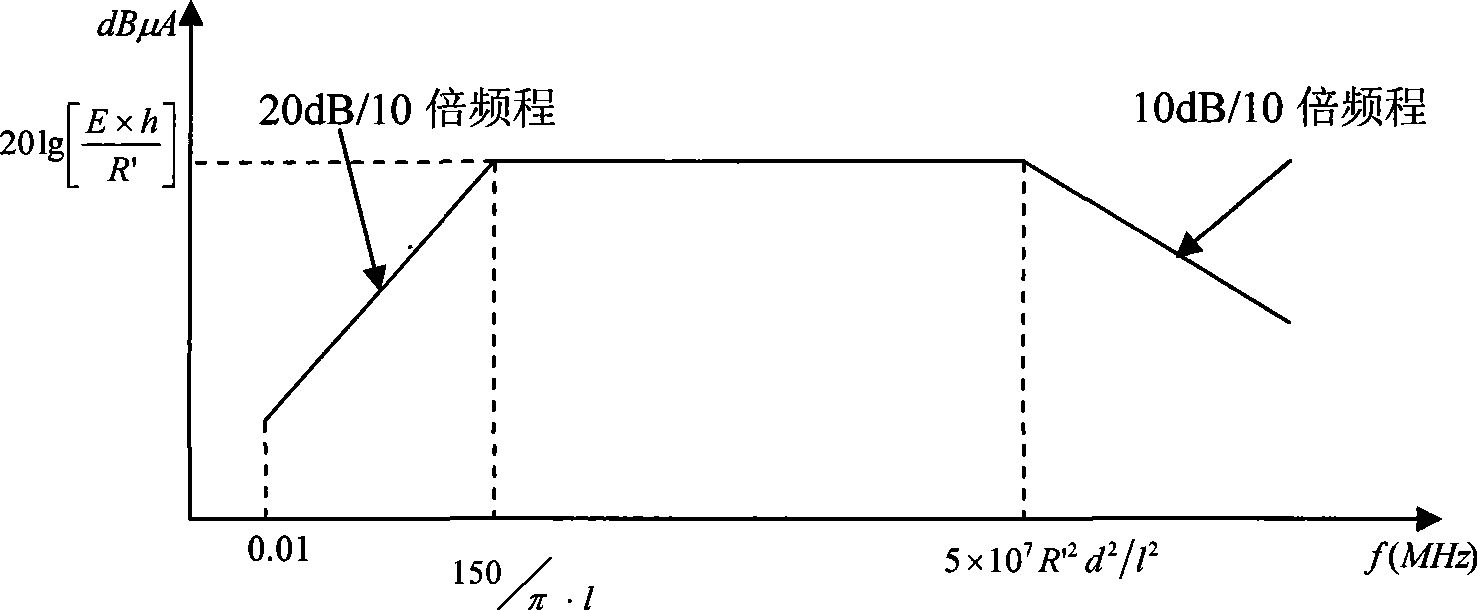
Method for detecting conducted susceptibility
ActiveCN101520480AComplete assessmentAccurate assessmentElectrical testingComputer scienceSusceptibility testing
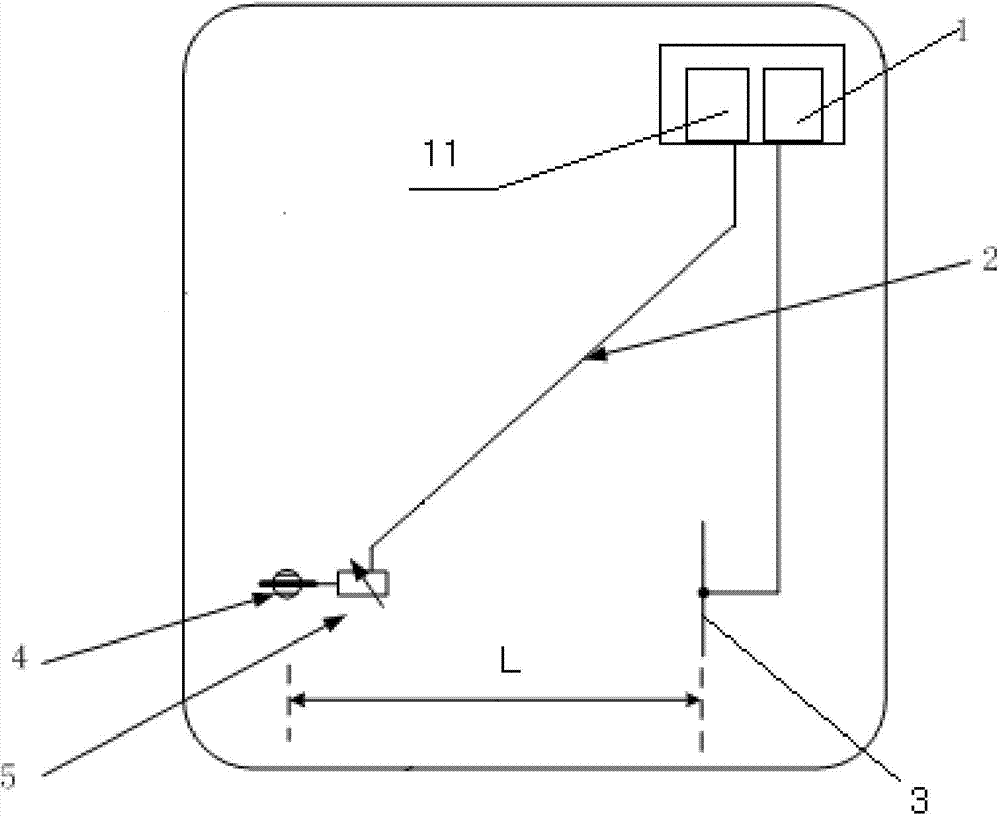
The invention discloses a method for detecting conducted susceptibility, which comprises the following steps: firstly, a conducted susceptibility testing principle model is established; secondly, an ultimate value curve is established; and thirdly, a sensitivity test is carried out by applying the ultimate value curve established in the second step. The invention can completely and accurately check a system level conducted sensitivity testing result, and can obtain the ultimate value curve and a sensitivity ultimate value electrical level, therefore, a system level conducted sensitivity test has an accurate testing basis and a testing standard, and the system level test check is more complete and accurate.
Owner:BEIHANG UNIV
Kit for quickly detecting drug resistance gene of pneumophila pathogenic bacteria
ActiveCN107475422AEasy to operateNucleotide librariesMicrobiological testing/measurementMethicillin resistanceAntibiotic Y
The invention relates to a kit for quickly detecting a drug resistance gene of pneumophila pathogenic bacteria, which comprises a gene chip capable of detecting 24 main drug resistance genes of pneumophila pathogenic bacteria and a reaction system for multiple asymmetric PCR reactions. The 24 drug resistance genes are derived from aminoglycosides, quinolones, extended spectrum beta-lactamases (ESBLs), cephalosporins (AmpC), carbapenems and drug resistance genes with vancomycin resistance and methicillin resistance causing membrane permeability transition. The kit can be used for directly detecting drug resistance genes of pneumophila pathogenic bacteria in clinical samples, is simple and quick to operate, does not need culture or drug sensitive tests, gains valuable time for reasonable application of antibiotics to a great extent, and is worthy of clinical popularization and application.
Owner:GENERAL HOSPITAL OF PLA +1
Method for quickly detecting drug resistance of bacteria
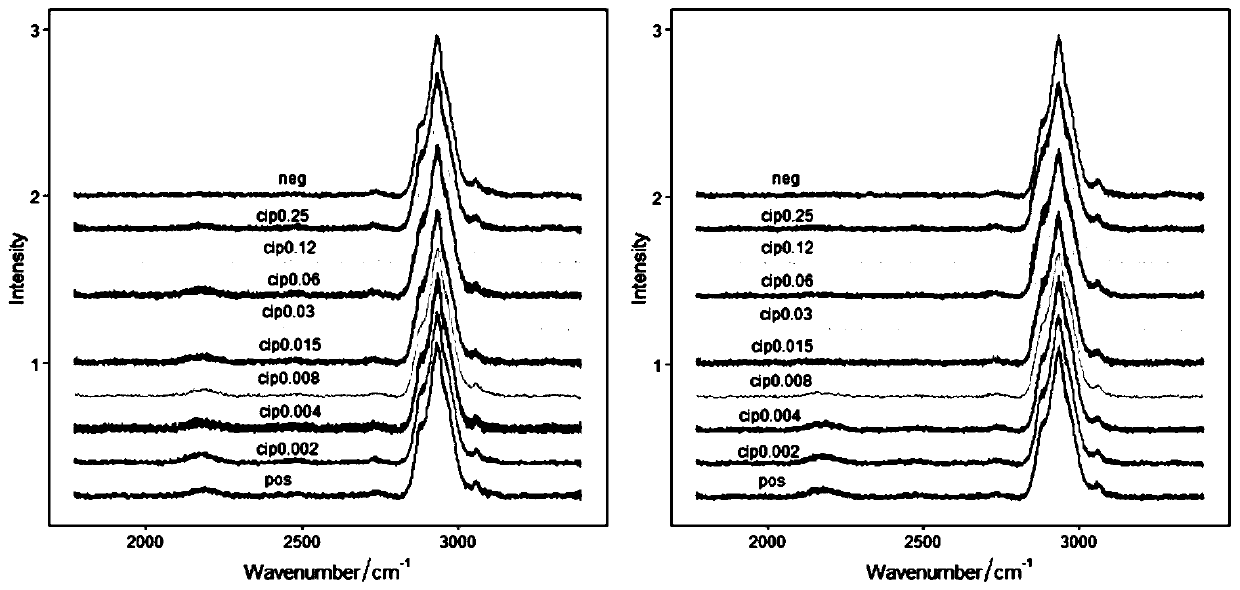
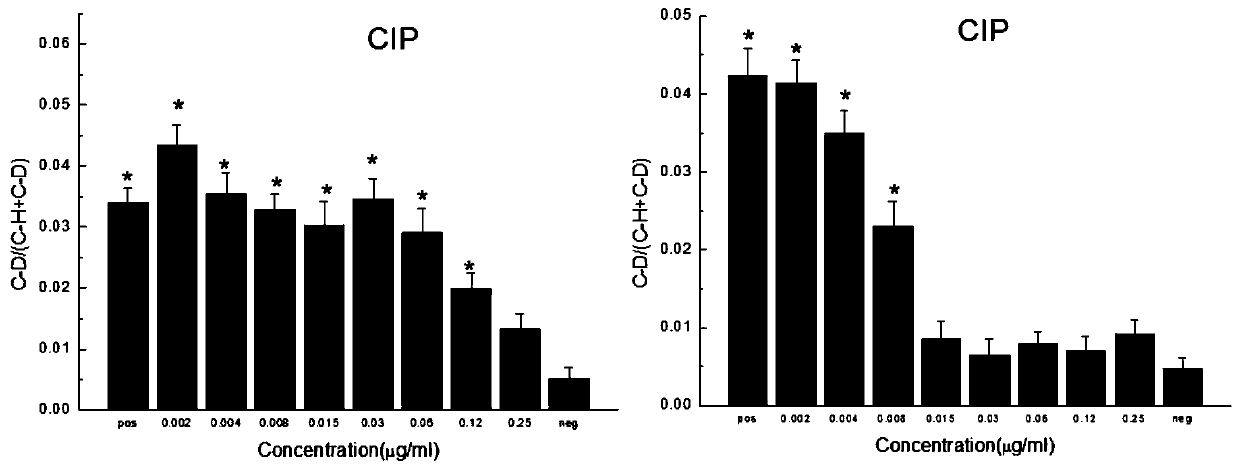
PendingCN110643675AStrong metabolic activityExactly give the sensitivityMicrobiological testing/measurementRaman scatteringMinimum inhibitory concentrationDrug resistance
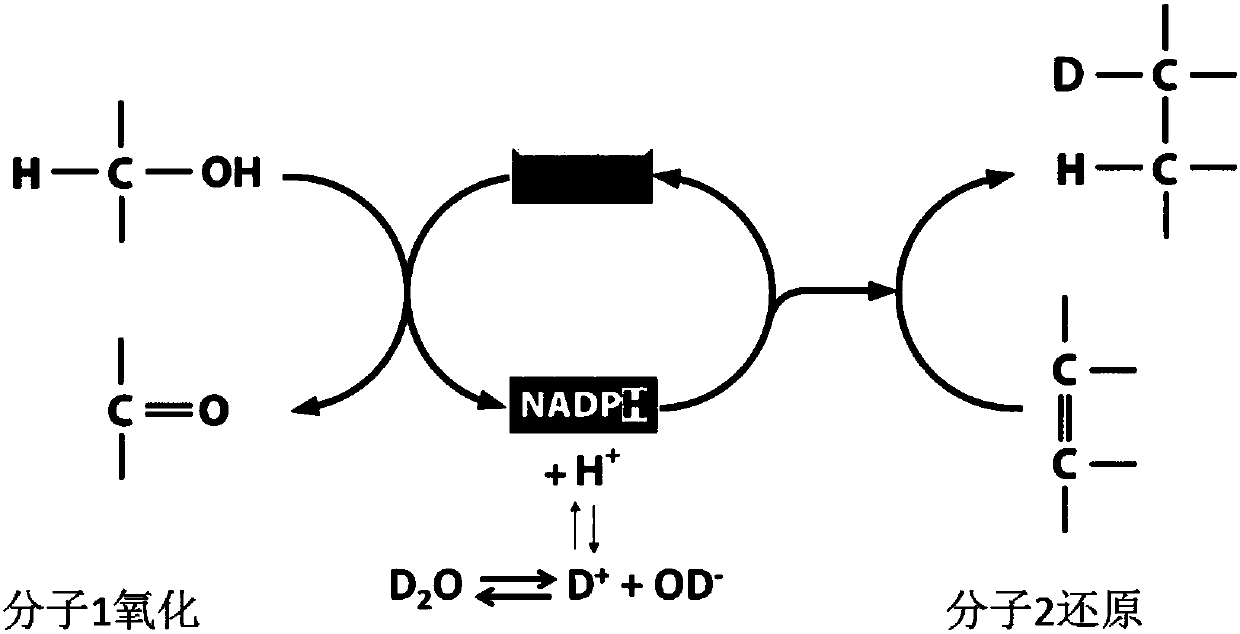
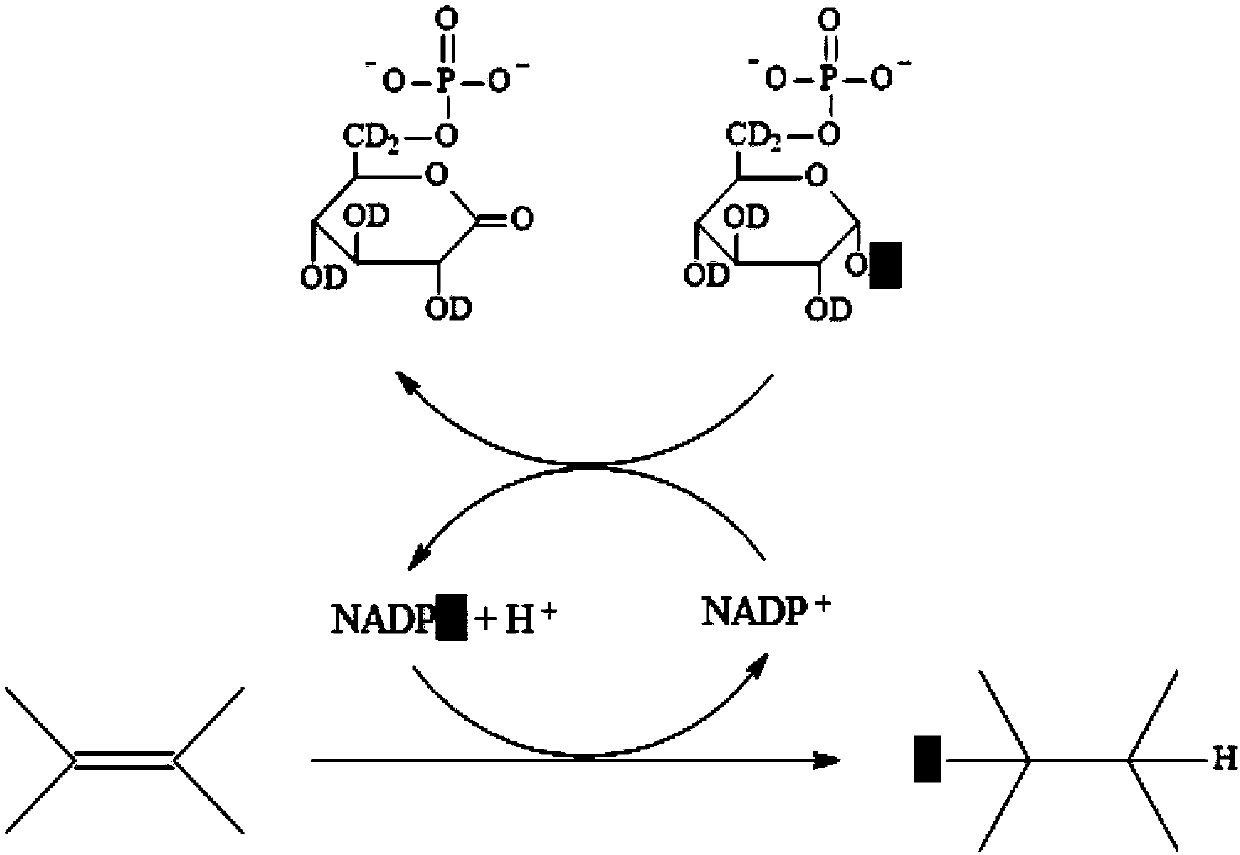
The invention relates to a method for quickly detecting drug resistance of bacteria. The method comprises the following steps: experimental groups of antibiotics with different concentrations, a positive control group and a negative control group are set, wherein the experimental groups of the antibiotics with the different concentrations refer to experimental groups of adding the antibiotics withthe different concentrations into culture mediums and adding heavy water in subsequent steps, the positive control group refers to the experimental group of adding antibiotics with a concentration of0 into a culture medium and adding heavy water in subsequent steps, and the negative control group refers to the experimental group of adding antibiotics with a concentration of 0 intp a culture medium without adding heavy water in subsequent steps, after incubation is performed, centrifugal washing is performed, samples are subjected to Raman detection, values of C-D / (C-D + C-H) are calculated respectively according to the obtained Raman spectra, a turning point of value decrease of C-D / (C-D + C-H) of the experimental groups of the antibiotics with different concentrations relative to the control groups is taken as a minimum inhibitory concentration of the antibiotics on the bacteria, and the minimum inhibitory concentration is compared with a breakpoint given in a CLSI susceptibility test standard to determine the bacterial sensitivity, intermediation or drug resistance. The method introduces a breakpoint comparison to more accurately give the sensitivity of the bacteria to the antibiotics.
Owner:上海氘峰医疗科技有限公司
In-situ rapid detection method for cell biological process
ActiveCN107741417ARealize in-situ rapid detectionQuick judgmentRaman scatteringHuman bodyActive cell
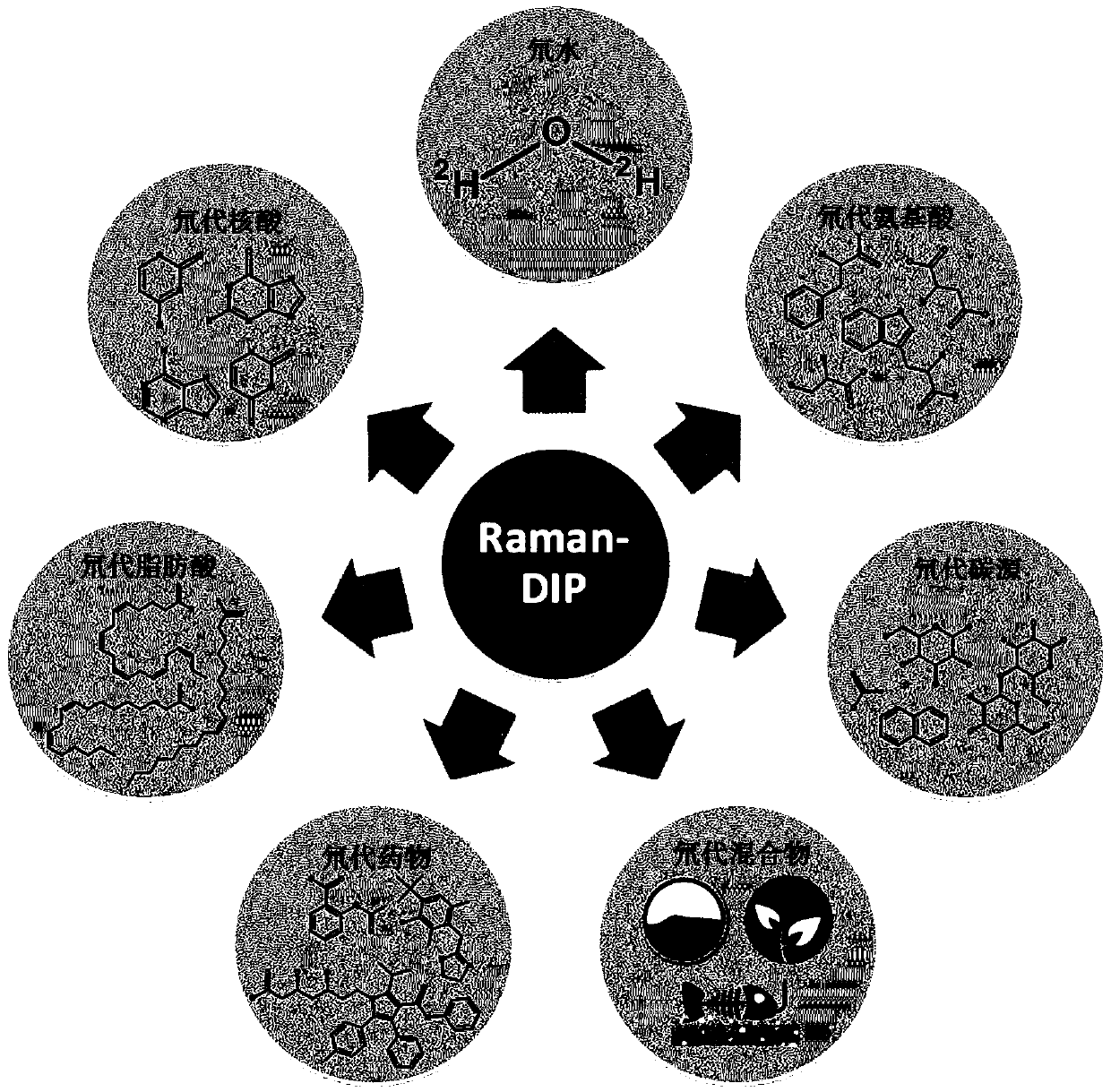
The invention relates to an in-situ rapid detection method for a cell biological process. After absorption, conversion, metabolism or storage for deuterium-containing matters based on active cells, in-situ marking for deuterium in cells can be realized in different stages of the cell biological process, the cells are detected through a Raman spectrum, whether deuterium exists in the cells is identified, and then the biochemical process, cell character or function related information about the cells is obtained. The method can be used for biological and medical fields such as bacterial infection drug susceptibility testing, environmental microbial metabolism and human body cell detection. Compared with the prior art, the method has the potential of detecting the activity and metabolism of the cells for most important biomolecules, and more complex deuteration matters are allowed to be researched and used, including natural products, medicines and a deuterization mixture containing various chemical matters. The method is extremely valuable for function research of the cells under natural conditions of single cell level.
Owner:上海氘峰医疗科技有限公司
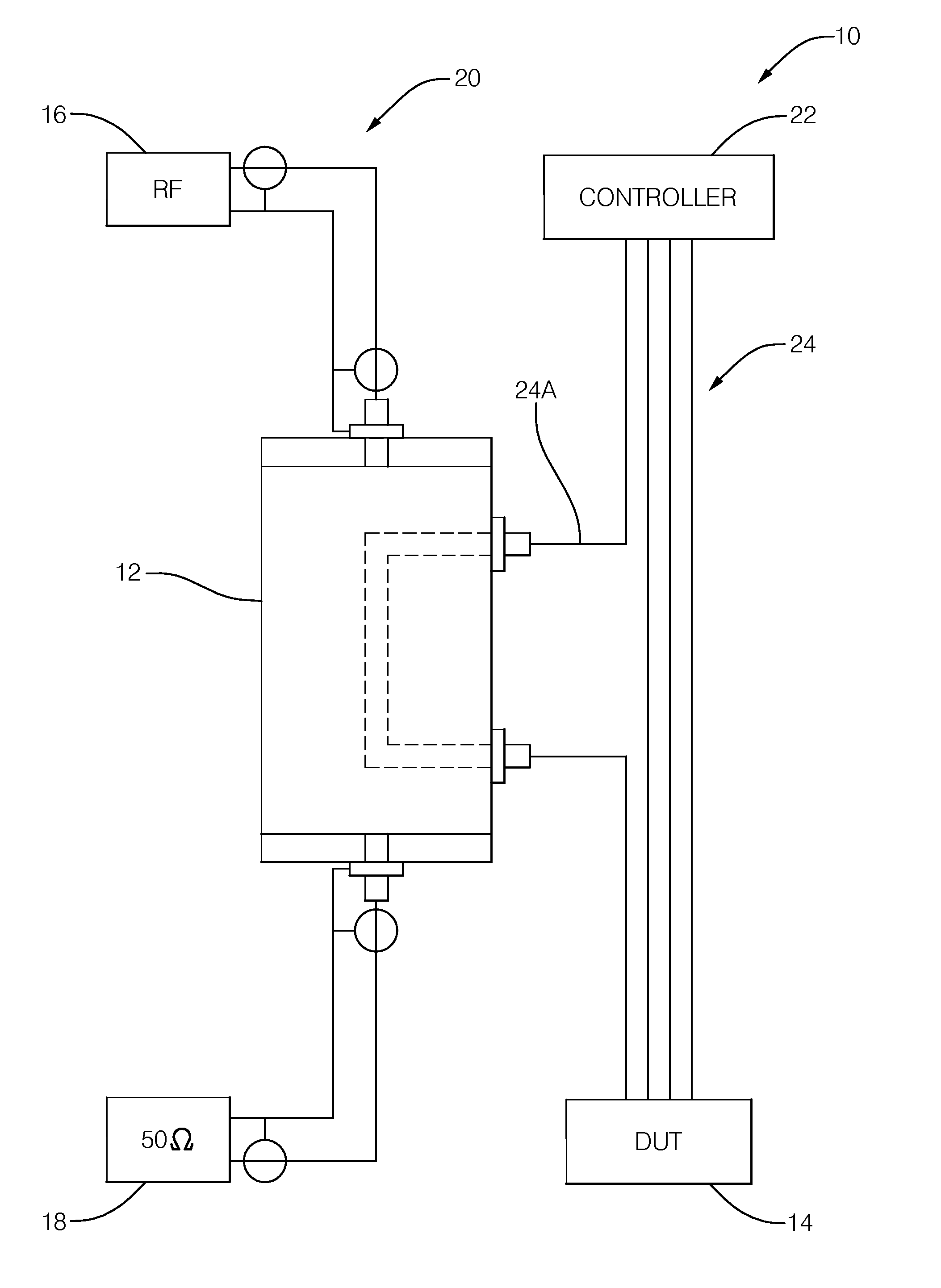
Electromagnetic interference (EMI) test apparatus
InactiveUS20150061698A1Resistance/reactance/impedenceTesting electric installations on transportEnergy couplingElectromagnetic interference
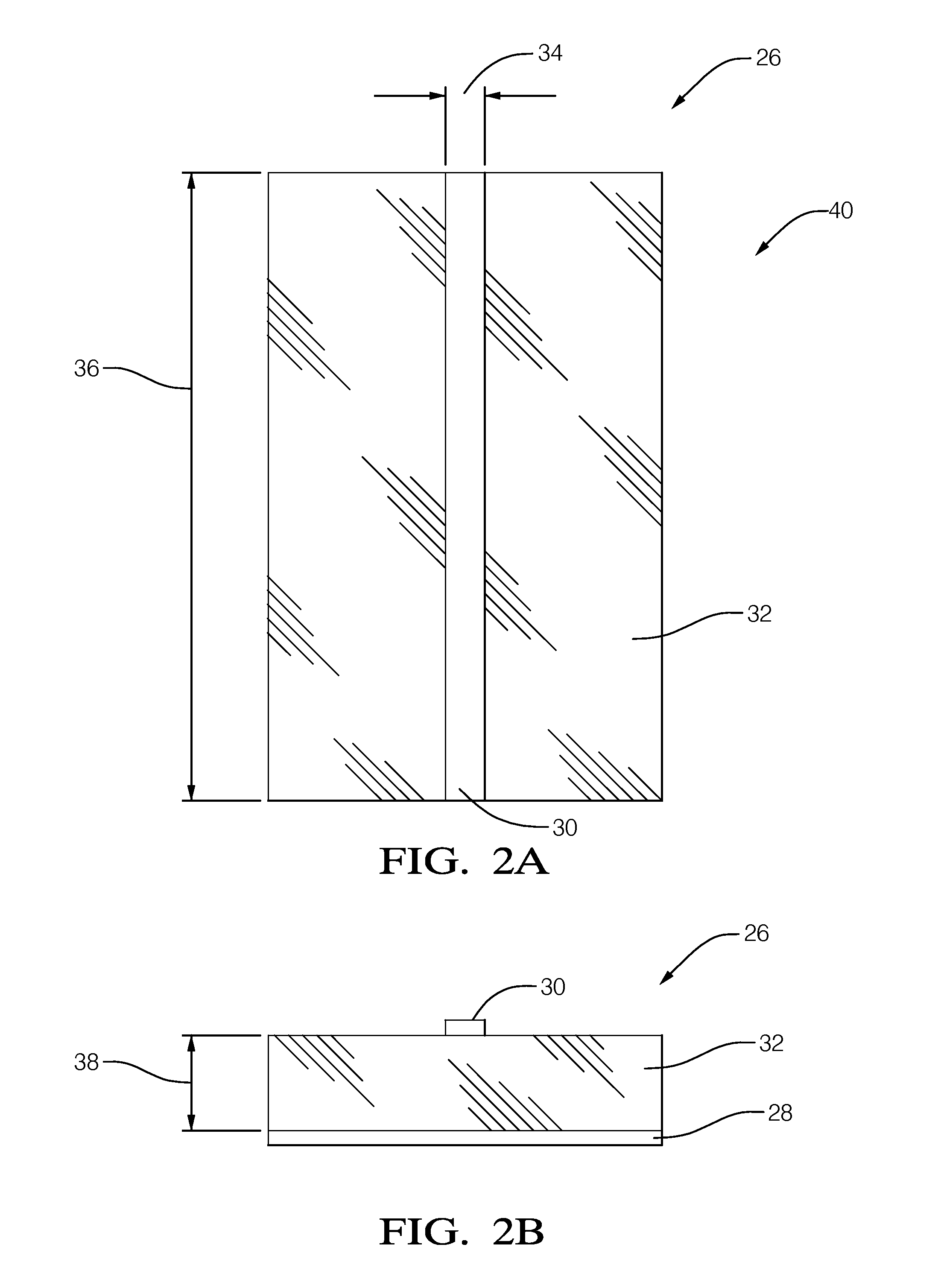
A radio-frequency (RF) energy coupling apparatus for electromagnetic interference (EMI) susceptibility testing of a device. The apparatus includes a ground-plane, a micro-strip, a first dielectric layer, a coupling-strip, and a second dielectric layer. The micro-strip overlies the ground-plane. The first dielectric layer is interposed between the ground-plane and the micro-strip. The combination of the ground-plane, the micro-strip, and the first dielectric layer cooperate to form a micro-strip transmission line configured to propagate RF energy from a RF generator to a termination load. The coupling-strip overlies the micro-strip opposite the first dielectric layer. The coupling-strip is configured to couple RF energy from the micro-strip to a harness wire connected to the device. The second dielectric layer is interposed between the coupling-strip and the micro-strip.
Owner:DELPHI TECH INC
High-throughput rapid antimicrobial susceptibility test kit for veterinary clinical application
PendingCN108866152AHigh speedEasy to observeMicrobiological testing/measurementAntibiotic YResazurin
The invention discloses a high-throughput rapid antimicrobial susceptibility test kit for veterinary clinical application and a method. The kit comprises an independently-packaged enrichment medium, an antimicrobial susceptibility test plate and reaction liquid; the enrichment medium is selected from a sterile 1640 medium or an MH medium with 1% sterile neonatal bovine serum and 0.1% sterile NAD added; the antimicrobial susceptibility test plate is a reaction plate coated with a plurality of antibiotics, each hole of the reaction plate is coated with an antibiotic, and the concentration of theantibiotics is 1-2 times of the maximum plasma concentration after the clinical administration of the antibiotics; and the reaction liquid is a 0.2% resazurin sterile solution. According to the kit and the method, an antimicrobial susceptibility test is carried out, the time for the result is short, more drugs are tested at a time, the kit and the method are easy to operate, and special instruments and equipment are not needed; the test process is a liquid-phase reaction, more accords with the physiological environment and is close to the actual production; and the result is visual, specifically, blue represents susceptible, red represents susceptible, and the closer the color is to blue, the more susceptible a drug is.
Owner:苏州艾可瑞动物检测技术服务有限公司 +2
Culture medium for rapid detection of mycobacterial growth by color change
InactiveUS20050079570A1Quick checkEasy to detectBacteriaMicrobiological testing/measurementMicroorganismAntimicrobial drug
The diagnosis of mycobacteria may be made by growing bacteria from clinical samples in a culture media. The culture medium enables rapid detection of mycobacterial growth by changing its color. It also differentiates mycobacterial growth from contamination by changing to a different color when other species of microorganisms grow. Different types of culture media may be obtained by adding antimicrobial drugs to either obtain a medium selective for mycobacteria or a medium for species differentiation or susceptibility testing of drugs.
Owner:SALUBRIS
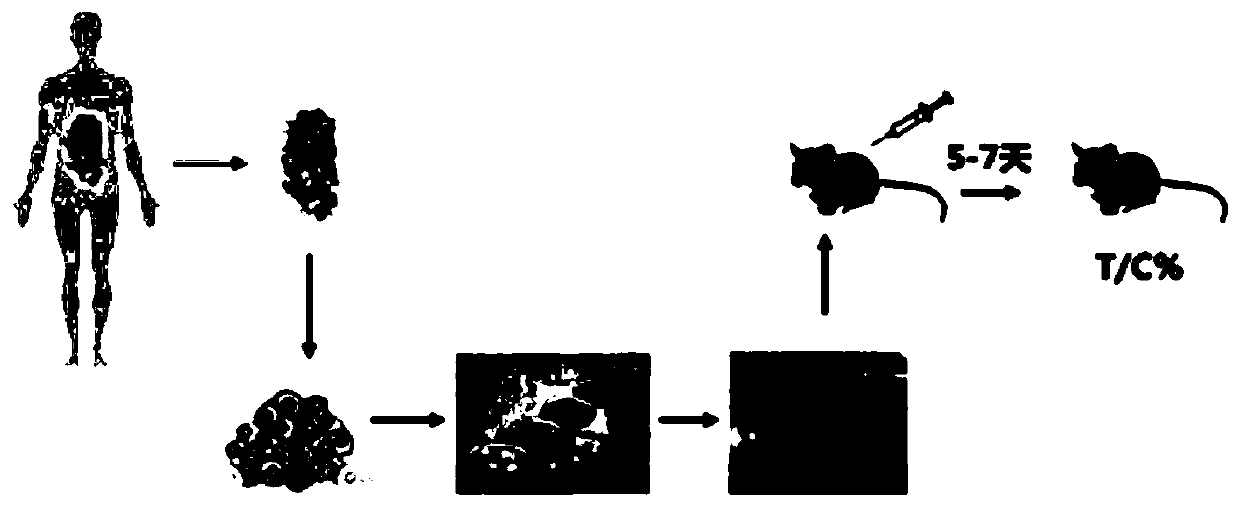
Tumor in-vitro culture method and clinical chemotherapeutic drug screening method
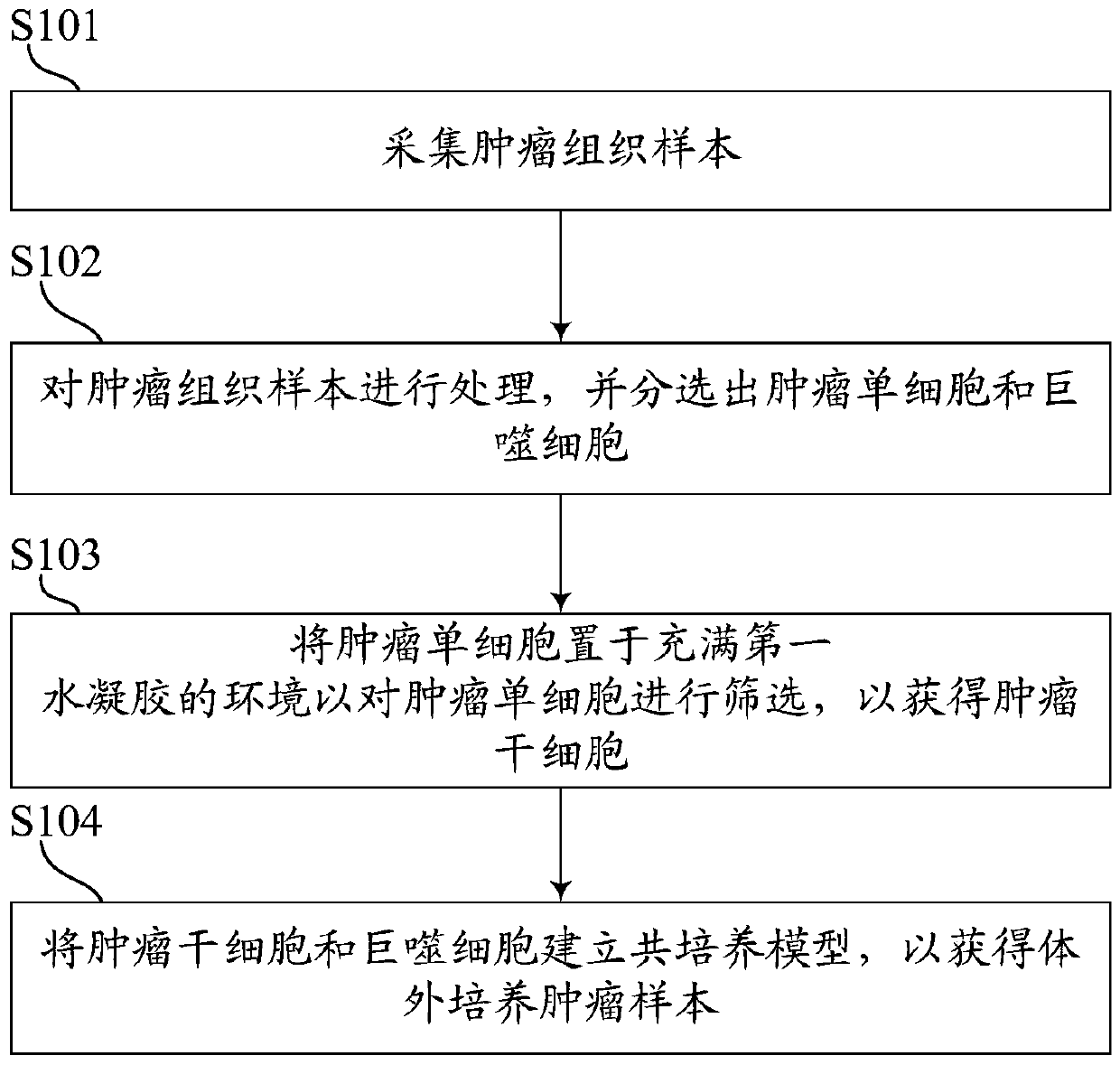
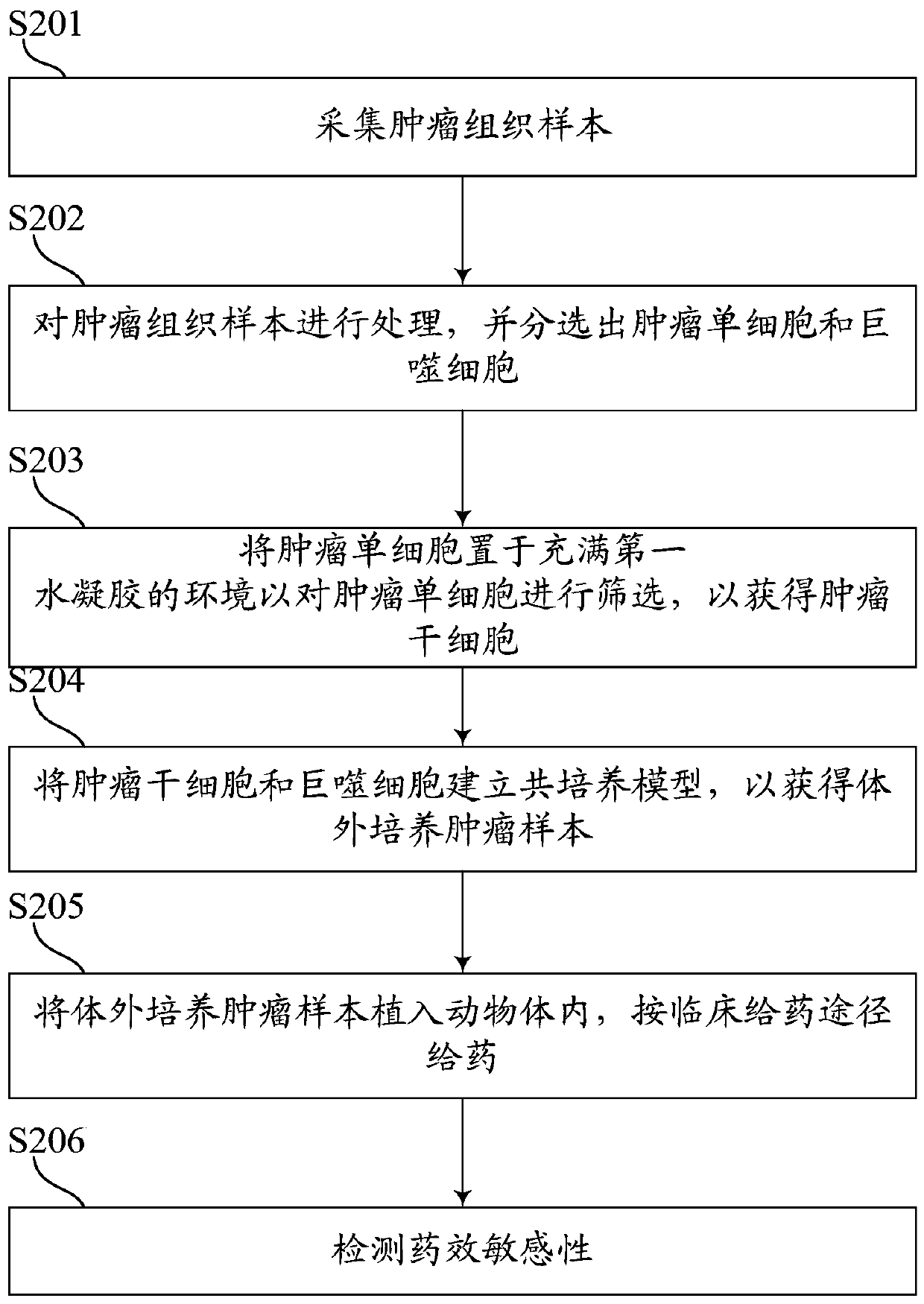
PendingCN109749999ALow costShorten tumor formation timeTumor/cancer cellsIn-vivo testing preparationsScreening methodWilms' tumor
The invention relates to the field of drug efficacy screening, and particularly discloses a tumor in-vitro culture method. The tumor in-vitro culture method includes the steps that tumor tissue samples are collected; the tumor tissue samples are treated, and single tumor cells and macrophages are sorted; the single tumor cells are placed in an environment full of hydrogel and then are screened toobtain tumor stem cells; the tumor stem cells and the macrophages are adopted to build a co-culture model to obtain in-vitro culture tumor samples. In this way, the tumor stem cells with high proliferation and differentiation capabilities can be screened out in vitro, therefore, the tumor stem cells are implanted into the animal body, the tumor formation time can be greatly shortened, the tumor formation rate is increased, and then susceptibility testing is conducted; therefore, the progress of susceptibility testing is speeded up, and time for treating patients is bought.
Owner:NANTONG UNIVERSITY
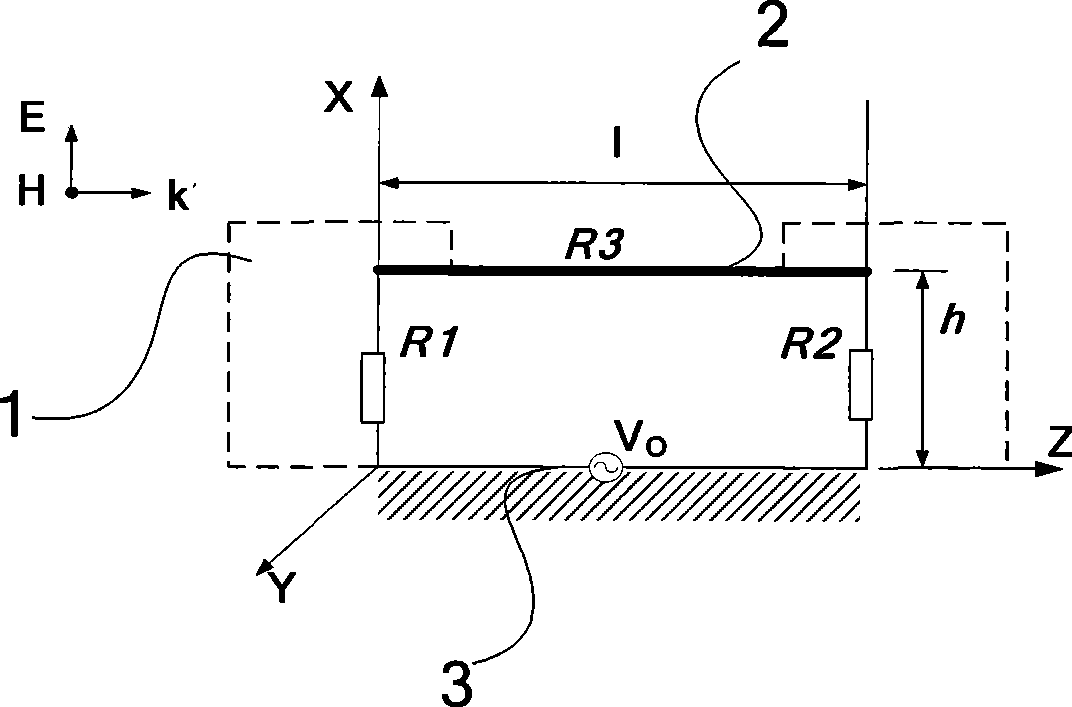
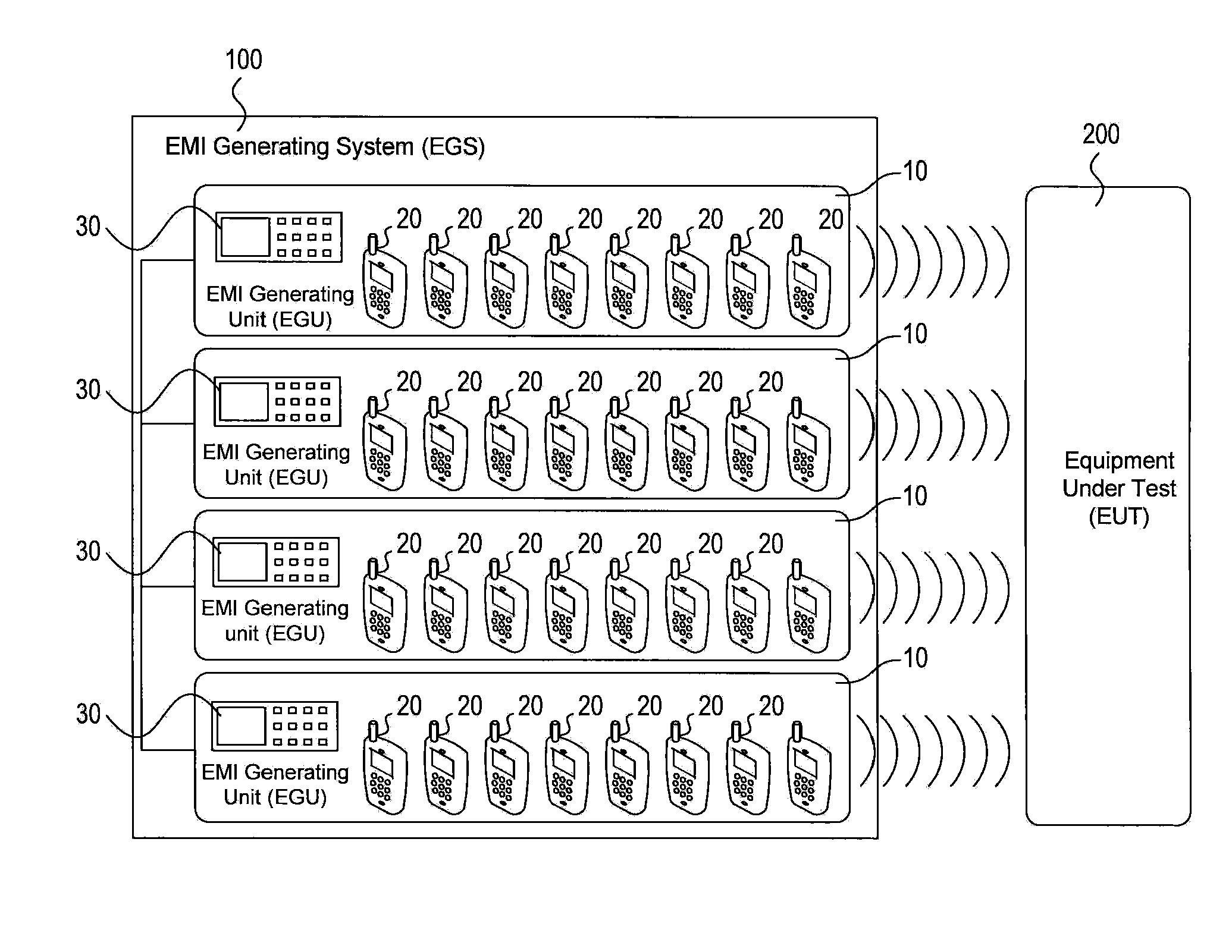
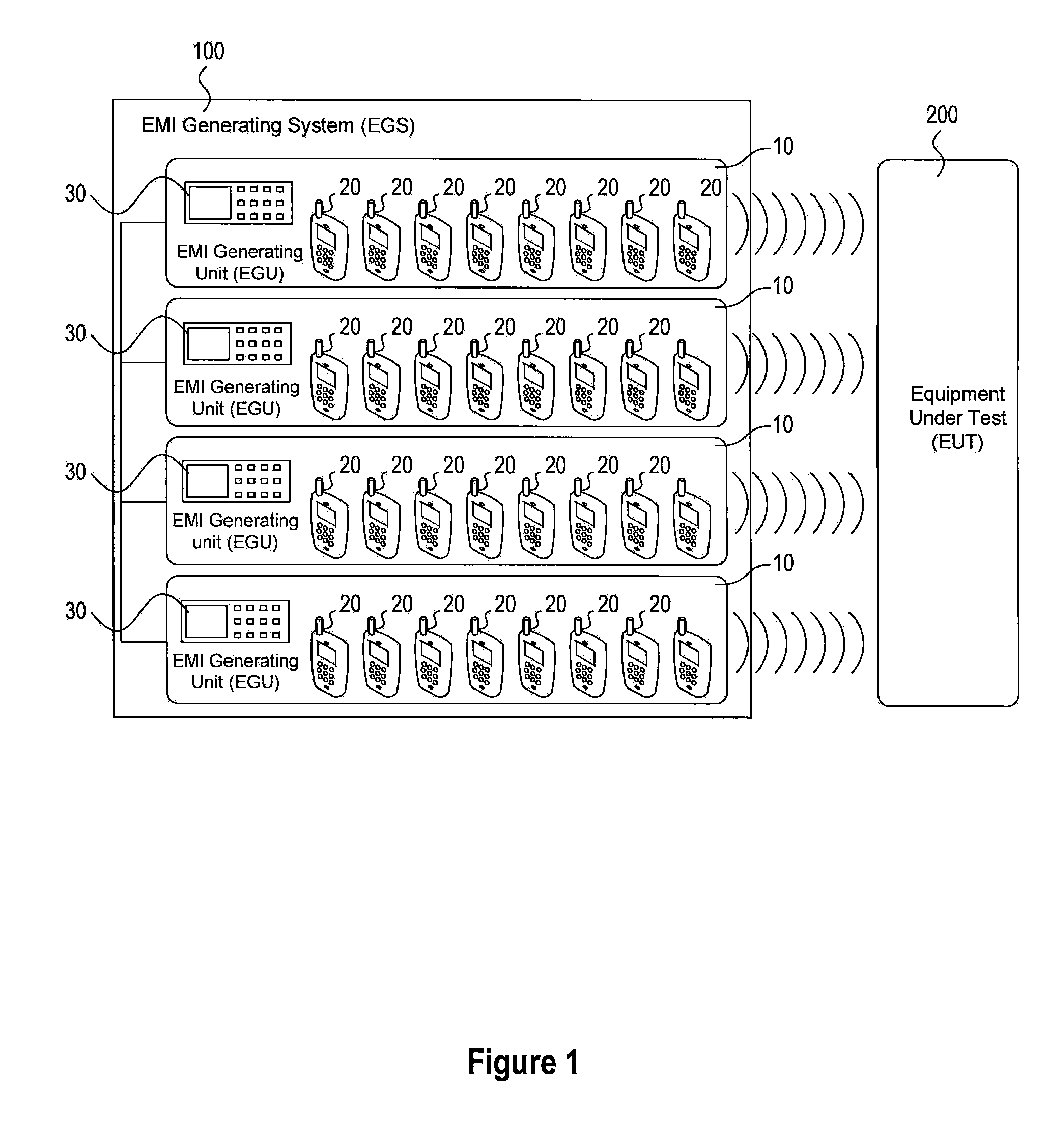
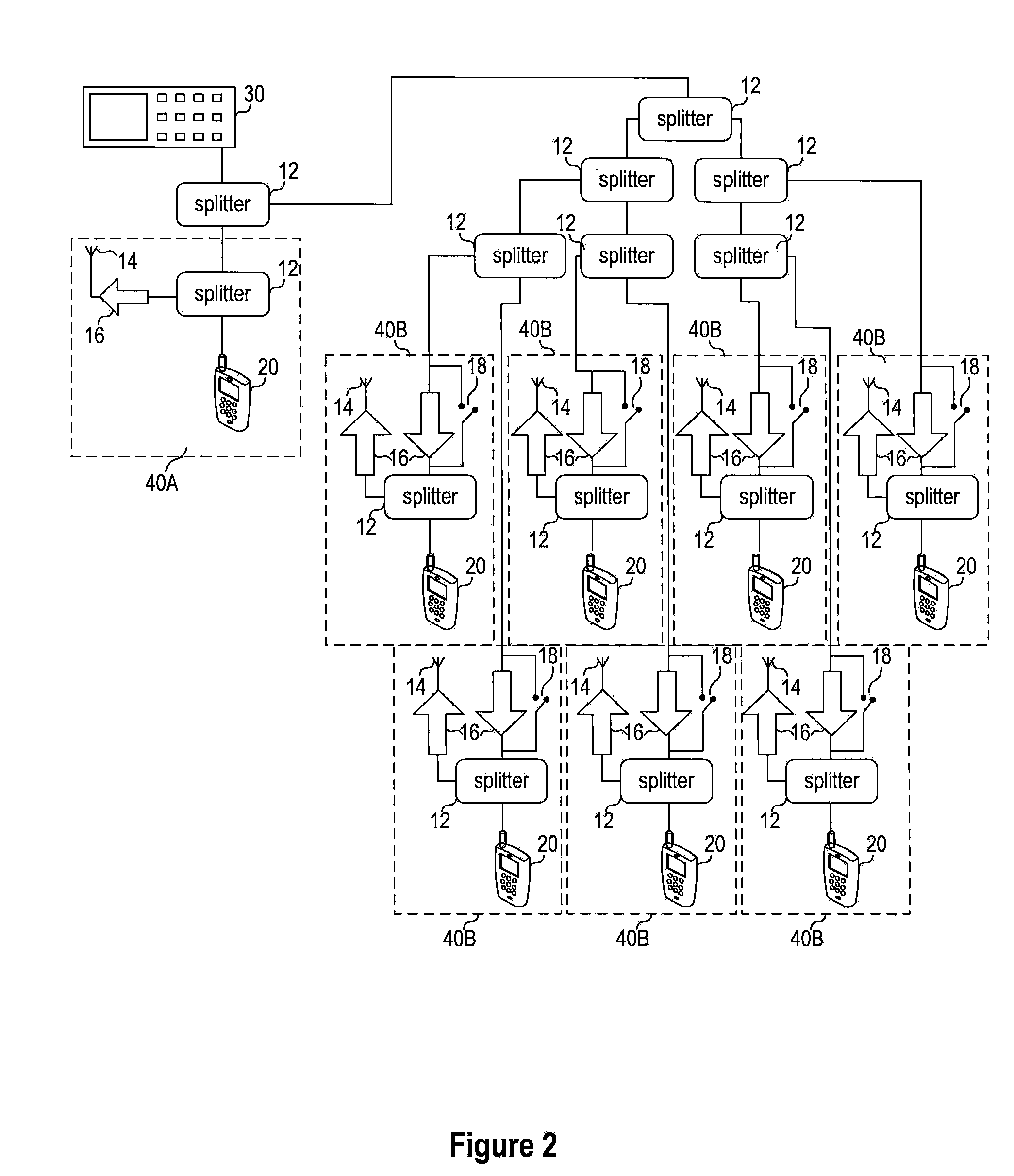
Systems and methods for conducting EMI susceptibility testing
ActiveUS20100283481A1Noise figure or signal-to-noise ratio measurementTransmission monitoringSusceptibility testingEmbedded system
System and methods for performing EMI susceptibility testing of a device is disclosed. A system may include an EMI generation unit that includes a plurality of EMI generating devices, where each EMI generating device generates EMI having substantially similar characteristics relative to EMI generated by other EMI generating devices in the system. Each EMI generating device is controlled by a controller that is configured to emulate at least partly a live cellular network.
Owner:FEDERAL EXPRESS CORP US
Fast bacteria detection and antibiotic susceptibility test by precision tracking of bacterial cells
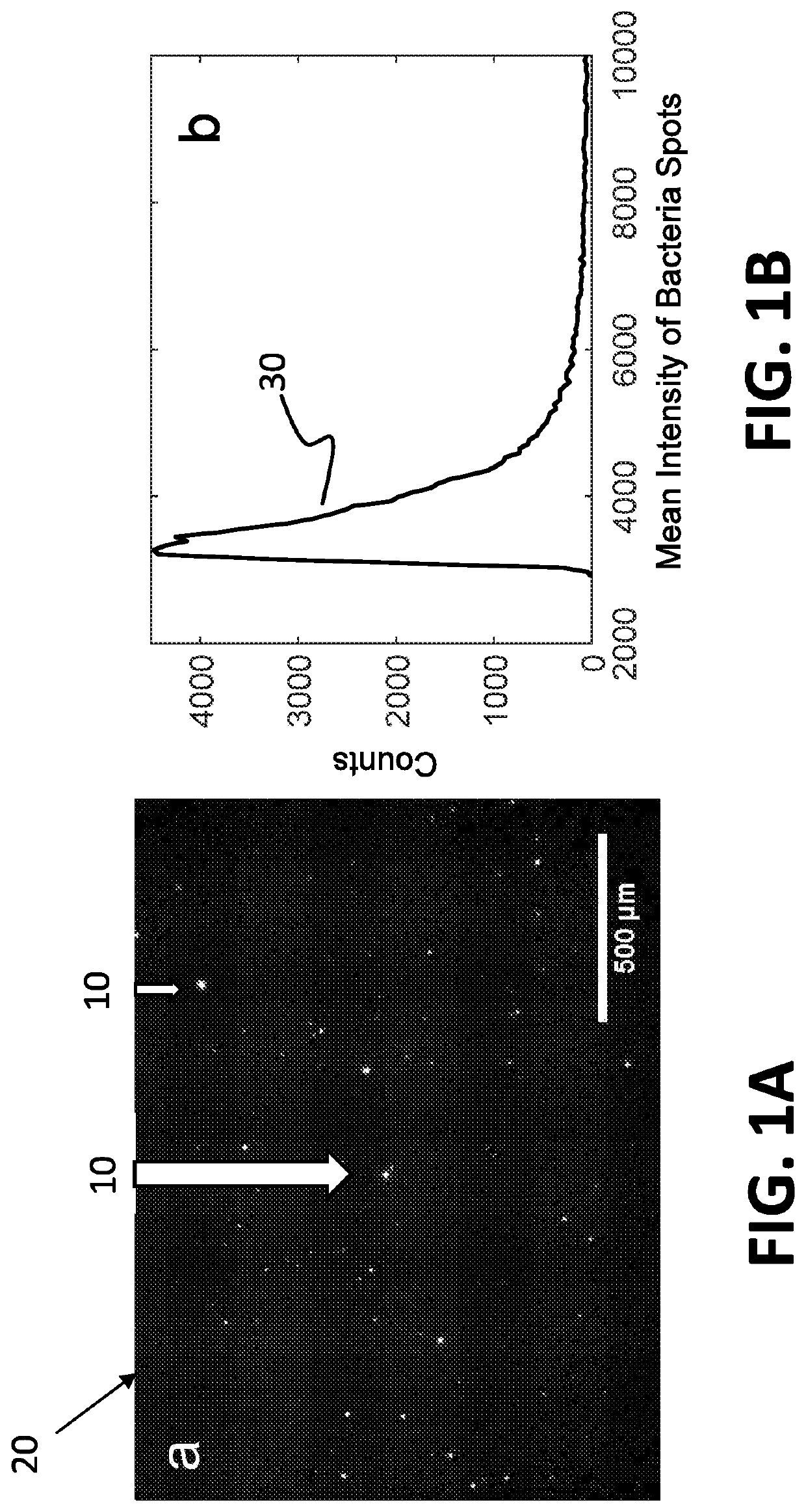
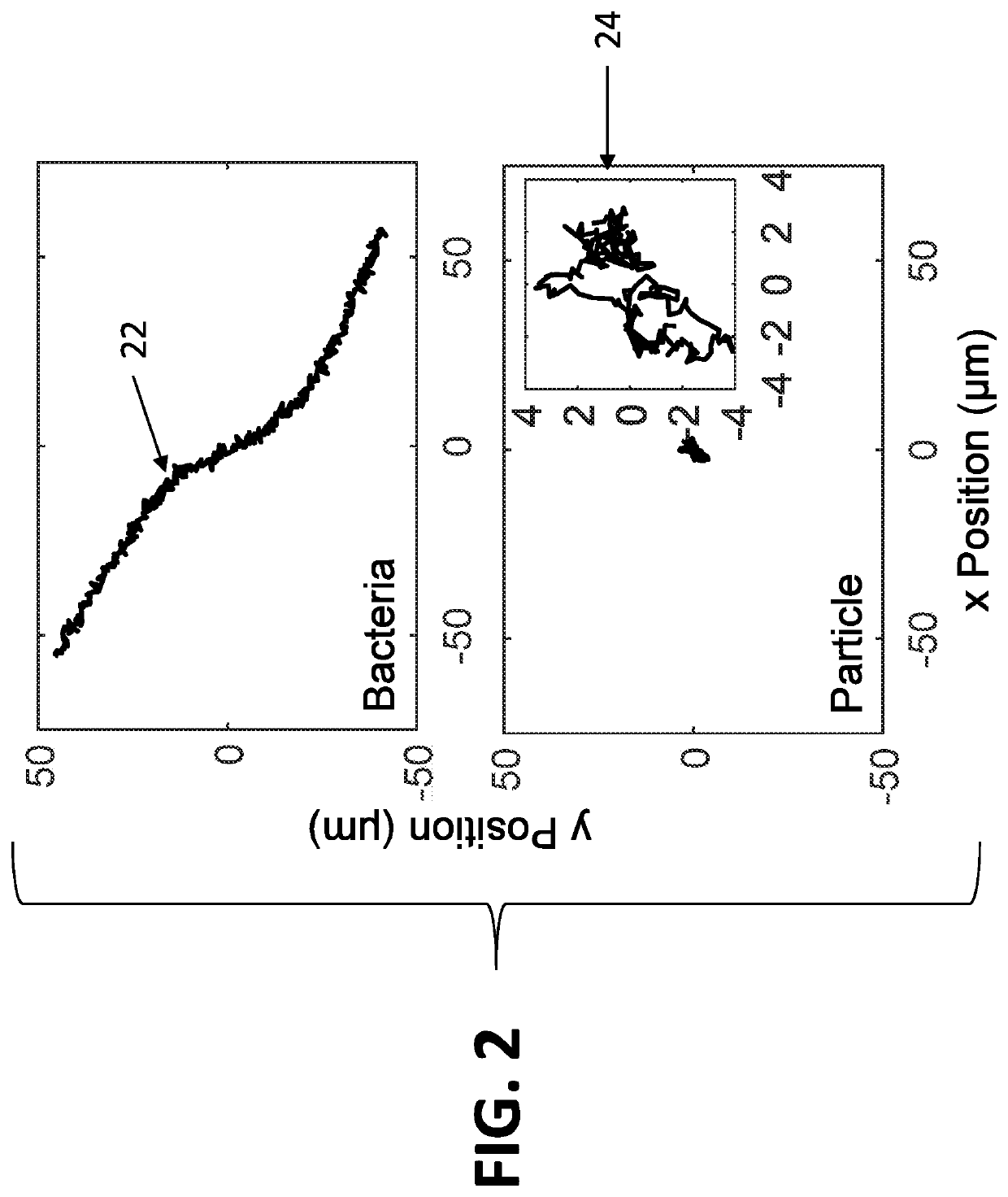
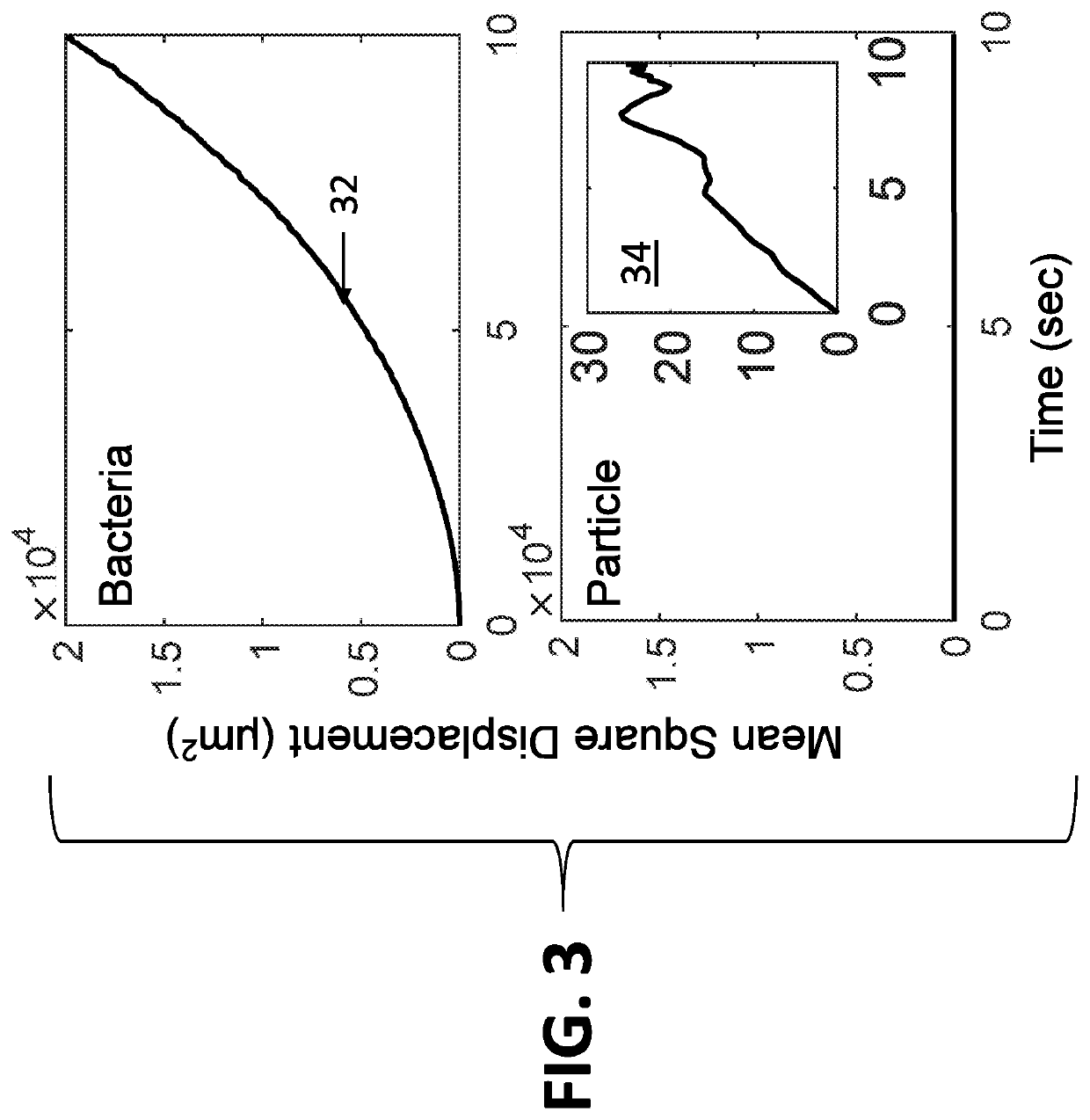
ActiveUS20210065368A1Minimal sample preparationMaximal phenotypic feature extractionImage enhancementTelevision system detailsControl signalArtificial intelligence
A system for identification of bacterial cells in free solution in a sample. A sample handler is adapted to position the sample. A light source illuminates a large volume of the sample. An imager is located to receive light scattered from the sample. A computer it is coupled to receive data transmitted from the imager. A controller is coupled to send control signals to the sample handler and the computer. The imager processes the scattered light to form images of the bacteria and transmits bacteria image information to the computer, wherein the bacteria image information includes intensity values and position data for the bacteria images from which the computer determines the presence of bacteria.
Owner:ARIZONA STATE UNIVERSITY
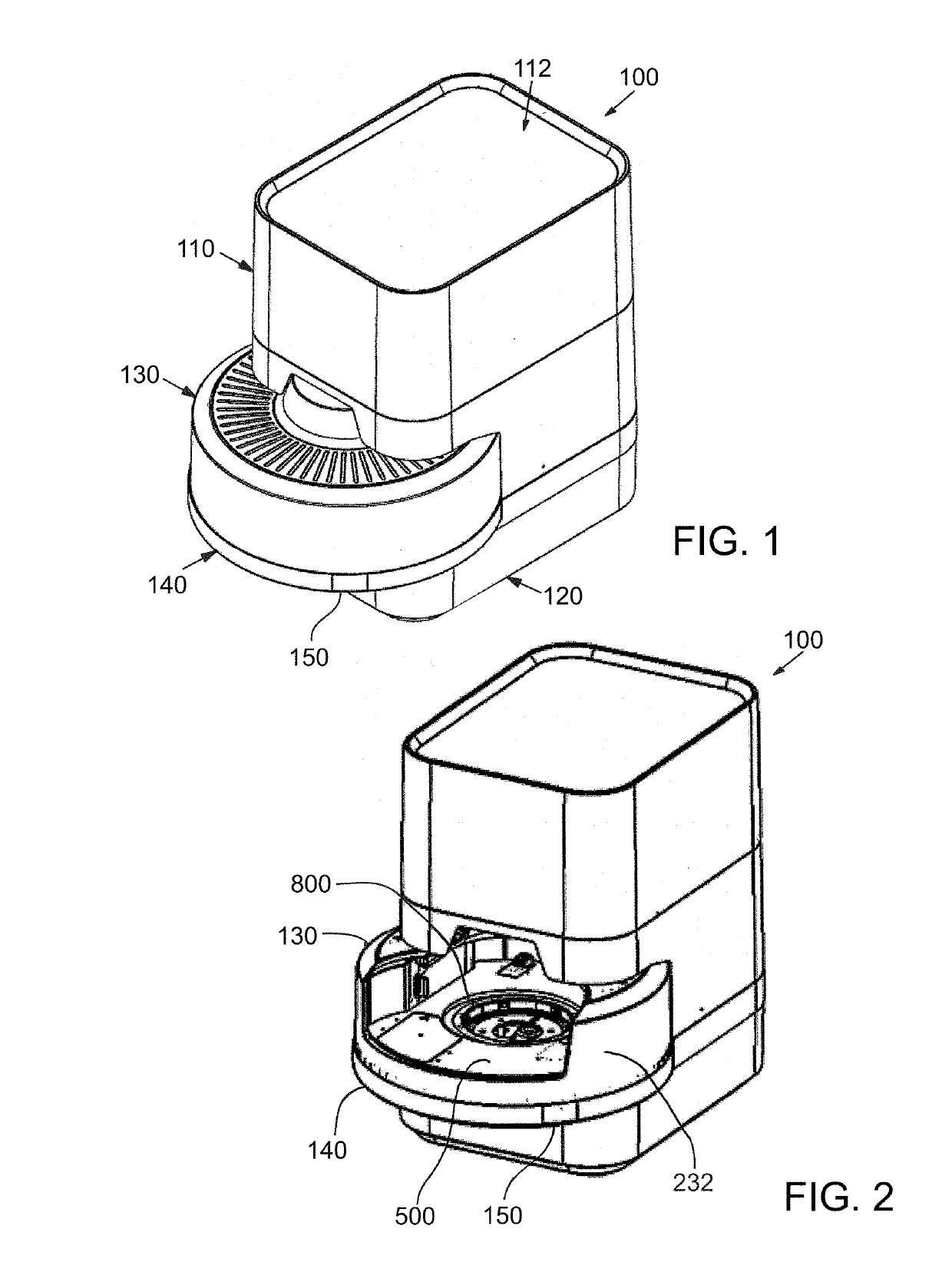
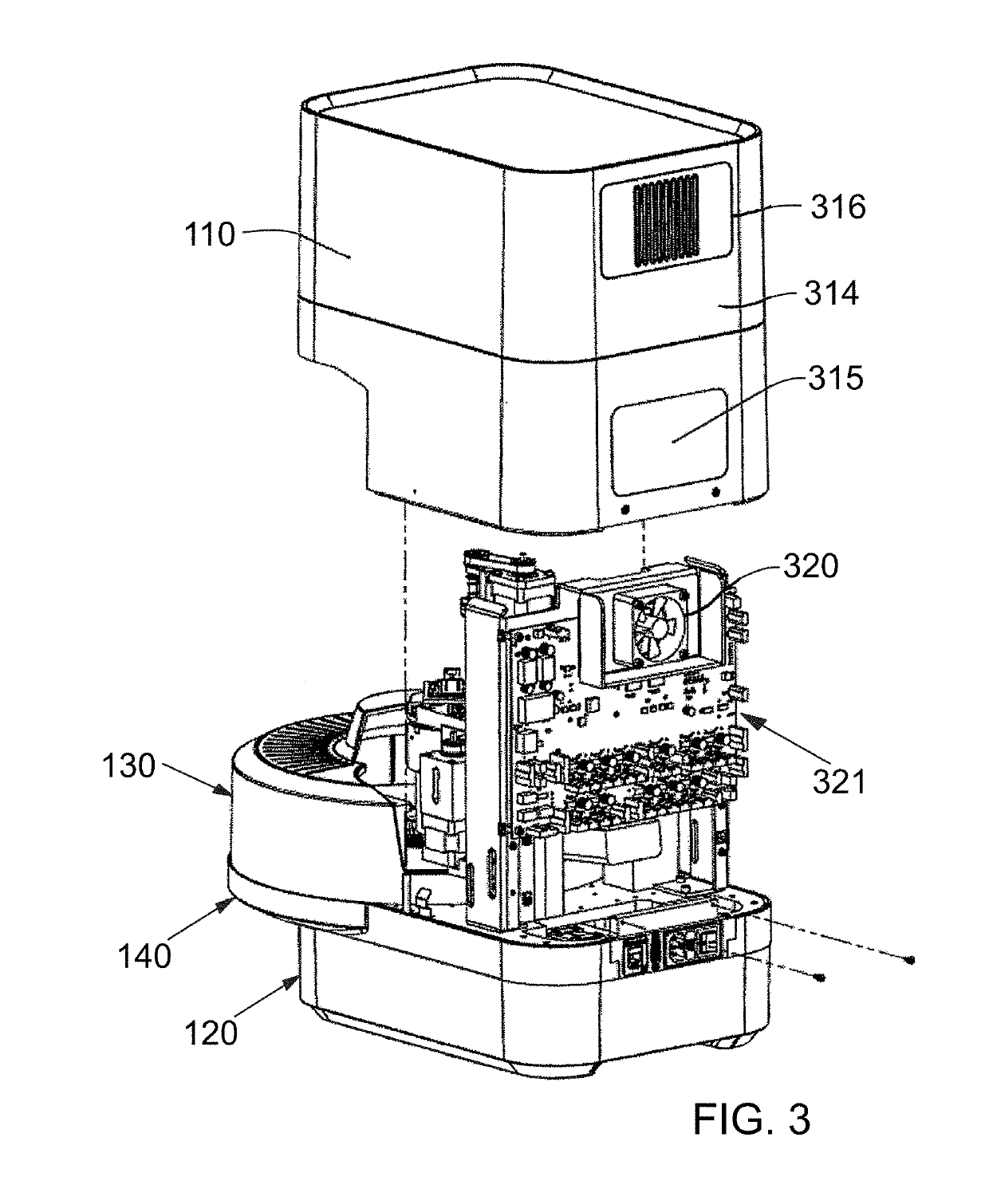
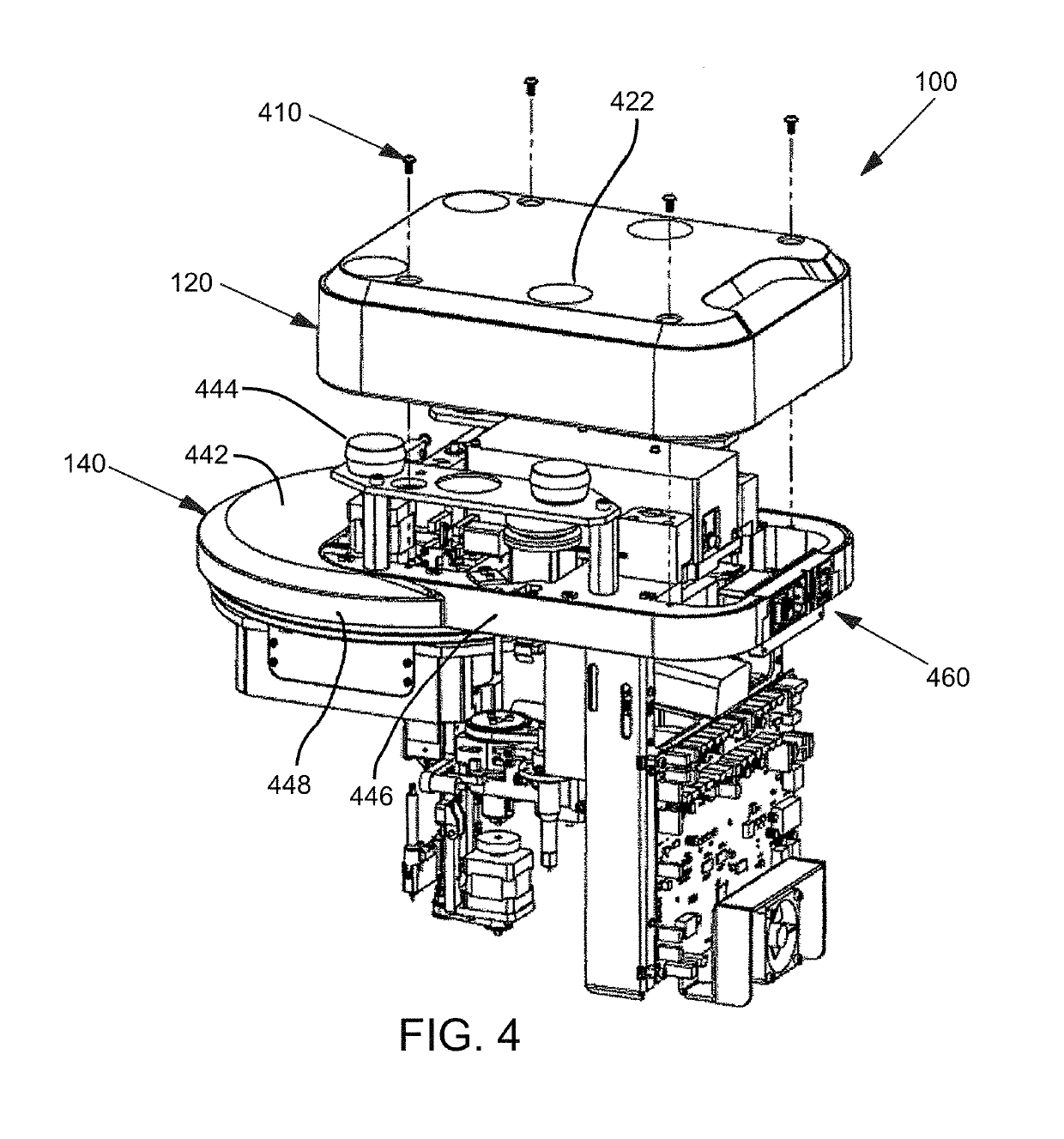
Instrument and system for rapid microorganism identification and antimicrobial agent susceptibility testing
ActiveUS10253355B2Bioreactor/fermenter combinationsTelevision system detailsMicroorganismMicrobial Susceptibility
A system for automated microorganism identification and antibiotic susceptibility testing comprising a reagent cartridge, a reagent stage, a cassette, a cassette, stage, a pipettor assembly, an optical detection system, and a controller is disclosed. The system is designed to dynamically adjust motor idle torque to control heat load and employs a fast focus process for determining the true focus position of an individual microorganism. The system also may quantify the relative abundance of viable microorganisms in a sample using dynamic dilution, and facilitate growth of microorganisms in customized media for rapid, accurate antimicrobial susceptibility testing. Automated quality control test components and methods of their use are also disclosed.
Owner:ACCELERATED MEDICAL DIAGNOSTICS INC
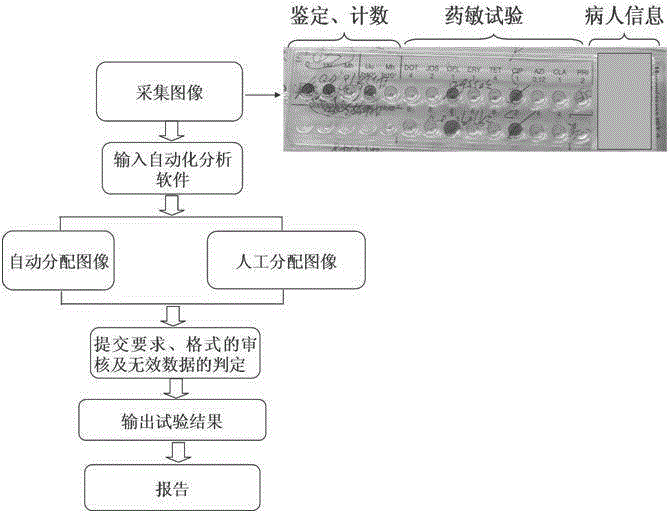
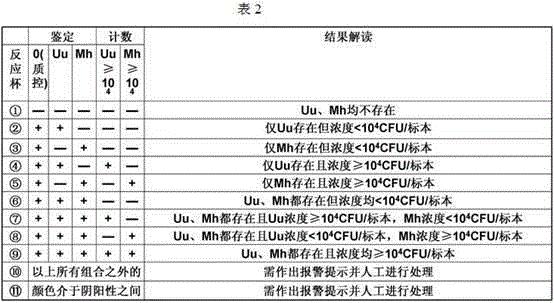
Automatic detection method for mycoplasma culture, identification, counting and drug sensitivity results
InactiveCN105296592ASolving automation problemsPerfect auto saveMicrobiological testing/measurementMicroorganism based processesMycoplasma cultureAntibiotic drug
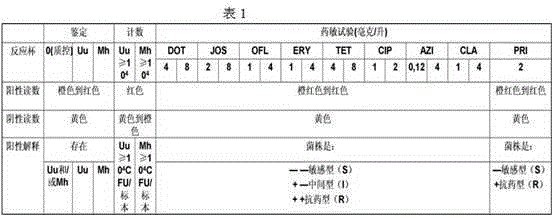
The invention discloses an automatic detection method for mycoplasma culture, identification, counting and drug sensitivity results. The method comprises the following steps: based on a visual image acquisition system and corresponding automatic analysis software, converting an optical signal of an image into a digital signal by using the visual image acquisition system; displaying images of mycoplasma culture, identification, counting and drug sensitivity results on a computer screen; allowing the automatic analysis software to automatically analyze permutation and combination of different colors according to preset conditions with the colors of the images and color collocation and combination as indexes; and displaying qualitative identification results of ureaplasma (Uu) and / or Mycoplasma hominis (Mh), semi-quantitative counting results of Uu and / or Mh and drug sensitive test results of antibiotics. The method provided by the invention can overcome problems in automatic monitoring of mycoplasma culture, identification, counting and drug sensitivity results, improves automatic storage of original pictorial information so as to facilitate retrospective query and check of an original result database and can rapidly, timely and accurately analyze and process large-batch original result data.
Owner:ZHEJIANG UNIV
Method and system for identifying bacteria and analyzing drug sensitivity
ActiveCN103760159AIdentification/Drug Susceptibility Test AccuracyImprove work efficiencyMaterial analysis by observing effect on chemical indicatorMicroorganismBacteria identification
The invention provides a method and a system for identifying bacteria and analyzing drug sensitivity, relating to the technical field of medical treatment reagent type detection. Through digital and automatic comparative analysis, a required result is obtained. Compared with the prior art, the invention provides a system for automatically identifying bacteria / experimenting drug sensitivity for the medical microorganism examination work so that the bacteria identification / drug experimenting is more accurate, simpler, micro and rapid.
Owner:聊城市鑫科金诺医疗器械有限公司
Device for tumor accurate treatment method based on CTC circulating tumor cells
PendingCN106957799APrecision Healing EffectAddressing drug resistanceBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenTherapeutic effect
The invention provides a device for a tumor accurate treatment method based on CTC circulating tumor cells. The device comprises CTC circulating tumor cell separation equipment for separating the CTC circulating tumor cells from added peripheral blood, CTC circulating tumor cell detection equipment for detecting the separated CTC circulating tumor cells, closable CTC circulating tumor cell culture equipment for carrying out in-vitro culture and proliferation on the separated CTC circulating tumor cells, and drug sensitivity detection equipment for carrying out a drug sensitivity experiment on the cultured CTC circulating tumor cells. According to the device, an in-vitro drug sensitivity test is carried out on the CTC circulating tumor cells of a patient and the device has an individualized accurate treatment effect on the patient; a parallel drug sensitivity test method is carried out and one preferred clinical medication scheme can selected each time so that the tumor cells are attacked by different types of medicines all the time, and furthermore, the medicine adaptation capability of tumors become weak and the problem of drug resistance caused by single clinical medication can be avoided.
Owner:郭昊伦
Culture medium for rapid detection of mycobacterial growth by color change
InactiveUS7754485B2Quick checkEasy to detectBacteriaMicrobiological testing/measurementBacteroidesMicroorganism
The diagnosis of mycobacteria may be made by growing bacteria from clinical samples in a culture media. The culture medium enables rapid detection of mycobacterial growth by changing its color. It also differentiates mycobacterial growth from contamination by changing to a different color when other species of microorganisms grow. Different types of culture media may be obtained by adding antimicrobial drugs to either obtain a medium selective for mycobacteria or a medium for species differentiation or susceptibility testing of drugs.
Owner:SALUBRIS
Betaines as adjuvants to susceptibility testing and antimicrobial therapy
The present invention is directed to methods of antimicrobial therapy for a patient infected with, or suspected of being infected with a microorganism that has mycolic acid structures in its outer membrane, said method comprising coadministering a betaine-like detergent and an antibiotic to said patient in an amount and for a length of time sufficient to kill said microorganism.
Owner:INTEGRATED RES TECH
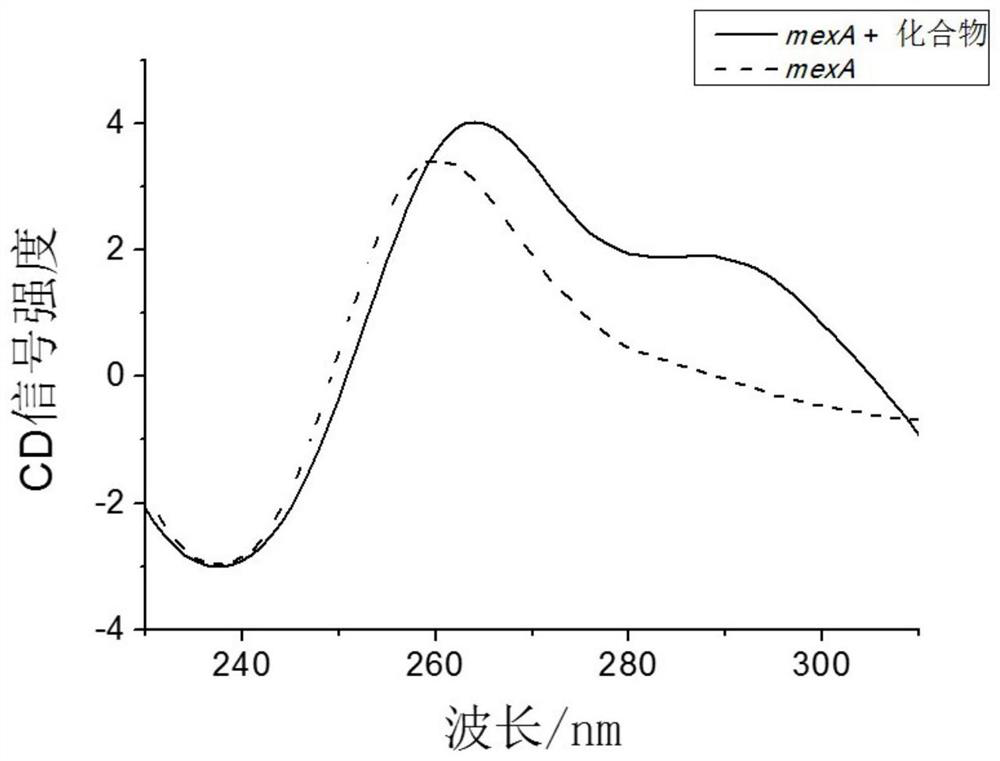
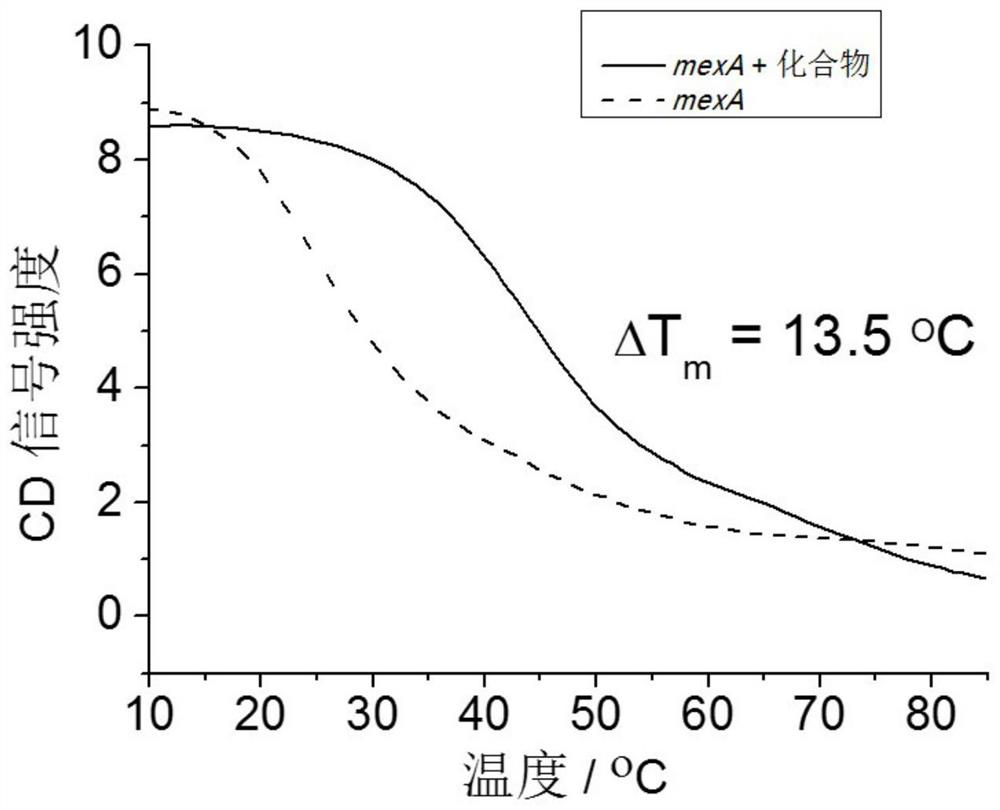
Application of 2,6-bis(2-benzimidazolyl) pyridine in preparation of carbapenem-resistant pseudomonas aeruginosa infection drug
ActiveCN111658646AInhibition of active effluxLow inhibitory concentrationAntibacterial agentsOrganic active ingredientsMeropenemBisbenzimidazole
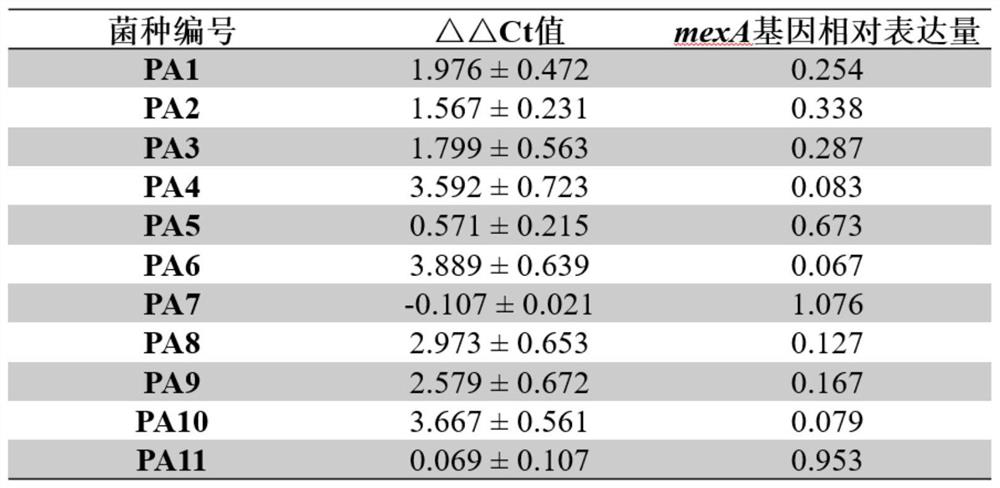
The invention discloses application of 2,6-bis(2-benzimidazolyl) pyridine in preparation of a carbapenem-resistant pseudomonas aeruginosa infection drug. Through circular dichroism, fluorescent real-time quantitative PCR and other methods, it is found that 2, 6-bis (2-benzimidazolyl) pyridine can form a G-quadruplex structure with a core sequence of a MexA gene in pseudomonas aeruginosa, thereby inhibiting expression of the MexA gene. A susceptibility testing verifies that 2, 6-bis (2-benzimidazolyl) pyridine can reduce the drug resistance of carbapenem-resistant pseudomonas aeruginosa to meropenem. When the usage amount of the 2, 6-bis (2-benzimidazolyl) pyridine is 5 <mu>M, the minimum inhibitory concentration of the carbapenem-resistant pseudomonas aeruginosa strain can be reduced to 2<mu> g / ml, and the effect of treating carbapenem-resistant pseudomonas aeruginosa infection by using meropenem is achieved.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Fiber-optic gyroscopemagnetic susceptibility testing system
InactiveCN103968857AHigh magnetic field accuracyImprove stabilitySagnac effect gyrometersFiberGyroscope
The invention relates to a fiber-optic gyroscopemagnetic susceptibilitytesting system. The system comprises a magnetic field generation device, a support, a vibration isolation platform, a power supply system, a gyroscope interface unit, a measurement and control computer and a direct-current stabilized power supply, wherein the magnetic field generation device is connectedwiththe direct-current stabilized power supply; thesupport is arranged in the magnetic field generation device, and a fiber-optic gyroscope is placed on the support; the vibration isolation platform is arranged at the bottom of the support; one end of the gyroscope interface unit is connected with thefiber-optic gyroscope, and the other end is connected with the measurement and control computer; the power supply system is connected with the gyroscope interface unit, the measurement and control computer and the direct-current stabilized power supplyrespectively; and the measurement and control computer is further connected onto the direct-current stabilized power supply. Thefiber-optic gyroscopemagnetic susceptibility testing system has the advantages of simple structure, capability of generating a three-dimensional magnetic field, accuracy in testing, conveniencein control and the like.
Owner:PEOPLES LIBERATION ARMY ORDNANCE ENG COLLEGE
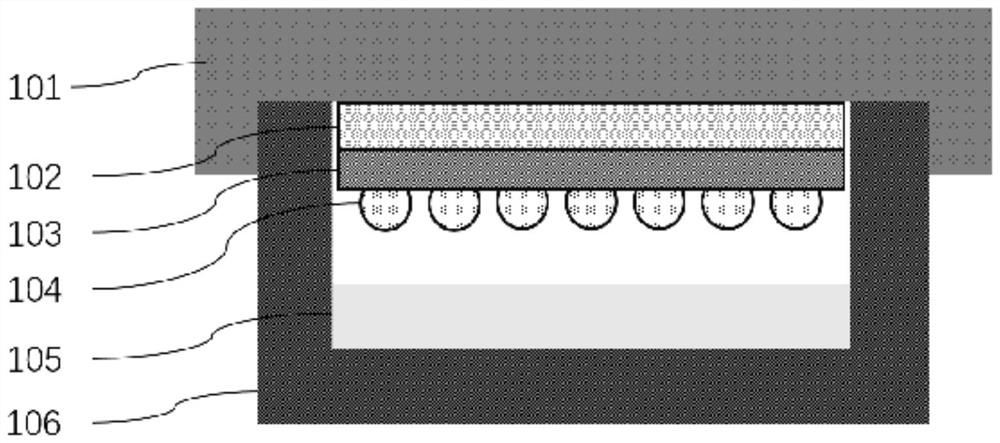
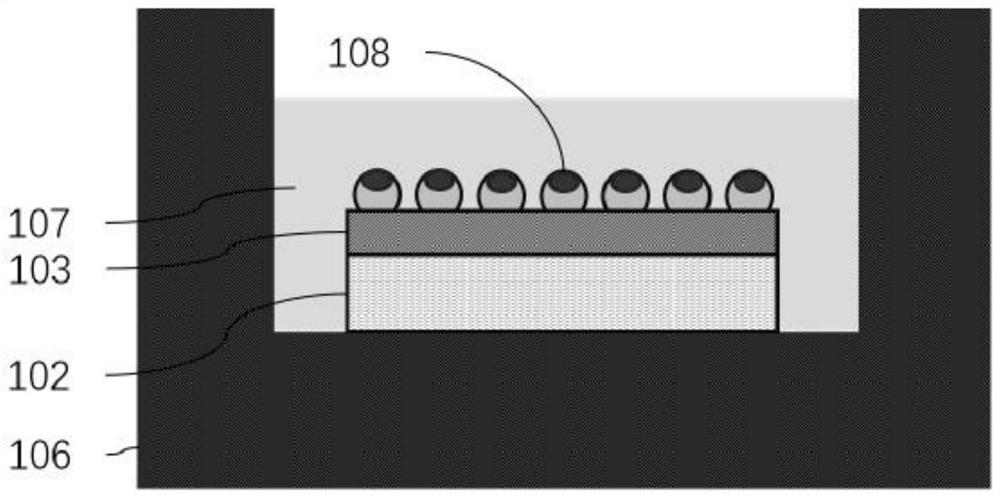
Novel bioactivity testing structure for single cell tracking using gelling agents
ActiveUS20170233786A1Rapid and simple drug susceptibility testingRapid and accurate and simple testingBioreactor/fermenter combinationsBiological substance pretreatmentsPre treatmentSusceptibility testing
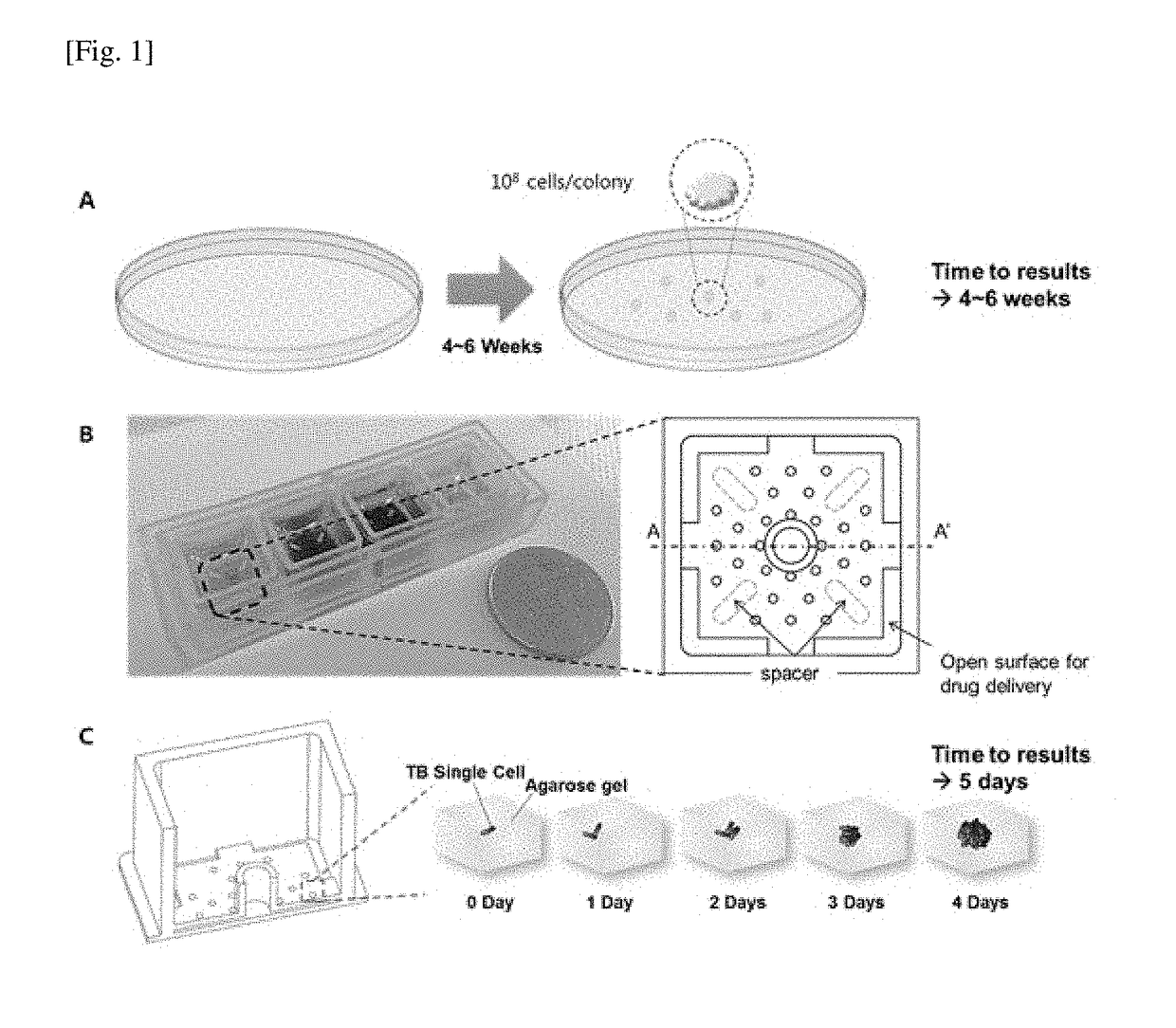
The present invention relates to a novel bioactivity testing structure for single cell tracking using a gelling agent and a bioactivity testing system including the testing structure. The present invention also relates to bioactivity testing, drug susceptibility testing, antibiotic screening, and diagnostic methods using the testing structure. The bioactivity testing structure of the present invention enables very rapid and simple drug susceptibility testing of bacteria, particularly Mycobacterium tuberculosis, drug screening, and bacterial diagnosis. Particularly, the use of the testing structure enables DST and diagnosis of bacteria only by pretreatment without the need to concentrate human sputum samples irrespective of inoculum effect, ensuring rapid, accurate, and simple testing compared to conventional tuberculosis diagnosis or DST systems. In addition, the testing structure of the present invention simultaneously enables the diagnosis and drug susceptibility testing of tuberculosis. Therefore, the present invention provides an effective alternative to the prior art.
Owner:QUANTA MATRIX
Method of drying drug sensitivity test card
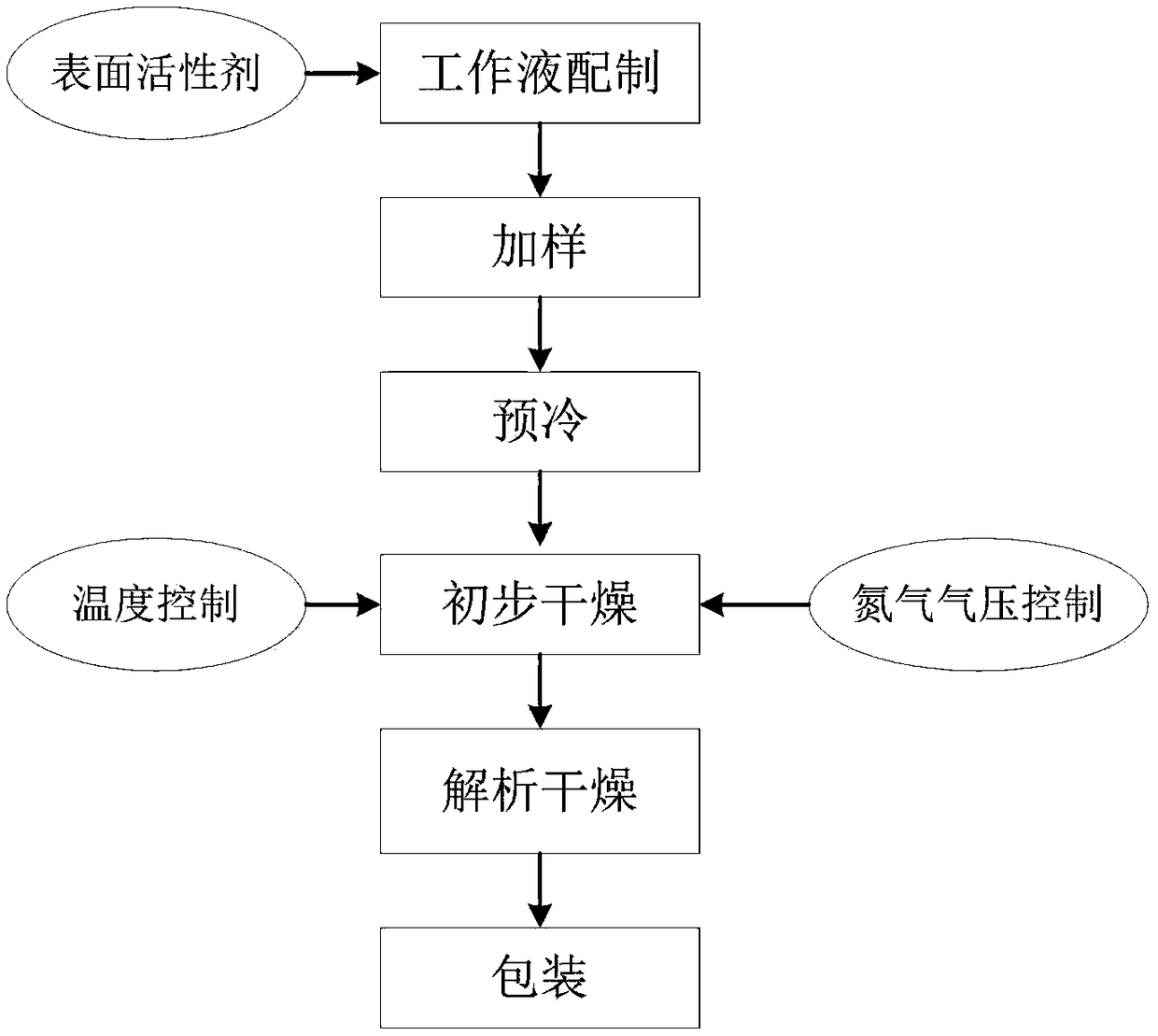
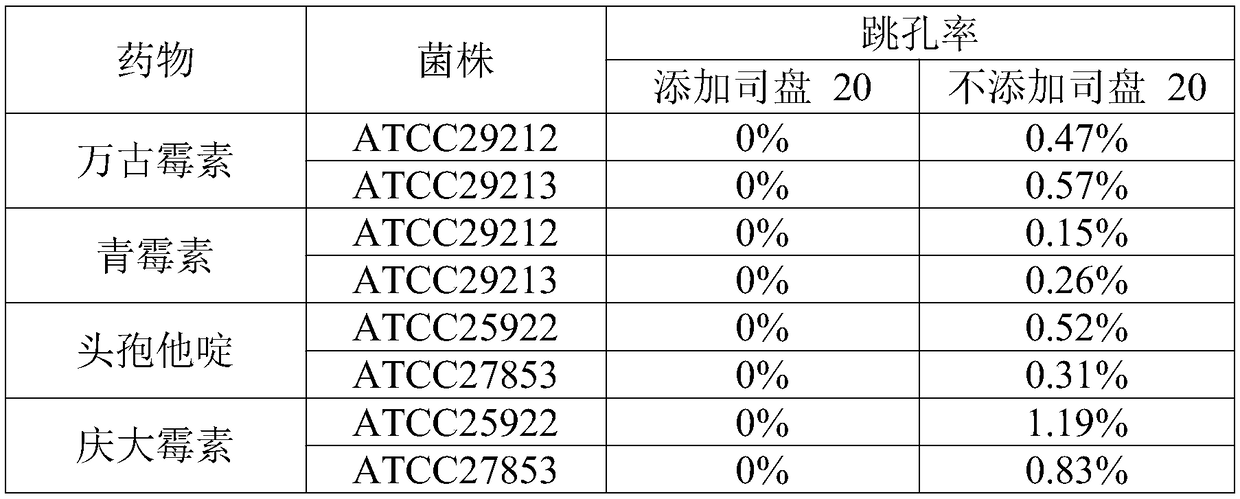
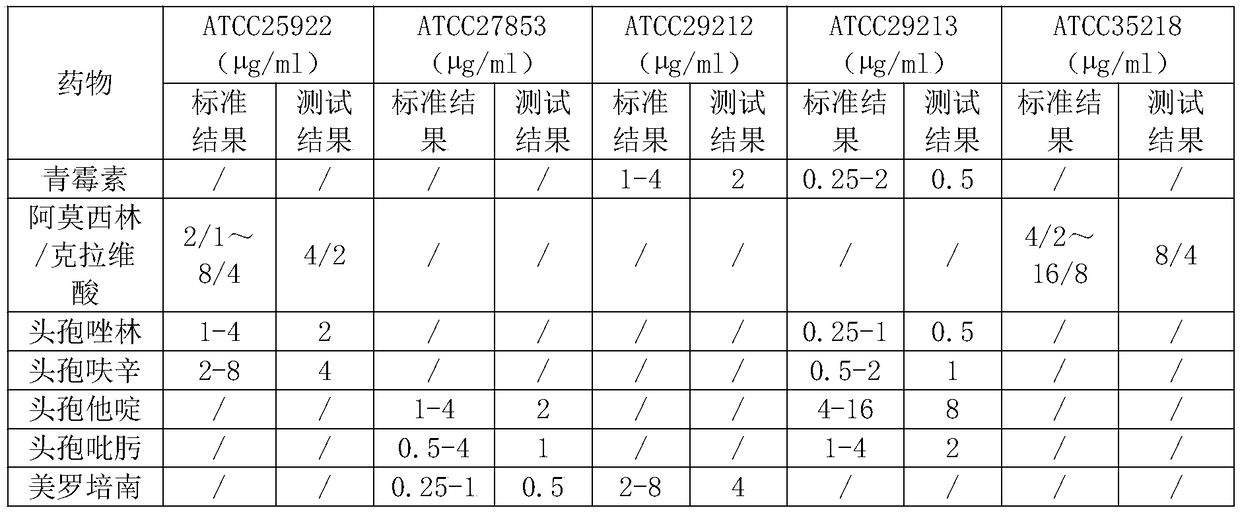
ActiveCN108917292AReduce surface tensionLower surface energyDrying solid materials with heatDrying solid materials without heatTest cardNitrogen
The invention discloses a method of drying a drug sensitivity test card. The method includes the steps of (1) preparing a drug and adding a sample, to be specific, adding a drug sensitivity solution in which a surfactant is added into a hole of a blank test card; (2) precooling a product to be dried; (3) drying preliminarily, to be specific, sealing a drying device of step (2), vacuumizing, charging nitrogen during vacuumizing, maintaining vacuity, heating the product, and holding the temperature; (4) resolving and drying, to be specific, reducing the vacuity to 100 pa and below, raising the temperature to 30 DEG C and above, and drying so that water content is reduced to 1% and below. The surfactant is added during the preparation of the drug herein; nitrogen is charged during drying; nosevere movement occurs in a liquid during the whole process; the problem is solved that the drug has poor stability and experiences hole skipping during drying; it is ensured that the dried drug is effectively attached in a test hole; the hole skipping problem is solved for the transporting process.
Owner:湖南迈瑞医疗科技有限公司
Stabilized susceptibility tests of aerobic pathogens
InactiveUS20050130252A1Improve efficiencyLow variabilityBacteriaMicrobiological testing/measurementAdjuvantAntibiotic Y
The present invention is directed to the field of microbiology and in particular to compositions and methods for determining susceptibility of aerobic pathogens to antibiotics. The addition of adjuvants to the compositions of the invention stabilize the test medium and eliminates inconsistencies in susceptibility testing, most especially testing involving the tetracycline family of antibiotics.
Owner:WYETH HOLDINGS CORP
Device for susceptibility testing of bacterial pathogens and vaccine strains in poultry
InactiveUS20050282243A1Convenience to workGuaranteed validityBioreactor/fermenter combinationsBiological substance pretreatmentsBacteroidesMicrotiter plate
Device for susceptibility testing of bacterial pathogens and vaccine strains in poultry with a microtiter plate comprising a plurality of wells and at least one well with a coating containing an antibiotic of a marker of a vaccine strain for the vaccination of poultry.
Owner:LOHMANN ANIMAL HEALTH
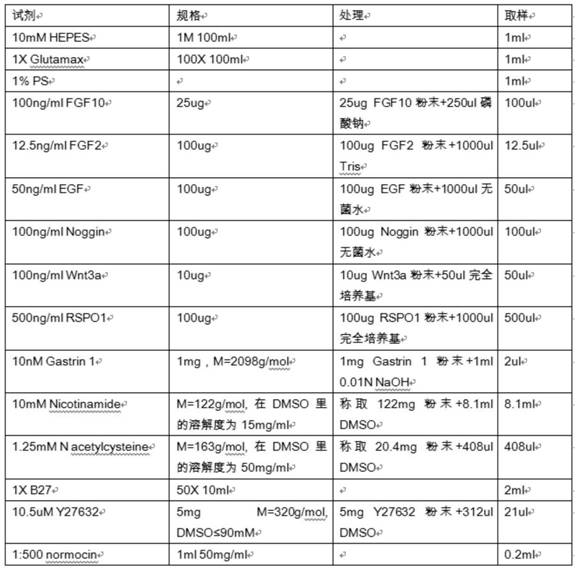
A kind of bladder cancer organoid culture medium and preparation method and application
ActiveCN112899230BReduce consumptionShorten the timeCell dissociation methodsSsRNA viruses positive-senseCell culture mediaCancer research
Owner:WUHAN UNIV
Method of quickly separating breast cancer primary tumor living cell
The invention belongs to the technical field of cell separation, and relates to a method of quickly separating breast cancer primary tumor living cells in batches at a high purity. The method includes the steps of: (1) obtaining an isolated breast cancer fresh tissue sample; (2) performing pretreatment and quality control of the tissue sample; and (3) quickly separating the breast cancer primary tumor living cells in batches at a high purity, so that the high-purity breast cancer primary tumor living cells are separated in batches quickly. According to the invention, living breast cancer cells are separated from breast cancer tissue blocks with the purity of the tumor cells being higher than 90%. The separated breast cancer primary tumor living cells can be used in drug sensitive tests. The method overcomes the defects that the breast cancer primary tumor living cells are low in separation purity and are very liable to be polluted by fibroblast and can provide reliable research object for clinical drug sensitive tests. The invention provides the new method and wider material sources for database foundation of DNA, RNA and protein of primary tumor living cells and tumor in-vivo researching.
Owner:上海兰卫医学检验所股份有限公司
Enzyme linked immunosorbent assay instrument testing method for penicillium marneffei mycelia-phase in-vitro susceptibility testing
InactiveCN106701888AInfectiousReduce dosageMicrobiological testing/measurementMicroorganism based processesPenicillium marneffeiNormal growth
The invention discloses an enzyme linked immunosorbent assay instrument testing method for penicillium marneffei mycelia-phase in-vitro susceptibility testing. The light transmittance of a bacterium-containing diluent with an antibiotic medicinal liquid is tested by using an enzyme linked immunosorbent assay instrument in the penicillium marneffei mycelia-phase in-vitro susceptibility testing process. The densities are different in different bacterium growth situations, the light transmittances are also different, results are judged by using the enzyme linked immunosorbent assay instrument, apparent phenomena can be converted into values, and the operation can be relatively convenient and accurate; secondly, as an enzyme linked immunosorbent assay instrument pore plate is used, material consumption can be reduced; only a solution less than 1ml is needed in each pore, so that the amount of medicines is small, and waste can be greatly reduced; in addition, penicillium marneffei has infectivity, so that the pollution property can be further reduced because of the small use amount. Finally, normal growth of the penicillium marneffei can be not affected by the enzyme linked immunosorbent assay instrument, the operation is simple, convenient and rapid, and precise in-vitro susceptibility data results can be obtained.
Owner:GUANGXI ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com