Method of drying drug sensitivity test card
A drying method and drug sensitivity test technology, which is applied in the field of medicine, can solve the problems of high drying energy consumption, long drying time, and easy drug failure, and achieve the effect of improving the drying rate, speeding up the drying rate, and avoiding the decomposition of easily oxidized drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
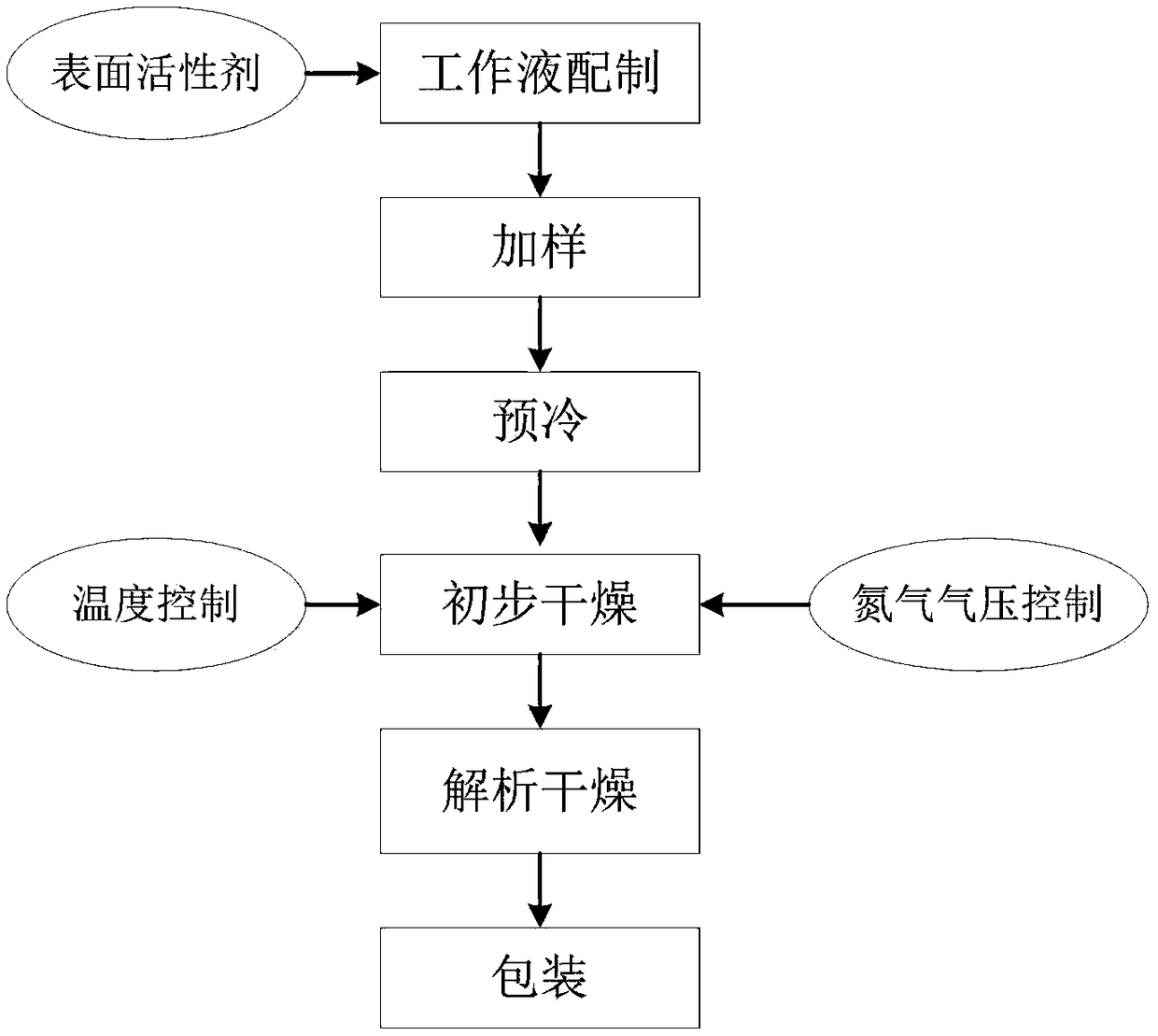
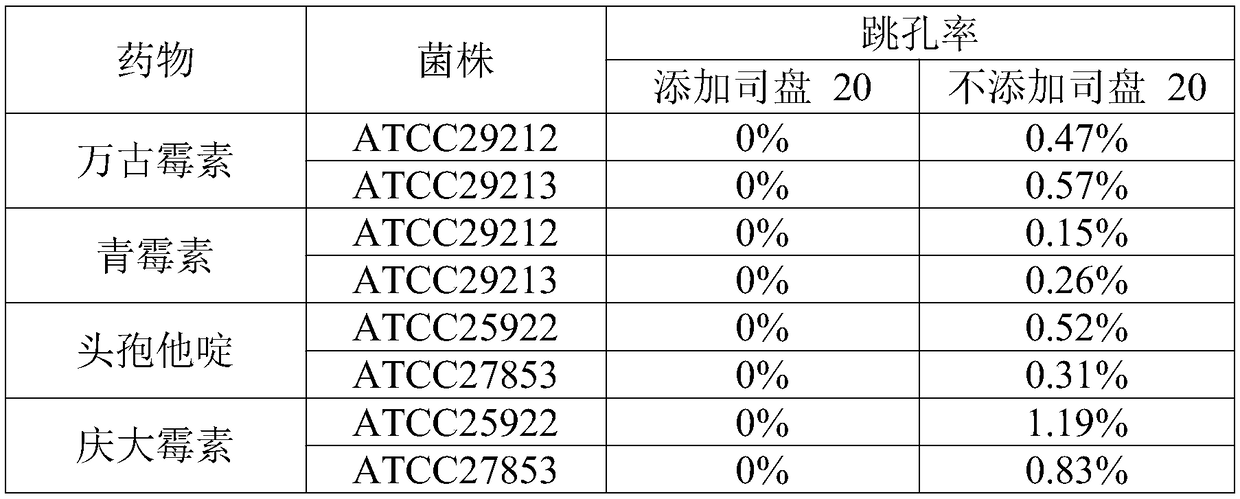
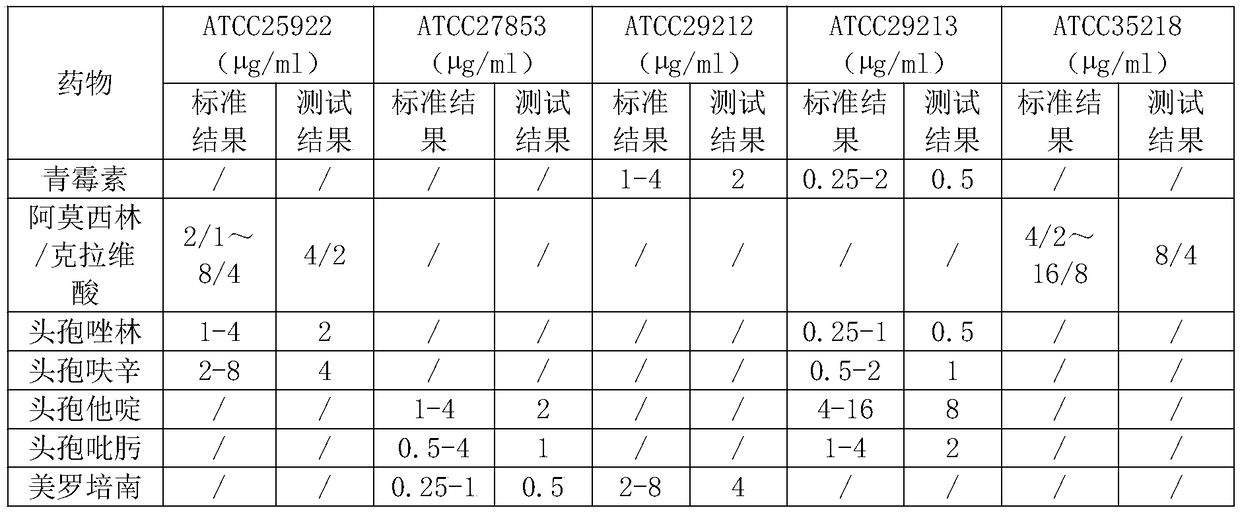
[0040] ①Preparation of working solution: prepare different concentrations and types of antibiotic solutions or biochemical working solutions required, and add non-ionic surfactant with HLB value of 3.0 to 20.0 during the preparation process;
[0041] ② Adding samples: add the prepared solution to the blank test card, and the amount added to each well is 25 μl;
[0042] ③ Pre-cooling: Put the test card that has added the sample into the partition of the drying box, close the door, cool the partition, and reduce the temperature of the product to 2-4 °C;
[0043] ④ Preliminary drying: Turn on the vacuum pump and nitrogen gas permeation switch, and set the program parameters according to the following procedures.
[0044]
[0045]
[0046] ⑤ Analyze and dry, close the nitrogen control solenoid valve, turn on the vacuum pump, and pump the vacuum to below 100Pa for 1 hour.
[0047] ⑥Packing: Vacuum-packed products in aluminum foil bags.
[0048] ⑦The entire drying time is no...
Embodiment 2
[0050] ①Preparation of working solution: prepare different concentrations and types of antibiotic solutions or biochemical working solutions required, and add non-ionic surfactant with HLB value of 3.0 to 20.0 during the preparation process;
[0051] ② Adding samples: add the prepared solution to the blank test card, and the amount added to each well is 25 μl;
[0052] ③ Pre-cooling: Put the test card that has added the sample into the partition of the drying box, close the door, cool the partition, and reduce the temperature of the product to 2-4 °C;
[0053] ④ Preliminary drying: Turn on the vacuum pump and nitrogen gas permeation switch, and set the program parameters according to the following procedures.
[0054]
[0055] ⑤ Analyze and dry, close the nitrogen control solenoid valve, turn on the vacuum pump, and pump the vacuum to below 100Pa for 1 hour.
[0056] ⑥Packing: Vacuum-packed products in aluminum foil bags.
[0057] ⑦The entire drying time is not longer tha...
Embodiment 3
[0059] ①Preparation of working solution: prepare different concentrations and types of antibiotic solutions or biochemical working solutions required, and add non-ionic surfactant with HLB value of 3.0 to 20.0 during the preparation process;
[0060] ② Adding samples: add the prepared solution to the blank test card, and the amount added to each well is 25 μl;
[0061] ③ Pre-cooling: Put the test card that has added the sample into the partition of the drying box, close the door, cool the partition, and reduce the temperature of the product to 2-4 °C;
[0062] ④ Preliminary drying: Turn on the vacuum pump and nitrogen gas permeation switch, and set the program parameters according to the following procedures.
[0063]
[0064] ⑤ Analyze and dry, close the nitrogen control solenoid valve, turn on the vacuum pump, and pump the vacuum to below 100Pa for 1 hour.
[0065] ⑥Packing: Vacuum-packed products in aluminum foil bags.
[0066] ⑦The entire drying time is not longer tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com