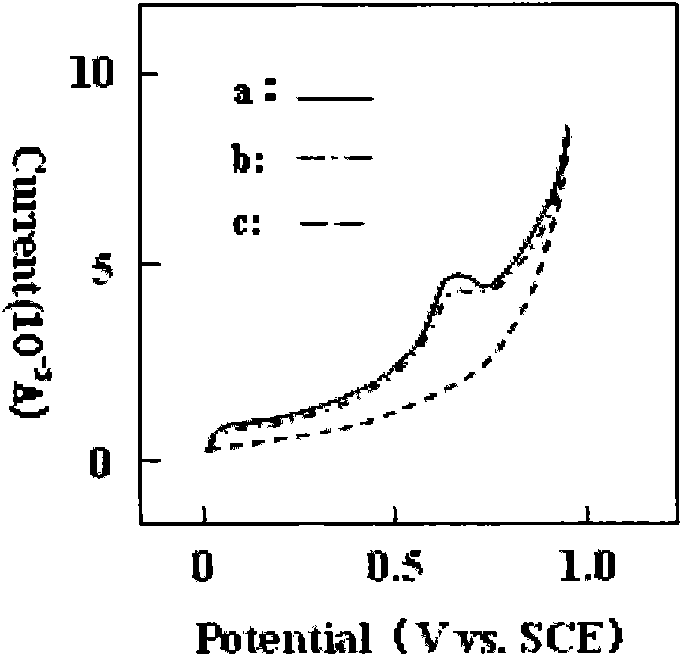
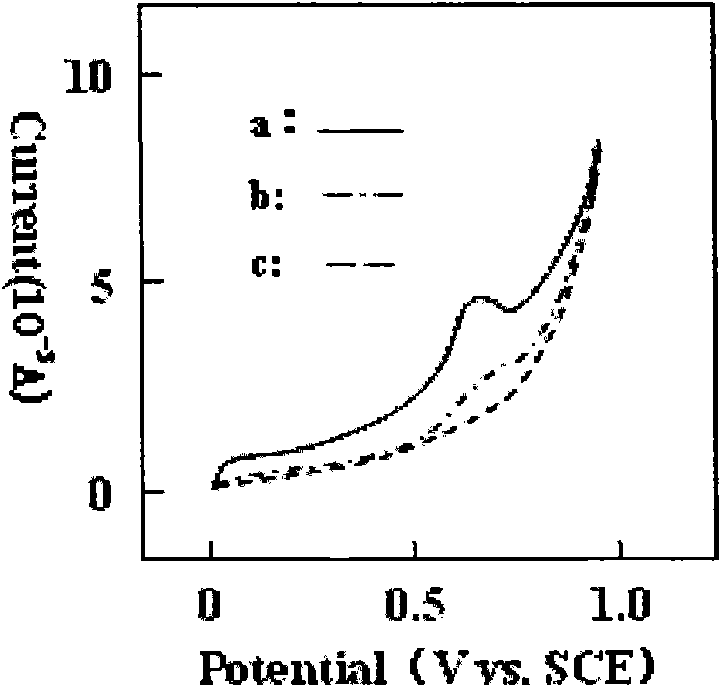
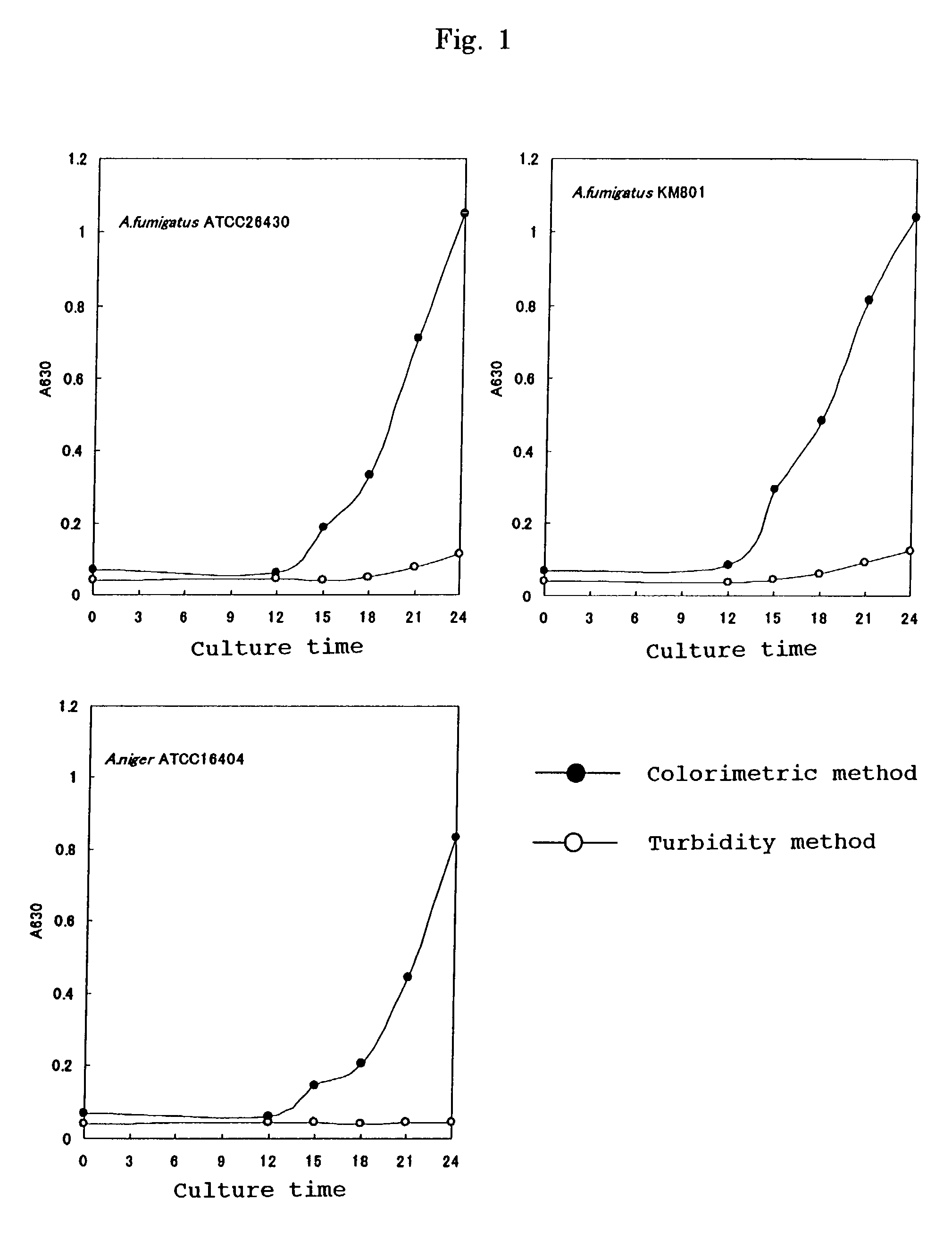
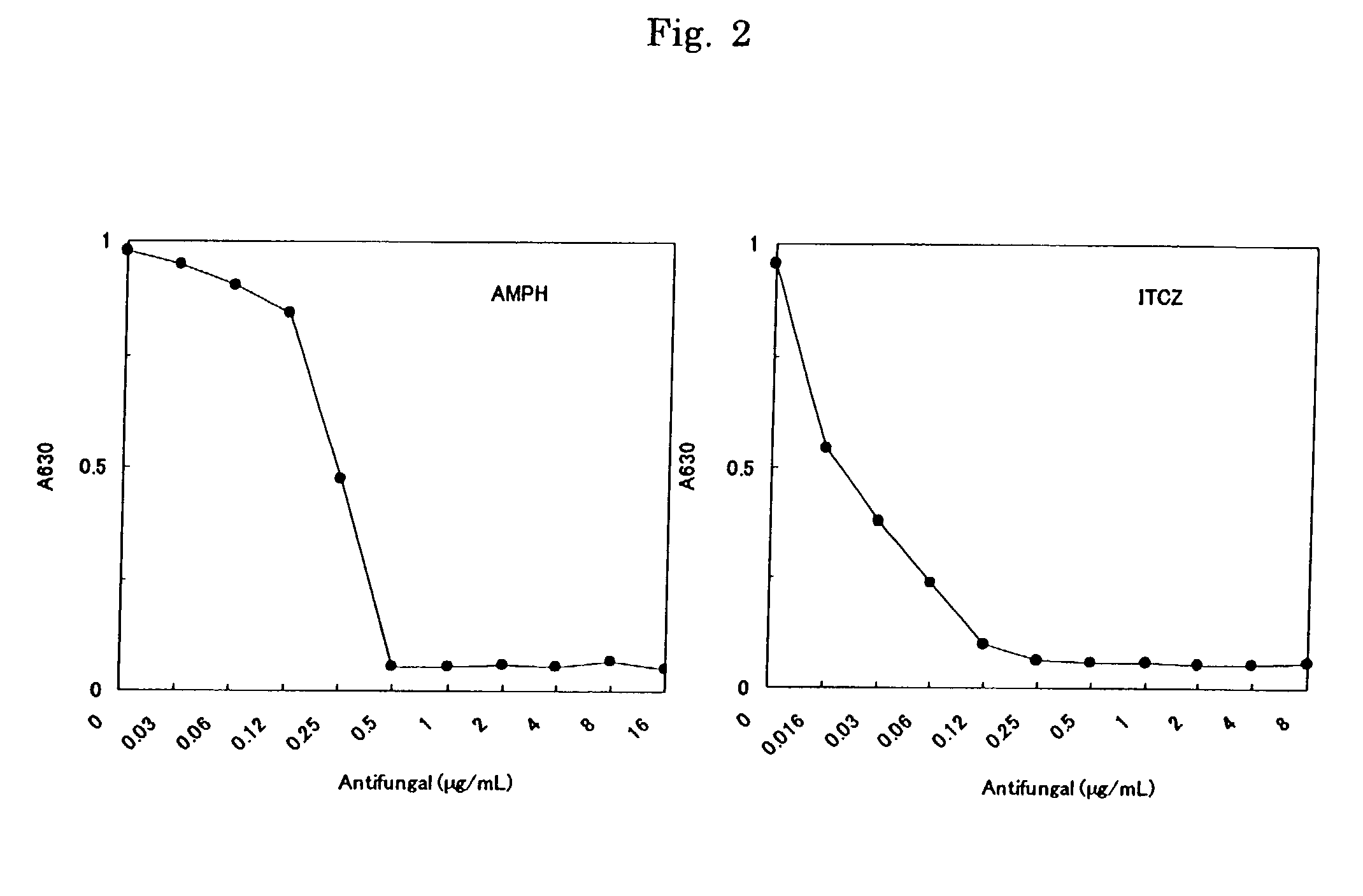
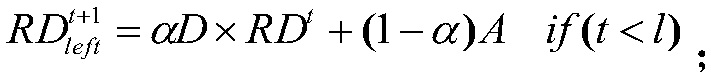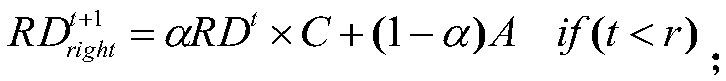Patents
Literature
132 results about "Drug susceptibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
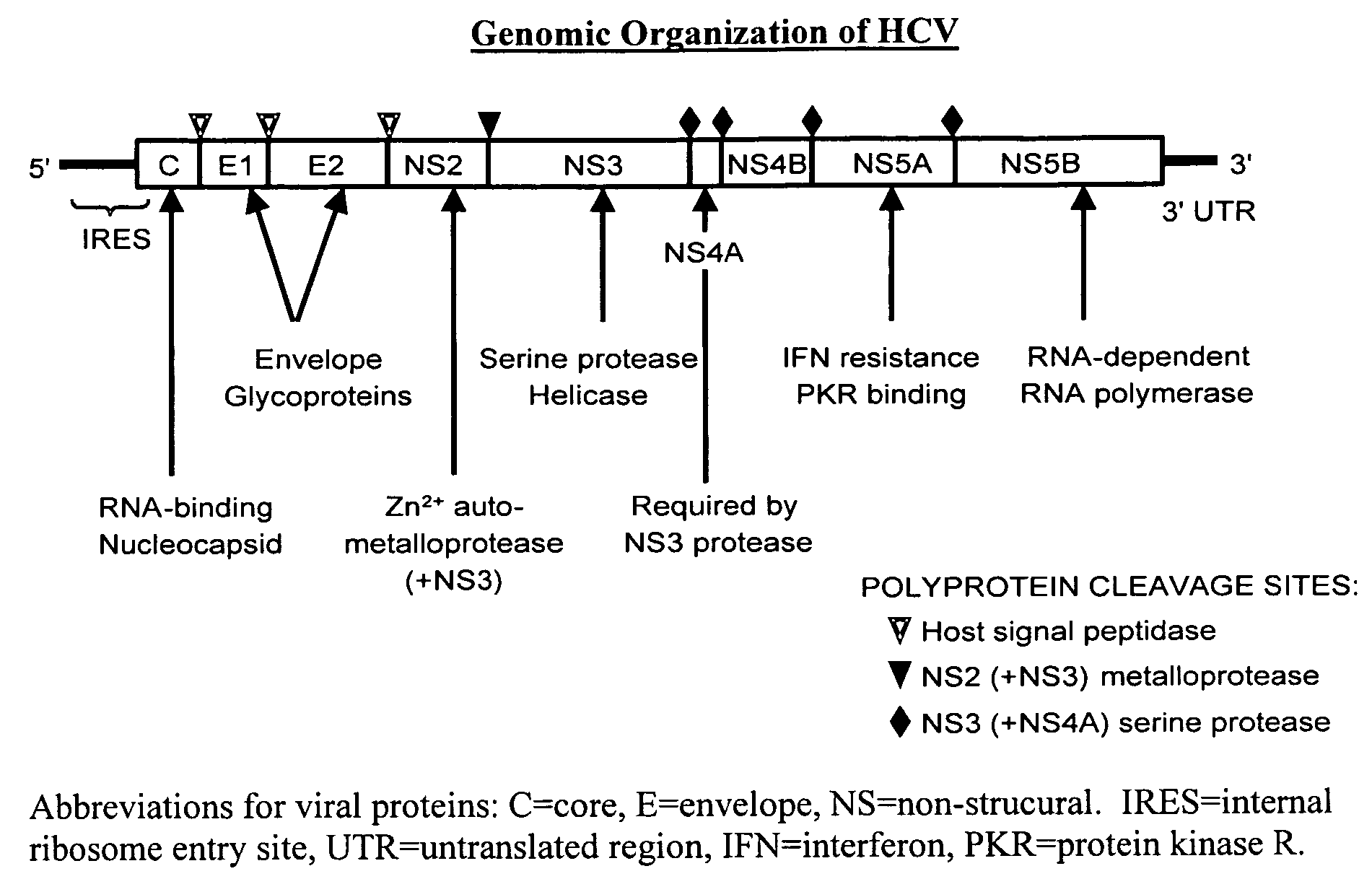
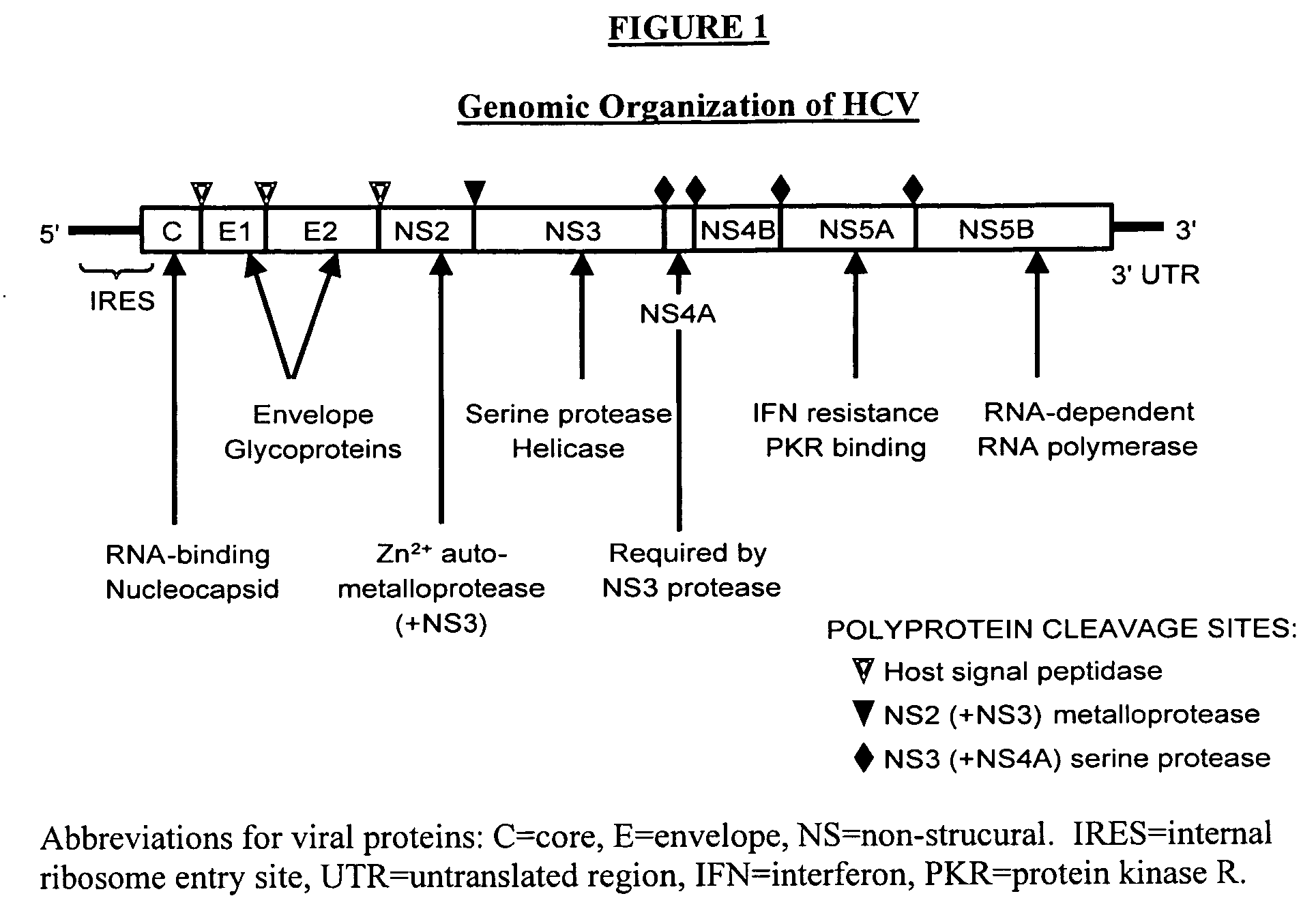
In microbiology, pharmacology, and medicine drug susceptibility is the ability of a microorganism to be inhibited or killed by the drug, as in antibiotic susceptibility, the susceptibility of microorganisms to antibiotics (often used synonymously with the lay term sensitivity)
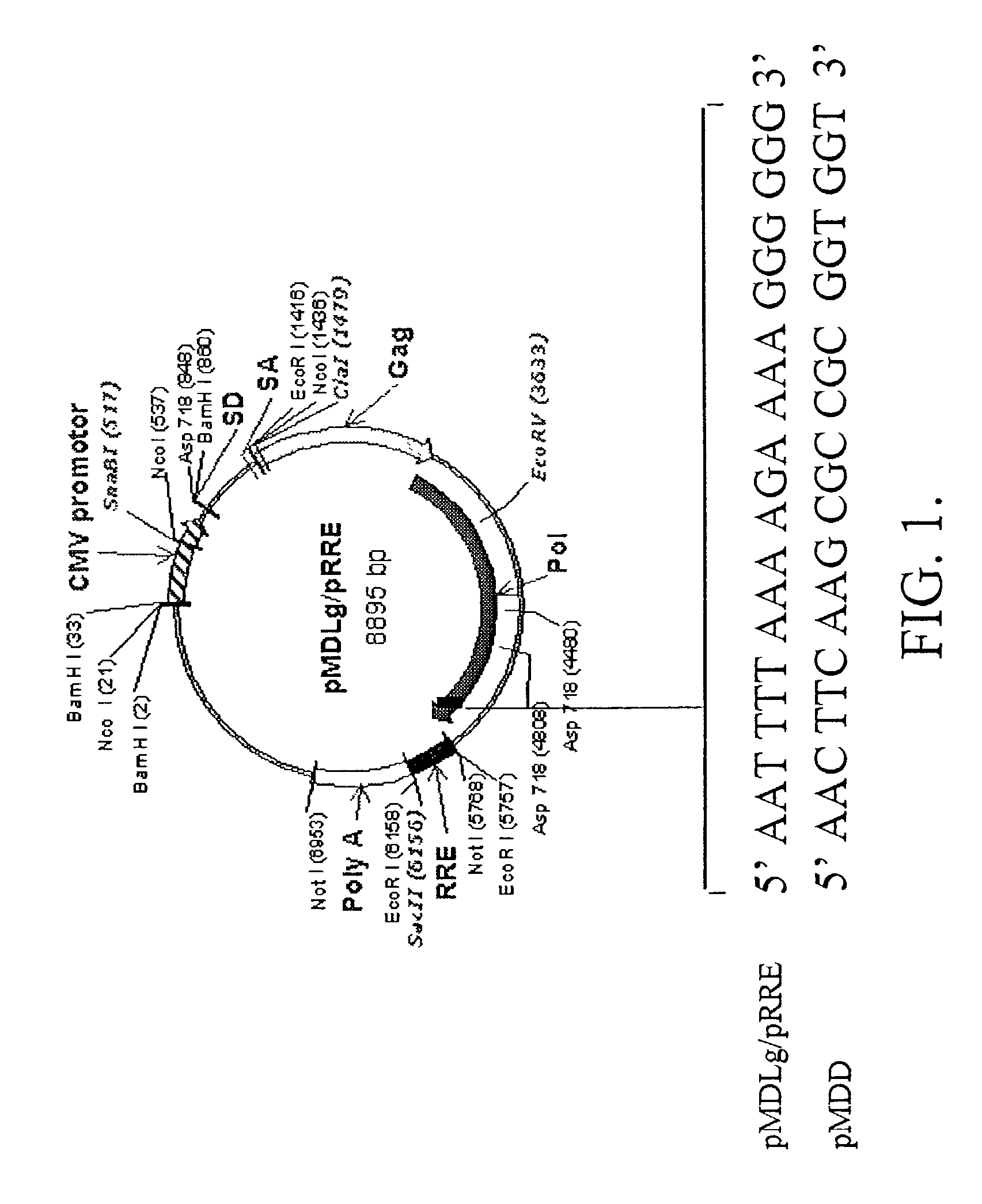
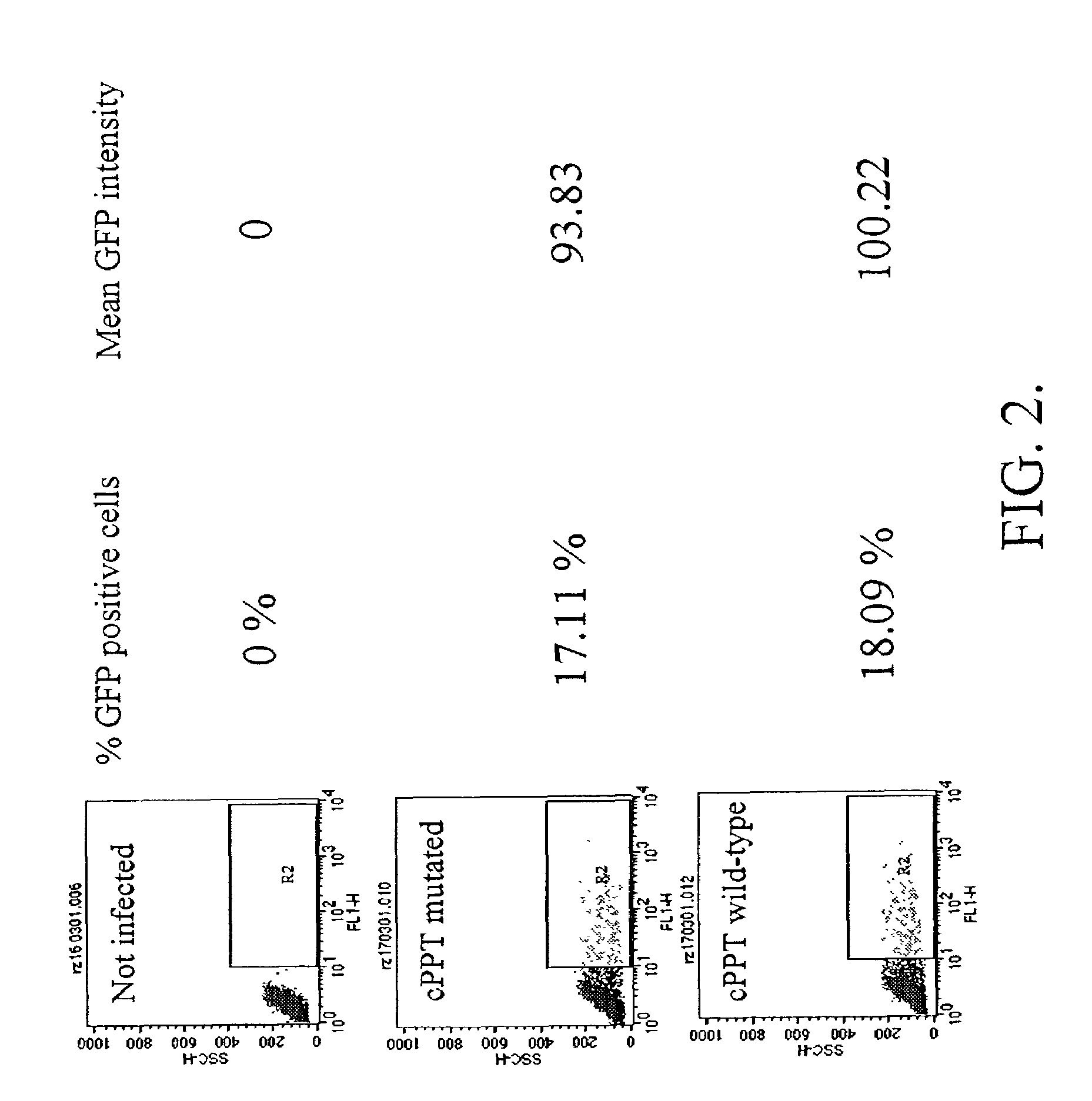
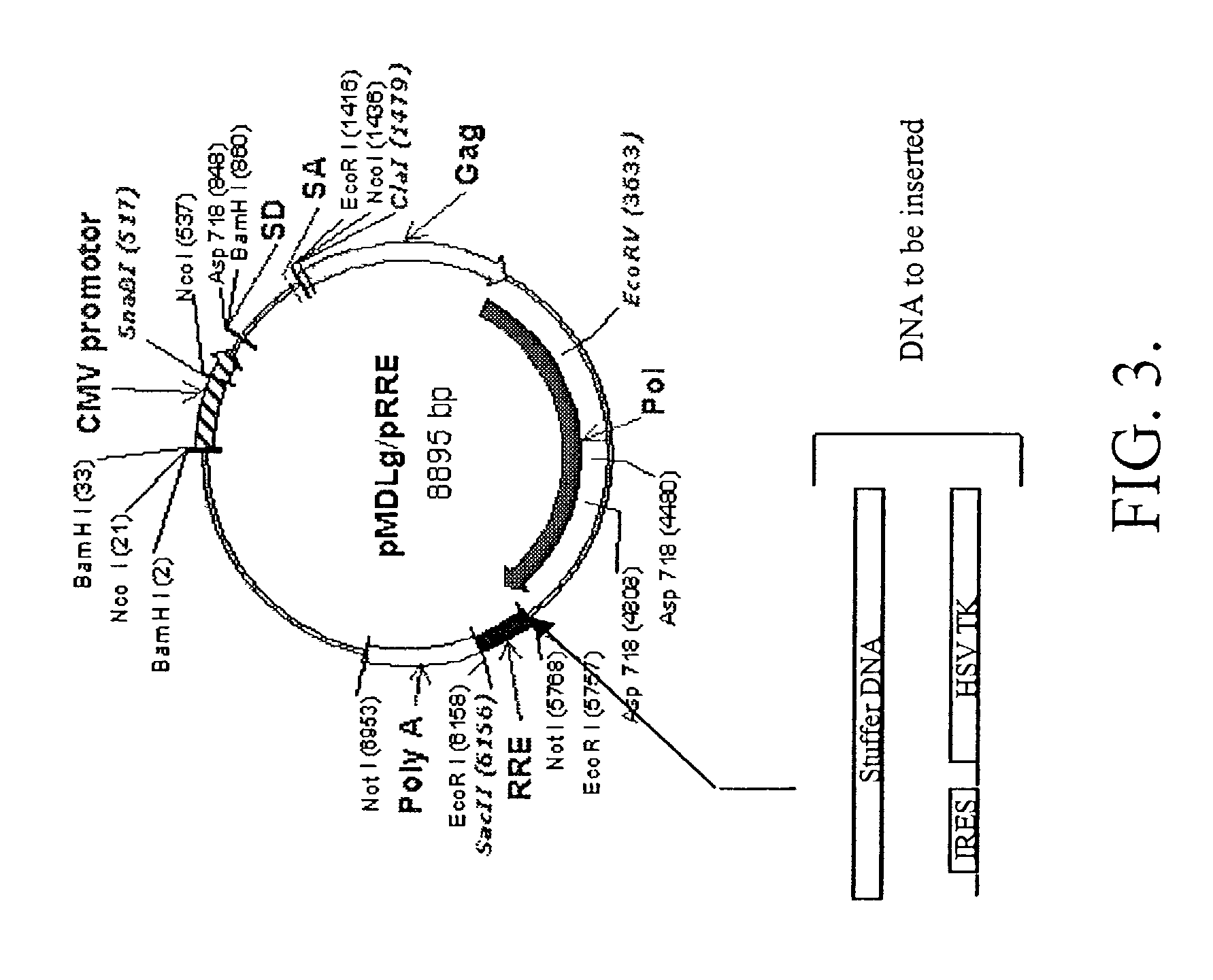
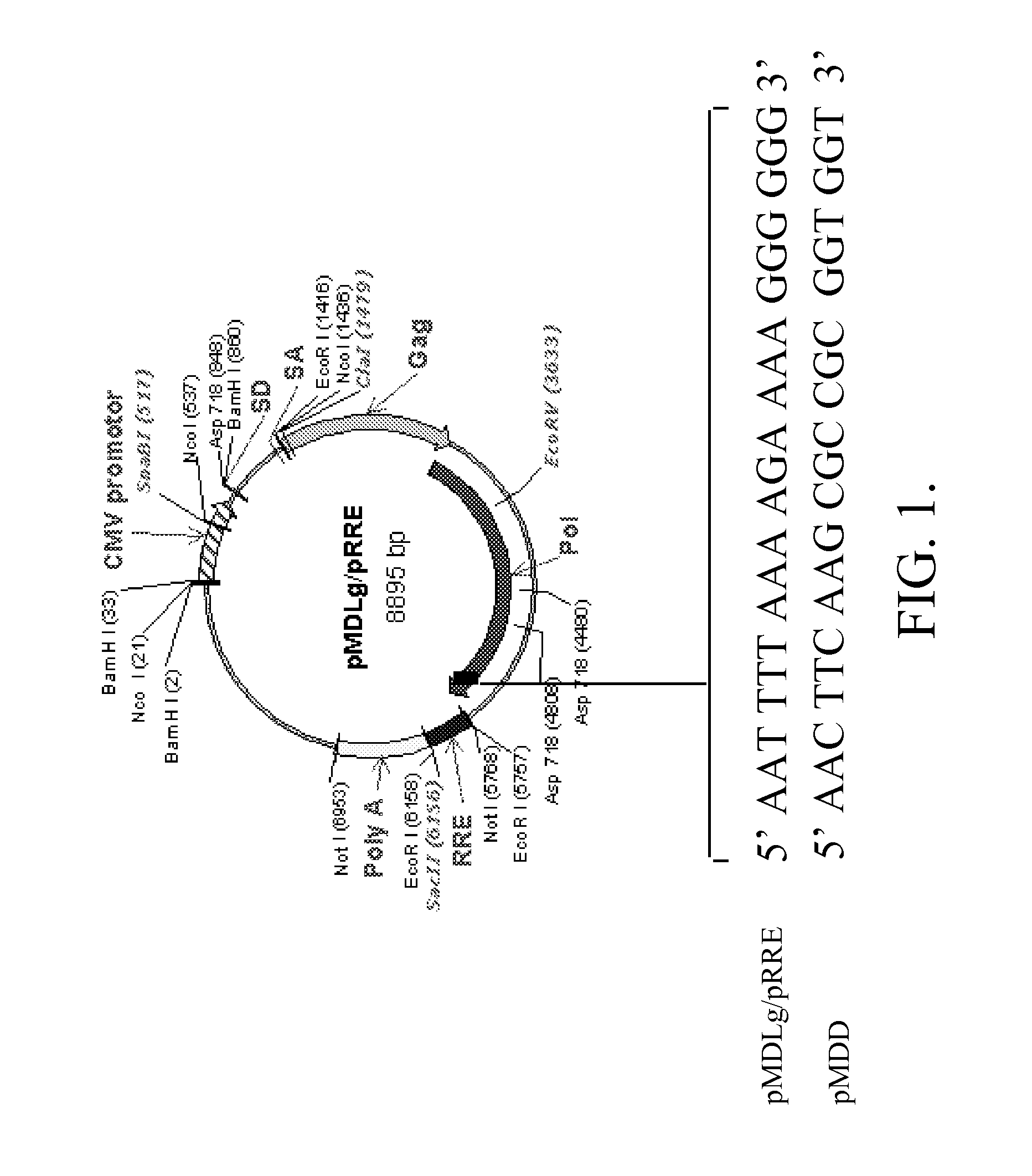
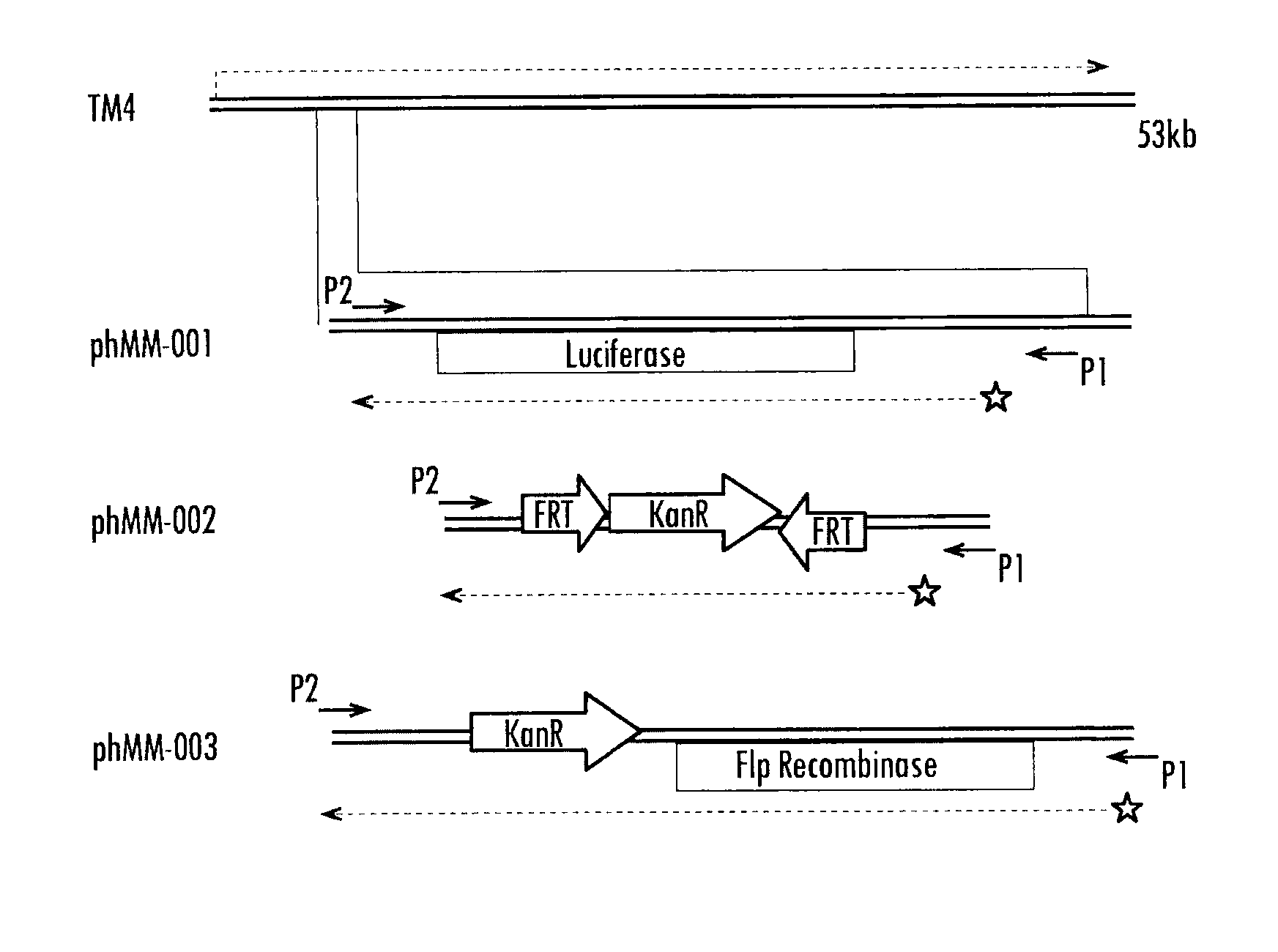
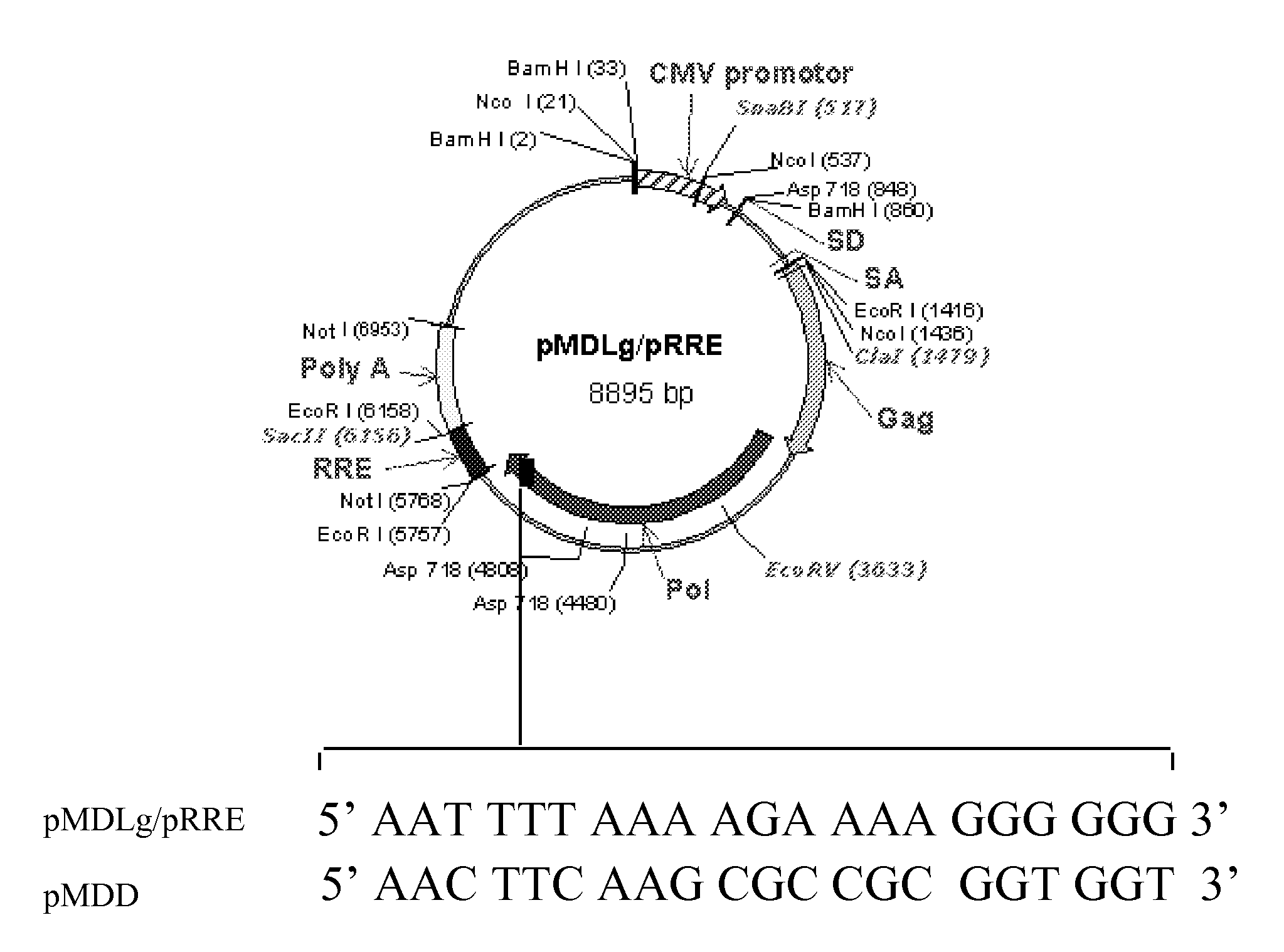
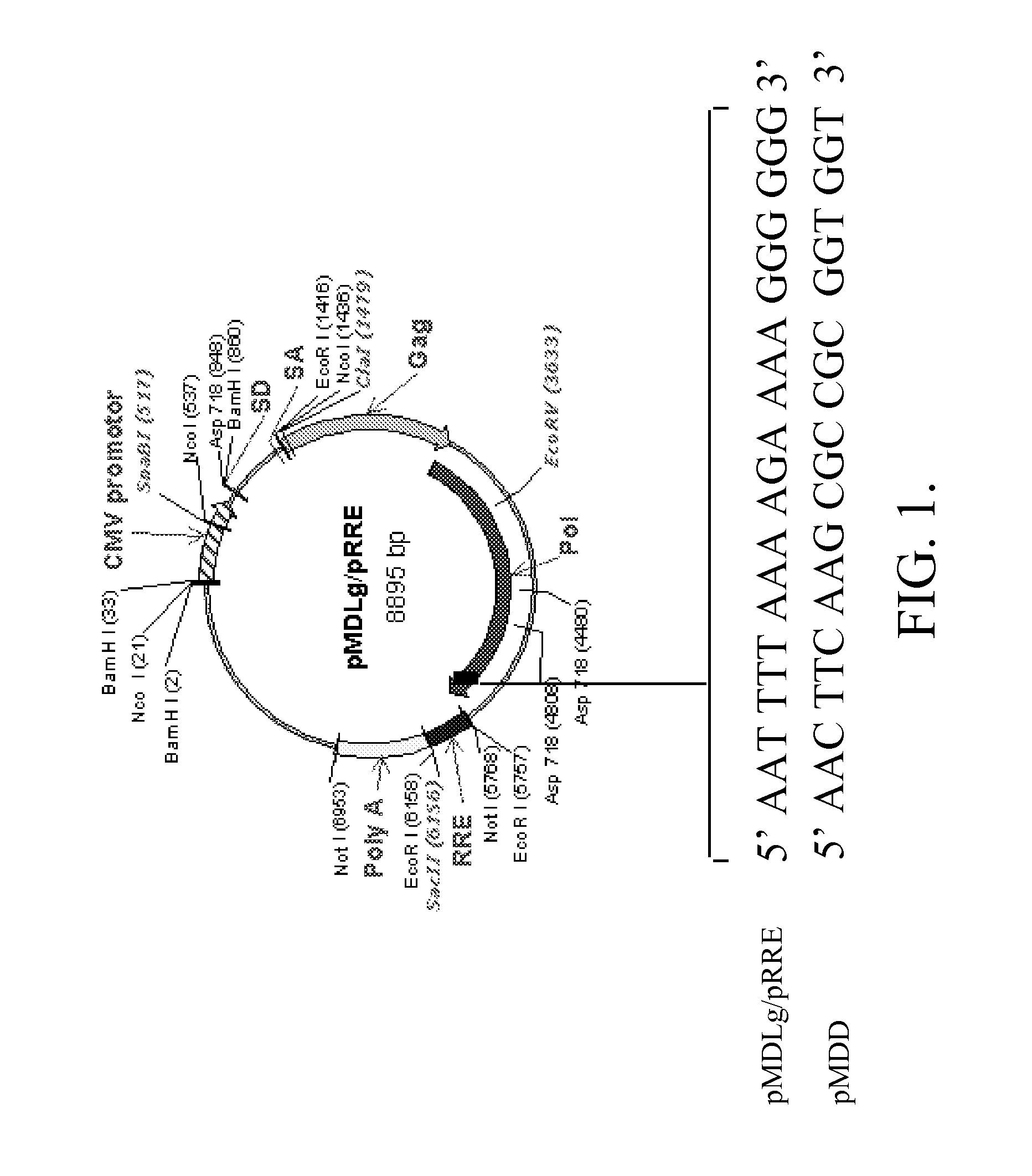
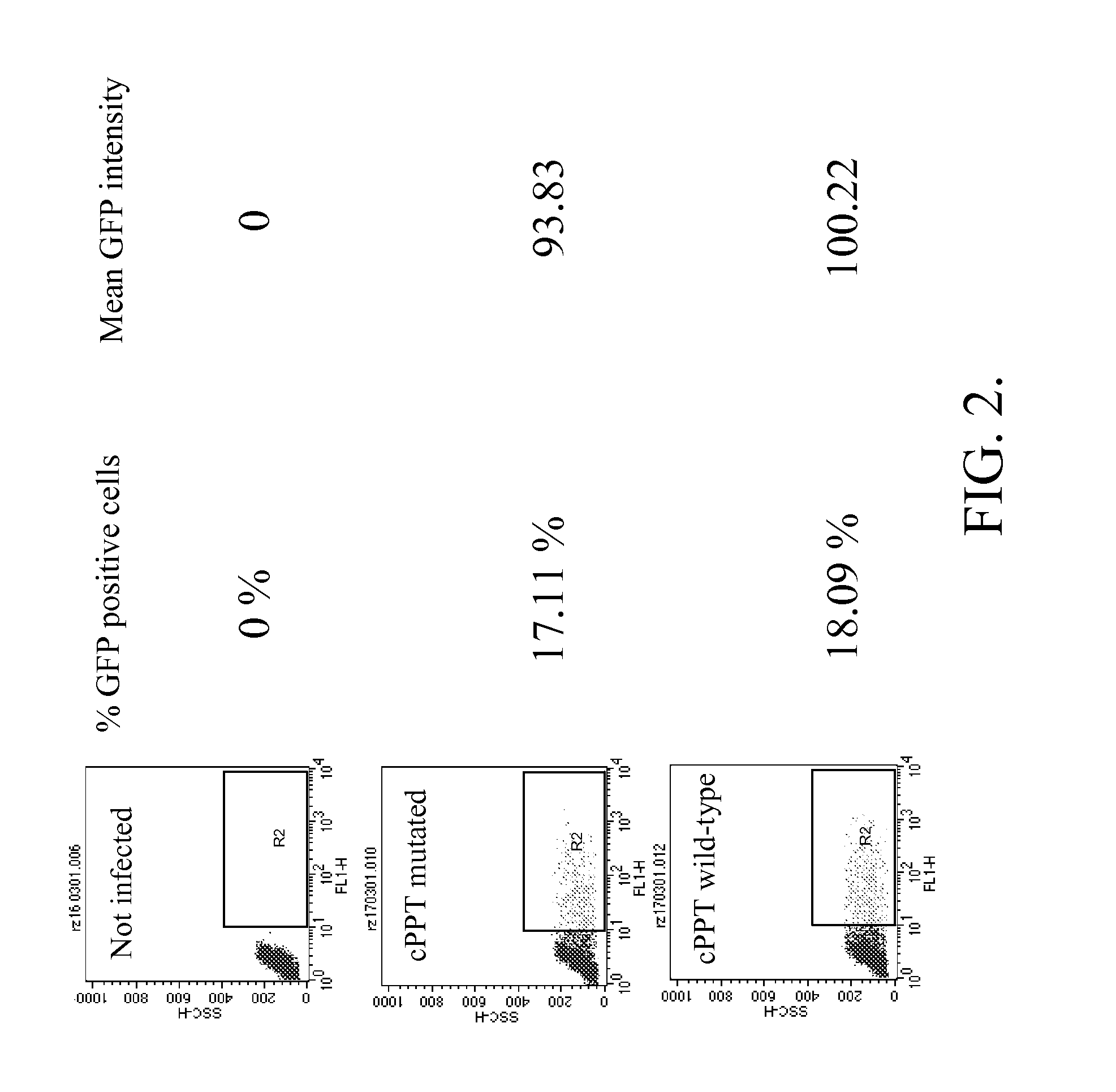
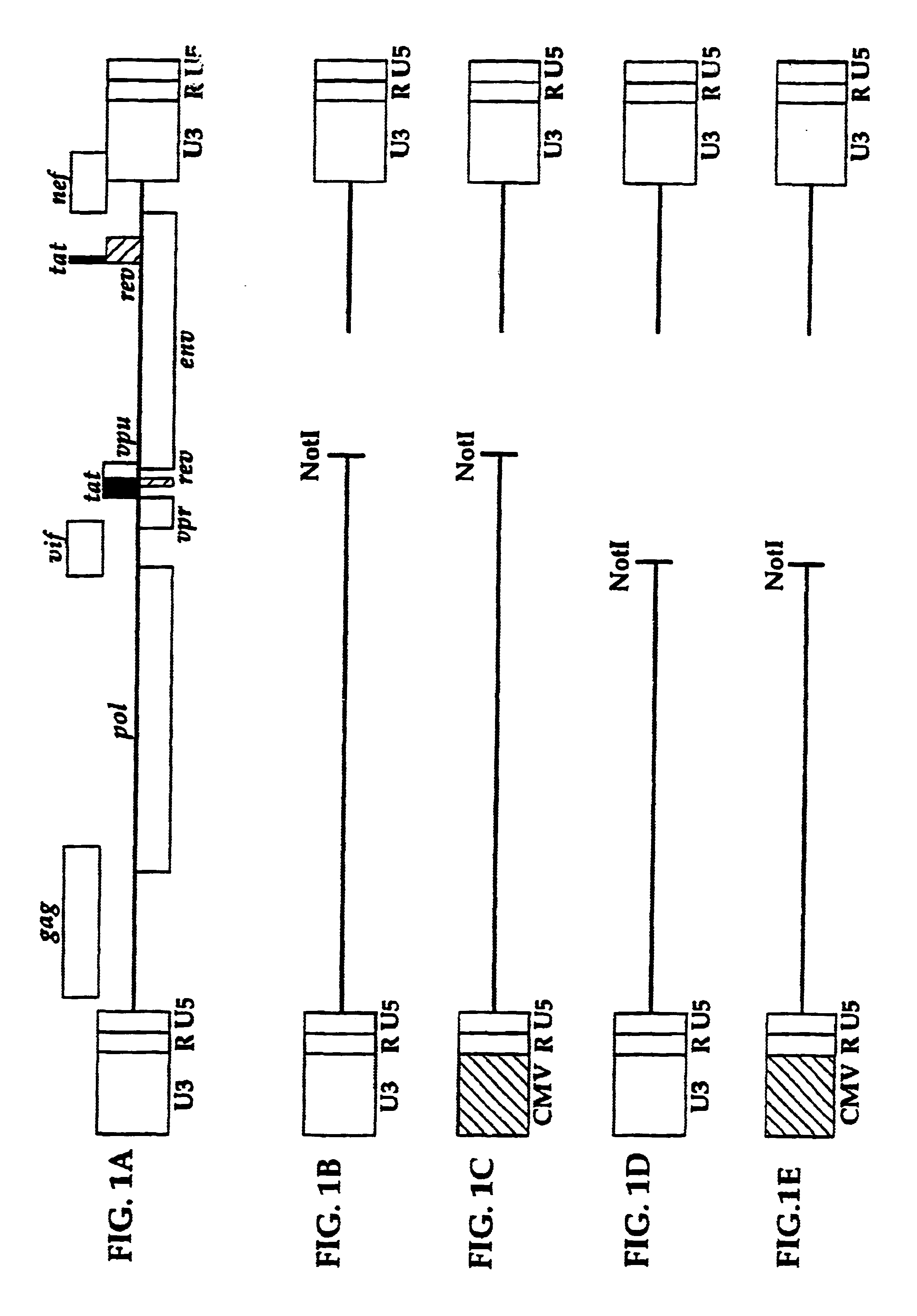
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS7629153B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsTranscriptional Regulatory ElementsTransgene
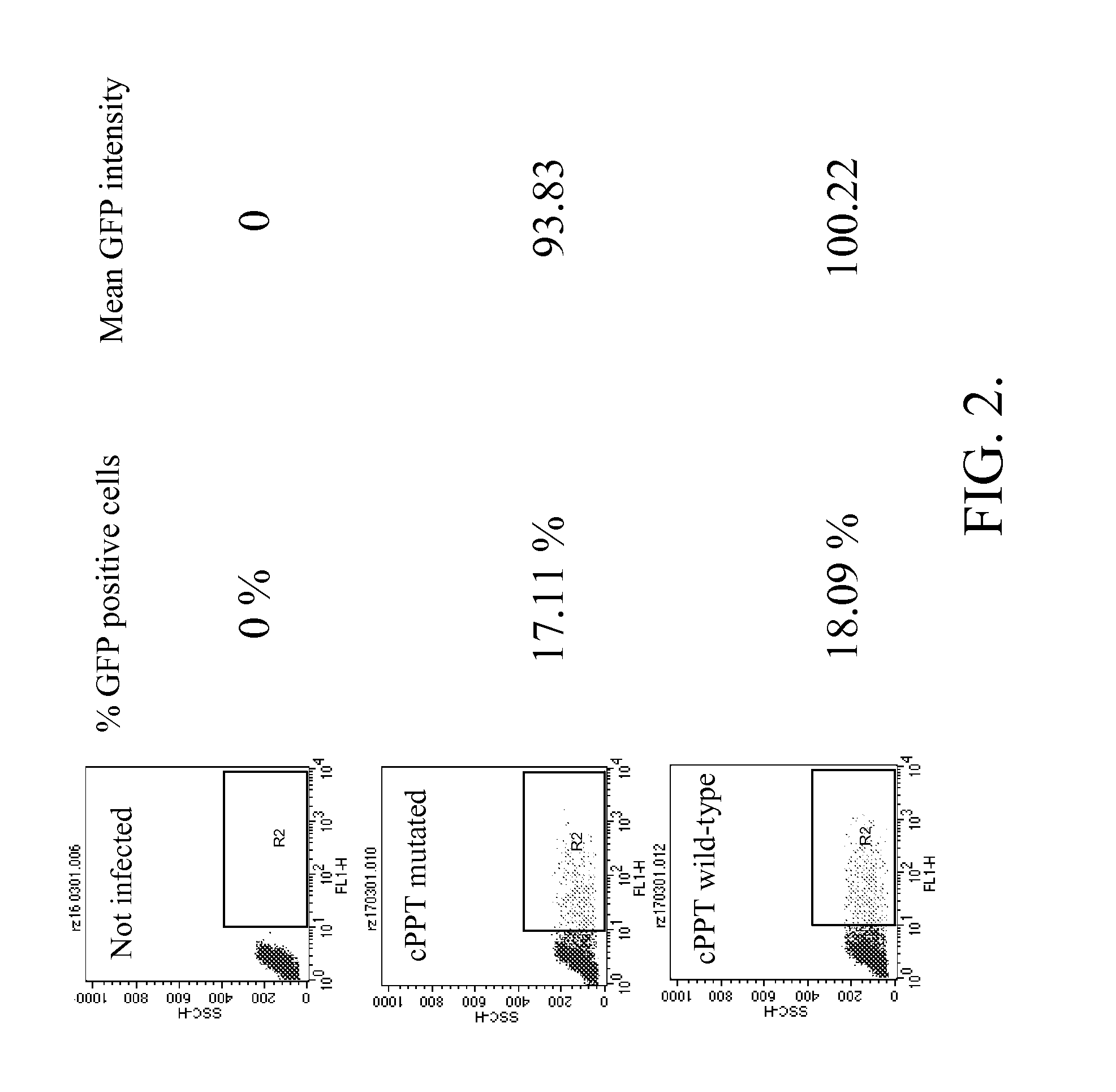
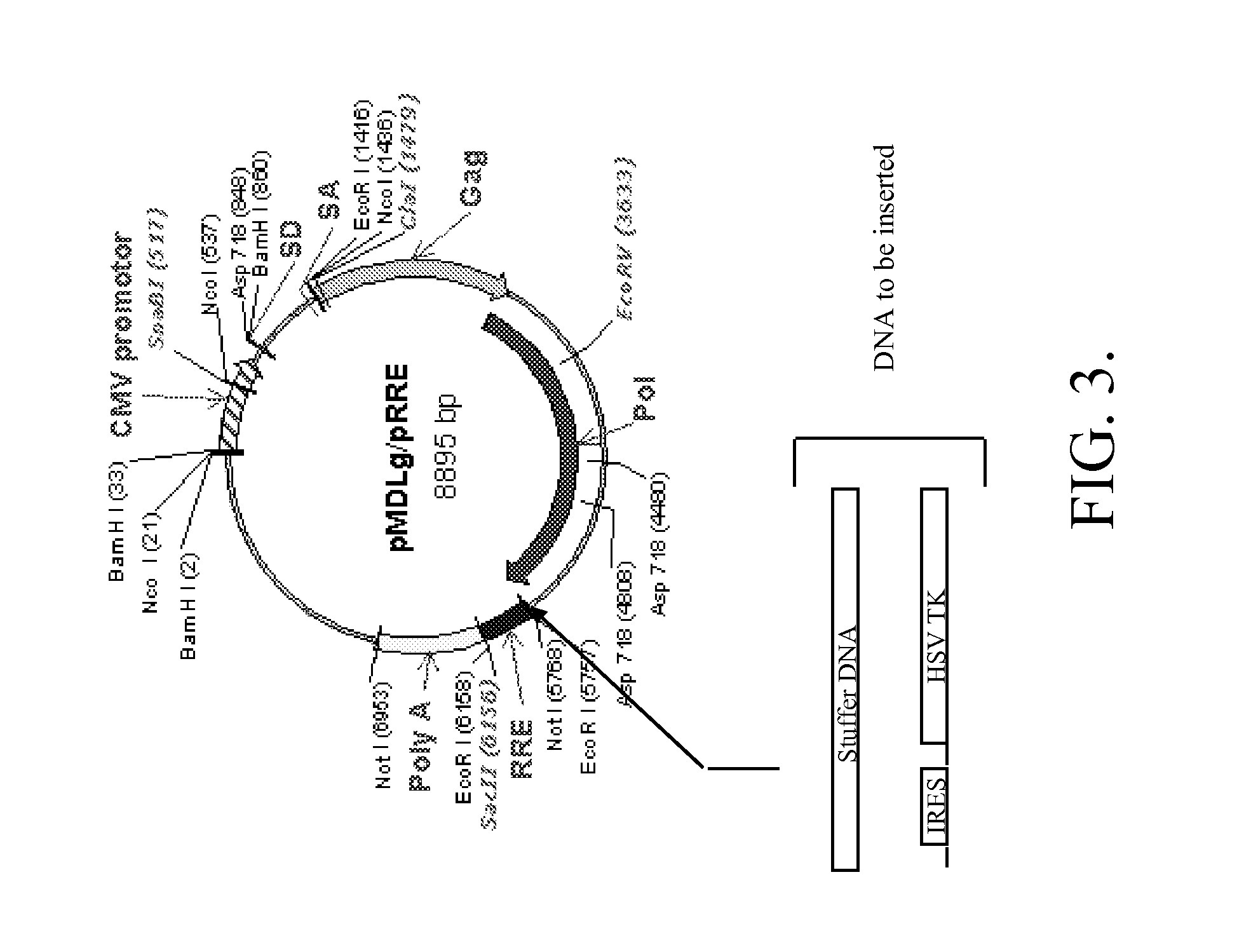
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosafety and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS8900858B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsDiseaseTranscriptional Regulatory Elements
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosaftey and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Human brain glioma cell line, and establishing method and application thereof
ActiveCN103627673AStable traitsStrong scientific research informationMicrobiological testing/measurementTumor/cancer cellsHuman gliomaIn vivo
The invention provides a human brain glioma cell line and an establishing method and application thereof. The human brain glioma cell line is preserved in China Center for Typical Culture Collection with an accession number of CCTCC No. C201115. The human brain glioma cell line is established by subjecting brain glioma cells originated from a clinic specimen to primary culture and subculture, can be directly used for in vitro drug screening or generation of human brain glioma in mammals and is used for establishing a human brain glioma animal model and screening candidate drugs used for treating human brain glioma. The human brain glioma cell line has stable properties, can realize stable multiple passages and maintain stable properties even after in vitro passage to the 50th generation; pathogenesis of human brain glioma, drug susceptibility, transitivity and other related characteristics can be analyzed in vitro and in vivo, so two correlated in-vitro and in-vivo anti-brain glioma drug screening platforms can be established, and novel experimental materials closer to clinic oncobiological characteristics are provided for research on human brain glioma.
Owner:SHANGHAI CHEMPARTNER CO LTD +1
Application of long-chain non-coding RNA in preparation of non-small cell lung cancer treatment drugs
ActiveCN103316359APromote apoptosisInhibit apoptosisGenetic material ingredientsAntineoplastic agentsApoptosisNon-coding RNA
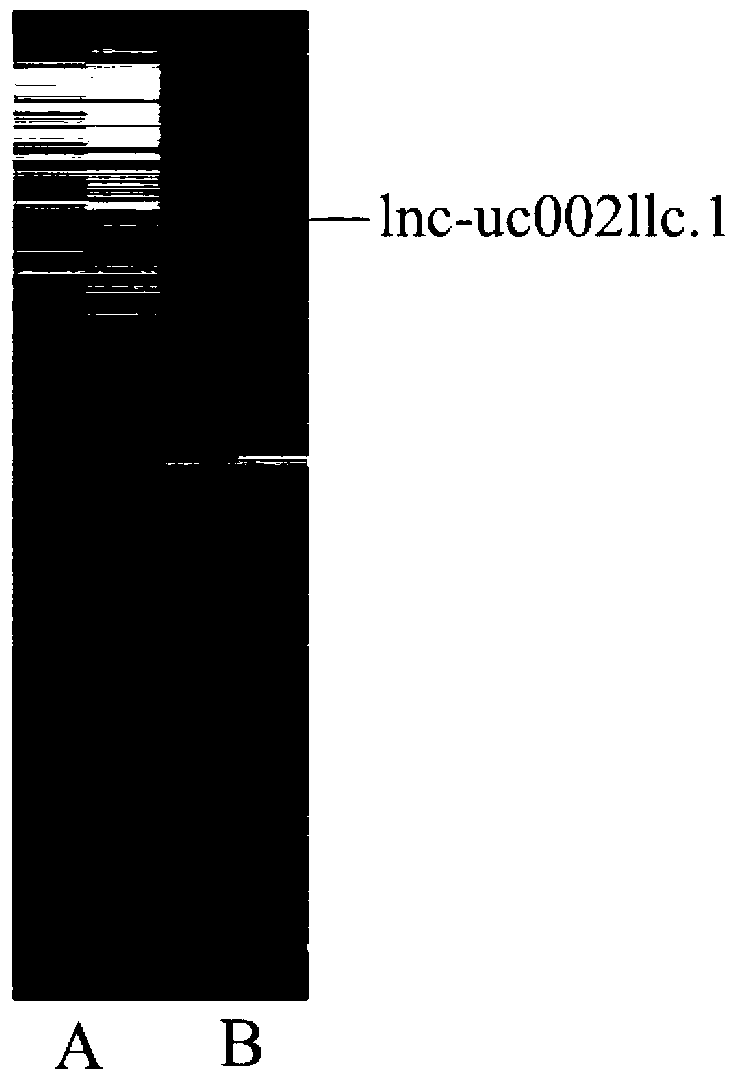
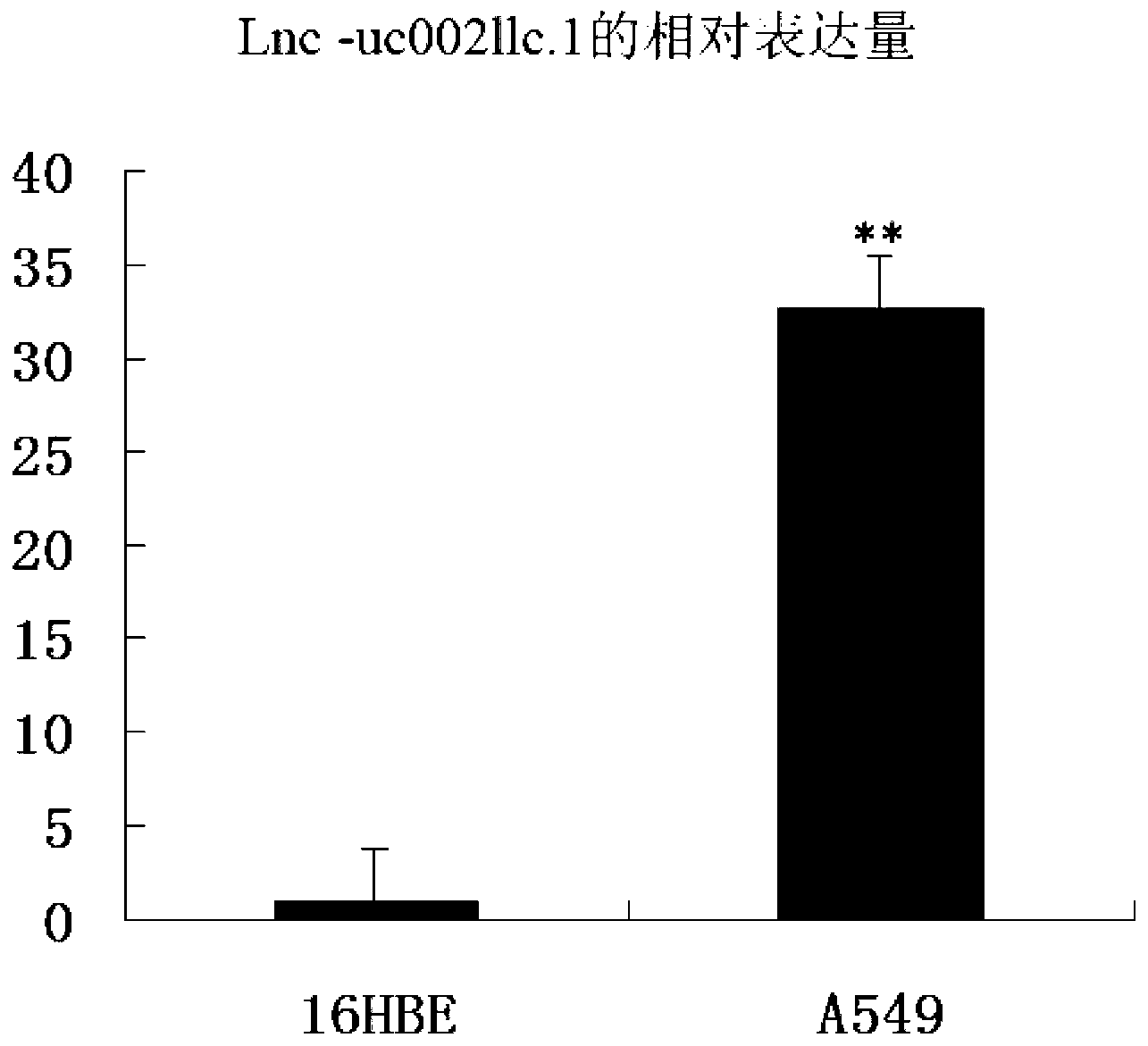
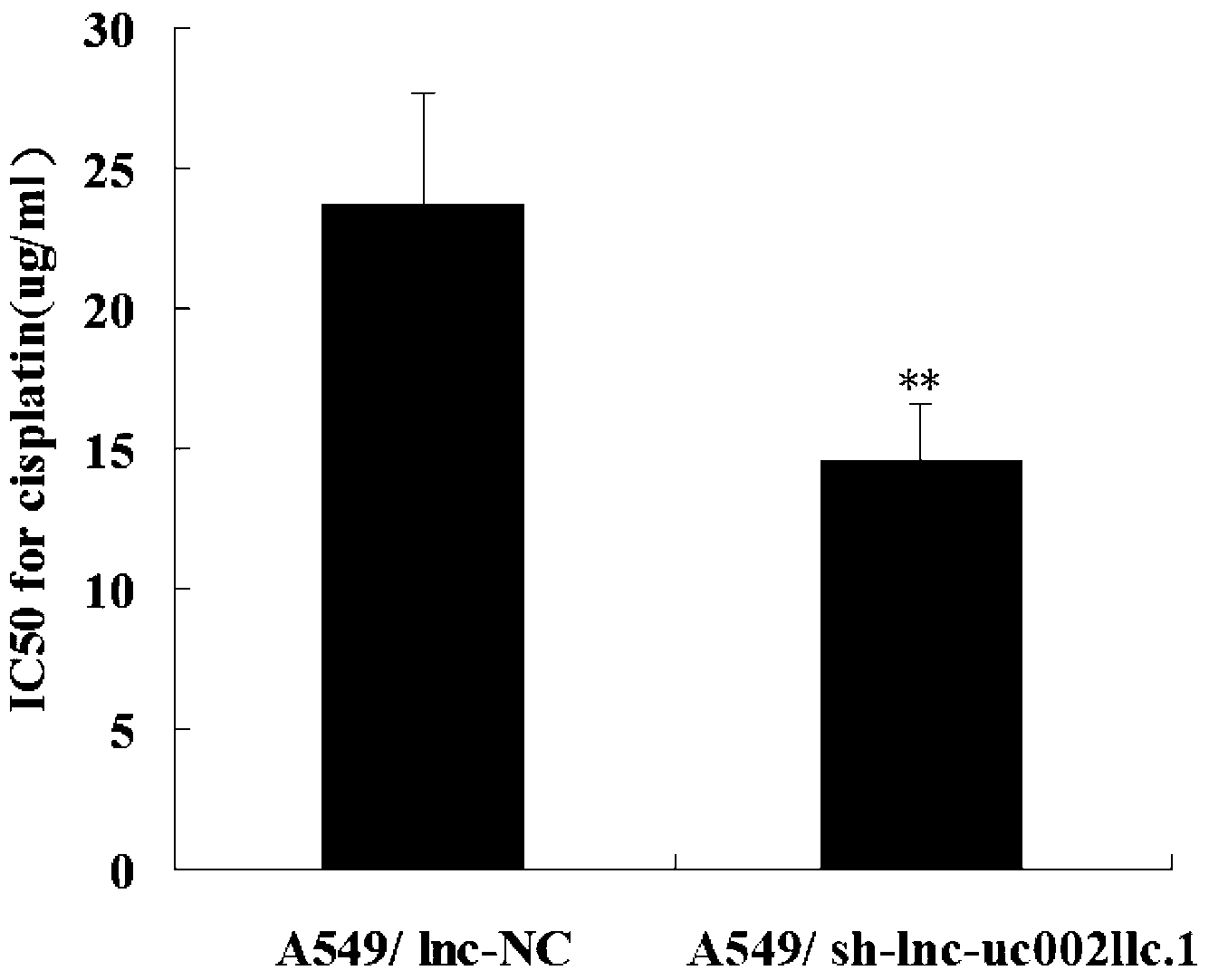
The invention belongs to the genetic engineering field, and especially relates to an application of long-chain non-coding RNA in the preparation of non-small cell lung cancer treatment drugs. The change of the lnc-uc002llc.1 expression has influences on the apoptosis, proliferation, the drug susceptibility and the like of non-small cell lung cancer cells, so the reduction of the lnc-uc002llc.1 expression can realize the apoptosis promotion, proliferation inhibition and chemotherapy drug susceptibility enhancement of the non-small cell lung cancer cells.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Method for detecting microorganisms and detection kit
InactiveUS20030068777A1FungiMaterial analysis by observing effect on chemical indicatorMicroorganismOxidation-Reduction Agent
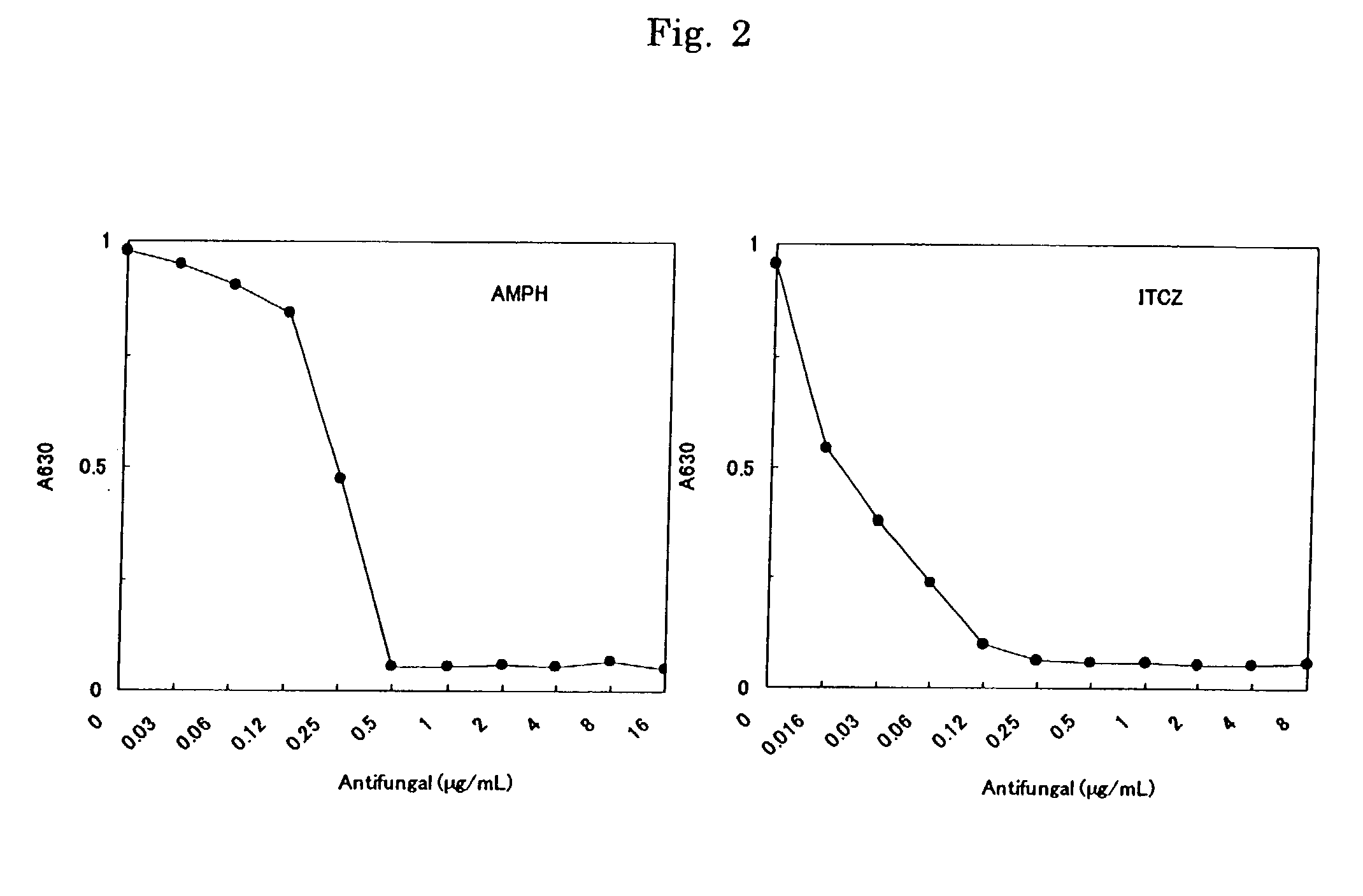
A method for detecting a microorganism by coloration is provided that includes adding and reacting, in a liquid culture medium, an alkaline sensitizing solution and a coloring reagent containing a redox dye, the liquid culture medium having been inoculated with a test sample, thereby detecting the microorganism by coloration in the reaction. There is also provided a method for testing drug susceptibility of a microorganism using above-mentioned method. Furthermore, kits used in these methods are provided. The invention is useful to assess readily and objectively the growth of microorganism when carrying out e.g. a detection of microorganism in foods and a test such as a drug susceptibility test.
Owner:KANTO CHEM CO INC
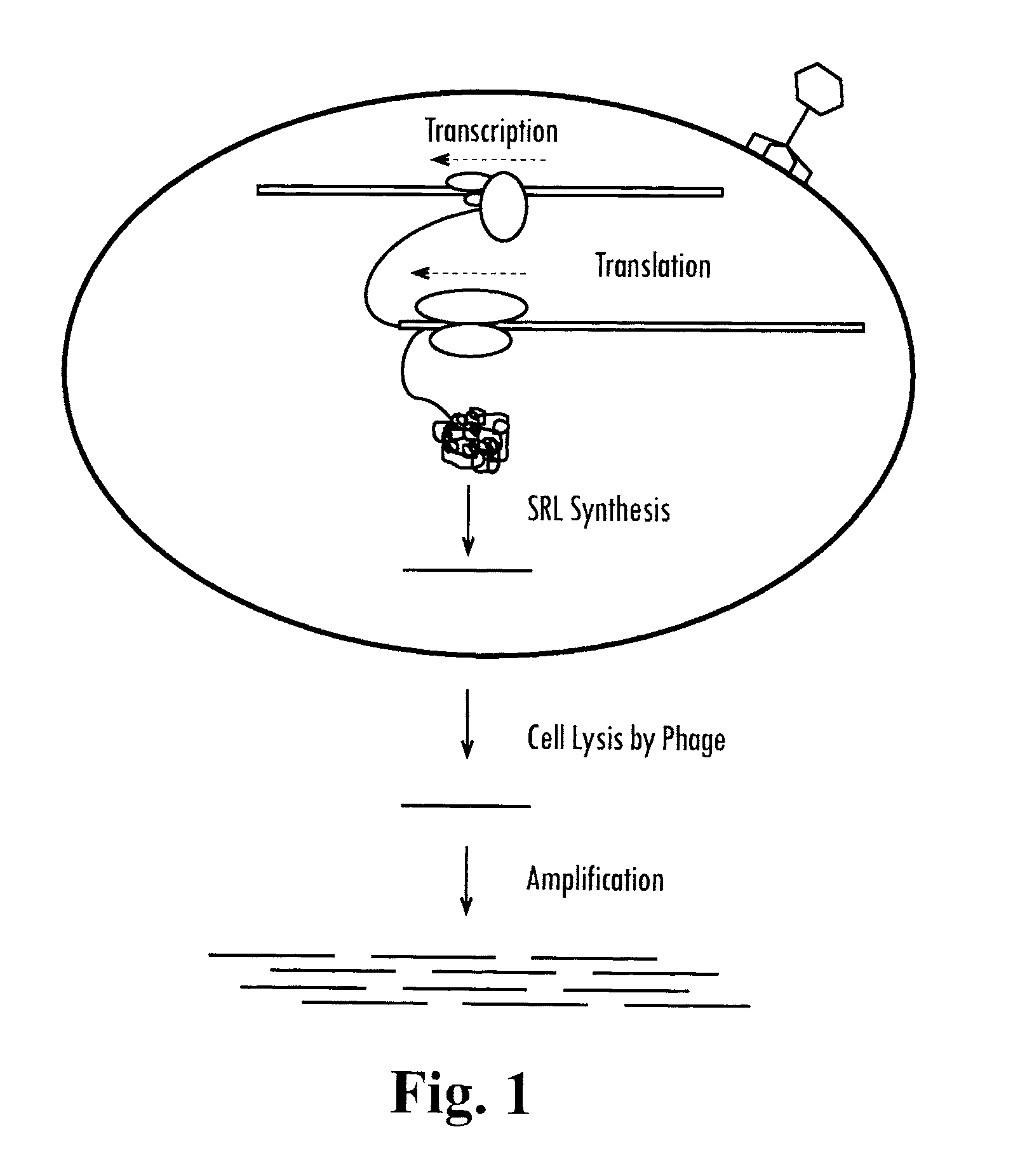
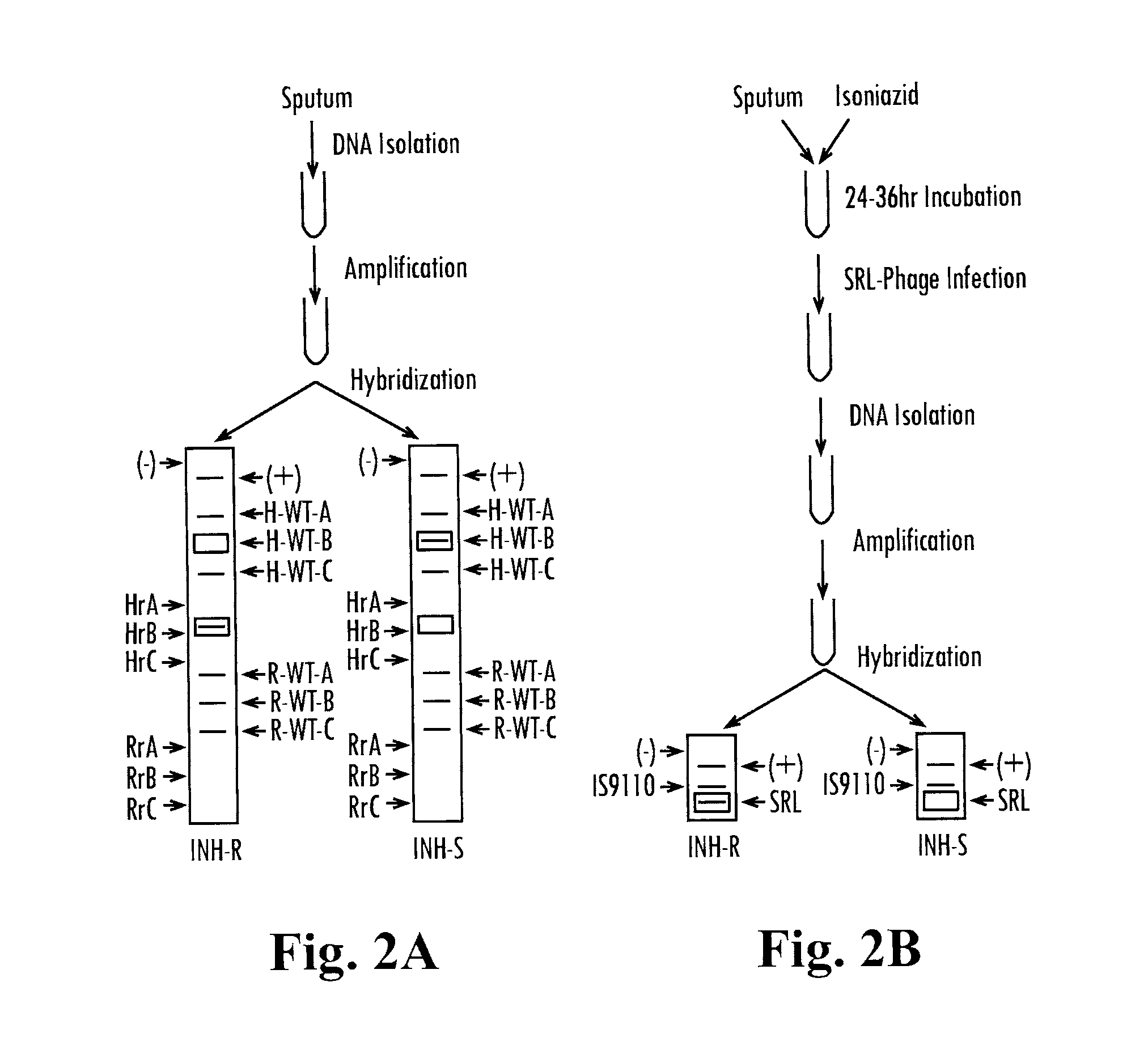
Methods and compositions for determining the pathogenic status of infectious agents
InactiveUS20090047658A1Effective therapyAssessing drug susceptibilityMicrobiological testing/measurementMycobacterium InfectionsMicrobiology
Methods and compositions for the detection of disease caused by infectious agents and microbes are provided. In particular, methods and compositions comprising novel combinations of nucleic acid amplification and drug susceptibility technologies are provided. In certain embodiments, the present invention enables the detection of infectious agents and microbes as well as providing information concerning the viability status of the agent or microbe. In one embodiment, the present invention is used for the detection of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA
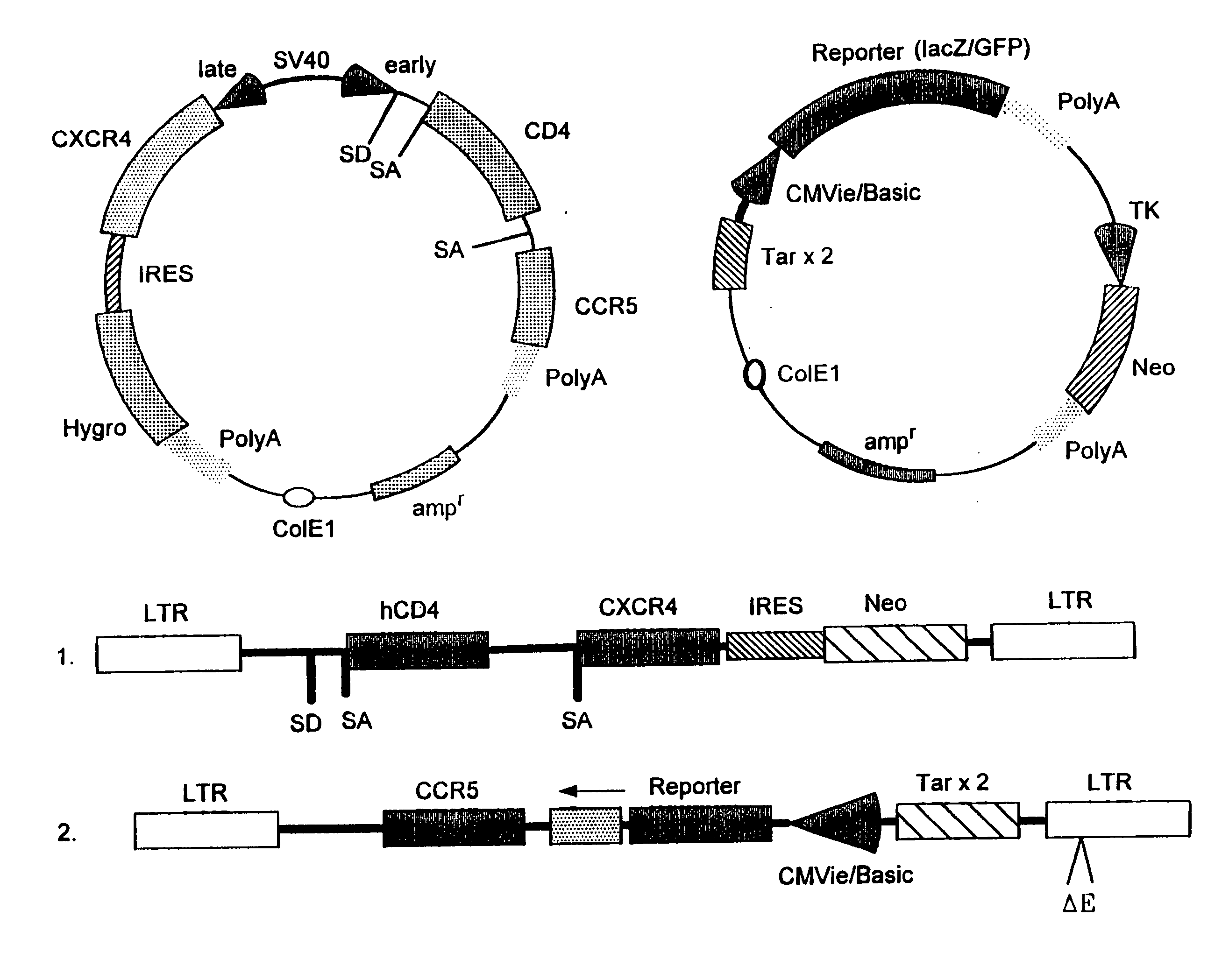
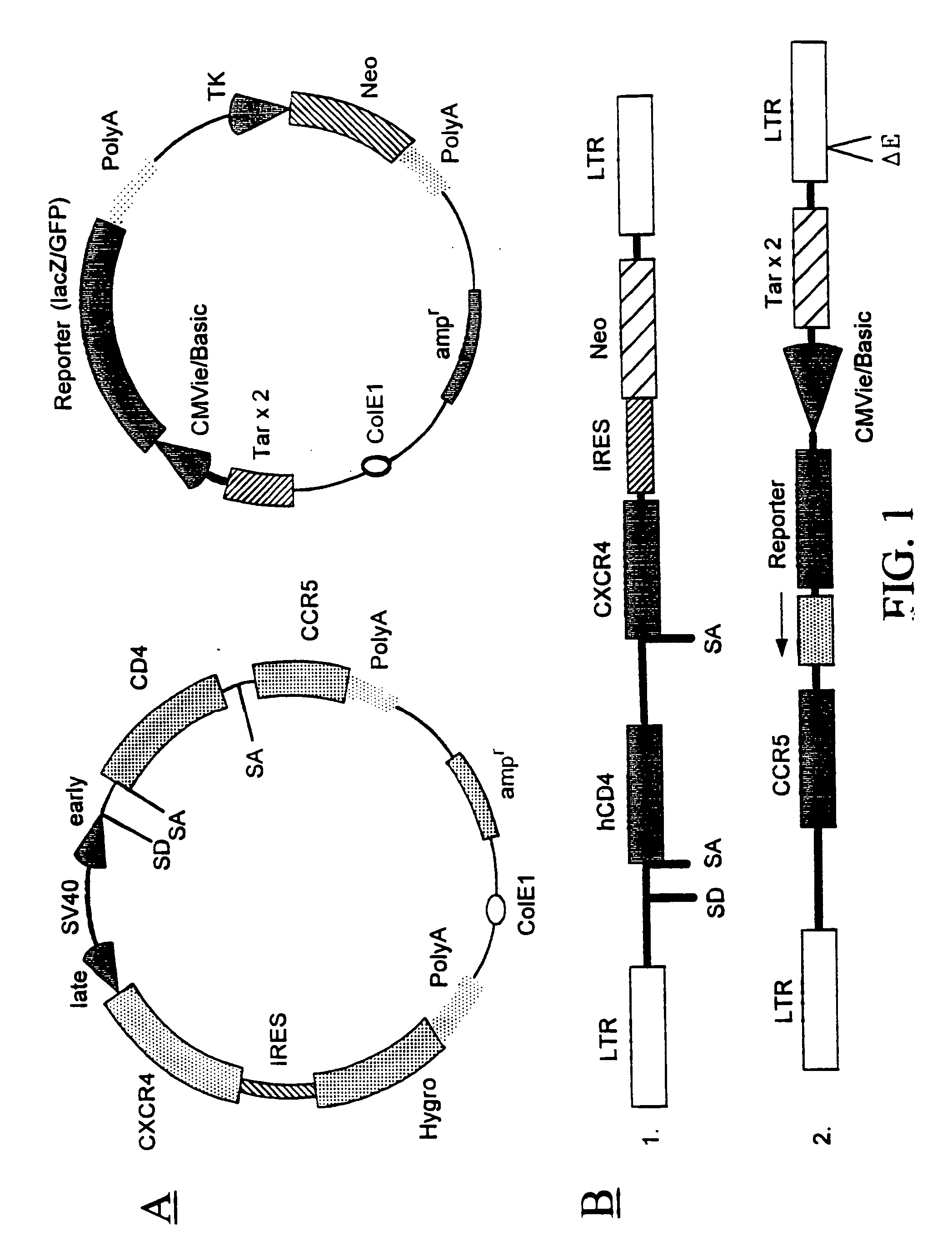
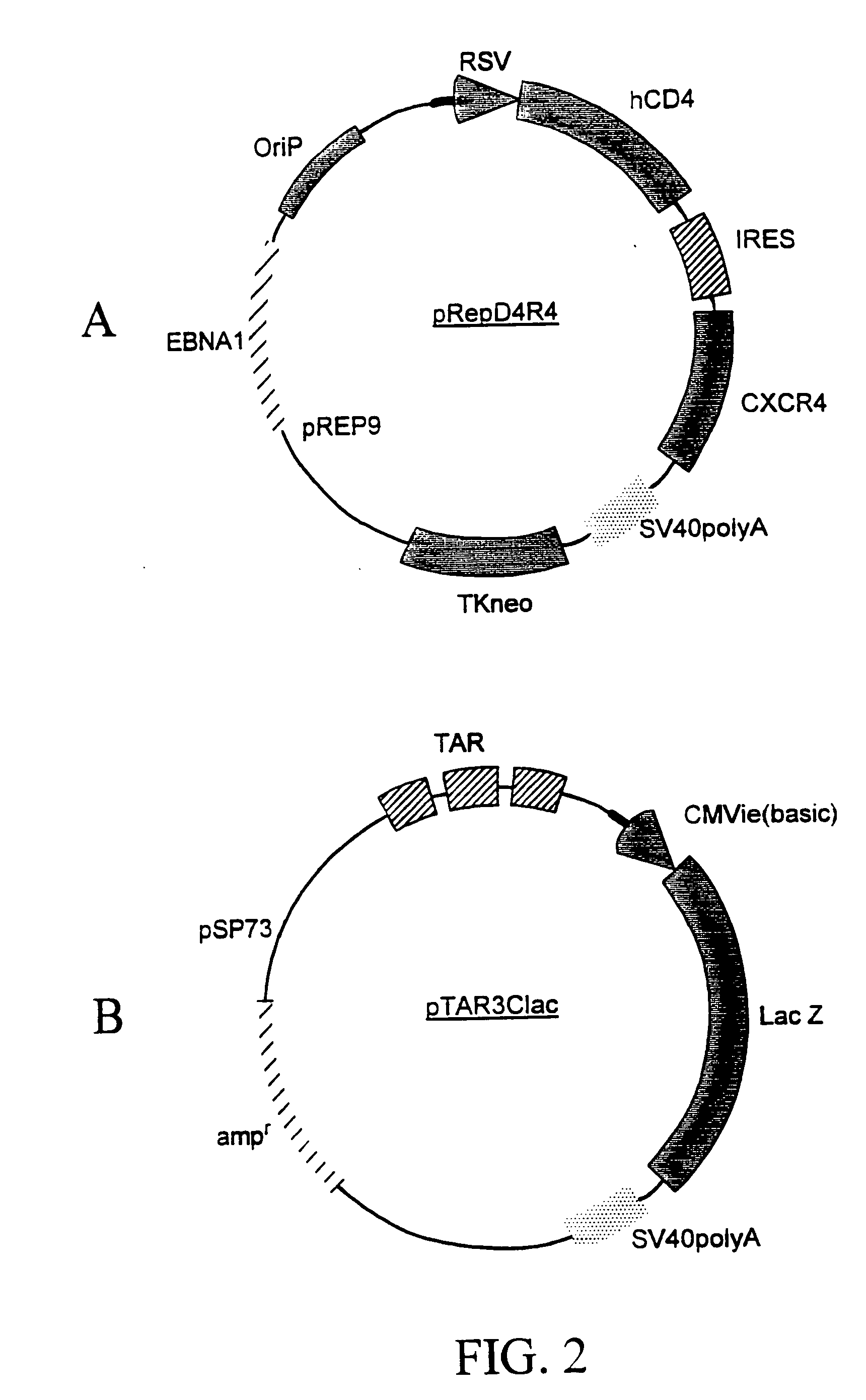
Method for testing drug susceptibility of HIV
InactiveUS20040106136A1Convenient and cost-effective and ultra sensitiveImprove throughputMicrobiological testing/measurementDisease diagnosisHigh-Throughput Screening MethodsHIV receptor
Methods, compositions and kits are provided for testing susceptibility of HIV to drug treatment, such as drug resistance of HIV and inhibition of HIV replication by a drug candidate. In one aspect of the invention, a method is provided for detecting drug resistance of HIV contained in a sample from an individual infected with HIV. In one embodiment, the method employs an indicator cell line which over-expresses CD4 and one or more co-receptors for HIV such as CXCR4 and CCR5 at high levels to render the cells susceptible to productive infection of various strains, subtypes or clades of HIV from both laboratory and clinical isolates. The methods, compositions and kits can be used for high throughput screening of HIV patient samples, anti-HIV agents, and for designing customized HIV therapy.
Owner:MUSC FOUND FOR RES DEV
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS20100062524A1Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsTranscriptional Regulatory ElementsTransgene
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosaftey and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Micro-fluidic chip and application thereof in authentication of pathogene and susceptibility testing
ActiveCN106238112AExpand the range of detection objectsGood application effectMicrobiological testing/measurementLaboratory glasswaresConcentration gradientDigestion
The invention discloses a micro-fluidic chip and application thereof in authentication of pathogene and susceptibility testing. The characteristic that an agar culture-medium has high-temperature digestion and low-temperature solidification is utilized, an authenticating culture medium is placed on the middle layer of the chip, an upper layer chip concentration gradient generator is utilized, and a drug to be studied is introduced; the drug is separated in different culture ponds, multiple pathogene analysis is achieved through the space resolving power of a culture pond array, pathogene authentication is achieved according to the specificity developing result, pathogene quantifying is achieved through real-time developing strength analysis, and the drug susceptibility is determined according to the lowest antibiotic concentration of a developing inhibiting reaction. The micro-fluidic chip is especially suitable for pathogene analysis under the deficient medical resource condition, and has wide application prospects.
Owner:QIQIHAR MEDICAL UNIVERSITY
Tumor chemotherapy drug susceptibility detection chip and using method thereof
ActiveCN104328040AGuaranteed not to touch each otherSave manpower and material resourcesBioreactor/fermenter combinationsBiological substance pretreatmentsAdditive ingredientNutrient solution
The invention discloses a tumor chemotherapy drug susceptibility detection chip and a using method thereof. The tumor chemotherapy drug susceptibility detection chip is formed by sealing a shell with a chip microstructure on the inner surface and a bottom carrier, wherein the chip microstructure comprises a nutrient solution perfusion unit, four cell culture units and a biological glue filling unit. According to the tumor chemotherapy drug susceptibility detection chip, multiple cell ingredients in in-vitro tumor tissue can be integrated on one micro chip for co-culture, biological factors generated by multiple cells can be diffused to the whole micro platform, heterocytotropic cells are ensured not to be contacted mutually, and the in-vivo growth microenvironment of the in-vitro tumor cell can be simulated to the maximum extent.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
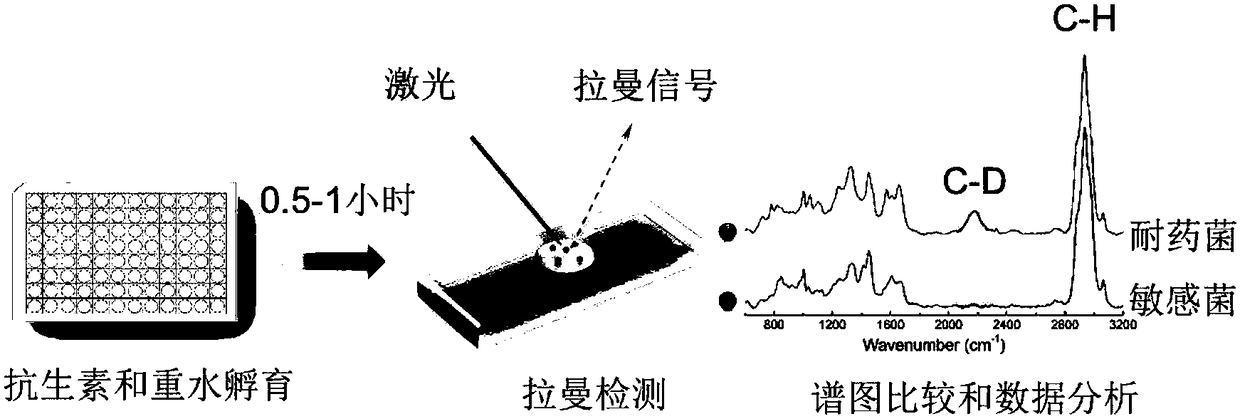
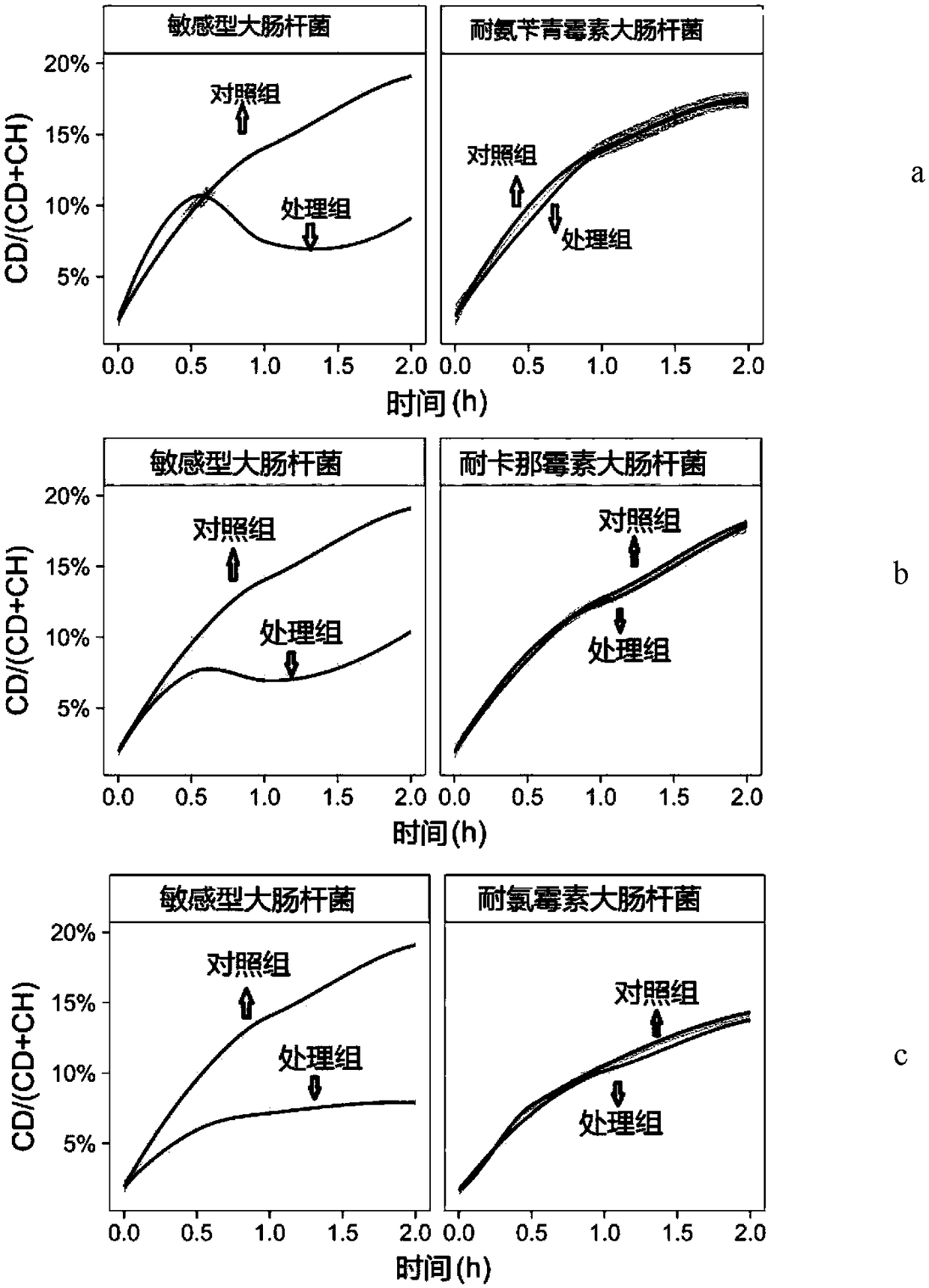
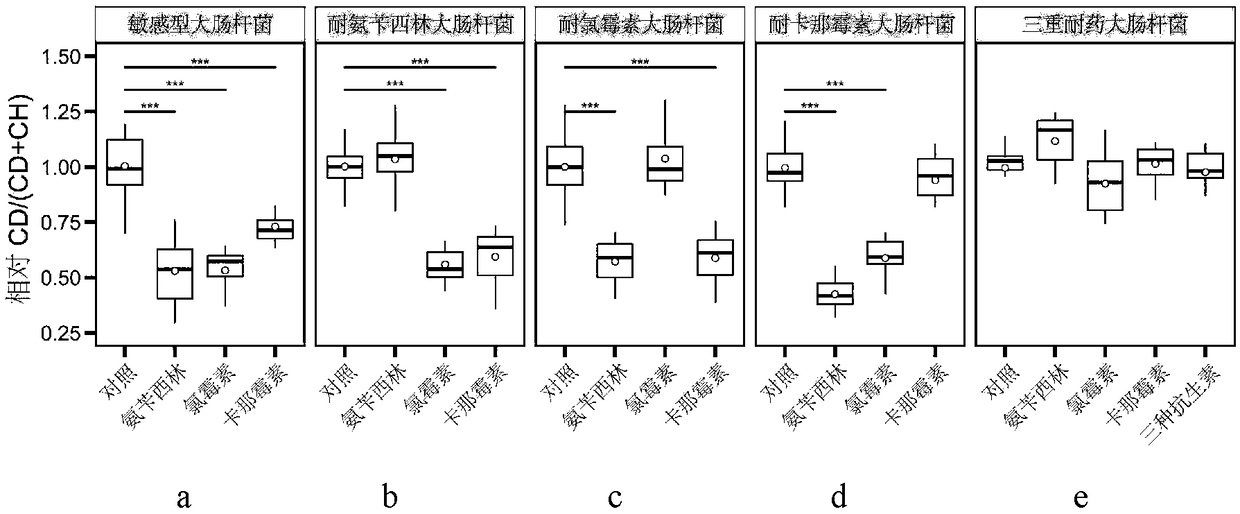
Raman spectroscopy-heavy water isotope labeling based rapid detection method for drug susceptibility of drug-resistant bacteria and judgment method for rational drug use
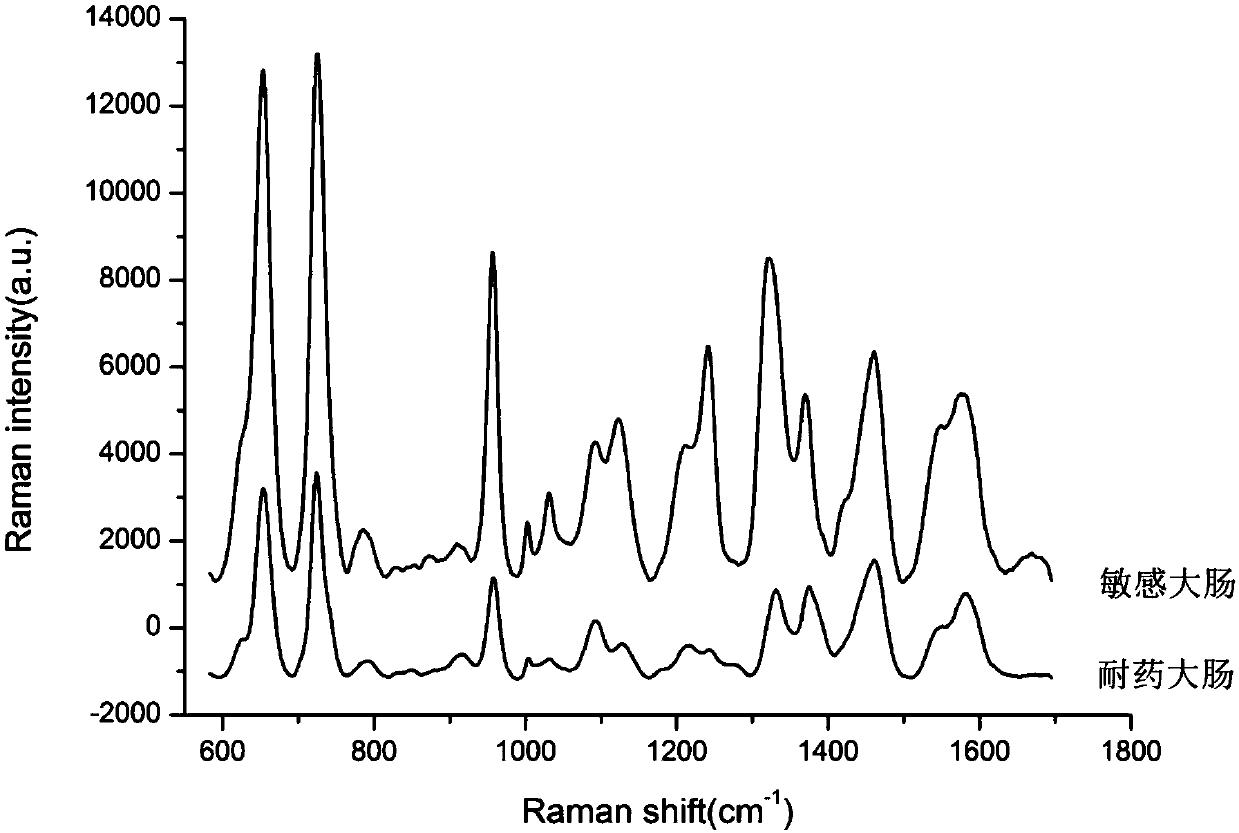
The invention discloses a Raman spectroscopy-heavy water isotope labeling based rapid detection method for the drug susceptibility of drug-resistant bacteria and a judgment method for rational drug use. Directed at the disadvantage of long time in traditional drug susceptibility detection, the invention utilizes the principle that drug-resistant bacteria and sensitive bacteria have different activities under the action of antibiotics and accordingly C-D Raman peaks have different intensities, and realizes rapid detection of drug susceptibility within 0.5-1h. A to-be-detected object is incubated in a culture solution containing heavy water, wherein the group containing antibiotics is adopted as the treatment group, and the group without antibiotics is adopted as the control group, the incubated to-be-detected object is subjected to centrifugal cleaning, then Raman detection is carried out, the C-D / (C-D+C-H) values of the treatment group and the control group are calculated respectively,and the ratio of the treatment group and the control group is taken as the judgment standard, if the value is smaller than or equal to 0.75, the detected object can be susceptible to antibiotics, andif the value is greater than 0.75, the detected object can be resistant to the antibiotics. The method is simple, is easy for operation and analysis, and is suitable for different antibiotics and different bacteria, thus having clinical application prospects.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI
Long non-coding RNA (Ribonucleic Acid) and application thereof
InactiveCN105177005APromote apoptosisPrevent proliferationGenetic material ingredientsAntineoplastic agentsApoptosisOncology
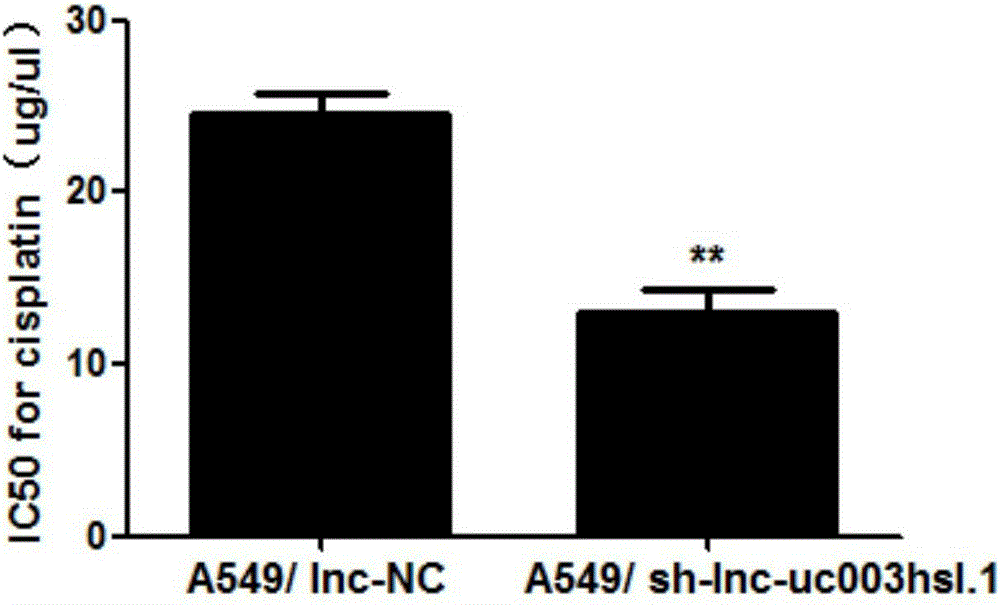
The invention belongs to the field of genetic engineering and particularly relates to application of long non-coding RNA (Ribonucleic Acid) uc003hsl.1 in preparation of drugs for treating non-small cell lung cancer. The influence on the apoptosis, propagation, drug susceptibility and the like of non-small cell lung cancer cells is caused through changing the expression of the long non-coding RNA uc003hsl.1, so that through lowering the expression of the long non-coding RNA uc003hsl.1, the apoptosis of the non-small cell lung cancer cells can be promoted, the propagation of the non-small cell lung cancer cells can be inhibited, and the susceptibility to chemotherapy drugs of the non-small cell lung cancer cells can be enhanced.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
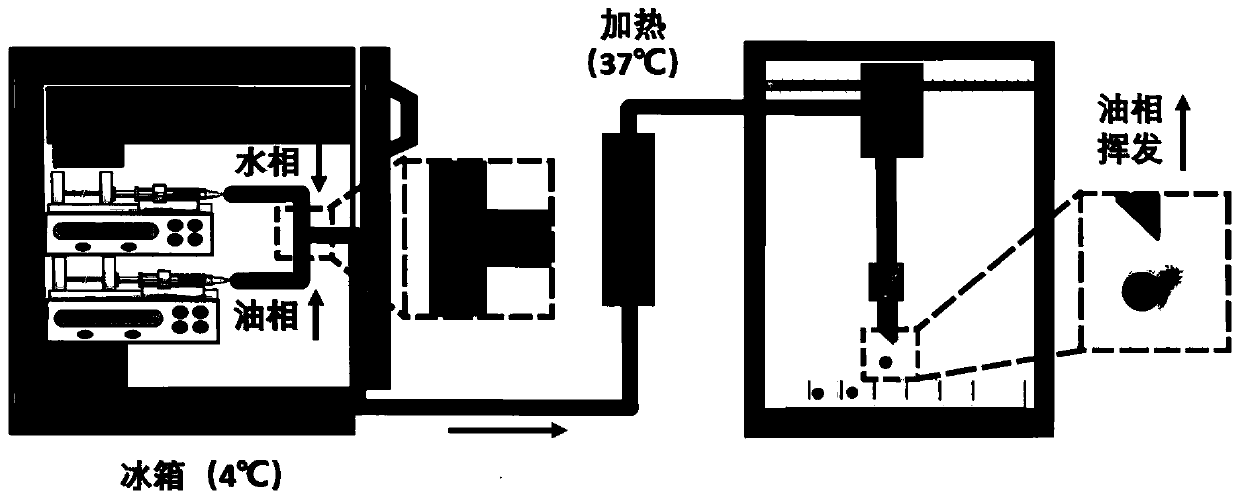
High-flux cultural method for organoid type spheroid
The invention provides a high-flux cultural method for an organoid type spheroid. The high-flux cultural method comprises the following steps of (1) respectively injecting cell-containing matrigel andfluorocarbon oil into a tee device to obtain the organoid type spheroid; and (2) heating the organoid type spheroid obtained in the step (1), linking the heated organoid type spheroid with a 3D printing platform, distributing the organoid type spheroid, and then culturing the organoid type spheroid. According to the high-flux cultural method provided by the invention, through micro-fluidic, an organoid is obtained, through combination with the 3D printing platform, the organoid can be simply, quickly and accurately printed to a culture carrier, the size and the structure of the organoid are appropriate and uniform and controllable, the number of the organoids in a culture cavity of each culture carrier is unchanged, and besides, drug susceptibility test and toxicity test are realized. Clinical pretest is accurate, the application prospect is favorable, and the high-flux cultural method has high application value.
Owner:TSINGHUA BERKELEY SHENZHEN INST
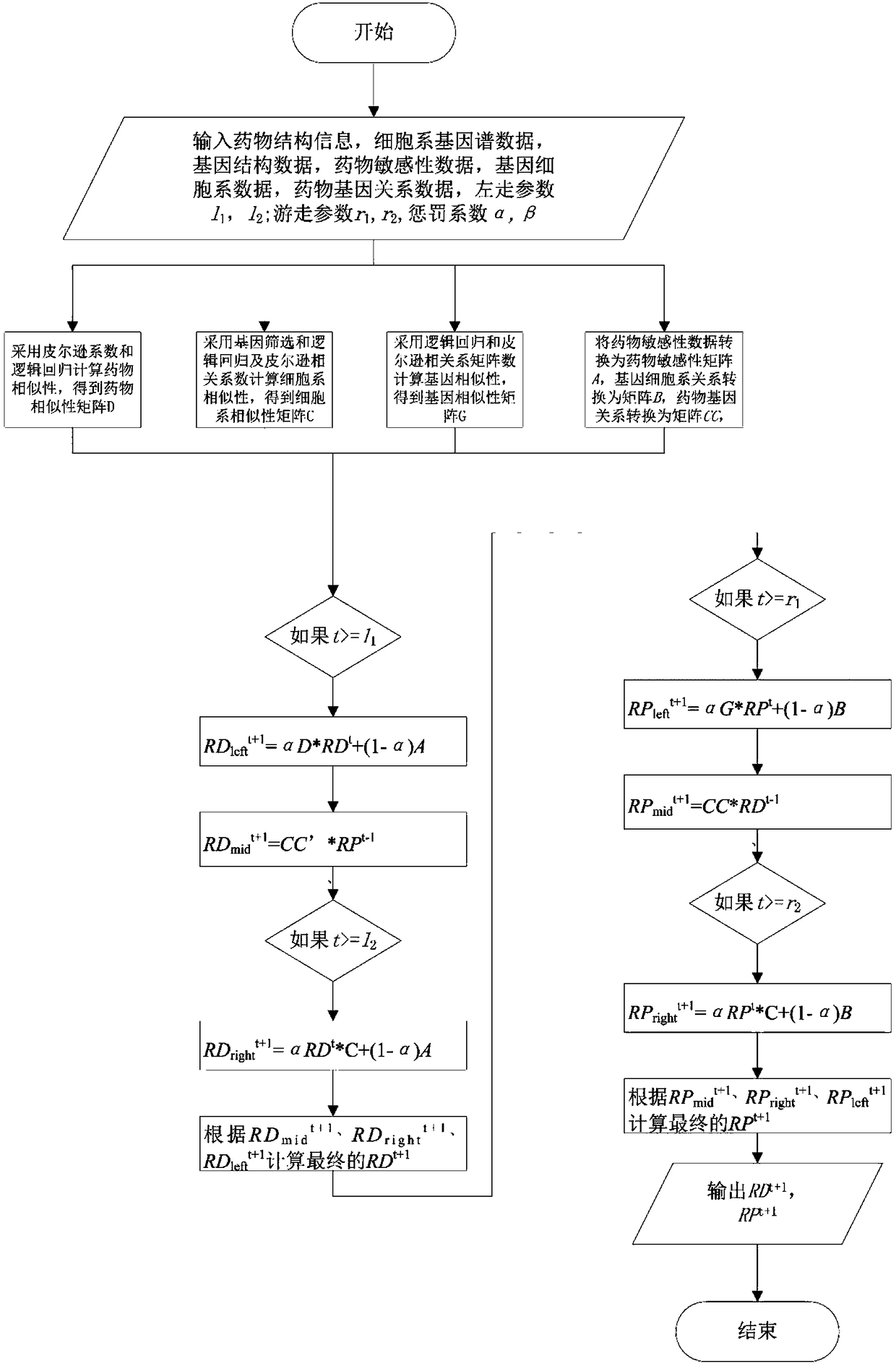
Drug susceptibility prediction method based on multi-similarity network
ActiveCN108877953AAccurate predictionImprove accuracyDrug referencesProtein structureComputer science
The present invention discloses a drug susceptibility prediction method based on a multi-similarity network. The method comprises the steps of: employing drug structure information to construct a drugsimilarity network, employing cell line gene expression profile data to construct a cell line similarity network after gene screening, and calculating a gene similarity network according to protein structure information; on this basis, establishing an association relation among drugs, the cell line and the genes, and performing three random walk in multiple networks formed by the constructed drugsimilarity network, the cell line similarity network and the gene similarity network to predict the drug susceptibility. On the basis of simpleness and practicability, the drug susceptibility prediction method based on a multi-similarity network can improve the drug susceptibility identification accuracy and can provide important reference for researchers to perform drug design.
Owner:CENT SOUTH UNIV
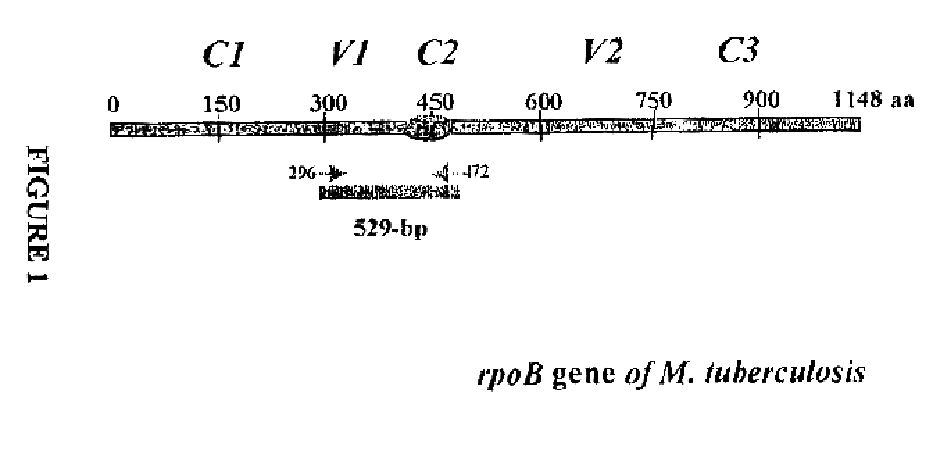
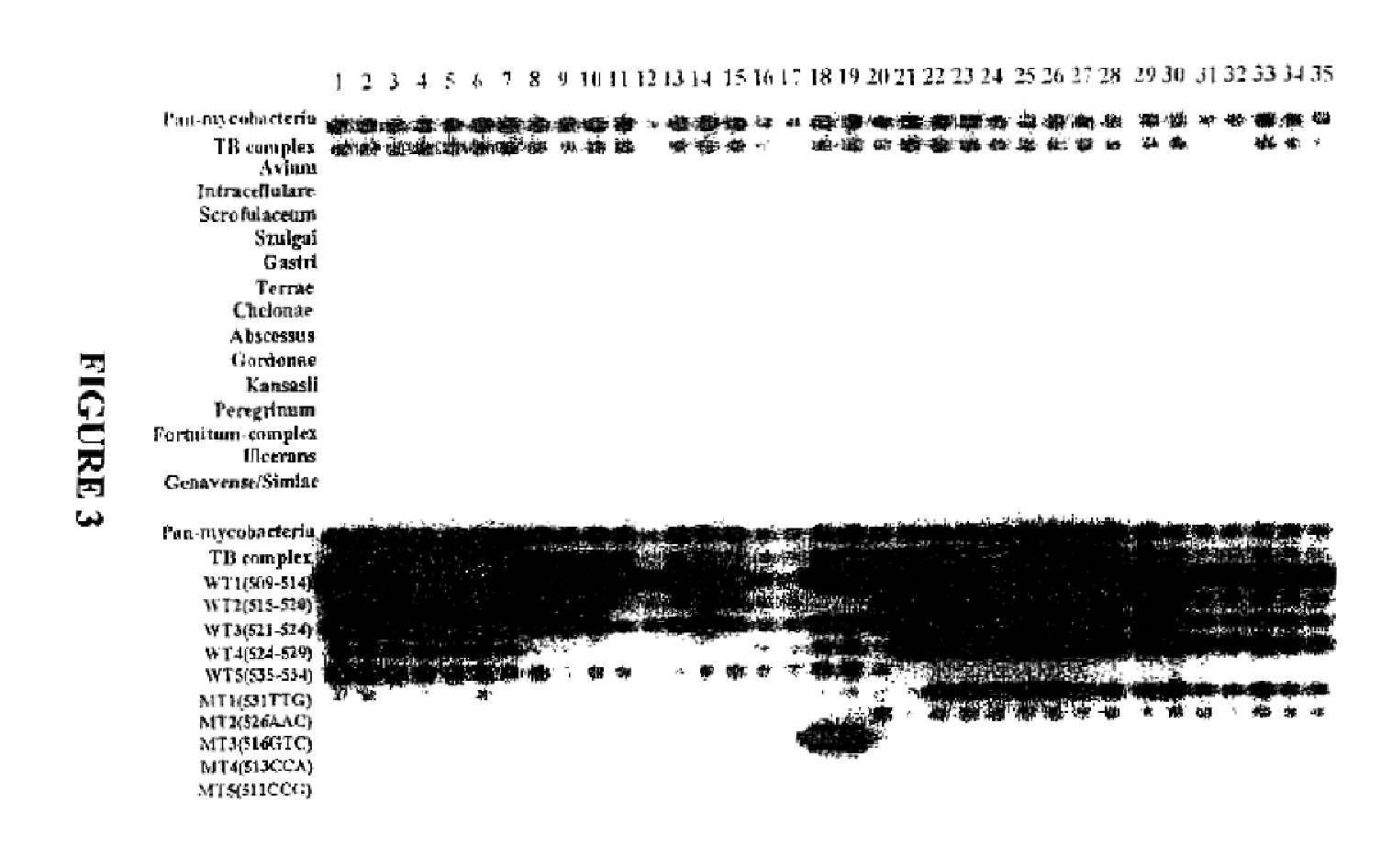
Method for identifying mycobacterium tuberculosis and mycobacteria other than tuberculosis, together with detecting resistance to an antituberculosis drug of mycobacteria obtained by mutation of rpob gene
The present invention provides a method for identifying Mycobacterium tuberculosis and non-tuberculosis Mycobacterium (MOTT), and for the determination of drug susceptibility of M. tuberculosis based on detection of mutations in the rpoB gene.
Owner:LEE HYEYOUNG +1
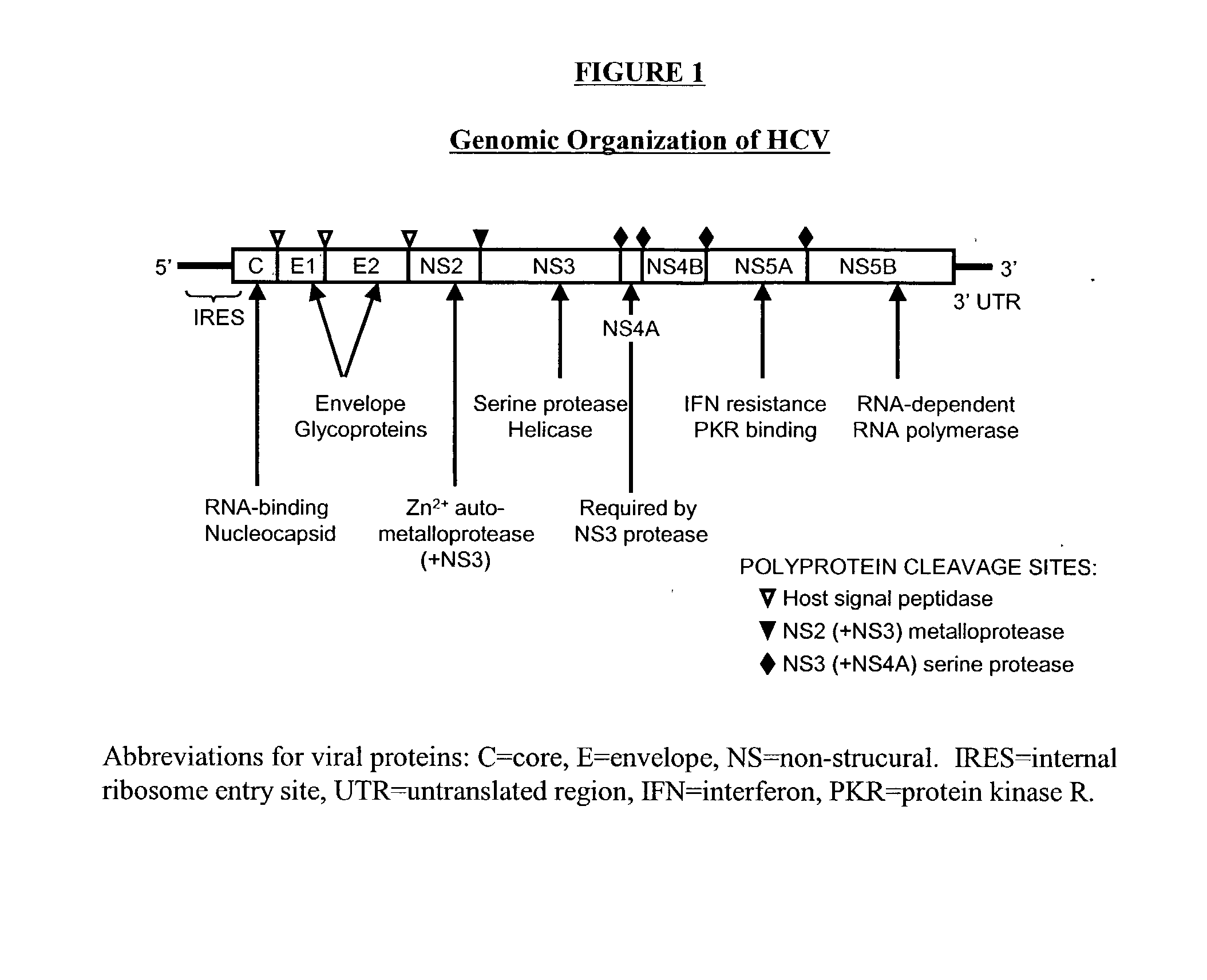
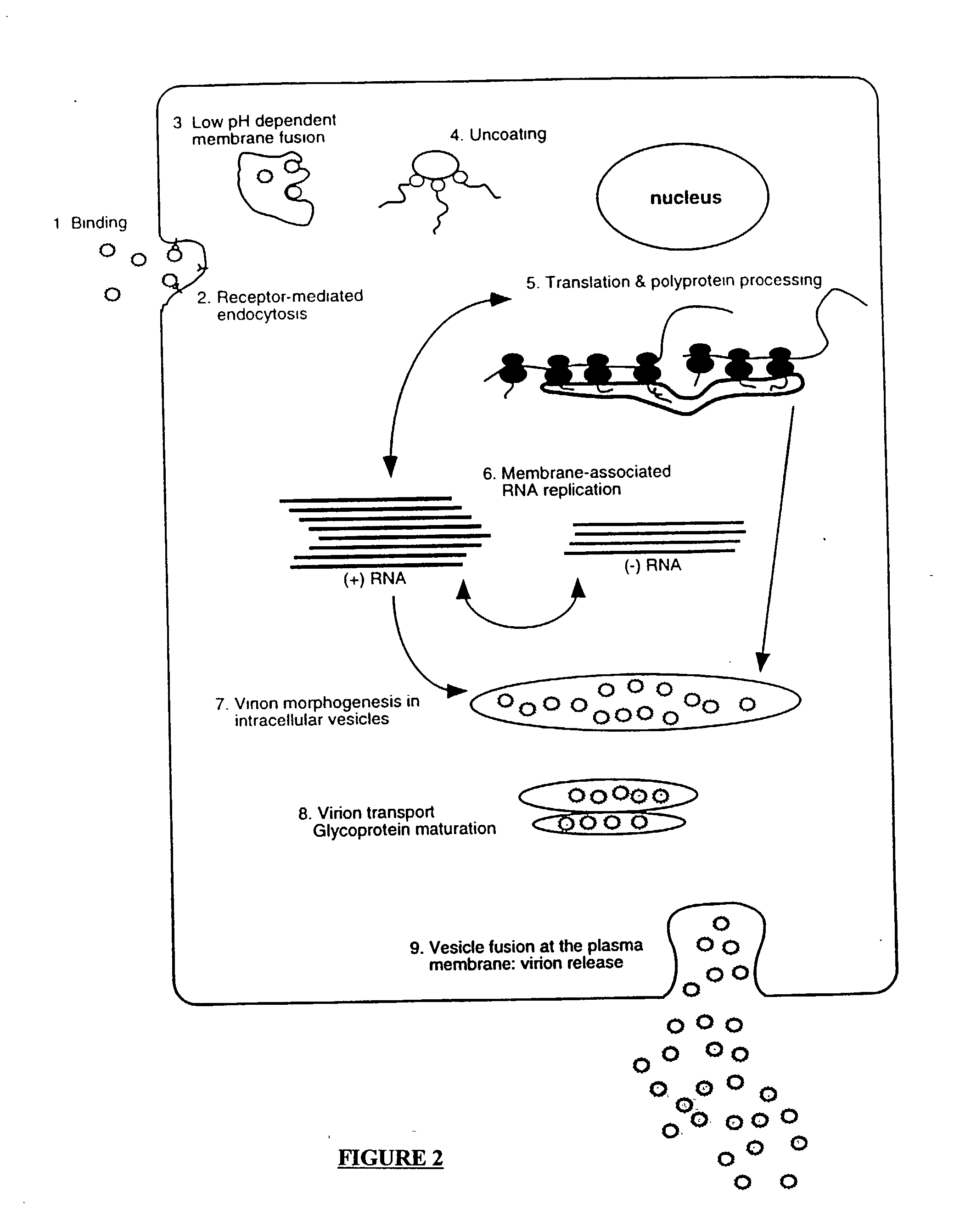
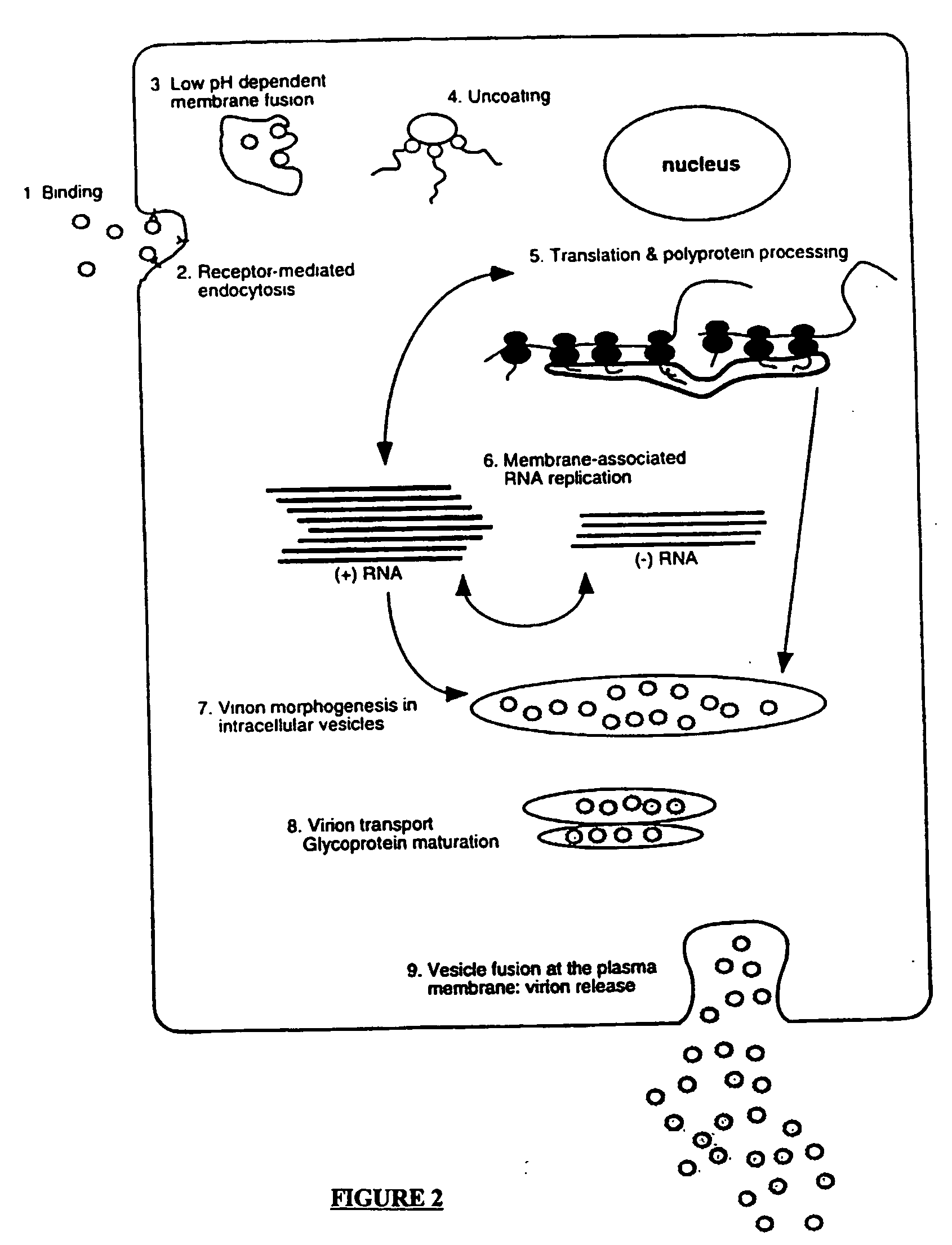
Compositions and methods for determining susceptibility of hepatitis C virus to anti-viral drugs
InactiveUS20030028011A1Change activityAltered susceptibilityOrganic active ingredientsSsRNA viruses positive-senseAntiviral drugDrug compound
The present invention provides methods for determining the susceptibility of a pathogenic flavivirus to anti-viral compounds. This invention also provides methods for determining anti-viral drug susceptibility in a patient infected with a flavivirus. This invention also provides a method for evaluating the biological effectiveness of a candidate anti-viral drug compound. The methods are useful for identifying effective drug regimens for the treatment of flaviviral infections, and identifying and assessing the biological effectiveness of potential therapeutic compounds. Compositions including resistance test vectors and host cells transformed with the resistance test vectors are provided.
Owner:VIROLOGIC INCORPORATED
Long noncoding RNA SNHG15 and application thereof in preparation of cancer diagnosis and treatment drugs
InactiveCN108707668APrevent invasionInhibit migrationMicrobiological testing/measurementCancers diagnosisColorectal cancer cell line
The invention belongs to the technical field of genetic engineering, and discloses long noncoding RNA SNHG15 and application thereof in preparation of cancer diagnosis and treatment drugs. Cancers include colorectal cancer, breast cancer, kidney cancer and the like. Expression of the long noncoding RNA SNHG15 in tumor tissues of patients suffering from the colorectal cancer, the breast cancer andthe kidney cancer is up-regulated. The patients with highly-expressed long noncoding RNA SNHG15 have poor prognosis. Through expression knock-down of the long noncoding RNA SNHG15 in colorectal cancercell lines, invasion, migration and multiplication capabilities of cells can be inhibited, and drug susceptibility of colorectal cancer cell lines to antitumor drugs including gemcitabine and cis-platinum can be improved.
Owner:PEKING UNIV
Rapid identification method for carbapenem drug susceptibility, based on Raman spectra technology
InactiveCN107586823AReduce dosageShort detection timeMicrobiological testing/measurementRaman scatteringStructure analysisRapid identification
The invention relates to a rapid identification method for carbapenem drug susceptibility, based on a Raman spectra technology. According to the method, a portable Raman detector is adopted, pathogenic bacteria to be detected is irradiated through laser, different types of nanostructures are taken as detection substrates, and scattered spectrum, the frequency of which is different from the frequency of incident light, is analyzed, so that information, such as molecular vibration, of a sample to be detected is obtained, corresponding molecular structure analysis is performed, obtained spectroscopic data of carbapenem drug sensitive bacteria and drug-resistance bacteria is analyzed through a chemometrics method, so that drug-resistance bacteria and sensitive bacteria are distinguished, and the method becomes an ultrasensitive pathogenic bacteria rapid detection tool. Compared with the clinically traditional drug sensitivity pathogenic microorganism detection method, the detection technology has the advantages that the quantity of samples is less, the detection time is short, the sensitivity is high, the detection time of clinical samples is shortened, and particularly as for detection samples with complex chemical and biochemical components, the detection efficiency is improved on the basis that the detection quality is guaranteed.
Owner:XUZHOU MEDICAL UNIV
Method for detecting multi-drug resistance
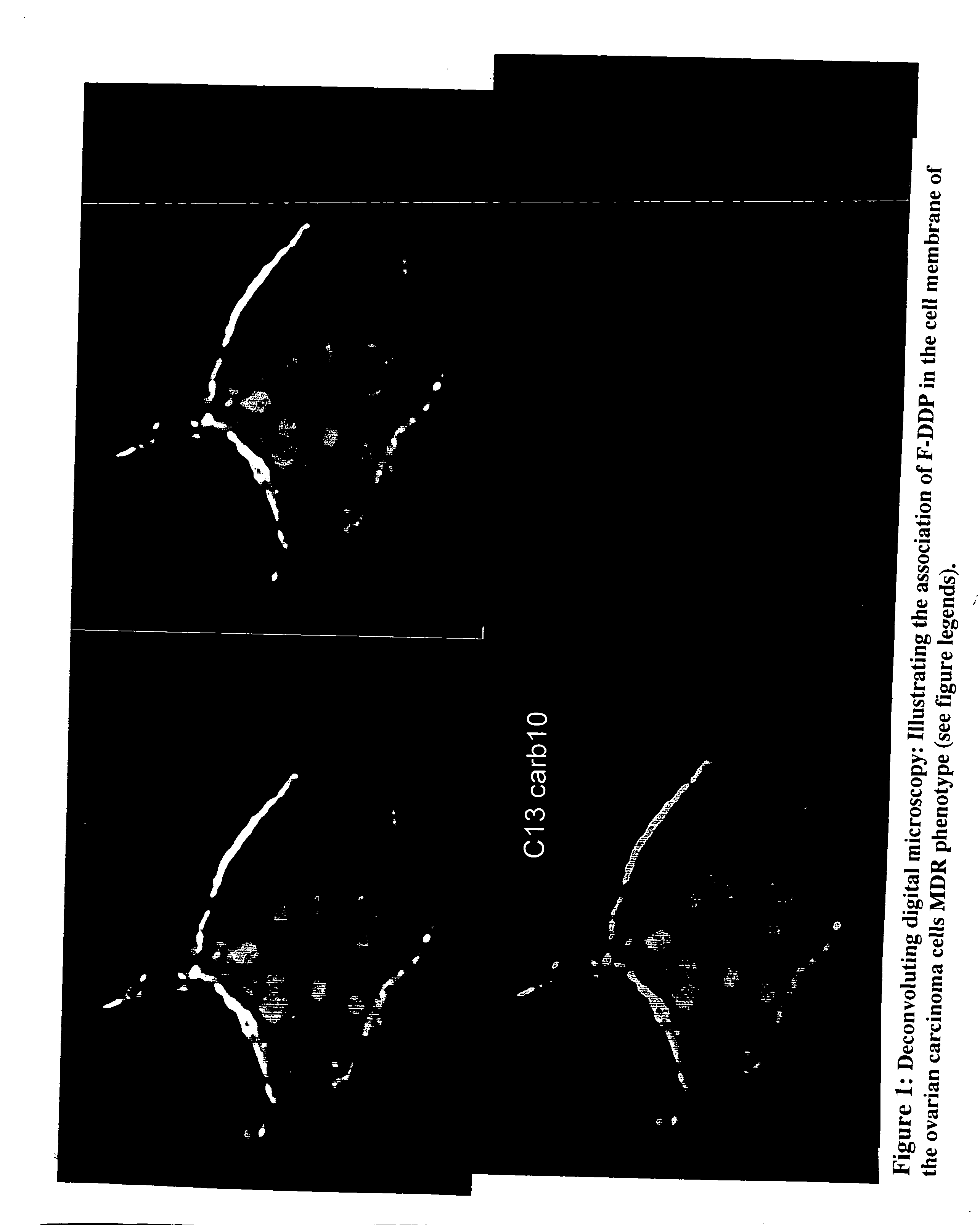
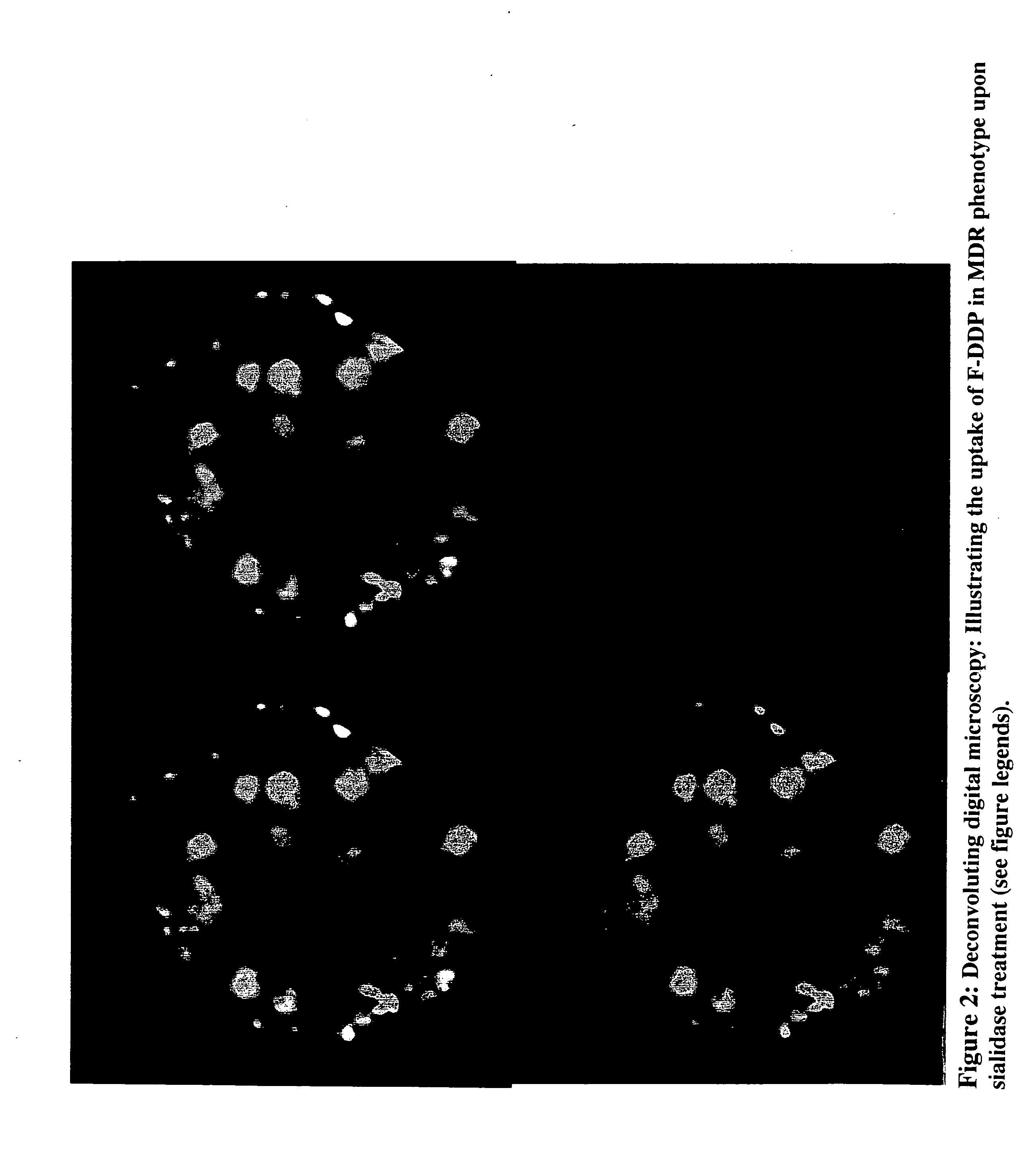
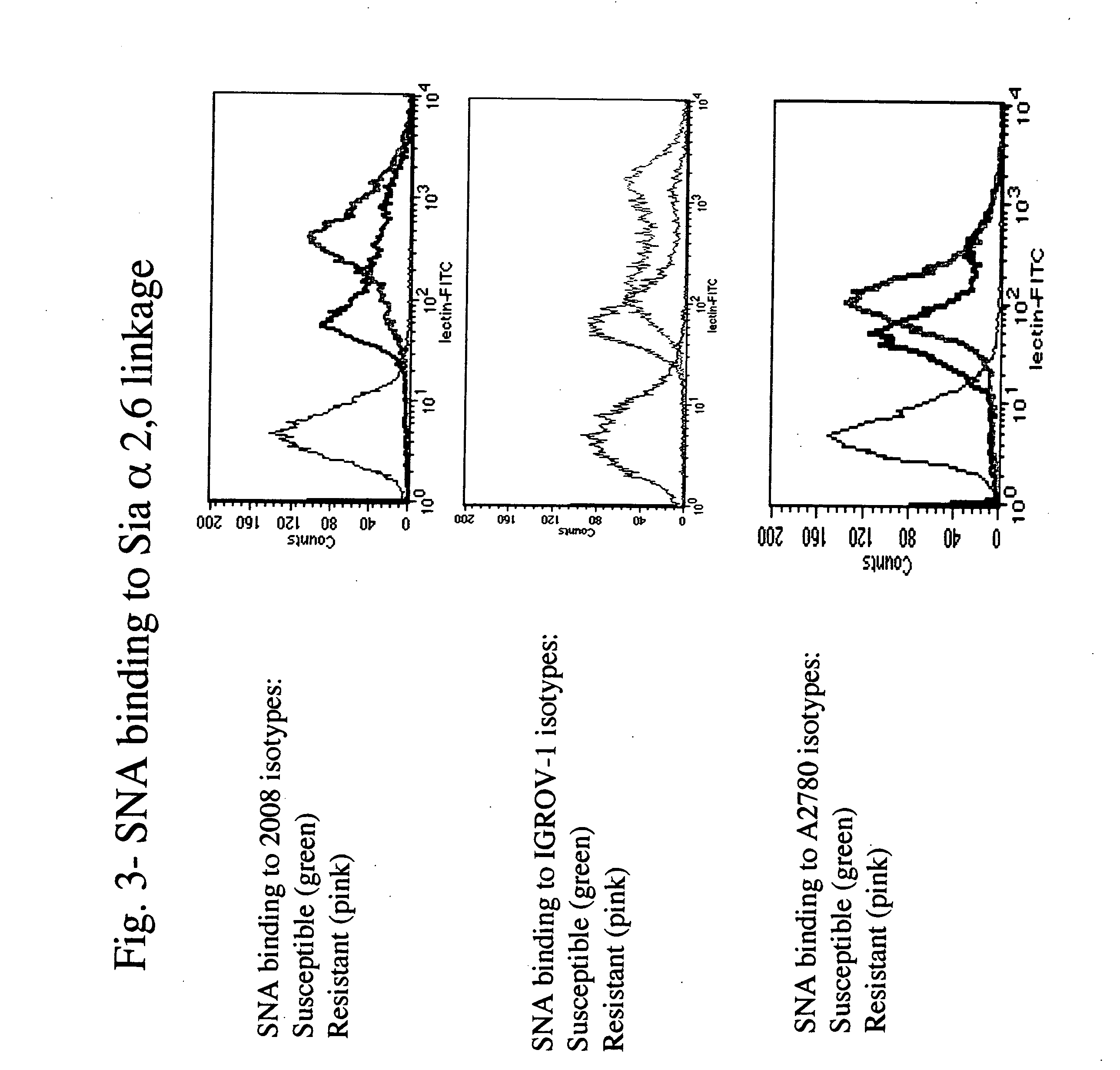
The present invention stems from the realization that the cell surface content of sialic acids is associated with drug susceptibility and resistance in neoplastic and damaged cells. A general decrease in the amounts of the α2-6 linked sialic acid has been confirmed in resistant phenotype compare to its corresponding sensitive parental cells. Treating the resistant phenotype with neuraminidase, an enzyme that removes the sialic acid from the sugar chain, facilitates the drug internalization and reinstalls the cell susceptibility to the drug. Based on these observations, methods for predicting the resistance to a number of drugs and detecting multi-drug resistanc in neoplastic and damaged cells have been invented.
Owner:RAZI NAHID
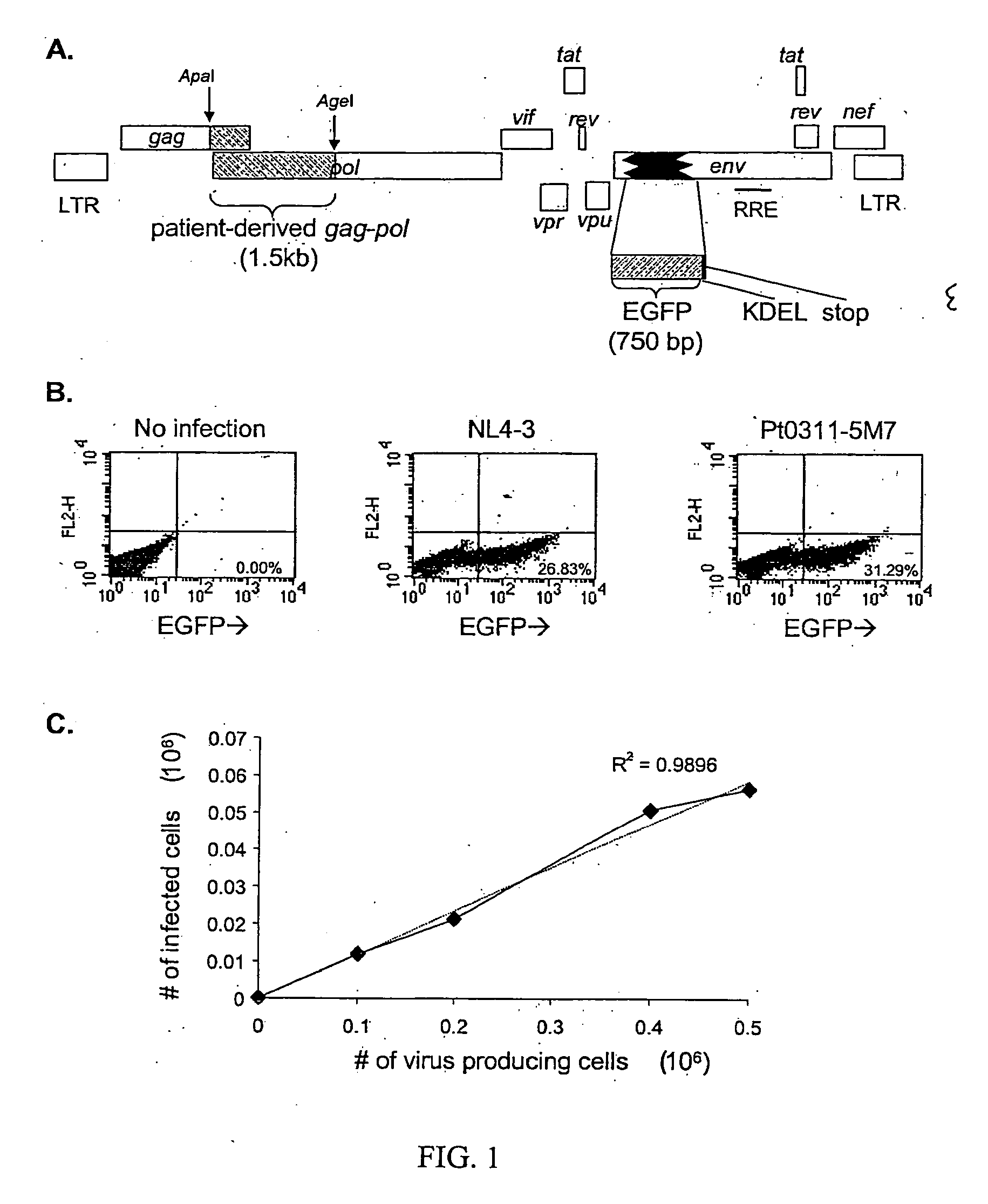
Single cell analysis of HIV replication capacity and drug resistance
ActiveUS20050244818A1Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementAssayResistant virus
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Single cell analysis of HIV replication capacity and drug resistance
ActiveUS7468274B2Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementResistant virusViral replication
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for extracting and purifying eimeria tenella merozoite
ActiveCN102796669AEasy to operateLow costProtozoaMicroorganism based processesEmoia loyaltiensisEimeria
The invention discloses a method for extracting and purifying eimeria tenella merozoite. The method comprises the steps of: releasing the merozoite and purifying the merozoite, wherein the merozoite release comprises mechanical grinding release and enzymic digestion release, 0.0625% pancreatin and 0.175% sodium taurodeoxylate are adopted as digestive juice in the enzymic digestion release process; digestion is moderate, which is beneficial to the activation of the merozoite, can eliminate erythrocytes to obtain pure merozoite. The method is convenient and simple to operate, is low in cost, and is ideal in separation and purification effects; the separated merozoites are large in quantity, are pure with few impurities and keep vigorous energy; and the method is suitable for research on drug susceptibility detection, vaccine research and other biological researches.
Owner:GUANGXI VETERINARY RES INST
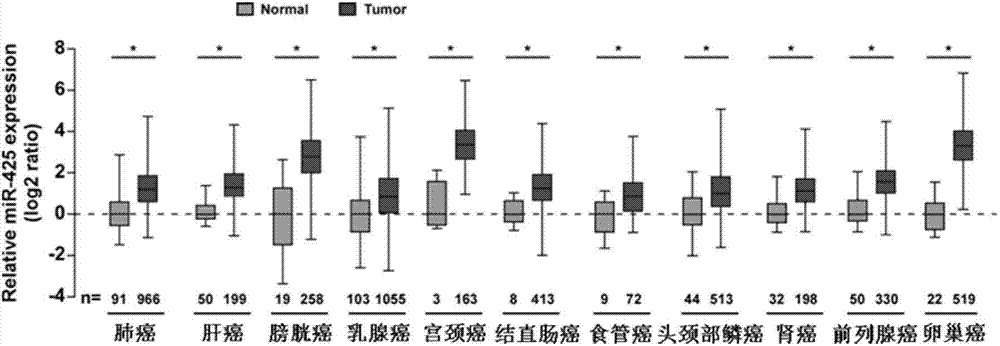
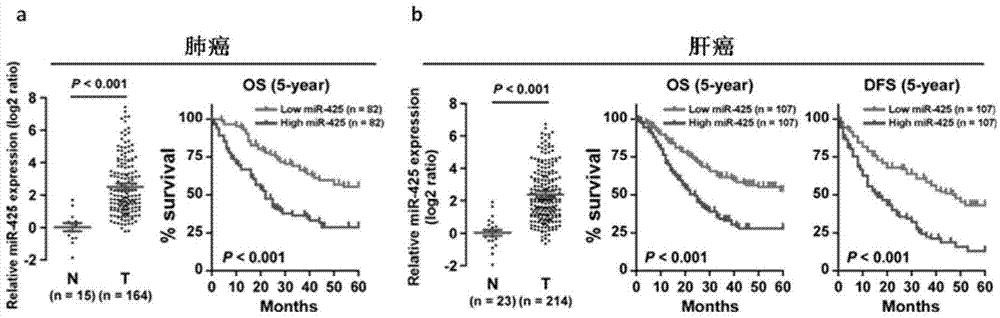
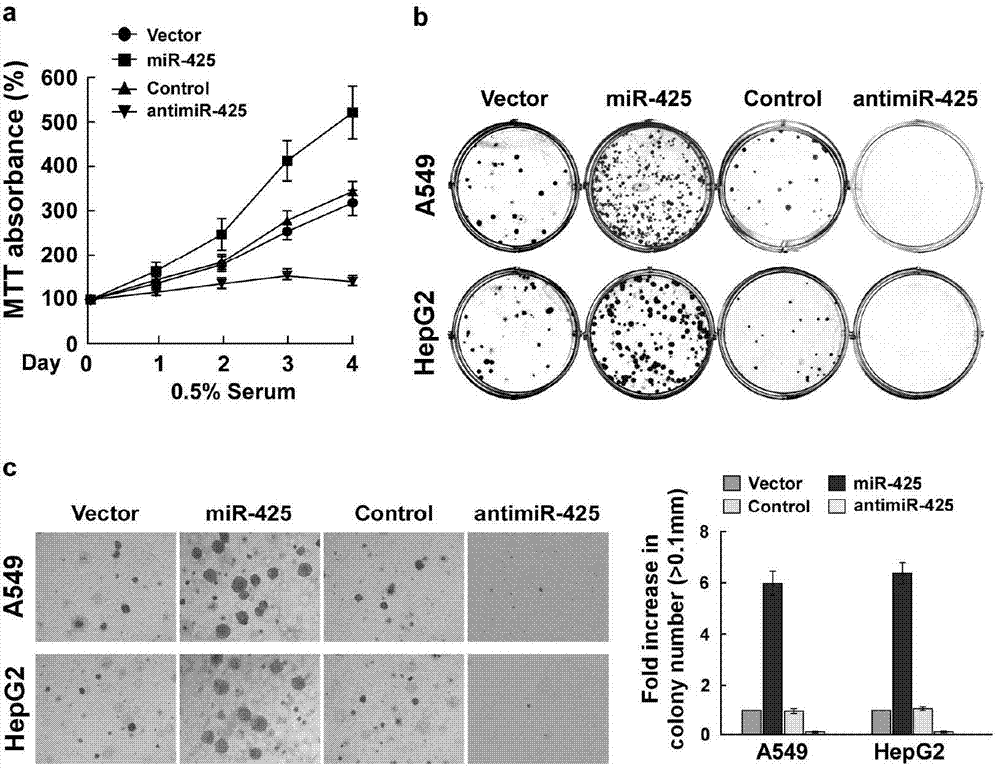
Application of miR-425 in tumor diagnosis, treatment and prognosis
ActiveCN104726584APrevent proliferationInhibit transferGenetic material ingredientsMicrobiological testing/measurementTumor therapyApoptosis
The invention discloses application of miR-425 in tumor diagnosis, treatment and prognosis. Multiple biological characteristics of tumor including apoptosis, proliferation, drug susceptibility and the like disclosed by the invention are closely related to the expression quantity of miR-425. MiR-425 antagonist antimiR-425 can be used for significantly inhibiting proliferation of multiple types of tumor cells and the tumorigenic ability of the tumor cells in a nude mouse, and enhancing the tumor treatment capacity of chemotherapy drug cisplatin and the like in the nude mouse. The invention discloses a kit for tumor assisted diagnosis and patient survival prognosis, and the kit contains a primer sequence for the quantitative determination of miR-425; the invention also discloses a pharmaceutical composition for treating tumor, wherein the composition contains the miR-425 antagonist antimiR-425. The invention provides a novel method for assisted diagnosis and prognosis diagnosis of cancer. The invention discovers that the antimiR-425 has high clinical application value in the preparation of a tumor-treating medicine, and particularly provides a novel medicine and a treatment method for the effective treatment of lung cancer and liver cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
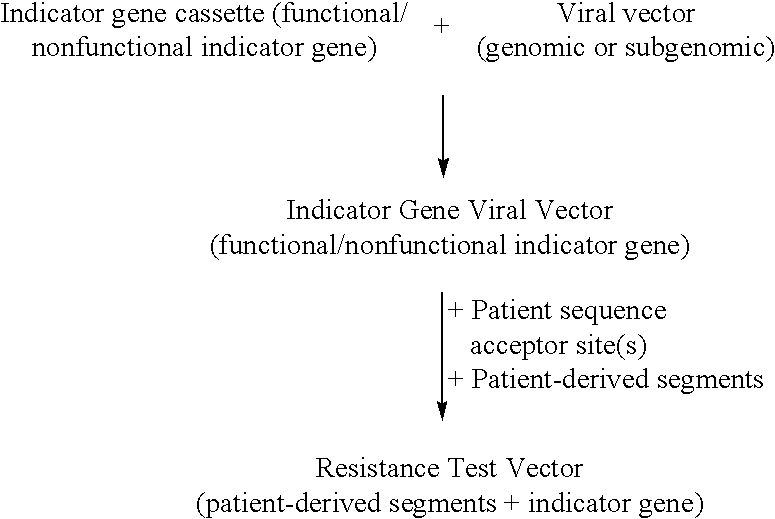
Compositions and methods for determining anti-viral drug susceptibility and resistance and anti-viral drug screening
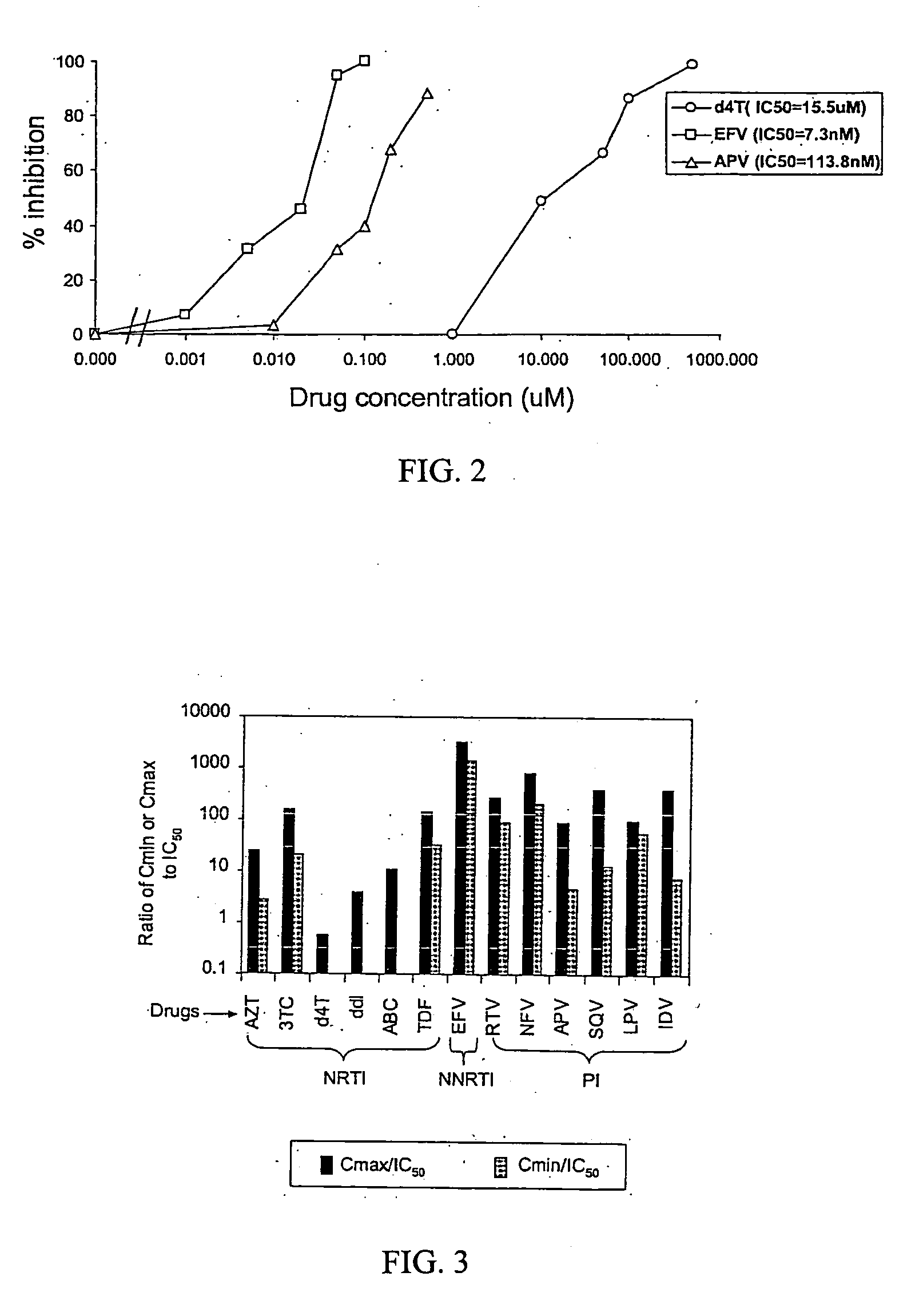
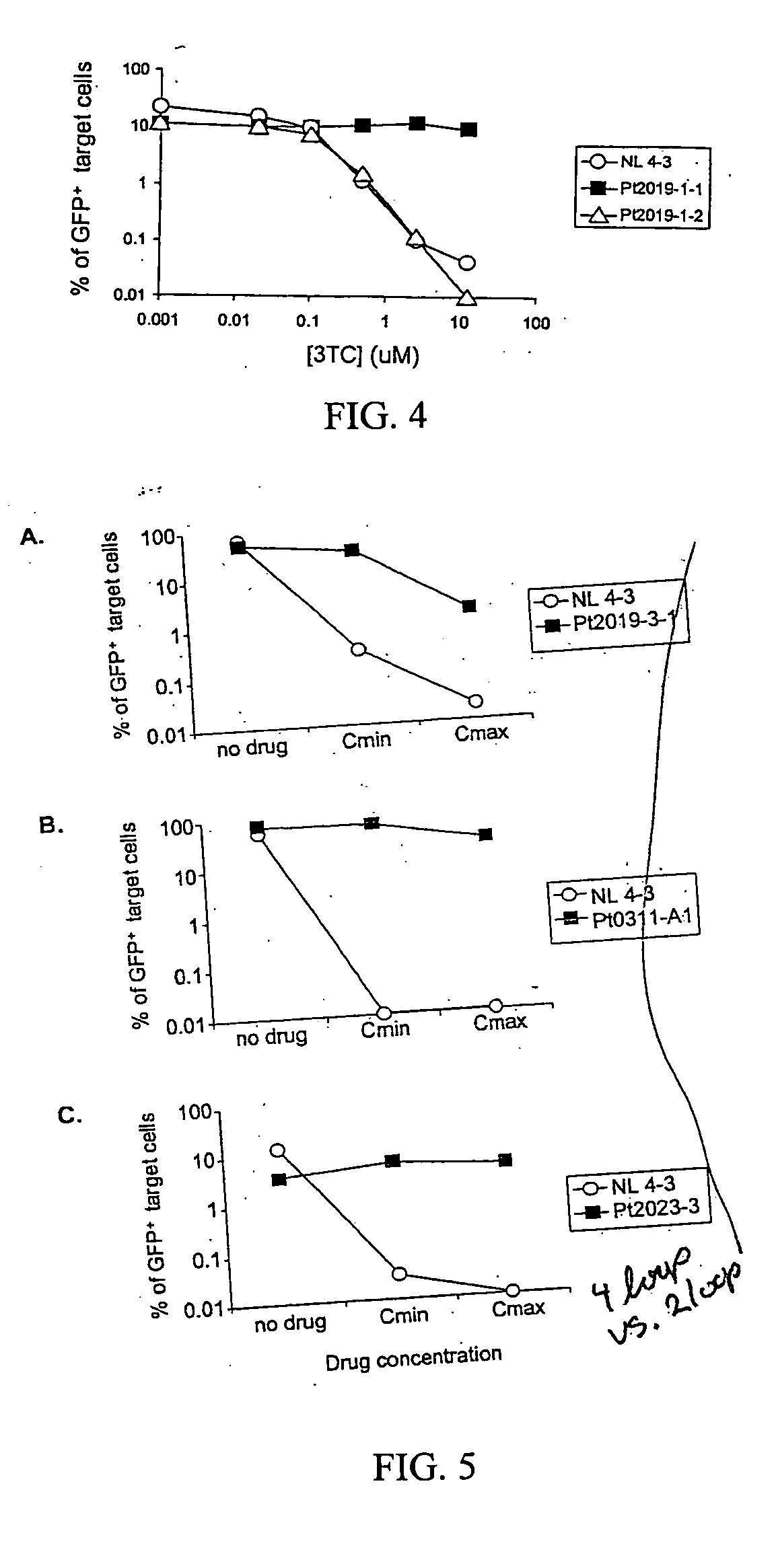
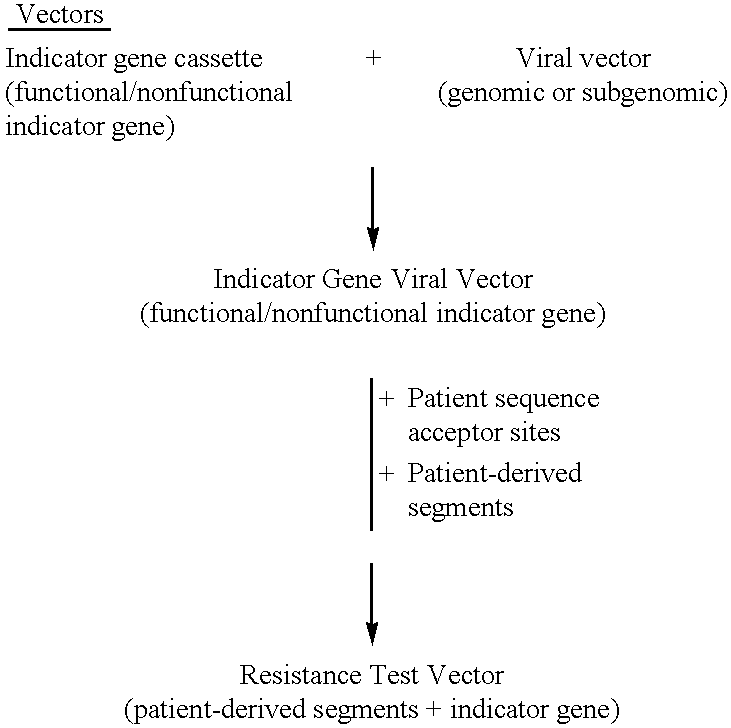
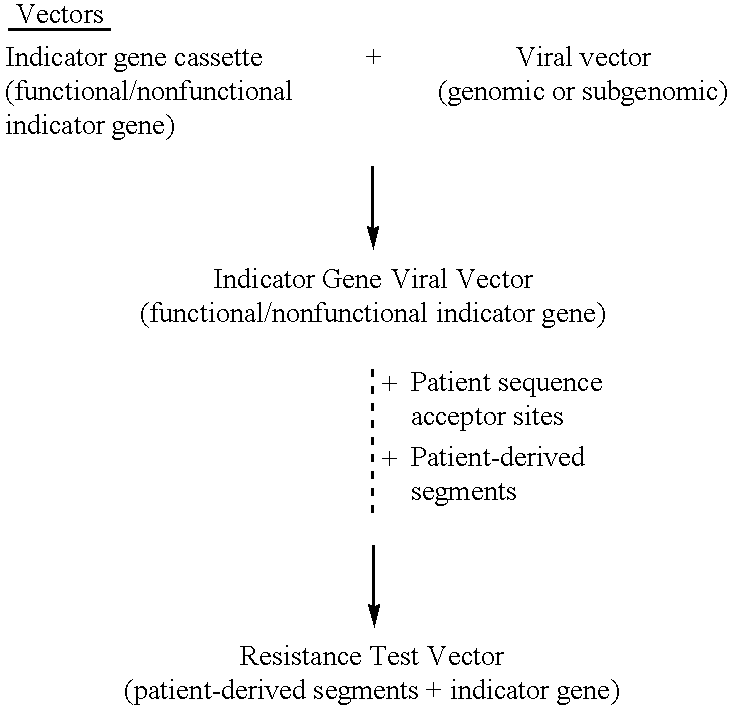
InactiveUS6942969B2More affordableRapid and effective and resistanceVectorsMicrobiological testing/measurementAntiviral drugDrug compound
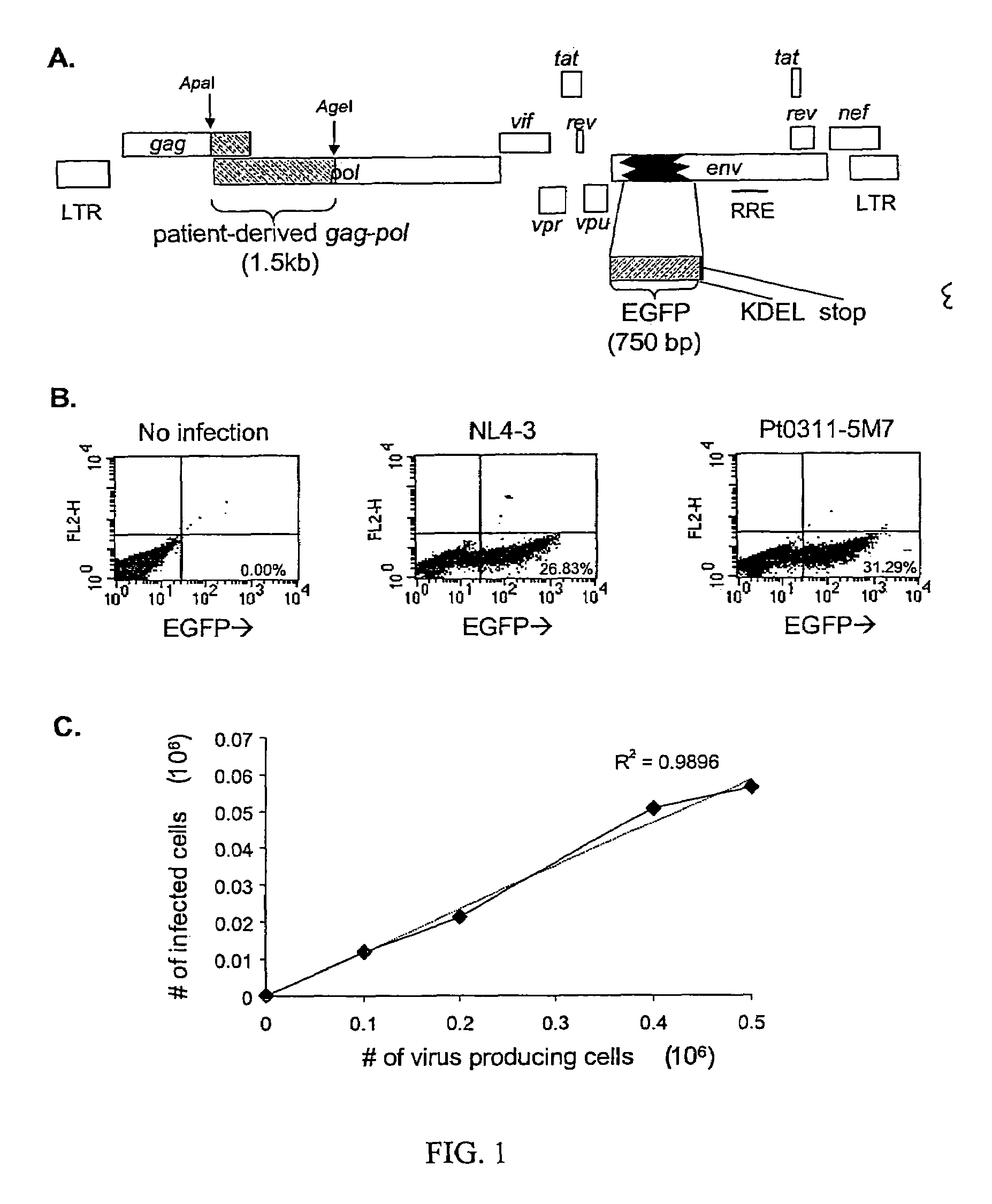
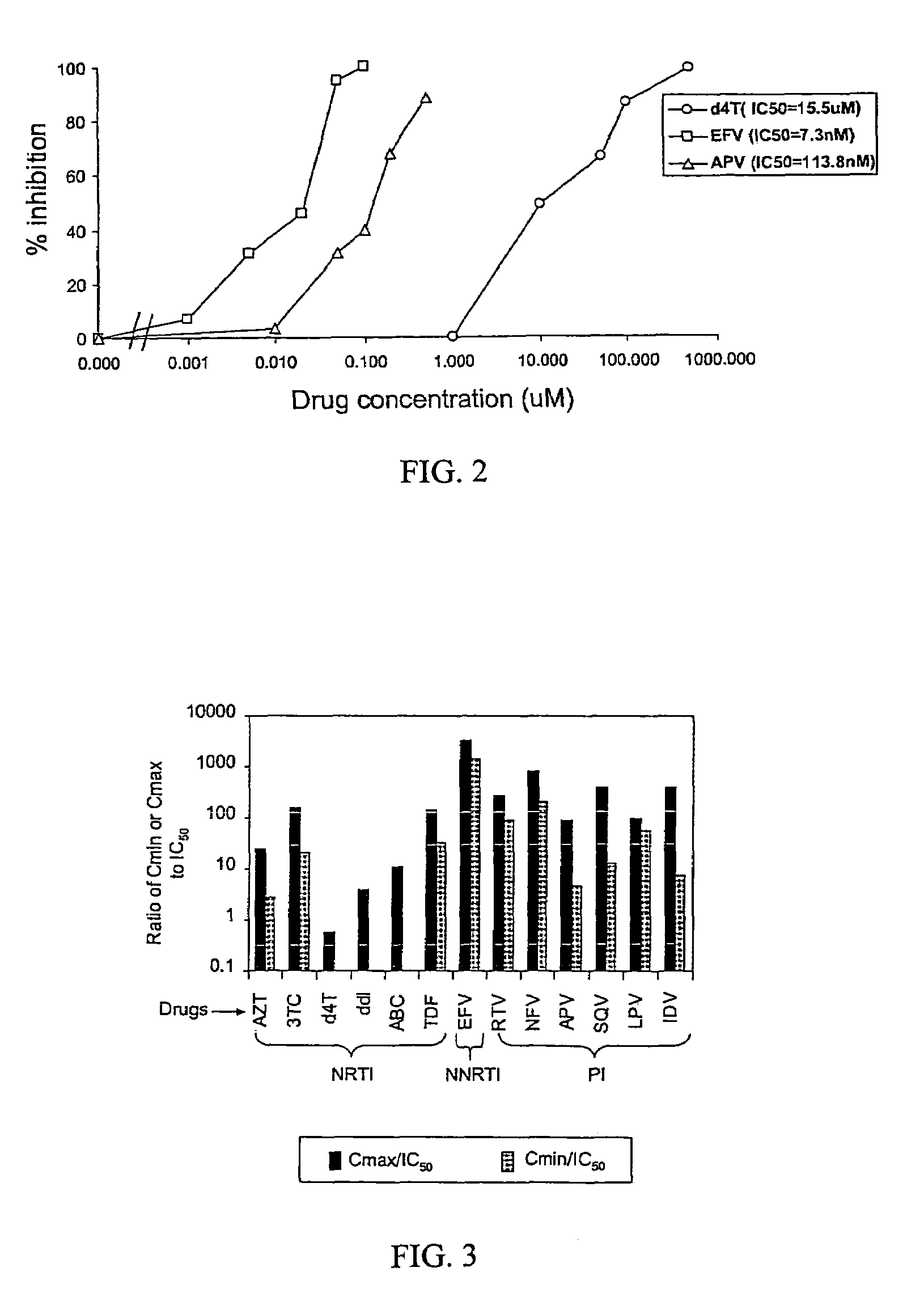
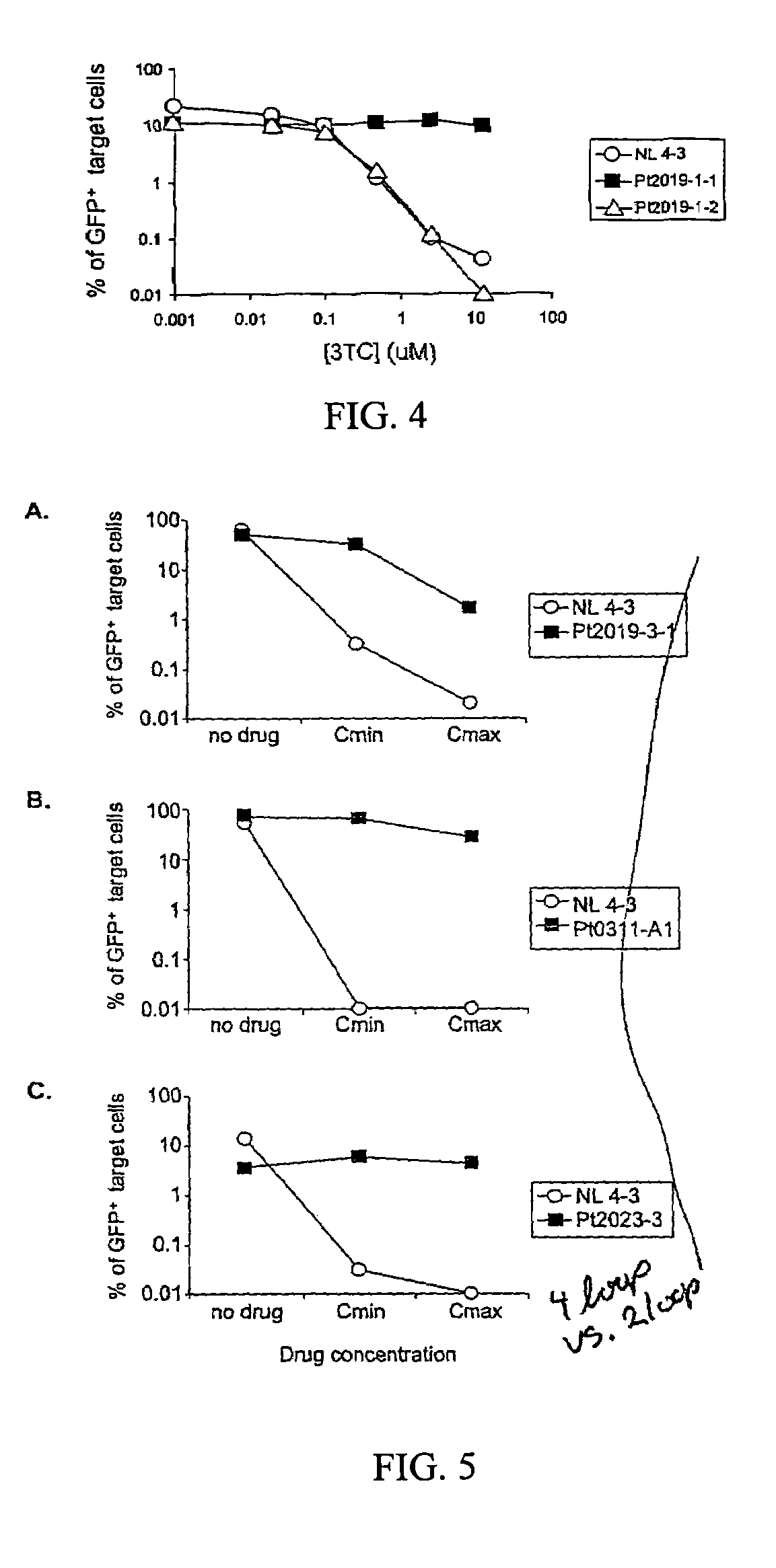
This invention provides a method for determining susceptibility for an anti-viral drug comprising: (a) introducing a resistance test vector comprising a patient-derived segment and an indicator gene into a host cell; (b) culturing the host cell from (a); (c) measuring expression of the indicator gene in a target host cell; and (d) comparing the expression of the indicator gene from (c) with the expression of the indicator gene measured when steps (a)-(c) are carried out in the absence of the anti-viral drug, wherein a test concentration of the anti-viral drug is present at steps (a)-(c); at steps (b)-(c); or at step (c). This invention also provides a method for determining anti-viral drug resistance in a patient comprising: (a) determining anti-viral drug susceptibility in the patient at a first time using the susceptibility test described above, wherein the patient-derived segment is obtained from the patient at about said time; (b) determining anti-viral drug susceptibility of the same patient at a later time; and (c) comparing the anti-viral drug susceptibilities determined in step (a) and (b), wherein a decrease in anti-viral drug susceptibility at the later time compared to the first time indicates development or progression of anti-viral drug resistance in the patient. This invention also provides a method for evaluating the biological effectiveness of a candidate anti-viral drug compound. Compositions including resistance test vectors comprising a patient-derived segment and an indicator gene and host cells transformed with the resistance test vectors are provided.
Owner:MONOGRAM BIOSCIENCES
Compositions and methods for determining susceptibility of hepatitis C virus to anti-viral drugs
InactiveUS20070099296A1High activityReduced activityOrganic active ingredientsSsRNA viruses positive-senseAntiviral drugDrug compound
The present invention provides methods for determining the susceptibility of a pathogenci flavirus to anti-viral compounds. This invention also provides methods for determining anti-viral drug susceptibility in a patient infected with a flavivirus. This invention also provides a method for evaluating the biological effectiveness of a candidate anti-viral drug compound. The methods are useful for identifying effective drug regimens for the treatment of flaviviral infections, and identifying and assessing the biological effectiveness of potentia therapeutic compounds. Compositions including resistance test vectors and host cells transformed with resistance test vectors are provided.
Owner:MONOGRAM BIOSCIENCES
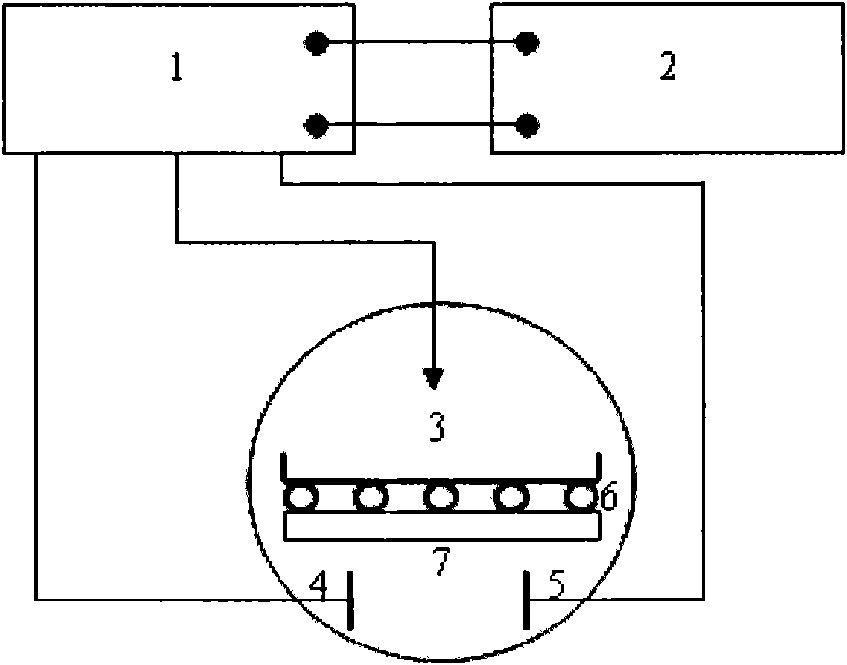
Device and method for judging bacterium drug susceptibility by utilizing oxidation reaction of bacterium
InactiveCN101852765AJudgment sensitivityMicrobiological testing/measurementMaterial electrochemical variablesBacteroidesElectron
The invention relates to a device for judging bacterium drug susceptibility by utilizing oxidation reaction of bacterium, comprising a virtual voltage signal generator, a computer, a working electrode and a pair electrode; the bacterium subject to medicine treatment is adhered on the working electrode; wherein the voltage signal generator is connected with the computer and can apply voltage changing in preset voltage range between the working electrode and the pair electrode under the control of the computer; and meanwhile the computer samples the current flowing through the working electrode and the pair electrode, thus obtaining the volt-ampere map of the voltage and the current. In the device and method in the invention, bacterium subject to medicine treatment is placed on the surface of the working electrode, a scanning voltage is applied between the electrodes, when the scanning voltage reaches the oxidation level of the bacterium, auxiliary enzyme A in the bacterium joins the oxidation reaction to release electron, thus judging bacterium drug susceptibility according to the fact whether a peak valley is existed in the volt-ampere map.
Owner:解宇
Method for testing drug susceptibility of Mycobacterium tuberculosis
A novel agar medium for the isolation, sub-cultivation, and indirect or direct drug-susceptibility testing of Mycobacterium tuberculosis is disclosed. Also disclosed are methods of isolating and growing Mycobacterium tuberculosis and methods of drug-resistance screening using the agar medium of the invention.
Owner:NAT JEWISH MEDICAL & RES CENT
Method for detecting microorganisms and detection kit
InactiveUS6984500B2Easy to operateEliminate needFungiMaterial analysis by observing effect on chemical indicatorMicroorganismOxidation-Reduction Agent
A method for detecting a microorganism by coloration is provided that includes adding and reacting, in a liquid culture medium, an alkaline sensitizing solution and a coloring reagent containing a redox dye, the liquid culture medium having been inoculated with a test sample, thereby detecting the microorganism by coloration in the reaction. There is also provided a method for testing drug susceptibility of a microorganism using above-mentioned method. Furthermore, kits used in these methods are provided. The invention is useful to assess readily and objectively the growth of microorganism when carrying out e.g. a detection of microorganism in foods and a test such as a drug susceptibility test.
Owner:KANTO CHEM CO INC
Tumor chemotherapy drug susceptibility detection kit
InactiveCN104111254AHigh activityProtect biological propertiesMaterial analysis by observing effect on chemical indicatorTumor chemotherapyPrimary cell
The invention discloses a tumor chemotherapy drug susceptibility detection kit which comprises a histocyte dispase, an antibiotic solution, an amphotericin solution, an EGTA-Trypsin (Ethylene Glycol Tetraacetic Acid) solution, a serum-containing culture medium DF10, a serum-free culture medium Kertin-SFM, a specimen preserving fluid, a specimen cleaning fluid, a gel coating culturing bottle, a cell fixing fluid, cell dispase, a cell filtering membrane, a neutral red dyeing solution and a collagen gel preparing reagent. A detection operation flow of the kit comprises the steps: primary cell culture, culture in collagen gel drops, and analysis of chemotherapy drug susceptibility. Compared with the prior art, the tumor chemotherapy drug susceptibility detection kit has the beneficial effects that the tumor chemotherapy drug susceptibility detection kit is capable of maximally protecting activity of tumor cells and shortening the drug susceptibility detection time. The tumor chemotherapy drug susceptibility detection kit contains all reagents required for tumor cell primary culture and chemotherapy drug detection; consumable items are disposable articles, thus bacterium pollution in an experiment process is reduced, and the convenience is brought for the use.
Owner:李维
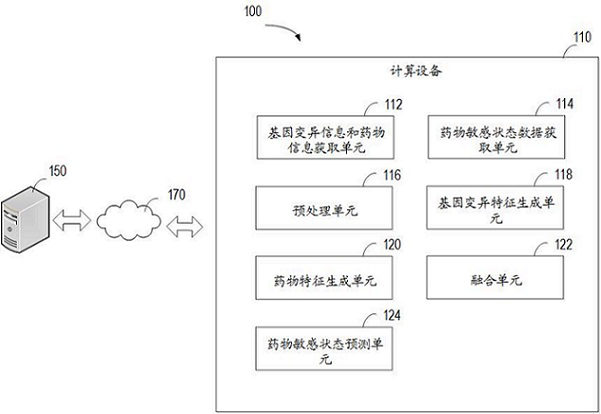
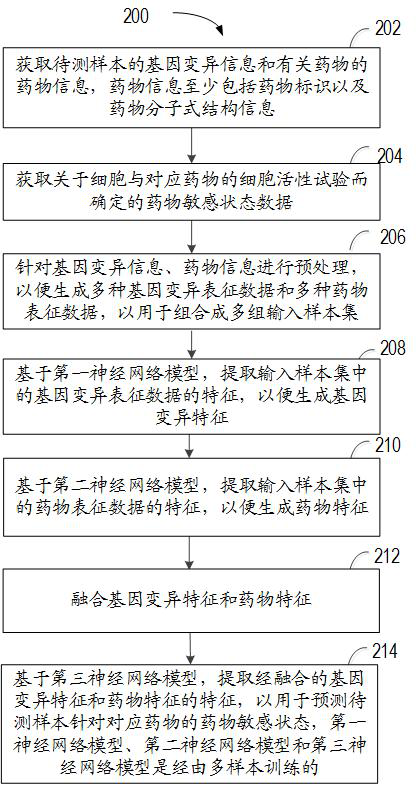
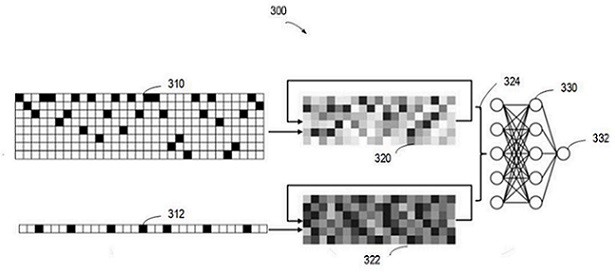
Method and device for predicting drug susceptibility state and storage medium
The invention relates to a method for predicting a drug susceptibility state, computing equipment and a storage medium. The method comprises the following steps: acquiring gene variation information of a to-be-detected sample and drug information of related drugs; acquiring drug susceptibility state data determined by a cell viability test about the cells and the corresponding drugs; preprocessing the gene variation information and the drug information so as to generate a plurality of gene variation characterization data and a plurality of drug characterization data for combining into a plurality of groups of input sample sets; generating gene variation features based on a first neural network model; generating drug features based on a second neural network model; and based on a third neural network model, extracting the fused gene variation features and drug features to predict the drug susceptibility state of the to-be-detected sample for the corresponding drug. According to the method and device, the drug susceptibility can be accurately predicted, and the method has good versatility.
Owner:SHANGHAI ORIGIMED CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com