Patents
Literature
832 results about "Hepatitis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral hepatitis is a liver disease that is caused by exposure to one of the five hepatitis viruses. Each virus is named after a letter of the alphabet, hepatitis A through E. Though other viruses can cause hepatitis, only the five are considered hepatitis viruses. Each of the five hepatotropic viruses are alike in many ways.
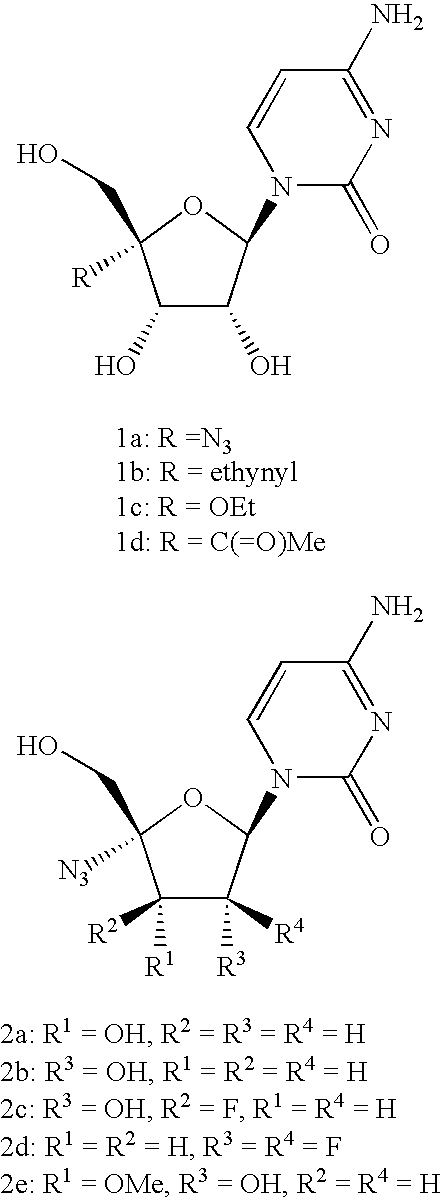
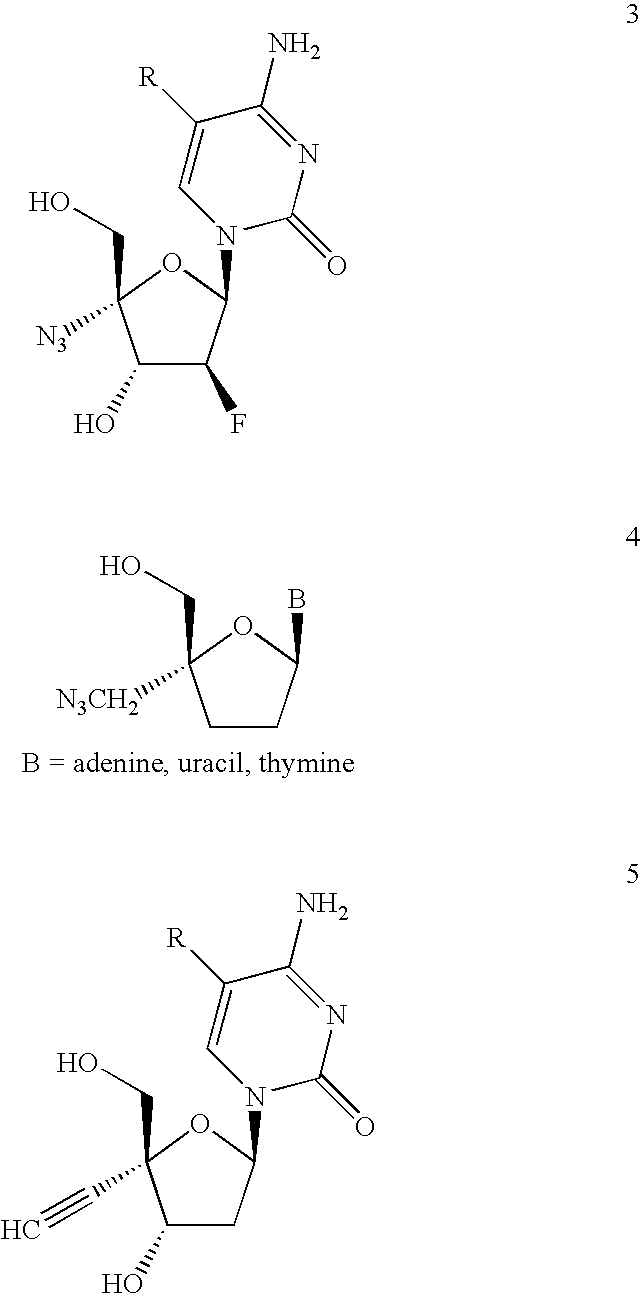
3′-or 2′-hydroxymethyl substituted nucleoside derivatives for treatment of hepatitis virus infections
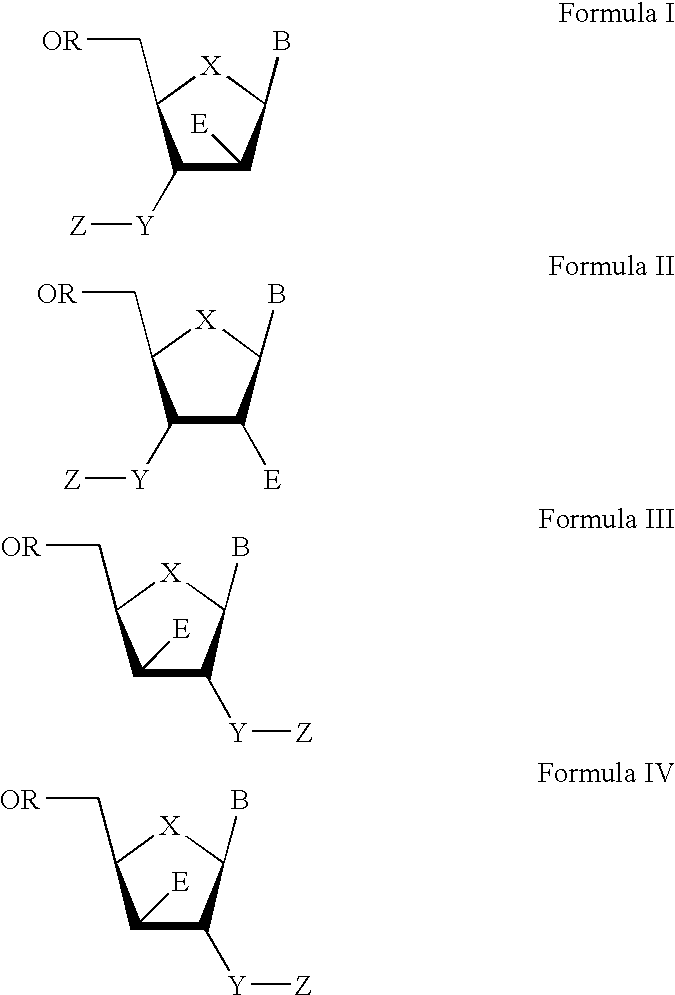
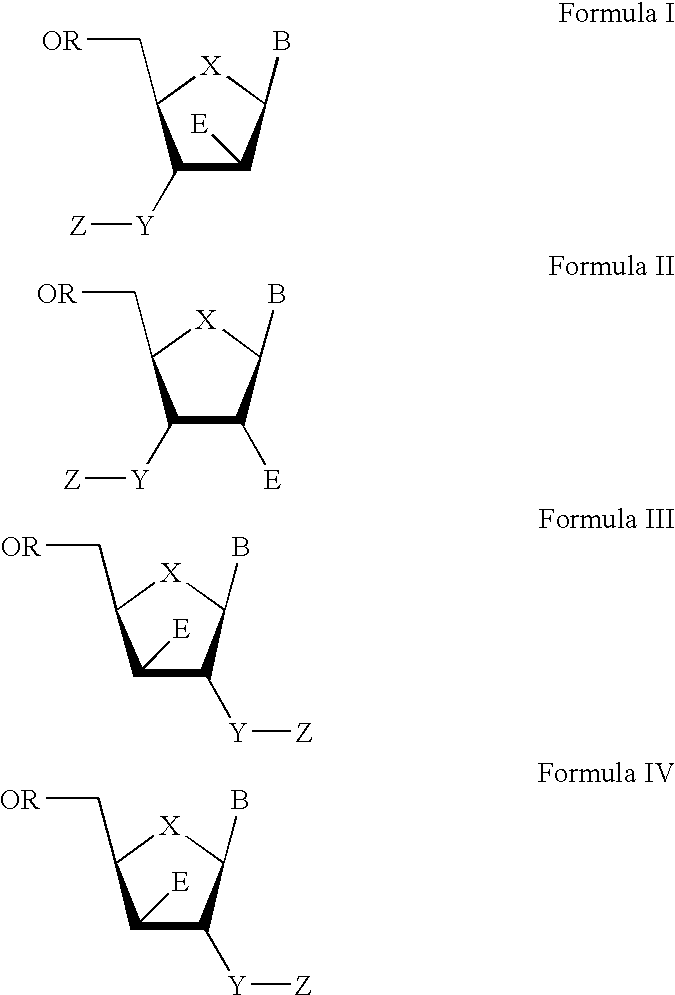
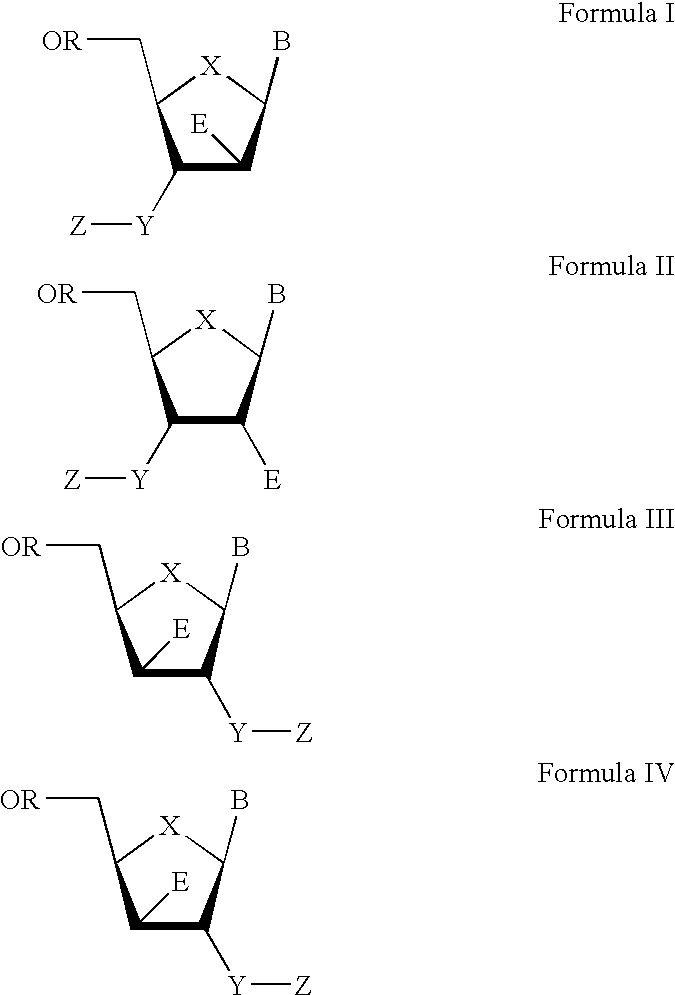
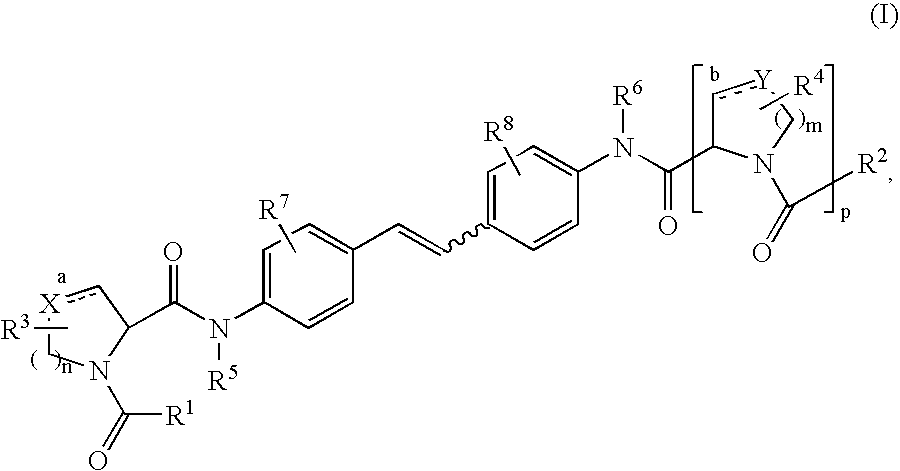
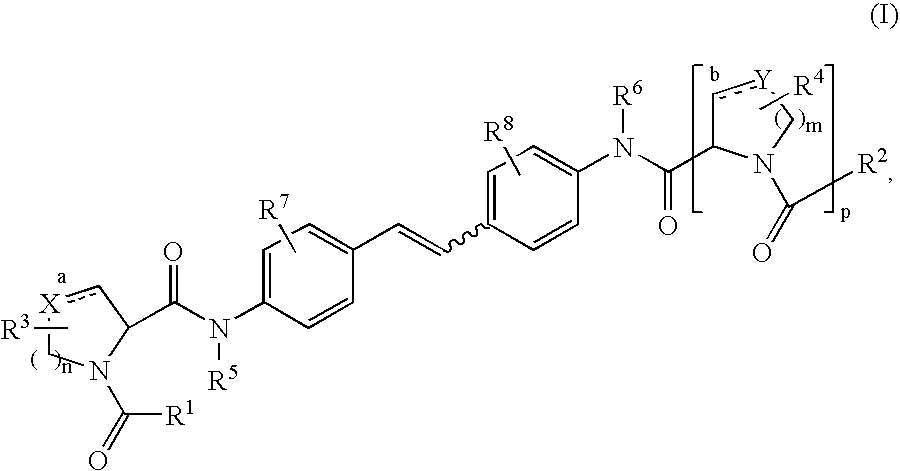
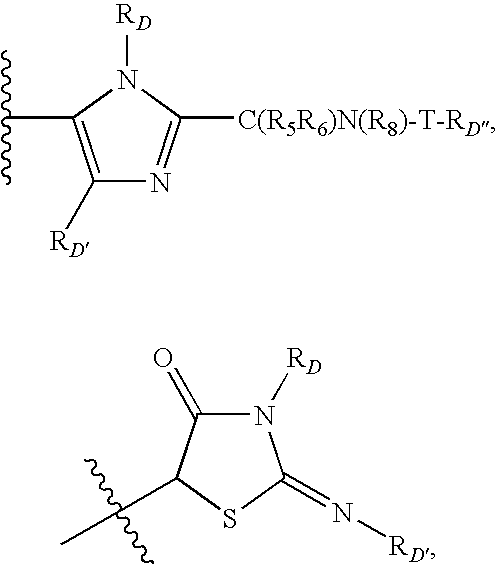
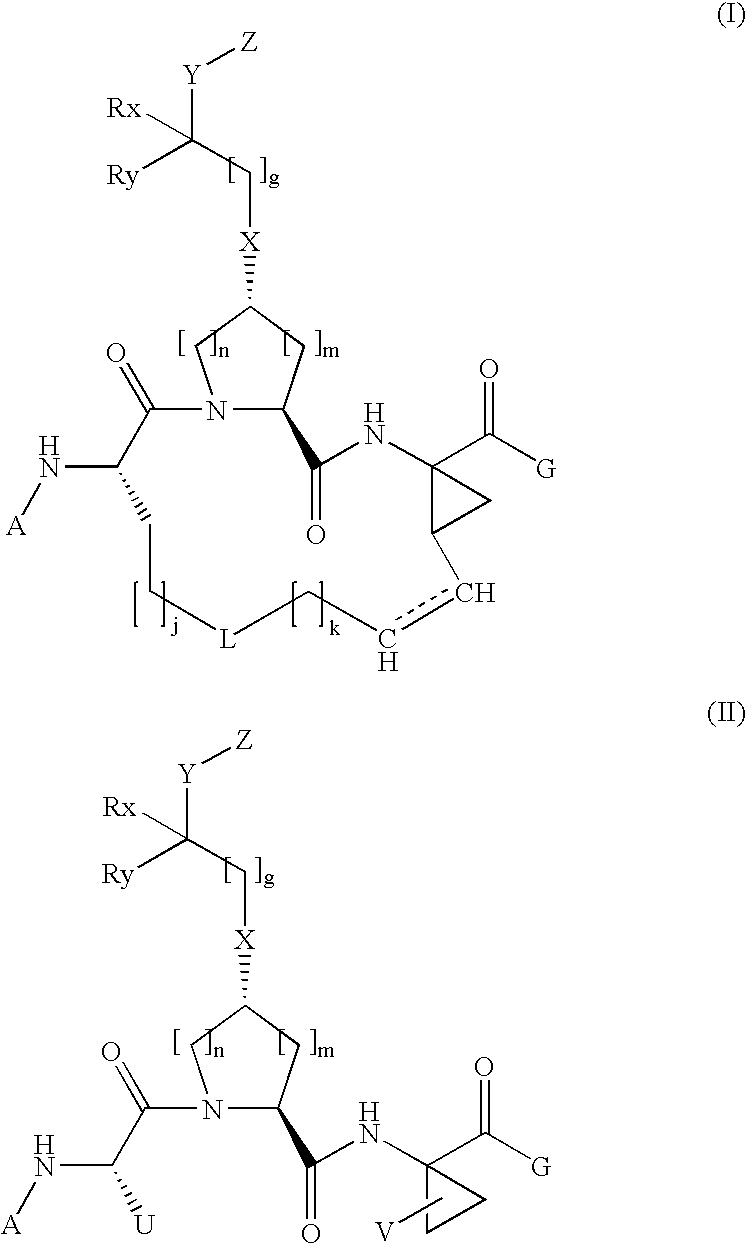
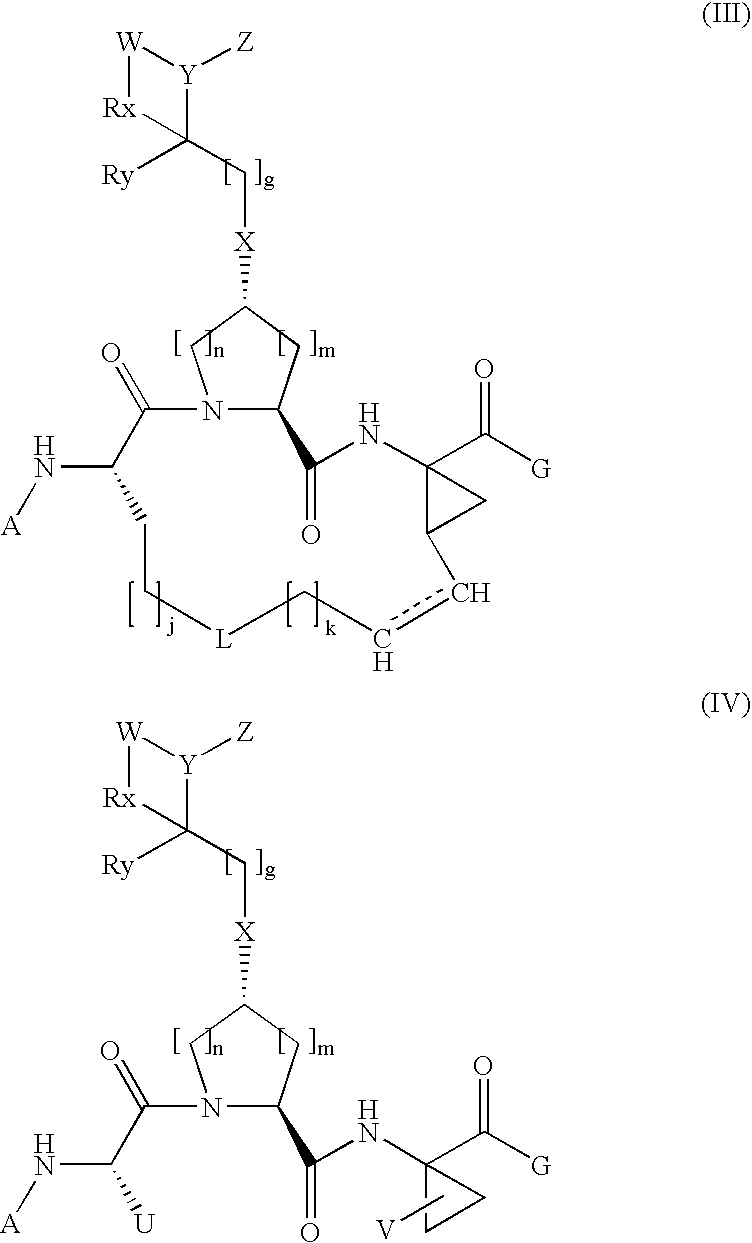
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]–[IV] below and mixtures of two or more thereof:wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
Inhibitors of HCV replication
ActiveUS20060276511A1Inhibit functioningEffective treatmentBiocideOrganic chemistryArrestinStereochemistry
Owner:BRISTOL MYERS SQUIBB CO
Macrocyclic NS3-serine protease inhibitors of hepatitis C virus comprising N-cyclic P2 moieties
The present invention discloses novel macrocyclic compounds which have HCV protease inhibitory activity as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising such macrocycles as well as methods of using them to treat disorders associated with the HCV protease.
Owner:SCHERING CORP
Pyrazolopyrimidines as protein kinase inhibitors
In its many embodiments, the present invention provides a novel class of pyrazolo[1,5-a]pyrimidine compounds as inhibitors of protein and / or checkpoint kinases, methods of preparing such compounds, pharmaceutical compositions including one or more such compounds, methods of preparing pharmaceutical formulations including one or more such compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more diseases associated with the protein or checkpoint kinases using such compounds or pharmaceutical compositions. The invention also relates to the inhibition of hepatitis C virus (HCV) replication. In particular, embodiments of the invention provide compounds and methods for inhibiting HCV RNA-dependent RNA polymerase enzymatic activity. The invention also provides compositions and methods for the prophylaxis and treatment of HCV infection.
Owner:MERCK SHARP & DOHME LLC
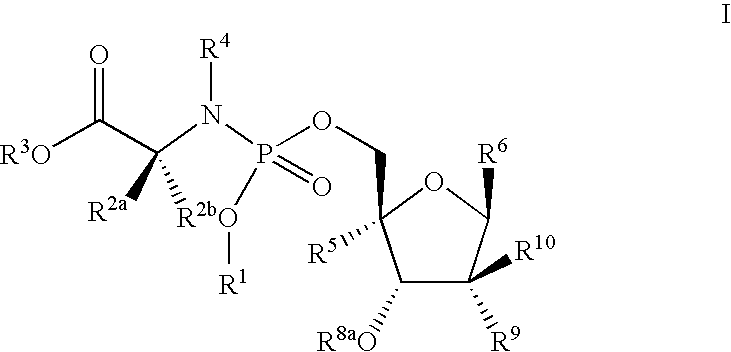
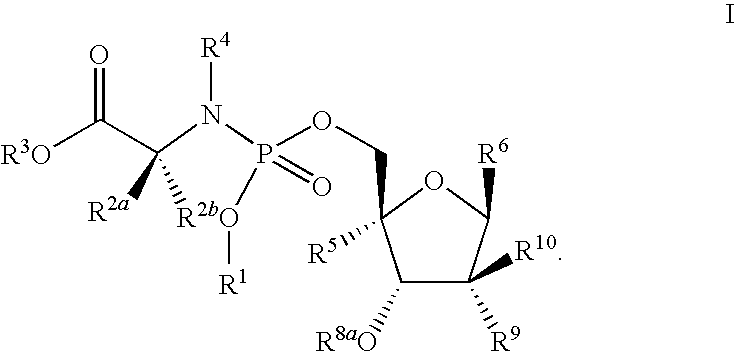
Antiviral phosphoramidates
The invention provides novel nucleoside compounds of formula I wherein R1, R2a, R2b, R3, R4, R5, R6, R8a, R9 and R10 are as defined herein which are useful for the treatment of Hepatitis C Virus (HCV) mediated diseases. The invention further provides methods for treatment or prophylaxis of HCV mediated diseases with compounds of formula I and pharmaceutical compositions comprising these compounds,
Owner:RIBOSCI
Anti-Viral Compounds
ActiveUS20100168138A1Lower Level RequirementsOrganic active ingredientsBiocideHepacivirusAnti virals
Owner:ABBVIE INC
Inhibitors of serine proteases, particularly HCV NS3-NS4A protease
InactiveUS7273885B2Inhibit HCV replicationReduce riskBiocidePeptide/protein ingredientsHepatitis c viralSerine Protease Inhibitors
The present invention relates to compounds that inhibit serine protease activity, particularly the activity of hepatitis C virus NS3-NS4A protease. As such, they act by interfering with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The invention further relates to compositions comprising these compounds either for ex vivo use or for administration to a patient suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a patient by administering a composition comprising a compound of this invention. The invention further relates to processes for preparing these compounds.
Owner:VERTEX PHARMA INC
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
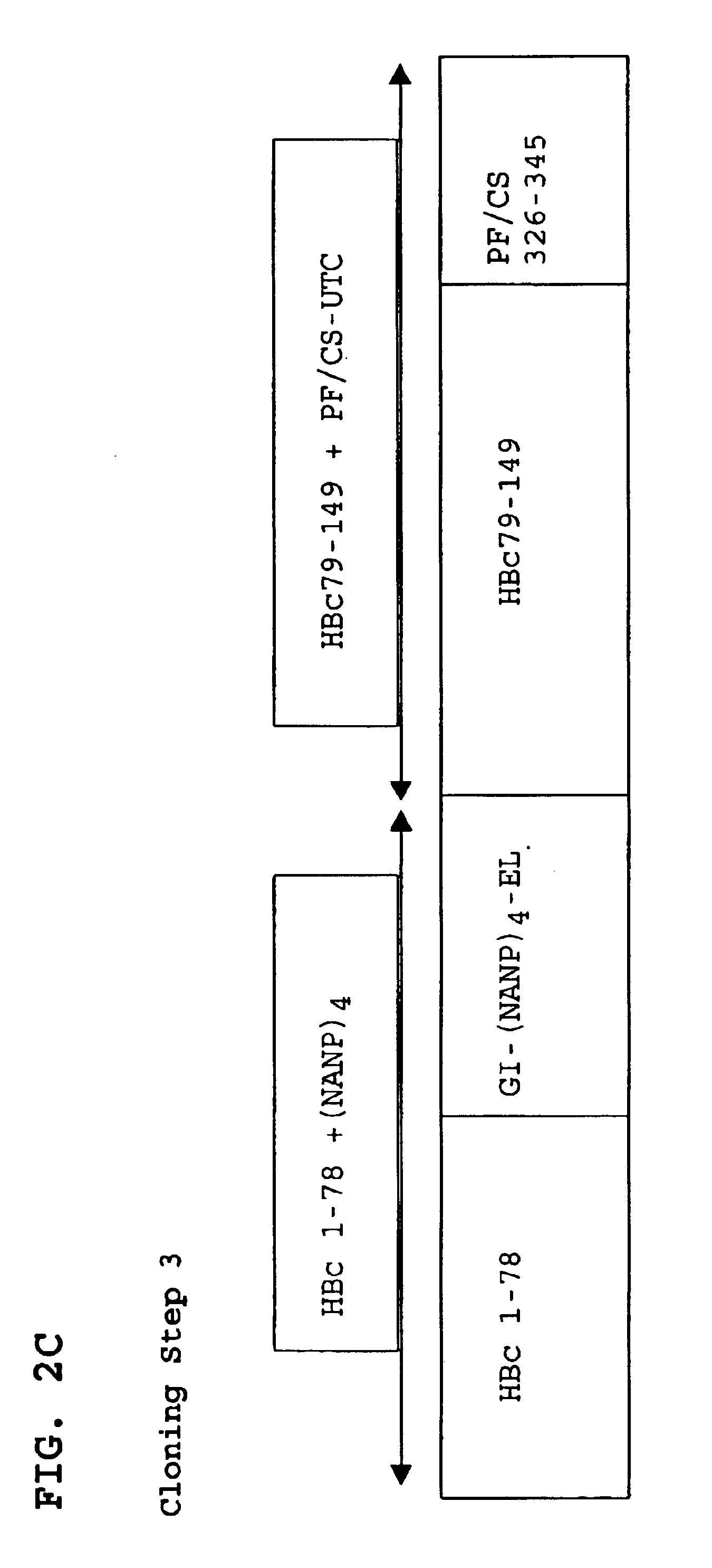
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
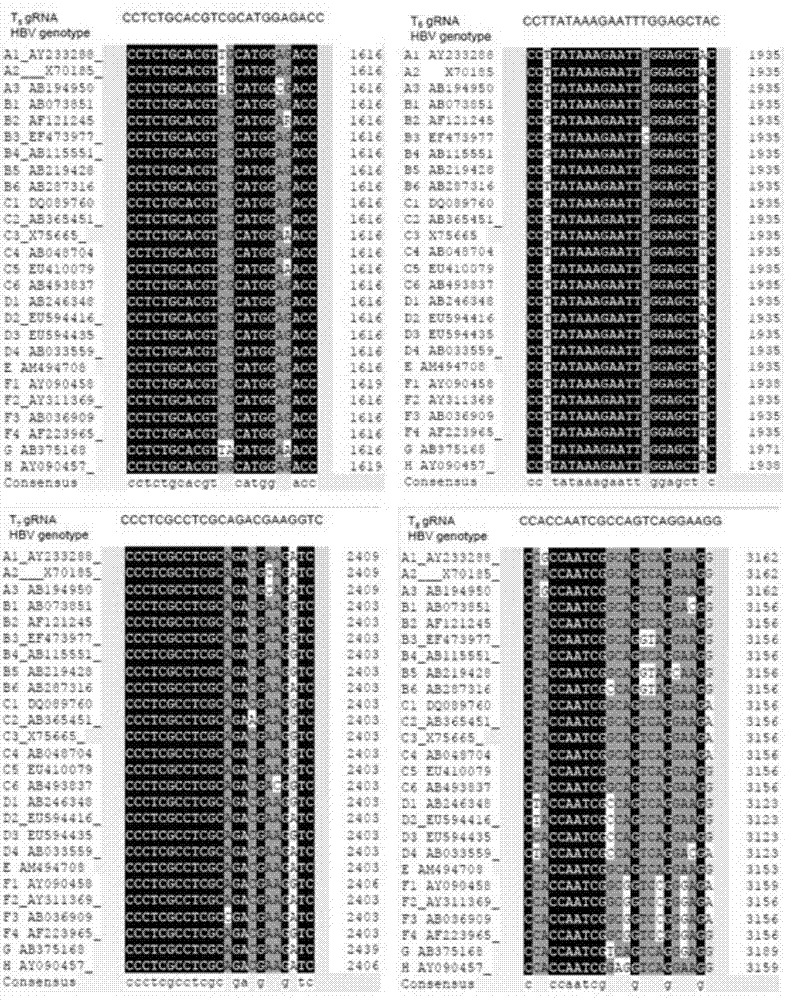
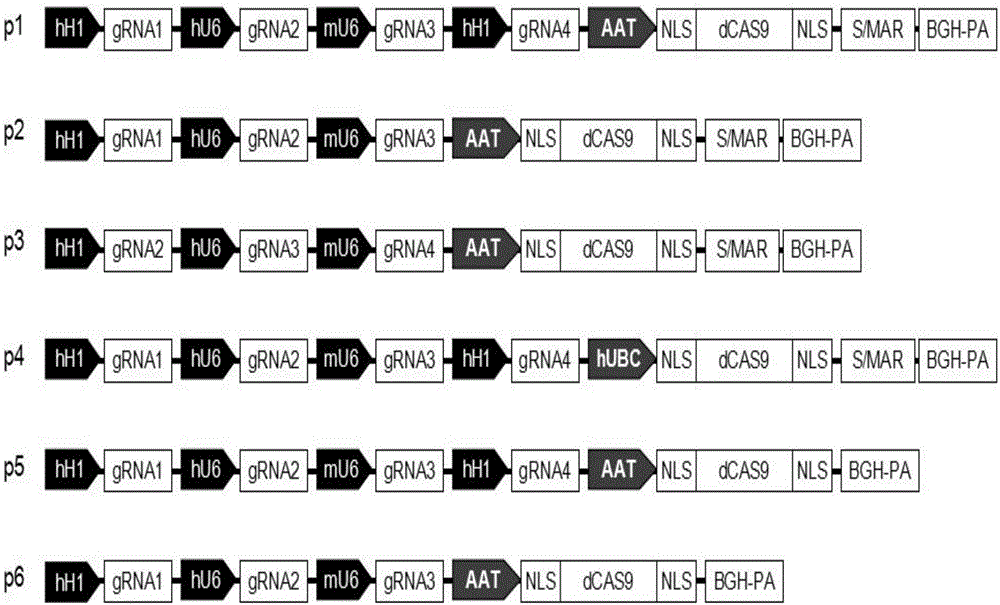
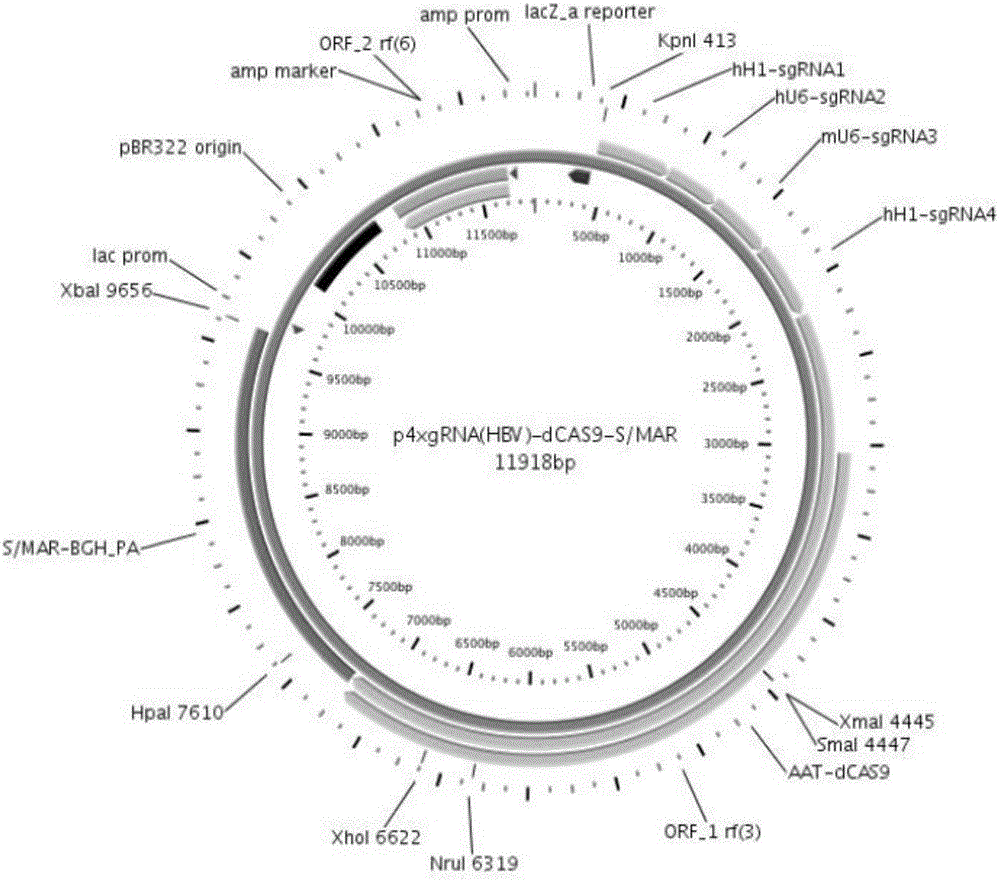
Method for specifically knocking out hepatitis B virus by CRISPR/Cas9 and gRNA applied to specific targeting HBV DNA
ActiveCN104498493AEfficient removalReduce escape from treatmentGenetic material ingredientsAntiviralsDrugRNA
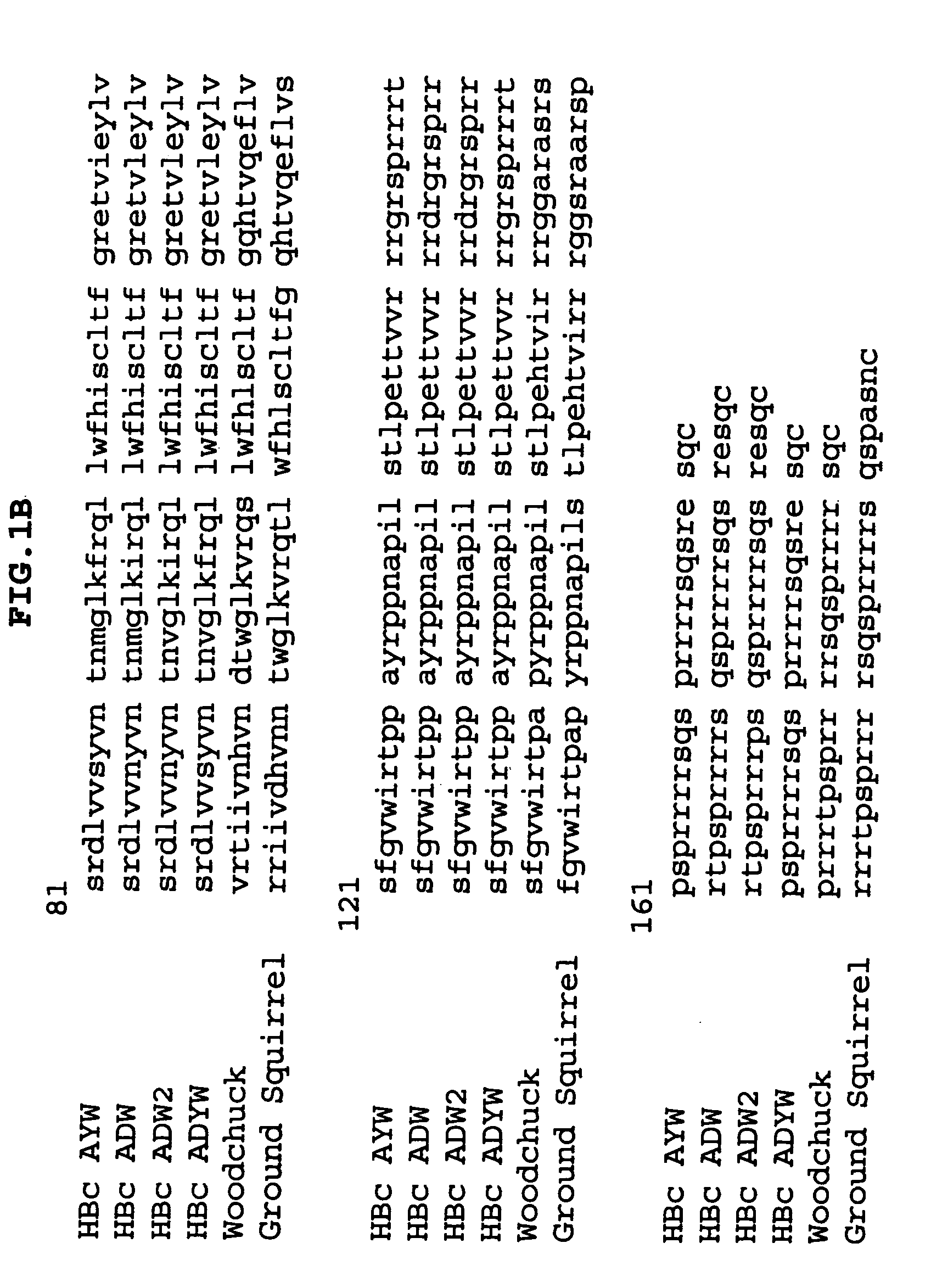
The invention relates to the technical field of molecular biology and biological medicines, and particularly relates to application of gRNA sequences and combination thereof based on a CRISPR system in treatment on hepatitis B virus. According to the method disclosed by the invention, eight types of guidance RNAs (gRNA) are designed according to design rules of CRISPR gRNA and a conservative region of different genotypes of HBV sequences, and the eight types of guidance RNAs are structured on a PX330 expression vector. By utilizing the eight gRNAs in a cell model, a mouse model and a CRISPR / Cas9 system guided by combination of the cell model and the mouse model, the expression and replication of the hepatitis B virus can be effectively inhibited. By united application of a plurality of gRNAs, a better effect can be achieved, and different genotypes of HBV replication can be better inhibited. The system has the characteristics of being easy to operate, high in inhibition efficiency on HBV replication and applicable to various genotypes. Therefore, the gRNA and the combination thereof related to the invention are expected to be applied in preparation of a novel drug for treating the hepatitis B virus.
Owner:浙江安维珞诊断技术有限公司
Antiviral phosphoramidates
The invention provides novel nucleoside compounds of formula I wherein R1, R2a, R2b, R3, R4, R5 R6, R8a, R9 and R10 are as defined herein which are useful for the treatment of Hepatitis C Virus (HCV) mediated diseases. The invention further provides methods for treatment or prophylaxis of HCV mediated diseases with compounds of formula I and pharmaceutical compositions comprising these compounds,
Owner:RIBOSCI
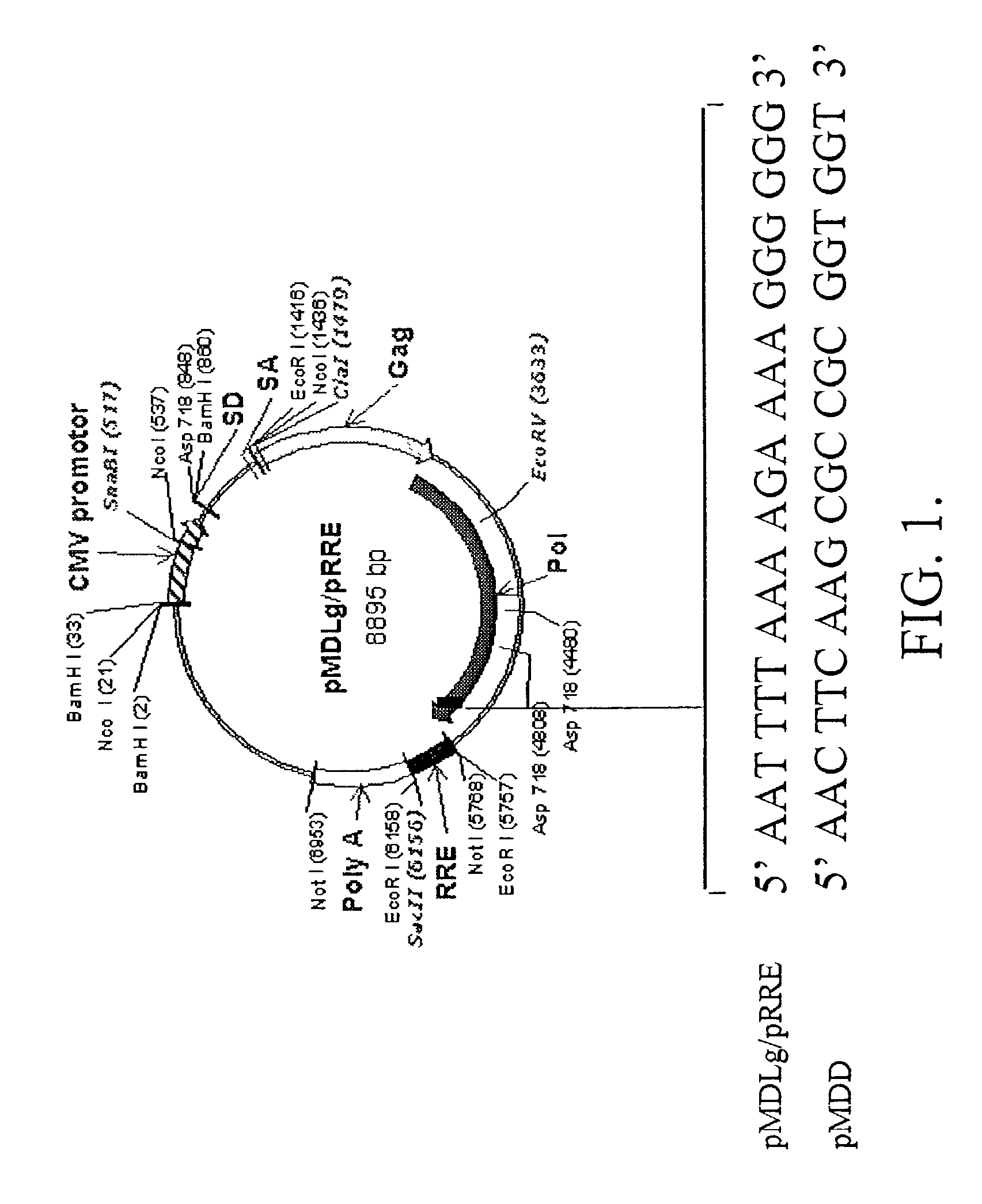
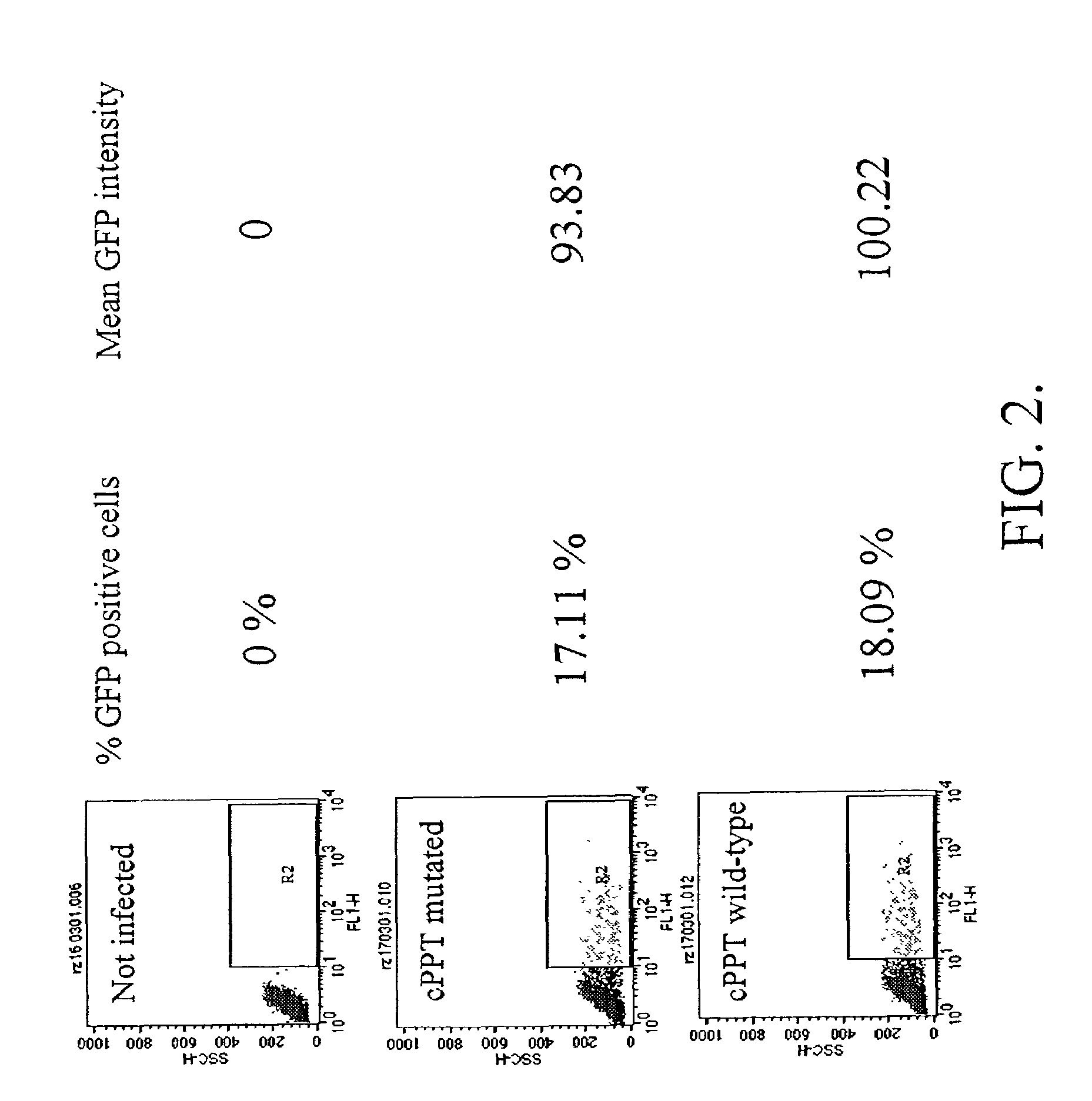
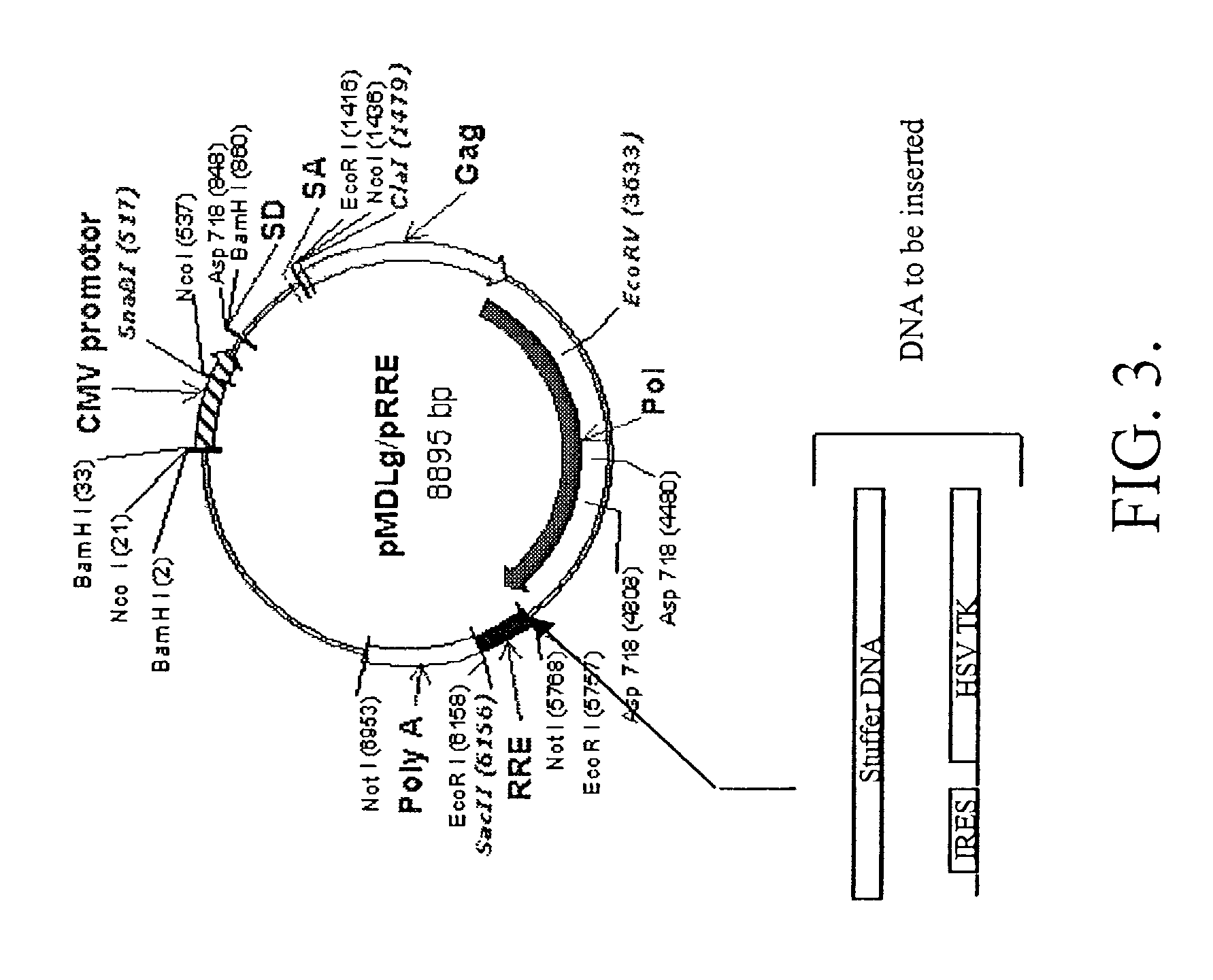
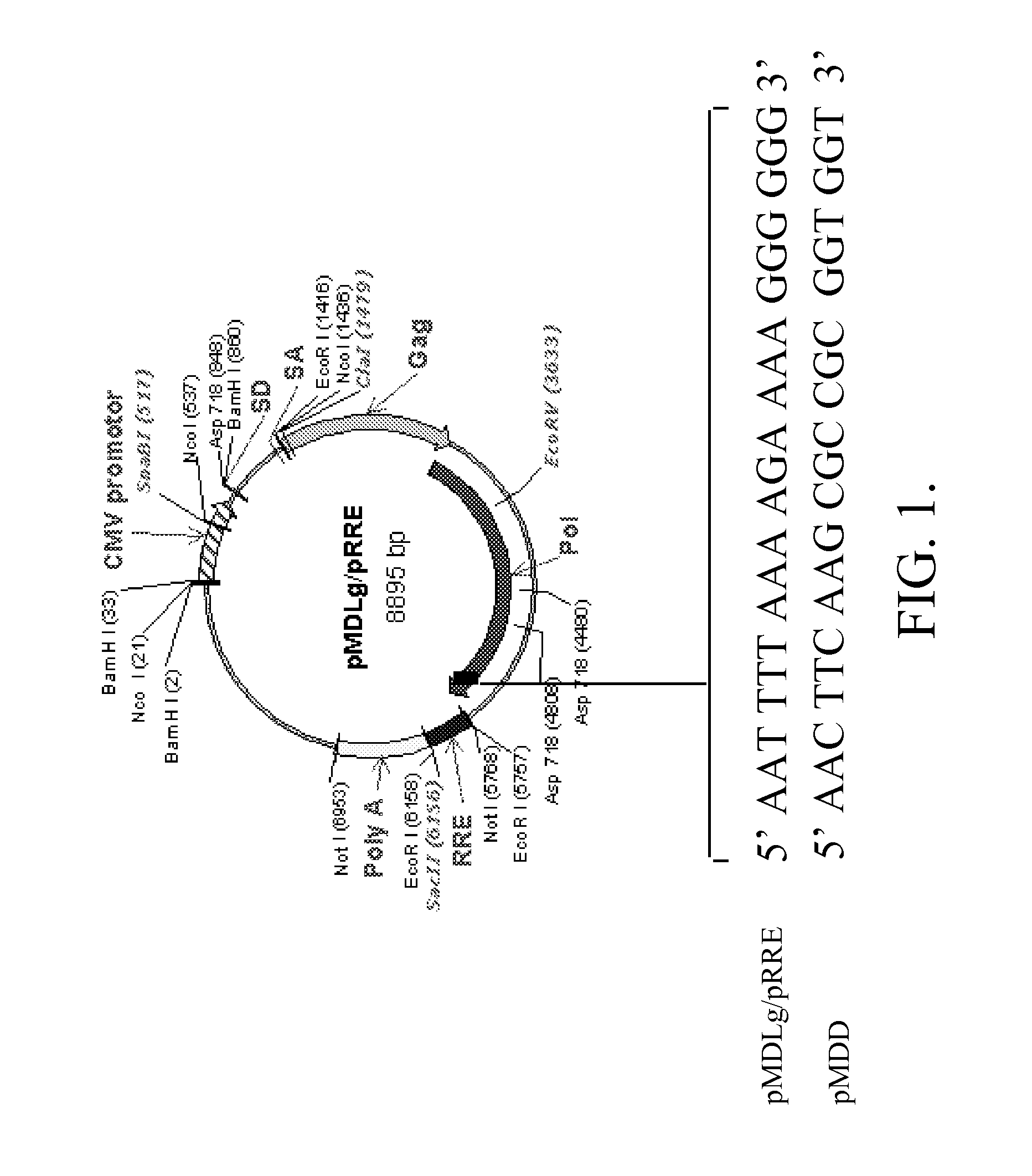
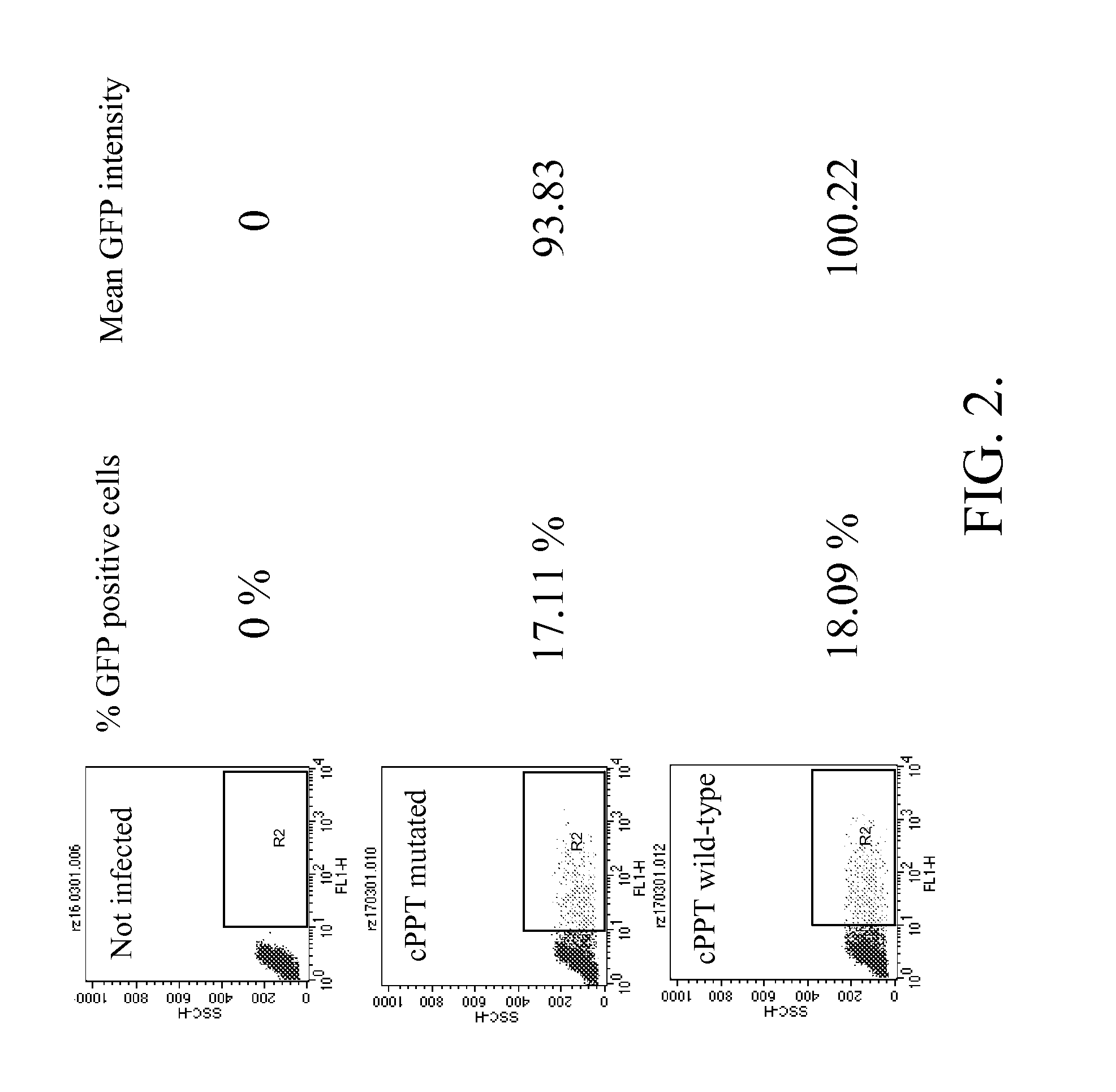
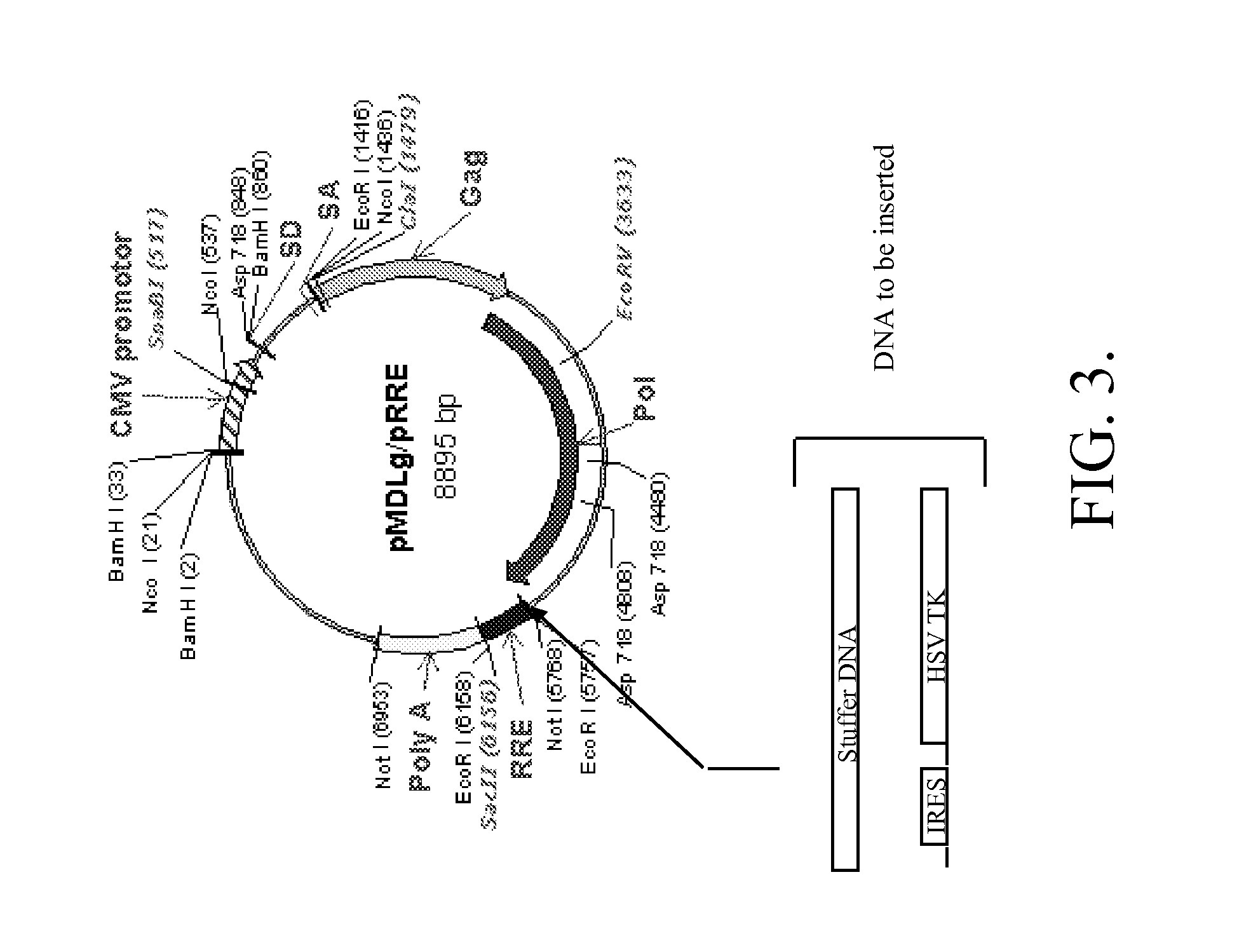
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS7629153B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsTranscriptional Regulatory ElementsTransgene
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosafety and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS8900858B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsDiseaseTranscriptional Regulatory Elements
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosaftey and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
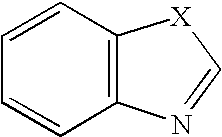
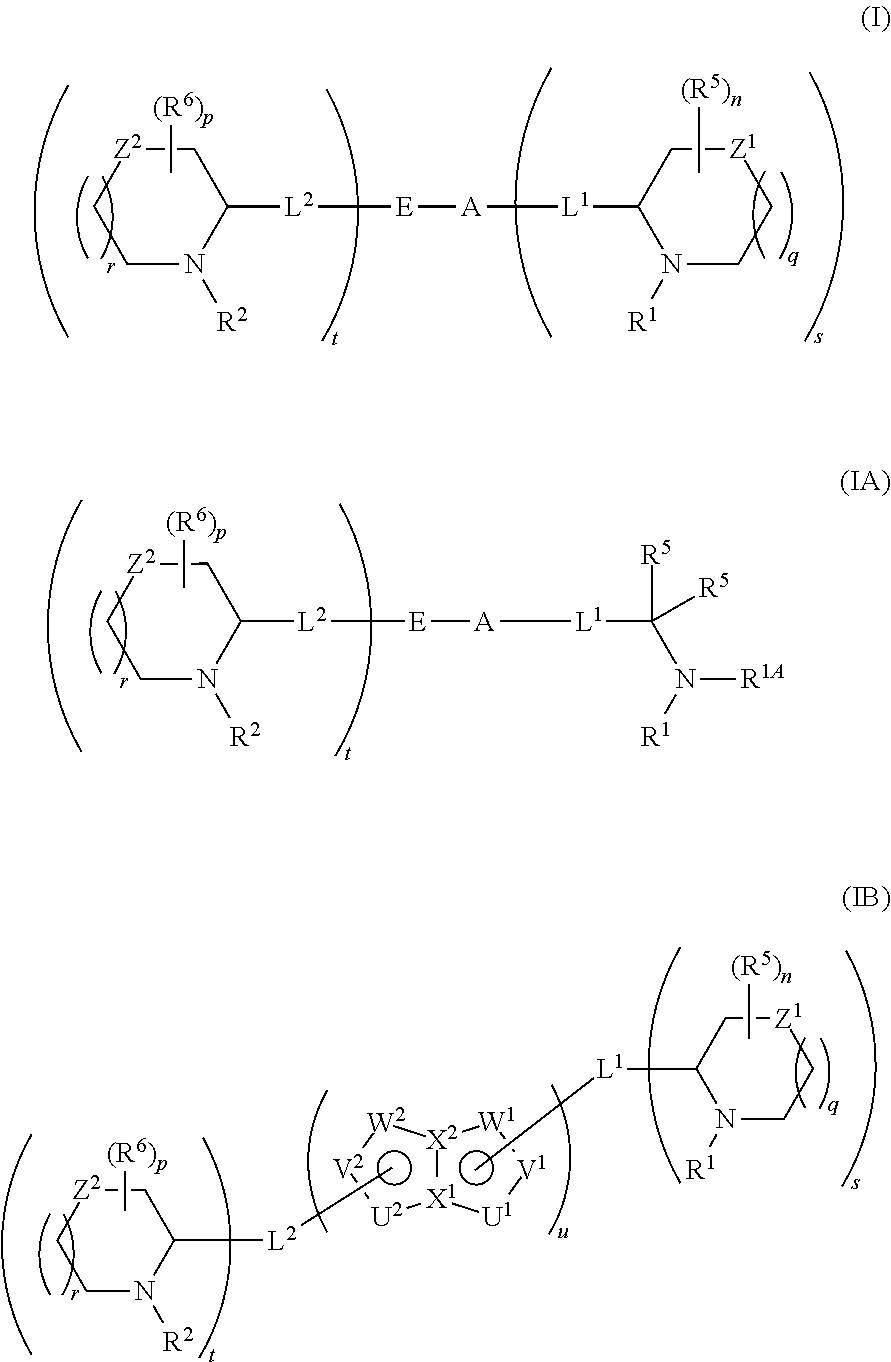
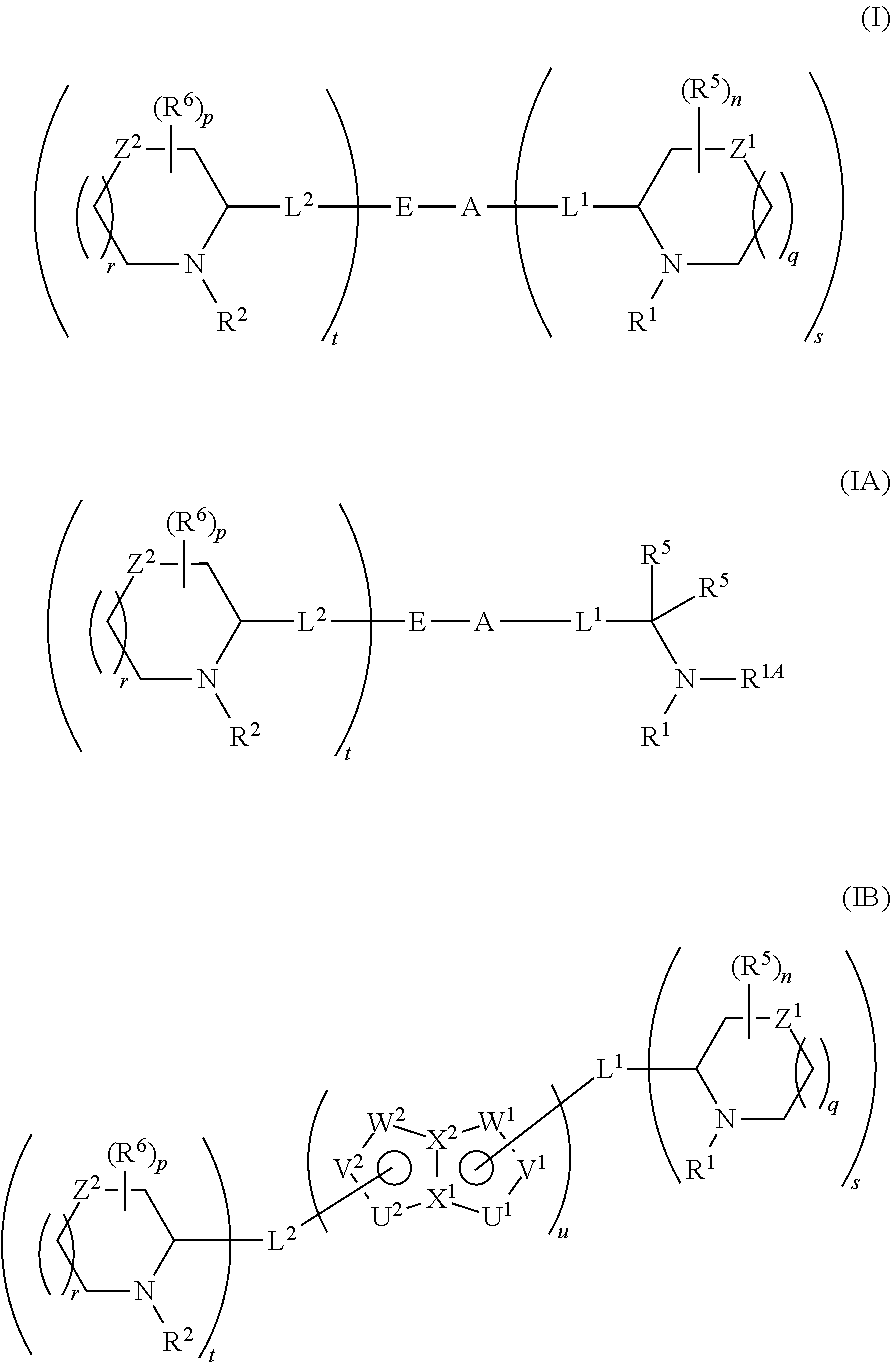
5,5-fused arylene or heteroarylene hepatitis C virus inhibitors
Provided herein are 5,5-fused heteroarylene hepatitis C virus inhibitor compounds, for example, of Formula I, IA, or IB, pharmaceutical compositions comprising the compounds, and processes of preparation thereof. Also provided are methods of their use for the treatment of an HCV infection in a host in need thereof.
Owner:INDENIX PHARM LLC
Compositions and Methods for Inhibiting Gene Expression of Hepatitis B Virus
ActiveUS20130005793A1Inhibit expressionReduce expressionOrganic active ingredientsAntipyreticDrugDouble stranded
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a Hepatitis B Virus gene. The invention also relates to a pharmaceutical composition comprising the dsRNA or nucleic acid molecules or vectors encoding the same together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Hepatitis B Virus infection using said pharmaceutical composition; and methods for inhibiting the expression of a Hepatitis B Virus gene in a cell.
Owner:ARROWHEAD PHARMA INC
Methods of treating hepatitis C virus
Compositions and therapeutic combinations are provided including at least one compound selected from the group consisting of compounds of Formulae I to XXVI as defined herein as well as methods of treatment, prevention or amelioration of one or more symptoms of hepatitis C, treating disorders associated with HCV virus, modulating activity of HCV protease, or inhibiting cathepsin activity in a subject using the same, in which the mean volume of distribution / bioavailability (Vd / F) of the compound as measured in the plasma of the subject is greater than about 1000 L.
Owner:SCHERING CORP
Macrocyclic hepatitis c virus serine protease inhibitors
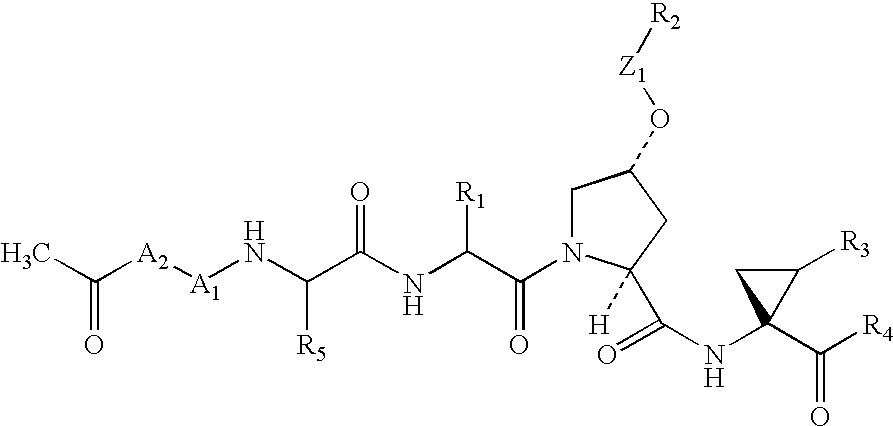
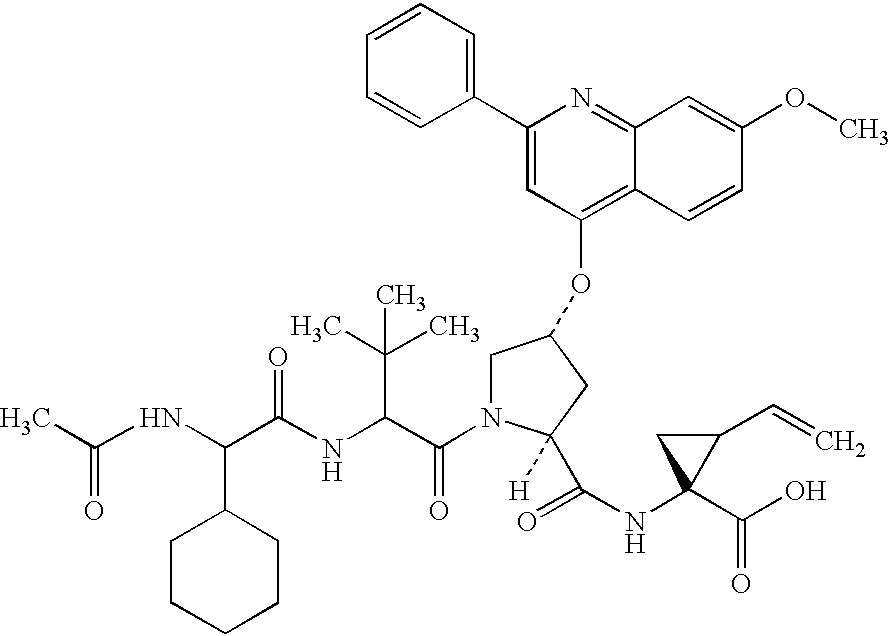
The present invention relates to compounds, including compounds of Formula I, or a pharmaceutically acceptable salt, ester, or prodrug, thereof: which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
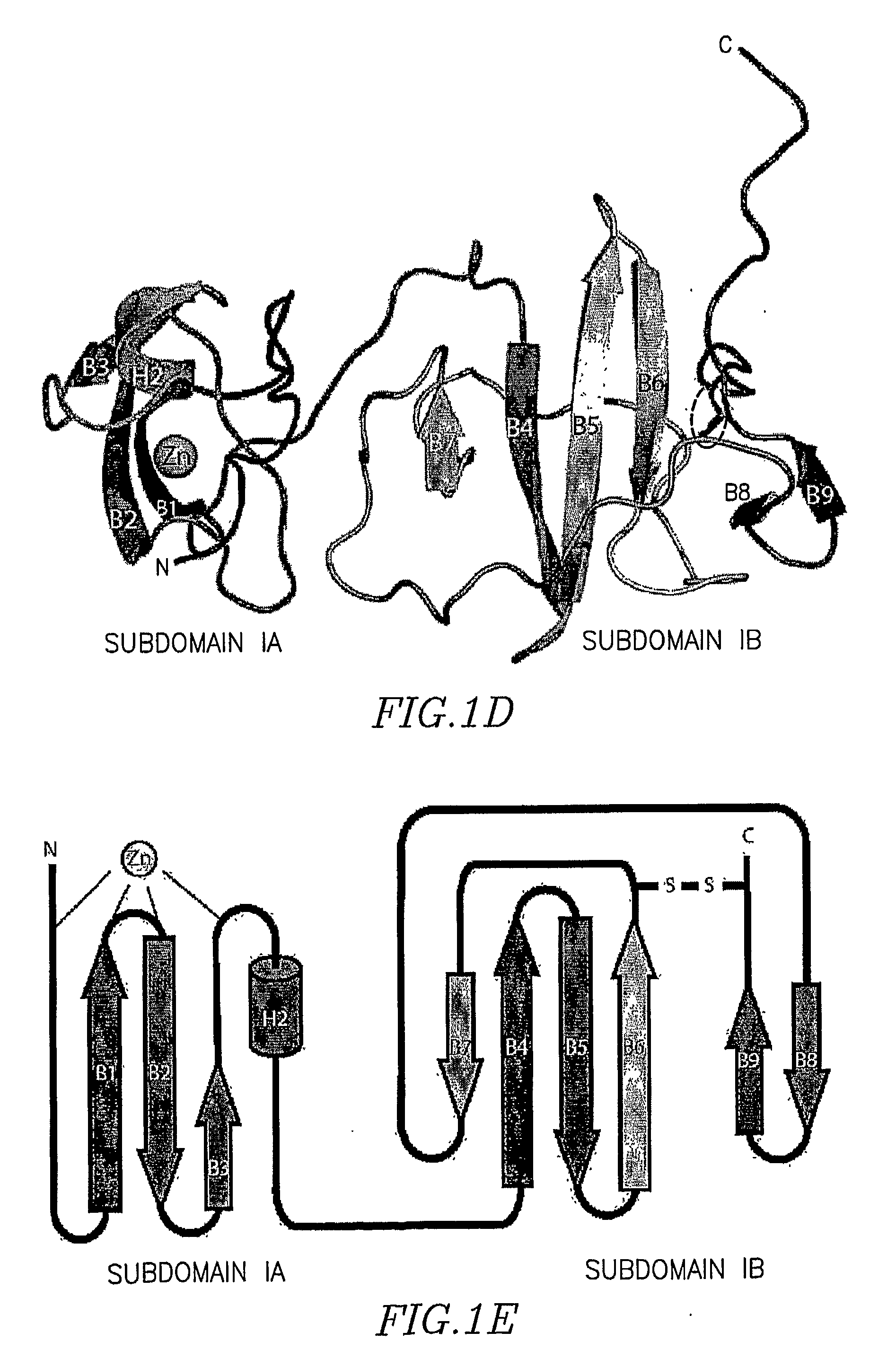
Structure of the Hepatitis C Ns5a Protein
The present invention provides a crystallized N-terminal domain of an NS5A protein of hepatitis C virus, methods of producing the same and methods of use thereof. The present invention also relates to structural elements of the N-terminal domain of hepatitis C virus NS5A protein, and methods of inhibiting hepatitis C virus infection, replication and / or pathogenesis, by interacting with the same.
Owner:THE ROCKEFELLER UNIV
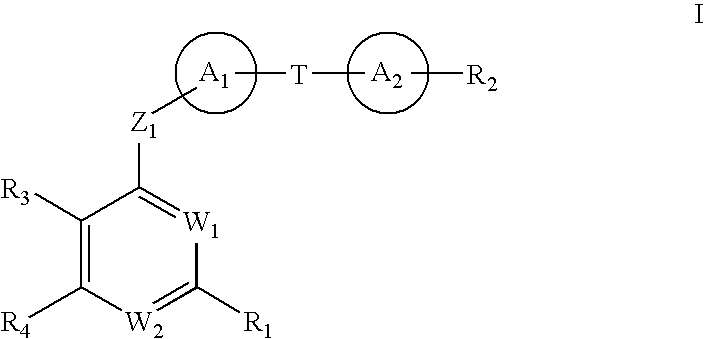
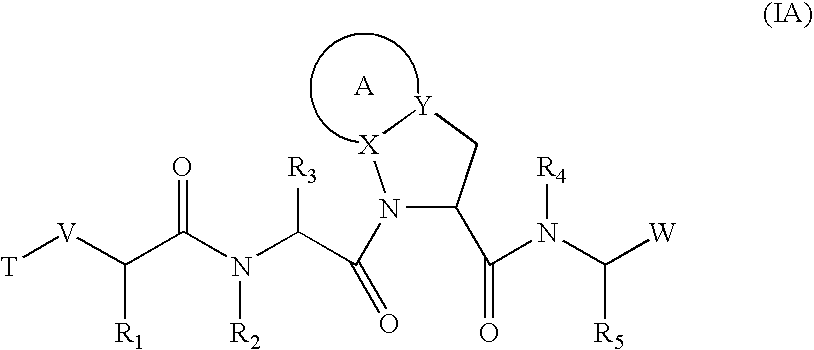
Hepatitis b antiviral agents
ActiveUS20170253609A1AntiviralsAmide active ingredientsHepatitis B immunizationPharmaceutical medicine
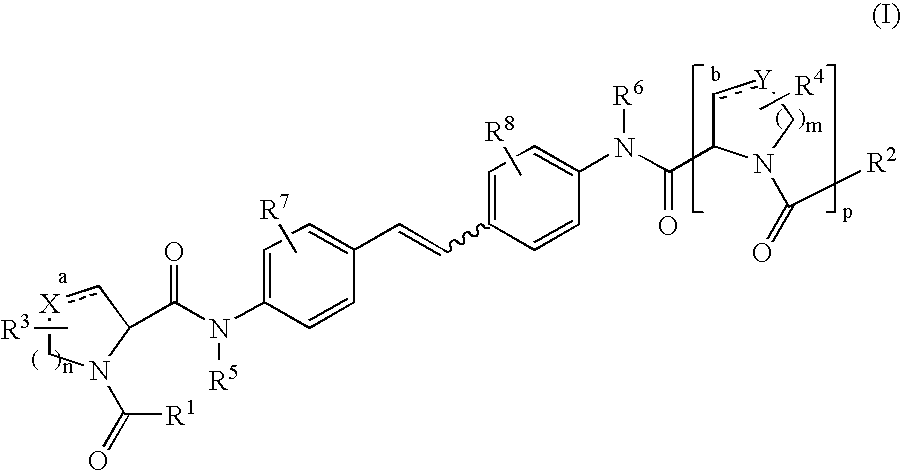
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:X-A-Y-L-R (I)which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
5,5-fused arylene or heteroarylene hepatitis c virus inhibitors
ActiveUS20110150827A1Inhibition of replicationBiocideDipeptide ingredientsPharmaceutical drugBioinformatics
Provided herein are 5,5-fused heteroarylene hepatitis C virus inhibitor compounds, for example, of Formula I, IA, or IB, pharmaceutical compositions comprising the compounds, and processes of preparation thereof. Also provided are methods of their use for the treatment of an HCV infection in a host in need thereof.
Owner:INDENIX PHARM LLC
Arylalkoxyl hepatitis c virus protease inhibitors
The present invention discloses compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering to the subject a pharmaceutical composition comprising a compound of the present invention.
Owner:ENANTA PHARM INC
Malaria immunogen and vaccine
InactiveUS6942866B2Easily preparedGreat stabilityHydrolasesAntibody mimetics/scaffoldsImmunogenicityPHA granule
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid protein (HBc) is disclosed that contains an immunogen for inducing the production of antibodies to malarial proteins. An immunogenic malarial epitope is expressed between residues 78 and 79 of the HBc immunogenic loop sequence. The chimer preferably contains a malaria-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:APOVIA INC
Liver cell membrane bionic liposome drug carrier as well as preparation method and application thereof
InactiveCN106109417AIncrease the number ofEnhanced generation abilityOrganic active ingredientsEnergy modified materialsCell membraneIn vivo
The invention relates to a liver cell membrane bionic liposome drug carrier, a preparation method of the liver cell membrane bionic liposome drug carrier, and application of the liver cell membrane bionic liposome drug carrier. The liver cell membrane bionic liposome drug carrier is characterized in that (1) the liposome drug carrier has a cell membrane protein component; (2) the liposome drug carrier is capable of loading drug in vitro, and is used for cell targeting fusion release; (3) the cell membrane protein component of the liposome comes from immortalized human liver cells, and is used for conveying targeted shear plasmids of a hepatitis virus genome; (4) the cell membrane protein component of the liposome comes from an immortalized liver tumor cell line, and is used for targeted drug delivery of liver tumor. The liver cell membrane bionic liposome drug carrier can be used for in vivo targeted hepatic cell transmission and drug delivery of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) gene targeting plasmids.
Owner:李因传
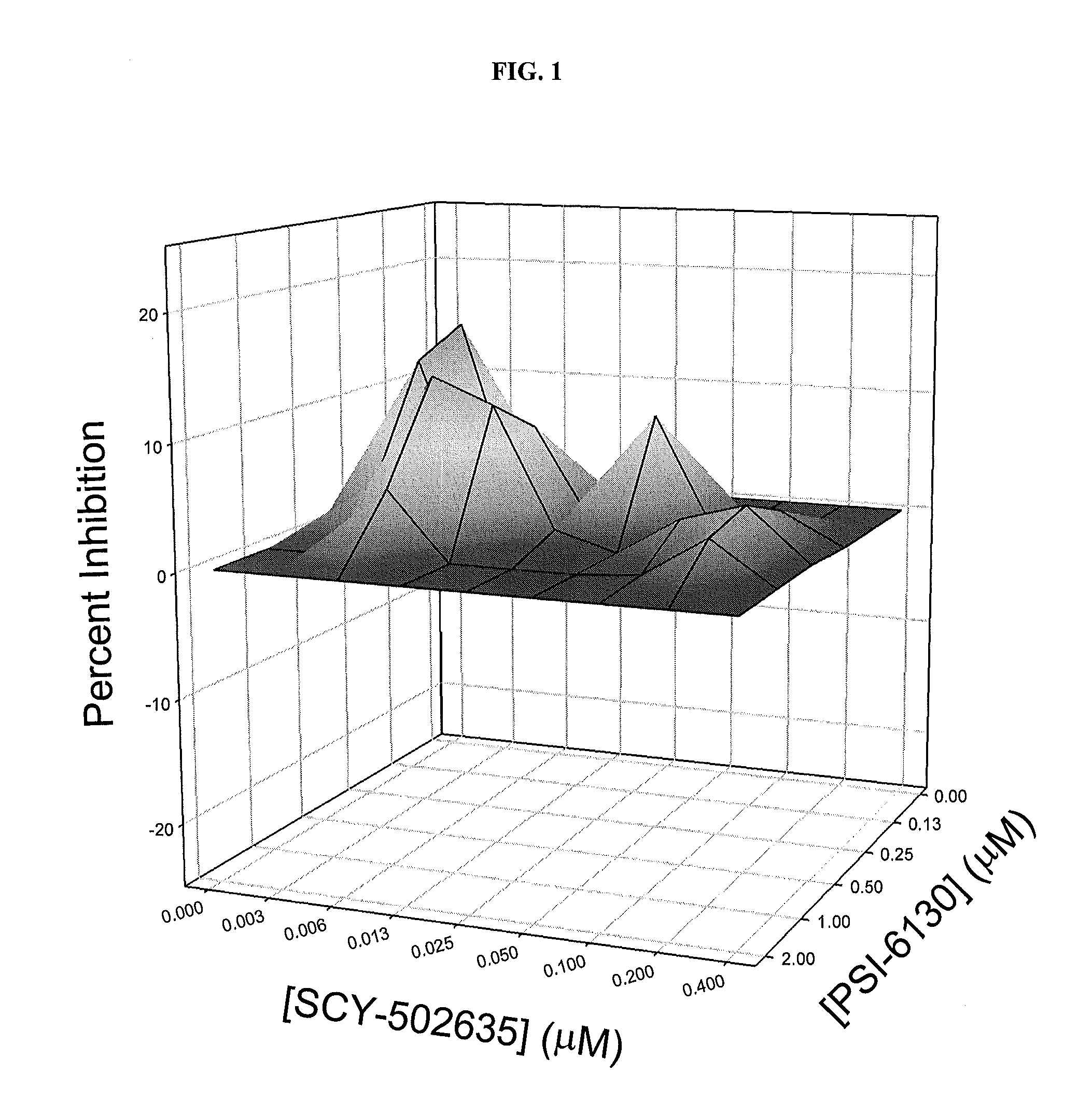
Pharmaceutical compositions
This invention relates to combinations comprising 3-[(R)-2-(N,N-dimethylamino)ethylthio-Sar]-4-(gammahydroxymethylleucine)cyclosporine, or a pharmaceutically acceptable salt, solvate or hydrate thereof; and certain nucleoside analogues, and their use in the treatment of hepatitis C virus.
Owner:SCYNEXIS INC
Pharmaceutical compositions for hepatitis C viral protease inhibitors
InactiveUS7157424B2Good chemical stabilityBiocideDigestive systemProteinase activityAdditive ingredient
Disclosed are pharmaceutical compositions of hepatitis C viral protease inhibitors, and methods of using these compositions for inhibiting the replication of the hepatitis C virus (HCV) and for the treatment of an HCV infection. These compositions are lipid based systems and comprise the hepatitis C viral protease inhibitor together with at least one pharmaceutically acceptable amine, at least one pharmaceutically acceptable base, at least one pharmaceutically acceptable oil and optionally one or more additional ingredients.
Owner:BOEHRINGER INGELHEIM INT GMBH
Inhibitors of serine proteases, particularly HCV NS3-NS4A protease
InactiveUS20080045480A1Reduce riskReduce severityBiocidePeptide/protein ingredientsSerine Protease InhibitorsProteinase activity
Owner:VERTEX PHARMA INC
Liver targeted antivirus precursor medicament annular phosphoester and use thereof
InactiveCN101475594AFew synthetic stepsEasy to operateOrganic active ingredientsGroup 5/15 element organic compoundsDrugPhosphate
The invention provides a prodrug of an antiviral drug for use in liver of cyclic phosphate of a general formula (I) and isomers, pharmaceutical salts, hydrates, solvates and pharmaceutical compositions of the same. The invention provides uses of the compounds singly or together with other antiviral drugs in the treatment of viruses, in particular of hepatitis B viruses (HBV), hepatitis C virus (HCV), HIV viruses and / or human cytomegaloviruses (HCMV).
Owner:廖国超
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
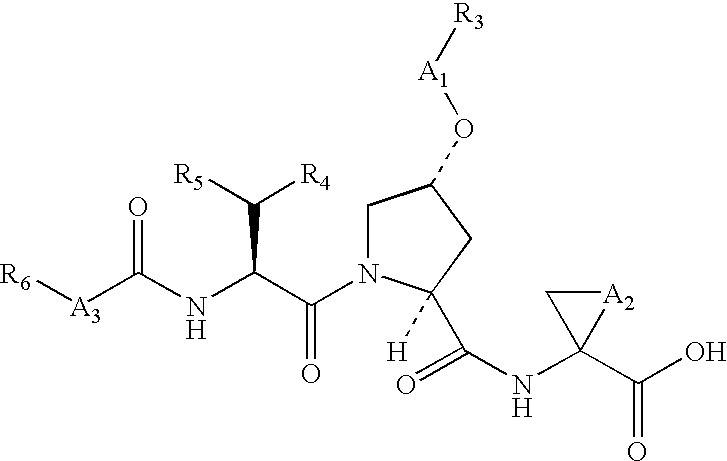
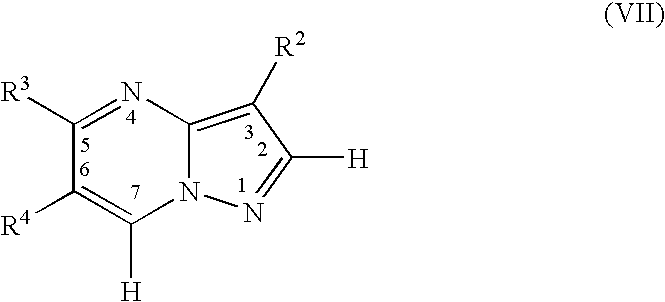
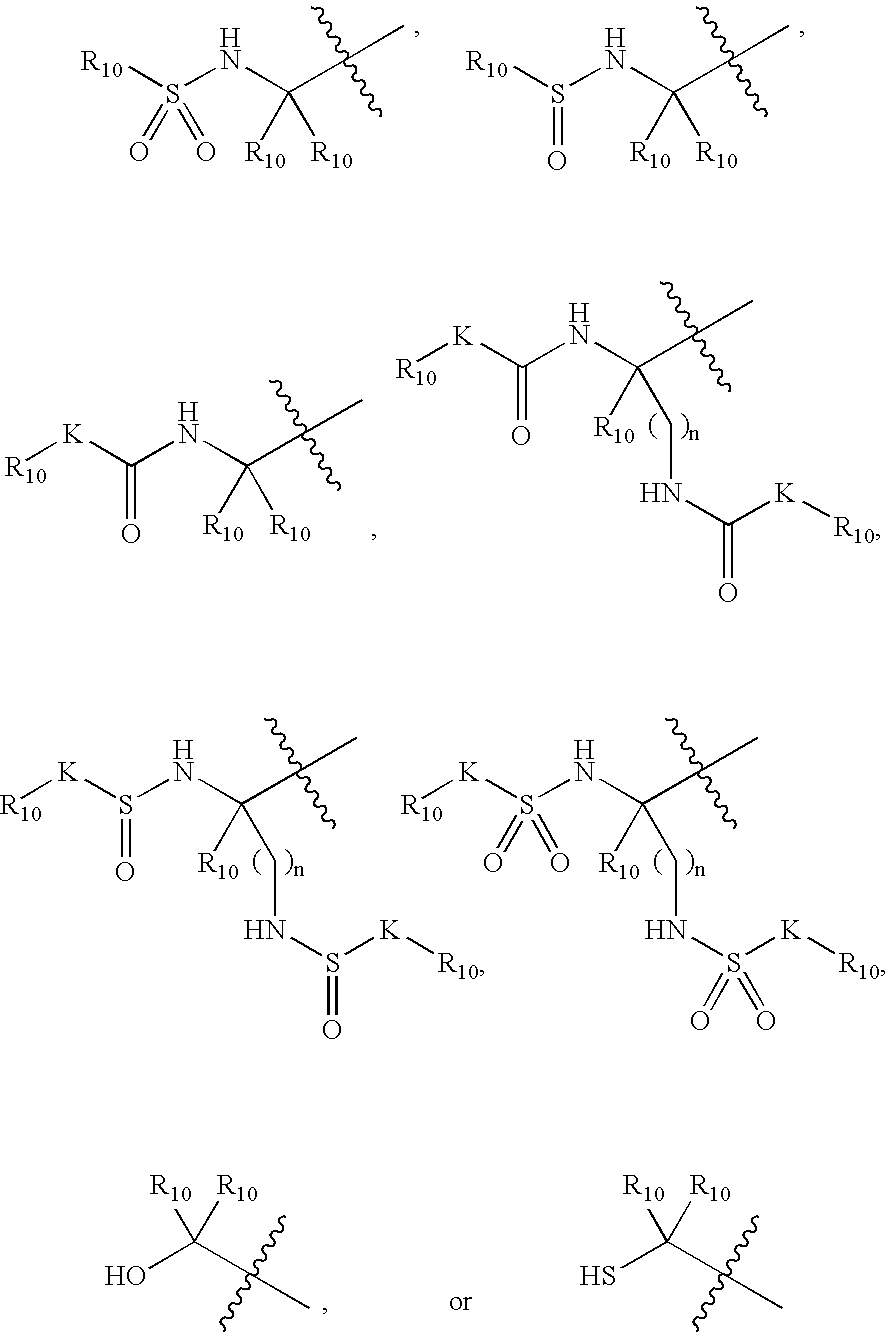
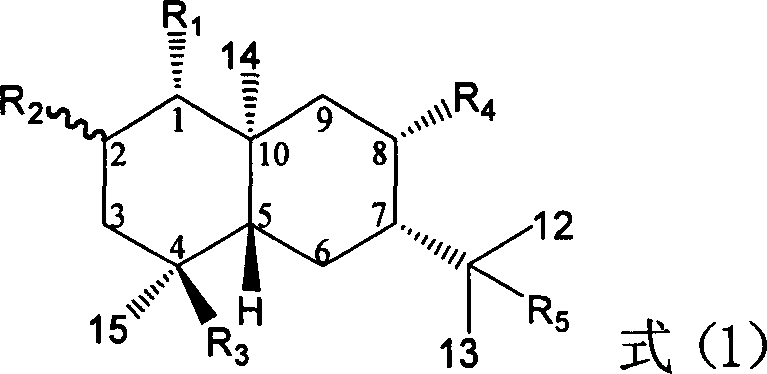
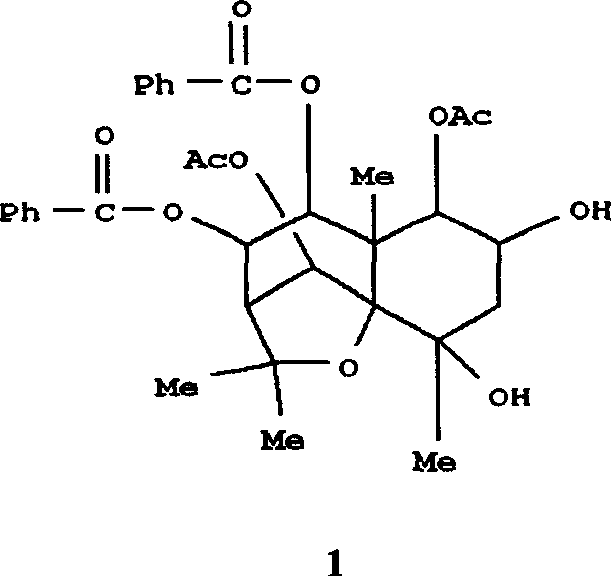
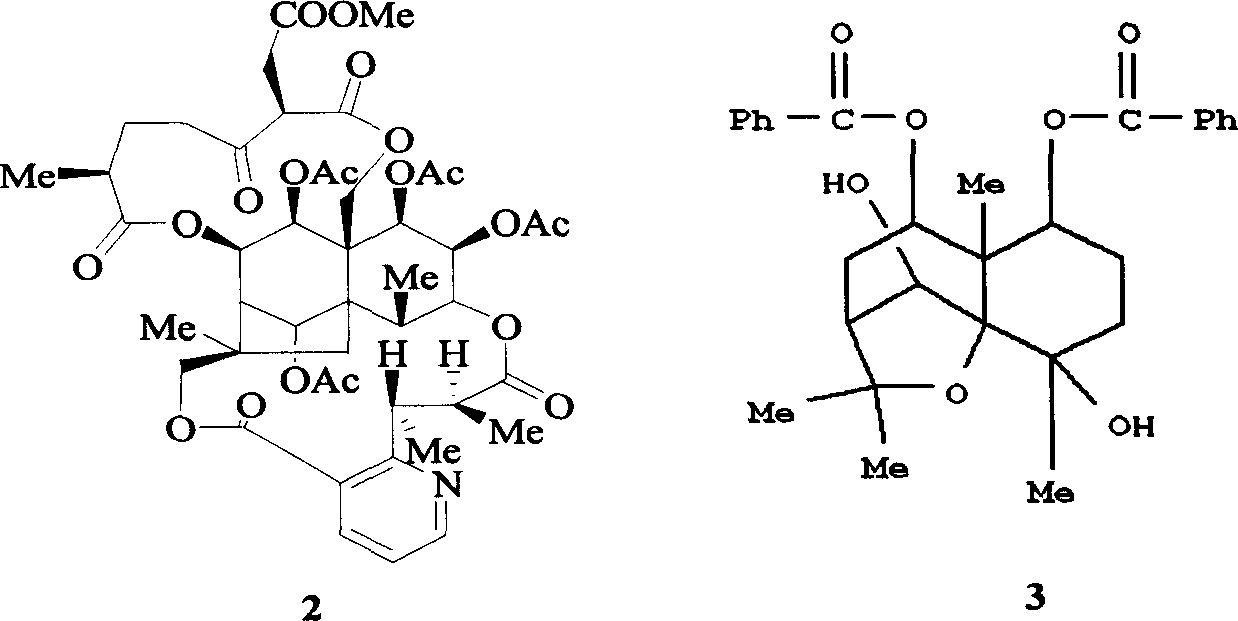
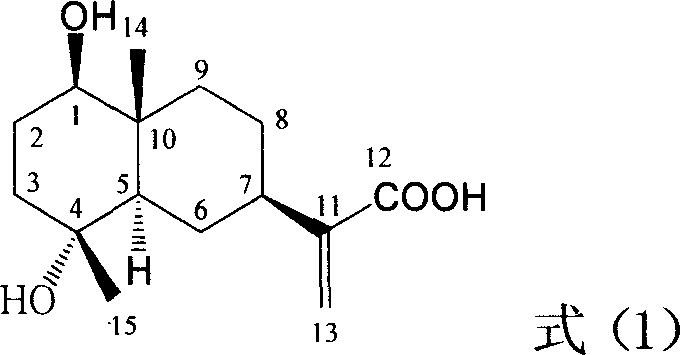
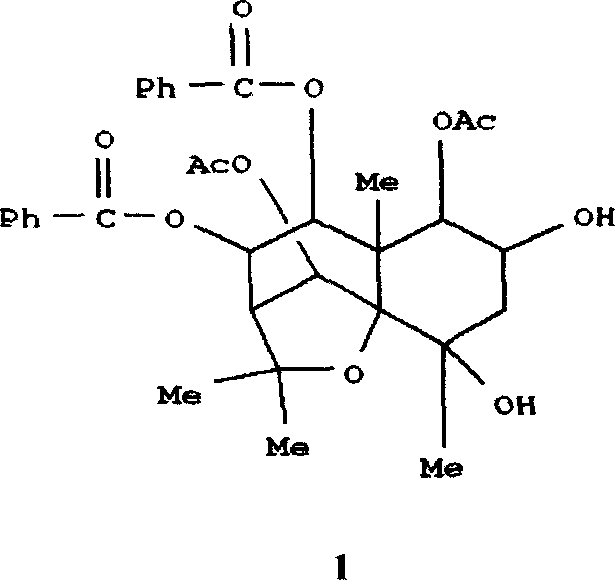
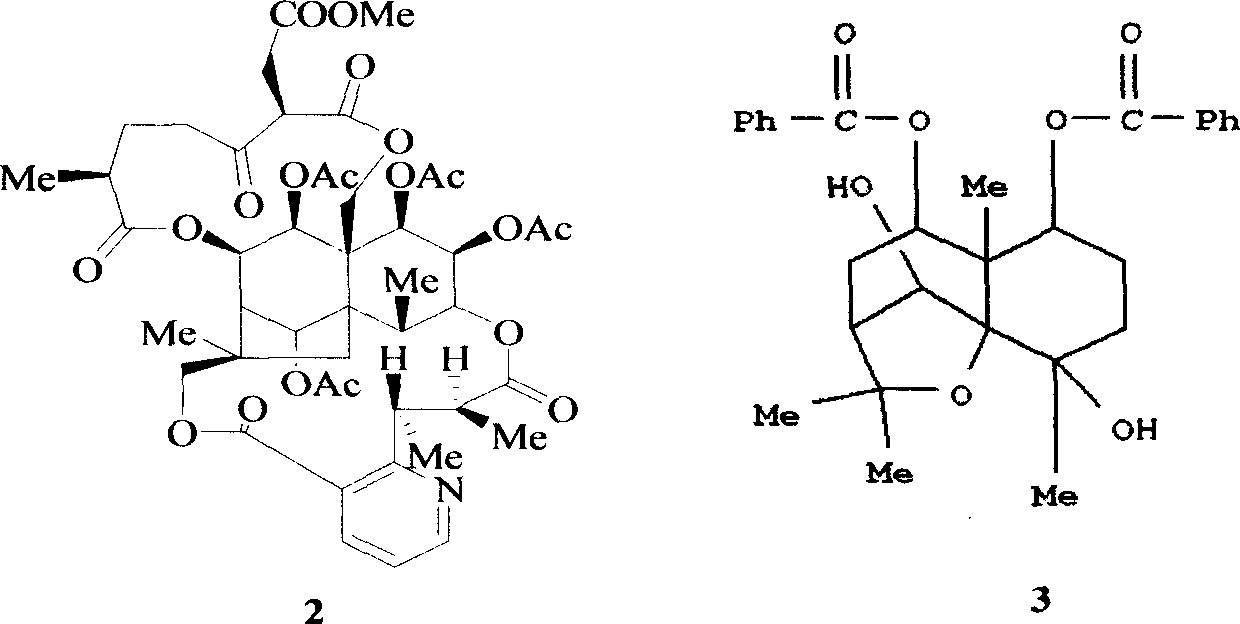


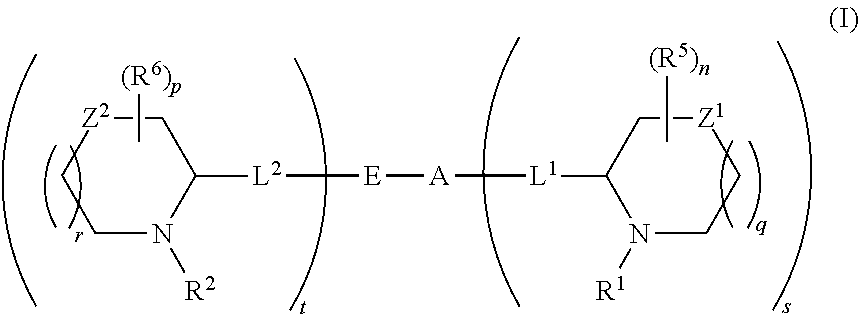
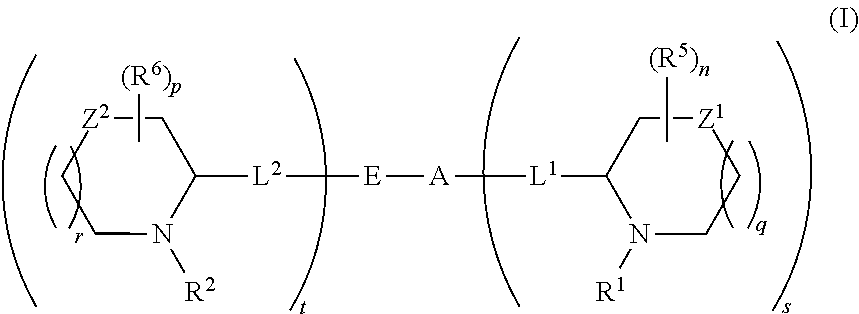


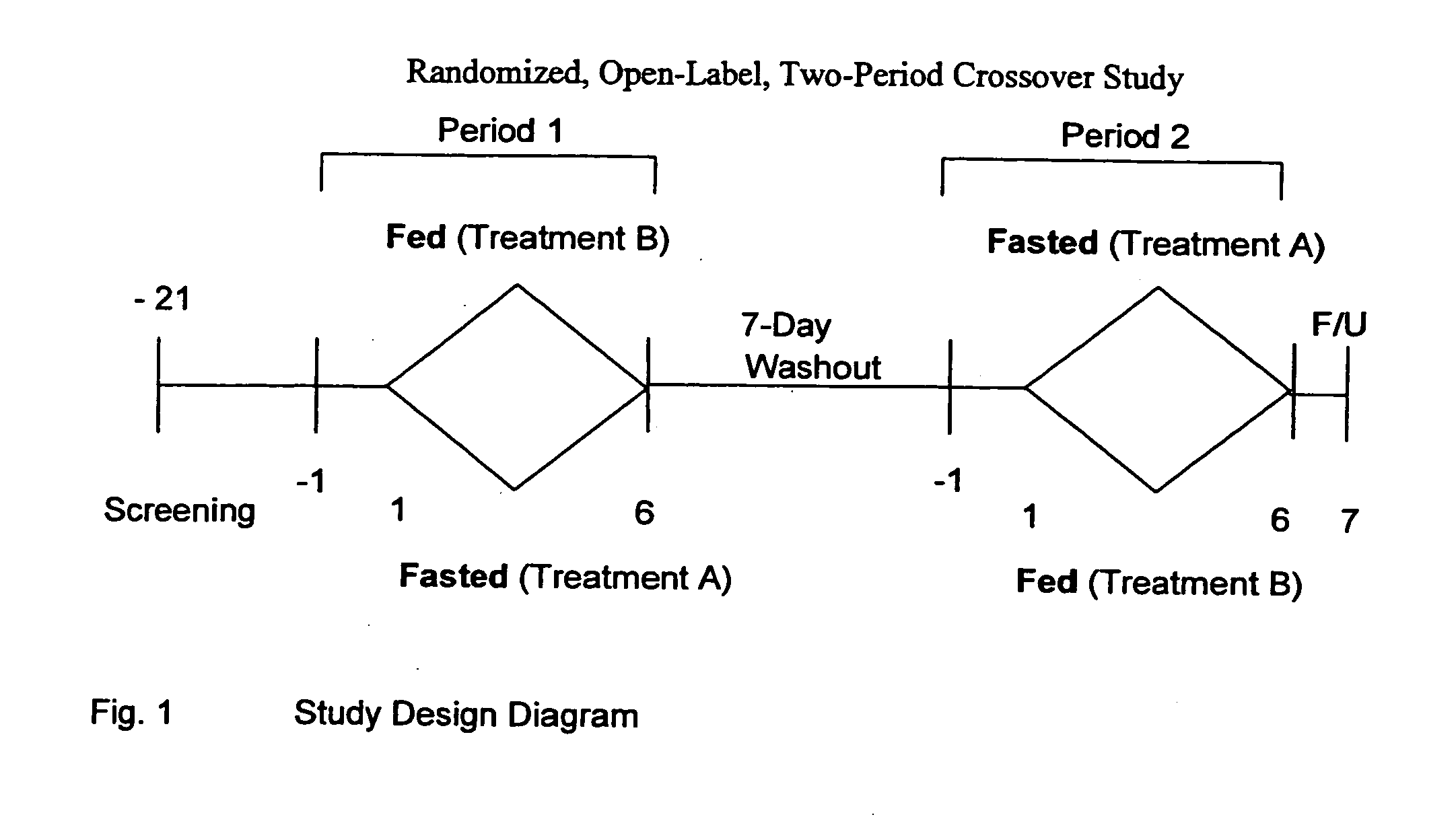
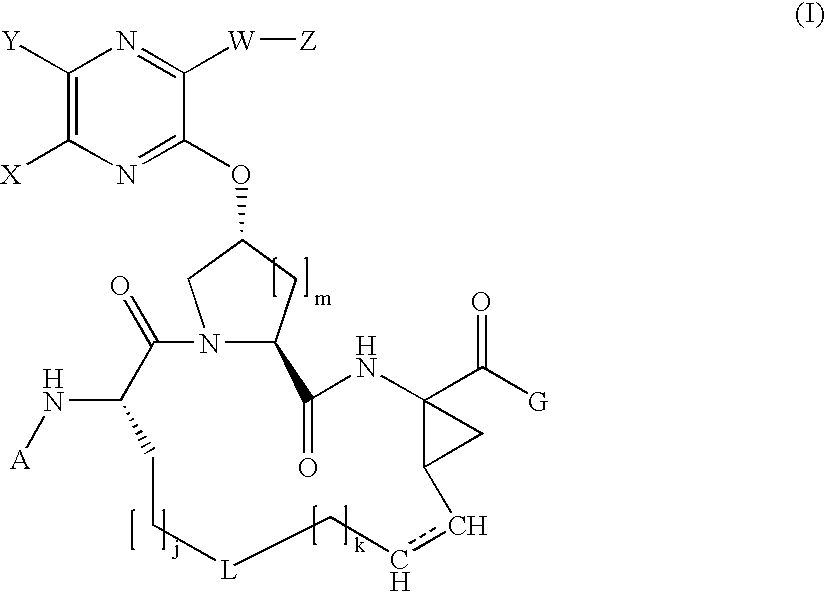
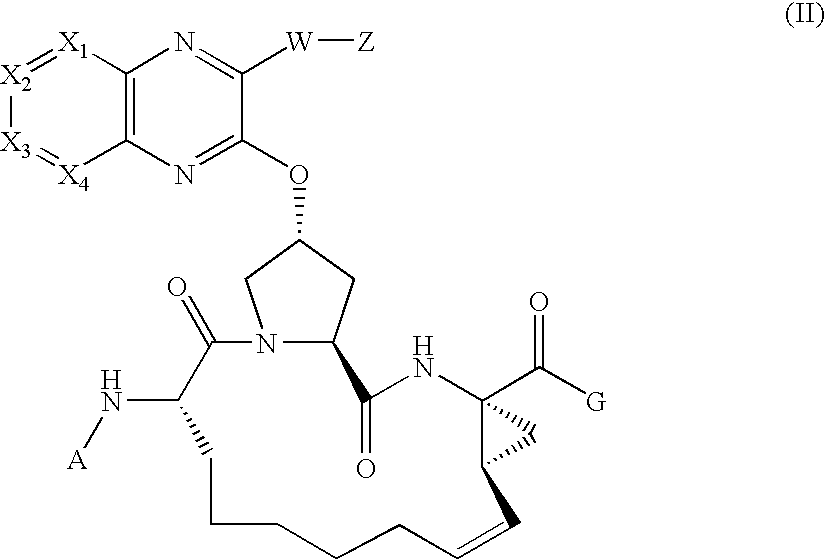
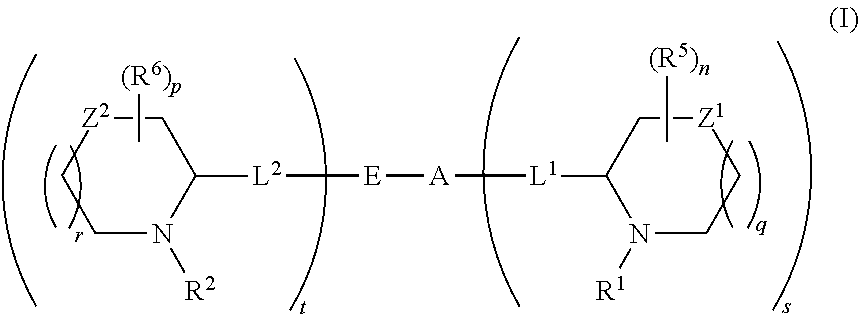
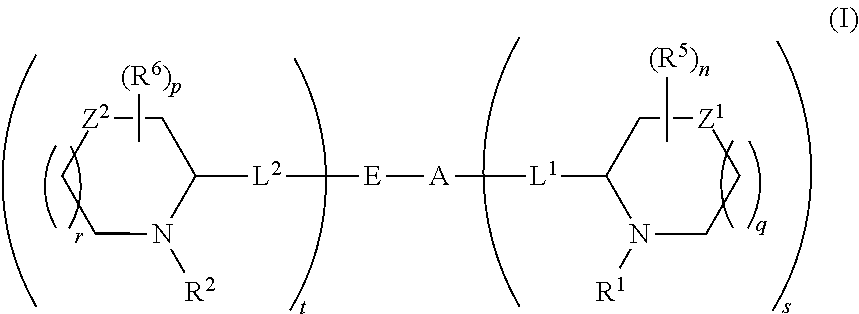

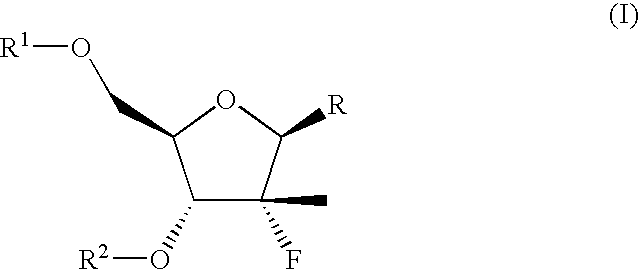
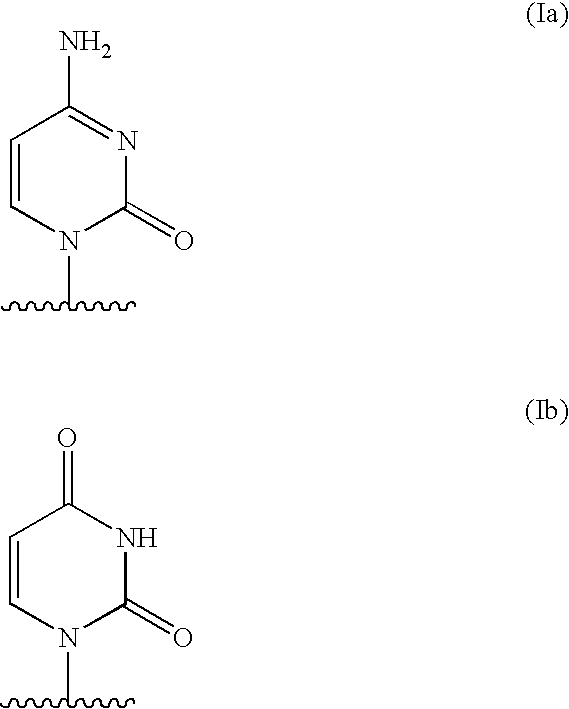
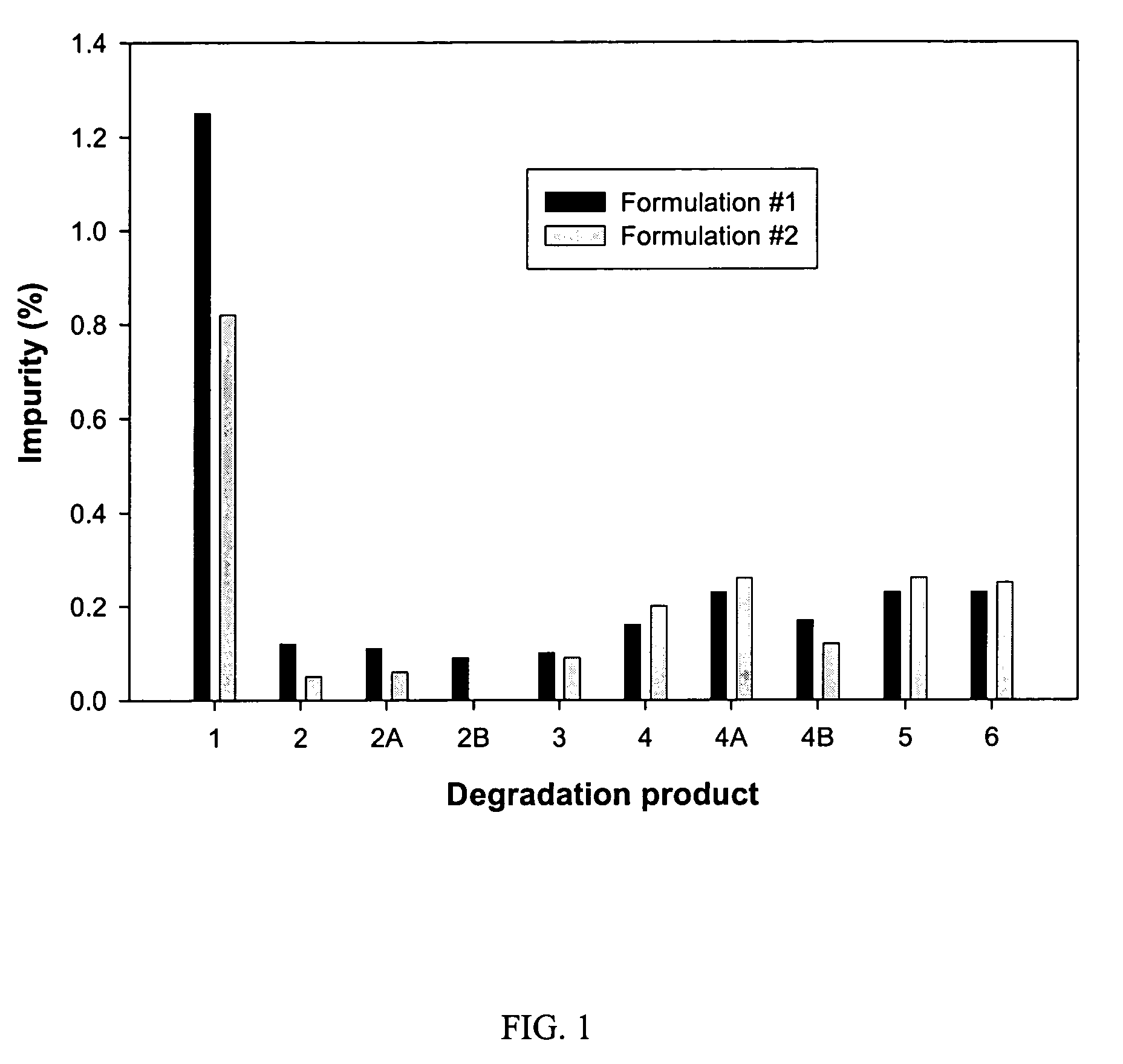
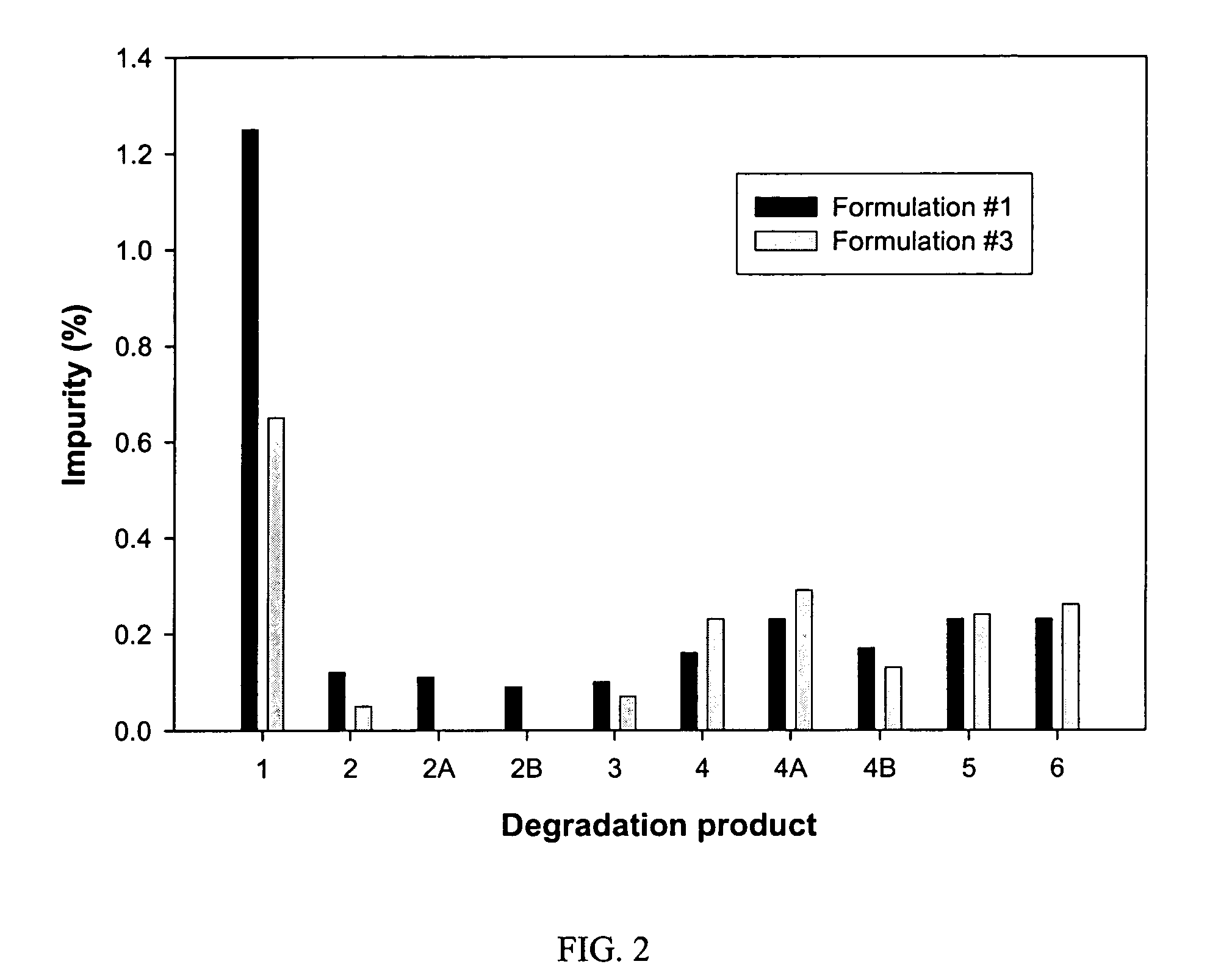
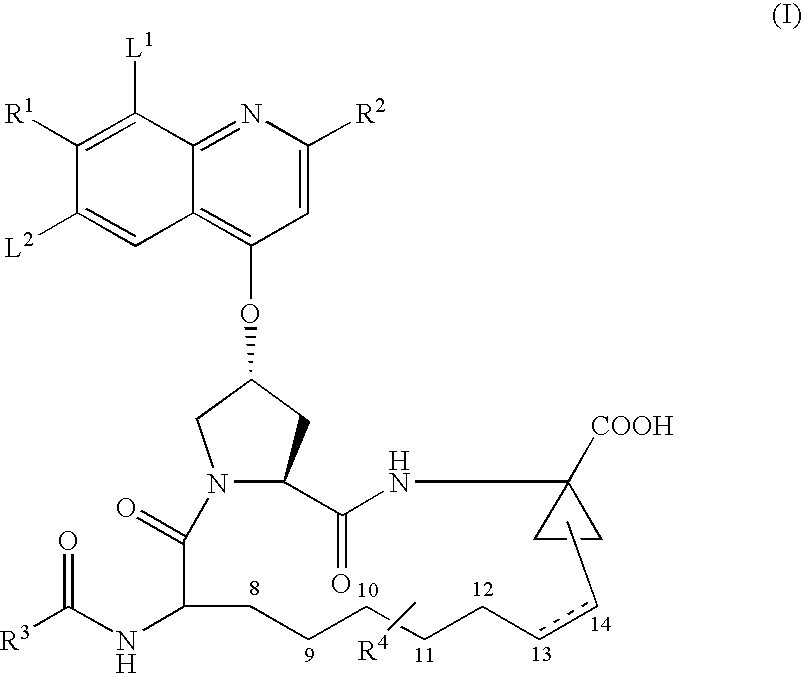
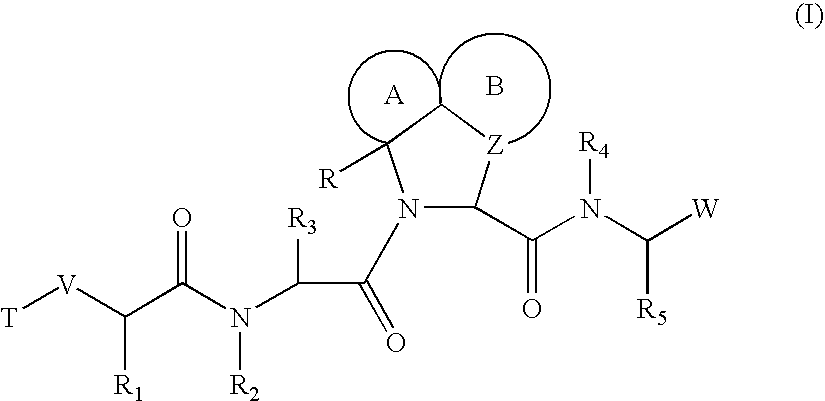
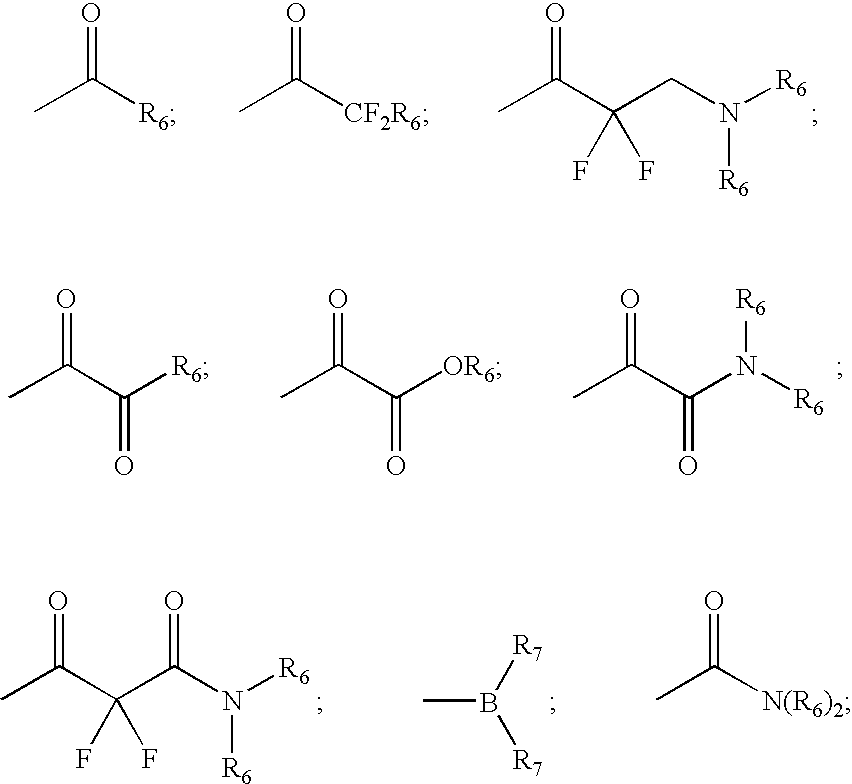
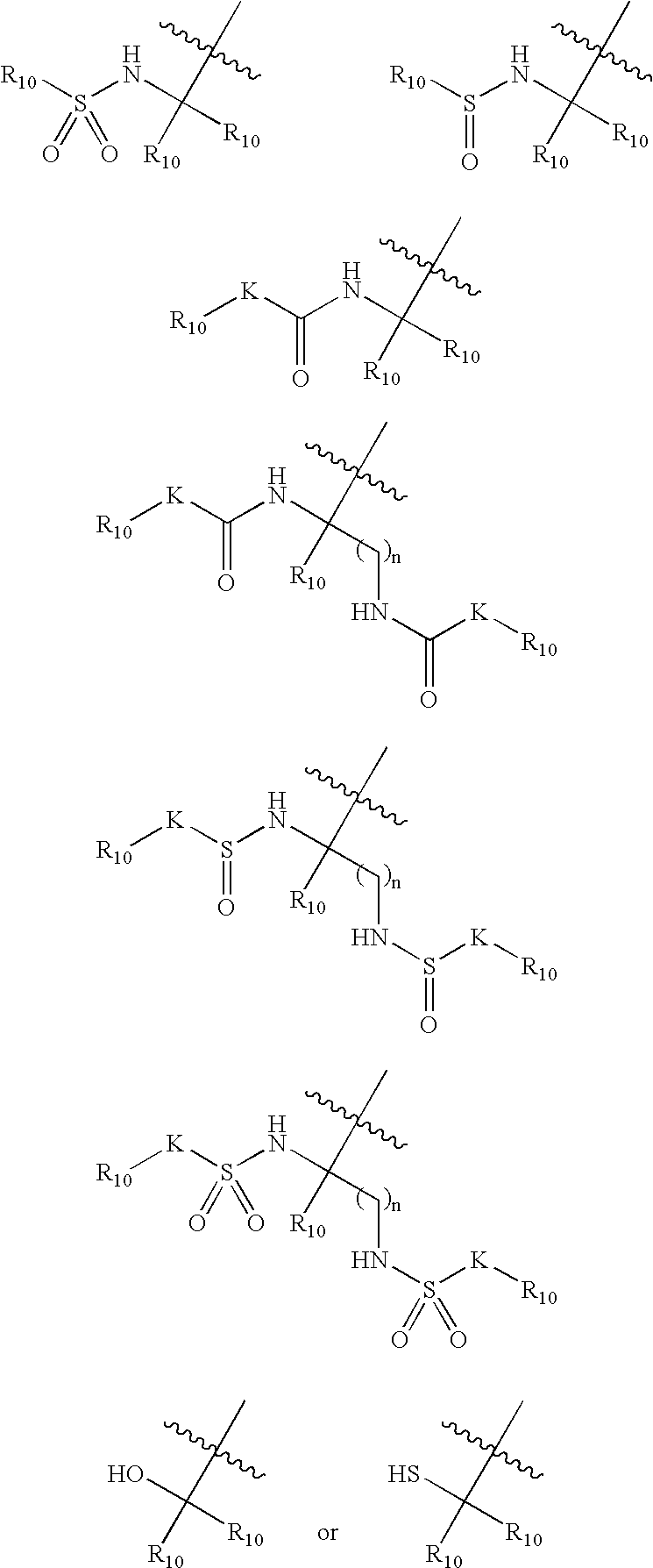
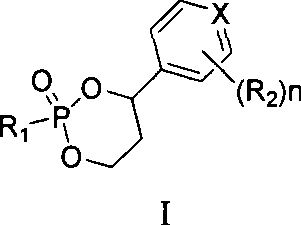
![Substituted triazolo[1,5-A]pyrimidines as antiviral agents Substituted triazolo[1,5-A]pyrimidines as antiviral agents](https://images-eureka.patsnap.com/patent_img/df3ffcef-a760-4864-a217-73f3cff6d885/US08609668-20131217-D00001.png)
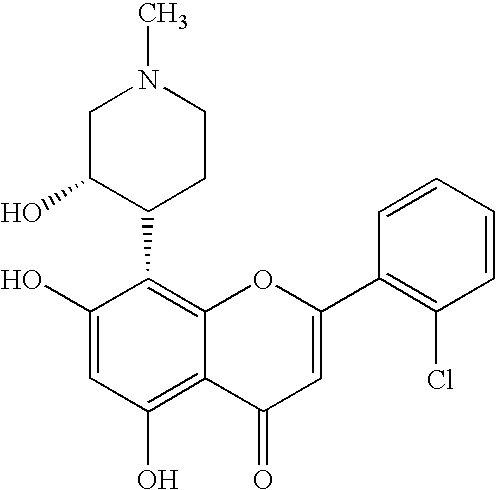
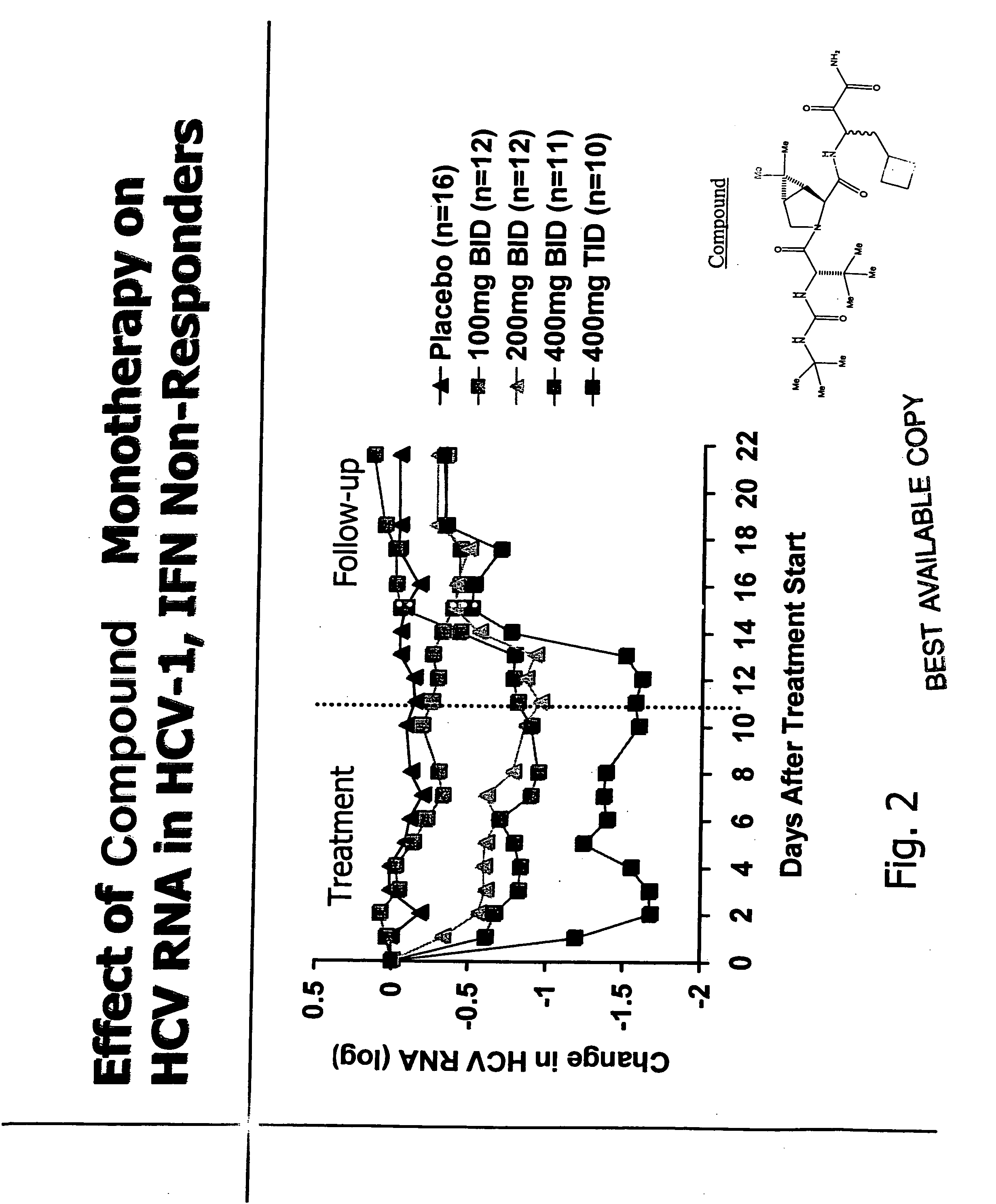
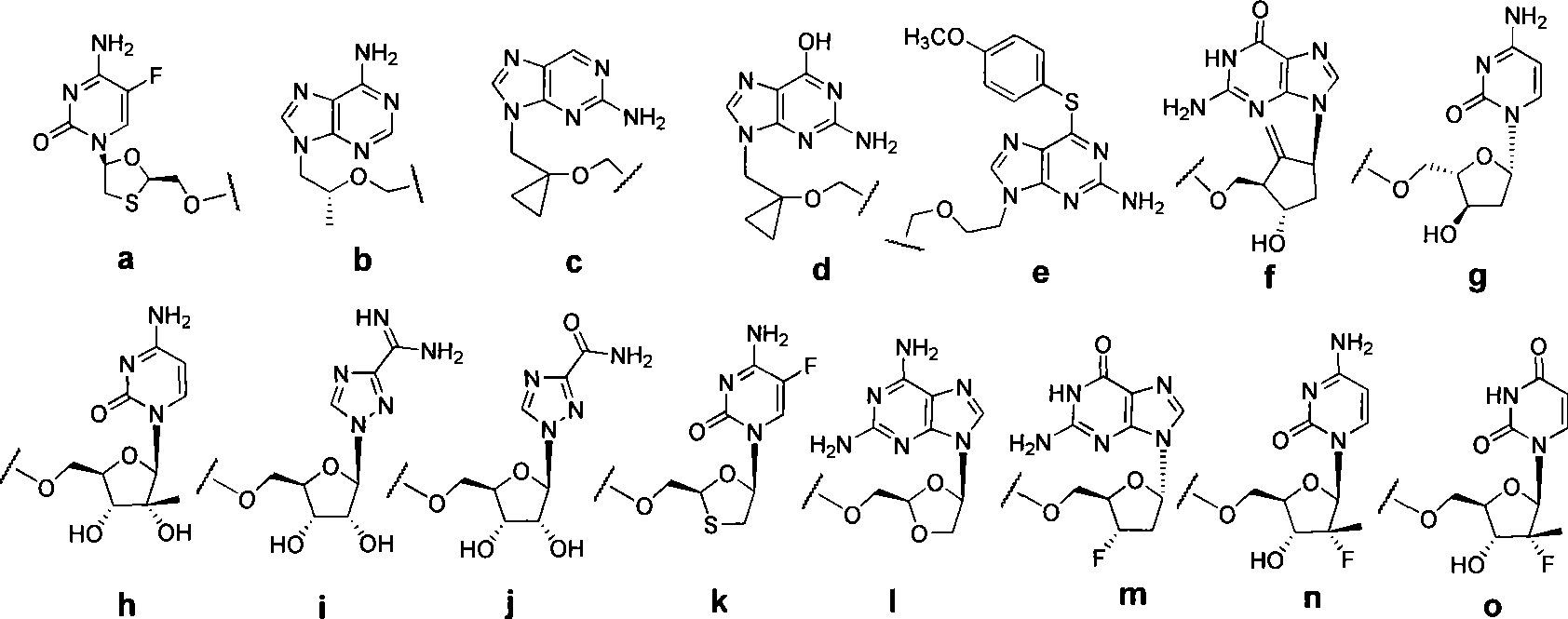
![Substituted triazolo[1,5-A]pyrimidines as antiviral agents Substituted triazolo[1,5-A]pyrimidines as antiviral agents](https://images-eureka.patsnap.com/patent_img/df3ffcef-a760-4864-a217-73f3cff6d885/US08609668-20131217-D00002.png)
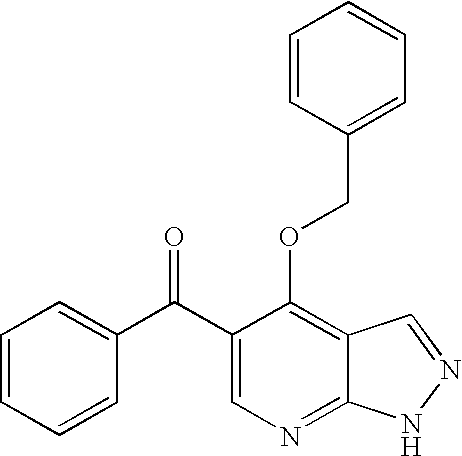
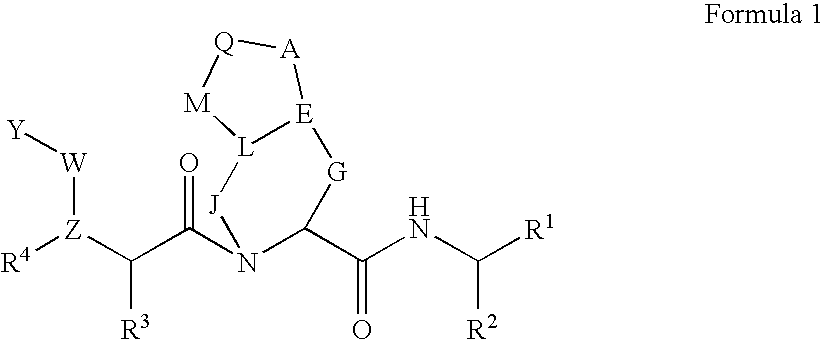
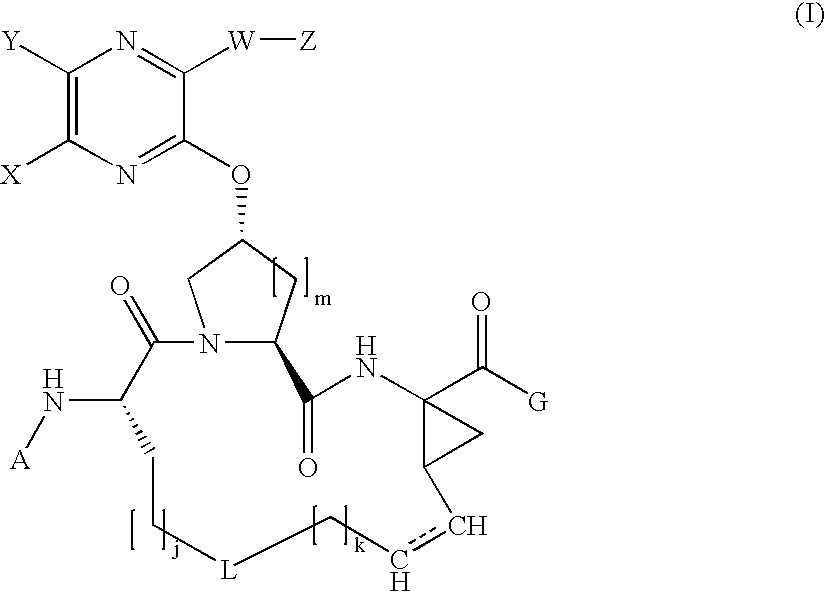
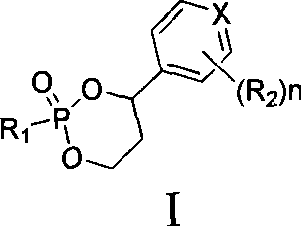
![Substituted triazolo[1,5-A]pyrimidines as antiviral agents Substituted triazolo[1,5-A]pyrimidines as antiviral agents](https://images-eureka.patsnap.com/patent_img/df3ffcef-a760-4864-a217-73f3cff6d885/US08609668-20131217-D00003.png)