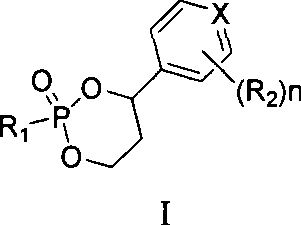
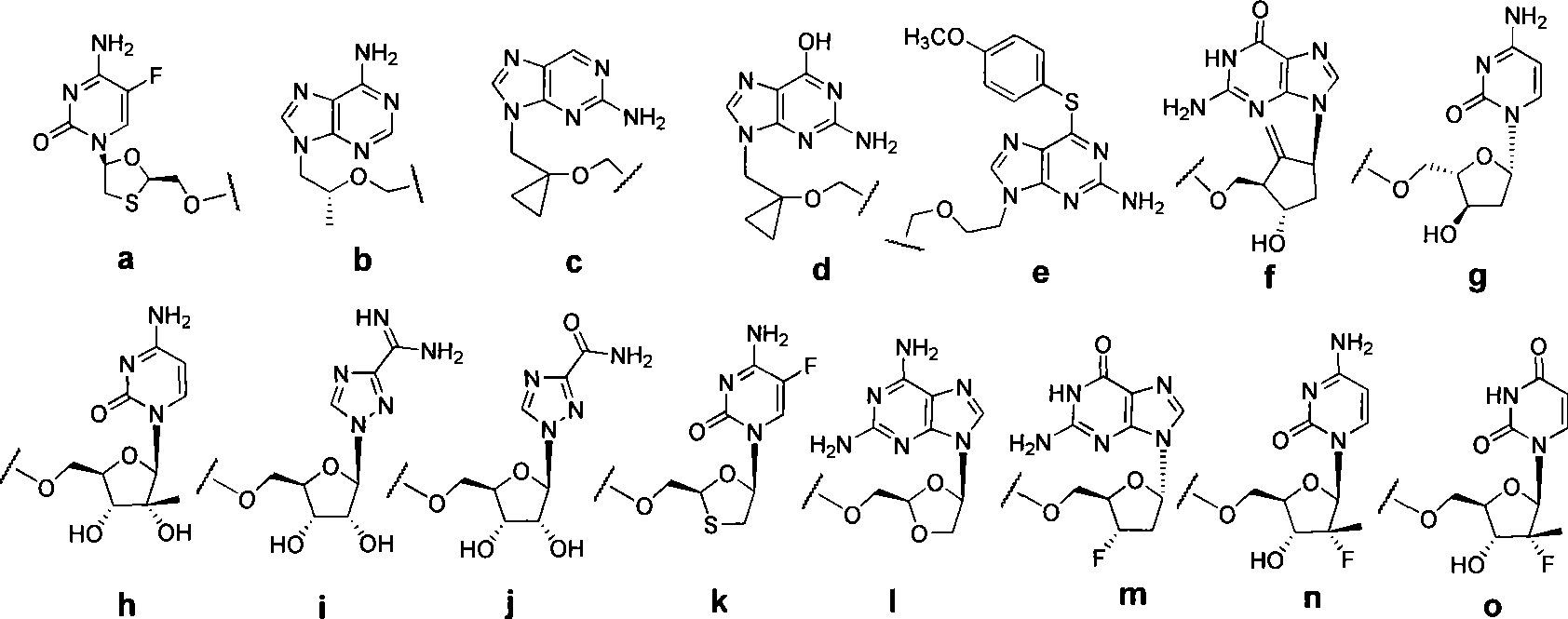
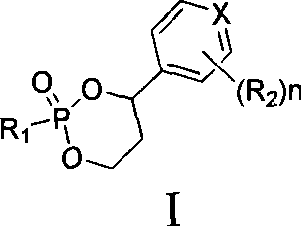
Liver targeted antivirus precursor medicament annular phosphoester and use thereof
A technology of phosphate esters and medicinal salts, applied in antiviral agents, drug combinations, pharmaceutical formulations, etc., can solve the problems of reducing curative effect and difficult to reach liver cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1 5-fluoro-1-(2R, 5S)-{2′,4′-cis-(4′S)-4′-[(3′-chlorophenyl)-2′-oxo- Preparation of 1', 3', 2'-dioxaphosphorin-2'-yl]oxymethyl-1,3-oxathione-5-yl}cytosine
[0099] In N,N-dimethylformamide (30ml) solution, add FTC (741.6mg, 3.0mmol), 4S-4-(3-chlorophenyl)-N,N-diisopropyl-1,3 , 2-dioxaphosphorinane-2-amine (1.45g, 4.5mmol) and 5-methylthiotetrazolium (527mg, 4.5mmol), cooled to minus 45°C, slowly added dropwise tert-peroxide Butanol, after reacting for 1 hour, warming up to room temperature for another 24 hours, distilling off the solvent under reduced pressure, silica gel column chromatography, eluting with methanol: dichloromethane = 1:18 to obtain 0.30 g of white powdery solid, yield 21.0% , melting point 98-100°C, R f =0.38 (methanol:dichloromethane=1:9). 1 HNMR (400MHz, DMSO), δ (ppm): 1.98-2.45 (m, 2H), 2.91-3.22 (m, 1H), 3.20-3.47 (m, 1H), 4.19-4.62 (m, 4H), 5.21- 5.60 (m, 2H), 6.16-6.32 (m, 1H), 7.20-7.51 (m, 4H), 8.22 (S, 1H); ESI-MS: 478.0 (M+1).
Embodiment 2
[0100] Example 2 5-fluoro-1-(2R, 5S)-{2', 4'-trans-(4'S)-4'-[(3'-chlorophenyl)-2'-oxo- Preparation of 1', 3', 2'-dioxaphosphorin-2'-yl]oxymethyl-1,3-oxathione-5-yl}cytosine
[0101] Prepared by the same method as in Example 1, 0.25 g of white powdery solid was obtained, yield 17.5%, melting point 92-94 ° C, R f =0.42 (methanol:dichloromethane=1:9). 1 HNMR (400MHz, DMSO), δ (ppm): 2.01-2.48 (m, 2H), 2.89-3.25 (m, 1H), 3.20-3.49 (m, 1H), 4.09-4.68 (m, 4H), 5.22- 5.59 (m, 2H), 6.18-6.30 (m, 1H), 7.20-7.51 (m, 4H), 8.22 (S, 1H); ESI-MS: 478.0 (M+1).
Embodiment 3
[0102] Example 3 5-fluoro-1-(2R, 5S)-{2′,4′-cis-(4′S)-[4′-(4′-pyridyl)-2′-oxo-1 Preparation of ', 3', 2'-dioxaphosphorin-2'-yl}oxymethyl-1,3-oxathione-5-yl]cytosine
[0103] Prepared by a method similar to Example 1, FTC (741.6mg, 3.0mmol), 4S-4-(4-pyridyl)-N,N-diisopropyl-1,3,2-dioxaphosphorus Heterocyclohexane-2-amine (1.27g, 4.5mmol) reacted with 5-methylthiotetrazolium (527mg, 4.5mmol) to obtain 0.28g brown powdery solid, yield 21.0%, R f =0.40 (methanol:dichloromethane=1:6). 1 HNMR (400MHz, DMSO), δ (ppm): 1 HNMR (400MHz, DMSO), δ (ppm): 2.01-2.38 (m, 2H), 2.91-3.26 (m, 1H), 3.22-3.50 (m, 1H), 4.10-4.67 (m, 4H), 5.20- 5.63(m, 2H), 6.19-6.35(m, 1H), 7.34(d, J=9.6Hz, 2H), 8.24(s, 1H), 8.56(d, J=9.6Hz, 2H); ESI-MS : 445.1 (M+1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com