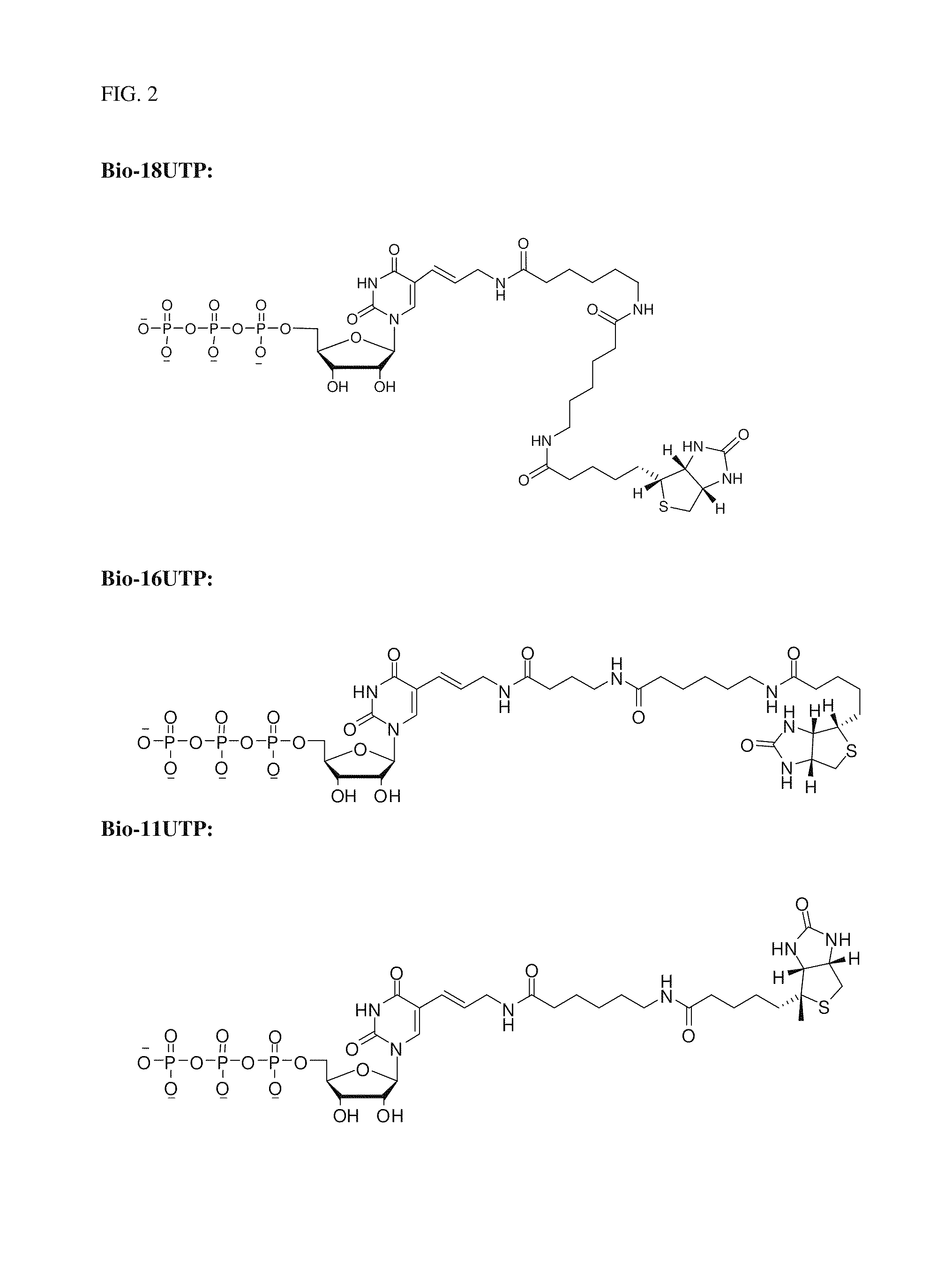
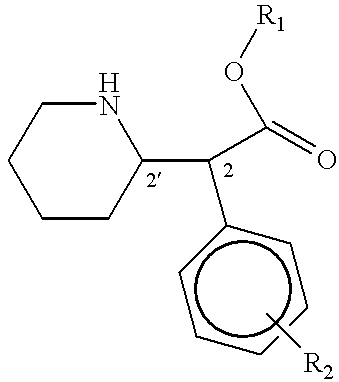
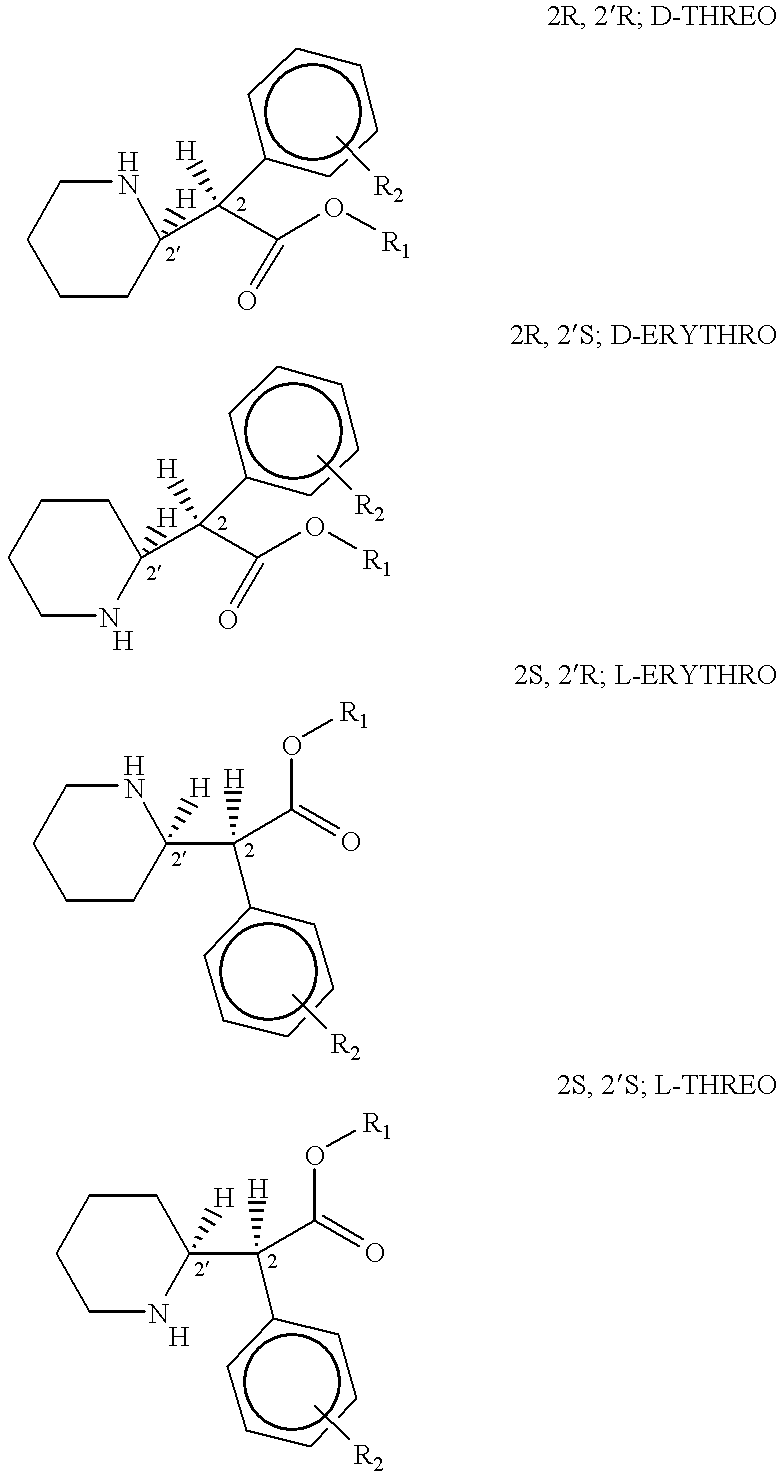
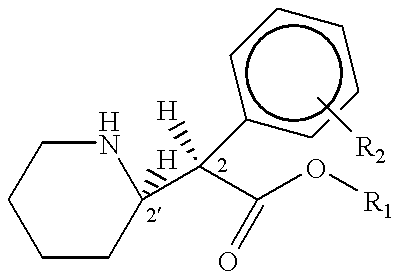
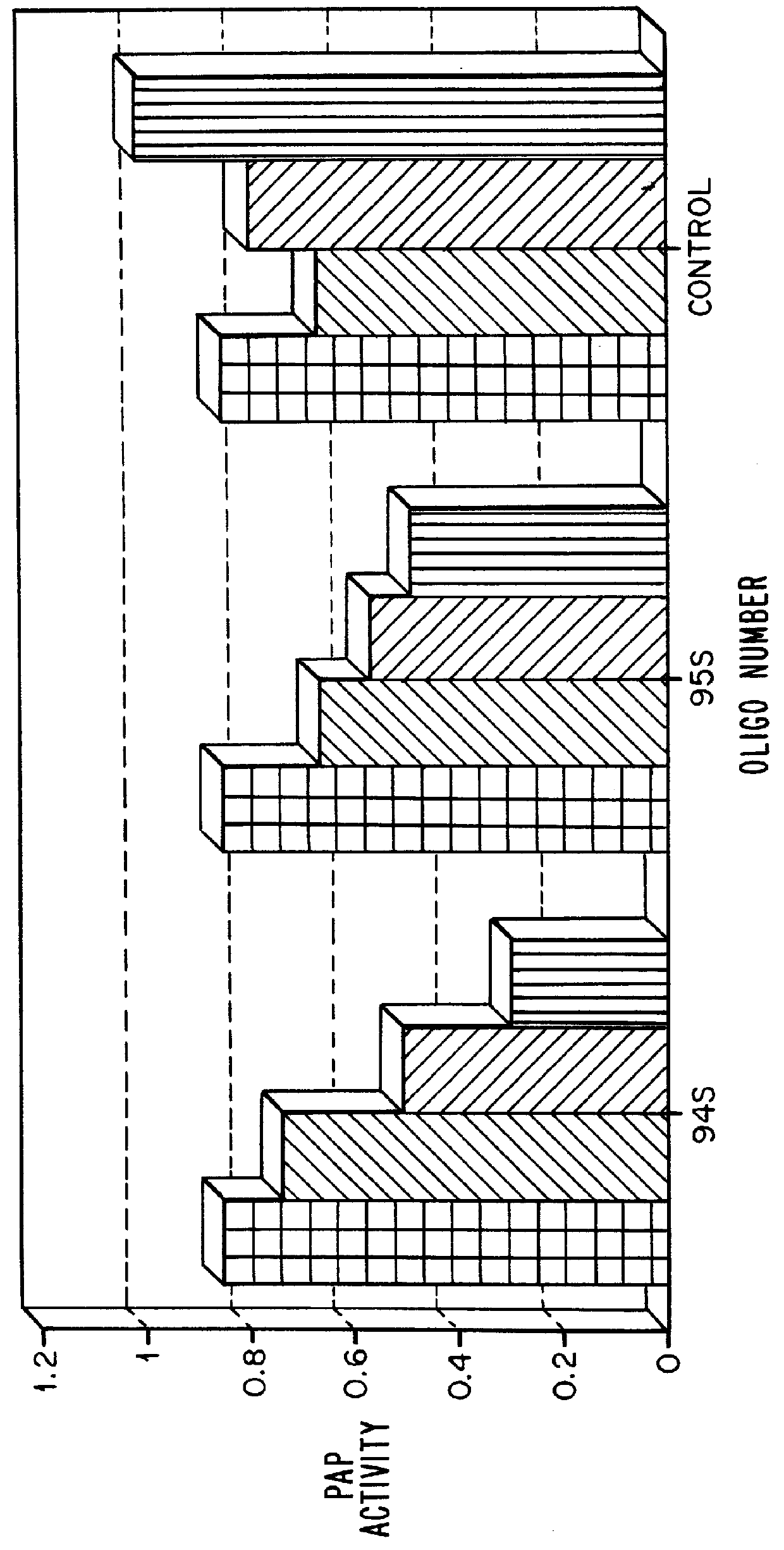
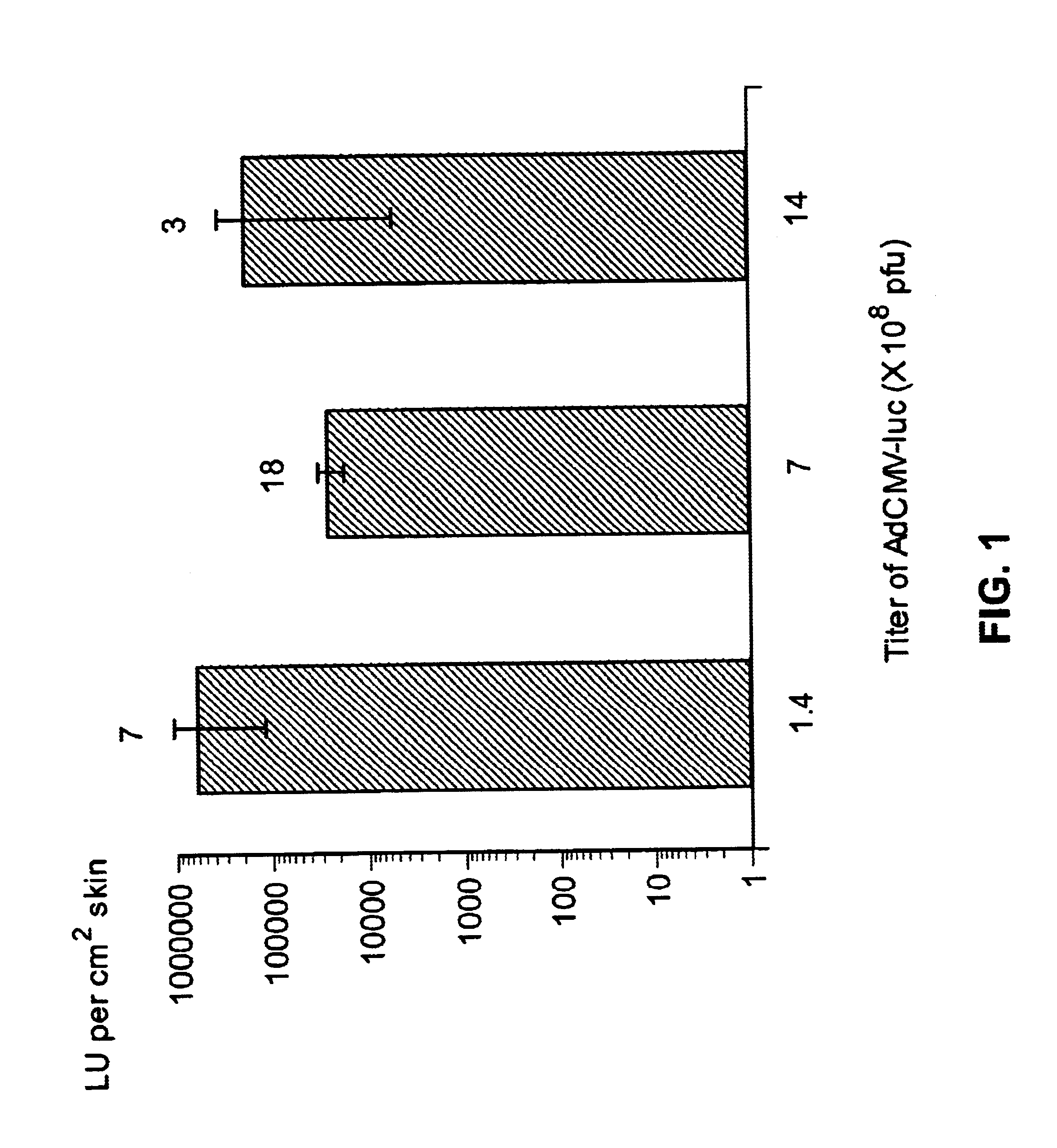
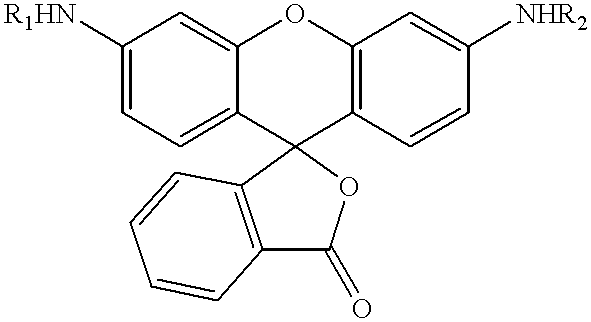
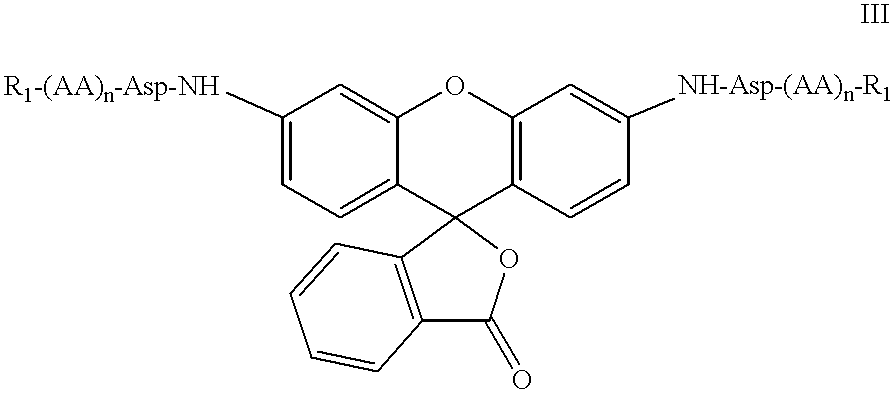
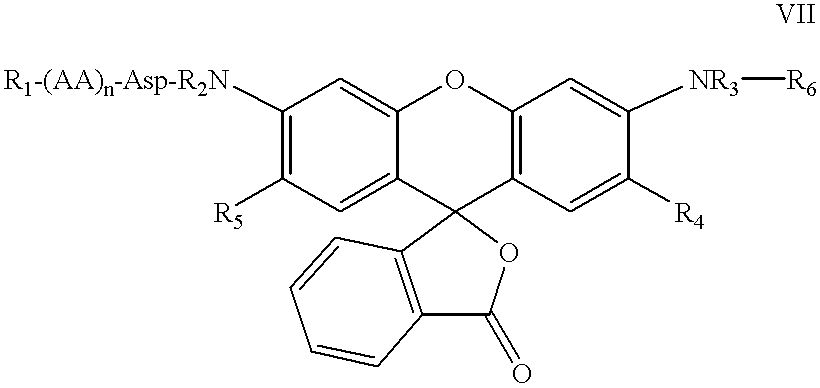
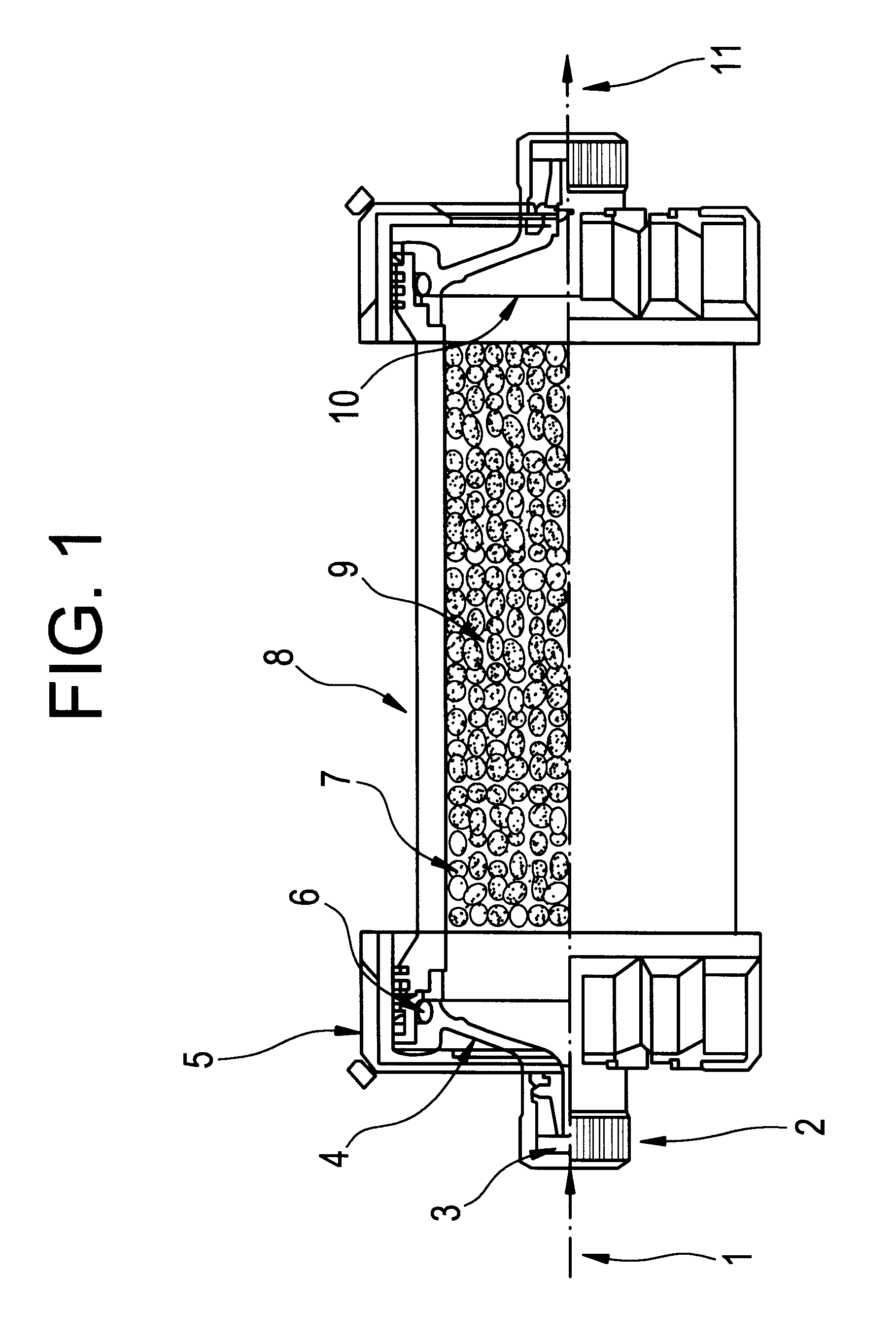
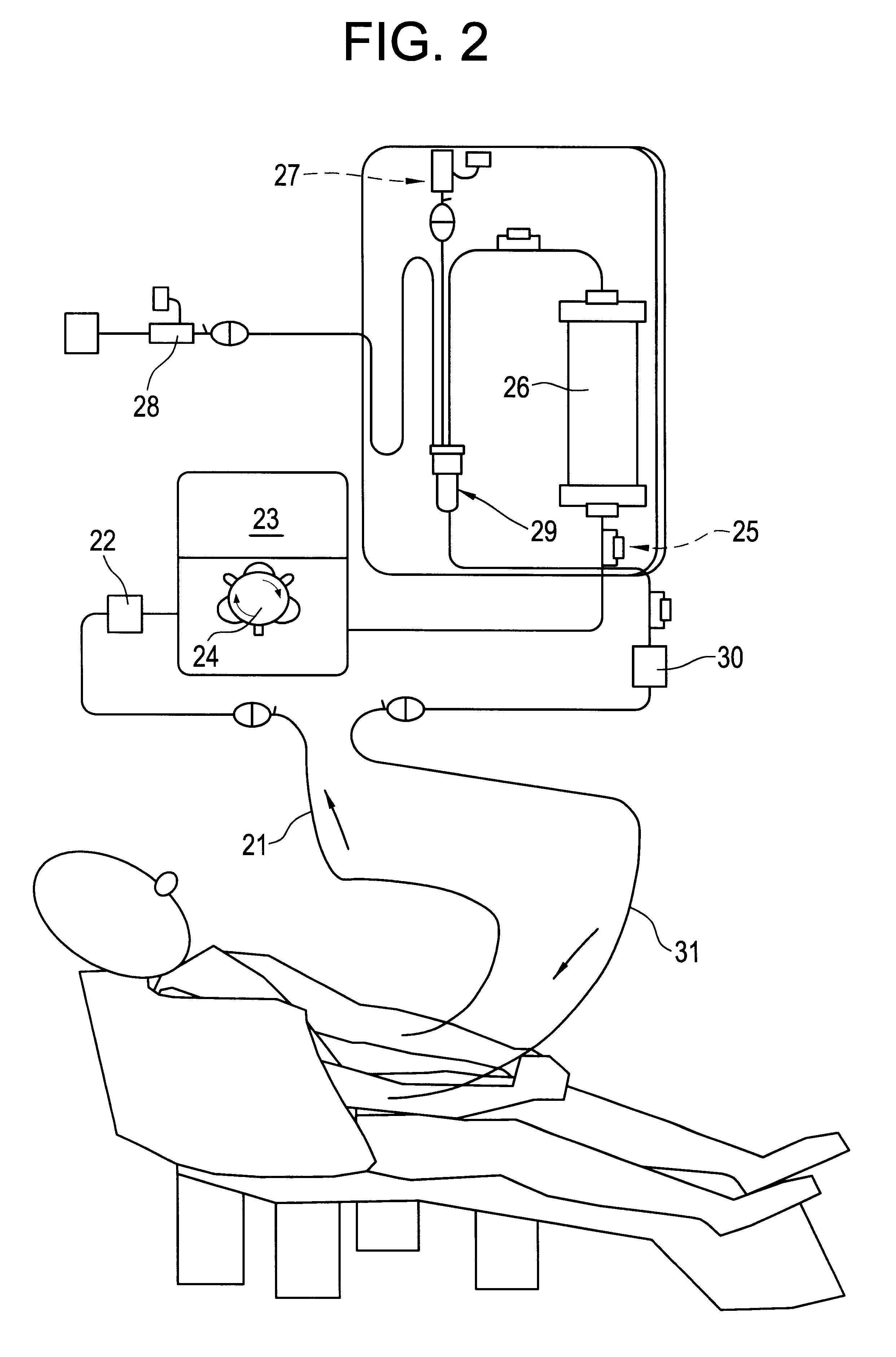
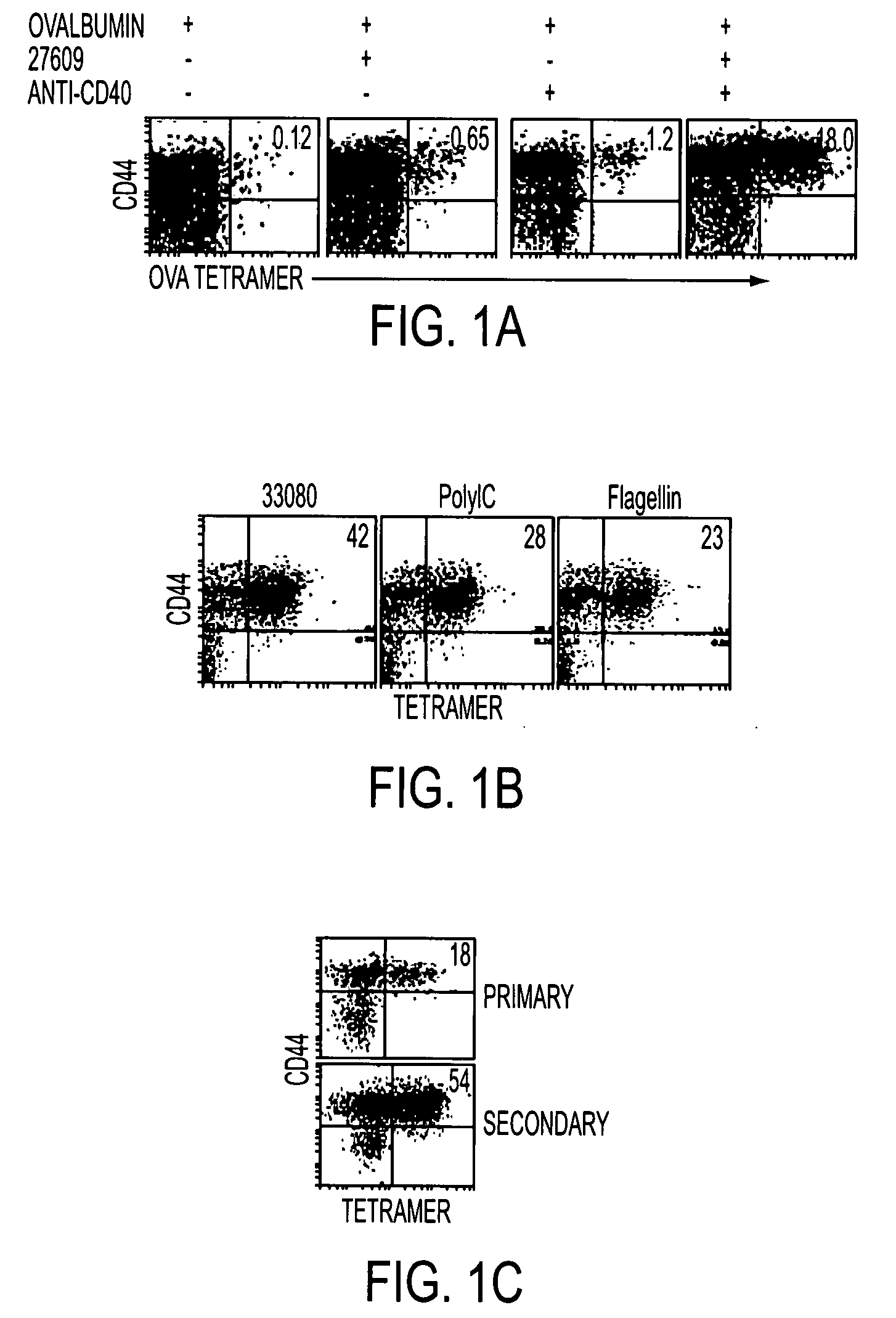
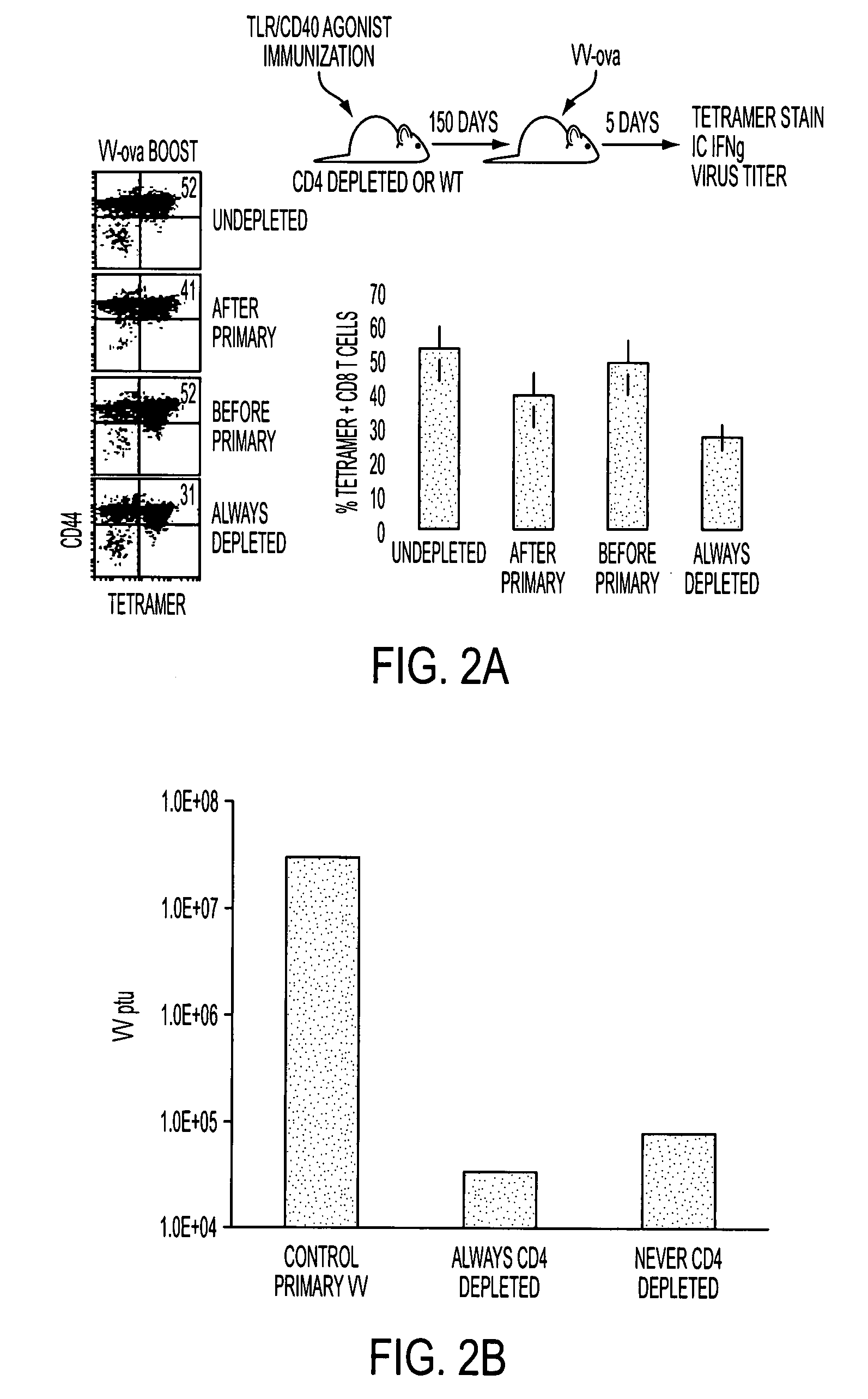
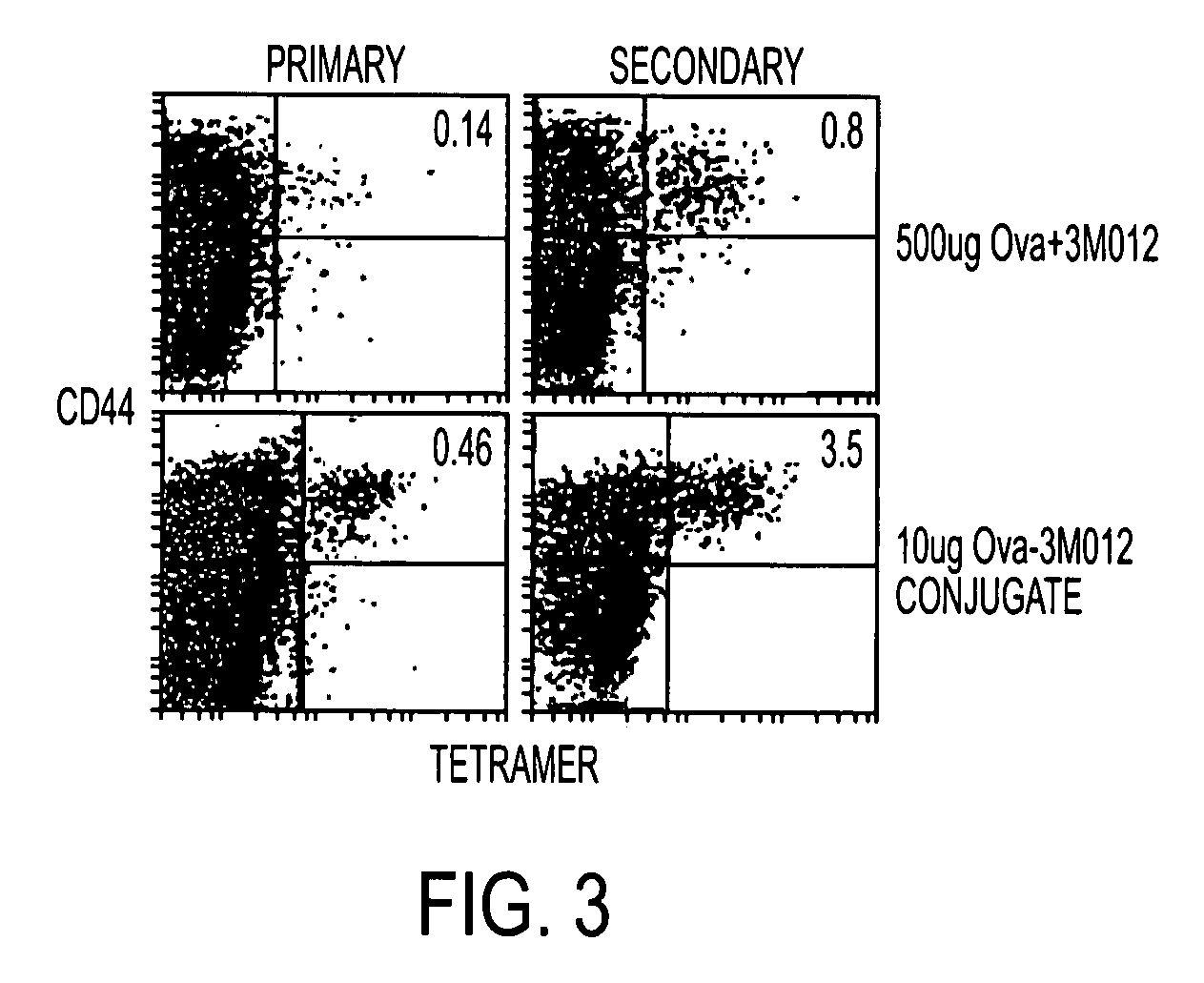
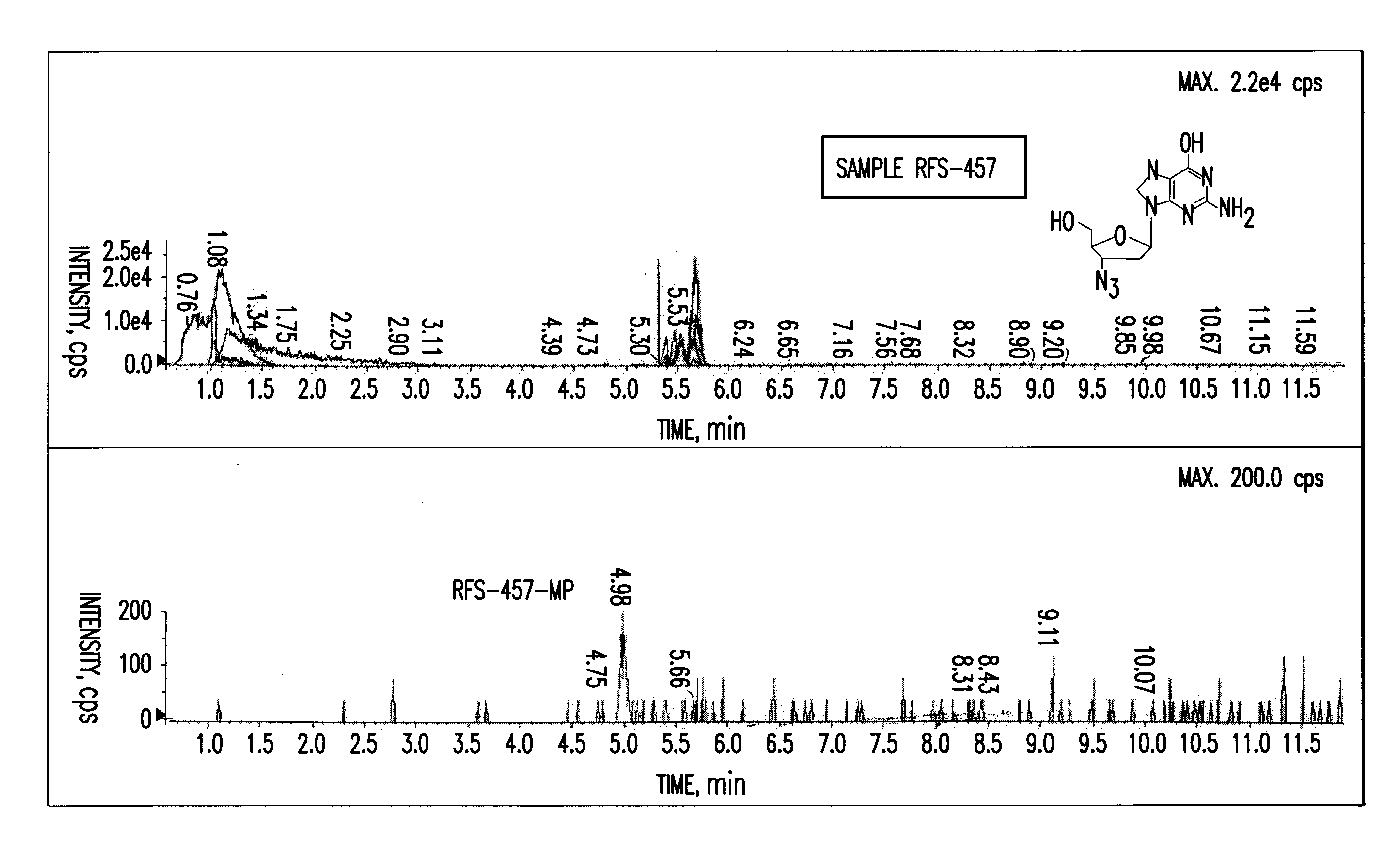
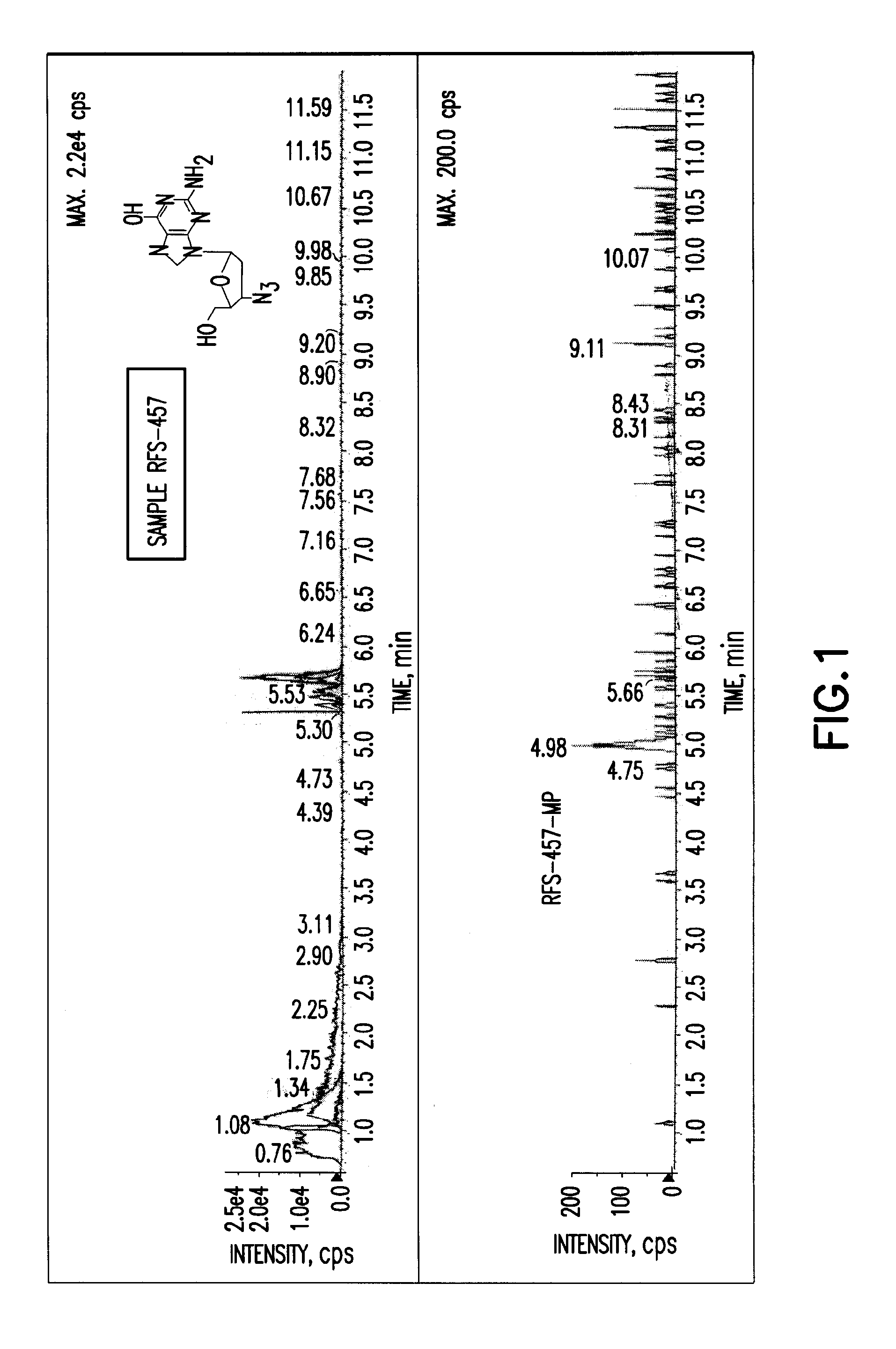
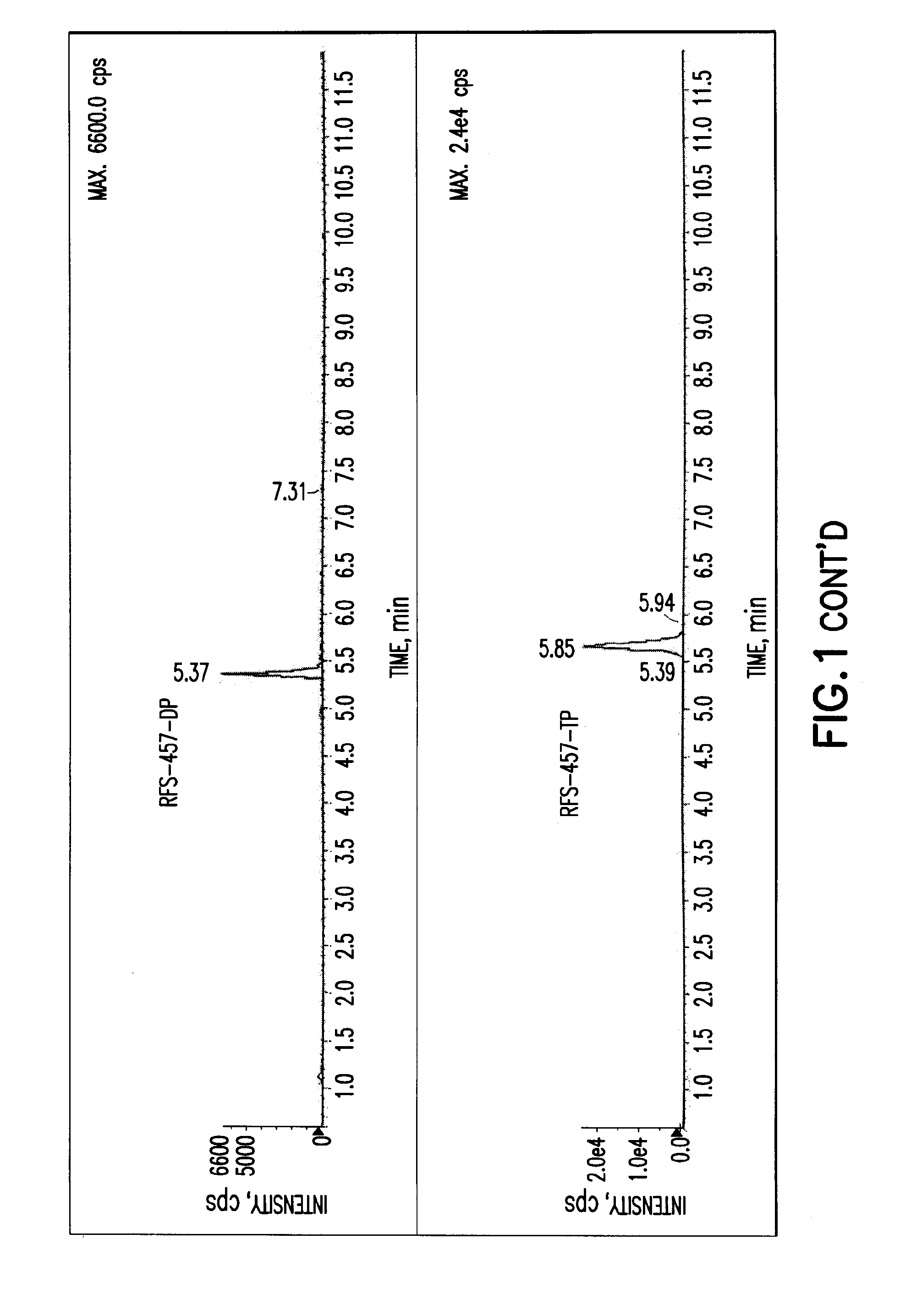
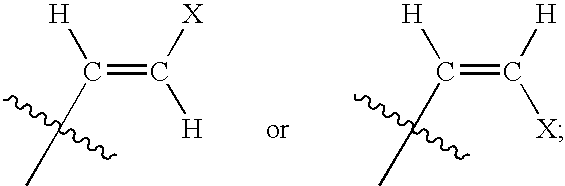Patents
Literature
2419 results about "Human immunodeficiency virus (HIV)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The human immunodeficiency viruses (HIV) are two species of Lentivirus (a subgroup of retrovirus) that infect humans. Over time they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype. In most cases, HIV is a sexually transmitted infection and occurs by contact with or transfer of blood, pre-ejaculate, semen, and vaginal fluids. Research has shown (for both same-sex and opposite-sex couples) that HIV is untransmittable through condomless sexual intercourse if the HIV-positive partner has a consistently undetectable viral load. Non-sexual transmission can occur from an infected mother to her infant during pregnancy, during childbirth by exposure to her blood or vaginal fluid, and through breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells.
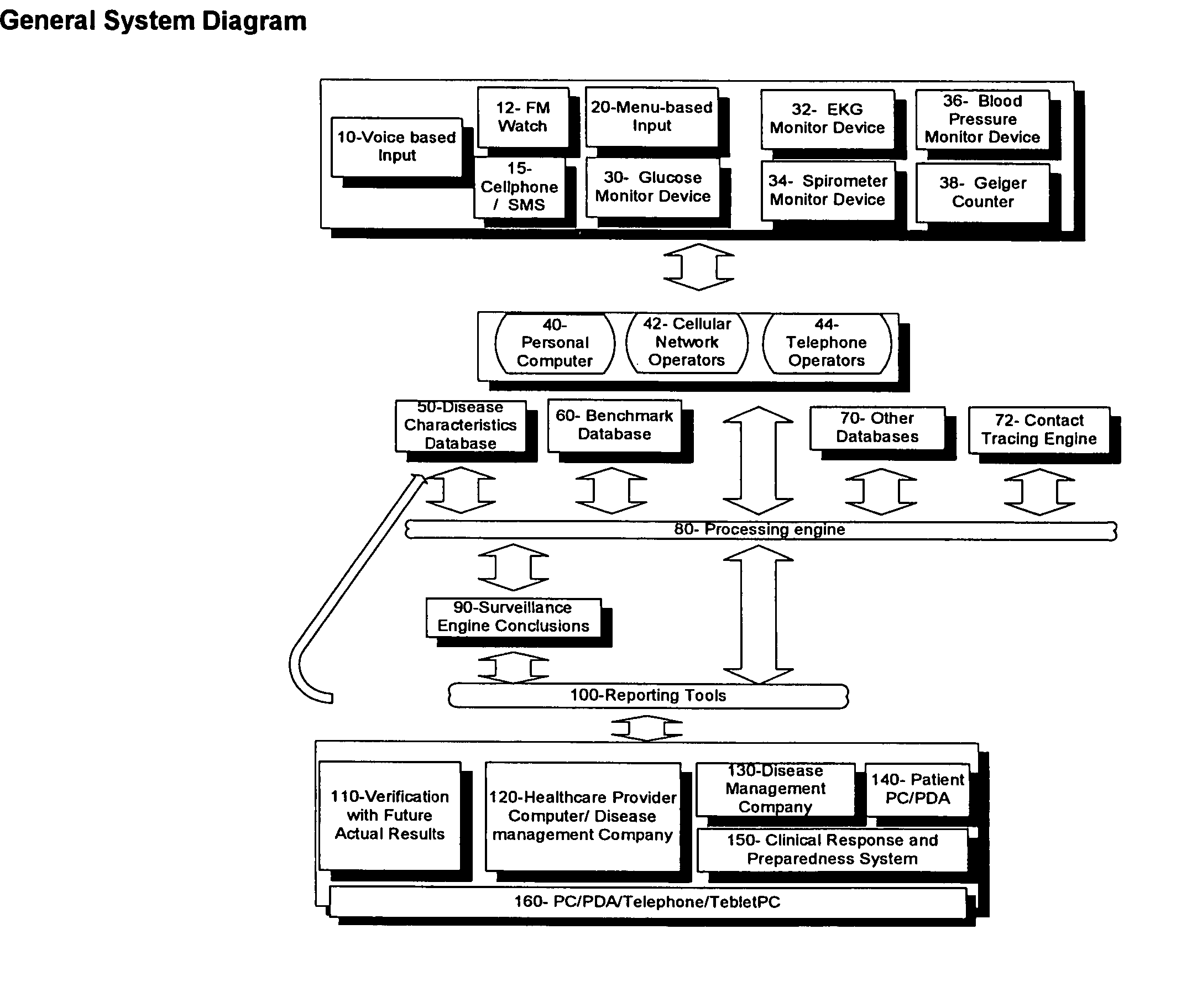
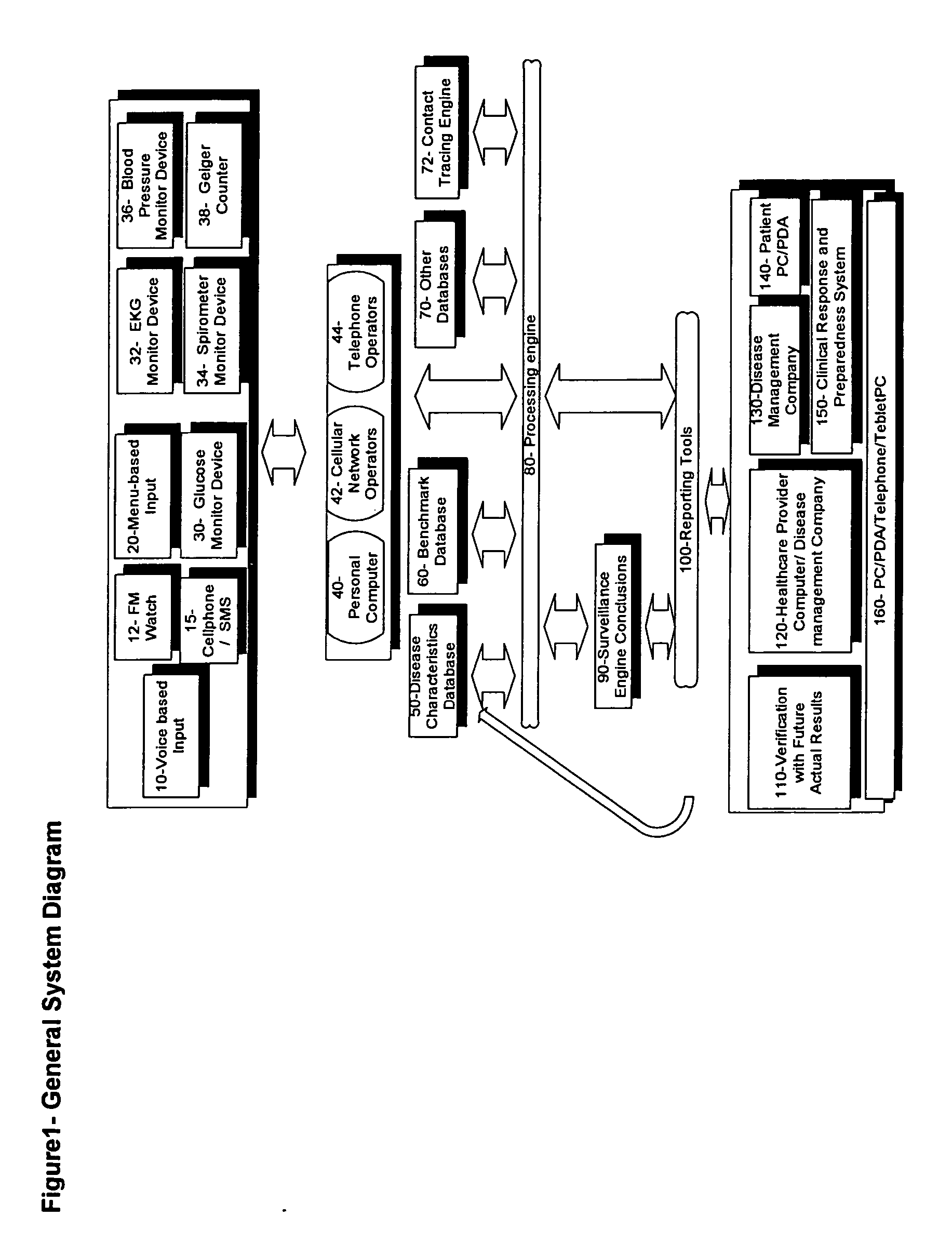
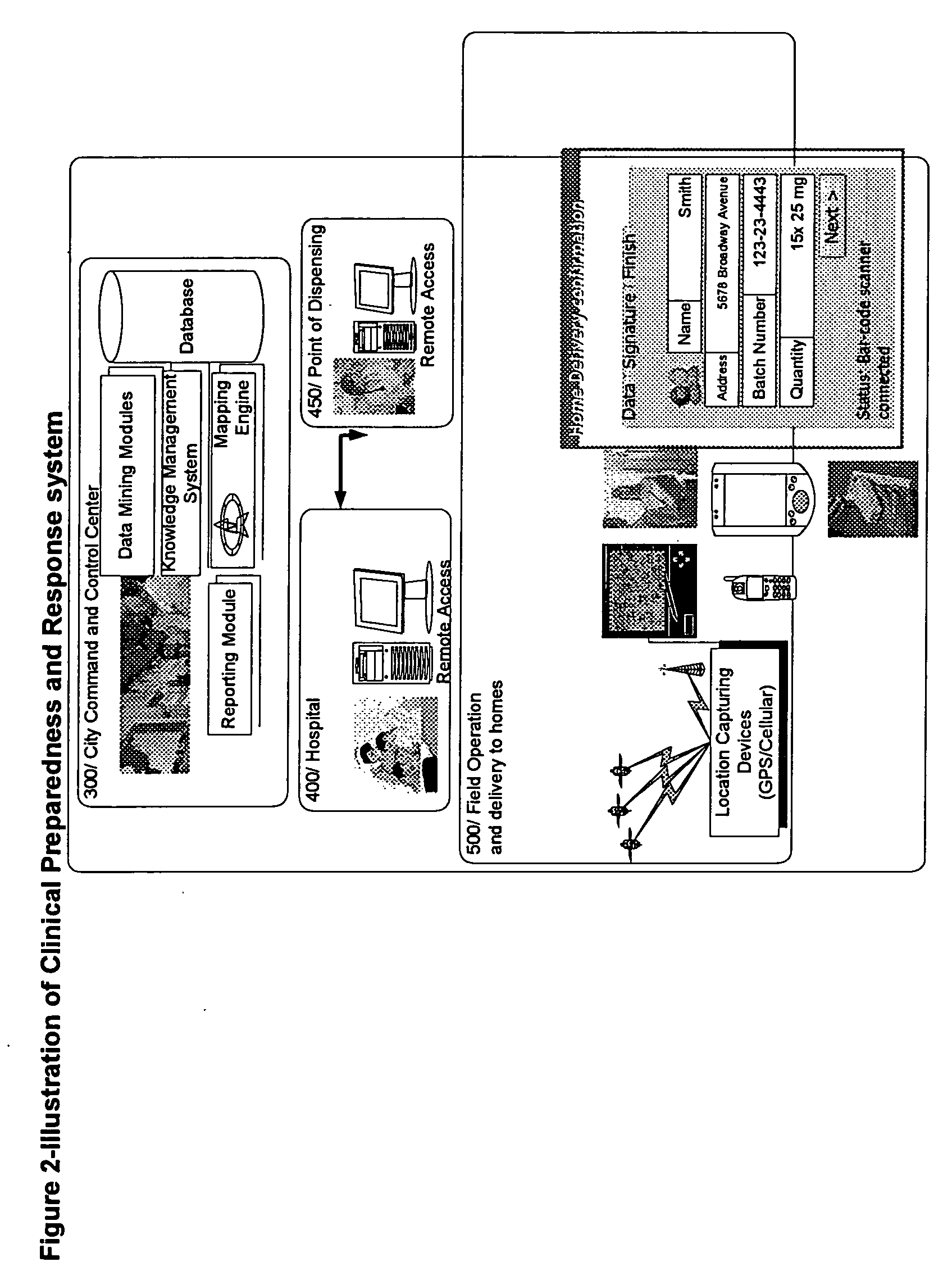
Method for accessing and analyzing medically related information from multiple sources collected into one or more databases for deriving illness probability and/or for generating alerts for the detection of emergency events relating to disease management including HIV and SARS, and for syndromic surveillance of infectious disease and for predicting risk of adverse events to one or more drugs
InactiveUS20060036619A1Medical data miningDigital data processing detailsMedical recordNatural state
The method of the present invention derives the illness probability of any selected person from a database of people stored in a computer and / or on a computer network using collected relational data from every person in the database, including whether a person has a contact relationship with another person in said database and utilizes a database of illnesses infection probability functions given different illnesses and states of nature including data relating to social relationship; type of disease; probability function of infection given a time unit; length of contact of the particular contact relationship link; and calculates at least one relational path between said person and each person in the data base with whom there is a contact relationship, direct or via other persons in the said database for deriving the illness probability of the selected person. In addition. the method of the present invention permits selecting the optimum treatment for a patient with an infectious disease based upon recommending a drug or drugs deemed optimum for treating the patient and permits generating alerts for the detection of emergency events such as the outbreak of an infectious disease or a biological, chemical or nuclear attack and for diseases management. Moreover, in accordance with the method of the present invention a given patient may compare his or her medical record with summary information of patients with similar defined criteria.
Owner:FUERST OREN +1
Small Molecule Modulators of HIV-1 Capsid Stability and Methods Thereof
InactiveUS20130165489A1Inhibiting and suppressing and preventing viral infectionBiocideOrganic chemistryViral infectionVirology
The present invention includes a method of inhibiting, suppressing or preventing a viral infection in a subject, comprising administering to the subject a pharmaceutical composition comprising one or more of the compounds useful within the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Therapy via targeted delivery of nanoscale particles
InactiveUS20050090732A1Destroying inhibiting vascularityAntibacterial agentsNervous disorderDiseaseProstate cancer
Disclosed are compositions, systems and methods for treating a subject's body, body part, tissue, body fluid cells, pathogens, or other undesirable matter involving the administration of a targeted thermotherapy that comprises a bioprobe (energy susceptive materials that are attached to a target-specific ligand). Such targeted therapy methods can be combined with at least one other therapy technique. Other therapies include hyperthermia, direct antibody therapy, radiation, chemo- or pharmaceutical therapy, photodynamic therapy, surgical or interventional therapy, bone marrow or stem cell transplantation, and medical imaging, such as MRI, PET, SPECT, and bioimpedance. The disclosed therapies may be useful in the treatment of a variety of indications, including but not limited to, cancer of any type, such as bone marrow, lung, vascular, neuro, colon, ovarian, breast and prostate cancer, epitheleoid sarcomas, AIDS, adverse angiogenesis, restenosis, amyloidosis, tuberculosis, cardiovascular plaque, vascular plaque, obesity, malaria, and illnesses due to viruses, such as HIV.
Owner:NANOTX INC
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
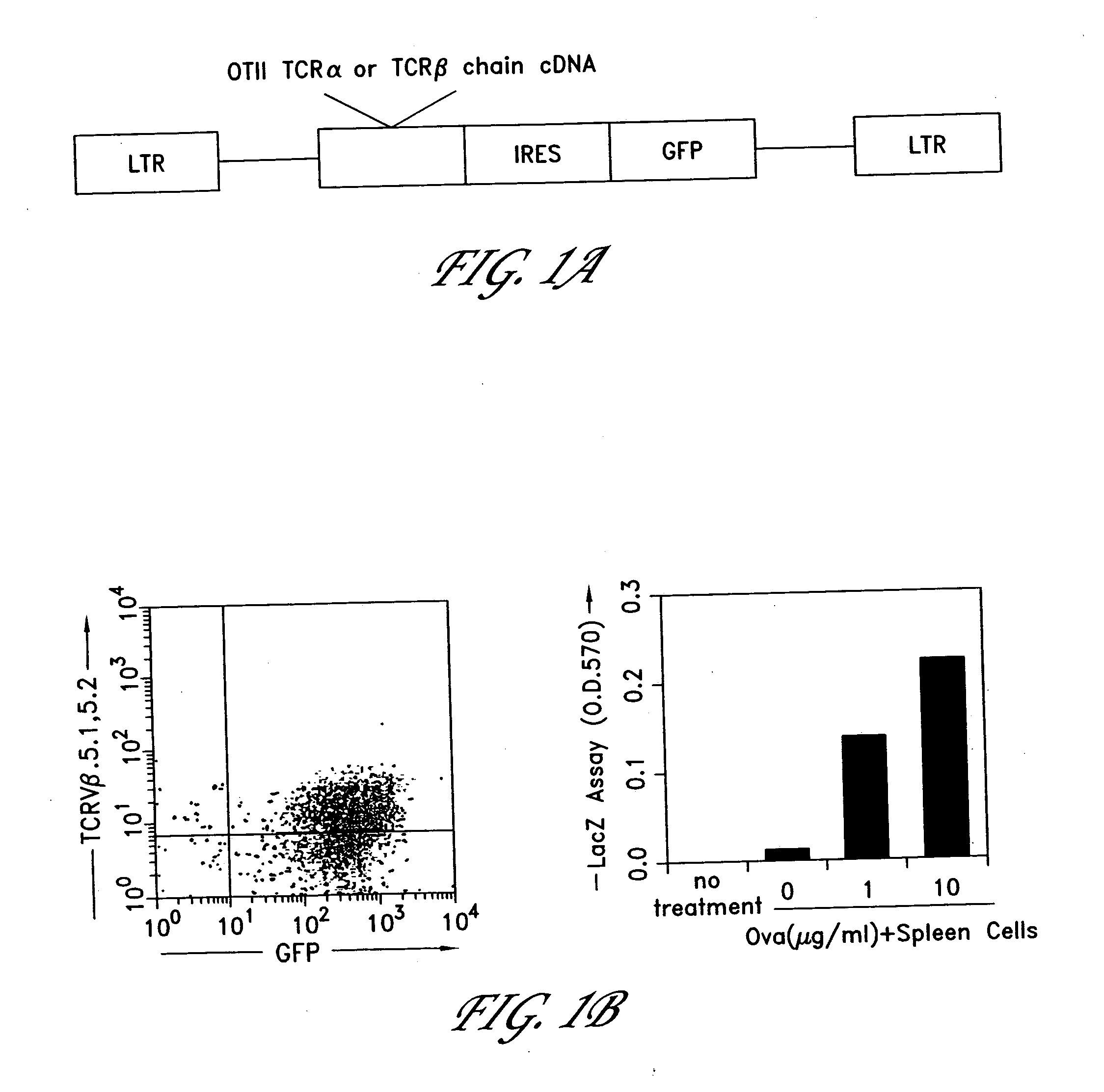
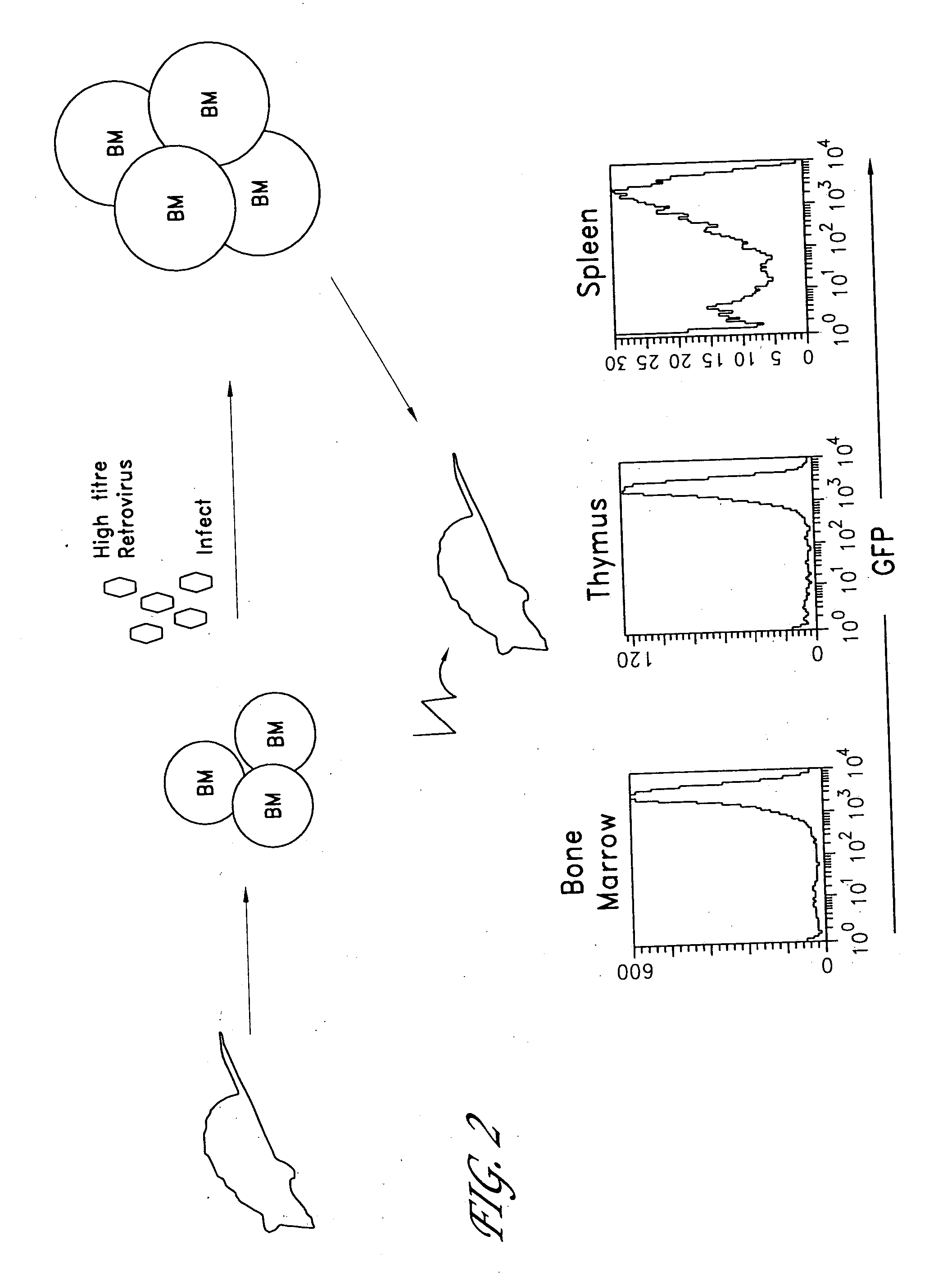
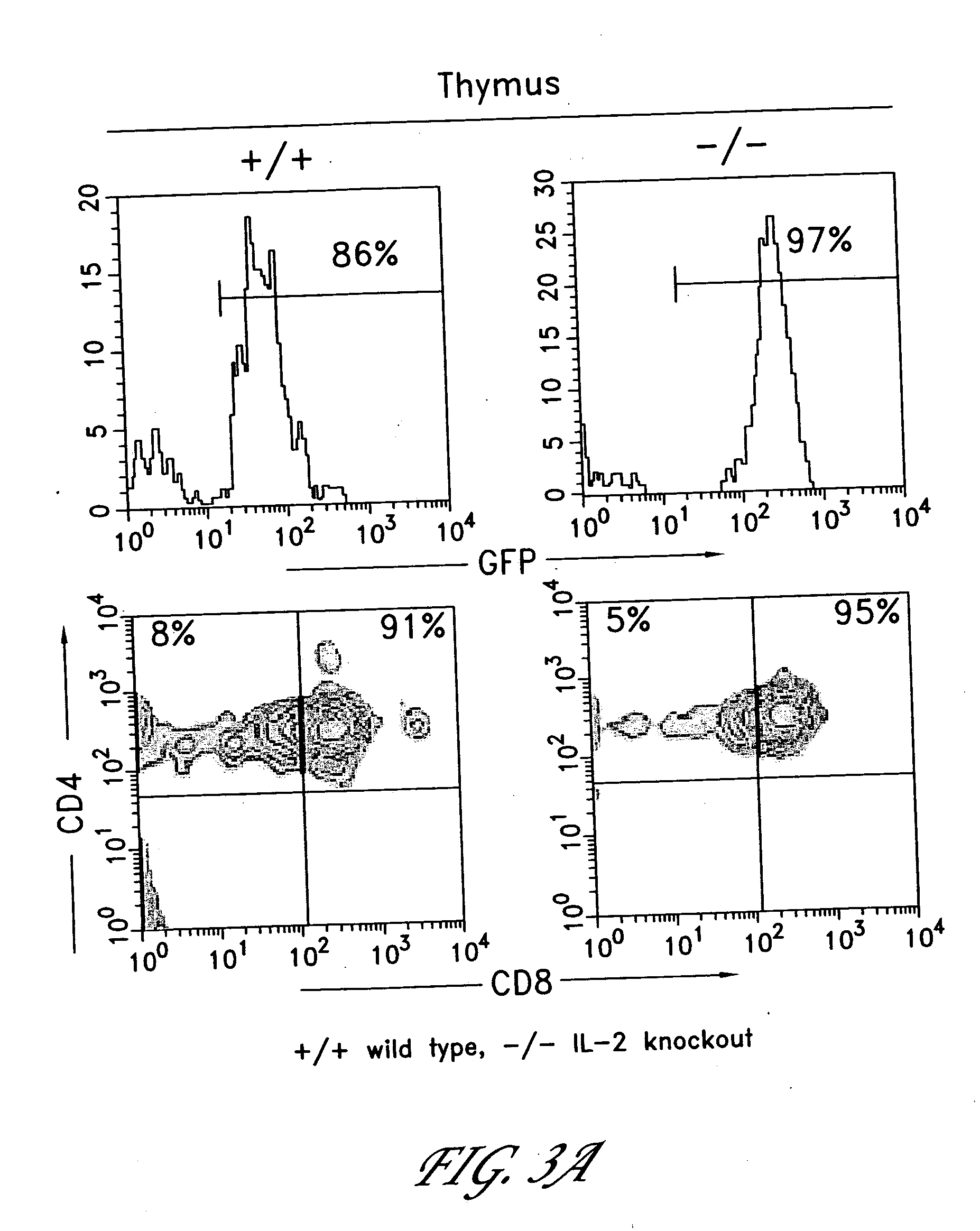
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Methods and algorithms for cell enumeration in low-cost cytometer
InactiveUS20060024756A1Simple designReduce operating costsBioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellCcd camera
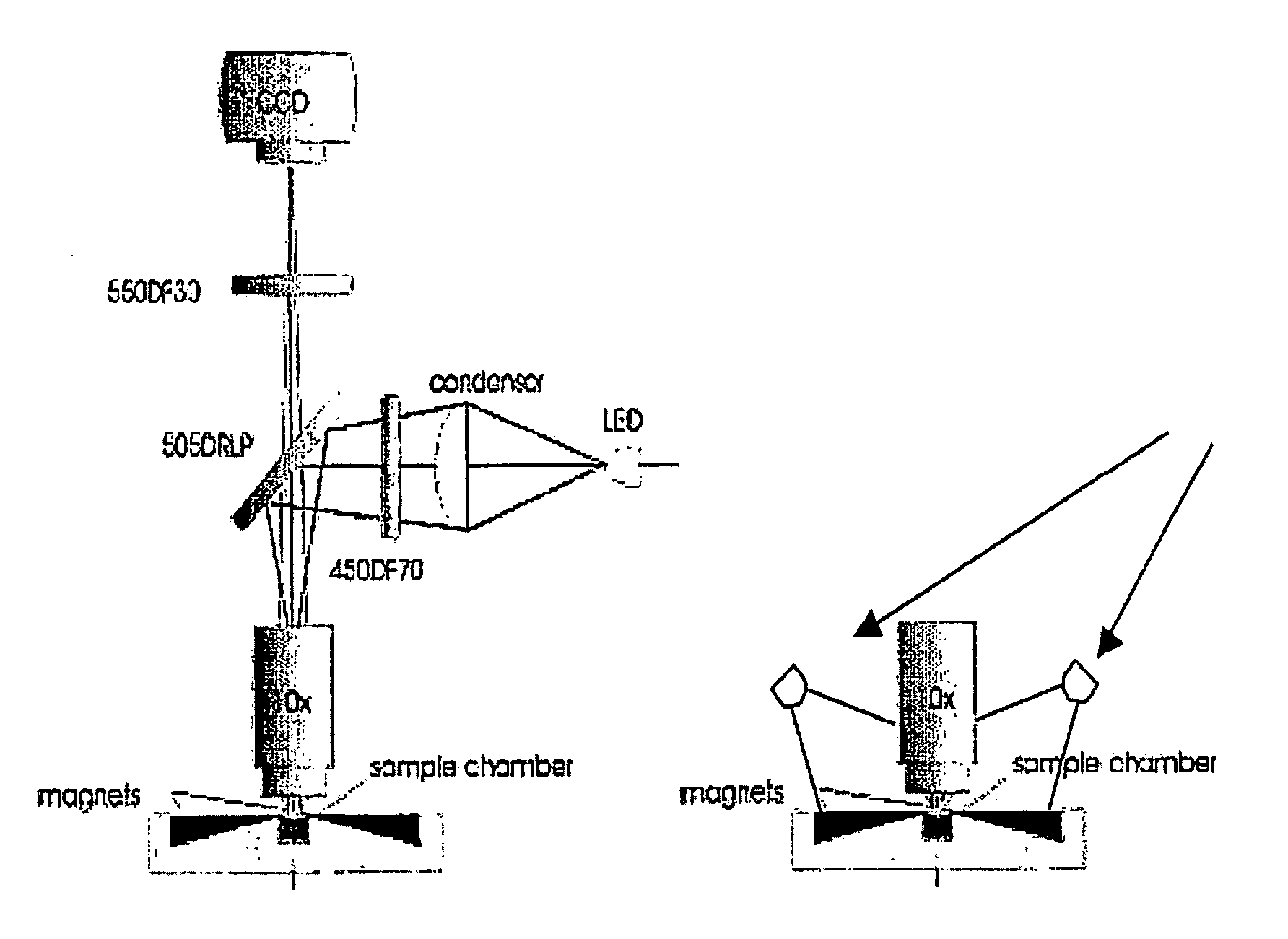
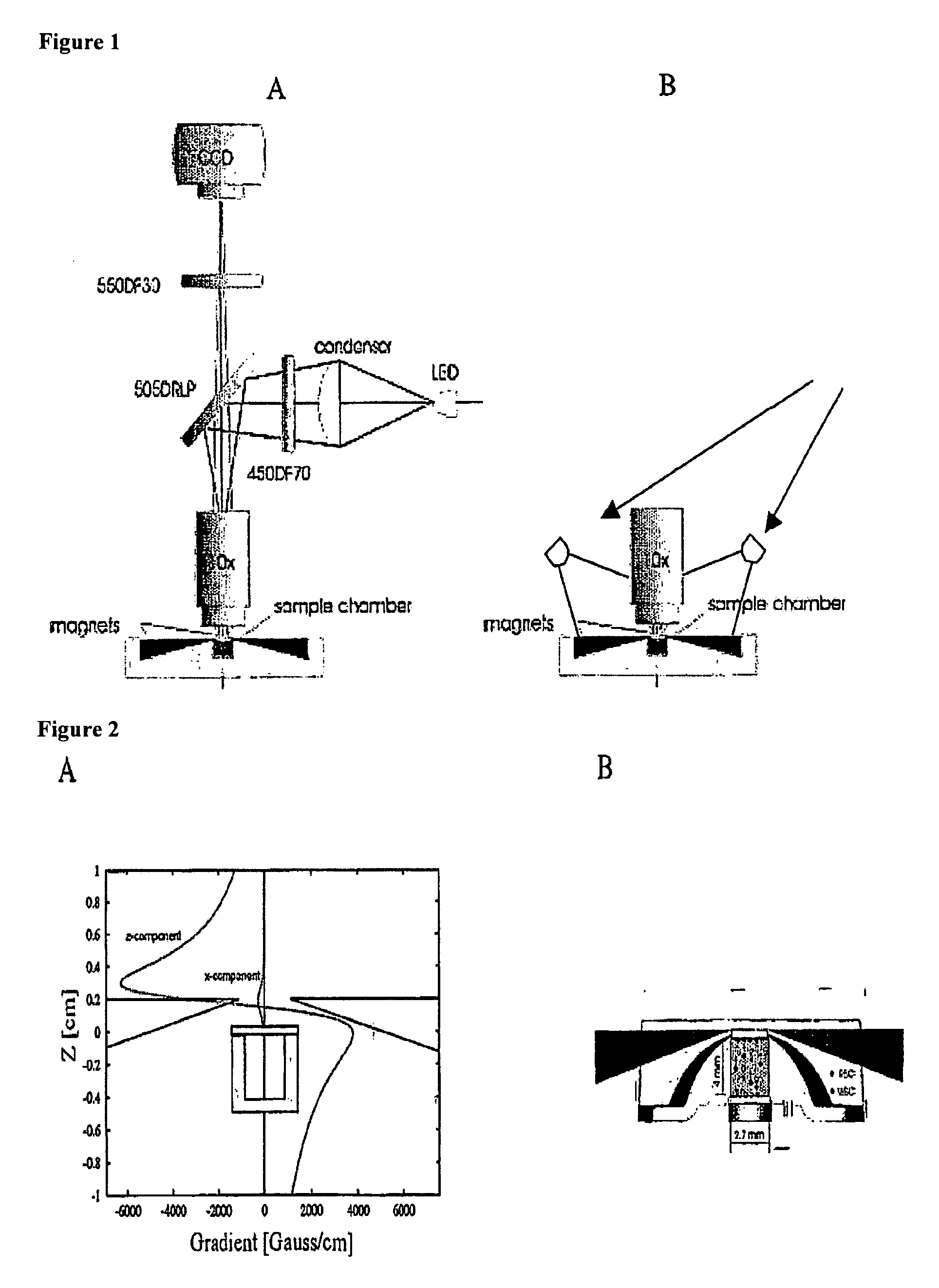
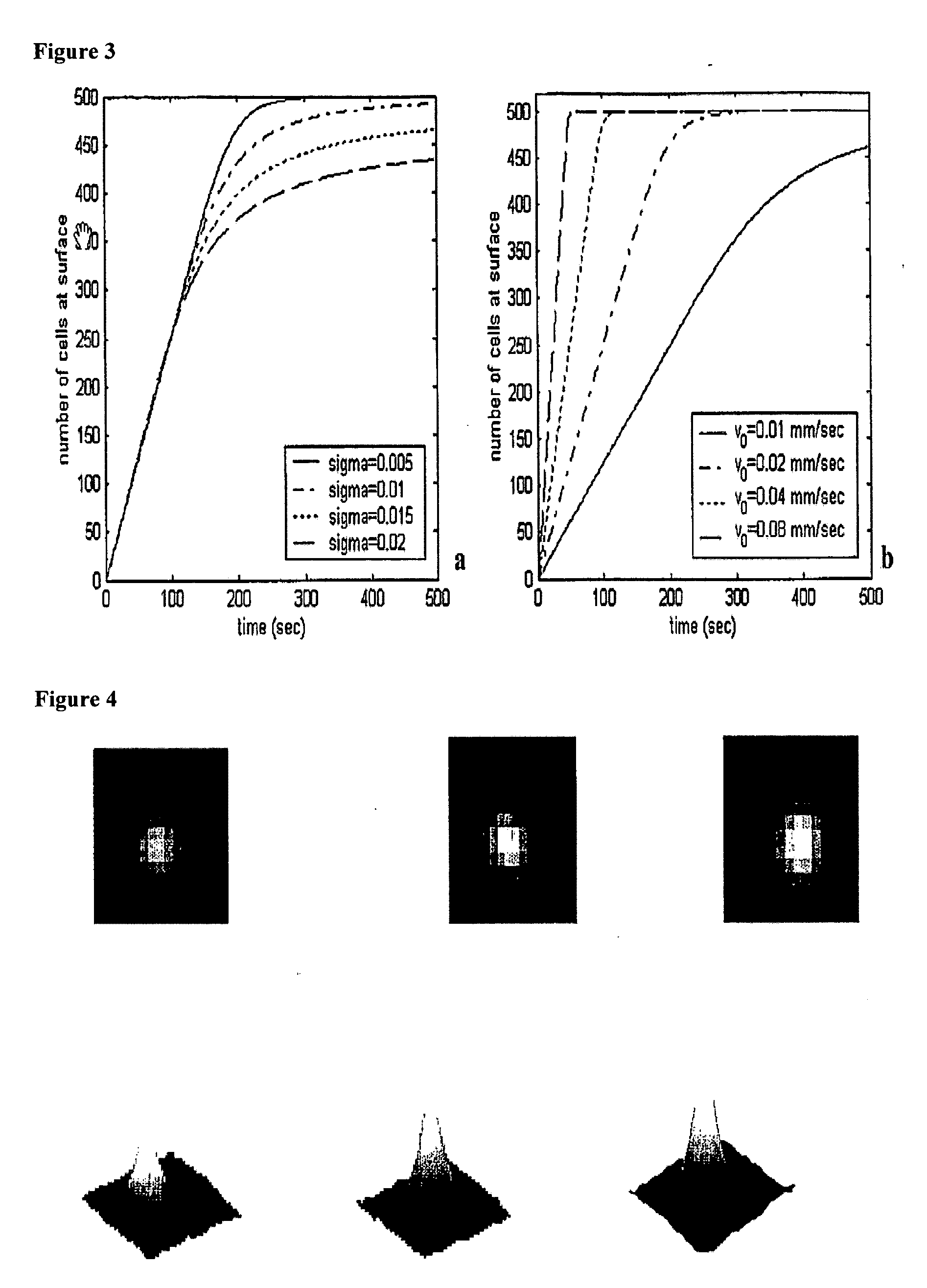
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and algorithms disclosed herein largely overcome these limitations. Briefly, all cells in a biological sample are fluorescently labeled, but only the target cells are also magnetically labeled. The labeled sample, in a chamber or cuvet, is placed between two wedge-shaped magnets to selectively move the magnetically labeled cells to the observation surface of the cuvet. An LED illuminates the cells and a CCD camera captures the images of the fluorescent light emitted by the target cells. Image analysis performed with a novel algorithm provides a count of the cells on the surface that can be related to the target cell concentration of the original sample. The compact cytometer system provides a rugged, affordable and easy-to-use technique, which can be used in remote locations.
Owner:UNIVERSITY OF TWENTE
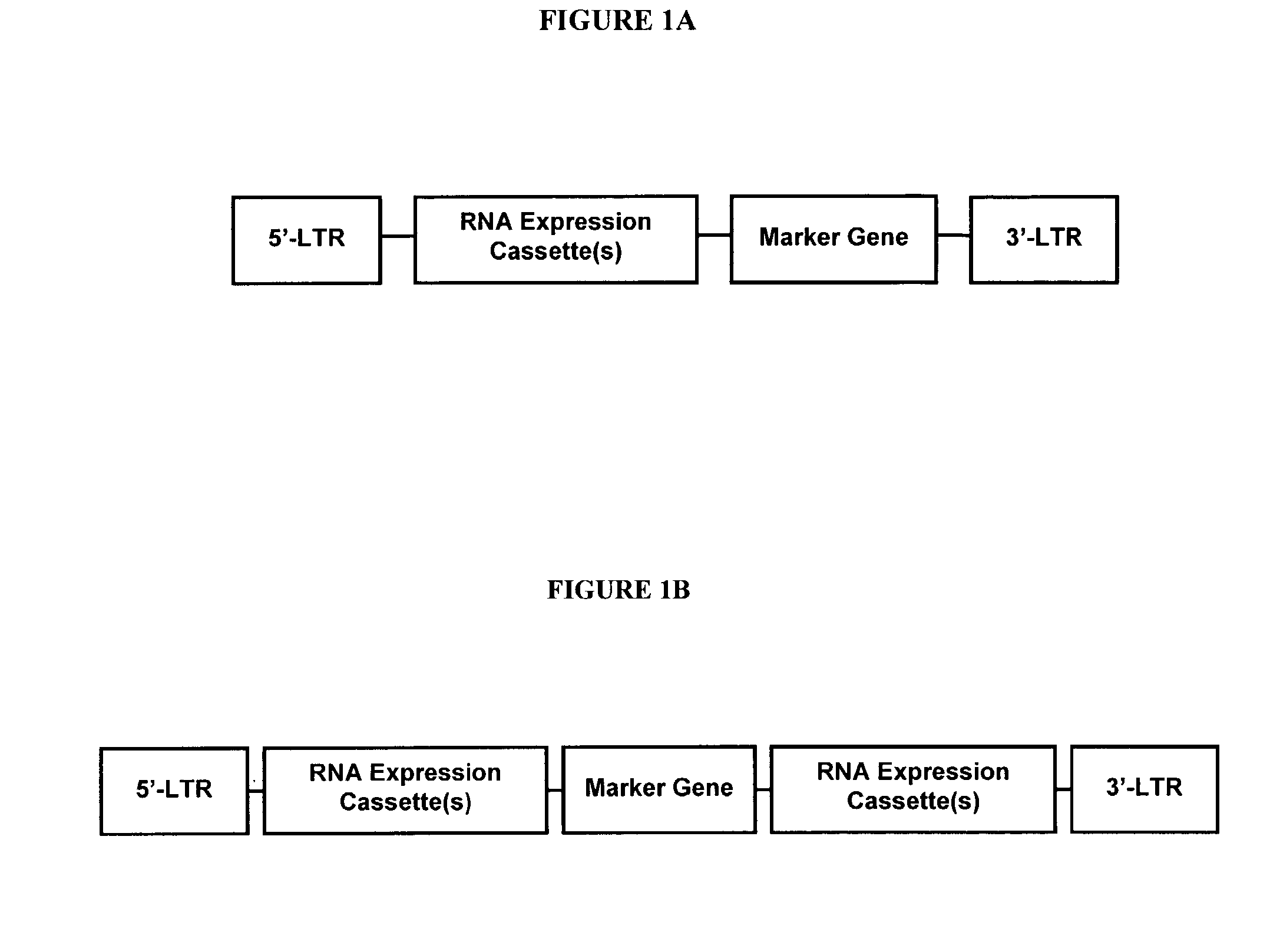
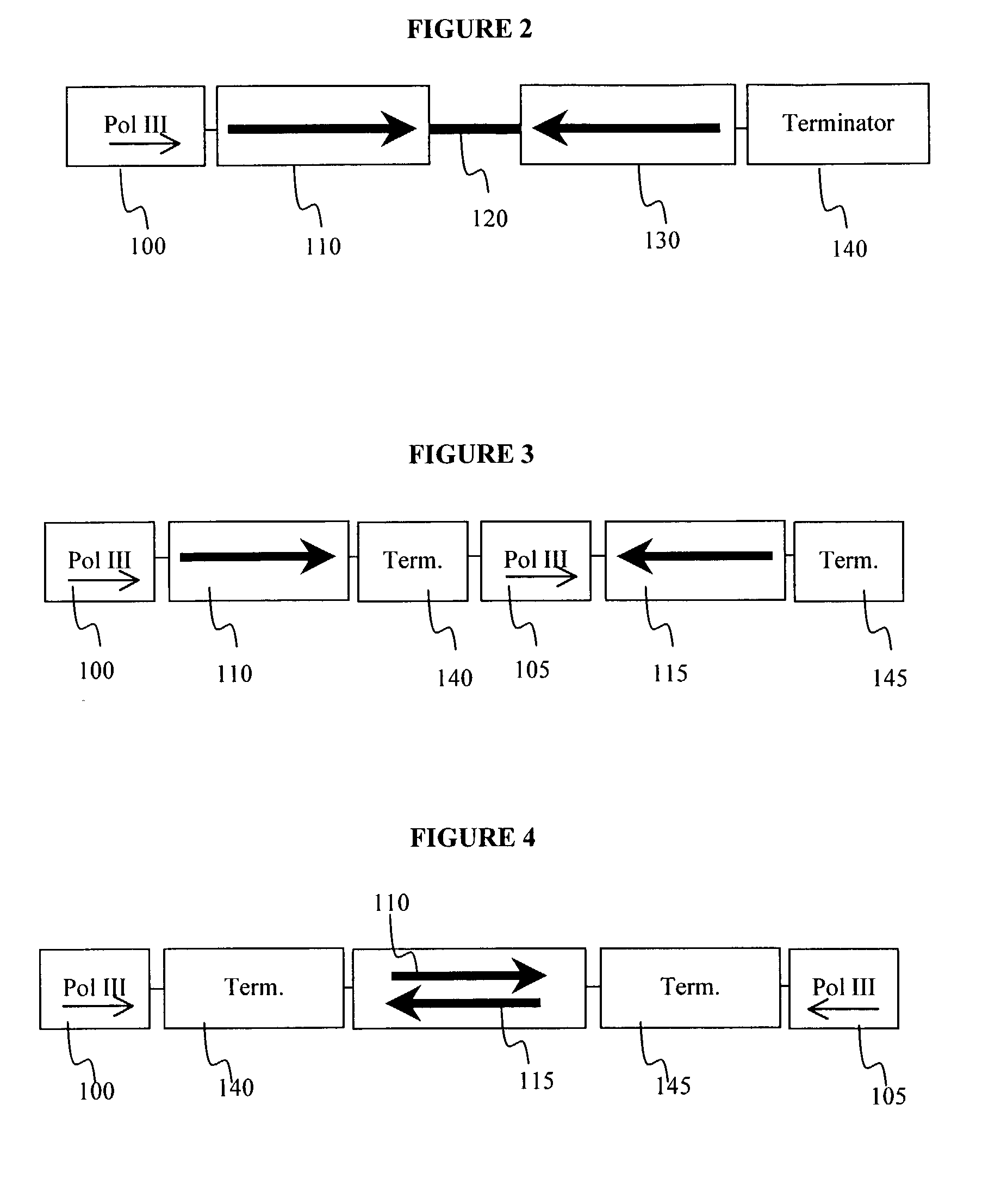
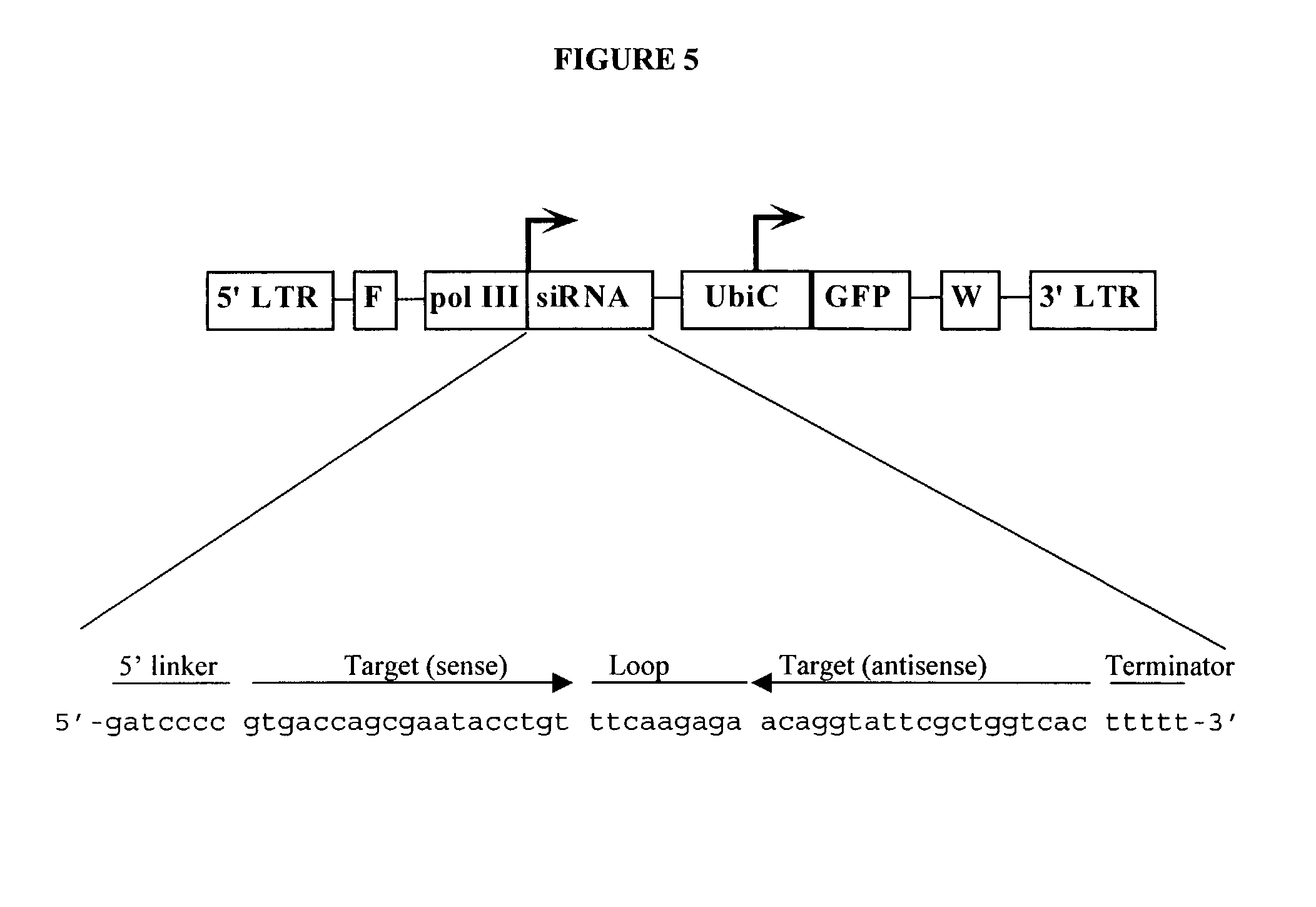
Method for expression of small antiviral RNA molecules within a cell
ActiveUS20030059944A1Prevents sequenceHigh expressionOrganic active ingredientsPeptide/protein ingredientsGeneticsViral life cycle
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV.
Owner:CALIFORNIA INST OF TECH
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for caspases and other enzymes and the use thereof
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Preparation and isolation of 5′ capped mRNA
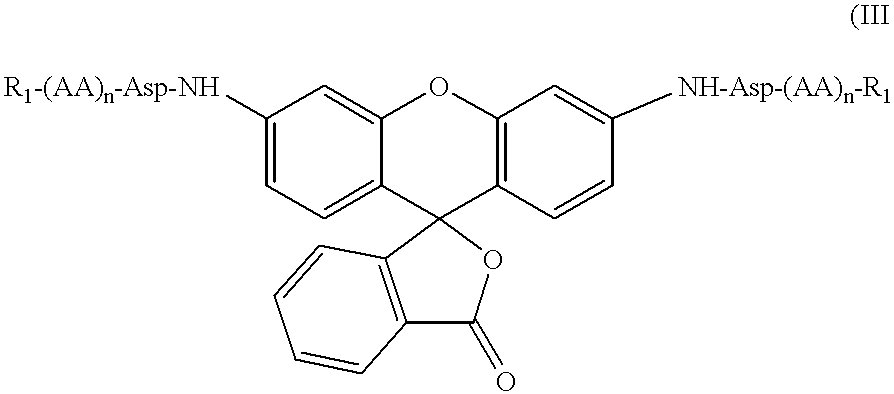
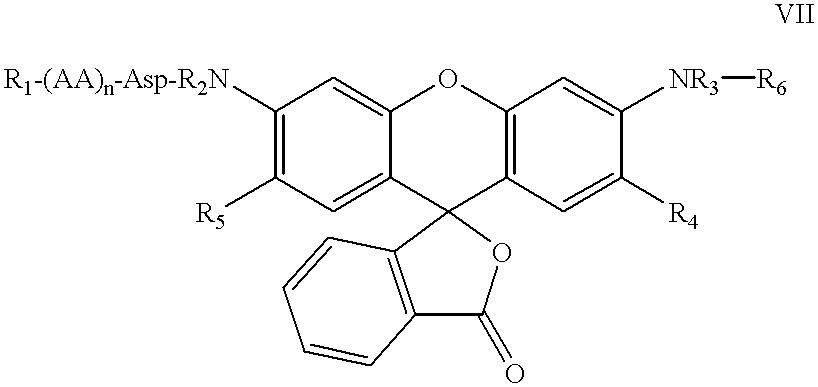
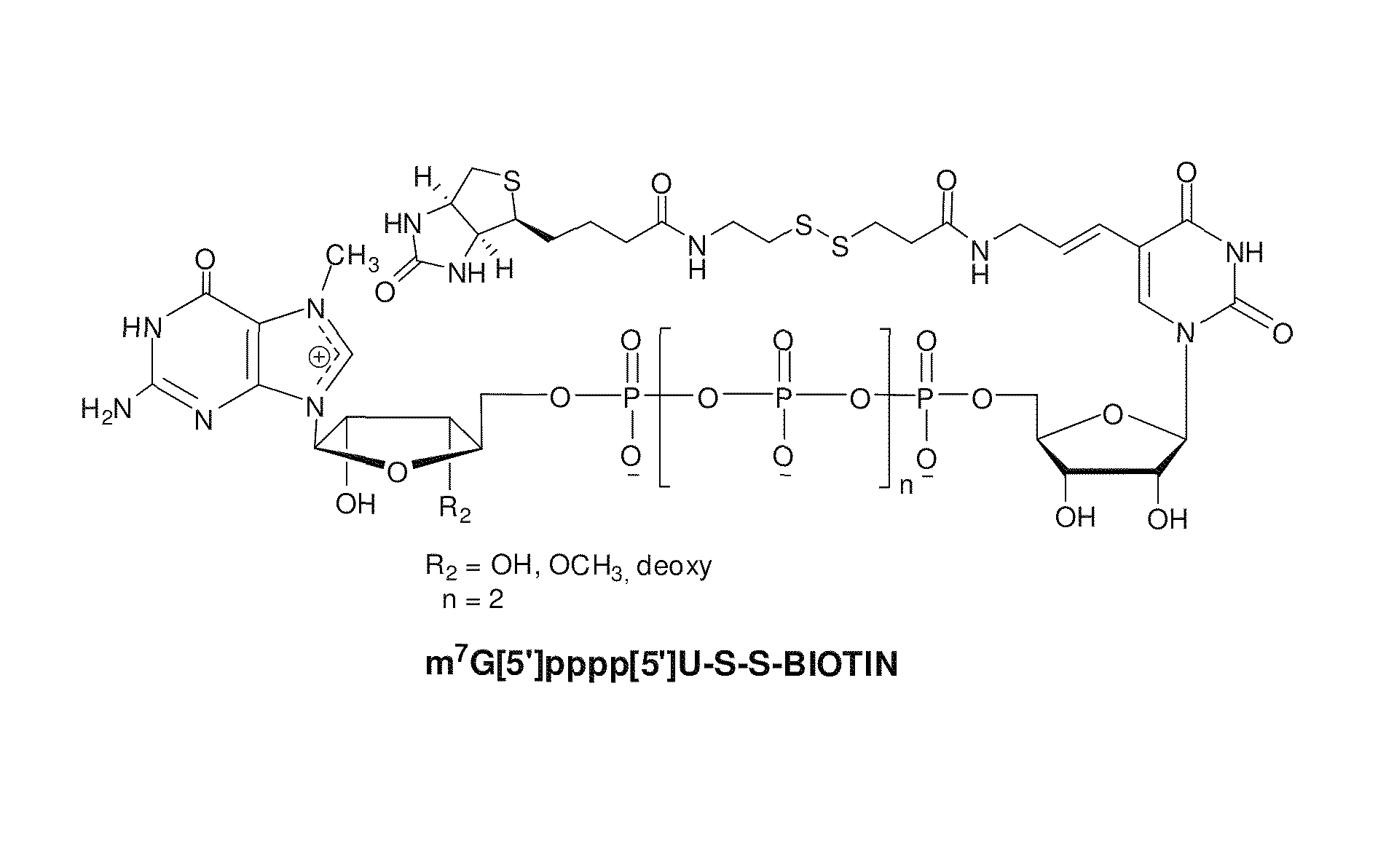
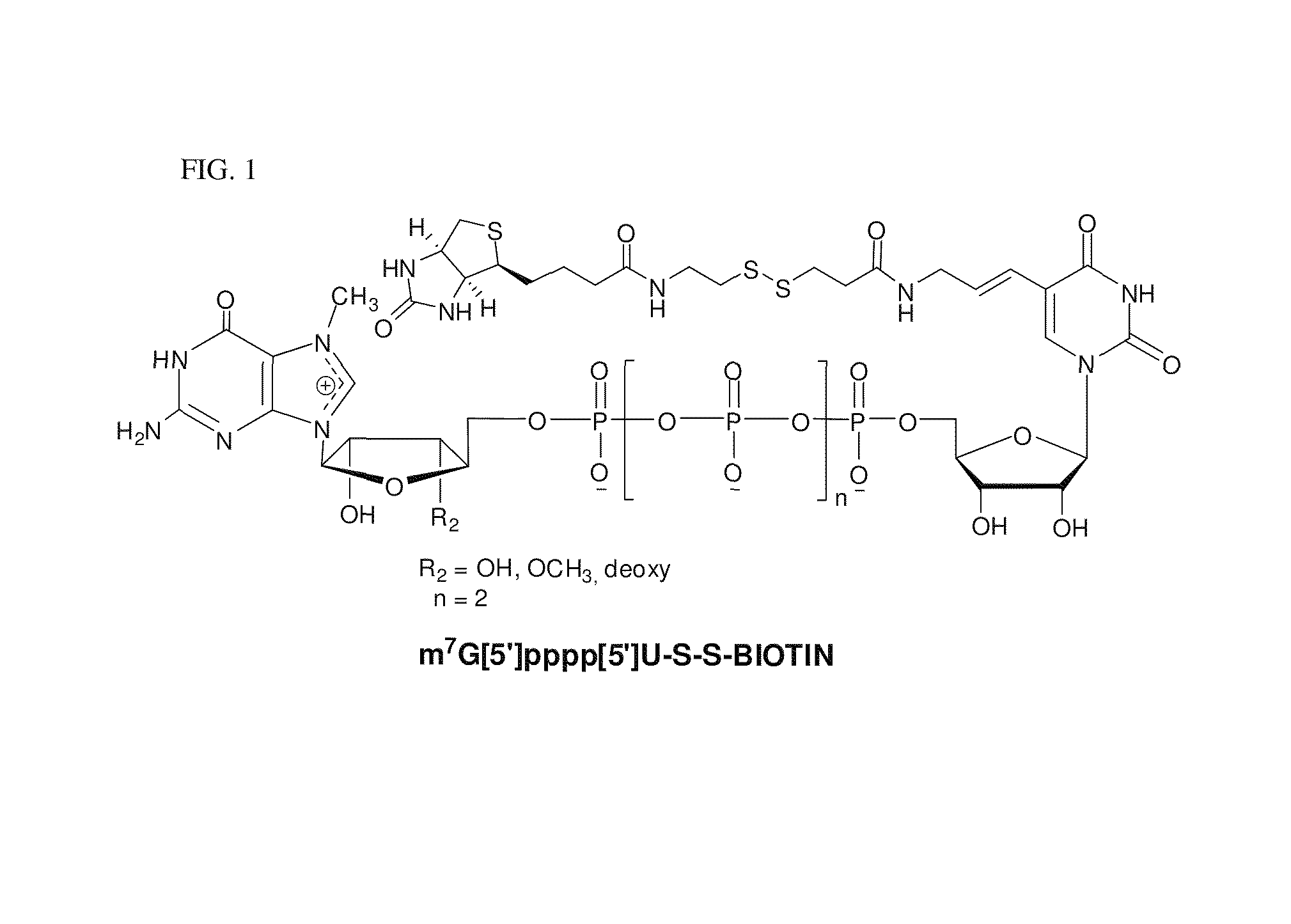
ActiveUS8093367B2Improve translationEasy to useSugar derivativesMicrobiological testing/measurementRNA-Protein InteractionRna protein
The synthesis of capped / tagged RNA, methods of use and kits providing same are contemplated. Tagged RNA permits isolation of RNA transcripts in vitro. The ability to isolate and purify capped RNA results in improved transcription and translation and provides a tool for identifying RNA-protein interactions. Such capped RNA finds use in therapeutic applications, diagnosis and prognosis and in the treatment of cancers and HIV.
Owner:APPL BIOSYSTEMS INC
Phenidate drug formulations having diminished abuse potential
InactiveUS6355656B1High serum levelHigh pharmaceutical efficacy levelBiocideOrganic chemistrySide effectMedicine
Phenidate drug formulations are provided having reduced potential for drug abuse. Dosage forms for treating Attention Deficit Disorder, Attention Deficit Hyperactivity Disorder, AIDS Dementia Complex and cognitive decline in HIV-AIDS are provided which minimize drug hypersensitivity, toxicity, side effects, euphoric effect, and drug abuse potential. Such dosage forms comprise <SMALLCAPS>D< / SMALLCAPS>-threo stereoisomer of a phenidate in the substantial absence of all other stereoisomers.
Owner:CELGENE CORP
DNA vaccines encoding antigen linked to a domain that binds CD40
InactiveUS7118751B1Improve abilitiesEasy to demonstrateAntibody mimetics/scaffoldsVirus peptidesPeptide antigenEukaryotic plasmids
Vaccines that target one or more antigens to a cell surface receptor improve the antigen-specific humoral and cellular immune response. Antigen(s) linked to a domain that binds to a cell surface receptor are internalized, carrying antigen(s) into an intracellular compartment where the antigen(s) are digested into peptides and loaded onto MHC molecules. T cells specific for the peptide antigens are activated, leading to an enhanced immune response. The vaccine may comprise antigen(s) linked to a domain that binds at least one receptor or a DNA plasmid encoding antigen(s) linked to a domain that binds at least one receptor. A preferred embodiment of the invention targets HIV-1 env antigen to the CD40 receptor, resulting in delivery of antigen to CD40 positive cells, and selective activation of the CD40 receptor on cells presenting HIV-1 env antigens to T cells.
Owner:HAYDEN LEDBETTER MARTHA S +1
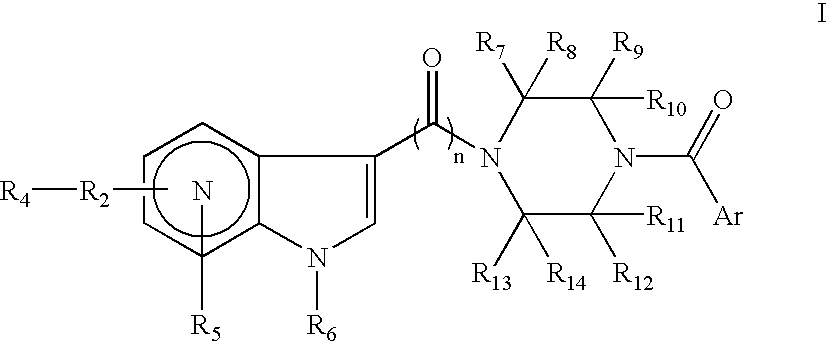
Antiviral azaindole derivatives
The invention comprises compounds of the class of azaindole piperazine diamide derivatives, compositions thereof and their use as anti-viral agents, and particularly for treating HIV infection.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
2'-O-alkylated oligoribonucleotides and phosphorothioate analogs complementary to portions of the HIV genome
InactiveUS6034233ARelieve symptomsSure easySugar derivativesVirus peptidesOligoribonucleotidesGenome
Owner:IONIS PHARMA INC
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
HIV env antibodies
InactiveUS7041293B1Reduce reverse transcriptase activityInhibit syncitia formationAnimal cellsMicrobiological testing/measurementReverse transcriptase activityCD4 antigen
The invention provides antibodies specific for HIV env, including monoclonal antibodies and related hybridomas. The antibodies block CD4 / g120 binding and reduce reverse transcriptase activity in vitro.
Owner:GENENTECH INC
Sugar modified oligonucleotides that detect and modulate gene expression
Compositions and methods are provided for the treatment and diagnosis of diseases amenable to modulation of the production of selected proteins. In accordance with preferred embodiments, oligonucleotides and oligonucleotide analogs are provided which are specifically hybridizable with a selected sequence of RNA or DNA wherein at least one of the 2'-deoxyfuranosyl moieties of the nucleoside unit is modified. Treatment of HIV, herpes virus, papillomavirus and other infections is provided.
Owner:IONIS PHARMA INC
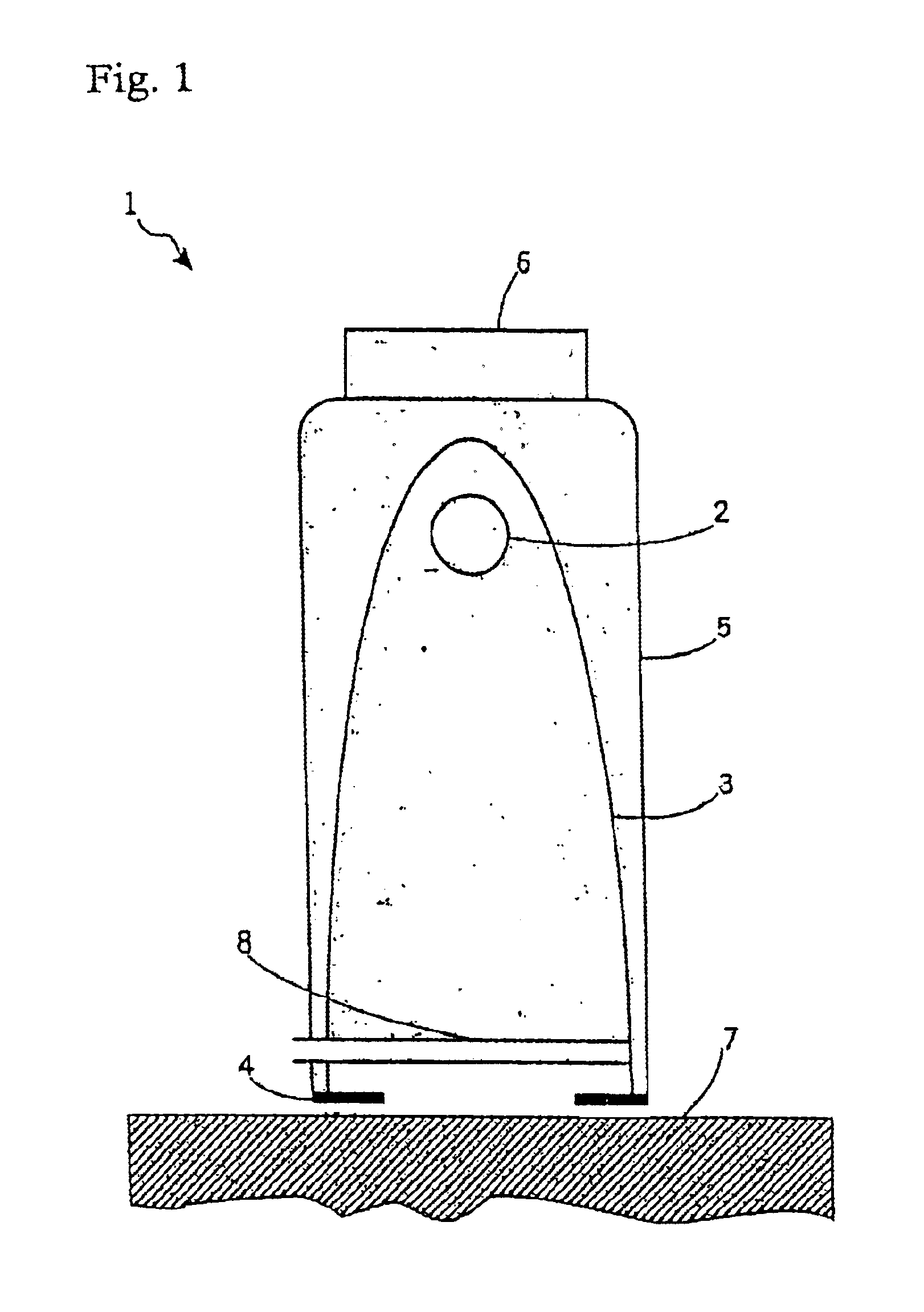
Irradiation device for therapeutic treatment of skin and other ailments
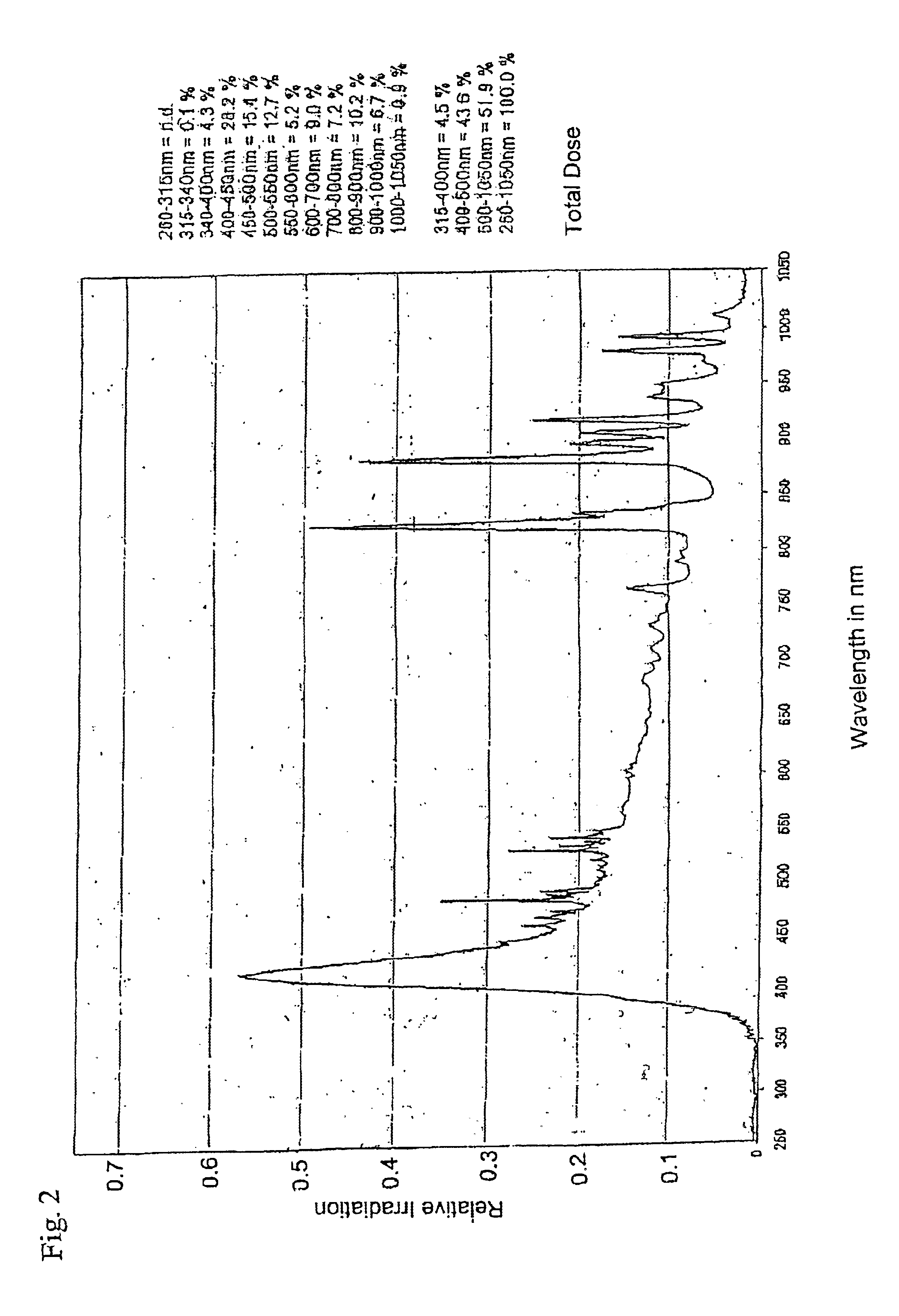
The invention relates to an irradiation device and method for the treatment of totally or partially cell-mediated inflammations of the skin, the connective tissue and the viscera, viral and other infectious diseases such as HIV and prionic infections, fungal infections of the skin and the mucous membranes, bacterial diseases of the skin and the mucous membranes as well hand eczema and anal eczema which comprises at least one irradiation device to irradiate a surface treatment area where the wavelength of the emitted radiation to a treatment area is longer than 400 nm and comprises at least one spectral band between 400-500 nm while the radiation device contains means for the generation of optical pulses towards a treatment area with a power density of the optical pulse peaks larger than 0.5 W / cm2 and smaller than 100 kW / cm2. The energy of one pulse relates to 0.05-10 J / cm2.
Owner:SPECTROMETRIX OPTOELECTRONICS SYST
Apparatus and methods for monitoring subjects
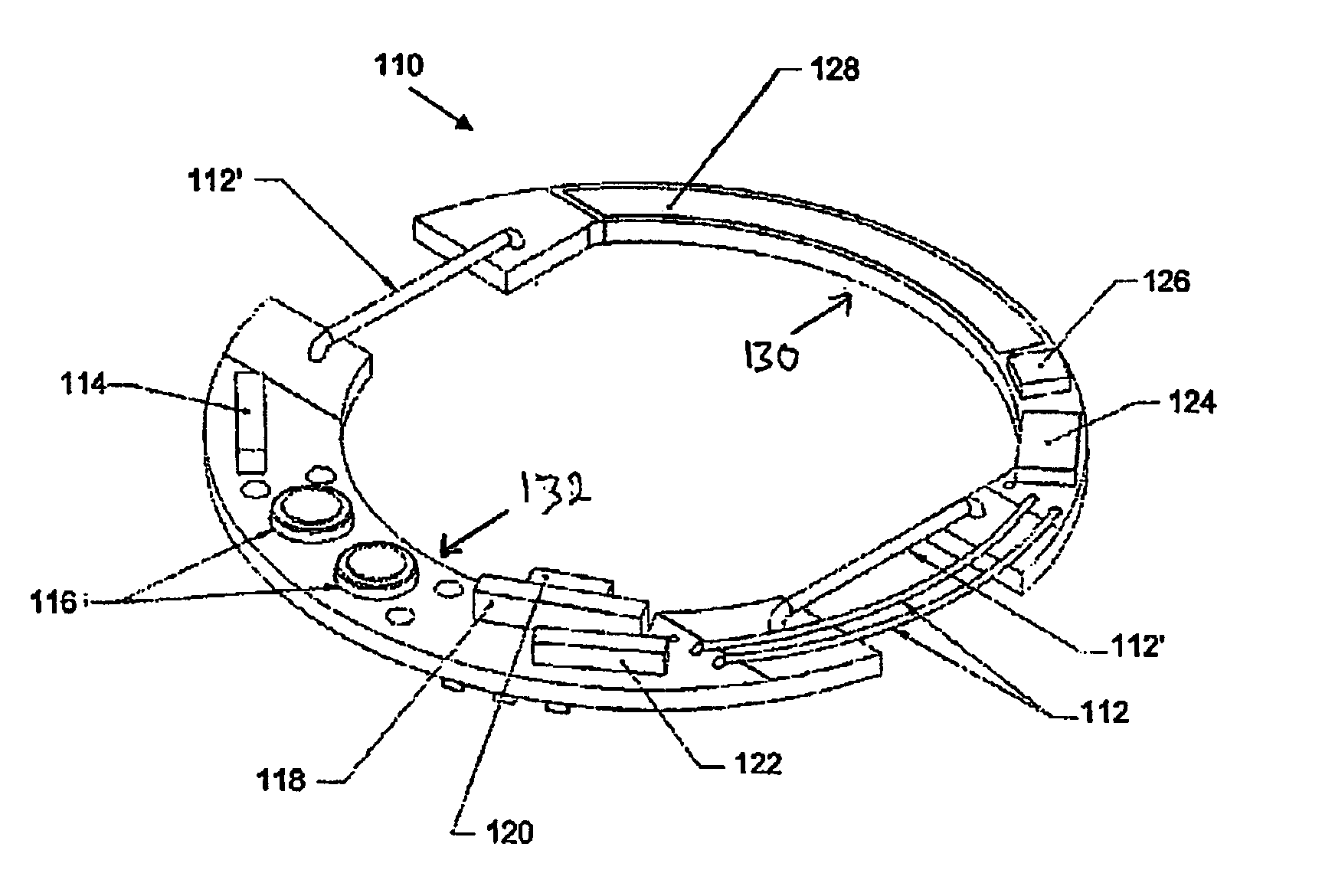
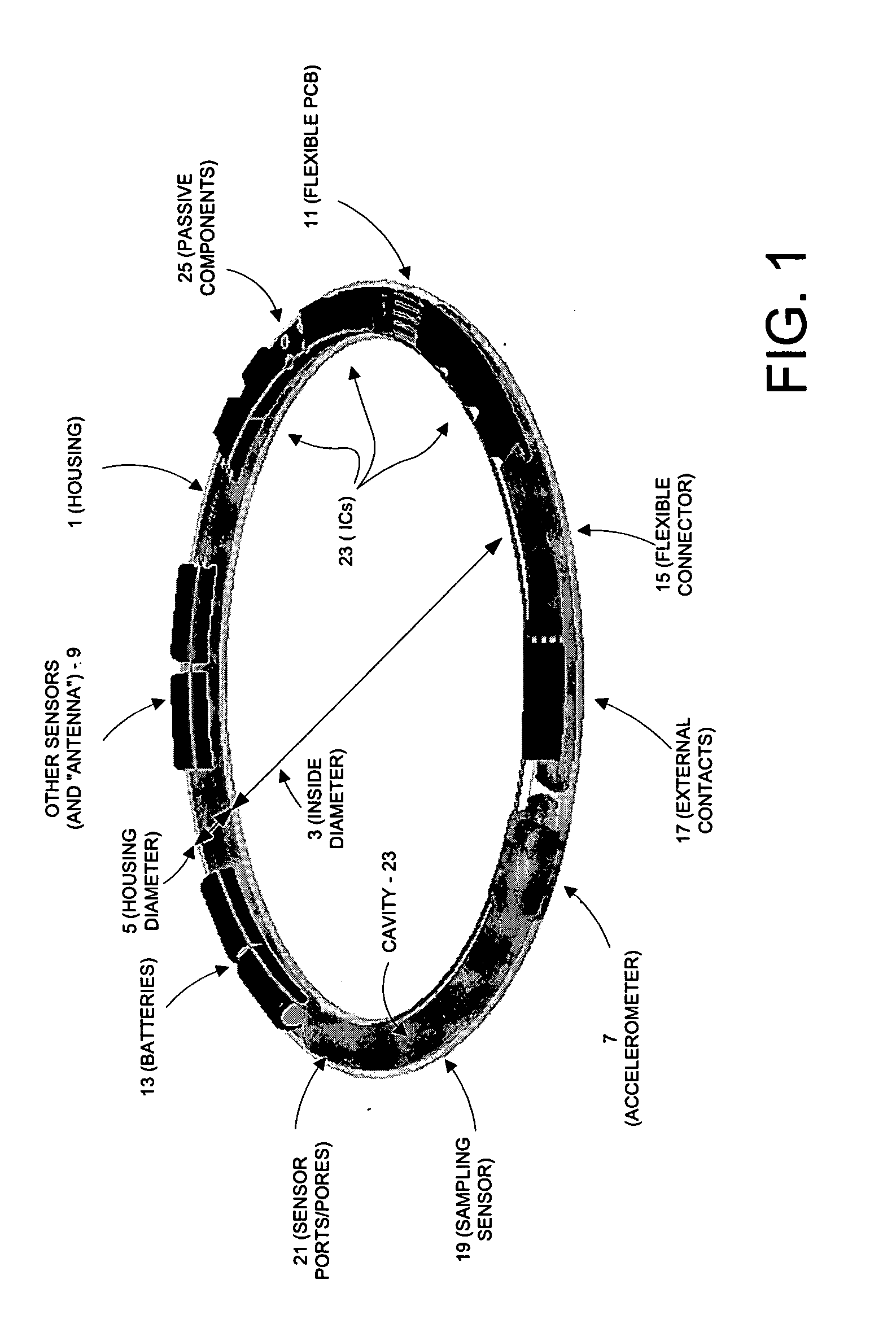
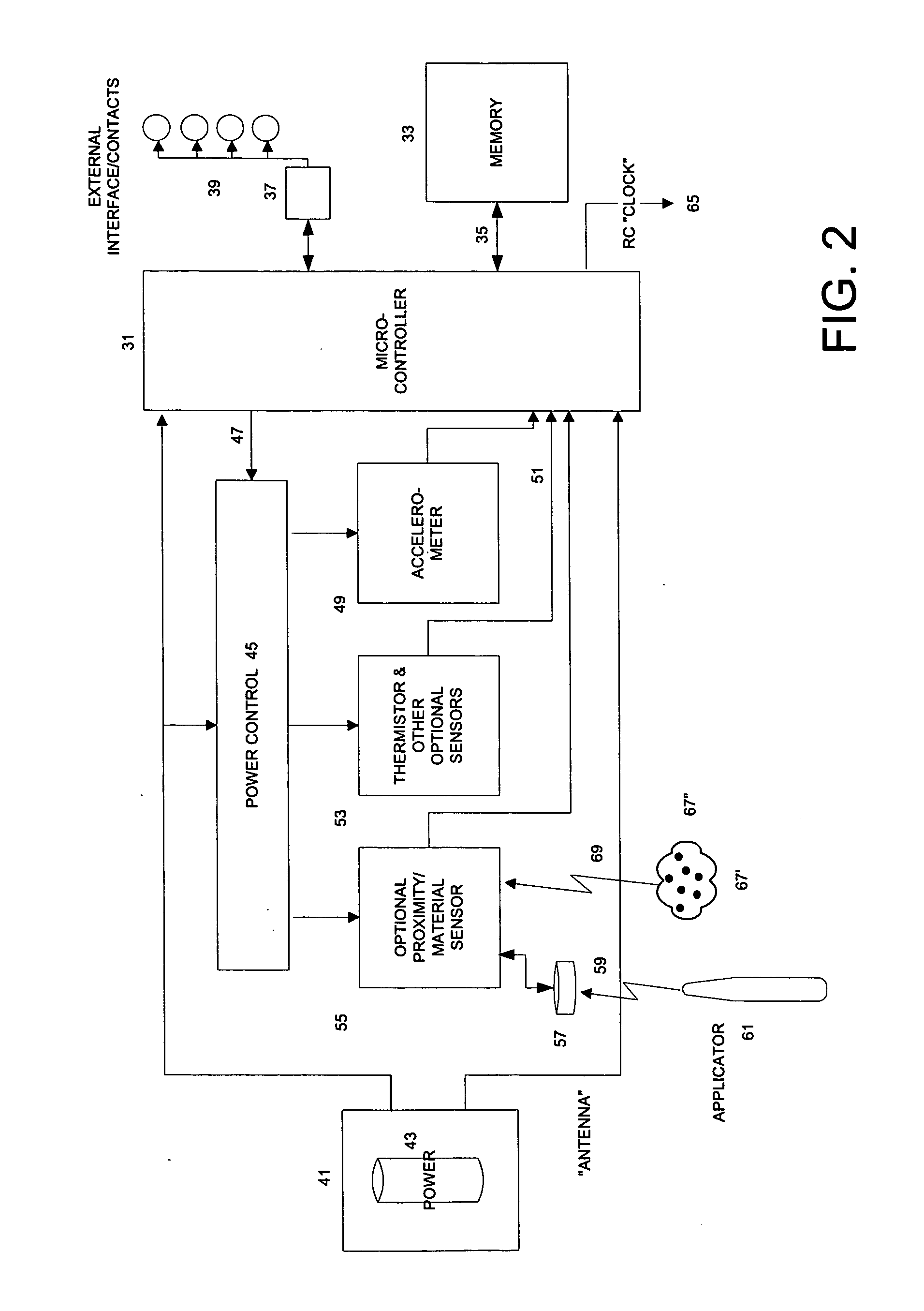
InactiveUS20060084848A1Improve battery lifeLow and controllable power consumptionPerson identificationInertial sensorsClinical trialComputer science
This invention provides a device, and method and system for its use, for monitoring participants in clinical trials so that participant self-reporting, which is known to be notoriously inaccurate, can be minimized or eliminated. In preferred embodiments, the device is self-contained and self-powered, resides on or in a body cavity of the participant, collects data monitoring medically relevant aspects of the participant's behavior and of the local device environment, and stores data in a memory on-board the device. An accompanying external station reads stored data and prepares it for use. Devices may include electrically-active sensors and non-electrical active sampling sensors. A preferred embodiment of the device is in clinical trials of microbicides inhibiting transmission of the HIV virus.
Owner:INT PARTNERSHIP FOR MICROBICIDES
Sulphonamide derivatives as prodrugs of aspartyl protease inhibitors
InactiveUS20050148548A1Good water solubilityImprove bioavailabilityBiocideSugar derivativesSulfur drugPatient compliance
The present invention relates to prodrugs of a class of sulfonamides which are aspartyl protease inhibitors. In one embodiment, this invention relates to a novel class of prodrugs of HIV aspartyl protease inhibitors characterized by favorable aqueous solubility, high oral bioavailability and facile in vivo generation of the active ingredient. This invention also relates to pharmaceutical compositions comprising these prodrugs. The prodrugs and pharmaceutical compositions of this invention are particularly well suited for decreasing the pill burden and increasing patient compliance. This invention also relates to methods of treating mammals with these prodrugs and pharmaceutical compositions.
Owner:VERTEX PHARMA INC
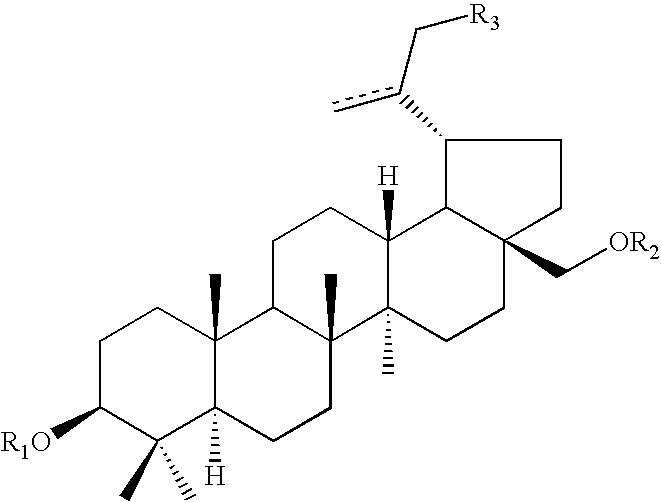
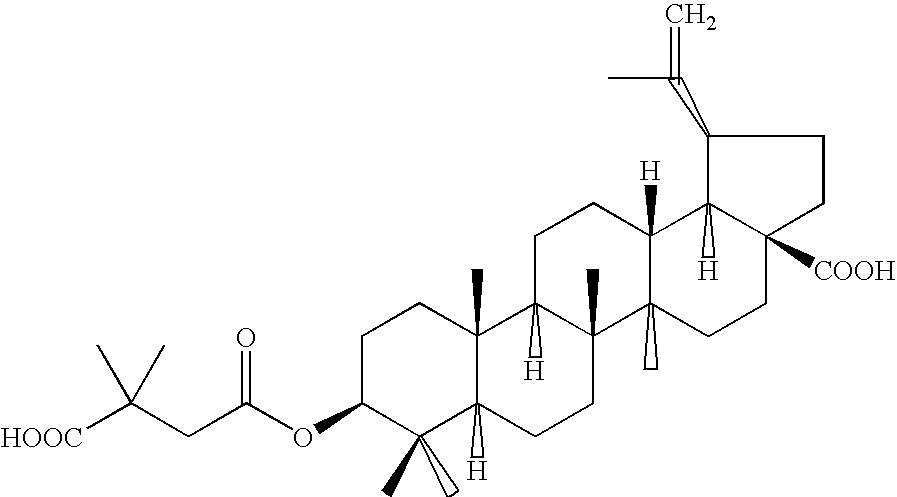
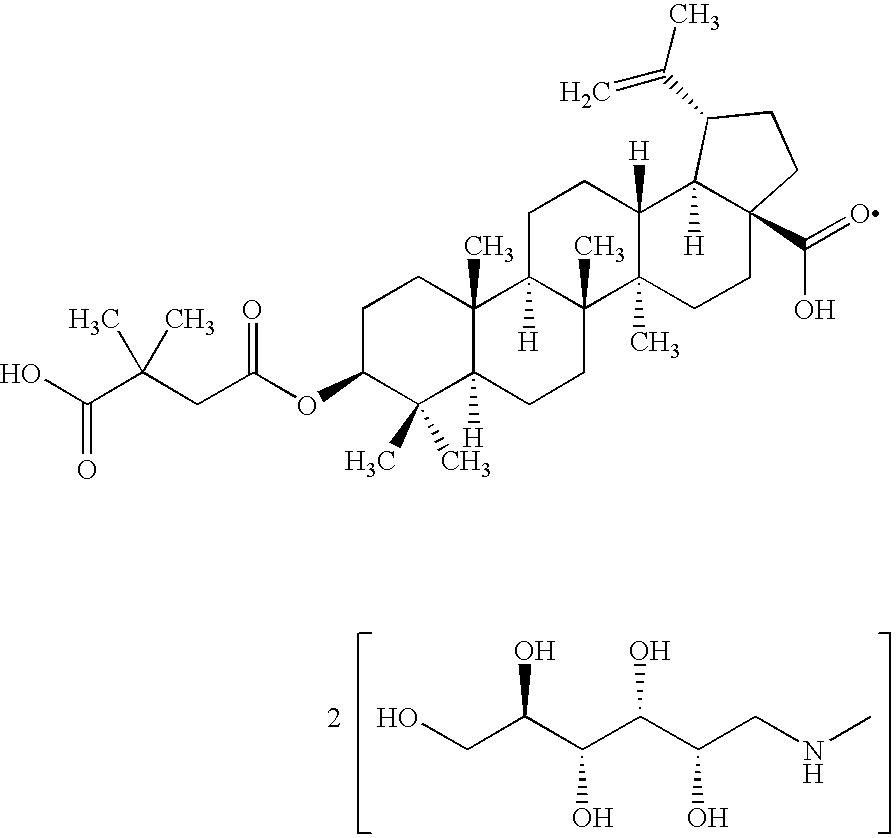
Pharmaceutical salts of 3-O-(3',3'-dimethylsuccinyl) betulinic acid
Salts of 3-O-(3′,3′-dimethylsuccinyl)Betulinic acid (DSB) are disclosed. Particularly, the preparation, pharmaceutical evaluation, and in vivo bioavailability evaluation of N-methyl-D-glucamine and alkali metal salt forms of DSB are disclosed. Pharmaceutical compositions including these salt forms are used in methods of treating HIV and related diseases. Methods of making the salts of DSB and the pharmaceutical compositions are also provided.
Owner:MYREXIS INC
Topical Antiviral Formulations
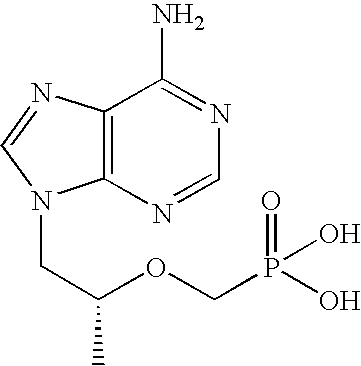
InactiveUS20080035155A1Reduce riskPrevent and reduce riskBiocideOrganic active ingredientsTopical antiviralReverse transcriptase
The present invention relates to formulations of nucleotide reverse transcriptase inhibitors (N(t)RTIs), preferably [2-(6-Amino-purin-9-yl)-1-methyl-ethoxymethyl]-phosphonic acid (tenofovir, PMPA), or a physiologically functional derivative thereof, suitable for topical application and their use in the prevention of HIV infections.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
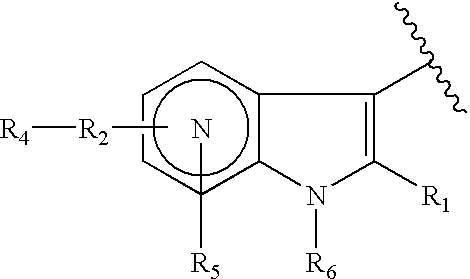
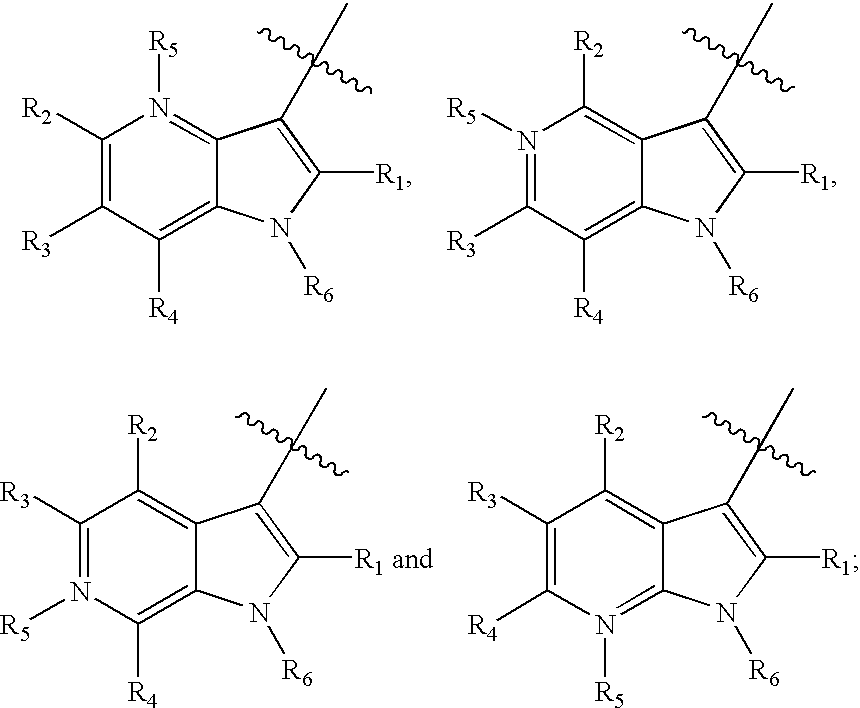
Indole, azaindole and related heterocyclic 4-alkenyl piperidine amides
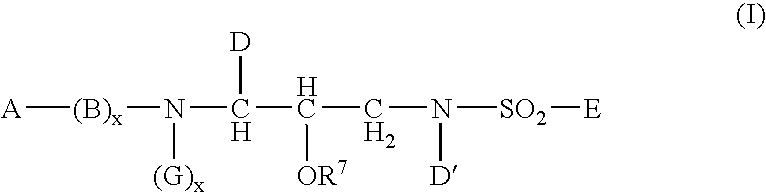
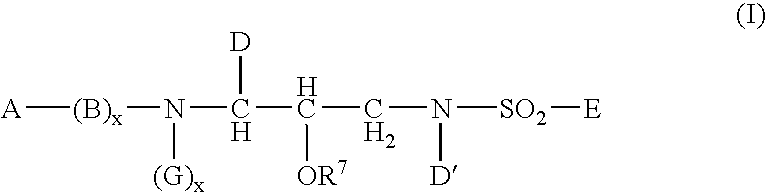
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with new piperidine 4-alkenyl derivatives that possess unique antiviral activity. More particularly, the present invention relates to compounds useful for the treatment of HIV and AIDS. The compounds of the invention for the general Formula I: wherein: Z is Q is selected from the group consisting of: -W- is
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for capsases and other enzymes and the use thereof
InactiveUS6342611B1Organic chemistryMicrobiological testing/measurementScreening proceduresApoptosis
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
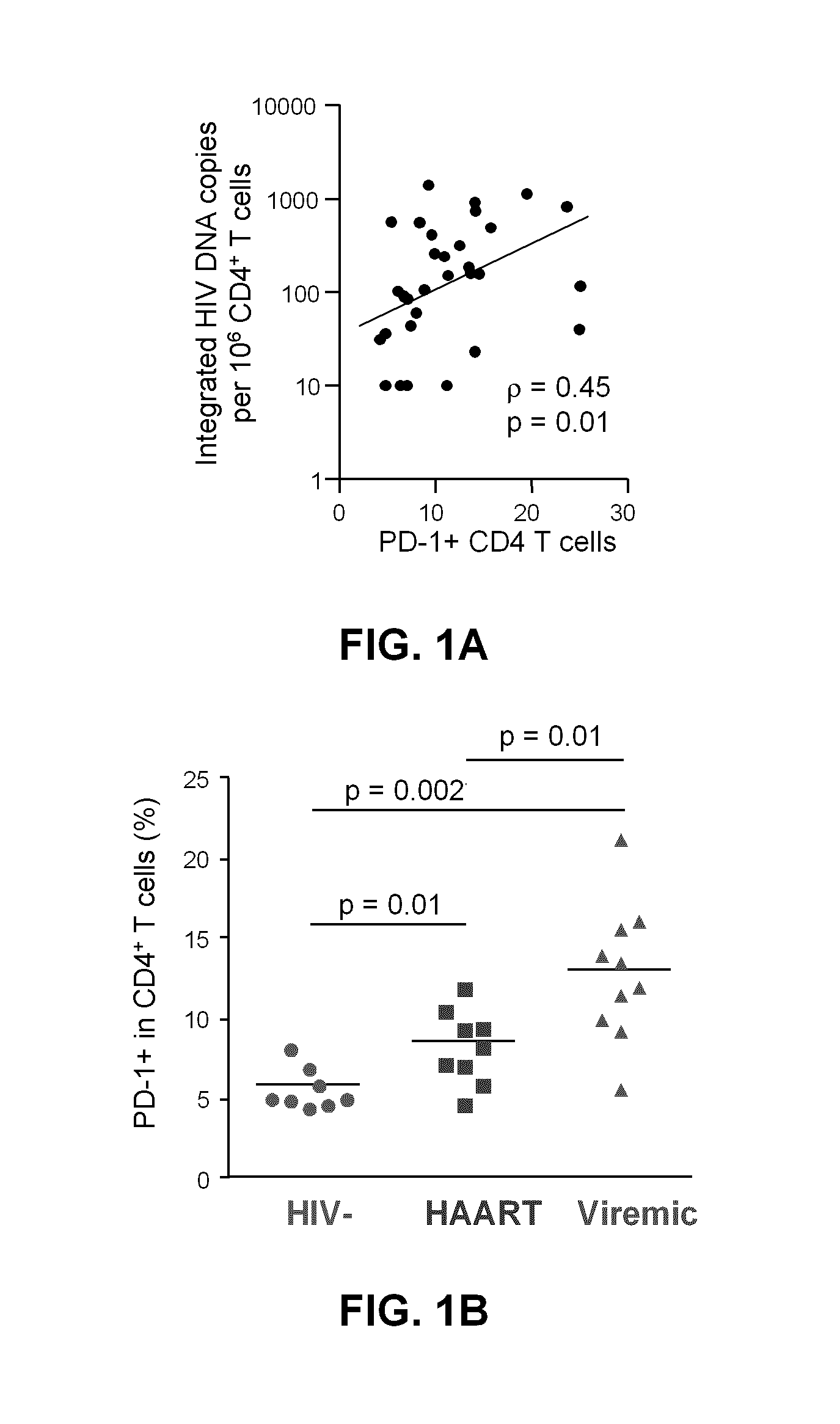
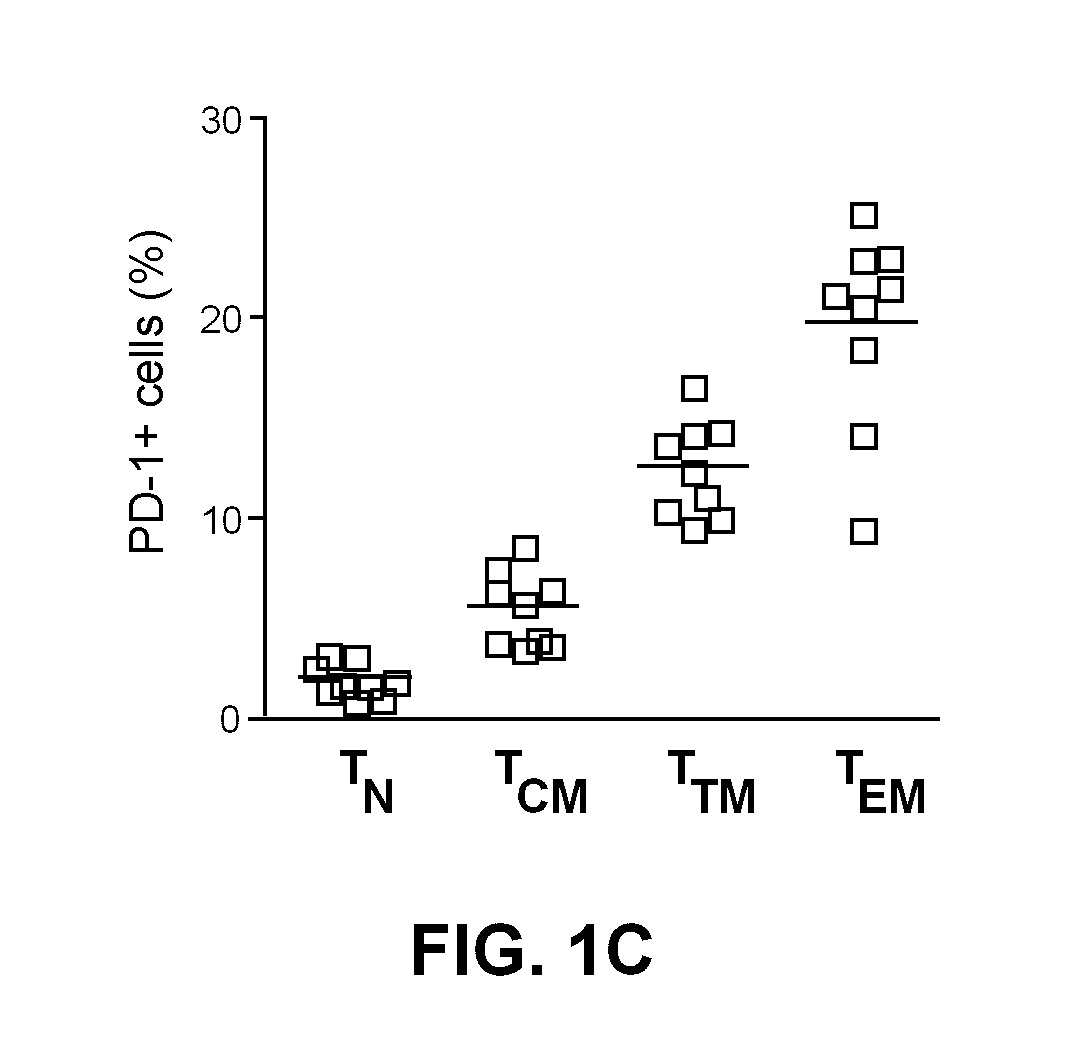
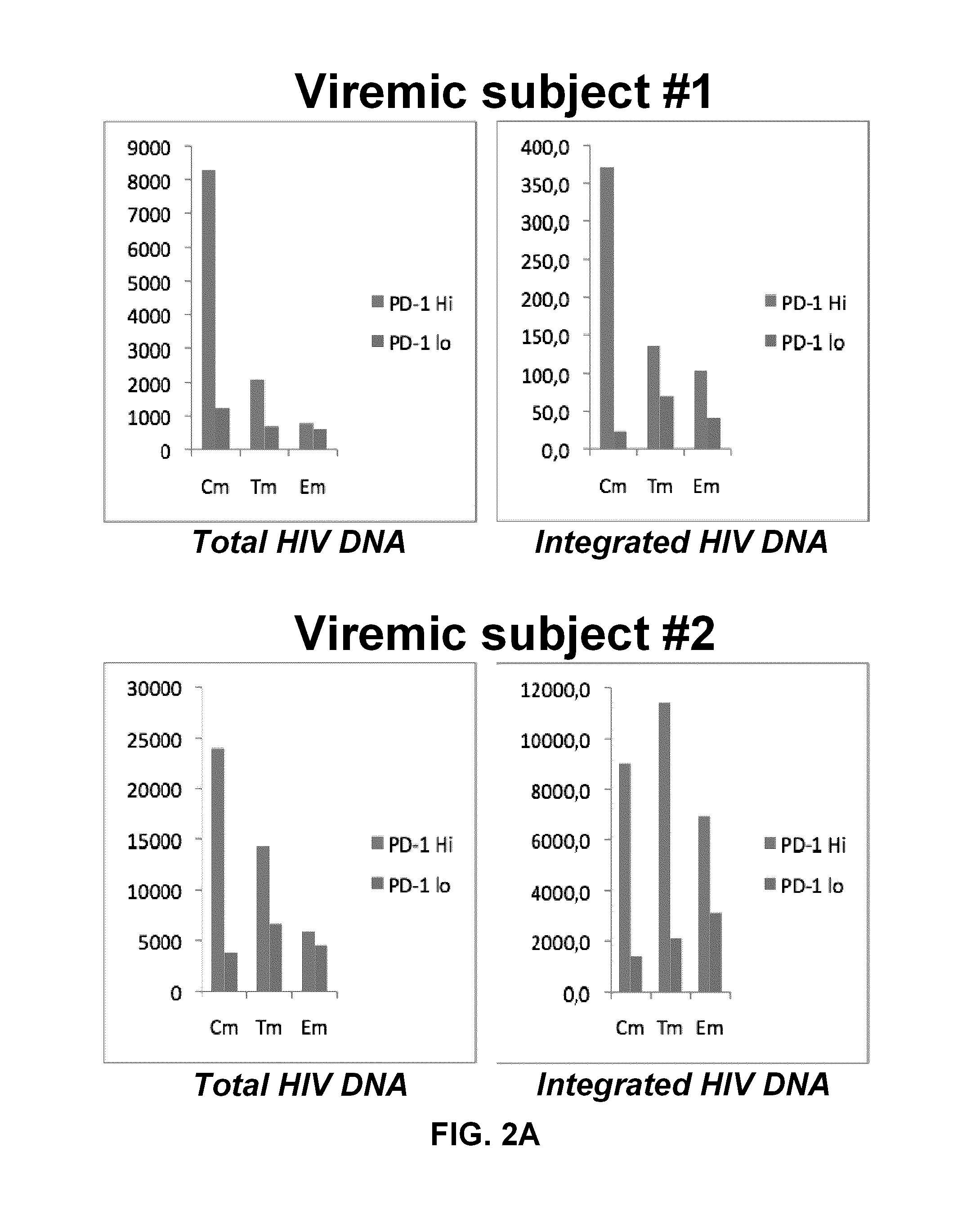
Pd-1 modulation and uses thereof for modulating HIV replication
InactiveUS20130202623A1Reduce in quantityPeptide/protein ingredientsAntibody mimetics/scaffoldsReplication methodHuman immunodeficiency virus (HIV)
Owner:OREGON HEALTH & SCI UNIV
Human monoclonal antibodies against human CXCR4
Compositions are provided that comprise antibody against membrane proteins such as chemokine receptors. In particular, monoclonal human antibodies against human CXCR4 are provided that are capable of inhibiting HIV infection and chemotaxis in human breast cancer cells. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection and cancer, for screening drugs, and for diagnosing diseases or conditions associated with interactions with chemokine receptors.
Owner:GENETASTIX CORP
Anti-viral nucleoside analogs and methods for treating viral infections, especially HIV infections
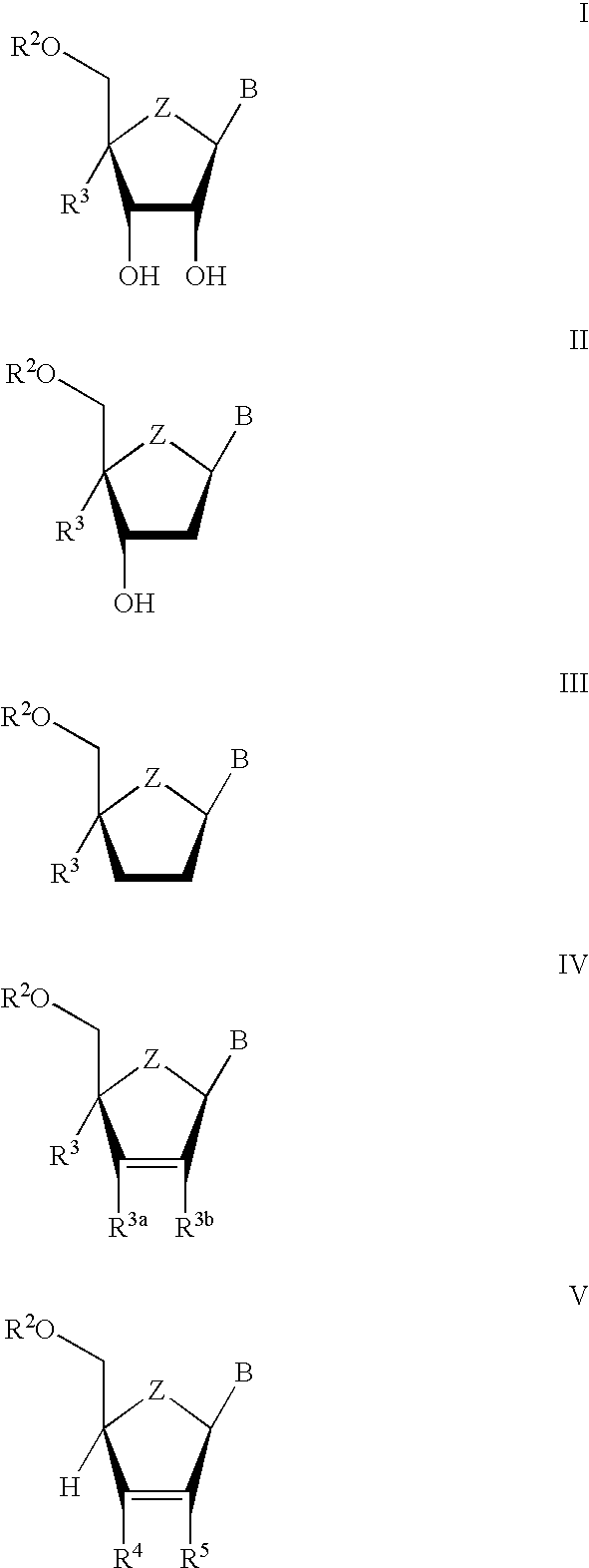
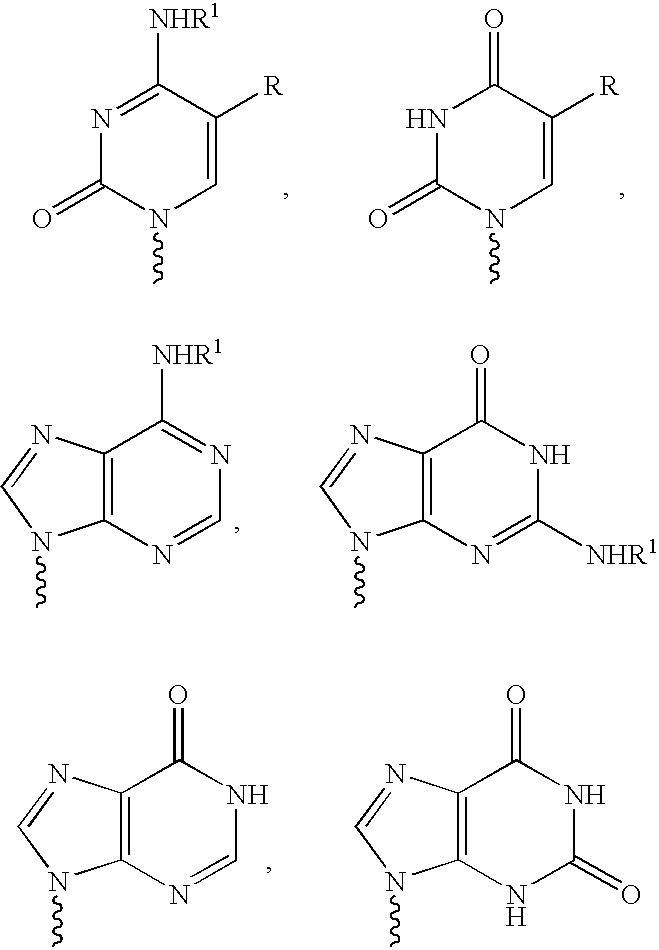
The present invention relates to novel compounds according to the to the general formulas I, II, III, IV or V: wherein B is nucleoside base according to the structure: R is H, F, Cl, Br, I, C1-C4 alkyl (preferably CH3), -C=N, -C=C-Ra, X is H, C1-C4 alkyl (preferably, CH3), F, Cl, Br or I; Z is O or CH2, with the proviso that Z is CH2 and not O when the compound is according to general formula II, R<3 >is -C=C-H and R<2 >is H or a phosphate, diphosphate, triphosphate or phosphotriester group; R<1 >is H, an acyl group, a C1-C20 alkyl or an ether group; R<2 >is H, an acyl group, a C1-C20 alkyl or ether group, a phosphate, diphosphate, triphosphate, phosphodiester group or a group; Nu is a radical of a biologically active antiviral compound such that an amino group or hydroxyl group from said biologically active antiviral compound forms a phosphate, phosphoramidate, carbonate or urethane group with the adjacent moiety; R<8 >is H, or a C1-C20 alkyl or ether group, preferably a C1-C12 alkyl group; k is 0-12, preferably, 0-2; R<3 >is selected from a C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, R<3a >and R<3b >are independently selected from H, F, Cl, Br or I; R<4 >and R<5 >are independently selected from H, F, Cl, Br, I, OH, C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, with the proviso that R<4 >and R<5 >are not both H; Ra is H, F, Cl, Br, I, or -C1-C4 alkyl, preferably H or CH3; Y is H, F, Cl, Br, I or -C1-C4 alkyl, preferably H or CH3; and n is 0, 1, 2, 3, 4 or 5, preferably 0, 1 or 2; and their anomers, pharmaceutically acceptable salts, solvates, or polymorphs thereof.
Owner:YALE UNIV
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Methods of treatment of disease using adsorbent carriers
InactiveUS6498007B1Increase productionReduce in quantityAntibacterial agentsSolvent extractionDiseaseAcetic acid
The invention relates to a method for the removal of leucocytes from blood which comprises bringing blood that comprises infected leucocytes into contact with an adsorbent carrier that has a greater affinity for infected, activated and / or defective leucocytes than for uninfected leucocytes especially cellulose acetate. The method can be used in the apheresis treatment of diseases caused by pathogenic organisms, for example, HIV, HCV or malaria. It is especially useful for treatment of HIV.
Owner:JIMRO
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
InactiveUS20090004194A1Improve immunityEnhance cellular immune responseAntibacterial agentsFungiDiseaseTlr agonists
Owner:UNIV OF COLORADO THE REGENTS OF
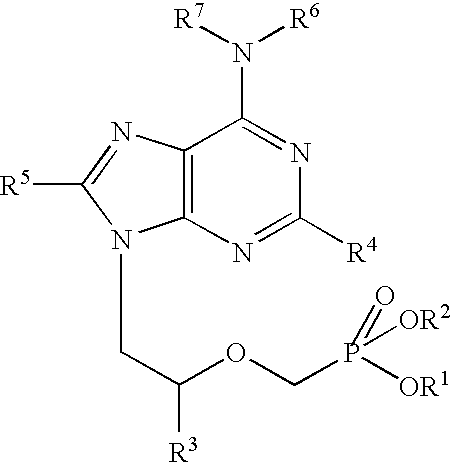
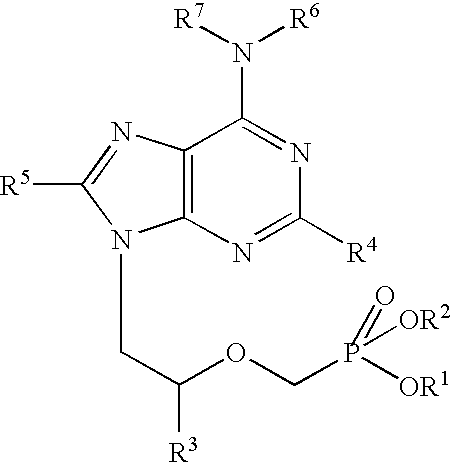
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com