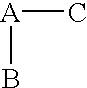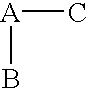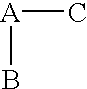Patents
Literature
210 results about "Chemotaxis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
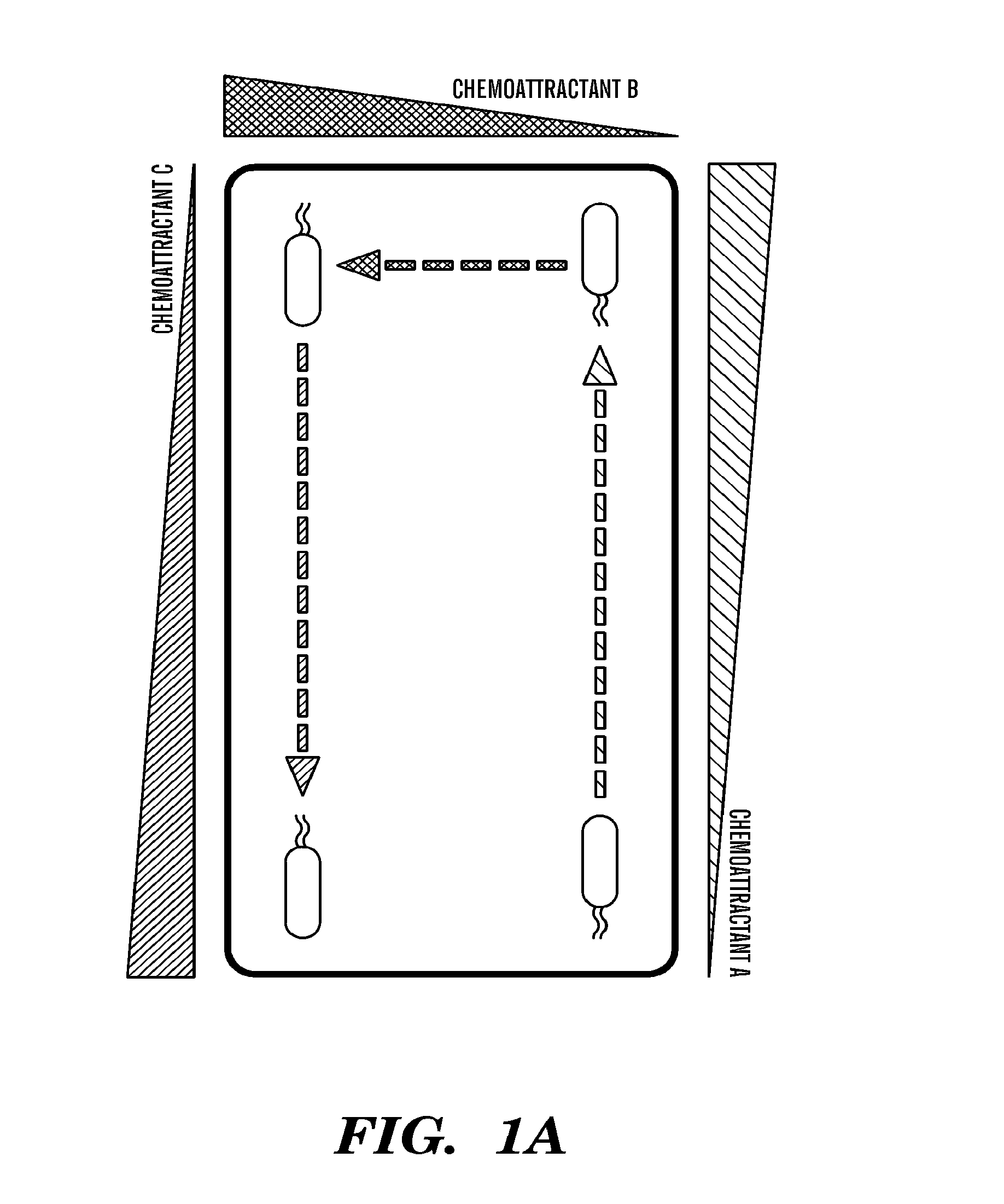
Chemotaxis (from chemo- + taxis) is the movement of an organism in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food (e.g., glucose) by swimming toward the highest concentration of food molecules, or to flee from poisons (e.g., phenol). In multicellular organisms, chemotaxis is critical to early development (e.g., movement of sperm towards the egg during fertilization) and subsequent phases of development (e.g., migration of neurons or lymphocytes) as well as in normal function and health (e.g., migration of leukocytes during injury or infection). In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthritis.
Injectable cross-linked polymeric preparations and uses thereof
InactiveUS20050003010A1Promote regenerationFunction increaseOrganic active ingredientsPowder deliveryCross-linkDamages tissue
A composition for promoting repair of damaged tissues, being a cross-linked alginate solution, which can be maintained in liquid form indefinitely (under constant conditions) and only gels in vivo. This cross-linked alginate solution is an ideal material to be used for tissue repair. Injection of said material into cardiac tissue post-myocardial infarct induced tissue regeneration. The invention provides such injectable solution, as well as compositions and method of preparation thereof. The invention also provides various methods and uses of the cross-linked alginate solution, for cardiac tissue regeneration, induction of neo-vascularization, enhancing SDF-1 expression and guiding stem cell chemotaxis, among others. A kit for tissue repair is also provided.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Human monoclonal antibodies against human CXCR4
Compositions are provided that comprise antibody against membrane proteins such as chemokine receptors. In particular, monoclonal human antibodies against human CXCR4 are provided that are capable of inhibiting HIV infection and chemotaxis in human breast cancer cells. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection and cancer, for screening drugs, and for diagnosing diseases or conditions associated with interactions with chemokine receptors.
Owner:GENETASTIX CORP
New effectors of dipeptidyl peptidase iv for topical use
InactiveUS20030092630A2Reduced activityOrganic active ingredientsPeptide/protein ingredientsDiseaseSide chain
Abstract of Disclosure The invention relates to compounds for topically influencing the activity of dipeptidyl peptidase of the general formula wherein A is an amino acid having at least one functional group in the side chain;B is a chemical compound covalently bound to a functional group of the side chain of A, chosen from the group consisting of (a) oligopeptides having a chain length of up to 20 amino acids, (b) homopolymers of glycine consisting of up to 6 glycine monomers, and (c) polyethylene glycols having molar masses of up to 20 000 g / mol; and C is a group amide-bonded to A chosen from the group consisting of thiazolidine, pyrrolidine, cyanopyrrolidine, hydroxyproline, dehydroproline or piperidine. The invention further relates to the use of said compounds for targeted intervention in local immunological processes (chemotaxis, inflammatory processes, autoimmune diseases), as well as effective and targeted treatment of pathophysiological and physiological processes related thereto (psoriasis, periodontitis, arthritis, allergies, inflammation), inter alia.
Owner:VIVORYON THERAPEUTICS NV
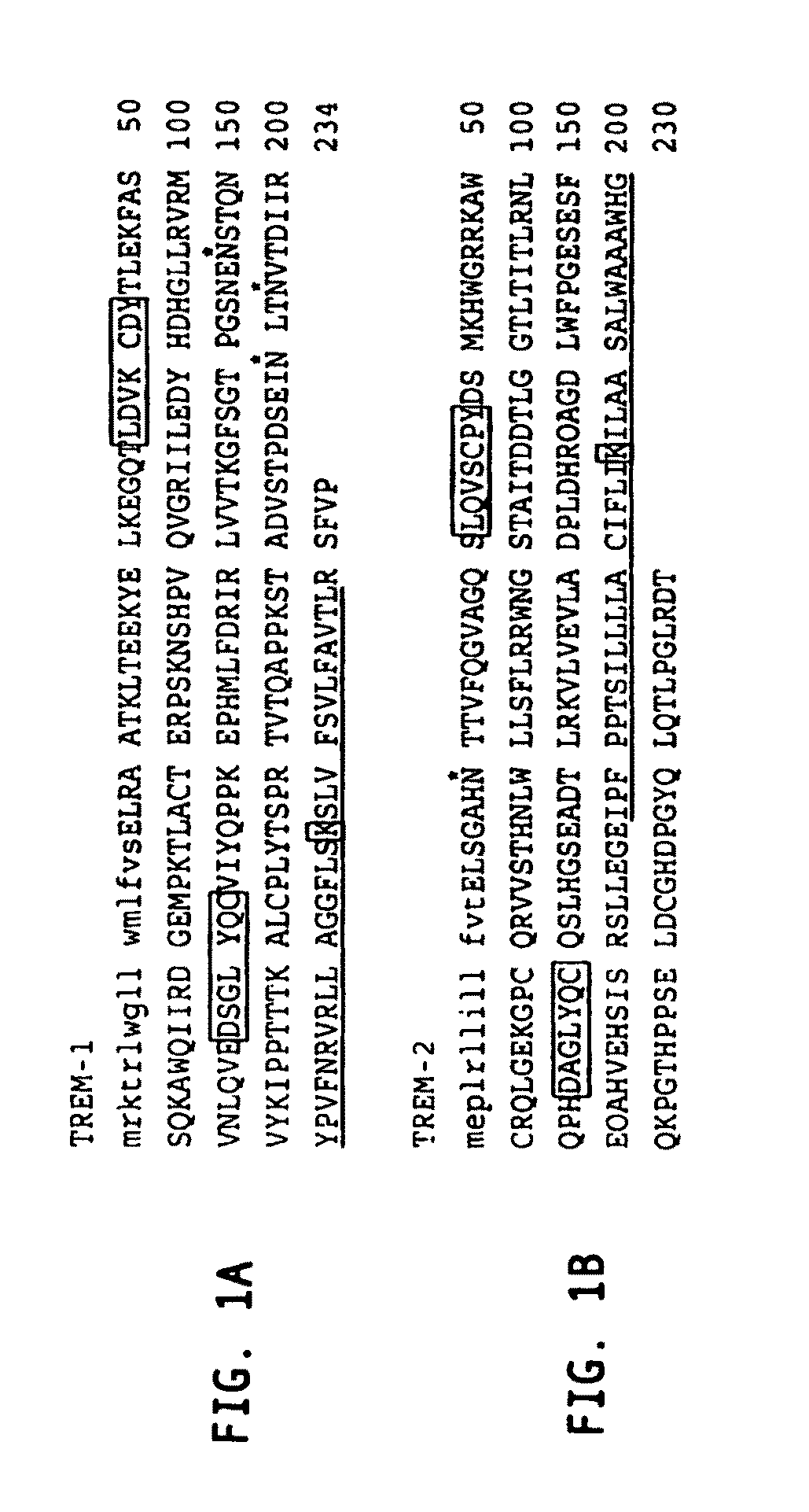
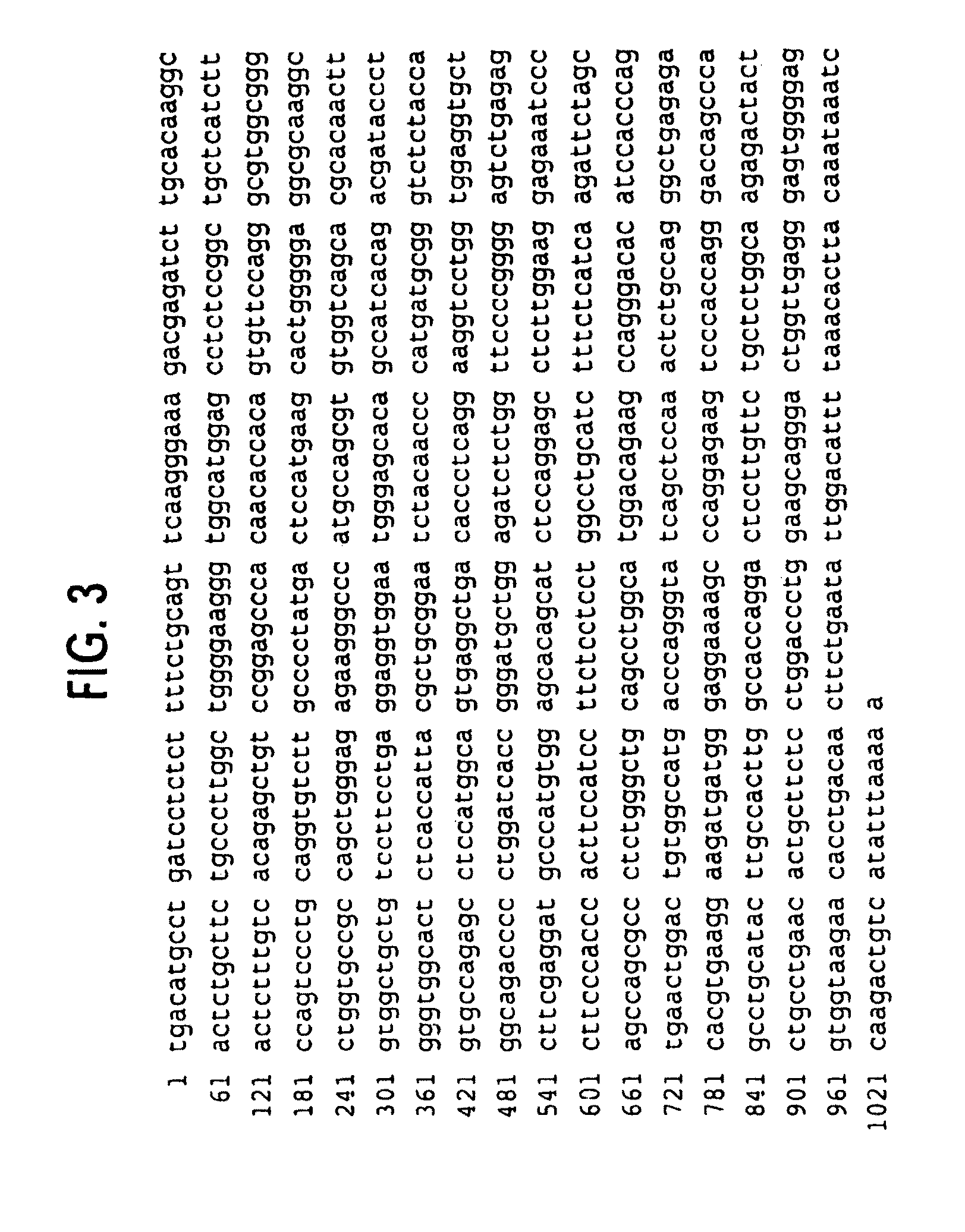
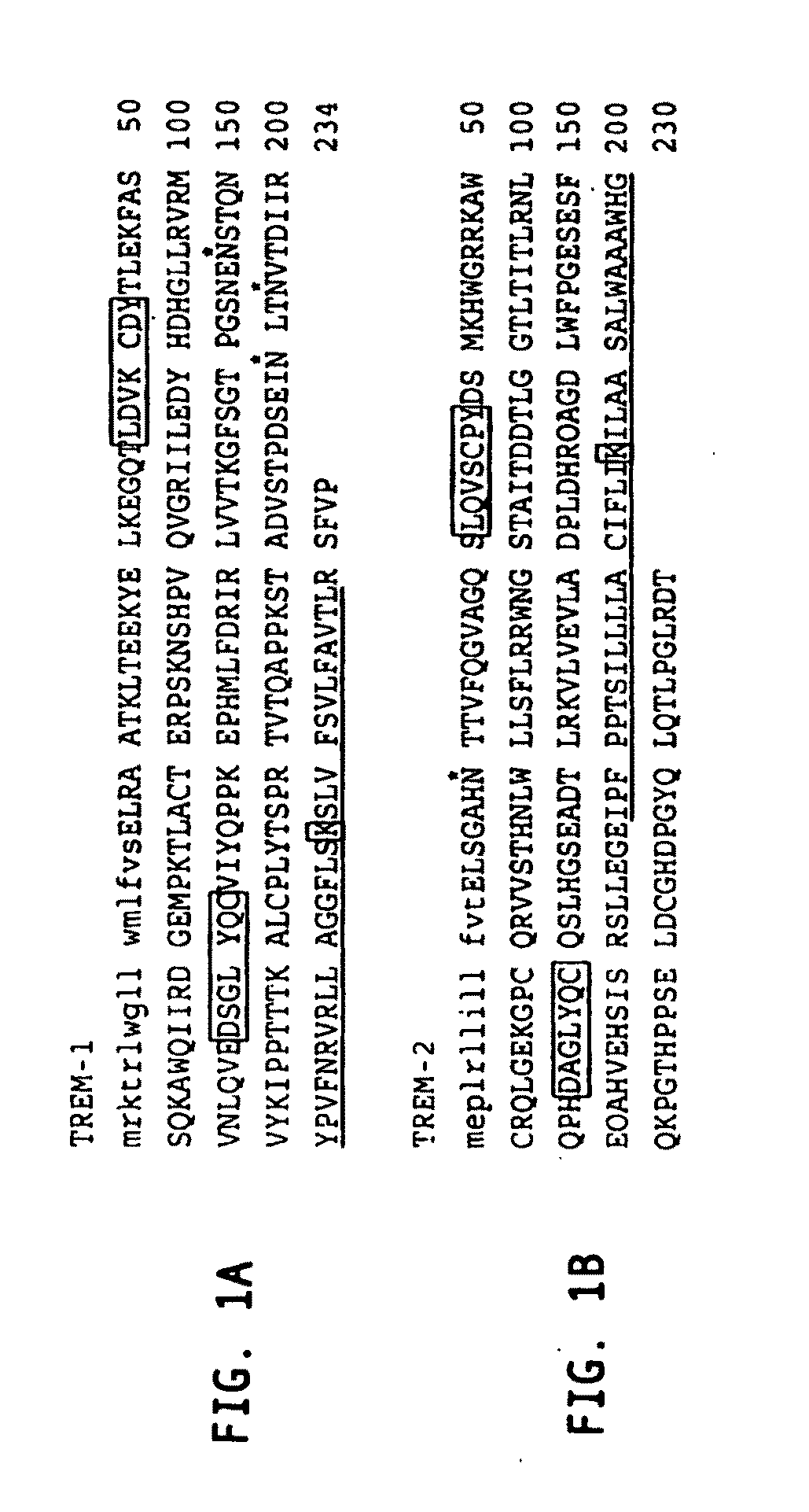
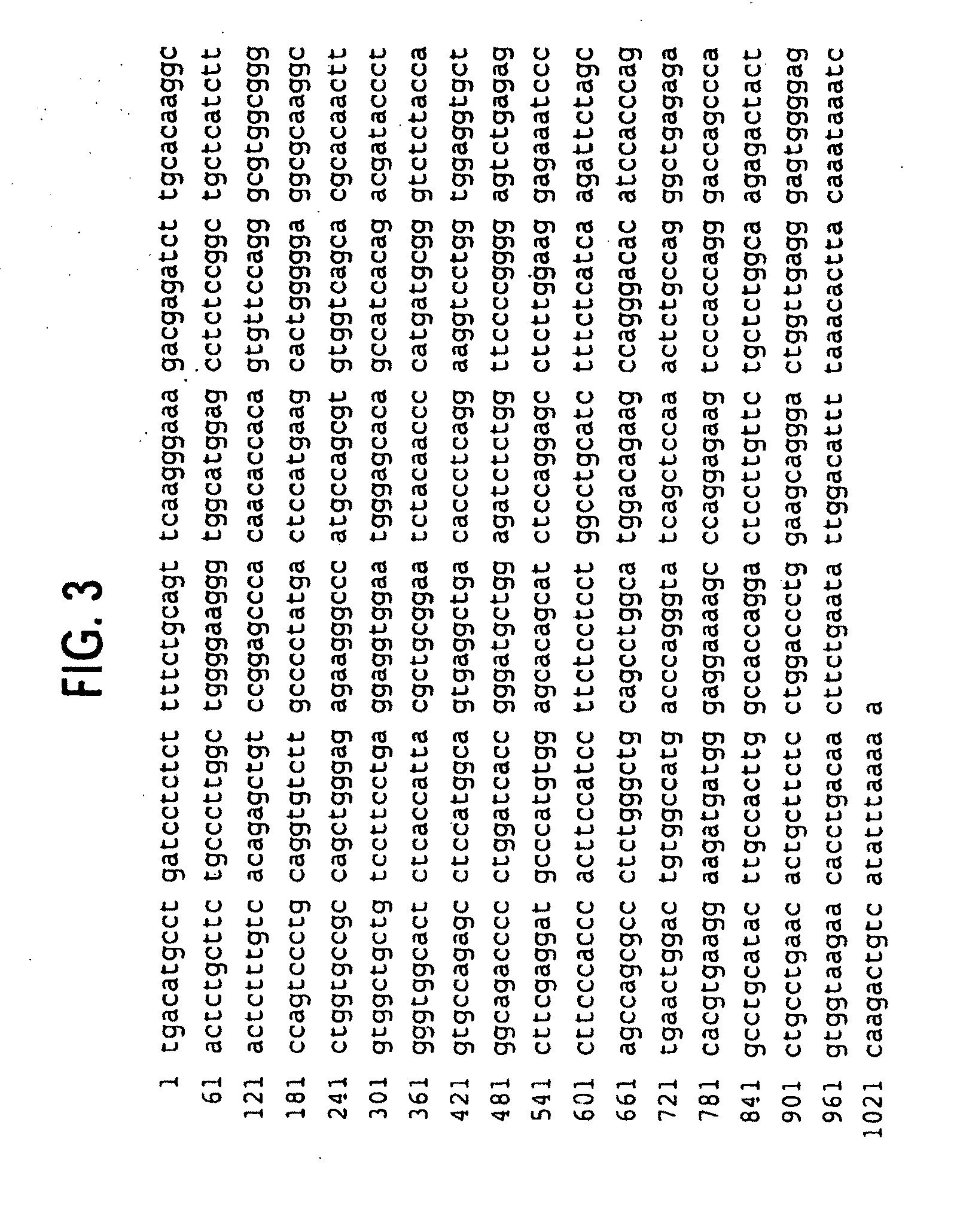
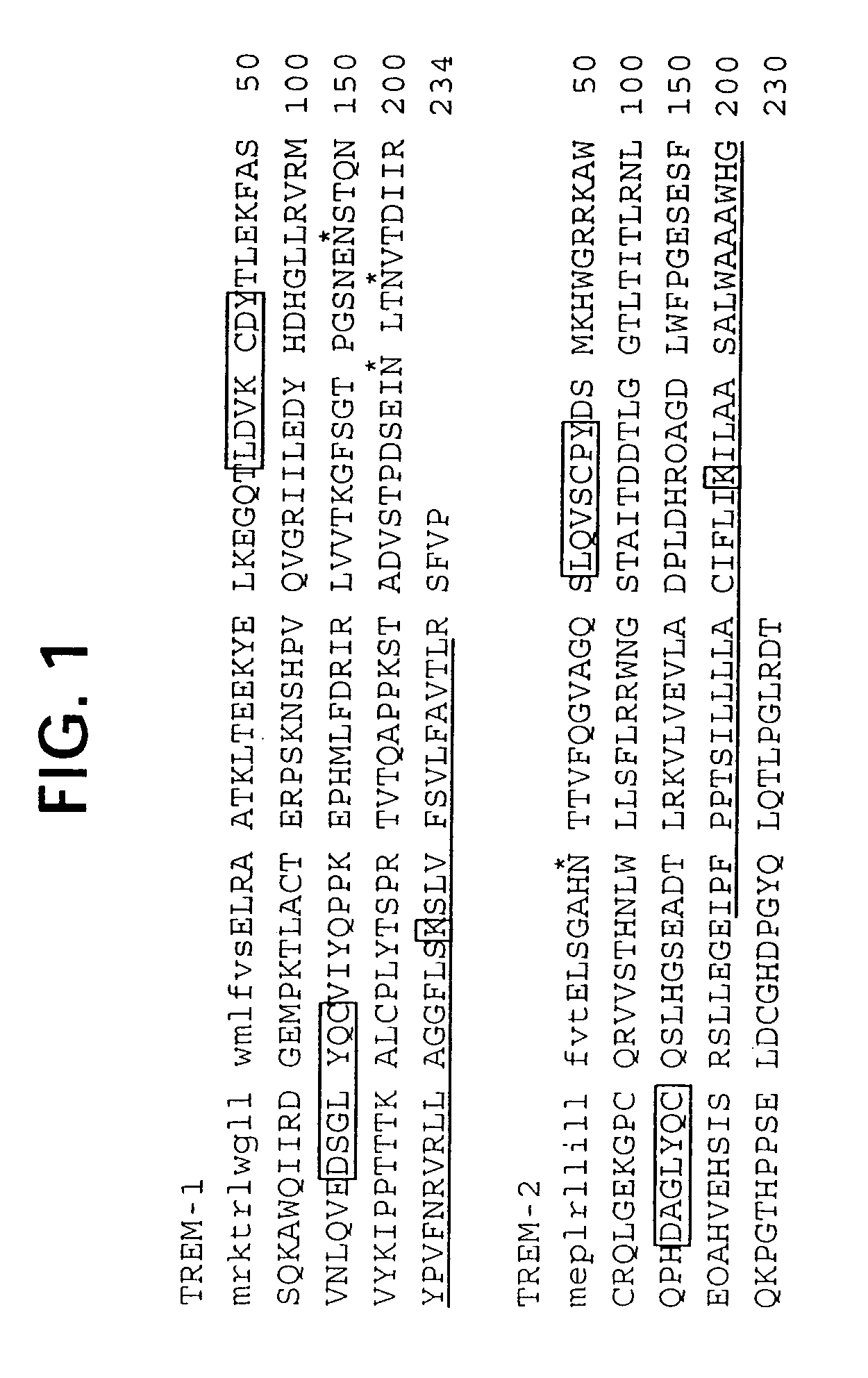
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20130150559A1Strong upregulationImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionDc maturation
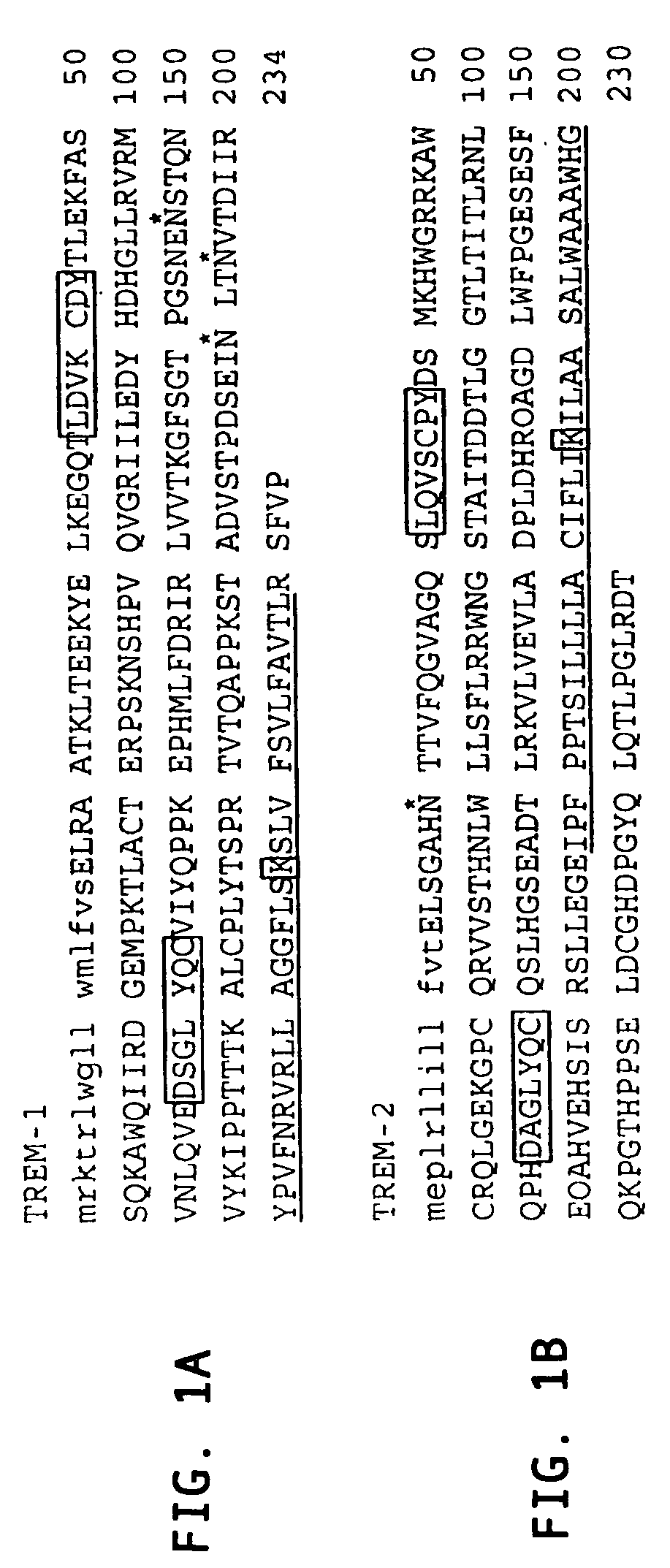
Novel activating receptors of the lg super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:NOVO NORDISK AS
Receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS8231878B2Strong upregulationImmunoglobulin superfamilyPeptide/protein ingredientsDc maturationAutoimmune disease
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:COSMO TECH LTD
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20100310560A1Strong upregulationOrganic active ingredientsImmunoglobulin superfamilyAutoimmune responsesDc maturation
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:COSMO TECH LTD
Inhibitory immunoglobulin polypeptides to human PDGF beta receptor
InactiveUS7060271B2Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman typeReceptor activation
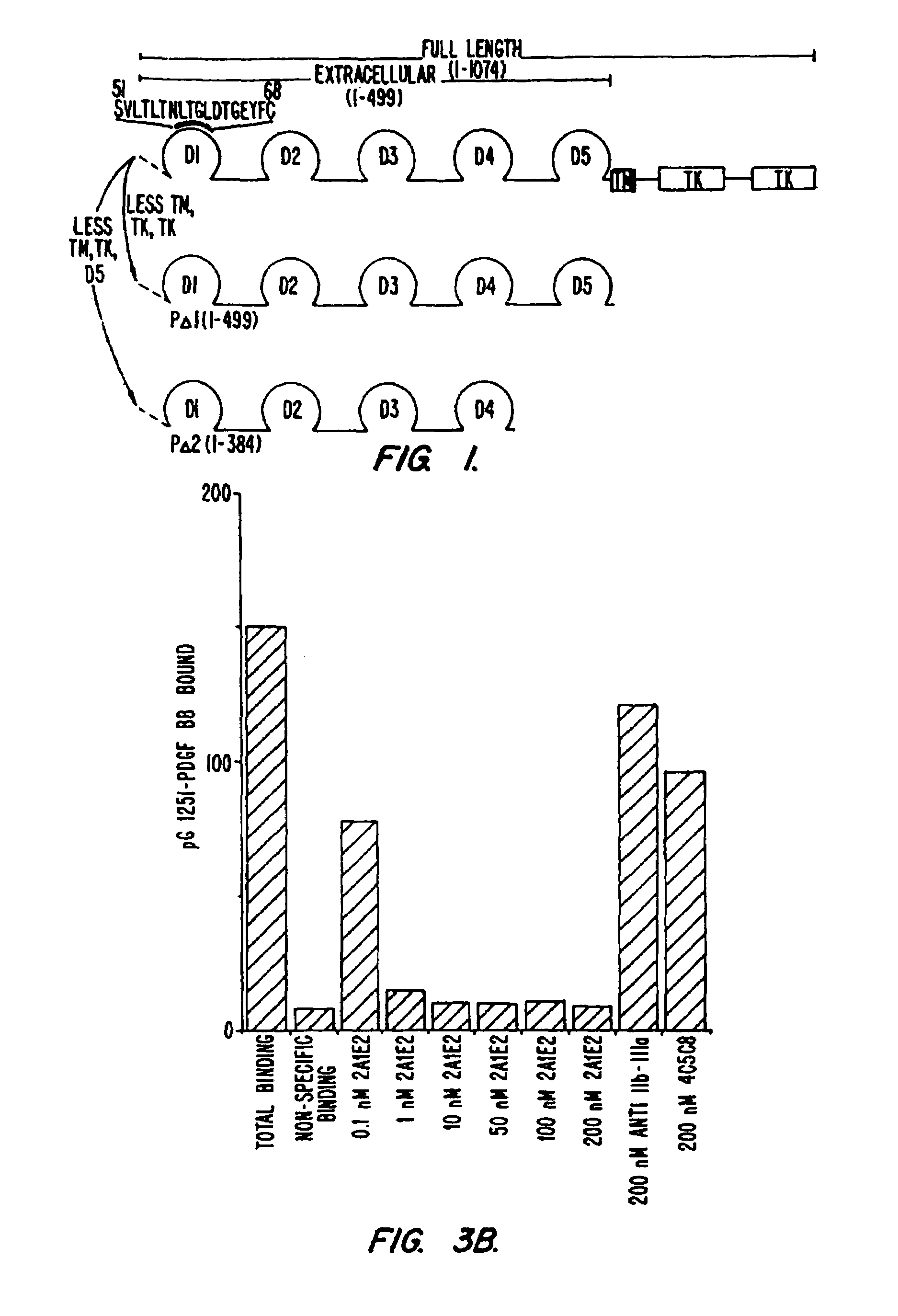
The present invention is directed towards immunoglobulin polypeptides that specifically bind to the extracellular domain of the human type beta PDGF receptor. The binding of the immunoglobulin polypeptides to the receptor inhibits PDGF-induced (or stimulated) receptor activation as indicated by inhibition of receptor phosphorylation and dimerization, and by inhibition of PDGF-mediated mitogenesis, chemotaxis and migration of cells displaying the human PDGF type beta receptor on the cell surface. Nucleic acids encoding the immunoglobulin polypeptides are also included in the invention. The immunoglobulin polypeptides have diagnostic and therapeutic uses.
Owner:MILLENNIUM PHARMA INC
Receptor TREM (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS8981061B2Strong upregulationImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsDc maturationApoptosis
Novel activating receptors of the lg super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:NOVO NORDISK AS
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20090081199A1Strong upregulationOrganic active ingredientsCompound screeningApoptosisAutoimmune disease
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:BIOXELL
Biological assays using gradients formed in microfluidic systems
InactiveUS7374906B2Bioreactor/fermenter combinationsMaterial nanotechnologyAssayBiological interaction
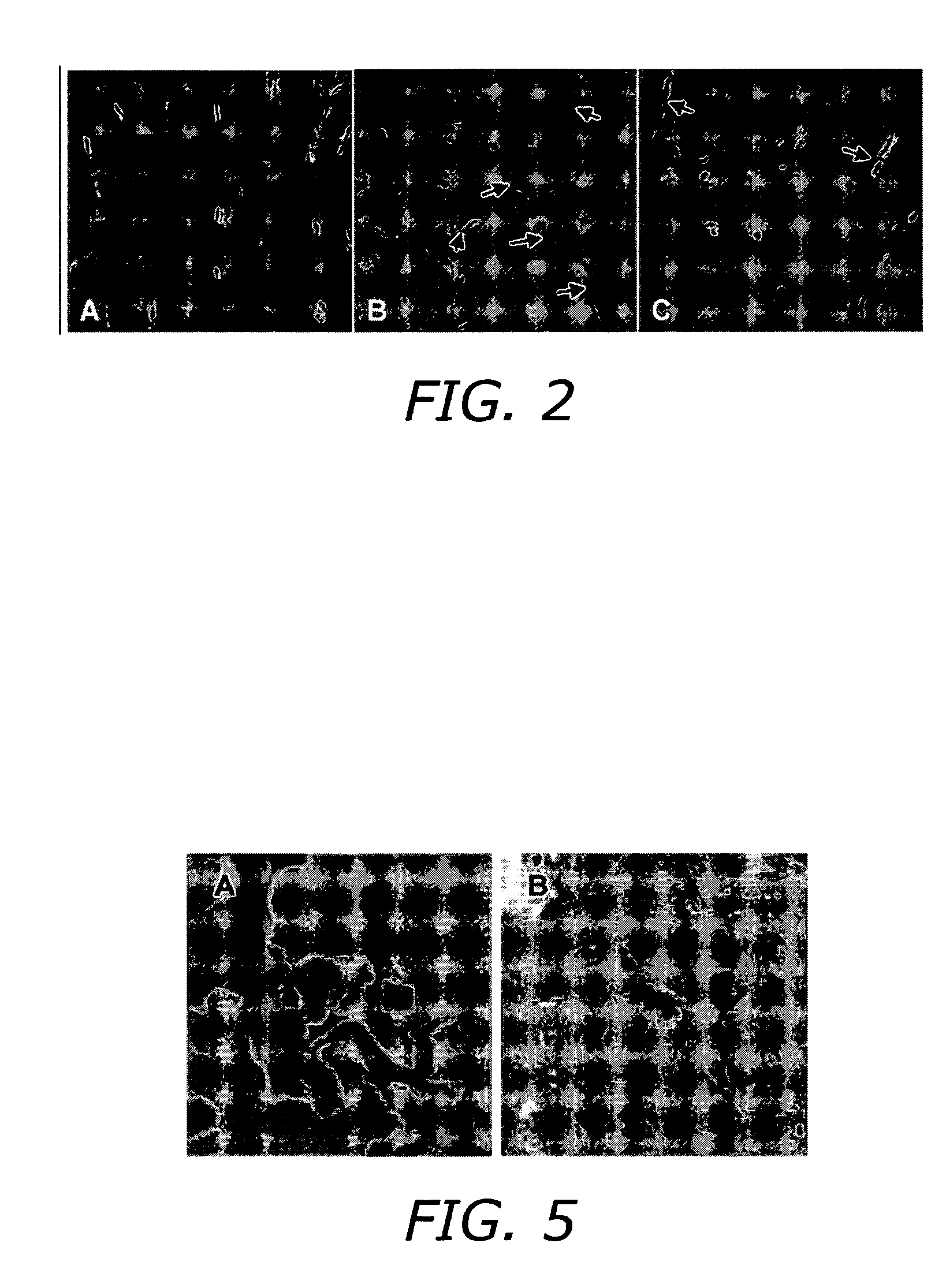
The present invention discloses a device for monitoring chemotaxis or chemoinvasion. The present invention further provides a flexible assay system and numerous assays that can be used to test biological interactions and systems. Laminar flow gradients are employed that mimic gradient situations present in vivo.
Owner:SURFACE LOGIX INC
Microfluidic device having stable static gradient for analyzing chemotaxis
ActiveUS20090123961A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringPressure difference
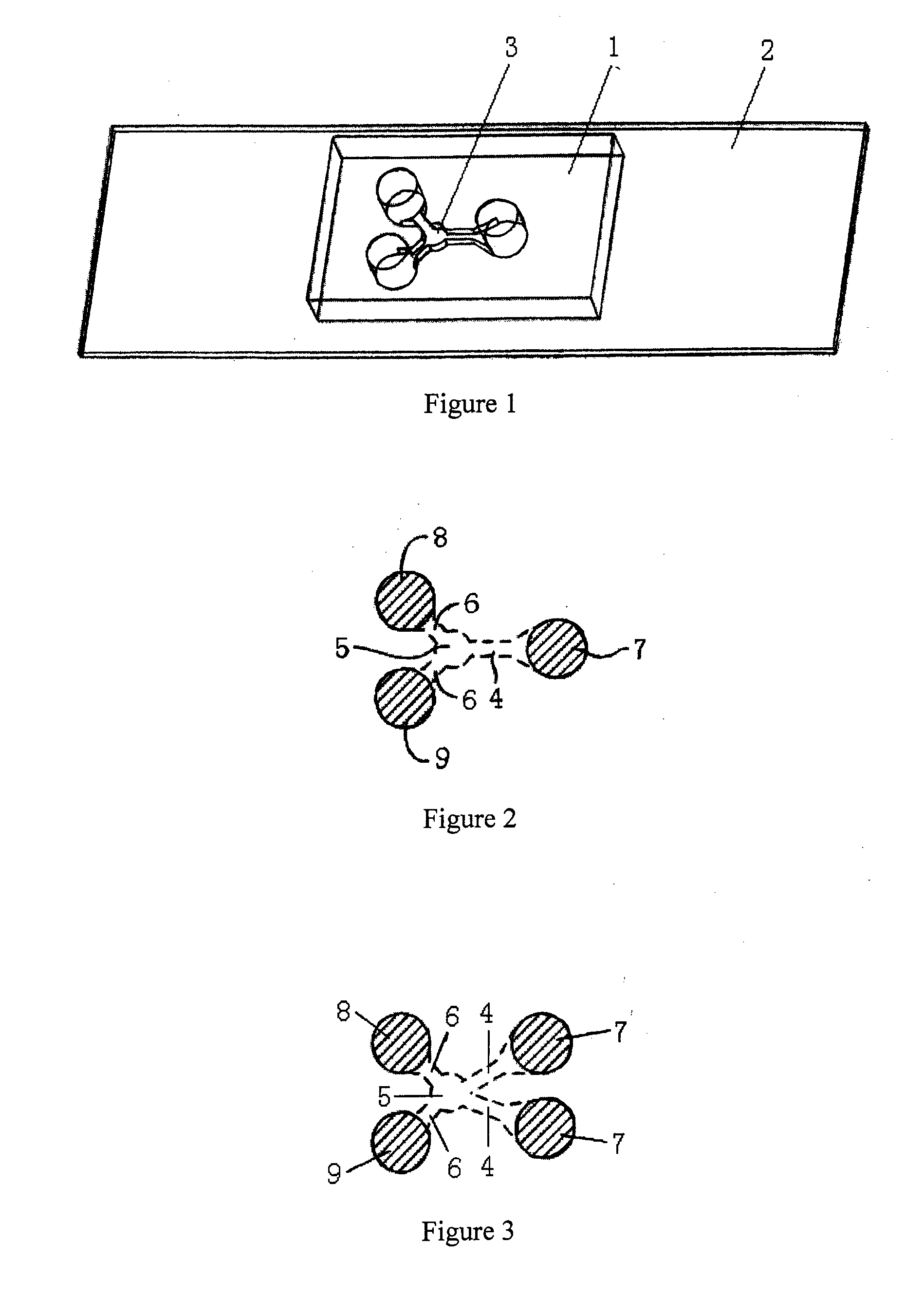
A microfluidic method and device for testing and analyzing chemotaxis by providing a stable, static fluid gradient. The device includes a sink reservoir for receiving biological cellular material and a source reservoir for receiving a chemoattractant. The biological cellular material migrates through a low fluid volume microfluidic gradient channel located between the source and sink reservoirs. The fluid in the gradient channel is static and stable due to a high fluid volume closed circuit bypass microfluidic channel also in fluid communication with the source and sink reservoirs, whereby the bypass channel relieves any pressure differential imparted across the gradient channel.
Owner:BELLBROOK LABS
Human monoclonal antibodies against membrane proteins
Compositions are provided that comprise antibody against membrane proteins such as chemokine receptors. In particular, monoclonal human antibodies against human CXCR4 are provided that are capable of inhibiting HIV infection and chemotaxis in human breast cancer cells. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection and cancer, for screening drugs, and for diagnosing diseases or conditions associated with interactions with chemokine receptors.
Owner:GENETASTIX CORP
Microfabricated cellular traps based on three-dimensional micro-scale flow geometries
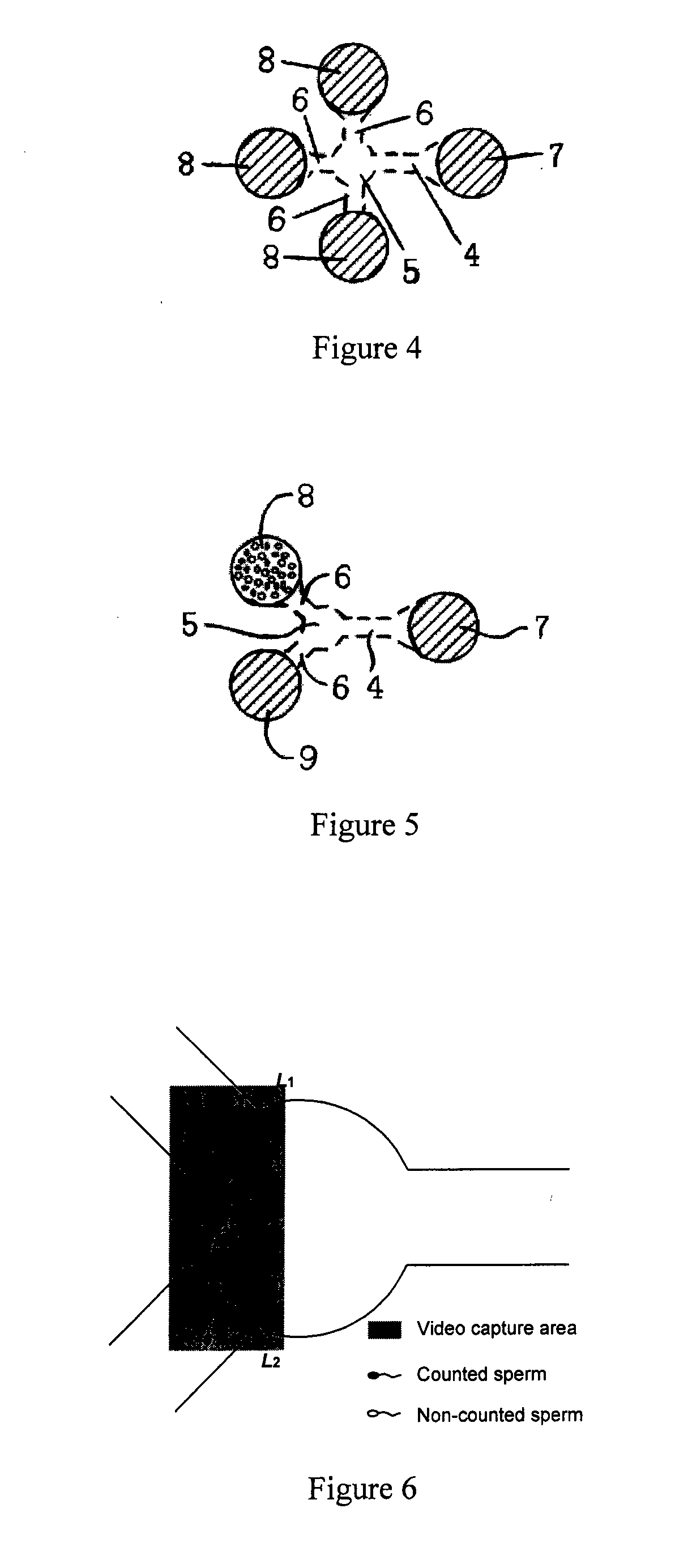
An improved microfluidic device and method of using the microfluidic device to measure natural motile response of a living moiety to a chemotactic agent. The invention comprises integrating microfluidic containment trenches within the microfluidic devices for cellular trapping, manipulation and real-time analysis of biological phenomena, including chemotaxis. The invention captures the design methodology for these traps, as well as the fabrication means and the associated external control mechanism that allows rapid, reversible and fully controlled changes in the chemical environment to which trapped cells are exposed.
Owner:KOSER HUR
Recombinant bacterium to decrease tumor growth
A recombinant bacterium capable of reducing tumor growth is provided, wherein said recombinant bacterium is capable of: a. increased expression of a nucleic acid encoding a chemoreceptor that directs chemotaxis towards tumors, b. accumulation in a quiescent tumor, c. hyper-invasion of a tumor, d. reduced fitness in normal tissue, e. enhanced stimulation of the host innate immune responses, f. delivering a tumor specific DNA vaccine vector to a tumor cell, and g. increased bacterium-induced host programmed cell death.
Owner:ARIZONA STATE UNIVERSITY
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20050260670A1Strong upregulationAntibacterial agentsAntimycoticsAutoimmune diseaseApoptosis
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-beta. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:COLONNA MARCO +1
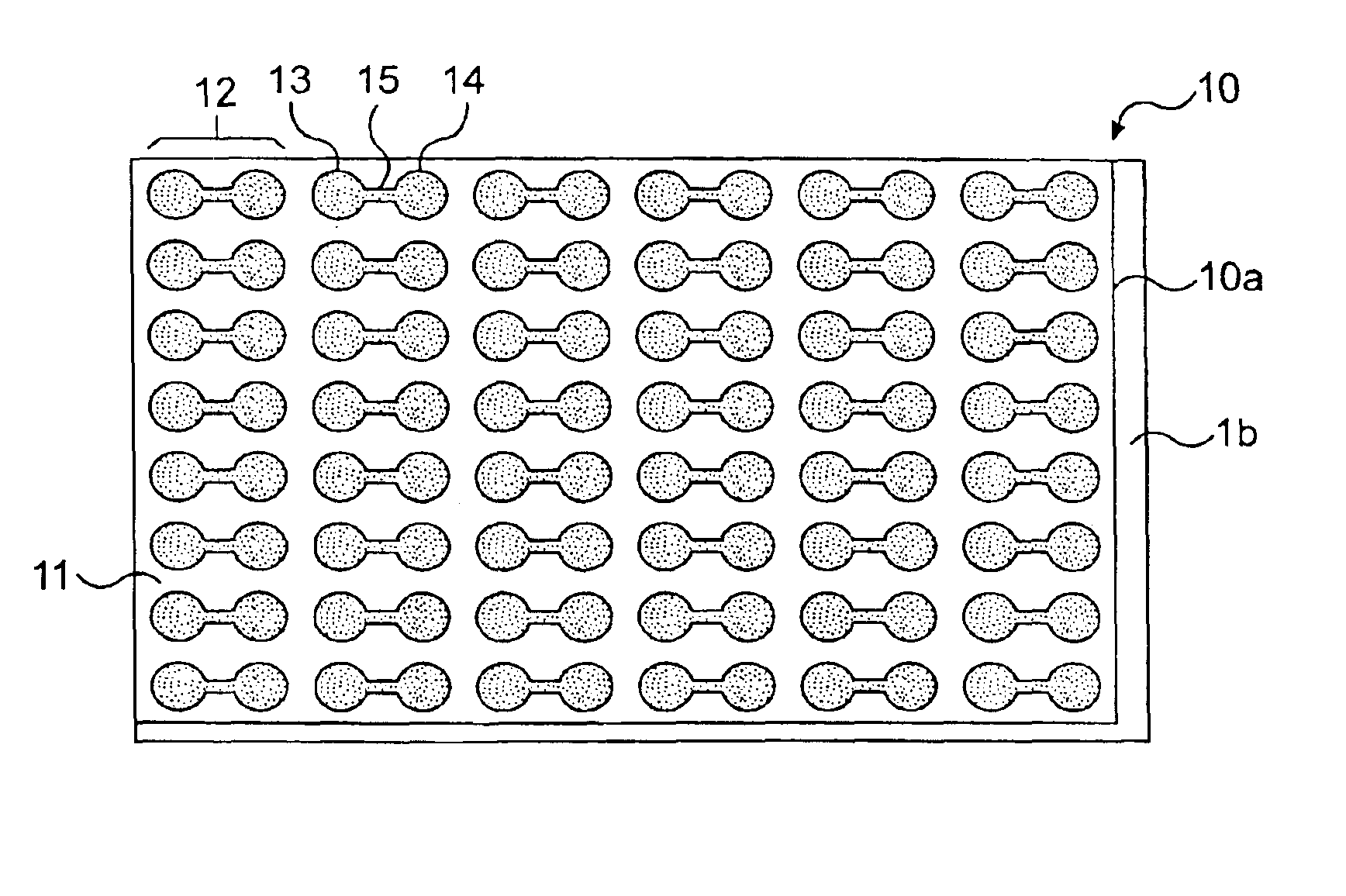
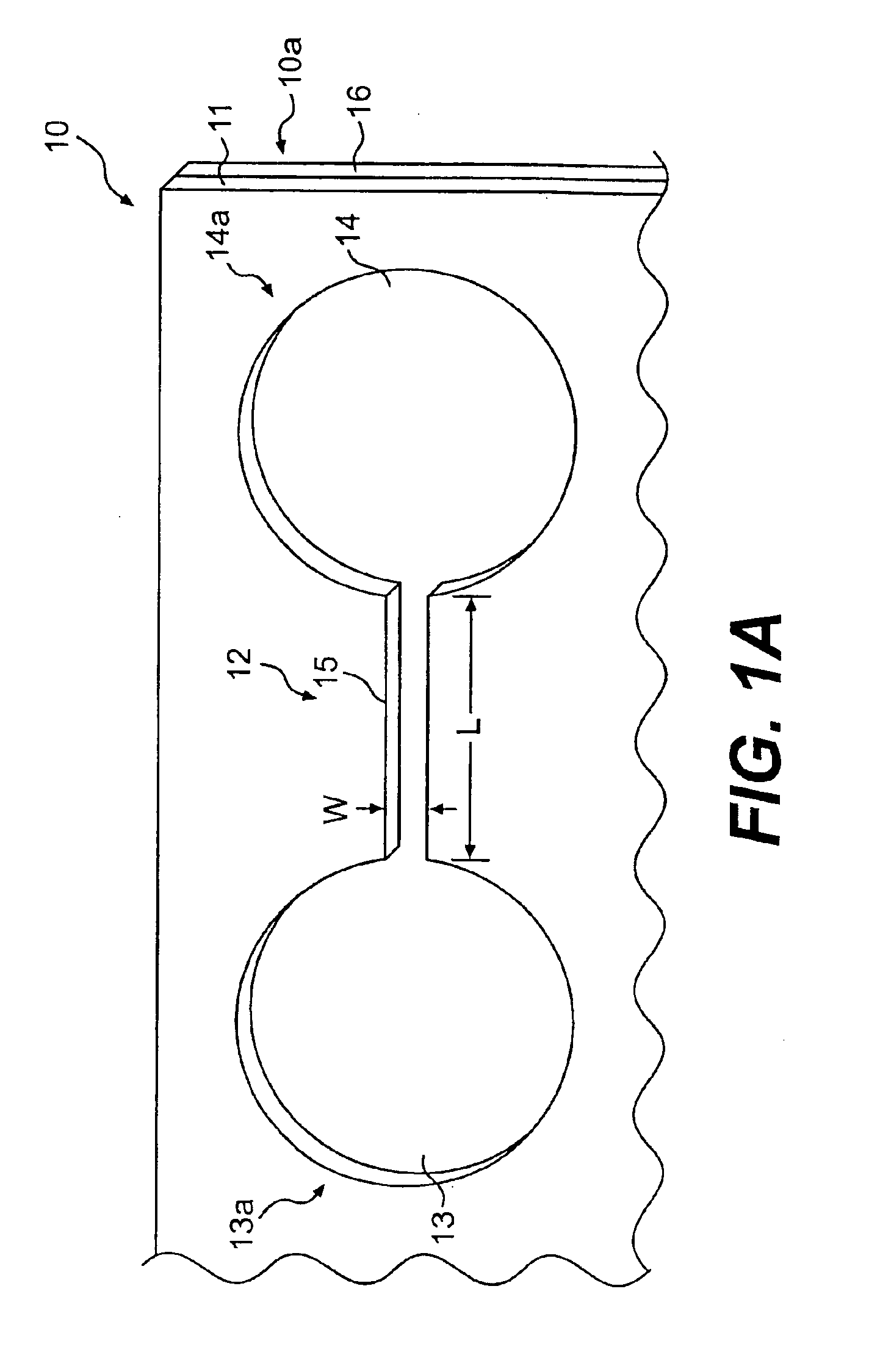
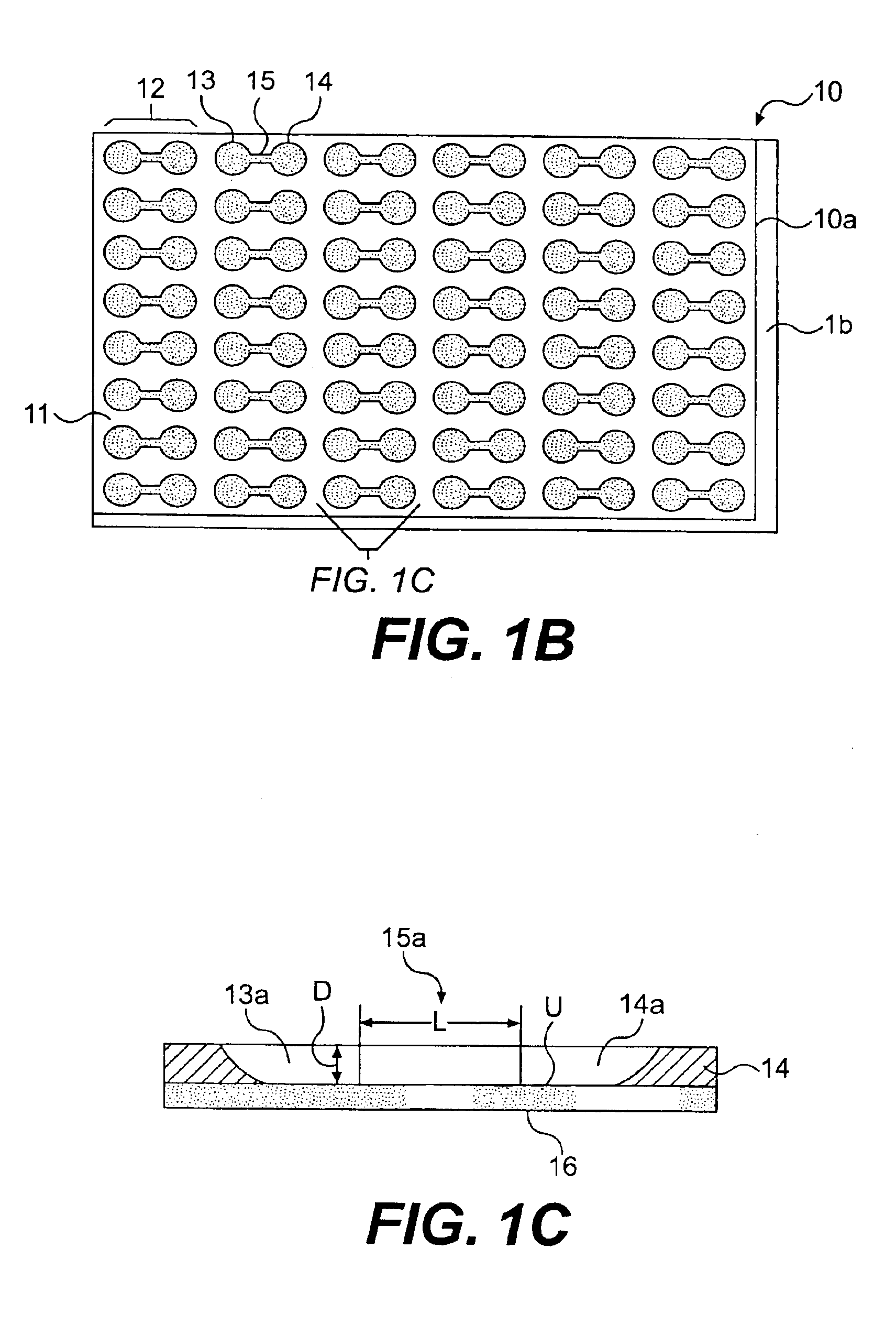
Cell motility and chemotaxis test device and methods of using same
InactiveUS6982171B2Reduce concentrationBioreactor/fermenter combinationsBiological substance pretreatmentsTest agentCell locomotion
The present invention discloses a device for monitoring chemotaxis or chemoinvasion including a housing comprising: a support member and a top member, the top member mounted to the support member by being placed in substantially fluid-tight conformal contact with the support member. The support member and the top member are configured such that they together define a discrete chamber adapted to allow a monitoring of chemotaxis or chemoinvasion therein. The discrete chamber includes a first well region including at least one first well, the at least one first well configured to received a test agent therein; a second well region including at least one second well, the at least one second well configured to receive a sample comprising cells therein; and a channel region including at least one channel connecting the first well region and the second well region with one another. The second well region is preferably horizontally offset with respect to the first well region in a test orientation of the device.
Owner:SURFACE LOGIX INC
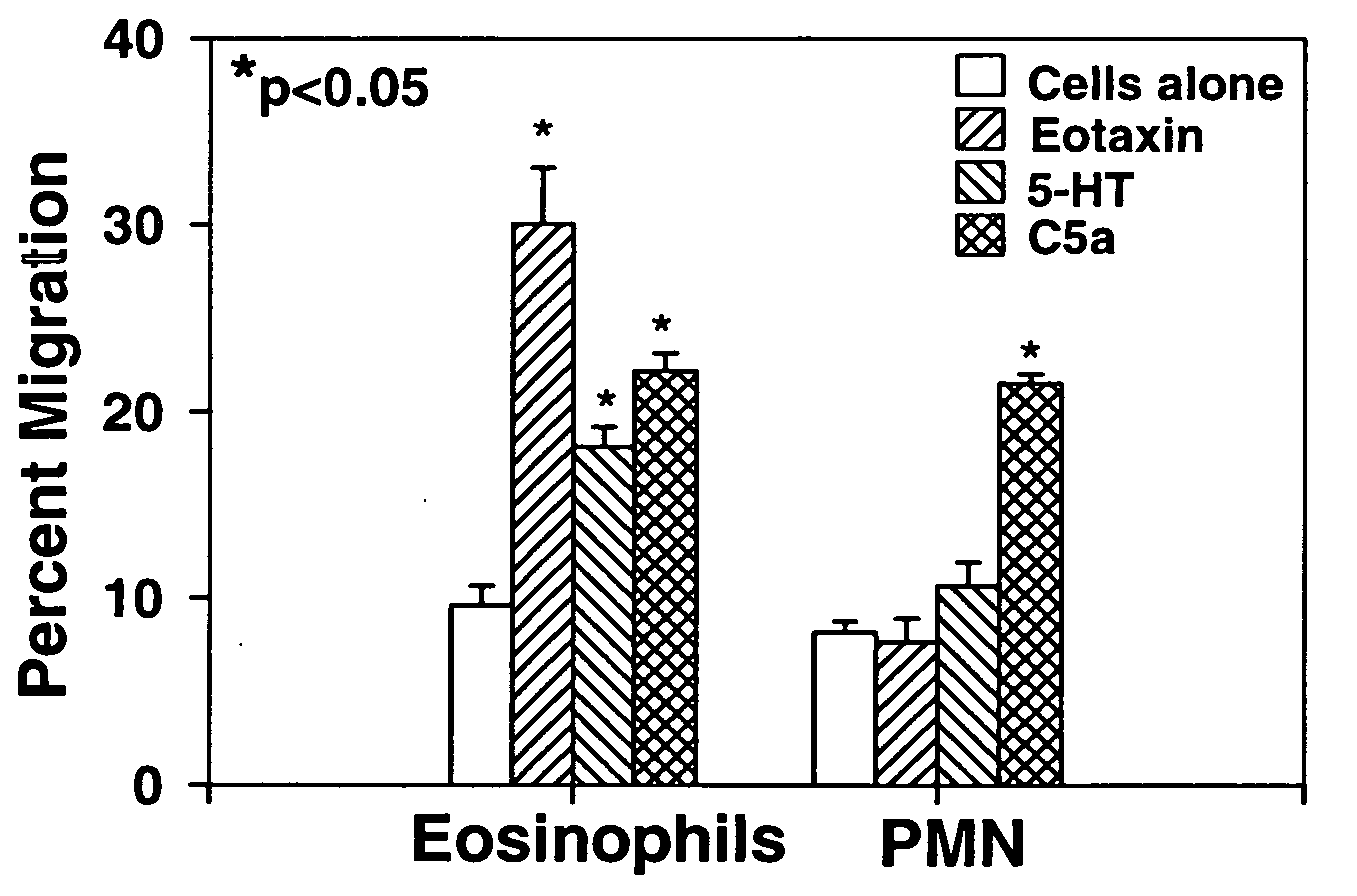
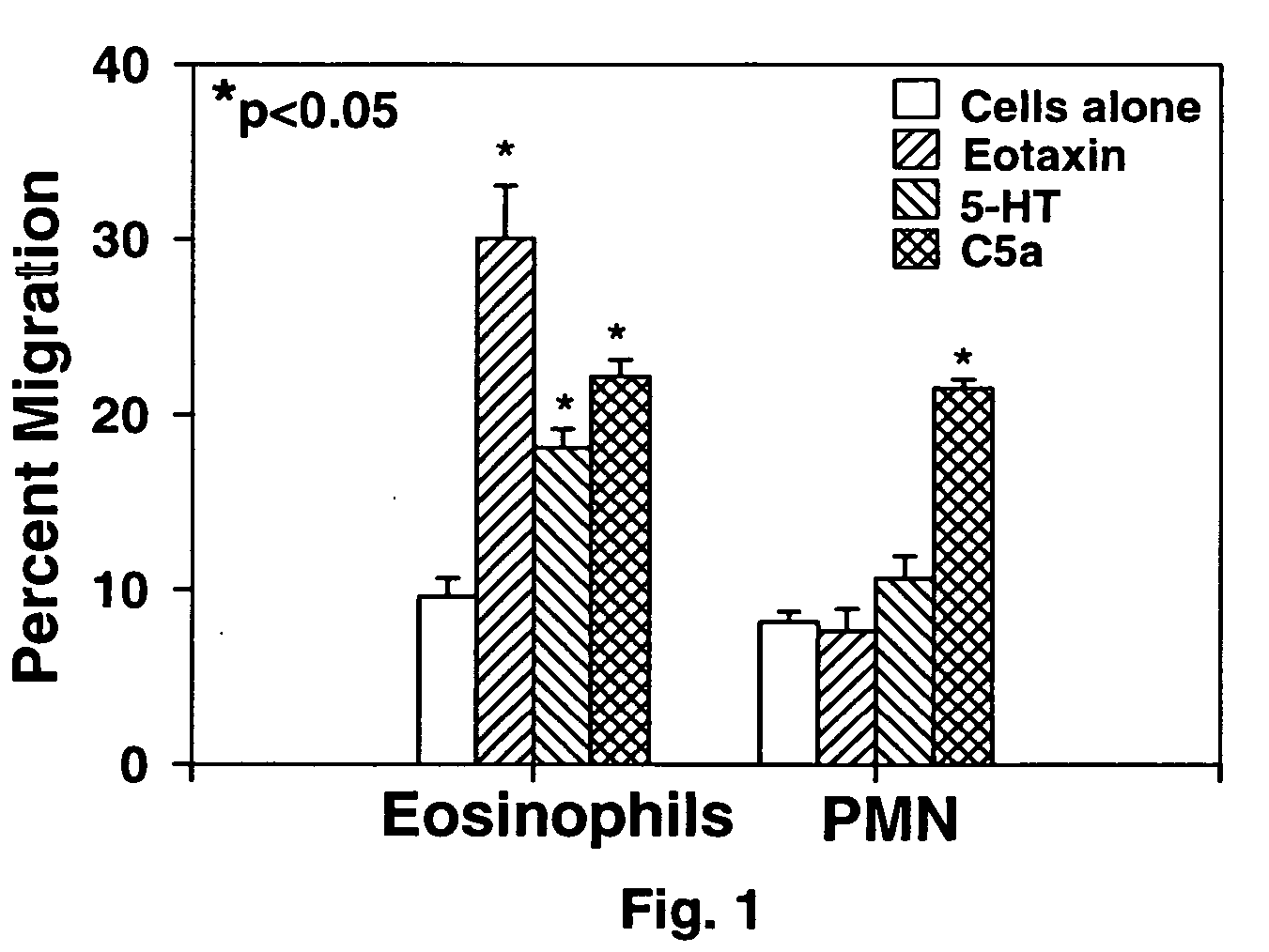
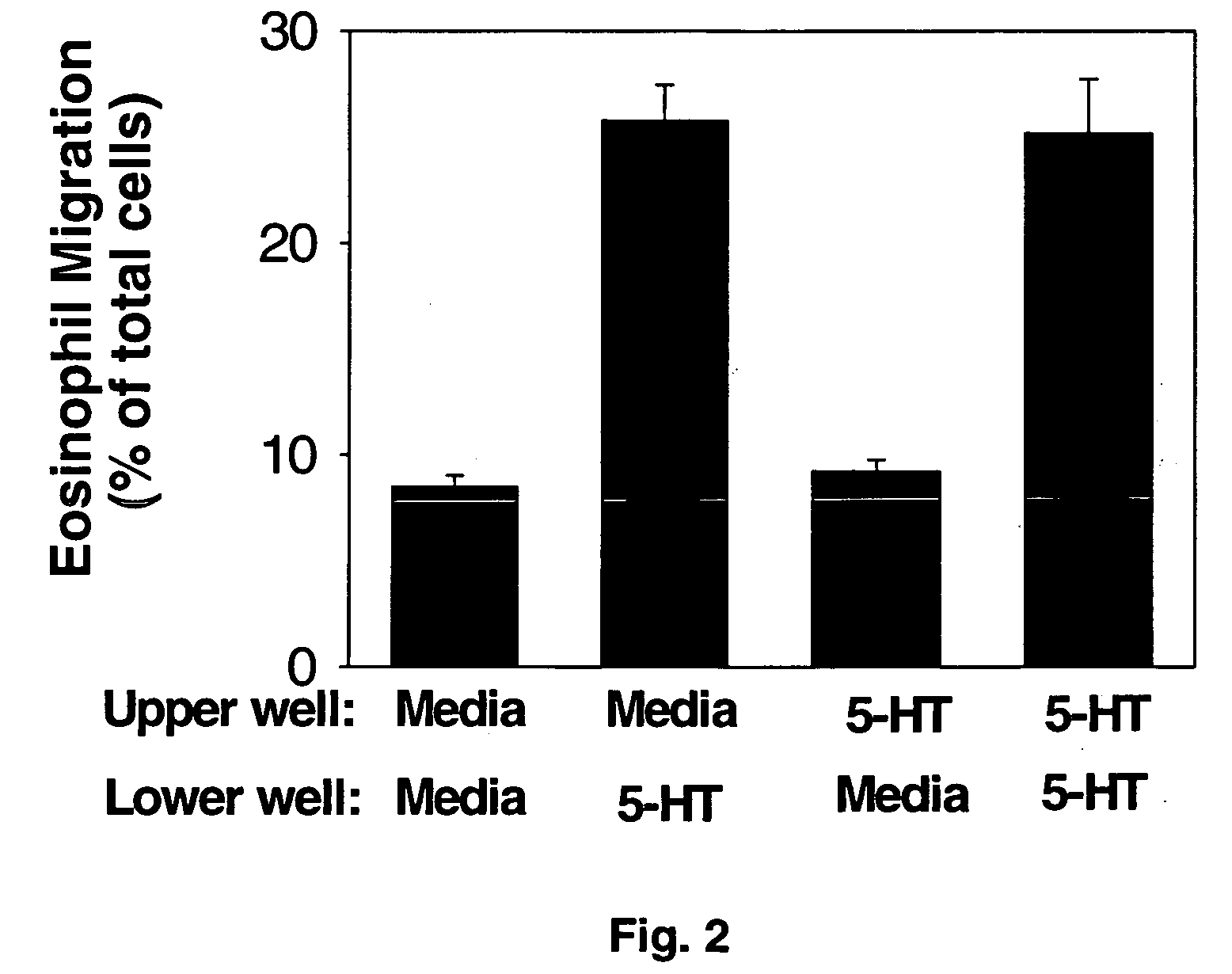
Eotaxin: an eosinophil chemoattractant
Disclosed is substantially pure eotaxin DNA sequence and eotaxin polypeptide, and methods of using such DNA and polypeptide to direct chemotaxis of eosinophils. Methods are provided for the treatment diseases and disorders such as inflammation and tumorigenesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Biological circuit chemotactic converters
ActiveUS20130034907A1Other foreign material introduction processesFermentationToggle switchModularity
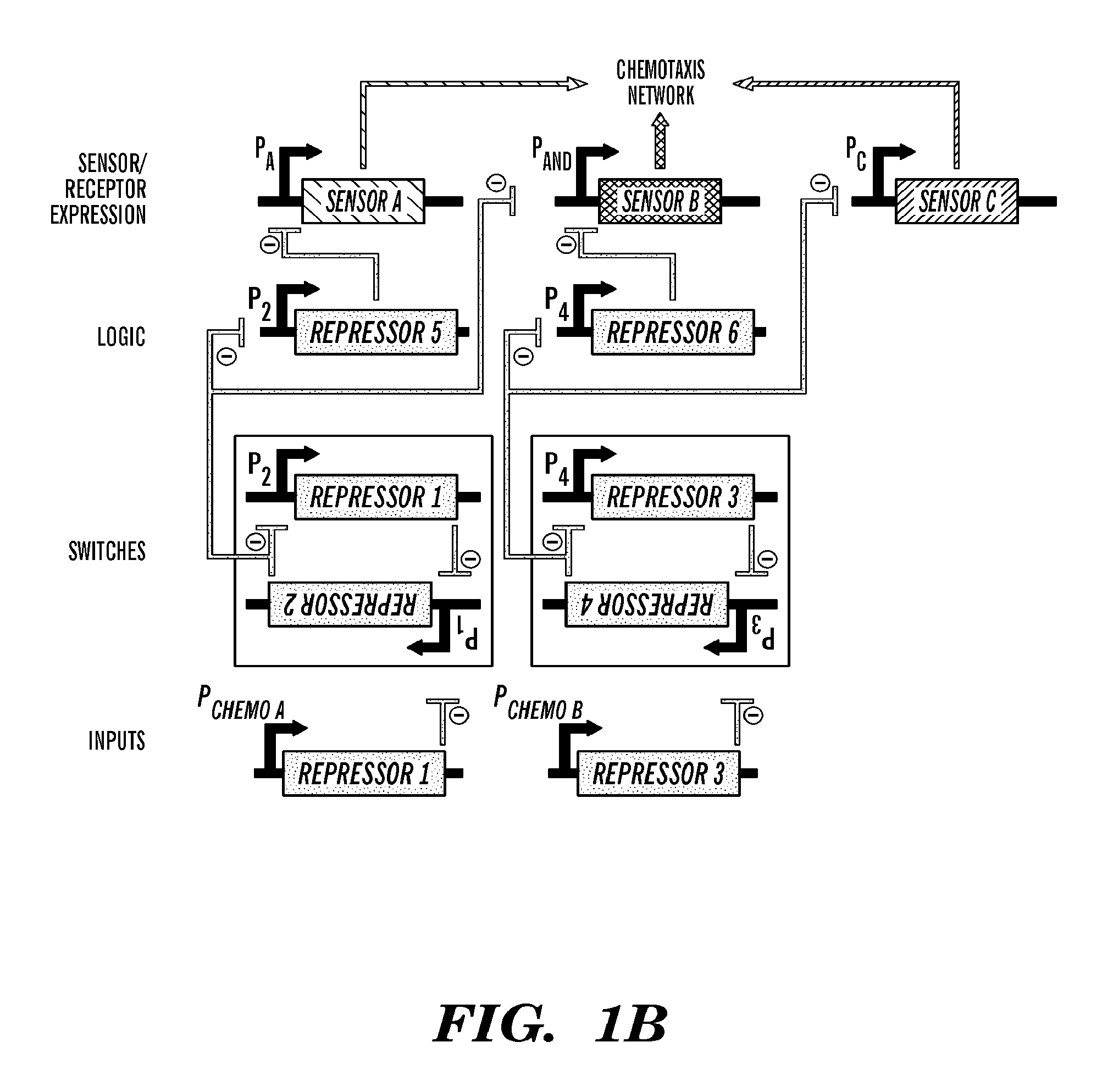
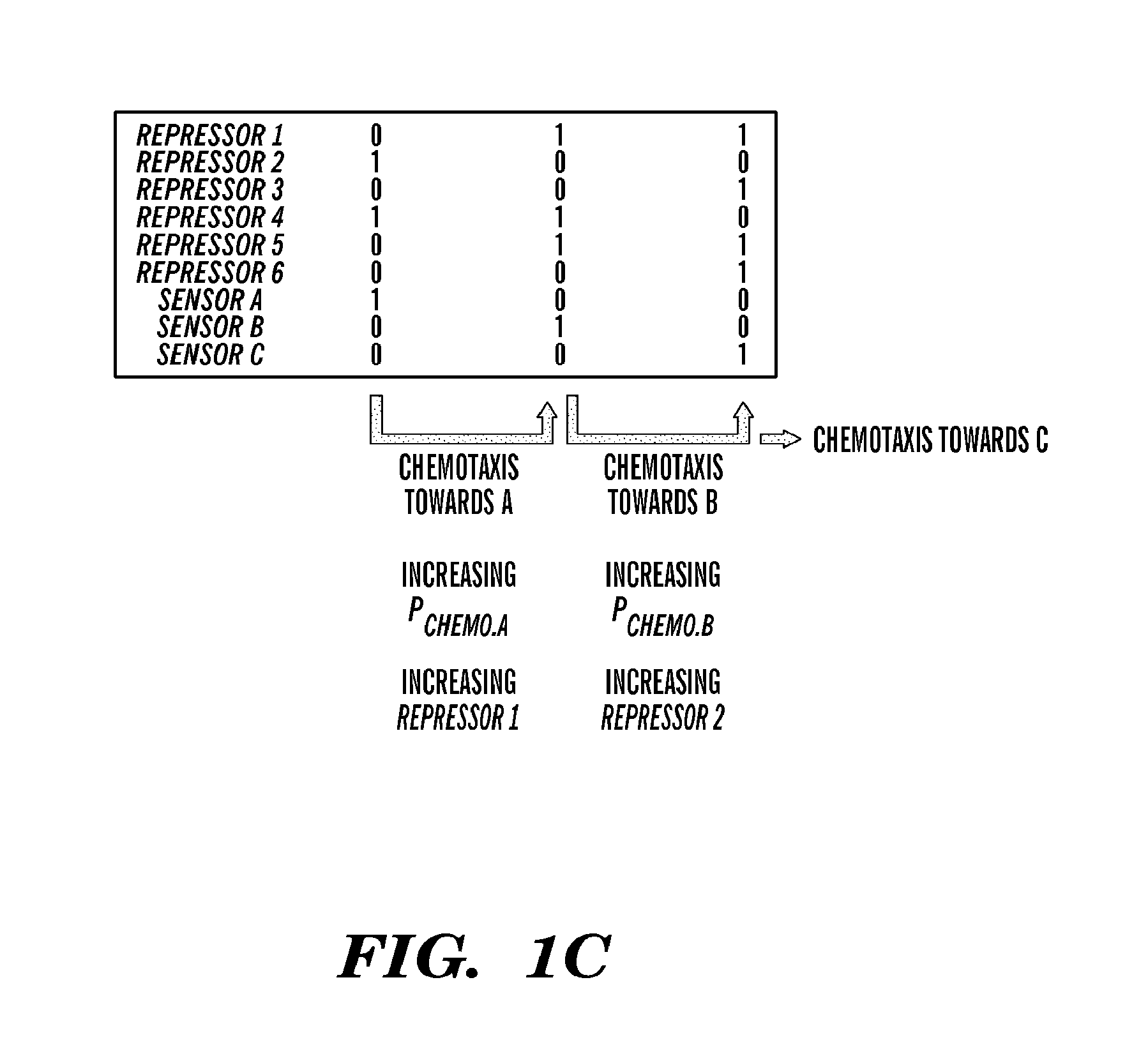
Described herein are novel biological circuit chemotactic converter that utilize modular components, such as genetic toggle switches and single invertase memory modules (SIMMs), for detecting and converting external inputs, such as chemoattractants, into outputs that allow for autonomous chemotaxis in cellular systems. Flexibility in these biological circuit chemotactic converter is provided by combining individual modular components, i.e., SIMMs and genetic toggle switches, together. These biological converter switches can be combined in a variety of network topologies to create network systems that regulate chemotactic responses based on the combination and nature of input signals received.
Owner:TRUSTEES OF BOSTON UNIV +1
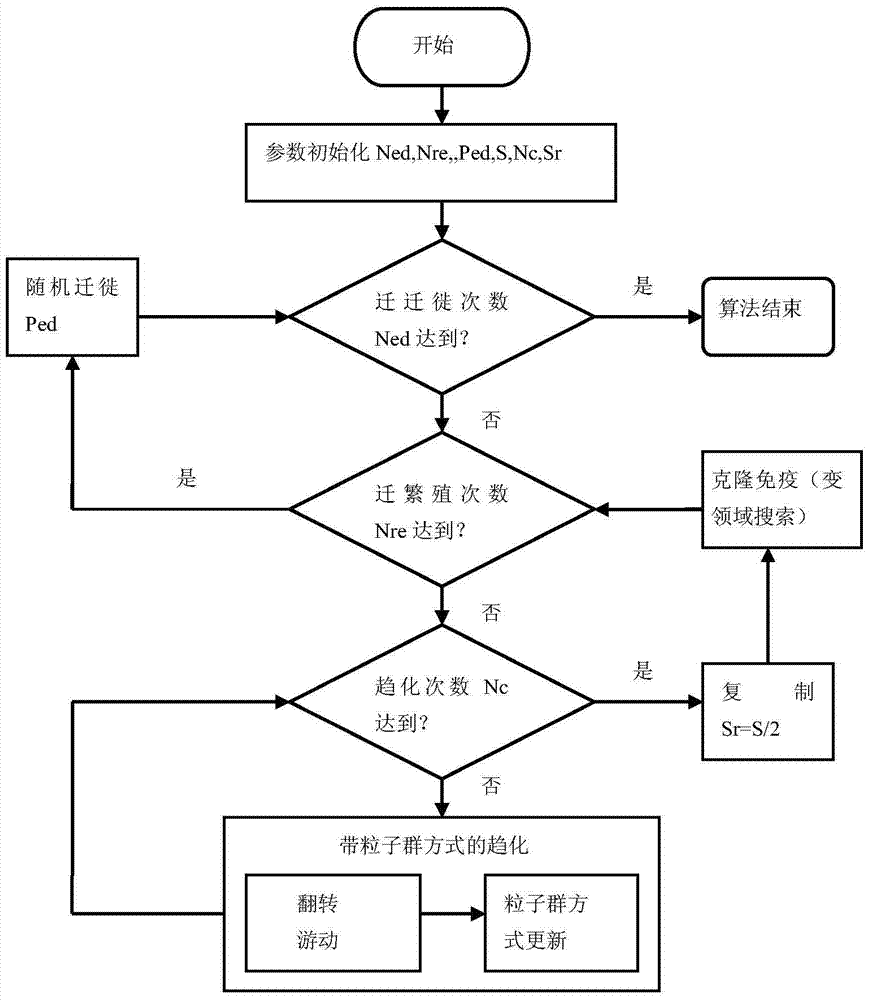
Container quay berth and quay crane distribution method based on bacterial foraging optimization method
InactiveCN103530709AImprove efficiencyShorten the timeGenetic modelsForecastingBacteria foraging algorithmParticle swarm algorithm
The invention discloses a container quay berth and quay crane distribution method based on a bacterial foraging optimization method. The method comprises the following steps: initializing and defining a solution space; defining a fitness function; randomly initializing the position and the speed of bacteria and selecting out the local and global optimal positions; allowing the bacteria to move in the solution space and performing chemotaxis circulation; after the chemotaxis times reach the set times, reproducing a certain proportion of individuals with high adaptive value to replace individuals with low adaptive value; performing cloning immunization on the individuals after reproduction; after the reproduction times reach the set times, performing individual migration; circulating. The invention has the benefits that the method is different from other single methods, is a new mixed algorithm combining a bacterial foraging algorithm, a particle swarm optimization, a cloning immunization algorithm and a variable field searching method, and has the advantages of the four algorithms. Through the adoption of the method, the efficiency of a wharf can be improved, resources are distributed reasonably, the congestion phenomenon is avoided, the information transfer time is shortened and the error rate of operation is reduced.
Owner:SHANGHAI MARITIME UNIVERSITY
Use of MDL-100,907 for treatment of allergic and eosinophil mediated diseases
Owner:THE LA JOLLA INST FOR MOLECULAR MEDICINE
Method of inhibiting leukocytes with human cxc chemokine receptor 3 antibody
InactiveUS20100061983A1Sugar derivativesPeptide/protein ingredientsExocytosisAntiendomysial antibodies
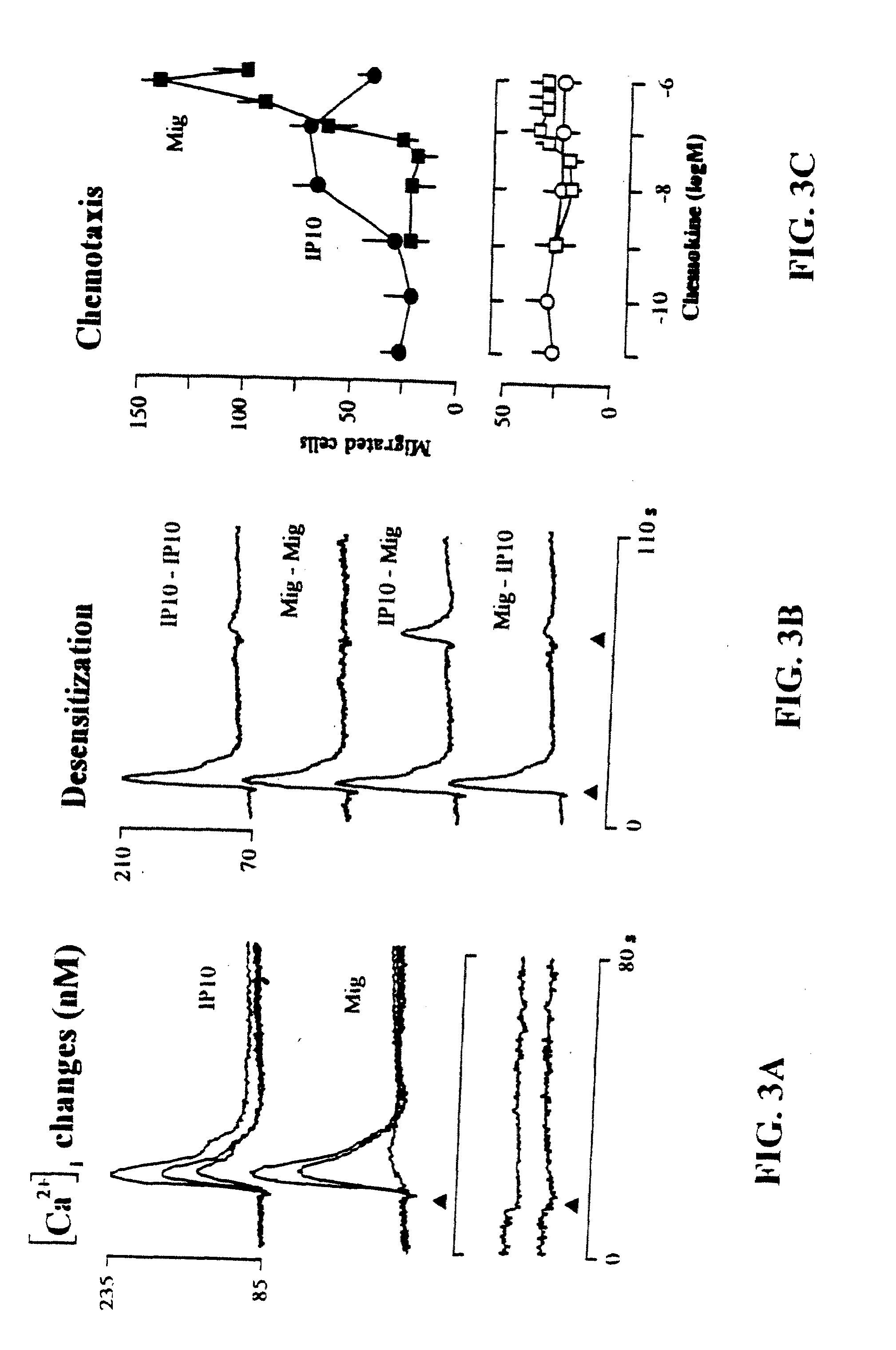
The present invention relates to proteins or polypeptides, referred to herein as isolated and / Or recombinant mammalian (e.g., human) IP-10 / Mig receptor proteins designated CXC Chemokine Receptor 3 (CXCR3) and variants thereof, including those characterized by selective binding of one or more chemokines (e.g., IP-10 and / or Mig), and / or the ability to induce a cellular response (e.g., chemotaxis, exocytosis). Antibodies reactive with CXCR3 receptors can be produced using the proteins or variants thereof or host cells comprising same as immunogen.
Owner:THEODOR KOCHER INST +1
Identification of allosteric peptide agonists of CXCR4
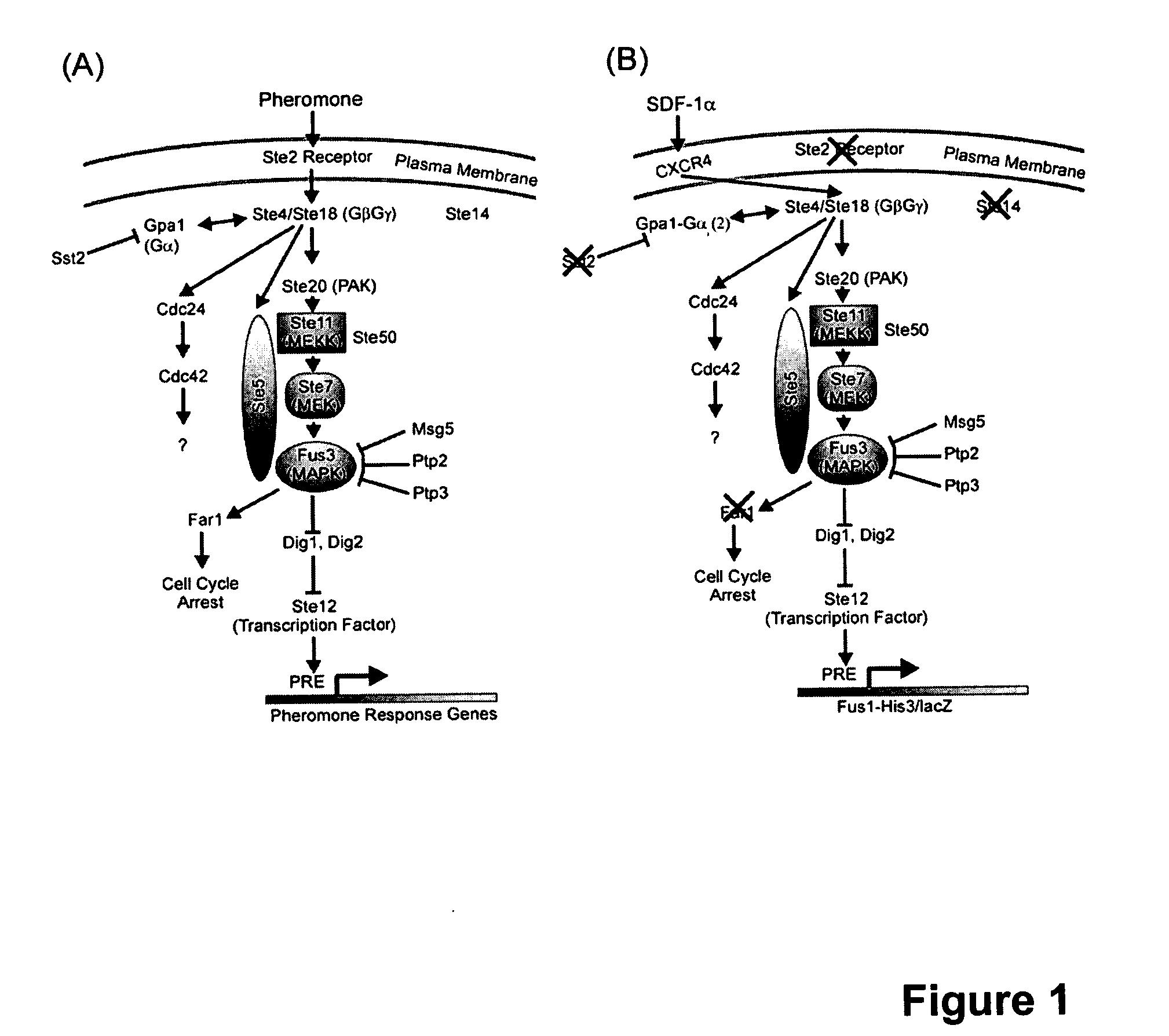
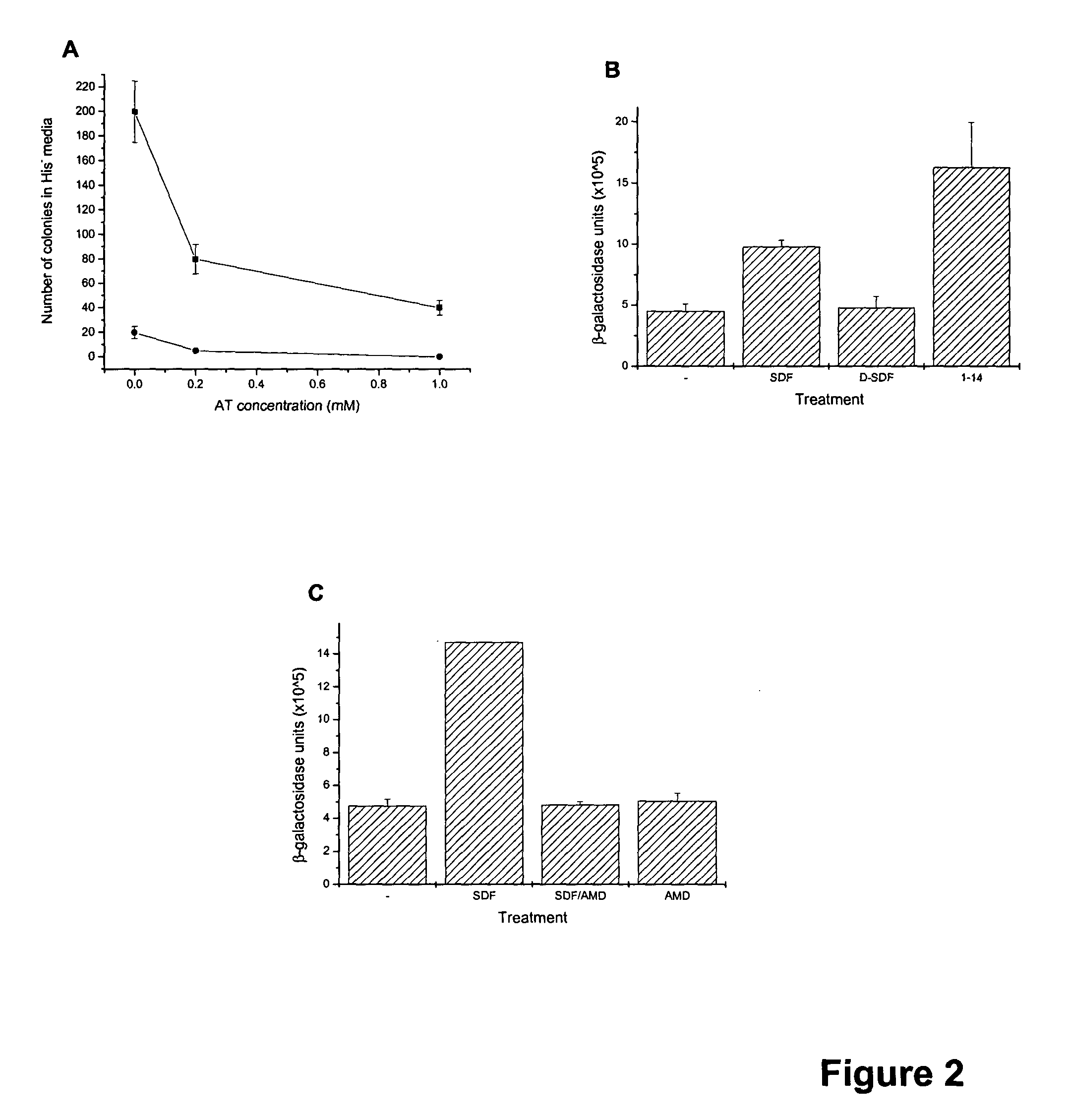
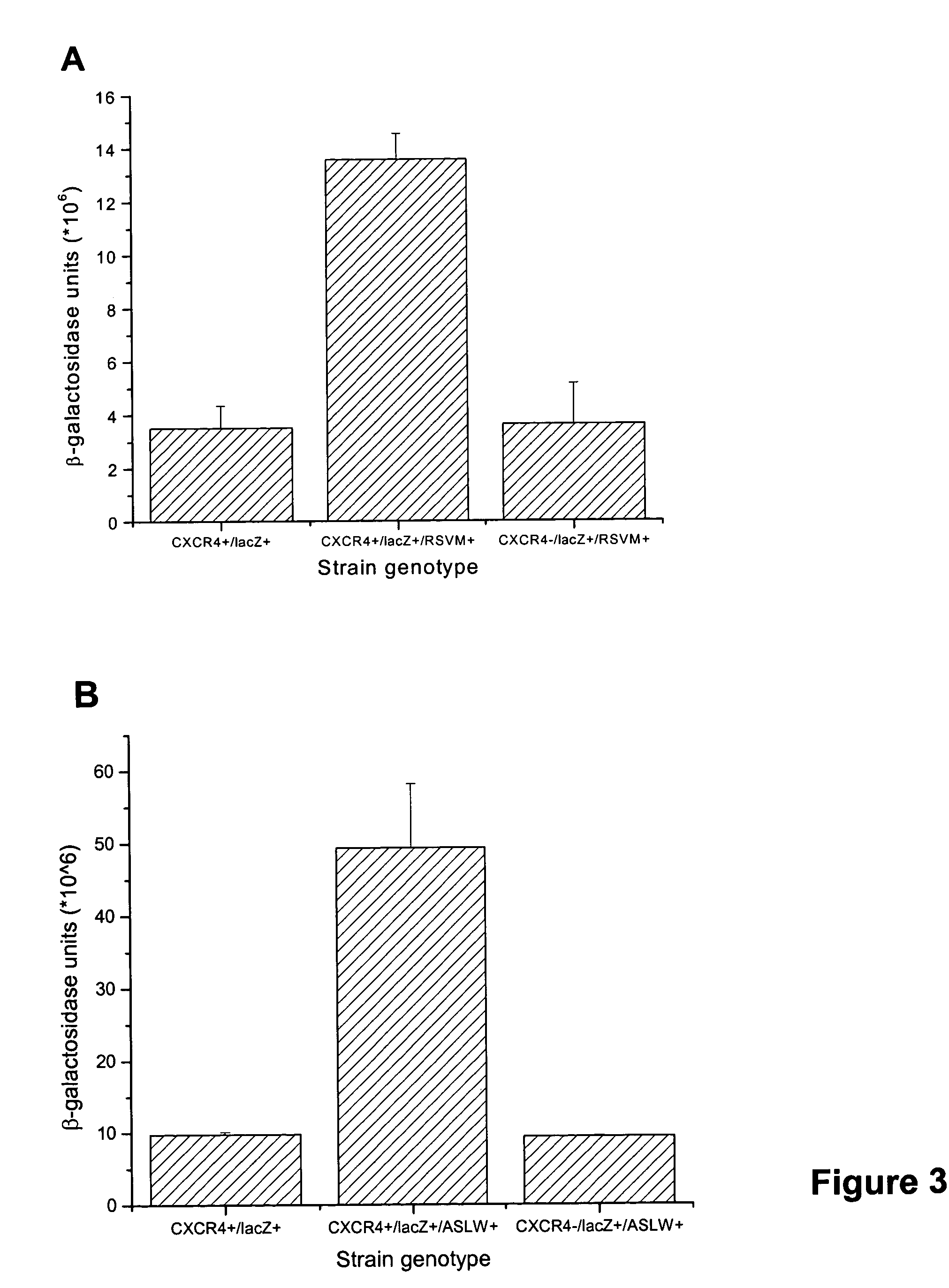
The chemokine receptor CXCR4 is a co-receptor for T-tropic strains of HIV-1. A number of small molecule antagonists of CXCR4 are in development, but all are likely to lead to adverse effects due to the physiological function of CXCR4. To prevent these complications, allosteric agonists may be therapeutically useful as adjuvant therapy in combination with small molecule antagonists. A synthetic cDNA library coding for 160,000 different SDF-based peptides was screened for CXCR4 agonist activity in a yeast strain expressing functional receptor. Peptides that activated CXCR4 in an autocrine manner induced colony formation. Two peptides, designated RSVM and ASLW, were identified as novel agonists that are insensitive to the CXCR4 antagonist AMD3100. In chemotaxis assays using the acute lymphoblastic leukemia cell line CCRF-CEM, RSVM behaves as a partial agonist and ASLW as a superagonist. The superagonist activity of ASLW may be related to its inability to induce receptor internalization. In CCRF-CEM cells, the two peptides are also not inhibited by another CXCR4 antagonist, T140, or the neutralizing monoclonal antibodies 12G5 and 44717.111. These results suggest that alternative agonist binding sites are present on CXCR4 that could be screened to develop molecules for therapeutic use.
Owner:LOLIS ELIAS +3
2-aryl-propionic acid and medicine composition containing same
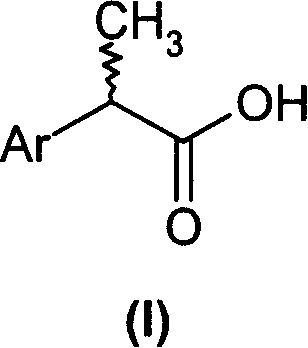
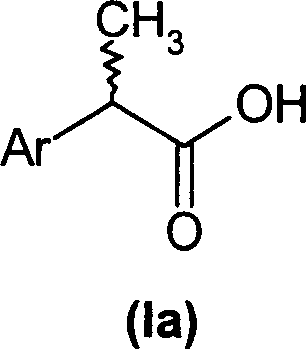
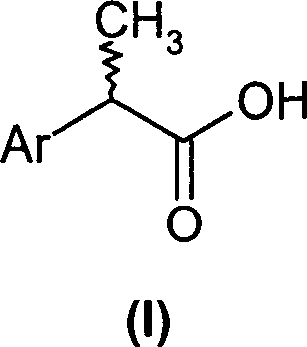
(R,S)-2-Aryl-propionic acids are useful as inhibitors of interleukin-8 induced human polymorphonucleated neutrophils (PMN) chemotaxis. (R,S)-2-Aryl-propionic acids of formula (I), their single (R) and (S) enantiomers or salts are useful as inhibitors of interleukin-8 (IL-8) induced human polymorphonucleated neutrophils (PMN) chemotaxis. [Image] Ar : phenyl ring (substituted by T in meta position, T1 in para position or T2 in ortho position); T : linear or branched 1-5C alkyl, 2-5C alkenyl, or 2-5C alkynyl (optionally substituted by 1-5C alkoxycarbonyl, optionally substituted phenyl, linear or branched 1-5C hydroxyalkyl or arylhydroxymethyl); T1benzoyloxy, benzoylamino, benzenesulfonyloxy, benzenesulfonylamino, benzenesulfonylmethyl (all optionally substituted), 1-5C acyloxy, 1-5C acylamino, 1-5C sulfonyloxy, 1-5C alkanesulfonylamino, 1-5C alkanesulfonylmethyl, 3-6C cycloalkyl, 2-furyl, 3-tetrahydrofuryl, 2- thiophenyl, 2-tetrahydrothiophenyl or ((1-8C)-alkanoyl, -cycloalkanoyl or -arylalkanoyl)-1-5C-alkylamino (preferably acetyl-N-methyl-amino or pivaloyl-N-ethyl-amino); and T2arylmethyl, aryloxy or acylamino (all optionally substituted by 1-4C alkyl, 1-4C-alkoxy, chlorine, fluorine or trifluoromethyl). The meta linear or branched 1-5C alkyl together with a substituent in ortho or para position and the benzene ring forms optionally saturated or optionally substituted bicyclo aryls. INDEPENDENT CLAIM are included for the following: (1) preparation of (I); and (2) use of (I) in the preparation of a medicament for the treatment of e.g. psoriasis, ulcerative colitis, melanoma and chronic obstructive pulmonary disease (COPD). - ACTIVITY : Antipsoriatic; Antiulcer; Respiratory-Gen.; Antirheumatic; Antiarthritic; Nephrotropic; Vasotropic; Antiinflammatory; Gastrointestinal-Gen.; Cytostatic. - MECHANISM OF ACTION : Interleukin-8 (IL-8) inhibitor; GROalpha inhibitor; CXCR2 agonist / antagonist. The ability of (R,S) 2-[(3'-isopropyl)phenyl]propionic acid (A) to inhibit IL-8 induced chemotaxis of human monocytes was tested as described in Van Damme J. et al. (Eur. J. Immunol., 19, 2367, 1989). (A) showed inhibition (%) of 51+-12.
Owner:DOMPE FARM SPA
High ferro and low frequency oscillation suppression method based on sliding mode control
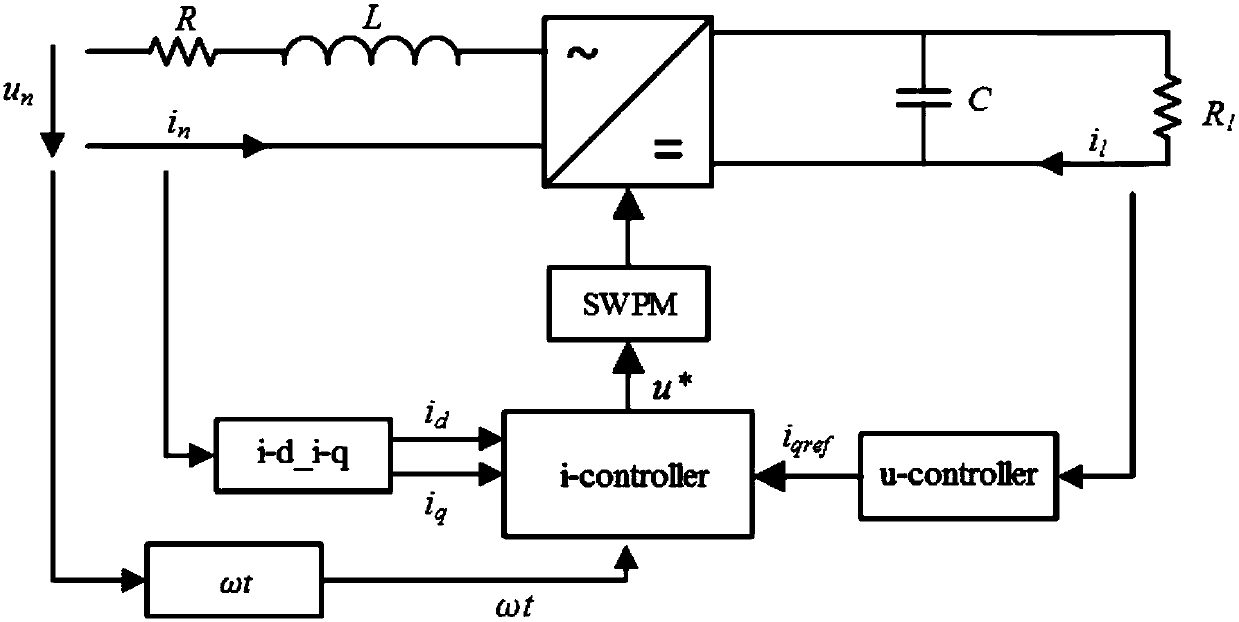
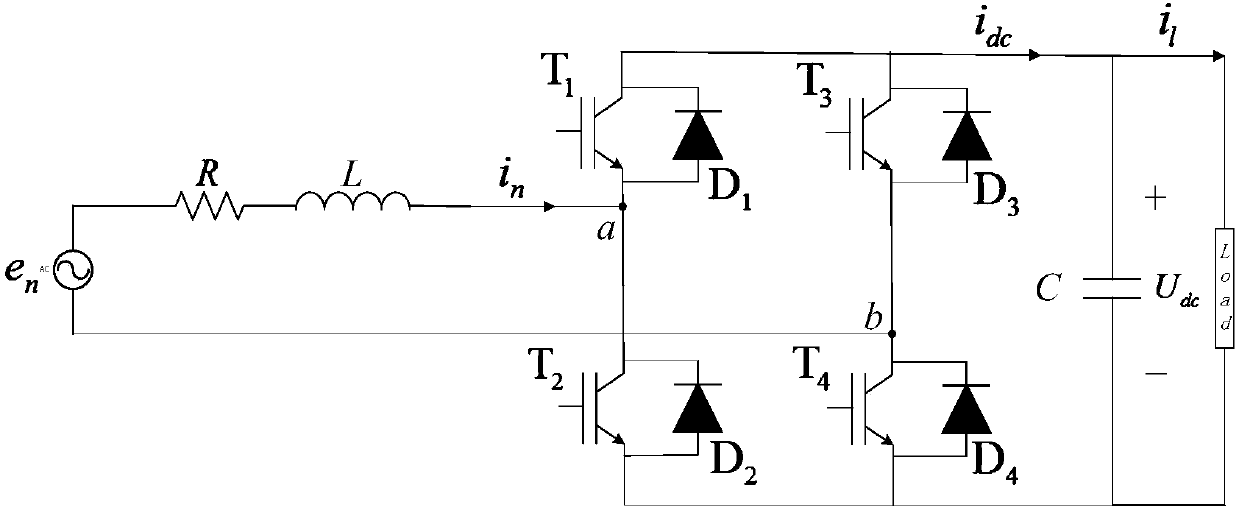
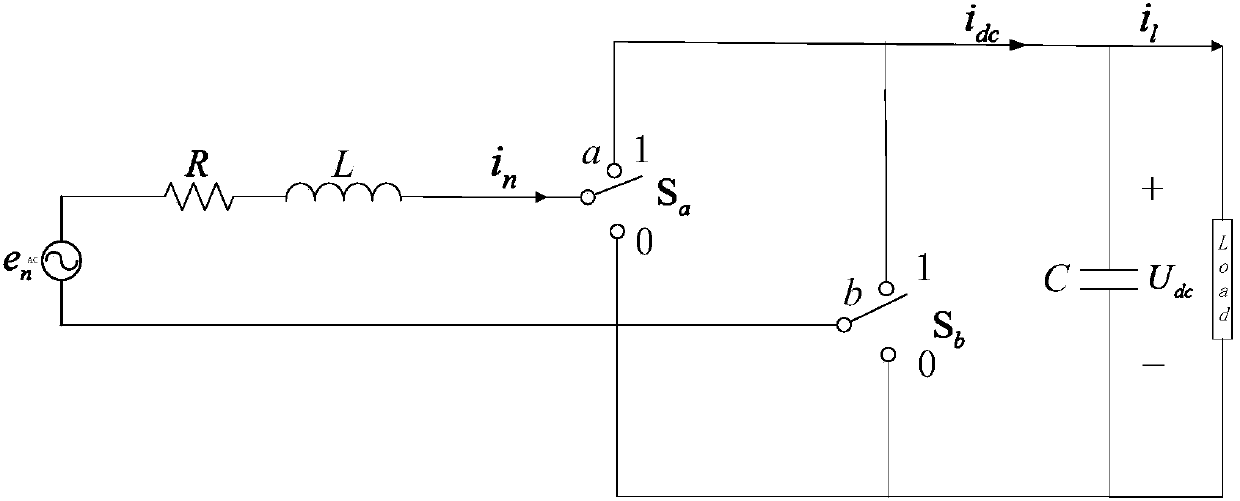
InactiveCN107565832AThe Problem of Damping Electric Quantity OscillationImprove robustnessAc-dc conversion without reversalAc network circuit arrangementsVoltage controlSurface expression
The invention discloses a high ferro and low frequency oscillation suppression method based on sliding mode control, comprising the steps of defining grid-side current orthogonal and establishing a state space model of a motor-car networking side rectifier under a dq rotating coordinate; selecting the output of a control system with the combination of a control object, and establishing two sliding-mode surfaces based on outer-ring voltage control, and obtaining a reference value of grid-side reactive component of current, and completing the calculating of a sliding-mode surface expression; selecting sliding mode control tendency rate for the two sliding mode surfaces, and substituting the two sliding mode surfaces into the sliding mode control tendency rate, and substituting the result into the state space model of a motor-car networking side rectifier under the dq rotating coordinate, obtaining a switch function, and multiplying the switch function with DC side voltage, obtaining control voltage; obtaining an Alfa Belta coordinate system component through the coordinate transformation of control voltage, and outputting control impulse through sine pulse width modulation. The invention is advantageous in that dynamic response and robustness of the rectifier can be effectively improved, and the low frequency oscillation of motor-car electrical quantities can be suppressed.
Owner:SOUTHWEST JIAOTONG UNIV
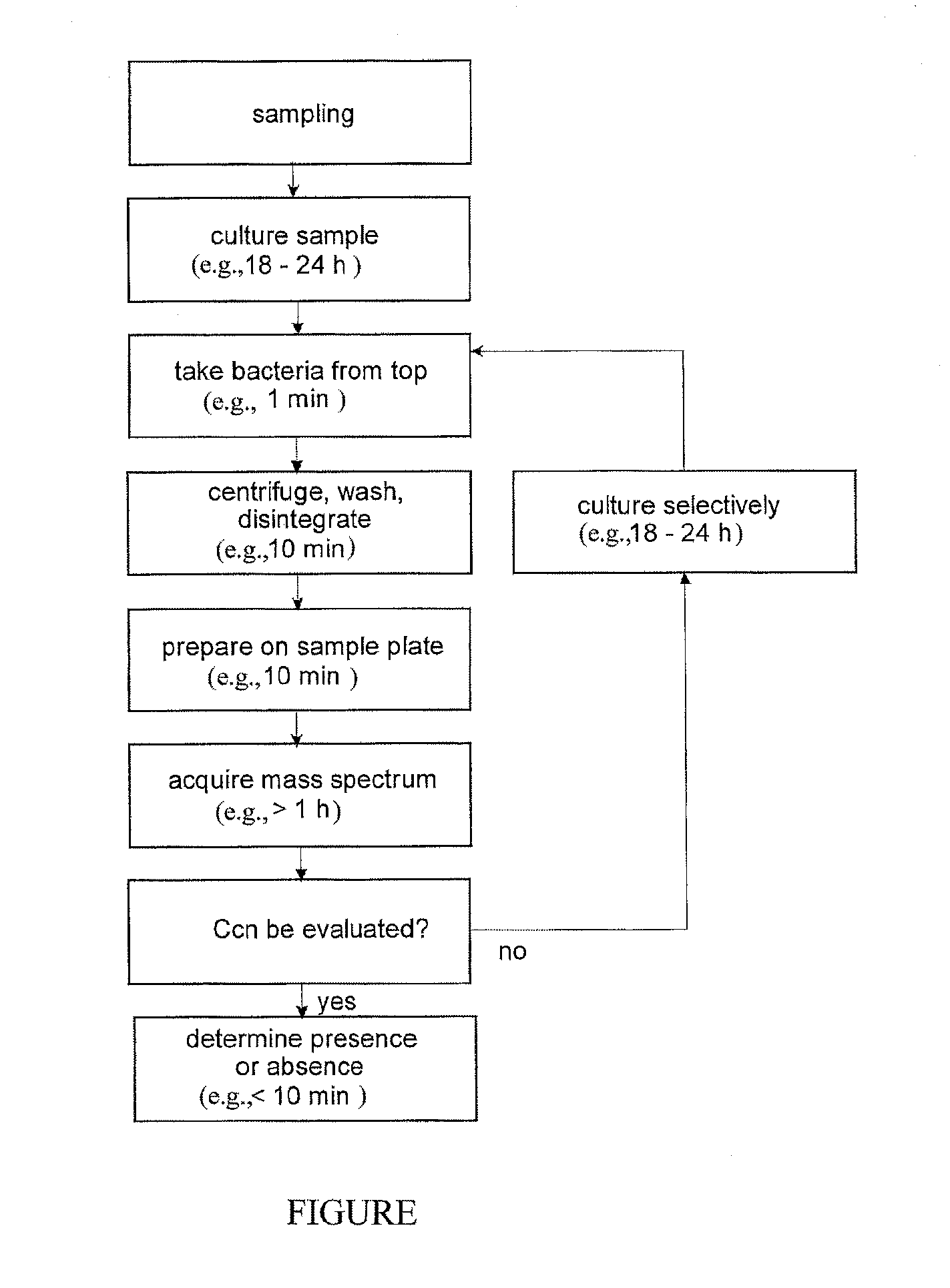
Mass spectrometric rapid detection of salmonella
ActiveUS20120107864A1Microbiological testing/measurementMass spectrometric analysisMicroorganismFeces
The invention relates to the detection of specified, flagellated bacteria, particularly Salmonella, in food and stool. A single culturing period of about 12 to 24 hours in a liquid nutrient medium without agitation is combined with a position-selective sampling of the flagellated microbes from the liquid of the culture, after which a mass spectrometric detection method is used which recognizes the target bacteria in mixtures. A second culture step is only necessary in exceptional cases. A species-selective or genus-selective culture medium is advantageous. Positional selection becomes possible because these bacteria use their flagella to counteract sedimentation by chemotaxis, and they collect near the surface. This provides a low-cost detection method that is several days faster than conventional methods
Owner:BRUKER DALTONIK GMBH & CO KG
Preparation method and application of a new tumor dendritic cell therapeutic vaccine
ActiveCN102258772APrevent relapseAvoid diversionBlood/immune system cellsAntibody medical ingredientsAbnormal tissue growthTumor therapy
The invention provides a preparation method and application of a new therapeutic vaccine for dendritic tumor cells. The therapeutic tumor vaccine is chemotherapeutic drug induced tumor antigen, wherein, the dendritic cells are loaded and activated. The chemotherapeutic medicament induced tumor antigen contains a plurality of immunostimulation molecules as well as tumor-associated and specific antigen, which can remarkably carry out chemotaxis and activation on immunocytes such as the dendritic cells, T-cells (thymus-dependent lymphocytes) and the like, stimulate the dendritic cells to be mature and express a plurality of cell factors and chemotactic factors, effectively induce immune response reaction with antigenic specificity and non-specificity and strengthen body immune function. The therapeutic vaccine provided by the invention can be used for preventing and treating tumors and has the characteristics of simple preparation process, low cost, strong specificity, obvious curative effect and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Isolation of endothelial progenitor cell subsets and methods for their use
InactiveUS20060035290A1Artificial cell constructsMammal material medical ingredientsFluorescenceAngiogenesis growth factor
A method is provided for the isolation of endothelial progenitor cells from a source of progenitor cells by isolating a population of lineage-negative cells and further isolating CD34+ cells from the lineage-negative population by fluorescence-activated cell sorting. Isolated populations of endothelial progenitor cells and therapeutic compositions containing CD34+ cells for the induction of blood vessels, induction of angiogenic responses in surrounding blood vessels and the chemotaxis of inflammatory cells are also provided.
Owner:MEDTRONIC INC
Microfluidic device for cell motility screening and chemotaxis testing
InactiveUS20130244270A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMotilityCell locomotion
Owner:CAPITALBIO CORP +1
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20160251434A1Strong upregulationImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsDc maturationApoptosis
Novel activating receptors of the lg super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:NOVO NORDISK AS
Compositions and methods for treating progressive myocardial injury due to a vascular insufficiency
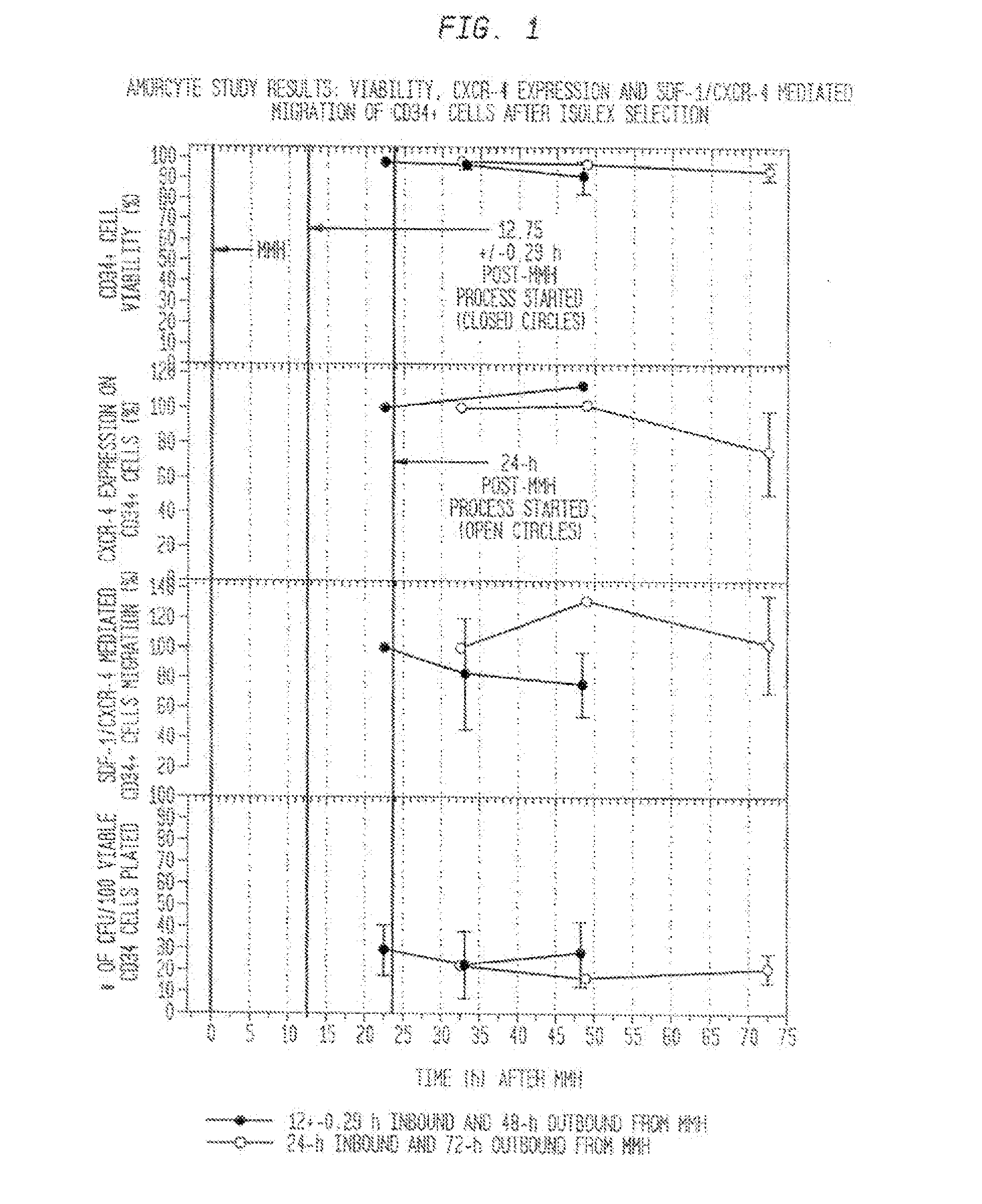
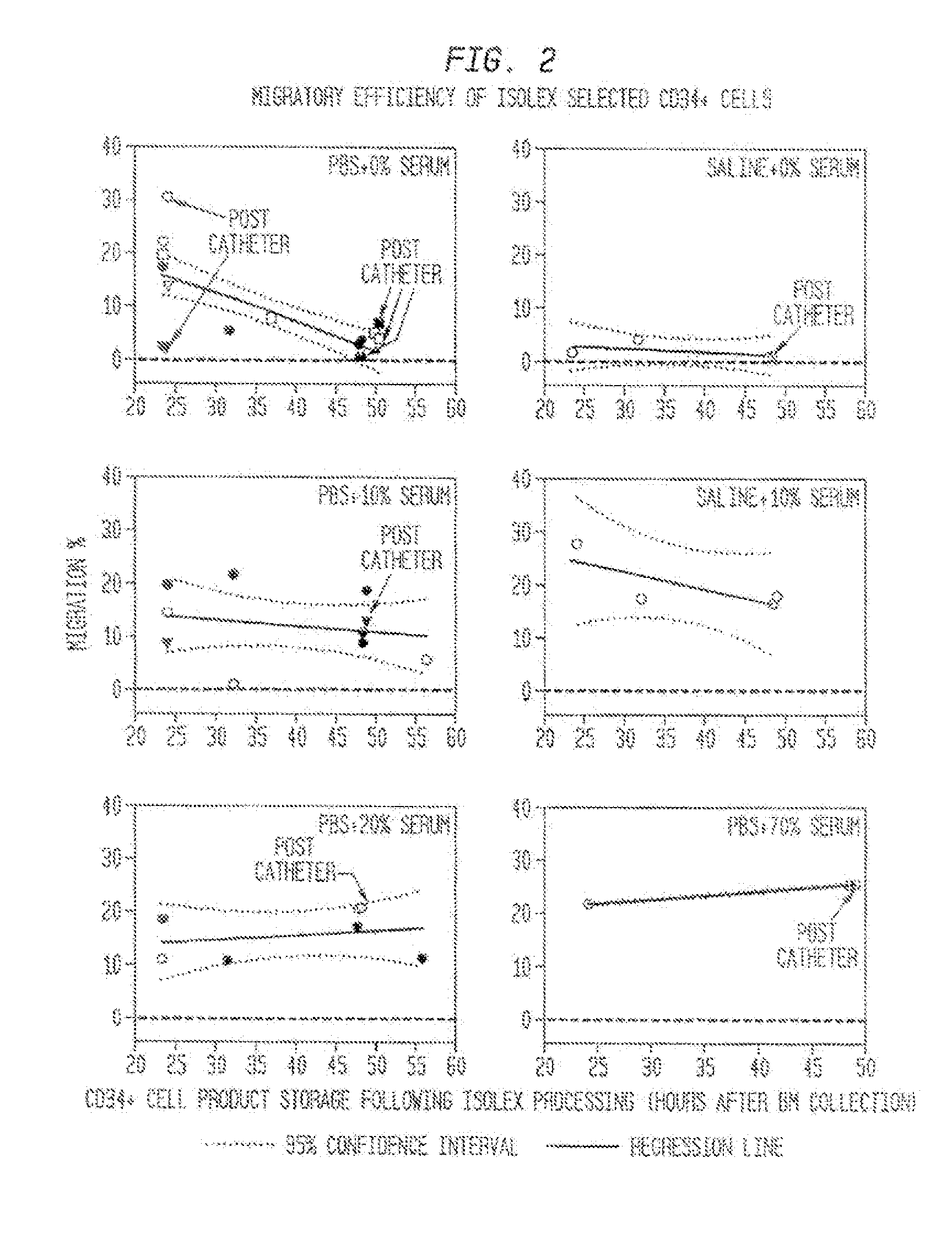
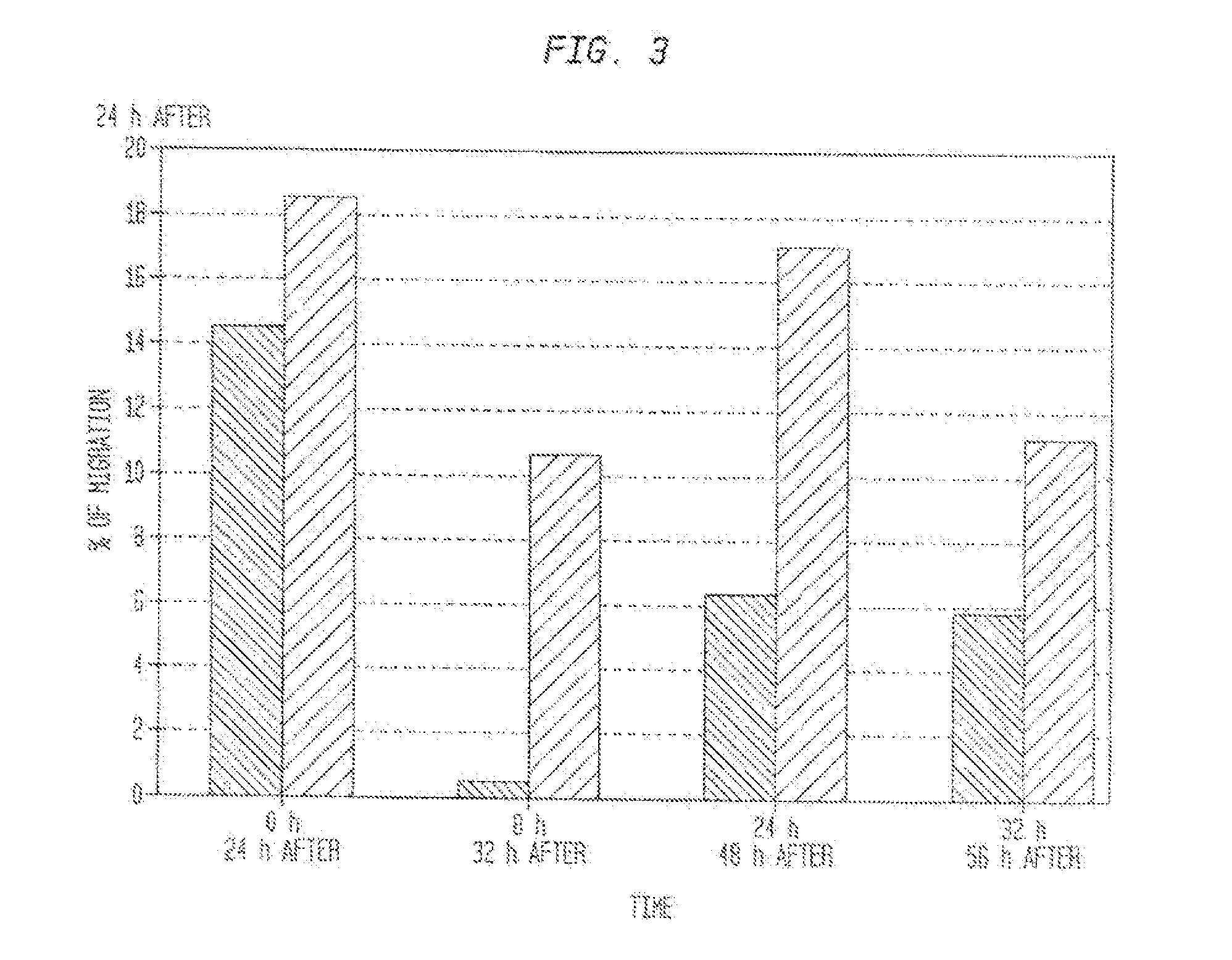
The described invention provides methods and regimens for treating adverse consequences of a persistent and progressive myocardial injury—due to a vascular insufficiency that occurs early or late in a subject in need thereof, and progressive myocardial injury-preventing compositions that contain a chemotactic hematopoietic stem cell product, and, optionally, an additional active agent.
Owner:AMORCYTE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com