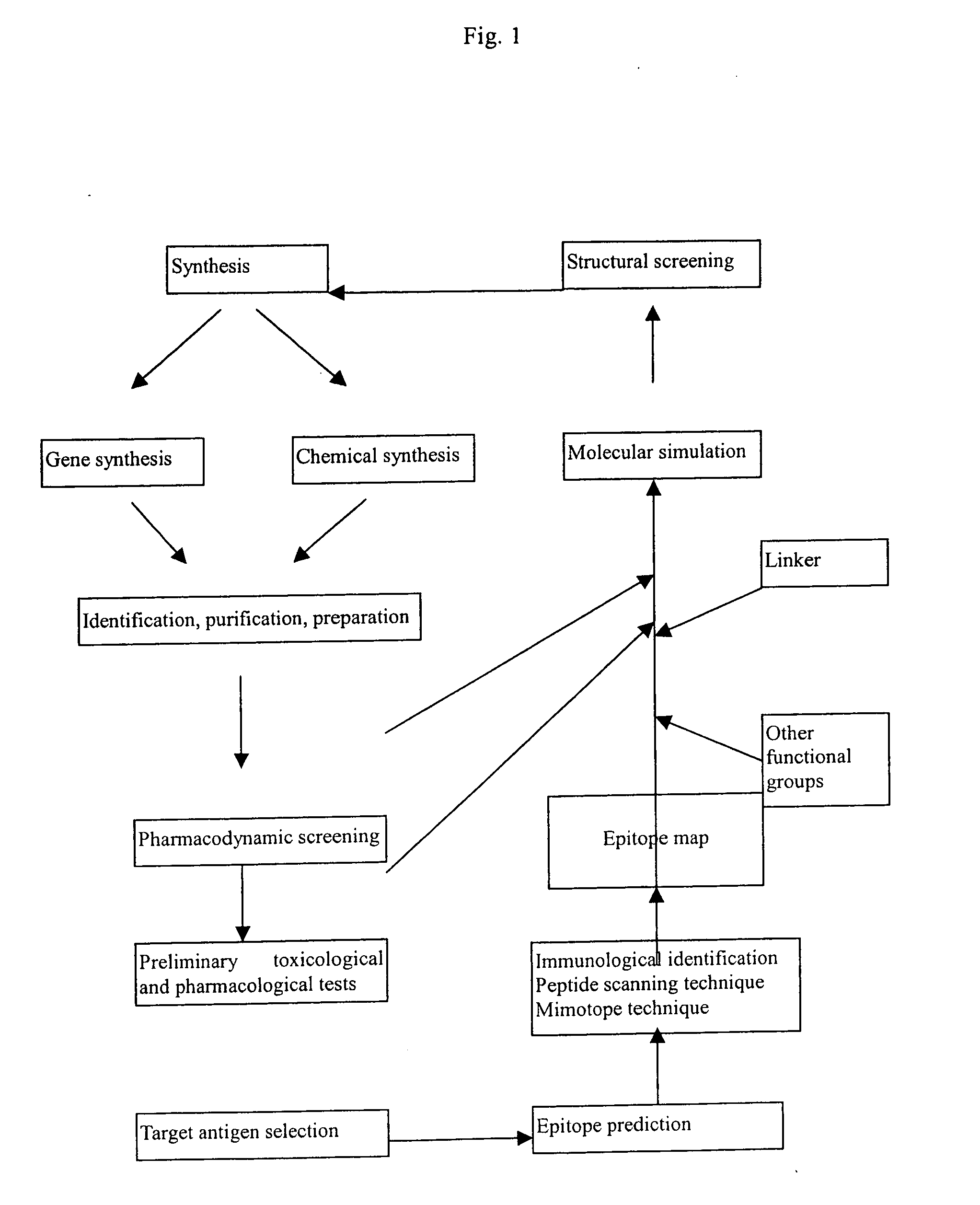
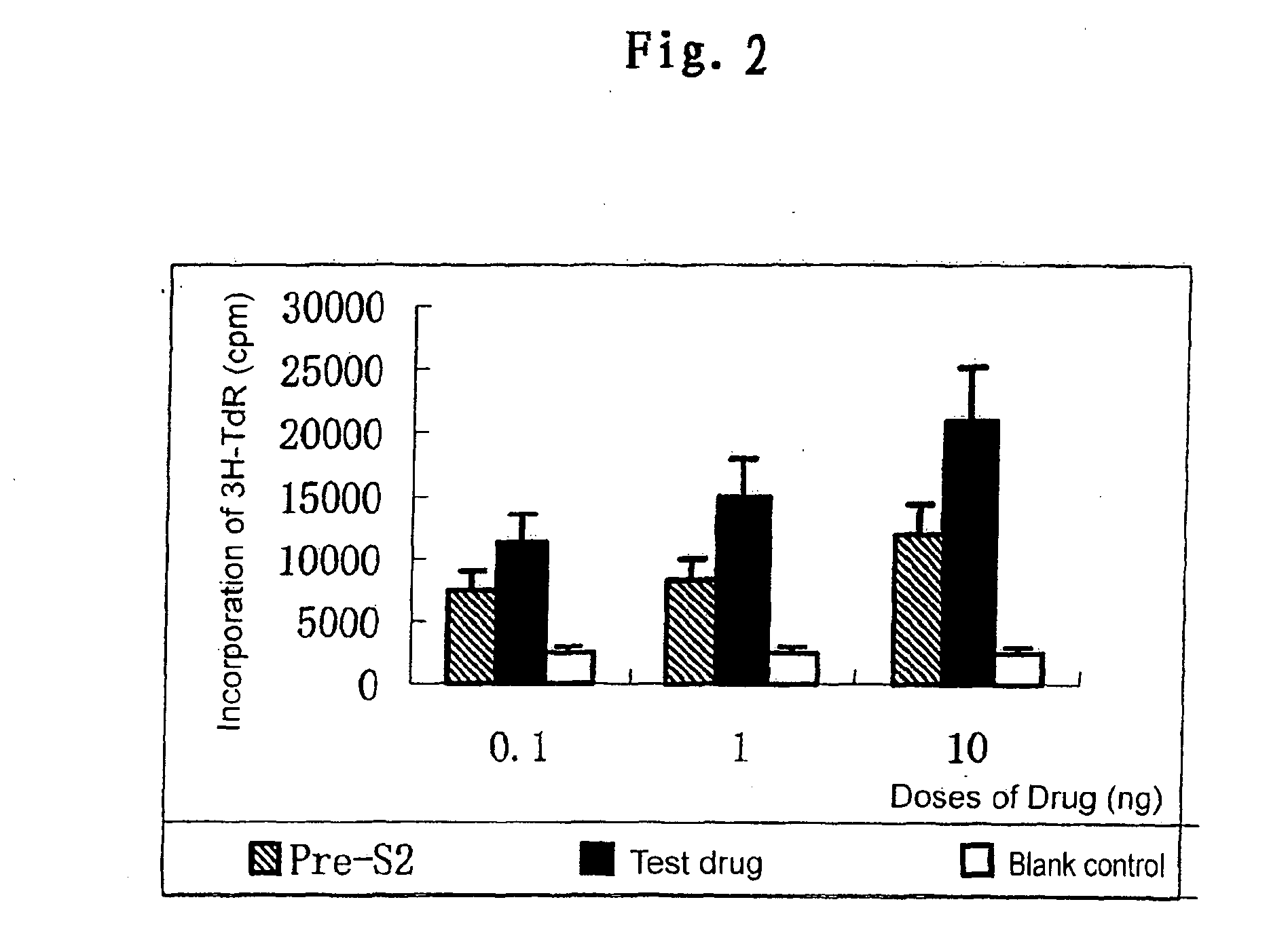
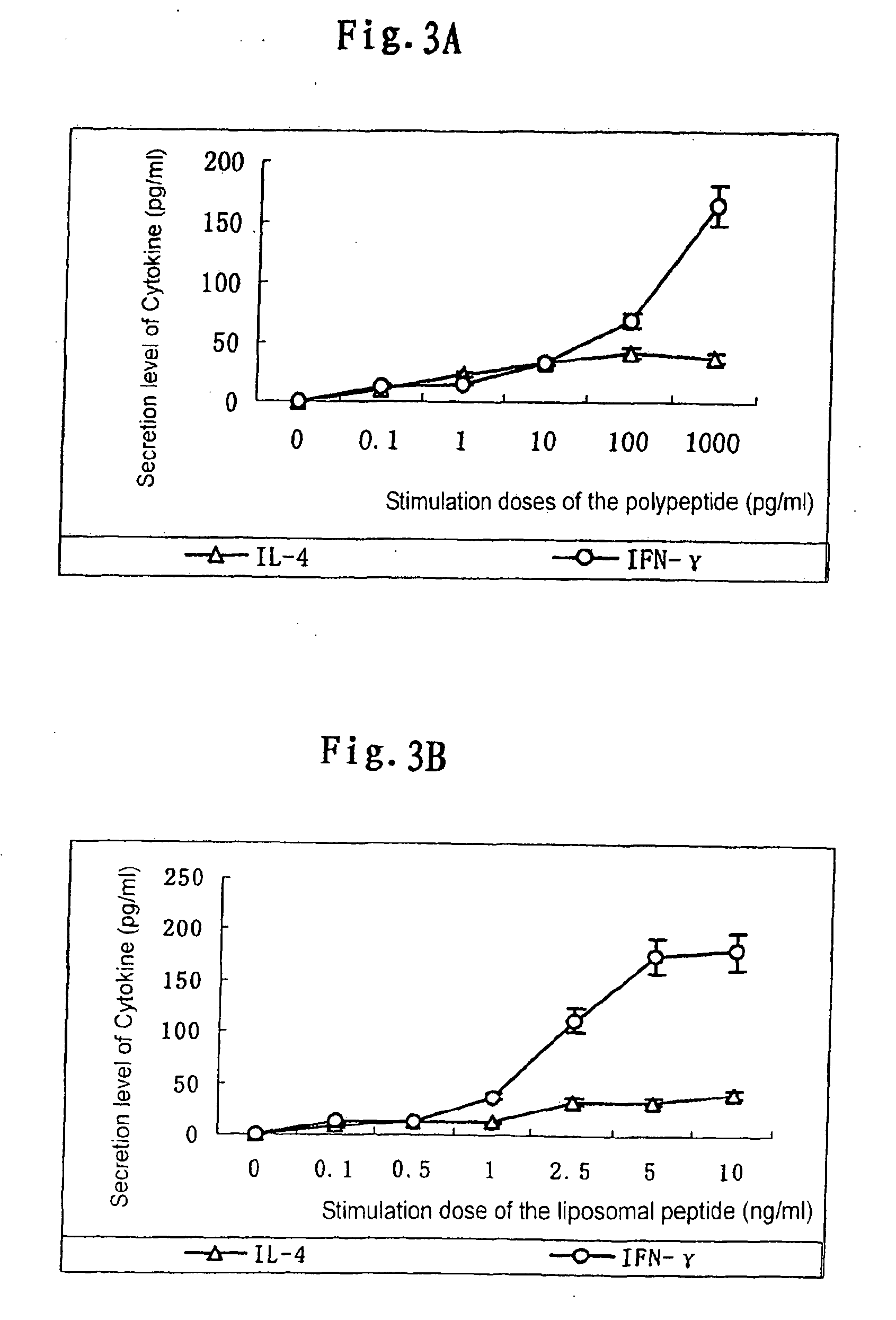
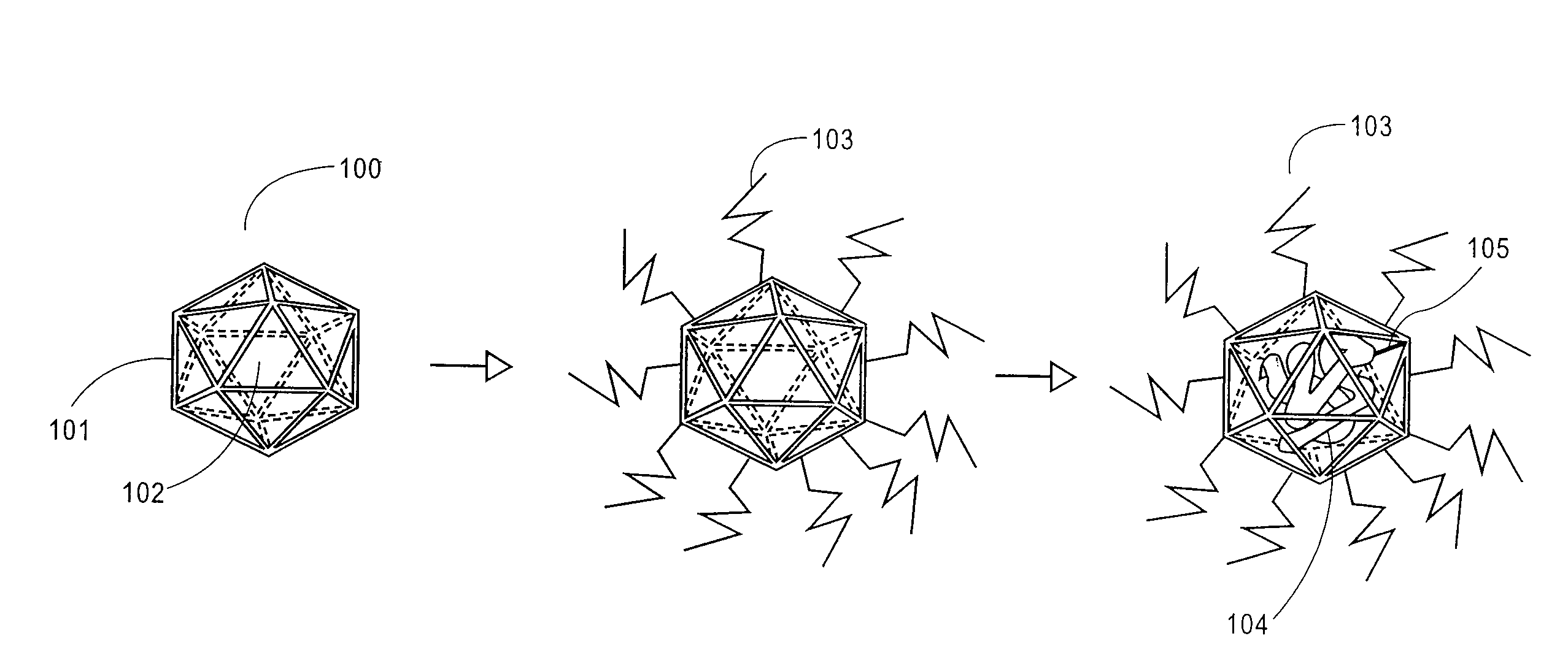
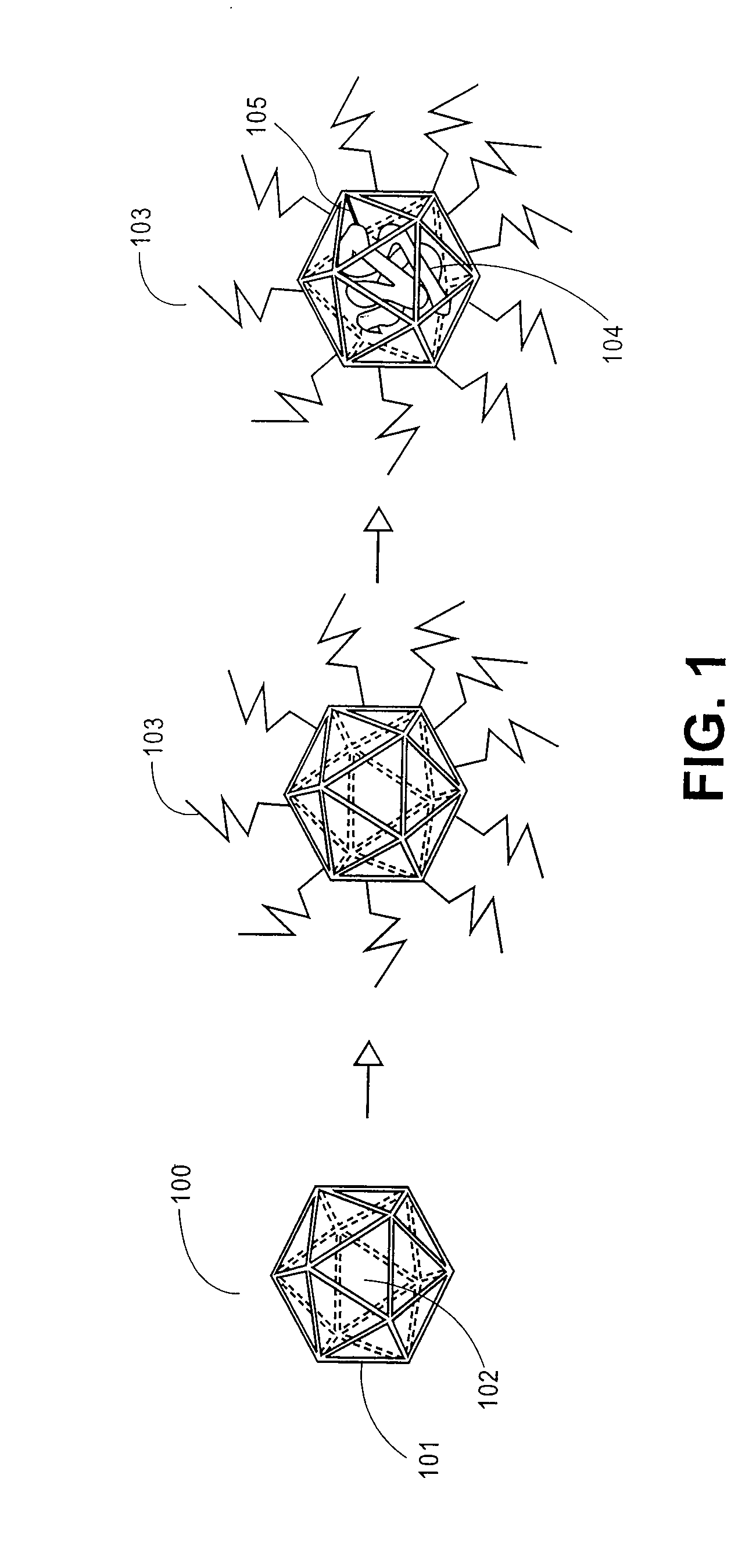
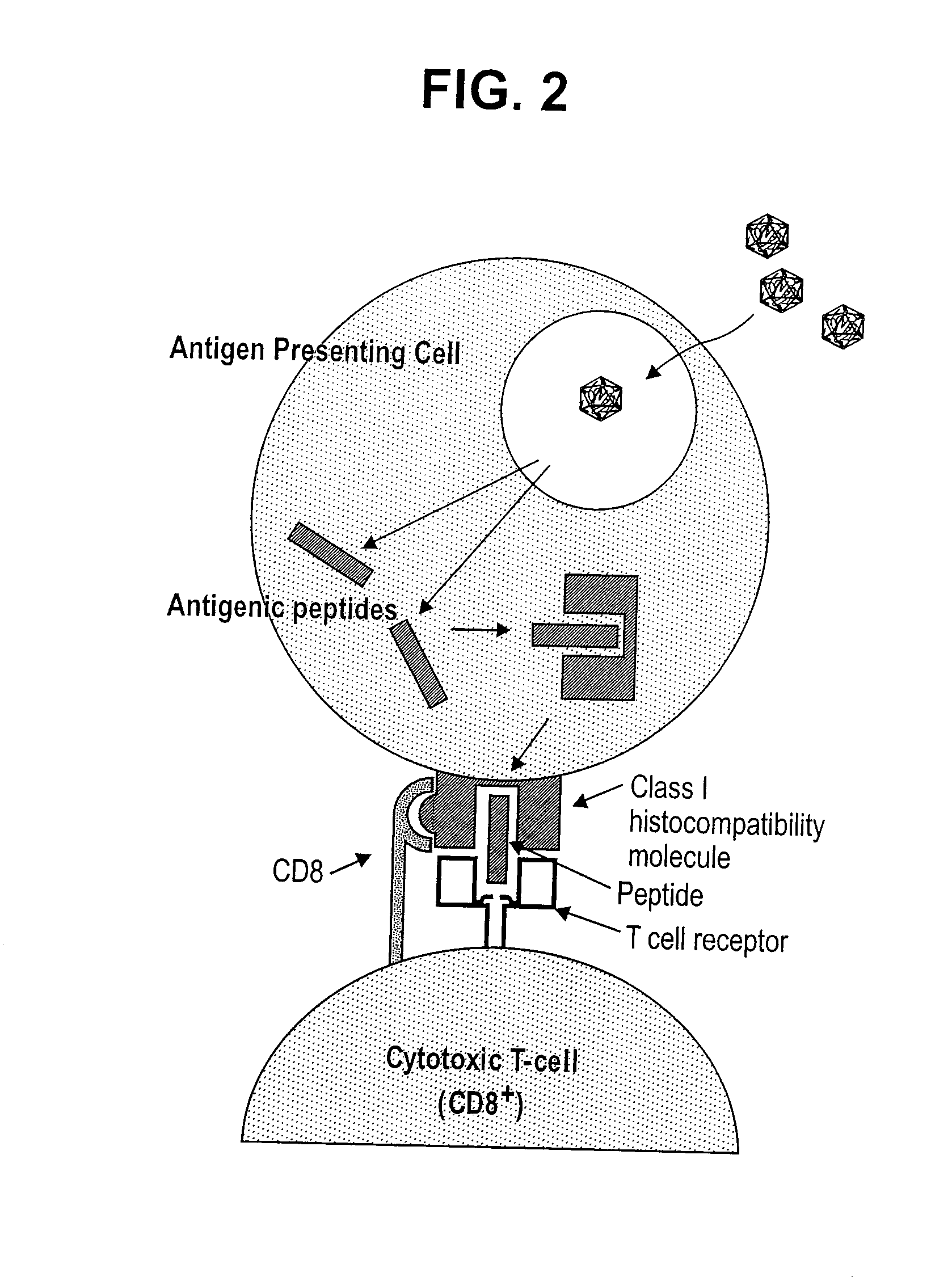
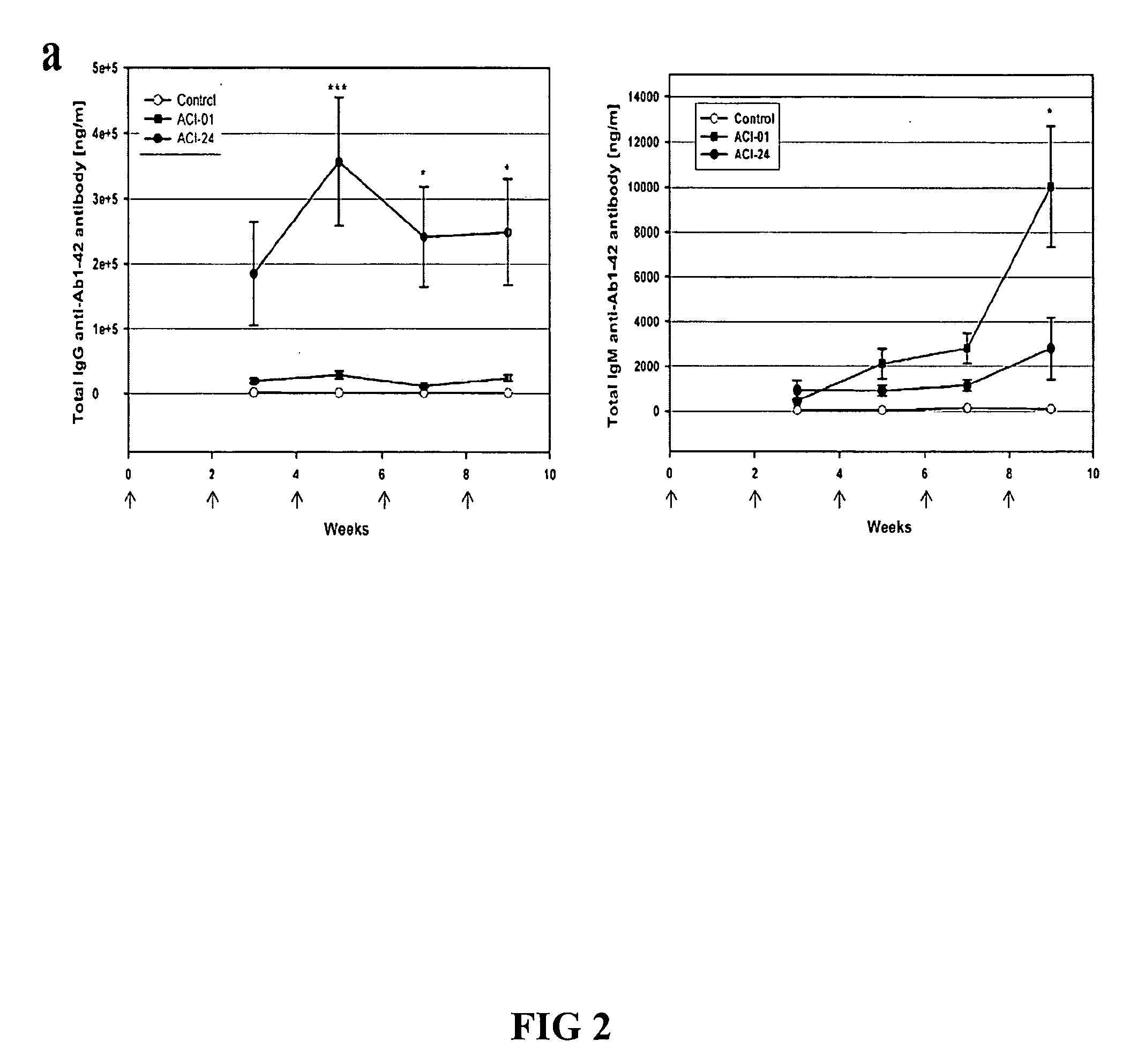
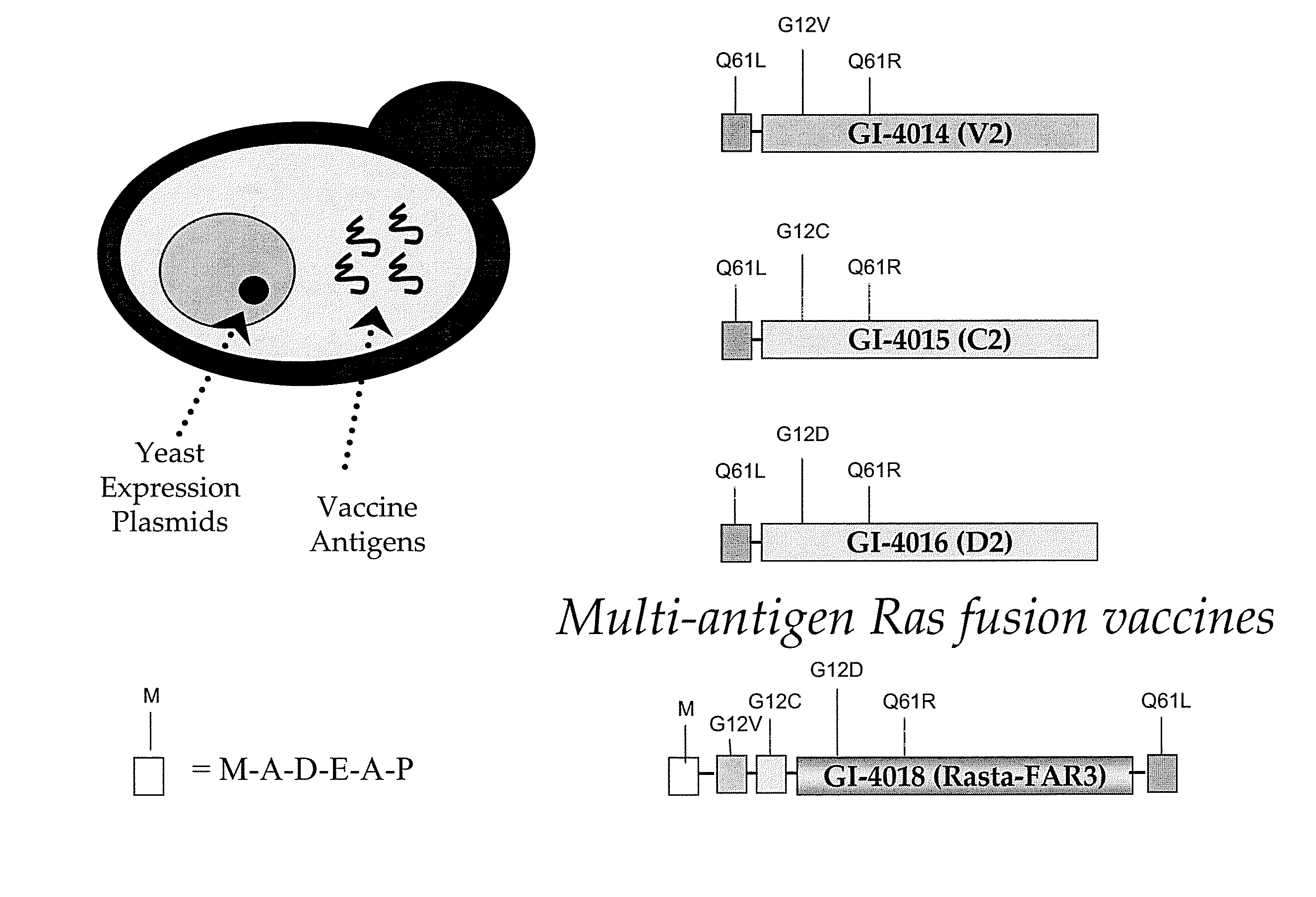
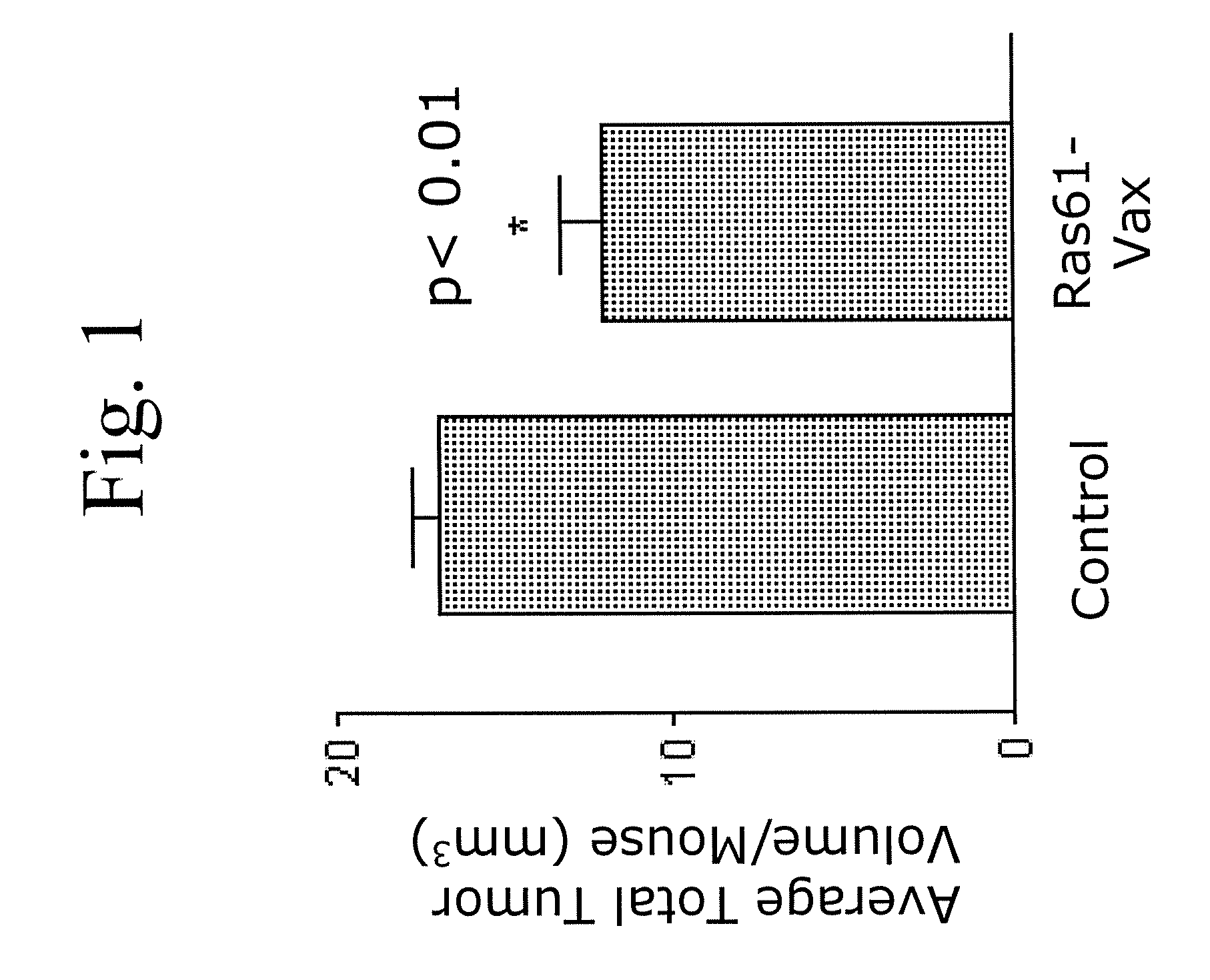
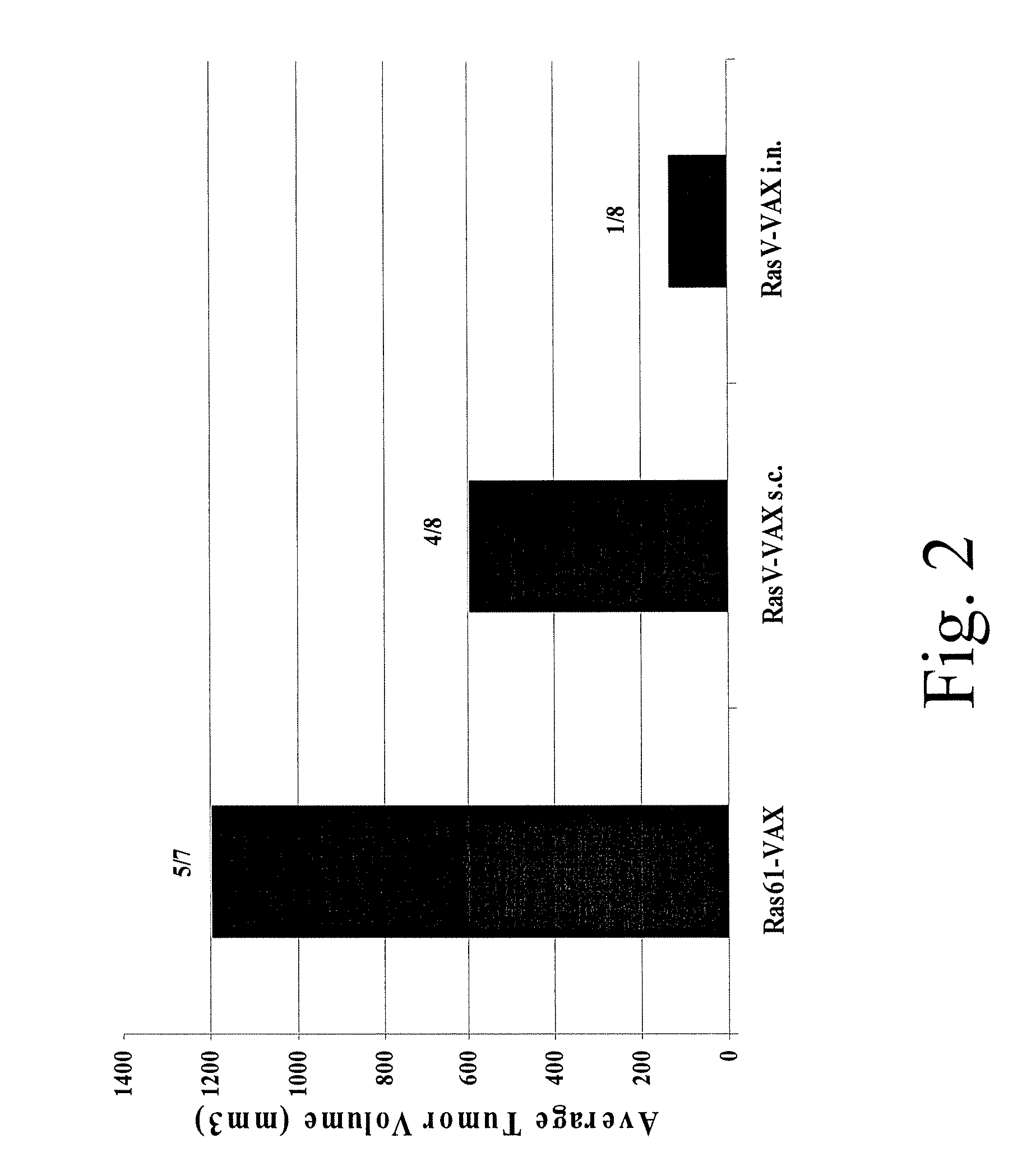
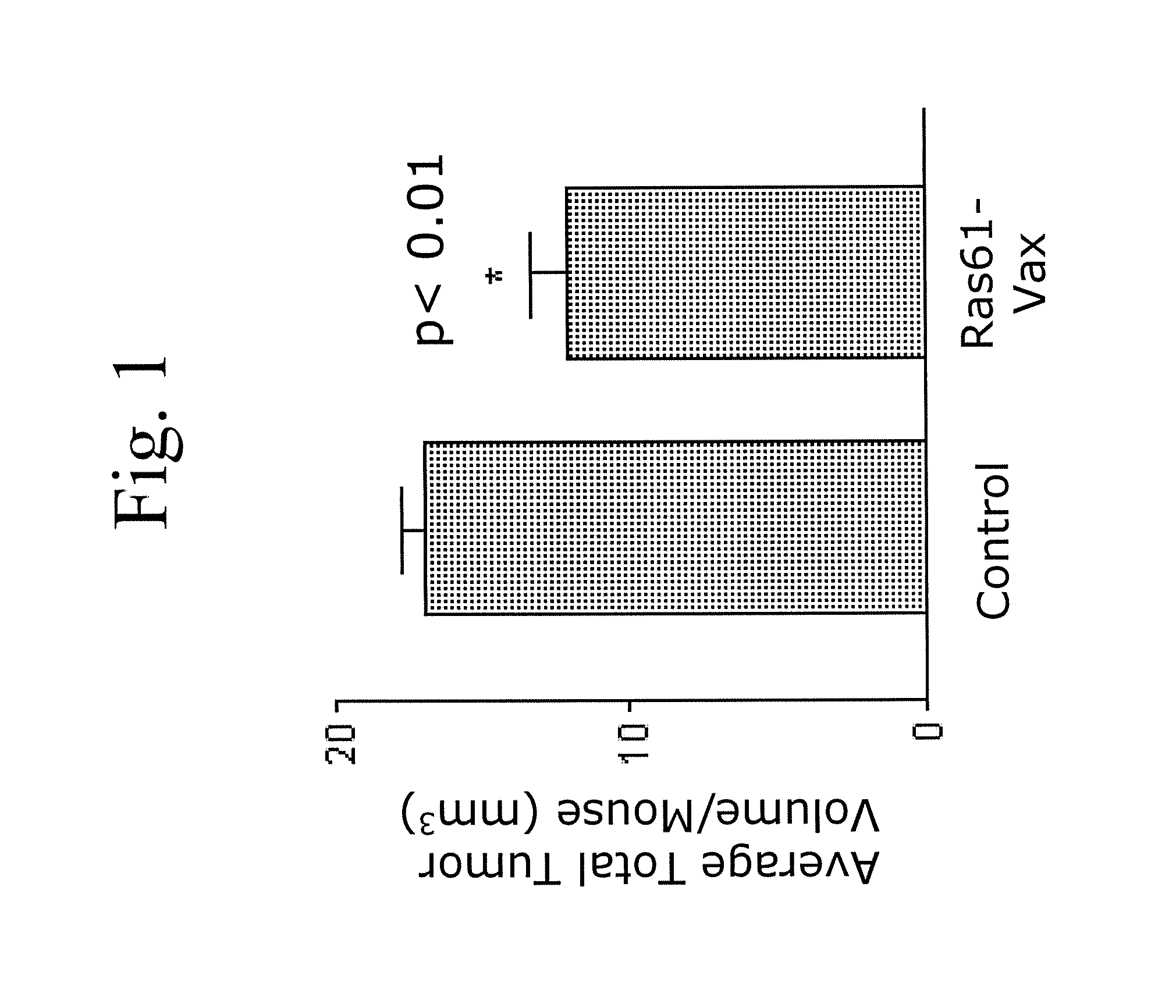
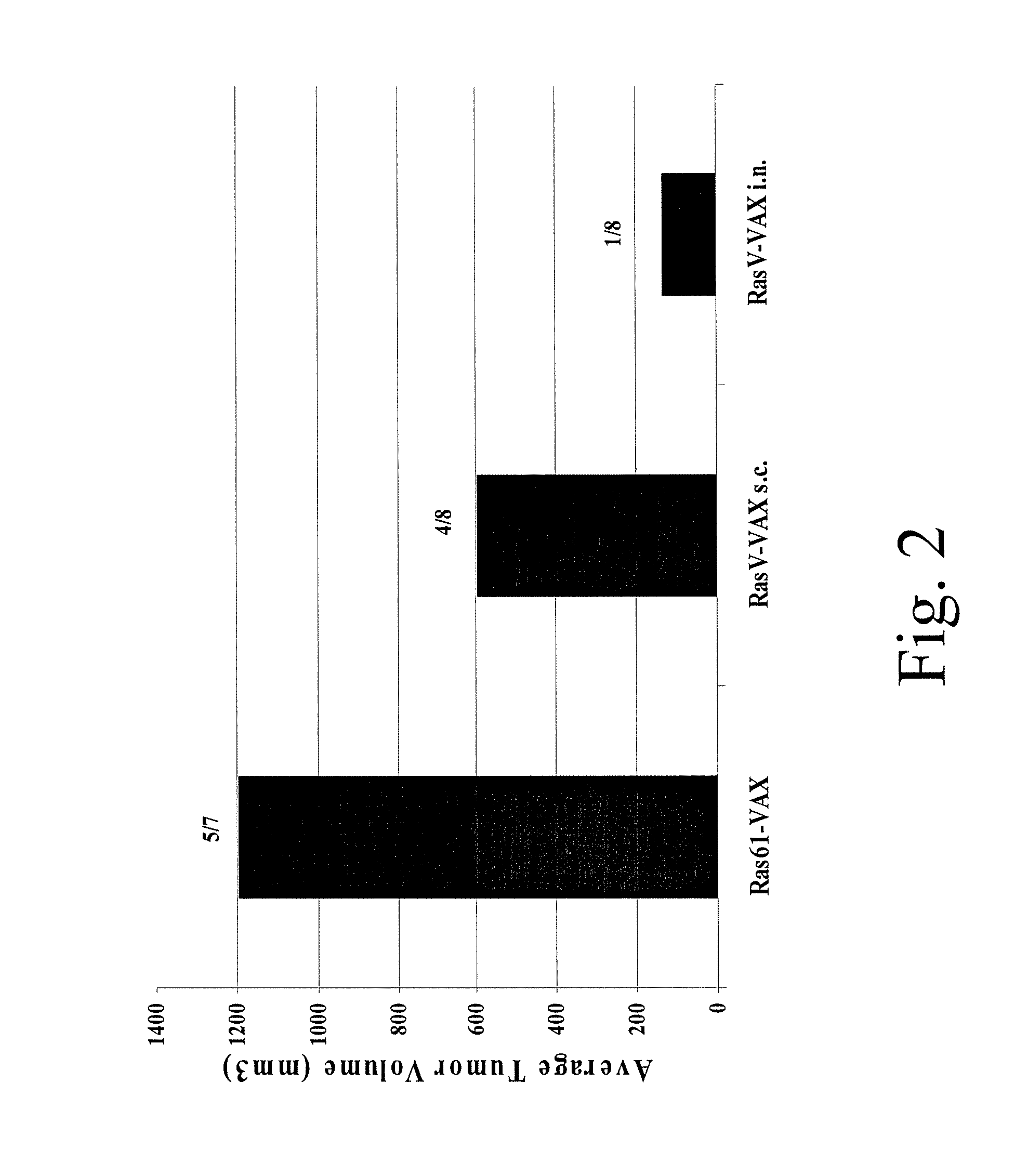
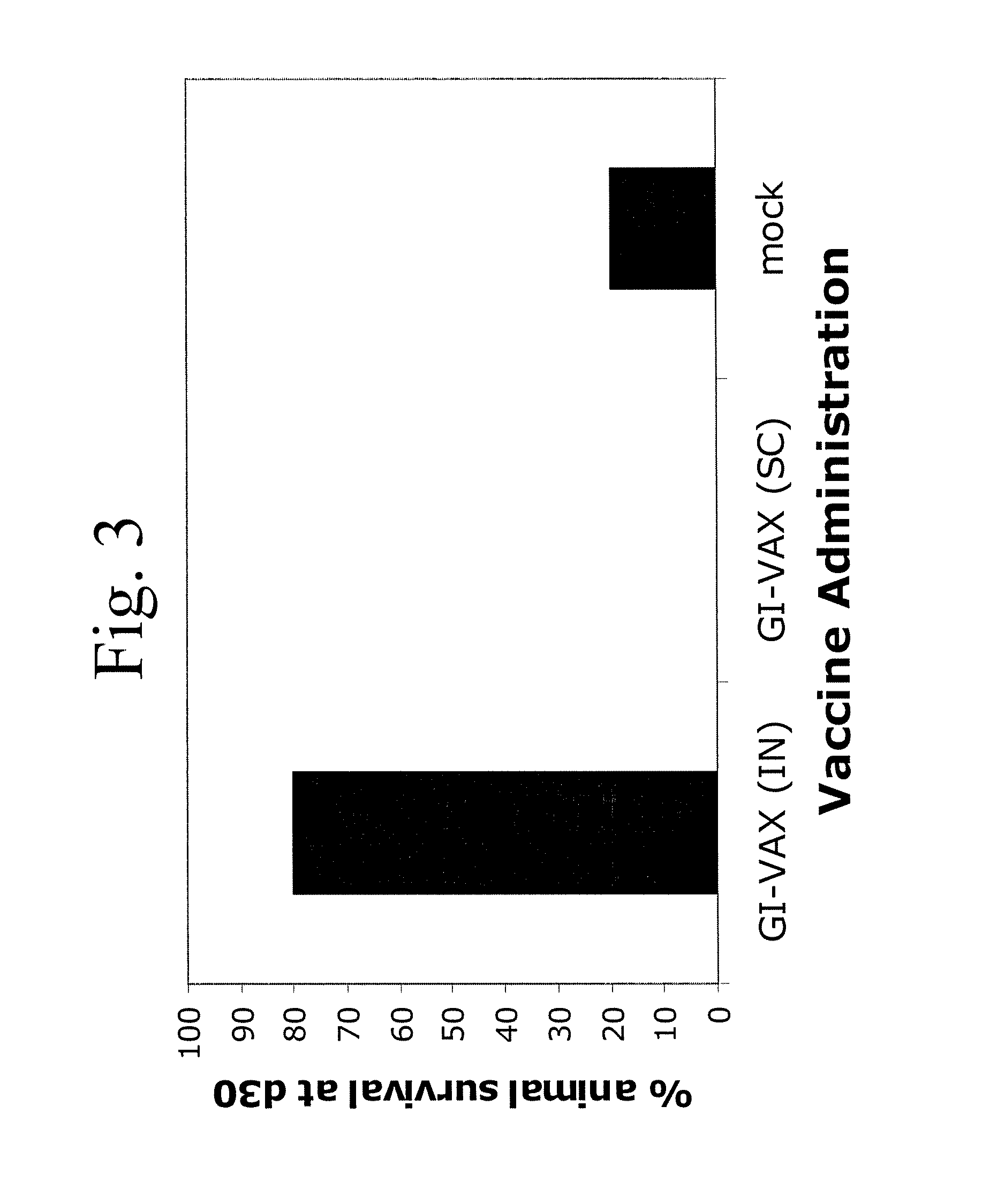
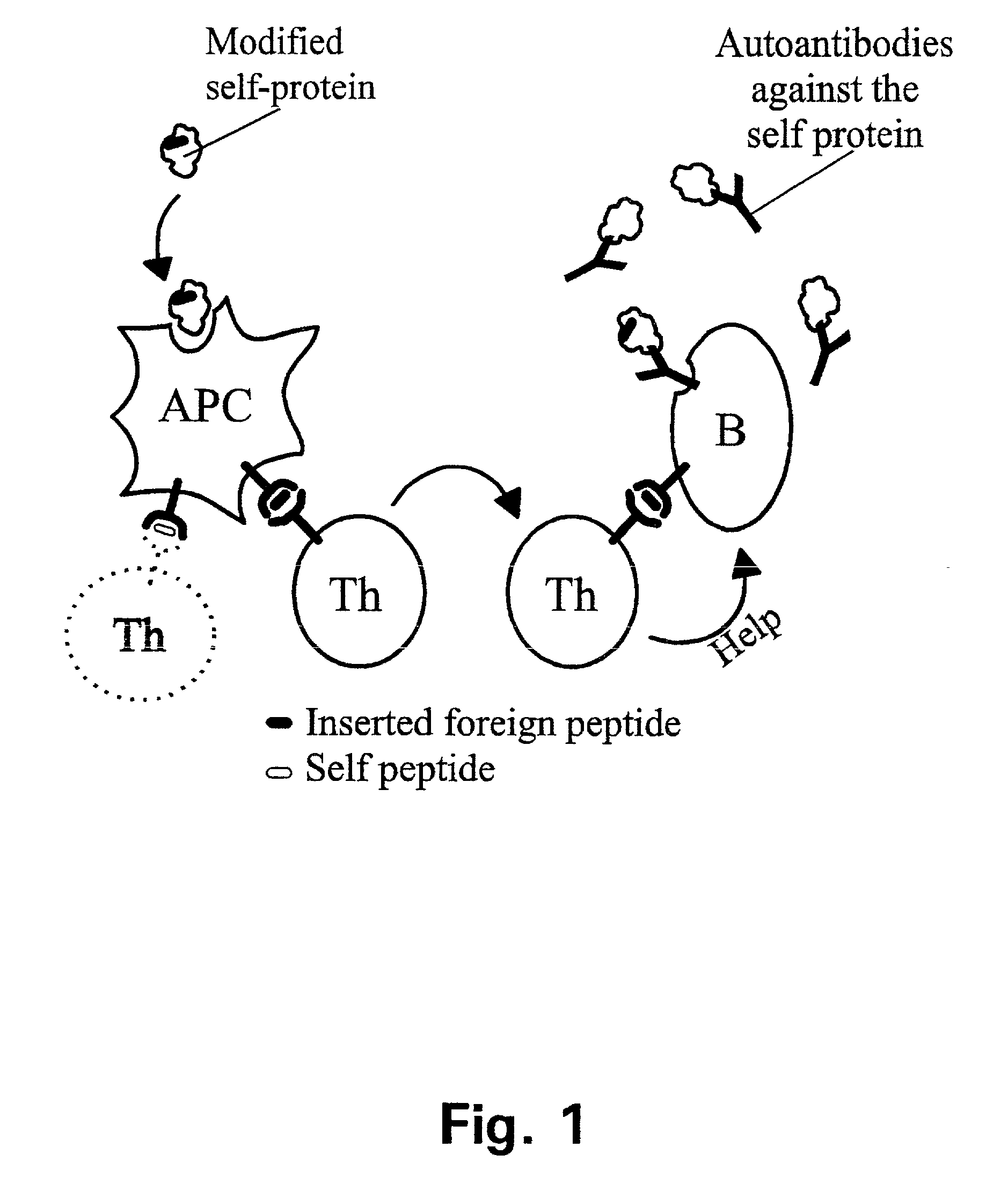
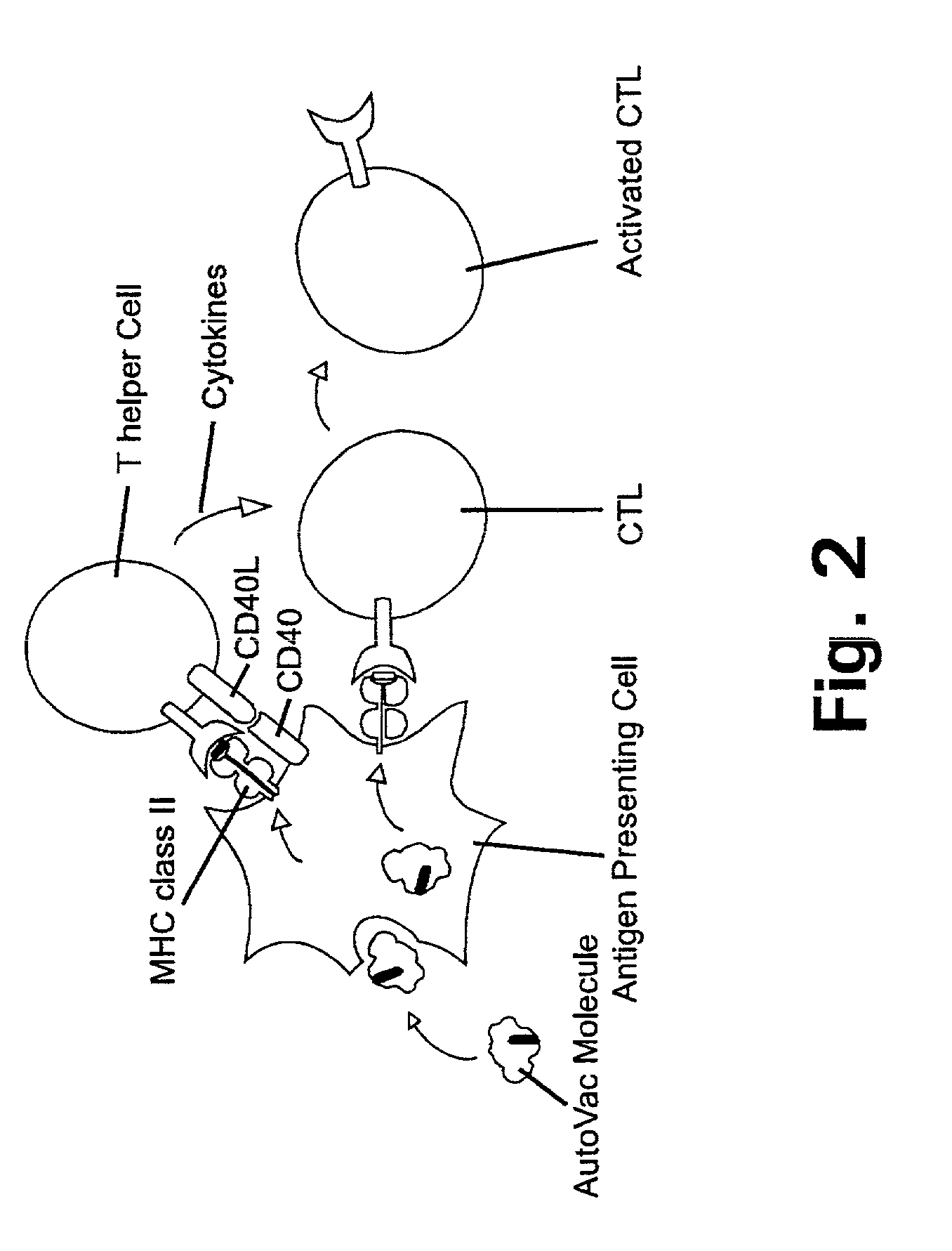
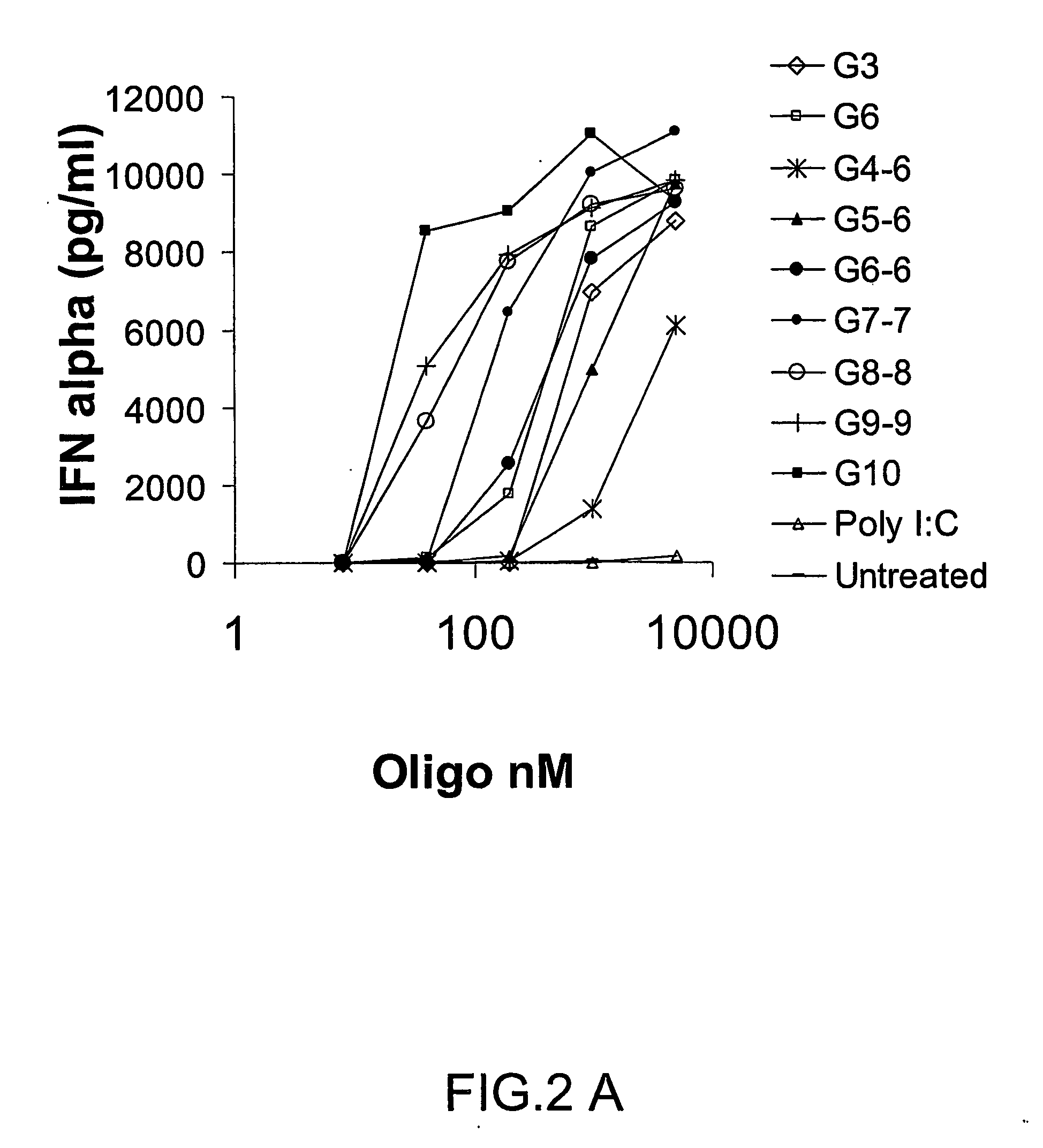
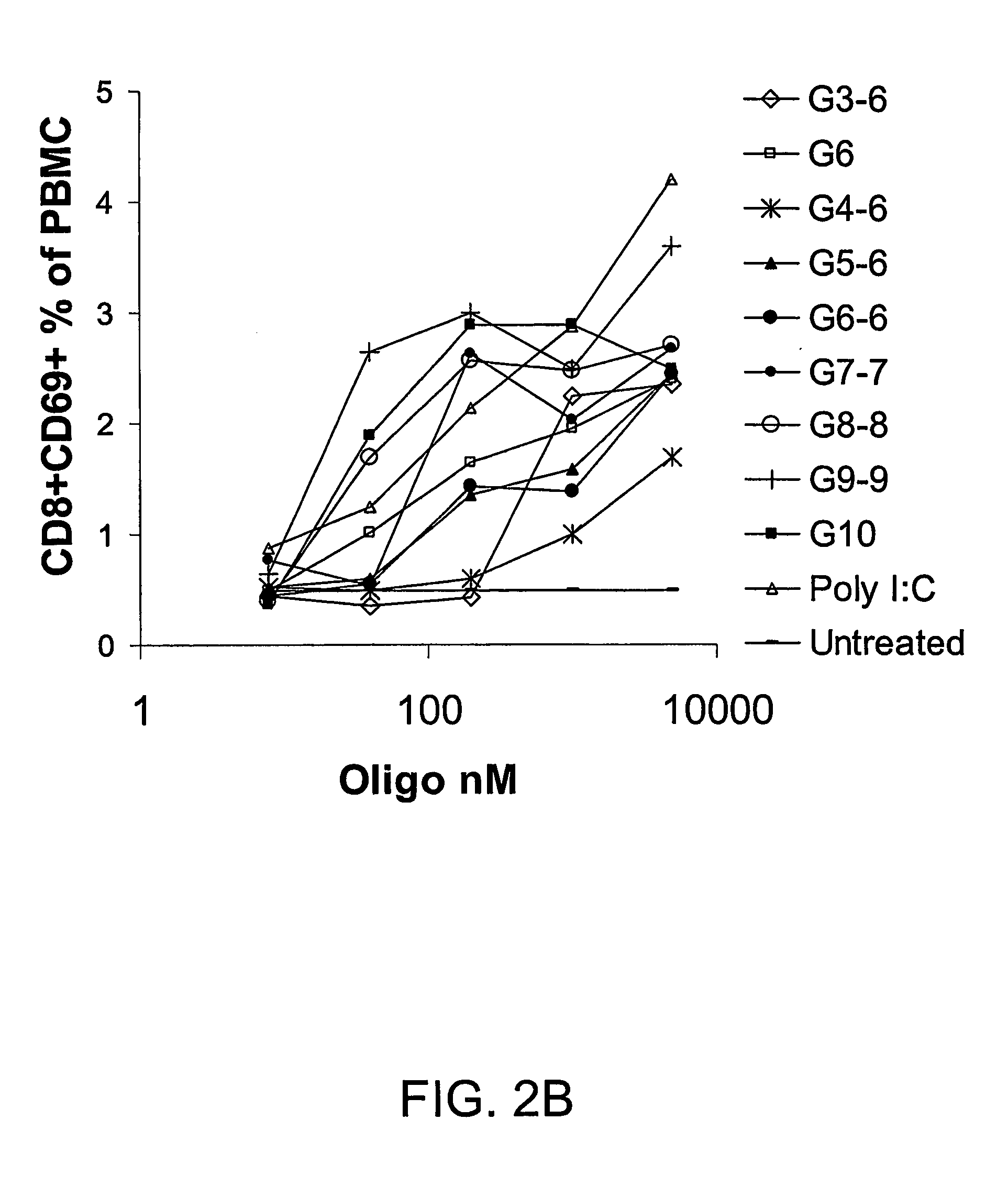
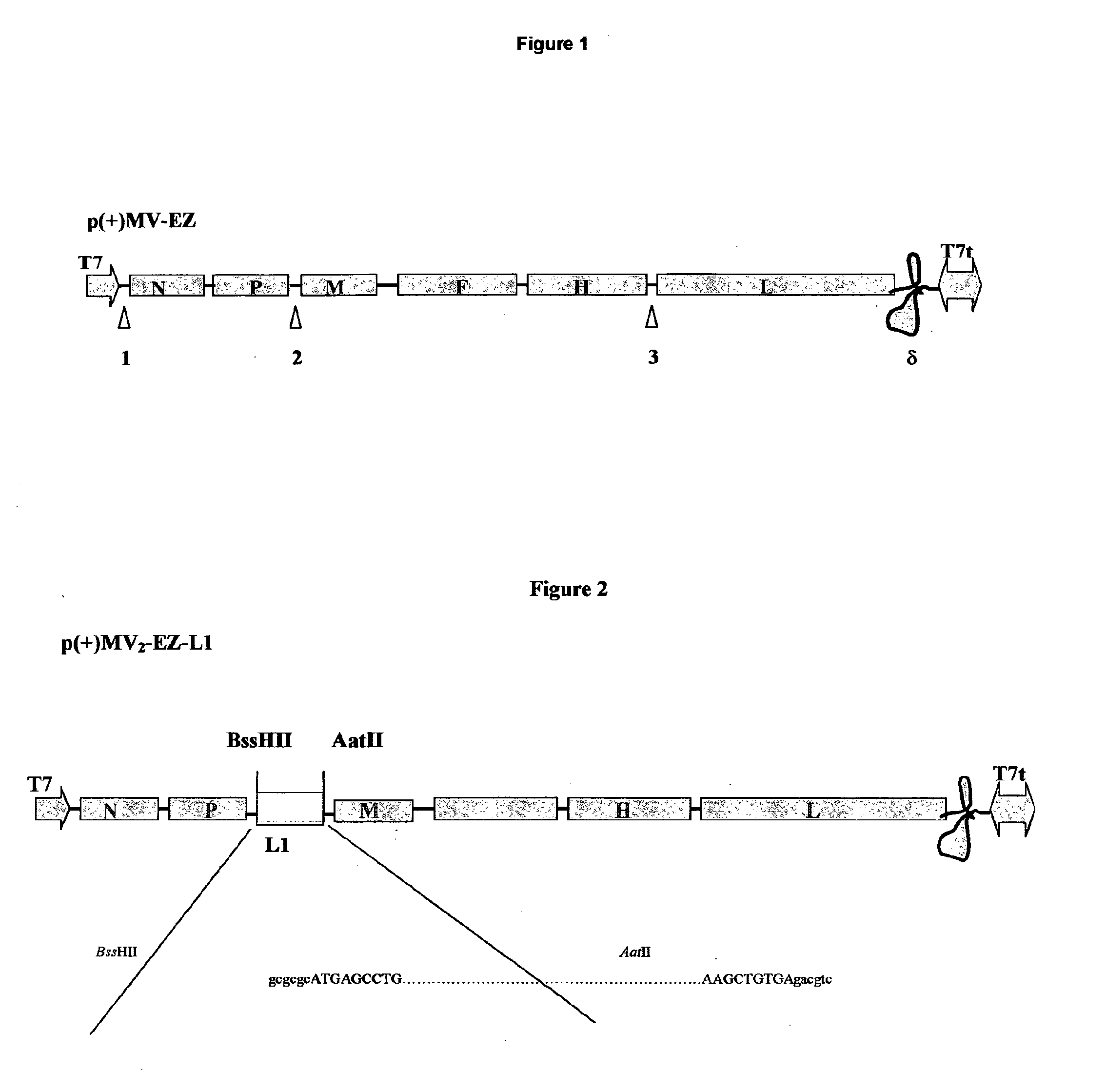
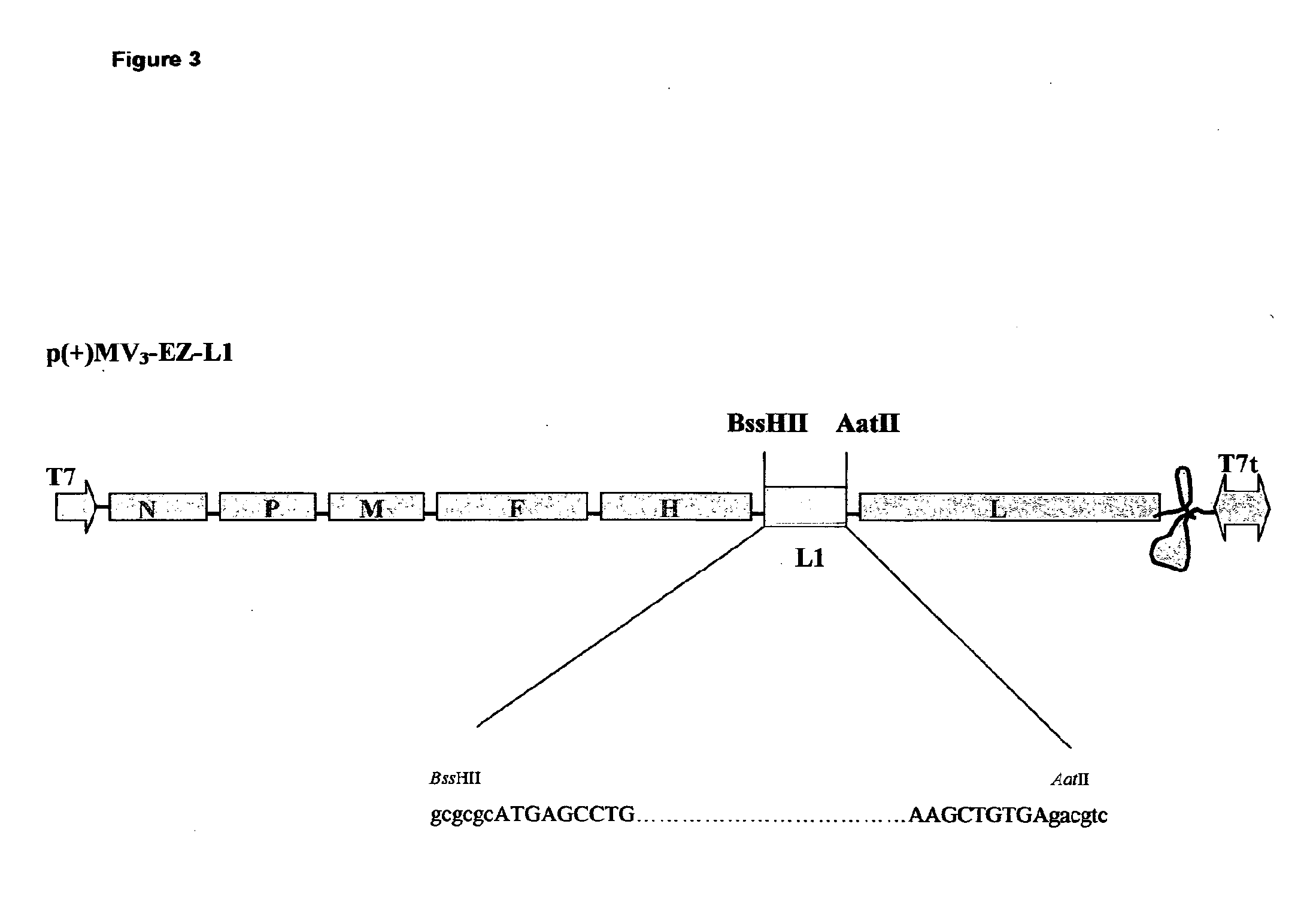
Patents
Literature
388 results about "Therapeutic vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic vaccines are vaccines which are intended to treat or cure a disorder or disease by stimulating the immune system. Therapeutic vaccines may be used to treat certain types of cancer, by stimulating the body's immune system to help it respond against certain cancer cells.
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
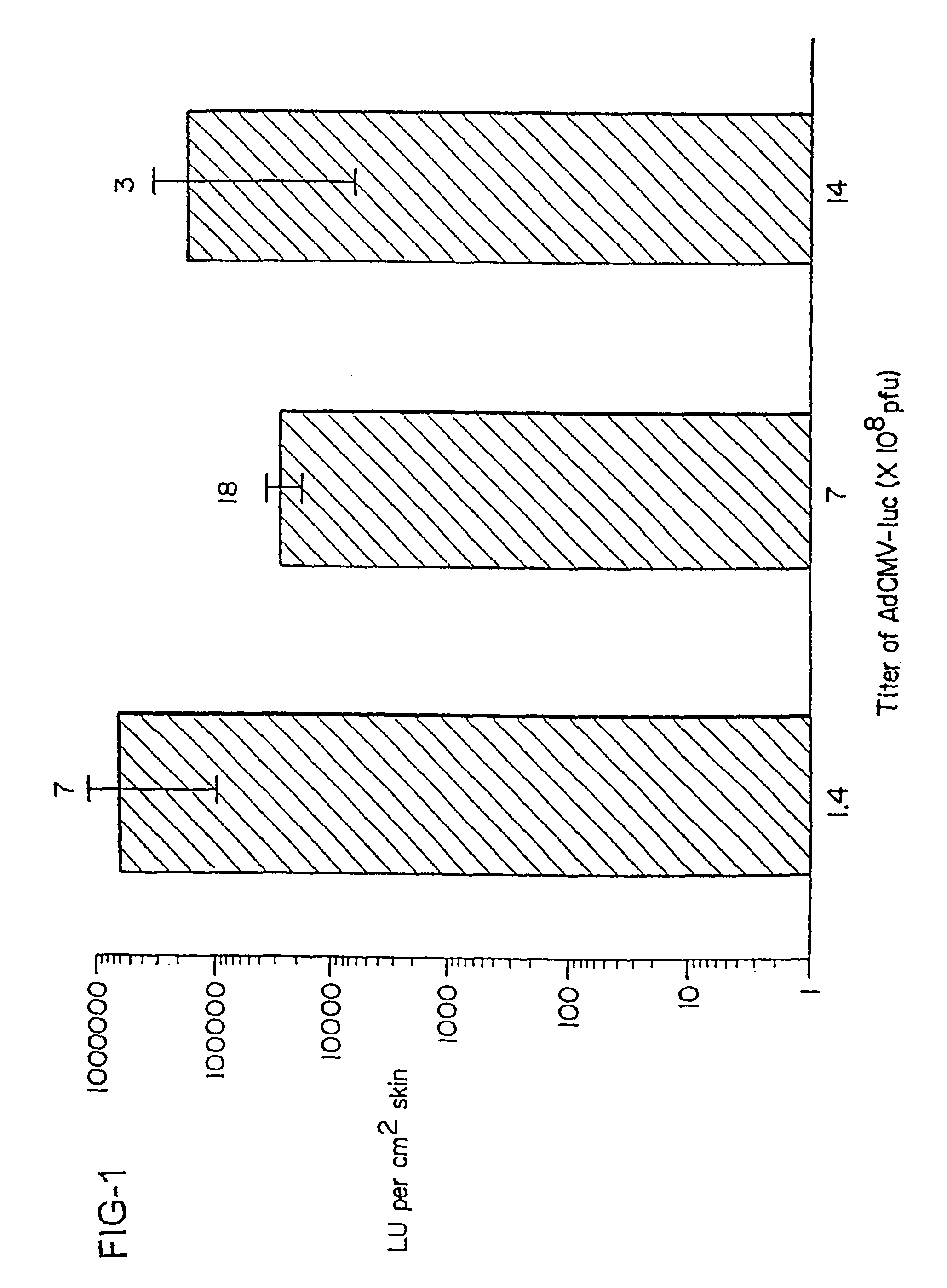
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Cyclic-Dinucleotides and Its Conjugates as Adjuvants and Their Uses in Pharmaceutical Compositions
ActiveUS20080286296A1Severe formLess immunomodulatory effectOrganic active ingredientsAntipyreticDiseaseAutoimmune responses
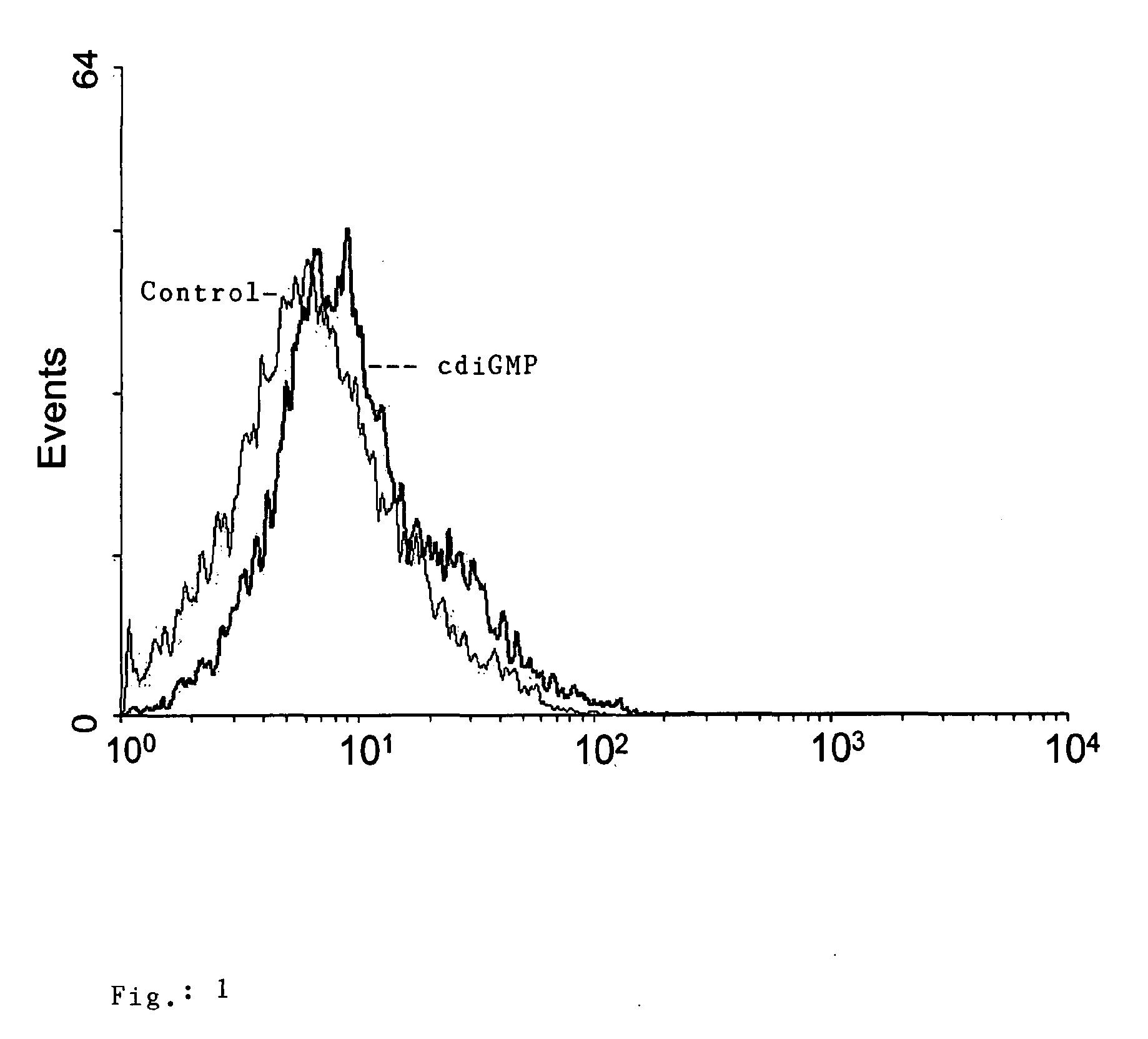
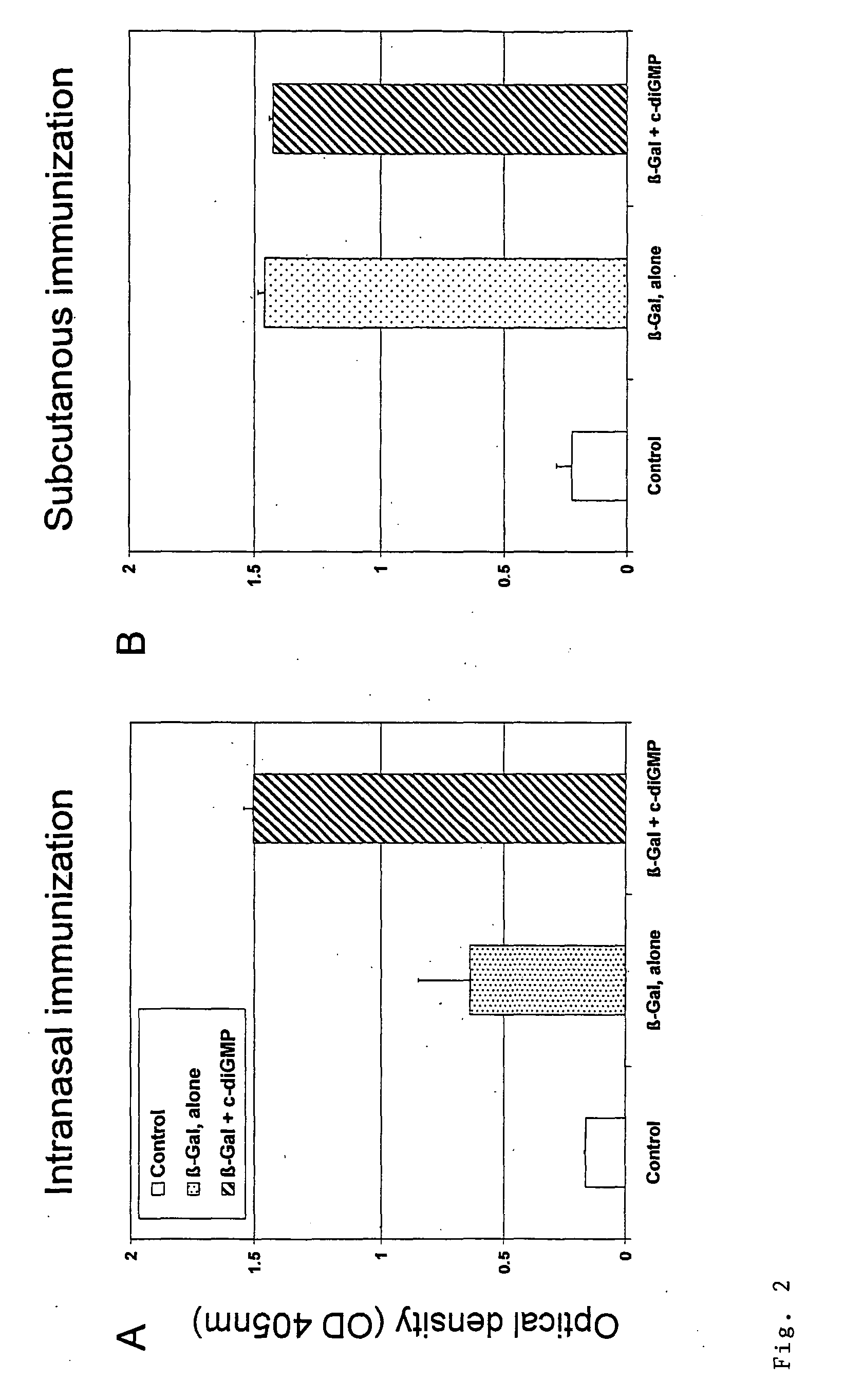
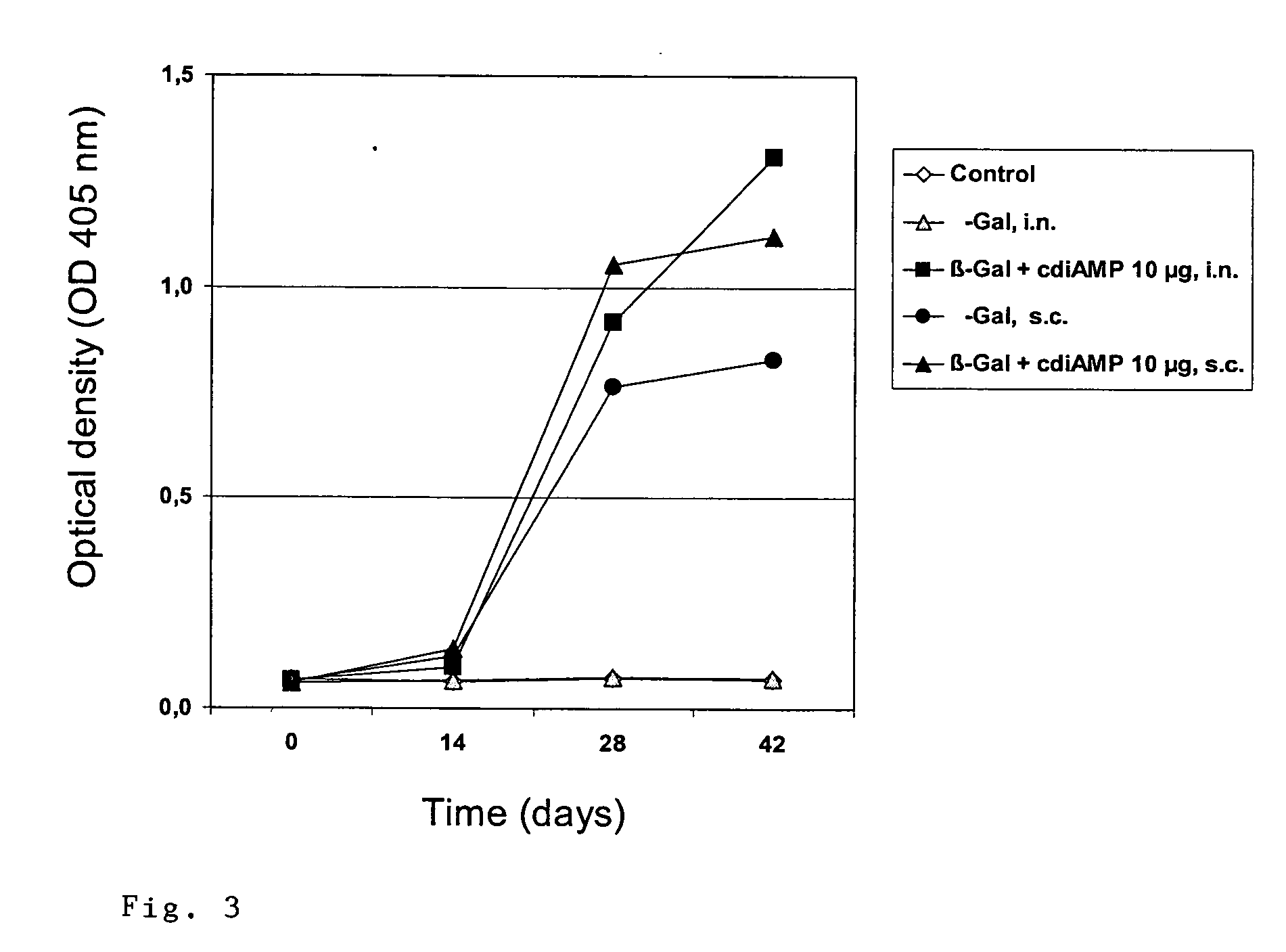
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumors, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
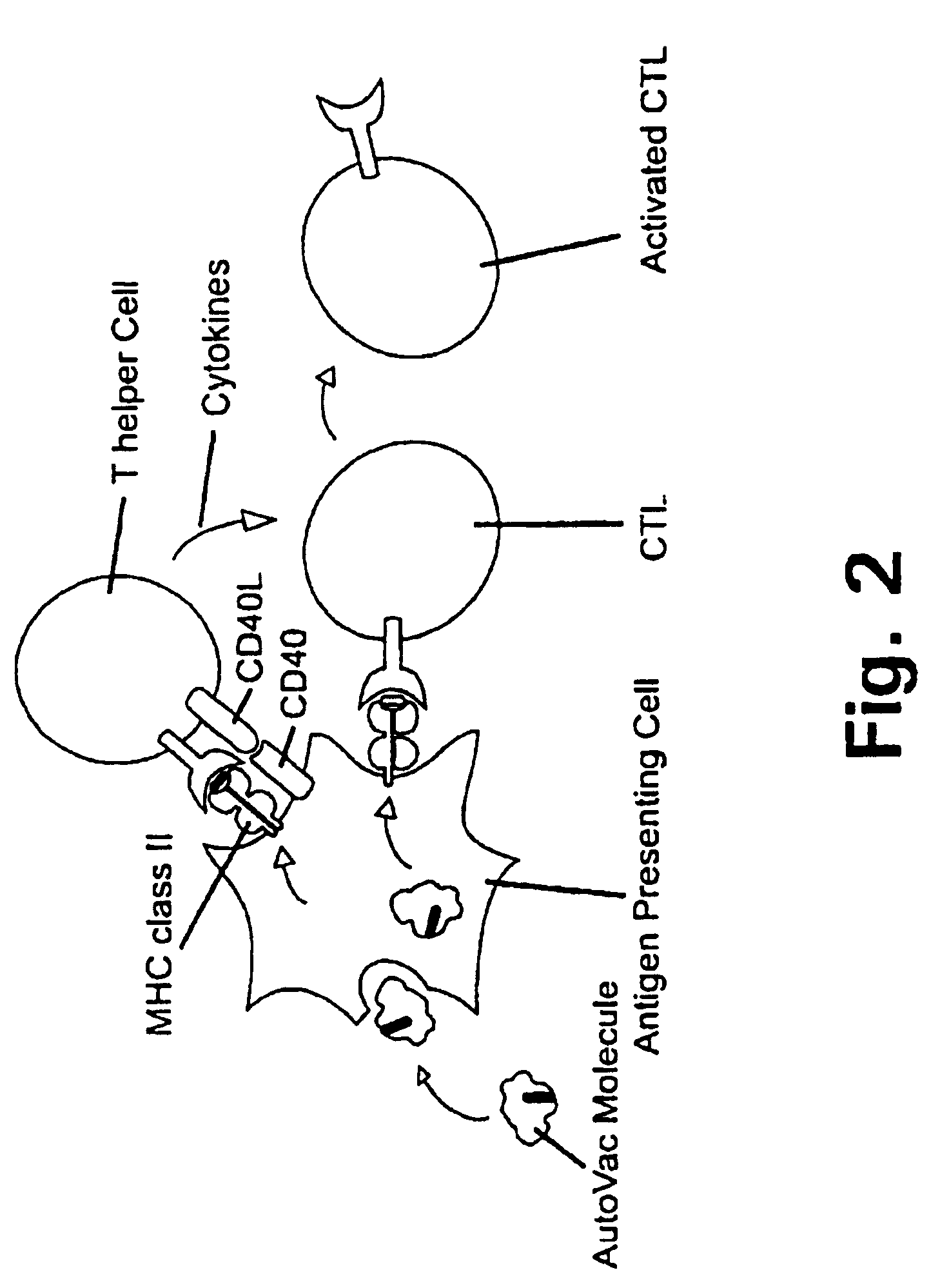
Novel methods for therapeutic vaccination
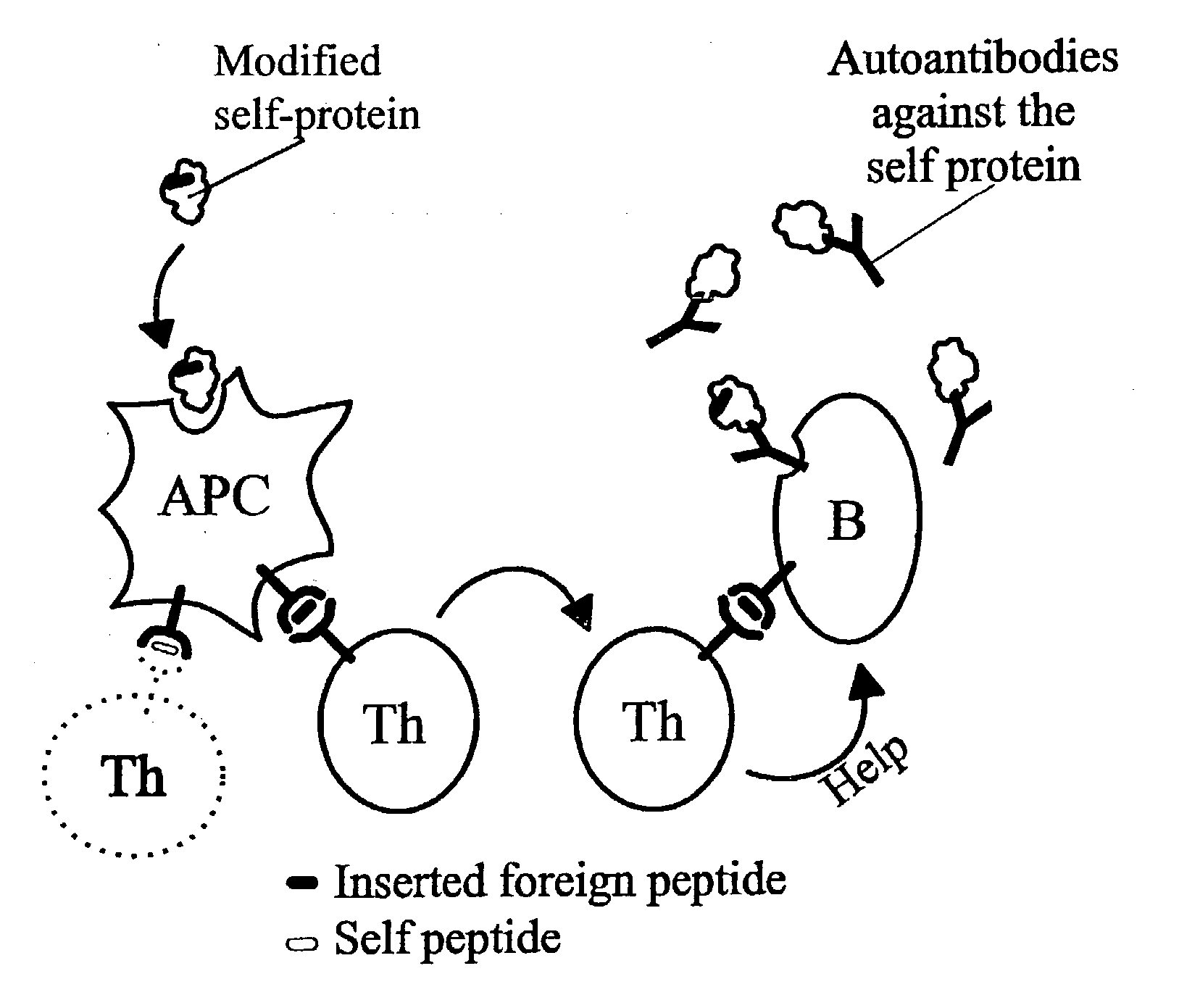
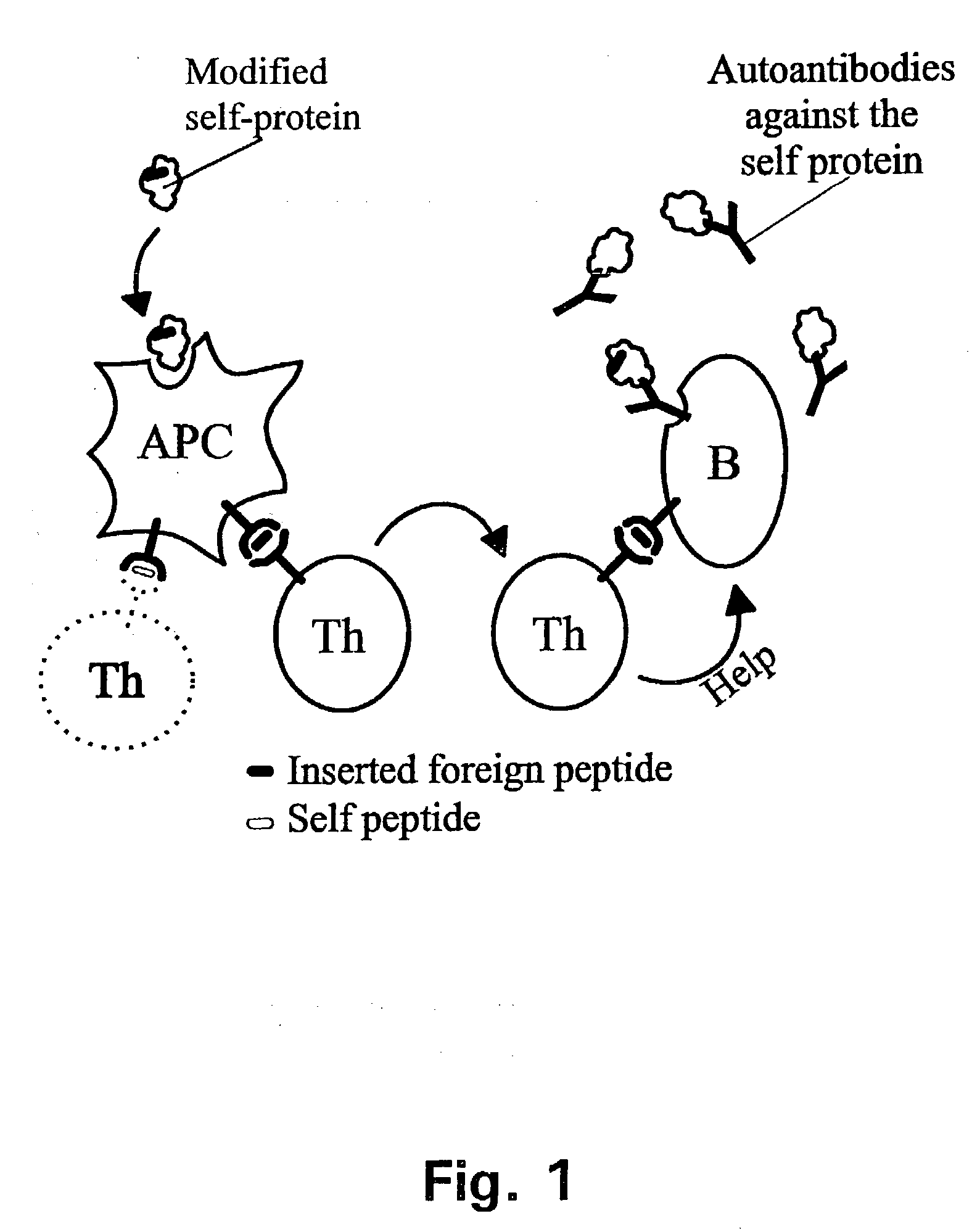
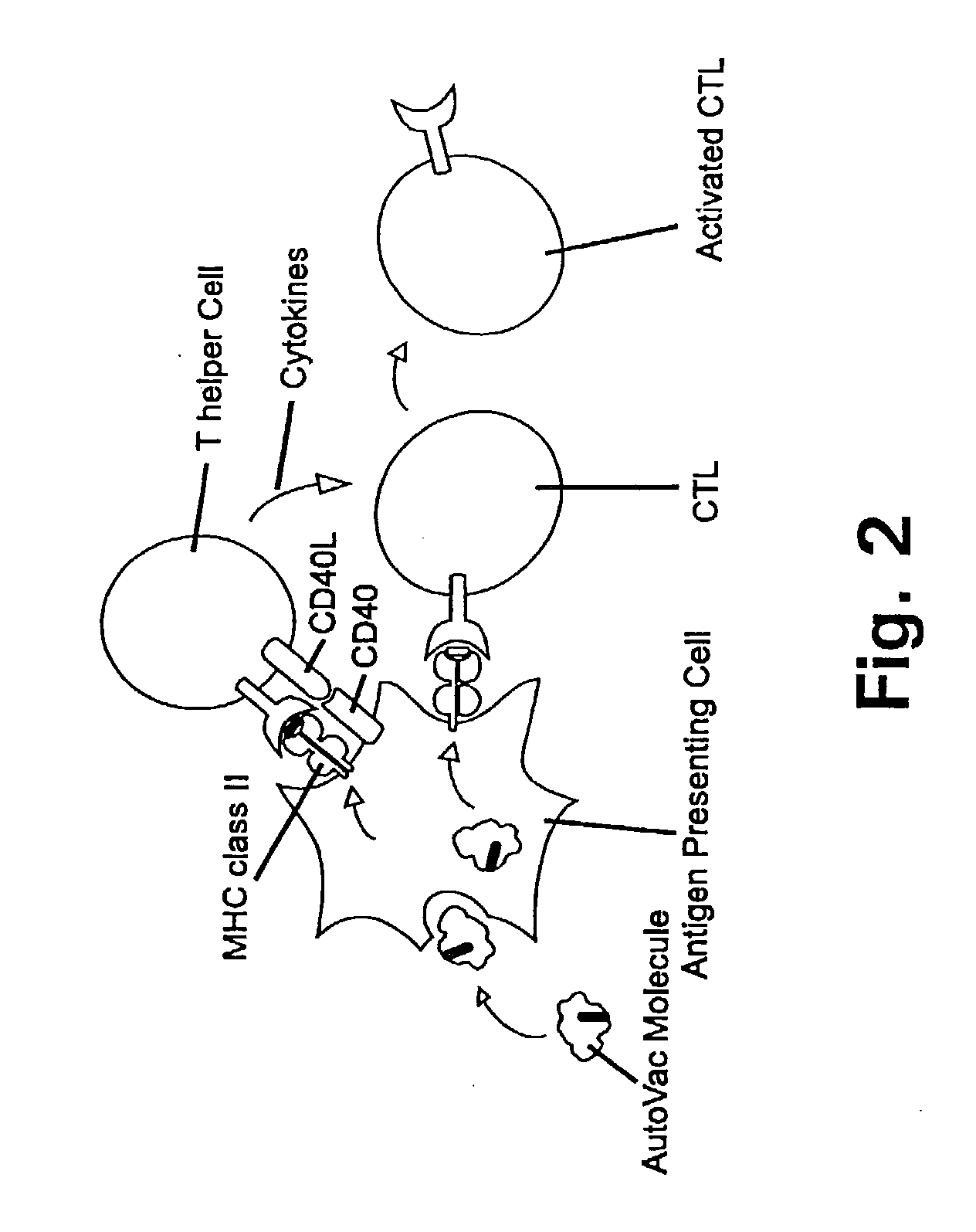
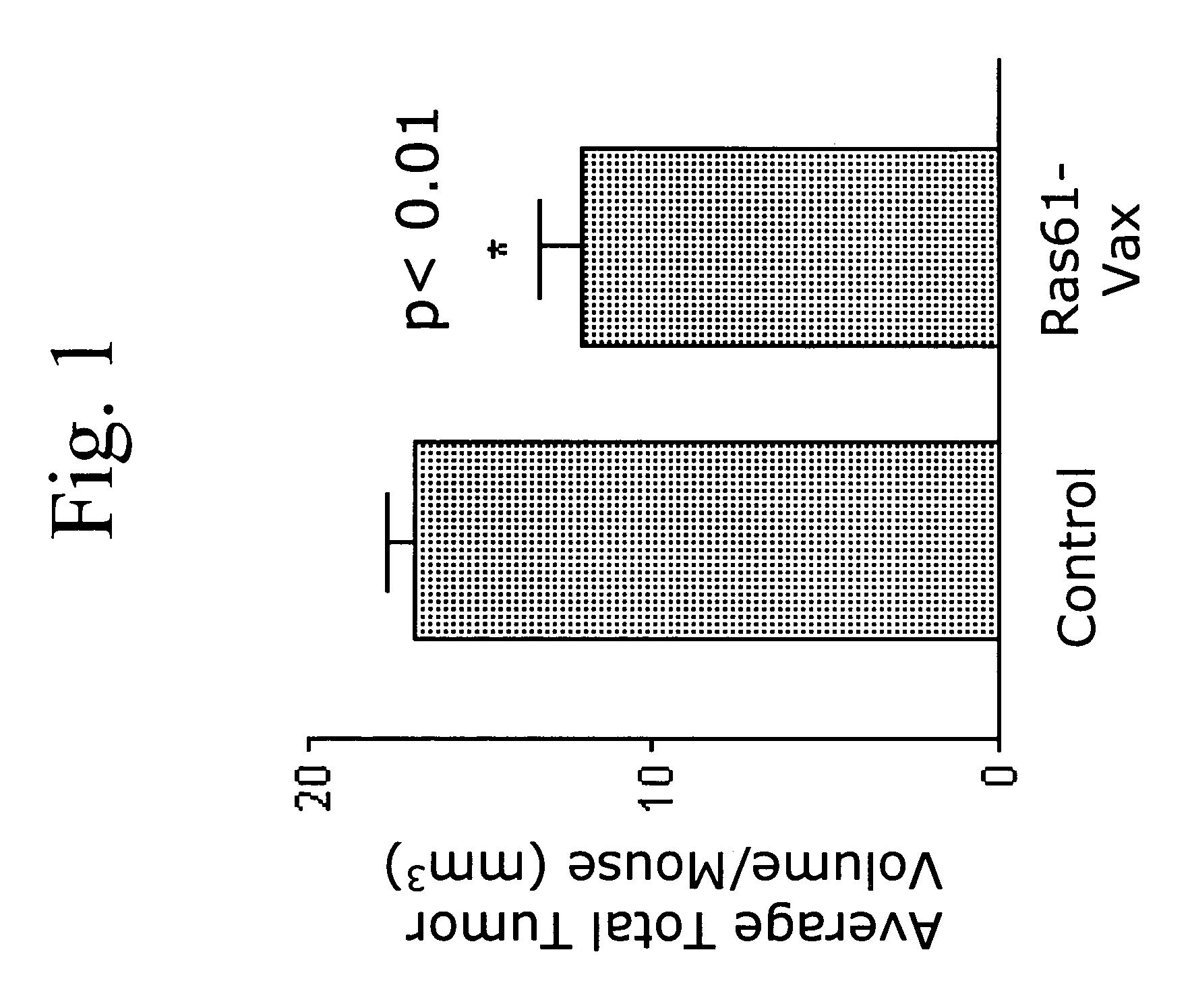
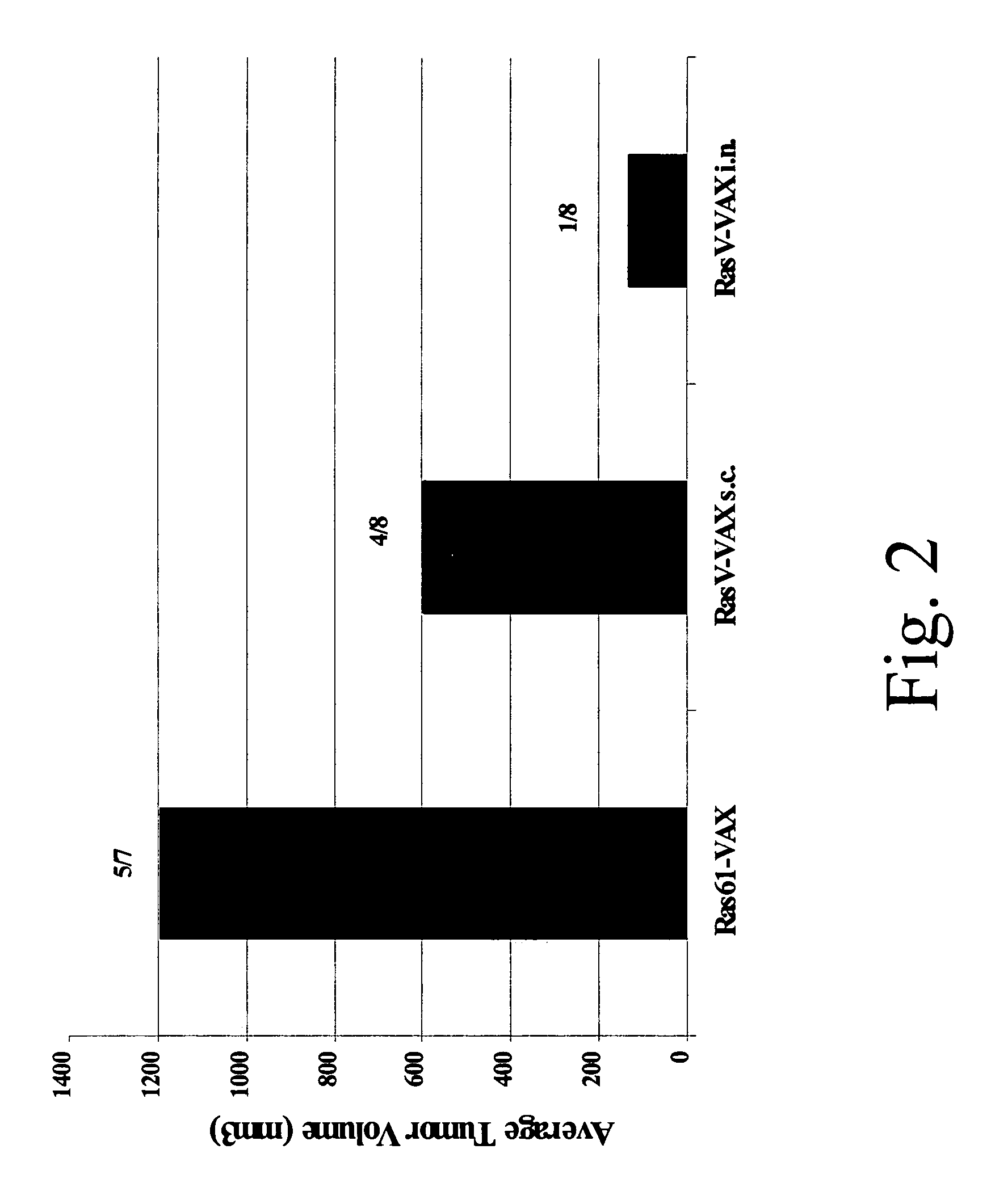
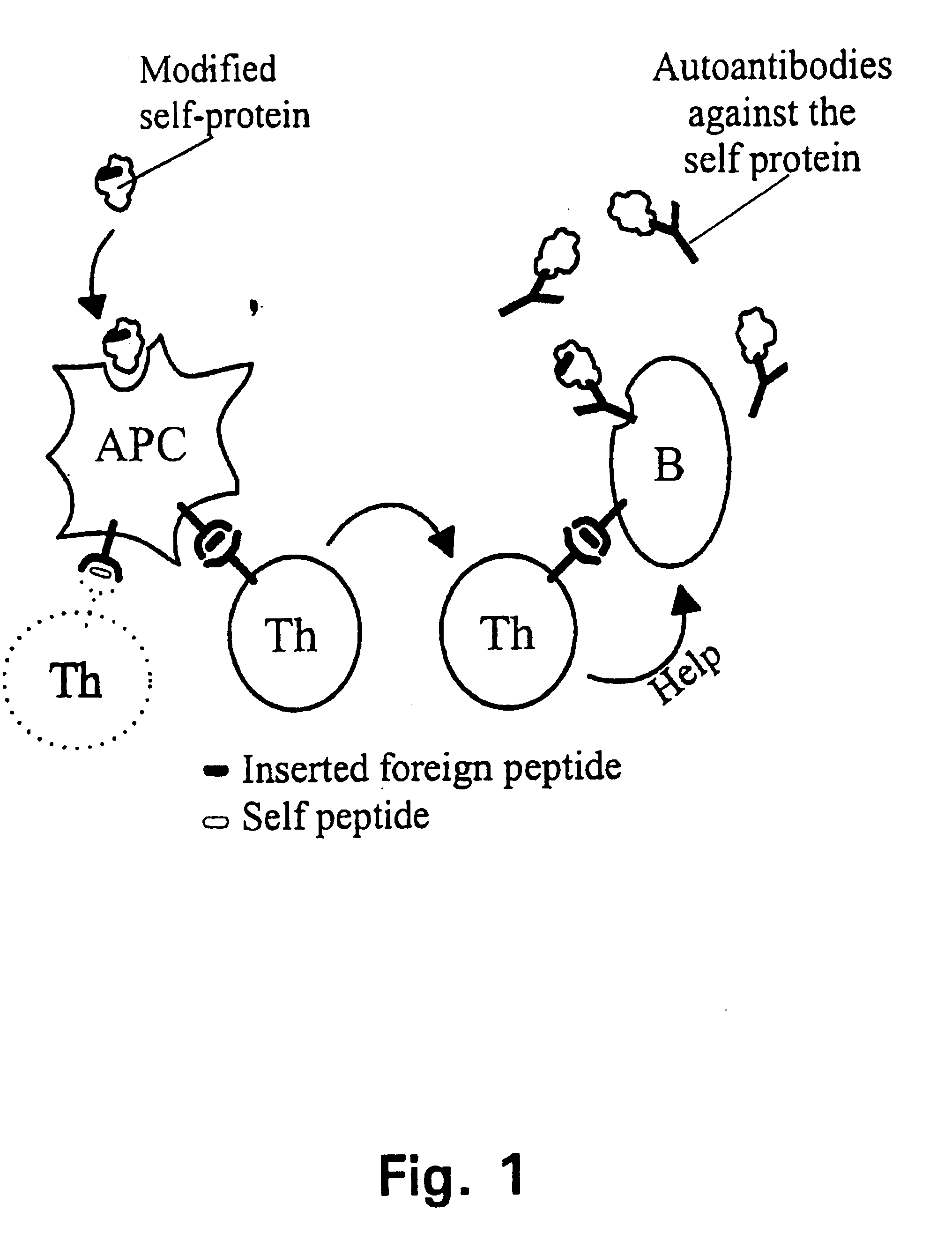
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Yeast-based vaccines as immunotherapy
InactiveUS7465454B2Enhance immune responseExtended half-lifeBiocideAntibody mimetics/scaffoldsYeastDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
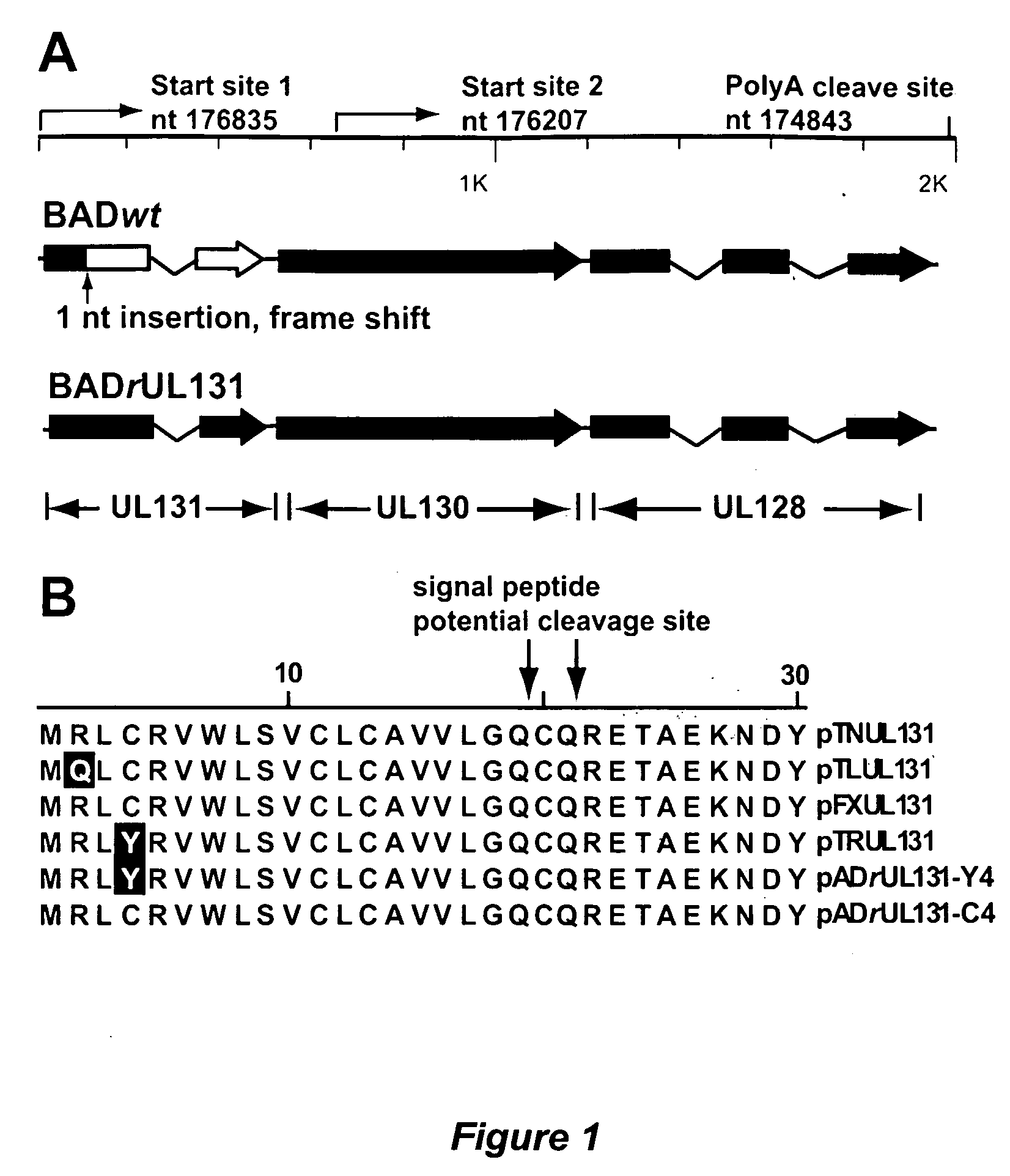
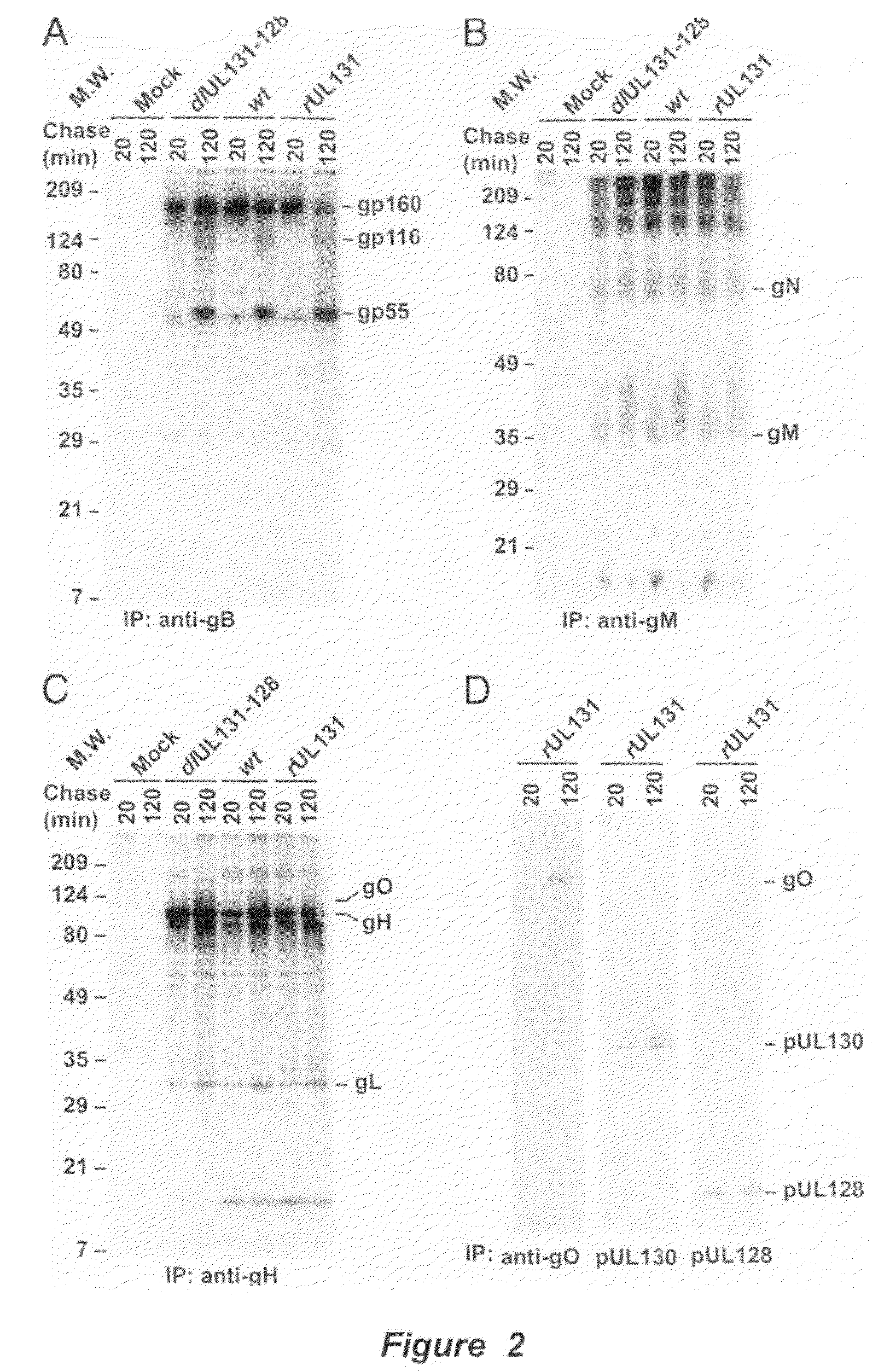
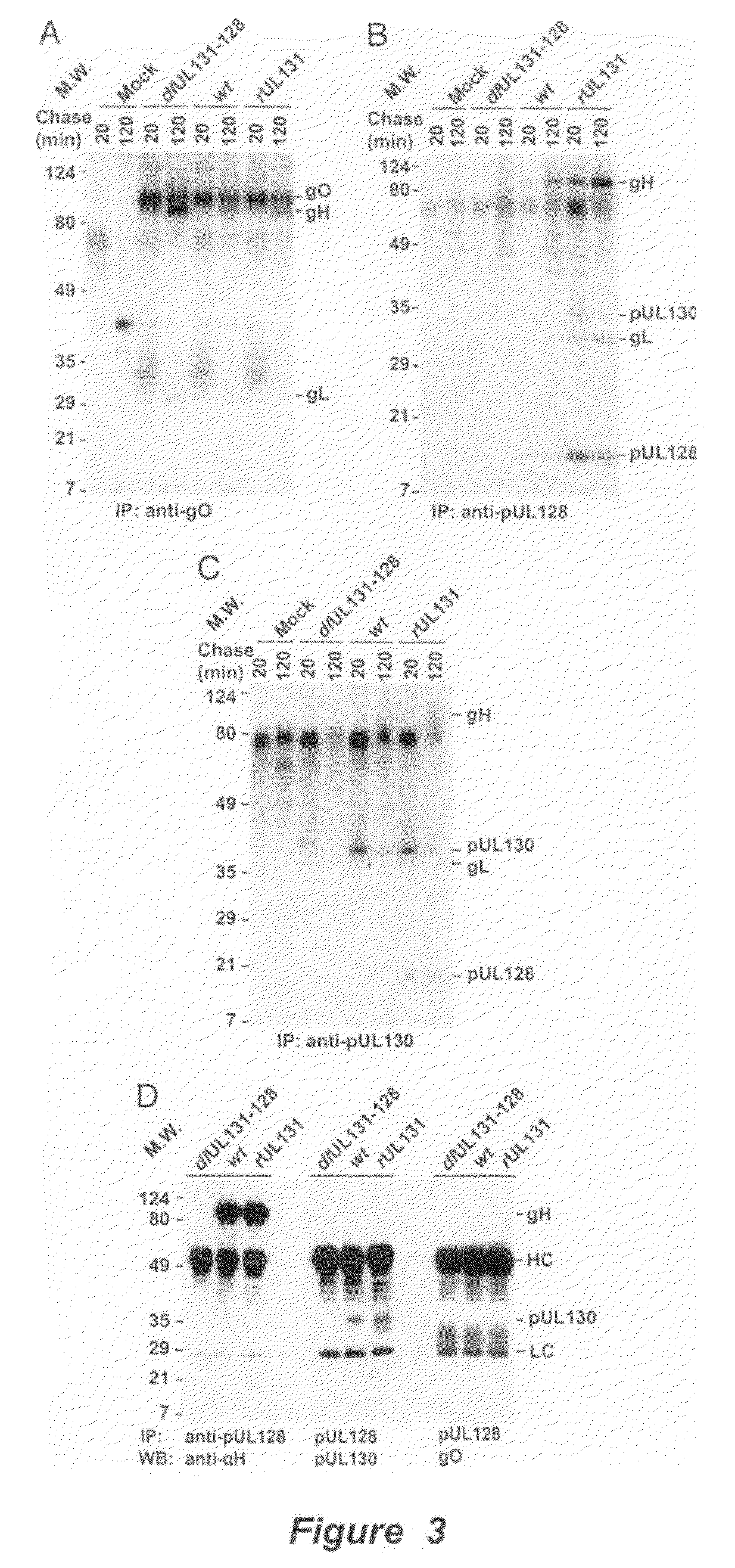
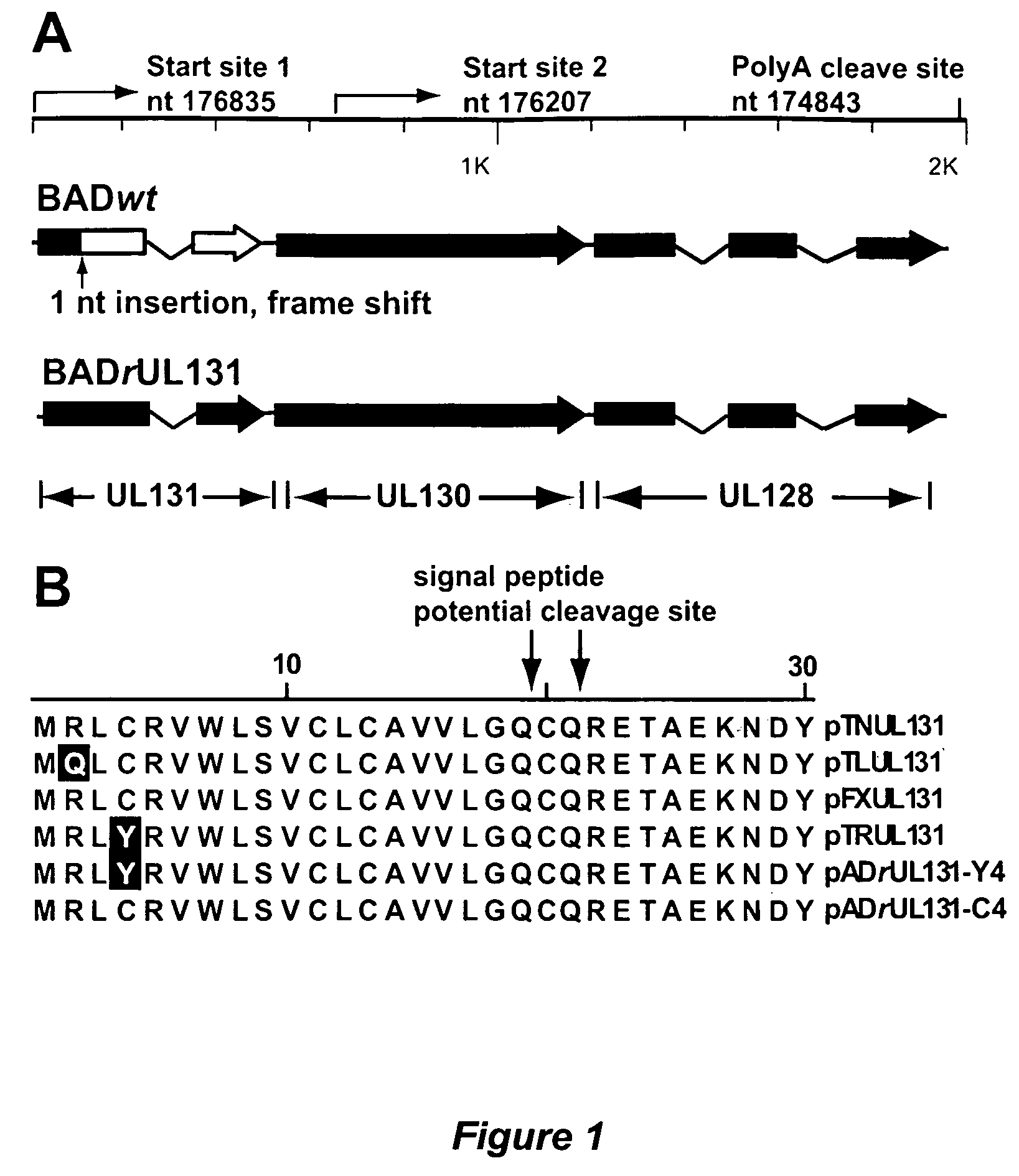
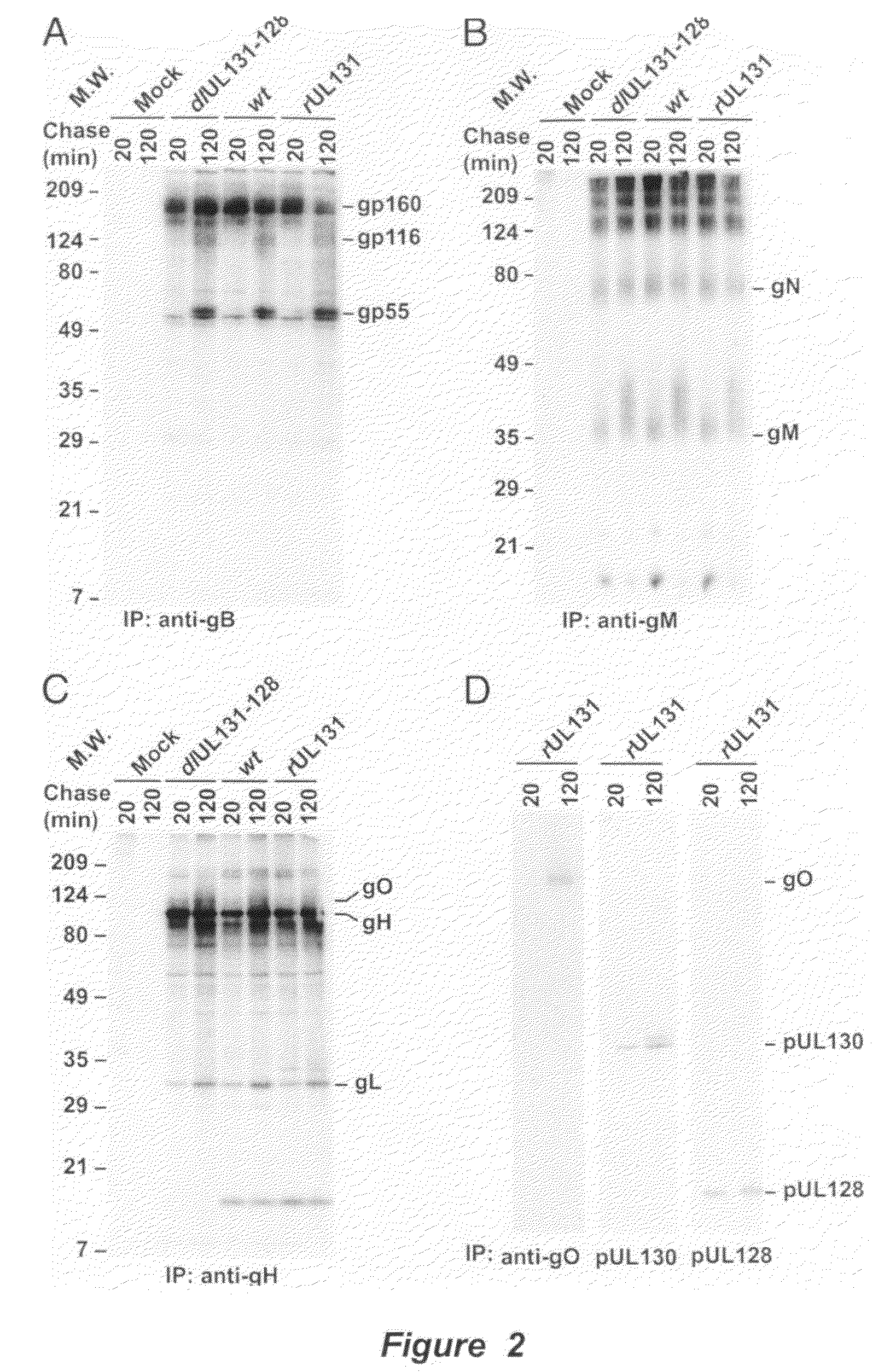
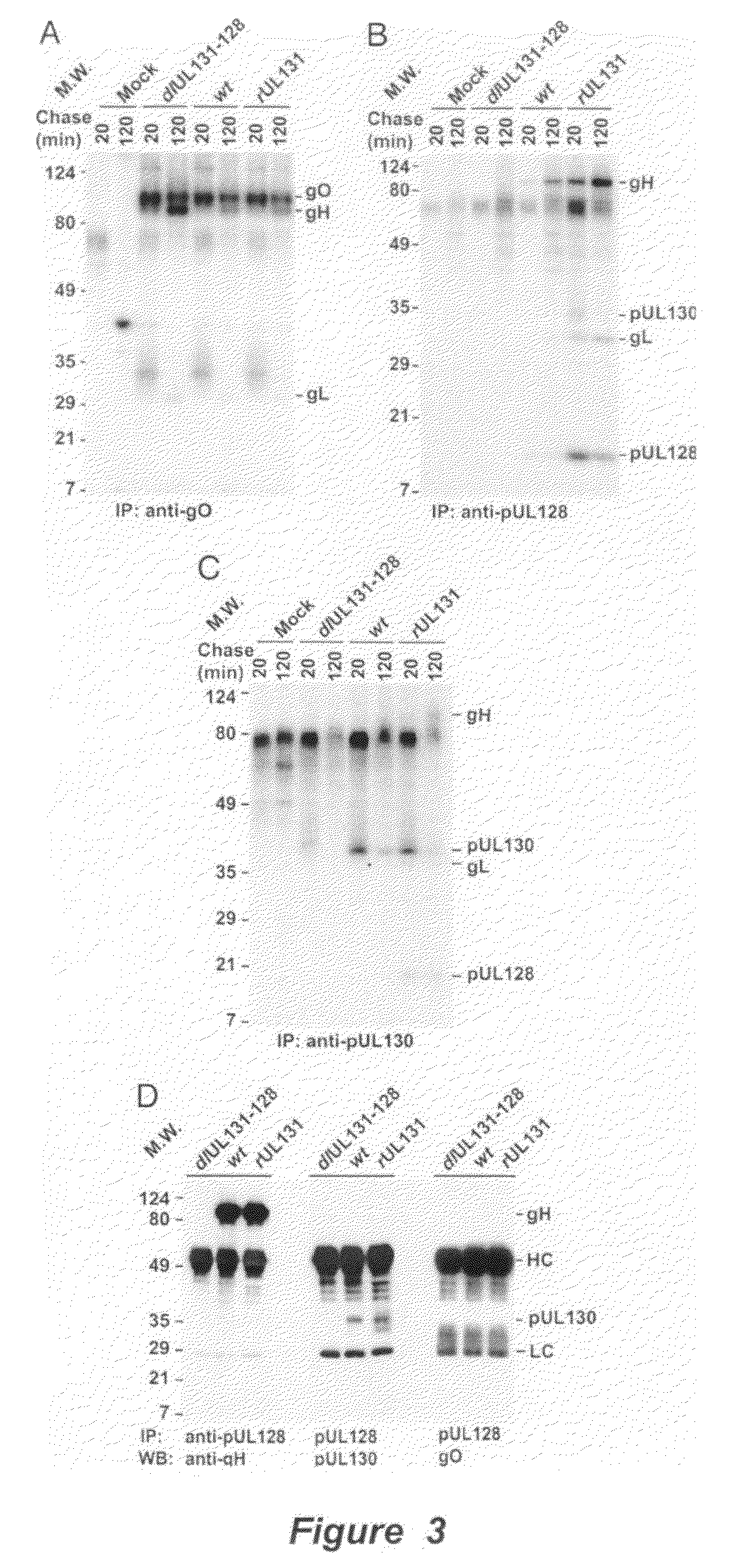
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Recombinant viral vectors
ActiveUS20130266611A1Escalate trimer abundanceImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsViral vectorImmunogenicity
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Microprojection array immunization patch and method
InactiveUS20050025778A1Adequate buffering capacityIncrease concentrationMicroneedlesSurgeryCurative effectVaccine antigen
Microprojection members (10) having a reservoir containing an antigenic agent and methods of using such members to vaccinate mammals (e.g., humans) are disclosed. The microprojection members are used to transdermally deliver an antigenic agent (e.g., a vaccine antigen) with substantially reduced skin reactions. This is achieved by delivering an induction amount and thereafter delivering one or more subsequent booster amounts. The induction amount is relatively larger than the booster amount. This technology has broad applicability for a wide variety of therapeutic vaccines to improve efficacy and convenience of use.
Owner:ALZA CORP
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
Methods and compositions for diseases associated with amyloidosis
The present invention generally relates to the detection, treatment or prevention of disease states. Specifically, the present invention relates to the detection, treatment or prevention of amyloidosis or amyloid-associated diseases. The present invention further comprises methods and compositions comprising therapeutic vaccines, antisera and molecular constructs, comprising expression vectors and fusion proteins encoded therein.
Owner:AVENTIS PHARMA SA +1
Melan-A- carrier conjugates
InactiveUS7537767B2Improve responseMore immunogenicSsRNA viruses negative-senseBiocideDiseaseAllergy
Owner:CYTOS BIOTECHNOLOGY AG
Immunoselection of recombinant vesicular stomatitis virus expressing hiv-1 proteins by broadly neutralizing antibodies
InactiveUS20130189754A1Simplifies enrichmentIncrease exposureViral/bacteriophage medical ingredientsElectrical/wave energy microorganism treatmentImmunogenicityVesicular stomatitis virus
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Immunogen for preparation of therapeutic vaccines or drugs for treatment of hepatitis b and the producing method and use thereof
Owner:NOVEL THERA RES INST LDT +1
Protein cage immunotherapeutics
The present invention provides compositions of heat shock protein cages for use in therapeutic vaccines. The heat shock protein cages of the invention have attached antigen, located either on the interior or exterior of the protein cage, and optionally an adjuvant.
Owner:MONTANA STATE UNIVERSITY
Therapeutic vaccine
ActiveUS20080267986A1Preventing and alleviating amyloidosisRestoring cognitive memory capacityBiocideSenses disorderNervous systemMammal
The present invention is related to methods and compositions for the therapeutic and diagnostic use in the treatment of diseases and disorders which are caused by or associated with amyloid or amyloid-like proteins including amyloidosis.In particular, the present invention provides novel methods and compositions for eliciting a highly specific and highly effective immune response in an organism, but particularly within an animal, particularly a mammal or a human, which is capable of preventing or alleviating amyloidosis, or the symptoms associated with amyloidosis, a group of diseases and disorders associated with amyloid plaque formation including secondary amyloidosis and age-related amyloidosis including, but not limited to, neurological disorders such as Alzheimer's Disease (AD), including diseases or conditions characterized by a loss of cognitive memory capacity such as, for example, mild cognitive impairment (MCI).
Owner:AC IMMUNE SA
Yeast-based vaccines as immunotherapy
ActiveUS20080069833A1Prevents posttranslational modificationEnhance immune responseBiocideAntibody mimetics/scaffoldsBody fluidHumoral immune reaction
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Yeast-Based Vaccines As Immunotherapy
ActiveUS20110150909A1Reduce or prevent symptomPrevent modifyingAntibacterial agentsBiocideImmunotherapyDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Novel therapeutic vaccine formulations
The present invention relates to a novel method and formulation for the induction of immune responses against polypeptide antigens. In particular, the invention provides a method and formulation for induction of cytotoxic T cell responses against a polypeptide antigen of choice. The formulations are characterized by containing chitosan in admixture with the polypeptide antigen, preferably in the form of microparticles that may be cross-linked.
Owner:PHARMEXA
Packaging of Immunostimulatory Substances into Virus-Like Particles: Method of Preparation and Use
InactiveUS20120301499A1More immunogenicEnhance immune responseAntibody mimetics/scaffoldsVirus peptidesVaccinationViral disease
The invention relates to the finding that virus like particles (VLPs) can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs). Such CpG-VLPs are dramatically more immunogenic than their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against antigens optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against the VLP itself. In addition, the T cell responses against both the VLPs and antigens are especially directed to the Th1 type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:KUROS US LLC
Packaging of Immunostimulatory Substances Into Virus-Like Particles: Method of Preparation and Use
InactiveUS20100098722A1Improve responseMore immunogenicSsRNA viruses negative-senseTumor rejection antigen precursorsDiseaseAllergy
The invention relates to the finding that virus like particles (VLPs) can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs). Such CpG-VLPs are dramatically more immunogenic than their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against antigens optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against the VLP itself. In addition, the T cell responses against both the VLPs and antigens are especially directed to the Th1 type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Hiv-peptide-carrier-conjugates
InactiveUS20060210588A1More immunogenicEnhance immune responseSsRNA viruses negative-senseViral antigen ingredientsAbnormal tissue growthAllergy
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a modified virus-like particle (VLP) comprising a VLP which can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs), and particular HIV peptides linked thereto. Such CpG-VLPs are dramatically more immunogenic that their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against HIV peptides optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against HIV peptides are especially directed to the Th1 type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Therapeutic vaccine targeted against p-glycoprotein 170 for inhibiting multidrug resistance in the treatment of cancers
InactiveUS20080026995A1High expressionSaccharide peptide ingredientsImmunoglobulinsPolyethylene glycolPhospholipid
The invention relates to conjugates comprising all or part of the amino acid sequences of at least one peptide derived from an extracellular loop of the P-170 protein. The peptide may be covalently attached to spacers which may be polyethyleneglycol (PEG), polyglycine, polylysine or any polymer chain suitable for human use and is coupled at its free end to a phospholipids, e.g., phosphatidylethanolamine or any other chemically suitable phospholipid.
Owner:AC IMMUNE SA
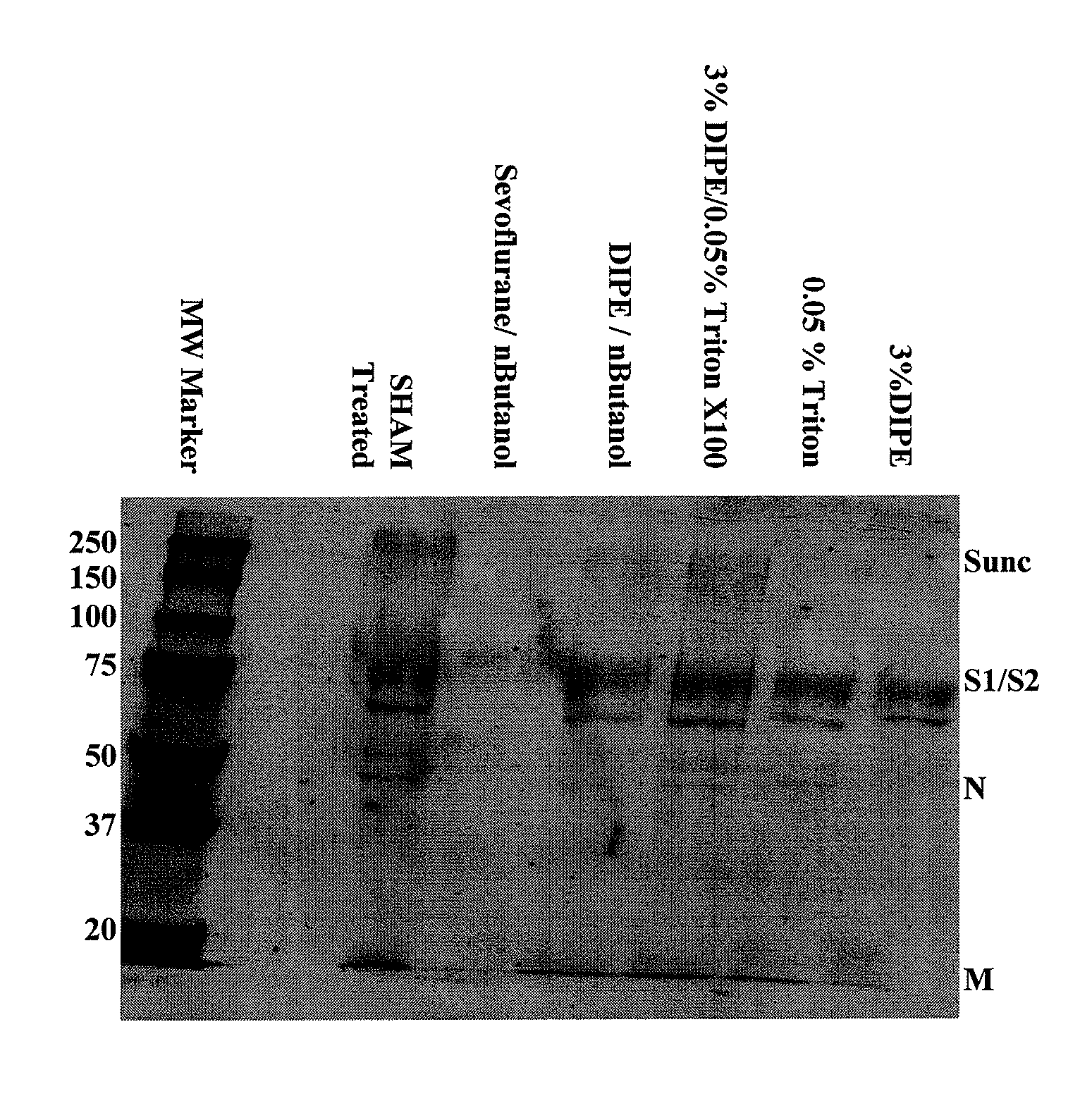
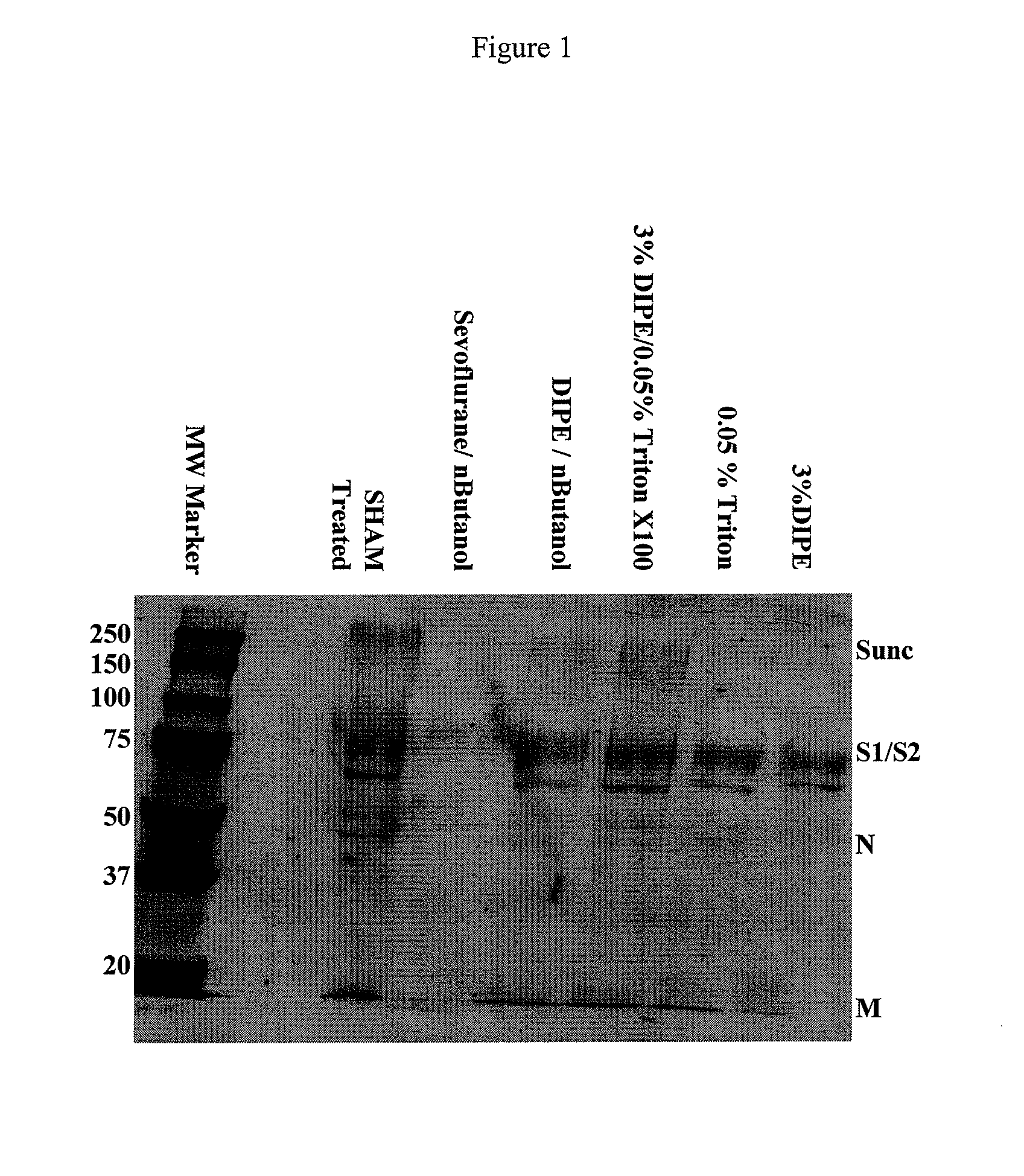
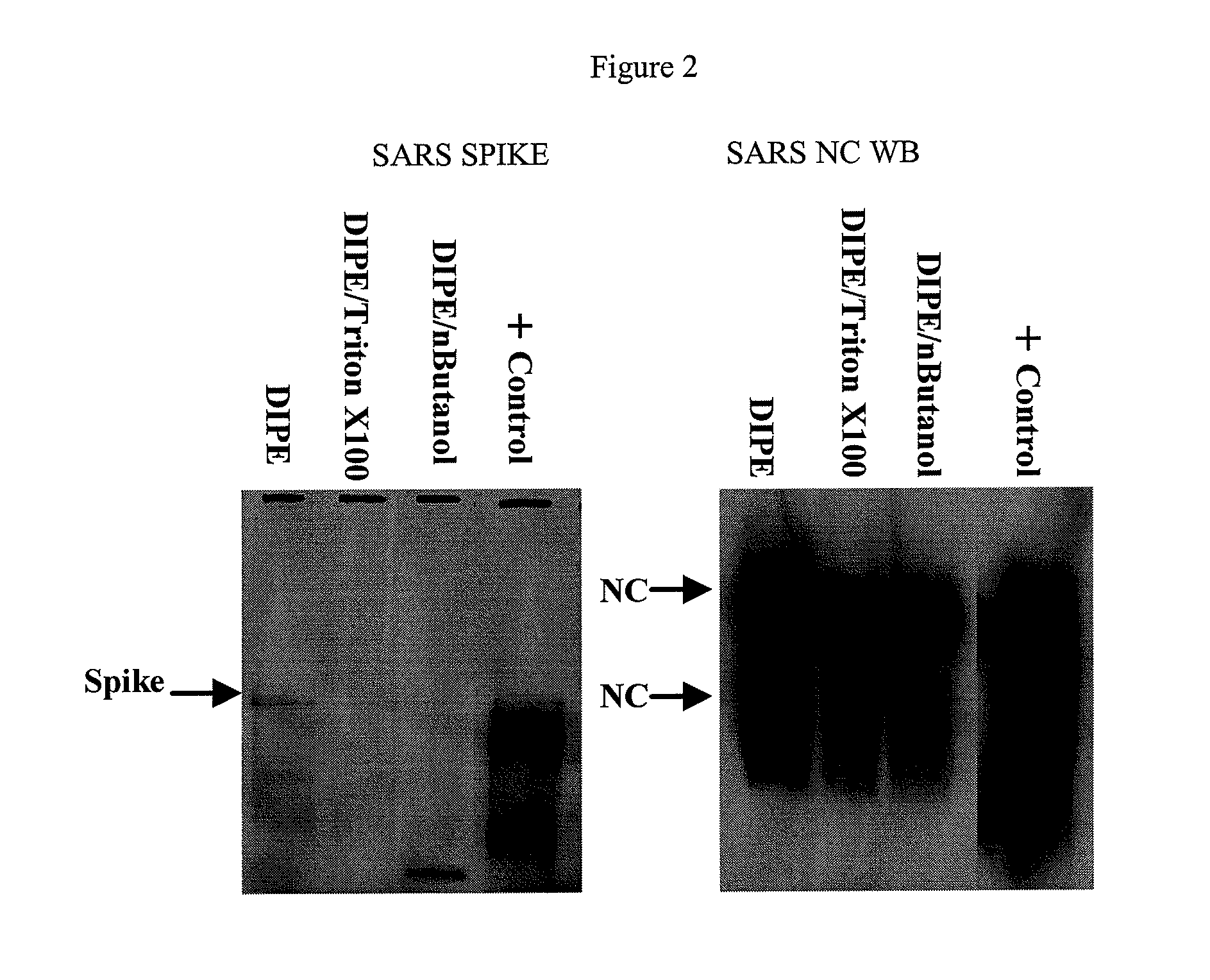
SARS Vaccine Compositions and Methods of Making and Using Them
InactiveUS20090017069A1The process is simple and effectiveThe method is simple and efficientSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Owner:ELI LILLY & CO +1
Melan-a peptide analogue-virus-like-particle conjugates
InactiveUS20060204475A1More immunogenicImprove responseSsRNA viruses negative-senseBiocideAbnormal tissue growthViral disease
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a modified virus-like particle (VLP) comprising a VLP which can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs), and particular peptides derived from MelanA linked thereto. Such CpGVLPs are dramatically more immunogenic than their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against MelanA peptide analogues optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against the VLP itself. In addition, the T cell responses against the MelanA peptide analogues are especially directed to the Thl type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Combined measles-human papilloma vacine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
Powerful vaccine composition comprising lipopeptide and poly I:C as an adjuvant
ActiveUS8216595B2Stimulate immune responseStrong immune responseSsRNA viruses negative-sensePeptide/protein ingredientsAdjuvantVirus
Owner:CHA VACCINE RES INST CO LTD
Immunogen by using mutant CRM197 of diphtheria toxin as carrier, preparation method, and application
ActiveCN101050236AGood effectHigh recovery ratePeptide/protein ingredientsPeptide preparation methodsN-HydroxysuccinimideCarrier protein
This invention relates to a method for preparing immunogen G17CRM197 containing diphtherin mutant CRM197 as carrier. The method comprises: crosslinking diphtherin mutant CRM197 with gastrin G17 through epsiv-maleimidyl caproic acid N-hydroxysuccinimide eater (EMCS). Diphtherin mutant CRM197 is used as a carrier, and has such advantages as high recovery rate and easy purification. The method has such advantages as high product crosslinking rate, short preparation time, and low cost. Immunogen G17CRM197 can be used as an effective component in therapeutic vaccines, or used in anti-tumor drugs with appropriate immunoadjuvants.
Owner:QILU PHARMA HAINAN
Powerful vaccine composition comprising lipopeptide and poly I:C as an adjuvant
ActiveUS20090155308A1Stimulate immune responseStrong immune responseSsRNA viruses negative-sensePeptide/protein ingredientsAdjuvantVirus
The present invention relates to an adjuvant comprising a lipopeptide and poly I:C. When the adjuvant of the present invention is used, the level of antigen specific antibody induction is synergistically increased and Th1 type immune response is also induced. Therefore, the adjuvant of the present invention can be very effectively used as an adjuvant in the formulation of preventive and therapeutic vaccines for viral or parasitic infection and cancer.
Owner:CHA VACCINE RES INST CO LTD
Vaccine complex for preventing or treating leishmaniases
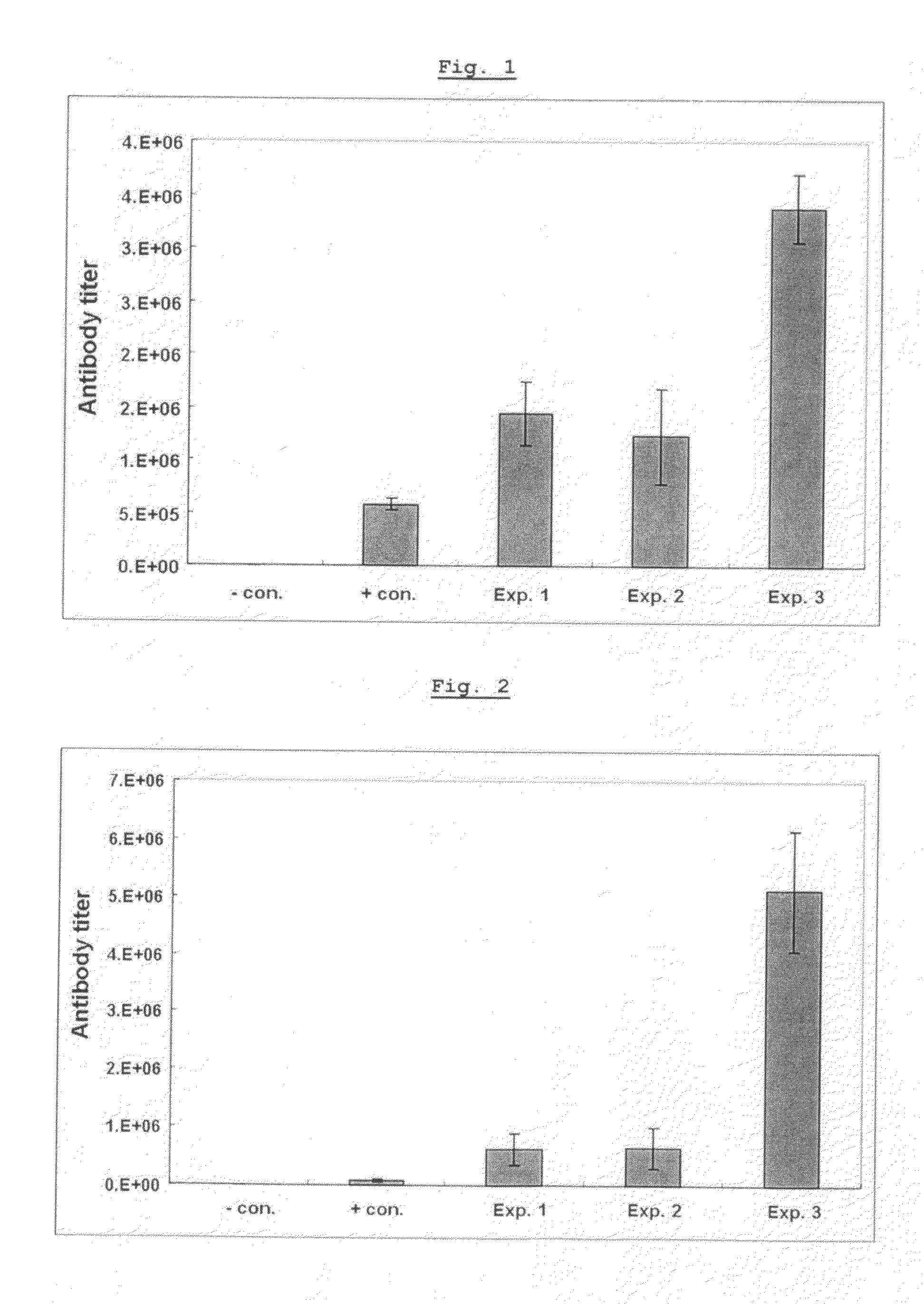
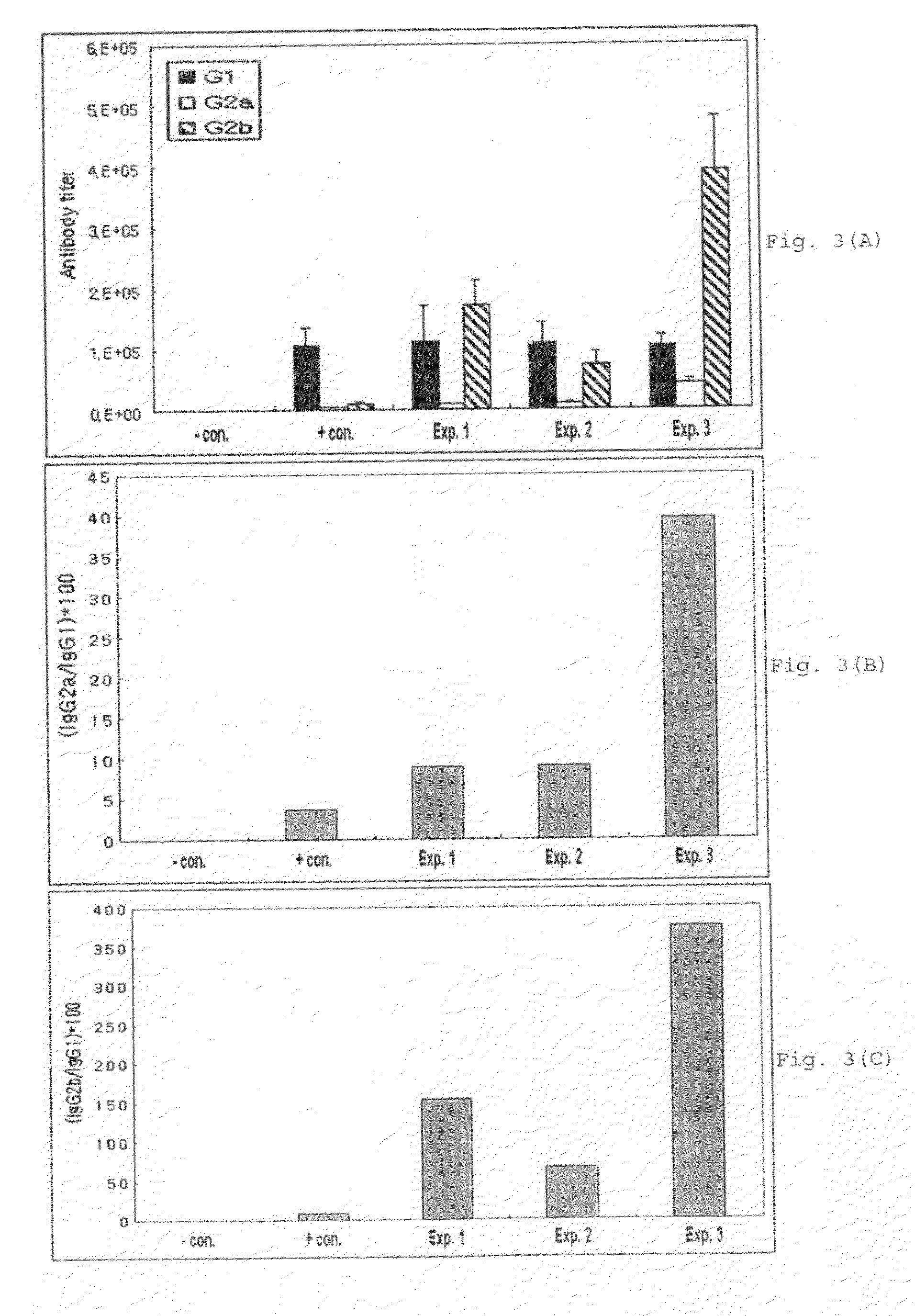
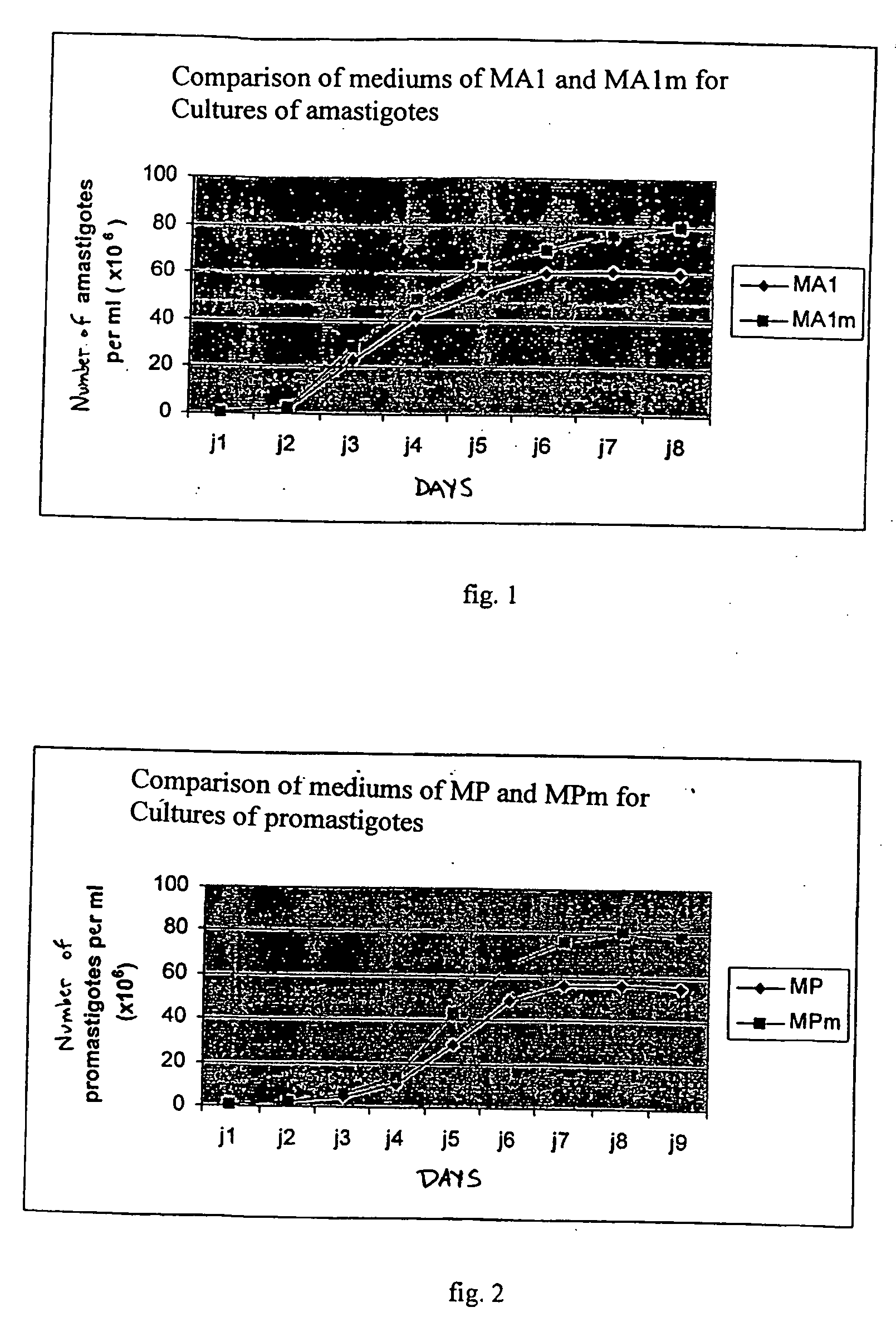
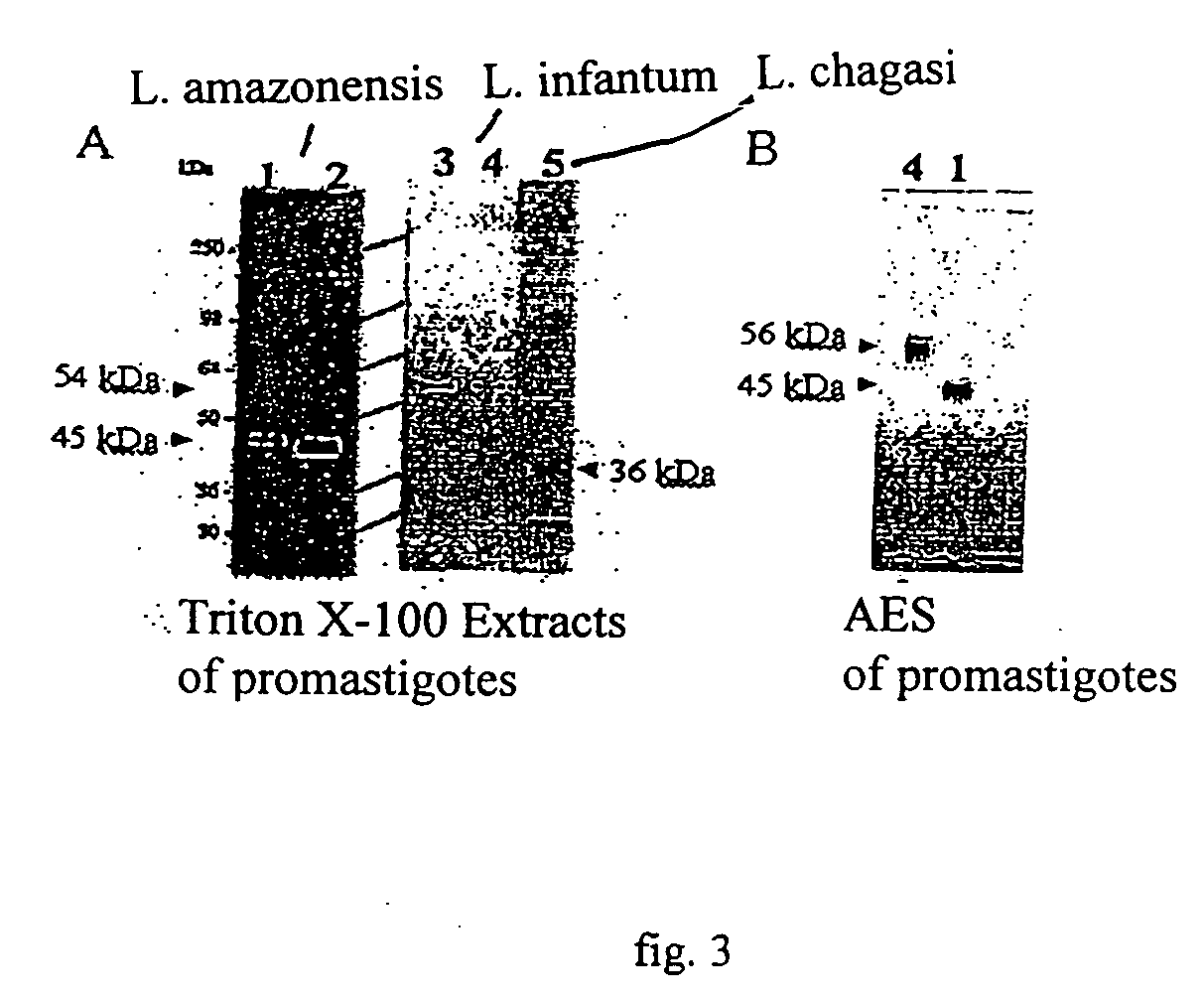
InactiveUS20060073170A1Facilitates emergenceInhibition of differentiationProtozoa antigen ingredientsAntiparasitic agentsSerum free mediaMammal
A therapeutic vaccine complex for preventing or treating leishmaniases and infections mediated by intracellular pathogenic micro-organism in mammals and in particular in humans, members of the dog, car and horse family. The invention is characterized in that it includes excretion secretion molecules derived from Leishmania sp. Promastigotes produced in a specific germ-free and serum-free medium.
Owner:BIO VETO TESTS BVT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com