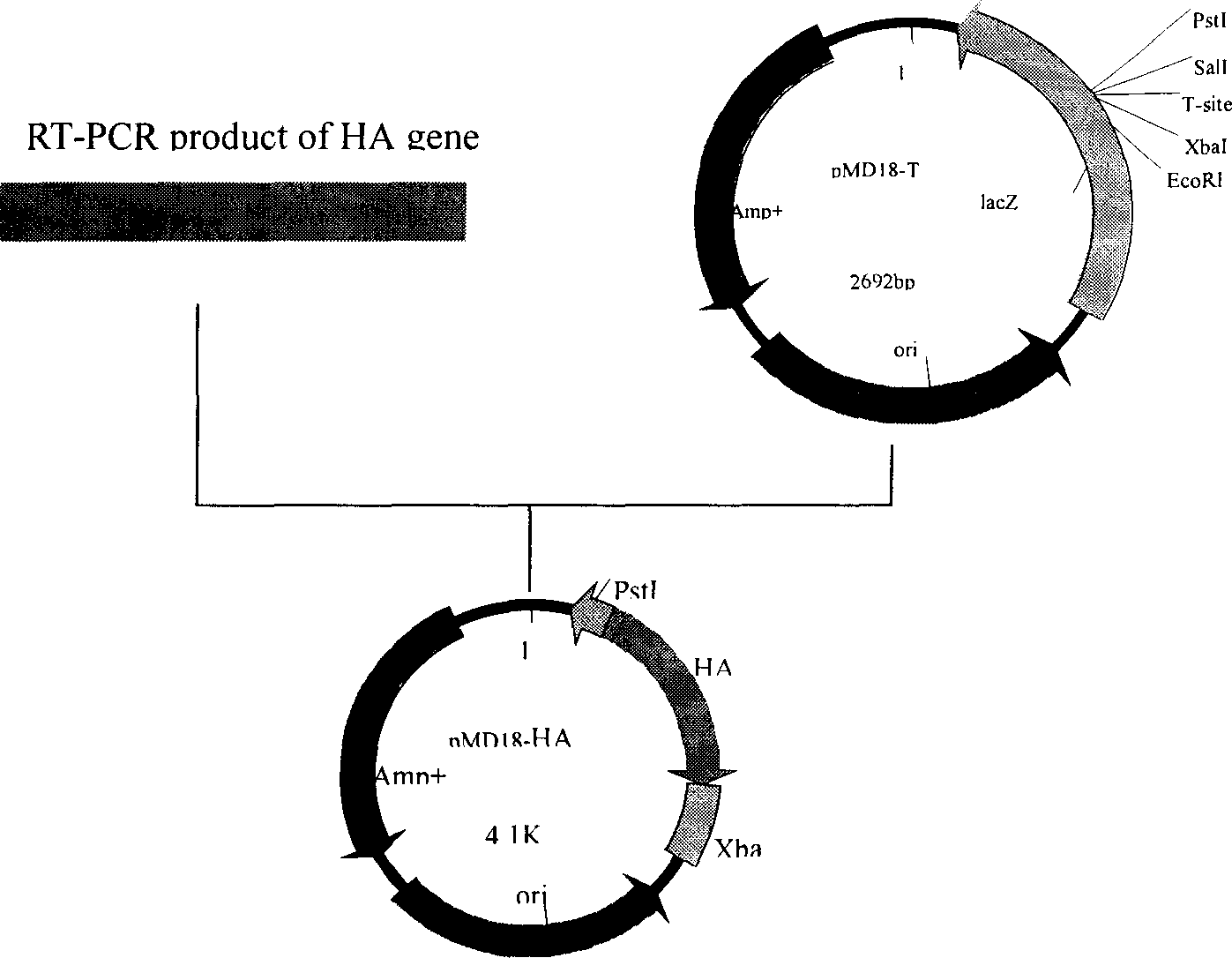
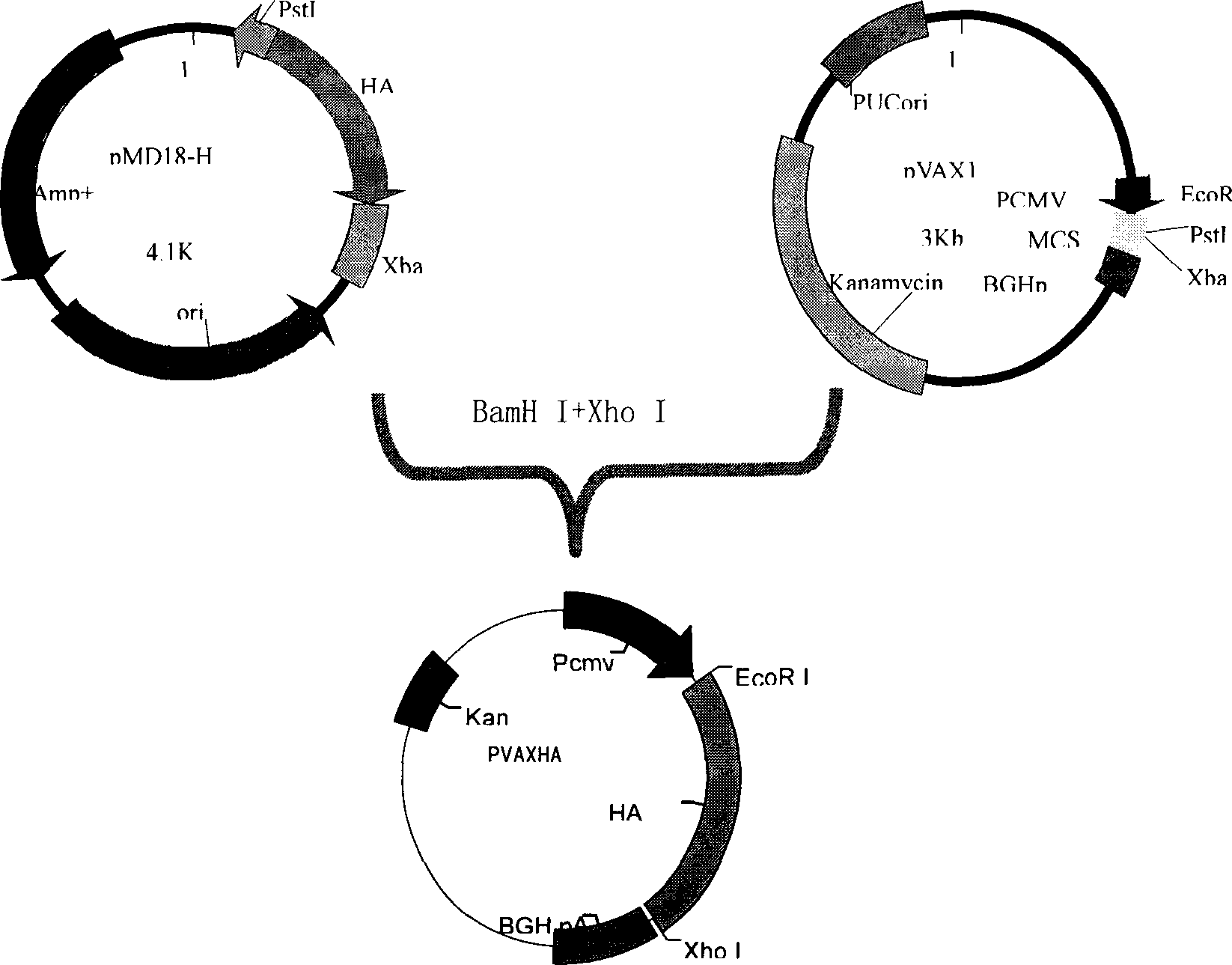
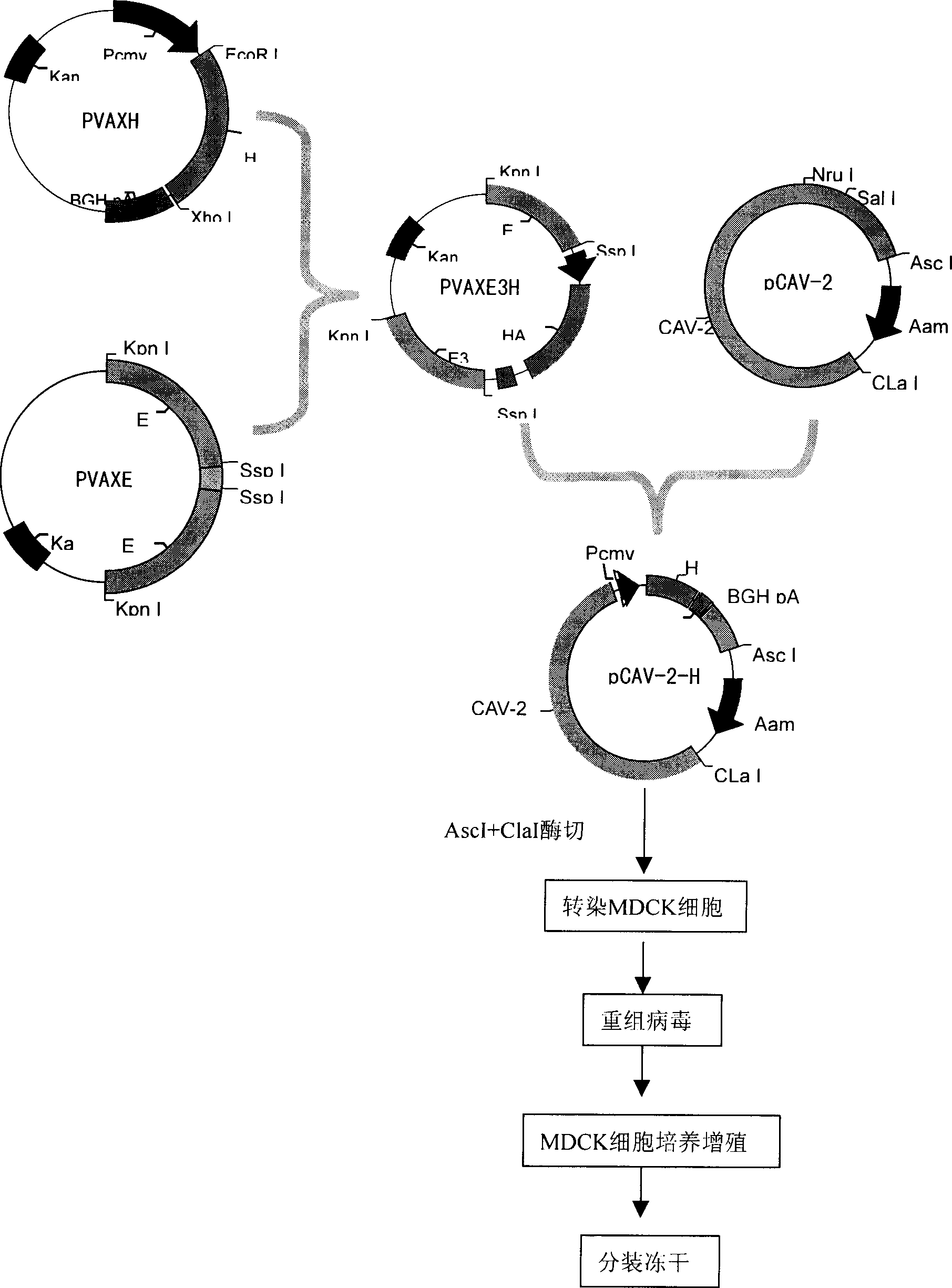
Patents
Literature
39 results about "Canine adenovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infectious canine hepatitis is a viral disease of that is caused by the canine adenovirus CAV-1, a type of DNA virus that causes upper respiratory tract infections.
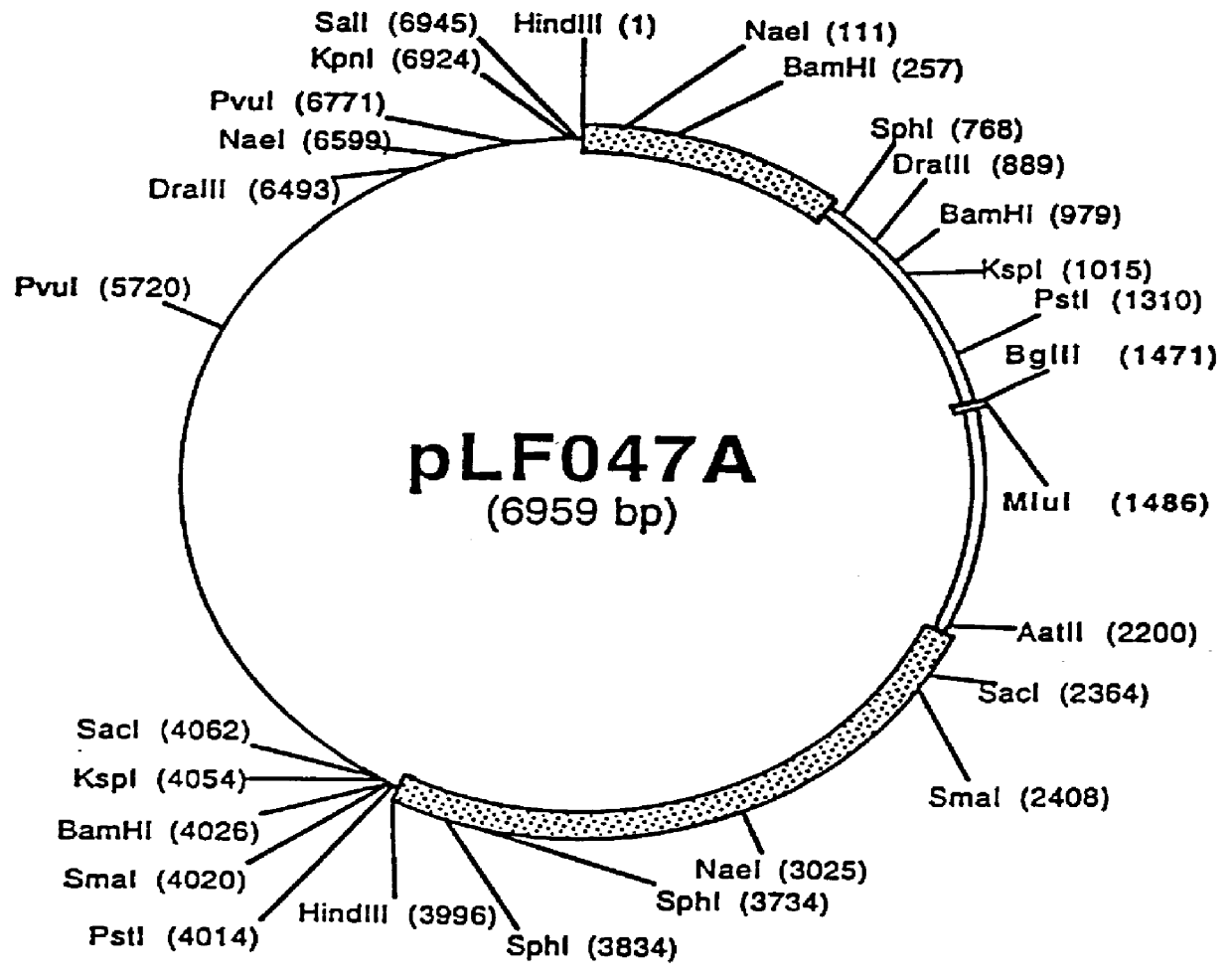
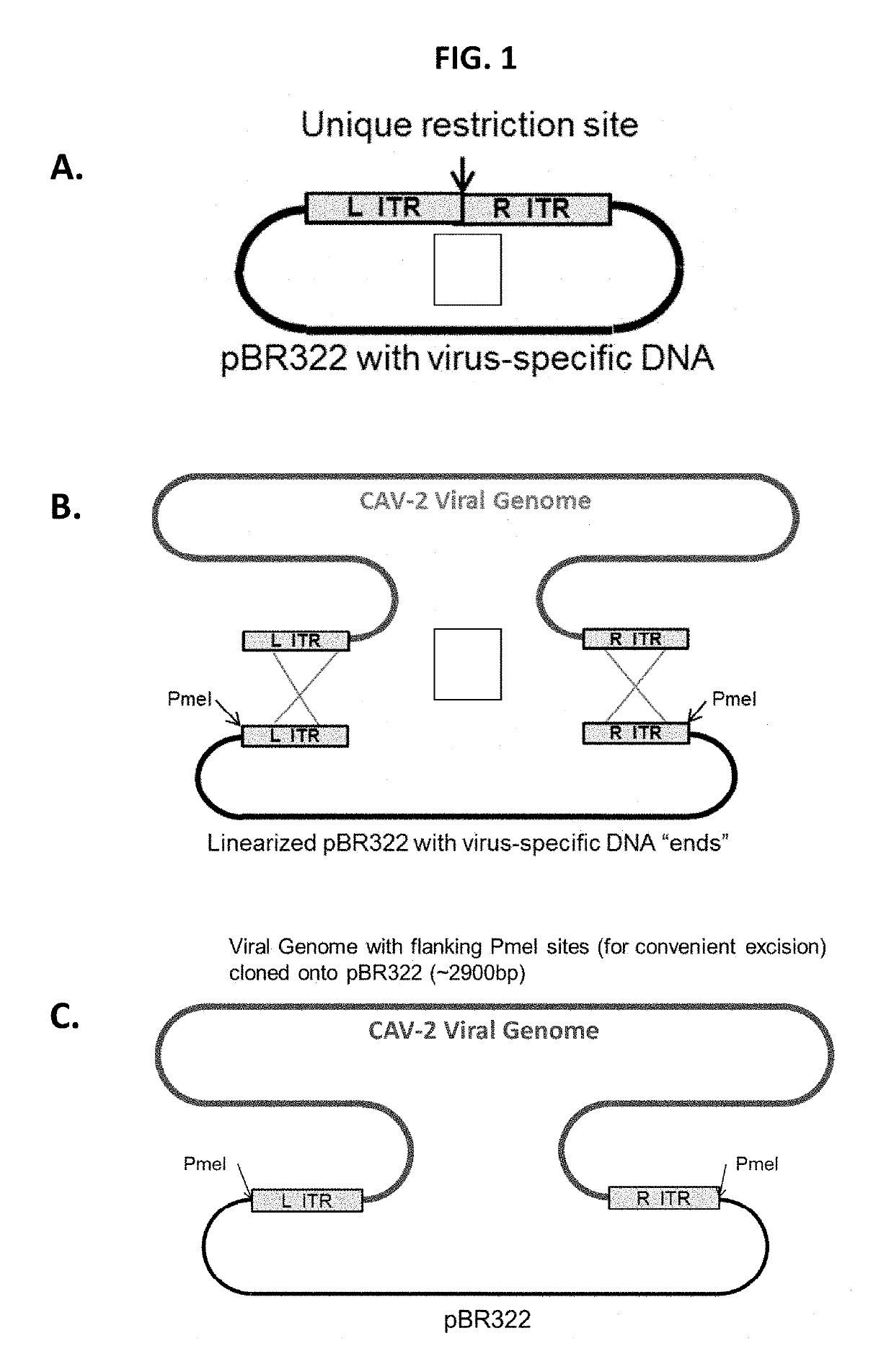
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
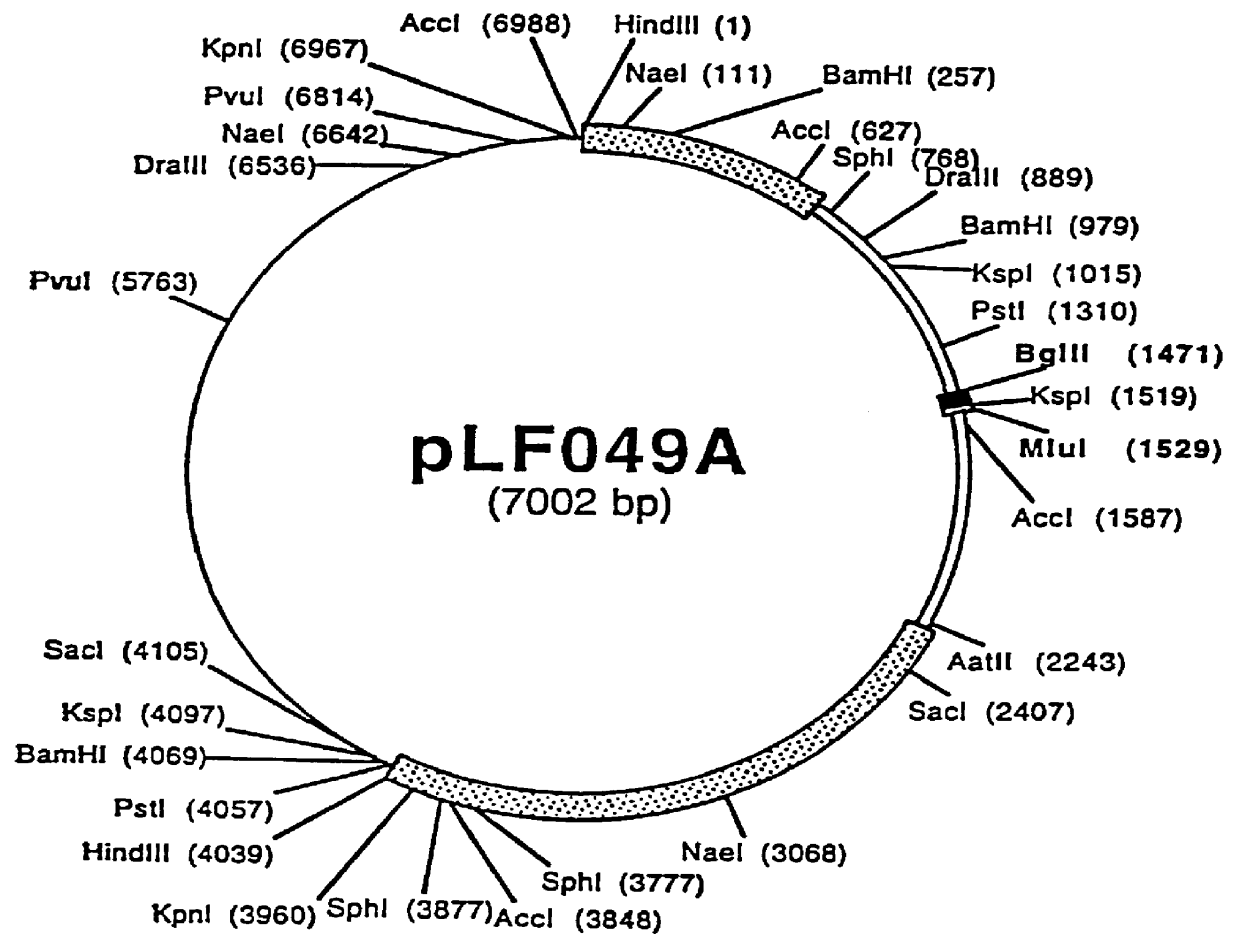
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
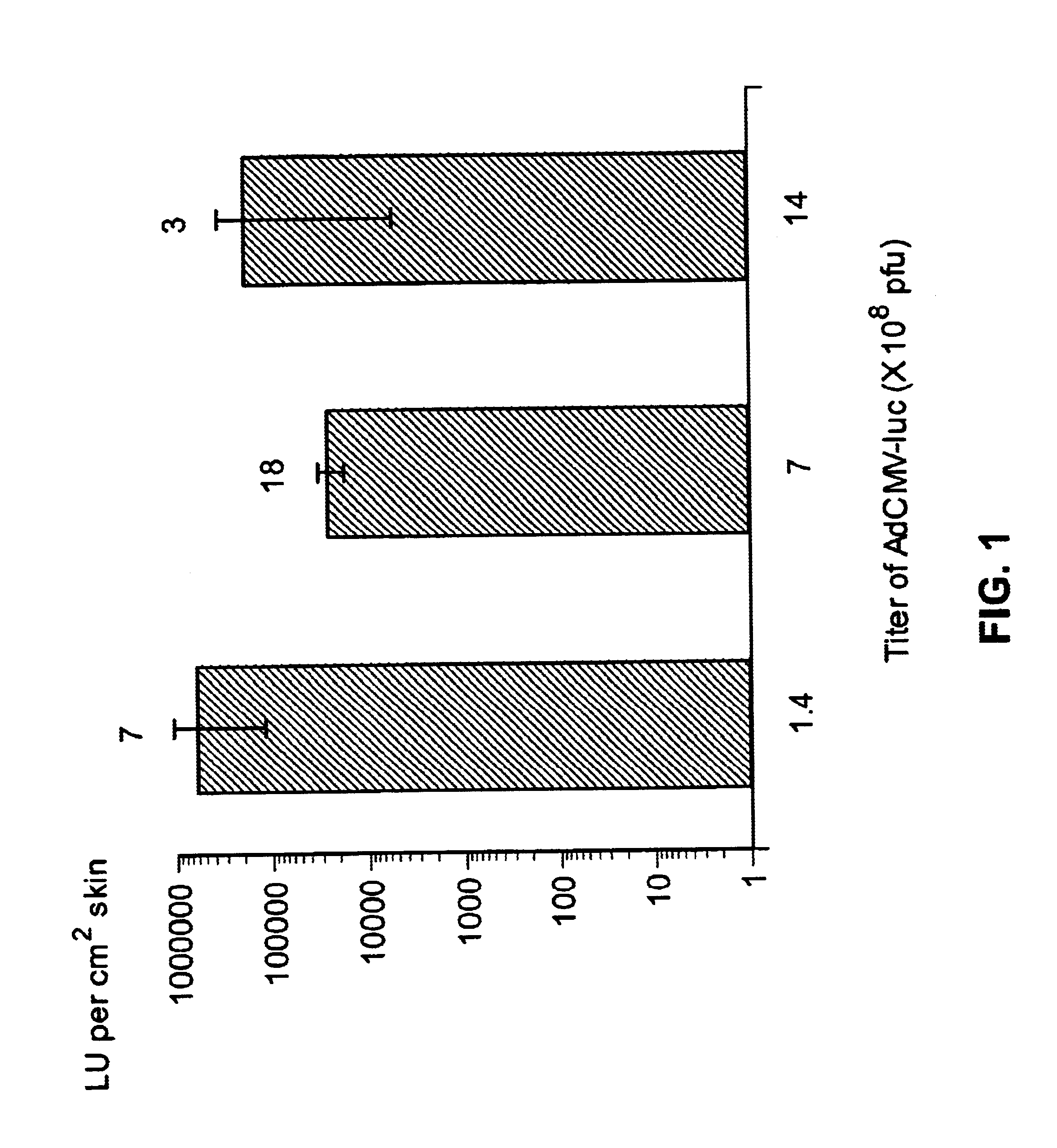
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Canine vaccines against Bordetella bronchiseptica
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
InactiveCN1827172AGood genetic stabilityEasy to storePowder deliveryGenetic material ingredientsAntigenVp4 gene
This invention supplies a series of production techniques of gene recombination live vaccine of swine virus contagion with canine ó� adenovirus as carrier and finished goods. The viral live vectors vaccine takes swine important virus zymad protective antigens gene as object gene, which are chosen from HCV-E1, E2 gene, FMDV-VP1íóVP2íóVP3íóVP4 gene, TGEV-SíóNíóM gene, PEDV-SíóNíóM geneú¼SIV-HAíóNA geneú¼RV-GíóN gene, etc. The produced vaccines contain recombined swine influenza virus HA gene adenovirus carrier live vaccine, swine plague virus E2 gene adenovirus carrier live vaccine and recombination swine AsiaI foot-and-mouth disease virus VPI gene adenovirus carrier live vaccine. Recombination virus has good inheritance stability, and vaccine immunization can induct pig develop differential antiviral neutralization antibody. It has good immune protection effect and has no toxic side effect. The goods are facilitating for preserve and transportú”it has long storage life and simple technics, and it fits for commercial manufacture.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Stable cell line MDCK for amplifying recombinant canine adenovirus (CAV2) and construction method of stable cell line
InactiveCN108342362ASolve problems that are difficult to expressImprove stabilityGenetically modified cellsVirus peptidesLentivirusTiter
The invention provides a stable cell line for expressing canine adenovirus genes E1A and E1B and a construction method and application of the stable cell line. The two genes E1A and E1B in an E1 region are constructed in a lentivirus carrier, then an MDCK cell stably expresses the genes E1A and E1B respectively, and the MDCK-E1A-E1B stable cell line is constructed. The E1 gene which is long in segment and not easy to construct is split into the genes E1A and E1B which are short in segment and easy to construct, so that the success rate of constructing stable cells is increased; the genes E1A and E1B carry fluorescent proteins with different colors so that the stability of the stable cells can be monitored in real time. Experiments prove that the MDCK-E1A-E1B stable cell line is higher in titer of the amplified canine adenovirus than that of an MDCK-E1 stable cell line, and a good foundation is laid for application of the canine adenovirus in the fields of virus live carrier vaccines, gene therapy, cancer treatment and the like.
Owner:BRAINVTA (WUHAN) CO LTD
Multiplex polymerase chain reaction (PCR) detection primers for simultaneously detecting canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus
InactiveCN102618666AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCanine distemper virus CDVCanine parvovirus
The invention discloses a set of multiplex polymerase chain reaction (PCR) detection primers for detecting canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus. The primers comprise three pairs of primers, wherein sequences of primers for detecting canine distemper virus are shown as SEQ ID NO:1 and SEQ ID NO:2; sequences of primers for detecting canine parvovirus are shown as SEQ ID NO:3 and SEQ ID NO:4; and sequences of primers for detecting type I canine adenovirus and type II canine adenovirus are shown as SEQ ID NO:5 and SEQ ID NO:6. The primers can simultaneously detect canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus through a multiplex PCR method, and have the advantages of high specificity, high sensitivity, high repeatability and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Immune colloidal gold test stripe and preparation method and application
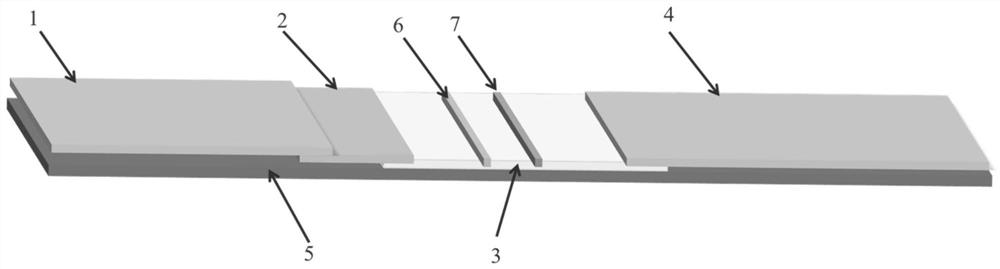
The invention discloses an immune colloidal gold test stripe and preparation method and application, and the immune colloidal gold test stripe comprises a rigid polyvinyl chloride backer board, a nitrocellulose film, a sample mat, a colloidal gold combination mat and a water absorption mat, the nitrocellulose film is pasted to the rigid polyvinyl chloride backer board, the colloidal gold combination mat is pasted to one end of the nitrocellulose film, the sample mat is pasted on the colloidal gold combination mat, the water absorption mat is located at the other end of the nitrocellulose film; the preparation method comprises the steps as follows: (1) having reaction on the trisodium citrate and chloroauric acid for preparing colloidal gold; (2) marking the canine adenovirus resisting virus II type monoclonal antibody on the colloidal gold test stripe; (3) covering the canine adenovirus resisting virus II type antibody on the nitrocellulose film; (4) orderly pasting together for obtaining colloidal gold test stripe. The application of the immune colloidal gold test stripe for detecting canine adenovirus virus II type, the test stripe is simple in operation, fast and sensitive in detection, clear in result and easy in judgment. The immune colloidal gold test stripe is convenient to carry and use for saving the sickness detection cost.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for labeling antibodies by colorful fluorescent granules and test paper strip prepared from antibodies
InactiveCN107132348AThe result is accurateRealize qualitative and quantitative simultaneous detectionBiological material analysisAnimal virusLeucosis
The invention provides a method for labeling antibodies by colorful fluorescent granules and a test paper strip prepared from the antibodies. Animal viruses including, but not limited to, canine distemper viruses, canine parvoviruses, canine adenoviruses, canine coronavirus, rabies viruses, feline leukemia viruses, Marek's disease viruses, Newcastle disease viruses and the like are used as targets, and the corresponding antibodies labeled by colorful fluorescent micro-spheres are prepared by the aid of chemical covalent processes and can be applied to detecting the animal viruses. The method for labeling the antibodies by the colorful fluorescent granules and the test paper strip prepared from the antibodies have the advantages that colorful detection lines with bright developed colors can appear during detection, and accordingly qualitative results can be obtained; the contents of the animal viruses in samples further can be subsequently quantitatively obtained, accordingly, test results are clear and are high in stability, and the test paper strip can be stored at the room temperature for a long time.
Owner:江苏雷森生物科技有限公司
Canine adenovirus vectors
ActiveUS20180080045A1Facilitate transgene expressionReduce in quantitySsRNA viruses negative-senseVectorsVector vaccineImmunogenicity
The present invention relates to the field of CAdV vector vaccines, and especially to promoters suitable to express target antigens from such vector vaccines. Disclosed and claimed are recombinant canine adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. Additionally, disclosed and claimed are truncated EHV4 promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Recombinant canine adenovirus type 2 transfer vector, construction method and application thereof
InactiveCN101358202AReduce workloadStable biological propertiesViruses/bacteriophagesFermentationPurification methodsFluorescence
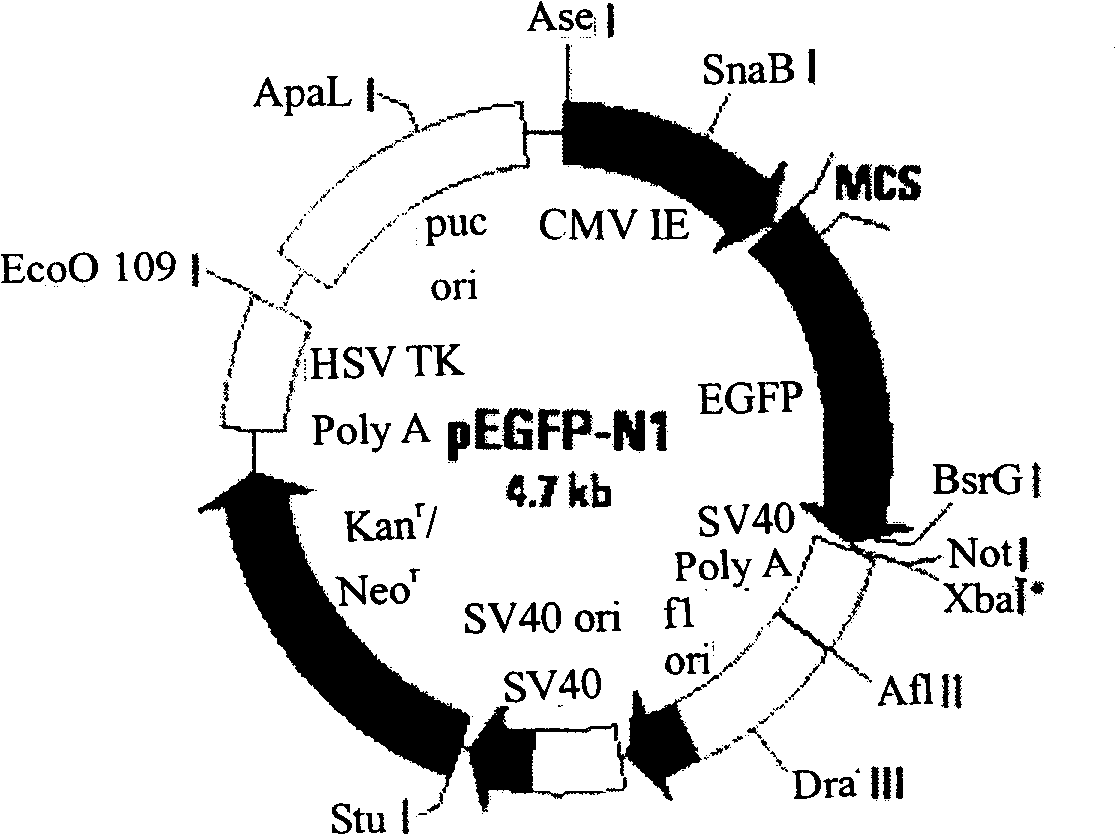
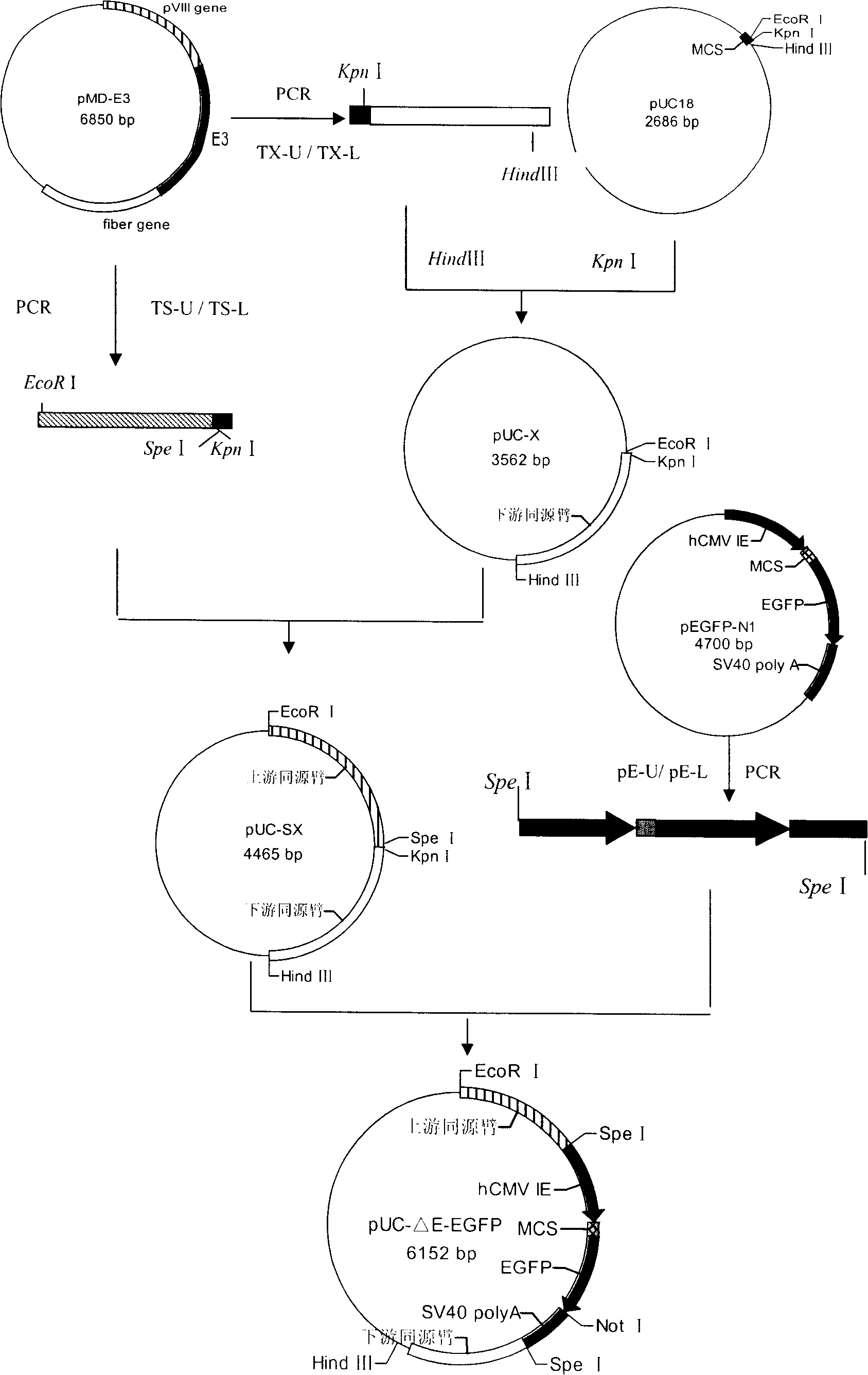
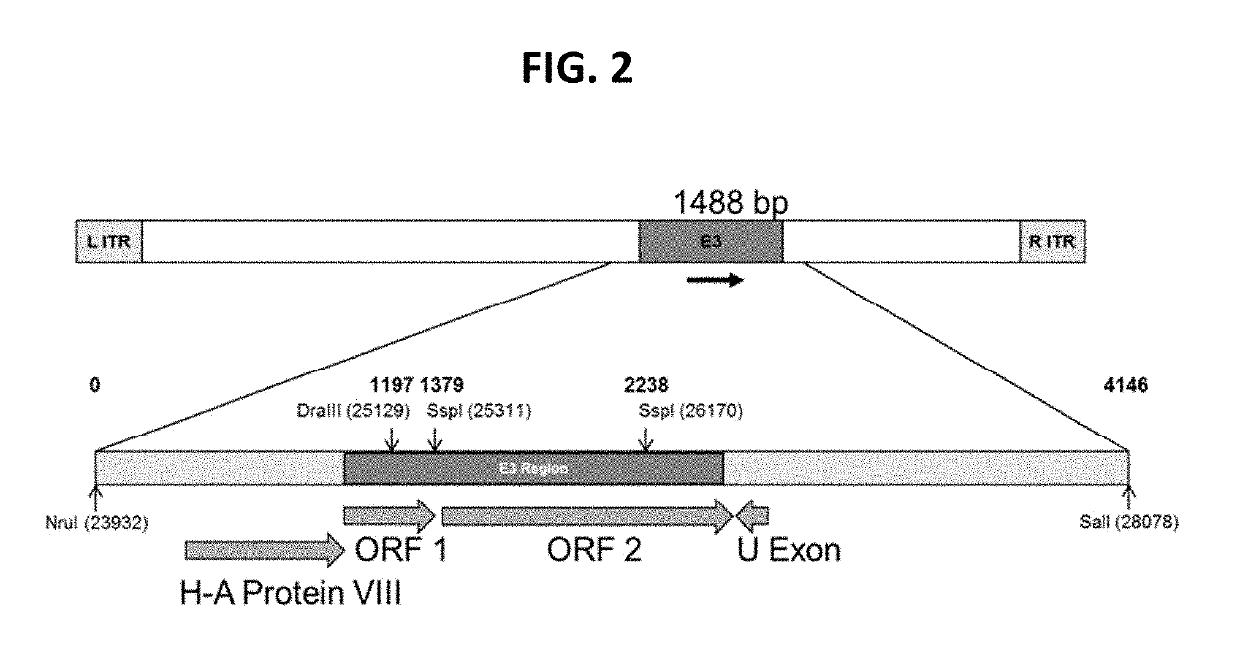
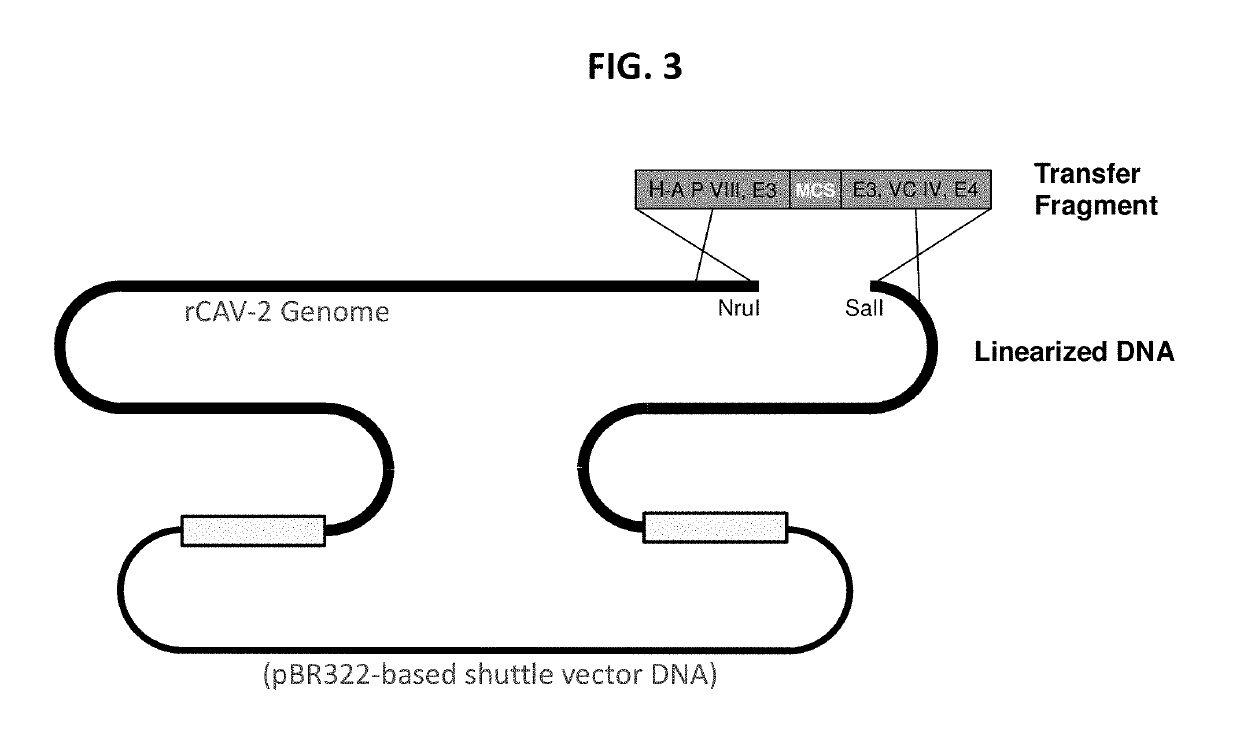
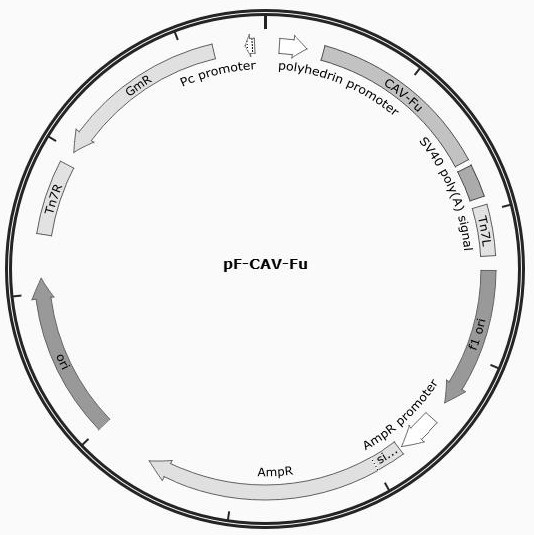
The present invention discloses a recombinant canine adenovirus-2 transfer vector, a method for constructing the recombinant canine adenovirus-2 transfer vector and an applications thereof. The recombinant canine adenovirus-2 transfer vector loses the 1412bp fragment of the E3 region, a large fragment of exogenous gene can be inserted, and moreover, a hCMV IE promoter, a multiple cloning site, an enhanced green fluorescent protein gene and a SV40 early transcribed Poly A signal sequence can be inserted in the position of the lost fragment of the E3 region. The method successfully constructs the lost recombinant CAV-2 transfer vector of the E3 region, obtains a purified canine adenovirus-2 strain containing EGFP reporter gene and optimizes the cloning and purification method thereof, an evaluation indicates that the biological property of the recombinant virus is stable, and therefore the present invention provides a technical platform for the further development of CAV-2 live vector vaccine and related fundamental researches.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116AGenetic material ingredientsViruses/bacteriophagesSwine Fever VirusRecombinant vaccines
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
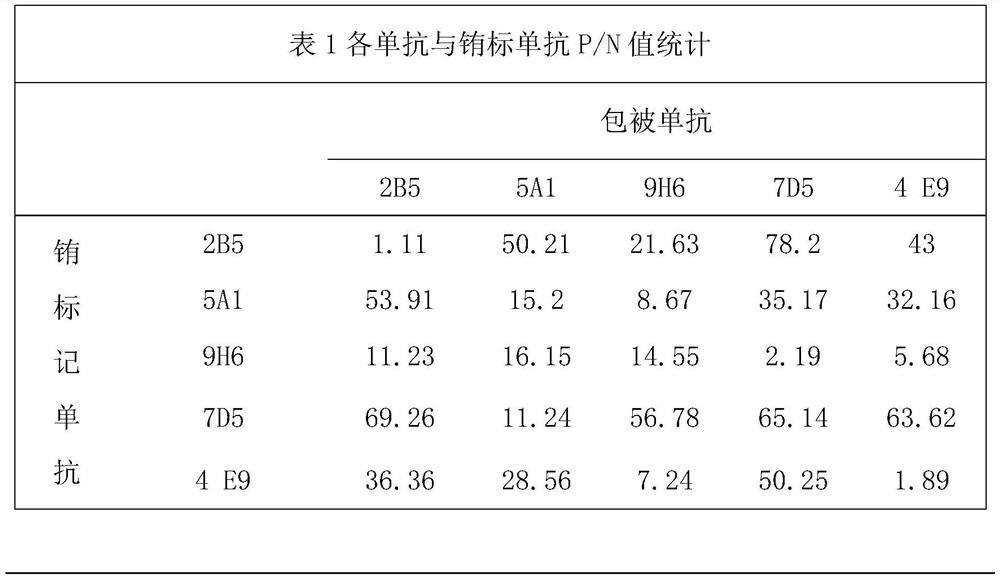
Monoclonal antibody specifically bound to CAV (canine adenovirus), pharmaceutical composition, kit and application of kit
ActiveCN109232734AOvercoming the problem of low detection sensitivityAvoid missing detectionBiological material analysisImmunoglobulins against virusesMouse monoclonal antibodyLeak detection
The invention provides a variable region sequence of a mouse monoclonal antibody specifically bound to a CAV (canine adenovirus) and an antibody specifically bound to the CAV. The antibody can be usedfor preparing a kit and pharmaceutical composition. According to the kit, the problem of low detection sensitivity in the prior art is solved, leak detection and false negative are avoided, various targets can be detected, and the kit has the advantages of being rapid, simple and accurate; the pharmaceutical composition can effectively prevent and treat disease caused by CAV-1 and CAV-2.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Novel vaccine for dog
InactiveUS20110091490A1Low costShorten the timeSsRNA viruses negative-senseViral antigen ingredientsCanine parvovirus infectionCanine distemper virus CDV
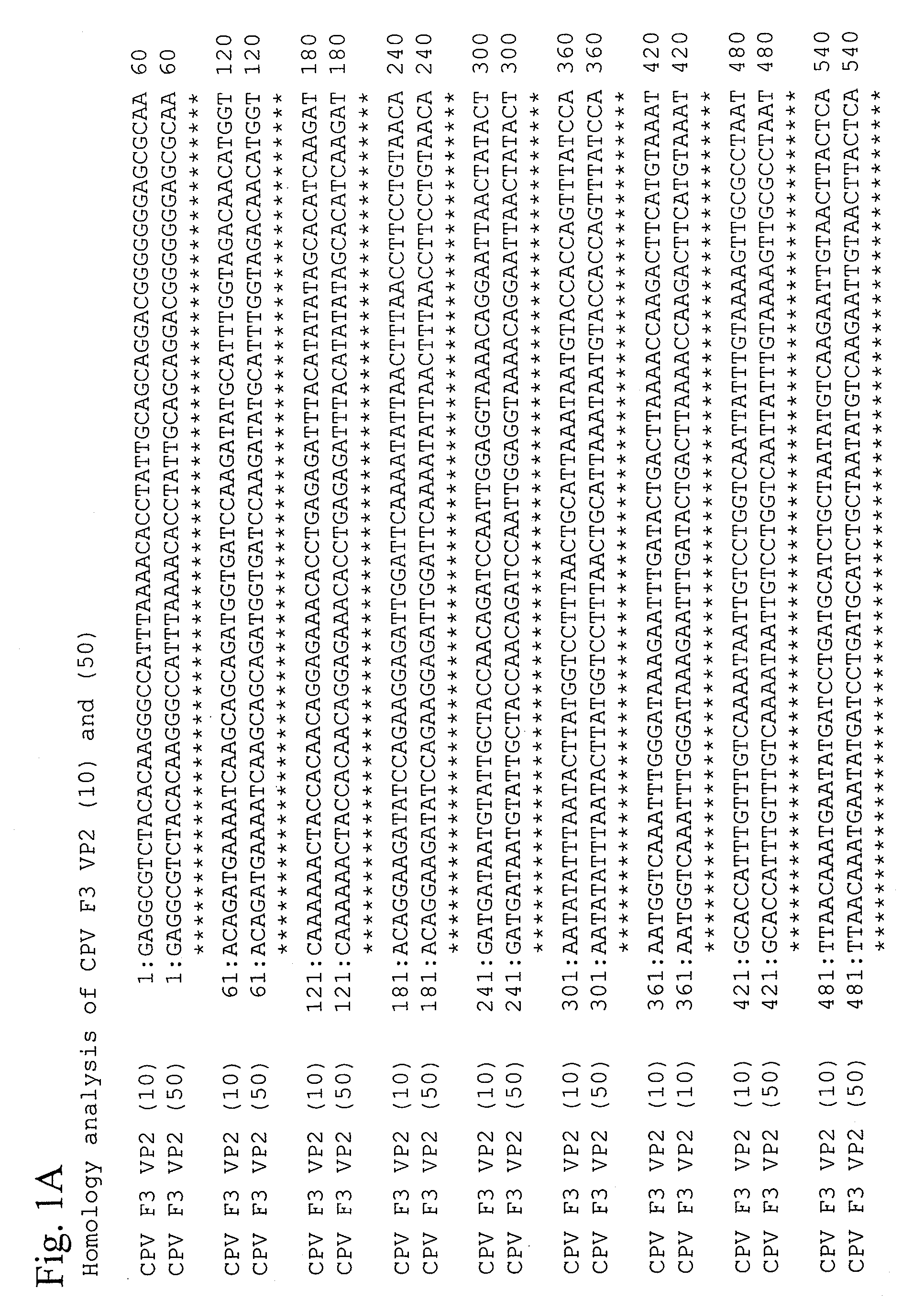
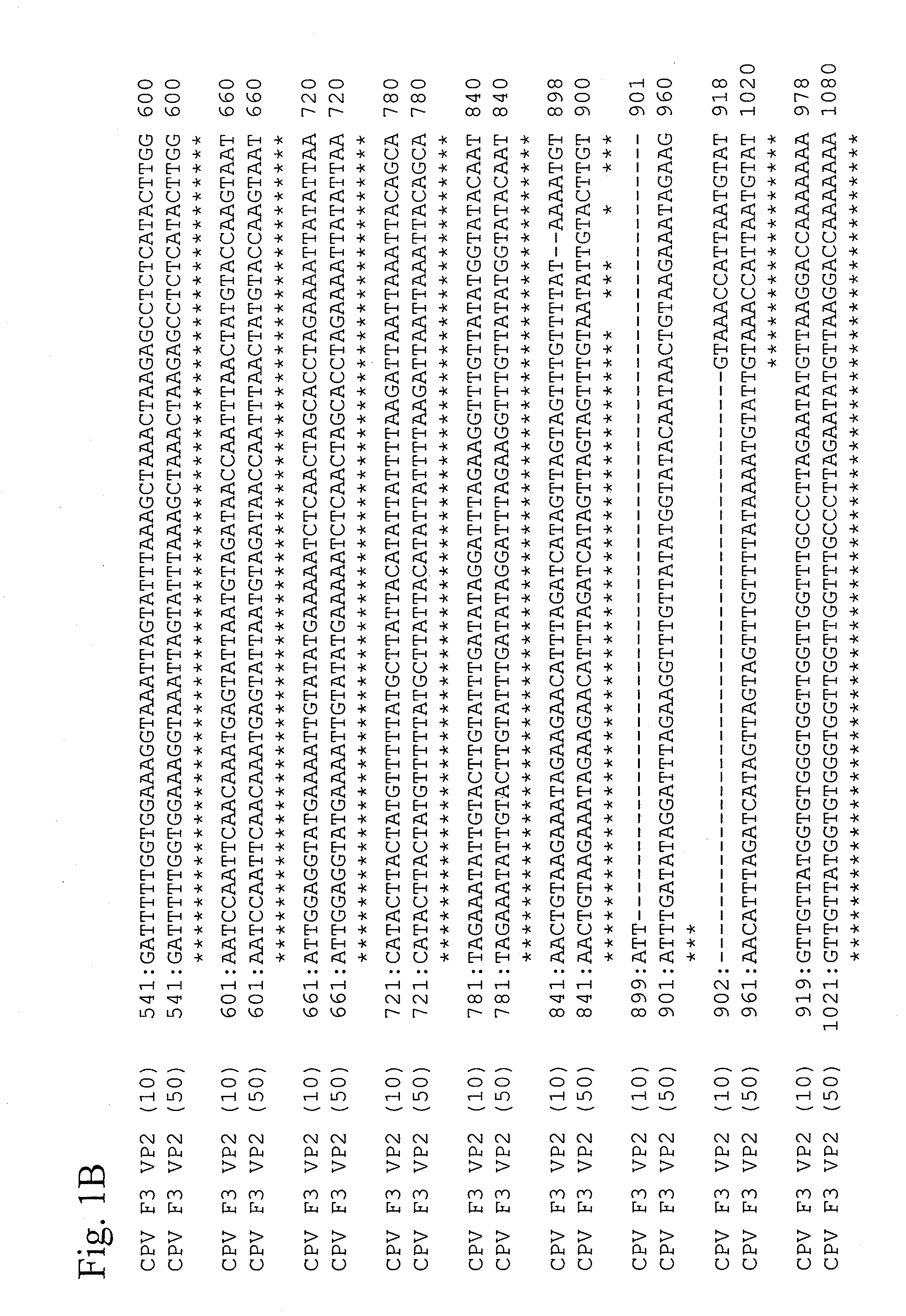
This invention provides vaccines against canine distemper virus infections, canine adenovirus type 2 infections, and canine parvovirus infections that can be orally administered. The invention also provides the attenuated canine distemper virus strain obtained by adapting the canine distemper virus 95-54 strains in cultured cells to attenuate the same, the attenuated canine adenovirus type 2 strain obtained by adapting the canine adenovirus type 2 F1 strain in cultured cells to attenuate the same, and an attenuated canine parvovirus strain obtained by adapting the canine parvovirus F3 strain in cultured cells to attenuate the same.
Owner:NIPPON ZENYAKU KOGYO CO LTD
Preparation of canine adenovirus II recombinant protein monoclonal antibody
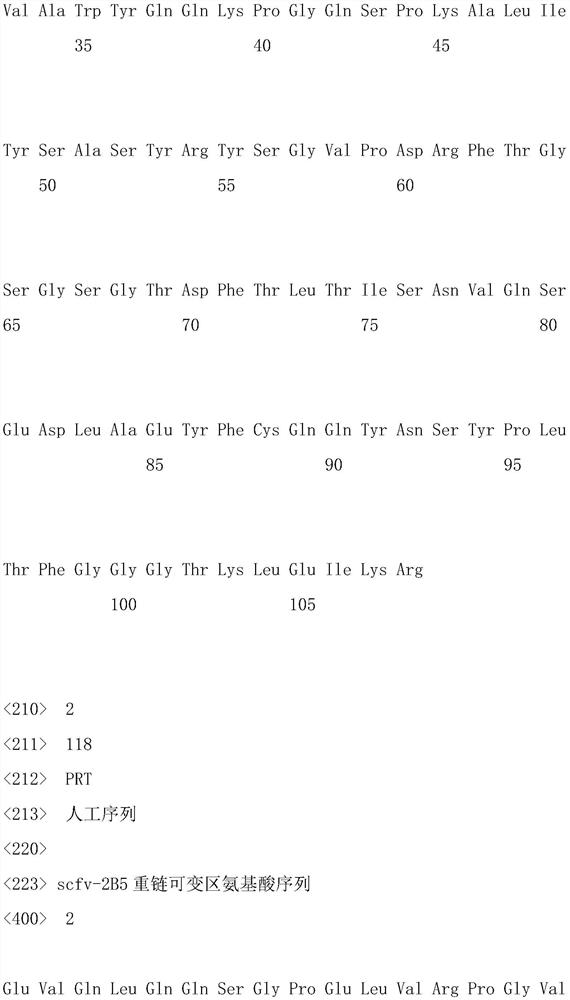
The invention belongs to the technical field of bioengineering. The invention relates to recombinant protein. The recombinant protein comprises two dominant epitopes of canine adenovirus II protein. In order to improve the yield of the recombinant protein in a prokaryotic expression system, preferred codons of escherichia coli are adopted to convert an amino acid sequence of the recombinant protein into a corresponding nucleotide sequence, the nucleotide sequence is chemically synthesized and a recombinant expression vector is constructed. The invention also relates to a method for establishing a phage library by immunizing a mouse with the recombinant protein. A corresponding canine adenovirus II protein single-chain antibody scfv sequence is obtained through panning and screening; the obtained scfv sequence is constructed into a complete mouse IgG1 antibody sequence expression vector; a monoclonal antibody is expressed by transiently transferring HEK293F cells; the monoclonal antibody is purified and europium ions (Eu<3+>) are marked; and an optimal monoclonal antibody pairing combination is determined through orthogonal experiments and can be used for early diagnosis of canine infectious laryngotracheitis and pneumonia.
Owner:杭州贤至生物科技有限公司
Nucleic acids and method for synchronous detection and distinguishing of five canine diarrhea viruses and kit
PendingCN109097488AReduce dosageRapid responseMicrobiological testing/measurementAgainst vector-borne diseasesHybridization probeCanine distemper virus CDV
The invention provides a group of nucleic acids for synchronously detecting and distinguishing five canine diarrhea viruses and also provides a multi-gene-chip high-flux molecular biological detectionmethod for the five canine diarrhea viruses. The nucleic acids include upper and lower primers and hybridization probes of a canine adenovirus type 1, a canine adenovirus type 2, canine coronaviruses, canine distemper viruses and canine parvoviruses of the five canine diarrhea viruses. By adopting the method, multi-gene-chip detection is performed by extracting the nucleic acids of the canine diarrhea viruses in samples to be detected, synchronous and accurate detection and distinguishing of the canine diarrhea viruses in samples to be detected are achieved, and the nucleic acids have the advantages of being good in specificity, high in sensitivity, good in stability, simple and convenient to operate and capable of achieving high-flux quick detection.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
CAV-1 monoclonal antibody, variable region sequence, hybridoma cell strain and application thereof
ActiveCN110632298ALSolve the inconvenience caused by complicated preparation proceduresMaterial analysisAgainst vector-borne diseasesFiberCanis lupus familiaris
The invention provides a variable region sequence of a mouse monoclonal antibody specifically bound with CAV-1 (Canine Adenovirus type I) Fiber protein, and an antibody specifically bound with the CAV-1 Fiber protein, wherein the antibody can be used for preparing a kit and a pharmaceutical composition. The kit provided by the invention overcomes the problem of low detection sensitivity in the prior art, avoids the occurrence of phenomena of missed detection and false negative, can detect various targets of dogs with high sensitivity, and can detect various targets of non-dog animals with highsensitivity; the kit has the advantages of rapidness, simpleness, convenience and accuracy; the pharmaceutical composition of the invention can effectively prevent and treat diseases caused by the CAV-1.
Owner:LUOYANG PULIKE WANTAI BIOTECH +1
Canine adenovirus type-1 antibody ELISA detection kit and application thereof
ActiveCN110568189ASensitiveIncreased sensitivityMaterial analysisAgainst vector-borne diseasesAntigenProkaryotic expression
The invention discloses a canine adenovirus type-1 (CAdV-1) antibody ELISA detection kit and application thereof. According to the detection kit, a pCold II prokaryotic expression system is utilized to prepare a PB (penton base) and Knob protein with good reactogenicity, the PB and the Knob protein are subjected to a large quantity of induced expressions, two types of protein after renaturation are used as envelop antigens respectively and compared with a purified CAdV-1 totivirus, and finally the Knob protein is determined as the optimal ELISA envelop antigen. On this basis, the ELISA detection kit with high efficiency, good sensitivity and good specificity for a CAdV-1 serum antibody is provided; results of simultaneous sample detection through the detection kit and an SN method show that the sensitivity of the ELISA detection kit is 97.14%, the specificity is 90.00%, and the SN coincidence rate is 93.57%; and the detection kit has the advantages of high sensitivity, good specificity, strong repeatability and the like.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Canine adenovirus type 2 monoclonal antibody, variable region sequence, hybridoma cell and application thereof
ActiveCN110627900AAvoid troubleAvoid poisoning riskBiological material analysisImmunoglobulins against virusesDiseaseFiber
The invention provides a variable region sequence of a mouse monoclonal antibody specifically binding to canine adenovirus type 2 fiber protein, and an antibody specifically binding to canine adenovirus type 2 fiber protein conformational epitope. The antibody can be used for preparing kits and pharmaceutical compositions. The kit provided by the invention overcomes the problem of low detection sensitivity in the prior art, avoids missing detection and false negative phenomena, can detect various targets, and has the advantages of rapidness, simplicity, convenience and accuracy; and the pharmaceutical composition disclosed by the invention can be used for effectively preventing and treating diseases caused by CAV-2.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Dog type II adenovirus live vector recombinant vaccine for displaying protective antigen of rabies virus
InactiveCN101406698AResist attackStrong immunityAntiviralsAntibody medical ingredientsDiseaseProtective antigen
The invention discloses a recombinant vaccine with canine adenovirus type-2 as a live vector for displaying a protective antigen of rabies virus, which relates to recombinant vaccines displaying the protective antigen or an antigenic epitope of the rabies virus respectively by utilizing the characteristics of different structural proteins of the canine adenovirus type-2. The protective antigen of the rabies virus or a ribonucleotide sequence expressing the antigenic epitope are inserted at a hydrophobic locus of a structural protein gene to ensure that an exogenous antigen or an antigenic epitope is subject to fusion expression with structural protein of the exogenous antigen or the antigenic epitope on the surfaces of adenoviruses. After the recombinant vaccines challenge, test animals are subject to closed and isolated rearing, feeding and disease conditions of the test animals are observed and recorded; and the results show that dogs taking any kind of recombinant vaccine by oral administration and intramuscular injection all can resist the attack of strong viruses, and the survival rate is more than 90 percent.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Multiplex polymerase chain reaction (PCR) detection primers for simultaneously detecting canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus
InactiveCN102618666BStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesGeneticsCanine distemper virus CDV
The invention discloses a set of multiplex polymerase chain reaction (PCR) detection primers for detecting canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus. The primers comprise three pairs of primers, wherein sequences of primers for detecting canine distemper virus are shown as SEQ ID NO:1 and SEQ ID NO:2; sequences of primers for detecting canine parvovirus are shown as SEQ ID NO:3 and SEQ ID NO:4; and sequences of primers for detecting type I canine adenovirus and type II canine adenovirus are shown as SEQ ID NO:5 and SEQ ID NO:6. The primers can simultaneously detect canine distemper virus, canine parvovirus, type I canine adenovirus and type II canine adenovirus through a multiplex PCR method, and have the advantages of high specificity, high sensitivity, high repeatability and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
RPA primer pair, probe, kit and detection method for detection of canine adenoviruses
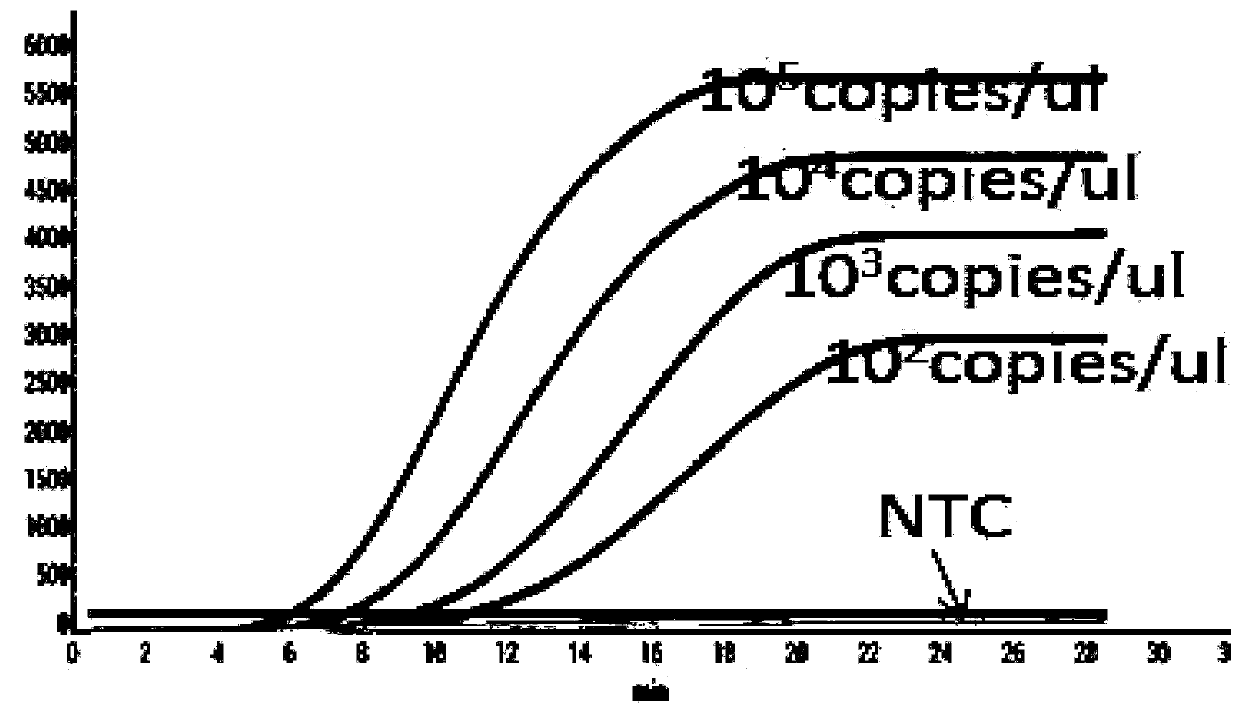
ActiveCN111334613AImprove amplification specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesDiagnosis laboratoryGene
The invention discloses a RPA primer pair, probe, kit and detection method for detection of canine adenoviruses, and belongs to the technical field of molecular biological detection. The real-time fluorescence RPA detection method for detection of canine adenoviruses has high amplification specificity and high sensitivity, and the sensitivity can reach 10<2> copies. The primer pair and probe are obtained through design and screening of gene sequences in E3 of the canine adenoviruses (CAV), so that amplification is more efficient, and the specificity is higher. The RPA method is simple, has lowrequirements for temperature and machines and a short consumption time, and is suitable for rapid detection in the laboratory or on-site detection, and a technical reference is provided for rapid detection, diagnosis and prevention of the canine adenoviruses.
Owner:GUANGDONG LAB ANIMALS MONITORING INST +1
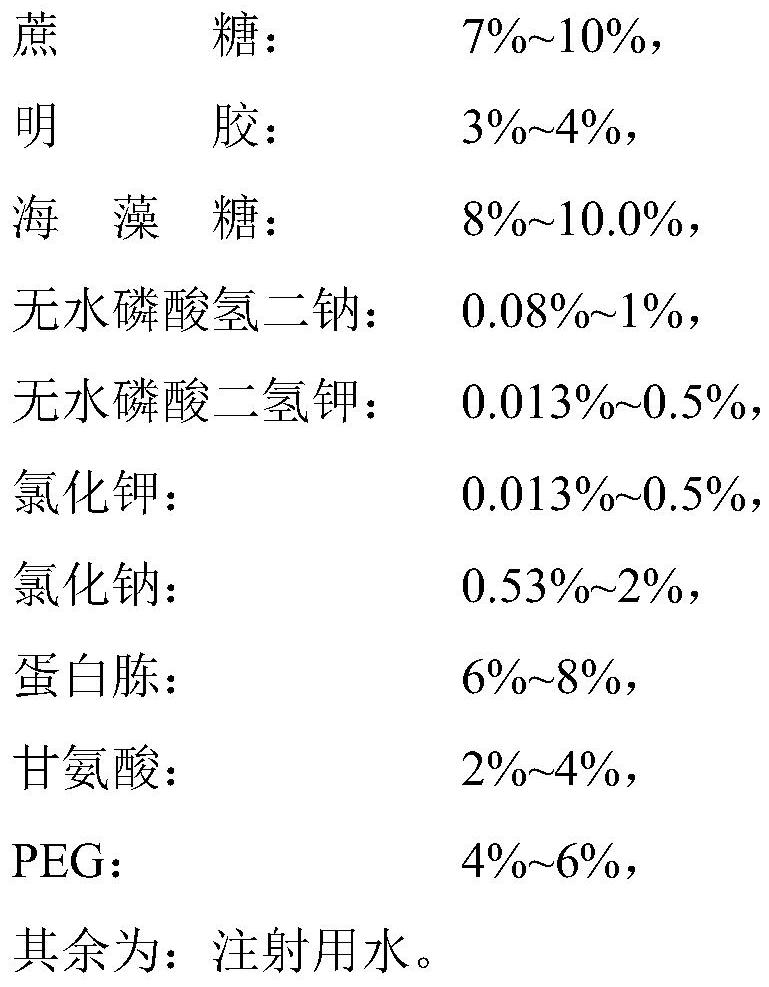
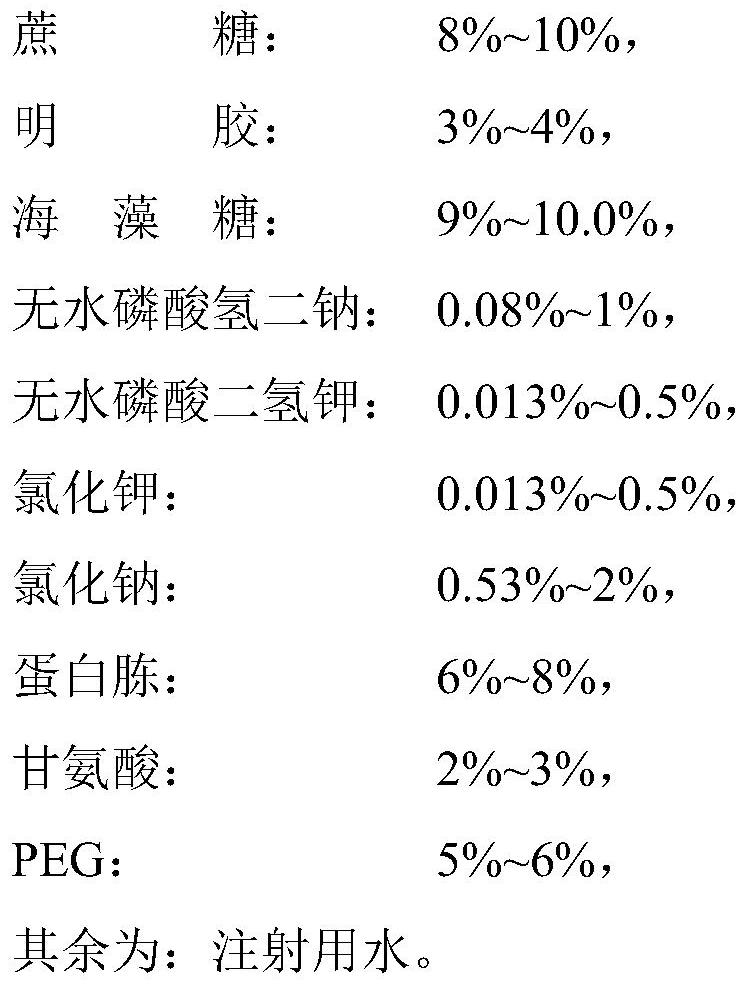
Heat-resistant protective agent for canine distemper, canine parvovirus, canine adenovirus and canine parainfluenza quadruple live vaccine as well as preparation method and application of heat-resistant protective agent
ActiveCN112370531AReduce manufacturing costEnsure safetySsRNA viruses negative-sensePowder deliveryGlycineSucrose
Owner:JINYUBAOLING BIO PHARMA CO LTD
Canine adenovirus vectors
ActiveUS10329586B2Reduce in quantityReduce incidenceSsRNA viruses negative-senseVectorsVector vaccineImmunogenicity
The present invention relates to the field of CAdV vector vaccines, and especially to promoters suitable to express target antigens from such vector vaccines. Disclosed and claimed are recombinant canine adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. Additionally, disclosed and claimed are truncated EHV4 promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
I-type canine adenovirus attenuated vaccine strain and application thereof
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
A kind of canine adenovirus type I inactivated vaccine and preparation method thereof
ActiveCN110841064BGood neutralizing antibody titerImprove immunityViral antigen ingredientsInactivation/attenuationSerum freeNeutralising antibody
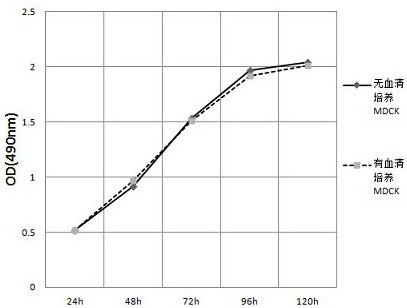
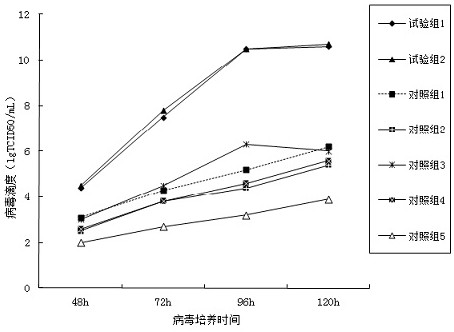
The invention relates to a canine adenovirus I-type inactivated vaccine and a preparation method thereof. The canine adenovirus I-type inactivated vaccine is prepared by a serum-free culture process,the immunogenicity of the canine adenovirus I-type inactivated vaccine is equivalent to that of a canine adenovirus I-type vaccine prepared by a serum-containing culture process, and the vaccine prepared by a canine adenovirus I-type cultured by the serum-free culture process can achieve better neutralizing antibody titer in an early immunity period as compared with a vaccine prepared by the serum-containing culture process.
Owner:HENGYANG NORMAL UNIV
Dog type II adenovirus live vector recombinant vaccine for displaying protective antigen of rabies virus
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Monoclonal antibody specifically binding to canine adenovirus, pharmaceutical composition, kit and application thereof
ActiveCN109232734BReduce morbidityReduce mortalityBiological material analysisImmunoglobulins against virusesPharmaceutical drugMouse monoclonal antibody
Owner:LUOYANG PULIKE WANTAI BIOTECH
Canine adenovirus genetic engineering subunit vaccine, its preparation method and application
ActiveCN112592410BImproving immunogenicityHigh expressionAntibody mimetics/scaffoldsInvertebrate cellsImmunogenicityGenetic engineering
The invention discloses a canine adenovirus genetic engineering subunit vaccine, its preparation method and application. The vaccine comprises a fusion protein and a pharmaceutically acceptable carrier, and the fusion protein has the sequence shown in SEQ ID NO:2. The vaccine provided by the present invention has high safety and good immunogenicity, and can generate strong humoral immunity in animals, and the immunized animals can resist the attack of strong viruses, and can also be suspended in a large scale without serum by using a bioreactor Culture preparation has the advantages of easy quality control, batch-to-batch stability, and low production cost.
Owner:苏州沃美生物有限公司
A detection kit for canine adenovirus type Ⅱ IgG antibody
ActiveCN105403699BConvenient for on-site testingEasy to operateMaterial analysisBottleImmuno detection
The invention discloses a canine adenovirus II type IgG antibody detection kit. The kit is internally provided with five 96-pore immune detection reaction plates covered with canine adenovirus antigens, one bottle of 120-mL 10-time concentrated scrubbing solution, one tube of 50-microliter positive blood serum, one tube of 50-microliter negative blood serum, one tube of 20-microliter horseradish peroxidase marked SPA enzyme-labeled antibody, one bottle of 60-mL substrate color developing solution A and two tubes of 1.5-mL substrate color developing solution B. The kit does not need special instruments and specific dyeing can be observed by naked eyes; non-specific dyeing is very easy to distinguish, and the kit is very convenient for laboratory detection and clinical field detection, and can be used for detecting flexibly, rapidly and accurately.
Owner:湖南普菲特动物保健有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com