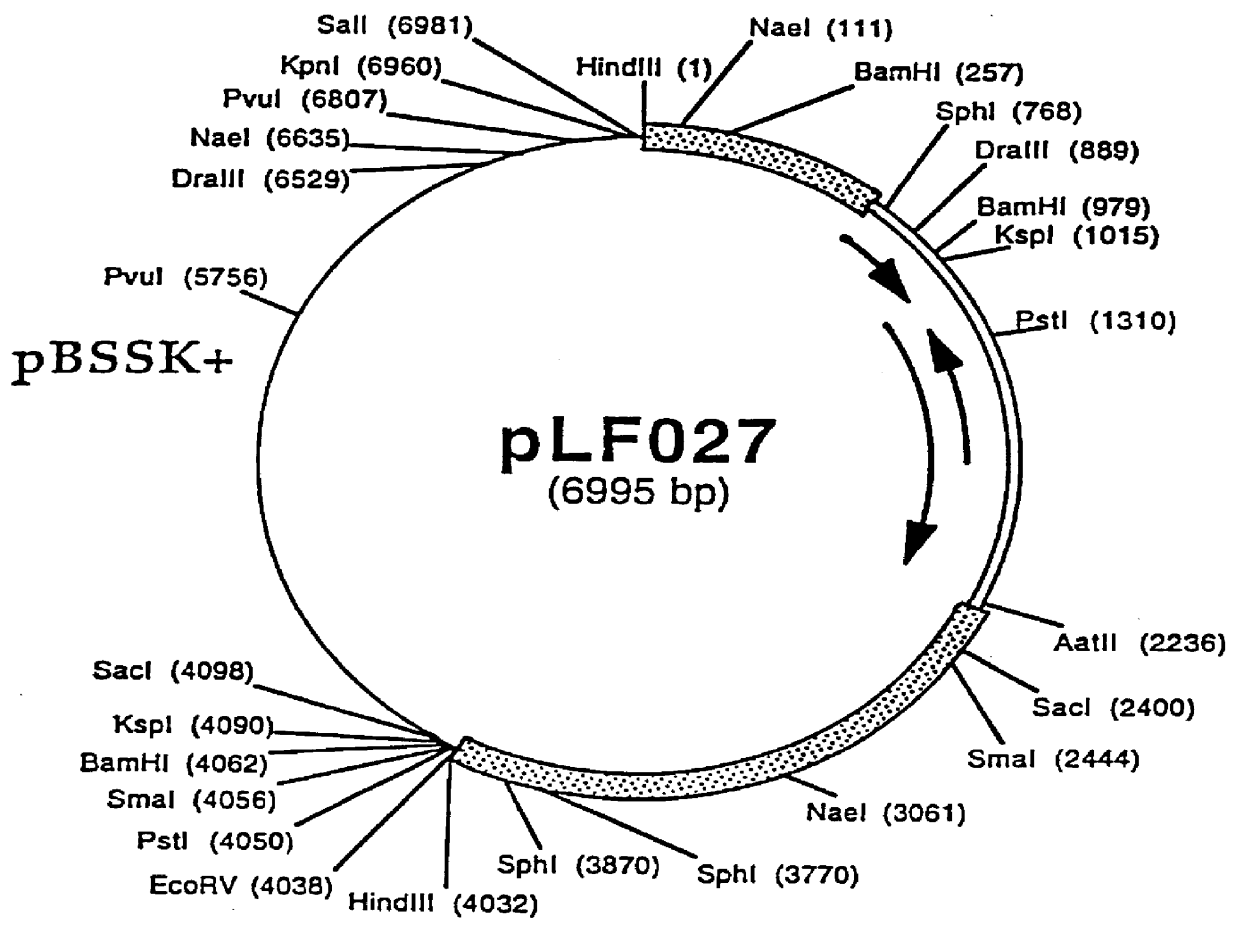
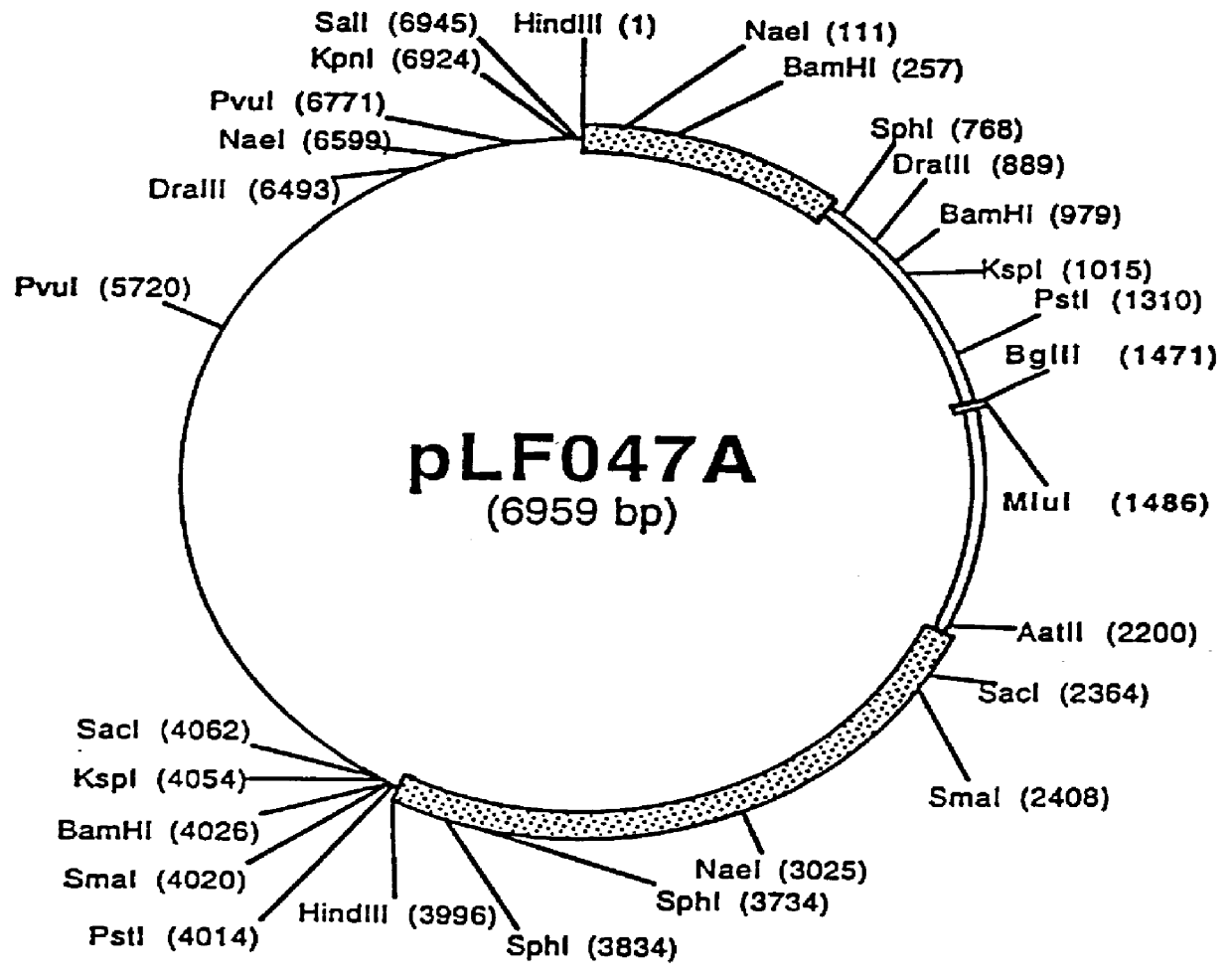
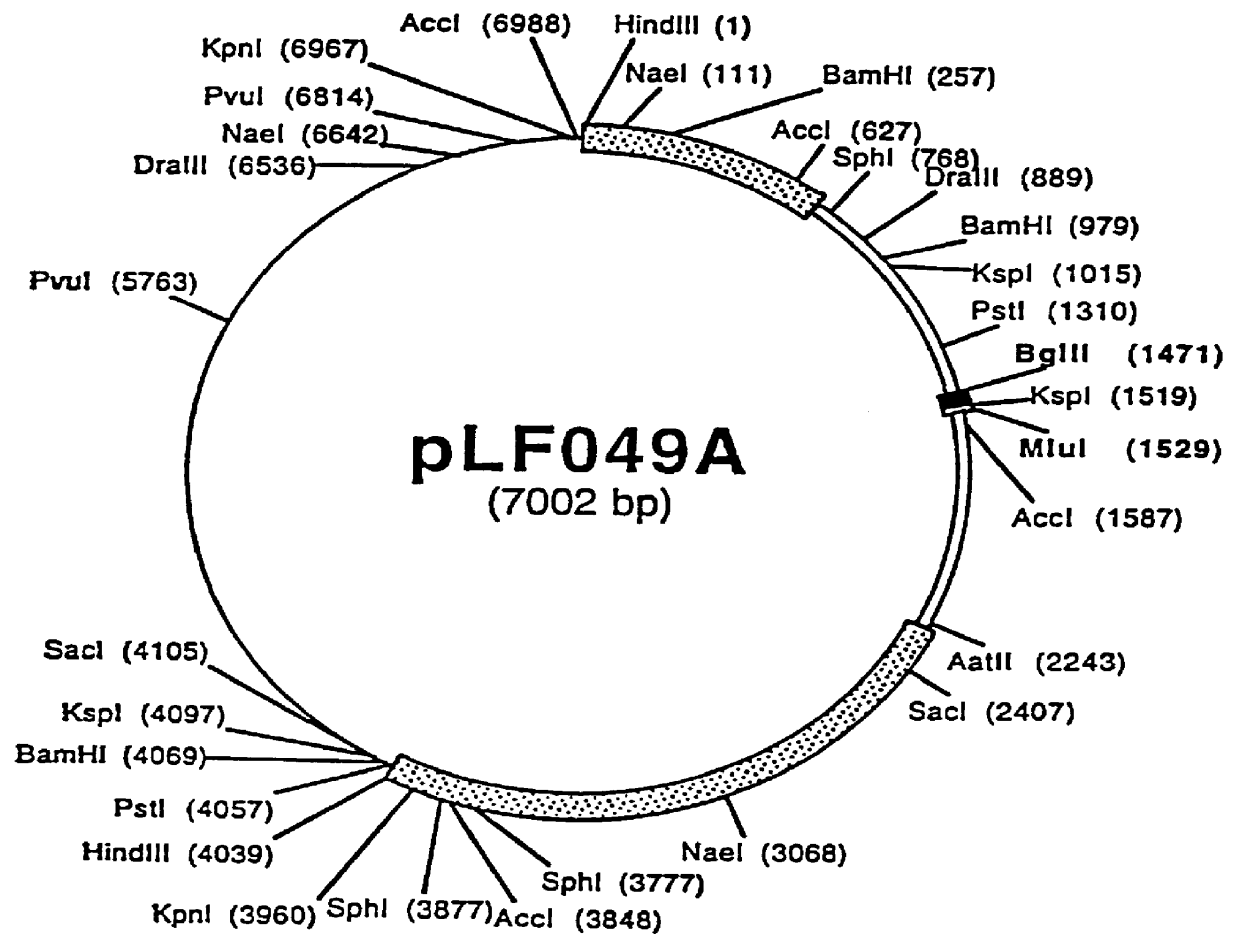
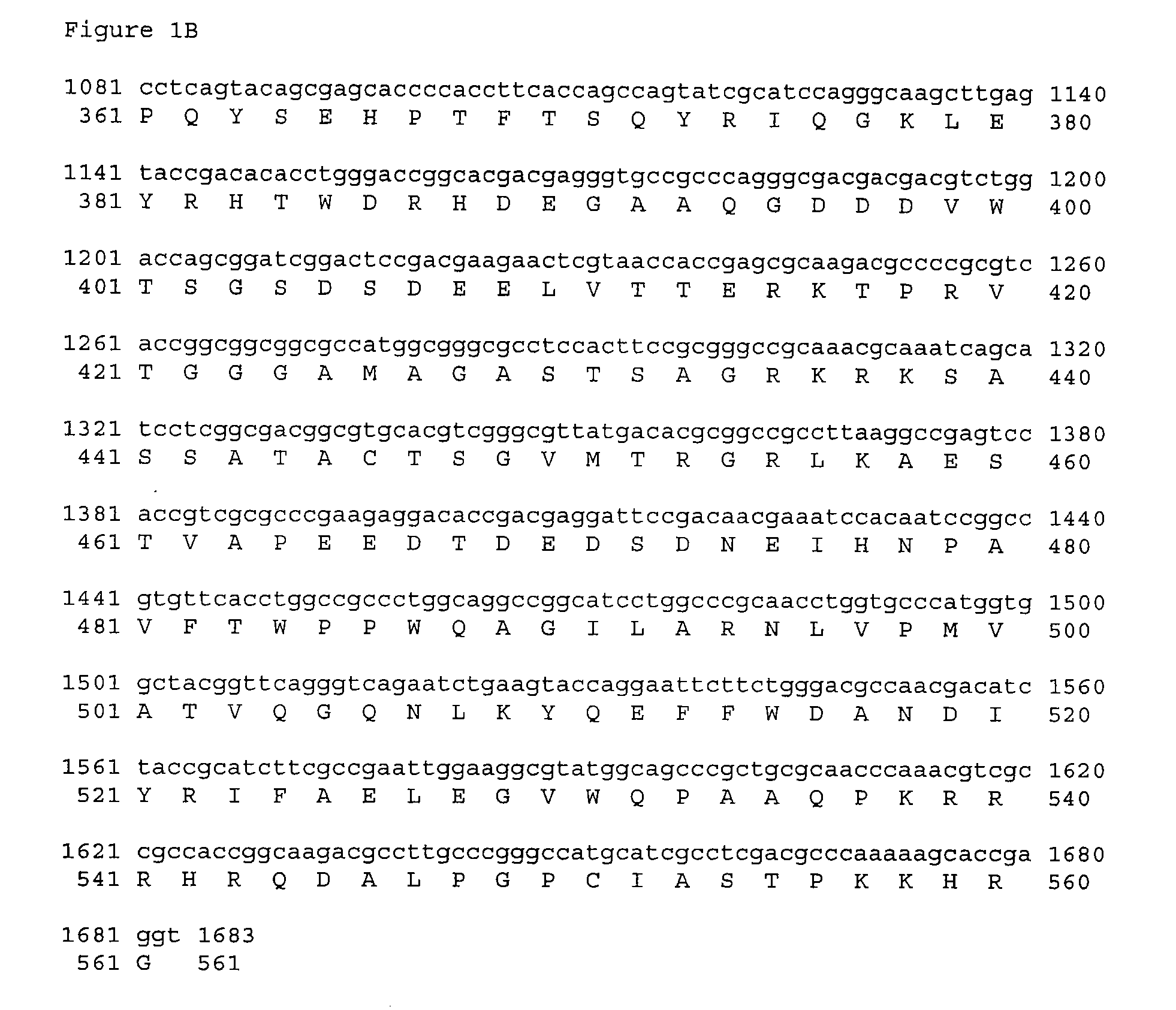
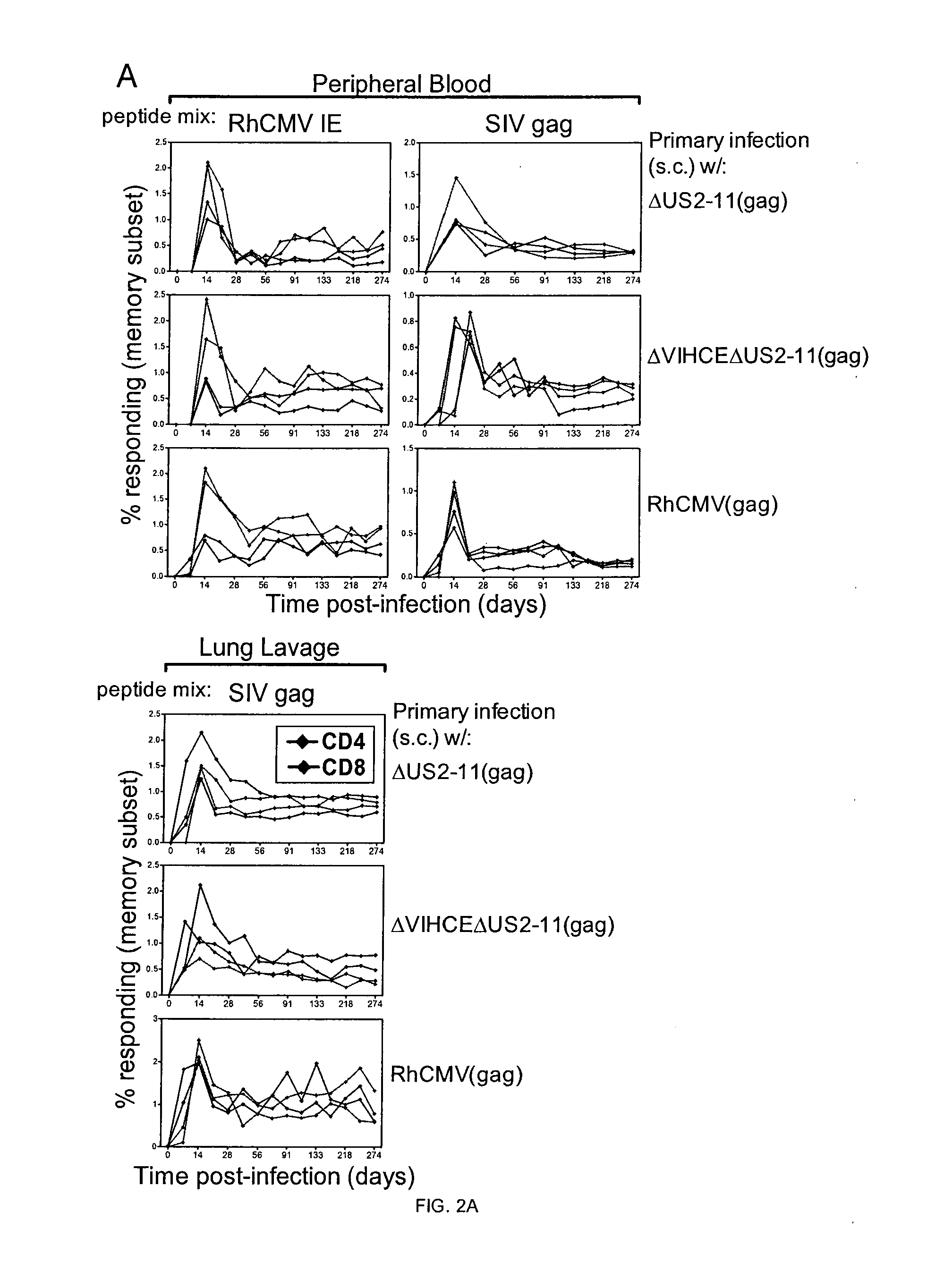
Patents
Literature
340 results about "Cytomegalovirus disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
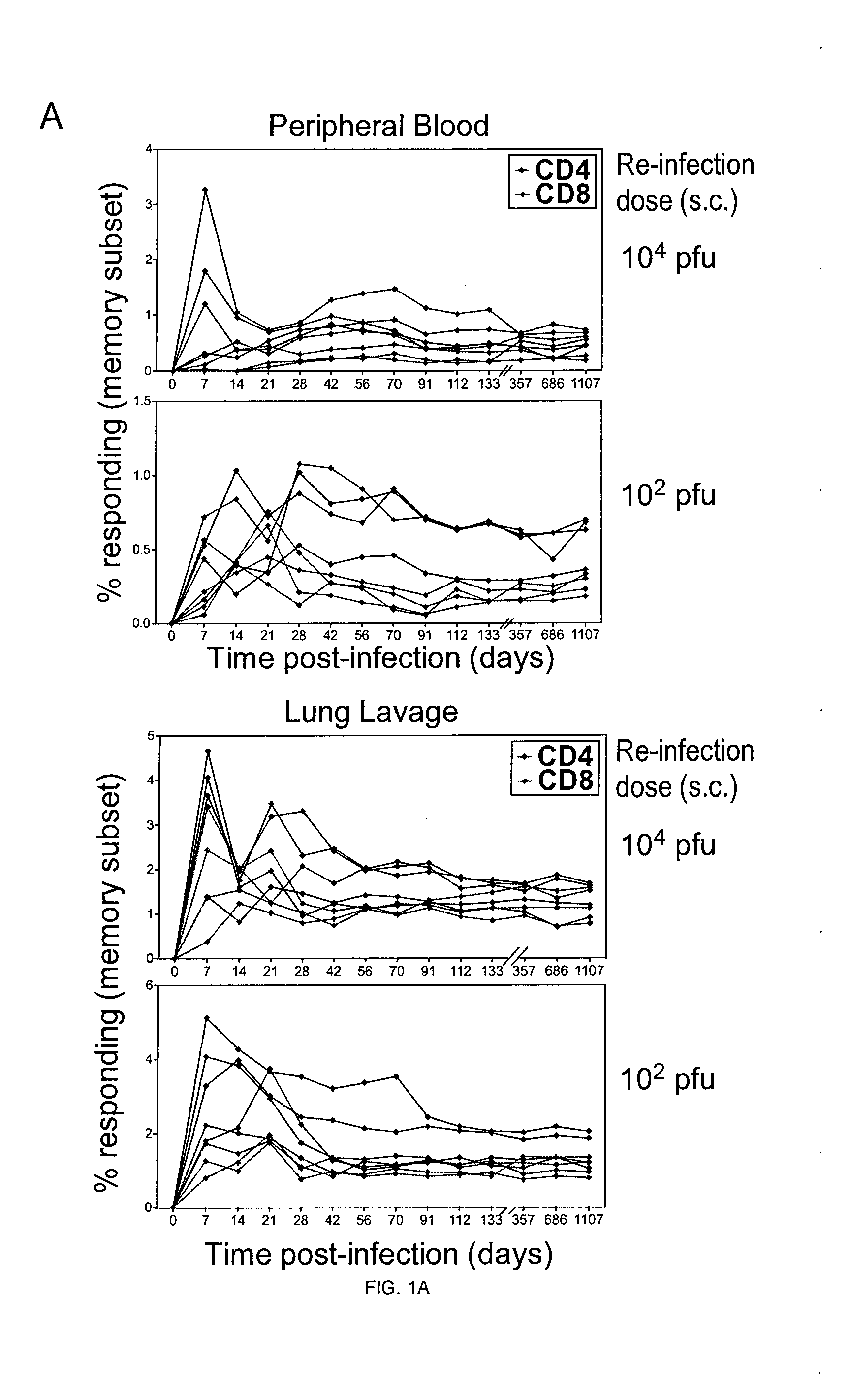
There are currently eight species in this genus including the type species, human cytomegalovirus (HCMV, human herpesvirus 5, HHV-5), which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis, and pneumonia.
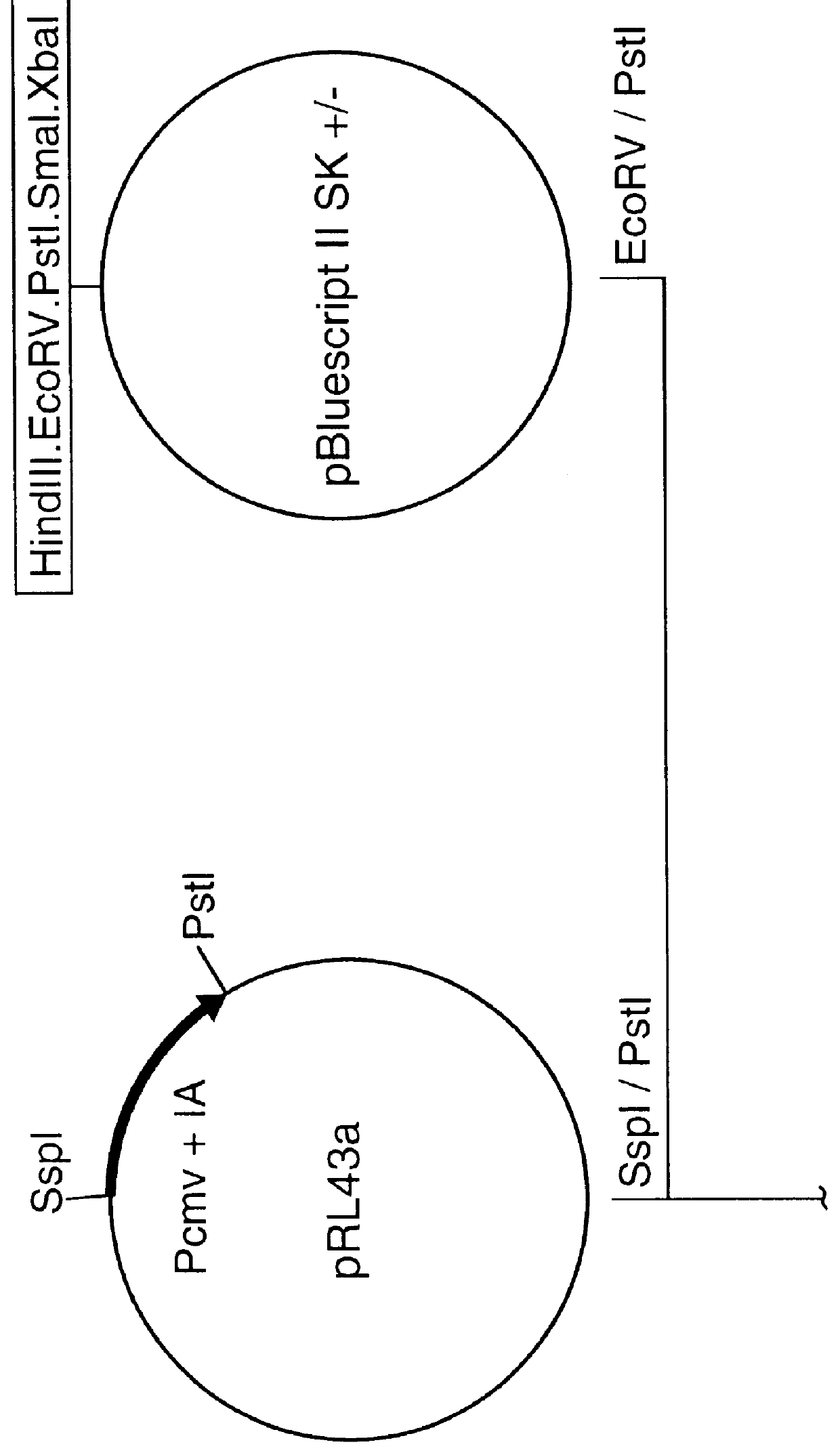
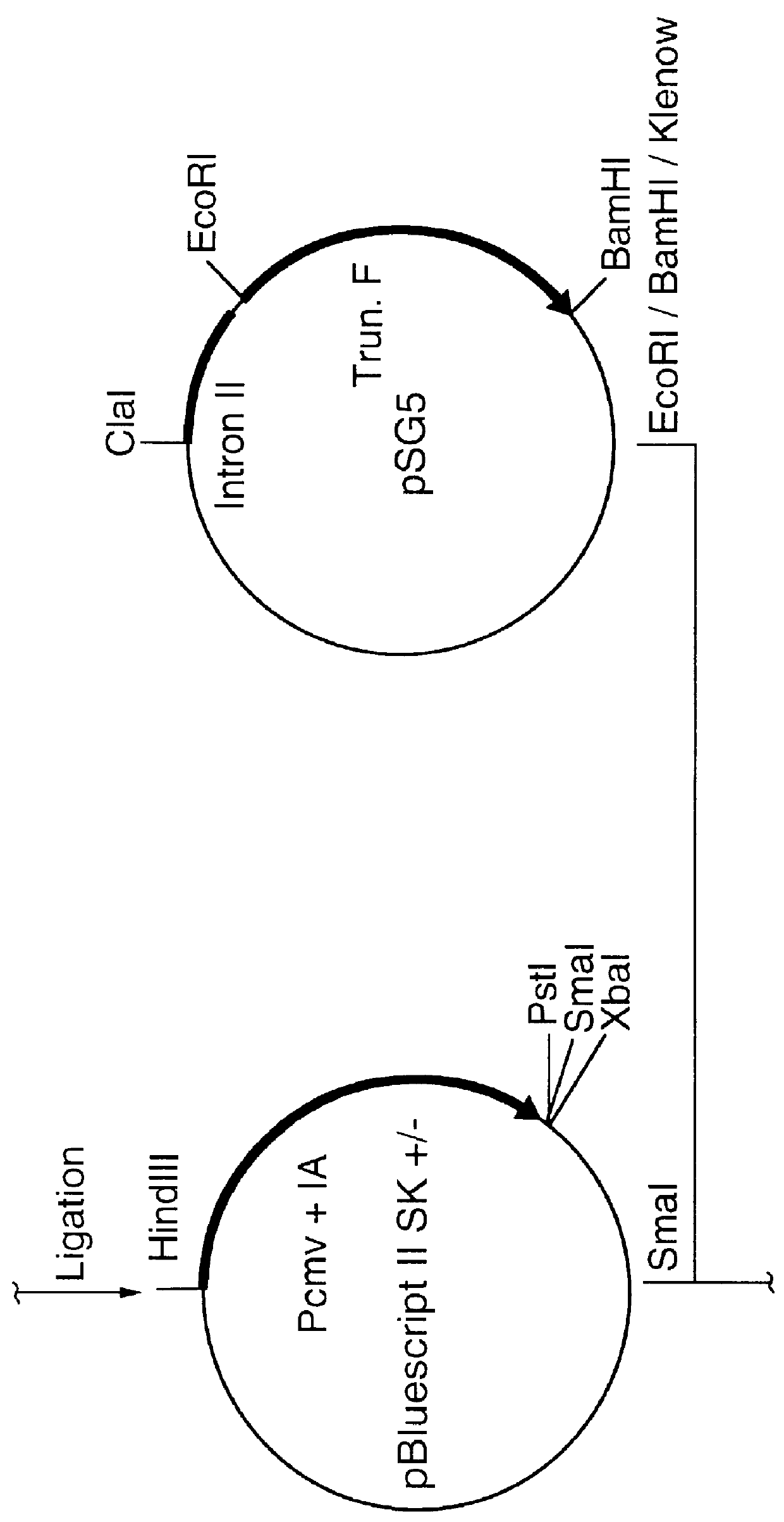
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Codon-optimized polynucleotide-based vaccines against human cytomegalovirus infection
InactiveUS20080085870A1Reduce in quantityDecreased immunological responseOrganic active ingredientsPeptide/protein ingredientsAntigenAdjuvant
The invention is related to polynucleotide-based cytomegalovirus vaccines. In particular, the invention is plasmids operably encoding HCMV antigens, in which the naturally-occurring coding regions for the HCMV antigens have been modified for improved translation in human or other mammalian cells through codon optimization. HCMV antigens which are useful in the invention include, but are not limited to pp65, glycoprotein B (gB), IE1, and fragments, variants or derivatives of either of these antigens. In certain embodiments, sequences have been deleted, e.g., the Arg435-Lys438 putative kinase in pp65 and the membrane anchor and endocellular domains in gB. The invention is further directed to methods to induce an immune response to HCMV in a mammal, for example, a human, comprising delivering a plasmid encoding a codon-optimized HCMV antigen as described above. The invention is also directed to pharmaceutical compositions comprising plasmids encoding a codon-optimized HCMV antigen as described above, and further comprising adjuvants, excipients, or immune modulators.
Owner:VICAL INC
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
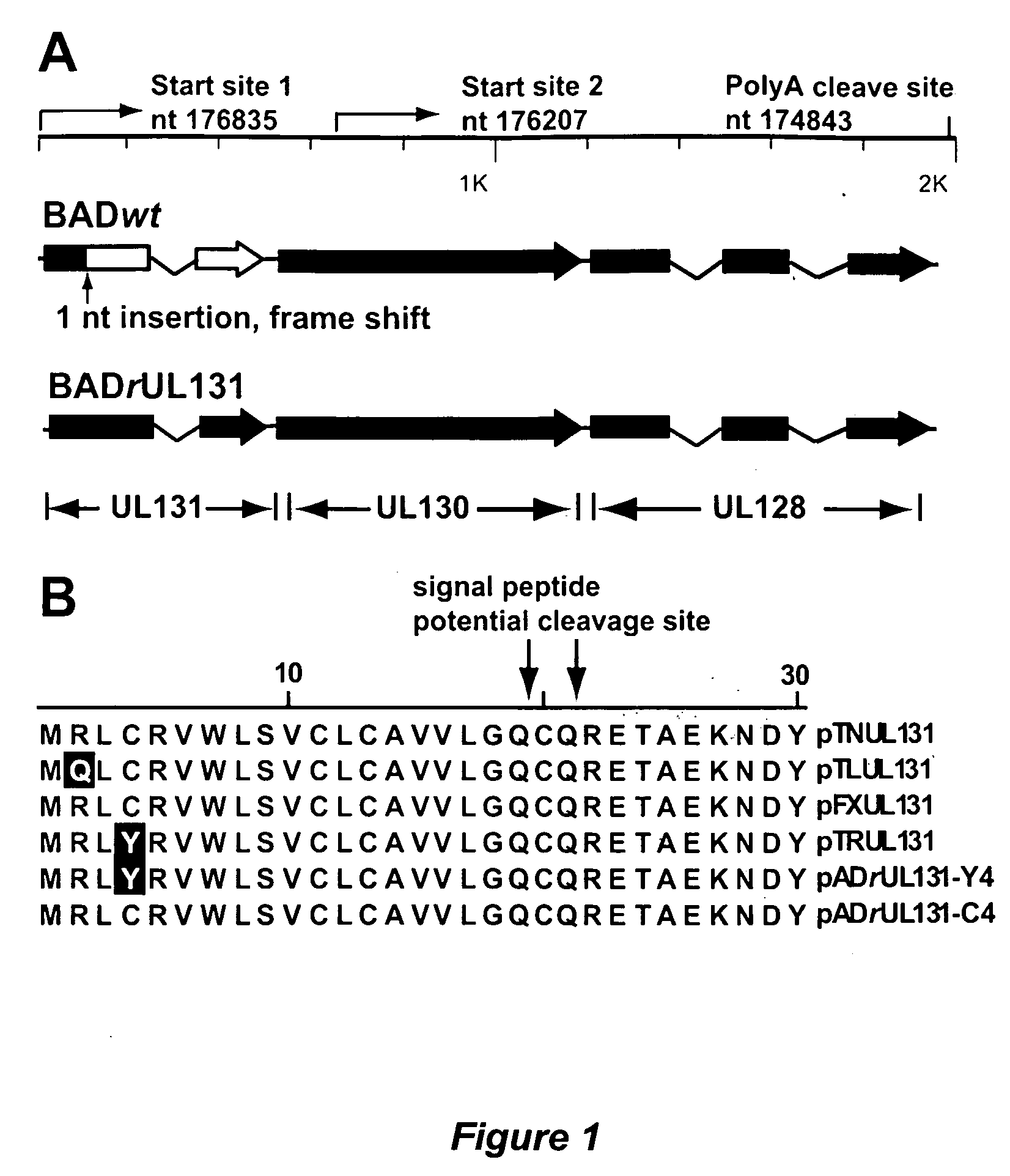
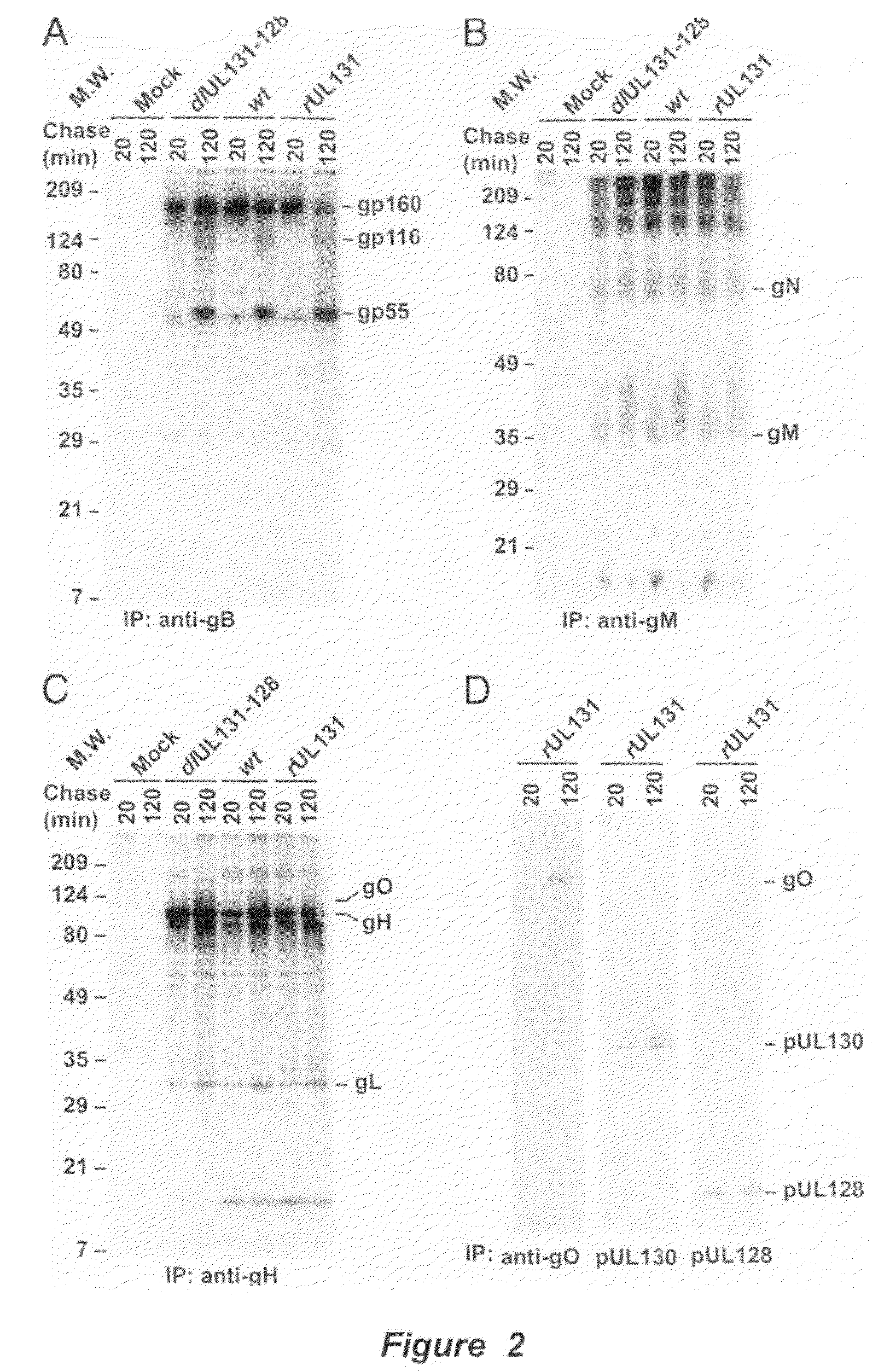
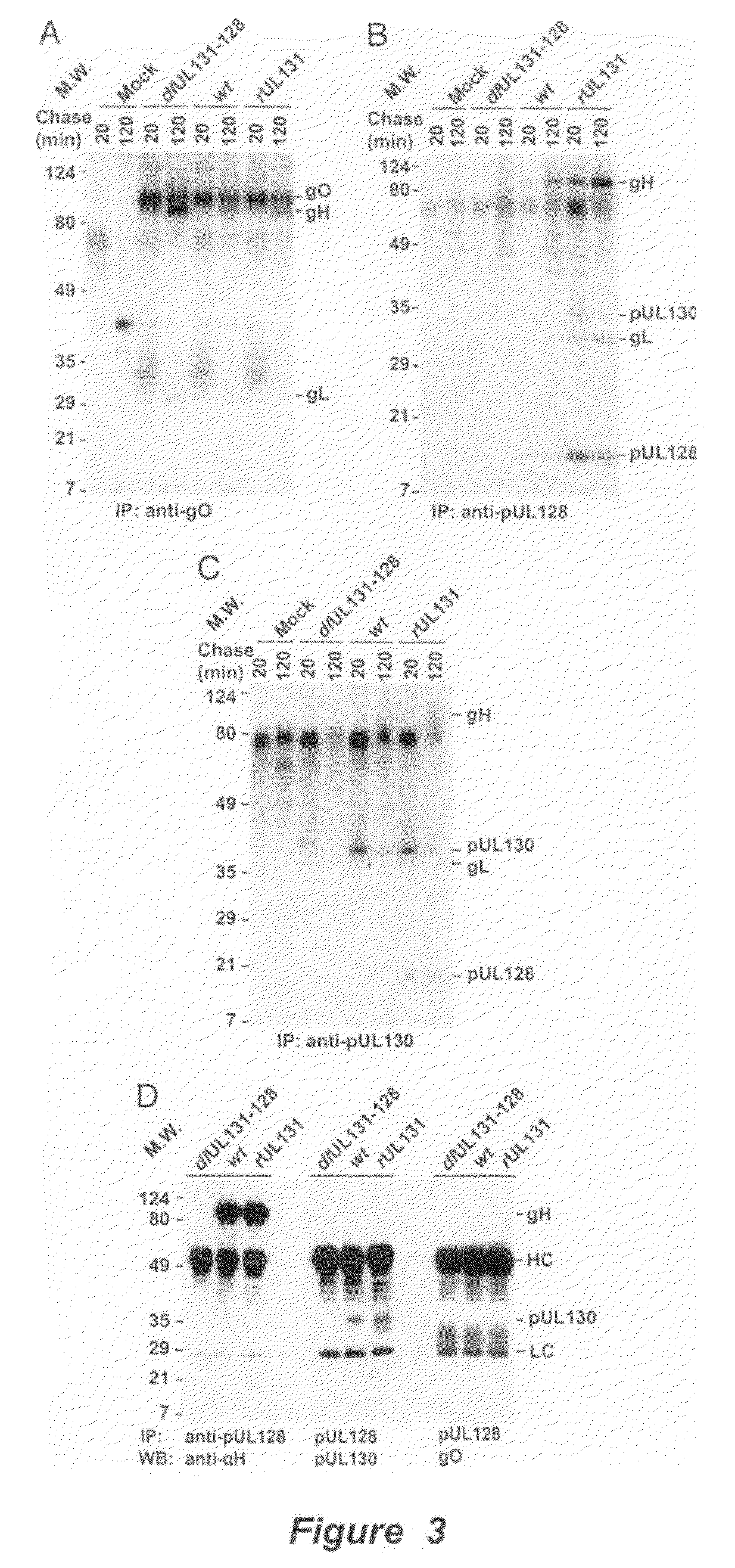
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Phospholipids for the treatment of infection by togaviruses, herpes viruses and coronaviruses
InactiveUS20050187192A1BiocidePhosphorous compound active ingredientsHerpes simplex virus DNACompound (substance)
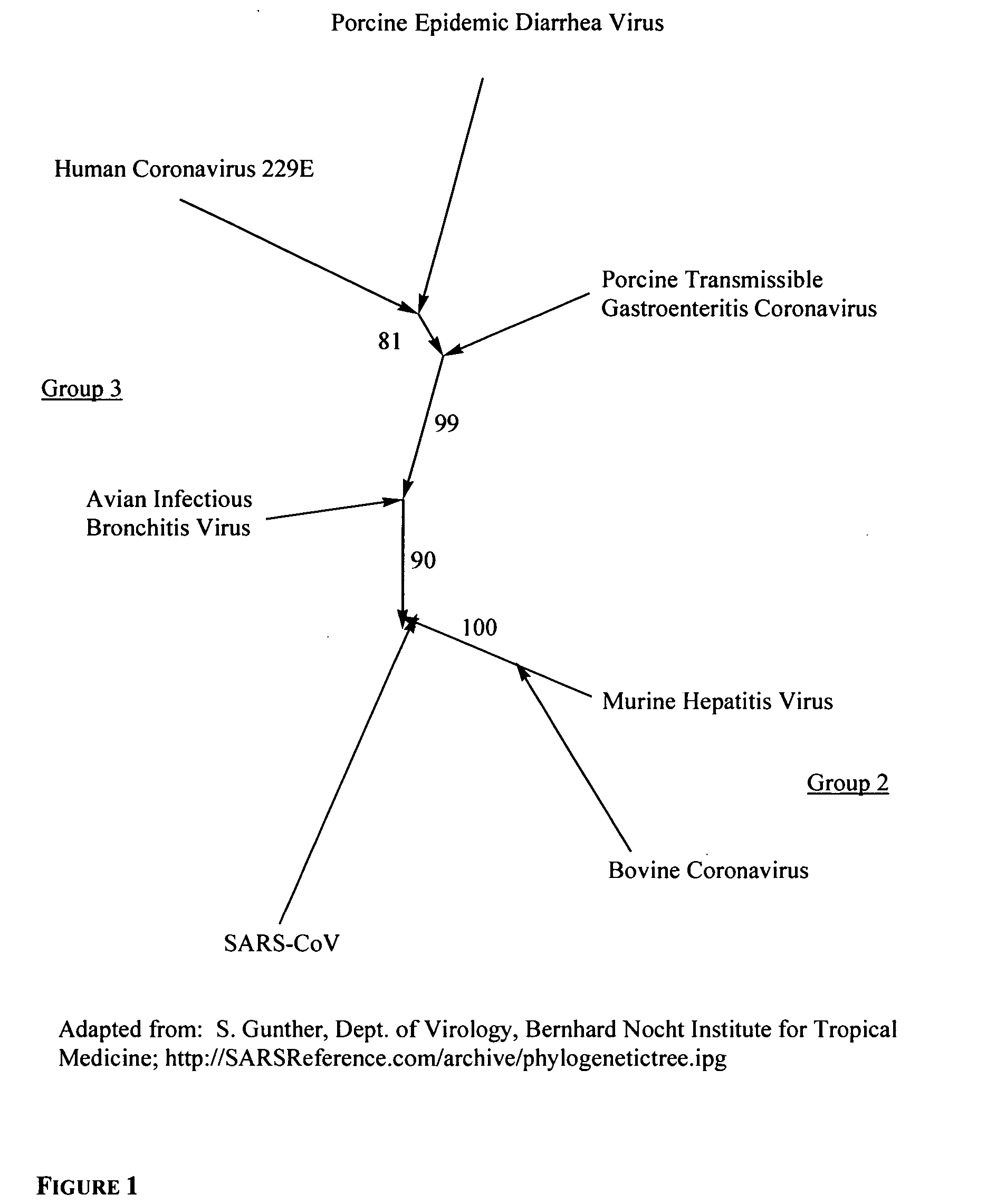
Provided are compounds, methods and pharmaceutical compositions for treating a host, especially a human, infected with a togavirus, herpes virus and / or coronavirus, and in particular SARS-CoV, cytomegalovirus or varicella-zoster virus. The method in one embodiment comprises administering to that host an effective amount of an anti-togavirus, anti-herpes virus and / or anti-coronavirus phospholipid or a pharmaceutically acceptable salt or prodrug thereof. The phospholipid compound is, e.g., a 3-alkylamido-2-alkoxypropylphosphocholine compound or salt thereof. The compound may be administered alone or in combination and / or alternation with one or more other anti-viral agents.
Owner:KUCERA PHARMA
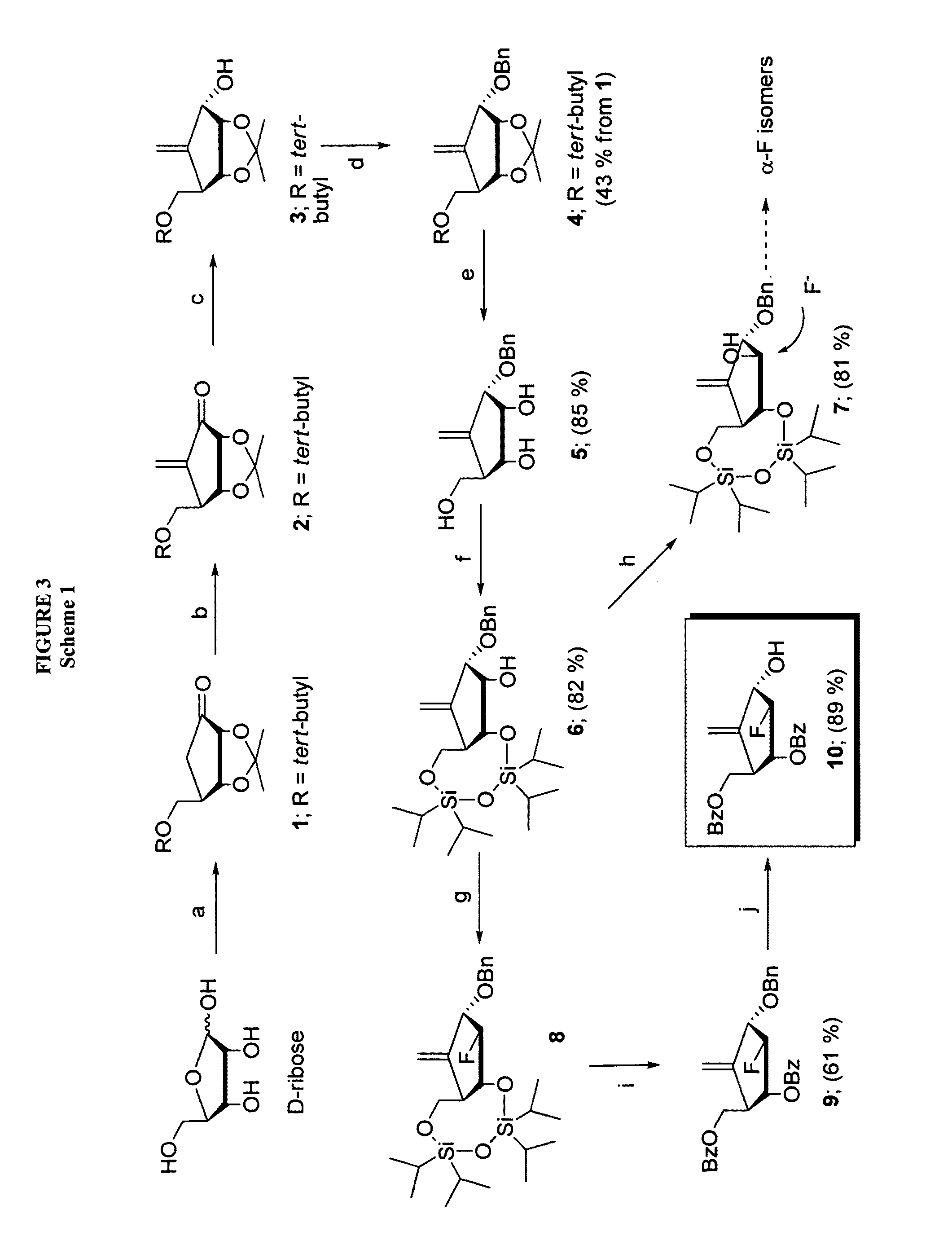
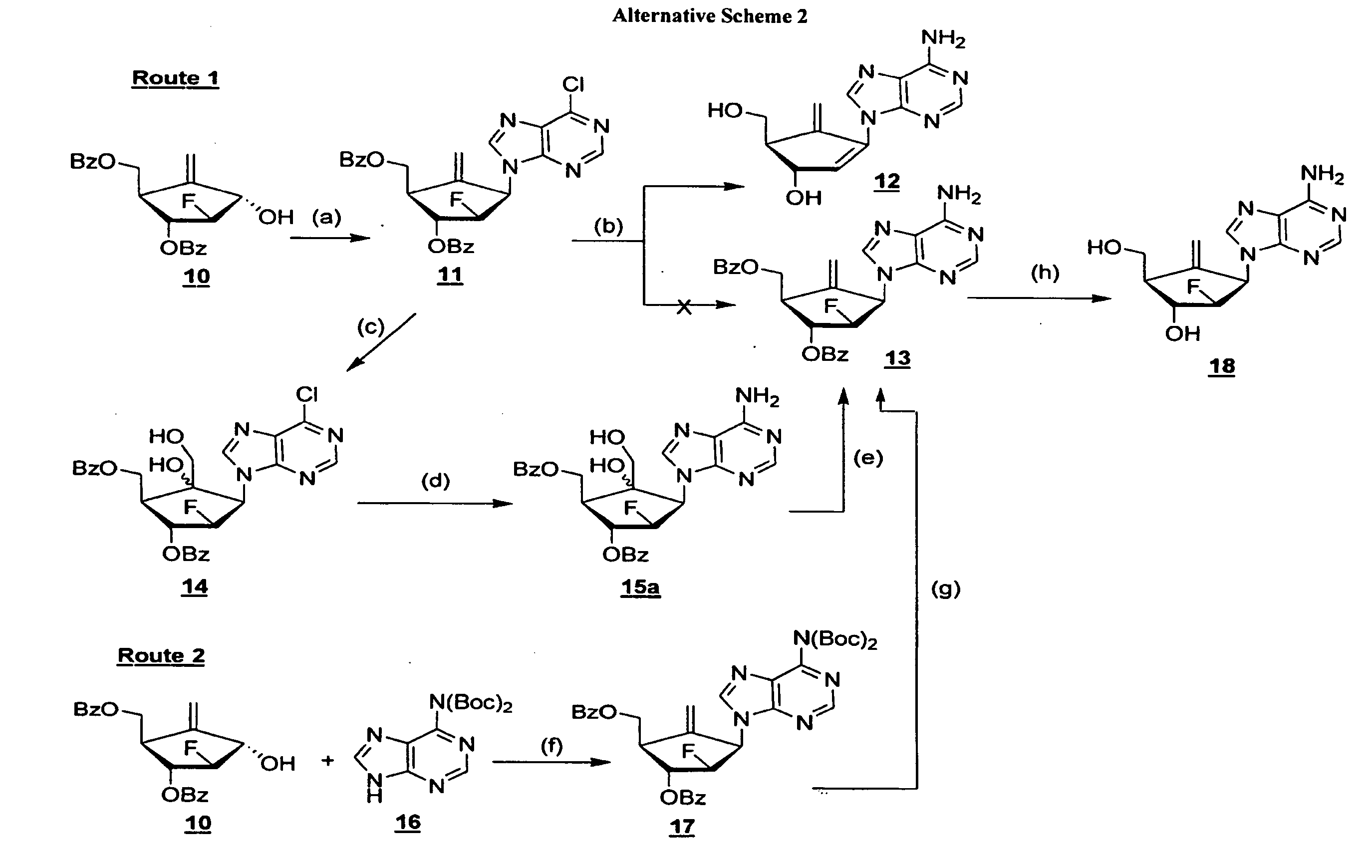
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
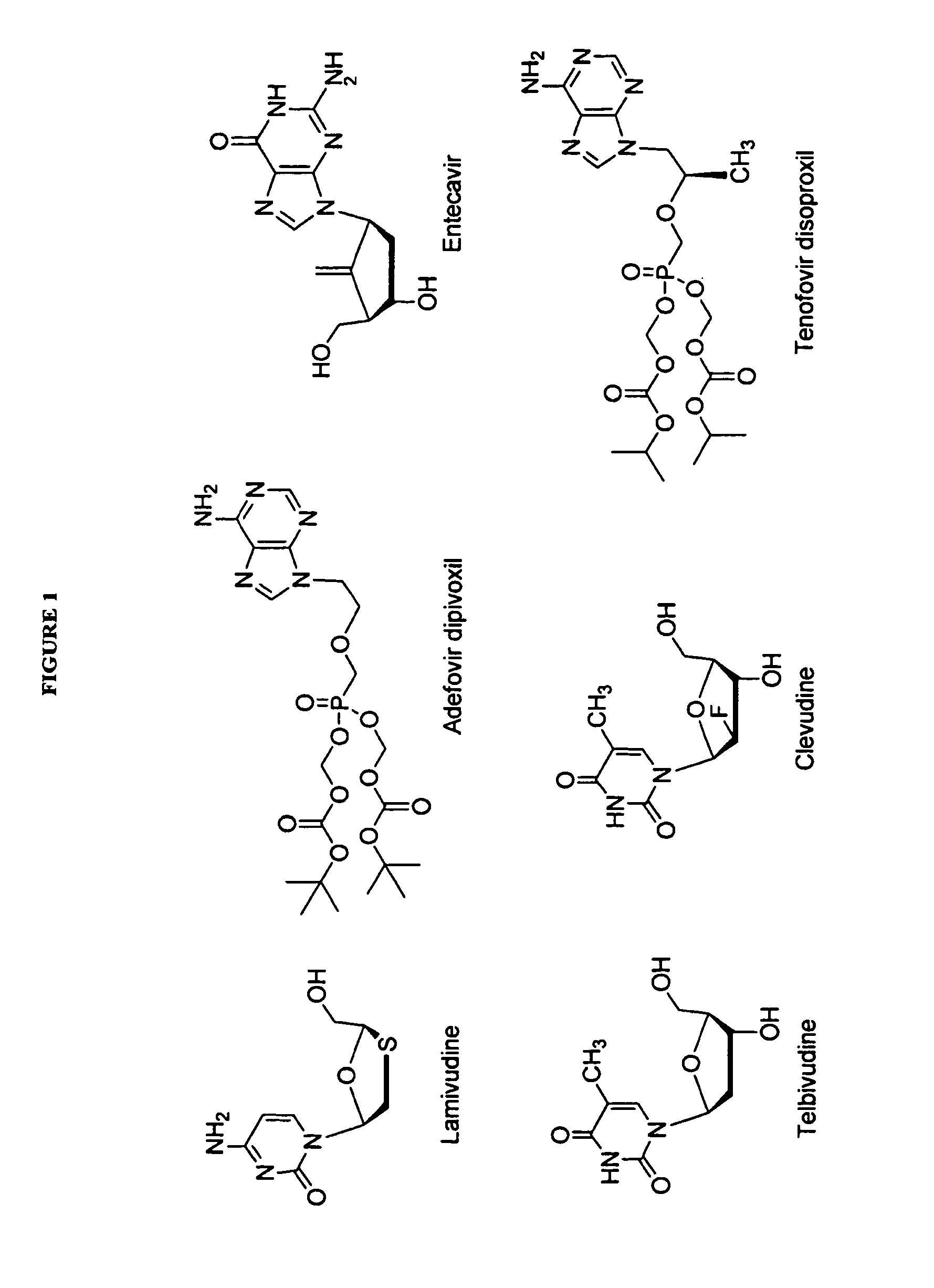
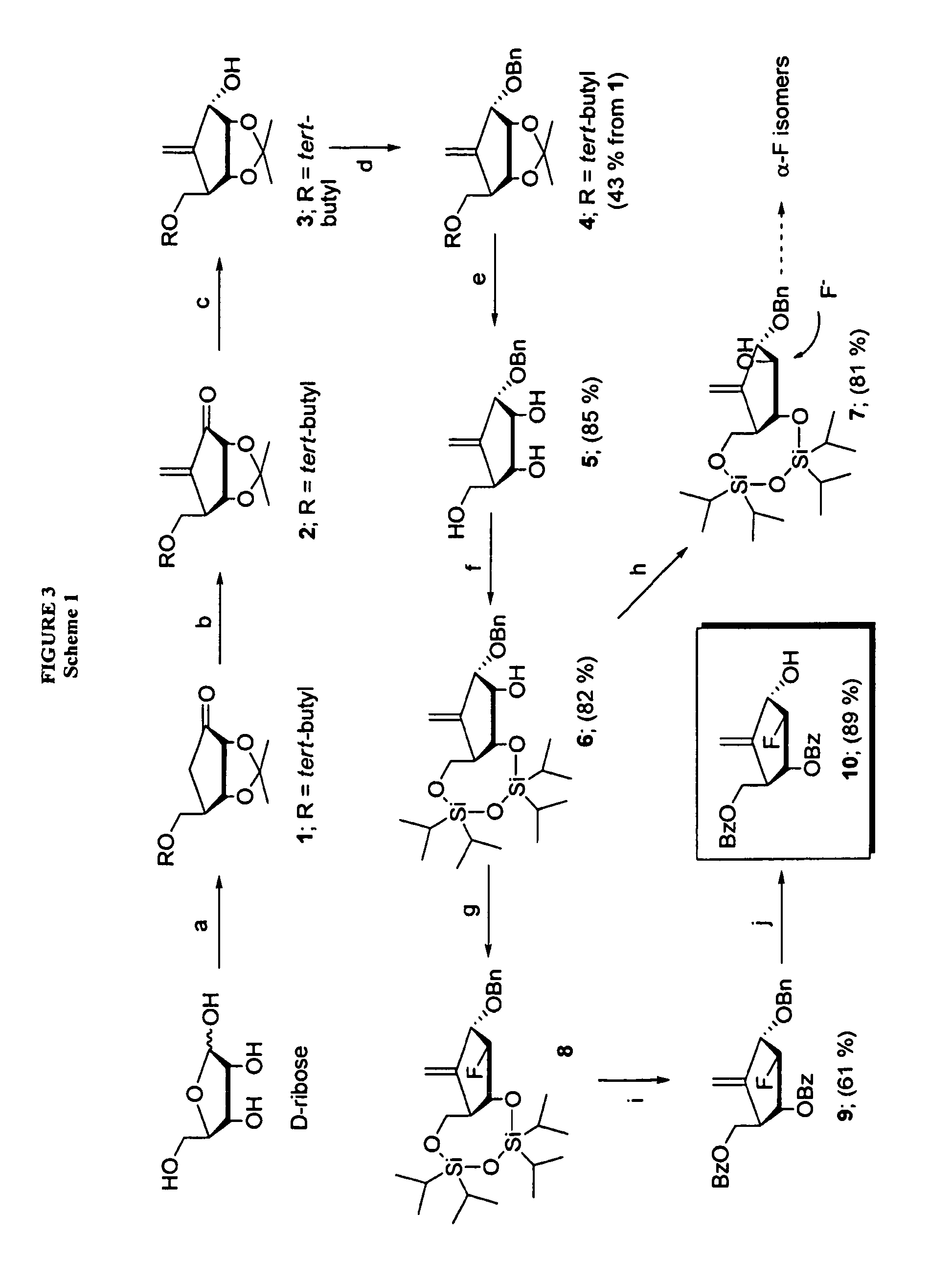
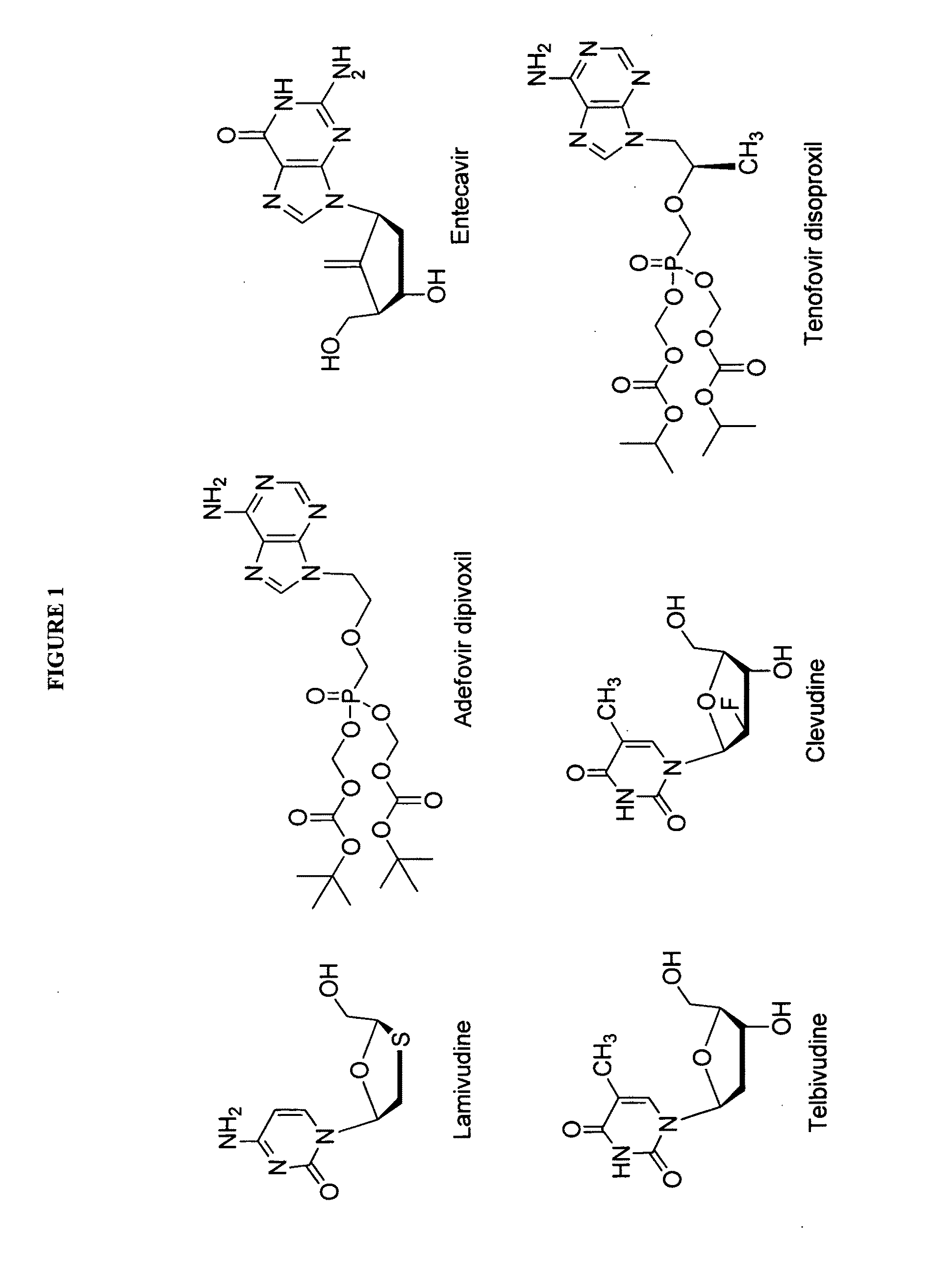
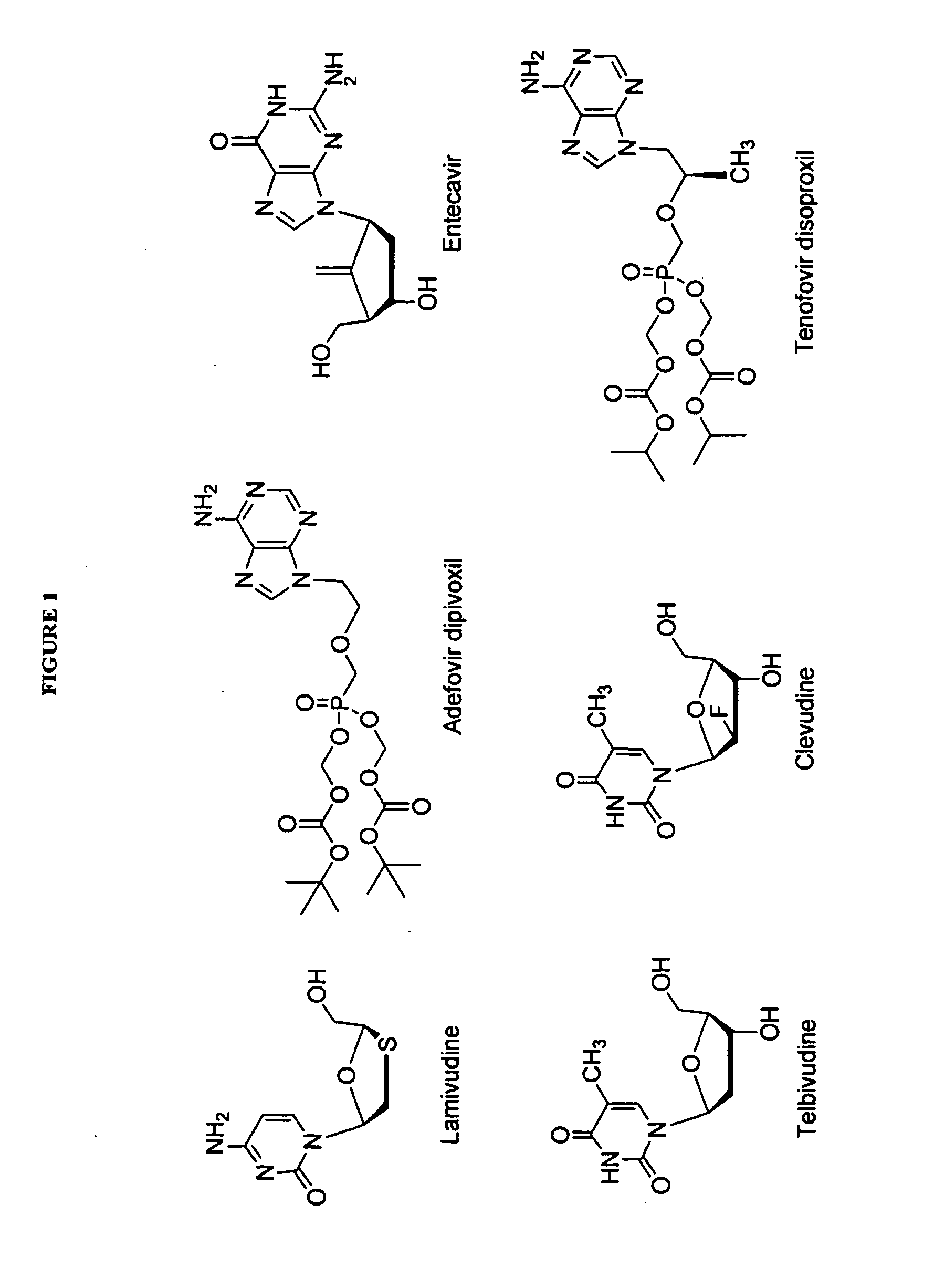
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Pyrimidine nucleosides and their monophosphate prodrugs for treatment of viral infections and cancer
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, or HBV in human patients or other animal hosts. The compounds are certain N4-hydroxycytidine nucleosides derivatives, modified monophosphate and phosphonates prodrugs analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
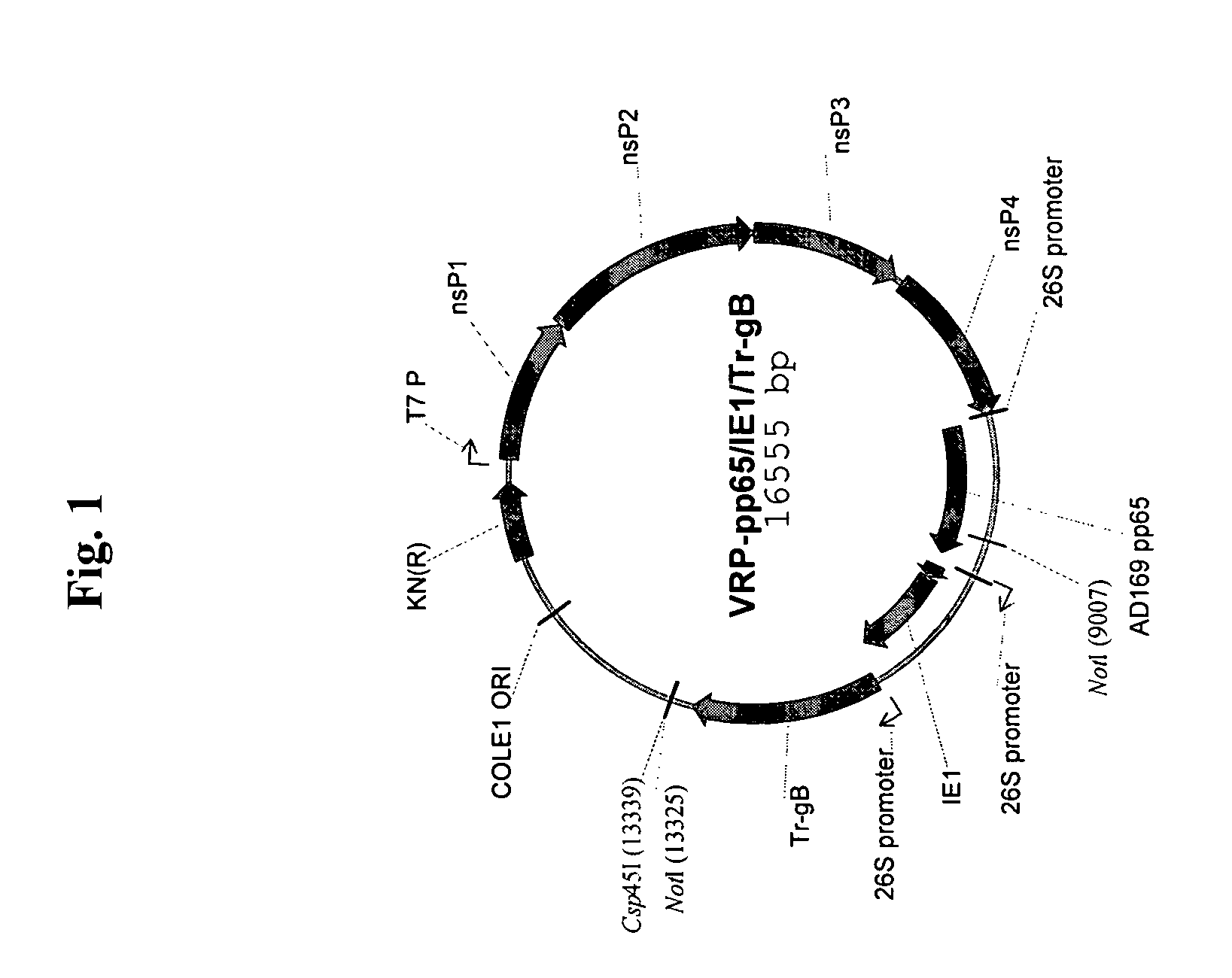
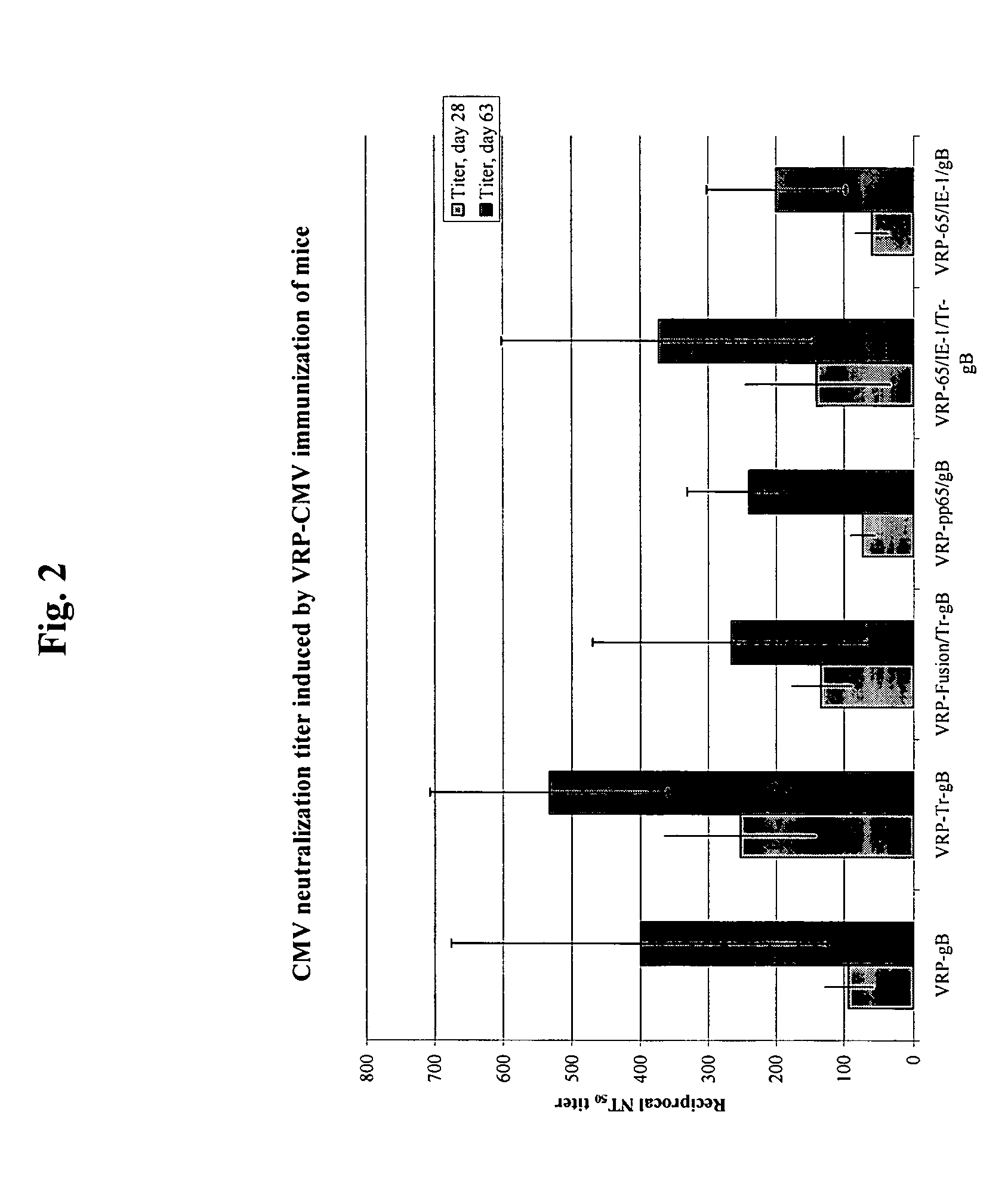
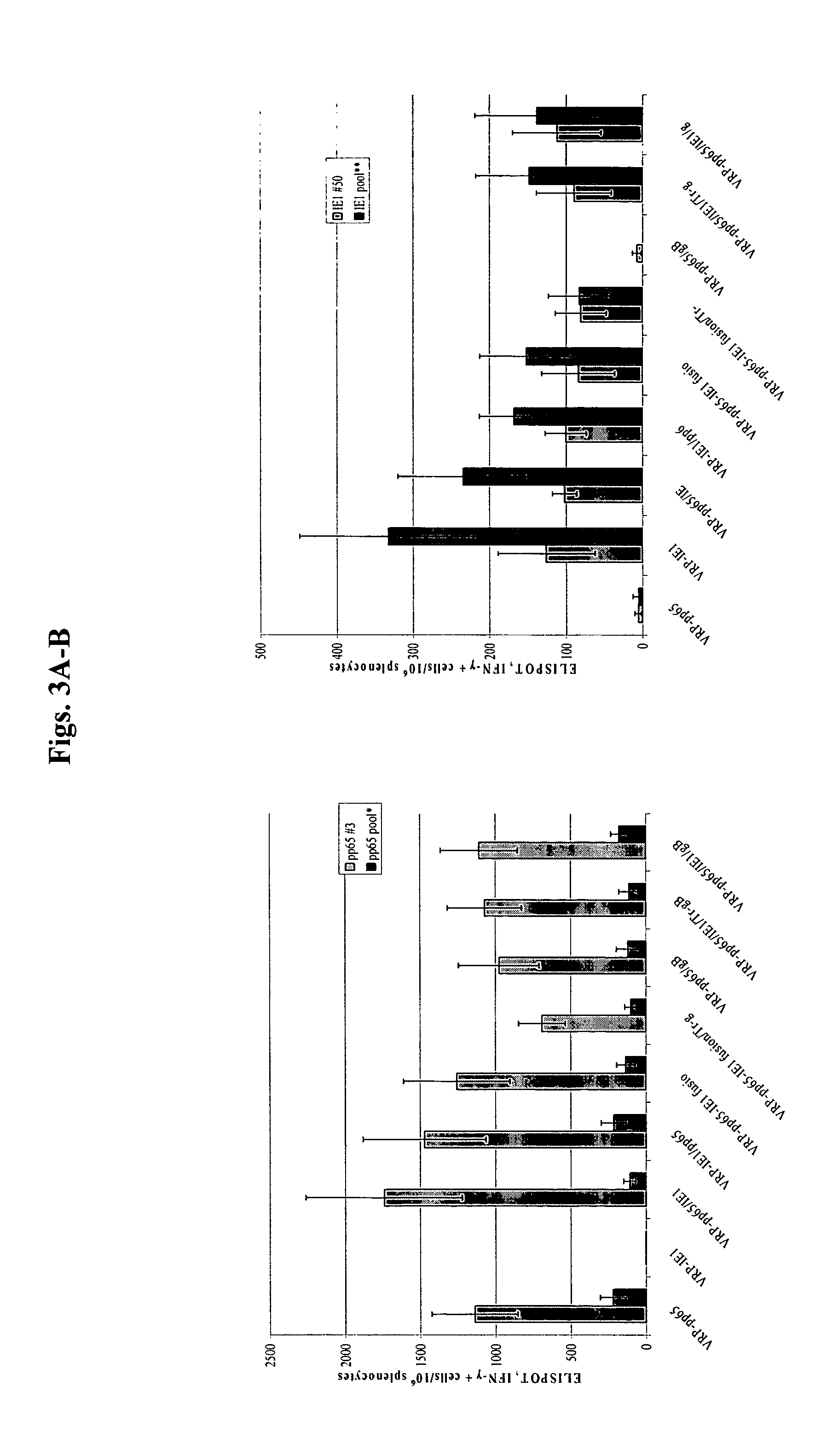
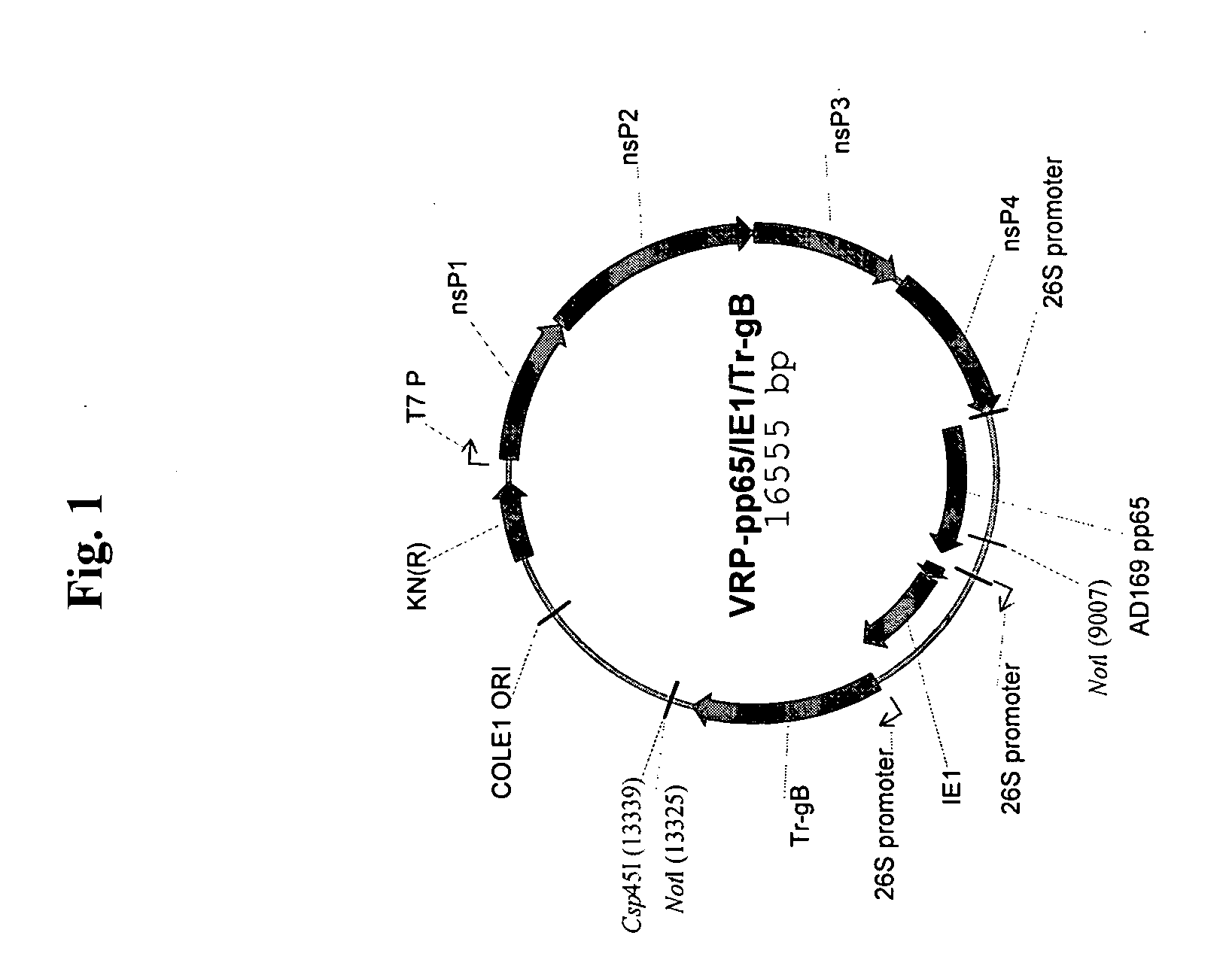
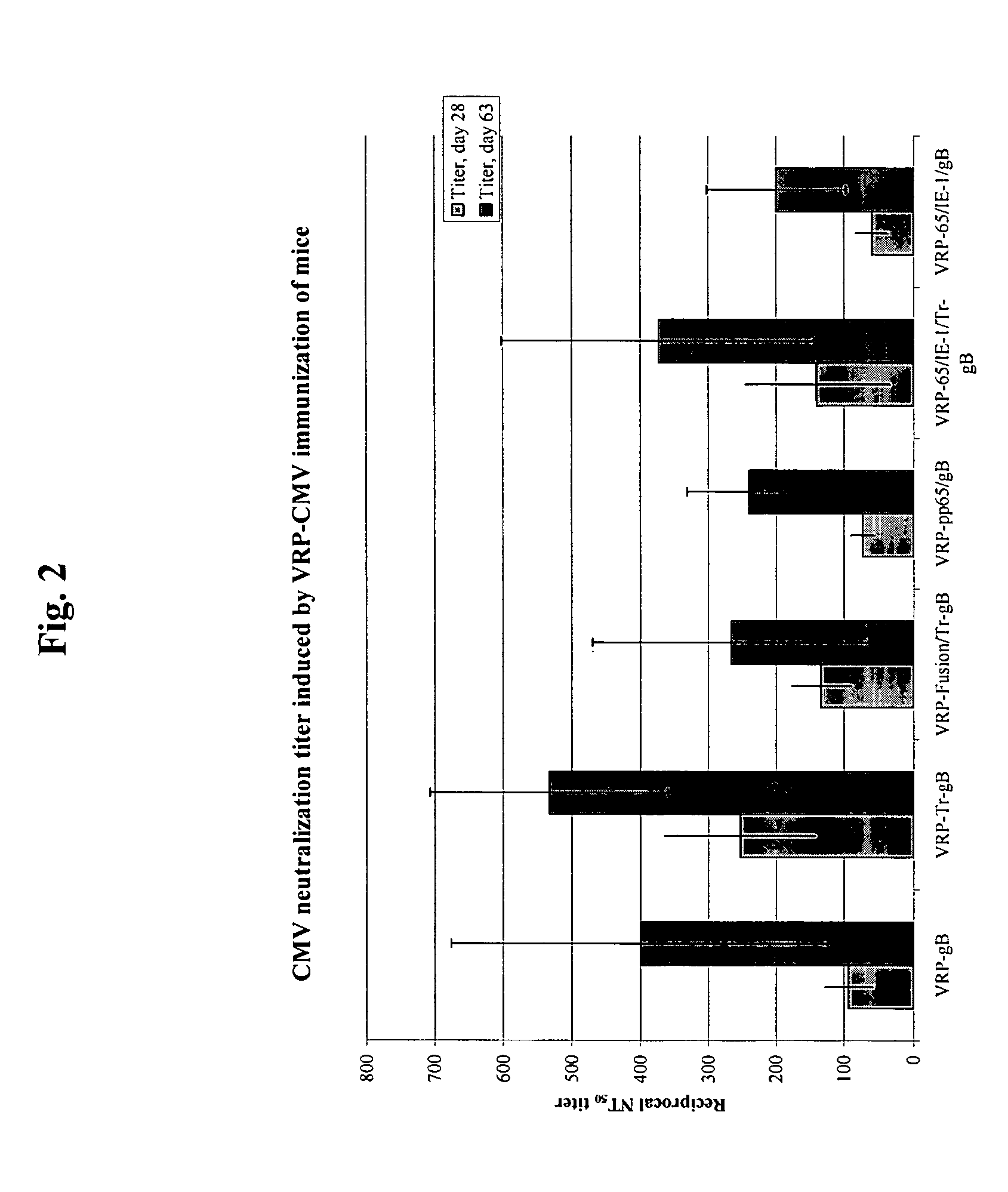
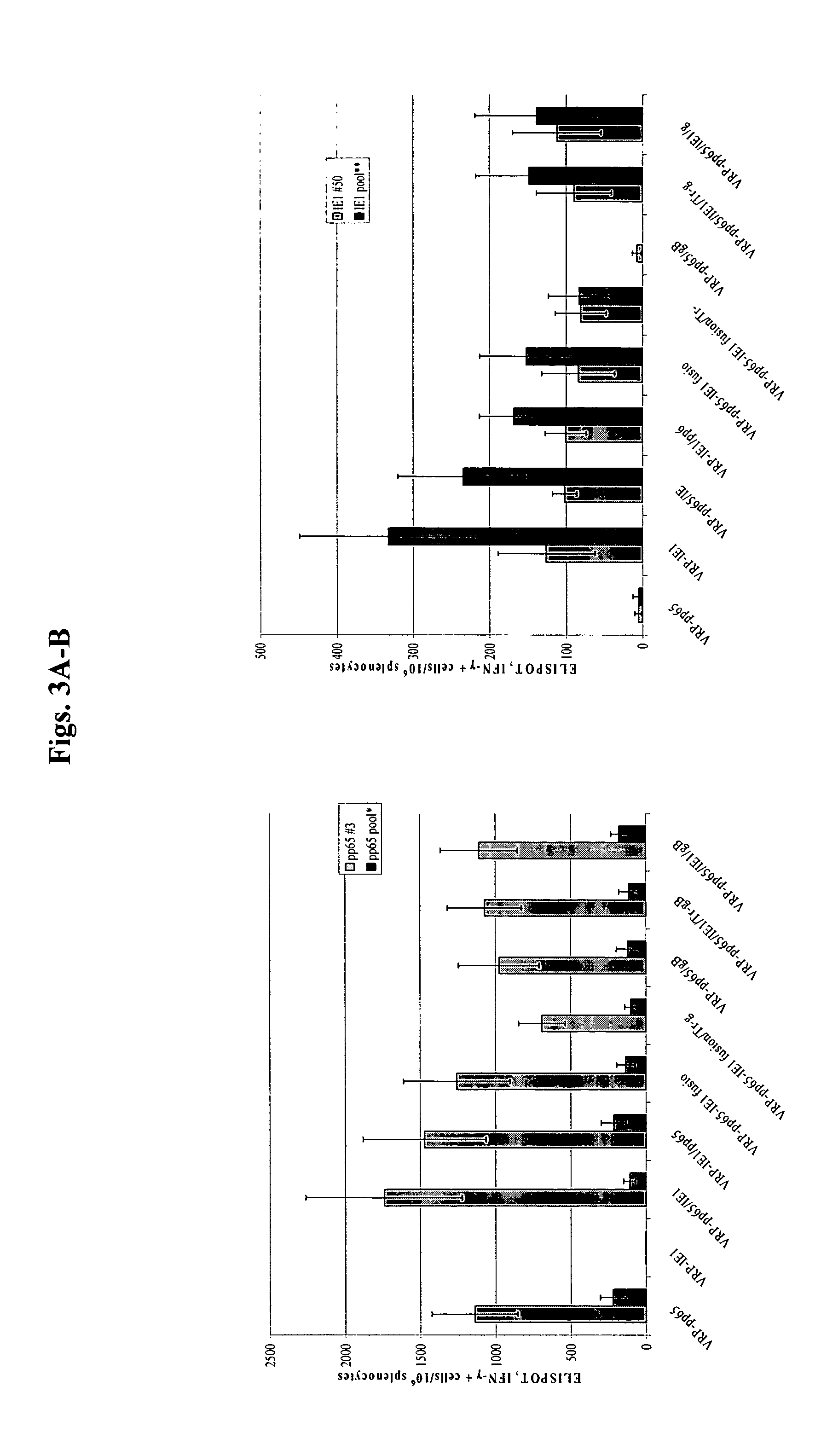
Alpha virus-based cytomegalovirus vaccines
The present invention provides methods and compositions comprising a population of alphavirus replicon particles comprising alphavirus replicon RNAs, wherein a first replicon RNA comprises nucleic acid encoding cytomegalovirus pp65 and IE1 protein or immunogenic fragments thereof, and a second replicon RNA comprises nucleic acid encoding cytomegalovirus gB protein or an immunogenic fragment thereof, and wherein each of the two replicon RNAs is contained within a separate alphavirus replicon particle.
Owner:ALPHAVAX INC
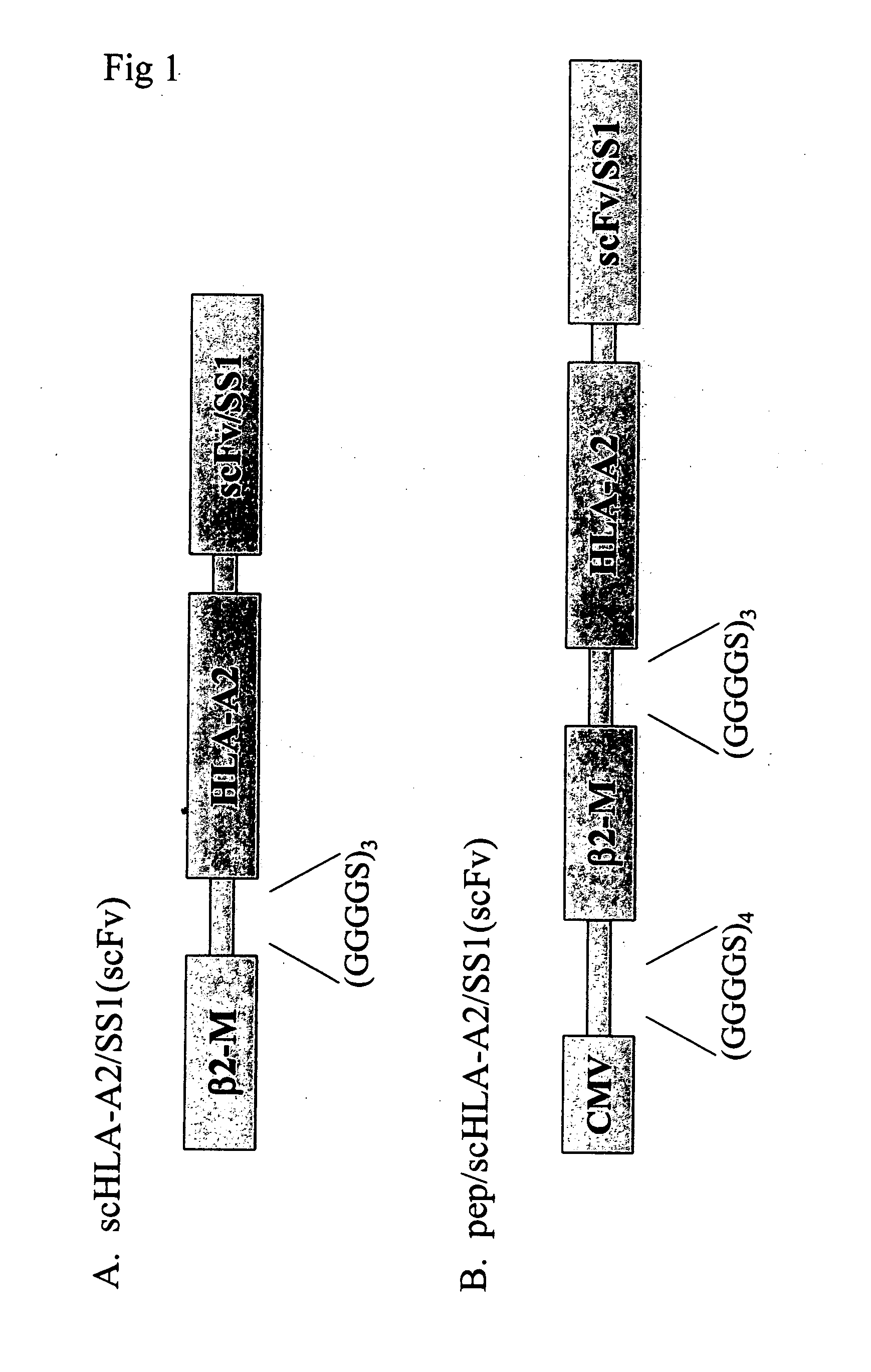
Fusion proteins, uses thereof and processes for producing same
InactiveUS20080014208A1Efficient expressionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAmino acid
This invention provides fusion proteins comprising consecutive amino acids which beginning at the amino terminus of the protein correspond to consecutive amino acids present in (i) a cytomegalovirus human MHC-restricted peptide, (ii) a first peptide linker, (iii) a human β-2 microglobulin, (iv) a second peptide linker, (v) a HLA-A2 chain of a human MHC class I molecule, (vi) a third peptide linker, (vii) a variable region from a heavy chain of a scFv fragment of an antibody, and (viii) a variable region from a light chain of such scFv fragment, wherein the consecutive amino acids which correspond to (vii) and (viii) are bound together directly by a peptide bond or by consecutive amino acids which correspond to a fourth peptide linker, wherein the antibody from which the scFv fragment is derived specifically binds to mesothelin. This invention provides nucleic acid constructs encoding same, processes for producing same, compositions, and uses thereof.
Owner:TECHNION RES & DEV FOUND LTD
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
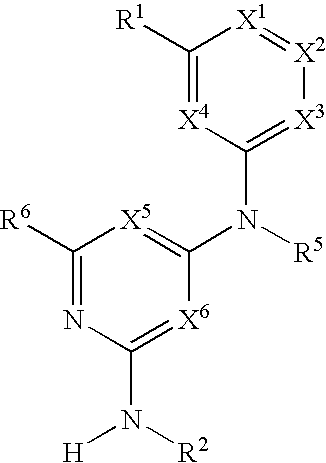
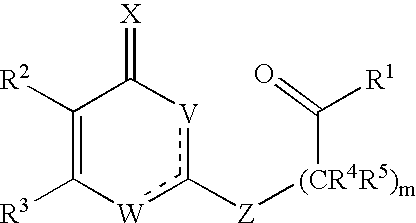
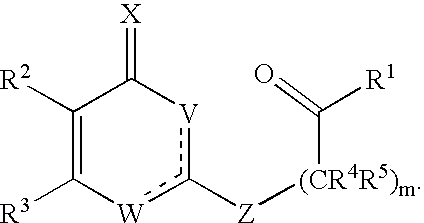
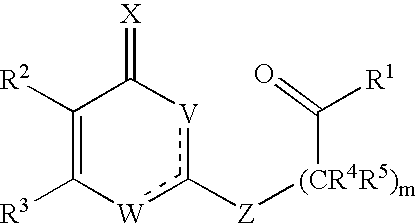
Substituted heterocyclic compounds and methods of use
The present invention relates to pyridines, pyrimidines and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Cmv glycoproteins and recombinant vectors
InactiveUS20130142823A1Bacterial antigen ingredientsViral antigen ingredientsBiotechnologyGlycoprotein
Owner:OREGON HEALTH & SCI UNIV
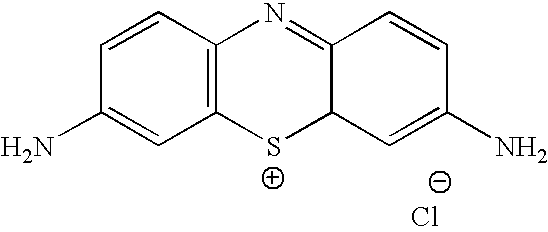
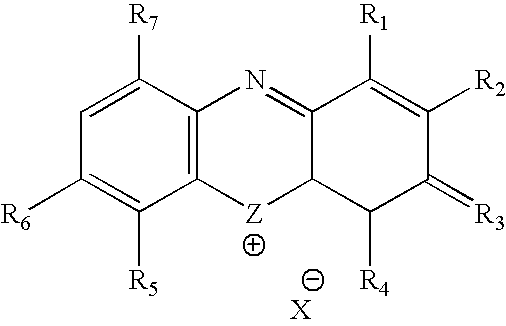
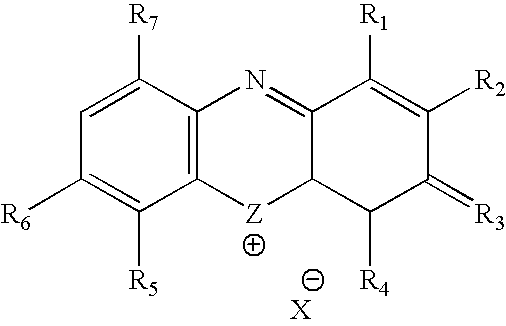
Methylene Blue Therapy of Viral Disease
InactiveUS20060264423A1Avoid infectionHeterocyclic compound active ingredientsAgainst vector-borne diseasesVirus typeHerpes zoster virus
A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, alone or in combination with low levels of light or other drugs, to selectively inactivate or inhibit hepatitis infection, has been developed. Clinical trial results demonstrate efficacy in a human clinical trial for treatment of hepatitis C by oral administration of methylene blue immediate release formulation, in a dosage of 65 mg twice daily, over a period of at least 100 days. A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, along or in combination with low levels of light or other drugs, to prevent or decrease reactivation of viruses, is also described. The preferred class of patient is infected with, or has been exposed to, viruses such as Herpes simplex virus type 1 & 2, Varicella zoster virus, Epstein-Barr virus, Cytomegalovirus, and Herpes virus type 6 & 7, Adenovirus, and Human polyoma viruses, e.g. JC virus and BK virus. In one embodiment the thiazine dye is administered to a patient experiencing symptoms or disease caused by reactivation of a virus. In a preferred embodiment the thiazine dye is administered to a patient at risk for or experiencing symptoms or disease caused by reactivation of a virus, prior to or during immunosuppression or chemotherapy.
Owner:BIOENVISION
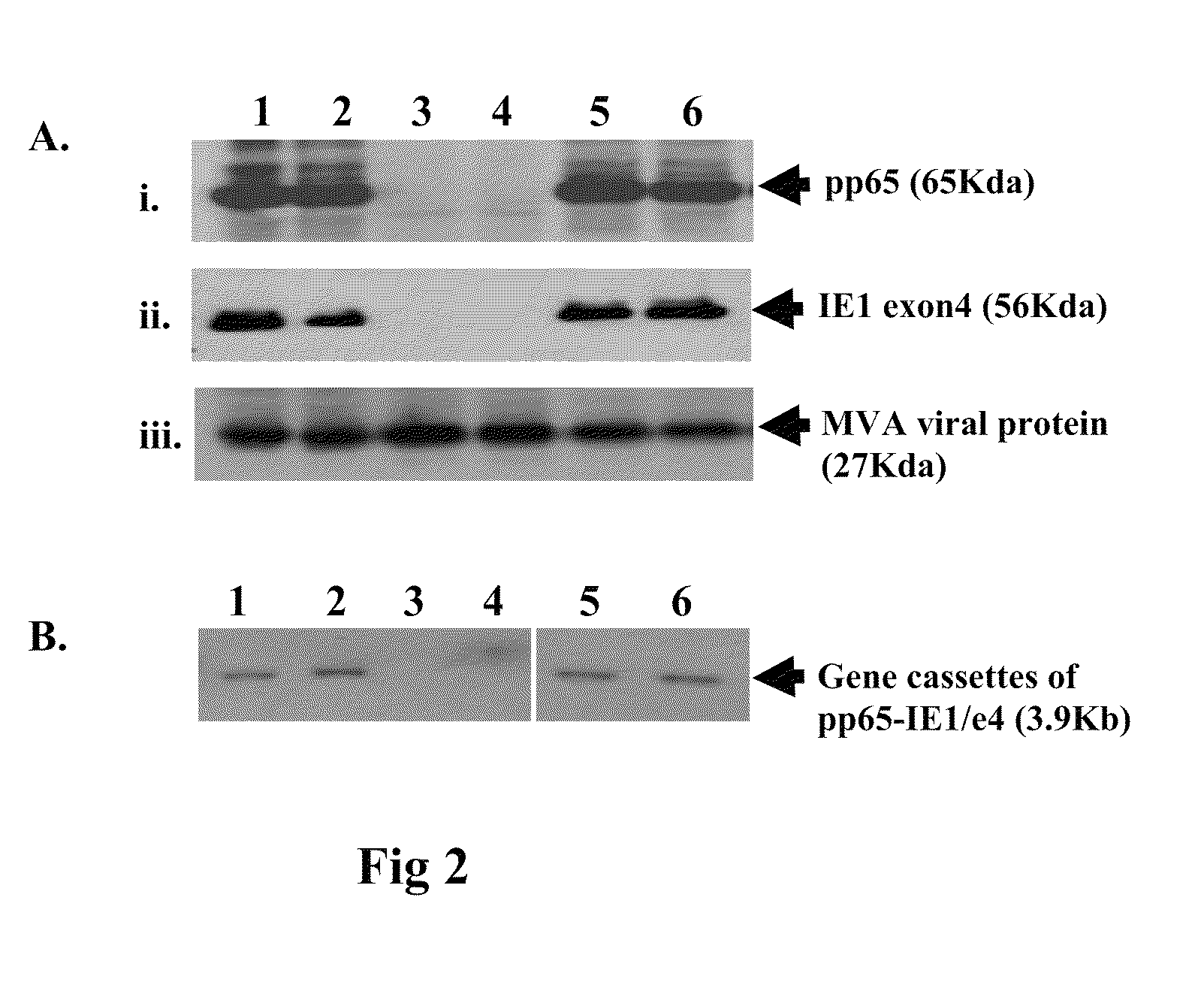
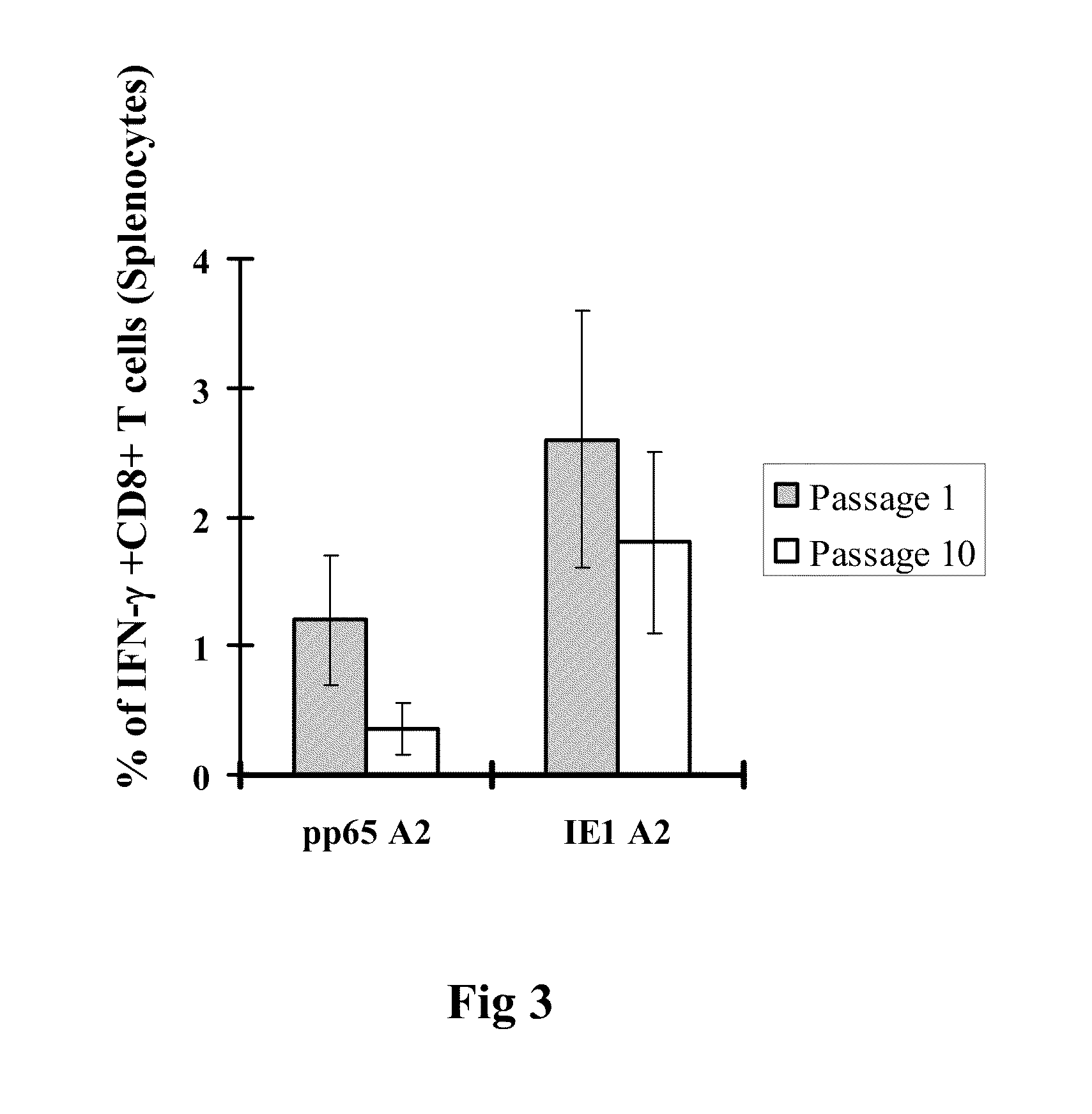
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
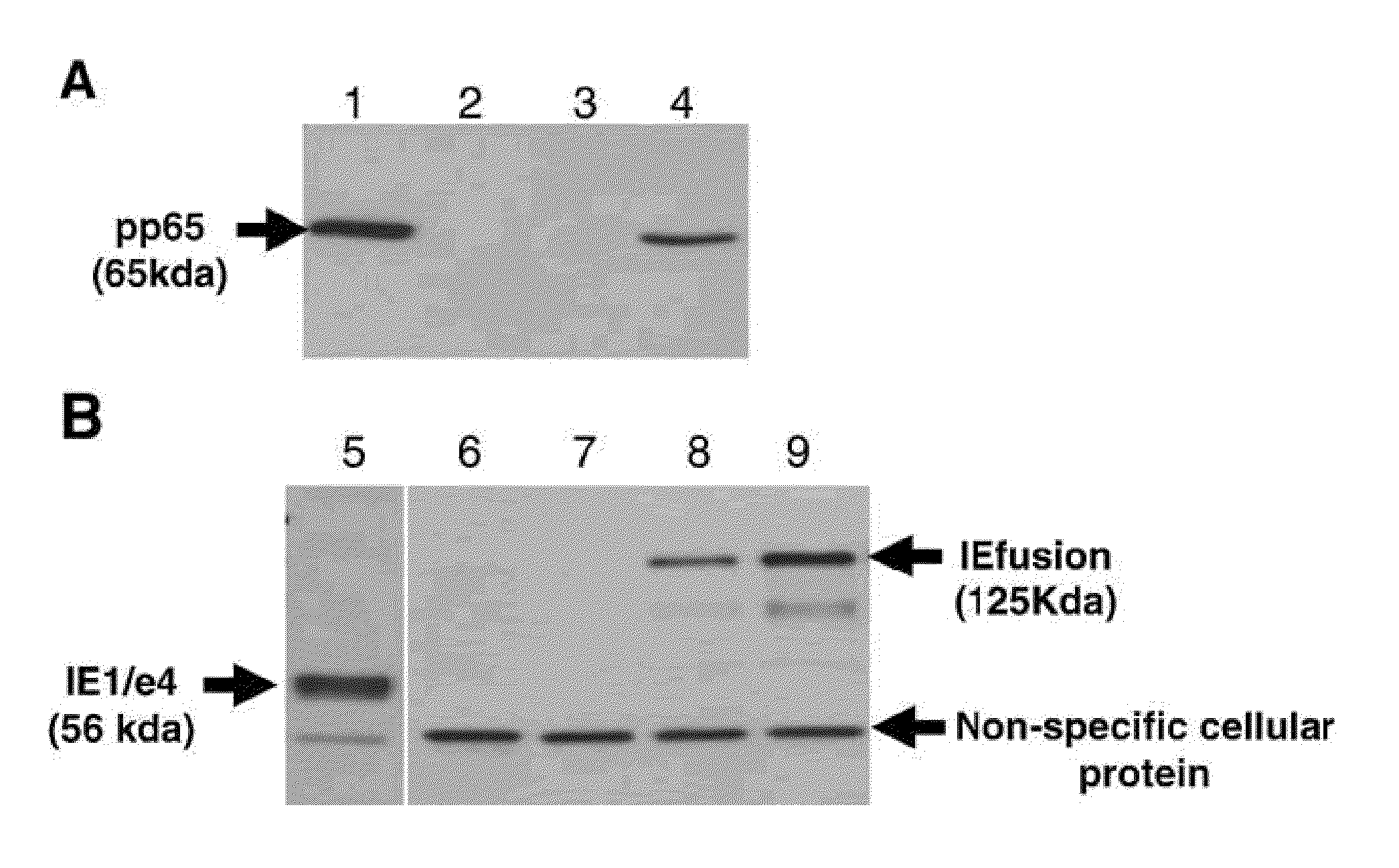
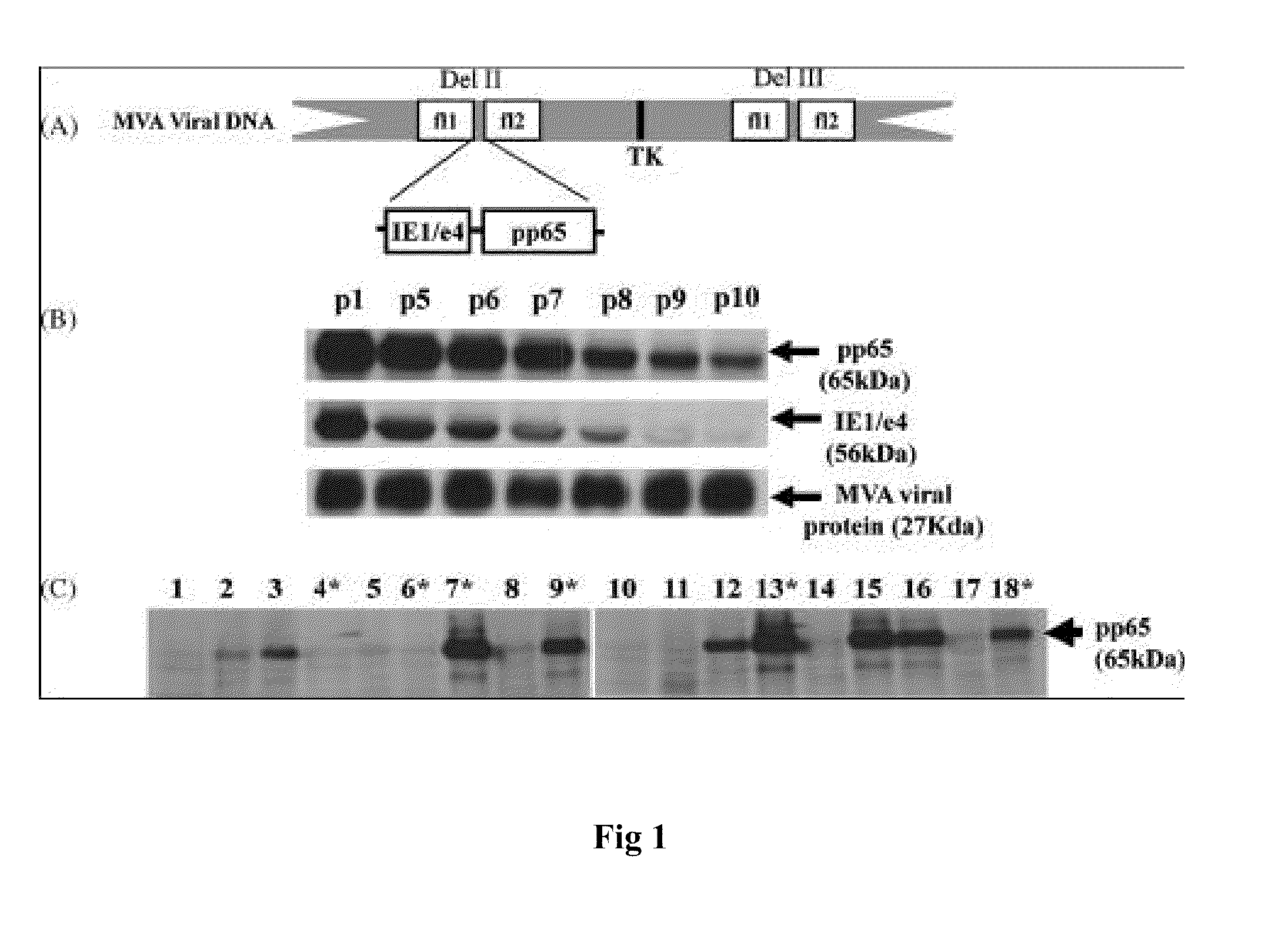
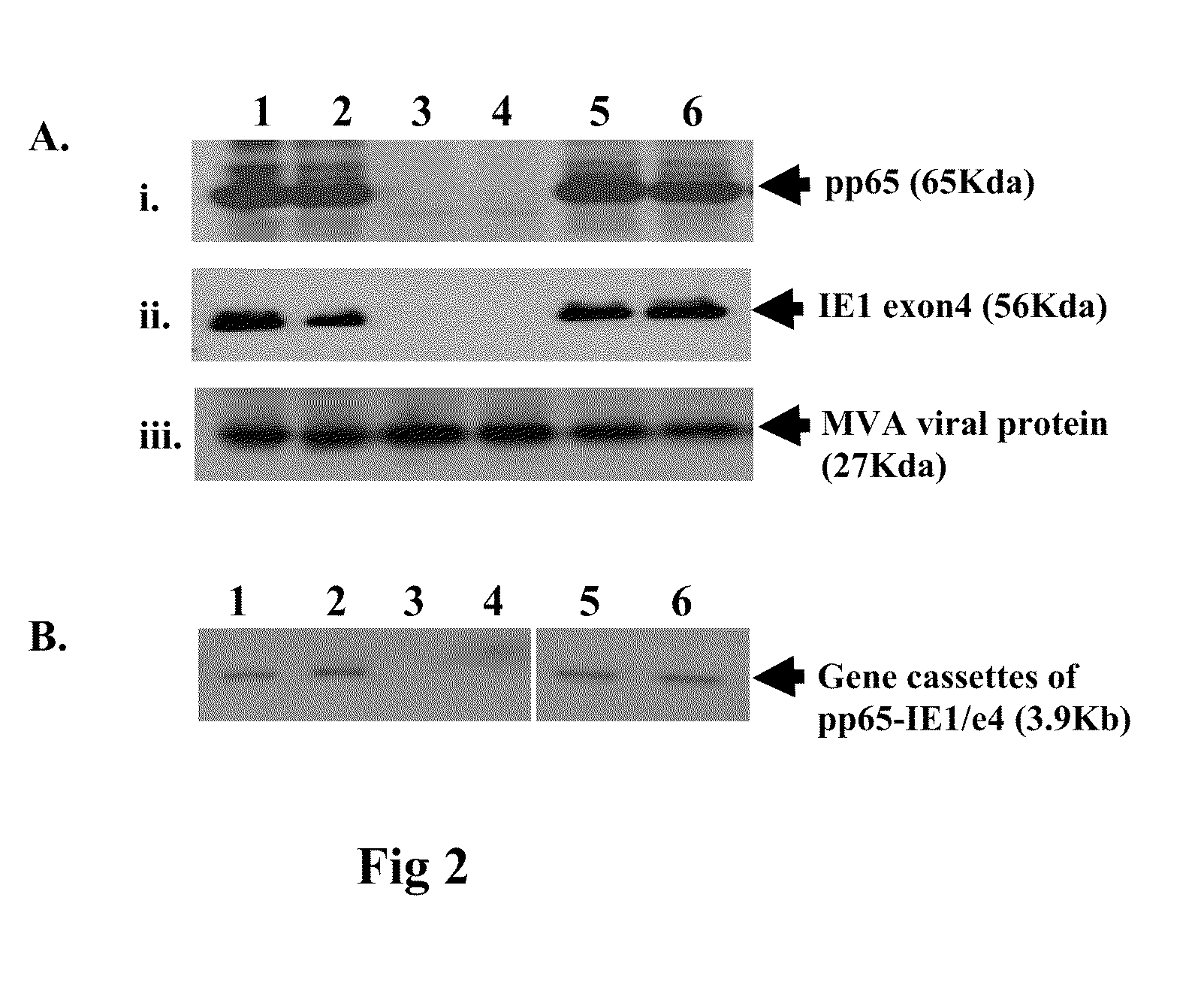
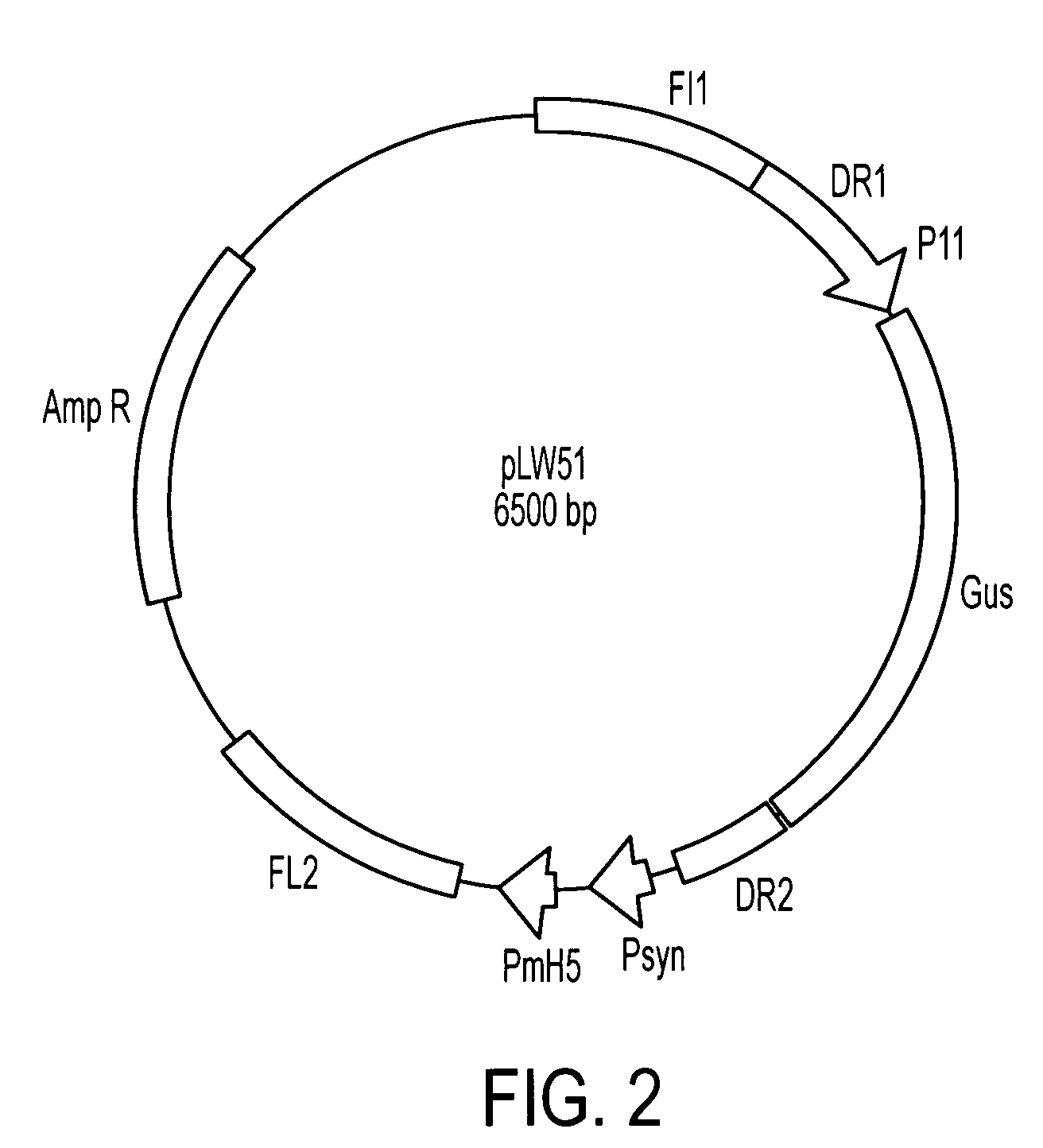
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Codon-optimized polynucleotide-based vaccines against human cytomegalovirus infection
InactiveUS7410795B2Enhance transfectionHigh expressionPeptide/protein ingredientsVirus peptidesAntigenAdjuvant
The invention is related to polynucleotide-based cytomegalovirus vaccines. In particular, the invention is plasmids operably encoding HCMV antigens, in which the naturally-occurring coding regions for the HCMV antigens have been modified for improved translation in human or other mammalian cells through codon optimization. HCMV antigens which are useful in the invention include, but are not limited to pp65, glycoprotein B (gB), IE1, and fragments, variants or derivatives of either of these antigens. In certain embodiments, sequences have been deleted, e.g., the Arg435-Lys438 putative kinase in pp65 and the membrane anchor and endocellular domains in gB. The invention is further directed to methods to induce an immune response to HCMV in a mammal, for example, a human, comprising delivering a plasmid encoding a codon-optimized HCMV antigen as described above. The invention is also directed to pharmaceutical compositions comprising plasmids encoding a codon-optimized HCMV antigen as described above, and further comprising adjuvants, excipients, or immune modulators.
Owner:VICAL INC
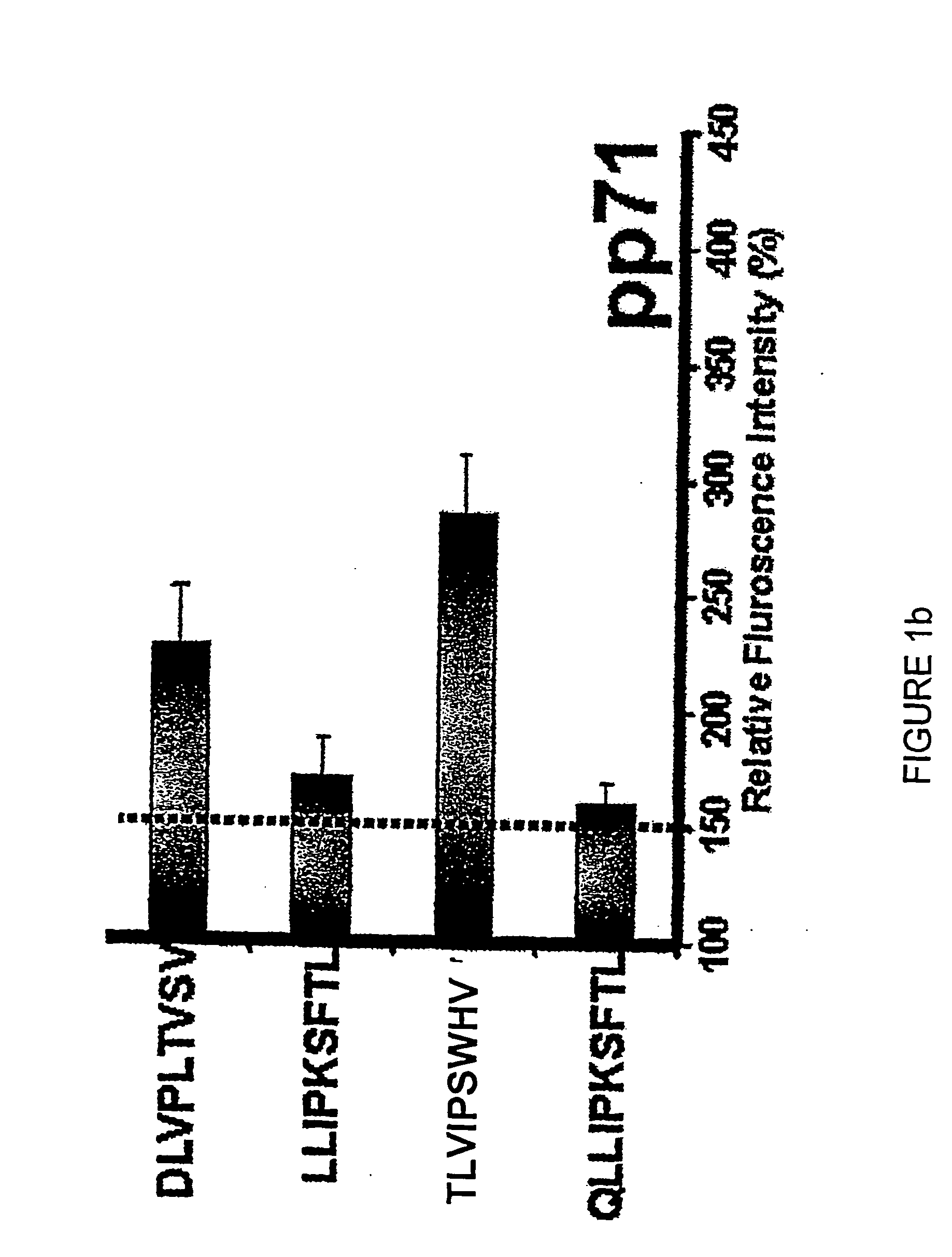
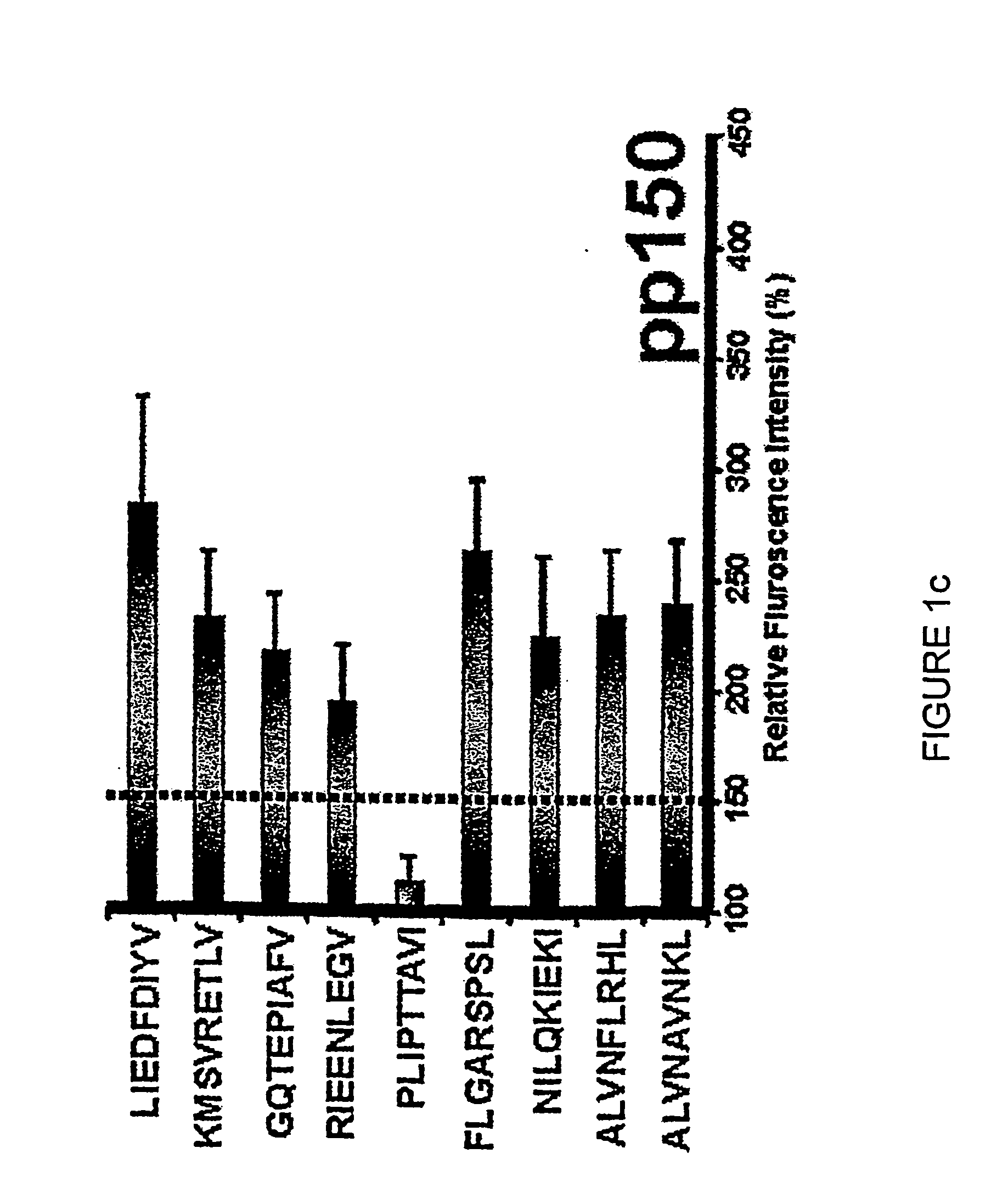
Novel human cytomegalovirus (hcmv) cytotoxic t cell epitopes, polyepitopes compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS20050019344A1Minimizing formulation difficultyReduce in quantityViral antigen ingredientsMicrobiological testing/measurementCtl epitopeVaccination
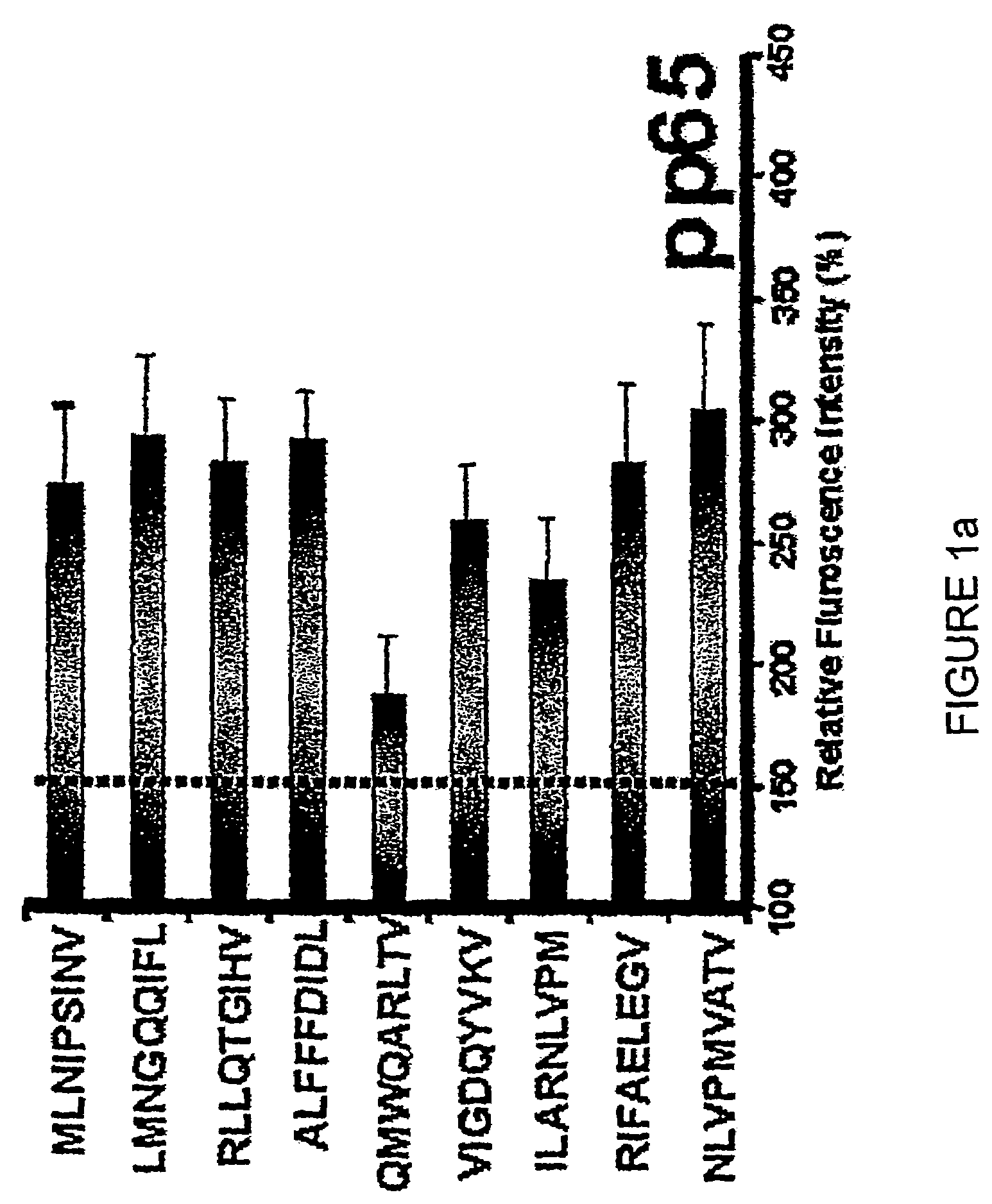
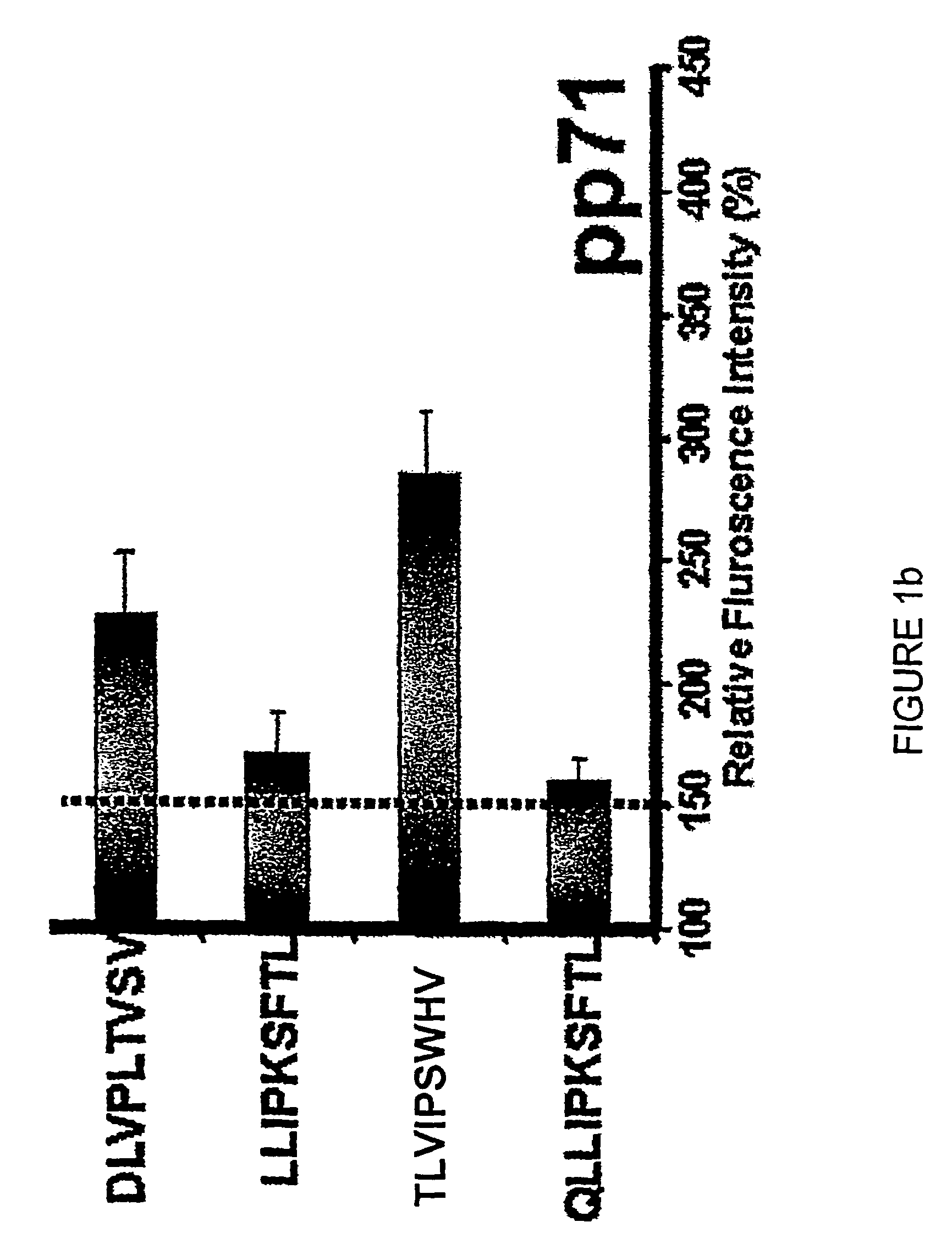
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the most commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine composiitons comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
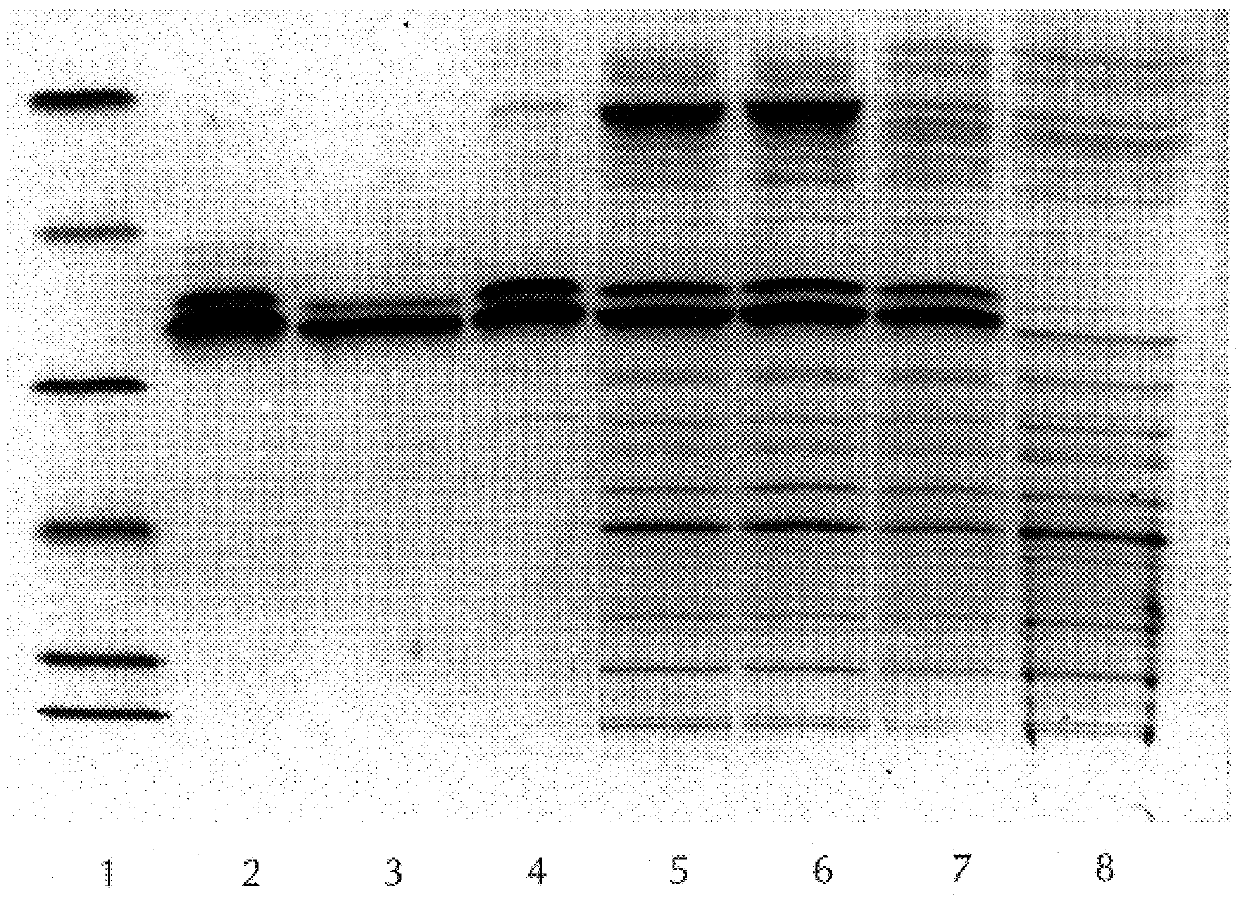
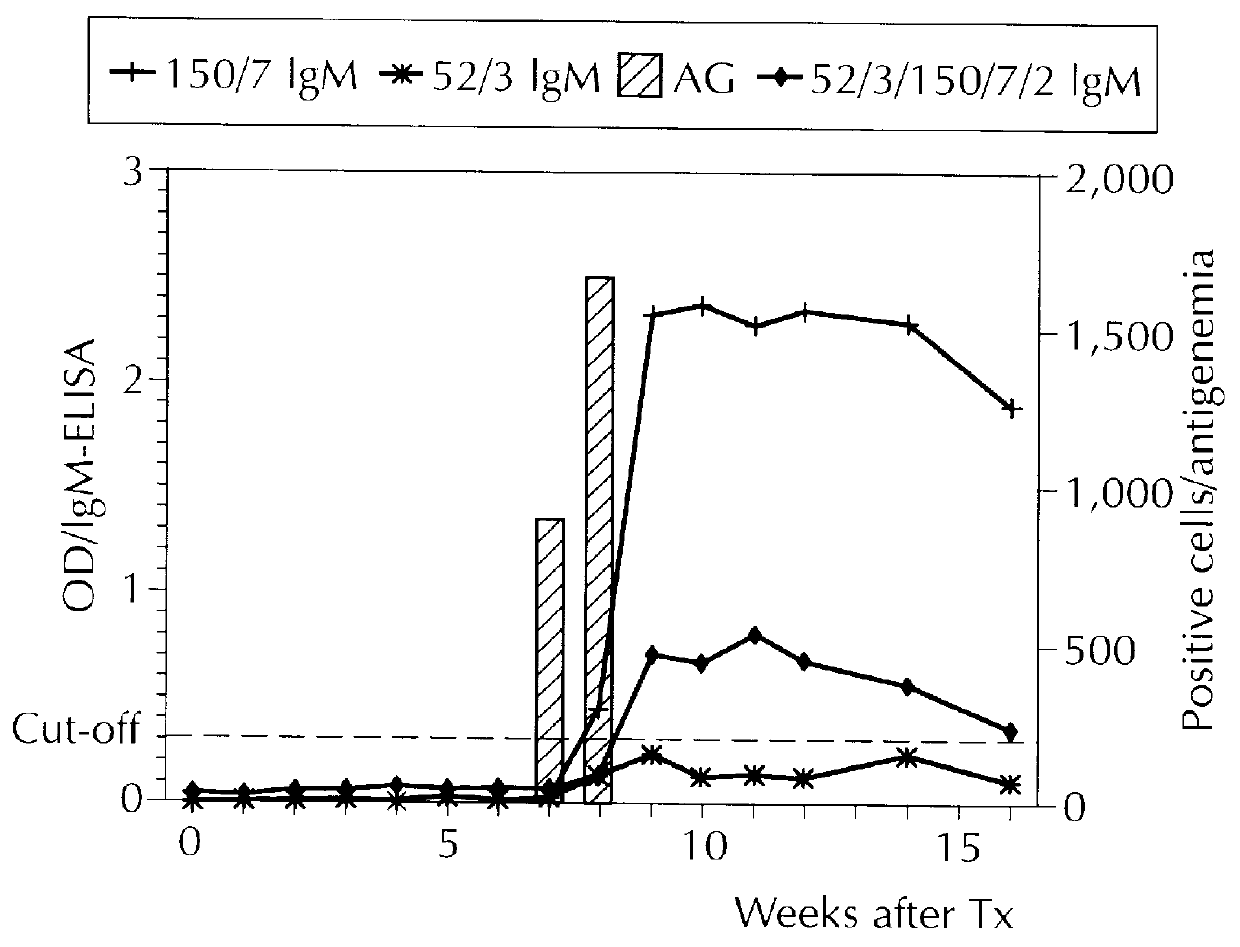
Isolated human cytomegalovirus polypeptides and uses thereof
InactiveUS6120989APeptide/protein ingredientsAntibody mimetics/scaffoldsIgm antibodyHuman cytomegalovirus
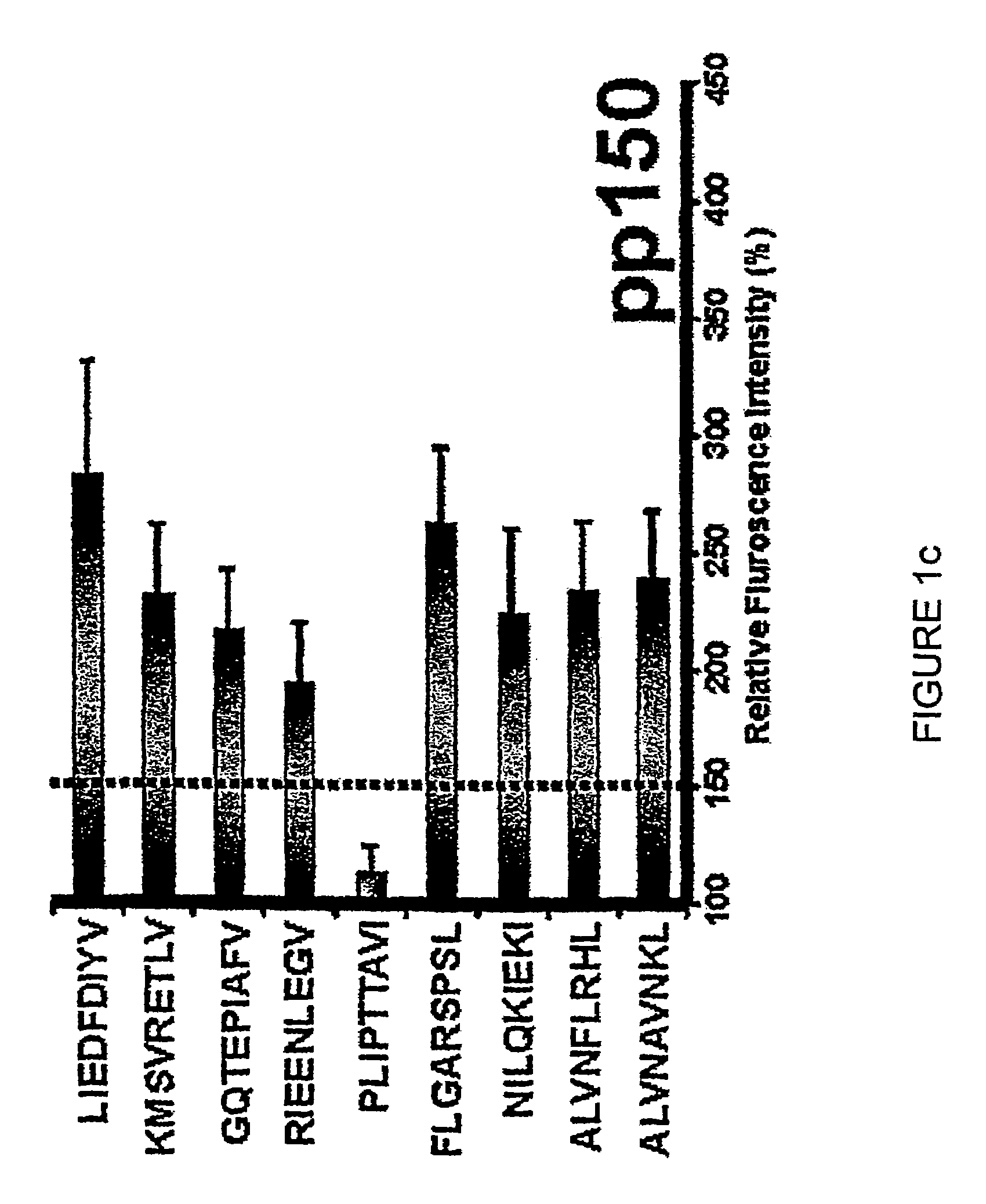
Diagnostically relevant polypeptides and fusion proteins comprising an amino acid sequence which originates from cytomegalovirus and corresponds to a region of the major DNA-binding protein or of the C-terminal region of the tegument protein pp150 fused with at least one further fragment from another antigenic protein of cytomegalovirus are disclosed. The major DNA-binding protein is encoded by the reading frame UL57. The poly-peptides and fusion proteins according to the invention can be used in an advantageous manner in diagnostic tests and methods for the detection of IgM antibodies against cytomegalovirus.
Owner:BIOTEST SERUM INST GMBH
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Enhanced production of recombinant proteins by transient transfection of suspension-growing mammalian cells
ActiveUS20050170450A1Increase transcriptional activityIncreasing nuclear importGenetically modified cellsVirus peptidesEpstein–Barr virusGene Variant
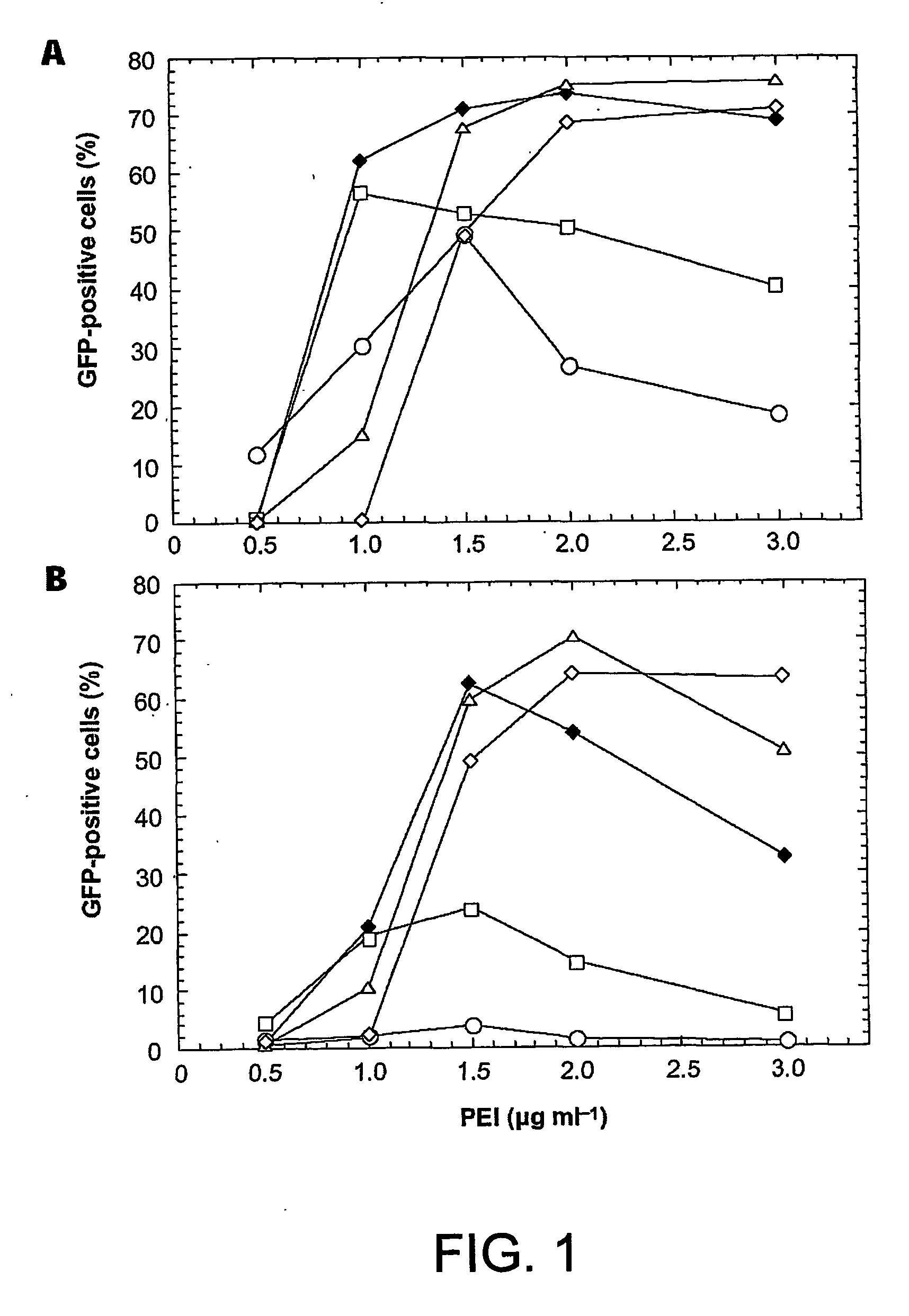
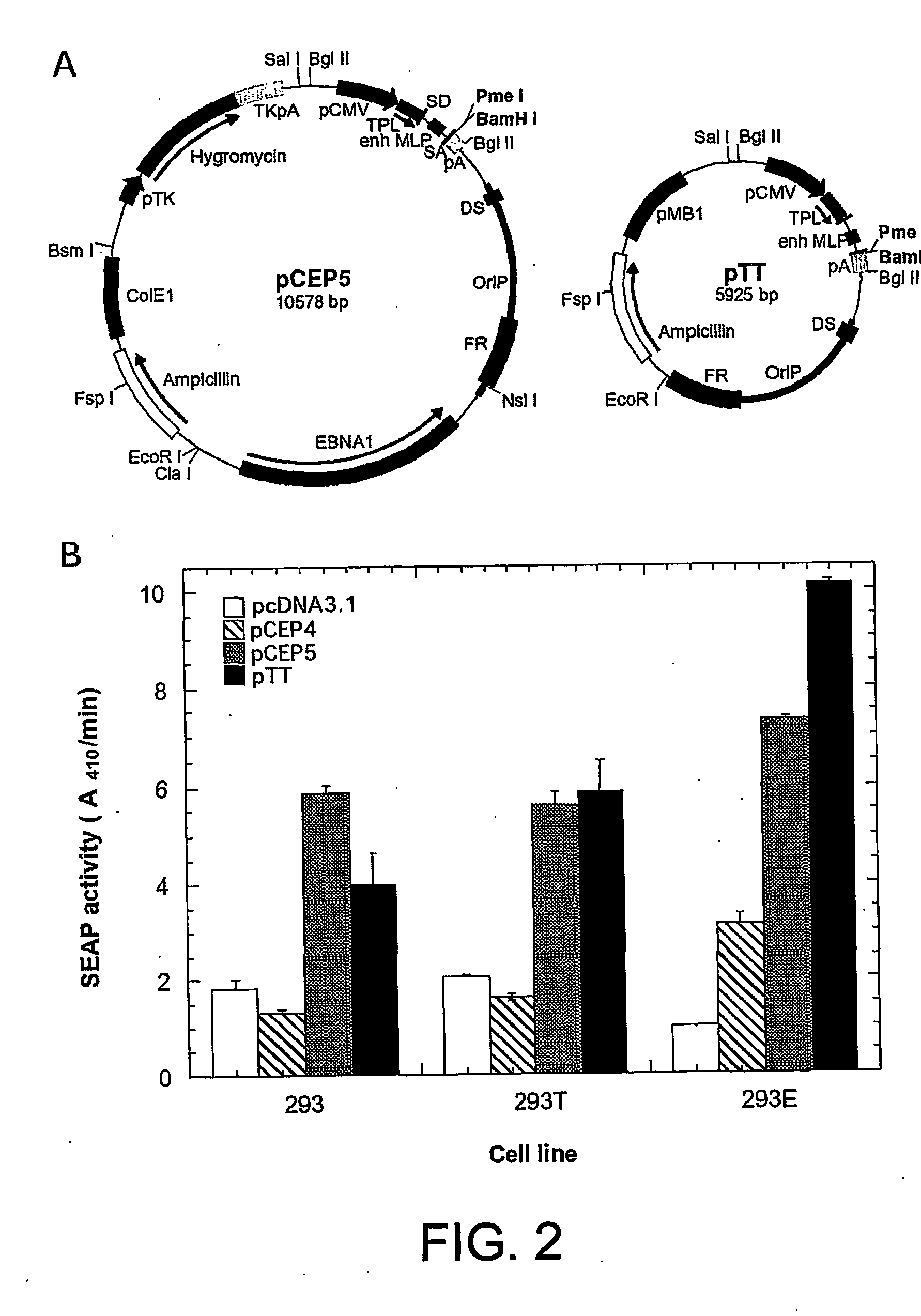
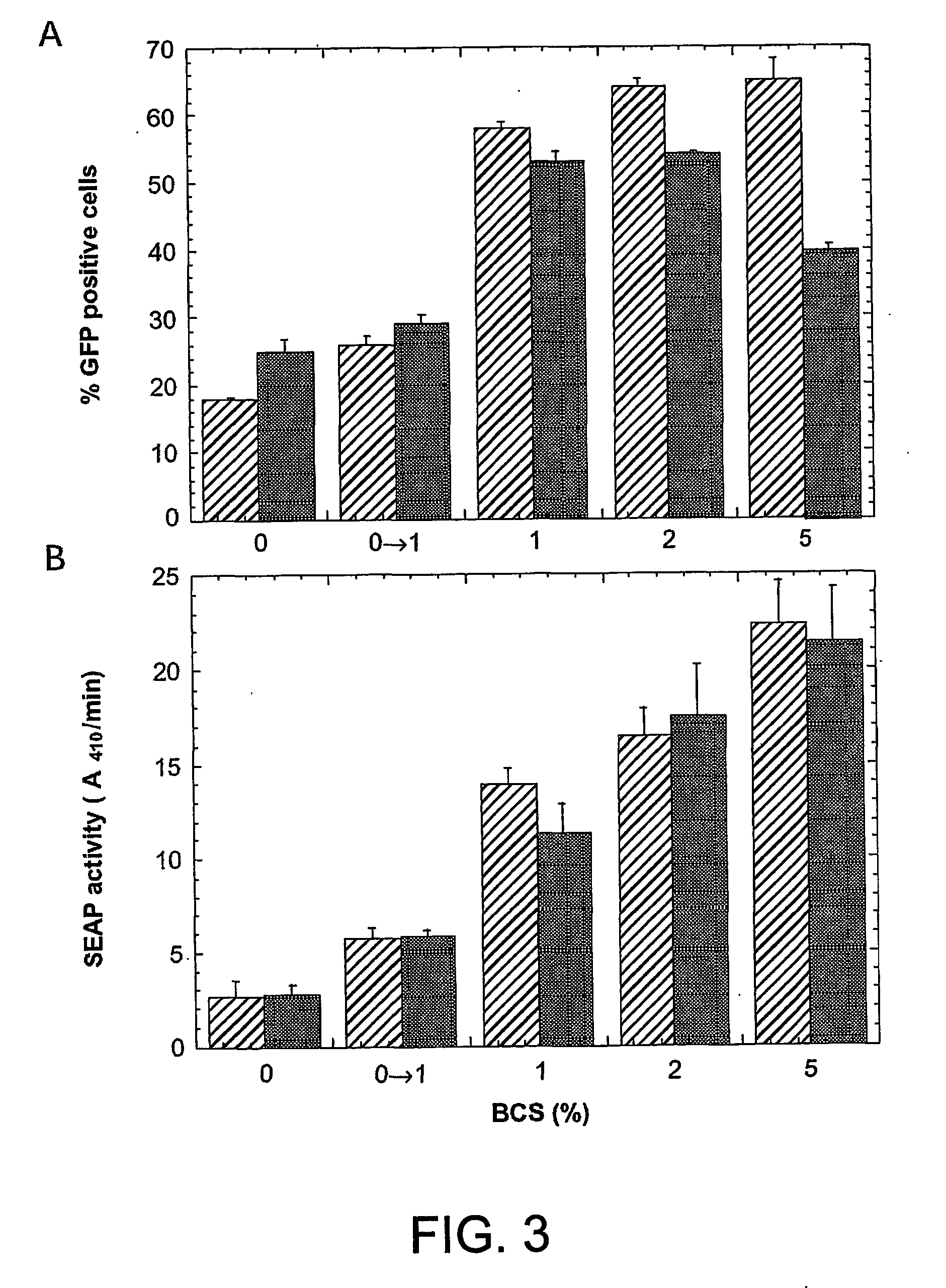
Disclosed is a new process for the production of recombinant proteins, by transient transfection of suspension-grown human embryonic kidney cells (293 cell line and its genetic variants) with an expression vector, using polyethylenimine (PEI) as a transfection reagent. In a preferred embodiment, the process uses 293E cells expressing the Epstein-Barr virus (EBV) EBNA 1 protein, in combination with an oriP-based episomal expression vector having an improved cytomegalovirus expression cassette comprising the CMV5 promoter. The process combines in a single step the cell growth, transfection and protein expression, is carried out without changing the culture medium, and allows to achieve high expression levels in a short period of time. The process may be carried out in a serum-free, low-protein culture medium, is easily scalable, compatible with continuous production processes, and fully adapted to high-throughput production of milligram quantities of recombinant proteins.
Owner:NAT RES COUNCIL OF CANADA
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6083925AGood immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
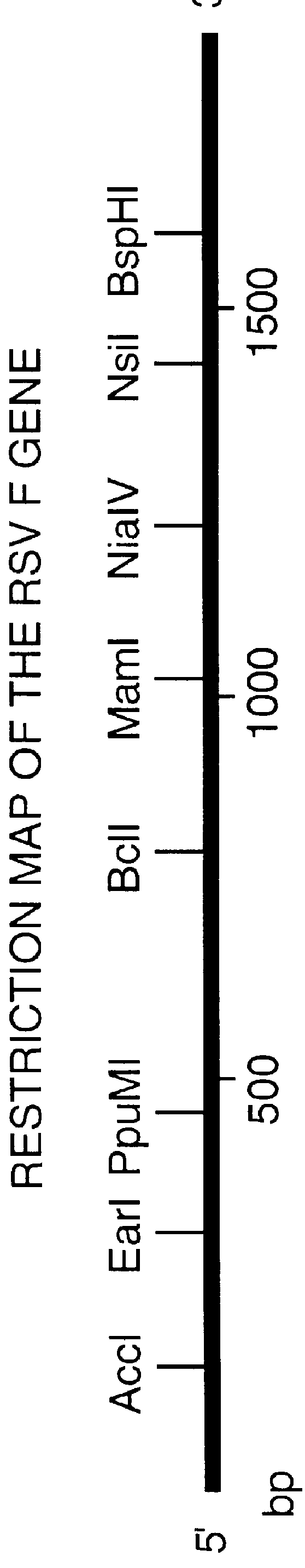
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
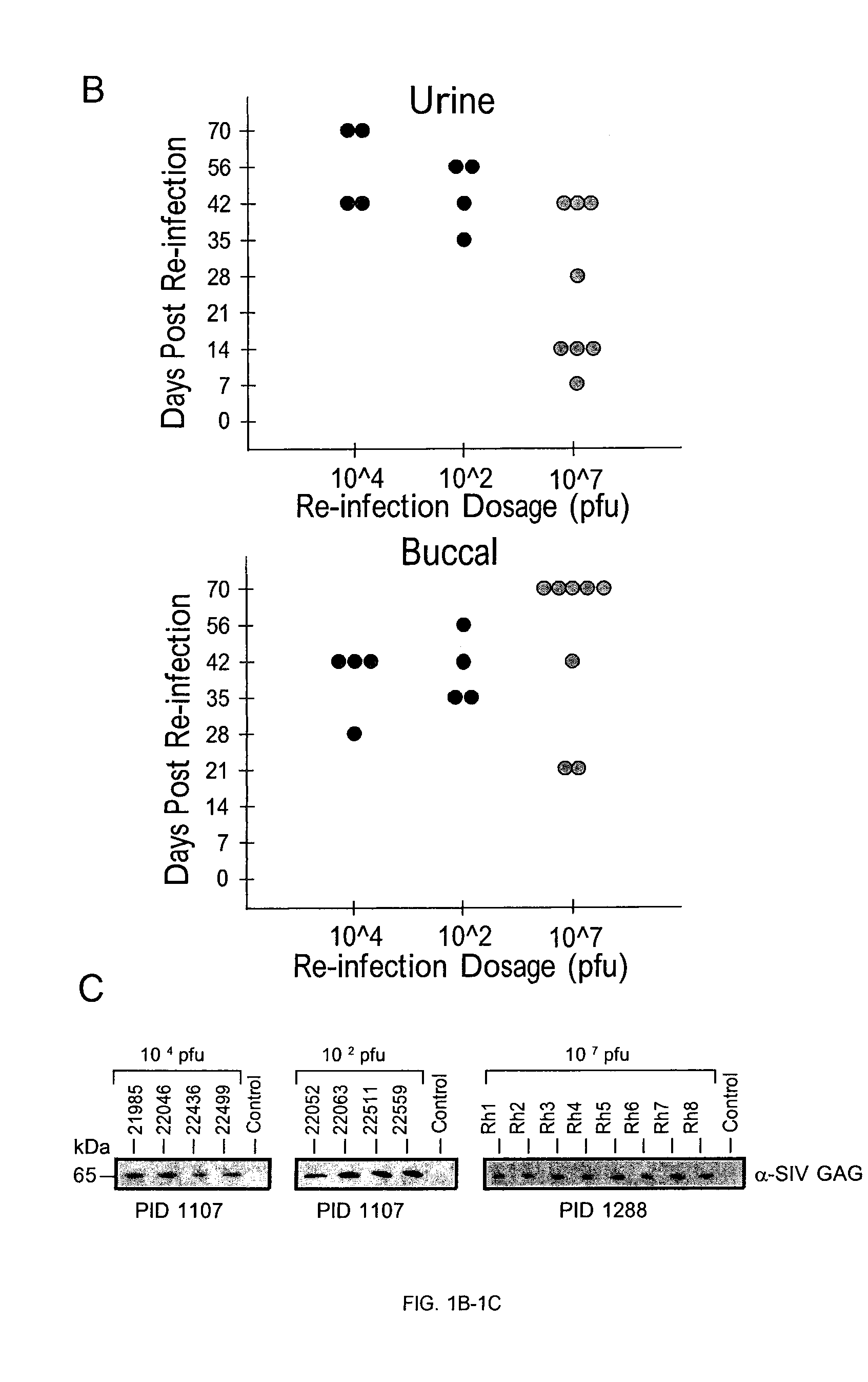
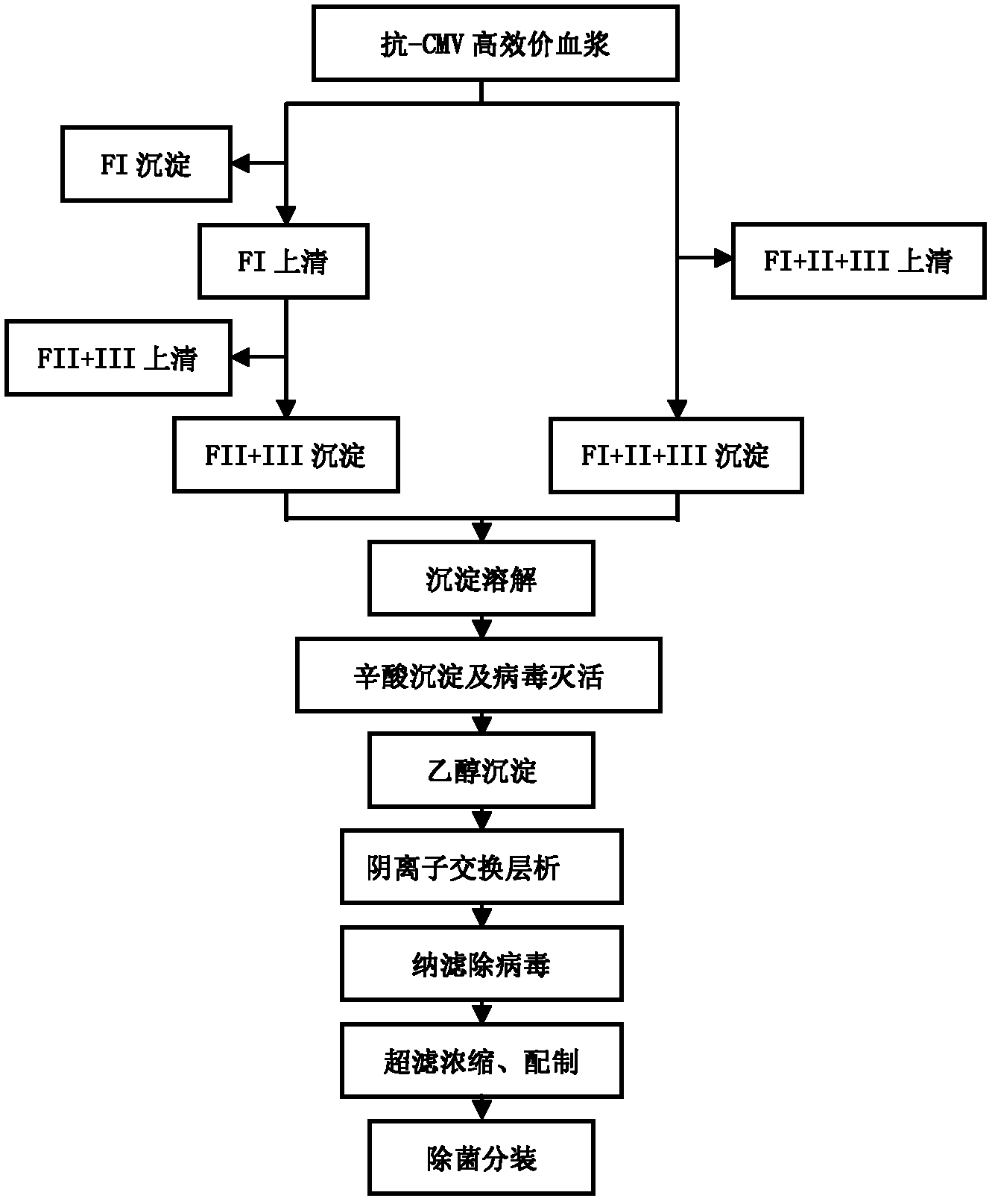
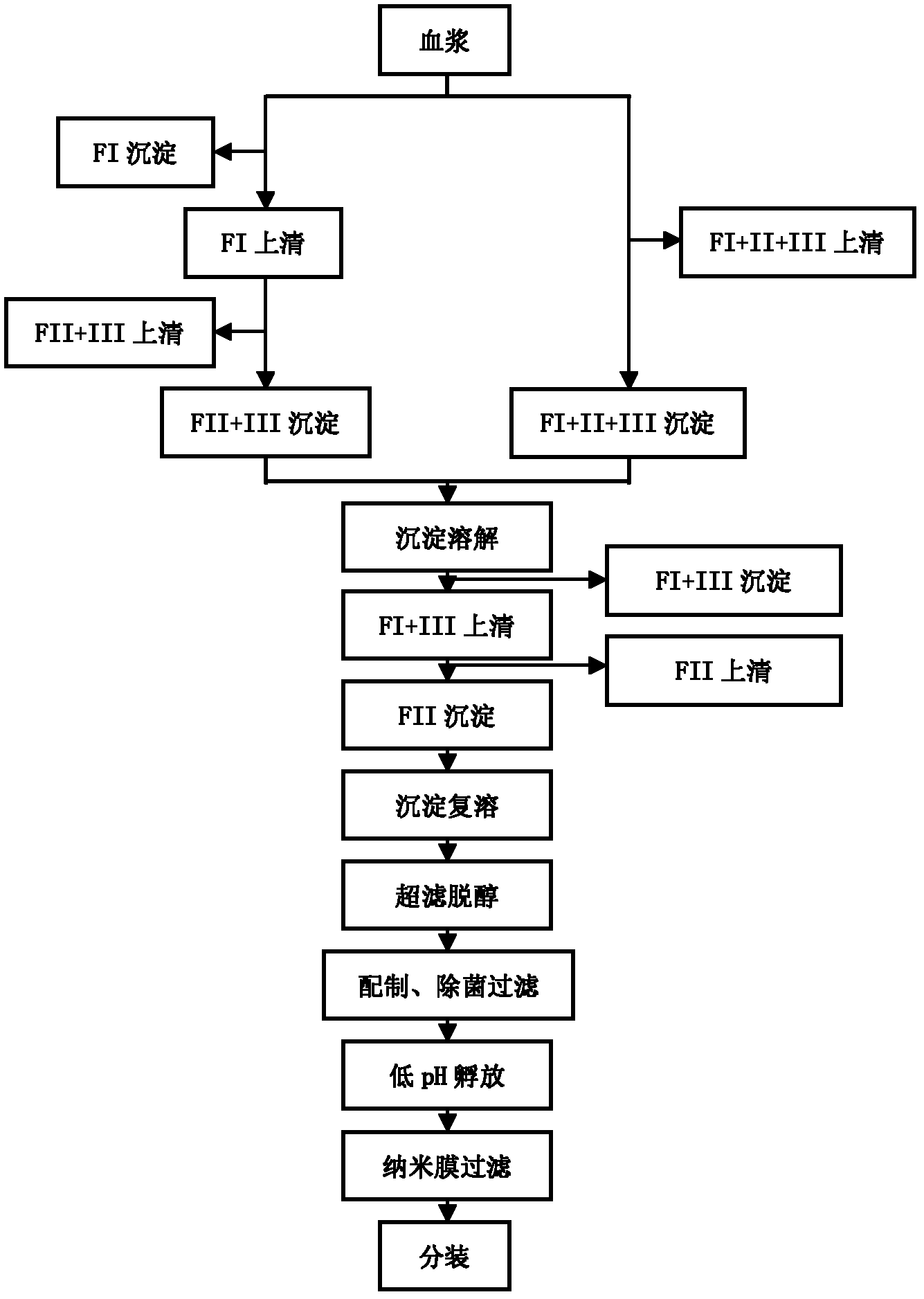
Intravenous injection of cytomegalovirus human immunoglobulin and its preparation method
ActiveCN102286099ASteps to reduce precipitationKeep aliveImmunoglobulins against virusesAntiviralsEthanol precipitationAnion-exchange chromatography
The invention discloses a human cytomegalovirus immunoglobulin for intravenous injection and a preparation method thereof, and aims to improve the purity, yield and safety of the product. In the invention, the specific activity of the human cytomegalovirus immunoglobulin for intravenous injection is not less than 2.5 PEI-U / mg, the anti-CMV titer is not less than 100 PEI-U / ml, the purity is greater than 98.2%, and the protein content is 51-55 mg / ml. Caprylic acid precipitation and anion exchange chromatography are used instead of the partial ethanol precipitation step in the traditional low-temperature ethanol method, thereby keeping IgG in the supernate all the time so as to keep the IgG activity; and processes of caprylic acid virus inactivation and nano film virus removal are used, thereby effectively ensuring the safety of the product. Researches show that the preparation method disclosed by the invention improves the purity, yield and safety of the product, saves the energy and reduces the production cost.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
Alpha virus-based cytomegalovirus vaccines
The present invention provides methods and compositions comprising a population of alphavirus replicon particles comprising alphavirus replicon RNAs, wherein a first replicon RNA comprises nucleic acid encoding cytomegalovirus pp65 and IE1 protein or immunogenic fragments thereof, and a second replicon RNA comprises nucleic acid encoding cytomegalovirus gB protein or an immunogenic fragment thereof, and wherein each of the two replicon RNAs is contained within a separate alphavirus replicon particle.
Owner:ALPHAVAX INC
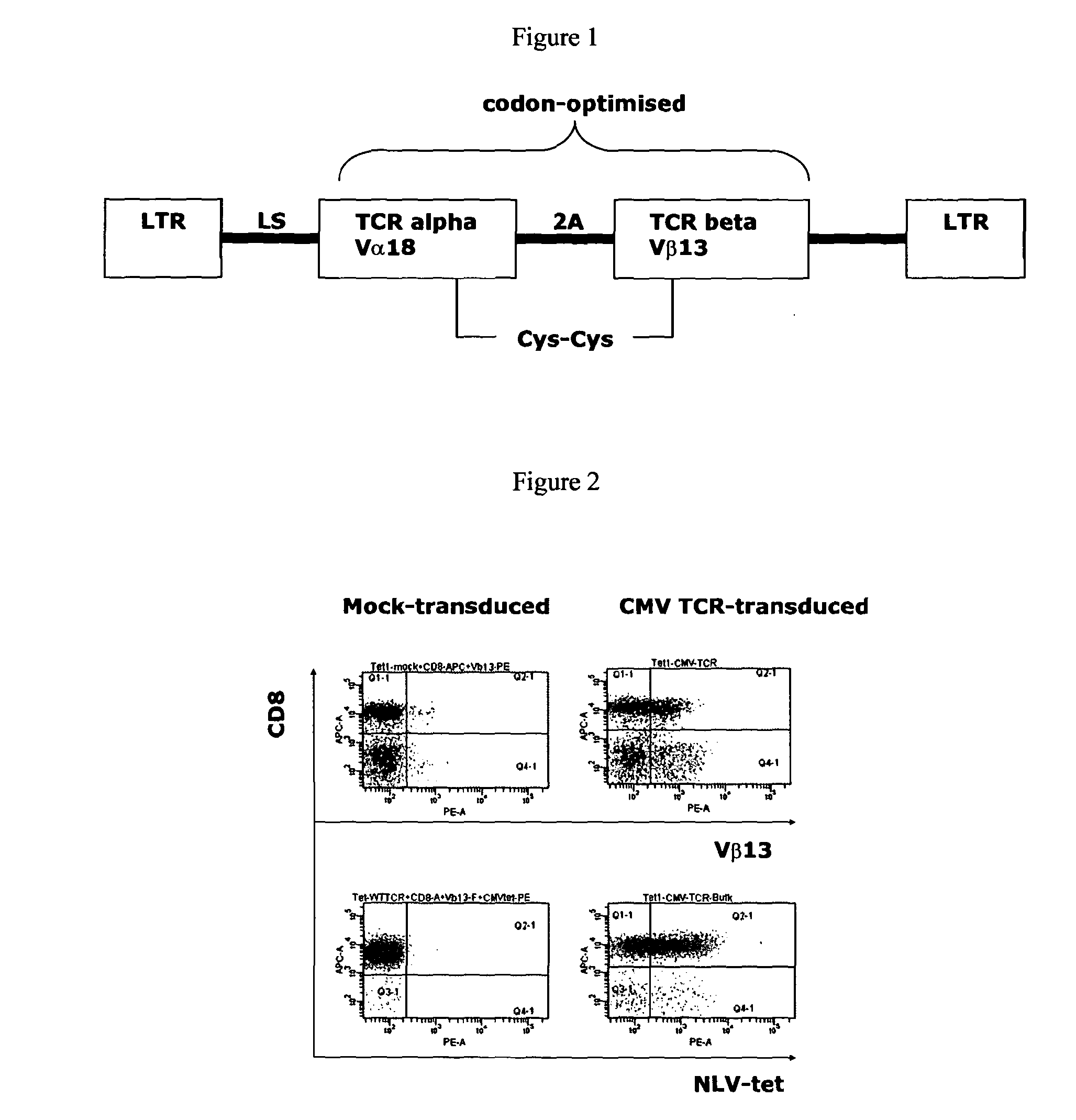
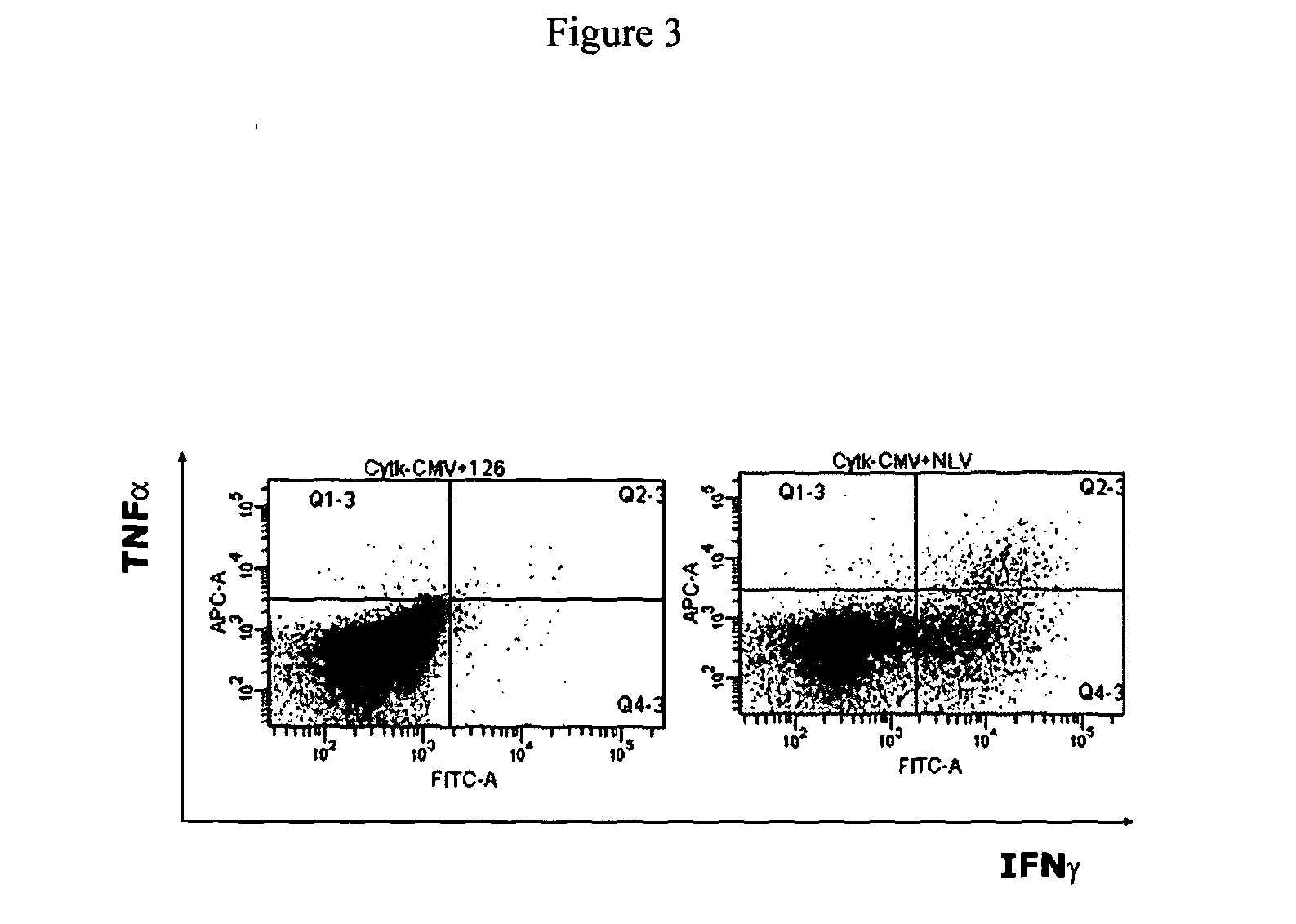
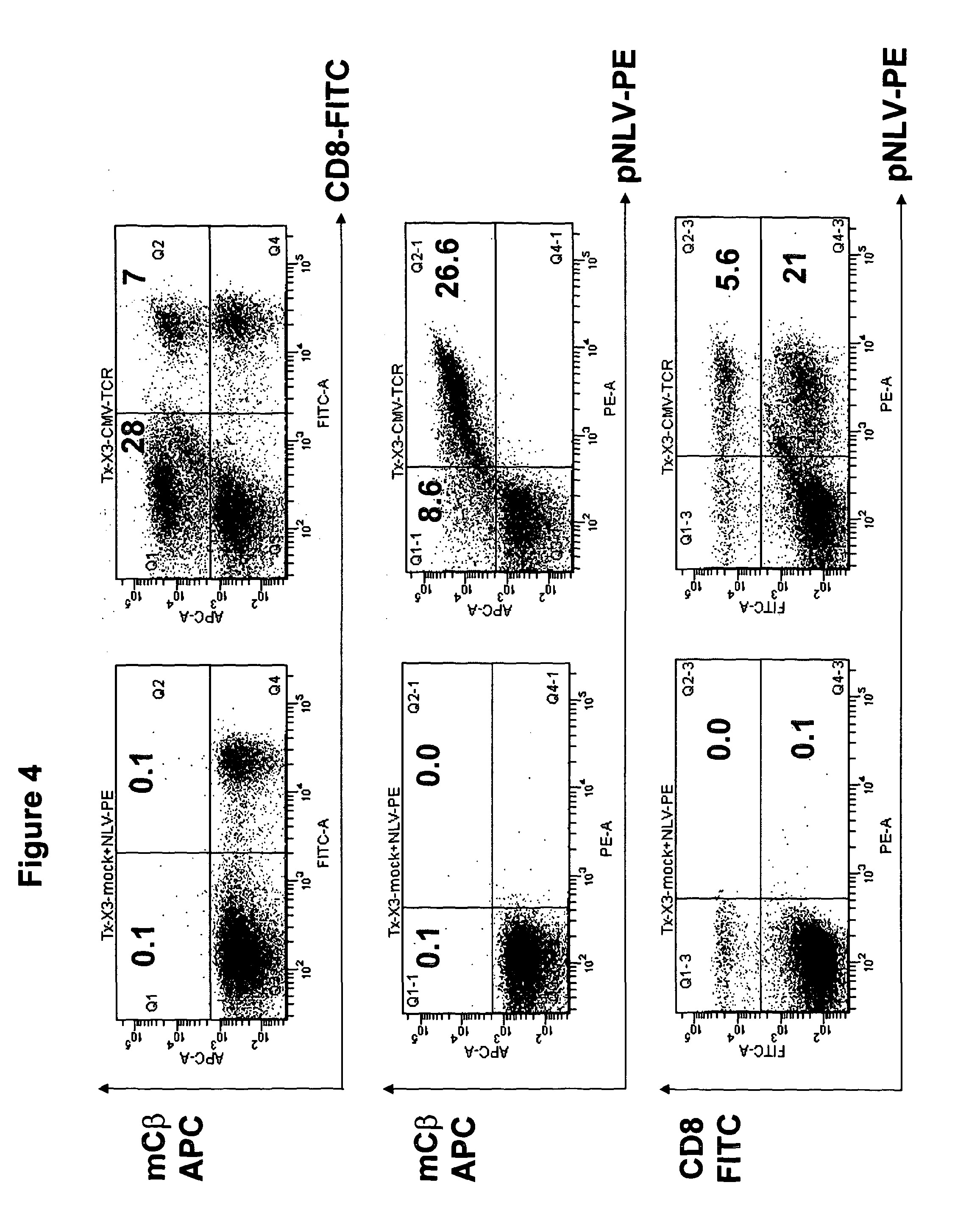
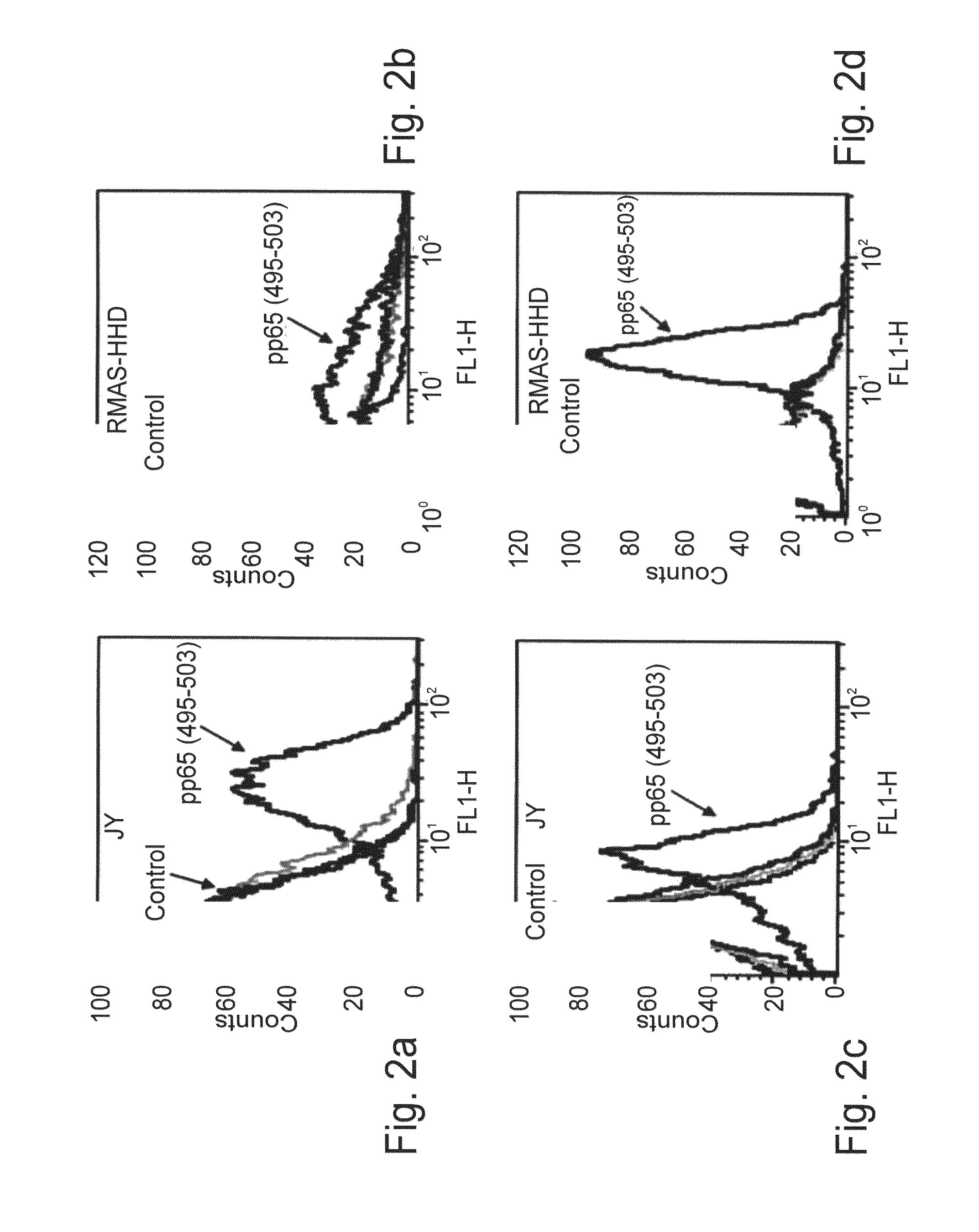
T-cell receptor capable of recognising an antigen from cytomegalovirus
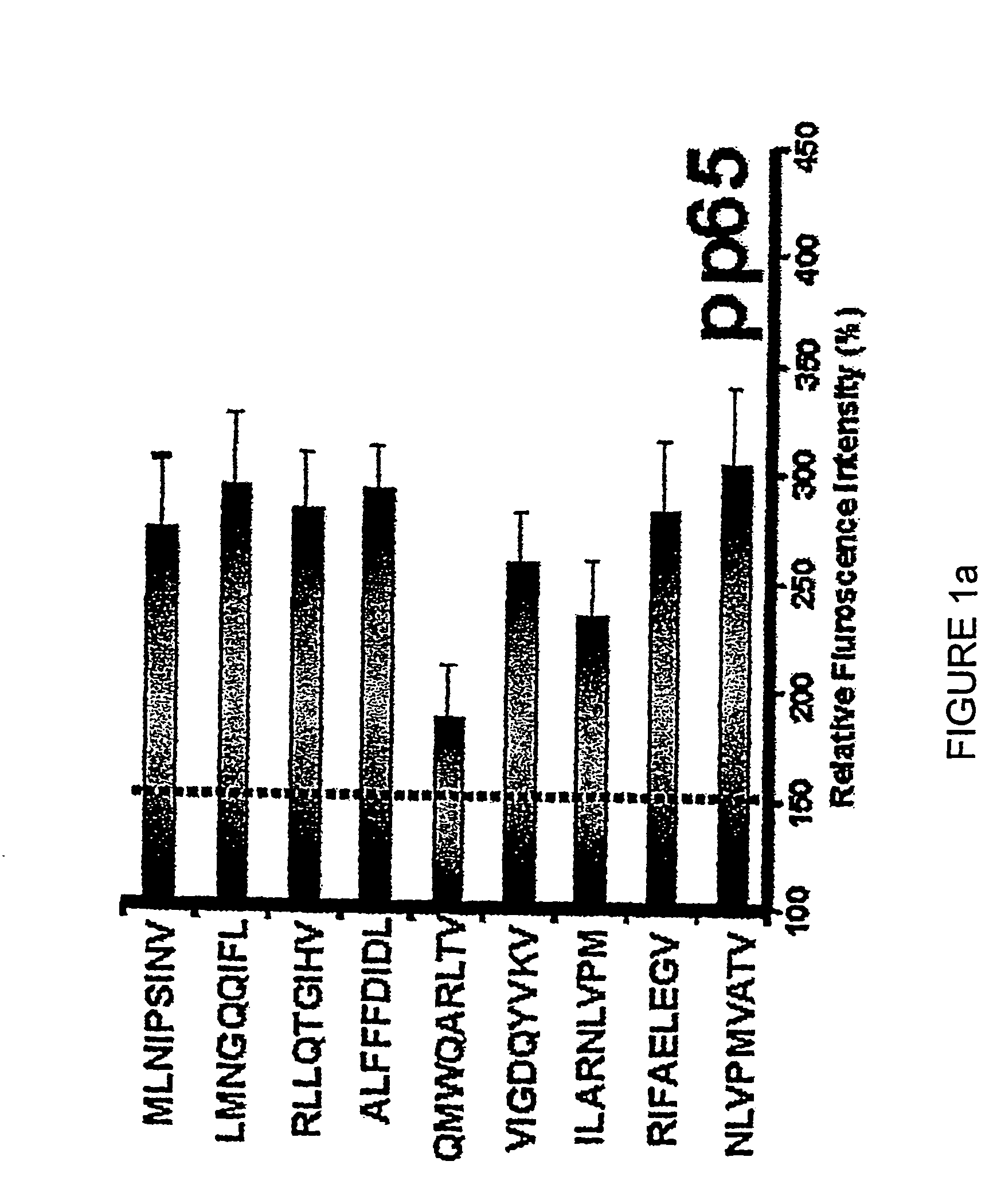
The present invention provides a T-cell receptor (TCR) which binds to a peptide from the cytomegalovirus (CMV) phosphoprotein pp65 having the amino acid sequence NLVPMVATV (SEQ ID No. 1) when presented by a major histocompatability complex (MHC) molecule. The present invention also provides a nucleotide sequence encoding such a TCR, a vector comprising such a nucleotide sequence and its use to produce a CMV-specific T-cell. The present invention also provides the use of CMV-specific T-cell for cellular immunotherapy.
Owner:UCL BUSINESS PLC
Antibodies and their uses for diagnosis and treatment of cytomegalovirus infection and associated diseases
InactiveUS8361473B2Microbiological testing/measurementAntibody ingredientsAnti-CMV AntibodyCytomegalovirus disease
Anti CMV antibodies are provided. Thus an antibody of the present invention comprises an antigen recognition domain capable of binding an MHC molecule being complexed with a cytomegalovirus (CMV) pp65 or pp64 peptide, wherein the antibody does not bind said MHC molecule in an absence of said complexed peptide, and wherein the antibody does not bind said peptide in an absence of said MHC molecule. Also provided are methods of using the antibodies.
Owner:TECHNION RES & DEV FOUND LTD
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
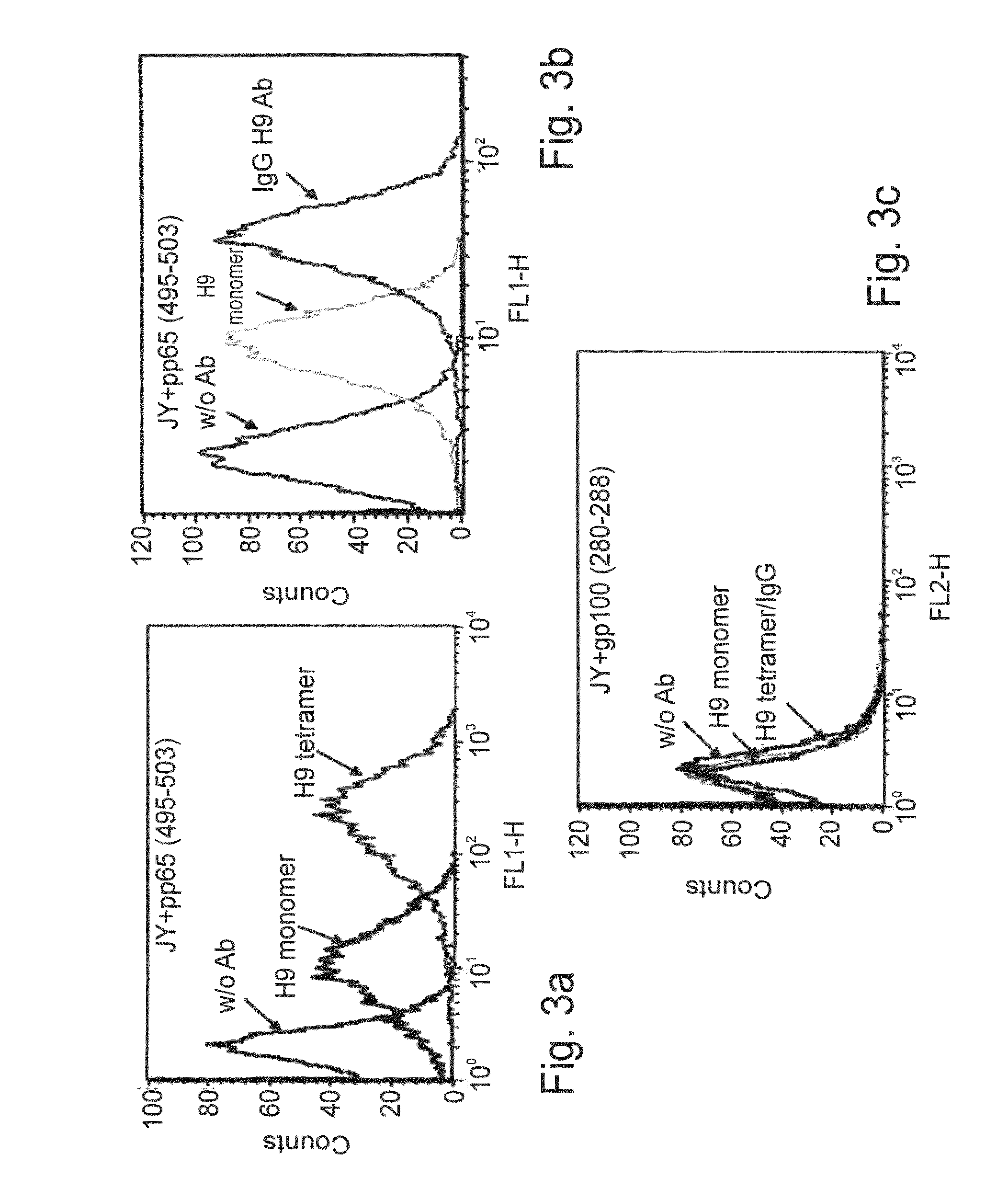
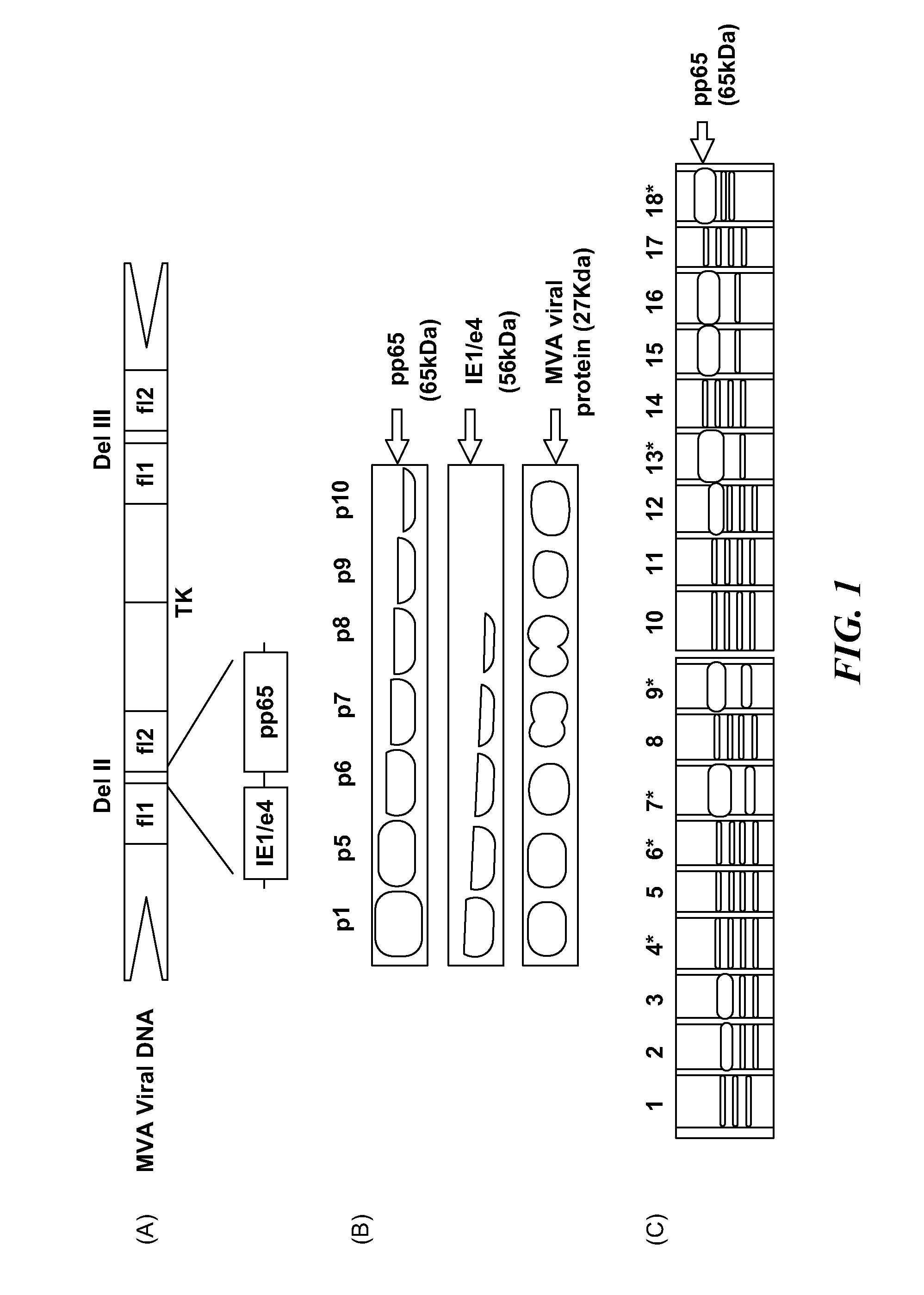
Human cytomegalovirus antigens expressed in MVA and methods of use
ActiveUS7163685B2Improve immunitySugar derivativesViral antigen ingredientsModified vaccinia AnkaraVaccine virus
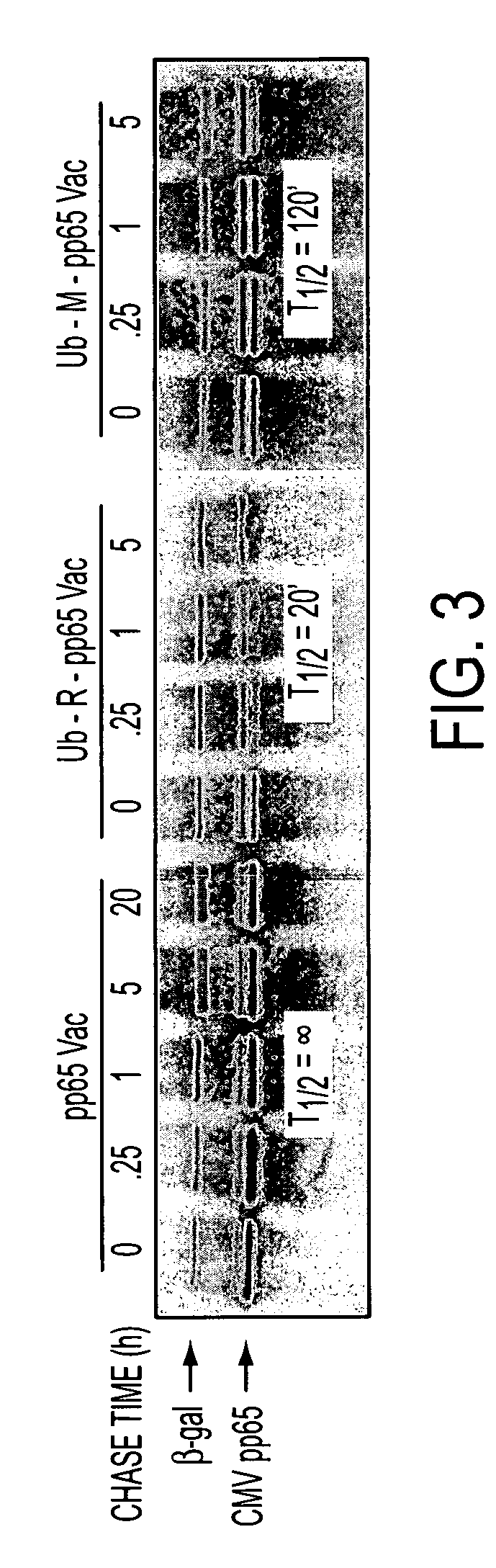
DNA and protein constructs useful in producing vaccines against human cytomegalovirus contain optionally N-end modified and N-terminal ubiquitinated human cytomegalovirus antigenic proteins, including pp65, pp150, IE1, gB and antigenic fragments thereof. Vaccine viruses, in particular poxviruses such as vaccinia and Modified Vaccinia Ankara, that express the constructs may be used as vaccines to augment the immune response to human cytomegalovirus, both prophylatically and in patients already carrying human cytomegalovirus, as well as to create and expand cytomegalovirus-reactive T cells for transfer of adoptive immunity.
Owner:CITY OF HOPE
Human cytomegalovirus (HCMV) cytotoxic T cell epitopes, polyepitopes compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS7524503B2Minimize difficultyReduce in quantityPeptide/protein ingredientsVirus peptidesCtl epitopeVaccination
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the most commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine compositions comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Substituted heterocyclic compounds and methods of use
The present invention relates to compounds having the general formula or a pharmaceutically acceptable salt thereof, wherein R1 is a saturated or unsaturated 5-, 6- or 7-membered, ring containing 0, 1, 2 or 3 atoms selected from N, O and S, wherein the ring may be fused with a benzo group, and is substituted by 0, 1 or 2 oxo groups, and wherein R1 is additionally substituted; and R2 is a substituted C1-6alkyl. Also included is a method of prophylaxis or treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com